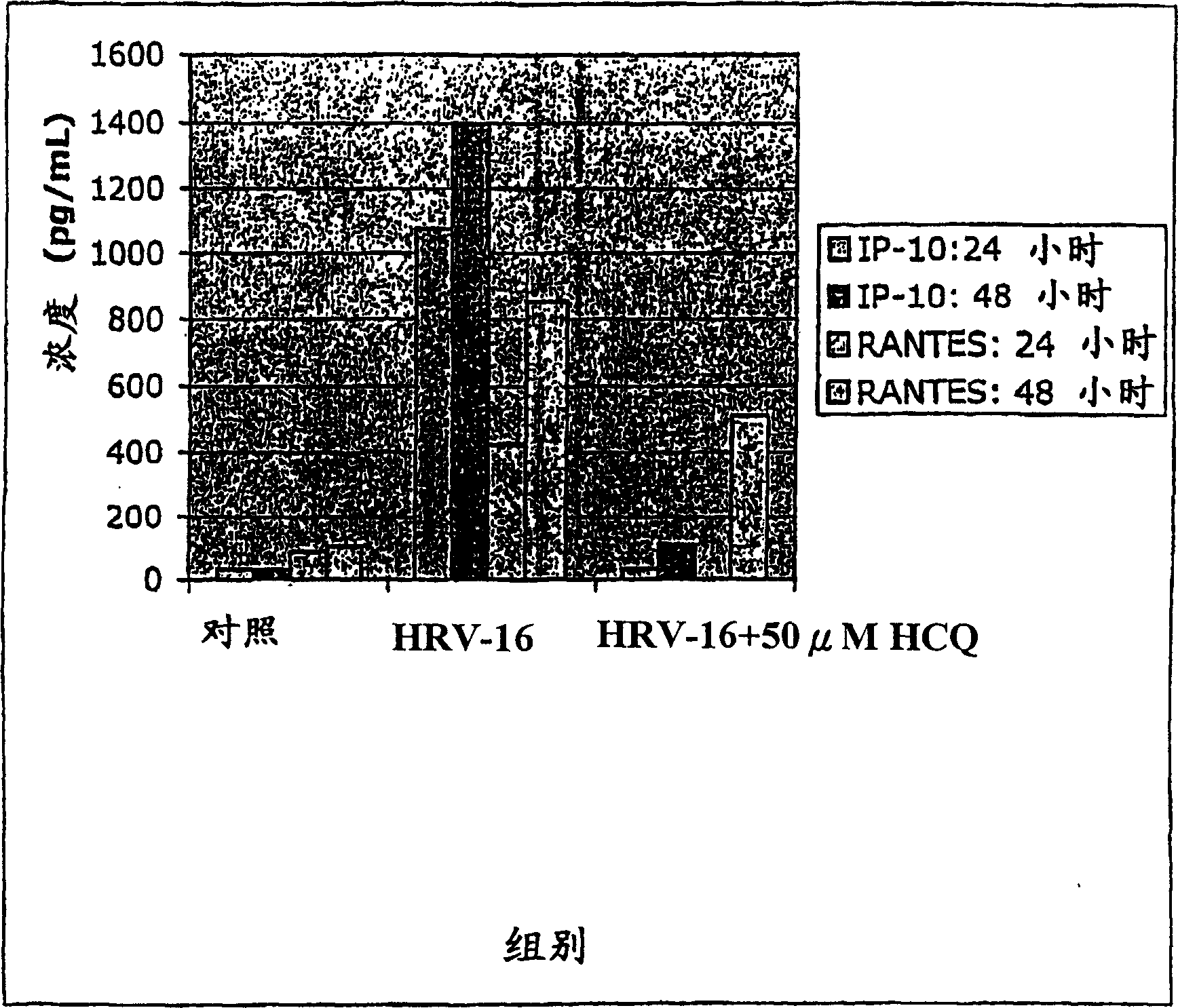
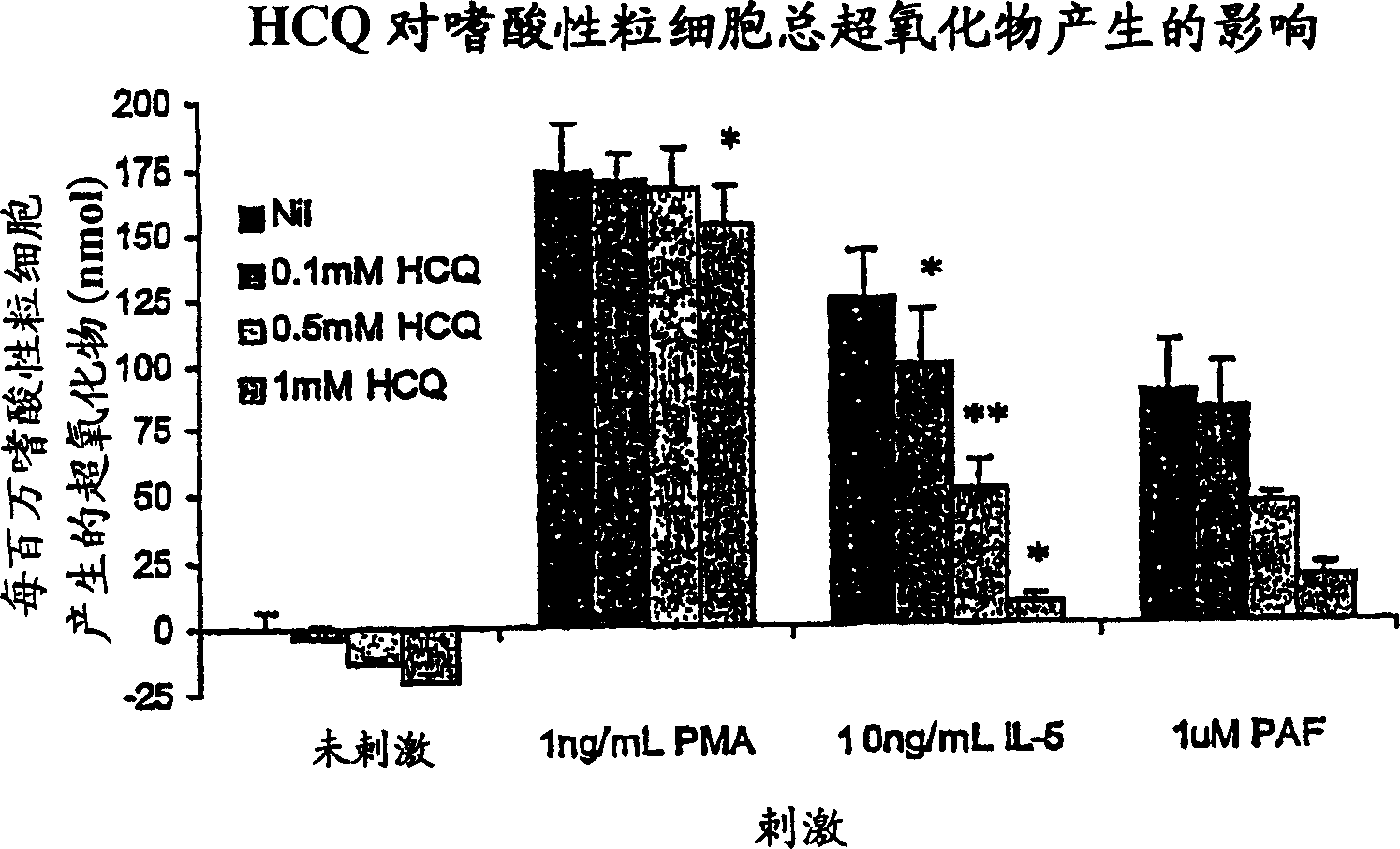
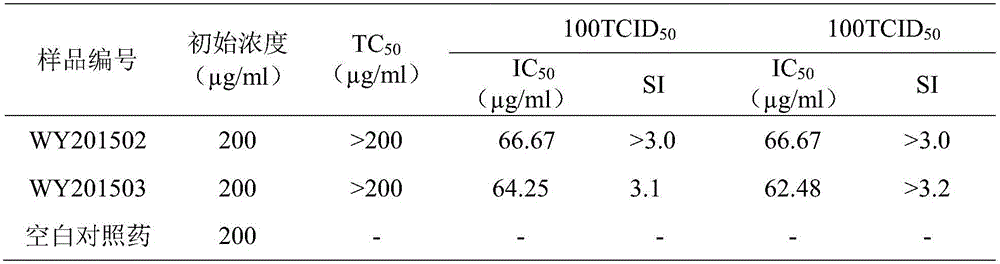
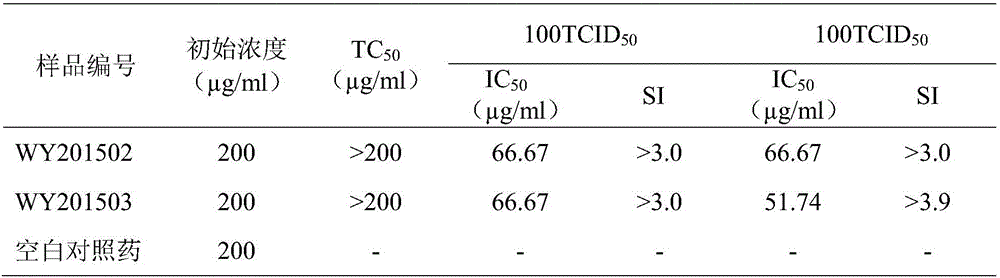
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Rhinovirus infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rhinovirus infection: Introduction. Rhinovirus infection: Infection with the rhinovirus which can be transmitted through direct contact with contaminated secretions from an infected person (e.g. sneezing or nasal or oral secretions on hands).
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
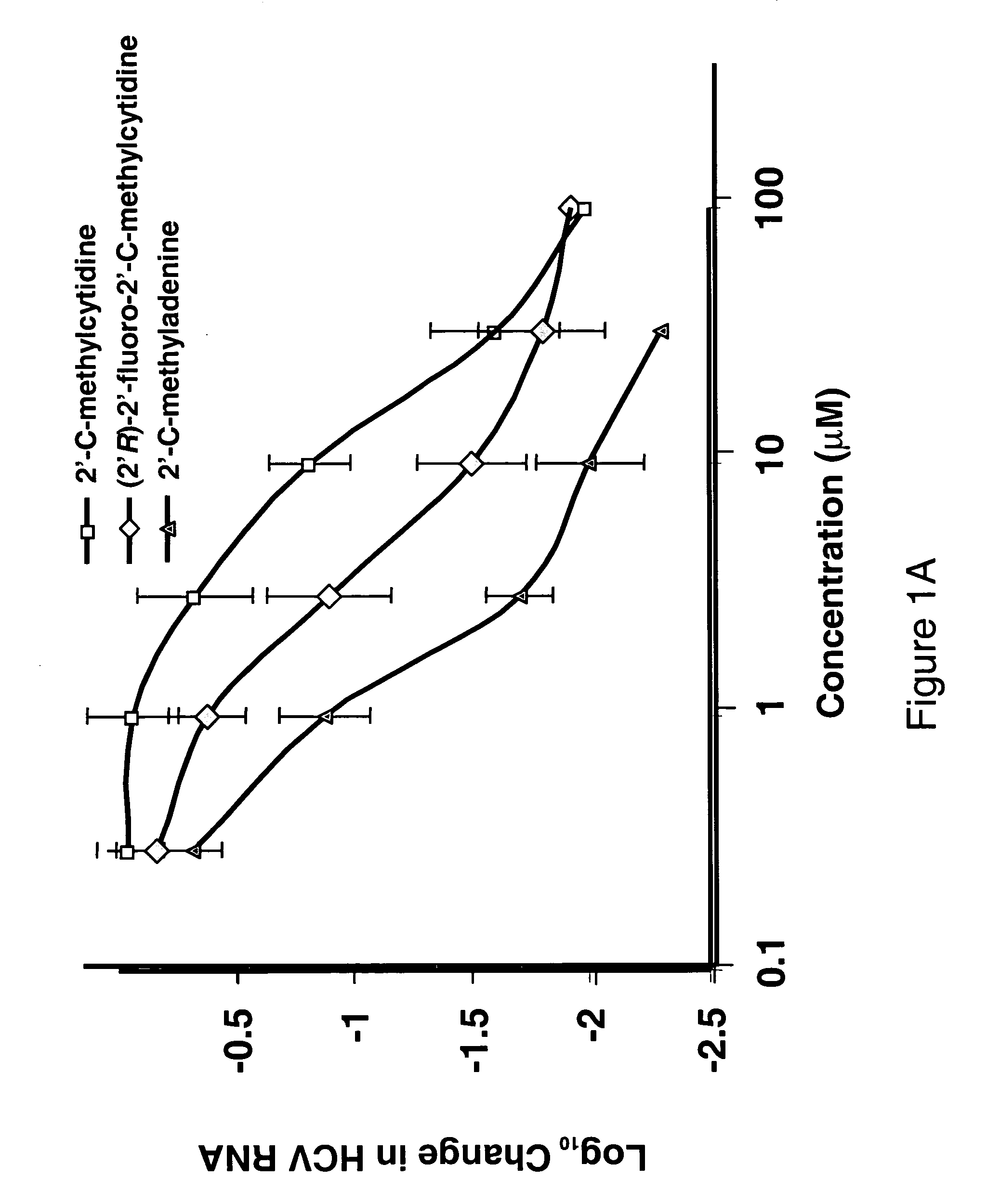
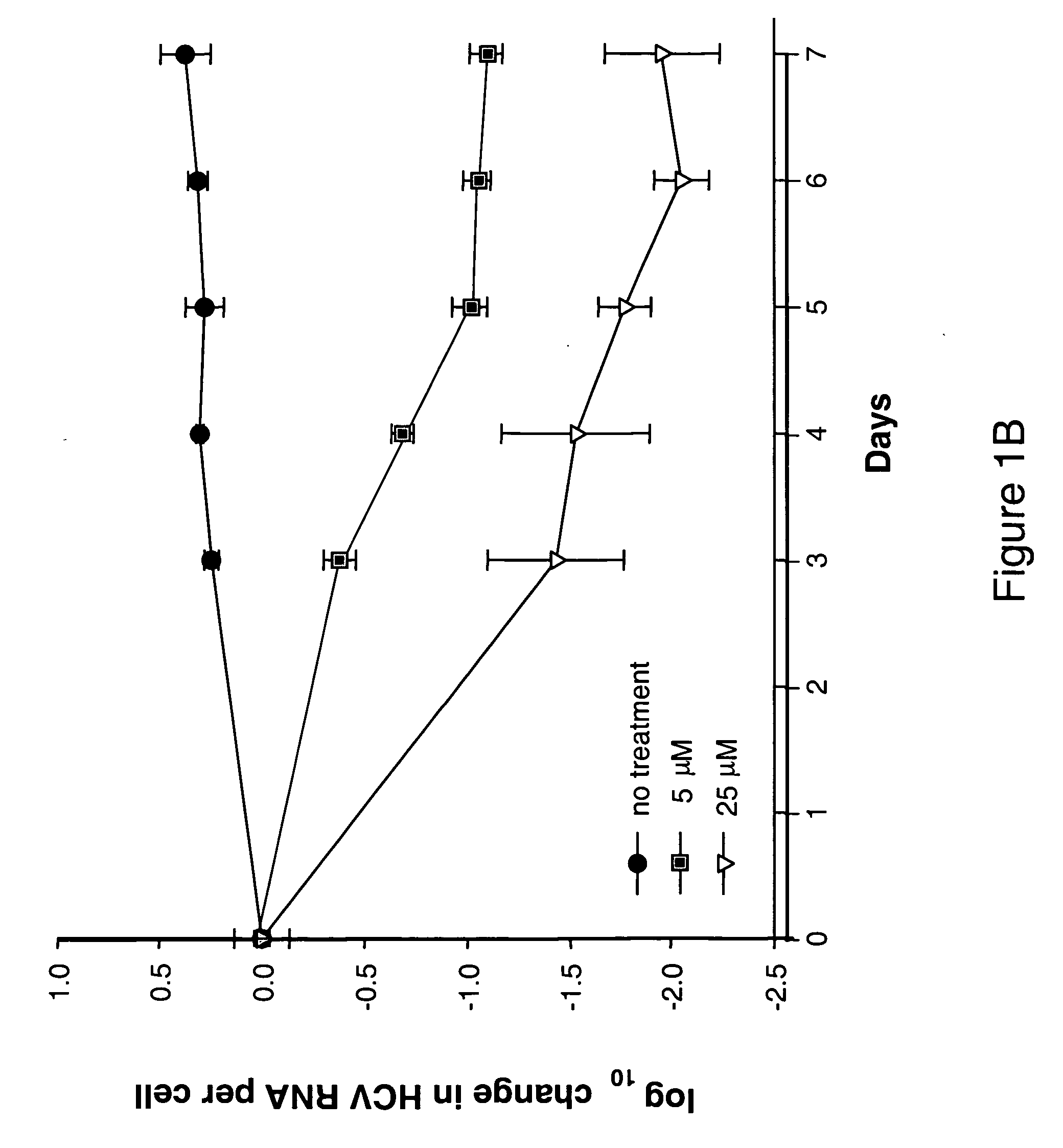
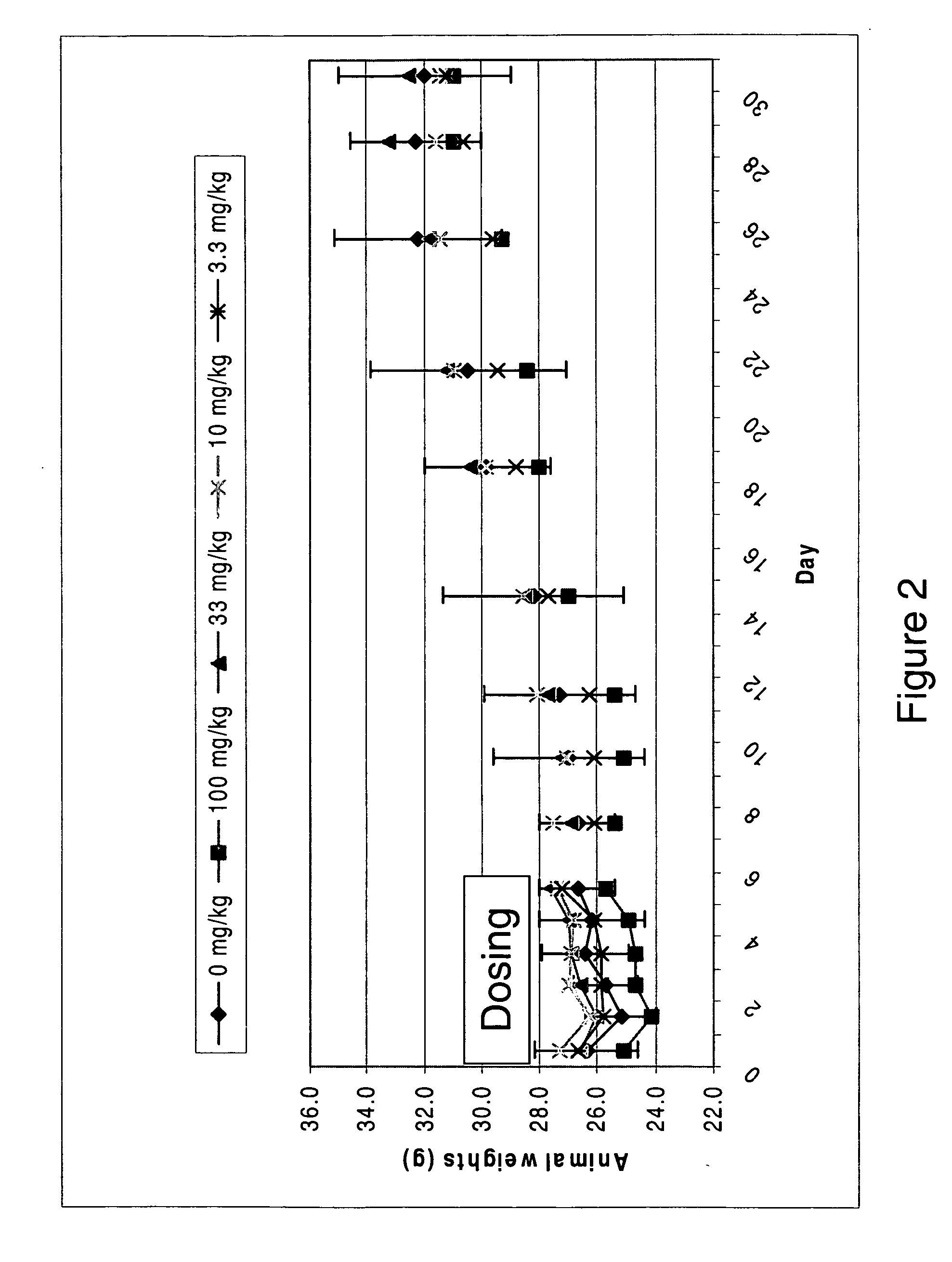
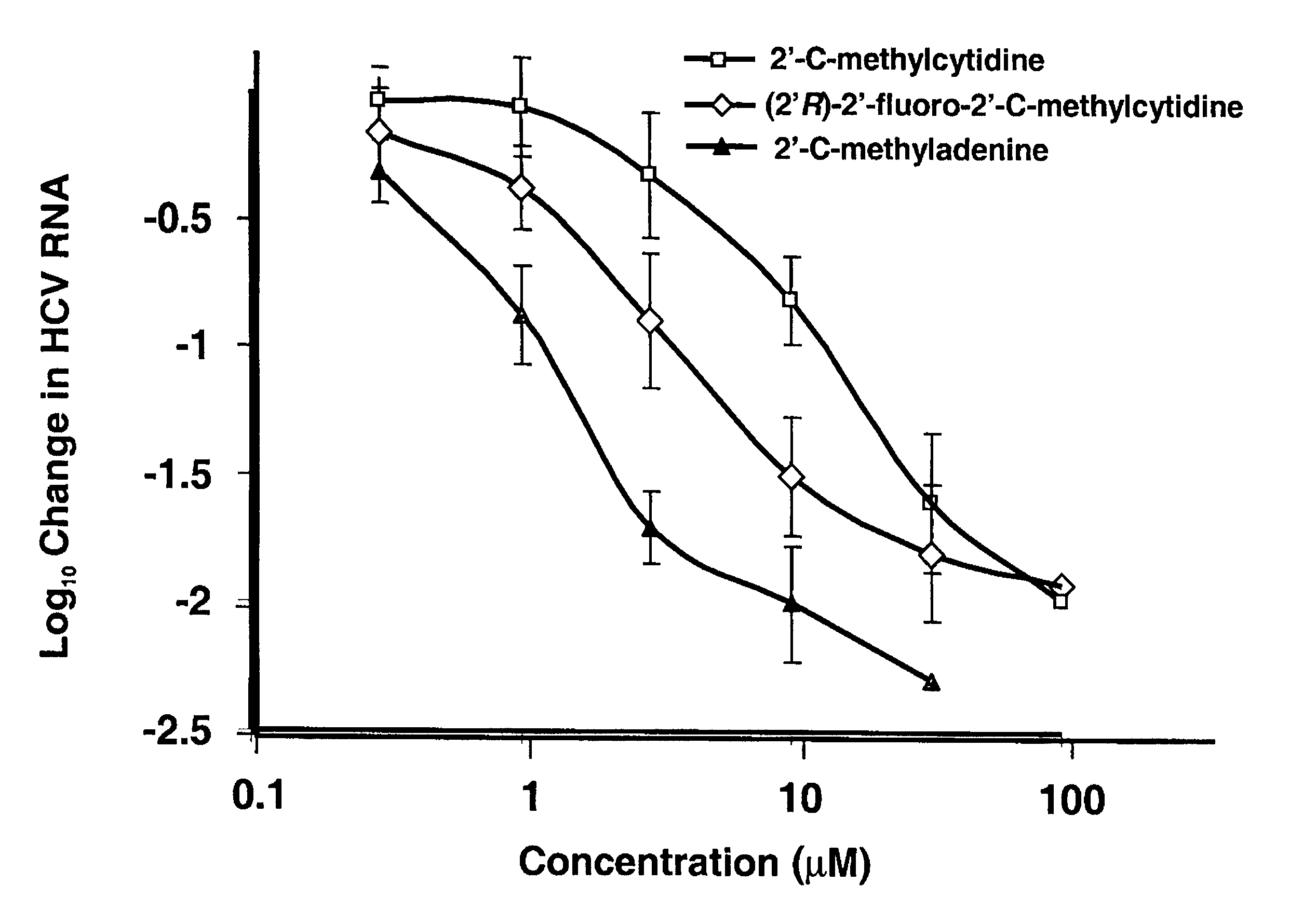
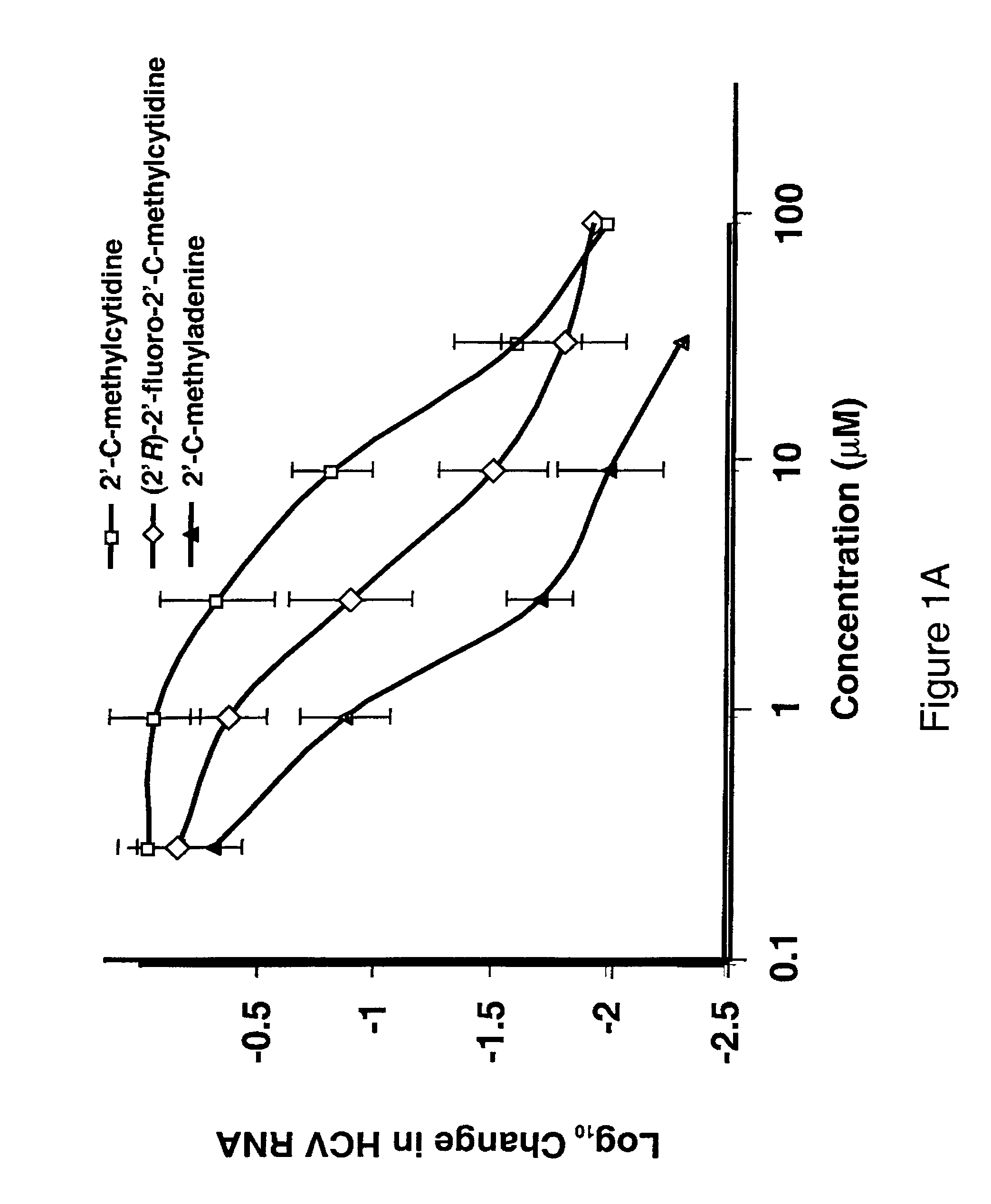
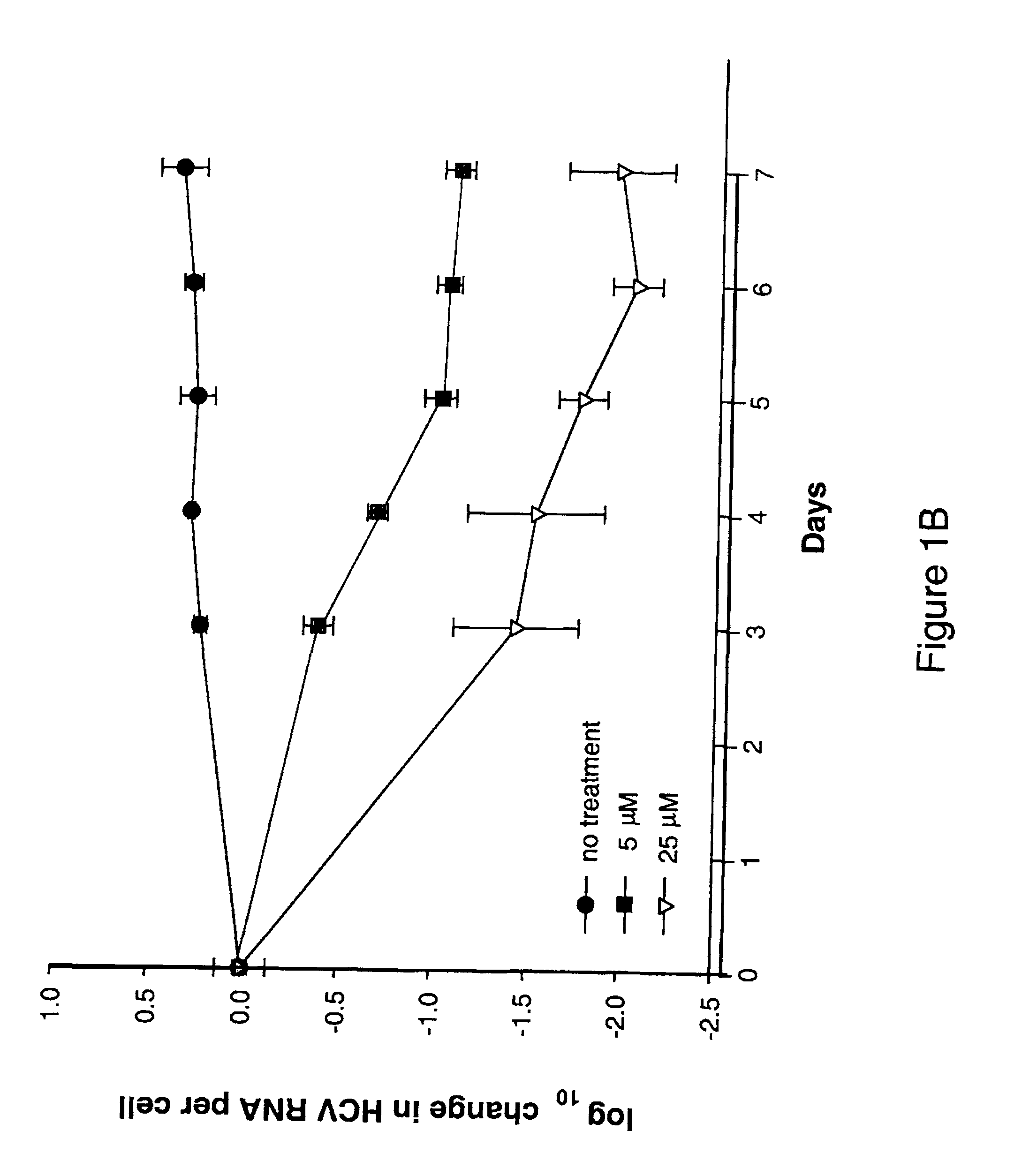
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
Modified fluorinated nucleoside analogues
ActiveUS20080070861A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
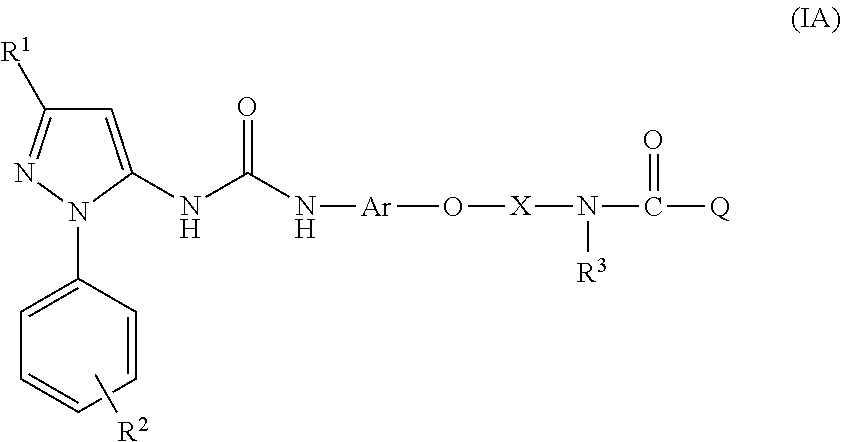
Ureido-pyrazole derivatives for use in the treatment of rhinovirus infections
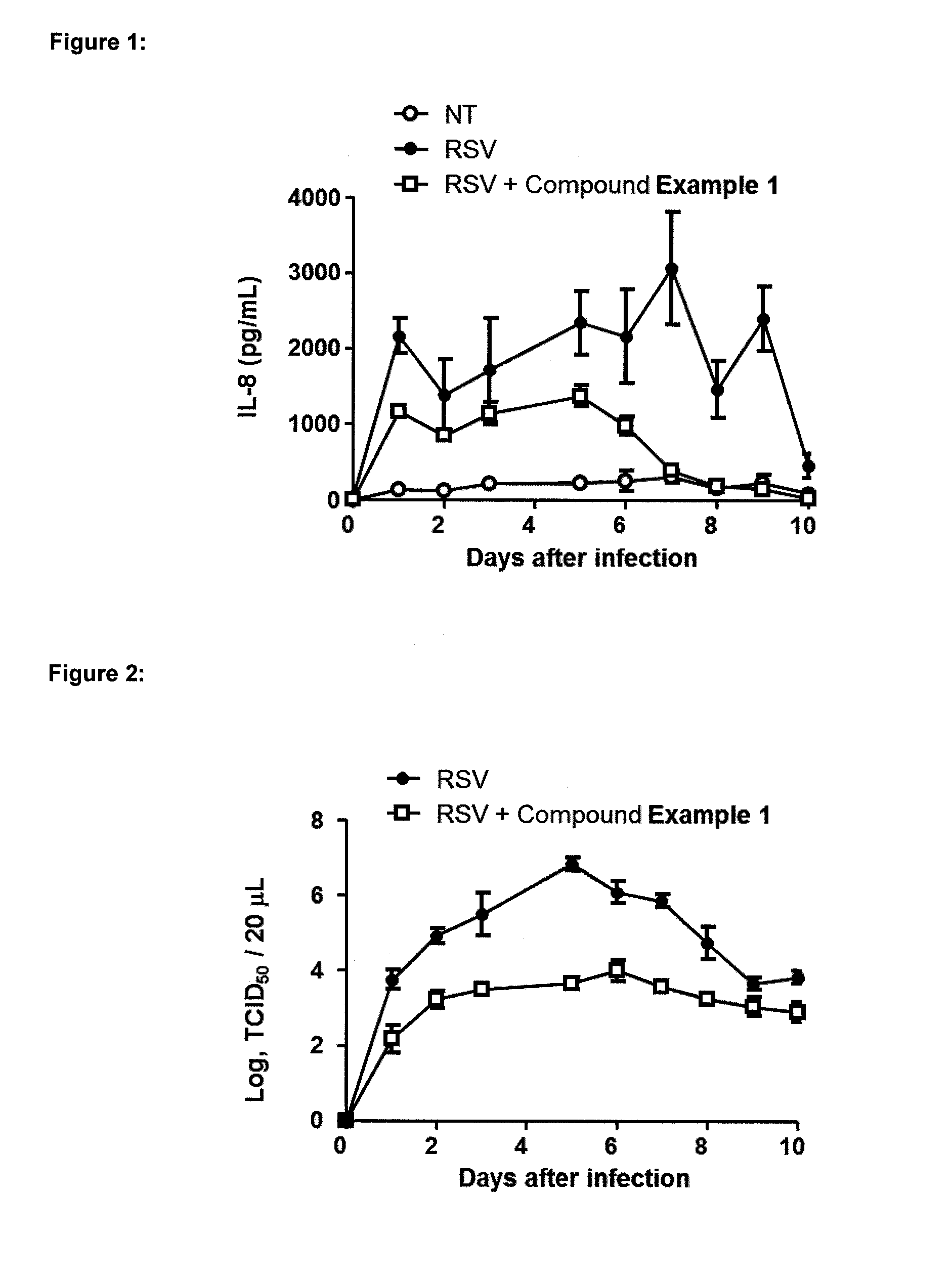
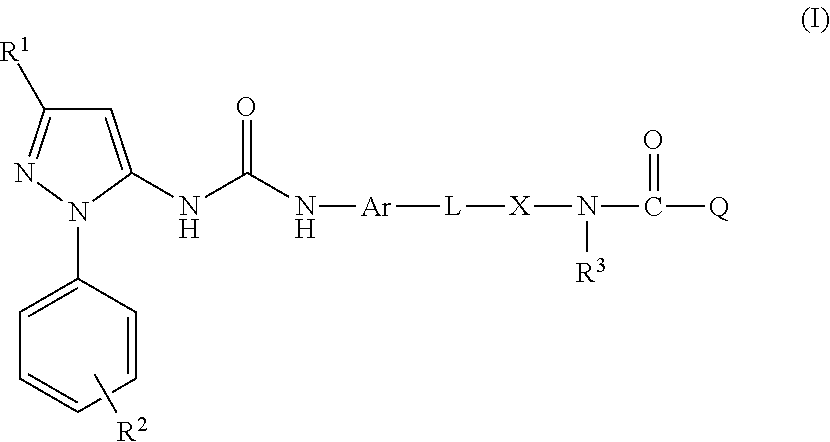
Medicinal use The disclosure relates to compounds of formula (I) for use in the treatment or prophylaxis of respiratory syncitial virus (RSV) infection in particular viral exacerbation of a respiratory disorder such as bronchitis, asthma, COPD and / or cystic fibrosis, methods of treating or preventing RSV infection employing said compounds or pharmaceutical composition comprising the same.
