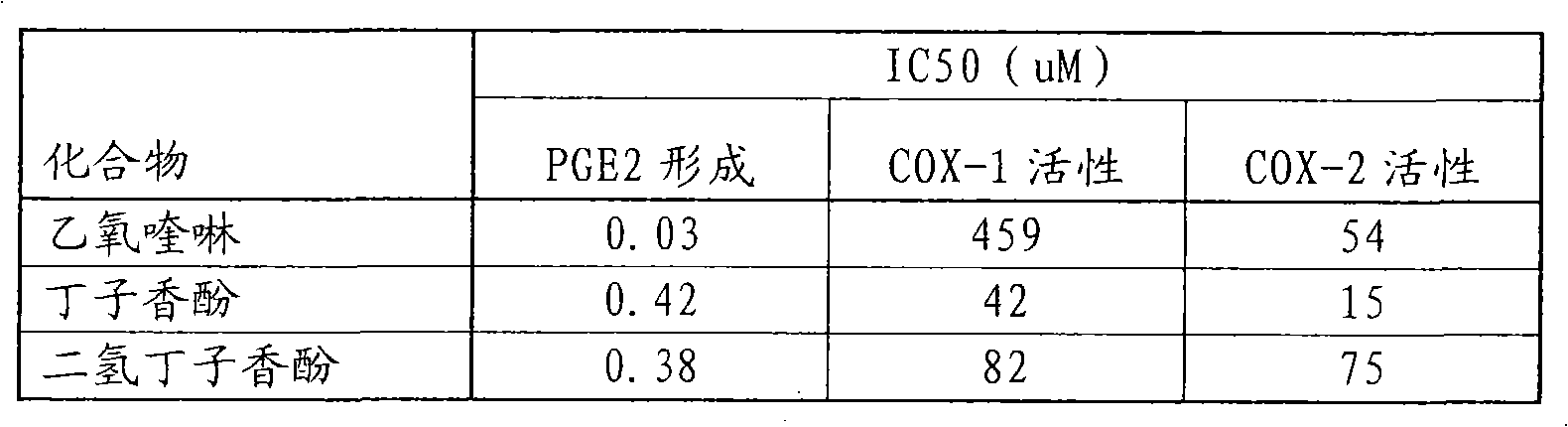
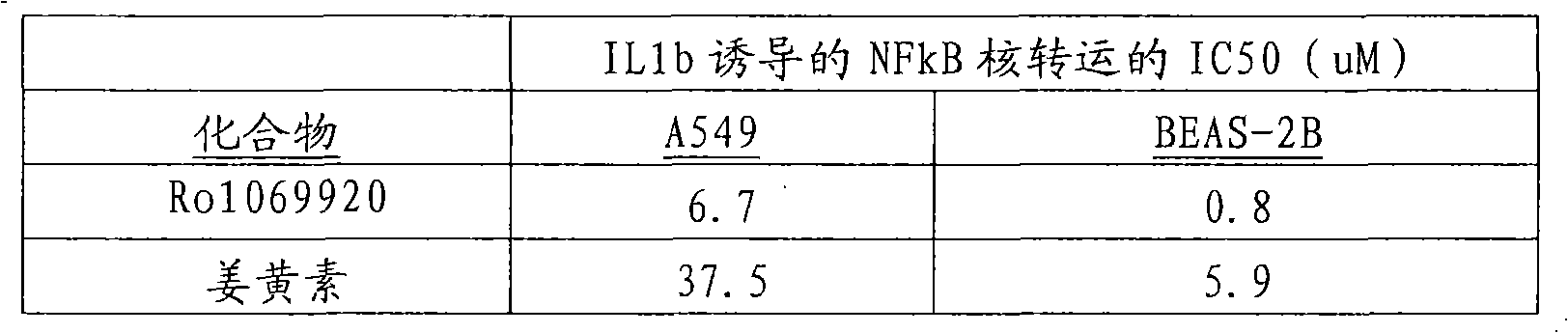
Methods and targets for identifying compounds for regulating rhinovirus infection
A technology for compounds and rhinoviruses, applied in the fields of compound screening, antiviral agents, biomaterial analysis, etc., can solve problems such as lack of screening target screening methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0152] The in vitro cell line of BEAS-2B cells can be infected with rhinovirus RV-16. The cells are then exposed to a variety of compounds and extracts, and then tested and analyzed for the content of respiratory biomarker proteins. By monitoring the content of respiratory biomarker protein after exposing infected cells to the extract and compound and comparing it with the content of respiratory biomarker protein in infected cells not exposed to the extract and compound, the extract and compound can be identified as A substance that regulates respiratory biomarker proteins.
[0153] In the described embodiment, the test component is the herb Andrographis paniculata extract or its main component andrographolide. The content of andrographolide in the test component is 5μM. The respiratory biomarker protein is IP-10 (CXCL10), a chemotactic agent.
[0154] Test ingredients
(5μM andrographolide content)
IP-10(CXCL10)
pg / mL
Control
61.99
18.07
And...
Embodiment B
[0159] The efficacy of the test compound during rhinovirus infection that naturally induces a cold can be evaluated by monitoring the content of respiratory protein biomarkers. A nasal wash was collected from subjects who showed the initial symptoms of a cold. The subject was then treated and samples of nasal wash were collected over the next few days.
[0160] The therapeutic agent consisted of standardized andrographis extract containing 20 mg of total andrographolide, 28.8 mg of Acanthopanax senticosus extract and 650 mg of curcumin (curcumin extract). This composition is taken three times a day. The respiratory biomarker protein is IP-10 (CXCL10), a chemotactic agent. In the few days after treatment, the content of respiratory biomarker protein was detected and analyzed, and the baseline value before treatment was used for statistical correction.
[0161] Test ingredients
IP-10(CXCL10)
pg / mL
Control
8183
Andrographis, Acanthopanax senticosus, and curcumin com...
Embodiment C
[0166] The efficacy of the test compound during rhinovirus infection that naturally induces a cold can be evaluated by monitoring the content of respiratory protein biomarkers. A nasal wash was collected from subjects who showed the initial symptoms of a cold. The subject was then treated and samples of nasal wash were collected over the next few days.
[0167] The therapeutic agent consisted of 400 mg ibuprofen and 4 mg chlorpheniramine maleate. The composition is taken three times a day. The respiratory biomarker protein is MCP1 (CCL2), a chemotactic agent. In the few days after treatment, the content of respiratory biomarker protein was detected and analyzed, and the baseline value before treatment was used for statistical correction.
[0168] Test ingredients
MCP1(CCL2)
pg / mL
Control
271
Combination of ibuprofen and chlorpheniramine maleate.
163
[0169] Ibuprofen was obtained from Wyeth Consumer Healthcare, Wilmington DE.
[0170] Chlorpheniramine maleate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com