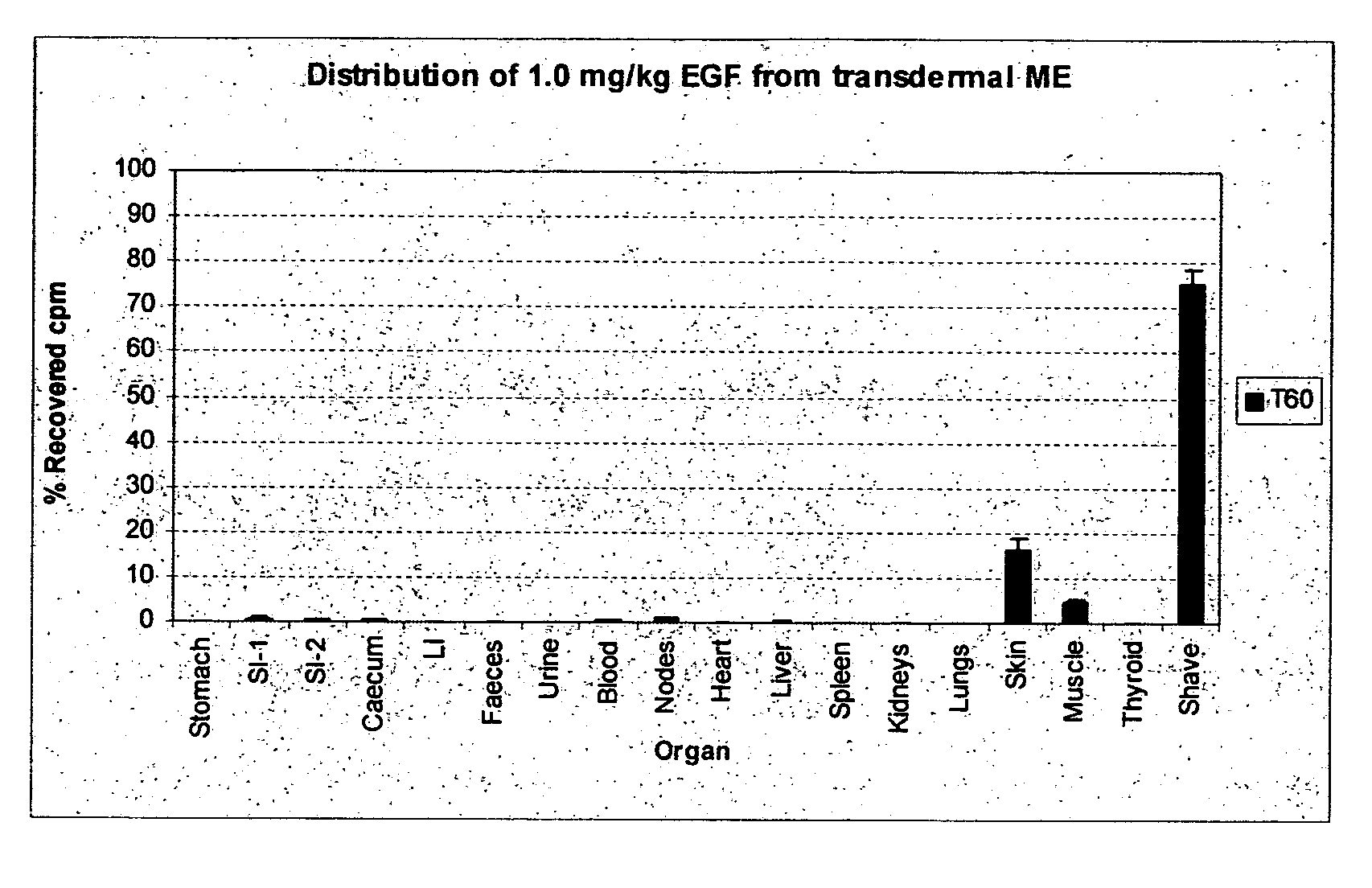
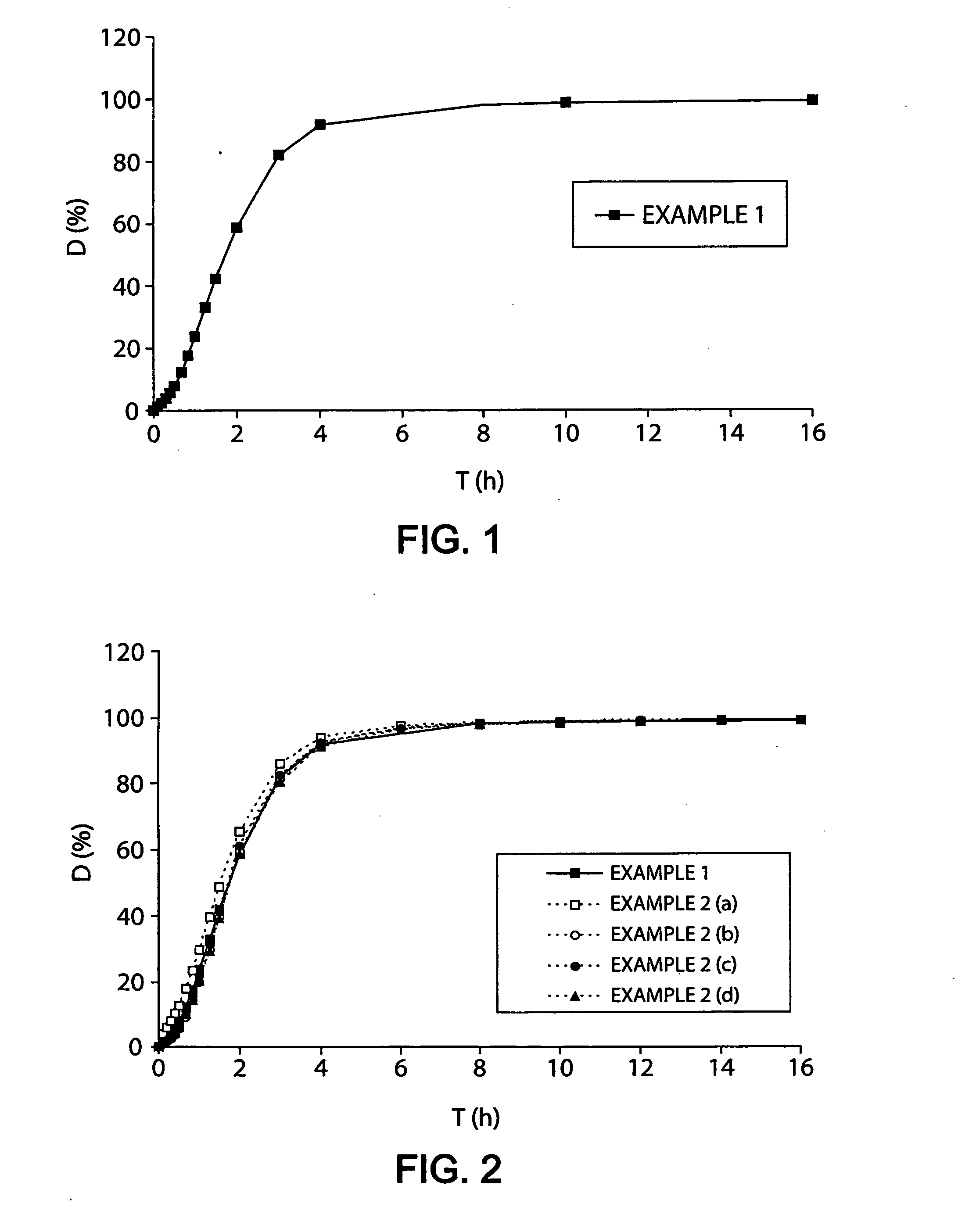
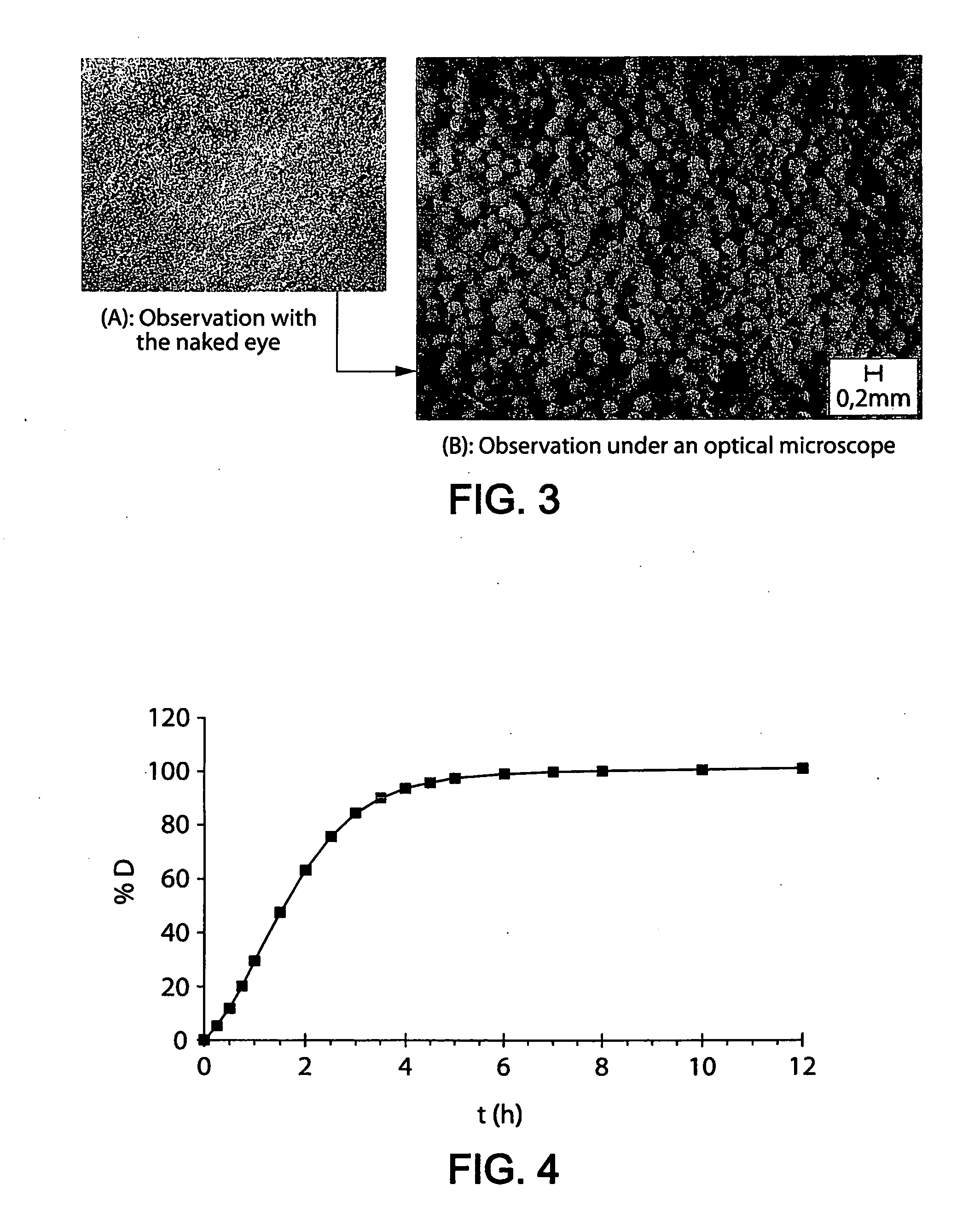
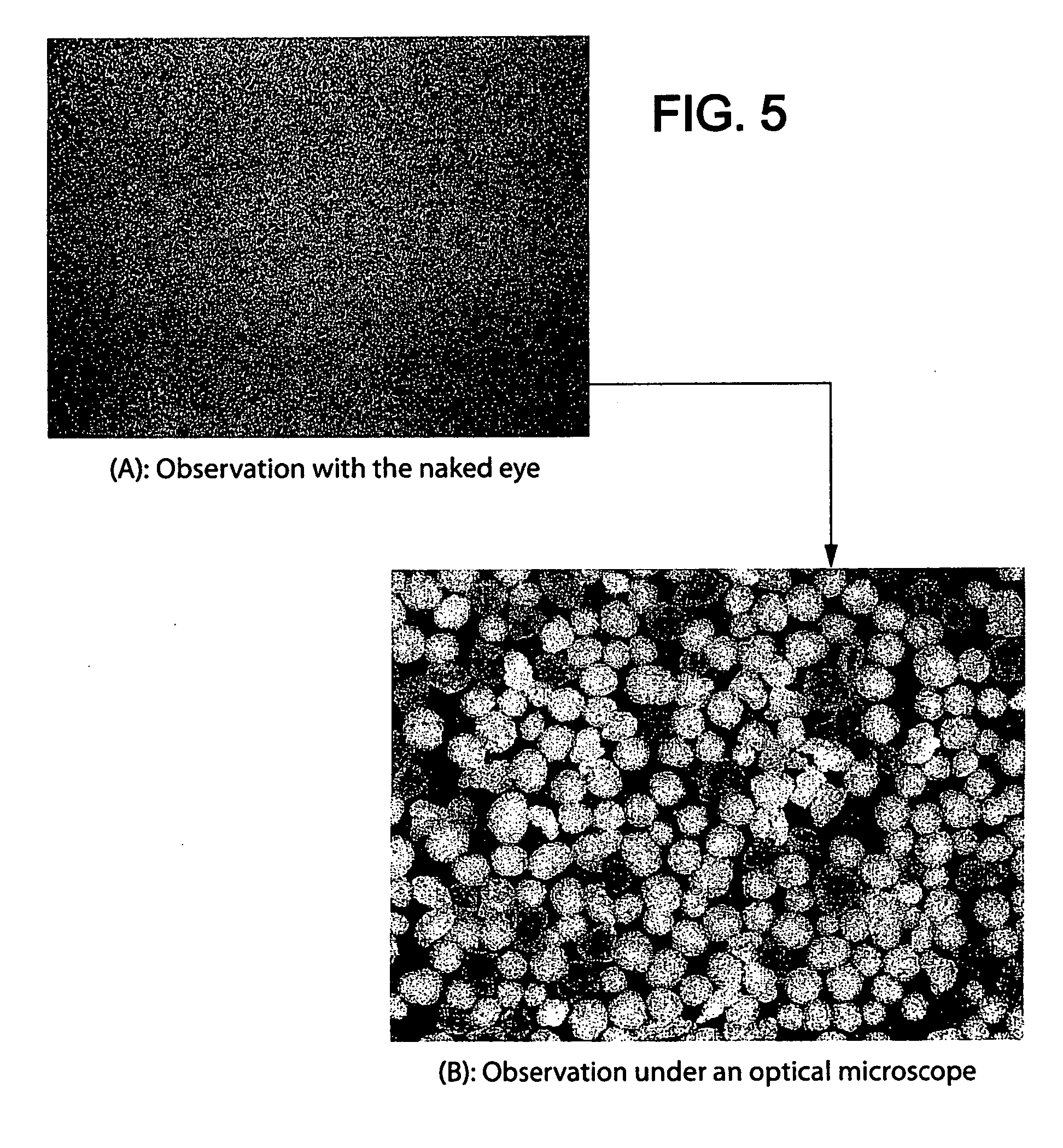
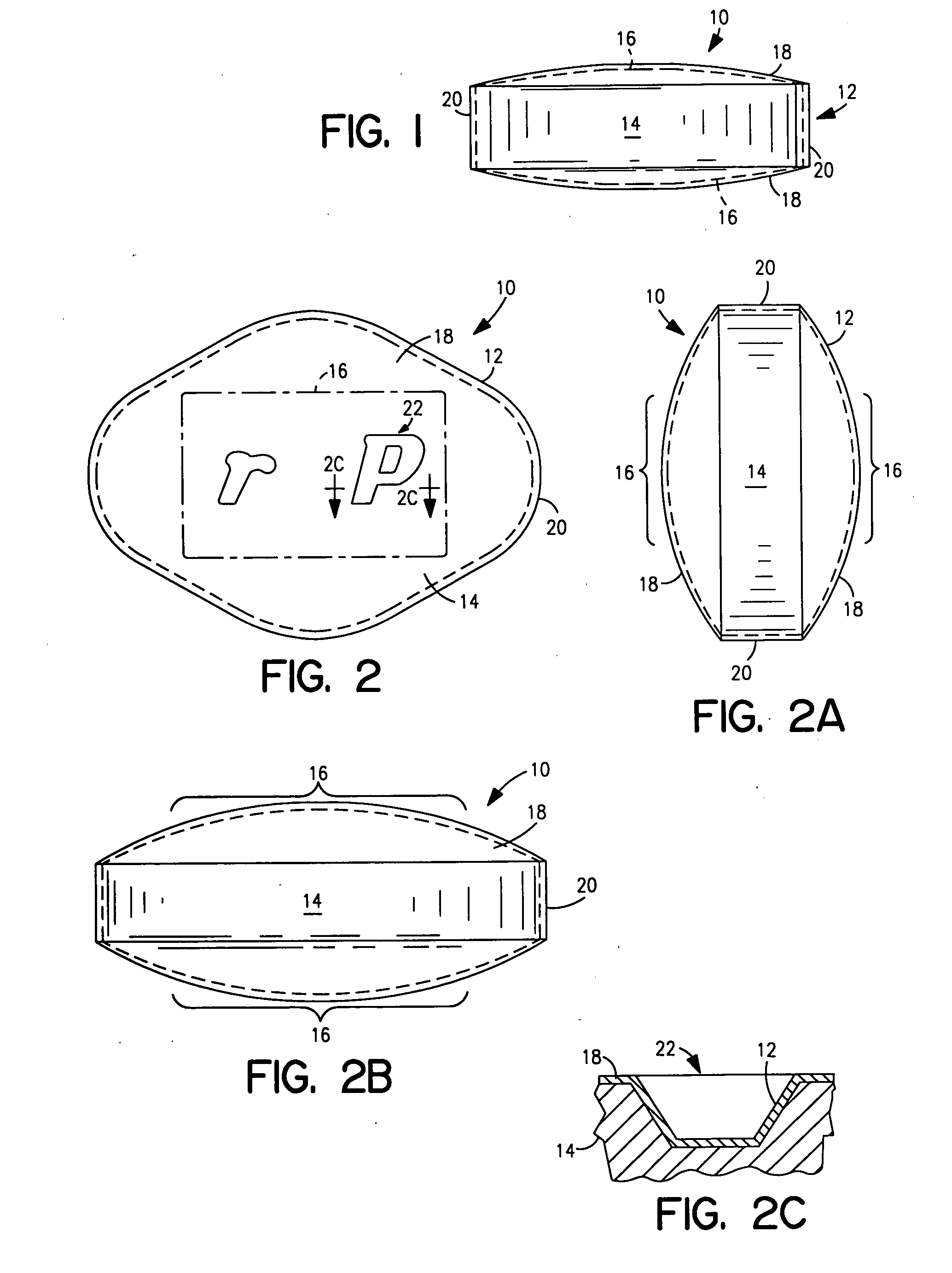
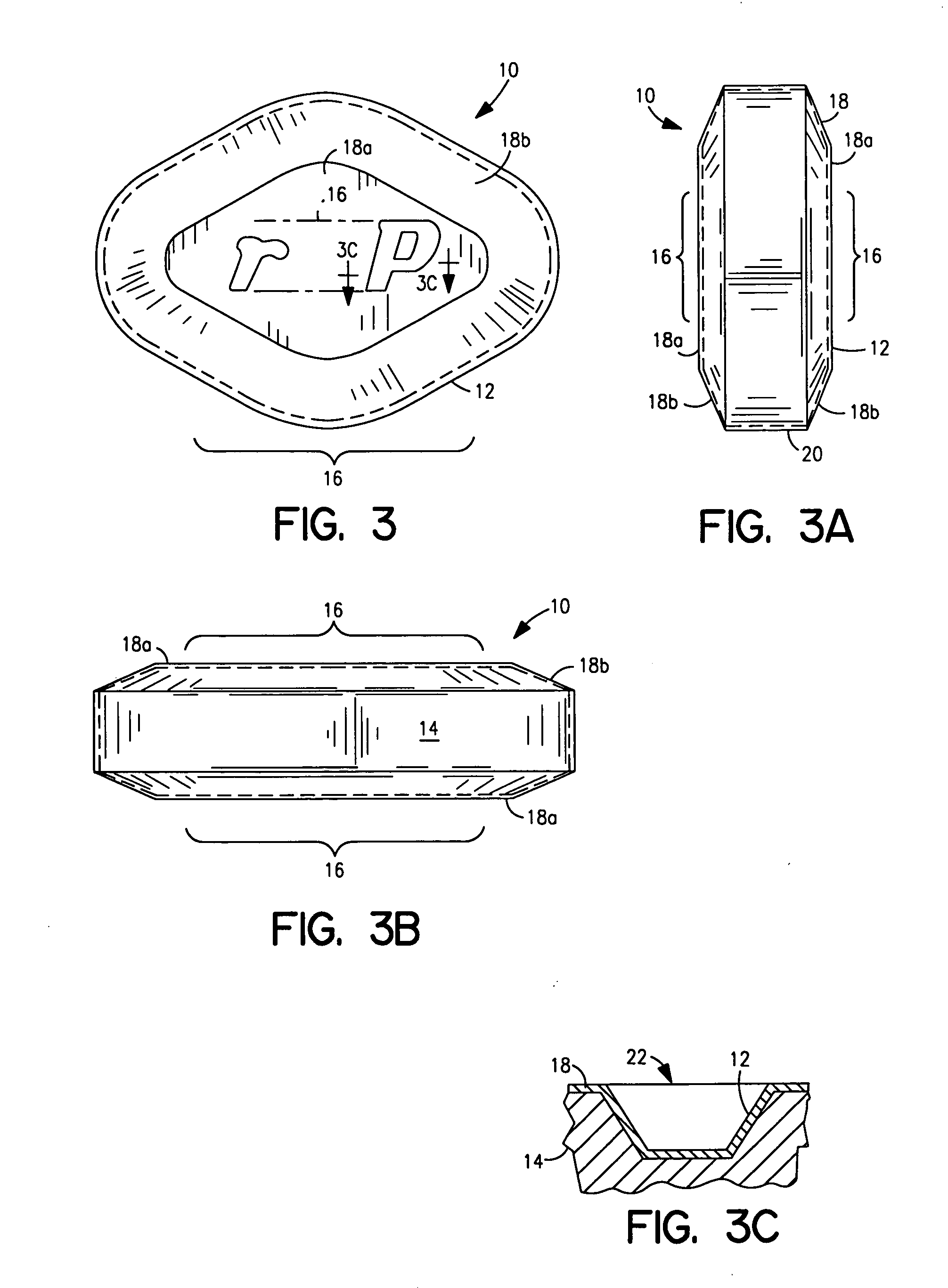
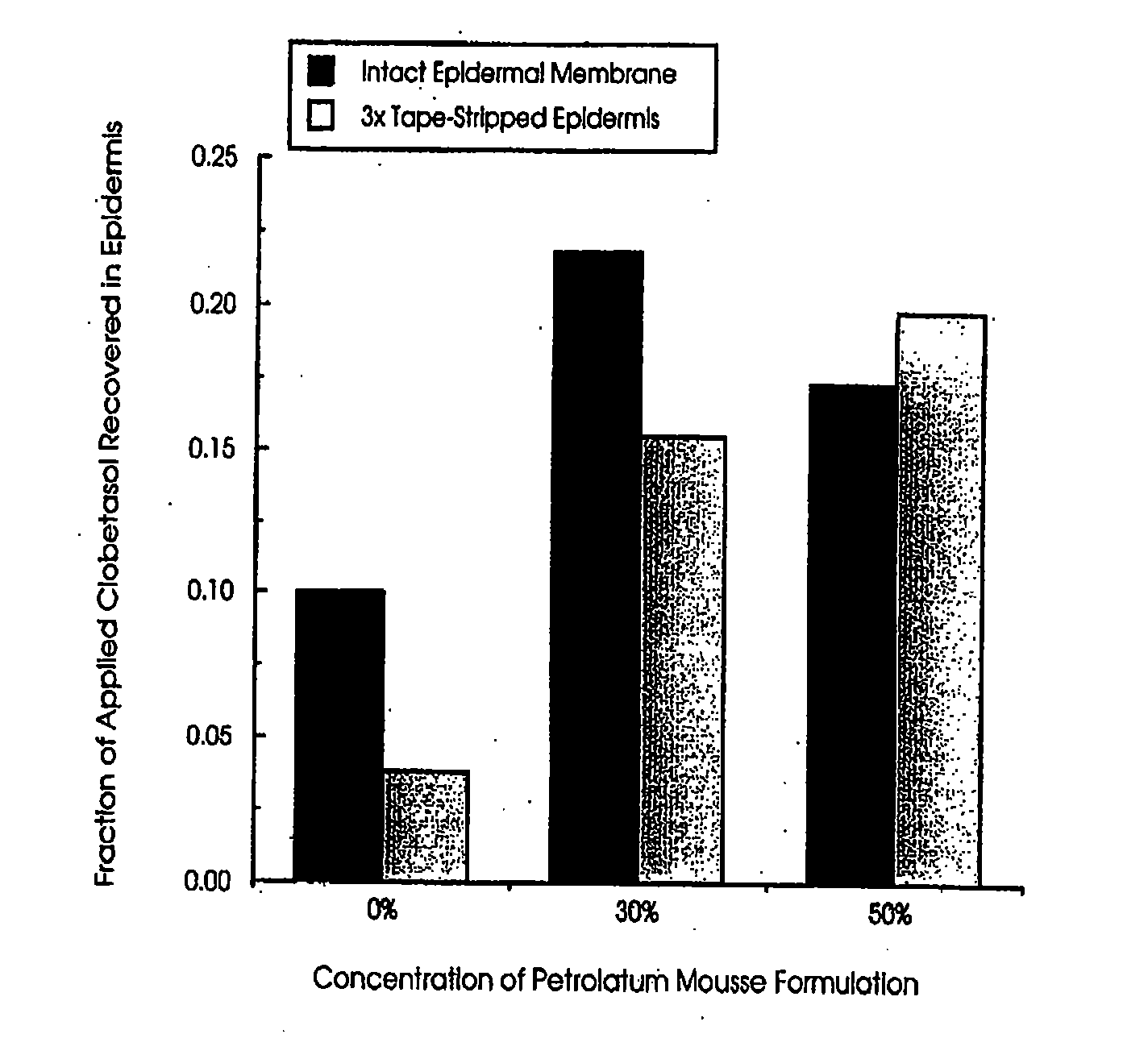
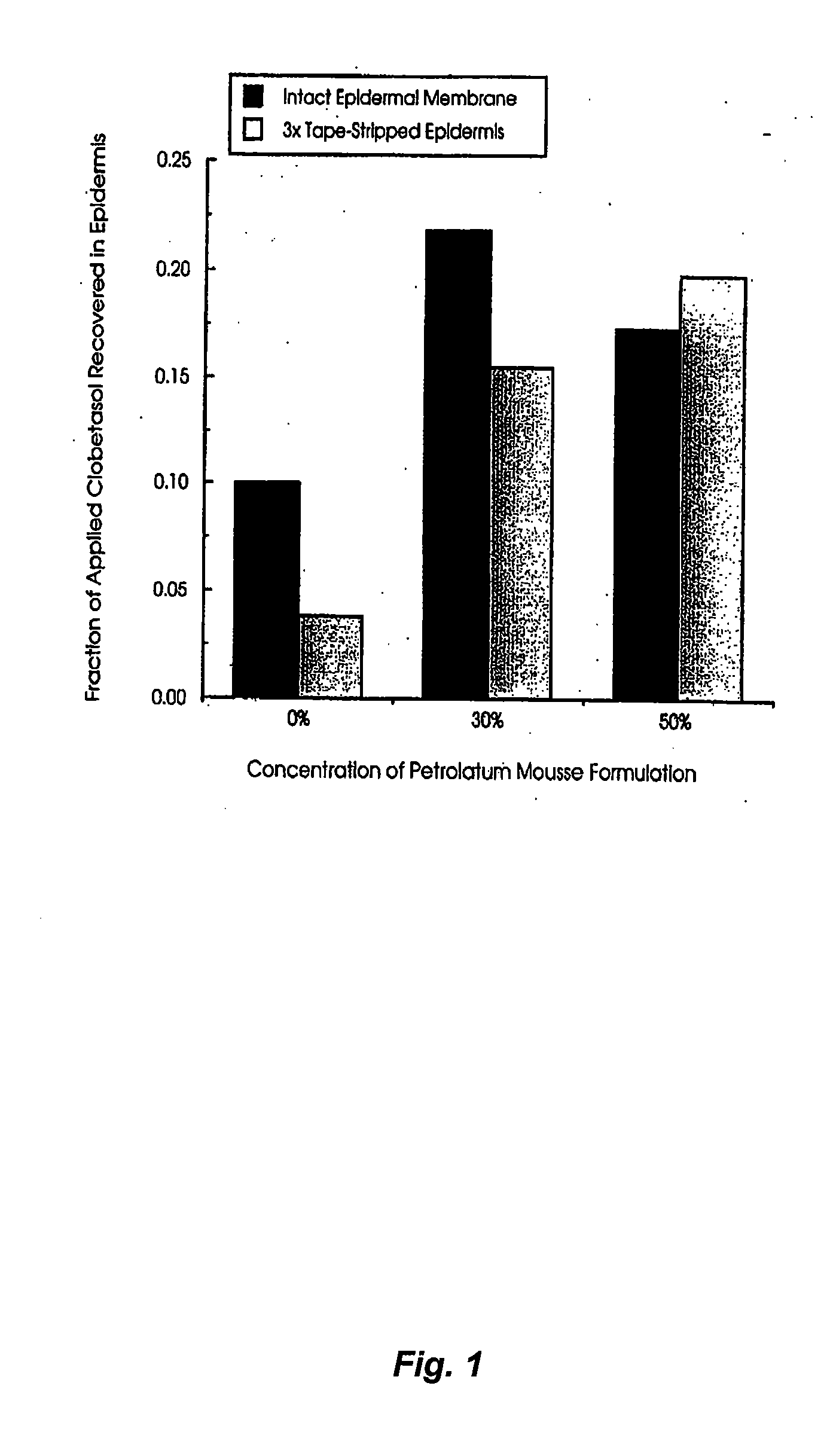
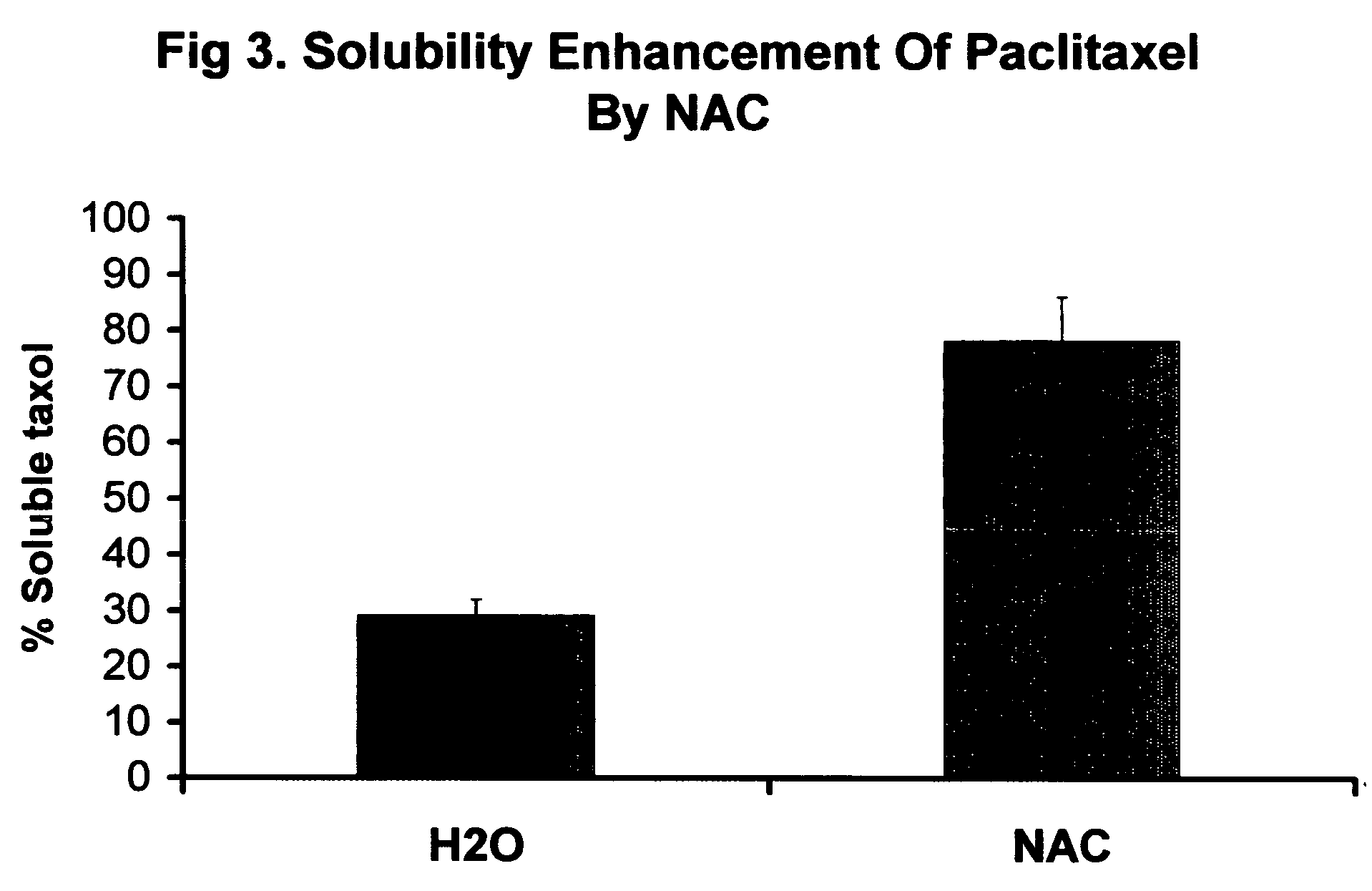
