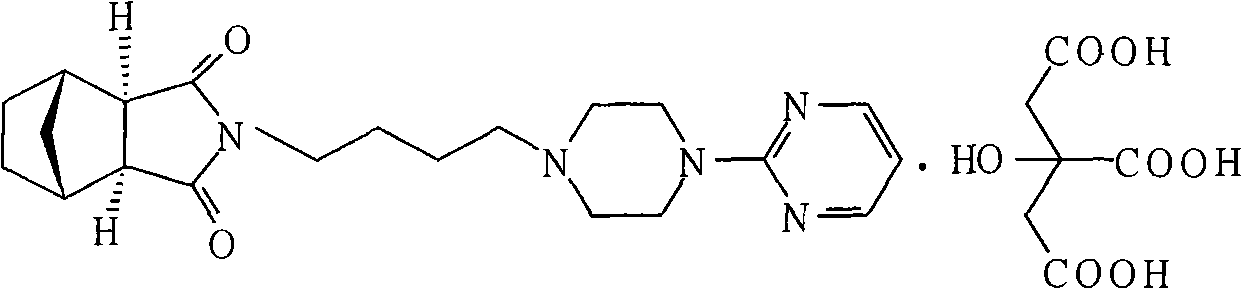
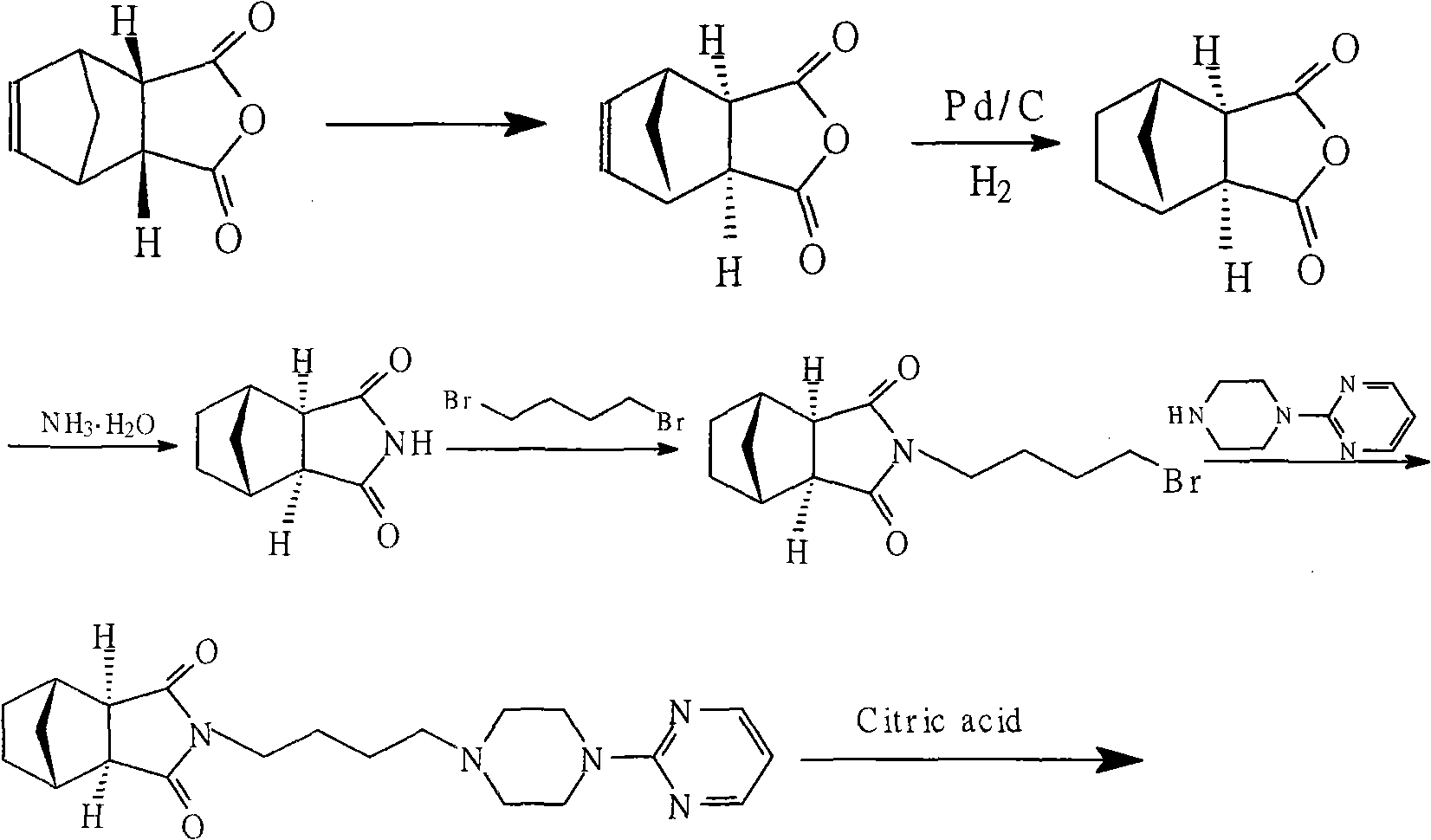
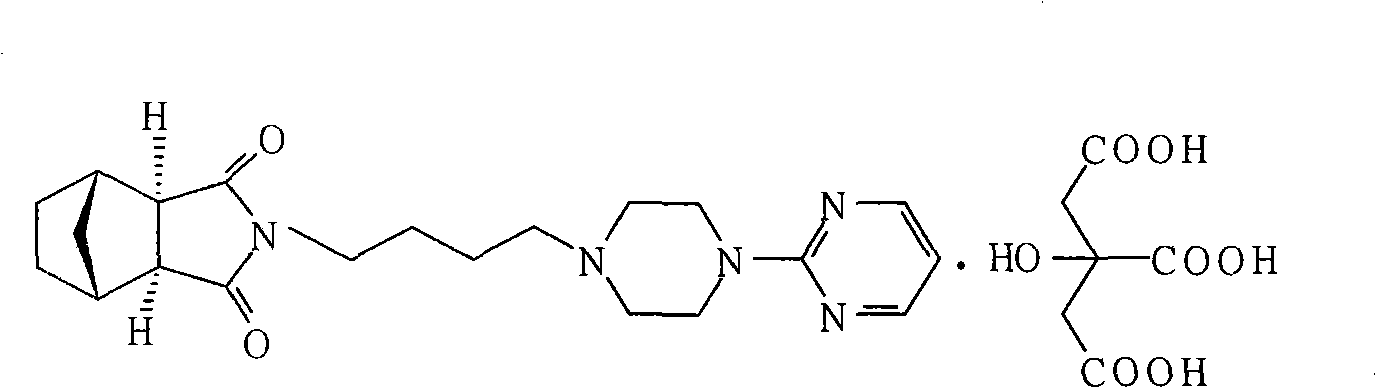
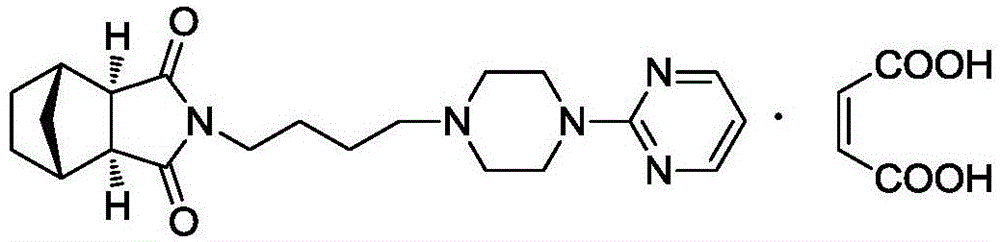
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Tandospirone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
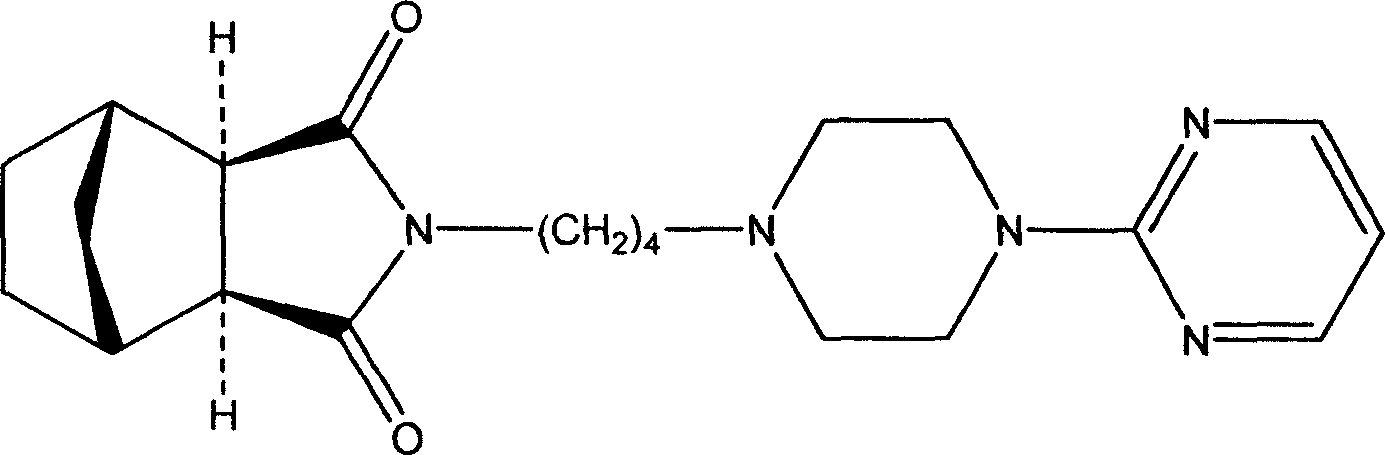
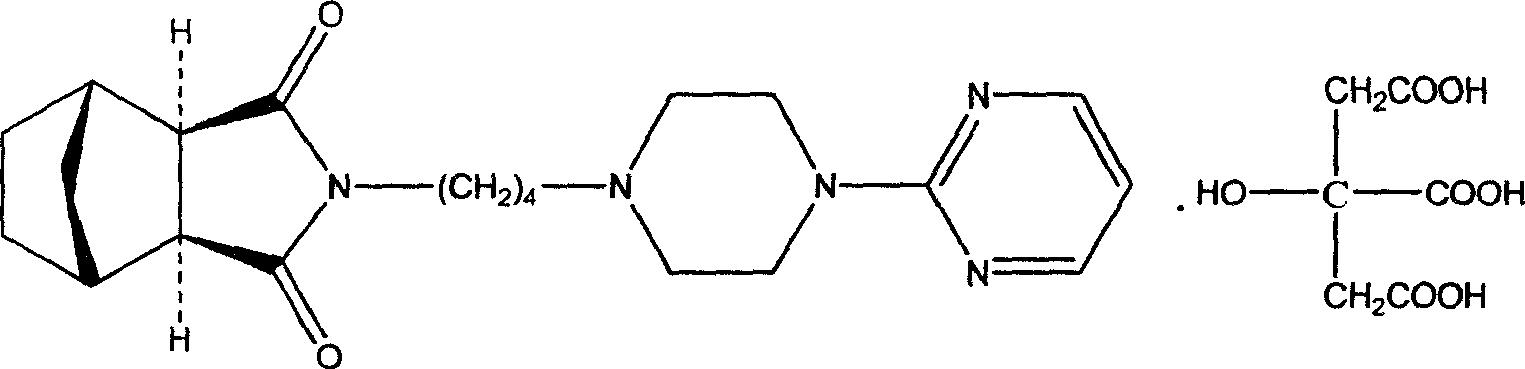
Tandospirone (brand name Sediel) is an anxiolytic and antidepressant drug used in China and Japan, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.
Method for preparing tandospirone and analogues of tandospirone
InactiveCN101880274AQuality improvementMild reaction conditionsOrganic chemistrySolventProtein translocation
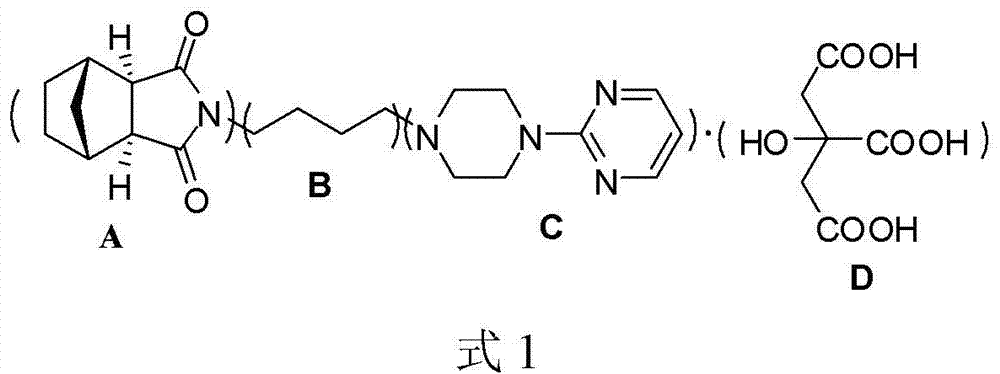
The invention discloses tandospirone and analogues of the tandospirone. In the method, NA anhydride generates translocation under an illumination condition, the product of the translocation and 1-(4-amniobutyl)-4-(2-pyrimidyl) piperazine are condensed, the product of the condensation is reduced to form tandospirone in the presence of Pd / C, and the tandospirone is reacted with an acid to form a salt of the tandospirone. The method has the advantages that: the reaction conditions are mild; special requirements on equipment are avoided; the yield is high; the used solvent can be recycled; the tandospirone and the analogues of tandospirone are of high quality; the single maximum impurity is less than 0.1 percent; and the prepared preparation has good medicament effect; the operation is simple; the purity is high; the cost is low; and large-scale product can be realized.
