Vaccines and methods to treat lyme disease in dogs
a technology for lyme disease and vaccines, applied in the field of veterinary medicine, can solve the problems of not purging the host of spirochetes and overcoming obstacles, and achieve the effect of preventing lyme diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
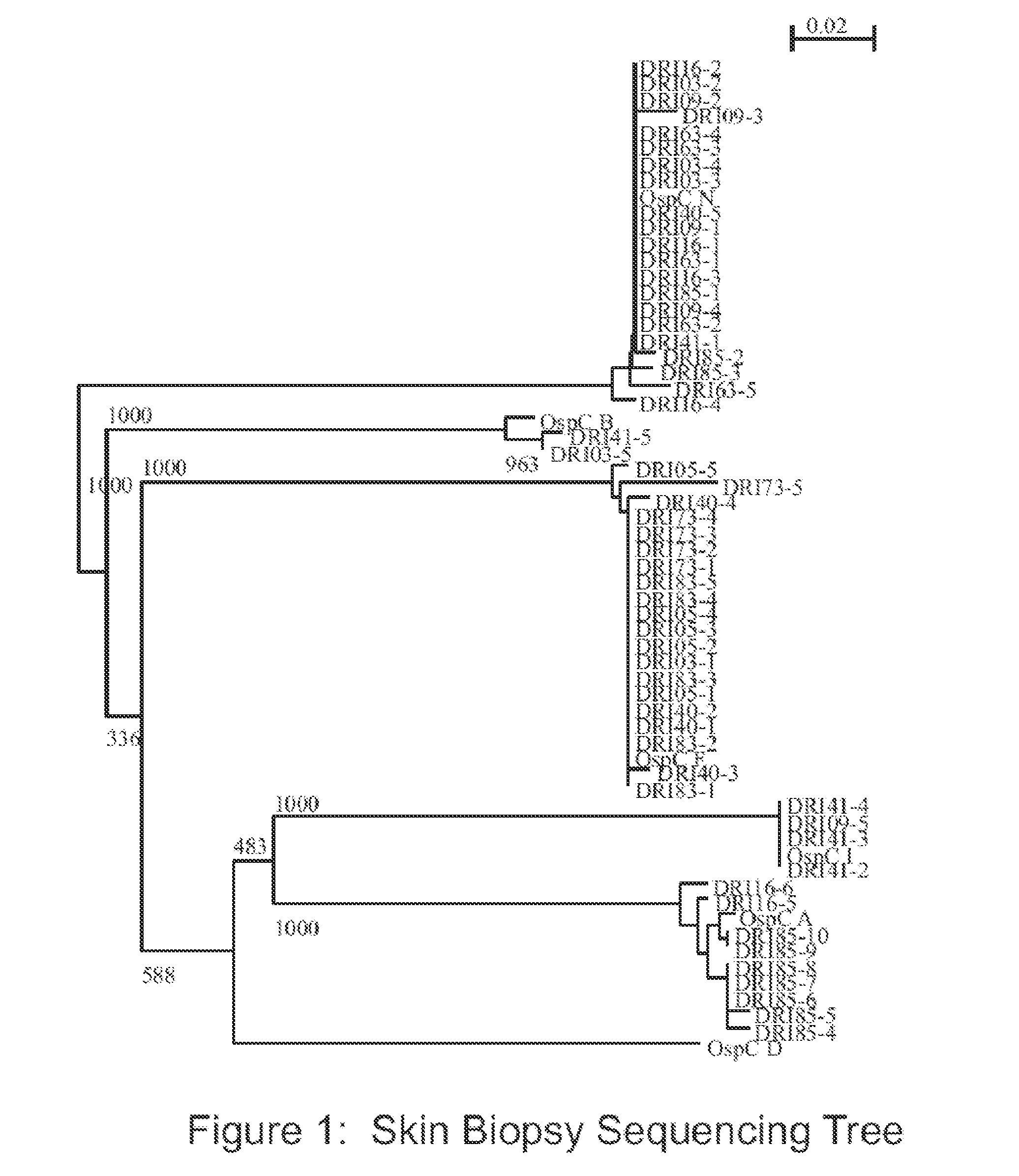
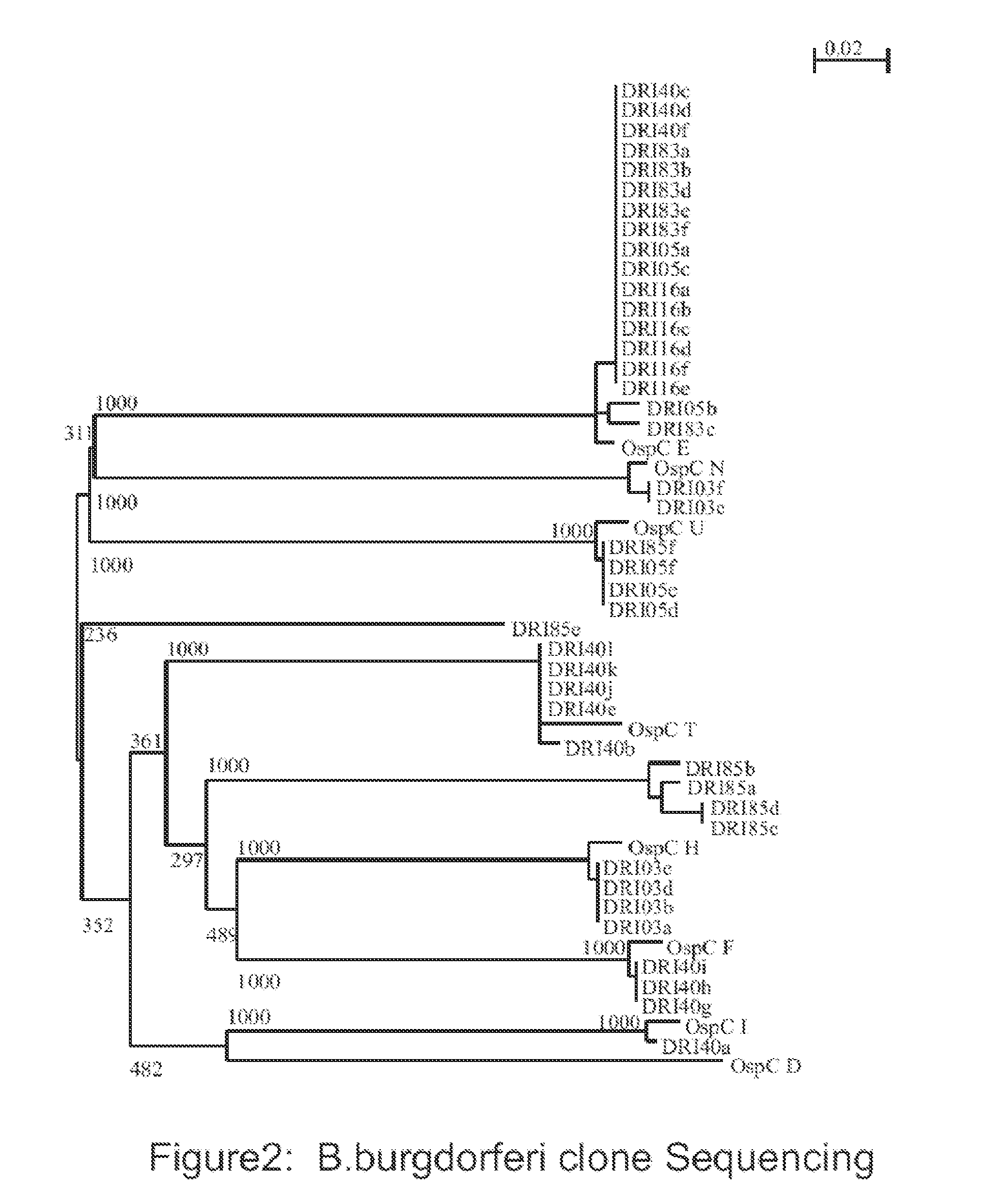
Determination of B. Burgdorferi OspC Phylotypes Associated with Lyme Disease in Dogs
[0093]Adult Ixodes scapularis ticks were collected in southern Rhode Island by flagging. The percentage of ticks infected with B. burgdorferi was determined through direct fluorescent microscopy using standard methods and labeled anti-B. burgdorferi antibody.
[0094]All procedures were conducted in compliance with regulations of the Animal Welfare Act and the dogs were maintained in accordance with Farm Canine Husbandry Standard Operating Procedures. Fifteen purpose-bred dogs of both sexes (7 males, 8 females; 9 to 10 weeks of age; Marshall Bioresources) were assigned identification numbers and divided into four study groups designated as T01 (n=4), T02 (n=4), T03 (n=4) and T04 (n=3). The dogs were fitted with Elizabethan collars and housed in one-over-one condo style cages. One day prior to tick infestation serum was collected from each dog. Dogs in study groups T01, T02, T03 and T04 were infested wit...
example 2
Efficacy of Recombinant Chimeric Borrelia burgdoferi OspC / OspA Vaccines in Dogs
[0105]Thirty dogs, all in good general health, were chosen for the study. Blood samples were collected prior to the initial vaccination. Dogs received one of the following vaccines, as described in Table 2: T01: PBS (control product); T02: ug / ml OspA+30 ug / ml A12CF (SEQ ID NO: 31); T03: 20 ug / ml OspA+30 ug / ml A10CF (SEQ ID NO: 30). (A12CF consists of epitopes from multiple OspC phylotypes, linked together to form a single polypeptide. A10CF also consists of epitopes from multiple OspC phylotypes; its design is similar to that of A12CF.) Dogs were vaccinated twice, at 8 and 11 weeks of age, and then challenged at 14 weeks of age. Following vaccination, dogs were observed for 20 minutes for reactions or abnormalities. Injection sites were observed on Days 1, 2, 3 and 22, 23, 24 for swelling, pain, heat, abscess, drainage, etc. Each dog was fitted with an Elizabethan [E] collar one day prior to placing the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com