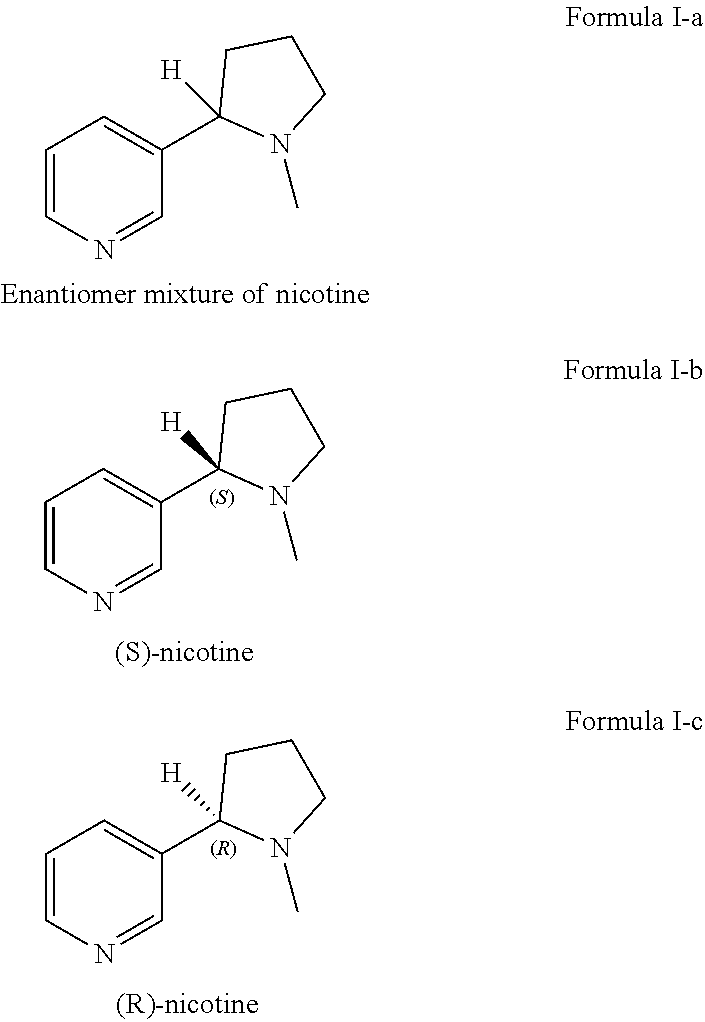
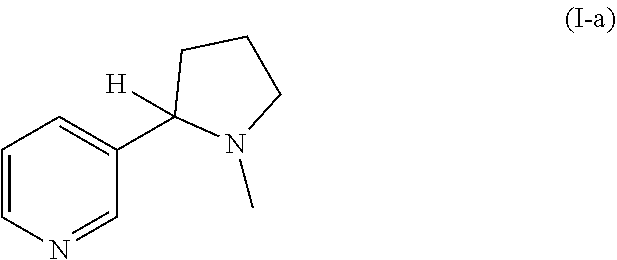
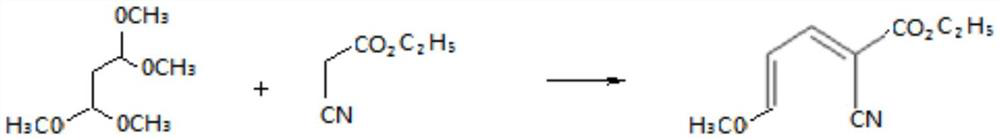Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Ethyl nicotinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
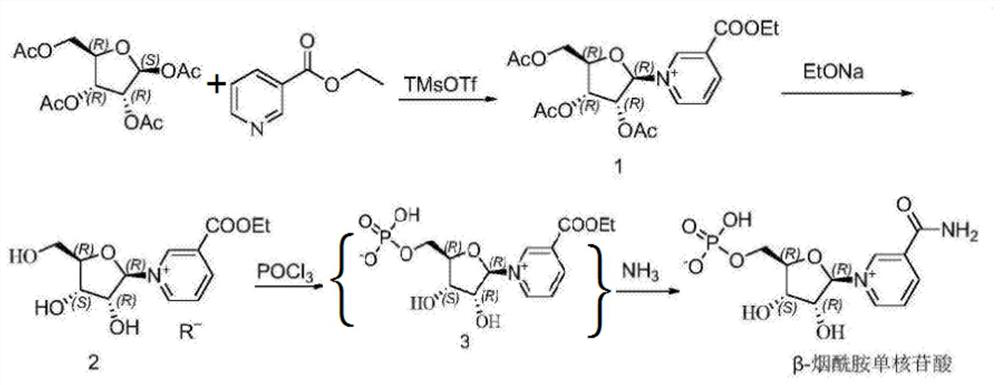
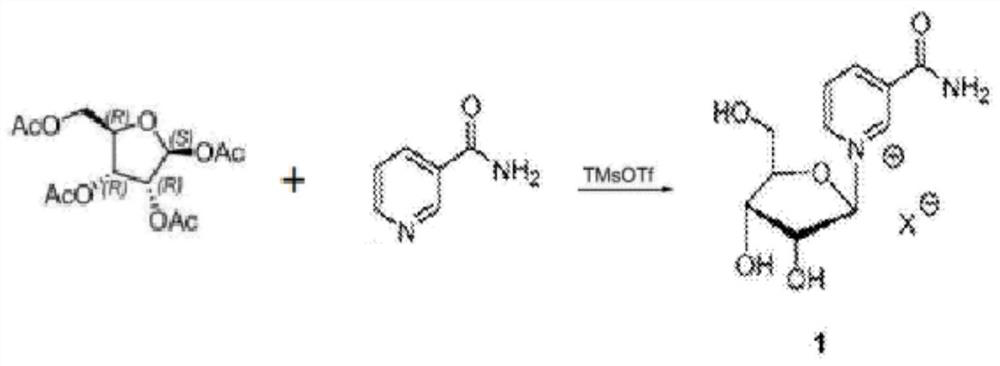
Method for preparing beta-niacinamide mononucleotide and application thereof
PendingCN110483601AEasy to operateEasy to zoom inOrganic active ingredientsNervous disorderChemical synthesisPhosphorylation
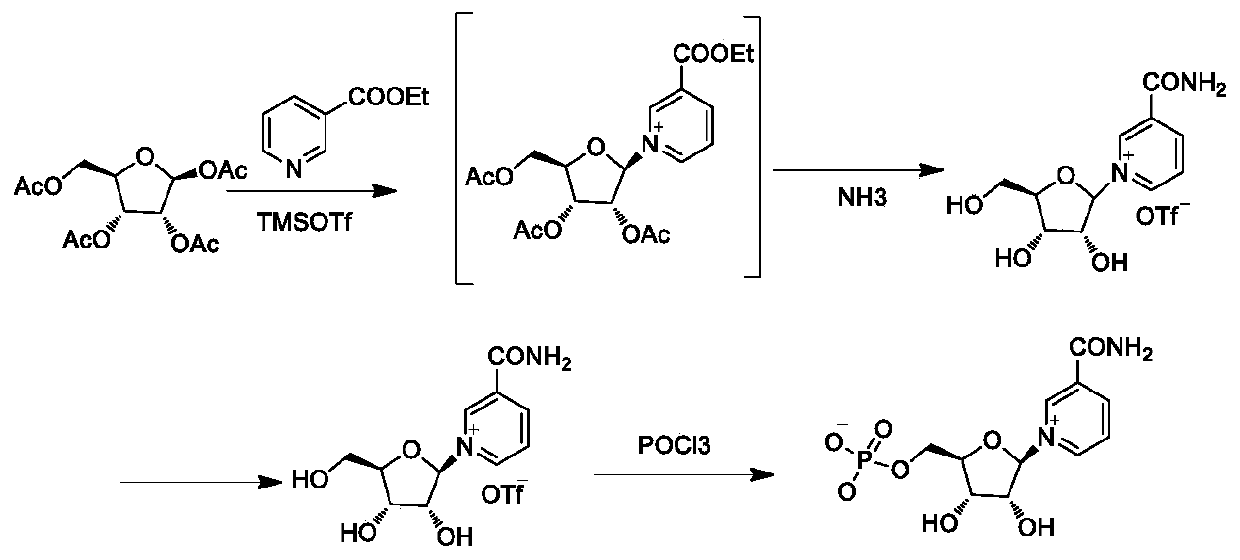
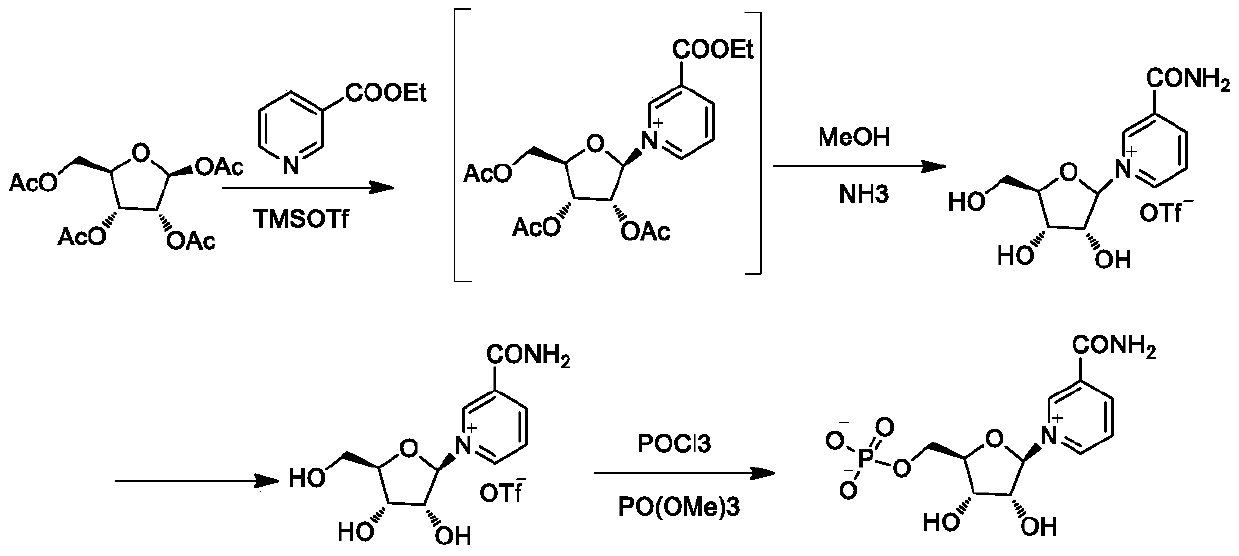
The invention belongs to the field of chemical synthesis, and in particular, relates to a method for preparing beta-niacinamide mononucleotide and an application thereof. The preparation method comprises the steps: S1, condensation reaction: carrying out condensation reaction of ethyl nicotinate, tetraacetylribose and a catalyst in a first solvent to obtain a solution containing ethyl nicotinate triacetylnucleoside; S2, ammonolysis reaction: carrying out ammonolysis reaction of the solution containing ethyl nicotinate triacetylnucleoside through an ammonia gas solution to obtain niacinamide riboside hydrochloride; and S3, phosphorylation reaction: carrying out reaction of niacinamide riboside hydrochloride with phosphorus oxychloride in a second solvent to obtain beta-niacinamide mononucleotide. Compared with a conventional method, the preparation method has the advantages of simple operation, easy amplification, easy purification, high yield and the like.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399AComplicated operationFew stepsOrganic chemistrySodium methoxideOrganic synthesis
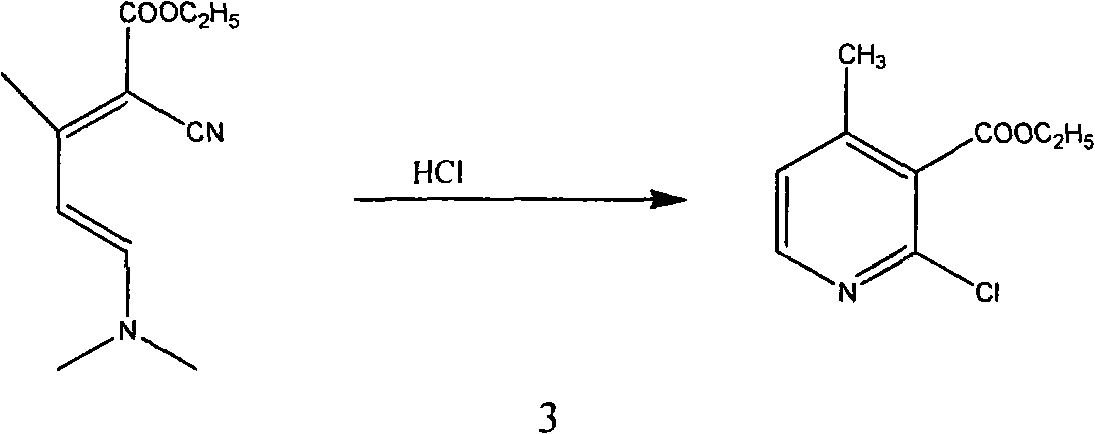
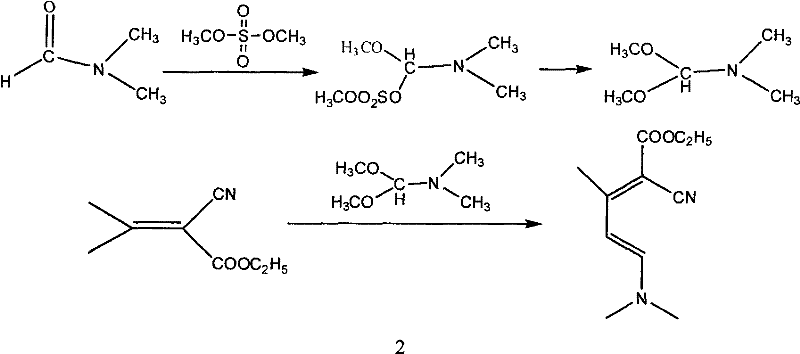
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing the reaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
Process preparation method of beta-nicotinamide mononucleotide
ActiveCN111548383AEasy to operateProcess scaling is easySugar derivativesSugar derivatives preparationBiochemical engineeringPhosphorylation
The invention provides a process preparation method of beta-nicotinamide mononucleotide. With tetraacetylribose and niacinamide or ethyl nicotinate as starting materials, through main process steps ofcondensation, ammonolysis, chemical resolution, phosphorylation and the like, and through optimization of the process method, the beta-nicotinamide mononucleotide is prepared; the method is based onthe purposes of quality control and safety of the beta-nicotinamide mononucleotide, industrial batch production feasibility of a preparation process and the like; the preparation method is improved onthe basis of an original process route, optimizes the selected materials and reagents, controls the material amount and the like, and has the advantages of simplicity in operation, easiness in process amplification, higher yield, controllable final product quality, controllable safety and the like compared with the original preparation process.
Owner:湖南和泰康瑞生物技术有限公司
Synthesis method of nicotinoyl hydrazone Schiff base compound as well as application of compound to bactericide
InactiveCN108840823AEasy to separateThe synthesis reaction is easy to operateBiocideOrganic chemistryOrganic synthesisFiltration
