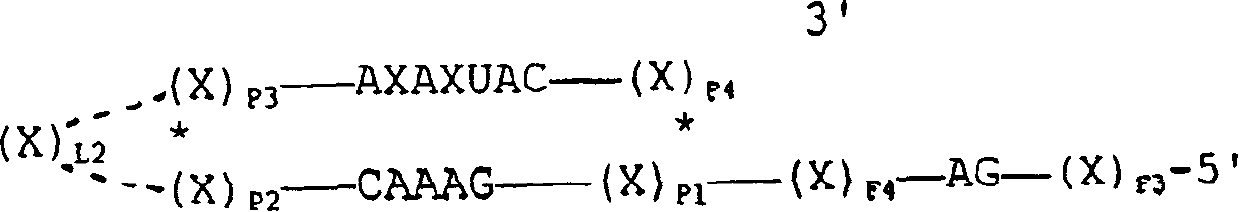Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Feline immunodeficiency virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Feline immunodeficiency virus (FIV) is a lentivirus that affects cats worldwide, with 2.5% to 4.4% of being infected. FIV differs taxonomically from two other feline retroviruses, feline leukemia virus (FeLV) and feline foamy virus (FFV), and is more closely related to human immunodeficiency virus (HIV). Within FIV, five subtypes have been identified based on nucleotide sequence differences coding for the viral envelope (env) or polymerase (pol). FIV is the only non-primate lentivirus to cause an AIDS-like syndrome, but FIV is not typically fatal for cats, as they can live relatively healthily as carriers and transmitters of the disease for many years. A vaccine is available, although its efficacy remains uncertain. Cats will test positive for FIV antibodies after vaccination.
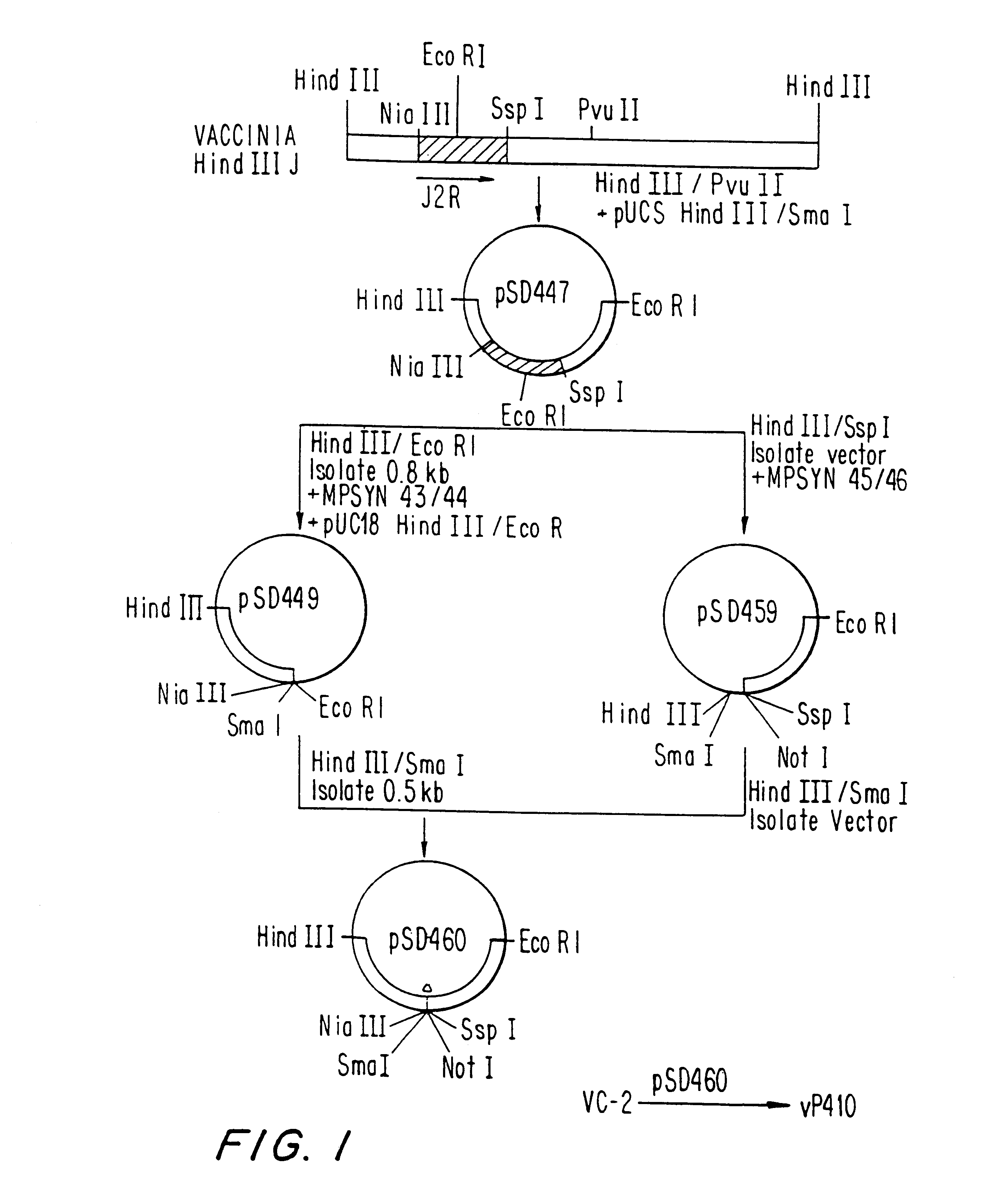
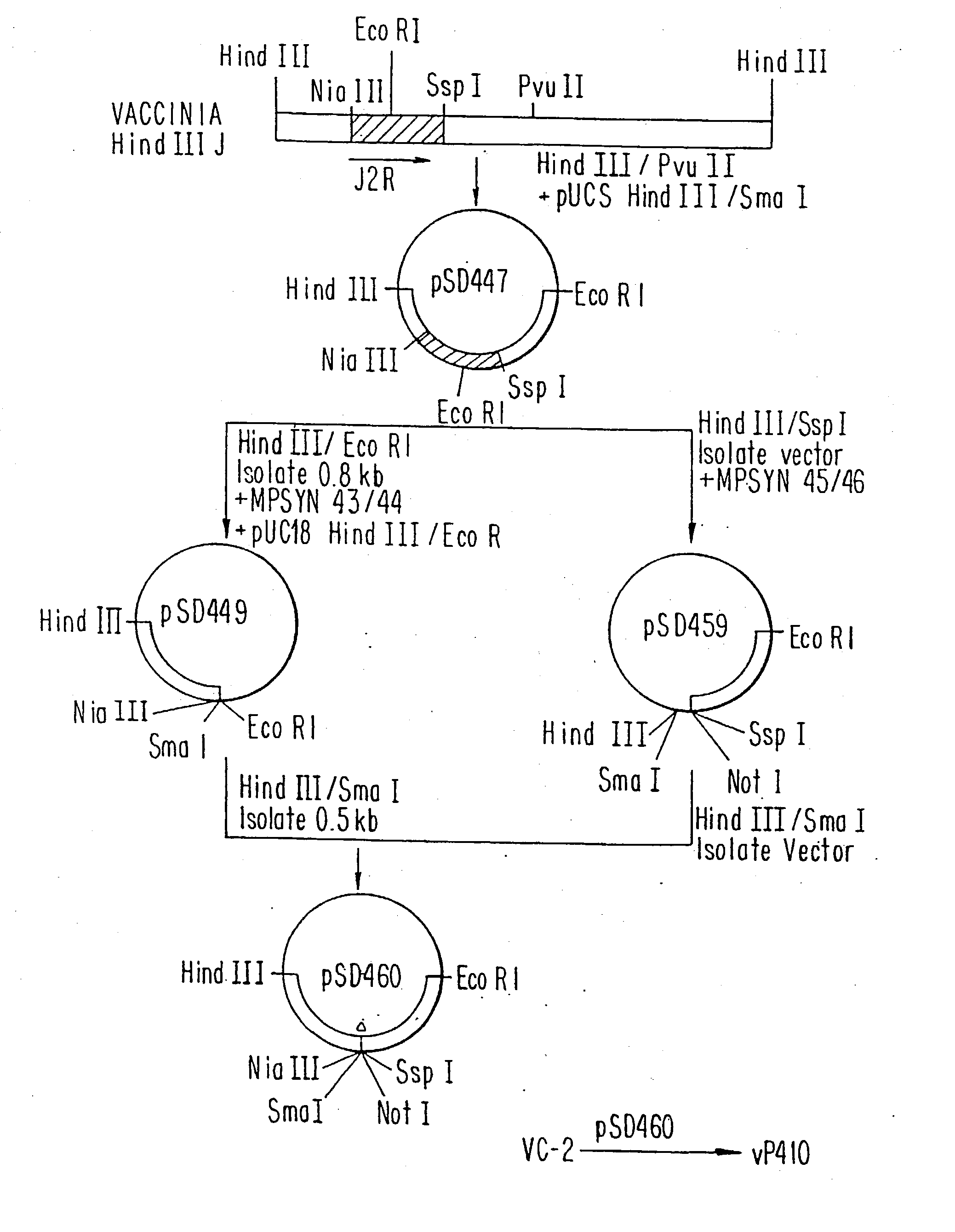
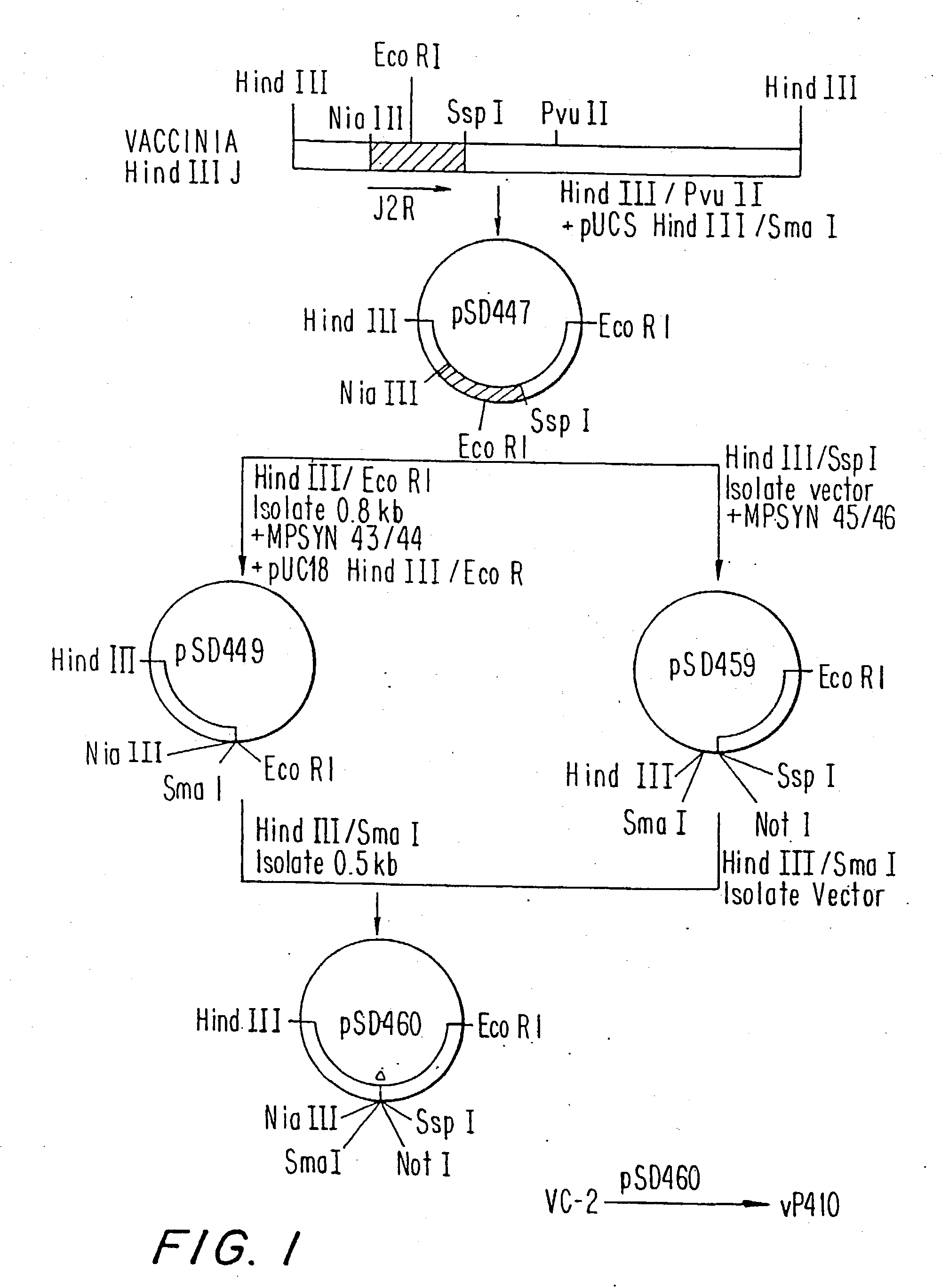
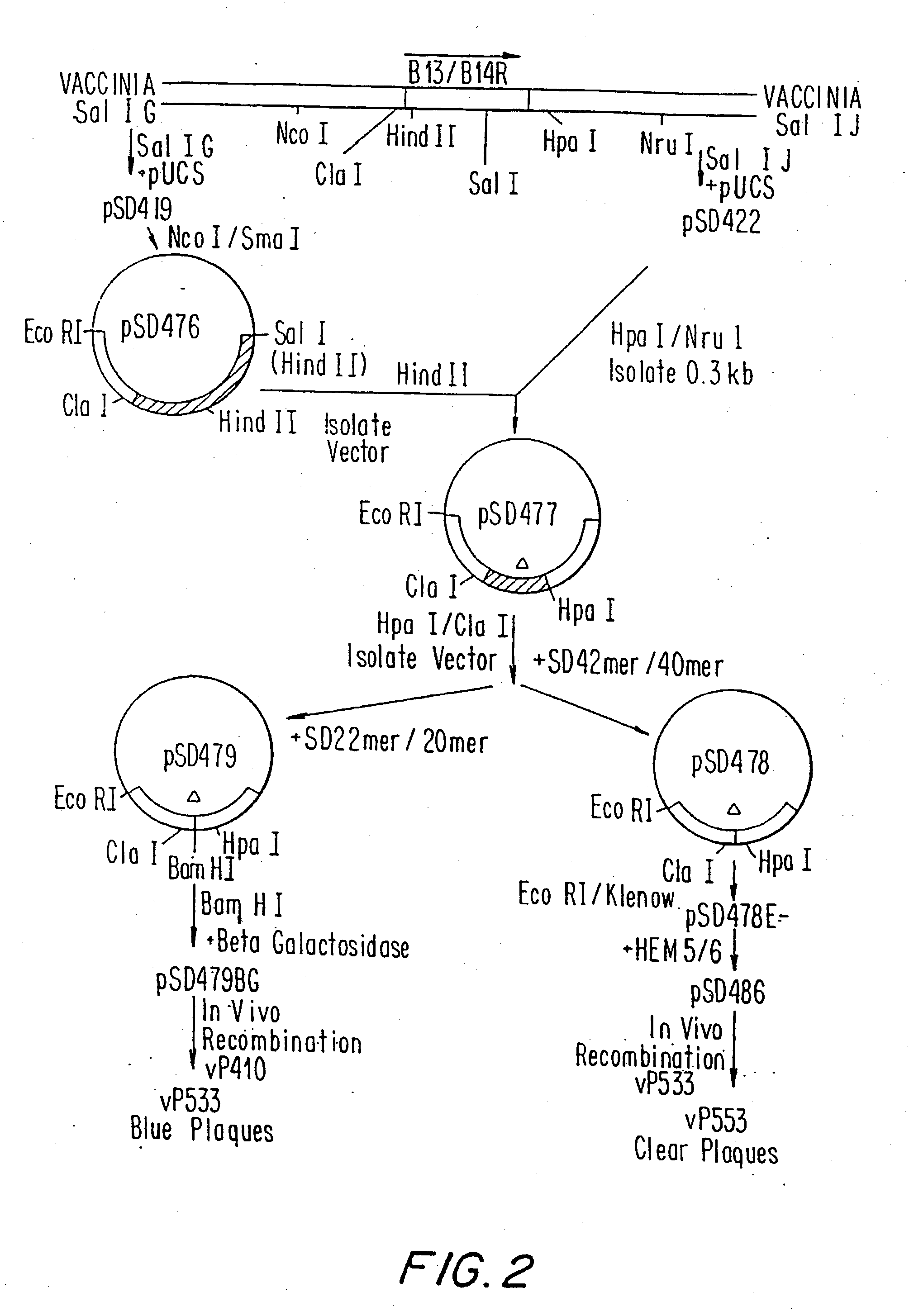
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
ALVAC/FIV constructs
InactiveUS7255862B1Improve security levelViral antigen ingredientsGenetic material ingredientsFeline immunodeficiency virusHeterologous
Recombinants containing and expressing lentivirus, retrovirus or immunodeficiency virus DNA and methods for making and using the same are disclosed and claimed. In an exemplified embodiment, attenuated recombinant viruses containing DNA encoding a feline immunodeficiency virus epitope such as an antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinants can be NYVAC or ALVAC recombinants. The DNA can encode at least one of: Env, Gag, Pol, or combinations thereof such as Gag and Pol or protease or Env, Gag and Pol or protease. The recombinants and gene products therefrom and antibodies generated by them have several preventive, therapeutic and diagnostic uses. DNA from the recombinants are useful as probes or, for generating PCR primers or for immunization. The immunogenicity and protective efficacy of immunization protocols involving ALVAC-FIV and priming with a recombinant canarypox virus ALVAC-FIV vaccine followed by a booster immunization with inactivated FIV-infected celled vaccine (ICV) was evaluated against FIV challenge in cats and the protocol was shown to effectively induce FIV-specific protective immune responses. Further, it was found that immunized cats were fully protected from an initial challenge with a slightly heterologous FIV strain (50CID50) and were partially protected from a second challenge with a distinctly heterologous FIV strain (75CID50) given eight months after the initial challenge without any intervening booster.