Owner:RESPIVERT
Method for treating enterovirus or rhinovirus infection using antisense antiviral compounds
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:7.
Owner:SAREPTA THERAPEUTICS INC
Antisense antiviral compound and method for treating picornavirus infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, including partially positively charged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:4.
Owner:AVI BIOPHARMA
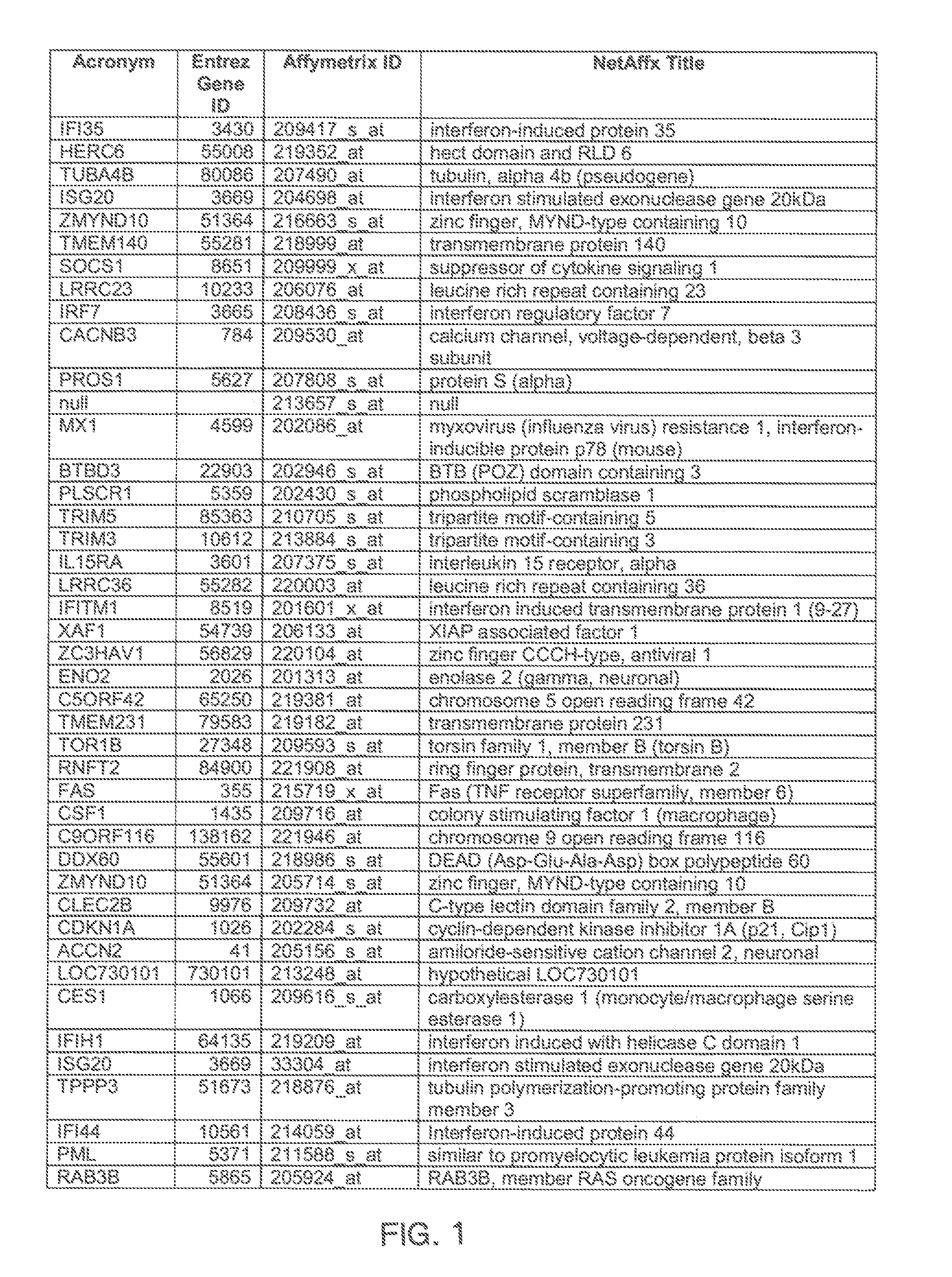
Methods for Detecting and Treating Rhinovirus Infection
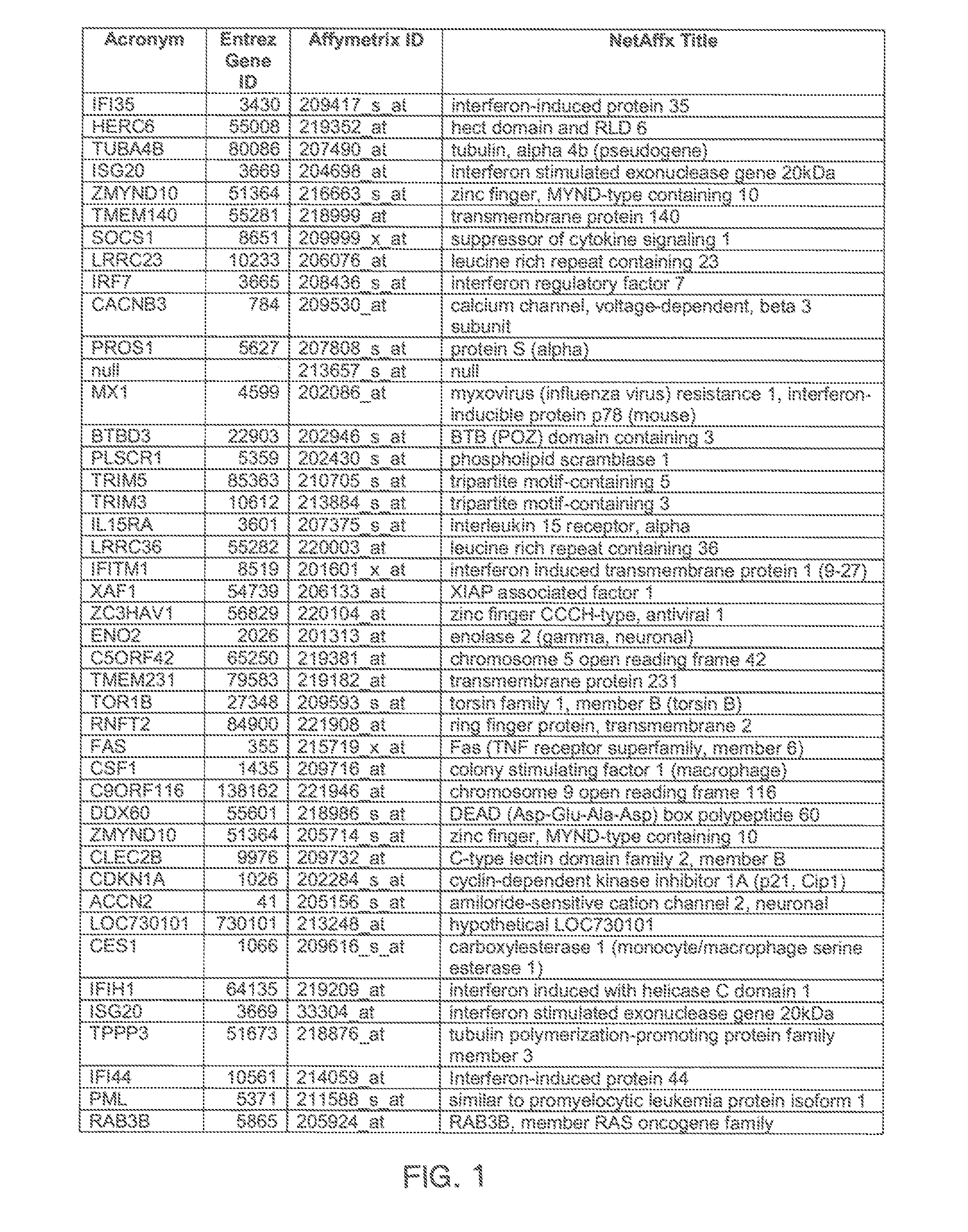
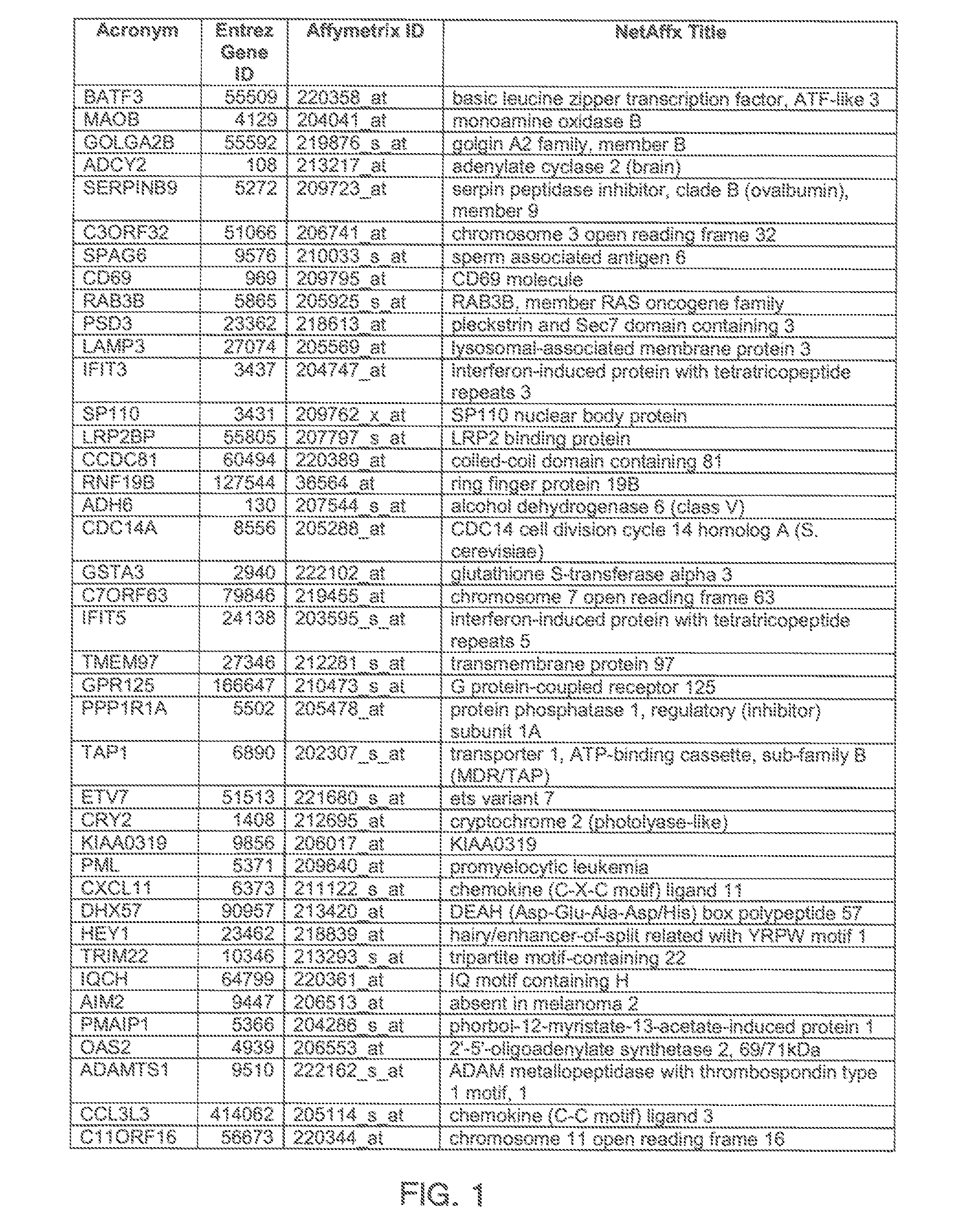
The invention provides a method for evaluating the activity of an agent for treating rhinovirus infection or a symptom thereof, a method of detecting or monitoring rhinovirus infection, and a method of treating rhinovirus infection or a symptom thereof. Various embodiments comprise measuring expression of (i) one or more genes selected from the group consisting of CRY2, B3GAT3, C10ORF95, and BATF3, and (ii) one or more genes selected from the group consisting of RNFT2, BTG4, PSD3, CAPN9, SULT1E1, HEY1, LRRC36, RAB3B, ALDH3B1, FAM134B, FAS, PLSCR1, CLEC2B, HAS2, MX1, SP110, GBP1, IFIT3, IFIT1, CXCL9, CXCL10, and