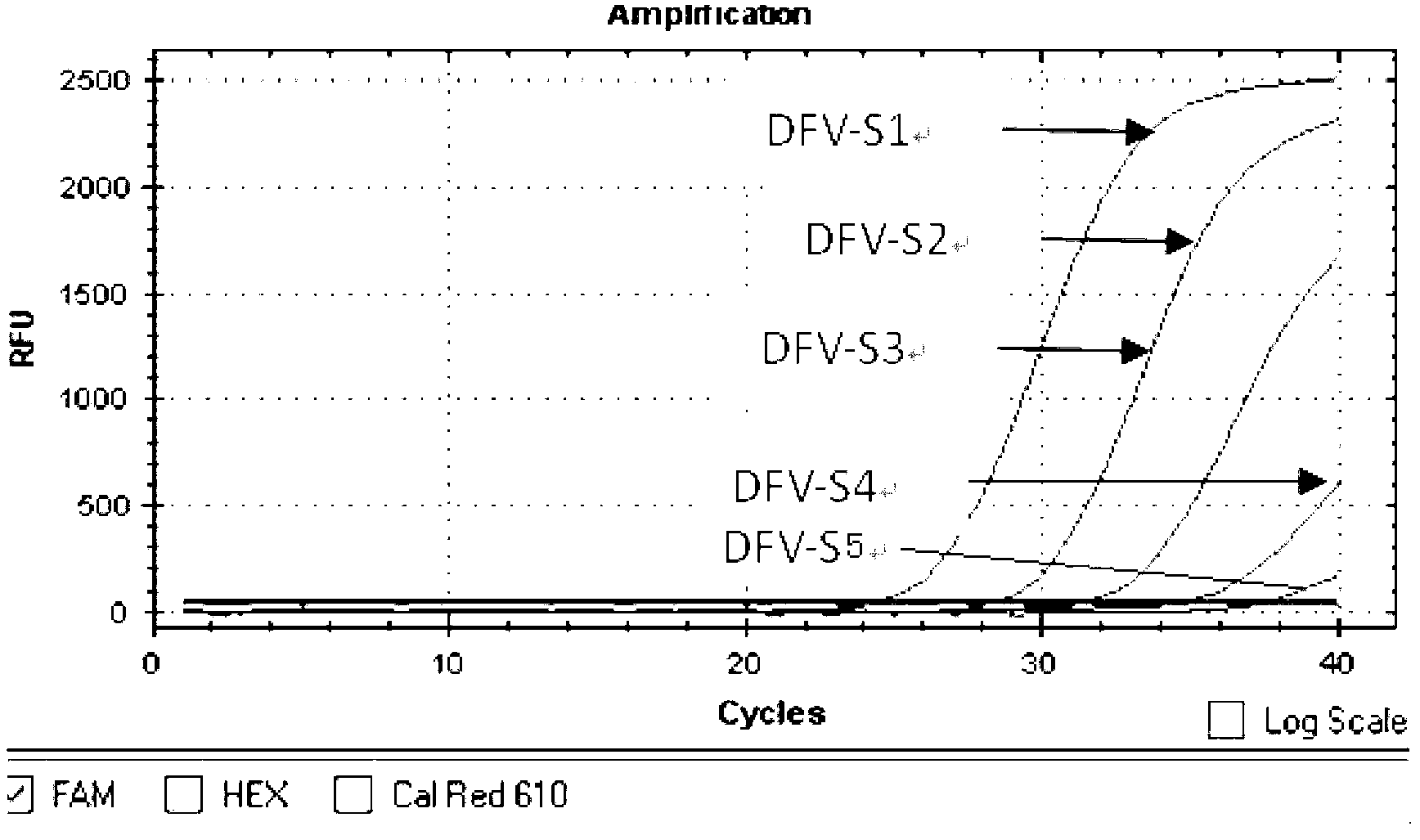
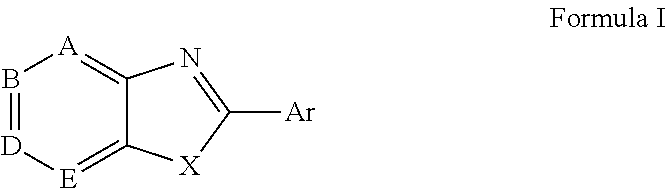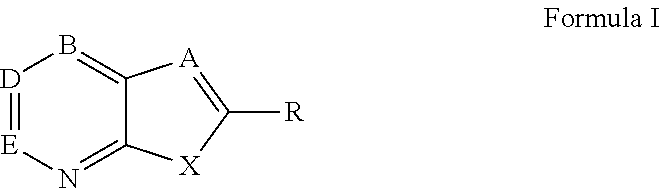Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Yellow fever viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Yellow fever is caused by a bite from a mosquito that is infected with the yellow fever virus. Understanding the Yellow Fever Virus. The yellow fever virus is a flavivirus (virus transmitted by mosquitoes) found in certain parts of Africa and South America.
