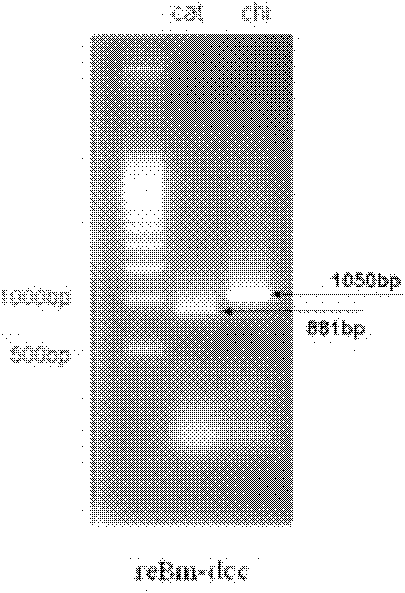
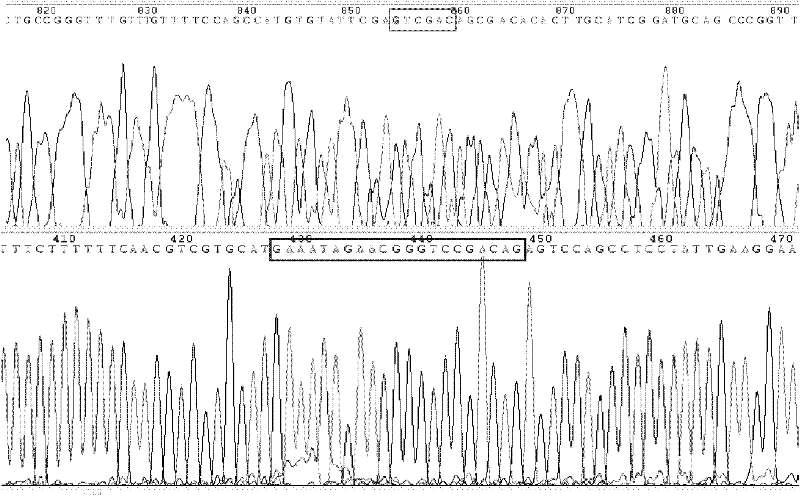
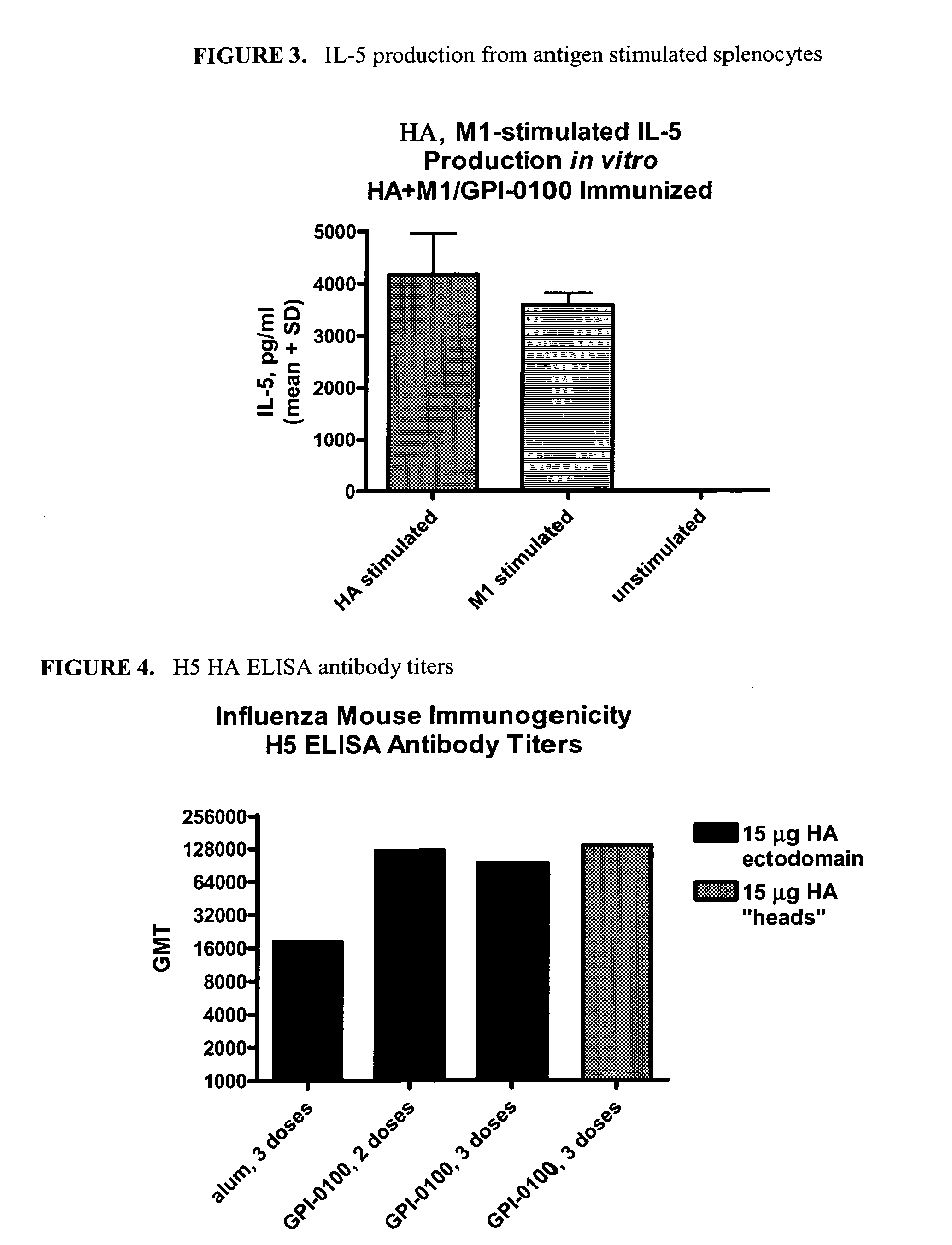
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
415 results about "Insect cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
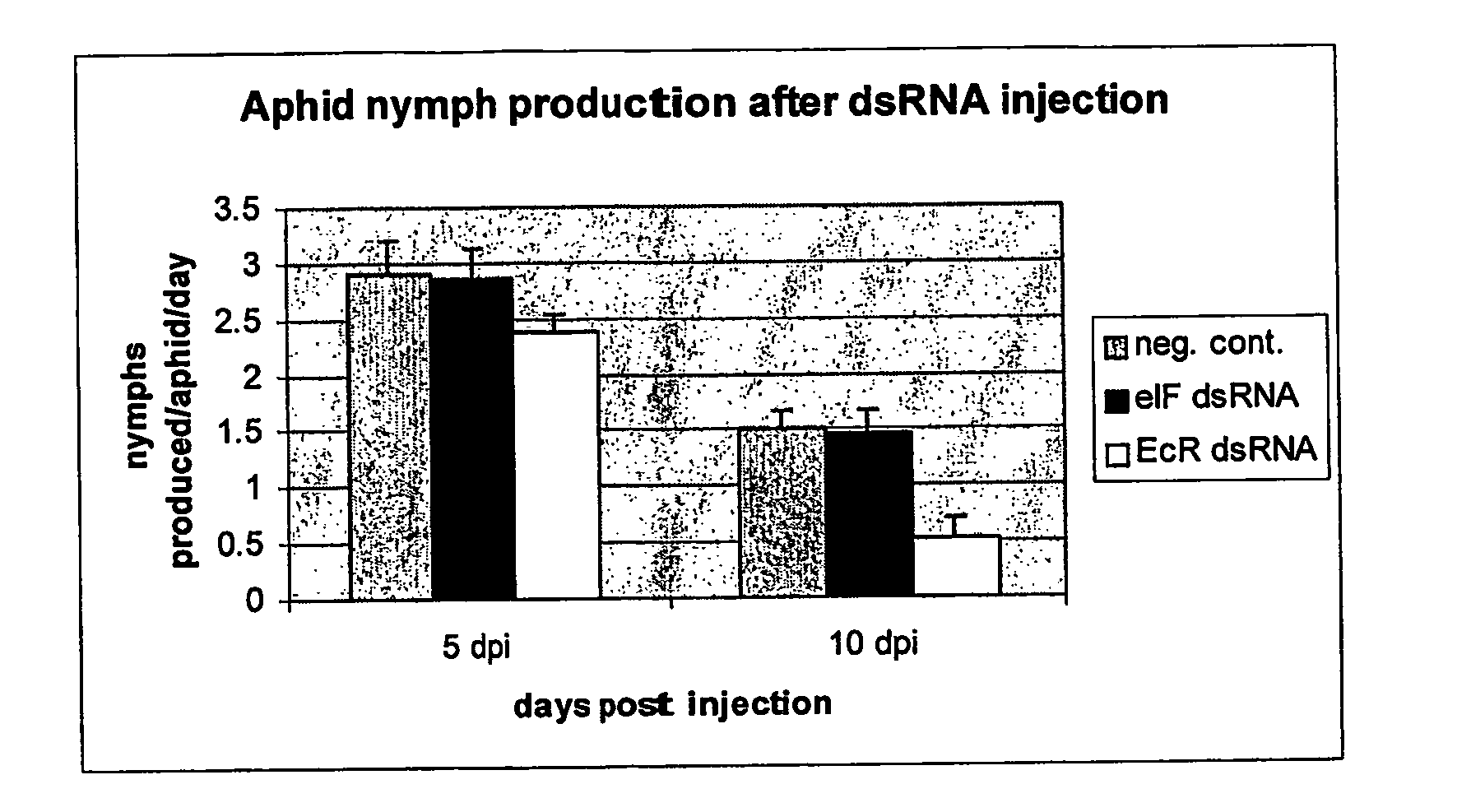
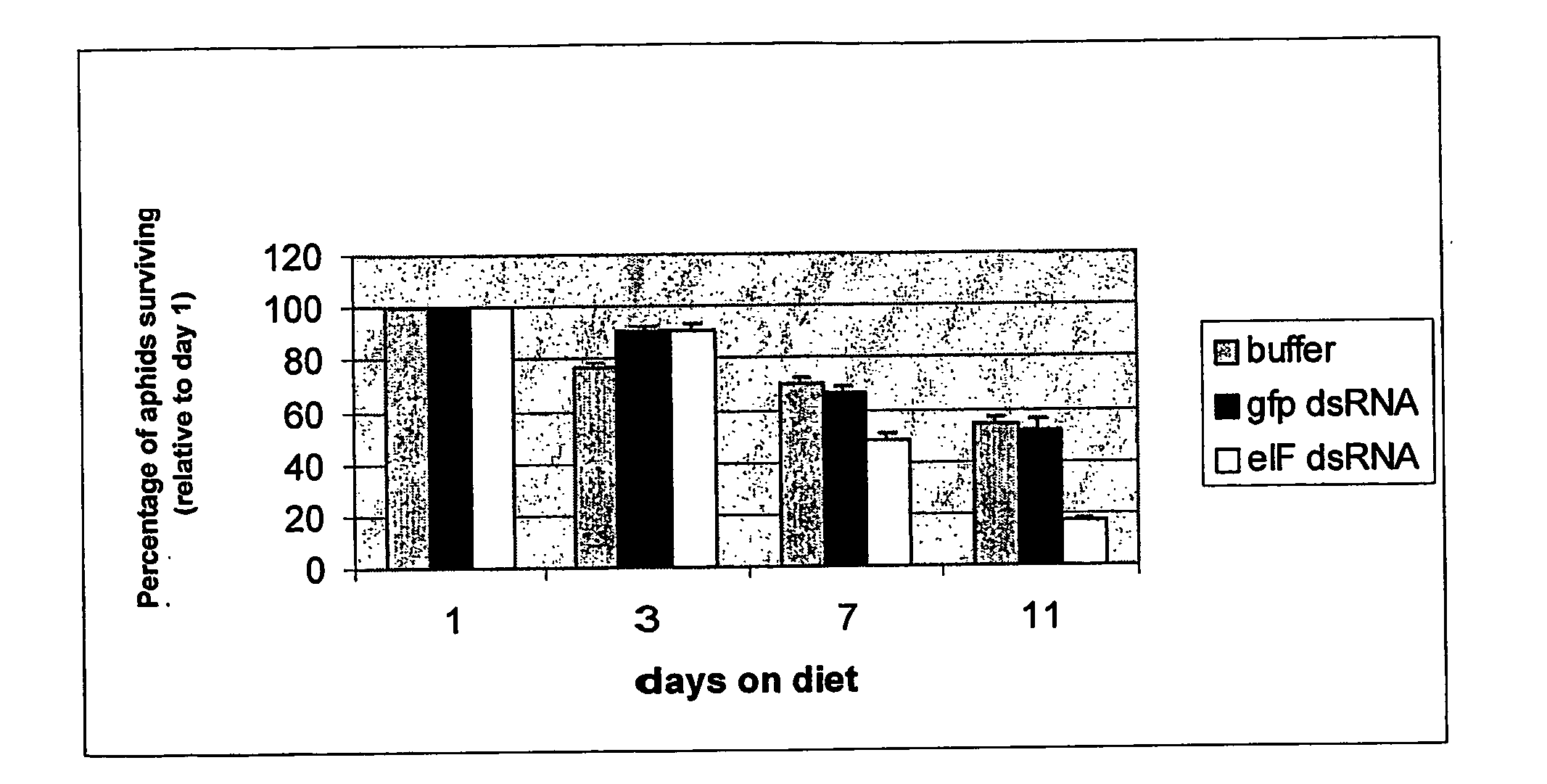
Insect resistance using inhibition of gene expression
The current invention provides methods to silence insect genes by using unpackaged dsRNA or siRNA, in one embodiment such dsRNA or siRNA is present in plant vascular tissue, preferably phloem, more particularly phloem sap, and the insect is a plant sap-sucking insect. Also provided are DNA sequences which when transcribed yield a double-stranded RNA molecule capable of reducing the expression of an essential gene of a plant sap-sucking insect, methods of using such DNA sequences and plants or plant cells transformed with such DNA sequences. Also provided is the use of cationic oligopeptides that facilitate the entry of dsRNA or siRNA molecules in insect cells, such as plant sap-sucking insect cells.
Owner:BASF AG
