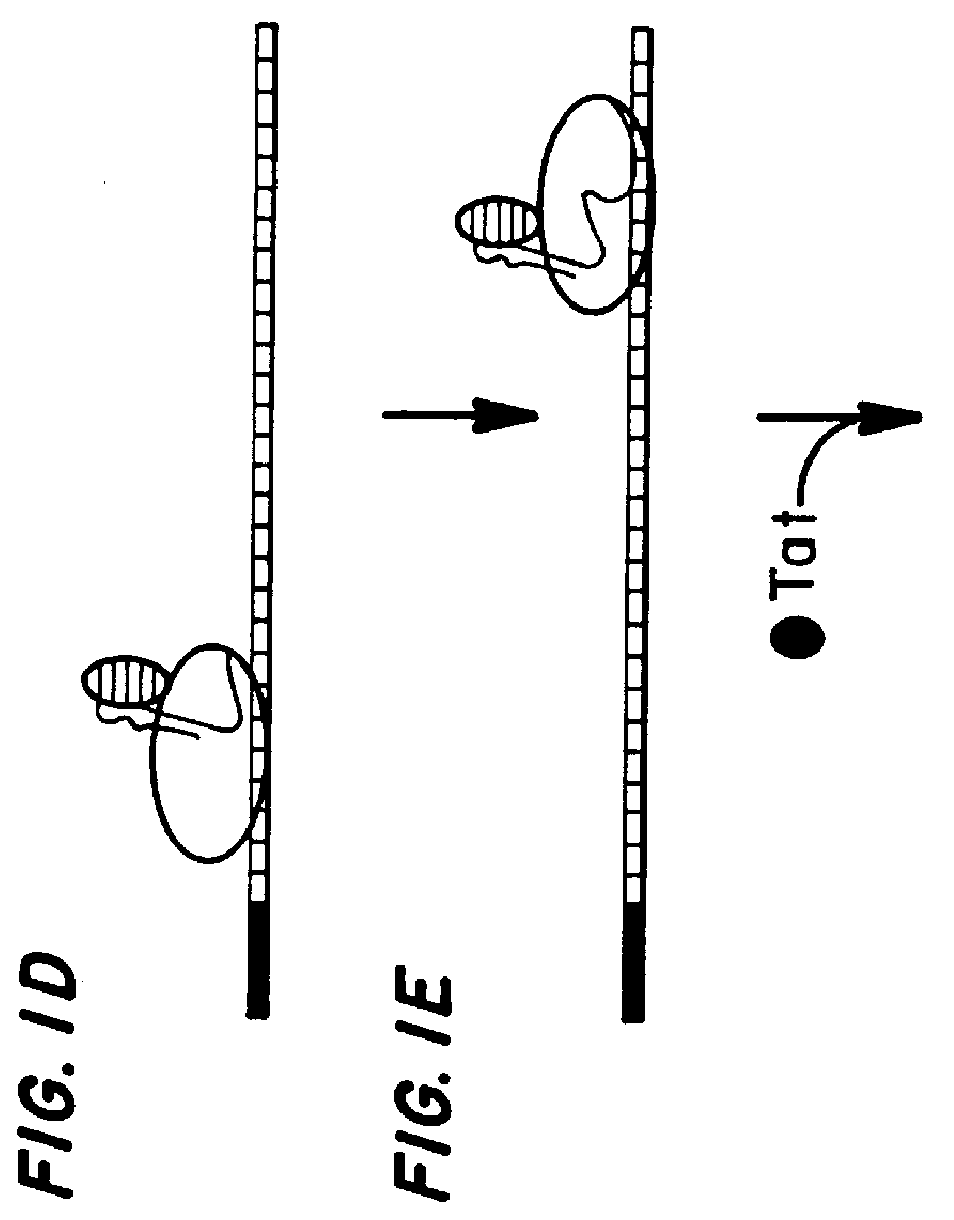
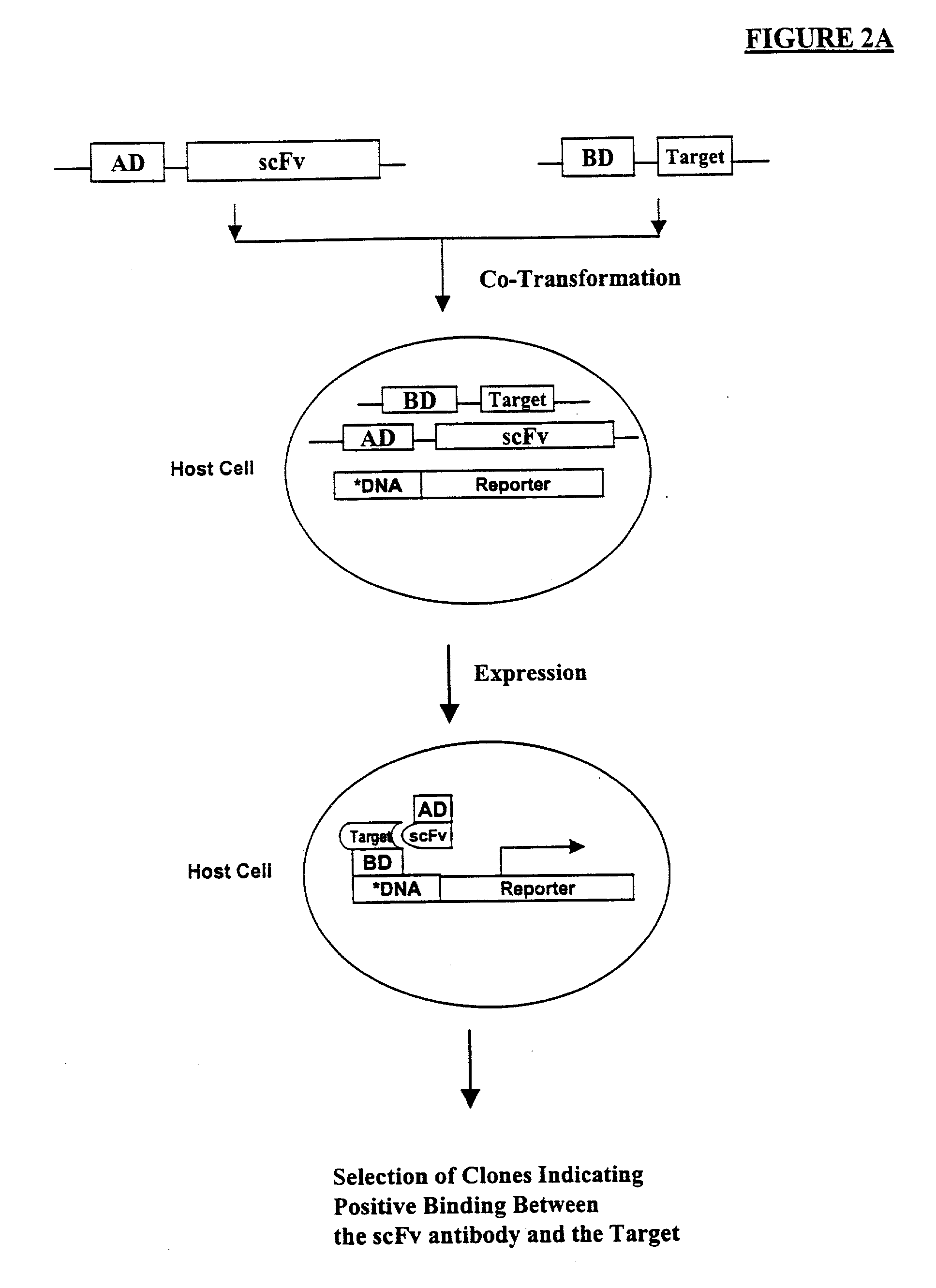
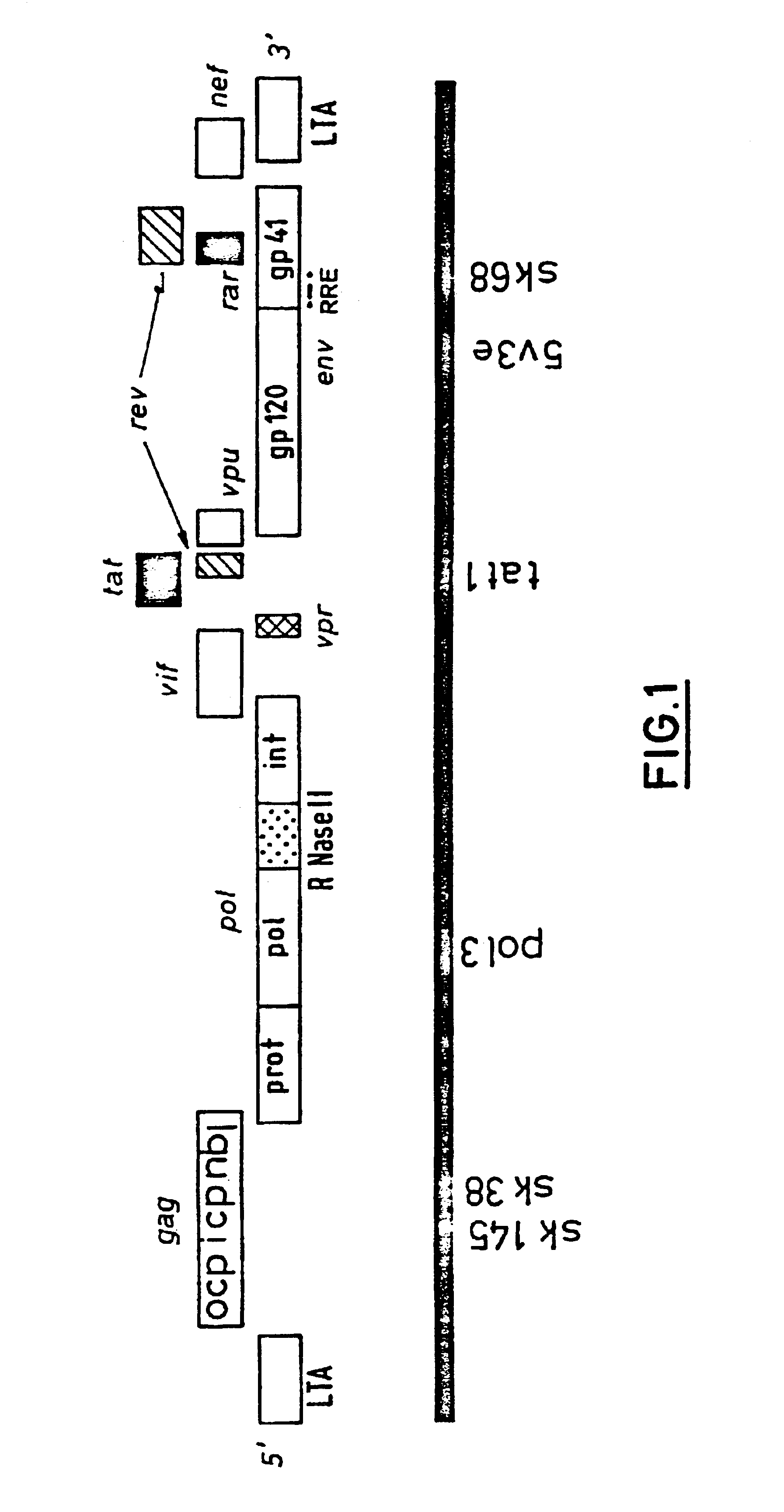
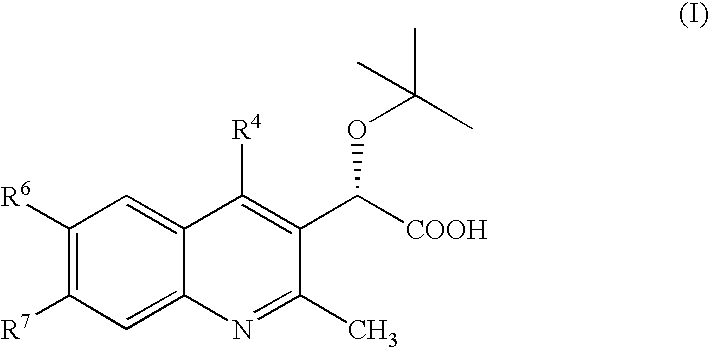
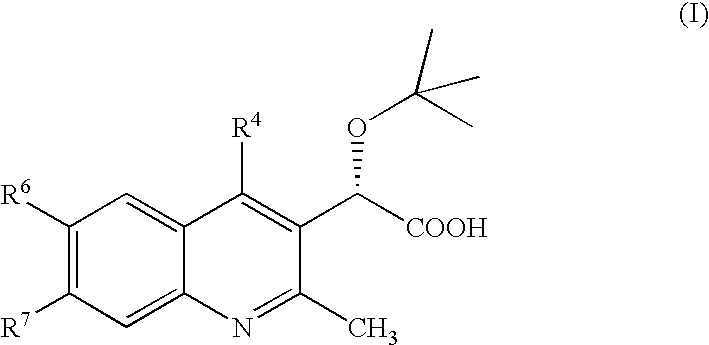
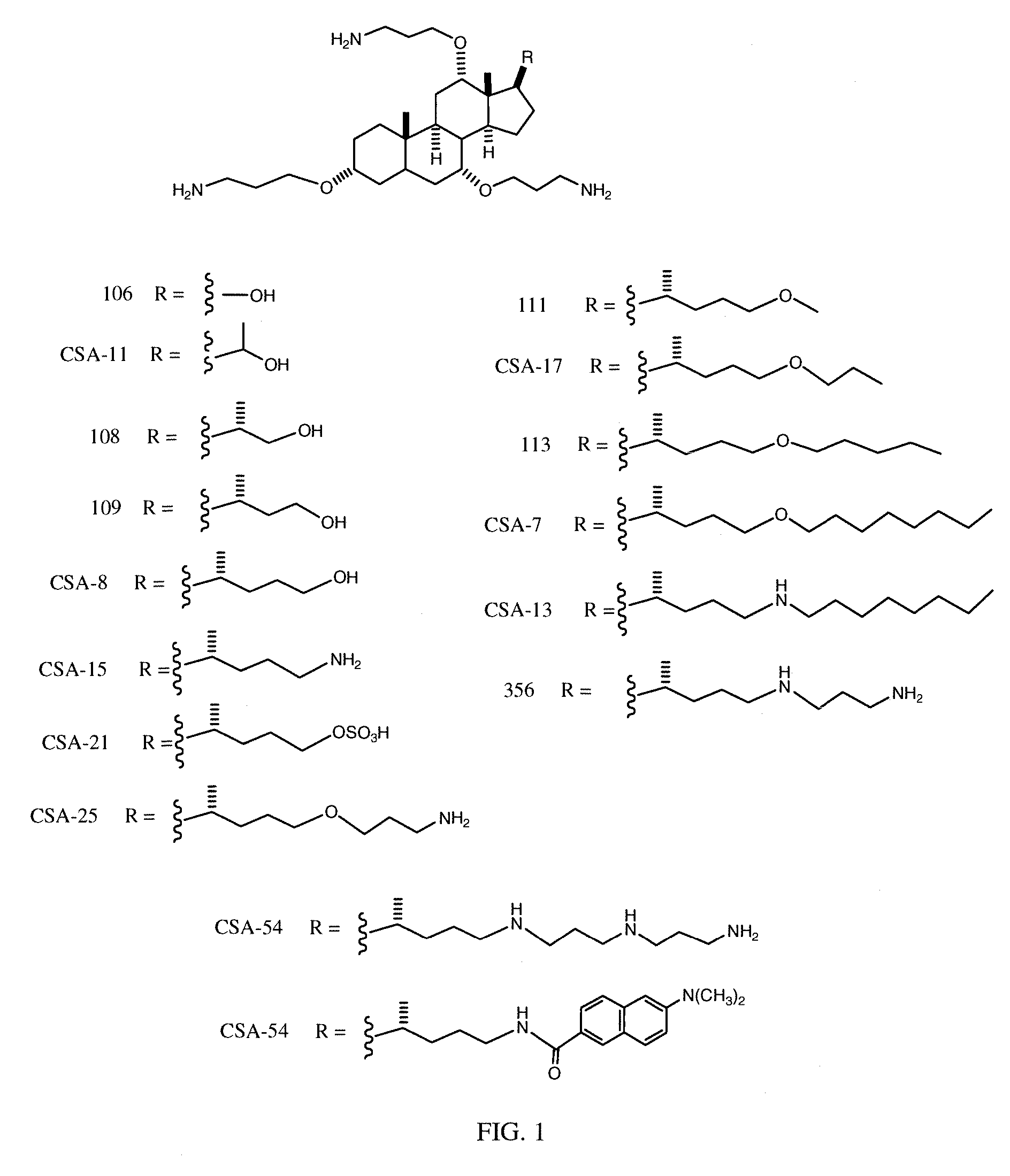Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
355 results about "Defective virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus which lacks a complete genome so that it cannot completely replicate or cannot form a protein coat; some are host-dependent defectives, meaning they can replicate only in cell systems which provide the particular genetic function which they lack; others, called satellite viruses, are able to replicate only when their genetic defect is complemented by a helper virus.
Method of eliminating inhibitory/instability regions from mRNA
InactiveUS6174666B1Microbiological testing/measurementVirus peptidesImmunodeficiency virusInstability
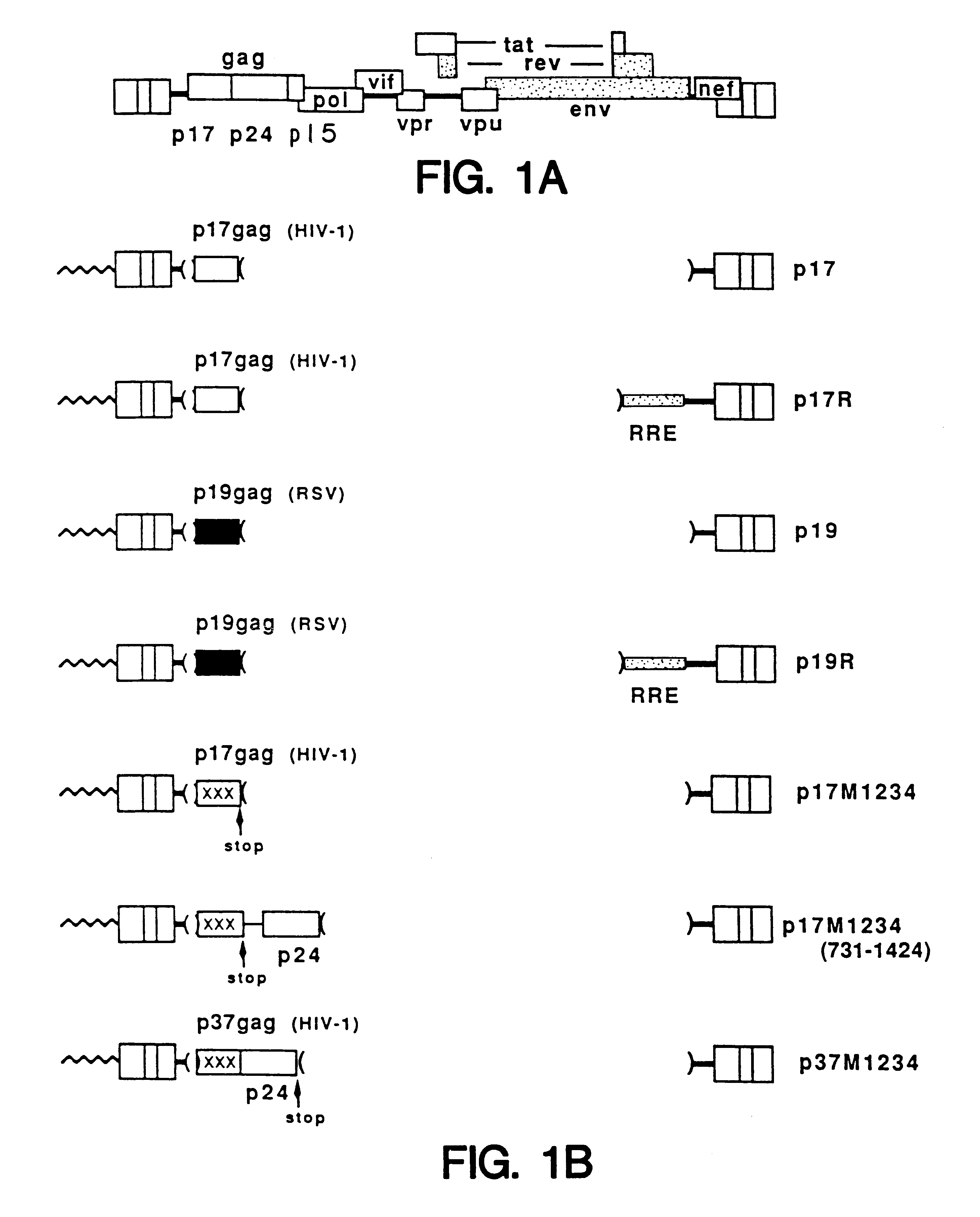
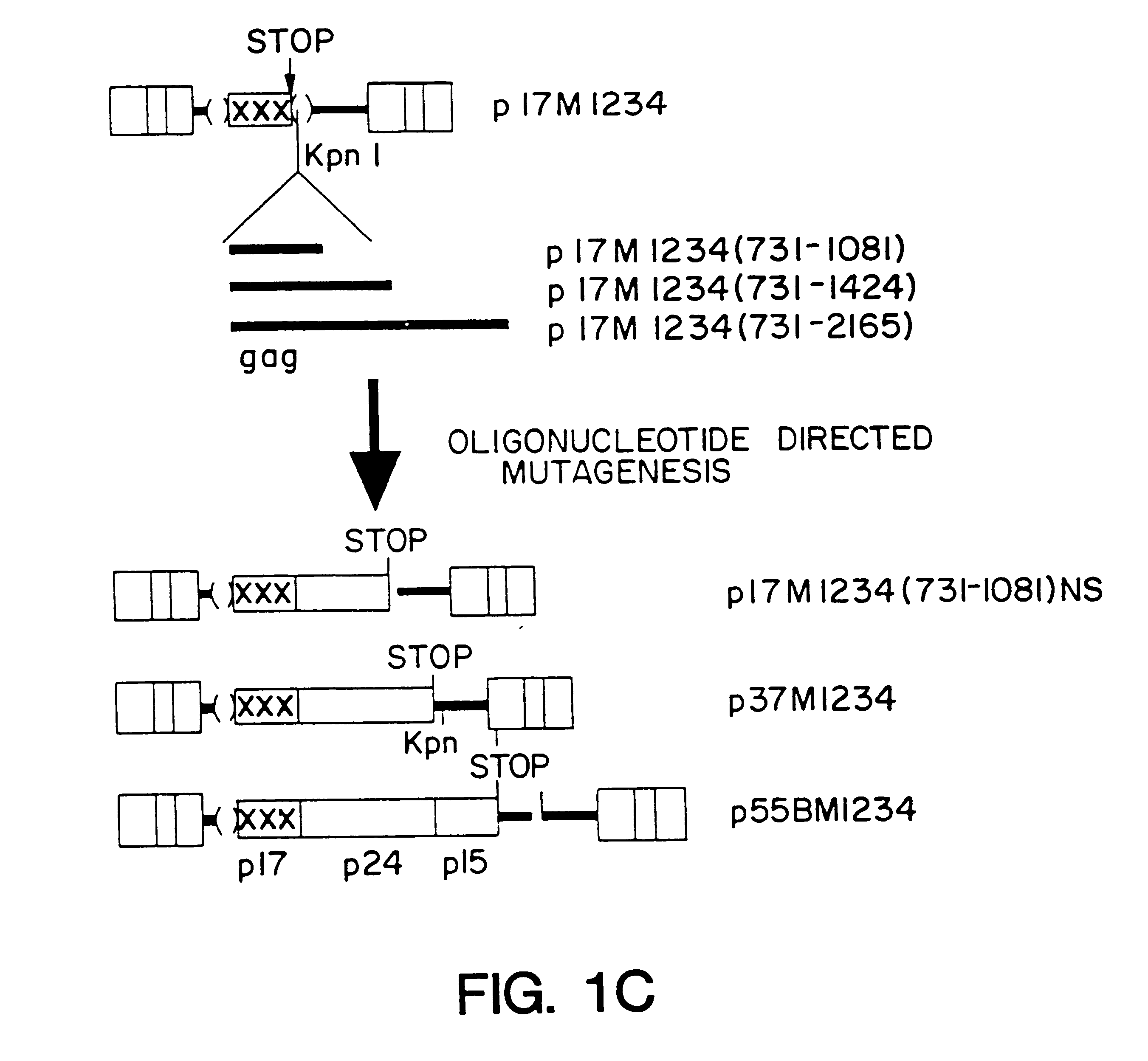
A method of locating an inhibitory / instability sequence or sequences within the coding region of an mRNA and modifying the gene encoding that mRNA to remove these inhibitory / instability sequences by making clustered nucleotide substitutions without altering the coding capacity of the gene is disclosed. Constructs containing these mutated genes and host cells containing these constructs are also disclosed. The method and constructs are exemplified by the mutation of a Human Immunodeficiency Virus-1 Rev-dependent gag gene to a Rev-independent gag gene. Constructs useful in locating inhibitory / instability sequences within either the coding region or the 3' untranslated region of an mRNA are also disclosed.