CXCL11, from at least one biological sample to produce a gene expression profile, and comparing the gene expression profile to a reference gene expression profile. Systems, computer readable media, compositions, and methods for maintaining or improving respiratory health also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
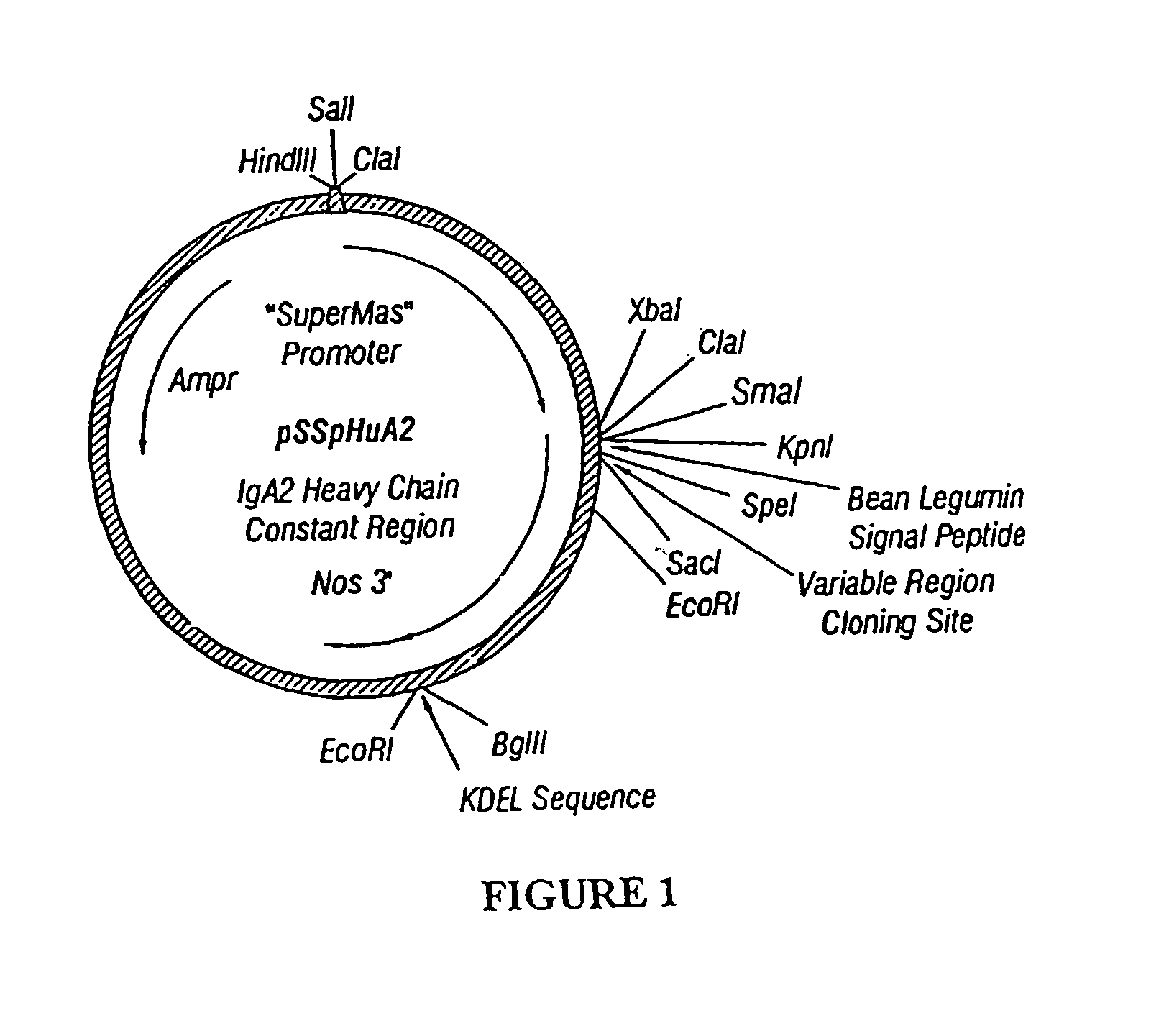
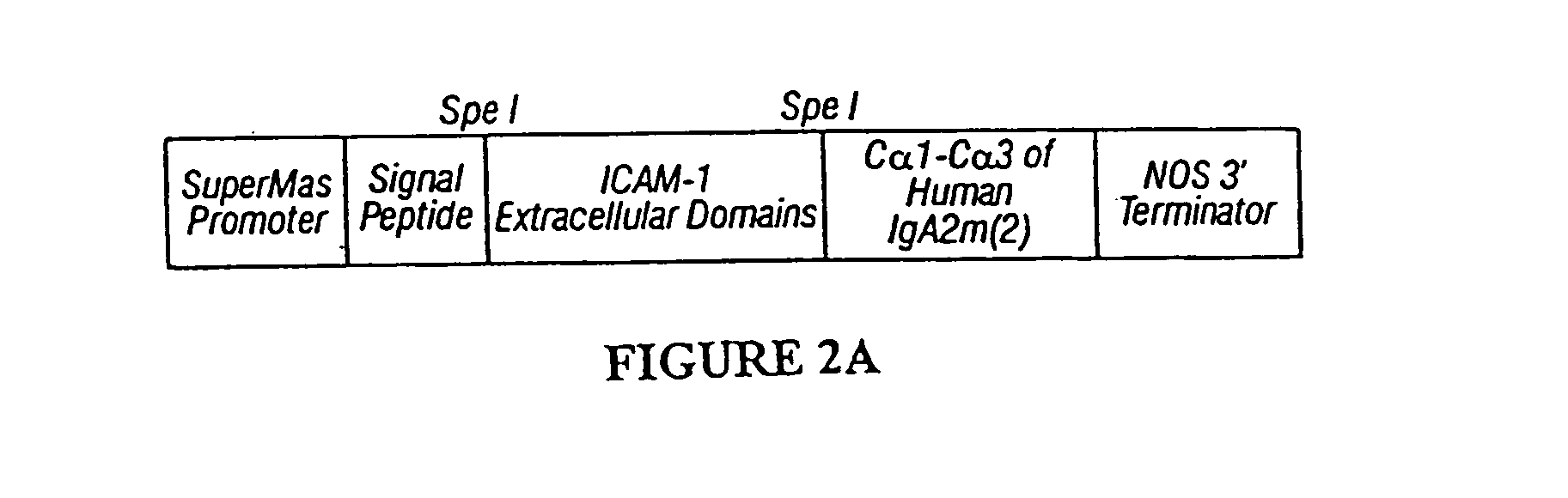
Immunoadhesin comprising a chimeric ICAM-1 molecule produced in a plant
InactiveUS7951378B2Improve efficiencyIncrease in valencySsRNA viruses positive-sensePeptide/protein ingredientsMammalADAMTS Proteins
The immunoadhesions of the present invention are useful in treating rhinovirus infections. The immunoadhesions contain a chimeric ICAM molecule and may optionally also contain J chain and secretory compounds. The chimeric ICAM molecule is a fusion protein that has a rhinovirus receptor protein linked to an immunoglobulin protein. This invention also includes the greatly increased and improved method of producing immunoadhesions in plants. Each of the components of an immunoadhesin is produced in a plant cell and thereby assembles within the plant cell. This method of producing the immunoadhesions of the present invention results in the efficient and economic production of these molecules. The present invention also contemplates the production of immunoadhesions in a variety of eukaryotic cells including plants and mammalian cells. The immunoadhesions of the present invention are useful as a therapeutic against the common cold in humans which is caused by rhinoviruses.
Owner:PLANET BIOTECH
Methods and Compositions for the Treatment of Rhinovirus Infections with Carrageenan
InactiveUS20080131454A1Easily incorporated into tissue materialPrevent and reduce riskOrganic active ingredientsBiocideCarrageenanAntiviral drug
The present invention provides the use of carrageenan or mixtures thereof for the manufacture of an antiviral pharmaceutical composition for the treatment of rhinovirus infections.
Owner:MARINOMED BIOTECHNOLOGIE GMBH
Methods and targets for identifying compounds for regulating rhinovirus infection
The present invention provides methods for identifying genes, expression regulators, receptors, protein product receptors, and proteins that may regulate rhinovirus infections. The genes identified may be used as markers for disease onset and progression and to measure efficacy of a therapeutic. The present invention also provides methods to screen agents that are capable of regulating rhinovirus infection. The present invention also provides methods of identifying therapeutic compounds that may treat various disorders by regulating the expression and activity of genes, expression regulators, receptors, protein product receptors, and proteins identified.
