Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "Diazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diazepine is a seven-membered heterocyclic compound with two nitrogen atoms (e.g., in ring positions 1 and 2).
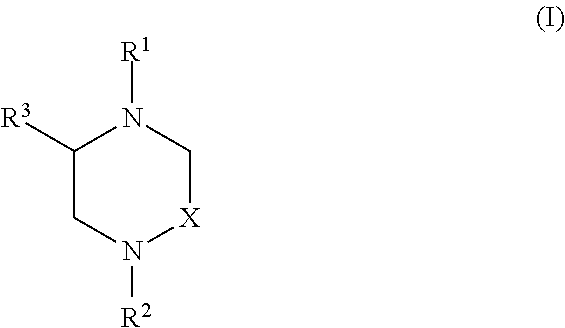
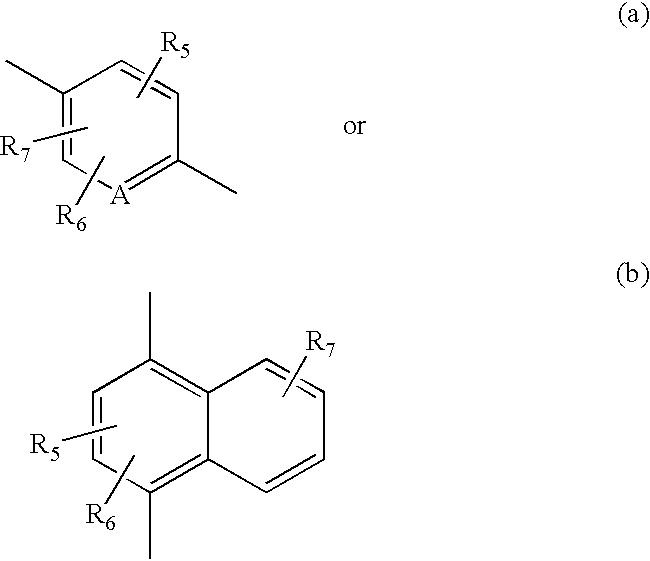
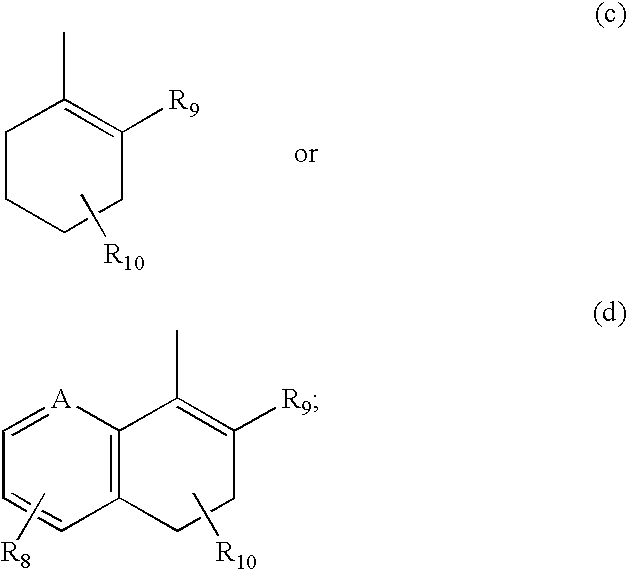
Hydroxy cyclohexenyl phenyl carboxamides tocolytic oxytocin receptor antagonists
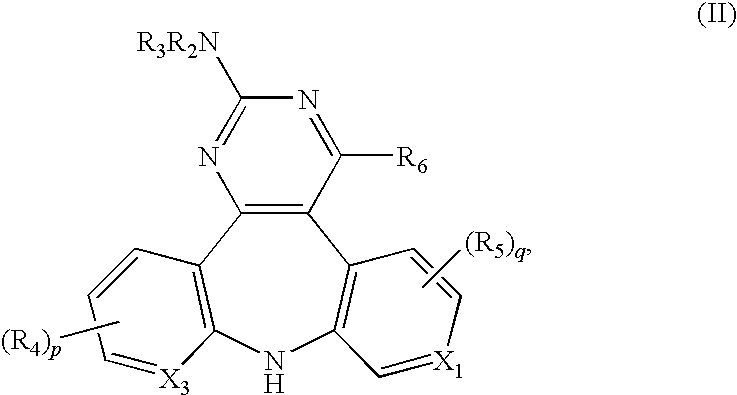
The present invention provides substituted 10,11-Dihydro-5H-benzo[e]-pyrrolo[1,2-a][1,4]diazepine and 9,10-Dihydro-4H-3a,5,9-triaza-benzo[f]azulene compounds as well as methods and pharmaceutical compositions utilizing these compounds for the treatment and / or prevention and / or suppression of disorders which may be remedied or alleviated by oxytocin antagonist activity, including prevention and / or suppression of preterm labor, suppression labor at term prior to caesarean deliver, and for the treatment of dysmenorrhea. These compounds are also useful in enhancing fertility rates, enhancing survival rates and synchronizing estrus in farm animals.
Owner:WYETH
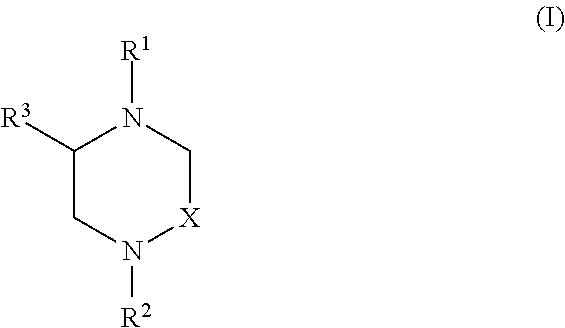
Tricyclic diazepines tocolytic oxytocin receptor antagonists
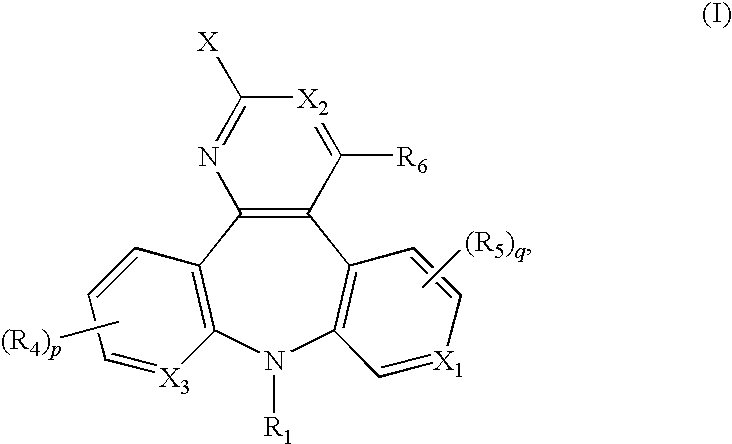
This invention provides novel tricyclic diazepine compounds as well as methods and pharmaceutical compositions utilizing these compounds for the treatment and / or prevention and / or suppression of disorders which may be remedied or alleviated by oxytocin antagonist activity, including treatment of preterm labor, dysmenorrhea, endometritis, and for suppressing labor prior to caesarean delivery. These compounds are also useful in enhancing fertility rates, enhancing survival rates and synchronizing estrus in farm animals; and may be useful in the prevention and treatment of disfunctions of the oxytocin system in the central nervous system including obsessive compulsive disorder (OCD) and neuropsychiatric disorders.
Owner:WYETH LLC
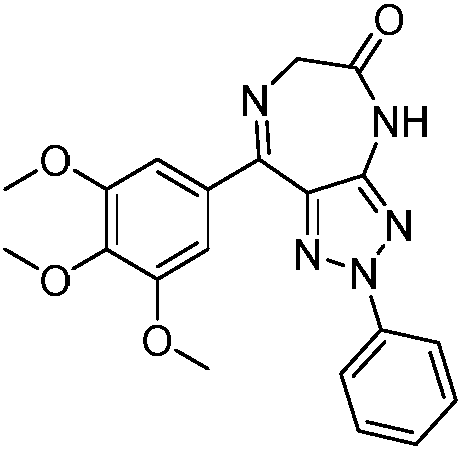
Pyrimido diazepine compound as well as pharmaceutical composition and application thereof
ActiveCN103374000AGrowth inhibitionOvercome drug resistanceOrganic active ingredientsOrganic chemistryErlotinibMedicine
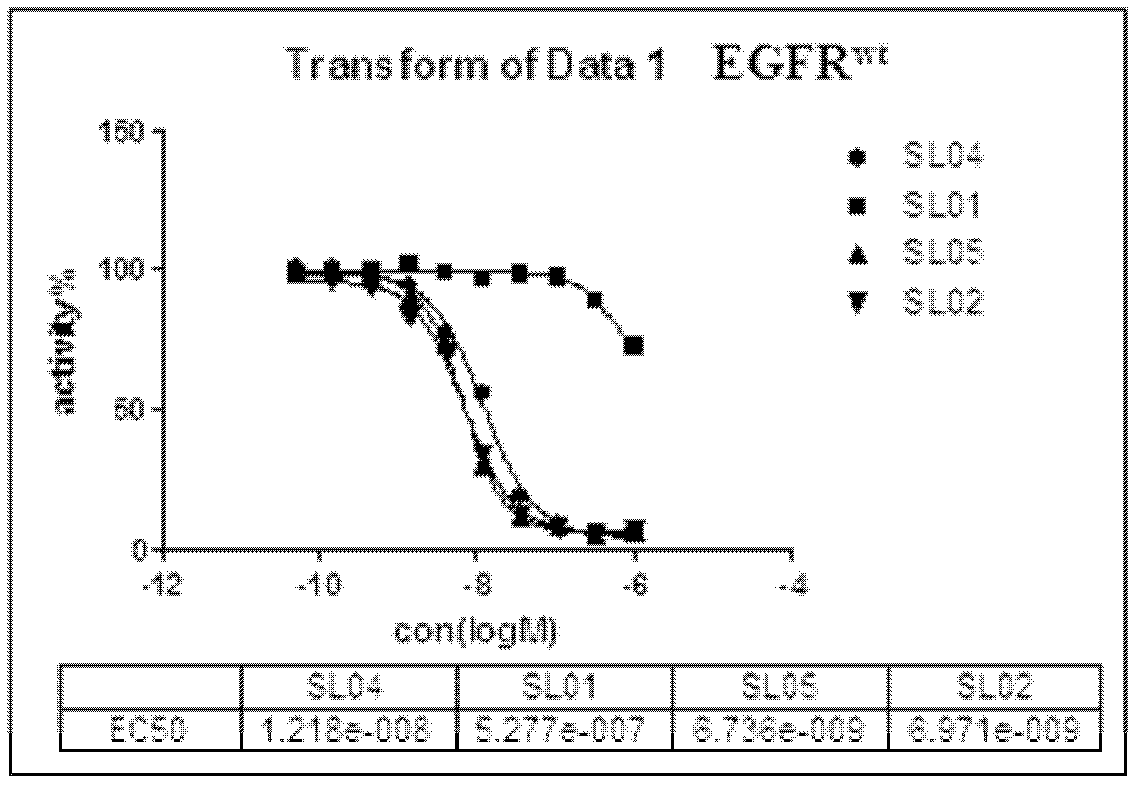
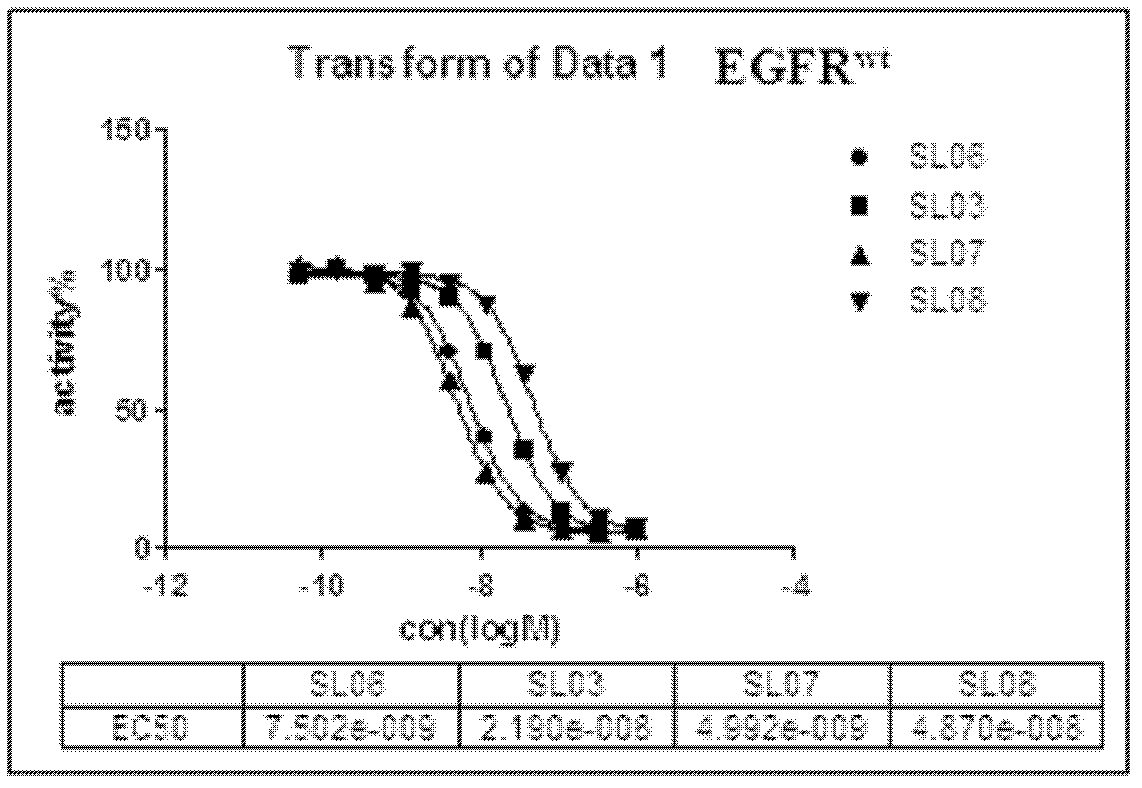
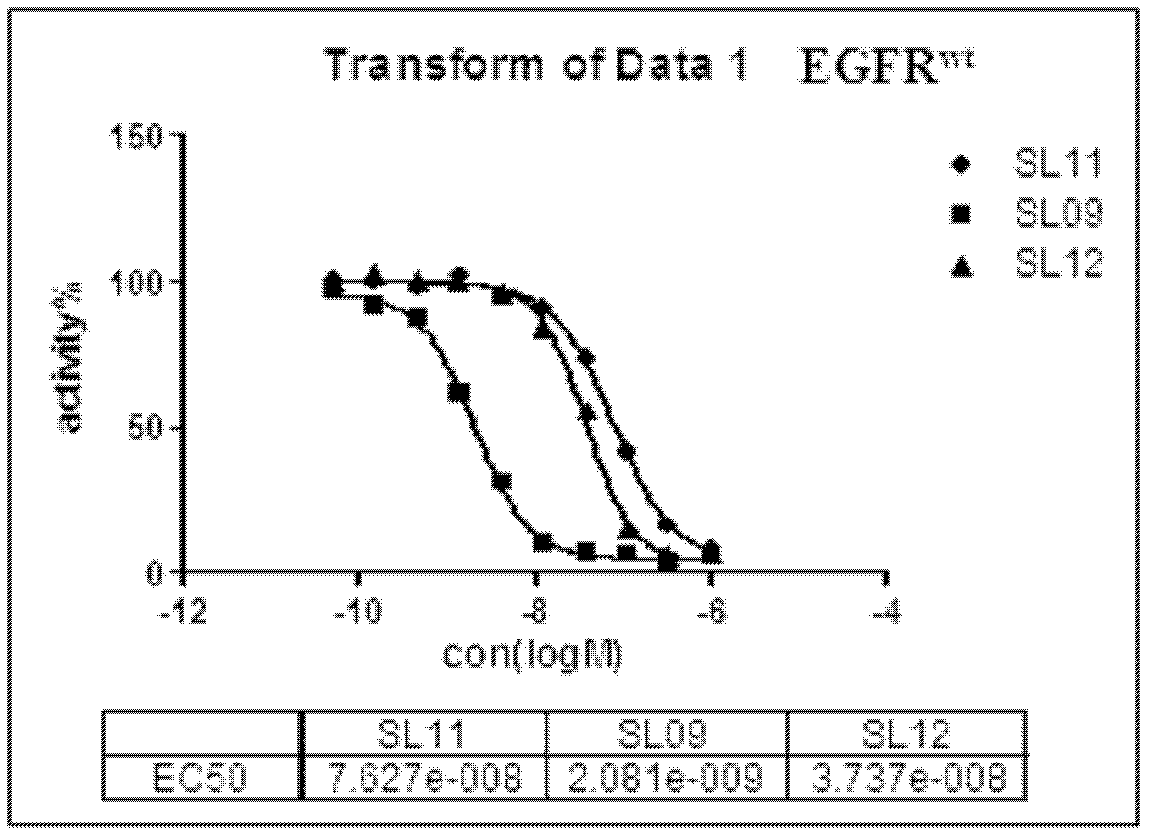
The invention discloses a 5,8-dioxo-pyrimido[4,5-e][1,4]diazepine compound of a structural formula (1) or a pharmaceutically acceptable salt or stereoisomer or prodrug molecule thereof. The 5,8-dioxo-pyrimido[4,5-e][1,4]diazepine compound has the effects of effectively inhibiting the growth of various tumor cells and the generation of the EGFR (epidermal growth factor receptor), can be used for preparing anticancer medicaments and can overcome the resistance of the existing medicaments including the gefitinib, the erlotinib and the like.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Nasal administration of benzodiazepines
