Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
281 results about "Viral Genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene therapy uses genetically modified viruses to deliver genes that can cure diseases in human cells.These viruses can deliver DNA or RNA genetic material to the targeted cells. Gene therapy is also used by inactivating mutated genes that are causing the disease using viruses.
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Methods and compositions for improved non-viral gene therapy
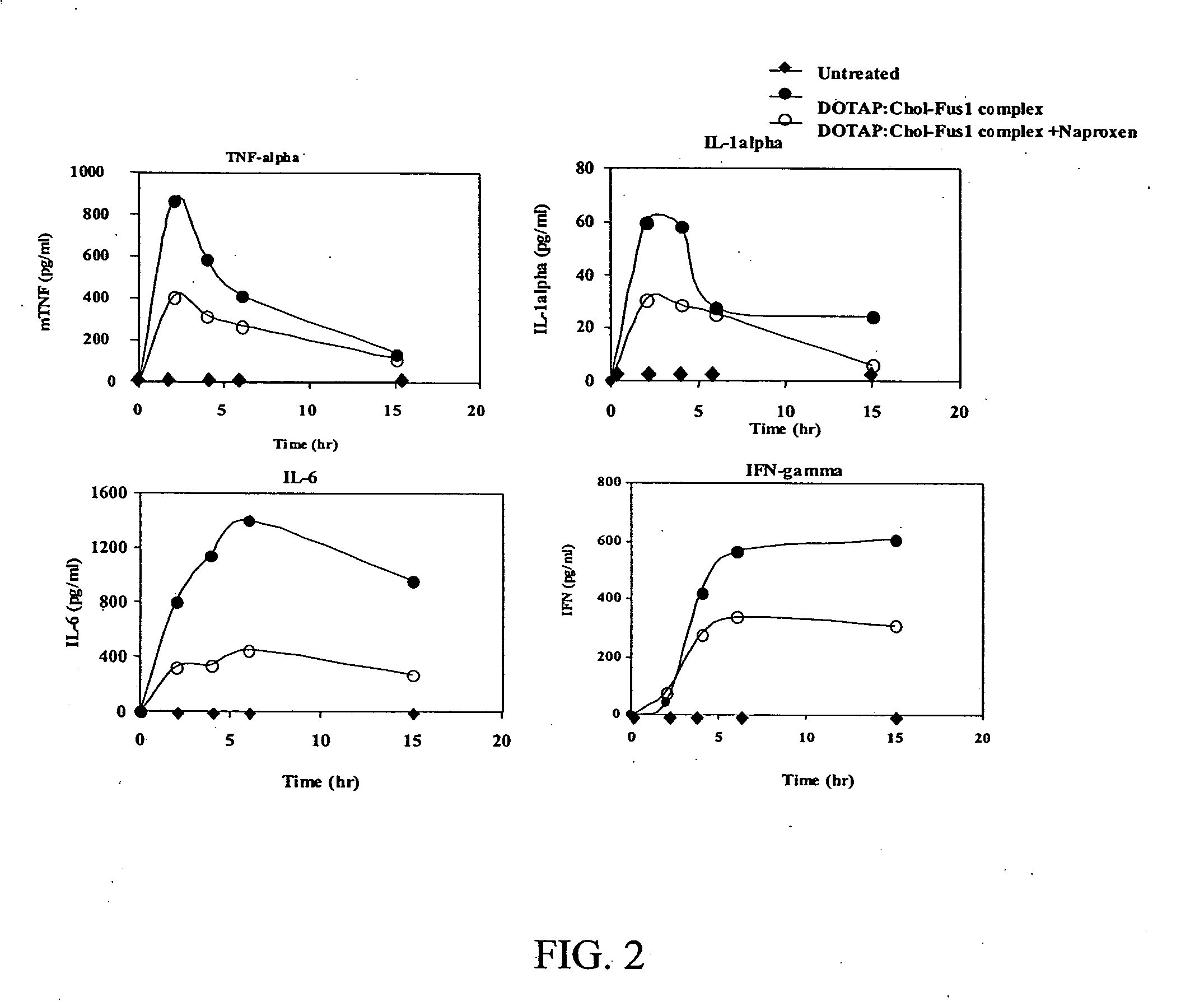
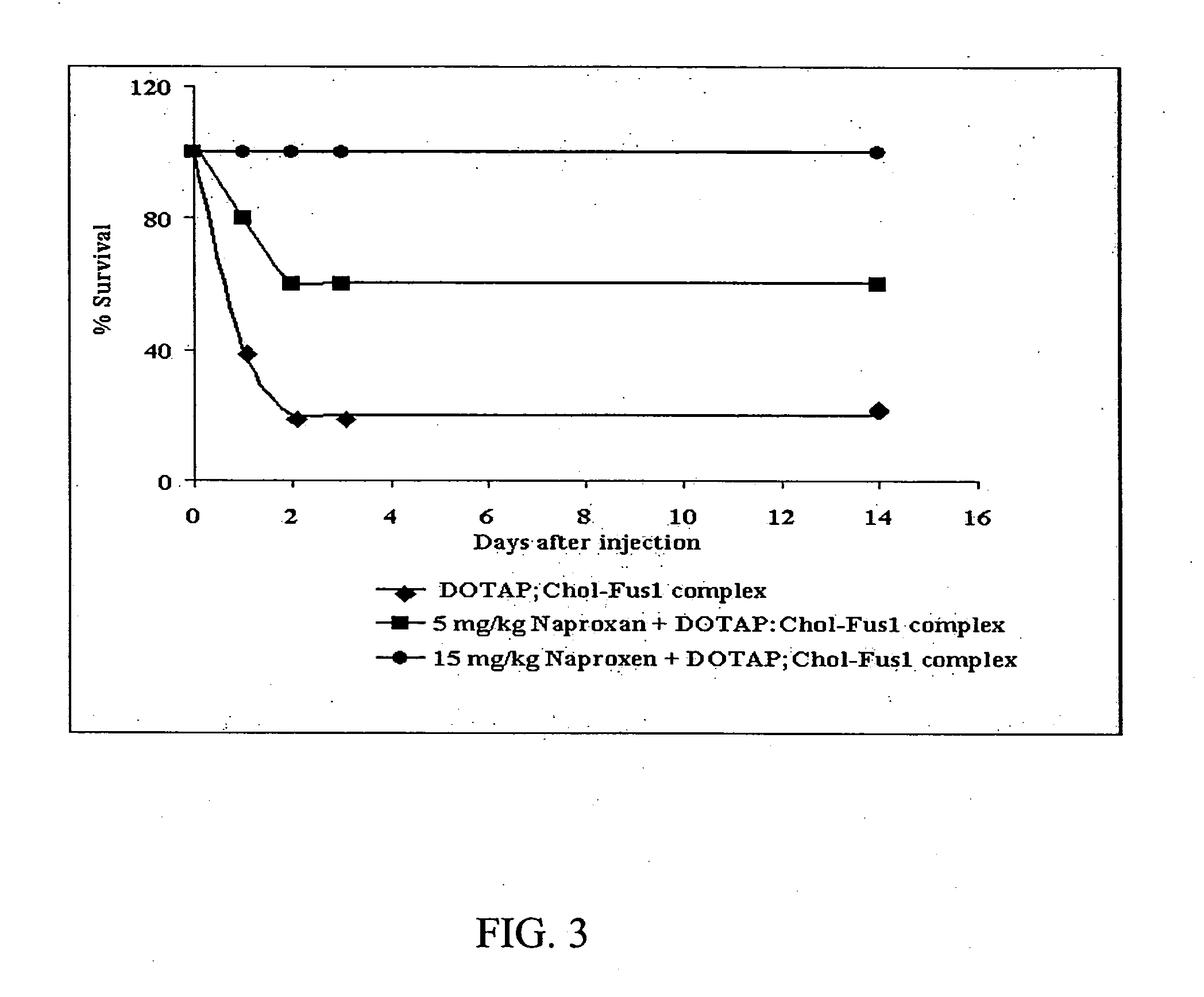
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
RNAi Agents Comprising Universal Nucleobases
InactiveUS20080213891A1Broad scopeReduce needSugar derivativesPolymorphism usesNitroimidazoleViral Genes
One aspect of the present invention relates to an oligonucleotide agent comprising at least one universal nucleobase. In certain embodiments, the universal nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the universal nucleobase is difluorotolyl. In certain embodiments, the oligonucleotide is double-stranded. In certain embodiments, the oligonucleotide is single-stranded. Another aspect of the present invention relates to a method of altering the expression level of a target in the presence of target sequence polymorphism. In a preferred embodiment, the oligonucleotide agent alters the expression of different alleles of a gene. In another preferred embodiment, the oligonucleotide agent alters the expression level of two or more genes. In another embodiment, the oligonucleotide agent alters the expression level of a viral gene from different strains of the virus. In another embodiment, the oligonucleotide agent alters the expression level of genes from different species.
Owner:ALNYLAM PHARMA INC
Transfection results of non-viral gene delivery systems by influencing of the innate immune system
The innate immune system of eukaryotes is able to recognise foreign genetic material by means of Toll-like receptors and to initiate signal transduction cascades that trigger an antiviral state of cell populations by way of an interferon response. That antiviral state is also a barrier for non-viral gene delivery systems. If the signal transduction cascade is interrupted intracellularly or intercellularly, transfection efficiencies of non-viral gene delivery systems can be increased and undesirable changes in the expression profile can be avoided. Since RNA-interference is to be attributed to the antiviral state, the RNAi machinery is likewise activated after activation of the innate immune system. In that way, knock-down efficiencies on transfection with siRNA can be increased.
Owner:BIONTEX LAB
Methods and means for targeted gene delivery