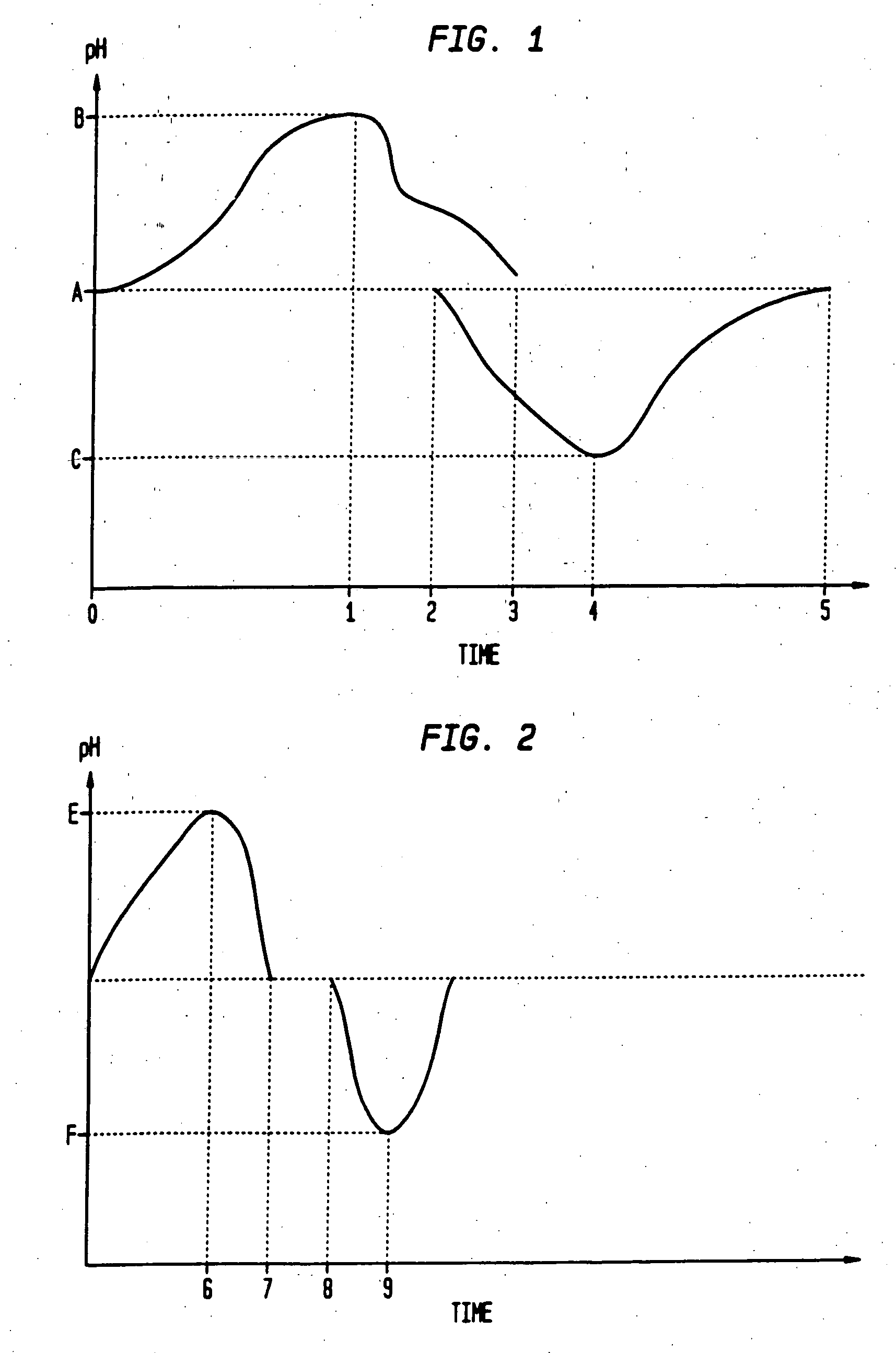
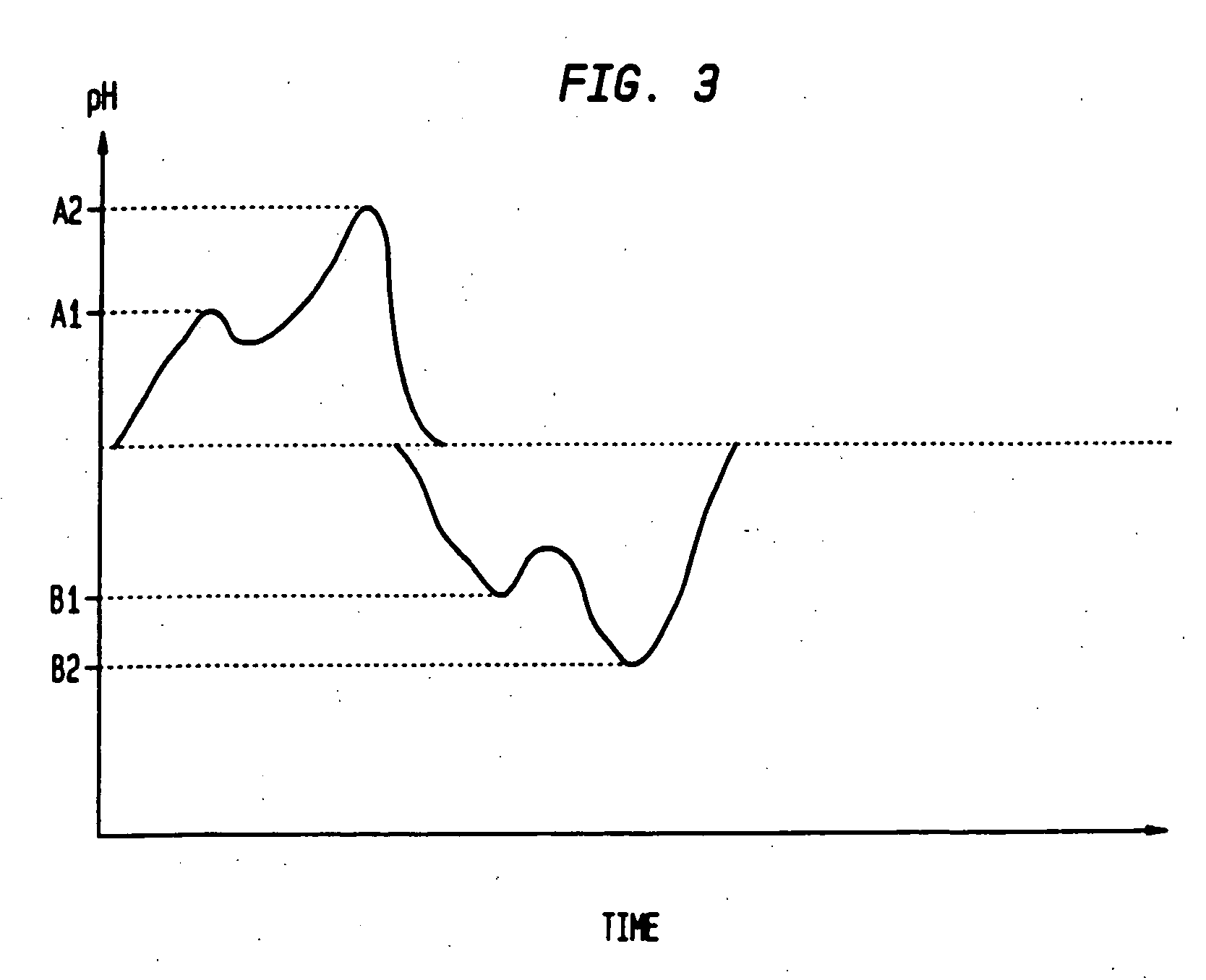
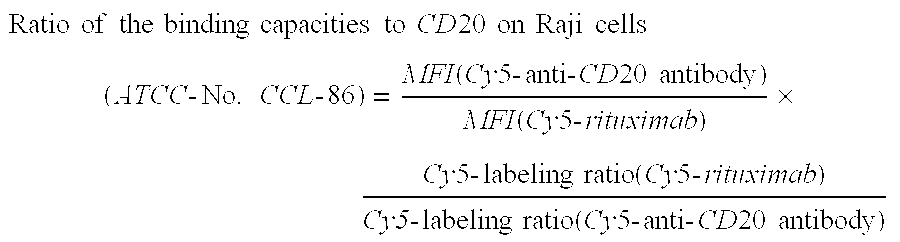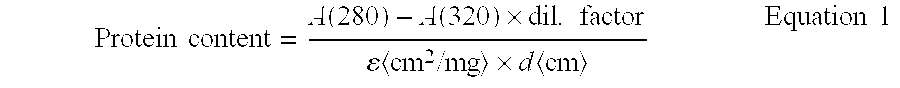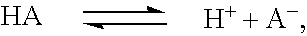Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1077 results about "Drug activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