Owner:PKU HEALTHCARE CORP LTD +2
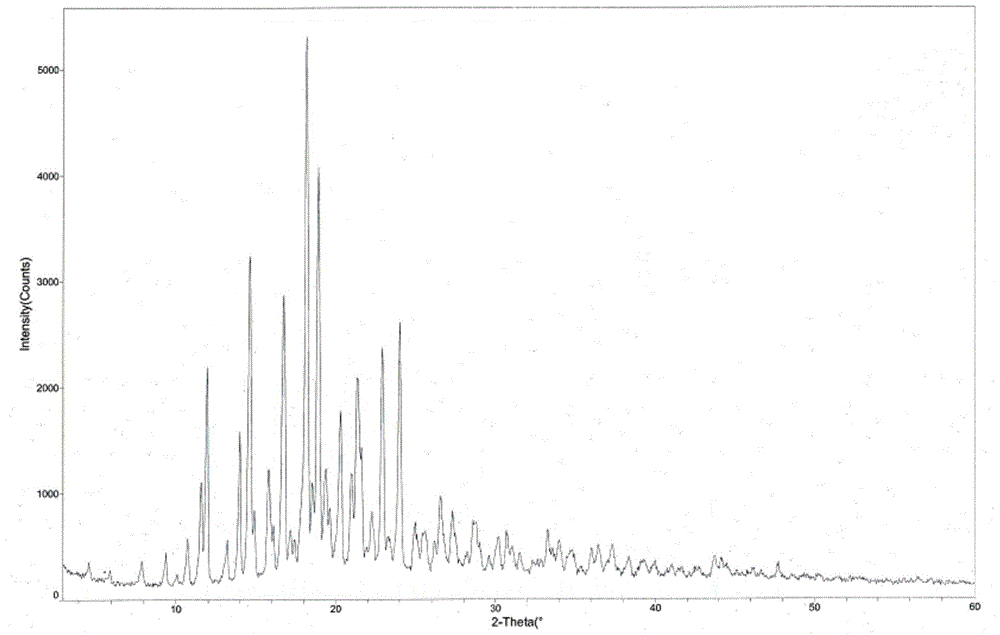
Tandospirone hydrochloride crystal form II and preparation method thereof
ActiveCN103664905AGood water solubilityImprove stabilityOrganic active ingredientsSenses disorderSolubilityWater soluble
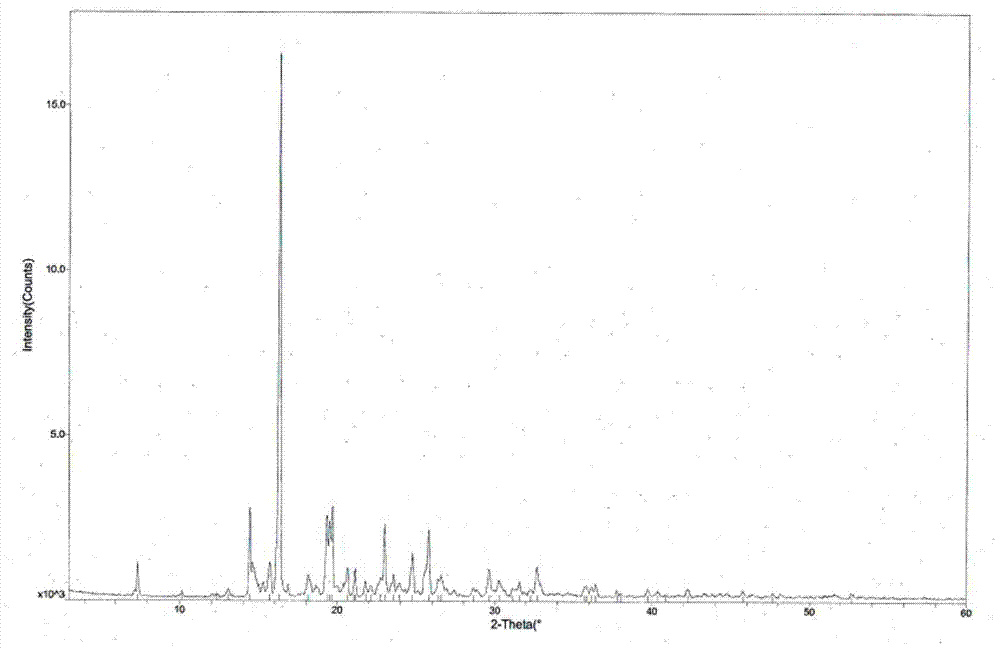
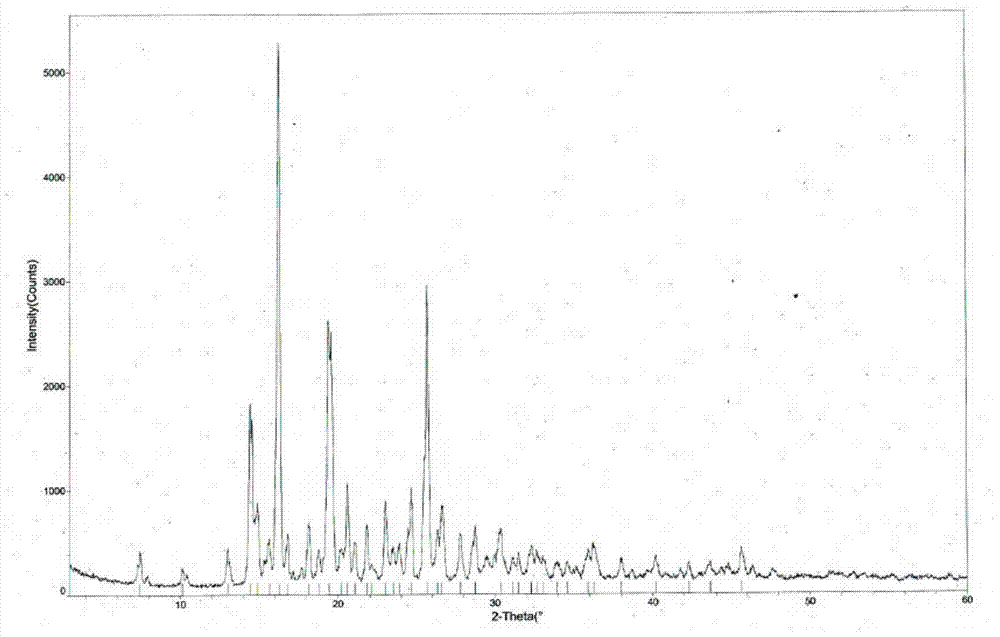
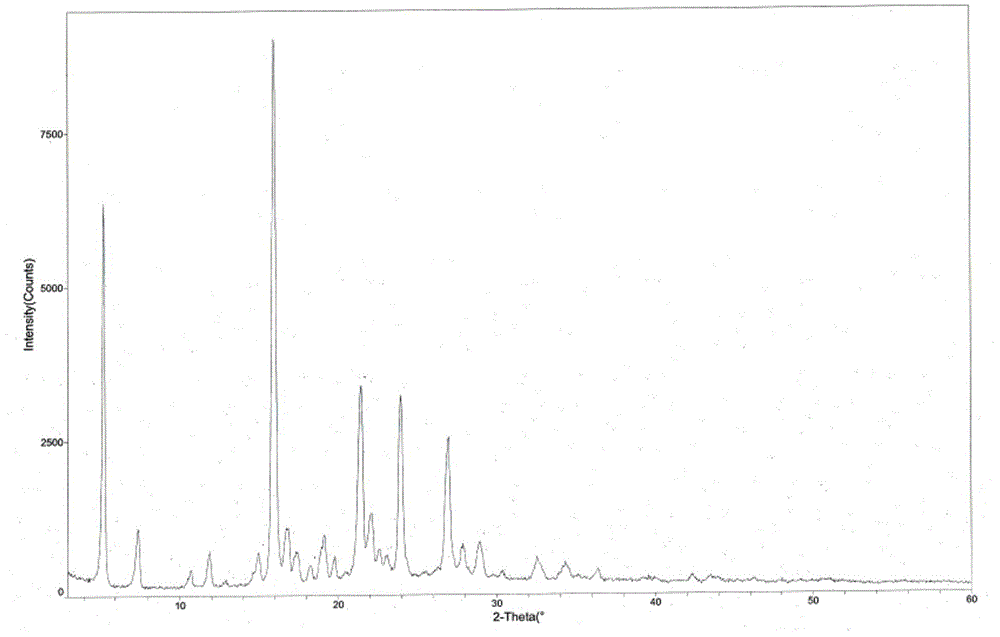
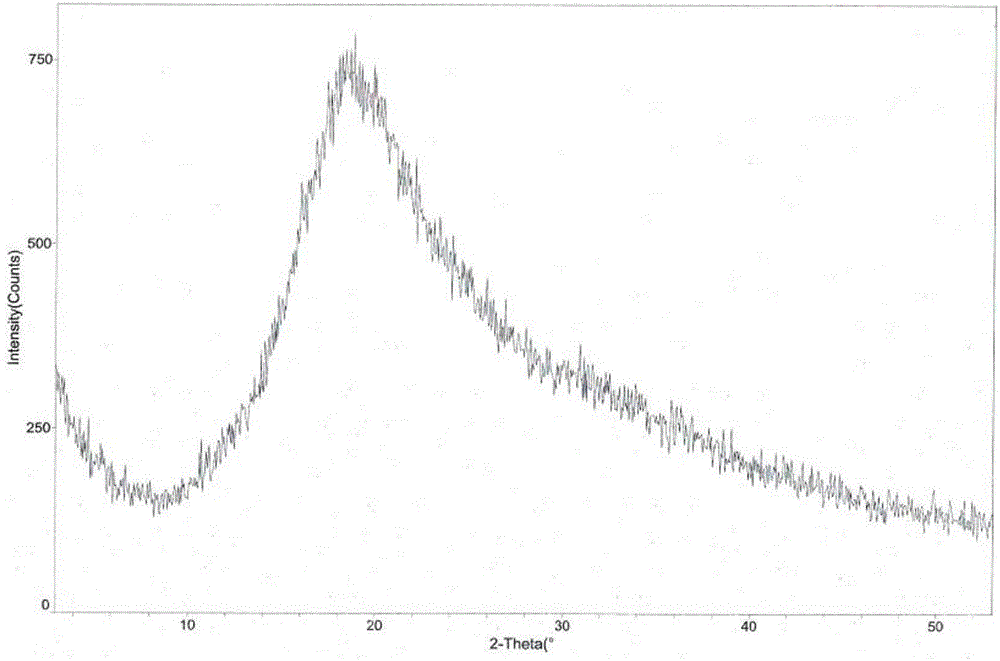
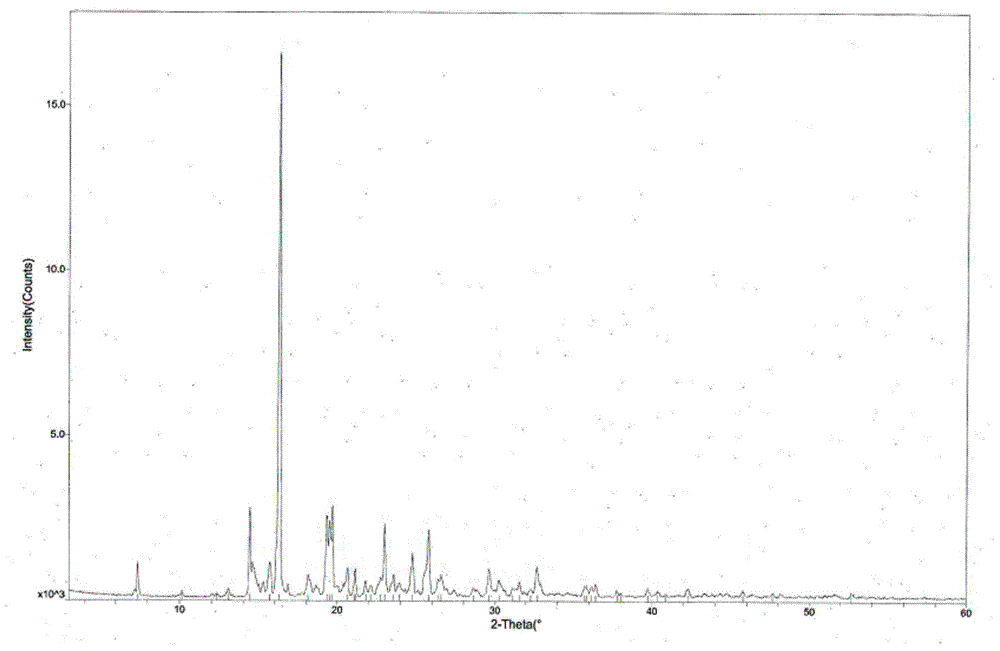
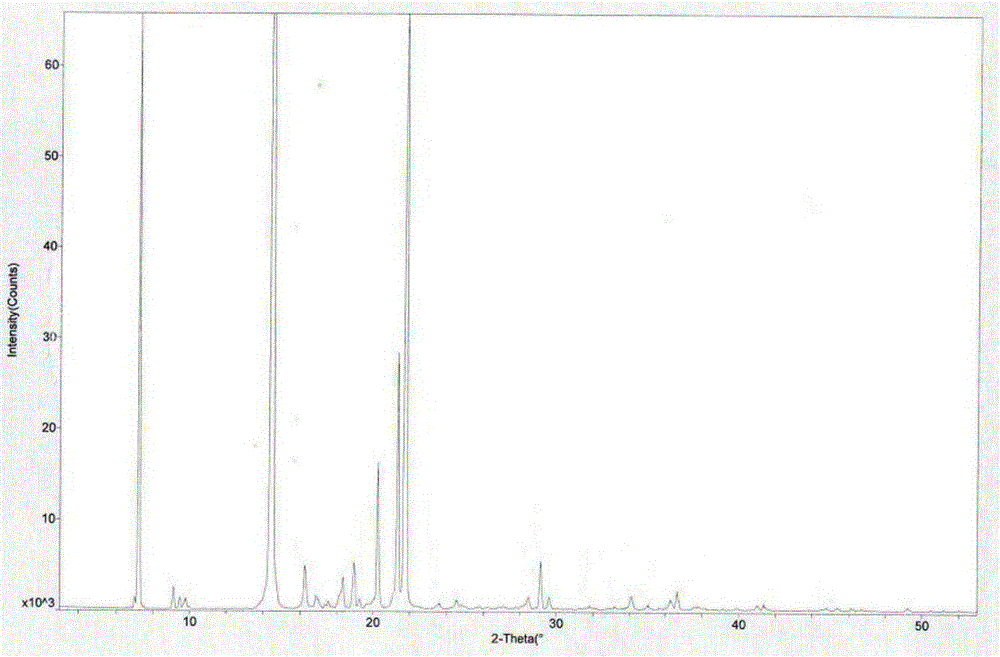
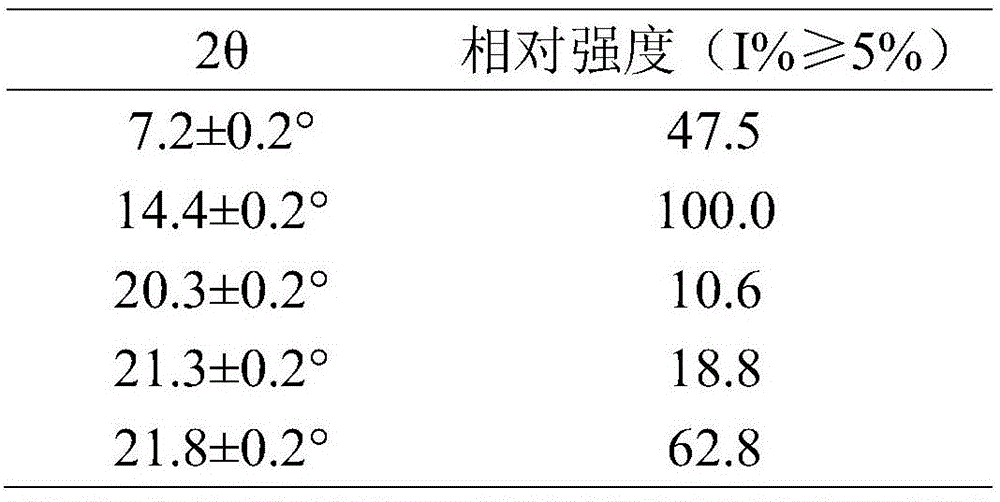
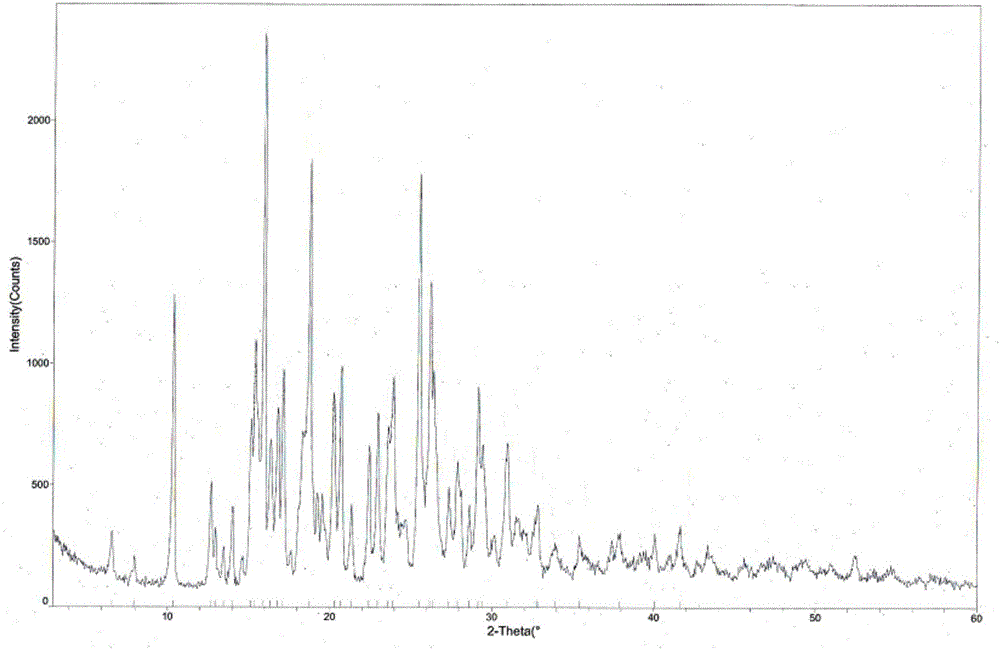
The invention provides a tandospirone hydrochloride crystal form II. The characteristic absorption peaks exist at the diffraction angle 2*theta within 2.127-2.527 degrees, 16.078-16.478 degrees, 19.157-19.557 degrees, 19.321-19.721 degrees, 19.499-19.899 degrees, 22.561-22.961 degrees, 29.437-29.837 degrees and 35.530-35.930 degrees in the X-ray powder diffraction diagram of the crystal form II. The invention further provides a preparation method of the crystal form II. Compared with the conventional tandospirone hydrochloride product, the tandospirone hydrochloride crystal form II provided by the invention is more excellent in water solubility and stability, and provides the possibility of improving bioavailability and safety of drugs; in addition, the preparation technology of the crystal form II is simple, the yield of the crystal form II is high, and the preparation method is suitable for industrial production.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
New usage of tandospirone and its derivative, and composition containing tandospirone
InactiveCN1915216APromote absorptionAvoid influenceOrganic active ingredientsNervous disorderWhole bodyAnxiety
An application of the tandospirone and its derivatives in preparing the locally applied medicine for treating anxiety by sublingual mucosa absorption is disclosed.
