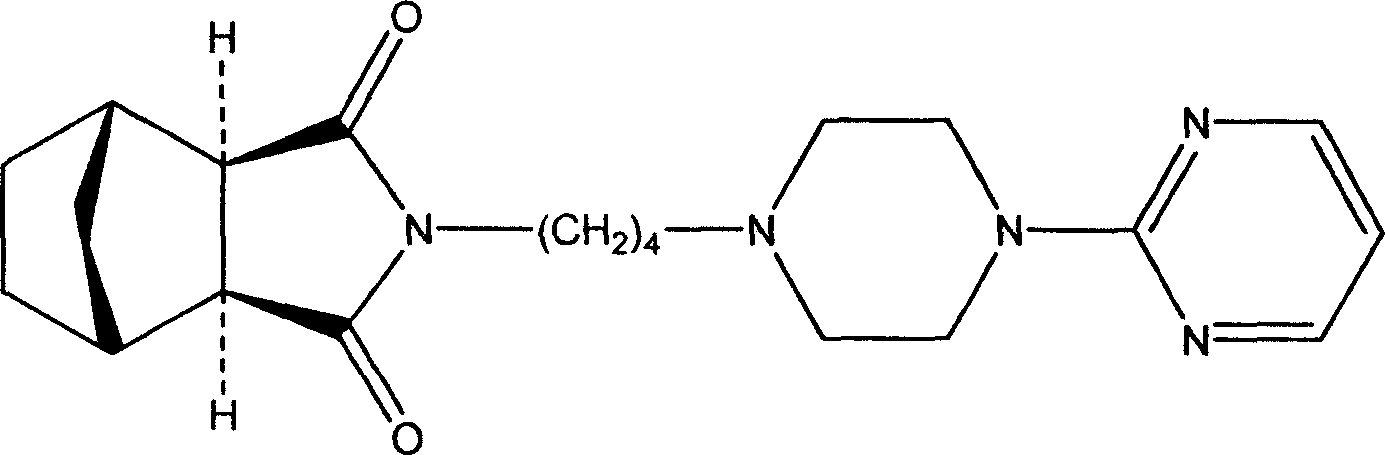
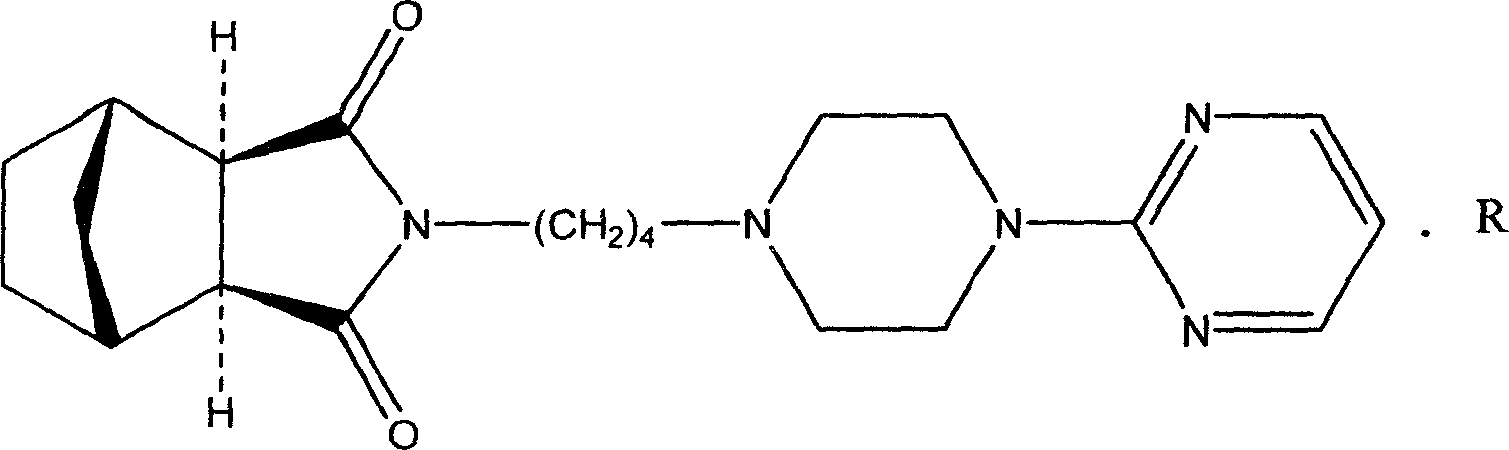
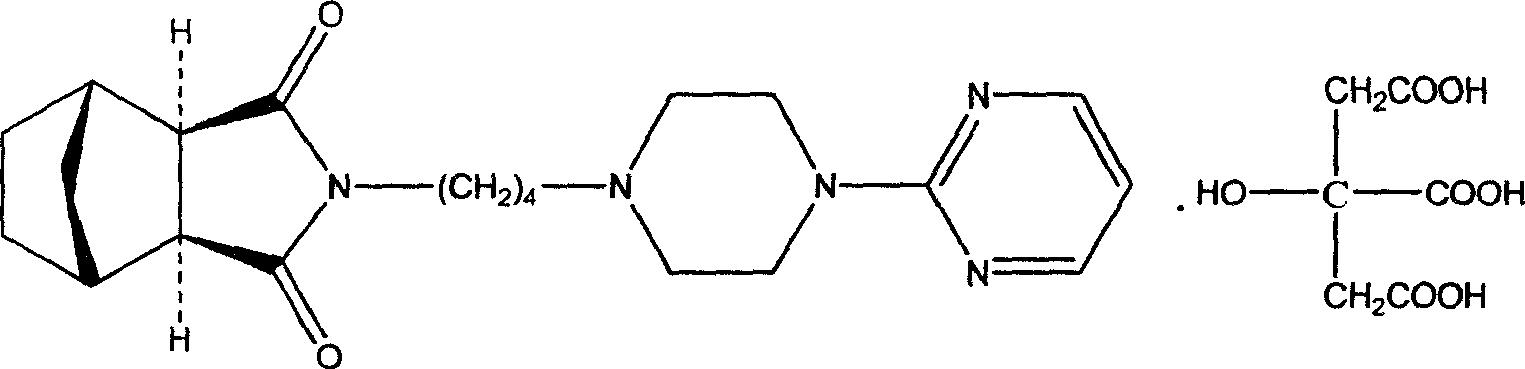
New usage of tandospirone and its derivative, and composition containing tandospirone
A technology of tandospirone and composition, which is applied in the field of compositions containing tandospirone to achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of orally disintegrating tablets of tandospirone
[0030] Tandospirone 3g (about 0.008mol)
[0031] Low-substituted hydroxypropyl methylcellulose 30g
[0033] Mannitol 79g
[0034] Sweet orange essence 2g
[0036] Preparation method: 1. Pass the raw and auxiliary materials through a 100-mesh sieve;
[0037] 2. Mix the prescribed amount of tandospirone citrate and low-substituted hypromellose in a mixer, and continue to add the remaining amount of low-substituted hypromellose in equal amounts, mix evenly, and then Add mannitol, lactose starch in turn, and finally add sweet orange essence and magnesium stearate and mix well;
[0038] 3. Determine the content, calculate the tablet weight, and directly compress the powder into tablets, making a total of 1,000 tablets, and controlling the hardness to 1.5-2.5kg;
[0039] ...
Embodiment 2
[0040] Example 2 Preparation of Sublingual Tablets of Tandospirone Citrate
[0041] Tandospirone citrate 5g (about 0.008mol)
[0042] Low Substituted Hydroxypropyl Methyl Cellulose 120g
[0044] Mannitol 10g
[0045] Sweet orange essence 2g
[0047] Preparation method: 1. Pass the raw and auxiliary materials through a 100-mesh sieve
