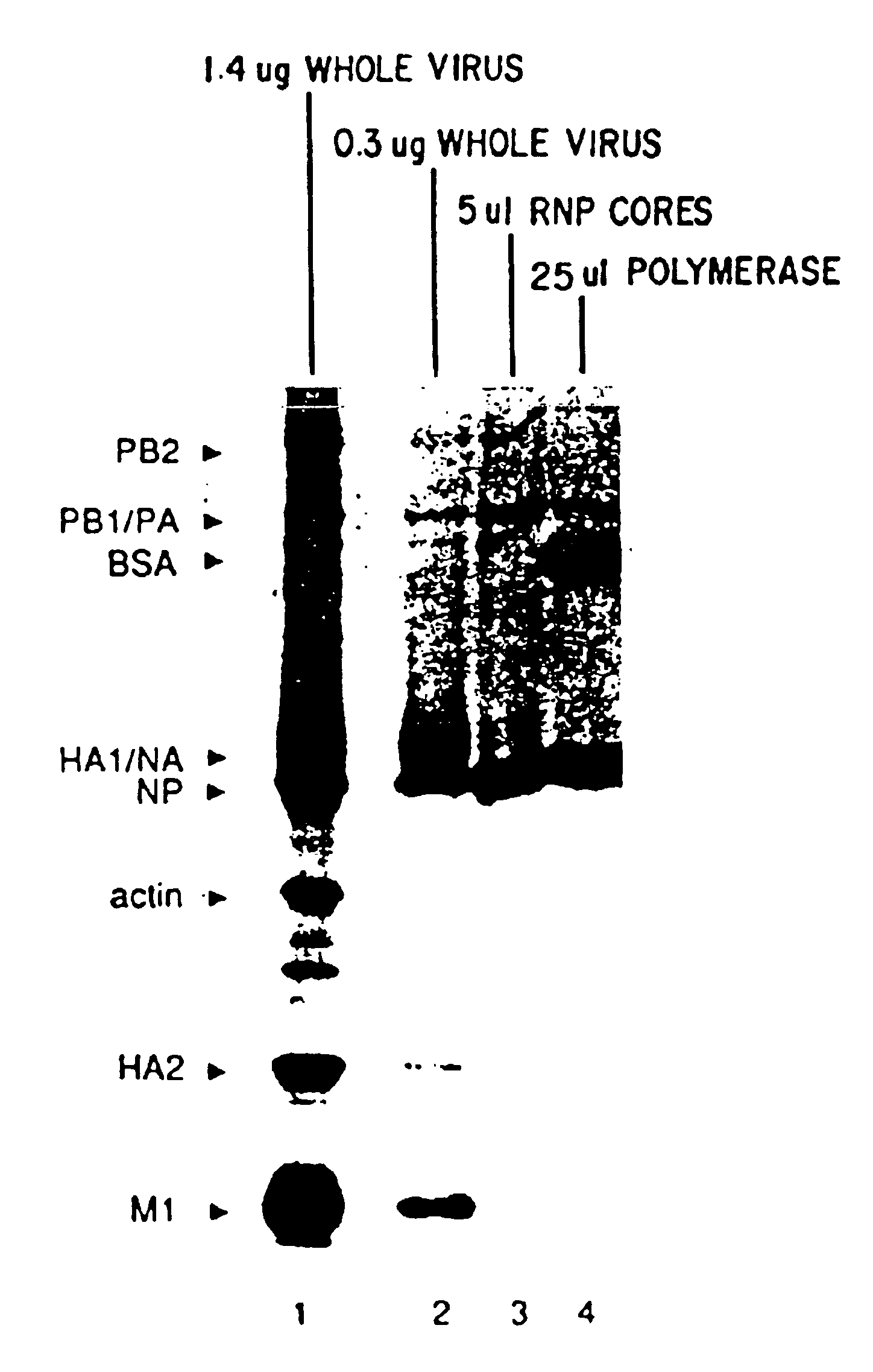
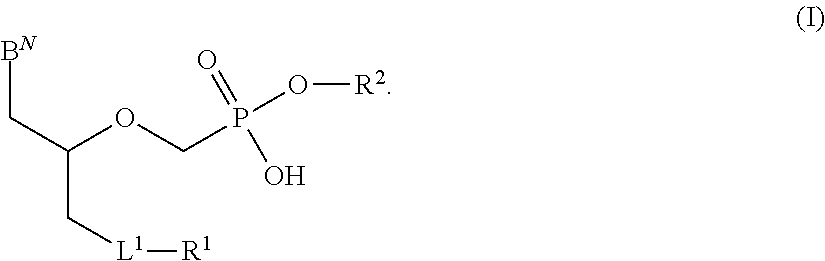Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
385 results about "Viral rna" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
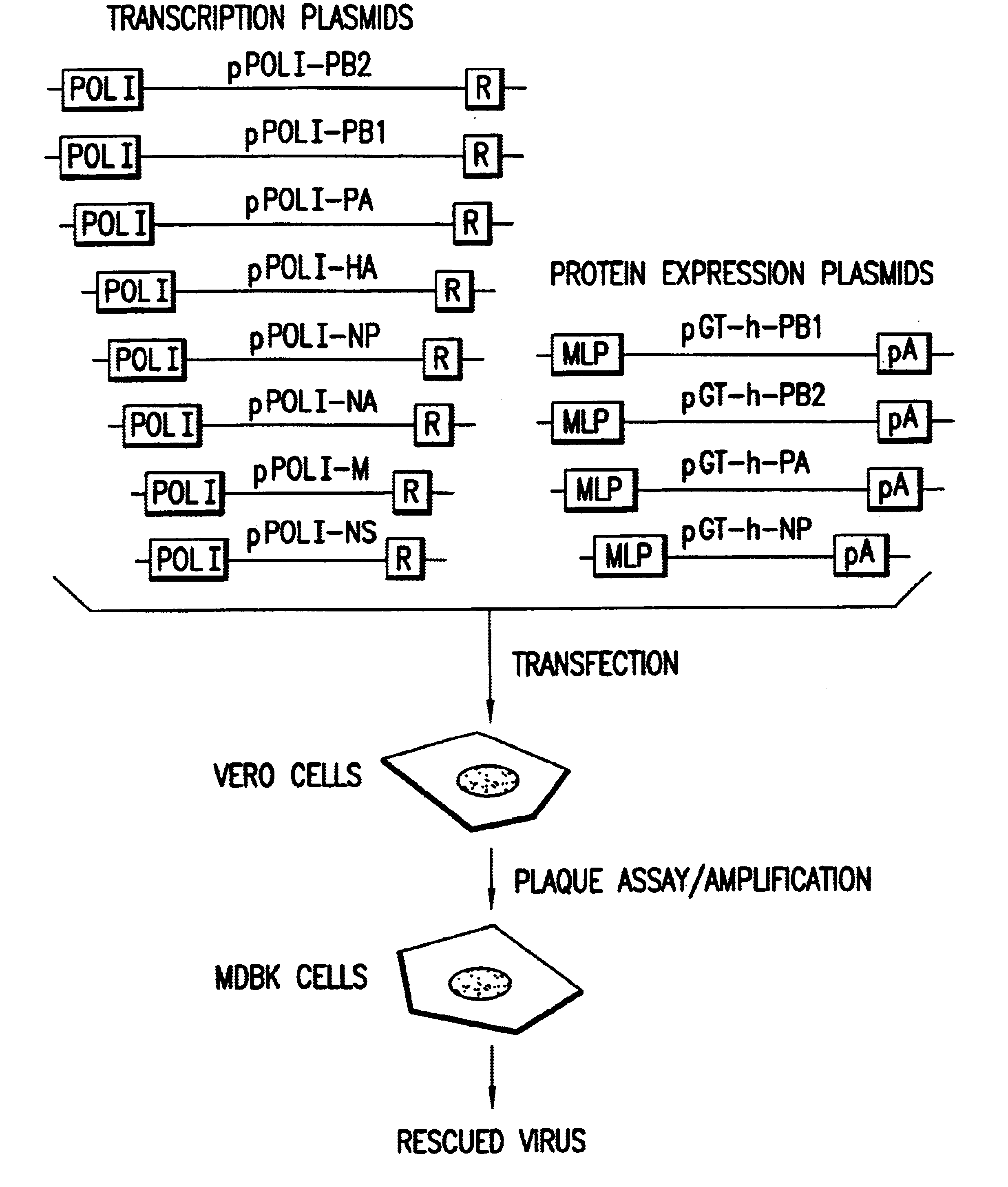
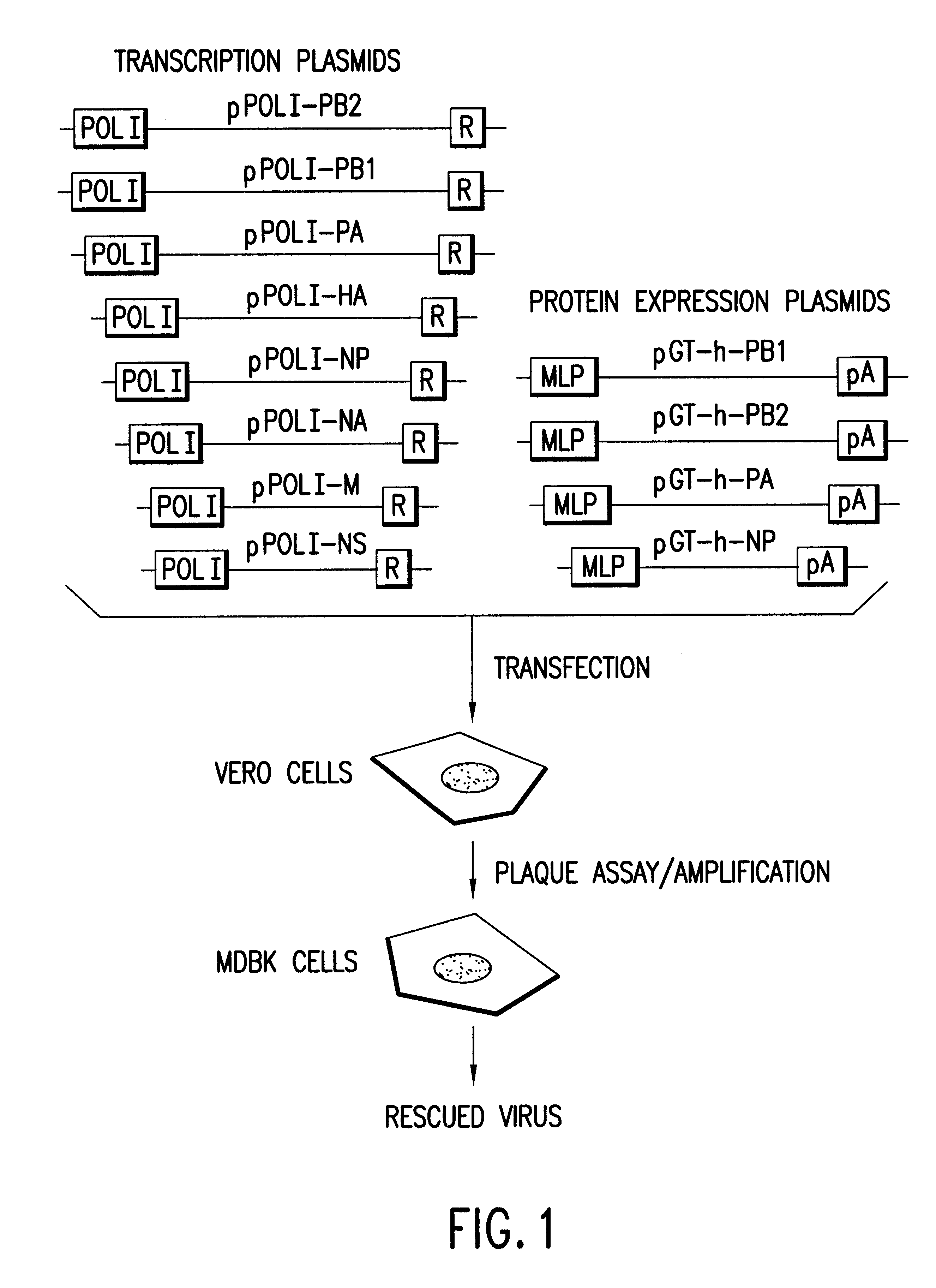
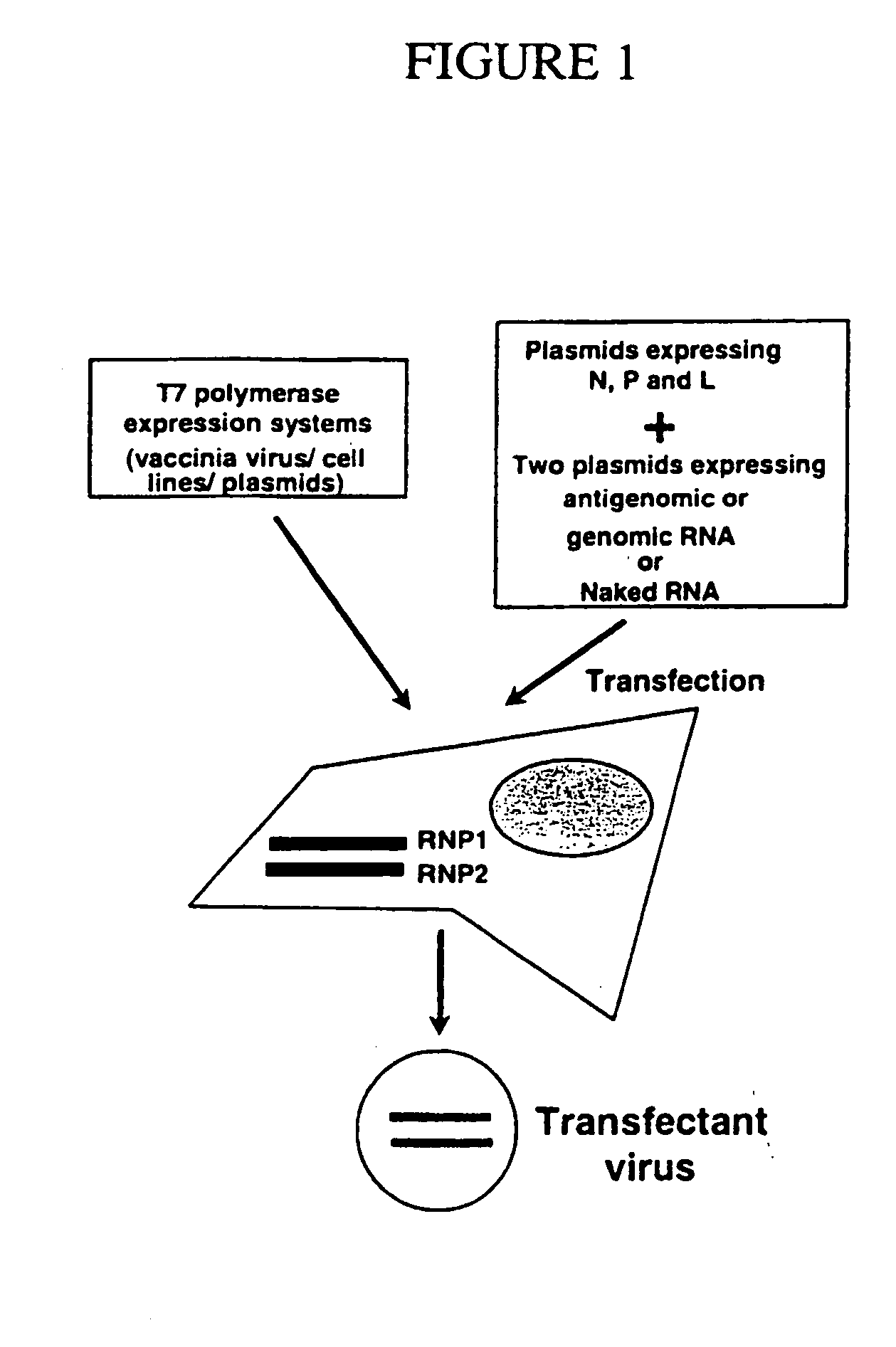
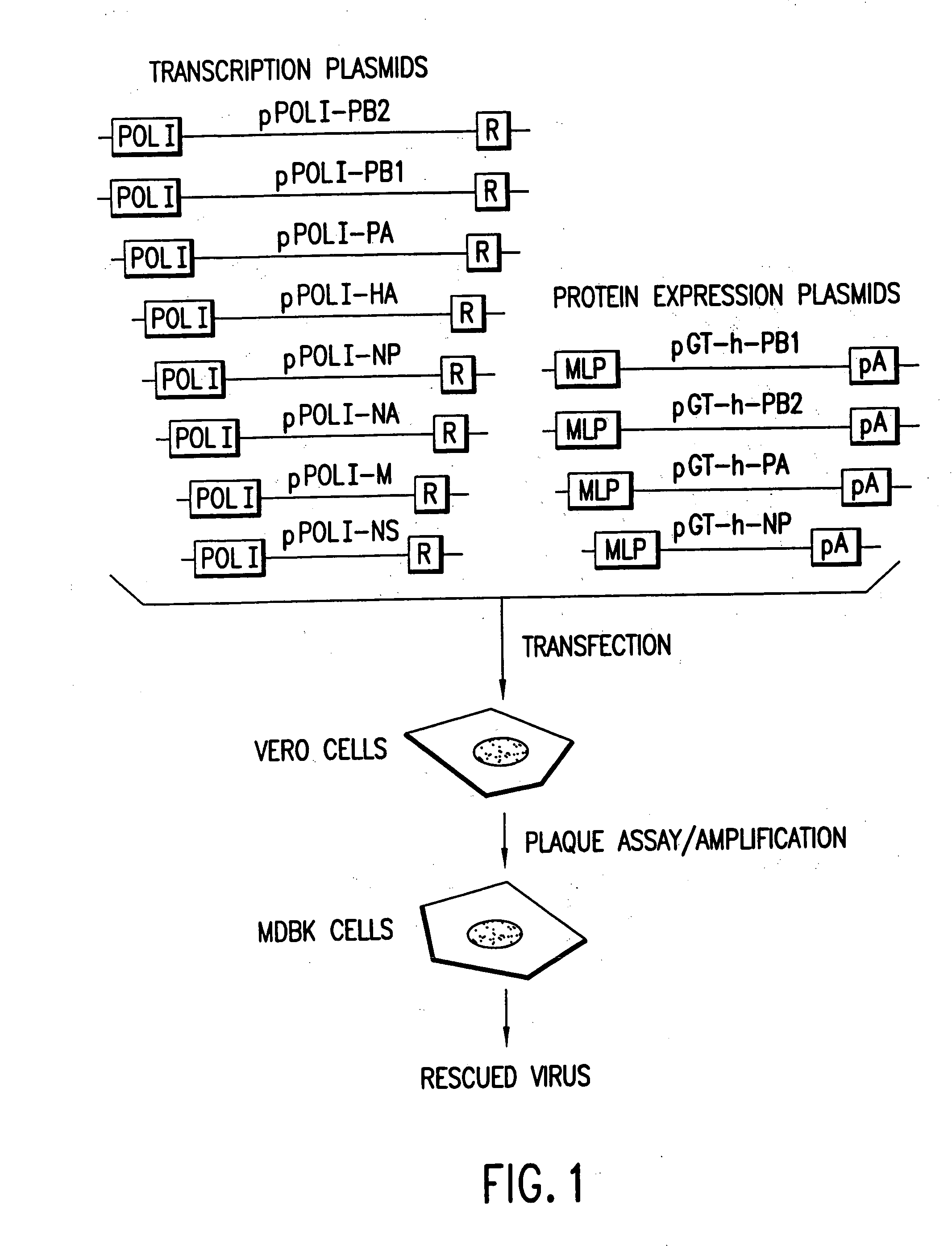
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
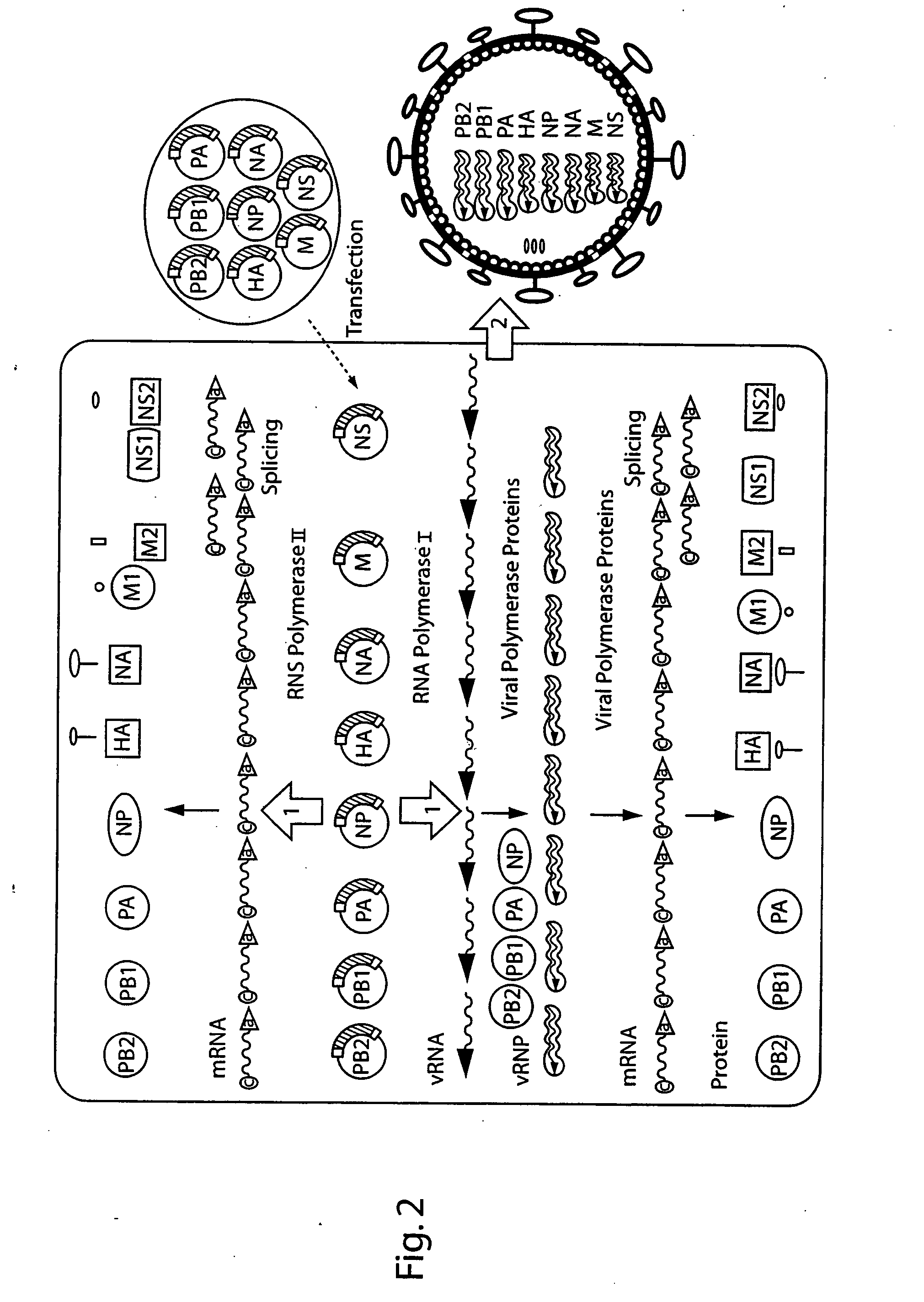
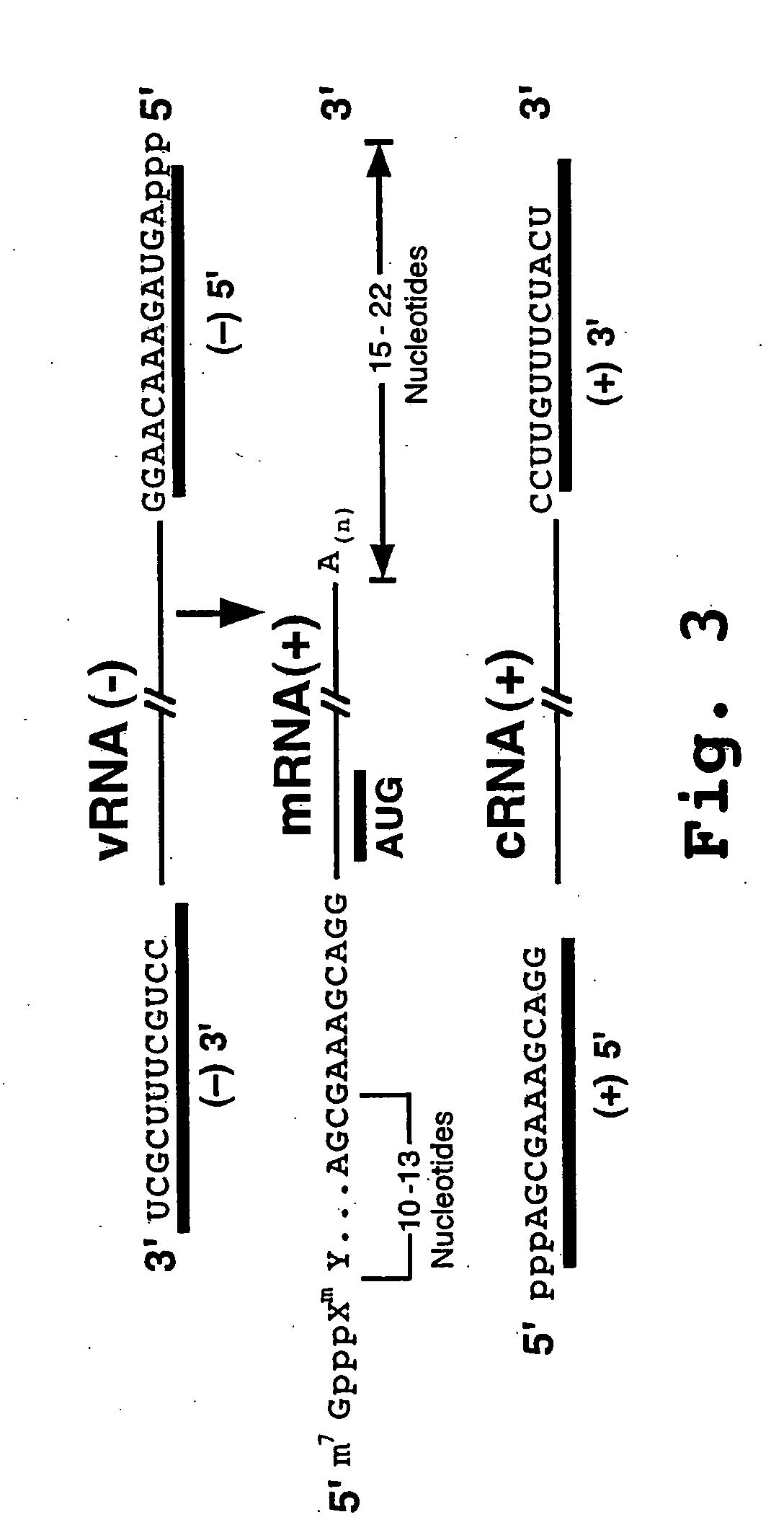
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
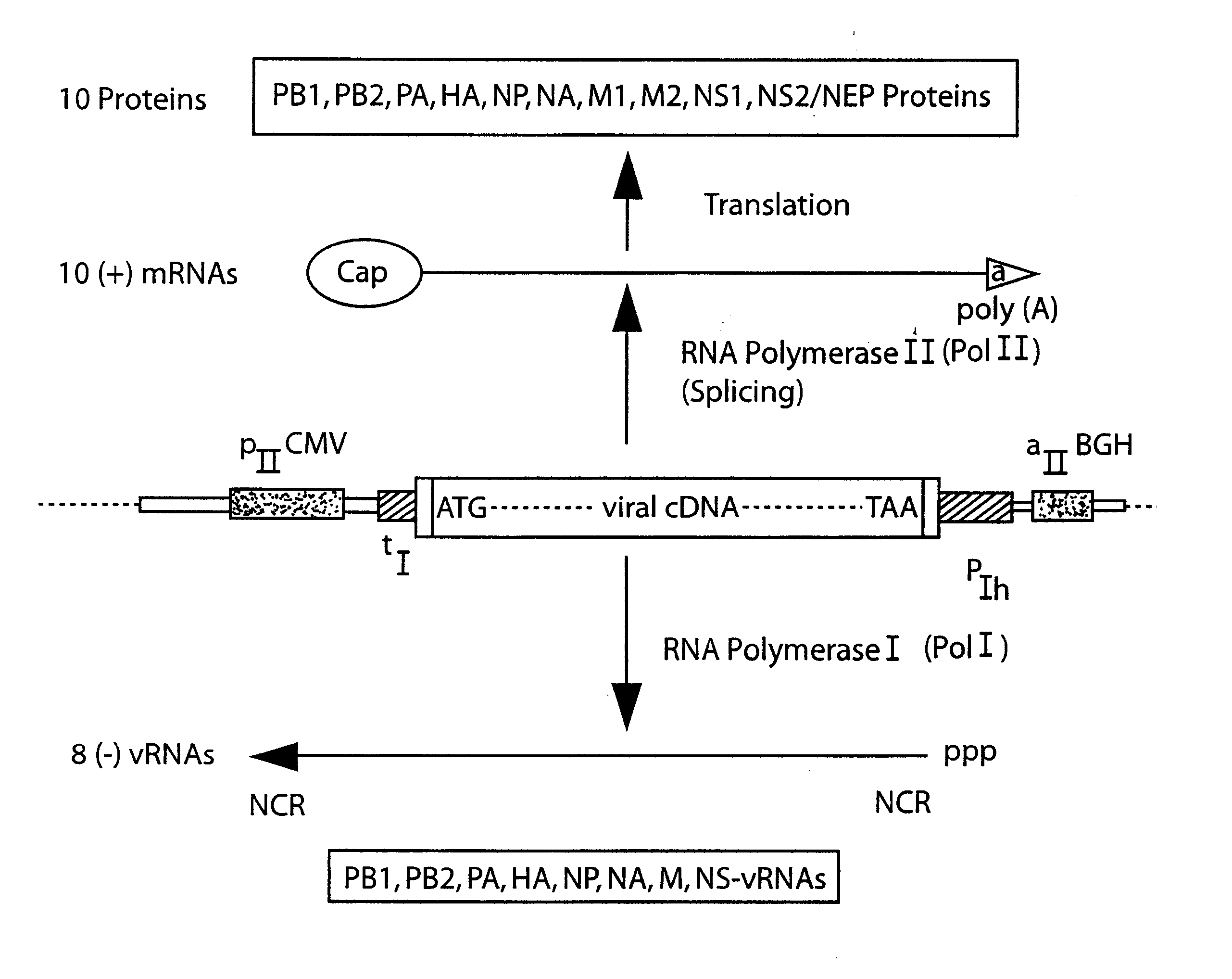
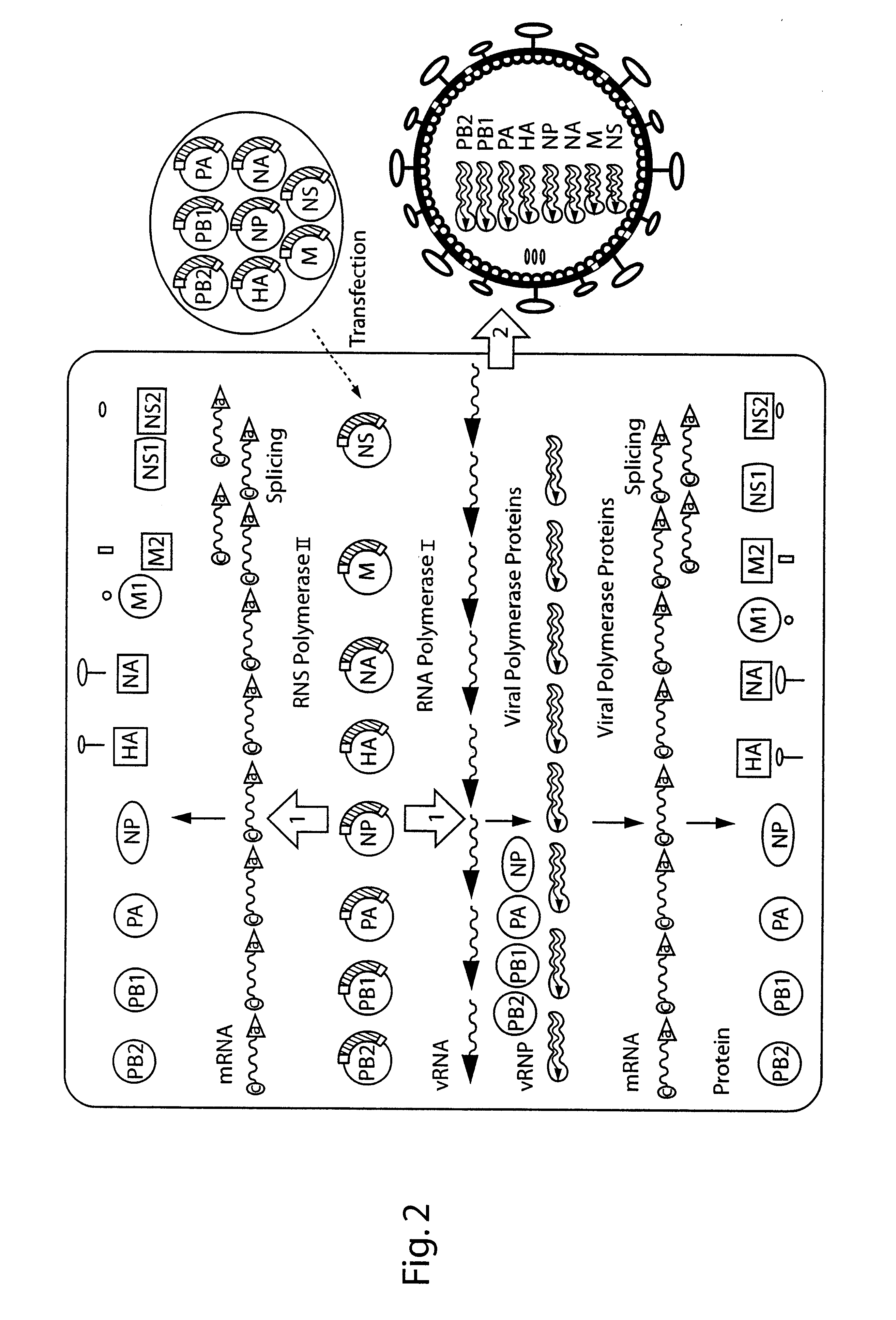
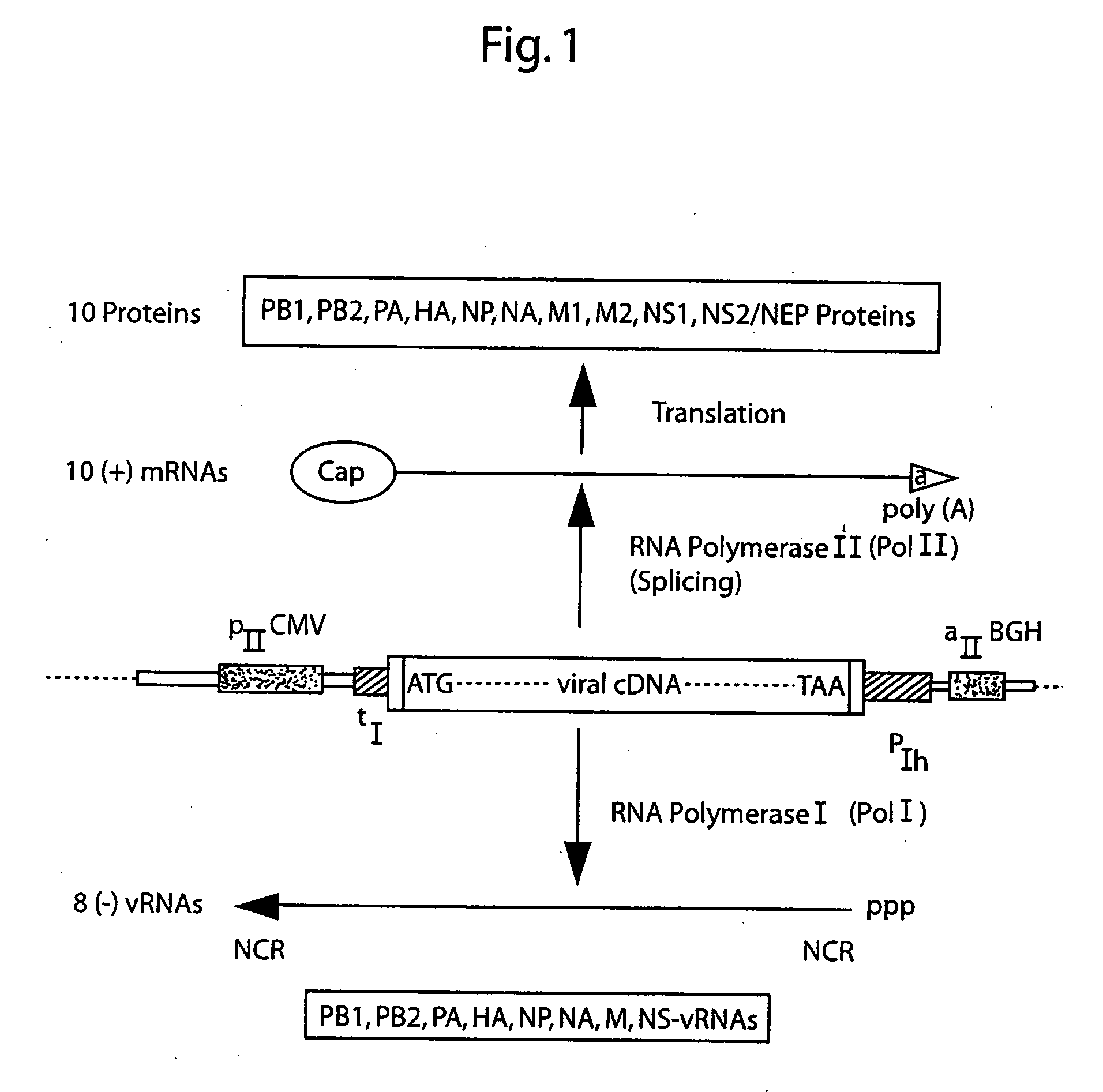
DNA transfection system for the generation of infectious influenza virus
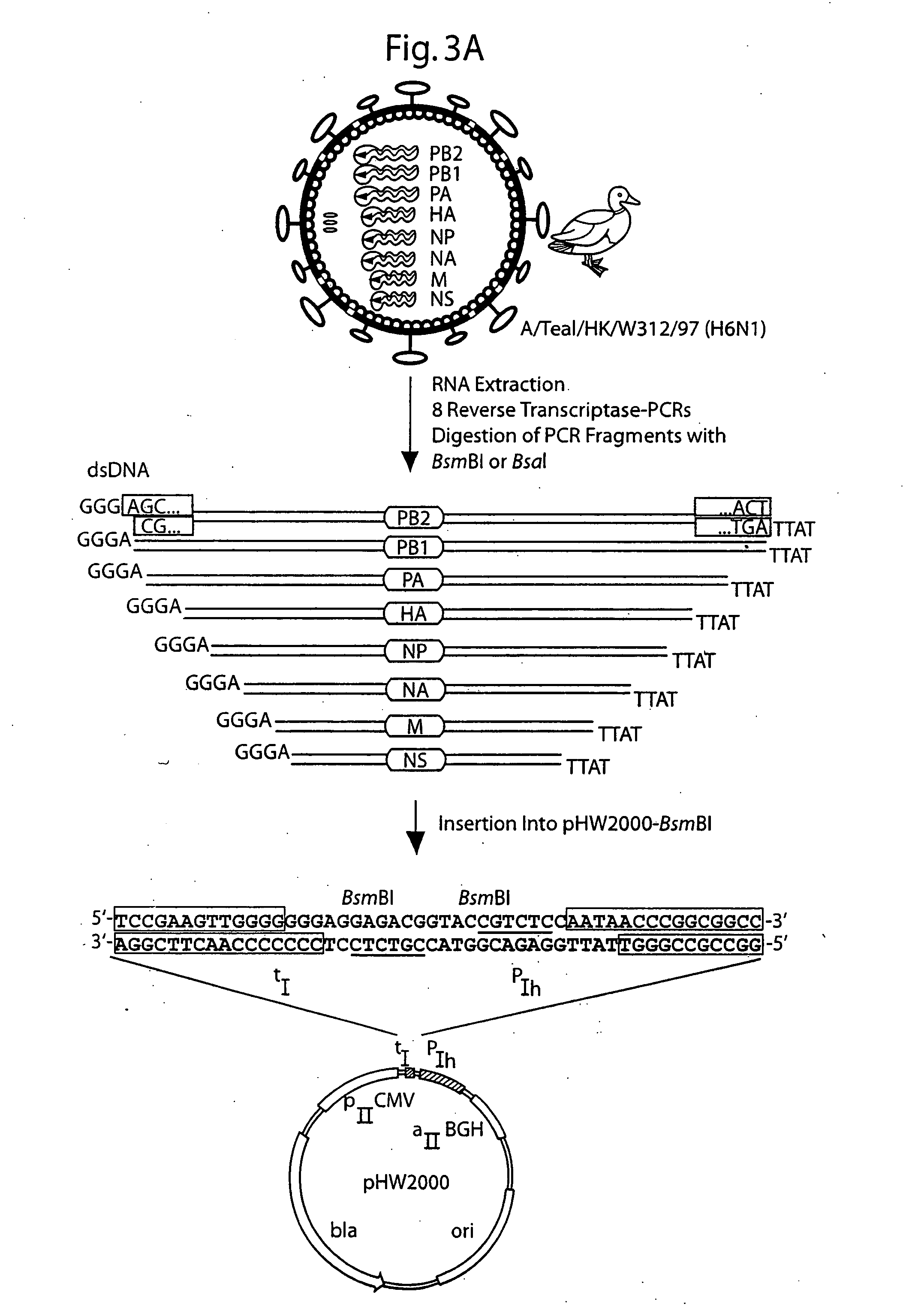
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Effecting virus resistance in plants through the use of negative strand RNAs
InactiveUS6617496B1Sugar derivativesOther foreign material introduction processesNegative strandPlant cell