Owner:SICHUAN CREDIT PHARMA
Adhesive Patch
InactiveUS20070232629A1Improve transdermal absorption rateBiocideOrganic active ingredientsOrganic acidMedicine
The object of the invention is to provide a patch that contains tandospirone or a pharmaceutically acceptable salt thereof as the drug component, and has a sufficiently high percutaneous absorption rate. A patch according to a preferred embodiment of the invention comprises a backing layer and a pressure-sensitive adhesive layer formed on one side of the backing layer, wherein the pressure-sensitive adhesive layer contains a drug component composed of tandospirone and / or a pharmaceutically acceptable salt thereof at 5 to 30 wt % based on the weight of the pressure-sensitive adhesive layer, an acrylic-based polymer and / or styrene-based block copolymer, and an organic acid and / or a salt thereof.
Owner:HISAMITSU PHARM CO INC
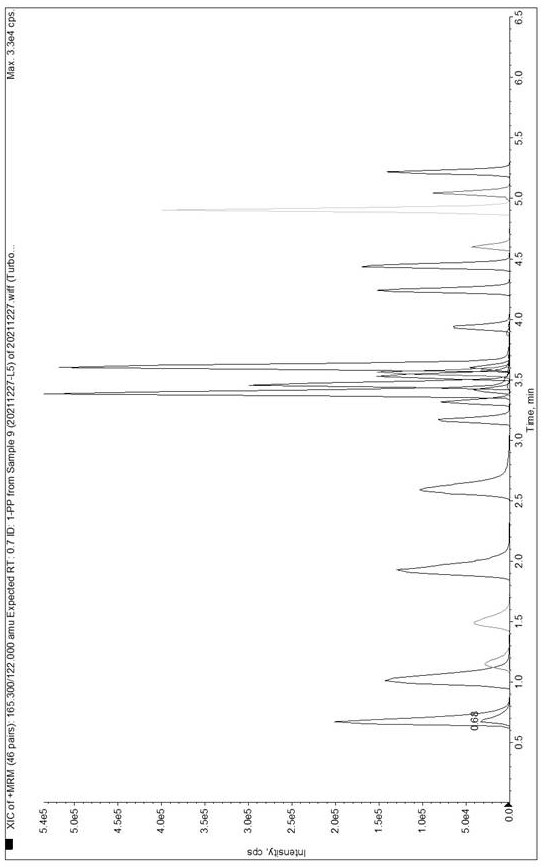
Method and kit for detecting 19 drugs and metabolites thereof in blood by liquid chromatography-tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 drugs and metabolites thereof in blood through liquid chromatography-tandem mass spectrometry. The substances to be detected comprise sulpiride, pentafluridol, mianserin, buspirone, tandospirone, hydroxyazine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, reboxetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, toluenesulfobutyl urea, glimepiride, 1-pyrimidinepiperazine, desmethylvenlafaxine, 6-hydroxy buspirone and normipramine, and the substances to be detected are selected from the group consisting of sulpiride, pentafluridol, mianserin, venlafaxine, metandospirone, metandospirone, hydroxazine, diazepam, venlafaxine, moclobemide, the pharmaceutical composition is prepared from noramitriptyline, nordiazepam and clopidogrel metabolite; the detection method comprises the following steps: calibrating a standard solution, treating a to-be-detected sample, and detecting the to-be-detected sample by adopting high performance liquid chromatography-mass spectrometry. The embodiment of the invention can quickly and accurately measure the content, and the sample treatment method is simple and easy to implement, high in sensitivity and accurate in quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Compositions of Topical Ocular Solutions to Deliver Effective Concentrations of Active Agent to the Posterior Segment of the Eye
The present invention relates to development of efficacious pharmaceutical compositions comprising an active agent, such as the free base or hydrochloride salt of tandospirone, for topical delivery to the eye for the treatment of retinal disorders.
Owner:ALCON RES LTD
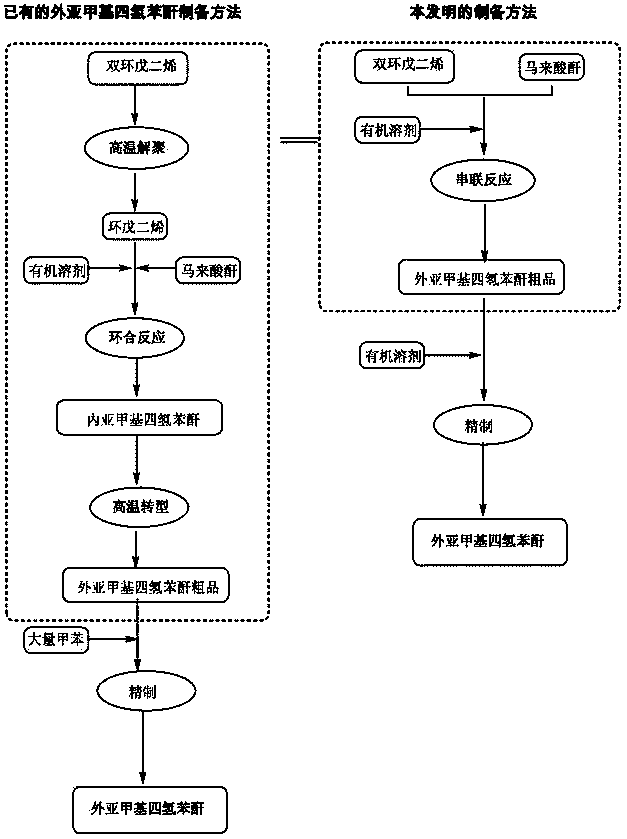
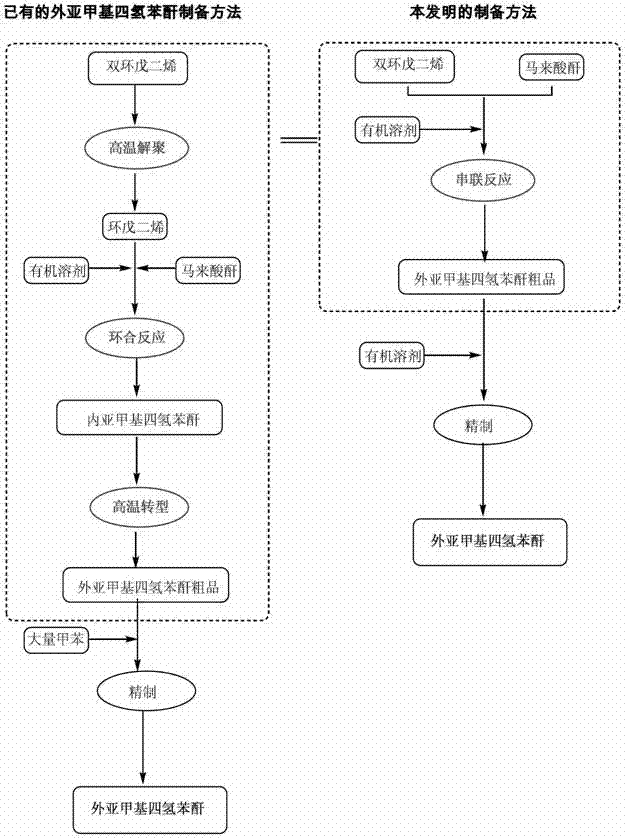
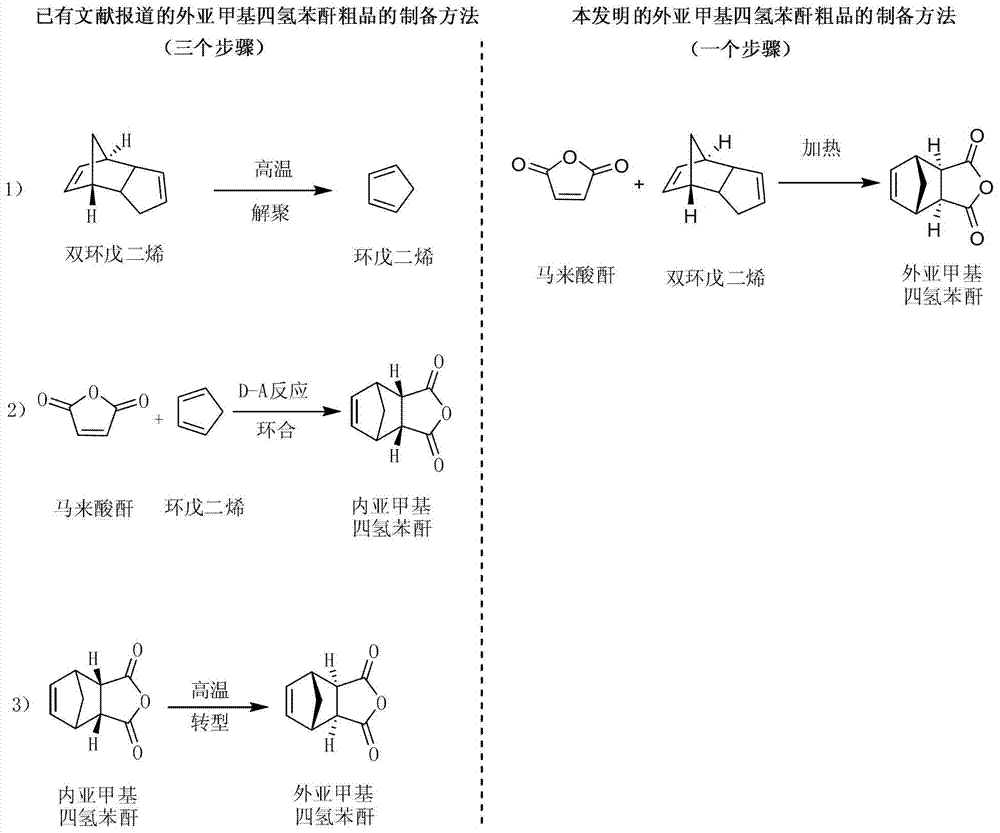
Preparation and refining method of exo-methylene tetrahydrophthalic anhydride and its use in preparation of tandospirone
ActiveCN103848801ASimple manufacturing processIncrease profitOrganic chemistryChemical recyclingDepolymerizationHigh energy
The invention relates to a preparation and refining method of exo-methylene tetrahydrophthalic anhydride and its use in preparation of tandospirone. The preparation and refining method realizes high-efficiency combination of depolymerization of dicyclopentadiene, cycloaddition of cyclopentadiene and maleic anhydride, and transformation of endo-methylene tetrahydrophthalic anhydride, is free of a single dicyclopentadiene depolymerization process, is free of a reaction intermediate product separation process, effectively solves the problem that the existing exo-methylene tetrahydrophthalic anhydride preparation method has defects of complex dicyclopentadiene depolymerization processes, low conversion rate, low yield, serious environmental pollution, high energy consumption, long production cycle and harsh storage condition requirements, and has the advantages of high yield, good quality, eco-friendly solvent, low cost and good industrial production applicability.
Owner:SICHUAN CREDIT PHARMA
Tandospirone micropore osmotic pump preparation
ActiveCN104706614AProlong the action timeReduce the number of daily dosesOrganic active ingredientsNervous disorderDrug releaseOsmotic pump
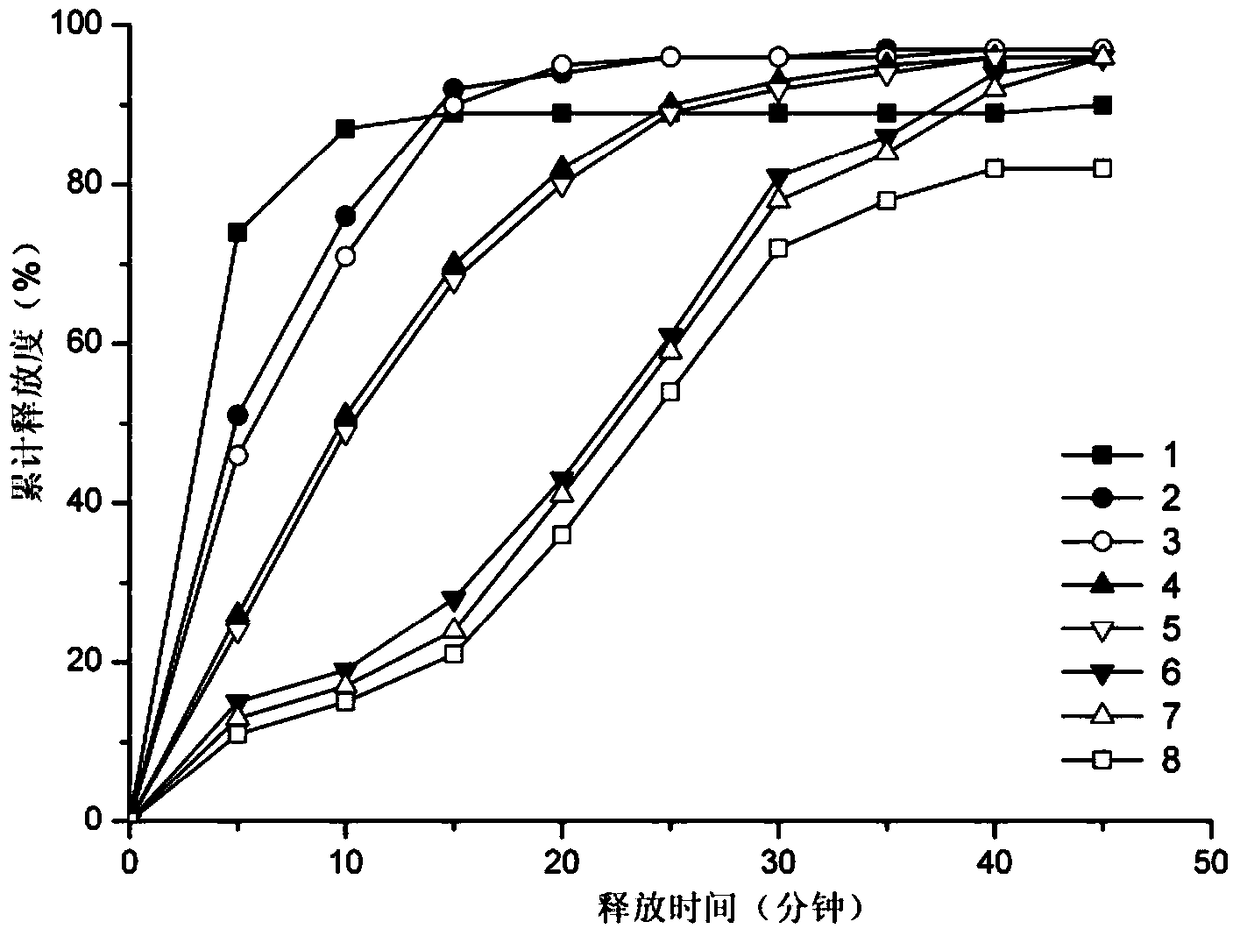
The invention provides a tandospirone micropore osmotic pump preparation. The osmotic pump preparation can reach zero-level release, reaches 24-hour drug release, can effectively prolong the time of drug action, makes patients reduce the number of times of taking medicine daily, and improves compliance.