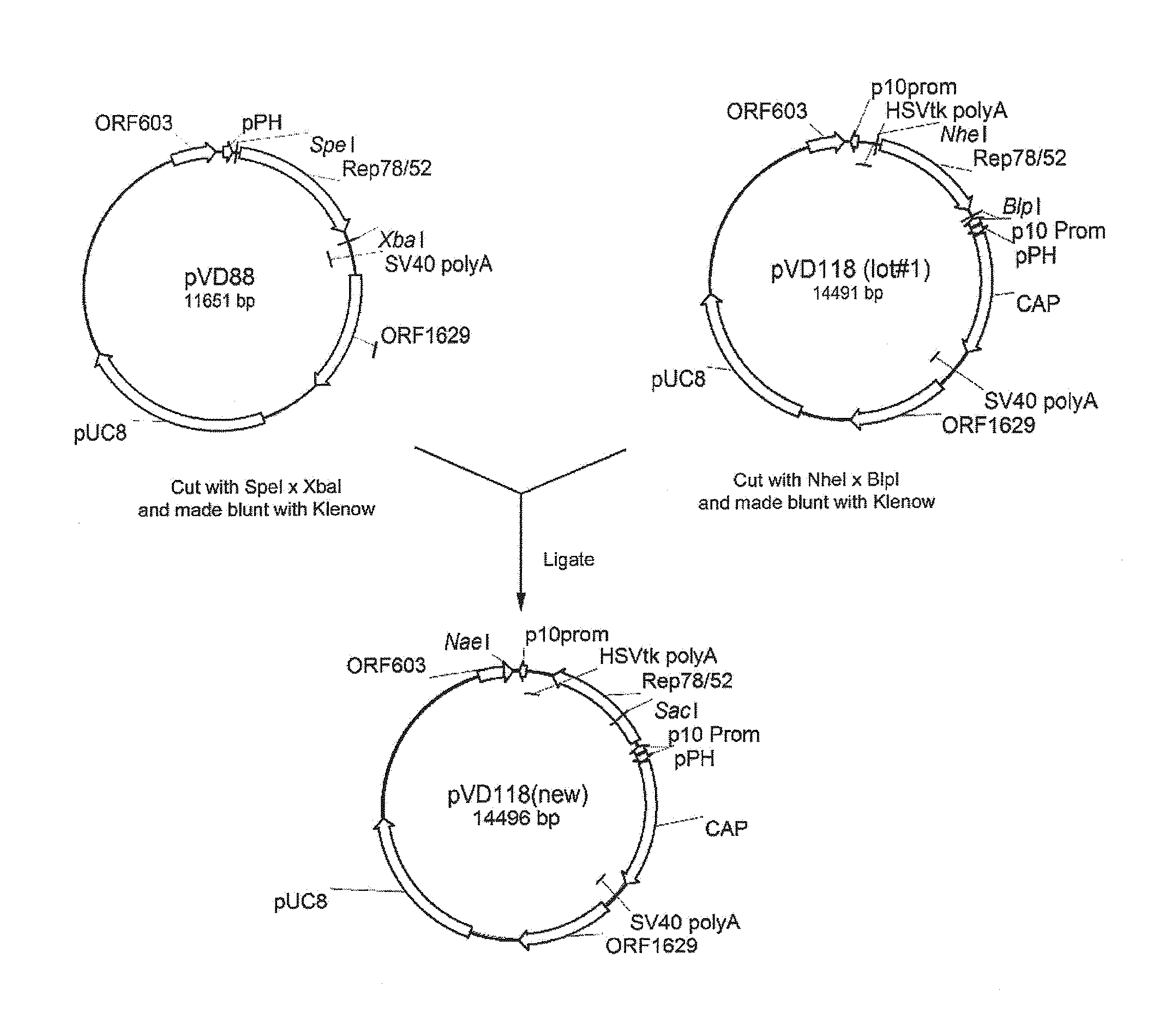
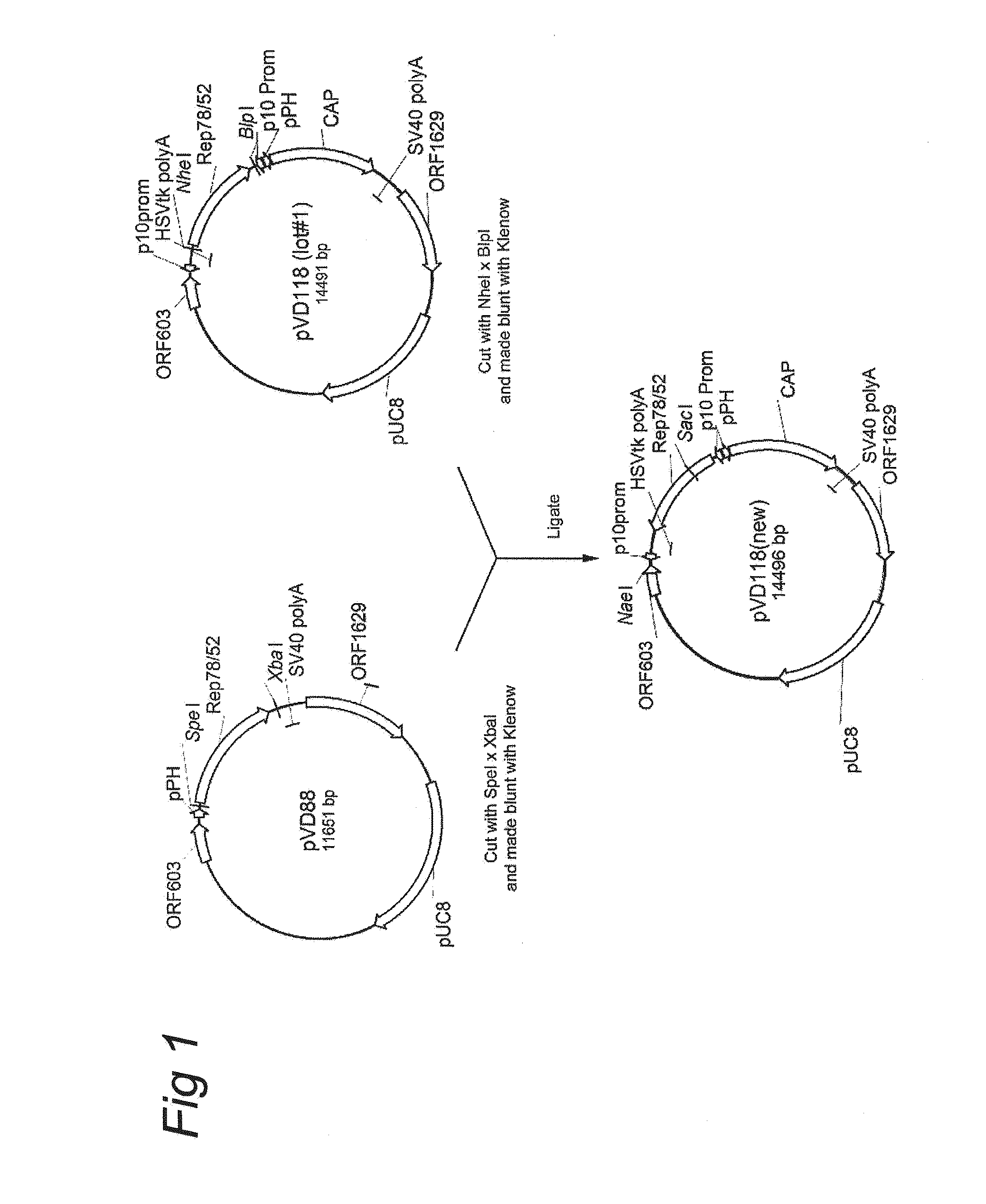
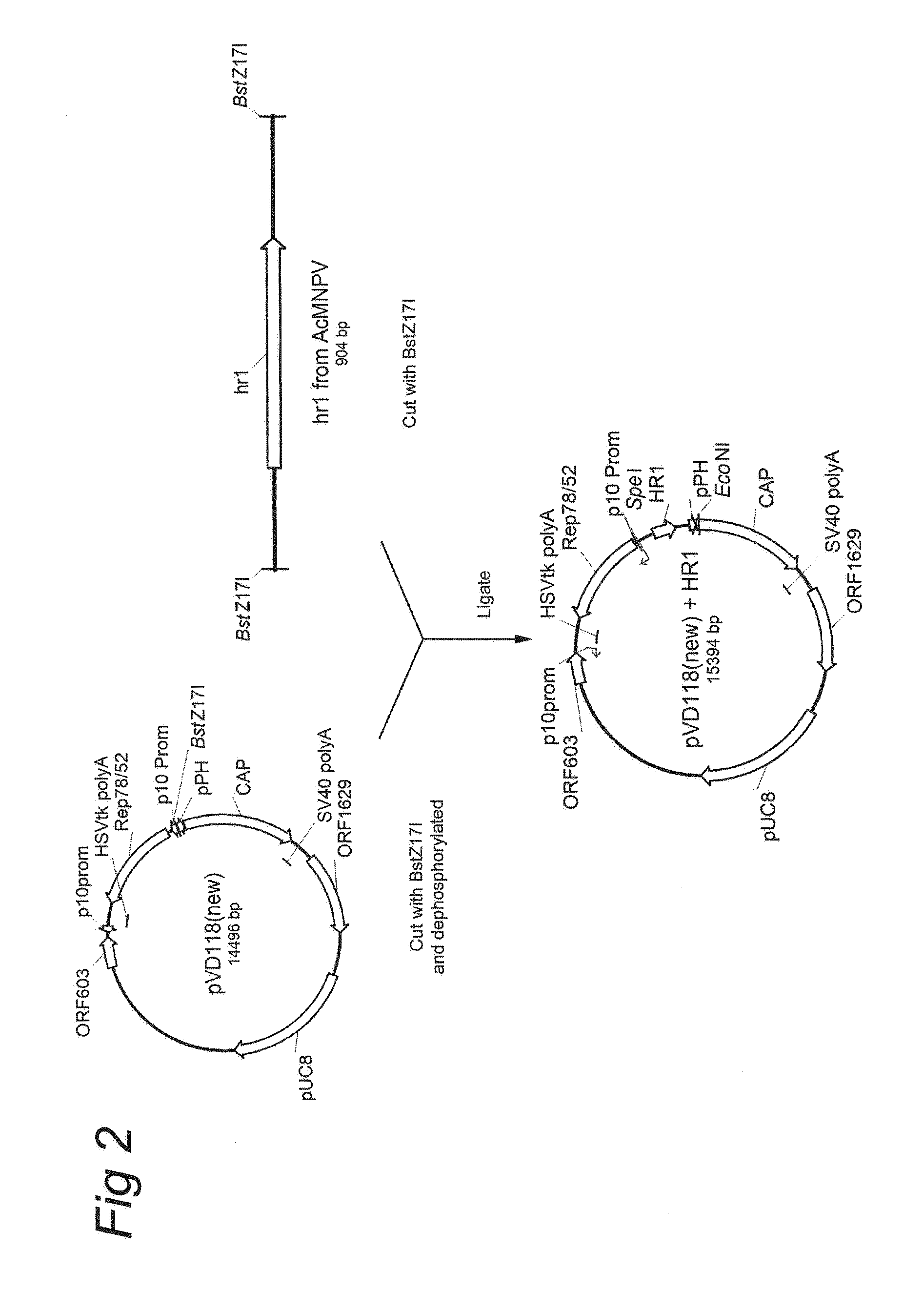
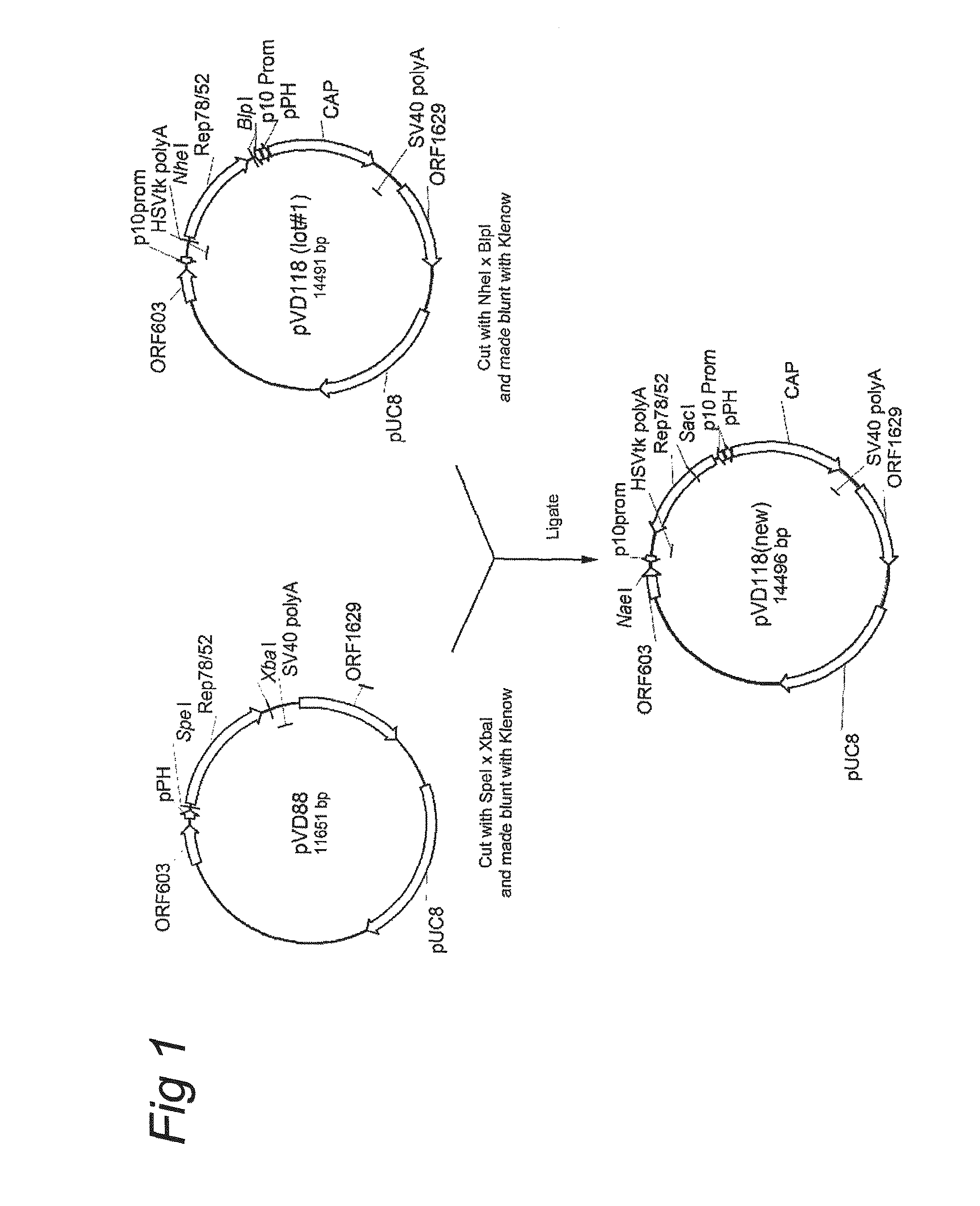
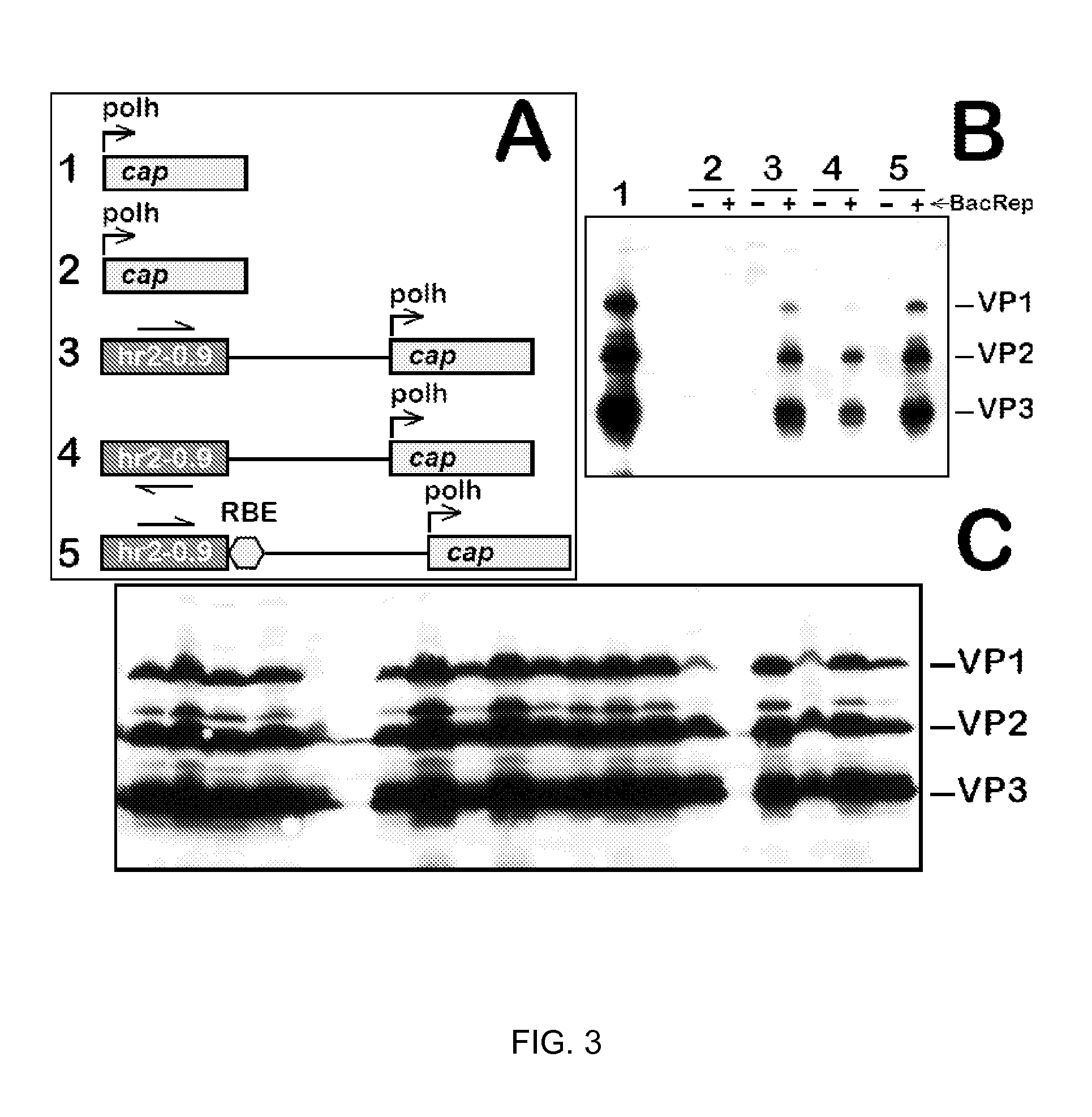
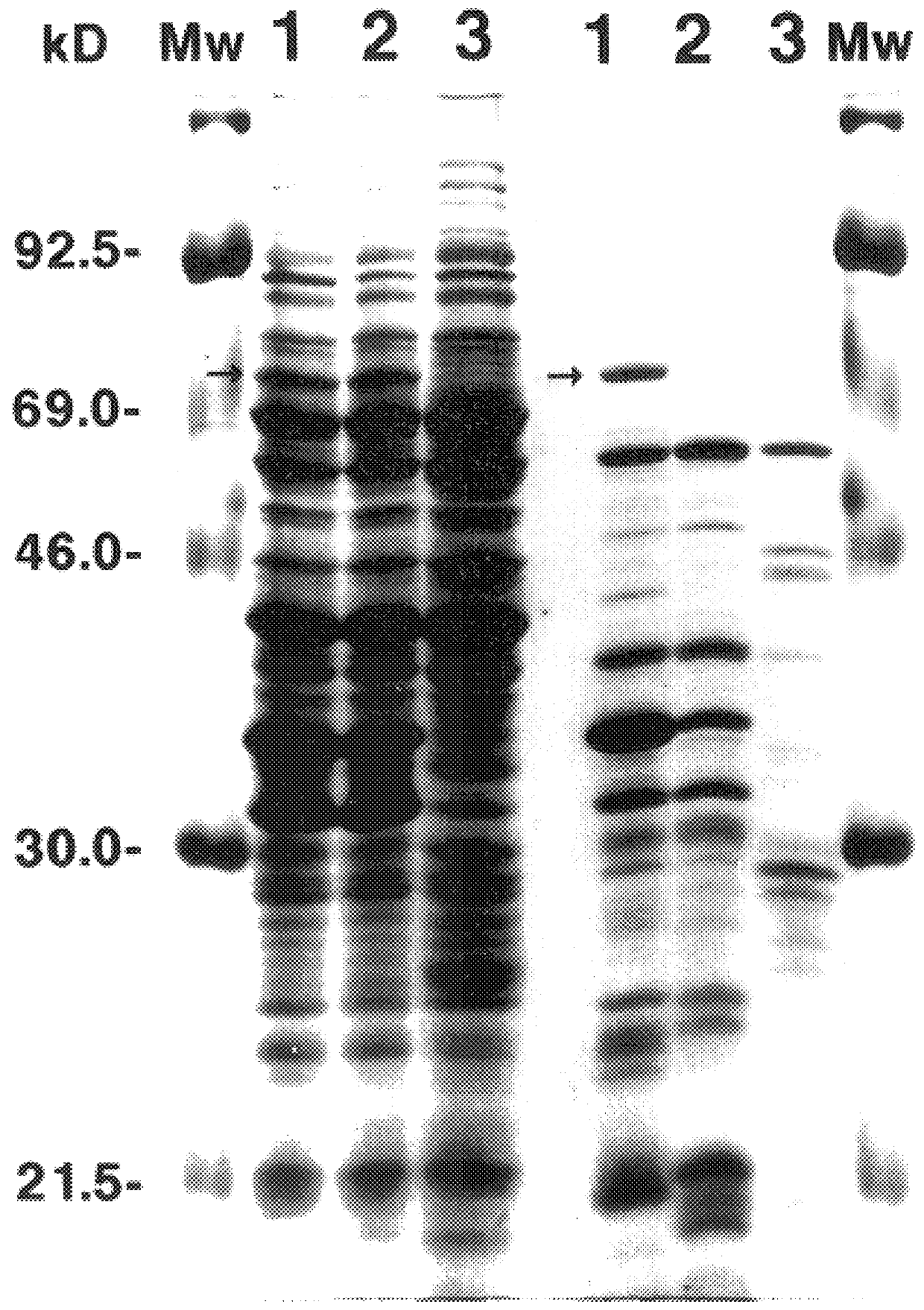
AAV vectors produced in insect cells
ActiveUS8163543B2Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
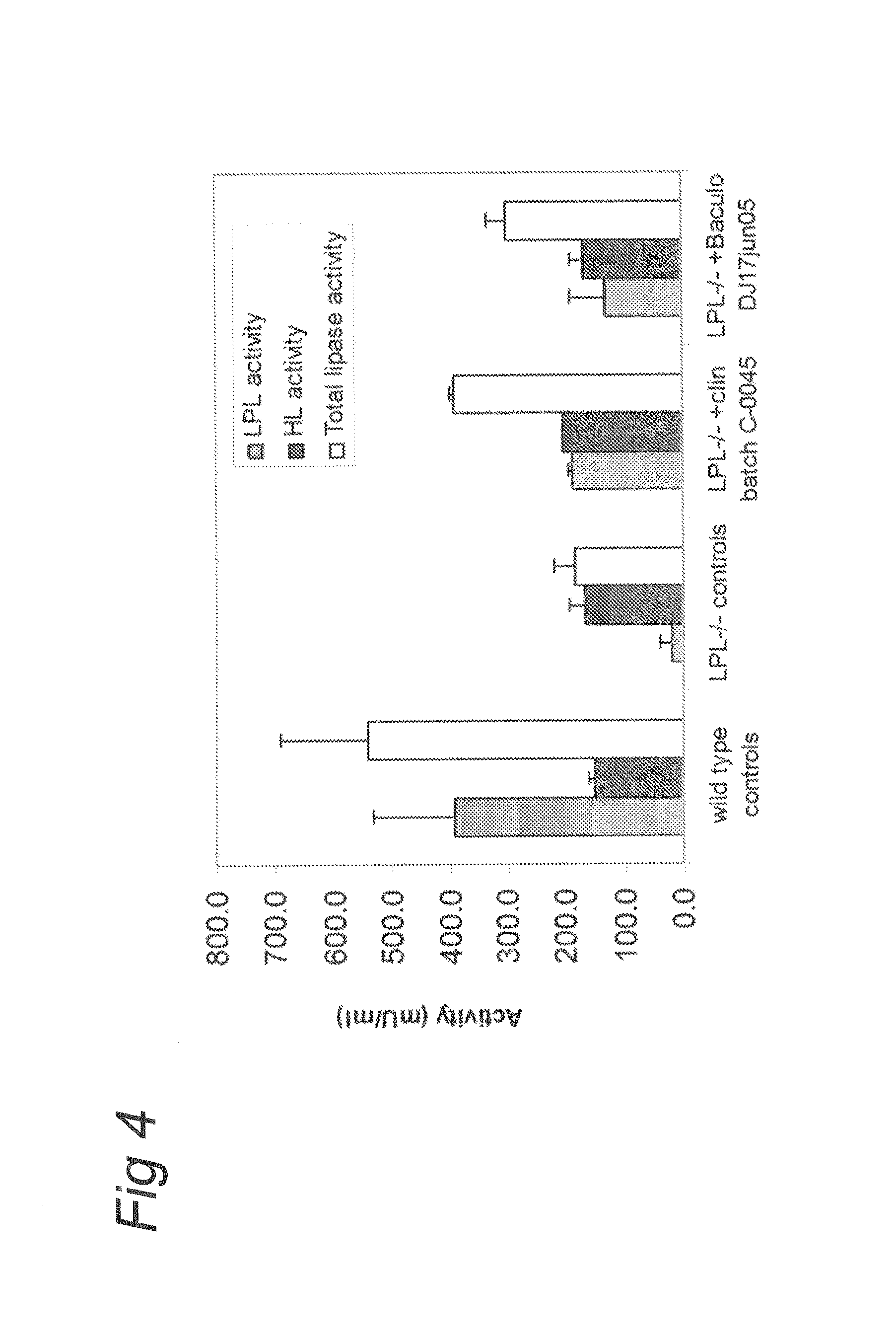
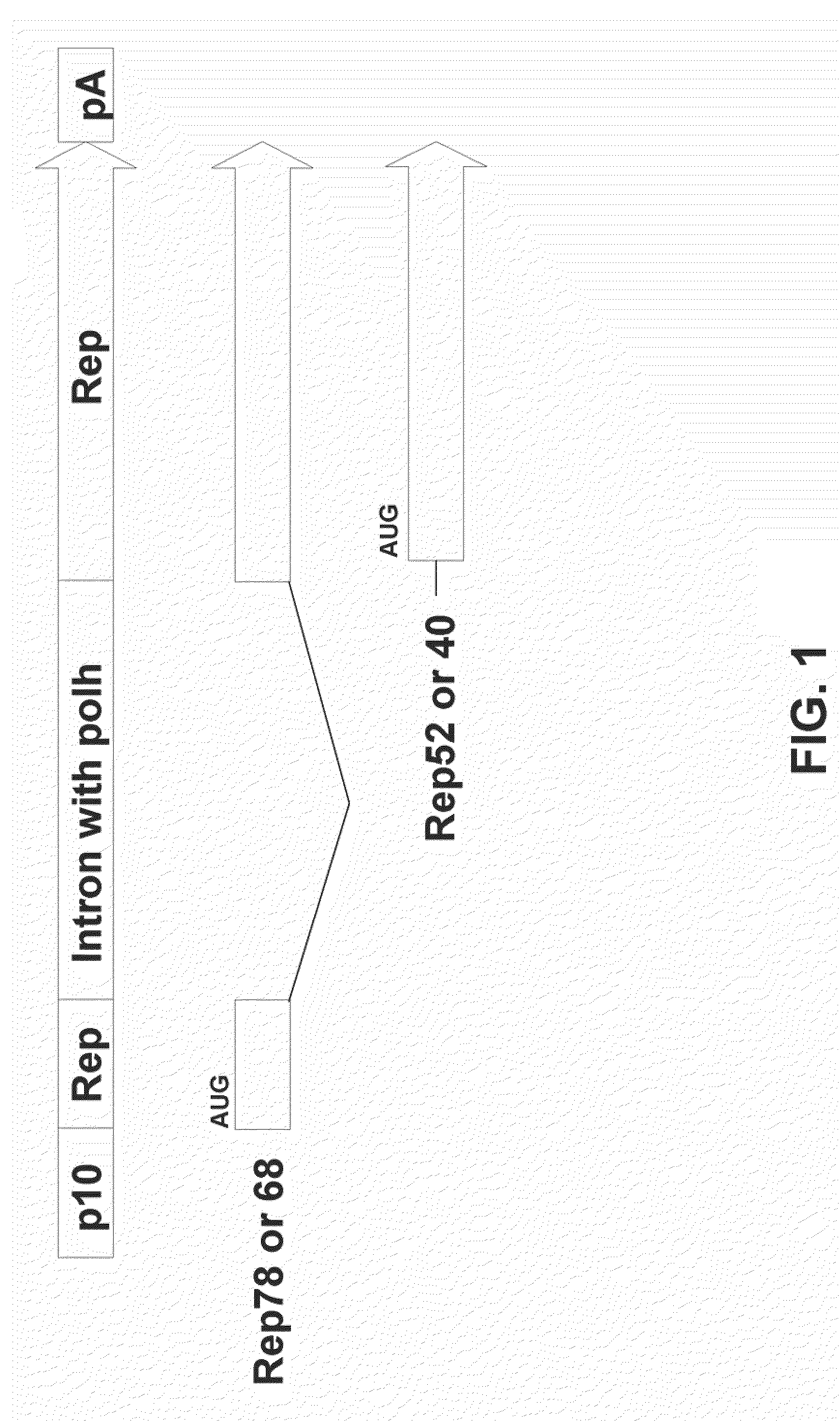
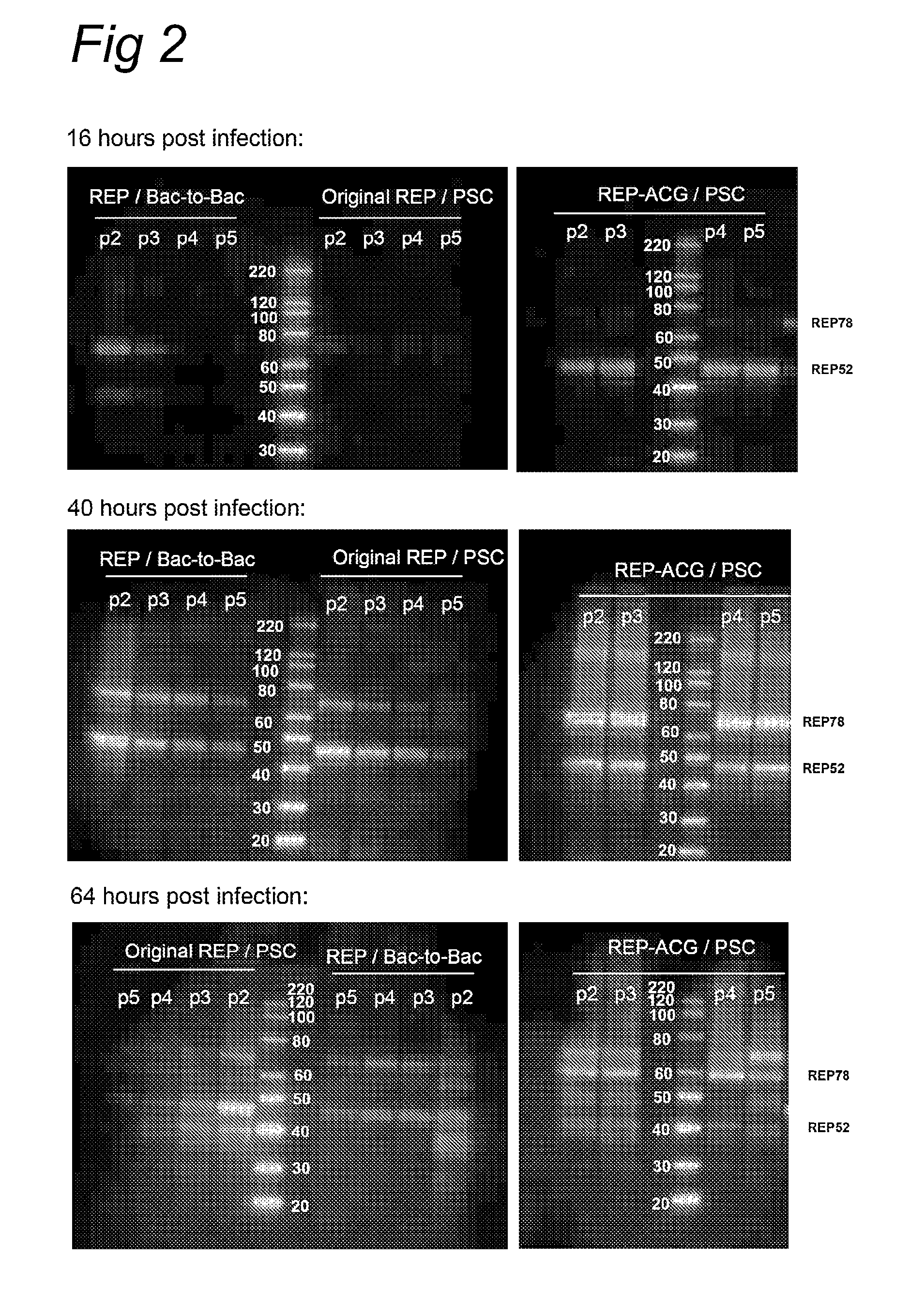
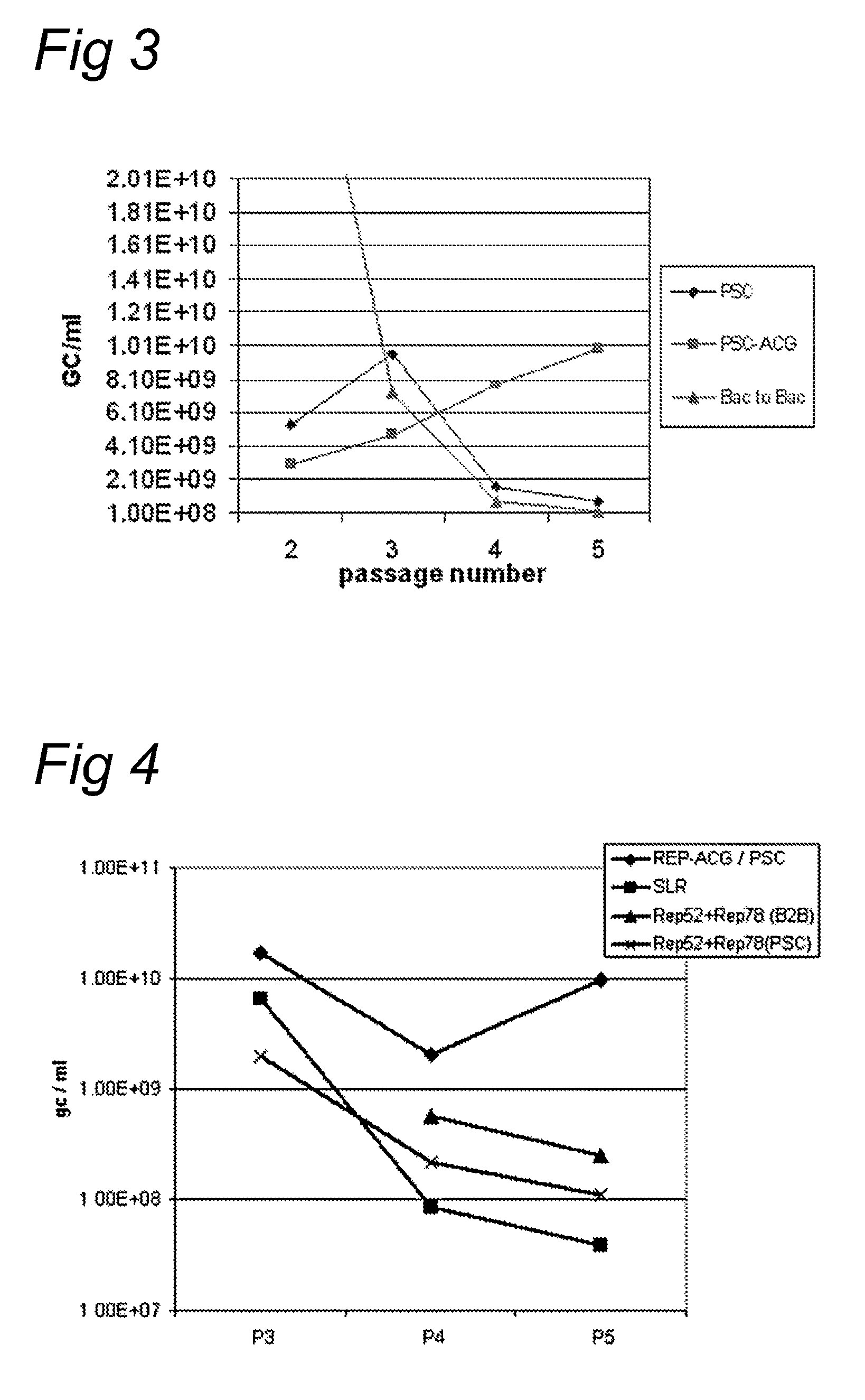
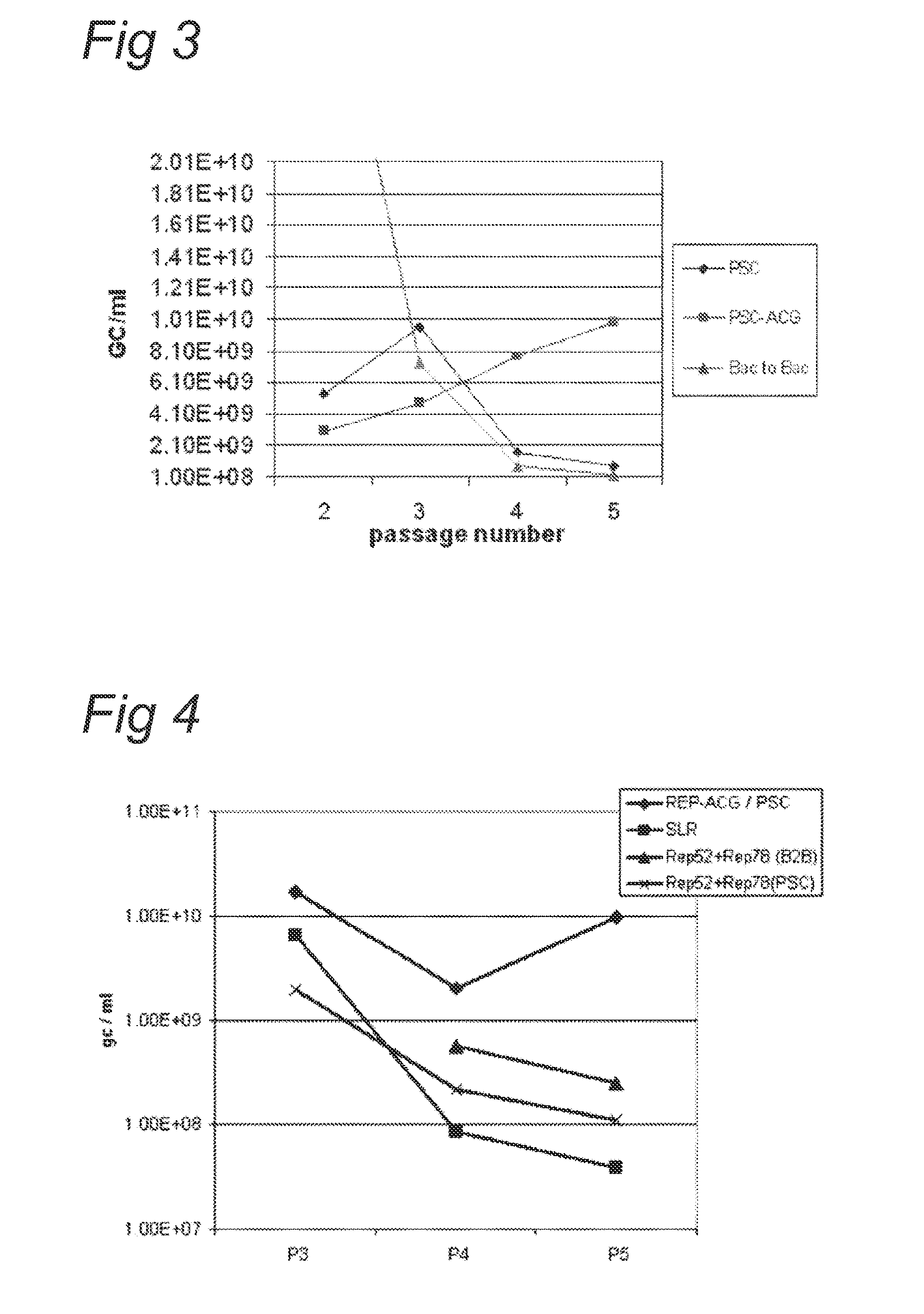
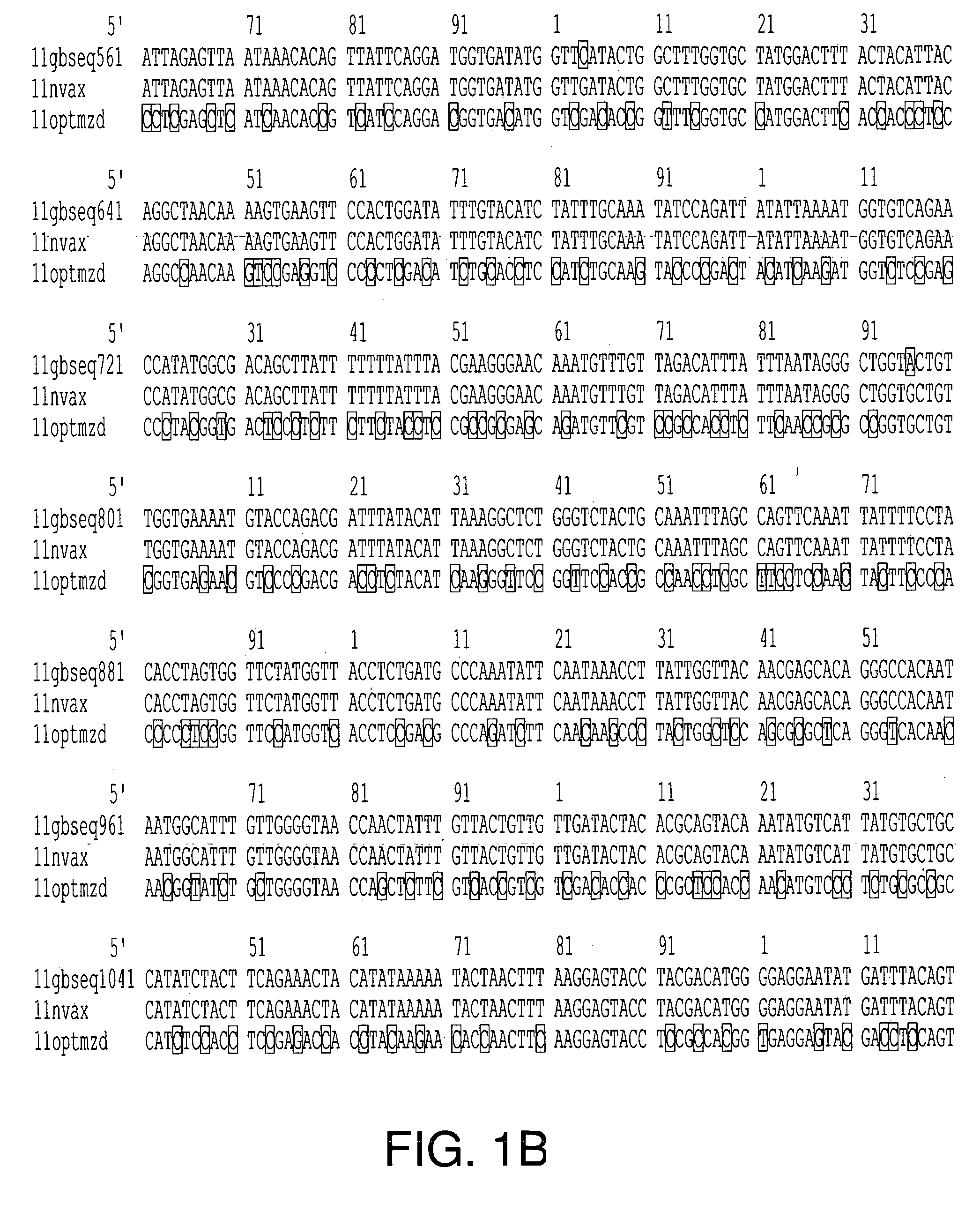
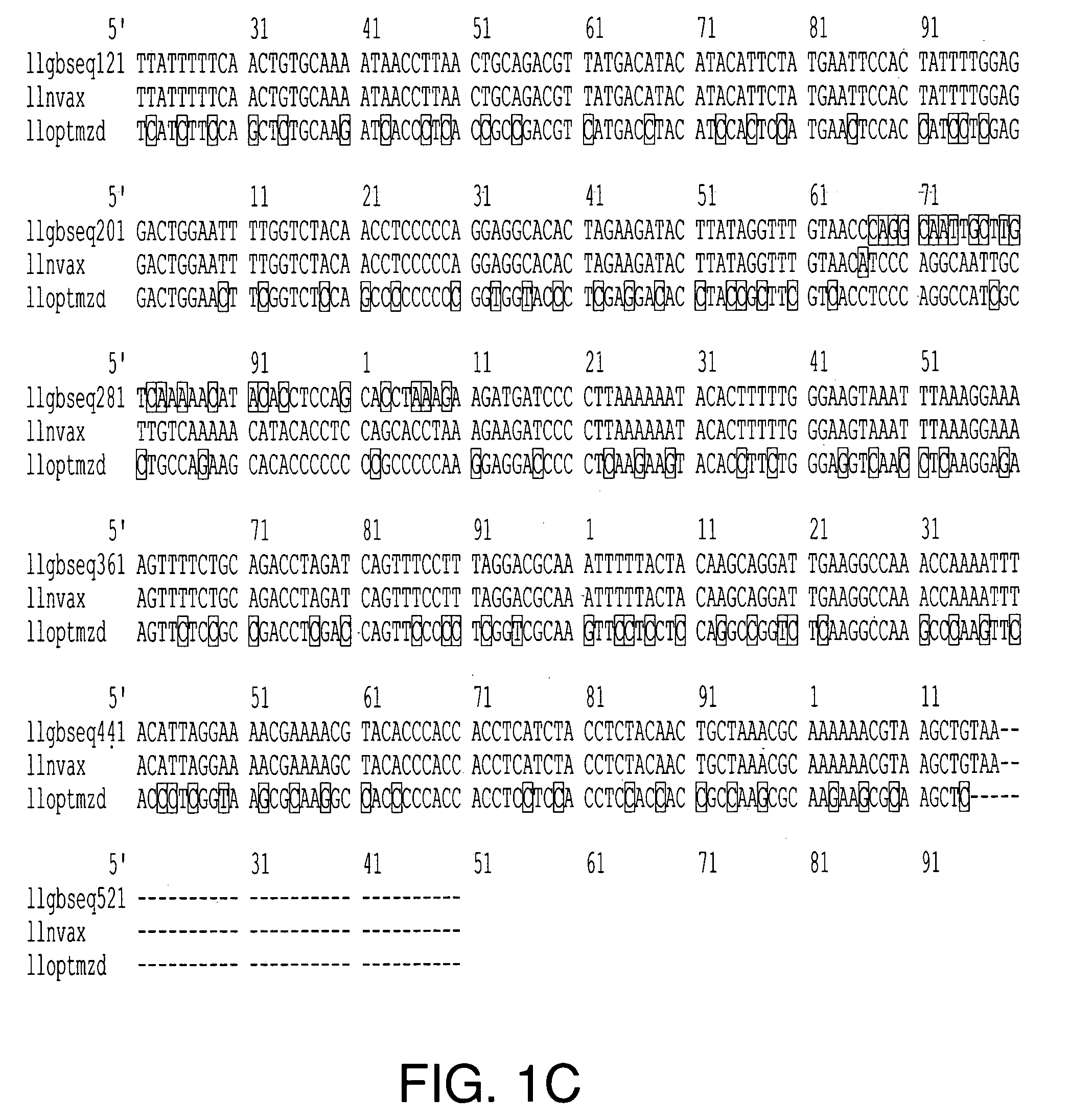
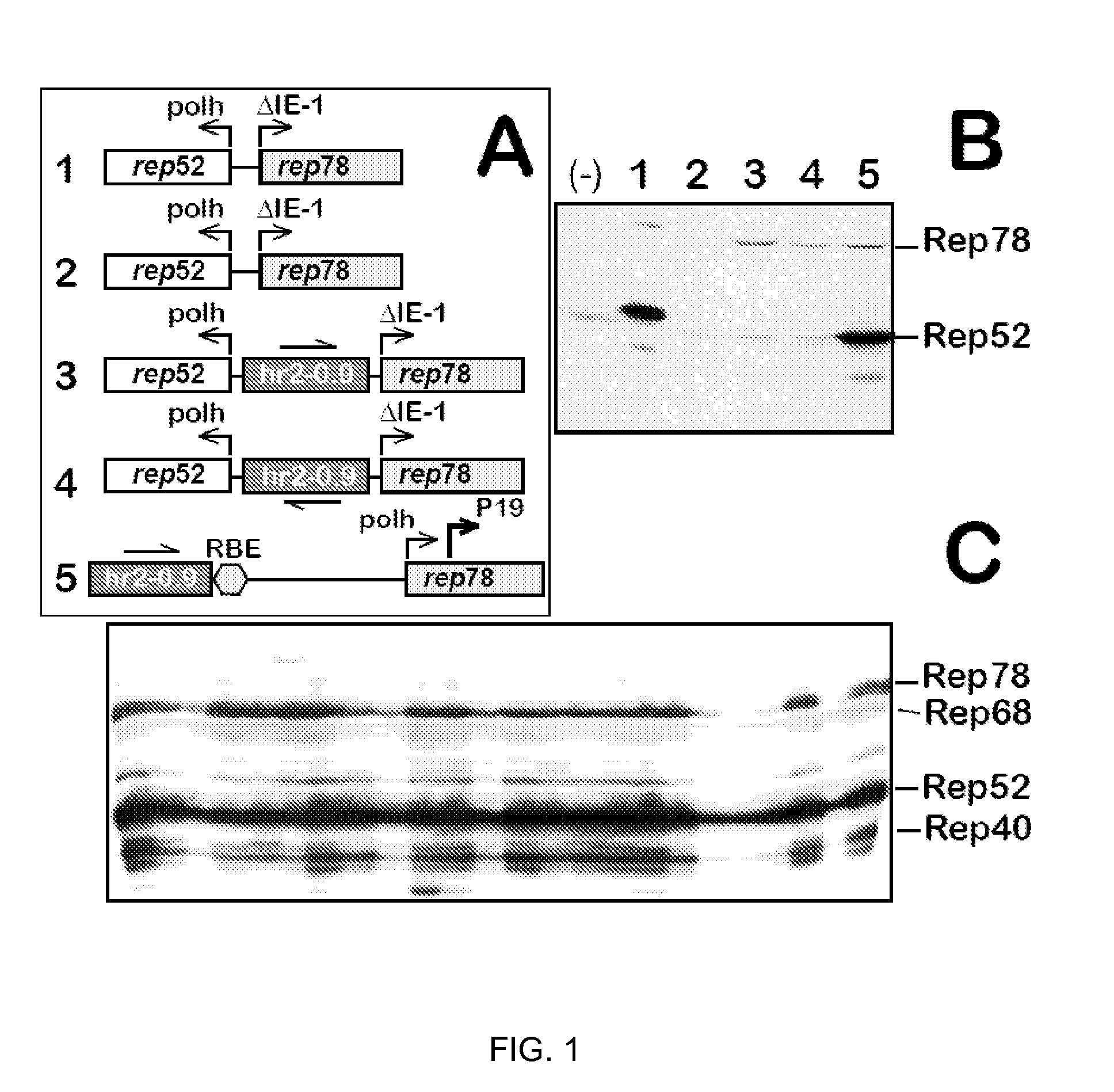
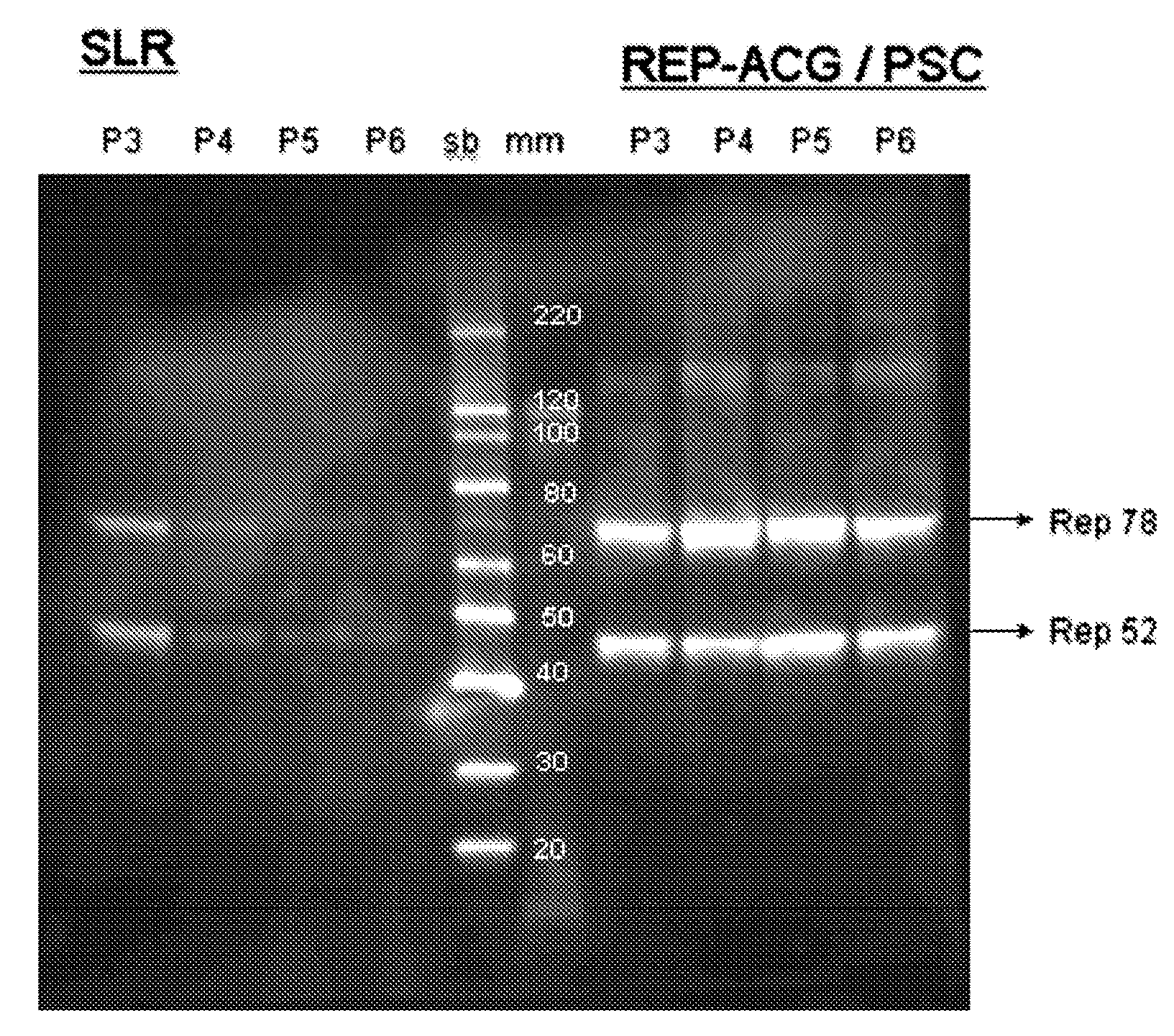
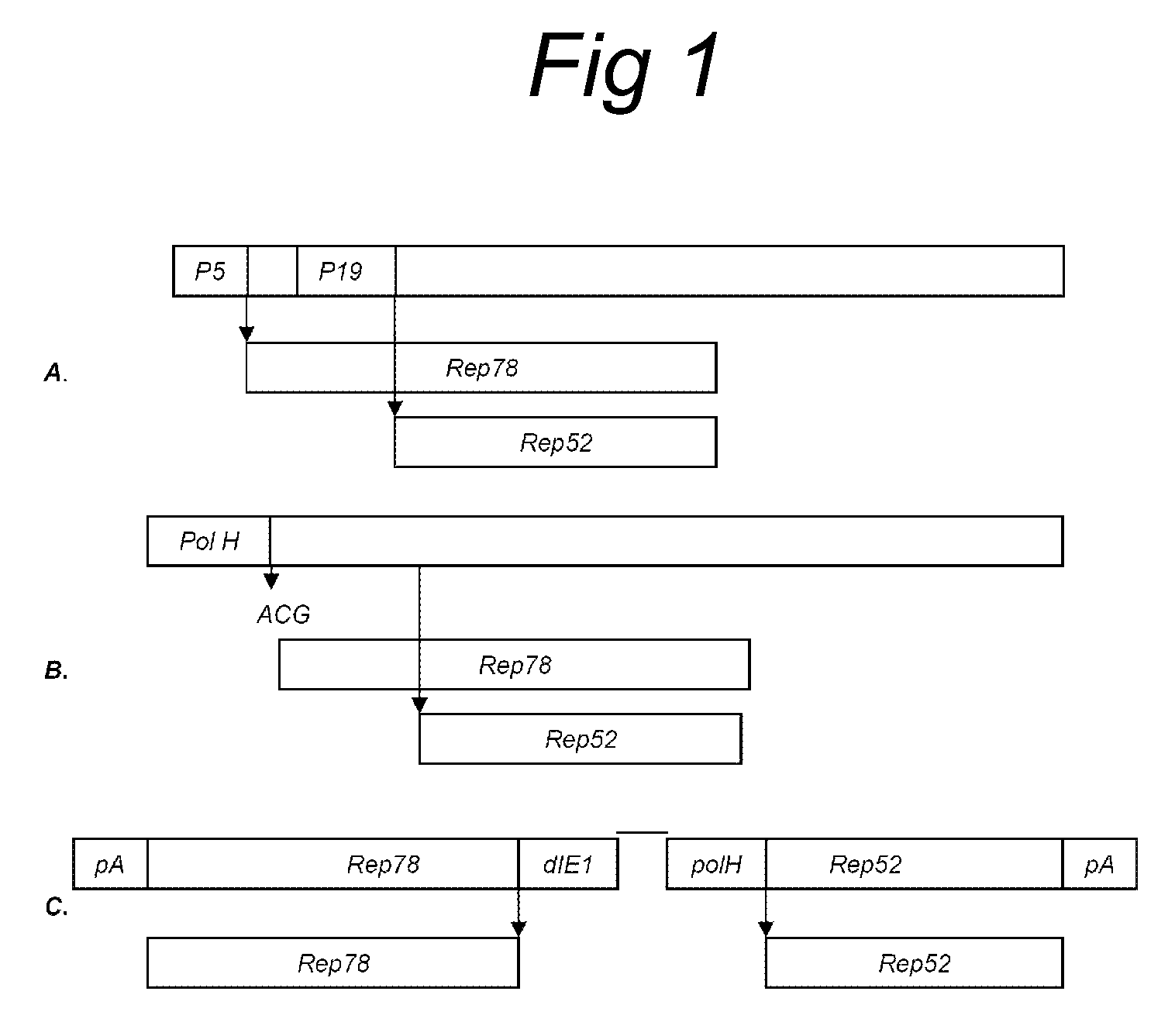
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
Inducible System for Highly Efficient Production of Recombinant Adeno-Associated Virus (rAAV) Vectors
ActiveUS20120100606A1High production costHigh yieldAnimal cellsNucleic acid vectorDiseaseClinical grade
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Engineering intracellular sialylation pathways