The invention relates to a synthesis method of a nicotinoyl hydrazone Schiff base compound as well as application of the compound to bactericide, and relates to the technical field of organic synthesis. The synthesis method of the nicotinoyl hydrazone Schiff base compound comprises two steps of synthesizing an intermediate nicotinoylhydrazine and synthesizing a target product, wherein the nicotinoylhydrazine is prepared by carrying out a refluxing stirring reaction among raw materials such as hydrazine hydrate, ethyl nicotinate and absolute ethanol, carrying out a reaction under the conditionof 80 DEG C for synthesis, and performing aftertreatment; the target product is prepared by performing refluxing stirring reaction on the prepared intermediate nicotinoylhydrazine as well as a small amount of glacial acetic acid and aldehyde compound under the condition of 65 DEG C, performing suction filtration and performing recrystallization. The synthesis reaction is simple to operate and theyield of products is high; furthermore, the synthesis method is low in equipment requirement, low in production cost and easy in industrialized large-scale application. In addition, the synthesized nicotinoyl hydrazone Schiff base compound can serve as the bactericide to selectively kill germs, and the prevention effect on wheat powdery mildew can reach to 83.1 percent.
Owner:HUAIHUA UNIV
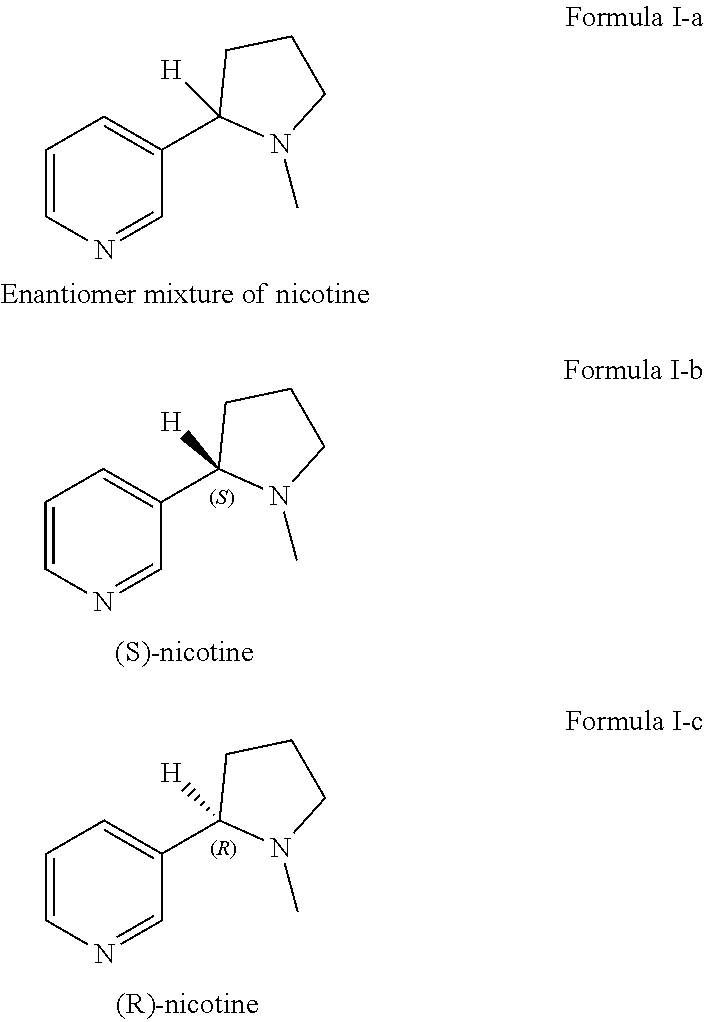
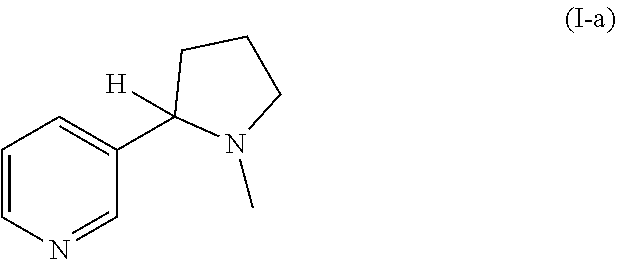
Preparation of racemic nicotine by reaction of ethyl nicotinate with n-vinylpyrrolidone in the presence of an alcoholate base and subsequent process steps
ActiveUS20200331884A1Organic baseOrganic active ingredientsNervous disorderPyrrolidinonesEthyl ester
The present invention relates to a method of preparing racemic nicotine comprising: (i) reacting ethyl nicotinate and N-vinylpyrrolidone in the presence of an alcoholate base to 3-nicotinoyl-1-vinylpyrrolidin-2-one; (ii) reacting the 3-nicotinoyl-1-vinylpyrrolidin-2-one with an acid to myosmine; (iii) reducing the myosmine to nornicotine using a reducing agent; and (iv) methylating the nornicotine to obtain the racemic nicotine.
Owner:CONTRAF NICOTEX TOBACCO GMBH +1
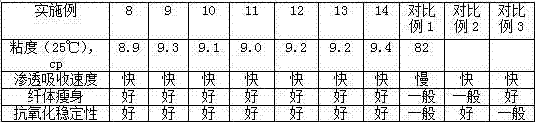
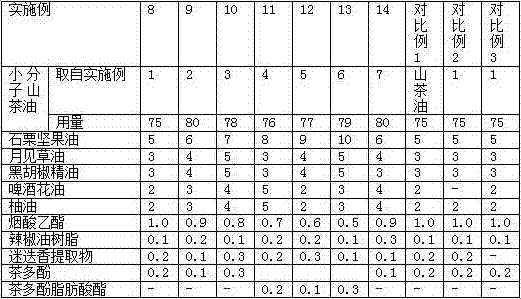
High-penetration slimming camellia japonica massage oil and preparation method thereof
InactiveCN107260608AThe preparation process is simple and reliableTo promote metabolismCosmetic preparationsToilet preparationsMetaboliteAdditive ingredient
The invention discloses high-penetration slimming camellia japonica massage oil, which is prepared by evenly mixing the following ingredients in parts by mass: 70 to 80 parts of micromolecule camellia japonica massage oil, 5 to 10 parts of candlenut nut oil, 3 to 5 parts of evening primrose seed oil, 3 to 5 parts of black pepper essential oil, 2 to 5 parts of hops oil, 2 to 5 parts of shaddock oil, 0.5 to 1 part of ethyl nicotinate, 0.1 to 0.3 part of capsicum oleoresin, 0.1 to 0.3 part of rosemary extract and 0.1 to 0.3 part of tea polyphenol or aliphatic ester of the tea polyphenol. The camellia japonica massage oil has no obvious greasy feeling, can effectively permeate to subcutaneous fat from skin, promotes internal circulation of blood and cells, dredges blood vessels, drains toxin and drains fat metabolite out of a body.