Owner:THE PROCTER & GAMBLE COMPANY
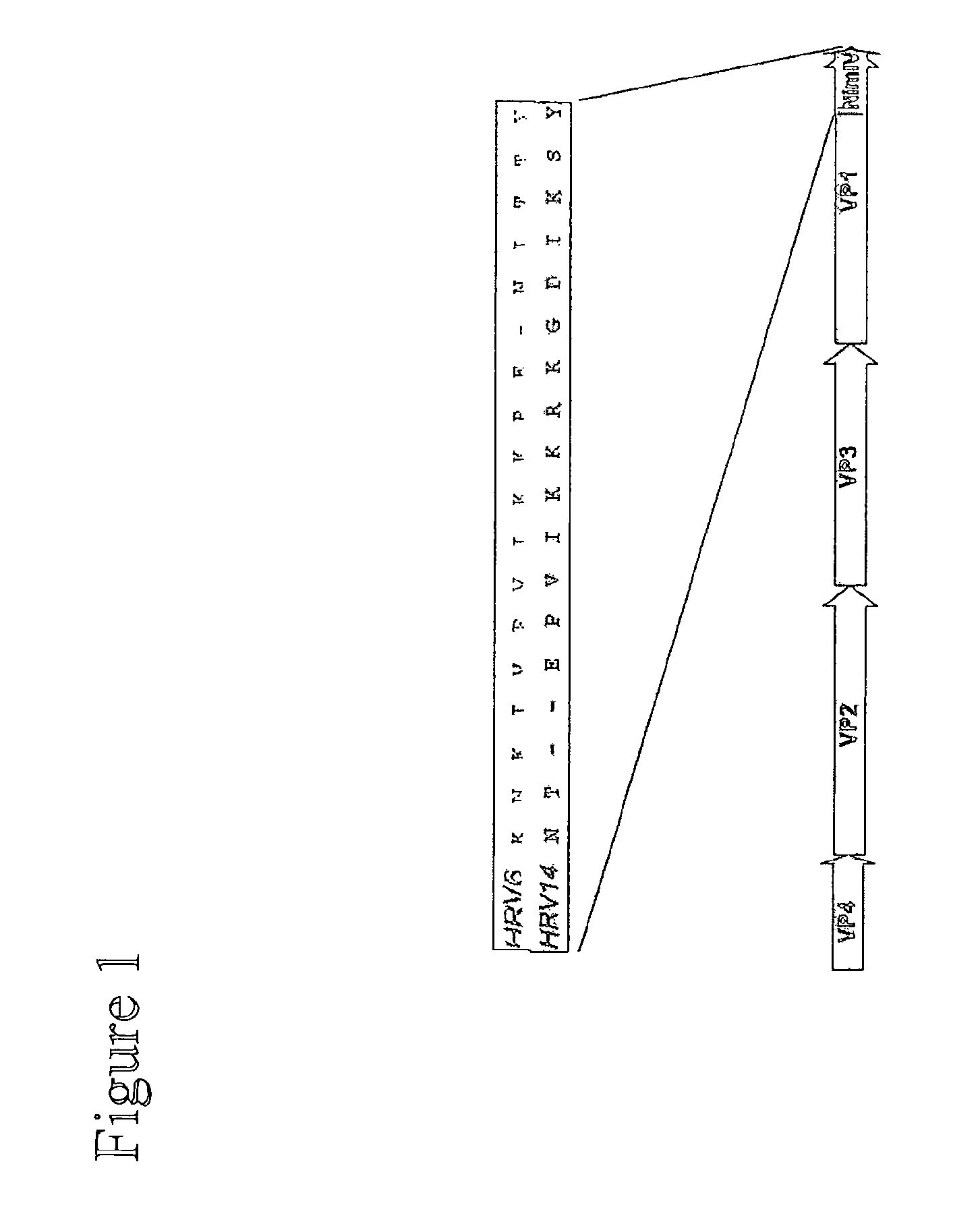
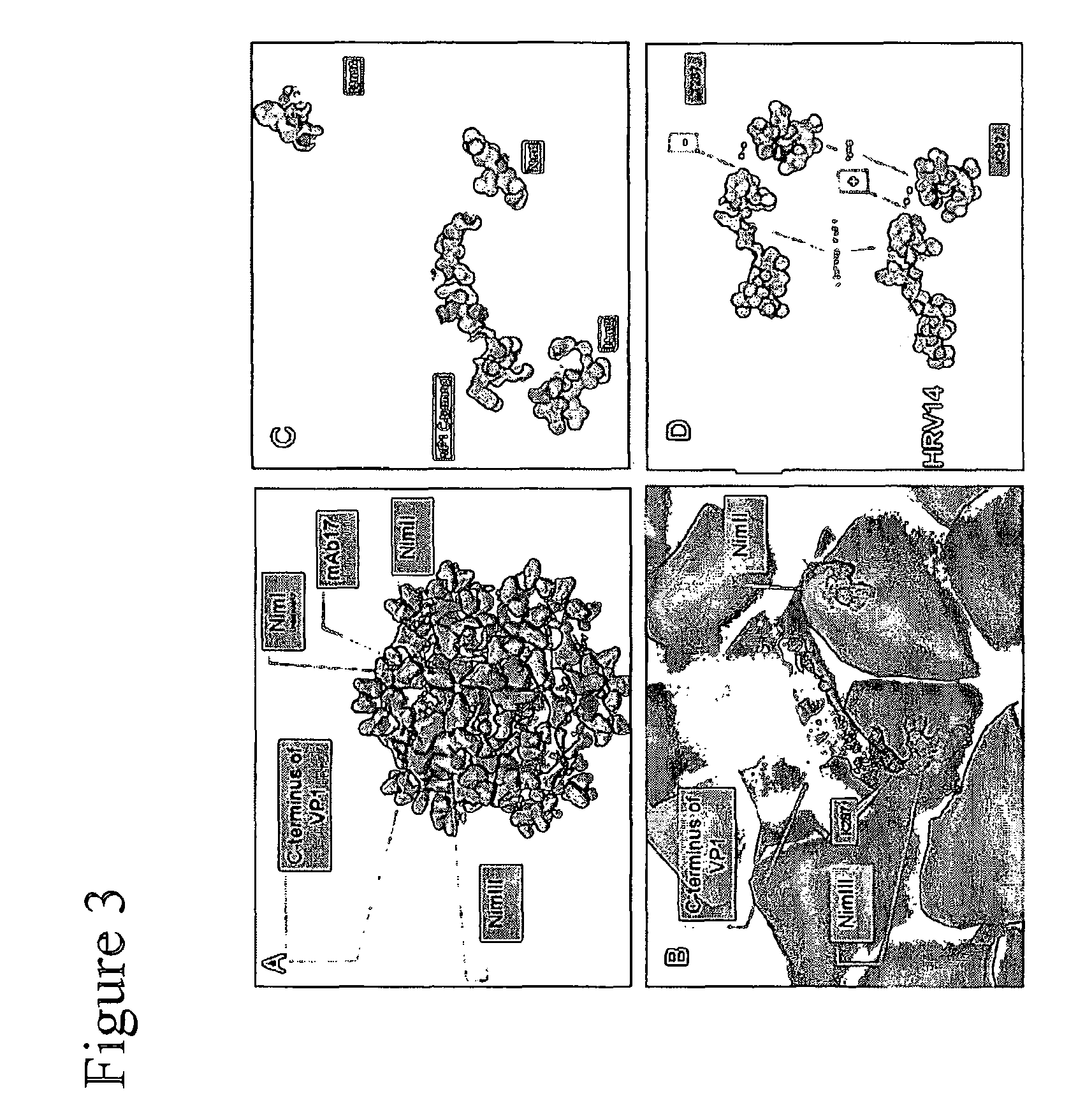
Novel neutralizing immunogen (NIMIV) of rhinovirus and its uses for vaccine applications
The invention relates to methods and compositions for preventing or treating human rhinovirus infection.
Owner:SANOFI PASTEUR BIOLOGICS CO
Method for detecting rhinovirus typing and enterovirus
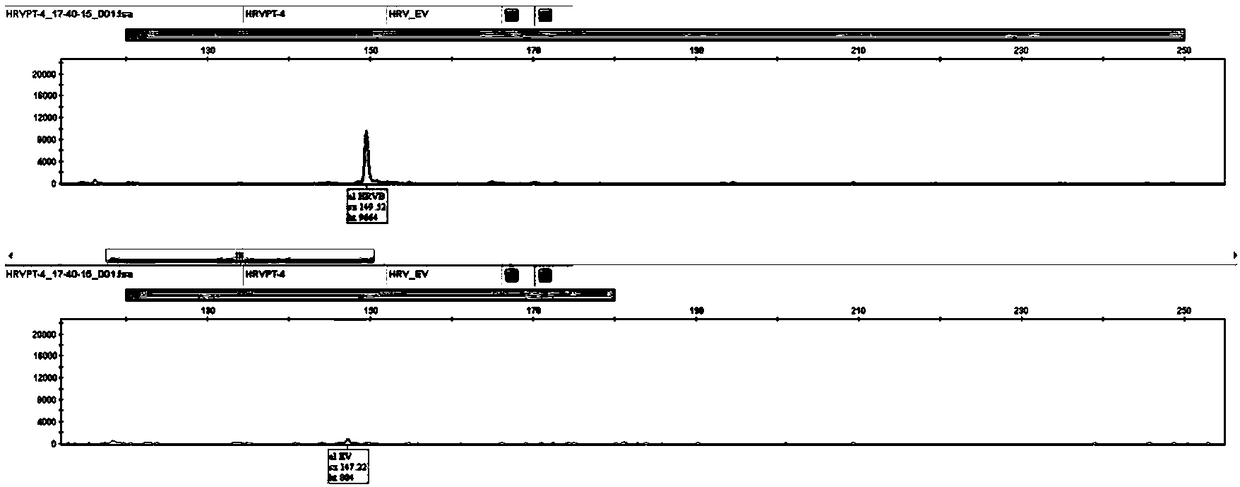
ActiveCN108504774AEasy to operateShorten the timeMicrobiological testing/measurementMicroorganism based processesBiologyRhinovirus infection
The invention provides a method for rapidly detecting HRV typing and EV by single-tube multiplex-RT-PCR. The method comprises the following steps: a) collecting a sample; b) extracting nucleic acid from the sample; c) adding the extracted nucleic acid to a premixed RT-PCR reagent; d) running an RT-PCR amplification program; and e) performing fragment analysis on the obtained RT-PCR amplification product, wherein the premixed RT-PCR reagent contains a primer specifically for HRV typing and a primer specifically for EV. The method has the advantages of simplicity in operation, short time and high flux, so a result can be obtained in 4 h at the soonest, and the method is suitable for clinic detection and scientific researches. The HRV virus typing result detected by the method can be used forguiding clinicians to medicate, and also can be used for the pathological study of rhinovirus infection.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Antiviral composition and method of use
ActiveUS20100040658A1Reduce the possibilityLower Level RequirementsBiocideOrganic active ingredientsAntiviral drugRhinovirus infection
A method for manufacturing an antiviral pharmaceutical composition for the prophylaxis or treatment of a rhinovirus infection that includes the utilization of iota-, kappa- or lambda-carrageenan or mixtures thereof.
Owner:MARINOMED BIOTECH AG
Use of medicine for treating virus infection of respiratory system
InactiveCN1994299AReduce mortalityPromote infectionOrganic active ingredientsAntiviralsMortality rateAntibody level
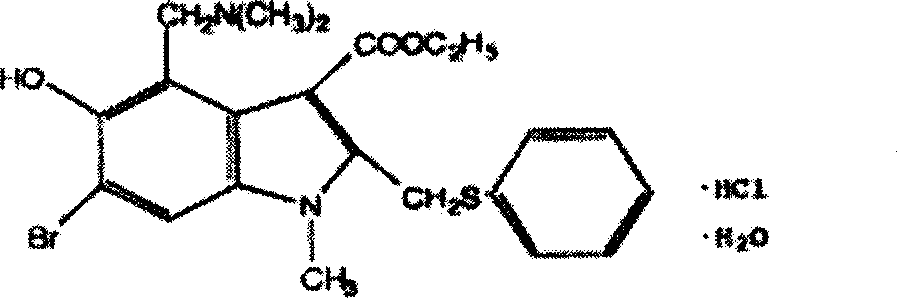
The invention relates to a method for producing drug that treats virus infection of respiratory tract, wherein it uses Arbidol Hydrochloride as 6-bromine-4-(dimethylamino)-5-hydroxyl group-1-methyl-2-(benzene sulfenyl)-1H-indole-3-glycolate-hydrates. The invention can reduce the mouse dead rate affected by virus B3, B5, adenovirus, etc, and improve antibody level, with wide application.