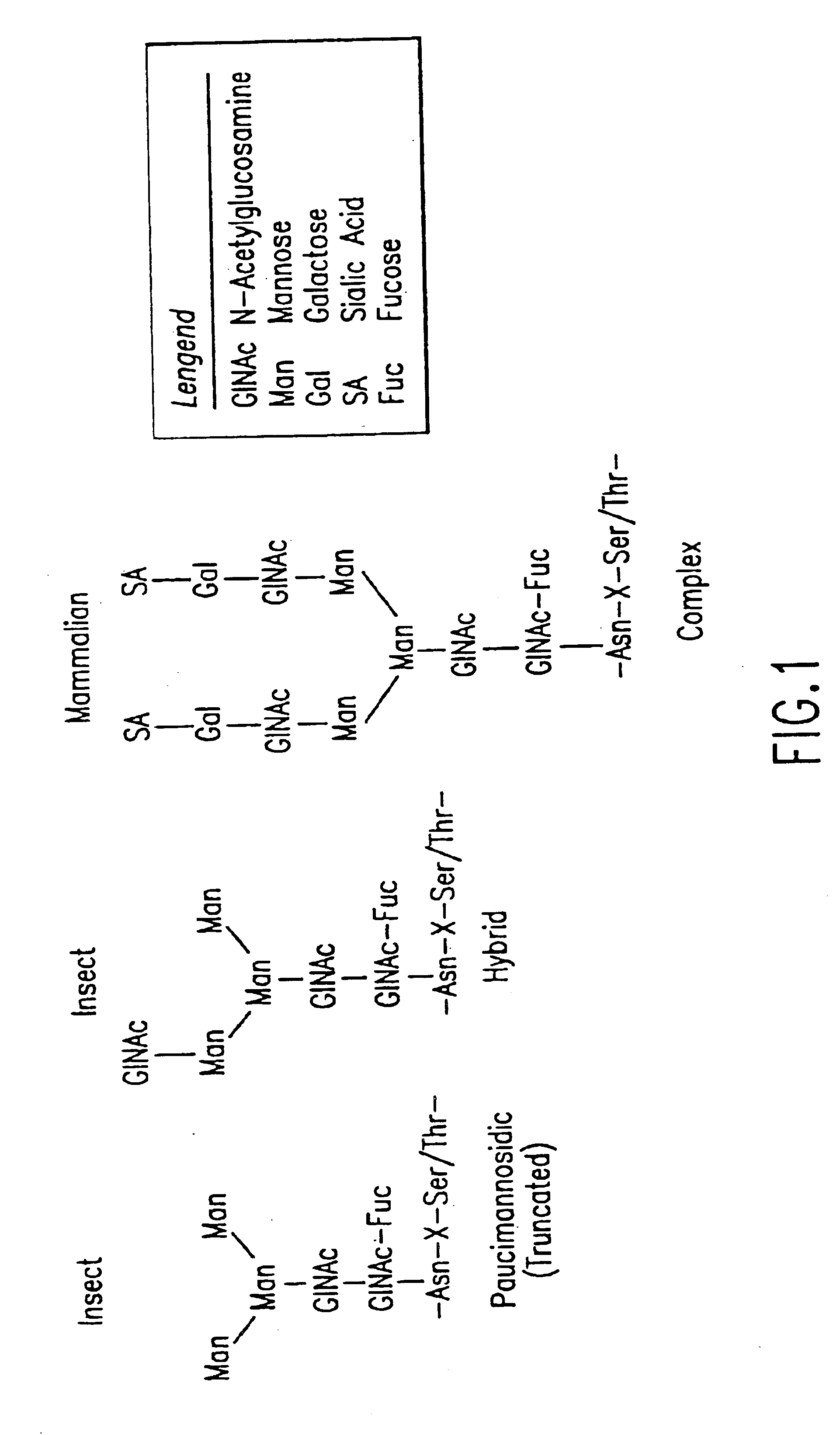
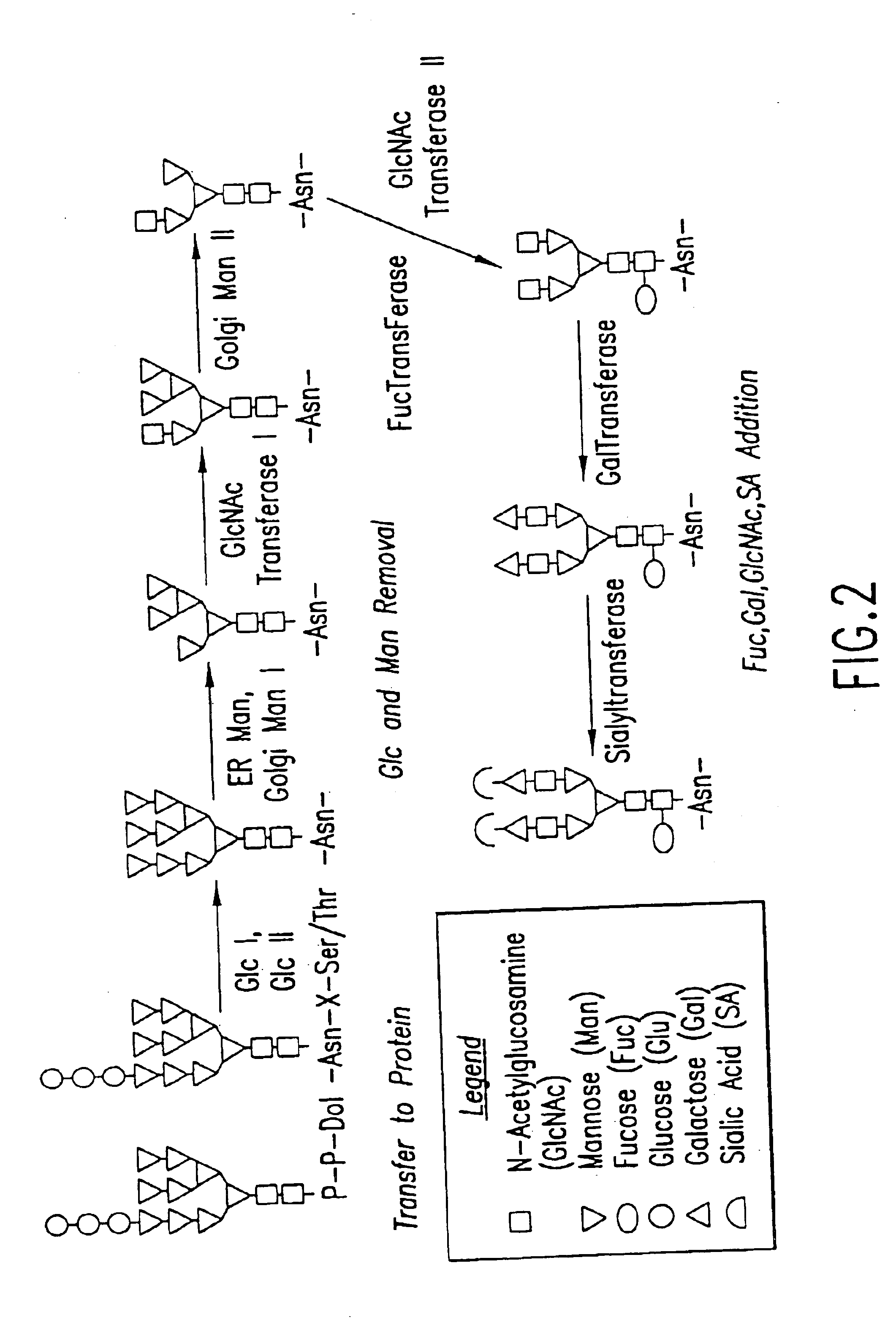
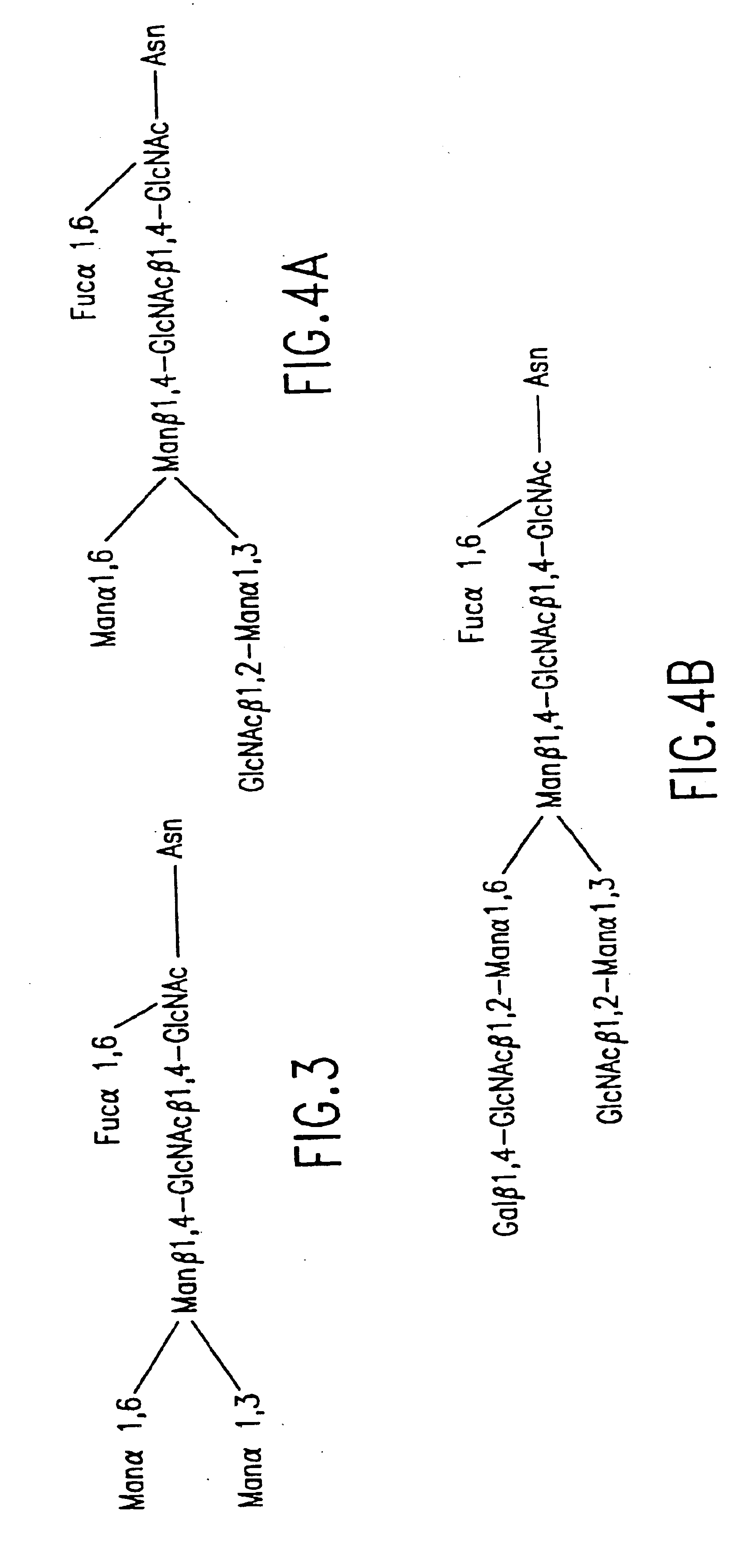
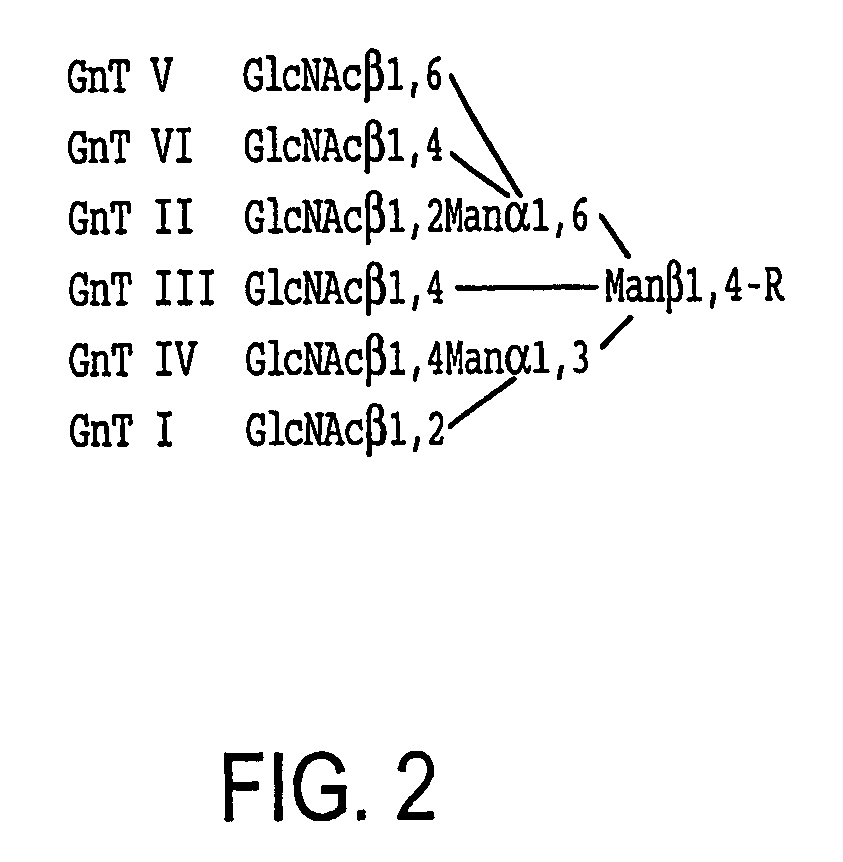
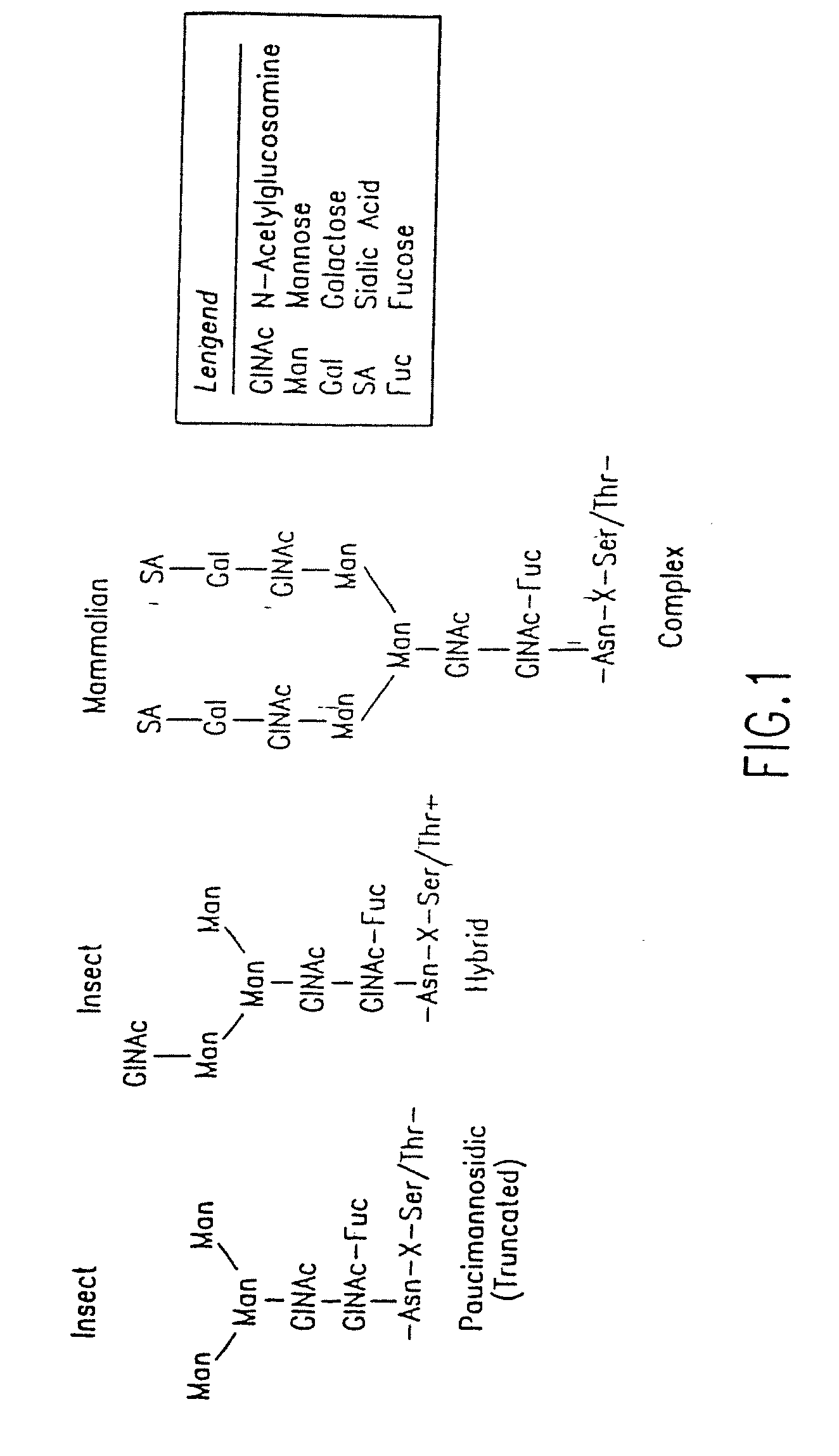
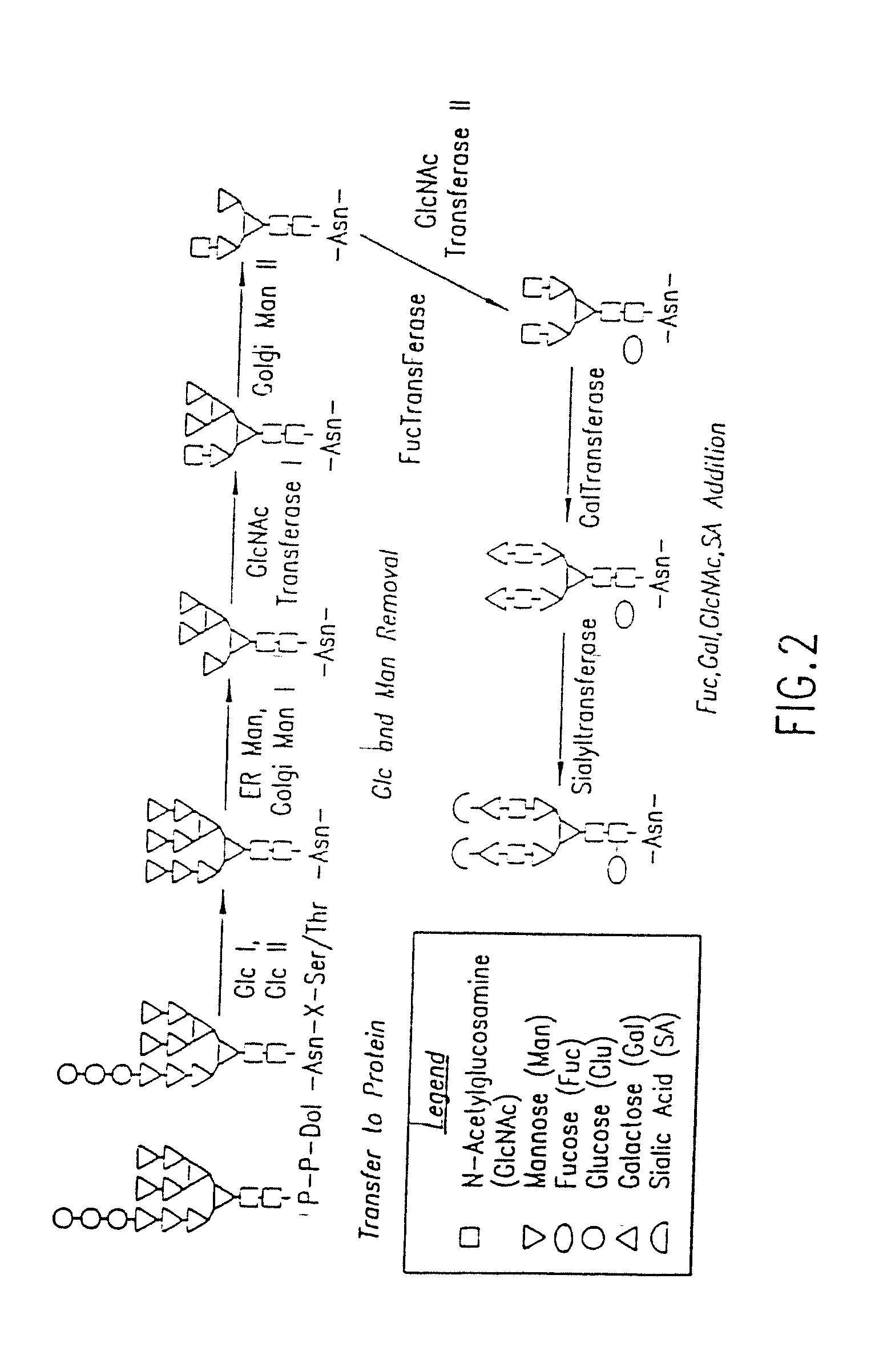
Methods for manipulating carbohydrate processing pathways in cells of interest are provided. Methods are directed at manipulating multiple pathways involved with the sialylation reaction by using recombinant DNA technology and substrate feeding approaches to enable the production of sialylated glycoproteins in cells of interest. These carbohydrate engineering efforts encompass the implementation of new carbohydrate bioassays, the examination of a selection of insect cell lines and the use of bioinformatics to identify gene sequences for critical processing enzymes. The compositions comprise cells of interest producing sialylated glycoproteins. The methods and compositions are useful for heterologous expression of glycoproteins.
Owner:HUMAN GENOME SCI INC +1
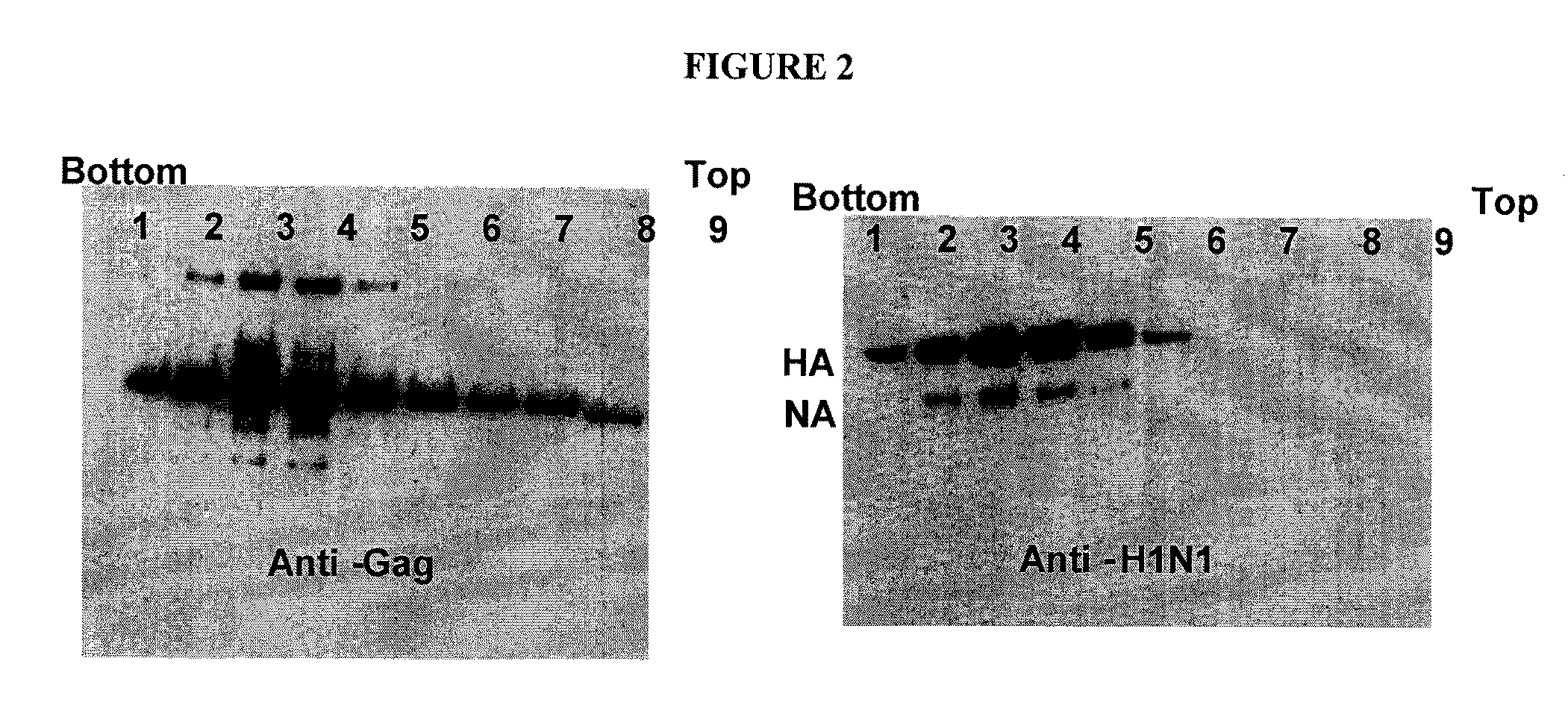
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Insect bioreactor expressing multiple exogenous genes and its construction method and application