InactiveUS7033834B2Efficient introductionEfficiently introducedBiocideGenetic material ingredientsGene deliveryDelivery vehicle
A method for producing viral gene delivery vehicles which can be transferred to pre-selected cell types by using targeting conjugates. The gene delivery vehicles comprise: 1) the gene of interest; and 2) a viral capsid or envelope carrying a member of a specific binding pair, the counterpart of which is not directly associated with the surface of the target cell. These vehicles can be rendered unable to bind to their natural cell receptor. The targeting conjugates include the counterpart member of the specific binding pair, linked to a targeting moiety which is a cell-type specific ligand (or fragments thereof). The number of the specific binding pair present on the viral vehicles can be, for example, an immunoglobulin binding moiety (e.g., capable of binding to a Fc fragment, protein A, protein G, FcR or an anti-Ig antibody), or biotin, avidin or streptavidin. The virus' outer membrane or capsid may contain a substance which mediates entrance of the gene delivery vehicle into the target cell. Due to the specificity of the ligand, the binding pair's high affinity, and the gene delivery vehicle's inability to be targeted when used alone, the universality of the method for gene delivery, together with its high cell type selectively can be achieved by using various targeting conjugates.
Owner:JANSSEN VACCINES & PREVENTION BV
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS6884414B1Quick changeAvoid problemsSsRNA viruses negative-senseBiocideTumor reductionIn vivo
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Methods of gene therapy using herpes viral vectors expressing GM-CSF
A genetically disabled mutant virus has a genome which is defective in respect of a selected gene that is essential for the production of infectious new virus particles, and which carries heterologous genetic material encoding an immunomodulatory protein such as GM-CSF, IL-2, or others, such that the mutant virus can infect normal host cells and cause expression of immunomodulatory protein, but the mutant virus cannot cause production of infectious new virus particles except when the virus infects recombinant complementing host cells expressing a gene that provides the function of the essential viral gene; the site of insertion of the heterologous genetic material encoding the immunomodulatory protein preferably being at the site of the defect in the selected essential viral gene. Uses include prophylactic and therapeutic use in generating an immune response in a subject treated therewith; use in the preparation of an immunogen such as a vaccine for use in tumor therapy; use in the in-vitro expansion of (e.g. virus-specific) cytotoxic T cells; and therapeutic or prophylactic use in corrective gene therapy.