Owner:UNITED STATES OF AMERICA
Simian immunodeficiency virus peptides with antifusogenic and antiviral activities
InactiveUS6017536ASsRNA viruses negative-sensePeptide/protein ingredientsSimian immunodeficiency viruses SIVDefective virus
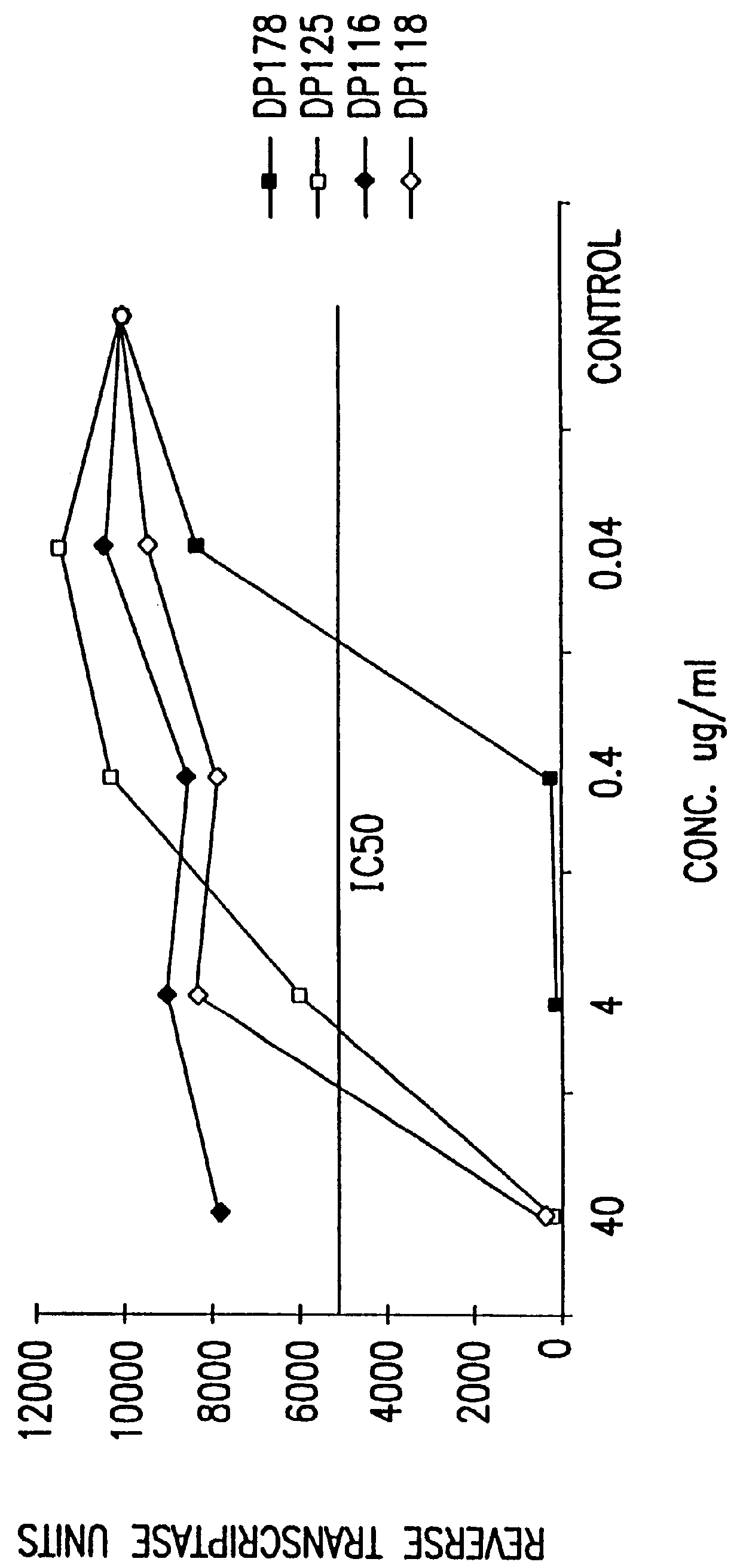
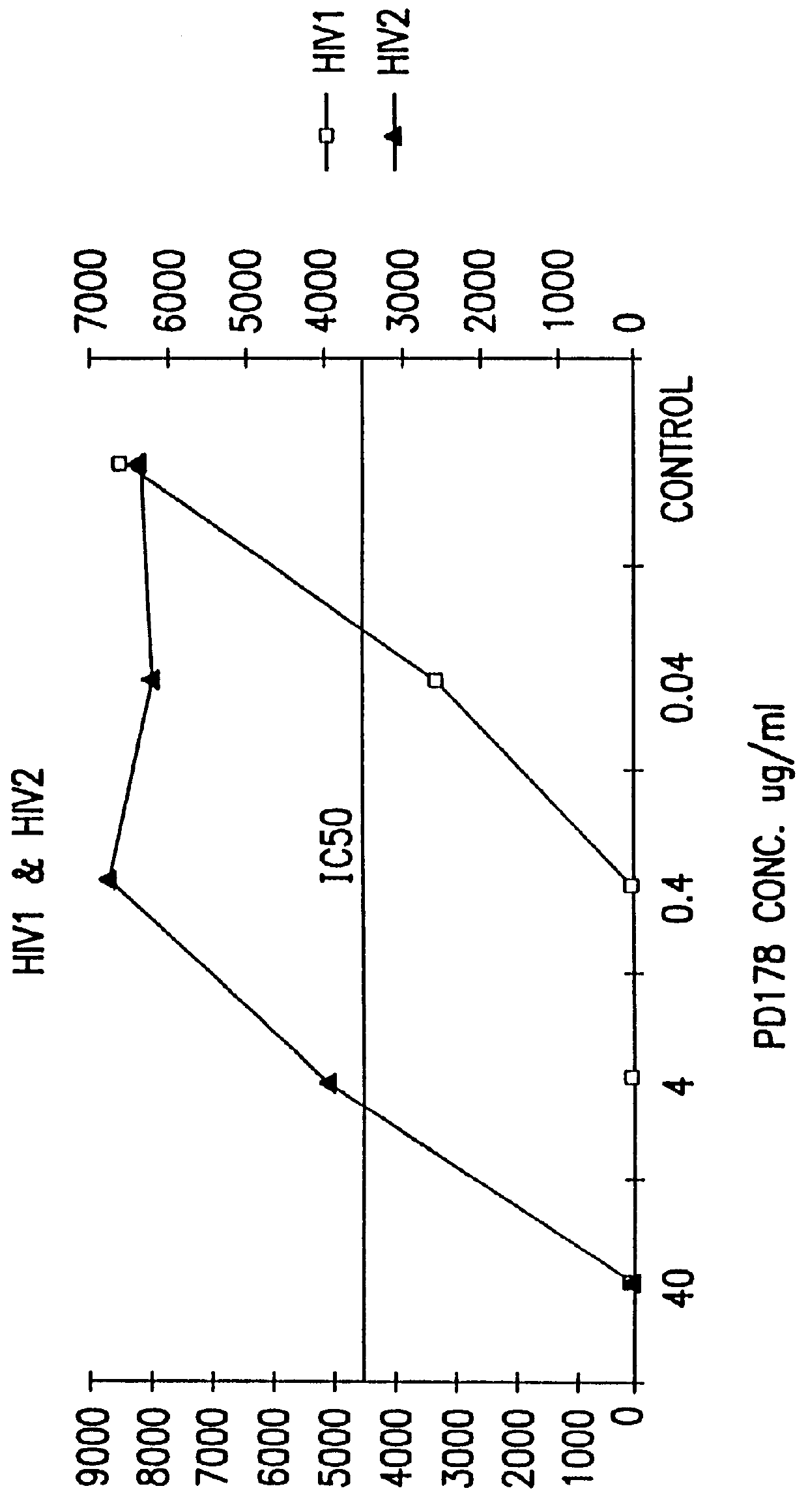
The present invention relates to peptides which exhibit antifusogenic and antiviral activities. The peptides of the invention consist of a 16 to 39 amino acid region of a simian immunodeficiency virus (SIV) protein. These regions were identified through computer algorithms capable of recognizing the ALLMOTI5, 107x178x4, or PLZIP amino acid motifs. These motifs are associated with the antifusogenic and antiviral activities of the claimed peptides.
Owner:TRIMERIS +1
Helper-free, totally defective adenovirus for gene therapy
InactiveUS6228646B1Encapsidation efficiencyMaximal recombinationInactivation/attenuationNucleic acid vectorIn vivoHelper virus
A method for producing in vivo packaged recombinant adenovirus vectors is provided. The recombinant Ad vectors do not contain any Adenovirus genes and are therefore useful for gene therapy. The recombinant Adenovirus vectors are packaged in vivo using a helper virus which is itself very inefficiently packaged, providing a recombinant viral preparation with very little or no contamination with helper virus. In particular, the method makes use of a helper virus in which the packaging site can be easily excised in vivo by recombination mediated by a recombinase. The helper virus is also useful for the in vivo construction of new recombinant adenovirus vectors containing substitutions in the E1 or other adenoviral region.
Owner:RGT UNIV OF CALIFORNIA
Aspartyl protease inhibitors
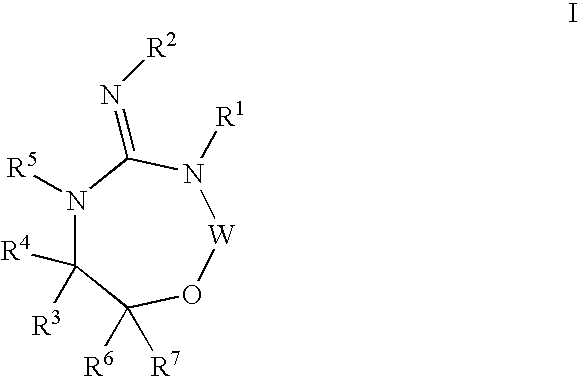
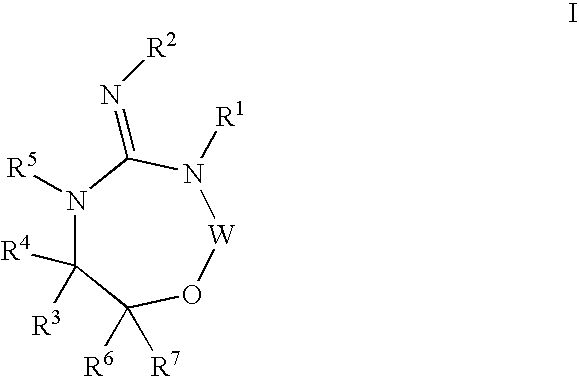
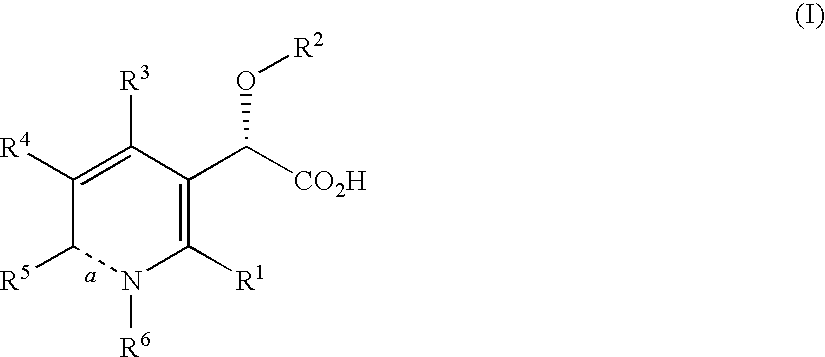
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W, R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:SCHERING CORP
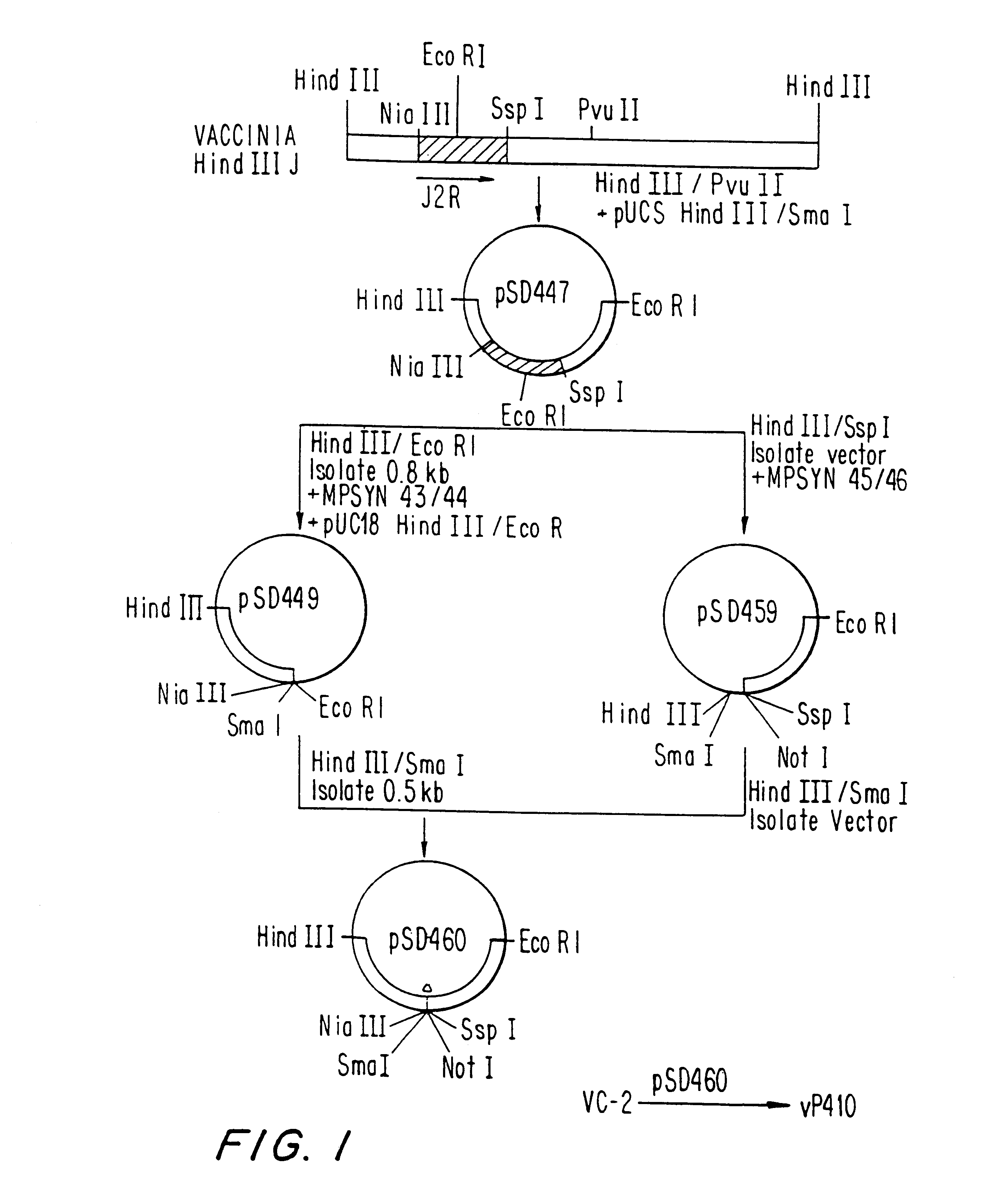
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
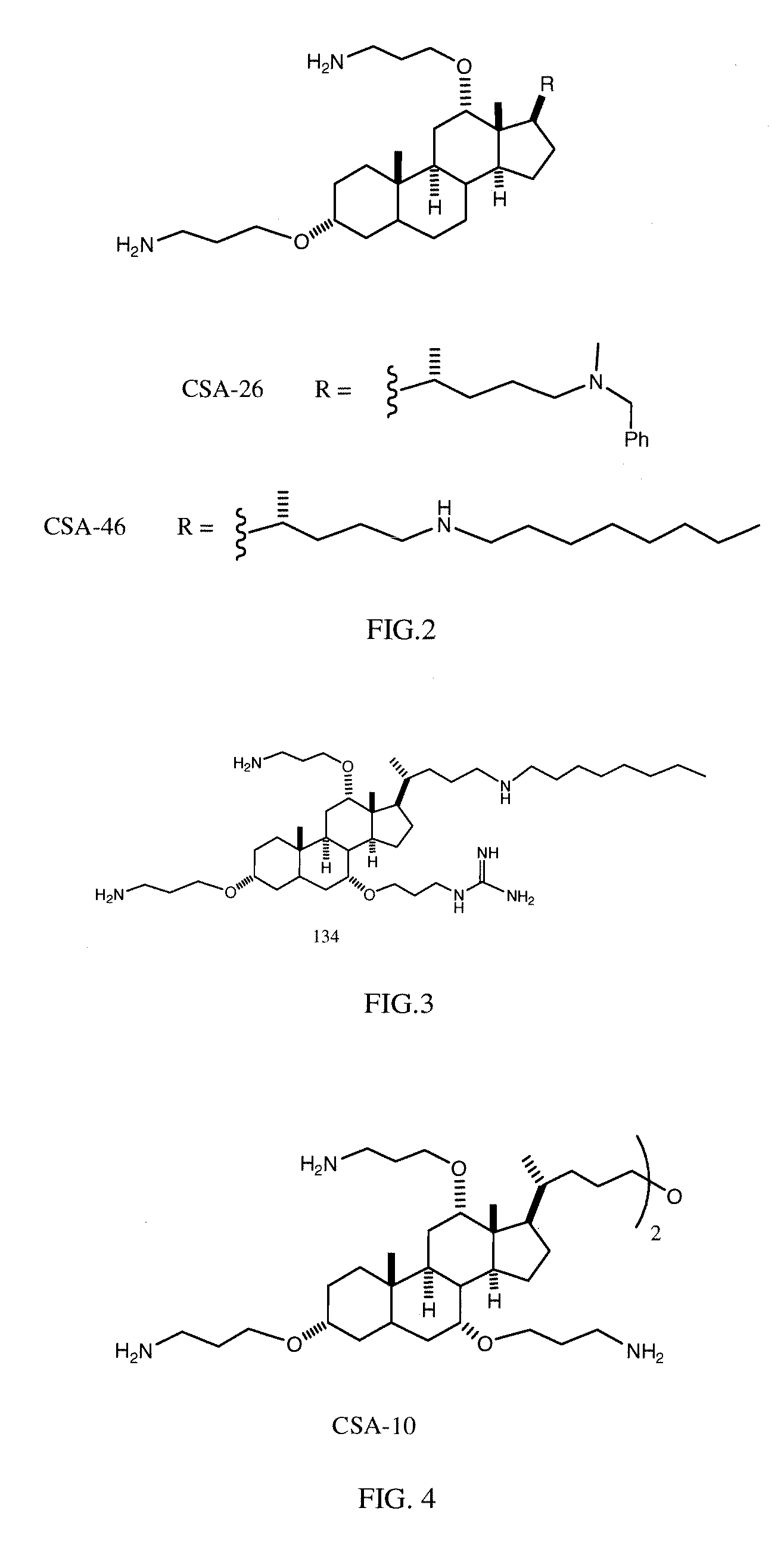
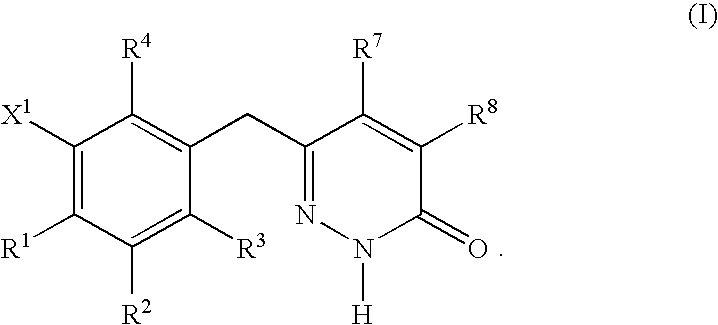
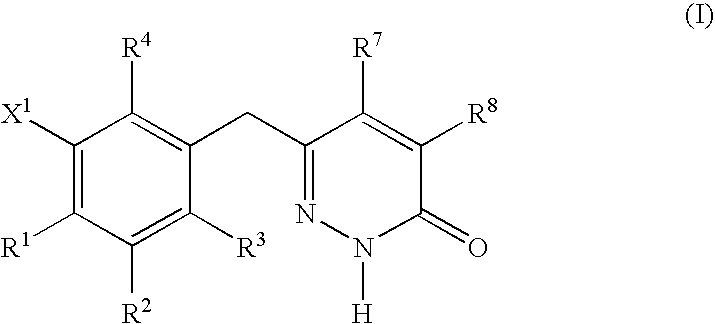
Cationic Steroid Antimicrobial Compositions and Methods of Use
The invention provides methods for decreasing or inhibiting human immunodeficiency virus (HIV) infection or pathogenesis (e.g., illness) of a cell in vitro, ex vivo or in vivo, a symptom or pathology associated with human immunodeficiency virus (HIV) infection or pathogenesis (e.g., illness) in vitro, ex vivo or in vivo, or an adverse side effect of human immunodeficiency virus (HIV) infection or pathogenesis (e.g., illness) in vitro, ex vivo or in vivo. In one embodiment, a method of the invention includes treating a subject with an invention compound (e.g., cationic steroid antimicrobial or CSA).
