Tandospirone hydrochloride crystal form II and preparation method thereof
A technology of tandospirone hydrochloride and tandospirone hydrochloride, applied in the field of tandospirone hydrochloride crystal form II and preparation thereof, can solve problems such as reports of tandospirone hydrochloride crystal form, and achieve water solubility and stability Good, simple preparation process, and the effect of improving bioavailability and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation method of tandospirone hydrochloride crystal form II
[0038] Weigh 2 kg of tandospirone, add 16 L of a mixed solution of tetrahydrofuran and acetonitrile (volume ratio 90:10), heat up to 80 ° C, after the dissolution is complete, add 2.6 L of 2 mol / L hydrochloric acid aqueous solution, stop heating, and naturally Cool to room temperature and stand for 2 hours, then stand at -5±5°C for 12 hours, filter with suction, wash, and dry to obtain 2.07 kg of tandospirone hydrochloride crystal form II with a yield of 94.8%.
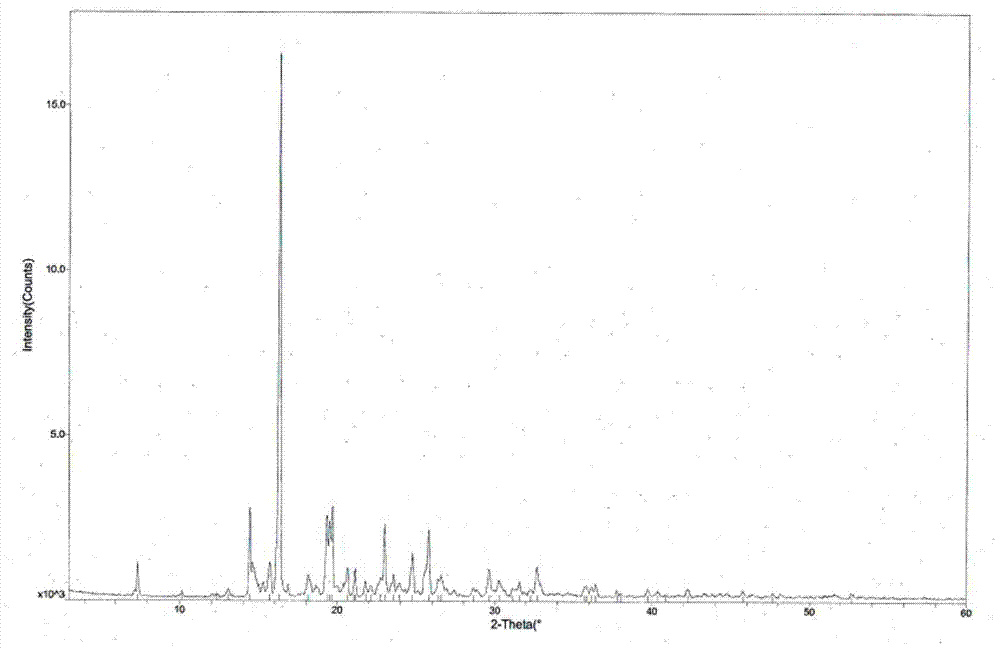
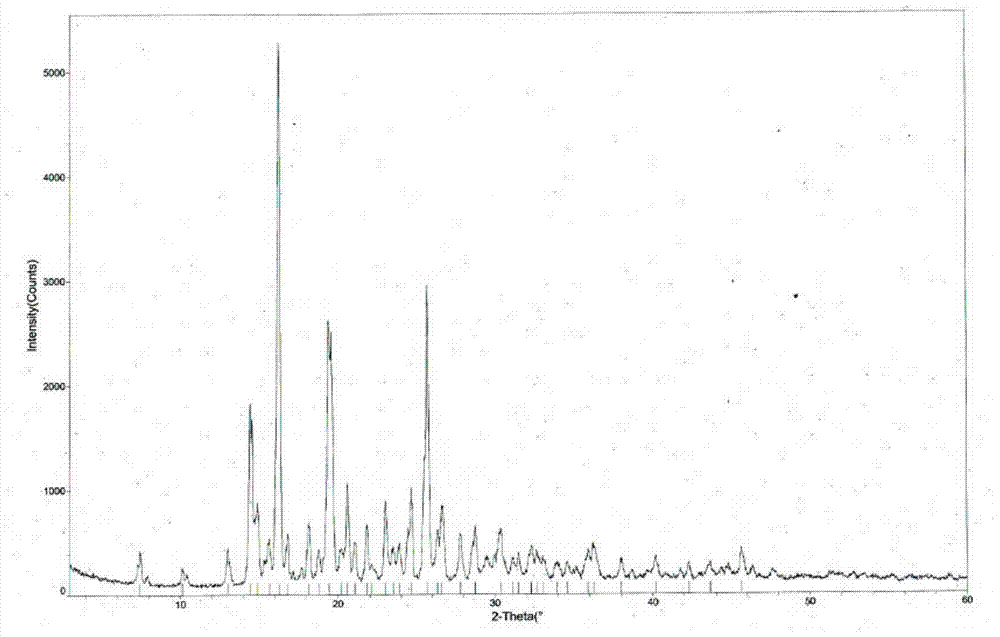
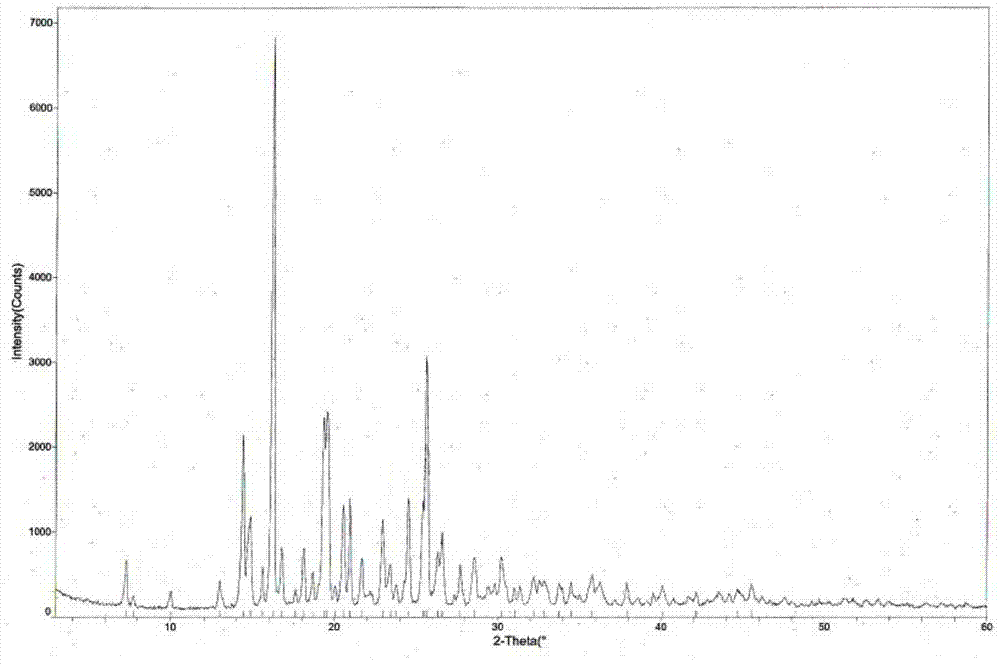
[0039] The measured melting point of tandospirone hydrochloride crystal form II is 225.5-226.5°C, and the X-ray powder diffraction pattern is shown in figure 1 (Using X’Pert Pro MPD Philips X-ray powder diffractometer to analyze the crystal phase of the sample, the radiation source Cu K α , graphite monochromator, tube voltage 40KV, tube current 35mA. ), the diffraction related data are shown in Table 1 (2θ measurement error is ±0.2)...
