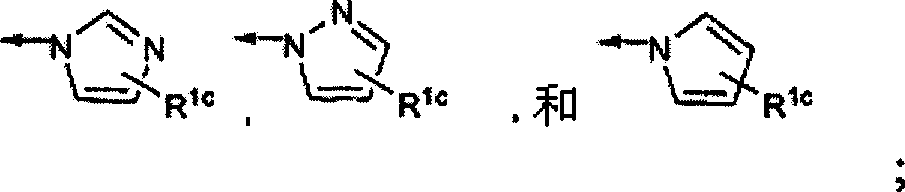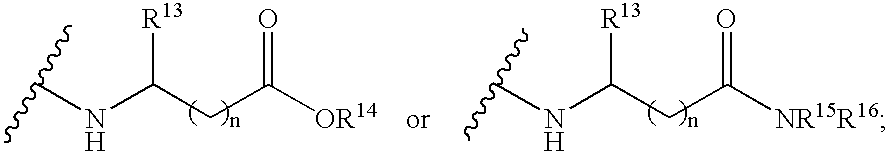Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
927 results about "Viral replication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral replication is the formation of biological viruses during the infection process in the target host cells. Viruses must first get into the cell before viral replication can occur. Through the generation of abundant copies of its genome and packaging these copies, the virus continues infecting new hosts. Replication between viruses is greatly varied and depends on the type of genes involved in them. Most DNA viruses assemble in the nucleus while most RNA viruses develop solely in cytoplasm.
Nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase
The present invention provides nucleoside derivatives which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as inhibitors of hepatitis C virus (HCV) NS5B polymerase, as inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such nucleoside derivatives alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the nucleoside derivatives of the present invention.
Owner:MERCK SHARP & DOHME LLC +1
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
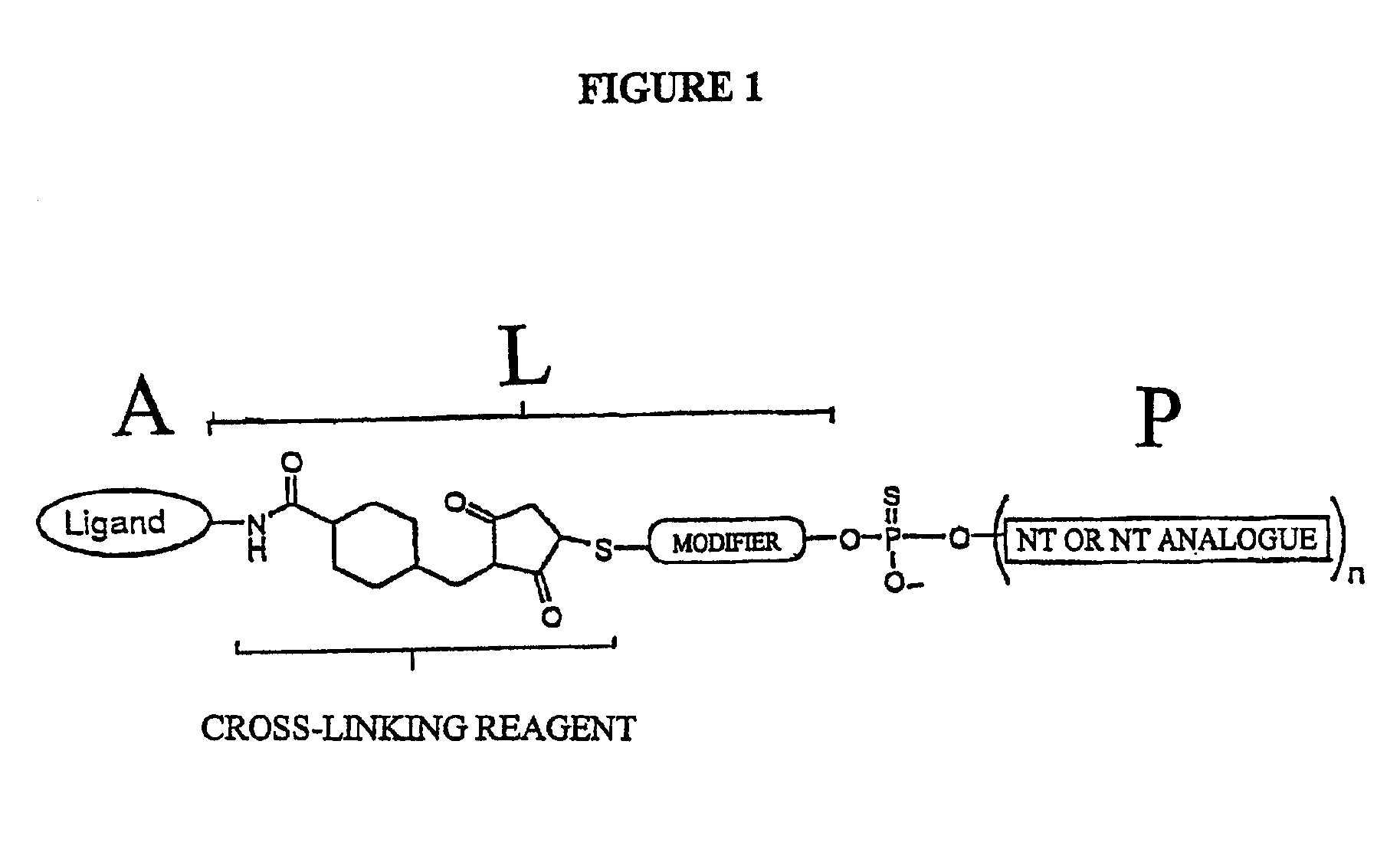
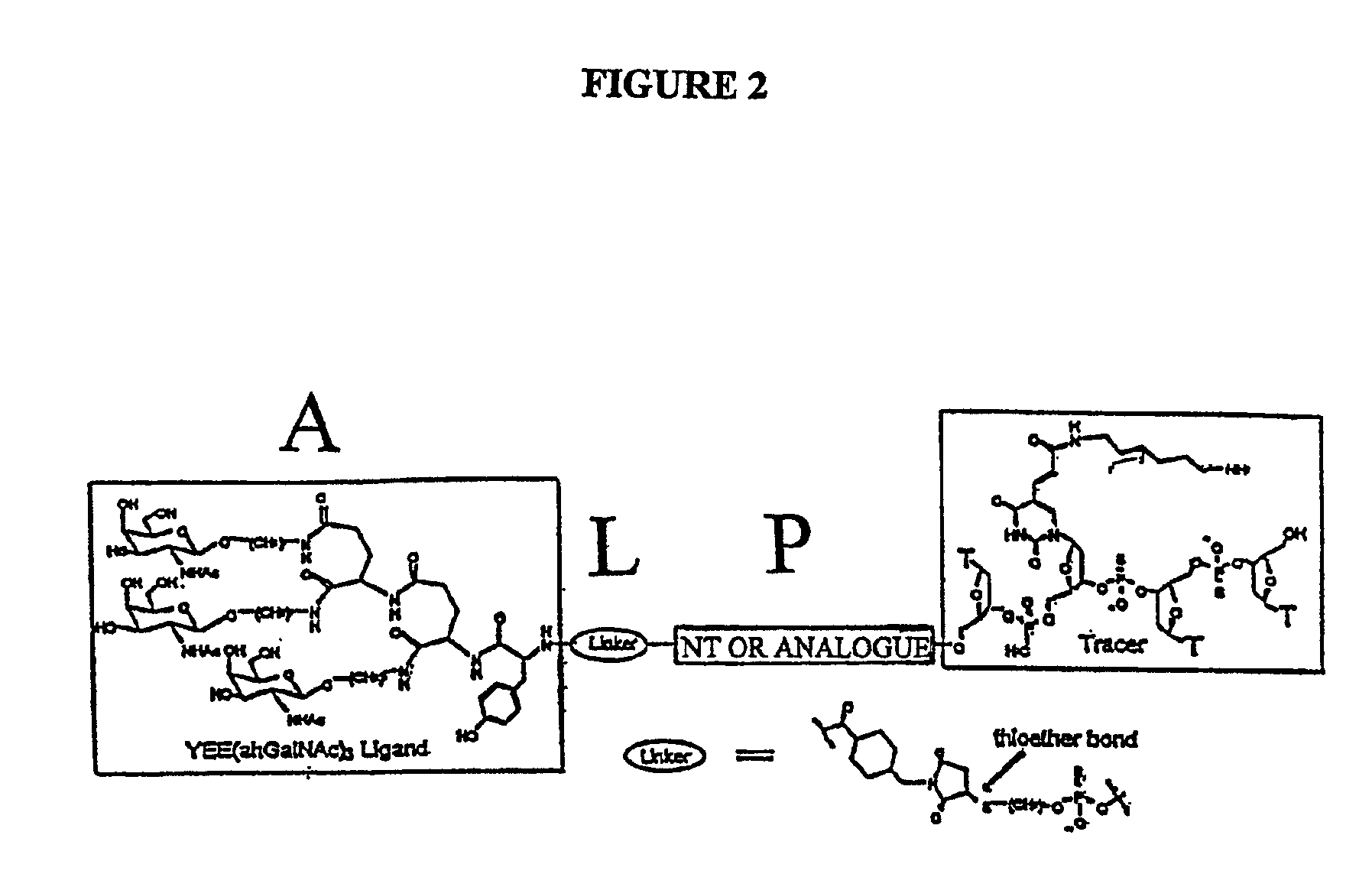
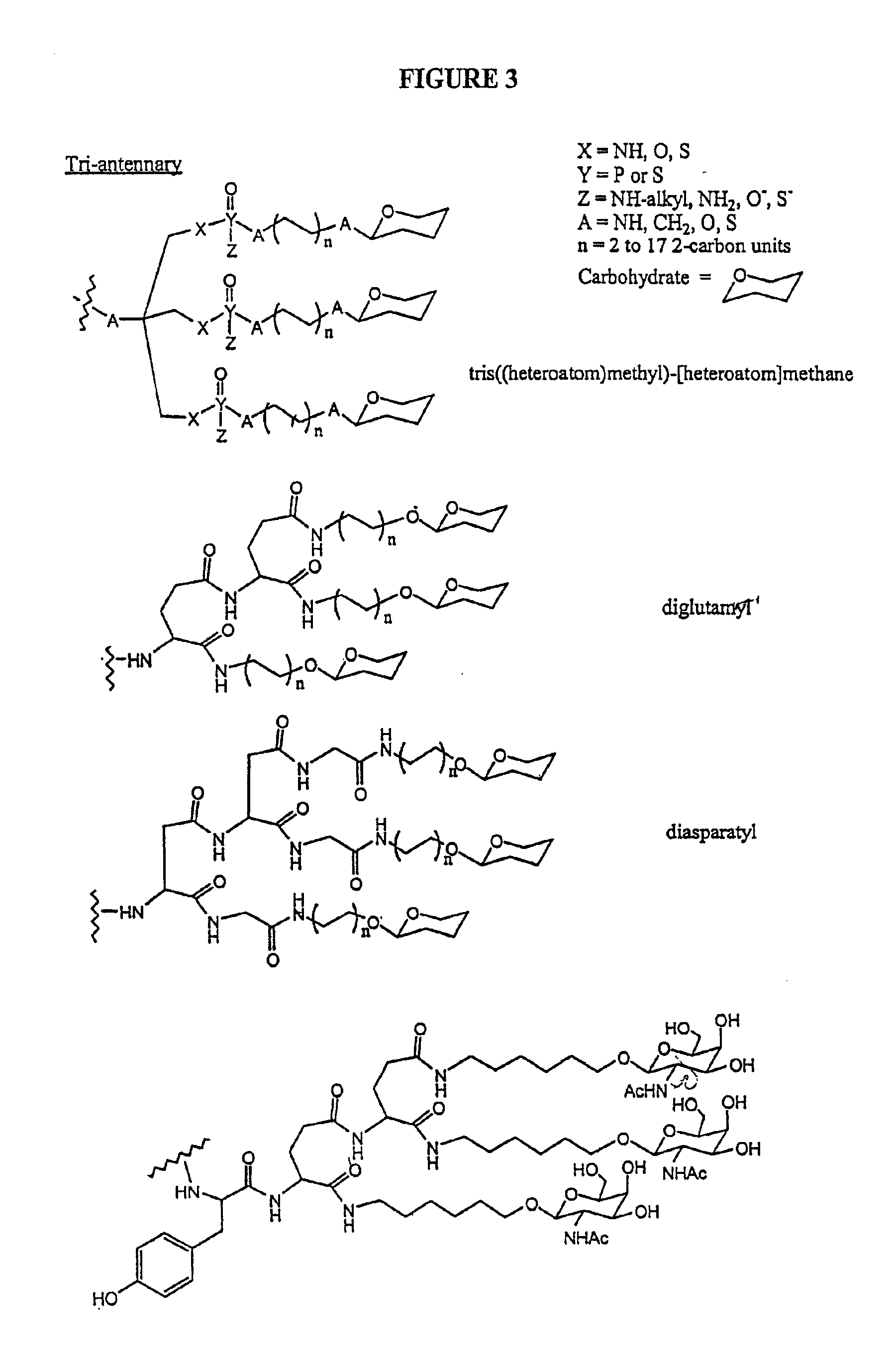
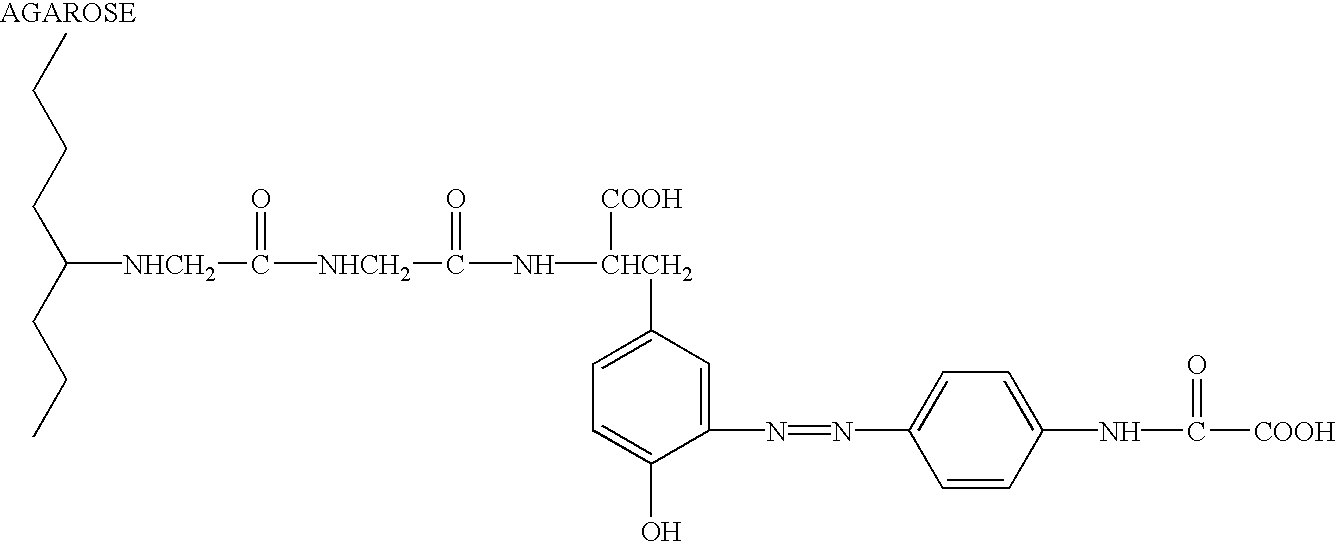
Conjugates of glycosylated/galactosylated peptide, bifunctional linker, and nucleotidic monomers/polymers, and related compositions and method of use
InactiveUS6906182B2Abnormal proliferationInhibition of replicationOrganic active ingredientsBiocideNucleotideEthylene Homopolymers
A conjugate of formula A-L-P, in which:A represents a glycosylated / galactosylated peptide that binds to a cell-surface receptor,L represents a bifunctional linker, which does not comprise a naturally occurring amino acid and is covalently bonded to A and P in a regiospecific manner, andP represents a monomer, homopolymer or heteropolymer comprising at least one nucleotide or an analogue thereof, which inhibits the intracellular biosynthesis of nucleotides or nucleic acids in a sequence-independent manner,wherein either or both of the covalent bond between A and L and the covalent bond between L and P can be cleaved intracellularly; a composition comprising such a conjugate; and a method of inhibiting abnormal cellular proliferation in a mammal; and a method of inhibiting replication of a virus in a mammal.