Particulate formulations of benzodiazepines, such as diazepam, are used for nasal administration of diazepine drugs to patients. Multimodal particulate formulations of benzodiazepines and methods for their use, e.g. by nasal administration for the treatment of seizure, are also provided.
Owner:AEGIS THERAPEUTICS LLC +1
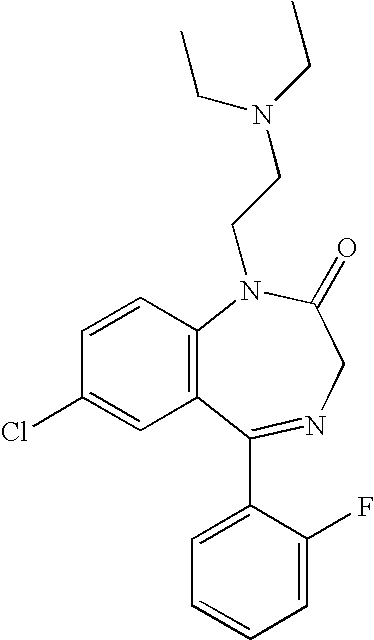
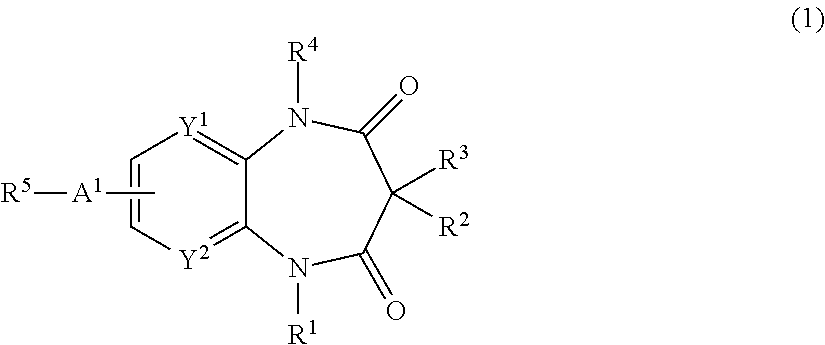
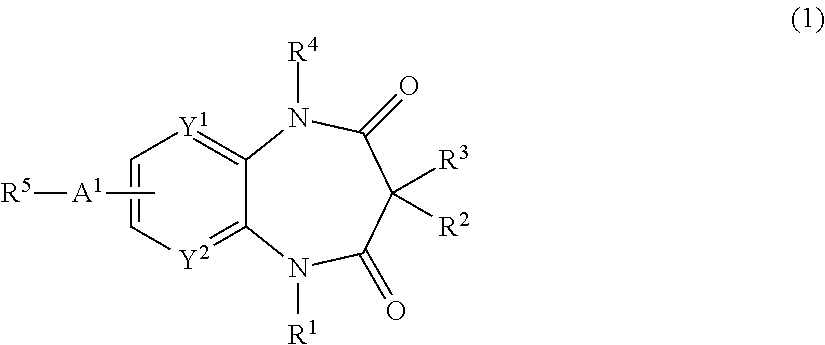
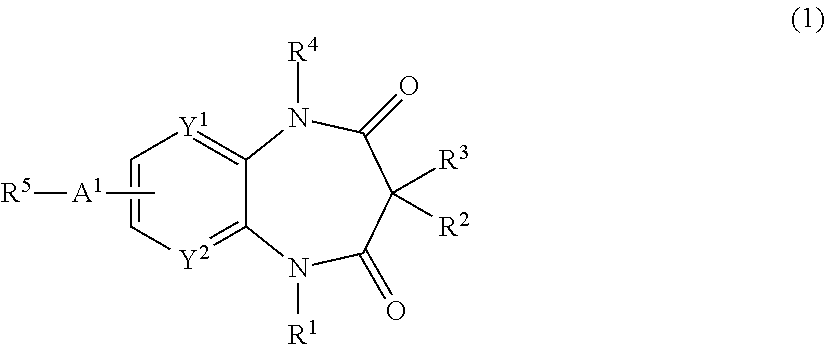
Nitrogen-containing compounds and pharmaceutical compositions thereof for the treatment of atrial fibrillation
InactiveUS20120225866A1Favorable to proceedImprove the blocking effectBiocideOrganic chemistryDiazepineAlkoxy group
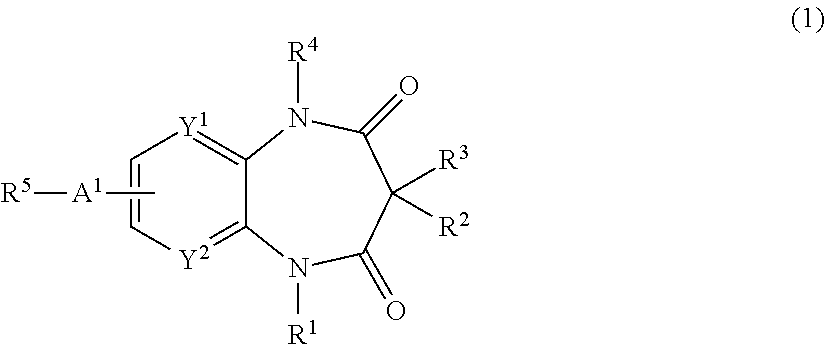
The present invention provides a novel diazepine compound that blocks the IKur current or the Kv1.5 channel potently and more selectively than other K+ channels. The present invention relates to a diazepine compound represented by General Formula (1)or a salt thereof,wherein R1, R2, R3, and R4 are each independently hydrogen, lower alkyl, cyclo lower alkyl or lower alkoxy lower alkyl;R2 and R3 may be linked to form lower alkylene;A1 is lower alkylene optionally substituted with one or more substituents selected from the group consisting of hydroxyl and oxo;Y1 and Y2 are each independently —N═ or —CH═;andR5 is group represented bywherein R6 and R7 are each independently hydrogen or organic group;R6 and R7 may be linked to form a ring together with the neighboring group —XA—N—XB—;XA and XB are each independently a bond, lower alkylene, etc.
Owner:OTSUKA PHARM CO LTD
Novel Diazepine Compounds as Ligands of the Melanocortin 1 and/or 4 Receptors
There is provided novel diazepines that function as agonists at the melanocortin 4 receptor and as agonists at the melanocortin 1 receptor, pharmaceutical compositions containing them, methods for their use in treatment, and processes for their preparation.
Owner:SMITHKLINE BECKMAN CORP
Nitrogen-containing compounds and pharmaceutical compositions thereof for the treatment of atrial fibrillation
InactiveUS8822453B2Improve the blocking effectGood effectBiocideOrganic chemistryDiazepineAlkoxy group
The present invention provides a novel diazepine compound that blocks the IKur current or the Kv1.5 channel potently and more selectively than other K+ channels. The present invention relates to a diazepine compound represented by General Formula (1)or a salt thereof,wherein R1, R2, R3, and R4 are each independently hydrogen, lower alkyl, cyclo lower alkyl or lower alkoxy lower alkyl;R2 and R3 may be linked to form lower alkylene;A1 is lower alkylene optionally substituted with one or more substituents selected from the group consisting of hydroxyl and oxo;Y1 and Y2 are each independently —N═ or —CH═;andR5 is group represented bywherein R6 and R7 are each independently hydrogen or organic group;R6 and R7 may be linked to form a ring together with the neighboring group —XA—N—XB—;XA and XB are each independently a bond, lower alkylene, etc.