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
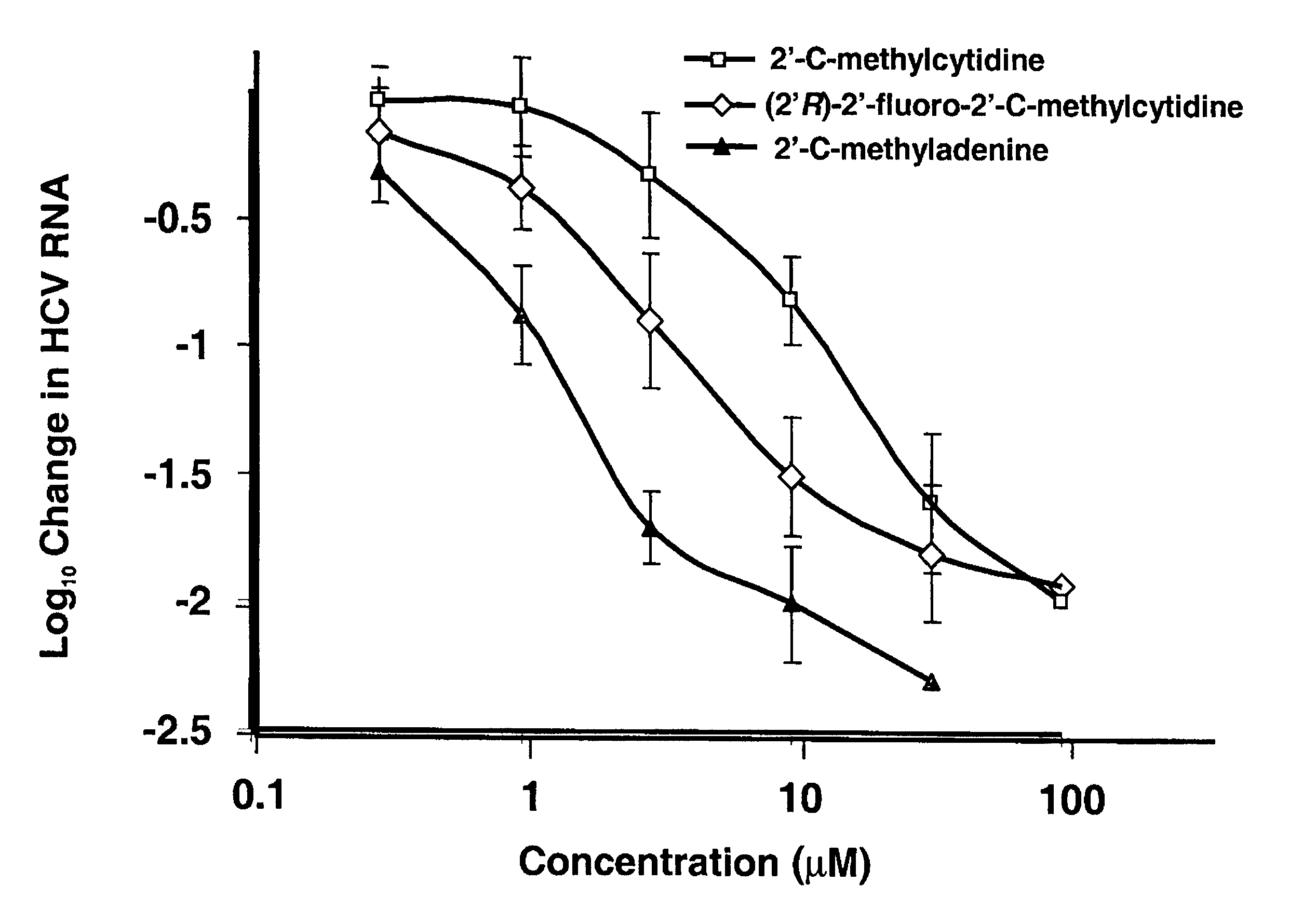
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
Modified fluorinated nucleoside analogues
ActiveUS20080070861A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
Chimeric flavivirus vaccines
InactiveUS6962708B1Easy to useImproving immunogenicityBiocideSsRNA viruses positive-senseNucleotideNucleotide sequencing
A chimeric live, infectious, attenuated virus containing a yellow fever virus, in which the nucleotide sequence for a prM-E protein is either deleted, truncated, or mutated, so that functional prM-E protein is not expressed, and integrated into the genome of the yellow fever virus, a nucleotide sequence encoding a prM-E protein of a second, different flavivirus, so that the prM-E protein of the second flavivirus is expressed.
Owner:SAINT LOUIS UNIVERSITY +1
Treatment and prevention of dengue virus infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain 2-aryl-benzothiazole or 2-heteroaryl-benzothiazole derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Needleless vaccination using chimeric yellow fever vaccine-vectored vaccines against heterologous flaviviruses
InactiveUS20040120964A1Good curative effectEfficient replicationSsRNA viruses positive-senseViral antigen ingredientsYellow fever vaccineVaccination
The present invention relates to a method of vaccinating a subject comprising delivering a chimeric yellow fever 17D strain vector expressing an envelope protein gene product of a heterologous flavivirus to the epidermal compartment or the intradermal compartment of the subject's skin. The invention encompasses vaccine compositions comprising the chimeric yellow fever viruses expressing an envelope protein gene product of a heterologous flavivirus. The compositions of the invention result in an enhanced therapeutic efficacy, e.g., enhanced protective immune response as they enhance the presentation and availability of the chimeric vaccine to the targeted compartment of the subject's skin.