Owner:VIROGENETICS
Recombinant FIV glycoprotein 160 and P24 gag protein
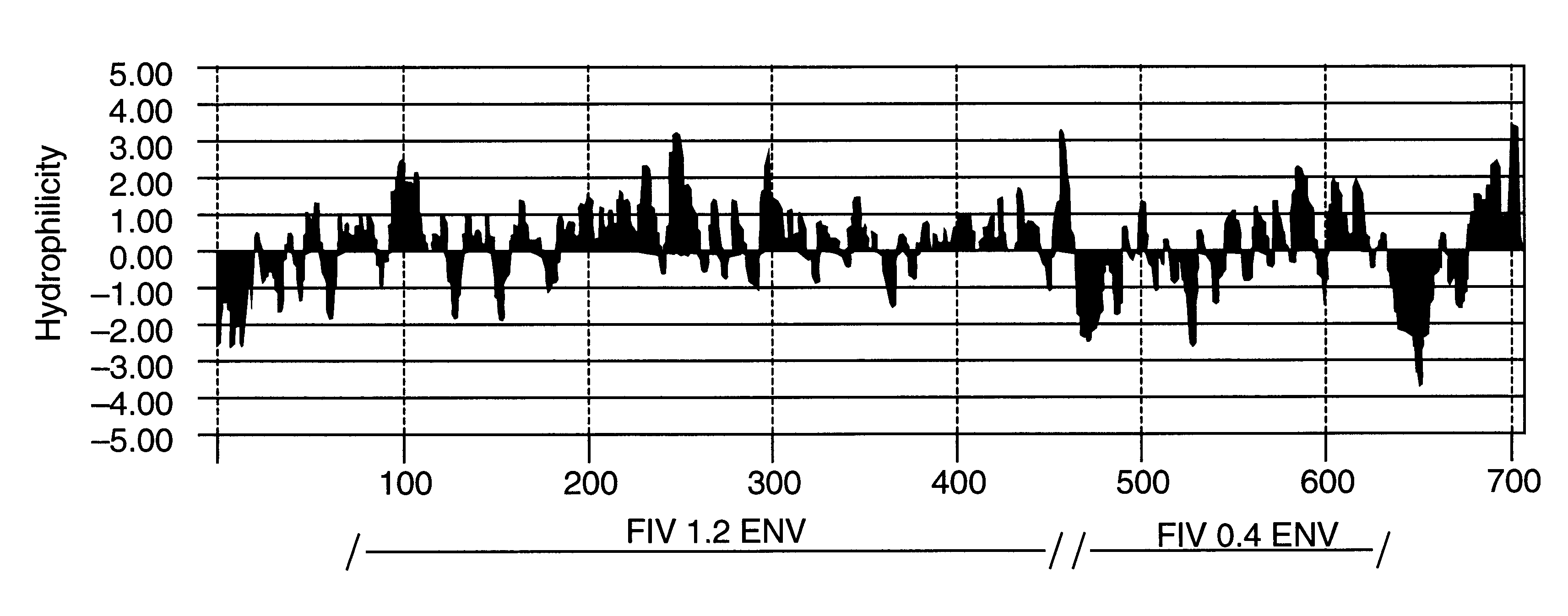
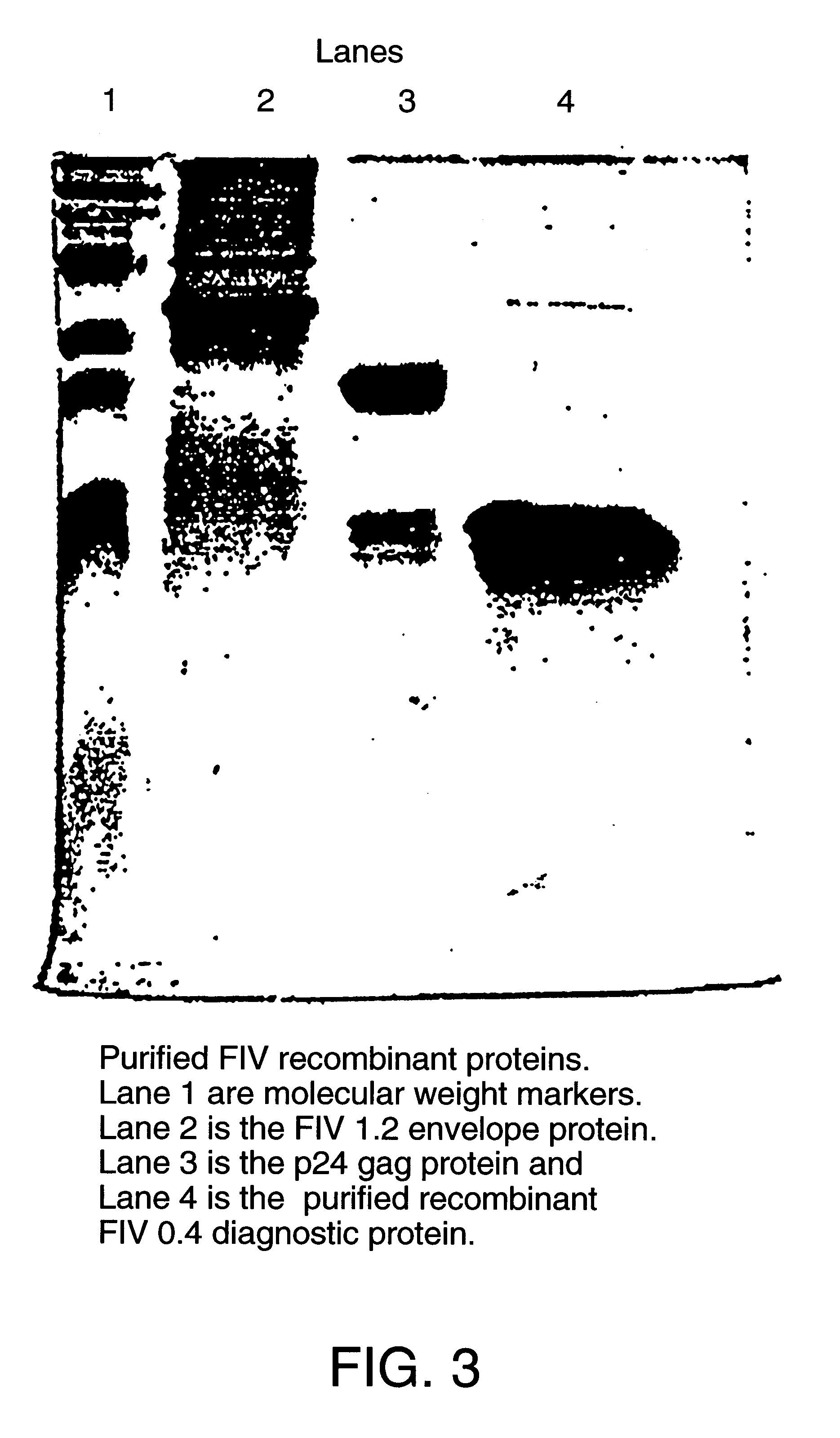
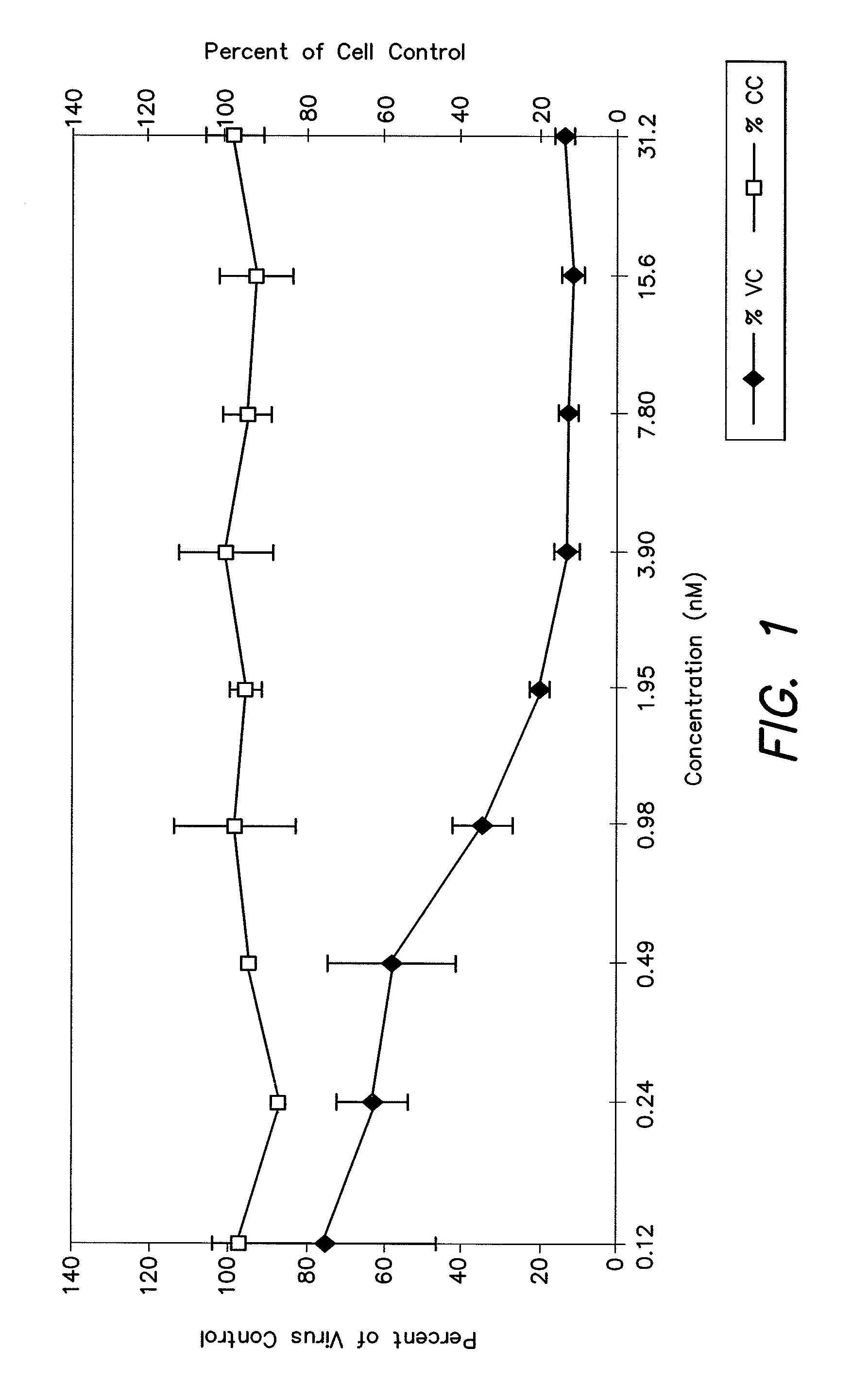
Feline immunodeficiency virus antigens from gp160 envelope protein, gp120 envelope protein and p24 gag protein, useful for the diagnosis, treatment, and prevention of FIV. The invention may also be used to purify FIV.
Owner:AQUILA BIOPHARMLS
Cysteic acid derivatives of Anti-viral peptides
InactiveUS20090088377A1Easy to processEasy to purifyPeptide/protein ingredientsVirus peptidesFeline immunodeficiency virusImmunodeficiency virus
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Modified venom and venom components as anti-retroviral agents
InactiveUS20060088858A1Avoid infectionInhibition of replicationPeptide/protein ingredientsMicrobiological testing/measurementFeline immunodeficiency virusAcquired immunodeficiency
The present invention relates to a class of proteins, and a method for treatment of neurological and viral diseases in humans and animals. More specifically it applies to the treatment of heretofore intractable diseases such as retro-viral infections including human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), bovine immunodeficiency virus (BIV) and equine acquired immunodeficiency virus (EAIV). The method of treatment comprises administering to the subject a disease mitigating amount of a detoxified modified venom composition.
Owner:RECEPTOPHARM
Modified venom and venom components as anti-retroviral agents
InactiveUS20050255097A1Strong antiviral activityAvoid infectionBiocideNervous disorderFeline immunodeficiency virusAcquired immunodeficiency
The present invention relates to a class of proteins, a process of production thereof, and a method for treatment of neurological and viral diseases in humans and animals. More specifically it applies to the treatment of heretofore intractable diseases such as retro-viral infections including human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), bovine immunodeficiency virus (BIV) and equine acquired immunodeficiency virus (EAIV). The
Owner:RECEPTOPHARM
Method for treating feline immunodefiency virus infections
InactiveUS20010007857A1BiocideHydrolysed protein ingredientsFeline immunodeficiency virusImmunodeficiency virus
The present invention discloses a method for treating patients having the FIV associated symptoms or patients carrying or infected by the FIV or having antibodies against the FIV is disclosed using Product R, a peptide-nucleic acid preparation.