Owner:OTSUKA PHARM CO LTD
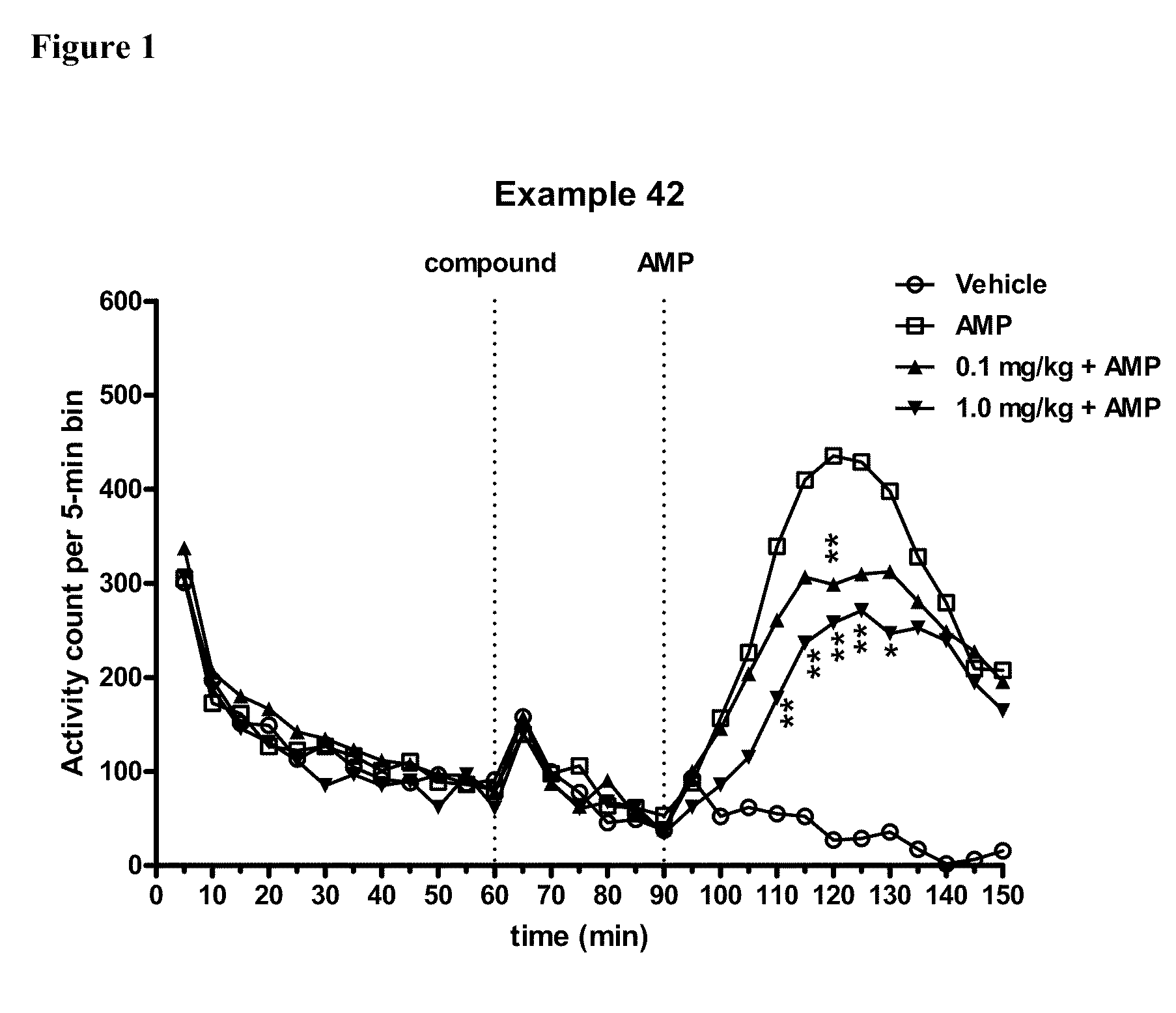
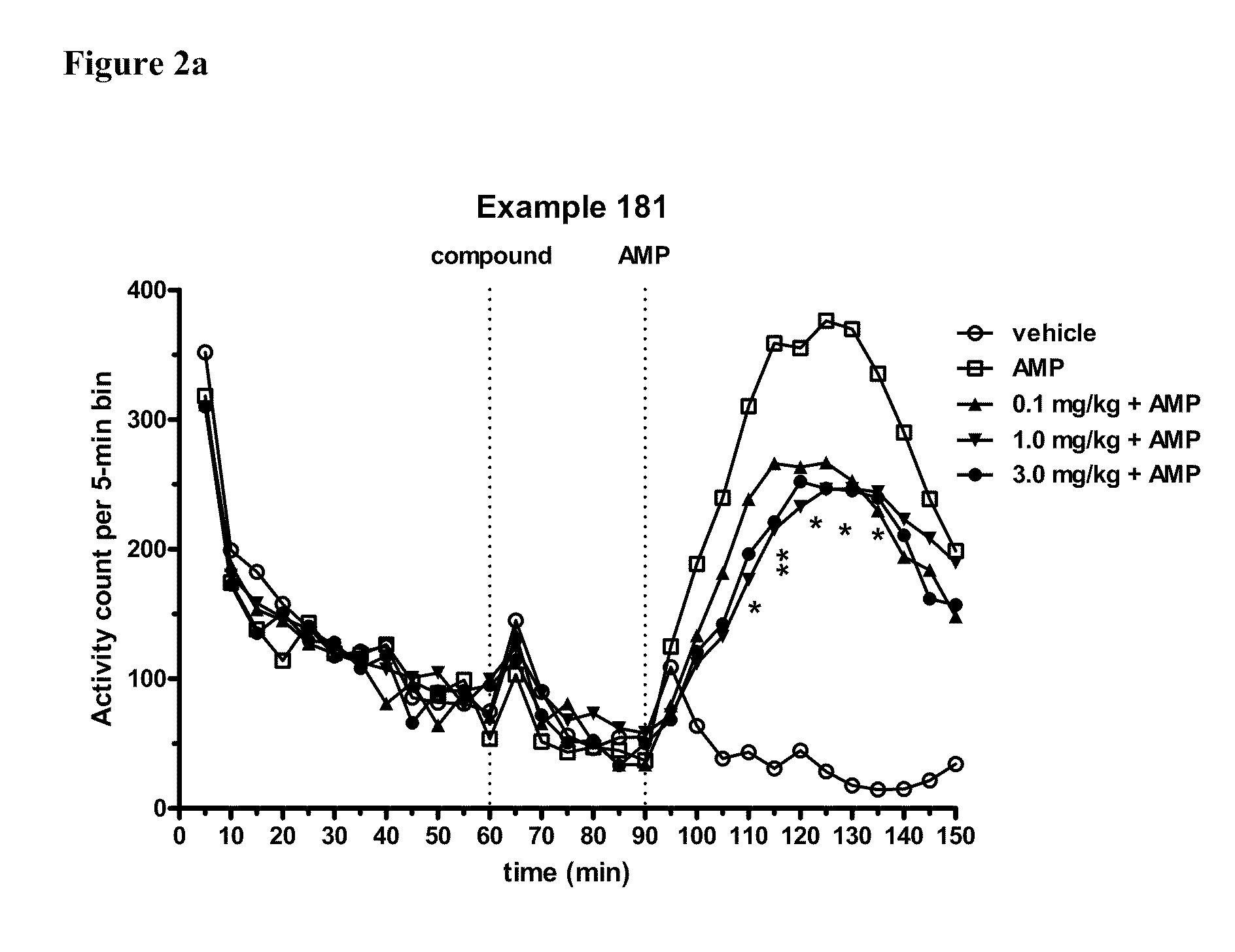
Substituted Benzo-Pyrido-Triazolo-Diazepine Compounds
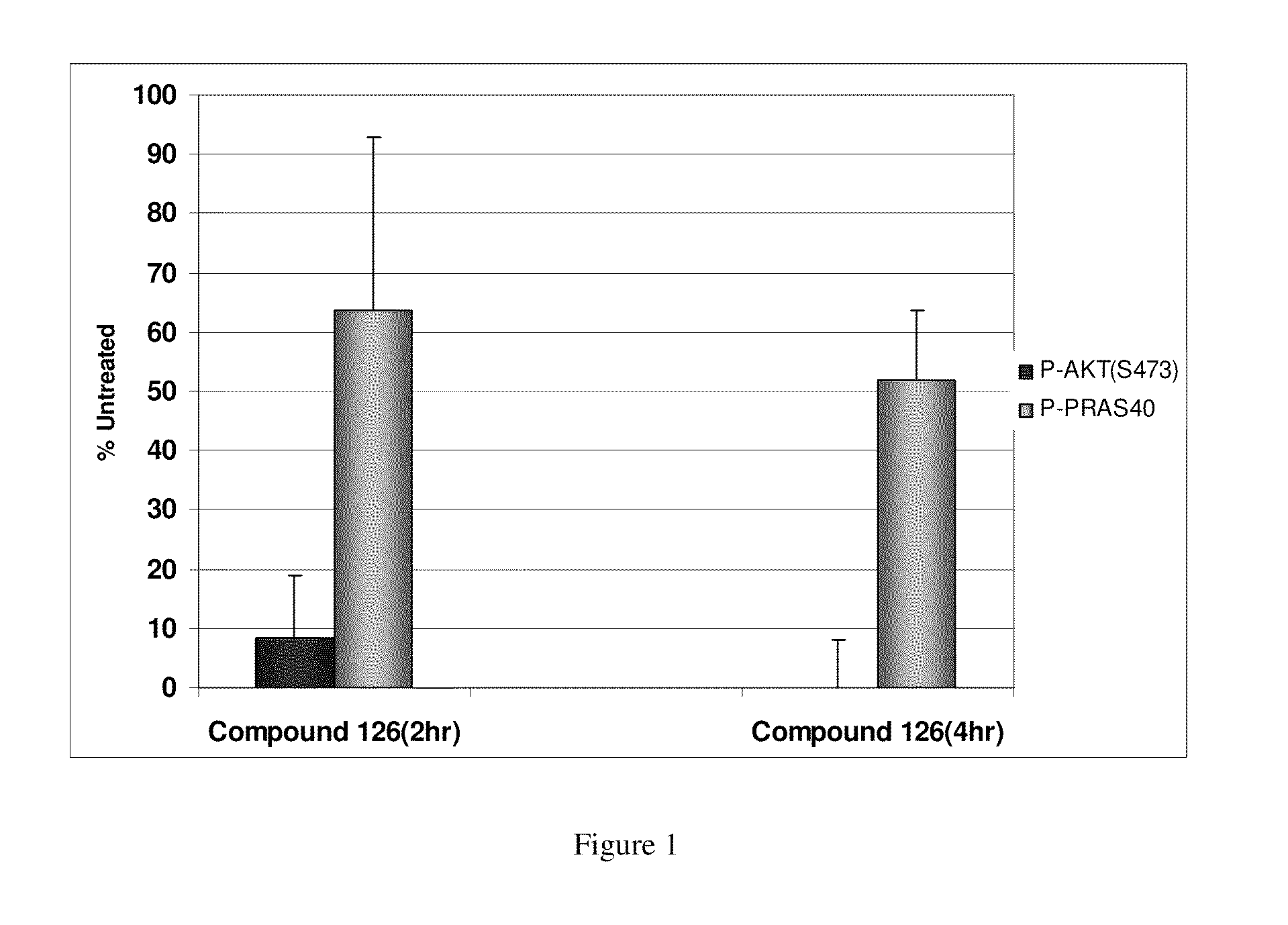
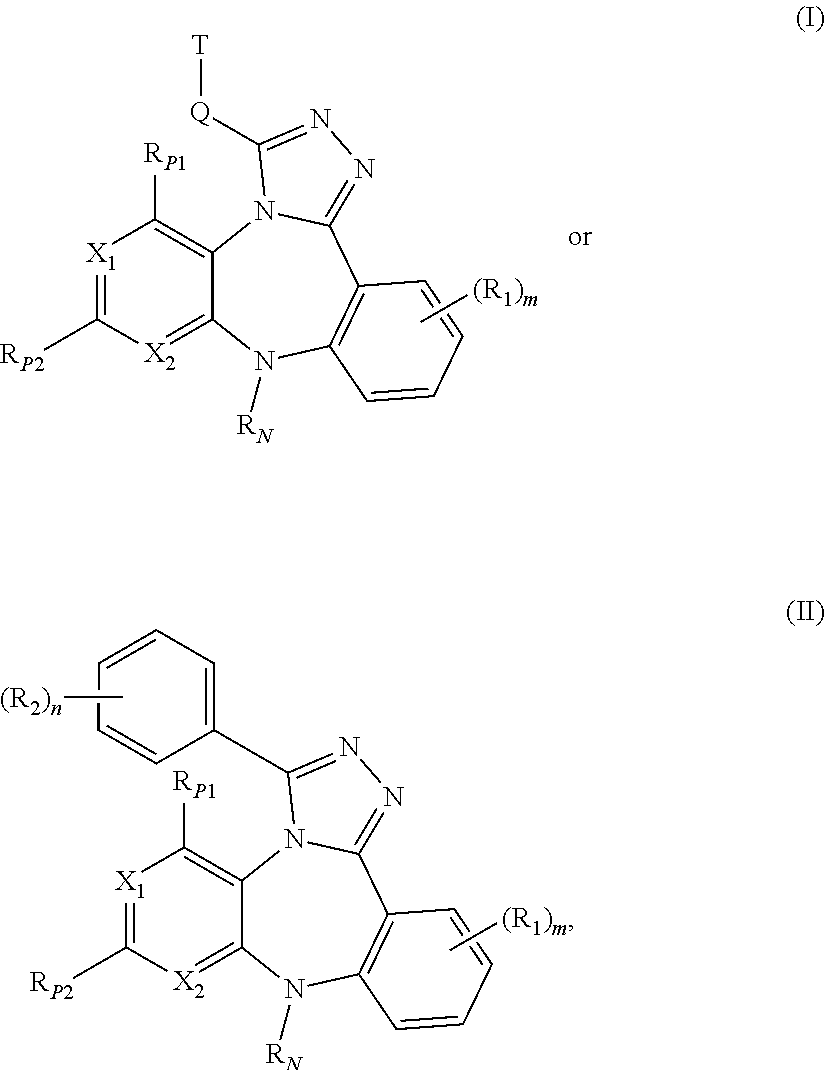
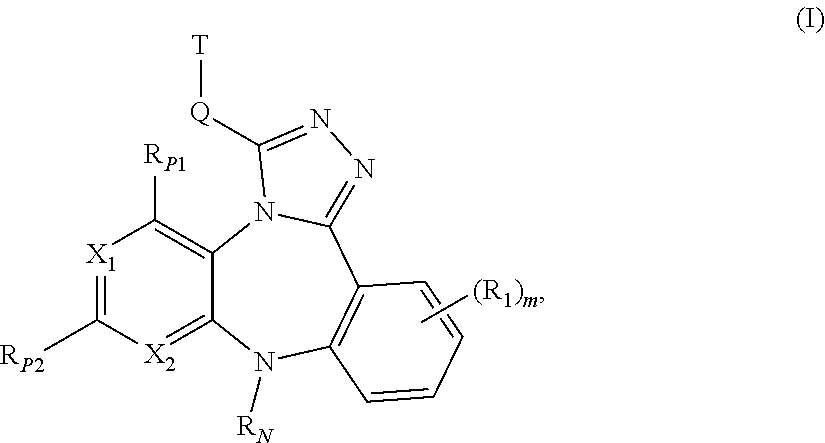
ActiveUS20110245236A1Reduce decreaseDecrease of AKT kinase activityBiocideOrganic chemistryMedicineDiazepine
The present invention relates to substituted benzo-pyrido-triazolo-diazepine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted benzo-pyrido-triazolo-diazepine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds or pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
Modulators of 5-ht receptors and methods of use thereof
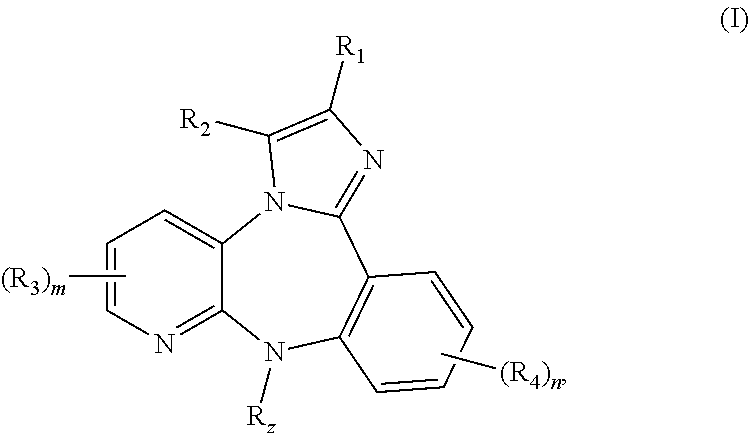
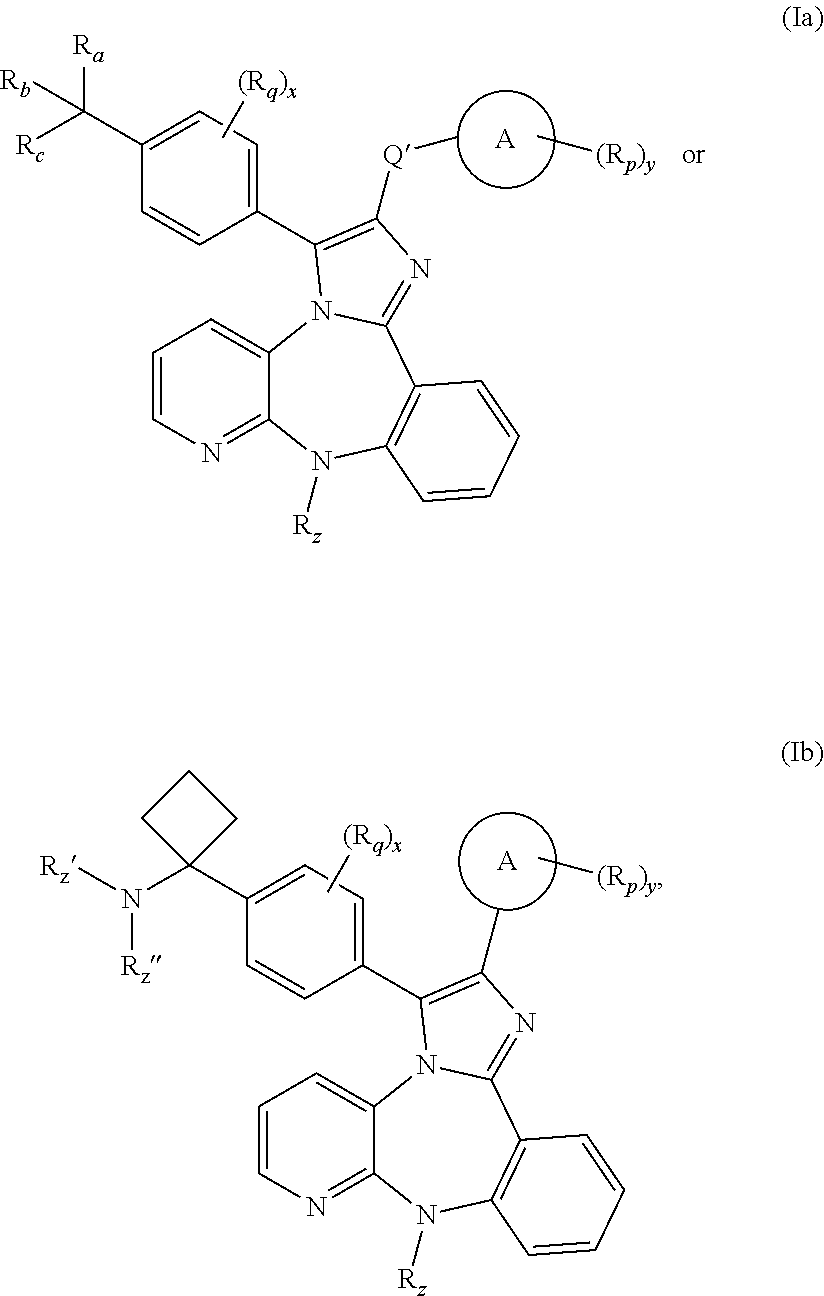
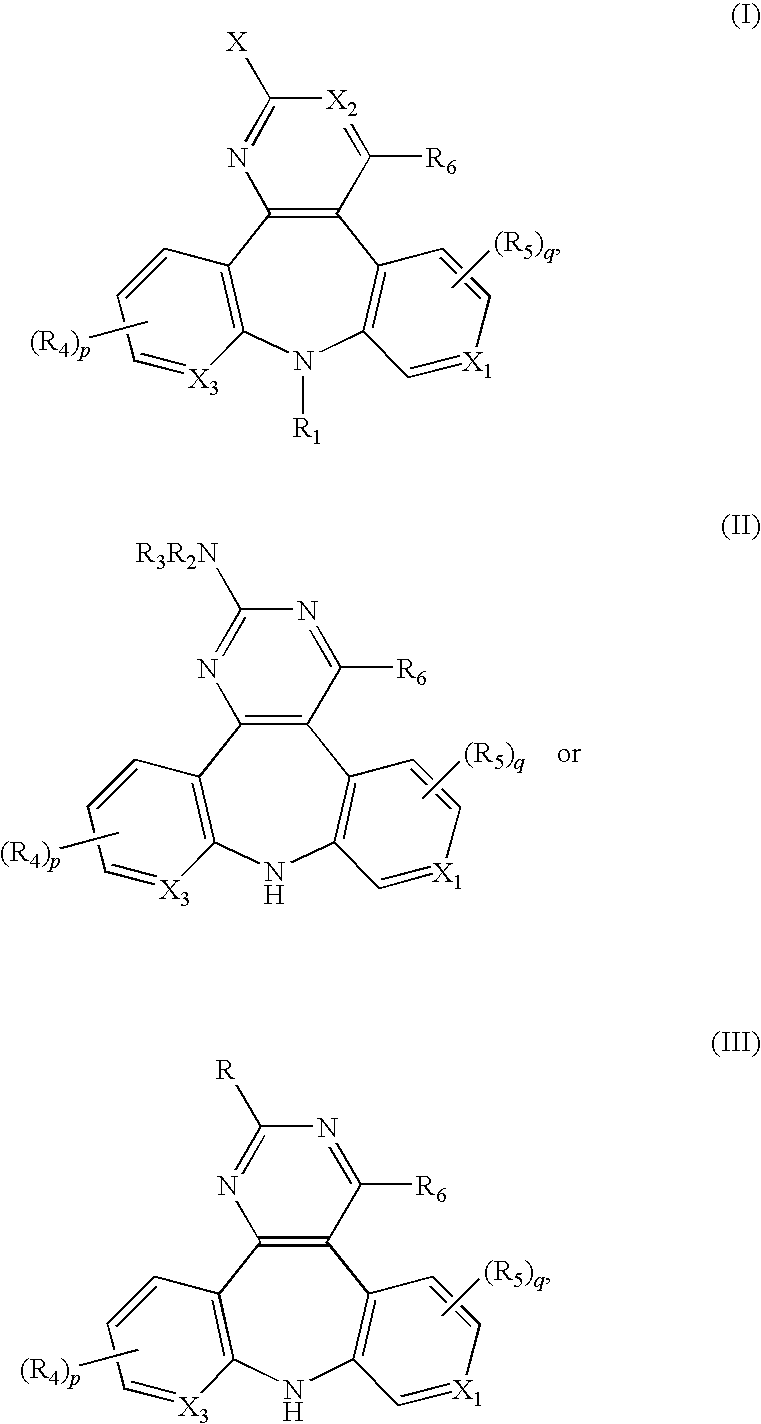
The present application relates to 1,2,3,4,4a,5,6,7-octahydropyrazino[1,2-a][1,4]benzodiazepine, 1,2,3,4,4a,5,6,7-octahydropyrazino[1,2-a][1,5]benzodiazepine, 2,3,4,4a,5,6,7,11b-octahydro-1H-pyrido[3,4-d][2]benzazepine, 1,2,3,4,4a,5,6,7-octahydropyrazino[1,2-a][1]benzazepine, 1,2,3,4,4a,5-hexahydro-7H-pyrazino[1,2-a][4,1]benzoxazepine, and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[2,1-d][1,5]benzoxazepine, and 5,6,7,7a,8,9,10,11-octahydropyrazino[1,2-d]pyrido[3,2-b][1,4]diazepine derivatives of formula (I)wherein R1, R2, R3, R4, R5, R6, X1, X2, X3, X4, Y1, Y2, and Y3 are as defined in the specification. The present application also relates to compositions comprising such compounds, and methods of treating disease conditions using such compounds and compositions, and methods for identifying such compounds.
