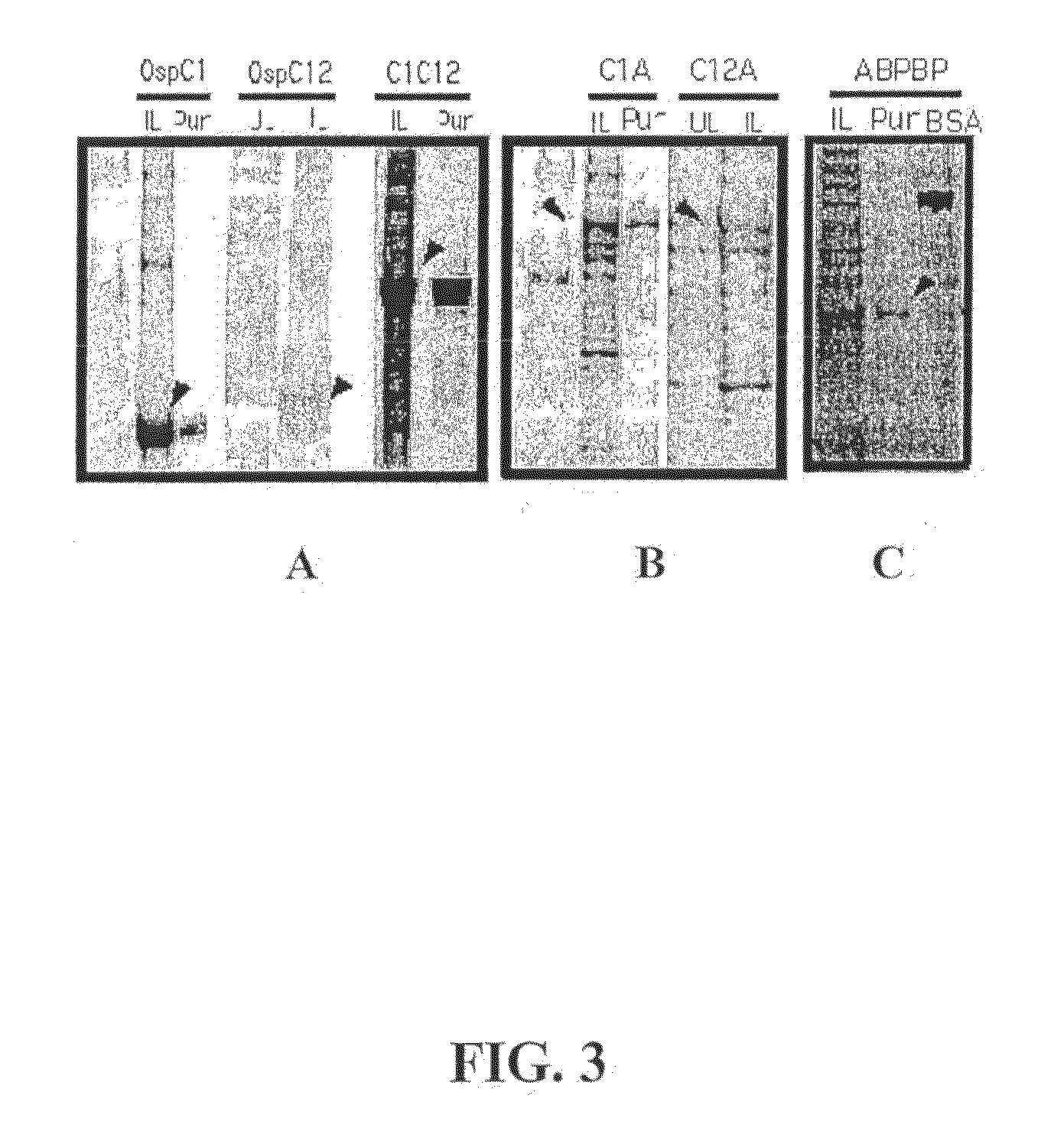
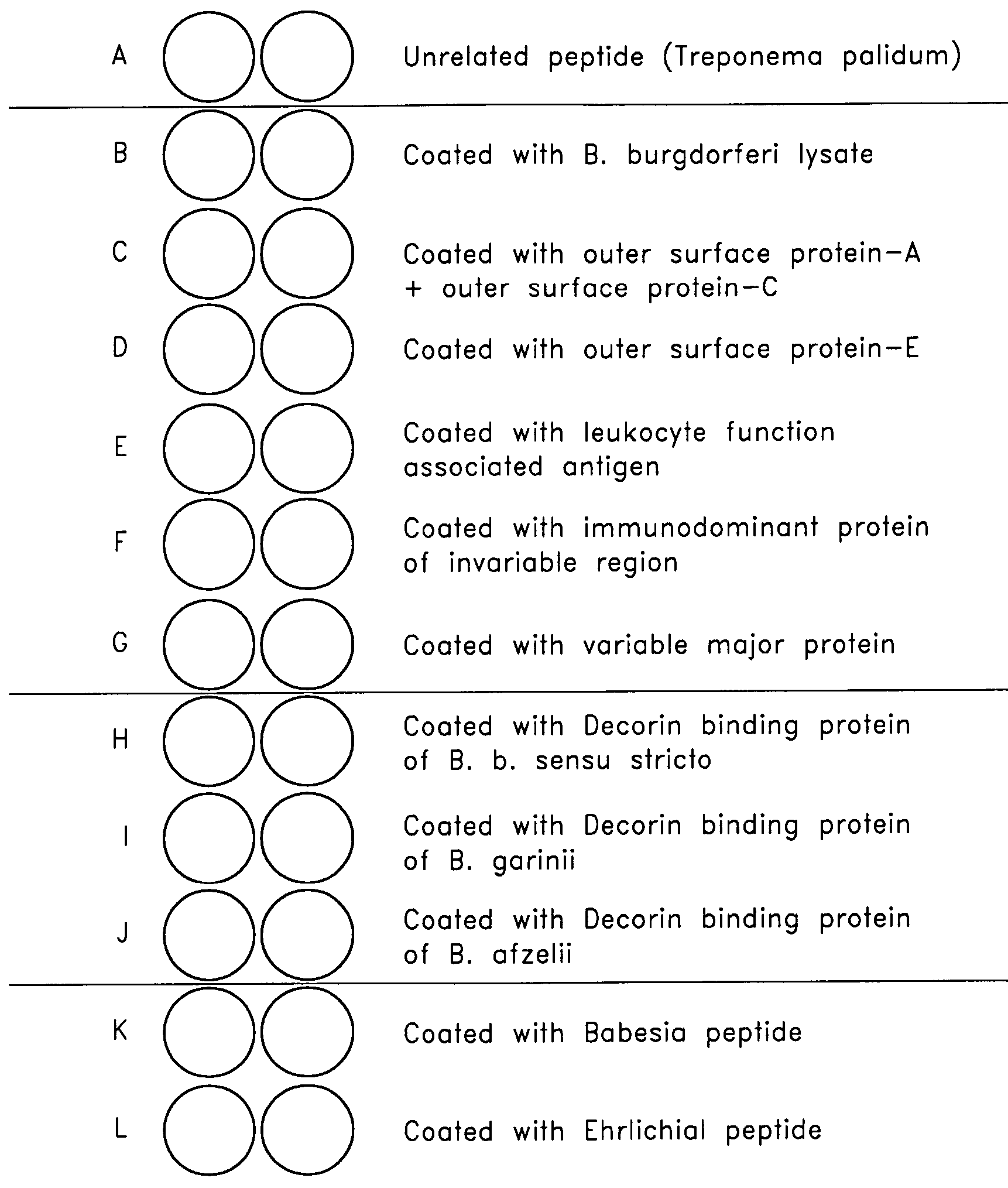
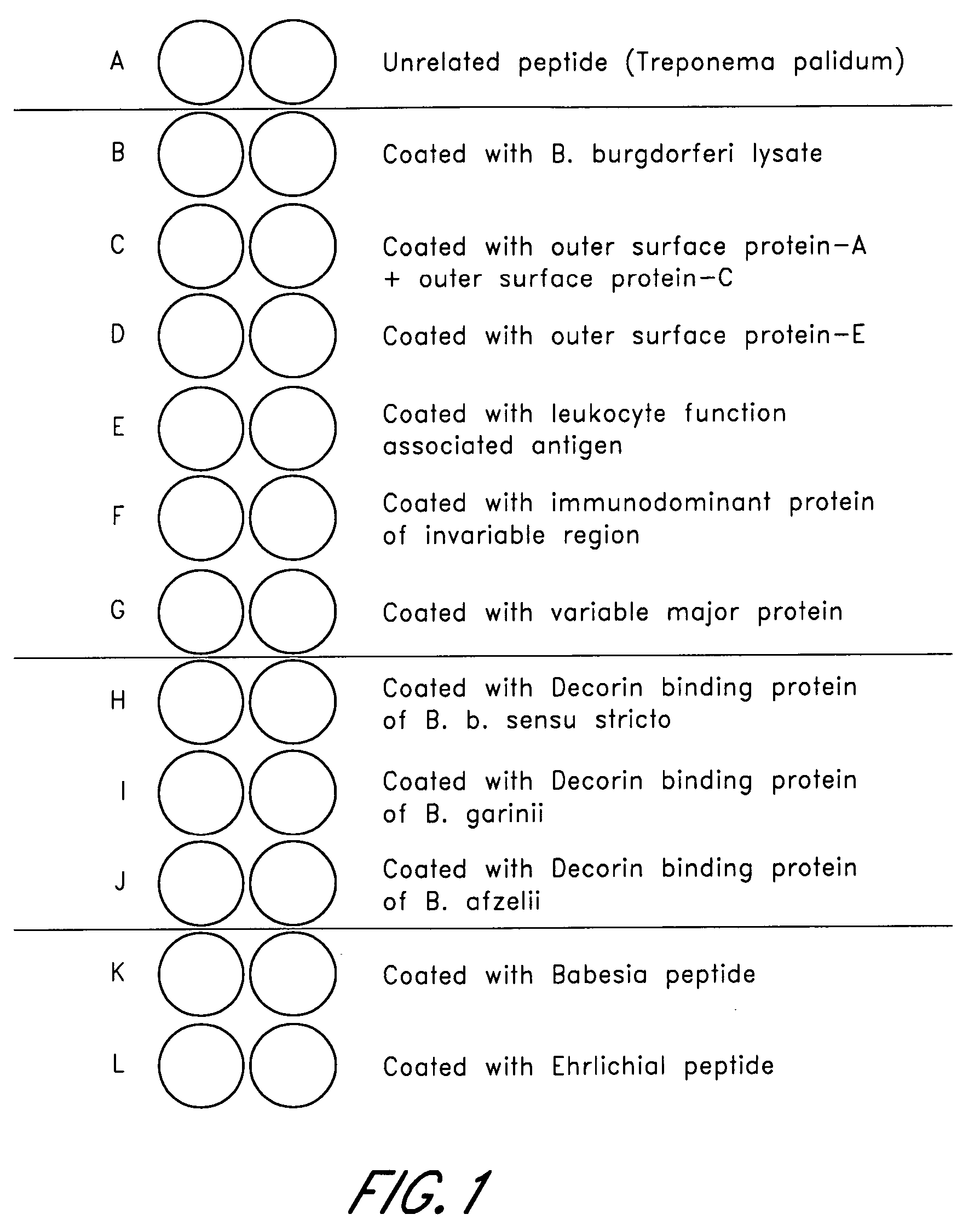
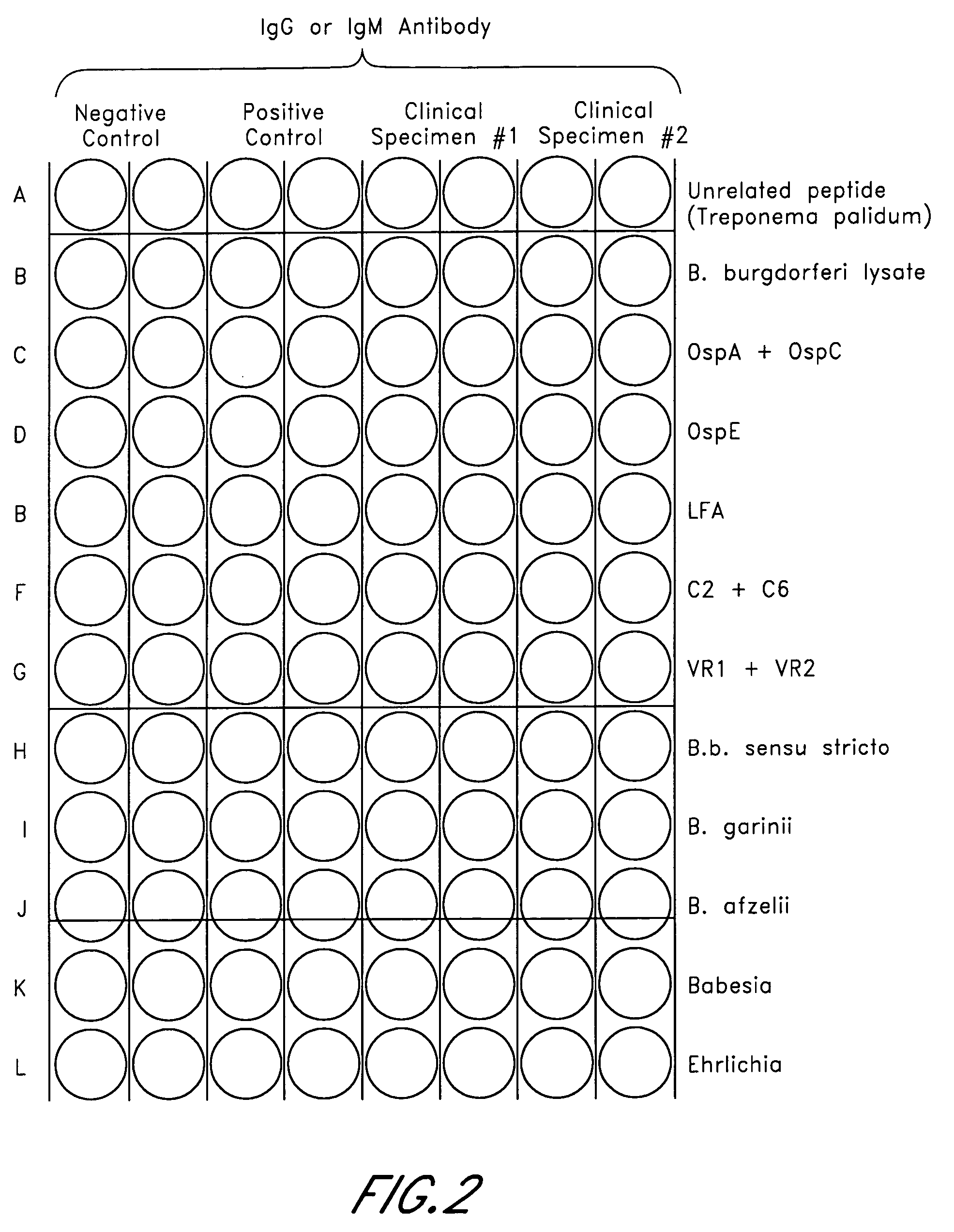
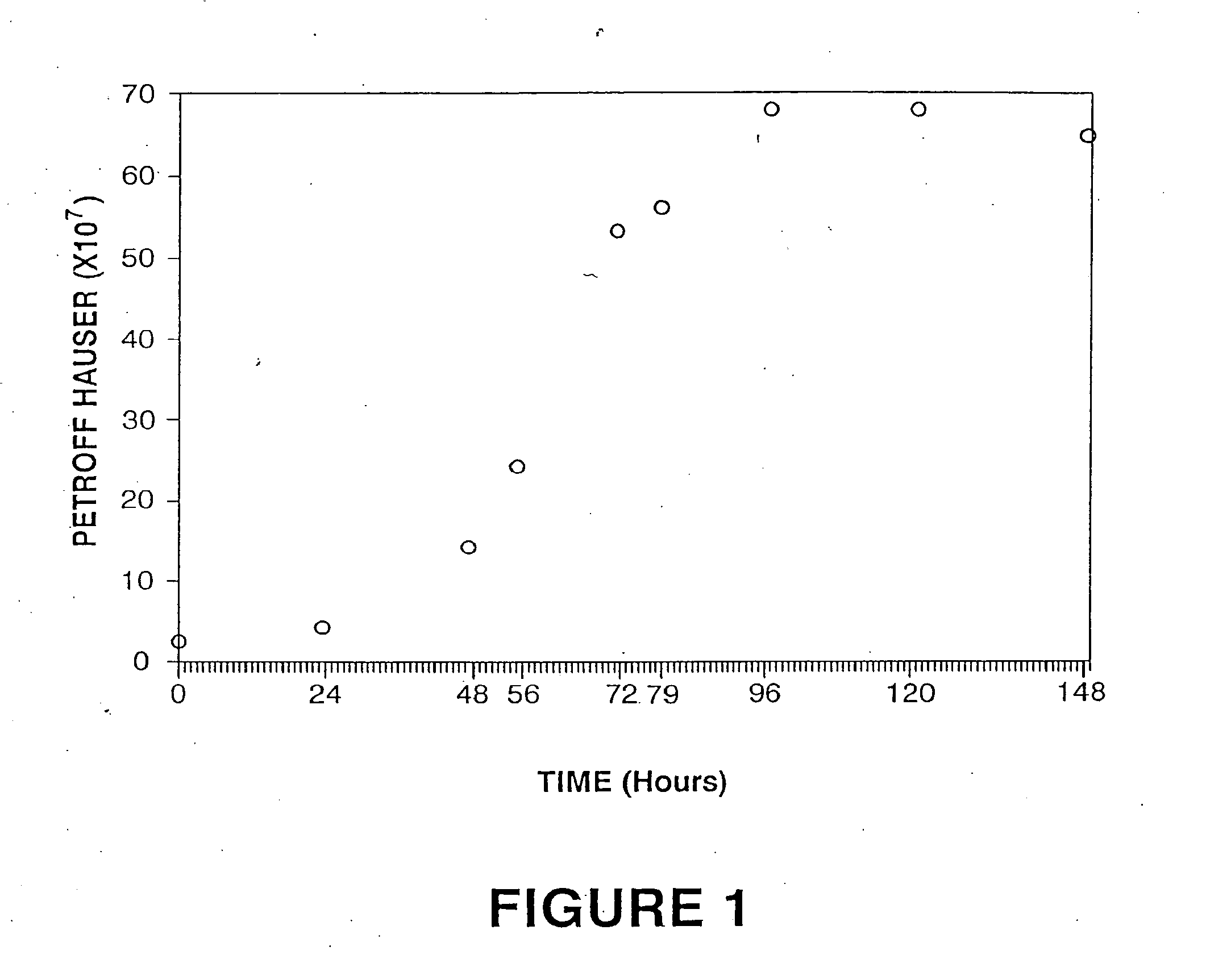
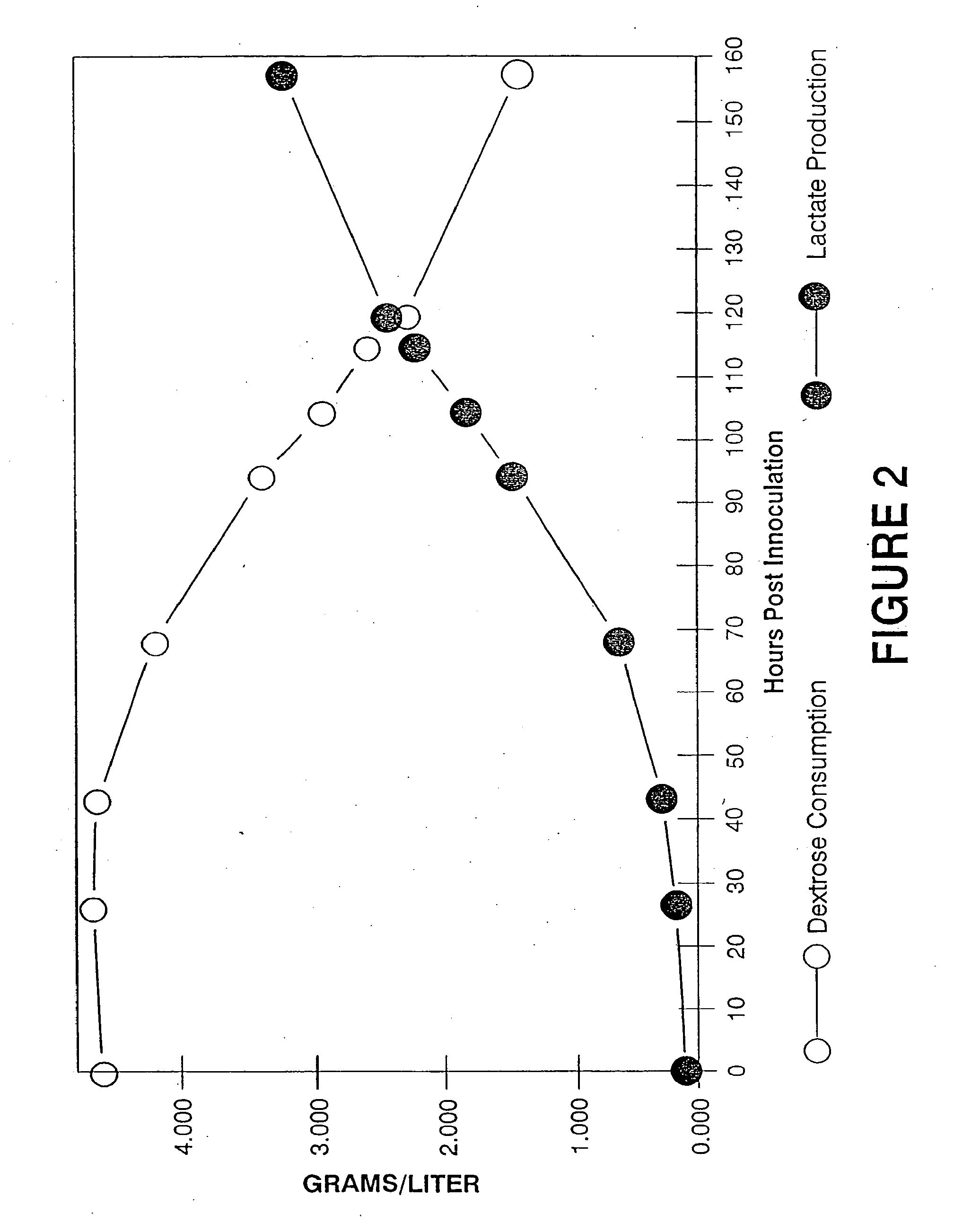