A strategy for effecting virus resistance in plants causes the transcription in the plant cells of negative RNA strands which are substantially complementary to a target RNA strand. The target RNA strand can be an mRNA transcript created in gene expression, a viral RNA, or other RNA present in the plant cells. The negative RNA strand is complementary to at least a portion of the target RNA strand to inhibit its activity in vivo.
Owner:AGRACETUS MIDDLETON WISCONSIN A GENERAL PARTNERSHIP OF NEW YORK +1
Recombinant negative strand RNA virus expression systems and vaccines
Owner:MEDIMMUNE VACCINES
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
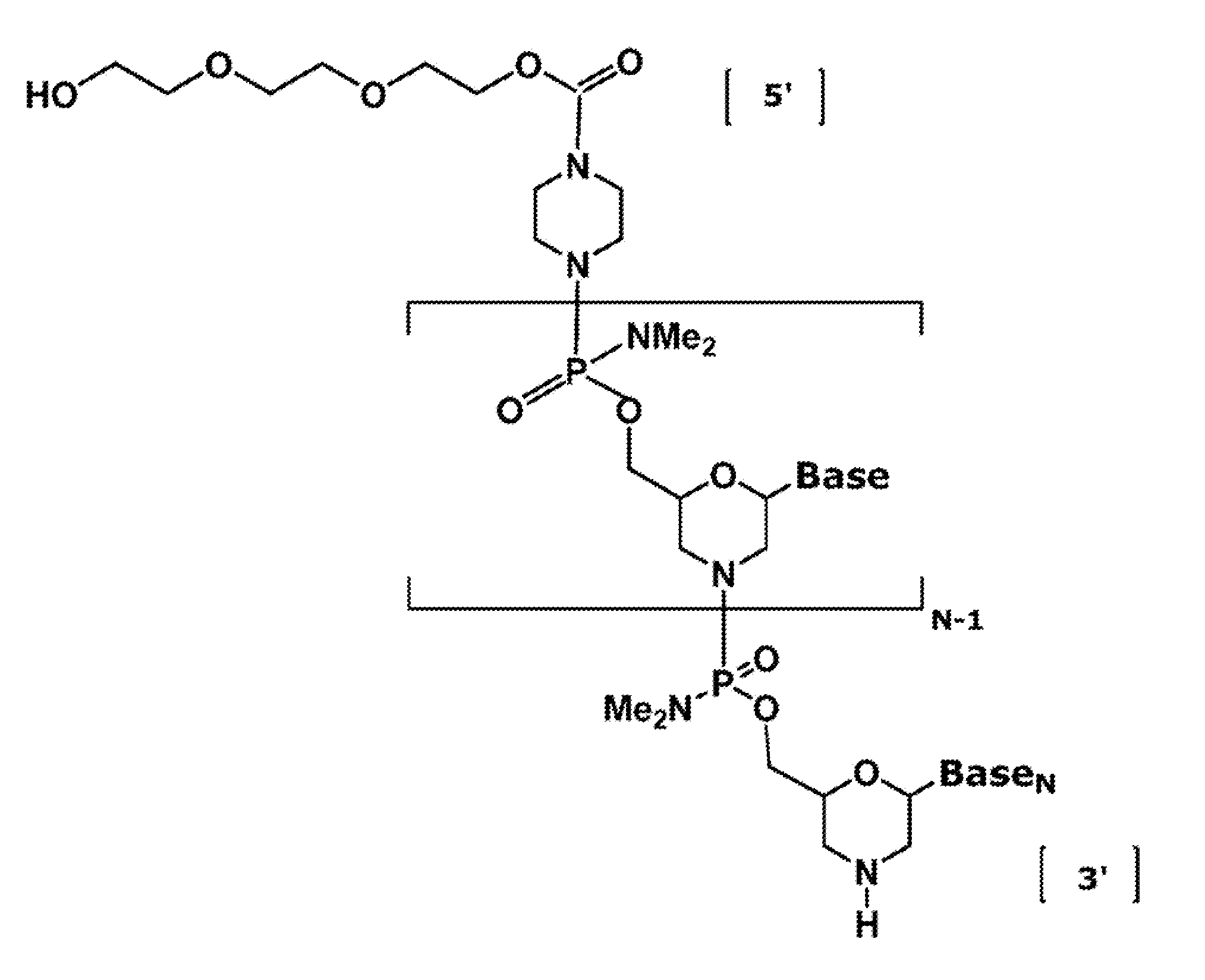
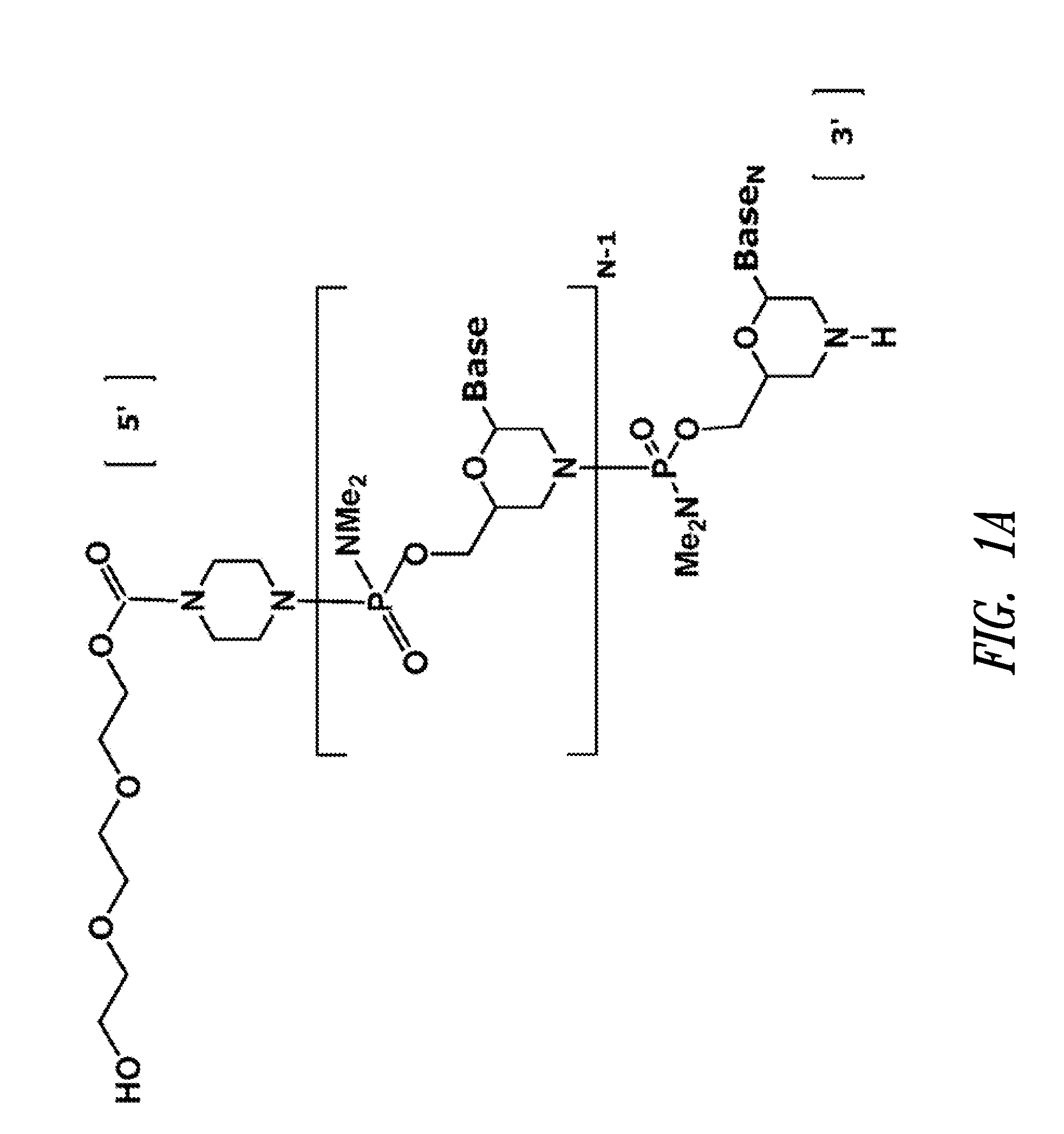
Antisense antiviral compound and method for treating influenza viral infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Orthomyxoviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of influenza virus infection in a mammal. The antisense antiviral compounds are substantially uncharged, including partially positively charged, morpholino oligonucleotides having 1) a nuclease resistant backbone, 2) 12-40 nucleotide bases, and 3) a targeting sequence of at least 12 bases in length that hybridizes to a target region selected from the following: a) the 5′ or 3′ terminal 25 bases of the negative sense viral RNA segment of Influenzavirus A, Influenzavirus B and Influenzavirus C; b) the terminal 25 bases of the 3′ terminus of the positive sense cRNA and; and c) the 50 bases surrounding the AUG start codon of an influenza viral mRNA.
Owner:MASSACHUSETTS INST OF TECH +1
Antisense antiviral compound and method for treating influenza viral infection
InactiveUS20060148747A1Inhibition of replicationPromote absorptionOrganic active ingredientsBiocideInfluenzavirus CInfluenzavirus B
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Orthomyxoviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of influenza virus infection in a mammal. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having 1) a nuclease resistant backbone, 2) 12-40 nucleotide bases, and 3) a targeting sequence of at least 12 bases in length that hybridizes to a target region selected from the following: a) the 5′ or 3′ terminal 25 bases of the negative sense viral RNA segment of Influenzavirus A, Influenzavirus B and Influenzavirus C; b) the terminal 25 bases of the 3′ terminus of the positive sense cRNA and; and c) the 50 bases surrounding the AUG start codon of an influenza viral mRNA.