Owner:ABBVIE INC +1
Substituted Benzo-Imidazo-Pyrido-Diazepine Compounds
The present invention relates to substituted benzo-imidazo-pyrido-diazepine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted benzo-imidazo-pyrido-diazepine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
Aryl-and heteroaryl-substituted tetrahydrobenzo-1,4-diazepines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
Owner:ALBANY MOLECULAR RESEARCH INC
Bifunctional chelating agents based on the 1,4-diazepine scaffold (DAZA) for non-invasive molecular imaging
ActiveUS20160136309A1Minimize interferenceGroup 5/15 element organic compoundsRadioactive preparation carriersDiazepineMolecular imaging
A compound fur radio metal complexation includes a chelator and one or more biological targeting vectors TV conjugated to said chelator, wherein the chelator has structure (A) or (B)or (C).based on 1,4-diazepine with groups R1, R2, R3, R4, X1, X2, X3. The compound is particularly suited for complexation of radio-isotopic metals, such as 66Ga(III), 67Ga(III) and 68Ga(III).
Owner:SPECIALCHEMICALS VERTRIEB GMBH
Diazepine compounds as ligands of the melanocortin 1 and/or 4 receptors
There is provided novel diazepines that function as agonists at the melanocortin 4 receptor and as agonists at the melanocortin 1 receptor, pharmaceutical compositions containing them, methods for their use in treatment, and processes for their preparation.