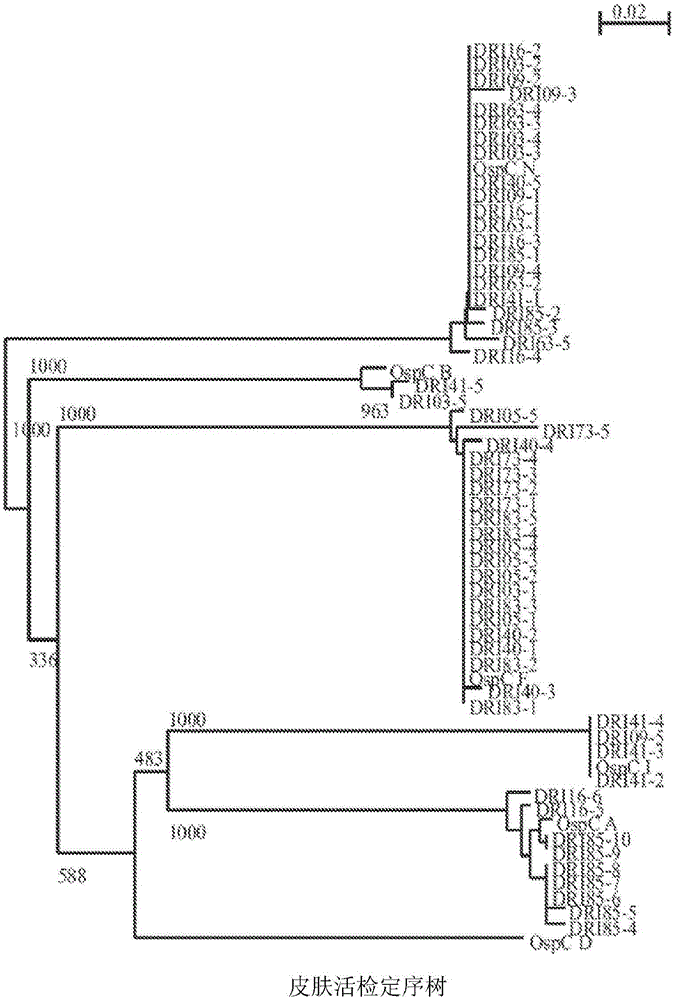
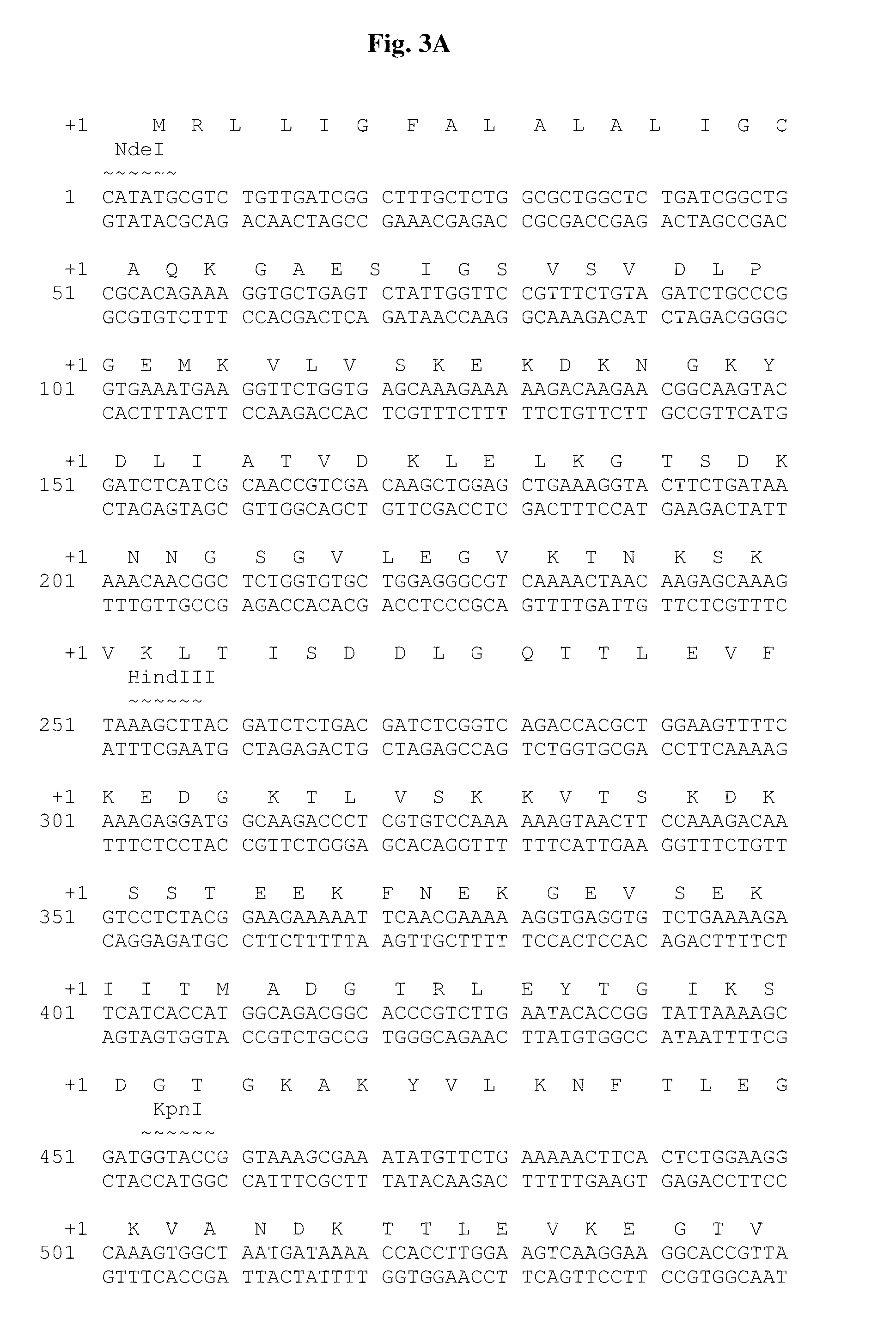Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Borrelia garinii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borrelia garinii is a spirochete bacterium in the genus Borrelia.