Owner:CELLECTIVE DX CORP
Nucleoside phosphoramidates
Disclosed herein are nucleoside phosphoramidates and their use as agents for treating viral diseases. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
Owner:GILEAD SCI INC
Nucleoside phosphoramidates
Disclosed herein are nucleoside phosphoramidates and their use as agents for treating viral diseases. These compounds are inhibitors of RNA-dependent 5 RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
Owner:GILEAD SCI INC
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Nucleoside aryl phosphoramidates for the treatment of RNA-dependent RNA viral infection
InactiveUS7879815B2Effective penetrationLess susceptibleBiocideSugar derivativesHepatitis c viralPhosphoramidate
Owner:MSD ITAL +1
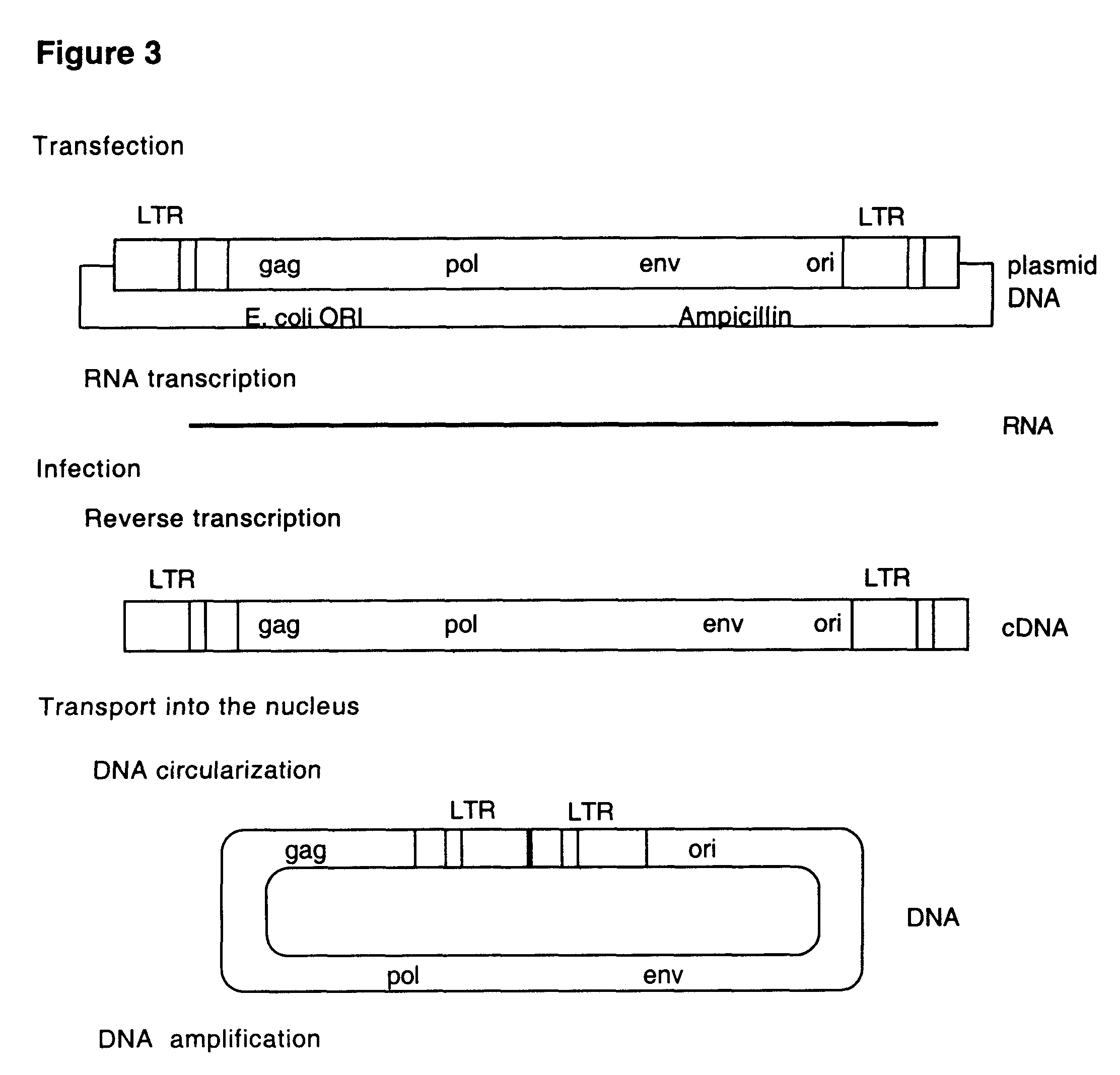
Retrovirus and viral vectors
InactiveUS6635472B1Prevent and attenuate diseaseEnhance immune responseGenetic material ingredientsVirus peptidesVirus-RetrovirusDna viral
This invention relates to the fields of genetic engineering, virus replication and gene transfer. More specifically, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors, wherein an ori derived from a DNA virus capable of replicating in vertebrate cells is inserted into the retrovirus, allowing the retrovirus following the reverse transcription to efficiently replicate as extrachromosomal or episomal DNA without the necessity of integration into the host cell chromosome. Additionally, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors replicating episomally without aid of an ori and related elements. Also, this invention encompasses preventive, therapeutic, and diagnostic applications employing said constructs, viruses and vectors.
Owner:RUBICON LABS
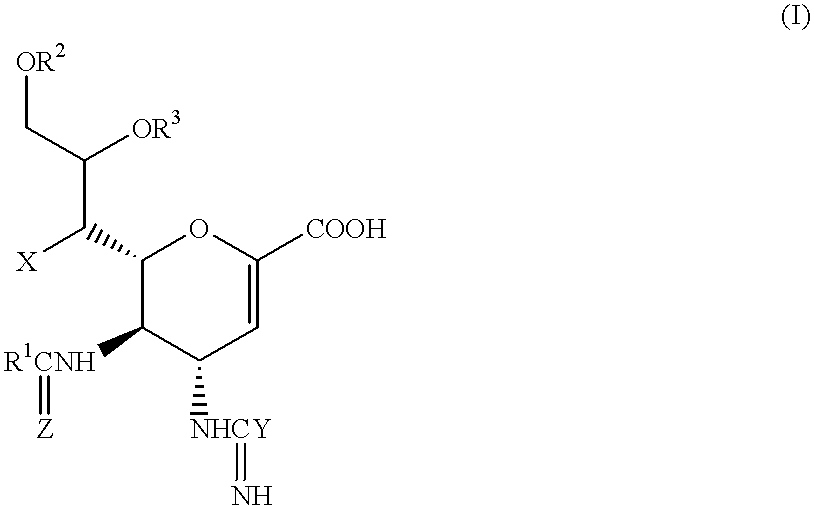
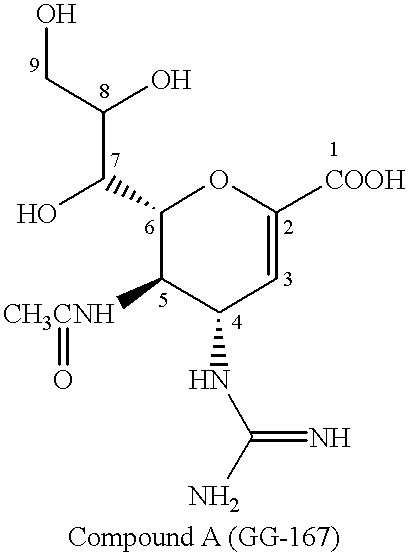
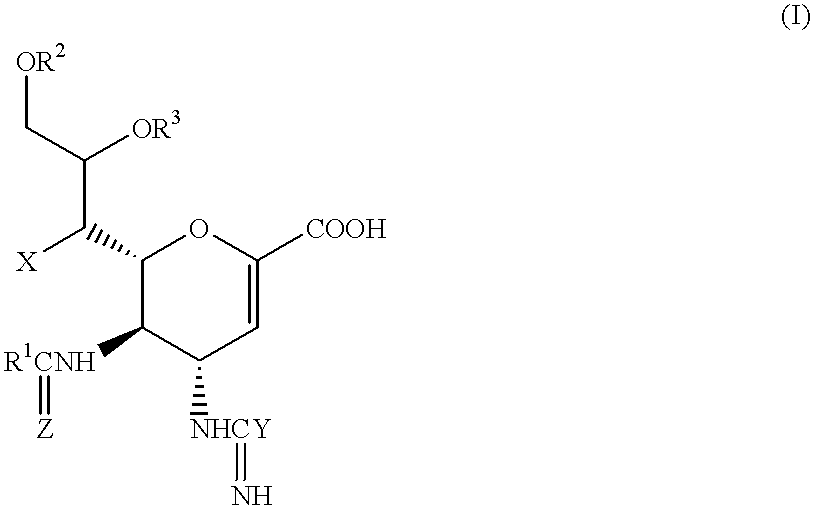
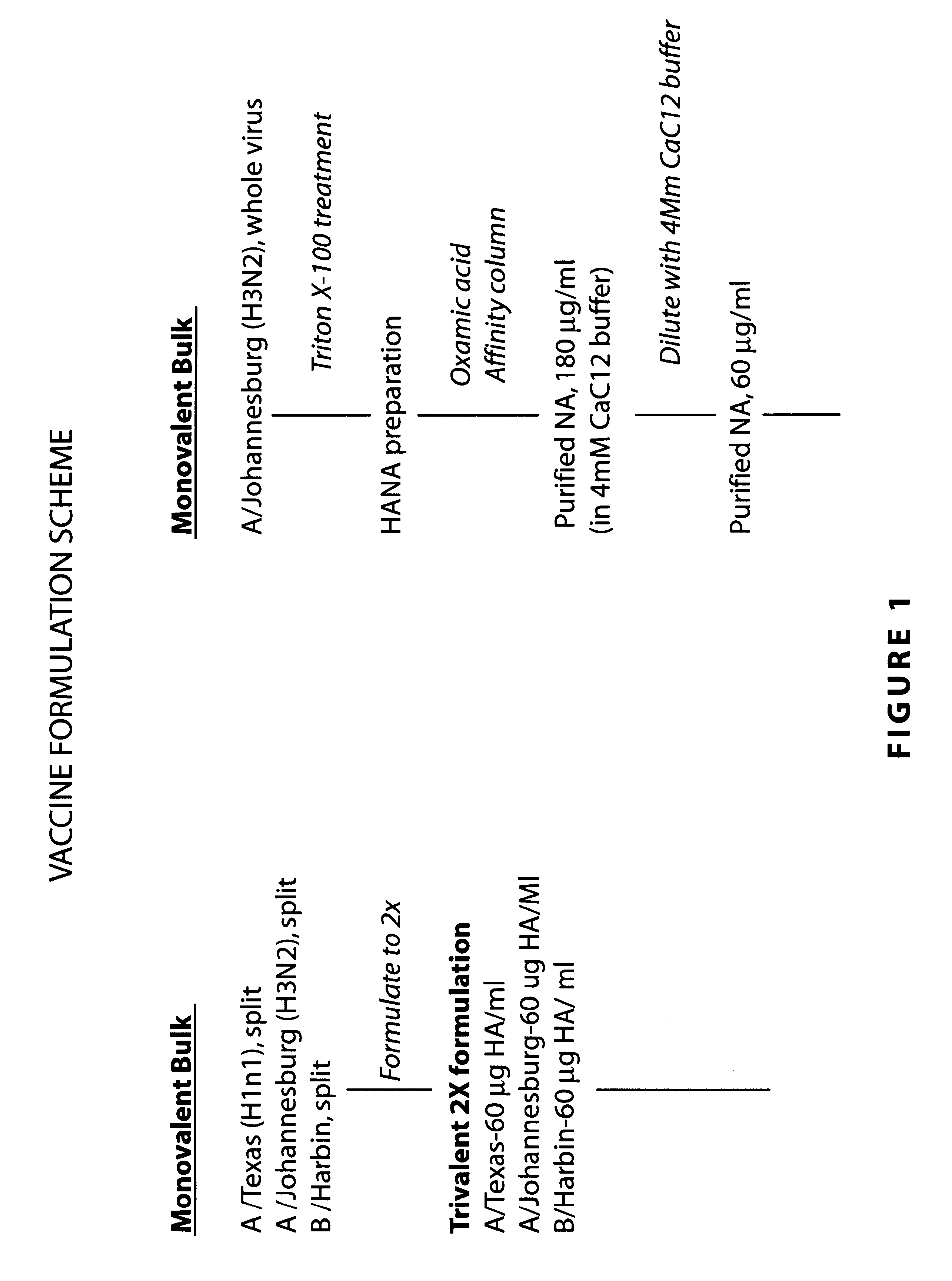
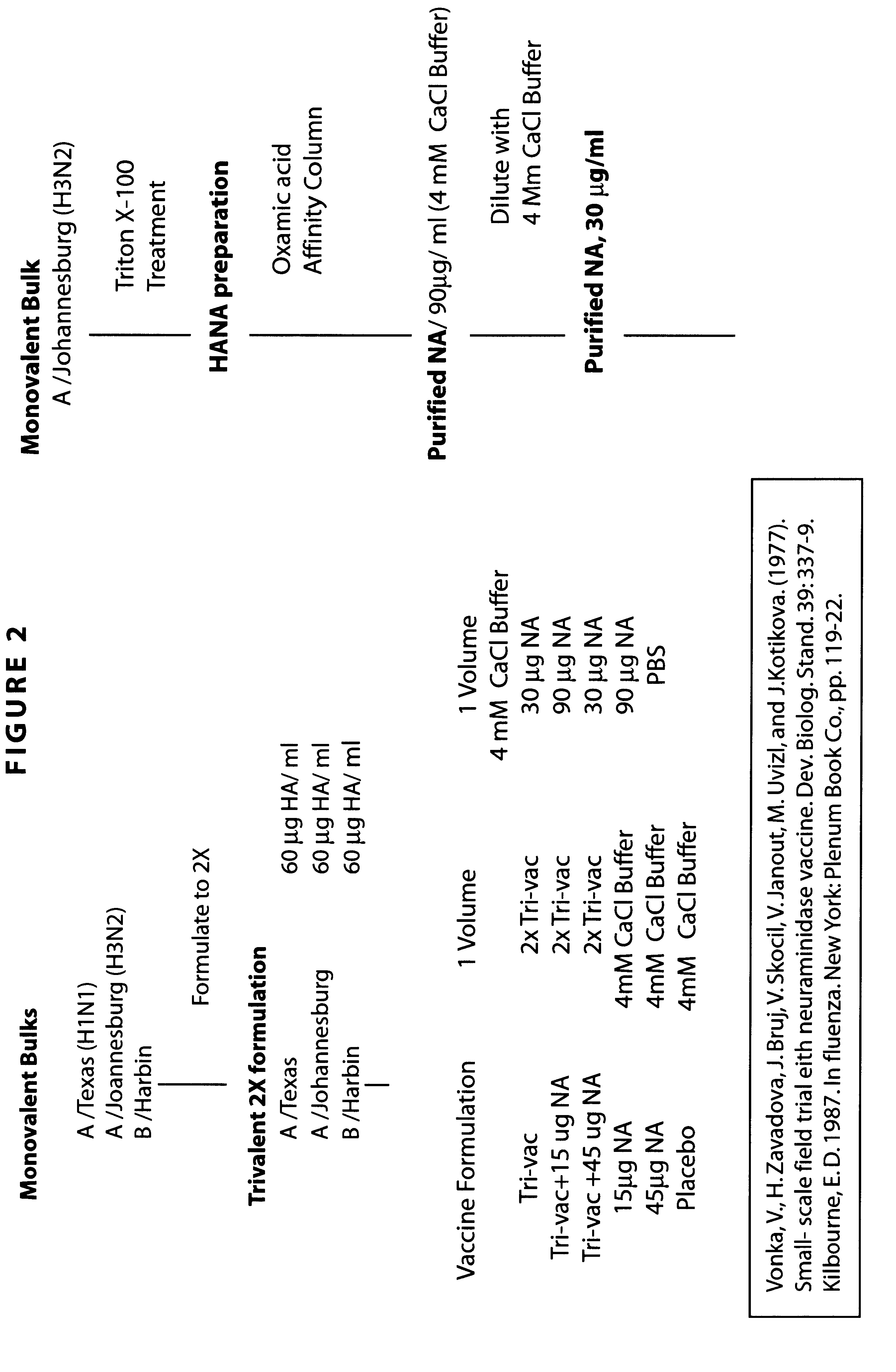
Neuraminidase-supplemented compositions
InactiveUS6485729B1Less importancePrevents and lessens HA immunodominanceSsRNA viruses negative-senseAntibody mimetics/scaffoldsNasal cavityAdjuvant
An anti-influenza vaccine composition wherein the improvement is that the vaccine includes, as an additive, neuraminidase (NA). The base anti-influenza vaccine can be any commercially available anti-influenza vaccine. The composition can include and be administered with an adjuvant. The vaccine composition provides protection in a host, animal or human, against influenza infection, including viral replication and systemic infection. Oral, nasal or other mucosal or per needle administration, including intracutaneous, intradermal, intramuscular, intravascular, and intravenous, are included.