Owner:ADVANCED VIRAL RES
Modified venom and venom components as anti-retroviral agents
InactiveUS20060088843A1Strong antiviral activityAvoid infectionPeptide/protein ingredientsMicrobiological testing/measurementFeline immunodeficiency virusAcoleareine
The present invention relates to a class of proteins, a process of production thereof, and a method for treatment of neurological and viral diseases in humans and animals. More specifically it applies to the treatment of heretofore intractable diseases such as retro-viral infections including human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), bovine immunodeficiency virus (BIV) and equine acquired immunodeficiency virus (EAIV). The method of treatment comprises administering to the subject a disease mitigating amount of a detoxified and neurotropically active modified venom composition containing alpha-neurotoxins that target a nicotinic acetylcholine receptor.
Owner:RECEPTOPHARM
Cocktail vaccine for anti immune tolerance and immunodeficiency virus and its application
InactiveCN101057975AGenetic material ingredientsAntiviralsFeline immunodeficiency virusImmunodeficiency virus
The invention provides a cocktail vaccine that is anti-immune tolerance and anti-immunodeficiency virus and its application. Said cocktail vaccine at least comprises two or more elements and any viral antigen recombinant protein and medical shaping. Said element is recombinant DNA and / or recombinant virus; it is chosen from (A) neutralizing antibody, receptor antagonist, membrane fusion blocking agent, cell factor coding sequence, and / or DNA interference sequence; (B) immunoadjuvants coded sequence; (C) antigen gene, viral antigen and immunoadjuvants fusion gene, viral antigen epitope coded sequence, multi-epitope fusion gene and / or viral antigen epitope -MHC-I SCT gene; (D) two seven-peptide multiplexed sequence HR1 and HR2 of membrana capsularis virus fusion protein and / or coded sequence of its polymer HR212. The expression carrier of all antiviral elements comprises recombinant DNA carrier, vaccinia virus carrier and / or adenoviral carrier.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Methods of treating immunodeficiency virus infection
ActiveUS20110305662A1Reducing reservoirBiocidePeptide/protein ingredientsFeline immunodeficiency virusImmunodeficiency virus
The present disclosure provides methods and compositions for reducing the reservoir of latent immunodeficiency virus in an individual, and for treating an immunodeficiency virus infection in an individual.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
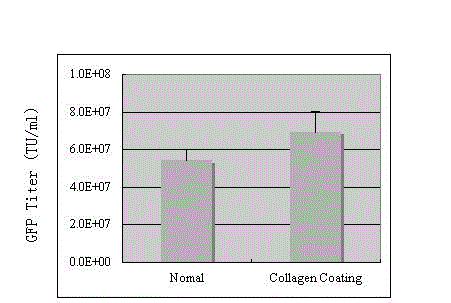
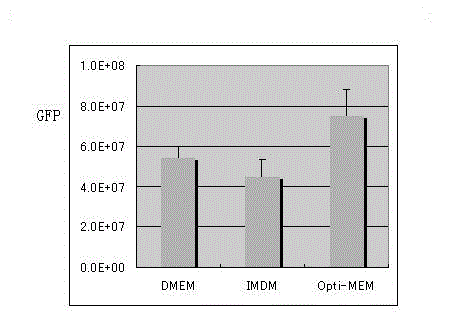
Preparation method of SIV (simian immunodeficiency virus) vector
InactiveCN103060378AIncrease productionImprove practicalityFermentationGenetic engineeringFeline immunodeficiency virusClinical efficacy
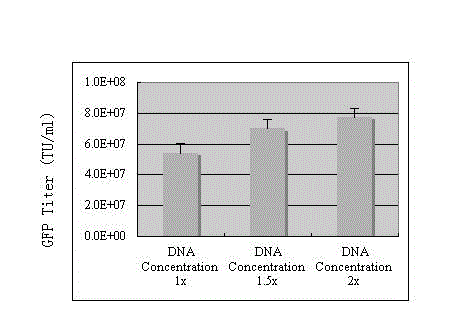
The invention discloses a preparation method of an SIV (simian immunodeficiency virus) vector. A culture dish is treated with collagen by a calcium phosphate coprecipitation transfection process; and in the transfection process, an Opti-MEM culture medium is used, and the DNA (deoxyribonucleic acid) concentration is increased. The invention has the advantages of ingenious concept and low production cost; and the prepared tolvaptan tablet has the advantages of high in-vitro dissolution, high drug bioavailability and favorable clinical curative effect.
Owner:SICHUAN BAILI PHARM CO LTD +1
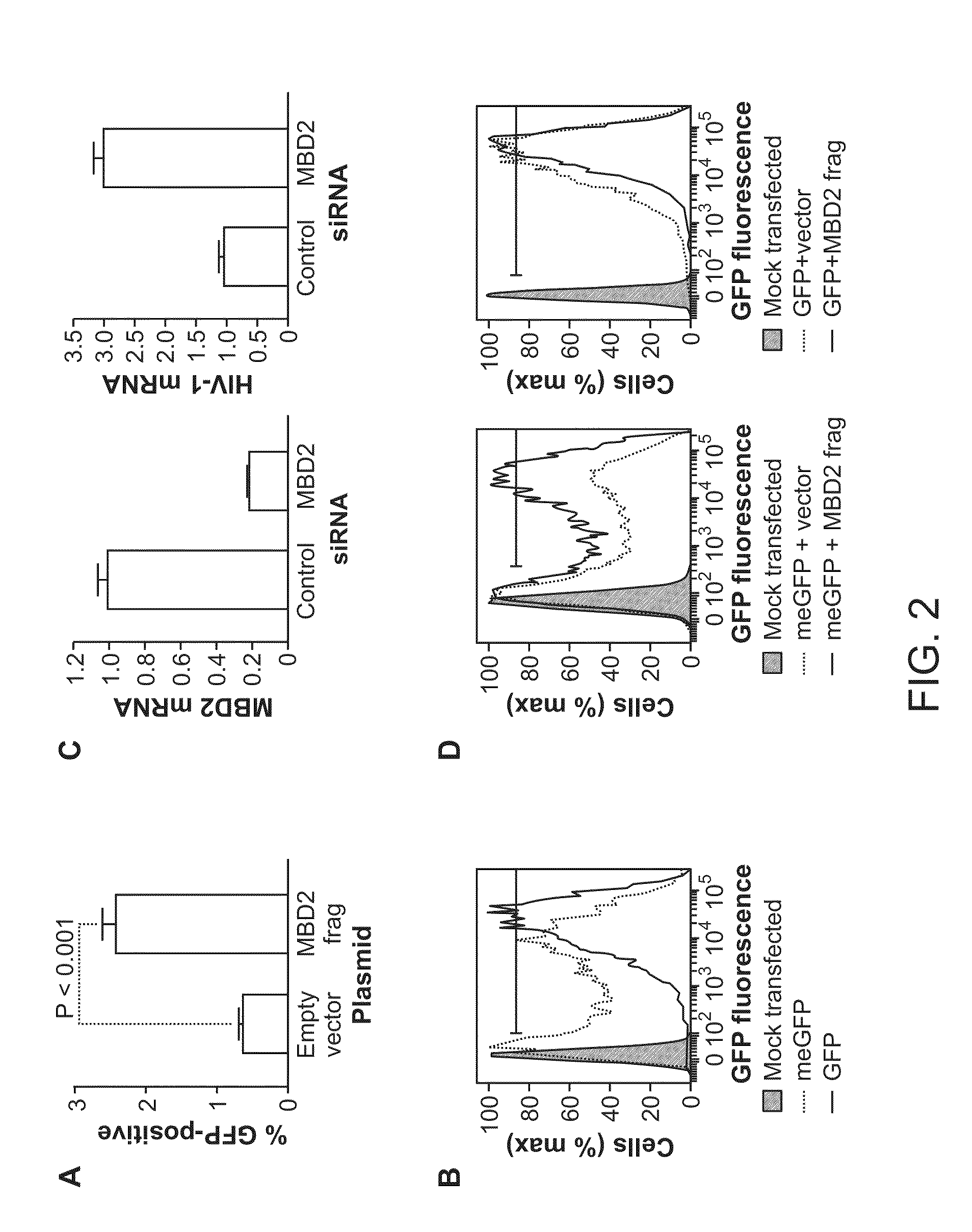
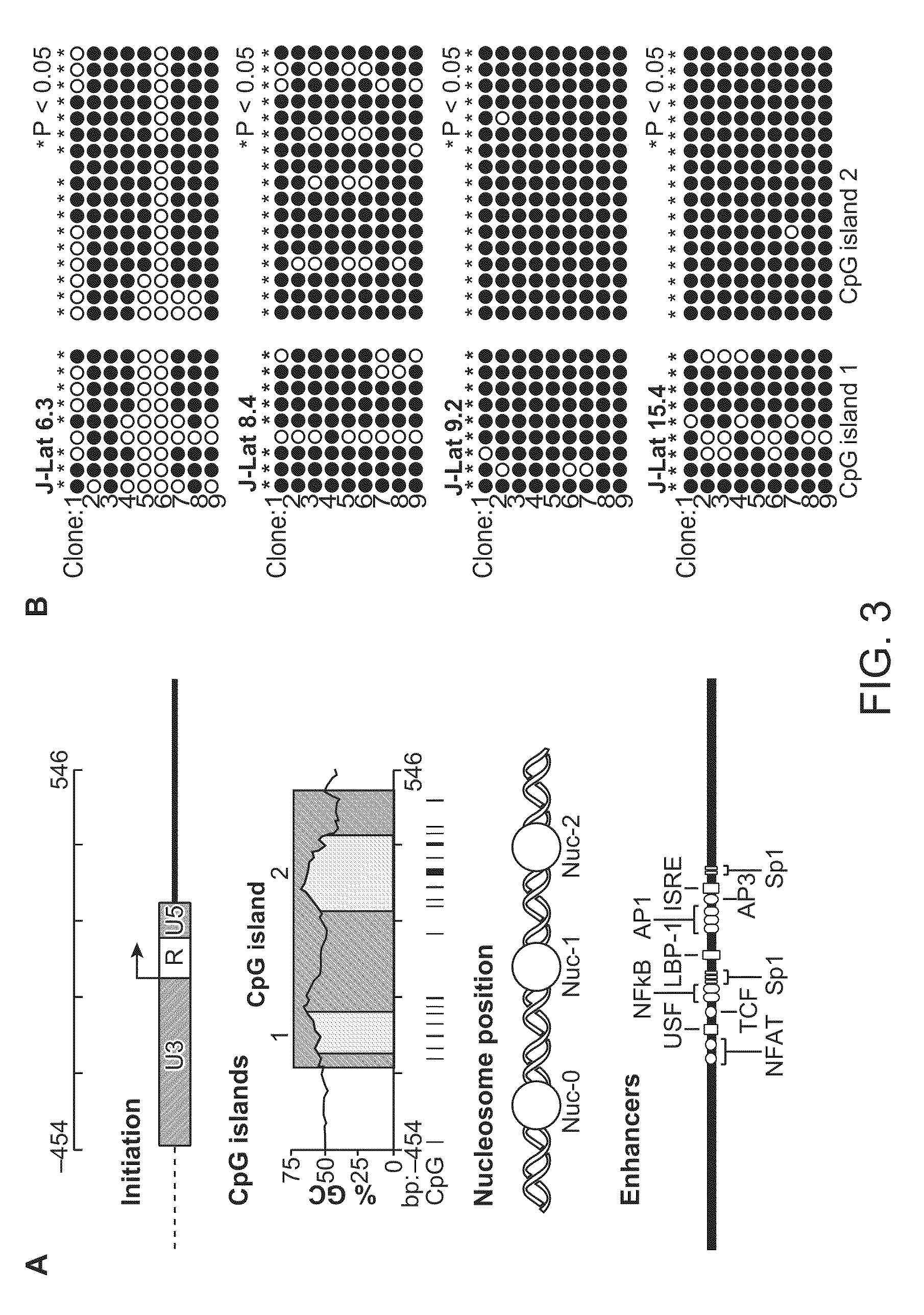
