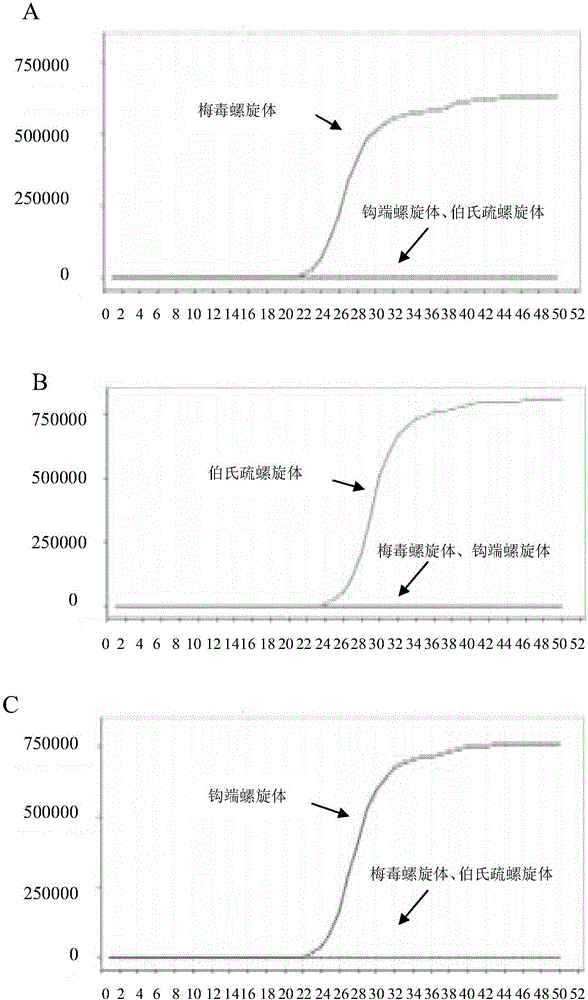
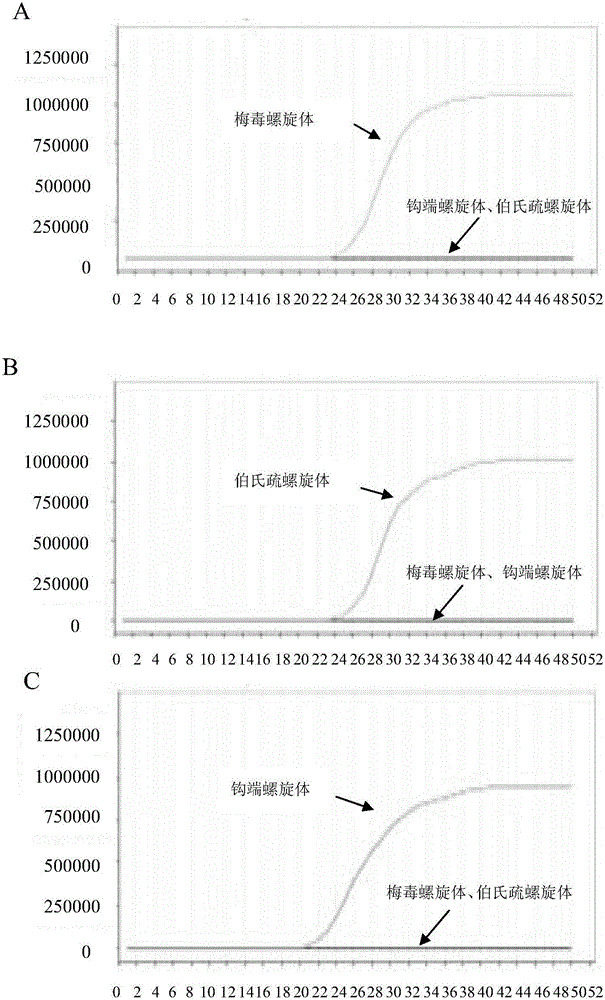
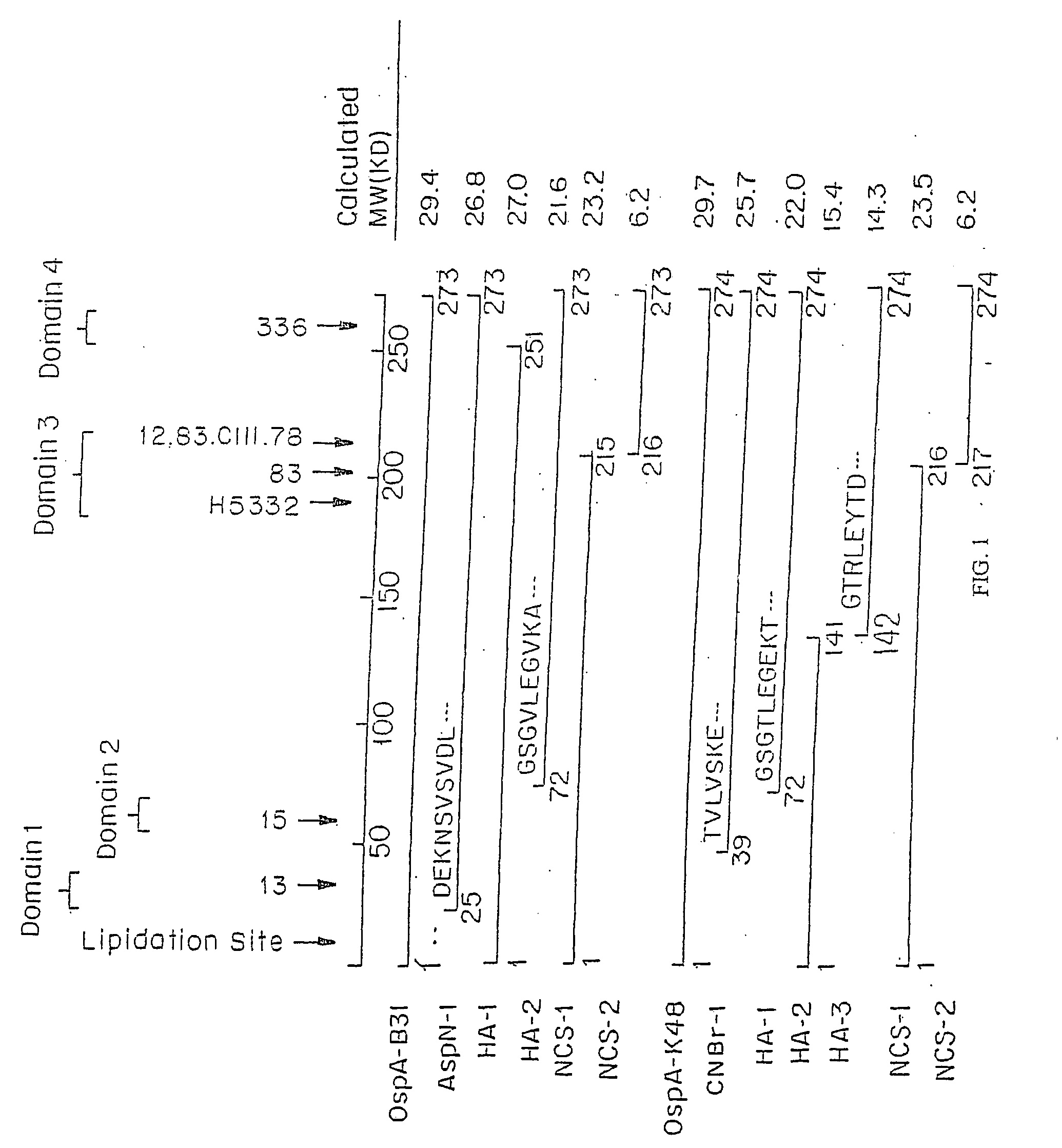
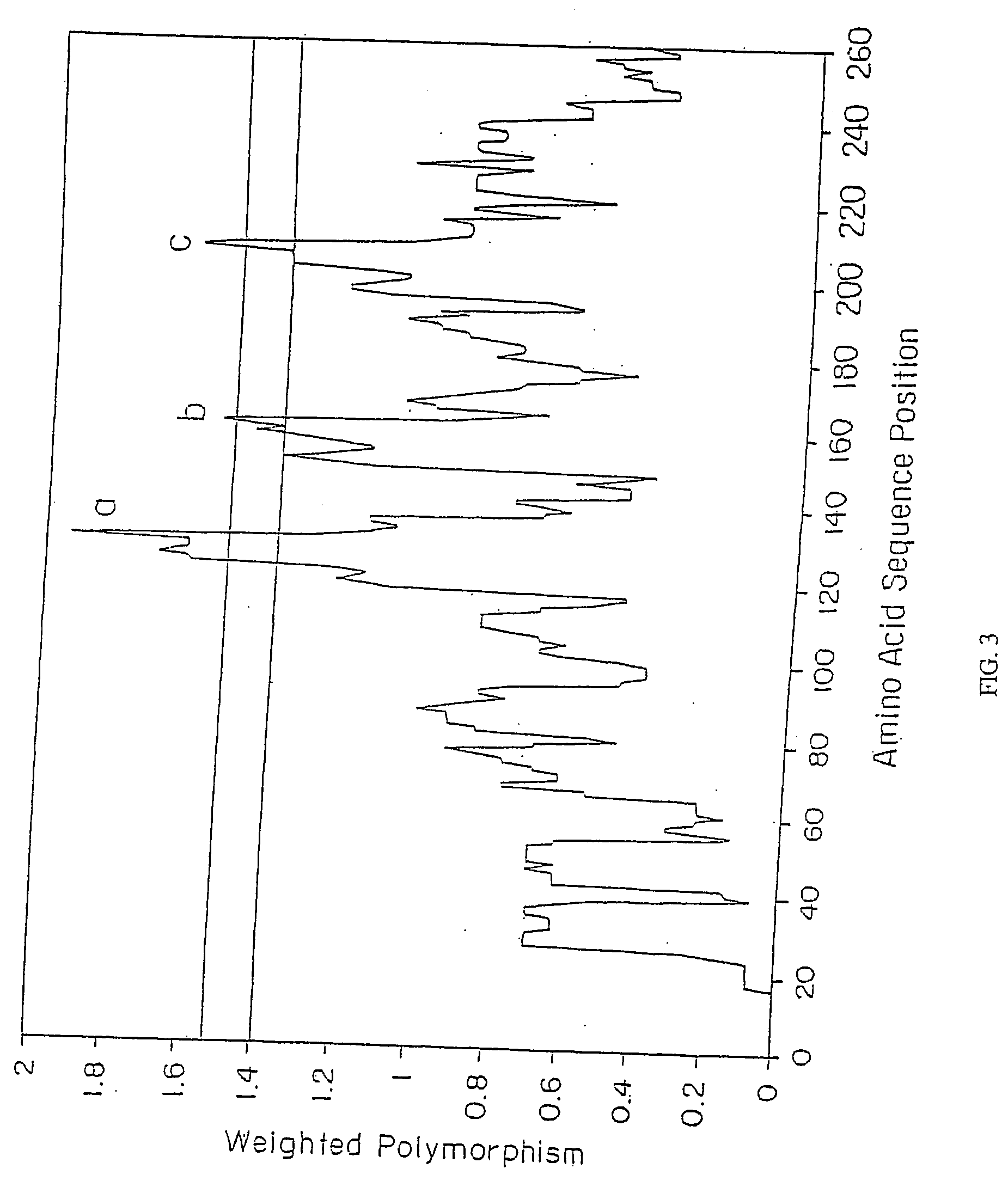
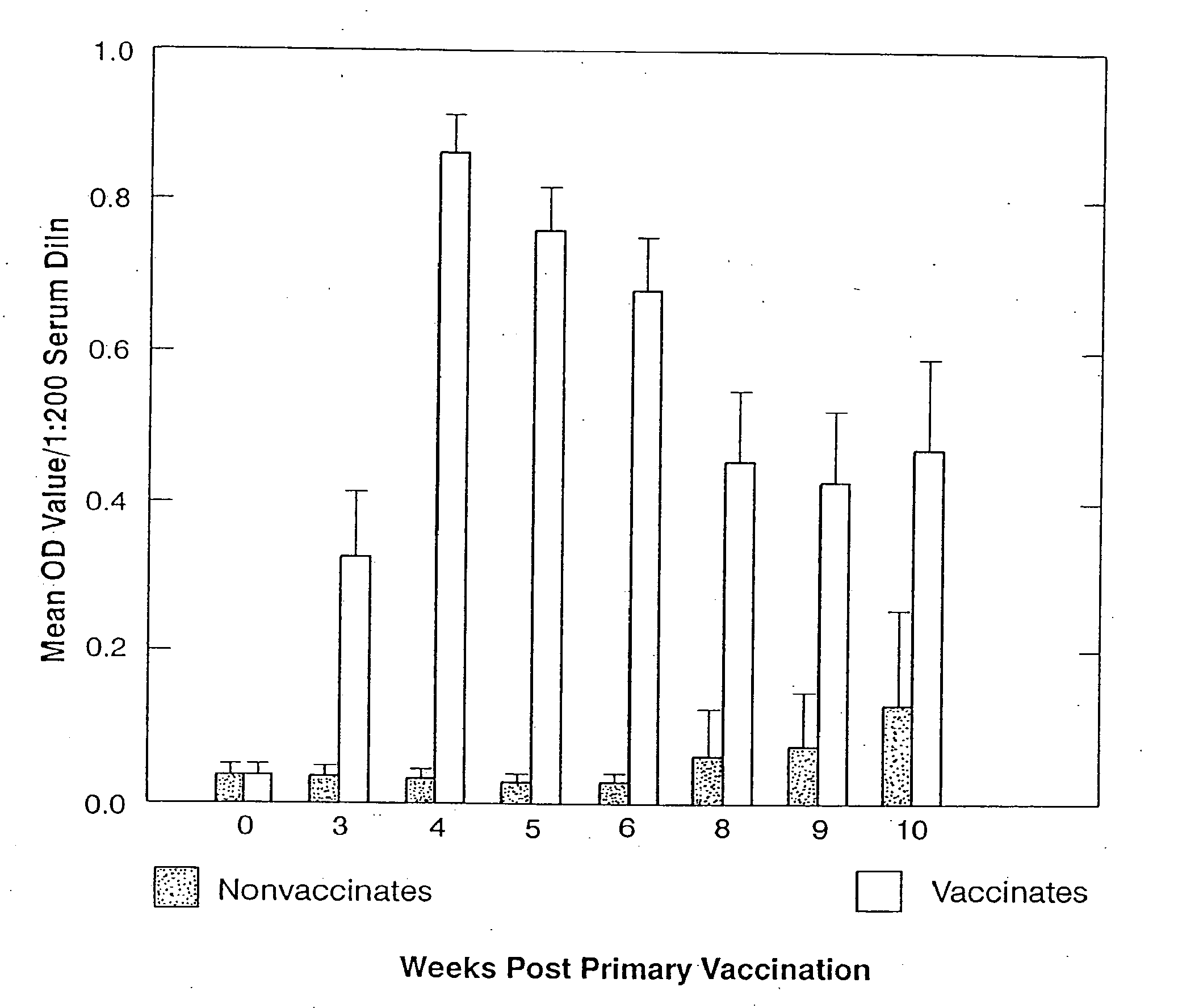
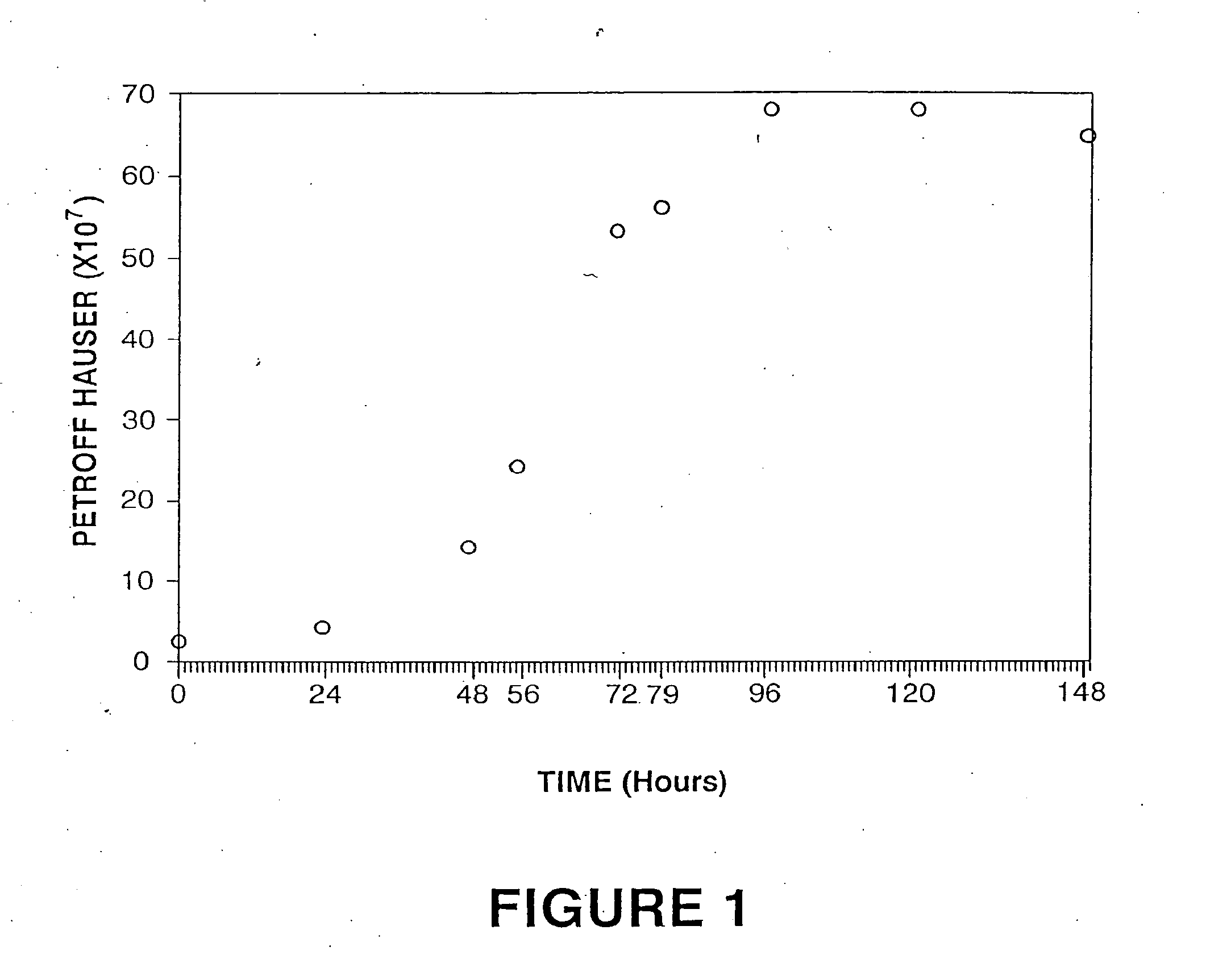
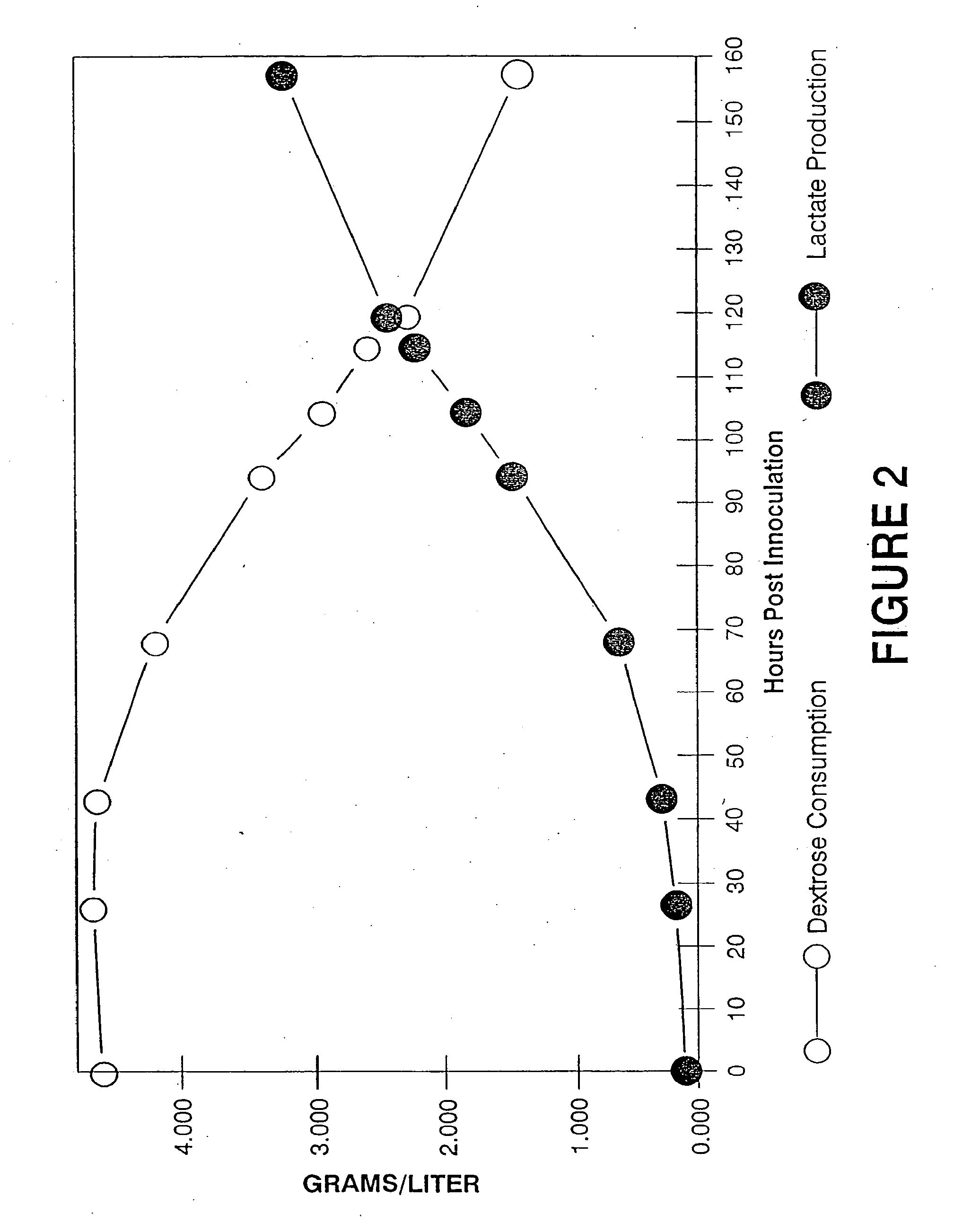