Owner:PROTEIN SCI
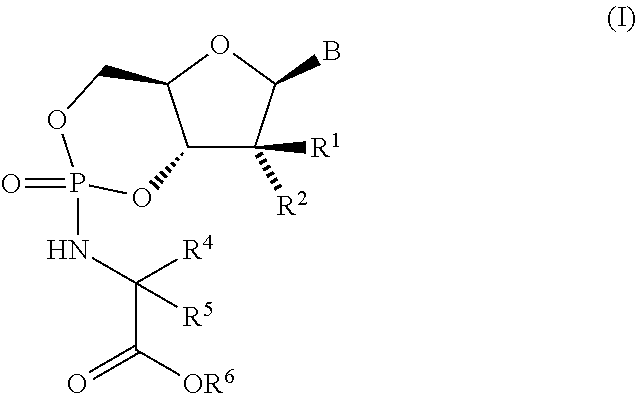
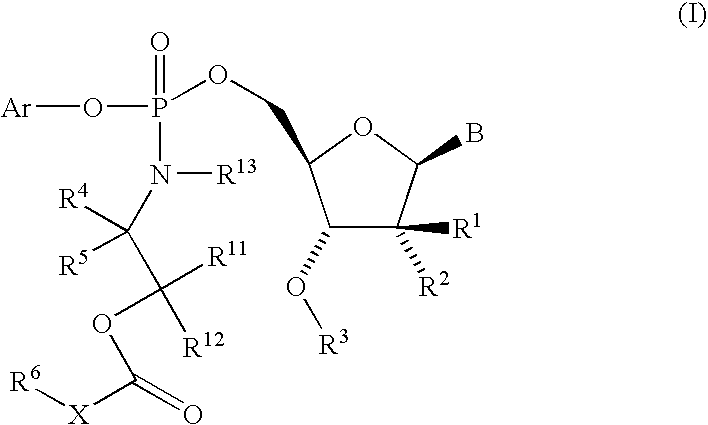
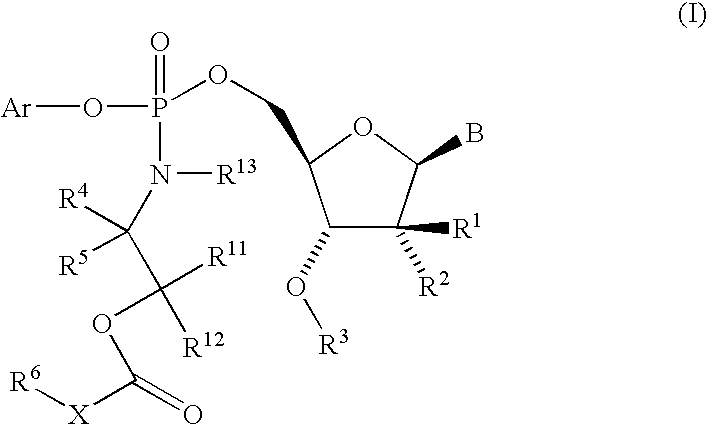
Nucleoside cyclic phosphoramidates for the treatment of RNA-dependent RNA viral infection
ActiveUS8148349B2Effective penetrationLess susceptibleBiocideSugar derivativesRNA Virus InfectionsRNA-dependent RNA polymerase
The present invention provides nucleoside cyclic phosphoramidates of formula (I), pharmaceutical compositions comprising the compounds of formula (I) and methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection using the compounds of formula (I).
Owner:MSD ITAL +1
Nucleoside aryl phosphoramidates for the treatment of RNA-dependent RNA viral infection
ActiveUS20100035835A1Efficient cell penetrationWider therapeutic indexBiocideSugar derivativesPolymerase LStructural formula
The present invention provides nucleoside aryl phosphoramidates of structural formula (I) which are precursors to inhibitors of RNA-dependent RNA viral polymerase. These compounds are precursors to inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as precursors to inhibitors of hepatitis C virus (HCV) NS5B polymerase, as precursors to inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such nucleoside aryl phosphoramidates alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the nucleoside aryl phosphoramidates of the present invention.
Owner:MSD ITAL +1
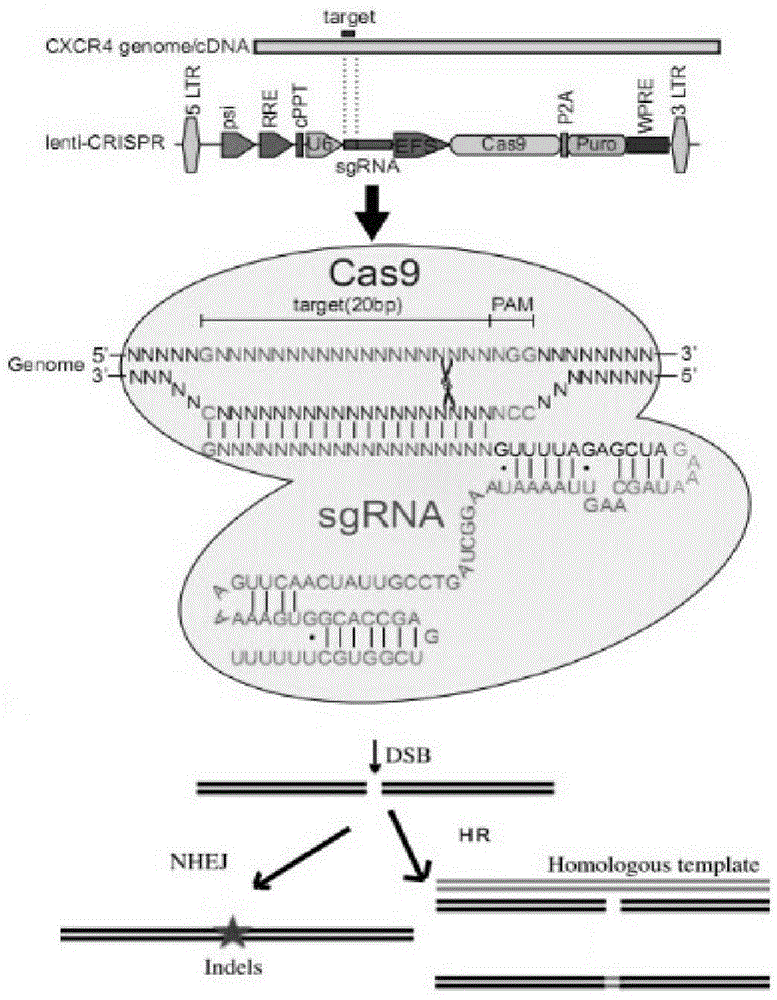
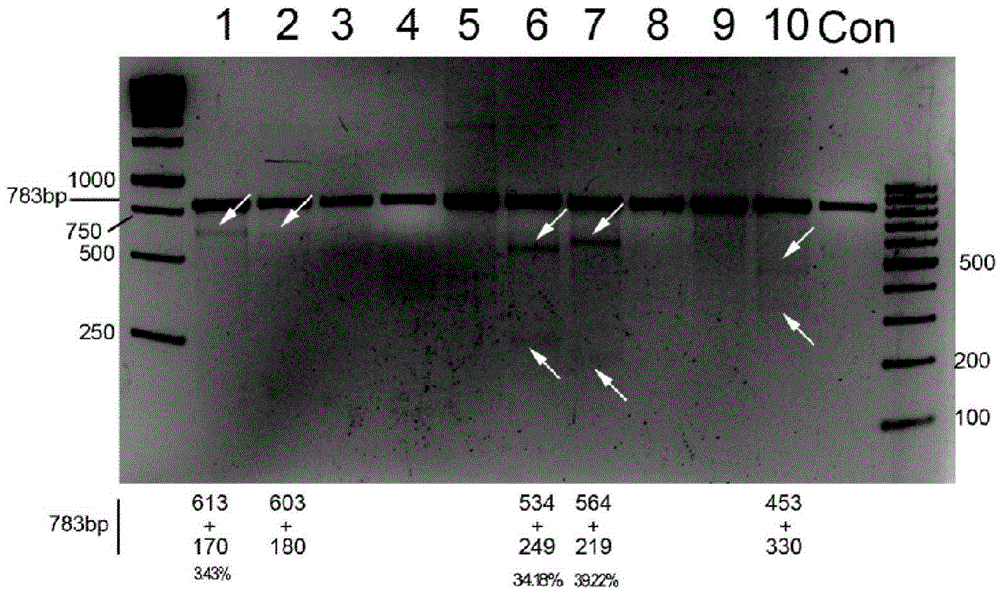
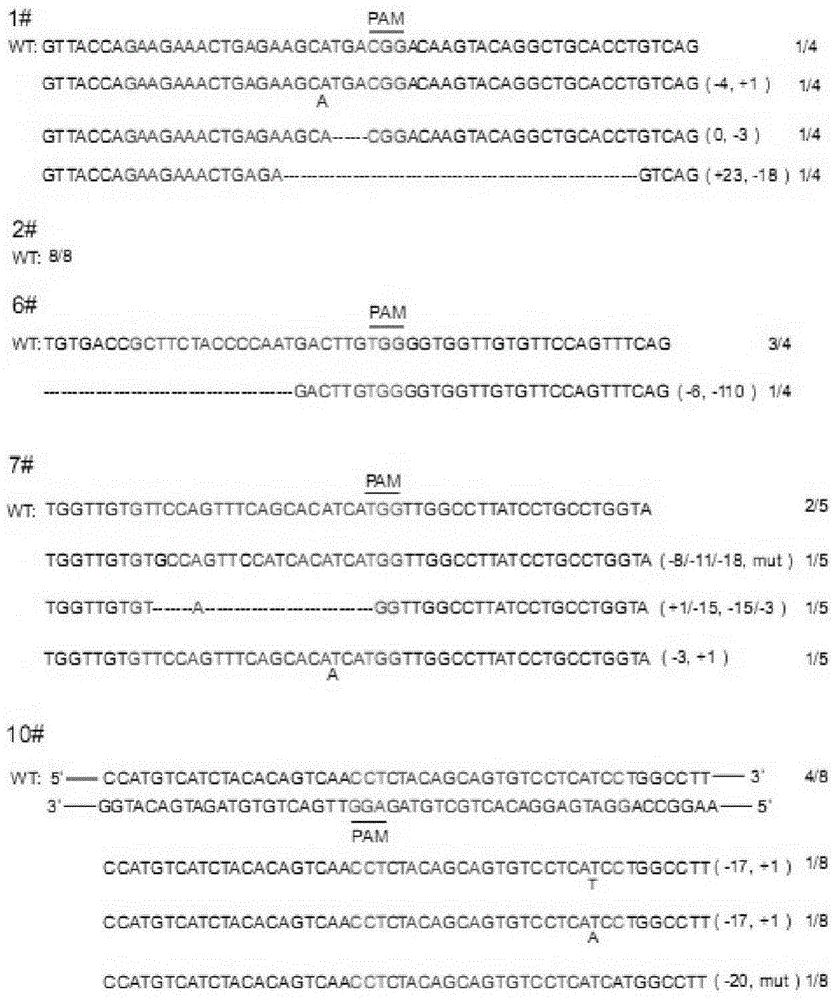
CRISPR/Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and lentivirus of CRISPR/Cas9 recombinant lentiviral vector
ActiveCN104480144AAvoid or delay intrusionInhibit the spread of infectionGenetic material ingredientsAntiviralsEnzyme digestionCXCR4
The invention belongs to the field of pharmaceutical and biological engineering, and relates to a CRISPR / Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and a lentivirus of the CRISPR / Cas9 recombinant lentiviral vector. The recombinant lentiviral vector is prepared by carrying out enzyme digestion on a lentiviral vectorlentiCRISPR by BsmBI and connecting into a BsmBI cohesive end-containing CXCR4 specific target sequence to recombine; the obtained CRISPR / Cas9 recombinant lentiviral vector is capable of mutating gene sequences at four different loci of ahuman immunodeficiency virusco-receptor CXCR4 and themutatuin rate is high and up to 25-75%. The cells transformed by the recombinant lentiviral vector cannot be infected by the human immunodeficiency virus. Compared with theRNAi-Knockdown, ZFN and TALEN technologies, the method has higher efficiency of suppressing the human immunodeficiency virus replication; the system is rapid to construct, simple and low in cost, is capable of preventing the invasion of the human immunodeficiency virus and is suitable for human immunodeficiency virus gene therapy.
Owner:WUHAN UNIV
Inhibitors of hepatitis c virus replication
The present invention relates to compounds of formula (I) that are useful as hepatitis C virus (HCV) NS5A inhibitors, the synthesis of such compounds, and the use of such compounds for inhibiting HCV NS5A activity, for treating or preventing HCV infections and for inhibiting HCV viral replication and / or viral production in a cell-based system.
Owner:MERCK SHARP & DOHME LLC
Nucleoside aryl phosphoramidates for the treatment of RNA-dependent RNA viral infection
ActiveUS8071568B2Effective penetrationLess susceptibleBiocideSugar derivativesPolymerase LPhosphoramidate
The present invention provides nucleoside aryl phosphoramidates of structural formula (I) which are precursors to inhibitors of RNA-dependent RNA viral polymerase. These compounds are precursors to inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as precursors to inhibitors of hepatitis C virus (HCV) NS5B polymerase, as precursors to inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such nucleoside aryl phosphoramidates alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the nucleoside aryl phosphoramidates of the present invention. (I)
Owner:MSD ITAL +1
Methods and compositions for efficient gene transfer using transcomplementary vectors
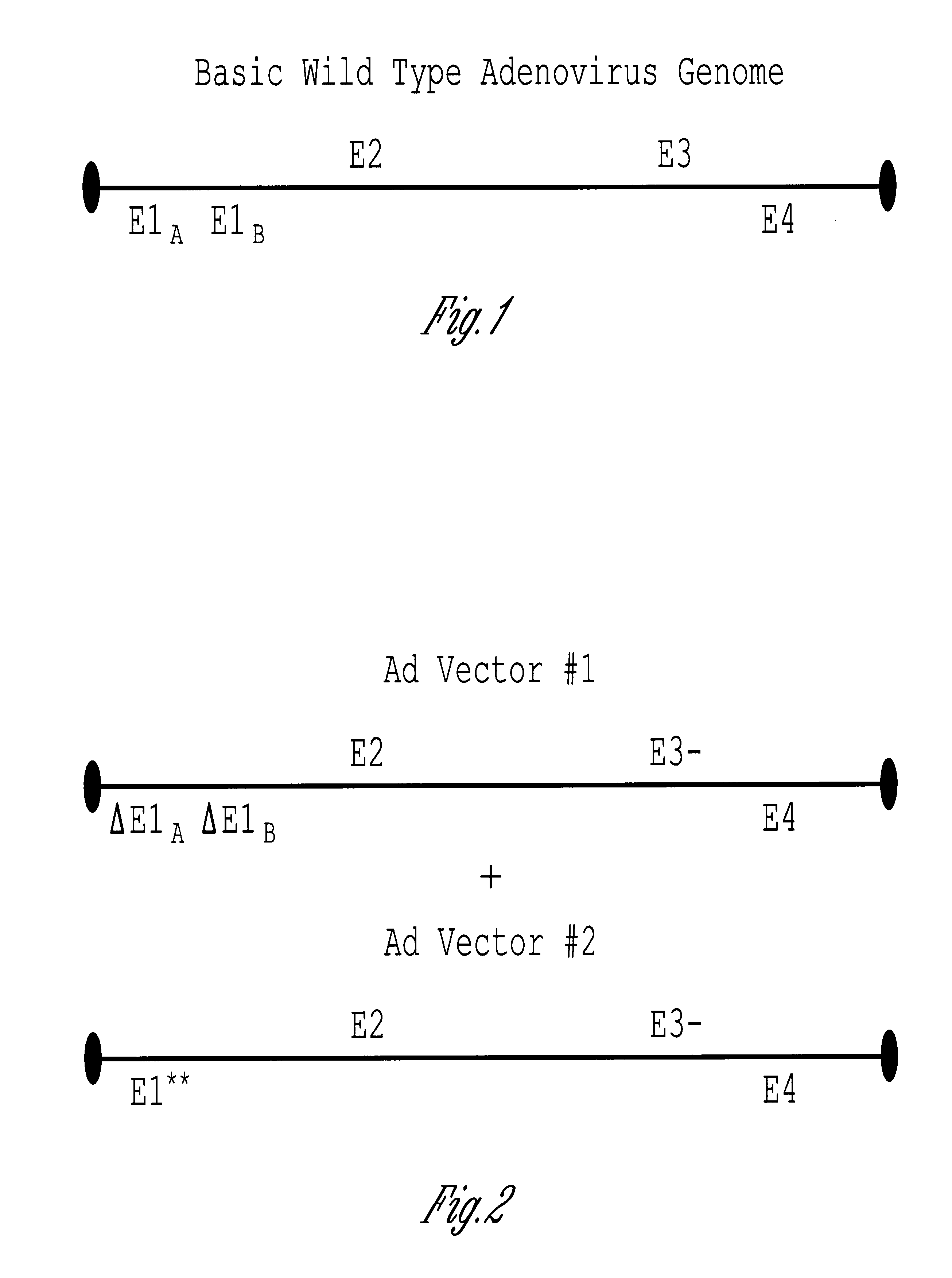
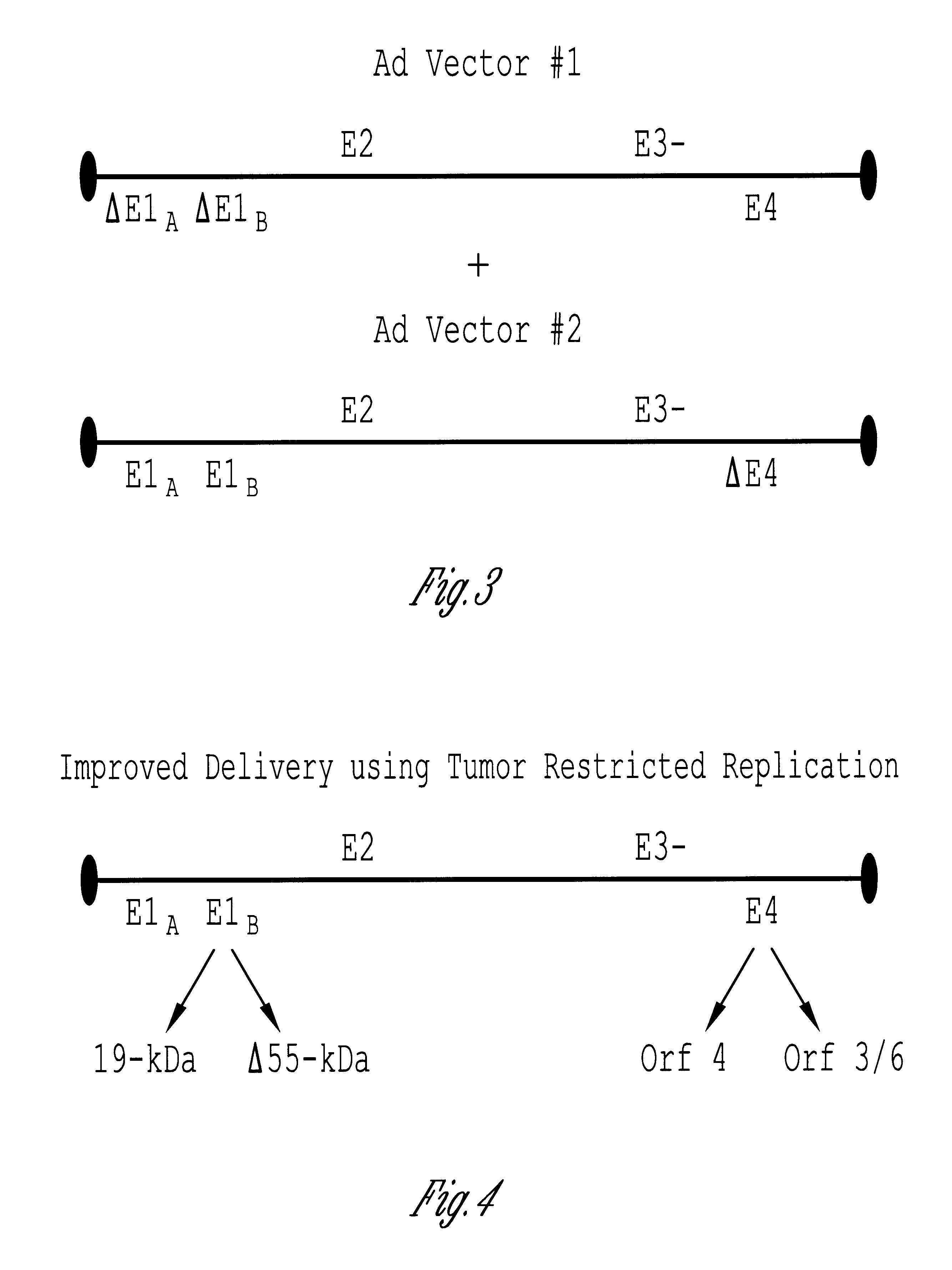
The invention includes a viral vector method and composition comprising transcomplementary replication incompetent viral vectors, preferably adenoviral vectors, which are cotransformed to a recipient cell. The two vectors complement each other and thus allow viral replication, in a synergistic combination which enhances both gene delivery and gene expression of genetic sequences contained within the vector.