Compositions and methods for administering Borrelia DNA
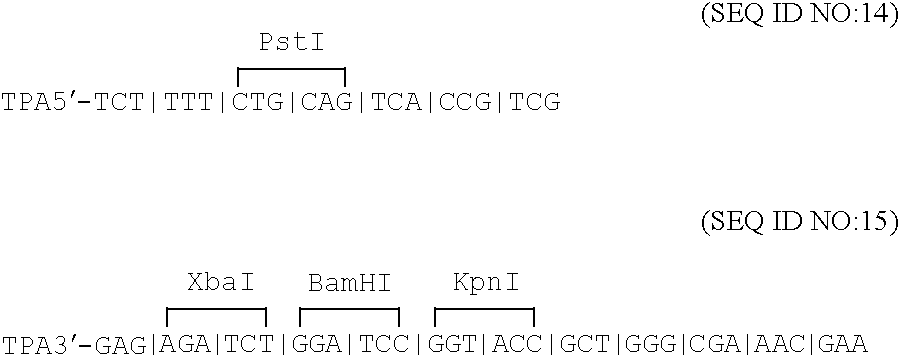
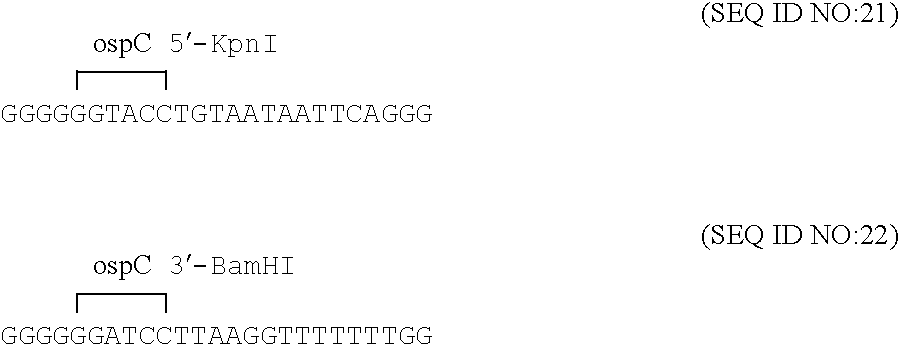
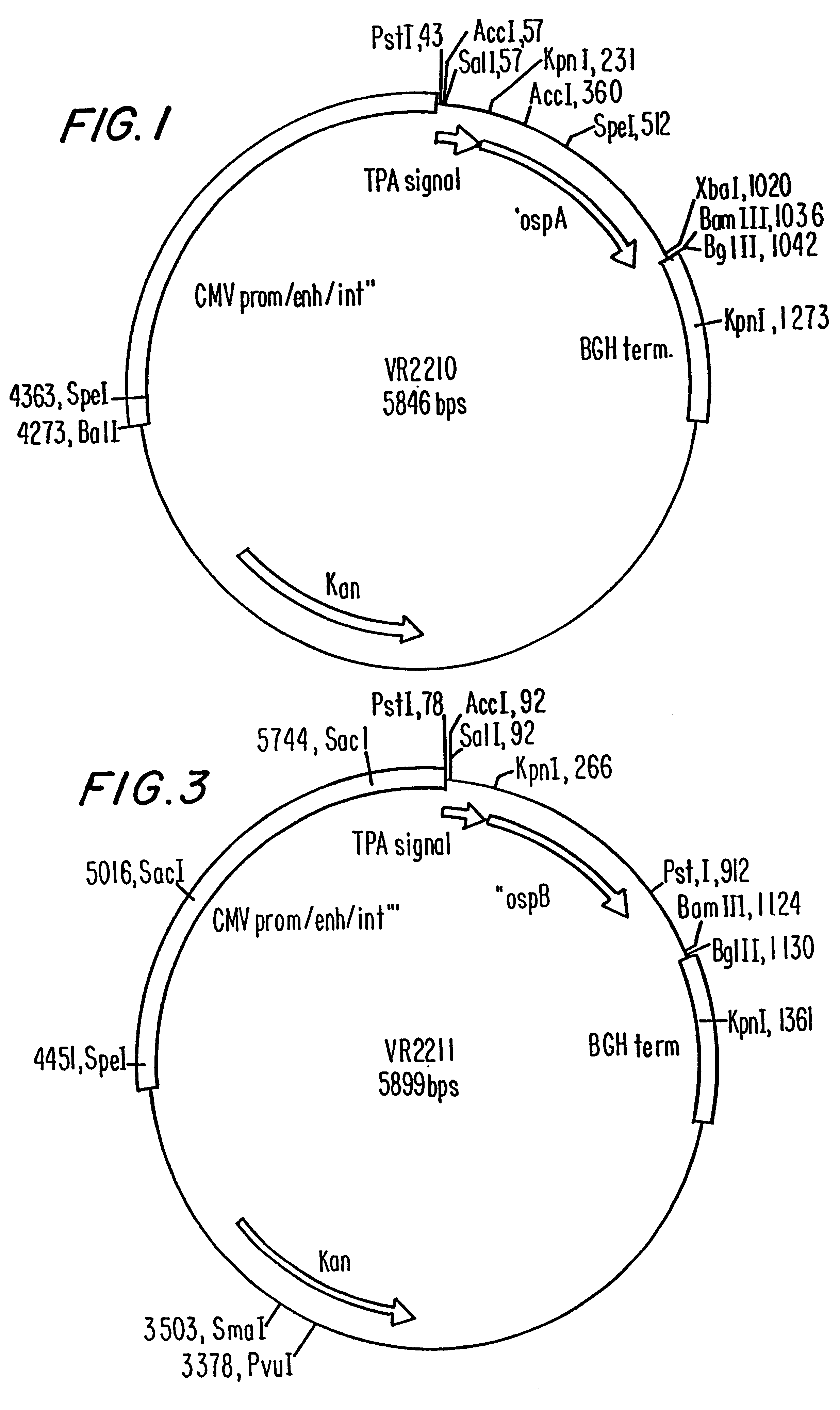
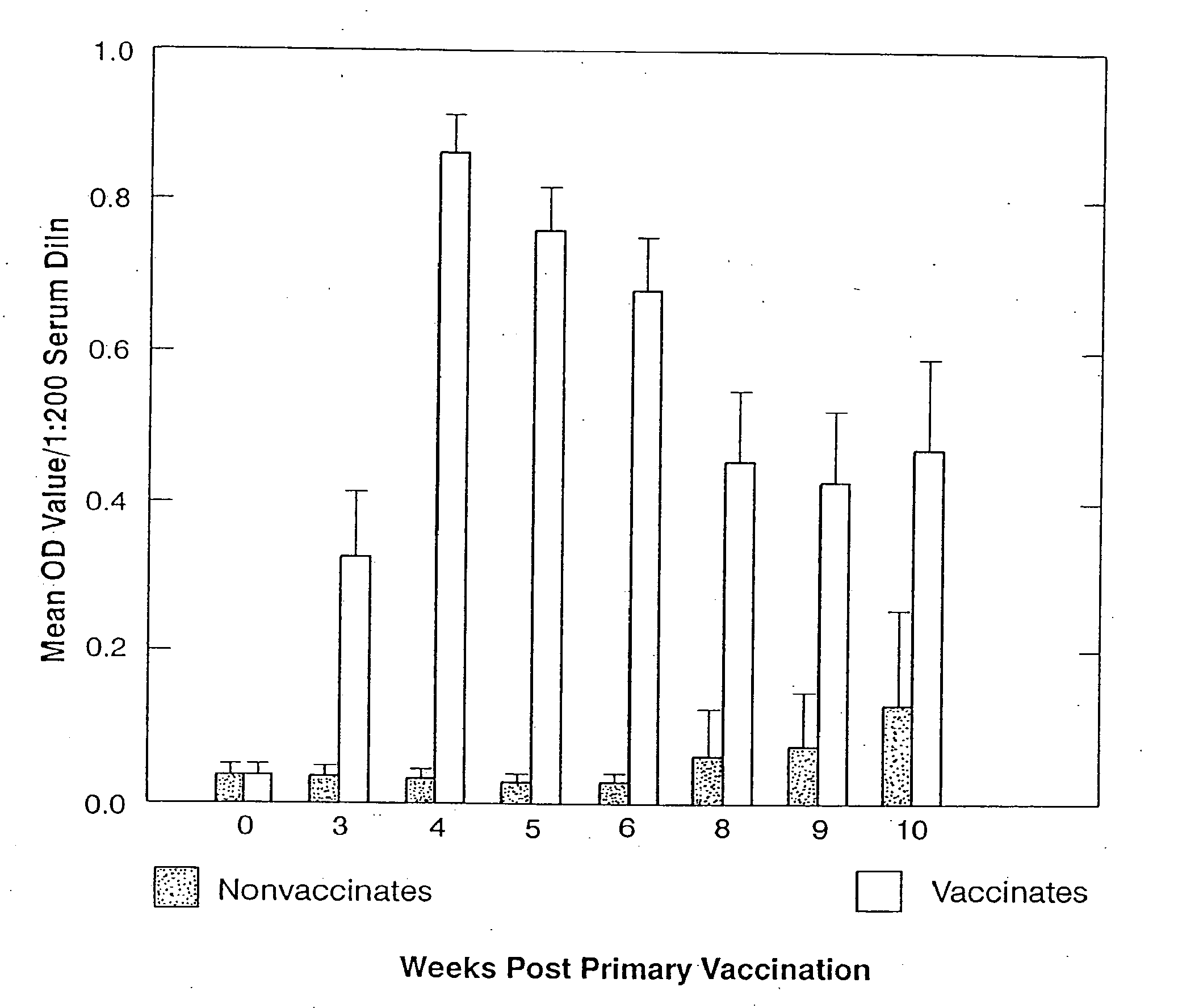
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
Nucleic acid amplification oligonucleotides and probes to Lyme disease associated Borrelia
InactiveUS6074826AReduced thermal stabilityMaximize differenceSugar derivativesMicrobiological testing/measurementBorrelia gariniiHybridization probe
The present invention discloses hybridization assay probes, amplification primers, nucleic acid compositions and methods useful for detecting Borrelia nucleic acids. Hybridization assay probes and amplification primers that selectively detect Lyme disease-associated Borrelia and distinguish those Borrelia from Borrelia hermsii are disclosed. Other hybridization probes selectively detect Borrelia hermsii and not Lyme disease-associated Borrelia are also described.
Owner:GEN PROBE INC
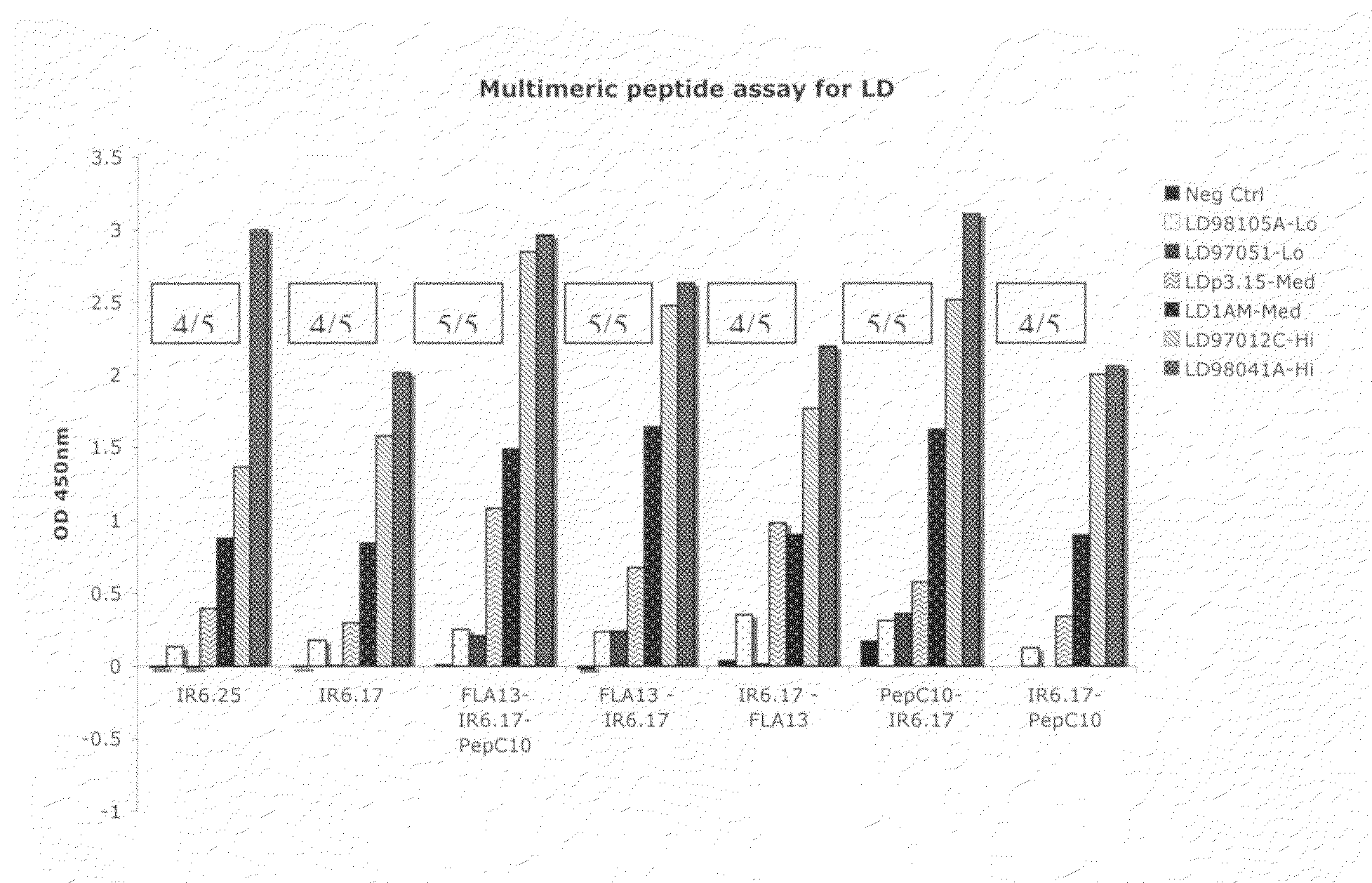
Peptide diagnostic agent for lyme disease
ActiveUS20090162875A1Bacterial antigen ingredientsPeptide/protein ingredientsEpitopeDiagnostic agent
The present invention relates, e.g., to an isolated peptide consisting of the sequence MKKDDQIAAAIALRGMA (SEQ ID NO:1) or an active variant thereof, wherein the peptide or active variant can bind specifically to an antibody induced by a causative agent of Lyme disease (a pathogenic Borrelia), e.g. in a sample from a subject having Lyme disease. Also disclosed are linear multimeric peptides that contain the peptide represented by SEQ ID NO:1 as well as one or more additional peptide epitopes from other Borrelia proteins that can also bind specifically to an antibody as above. Compositions and diagnostic kits comprising a peptide of the invention are described, as are diagnostic assays using the peptide(s).
Owner:BIOPEPTIDES LLC
Peptide diagnostic agent for lyme disease
The present invention relates, e.g., to an isolated peptide consisting of the sequence MKKDDQIAAAIALRGMA (SEQ ID NO:1) or an active variant thereof, wherein the peptide or active variant can bind specifically to an antibody induced by a causative agent of Lyme disease (a pathogenic Borrelia), e.g. in a sample from a subject having Lyme disease. Also disclosed are linear multimeric peptides that contain the peptide represented by SEQ ID NO:1 as well as one or more additional peptide epitopes from other Borrelia proteins that can also bind specifically to an antibody as above. Compositions and diagnostic kits comprising a peptide of the invention are described, as are diagnostic assays using the peptide(s).