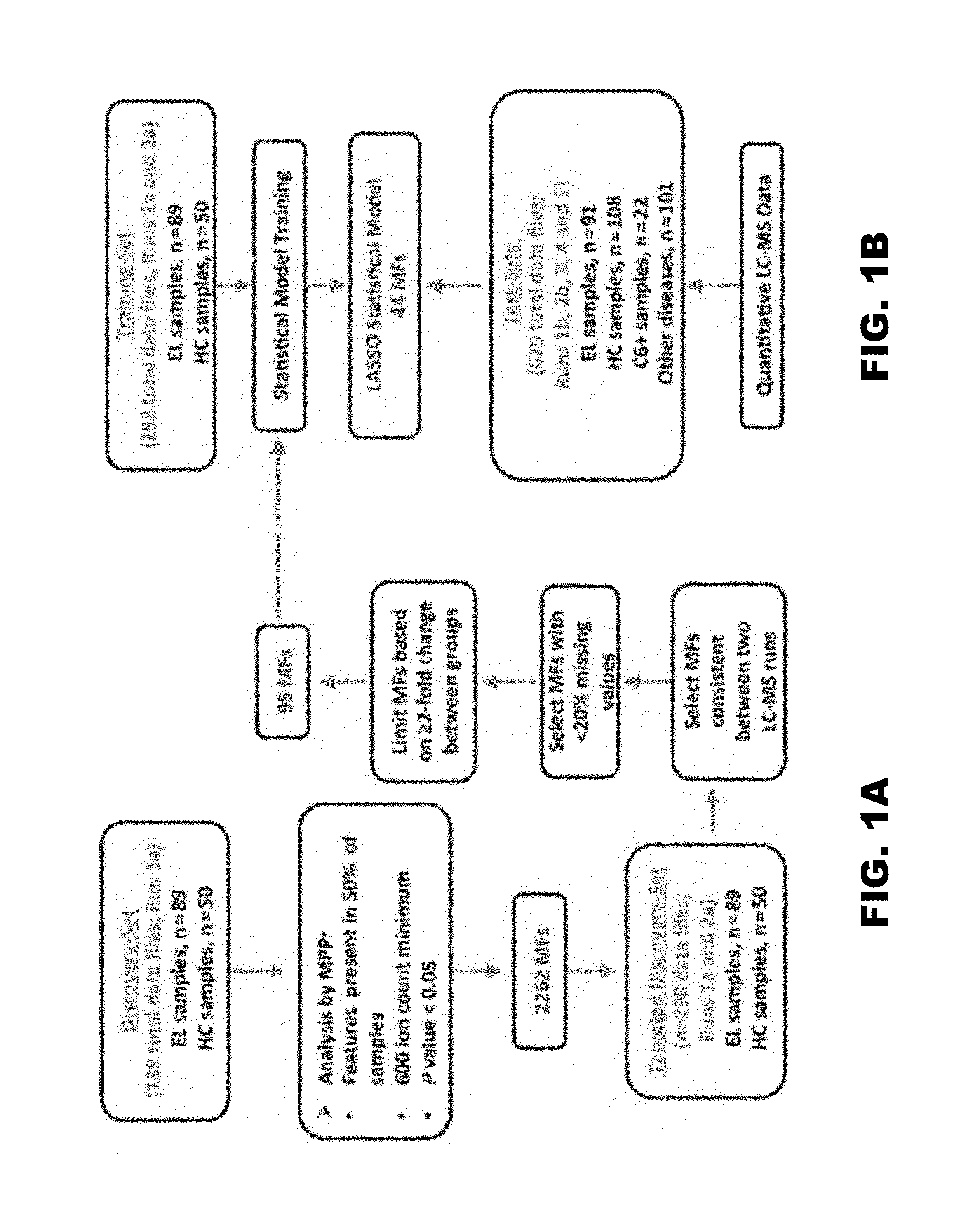
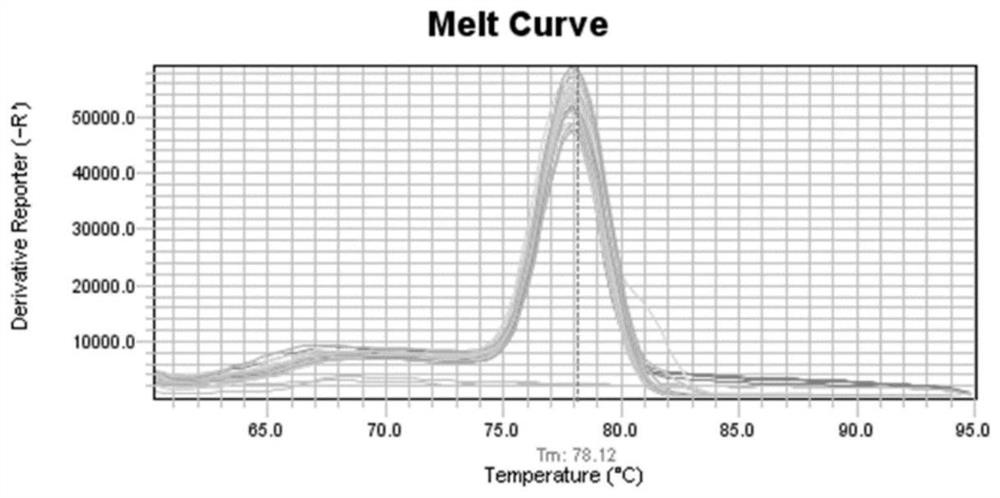
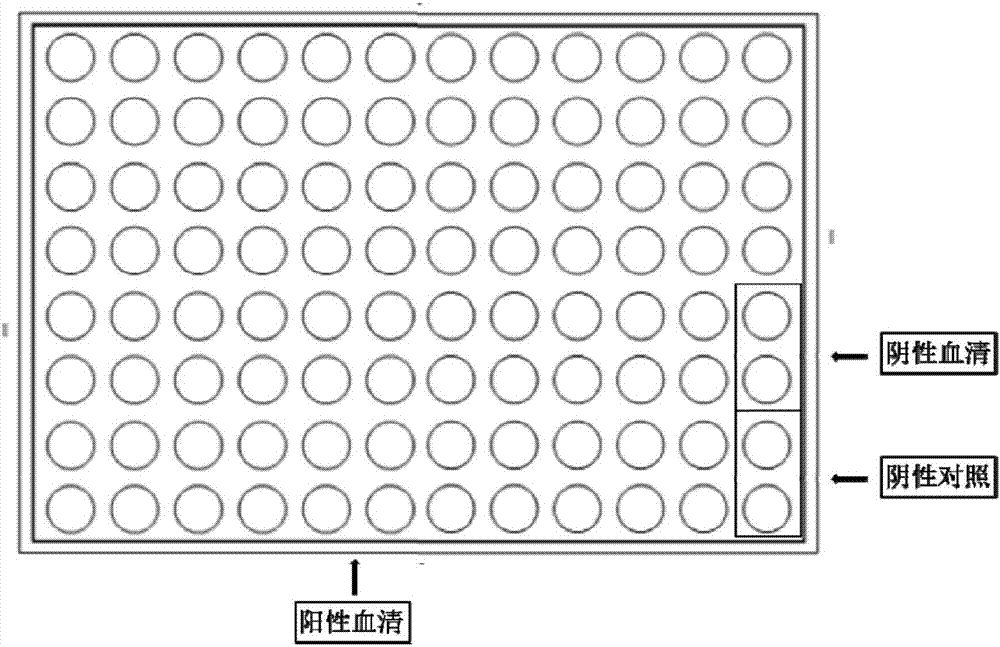
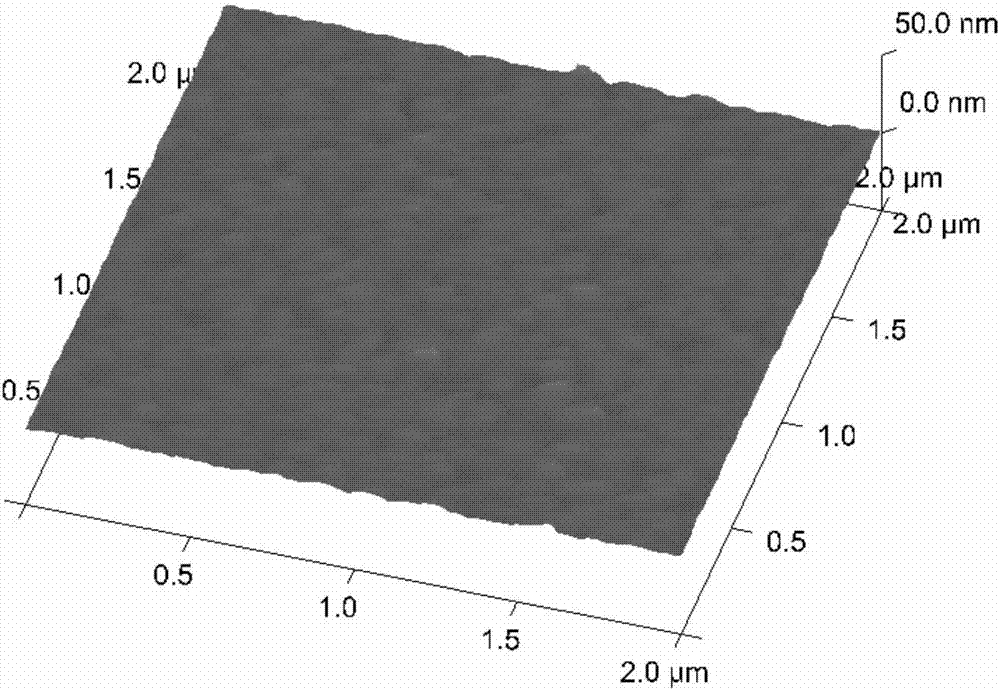
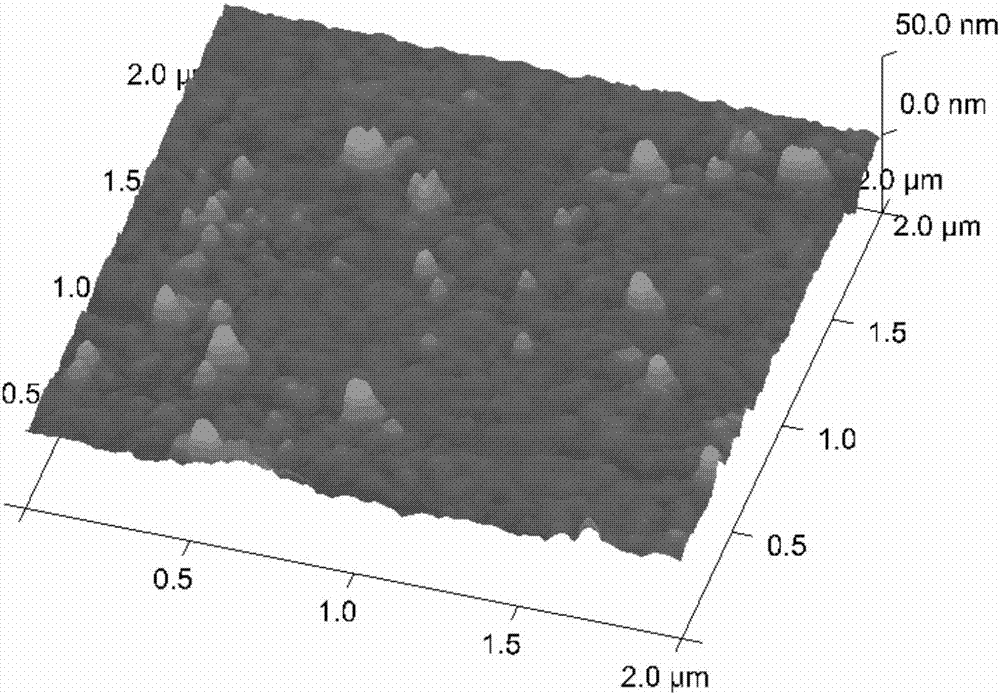
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Borreliella burgdorferi" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borrelia burgdorferi is a bacterial species of the spirochete class of the genus Borrelia. B. burgdorferi exists in North America and Europe and is the only causative agent of Lyme disease in the United States.