Owner:HUMAN GENE THERAPY RES INST
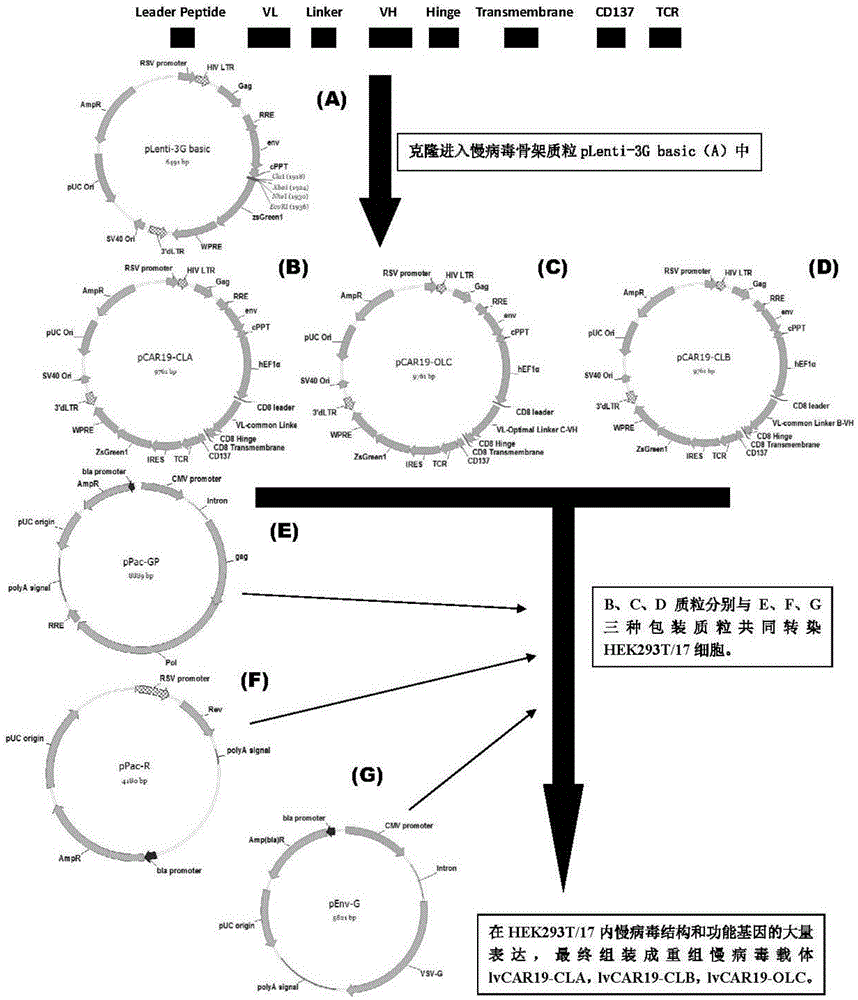
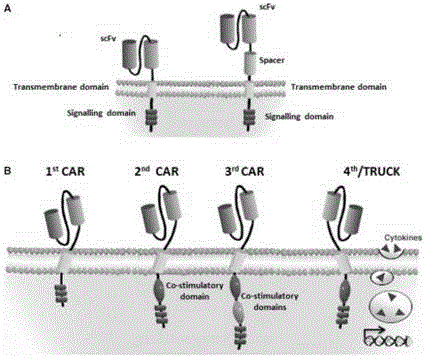
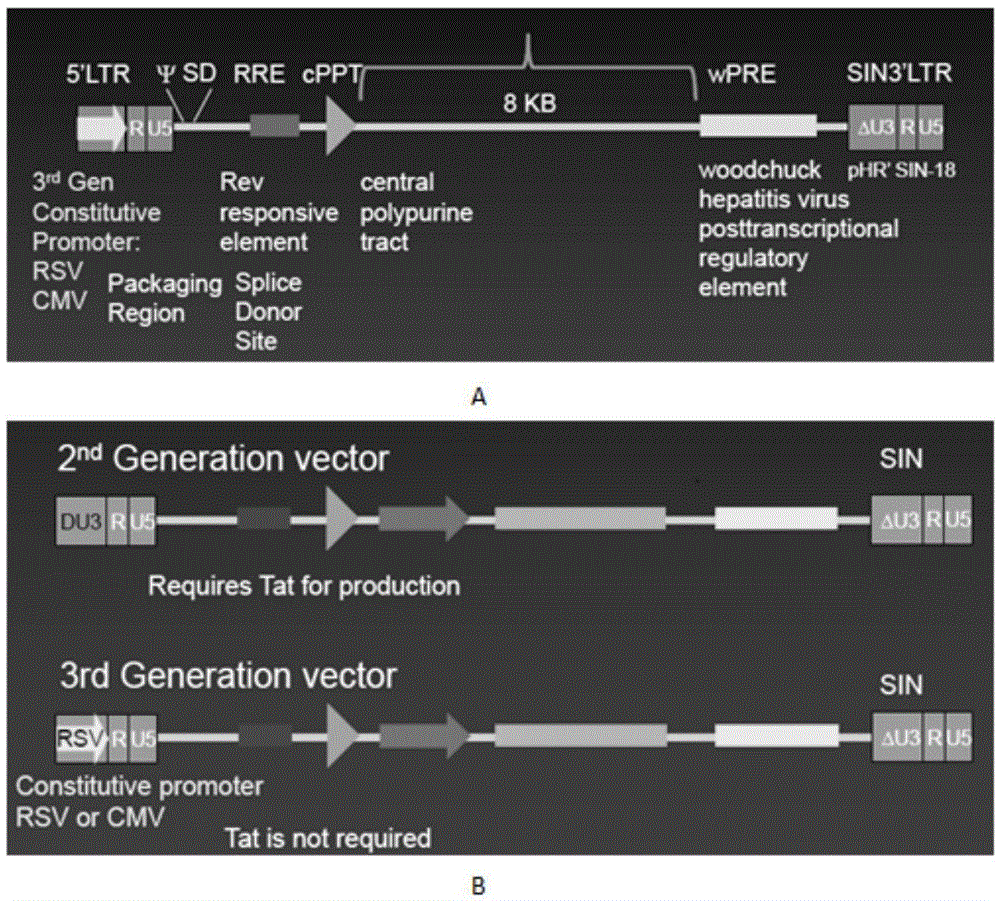
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Insect bioreactor expressing multiple exogenous genes and its construction method and application
The invention discloses an insect bioreactor capable of expressing multiple exogenous genes, and a construction method and application thereof. The construction method comprises the following steps: (1) introducing multicopy high-efficiency bacteria DNA (deoxyribonucleic acid) replication initiator into chitinase and cysteine proteinase genes of a baculovirus genome to obtain a baculovirus shuttle plasmid; (2) replacing virus duplicated essential gene downstream the polyhedrosis gene of the baculovirus shuttle plasmid with antibiotic gene to obtain a baculovirus plasmid DNA; and (3) replacingother virus duplicated and infected nonessential genes in the baculovirus shuttle plasmid with reverse selection marker gene to obtain the insect bioreactor. The antibiotic gene or reverse selection marker gene in the insect bioreactor which is constructed by replacing the exogenous target genes can express multiple exogenous genes in a host insect or insect cell. The insect bioreactor disclosed by the invention can efficiently expressing one or more exogenous genes in an insect body at the same time, and can produce massive recombinant proteins at low cost.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
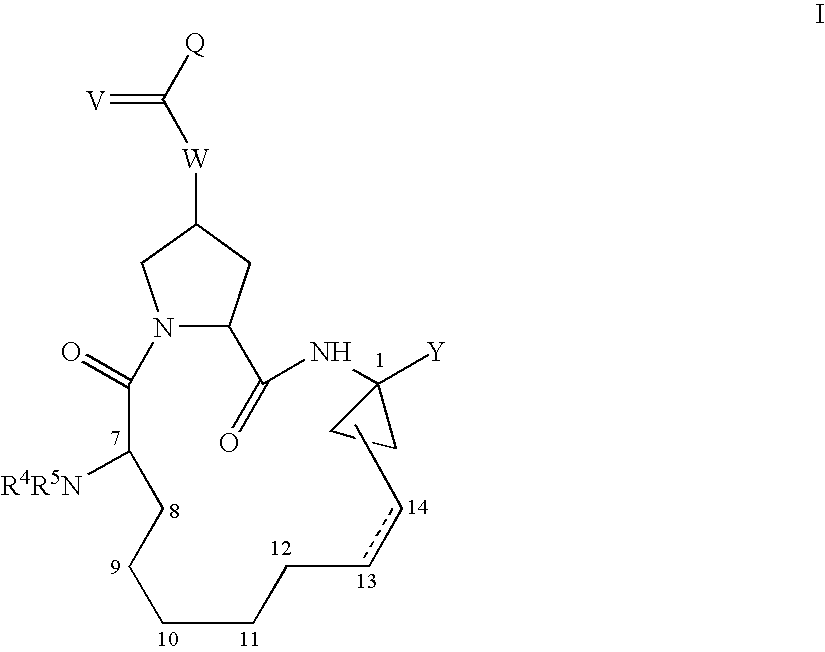
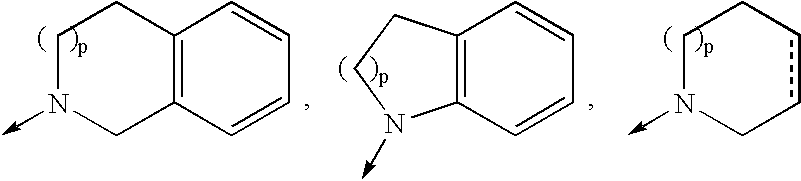
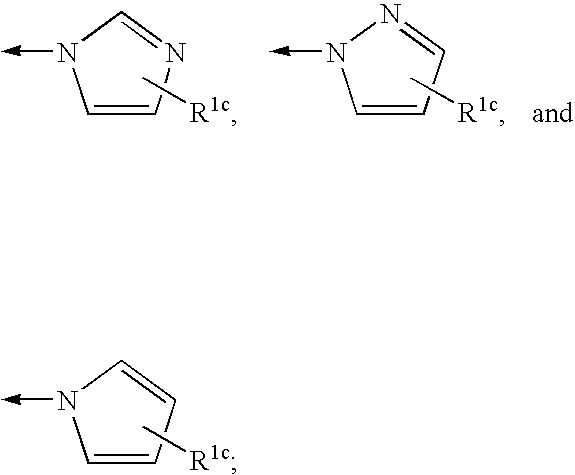
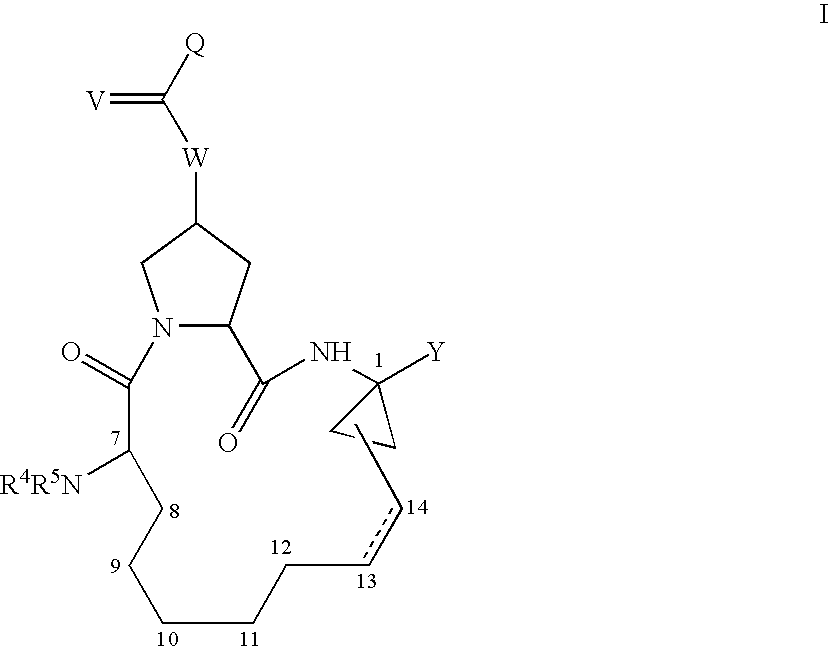
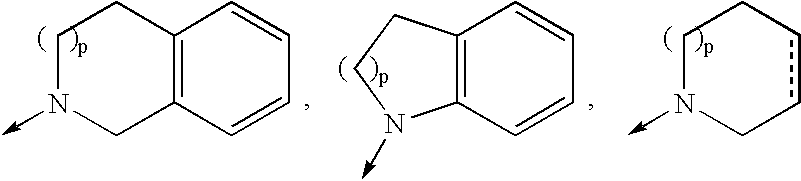
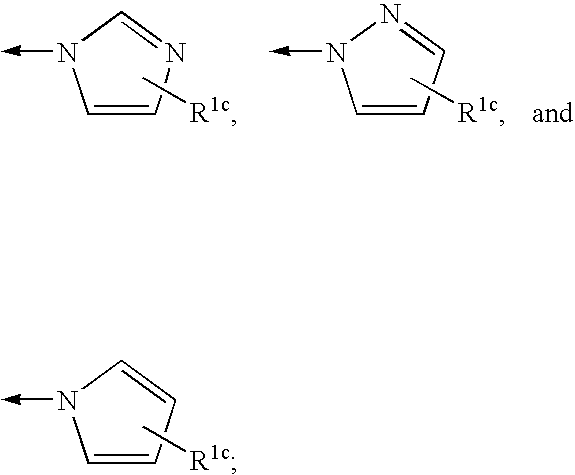
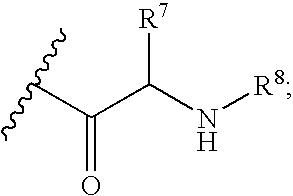
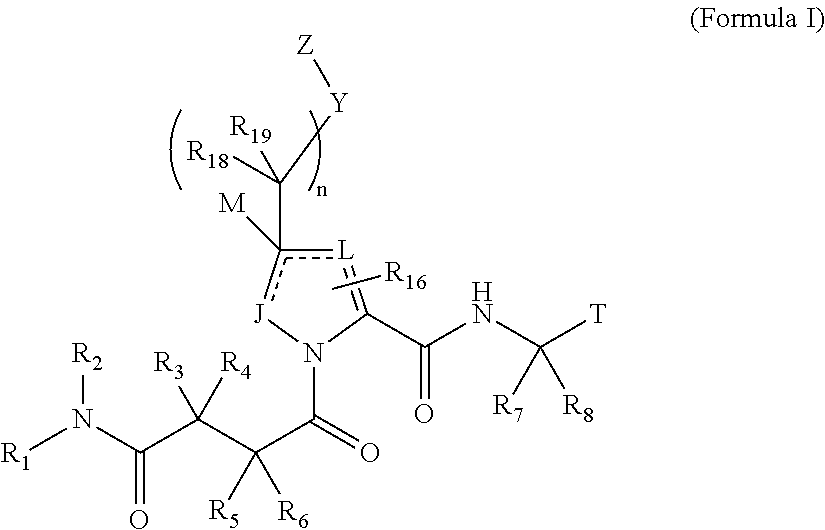
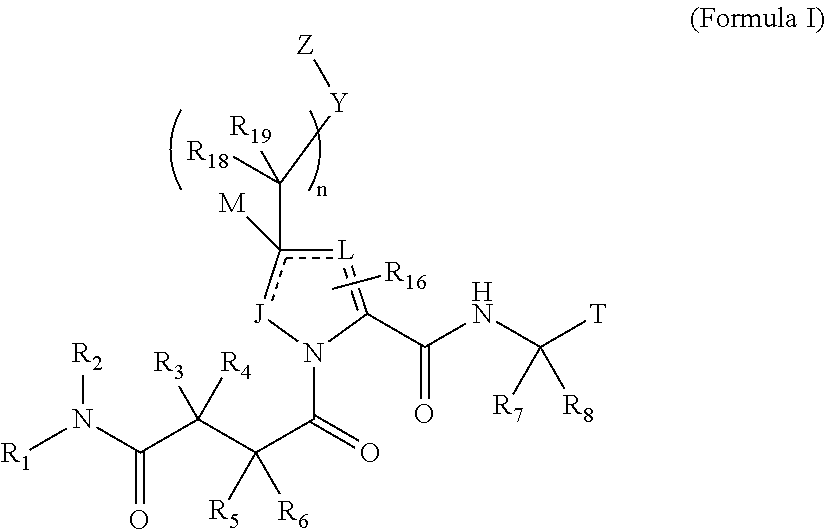
4-amino-4-oxobutanoyl peptides as inhibitors of viral replication
The invention provides 4-amino-4-oxobutanoyl peptide compounds of Formula Iand the pharmaceutically salts and hydrates thereof.The variables R1-R9, R16, R18, R19, n, M, n, M, and Z are defined herein. Certain compounds of Formula I are useful as antiviral agents. Certain 4-amino-4-oxobutanoyl peptide compounds disclosed herein are potent and / or selective inhibitors of viral replication, particularly Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more 4-amino-4-oxobutanoyl peptide compounds and one or more pharmaceutically acceptable carriers. Such pharmaceutical compositions may contain 4-amino-4-oxobutanoyl peptide compound as the only active agent or may contain a combination of 4-amino-4-oxobutanoyl peptide containing peptides compound and one or more other pharmaceutically active agents. The invention also provides methods for treating viral infections, including Hepatitis C infections, in mammals.
