Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158 results about "Diazepam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
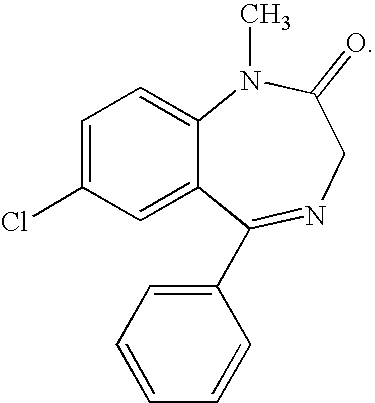
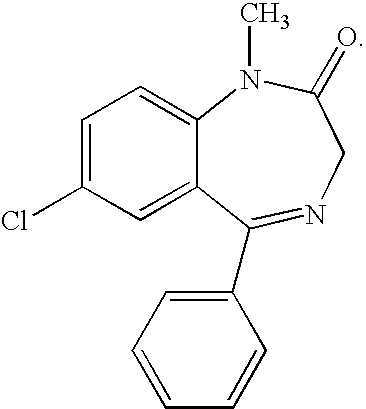
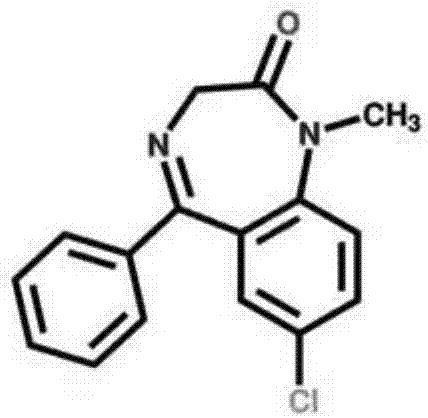
Diazepam is used to treat anxiety, alcohol withdrawal, and seizures. It is also used to relieve muscle spasms and to provide sedation before medical procedures.
Delivery of diazepam through an inhalation route
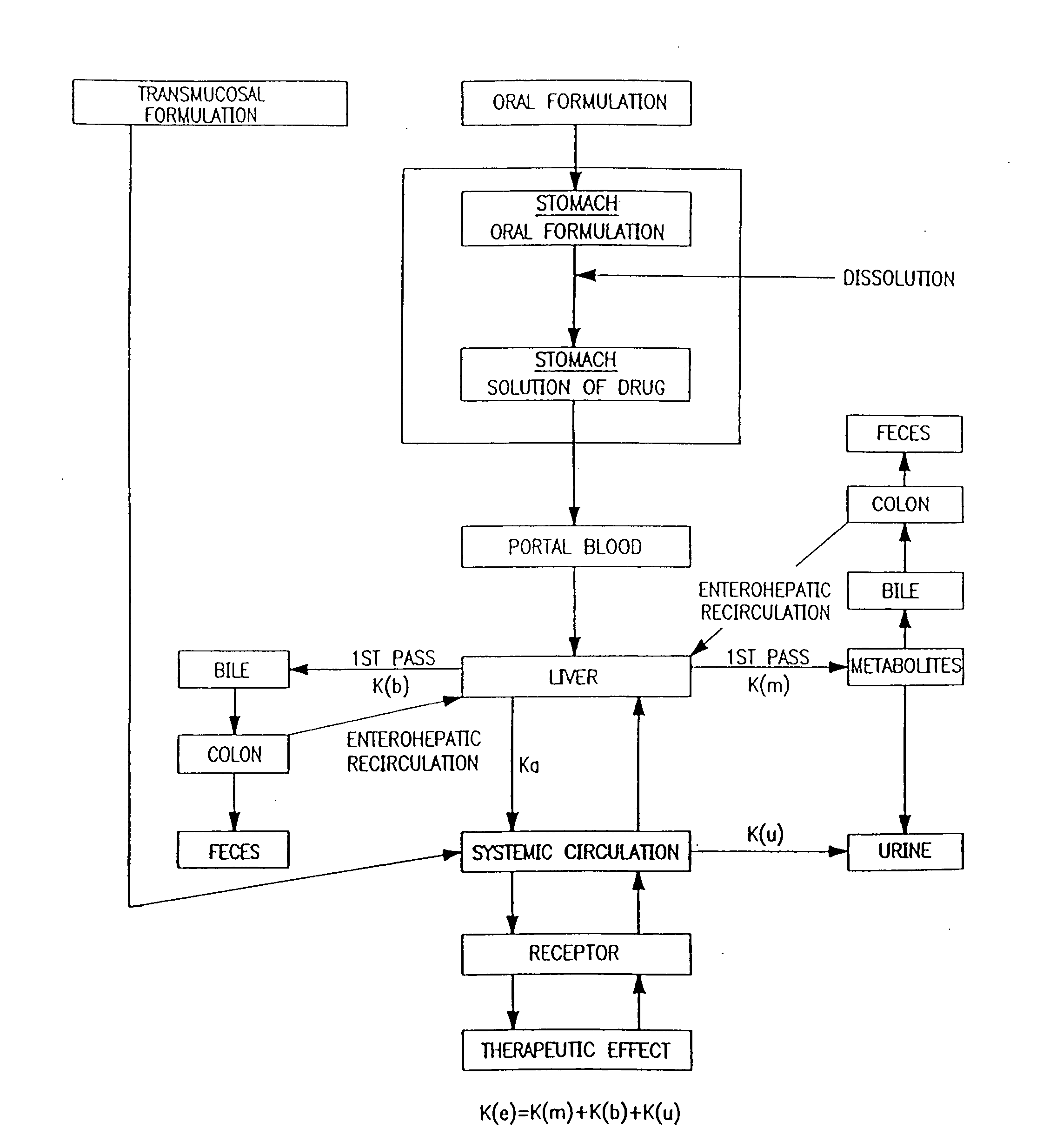
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a composition, comprising diazepam to form a vapor; and, b) allowing the vapor to cool, thereby forming a condensation aerosol with less than 5% of the drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol
Owner:ALEXZA PHARMA INC
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing diazepam that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a thin layer of diazepam on a solid support to form a vapor; and, b) passing air through the heated vapor to produce aerosol particles having less than 5% drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
Buccal, polar and non-polar spray containing diazepam
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide diazepam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, diazepam, and optional flavoring agent; formulation II: aqueous polar solvent, diazepam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, diazepam, and optional flavoring agent; and formulation IV: non-polar solvent, diazepam, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, diazepam, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, diazepam, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Methods and compositions for improving drug safety
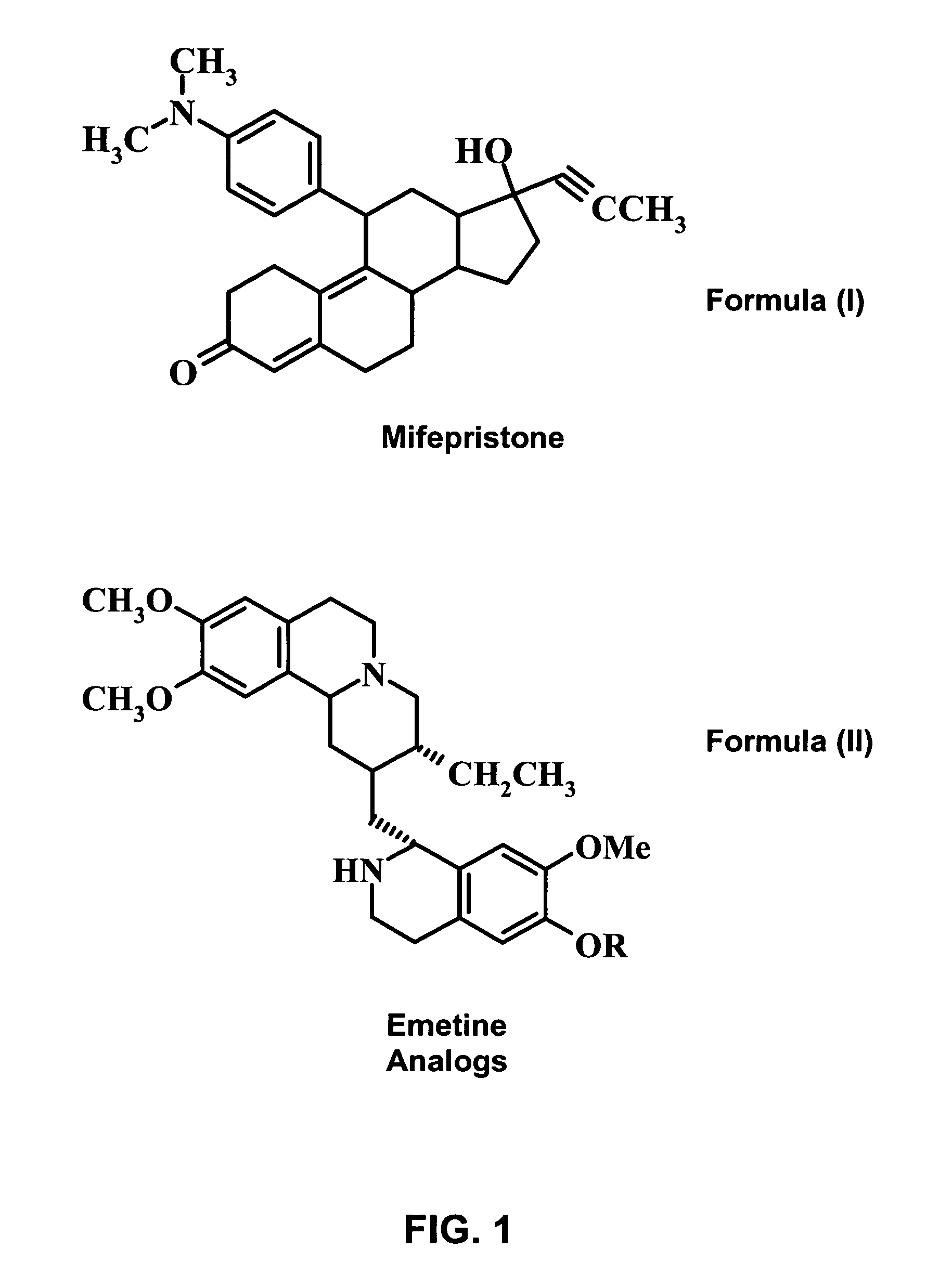
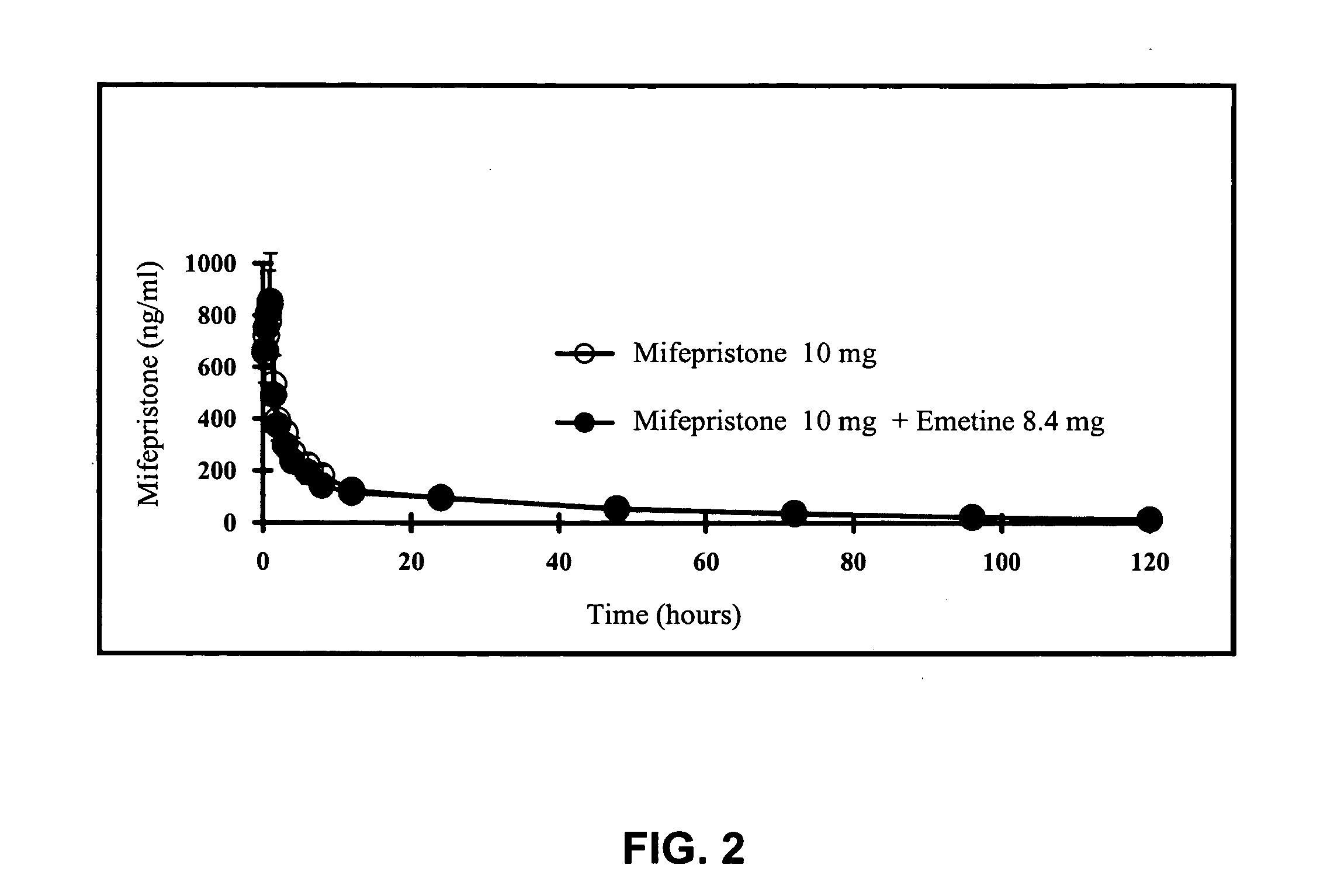
A pharmaceutical composition with improved safety includes a selected amount of a vomit-inducing agent, wherein the selected amount is less than an amount needed to induce vomit in a user; and a therapeutic agent. The therapeutic agent may be selected from a sleeping pill, an anxiolytic, a hypnotic, a contraceptive agent. The therapeutic agent may also be selected from diazepam, flunitrazepam, alprazolam, triazolam, fludiazepam, midazolam, estazolam, zopiclone, and a combination thereof. The vomit-inducing agent may be selected from emetine, cephaeline, and a combination thereof.
Owner:LOTUS PHARMA CO LTD
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing diazepam that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a thin layer of diazepam on a solid support to form a vapor; and, b) passing air through the heated vapor to produce aerosol particles having less than 5% drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
Buccal, polar and non-polar spray containing diazepam
InactiveUS20060216241A1Rapid onsetFast absorptionOrganic active ingredientsNervous disorderDiazepamSolvent
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide diazepam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, diazepam, and optional flavoring agent; formulation II: aqueous polar solvent, diazepam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, diazepam, and optional flavoring agent; and formulation IV: non-polar solvent, diazepam, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, diazepam, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, diazepam, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Medicine for preventing and treating acute altitude stress
InactiveCN104721202APrevention and treatment of acute hypertensive reactionsAntinoxious agentsHeterocyclic compound active ingredientsDiazepamMedicine
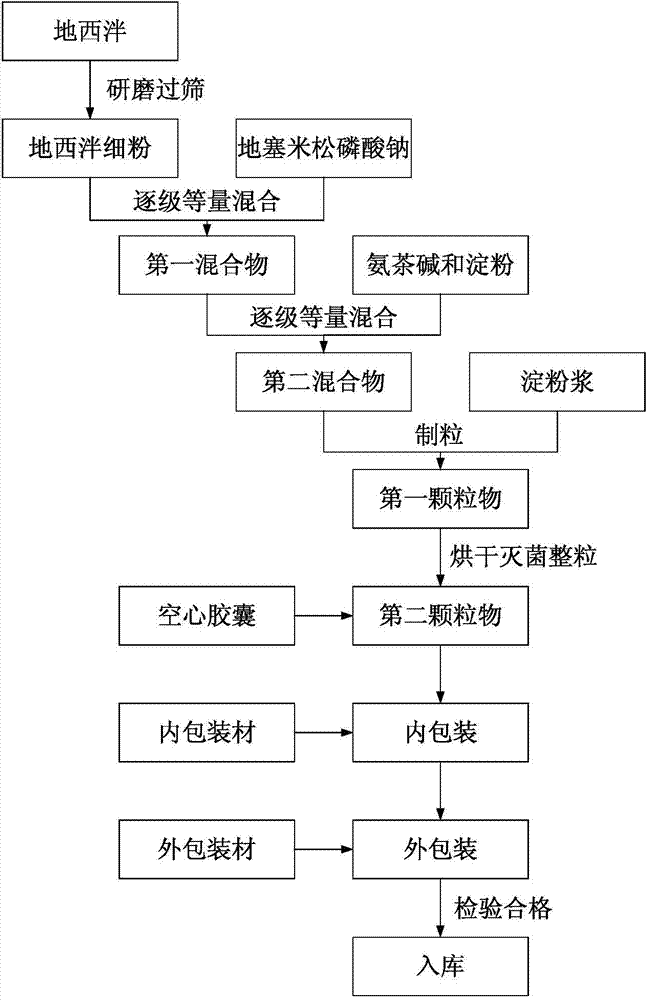
The invention discloses a medicine for preventing and treating acute altitude stress. The medicine comprises the following raw materials in percentage by mass: 0.23-1.15 percent of dexamethasone sodium phosphate, 1.14-5.68 percent of diazepam, 45.45-48.41 percent of aminophylline and a pharmaceutically acceptable excipient. Since capsules for treating acute altitude stress comprise 0.23-1.15 percent of dexamethasone sodium phosphate, 1.14-5.68 percent of diazepam and 45.45-48.41 percent of aminophylline, acute altitude stress can be treated effectively, the effective rate of treatment can be above 87%, and the maximum effective rate of treatment is up to 92.5%.
Owner:中国人民解放军西藏军区总医院
Nasal administration of benzodiazepines
Particulate formulations of benzodiazepines, such as diazepam, are used for nasal administration of diazepine drugs to patients. Multimodal particulate formulations of benzodiazepines and methods for their use, e.g. by nasal administration for the treatment of seizure, are also provided.
