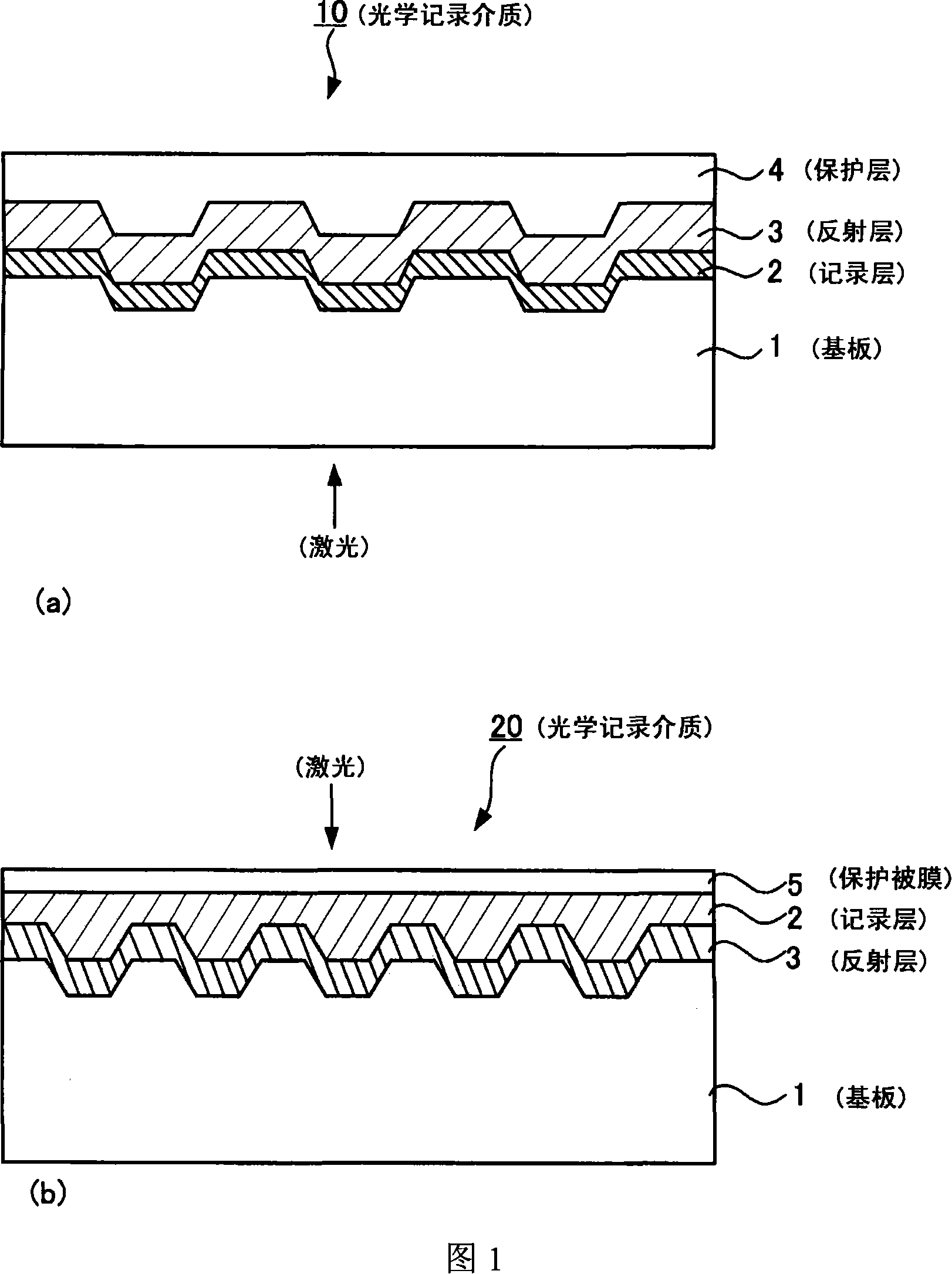
Optical recording medium, metal complex compound and organic dye compound
A technology of optical recording media and metal complexes, which is applied in the direction of nickel organic compounds, azo dye complex metal compounds, optical recording carriers, etc., can solve the problems of low reflectivity, inability to record and read, etc., and achieve high The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0234] (a-1)
[0235] With 22.63g (0.2 mol) of ethyl cyanoacetate represented by the following structural formula [1], 3-oxobutyric acid ethyl ester represented by the structural formula [2] of 26.03g (0.2 mol) and 14.63g (0.2 mol) The n-butylamine represented by the structural formula [3] of ) was dissolved in 20ml of ethanol, and after dripping 6ml of piperidine, half reflux was carried out under stirring conditions for 26 hours. After the reaction solution was cooled, it was stirred in 200 ml of 10% hydrochloric acid aqueous solution, and then a solid was precipitated. After the precipitated solid was filtered and washed with water, it was suspended in 100 ml of hexane solution, filtered after stirring for about 30 minutes, and the filtrate was heated and dried in vacuum to obtain 11.3 g (yield 27.4%) of the following compound [4 ] (1-n-butyl-3-cyano-4-methyl-6-hydroxy-2-pyridone).