Owner:ACHILLION PHARMA INC
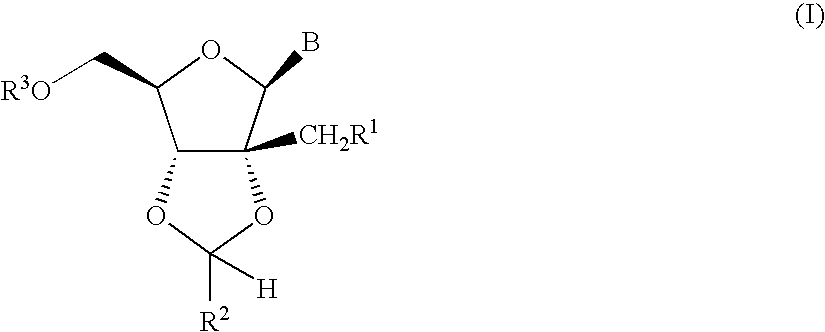
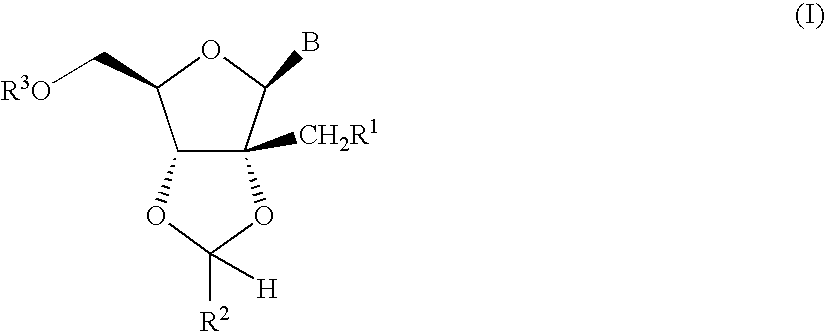
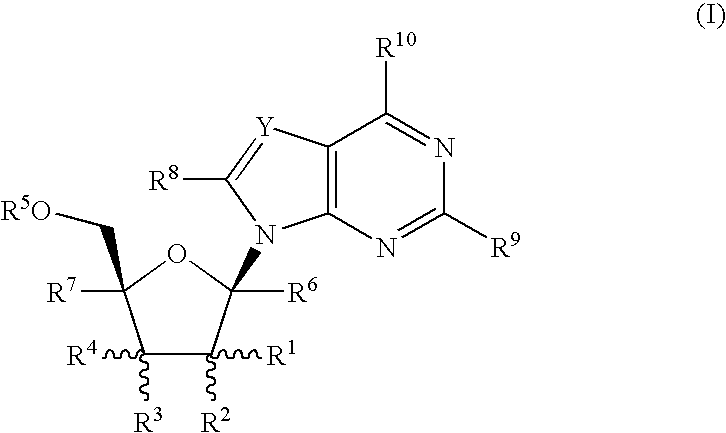
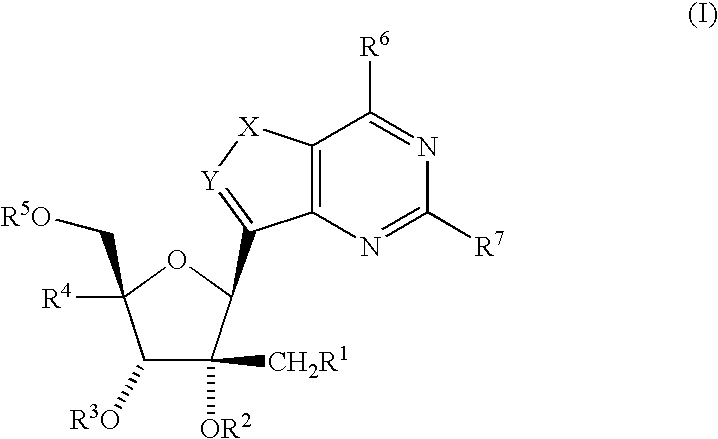
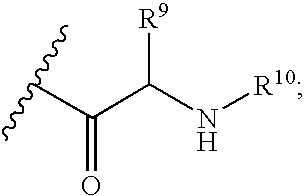
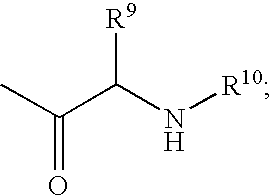
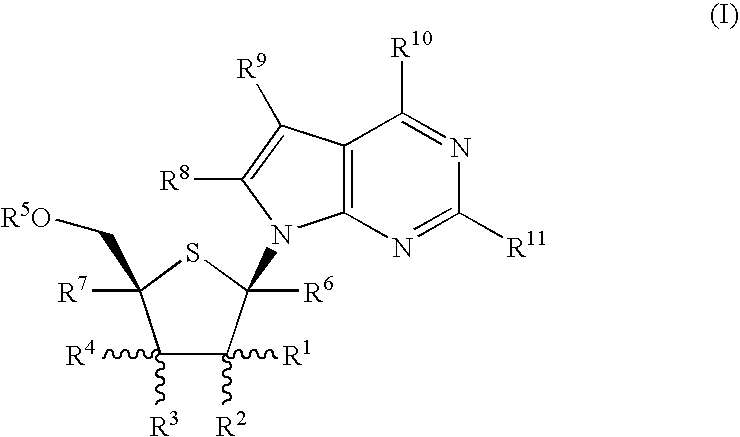
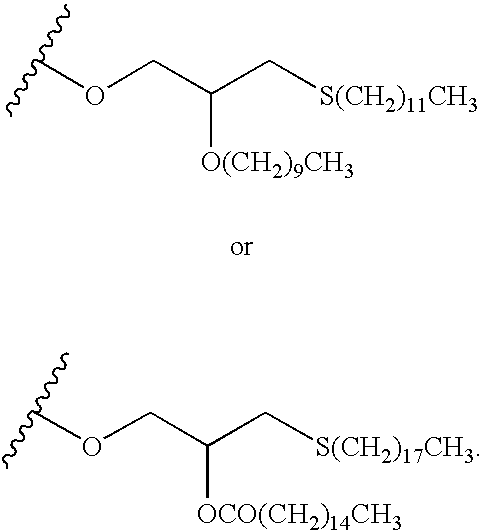
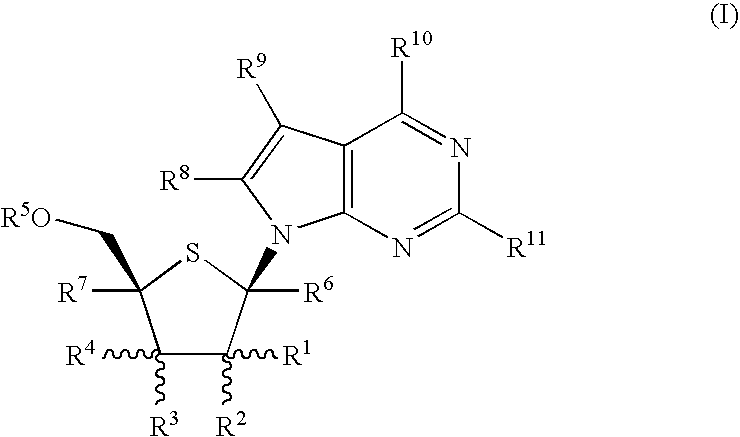
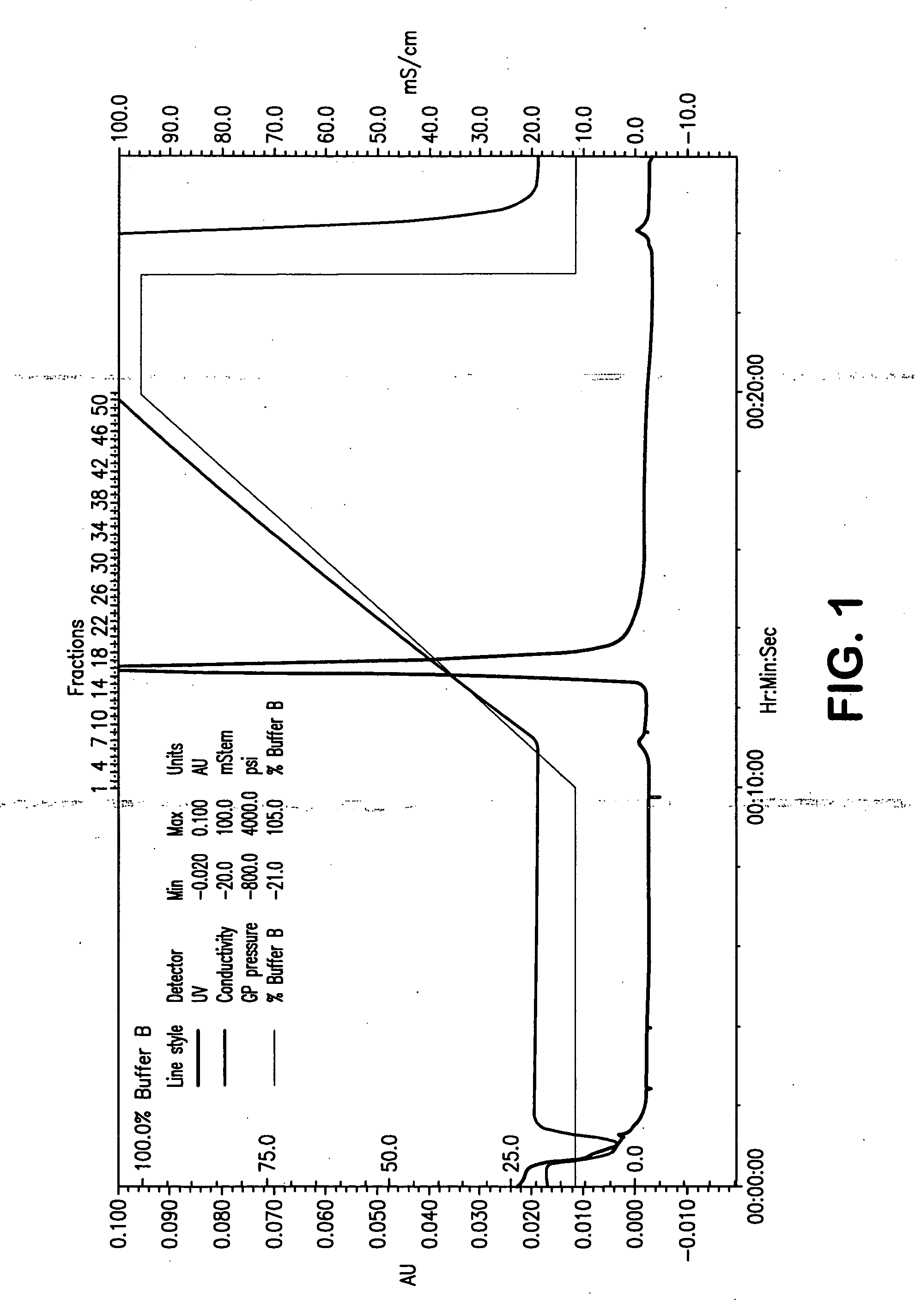
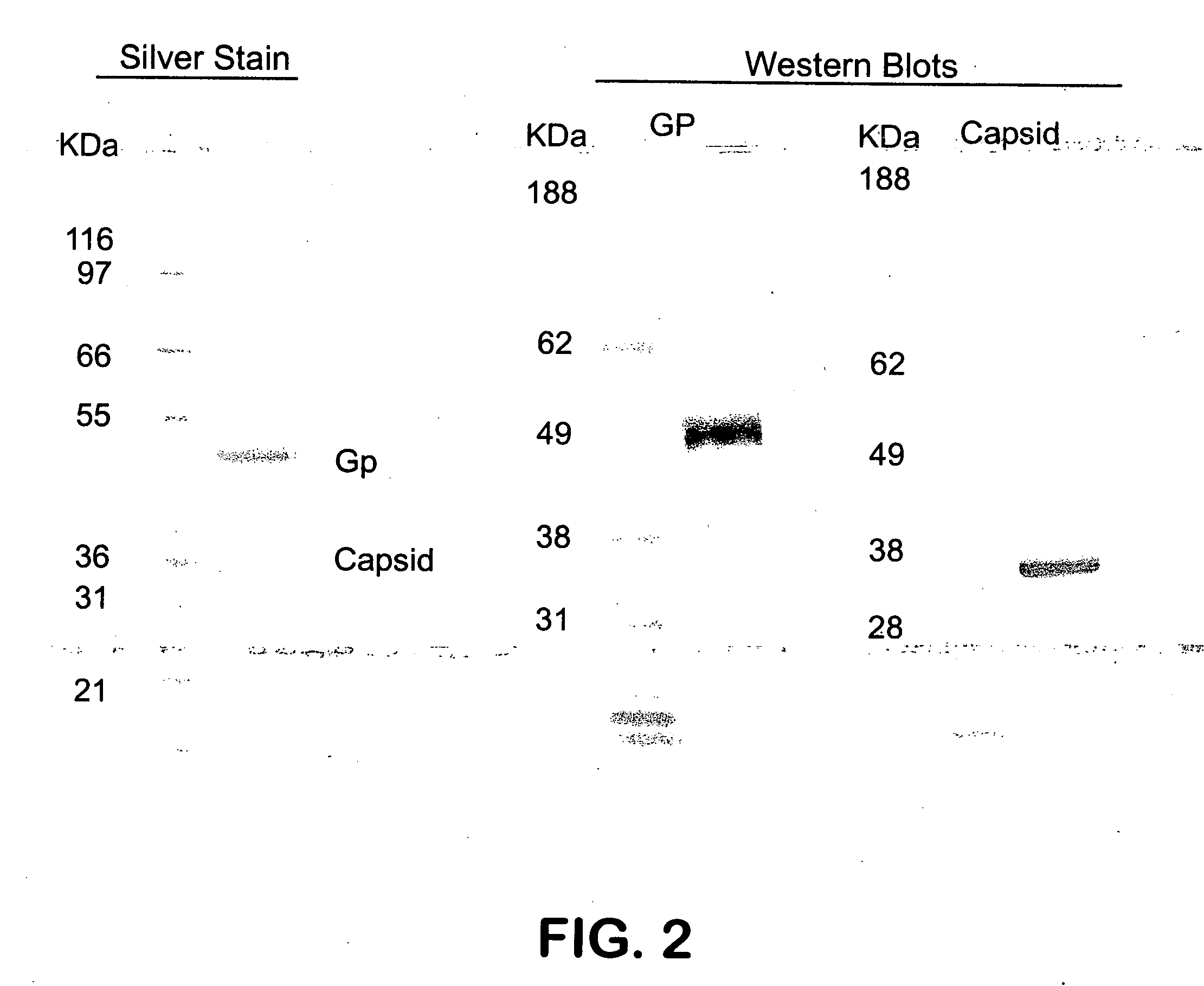
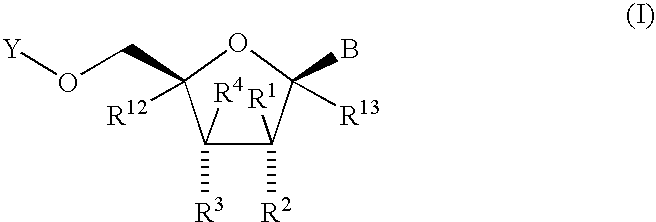
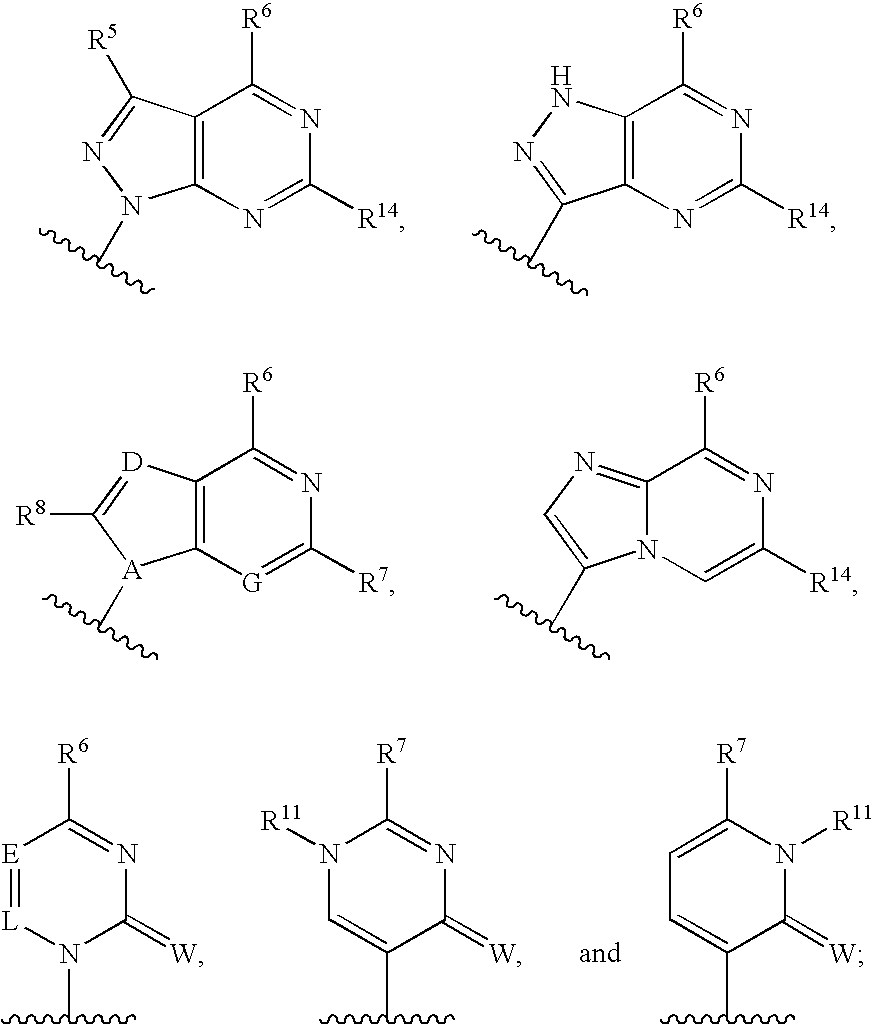
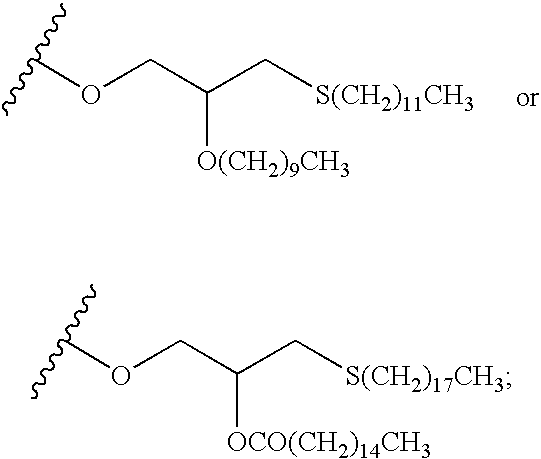