Owner:AEGIS THERAPEUTICS LLC +1
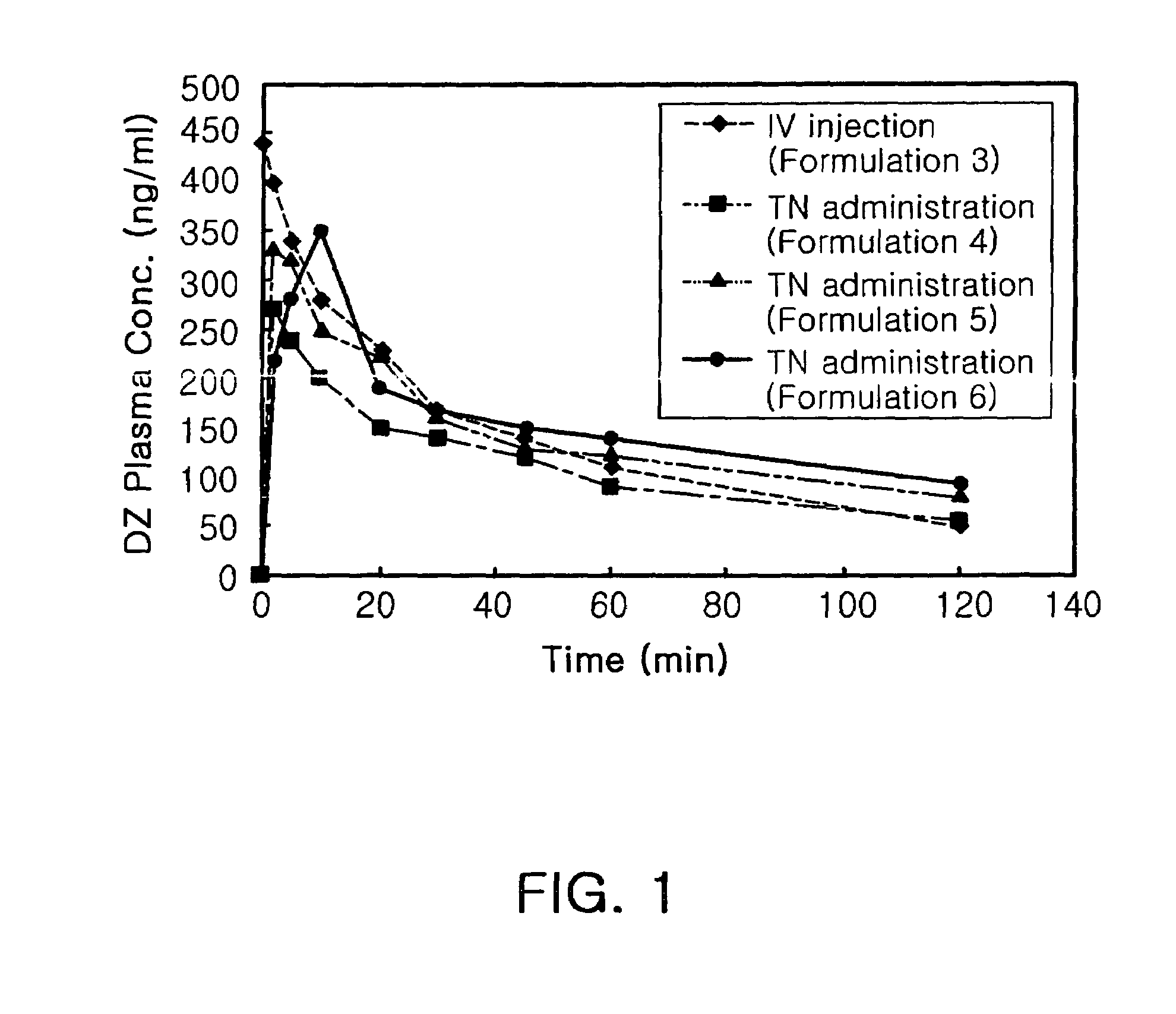
Transnasal microemulsions containing diazepam
InactiveUS20050002987A1High plasma concentrationHigh concentrationPowder deliveryNervous disorderNasal cavityBlood plasma
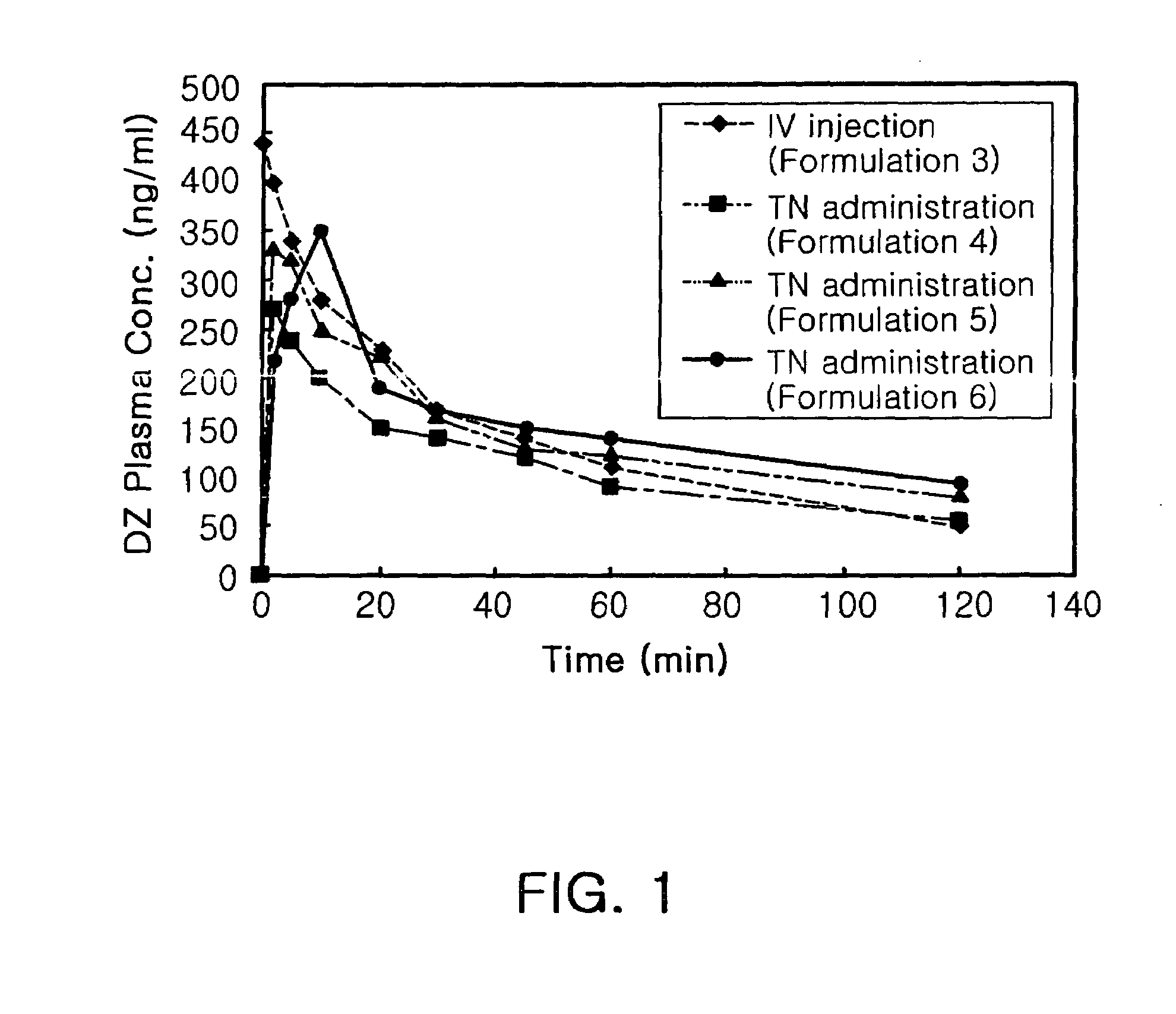
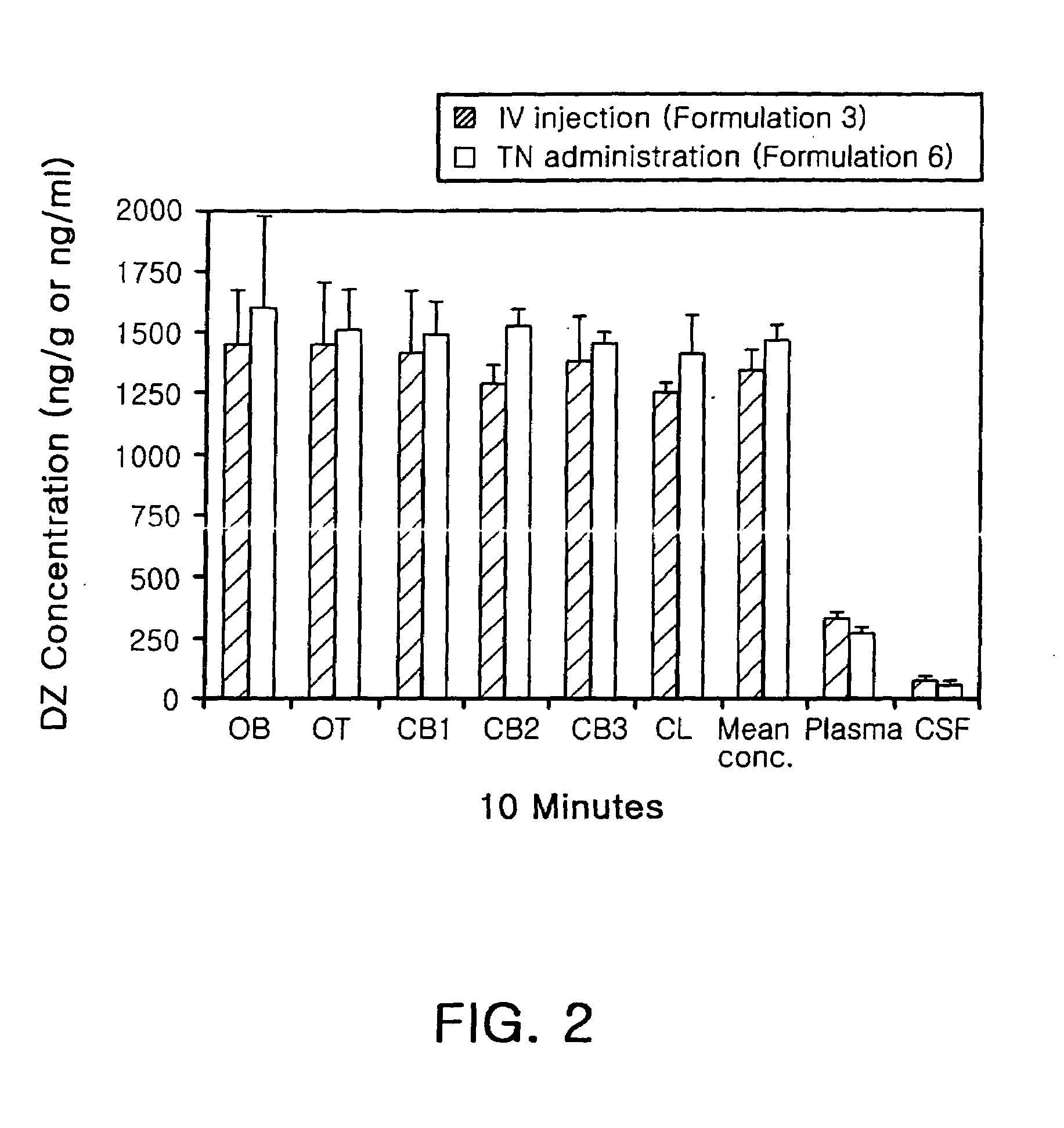
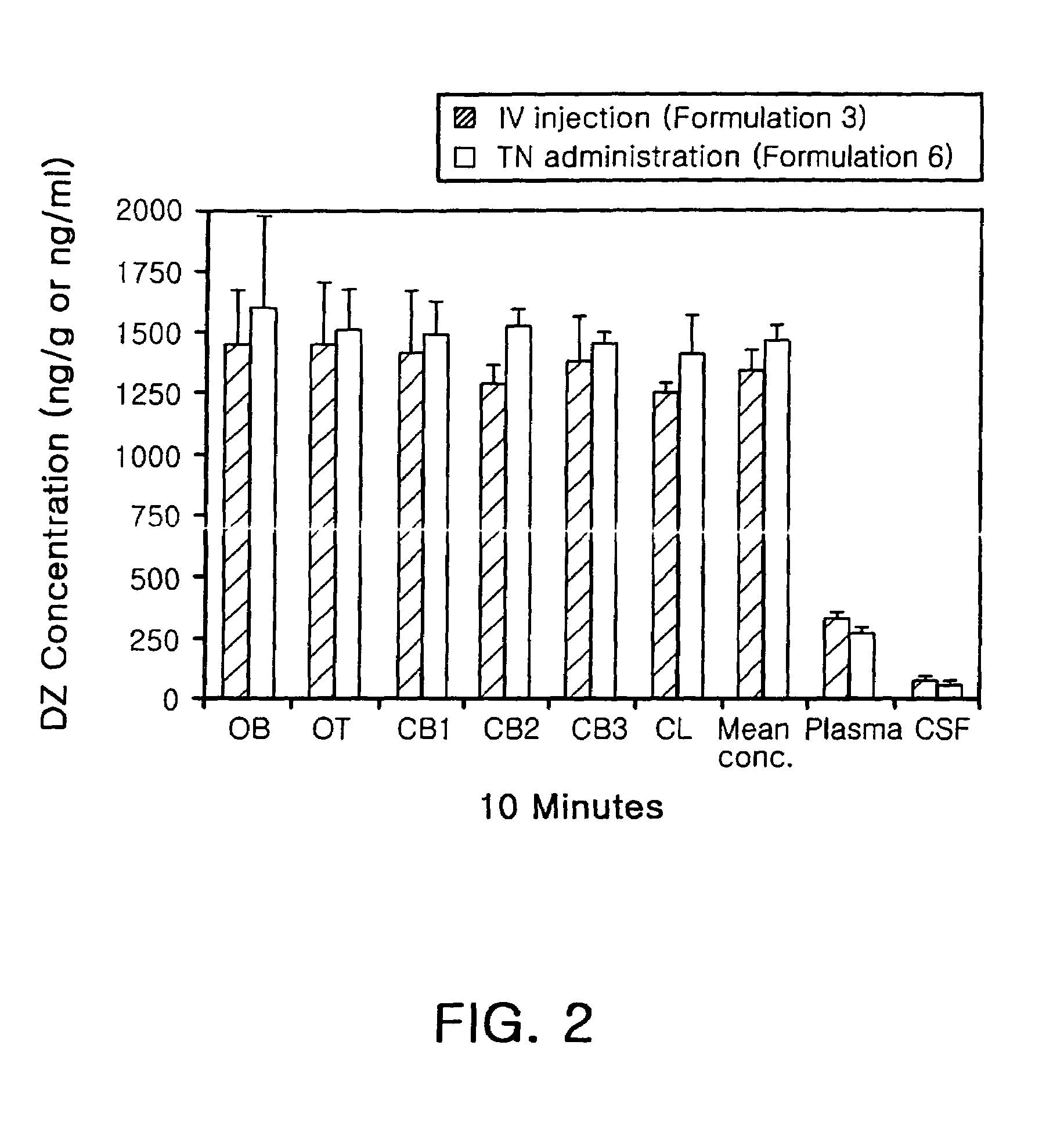
Diazepam is administered intranasally in the form of specific microemulsions having advantageous properties. The microemulsions are comprised of about equal quantities of a fatty acid and water with the remainder being a hydrophilic surfactant, a polar solvent and an alcohol in a weight ratio such that alcohol is present in a greater quantity by weight than either of the other two. Nasal administration of the subject microemulsions produces a high plasma concentration of diazepam nearly as fast as intravenous administration. The present microemulsions are particularly suitable for a prompt and timely treatment of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:SK
Method for simultaneously detecting five medicaments in water
InactiveCN103323550AHigh selectivityHigh sensitivityComponent separationTrimethoprimSolid phase extraction
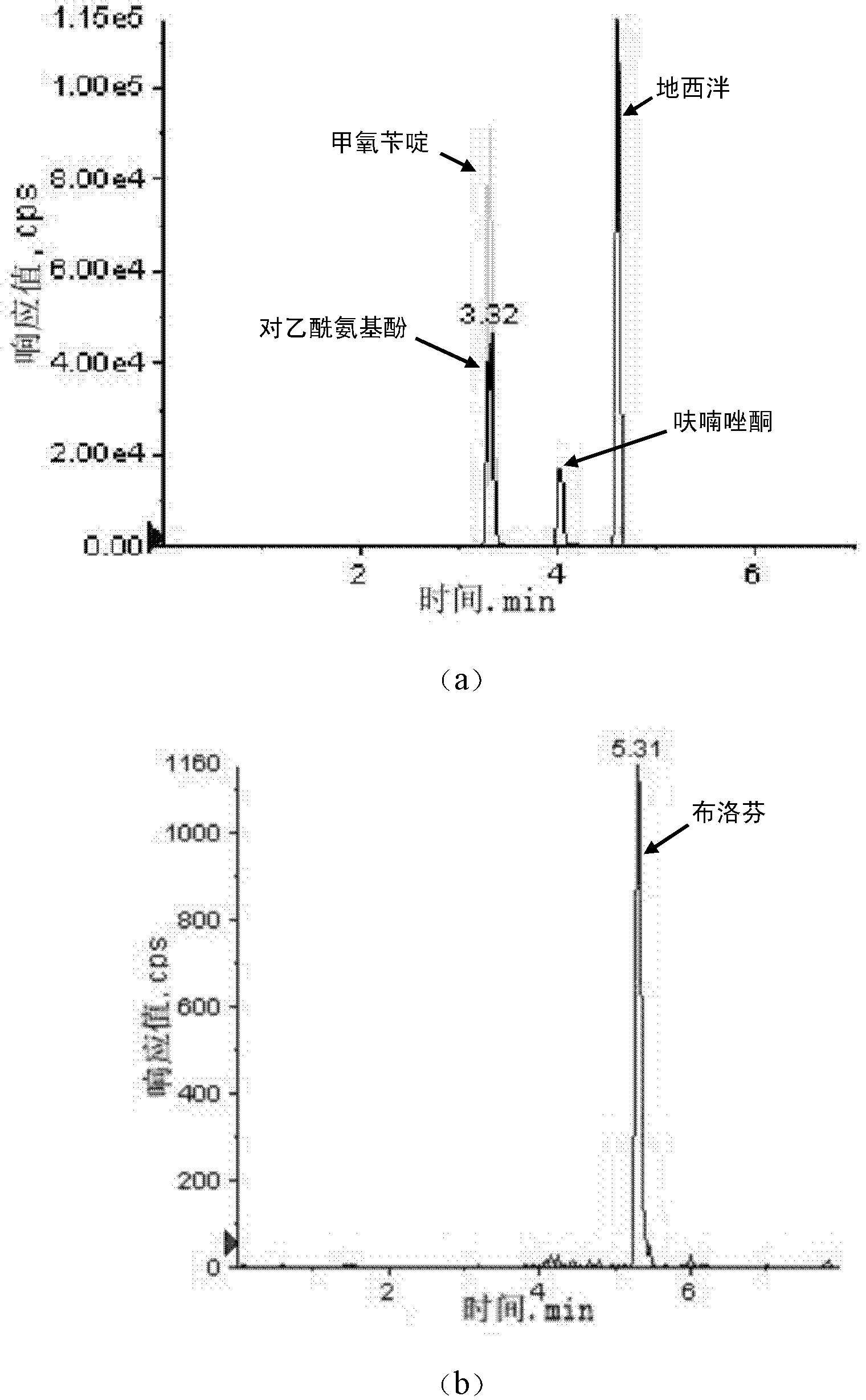
The invention discloses a method for simultaneously detecting the medicaments such as furazolidone, diazepam, trimethoprim, acetaminophen and ibuprofen in water. The method comprises the following steps of: filtering a water sample to remove suspended matter; adjusting the pH of the water sample to be 2 to 4; activating hydrophile-lipophile balance (HLB) solid phase extraction column by using acetone, methanol, ammonium acetate-containing formic acid aqueous solution and ultrapure water in sequence; after the enrichment is finished, drying the HLB solid phase extraction column under the protection of nitrogen and eluting the HLB solid phase extraction column; collecting eluant, and drying the eluant by blowing a nitrogen flow; adding acetonitrile to dissolve the residue; and quantitatively detecting the concentration of the five medicaments. By the method, the water sample pretreatment is environment-friendly, easy to operate, large in enrichment factor, and high in reproducibility; and the content of the five common medicaments in a water environment can be analyzed quickly and accurately.