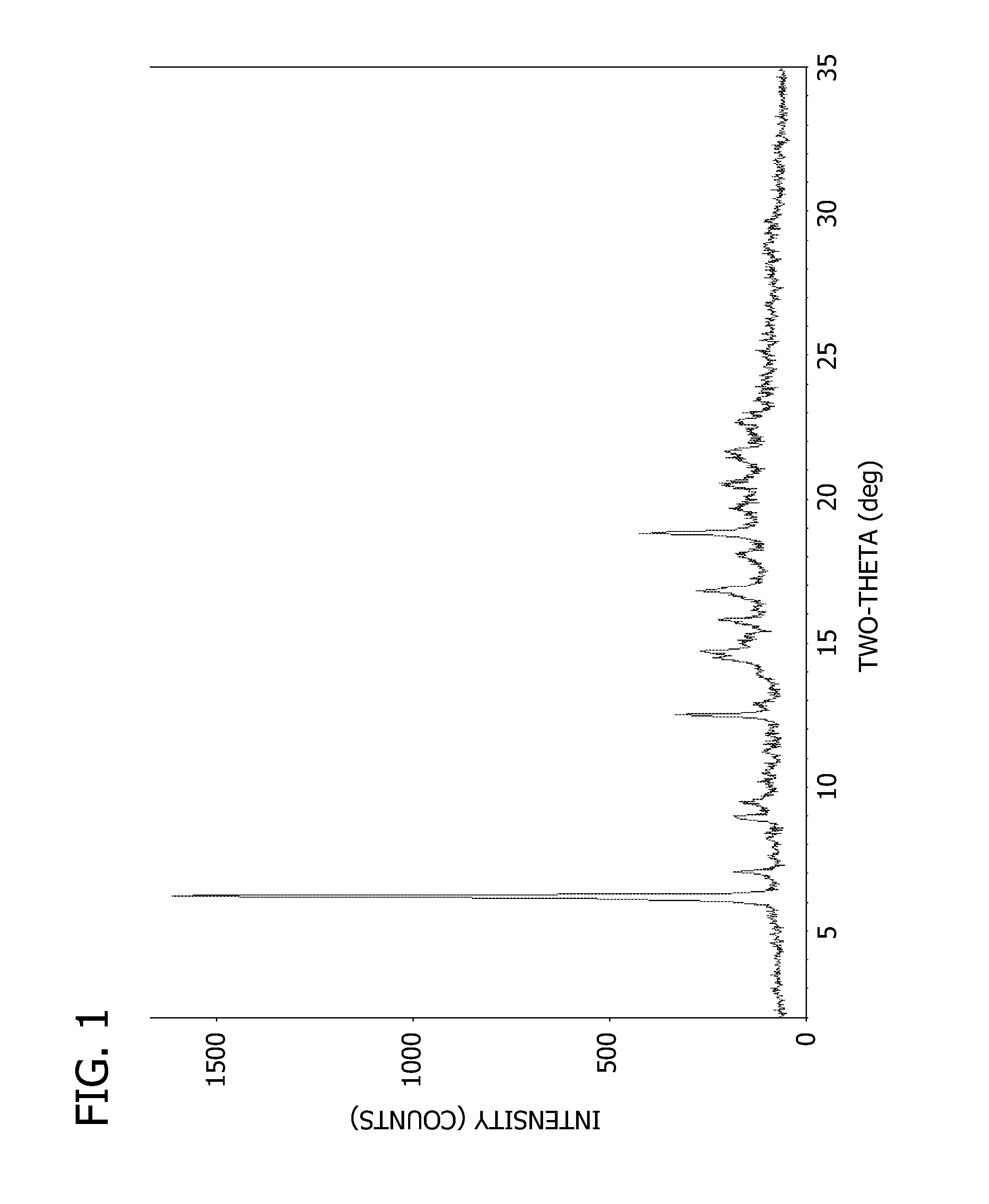
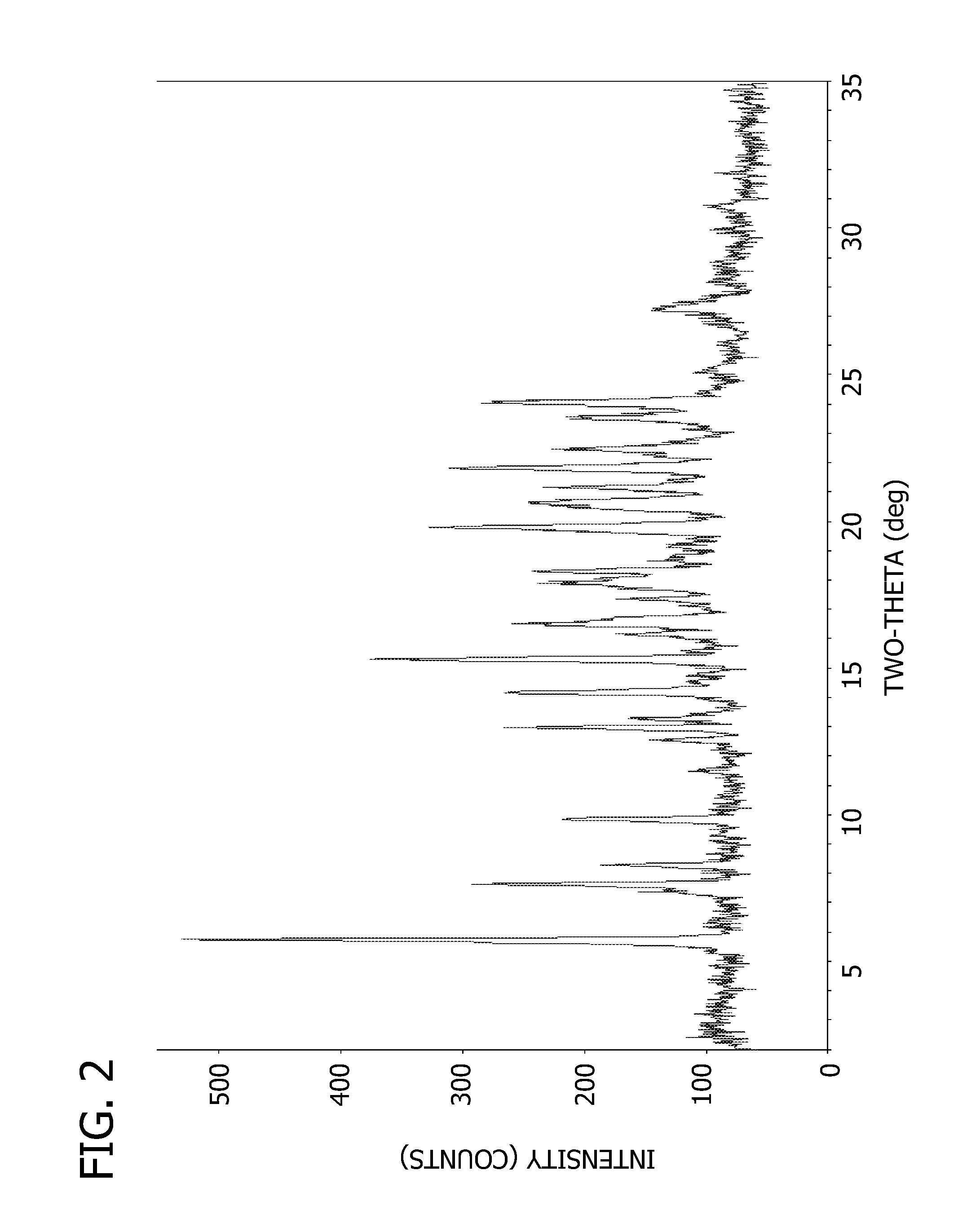
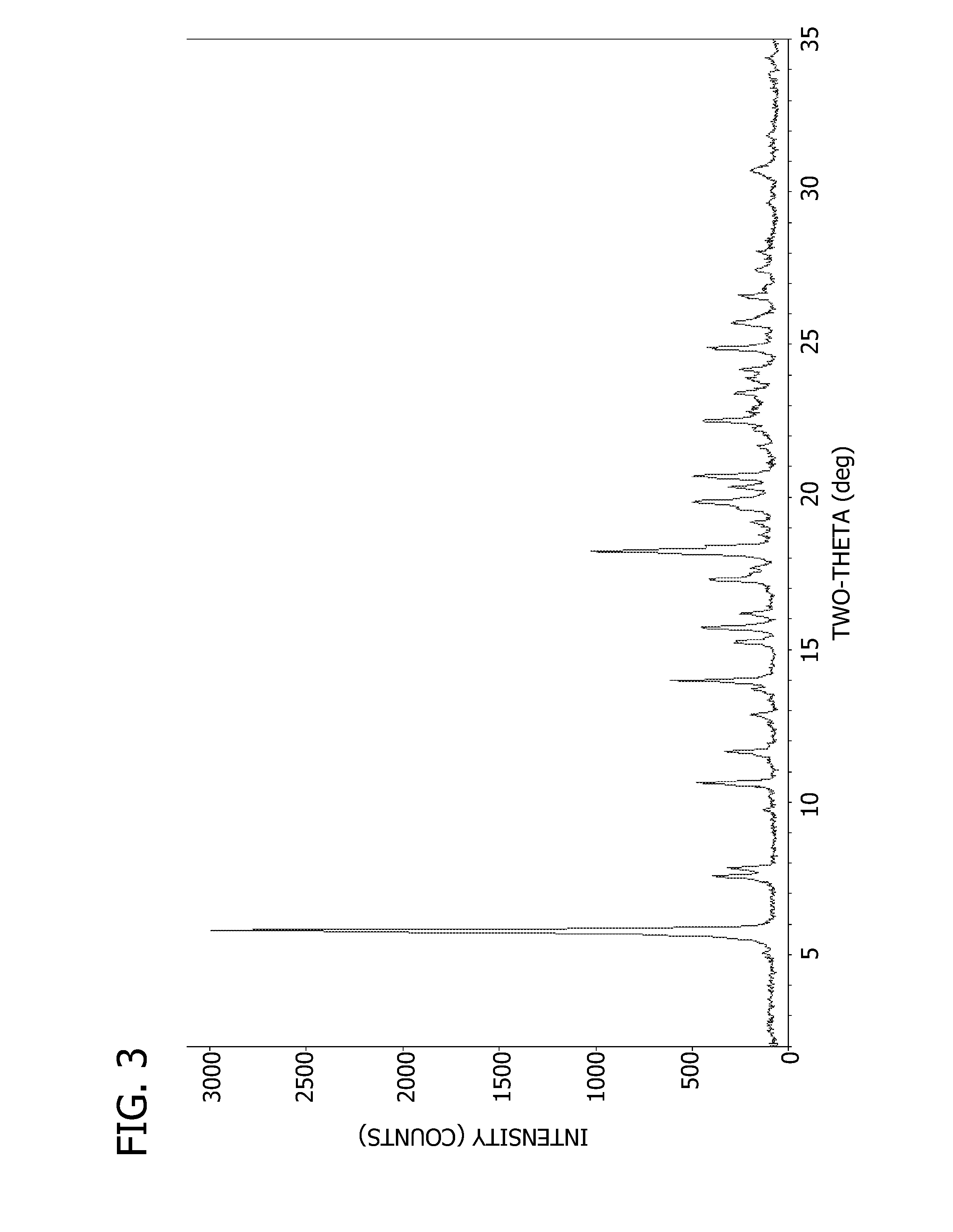
Drug activity: A measure of the physiological response that a drug produces. A less active drug produces less response, and a more active drug produces more response. CONTINUE SCROLLING OR CLICK HERE FOR RELATED ARTICLE. Reviewed on 12/4/2018.