The invention discloses an insect bioreactor capable of expressing multiple exogenous genes, and a construction method and application thereof. The construction method comprises the following steps: (1) introducing multicopy high-efficiency bacteria DNA (deoxyribonucleic acid) replication initiator into chitinase and cysteine proteinase genes of a baculovirus genome to obtain a baculovirus shuttle plasmid; (2) replacing virus duplicated essential gene downstream the polyhedrosis gene of the baculovirus shuttle plasmid with antibiotic gene to obtain a baculovirus plasmid DNA; and (3) replacingother virus duplicated and infected nonessential genes in the baculovirus shuttle plasmid with reverse selection marker gene to obtain the insect bioreactor. The antibiotic gene or reverse selection marker gene in the insect bioreactor which is constructed by replacing the exogenous target genes can express multiple exogenous genes in a host insect or insect cell. The insect bioreactor disclosed by the invention can efficiently expressing one or more exogenous genes in an insect body at the same time, and can produce massive recombinant proteins at low cost.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Expression in insect cells of genes with overlapping open reading frames, methods and compositions therefor
ActiveUS20090203071A1Reduce riskEfficient infectionAnimal cellsSugar derivativesOpen reading framePromoter
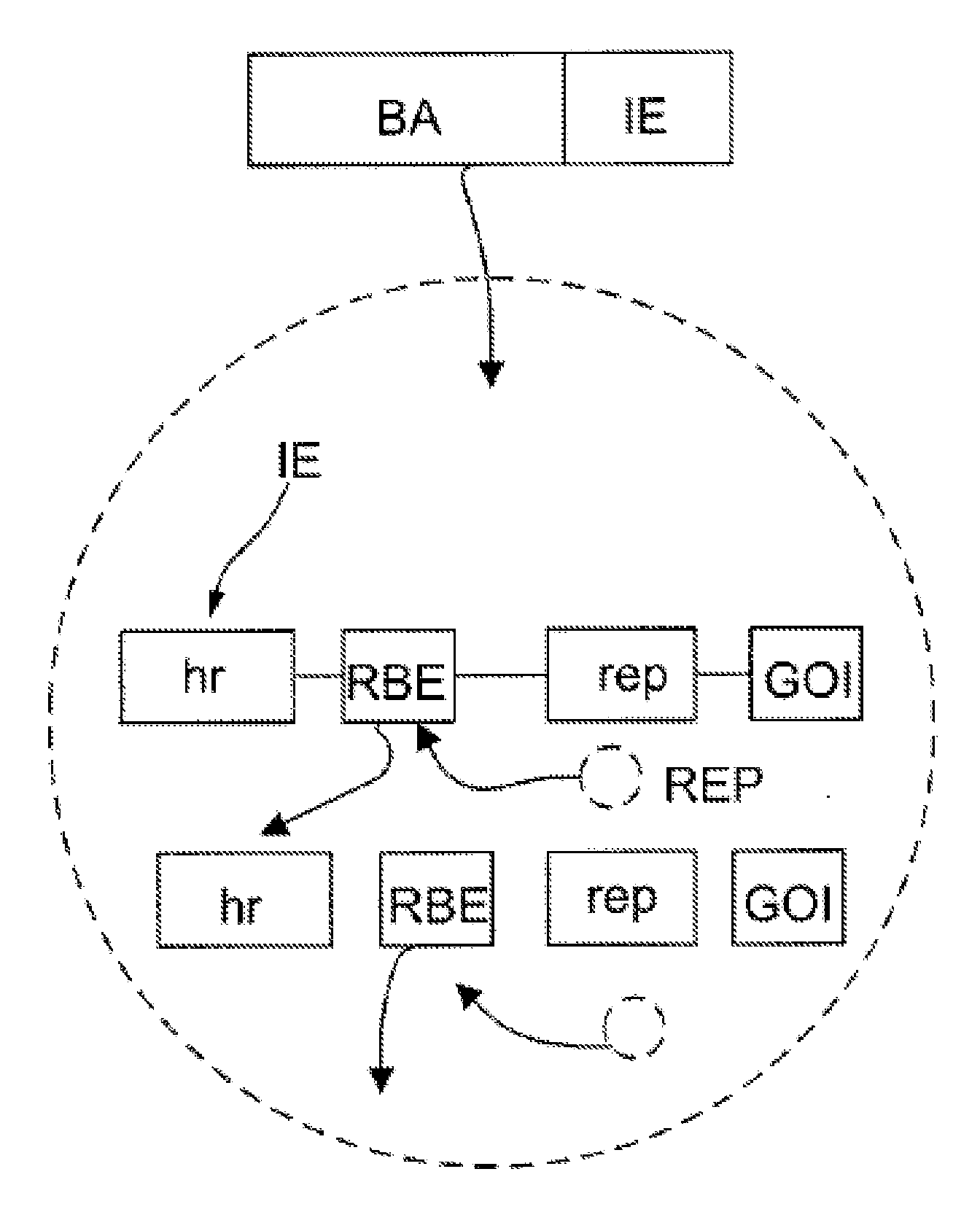
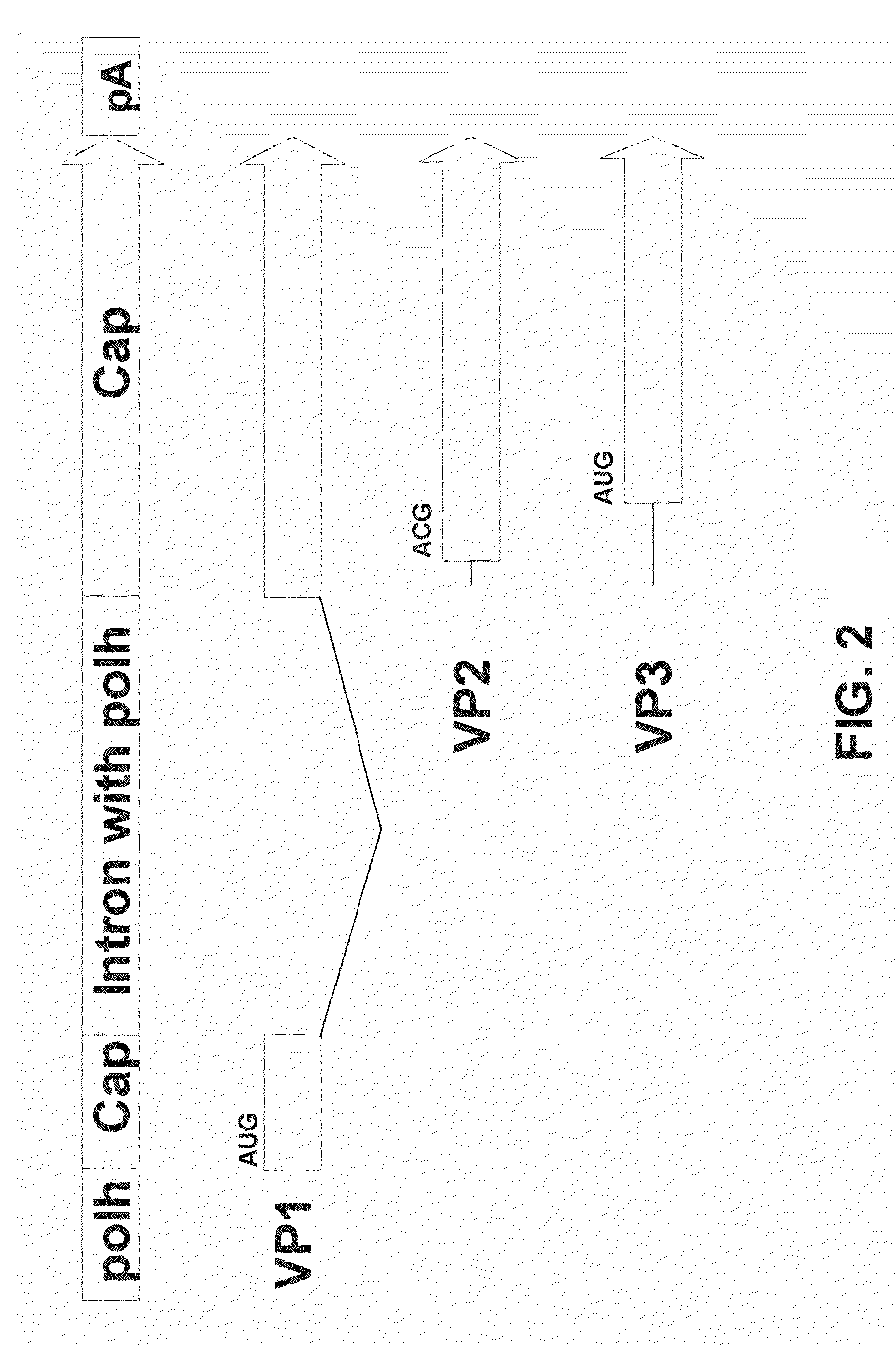
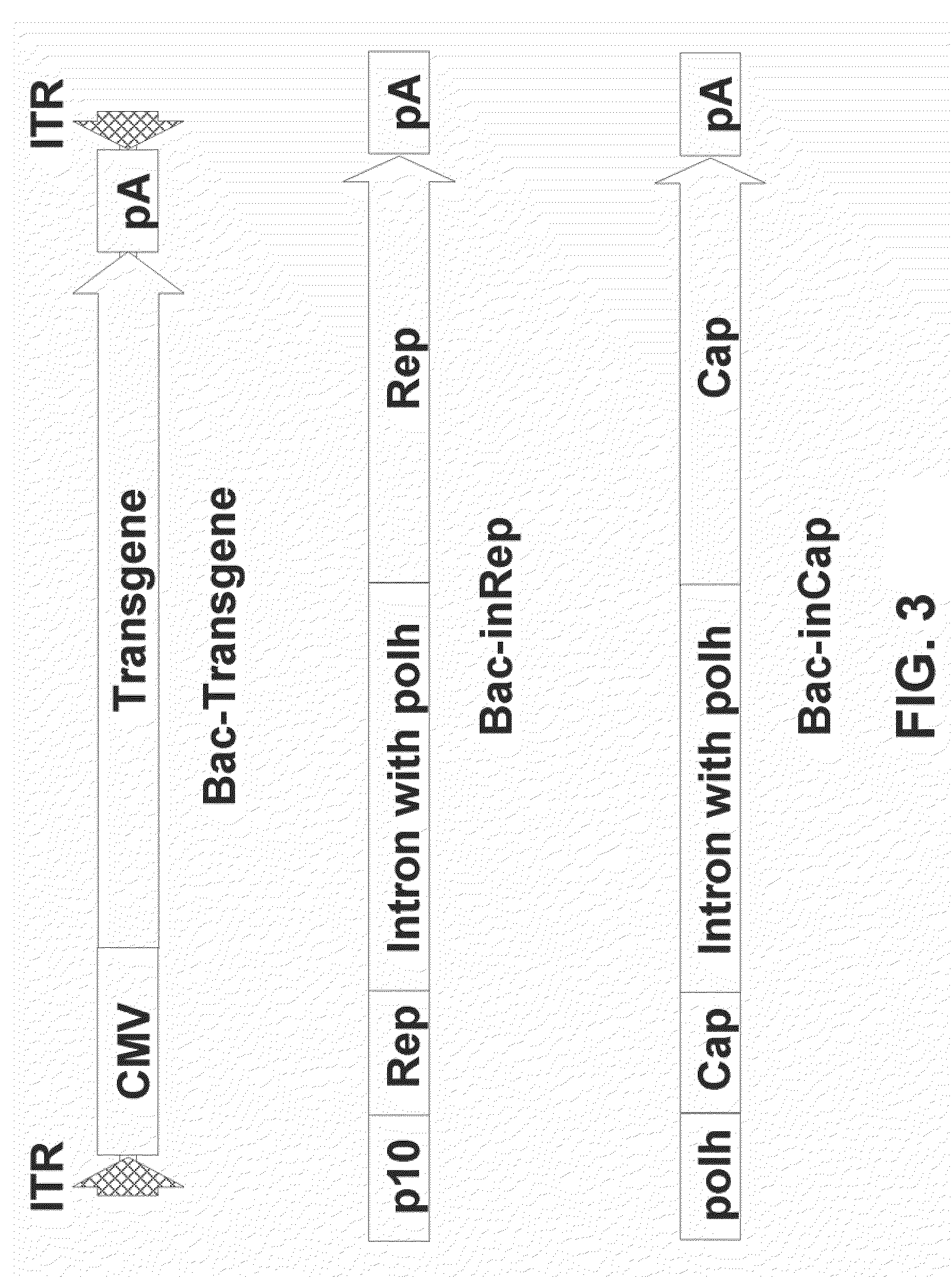
The present teachings disclose nucleic acid cassettes for expressing in an insect cell a plurality of polypeptides encoded by a gene comprising overlapping open reading frames (ORFs). A cassette comprises, in 5′ to 3′ order, a) a first insect cell-operable promoter, b) a 5′ portion of a gene comprising a first ORF of the gene, c) an intron comprising a second insect cell-operable promoter, and d) a 3′ portion of the gene comprising at least one additional ORF. Vectors and insect cells comprising the cassettes are also disclosed, as well as methods for production of recombinant adeno-associated virus in insect cells using the cassettes.