Owner:CHINESE RES ACAD OF ENVIRONMENTAL SCI
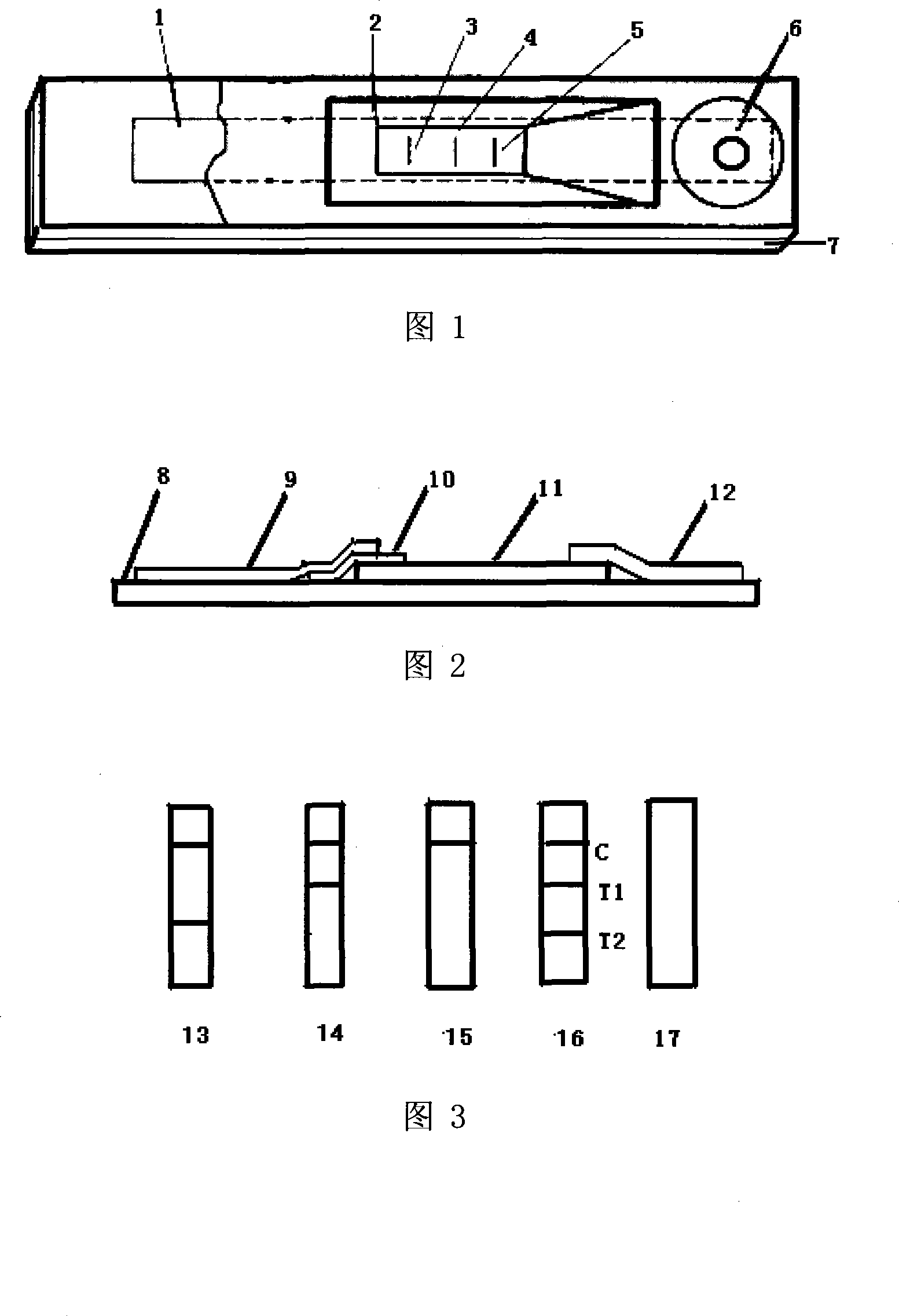
Chlorpromazine-diazepam dual detection card and its detection method
The invention relates to a device for detecting chlorpromazine and diazepam class medicines, in particular to a chlorpromazine-diazepam bigeminy test card. The chlorpromazine-diazepam bigeminy test card comprises a casing and a test paper strip. The test paper strip comprises a base plate, and a sample absorption cushion, a colloidal gold label cushion, a detection reaction area and an absorption cushion which are adhered in order on the base plate, wherein the colloidal gold label cushion contains a colloidal gold label of a chlorpromazine antibody and a diazepam antibody, the detection reaction area at least comprises a quality control line and two detection lines, and the two detection lines are respectively coated with coupling compound formed by the chlorpromazine diazepam coupled with carrier protein. The chlorpromazine-diazepam bigeminy test card has the advantages of capability of simultaneously detecting the chlorpromazine and diazepam in urine or fodder, animal tissues, meat and liver, easy preparation, detection cost saved, convenient use, quick detection, high sensitivity and correct results.
Owner:CHINA JILIANG UNIV +1
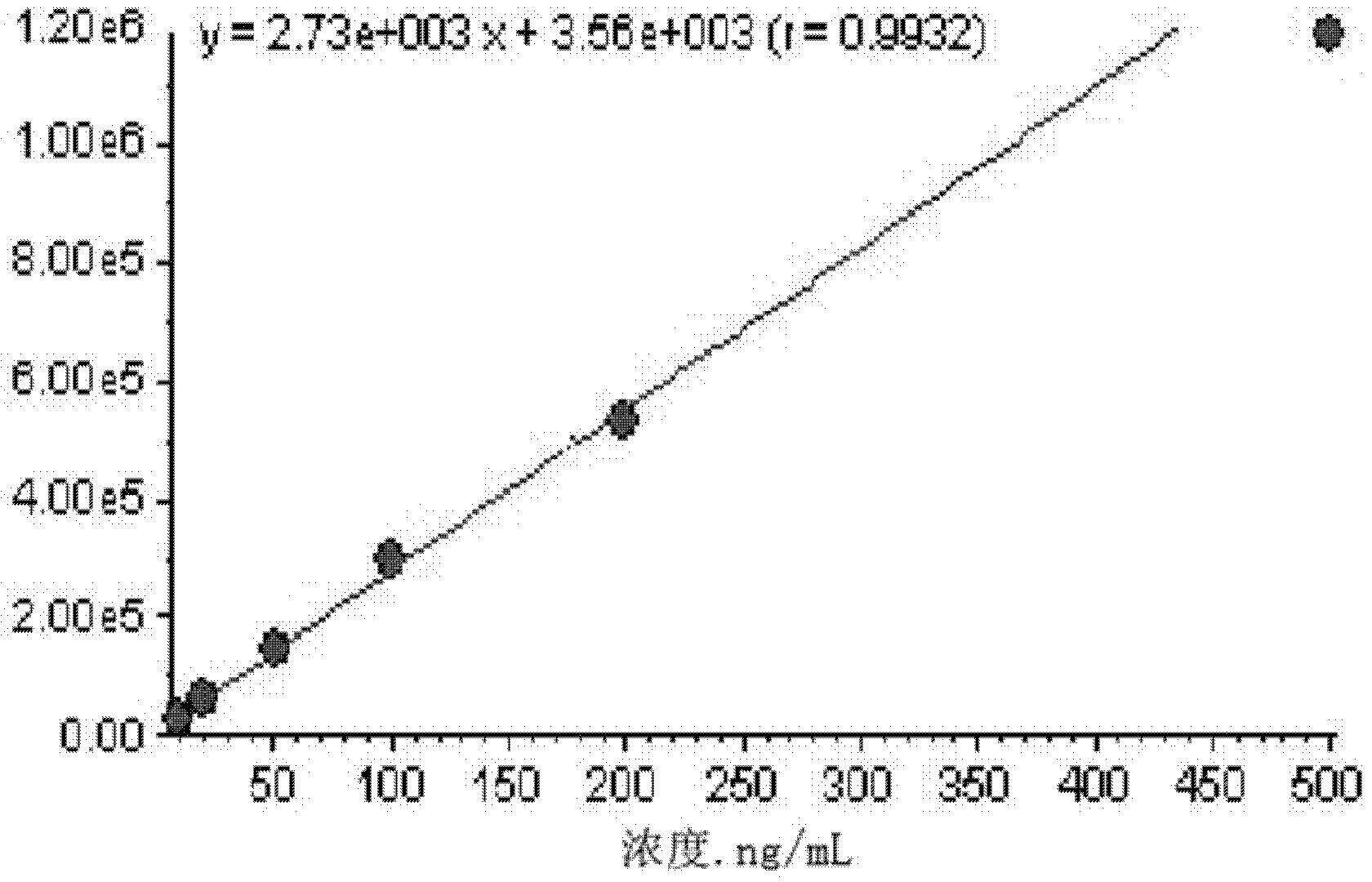
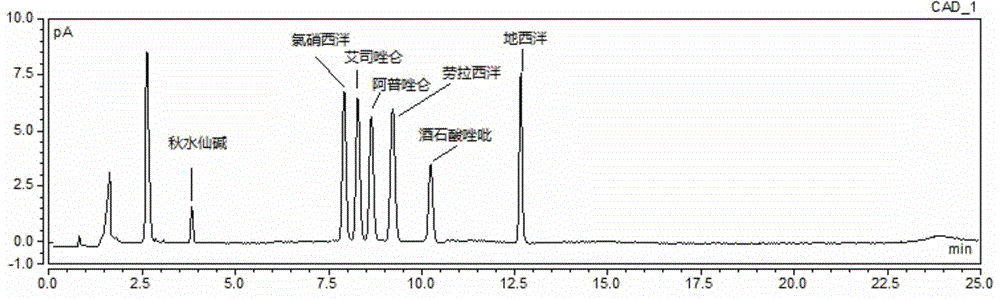
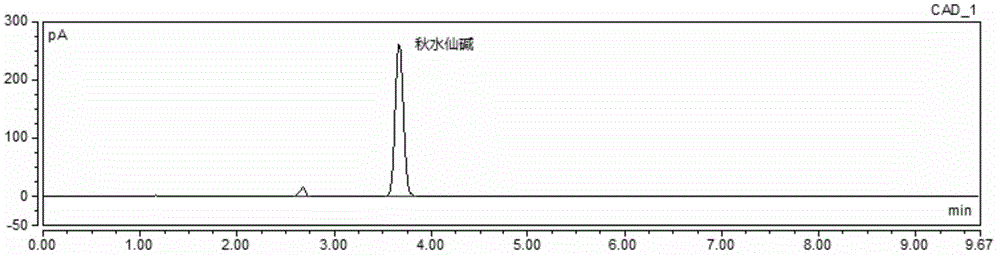
Method for simultaneous determination of contents of 6 kinds of sedative-hypnotic drugs in serum by UHPLC-CAD technology
ActiveCN104133030ASimultaneous measurementShort analysis timeComponent separationSide effectSedative/hypnotic