[0236] Chemical formula 28
[0237]
[0238] Chemical formula 29
[0239]
[0240...
manufacture example
[0266] Suspend 18.61 g of the following citrazinic acid [7] in 500 ml of ethanol, drip 27 ml of concentrated sulfuric acid at room temperature under stirring, and then reflux for 4 hours. After the reaction solution was cooled, it was put into 1000 ml of water to precipitate solid. The precipitate was filtered, washed with water, and dried in vacuum to synthesize 14.88 g of the following compound [8] (67.7% yield).
[0267] Chemical formula 33
[0268]
[0269] Chemical formula 34
[0270]
[0271] Then, 3.5g (0.025mol) of 3-amino-5-tert-butylisoxazole represented by the following structural formula [9] was stirred and dissolved in 25ml of acetic acid, 8.3ml of propionic acid, and 1ml of concentrated sulfuric acid solution, and cooled to 0~ 5°C. 8.85g of 43% nitrosyl sulfuric acid was dropped therein to keep the temperature below 10°C for diazotization to prepare a diazonium solution.
[0272] Chemical formula 35
[0273]
[0274] On the other hand, in another co...
Embodiment 3~ Embodiment 16
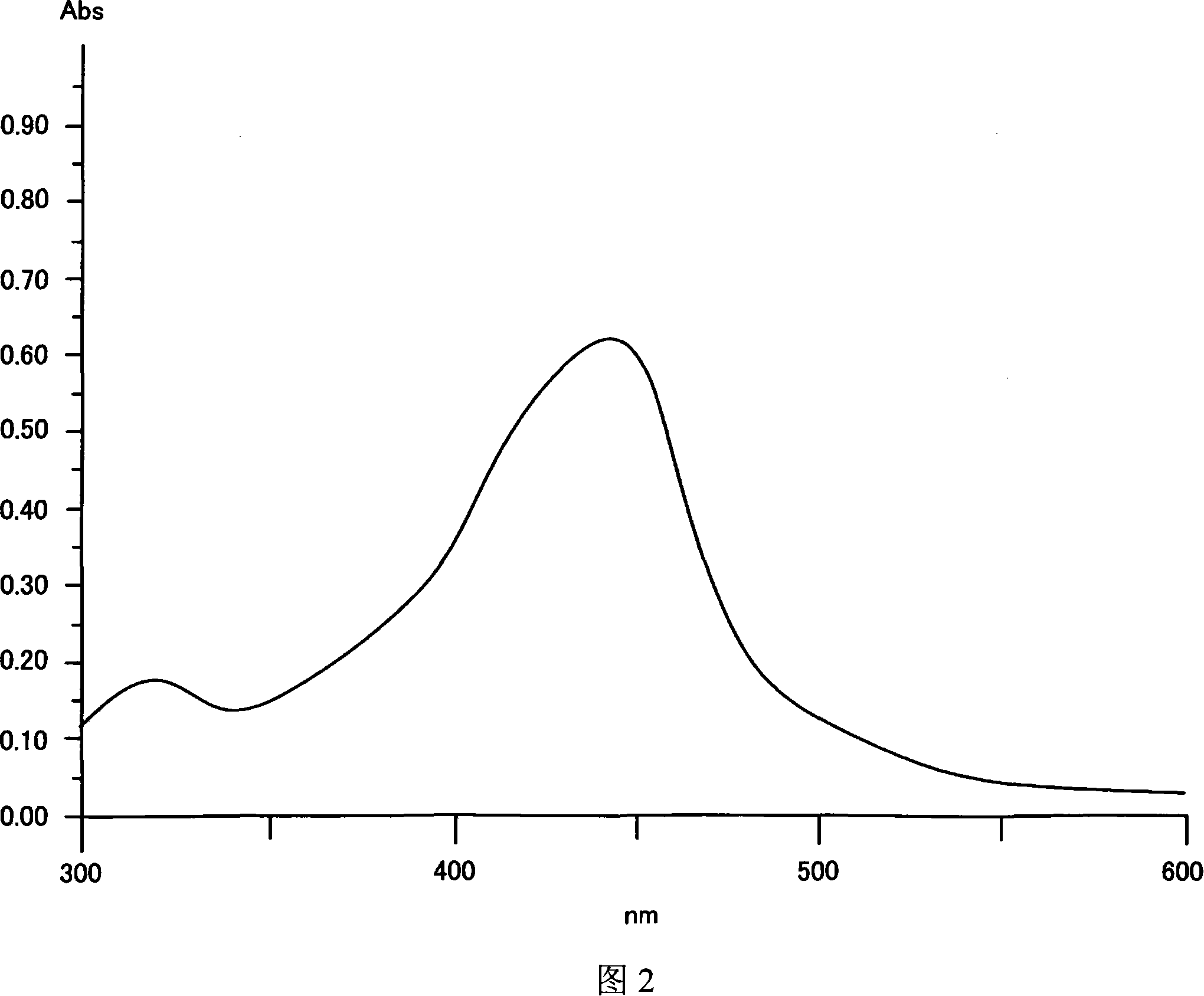
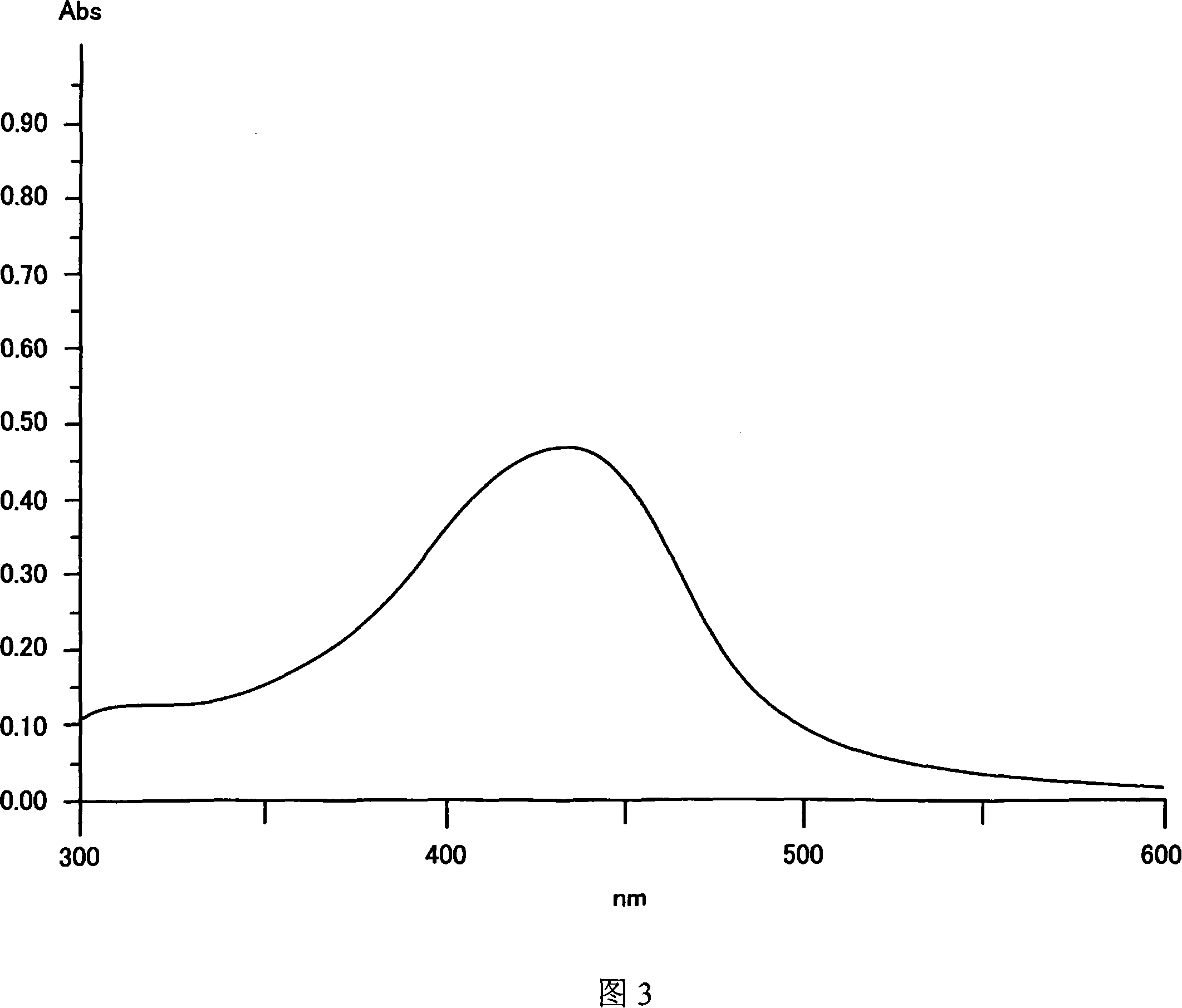
[0291] Next, metal complex (60) to metal complex (73) were synthesized by the same method as the above-mentioned synthesis method, and a coating film was formed in the same manner as in Example 1, and the absorption spectrum of the coating film was measured. The maximum absorption wavelength (λmax) in solution (chloroform), the molar absorptivity, and the maximum absorption wavelength (λmax) of the coating film of these compounds were measured (octafluoro-n-pentanol was used as the coating solvent).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com