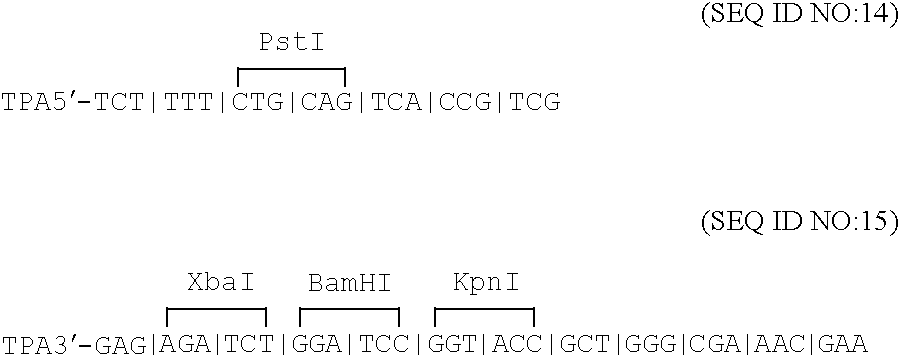
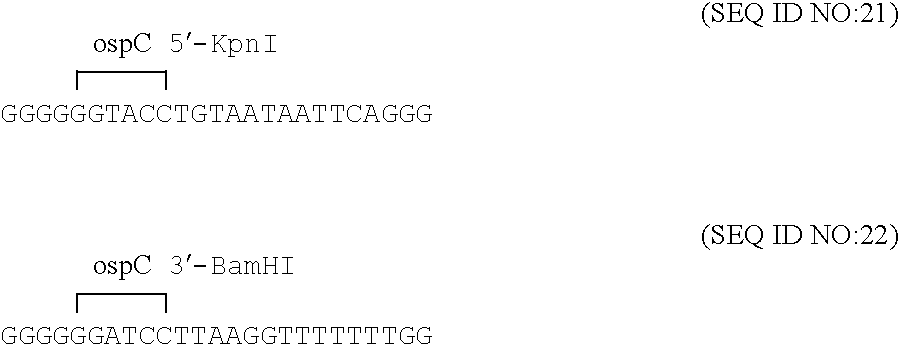
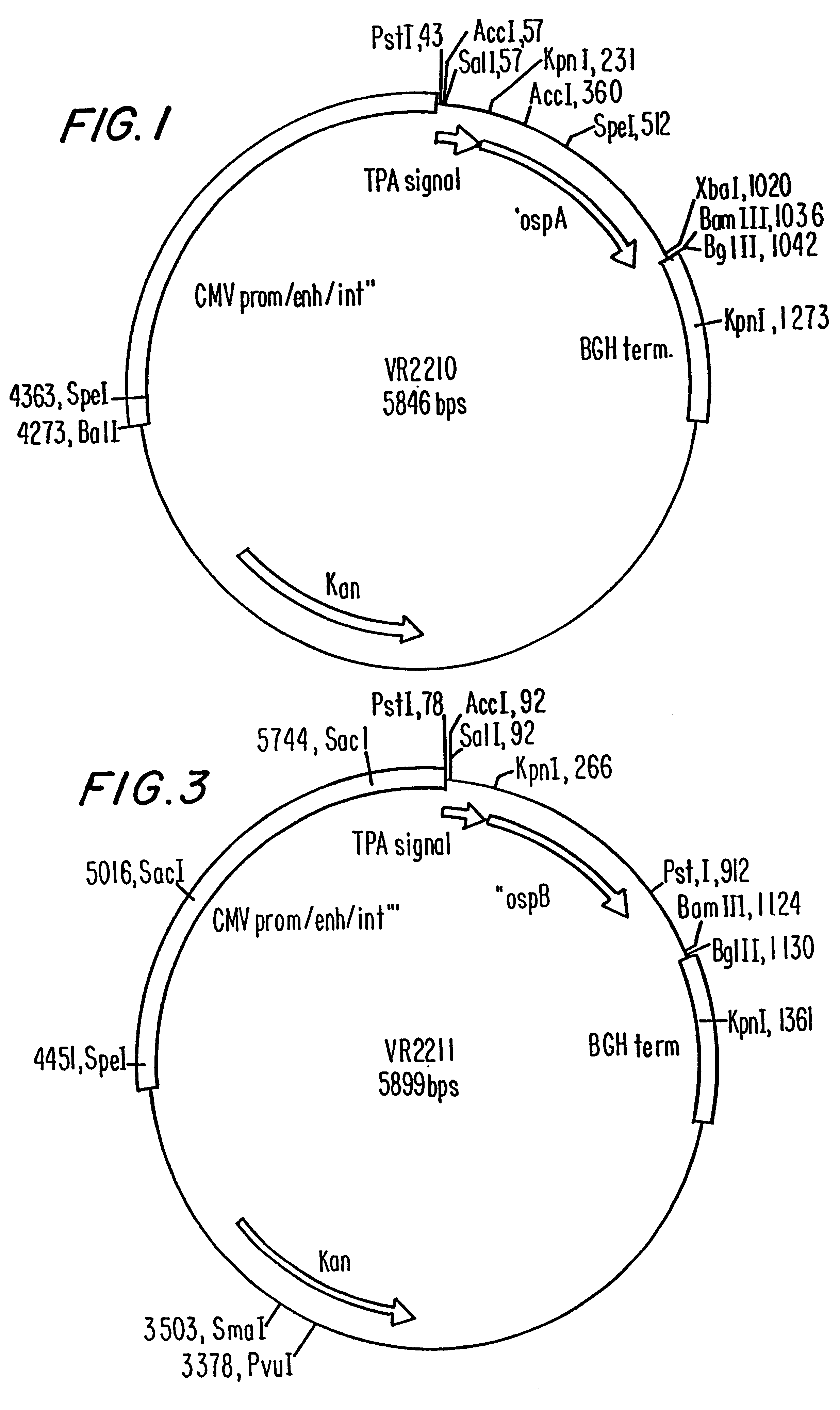
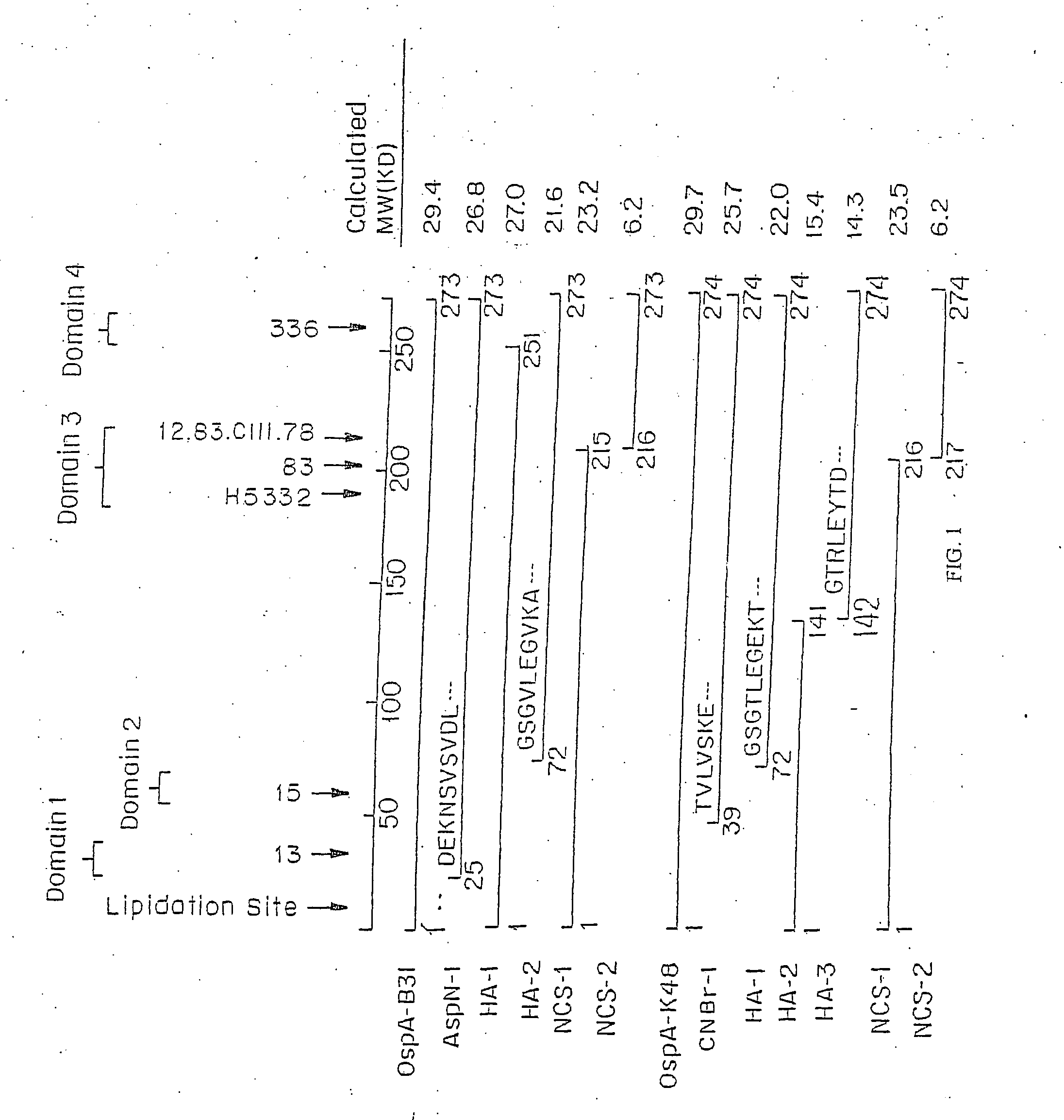
Compositions and methods for administering Borrelia DNA
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Recombinant constructs of Borrelia burgdorferi
InactiveUS20070020286A1Improve overall utilizationAvoid infectionBacteriaAntibody mimetics/scaffoldsImmunodiagnosticsBorreliella burgdorferi
Novel chimeric nucleic acids, encoding chimeric Borrelia proteins comprising OspC or an antigenic fragment thereof and OspA or an antigenic fragment thereof, are disclosed. Chimeric proteins encoded by the nucleic acid sequences are also disclosed. The chimeric proteins are useful as vaccine immunogens against Lyme borreliosis, as well as for immunodiagnostic reagents.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
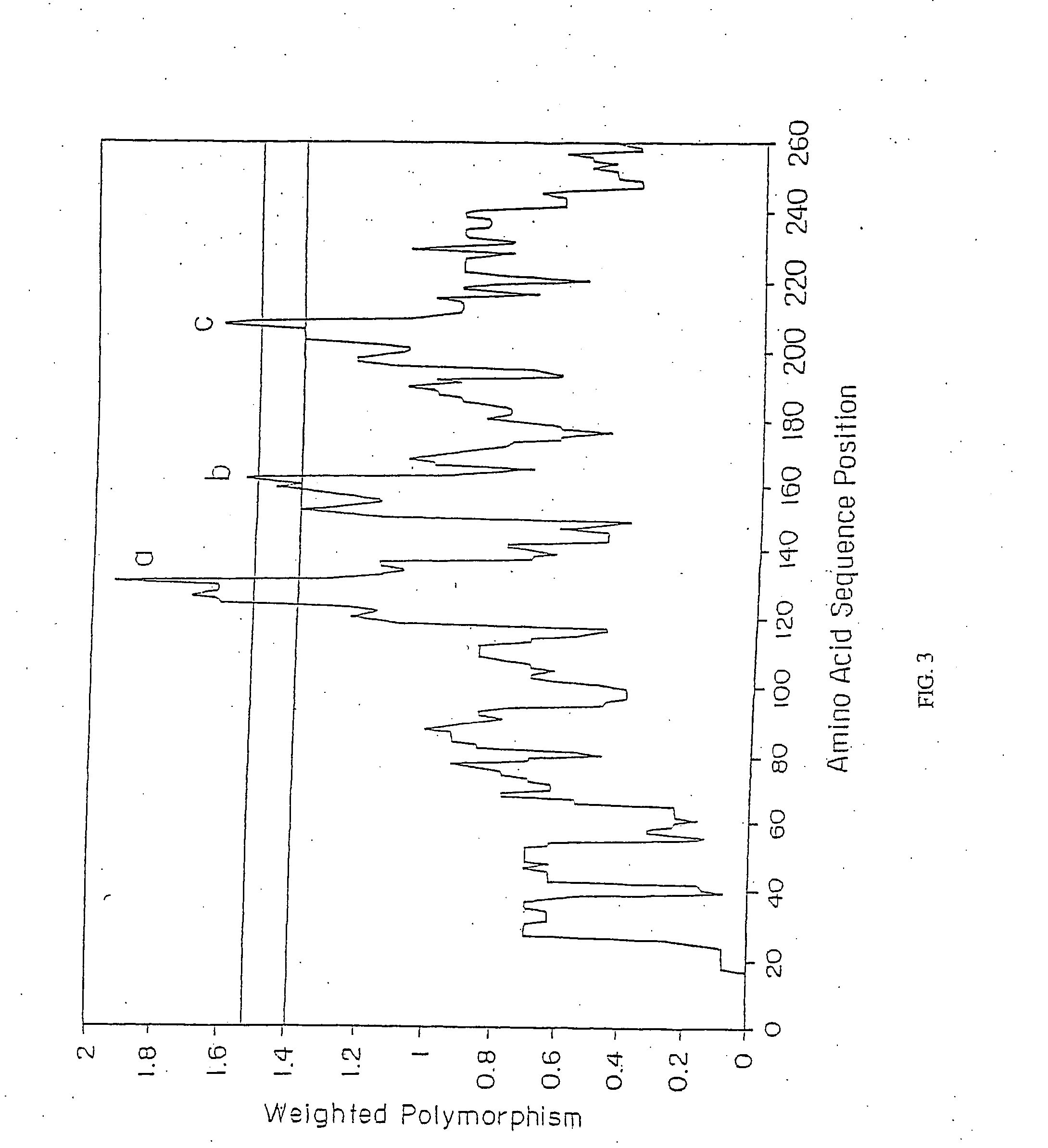
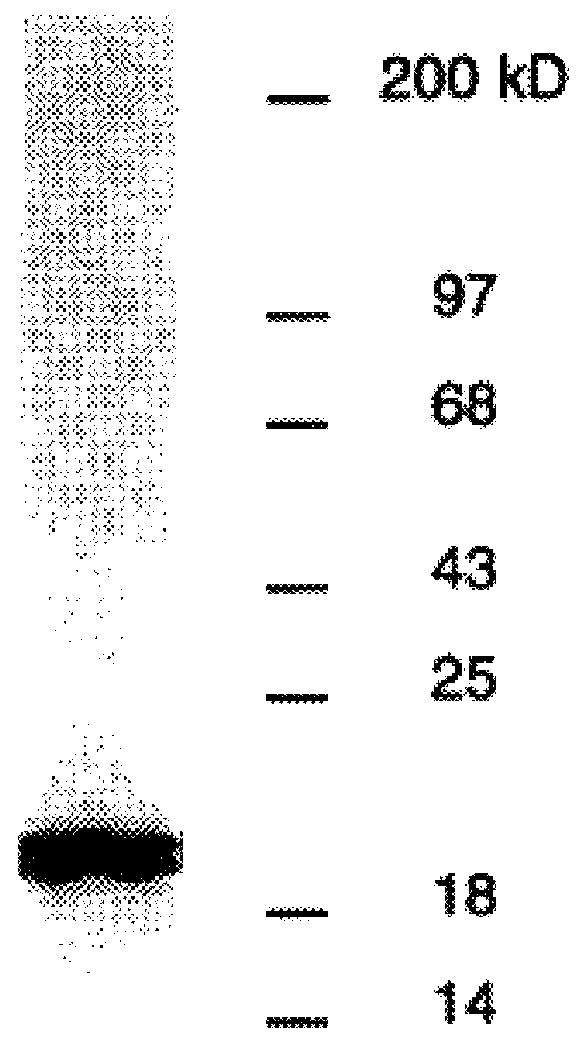
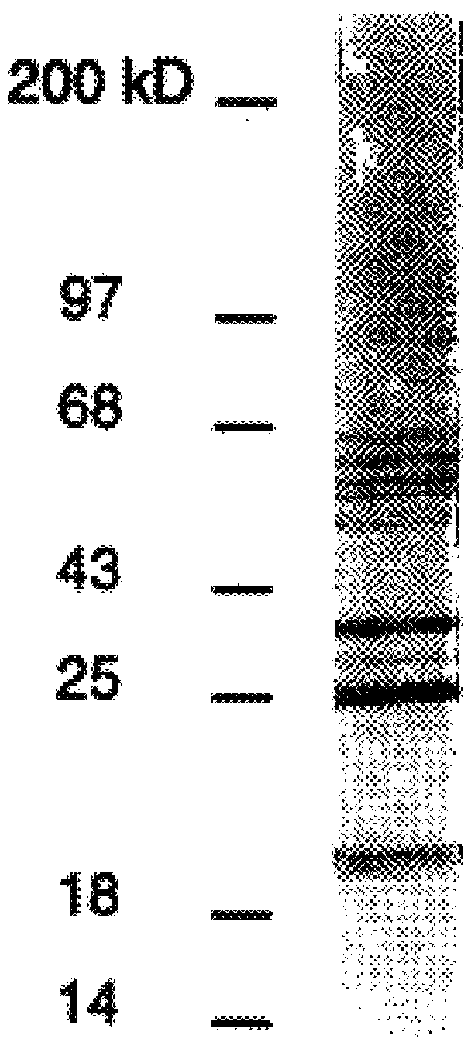
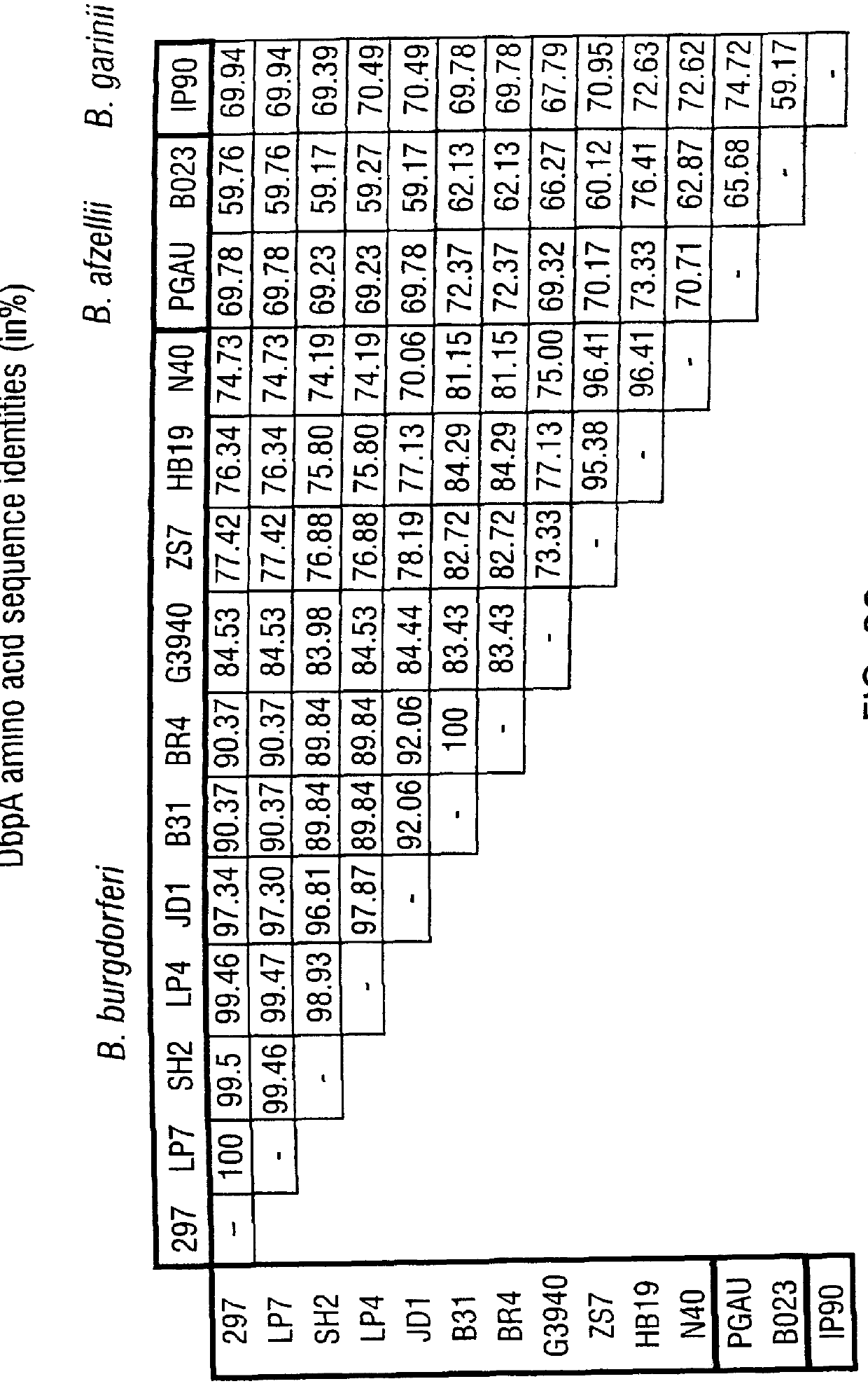
DbpA compositions
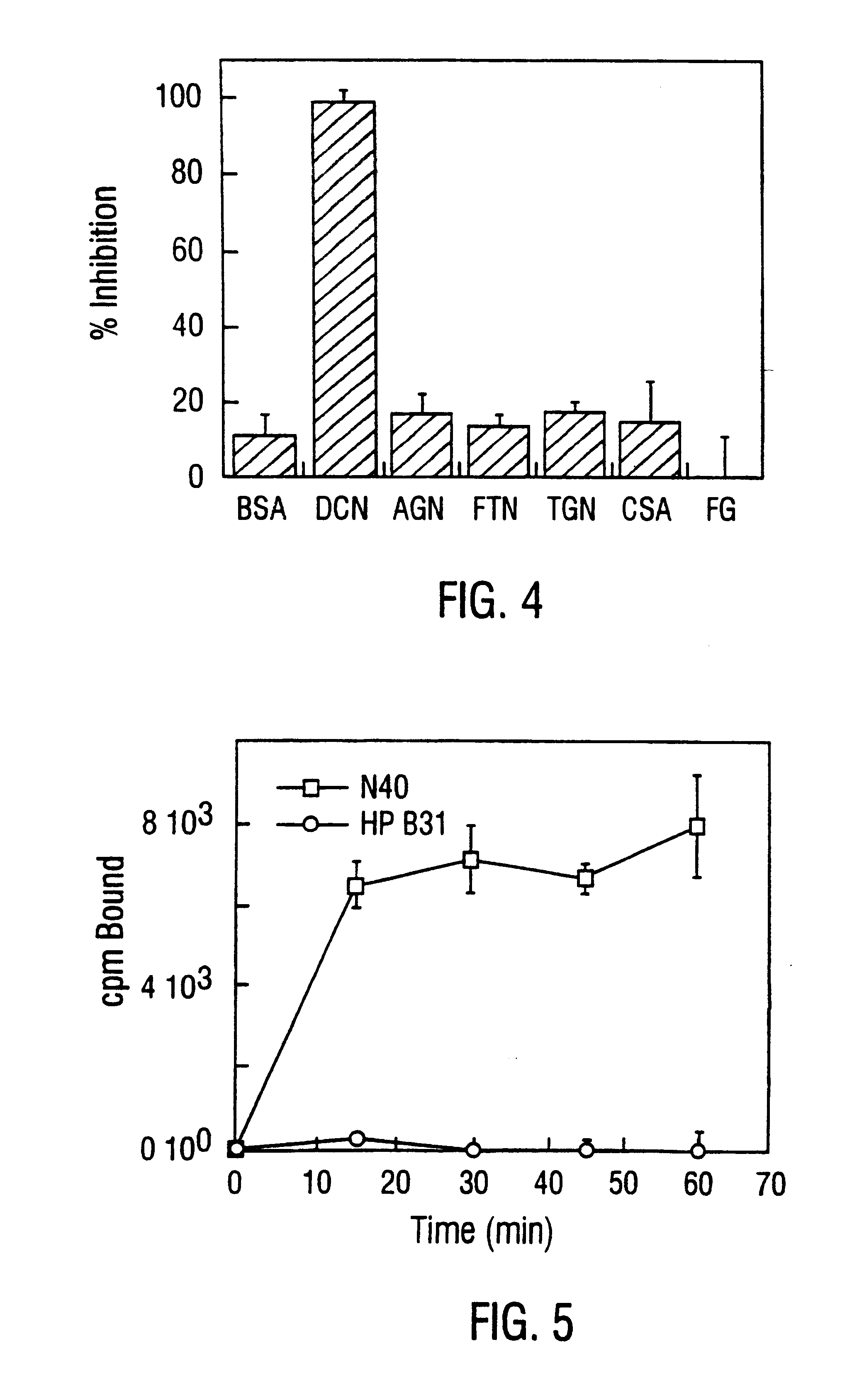
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:MEDIMMUNIE +1
Test for lyme disease
InactiveUS6838247B2Bacterial antigen ingredientsMicrobiological testing/measurementBorrelia antigenBorrelia burgdorferi