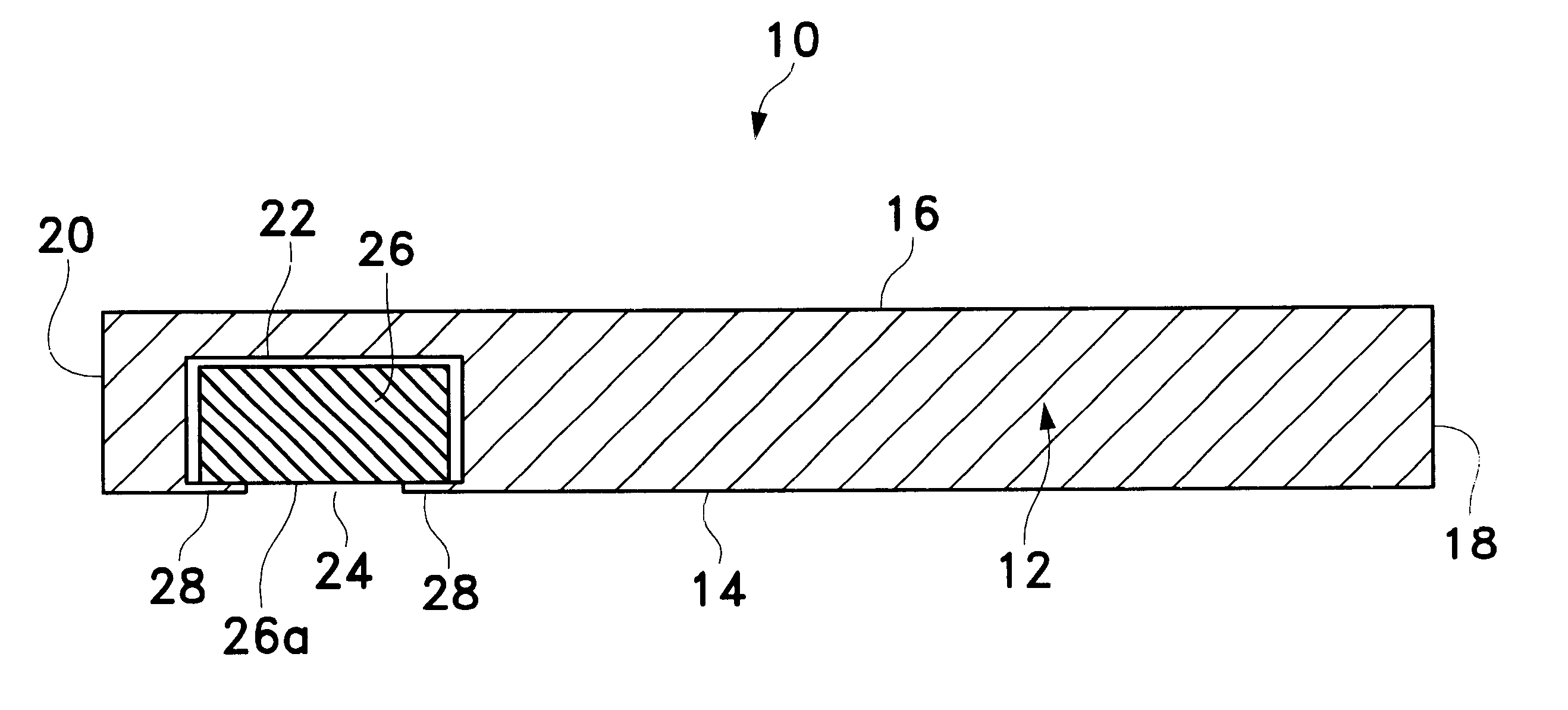
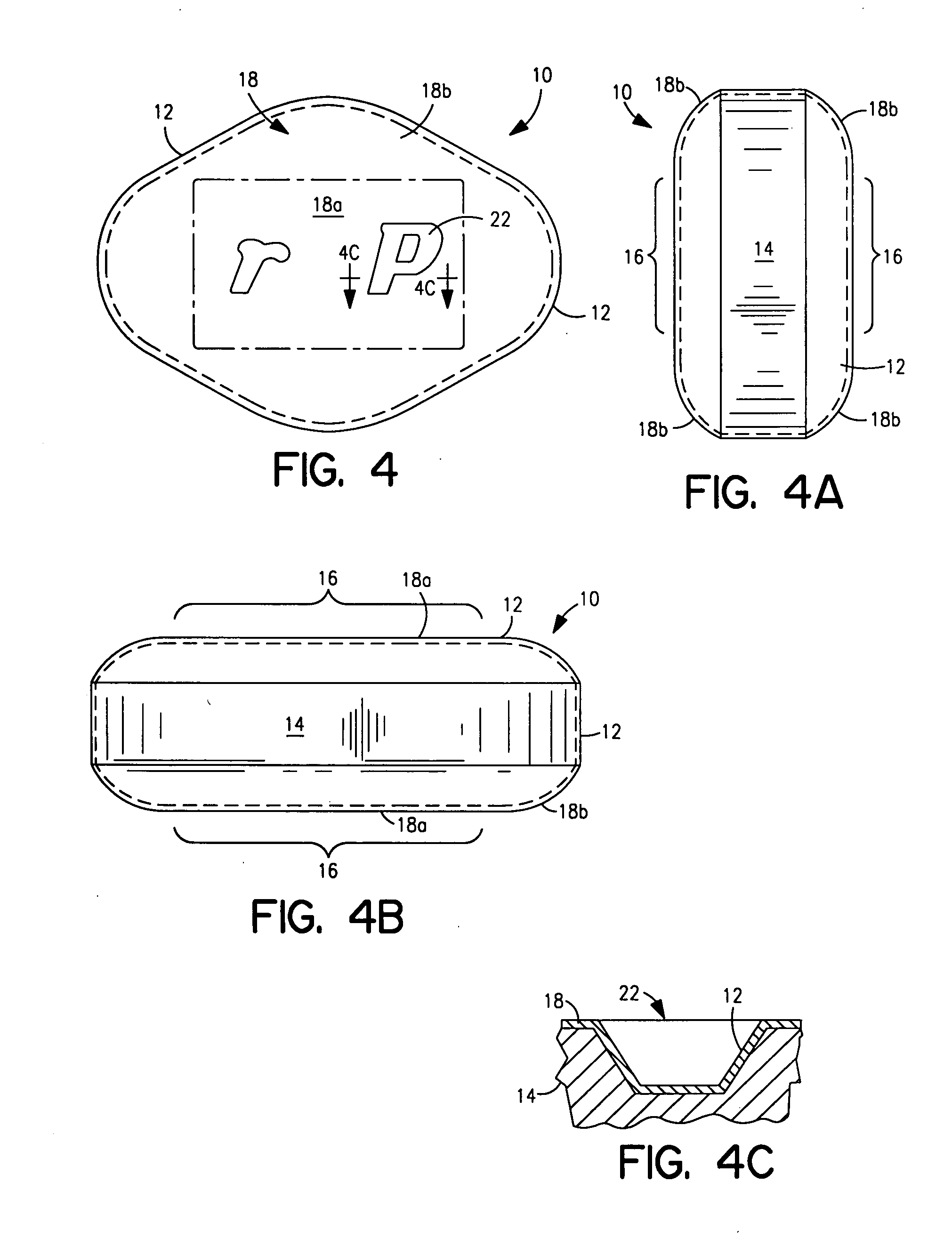
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
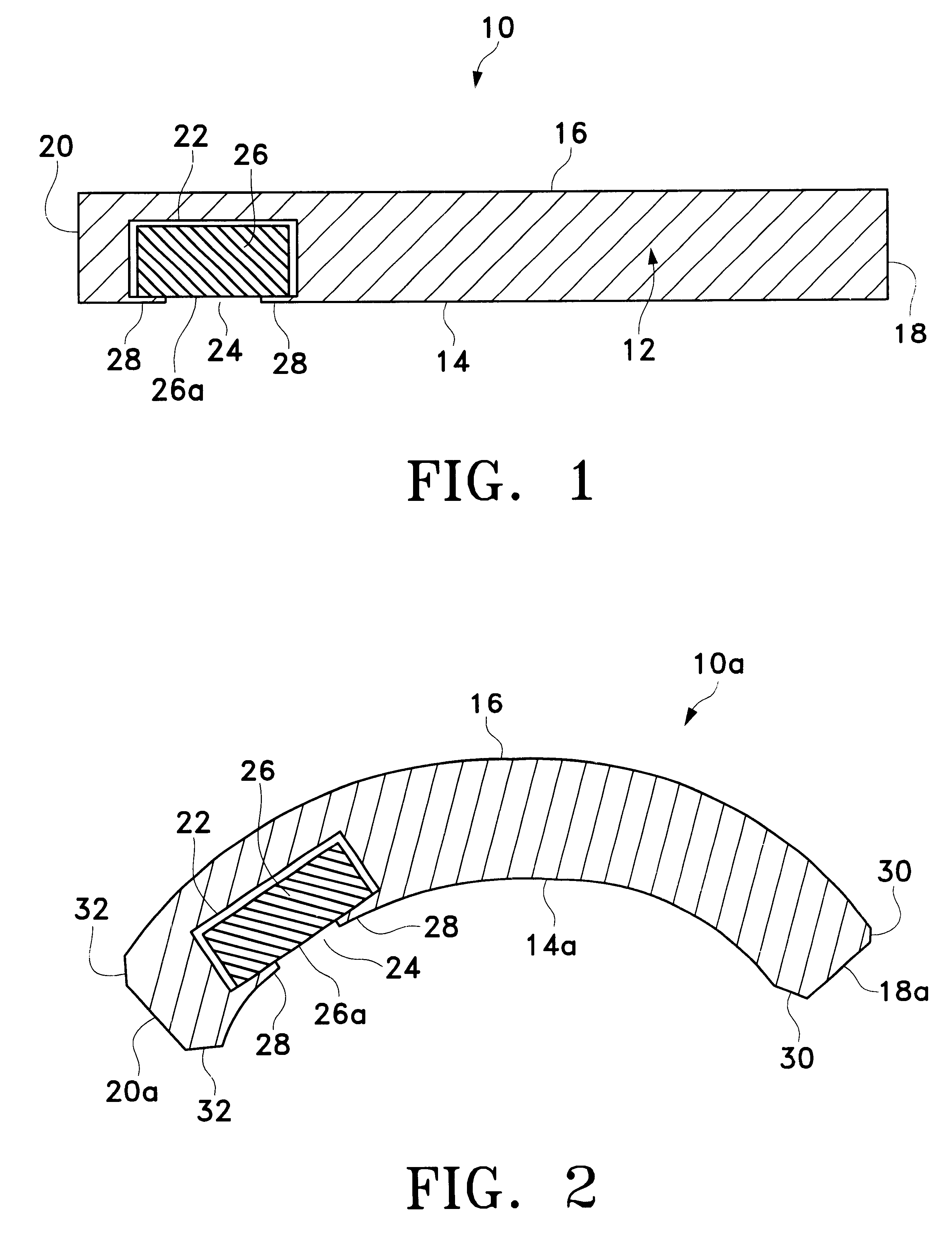
Drug delivery device
Drug delivery devices, and methods of delivering pharmaceutically active agents to a target tissue within a body using such devices, are disclosed. One drug delivery device includes a body having an internal surface for placement proximate a target tissue and a well having an opening to the internal surface. An inner core comprising a pharmaceutically active agent is disposed in the well.
Owner:NOVARTIS AG
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Transdermal delivery of pharmaceutical agents
InactiveUS20070243132A1Improve permeabilityEfficient use ofAntibacterial agentsOrganic active ingredientsActive agentGenetic molecular
The present invention generally relates to a vehicle useful for delivering a pharmaceutically active compound including a genetic molecule or composition. More particularly, the present invention provides microemulsions for transdermal delivery of pharmaceutically active agents to a subject.
Owner:APOLLO LIFE SCI
Anti-misuse microparticulate oral pharmaceutical form
InactiveUS20070224129A1Avoid misusePrevent misuse by short liquid extraction and/or crushingOrganic active ingredientsDrug compositionsPublic healthMicroparticle
The present invention relates to solid microparticulate oral pharmaceutical forms whose composition and structure make it possible to avoid misuse of the pharmaceutical active principle (AP) they contain. The object of the present invention is to prevent solid oral drugs from being misappropriated for any use other than the therapeutic use(s) officially approved by the competent public health authorities. In other words, the object is to avoid the voluntary or involuntary misuse of solid oral drugs. The invention relates to a solid oral pharmaceutical form which is characterized in that it contains anti-misuse means, in that at least part of the AP it comprises is contained in coated microparticles for modified release of the AP, and in that the coated microparticles of AP have a coating layer (Ra) which assures the modified release of the AP and simultaneously imparts crushing resistance to the coated microparticles of AP so as to avoid misuse.
Owner:FLAMEL IRELAND
Edible holographic products, particularly pharmaceuticals and methods and apparatus for producing same
InactiveUS20060068006A1Preserve product integrityCheap productionPretreated surfacesDiffraction gratingsCoated tabletsPlasticizer
An edible product such as a unit dosage form of a pharmaceutically active substance includes a layer of a material that can receive and retain a high resolution microrelief that can convey information. The microrelief is themo-formable, preferably formed from an aqueous solution of HPMC and / or HPC plus a plasticizer and colorant. Other additives such as strengtheners, surfactants and adherents may be used depending on the application. The materials are selected and proportioned to control the fading or change in color of the visual image or effect produced by the relief to indicate exposure to an unacceptable degree of heat or humidity. The dosage form can be the relief-containing layer itself with the pharmaceutical carried therein. In a preferred form, the layer is an outer coating over a core containing the pharmaceutically active substance. Coated tablets are configured to resist twinning. To produce such dosage forms, the coated core is transported in unison with a flexible mold or transfer plate that can heat-replicate the microrelief on the outer layer of the dosage form, followed by a cooling and release of the transfer plate from the coating.
Owner:EDWARDS ANGELL PALMER & DODGE
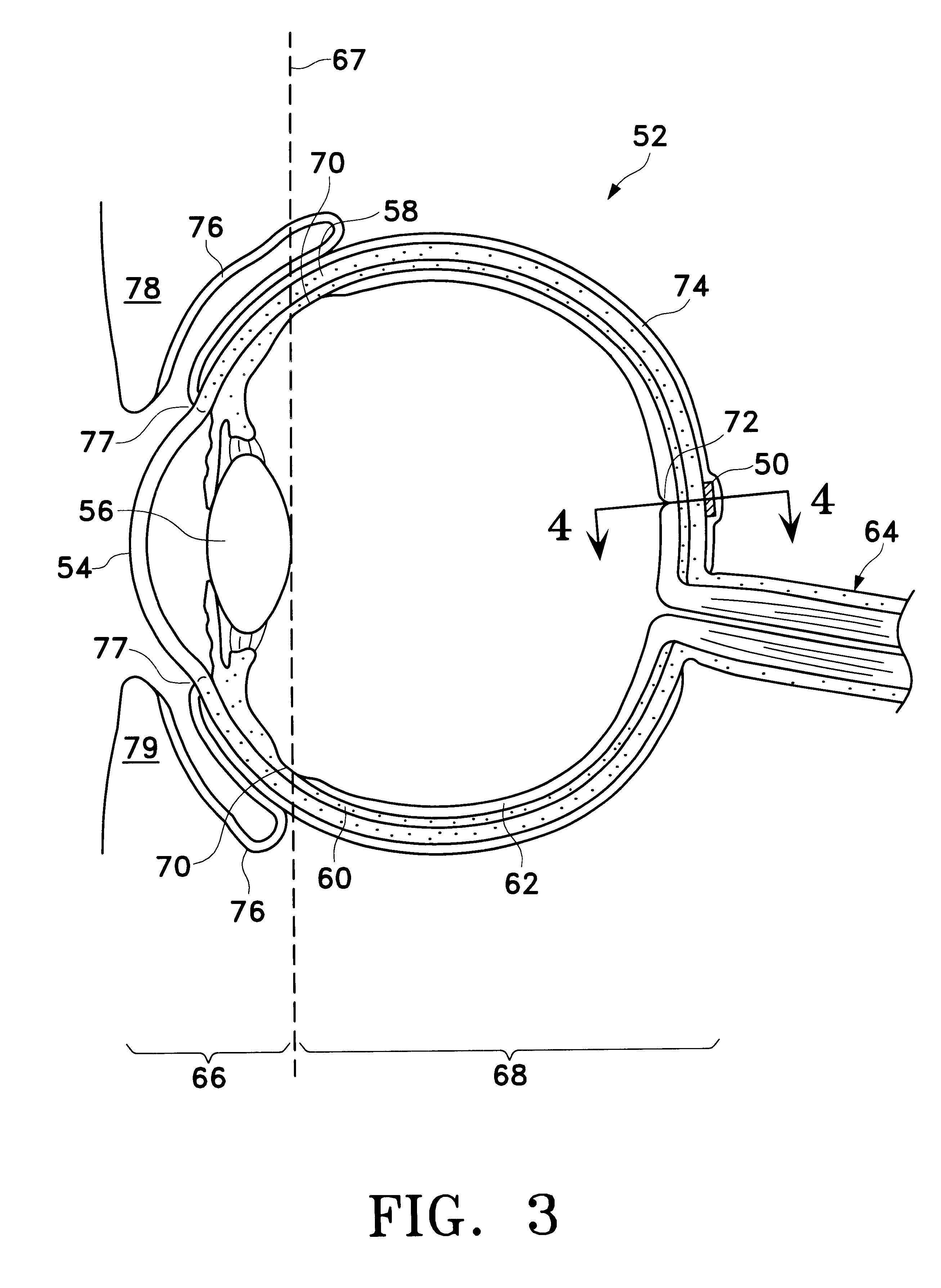
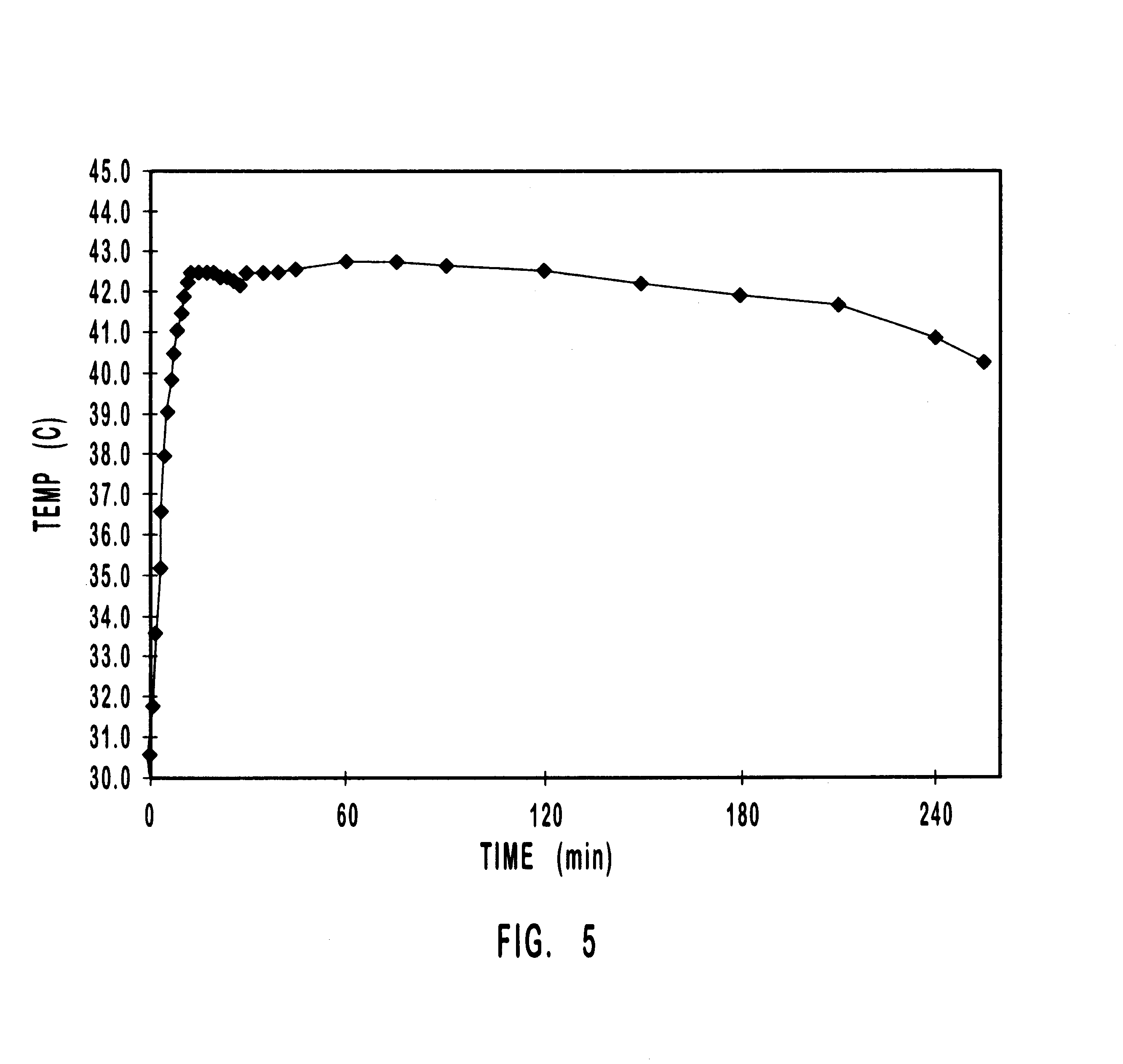
Apparatus for heating to a desired temperature for improved administration of pharmaceutically active compounds
Methods and apparatus for improving administration of drugs through the use of heat and other physical means. The present invention relates to the use of heat and other physical means in conjunction with specially designed dermal drug delivery systems, conventional commercial dermal drug delivery systems, or drugs delivered into a sub-skin depot site via injection and other methods to alter, mainly increase, the drug release rate from the dermal drug delivery systems or the depot sites to accommodate certain clinical needs.