Owner:湖南大三湘油茶生态产业有限公司
Preparation method of 3, 4, 5-trimethylhydroquinone dialkanoate
ActiveCN111393290AHigh selectivityHigh yieldPreparation rom asymmetrical anhydridesPreparation from carboxylic acid halidesO-Phosphoric AcidPtru catalyst
The invention provides a preparation method of 3, 4, 5-trimethylhydroquinone dialkanoate. The preparation method comprises the following steps: reacting in the presence of a catalyst and a cocatalystand carrying out esterification and rearrangement reaction on 2, 6, 6-trimethylcyclohexyl-2-ene-1, 4-diketone and an acylating agent to obtain the product, wherein the catalyst is selected from sulfuric acid, hydrochloric acid, phosphoric acid, fluoroboric acid, p-toluenesulfonic acid, benzenesulfonic acid, methanesulfonic acid, chloroacetic acid, BF3, BF3.OEt2, AlCl3, FeCl3, ZnCl2, TiCl4, SnCl2 and the like, and the cocatalyst is selected from nicotinic acid, 3-pyridylaldehyde, phenyl nicotinate, furoate nicotinate, 5-hydroxy-6-methyl nicotinic acid and 5-hydroxy-6-methyl ethyl nicotinate. The process has the advantages of easiness in industrialization, good selectivity, high product yield and the like.
Owner:WANHUA CHEM GRP CO LTD
Composition containing attractant of noxious arthropod comprising plant-derived component and analogue of same
InactiveUS20160227776A1Superior noxious arthropod attracting effectEfficient captureBiocideDrug compositionsBenzaldehydeEugenol
Owner:KYOYU AGRI
Preparation method of beta-nicotinamide mononucleotide and purification method
ActiveCN111253448AHigh yieldHigh selectivitySugar derivativesSugar derivatives preparationPhosphorylationNucleotide
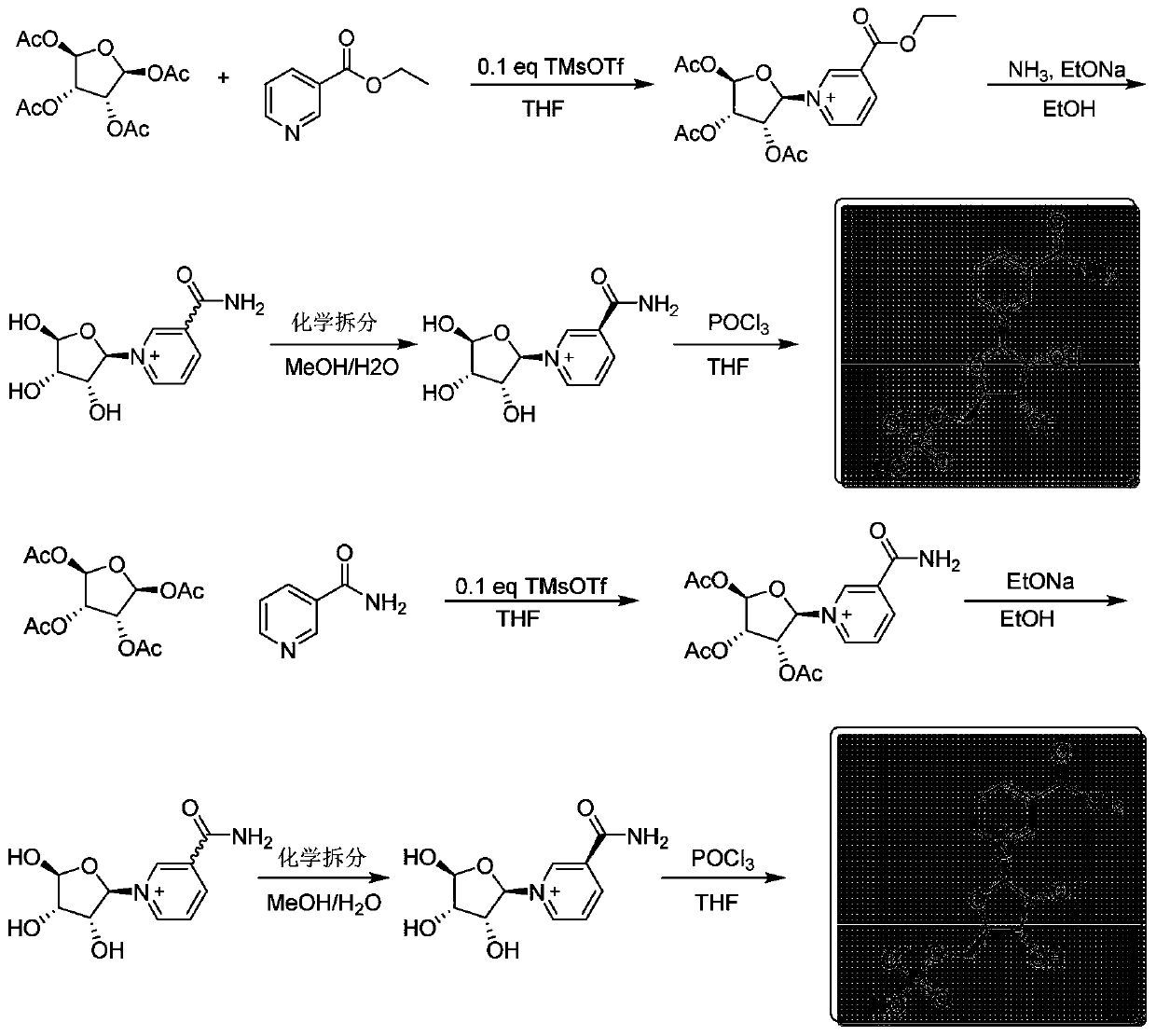
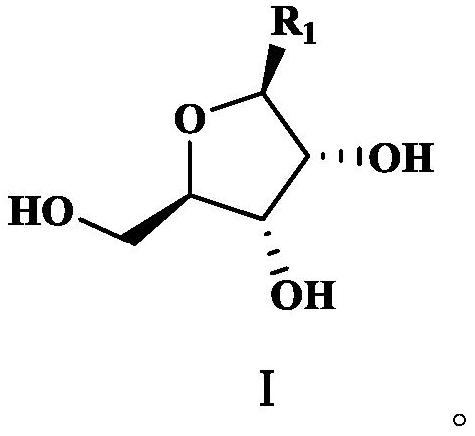
The invention discloses a preparation method of beta-nicotinamide mononucleotide and a purification method. The purification method of a furanose substrate intermediate material comprises the following steps: (1) extracting the furanose substrate intermediate material serving as a raw material by adopting a water phase and an oil phase, and collecting a water phase containing a compound shown in aformula I; and (2) extracting the water phase containing the compound shown in the formula I in the step (1) by adopting a phosphorylation auxiliary agent, and collecting a phosphorylation auxiliaryagent phase containing the compound shown in the formula I to obtain a purified furanose substrate intermediate material, wherein the raw material furanose substrate intermediate material is a mixed material containing the compound shown in the formula I, and R1 is one or more of acetyl, bromine, nicotinamide, ethyl nicotinate and butyl nicotinate. The preparation method of the beta-nicotinamide mononucleotide can effectively improve the total yield and purity, the reaction raw materials are cheap and easy to obtain, and the preparation process is simple and suitable for industrial production.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
Method for preparing tazarotene without copper iodide
ActiveCN109081835AEasy to recycleShort reaction timeOrganic chemistryPalladium catalystReaction temperature
The invention provides a method for preparing tazarotene without copper iodide. Palladium catalysts and triphenylphosphine are added into specific organic solvents and stirred, replacement in nitrogenis performed, 4, 4-dimethyl thiochroman-6-radical-acetylene, 6-chloro-ethyl nicotinate and acid-binding agents are added to perform reaction. The method is environmentally friendly, the catalysts areeasily recycled, and waste water containing heavy metal is not generated. Compared with the prior art, the method is short in reaction time and reaction temperature. A product prepared by the methodis high in yield and purity and stable in quality.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Preparation method of high-purity L-nicotine medical intermediate
The invention discloses a preparation method of a high-purity L-nicotine medical intermediate, which comprises the following steps: synthesis of myosmin: pumping dry xylene into a condensation reaction kettle, adding metal sodium and tert-butyl alcohol, heating, preserving heat, and cooling for later use; the method comprises the following steps: pumping xylene, ethyl nicotinate and N-vinylpyrrolidone into a reaction kettle, carrying out heat preservation, carrying out stirring until the solution is clear, and pumping the solution into a high position; dropwise adding the high-order reaction liquid into a condensation reaction kettle, heating after dropwise adding, and cooling for later use; adding concentrated hydrochloric acid and water into an acidolysis kettle, stirring and cooling, transferring a reaction solution in the condensation reaction kettle into the acidolysis kettle, transferring a layered lower water layer into a reflux kettle for reflux, transferring into a desolventizing kettle, transferring a desolventizing kettle bottom solution into a myosmin distillation kettle, and distilling off myosmin under reduced pressure; and preparing the L-nicotine. By adopting the preparation method of the high-purity L-nicotine medical intermediate, the high-purity L-nicotine is obtained, the consumption of acid, alkali and organic matters is reduced, and the cost is greatly reduced.
Owner:仙居两山生物科技有限公司
Method for improving ring crush compression resistance of corrugated paper
InactiveCN109137609AEnhance ring sub-strengthImprove stabilityCoatings with pigmentsWater-repelling agents additionMethacrylateBetaine
The invention belongs to the technical field of corrugated paper, and particularly relates to a method for improving the ring crush compression resistance of corrugated paper. A surface reinforcing adhesive is applied to the surface of the corrugated paper, and the surface reinforcing adhesive comprises modified starch, alkali-activated metakaolin-based oligomer, (methyl)methacrylate, 2-hydroxyl-4-methoxyl benzophenone, ethyl nicotinate, fatty alcohol-polyoxyethylene ether, organic amine, an additive, polycaprolactone polyhydric alcohol, sulfopropyl glycine betaine and water. Compared with theprior art, the method has the advantages that the alkali-activated metakaolin-based oligomer and the modified starch cooperate, and the ring crush compression resistance of the corrugated paper can be effectively enhanced; ethyl nicotinate, fatty alcohol-polyoxyethylene ether, organic amine and polycaprolactone polyhydric alcohol cooperate, the stability of the surface reinforcing adhesive is improved, the water resistance of the corrugated paper is improved in cooperation with sulfopropyl glycine betaine and the additive, and the application range of the corrugated paper can be effectively widened.