Cell lines with latent immunodeficiency virus and methods of use thereof
ActiveUS7232685B2VectorsGenetically modified cellsFeline immunodeficiency virusImmunodeficiency virus
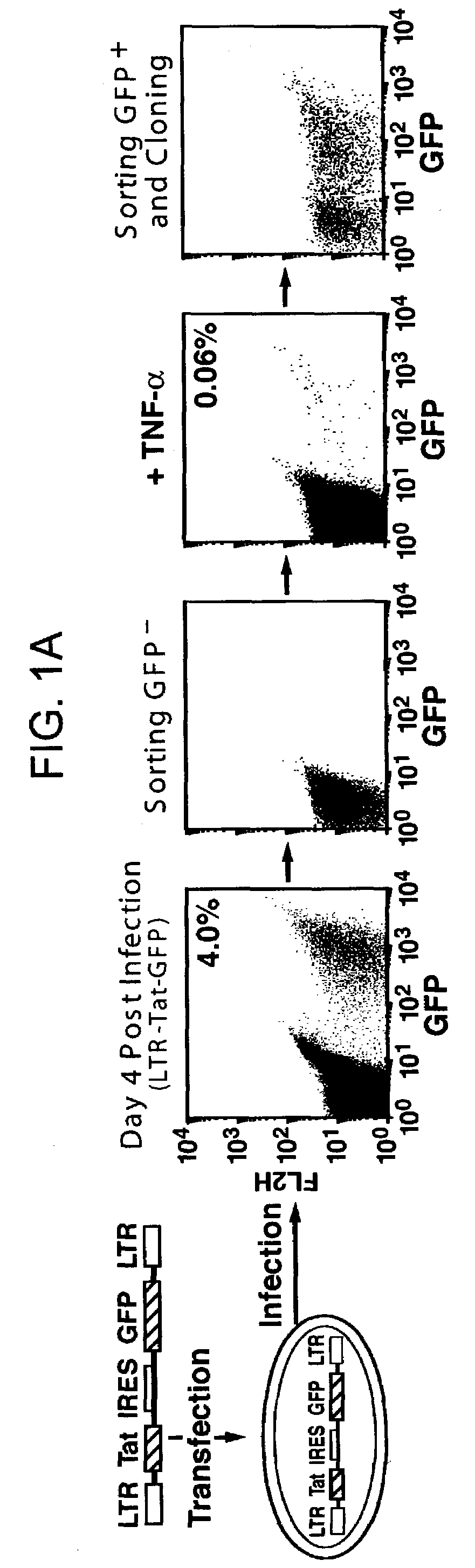
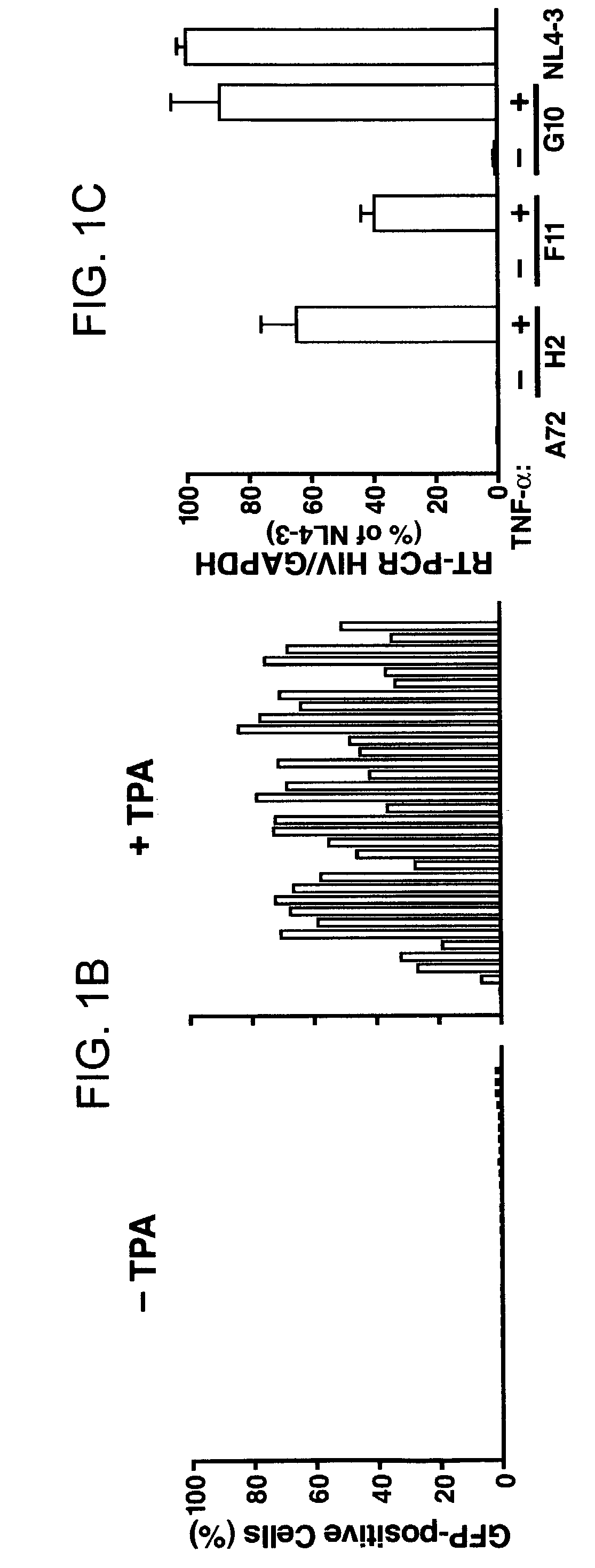
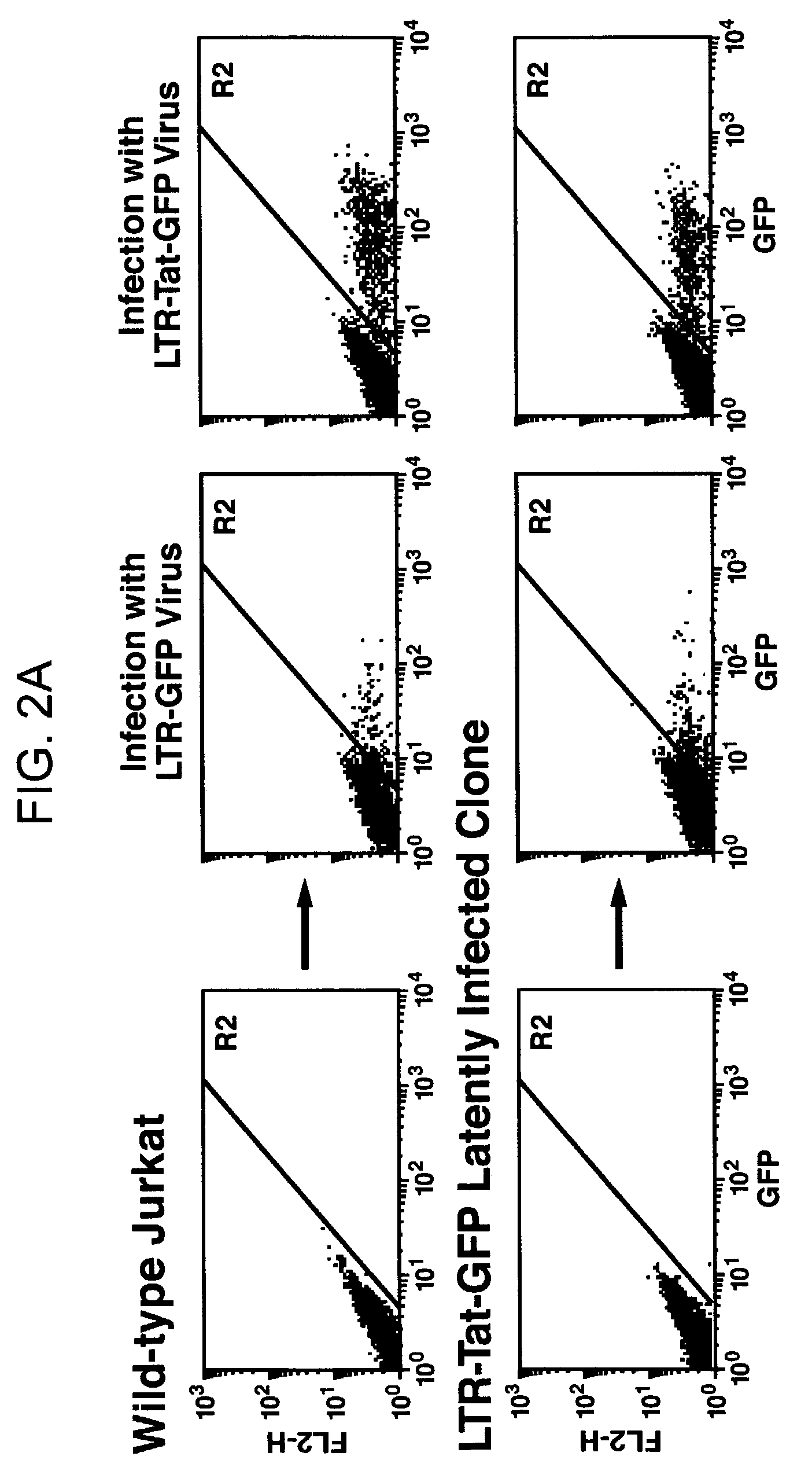
The present invention provides isolated cells that comprise, integrated into the genome of the cell, a transcription-competent immunodeficiency virus or a transcription-competent immunodeficiency virus-based retroviral vector. Under basal in vitro culture conditions, the immunodeficiency virus is latent, and the expression of the latent immunodeficiency virus can be reactivated. The invention further provides methods of making a subject cell. The invention further provides screening methods for identifying agents that activate a latent immunodeficiency virus; and screening method for identifying agents that block reactivation of latent immunodeficiency virus expression in response to T cell activation signals. The invention further provides agents identified in the subject screening assays. The invention further provides methods of treating an immunodeficiency virus infection.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Compounds for use in the treatment of feline retroviral infections
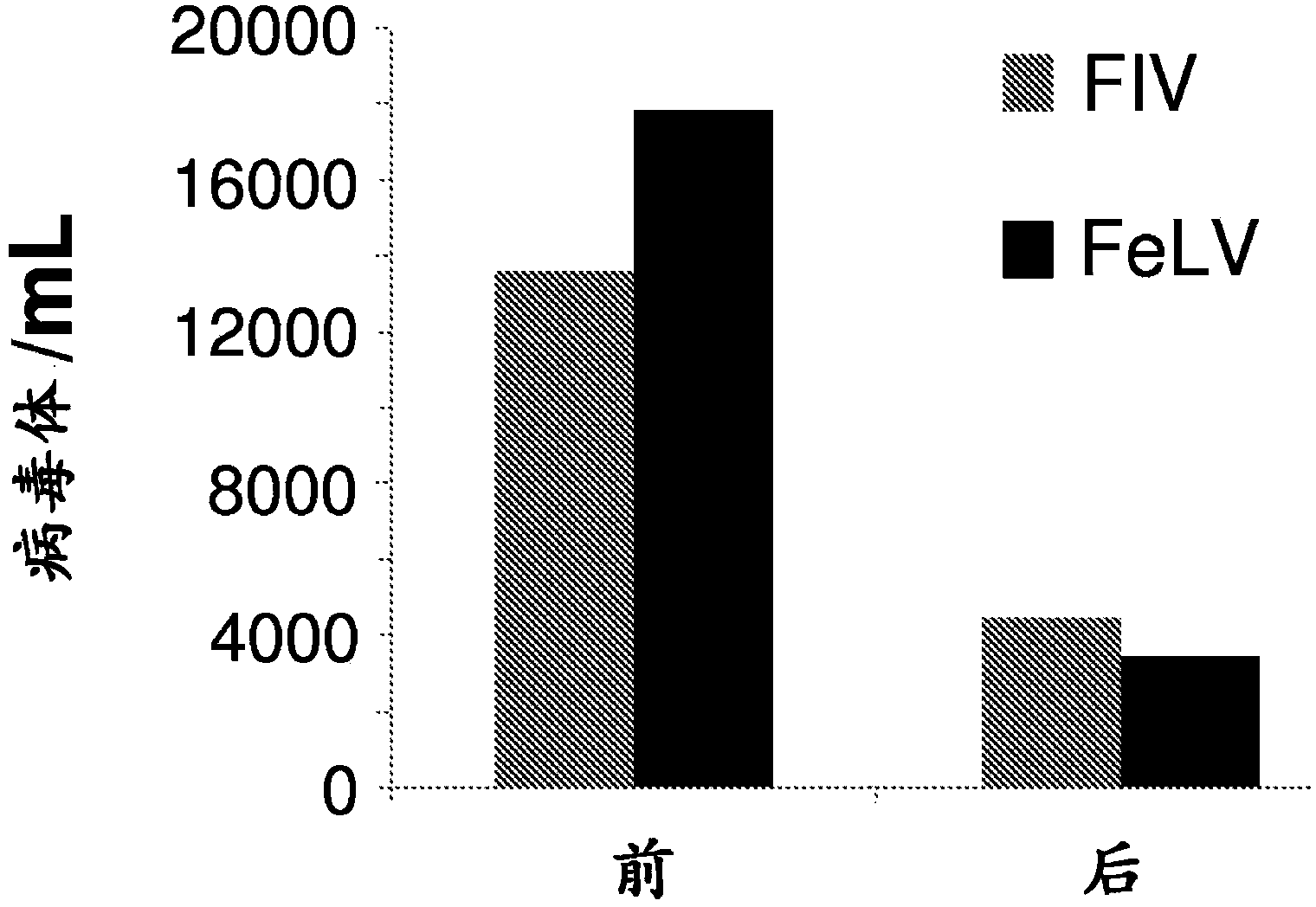
InactiveCN103917237AOrganic active ingredientsGroup 5/15 element organic compoundsFeline immunodeficiency virusImmunodeficiency virus
The present invention relates tocompounds and compositions for use in the treatment or prevention of retroviral infections in felines, in particular cats, and to the use of said derivatives for the manufacture of a medicament for the treatment or prevention of feline leukemia virus (FeLV) infections occurring alone or together with feline immunodeficiency virus (FIV) infections in cats.
Owner:阿拉塔纳医疗有限公司
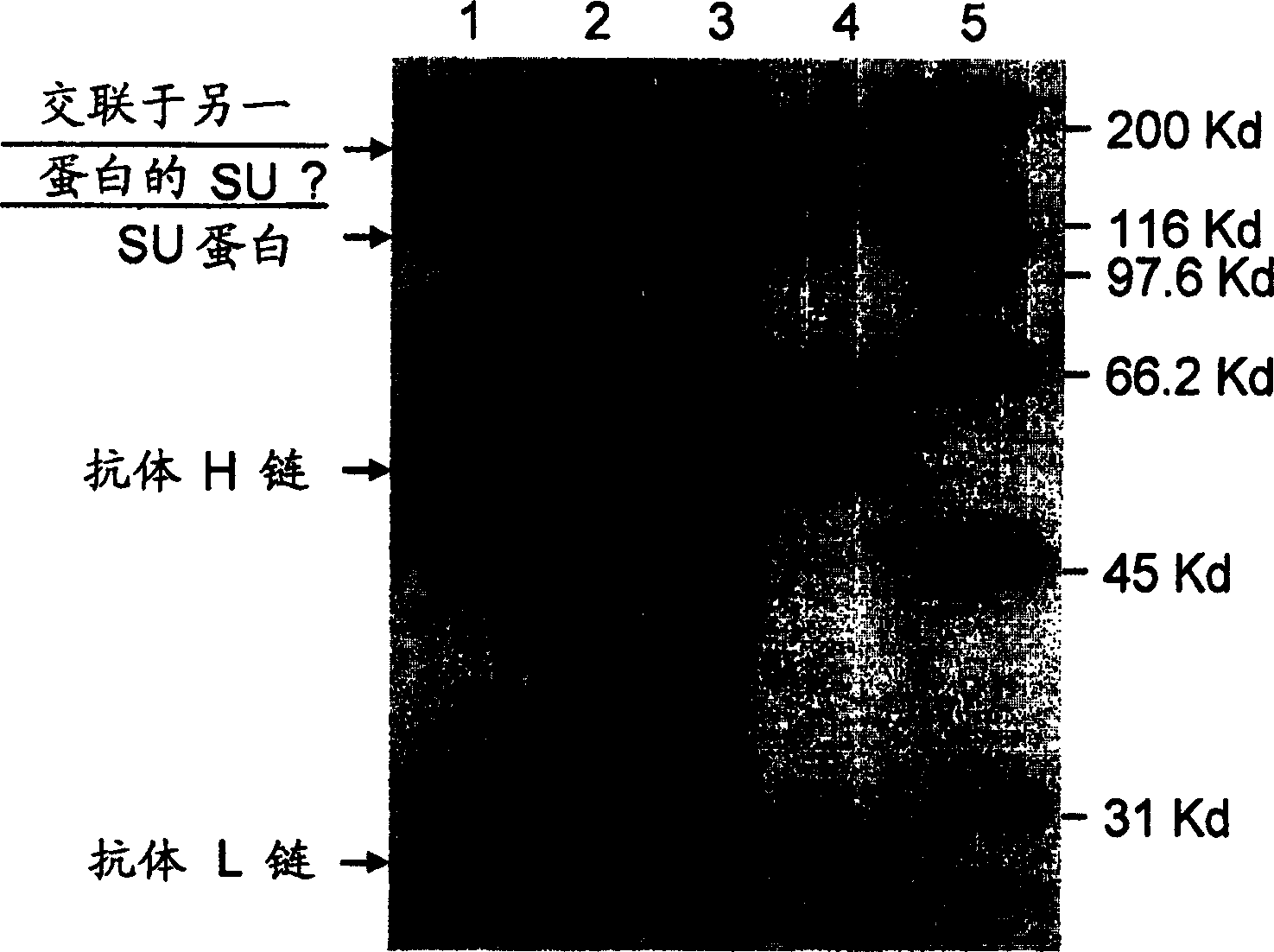
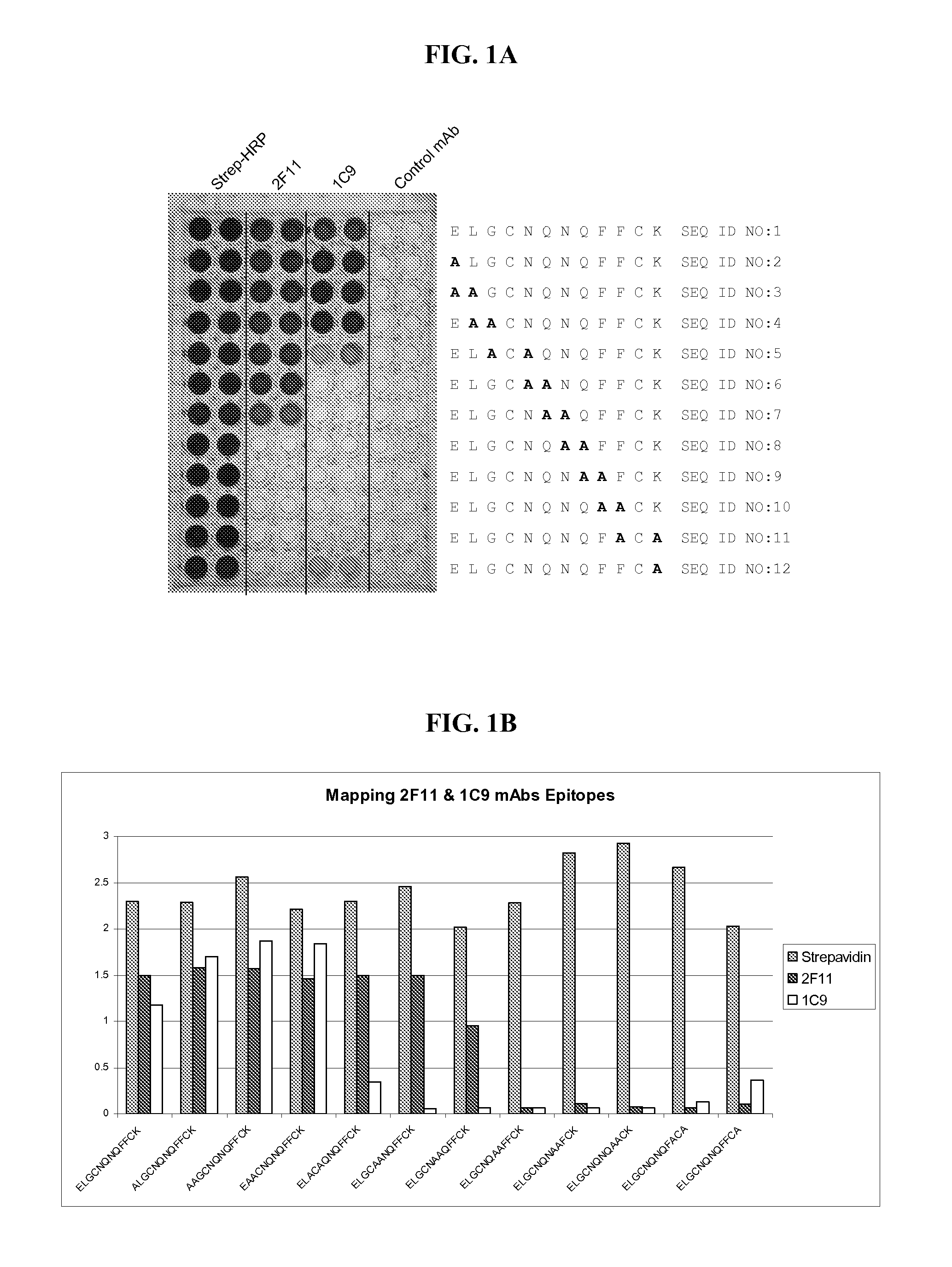
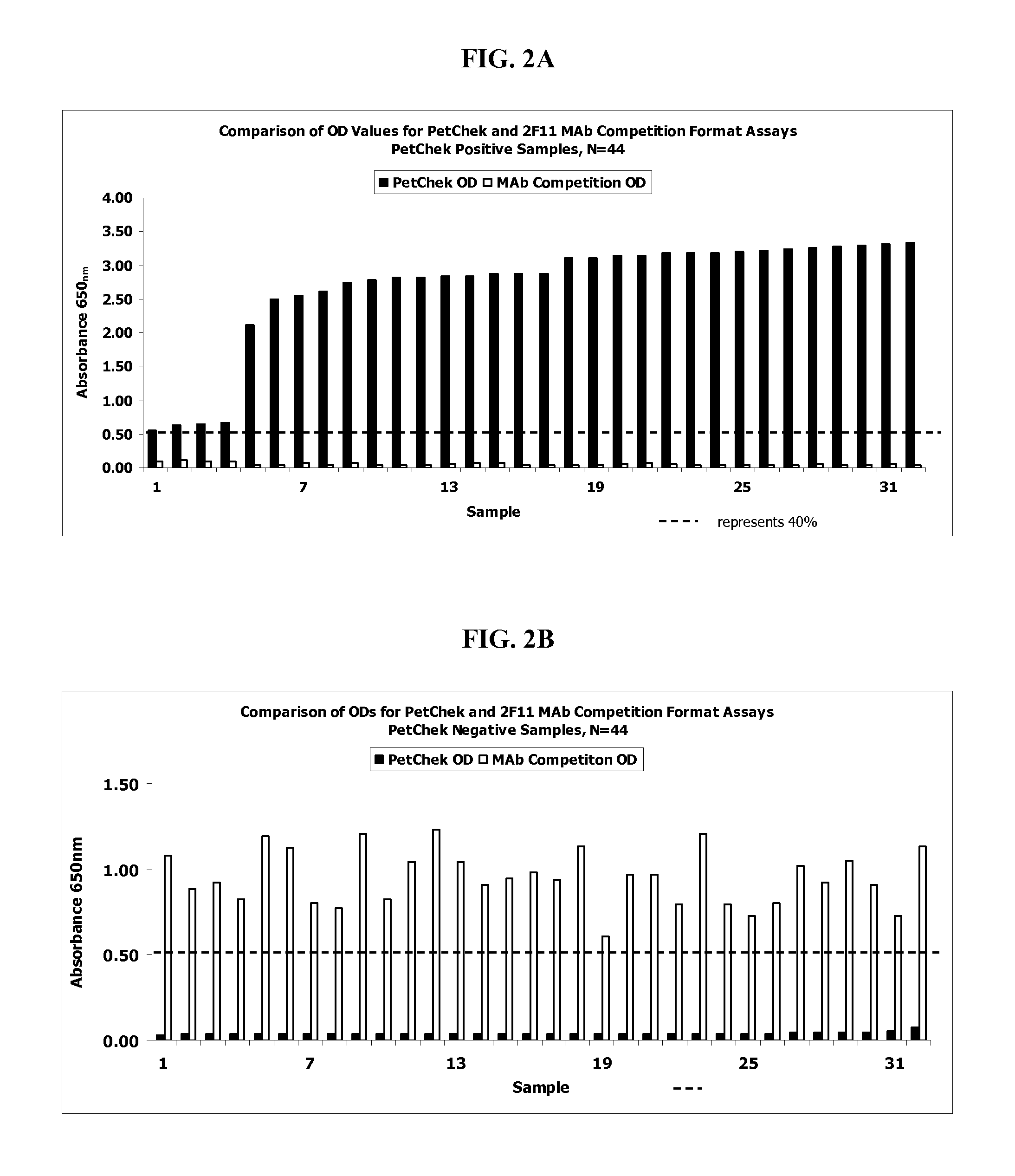
Specific monoclonal antibody against epitope of clygoprotein coding inactivated feline immunodeficiency virus
InactiveCN1528791AViral antigen ingredientsHybrid cell preparationFeline immunodeficiency virusEpitope
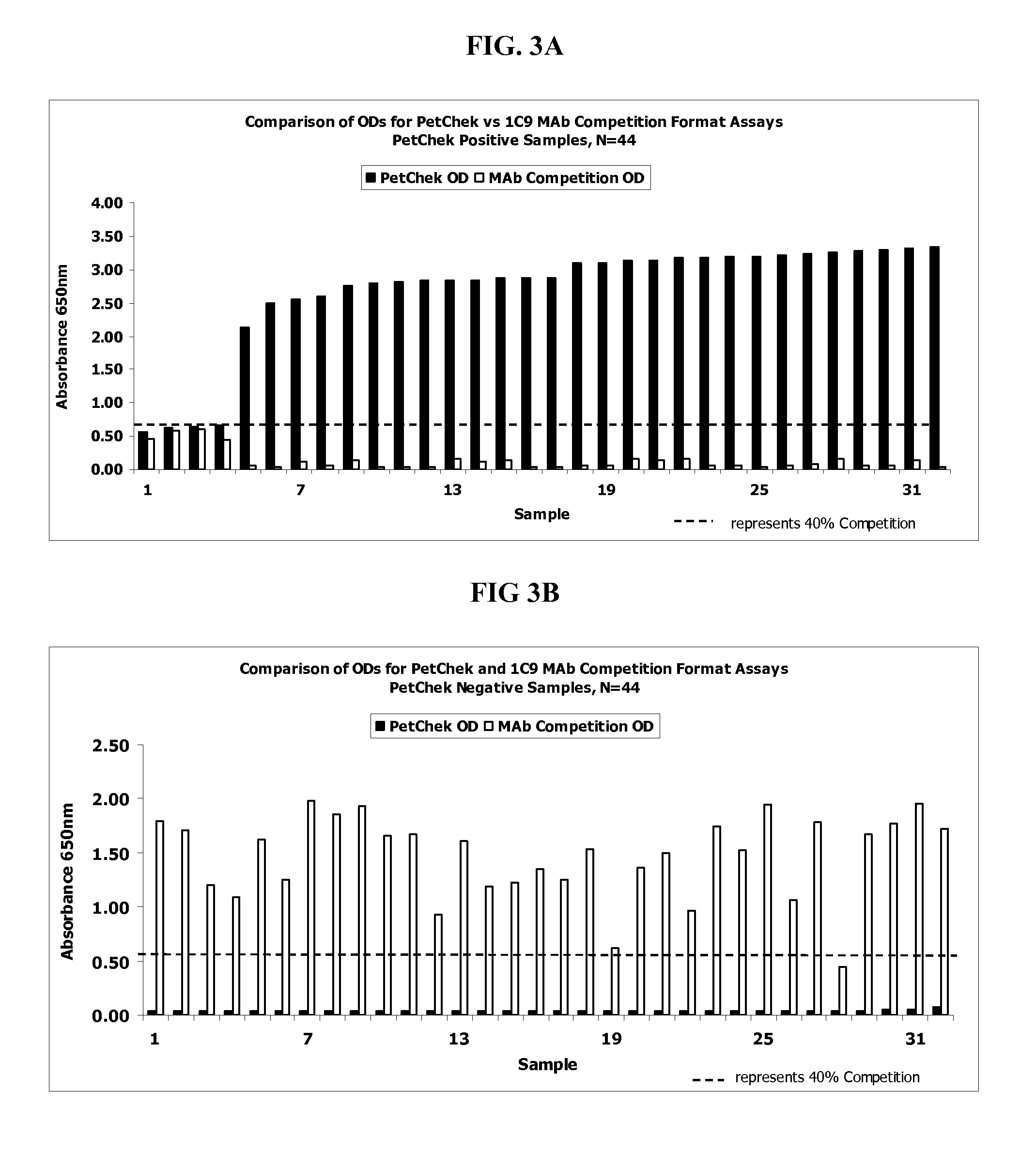
The present invention provides a monoclonal antibody specific for an epitope which is unique to the surface protein component of an inactivated feline immunodeficiency virus (FIV) envelope glycoprotein. Said antibody is useful for the quantification of inactivated FIV or the determination of the potency of an inactivated FIV vaccine.
Owner:ZOETIS W LLC
Detection of feline Immunodeficiency Virus
ActiveUS20130101987A1Microbiological testing/measurementVirus peptidesFeline immunodeficiency virusImmunodeficiency virus
Method, device and kit for the detection of antibodies directed to Feline Immunodeficiency Virus (FIV). The method includes contacting the felid biological sample with FIV env polypeptide and detecting whether the polypeptide substantially binds to the antibody in the biological sample. The method will detect FIV antibodies in a sample from animals that have been naturally infected but the method will not detect antibodies in a sample from animals that have not been infected and that have not been vaccinated with an FIV vaccine after within about the previous five to eight weeks.
Owner:IDEXX LABORATORIES
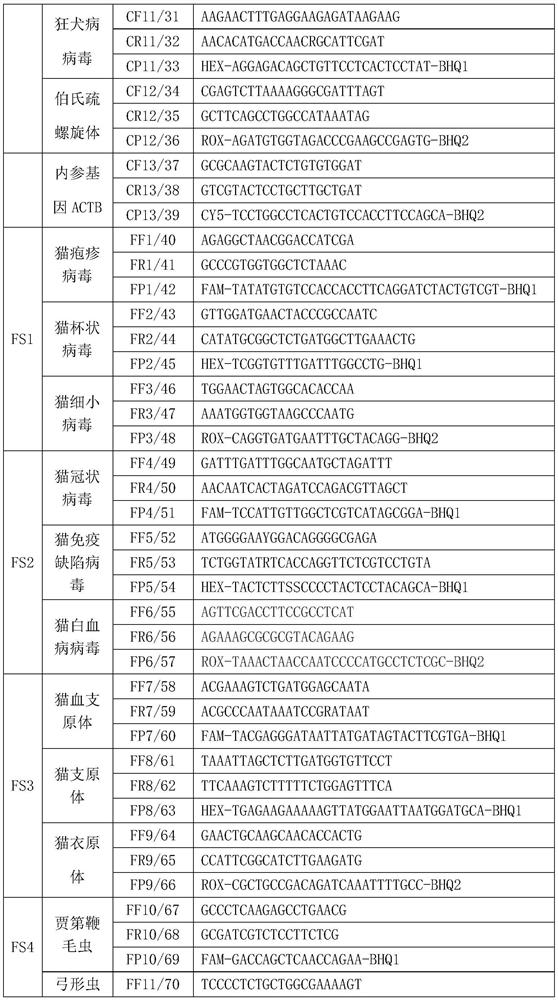
Nucleic acid combination product, detection kit and micro-fluidic chip
PendingCN113061665ABioreactor/fermenter combinationsBiological substance pretreatmentsFeline parvovirusFeline immunodeficiency virus