Owner:NUVO RES
Highly Concentrated Pharmaceutical Formulations
InactiveUS20110076273A1Peptide/protein ingredientsAntibody ingredientsAntibody moleculeSubcutaneous injection
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-CD20 antibody, such as e.g. Rituximab, Ocrelizumab, or HuMab<CD20>, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-CD20 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The said formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Vitamin formulation
A pharmaceutical aerosol foam composition, comprising: an effective amount of a pharmaceutically active ingredient, wherein said pharmaceutically active ingredient is a vitamin or analogue thereof; an occlusive agent; an aqueous solvent; an organic cosolvent; wherein the pharmaceutically active ingredient is insoluble in both water and the occlusive agent; and the occlusive agent being present in an amount sufficient to form an occlusive layer on the skin, in use. In a second embodiment, an oil-in water emulsion having a vitamin, an occlusive agent; an aqueous solvent; and an organic cosolvent, wherein the occlusive agent is present in an amount sufficient to form an occlusive layer on the skin.
Owner:STIEFEL WEST COAST
Method for improving the bioavailability of orally delivered therapeutics
The disclosed invention is a method and composition for improving the bioavailability of a pharmaceutically active ingredient comprising an oral dosage form consisting essentially of a granulation of active ingredient, amino acid, and hydrophilic polymer, wherein the granulation is dispersed in an immediate release or extended release excipient.
Owner:SCOLR PHARMA
Methods and devices for the sustained release of multiple drugs
ActiveUS20070196433A1Easy to produceSustained releaseOrganic active ingredientsPowder deliveryPost menopausal periodPost menopausal
The invention relates to an drug delivery device and a method for delivering multiple drugs over a prolonged period of time. The drug delivery device has two or more unitary segments comprising a drug-permeable polymeric substance, wherein at least one of the segments further comprises a pharmaceutically active agent. The invention also relates to a method for the treatment of a benign ovarian secretory disorder in a female mammal, a method of contraception, and a method of relieving the symptoms associated with menopausal, perimenopausal and post-menopausal periods in a woman.
Owner:MASSACHUSETTS INST OF TECH
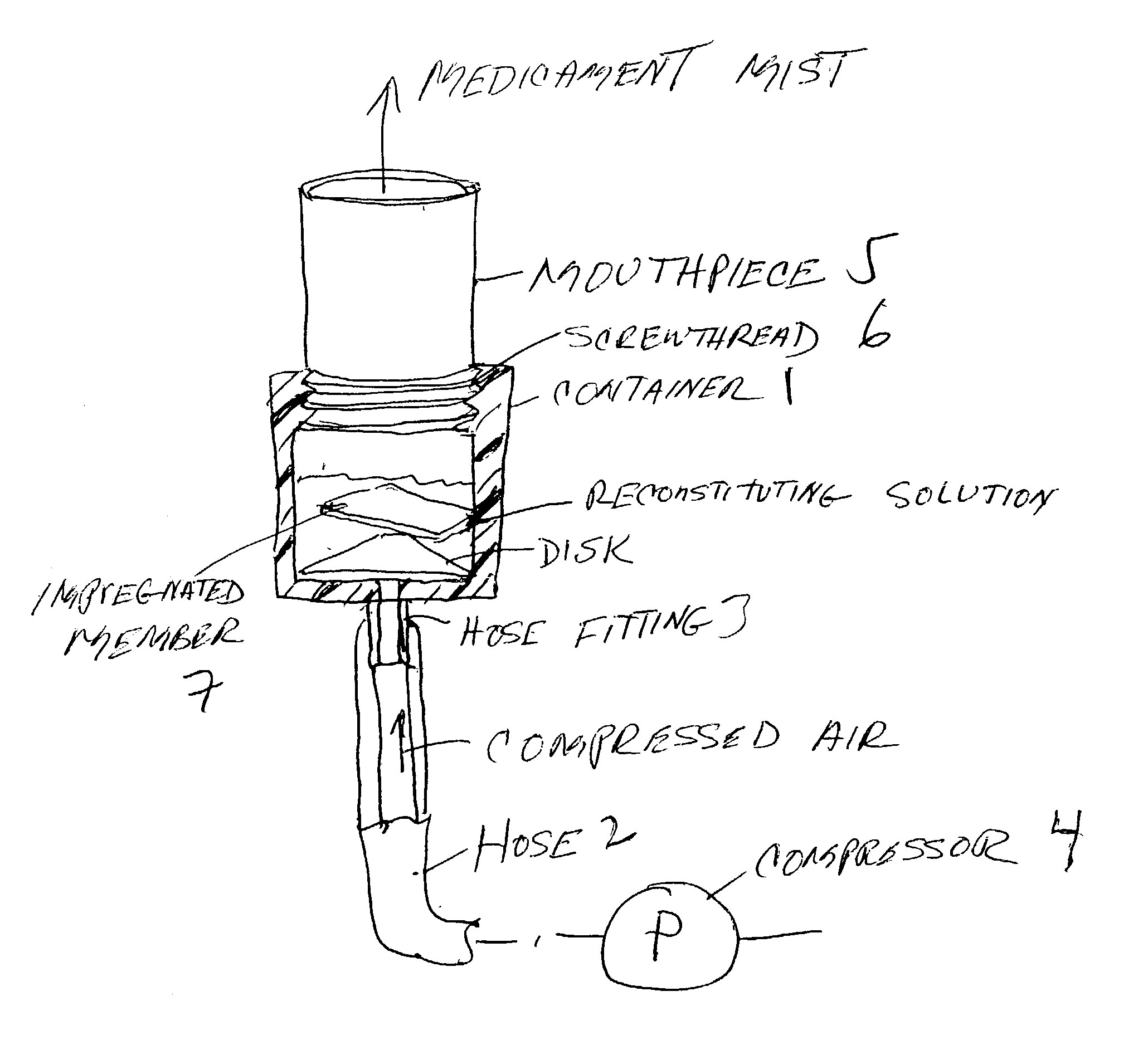
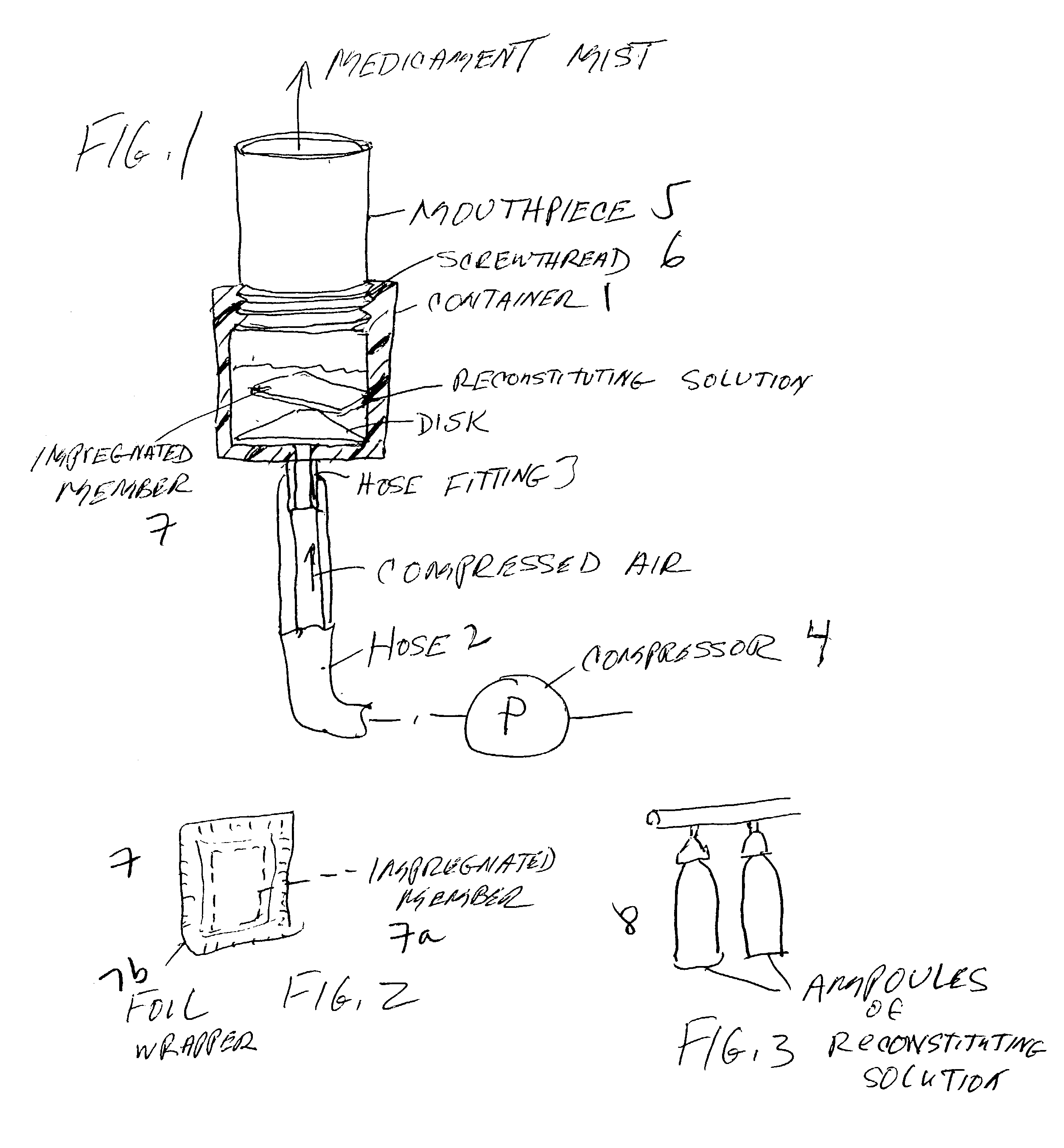
Pharmaceutical delivery system for oral inhalation through nebulization consisting of inert substrate impregnated with substance (S) to be solubilized or suspended prior to use
InactiveUS20040045546A1Good chemical stabilitySolvent evaporates quicklyDispersion deliverySolution deliveryBULK ACTIVE INGREDIENTDelivery system
A pharmaceutical delivery system for oral inhalation is disclosed through nebulization consisting of an inert supporting material impregnated with or deposited with pharmaceutically active ingredient which must be solubilized or suspended in a pharmaceutical solvent to form a solution or suspension prior to administration. Each pharmaceutical delivery unit dosage form comprises one or more therapeutically effective and safe amounts of pharmaceutically active ingredient uniformly impregnated in or deposited on a supporting material which is a natural or synthetic polymer, woven or non-woven fabrics, inert paper, inorganic materials such as foil and combination thereof in a single or multi-layer lamination in a form of a sheet or strip or film or membrane or sponge-like or cup or well. The dosage form of this invention is to be administered to a patient through oral or nasal inhalation using a nebulizer after reconstitution with a reconstituting solvent.
Owner:COLLEGIUM PHARMA INC
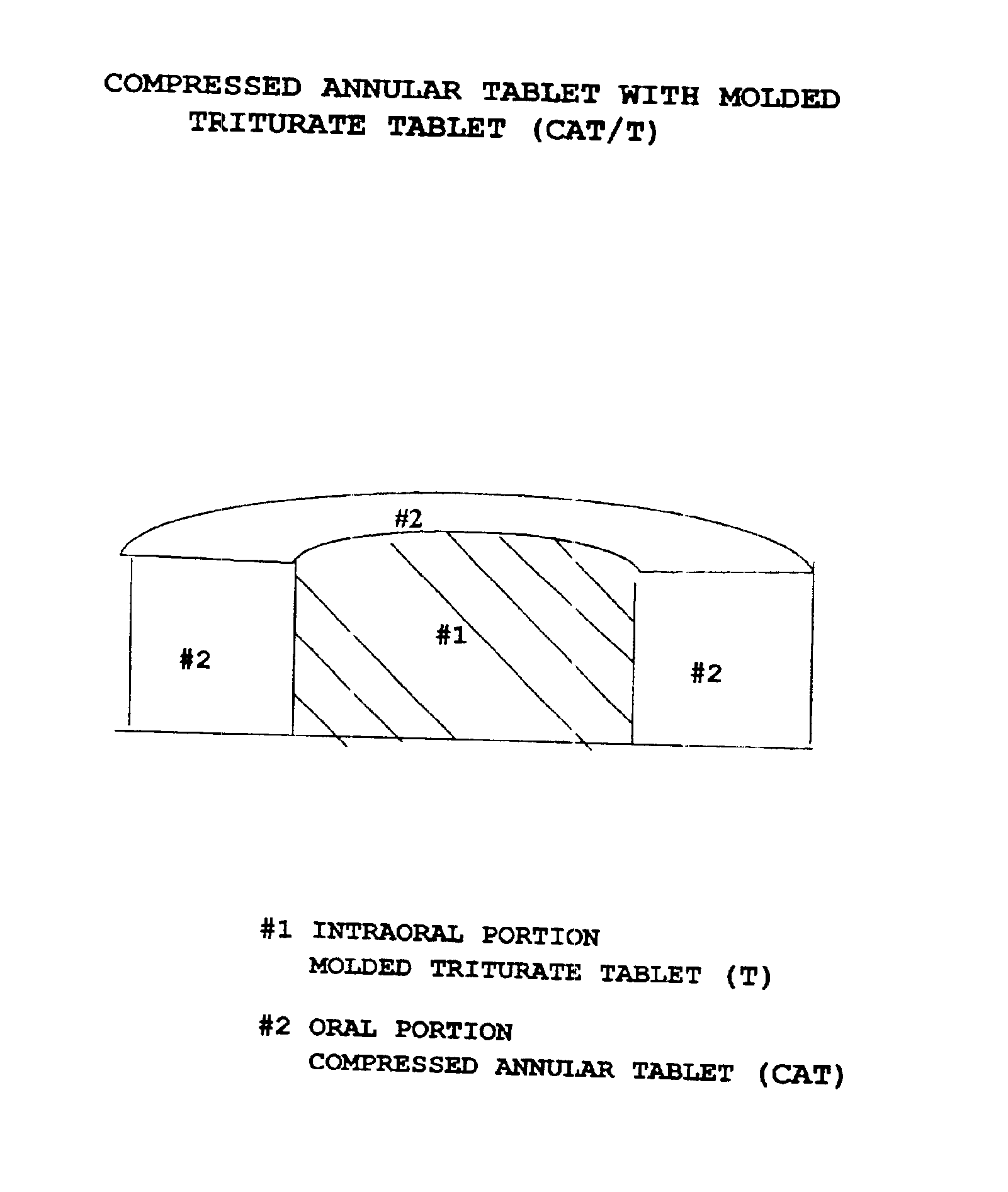
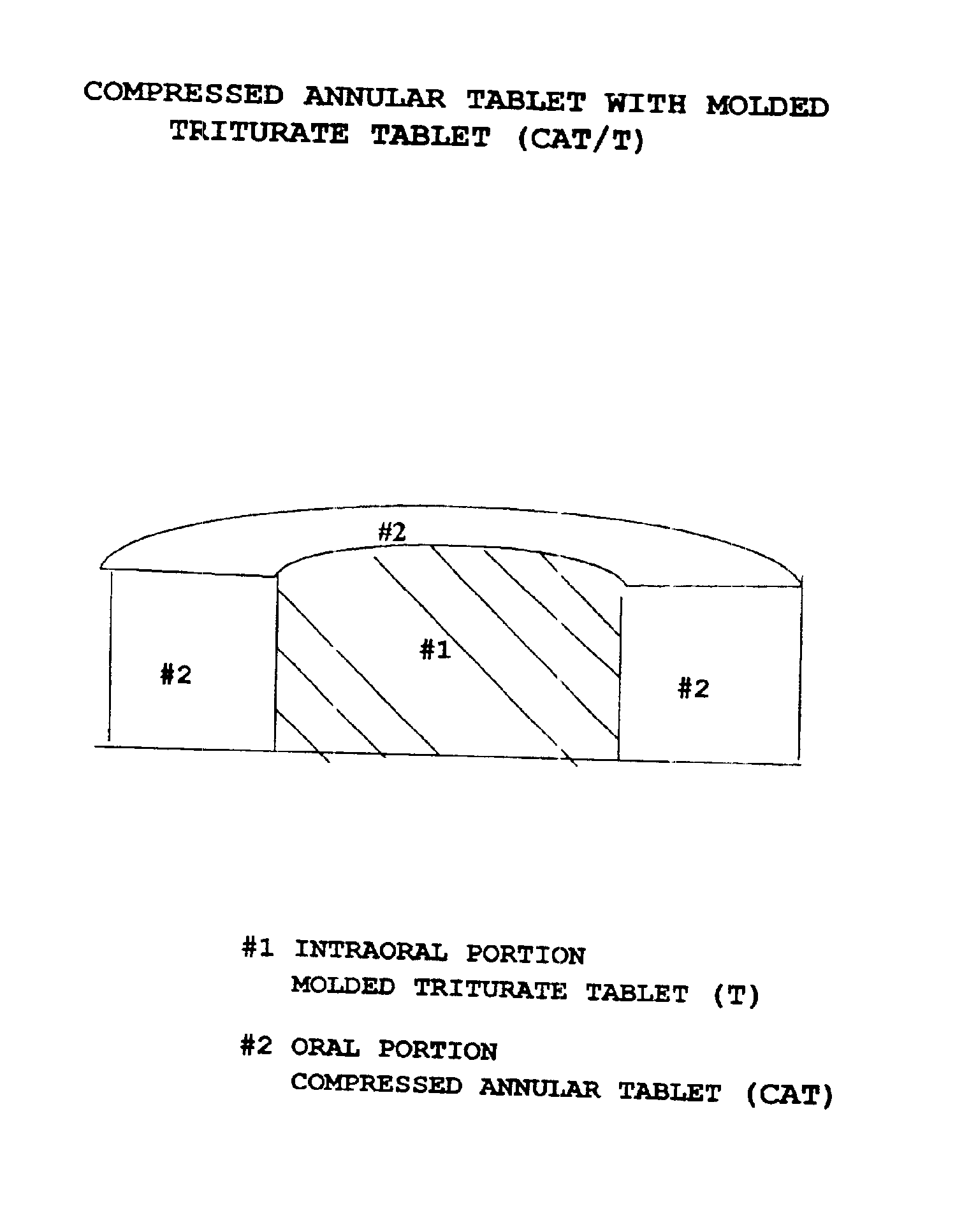
Pharmaceutical composition for compressed annular tablet with molded triturate tablet for both intraoral and oral administration
InactiveUS6863901B2Easily administrable to a patientMaximize the effect of treatmentNervous disorderSkeletal disorderOral medicationPharmaceutical drug
New pharmaceutical compositions in unit dosage form are disclosed for both intraoral and oral administration to a patient, said unit dosage form configured to be placed intraorally of said patient, which comprises:(a) as a first portion, at least one discrete molded triturate tablet comprising a therapeutically effective amount of at least one pharmaceutically active ingredient capable of intraoral administration; and(b) as a second portion located around the said first portion, a therapeutically effective amount of at least one pharmaceutically active ingredient capable of oral administration and which is releasable and orally ingestible by the patient after the molded triturate tablet has disintegrated or has dissolved intraorally.
