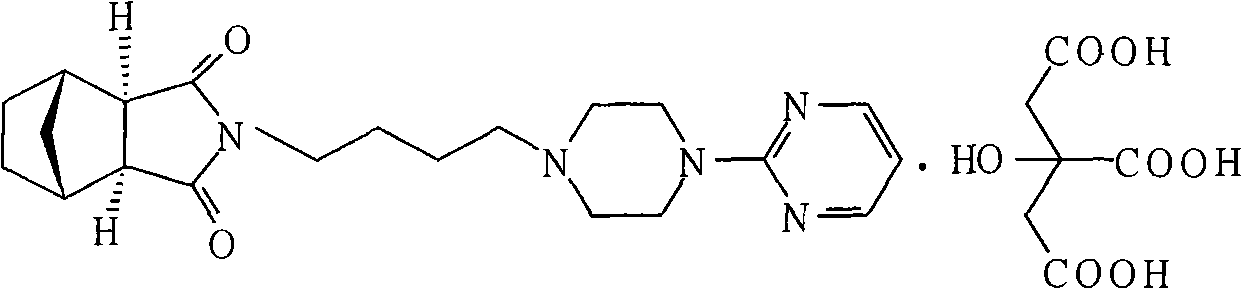
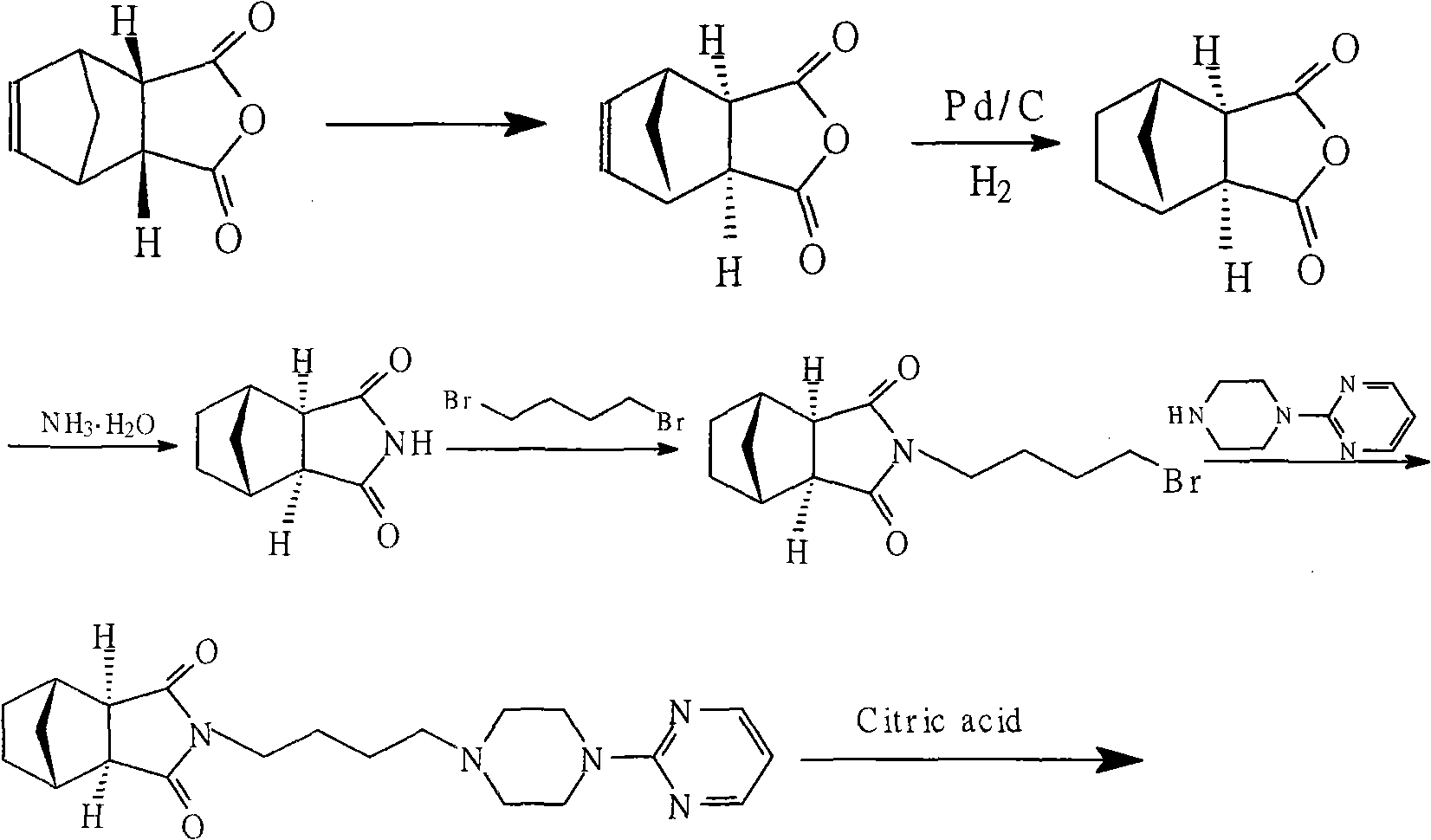
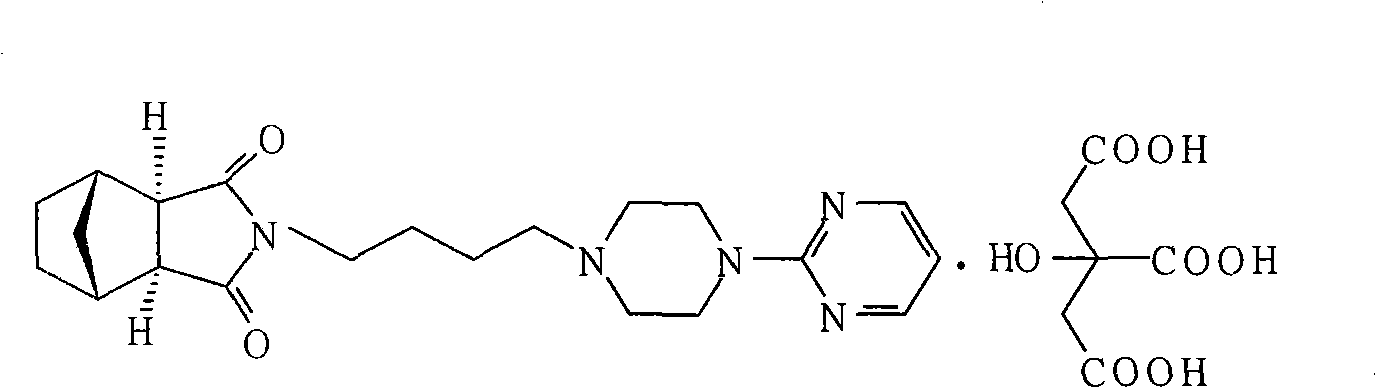
Method for preparing tandospirone and analogues of tandospirone
A technology for tandospirone and analogs, which is applied in the field of preparation of tandospirone citrate, can solve the problems of poor product quality, difficulty in purifying intermediates, and difficulty in meeting medicinal requirements, and achieves low cost and good quality. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of exo-cis-bicyclo[2,2,1]heptane-5-ene-2,3-dicarboxylic anhydride In a reaction flask, add 300 grams of NA anhydride, 2100 ml of ethyl acetate, and heat to Reflux, under the irradiation of sodium lamp 500W, react for 2 hours, evaporate the solvent, and refine exo-cis-bicyclo[2,2,1]heptane-5-ene-2,3-dicarboxylic acid anhydride 274.2 grams, received Rate 91.4%, mp 141.0-142.5°C.
Embodiment 2
[0023] Example 2: N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-exo-cis-bicyclo[2,2,1]heptane-5-ene-2, Preparation of 3-dicarboximide (abbreviated as condensate, the same below)
[0024] In the reaction flask, 200 grams of exo-cis-bicyclo[2,2,1]heptane-5-ene-2,3-dicarboxylic anhydride, 1-(4-aminobutyl)-4-(2 -320 grams of pyrimidinyl) piperazine, 1600 ml of toluene, 320 grams of sodium methoxide, reacted at 110 ° C for 10 hours, evaporated the solvent, and purified 425.8 grams with ethyl acetate, yield 91.6%, mp 110.5~111.8 ° C, HPLC 98.7 %.
Embodiment 3
[0025] Embodiment 3: the preparation of condensate
[0026] In the reaction flask, add 80 grams of exo-cis-bicyclo[2,2,1]heptane-5-ene-2,3-dicarboxylic anhydride, 1-(4-aminobutyl)-4-(2- Pyrimidinyl)piperazine 160g, DMF700ml, sodium carbonate 120g, reacted at 140°C for 7 hours, evaporated the solvent, and purified with ethyl acetate to obtain 176.8g, yield 95.1%, mp 110.0~112.0°C, HPLC 99.1% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com