The invention relates to a nucleic acid combination product, a detection kit and a micro-fluidic chip. The nucleic acid combination product comprises at least two of the following detection primer pairs: a feline parvovirus primer pair, a feline coronavirus primer pair, a feline herpesvirus type I primer pair, a feline calicivirus primer pair, a feline leukemia virus primer pair, a feline immunodeficiency virus primer pair, a feline astrovirus primer pair, a feline rotavirus primer pair, a cat vaccinia virus primer pair and a cat reovirus primer pair. The nucleic acid combination product can be used for detecting at least two feline pathogenic viruses in the sample to be detected at one time, and is good in specificity and relatively high in detection sensitivity.
Owner:深圳市刚竹医疗科技有限公司
AIDS vaccine and preparation method thereof
InactiveCN108823232AImprove immunityStimulate maturityBacteriaViral antigen ingredientsFeline immunodeficiency virusAntigen
The invention discloses a recombinant plasmid. The recombinant plasmid contains a gp120 gene of the monkey immunodeficiency virus and at least one cell wall carrier protein gene of Mycobacterium tuberculosis. The invention also discloses recombinant mycobacterium smegmatis which can stably express the gp120 gene of the monkey immunodeficiency virus and at least one cell wall carrier protein gene of Mycobacterium tuberculosis. The invention also discloses an AIDS vaccine and a preparation method thereof. The AIDS vaccine contains the recombinant mycobacterium smegmatis, is easy to culture, grows fast and saves time, labor, material resources and financial resources. The vaccine comprising three strains of the recombinant mycobacterium smegmatis ZWY2 exhibiting the SIV gp120 antigen proteinon the surface can stably exhibit the SIV gp120 antigen protein on the surface of the mycobacterium smegmatis ZWY2 and has the immunological effect in C57 mice far superior to that of intracellular expression of the antigen protein gp120 and that of the already published AIDS vaccine exhibiting gp120 on the surface based on adenovirus vector construction.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
PCR (Polymerase Chain Reaction) detection kit for cat and/or dog pathogens, detection method and application
PendingCN114277189AShorten detection timeSave human resourcesMicrobiological testing/measurementAgainst vector-borne diseasesFeline parvovirusLeucosis
The invention relates to the technical field of molecular biomedicine, in particular to a PCR (Polymerase Chain Reaction) detection kit for cat and / or dog pathogens, a detection method and application. The kit is used for detecting canine distemper virus, canine influenza A virus, canine parainfluenza virus, canine parvovirus, canine coronavirus, canine rotavirus, canine babesia, canine ascaris, canine Ehrlichia, canine brucella, rabies virus, borrelia burgdorferi, reference gene ACTB, feline herpes virus, feline calicivirus and feline parvovirus. The kit comprises primers and probes of feline coronavirus, feline immunodeficiency virus, feline leukemia virus, feline mycoplasma, feline mycoplasma, feline chlamydia, giardia, toxoplasma, bartonella and reference gene GAPDH, collected DNA and RNA are added into the kit, a real-time fluorescence PCR instrument is adopted for PCR reaction, FAM, HEX, ROX and CY5 fluorescence signals are collected in each cycle, analysis of related pathogens is carried out, and the kit can be used for detecting the feline and the canine. Compared with a traditional detection method, the method has the advantages of higher specificity and higher sensitivity.