Owner:SAREPTA THERAPEUTICS INC +1
Antisense antiviral compound and method for treating picornavirus infection
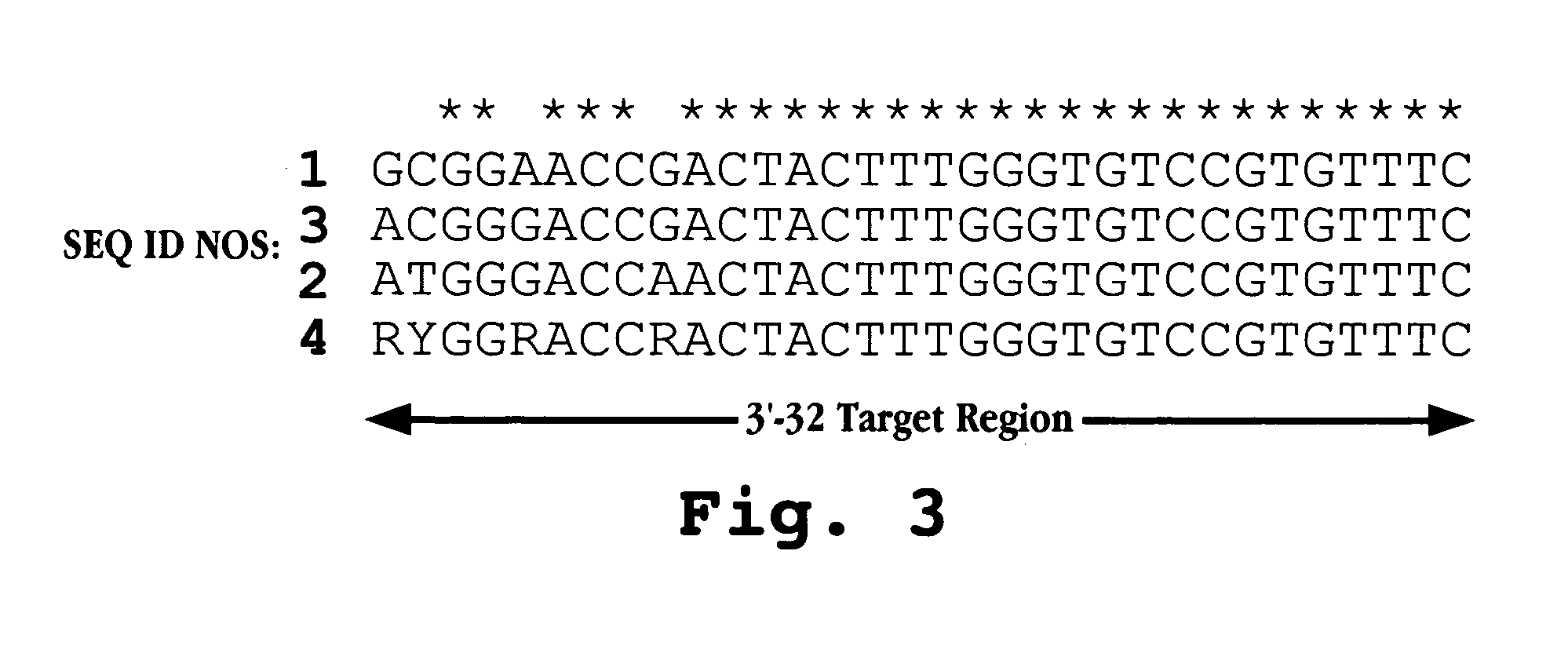
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:7.
Owner:SAREPTA THERAPEUTICS INC
Antisense antiviral compound and method for treating arenavirus infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Arenaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Arenavirus infection in a mammal. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 19 nucleotide region of the 5′-terminal regions of the viral RNA, viral complementary RNA and / or mRNA identified by SEQ ID NO:1.
Owner:THE SCRIPPS RES INST +1
Antisense antiviral compound and method for treating picornavirus infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, including partially positively charged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:4.
Owner:AVI BIOPHARMA
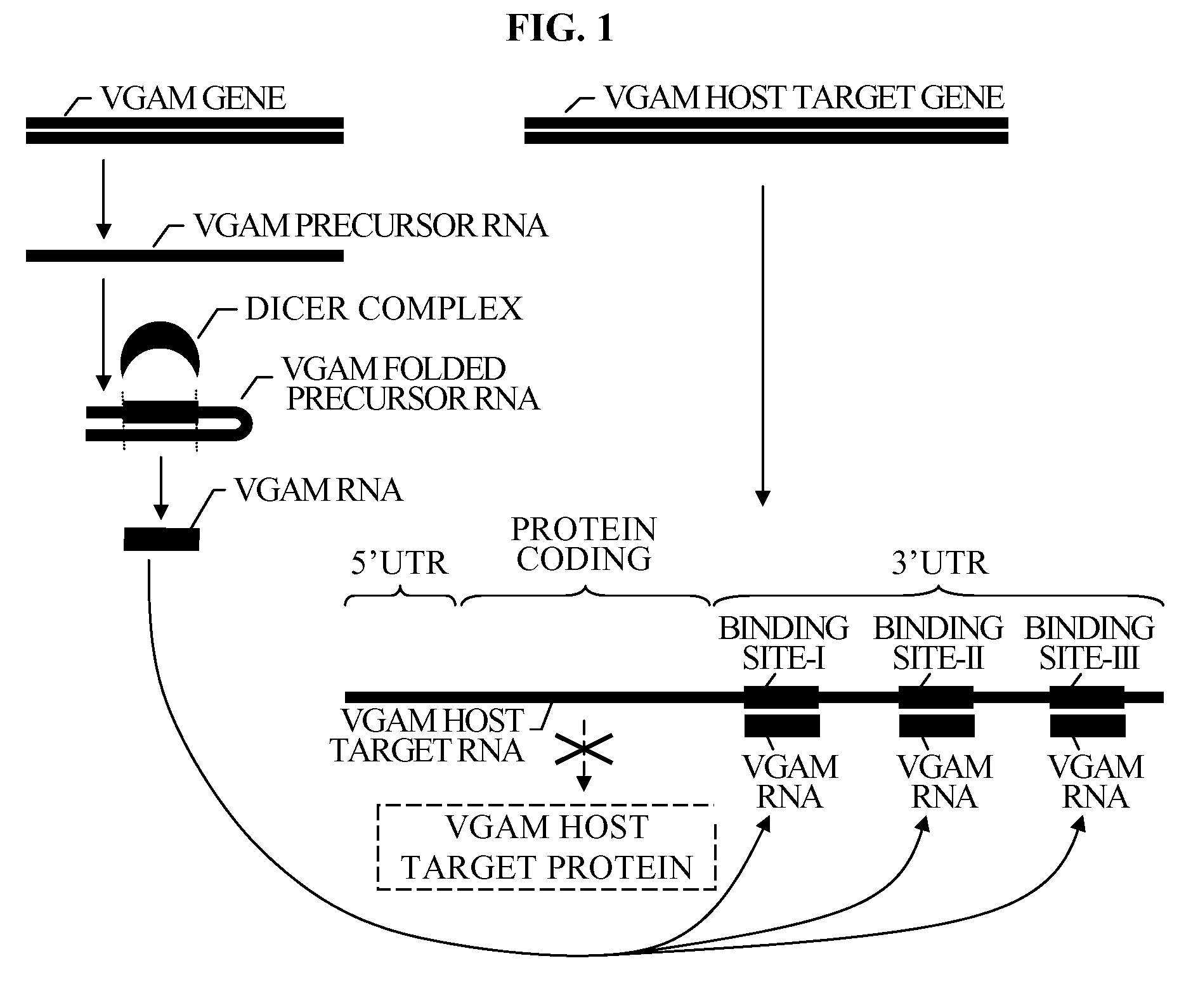
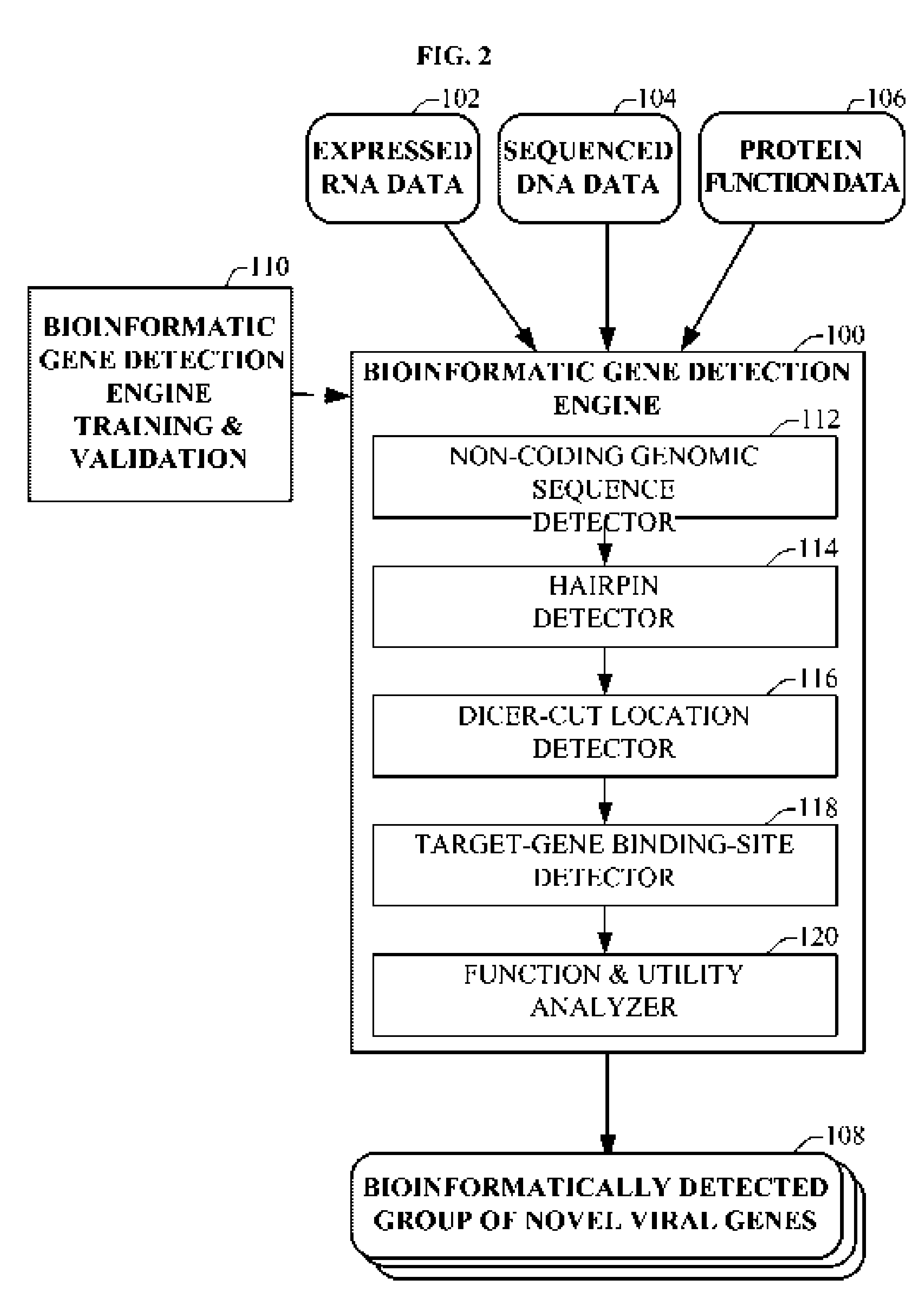
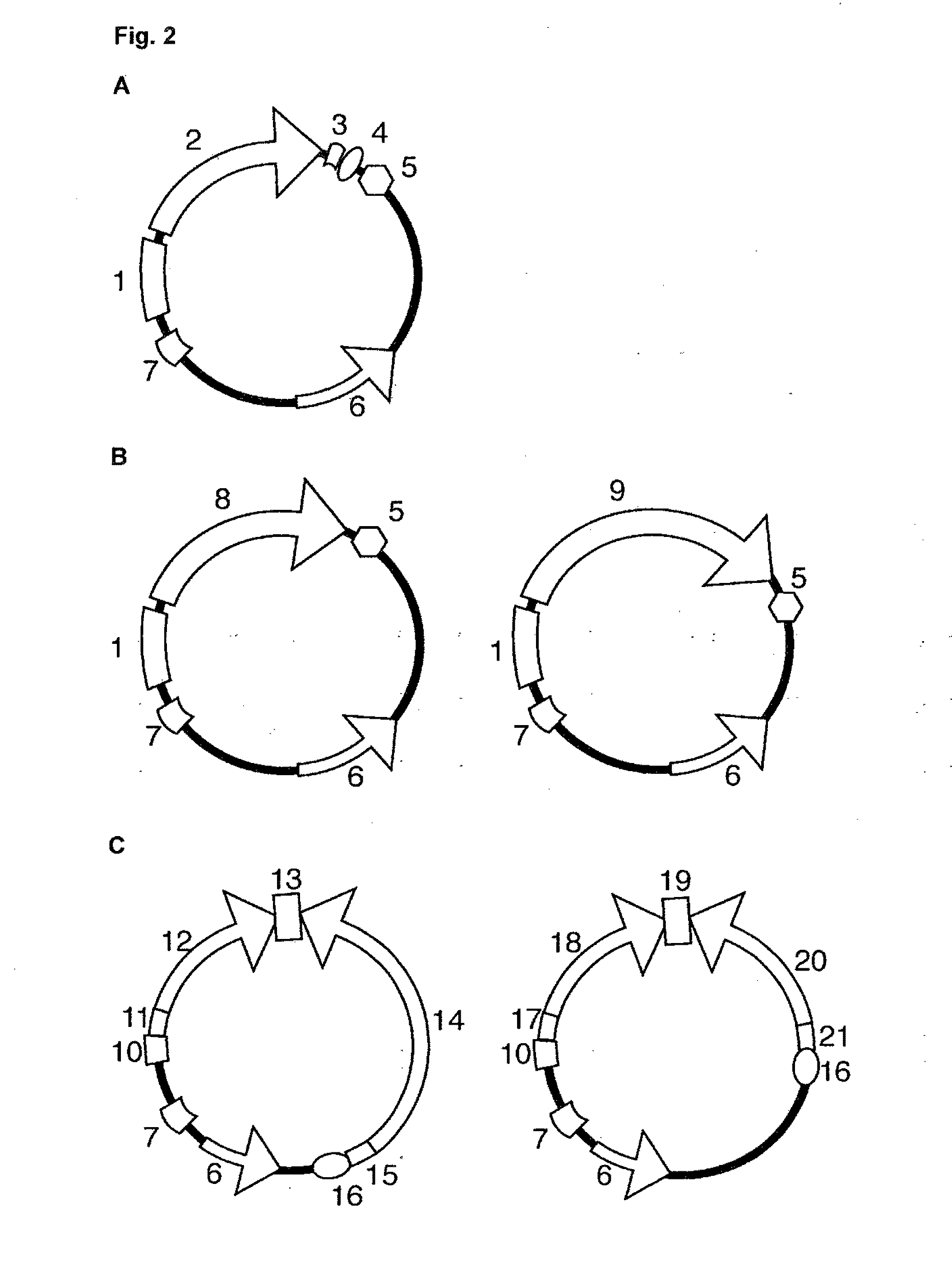
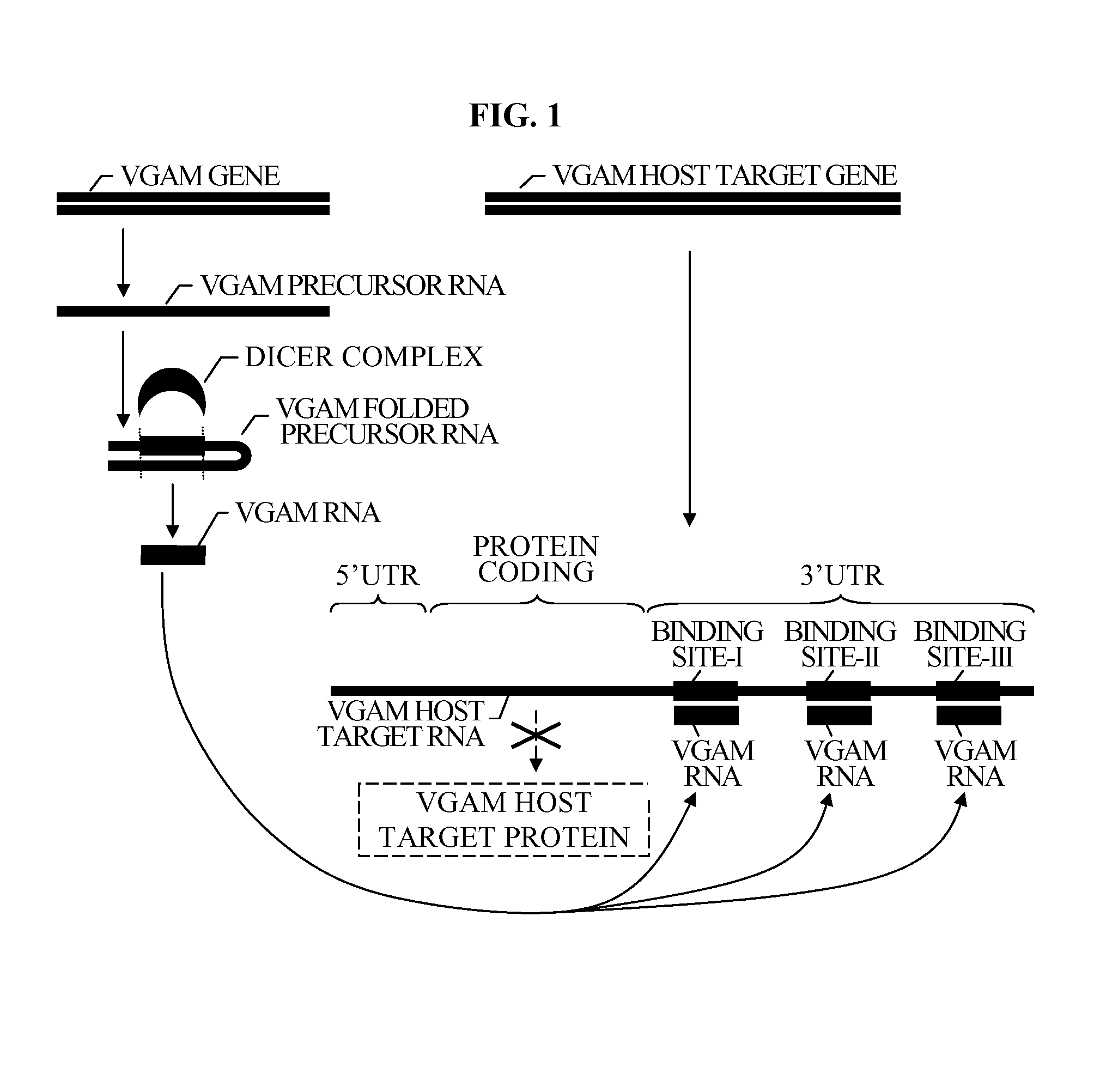
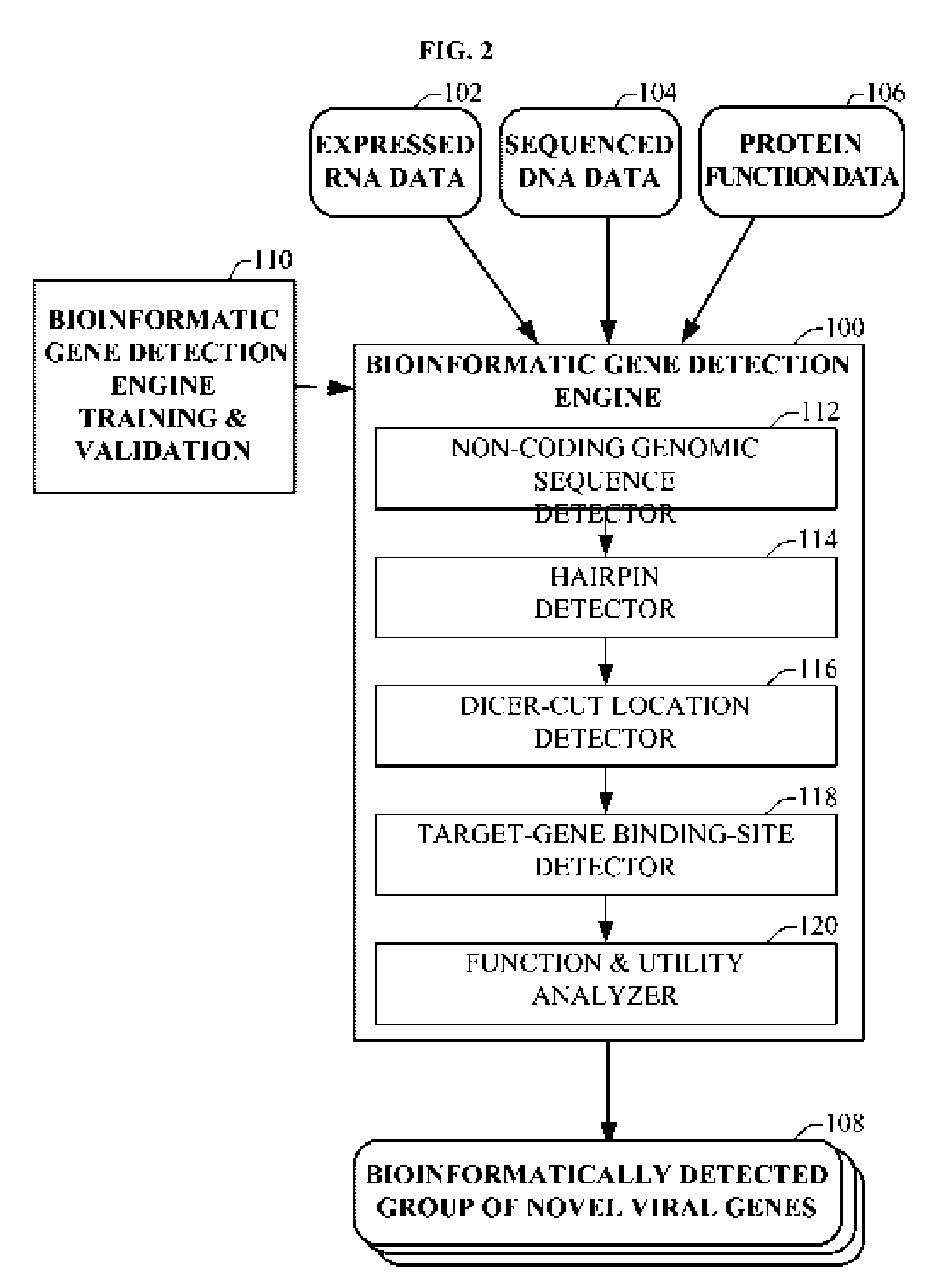
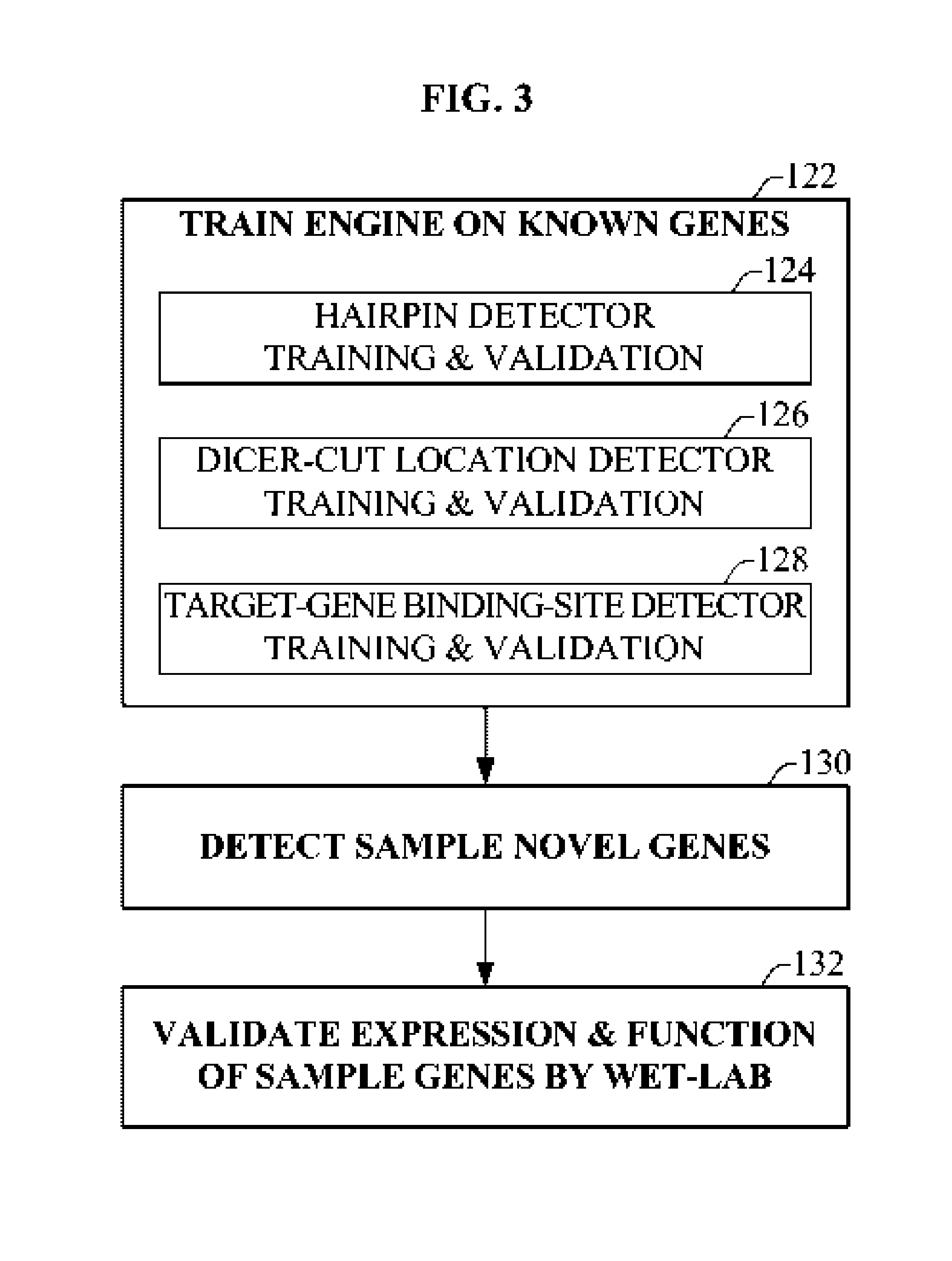
Bioinformatically detectable group of novel HIV regulatory genes and uses thereof
InactiveUS7217807B2Preventing and treating viral diseasesSugar derivativesMicrobiological testing/measurementViral diseaseOperon
The present invention relates to a group of novel viral RNA regulatory genes, here identified as “viral genomic address messenger genes” or “VGAM genes”, and as “genomic record” or “GR” genes. VGAM genes selectively inhibit translation of known host target genes, and are believed to represent a novel pervasive viral attack mechanism. GR genes encode an operon-like cluster of VGAM genes. VGAM and viral GR genes may therefore be useful in diagnosing, preventing and treating viral disease. Several nucleic acid molecules are provided respectively encoding several VGAM genes, as are vectors and probes, both comprising the nucleic acid molecules, and methods and systems for detecting VGAM genes, and for counteracting their activity.
Owner:ROSETTA GENOMICS
Antisense antiviral compound and method for treating influenza viral infection
ActiveUS20110118334A1Improve throughputLimit deliveryAntibacterial agentsSugar derivativesInfluenzavirus CStart codon
The present invention relates to antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Orthomyxoviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of influenza virus infection in a mammal. Exemplary antisense antiviral compounds are substantially uncharged, or partially positively charged, morpholino oligonucleotides having 1) a nuclease resistant backbone, 2) 12-40 nucleotide bases, and 3) a targeting sequence of at least 12 bases in length that hybridizes to a target region selected from the following: a) the 5′ or 3′ terminal 25 bases of the negative sense viral RNA segment of Influenzavirus A, Influenzavirus B and Influenzavirus C; b) the terminal 30 bases of the 5′ or 3′ terminus of the positive sense vcRNA; c) the 45 bases surrounding the AUG start codon of an influenza viral mRNA and; d) 50 bases surrounding the splice donor or acceptor sites of influenza mRNAs subject to alternative splicing.