Owner:BRIGHAM YOUNG UNIV +1
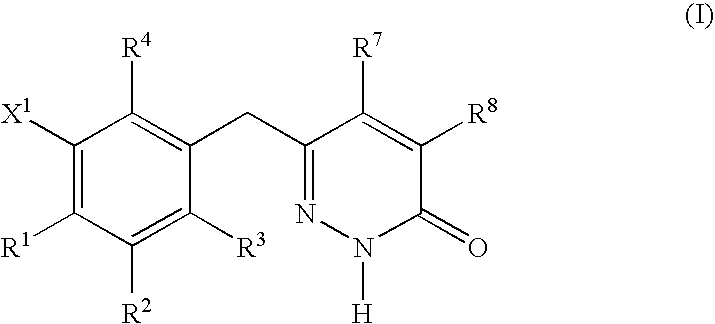
Non-nucleoside reverse transcriptase inhibitors
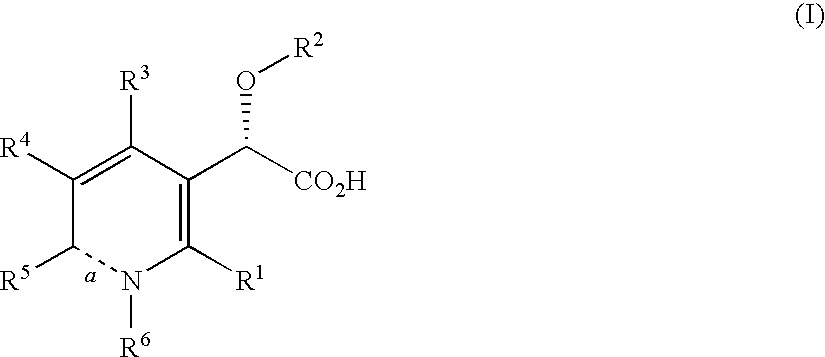
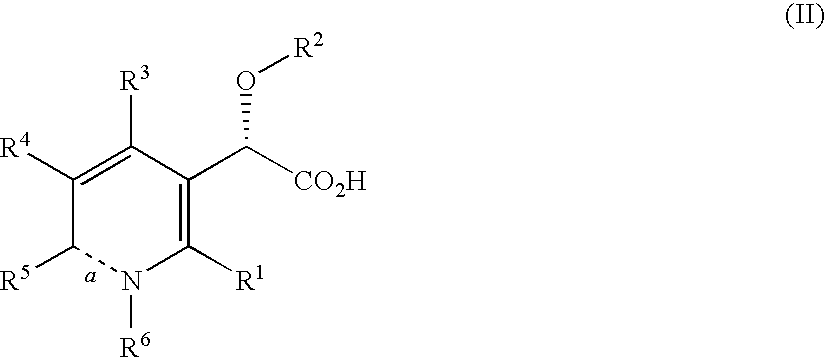
This invention relates to novel pyridazinone derivatives of formula I wherein R1–R4, R7, R8 and X1 are as defined in the summary and pharmaceutically acceptable salts and solvates thereof, methods to inhibit or modulate Human Immunodeficiency Virus (HIV) reverse transcriptase with compounds of formula I, pharmaceutical compositions containing of formula I admixed with at least one solvent, carrier or excipient and processes to prepare compounds of formula I. The compounds are useful for treating disorders in which HIV and genetically related viruses are implicated
Owner:ROCHE PALO ALTO LLC
Recombinant Newcastle disease virus RNA expression systems and vaccines
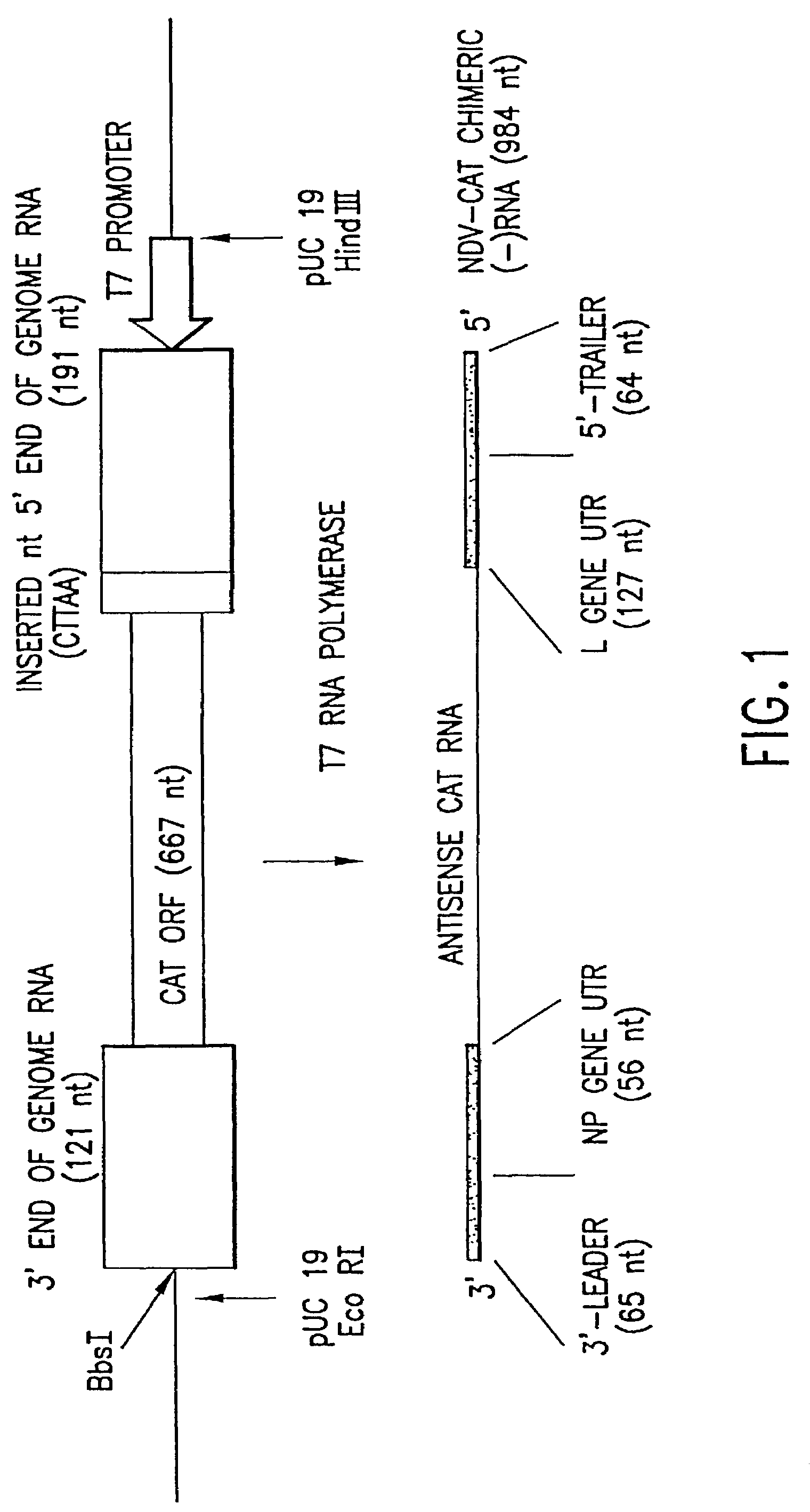
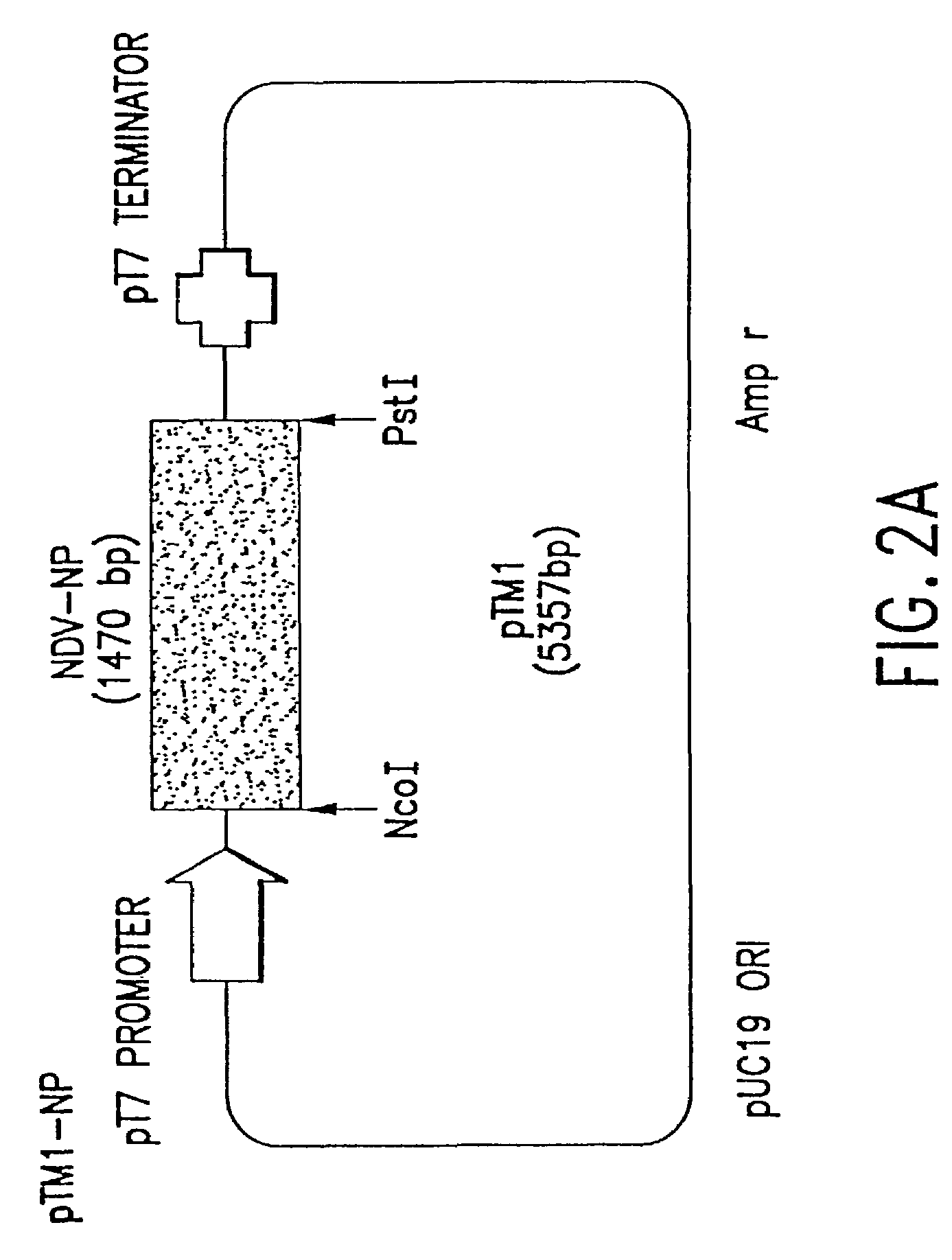
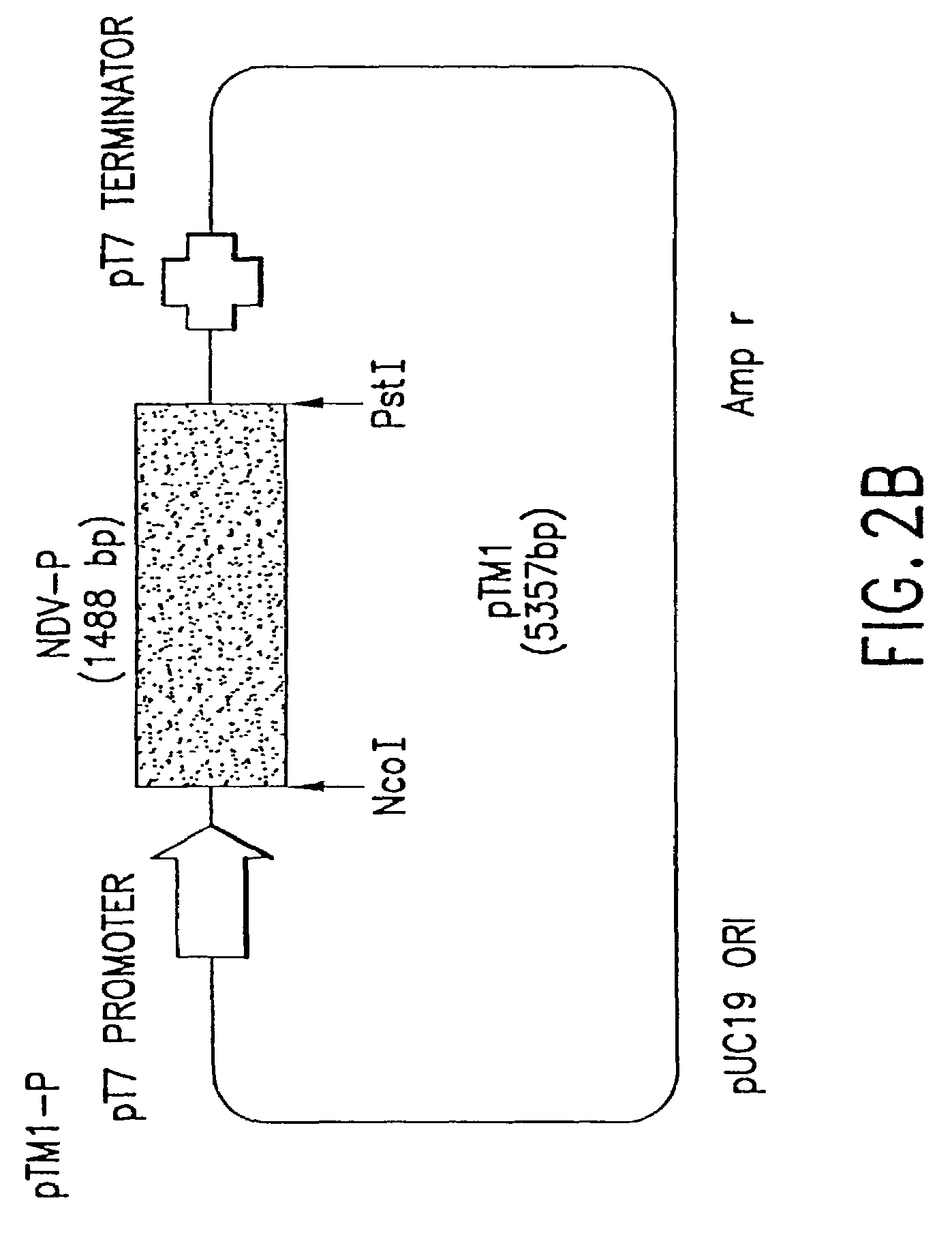
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Inhibitors of human immunodeficiency virus replication
ActiveUS20100292227A1Reduce HIV replicationInhibitory activityBiocideOrganic chemistryMedicineDefective virus
Owner:GILEAD SCI INC
HIV antibody and antigen combined rapid detection reagent kit
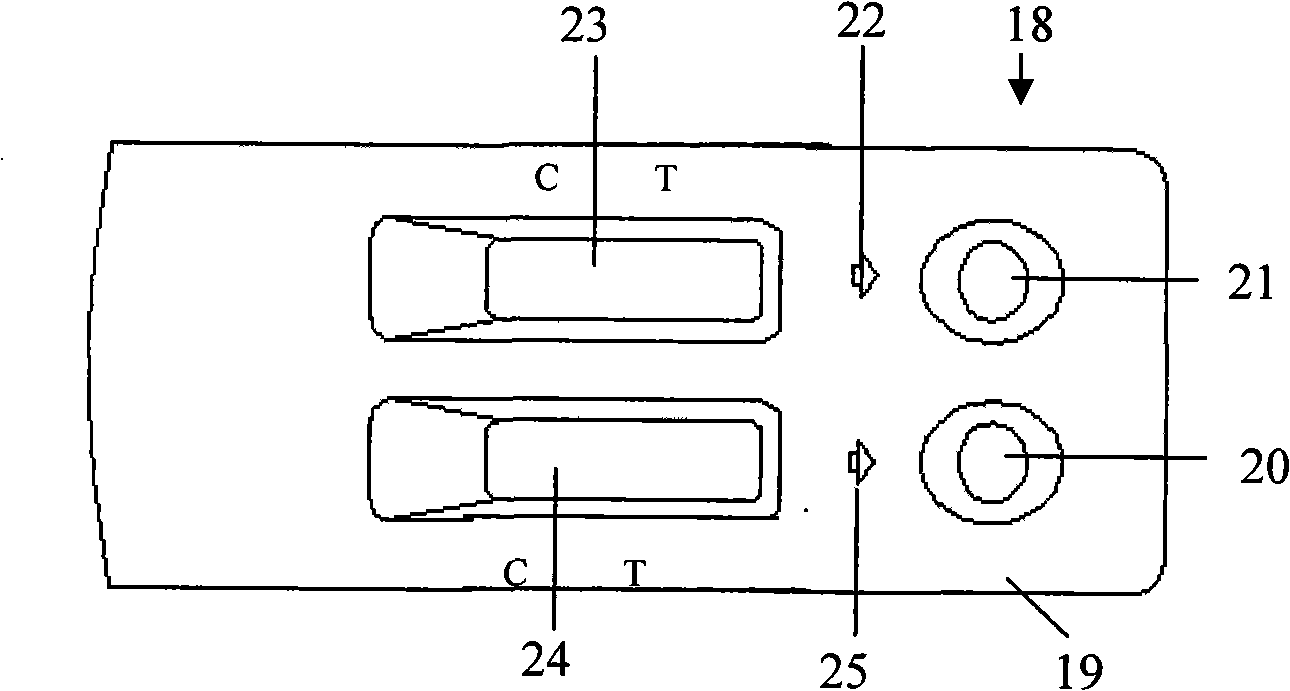
ActiveCN101266246AImprove the detection rateShorten the windowMaterial analysisDiseaseDirect observation
The invention relates to biology applied technology field, especially relates to a immune chromatography assay for quickly detecting the human immunologic deficiency disease (HIV) antibody and antigen. The assay comprises two independent test papers A and B and plastic case C for storing the test paper, wherein the test paper A is used for detecting the HIV antibody and the test paper B is used for detecting the HIV p24 antigen. The test paper A and test paper B are parallely encased in the plastic case to constitute the assay. On detecting, the detected sample is added on the sample pad of the test paler and the immunoreaction result is directly observed to perform detection. The assay is used for screening or clinical diagnosis of the HIV infection and at the same time the HIV antigen and antibody are detected, the simpler antibody detection can effectively reduce the window phase for detecting the HIV, with features of quick reaction, easy operation, economy and practicality, suitable for insitu detecting.