The invention relates to a method for simultaneous determination of the contents of 6 kinds of sedative-hypnotic drugs in serum by a UHPLC-CAD combined technology, wherein the 6 kinds of sedative-hypnotic drugs are diazepam, lorazepam, alprazolam, estazolam, clonazepam and zolpidem tartrate. The method is characterized by including the following steps: 1) reference substance preparation, 2) internal standard solution preparation, 3) sample solution preparation, 4) mobile phase solution preparation, 5) chromatographic condition setting, 6) electrospray detector condition optimization, 7) sample determination, 8) gradient-concentration reference substance solution preparation, 9) standard curve preparation, and 10) data calculation and analysis. The method is advanced, has good clinical application prospects and effects, can rationally use drugs for clinic, increases drug curative effects, lowers toxic and side effects and drug costs, and plays a positive role.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
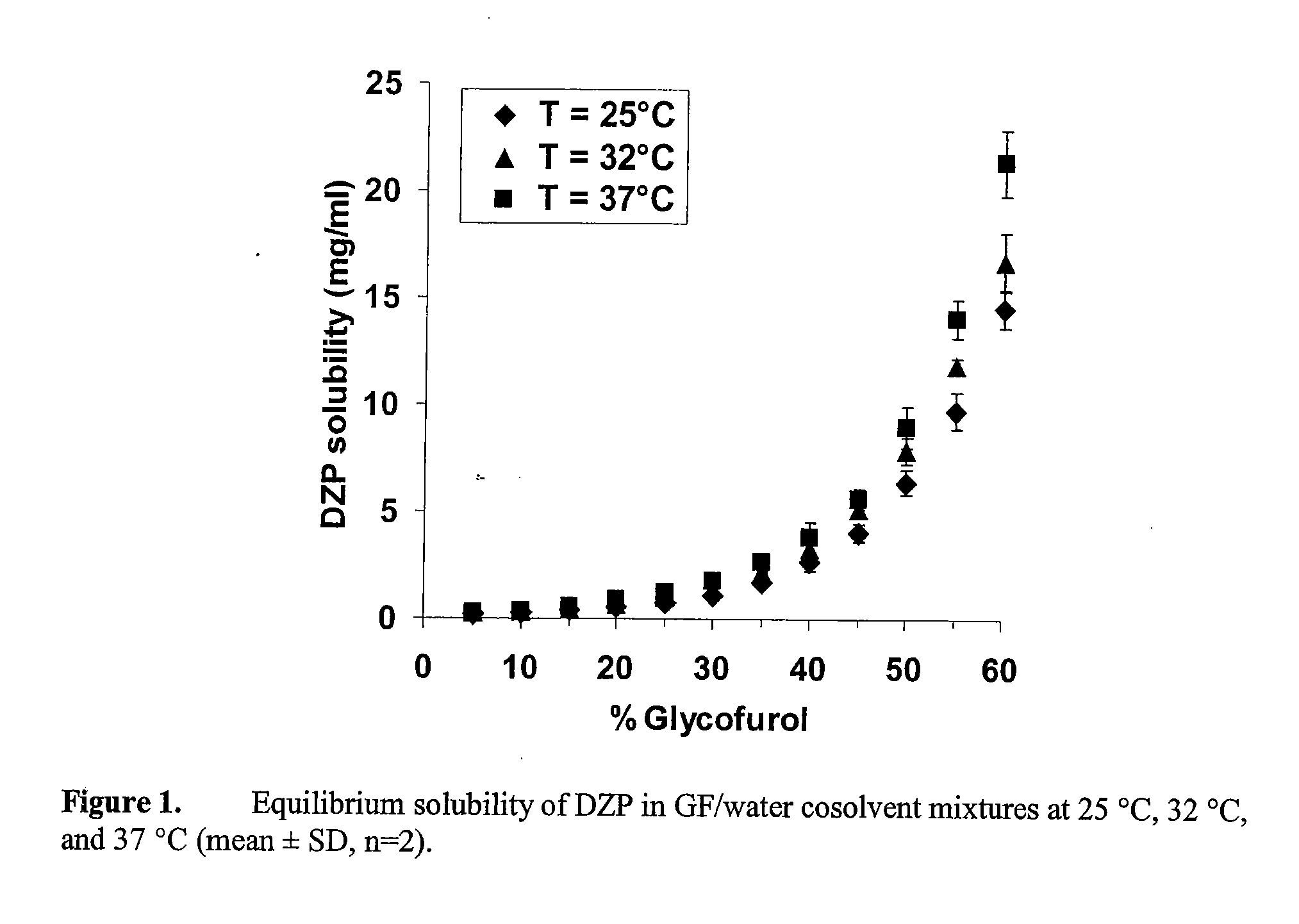
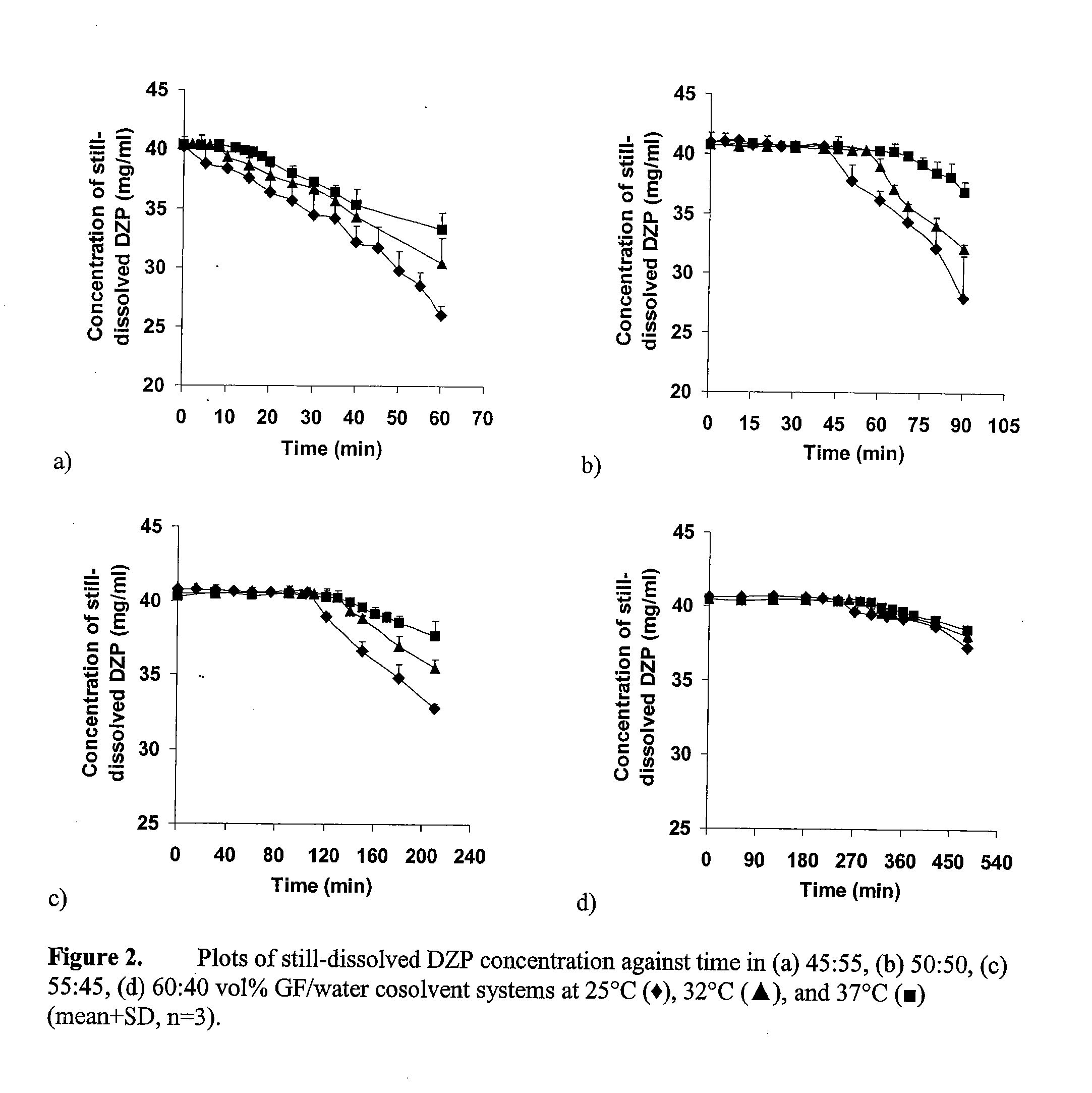
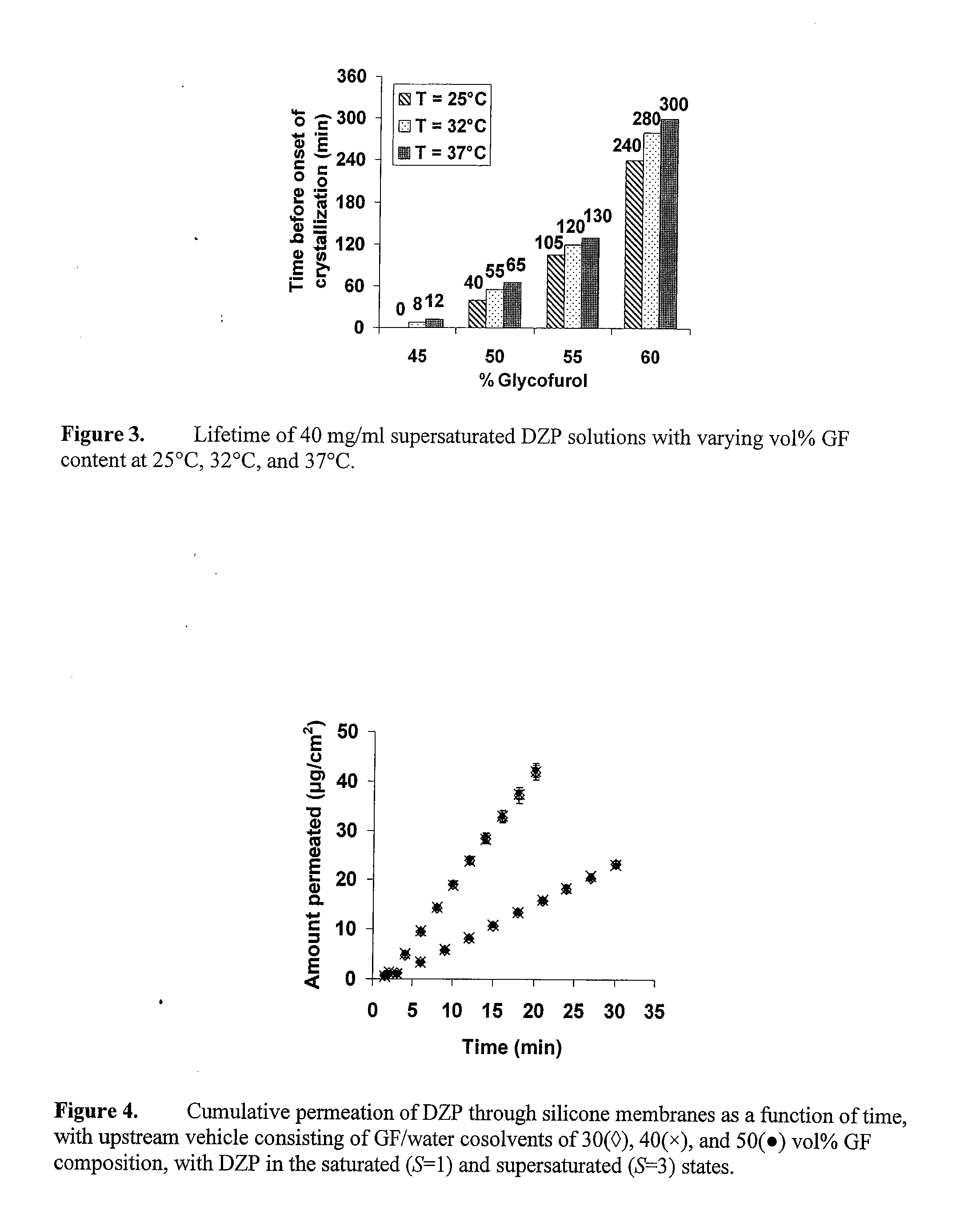
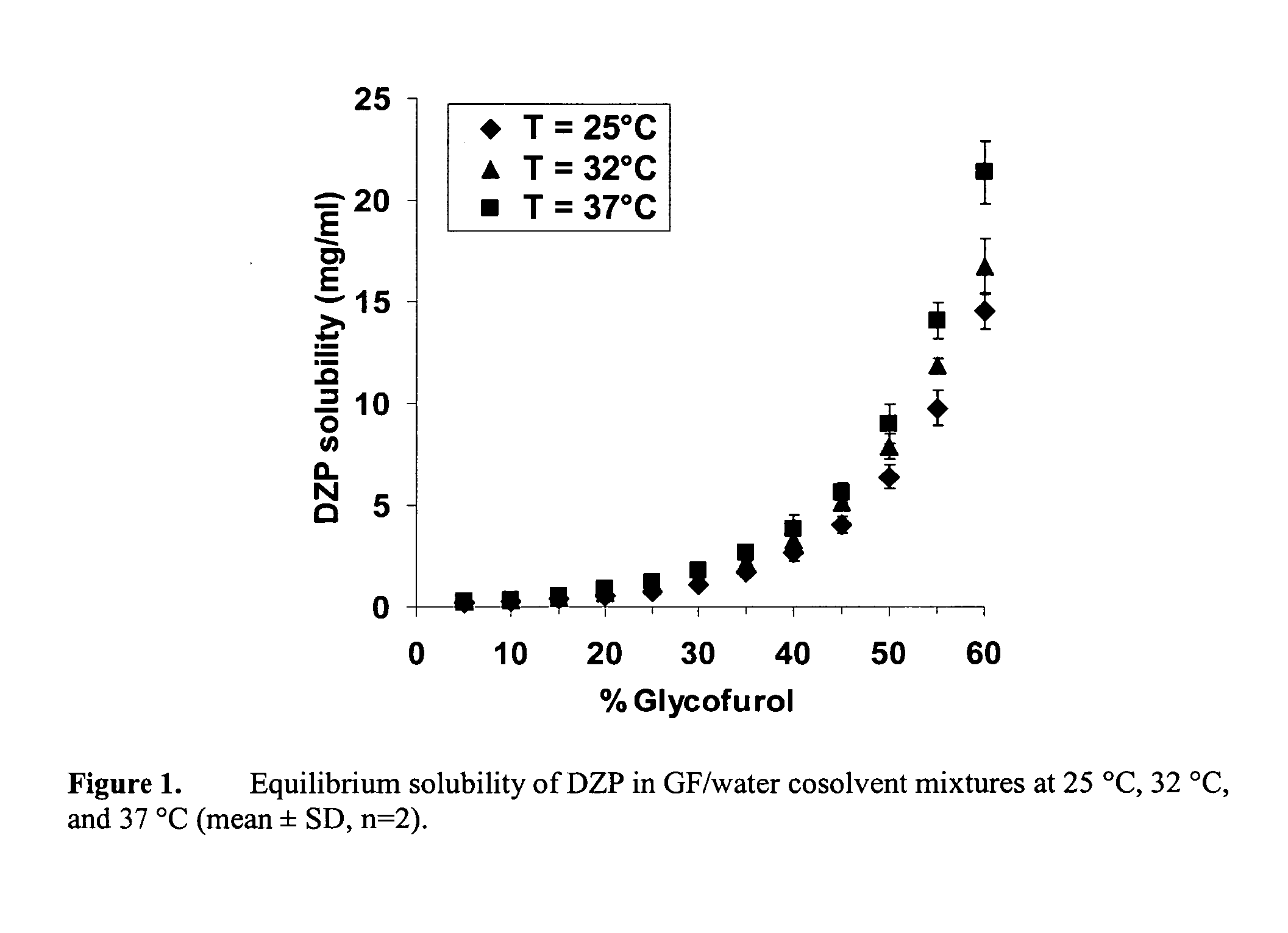
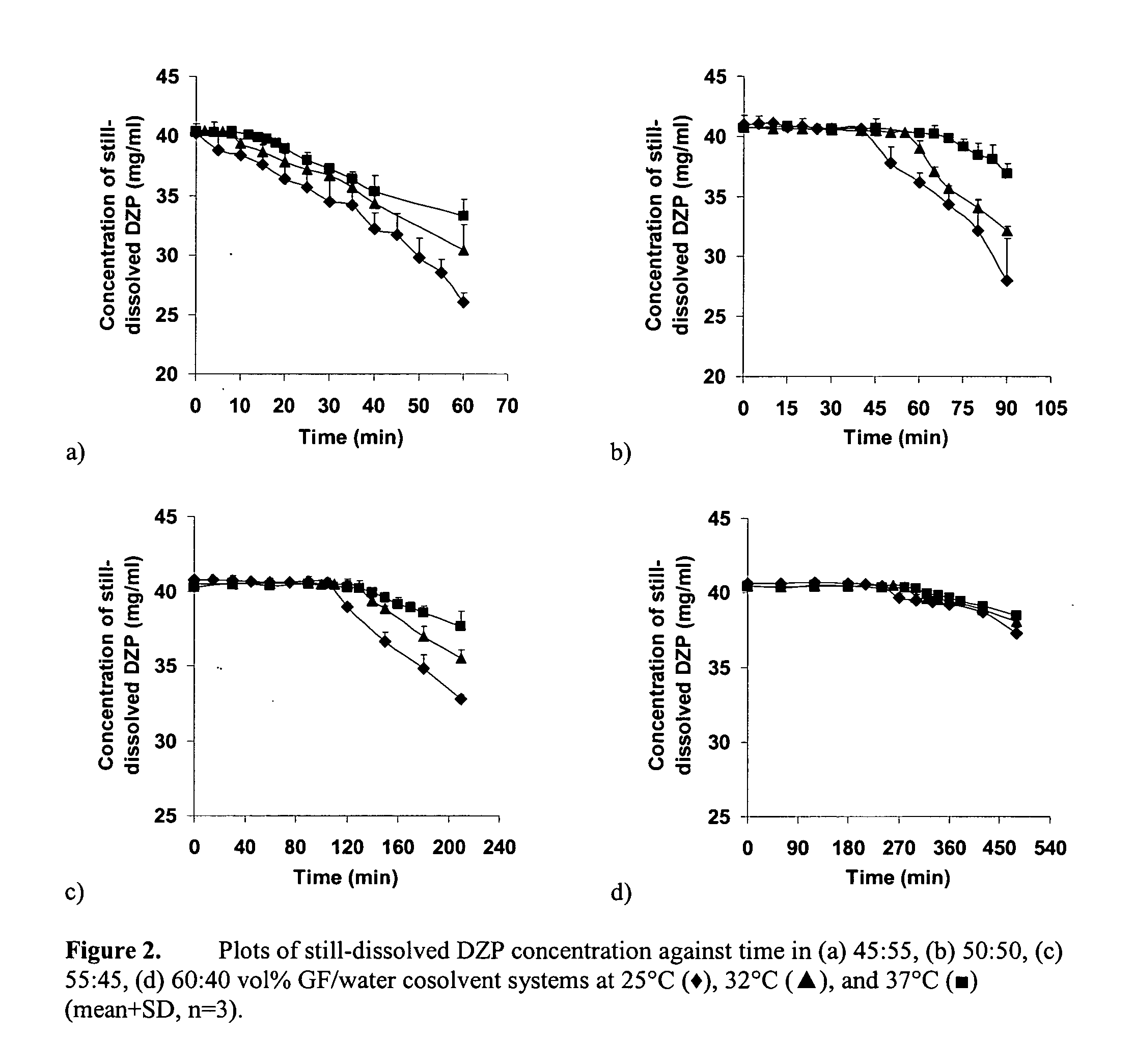
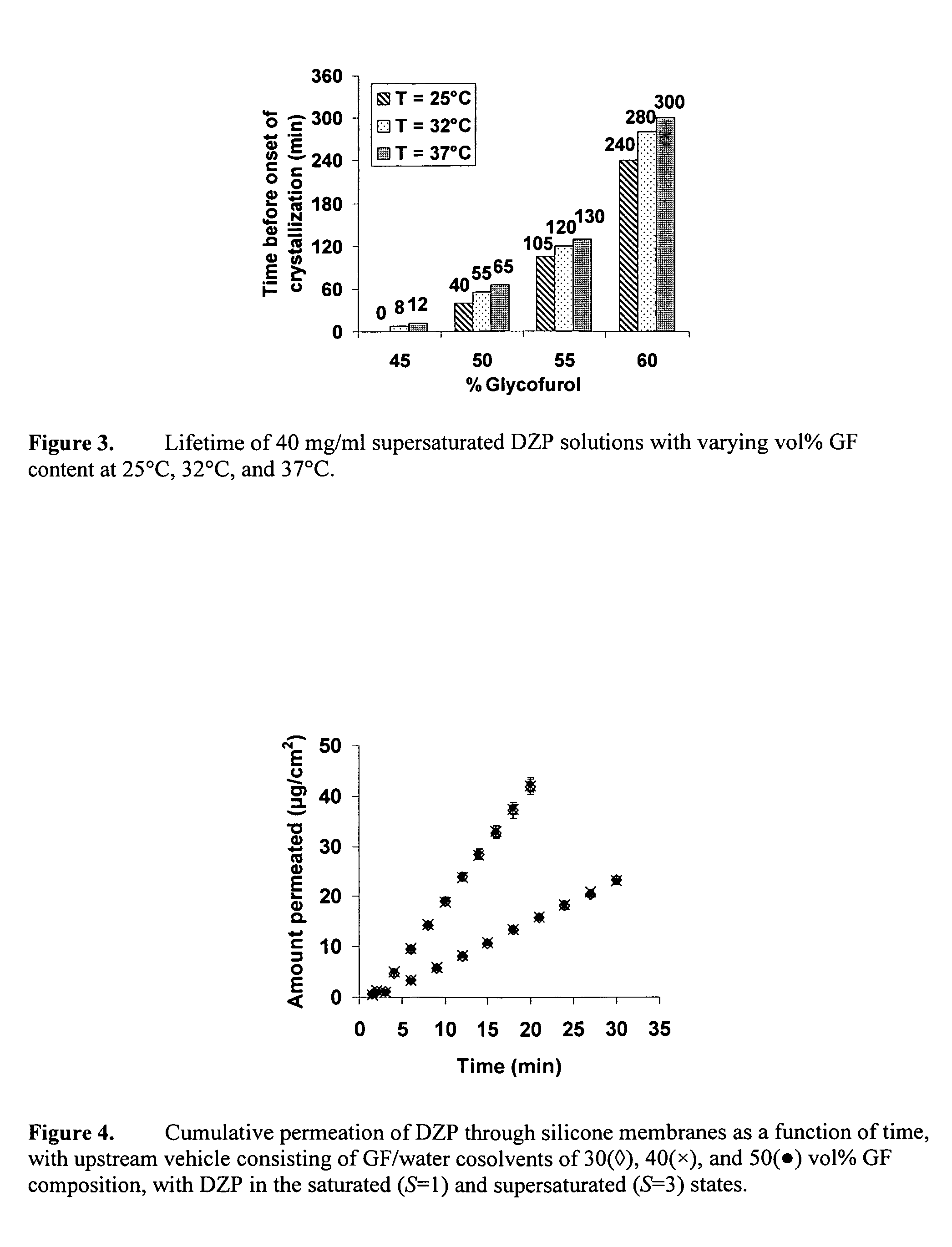
Supersaturated Benzodiazepine Solutions and Their Delivery
InactiveUS20070208011A1Fast absorptionAvoid excessive injuryBiocidePharmaceutical delivery mechanismBenzodiazepineDisease
The invention describes supersaturated solutions of benzodiazepines, such as diazepam, glycofurol and water and their use for intranasal (NS) administration to combat various disorders.