Owner:BENGBU AOTE CARTON MACHINERY
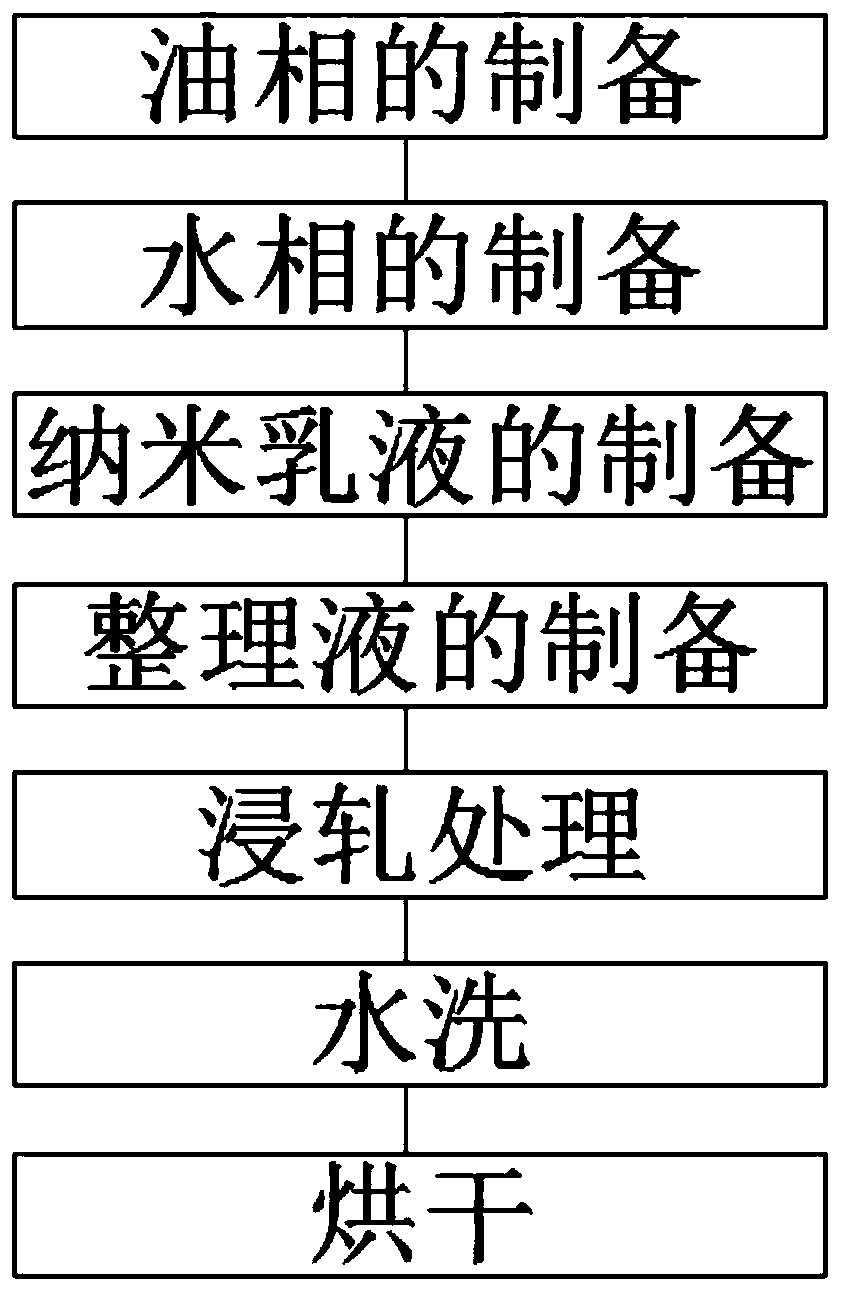
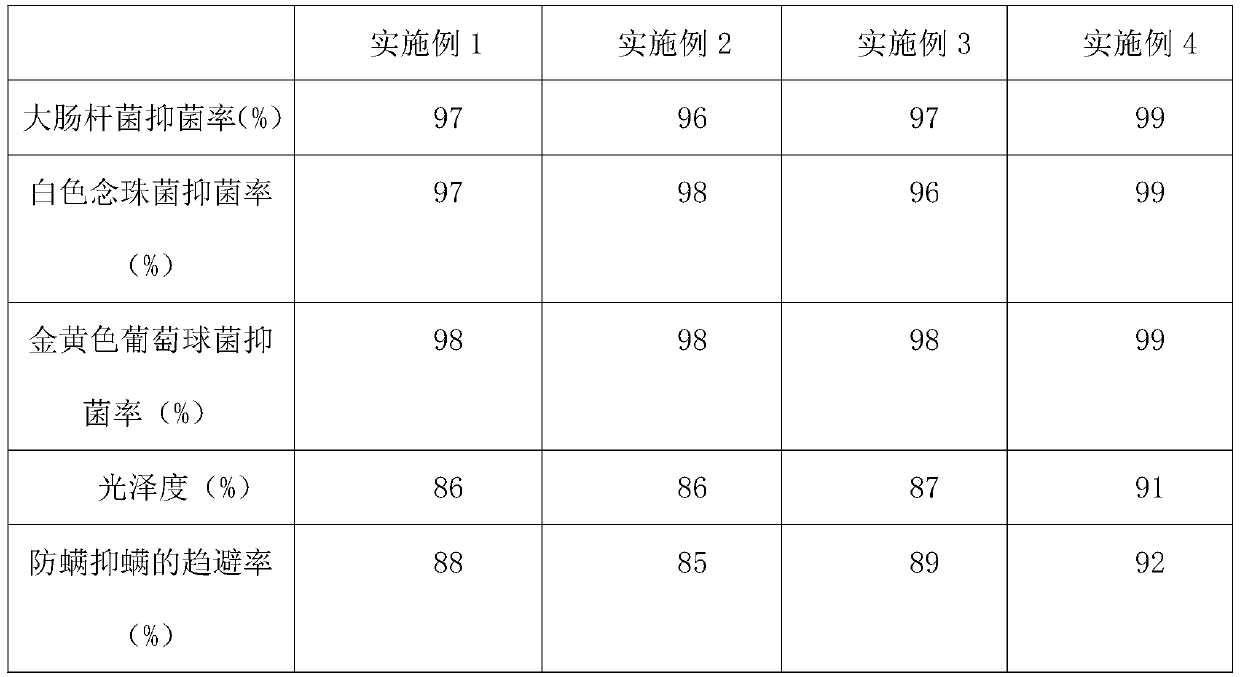
After-finishing technology for home textile fabric based on shea butter
InactiveCN111088694AHigh glossAchieve anti-mite effectBiochemical fibre treatmentBiotechnologyEngineering
The invention discloses an after-finishing technology for home textile fabric based on shea butter, and belongs to the home textile fabric processing technologies. The after-finishing technology for home textile fabric based on shea butter is completed via the steps of preparation of oil phase, preparation of aqueous phase, preparation of nano-emulsion, preparation of finishing liquid, padding processing, washing and drying. The nano-emulsion is added into the finishing liquid, the nano-emulsion is prepared by using shea butter, and then the shea butter is combined with the textile fabric viathe after-finishing technology, so that when skin of a consumer is in contact with the textile fabric, the effect of moistening skin and preserving moisture is achieved; hyaluronic acid, butyl ethyl nicotinate and acetyl hydroxyproline are added, so that glossiness of the fabric is improved; meanwhile, a mite suppression agent is added, so that the mote suppression effect of the textile fabric isrealized; and due to addition of chitin, the fabric has the anti-microbial and bacteriostatic effects.
Owner:李慧峰
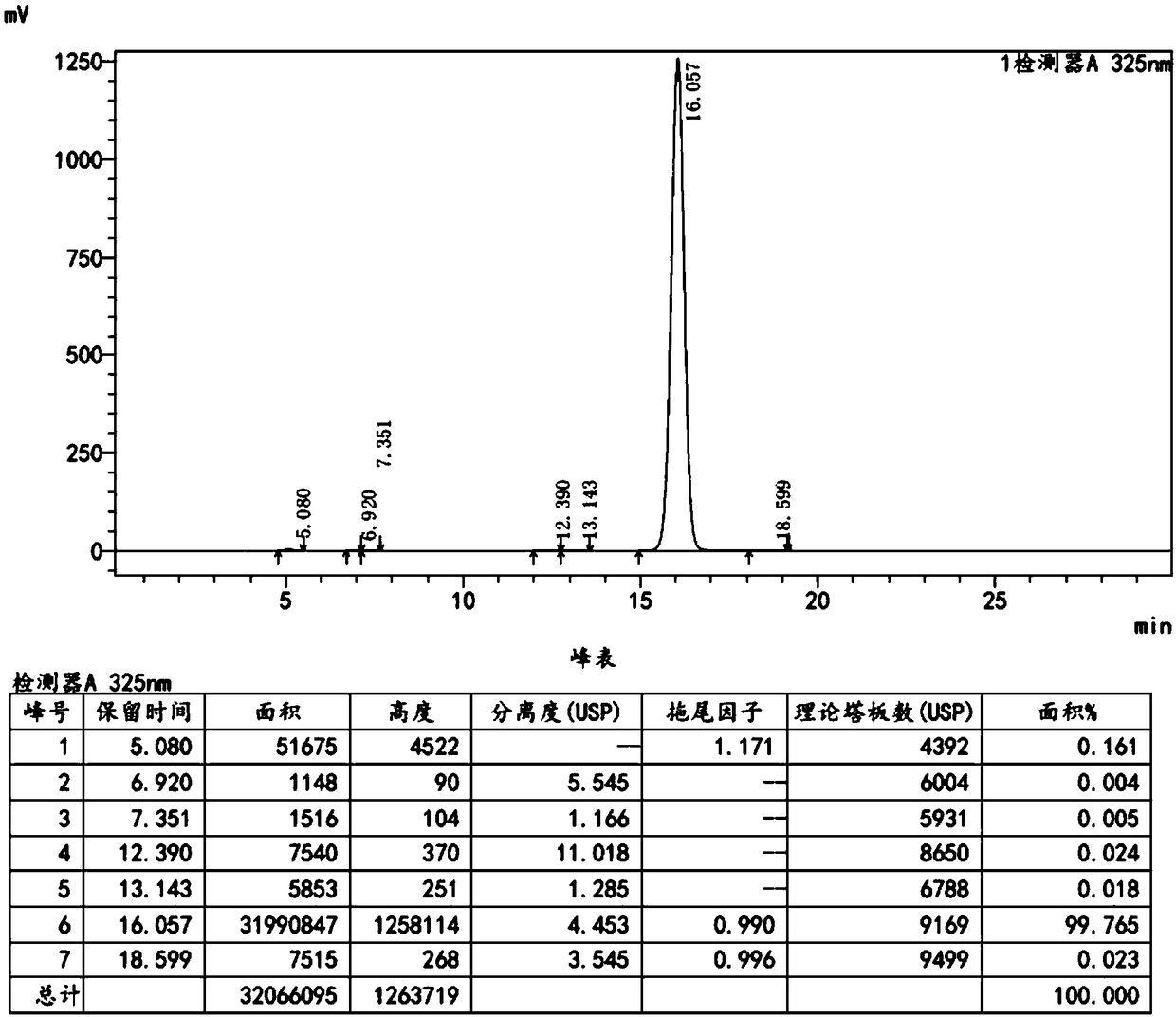
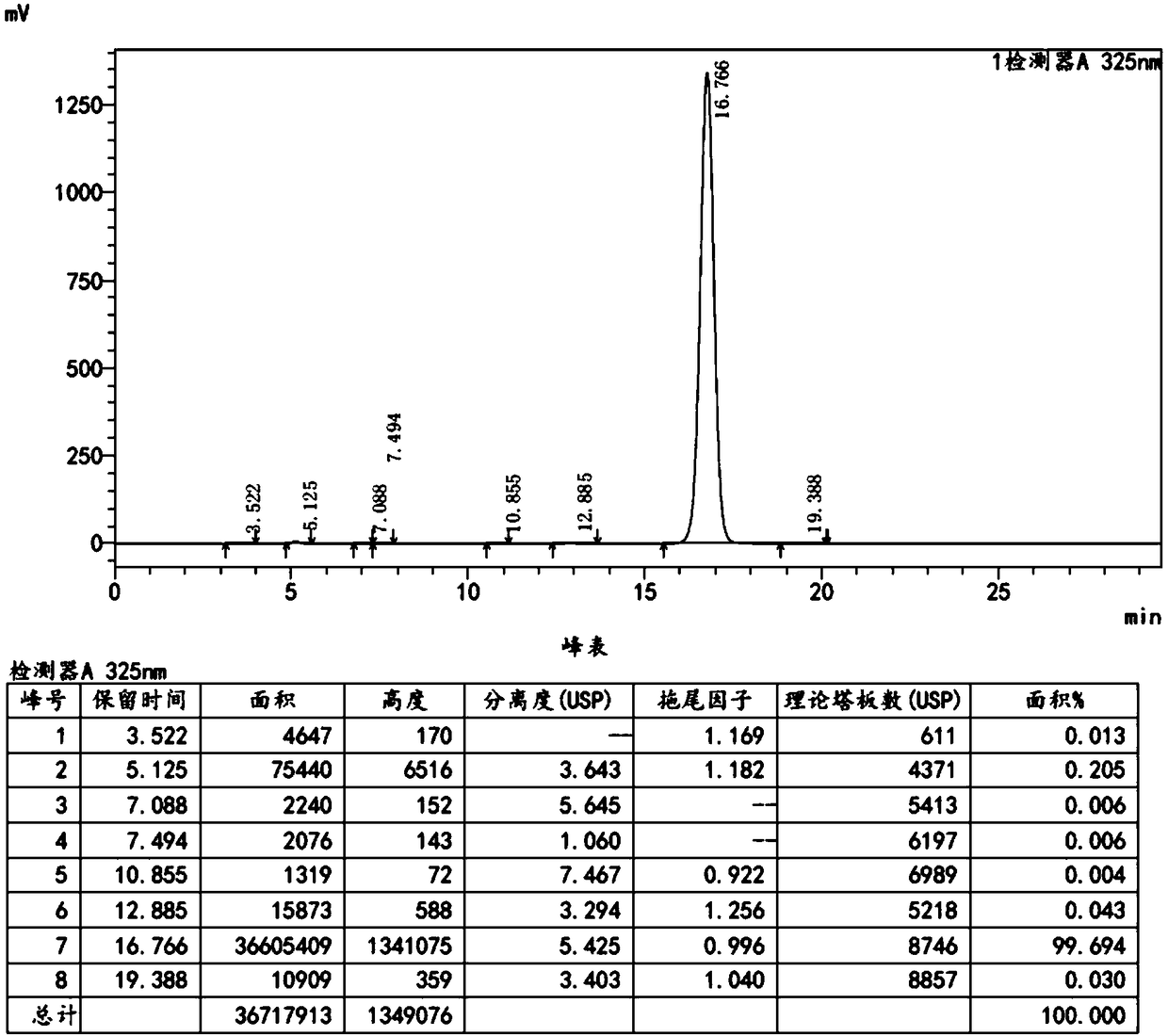
Preparation method of ethyl nicotinate
InactiveCN106957262AReduce generationSimple post-processingOrganic chemistryChemical recyclingAnhydrous ethanolAlcohol
The invention relates to a preparation method of ethyl nicotinate. The preparation method is characterized by comprising the following steps of: adding nicotinic acid, absolute ethyl alcohol and an esterification reaction solid acid catalyst into methylbenzene; stirring the mixture to react for 3-6h at 50-65 DEG C; then raising the temperature to return and divide water; finishing the reaction till no water is divided; reducing the temperature to room temperature; filtering and recovering the esterification reaction solid acid catalyst; recovering methylbenzene at a reduced pressure from the filtrate; recovering the methylbenzene to obtain a light yellow transparent liquid which is the finished product ethyl nicotinate, wherein the molar ratio of nicotinic acid and absolute ethyl alcohol is 1: 1 to 1: 2; the weight ratio of nicotinic acid and methylbenzene is 1:0.3 to 1:8; the adding amount of the esterification reaction solid acid catalyst is 0.01-0.1 time of the weight of the nicotinic acid; the esterification reaction solid acid catalyst is an HND230 solid catalyst. The preparation method provided by the invention has the advantages of reducing generation of highly polluted wastewater, reducing the manual and equipment costs and being relatively suitable for industrial production on a large scale.