Owner:SAREPTA THERAPEUTICS INC
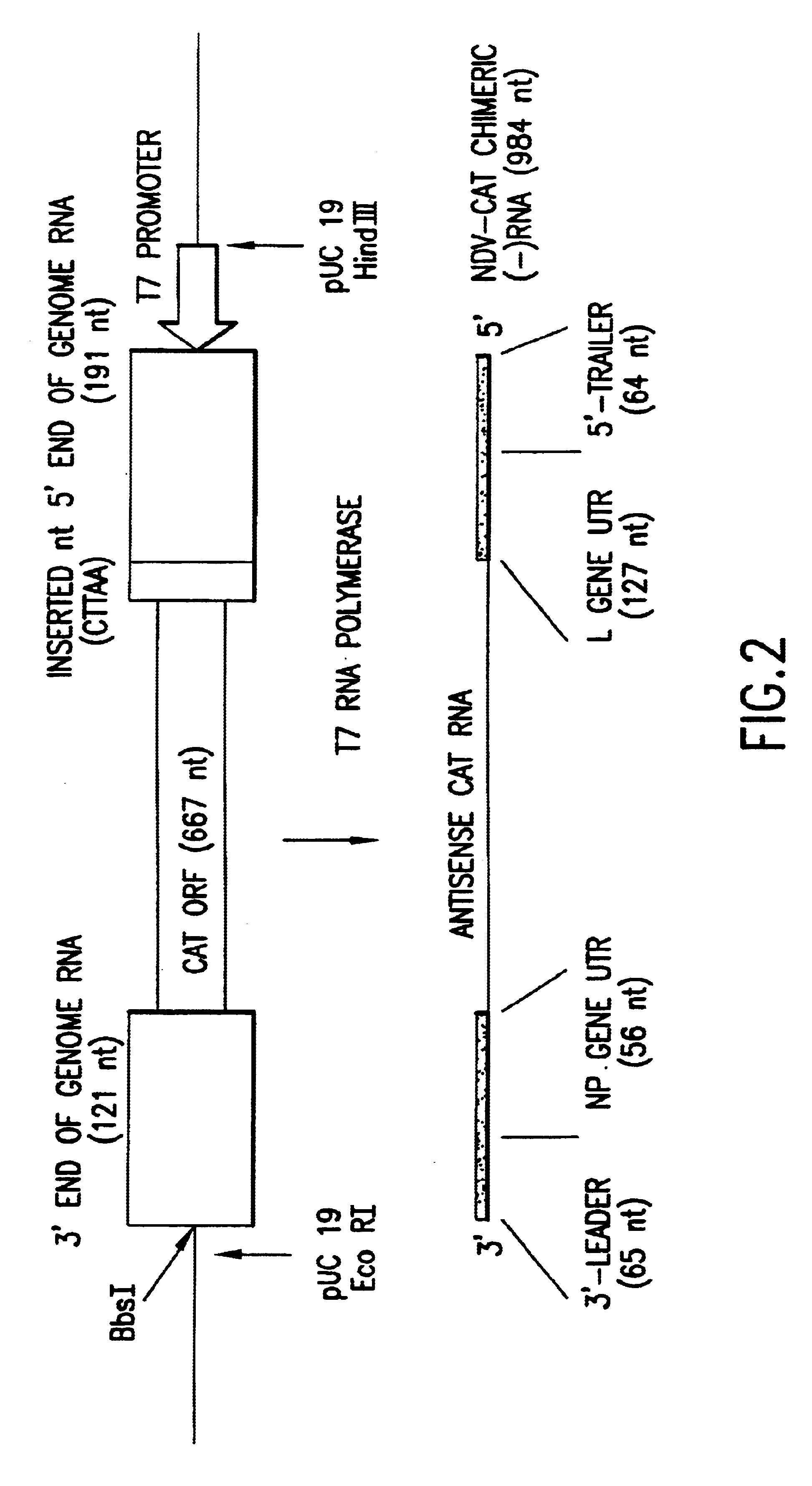
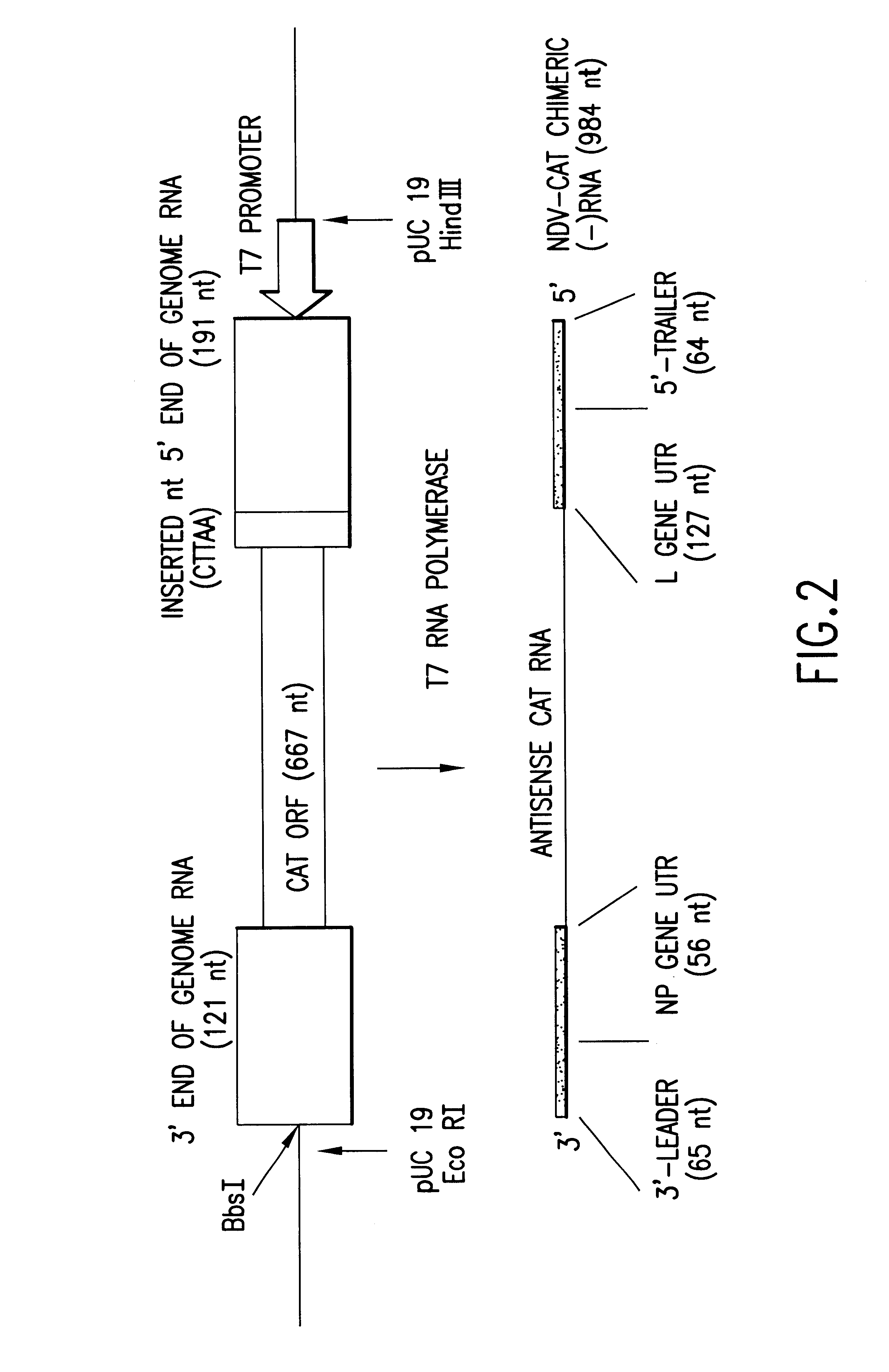
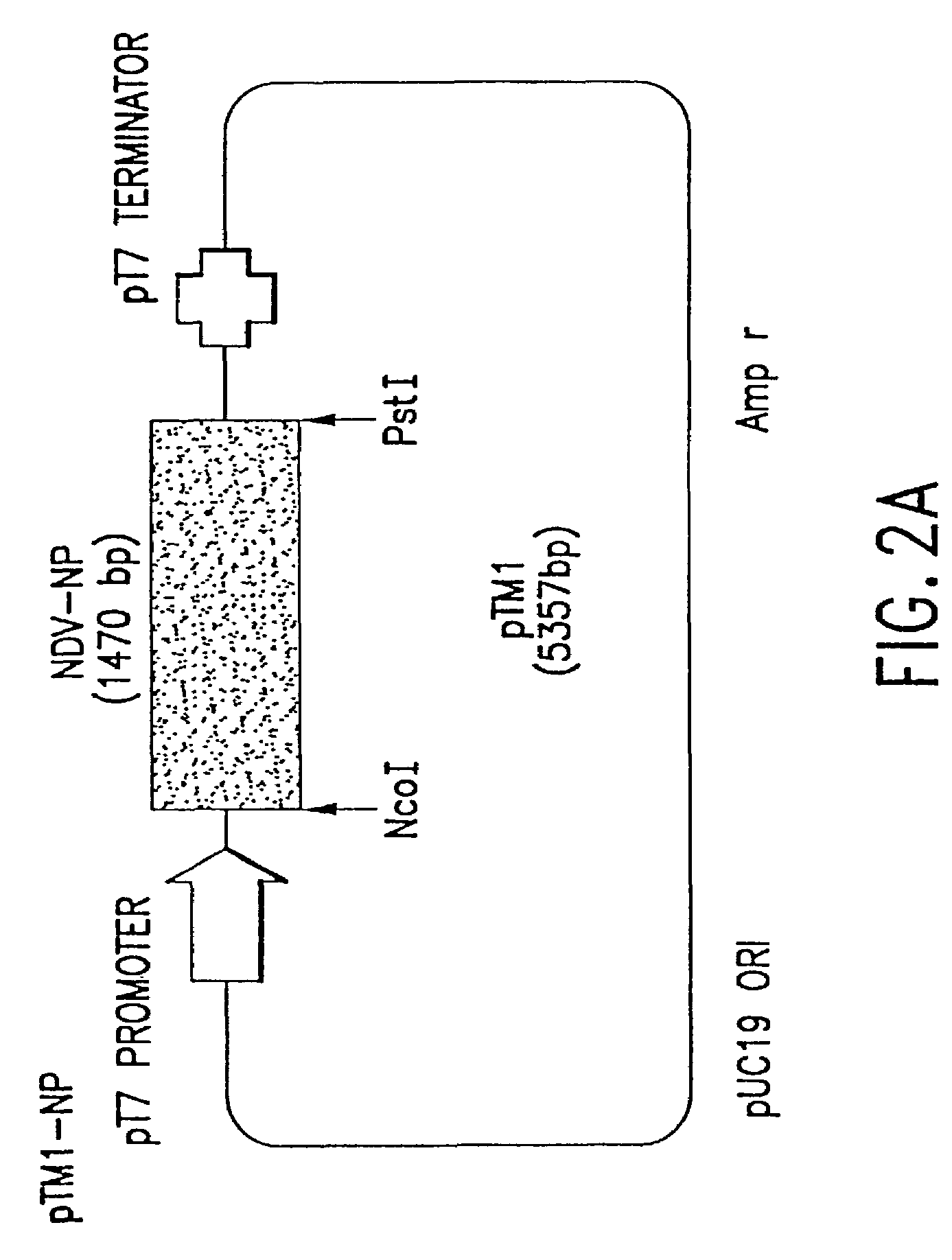
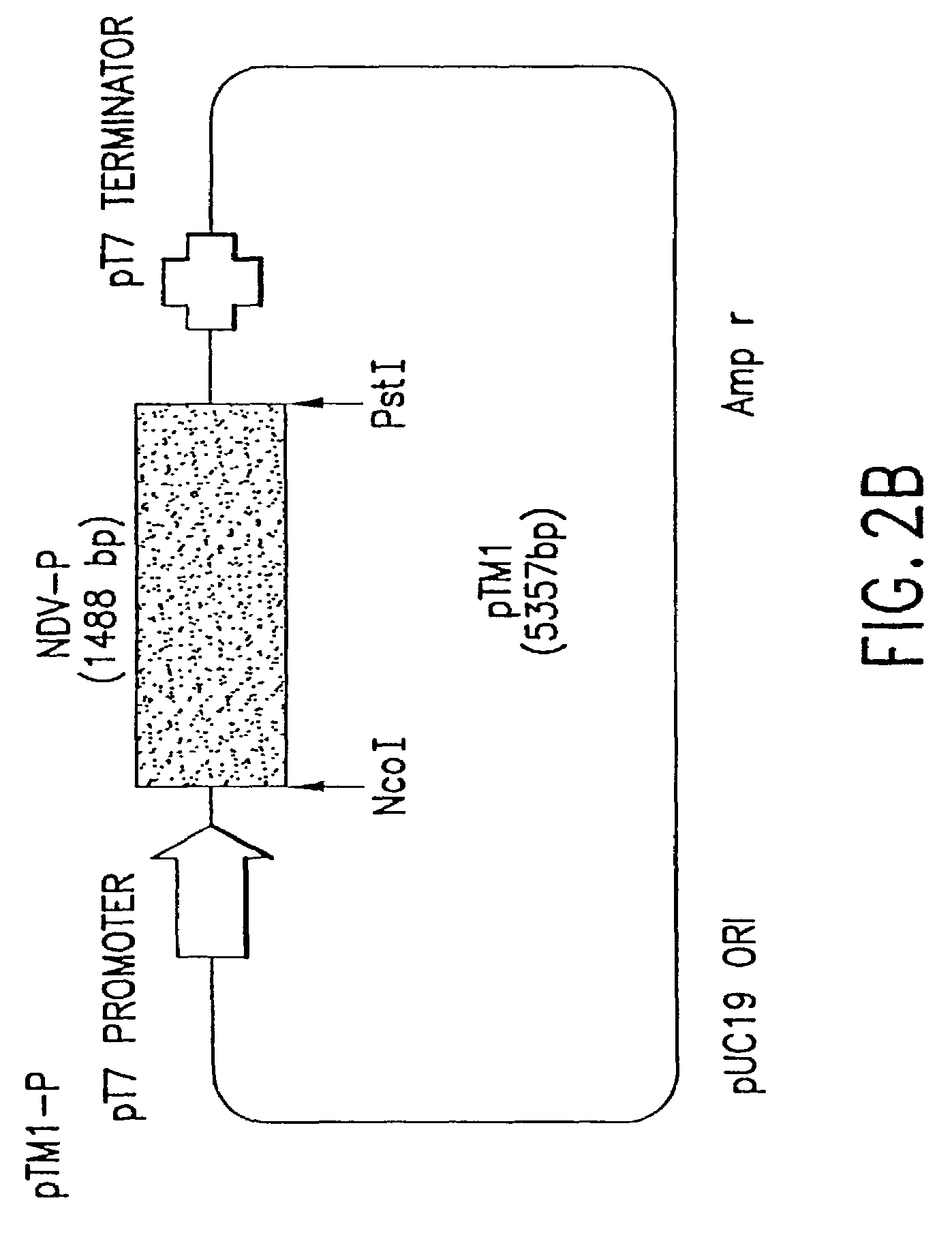
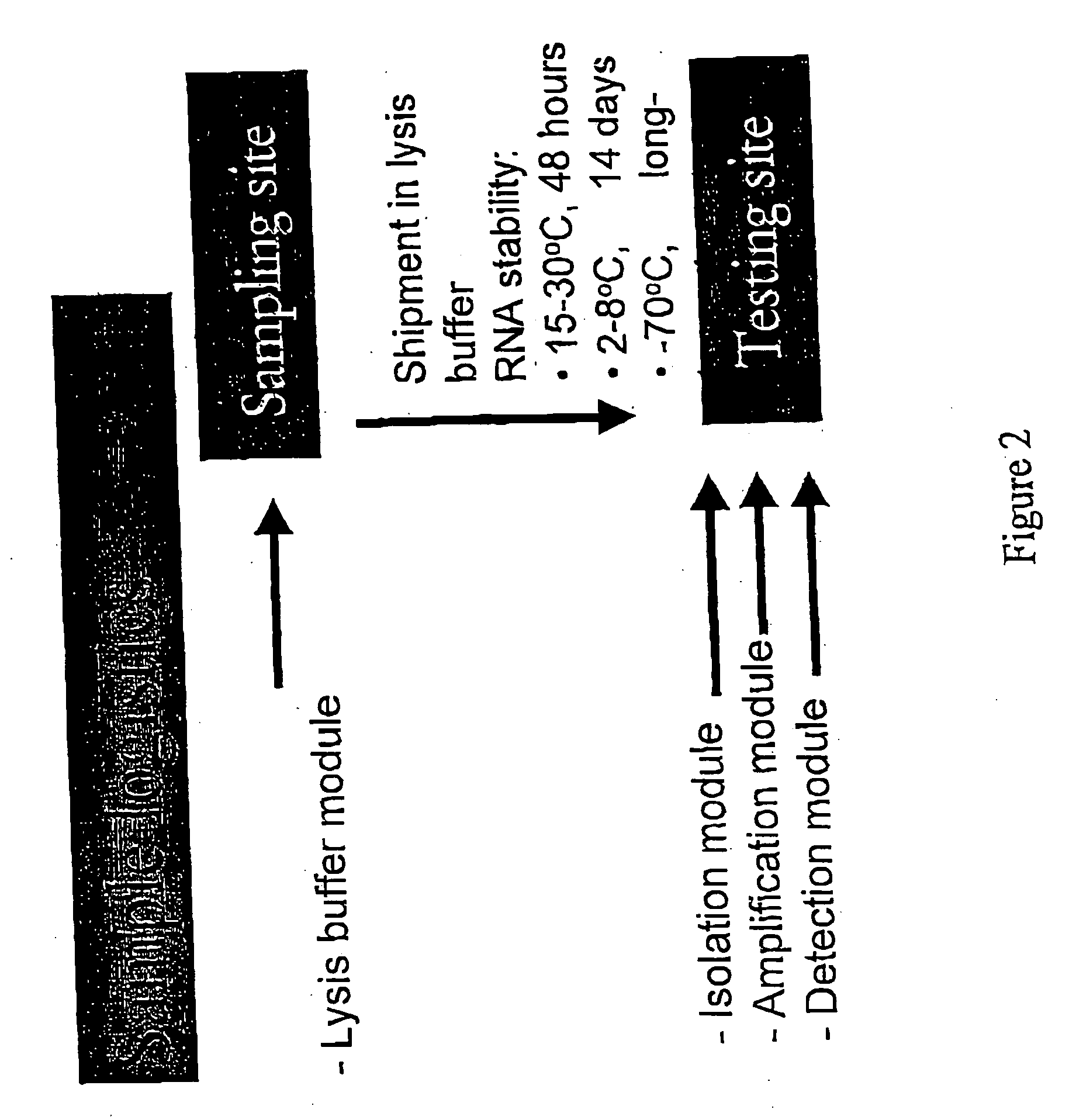
Recombinant Newcastle disease virus RNA expression systems and vaccines
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Modified polynucleotides and uses thereof
InactiveUS6867290B2Improve stabilityReduce potential blockSugar derivativesMicrobiological testing/measurementOligoribonucleotidesPolyribonucleotides
Provided is a polynucleotide comprising mRNA, rRNA or viral RNA, comprising ribose rings that are covalently modified at the 2′-OH position. Further provided are methods for producing a double-stranded oligo- or polynucleotide from a template comprising an oligo- or polyribonucleotide, a proportion of the ribose rings of which are covalently modified at the 2′-OH position to bear a substituent which enables replication of the template by the nucleic acid polymerase. Also provided is use of a poly-nucleotide comprising mRNA, rRNA or viral RNA, a proportion of the ribose rings of which are covalently modified at the 2′-OH position, in a hybridisation reaction.
Owner:CYCLOPS GENOME SCI
Superior molecular vaccine based on self-replicating RNA, suicidal DNA or naked DNA vector, that links antigen with polypeptide that promotes antigen presentation
InactiveUS7557200B2Efficiently presentedImprove effectivenessSsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseMHC class I
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Antisense antiviral compound and method for treating influenza viral infection
ActiveUS8697858B2Improve throughputLimit deliveryAntibacterial agentsSugar derivativesInfluenzavirus CBase J
The present invention relates to antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Orthomyxoviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of influenza virus infection in a mammal. Exemplary antisense antiviral compounds are substantially uncharged, or partially positively charged, morpholino oligonucleotides having 1) a nuclease resistant backbone, 2) 12-40 nucleotide bases, and 3) a targeting sequence of at least 12 bases in length that hybridizes to a target region selected from the following: a) the 5′ or 3′ terminal 25 bases of the negative sense viral RNA segment of Influenzavirus A, Influenzavirus B and Influenzavirus C; b) the terminal 30 bases of the 5′ or 3′ terminus of the positive sense vcRNA; c) the 45 bases surrounding the AUG start codon of an influenza viral mRNA and; d) 50 bases surrounding the splice donor or acceptor sites of influenza mRNAs subject to alternative splicing.
Owner:SAREPTA THERAPEUTICS INC
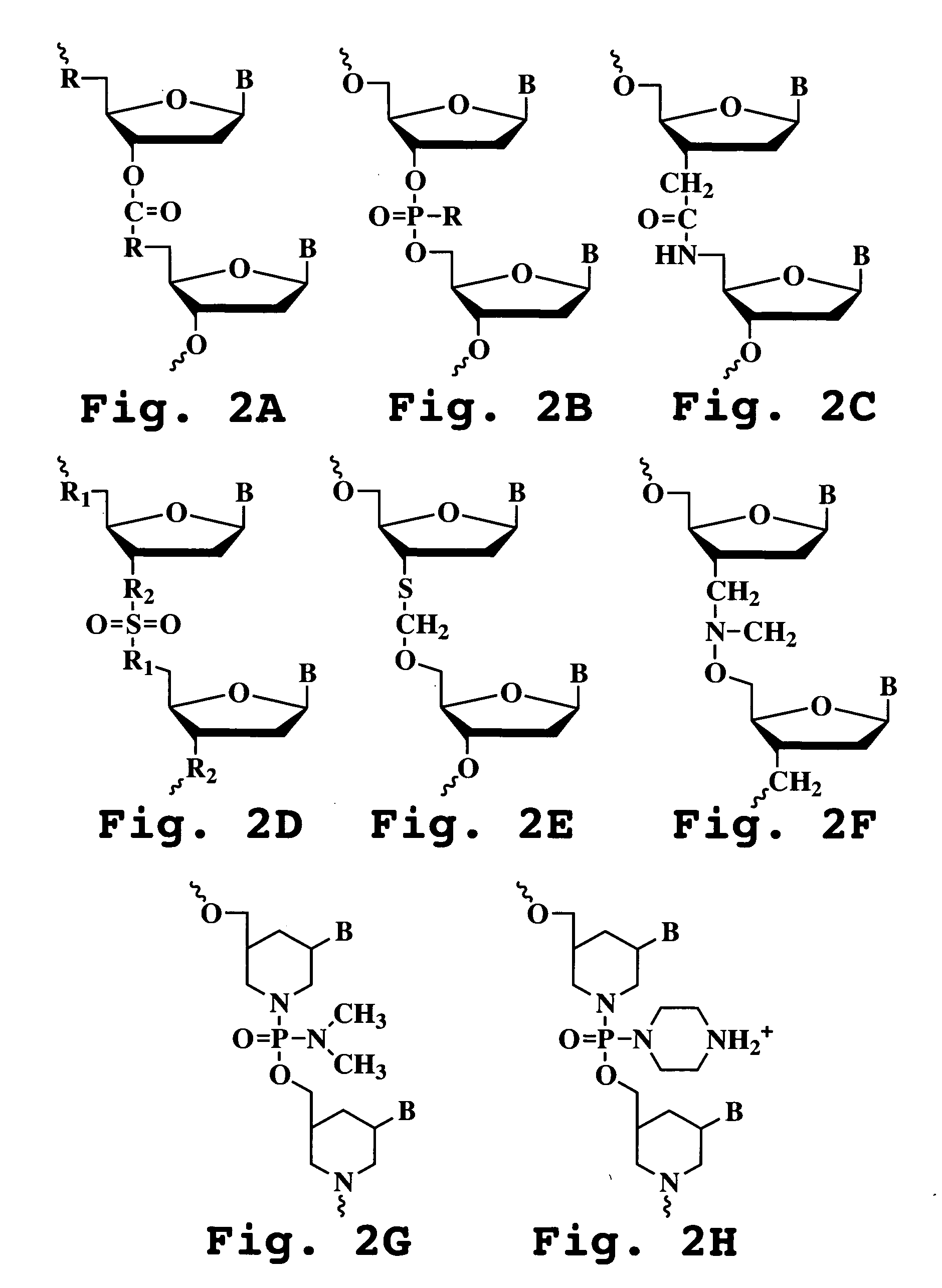
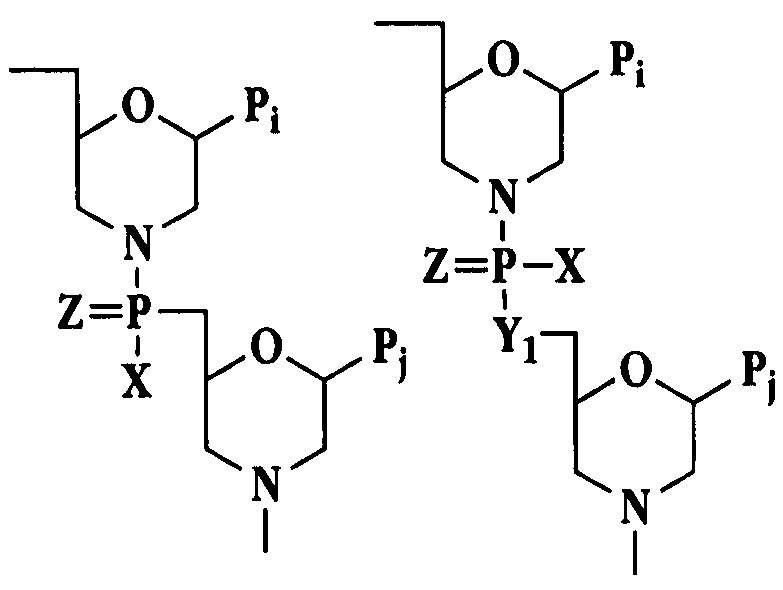
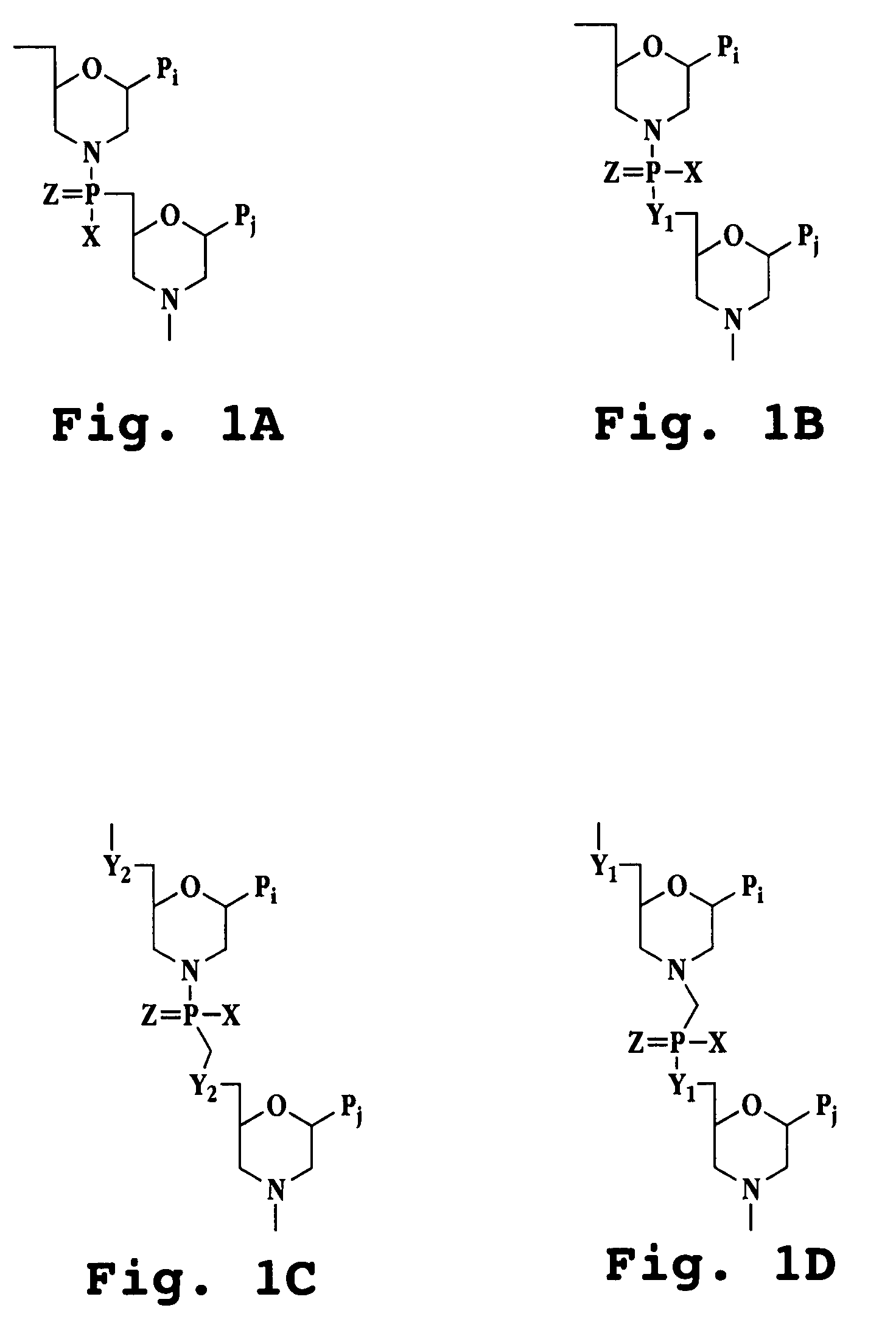
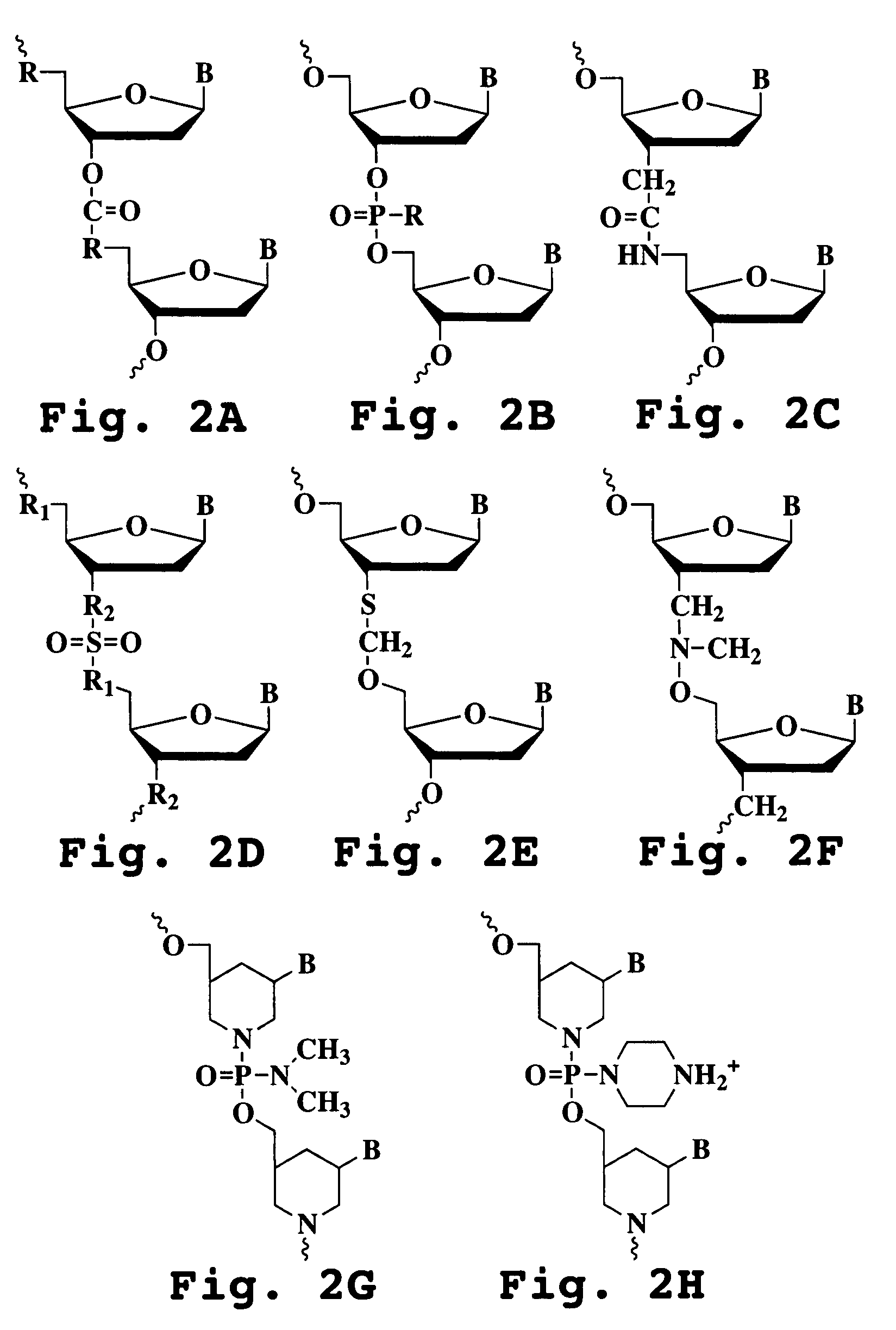
Phosphonates with reduced toxicity for treatment of viral infections