Owner:RGT UNIV OF MINNESOTA
Method for detecting residual tranquilizer medicines in meat product
The invention discloses a method for detecting residual tranquilizer medicines in a meat product. The method comprises the following steps: extracting and purifying by adopting a C18 solid phase extracting column, and then detecting by adopting a gas chromatography-mass spectrometer (GC-MS), so as to rapidly and exactly detect out the variety and the content of the residual tranquilizer medicines, including seven tranquilizer medicines, namely, diazepam, oxazepam, estazolam, alprazolam, triazolam, phenobarbital and promethazine, in the meat product. The method is suitable for detecting multiple tranquilizer medicine residues in complex substrate meat products such as sausage; the method has the advantages of being low in detection limit, accurate, fast, easy and convenient in operation and economic in detection cost, and can be developed and applied in detection mechanisms.
Owner:广东省中山市质量计量监督检测所
Transnasal anticonvulsive pharmaceutical composition
InactiveUS20080113970A1Improve permeabilityImprove solubilityBiocideNervous disorderConvulsionSolubility
Disclosed herein is a transnasal anticonvulsive pharmaceutical composition comprising diazepam as an active ingredient, water, a fatty acid ester, diethylene glycol monoethyl ether, ethanol and sodium glycocholate, wherein the weight of the fatty acid ester is at least 2-fold higher than that of water and is at least 2-fold higher than that of ethanol.The anticonvulsive pharmaceutical composition for transmucosal delivery of diazepam according to the present invention includes a minimized content of water and ethanol, a fatty acid ester as a main ingredient and no use of a polar solvent, e.g. glycol, and, exhibits improved diazepam solubility and transmucosal permeability due to using a small amount of water and ethanol. The present invention also includes treatment of convulsions by transnasally administering to a patient in need thereof a therapeutically effective amount of the disclosed compositions.
Owner:BIOPHARM
Medication and treatment for disease
Owner:ALTMAN ENTERPRISES
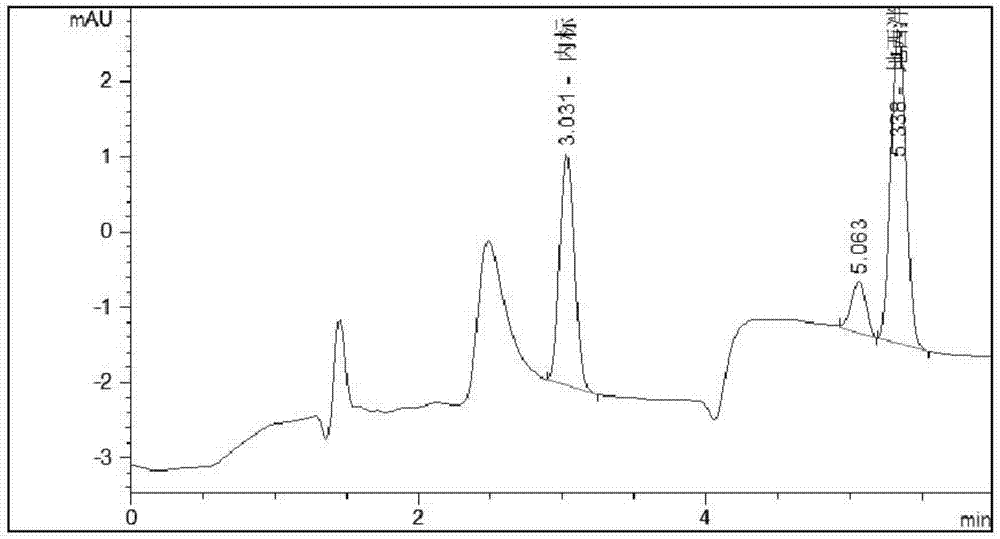
Liquid chromatographic analysis method for detecting content of diazepam in blood
InactiveCN107991421AImprove accuracyEliminate errorsComponent separationWorking fluidInstrumentation
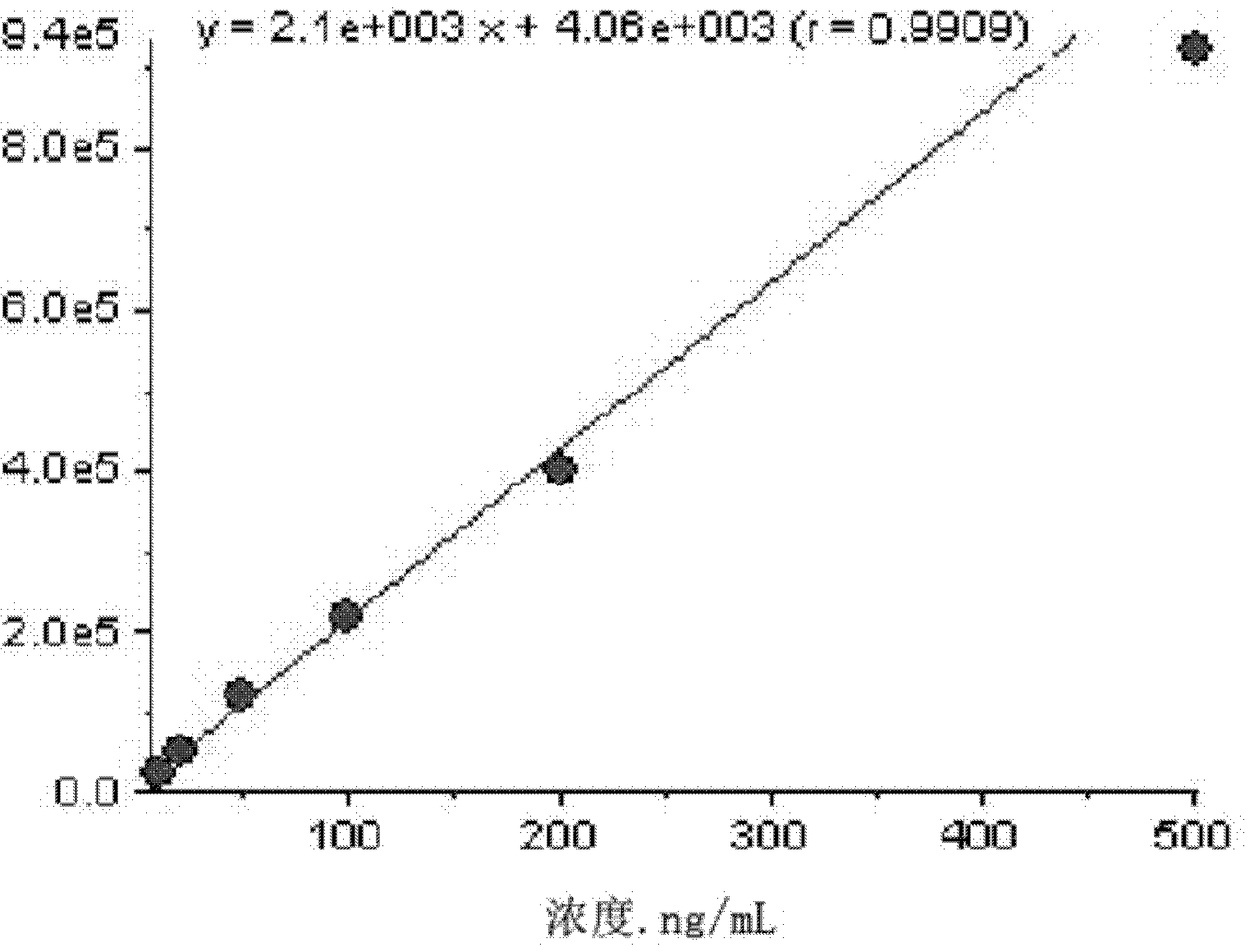
The invention discloses a method for detecting a drug concentration of diazepam in blood. According to the method, a standard solution is calibrated by using a liquid chromatographic analytic instrument and an ultraviolet detector; a standard curvilinear equation which is y=a*x+b is fitted and obtained; a to-be-detected blood sample is taken; after to-be-detected blood is treated, a to-be-detectedsample is detected by similarly using the liquid chromatographic analytic instrument and the ultraviolet detector, so as to obtain a value y of the to-be-detected blood; the y of the to-be-detected blood is substituted into the standard curvilinear equation; a relative concentration x of a target substance in the to-be-detected blood sample is obtained through calculation; a concentration of working fluid of an internal standard substance is known; thus, the drug concentration of the diazepam in the to-be-detected blood in the sample is calculated and obtained; according to the method, an internal standard method is combined with high performance liquid chromatography; the accuracy of a quantitative result is improved; a system error is eliminated; an analysis time is effectively shortened; a detection process is enabled to be simple, convenient and quick, and the monitoring carried out on a blood concentration of the diazepam in the body of a patient in clinical treatment is more facilitated.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
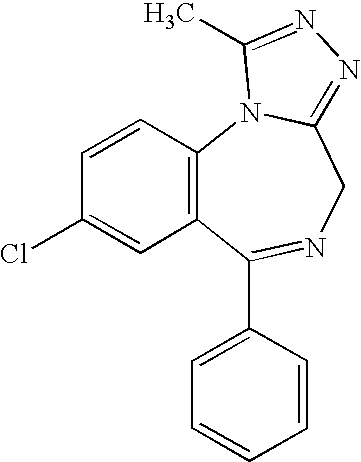
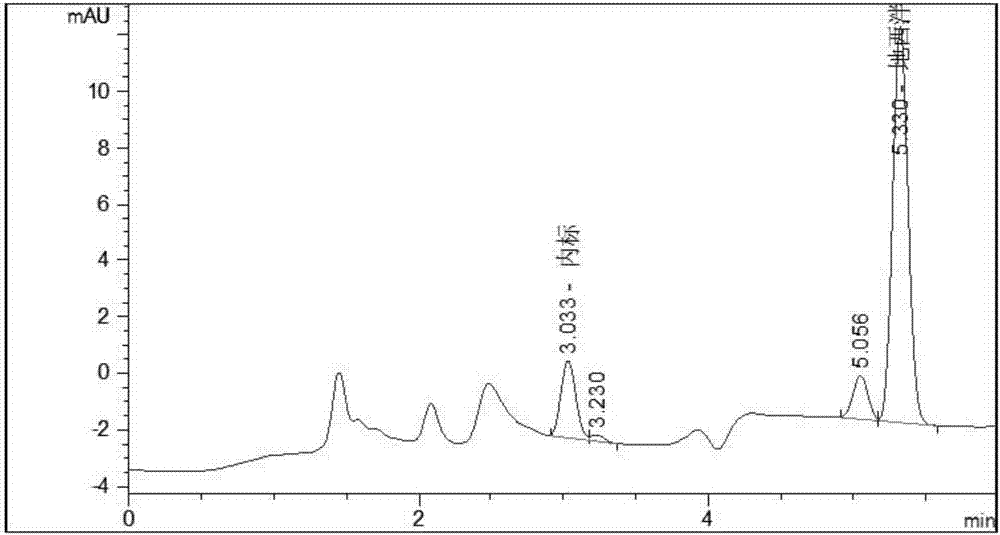
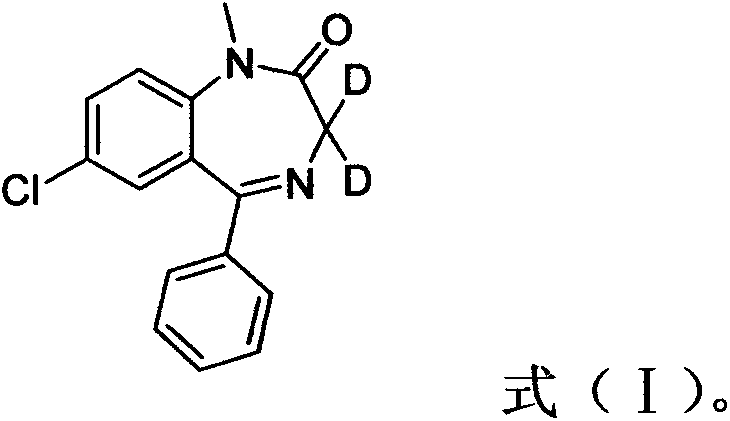
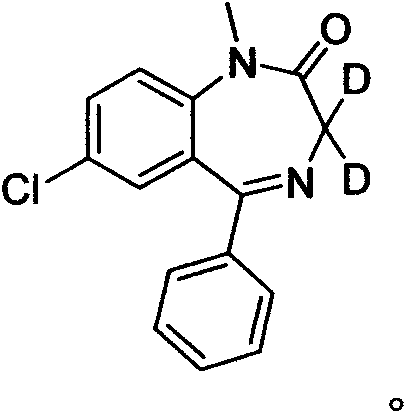
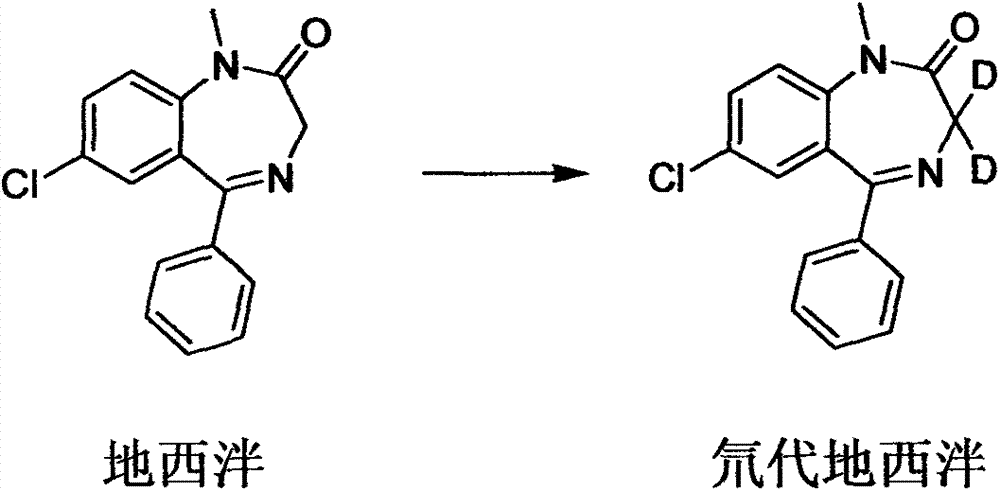
Deuterated diazepam and preparation method thereof
InactiveCN103204819ASimple reaction conditionsEasy to operateOrganic chemistryBenzodiazepineDeuterated chloroform