Owner:MIKSZTA JOHN A +5
Flavivirus human monoclonal antibody and application
ActiveCN106589116AImprove bindingViral antigen ingredientsImmunoglobulins against virusesFlaviviridaeMonoclonal antibody
The invention discloses a flavivirus human monoclonal antibody and application, which belong to the technical field of medicine. According to the flavivirus human monoclonal antibody and the application provided by the invention, three antibodies capable of protein-binding with ZIKV-E are acquired, and binding sites of the three antibodies are confirmed. The three antibodies are completely different from a reported ZIKV antibody sequence, and are three new-found antibodies. The binding constants of the three antibodies and the ZIKV-E are 39.9pM (Z5), 44.7pM (Z6) and 200pM (Z7), which show that the three human monoclonal antibodies have a higher ZIKV-E protein-binding capacity. Through competiton experiments, the binding sites of the three antibodies have competitive relationships with 2A10G6, and the phenomenon shows that the binding sites of the three antibodies are near FL, and E protein of flaviviridae is highly conserved at the FL part. The antibody provided by the invention effectively detects the infection of common flaviviridae viruses: ZIKV, dengue 1-4 type and yellow fever viruses, and has huge application values on clinic detection and fundamental research.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Thienopyridine Derivatives for the Treatment and Prevention of Dengue Virus Infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain thienopyridine derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Humanized monoclonal antibody and application thereof
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
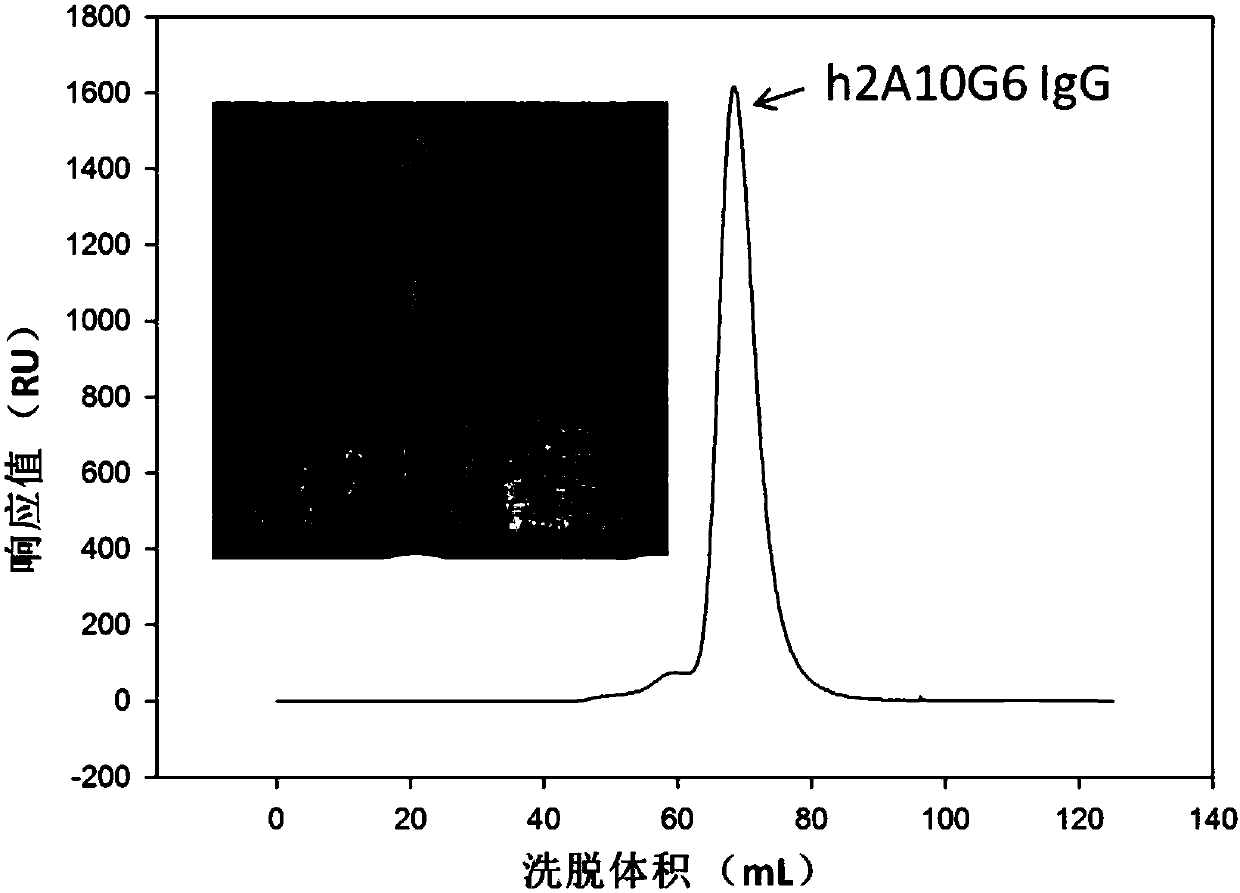
