Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
228 results about "Ethyl cyanoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
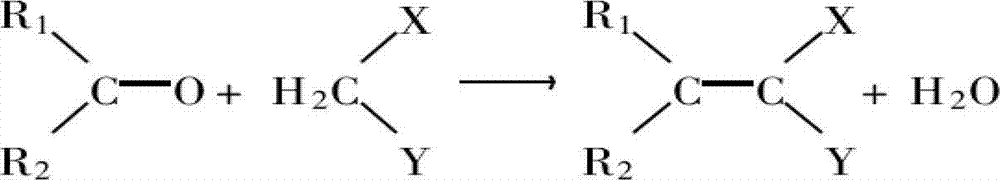
Ethyl cyanoacetate is an organic compound that contains a carboxylate ester and a nitrile. It is a colourless liquid with a pleasant odor. This material is useful as a starting material for synthesis due to its variety of functional groups and chemical reactivity.
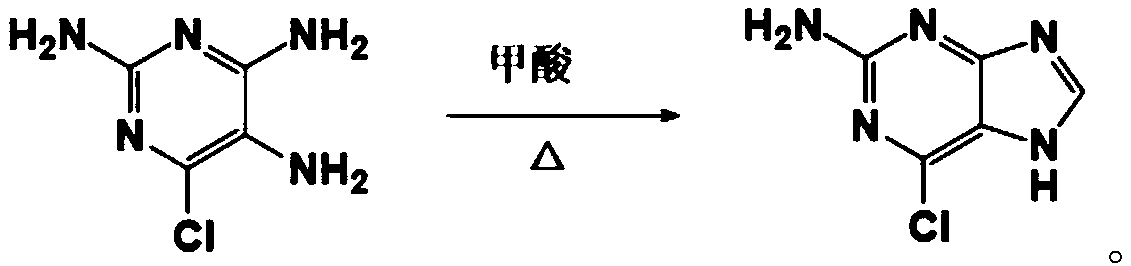
Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN104860950AHigh puritySolve the problem of complicated and refined synthesisOrganic chemistryAcetic acidAlcohol
The invention discloses a novel method used for preparing high-purity 4-chloropyrrolo[2,3-d]pyrimidine in industrialization scale. According to the method, ethyl cyanoacetate, bromo-acetal, formamidine acetate, and sodium alcoholate are taken as raw materials; N,N-dimethylformamide, ethyl alcohol, and phosphorus oxychloride are taken as solvents; and 4-chloropyrrolo[2,3-d]pyrimidine is obtained via three-step approach. The process route of the method is simple; time is short; next step feed can be carried out directly without complex purification process of intermediates; no toxic agent is used; the method is safe and reliable, and is low in cost; total yield is higher than 30%; and the purity of obtained products is as high as 99.2%.
Owner:CHONGQING PHARMA RES INST
Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN105622616AIncrease productivityImprove product qualityOrganic chemistryCyanoacetic acidFormamidine acetate
The invention relates to a preparation method of 4-chloropyrrolo[2,3-d]pyrimidine.The method includes the following steps of obtaining 2-cyano-3-(1,3-dioxolan)ethyl propionate after 2-bromomethyl-1,3-dioxolane and ethyl cyanoacetate which are used as the raw materials react with alkaline matter as the catalyst; conducting cyclization on obtained 2-cyano-3-(1,3-dioxolan)ethyl propionate and formamidine acetate with alkaline matter as the catalyst, and adding hydrochloric acid for hydrolysis cyclization to obtain pyrrolo[2,3-d]pyrimidin-4-ol; making obtained pyrrolo[2,3-d]pyrimidin-4-ol react with phosphorus oxychloride to obtain 4-chloropyrrolo[2,3-d]pyrimidine.The method for preparing 4-chloropyrrolo[2,3-d]pyrimidine is simple in technological process, the requirement for production conditions is low, the product is easy to purify and high in yield, and the production efficiency and product quality of 4-chloropyrrolo[2,3-d]pyrimidine are remarkably improved.
Owner:ABA CHEM SHANGHAI
Organic solid base catalyst for synthesizing alpha-cyanoethyl cinnamate, and preparation method and application thereof
InactiveCN102728403ANot easy to polluteEasy to prepareCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeEthyl cyanoacetate
The invention discloses an organic solid base catalyst for synthesizing alpha-cyanoethyl cinnamate. The preparation method of the organic solid base catalyst for synthesizing alpha-cyanoethyl cinnamate comprises the following steps: by using chloropropyltrimethoxysilane as a coupling agent and cyclohexane as a solvent, grafting an N-butylimidazole basic ionic liquid onto a silica gel supporter by ultrasonic technology, thereby obtaining the organic solid base catalyst for synthesizing alpha-cyanoethyl cinnamate. The method for synthesizing alpha-cyanoethyl cinnamate by using the catalyst comprises the following steps: evenly mixing benzaldehyde and ethyl cyanoacetate in a mol ratio of 1:(0.9-1.1), adding the organic solid base catalyst which accounts for 2 wt% of the reactants into the reaction kettle, heating to 80-110 DEG C while stirring to react for 5-12 hours, and centrifugally settling to obtain the product, wherein the organic solid base catalyst can be recycled and used repeatedly. The catalyst disclosed by the invention has high catalytic activity and can be used repeatedly.
Owner:SHIJIAZHUANG UNIVERSITY
Method for preparing solid base catalyst with high specific surface by hybrid composite precursors
InactiveCN101554596APore Structure RegulationControl areaCarboxylic acid nitrile preparationOrganic compound preparationAcetic acidBenzaldehyde
Owner:BEIJING UNIV OF CHEM TECH
Process of one-step hydrogenation synthesis of alpha-substd. beta-amino acid
InactiveCN1435408AEasy to operateOrganic compound preparationAmino-carboxyl compound preparationSodium acetateAcetic anhydride
A one-step hydrogenating process for synthesizing beta-amino acid with substituent at its alpha position includes proportionally feeding aldehyde and ethyl cyanoacetate into flask, adding absolute alcohol, stirring while adding dropwise diethylamine, standing for 3-24 hr, adding water, stirring, filtering, drying, adding Raney Ni, acetic anhydride and sodium acetate, hydrogenating at 50-80 deg.C and 5-8 MPa for 16-24 hr, filtering to remove Raney Ni, evaporating out solvent, adding concentrated sulfuric acid, reflux for 0-16 hr, neutralizing with solution of sodium hydroxide, washing and drying.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Technology of synthesizing 2,3-dicyano ethyl propionate
InactiveCN1785966AHigh yieldImprove product qualityPreparation by cyanide reactionParaformaldehydeEthyl fumarate
The present invention relates to a process for synthesizing ethyl-2,3-dicyano-propionate which is used for produce pesticide intermediate. The basic method of said process includes the following steps: using the raw materials of ethyl cyanoacetate, paraformaldehyde and sodium cyanide to synthesize ethyl-2,3-dicyano-propionate in the medium dimethyl sulfoxide; after the ethyl-2,3-dicyano-propionate is synthesized, using solvent dichloromethane to extract the synthesized ethyl-2,3-dicyano-propionate from medium dimethyl sulfoxide, reduced pressure desolventizing to obtain crude product, then rectifying said crude product so as to obtain the refined product ethyl-2,3-cyano-propionate. Said invention also provides the concrete requirements of the above-mentioned every step. The purity of the obtained product is greater than 98%.
Owner:栾忠岳
Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN101830904AShort process routeShorten the production cycleOrganic chemistryChemical synthesisSodium acetate
The invention provides a novel method for synthesizing 4-chloropyrrolo[2,3-d]pyrimidine, belonging to the technical field of chemical synthesis. The method comprises the steps of carrying out four-step reaction by using ethyl cyanoacetate, thiourea, caustic alcohol, 2-chloroacetaldehyde and sodium acetate as raw materials and using alcohol, ammonia water, water and phosphorus oxychloride as solvents as well as using active nickel as a catalyst to obtain the 4-chloropyrrolo[2,3-d]pyrimidine as a target product; and carrying out data representation by liquid chromatogram, nuclear magnetism spectrogram and mass spectrum. The invention has short process route and production cycle, low synthesis cost, safe and reliable synthesis and simple and convenient posttreatment method, and avoids the use of dangerous goods of sodium hydride. The product prepared by the method has high yield (50-58 percent) and high purity (98-99 percent).
Owner:兰州五龙新能源科技有限公司
Preparation method of Cangrelor intermediate
InactiveCN106008632AReduce processing costsLow costSugar derivativesSugar derivatives preparationThioureaNitration
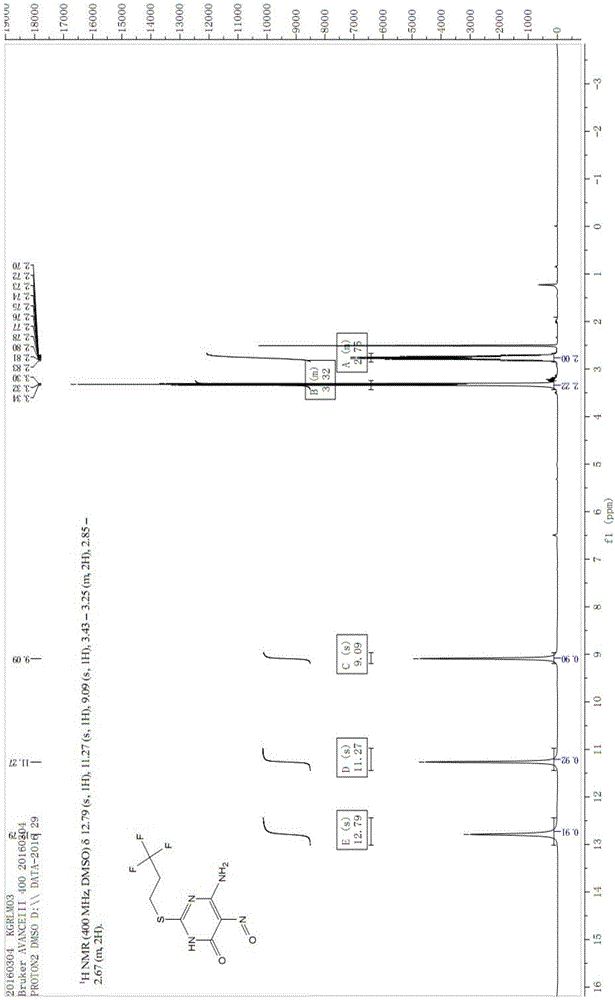
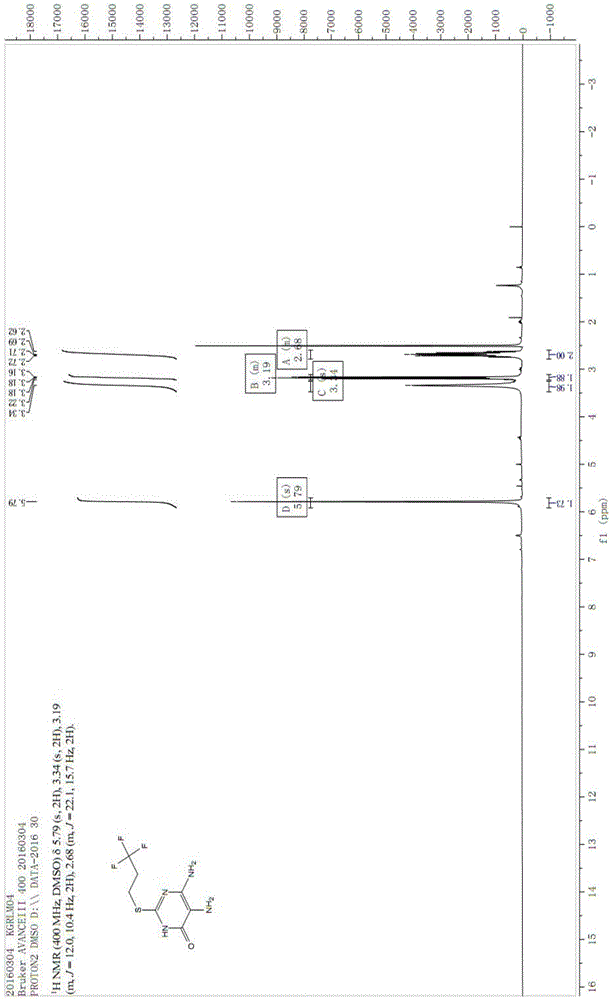
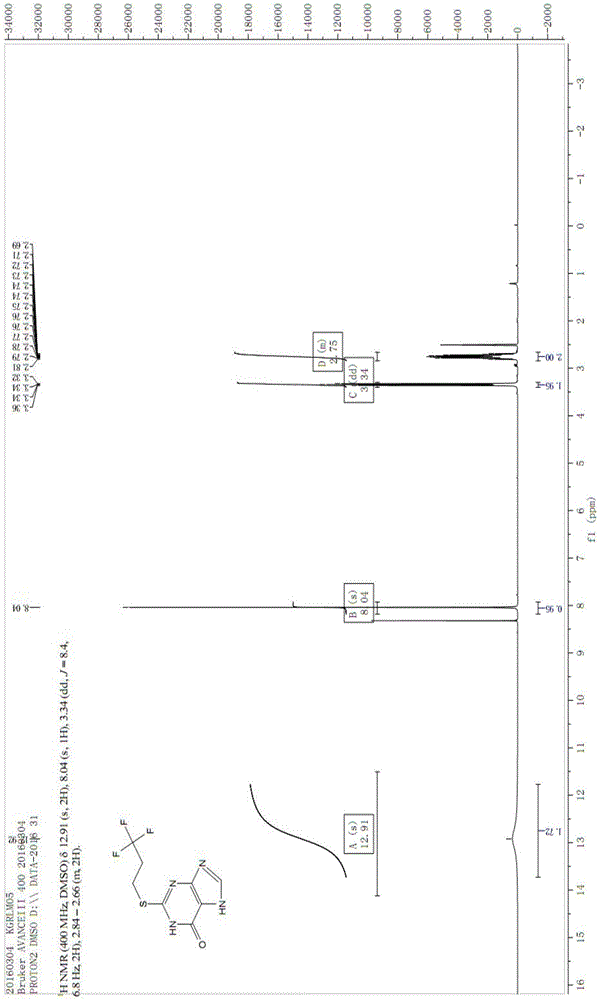
The invention discloses a preparation method of a Cangrelor intermediate. The preparation method comprises the following steps of enabling ethyl cyanoacetate and thiourea to perform closed-loop reaction to generate a product under the alkaline condition, reacting the product and trifluoropropane under the alkaline condition, performing nitration reaction and reduction reaction, and performing closed-loop reaction with formic acid; chlorinating the generated product, and performing condensation reaction on the product and 2-(thiomethyl)ethylamine under the alkaline condition; reacting the obtained product and 1,2,3,5-tetraacetyl-beta-D-ribofuranose under the actions of alkylating agent and TMSOTF (trimethylsilyl trifluoromethanesulfonate), and hydrolyzing the product under the alkaline condition, so as to obtain the Cangrelor intermediate. The preparation method has the advantages that the silica-gel column chromatography is not needed, so that the technology cost is greatly reduced; the carbon disulfide, fuming nitric acid or concentrated sulfuric acid is not used in the preparation process, so that any danger is avoided; the noble metal hydrogenating reducing agent is not needed, so that the cost is reduced, and the operation danger is decreased; the operation is easy, safe and reliable, and the preparation method is suitable for large-scale industrial production.