Owner:SMITHKLINE BECKMAN CORP
Substituted dipyrido-pyrimido-diazepine and benzo-pyrido-pyrimido compounds
The present invention relates to substituted dipyrido-pyrimido-diazepine compounds, substituted benzo-pyrido-pyrimido-diazepine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted dipyrido-pyrimido-diazepine compounds, substituted benzo-pyrido-pyrimido-diazepine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
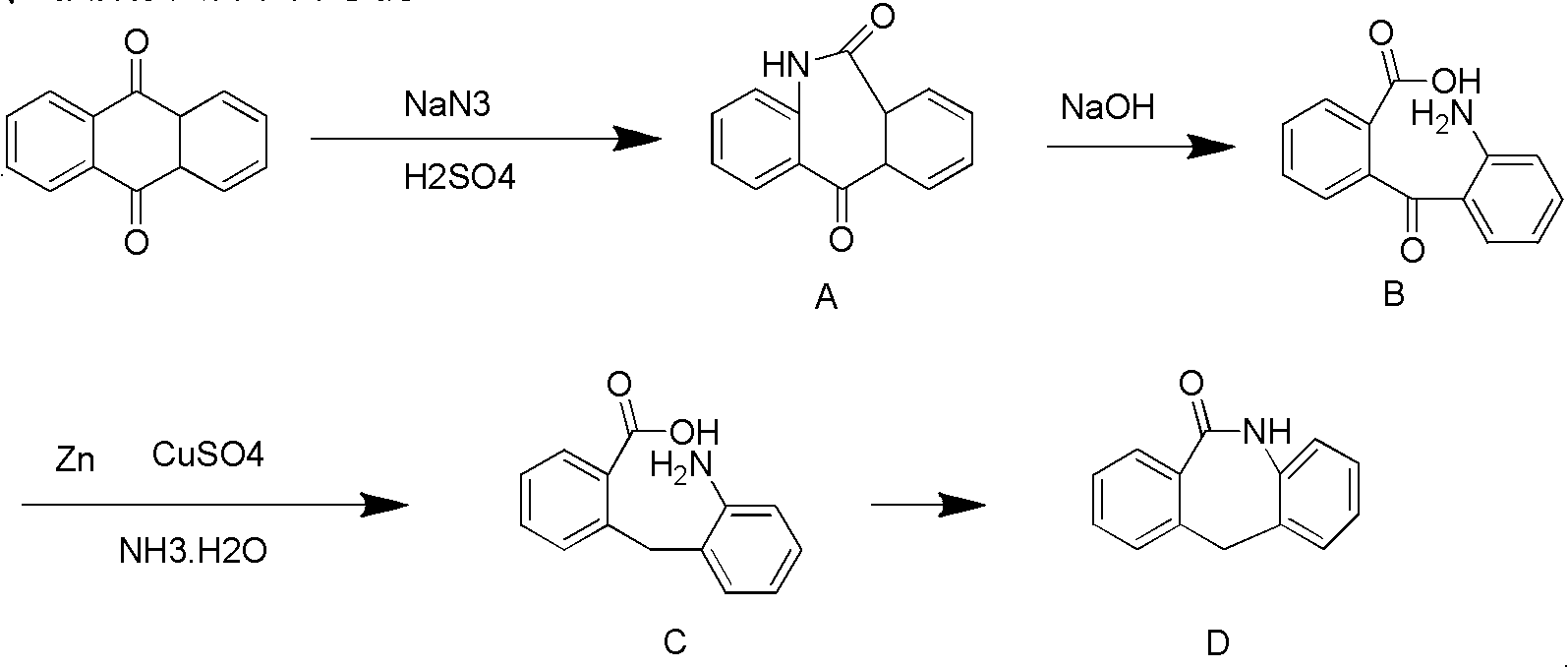
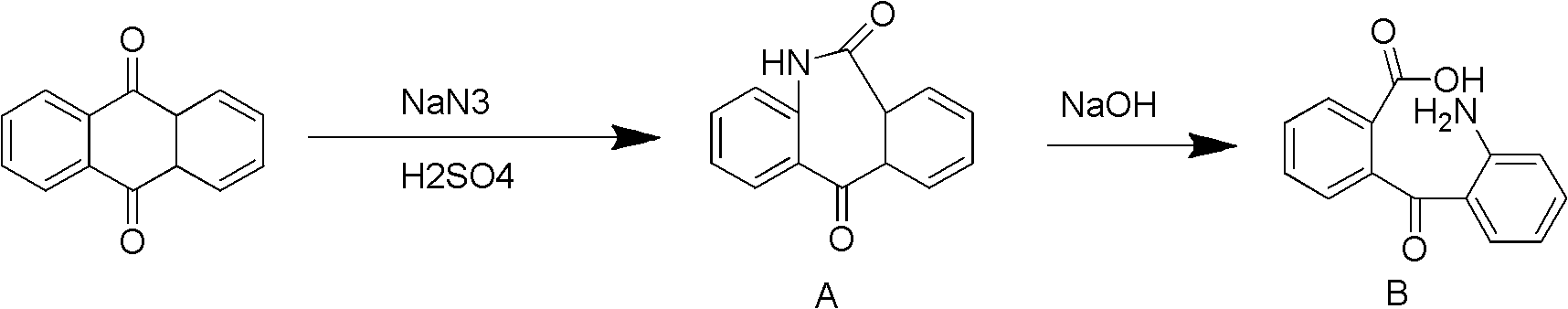
Preparation method of 2-benzo diazepine anthrone
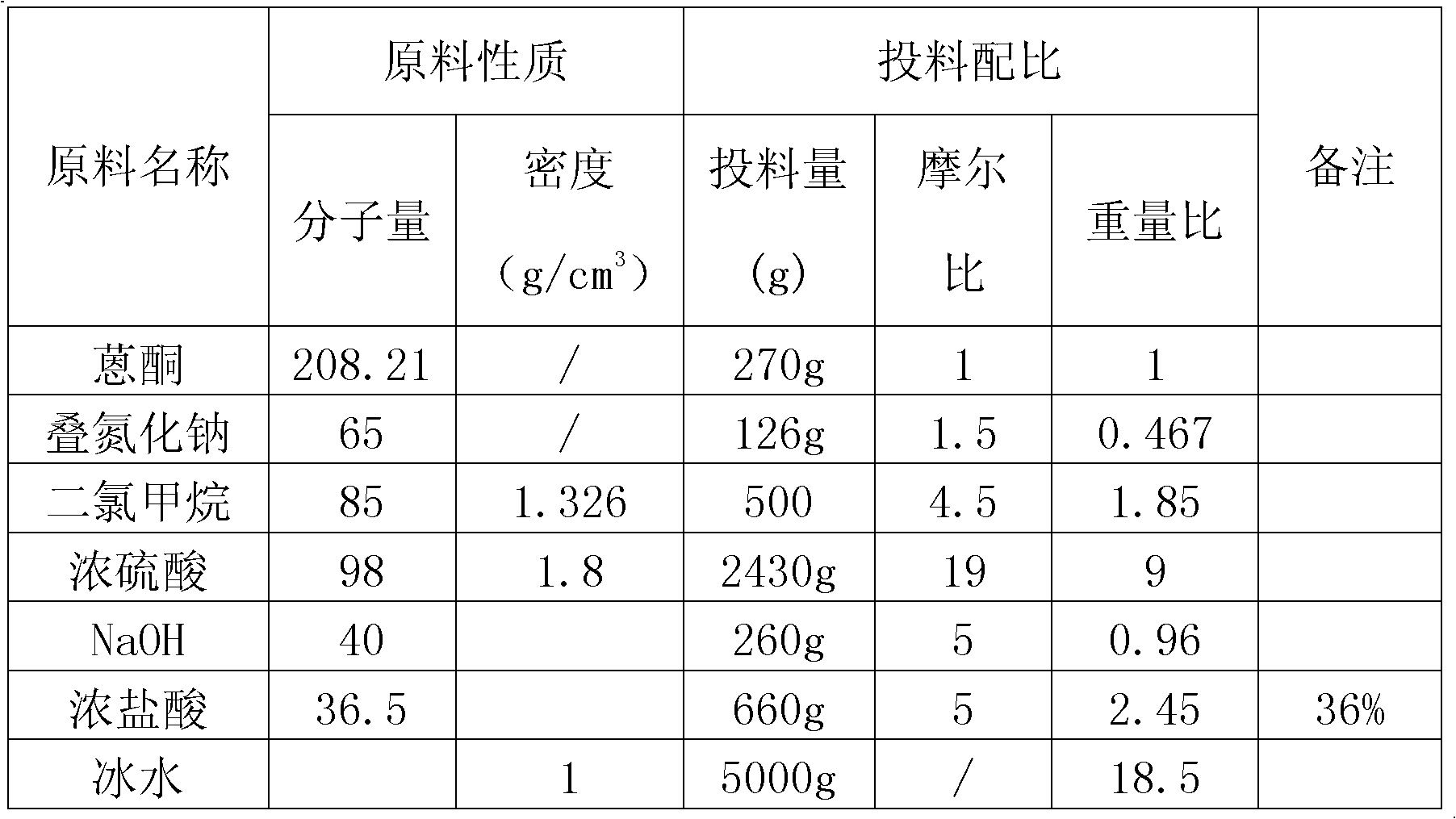
The invention discloses a preparation method of 2-benzo diazepine anthrone. The method comprises the following steps: anthranone, dichloromethane and concentrated sulfuric acid are filled in a reaction bulb according to a certain proportion, mechanical stirring is carried out, and sodium azide is slowly filled in the reaction bulb under room temperature to obtain yellow solid; the yellow solid and ammonia water are put in the reaction bulb to heat up to 80 DEG C to 90 DEG C, then zinc powder and copper sulfate are filled in batches, and the solution is stirred for 24 hours to 40 hours. Thin-layer chromatography (TLC) detection is carried out to confirm that no raw materials is left, the solution is cooled to room temperature, the zinc power is filtered out, and the zinc powder is washed for 3 to 4 times by ammonia water of 10%. PH is adjusted to 4 to 5 through acid under the room temperature, the solution is cooled to the temperature lower than 5 DEG C, and is stirred, and suction filtration is carried out, mother solution is extracted through ethyl acetate, organic phase is washed with Na2S solution, Na2SO4 is dried and concentrated, and crude products are obtained through filter cakes and the organic phase; and the crude products and toluene are put into the reaction bulb, and products are obtained after a series of reactions. The purity of the raw materials of the preparation method of the 2-benzo diazepine anthrone is high, and the yield coefficient of the obtained product is quite high.
Owner:XUZHOU B&C CHEM CO LTD
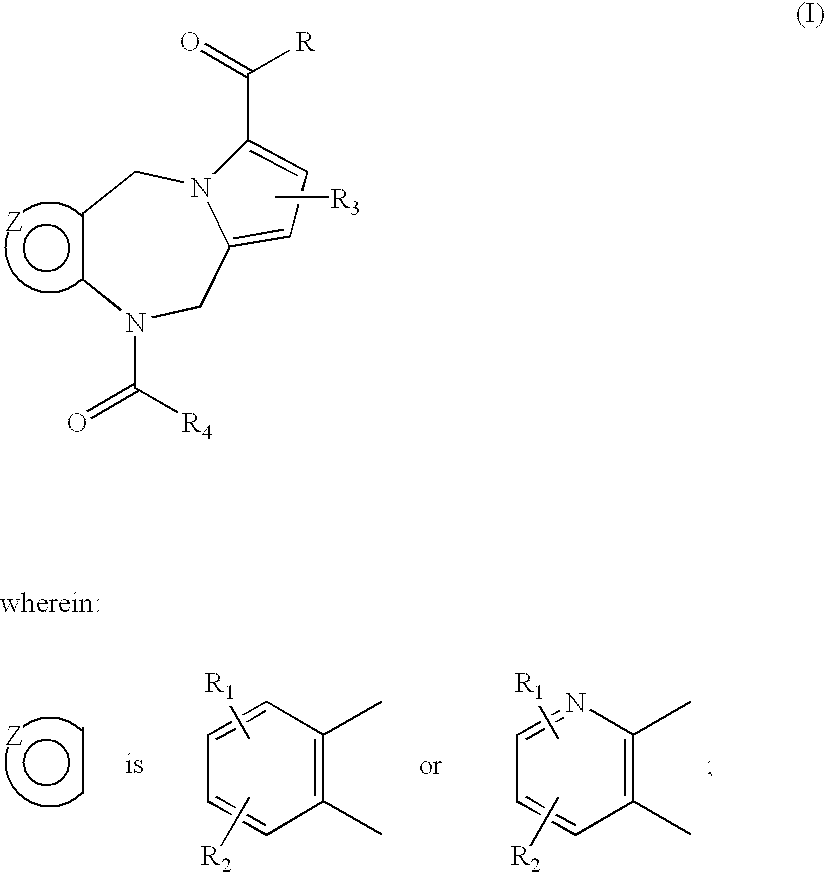
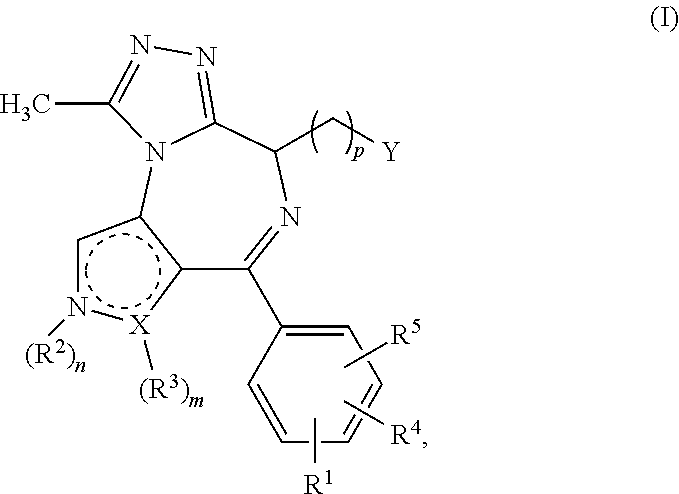
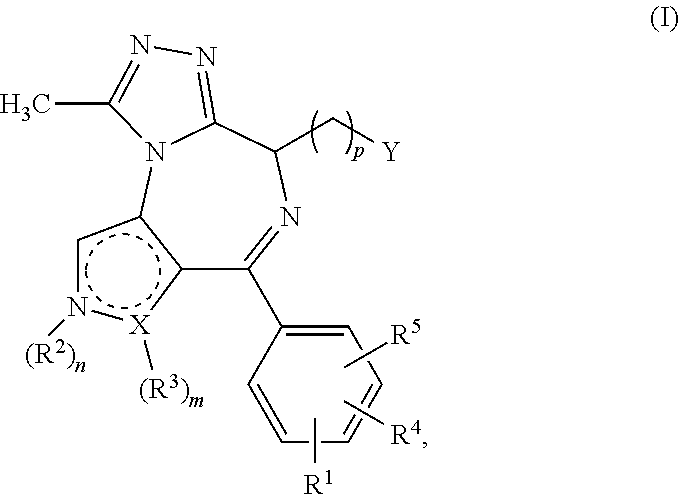
4-substituted pyrrolo- and pyrazolo-diazepines
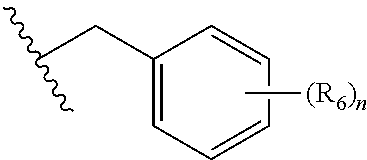
BET protein-inhibitory, especially BRD2-, BRD3- and BRD4-inhibitory, 4-substituted pyrrolo- and pyrazolodiazepines of the general formula Iare described, in which X, Y, n, m, p, R1, R2, R3, R4 and R5 are each as defined in the description, as are pharmaceutical compositions comprising the inventive compounds, and the prophylactic and therapeutic use thereof in the case of hyperproliferative disorders, especially in the case of neoplastic disorders. Also described is the use of the inventive compounds as BET protein inhibitors in benign hyperplasias, in atherosclerotic disorders, in sepsis, in autoimmune disorders, in vascular disorders, in viral infections, in neurodegenerative disorders, in inflammatory disorders and in male fertility control.