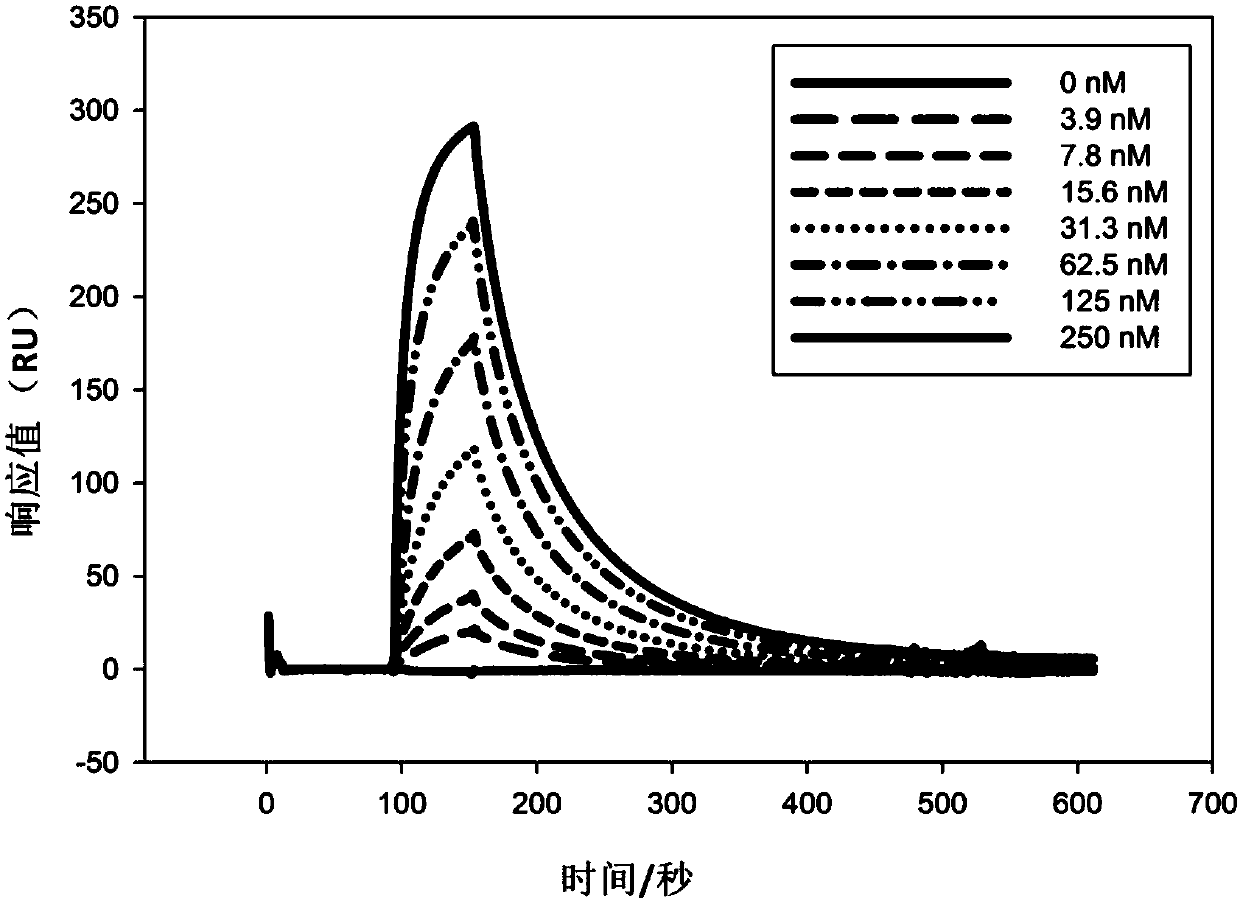
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Antiviral Drugs for Treatment or Prevention of Dengue Infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Samll molecule inhibitors for the treatment or prevention of dengue virus infection
Methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
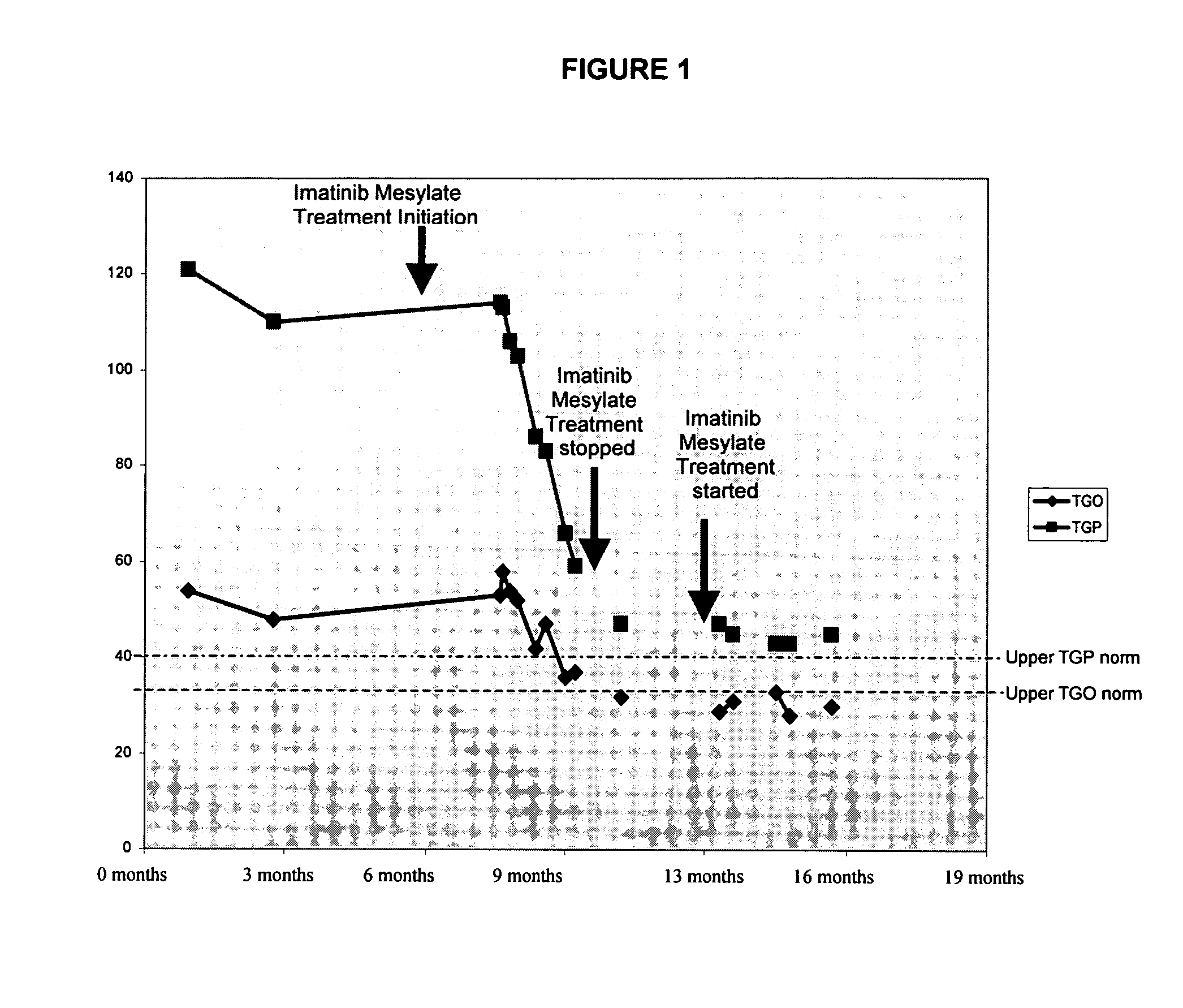
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
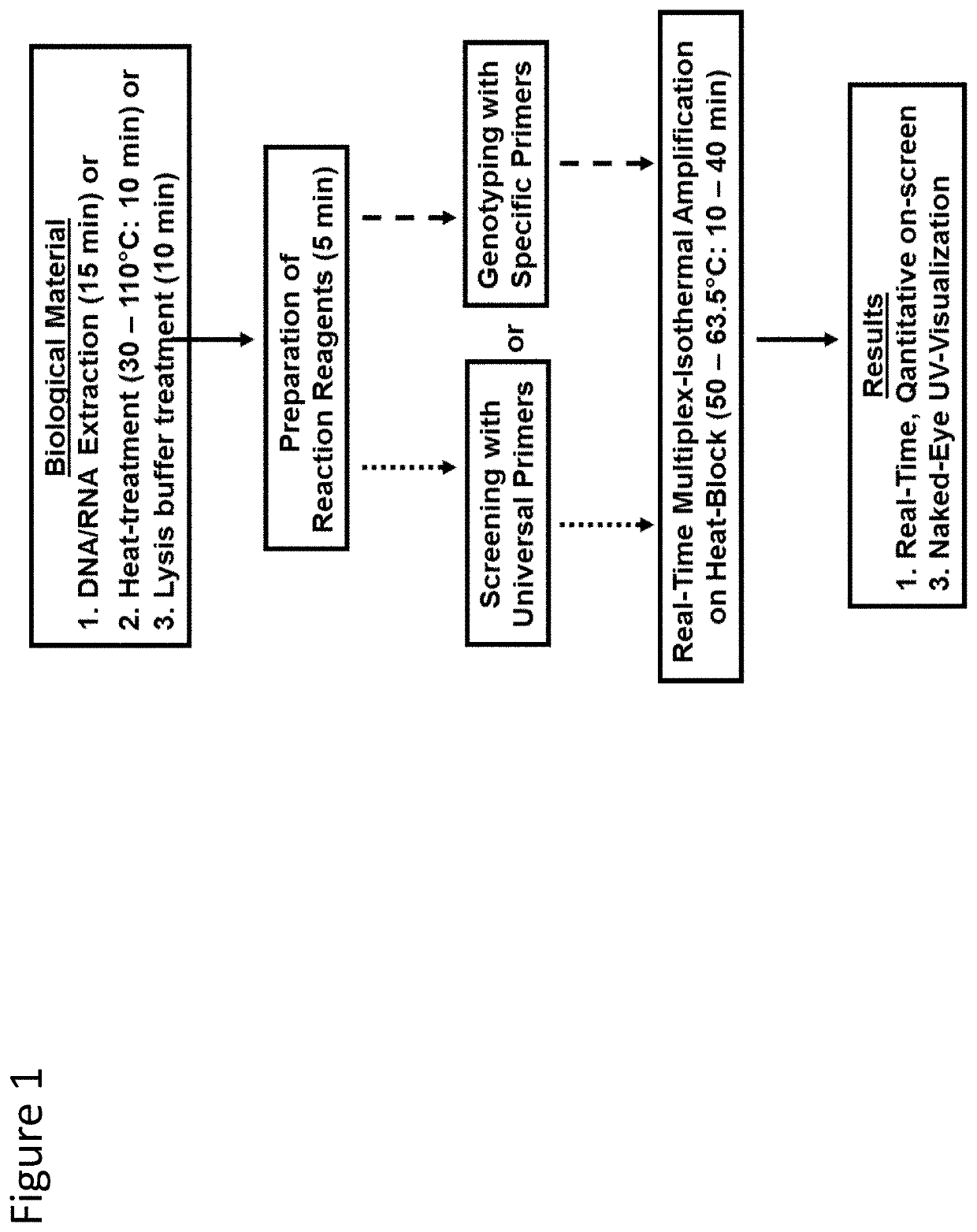
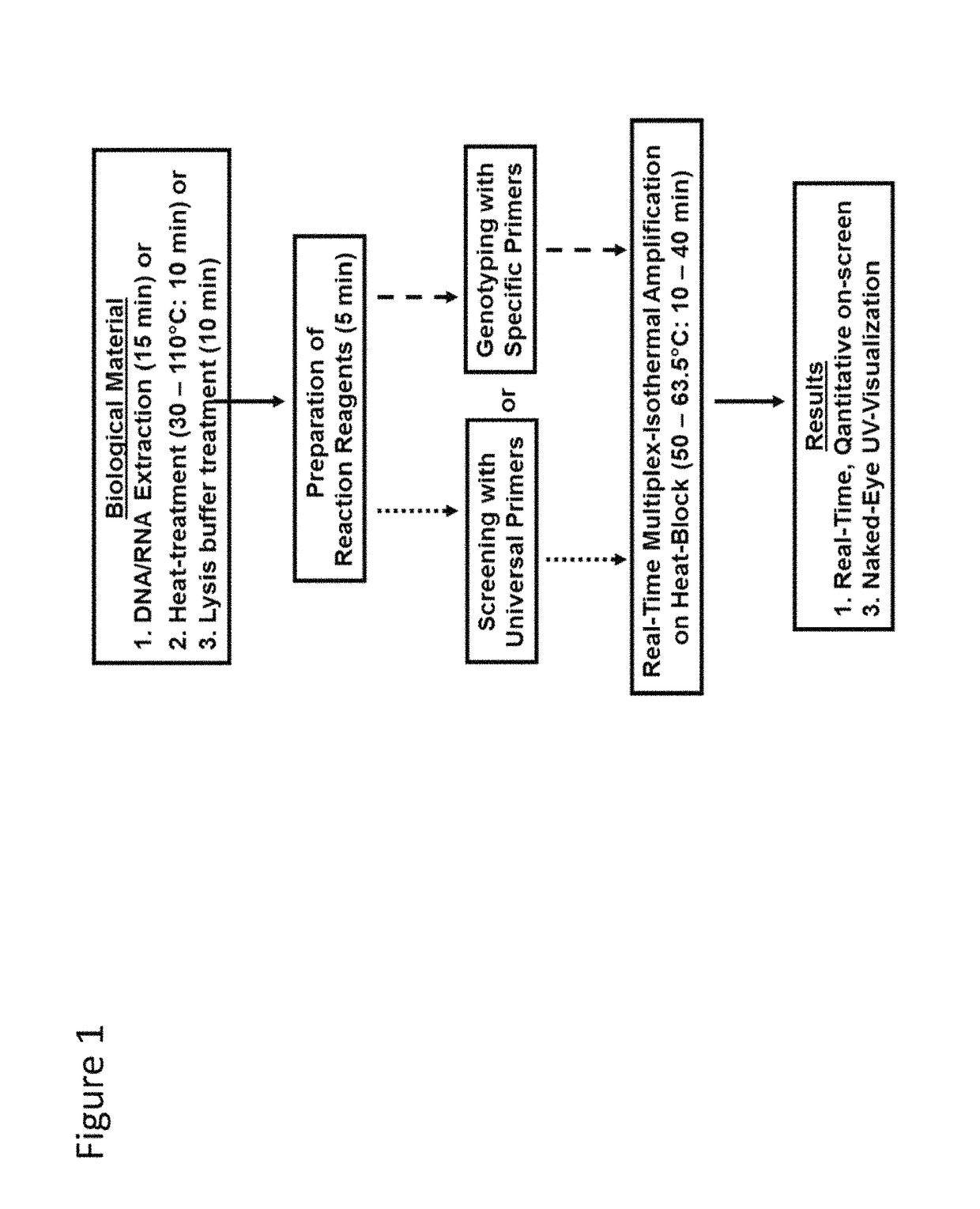
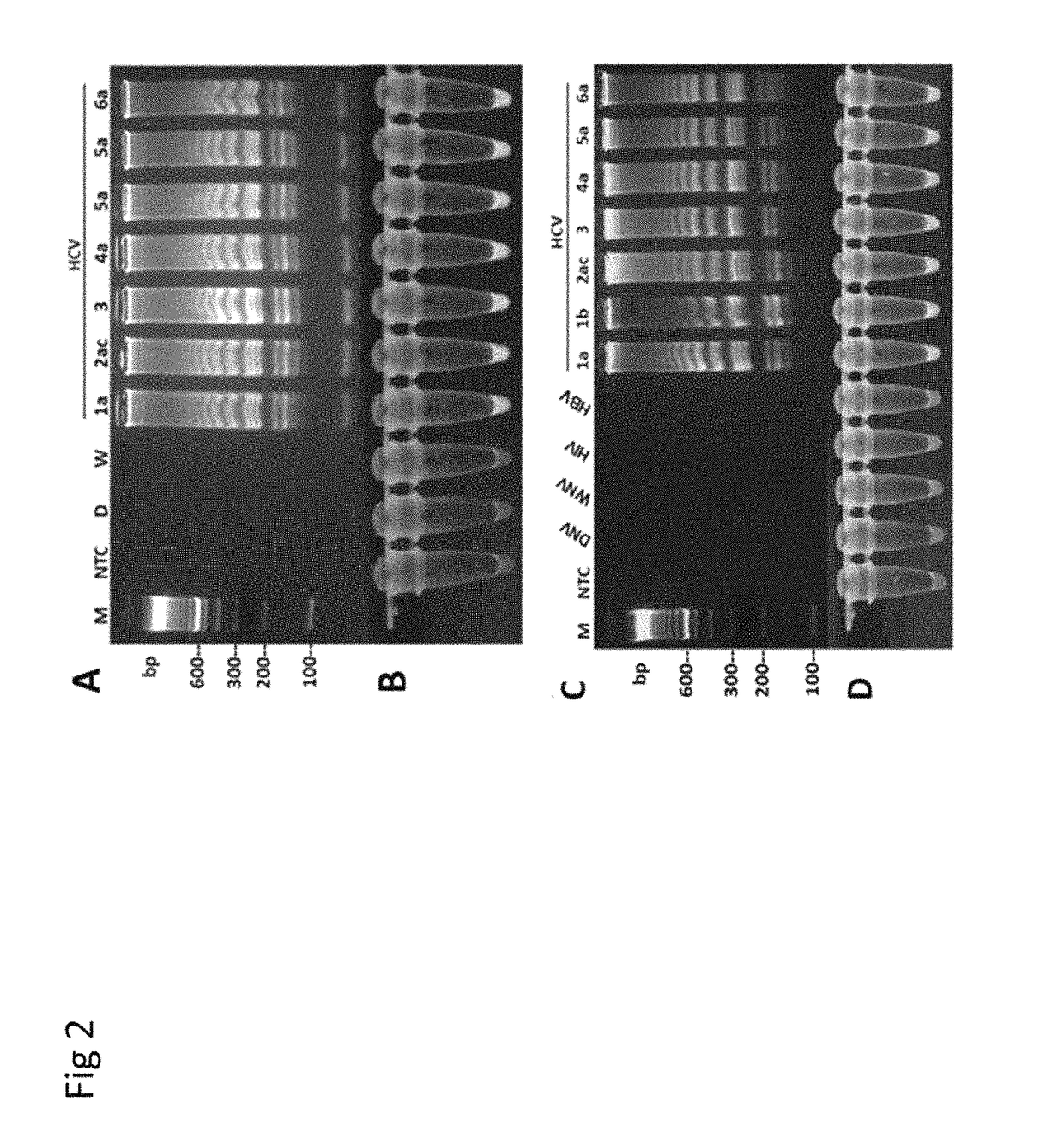
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7417136B1Easy to administerEasy to prepareOrganic active ingredientsVirusesActive agentEncephalitis Viruses
The invention encompasses nucleic acid molecules containing transcription units which encode the flavivirus M and E protein antigens. The flaviviruses include Japanese encephalitis virus, dengue, yellow