Owner:北京信益泰医药科技开发有限公司
Preparation method of carbon nitride nanosheet catalyst and Knoevenagel condensation reaction method based on catalyst
InactiveCN107824211ARaw materials are easy to getLow reaction temperatureCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeSolvent
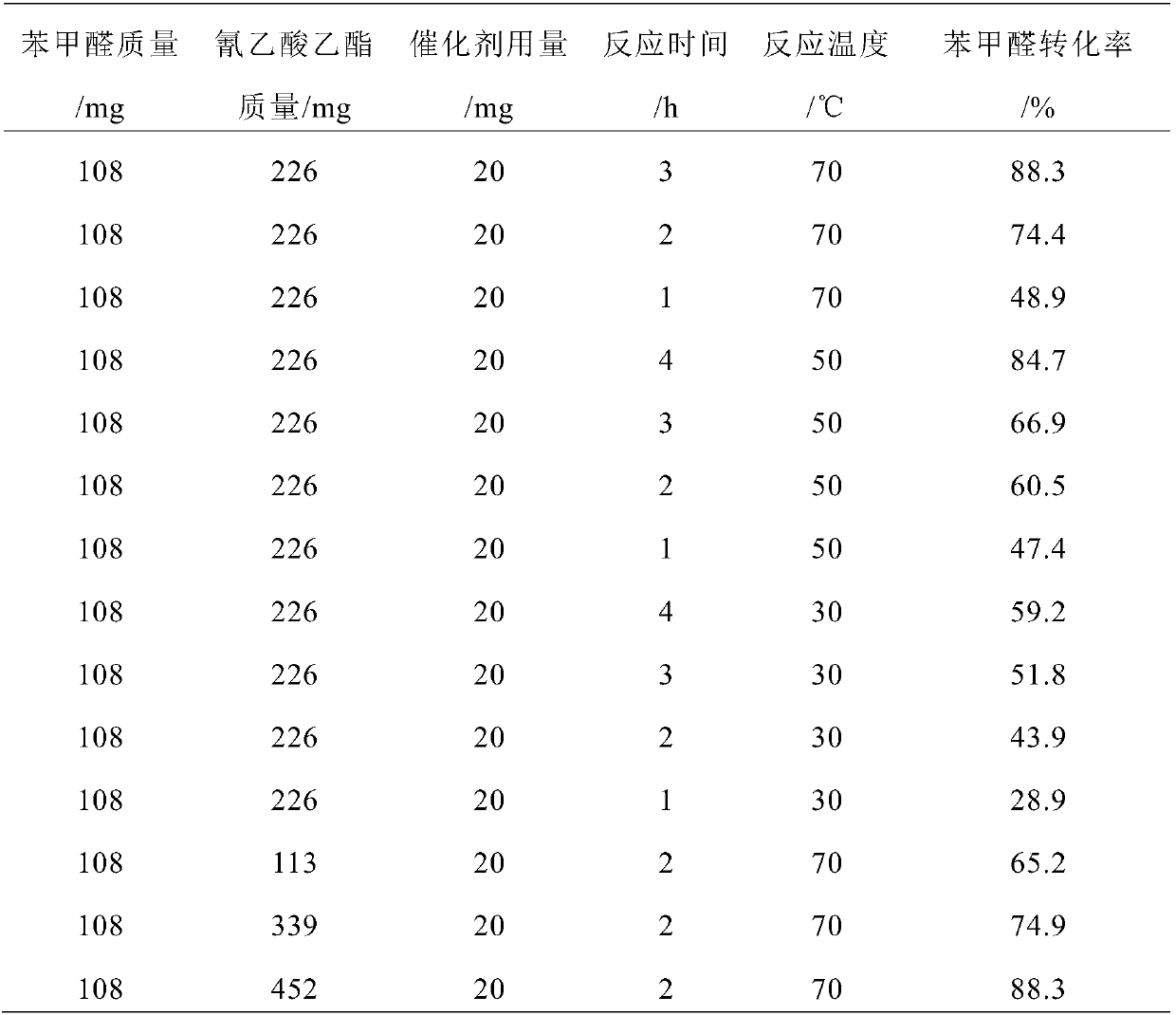
The invention discloses a preparation method of a carbon nitride nanosheet catalyst. The preparation method comprises mixing a chloride salt, a carbon nitrogen source and a solvent in a mass ratio of(0.1-28):(2-6):(100-500), and performing uniform stirring to form a mixture; removing the solvent in the mixture, performing drying and roasting, performing repeated water washing on the roasted solid, performing separation and drying to obtain the carbon nitride nanosheet catalyst. The invention also discloses a condensation reaction method by using the carbon nitride nanosheet catalyst. According to the invention, when the carbon nitride nanosheet is prepared, the reactants are thoroughly mixed in an aqueous solution, and after one-step pyrolysis, the metal cations and chloride ions in the chloride salts destroy the acting force between layers of the laminar carbon nitride to achieve thermal stripping and further to form laminar carbon nitride nanosheets, thereby improving the catalyticperformance. When the catalyst is applied to a condensation reaction of benzaldehyde and ethyl cyanoacetate, the temperature can be as low as 30 DEG C, and the required longest reaction time is 4 h.
Owner:HUBEI UNIV
Malononitrile synthesis method
InactiveCN104945278AReduce dosageReduce pollutionPreparation by carboxylic acid amide dehydrationFiltrationSynthesis methods
The invention relates to a malononitrile synthesis method and belongs to the technical field of malononitrile synthesis. The malononitrile synthesis method comprises the following steps of 1, adding methanol or ethanol into a reactor, feeding ammonia gas into the reactor at a temperature of -20 to 50 DEG C, dropwisely adding methyl cyanoacetate or ethyl cyanoacetate into the reactor at a temperature of -20 to 50 DEG C, after dropwise addition, carrying out heat-preservation stirring, carrying out reduced pressure degasification until a temperature is reduced to below -10 DEG C and carrying out filtration and drying to obtain cyanoacetamide, and 2, mixing a dehydrant, a catalyst, cyanoacetamide and a solvent, heating the mixture for a reflux reaction lasting for 6h, carrying out cooling and filtration, removing the solvent to obtain a crude product, and carrying out reduced pressure rectification to obtain a malononitrile product. The preparation method has less reaction processes, can be operated simply, has a high yield and good product purity, utilizes cheap and easily available raw materials, greatly reduces a production cost, is free of an adsorbent, has a less phosphorous oxychloride use amount, greatly reduces three wastes, realizes easy recovery and recycle of the solvent, is suitable for industrial production and solves the problems of the existing a malononitrile synthesis method.
Owner:JINGZHOU HELE IND SCI & TECH CO LTD
Synthesis technique of alpha-cyanoacrylate monomer
ActiveCN102603564AHigh yieldSave energyCarboxylic acid nitrile preparationOrganic compound preparationHexahydropyridineCyanoacetic acid
The invention belongs to the field of adhesives, and particularly relates to a synthesis technique of an alpha-cyanoacrylate monomer, which comprises the following steps: (1) directly reacting cyanoacetate and polyformaldehyde, which are used as raw materials, under solventless conditions by using a basic catalyst; (2) naturally cooling, keeping the temperature, and dehydrating to obtain the crude product; and (3) in the presence of a polymerization inhibitor, preparing the alpha-cyanoacrylate monomer, wherein the basic catalyst in the step (1) is one or mixture of hexahydropyridine, pyridine and KOH, the cyanoacetate is one or mixture of methyl cyanoacetate, ethyl cyanoacetate and n-butyl cyanoacetate, the polymerization inhibitor in the step (3) is hydroquinone and sulfur dioxide, a dehydrating agent is used for dehydration in the step (2); and the dehydrating agent is one or mixture of benzene, methylbenzene and methanol. The reaction process has the advantages of no need of organic solvent, low energy consumption, obvious environmental protection effect and high target product yield.