Owner:天津中新科炬生物制药股份有限公司
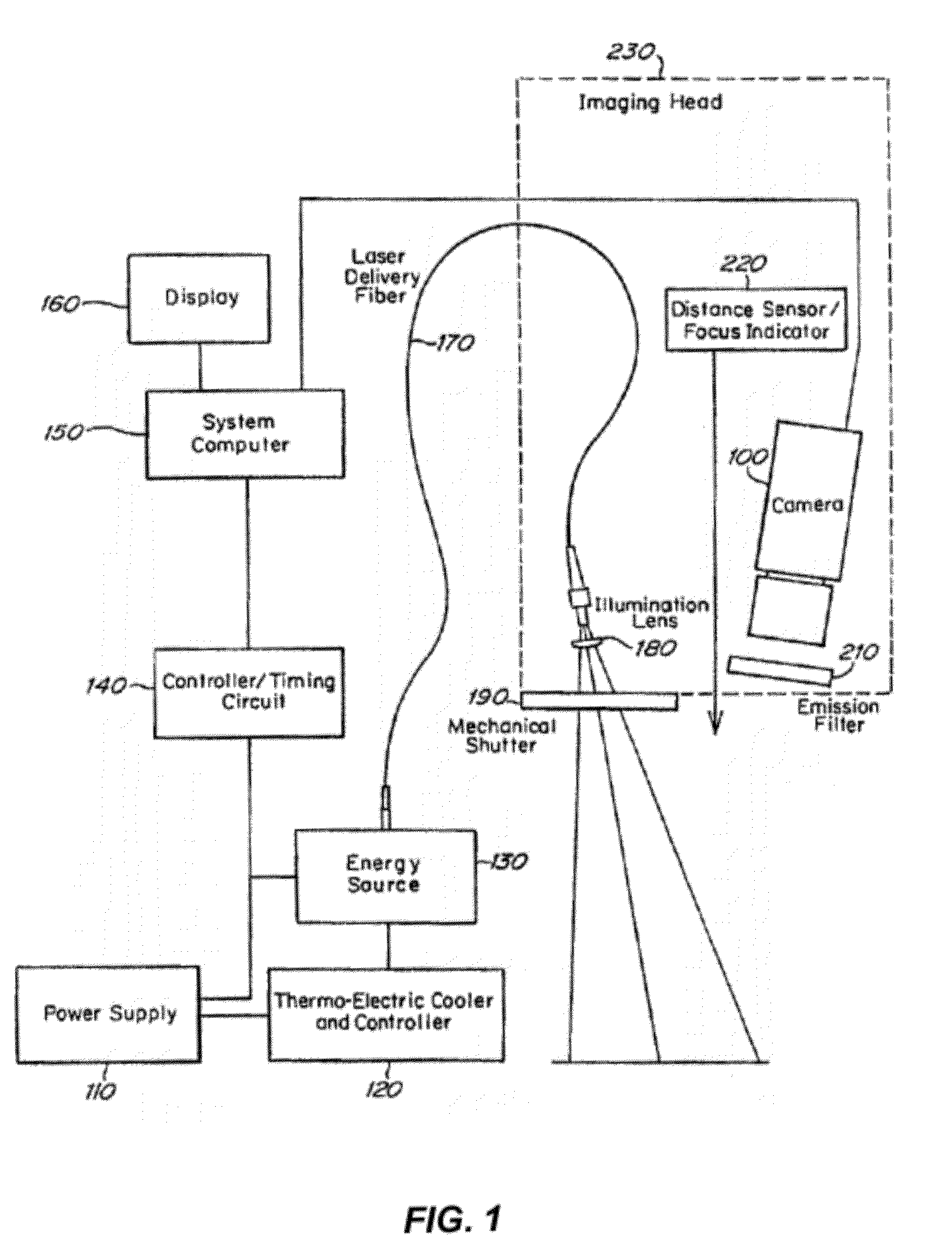
Imaging methods and compositions comprising fluorescent dyes associated with viral components for nerve imaging
Disclosed herein are compositions and methods for imaging nerve cells. The composition comprises a fluorescent dye; and a viral component selected from a neurotropic, replication-defective virus, a viral protein of a neurotropic virus, and a capsid of a neurotropic virus. Although the fluorescent dye in itself cannot penetrate nerve cells, the fluorescent dye is bound to the viral component to form a dye / viral component complex that is capable of penetrating nerve cells.
Owner:STRYKER CORP
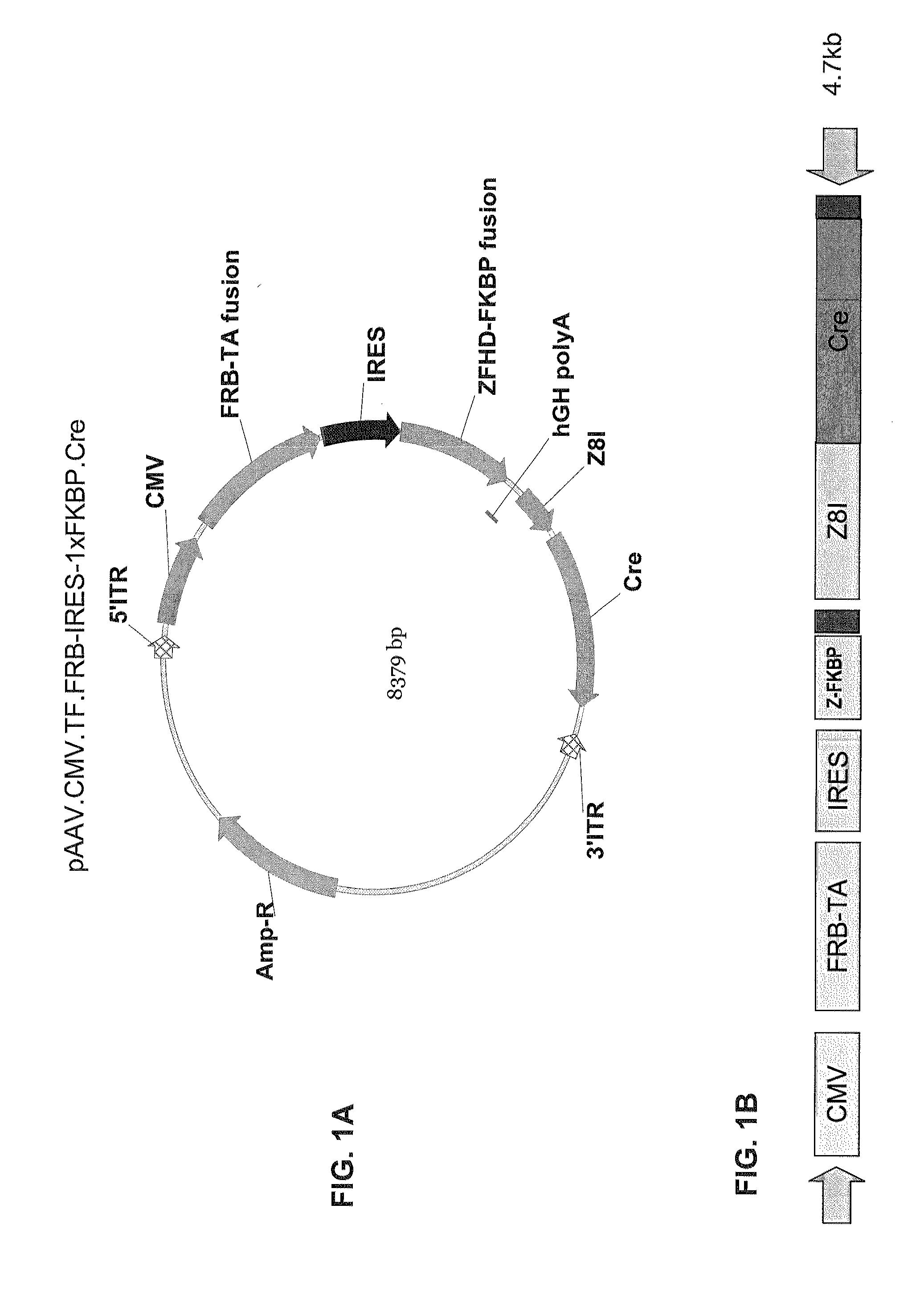
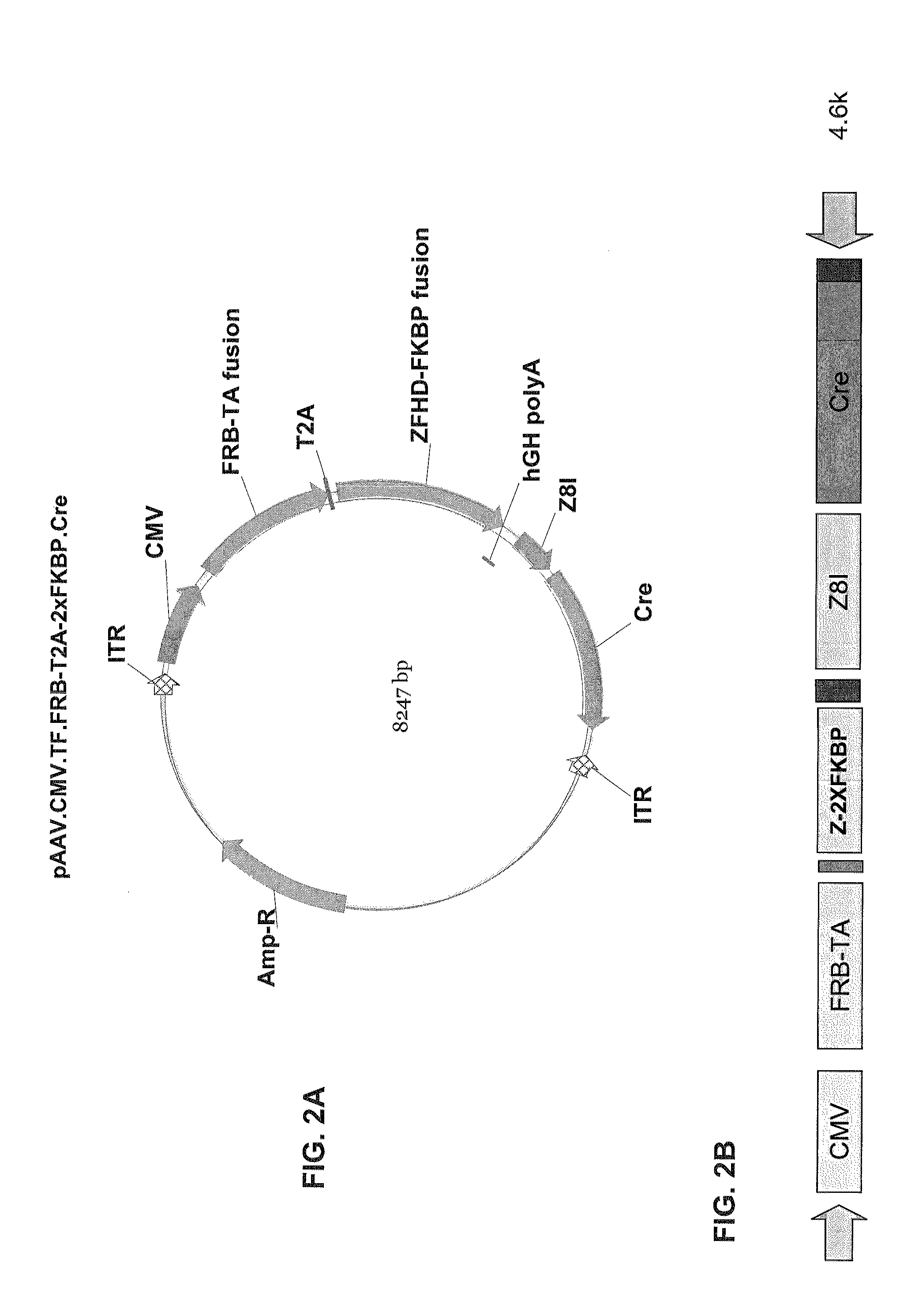
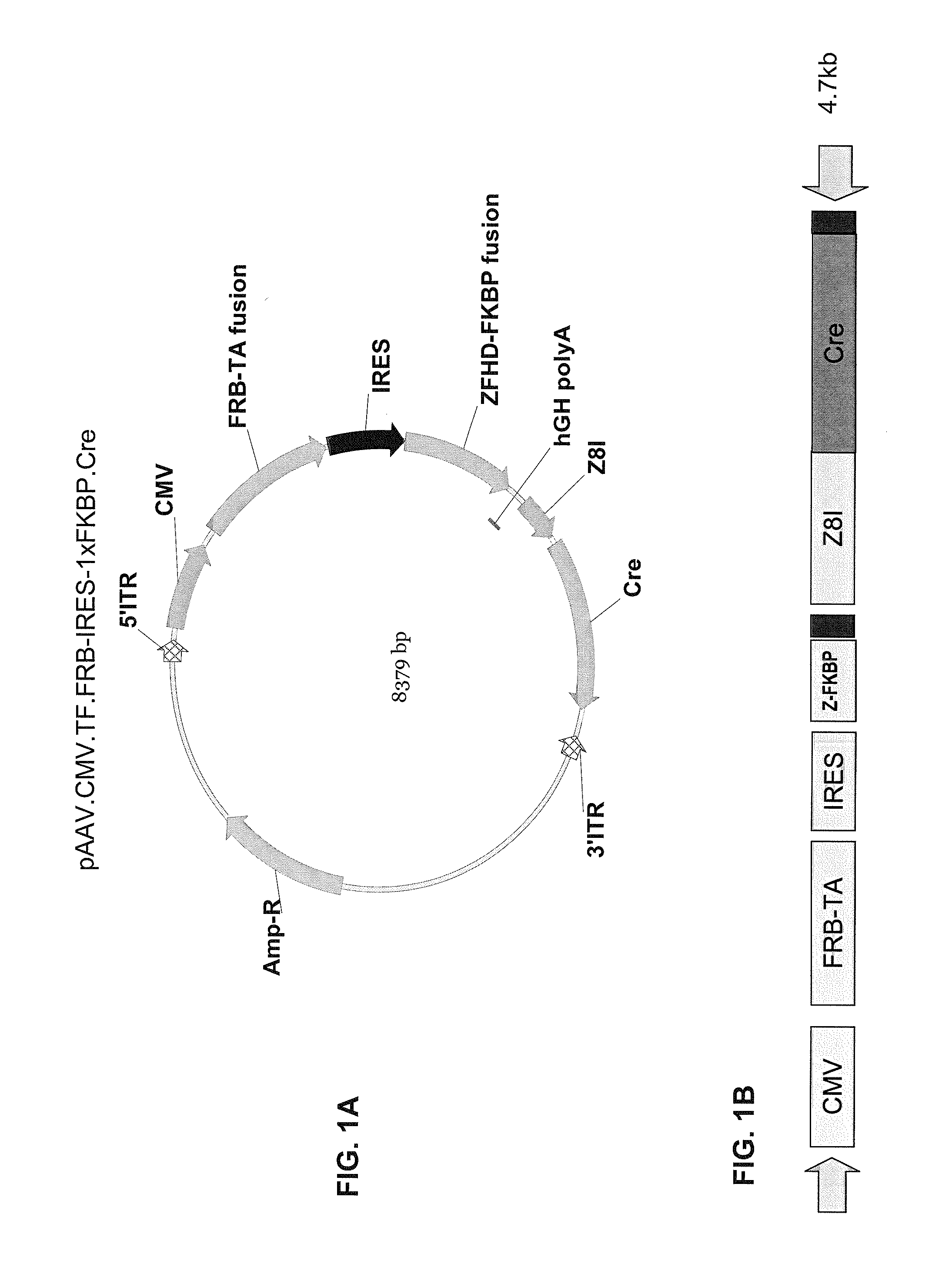
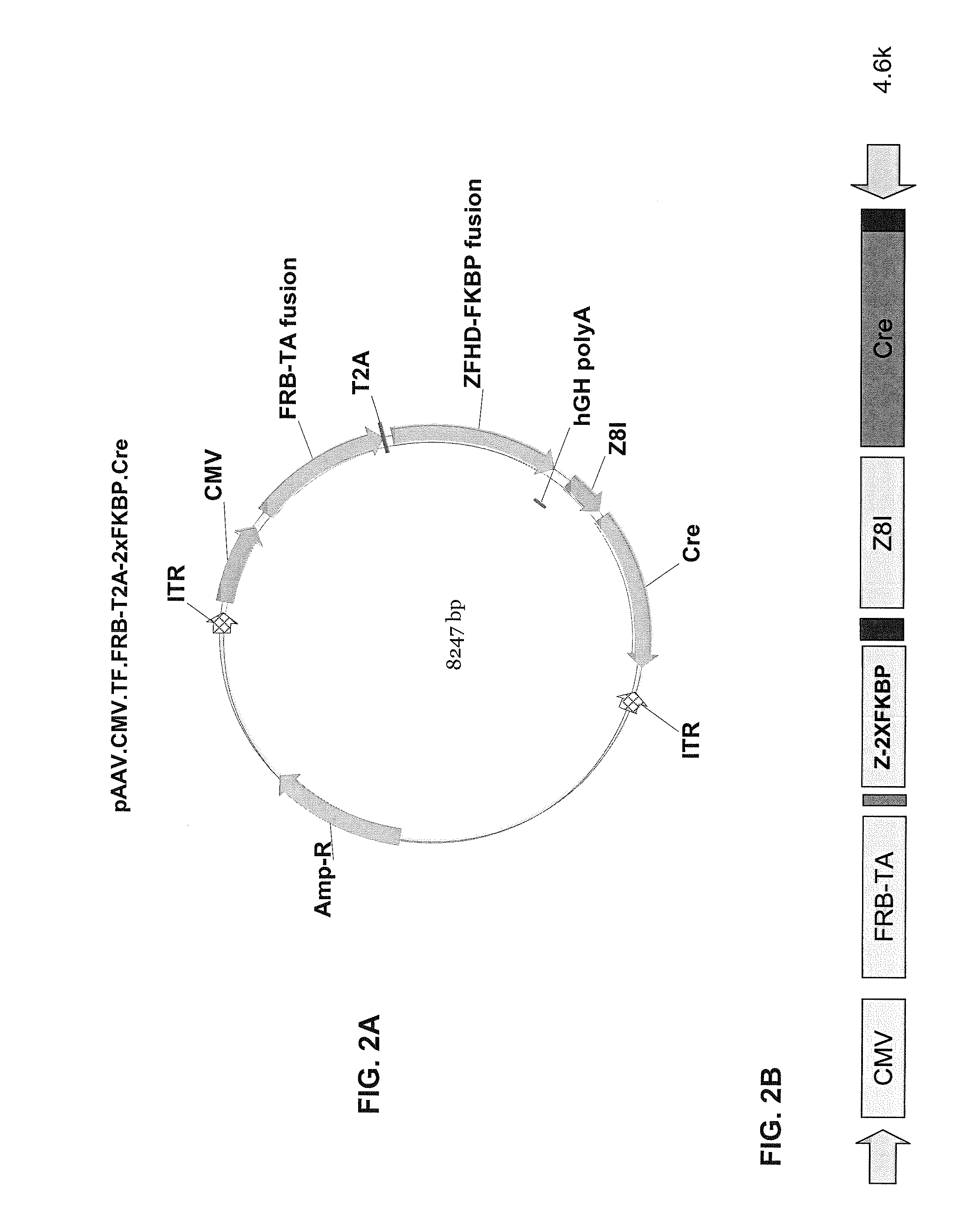
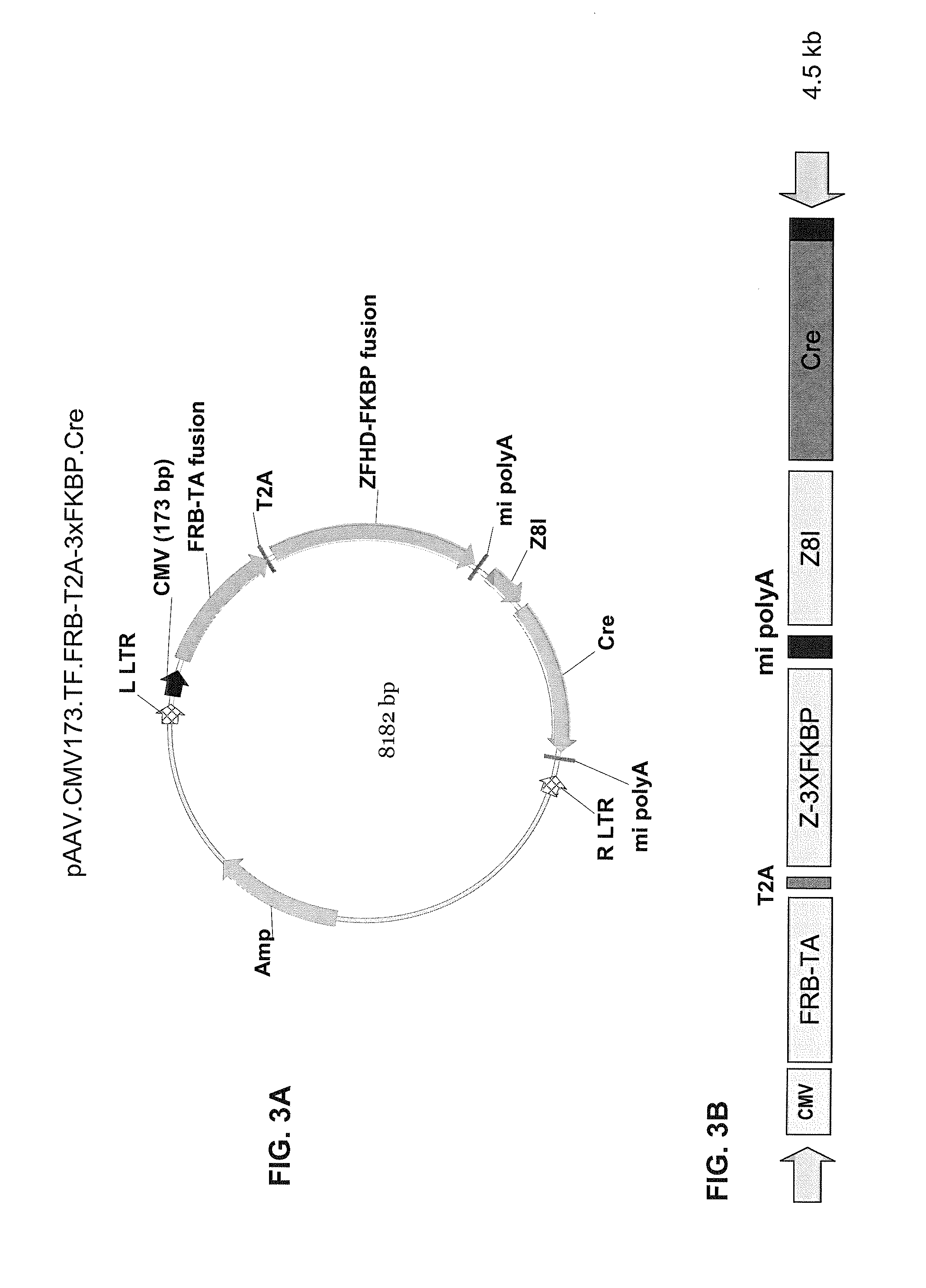
Pharmacologically induced transgene ablation system
The present invention relates to gene therapy systems designed for the delivery of a therapeutic product to a subject using replication-defective virus composition(s) engineered with a built-in safety mechanism for ablating the therapeutic gene product, either permanently or temporarily, in response to a pharmacological agent—preferably an oral formulation, e.g., a pill. The invention is based, in part, on the applicants' development of an integrated approach, referred to herein as “PITA” (Pharmacologically Induced Transgene Ablation), for ablating a transgene or negatively regulating transgene expression. In this approach, replication-deficient viruses are used to deliver a transgene encoding a therapeutic product (an RNA or a protein) so that it is expressed in the subject, but can be reversibly or irreversibly turned off by administering the pharmacological agent; e.g., by administration of a small molecule that induces expression of an ablator specific for the transgene or its RNA transcript.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Inhibitors of serine protease activity, methods and compositions for treatment of viral infections
InactiveUS6849605B1Inhibit productionIncrease in p2 antigen productionBiocideDipeptide ingredientsImmunodeficiency virusRetroviral infection
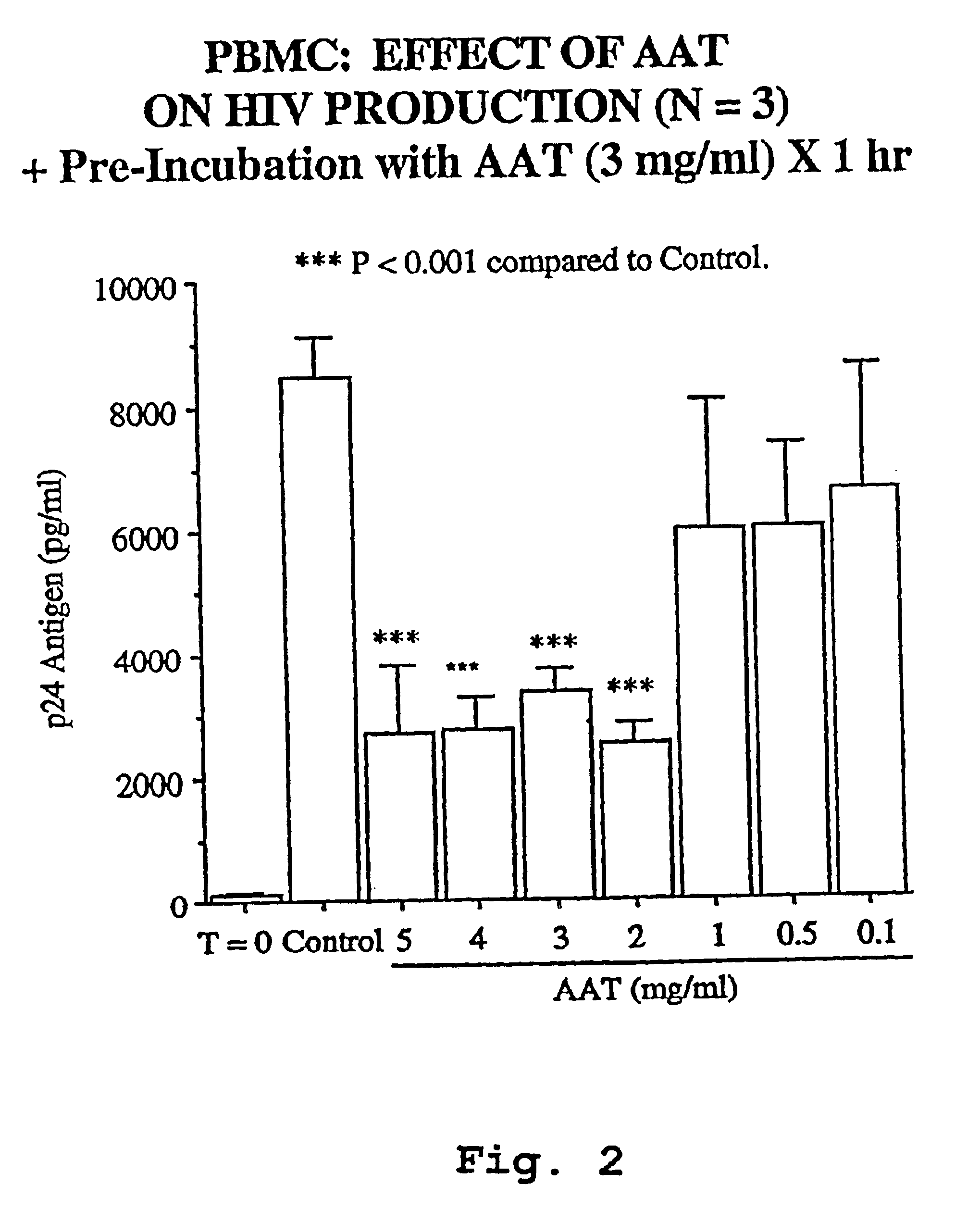
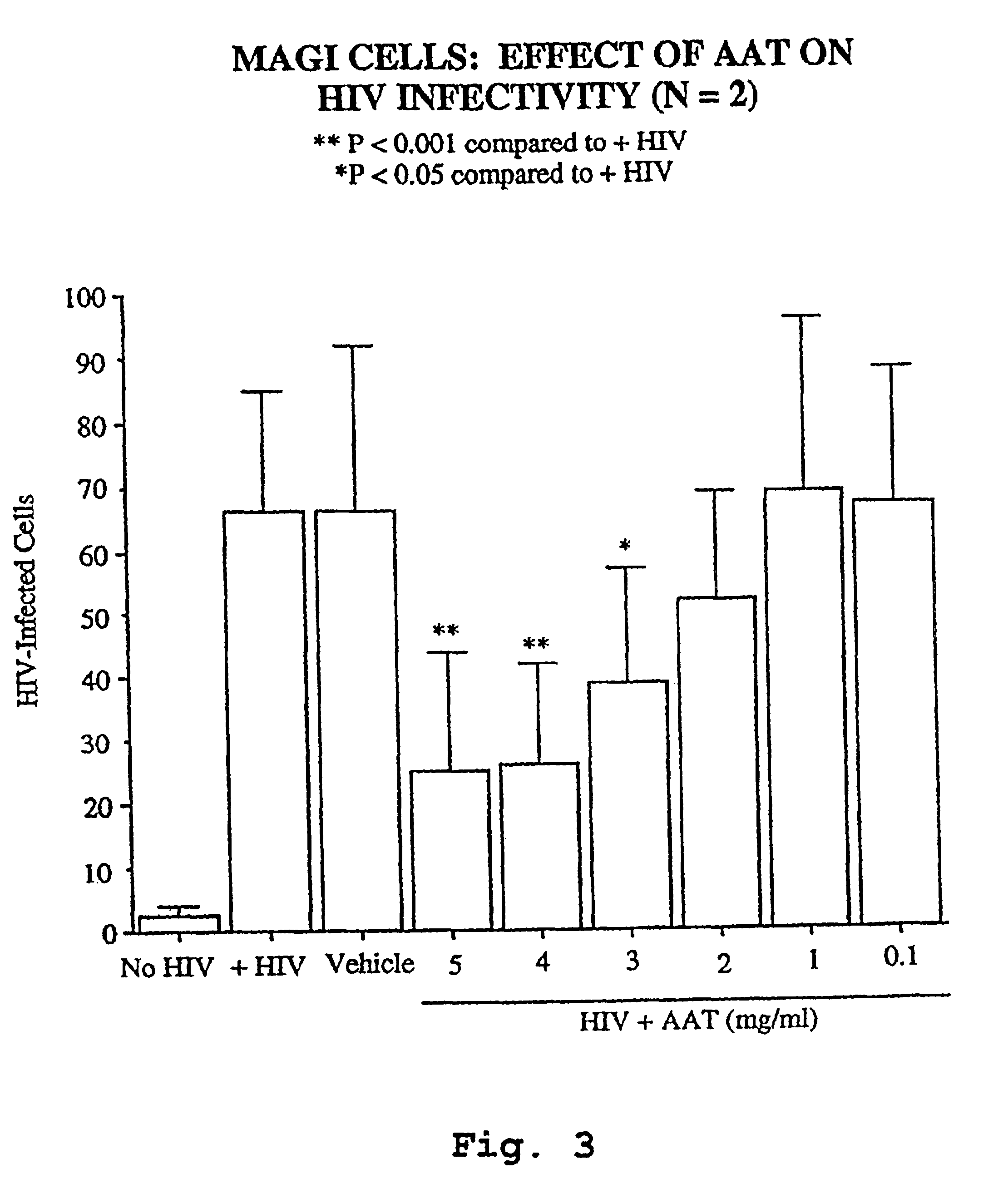
A novel method of treating and preventing viral infection is provided. In particular a method of blocking viral infection facilitated by a serine proteolytic (SP) activity is disclosed, which consists of administering to a subject suffering or about to suffer from viral infection a therapeutically effective amount of a compound having a serine protease inhibitory or serpin activity. Among compounds are α1-antitrypsin (AAT), peptide derivatives from the carboxyterminal end of AAT, and man-made, synthetic compounds mimicking the action of such compounds. The preferred viral infections include retroviral infection such as human immunodeficiency virus (HIV) infection.