Owner:BAYER PHARMA AG
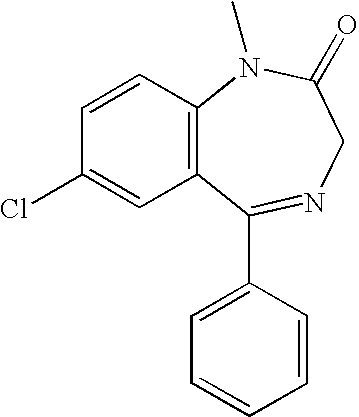
ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES
The present invention provides one step processes for the preparation of substituted dibenzo[b,e][1,4]-diazepine-11-ones by the reaction of substituted isatoic anhydrides with substituted 1,2-phenylenediamines in the presence of aqueous acetic acid.
Owner:COUNCIL OF SCI & IND RES
Synthesis method of suvorexant intermediate
The invention provides a synthesis method of a suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester. The synthesis method of the suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester comprises the following steps: (1) enabling a compound Suvor-1 to react with vitride solution to obtain a compound Suvor-2; enabling the compound Suvor-2 to react with 4,4-dimethoxy-2-butanone to obtain Suvor-3; (3) enabling the compound Suvor-3 to react with methanesulfonic acid to obtain a compound Suvor-4; (4) enabling the compound Suvor-4 to react with di-tert-butyl dicarbonate to obtain a compound Suvor-5; (5) enabling the compound Suvor-5 to react with hydrogen to obtain the suvorexant intermediate. The synthesis method of the suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester provided by the invention has the advantages that the reaction is simple, starting materials are cheap and easy to obtain, the reaction condition is mild, and the production safety is high; in addition, the reaction steps are less, the reaction yield is high, and the production cost is lower; moreover, by introducing a chiral functional group, the purity of a target product obtained after induced synthesis and ring closure is high, the amount of impurities in enantiomer is small, the ee% is greater than97%, and the method is suitable for commercial large-scale production.
Owner:成都美域高制药有限公司
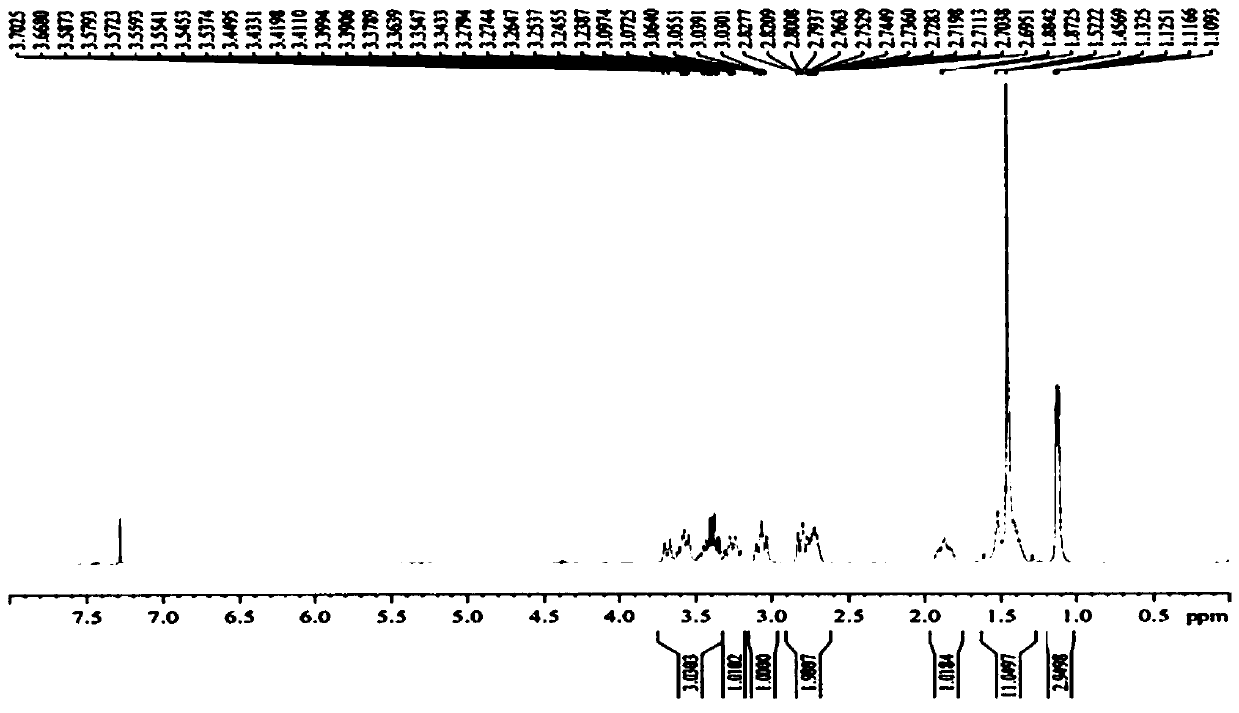
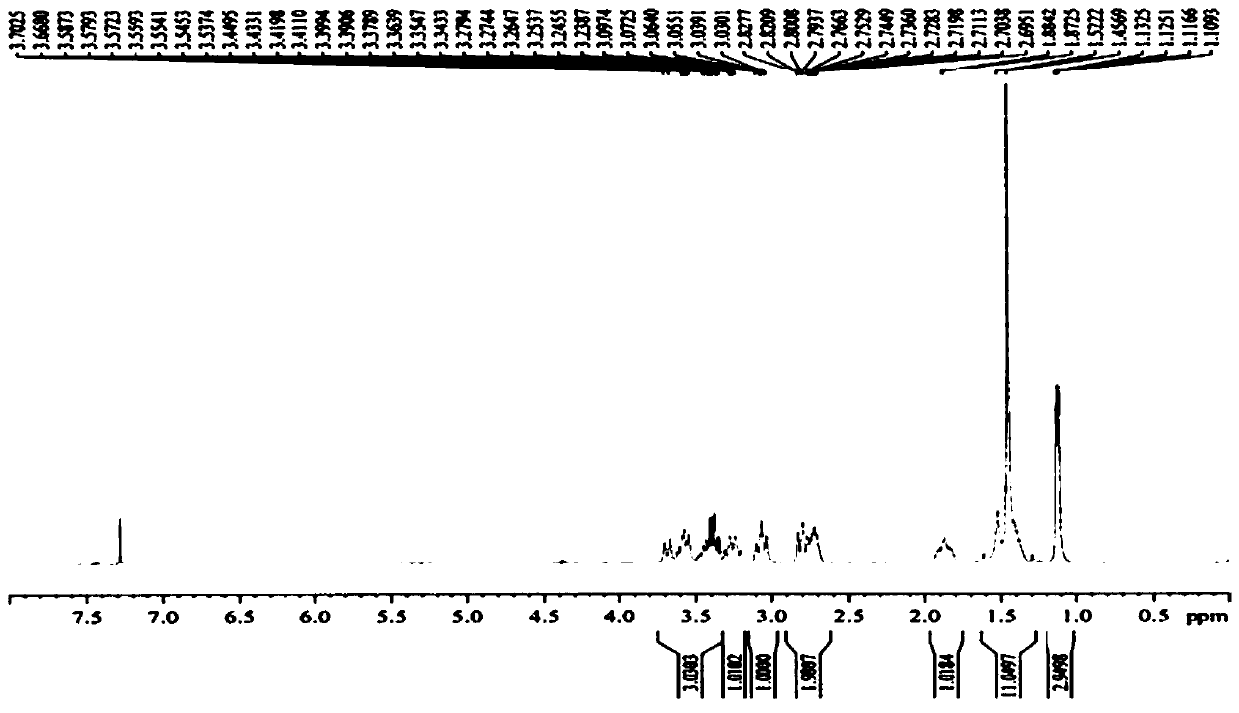
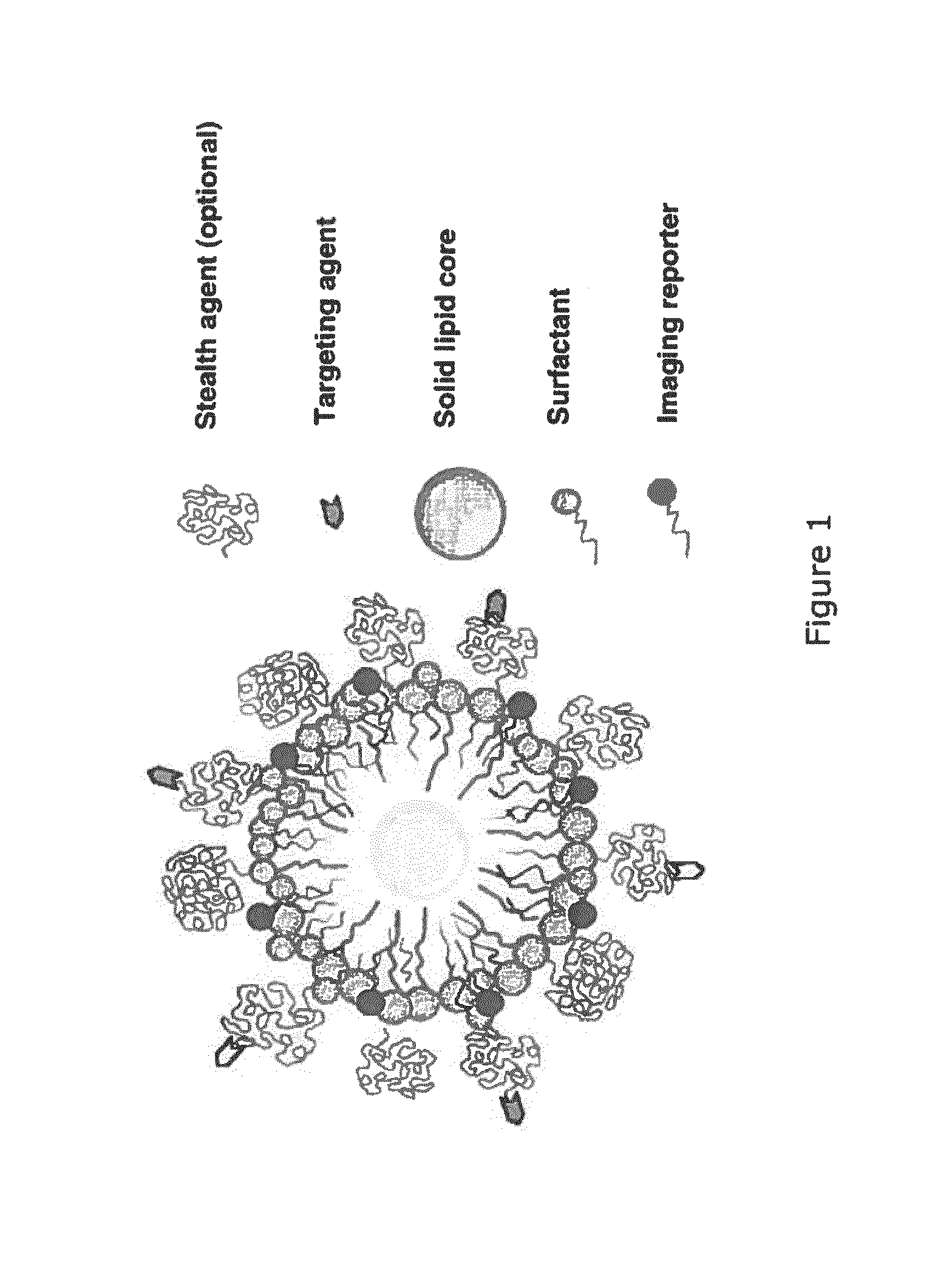
Paramagnetic Solid Lipid Nanoparticles (pSLNs) Containing Metal Amphiphilic Complexes For MRI
ActiveUS20150258221A1Improve stabilityOrganic chemistryNMR/MRI constrast preparationsLipid formationDodecane
The present invention relates to paramagnetic solid lipid nanoparticles (pSLNs) comprising an amphiphilic paramagnetic metal chelating moiety selected from: a diazepine derivative of Formula I and a tetraazocyclododecane derivative of Formula (II): being said chelating moiety complexed to a paramagnetic metal ion selected from the group consisting of: Gd(III), Mn(II), Cr(III), Cu(II), Fe(III), Pr(III), Nd(III), Sm(III), Tb(III), Yb(III), Dy(III), Ho(III) and Er(III), or salts thereof. The invention further relates to the process for preparation of said solid lipid nanoparticles comprising amphiphilic complexes of paramagnetic metals (pSLNs) and to the use of pSLNs as MRI contrast agents in the diagnostic field.