Owner:CANTAB PHARMA RES
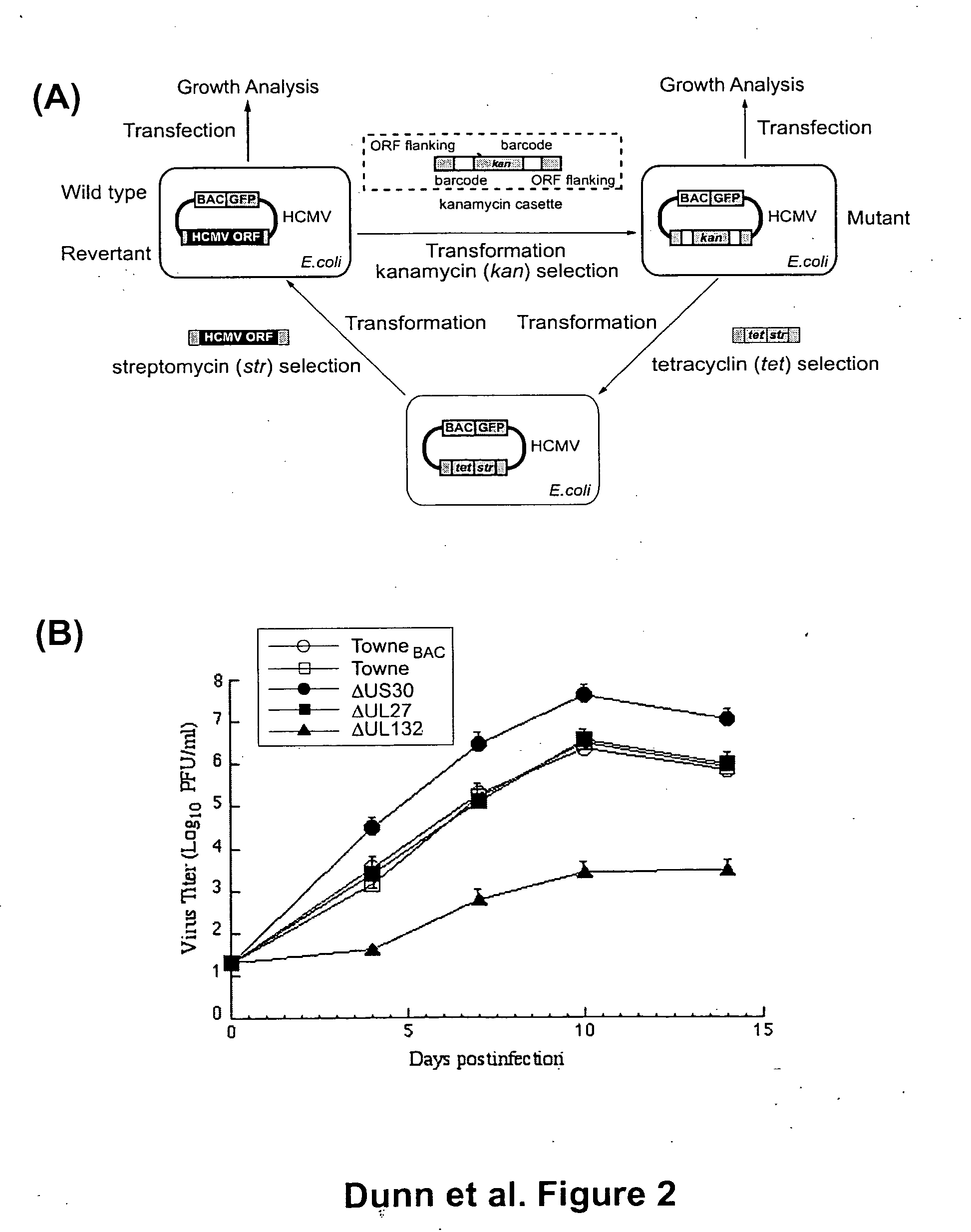
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
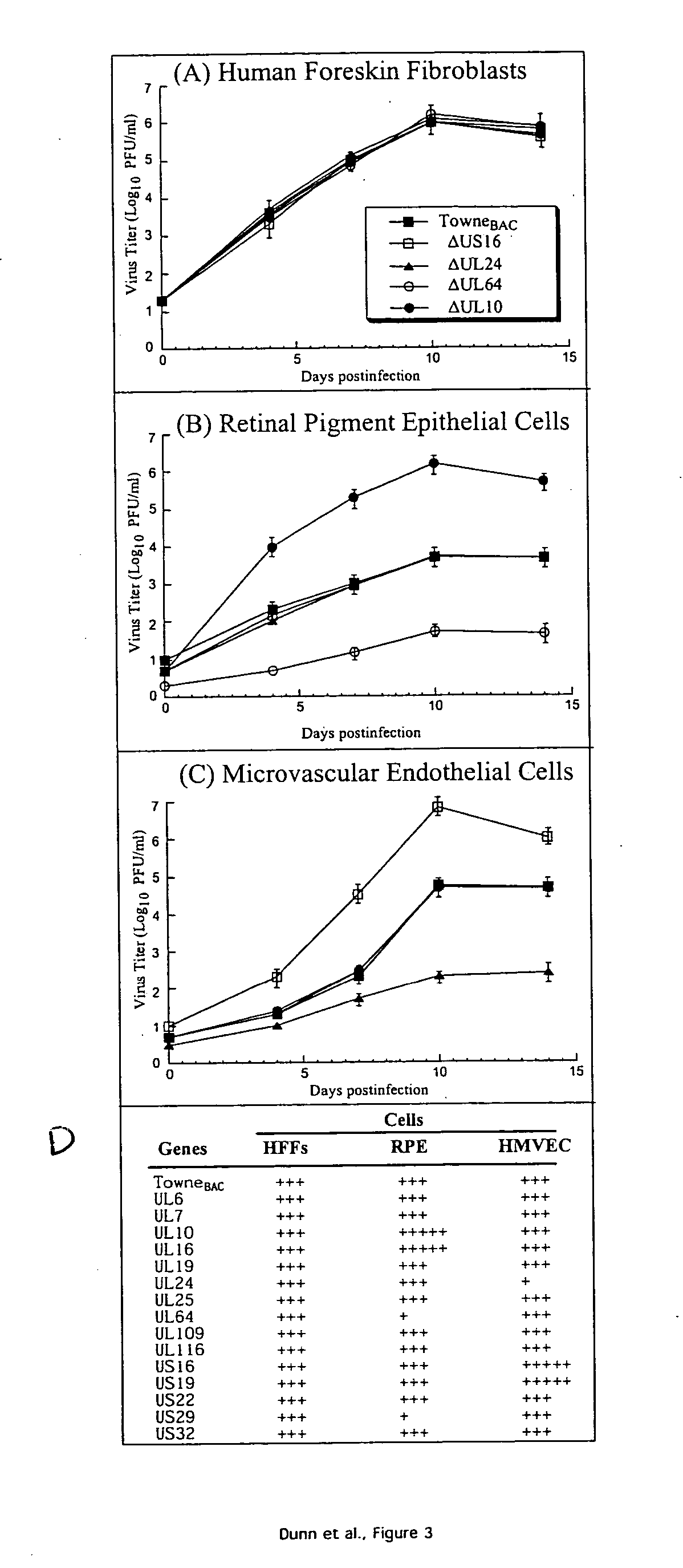
ActiveUS20050064394A1Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
Recombinant Newcastle disease virus RNA expression systems and vaccines
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS20040253273A1Prevent tumor formationQuick changeSsRNA viruses negative-senseAntibody mimetics/scaffoldsTumor reductionEpitope
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:PALESO PETER +2
Therapeutic use of cis-element decoys in vivo
The invention provides for the use of oligodeoxynucleotide decoys for the prophylactic or therapeutic treatment of diseases associated with the binding of endogenous transcription factors to genes involved in cell growth, differentiation and signalling or to viral genes. By inhibiting endogenous trans-activating factors from binding transcription regulatory regions, the decoys modulate gene expression and thereby regulating pathological processes including inflammation, intimal hyperplasia, angiogenesis, neoplasia, immune responses and viral infection. The decoys are administered in amounts and under conditions whereby binding of the endogenous transcription factor to the endogenous gene is effectively competitively inhibited without significant host toxicity. The subject compositions comprise the decoy molecules in a context which provides for pharmacokinetics sufficient for effective therapeutic use.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