Owner:GLEIHOW NEW MATERIALS CO LTD
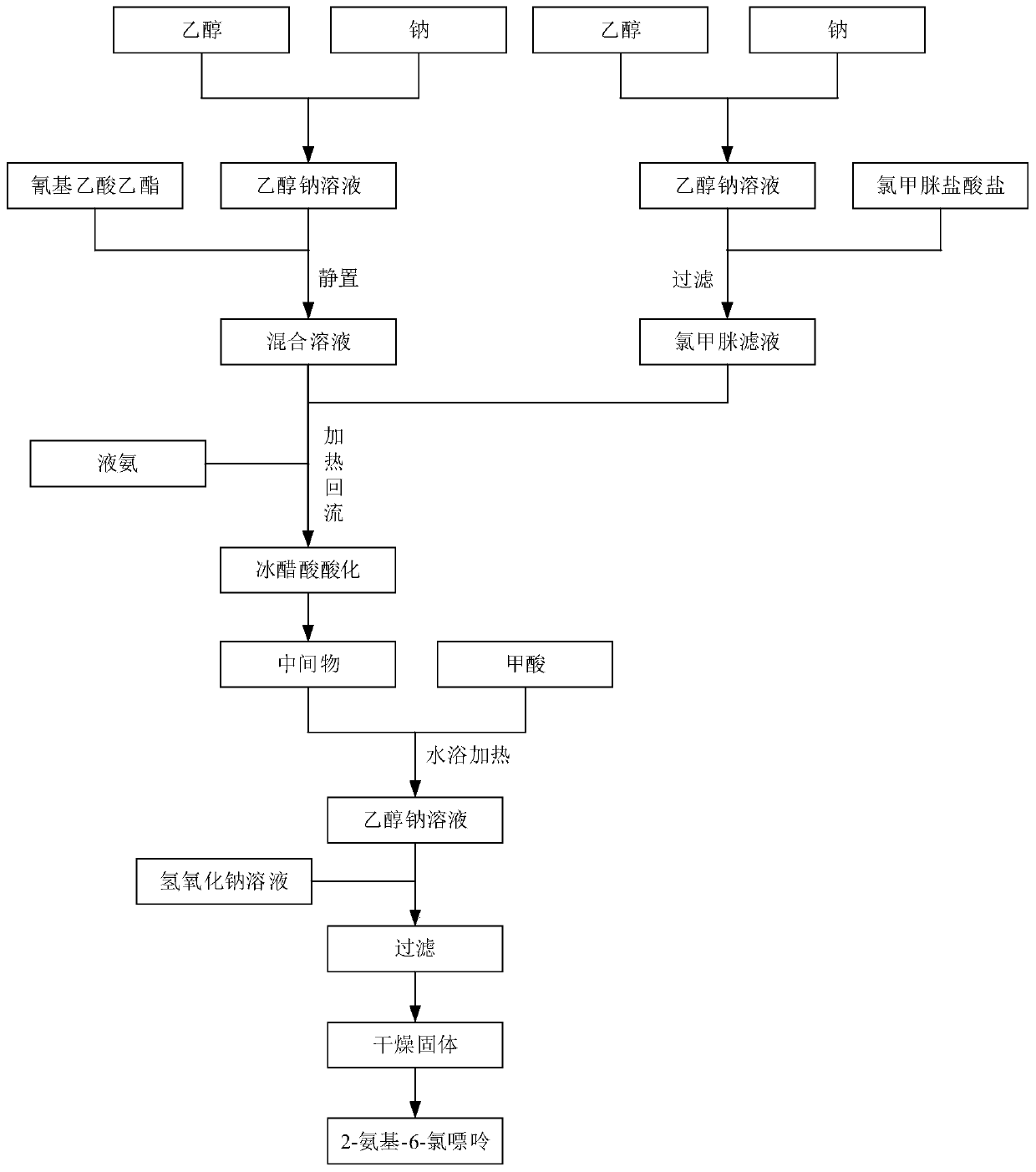
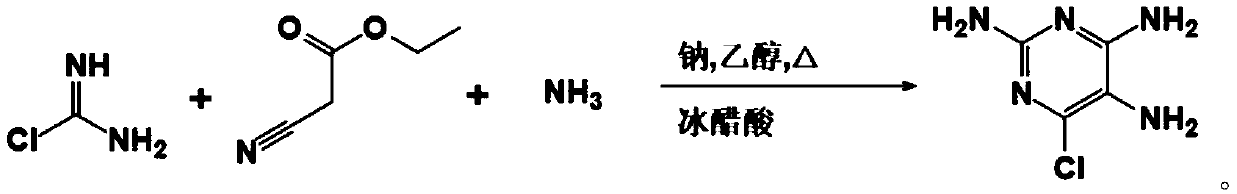
2-amino-6-chloropurine as well as synthesis method, intermediate and application thereof
ActiveCN110627729ANothing producedRaw materials are cheap and easy to getOrganic chemistrySynthesis methods2-amino-6-chloropurine
The invention belongs to the technical field of medicines and in particular relates to 2-amino-6-chloropurine as well as a synthesis method, an intermediate and application thereof. The synthesis method of the 2-amino-6-chloropurine comprises the following steps: performing a primary reaction, namely mixing ethyl cyanoacetate, chloroformamidine hydrochloride, liquid ammonia and sodium to implementa reaction so as to generate an intermediate; performing a secondary reaction, namely mixing the intermediate with formic acid to implement a reaction; adding a sodium hydroxide solution into the product of the secondary reaction till alkali; and performing filtering and drying, so as to obtain 2-amino-6-chloropurine. Raw materials are low in price and easy to obtain, reaction steps are small, the reaction time is short, a large amount of wastewater is not generated, and industrial production can be facilitated.
Owner:NANJING REDWOOD FINE CHEM CO LTD
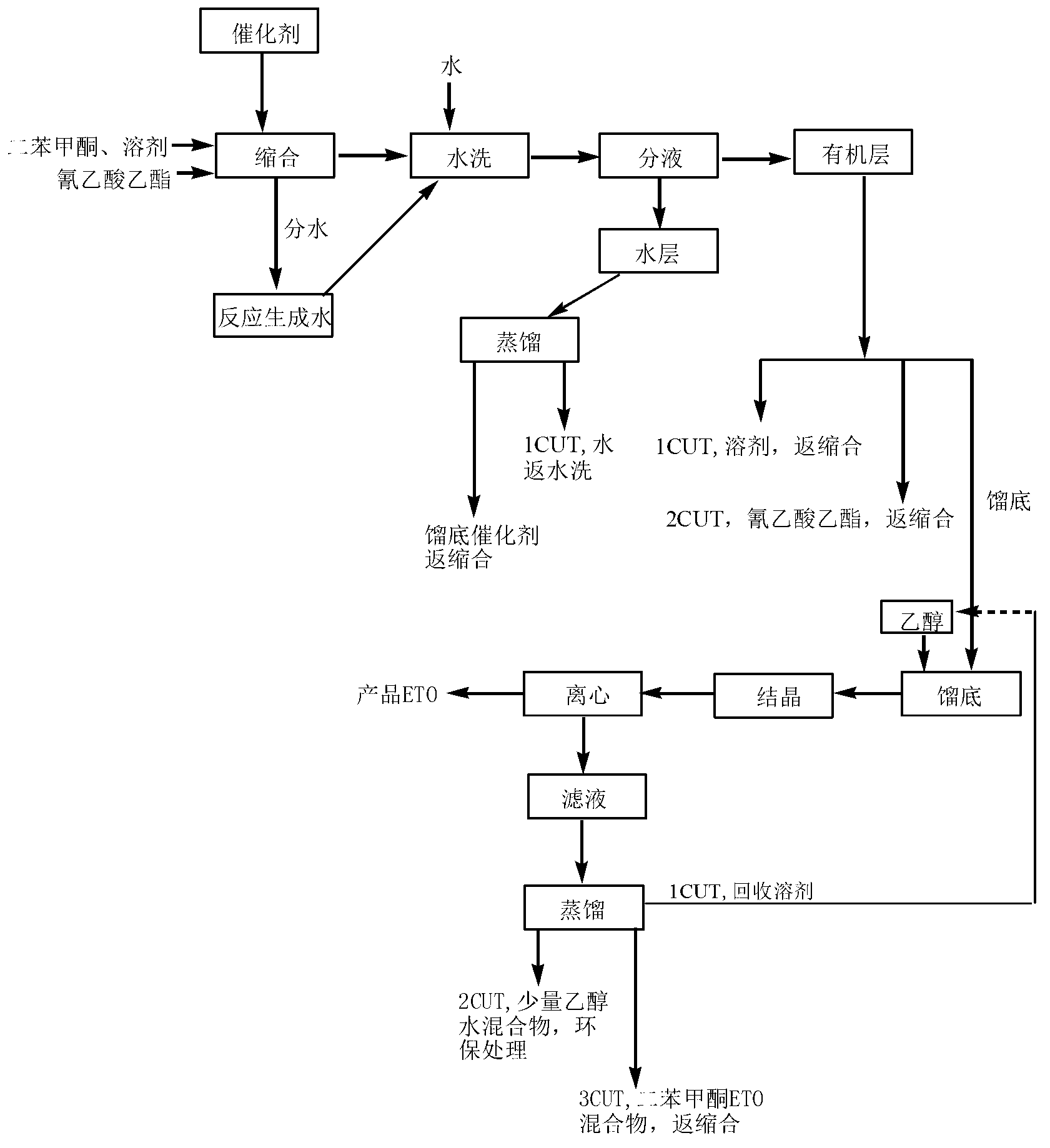
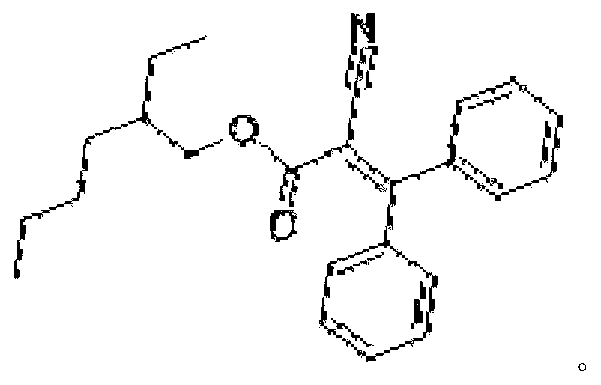
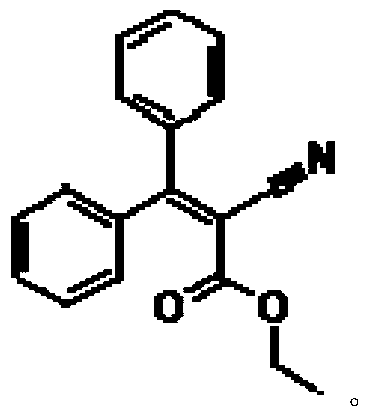
Preparation method of ultraviolet absorbent intermediate etocrilene (ETO)
ActiveCN103242197AHarm reductionReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationDistillationEthyl cyanoacetate
The invention relates to a preparation method of an ultraviolet absorbent intermediate etocrilene (ETO). The preparation method comprises the following steps of: 1, adding benzophenone, ethyl cyanoacetate, a catalyst and a water-insoluble solvent into a condensation reaction kettle, carrying out backflow water distribution reaction for 12-15h, and then, cooling to 50-55 DEG C, wherein the excessive equivalent of the ethyl cyanoacetate relative to the benzophenone is 0.2-5; 2, washing the reaction liquid by using water, and separating the liquid to respectively obtain a water layer and an organic layer; distilling the recycled water at the water layer at normal pressure, dissolving distillation tailings by using a solvent same as the solvent used in the step 1, and then, replenishing a proper quantity of catalyst to obtain a product, wherein the distilled recycled water is used in the step 2, and the product is used in the step 1; and distilling the recycled solvent at the organic layer at normal pressure, and carrying out reduced pressure distillation to recycle the excessive ethyl cyanoacetate, wherein the recycled solvent and ethyl cyanoacetate are used in the step 1; 3, carrying out reduced pressure distillation on the distillation tailings, adding 75-100% of ethanol for crystallizing, and filtering to obtain the etocrilene; and 4, filtering a mother solution obtained in the step 3, recycling the ethanol at normal pressure, and carrying out reduced pressure recycle on the benzophenone and the etocrilene, wherein the recycled ethanol is used in the step 3, and the recycled benzophenone and etocrilene are used in the step 1. The preparation method has the advantages of simplicity in operation, short production period, little environment pollution, low energy consumption, realization of material recycling, and the like.