Owner:北京迈基诺基因科技股份有限公司
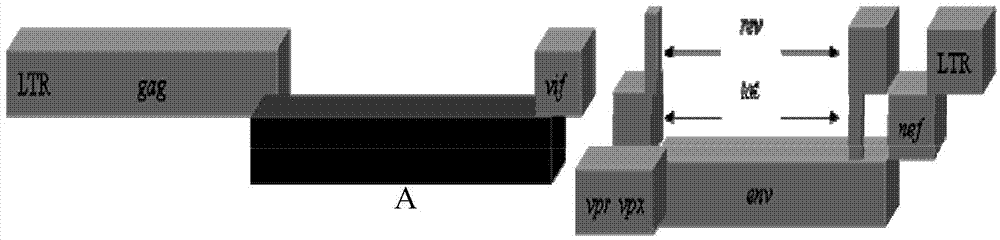
Recombinant immunodeficiency plasmid and virus and application thereof
ActiveCN104263745AEfficient replication capabilityViruses/bacteriophagesVector-based foreign material introductionFeline immunodeficiency virusImmunodeficiency virus
The invention relates to the technical field of biology, and particularly discloses a recombinant immunodeficiency plasmid and virus and application thereof. The immunodeficiency plasmid comprises LTR, gag gene, nucleotide sequence disclosed as SEQ ID No.1, vif gene, vpr gene, vpx gene, tat gene, rev gene, env gene and nef gene. The nucleotide sequence disclosed as SEQ ID NO.1 is introduced to the pathogenic SIVmac239 full-length plasmid frame to construct the immunodeficiency plasmid comprising a plurality of drug target spots, and packaging is performed in the 293T cell to generate immunodeficiency virus. The virus has the capacity for infecting target cells and the capacity of efficient replication, and is applicable to researching human immunodeficiency virus (HIV) in-vivo infection process, pathogeny characteristic, pathogenesis and immunoreaction and screening anti-HIV drugs and anti-HIV drug application strategies.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Immunodeficiency recombinant poxvirus
InactiveUS20030223987A1Improve securityImprove security levelSsRNA viruses negative-senseSsRNA viruses positive-senseCtl epitopeFeline immunodeficiency virus
Owner:PAOLETTI ENZO +4
Method of treating human-immunodeficiency virus (HIV) disease infection
InactiveUS8110204B2Relieving symptoms of HIVReduce developmentBiocideBacterial antigen ingredientsFeline immunodeficiency virusSide effect
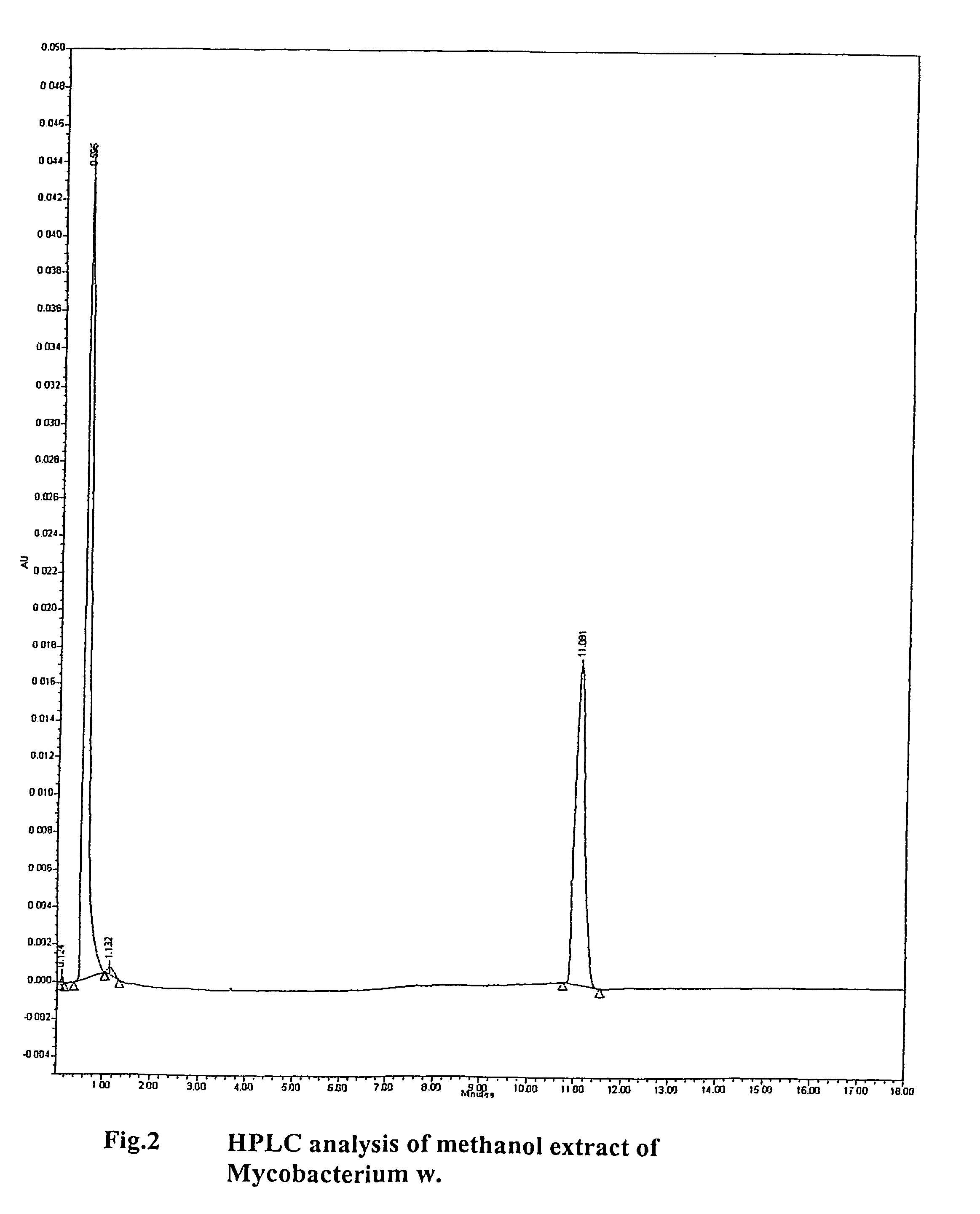
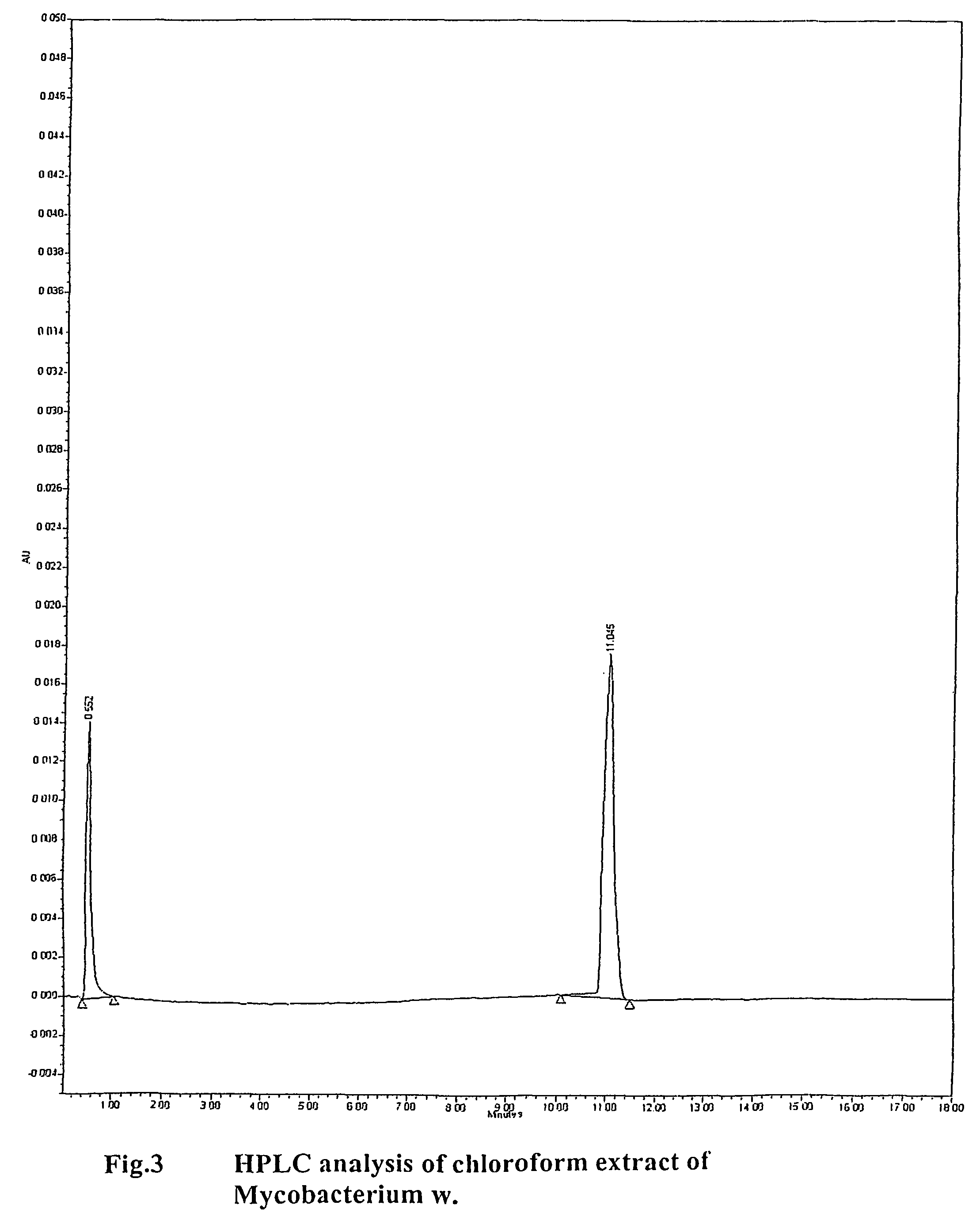
Human Immunodeficiency virus causes depletion of CD4 cells. The depletion of CD4 cells results in decrease in immunity of an infected individual. Due to decrease immunity various opportunistic infections occur. These infections are cause for morbidity and mortality in HIV infected individuals. The treatment of HIV these includes antiretroviral drugs. These drugs have their own side effects and immune reconstitution achieved is delayed and slow. Various attempts have been made to improve CD4 count, use of IL-2 is one of them. It is associated with systemic side effects during the period of its administration. The present invention provides method of using mycobacterium w for the management of HIV. According to present invention mycobacterium w when given intradermally is effective in prophylaxis and treatment of AIDS or AIDS related complex (ARC). It is found to improve immunity as well as CD4 count. It is found to eliminate symptoms like fever, diarrhea. The effect is seen even when no antiretrovirals are used.
Owner:CADILA PHARMA
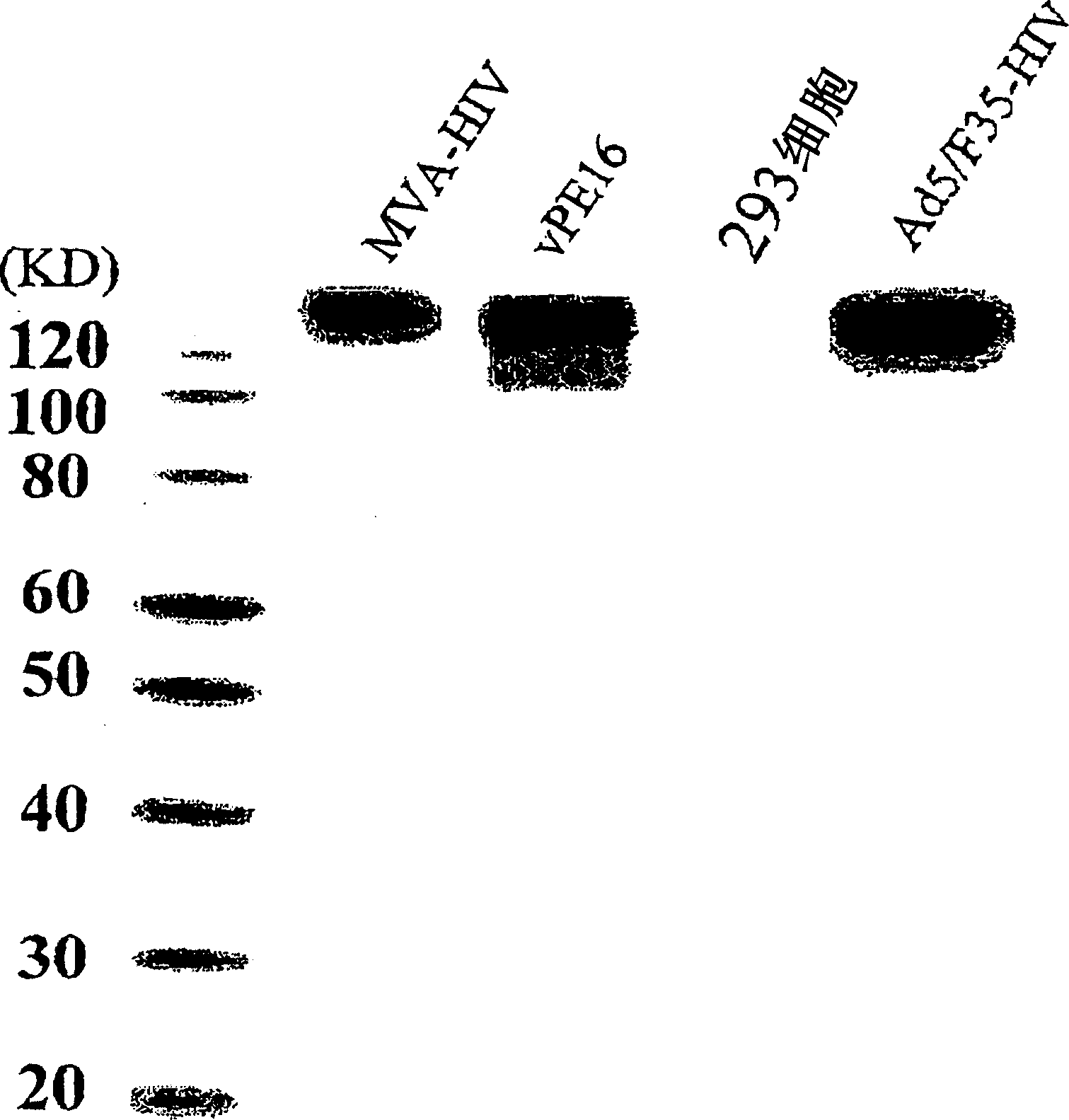
Chimeric type 5/type 11 or type 35 adenovirus vector for preventing infection with antihuman immunodeficiency virus
InactiveCN1886511ALow toxicityHigh affinitySsRNA viruses positive-sensePeptide/protein ingredientsFeline immunodeficiency virusImmunodeficiency virus
A chimeric type 5 / type 11 or type 35 adenovirus vector, wherein a gene encoding the envelope protein of human immunodeficiency virus or its mutant having an equivalent function is integrated into a nonproliferation type 5 adenovirus in such a manner as allowing the expression and a gene encoding the fiber protein of the type 5 adenovirus is substituted by a gene encoding the fiber protein of a type 11 or type 35 adenovirus or a mutant having an equivalent function, has a lessened toxicity on the liver and can induce an extremely potent HIV-specific immune response. Thus, it is highly efficacious as a drug for preventing HIV-infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Long lasting fusion peptide inhibitors for HIV infection
InactiveUS20110166061A1Prolong half-life in vivoBiocidePeptide/protein ingredientsFeline immunodeficiency virusImmunodeficiency virus
The present invention is concerned with This invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Viral chemokine-antigen fusion proteins
InactiveUS7897152B2Organic active ingredientsVirusesFeline immunodeficiency virusImmunodeficiency virus
The present invention relates to a vaccine for increasing the immunogenicity of a tumor antigen thus allowing treatment of cancer, as well as a vaccine that increases the immunogenicity of a viral antigen, thus allowing treatment of viral infection, including immunodeficiency virus (HIV) infection. In particular, the present invention provides a fusion protein comprising a viral chemokine fused to either a tumor antigen or viral antigen which is administered as either a protein or nucleic acid vaccine to elicit an immune response effective in treating cancer or effective in treating or preventing viral infection.