Owner:常州沃腾化工科技有限公司
Rheumatism expelling bath salt and preparing method thereof
InactiveCN104997712APrevention and treatment ofPrevention and Treatment of RelapseCosmetic preparationsAntipyreticCelluloseSodium stearate
The invention relates to rheumatism expelling bath salt and a preparing method thereof. The rheumatism expelling bath salt comprises, by mass, 80-110 parts of anhydrous sodium sulfate, 2-8 parts of potassium chloride, 4-12 parts of sodium stearate, 15-25 parts of ethyl nicotinate, 30-40 parts of beta-lactose, 25-35 parts of sodium chloride, 10-19 parts of fat alkylamide propyl dimethyl amine oxide, 1.5-6.5 parts of kojic acid, 0.1-0.5 part of pigment, 2-5 parts of jasmine essence, 4-9 parts of carboxy methyl cellulose, 12-20 parts of Chinese starjasmine stem, 10-18 parts of loofah sponge and 8-15 parts of long-noded pit viper. The bath salt can be used for effectively removing dirt on the surface of the skin to make the skin become white, tender, smooth and rich in elasticity, meanwhile, the effect of expelling rheumatism is achieved, and after the bath salt is used for a long time, generation and relapses of rheumatism can be prevented effectively, and rheumatism can be effectively treated.
Owner:JIANGSU QILIKANG SKIN PHARMA
Mid-temperature e-cigarette
A mid-temperature electronic cigarette that heats ingredients to a temperature range between 125 and 145 degrees Fahrenheit. The temperature range is critical for a consistent cigarette-like taste, aroma, and physiological satisfaction. The ingredients can include tobacco extract, ethyl nicotinate, ethanol, nicotine and ethanol-based flavors, DMEA, but not propylene glycol (PG) or vegetable glycerin (VG). Due to the consistent temperature range, the mid-temperature electronic cigarette can consistently deliver 8-10 μg nicotine per puff, for at least 10 puffs, over 2-8 hours.
Owner:BANKERT TIMOTHY J +2
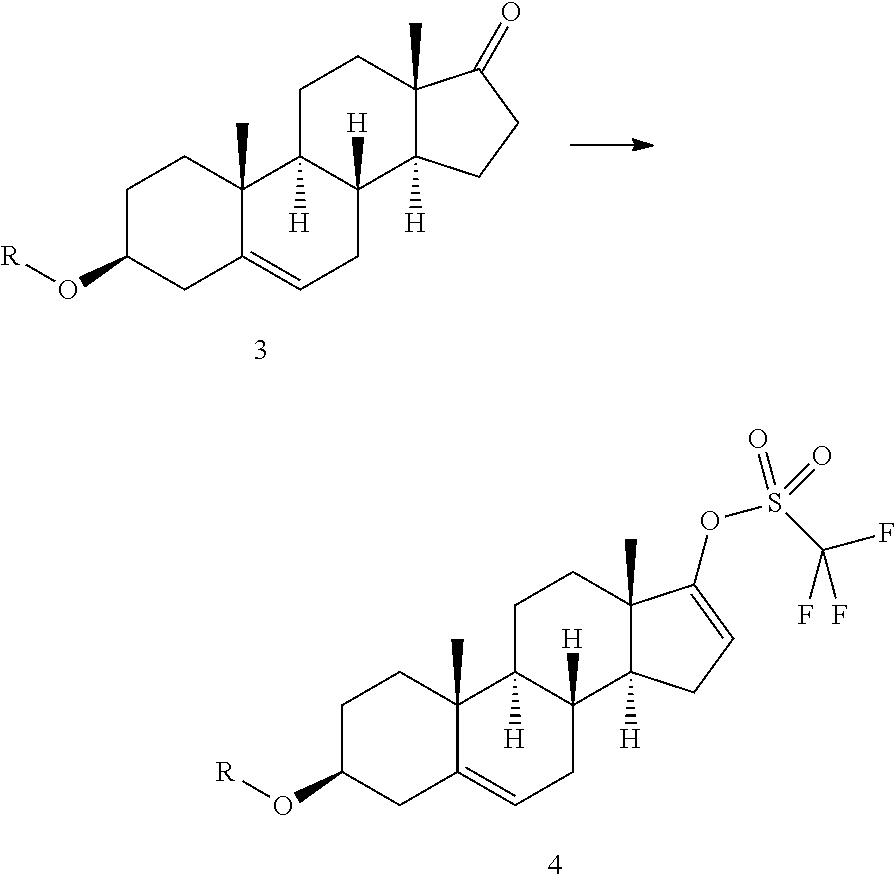
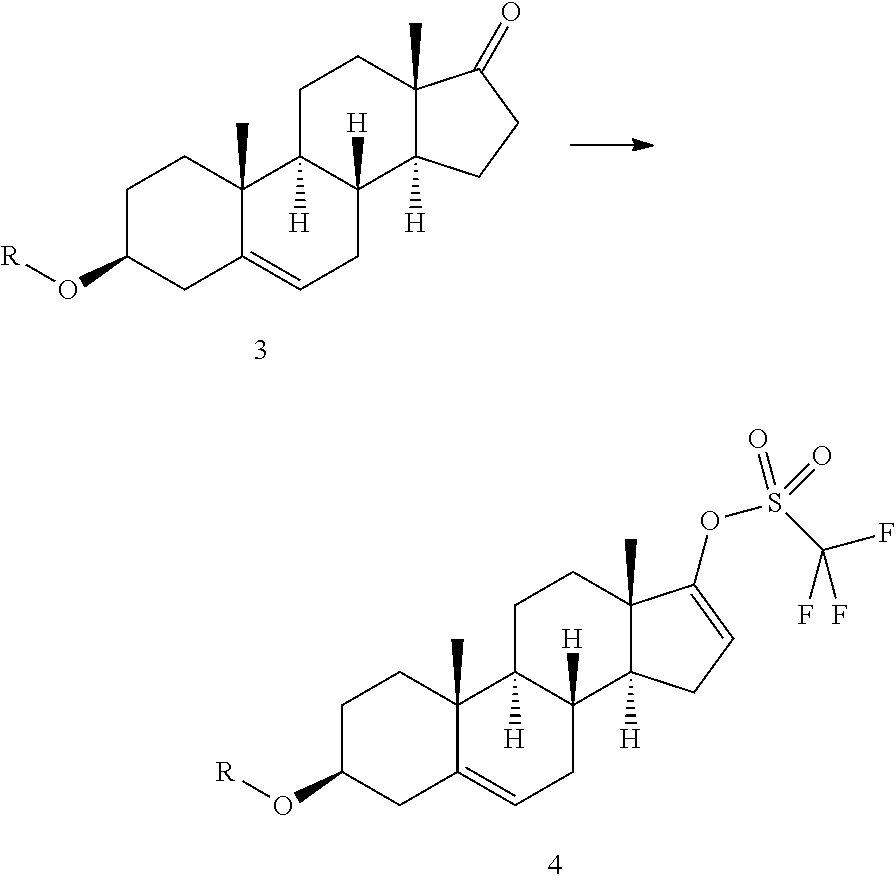
Process for the preparation of unsaturated trifluoromethanesulfonate steroid derivatives
Disclosed is a method for the conversion of a compound of formula 3 to a compoundof formula 4, wherein R is an acetyl group or an alcohol-protecting group. The process involves reacting 3 with a triflating agent in the presence of a nicotinate (3-pyridinecarboxylate) of a C1-C4 alcohol, preferably methyl nicotinate (methyl 3-pyridinecarboxylate) or ethyl nicotinate (ethyl 3-pyridinecarboxylate), to give 4. The method can be conveniently used in a process for the preparation of Abiraterone or Abiraterone acetate.