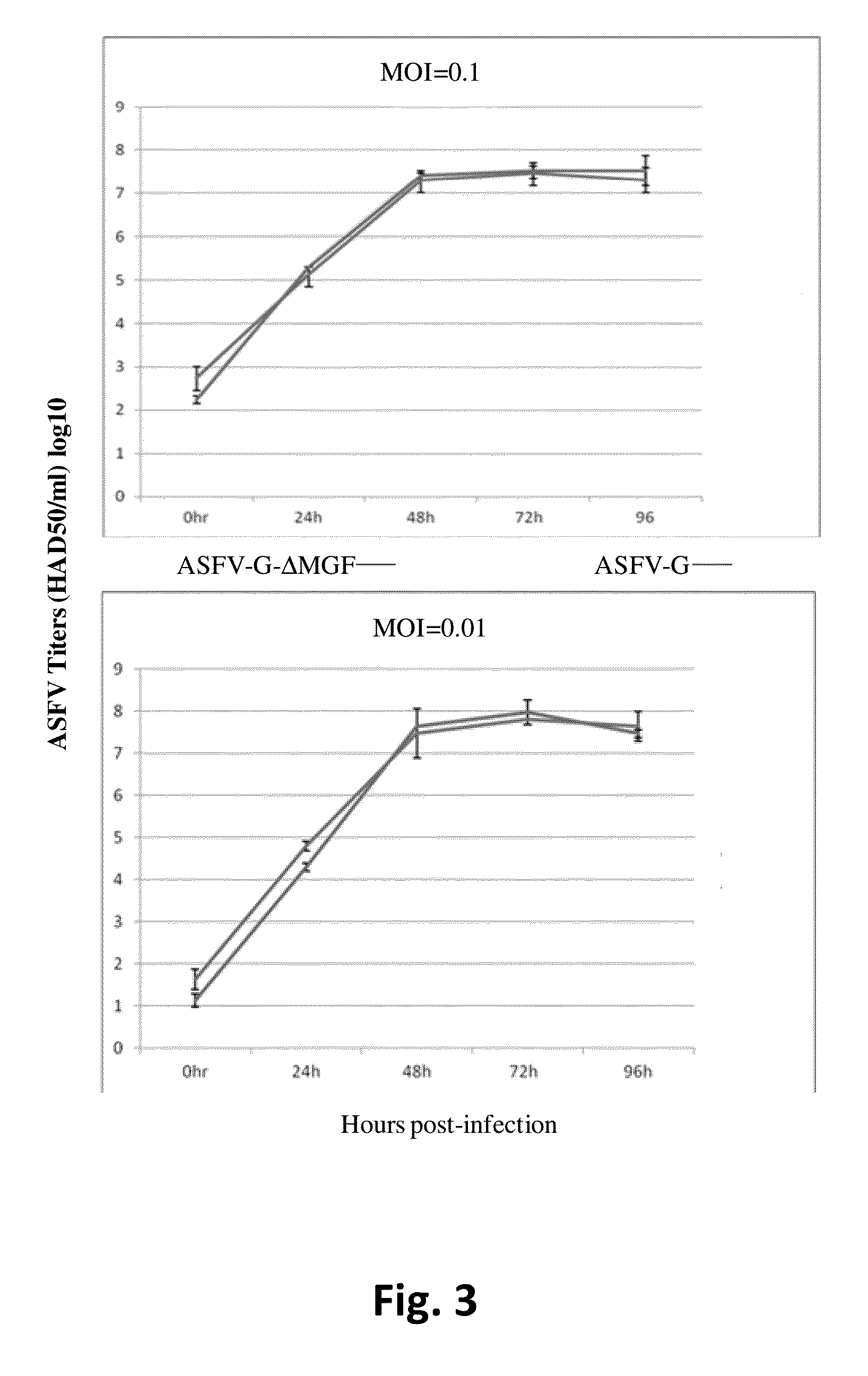
Attenuated African Swine Fever Virus Vaccine Based in the Deletion of MGF Genes
ActiveUS20160130562A1Viral antigen ingredientsMicrobiological testing/measurementVirulent characteristicsDomestic pig
African swine fever virus (ASFV) is the etiological agent of a contagious and often lethal viral disease of domestic pigs. Control of ASF has been hampered by the unavailability of vaccines. Experimental vaccines have been derived from naturally occurring, cell culture-adapted, or genetically modified live attenuated ASFVs; however, these vaccines are only successful when protecting against homologous viruses. Among viral genes reported to be involved in virulence are components of the multi gene family (MGF). Here we report the construction of a recombinant ΔMGF virus derived from the highly virulent ASFV Georgia 2007 (ASFV-G) isolate. In vivo, ASFV-G ΔMGF administered intramuscularly (IM) to swine at either 102 or 104 HAD50 are completely attenuated; the inoculated animals are completely asymptomatic. Animals infected with 102 or 104 HAD50 of ASFV-G ΔMGF are protected against the presentation of clinical disease when challenged at 28 days post infection with the virulent parental strain Georgia 2007.