Owner:UNITED STATES OF AMERICA
Immuno comb array test paper for detecting antibody of SIV (simian immunodeficiency virus) as well as preparation method and application
InactiveCN105647913ASimple customs clearance inspectionFast customs clearance detectionBiological material analysisNucleic acid vectorFeline immunodeficiency virusSerum ige
The invention mainly relates to immuno comb array test paper for detecting an antibody of an SIV (simian immunodeficiency virus) as well as a preparation method and an application. A recombinant plasmid pGEX-4T-SIV for establishment of an immuno comb array is shown in the sequence table Seq No.3. An upper primer SIVF in a primer pair for establishment of the recombinant plasmid is shown in the sequence table Seq No.1, and a lower primer SIVR of the primer pair is shown in the sequence table Seq No.2. The establishment method comprises the following steps: the SOE (splicing overlapping extension)-PCR (polymerase chain reaction) primer pair is designed according to the nucleotide sequence of the SIV, SIV genes are amplified through SOE-PCR, and the recombinant plasmid pGEX-4T-SIV is established; finally, the immuno comb array for quickly detecting the antibody in blood or serum of an experimental monkey is prepared with a film chromatographic technique according to the enzyme linked immunosorbent assay principle and has good stability, specificity, sensitivity and repeatability.
Owner:海南出入境检验检疫局检验检疫技术中心
Cocktail vaccine for anti immune tolerance and immunodeficiency virus and its application
InactiveCN101057975BGenetic material ingredientsAntiviralsFeline immunodeficiency virusImmunodeficiency virus
The invention provides a cocktail vaccine that is anti-immune tolerance and anti-immunodeficiency virus and its application. Said cocktail vaccine at least comprises two or more elements and any viral antigen recombinant protein and medical shaping. Said element is recombinant DNA and / or recombinant virus; it is chosen from (A) neutralizing antibody, receptor antagonist, membrane fusion blocking agent, cell factor coding sequence, and / or DNA interference sequence; (B) immunoadjuvants coded sequence; (C) antigen gene, viral antigen and immunoadjuvants fusion gene, viral antigen epitope coded sequence, multi-epitope fusion gene and / or viral antigen epitope -MHC-I SCT gene; (D) two seven-peptide multiplexed sequence HR1 and HR2 of membrana capsular is virus fusion protein and / or coded sequence of its polymer HR212. The expression carrier of all antiviral elements comprises recombinant DNA carrier, vaccinia virus carrier and / or adenoviral carrier.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
AIDS vaccines
InactiveUS20080267993A1Virus peptidesAntiviralsFeline immunodeficiency virusPrimate immunodeficiency viruses
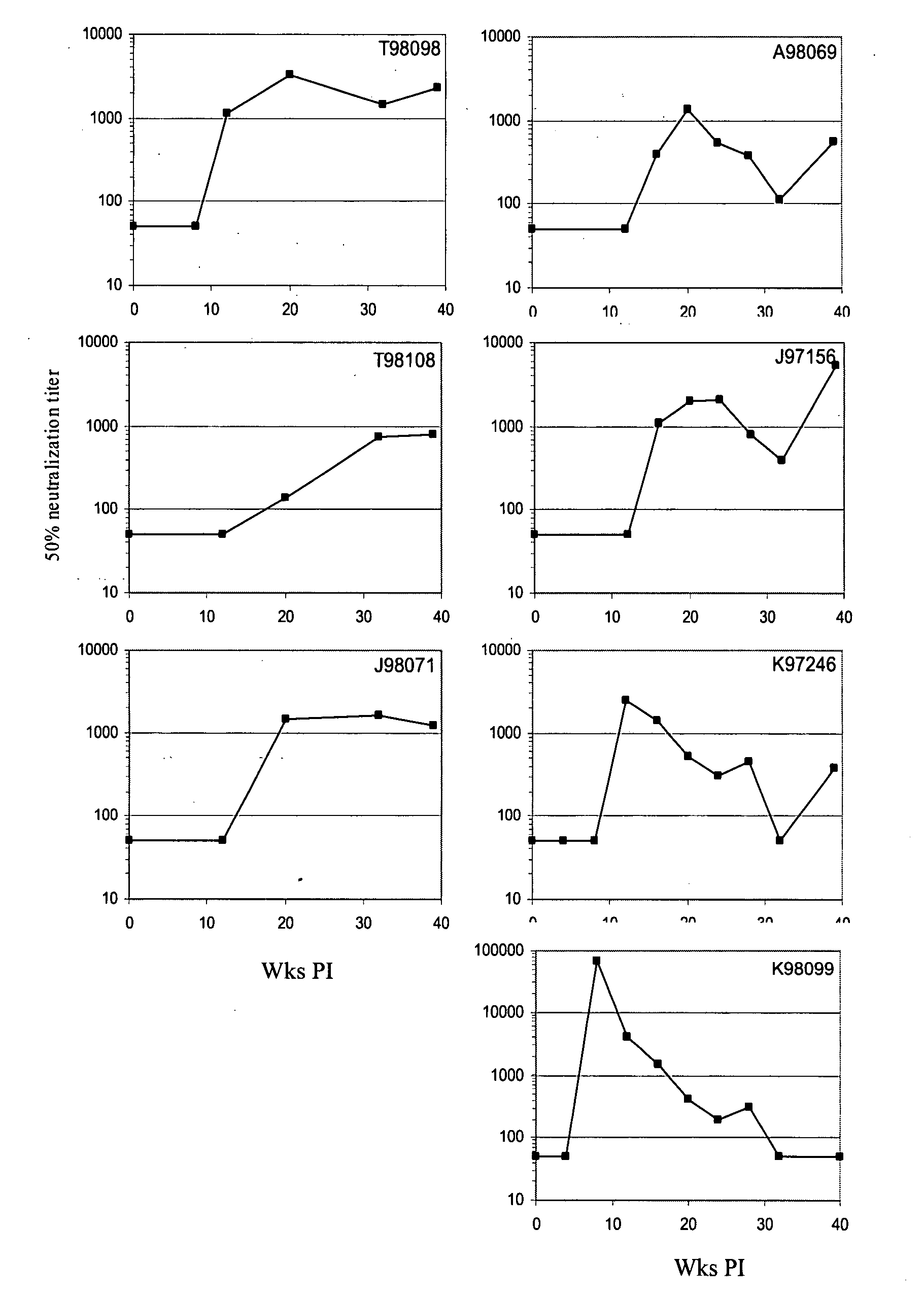
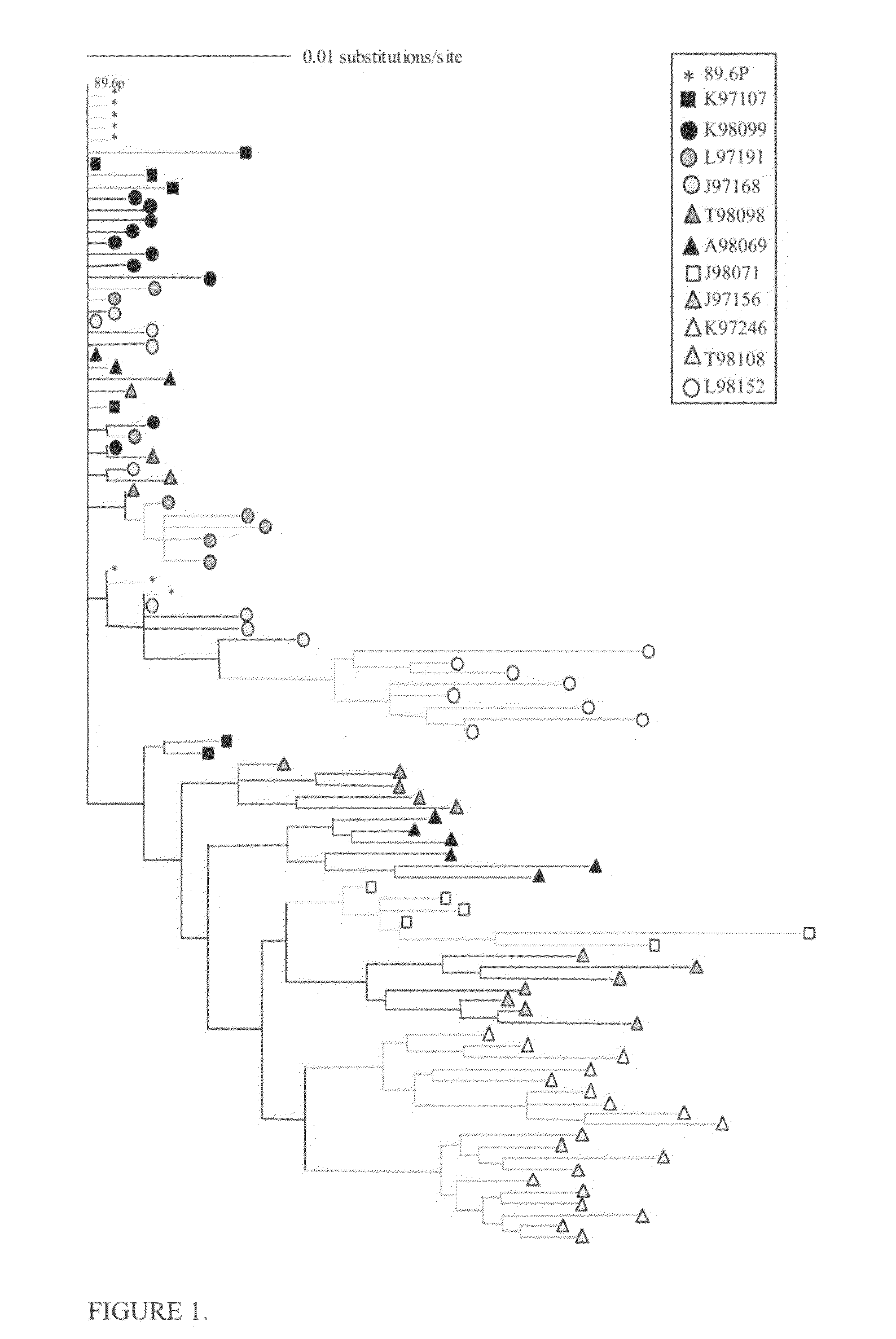
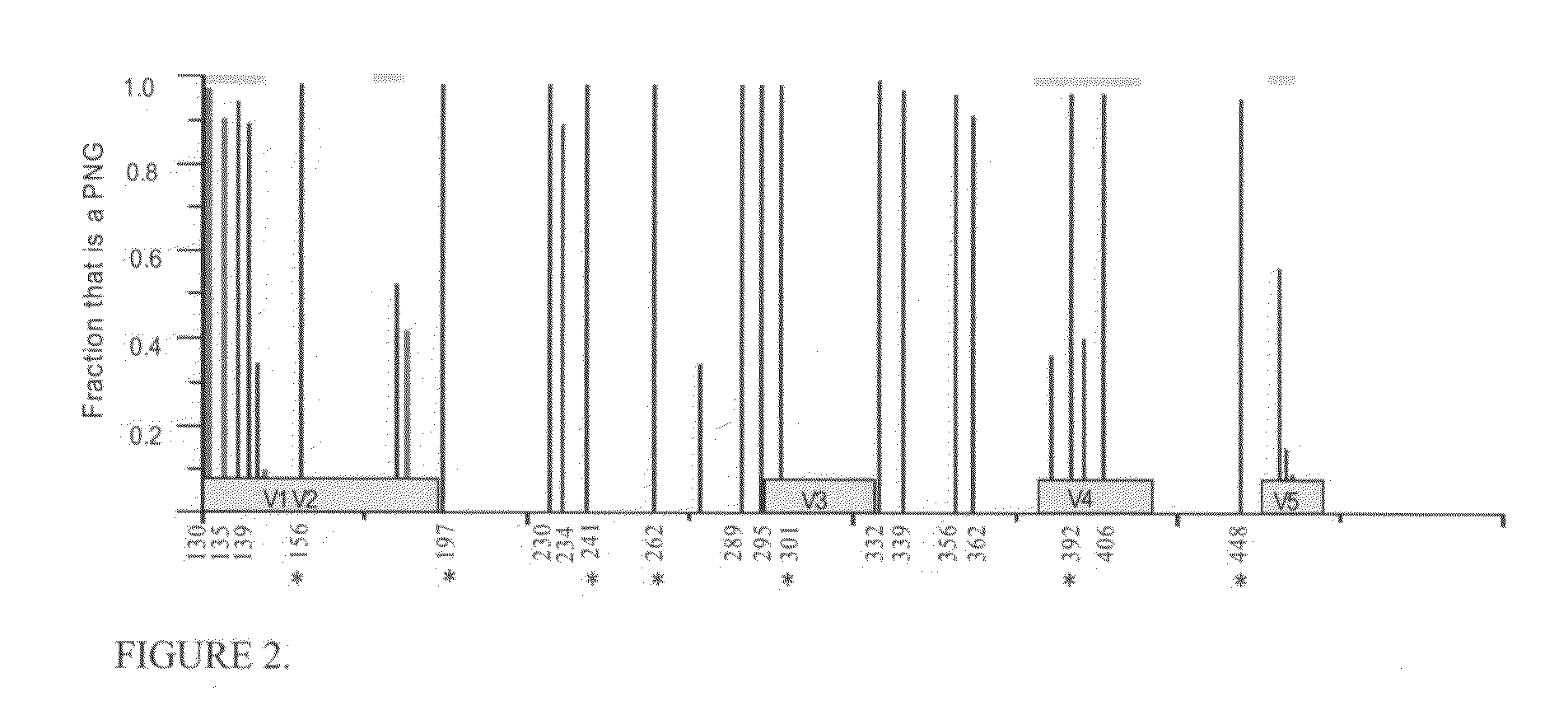
The invention provides vaccination protocols for administering immunogens to a primate host in order to promote the formation of neutralizing antibodies (NAbs) against primate immunodeficiency viruses. In some embodiments, the vaccination protocols comprise the step of administering to a primate host a first immunogen comprising at least one primate immunodeficiency virus Envelope (env) sequence having a first set of consensus glycosylation sequences, followed by a second immunogen comprising at least one primate immunodeficiency virus env sequence having a second set of consensus glycosylation sequences, wherein the differences between the first set of consensus glycosylation sequences and the second set of consensus glycosylation sequences comprise differences in consensus glycosylation sequences observed in HIV isolates obtained at different time points of a natural infection.