Owner:BIOPEPTIDES LLC
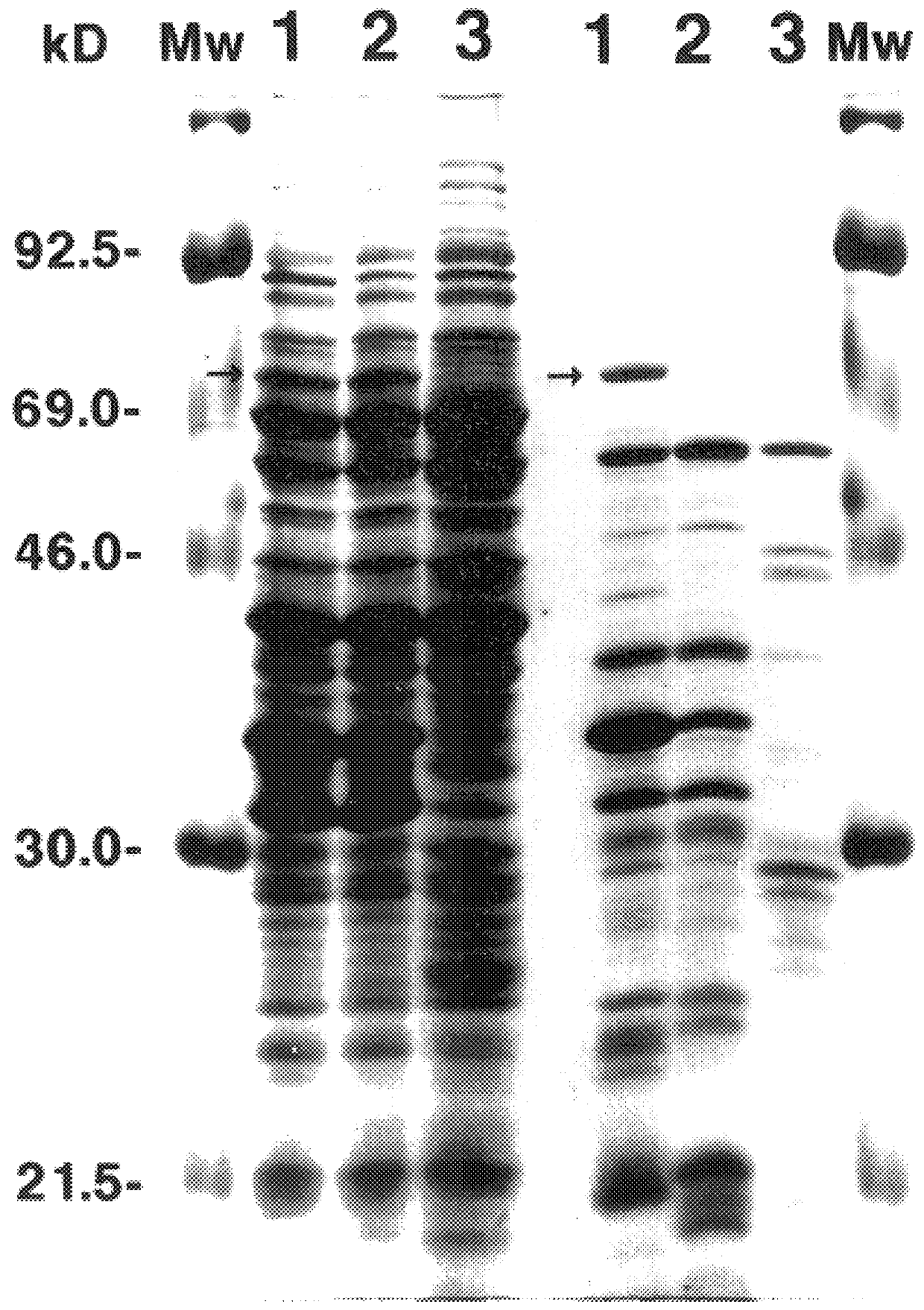
66 kDa antigen from Borrelia
InactiveUS6054296AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsProtozoaAntigen
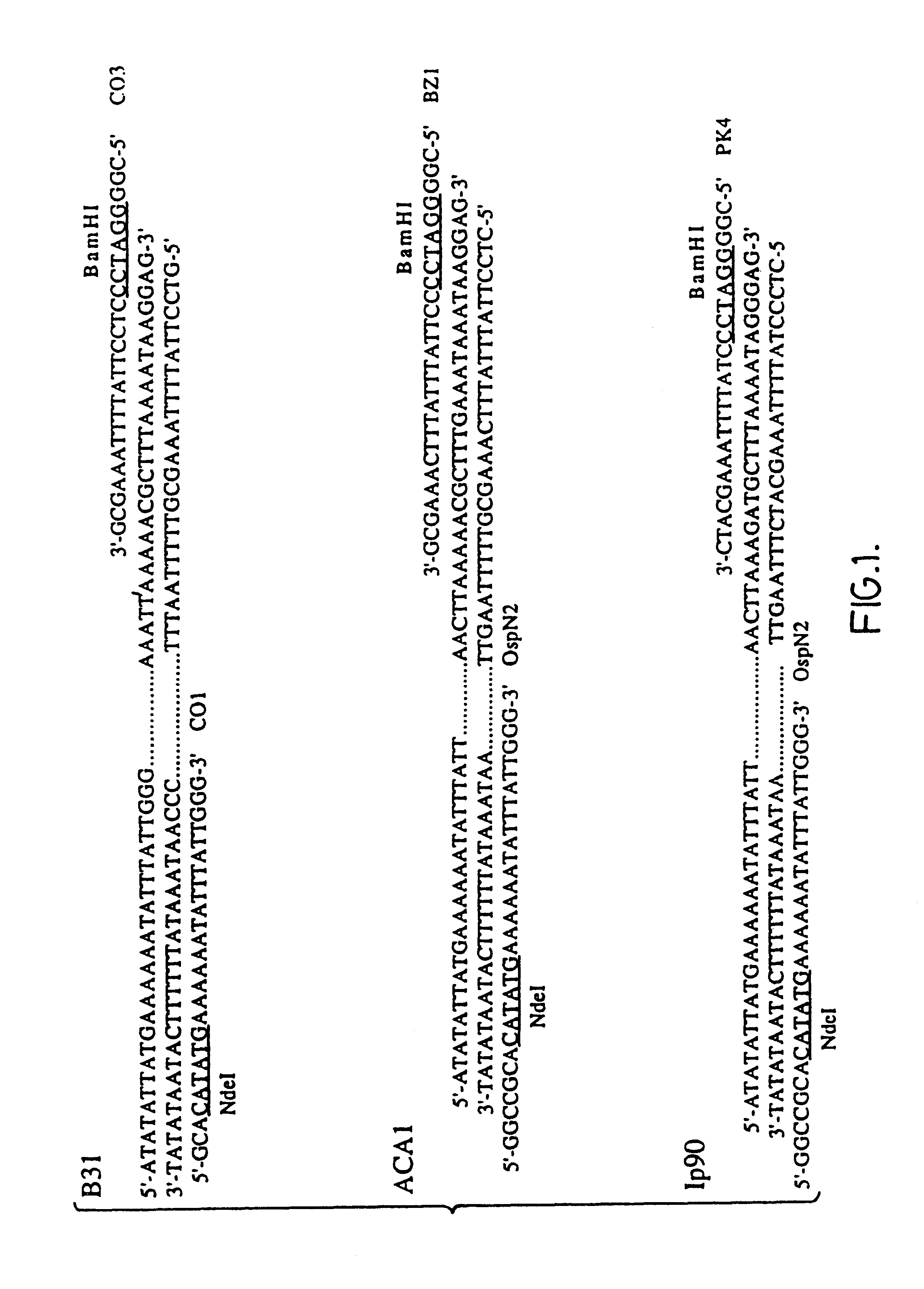
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
66 kDa antigen from Borrelia
InactiveUS6090586AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsAntigenProtozoa
Owner:SYMBICOM
66 kDa antigen from Borrelia
InactiveUS6068842AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsAntigenProtozoa
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
Diagnostic test for borrelia infection
InactiveUS6617441B1Inhibit digestionSugar derivativesAntibody mimetics/scaffoldsDiseaseLone star ticks
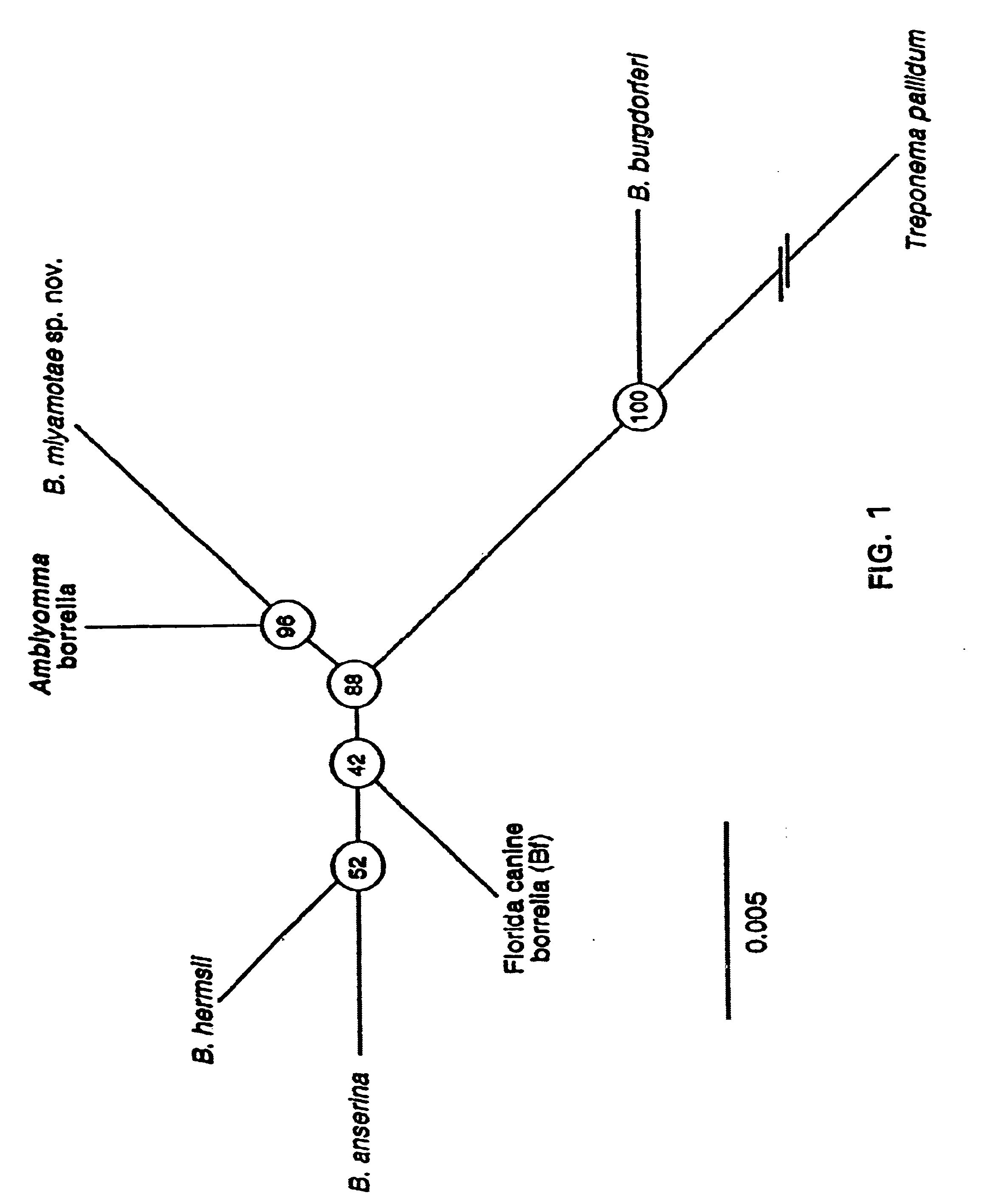
Bites from Amblyomma americanum, a hard tick, have been associated with a Lyme disease-like illness in the southeastern and south-central United States. Present in 2% of ticks collected in four states were uncultivable spirochetes. Through use of the polymerase chain reaction, partial sequences of the flagellin and 16s rRNA genes of microorganisms from Texas and New Jersey were obtained. The sequences showed that the spirochete was a Borrelia sp. but distinct from other known members of this genus, including B. burgdorferi, the agent of Lyme disease. Species-specific differences in the sequences of the flagellin protein, the flagellin gene and the 16s rRNA gene between the new Borrelia species and previously known species provide compositions and methods for assay for determining the presence of this new spirochete, or for providing evidence of past or present infection by this spirochete in animal reservoirs and humans.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
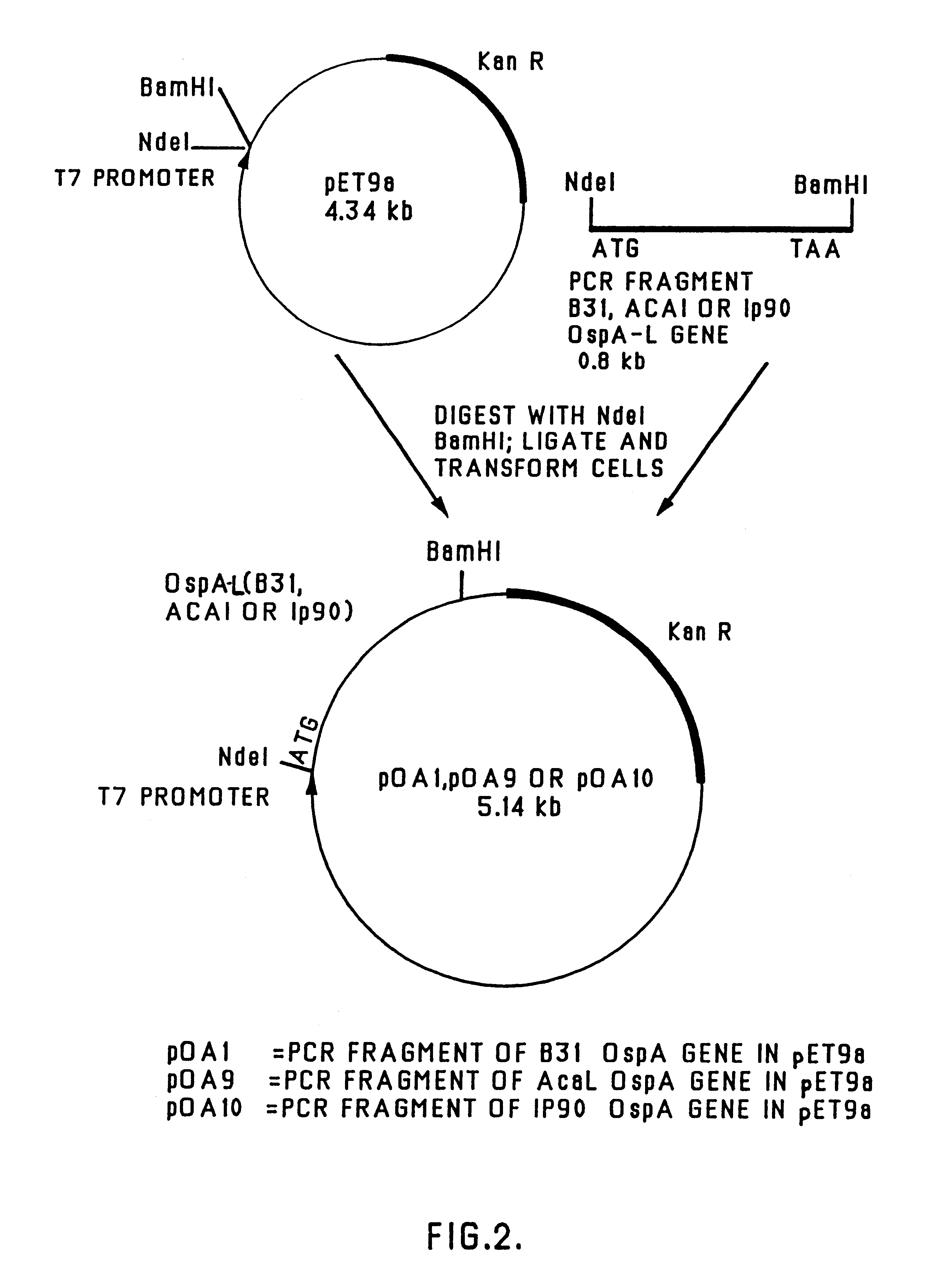
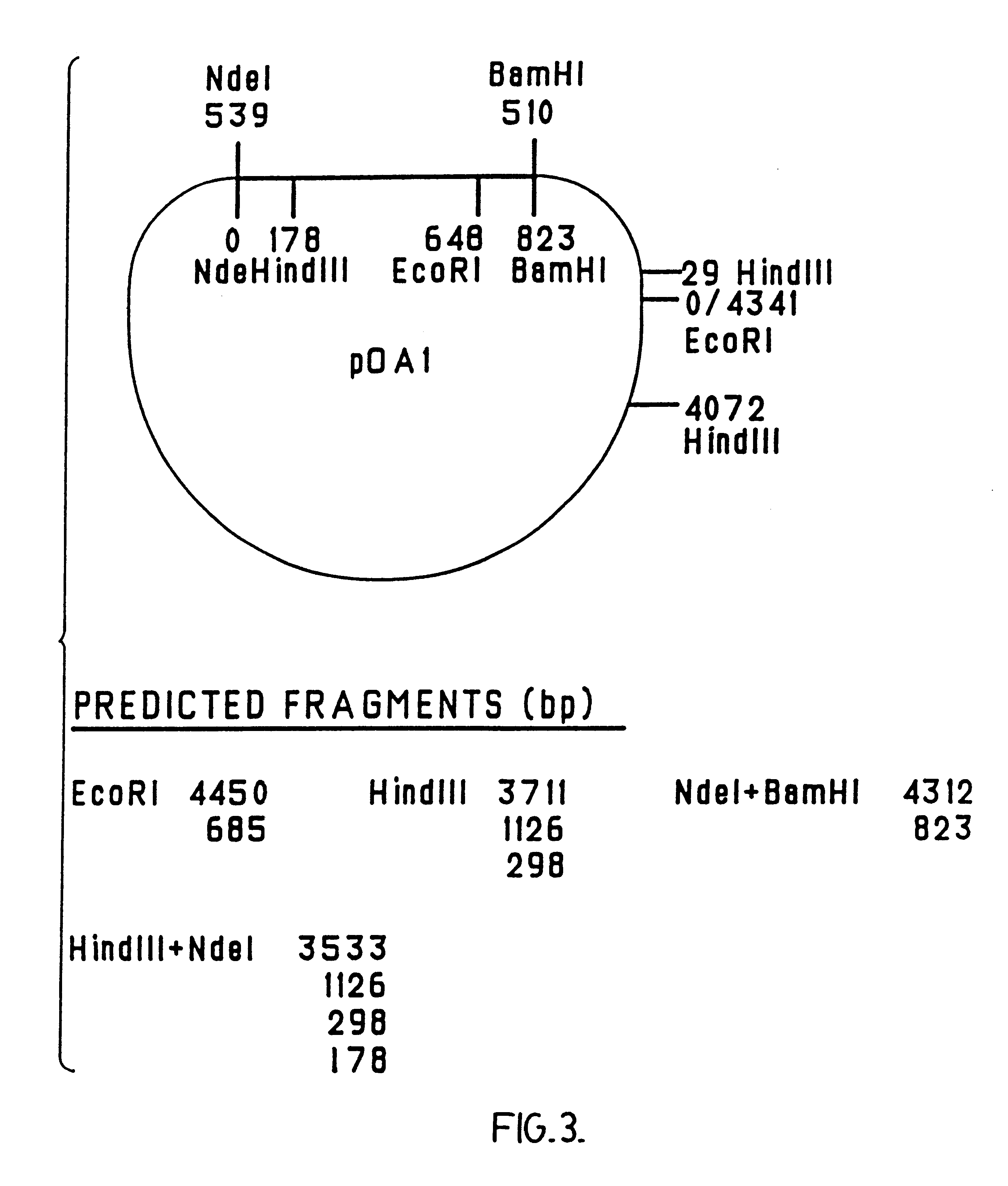
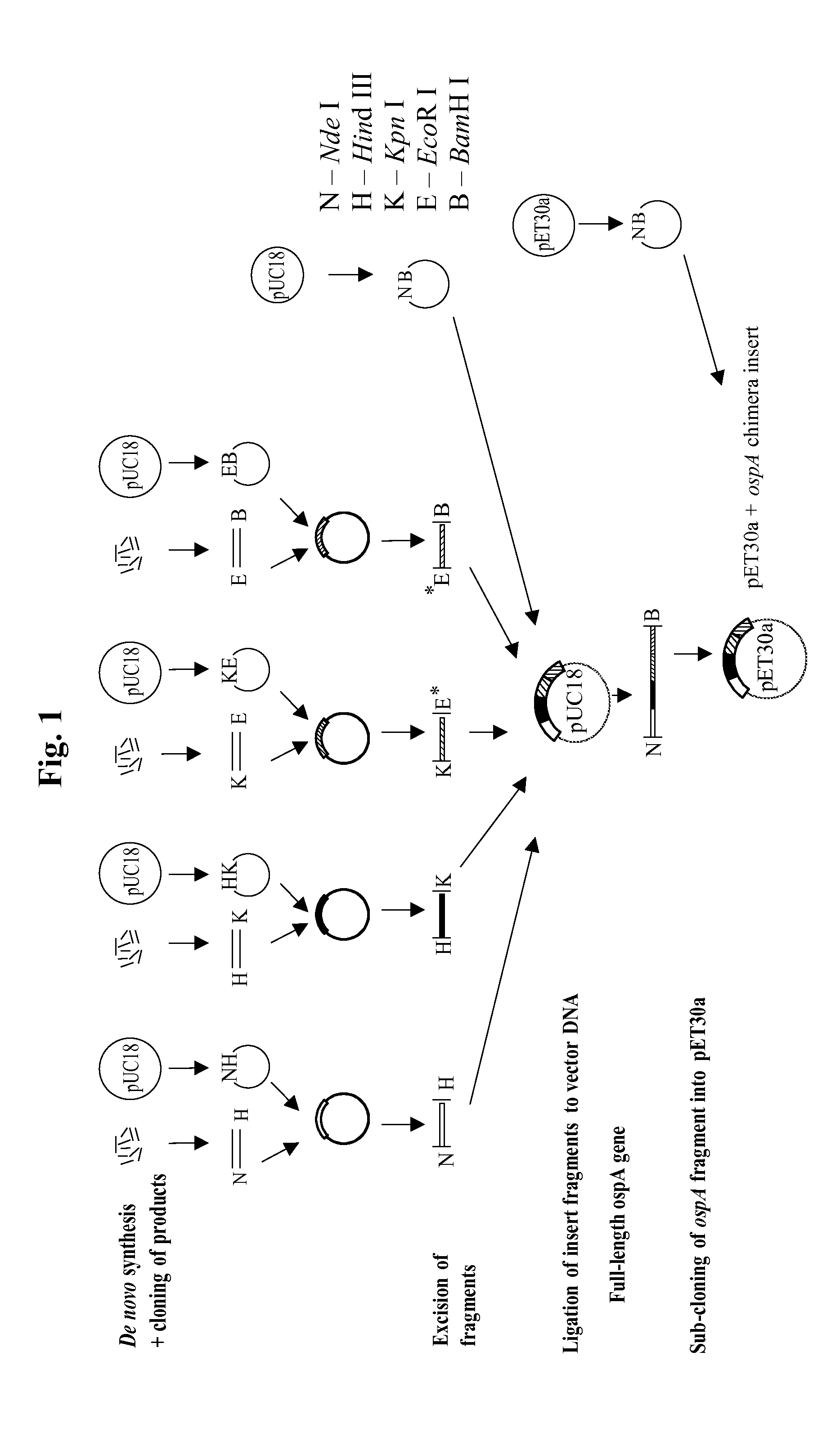
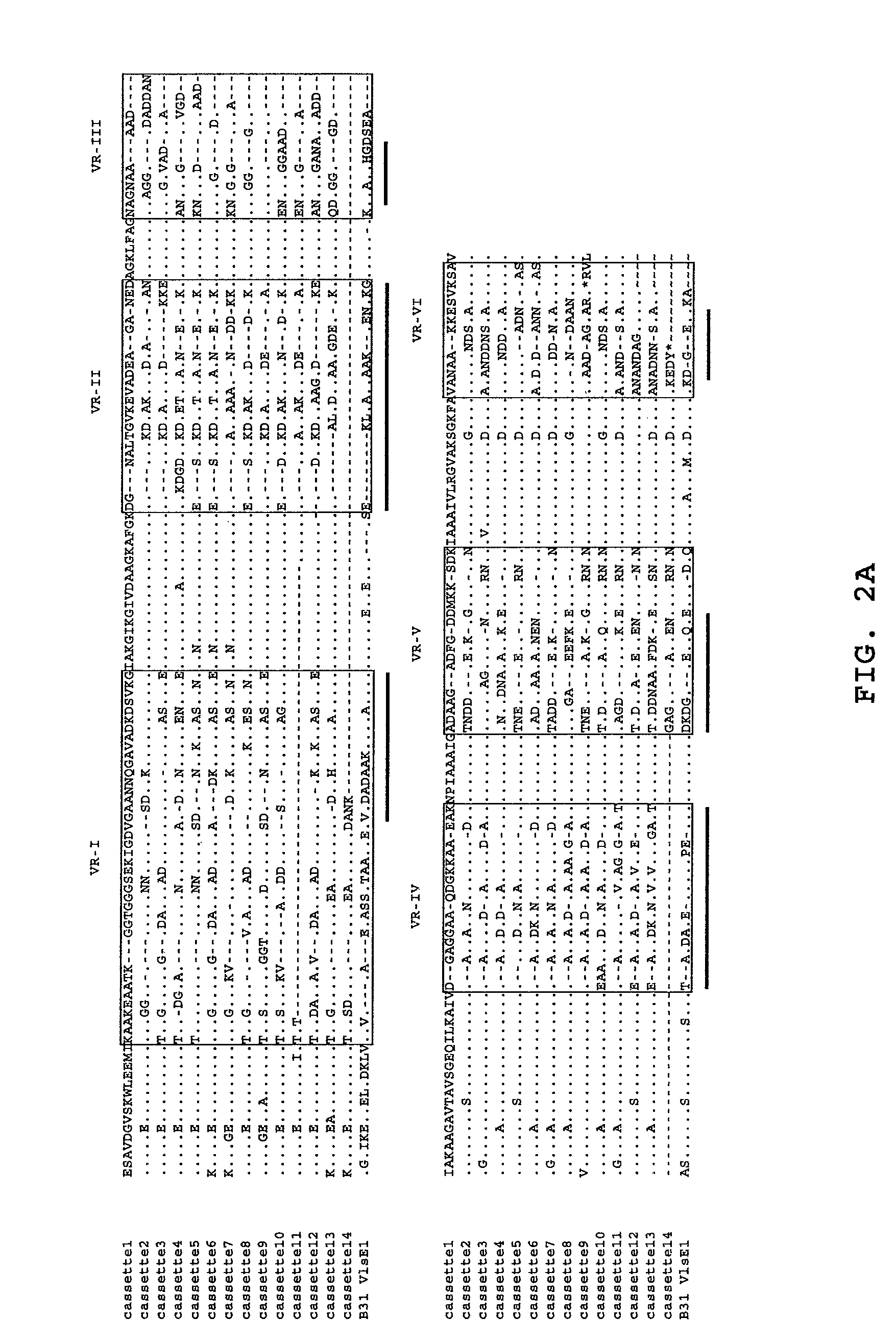
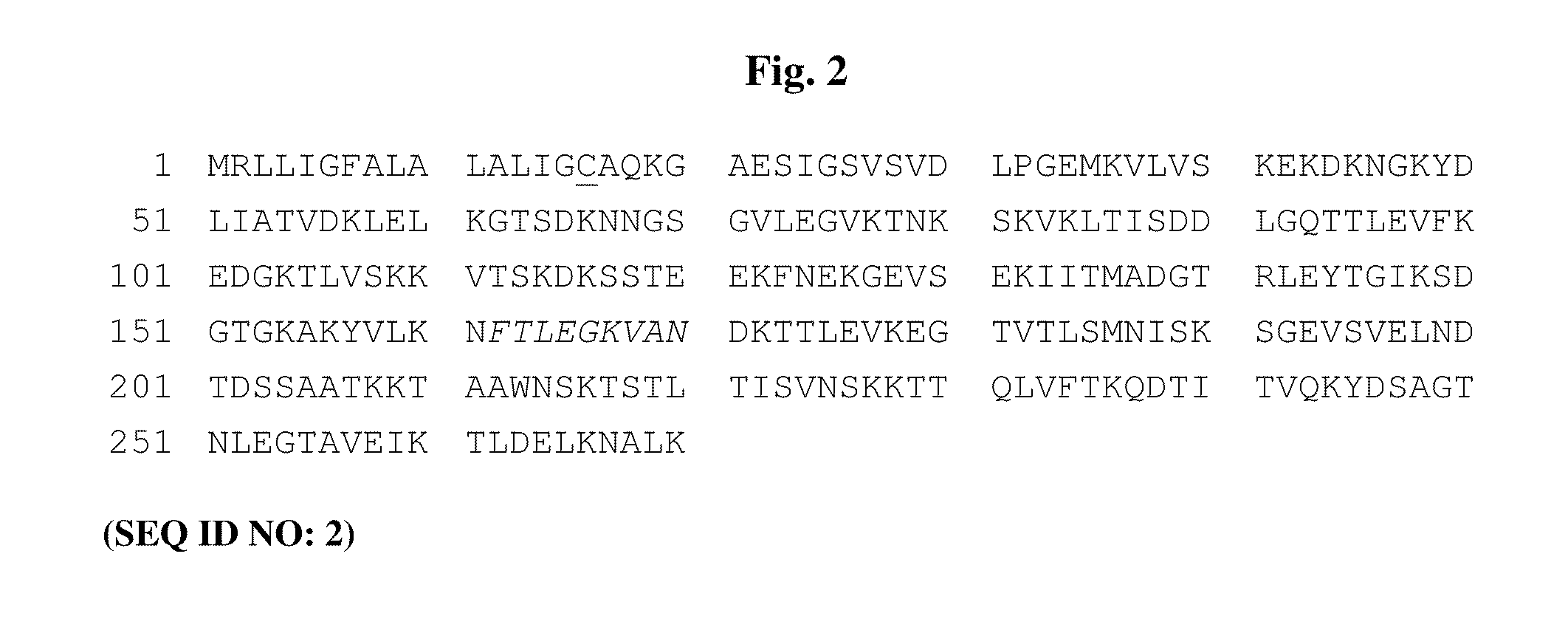
Chimeric ospa genes, proteins, and methods of use thereof
The invention relates to the development of chimeric OspA molecules for use in a new Lyme vaccine. More specifically, the chimeric OspA molecules comprise the proximal portion from one OspA serotype, together with the distal portion from another OspA serotype, while retaining antigenic properties of both of the parent polypeptides. The chimeric OspA molecules are delivered alone or in combination to provide protection against a variety of Borrelia genospecies. The invention also provides methods for administering the chimeric OspA molecules to a subject in the prevention and treatment of Lyme disease or borreliosis.
Owner:BAXALTA INC +2
VMP-like sequences of pathogenic Borrelia species and strains
The present invention relates to DNA sequences encoding Vmp-like polypeptides of pathogenic Borrelia, the use of the DNA sequences in recombinant vectors to express polypeptides, the encoded amino acid sequences, application of the DNA and amino acid sequences to the production of polypeptides as antigens for immunoprophylaxis, immunotherapy, and immunodiagnosis. Also disclosed are the use of the nucleic acid sequences as probes or primers for the detection of organisms causing Lyme disease, relapsing fever, or related disorders, and kits designed to facilitate methods of using the described polypeptides, DNA segments and antibodies.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chimeric ospa genes, proteins, and methods of use thereof
The invention relates to the development of chimeric OspA molecules for use in a new Lyme vaccine. More specifically, the chimeric OspA molecules comprise the proximal portion from one OspA serotype, together with the distal portion from another OspA serotype, while retaining antigenic properties of both of the parent polypeptides. The chimeric OspA molecules are delivered alone or in combination to provide protection against a variety of Borrelia genospecies. The invention also provides methods for administering the chimeric OspA molecules to a subject in the prevention and treatment of Lyme disease or borreliosis.