Owner:US SEC AGRI +1
ENABLING THE USE OF LONG dsRNA FOR GENE TARGETING IN MAMMALIAN AND OTHER SELECTED ANIMAL CELLS
ActiveUS20130330824A1Simplified and advanced gene targetingRegulate expressionSpecial deliveryAntiviralsMammalGene silencing
Owner:1GLOBE BIOMEDICAL CO LTD
Glutathione-modified chitosan copolymer serving as non-viral gene carrier material and preparation and application thereof
ActiveCN102140171AGood biocompatibilityReduce immune rejectionVector-based foreign material introductionActivation methodPolyethylene glycol
The invention discloses a glutathione-modified chitosan copolymer serving as a non-viral gene carrier material and preparation and application thereof, which belong to the fields of gene therapy and novel materials. A preparation method of the copolymer comprises the following steps of: (1) synthesizing an allyl-modified chitosan derivative; (2) synthesizing brush-like PEG (Polyethylene Glycol) polymer chains with different molecular weights through RAFT (Reversible Addition-Fragmentation Chain Transfer); (3) grafting the brush-like PEG onto a chitosan framework by adopting a free radical coupling method; and (4) linking glutathione to the chain end of the brush-like PEG by adopting an EDC (1-Ethyl-3-(3-Dimethyllaminopropyl) Carbodiimide hydrochloride) / NHS (N-hydroxysuccinimide) activation method to obtain a glutathione-modified chitosan copolymer carrier material. The glutathione-modified chitosan copolymer obtained by adopting the technology serves as the non-viral gene carrier material. By adopting the copolymer, the endocytosis function of a composite nanoparticle formed from the copolymer and DNA (Deoxyribonucleic Acid) can be remarkably enhanced, the releasing mechanism of DNA from a composite particle after cell entrance is improved, and the non-viral gene carrier material with a high transfection efficiency is further obtained.
Owner:NANKAI UNIV
Multiple RNA polymerase III promoter expression constructs
Expression constructs comprising at least two different RNA polymerase III promoters, wherein each promoter is operably linked to a nucleic acid sequence encoding an RNA effector molecule, are disclosed herein. Further provided are expression constructs comprising multiple polymerase III promoters operably linked to sequences encoding short hairpin RNA molecules, which may comprise single and / or multiple fingers. The provided constructs are useful for in vivo delivery of RNA molecules effective in gene silencing, including of viral genes including HBV and HCV.
Owner:ALNYLAM PHARMA INC
Polymer micelle complex including nucleic acid
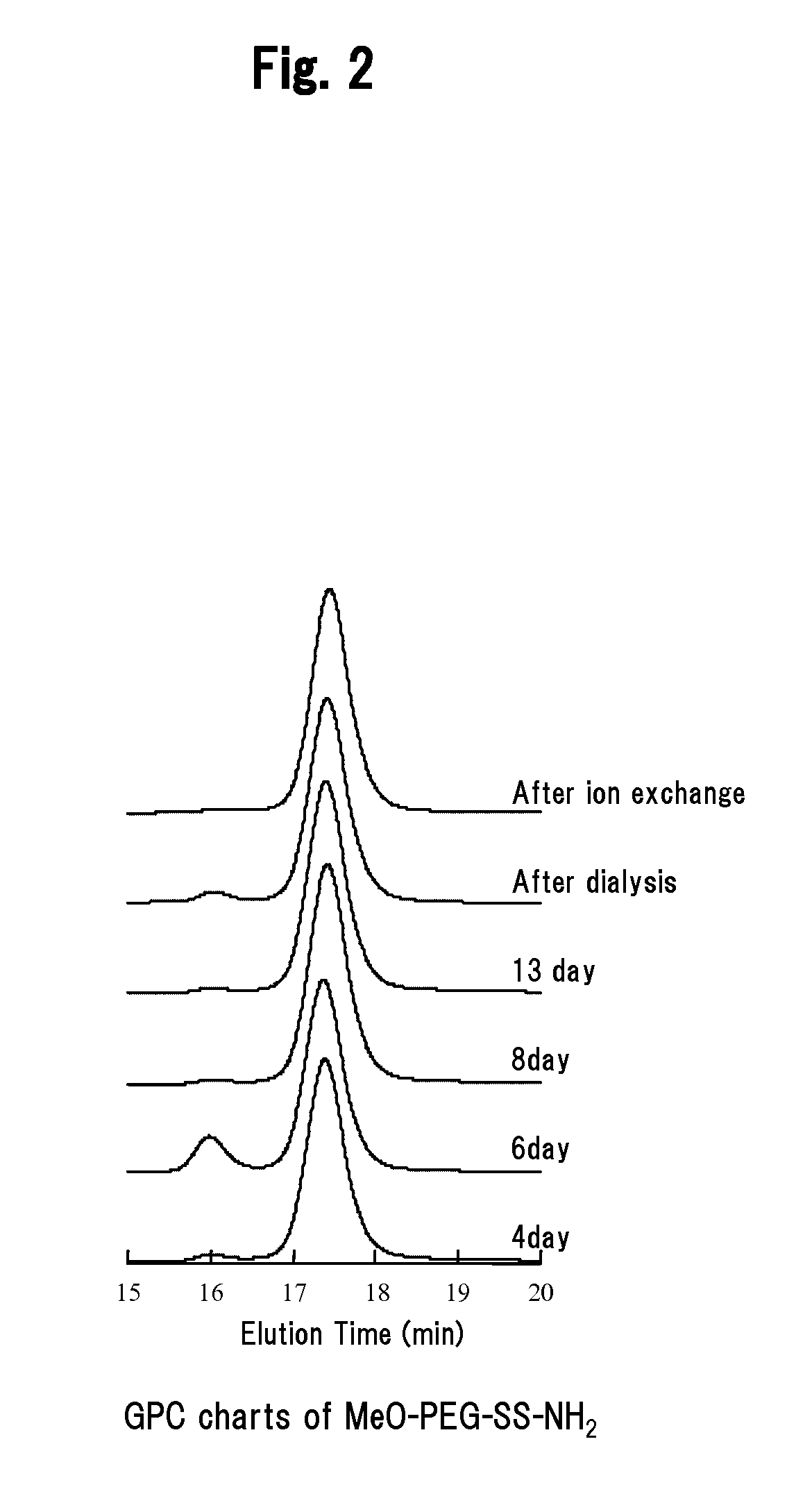
ActiveUS20090258416A1Efficient expressionMicroencapsulation basedGenetic material ingredientsViral GenesPolyethylene glycol
It is an object of the present invention to provide a polyion complex used as a non-viral gene vector, which achieves sufficiently high gene expression efficiency to a target cell. The polyion complex of the present invention comprises a block copolymer formed by binding polyethylene glycol to polycation via a disulfide group and a nucleic acid.