Owner:WUHAN UNIV
Application of recombinant alpha-interferon in preparing medicine for preventing and treating respiratory system infection
InactiveCN1548149AEnhanced inhibitory effectPeptide/protein ingredientsDigestive systemDiseaseInterferon alpha
The alpha-interferon has been used mainly in treating chronic viral infection. The present invention uses coronavirus or rhinovirus infected human cervical cancer cell as estimation model in observing the inhibition effect of alpha-interferon on common virus causing respiratory system infection. The results show that alpha-interferon may be used in preventing and treating coronavirus and rhinovirus caused diseases, including SARS. The integrated interferon, its mutant and interferon alpha-1b have even high inhibiting effect on new SARS virus.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD +1
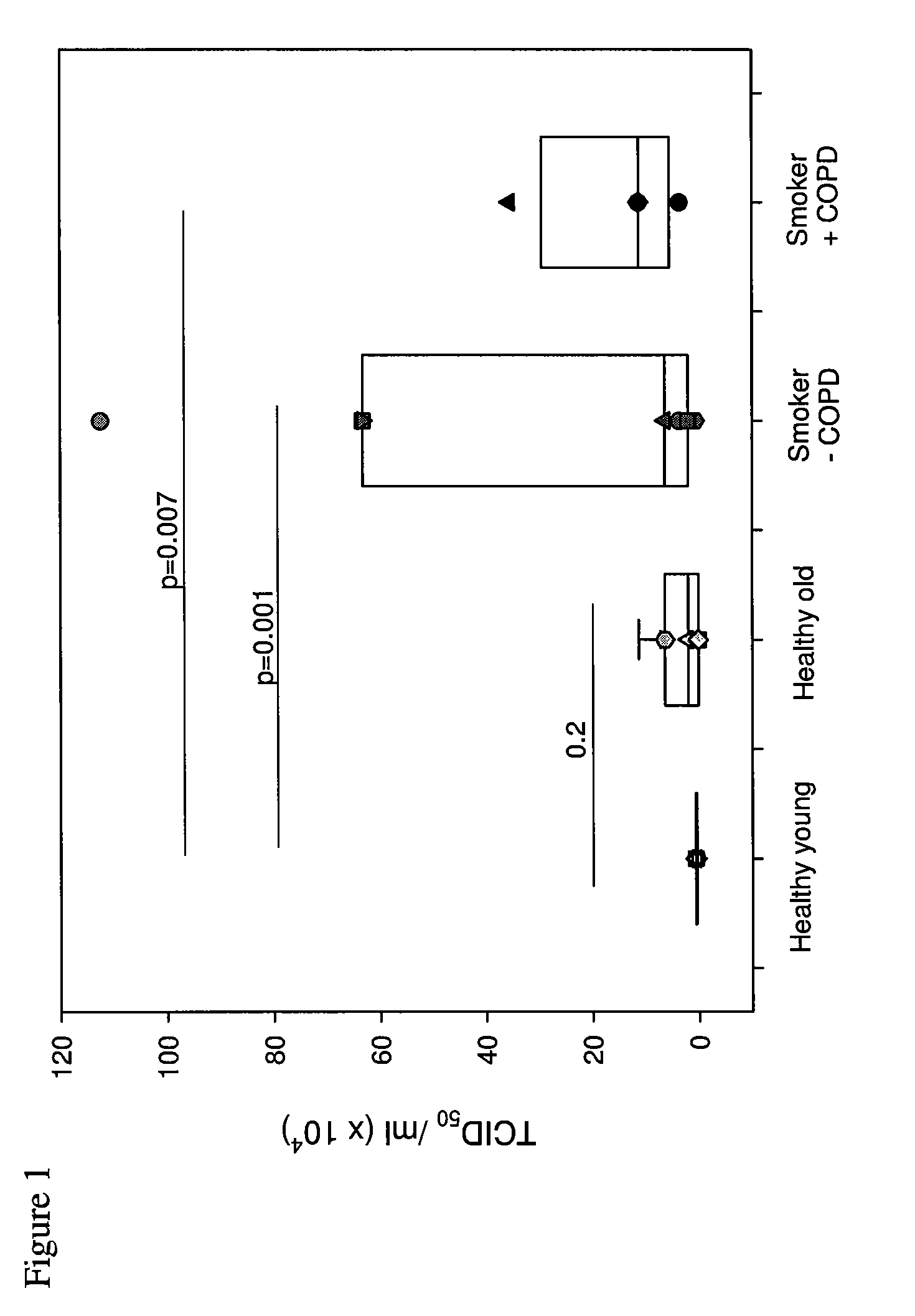
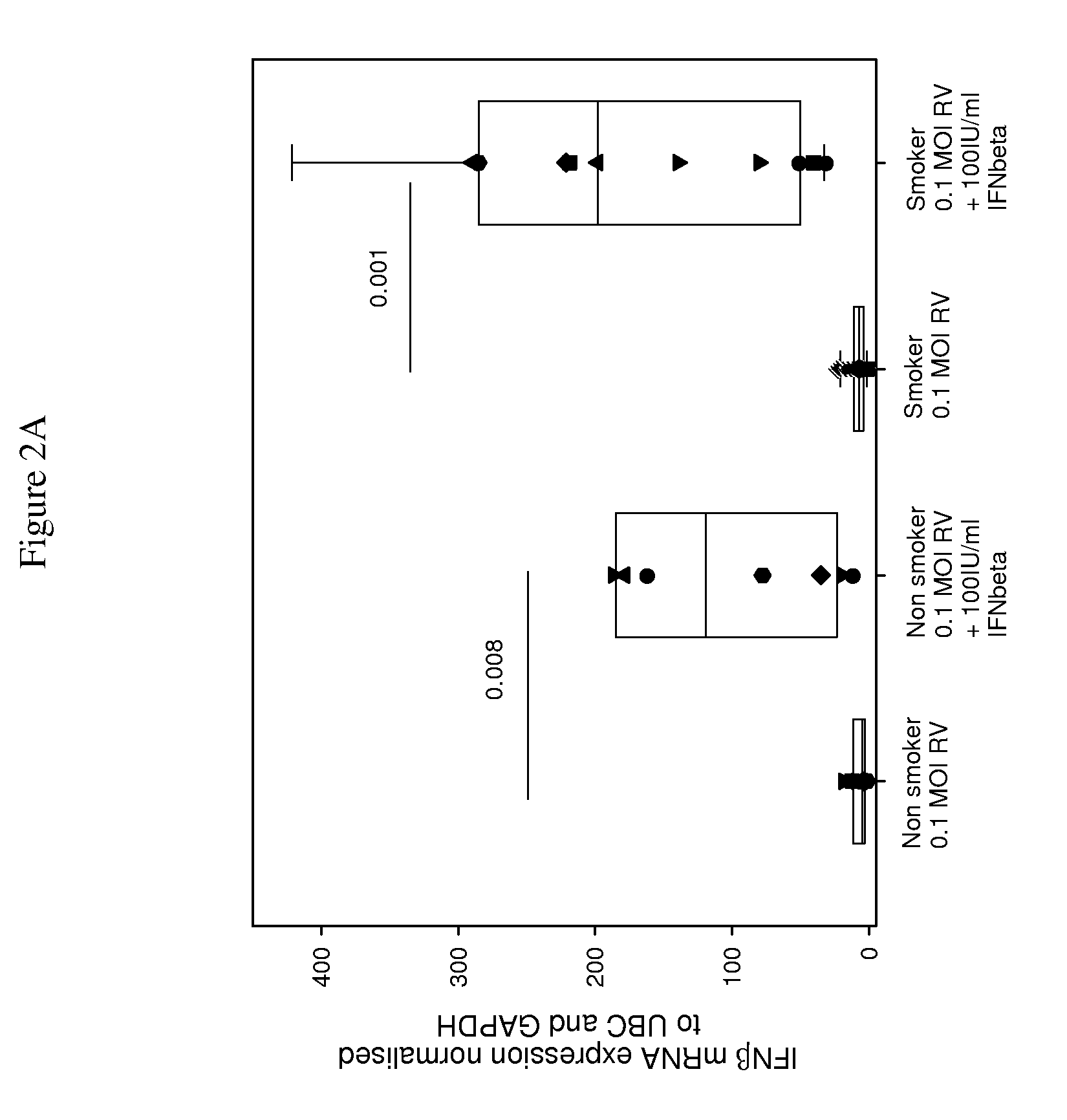
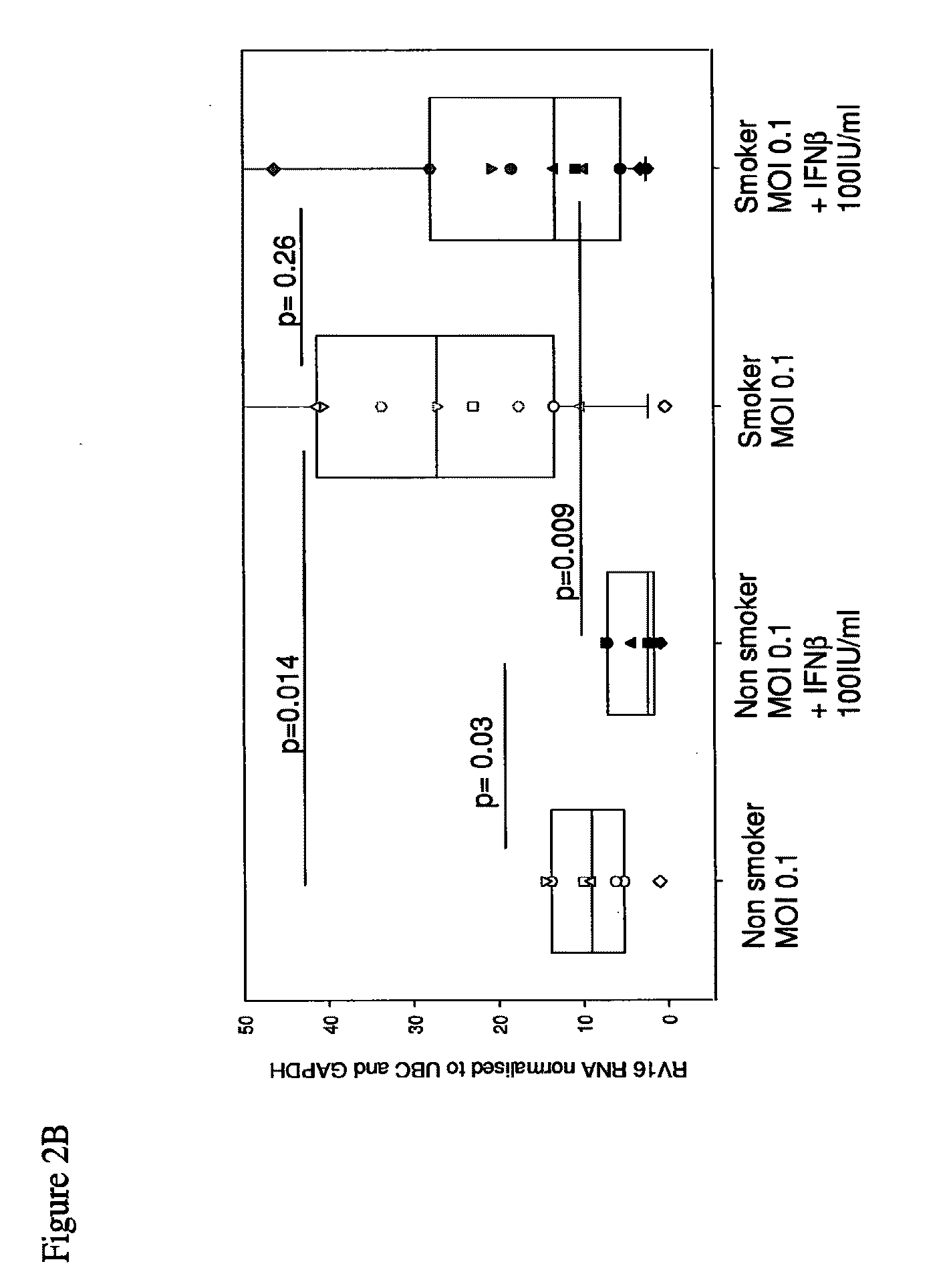
Interferon-beta and/or lambda for use in treating rhinovirus infection in the elderly
ActiveUS7871603B2Improve efficiencyOrganic active ingredientsPeptide/protein ingredientsMortality rateSevere complication
Use of interferon-beta (IFN-β) and / or IFN-λ for treating rhinovirus (RV) infection in elderly people, particularly elderly people who are, or have been long-term smokers, especially those who have a clinical history of recurrent RV infections, and may have other medical conditions, such as cardiac or circulation problems, and who are liable to suffer severe complications / high mortality from poor innate ability to fight such a viral infection.
Owner:SYNAIRGEN RES
Neutralizing immunogen (NimIV) of rhinovirus and its use for vaccine applications
InactiveUS8652486B2SsRNA viruses positive-sensePeptide/protein ingredientsMedicineRhinovirus infection
The invention relates to methods and compositions for preventing or treating human rhinovirus infection.
Owner:SANOFI PASTEUR BIOLOGICS CO
Nitric oxide inhibits rhinovirus infection
Nitric oxide generating compounds or compounds which induce in situ synthesis of nitric oxide can be used to inhibit rhinovirus infection. Nitric oxide has the ability to inhibit both viral replication as well as the synthesis of cytokines, in particular the proinflammatory cytokines. Thus the symptoms of rhinovirus infections can be ameliorated by treatments to increase nitric oxide in the respiratory tract.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Novel Neutralizing Immunogen (NIMIV) of Rhinovirus and its Use for Vaccine Applications
InactiveUS20100297169A1Maintain immunogenicitySsRNA viruses positive-sensePeptide/protein ingredientsRhinovirus infectionViral infection
The invention relates to methods and compositions for preventing or treating human rhinovirus infection.