Owner:ANHUI SHENGNUOBEI CHEM TECH
Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof
The invention relates to an oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof. Ethyl cyanoacetate is used as a raw material, a hydroxylamine compound (A) is generated under the action of sodium nitrite and phosphoric acid, 2-amino ethyl cyanoacetate (B) is obtained through reduction with sodium hydrosulfite, the 2-amino ethyl cyanoacetate (B) reacts with different substituted acyl chlorides under alkali conditions, and oxazole compounds (D1-D48) of different substituted 5-amino-4-formate are generated under the action of trifluoroacetic acid, and then react with valerolactam under the action of phosphorus oxychloride to obtain the oxazolo[5,4-d]pyrido[1,2-a]pyrimidone compounds (E1-E48). The inhibitory activity of the 48 compounds on Hela cervical cancer cells, MCF-7 breast cancer cells and A549 lung cancer cells is investigated, and the result shows that 11 compounds have the inhibitory activity on the Hela cervical cancer cells; eight compounds have inhibitory activity on MCF-7 breast cancer cells; and five compounds have inhibitory activity on A549 lung cancer cells. E32, E33, E45, E46 and E47 have inhibitory activity on three tumor cells; and E29 and E42 have inhibitory activity on Hela cervical cancer cells and MCF-7 breast cancer cells.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
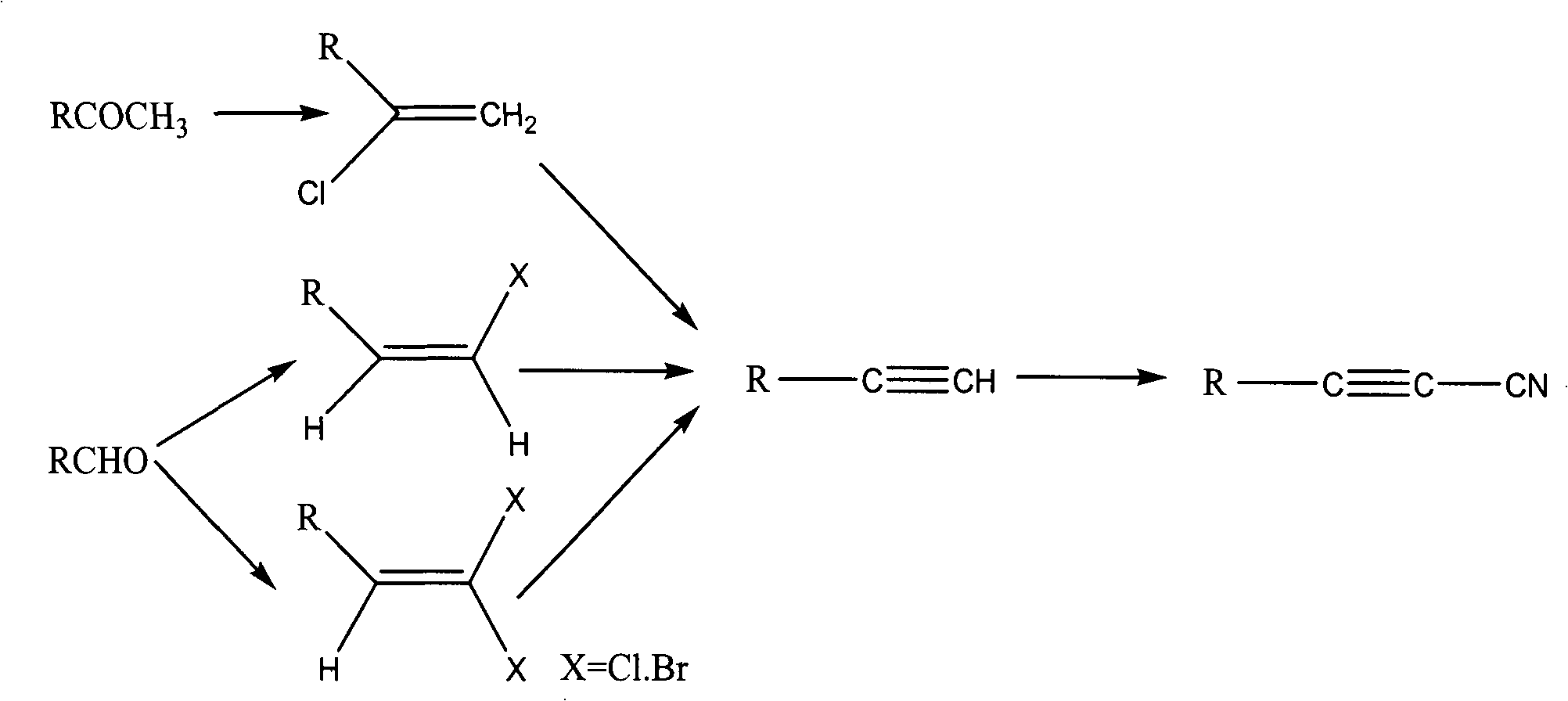
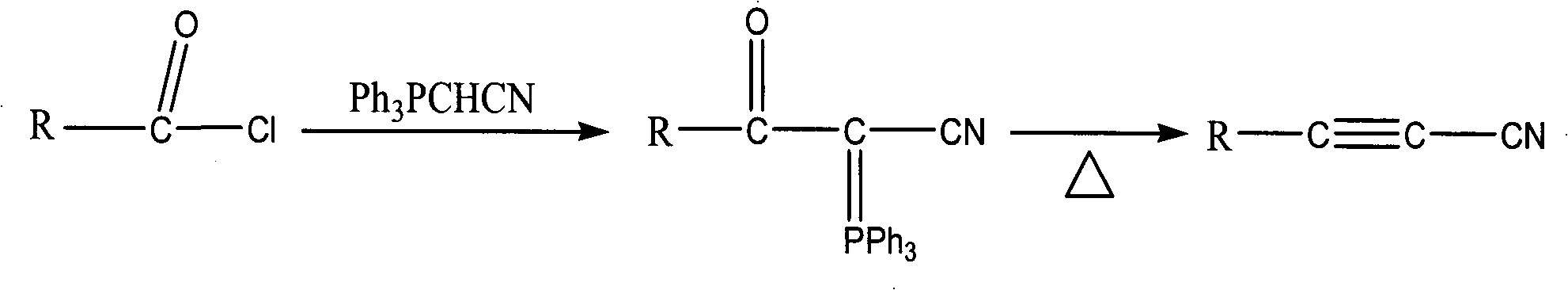
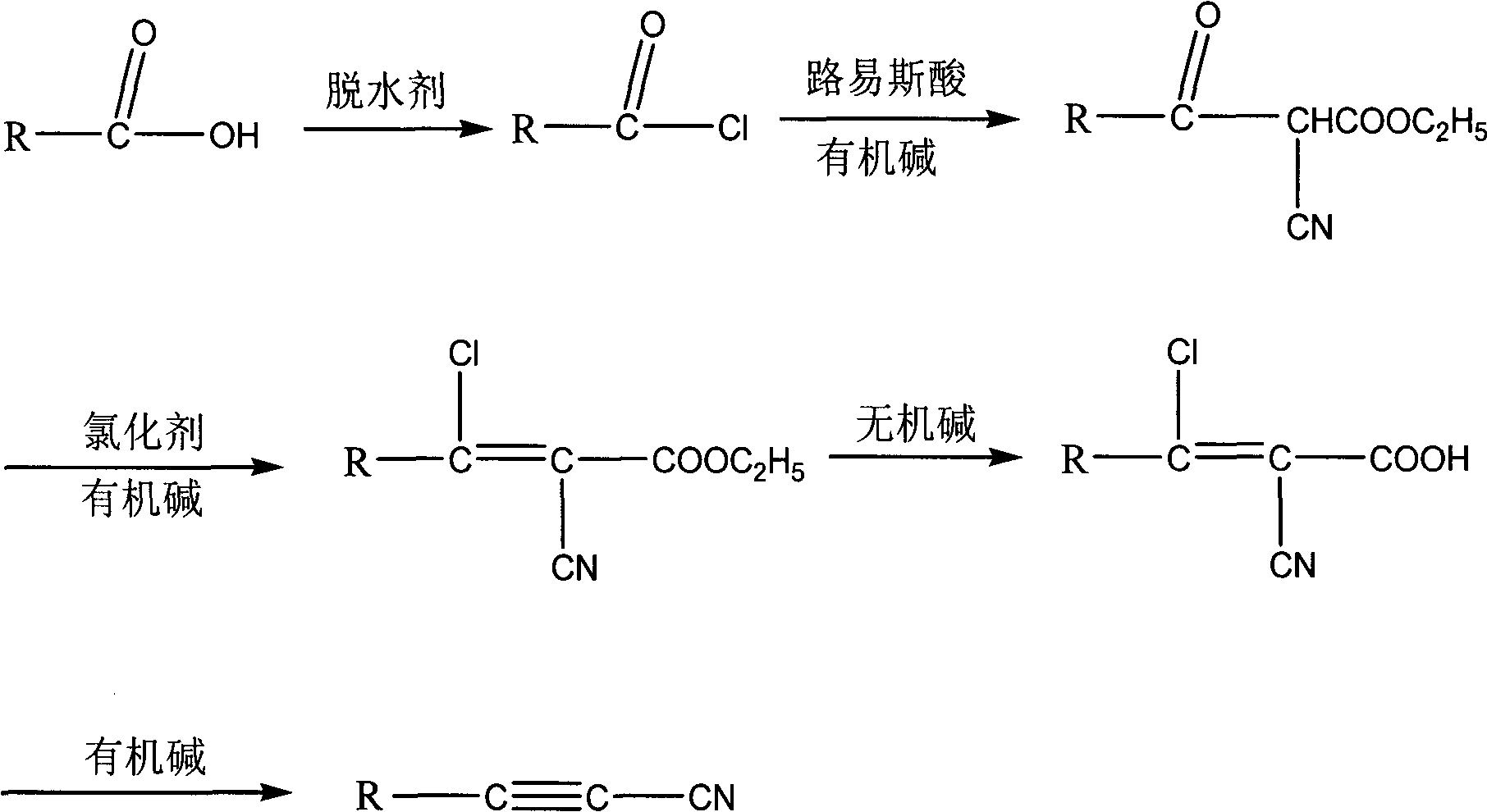
Method for synthesizing cyanoacetylene derivatives
ActiveCN101671270ASimple post-processingReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationEthyl fumarateElimination reaction
The invention discloses a method for synthesizing cyanoacetylene derivatives. The prior preparation method has high production cost and low yield, and is not suitable for industrial production. The method comprises that: in the presence of a dehydrating agent, organic formic acid generates organic formyl chloride; in the presence of Lewis acid and organic alkali, the organic formyl chloride is reacted with ethyl cyanoacetate to generate alpha-cyano-beta-carbonyl ethyl propionate compounds; in the presence of chlorinating agent and organic alkali, the alpha-cyano-beta-carbonyl ethyl propionatecompounds are subjected to chlorination reaction to generate alpha-cyano-beta-chloroacrylate ethyl ester compounds; in the presence of inorganic alkali and lower alcohol solution, the alpha-cyano-beta-chloroacrylate ethyl ester compounds are hydrolyzed to generate alpha-cyano-beta-chloroacrylate compounds; and in the presence of organic alkali, the alpha-cyano-beta-chloroacrylate compounds are subjected to decarboxylation and dehalogenation elimination reaction to generate the cyanoacetylene derivatives. The whole process operation is simple and convenient, the post treatment is simple and the yield is high, so the method is quite suitable for industrialized production.
Owner:HANGZHOU ALLSINO CHEM
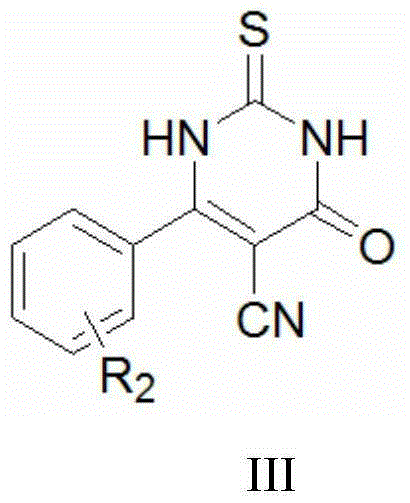
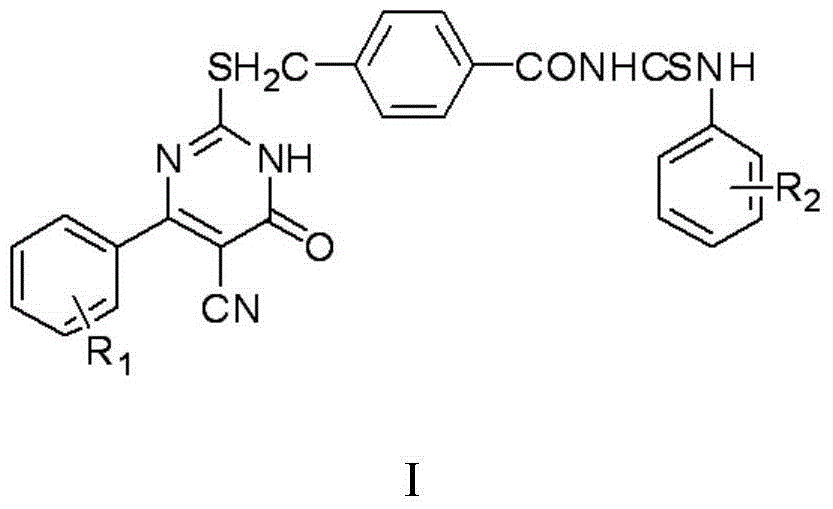
Thiouracil derivative, preparation method and application thereof
InactiveCN104876881ABroaden your optionsOvercoming the drawbacks of drug resistanceAntibacterial agentsOrganic active ingredientsThioureaPotassium thiocyanate
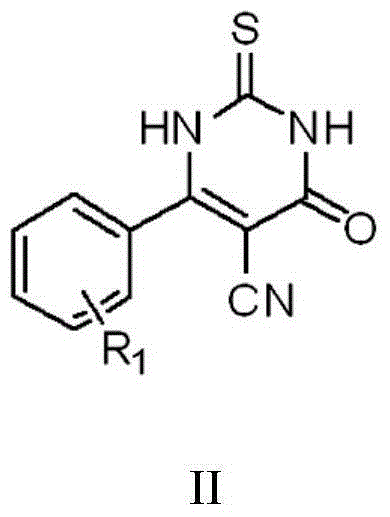
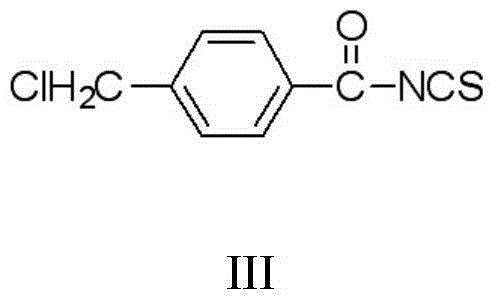
The invention discloses a thiouracil derivative. The chemical formula is shown in the formula I, wherein R1 is hydrogen, halogen or aromatic group, R2 is hydrogen, halogen or alkyl group from C1 to C6. Simultaneously, the invention also discloses a method for synthesizing the derivative. The method comprises the following steps : adopting aromatic aldehyde or an aromatic amine compound as a starting material, and reacting the aromatic aldehyde, ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound II; reacting chloromethyl-benzoyl chloride and potassium thiocyanate in a methylbenzene / water / TBAB system to obtain a compound III; reacting the compound III and aromatic amine in an acetonitrile solvent to obtain a compound IV, reacting the compound II and the compound IV with the equal mole in the acetonitrile solvent under the catalytic action of potassium carbonate to obtain a target product, i.e., a compound I. The method adopted is simple, the operation is easy, and the large-scale production is easy; and verified by experiment, the prepared compound I has stronger bacteriostatic activity and can be widely applied in bacteriostatic medicine preparations.