Owner:BRACCO IMAGINIG SPA
Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines
An improved process for preparing 6-aryl-4H-s-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines of formula I, wherein:R1 is a hydrogen or halogen atom or a C1-C6 alkyl group,R2 is a hydrogen or halogen atom or a C1-C6 alkyl, C1-C6 hydroxyalkyl, C3-C6 cycloalkyl group or a 5- or 6-membered oxygen-, sulphur- or nitrogen-containing heterocyclic group which may optionally be substituted at the nitrogen atom by a C1-C3 alkyl group, andR3 is a hydrogen or halogen atom.
Owner:BOEHRINGER INGELHEIM PHARM KG
Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester
ActiveCN101875658AImprove conversion rateReaction raw materials are cheap and easy to obtainOrganic chemistryRaney catalystsTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention relates to a synthesis method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester, mainly solving the technical problems of difficult reactant purification and the like in the traditional synthesis method and additionally providing a route for synthesizing an important intermediate, i.e. 8-(tert-butoxycarbonyl)-3-carbonyl-2,8-diazepine helix[4.5]decane-4-carboxylic acid. The synthesis method comprises the steps of: carrying out Micheal addition on an intermediate, i.e. tertiary butyl-4-(2,2-dimethyl-4,6-dicarbonyl-1,3-dioxan-5-ylene)piperidine-1-carboxylic ester and nitromethane to obtain a compound, i.e. tertiary butyl-4-(2,2-dimethyl-4,6-dicarbonyl-1,3-dioxan-5-group)-4-(nitromethyl)piperidine-1-carboxylic ester, then carrying out catalytic hydrogenation and lactamization to obtain a compound, i.e. 8-(butylcarbonyl)-3-carbonyl-2,8-diazepine helix[4.5]decane-4-carboxylic acid, and finally carrying out decarboxylation to obtain the final product, i.e. 3-carbonyl-2,8-diazepine helix[4.5]decane--8-carboxylic acid tert-butyl ester. The product of the invention is used as a template micromolecule for synthesizing various compound libraries.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
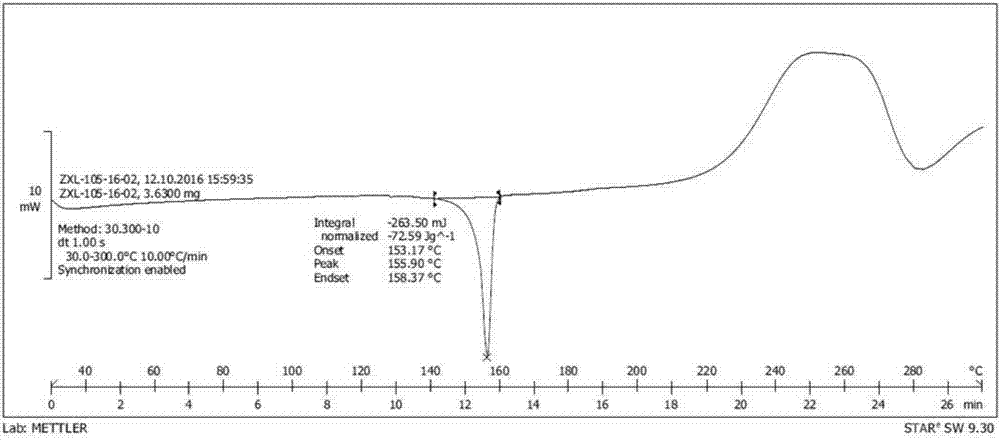
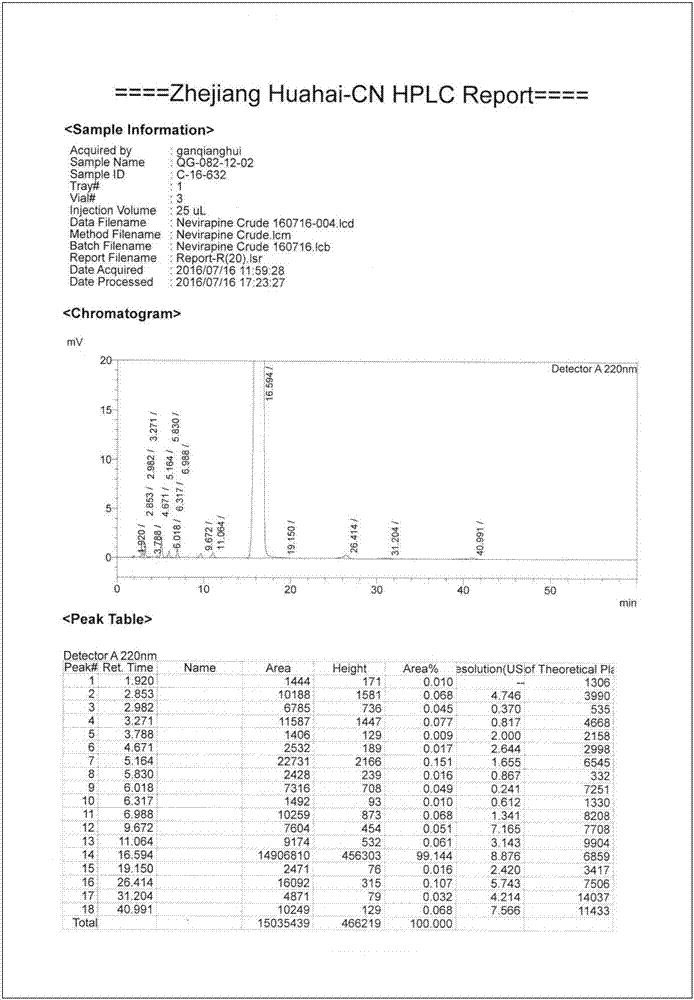
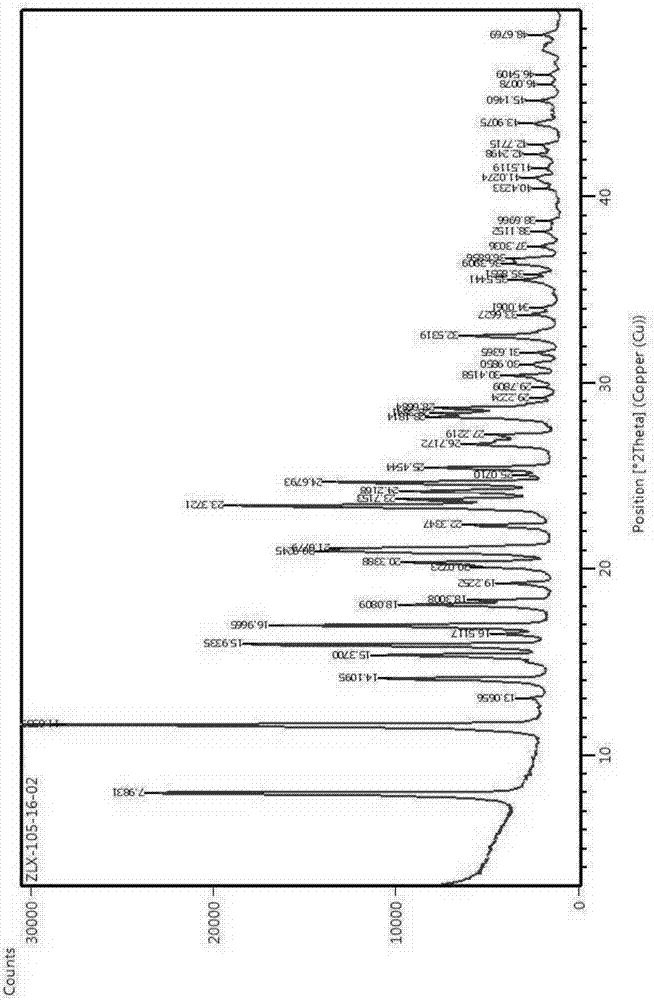
Preparation method of nevirapine intermediate spherical crystal
InactiveCN106938981ALow suspension densityImprove liquidityOrganic chemistry methodsTemperature controlState of art
The invention provides a preparation method of a nevirapine intermediate spherical crystal. The method concretely comprises the following steps: heating a reaction solution of a nevirapine intermediate 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido-[3,2-b:2,3-e][1,4]diazepine to make the solution clarified, slowly dropwise adding the reaction solution to a crystallization solvent with the temperature controlled to be 10-40 DEG C, controlling the temperature of the crystallization system to be 0-50 DEG C during the dropwis adding, cooling the system to -10-10 DEG C after the dropwise adding is finished, filtering the solution, and drying the filtered solution to obtain the nevirapine intermediate spherical crystal. The method fills the gap in the prior art, the prepared spherical crystal has the advantages of small density and good fluidity of a crystal slurry, easiness in separation and drying, single solvent, low solvent rate, high crystallization yield, simple preparation method and high purity, and the preparation method has the advantages of easiness in commercial large-scale production, and very high promotion and application values.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Synthesis method of tert butyl-7-(hydroxymethyl)-7, 8-dihydrogen-4H-pyrazolo diazepine-5(6H)-formyl ester
ActiveCN107216332AReasonable reaction process designLower synthesis costOrganic chemistrySynthesis methodsDiazepine
The present invention relates to a synthesis method of tert butyl-7-(hydroxymethyl)-7, 8-dihydrogen-4H-pyrazolo[1,5-a][1,4] diazepine-5(6H)-formyl ester, and mainly solves the technical problems of low imperfect rate, no easiness in control of reaction, experimental operation inconvenience and the like of a middle-yield route of the synthesis process in the prior art. The tert butyl-7-(hydroxymethyl)-7, 8-dihydrogen-4H-pyrazolo[1,5-a][1,4] diazepine-5(6H)-formyl ester is prepared from raw material1H-pyrazole-3-formaldehyde which is cheap and easily available and capable of large-scale production by five steps, synthesis cost is saved, and mass production can be realized. The reaction formula is as shown in the specification, and the product obtained by the method is a useful intermediate or product for synthesis of many pharmaceuticals.
Owner:上海药明康德新药开发有限公司 +4
P2X4 receptor antagonist
The present invention relates to a diazepine derivative represented by the following general formula (I) (in the formula, R1 and R2 represent hydrogen atom and the like, or R1 and R2 bind together to form a naphthalene ring and the like together with the benzene ring to which they bind, R3 and R4 represent hydrogen atom and the like, R5 represents hydrogen atom and the like, R6 and R7 represent hydrogen atom and the like, X represents C, CH or N, Y represents N, NH or C(═O), provided that when X is N, Y is not N or NH, and when X is C or CH, Y is not C(═O), Z represents oxygen atom or sulfur atom, A represents benzene ring and the like, B represents NHC(═O) and the like, D represents an atomic bond and the like, E represents an atomic bond and the like, G represents benzene which may be substituted and the like, and m represents an integer of 0 to 5) or a pharmacologically acceptable salt thereof, and a P2X4 receptor antagonist.