Owner:UNIV TECH
Chemokines that inhibit immunodeficiency virus infection and methods based thereon
InactiveUS6214540B1Prevent diseaseInhibition of replicationOrganic active ingredientsPeptide/protein ingredientsCC chemokineImmunodeficiency virus
The present invention relates to therapeutic compositions and methods for treating and preventing infection by an immunodeficiency virus, particularly HIV infection, using chemokine proteins, nucleic acids and / or derivatives or analogues thereof.
Owner:UNIV OF MARYLAND BALTIMORE
Pharmacologically induced transgene ablation system
InactiveUS20130023033A1Nervous disorderFusion with DNA-binding domainSystems designPharmacological chaperone
The present invention relates to gene therapy systems designed for the delivery of a therapeutic product to a subject using replication-defective virus composition(s) engineered with a built-in safety mechanism for ablating the therapeutic gene product, either permanently or temporarily, in response to a pharmacological agent—preferably an oral formulation, e.g., a pill. The invention is based, in part, on the applicants' development of an integrated approach, referred to herein as “PITA” (Pharmacologically Induced Transgene Ablation), for ablating a transgene or negatively regulating transgene expression. In this approach, replication-deficient viruses are used to deliver a transgene encoding a therapeutic product (an RNA or a protein) so that it is expressed in the subject, but can be reversibly or irreversibly turned off by administering the pharmacological agent; e.g., by administration of a small molecule that induces expression of an ablator specific for the transgene or its RNA transcript.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Long lasting fusion peptide inhibitors for hiv infection
InactiveUS20050065075A1Prolong half-life in vivoBiocidePeptide/protein ingredientsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Imaging methods and compositions comprising fluorescent dyes associated with viral components for nerve imaging
Disclosed herein are compositions and methods for imaging nerve cells. The composition comprises a fluorescent dye; and a viral component selected from a neurotropic, replication-defective virus, a viral protein of a neurotropic virus, and a capsid of a neurotropic virus. Although the fluorescent dye in itself cannot penetrate nerve cells, the fluorescent dye is bound to the viral component to form a dye / viral component complex that is capable of penetrating nerve cells.
Owner:STRYKER EUROPEAN OPERATIONS LIMITED
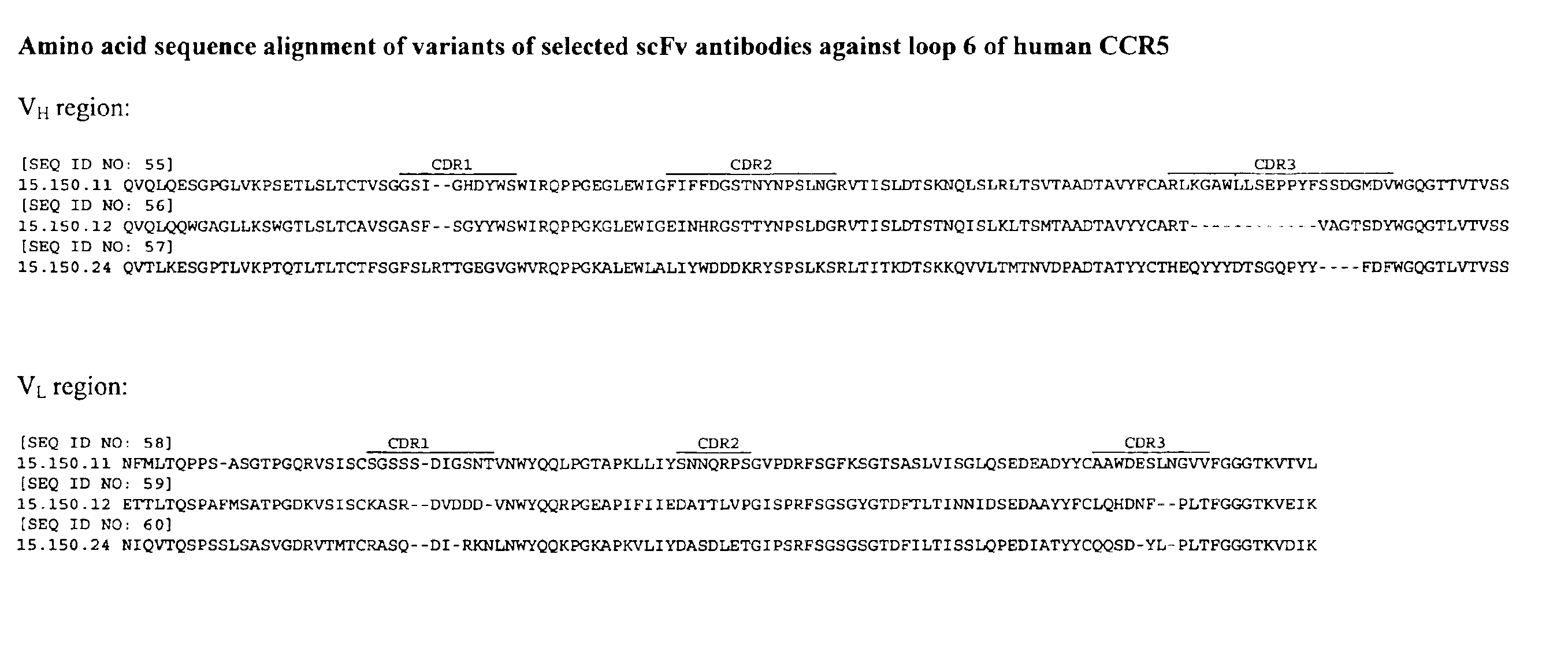
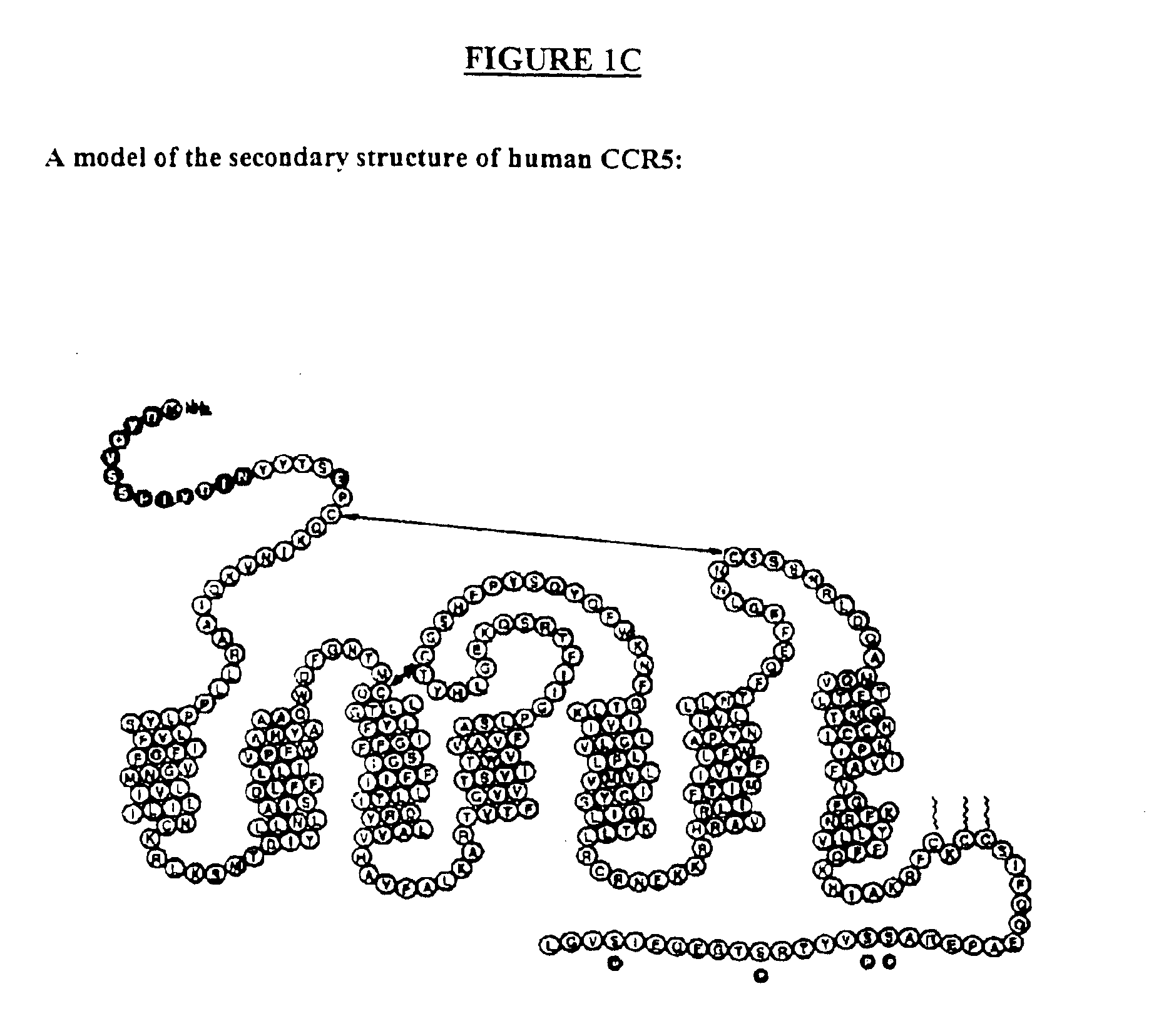