Owner:THE UNIV OF TOKYO
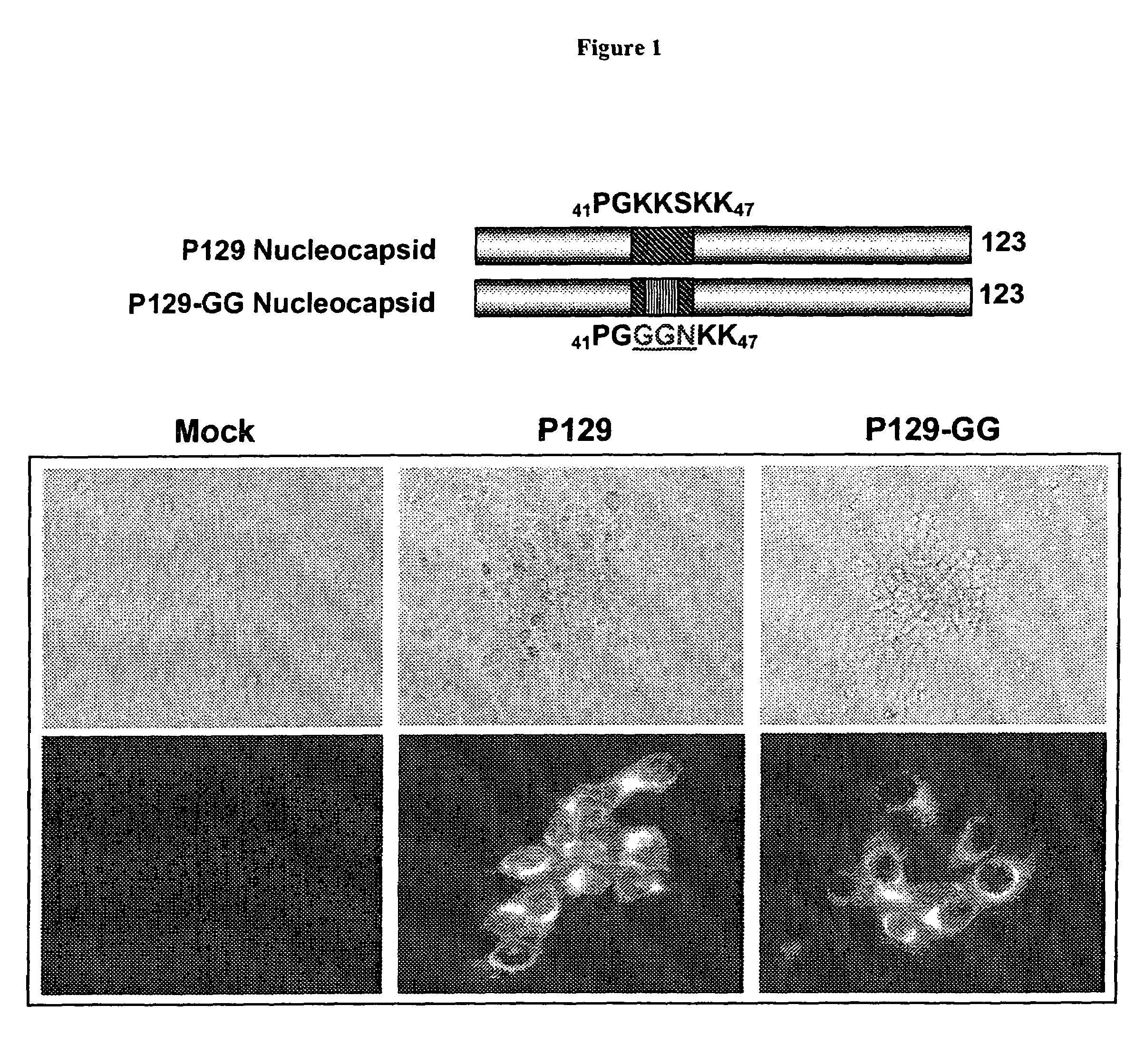
N protein mutants of porcine reproductive and respiratory syndrome virus
ActiveUS7544362B1Minimizes probabilityRestoring a functional NLS motifSsRNA viruses positive-senseSugar derivativesViral GenesNucleotide
Owner:ZOETIS SERVICE LLC
ENABLING THE USE OF LONG dsRNA FOR GENE TARGETING IN MAMMALIAN AND OTHER SELECTED ANIMAL CELLS
InactiveUS20110111481A1Simplified and advanced gene targetingRegulate expressionSpecial deliveryBacteriaMammalGene silencing
The present invention provides a method of enabling the use of long dsRNA for gene silencing in mammalian cells through bacteria, preferably non-pathogenic or therapeutic strains of bacteria. DNA that encodes long double-strand RNAs are transformed into bacteria and processed in the bacterial cells into a mixture of smaller RNA duplexes and then released into the cytoplasm of the target cells, resulting in modulation of gene expression in the target cells. The methods overcome the incompatibility between long strong dsRNA and mammalian cells by eliminating, or mitigating, the non-specific innate immune response. The eukaryotic cells can be mammalian cells or avian cells. The gene of interest can be a mammalian, avian, bacterial, eukaryotic, or viral gene.