There are provided, inter alia, acyclic nucleoside phosphonate compounds having reduced toxicity and enhanced antiviral activity, and pharmaceutically accepted salts and solvates thereof. There are also provided methods of using the disclosed compounds for inhibiting viral RNA-dependent RNA polymerase, inhibiting viral reverse transcriptase, inhibiting replication of virus, including hepatitis C virus or a human retrovirus, and treating a subject infected with a virus, including hepatitis C virus or a human retrovirus.
Owner:RGT UNIV OF CALIFORNIA
Method for treating enterovirus or rhinovirus infection using antisense antiviral compounds
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:7.
Owner:SAREPTA THERAPEUTICS INC
CRISPRs
A composition for treating a lysogenic virus, including a vector encoding isolated nucleic acid encoding two or more gene editors chosen from gene editors that target viral DNA, gene editors that target viral RNA, and combinations thereof. A composition for treating a lytic virus, including a vector encoding isolated nucleic acid encoding at least one gene editor that targets viral DNA and a viral RNA targeting composition. A composition for treating both lysogenic and lytic viruses, including a vector encoding isolated nucleic acid encoding two or more gene editors that target viral RNA. A composition for treating lytic viruses. Methods of treating a lysogenic virus or a lytic virus, by administering the above compositions to an individual having a virus and inactivating the virus.
Owner:EXCISION BIOTHERAPEUTICS INC
Kit for detecting non-pathogenic or pathogenic influenza a subtype h5 virus
InactiveUS20040142319A1High sensitivityStrong specificitySugar derivativesMicrobiological testing/measurementReverse transcriptaseHaemagglutination inhibition
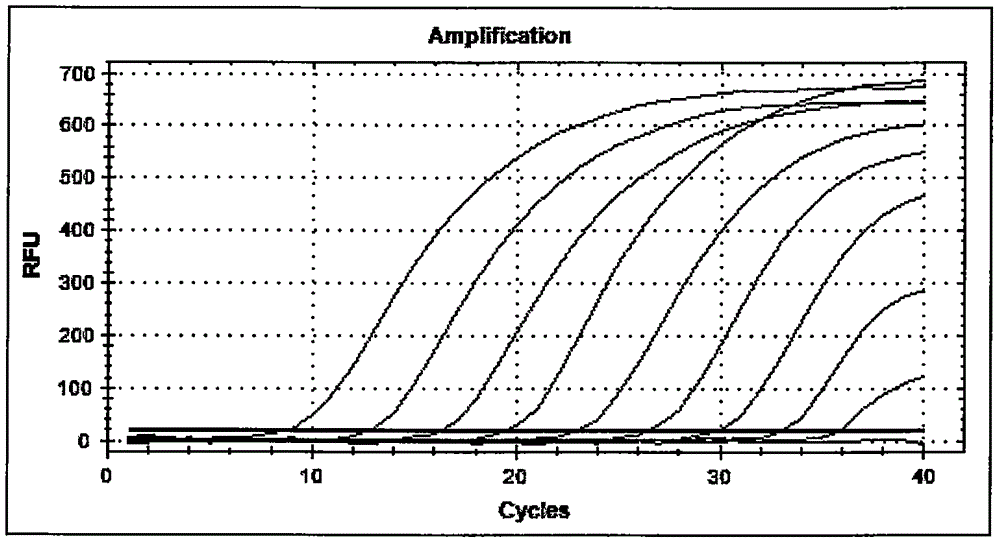
Current methods for detecting influenza A subtype H5 virus, for example cell culture, haemagglutination-inhibition, fluorescent antibody and enzyme immunoassay, and reverse transcriptase polymerase chain reaction (RT-PCR) may have the disadvantages of low sensitivity and low specificity. Furthermore, such methods are relatively difficult to use, and may not be suitable for routine detection on a daily basis. The kit for detecting H5 virus of this invention may provide a user-friendly alternative that is relatively more sensitive and specific to H5 virus. The detection kit utilizes two specially designed primers A and B for the replication of H5 virus, and a specific capture probe for immobilizing the amplified viral RNA. An additional primer C is also designed for the detection of pathogenic H5 virus. The detection of H5 virus by the detection kit may be accomplished within one day if desired.
Owner:HAI KANG LIFE
Recombinant negative strand virus rna expression systems and vaccines
ActiveUS20050221489A1Improving immunogenicityAttenuated phenotypeSsRNA viruses negative-senseAntibacterial agentsHeterologousNegative strand