Owner:VIROVEK
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
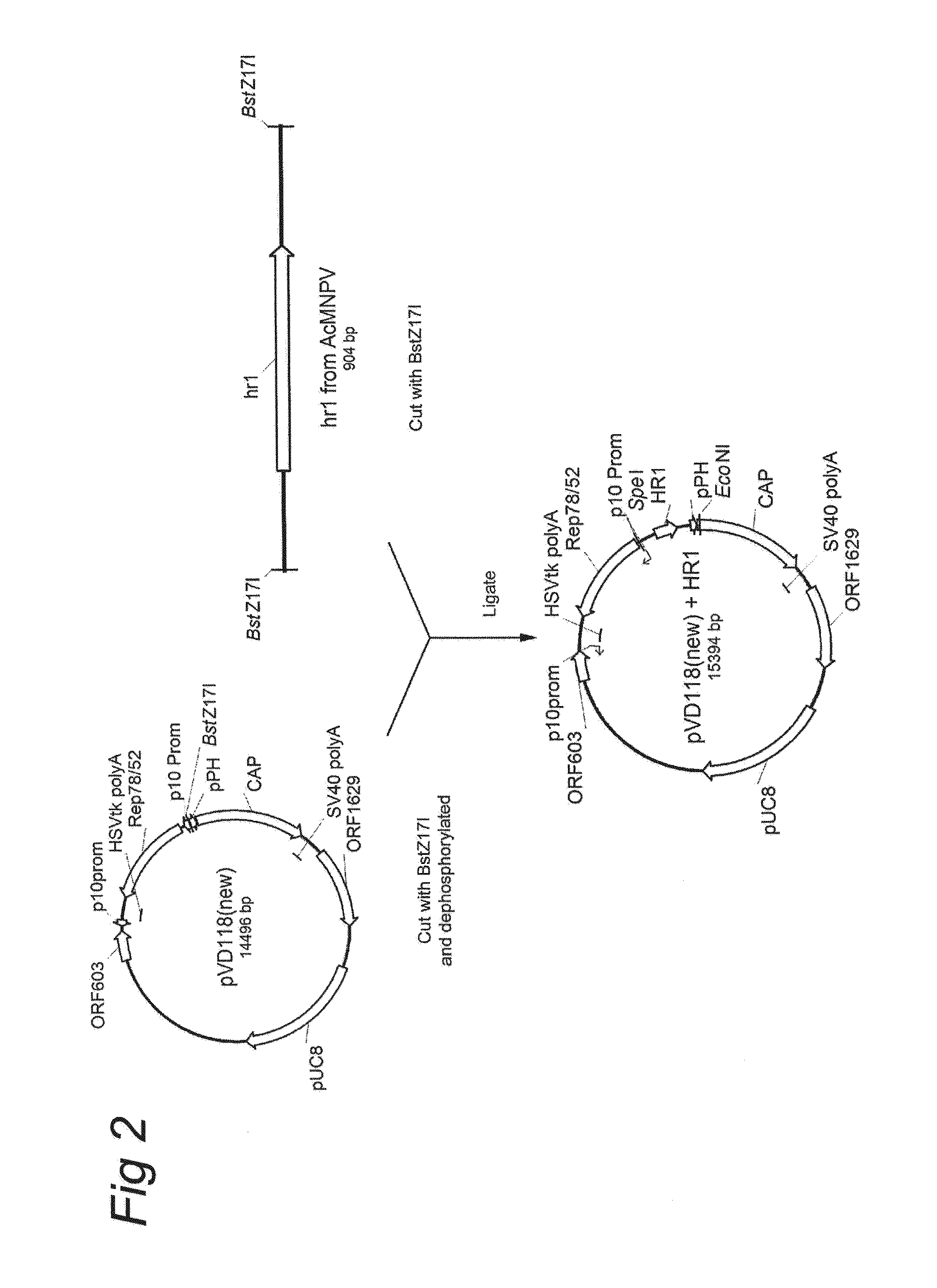
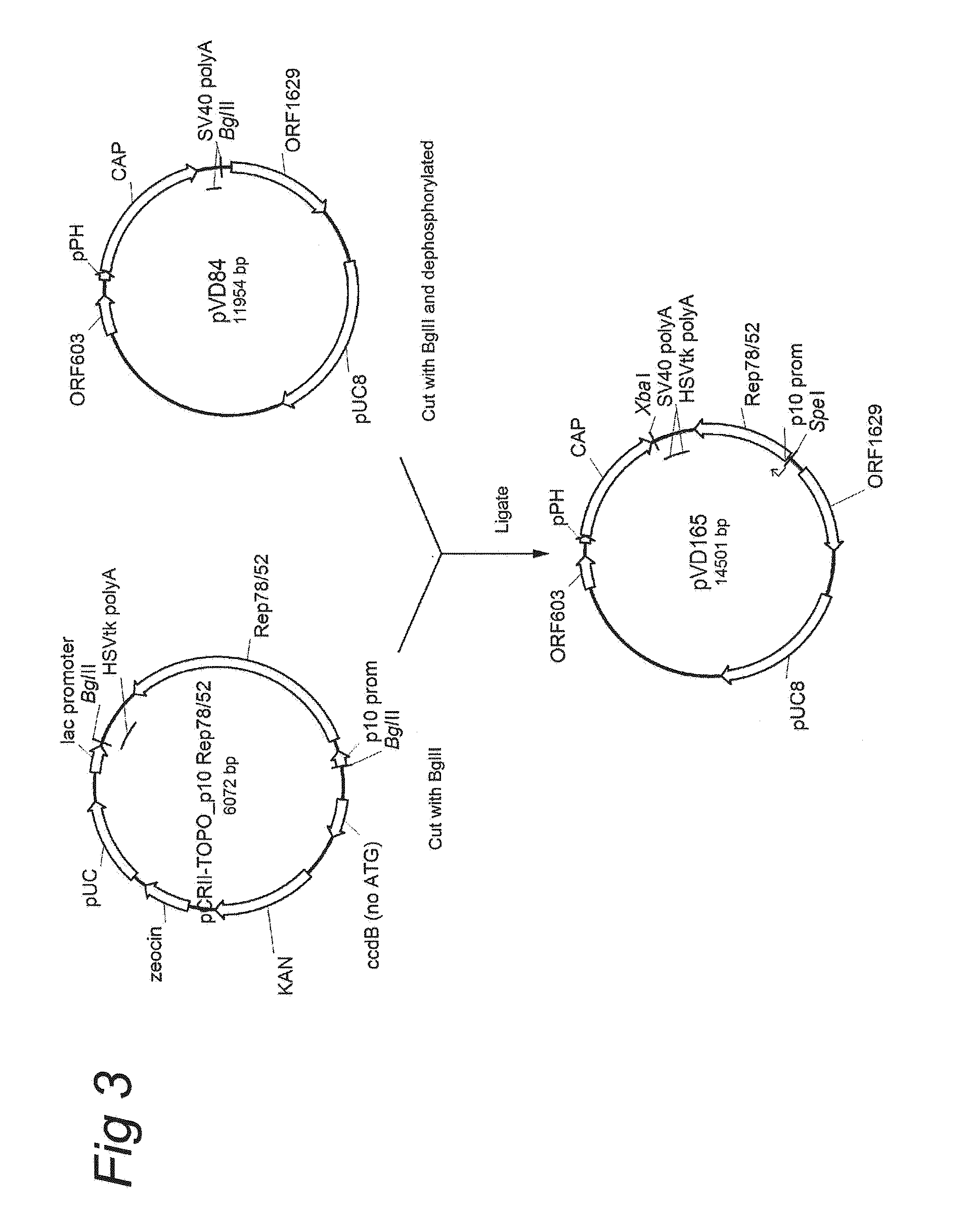
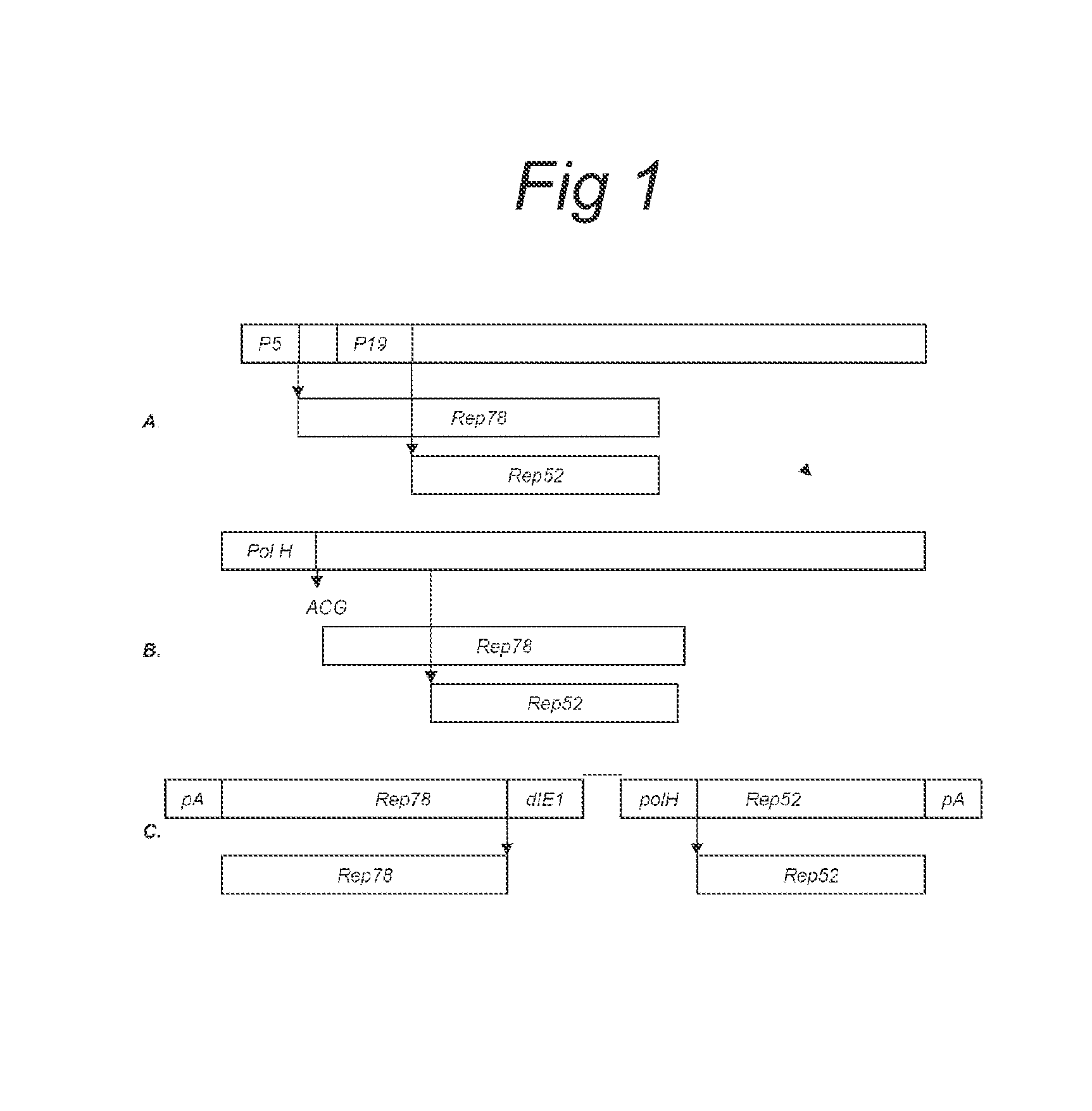
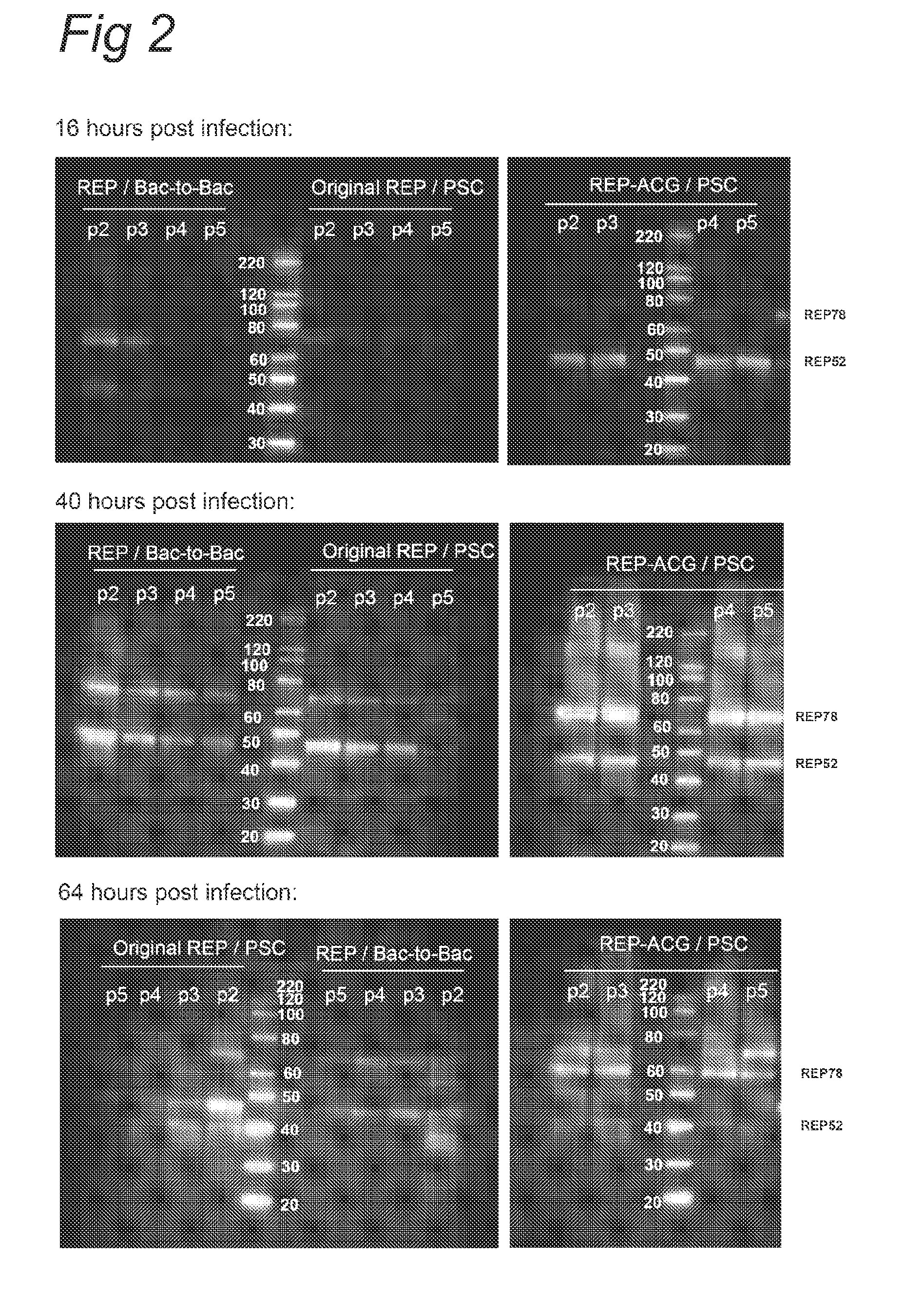
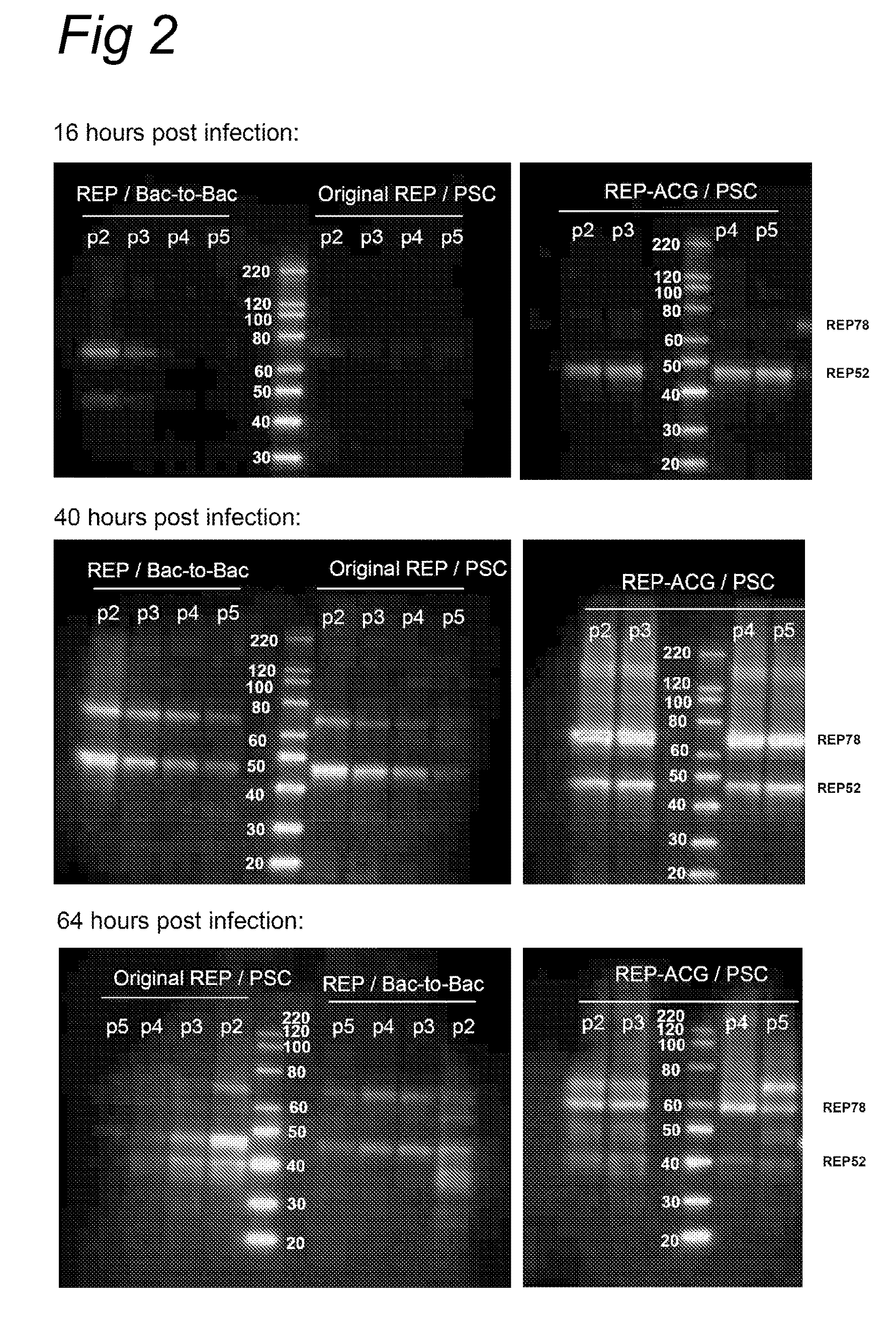
Vectors with modified initiation codon for the translation of AAV-Rep78 useful for production of AAV
ActiveUS8512981B2Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorStart codonNucleotide
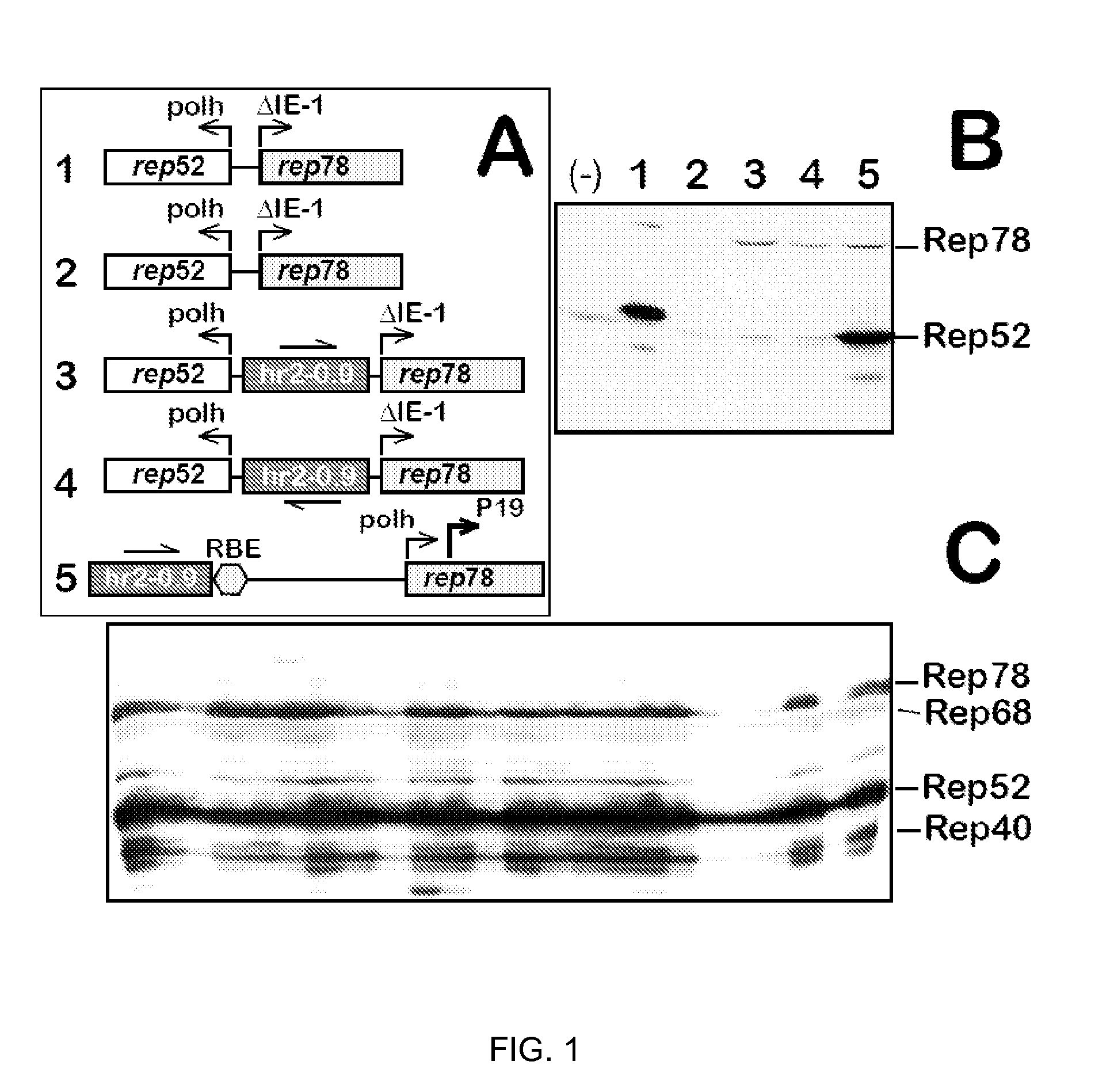
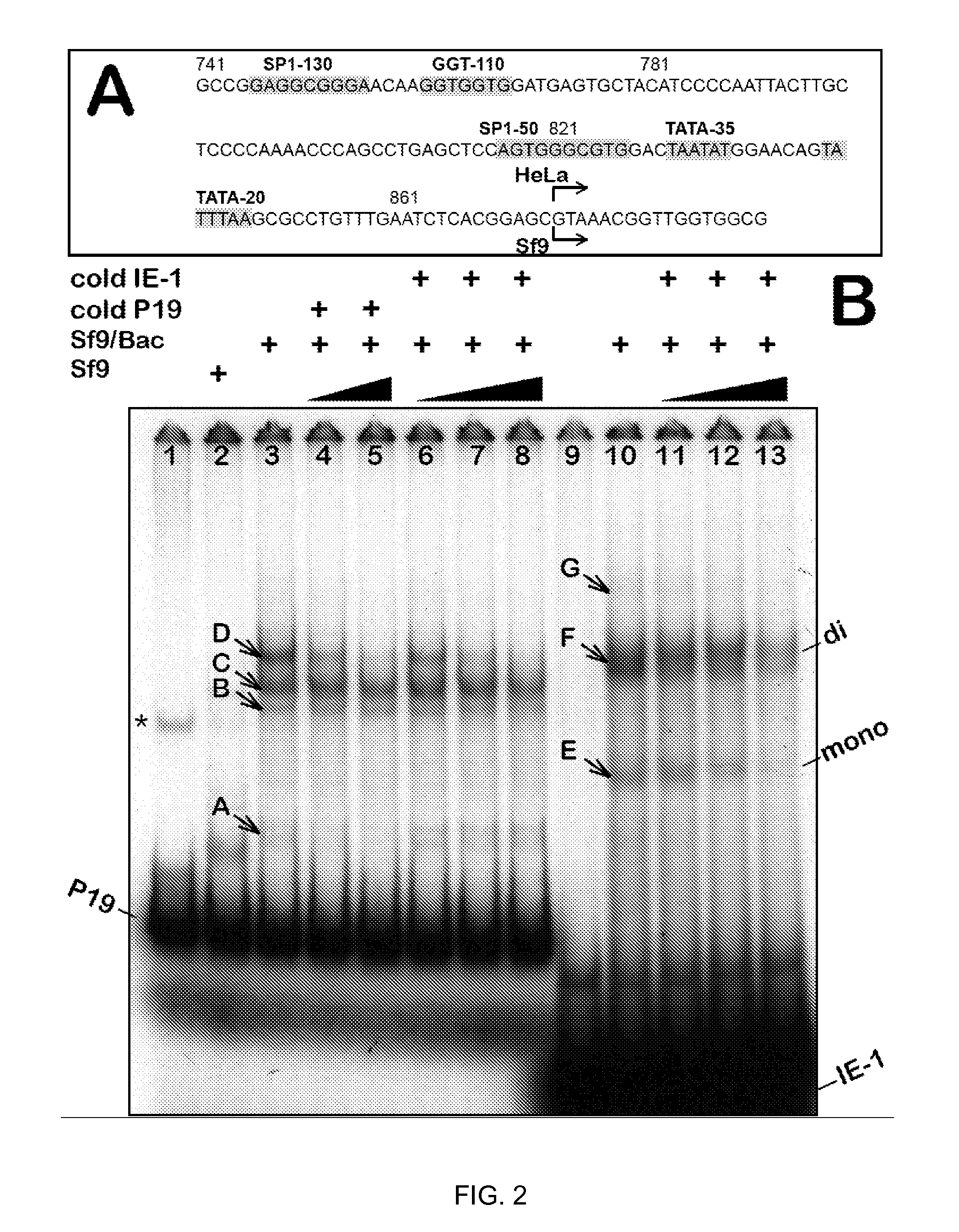
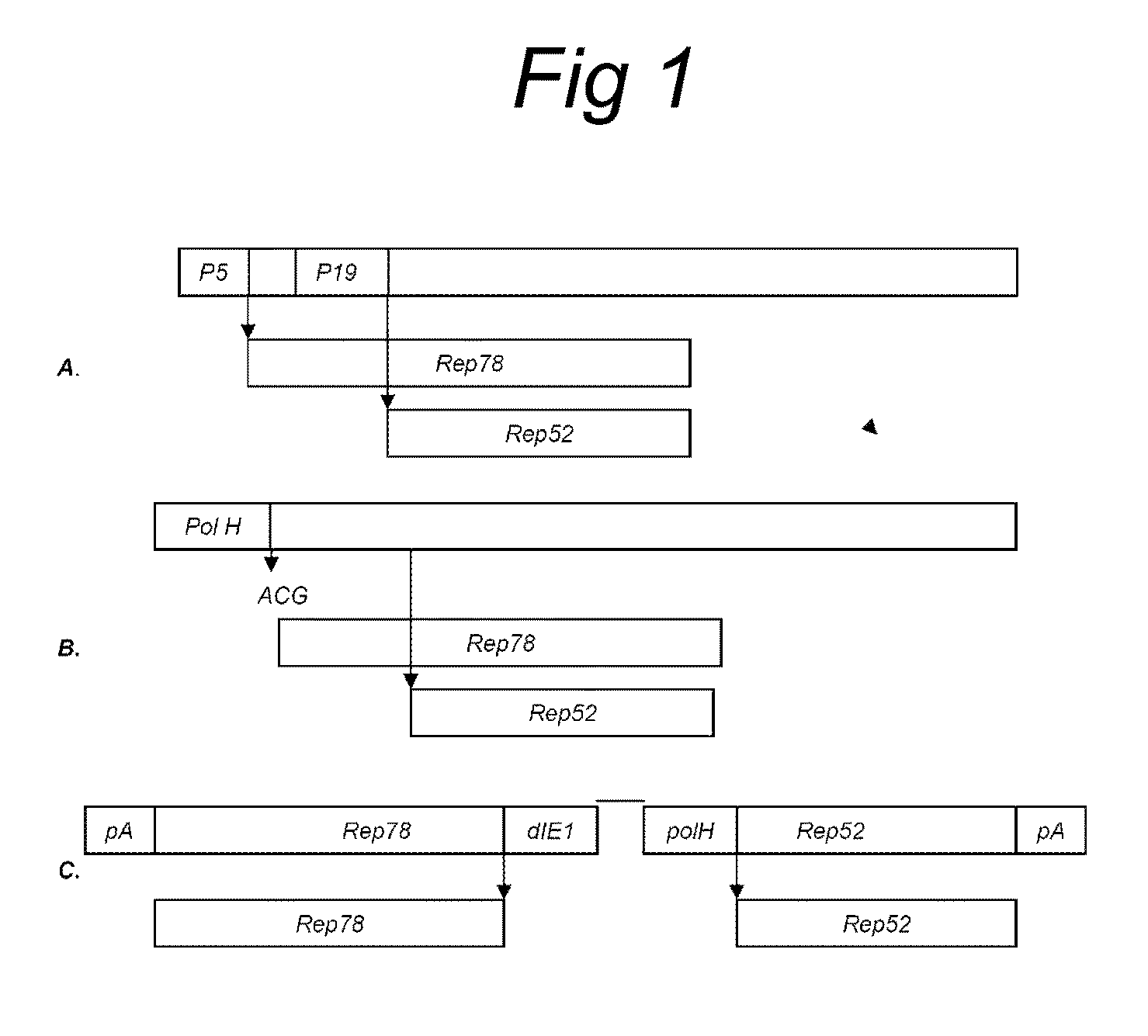
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Influenza recombinant subunit vaccine
InactiveUS20070042002A1Improving immunogenicityImprove efficacySsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
Baculoviral vectors comprising repeated coding sequences with differential codon biases
ActiveUS8697417B2Improve stabilityReduced expression levelAnimal cellsSugar derivativesViral vectorParvovirus
The present invention relates to production of proteins in insect cells whereby repeated coding sequences are used in baculoviral vectors. In particular the invention relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Optimisation of expression of parvoviral rep and cap proteins in insect cells
ActiveUS20110136227A1Increased production titreGreat proportionAnimal cellsVirus peptidesViral vectorParvovirus
The present invention relates to the improved production of recombinant parvoviral virions in insect cells. In particular, the invention relates to an improved process for the production of recombinant parvoviral virions in insect cells, wherein the full / empty parvoviral virion ratio is increased. The invention also relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral Rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Optimization of expression of parvoviral rep and cap proteins in insect cells
The present invention relates to the improved production of recombinant parvoviral virions in insect cells. In particular, the invention relates to an improved process for the production of recombinant parvoviral virions in insect cells, wherein the full / empty parvoviral virion ratio is increased. The invention also relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral Rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20130296532A1Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorNucleotideCapsid
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:UNIQURE IP BV
Compositions and methods for controlling insects involving the tyramine receptor
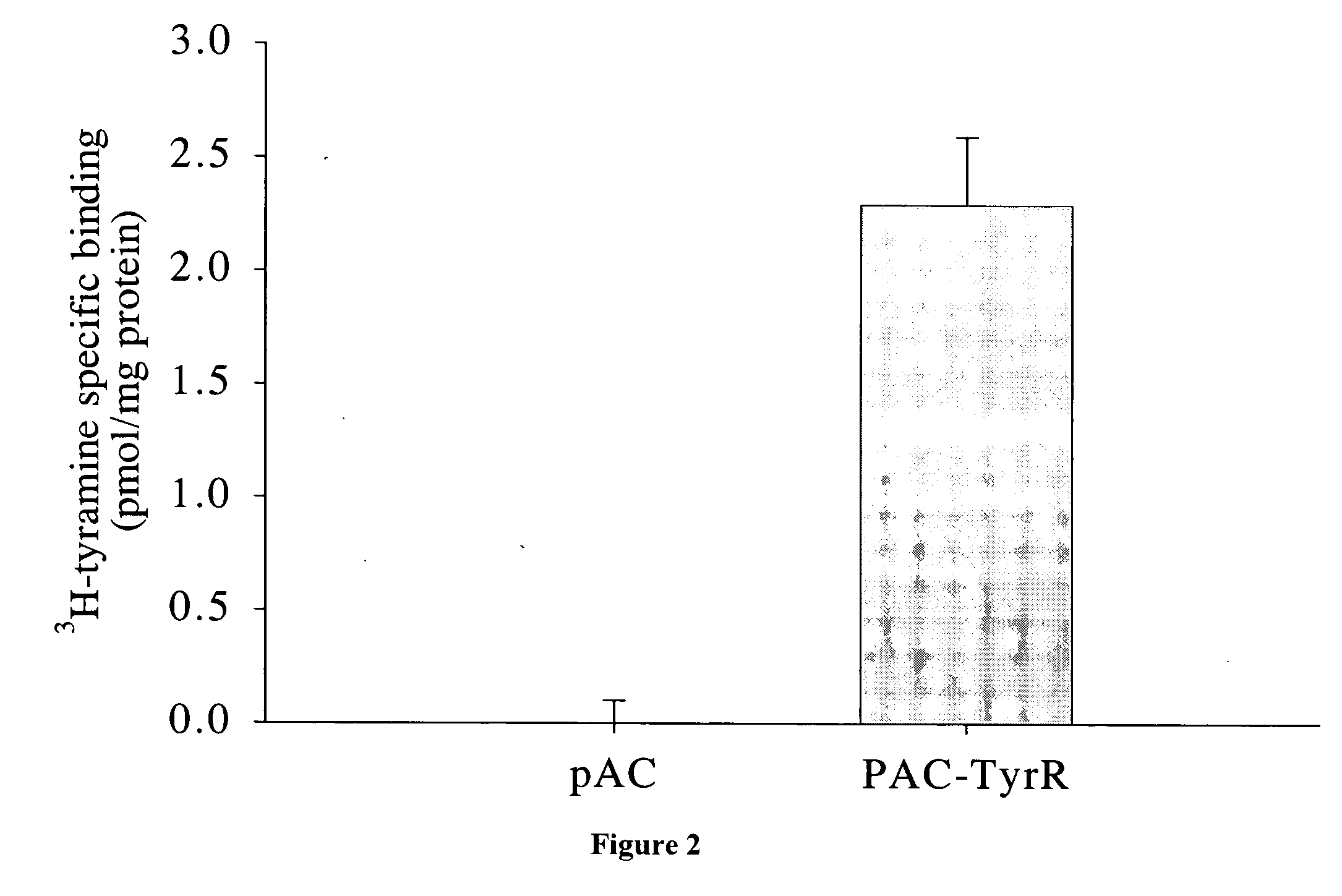
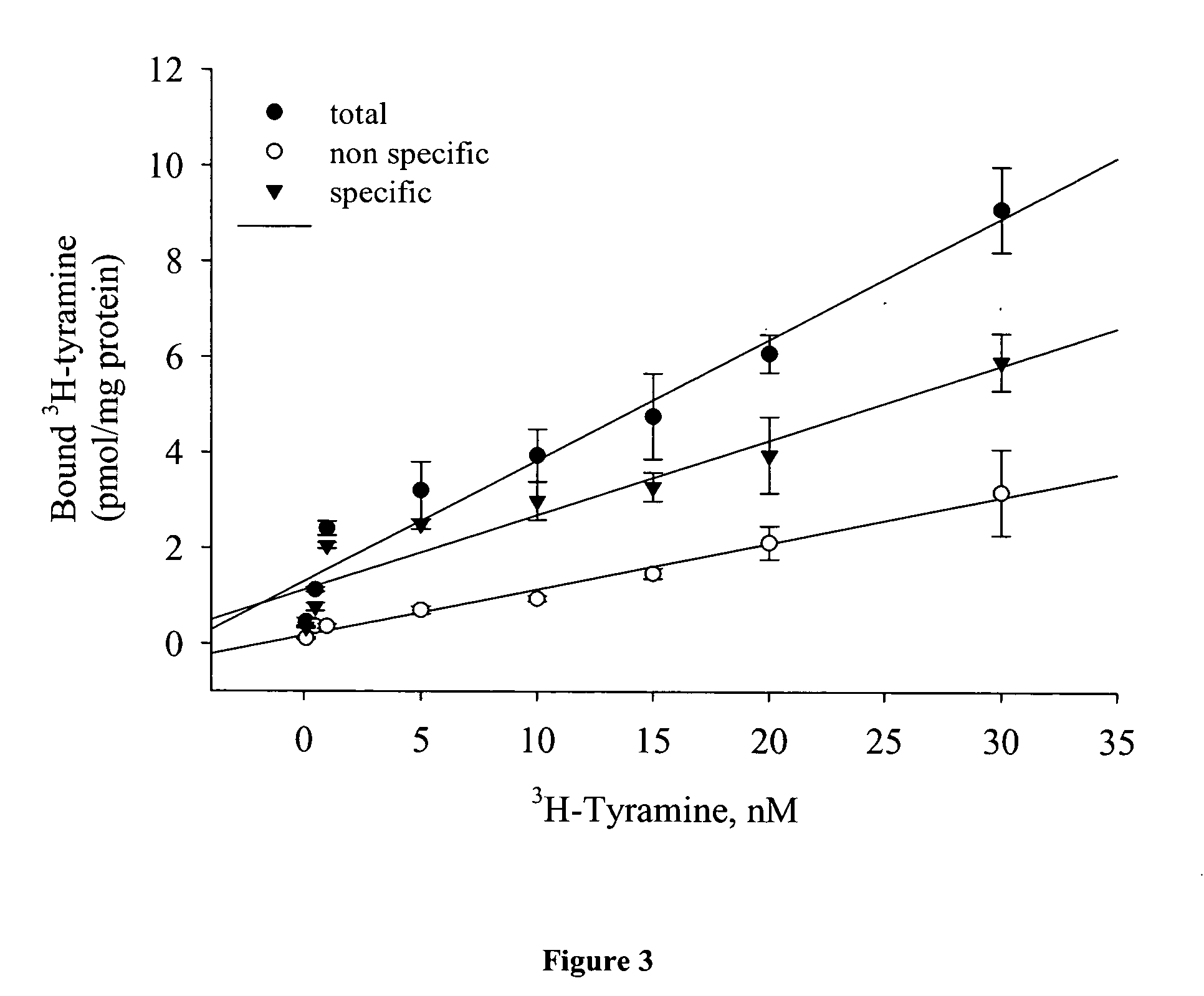