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS7005503B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Immunodeficiency virus
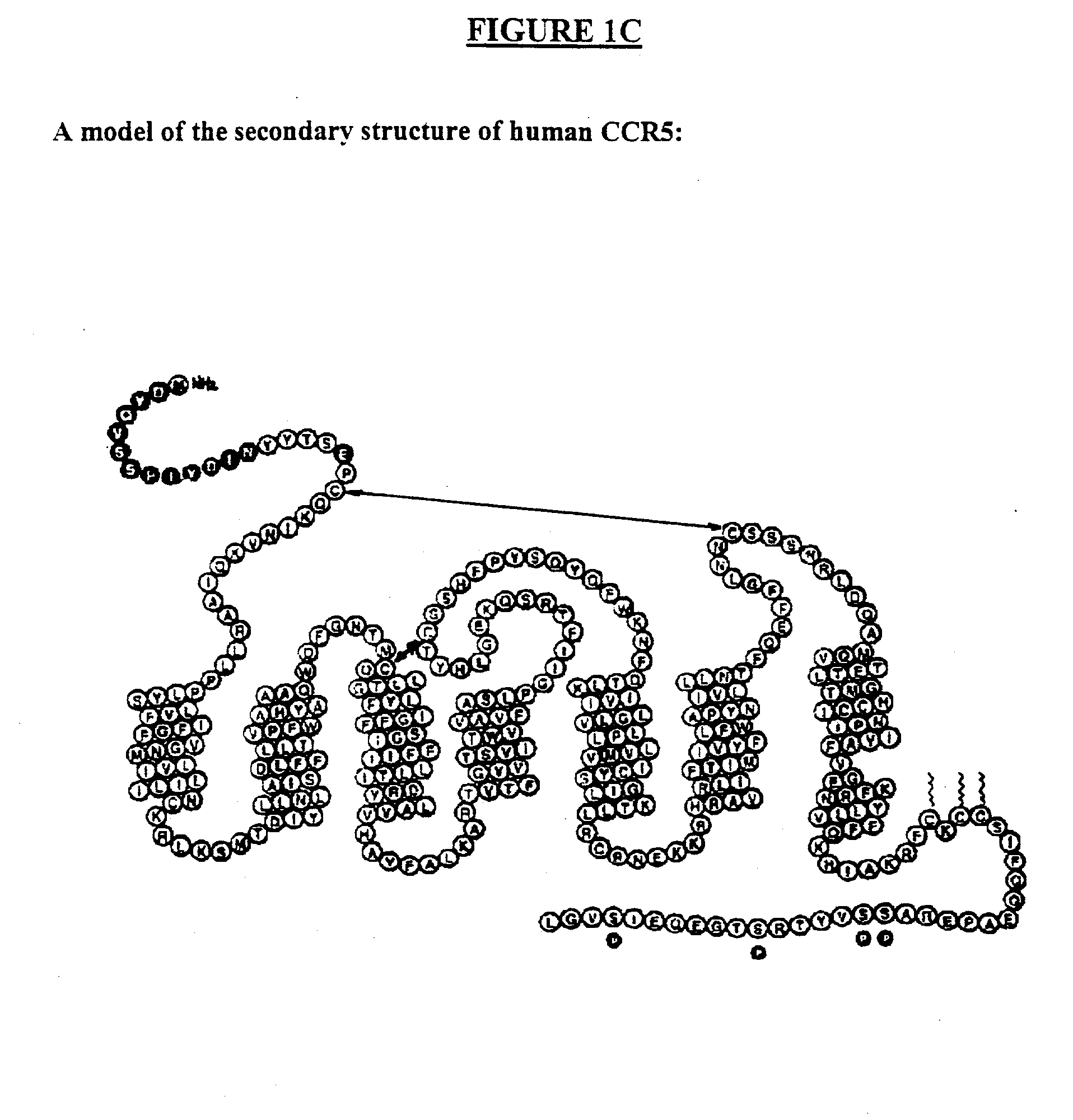
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
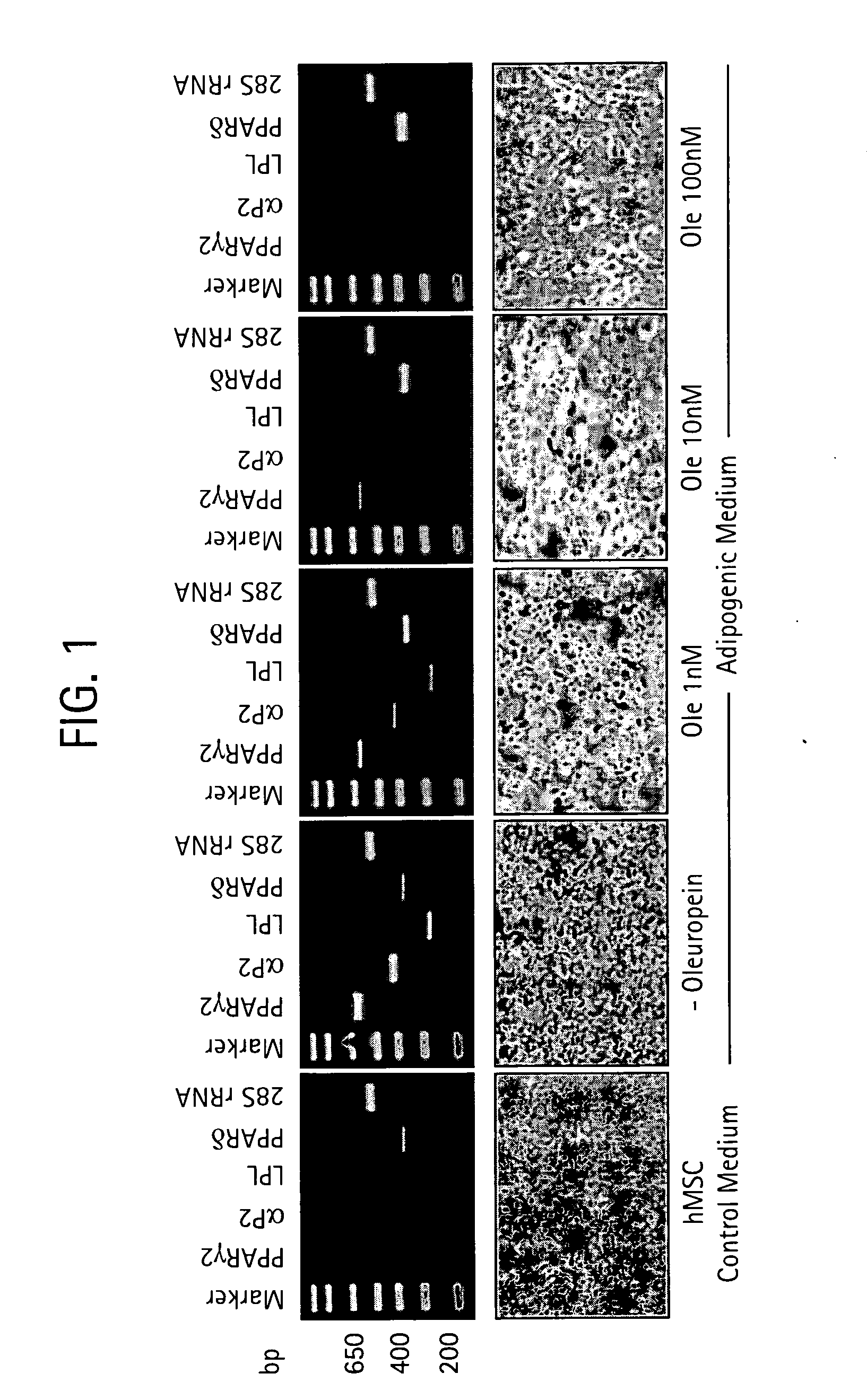
Compositions and methods for treating obesity, obesity related disorders and for inhibiting the infectivity of human immunodeficiency virus
InactiveUS20090061031A1Modulate adipogenesisModulate lipodystrophyBiocideHydroxy compound active ingredientsMetaboliteSecoiridoid Glycosides
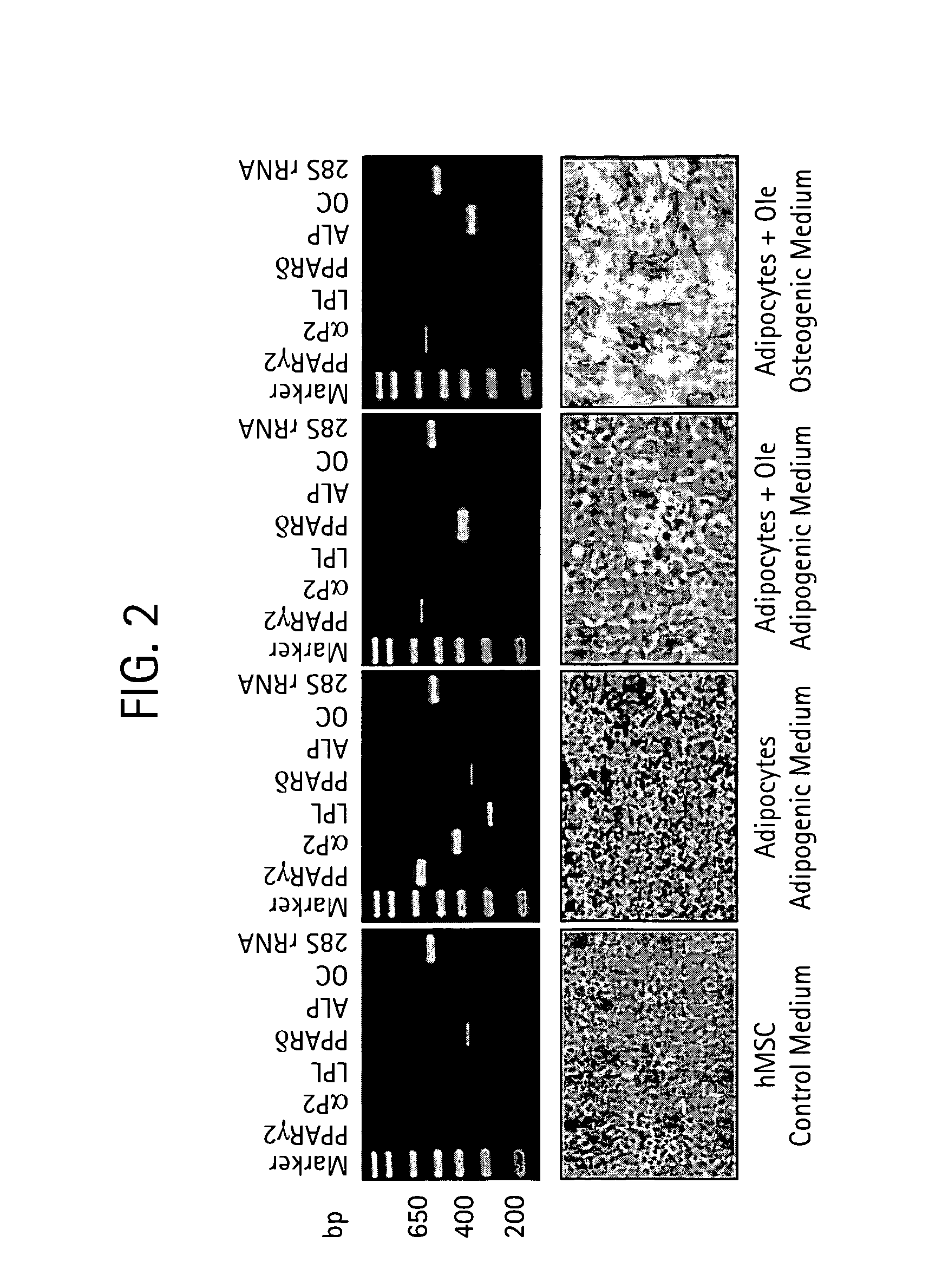
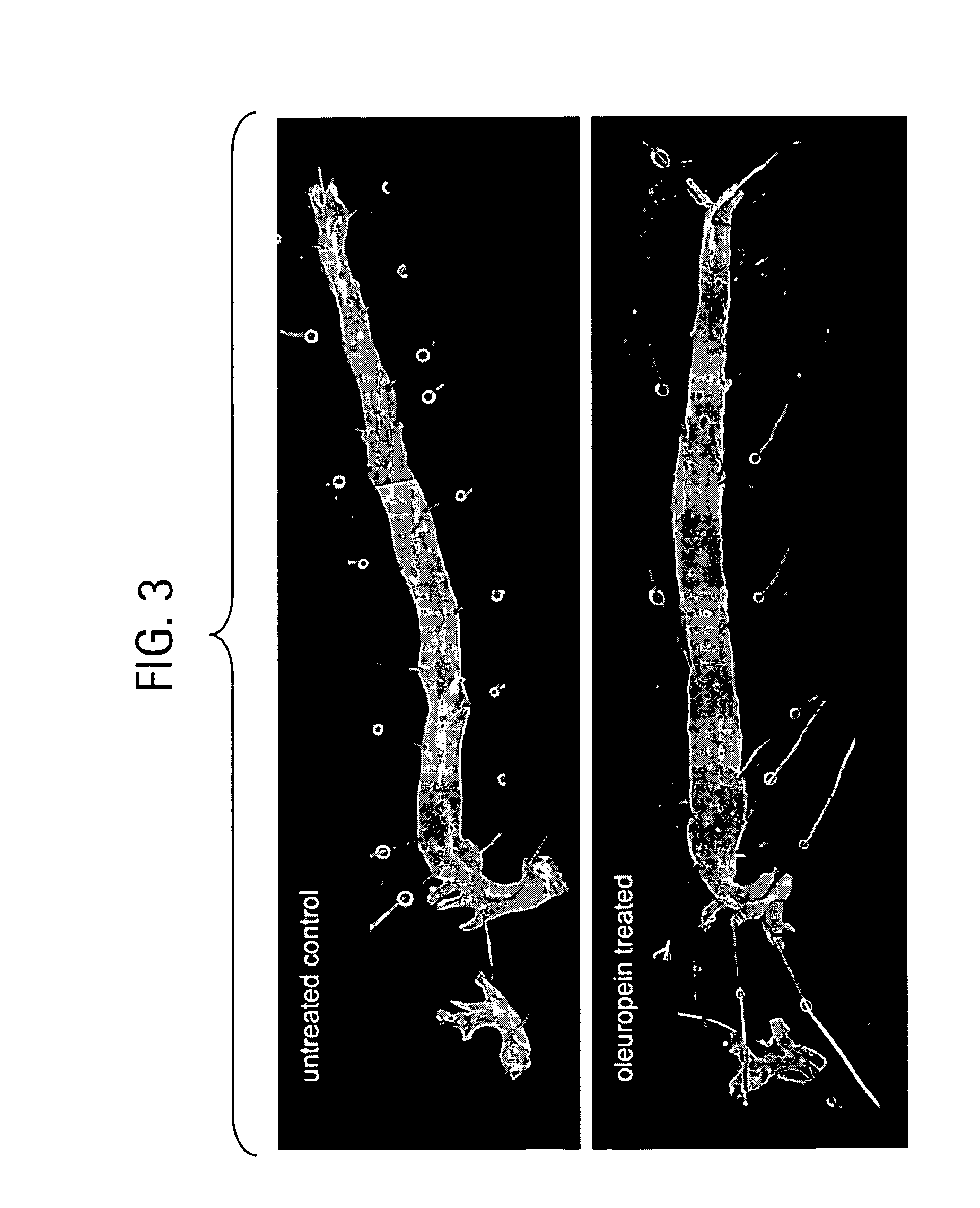
The present invention relates to methods and pharmaceutical compositions for treating obesity or obesity-related disorders in a subject suffering from or predisposed to developing obesity or an obesity-related disorder, or for inhibiting the infectivity of HIV, by administering oleuropein, an analogue or derivative thereof, or the major metabolites of oleuropein including oleuropein aglycone, hydroxytyrosol, and elenolic acid or their analogues, or derivatives thereof, an iridoid glycoside, or a secoiridoid glycoside or analogues or derivatives thereof, or any combination of the foregoing including olive leave extract. The invention also relates to methods for screening / diagnosing a subject having, or predisposed to having obesity or a related disorder by measuring the expression profiles of an adipogenic gene selected from PPARγ2, LPL and αP2 gene and gene product, or other adipogenic, lipogenic, or lipolytic genes and gene products in an individual. The invention further provides for screening for novel oleuropein analogues.
Owner:NEW YORK UNIV +1
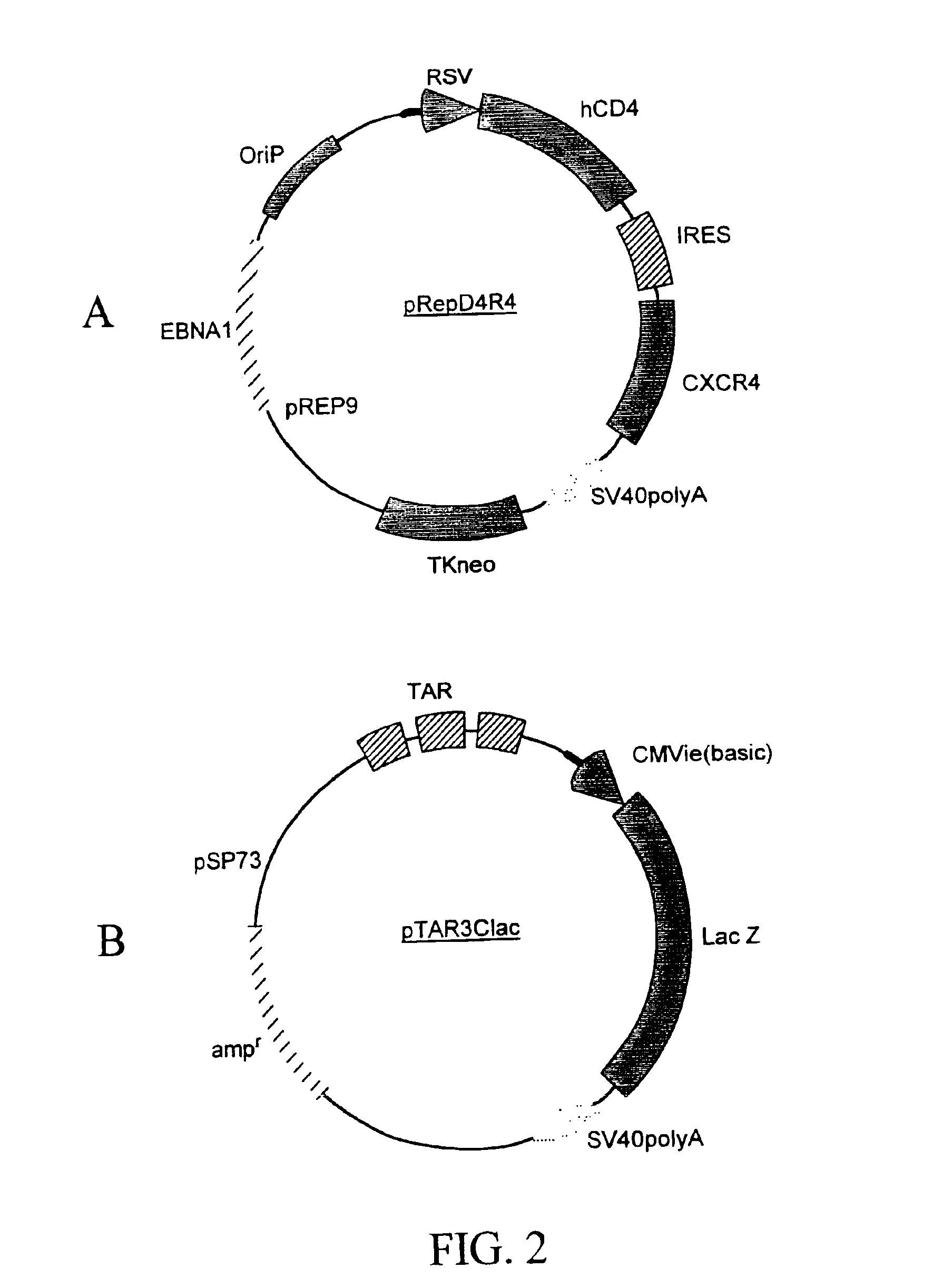
Marker, method and an assay for detection and monitoring of latency and activation of human immunodeficiency virus
A TAR marker, method for detection and monitoring of HIV latency and activation and an assay for detection of the marker. The assay sensitively detects HIV transcription and monitors HIV transcriptional activity by detecting the presence of TAR fragments and full length transcripts, quantifying both and determining the ratio of short to long transcripts. A low ratio correlates with a latent-type transcriptional activity of HIV whereas the appearance of long transcripts signifies increased efficiency of transcriptional activity of HIV and the transition from latency to activation. The size difference between the TAR fragments appearing predominantly in latency and the full length transcripts appearing predominantly during the HIV activation is detected by RT-PCR assay that utilizes novel primers and probes. The obtained ratio is a sensitive tool in detection of HIV infection, the analysis of load of latent and active virus and monitoring the transition from the latent to active state of HIV replication.