Owner:SICHUAN CREDIT PHARMA
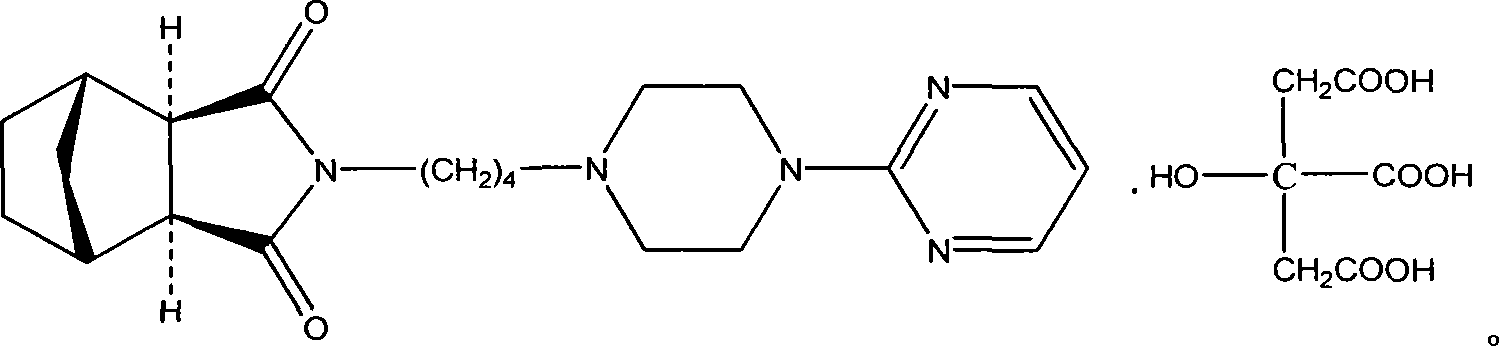
L-tandospirone tartrate compound
InactiveCN105218528AStable in natureGood water solubilityOrganic active ingredientsSenses disorderSolubilityWater soluble
The invention provides an L-tandospirone tartrate compound. The invention also provides a preparation method for the compound, a pharmaceutical composition and application. The L-tandospirone tartrate compound provided by the invention exists in a crystal form, has excellent water-solubility and stability, and provides possibility to improve bioavailability and safety of medicines; in addition, the compound provided by the invention has the advantages of simple preparation process, high yield, and applicability to industrial production.
Owner:SICHUAN CREDIT PHARMA
Tandospirone enteric-coated tablets and preparation method thereof
ActiveCN106176659AQuality improvementGood disintegration and dissolutionOrganic active ingredientsNervous disorderModerate depressionDisease
The invention provides application of tandospirone in anti-anxiety drugs for intestinal administration and further provides tandospirone enteric-coated tablets and a preparation method thereof. The tandospirone enteric-coated tablets are prepared by using tandospirone or pharmaceutically-acceptable salt thereof as an active ingredient and adding pharmaceutically-acceptable excipients. The preparation method includes: mixing tandospirone or the pharmaceutically-acceptable salt and the excipients, granulating, and tabletting to obtain tablet cores; sequentially performing isolation layer coating, enteric layer coating and modification layer coating on the tablet cores, finally polishing and waxing to obtain the tandospirone enteric-coated tablets. The tandospirone enteric-coated tablets prepared by the method are stable in quality, high in disintegration and dissolution performance, little in damage to digestive tract mucous membranes, little in side effect and high in administration compliance, can be used for treating anxiety state caused by various neuroses, anxiety state accompanied by body diseases like primary hypertension and digestive ulcer and light-and-moderate depression, and is especially suitable for patients with anxiety, gastric mucosal lesion or proneness to bleeding caused by digestive system diseases and old patients needing long-time administration.
Owner:SICHUAN CREDIT PHARMA
Tandospirone oxalate compound
InactiveCN105175398AStable in natureGood water solubilityOrganic active ingredientsSenses disorderSolubilityOxalate
The invention provides a tandospirone oxalate compound. The invention also provides a preparation method of the tandospirone oxalate compound, a pharmaceutical composition containing the compound and a use of the compound. The tandospirone oxalate compound provided by the invention exists in a form of crystals, has stable properties and good solubility in water, and provides an effective solution way for improving the drug bioavailability and safety; and in addition, the preparation process of the compound is simple, high in yield, and suitable for industrialized production.
Owner:SICHUAN CREDIT PHARMA
Tandospirone dispersible tablets or coating dispersible tablets, and preparation method thereof
ActiveCN104997740AFast disintegrationHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsOlder peopleFiller Excipient
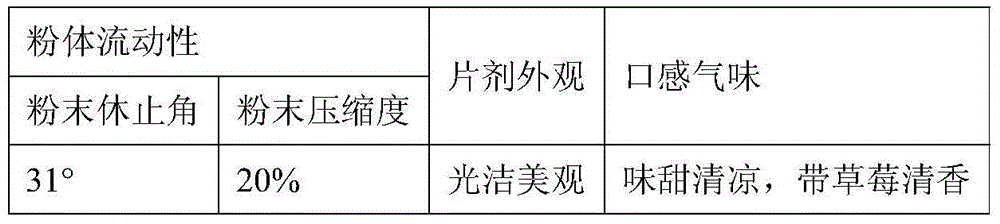
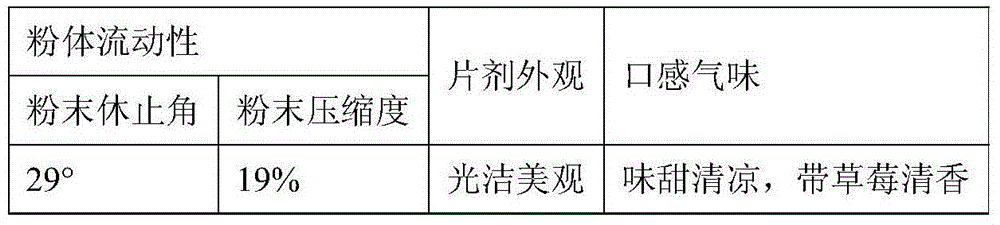
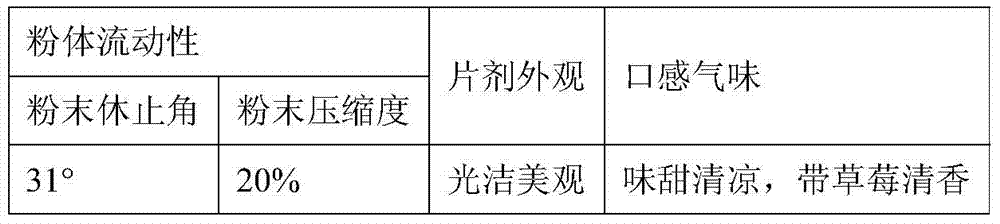
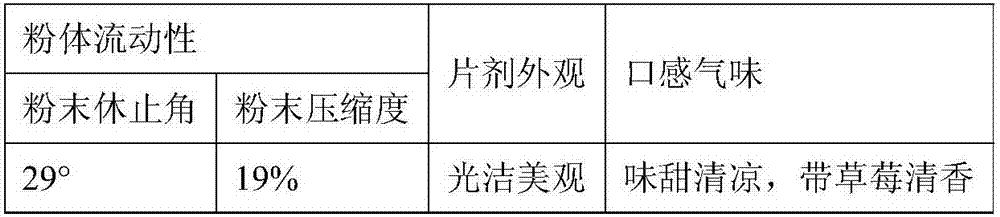
The present invention provides tandospirone dispersible tablets, which is prepared from the following raw materials and auxiliary materials: 1-8% by weight of tandospirone or a pharmaceutically acceptable salt, 65-90% by weight of a filler, and 4-30% of a disintegrant. According to the present invention, the prepared tandospirone dispersible tablets have characteristics of rapid disintegration rate and high dissolution rate compared with the conventional tablets, can be efficiently absorbed in the digestive tract so as to obtain high bioavailability, can be taken after being dispersing in water, has fragrant and sweet taste, and is more suitable for people with poor absorption of oral formulations, people with swallowing difficulty, and old people or children with low medication compliance.
Owner:SICHUAN CREDIT PHARMA
Tandospirone pharmaceutical composition as well as preparation method and application thereof
ActiveCN113018271AFloat quicklyExtended stayOrganic active ingredientsSenses disorderPharmaceutical drugPharmaceutical medicine
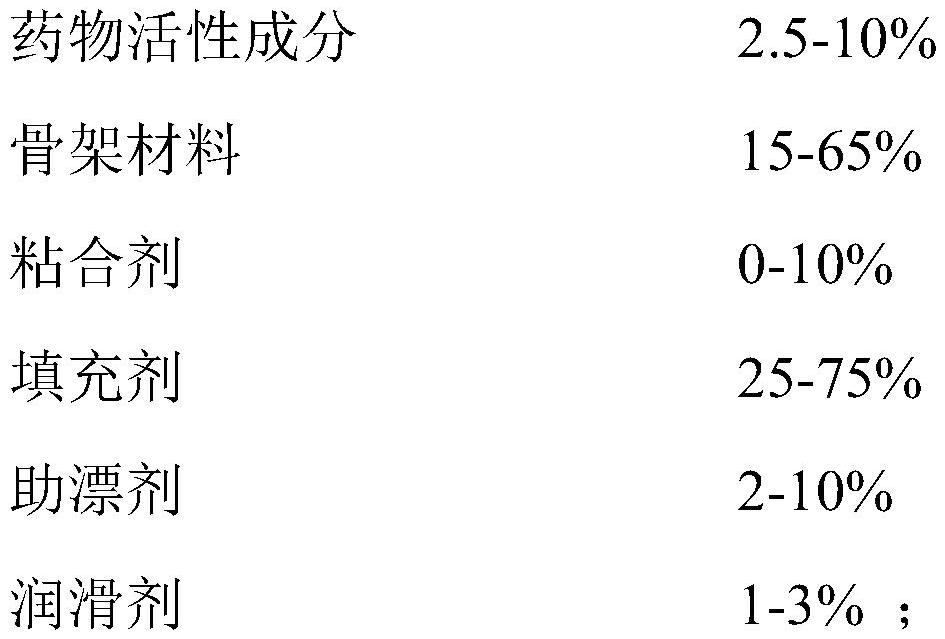
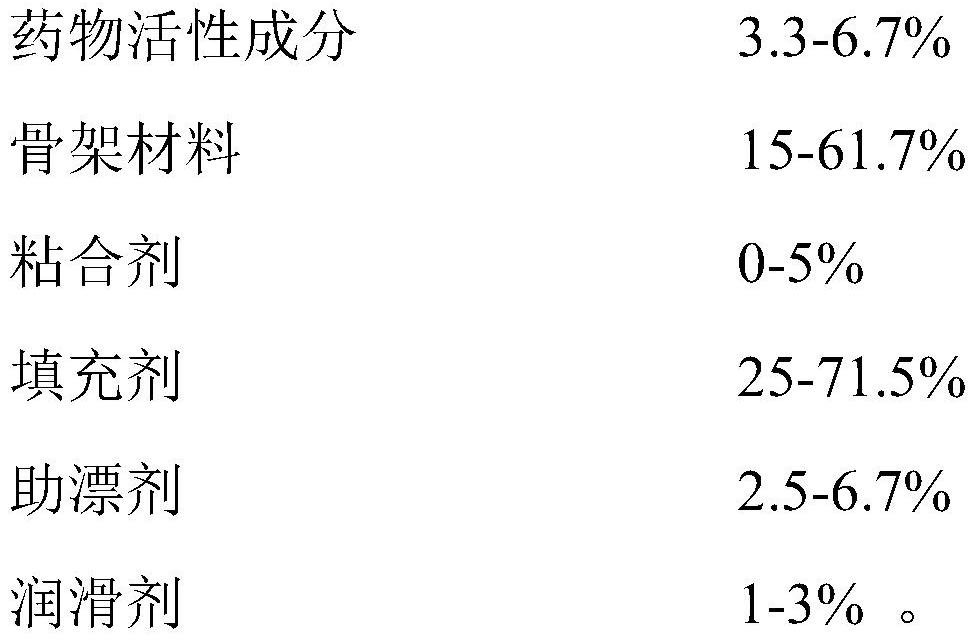
The invention discloses a tandospirone pharmaceutical composition as well as a preparation method and an application thereof. The tandospirone pharmaceutical composition comprises an active pharmaceutical ingredient, a framework material, a filler, a bleaching aid, a lubricant and an optional adhesive, and the active pharmaceutical ingredient is tandospirone, pharmaceutically acceptable salt thereof or a solvate of the tandospirone or the pharmaceutically acceptable salt thereof. The framework material comprises one or a combination of a plurality of materials selected from polyoxyethylene WSR 303, polyoxyethylene WSR 1105, polyoxyethylene WSR 301, polyoxyethylene WSR 205 and polyoxyethylene N-80, and the bleaching aid is selected from one or a combination of more of sodium carbonate, sodium bicarbonate, potassium bicarbonate, magnesium carbonate and calcium carbonate. According to the tandospirone pharmaceutical composition disclosed by the invention, the residence time of the medicine in the stomach is prolonged, so that the medicine can be fully released and absorbed, and the bioavailability of the medicine is greatly improved.