Owner:SANOFI PASTEUR BIOLOGICS CO
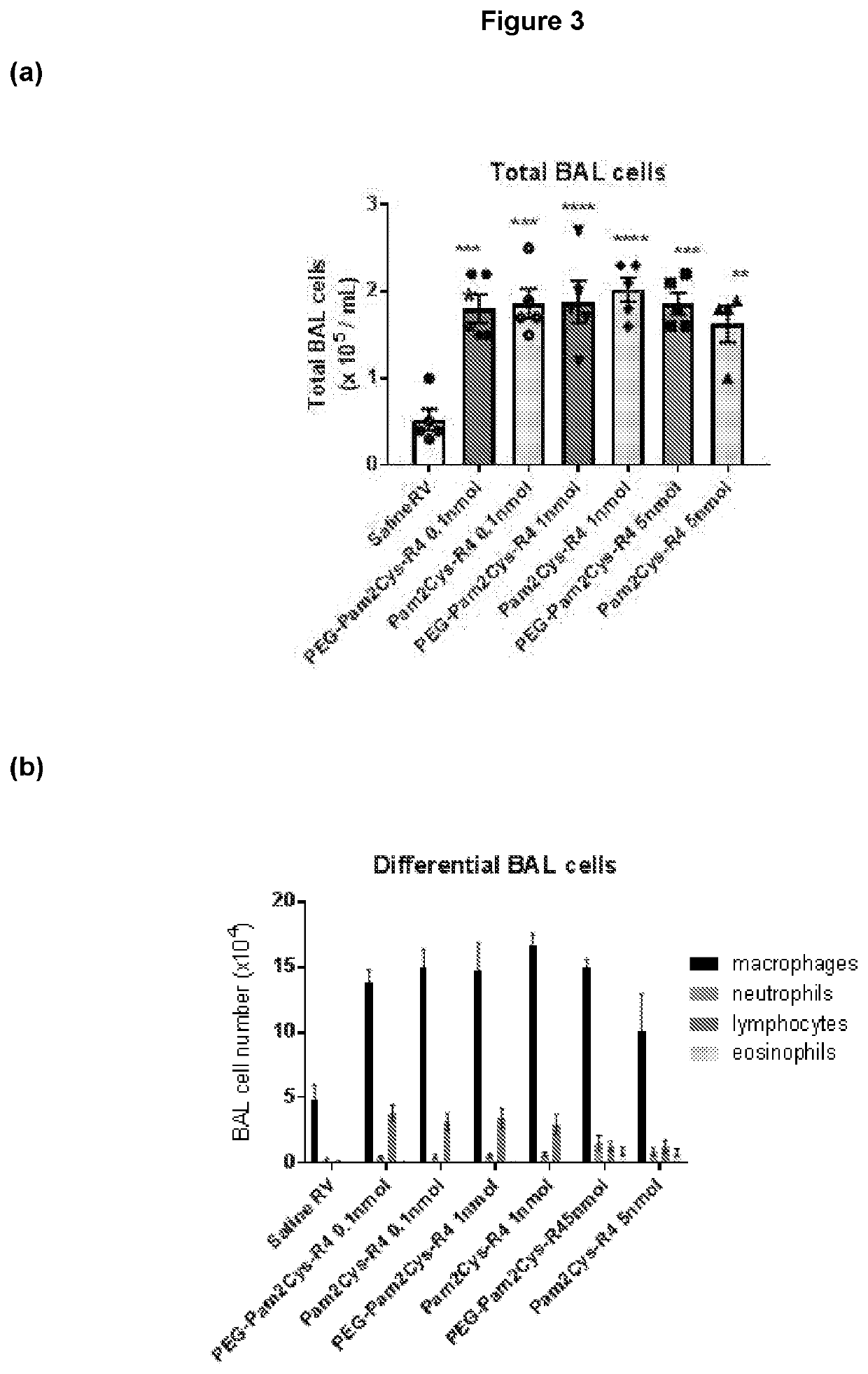
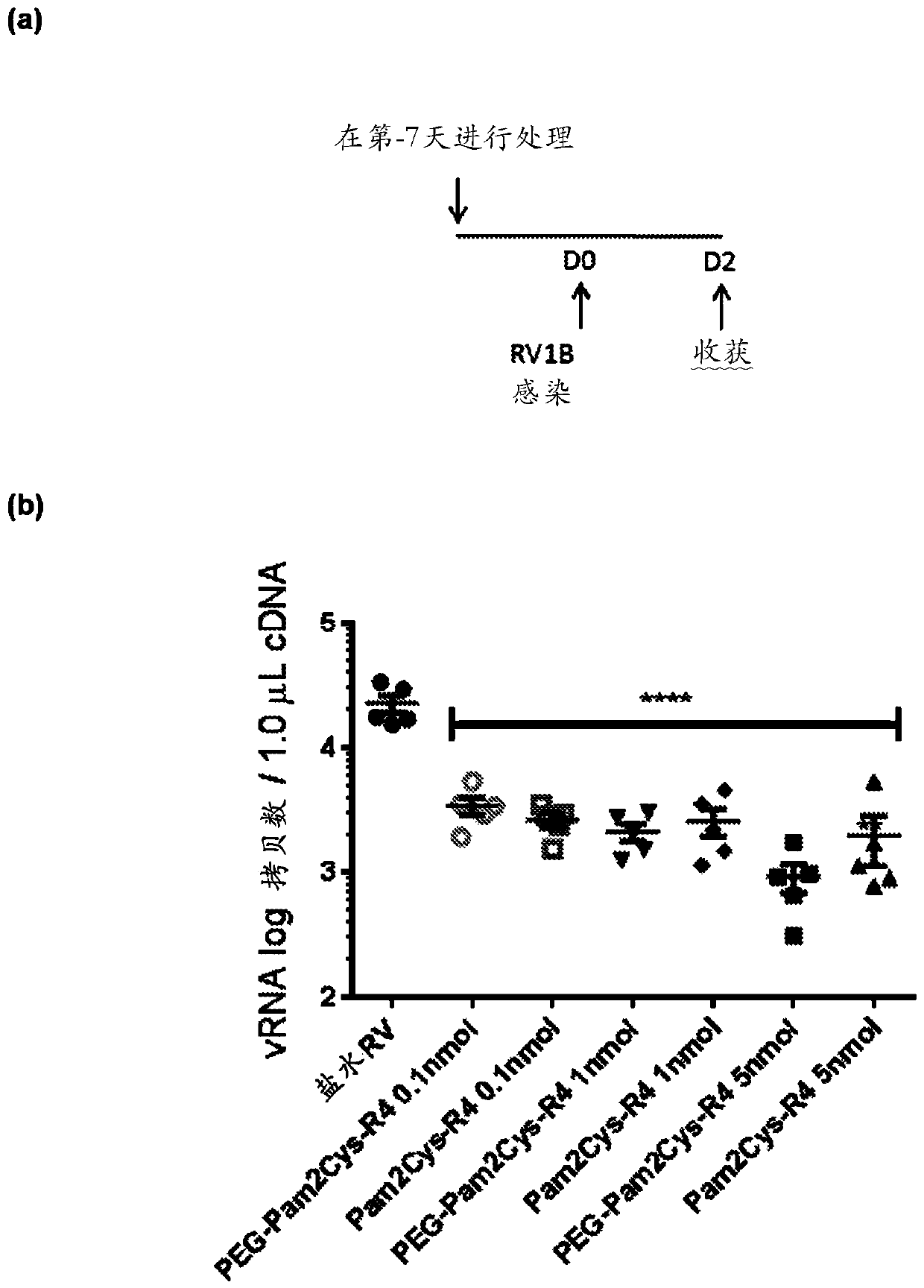
Treatment of respiratory infection with a tlr2 agonist
PendingUS20200147028A1Viral load in subjectReduce viral loadPowder deliverySsRNA viruses positive-sensePharmaceutical medicineRhinovirus infection
The present invention relates to methods, compositions and kits for the treatment or prevention of respiratory conditions. In particular, the methods, compositions and kits are particularly useful, but not limited to, the prevention and / or treatment of rhinovirus infection and the prevention and / or treatment of asthma exacerbation. The invention provides a method inhibiting a rhinovirus infection in a subject comprising administering a composition consisting of a compound comprising a TLR2 agonist and a pharmaceutically acceptable carrier.
Owner:ENA RESPIRATORY PTY LTD +2
Methods for Detecting and Treating Rhinovirus Infection
The invention provides a method for evaluating the activity of an agent for treating rhinovirus infection or a symptom thereof, a method of detecting or monitoring rhinovirus infection, and a method of treating rhinovirus infection or a symptom thereof. Various embodiments comprise measuring expression of (i) one or more genes selected from the group consisting of CRY2, B3GAT3, C10ORF95, and BATF3, and (ii) one or more genes selected from the group consisting of RNFT2, BTG4, PSD3, CAPN9, SULT1E1, HEY1, LRRC36, RAB3B, ALDH3B1, FAM134B, FAS, PLSCR1, CLEC2B, HAS2, MX1, SP110, GBP1, IFIT3, IFIT1, CXCL9, CXCL10, and CXCL11, from at least one biological sample to produce a gene expression profile, and comparing the gene expression profile to a reference gene expression profile. Systems, computer readable media, compositions, and methods for maintaining or improving respiratory health also are provided.
Owner:PROCTER & GAMBLE CO
Treatment of respiratory infection with a tlr2 agonist
PendingCN110461351AAvoid infectionExcellent anti-virusPowder deliverySsRNA viruses positive-sensePharmaceutical medicineRhinovirus infection
Owner:艾娜呼吸私人有限公司
Electronic detection device and kit for influenza viruses and rhinoviruses
The invention relates to an electronic detection device and kit for influenza viruses and rhinoviruses and relates to the field of respiratory virus detection. The electronic detection device comprises a photoelectric detection module, a data processing module and colloidal gold detection test paper, wherein the colloidal gold test paper comprises a bottom lining, a nitrocellulose film, a gold padand a sample pad, the nitrocellulose film is fixed on the bottom lining, the gold pad is arranged between the nitrocellulose film and the sample pad, one side of the gold pad is partially overlappedwith the nitrocellulose film, the other side of the gold pad is partially overlapped with the sample pad, the nitrocellulose film coats a detection line and a quality control line close to one side ofthe gold pad, the detection line comprises a plurality of virus detection lines, the gold pad is made of mixture of multiple gold-labeled virus monoclonal antibodies, the photoelectric detection module is used for detecting color changes of the quality control line and the detection line, and the data processing module is used for judging whether influenza virus or rhinovirus infection exists ornot according to the color change detected by the photoelectric detection module. The electronic detection device is advantaged in that purposes of rapidness, convenient operation and low-cost detection can be achieved.
Owner:石家庄洹众生物科技有限公司
Protection against human rhinovirus infection
ActiveUS9121048B1Microbiological testing/measurementBiological material analysisCotton ratRhinovirus infection
Methods, kits, and compositions are disclosed for vaccinating a human or animal against human rhinovirus infection. Also provided are methods of using a cotton rat model for identifying and testing vaccines and therapeutic agents that prevent or ameliorate human rhinovirus infection.
Owner:LOVELACE RESPIRATORY RES INST +1
A method for detecting rhinovirus typing and enterovirus
ActiveCN108504774BEasy to operateShorten the timeMicrobiological testing/measurementMicroorganism based processesEnterovirusRhinovirus infection