Embodiment 2
[0042] Example 2 Preparation method of tandospirone hydrochloride crystal form II
[0043] Weigh 2 kg of tandospirone, add 10 L of a mixed solution of tetrahydrofuran and acetonitrile (volume ratio 75:25), heat up to 60 °C, and after the dissolution is complete, add 3.9 L of 2 mol / L hydrochloric acid aqueous solution, stop heating, and naturally Cool to room temperature and stand for 5 hours, then stand at -5±5°C for 5 hours, filter with suction, wash and dry to obtain 2.05 kg of tandospirone hydrochloride crystal form II with a yield of 93.9%. The structural analysis results of the obtained product are not significantly different from those of Example 1.
Embodiment 3
[0044] Example 3 Preparation method of tandospirone hydrochloride crystal form II
[0045] Weigh 2 kg of tandospirone, add 30 L of a mixed solution of tetrahydrofuran and acetonitrile (volume ratio 50:50), heat up to 40 ° C, after the dissolution is complete, add 5 L of 2 mol / L hydrochloric acid aqueous solution, stop heating, and let it cool naturally Stand at room temperature for 3 hours, then place at -5±5°C for 8 hours, filter with suction, wash, and dry to obtain 2.03 kg of tandospirone hydrochloride Form II, with a yield of 92.9%. The structural analysis results of the obtained product are not significantly different from those of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com