Owner:SICHUAN CREDIT PHARMA
Method and kit for the detection of 19 drugs and their metabolites in blood by liquid chromatography tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 kinds of drugs and their metabolites in blood by liquid chromatography tandem mass spectrometry. Substances to be tested include sulpiride, penfluridol, mianserin, buspirone, tandospirone, hydroxyzine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, rebo Cetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, tolbutamide, glimepiride, 1-pyrimidine piperazine, desvenlafaxine, 6- Hydroxybuspirone, norimipramine, noramitriptyline, nordiazepam, clopidogrel metabolites; detection methods include: calibrating standard solutions, processing samples to be tested, using high performance liquid chromatography‑ Mass spectrometry detects the sample to be tested. The embodiment of the present invention can quickly and accurately measure the content, the sample processing method is simple and easy, and has high sensitivity and accurate quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
A kind of tandospirone dispersible tablet or coated dispersible tablet and preparation method thereof
ActiveCN104997740BIngenious designImprove the disintegration effectOrganic active ingredientsPill deliveryDrug complianceOrganic chemistry
Owner:SICHUAN CREDIT PHARMA
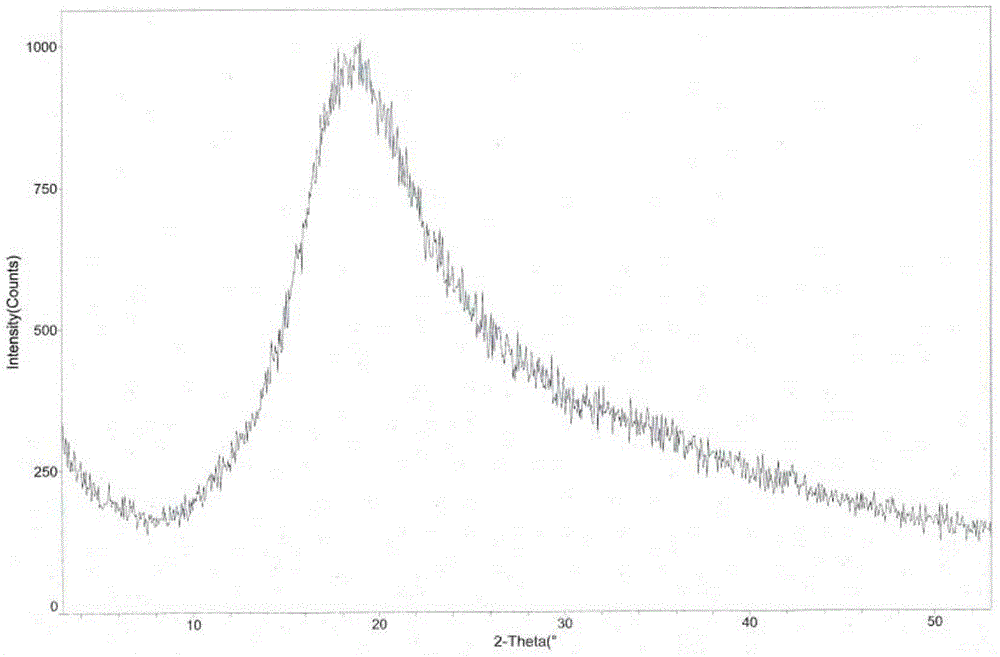
L-tartaric acid tandospirone compound
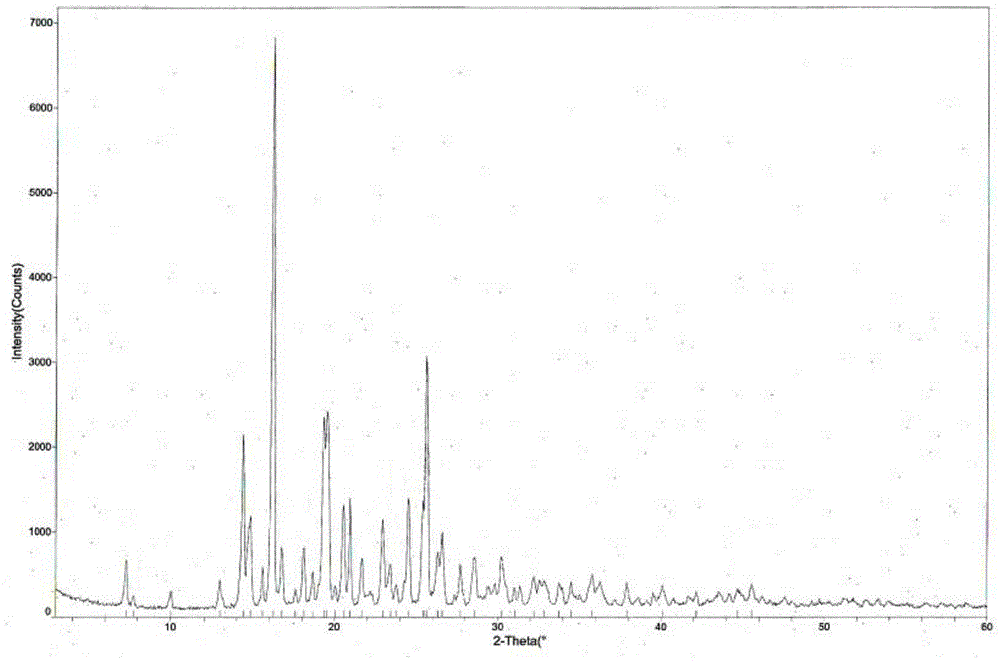
InactiveCN106349226AStable in natureGood water solubilityOrganic active ingredientsSenses disorderCombinatorial chemistryAqueous solubility
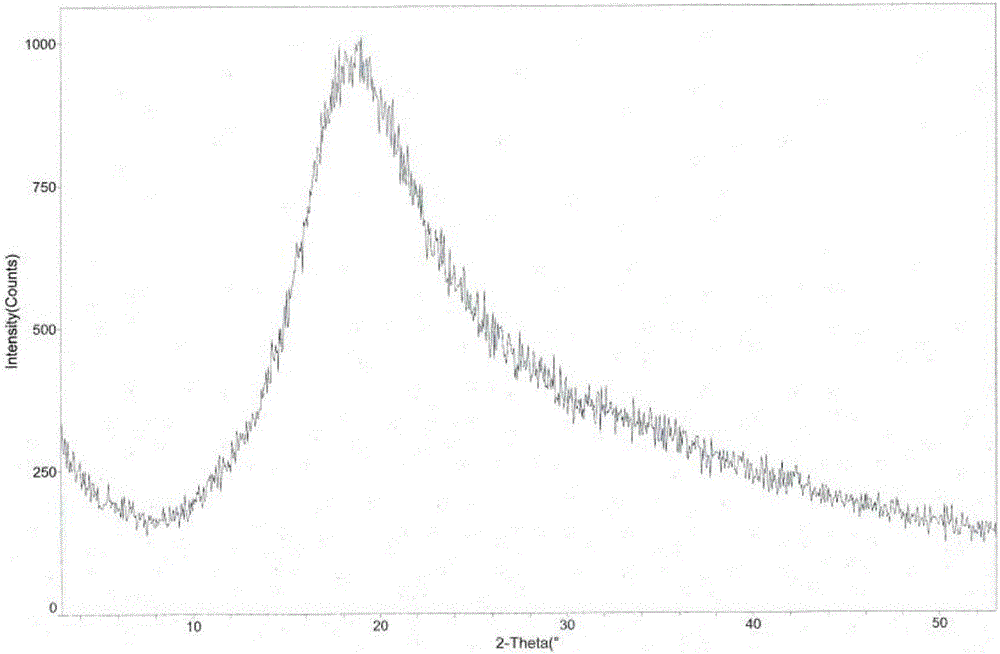
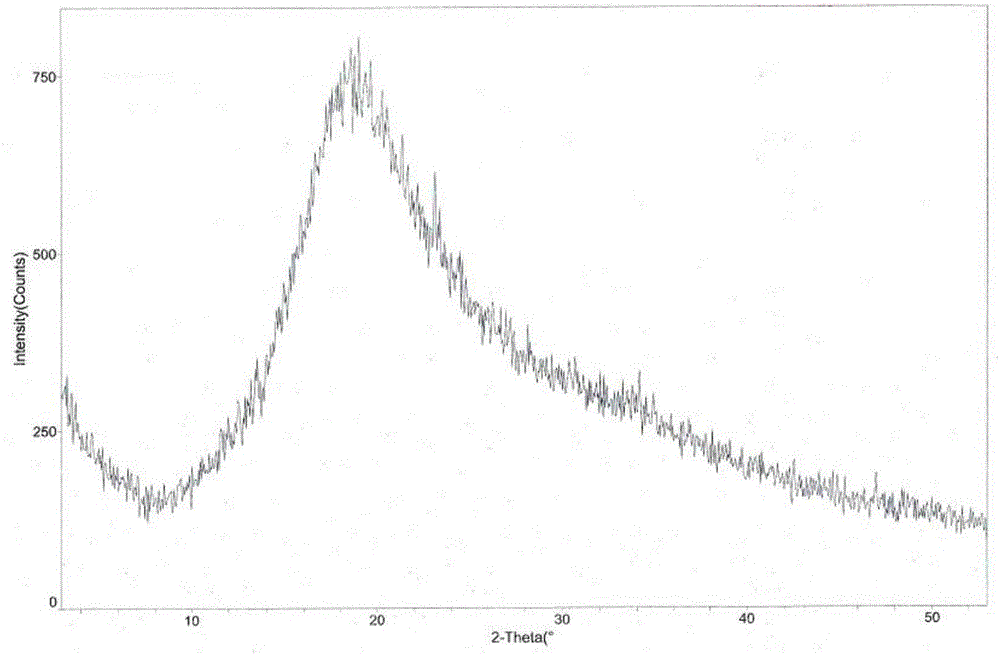
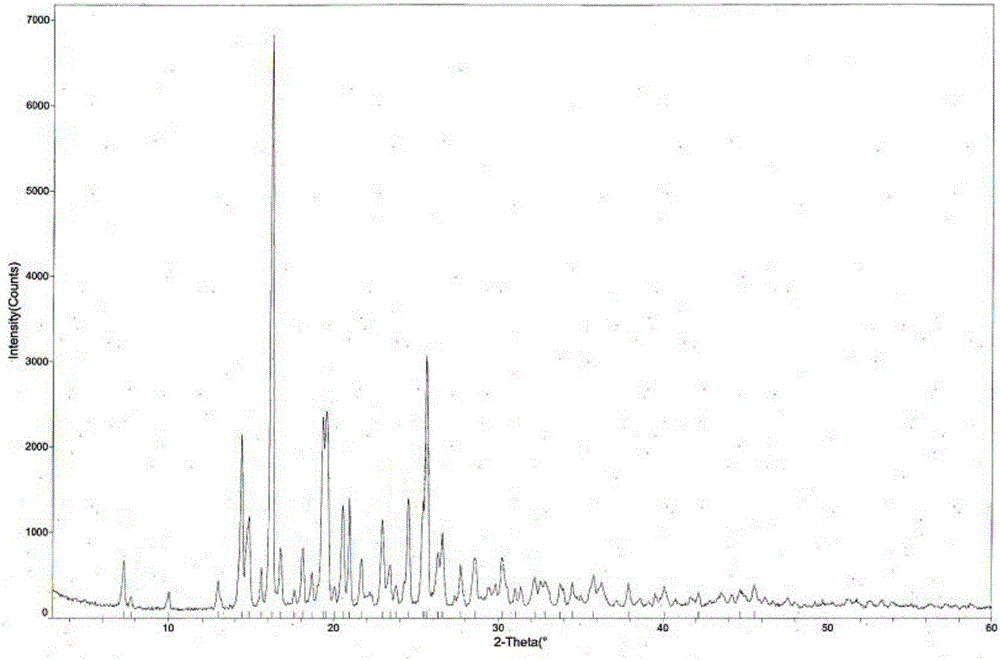
The invention provides an L-tartaric acid tandospirone compound. The invention further provides a preparation method, a pharmaceutical composition and application of the compound. The L-tartaric acid tandospirone compound provided by the invention exists in a crystal form, and is characterized in that the compound exists in an amorphous form and has no characteristic peaks in an X-ray powder diffraction pattern. The L-tartaric acid tandospirone compound provided by the invention has very good water solubility and stability and provides the possibility of improving the bioavailability and the safety of medicines; furthermore, the compound has a simple prepapration process and high yield and is suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA
Uses of tandospirone and derivative thereof in improving memory and cognitive uses
ActiveCN101224208AEnhance memoryImprove cognitive functionOrganic active ingredientsNervous disorderClinical trialPoor memory
The invention provides the usage of tandospirone and derivatives thereof in the preparation of drugs for improving memory capability and recognition function. The invention also provides a drug combination for improving memory capability and recognition function. The tandospirone and the derivatives thereof can improve memory capability and recognition function and in particular has evident medical treatment effect on poor memory due to neurasthenia or senile dementia. The medicine of the invention can also treat neurasthenia and senile dementia; proved by clinical experiments, the medical effect is remarkable.
Owner:SICHUAN CREDIT PHARMA
A serotonin receptor agonist
ActiveCN106800550BStable in natureGood water solubilityOrganic active ingredientsSenses disorderSerotonin receptor agonistTryptamine Receptors
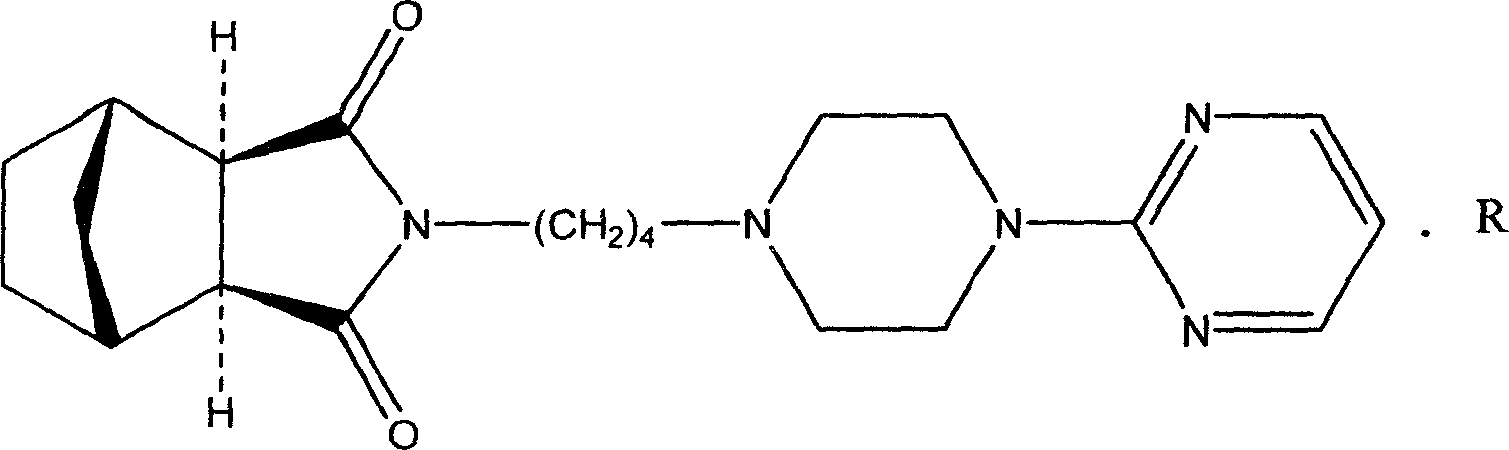
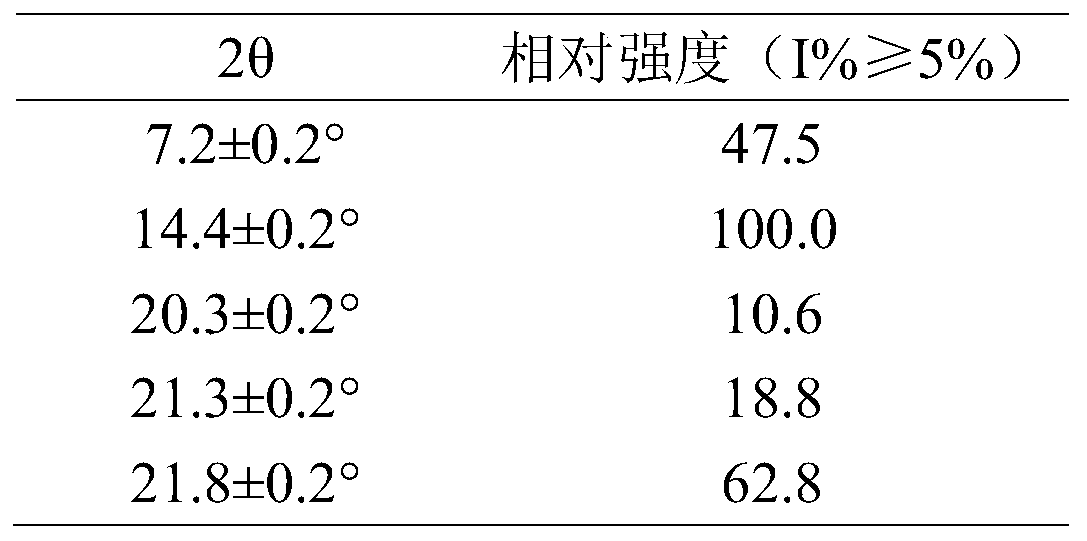
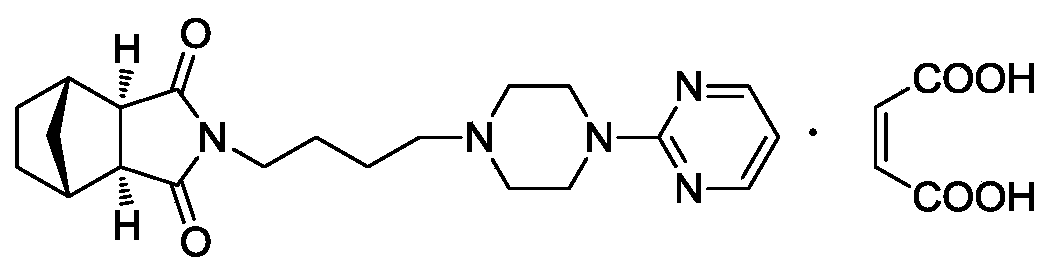
The invention provides a serotonin receptor agonist. Specifically, a tandospirone maleate compound is provided. The invention also provides the preparation method of the compound, as well as the pharmaceutical composition and application containing the compound. Tandospirone maleate provided by the present invention exists in the form of crystals, has stable properties, good water solubility, and significantly improved bioavailability, providing an effective solution for improving the safety and effectiveness of drugs; in addition , the preparation process of the compound of the present invention is simple, the yield is high, and it is suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA +1
A kind of tandospirone pharmaceutical composition and its preparation method and use
ActiveCN113018271BFloat quicklyExtended stayOrganic active ingredientsSenses disorderPolyethylene oxidePharmaceutical medicine
Tandospirone pharmaceutical composition and preparation method and use thereof. The tandospirone pharmaceutical composition comprises a pharmaceutical active ingredient, a matrix material, a filler, a bleaching aid, a lubricant and an optional binder, and the pharmaceutical active ingredient is tandospirone, which is pharmaceutically acceptable. The salt or the solvate of tandospirenone or a pharmaceutically acceptable salt thereof, the framework material includes selected from polyethylene oxide WSR 303, polyethylene oxide WSR 1105, polyethylene oxide WSR 301, polyethylene oxide WSR 205 , the combination of one or more in polyoxyethylene N-80; Described bleaching aid is selected from the combination of one or more in sodium carbonate, sodium bicarbonate, potassium bicarbonate, magnesium carbonate, calcium carbonate. The tandospirone pharmaceutical composition of the present invention prolongs the retention time of the drug in the stomach, so that the drug can be fully released and absorbed, and its bioavailability is greatly improved.