Owner:1GLOBE BIOMEDICAL CO LTD
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
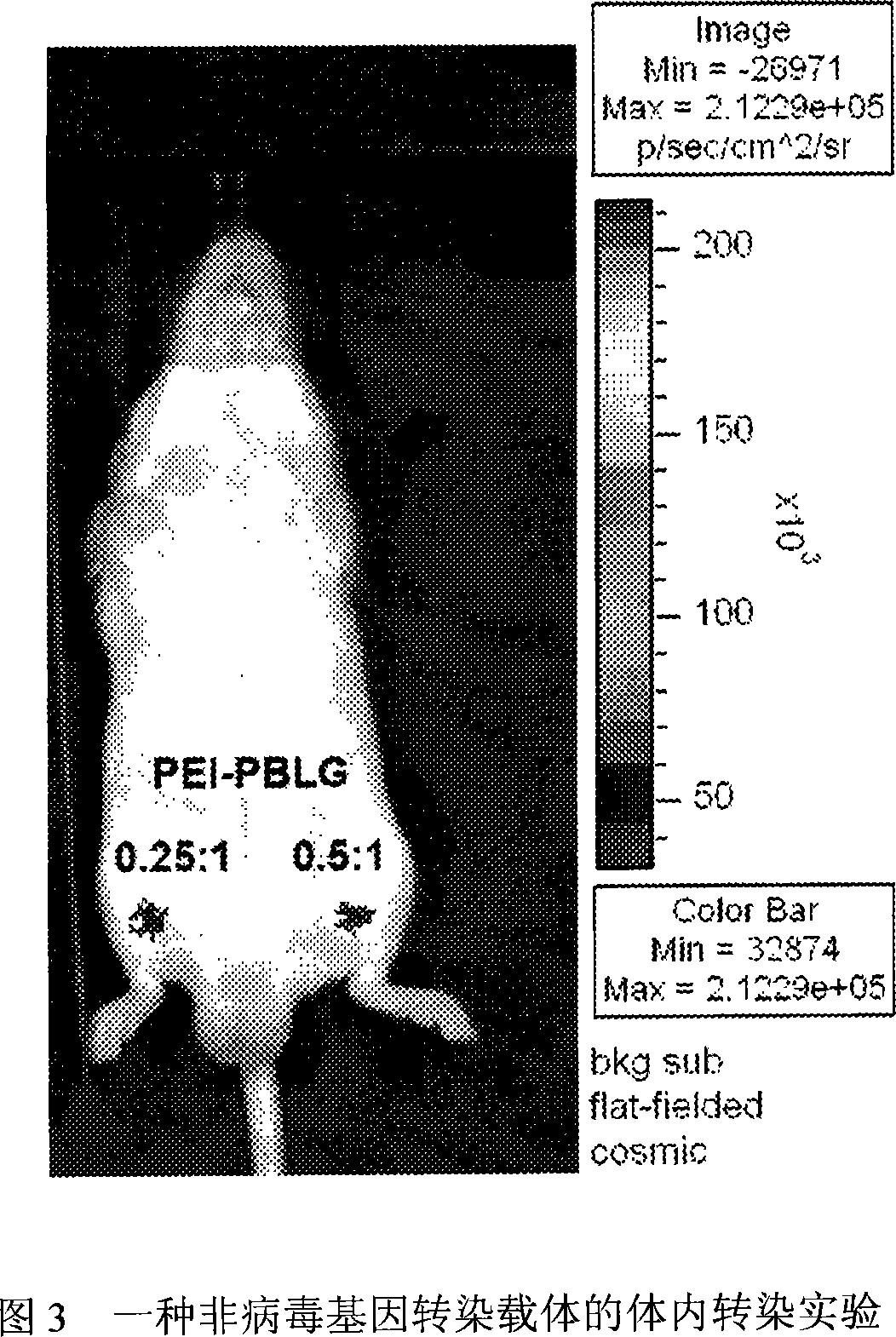
Non-virogene transfection carrier, complex particles of the same and plasmid DNA, preparing method and using method
InactiveCN101085356ALow toxicityHigh transfection efficiencyGenetic material ingredientsPharmaceutical non-active ingredientsPlasmid dnaIn vivo
The invention belongs to technology field of carrier material for non-viral gene transfection, and relates to a carrier material for non-viral gene transfection, plasmid DNA compound particle and preparation method and administration. The weight ratio of polyethylene imine and hydrophobic unit containing benzene ring is 1:9-4:6.The polyethylene imine is superbranched polyethylene imine (PEI) with average molecular weight of 1.8K-25K.The hydrophobic unit containing benzene ring is one or more of gamma -benzyl-L-glutamic acid, epsi -carbobenzoxy lysine and phenylalanine at any ratio. The water soluble polyethylene imine modified substance of hydrophobic unit containing benzene ring can compound with genic material to form a particle with positive charge on surface and particle diameter of 50-300nm.The compound particle can transfer loaded genic material into cell to realize expression of genic material and complete transfection process. The invention prepared non-gene transfection carrier material has the advantages of low toxicity and high up transfection efficiency; and can be used as transfection agent in vivo and in vitro.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Methods and compositions for the production of recombinant virus vectors
A method for the production of a replication-deficient recombinant virus vector is disclosed. The replication-deficient recombinant virus vector has a recombinant virus genome with one or more defective viral genes. The method comprises infecting a host cell with a carrier virus having a carrier virus genome encoding one or more trans factors or variants thereof, incubating the infected host cell for a desired period of time, and isolating the replication-deficient recombinant virus vector. The carrier virus is a cytoplasmic virus that retains the carrier virus genome in the cytoplasm of the host cell. The host cell contains the recombinant viral genome and retains the recombinant viral genome in a nucleus of the host cell. Also disclosed is a carrier virus for the production of a replication-deficient recombinant virus vector.