Owner:BAXALTA GMBH
Live bacterial vaccine
The present invention relates, e.g., to a Lactobacillus bacterium, which (1) expresses a recombinant polypeptide containing a lipoprotein signal sequence from the OspA protein of Borrelia burgdorferi, or an active variant of the leader sequence, operably linked to one or more heterologous polypeptide(s) of interest and / or (2) which comprises an expressible polynucleotide encoding a recombinant polypeptide, wherein the polynucleotide encodes a lipoprotein signal from the OspA protein of Borrelia burgdorferi, or an active variant thereof, which is operably linked to one or more heterologous polypeptide(s) of interest. In one embodiment, the heterologous polypeptide is from Yersinia pestis, the etiologic agent of plague. In another embodiment, the heterologous polypeptide is from Borrelia burgdorferi, the etiologic agent of Lyme disease. Also described are immunogenic compositions, such as live bacterial vaccines, comprising the bacterium; methods for eliciting an immune response against the polypeptide using the bacterium; and kits comprising the bacterium.
Owner:LACTRYS OCTROOI +1
Oral vaccine for Borrelia
The present invention relates to vaccines for control of Borrelia infections in animal and human populations. In particular, the present invention provides compositions and methods comprising recombinant bacteria engineered to express one or more Borrelia burgdorferi antigens for use as Lyme disease vaccines. In some embodiments, the recombinant bacteria are freeze-dried.
Owner:US BIOLOGIC INC
Methods and kit for diagnosing tick borne illnesses
InactiveUS7390626B2High sensitivityStrong specificitySamplingAnalysis by subjecting material to chemical reactionDiseaseLeukocyte function
Owner:IMMUNOSCI LAB
Borrelia diagnostics and screening methods
InactiveUS20100278866A1Antibacterial agentsBacterial antigen ingredientsBorrelia gariniiScreening method
Compositions and methods of detecting Borrelia proteins, nucleic acid sequences encoding these proteins, and subject antibodies to these proteins in a sample are disclosed.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for vaccination comprising nucleic acid and/or polypeptide sequences of the genus Borrelia
The invention relates to antigens and nucleic acids encoding such antigens obtainable by screening a Borrelia genome, in particular an B. burgdorferi genome. In more specific aspects, the invention relates to methods of isolating such antigens and nucleic acids and to methods of using such isolated antigens for producing immune responses. The ability of an antigen to produce an immune response may be employed in vaccination or antibody preparation techniques.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method for diagnosing early and late lyme borreliosis
InactiveUS20060034862A1High sensitivityBacterial antigen ingredientsBiological material analysisAntigenErythema migrans
The present invention relates to a method for detecting Borrelia burgdorferi sensu lato infection or the presence of antibodies against Borrelia species in a body fluid from a suspected infected or vaccinated human or other mammal, to a diagnostic kit useful in said method and to an immunoassay method for diagnosing early and late Lyme borreliosis, especially for diagnosing erythema migrans. The methods according to the invention are characterized in that recombinant BBK32 proteins, optionally other immunogenic borrelial proteins, or their fragments are used as antigens.
Owner:BORTECH
Topical antibiotic composition for the prevention of Lyme disease
The invention relates to topical pharmaceutical compositions and methods related to Borrelia burgdorferi toxins, in particular, the present invention provides compositions and methods for the treatment of infections caused by Borrelia burgdorferi and in particular for the prevention of Lyme disease.