Owner:RGT UNIV OF CALIFORNIA
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
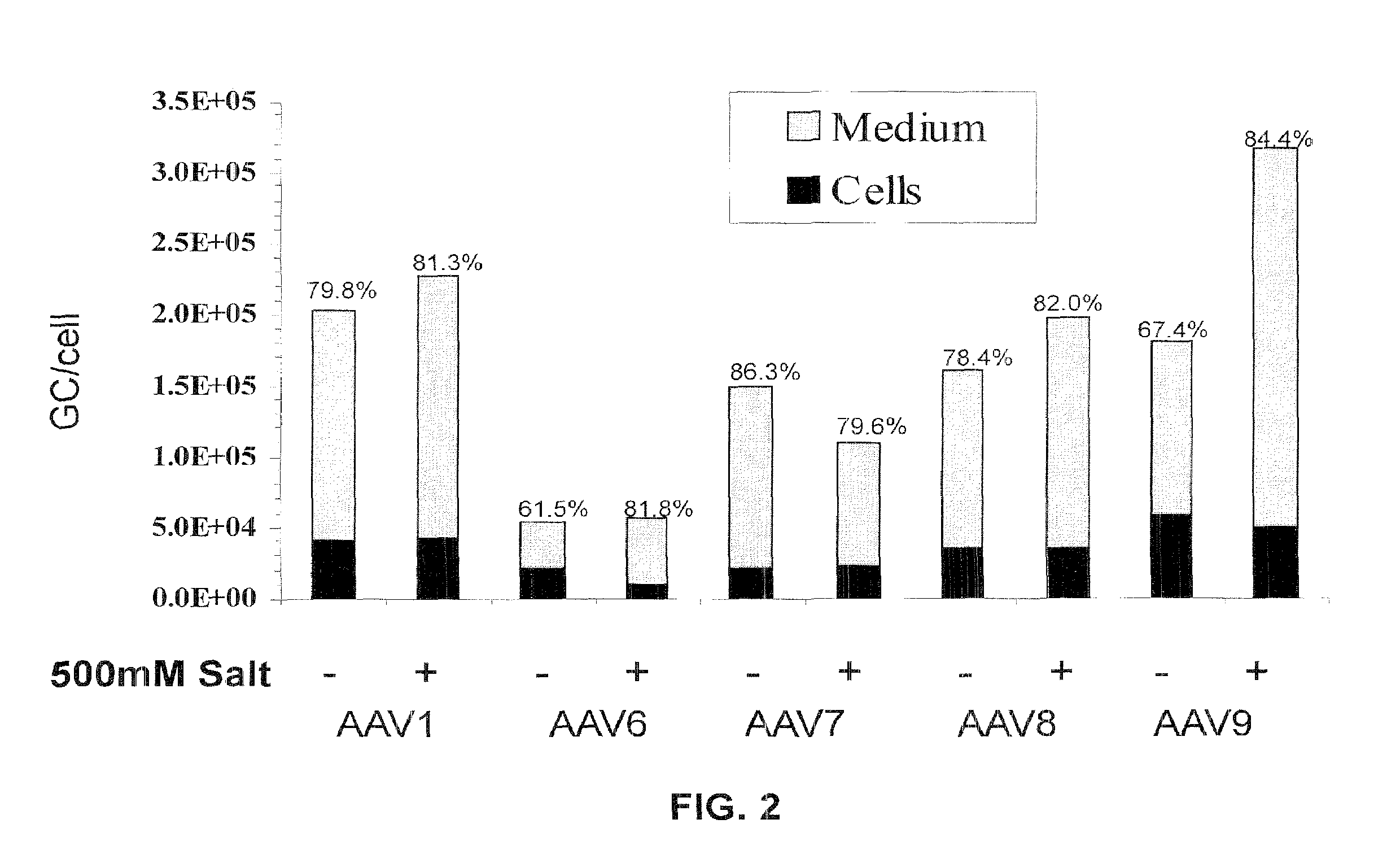
Adeno-associated virus materials and methods
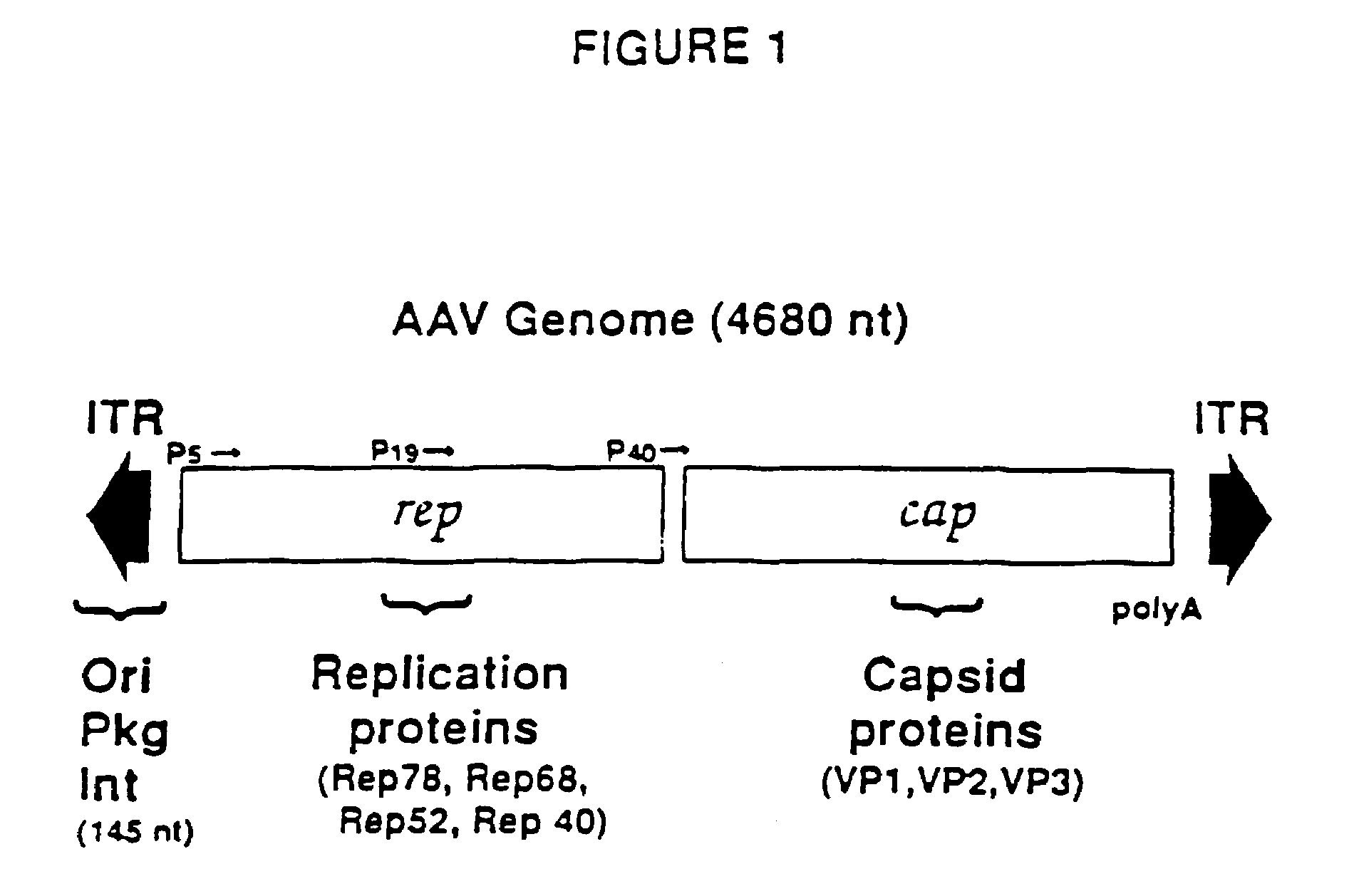
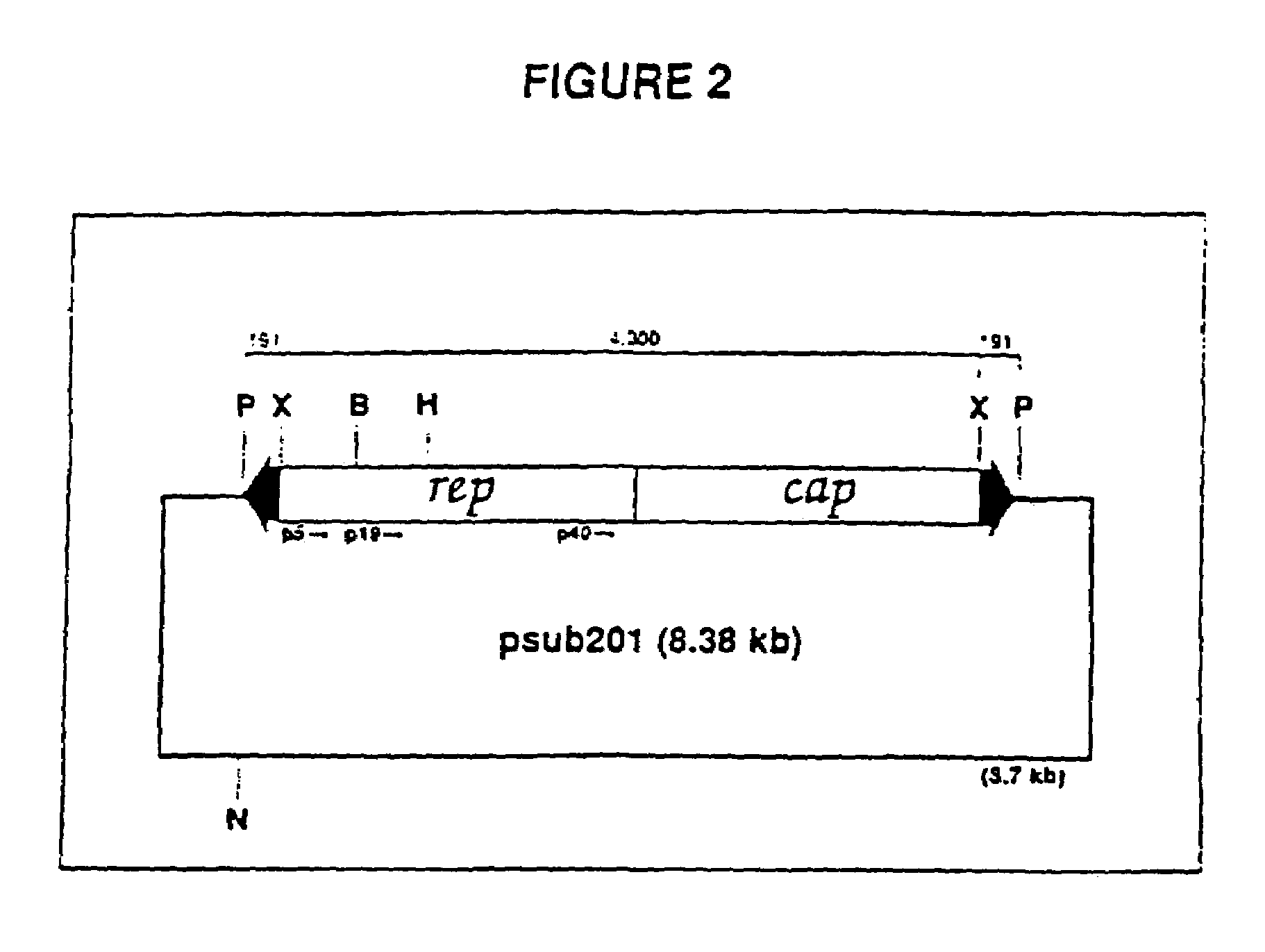
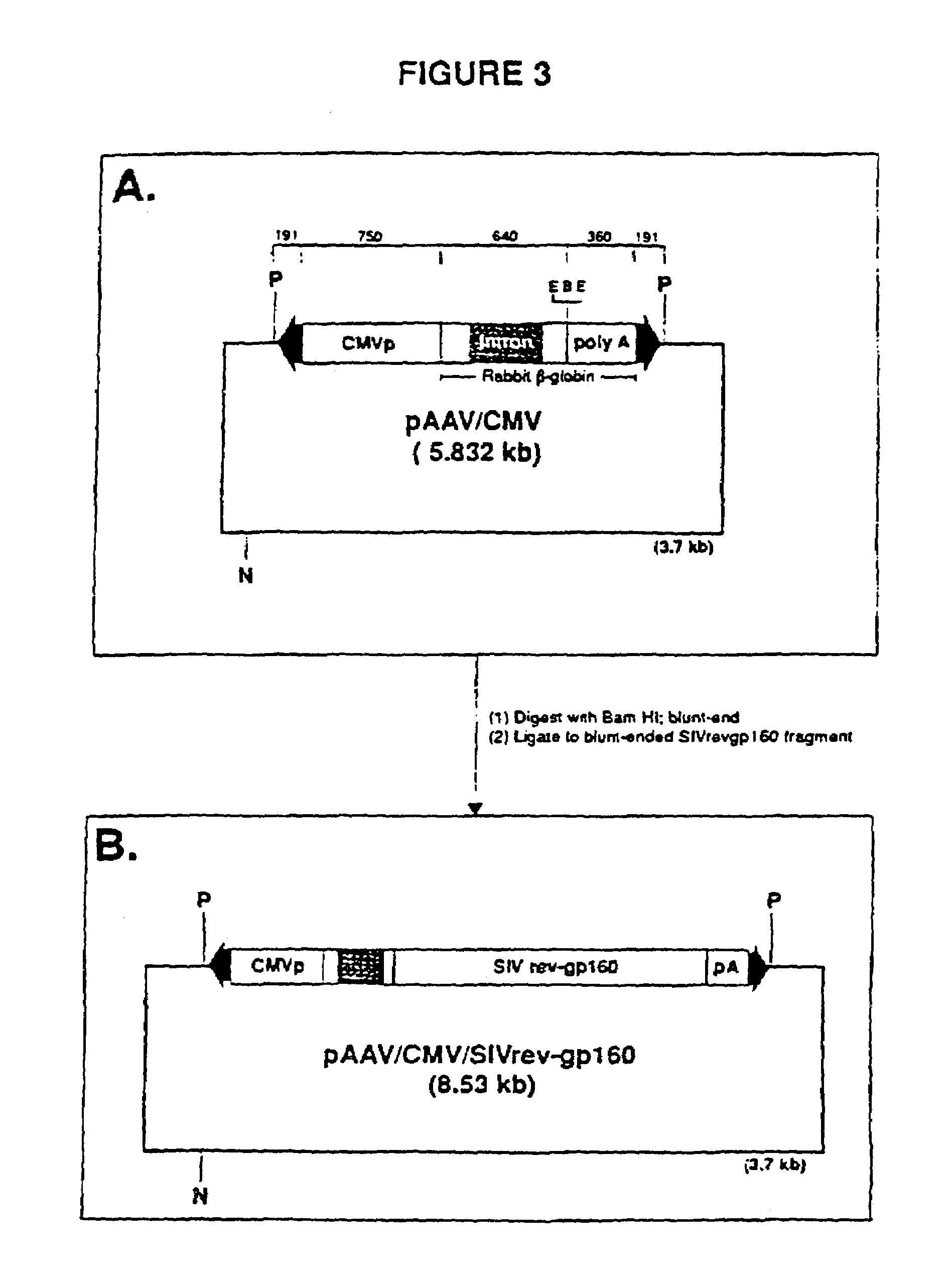
The present invention provides adeno-associated virus (AAV) materials and methods which are useful for DNA delivery to cells. More particularly, the invention provides recombinant AAV (rAAV) genomes, methods for packaging rAAV genomes, stable host cell lines producing rAAV and methods for delivering genes of interest to cells utilizing the rAAV. Particularly disclosed are rAAV useful in generating immunity to human immunodeficiency virus-1 and in therapeutic gene delivery for treatment of neurological disorders.