fever virus and St. Louis encephalitis virus. The nucleic acids function to provide the M and E protein antigens when the nucleic acid resides in an appropriate host cell, especially when the host cell is the cell of a subject. The invention also encompasses a vaccine whose active agent is the nucleic acid. The invention further encompasses the cultured host cells when they contain within them nucleic acid molecules containing the transcription units. The invention in addition encompasses a method of immunizing a subject against flavivirus infection by administering to the subject an effective amount of a vaccine containing a nucleic acid molecule containing the transcription unit of the invention.
Owner:HEALTH & HUMAN SERVICES DEPT OF UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE
Kit for detecting mosquito borne pathogens and detection method thereof
InactiveCN103911463AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic encephalitisMultiplex polymerase chain reaction
The invention discloses a kit for detecting a plurality of mosquito borne pathogens and a detection method thereof. The plurality of mosquito-borne infectious pathogens are detected by using multiplex polymerase chain reaction (PCR) combined with a liquid chip, and a yellow fever virus (YFV), dengue fever viruses (DV) I-IV type, epidemic encephalitis B (japanese encephalitis) virus (JEV), plasmodium (Plasmodium), (including plasmodium vivax (PV), plasmodium falciparum (PF), plasmodium malariae (PM), plasmodium ovale (PO)), a west nile virus (MNV) and a chikungunya virus (CHIKV) can be detected in parallel one time. The kit has the advantages of being large in flux, high in specificity and sensitivity, stable in result, good in repeatability, simple to operate and fast in detection speed.