Owner:SICHUAN REAL&BEST BIOTECH CO LTD
Novel cationic graft copolymer, and preparation method and application of multiple composite non-viral gene vector
InactiveCN103665384AHas clinical application valueHigh transfection rateGenetic material ingredientsPharmaceutical non-active ingredientsTumor targetingPolycaprolactone
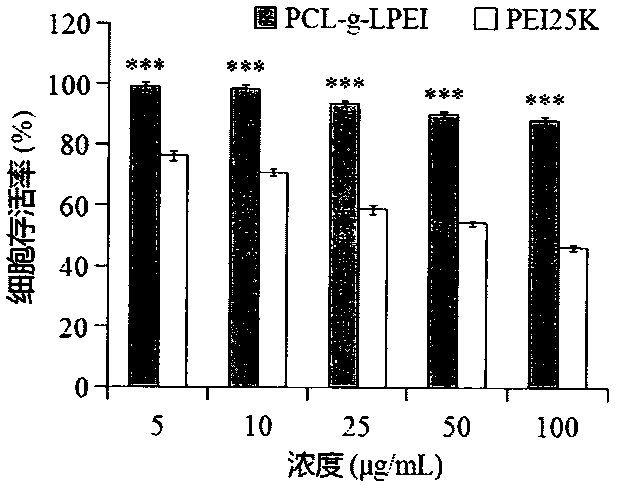
The invention relates to a novel cationic graft copolymer, and a preparation method and an application of a multiple composite non-viral gene vector. The novel cationic graft copolymer PCL-g-LPEI (polycaprolactone-g-linear polyethylenimine) is synthesized by ring-opening copolymerization and amidation reaction. The multiple composite non-viral gene vector comprises: (A) a plasmid DNA (deoxyribonucleic acid)-supported PCL-g-LPEI cationic compound serving as a kernel of a multiple gene compound; (B) a tumor-targeting ligand HA (hyaluronic acid) wrapping the cationic compound under the interaction of positive and negative charges to form a shell of the multiple gene compound. A multiple composite gene constructed by the non-viral gene vector has the advantages of low cytotoxicity, high blood compatibility, high transfection efficiency, tumor targeting effects and the like, and is expected to be applied to clinical gene therapy.
Owner:CHINA PHARM UNIV
Biodegradable cationic polymer gene transfer compositions and methods of use
The invention provides biodegradable, cationic compositions based on cationic α-amino acid-containing PEA, PEUR and PEU polymers for use in preparation of non-viral gene transfer compositions. In the invention gene transfer compositions a poly nucleic acid is condensed with the polymer to form a soluble unit wherein the electrical charge of the poly nucleic acid is neutralized by the polymers. The invention gene transfer compositions can be used to transfect target cells by contact with the target cells.
Owner:CORNELL UNIVERSITY +1
Effective vaccination against porcine reproductive and respiratory syndrome (PRRS) virus prior to weaning
ActiveUS20130309263A1Reduce inhibitionEfficient productionSsRNA viruses positive-senseViral antigen ingredientsVaccinationViral Genes
The invention provides isolated polynucleotide molecules that comprise a DNA sequence encoding an infectious RNA sequence encoding a genetically-modified North American PRRS virus, methods to make it and related polypeptides, polynucleotides, and various components. Vaccines comprising the genetically modified virus and polynucleotides and a diagnostic kit to distinguish between naturally infected and vaccinated animals are also provided.
Owner:ZOETIS SERVICE LLC
Tetravinyl-based Gemini type amphiphilic compound as well as preparation method and application thereof
ActiveCN106432203AHas aggregation-induced luminescent propertiesFacilitates the study of transfection mechanismsOrganic chemistryOther foreign material introduction processesFluorescenceMass spectrometry
The invention discloses a tetravinyl-based Gemini type amphiphilic compound as well as a preparation method and application thereof. The compound disclosed by the invention is mainly prepared through McMurry coupling reaction, nucleophilic substitution reaction and Click reaction; the structure of the compound is also confirmed through infrared, nuclear magnetic and mass-spectrum ways; in an aqueous solution, molecules of a derivative of the compound disclosed by the invention self-assemble to form aggregation-induced fluorescence-enhanced micelles (AIE micelles); the compound acts with a nucleic acid, and then can co-aggregate to form nano particles easy for cellular uptake; green fluorescent protein (GFP) and luciferase (Luciferase) expression assays prove that the compound self and a liposome formed with dioleoyl phosphatidyl ethanolamine (DOPE) can be used as non-viral gene vectors; meanwhile, the derivative is successfully used for tracing the cellular uptake and release processes of pGL-3 and FAM-DNA by utilizing the reversible transformation between the self-assembly of the compound and the co-assembly with DNA (Deoxyribonucleic Acid).
Owner:BEIJING NORMAL UNIVERSITY
Target quaternary ammonium salt cationic polymer lipid gene carrier, preparation method and application thereof
InactiveCN102234658ARich varietyHigh transfection efficiencyGenetic material ingredientsMacromolecular non-active ingredientsPositive controlQuaternary ammonium cation
The invention discloses a target quaternary ammonium salt cationic polymer lipid gene carrier, a preparation method and an application thereof. The target quaternary ammonium salt cationic polymer lipid genetic carrier is characterized in that: a polymeric quaternary ammonium salt and lipid are adopted for preparing a quaternary ammonium salt cationic polymer lipid genetic carrier according to a mass ratio, wherein the mass ratio of the polymeric quaternary ammonium salt to the lipid is 0.05-20:1; then a assembly method or a modification method is adopted for modifying to prepare a folic acid or EGFR antibody modified cationic polymer lipid gene carrier. Results of gene transfection experiments show that: gene transfection efficiencies of the target quaternary ammonium salt cationic polymer lipid gene carrier in 293T cells and NIH-3T3 cells are the same as the gene transfection efficiencies of positive control lipofectamine of lipofectamine<TM>2000 in the 293T cells and the NIH-3T3 cells; the gene transfection efficiencies of the EGFR antibody modified cationic polymer lipid genetic carrier in liver cancer Huh-7 cells and breast cancer MCF-7 cells are higher than the gene transfection efficiencies of the lipofectamine<TM>2000 in the liver cancer Huh-7 cells and the breast cancer MCF-7 cells. The cationic polymer lipid genetic carrier system provided by the present invention has good biocompatibility and low cytotoxicity, and can be as an excellent non-viral gene delivery carrier.
Owner:SHANGHAI INST OF ONCOLOGY
siRNA COMPOSITIONS AND METHODS FOR POTENTLY INHIBITING VIRAL INFECTION
ActiveUS20100166661A1Improve the protective effectLess antiviral effectOrganic active ingredientsSugar derivativesRegimenH5N1 virus
No antiviral regimen has been consistently successful in treating H5N1 virus infection. We demonstrate that a group of highly effective siRNAs targeting different H5N1 viral genes shares a unique motif, GGAGU / ACUCC. We further demonstrate that the effectiveness of siRNAs containing this motif is not sequence specific. The results suggested that the structure of the unique motif is critical in determining the potency of siRNA-mediated protective effects against viral infection and this potent in vivo protection is associated with early productions of β-defensin and IL-6 induced by the motif. Provided are methods and prophylactic and therapeutic agents useful against other viral infections in addition to the H5N1 influenza virus.
Owner:KWUN TONG KOWLOON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com