Owner:IXODES
Oligonucleotides and methods for detecting Borrelia burgdorferi
InactiveUS20070172829A1Positive resistSugar derivativesMicrobiological testing/measurementBorrelia gariniiTest sample
The present invention provides methods and compositions for determining the presence and / or amount of Borrelia burgdorferi nucleic acids in a test sample related to Lyme disease. In particular, substantially purified oligonucleotide primers and probes are described that can be used for qualitatively and quantitatively detecting Borrelia burgdorferi nucleic acid in a test sample by amplification methods. The present invention also provides primers and probes for generating and detecting control nucleic acid sequences that provide a convenient method for assessing internal quality control of the Borrelia burgdorferi assay.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Lyme disease vaccine
InactiveUS20110262475A1Broad protectionAntibacterial agentsPeptide/protein ingredientsImmunodominant EpitopesBorrelia garinii
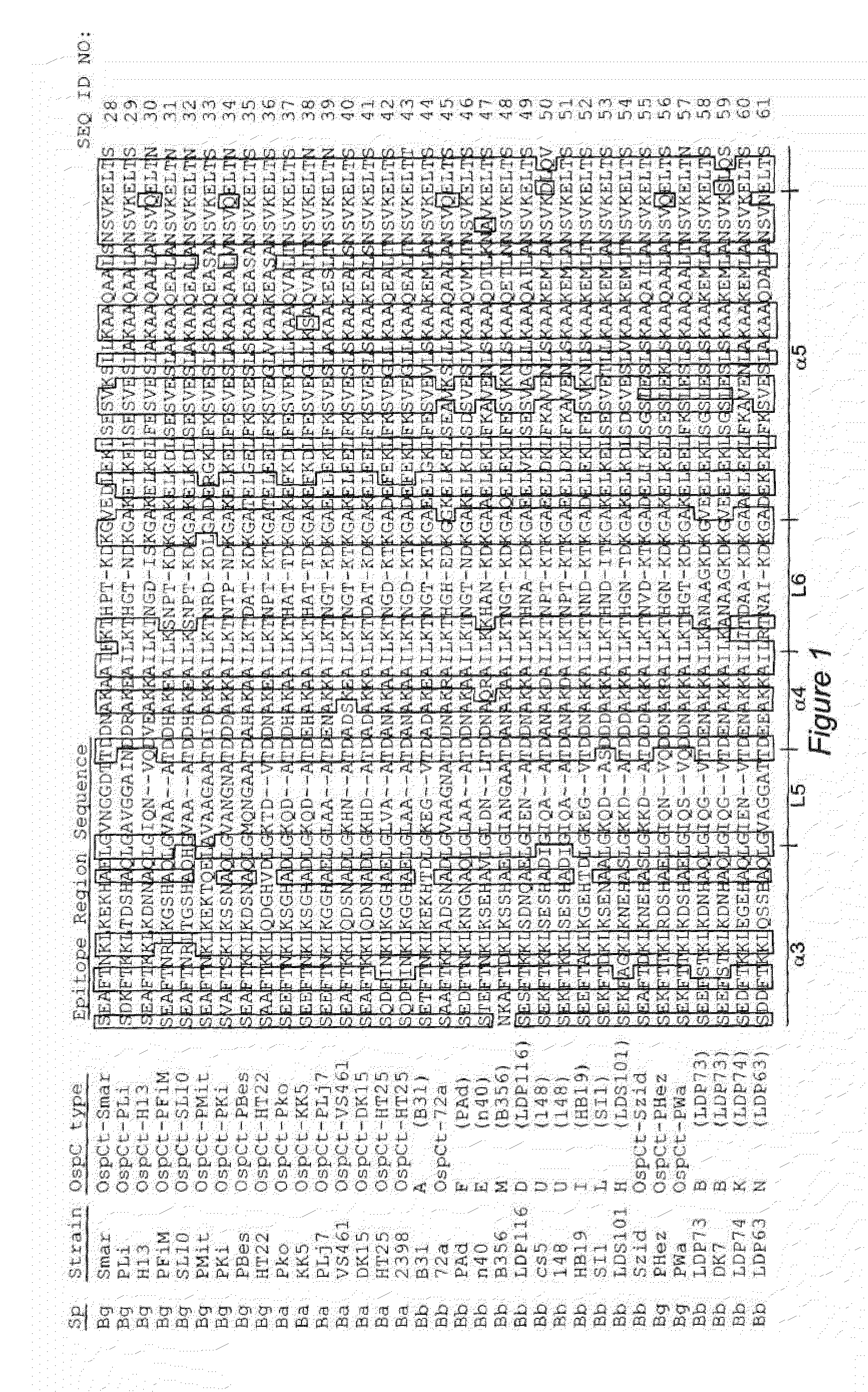
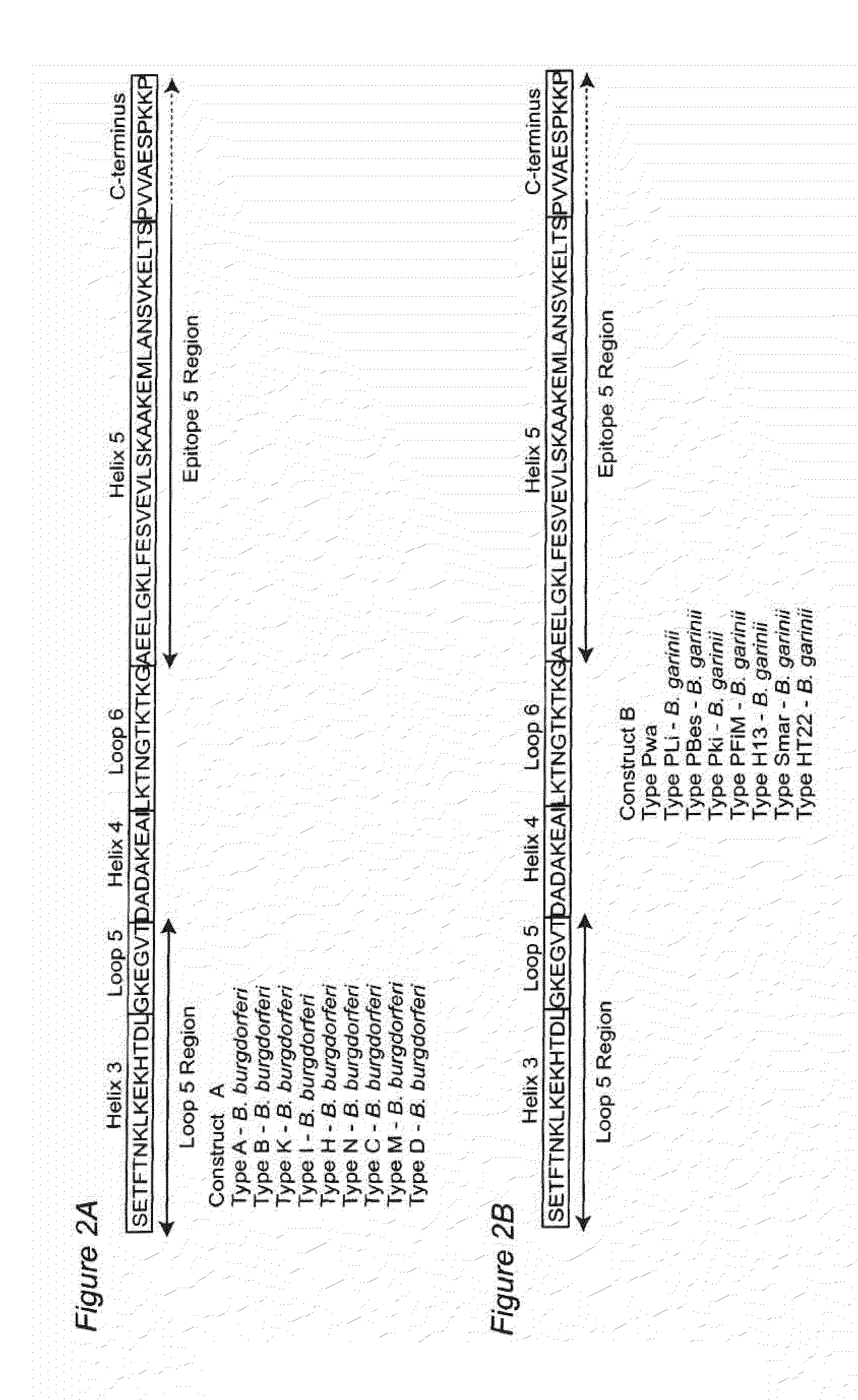
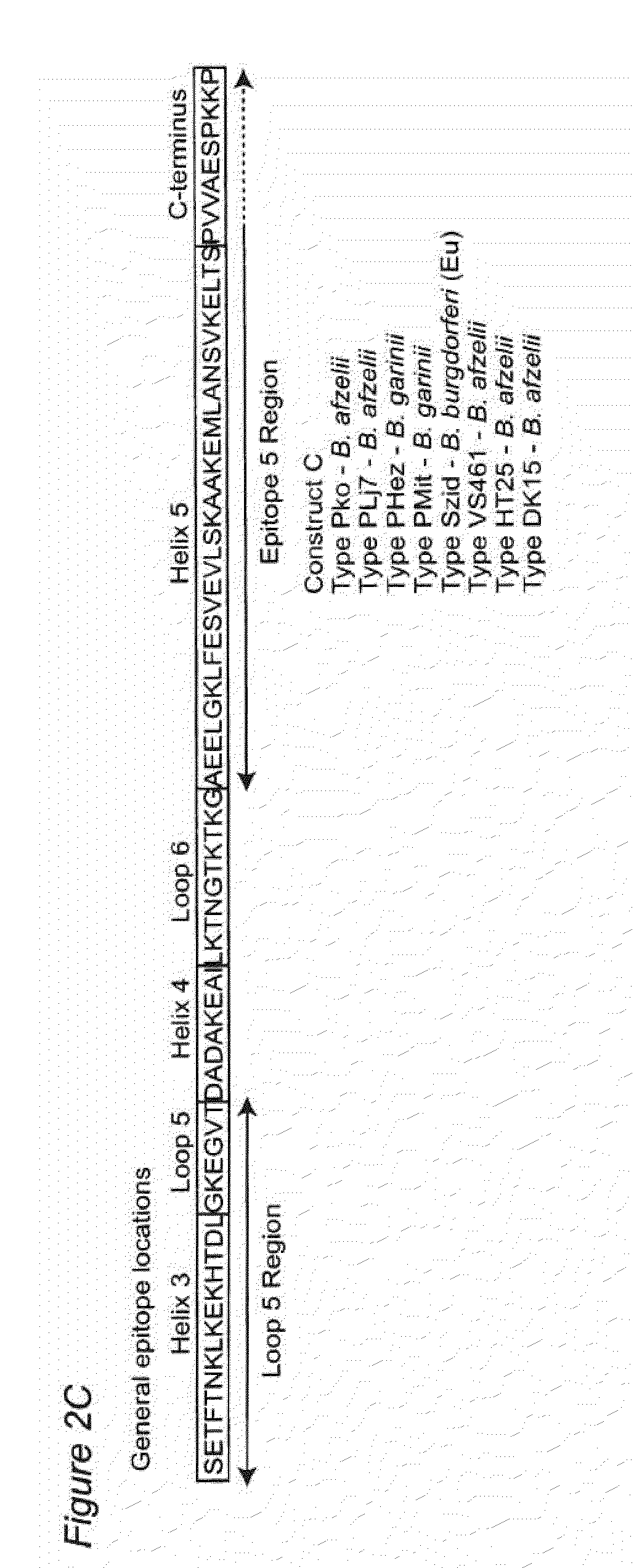
Antigenic polypeptides comprising linear immunodominant epitopes of Borrelia outer surface protein A (OspA) or Borrelia outer surface protein C (OspC) are useful as vaccines against Lyme disease, and as diagnostics for detecting Borrelia infections. The OspA and OspC antigenic polypeptides typically comprise a plurality of peptides representing epitope containing regions from multiple distinct phyletic groups. The antigenic polypeptides may also include epitopes from both Borrelia OspA and Borrelia OspC.
Owner:VIRGINIA COMMONWEALTH UNIV
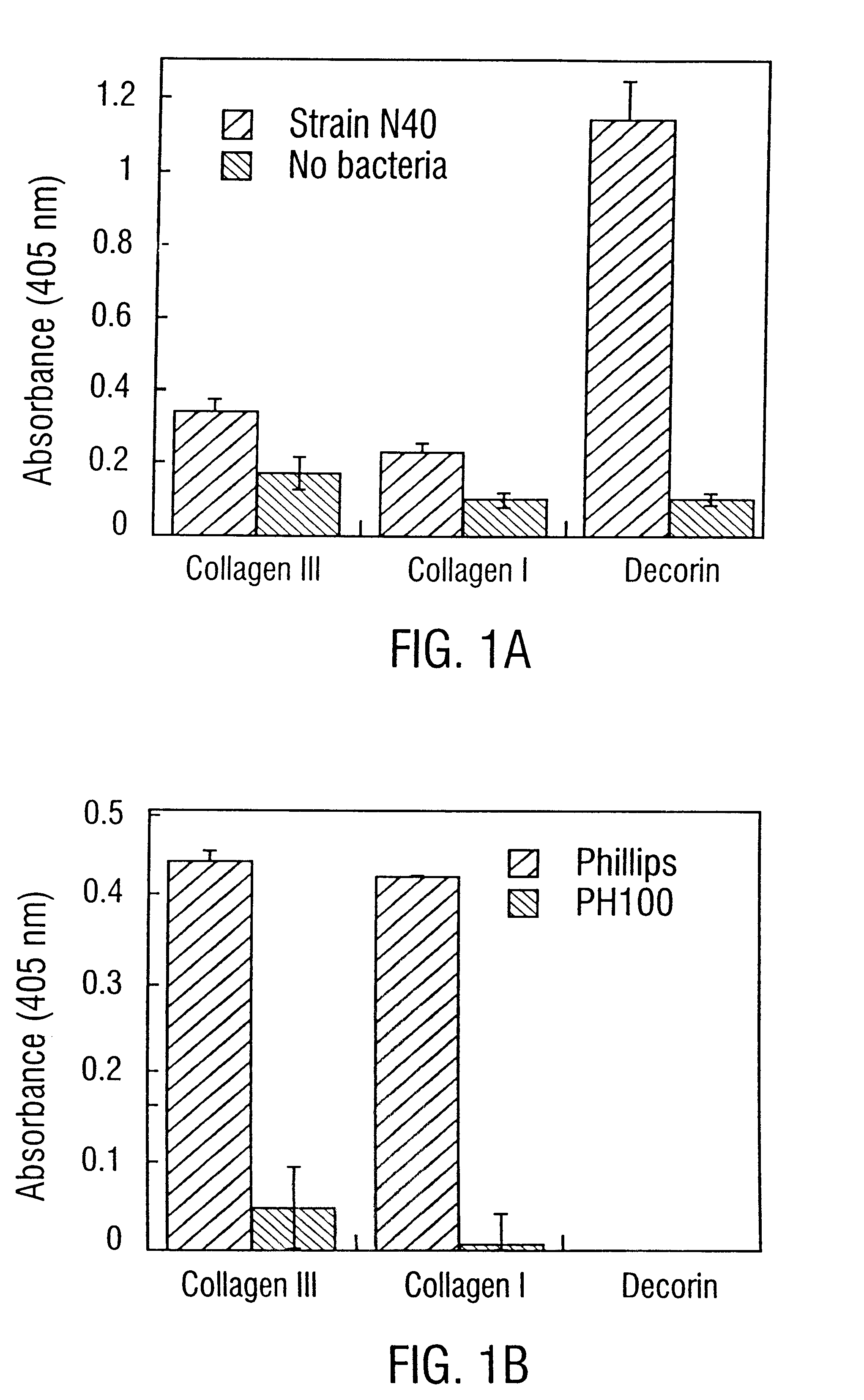
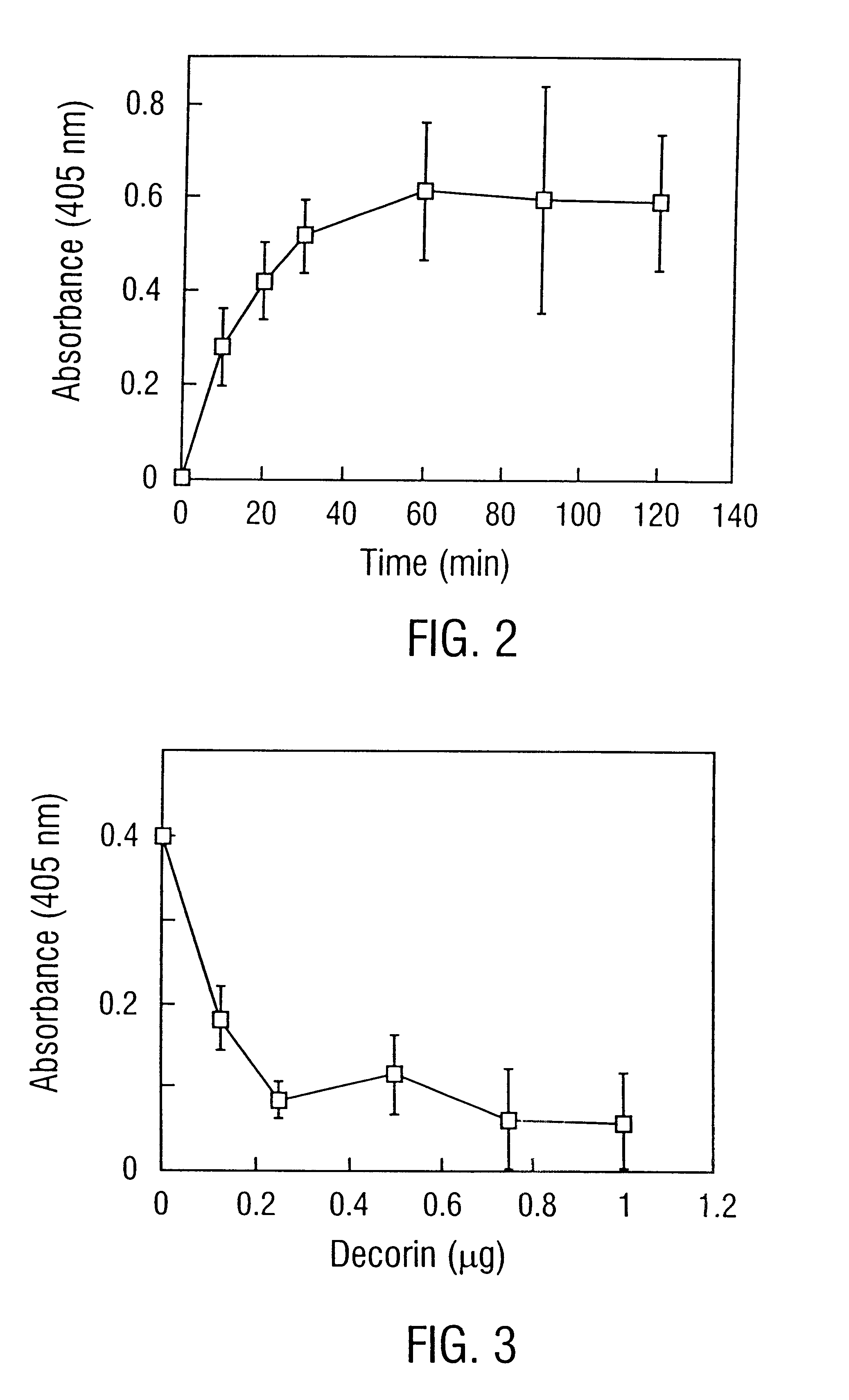
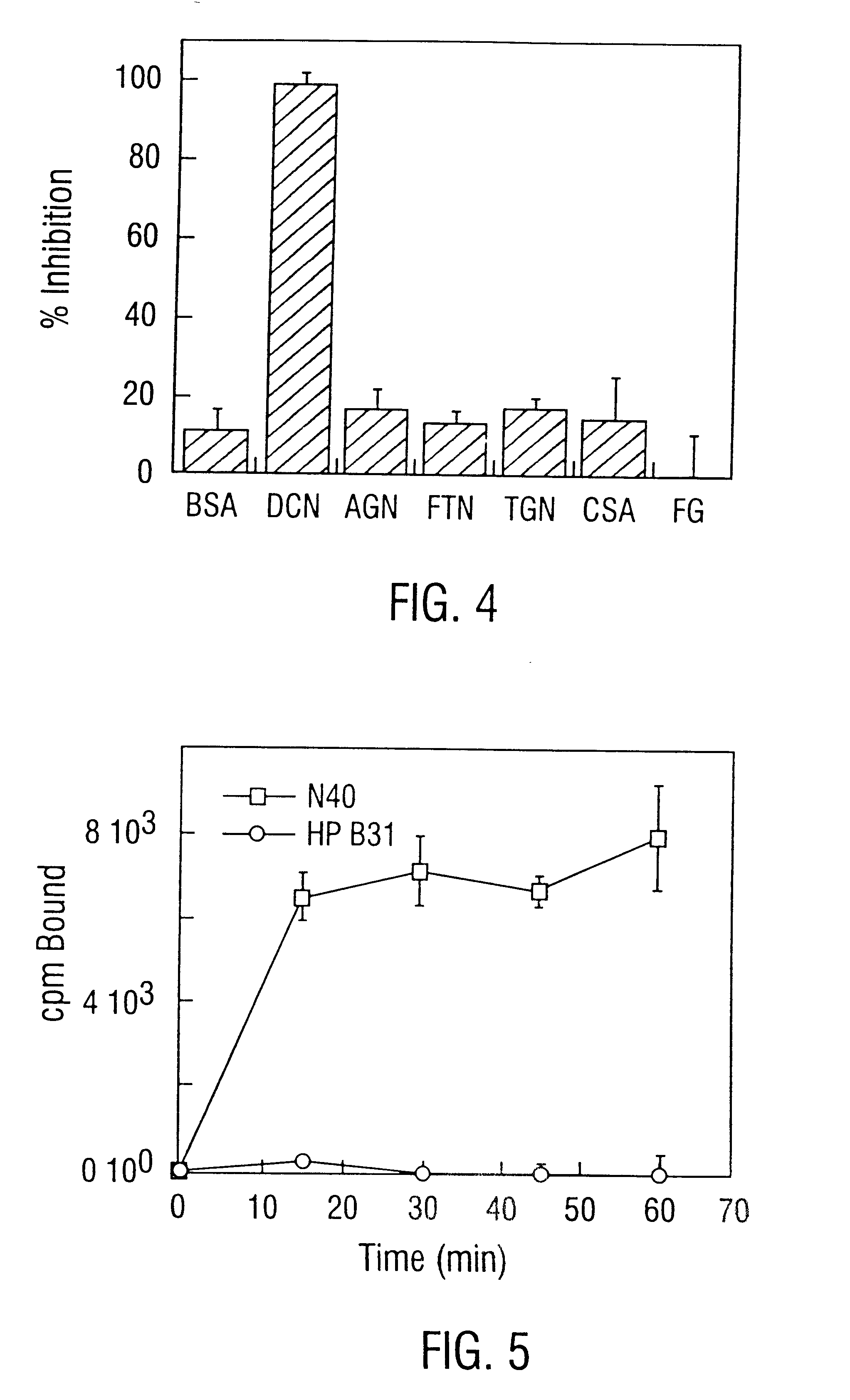
Decorin binding protein compositions
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:TEXAS A&M UNIVERSITY
Borrelia Provocation Procedure Kit
ActiveUS20150353988A1Effective treatmentEffective and timely interventionBiocideMicrobiological testing/measurementBacteroidesSpiroplasma
The testing for the Lyme disease pathogen, (Borrelia bergdorferi (Bb) and all other Lyme Borrelia), is notoriously difficult. Bb is not by nature a blood loving bacteria, and prefers the climate of avascular tissues such as cartilage. The Borrelia Provocation technology disclosed herein proposes to uniquely lure the Lyme Borrelia spirochetes into the blood through a simple procedure of sub-dermal injection of benign tick saliva and co-factors. Lyme Borrelia can then be tested accurately after the provocation injection, utilizing any Lyme Borrelia detection test such as a specific PCR (polymerase chain reaction) test for the most accurate results, as there will be abundantly available DNA present in the blood if there is an existing infection. Treatment is more effective after provocation as well. The provocation protocol taught herein makes accurate, non-invasive, active direct Lyme infection testing possible, and creates a platform for an effective treatment as well.
Owner:TITAN COLLABORATIVE KITHE LLC
Recombinant constructs of Borrelia burgdorferi
InactiveUS20050271682A1Improve overall utilizationAvoid infectionBacteriaPeptide/protein ingredientsBorrelia gariniiBorreliella burgdorferi
Novel chimeric nucleic acids, encoding chimeric Borrelia proteins comprising OspC or an antigenic fragment thereof and OspA or an antigenic fragment thereof, are disclosed. Chimeric proteins encoded by the nucleic acid sequences are also disclosed. The chimeric proteins are useful as vaccine immunogens against Lyme borreliosis, as well as for immunodiagnostic reagents.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Borrelia burgdorferi bacterin