Owner:SICHUAN CREDIT PHARMA
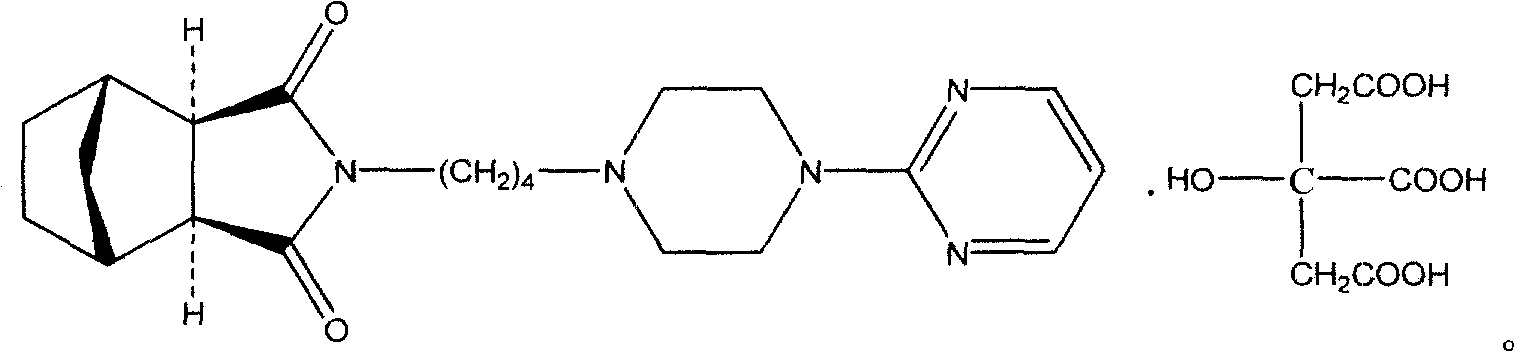
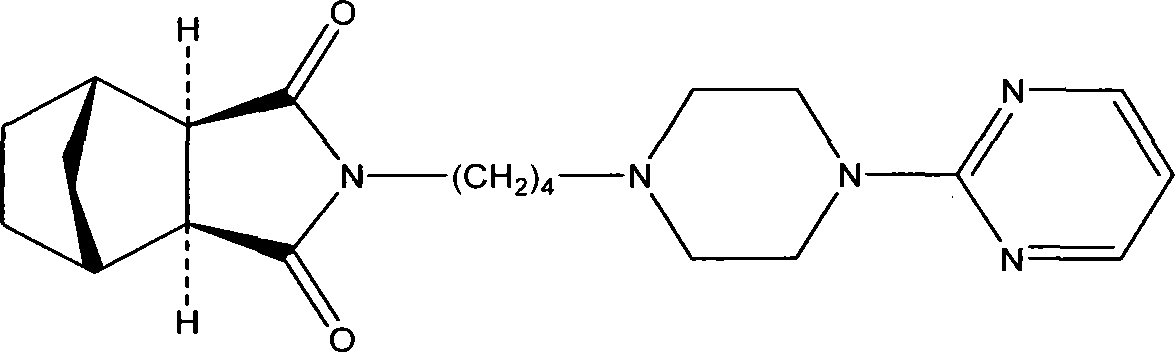
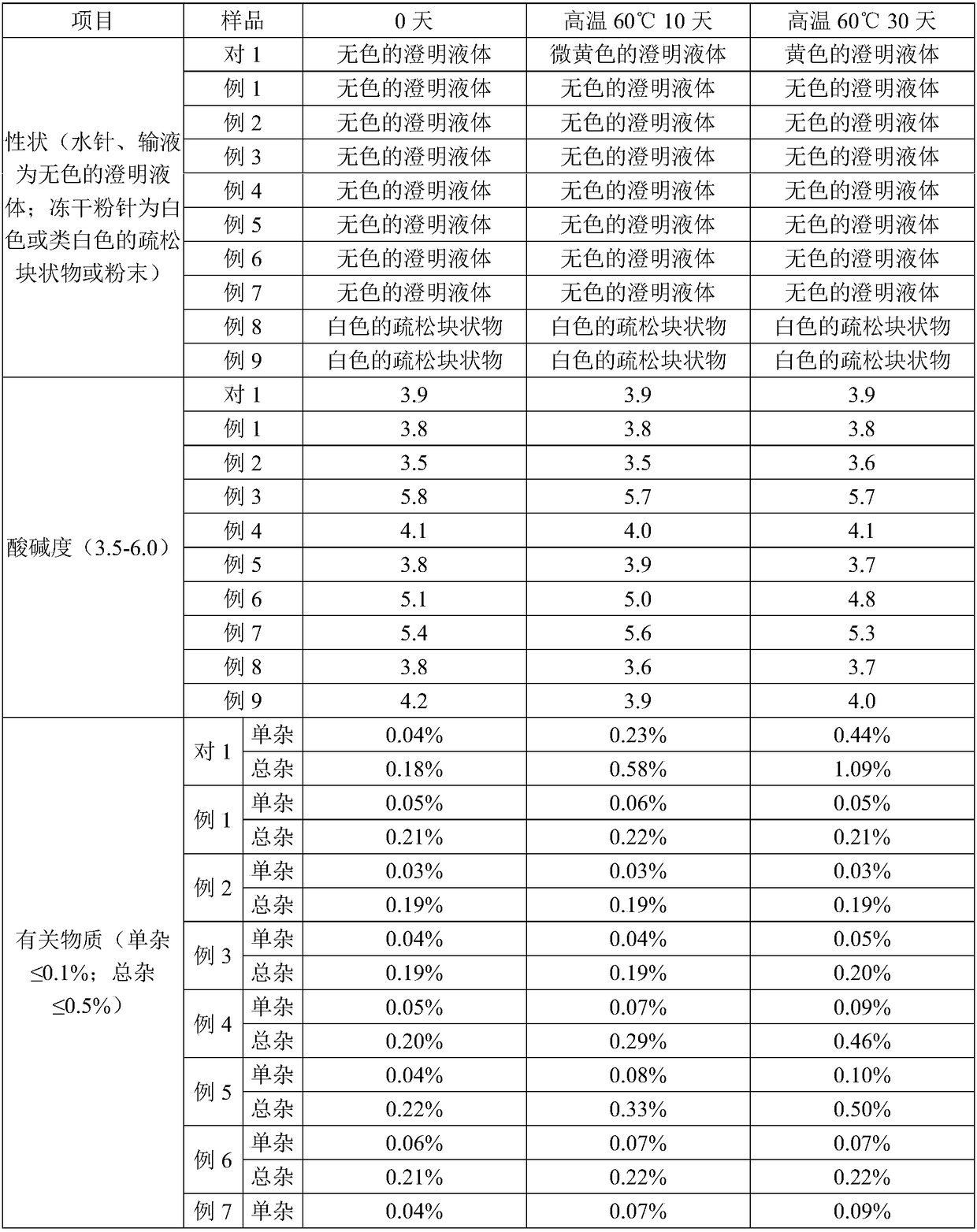
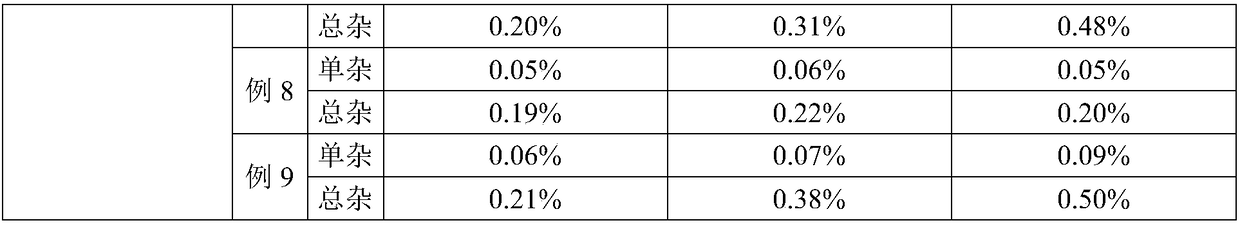
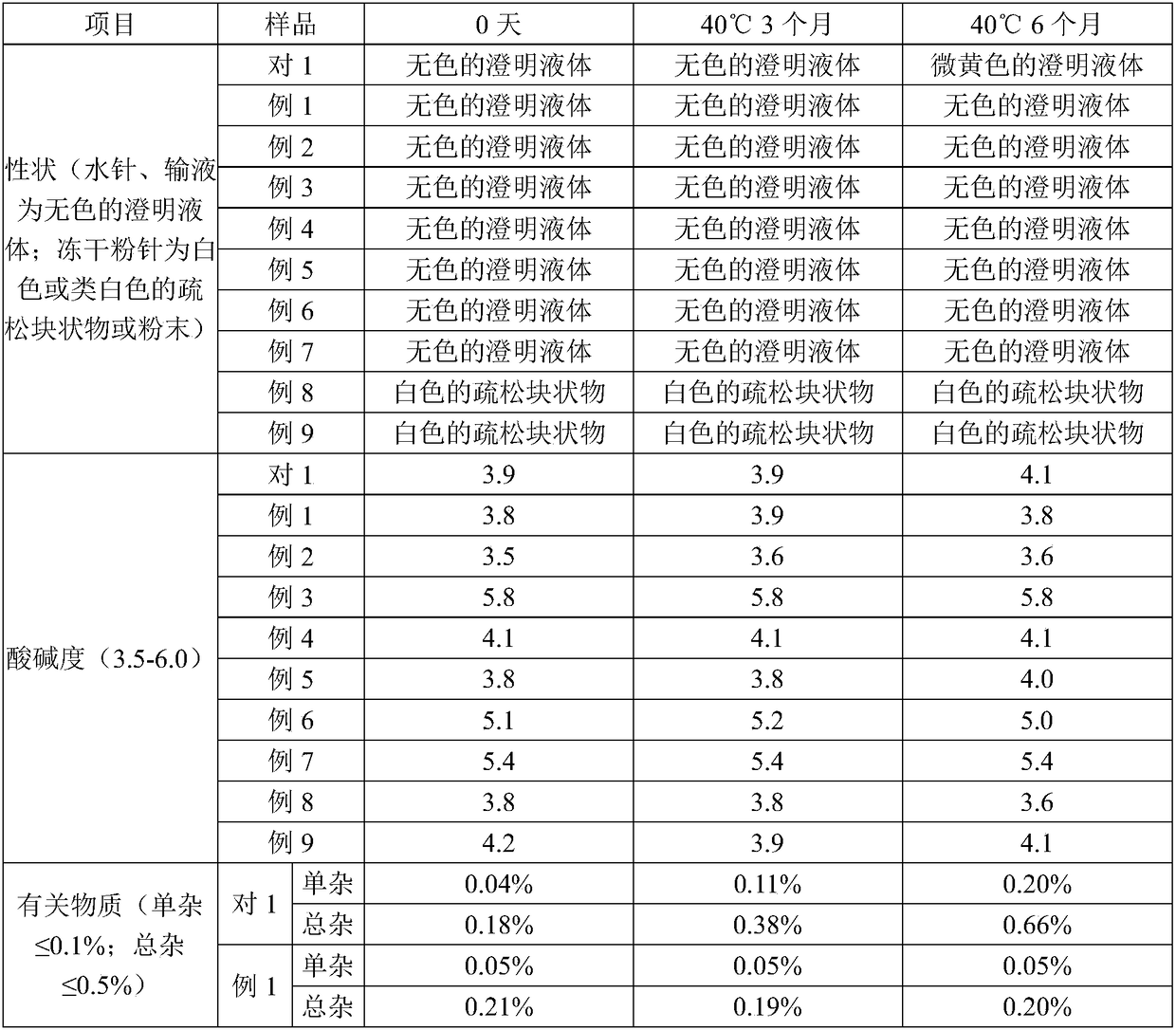
Azaspirone drug injection and its preparation method and application
ActiveCN108619100BImprove stabilityGood anti-anxiety effectPowder deliveryOrganic active ingredientsDrug injectionIrritation
Owner:SICHUAN CREDIT PHARMA
Uses of tandospirone and derivative thereof in improving memory and cognitive uses
ActiveCN101224208BEnhance memoryImprove cognitive functionOrganic active ingredientsNervous disorderClinical trialPoor memory
The invention provides the usage of tandospirone and derivatives thereof in the preparation of drugs for improving memory capability and recognition function. The invention also provides a drug combination for improving memory capability and recognition function. The tandospirone and the derivatives thereof can improve memory capability and recognition function and in particular has evident medical treatment effect on poor memory due to neurasthenia or senile dementia. The medicine of the invention can also treat neurasthenia and senile dementia; proved by clinical experiments, the medical effect is remarkable.