The invention discloses deuterated diazepam and a preparation method thereof. The preparation method of deuterated diazepam comprises the following steps of: (1) mixing 7-chlorine-1-methyl-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-ketone and N,N-dimethyl formamide, and stirring; (2) adding potassium carbonate and deuterated chloroform, heating the mixture to more than 40 DEG C and stirring; and (3) carrying out separation to obtain the deuterated diazepam. The preparation method provided by the invention is short, simple and convenient to operate, low in cost and easy to purify. The commercially available non-deuterated diazepam uses a small amount of deuterated reagents as deuterium sources in a non-deuterated solvent atmosphere, so that deuterated diazepam is obtained in a relatively short period of time, and through simple column chromatography purification, a purified product is purified. The deuterated diazepam standard product prepared according to the invention is high in purity and stable in chemical property; and the preparation of the standard product for analysis is convenient. The preparation method provided by the invention can be used for producing deuterated internal standard substance used for analyzing and detecting diazepam.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Diazepam monoclonal antibody screening and application
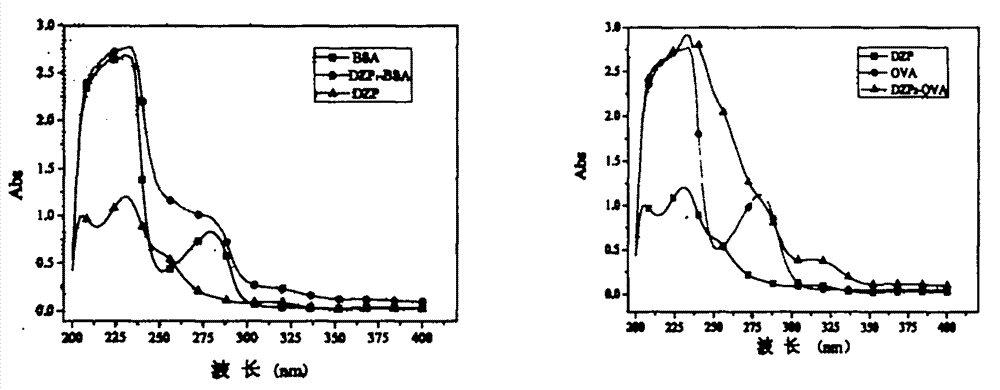
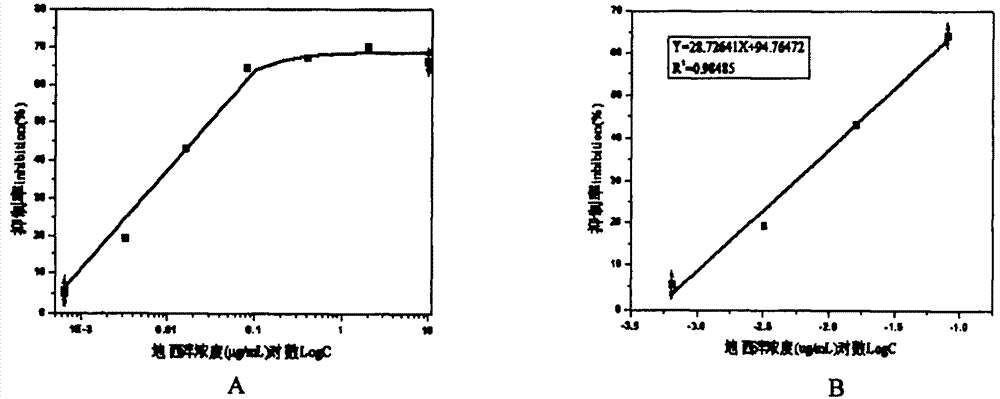
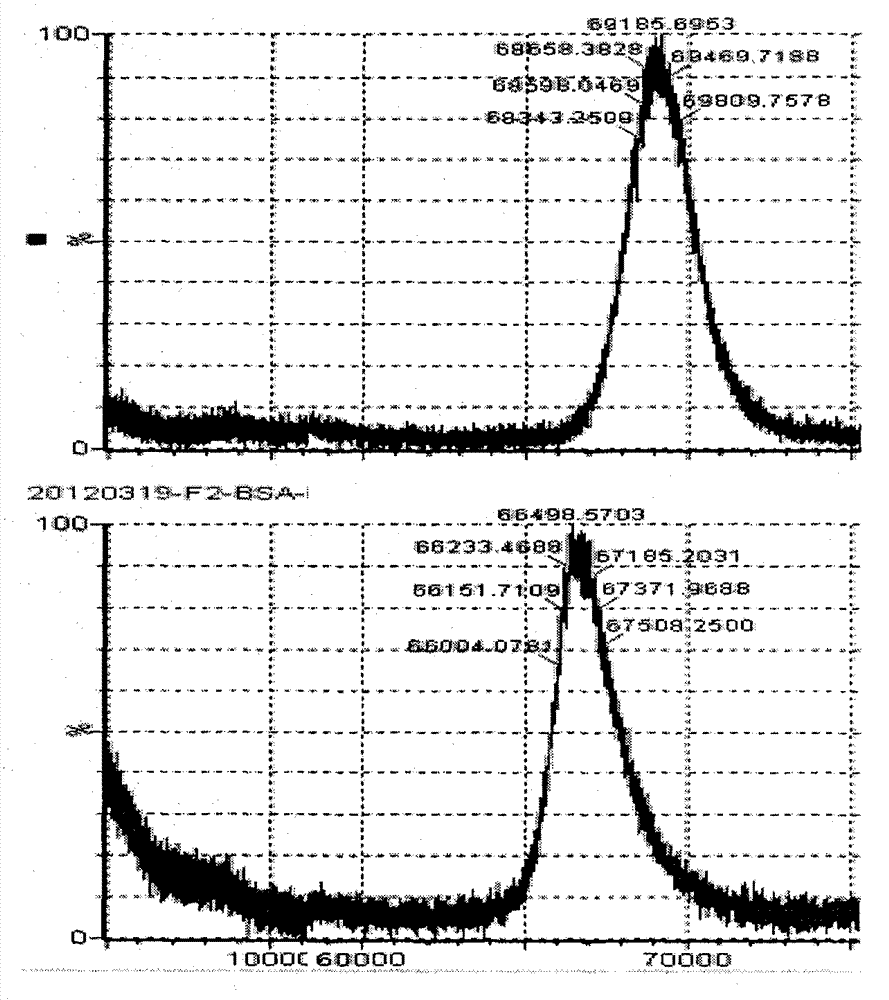
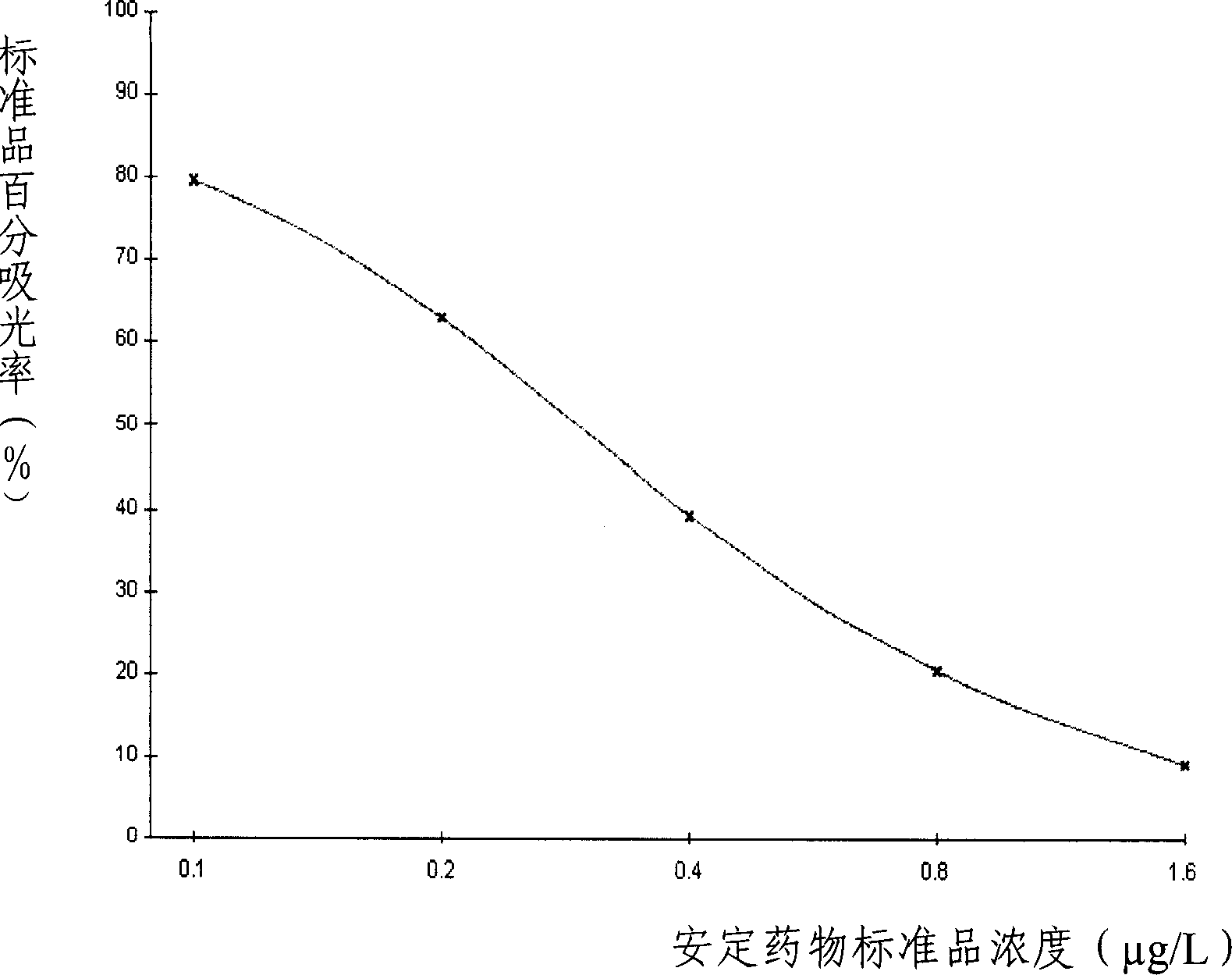
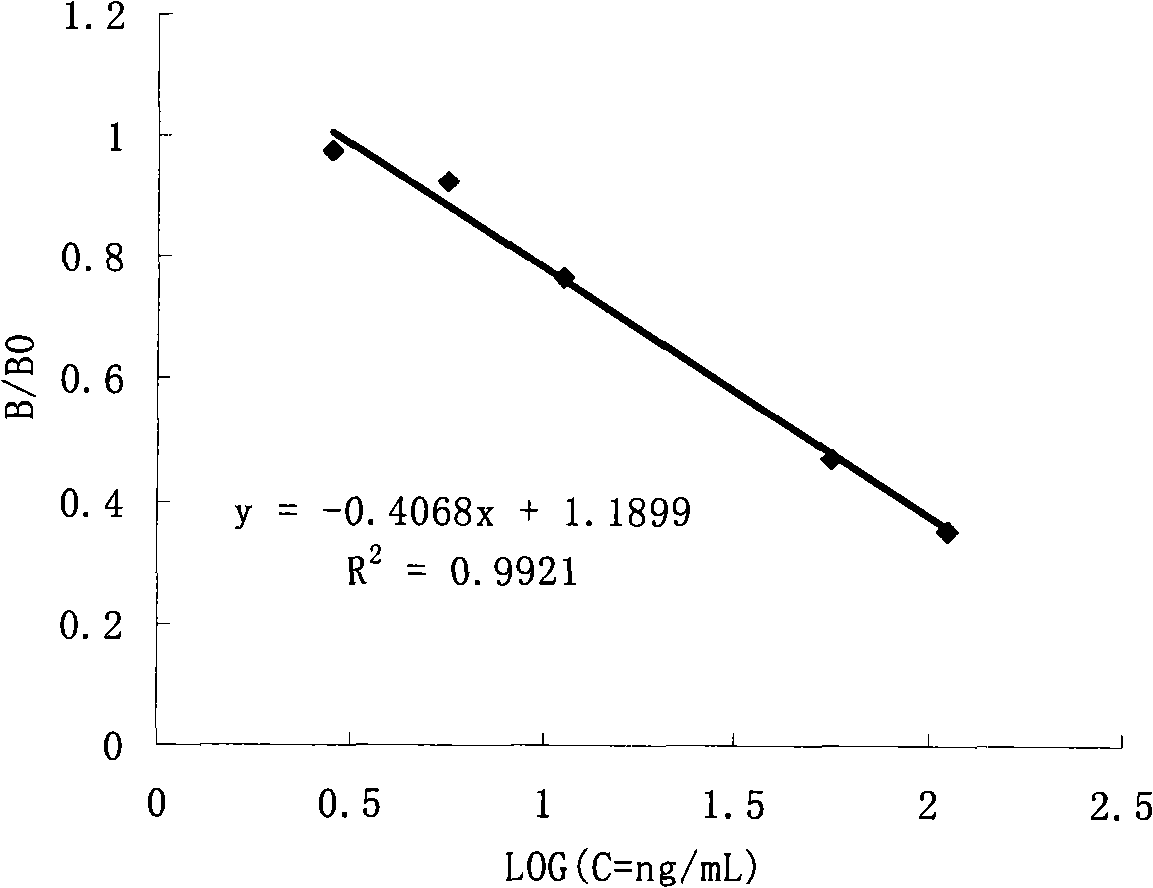
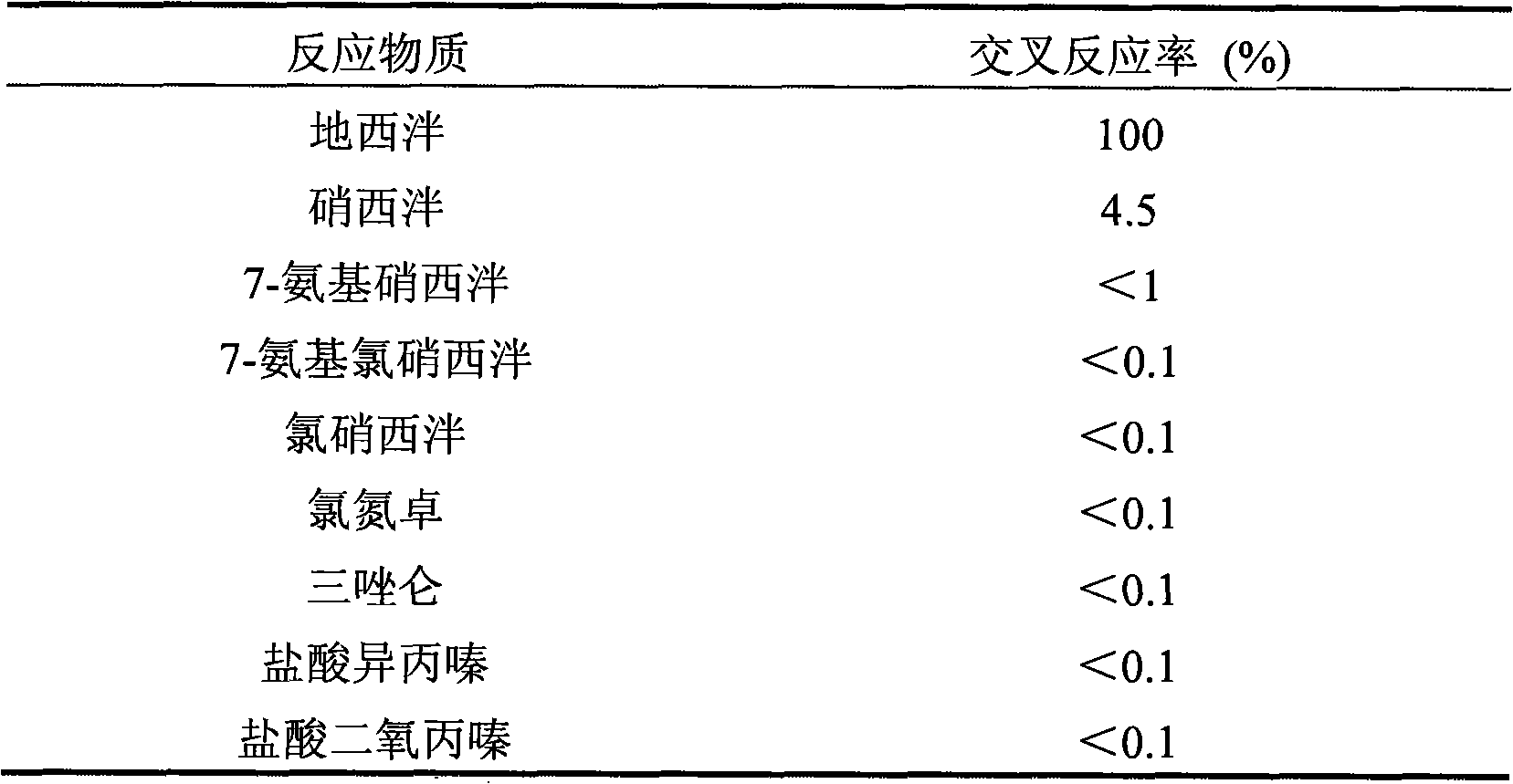
Belonging to the field of biotechnologies, the invention discloses a preparation method of a diazepam monoclonal antibody, and especially discloses application of the diazepam monoclonal antibody in the rapid detection method of ELISA. The method includes: coupling carboxylation reformed diazepam with carrier protein by an EDC technique, immunizing 5-6-week-old Balb / c mice by intraperitoneal injection, taking spleen from the mouse with positive binding activity and competitive activity to conduct cell fusion and carrying out screening to prepare the monoclonal antibody, and optimizing reaction conditions, determining the concentration of optimal coating antigen DZP-OVA at 1.25 microgram / mL and the optimal antibody dilution factor at 1:16000, thus establishing the ELISA method for the rapid detection of diazepam. The lowest detection limit IC10 of the method is 0.0011 microgram / mL. The invention provides the basic reagent antibody for immunoassay of diazepam and the detection method.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Online SPE LC-MS/MS (solid phase extraction liquid chromatography/mass spectrometry) analysis method for diazepam and metabolites thereof in human saliva
InactiveCN107271599AMeet the requirements of the analytical methodGood linear relationshipComponent separationChromatographic separationSaliva sample
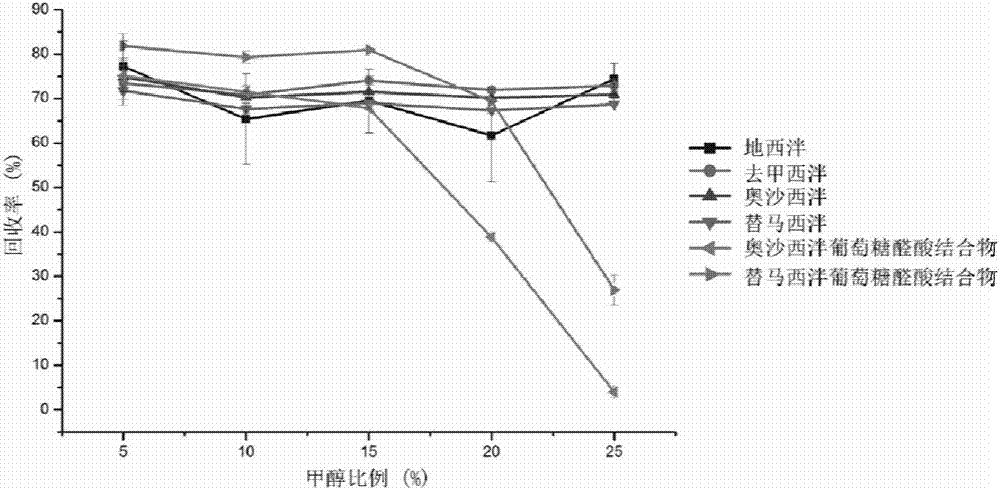
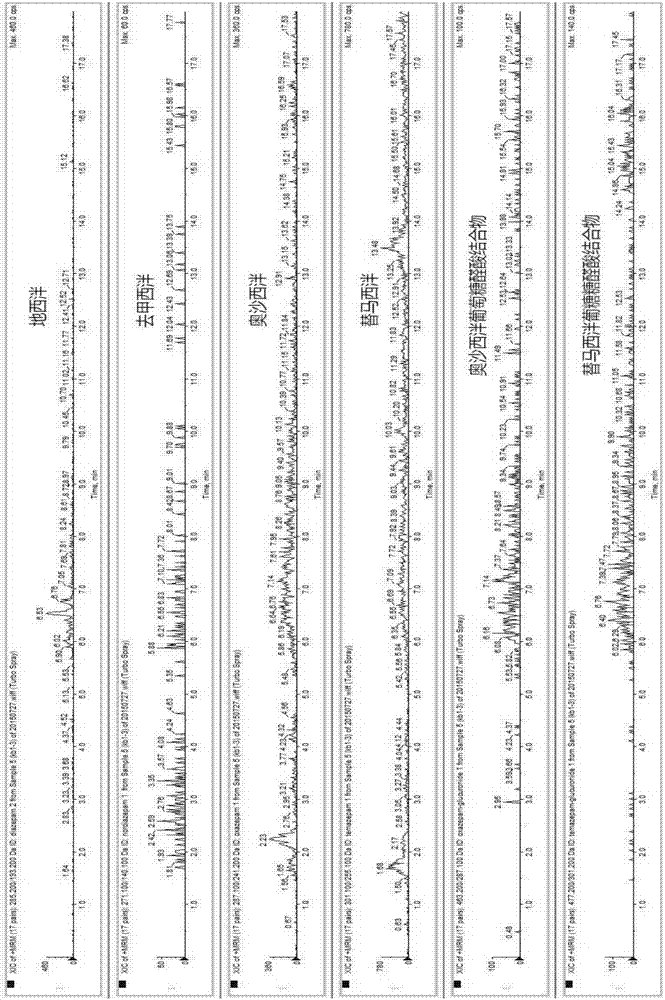
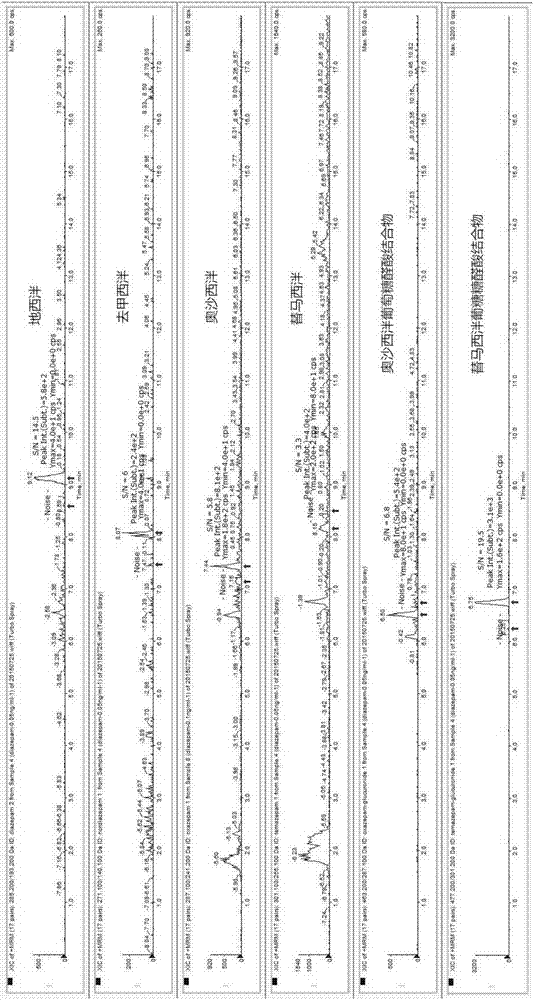
The invention provides an online PE LC-MS / MS (solid phase extraction liquid chromatography / mass spectrometry) analysis method for diazepam and metabolites thereof in human saliva. The analysis method comprises the steps as follows: a saliva sample is subjected to online solid phase extraction, and a HySphere Resin GP (10 mm x 2 mm) column is selected in the process of solid phase extraction; a C18 chromatographic column is used for chromatographic separation, and mobile-phase water (containing 2-mmol / L ammonium formate and 0.1 % formic acid) and mobile-phase acetonitrile (containing 2-mmol / L ammonium formate and 0.1 % formic acid) are used for gradient elution; ESI (electrospray ionization) and MRM (multi-reaction monitoring) are used for mass spectrum analysis for detecting target compounds. The simple, sensitive, stable and full-automatic online SPE LC-MS / MS analysis method is established for the first time to be used for quantitating diazepam and five metabolites (nordazepam, oxazepam, temazepam, oxazepam glucuronic acid conjugates and temazepam glucuronic acid conjugates) of diazepam in the human saliva.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST
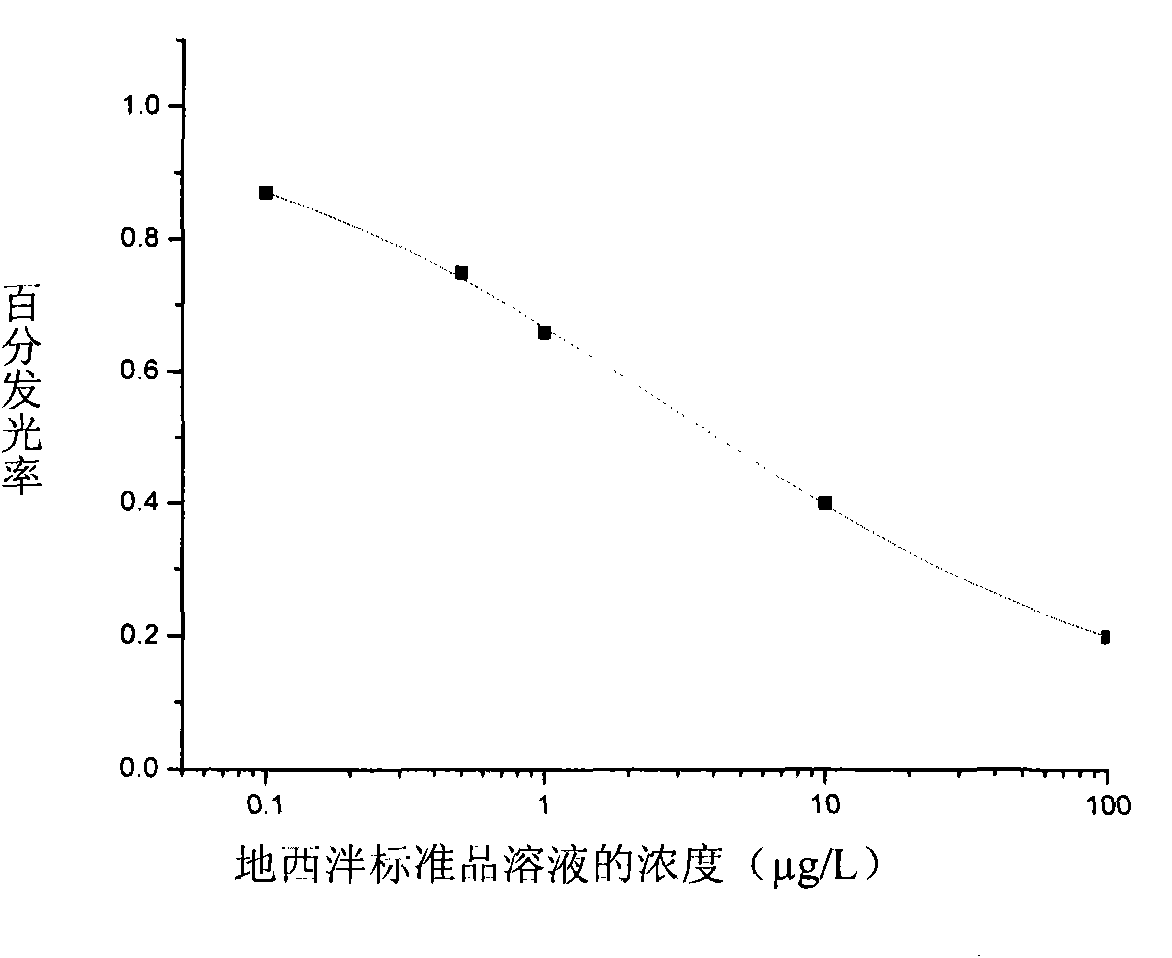
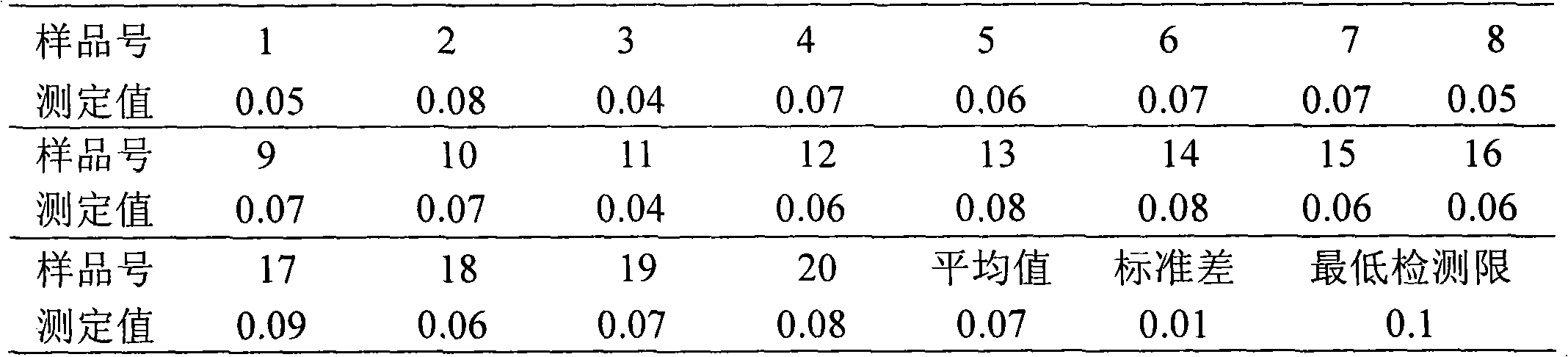
Method for detecting diazepam and chemoluminescence immunoassay kit special for same
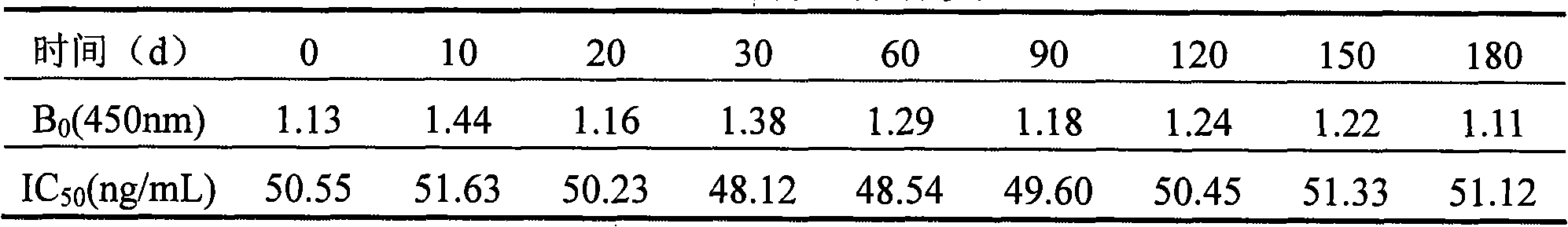
ActiveCN101936986AConducive to preservationImprove stabilityMicroorganism based processesTissue cultureDiazepamSimple sample
The invention discloses a method for detecting diazepam and a chemoluminescence immunoassay kit special for the same. The chemoluminescence immunoassay kit special for detecting the diazepam provided by the invention comprises a diazepam specific antibody, a coating antigen and standard substance solution, wherein the coating antigen is a conjugate of a diazepam semi-antigen and a carrier protein. The detection method of the invention has the characteristics of simple sample pretreatment process, simple and convenient operation, low cost, high specificity, high sensitivity, high accuracy and the like, can be monitored on site and is suitable for screening a large number of samples.