Owner:HIRSH JANE +1
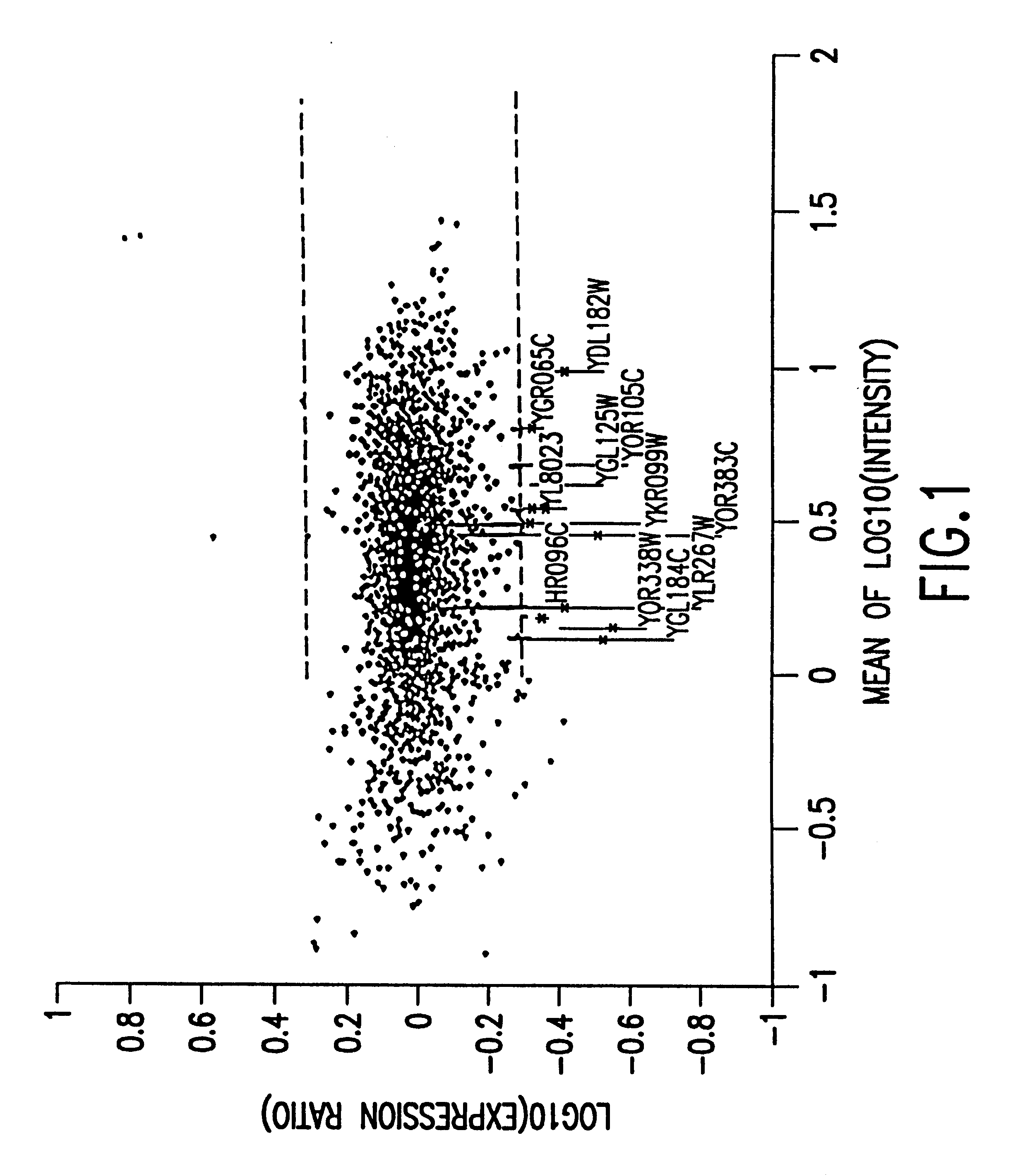
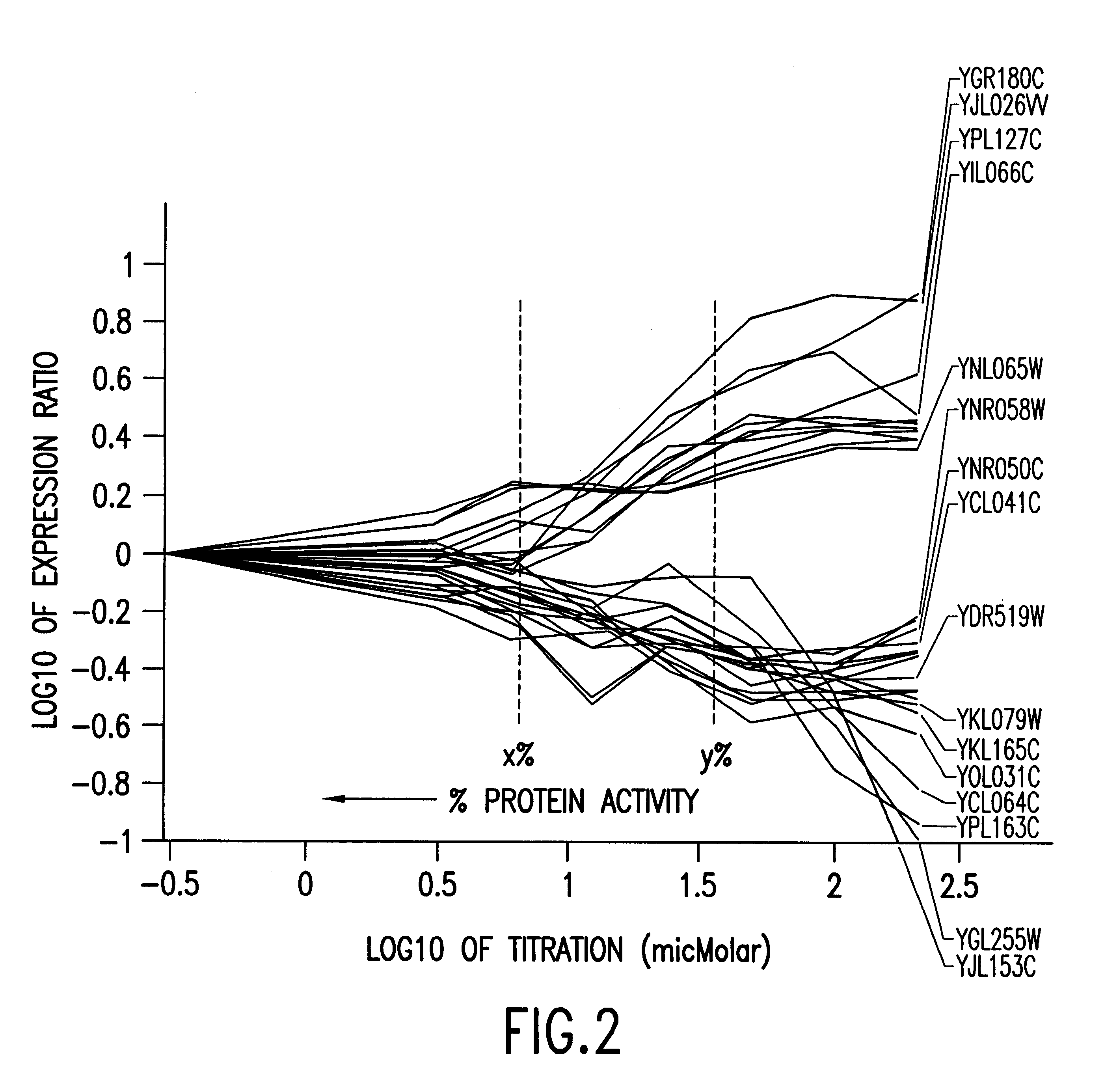
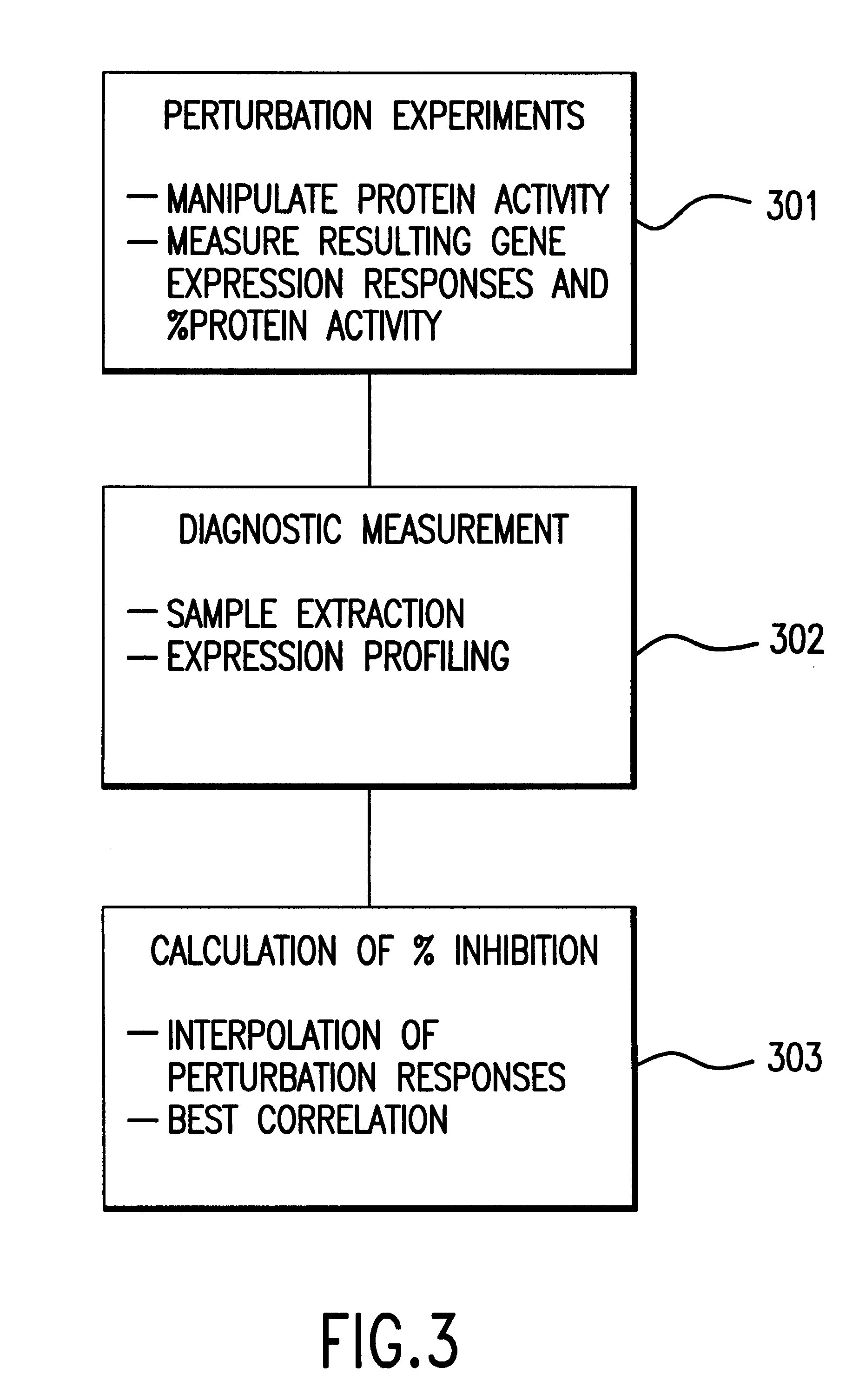
Methods of determining protein activity levels using gene expression profiles
InactiveUS6324479B1High similaritySugar derivativesPeptide/protein ingredientsProtein insertionDrug activity
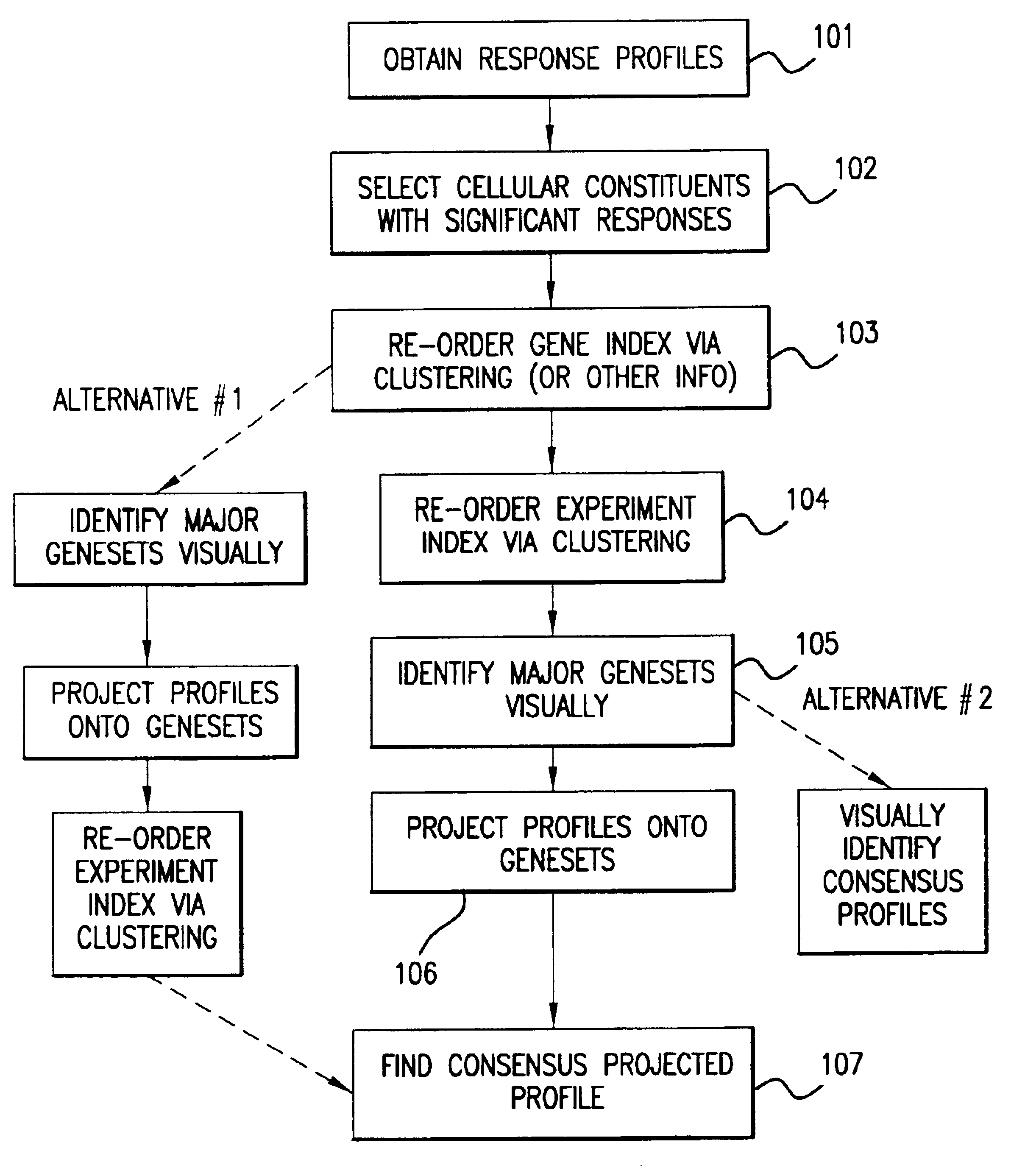
The present invention provides methods for determining the level of protein activity in a cell by: (i) measuring abundances of cellular constituents in a cell in which the activity of a specific protein is to be determined so that a diagnostic profile is thus obtained; (ii) measuring abundances of cellular constituents that occur in a cell in response to perturbations in the activity of said protein to obtain response profiles and interpolating said response profiles to generate response curves; and (iii) determining a protein activity level at which the response profile extracted from the response curves best fits the measured diagnostic profile, according to some objective measure. In alternative embodiments, the present invention also provides methods for identifying individuals having genetic mutations or polymorphisms that disrupt protein activity, and methods for identifying drug activity in vivo by determining the activity levels of proteins which interact with said drugs.
Owner:MICROSOFT TECH LICENSING LLC
Anti-misuse microparticulate oral pharmaceutical form
InactiveUS20100092553A1Prevent misuse by short liquid extraction and/or crushingPowder deliveryBiocidePublic healthMicroparticle
Owner:FLAMEL IRELAND
Fast dissolving orally consumable films containing a sweetener
InactiveUS20030211136A1Fast deliverySufficient toleranceBiocideCosmetic preparationsActive agentSweetness
A consumable film adapted to adhere to and dissolve in the oral cavity of a warm-blooded animal including humans, comprising at least one water soluble polymer, a taste masking effective amount of a sweetener, and a pharmaceutically active agent having a sufficiently unpleasant taste that it is desirably masked by the sweetener.
Owner:MCNEIL PPC INC
Controlled release delivery device for pharmaceutical agents incorporating microbial polysaccharide gum
The present invention provides a controlled release device for sustained or pulsatile delivery of pharmaceutically active substances for a predetermined period of time. This invention further provides such device in which sustained or pulsatile delivery is obtained by the unique blend and intimate mixture of pharmaceutically active substances with a microbial polysaccharide and uncrosslinked linear polymer and optionally a crosslinked polymer and / or lipophillic polymer and / or saturated polyglycolyzed glyceride. The invention also provides a process for the manufacture of such devices and pharmaceutical compositions containing the same.