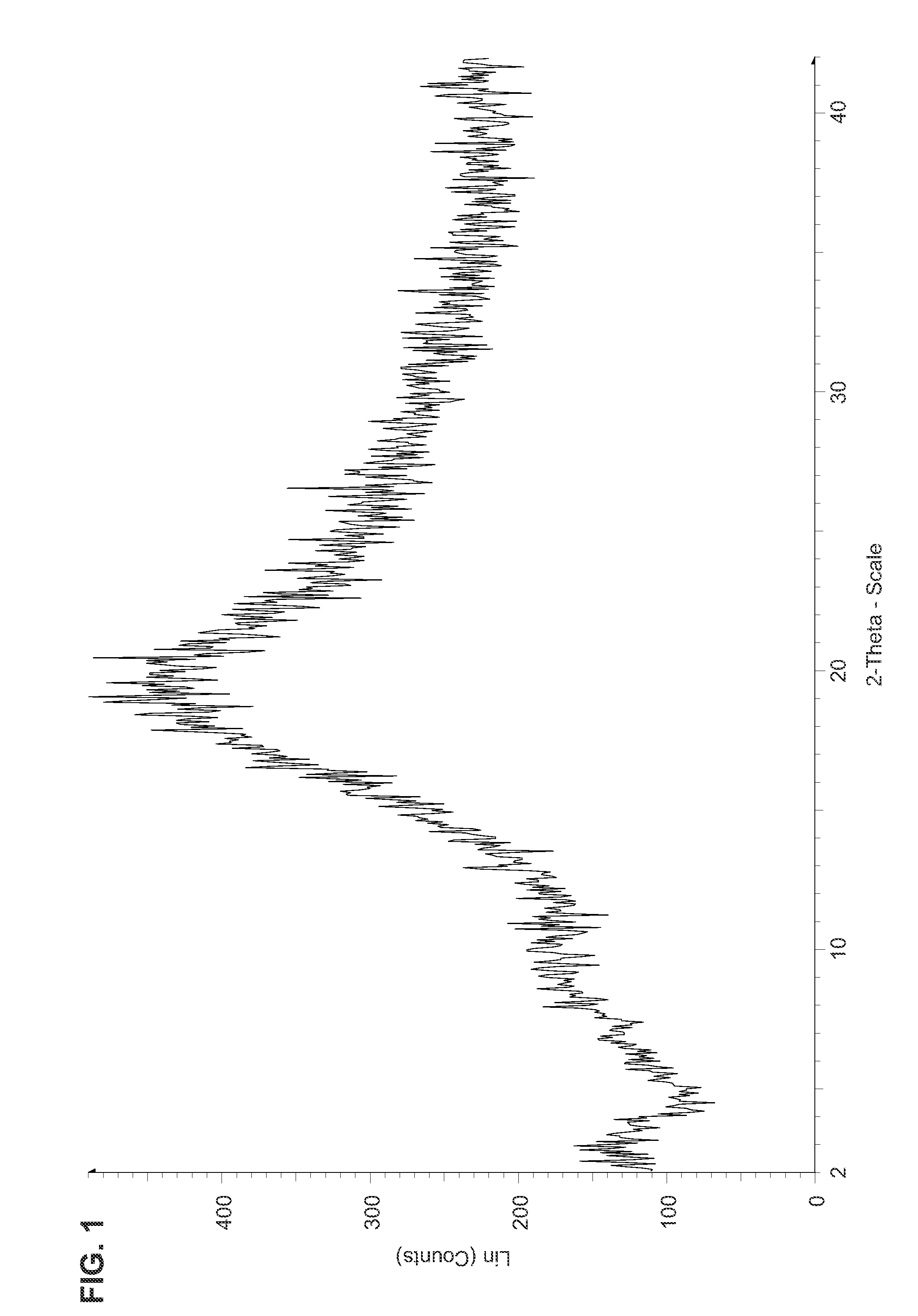
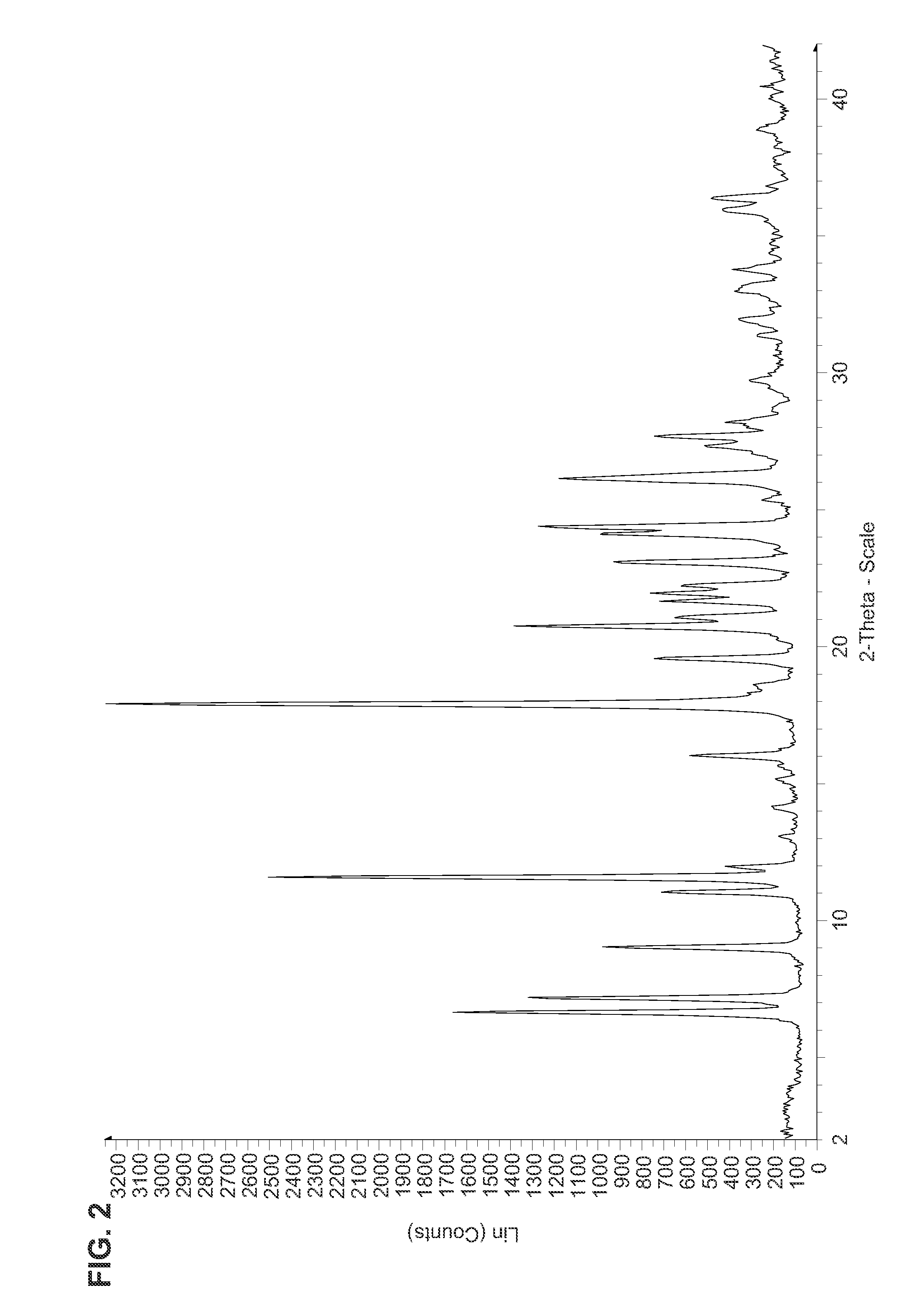
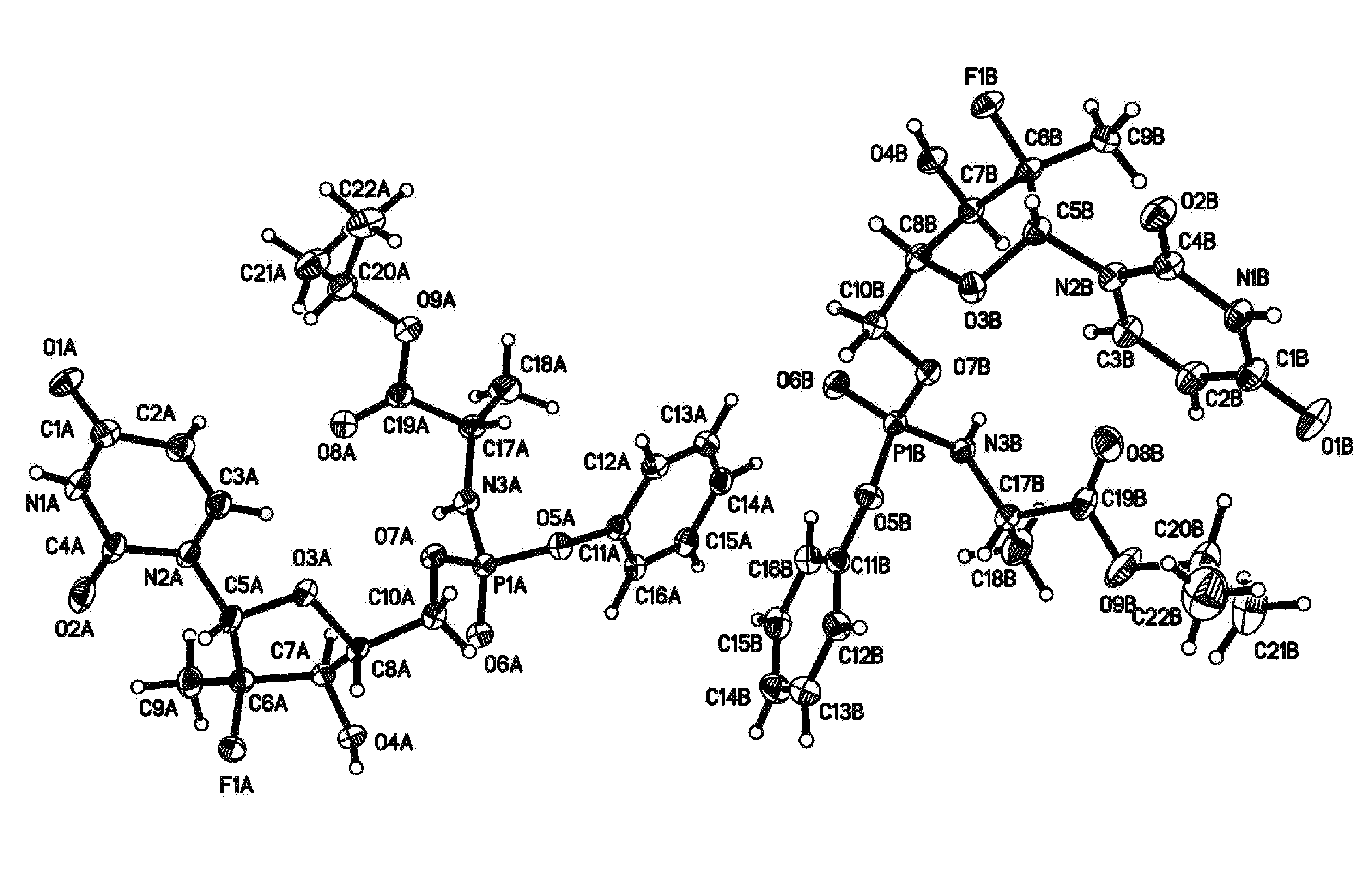
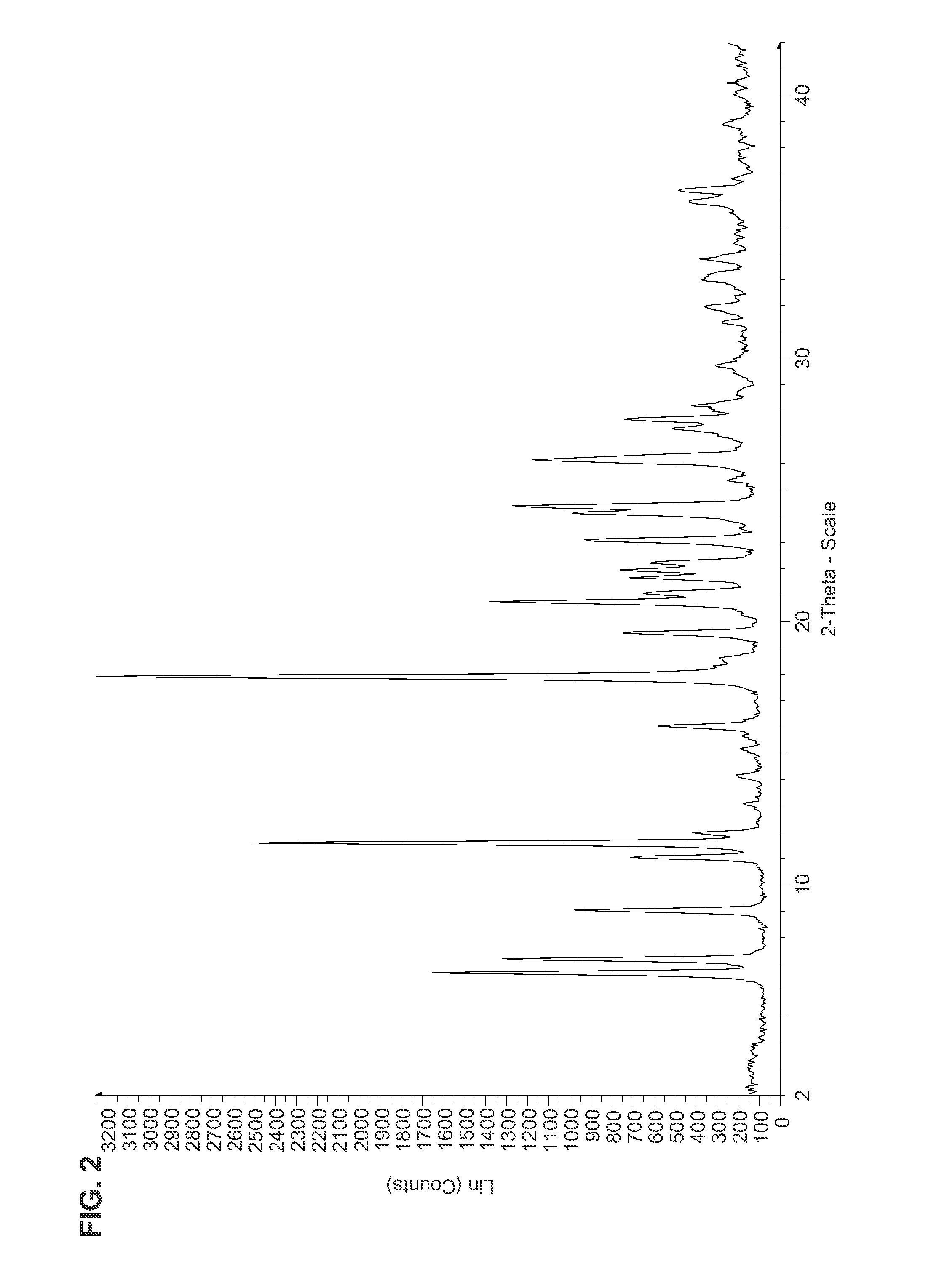
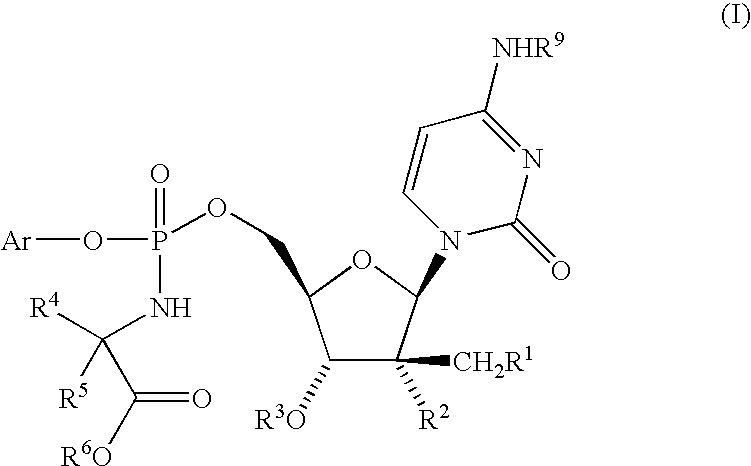
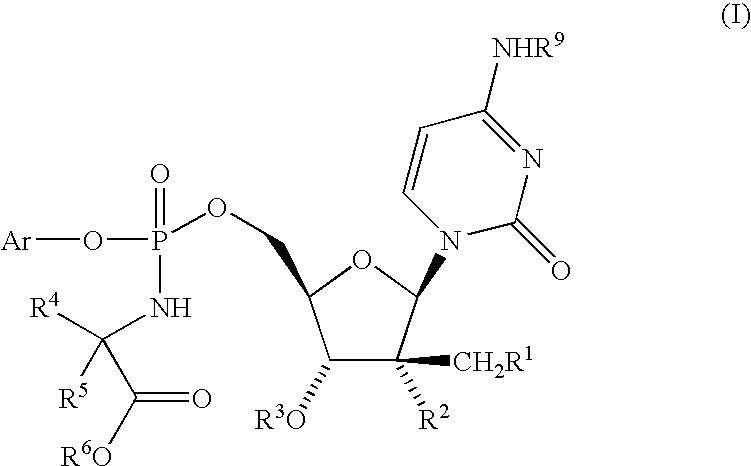
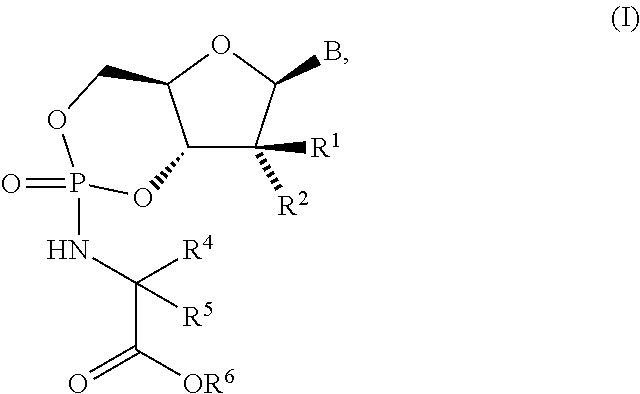
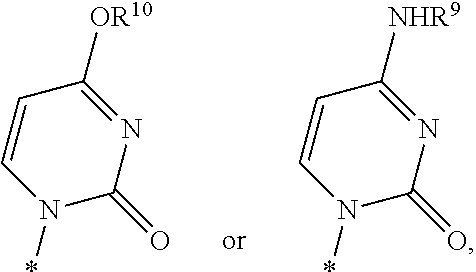
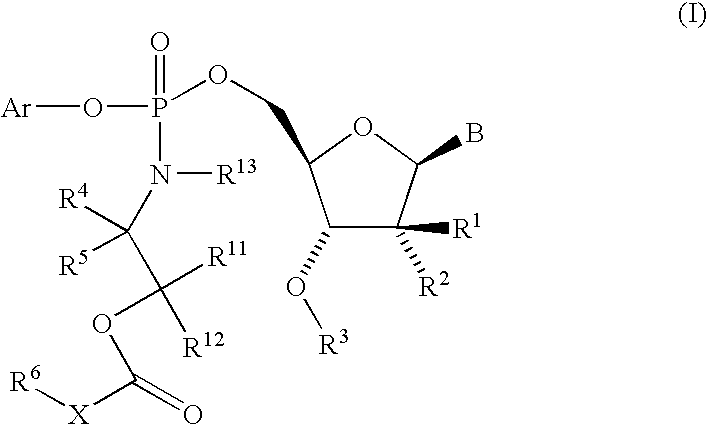
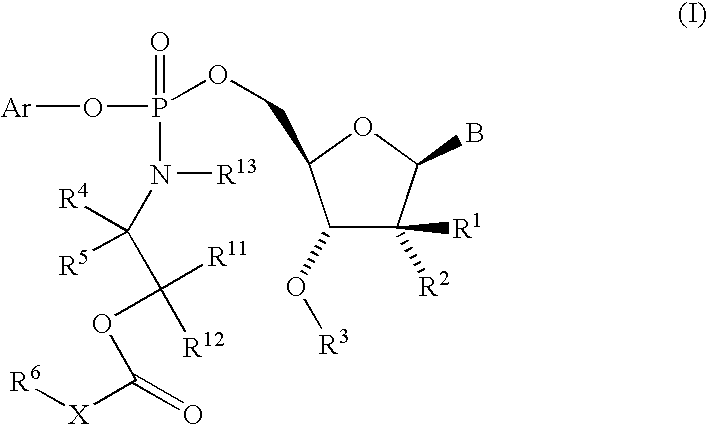
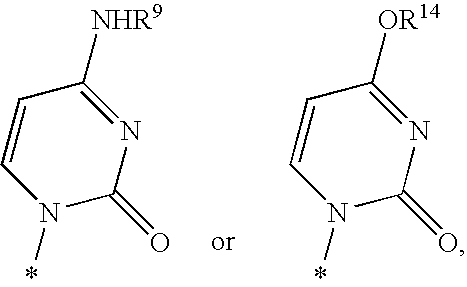
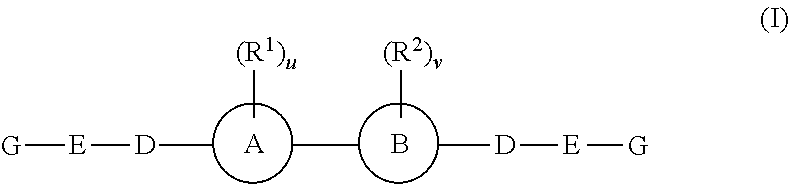
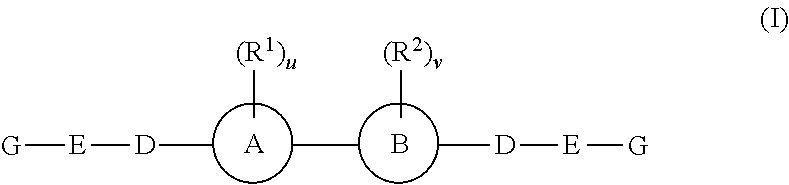
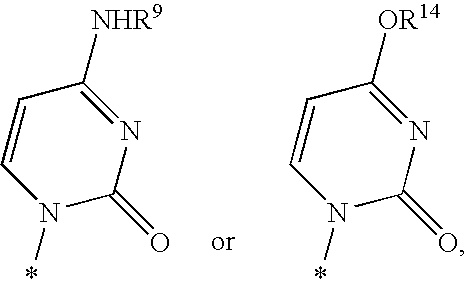
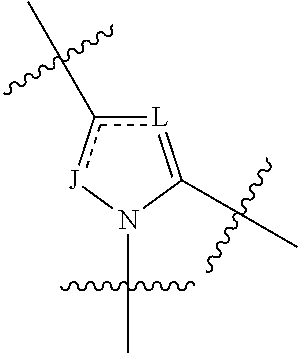
Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection
The present invention provides fluorinated pyrrolo[2,3,d]pyrimidine nucleoside compounds which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as precursors to inhibitors of hepatitis C virus (HCV) NS5B polymerase, as precursors to inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such fluorinated pyrrolo[2,3-d]pyrimidine nucleoside alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the fluorinated pyrrolo[2,3-d]pyrimidine nucleoside of the present invention.
