Tandospirone dispersible tablets or coating dispersible tablets, and preparation method thereof
A technology of tandospirone and tandospirone hydrochloride, which is applied in the field of tandospirone dispersible tablets or coated dispersible tablets and its preparation, can solve the problem of difficulty in increasing blood drug concentration, increasing adverse reactions, affecting drug absorption, etc. problem, achieve excellent disintegration effect, prevent the influence of moisture on the tablet core, and achieve high disintegration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Tablet composition (1000 tablets):
[0047] Tandospirone Citrate 10g
[0048] Sucrose 240g
[0049] Mannitol 45g
[0050] Pregelatinized starch 50g
[0051] Microcrystalline Cellulose 80g
[0052] Low-substituted hydroxypropyl cellulose 70g
[0053] Sodium Laureth Sulfate 3g
[0054] Micronized silica gel 2g
[0055] Coating liquid composition:
[0056] Hypromellose 12g
[0057] Mannitol 10g
[0058] Glycerin 12ml
[0059] Strawberry essence 2g
[0060] Chili red 3g
[0061] water 600ml
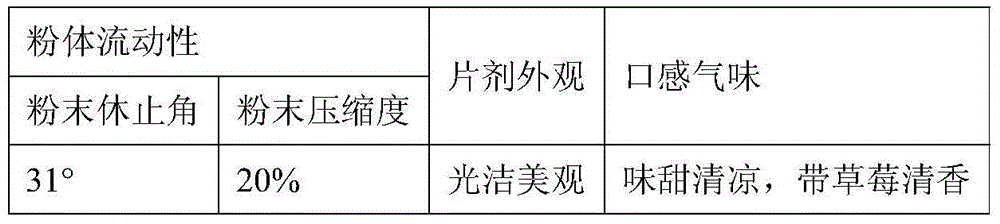
[0062] Evaluation indicators:
[0063]
[0064] Preparation Process:
[0065] Tablet core compression: pass tandospirone or its pharmaceutically acceptable salt, filler, and disintegrant respectively through a 100-mesh sieve, and mix uniformly; According to the content of dospirone, the weight of the tablet is calculated, and the tablet is directly compressed with a tablet machine.
[0066] Preparation of coating liquid: first add sugar alcohol, glycerin, flavoring age...
Embodiment 2
[0070] Tablet composition (1000 tablets):
[0071] Tandospirone Citrate 10g
[0072] Glucose 240g
[0073] Mannitol 45g
[0074] Pregelatinized starch 50g
[0075] Microcrystalline Cellulose 60g
[0076] Croscarmellose Sodium 90g
[0077] Sodium stearyl fumarate 3g
[0078] Micronized silica gel 2g
[0079] Coating liquid composition:
[0080] Hypromellose 12g
[0081] Mannitol 10g
[0082] Glycerin 12ml
[0083] Strawberry essence 2g
[0084] Chili red 3g
[0085] water 600ml
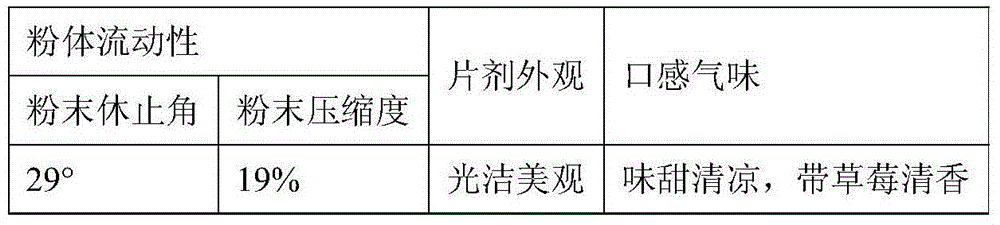
[0086] Evaluation indicators:
[0087]
Embodiment 3
[0089] Tablet composition (1000 tablets):
[0090] Tandospirone Citrate 10g
[0092] Mannitol 45g
[0093] Pregelatinized starch 50g
[0094] Microcrystalline Cellulose 80g
[0095] Crospovidone 70g
[0096] Sodium Polyoxyethylene Lauryl Sulfate 3g
[0097] Micronized silica gel 2g
[0098] Coating liquid composition:
[0099] Hypromellose 12g
[0100] Mannitol 10g
[0101] Glycerin 12ml
[0102] Apple essence 2g
[0103] Pine leaf orchid 5g
[0104] water 600ml
[0105] Evaluation indicators:
[0106]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com