Owner:INTELLIPHARMACEUTICS
Oral capsule formulation with increased physical stability
InactiveUS7011846B2Improve capsule stabilityImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryMonoglycerideOral medication
A formulation for a stabilized capsule for oral administration of a hydrophobic pharmaceutically active agent; comprising a non-aqueous solubilizer selected from 2-pyrrolidone, N-alkylpyrrolidones and combinations thereof; and a capsule stabilizing agent selected from mono-, di- and triglycerides, mono- and di-fatty esters of polyethylene glycol, fatty acids and combinations thereof wherein capsule integrity is maintained for at least 24 hours is disclosed.
Owner:SUPERNUS PHARM INC
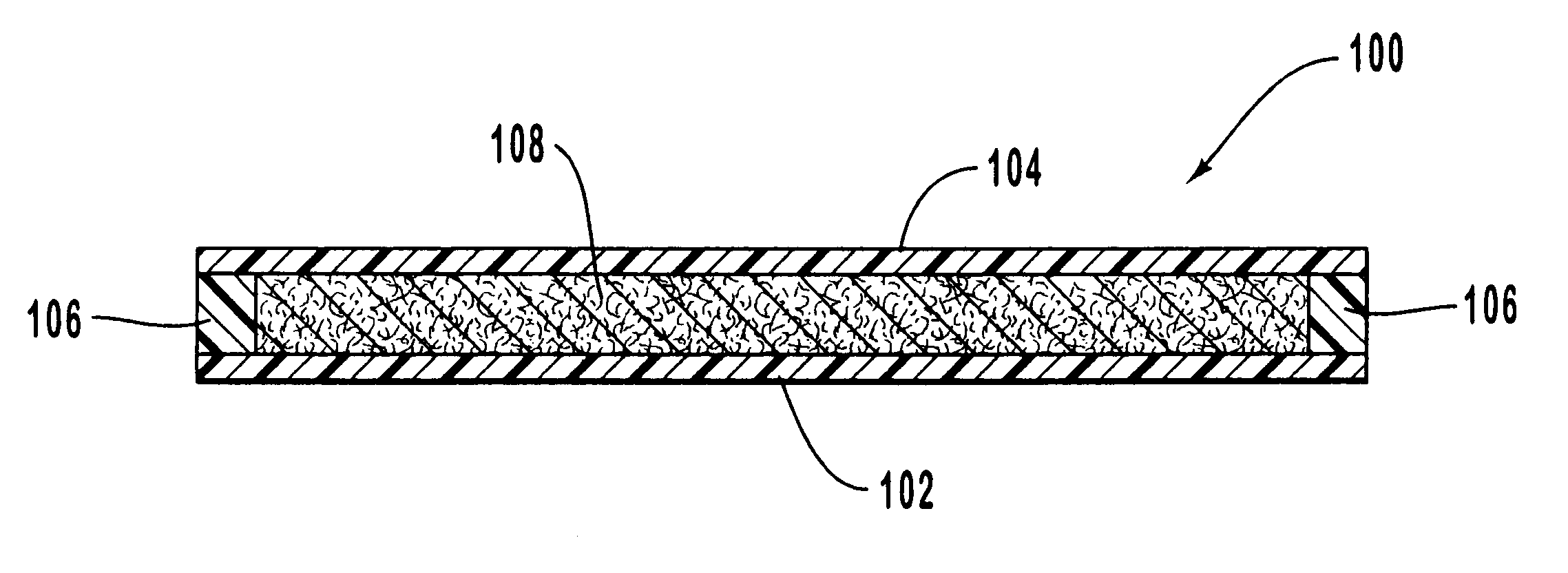
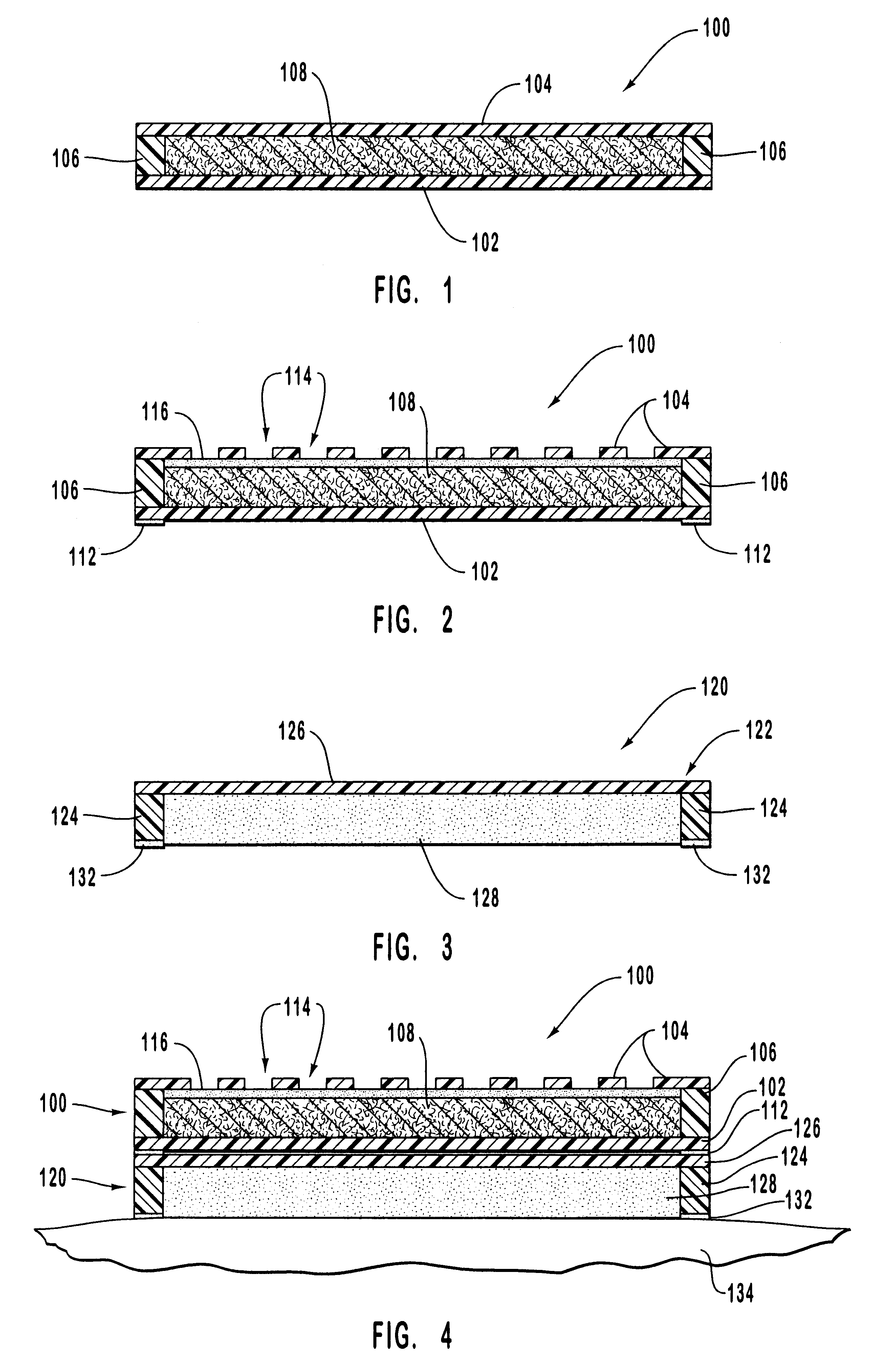
Drug delivery system
The subject invention provides a drug delivery system comprising at least one compartment consisting of (i) a drug-loaded thermoplastic polymer core, (ii) a drug-loaded thermoplastic polymer intermediate layer and (iii) a non-medicated thermoplastic polymer skin covering the intermediate layer, wherein said intermediate layer is loaded with (a) crystals of a first pharmaceutically active compound and with (b) a second pharmaceutically active compound in dissolved form and wherein said core is loaded with said second compound in dissolved form.
Owner:MERCK SHARP & DOHME BV
Prodrugs and methods of making and using the same
InactiveUS20080318905A1Improve featuresExtended half-lifeGroup 4/14 element organic compoundsOrganic active ingredientsDrug activityProdrug
Prodrugs of parent drugs and methods of making and using the same are described. The prodrugs comprise an amine-containing parent drug moiety and a prodrug moiety, such as methoxyphosphonic acid or ethoxyphosphonic acid. The prodrugs may be employed in therapy for the treatment of various indications, such as pain, and in methods of decreasing the abuse potential of abuse-prone drugs and / or delaying the onset of parent drug activity and / or prolonging parent drug activity as compared to administration of a parent drug.
Owner:NEUROGESX INC
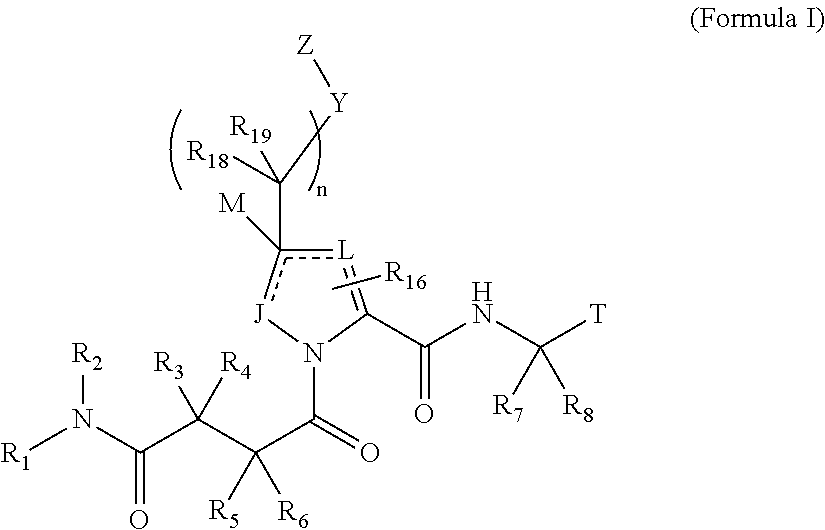
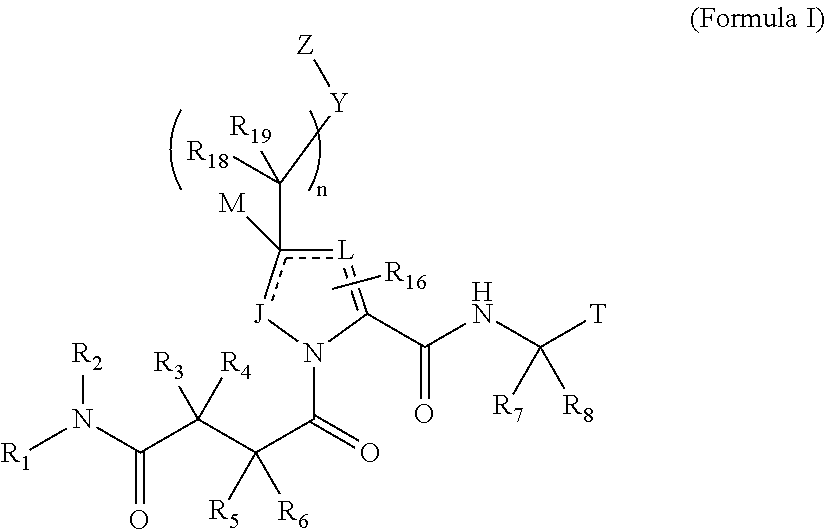
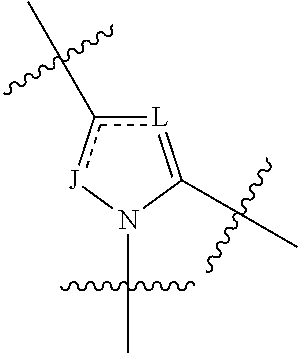
4-amino-4-oxobutanoyl peptides as inhibitors of viral replication
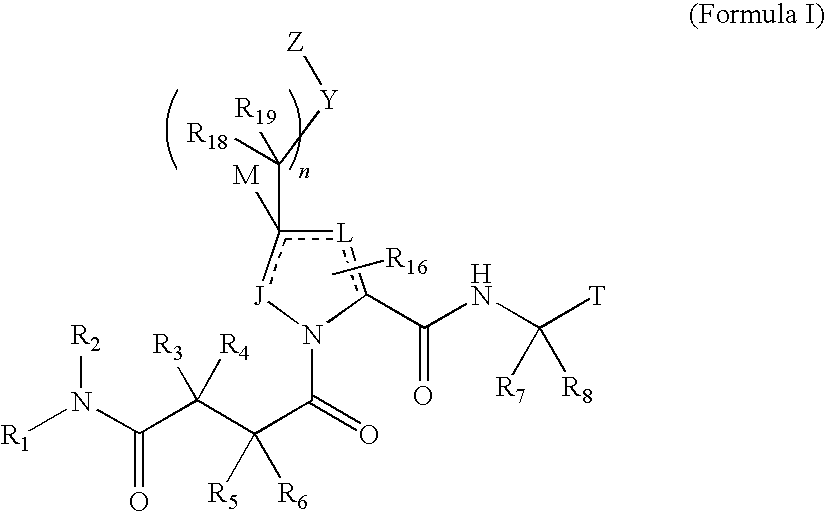
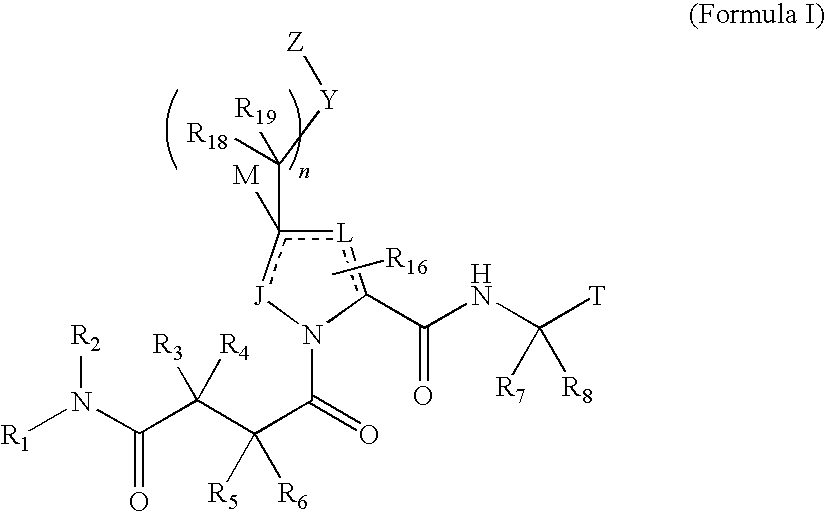
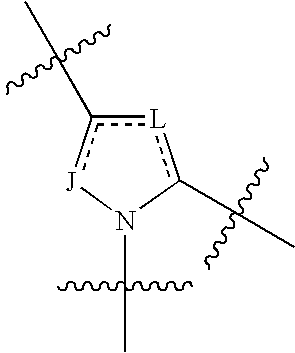
The invention provides 4-amino-4-oxobutanoyl peptide compounds of Formula Iand the pharmaceutically salts and hydrates thereof.The variables R1-R9, R16, R18, R19, n, M, n, M, and Z are defined herein. Certain compounds of Formula I are useful as antiviral agents. Certain 4-amino-4-oxobutanoyl peptide compounds disclosed herein are potent and / or selective inhibitors of viral replication, particularly Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more 4-amino-4-oxobutanoyl peptide compounds and one or more pharmaceutically acceptable carriers. Such pharmaceutical compositions may contain 4-amino-4-oxobutanoyl peptide compound as the only active agent or may contain a combination of 4-amino-4-oxobutanoyl peptide containing peptides compound and one or more other pharmaceutically active agents. The invention also provides methods for treating viral infections, including Hepatitis C infections, in mammals.
Owner:ACHILLION PHARMA INC
Nucleic acid carriers for delivery of therapeutic agents
InactiveUS20070225213A1Improve solubilityLow biological effectHeavy metal active ingredientsBiocideIn vivoProliferation rate
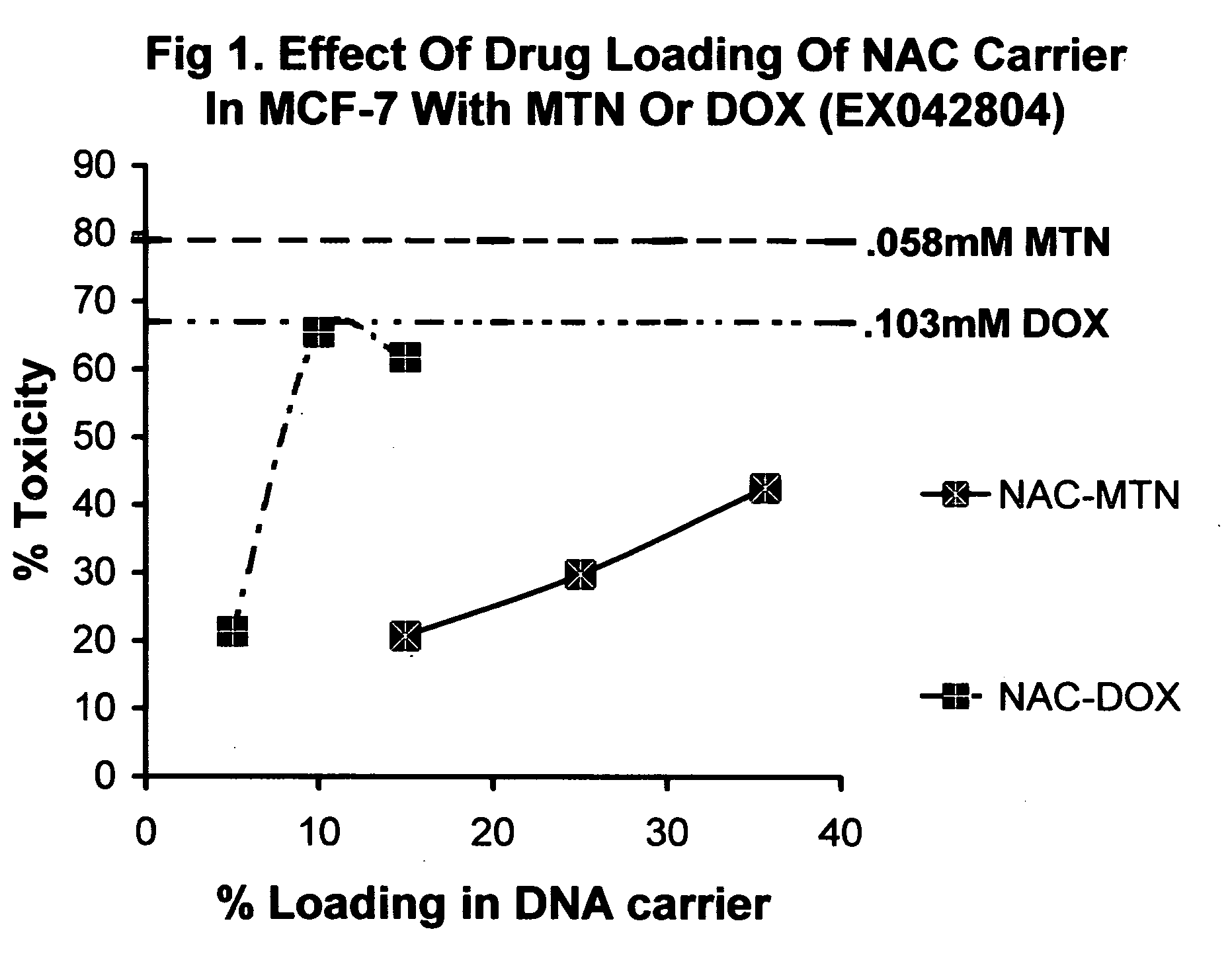
Nucleic acid drug carriers comprise a nucleic acid carrier complexed with a drug, wherein the nucleic acid carrier and the drug are associated non-covalently, and optionally other agents such as spacer, transfection agents, and targeting agents. The nucleic acid drug complex are discovered to have permissive or refractory uptake depending on many factors including cell type, proliferation rate, among others. The refractive uptake of the nucleic acid drug complex are shown to be useful in the nucleic acid targeting of drugs, both in vitro and in vivo. Novel drug compositions are disclosed that effectively reduce the toxicity of drugs while maintaining drug activity and enhancing a drug's therapeutic index.