The present invention provides an accurate method to identify and quantify the Borrelia burgdorferi (Bb) antigen, the cause of Lyme Disease, in a sample of whole blood, body tissues and fluids of a subject, a human or animal subject. The qualitative method provides a quick, easy and accurate method of detection of the Bb antigen. The quantitative method allows for monitoring of treatment in conjunction with severity of clinical signs and symptoms.
Owner:BOWEN RES & TRAINING
Protein chip for lyme disease flagellin antigen immunoserology diagnosis and preparation method and application of protein chip
InactiveCN104374921AHigh sensitivityStrong specificityBiological testingAgainst vector-borne diseasesSerodiagnosesADAMTS Proteins
The invention discloses a protein chip for lyme disease flagellin antigen immunoserology diagnosis and a preparation method and application of the protein chip. The protein chip is characterized in that borrelia burgdorferi recombination flagellin antigen probes are fixed on the surface of a solid phase carrier in a dot matrix mode; the solid phase carrier is a 16-amino-1-hexadecyl mercaptan modified gold foil chip which is combined with 4-(N-maleinimide methyl) cyclohexane-1-carboxylic acid succinimide ester and 4-(dimethylamino) pyridine. The protein chip disclosed by the invention can accurately detect an anti-flagellin antigen IgG antibody and an anti-flagellin antigen IgM antibody in serums of lyme disease patients, the operation is simple, and the detection result is stable.