Owner:HEBEI UNIVERSITY
Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof
The invention relates to a dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application. The preparation method comprises the following steps: by taking ethyl cyanoacetate as an initial raw material, generating a hydroxylamine compound (A) under the action of sodium nitrite and phosphoric acid, reducing with sodium hydrosulfite to obtain ethyl 2-aminocyanoacetate (B), reacting with different substituted acyl chlorides respectively under an alkali condition, generating different substituted oxazole compounds D1-D48 of 5-amino-4-formate under the action of trifluoroacetic acid, and reacting with pyrrolidone under the action of phosphorus oxychloride to obtain a dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one compound E1-E48. According to the invention, the inhibition activity of 48g of the compounds on Hela cervical cancer cells, MCF7 breast cancer cells and A549 lung cancer cells is investigated, and results show that the compounds E5, E10, E13, E16, E18, E19, E24, E42 and E43 have inhibition activity on Hela cervical cancer cells, the compounds E22, E24, E47 and E48 have inhibitory activity on MCF7 breast cancer cells, and the compounds E18 and E20 have inhibitory activity on A549 lung cancer cells.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
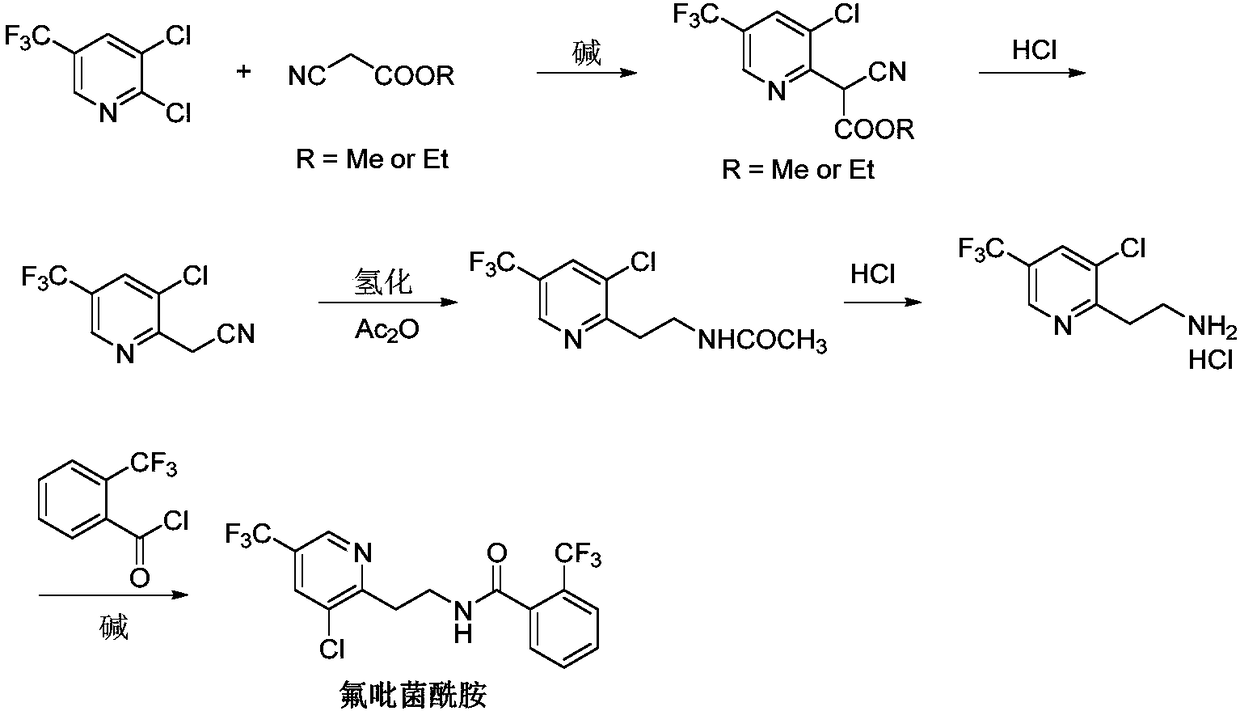
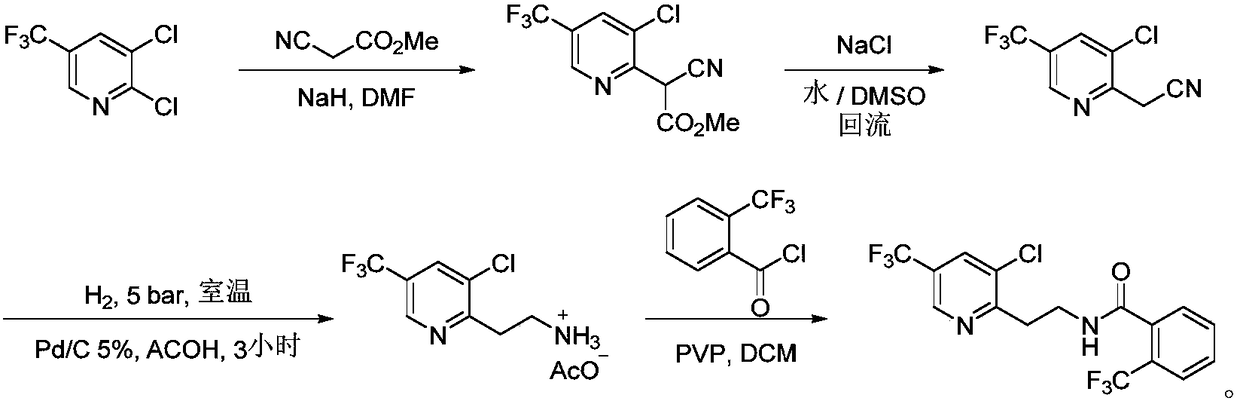
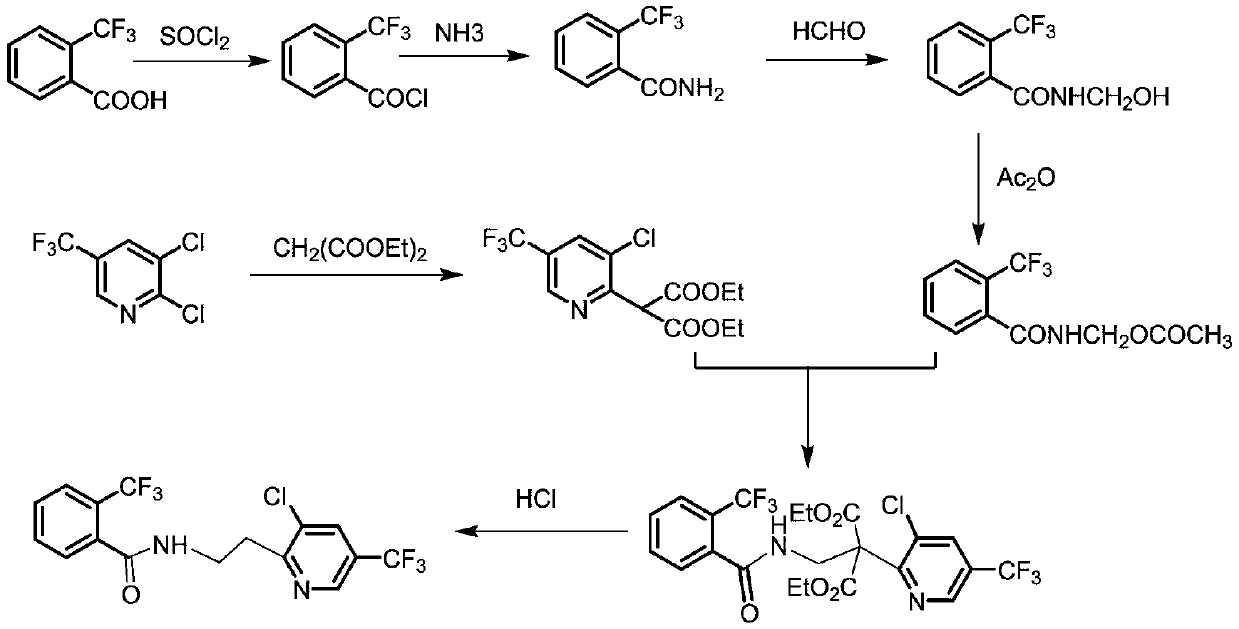
Fluopyram preparation method
ActiveCN109293565AShort stepsAvoid protection‐deprotection stepsOrganic chemistryHydrogenCyanoacetic acid
The invention relates to a fluopyram preparation method, which sequentially comprises: (1) carrying out a substitution reaction on 2,3-dichloro-5-trifluoromethylpyridine and ethyl cyanoacetate or methyl cyanoacetate at a temperature of 30-160 DEG C in the presence of an alkali and a solvent, adjusting the reaction liquid to an acidic pH value after completing the reaction, and carrying out a decarboxylation reaction at a temperature of 80-160 DEG C to obtain 3-chloro-5-(trifluoromethyl)-2-acetonitrilepyridine; and (2) carrying out tandem hydrogenation and condensation reaction on the 3-chloro-5-(trifluoromethyl)-2-acetonitrilepyridine prepared in the step (1) and o-trifluoromethylbenzoyl chloride at a temperature of 20-100 DEG C in the presence of a catalyst, hydrogen, an alkali and a solvent to obtain fluopyram. According to the present invention, the preparation method has the short steps, avoids the unnecessary protection-deprotection step, is economical and environmentally friendly, and is suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Thiouracil derivatives containing oxadiazole/thiadiazole and preparation method and application of thiouracil derivatives
InactiveCN105153143AEasy to operateEase of mass productionAntibacterial agentsOrganic active ingredientsThioureaThiadiazoles
The invention discloses thiouracil derivatives containing oxadiazole / thiadiazole, and further provides a method for synthesizing the thiouracil derivatives. The general chemical formula of the thiouracil derivatives is shown in figure I or figure II, wherein R1, R2 and R3 are hydrogen or halogen or aryl-. The method includes the steps of making aromatic aldehyde react with ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound III, making aromatic aldehyde react with semicarbazide to obtain a compound IV, making the compound IV react with bromine to obtain a compound V, making the compound V react with benzoyl chloride to obtain a compound VI, making the compound VI and the compound III have a reflux reaction under the catalysis of potassium carbonate to obtain the general chemical formula I, making aromatic acid react with thiosemicarbazide to obtain a compound VII, making the compound VII react with benzoyl chloride to obtain a compound VIII, and making the compound VIII react with the compound III under the catalysis of potassium carbonate to obtain the general chemical formula II. The adopted method is simple, easy to operate and beneficial to large-scale production; it is verified that the prepared compound has high bacteriostatic activity and can be widely applied in bacteriostatic drug preparations.