Owner:OLON
Preparation of racemic nicotine by reaction of ethyl nicotinate with N-vinylpyrrolidone in the presence of an alcoholate base and subsequent process steps
Owner:CONTRAF NICOTEX TOBACCO GMBH +1
Method for closed-loop synthesis of nicosulfuron by using hydrogen sulfide
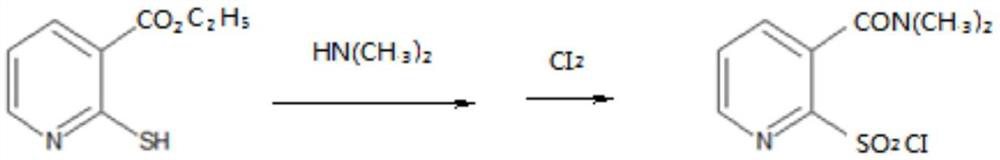
ActiveCN113461667AMild and controllable reactionSimple process equipmentOrganic chemistryReaction stepEthyl nicotinate
The invention belongs to the technical field of nicosulfuron original medicine production, and particularly relates to a method for closed-loop synthesis of nicosulfuron by using hydrogen sulfide, wherein the method comprises the following steps: reacting tetramethoxypropane with ethyl cyanoacetate to generate 1-cyano-4-methoxy-1-ethoxycarbonyl-1,3-butadiene (cyanoene for short, the same below); reacting cyano alkene with hydrogen sulfide to generate ethyl 2-mercaptonicotinate (sulfydryl substance for short) in a closed-loop manner; reacting the sulfydryl substance with dimethylamine, and then carrying out oxychlorination reaction to obtain sulfonyl chloride; carrying out ammonolysis reaction on the sulfonyl chloride and ammonia gas to obtain sulfonamide; and reacting sulfonamide with solid light and pyrilamine to obtain the nicosulfuron. According to the method for closed-loop synthesis of nicosulfuron by using hydrogen sulfide, each reaction step is mild and controllable, process equipment is simple, the production cost is low, the product quality is good, three wastes are reduced, energy is saved, the production environment is improved, and the goal of carbon neutralization is favorably realized.
Owner:ZIBO NAB AGROCHEM
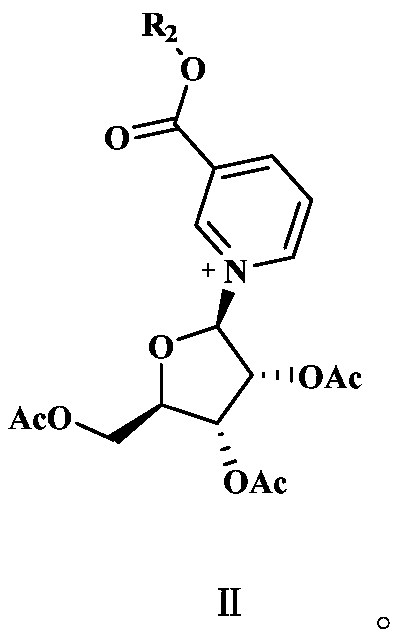
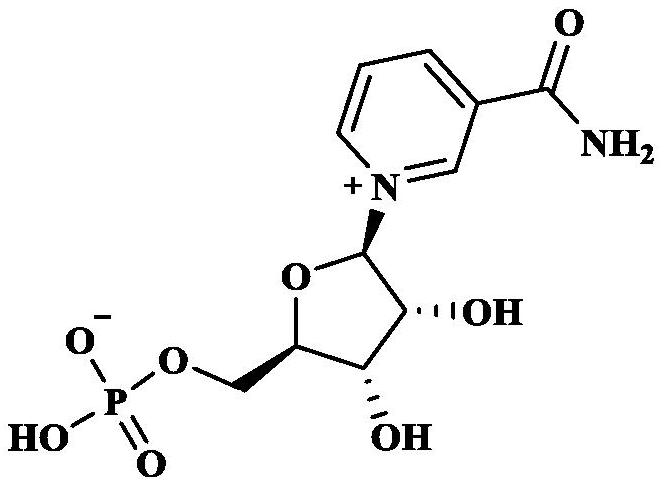
Methyl and ethyl nicotinate-riboside-5-phosphates, preparation thereof and methods of use thereof
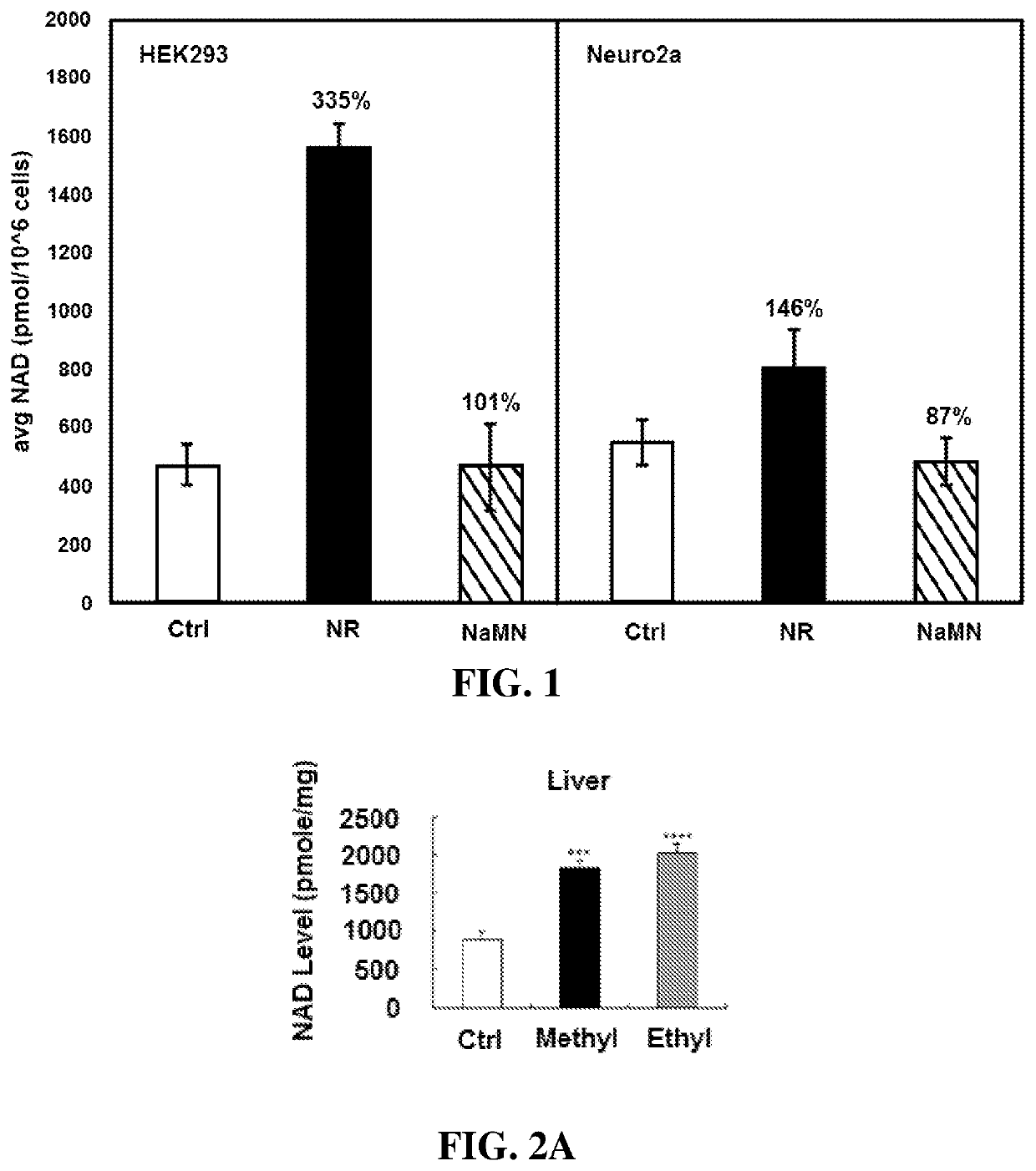
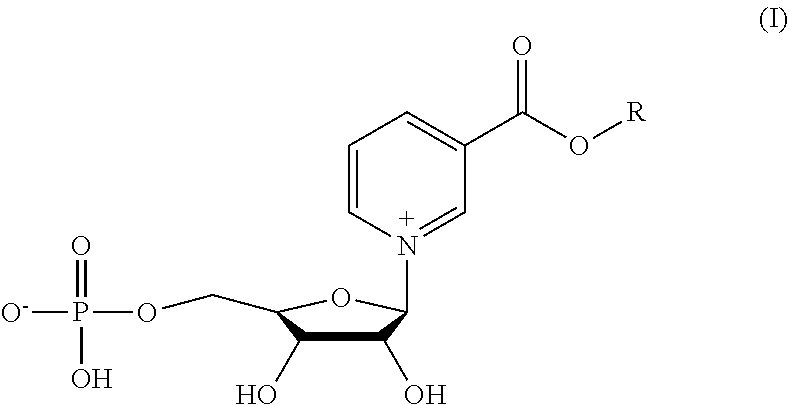
The invention provides a compound of formula (I): (I) wherein R is methyl or ethyl. The invention also provides a process for the preparation of the compound. The invention further provides a method for increasing cell NAD+ production or improving mitochondrial densities in a cell, wherein the method comprises administering to the cell a compound or salt of the invention.