Owner:ANHUI MEDICAL UNIV
Compositions and methods to detect various infectious organisms
The invention relates to compositions and methods for the detection of various infectious organisms, including heartworm (Dirofilaria immitis), Ehrlichia Canis, Anaplasma phagocytophilum, and Borrelia burgdorferi. More particularly, this invention relates to antibodies that bind to a heartworm antigen, the E. Canis gp36 polypeptide, the A. phagocytophilum p44 polypeptide, the B. burgdorferi OspA, OspC, OspF, p39, p41 and VlsE polypeptides, and uses thereof.
Owner:VCA
Methods for Diagnosing Lyme Disease
ActiveUS20130273572A1Biological material analysisPeptide preparation methodsOuter surface proteinBorrelia burgdorferi
A method for diagnosing Lyme disease status in a mammal is provided. The method entails, in a biological sample obtained or derived from a mammal, determining antibodies to Borrelia burgdorferi (B. burgdorferi) outer surface proteins (Osp) OspA, OspC, and OspF. Based upon determining the OspA, OspC, and OspF antibodies, the mammal can be diagnosed as vaccinated, not vaccinated, infected or not infected with B. burgdorferi. Mammals that have early, intermediate or chronic B. burgdorferi infection can also be identified. The method is particularly suited for use with horses and dogs. Isolated or recombinant B. burgdorferi antigens and compositions that contain them are also provided.
Owner:CORNELL UNIVERSITY
Decorin binding protein compositions and methods of use
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:TEXAS A&M UNIVERSITY
Borrelia burgdorferi bacterial antigen diagnosic test using polymeric bait containing capture particles
InactiveUS20130085076A1Improve abilitiesReliable, rapid, inexpensive and non-invasiveLibrary screeningImmunoassaysDiagnostic testSeroconversion
The invention relates to both a sensitive method for the capture and detection of low-abundance Borrelia burgdorferi (Bb) bacterial antigens allowing for the diagnosis of Lyme Disease using standard immunoassays. Furthermore, this invention allows the antigen to be identified in a sample of urine, serum, or other biological fluids isolated from humans and animals. The invention provides a method to capture, concentrate, separate and specifically quantify the abundance of Bb antigens using immunoassays. The detection of Bb Outer Surface Protein A is presented as an example of the disclosed invention. High sensitivity levels, low cost and easily collected biofluids allow this technology to reach patients in clinics as well as POC applications for the early detection of Lyme disease prior to seroconversion. A kit containing necessary reagents and the method for diagnosis, monitoring or assessing lyme disease using an immunoassay such as an ELISA, western blot or RPPMA is disclosed.
Owner:GEORGE MASON INTPROP INC +1
LAMP primer combination for detecting three ophthalmic infection spirochetes and application
ActiveCN105861727AMicrobiological testing/measurementMicroorganism based processesSpiroplasmaBorrelia burgdorferi
The invention discloses an LAMP primer combination for detecting three ophthalmic infection spirochetes and application. The primer combination is composed of eighteen single-stranded DNA molecules shown from the sequence 1 to the sequence 18. The invention further provides application of the primer combination. The invention furthermore provides a method of using the primer combination for identifying whether a spirochete to be detected is treponema pallidum, or borrelia burgdorferi or leptospira, a method for identifying whether the spirochete to be detected is treponema pallidum, or borrelia burgdorferi or leptospira and a method for identifying whether a sample to be detected is infected by treponema pallidum and / or borrelia burgdorferi and / or leptospira. Treponema pallidum, borrelia burgdorferi and leptospira can be fast and accurately detected by using the method.
Owner:智德科技(无锡)有限公司
Recombinant constructs of Borrelia burgdorferi
InactiveUS20050271682A1Improve overall utilizationAvoid infectionBacteriaPeptide/protein ingredientsBorrelia gariniiBorreliella burgdorferi
Novel chimeric nucleic acids, encoding chimeric Borrelia proteins comprising OspC or an antigenic fragment thereof and OspA or an antigenic fragment thereof, are disclosed. Chimeric proteins encoded by the nucleic acid sequences are also disclosed. The chimeric proteins are useful as vaccine immunogens against Lyme borreliosis, as well as for immunodiagnostic reagents.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Luciferase co-immunoprecipitation kit for detecting mammalian lyme diseases
ActiveCN106771250AHigh sensitivityImprove signal-to-noise ratioBiological material analysisBiological testingAntigenSurface membrane
The invention discloses a luciferase co-immunoprecipitation kit for detecting a mammalian lyme disease. The luciferase co-immunoprecipitation kit comprises the following components: (1) a sample diluent; (2) a fusion protein solution composed of ranilla luciferase and a borrelia burgdorferi surface membrane protein OspC antigen; (3) a proteinA / G-coated ELISA plate; (4) washing liquid; (5) a luciferase substrate. The luciferase co-immunoprecipitation kit disclosed by the invention adopts a luciferase co-immunoprecipitation method and can be used for detecting the lyme disease and the onset period thereof according to the fluorescence intensity. The method is quick, simple and convenient, high in sensitivity, high in signal-noise ratio, and stable and reliable in measured C:\Program Files\Youdao\Dict\7.1.0.0421\resultui\dict\value.
Owner:杭州北角医学检验所有限公司
Borrelia burgdorferi bacterin
InactiveUS20080026009A1Improving immunogenicityAntibacterial agentsBacterial antigen ingredientsBorrelia gariniiAdjuvant
A bacterin including effective immunizing amounts of two non-crossprotective isolates of inactivated Borrelia burgdorferi, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi isolates and a suitable carrier is provided herein. The bacterin may also contain a third non-crossprotective isolate. A bacterin including effective immunizing amounts of an antigenic subunit derived from a first Borrelia burgdorferi isolate and a second, non-crossprotective Borrelia burgdorferi isolate, an adjuvant in an amount effective to enhance the immunogenicity of the antigenic subunits and a suitable carrier is also provided. The bacterin may also contain an effective immunizing amount of an antigenic subunit of a third Borrelia burgdorferi. Further provided is a bacterin which includes effective immunizing amounts of two non-crossprotective isolates of inactivated Borrelia burgdorferi and one or more antigenic subunits from the non-crossprotective isolates, an adjuvant in an amount effective to enhance the immunogenicity of the inactivated Borrelia burgdorferi and antigenic subunits and a suitable carrier.
Owner:SCHERING CORP
High sensitivity method for early lyme disease detection
ActiveUS20160237470A1Accurate distinctionComponent separationMicrobiological testing/measurementBorreliella burgdorferiBorrelia Infections
The present disclosure provides methods for detecting early Lyme disease. The present disclosure provides a biosignature indicative of the presence or absence of Borrelia burgdorferi infection.
Owner:NEW YORK MEDICAL COLLEGE +2
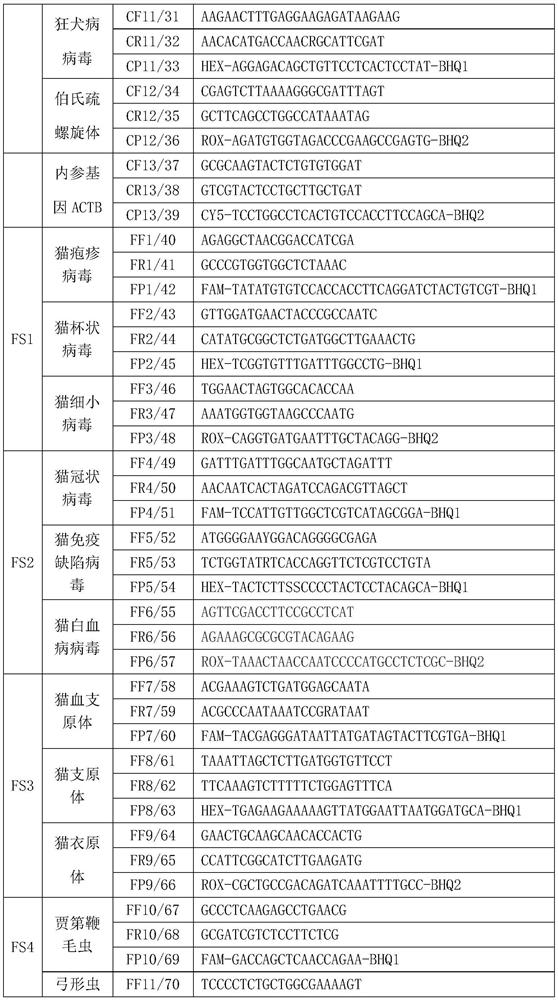
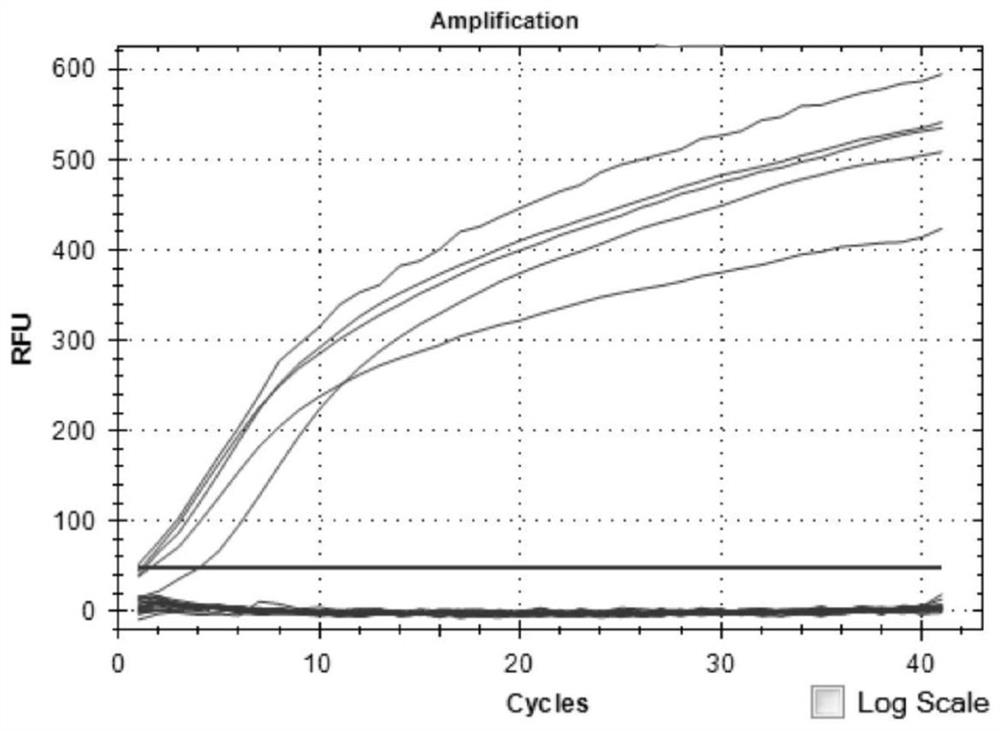
PCR (Polymerase Chain Reaction) detection kit for cat and/or dog pathogens, detection method and application
PendingCN114277189AShorten detection timeSave human resourcesMicrobiological testing/measurementAgainst vector-borne diseasesFeline parvovirusLeucosis
The invention relates to the technical field of molecular biomedicine, in particular to a PCR (Polymerase Chain Reaction) detection kit for cat and / or dog pathogens, a detection method and application. The kit is used for detecting canine distemper virus, canine influenza A virus, canine parainfluenza virus, canine parvovirus, canine coronavirus, canine rotavirus, canine babesia, canine ascaris, canine Ehrlichia, canine brucella, rabies virus, borrelia burgdorferi, reference gene ACTB, feline herpes virus, feline calicivirus and feline parvovirus. The kit comprises primers and probes of feline coronavirus, feline immunodeficiency virus, feline leukemia virus, feline mycoplasma, feline mycoplasma, feline chlamydia, giardia, toxoplasma, bartonella and reference gene GAPDH, collected DNA and RNA are added into the kit, a real-time fluorescence PCR instrument is adopted for PCR reaction, FAM, HEX, ROX and CY5 fluorescence signals are collected in each cycle, analysis of related pathogens is carried out, and the kit can be used for detecting the feline and the canine. Compared with a traditional detection method, the method has the advantages of higher specificity and higher sensitivity.