InactiveUS20050042235A1Improving immunogenicityAntibacterial agentsBacterial antigen ingredientsAdjuvantBorrelia garinii
A bacterin including effective immunizing amounts of two non-crossprotective isolates of inactivated Borrelia burgdorferi, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi isolates and a suitable carrier is provided herein. The bacterin may also contain a third non-crossprotective isolate. A bacterin including effective immunizing amounts of an antigenic subunit derived from a first Borrelia burgdorferi isolate and a second, non-crossprotective Borrelia burgdorferi isolate, an adjuvant in an amount effective to enhance the immunogenicity of the antigenic subunits and a suitable carrier is also provided. The bacterin may also contain an effective immunizing amount of an antigenic subunit of a third Borrelia burgdorferi. Further provided is a bacterin which includes effective immunizing amounts of two noncrossprotective isolates of inactivated Borrelia burgdorferi and one or more antigenic subunits from the non-crossprotective isolates, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi and antigenic subunits and a suitable carrier.
Owner:SCHERING CORP
Borrelia burgdorferi bacterin
InactiveUS20080026009A1Improving immunogenicityAntibacterial agentsBacterial antigen ingredientsBorrelia gariniiAdjuvant
A bacterin including effective immunizing amounts of two non-crossprotective isolates of inactivated Borrelia burgdorferi, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi isolates and a suitable carrier is provided herein. The bacterin may also contain a third non-crossprotective isolate. A bacterin including effective immunizing amounts of an antigenic subunit derived from a first Borrelia burgdorferi isolate and a second, non-crossprotective Borrelia burgdorferi isolate, an adjuvant in an amount effective to enhance the immunogenicity of the antigenic subunits and a suitable carrier is also provided. The bacterin may also contain an effective immunizing amount of an antigenic subunit of a third Borrelia burgdorferi. Further provided is a bacterin which includes effective immunizing amounts of two non-crossprotective isolates of inactivated Borrelia burgdorferi and one or more antigenic subunits from the non-crossprotective isolates, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi and antigenic subunits and a suitable carrier.
Owner:SCHERING CORP
Vaccines and methods to treat lyme disease in dogs
InactiveCN104379164AAntibacterial agentsBacterial antigen ingredientsBorrelia gariniiBorrelia burgdorferi
The instant invention provides an immunogenic composition comprising an antigenic fragment of OspA protein of Borrelia burgdorferi and a chimeric protein containing antigenic fragments of different phylotypes of OspC protein of Borrelia burgdorferi. Vaccines incorporating the immunogenic composition of the invention, as well as methods of preventing Lyme disease in dogs and / or protecting dogs from Lyme disease using the vaccines are also provided.
Owner:ZOETIS SERVICE LLC +1
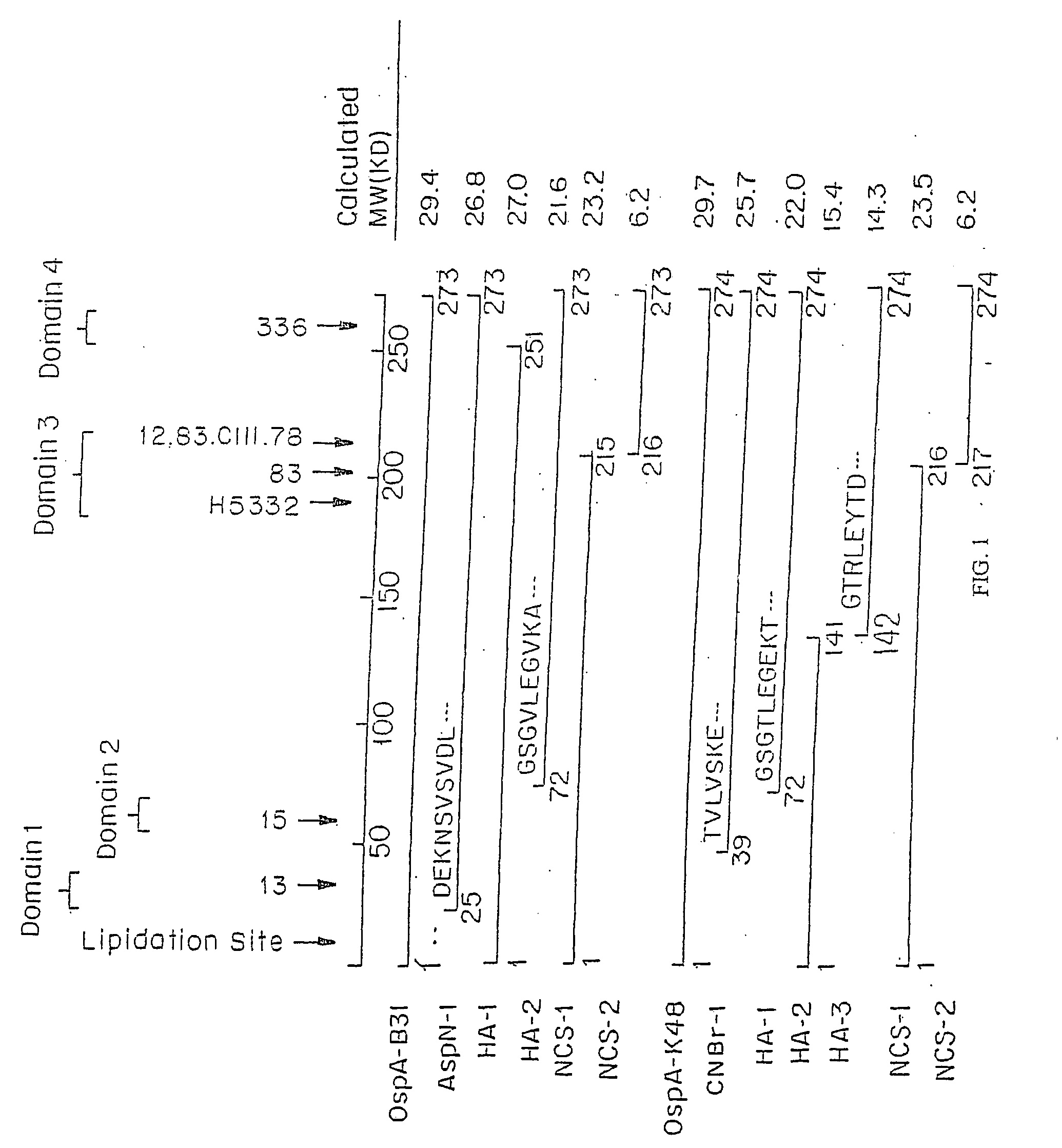
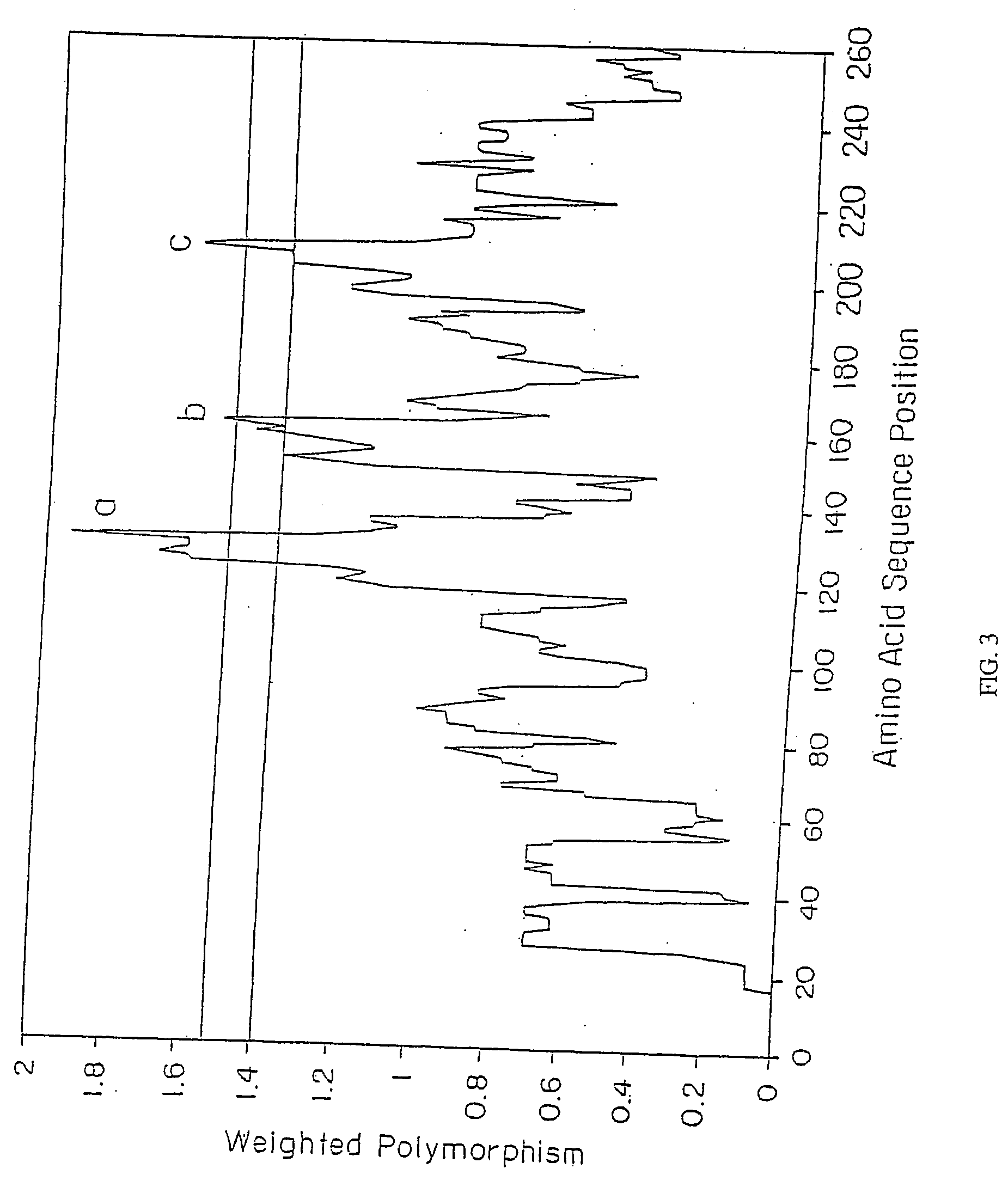
Borrelia garinii OspA protein C-terminal peptide fragment and application thereof
ActiveCN110483624AGood immune protectionNo adverse side effectsAntibacterial agentsBacterial antigen ingredientsSide effectBorrelia garinii
The invention provides a Borrelia garinii OspA protein C-terminal peptide fragment and an application thereof. The OspA peptide fragment (126-274aa), containing the His label, of the Borrelia gariniiprovided by the invention has good immune protection on rabbits, and does not generate adverse side effects on organisms. The invention provides an effective means for treating or preventing Borreliaburgdorferi infection.