Owner:HEBEI UNIVERSITY
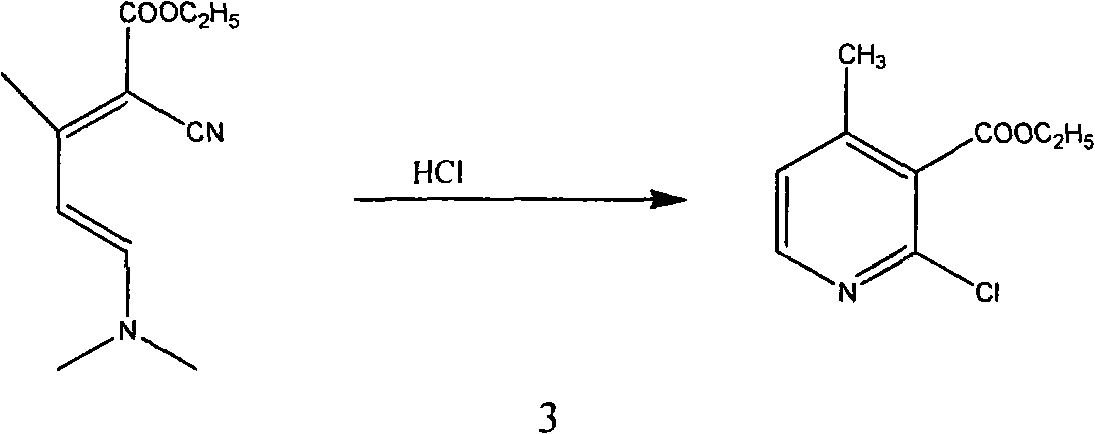
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399AComplicated operationFew stepsOrganic chemistrySodium methoxideOrganic synthesis
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing the reaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
Glue production process
InactiveCN106349984ALess albinismImprove product qualityCarboxylic acid nitrile preparationOrganic compound preparationCyanoacetic acidPhosphoric acid
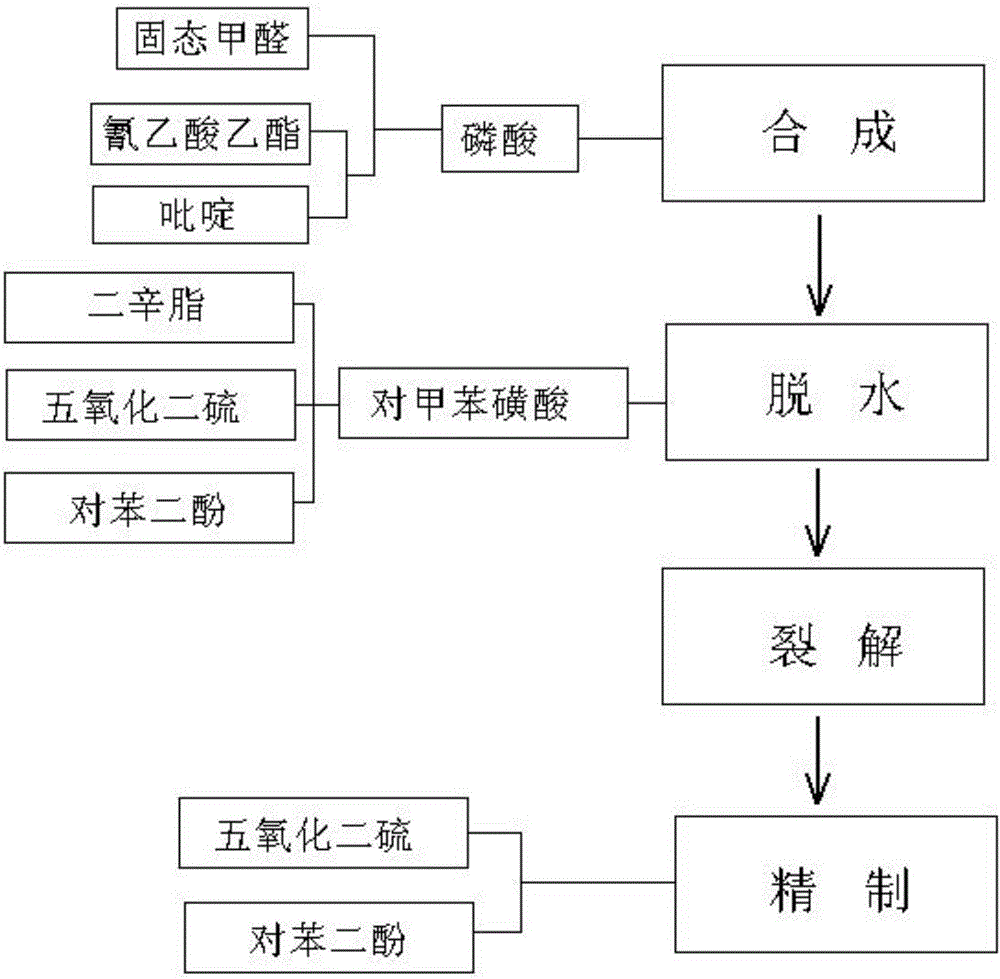
The invention disclsoes a glue production process. The glue production process comprises the following steps: S1: synthesizing: after liquefying solid-state formaldehyde, mixing the liquefied solid-state formaldehyde with an organic solvent; dropwise adding a mixture composed of ethyl cyanoacetate and pyridine; keeping heat, and adding phosphoric acid into the materials, and uniformly stirring; standing and layering; S2: dehydrating: performing reflux condensation dehydrating on the materials through adding an organic solvent into the materials from the top of a container; after dehydrating, raising the temperature, and drawing the materials in vacuum to obtain crude glue; adding a mixture of dioctyl phthalate, disulfur pentoxide and hydroquinone into the crude glue; meanwhile, adding p-toluene sulfonic acid into the container; S3: cracking: heating the inner part of the container and raising the temperature; stirring, vacuumizing and starting external cooling circulating water of the container; increasing the temperature in the container to separate out secondary glue; S4: refining: adding phosphorus pentoxide and hydroquinone into the secondary glue; distilling and refining the secondary glue under a vacuum condition, and purifying and separating out refined glue. According to the glue production process, the production quality of the glue can be effectively enhanced, the rate of finished products can also be remarkably improved, and the production cost of the glue is reduced.
Owner:湖南浩森胶业有限公司
Synthesizing method of L-carnosine
InactiveCN101735305ASimple processEasy to operateNervous disorderDigestive systemCyanoacetic acidL-Carnosine
The invention discloses a production method of carnosine, which is characterized by comprising the following steps of: synthesizing N-acetyl cyanide-L-histidine through ammonolysis reaction by using L-histidine and ethyl cyanoacetate as raw materials and sodium alcoholate as a catalyst; and then obtaining L-carnosine through the intermediate by using stronger ammonia water and alcohol as a solvent in a high-pressure reaction kettle and using a Pd-Rh / C composite catalyst for catalytic hydrogenation under the protection of nitrogen, wherein the overall yield of two-step reaction achieves 72 percent. The invention has simple process, easy operation, low cost, and the like and is very suitable for industrialized production.
Owner:SOUTH CHINA NORMAL UNIVERSITY +1
Instant adhesive
InactiveCN102994022AExcellent low temperature adhesionLow priceNon-macromolecular adhesive additivesEster polymer adhesivesPolymer scienceDistillation
The invention discloses an instant adhesive. The instant adhesive is prepared from the following raw materials: polymethyl methacrylate, ethyl cyanoacetate, 30-35% formaldehyde, piperidine, dichloroethane, a hydroquinone polymerization inhibitor and modified sepiolite. The instant adhesive is prepared by the following steps of: based on parts by weight, mixing the polymethyl methacrylate, the piperidine, the ethyl cyanoacetate and the 35% formaldehyde and then adding the obtained mixture into a reaction kettle to react for 1 hour at 70 DEG C when heated; and then adding the hydroquinone polymerization inhibitor, the modified sepiolite and the dichloroethane for reduced pressure distillation at 180 DEG C. The instant adhesive disclosed by the invention is an adhesive which has single component and is instantly cured on meeting moisture, and is convenient to use, the adhered surface needs no special pretreatment, heating and pressurizing are not necessary, and the adhesive is widely applied to the adhesion and location between plastics, and between the plastic and metal, is water-fast, cannot be combusted, is good in low-temperature adhesion property, and is low in cost.
Owner:ANHUI DONGFANGJINHE PRECISION MACHINERY MFG +1
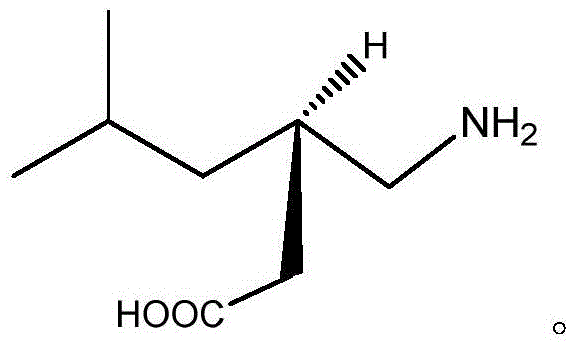
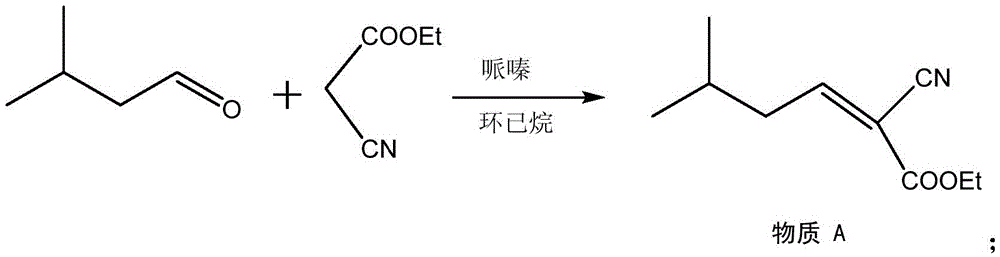
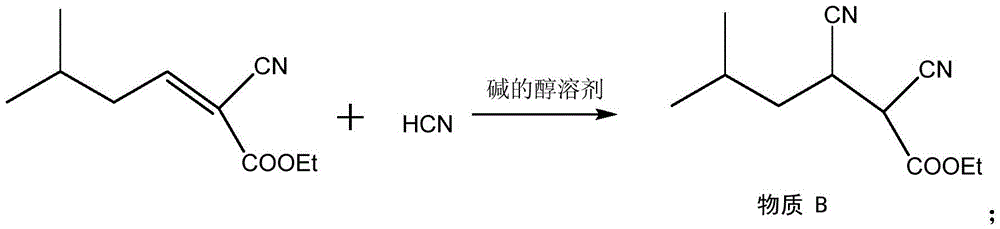
Method for synthesizing pregabalin with isobutyl butanedinitrile as intermediate
InactiveCN105463037AGuaranteed yieldGuaranteed purityOrganic compound preparationOrganic chemistry methodsCyanide hydrataseSolvent
The invention discloses a method for synthesizing pregabalin with isobutyl butanedinitrile as the intermediate. The method includes the steps of conducting the Knoevenagel condensation reaction on isovaleraldehyde and ethyl cyanoacetate in cyclohexane solvent with piperazine as the catalyst, conducting Michael addition on the product obtained in the first step and cyanic acid in alkaline alcohol solvent, conducting the decarboxylic reaction on the product obtained in the second step in isopropanol solvent under the heating condition to obtain the isobutyl butanedinitrile solvent, conducting hydrolysis on the intermediate under catalysis of cyanide hydratase AtNiTl, and conducting catalytic hydrogenation with raney nickel as the catalyst. According to the method, isoamyl aldehyde and ethyl cyanoacetate which are low in price and easy to obtain are used as raw materials, and the isobutyl butanedinitrile intermediate is obtained through the Knoevenagel condensation reaction, Michael addition and the decarboxylic reaction. The intermediate is then catalyzed, hydrolyzed and reduced through cyanide hydratase AtNiTl, and pregabalin is obtained. The reaction route is simple, the yield of each step of reaction is high, and therefore the final total recovery and purity of pregabalin are ensured.
Owner:TAICANG YUNTONG BIOCHEM ENG
Method for synthesizing 3-(5'-substitution-2-benzoxazole group)-7-diethyl amino group H-1-benzopyrans-2-ketone
InactiveCN1760194AFew reaction stepsThe synthesis process is simpleOrganic chemistryBenzopyranBenzoxazole
A process for synthesizing 3-(5í»-substituent-2-benzoxazolyl)-7-diethylamino-2H- 1-benzopyran-2-one includes such steps as the reflux reaction between ethyl cyanoacetate, 4-N,N-diethylamino salicylaldehyde and 0-aminophenol derivative in non-protonic polar solvent for 10-24 hr under the action of catalyst, and post-treating.