Owner:北京迈基诺基因科技股份有限公司
Nested fluorescent quantitative PCR (Polymerase Chain Reaction) detection method for Lyme disease spirochetes
PendingCN113512605AEasy to distinguishStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesBorreliella burgdorferiSpirochaete
The invention provides a nested fluorescent quantitative PCR (polymerase chain reaction) detection method for Lyme disease spirochetes. According to the invention, rrf-rrl is taken as a target gene, nested PCR primers and probes for detecting lyme disease spirochetes are designed, the nested PCR primers comprise a first round of PCR primers and a second round of PCR primers, and the probes are introduced in the second round of PCR reaction so as to improve the detection sensitivity. The nested fluorescent quantitative PCR detection method for Lyme disease spirochetes provided by the invention has better specificity and high sensitivity, and the lower detection limit reaches 0.01 pg / [mu]L. According to the method disclosed by the invention, a positive sample can be subjected to single-point typing, so that the effective discrimination of four types of Borrelia burgdorferi, namely, Borrelia burgdorferi sensu stricto (B.b.s.s), Borrelia garinii (B.g), Borrelia afzelii (B.a), and Borrelia valaisiana (B.v) can be realized.
Owner:ICDC CHINA CDC
High sensitivity method for early Lyme disease detection
ActiveUS10669567B2Component separationMicrobiological testing/measurementBorrelia burgdorferiBorreliella burgdorferi
The present disclosure provides methods for detecting early Lyme disease. The present disclosure provides a biosignature indicative of the presence or absence of Borrelia burgdorferi infection.
Owner:NEW YORK MEDICAL COLLEGE +2
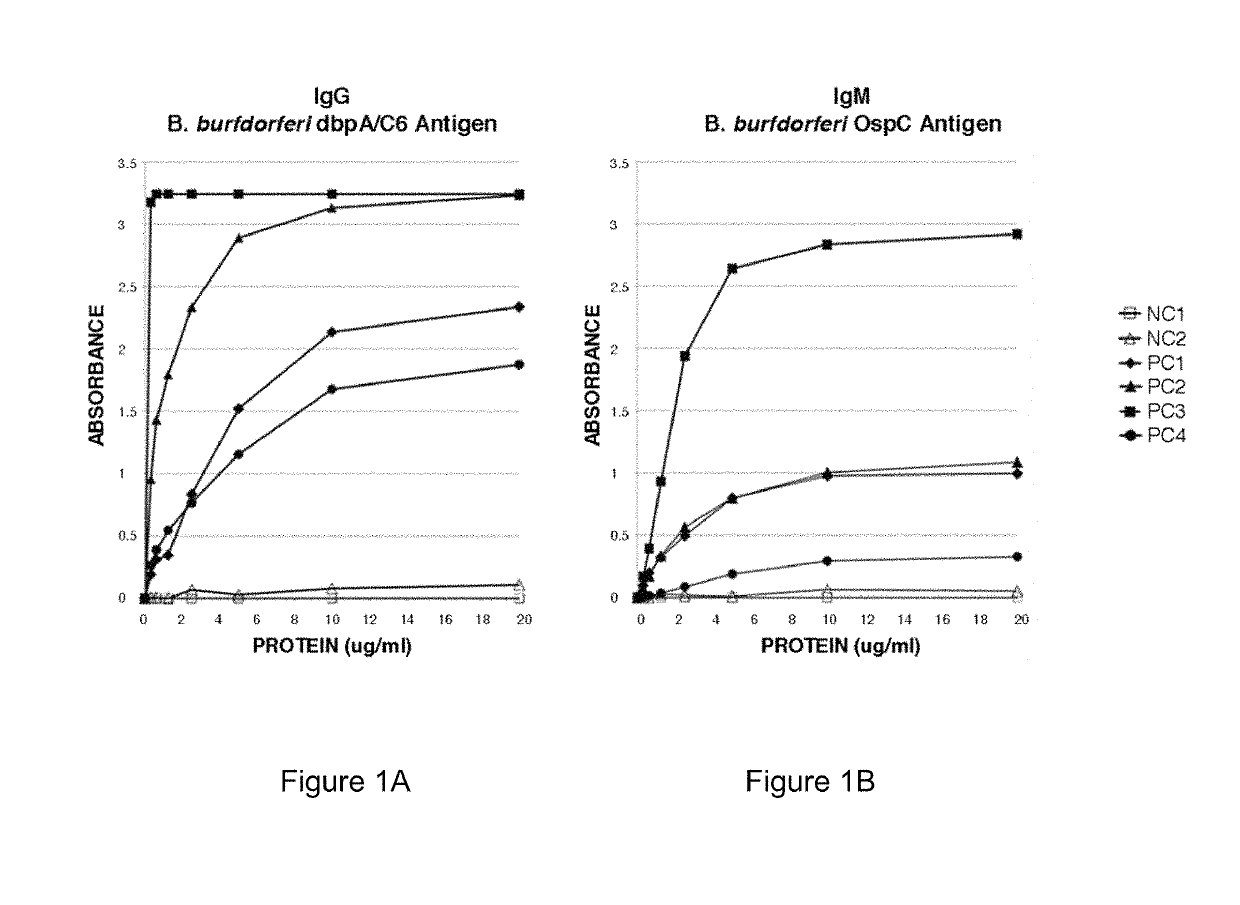
Serodiagnosis of lyme disease by use of two recombinant proteins in ELISA
ActiveUS10401358B1Great capacity to identifyFast and easy to performAntibody mimetics/scaffoldsDepsipeptidesSerodiagnosesEscherichia coli
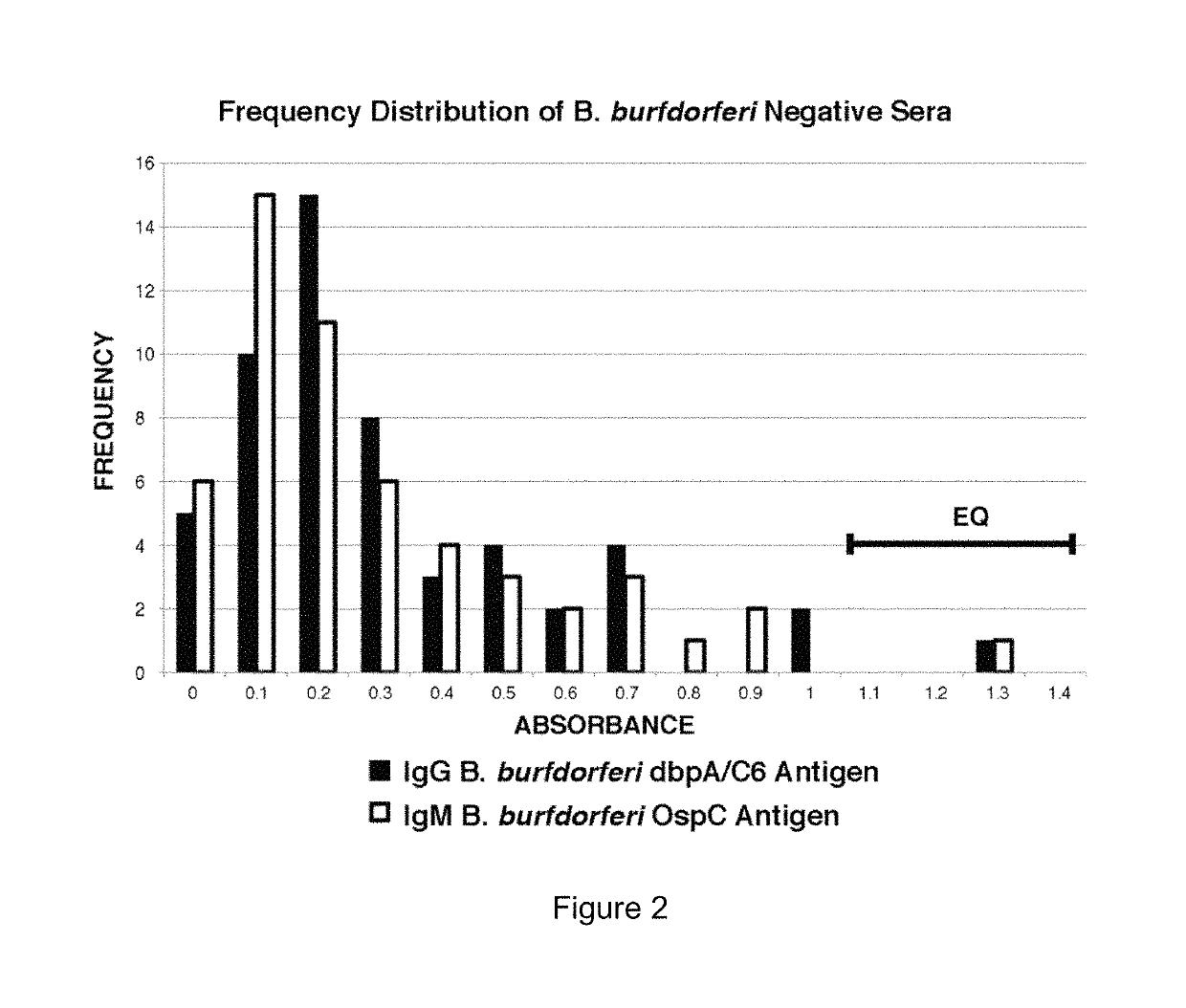
Two Borrelia burgdorferi recombinant proteins were expressed in E. coli. These two proteins were generated from (a) the full length dbpA gene combined with the invariable region 6 of the VlsE gene (dbpA / C6), and (b) the full length OspC gene combined with the coding sequence for amino acids 1-121 of the E. coli maltose binding protein gene (OspC / MBP). Methods of using these recombinant proteins for detecting anti-Borrelia burgdorferi antibodies in patient sera and diagnosis of Lyme Disease are described.
Owner:ROSS SOUTHERN LAB
Method of treating central nervous system disorders with Borrelia burgdorferi antigen
InactiveUS20150320849A1Bacterial antigen ingredientsPeptide/protein ingredientsBorrelia antigenAntigen
The present invention relates to a treatment for mammalian subjects suffering from a central nervous system (CNS) disorder by administering a composition comprising an Borrelia burgdorferi antigen at a sub-vaccine level effective to alleviate symptoms of the CNS disorder.
Owner:BEECH TREE LABS
Altered OSPA of Borrelia Burgdorferi
InactiveUS20140030285A1Improve stabilityLow cross-reactivityBacterial antigen ingredientsSugar derivativesBorrelia gariniiAntigenicity
Provided herein are OspA polypeptides from Lyme Disease-causing Borrelia having certain alteration(s). In one embodiment, the alteration(s) increase the conformational stability of the OspA polypeptide containing the alteration(s) while maintaining at least some of the antigenicity of the corresponding unaltered OspA polypeptide. In another embodiment, the altered OspA polypeptide has reduced cross-reactivity to hLFA-1, as compared to the corresponding unaltered OspA polypeptide.
Owner:BROOKHAVEN SCI ASSOCS +2
Serodiagnosis of Lyme disease by use of two recombinant proteins in ELISA
Two Borrelia burgdorferi recombinant proteins were expressed in E. coli. These two proteins were generated from (a) the full length dbpA gene combined with the invariable region 6 of the VlsE gene (dbpA / C6), and (b) the full length OspC gene combined with the coding sequence for amino acids 1-121 of the E. coli maltose binding protein gene (OspC / MBP). Methods of using these recombinant proteins for detecting anti-Borrelia burgdorferi antibodies in patient sera and diagnosis of Lyme Disease are described.
Owner:ROSS SOUTHERN LAB
Compositions and Methods for the Detection of Bacterial Infections Associated with Lyme Disease
InactiveUS20150219646A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAntigenAntibody
The invention is directed to methods of assaying biological samples for the presence of antibodies or antigens indicative of infection by a bacterium of the Borrelia burgdorferi sensu lato complex. Also included in the invention are devices that can be used in carrying out these methods. The methods and devices may be used to help identify subjects that have Lyme disease.
Owner:THE GENERAL HOSPITAL CORP
Borrelia burgdorferi detection kit and application thereof
InactiveCN109097438AQuick checkObvious discolorationMicrobiological testing/measurementAgainst vector-borne diseasesSpiroplasmaRapid testing
The invention discloses a Borrelia burgdorferi detection kit. The Borrelia burgdorferi detection kit comprises a PCR (polymerase chain reaction) primer for detecting Borrelia burgdorferi, and a Borrelia burgdorferi detection system is provided; a bacteria solution is diluted 1 / 10<8> times in a PCR-immune colloidal gold test strip detection method, a detection band changes color obviously, a positive result can be judged clearly, and the detection kit is proved to be higher in detection sensitivity; by combining a high-sensitivity and high-specificity method of PCR in nucleic acid testing withan immune colloidal gold rapid testing technique in immunological detection and designing the unique primer, extracted target DNA is subjected to specific amplification, an amplification product in adeveloping solution binds with a gold labelled antibody fixed on the test strip, a stable and visible detection band and a quality control band are formed, and rapid detection of Borrelia burgdorferiis realized.