Owner:NIPPON CHEMIPHAR CO LTD
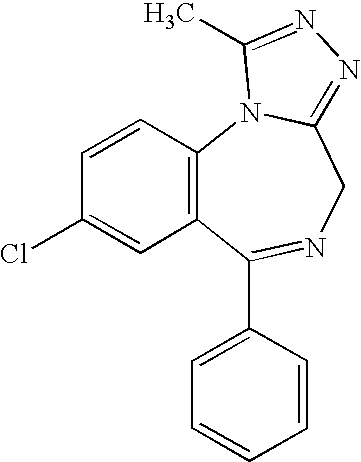
Deuterated estazolam and preparation method thereof
InactiveCN103864798AEasy to prepareSimple and fast operationOrganic chemistryDeuterated chloroformEstazolam
The invention discloses deuterated estazolam and a preparation method thereof. The preparation method comprises following steps of (1) adding 6-phenyl-8-chlorine-4H-[1,2,4] triazole-[4,3-a][1,4] benzo-diazepine into dimethyl sulfoxide or N,N-dimethylformamide, mixing and stirring; (2) adding a catalyst cesium carbonate or potassium carbonate as well as deuterated chloroform, stirring and heating up to over 40DEG C; and (3) separating so as to obtain deuterated estazolam. The preparation method is concise, is simple and convenient to operate, has low cost, and is easy for purification. Commercially available non-deuterated estazolam can be converted into deuterated estazolam by a little deuterated reagent used as a deuterium source in the non-deuterated solvent atmosphere in shorter time, and a pure product can be obtained through simple purification by column chromatography. The standard deuterated estazolam prepared by the method has high purity and stable chemical property, and is convenient for preparing standard substances for analysis. The preparation method can be used for producing, analyzing and detecting the deuterated internal standard substance used by estazolam.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Heterocyclic Compounds and Methods For Their Use
InactiveUS20150011565A1Treating or preventing impaired nerve conduction velocityOrganic active ingredientsNervous disorderBone formationNormal nerve conduction velocities
The present invention relates to heterocyclic compounds useful for antagonizing angiotensin II Type 2 (AT2) receptor. More particularly the invention relates to piperazine and diazepine compounds, compositions containing them and their use in methods of treating or preventing disorders or diseases associated with AT2 receptor function including neuropathic pain, inflammatory pain, conditions associated with neuronal hypersensitivity, impaired nerve conduction velocity, cell proliferation disorders, disorders associated with an imbalance between bone resorption and bone formation and disorders associated with aberrant nerve regeneration.
Owner:NOVARTIS AG
Modulators of 5-HT receptors and methods of use thereof
The present application relates to 1,2,3,4,4a,5,6,7-octahydropyrazino[1,2 -a][1,4]benzodiazepine, 1,2,3,4,4a,5,6,7-octahydropyrazino[1,2-a][1,5]benzodiazepine, 2,3,4,4a,5,6,7,11b-octahydro-1H-pyrido[3,4-d][2]benzazepine, 1,2,3,4,4a,5,6,7 -octahydropyrazino[1,2-a][1]benzazepine, 1,2,3,4,4a,5-hexahydro-7H-pyrazino[1,2 -a][4,1]benzoxazepine, and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[2,1-d][1,5]benzoxazepine, and 5,6,7,7a,8,9,10,11-octahydropyrazino[1,2-d]pyrido[3,2-b][1,4]diazepine derivatives of formula (I)wherein R1, R2, R3, R4, R5, R6, X1, X2, X3, X4, Y1, Y2, and Y3 are as defined in the specification. The present application also relates to compositions comprising such compounds, and methods of treating disease conditions using such compounds and compositions, and methods for identifying such compounds.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
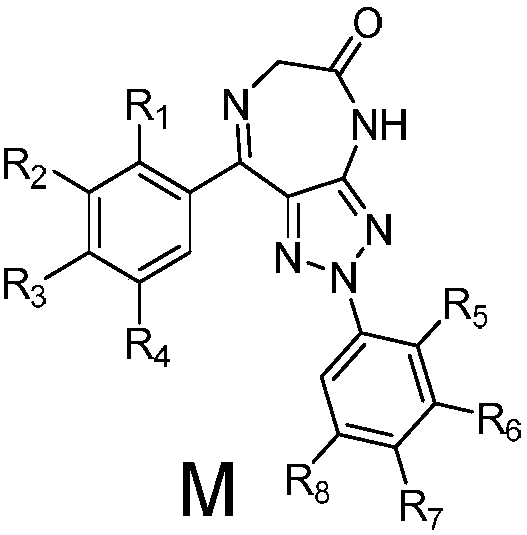
Triazole-diazepine-5-ketone compound
The invention belongs to the technical field of drugs, and relates to a triazole-diazepine-5-ketone compound and application thereof, in particular to application of the compound, which serves as a tumor cell proliferation inhibitor, in the aspect of preparing antineoplastic drugs. According to the compound, the pharmaceutically accepted salt, solvate or isomer thereof has a structural formula asshown in the description, wherein the definitions of R1 to R8 are as described in the claims and the description.
Owner:SHENYANG PHARMA UNIVERSITY
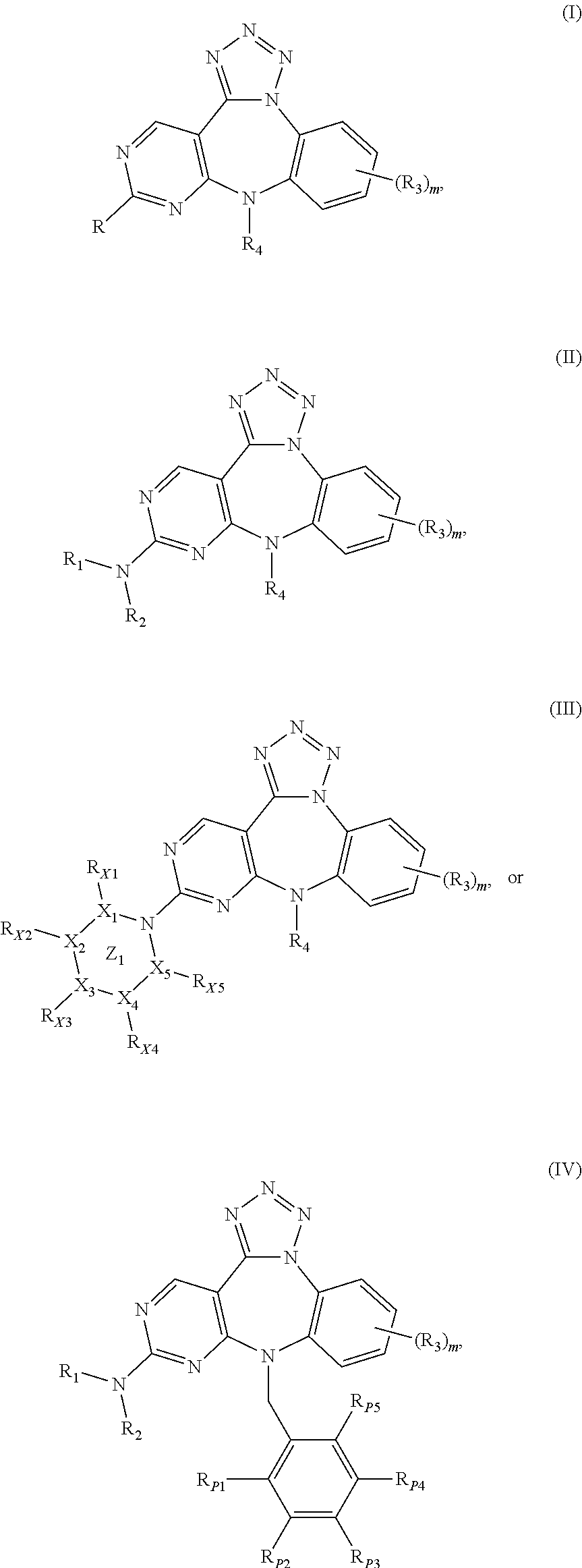
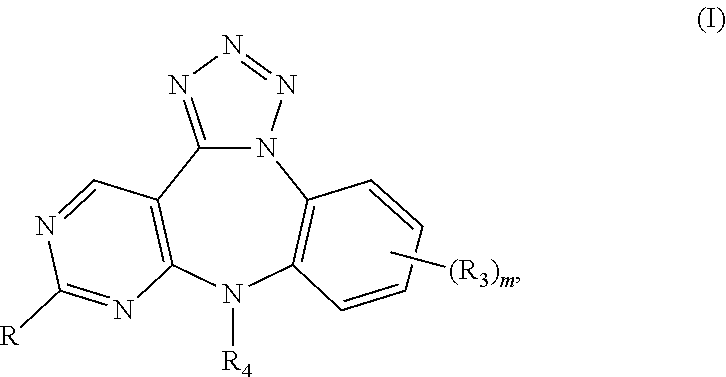
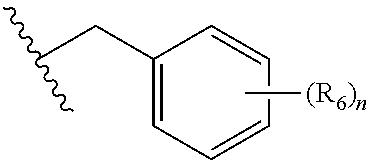
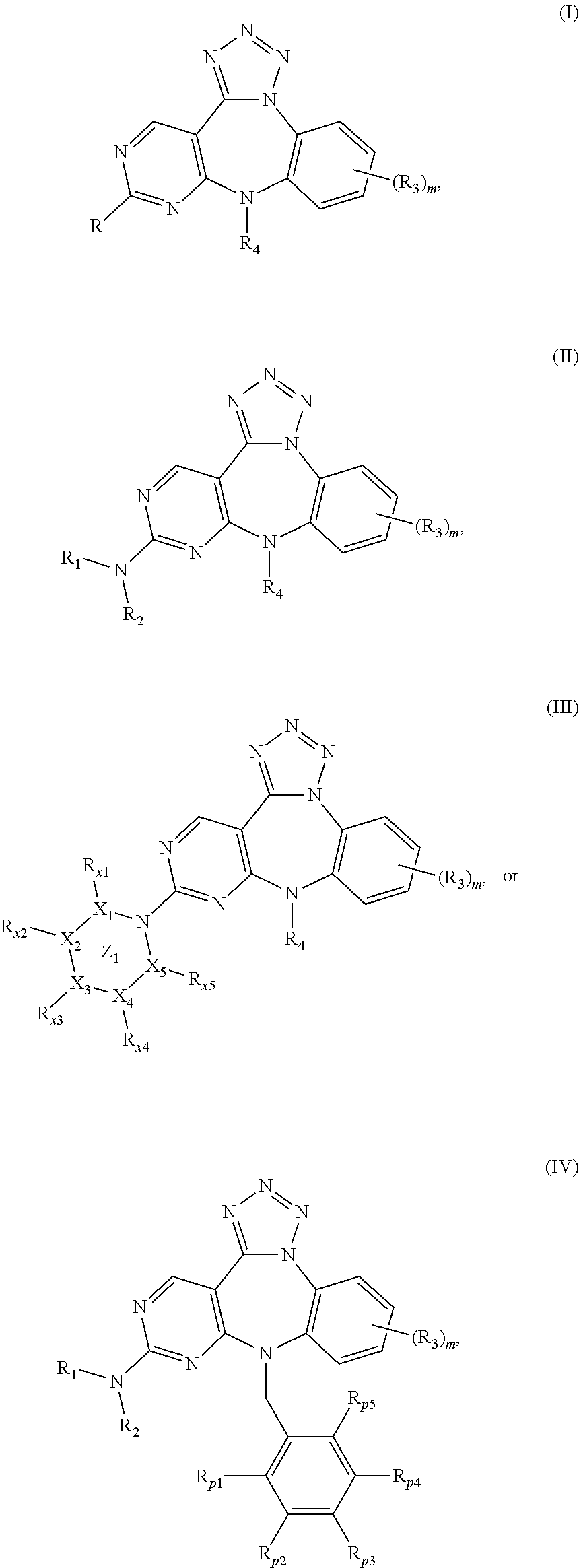
Substituted benzo-pyrimido-tetrazolo-diazepine compounds
The present invention relates to substituted benzo-pyrimido-tetrazolo-diazepine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted benzo-pyrimido-tetrazolo-diazepine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
Substituted Benzo-Pyrimido-Tetrazolo-Diazepine Compounds
The present invention relates to substituted benzo-pyrimido-tetrazolo-diazepine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted benzo-pyrimido-tetrazolo-diazepine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

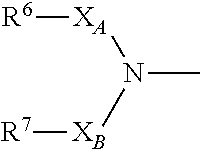


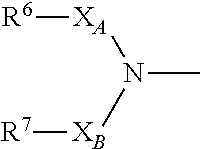








![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00001.png)
![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00002.png)
![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00003.png)

![Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cf0344f-6018-40aa-8fa4-02d0229ce6e3/US06884886-20050426-C00001.png)
![Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cf0344f-6018-40aa-8fa4-02d0229ce6e3/US06884886-20050426-C00002.png)
![Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines Process for preparing 6-aryl-4H-S-triazolo[3,4-c]-thieno[2,3-e]-1,4-diazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cf0344f-6018-40aa-8fa4-02d0229ce6e3/US06884886-20050426-C00003.png)
![Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd238a20-d894-48f3-a637-f2b54edfca6f/B2009100571420D0000011.PNG)
![Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd238a20-d894-48f3-a637-f2b54edfca6f/B2009100571420D0000021.PNG)
![Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester Preparation method of 3-carbonyl-2,8-diazepine helix[4.5]decane-8-carboxylic acid tert-butyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd238a20-d894-48f3-a637-f2b54edfca6f/F2009100571420C0000011.PNG)