Owner:SICHUAN CREDIT PHARMA
Azaspirone drug injection, preparation method and uses thereof
ActiveCN108619100AImprove stabilityHigh stability, good anti-anxiety effectPowder deliveryOrganic active ingredientsForeign matterIrritation
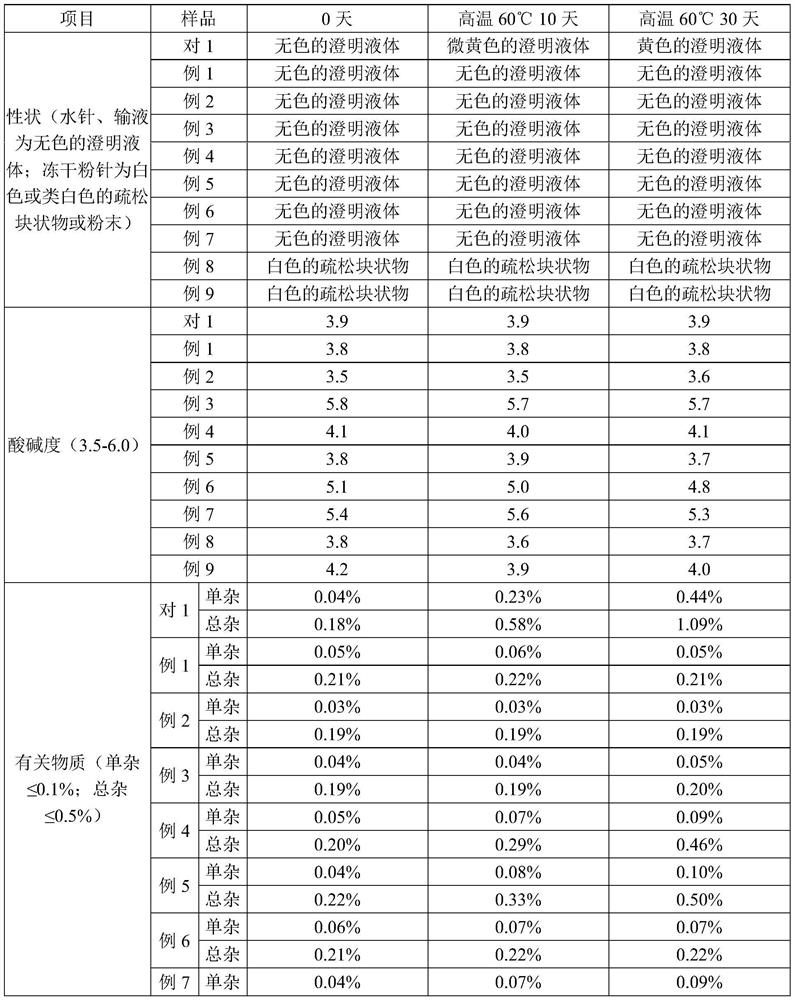
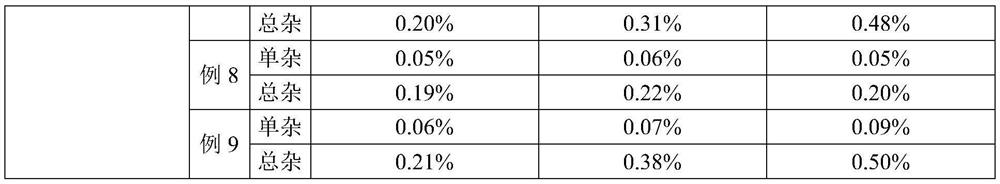
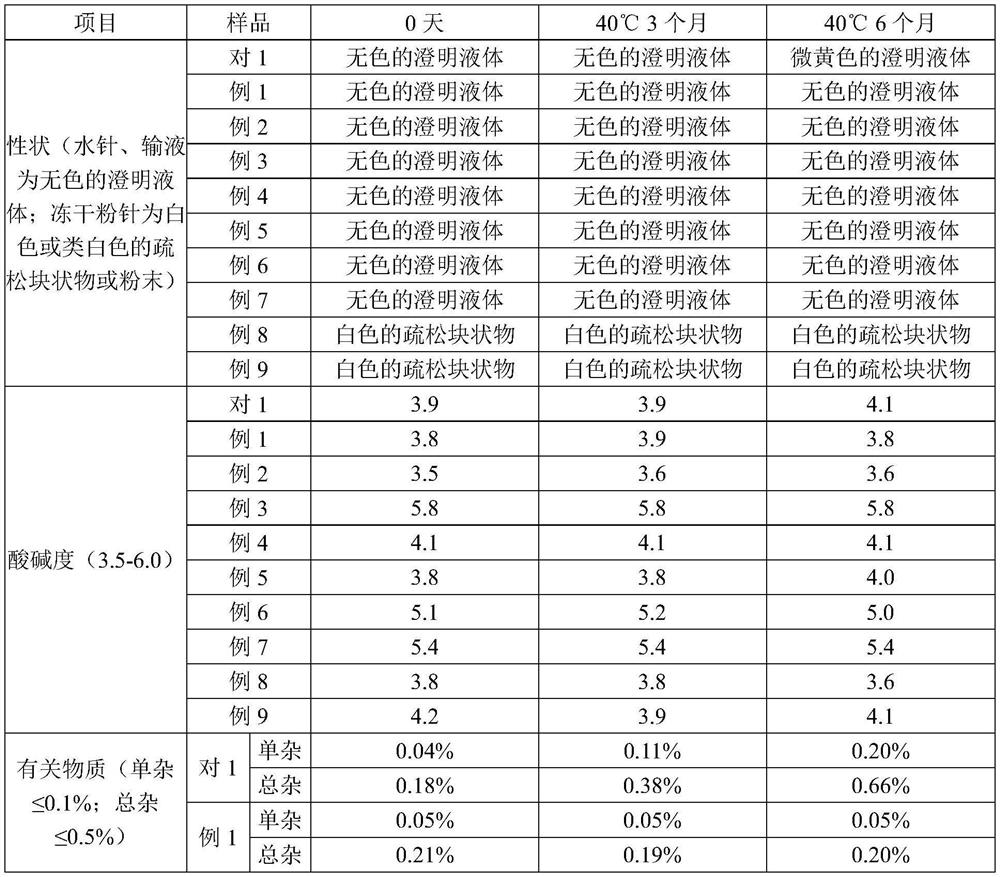
The invention relates to an azaspirone drug injection, a preparation method and uses thereof, wherein the azaspirone drug injection contains a tandospirone active component and a stabilizer. Accordingto the present invention, the azaspirone drug injection has characteristics of high stability and good anti-anxiety effect, and can meet the needs of clinical patients; the problem that children, orpatients with digestive system disorder, or patients in coma, tic, convulsion, and other states, and patients with dysphagia (such as elderly patients and bedridden patients) are not suitable for theoral administration of tandospirone tablets or capsules can be effectively solved with the azaspirone drug injection; and the azaspirone drug injection further has advantages of low cost, convenient use and the like, wherein the solution of the azaspirone drug injection is clarified, the insoluble particles meet the standard and have good stability, the visible foreign matter does not exist, the azaspirone drug injection does not have abnormal toxicity, and the concentration of the azaspirone drug injection cannot cause toxicity and obvious irritation.
Owner:SICHUAN CREDIT PHARMA
Tandospirone hydrochloride crystal form II and preparation method thereof
ActiveCN103664905BGood water solubilityImprove stabilityOrganic active ingredientsSenses disorderSolubilityDrospirenone
The invention provides a tandospirone hydrochloride crystal form II. The characteristic absorption peaks exist at the diffraction angle 2*theta within 2.127-2.527 degrees, 16.078-16.478 degrees, 19.157-19.557 degrees, 19.321-19.721 degrees, 19.499-19.899 degrees, 22.561-22.961 degrees, 29.437-29.837 degrees and 35.530-35.930 degrees in the X-ray powder diffraction diagram of the crystal form II. The invention further provides a preparation method of the crystal form II. Compared with the conventional tandospirone hydrochloride product, the tandospirone hydrochloride crystal form II provided by the invention is more excellent in water solubility and stability, and provides the possibility of improving bioavailability and safety of drugs; in addition, the preparation technology of the crystal form II is simple, the yield of the crystal form II is high, and the preparation method is suitable for industrial production.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
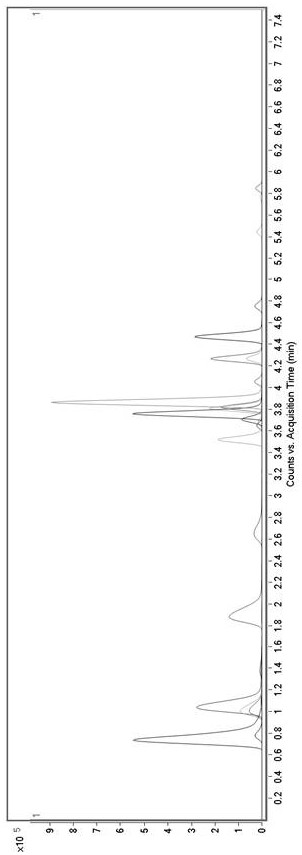
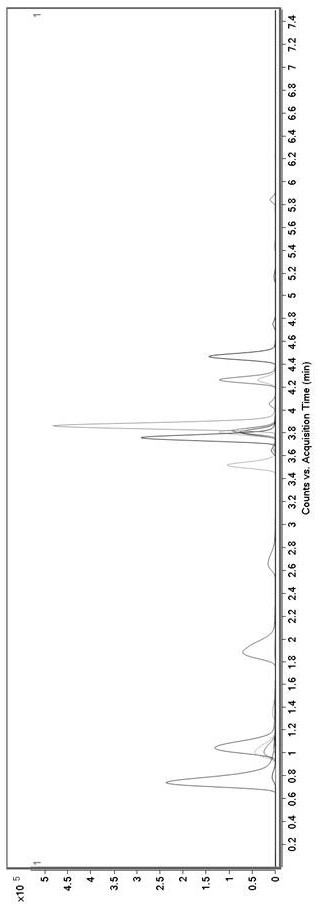
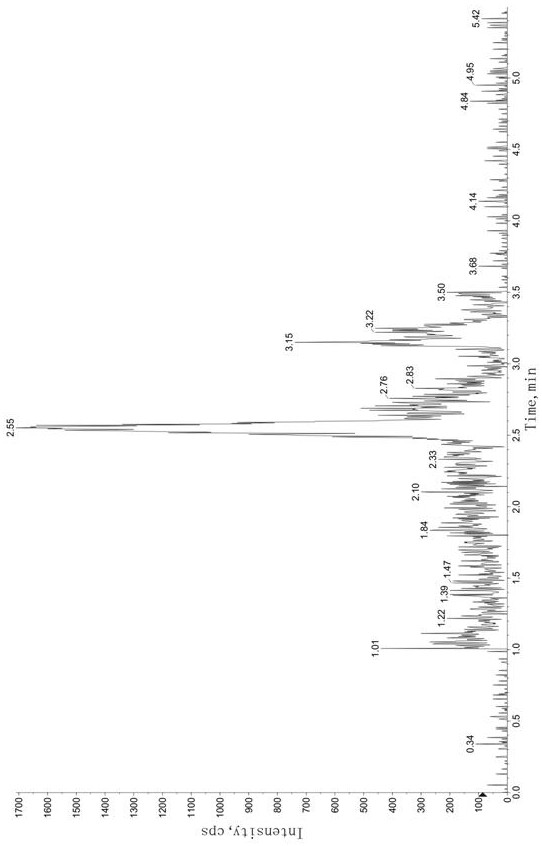
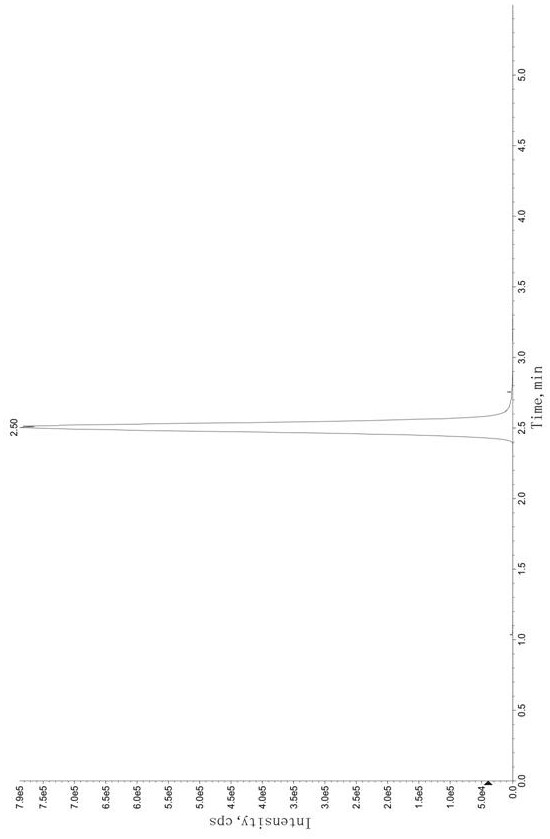
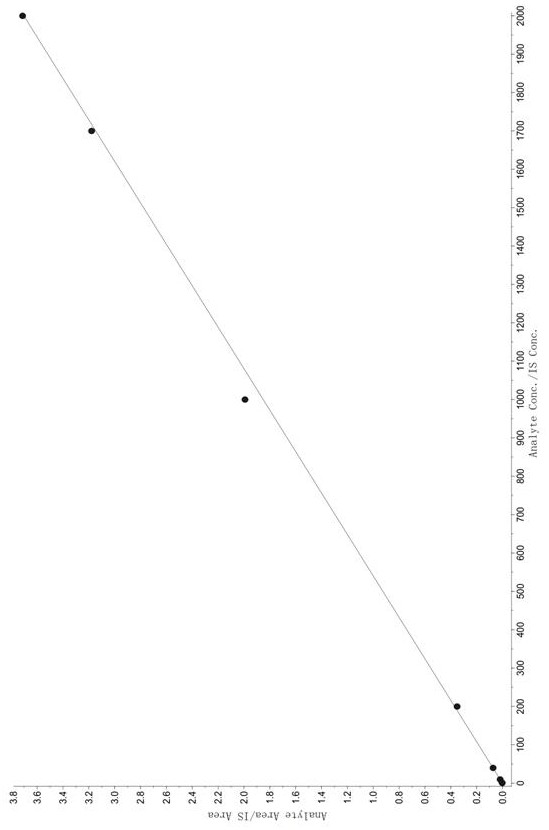
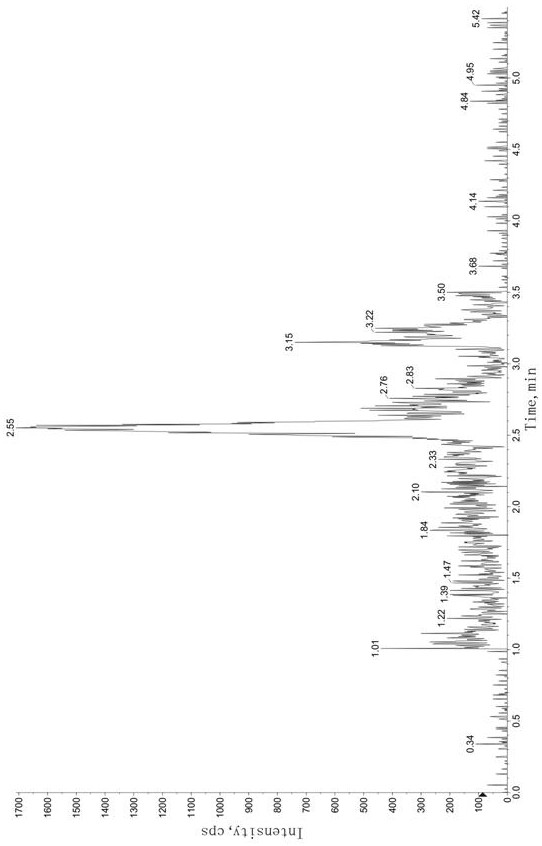
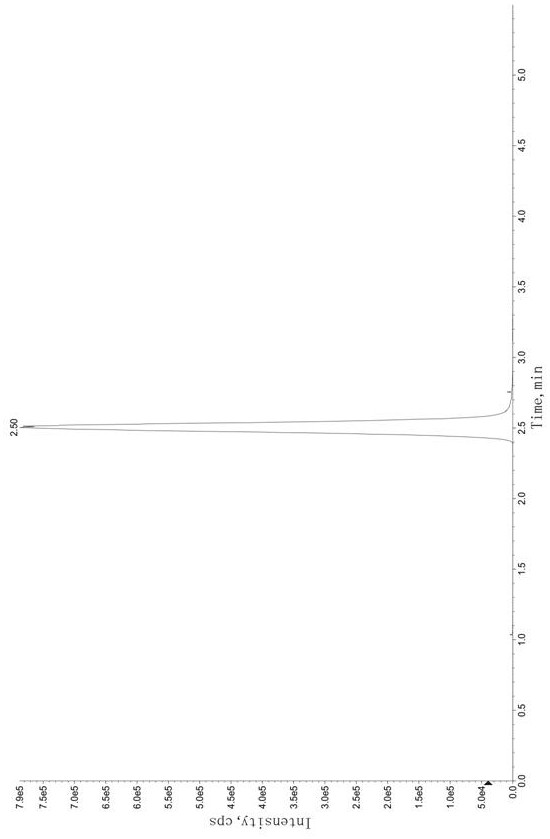
Method for determining concentration of tandospirone in human plasma
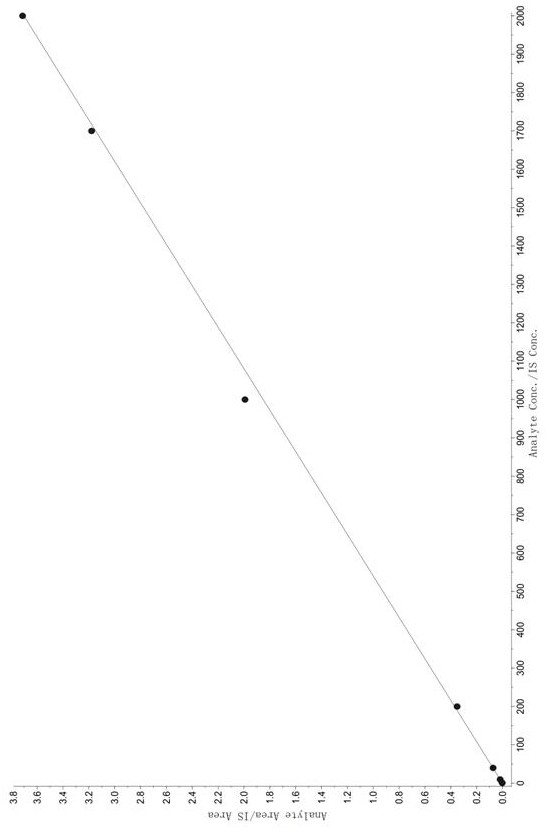
ActiveCN112986452AEfficient separationEfficient identificationComponent separationTandospironeStandard curve
The invention belongs to the technical field of medicine detection, and discloses a method for determining the concentration of tandospirone in human plasma, which comprises the following steps: detecting tandospirone in a pretreated human plasma sample by adopting a high performance liquid chromatography-mass spectrometry technology, separating tandospirone from impurities by utilizing high performance liquid chromatography, quantifying by utilizing an isotope internal standard method, establishing a standard curve regression equation by taking the concentration ratio of the standard substance in the standard solution to the internal standard substance in the internal standard working solution as an X axis and the peak area ratio of the standard substance in the standard solution to the internal standard substance in the internal standard working solution as a Y axis, and performing linear least square regression calculation on the theoretical concentration ratio of the standard substance to the internal standard substance in the standard curve regression equation according to the peak area ratio of the substance to be detected to the internal standard substance, and calculating the actually measured concentration of tandospirone in the human plasma sample according to the obtained standard curve regression equation. According to the invention, the defect of accurate quantitative detection at a relatively low concentration level is overcome, and the requirement of measuring the content in a living body when the dosage is relatively small can be met.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Preparation and purification method of exomethylenetetrahydrophthalic anhydride and its application in the preparation of tandospirone
ActiveCN103848801BSimple manufacturing processIncrease profitOrganic chemistryChemical recyclingDepolymerizationHigh energy
The invention relates to a preparation method of exomethylenetetrahydrophthalic anhydride, a refining method thereof and an application thereof in a method for preparing tandospirone. The present invention combines the depolymerization of dicyclopentadiene, the cycloaddition of cyclopentadiene and maleic anhydride, and the transformation of endomethylene tetrahydrophthalic anhydride in series to complete the three-step reaction efficiently in one step, and the method does not need to carry out dicyclopentadiene separately. The depolymerization of dienes without separation of intermediate products generated by the reaction effectively solves the complicated depolymerization of dicyclopentadiene, low conversion rate and low yield in the existing preparation method of exomethylenetetrahydrophthalic anhydride , serious environmental pollution, high energy consumption, long production cycle, harsh storage conditions and other defects, and has the advantages of high yield, good quality, environmentally friendly use of solvents, low cost, and is suitable for industrialized large-scale production.