Owner:CHINA AGRI UNIV
Medication and Treatment for Disease
ActiveUS20110217278A1Shorten the progressPromote formationOrganic active ingredientsNervous disorderDiseaseVitamin B12
A treatment is described for diseases with symptoms that can include fatigue, muscle aches and spasms, weakness, demylenation, and nerve pain. Diseases can include fibromyalgia, depression, and auto-immune and immuno-suppressive diseases, such as MS. The treatment comprises about 1-10 mg naltrexone, at least about 20 μg vitamin B12, at least about 5 mg vitamin B6, at least about 2 mg coenzyme Q, and preferably at least one ancillary medication selected from the group consisting of diazepam, cyclcobenzaprine, clonazepam, alprazolam, 9-tetrahydrocannibinol, fumarate, caffeine, and combinations thereof. The treatment can be administered orally, and can decrease mental and physical symptoms such as, for example, fatigue, gait problems, visual dysfunction, and pain while improving cognitive skills.
Owner:ALTMAN ENTERPRISES
Supersaturated benzodiazepine solutions and their delivery
InactiveUS20070021411A1Fast absorptionAvoid excessive injuryBiocidePharmaceutical delivery mechanismBenzodiazepineDisease
The invention describes supersaturated solutions of benzodiazepines, such as diazepam, glycofurol and water and their use for intranasal (NS) administration to combat various disorders.
Owner:RGT UNIV OF MINNESOTA
ELISA kit for detecting diazepam residue and detection method thereof
The invention provides an enzyme immune agent box for detecting atilen residual quantity of animal foodstuff which comprises: enzyme mark plate which coats atilen antigen or antibody, enzyme mark material, atilen peculiar antibody, atilen standard solution, color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:贵州勤邦食品安全科学技术有限公司
ELISA detection reagent kit suitable for diazepam relict analysis
InactiveCN101315374ALong storage timeNo radioactive contaminationMaterial analysisBenzodiazepineRetention time
The invention relates to an enzyme-linked immune detection kit suitable for the analysis of diazepam residues and belongs to the technology field of the enzyme-linked immune adsorption analysis kit. The kit comprises an enzyme label plate coated with the envelope antigen of the diazepam, a sponge support, a diazepam standard, a diazepam polyclonal antibody, an enzyme sign second antibody, a concentrating and washing liquid, a colored solution and a reaction stopping solution; the envelope antigen of the diazepam is the coupling compound of 3-half succinate diazepam and an egg-white protein; and the enzyme sign second antibody is a horseradish peroxidase labeled goat anti-rabbit antibody. The kit adopts the diazepam polyclonal antibody, and can accurately and sensitively detect the diazepam residues or other structurally similar benzodiazepine residues in urine or tissue; the process of pretreating samples is simple; the time consumption is low; a large quantity of samples can be tested simultaneously; furthermore, the cost for sample detection is lower than that of the traditional instrument detection method; the kit has a long retention time, no radioactive pollution, and practical significance for realizing on-site monitoring of the diazepam residues of the large quantity of samples.
Owner:JIANGNAN UNIV
Medicine composition for treating insomnia
InactiveCN102847075AImprove efficacyQuick resultsOrganic active ingredientsNervous disorderSide effectPropranolol Hydrochloride
The invention relates to medicine composition for treating insomnia, which consists of following ingredients in parts by weight: 15 to 35 parts of Chinese angelica, 10 to 30 parts of roots of red-rooted salvia, 15 to 30 parts of radix curcumae, 10 to 25 parts of polygala tenuifolia, 5 to 23 parts of liquorice, 10 to 28 parts of poria with hostwood, 15 to 33 parts of fried spina date seeds, 1 to 15 parts of fructus alpiniae oxyphyllae, 5 to 23 parts of pericarpium citri reticulatae, 4 to 22 parts of platycladi seeds, 6 to 24 parts of calamus, 5 to 23 parts of dragon bone, 15 to 33 parts of rheum officinale, 1 to 15 parts of ligusticum wallichii, 2 to 18 parts of schisandra chinensis, 5 to 23 parts of radix paeoniae alba, 2 to 18 parts of Cape jasmine, 10 to 28 parts of cortex albiziae, 0.4 to 0.7 part of propranolol hydrochloride, 0.4 to 0.7 part of oryzanol and 0.2 to 0.3 part of diazepam tablets. The medicine composition has a function for treating nervous insomnia caused by various reasons, refractory insomnia and insomnia with unknown reasons; and moreover, the medicine composition is remarkable in curative effect, short in treatment process, fast in effectiveness and free from side effect.
