A kind of tandospirone pharmaceutical composition and its preparation method and use
A technology for tandospirenone and composition, applied in the field of tandospirenone pharmaceutical composition and its preparation and use, can solve the problems of large fluctuation of blood drug concentration, poor drug compliance, large drug side effects and the like, and achieves blood drug concentration The effect of less fluctuation, less side effects and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-20
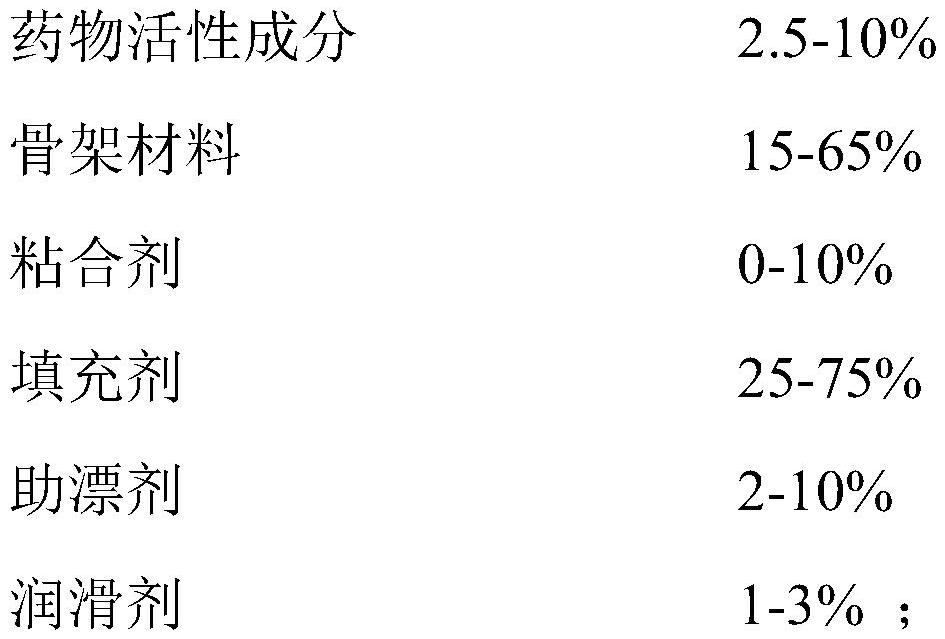
[0070] The tandospirone pharmaceutical composition was prepared according to the components and proportions shown in Tables 1-3.
[0071] Table 1. Tandospirone Pharmaceutical Compositions - Examples 1-8
[0072]
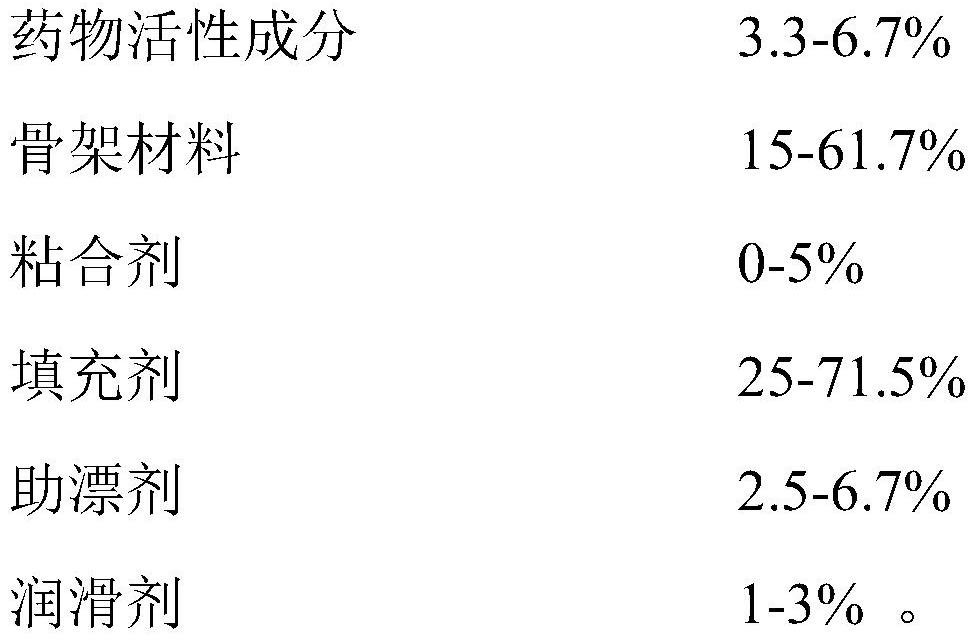
[0073] Table 2. Tandospirone Pharmaceutical Compositions - Examples 9-14
[0074]
[0075] Table 3. Tandospirone Pharmaceutical Compositions - Examples 15-20
[0076]
[0077]
Embodiment 1-7
[0078] For embodiment 1-7, 9-12 and 15-18, its preparation process is as follows:
[0079] A, magnesium stearate is crossed 60 mesh sieves, each component except magnesium stearate is crossed 20 mesh sieves respectively, get Tandospirone citrate, skeleton material, adhesive and filler, mix 15 minutes ;
[0080] b. Add bleaching aid and mix for 10 minutes; then add magnesium stearate and mix for 3 minutes to obtain mixed powder;
[0081] c. Use a punch of 16.4 mm×7.9 mm to directly compress the mixed powder obtained in step b to obtain a tablet.
Embodiment 8
[0082] For embodiment 8, 13-14 and 19-20, its preparation process is as follows:
[0083] a. Pass the tandospirone citrate, the framework material, the binder and the filler through a 20-mesh sieve, respectively, and mix for 10 minutes; add water to prepare a soft material, and dry the wet granules at 50 ° C and 35 cfm for 15 minutes, and the whole The granules are passed through a 20-mesh sieve to obtain dry granules;
[0084] b, the bleaching aid is crossed through a 20-mesh sieve, and mixed with the dry granules obtained in step a for 5 minutes; then add magnesium stearate (cross a 60-mesh sieve) and mix for 3 minutes to obtain mixed granules;
[0085] c. Use a punch of 16.4 mm×7.9 mm to compress the mixed granules obtained in step b to obtain a tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com