InactiveUS20060263403A1Improve the level ofHigh affinityBiocideCompound screeningCompound (substance)Tyramine receptors
An exemplary method of screening compositions for insect control activity includes, providing an insect cell expressing a receptor of the insect olfactory cascade or fragment thereof, contacting a test composition to the insect cell, measuring at least one parameter selected from olfactory cascade receptor binding affinity, intracellular cAMP levels, and intracellular Ca2+ levels, and selecting a compound capable of altering at least one of parameter selected from increased olfactory cascade receptor binding affinity, altered intracellular cAMP levels, and altered intracellular Ca2+ levels. An exemplary isolated eukaryotic cell is transformed with a nucleic acid encoding an insect olfactory cascade receptor protein or fragment thereof. An exemplary method for controlling an insect includes, contacting a composition including a compound having a binding affinity for an olfactory cascade receptor of an insect.
Owner:TYRATECH
Optimization of gene sequences of virus-like particles for expression in insect cells
InactiveUS20040121465A1Improve the level ofMinimize the numberAnimal cellsViral antigen ingredientsPolynucleotideTGE VACCINE
Codon optimized polynucleotides for optimal expression of recombinant proteins in eukaryotic cells are provided. The codon optimized polynucleotides encode a viral capsid protein that self assembles into a virus-like particle. The virus-like particle is expressed extracellularly and exhibits conformational antigenic epitopes capable of raising neutralizing antibodies. Pharmaceutical compositions, vaccines, and diagnostic test kits containing the gene products of the codon-optimized polynucleotides are also provided.
Owner:NOVAVAX
Inducible system for highly efficient production of recombinant Adeno-associated virus (rAAV) vectors
ActiveUS8679837B2High production costHigh yieldBiocideGenetic material ingredientsClinical gradeDisease
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
DsRNA as insect control agent
The present invention concerns methods for controlling insect infestation via RNAi- mediated gene silencing, whereby the intact insect cell(s) are contacted with a double-stranded RNA from outside the insect cell(s) and whereby the double-stranded RNA is taken up by the intact insect cell(s). In one particular embodiment, the methods of the invention are used to alleviate plants from insect pests. Alternatively, the methods are used for treating and / or preventing insect infestation on a substrate or a subject in need of such treatment and / or prevention. Suitable insect target genes and fragments thereof, dsRNA constructs, recombinant constructs and compositions are disclosed.