The present invention provides a kind of single-tube multiple RT-PCR rapid detection method for HRV typing and EV, comprising the steps of: a) collecting samples; b) extracting nucleic acids from the samples; c) adding the extracted nucleic acids to In the premixed RT-PCR reagent; d) run the RT-PCR amplification program; e) fragment analysis is carried out to the RT-PCR amplified product; wherein, the premixed RT-PCR reagent comprises specific primers for HRV typing and primers specific for EVs. The method is simple in operation, short in time, can produce results within 4 hours at the fastest, and has high throughput and is suitable for clinical testing and scientific research. The HRV virus typing result detected by the method can guide clinicians to use medicine, and can also be used for pathological research of rhinovirus infection.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Interferon-beta and/or lambda for use in treating rhinovirus infection in the elderly
InactiveUS20110177028A1Improve efficiencyBiocidePeptide/protein ingredientsRhinovirus infectionViral infection
Use of interferon-beta (IFN-β) and / or IFN-λ for treating rhinovirus (RV) infection in elderly people, particularly elderly people who are, or have been long-term smokers, especially those who have a clinical history of recurrent RV infections, and may have other medical conditions, such as cardiac or circulation problems, and who are liable to suffer severe complications / high mortality from poor innate ability to fight such a viral infection.
Owner:DAVIES DONNA +2
Esters of hydroxy-benzoic acids for use in the treatment of rhinovirus
PendingUS20210213112A1Improve biological effectAugmenting and prolonging therapeutic effectOrganic active ingredientsPeptide/protein ingredientsBenzoic acidHydroxybenzoates
The present invention provides ester derivatives of hydroxybenzoic acid for use in the treatment or prevention of a rhinovirus infection in a mammal wherein R represents a C1-10 alkyl group, X1, X2, X3, X4 and X5 independently represent —H or —OH and wherein at least one of X1, X2, X3, X4 and X5 is —OH.
Owner:ENZYMATICA
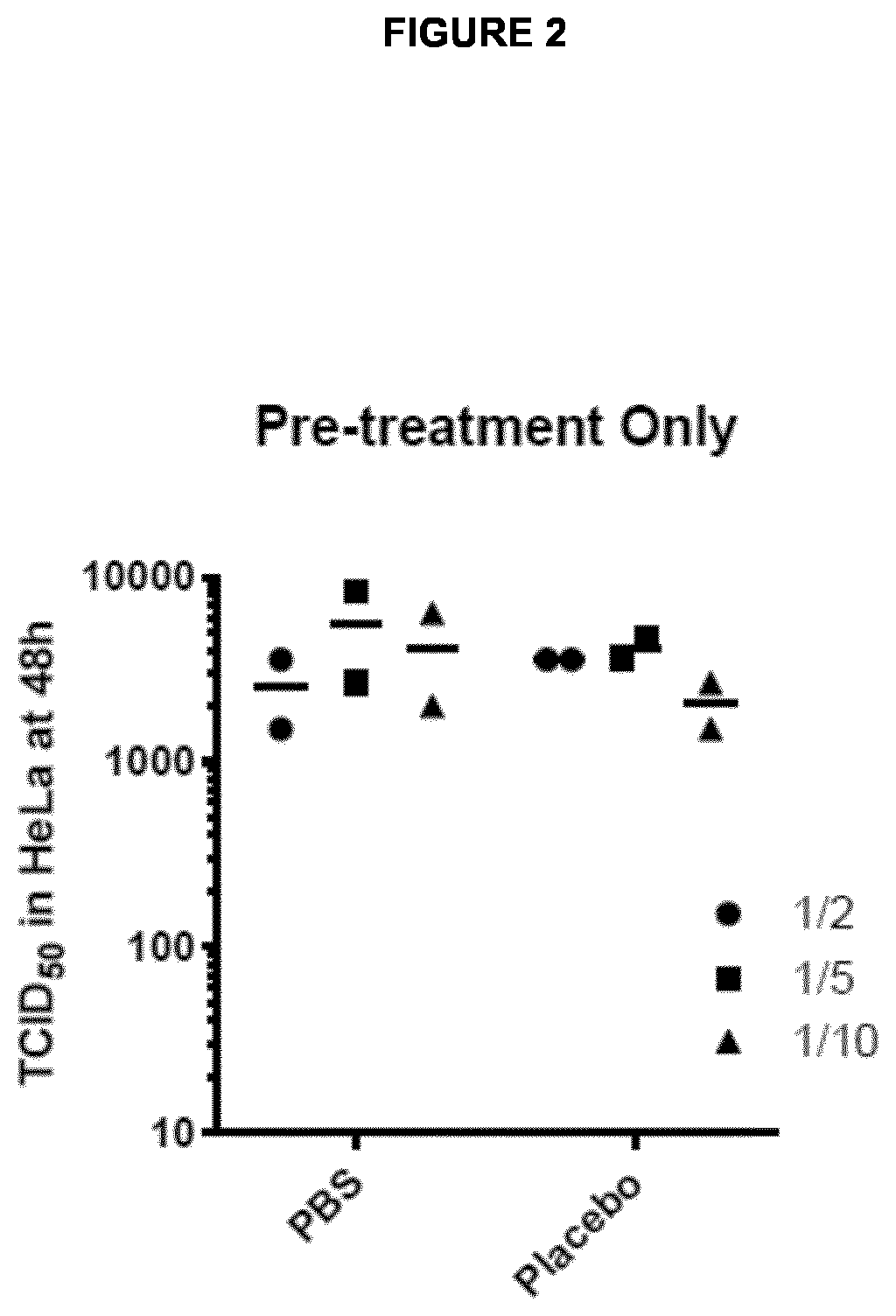
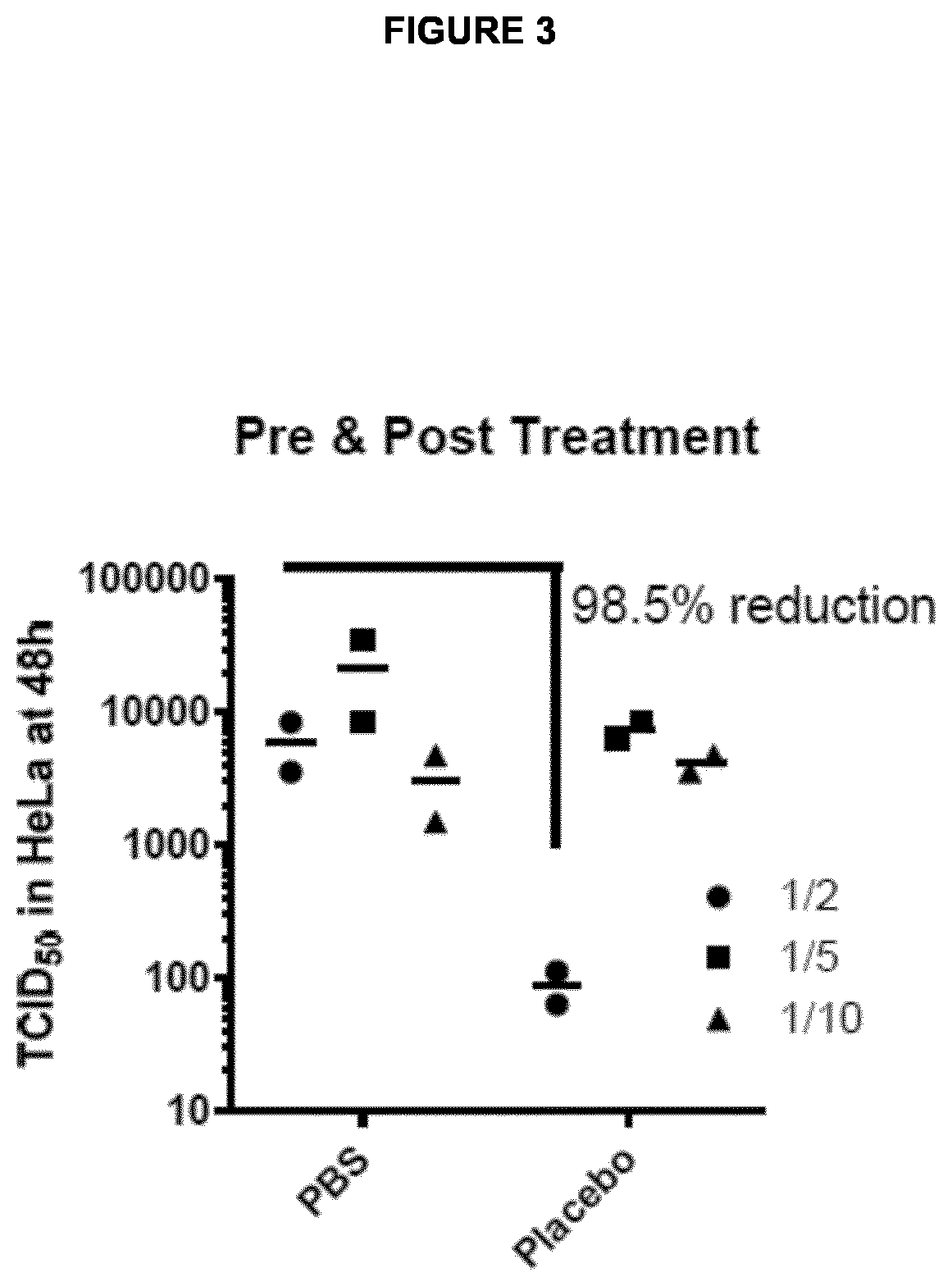
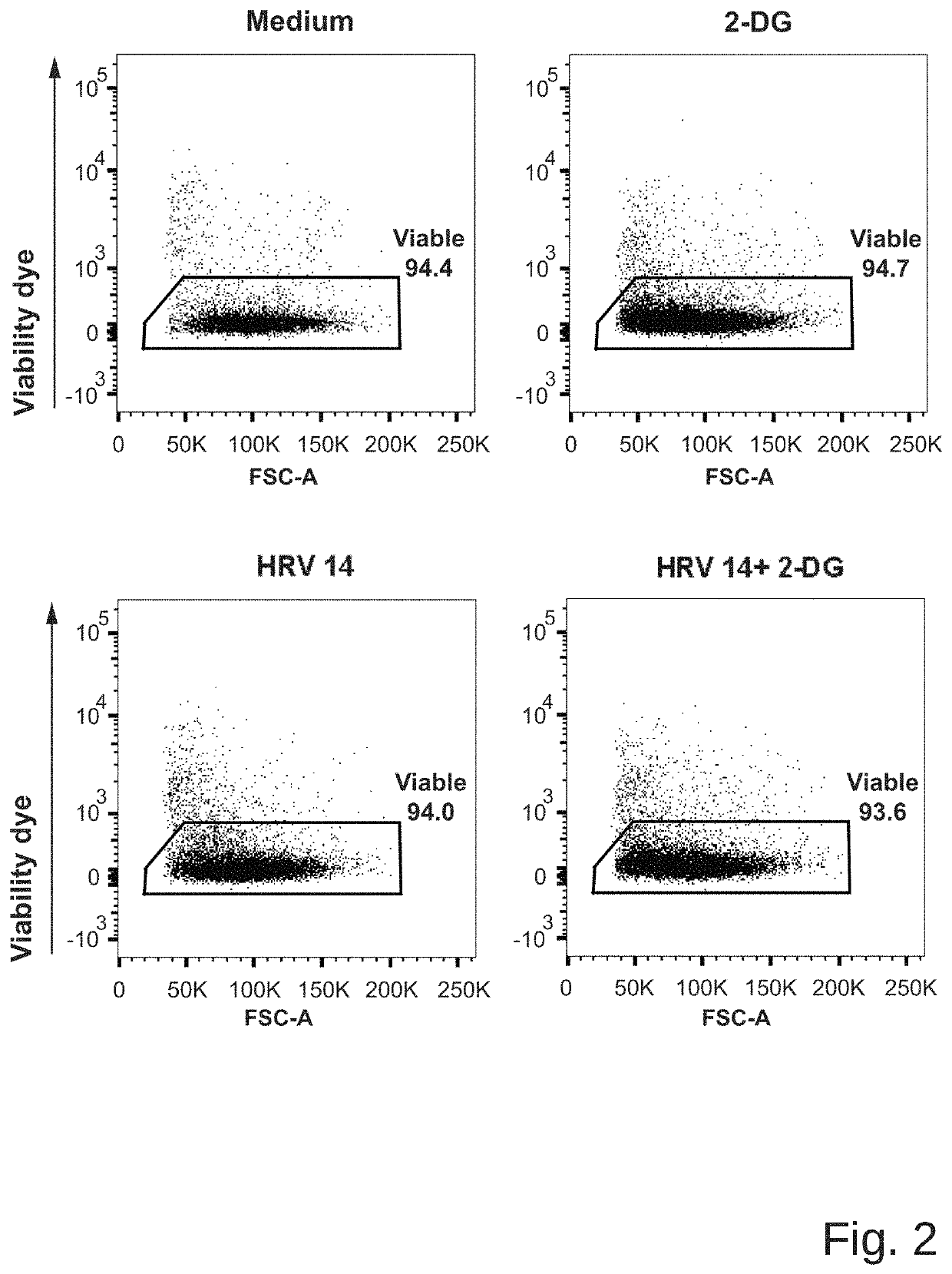
Medicament for prevention of treatment of rhinovirus infection
ActiveUS11413298B2Safe and effective HRV infectionPowder deliveryOrganic active ingredientsPowder InhalerPharmacology
Owner:MEDIZINISCHE UNIVET WIEN
Uses for anti-malarial therapeutic agents
The present invention is directed to the use of anti-malarial compound for the treatment and prophylaxis of infections by adenovirus or rhinovirus.
Owner:APT PHARMA INC
Application of Zukamu granules to preparation of drugs for resisting coxsakievirus and rhinovirus infection
InactiveCN106620461ARigorous structureSmall side effectsAntiviralsPlant ingredientsCoxsackievirusColds coughs
The invention discloses Zukamu granules. The Zukamu granules are prepared from rhizome kaempferiae, nymphaea tetragona, mint, jujubes, chamomile, cordia dichotoma fruits, liquorice, hollyhock seeds, rheum officinale and pericarpium papaveris. The Zukamu granules are used for resisting coxsakievirus and / or rhinovirus infection. The Zukamu granules can effectively inhibit activity of coxsakievirus and rhinovirus, regulate abnormal constitution, clear heat, promote sweating and open orifices and is used for treating cold coughs, fever adiaphoresis, sore throat and nasal congestion.
Owner:新疆维吾尔药业有限责任公司 +1
Medicament for prevention of treatment of rhinovirus infection
ActiveUS20200397805A1Safe and effective HRV infectionPowder deliveryOrganic active ingredientsPowder InhalerPharmacology
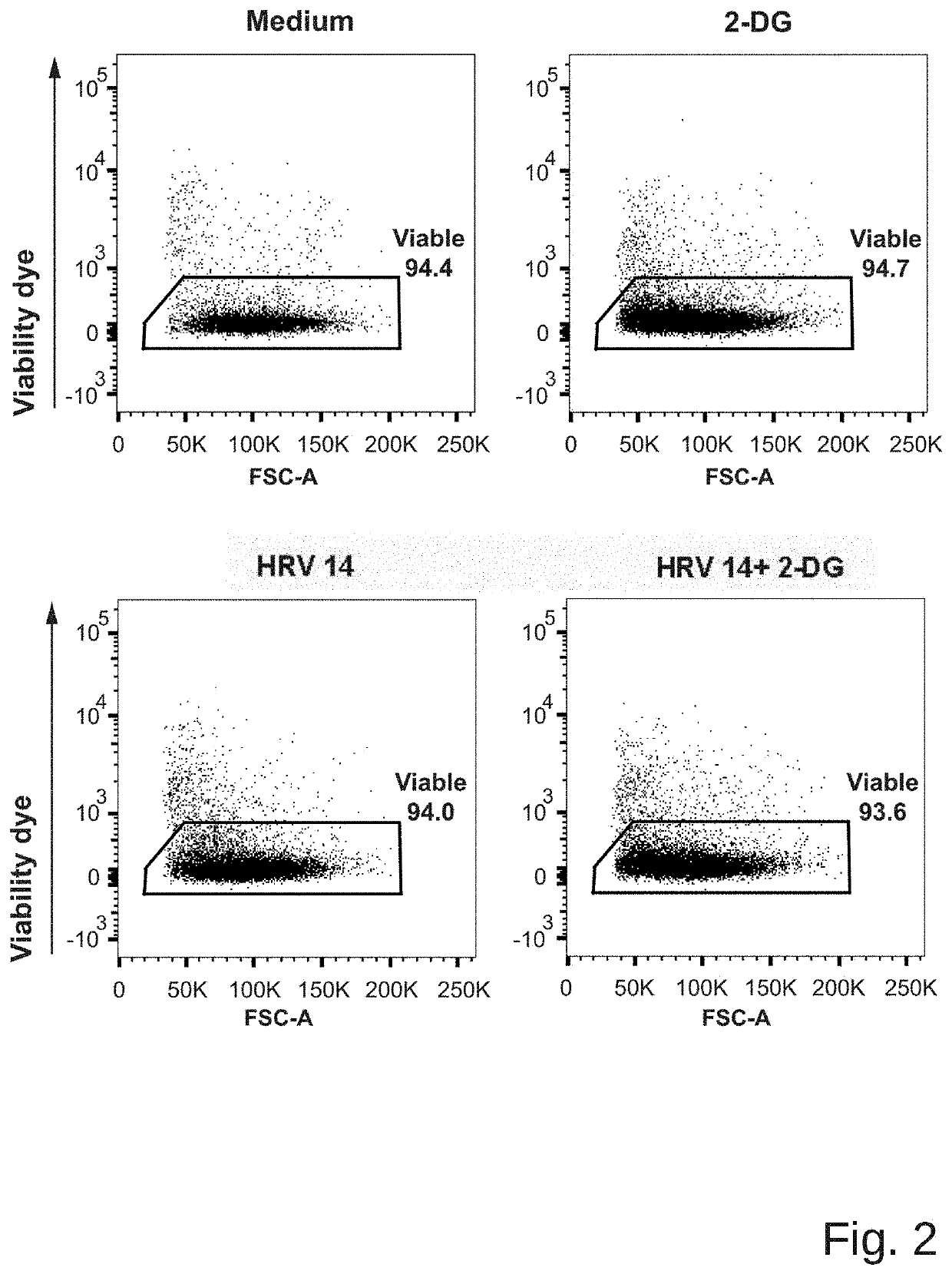
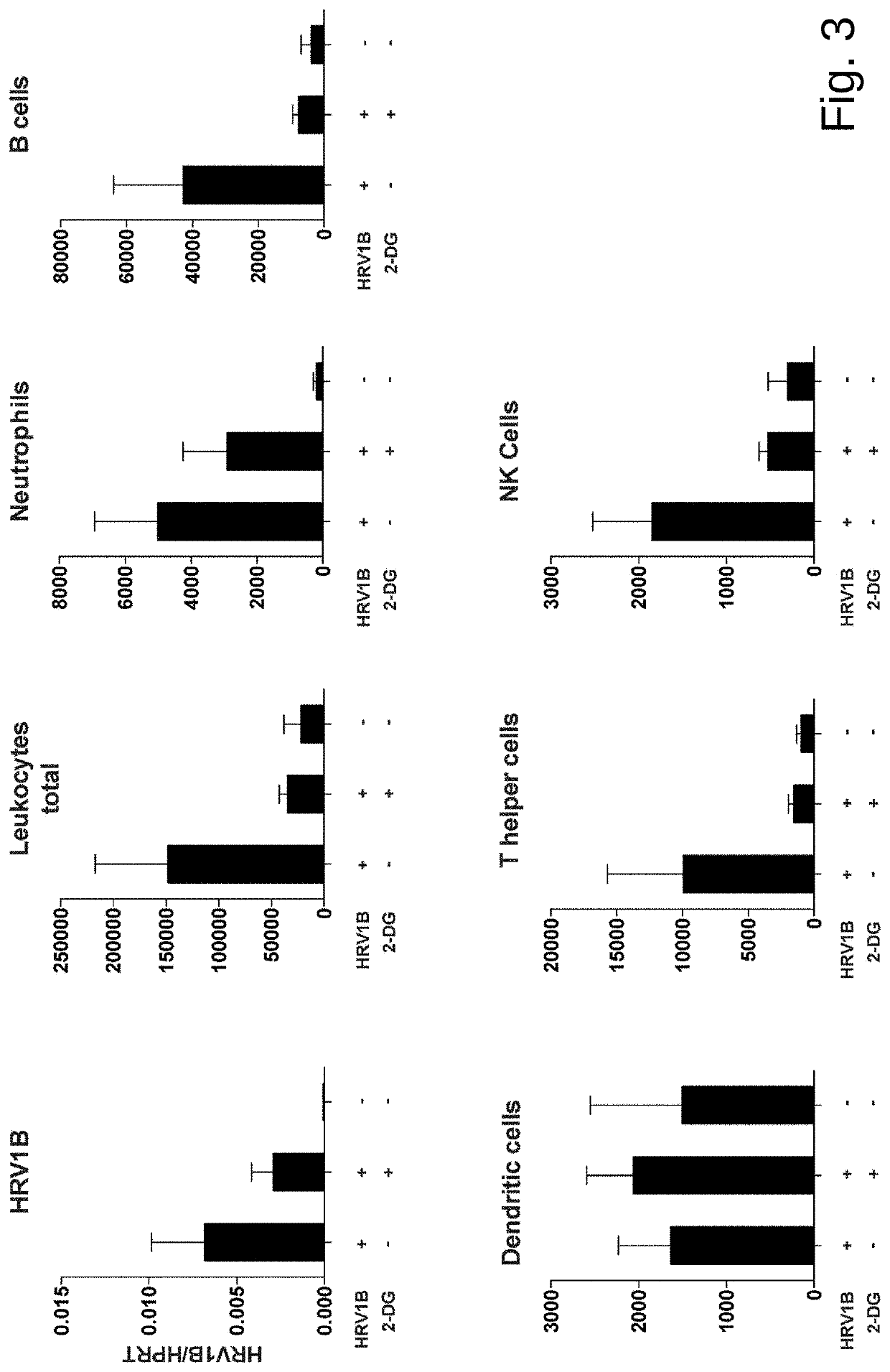
The present invention provides a pharmaceutical composition for use in prevention or treatment of a human rhinovirus (HRV) infection. The composition comprises an aldohexose, wherein the hydroxyl group at carbon 2 of the aldohexose is replaced by any one of H, F, Cl, Br, I, SH, Me, OMe and SMe, such as a 2-deoxy-glucose. Furthermore, a dispenser for intranasal administration, such as a nasal spray or nose drop applicator containing said pharmaceutical composition is provided. In addition, an inhalation device, such as a metered-dose inhaler, a dry-powder inhaler or a nebuliser, comprising said composition is provided.
Owner:MEDIZINISCHE UNIVET WIEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com