Owner:ZHEJIANG UNIV OF TECH
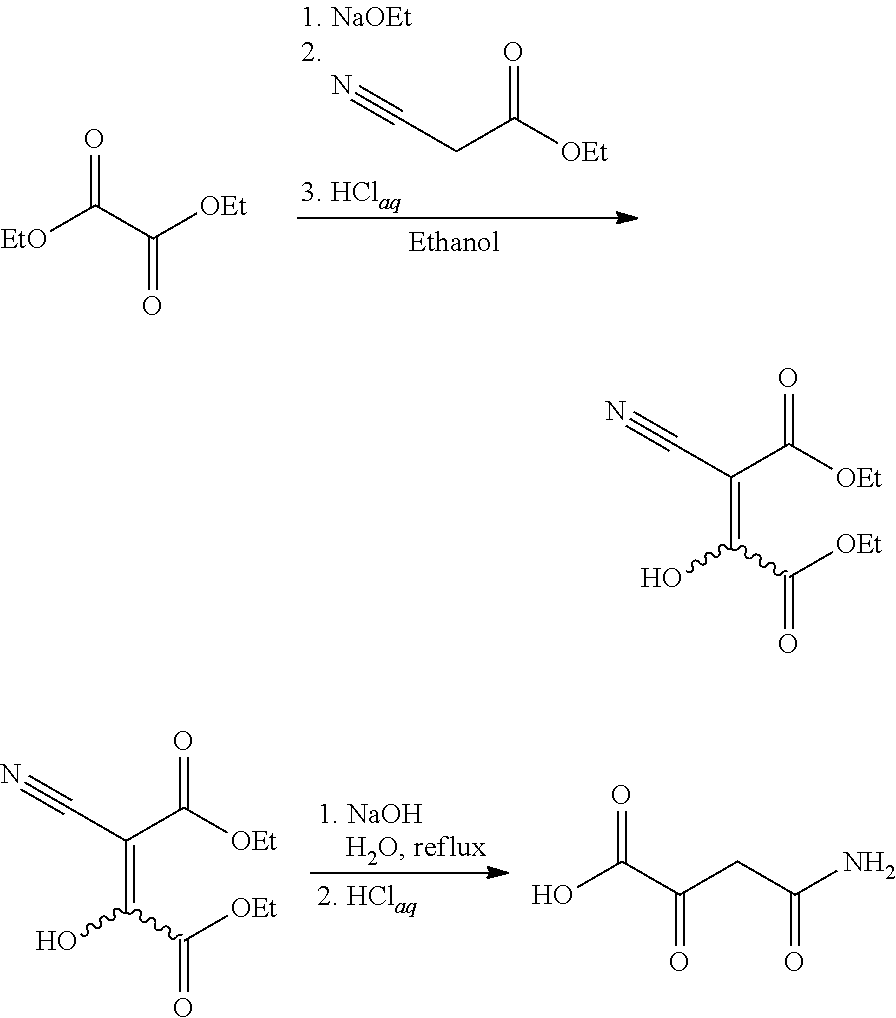
Preparation of 4-amino-2,4-dioxobutanoic acid
ActiveUS9045392B2Carboxylic acid nitrile preparationOrganic compound preparationAqueous sodium hydroxideEthyl cyanoacetate
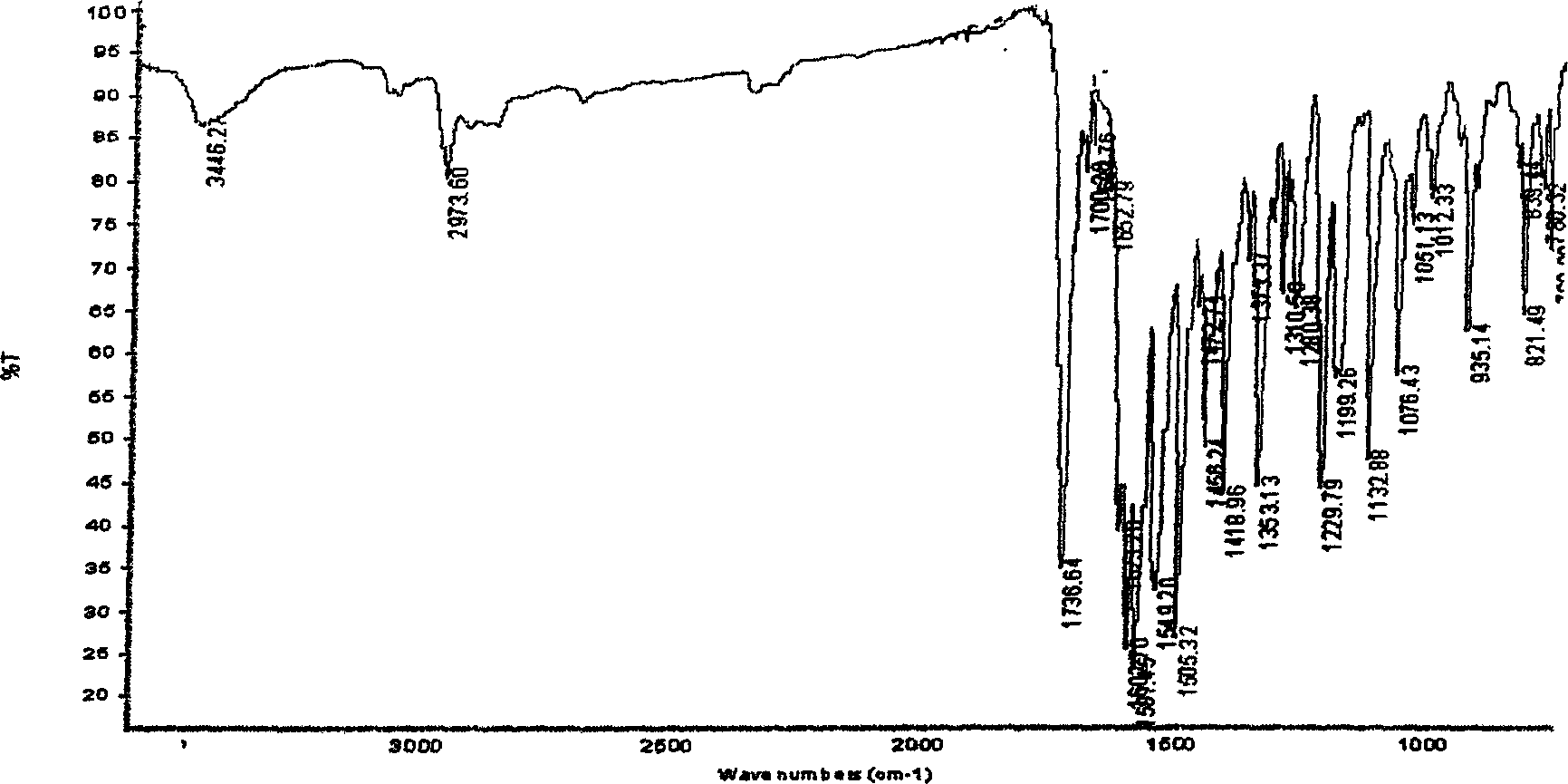
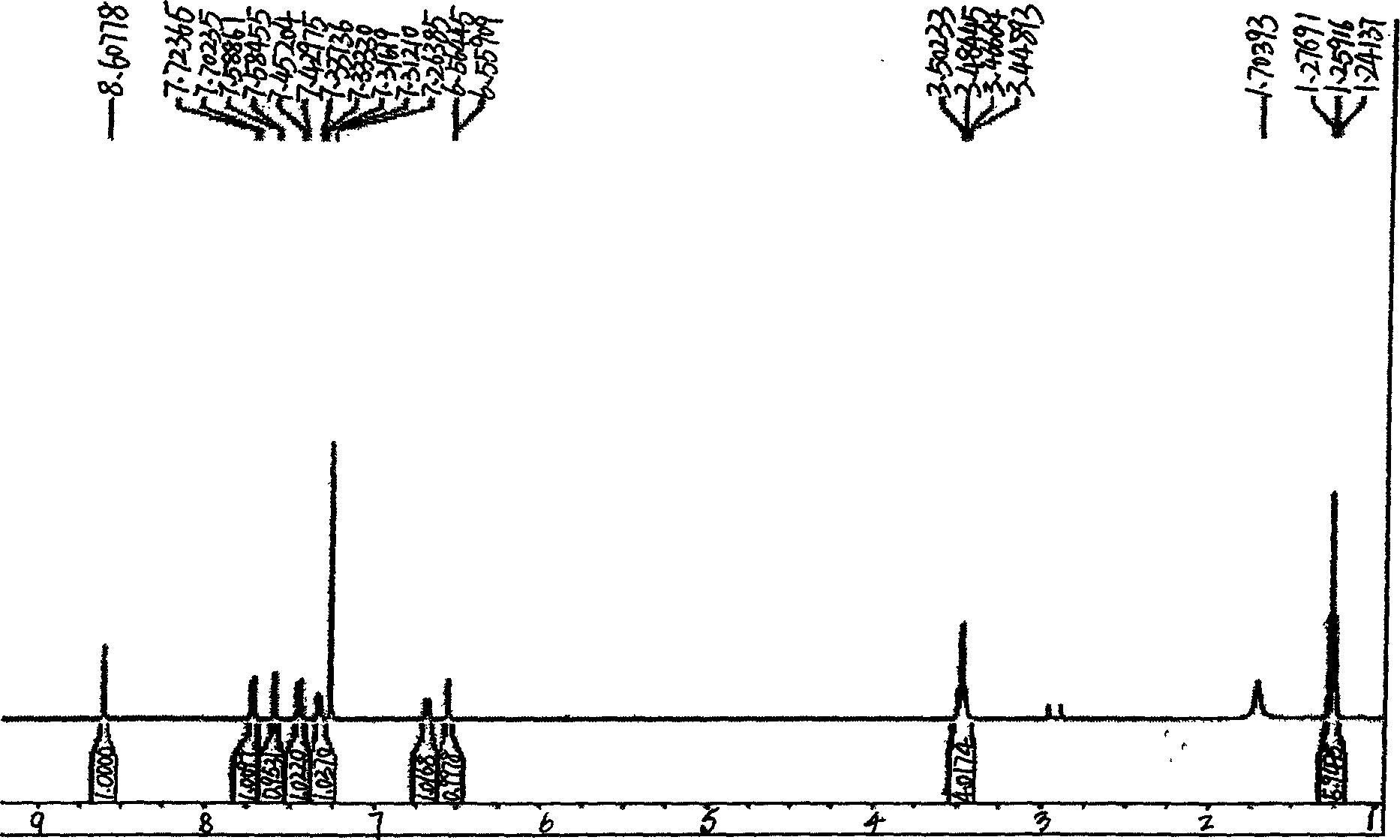
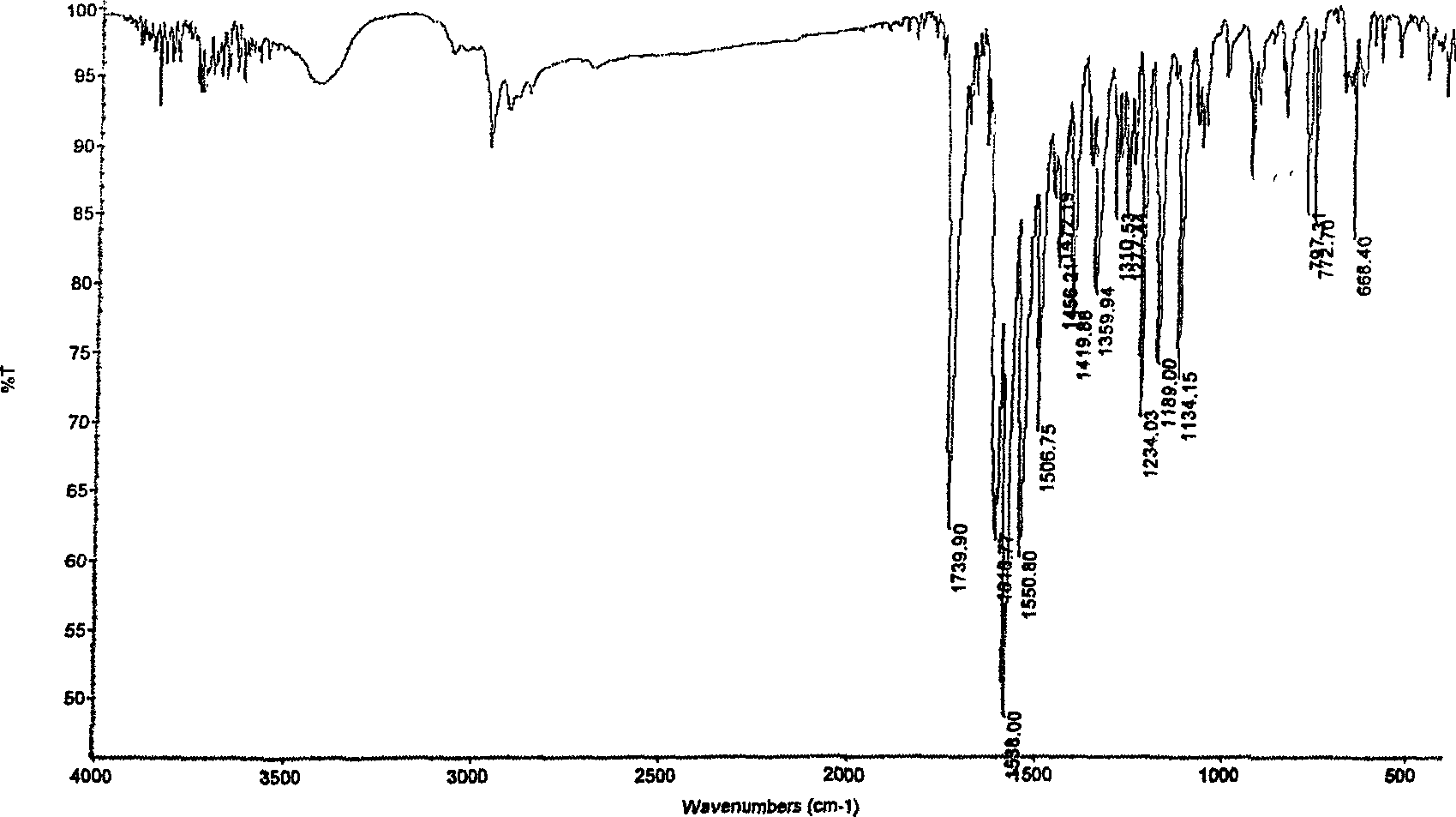
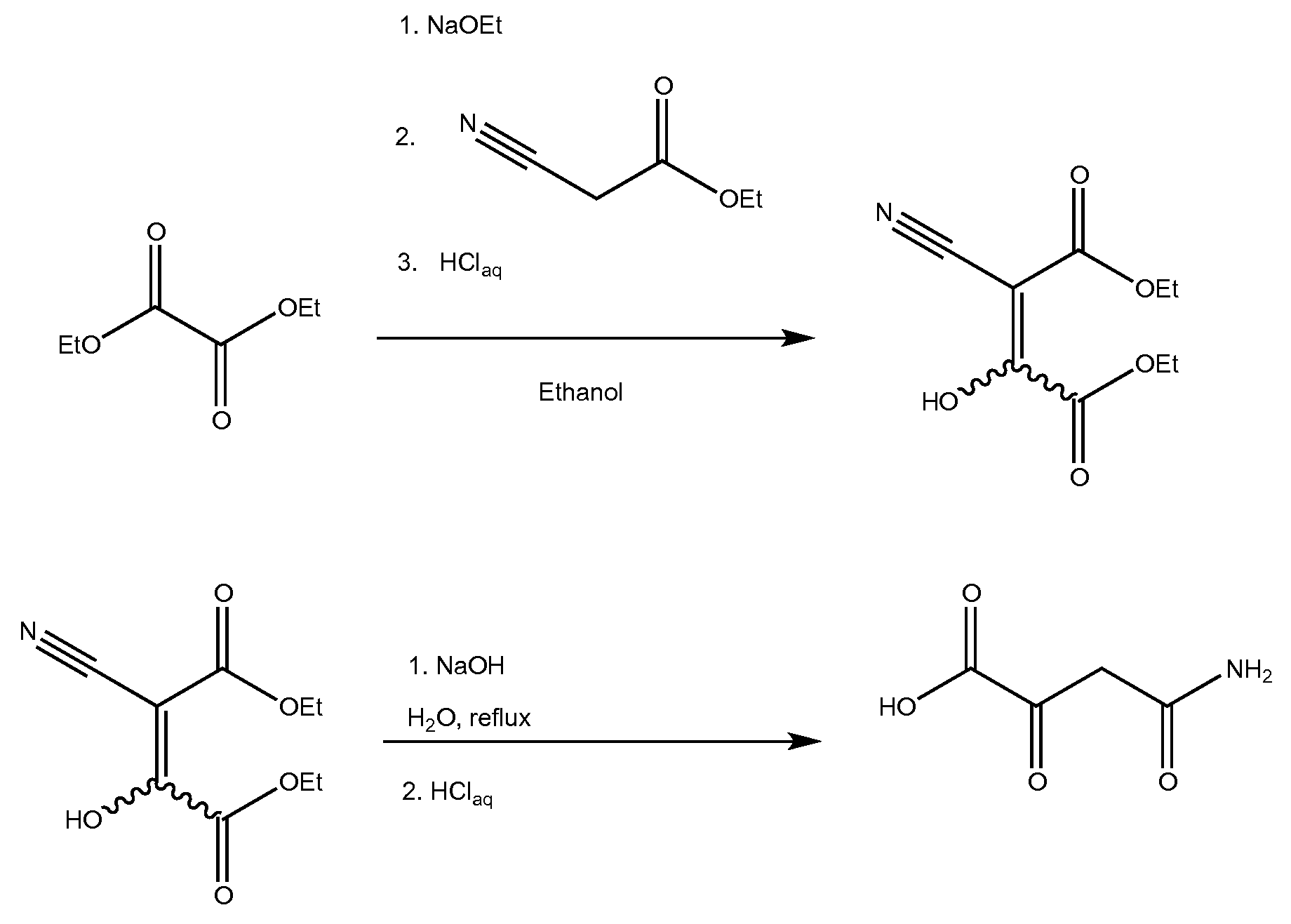
A process for synthesizing 4-amino-2,4-dioxobutanoic acid involves reacting diethyl oxalate with sodium ethoxide in ethanol to form a reaction mixture, and afterward adding ethyl cyanoacetate to the reaction mixture and allowing a reaction to proceed under conditions suitable to form a first reaction product of the formula diethyl-2-cyano-3-hydroxy-butenedioate, and then isolating the diethyl-2-cyano-3-hydroxybutenedioate, and afterward reacting the diethyl-2-cyano-3-hydroxy-butenedioate with aqueous sodium hydroxide under conditions suitable to form 4-amino-2,4-dioxobutanoic acid.
Owner:TRIAD NAT SECURITY LLC +1
Synthesis method of cytosine
The invention discloses a synthesis method of cytosine. According to the method, ethyl cyanoacetate, urea and triethyl orthoformate are utilized as raw materials, ethyl 3-cyano-2-ureido-acrylate, 5-ethoxycarbonyl cytosine, and 5-carboxyl cytosine are sequentially synthesized, decarboxylation is performed to synthesize cytosine and refining, correction, perfection and other various process steps are sequentially performed. Therefore, the yield of the synthesis method is high, the highest total yield can achieve 75.14%, the comprehensive benefits are finally improved and the method has the advantages of simplicity in operation, small difficulty in actual production and convenience in large-scale application.
Owner:NANYANG NORMAL UNIV
Production process for alpha-cyanoethyl acrylate adhesive
ActiveCN105439902AExtended shelf lifeEasy to removeOrganic compound preparationCarboxylic acid nitrile purification/separationAdhesiveCracking reaction
The invention provides a production process for an alpha-cyanoethyl acrylate adhesive. The production process comprises the following steps: under the action of a catalyst, subjecting ethyl cyanoacetate, solid formaldehyde and an organic solvent to condensation polymerization in a reaction vessel so as to obtain a reaction system; then adding an organic solvent into the reaction system from the bottom of the reaction vessel and then carrying out condensation, reflux and dehydration; and finally, adding an auxiliary agent into the reaction system obtained in the previous step and carrying out a cracking reaction so as to obtain the alpha-cyanoethyl acrylate adhesive. The production process employs specific reaction steps, so yield is further improved, and production cost is reduced; and in particular, after completion of condensation polymerization and synthesis reaction of materials, the organic solvent is added from the bottom of reaction vessel and an external reflux and dehydration manner is employed, so dehydration is more thorough, removal of a trace amount of moisture is much easier, stability of the adhesive is improved, and the shelf-life of the adhesive is further prolonged.
Owner:湖南浩森胶业有限公司
Synthesis method of fluopyram