Owner:吉林出入境检验检疫局检验检疫技术中心
Borrelia burgdorferi peptidoglycan as a diagnostic and target for therapeutic intervention of lyme disease-related pathologies
PendingUS20210364514A1Excessive levelLower Level RequirementsAntibacterial agentsPeptide/protein ingredientsAntiendomysial antibodiesBorrelia burgdorferi
The present disclosure relates to a method of diagnosing Lyme disease in a subject comprising measuring the level of B. burgdorferi peptidoglycan or the level of an antibody that specifically binds to B. burgdorferi peptidoglycan (“anti-peptidoglycan agent”). The present disclosure also relates to a method of treating a Lyme disease in a subject in need thereof comprising administering to the subject an antagonist against B. burgdorferi peptidoglycan (e.g., an anti-peptidoglycan antibody or a peptidoglycan-specific hydrolase). Antagonists (e.g., anti-peptidoglycan antibodies) suitable for the present methods are also disclosed.
Owner:VIRGINIA TECH INTPROP INC
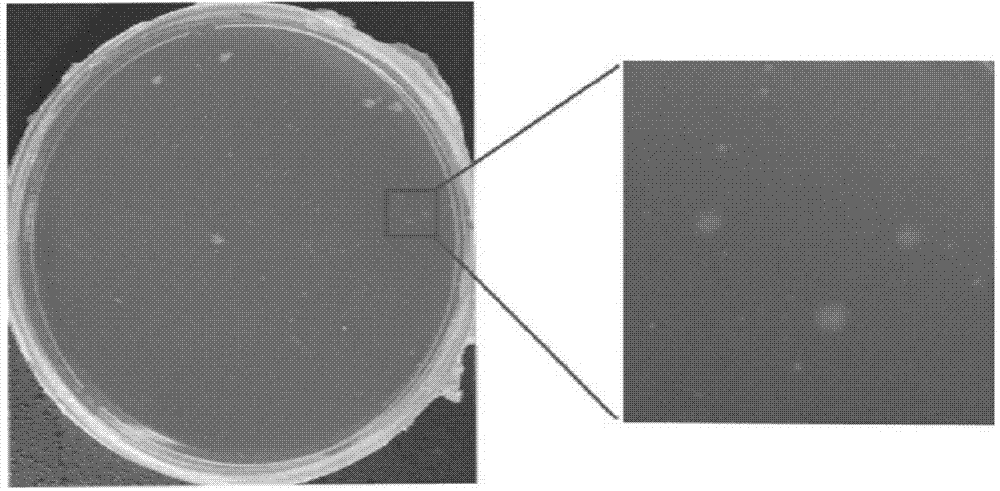
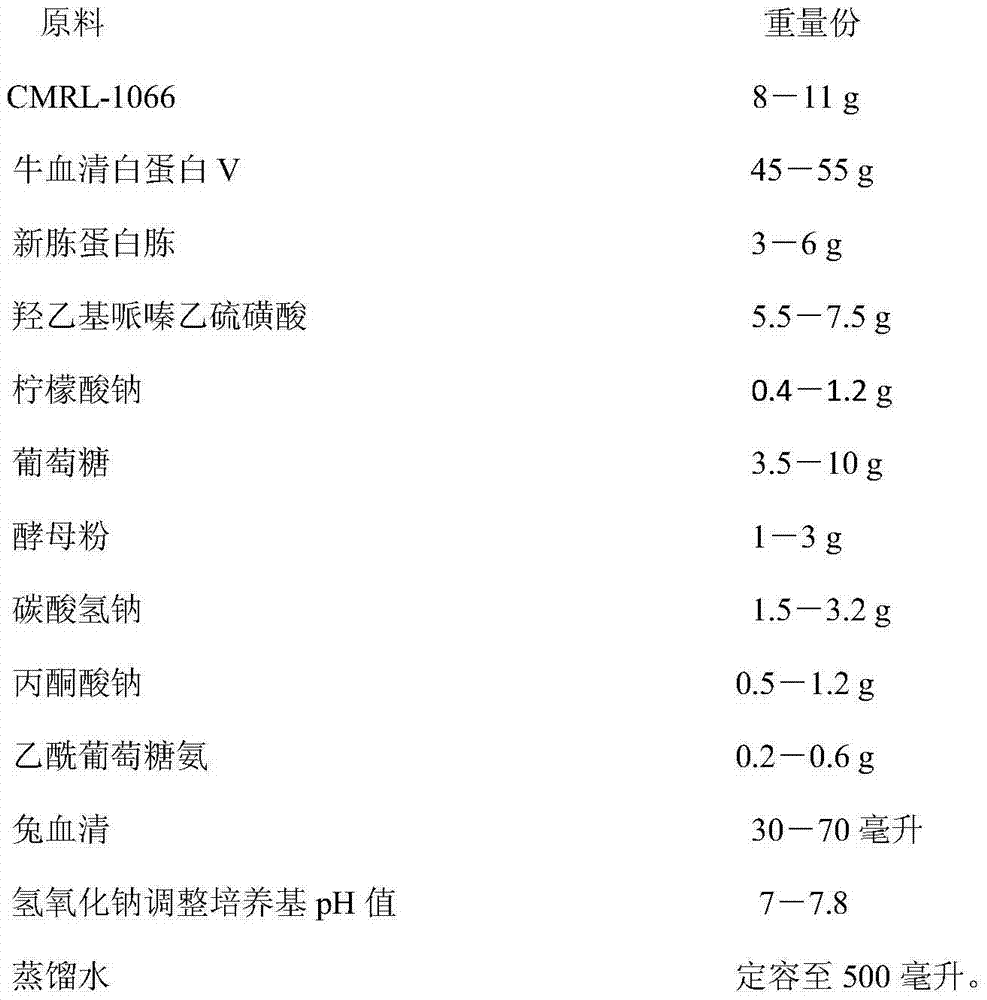
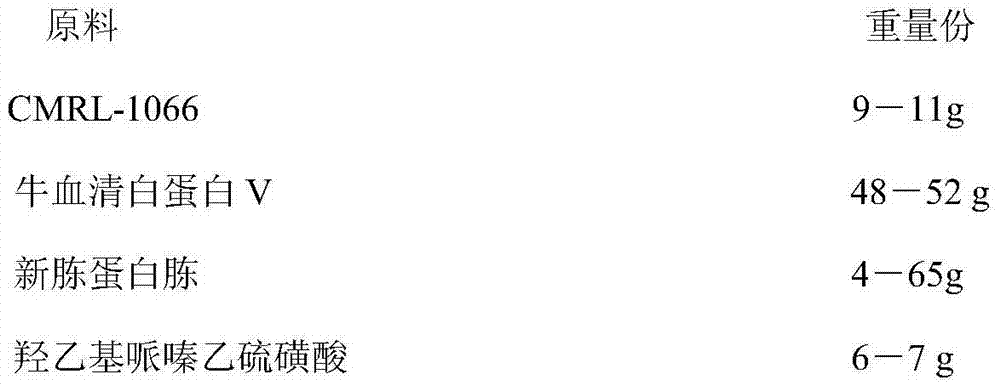
Borrelia BSK storage liquid culture medium and single colony separating and purifying method and application thereof
ActiveCN103409356BPromote growthPromote proliferationBacteriaMicroorganism based processesSodium bicarbonateBovine serum albumin
The invention discloses a borrelia BSK storage liquid culture medium and a single colony separating and purifying method and application thereof. The borrelia BSK storage liquid culture medium comprises CMRL-1066, bovine serum albumin V, new peptone, hydroxyethyl piperazine ethanesulfonic acid, sodium citrate, glucose, yeast powder, sodium bicarbonate, sodium pyruvate, acetyl glucosamine, rabbit serum, sodium hydroxide, and the like. The single colony separating and purifying method comprises the following steps of: preparing the BSK storage liquid culture medium, preparing sepharose gel, sterilizing at high pressure, gradually melting, uniformly mixing with the BSK storage liquid culture medium in proportion, and pouring to prepare a lower solid culture medium; mixing a borrelia burgdorferi bacterium suspension diluted in a gradient way and the BSK storage liquid culture medium, pouring into an upper culture medium so that a macroscopic single colony appears; raising the single colony by using a toothpick, inspecting by using a dark-field microscope to observe a representative burgdorferi bacterium strain. The method disclosed by the invention has the advantages of easiness and fastness for operation and clarity in result. According to the invention, the representative burgdorferi bacterium strain is uniform in genetic background without heterogeneity, suitable for subsequent scientific research and used as a standard strain.
Owner:WENZHOU MEDICAL UNIV
Compositions and methods for culturing spirochetes
The present invention relates to methods for culturing spirochetes, in particular Borrelia burgdorferi. The present invention also provides methods of identifying spirochetes present in a biological sample. The present invention further provides methods of diagnosing diseases cause by a spirochete infection, such as Lyme disease, syphilis, and multiple sclerosis. The present invention further provides methods for identifying spirochete susceptibilities to antimicrobials and antimicrobial compositions and cocktails. The present invention also provides methods for treating subjects suspected of having a spirochete infection.
Owner:ADVANCED LAB SERVICES
Compositions and methods for culturing spirochetes
The present invention relates to methods for culturing spirochetes, in particular Borrelia burgdorferi. The present invention also provides methods of identifying spirochetes present in a biological sample. The present invention further provides methods of diagnosing diseases cause by a spirochete infection, such as Lyme disease, syphilis, and multiple sclerosis. The present invention further provides methods for identifying spirochete susceptibilities to antimicrobials and antimicrobial compositions and cocktails. The present invention also provides methods for treating subjects suspected of having a spirochete infection.
Owner:ADVANCED LAB SERVICES
Vaccines and methods to treat lyme disease in dogs
ActiveUS20160083435A9Bacterial antigen ingredientsAntibody mimetics/scaffoldsBorrelia burgdorferiOspA protein
The instant invention provides an immunogenic composition comprising an antigenic fragment of OspA protein of Borrelia burgdorferi and a chimeric protein containing antigenic fragments of different phylotypes of OspC protein of Borrelia burgdorferi. Vaccines incorporating the immunogenic composition of the invention, as well as methods of preventing Lyme disease in dogs and / or protecting dogs from Lyme disease using the vaccines are also provided.
Owner:ZOETIS SERVICE LLC +1
Dual fluorescent quantitative PCR primer and kit for Escherichia coli disease and Lyme disease
ActiveCN112725488AQuick checkEfficient detectionMicrobiological testing/measurementMicroorganism based processesEscherichia coliBorrelia
The invention discloses a dual fluorescent quantitative PCR primer and a kit for Escherichia coli disease and Lyme disease. The dual fluorescent quantitative PCR primer for the Ehrlichi disease and the Lyme disease comprises a primer for detecting the Ehrlichi disease and a primer for detecting the borrelia burgdorferi; the kit contains the Ehrlichia body disease and Lyme disease dual fluorescent quantitative PCR primer. The kit disclosed by the invention can be used for simultaneously detecting Ehrlichia and Borrelia borreliae, and has the advantages of rapidness in detection, high efficiency, accuracy in quantification, high sensitivity and strong specificity.
Owner:JILIN UNIV
A succinyl-β-cyclodextrin-modified protein chip for Lyme disease detection and its preparation and application
ActiveCN105548577BLow biocompatibilityAvoid false positivesBiological material analysisBiological testingSerum samplesBorrelia burgdorferi
The invention belongs to the field of biomedicine detection, and particularly relates to a protein chip modified by succinyl-beta-cyclodextrin for detecting lyme disease and preparation and application of the protein chip. On the one hand, the invention provides a protein chip for detecting the lyme disease, comprising a solid phase carrier and a capture molecule fixed on the solid phase carrier, wherein the capture molecule contains a principal protein-like sequence expression E protein (VisE) of borrelia burgdorferi variable. In detection, each chip can detect multiple serum samples simultaneously, and is relatively high in flexibility and specificity, so that the occurrence probability of false positive detection and false negative detection among groups is reduced. Compared with a traditional method, the protein chip is applied to screening population at high-risk areas on a large scale; relatively simple, convenient and reliable detection methods can be provided; the screening efficiency as well as the positive detecting rate and accuracy are enhanced.
Owner:ANHUI MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com