Owner:SICHUAN CREDIT PHARMA
A kind of tandospirone enteric-coated pellets and its preparation method and application
ActiveCN106344519BQuality improvementLarge distribution areaOrganic active ingredientsNervous disorderDiseaseModerate depression
The invention provides a tandospirone enteric-coated pellet and a preparation method thereof. The enteric-coated pellet is prepared by adding tandospirone or a pharmaceutically acceptable salt thereof as an active ingredient and adding pharmaceutically acceptable auxiliary materials . The present invention also provides the use of the tandospirone enteric-coated pellets in the preparation of an anti-anxiety drug for enteral administration. The tandospirone enteric-coated pellets prepared by the invention have stable quality, no disintegration in the stomach, large distribution area in the intestinal tract, good disintegration and dissolution properties, high bioavailability, less irritation to the digestive tract mucosa, and less side effects , The patient's medication compliance is high. It can be used to treat patients with the following diseases: anxiety state caused by various neurosis, anxiety state accompanied by physical diseases such as essential hypertension and peptic ulcer, mild and moderate depression; especially suitable for digestive system diseases Patients who cause anxiety, patients with gastric mucosal injury or easy bleeding, and elderly patients who need long-term medication.
Owner:SICHUAN CREDIT PHARMA
5-hydroxytryptamine receptor agonist
ActiveCN106800550AStable in natureGood water solubilityOrganic active ingredientsSenses disorderSolubilityWater soluble
The invention provides a 5-hydroxytryptamine receptor agonist. Particularly, provided is a tandospirone maleate compound. The invention also provides a preparation method of the compound, and a pharmaceutical composition containing the compound and a use of the compound. The tandospirone maleate provided by the invention exists in a crystal form, has stable properties and good water solubility, has the bioavailability significantly improved, and provides an effective solving way for improving the safety and effectiveness of drugs; in addition, the compound is simple in preparation technology, high in yield and suitable for industrialized production.
Owner:SICHUAN CREDIT PHARMA +1
Tandospirone hydrochloride crystal form I and preparation method thereof
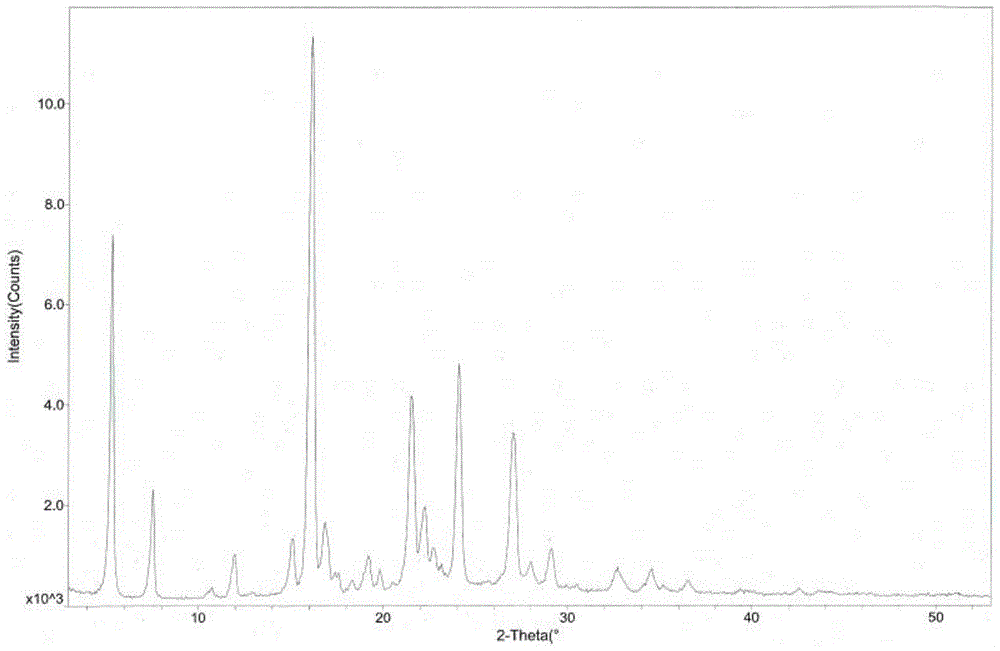
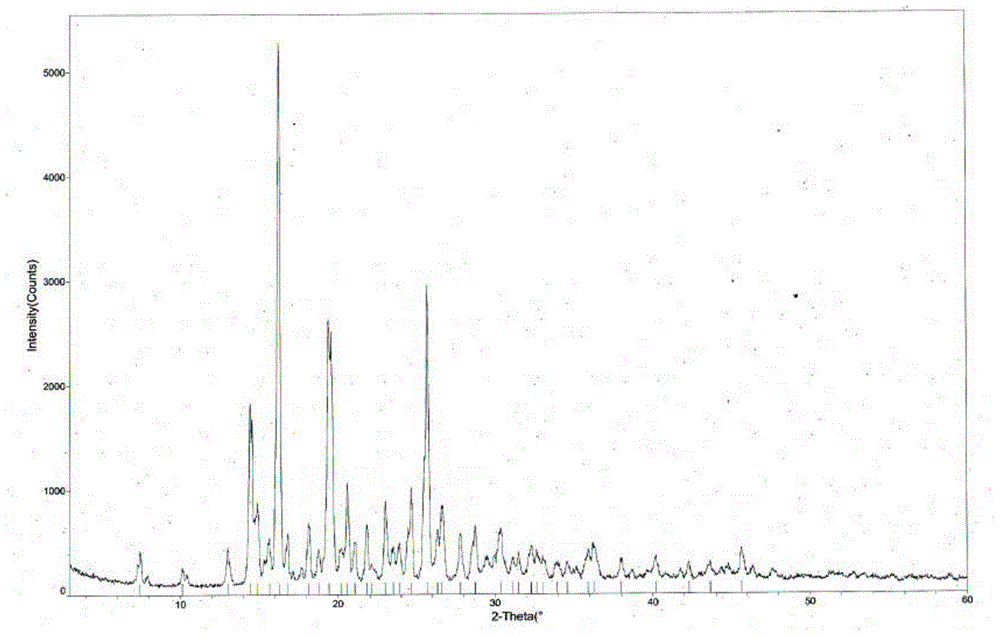
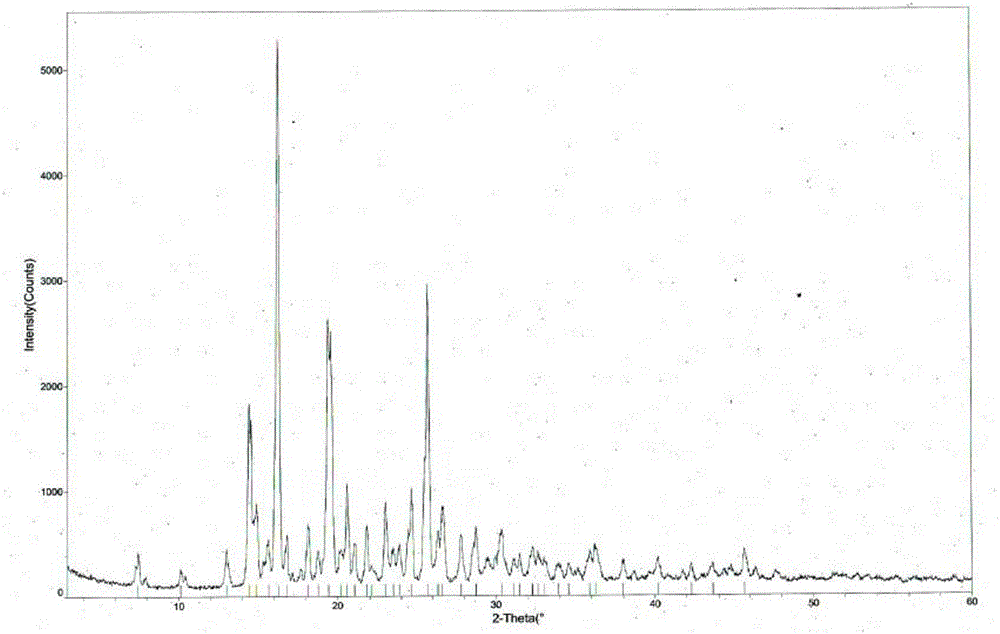
ActiveCN103755687BGood water solubilityImprove stabilityOrganic active ingredientsSenses disorderSolubilityDrospirenone
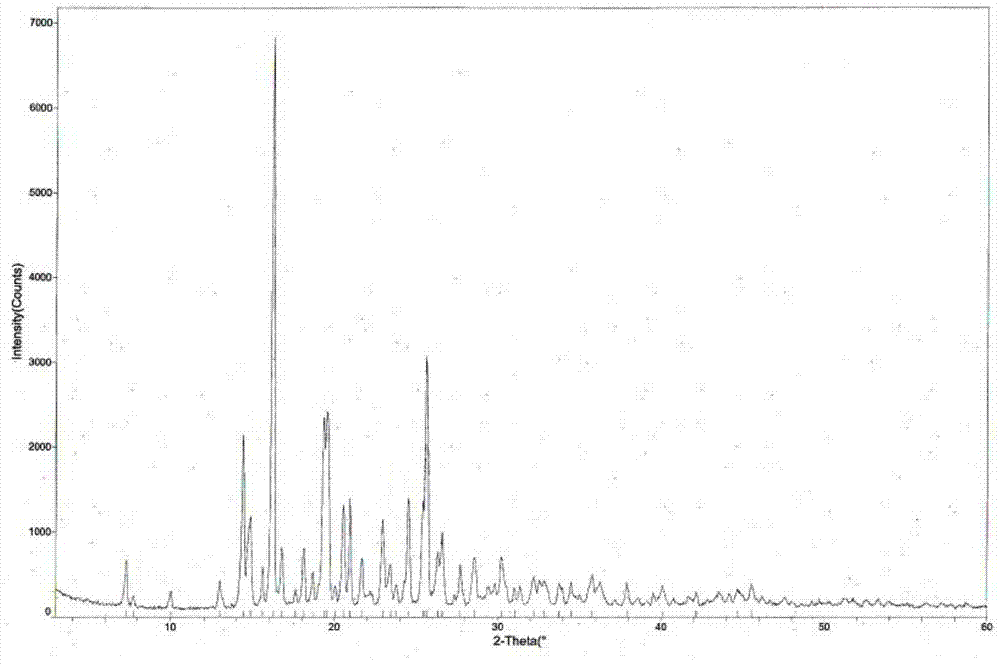
The invention provides a hydrochloric tandospirone crystal form I. The hydrochloric tandospirone crystal form I is characterized in that an X-ray powder diffraction pattern of the crystal form I shows that characteristic absorption peaks appear at 2 theta diffraction angles of 6.597+ / -0.2, 10.352+ / -0.2, 12.676+ / -0.2, 15.362+ / -0.2, 15.922+ / -0.2, 17.122+ / -0.2, 18.752+ / -0.2, and 25.522+ / -0.2 degrees. The invention also provides a preparation method of the crystal form I. Compared with the existing hydrochloric tandospirone product, the hydrochloric tandospirone crystal form I, provided by the invention, has the advantages that the water solubility and the stability are improved, and the bioavailability and the safety of a drug can be improved; in addition, the crystal form is simple in preparation process, high in yield, and suitable for industrial production.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
Tandospirone Microporous Osmotic Pump Preparation
ActiveCN104706614BProlong the action timeReduce the number of daily dosesOrganic active ingredientsNervous disorderOsmotic pumpDrug release
The invention provides a tandospirone micropore osmotic pump preparation. The osmotic pump preparation can reach zero-level release, reaches 24-hour drug release, can effectively prolong the time of drug action, makes patients reduce the number of times of taking medicine daily, and improves compliance.
Owner:SICHUAN CREDIT PHARMA
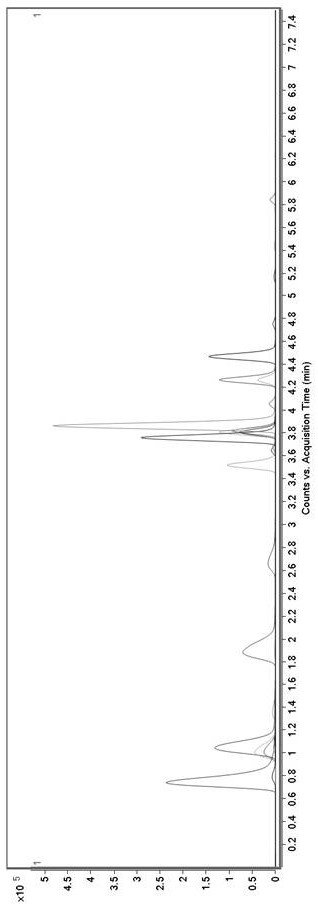
Method for Determination of Tandospirone Concentration in Human Plasma
ActiveCN112986452BEfficient separationEfficient identificationComponent separationBiological bodyFluid phase
The invention belongs to the technical field of medical detection, and discloses a method for measuring the concentration of tandospirone in human plasma, using high performance liquid phase tandem mass spectrometry technology to detect tandospirone in pretreated human plasma samples, and using high performance liquid chromatography to detect tandospirone Tandospirone is separated from impurities, and then quantified by the isotope internal standard method. The concentration ratio of the standard in the standard solution and the internal standard in the internal standard working solution is the X axis, and the internal standard in the standard solution and the internal standard in the working solution are The peak area ratio of the standard substance is the Y axis, and the standard curve regression equation is established, and the linear least squares method is used to calculate the theoretical concentration ratio of the standard substance and the internal standard substance in the standard curve regression equation based on the peak area ratio of the analyte and the internal standard substance. , the measured concentration of tandospirone in human plasma samples was calculated by the obtained standard curve regression equation. The invention overcomes the defect of precise quantitative detection at a lower concentration level, and can meet the content determination requirement in a living body when a small dosage is administered.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com