Owner:MERCK & CO INC
Compositions and methods for helper-free production of recombinant adeno-associated viruses
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
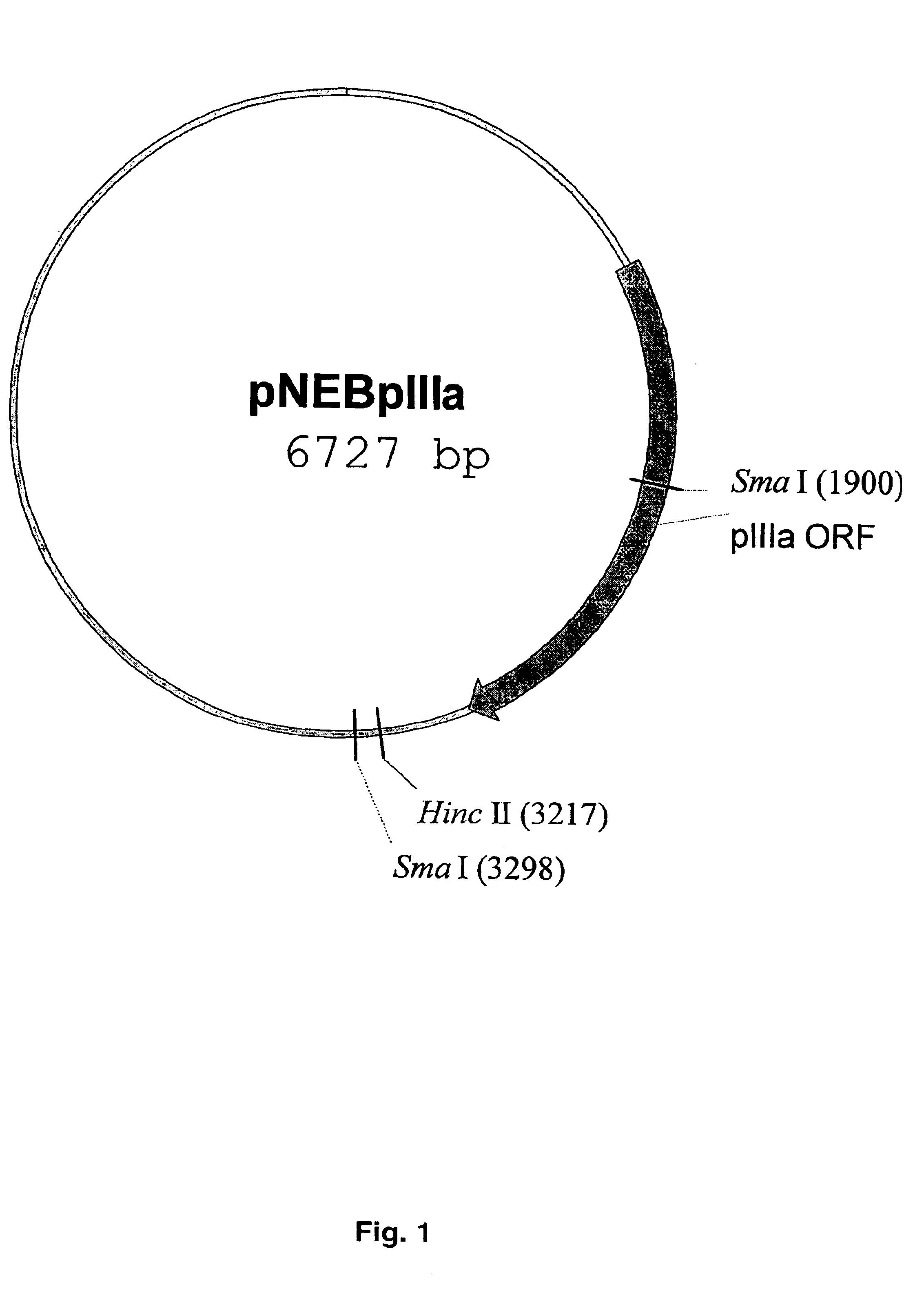
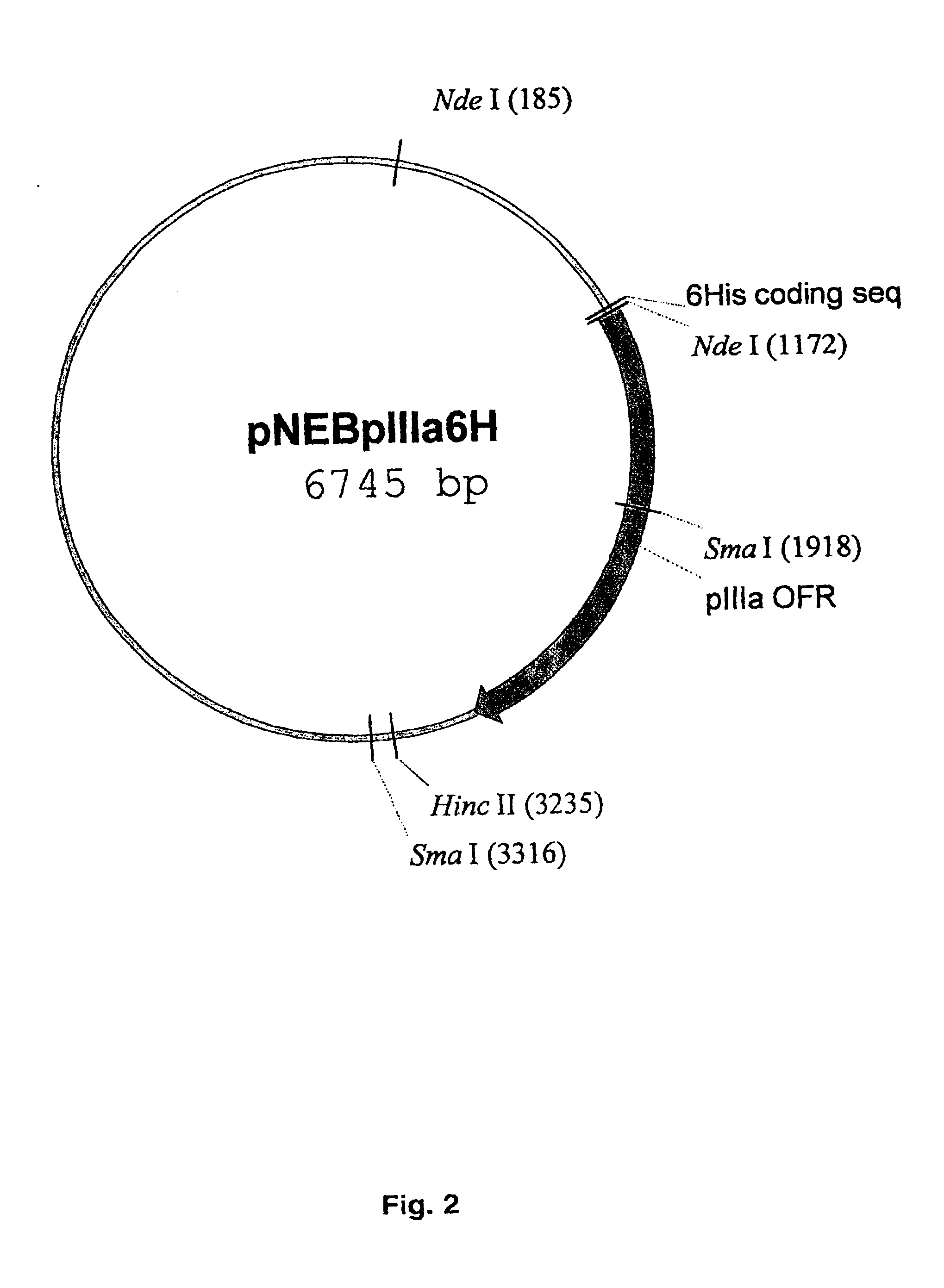
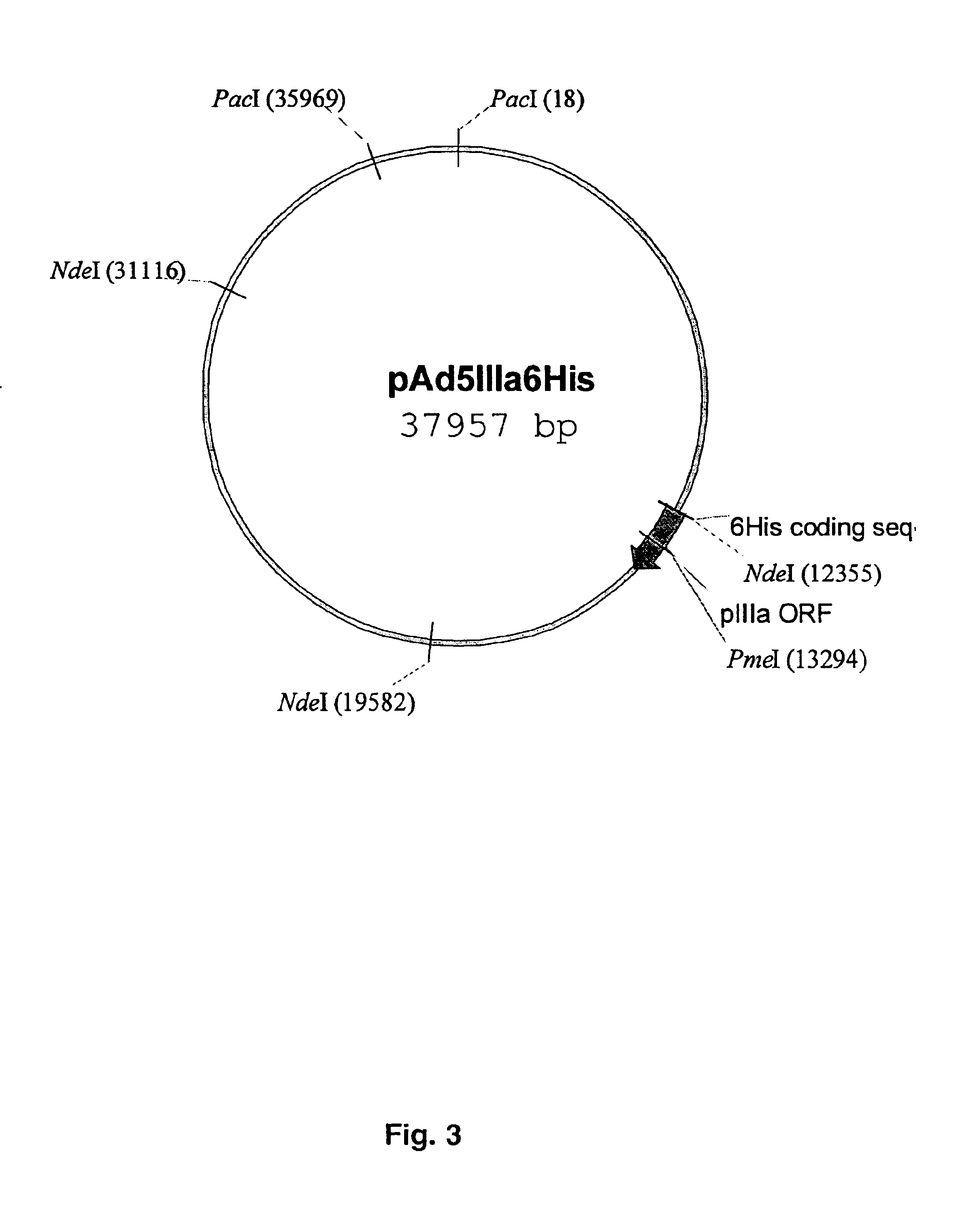
Capsid-modified recombinant adenovirus and methods of use
InactiveUS6955808B2Low yieldEfficiencyBiocideAntibody mimetics/scaffoldsSingle-Chain AntibodiesNoninvasive imaging
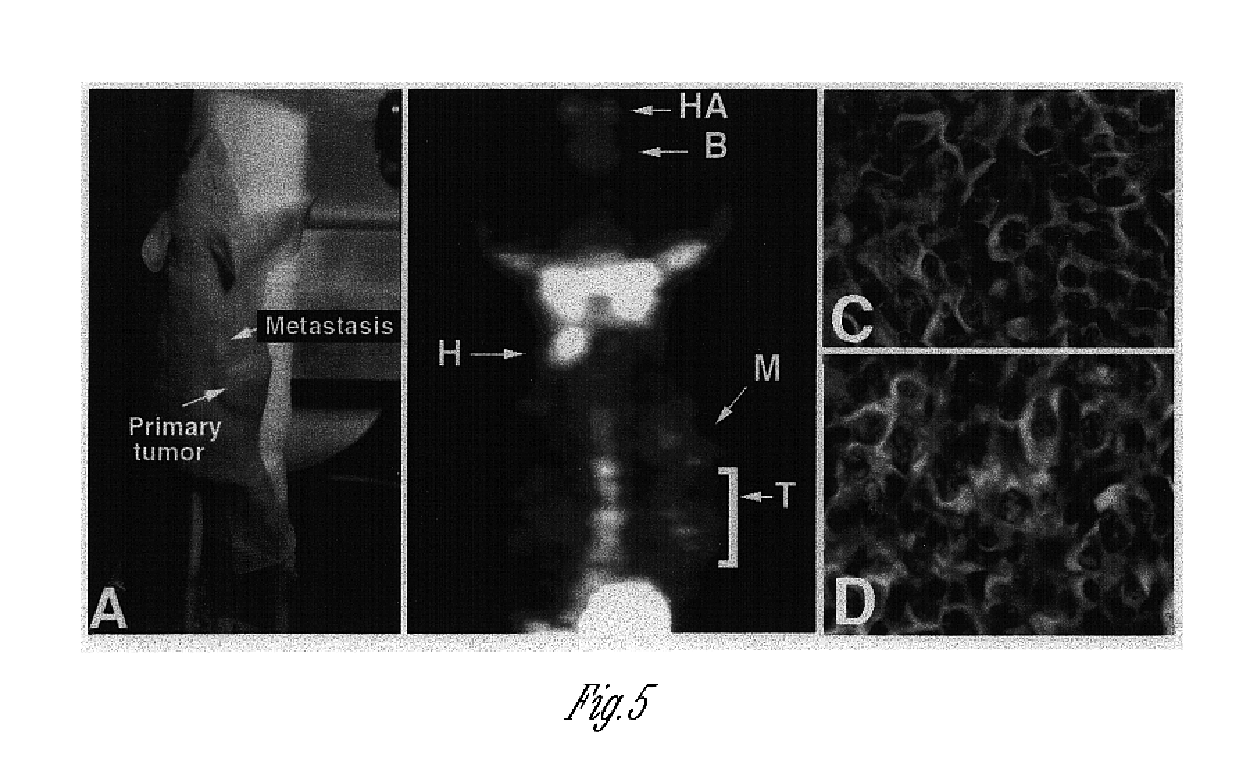
The present invention describes recombinant adenoviral vectors modified by incorporating targeting ligands or label into viral capsid or structural proteins. In one embodiment, single-chain antibody was introduced into the minor capsid proteins pIIIa or pIX so that the adenoviral vector can be targeted to a particular cell type. In another embodiment, there is provided a noninvasive imaging strategy useful for monitoring the replication and spread of conditionally replicative adenoviral vectors. Viral structural proteins such as pIX capsid protein, core proteins mu, V and VII were expressed as fusion protein with a fluorescent label. Once incorporated into the virions, detection of the structural fusion protein label would indicate the localization of the disseminated viral progeny. The detected fluorescent signals also closely correlate with the level of viral replication and progeny production.
Owner:UAB RES FOUND
Ribonucleoside cyclic acetal derivatives for the treatment of RNA-dependent RNA viral infection
The present invention provides ribonucleoside 2′,3′-cyclic acetals of structural formula I which are precursors or prodrugs of inhibitors of RNA-dependent RNA viral polymerase. These compounds are precursors of inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as precursors or prodrugs of inhibitors of hepatitis C virus (HCV) NS5B polymerase, as precursors or prodrugs of inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such ribonucleoside 2′,3′-cyclic acetals alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the ribonucleoside 2′,3′-cyclic acetals of the present invention.
Owner:MERCK SHARP & DOHME CORP
Inhibitors of hepatitis C virus replication
ActiveUS8871759B2Organic active ingredientsDipeptide ingredientsHcv hepatitis c virusViral replication
The present invention relates to compounds of formula (I) that are useful as hepatitis C virus (HCV) NS5A inhibitors, the synthesis of such compounds, and the use of such compounds for inhibiting HCV NS5A activity, for treating or preventing HCV infections and for inhibiting HCV viral replication and / or viral production in a cell-based system.
Owner:MERCK SHARP & DOHME LLC
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:INTERMUNE INC
Nucleoside derivatives as inhibitors of rna-dependent rna viral polymerase
The present invention provides nucleoside compounds and certain derivatives thereof which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as inhibitors of hepatitis C virus (HCV) NS5B polymerase, as inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such nucleoside compounds alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the nucleoside compounds of the present invention.
Owner:OLSEN DAVID B +3
C-purine nucleoside analogs as inhibitors of RNA-dependent RNA viral polymerase
ActiveUS7534767B2BiocideSaccharide with heterocyclic radicalsRNA Virus InfectionsRNA-dependent RNA polymerase
The present invention provides C-purine nucleoside analogs and certain derivatives thereof which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as inhibitors of hepatitis C virus (HCV) NS5B polymerase, as inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such C-nucleoside compounds alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the C-nucleoside compounds of the present invention.
Owner:IONIS PHARMA INC +1
Compositions and methods for inhibiting viral replication
The present invention relates to a double-stranded ribonucleic acid (dsRNA) having a nucleotide sequence which is less that 30 nucleotides in length and which is substantially identical to at least a part of a 3′-untranslated region (3′-UTR) of a (+) strand RNA virus, such as HCV, as well as pharmaceutical compositions comprising the dsRNA, together with a pharmaceutically acceptable carrier. The pharmaceutical compositions are useful for treating infections and diseases caused by the replication or activity of the (+) strand RNA virus, as well as methods for inhibiting viral replication.
Owner:ALNYLAM PHARMA INC
Thionucleoside derivatives as inhibitors of rna-dependent rna viral polymerase
The present invention provides thionucleoside compounds and certain derivatives thereof which are inhibitors of RNA-dependent RNA viral polymerase. These compounds are inhibitors of RNA-dependent RNA viral replication and are useful for the treatment of RNA-dependent RNA viral infection. They are particularly useful as inhibitors of hepatitis C virus (HCV) NS5B polymerase, as inhibitors of HCV replication, and / or for the treatment of hepatitis C infection. The invention also describes pharmaceutical compositions containing such thionucleoside compounds alone or in combination with other agents active against RNA-dependent RNA viral infection, in particular HCV infection. Also disclosed are methods of inhibiting RNA-dependent RNA polymerase, inhibiting RNA-dependent RNA viral replication, and / or treating RNA-dependent RNA viral infection with the thionucleoside compounds of the present invention.
Owner:IONIS PHARMA INC +1
TC-83-derived alphavirus vectors, particles and methods
InactiveUS20050266550A1Reduce expressionMaximizing expressionSsRNA viruses positive-senseGenetic material ingredientsViral replicationImmunogenicity
The present disclosure provides TC-83 VEE-derived replicons, alphaviral replicon particles and immunogenic compositions containing TC-83 alphaviral replicon particles which direct the expression of at least one antigen when introduced into a suitable host cell. The TC-83 VEE-derived ARPs described herein are improved in that they are subject to a lower vector-specific immune response than prior art ARPs.
Owner:ALPHAVAX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ff07340-b9e8-44bf-b258-f448d1aa2f35/US20080280842A1-20081113-C00001.png)
![Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ff07340-b9e8-44bf-b258-f448d1aa2f35/US20080280842A1-20081113-C00002.png)
![Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection Fluorinated Pyrrolo[2,3-D]Pyrimidine Nucleosides for the Treatment of Rna-Dependent Rna Viral Infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ff07340-b9e8-44bf-b258-f448d1aa2f35/US20080280842A1-20081113-C00003.png)