Owner:郭秀翠
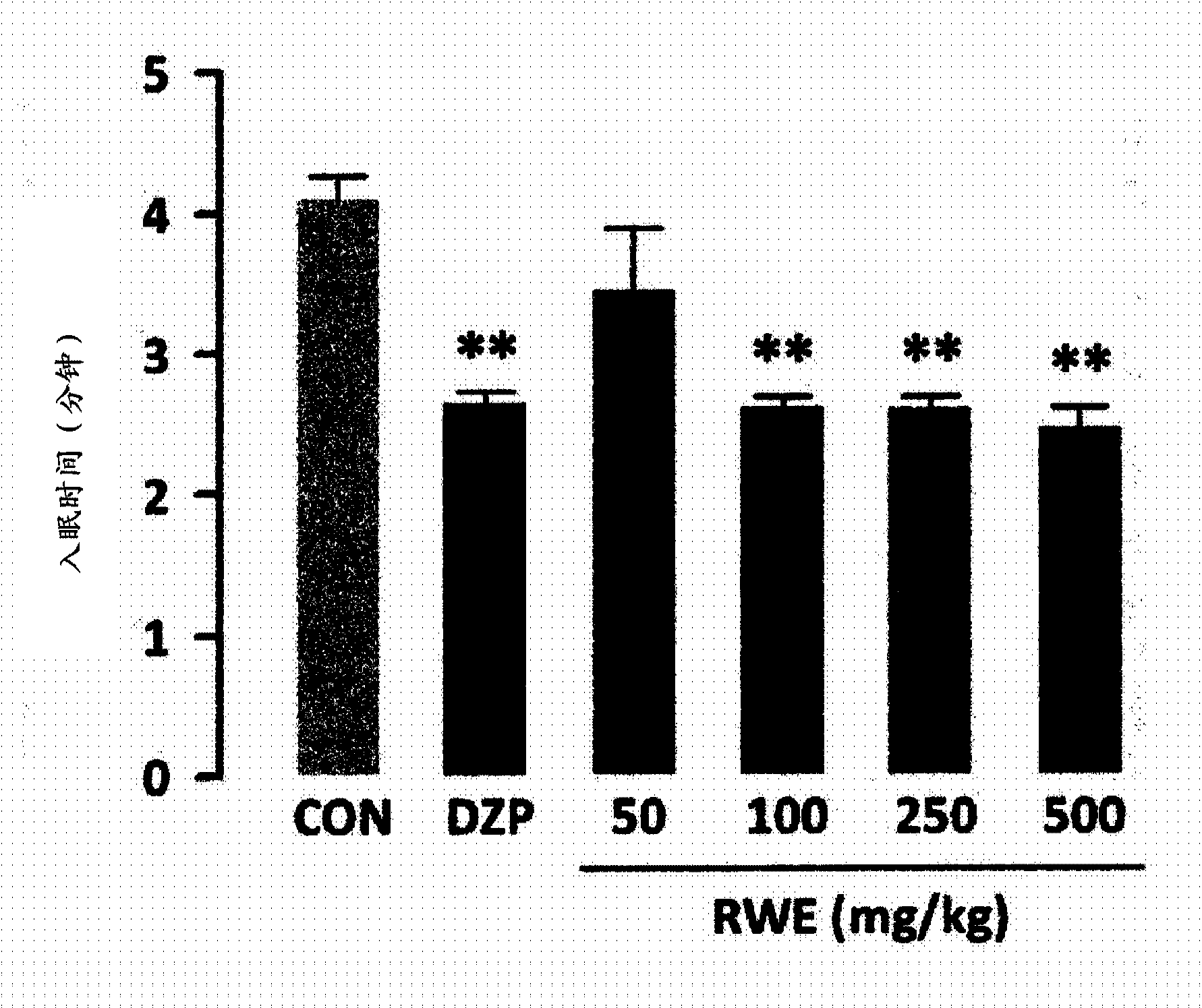
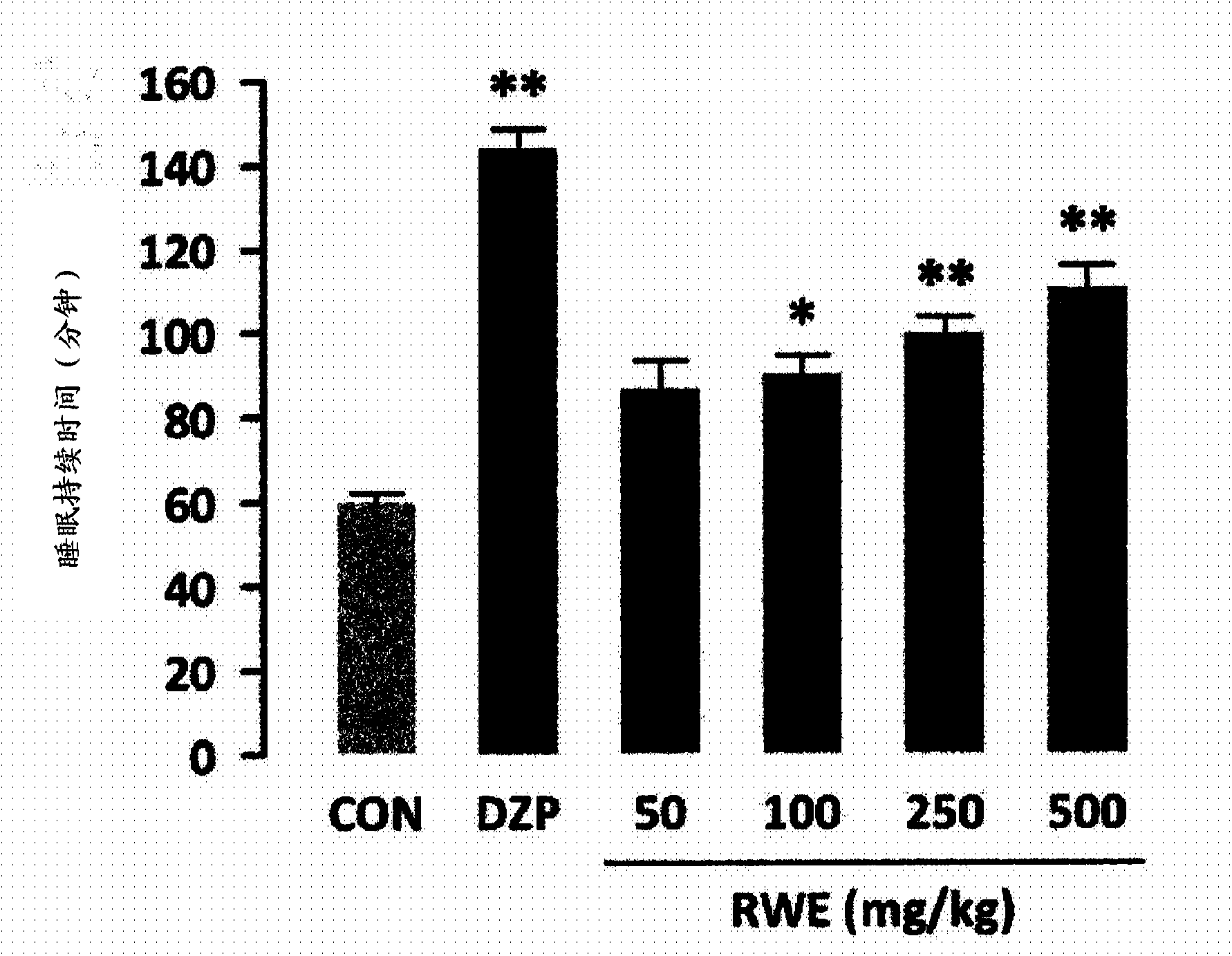
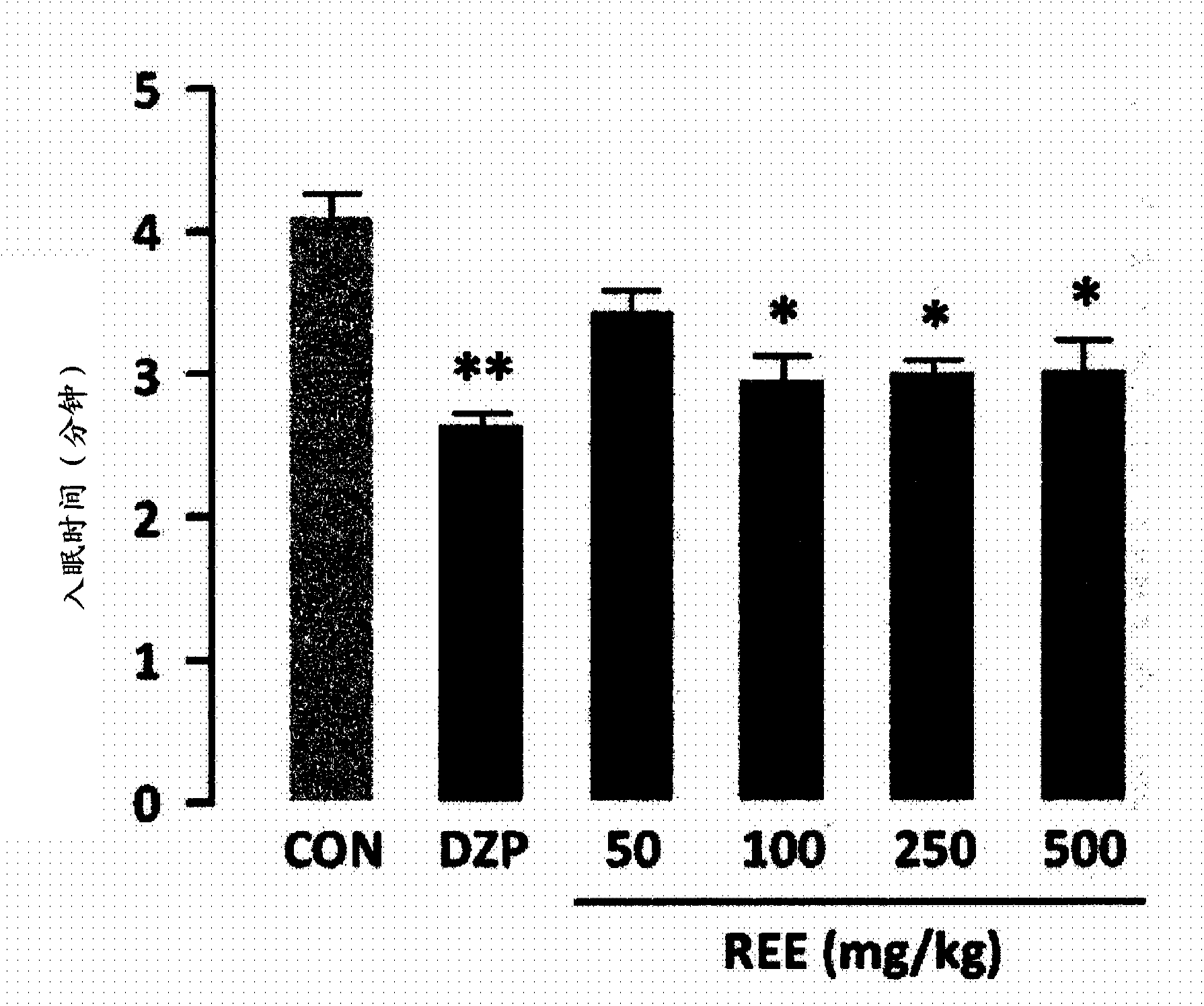
Novel usage of rice, rice bran, or chaff extract as histamine receptor antagonist
InactiveCN103561755APrevent or improve sleep disordersNervous disorderDigestive systemNausea sicknessSide effect
Disclosed is a novel use of rice, rice bran or rice hull extract as a histamine receptor antagonist. The rice, rice bran or rice hull extract may be used as a natural antihistamine to prevent or treat allergic rhinitis, inflammatory bowel disease, asthma, bronchitis, nausea, gastric and duodenal ulcer, gastroesophageal reflux disease, sleep disorder, anxiety and depression. It provides comparable or better effect of decreasing sleep latency, increasing sleep duration and increasing non-REM sleep as compared to diazepam, which is currently used as sleeping drug. Derived from the natural product rice, rice bran or rice hull, it has no side effect such as cognitive impairment, resistance or dependency even after long-term use.
Owner:KOREA FOOD RES INST
Transnasal anticonvulsive pharmaceutical composition
InactiveUS7745430B2Improve permeabilityImprove solubilityBiocideNervous disorderSolubilityConvulsion
Owner:BIOPHARM
Method and kit for detecting 19 drugs and metabolites thereof in blood by liquid chromatography-tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 drugs and metabolites thereof in blood through liquid chromatography-tandem mass spectrometry. The substances to be detected comprise sulpiride, pentafluridol, mianserin, buspirone, tandospirone, hydroxyazine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, reboxetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, toluenesulfobutyl urea, glimepiride, 1-pyrimidinepiperazine, desmethylvenlafaxine, 6-hydroxy buspirone and normipramine, and the substances to be detected are selected from the group consisting of sulpiride, pentafluridol, mianserin, venlafaxine, metandospirone, metandospirone, hydroxazine, diazepam, venlafaxine, moclobemide, the pharmaceutical composition is prepared from noramitriptyline, nordiazepam and clopidogrel metabolite; the detection method comprises the following steps: calibrating a standard solution, treating a to-be-detected sample, and detecting the to-be-detected sample by adopting high performance liquid chromatography-mass spectrometry. The embodiment of the invention can quickly and accurately measure the content, and the sample treatment method is simple and easy to implement, high in sensitivity and accurate in quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Detection method of diazepam in fresh water
InactiveCN109765306AAccurate judgmentThe advantage of enrichment efficiency is obviousComponent separationDiazepamFresh water
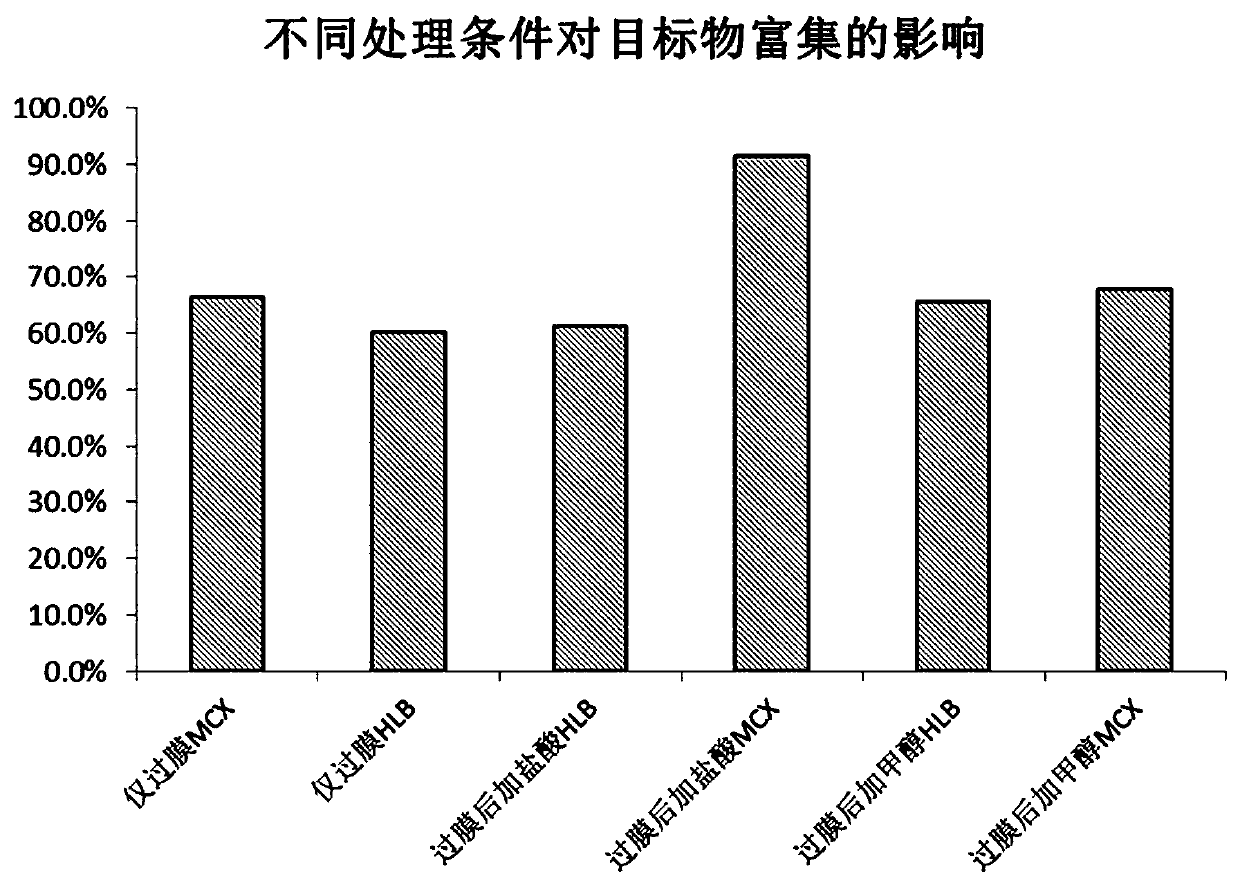
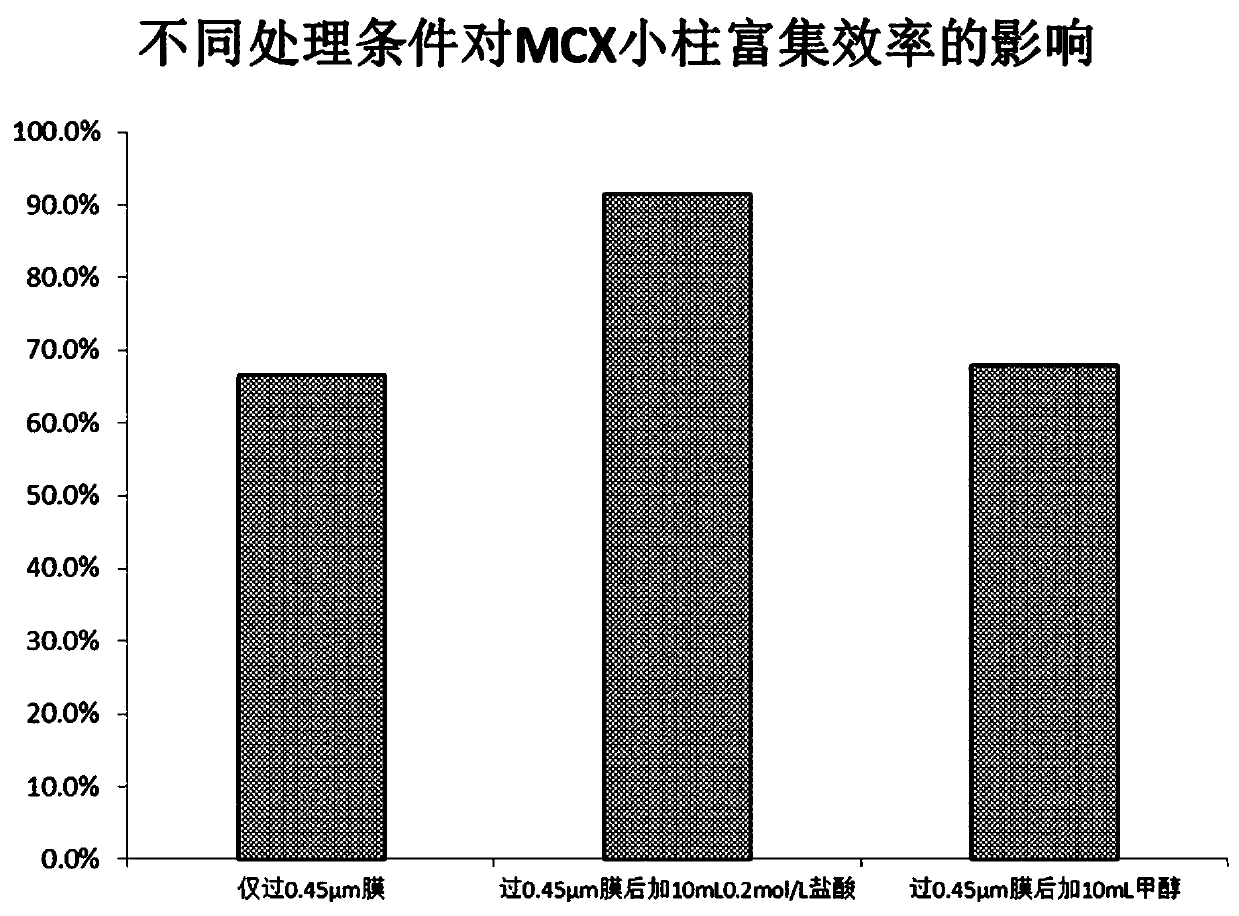
The invention relates to a detection method of diazepam in fresh water and belongs to the technical field of water environment pollutant detection. After a detecting water sample is filtered by a filter membrane, hydrochloric acid is added, the detecting water sample is treated through an MCX small column, the enrichment efficiency of the diazepam has obvious advantages, the enrichment efficiencyand the purification effect are better, the lower detection limit and better stability can be achieved, the detection limit of the diazepam can reach 0.5 ng / L, thus the content of the diazepam in a water body can be detected rapidly and effectively, the pollution situation of the water body environment is judged accurately, and risk early-warning is conveniently established in advance.
Owner:山东省海洋资源与环境研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com