Owner:KOSAK MATTHEW K
Active substances for use in cosmetic and/or pharmaceutical products, obtainable from the fermentation of plant components and/or plant extracts
Processes for producing cosmetic and / or pharmaceutical active components which comprise: (a) providing a fermentation broth comprising a plant component selected from the group consisting of plant constituents, plant extracts and mixtures thereof; (b) inoculating the fermentation broth with a microorganism; and (c) fermenting the microorganism-containing fermentation broth to produce an active component; are described along with cosmetic and / or pharmaceutical preparations containing such active components and methods of using the same to treat the skin and / or hair.
Owner:COGNIS FRANCE SA
Sequential drug delivery systems
InactiveUS20050031677A1Promote absorptionImprove solubilityGogglesInorganic non-active ingredientsDissolutionDrug activity
The invention relates to methods and composition for improving absorption and dissolution of active ingredients of drugs. The invention provides a method of administration of an active ingredient to a mammal through a transmucosal route that includes delivering the active ingredient to a desired site in a body of the mammal, and, sequentially, at the desired site, promoting dissolution and absorption of the active ingredient. In a preferred embodiment, the pH of the localized environment of the active ingredient is sequentially modified to promote dissolution and absorption.
Owner:CIMA LABS
Salts and crystalline forms of an apoptosis-inducing agent
Salts and crystalline forms of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}-sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide are suitable active pharmaceutical ingredients for pharmaceutical compositions useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
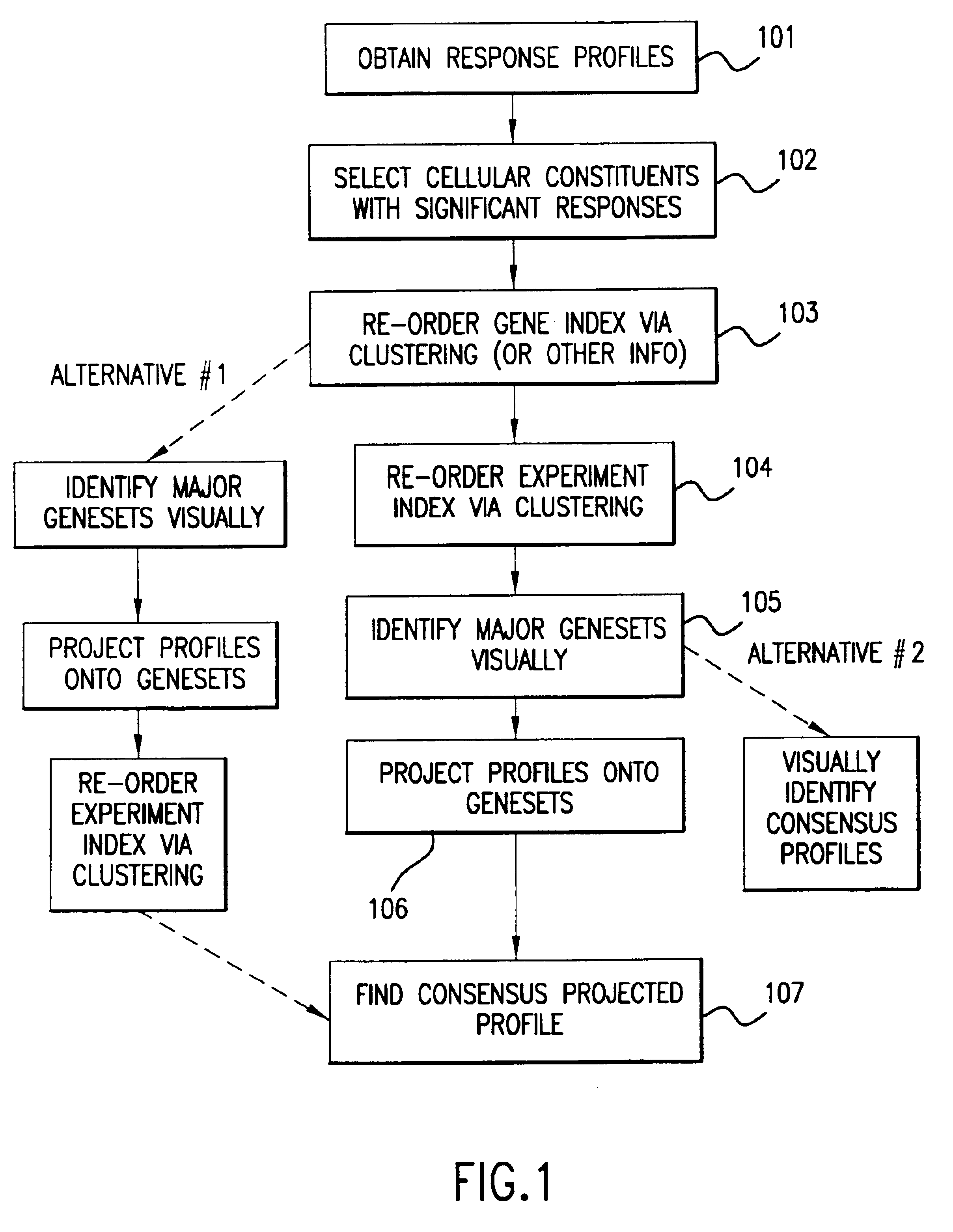
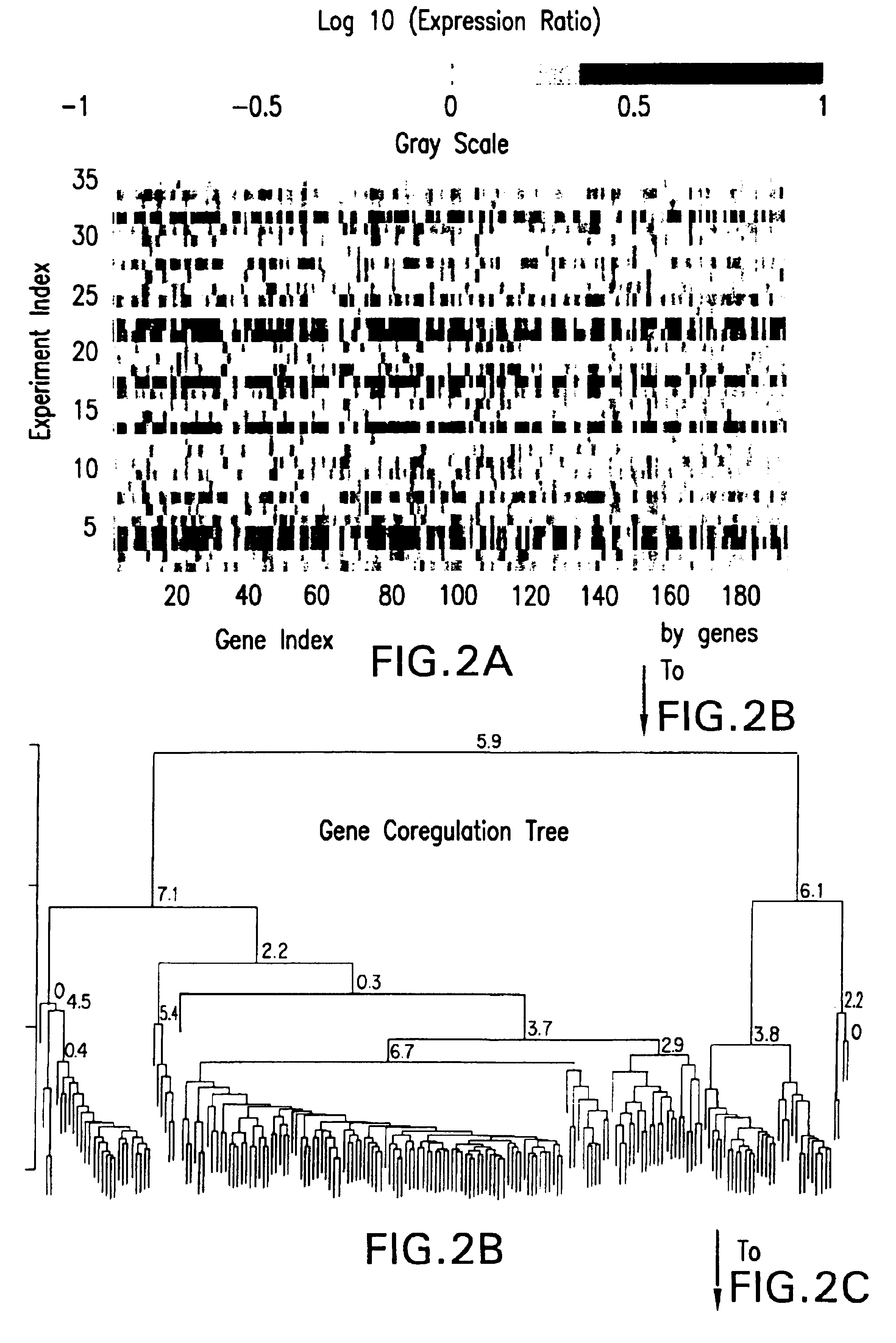
Methods of characterizing drug activities using consensus profiles
InactiveUS6801859B1Good detection and classification and comparisonSimple structureMicrobiological testing/measurementAnalogue computers for chemical processesNew medicationsPharmaceutical drug
The present invention provides methods for enhanced detection of biological response profiles. In particular, the methods of this invention allow for the detection of biological response patterns, such as gene expression patterns, in response to different drug treatments. The methods of the invention also allow the determination of a "consensus profile" which describes a particular class or type of biological response. In certain embodiments the consensus profile may describe the biological response of a particular group or class of drugs. In other embodiments, the consensus profile may describe an "ideal" biological response such as one associated with a desired therapeutic effect. The methods of the present invention also allow for the comparison of different biological responses. Thus, the methods of the invention may be used, e.g., to identify and / or study new drugs.
Owner:MICROSOFT TECH LICENSING LLC
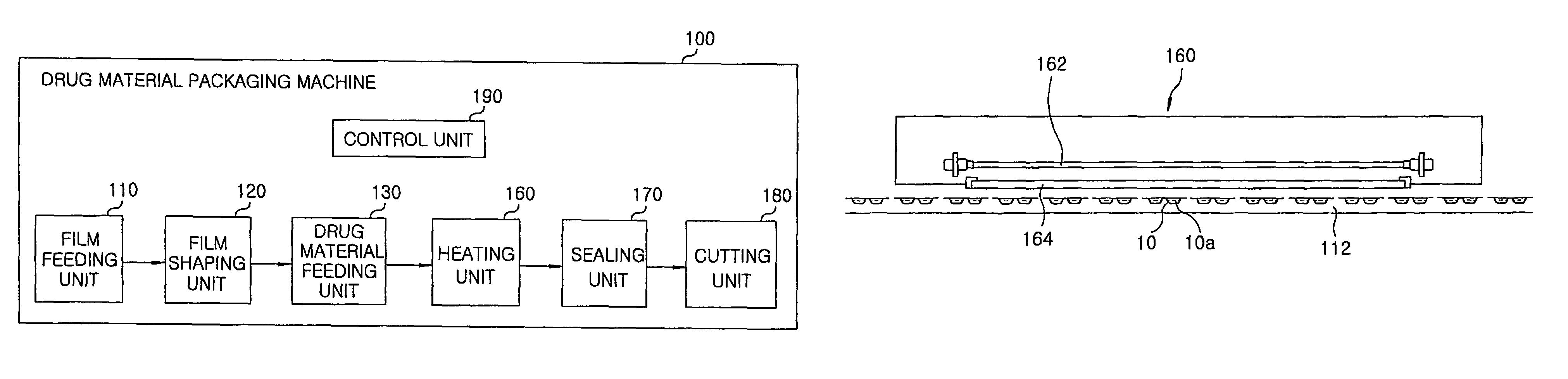
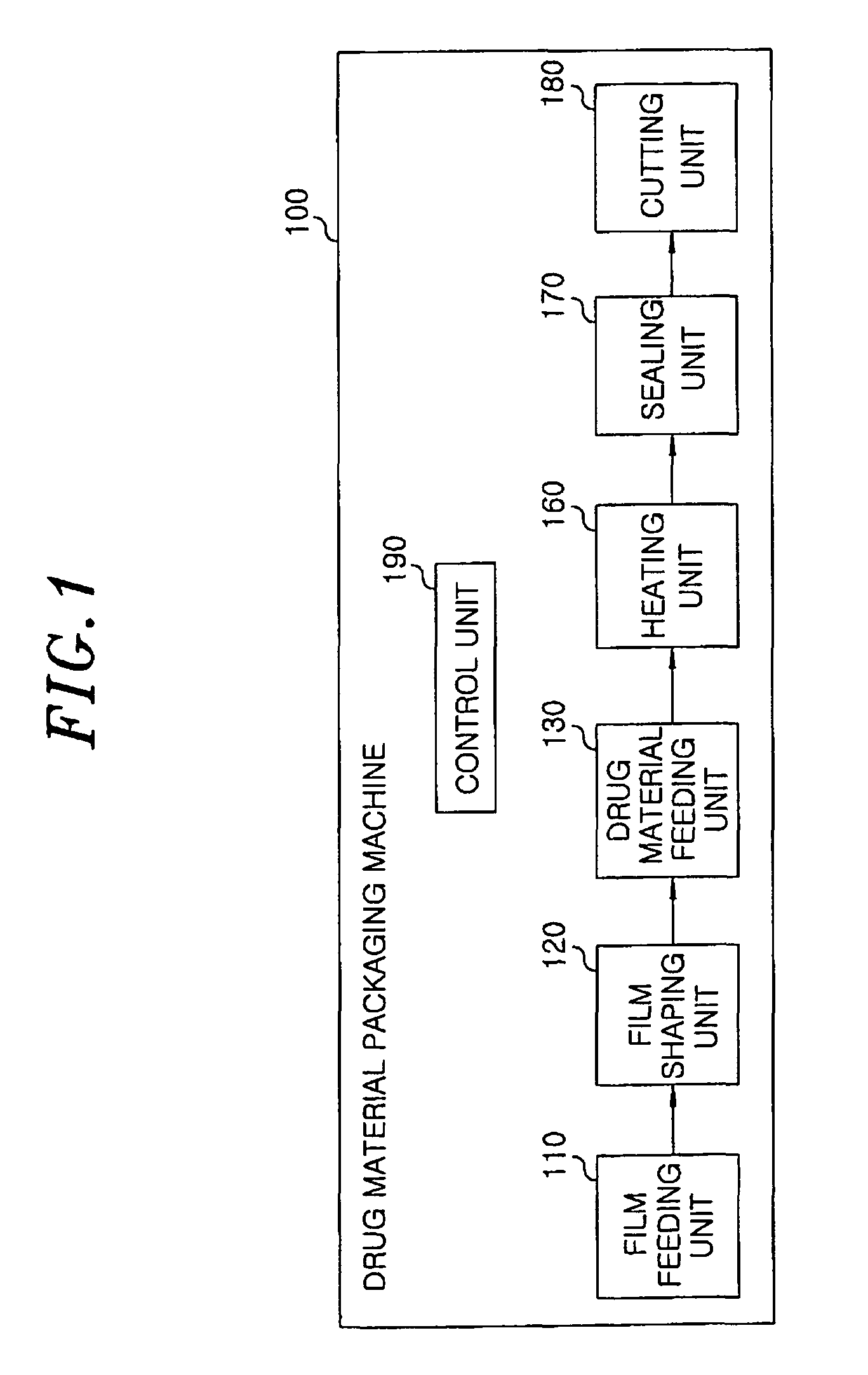
Method for preparing rapidly disintegrating formulation for oral administration and apparatus for preparing and packing the same
InactiveUS8127516B2Disintegrates quicklyEnhanced patient comfortAntibacterial agentsNervous disorderPowder mixtureOral medication
A method and packaging machine for preparing rapidly disintegrating formulations for oral administration are disclosed. The present invention is characterized in that a powdery mixture including a pharmaceutically active ingredient and a sugar or a sugar alcohol powder is filled into a packaging material and, thereafter, the mixture, filled in the packaging material, is heated. The present invention can simply and economically prepare an oral formulation which undergoes rapid disintegration in the oral cavity and provides for high-quality administration to patients.
Owner:HANMI PHARMA
Serum half-life extension using igbd
InactiveUS20170145062A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeHeavy chain
Owner:UNIV STUTTGART
4-amino-4-oxobutanoyl peptides as inhibitors of viral replication
The invention provides 4-amino-4-oxobutanoyl peptides of Formula Iand the pharmaceutically salts and hydrates thereof. The variables R, R1, R6-R8, R16, R18, R19, M, n, T, Y, and Z are defined herein. Certain compounds of Formula I are useful as antiviral agents. The 4-amino-4-oxobutanoyl peptides disclosed herein are potent and / or selective inhibitors of viral replication, particularly Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more 4-amino-4-oxobutanoyl peptides and one or more pharmaceutically acceptable carriers. Such pharmaceutical compositions may contain a 4-amino-4-oxobutanoyl peptides as the only active agent or may contain a combination of a 4-amino-4-oxobutanoyl peptides and one or more other pharmaceutically active agents. The invention also provides methods for treating viral infections, including Hepatitis C infections.
Owner:ACHILLION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com