The present invention relates to recombinant RNA virus templates derived from and applicable to negative strand naturally non-segmented viruses, including the families Bornaviridae, Filoviridae, and Paramyxoviridae, and methods for generating such recombinant RNA virus templates, wherein the templates are RNA generated from two or more recombinant RNA molecules. The invention relates to the use of segmented recombinant RNA virus templates for naturally non-segmented RNA viruses to express heterologous gene products in appropriate host cell systems and / or to construct recombinant viruses taken from that family and that express, package, and / or present the heterologous gene product. The invention includes the expression products and recombinant and chimeric viruses thus prepared and vaccine and therapeutic formulations comprising the recombinant RNA viruses.
Owner:MT SINAI SCHOOL OF MEDICINE
Induction of viral mutation by incorporation of miscoding ribonucleoside analogs into viral RNA
InactiveUS20050187180A1High mutation rateReduced viabilityOrganic active ingredientsSsRNA viruses positive-senseRibonucleosideViral replication
Owner:UNIV OF WASHINGTON
Replication-defective arenavirus vectors
ActiveUS20100297172A1Enhance protein expressionHigh expressionAntibacterial agentsSsRNA viruses negative-senseAntigenDisease
The invention relates to an infectious arenavirus particle that is engineered to contain a genome with the ability to amplify and express its genetic information in infected cells but unable to produce further infectious progeny particles in normal, not genetically engineered cells. One or more of the four arenavirus open reading frames glycoprotein (GP), nucleoprotein (NP), matrix protein Z and RNA-dependent RNA polymerase L are removed or mutated to prevent replication in normal cells but still allowing gene expression in arenavirus vector-infected cells, and foreign genes coding for an antigen or other protein of interest or nucleic acids modulating host gene expression are expressed under control of the arenavirus promoters, internal ribosome entry sites or under control of regulatory elements that can be read by the viral RNA-dependent RNA polymerase, cellular RNA polymerase I, RNA polymerase II or RNA polymerase III. The modified arenaviruses are useful as vaccines and therapeutic agents for a variety of diseases.
Owner:UNIV ZURICH
Bioinformatically detectable human herpesvirus 5 regulatory gene
ActiveUS7696334B1Preventing and treating viral diseasesSugar derivativesMicrobiological testing/measurementOperonVirus attack
The present invention relates to a group of novel viral RNA regulatory genes, here identified as “viral genomic address messenger genes” or “VGAM genes”, and as “genomic record” or “GR” genes. VGAM genes selectively inhibit translation of known host target genes, and are believed to represent a novel pervasive viral attack mechanism. GR genes encode an operon-like cluster of VGAM genes. VGAM and viral GR genes may therefore be useful in diagnosing, preventing and treating viral disease. Several nucleic acid molecules are provided respectively encoding several VGAM genes, as are vectors and probes, both comprising the nucleic acid molecules, and methods and systems for detecting VGAM genes, and for counteracting their activity.