Owner:DEVGEN PTE LTD
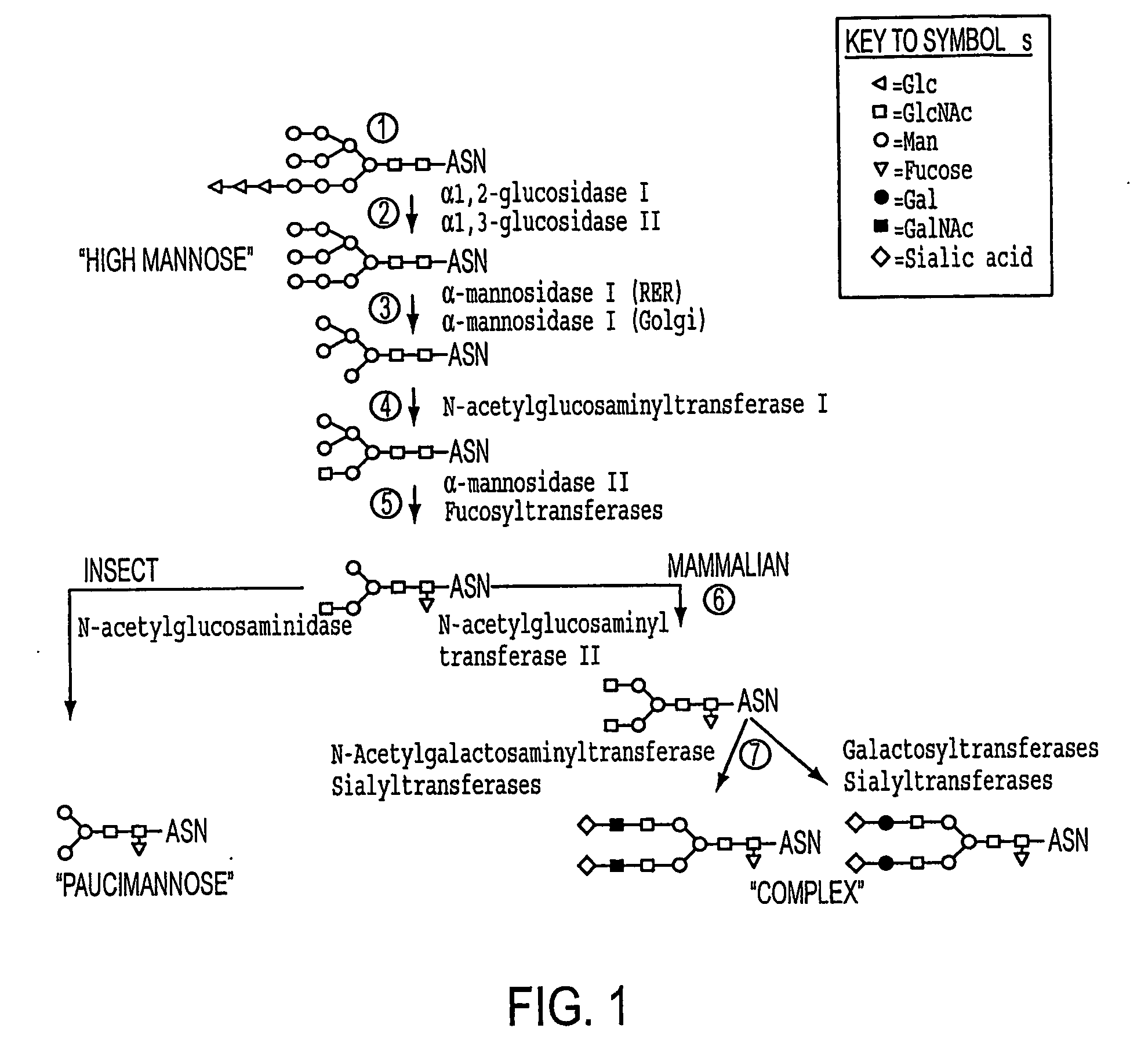
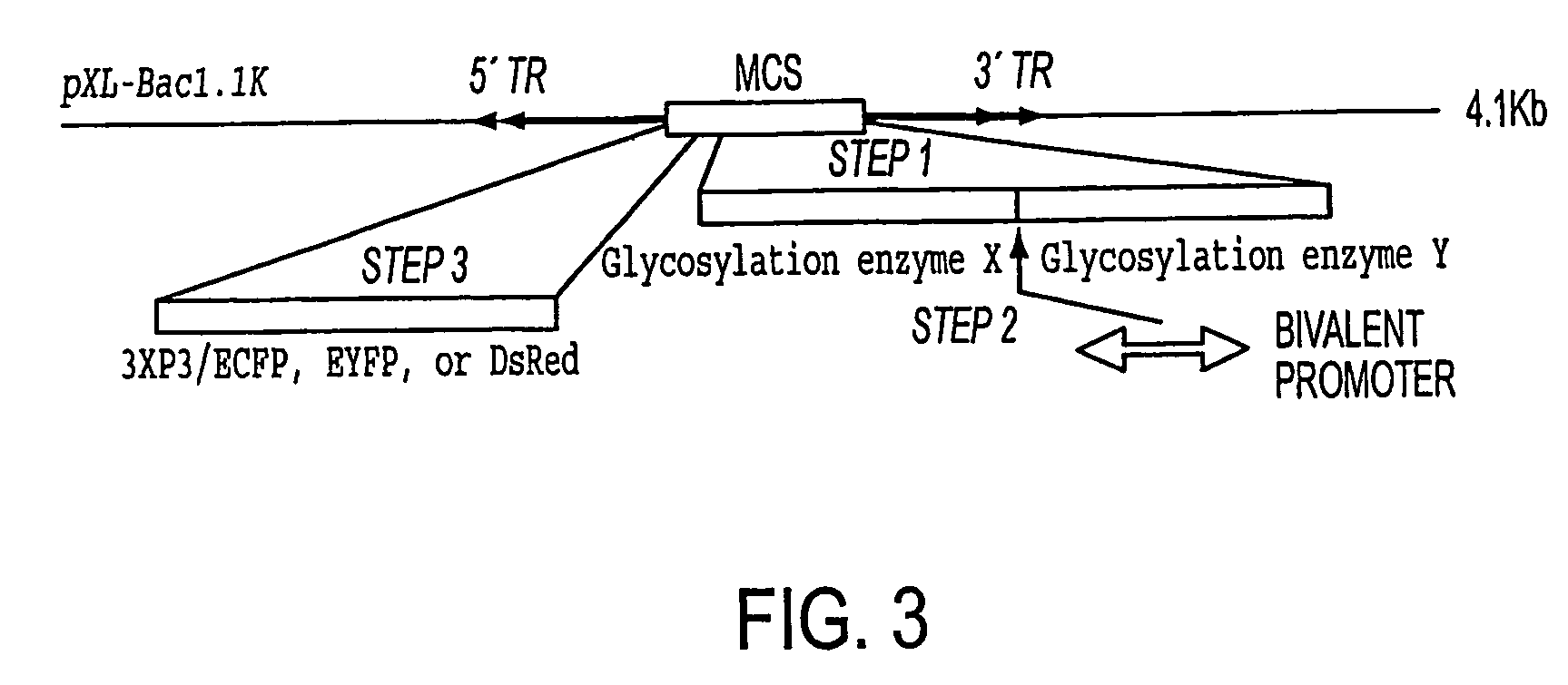
Production of human glycosylated proteins in transgenic insects
This invention relates, e.g., to transgenic insects, or progeny thereof, whose cells contain at least one genomically integrated, expressible, nucleic acid encoding two or more of a set of Nglycosylation enzymes that can glycosylate a heterologous protein with a mammalianized (e.g., humanized) glycosylation pattern. The glycosylation genes are preferably expressed in the insect cells in catalytic amounts. Also described are methods to use such a transgenic insect to produce heterologous, mammalianized polypeptides of interest.
Owner:CHESAPEAKE PERL
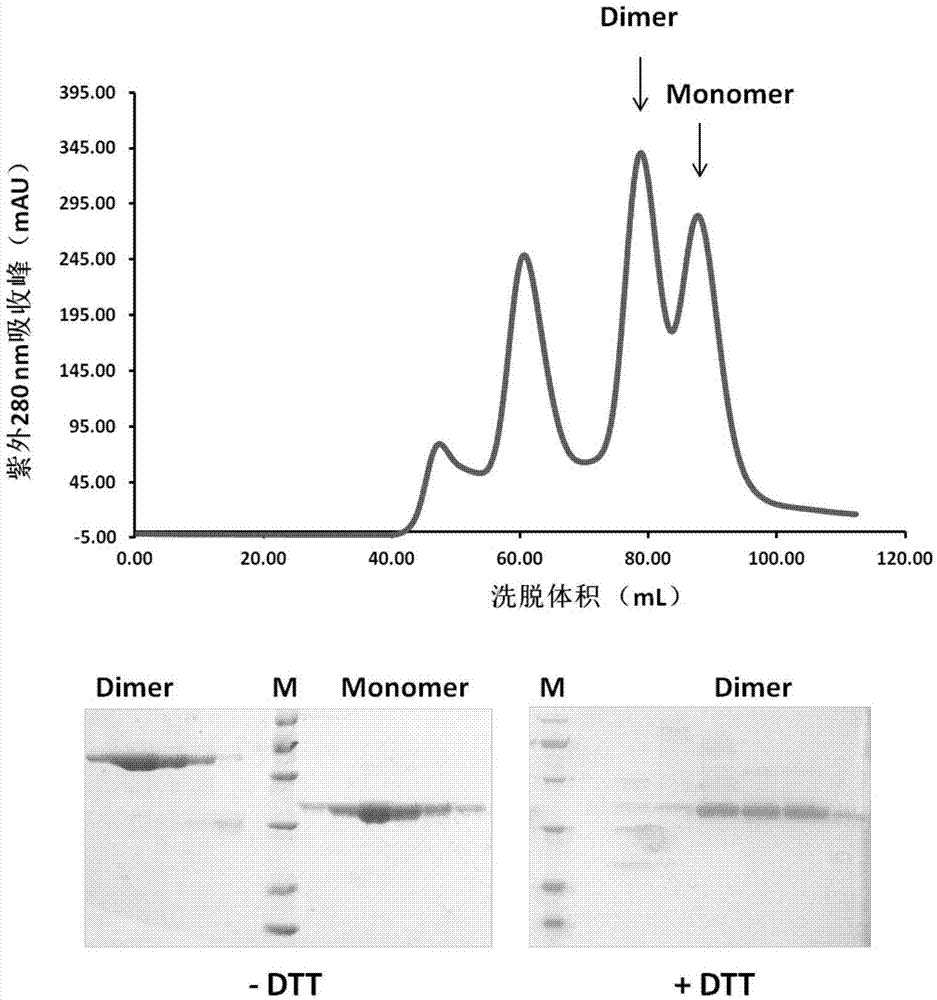
Subunit coronavirus vaccine for dimerization-based receptor binding domains
ActiveCN106928326AOvercoming the disadvantage of insufficient immunogenicityIncrease neutralizing antibody productionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationMiddle East respiratory syndrome coronavirus
The invention discloses a subunit coronavirus vaccine for dimerization-based receptor binding domains and belongs to the technical field of medicine. A baculovirus expresses RBD (receptor binding domain (E367-Y606) of MERS-CoV (middle east respiratory syndrome coronavirus) protein and RBD (R294-F515) of SARS-CoV (severe acute respiratory syndrome coronavirus) in insect cells, the RBDs may form a dimer through cysteine residue at 603 of S (spike) protein or form a dimer through cysteine residue at 512 of the S protein, and purified RBD protein dimer and monomer are used respectively to immunize Bald / c mice. The dimerized RBDs have the advantages that the defect that RBD monomers have poor immunogenicity is overcome and the generation of neutralizing antibodies in against MERS-CoV is increased greatly.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
Influenza recombinant subunit vaccine
InactiveUS20080008725A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseViral antigen ingredientsAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:MERCK SHARP & DOHME CORP
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Influenza recombinant subunit vaccine
InactiveUS20070042001A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
Preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV) and its application
InactiveCN102321639ANeutralizing activityViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
The invention relates to a preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV). The method comprises the steps of: modifying genetic elements of a structural protein encoding gene C-E3-E2-6K-E1 of CHIKV, cloning the modified genetic elements into the expression vector of an insect cell, then transfecting the obtained recombined expression vector and baculovirus linear DNA respectively to an SF9 insect cell and making the cell secrete and express CHIKV VLPs. Additionally, the invention also makes preliminary studies on the immune effects of CHIKV VLPs and applicationof CHIKV VLPs in virus specific antibody detection, thus laying a foundation for research and preparation of immunological detection reagents and even vaccines based on CHIKV VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Method and composition for altering a B cell mediated pathology
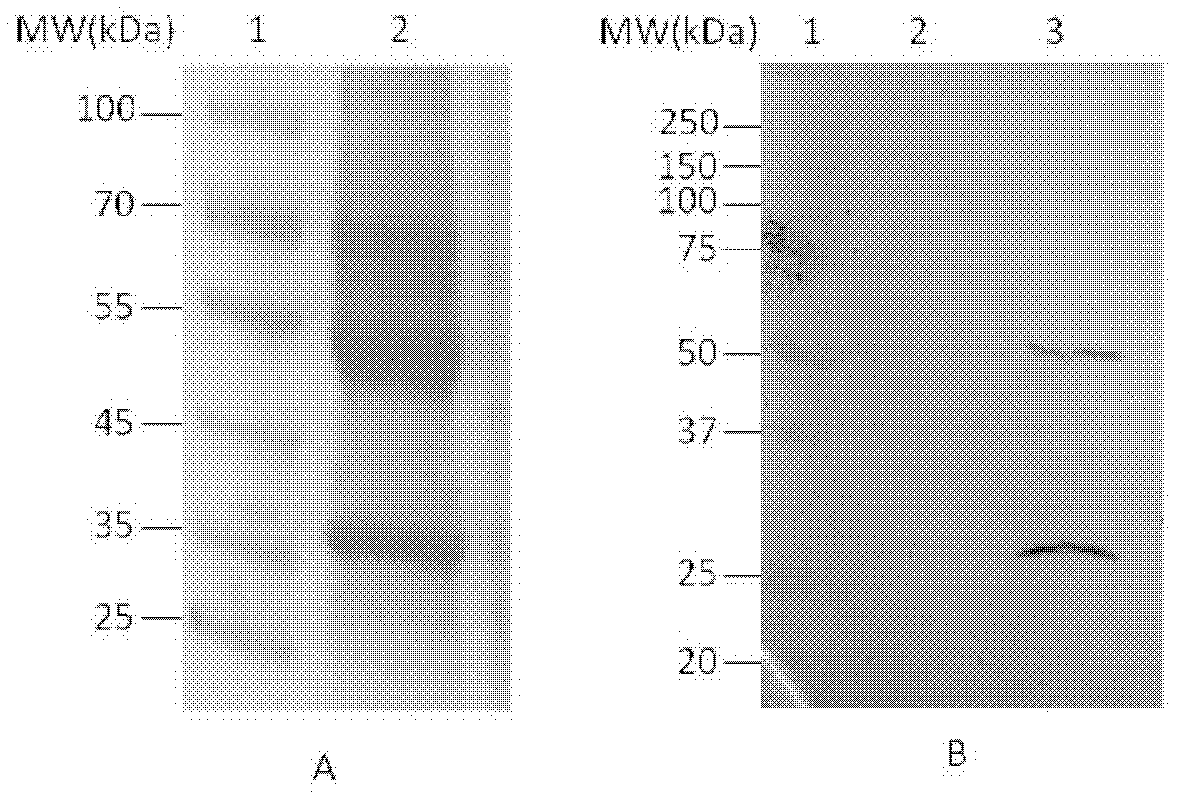
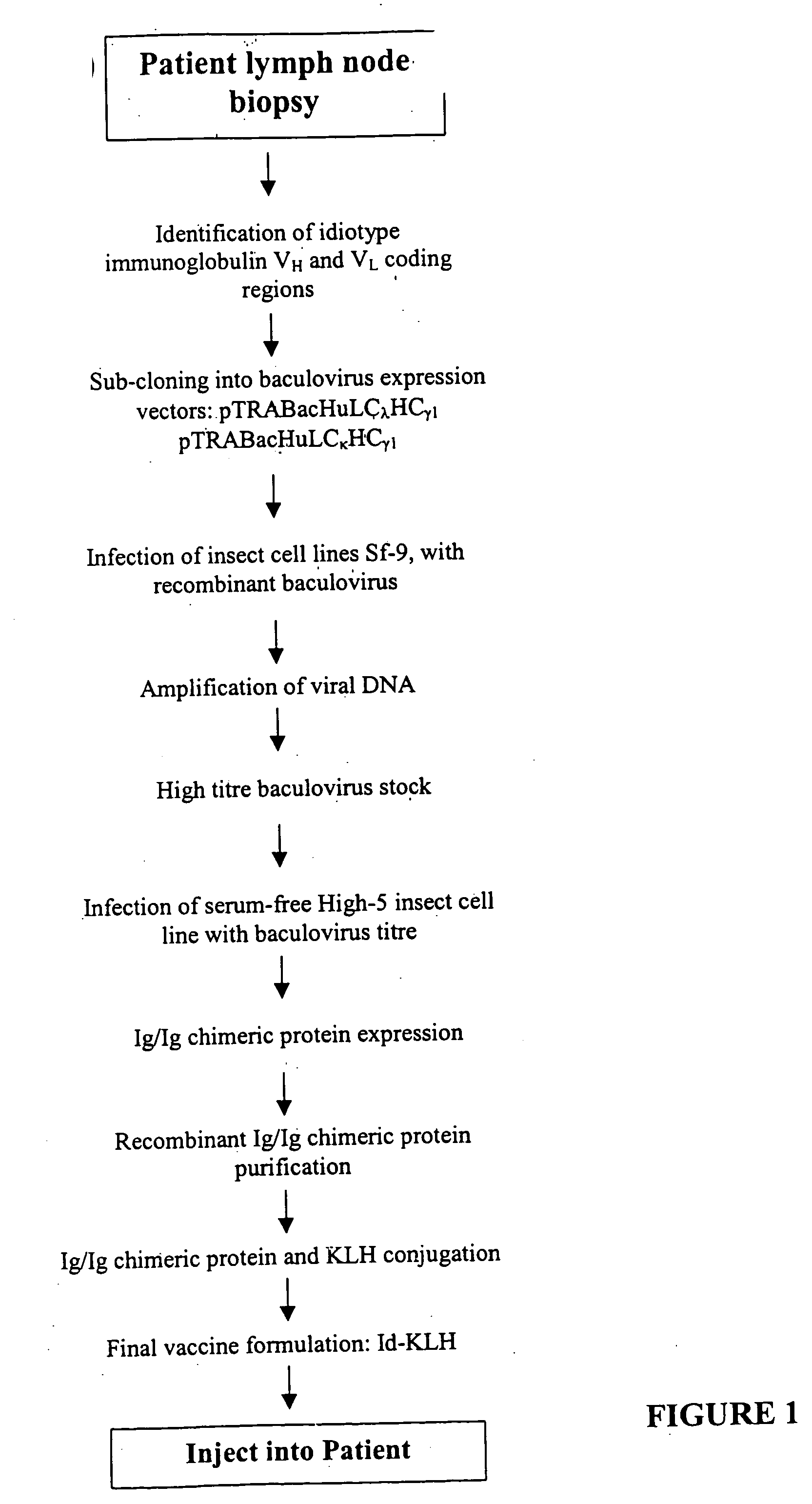
InactiveUS20050238645A1BiocidePeptide/protein ingredientsAntibody AffinitiesKeyhole-limpet haemocyanin
The present invention provides a method for altering a B cell mediated pathology in a patient. This method comprises administering a composition comprising at least one and / or two chimeric proteins. Each chimeric protein comprises at least a portion of either the VH or VL region of a immunoglobulin molecule from particular B cells from a patient having a B cell mediated pathology, and an immunoglobulin constant region. The genes encoding VH and / or VL regions and the genes encoding immunoglobulin constant regions are isolated and inserted into an expression vector. The chimeric proteins are produced by introducing the expression vectors into insect cell lines. The chimeric proteins are purified using antibody affinity columns, and then chemically conjugated to an immunogenic carrier, keyhole-limpet hemocyanin (KLH). Since the conjugates comprise chimeric proteins made specifically from particular B cells from a patient having B cell mediated pathology, when it is administered to such a patient, with or without a cytokine, such as granulocyte-macrophage-CSF, or a chemokine, it can induce immune responses to alter such a B cell mediated pathology.
Owner:MMRGLOBAL
Engineering Intracellular Sialylation Pathways
Methods for manipulating carbohydrate processing pathways in cells of interest are provided. Methods are directed at manipulating multiple pathways involved with the sialylation reaction by using recombinant DNA technology and substrate feeding approaches to enable the production of sialylated glycoproteins in cells of interest. These carbohydrate engineering efforts encompass the implementation of new carbohydrate bioassays, the examination of a selection of insect cell lines and the use of bioinformatics to identify gene sequences for critical processing enzymes. The compositions comprise cells of interest producing sialylated glycoproteins. The methods and compositions are useful for heterologous expression of glycoproteins.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Production method of insect specimen
InactiveCN1918983AMaintain the structureAvoid Polymerization DamageDead animal preservationEducational modelsAlcoholInsect
The invention discloses a preparing method of insect sample, which comprises the following steps: fixing living insect, dehydrating through alcohol, replacing, permeating, solidifying or polymerizing to obtain the insect sample, fitting for studying microscopic structure and super-microscopic structure of insect cell.
Owner:SHANXI UNIV
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20090191588A1Improve stabilityReduced expression levelAnimal cellsSugar derivativesStart codonNucleotide
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Chimeric virus-like particles
InactiveUS20100047266A1SsRNA viruses negative-senseAntibacterial agentsVirus-like particleChimeric virus
Chimeric virus-like particles including gag polypeptides are described. Virus-like particles are generated with a gag polypeptide and lipid raft-associated polypeptide linked to an antigen that is not naturally associated with a lipid raft. Preferred methods of generation include expression in insect cells.
Owner:TAKEDA VACCINES INC
66 kDa antigen from Borrelia
InactiveUS6054296AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsProtozoaAntigen
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com