Owner:SEATTLE BIOMEDICAL RES INST +1
Ribozymes targeting retroviral packaging sequence expression constructs and recombinant retroviruses containing such constructs
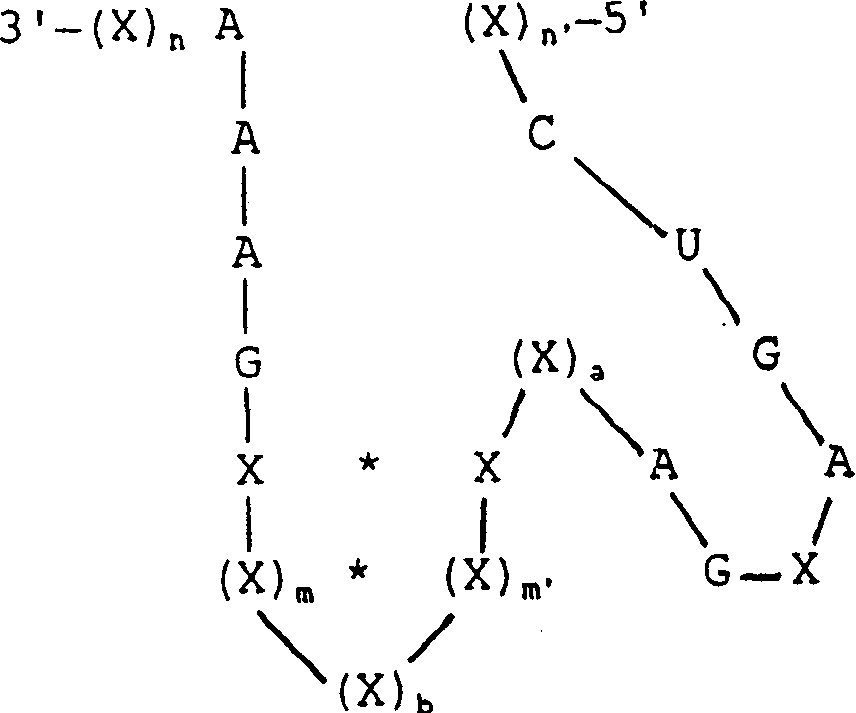
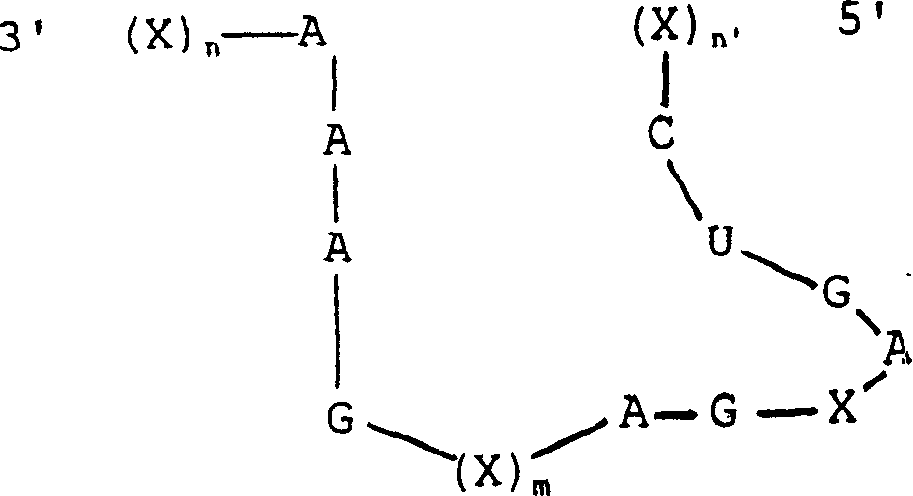
This invention is directed to a synthetic non-naturally occurring oligonucleotide compound which comprises nucleotides whose sequence defines a conserved catalytic region and nucleotides whose sequence is capable of hybridizing with a predetermined target sequence within a packaging sequence of an RNA virus. Preferably, the viral packaging sequence is a retrovirus packaging sequence or the HIV-1 Psi packaging sequence. The RNA virus may be HIV-1, Feline Leukemia Virus, Feline Immunodeficiency Virus or one of the viruses listed in Table I. The conserved catalytic region may be derived from a hammerhead ribozyme, a hairpin ribozyme, a hepatitis delta ribozyme, an RNAase P ribozyme, a group I intron, a group II intron. The invention is also directed to multiple ribozymes, combinations of ribozymes, with or without antisense, and combinations of ribozymes, with antisense, and TAR decoys, polyTARs and RRE decoys targeted against the RNA virus. Vectors are also described. Further, methods of treatment and methods of use both in vivo and ex vivo are described.
Owner:GENE SHEARS PTY LTD
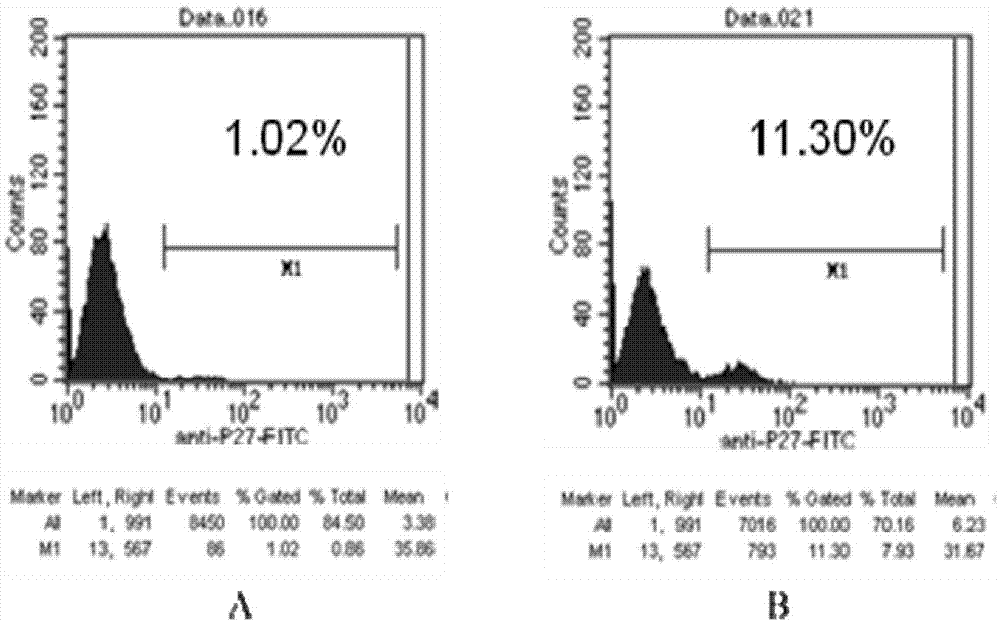
BIV indication cell line using enhanced green fluorescence protein as reporting gene
InactiveCN101100675BEasy to operateUnderstanding the Dynamics of InfectionVector-based foreign material introductionForeign genetic material cellsInfected cellFeline immunodeficiency virus
A Bovine Immunodeficiency Virus BIV infected cell system and use are disclosed. The process is carried out by expressing reinforced EGFP under BIV infectious specific condition, constructing respondent Bovine Immunodeficiency Virus BIV infected reporting plasmid with nucleotide acid sequence in sequence table, transfecting it to BHK-21 cell, screening expressed cell system, mono-cloning and selecting high-expression EGFP negative cloning. It can be used to indicate BIV infection and activate BIV trans factor.
Owner:NANKAI UNIV
Compounds for use in the treatment of feline retroviral infections
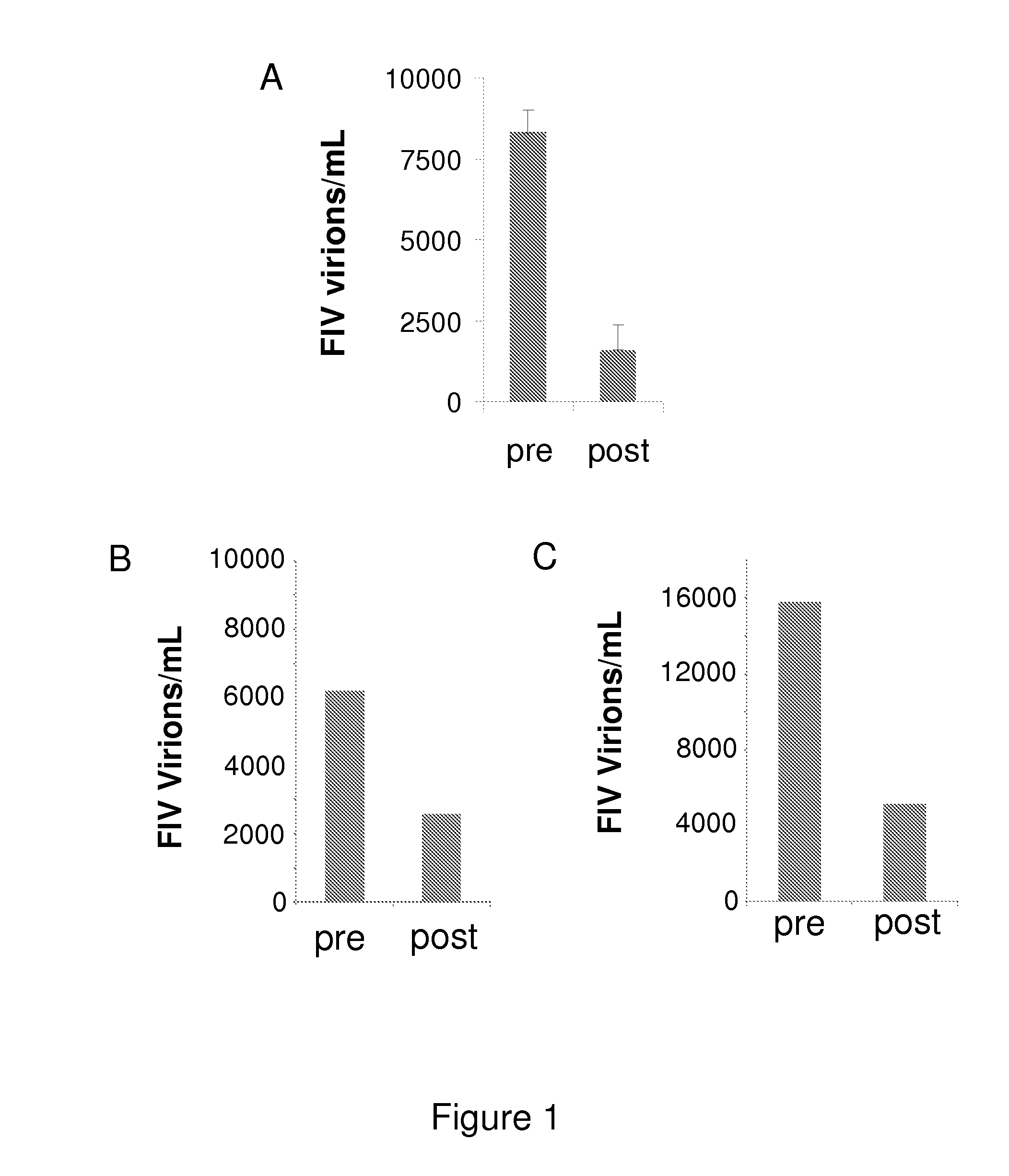
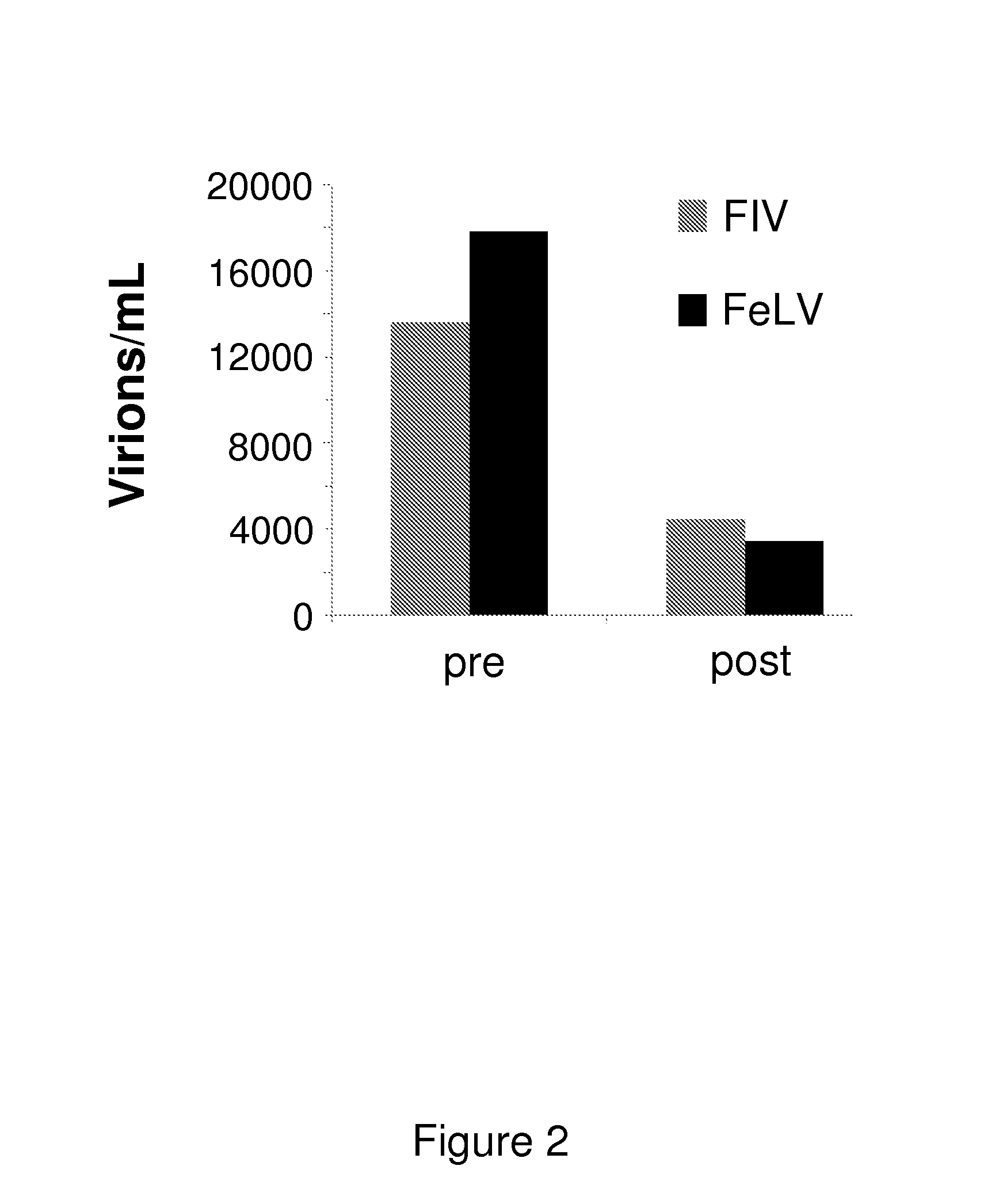
InactiveUS20140073607A1Reduce loadRelief of clinical symptomsBiocideOrganic active ingredientsFeline immunodeficiency virusRetroviral infection
The present invention relates to compounds and compositions for use in the treatment or prevention of retroviral infections in felines, in particular cats, and to the use of said derivatives for the manufacture of a medicament for the treatment or prevention of feline leukemia virus (FeLV) infections occurring alone or together with feline immunodeficiency virus (FIV) infections in cats.
Owner:ARATANA THERAPEUTICS NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com