[0048] 2. Mix the prescribed amount of tandospirone citrate and low-substituted hypromellose in a mixer, and continue to add the remaining amount of low-substituted hypromellose in equal amounts, mix evenly, and then Add mannitol, lactose starch in turn, and finally magnesium stearate and mix well.
[0049] 3. Measure the content, calculate the tablet weight, and directly compress the powder into tablets, making a total of 1000 tablets, controlling the hardness to 1.5-2.5kg, and the tablet weight to 0.3g.
[0050] ...
Embodiment 3
[0051] Example 3 Preparation of Sublingual Tablets of Tandospirone
[0052] Tandospirone 10g (about 0.027mol)
[0053] Low Substituted Hypromellose 110g
[0054] Lactose starch 63g
[0055] Mannitol 12g
[0056] Sweet orange essence 2g
[0057] Magnesium stearate 1g.
[0058] Preparation method: 1. Pass the raw and auxiliary materials through a 100-mesh sieve
[0059] 2. Mix the prescribed amount of tandospirone citrate and low-substituted hypromellose in a mixer, and continue to add the remaining amount of low-substituted hypromellose in equal amounts, mix evenly, and then Add mannitol, lactose starch in turn, and finally sweet orange essence and magnesium stearate and mix well.
[0060] 3. Measure the content, calculate the tablet weight, and directly compress the powder into tablets, making a total of 1000 tablets, controlling the hardness to 1.5-2.5kg, and the tablet weight to 0.3g.
[0061] 4. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com