Owner:CORNELL UNIVERSITY
Method for synthesis of 4-OH substituted anabaseine derivative
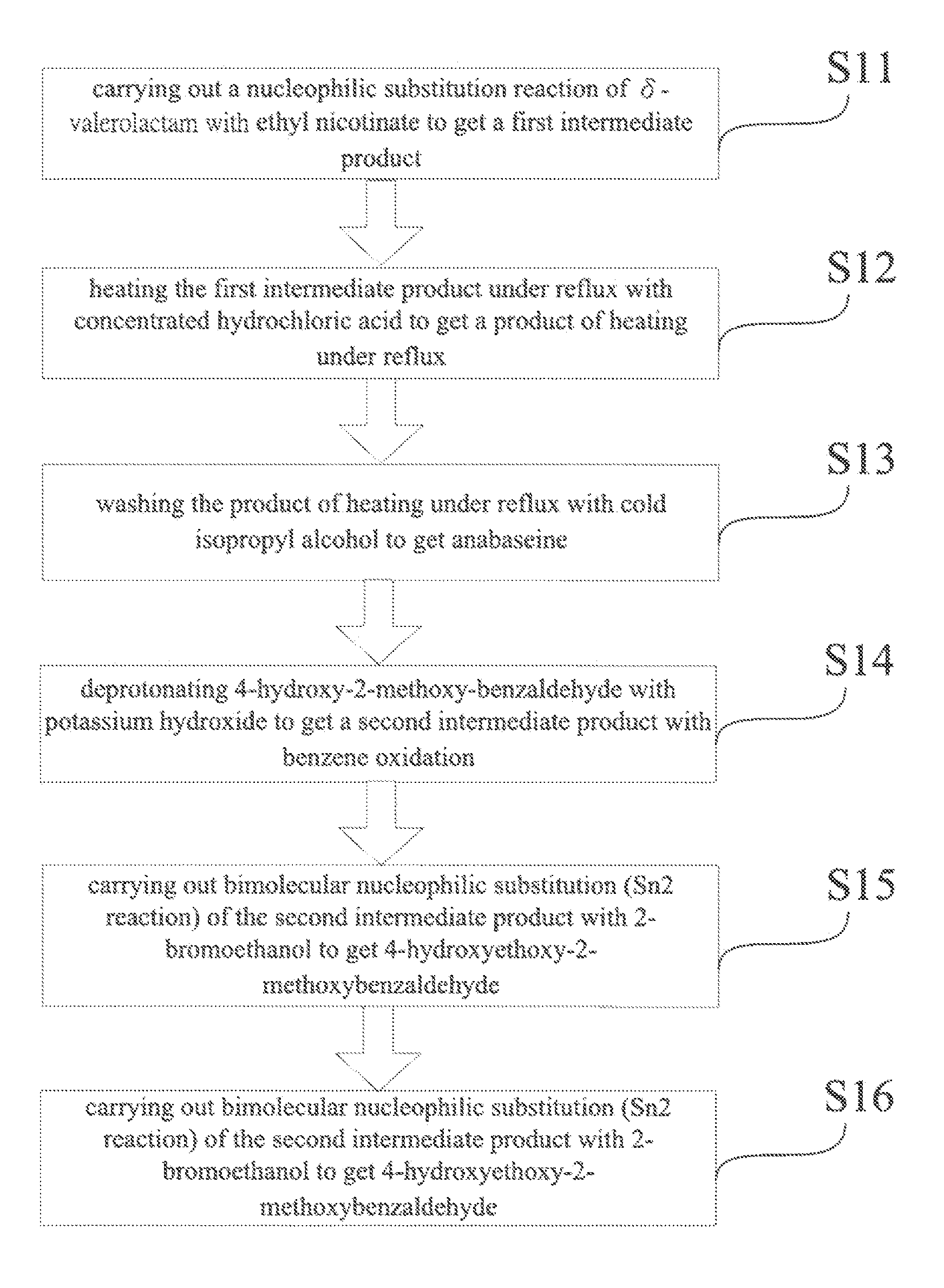
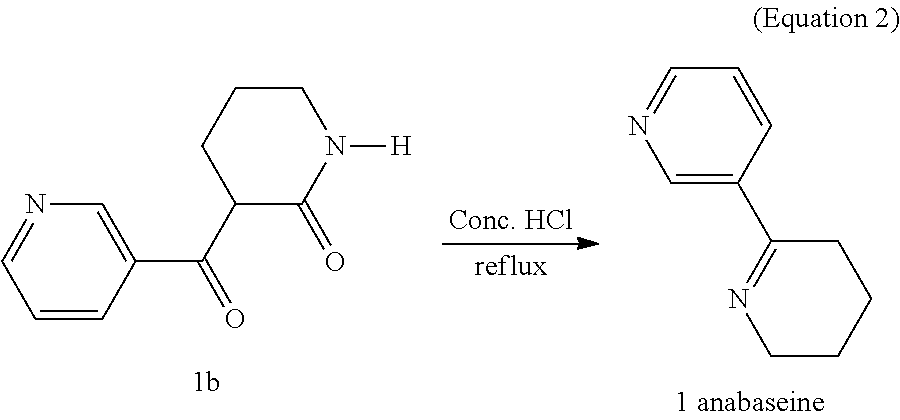
A method for synthesis of 4-OH substituted anabaseine derivative which is used as an α7 receptor agonist is revealed. A nucleophilic substitution reaction of δ-valerolactam with ethyl nicotinate is carried out to get an intermediate product. Then heat the intermediate product under reflux with concentrated hydrochloric acid to get a cyclized product-anabaseine. Next anabaseine and 4-hydroxyethoxy-2-methoxybenzaldehyde are reacted under concentrated hydrochloric acid catalysis to get a 4-OH anabaseine derivative 3-[(4-Hydroxyethoxy-2-methoxy)-benzylidene]anabaseine. The 4-OH anabaseine derivative is synthesized and prepared easily by the present invention. Not only the steps for synthesis are simplified, the yield is as high as 60%. The product can be mass-produced.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Process for the preparation of unsaturated trifluoromethanesulfonate steroid derivatives
Disclosed is a method for the conversion of a compound of formula 3 to a compoundof formula 4, wherein R is an acetyl group or an alcohol-protecting group. The process involves reacting 3 with a triflating agent in the presence of a nicotinate (3-pyridinecarboxylate) of a C1-C4 alcohol, preferably methyl nicotinate (methyl 3-pyridinecarboxylate) or ethyl nicotinate (ethyl 3-pyridinecarboxylate), to give 4. The method can be conveniently used in a process for the preparation of Abiraterone or Abiraterone acetate.
Owner:OLON
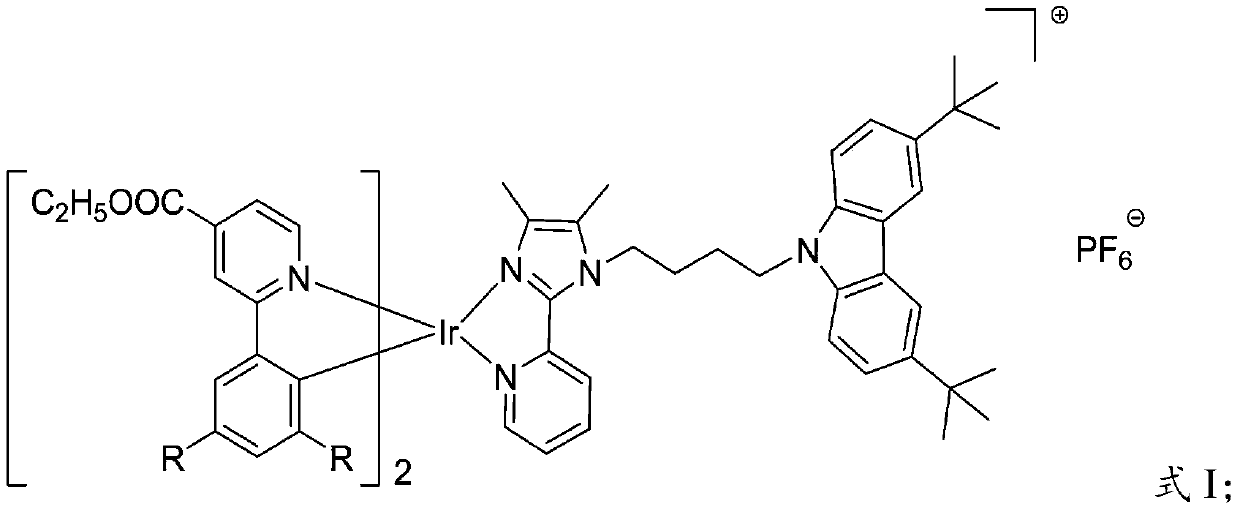
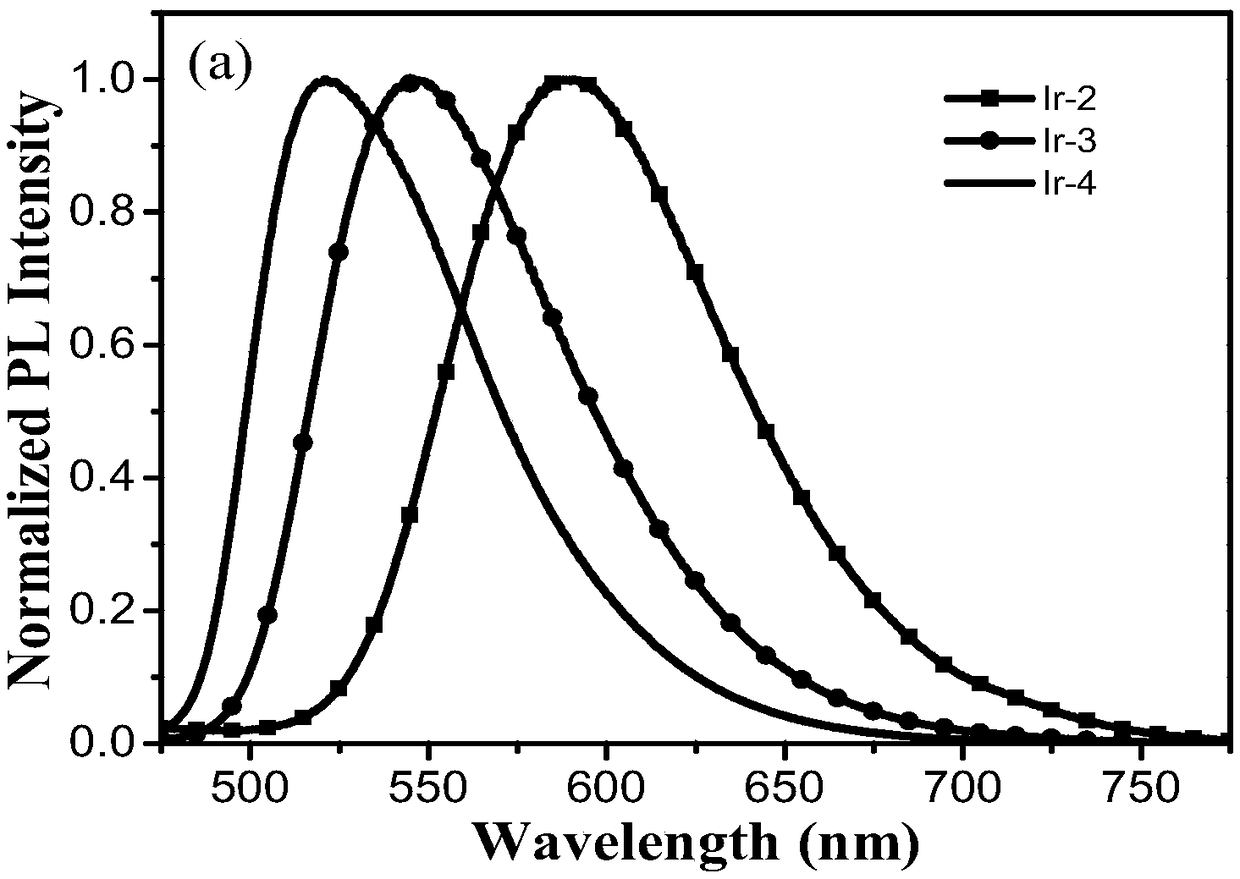
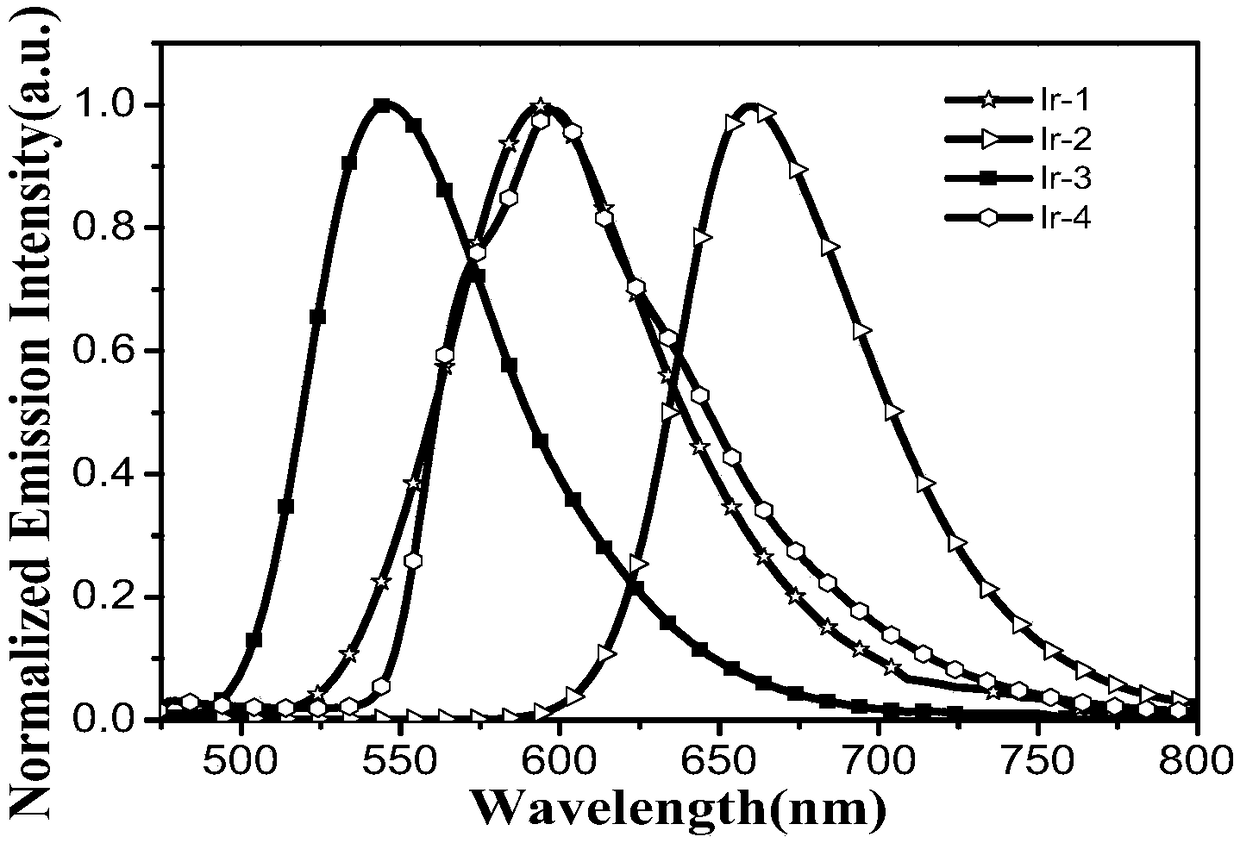
A kind of iridium (Ⅲ) complex and its preparation method and application
ActiveCN109053815BGood light stabilityImprove luminous efficiencyIndium organic compoundsFluorescence/phosphorescenceCarbazolePhoto stability
The invention provides an iridium (III) complex. A 2-phenylethyl isonicotinate derivative is used as the main ligand, and 3,6-di-tert-butyl -9-(4-(4,5)-dimethyl-2-(pyrid-2-yl)-1H-imidazol-1-yl) butyl)-9H-carbazole is used as the auxiliary ligand. The iridium (III) complex has good light stability, large Stokes shift, high luminous efficiency and aggregation-induced emission property, and can be applied to the field of cell imaging.
Owner:NANJING TECH UNIV
A preparation method and purification method of β-nicotinamide mononucleotide
ActiveCN111253448BHigh yieldHigh selectivitySugar derivativesSugar derivatives preparationPhosphorylationNucleotide
The invention discloses a preparation method and purification method of β-nicotinamide mononucleotide. The method for purifying the furanose substrate intermediate material comprises the following steps: (1) extracting the raw material furanose substrate intermediate material with water phase and oil phase, and collecting the water phase containing the compound shown in formula I; (2) using phosphorylation The auxiliary agent extracts the aqueous phase containing the compound shown in formula I in step (1), collects the phosphorylation auxiliary phase containing the compound shown in formula I, and obtains the purified furanose substrate intermediate material; wherein, the raw material furanose substrate The intermediate material of the product is a mixed material comprising a compound represented by formula I, wherein R1 is one or more of acetyl, bromo, nicotinamide, ethyl nicotinate and butyl nicotinate. The preparation method of β-nicotinamide mononucleotide in the present invention can effectively improve the total yield and purity, the reaction raw materials are cheap and easy to obtain, the preparation process is simple, and it is suitable for industrial production.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399BComplicated operationFew stepsOrganic chemistryCyanoacetic acidOrganic synthesis
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing thereaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
