Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
A technology of ethyl cyanoacetate and picoline, applied in the field of organic synthesis, can solve problems such as rising cost pressure, and achieve the effects of reducing steps, complicated operations, and reducing raw materials and operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
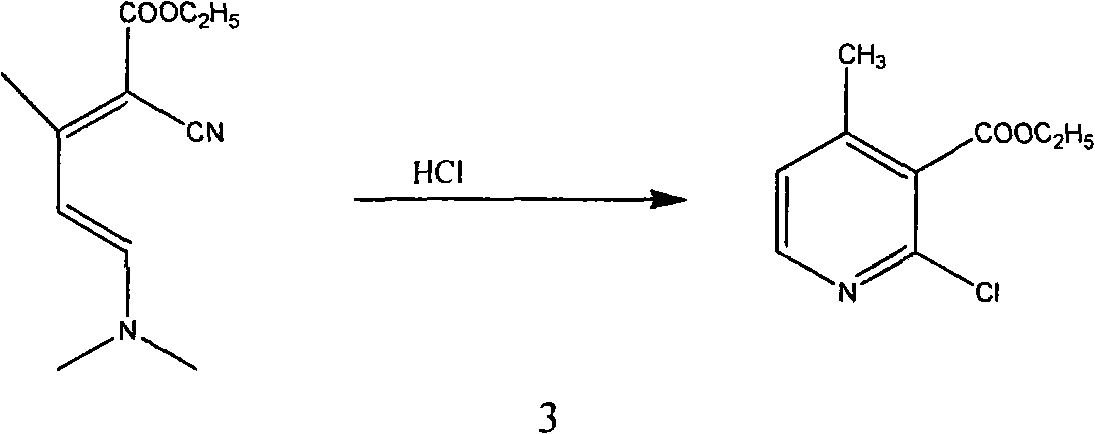
[0053] The first step, synthesis condensate I
[0054] Add 55g (0.49mol) of ethyl cyanoacetate (0.49mol), 90g of benzene, and 1.6g of piperidine into the reaction flask, heat up to reflux, add dropwise a mixture of 46g of acetone and 9.4g of glacial acetic acid, and reflux to separate water while adding dropwise. Then continue to reflux and divide the water until all the water is evaporated. After the reaction, cool down to 25°C, wash and separate the layers with 25ml of water, adjust the pH value of the organic phase to 8-9 with saturated sodium carbonate, and separate the layers at rest. Extract the aqueous phase with 50ml of benzene. The organic phases were combined and washed once with saturated sodium chloride solution, concentrated to dryness to obtain 71.5 g of condensate I (92% content, 95.4% molar yield), and 20 g of methanol was added after the condensate I was cooled;
[0055] Step 2: Synthesis of Condensate II
[0056] In a reaction bottle, under the protection of...
Embodiment 2
[0066] The other steps are the same, only the fourth step is changed:
[0067] Add 48g (0.24mol) of ethyl 2-chloro-4-methyl-nicotinate and 80g of ethylene glycol into the reaction device, raise the temperature to 120°C, pass ammonia gas for 24h, monitor the end point of the reaction by TLC, cool to room temperature, add 20g of water, separated into layers, the aqueous layer was extracted with 20ml of dichloromethane×3, the organic phases were combined and concentrated to obtain 34.8g of 2-chloro-4-methyl-nicotinamide (content 90.2%, molar yield 85.0%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com