Owner:ROSETTA GENOMICS
Superior molecular vaccine based on self-replicating rna, suicidal dna or naked dna vector, that links antigen with polypeptide that promotes antigen presentation
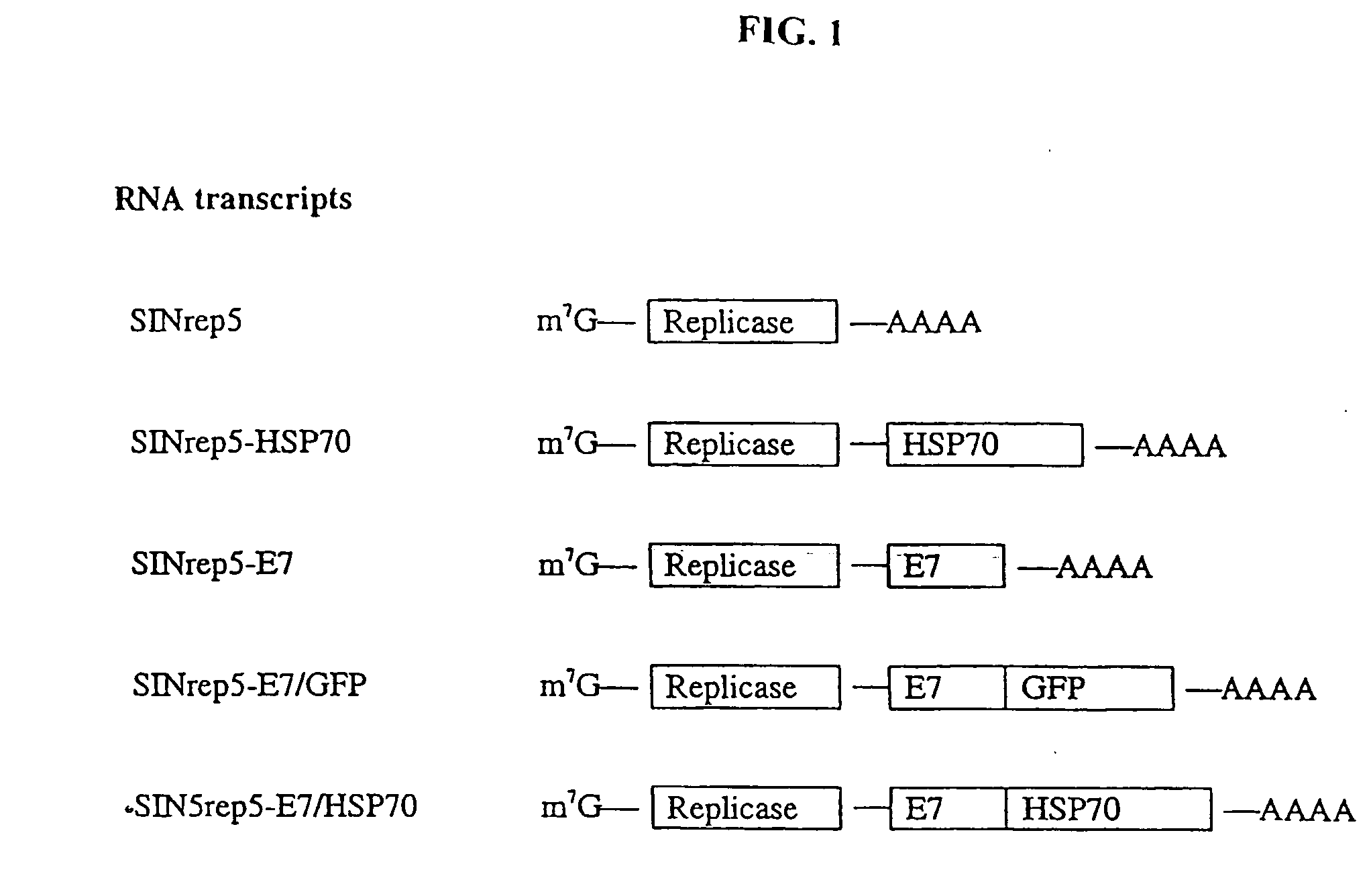
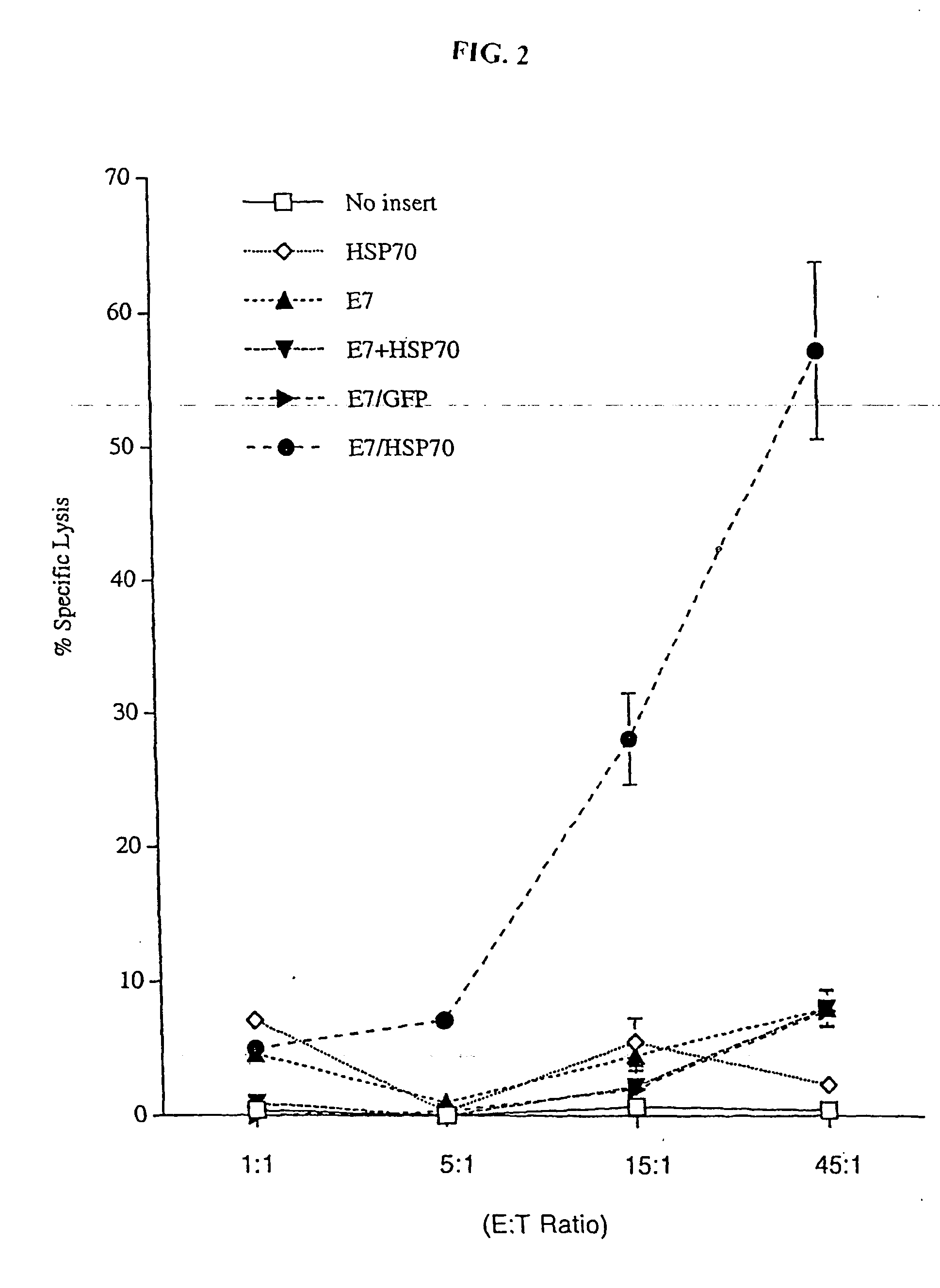
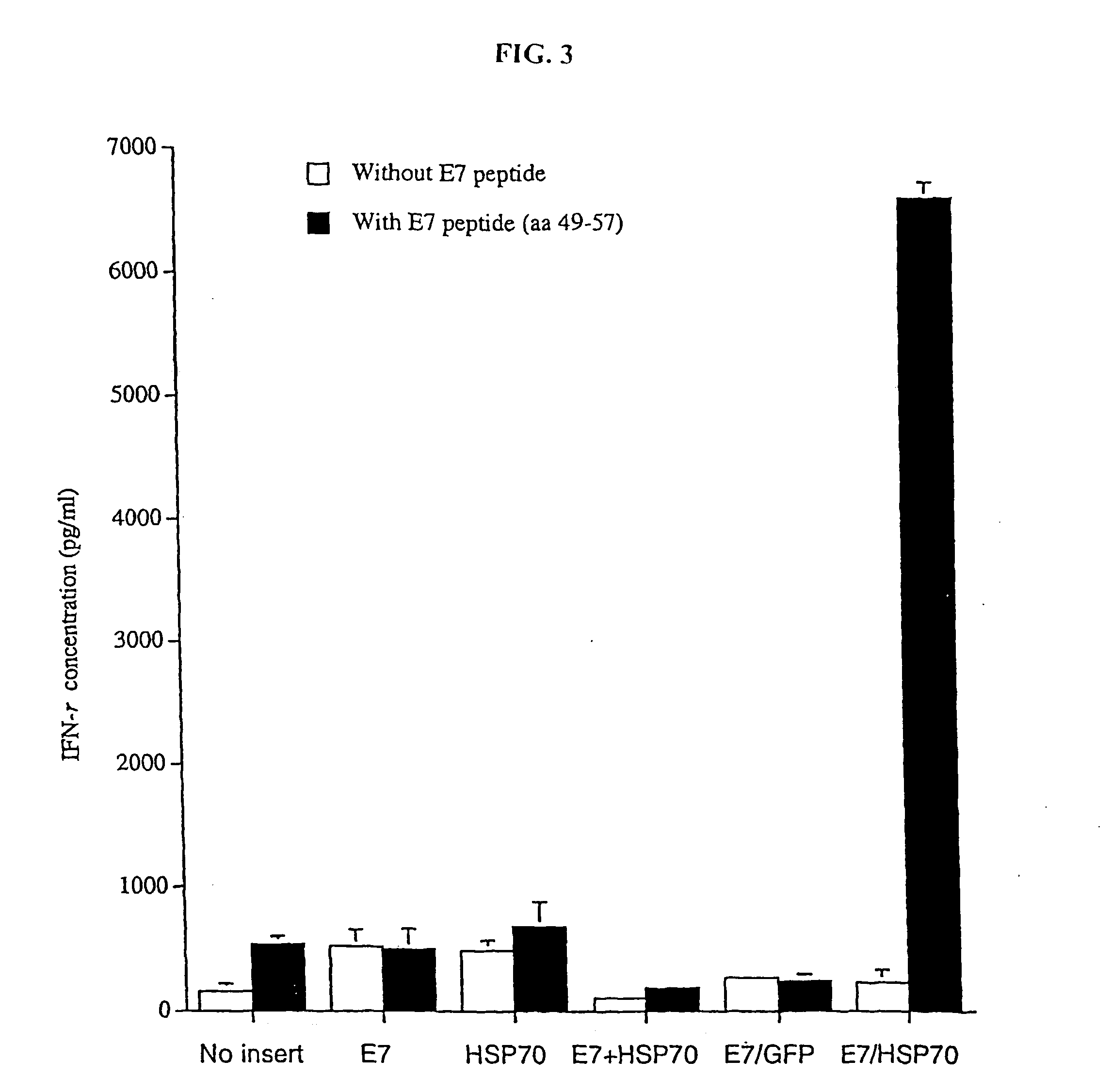
InactiveUS20050277605A1Great E7-specific T cell-mediated immunityEfficiently presentedSsRNA viruses positive-senseAntibody mimetics/scaffoldsMHC class IDisease
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Primers, probes and detection kits for detection of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN102277454AShorten the timeSave costsMicrobiological testing/measurementFluorescence/phosphorescenceDuplex pcrDiarrhea
The invention discloses a primer, a probe and a detection kit for detecting porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The detection primers and probes are SEQ ID NO: 1 to SEQ ID NO: 6 in the sequence table, wherein the sequences SEQ ID NO: 1 and SEQ ID NO: 2 are sense primers and antisense primers for detecting porcine transmissible gastroenteritis virus respectively, and the sequence SEQ ID NO: 3 For detecting the fluorescent probe of porcine transmissible gastroenteritis virus, sequence SEQIDNO: 4 and SEQIDNO: 5 are sense primer and antisense primer for detecting porcine epidemic diarrhea virus respectively, and sequence SEQIDNO: 6 is the detection primer of porcine epidemic diarrhea virus fluorescent probe. The invention also provides detection kits for porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The primers and probes selected by the present invention have very strong specificity. The total viral RNA extracted from porcine diarrhea does not need to be transcribed into cDNA first. The synthesis of the first strand of cDNA and double PCR are completed in one step, and two viruses can be detected at one time. ,Improve efficiency.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Zika virus fluorescent PCR detecting kit
InactiveCN105734171AAccurate detectionEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention provides a Zika virus fluorescent PCR detecting kit, and relates to a kit for detecting Zika virus, in particular to simultaneous detection of Zika virus RNA by using a real-time fluorescent polymerase chain type reaction technology.The kit mainly comprises RT-PCR reaction liquid, primer probe mixed liquid, an RT-PCR reaction enzyme system, DEPC H2O and a packaging box for separating and packaging reagent bottles or tubes in a centralized manner.The kit adopts a Taqman probe detection mode and a one-step real-time fluorescent PCR reaction mode, can accurately and quickly detect the Zika virus RNA, and can be widely applied to various fields such as Zika virus disease clinic early diagnosis, disease prevention and scientific research.
Owner:DAAN GENE CO LTD
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant negative strand RNA virus expression systems and vaccines
InactiveUS20050032043A1Equal efficiencySsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
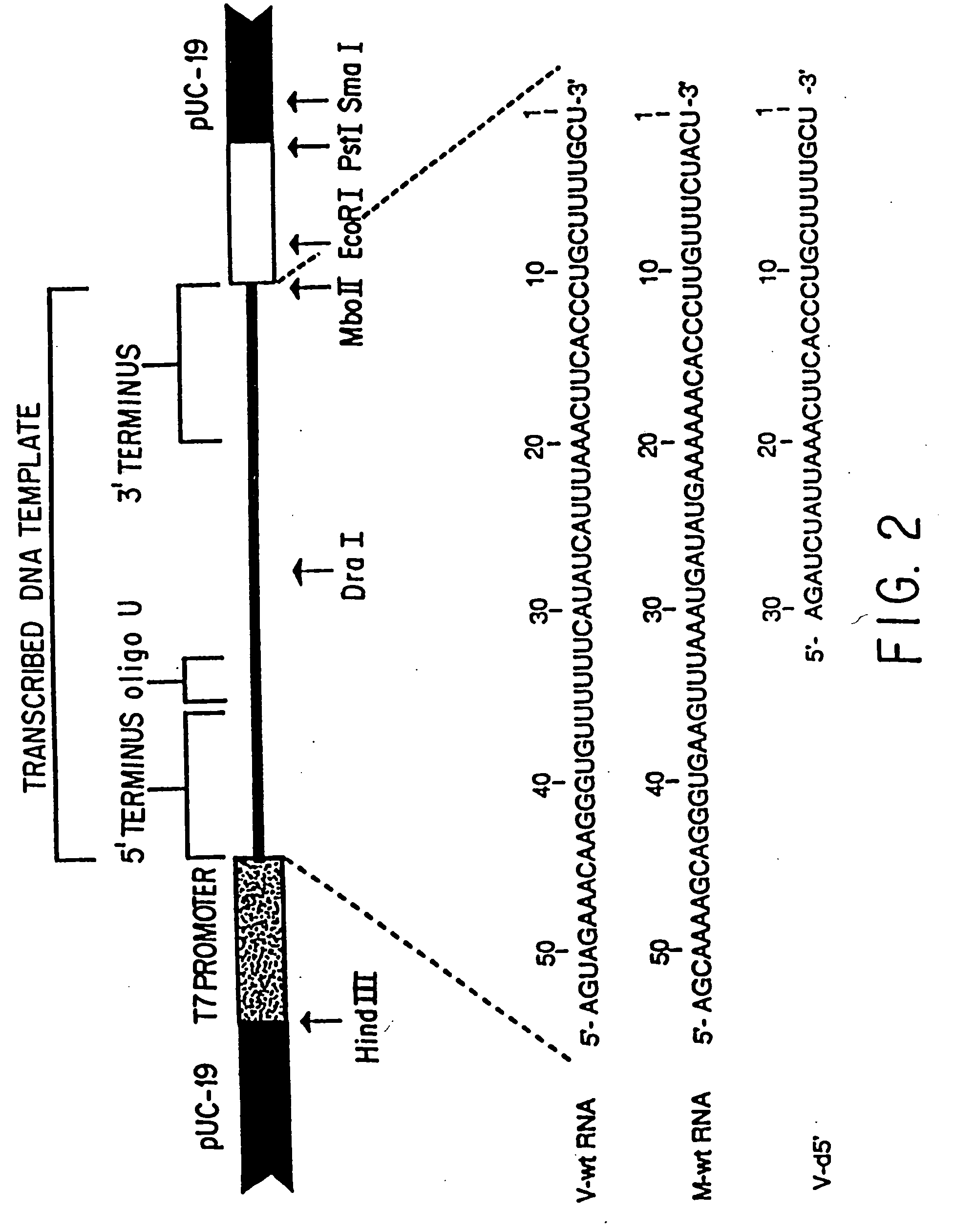
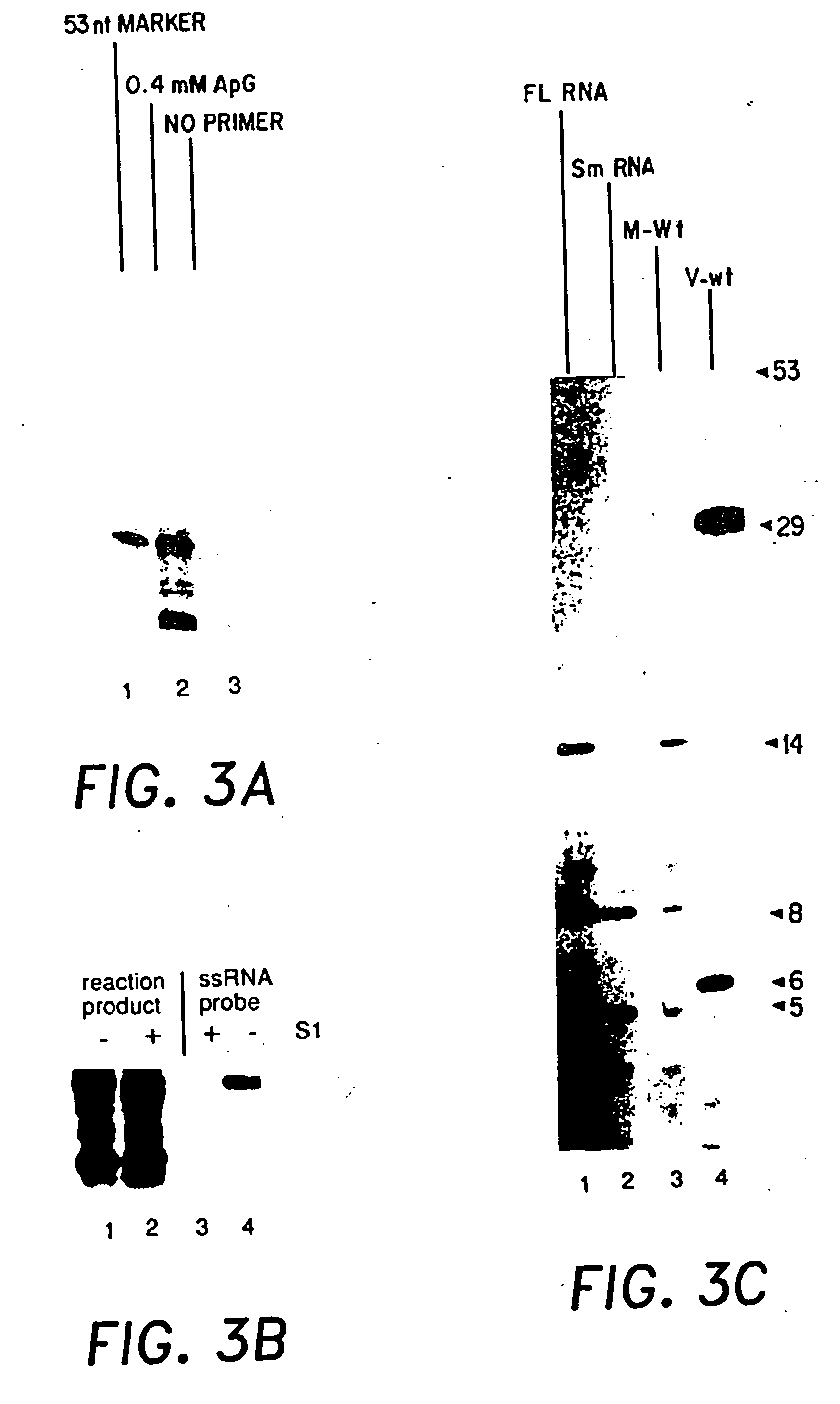
Recombinant negative-strand viral RNA templates are described which may be used with purified RNA-directed RNA polymerase complex to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. The RNA templates are prepared by transcription of appropriate DNA sequences with a DNA-directed RNA polymerase. The resulting RNA templates are of the negative-polarity and contain appropriate terminal sequences which enable the viral RNA-synthesizing apparatus to recognize the template. Bicistronic mRNAs can be constructed to permit internal initiation of translation of viral sequences and allow for the expression of foreign protein coding sequences from the regular terminal initiation site, or vice versa.
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com