A kind of preparation method of tazarotene without using cuprous iodide
The invention provides a method for preparing tazarotene without the participation of cuprous iodide. First, a palladium catalyst and triphenylphosphine are added to a specific organic solvent, stirred, replaced by nitrogen, and then added 4,4-dimethyl Benzothiopyran-6-yl-acetylene, ethyl 6-chloronicotinate and acid-binding agent are reacted. The method is environmentally friendly, the catalyst is easy to recycle, no waste water containing heavy metals is produced, and the reaction time is shorter and the reaction temperature is lower than the prior art. The product obtained by the method has high yield, high purity and stable quality.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Iridium (III) complex, preparation method and application thereof
ActiveCN109053815AGood light stabilityImprove luminous efficiencyIndium organic compoundsFluorescence/phosphorescenceIridiumAggregation-induced emission
The invention provides an iridium (III) complex. A 2-phenylethyl isonicotinate derivative is used as the main ligand, and 3,6-di-tert-butyl -9-(4-(4,5)-dimethyl-2-(pyrid-2-yl)-1H-imidazol-1-yl) butyl)-9H-carbazole is used as the auxiliary ligand. The iridium (III) complex has good light stability, large Stokes shift, high luminous efficiency and aggregation-induced emission property, and can be applied to the field of cell imaging.
Owner:NANJING UNIV OF TECH
Synthesis method and synthesis device of beta-nicotinamide mononucleotide
InactiveCN111943992APrevent leakageOperational securitySugar derivativesSugar derivatives preparationPtru catalystPhosphorylation
The invention provides a synthesis method of beta-nicotinamide mononucleotide. The synthesis method of the beta-nicotinamide mononucleotide comprises the following steps: S1, condensation reaction: adding ethyl nicotinate, tetraacetylribose and a solvent A into a reaction kettle, performing stirring and dissolving at room temperature, dropwise adding a solution of a catalyst A, carrying out condensation reaction at 50 DEG C for 1 hour, cooling to room temperature after the reaction is finished, adding ethanol for quenching reaction, and removing the solvent under reduced pressure, and obtaining triacetyl nucleoside containing ethyl nicotinate; and S2, deacetylation: dissolving the ethyl nicotinate triacetyl nucleoside obtained in the step S1 into ethanol, and dropwise adding organic alkaliat 0 DEG C. The synthesis method of beta-nicotinamide mononucleotide provided by the invention has the advantages that two steps of phosphorylation and ammonolysis are integrated into one step, leakage of dangerous materials such as phosphorus oxychloride and ammonia gas and the like is avoided, the operation is safe and reliable, and the production cost is greatly reduced.
Owner:青岛新通道合成技术有限公司
A kind of synthetic method of 2-trifluoromethyl-3-fluoropyridine
ActiveCN102977009BLow costSimple and fast operationOrganic chemistryTrifluoromethylationChemical industry
The invention relates to the field of medicine and chemical industry. Aims at solving problems of high trifluoromethylation reagent toxicity, or high cost, complicated reaction, and low conversion rate existing in synthesizing reactions of trifluoromethylation similar compounds, the invention provides a synthesizing method of 2-trifluoromethyl-3-fluoropyridin. The method comprises the steps that: (2) with 2-trifluoromethyl ethyl nicotinate as a raw material, 2-trifluoromethyl-3-aminopyridine is produced through a reaction; and (2) the product obtained in the step (1) is subjected to a reaction with fluoboric acid, such that 2-trifluoromethyl-3-fluoropyridin is obtained. According to the invention, the raw materials have low cost, and are easy to obtain. Also, post treatment is simple. The method is suitable for laboratory small-scale preparation, and is suitable for large-scale industrialized production.
Owner:HANGZHOU ALLSINO CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com