The invention discloses a synthesis method of fluopyram and belongs to the technical field of fine chemical engineering. The synthesis method comprises the following steps: by taking 2,3-dichloro-5-trifluoromethylpyridine as a raw material, performing condensation and hydrolysis decarboxylation with ethyl cyanoacetate in the presence of an alkali so as to obtain 2-acetonitrile-3-chloro-5-trifluoromethylpyridine; performing catalytic hydrogenation reduction so as to obtain 2-ethylamino-3-chloro-5-trifluoromethylpyridine, performing protection by using o-trifluoromethyl benzoic anhydride or o-trifluoromethyl benzoic acid-trimethylacetic anhydride, so as to obtain a target product, namely fluopyram. The synthesis method is reasonable and simple and convenient in route selection and is applicable to industrial amplification, the synthesis method breaks through a conventional process, a step that hydrogenation reduction is implemented and at the same time amino is protected by using other acylation reagents firstly, and acylation of the amino with o-trifluoromethyl benzoyl chloride is implemented later is avoided, a step that hydrogenation is implemented and at the same time protectionis implemented to obtain the target product amide is implemented instead, and process procedures can be simplified.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
Bifunctional fluorescent probe for detecting HSO3- and hydrazine hydrates and preparation method and application of probe
InactiveCN110172336AMaterial analysis by observing effect on chemical indicatorGroup 3/13 element organic compoundsFluorescenceDiethyl ether
The invention discloses a bifunctional fluorescent probe for detecting HSO3- and hydrazine hydrates and a preparation method and application of the probe. The preparation method comprises the steps ofcarrying out a condensation reaction on 3-(4'-formyl-1',1'-biphenyl-4'-carbonyl) norpinone and 2-ethyl cyanoacetate to obtain 3-(4'-((2-cyano) ethyl acrylate)-1',1'-biphenyl-4'-carbonyl) norpinone; making 3-[4'-(1-cyano-2-ethoxycarbonyl)vinyl-1',1'-biphenyl-4'-carbonyl] norpinone react with boron trifluoride diethyl ether to obtain a 3-[4'-(1-cyano-2-ethoxycarbonyl)vinyl-1',1'-biphenyl-4'-carbonyl] norpinone-based fluorine-boron complex. After the 3-(4'-((2-cyano) ethyl acrylate)-1',1'-biphenyl-4'-carbonyl) nopinone fluorine-boron complex prepared by means of the method is complexed with HSO3- ions, the fluorescence color of a solution is changed from blue green to blue. After a reaction with the hydrazine hydrates, the fluorescence color of the solution is changed from blue green to yellow, and the probe can be used as a bifunctional fluorescence probe and has a good application prospect in the aspect of identifying the HSO3- ions and the hydrazine hydrates.
Owner:NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/314a8975-9605-4db5-b49d-381a5f6f289a/2014100615097100002DEST_PATH_IMAGE001.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000011.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000012.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000021.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e595af4f-6fd7-4780-8b9f-d31fa561dd1a/GSA00000123074400021.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e595af4f-6fd7-4780-8b9f-d31fa561dd1a/GSA00000123074400031.PNG)


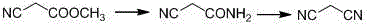

![Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/39801a43-5e9a-4c45-872d-56f508a38e56/FDA0002912820410000011.png)
![Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/39801a43-5e9a-4c45-872d-56f508a38e56/FDA0002912820410000021.png)
![Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof Oxazolo[5,4-d]pyrido[1,2-a]pyrimidone derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/39801a43-5e9a-4c45-872d-56f508a38e56/BDA0002912820420000041.png)
![Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c535610a-4024-4bd7-ba03-8c12e632f709/FDA0002912815240000011.png)
![Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c535610a-4024-4bd7-ba03-8c12e632f709/FDA0002912815240000021.png)
![Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof Dihydrooxazolo[5,4-d]pyrrolo[1,2-a]pyrimidine-9(5H)-one derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c535610a-4024-4bd7-ba03-8c12e632f709/BDA0002912815250000041.png)