Owner:ICDC CHINA CDC
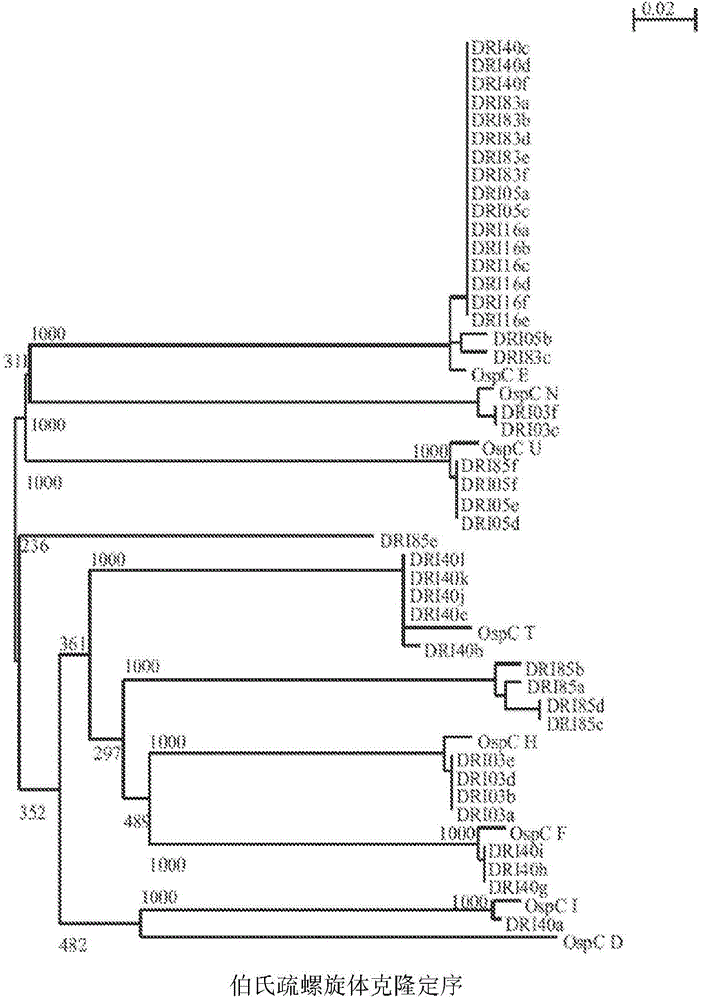
B.garinii polyclonal antibody and application
InactiveCN106432487AStrong specificityHigh potencySerum immunoglobulinsImmunoglobulins against bacteriaAdjuvantNew Zealand white rabbit
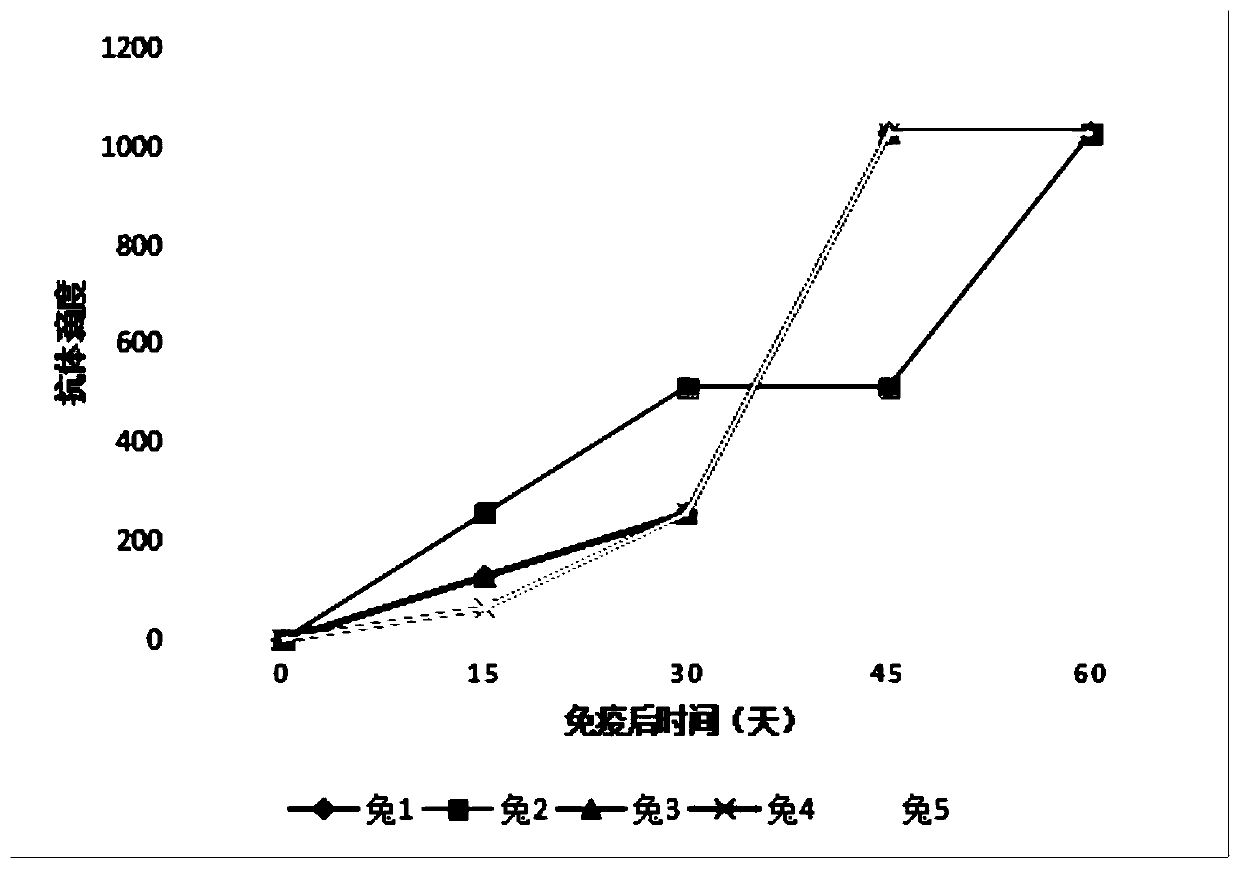
The invention discloses a B.garinii polyclonal antibody and an application. A BSK-II culture medium is inoculated with a B.garinii strain for enlargement culture, and a somatic antigen is obtained after inactivation; after the obtained somatic antigen and an adjuvant are fully emulsified, a New Zealand white rabbit is immunized by using multi-point intradermal injection; the immunized New Zealand white rabbit is anesthetized with chloral hydrate, whole blood is solidified at the room temperature after being collected, and serum containing the B.garinii polyclonal antibody is subjected to centrifugal separation. The B.garinii polyclonal antibody prepared with the method has good specificity and high titer, can effectively detect Lyme disease spirochete and has brighter application prospect.
Owner:杭州贝英福生物科技有限公司
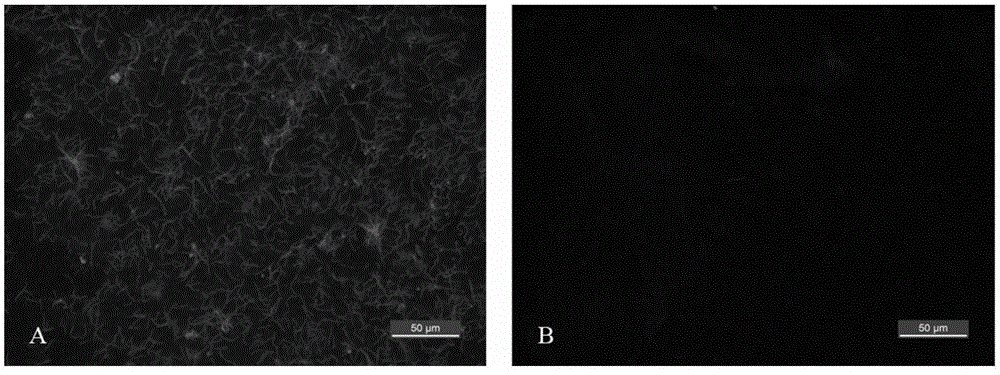
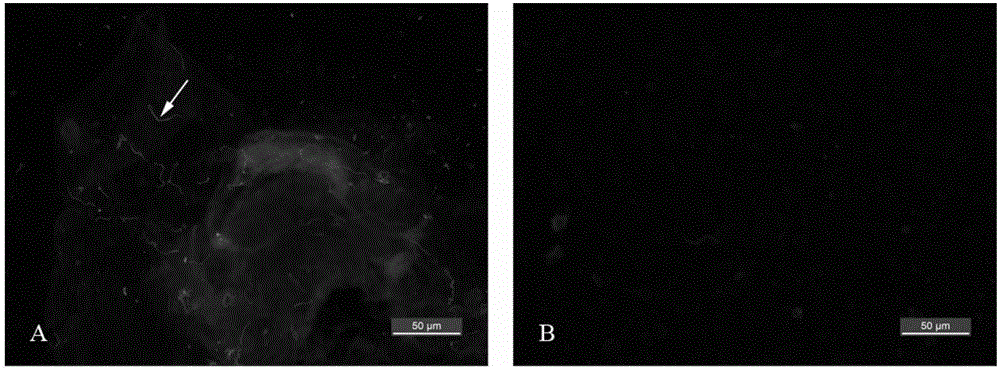
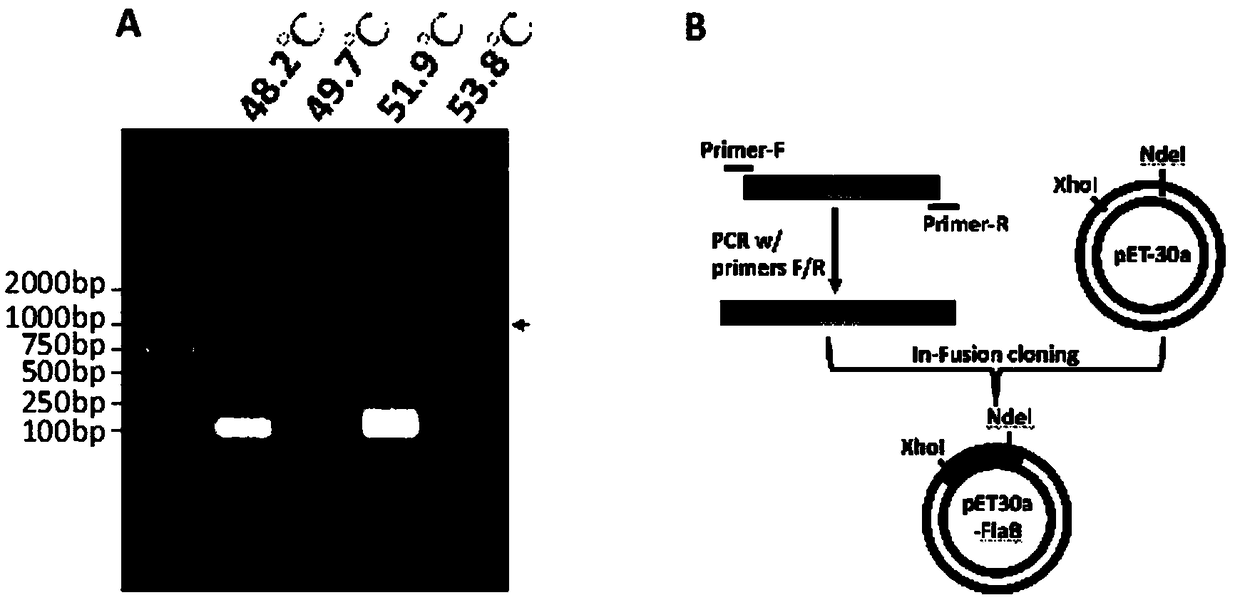
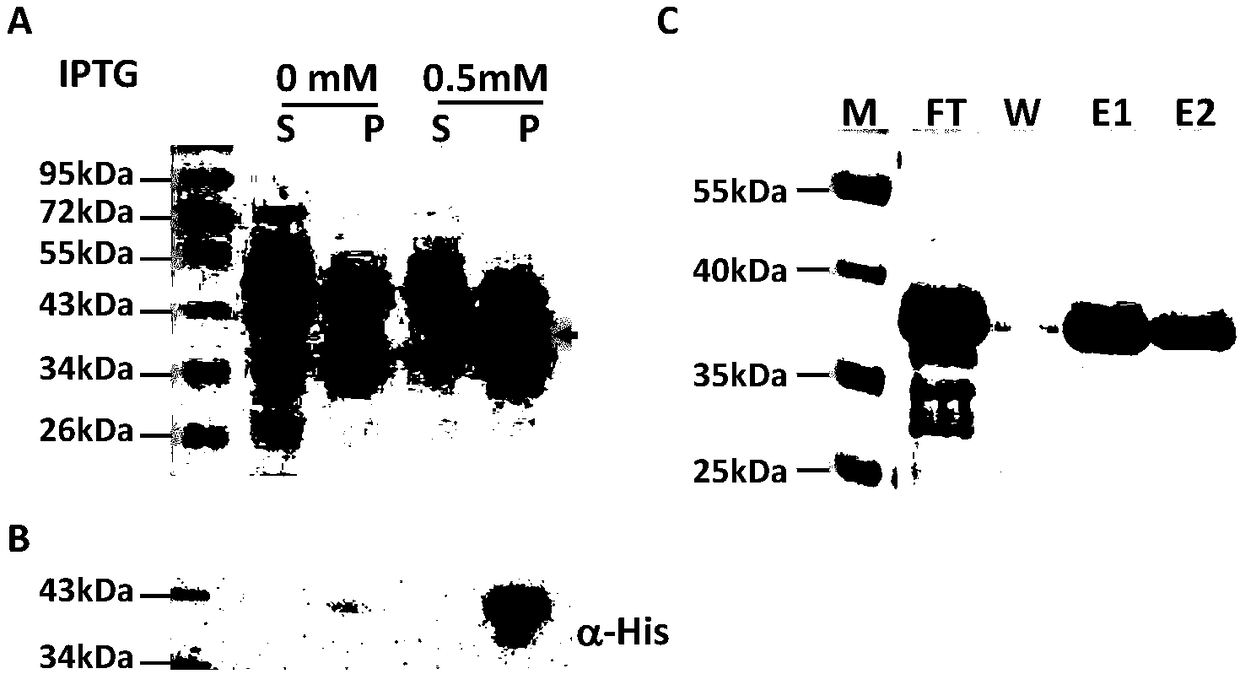
Obtaining mode and application of borrelia burgdorferi flagellin FlaB antibody
InactiveCN109134649AHigh purityHigh potencySerum immunoglobulinsBiological material analysisEscherichia coliSpiroplasma
The invention relates to preparation and application of a borrelia burgdorferi flagellin FlaB antibody. By means of a PCR method, a gene segment of FlaB is obtained from a borrelia burgdorferi genomethrough amplification, cloned into a prokaryotic expression vector pET30a containing a histidine tag and then transferred into escherichia coli BL21 competent cells. Induction expression is carried out through IPTG, affinity purification is performed to obtain FlaB protein, immunized mice obtain the borrelia burgdorferi flagellin FlaB antibody, and a solid foundation is laid for detection and deepresearch of borrelia burgdorferi.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com