Owner:CHILDRENS HOSPITAL
Treatment and prevention of immunodeficiency virus infection by administration of non-pyrogenic derivatives of lipid A
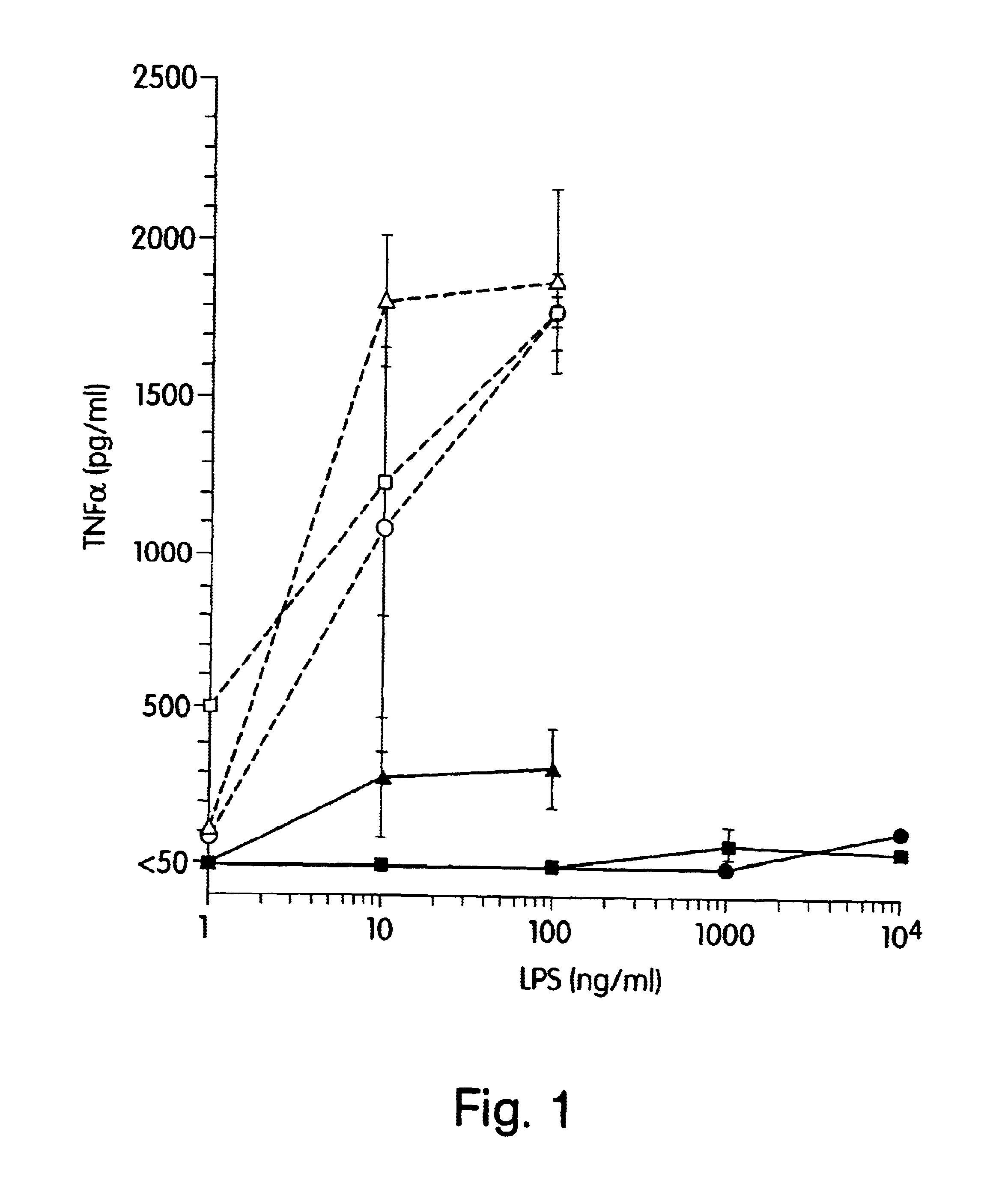
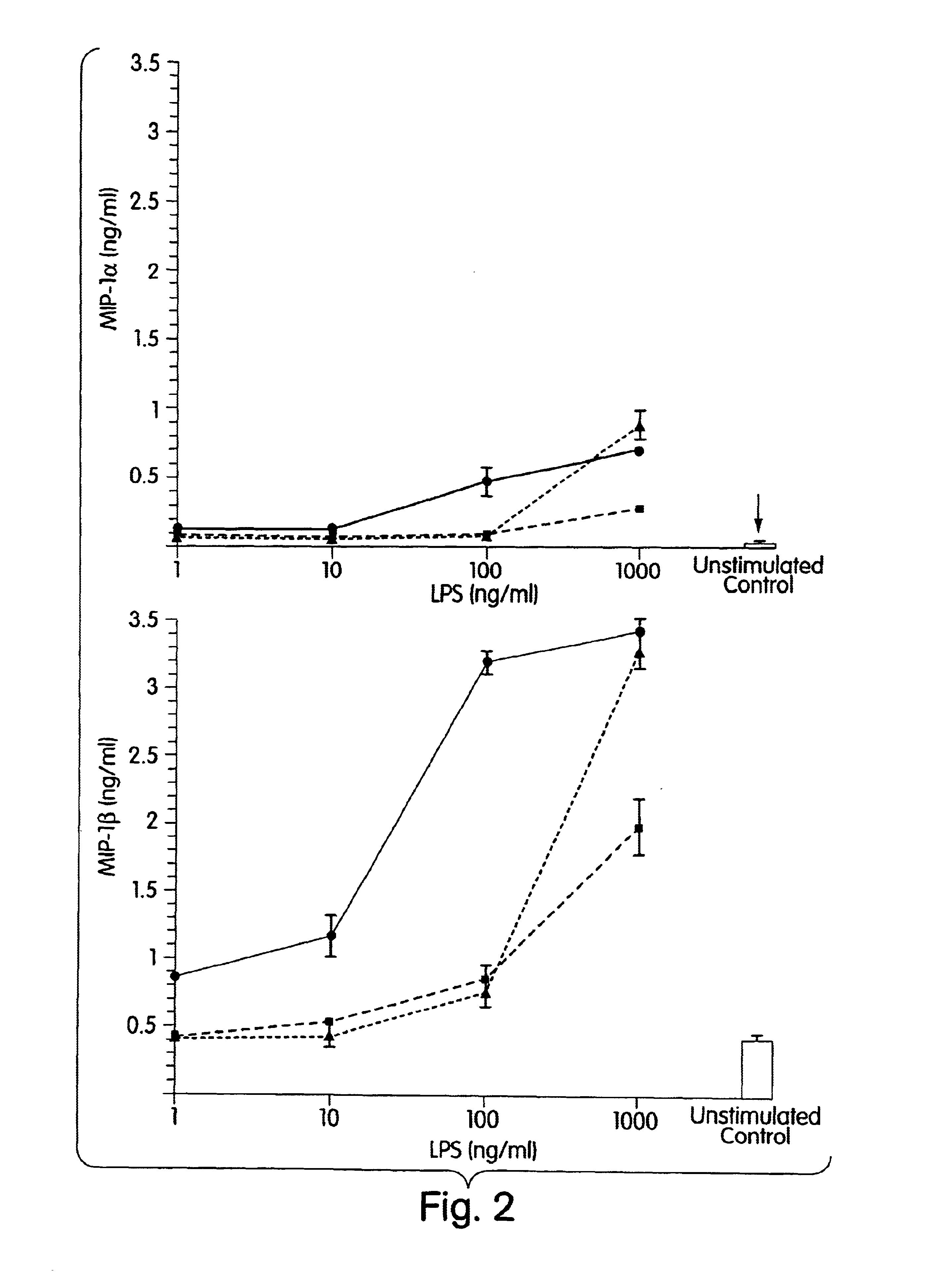
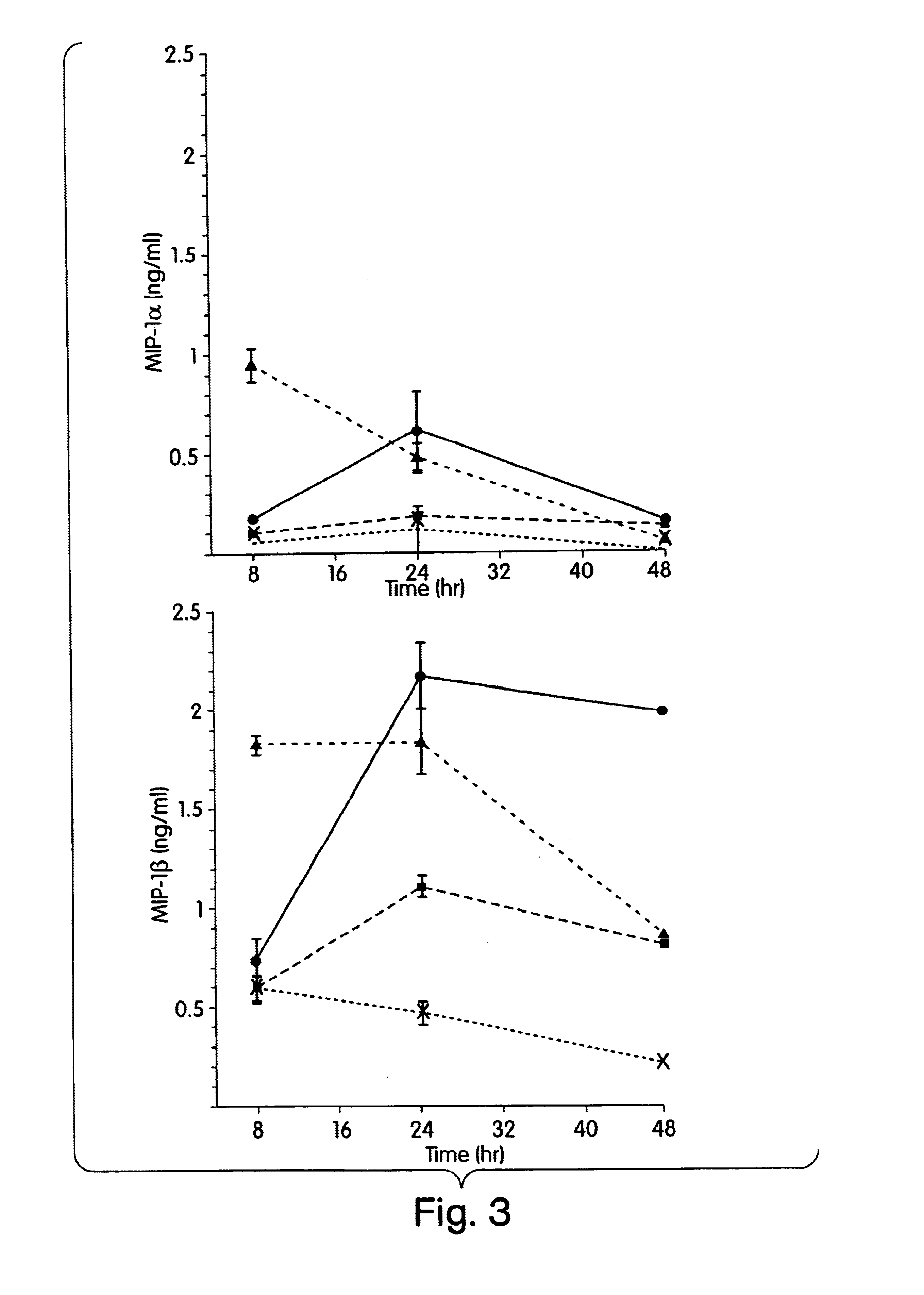
The present inventors have found that certain preparations containing LPS and / or lipid A variants, derivatives, and / or analogs demonstrate non-pyrogenic properties and exhibit anti-viral activities. In particular, non-pyrogenic preparations of LPS, lipid A, LPS antagonists and lipid A antagonists, and derivatives thereof induce β chemokine secretion, such as MIP-1β, but not proinflammatory cytokines, such as TNFα, IL-1β and IL-6. Non-pyrogenic preparations of the invention have been demonstrated by the Applicant to suppress HIV replication in human peripheral blood monocytes, as described by way of example herein. The present invention provides preparations of LPS or lipid A variants, analogs and derivatives of decreased or absent pyrogenicity which can be used as therapeutics for the treatment or prevention of immunodeficiency virus infection and its consequences.
Owner:UNIV OF MARYLAND BIOTECH INST
Compositions and methods for detecting human immunodeficiency virus
InactiveUS6900010B2Robust and efficient and sensitive in detectionEarly diagnosisBiocideGenetic material ingredientsHeterologousInfected cell
Owner:MUSC FOUND FOR RES DEV
Cell lines for production of replication-defective adenovirus
ActiveUS20060270041A1Promote infectionIncrease productionSugar derivativesGenetically modified cellsPolymerase LDefective virus
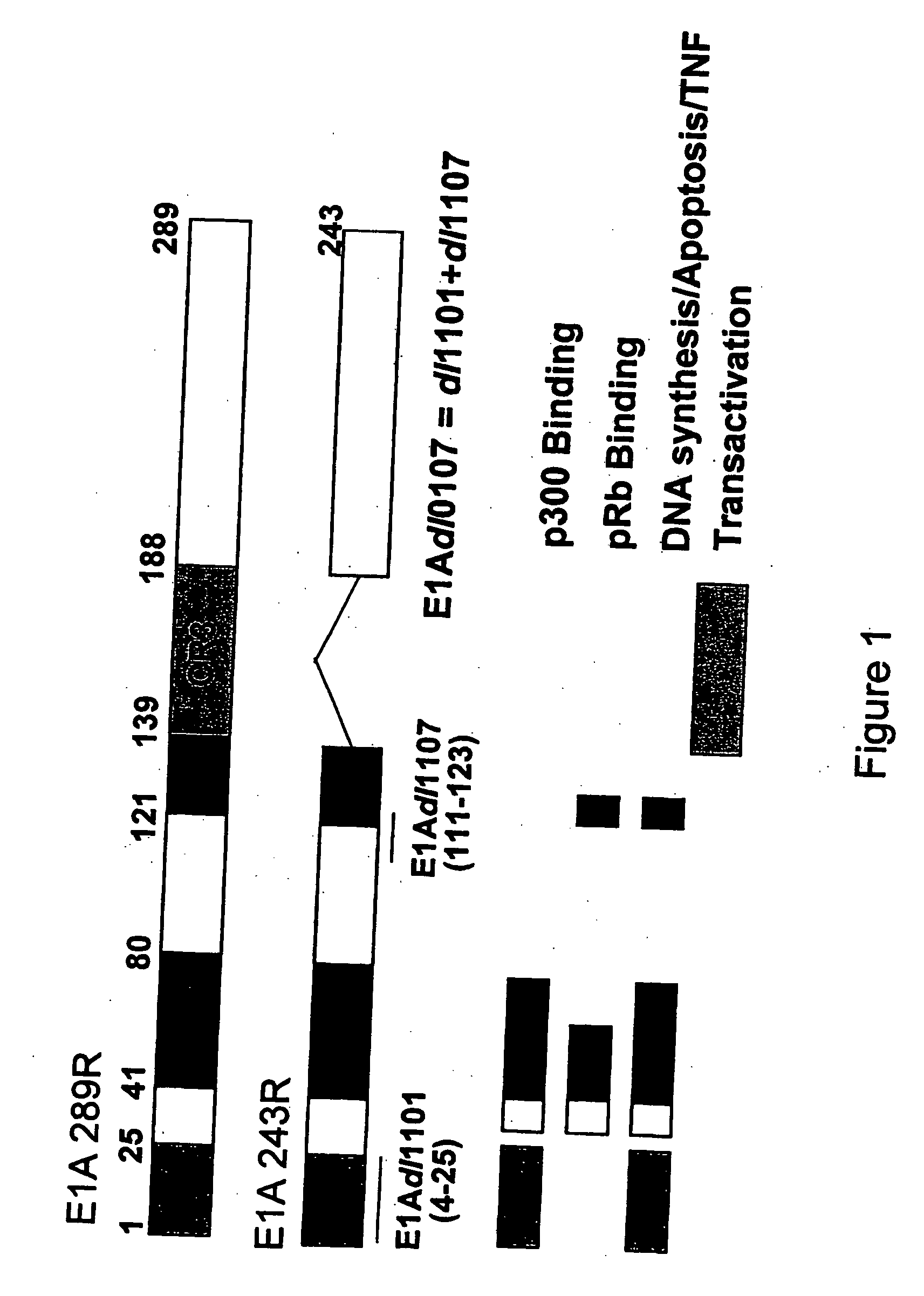
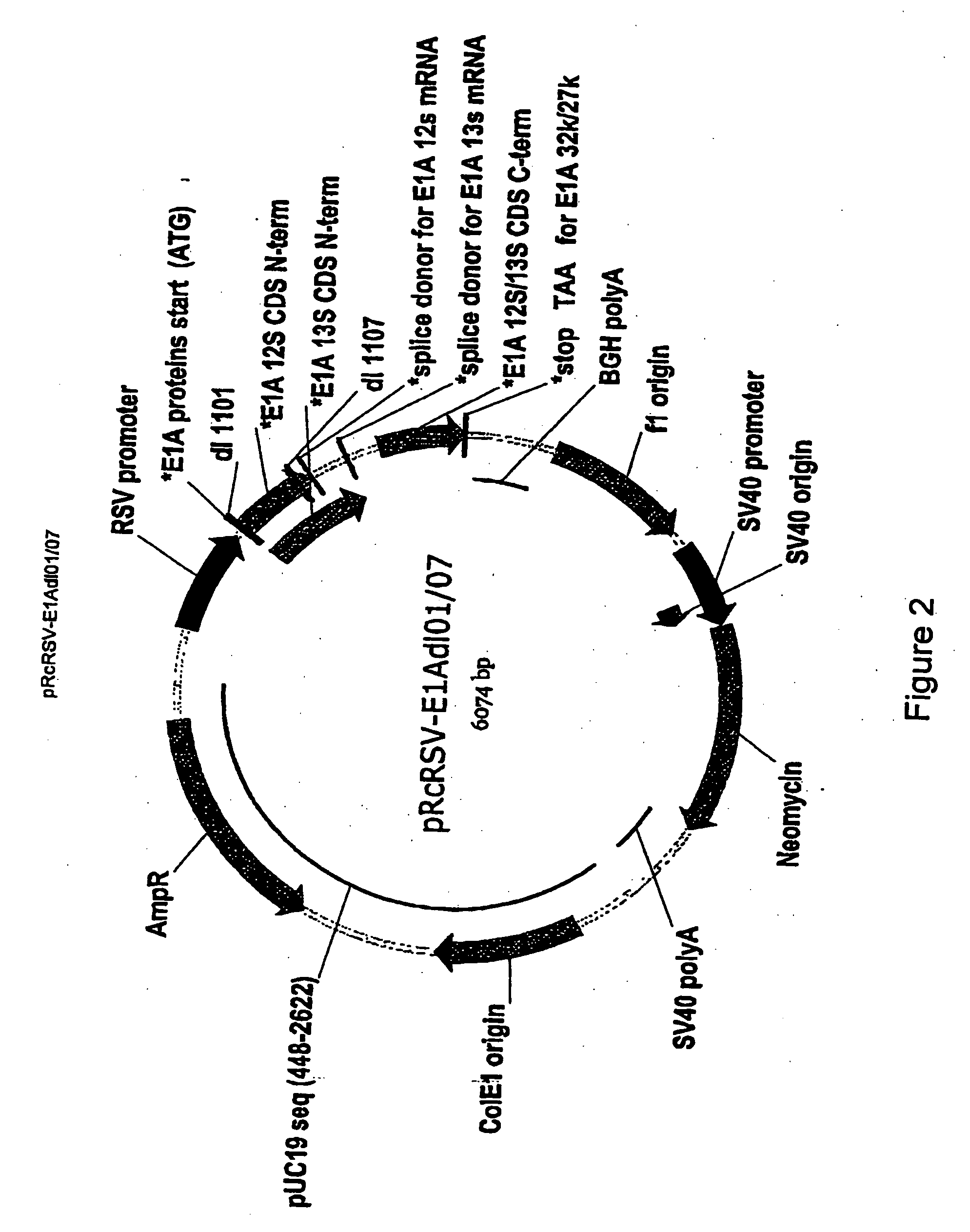
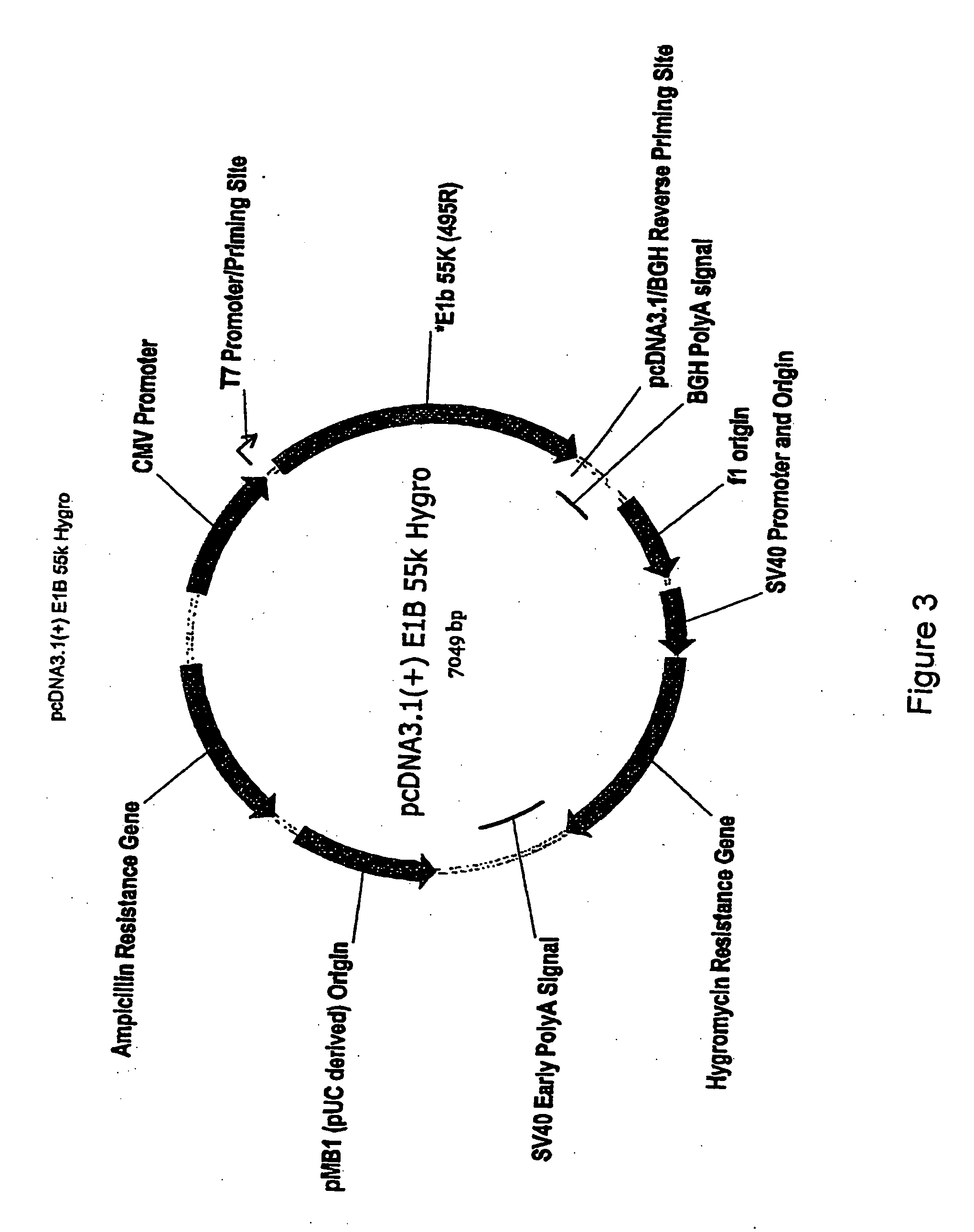
The present invention provides cell lines for the production of E1-deleted adenovirus (rAd) vectors that complement E1A and E1B functions. The present invention also provides cell lines for the production of E1- and E2-deleted adenovirus vectors that complement E1A, E1B and E2B polymerase functions. The invention provides particular cell lines that complement E1A function by insertion of an E1A sequence containing mutations in the 243R and 289R proteins and an E1B sequence comprising the E1B-55K gene. Production yields in the resulting producer cell lines, designated SL0003 and SL0006, were similar to those obtained from 293 cells without generation of detectable recombinant replication competent adenovirus (“RCA”).
Owner:CANJI
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS20030152913A1Microbiological testing/measurementImmunoglobulins against virusesCXCR4Immunodeficiency virus
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
Retrovirus from the HIV type O and its use (MVP-2901/94)
A novel HIV type O immunodeficiency virus is disclosed which has the designation MVP-2901 / 94 and which has been deposited with the European collection of animal Cell Cultures (ECACC) under No. V 950121601. The characteristic antigens which can be obtained from the virus and which can be employed for detecting antibodies against retroviruses which are associated with immunodeficiency diseases are also disclosed, as are the partial DNA and amino acid sequences of the virus.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Inhibitors of human immunodeficiency virus replication
ActiveUS20100311735A1Inhibitory activityReduce HIV replicationBiocideOrganic chemistryMedicineDefective virus
Owner:GILEAD SCI INC
Pharmacologically induced transgene ablation system
The present invention relates to gene therapy systems designed for the delivery of a therapeutic product to a subject using replication-defective virus composition(s) engineered with a built-in safety mechanism for ablating the therapeutic gene product, either permanently or temporarily, in response to a pharmacological agent—preferably an oral formulation, e.g., a pill. The invention is based, in part, on the applicants' development of an integrated approach, referred to herein as “PITA” (Pharmacologically Induced Transgene Ablation), for ablating a transgene or negatively regulating transgene expression. In this approach, replication-deficient viruses are used to deliver a transgene encoding a therapeutic product (an RNA or a protein) so that it is expressed in the subject, but can be reversibly or irreversibly turned off by administering the pharmacological agent; e.g., by administration of a small molecule that induces expression of an ablator specific for the transgene or its RNA transcript.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com