Owner:河北国际旅行卫生保健中心
Kit for flavivirus quick typing and virus load detection
InactiveCN106086242ANo cross reactionAchieve quantitative goalsMicrobiological testing/measurementAgainst vector-borne diseasesZika virusMicroorganism
The invention belongs to the field of microbial molecular detection, and particularly relates to a kit for flavivirus quick typing and virus load detection. The kit for flavivirus quick typing and virus load detection comprises specific primers and probes for flaviviruses, including specific primers and a probe for dengue virus, specific primers and a probe for Zika virus, specific primers and a probe for yellow fever virus, and specific primers and a probe for Chikungunya virus. The kit for flavivirus quick typing and virus load detection has the advantages of high detection speed and accurate detection and quantification results, can simultaneously detect 3 different flavivirus pathogens and 1 togavirus pathogen capable of causing similar clinical symptoms at one time, can detect and diagnose flavivirus-pathogen-infected suspicious cases in time, and enhances the detection accuracy of flavivirus pathogen infection.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
Chimeric flavivirus vaccines
InactiveUS20100278773A1Improving immunogenicityEffective vaccineSsRNA viruses positive-sensePeptide/protein ingredientsNucleotideViral Vaccine
A chimeric live, infectious, attenuated virus containing a yellow fever virus, in which the nucleotide sequence for a prM-E protein is either deleted, truncated, or mutated, so that functional prM-E protein is not expressed, and integrated into the genome of the yellow fever virus, a nucleotide sequence encoding a prM-E protein of a second, different flavivirus, so that the prM-E protein of the second flavivirus is expressed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Flavivirus vaccine
PendingUS20210069315A1Eliminate and prevent growthStrong attackSsRNA viruses positive-senseViral antigen ingredientsDiseaseImmunogenicity
The present invention is directed to an artificial nucleic acid and to a polypeptide suitable for use in the treatment or prophylaxis of an infection with a flavivirus, in particular an infection with yellow fever virus or with dengue virus, or of a disorder related to such an infection. The present invention is also directed to a composition, preferably an immunogenic composition, comprising the artificial nucleic acid or the inventive polypeptide. In particular, the present invention concerns an immunogenic composition against a flavivirus, such as yellow fever virus or dengue virus. Further, the invention concerns a kit, particularly a kit of parts, comprising the artificial nucleic acid, polypeptide or (immunogenic) composition. The invention is further directed to a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial nucleic acid, polypeptide, composition, in particular the first and second medical uses of the immunogenic composition according to the invention.
Owner:CUREVAC SE +1
Recombinant antigen protein for detecting yellow fever virus antibody, kit and application of recombinant antigen protein
ActiveCN102827260AStrong specificityEfficient expression effectVirus peptidesBiological testingFhit geneProtein C
The invention discloses a recombinant antigen protein for detecting a yellow fever virus antibody. The sequence of the recombinant protein is shown as SEQ ID NO.1; the sequence of an encoding gene of the recombinant antigen protein is shown as SEQ ID NO.2, and comprises an extension segment for encoding a flexible arm of the recombinant antigen protein; and the flexible arm can be used for eliminating masking of a peptide epitope by a fusion protein of an expression carrier, so that the expressed recombinant antigen protein is folded correctly. The invention further discloses a preparation method of the recombinant antigen protein. Moreover, a yellow fever virus kit disclosed by the invention comprises an antigen detection plate coated with the recombinant antigen protein and an ELISA (Enzyme-Linked Immunosorbent Assay) reaction liquid. The recombinant antigen protein provided by the invention has the advantages of high specificity and high affinity, does not undergo any serological cross reaction with other congenial arthropod-borne viruses, has ultrahigh affinity with the yellow fever virus antibody, and can be used for detecting the yellow fever virus antibody rapidly and accurately for diagnosing the infection condition of a yellow fever virus.
Owner:中国人民解放军南部战区疾病预防控制中心
Reagent and method for detecting yellow fever virus
InactiveCN102952900AGuaranteed specificitySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceAgricultural scienceNucleotide sequencing
The invention provides a reagent for detecting yellow fever virus. The reagent comprises an outer primer F3, an outer primer B3, an inner primer FIP, an inner primer BIP, wherein primer gene sequences are as follows: the outer primer F3: TGGGAGAGGAGATTCACGT; the outer primer B3: TCAAGCCGCCAAATAGCC; the inner primer FIP: CCACGCCTTTCATGGTCTGAGTTTACCAGTGGCACAAAGAGG; and the inner primer BIP: AGACACCGCCTGGGATTTYAGGCAGAGCCAAACACCGTATG. The primers do not have isogeny with nucleotide sequences of other species, so that the specificity of a detection method is ensured. The method for detecting the yellow fever virus through isothermal amplification established by the primers has the advantages of simplicity and convenience in operation, high efficiency, quickness and specificity. Due to the establishment of the method, a blank of the yellow fever virus isothermal nucleic acid amplification detection method is filled, the detection on a sample can be completed by taking around one hour, and significance is provided for preventing the introduction of yellow fever virus in frontier ports.
Owner:浙江国际旅行卫生保健中心
Methods of treatment and diagnosis using modulators of virus-induced cellular gene sequences
InactiveUS20070087982A1Reduce expressionReducing and preventing expression of mRNABiocideGenetic material ingredientsDiseaseImmunodeficiency virus
Applicants have used microarrays, gene expression profiling, and gene silencing methods to identify and provide a plurality of ‘validated’ virus-induced cellular gene sequences (e.g., HMG20B, HRH1, NP and c-YES (src family kinases)) and pathways useful as therapeutic targets for modulation of viral-mediated cellular effects. Particular embodiments provide therapeutic compositions, and methods for modulation of viral infection, replication, maturation, progression, or other virally-related conditions or diseases, comprising inhibition of virally-induced gene sequences and gene products. Additional embodiments provide screening assays for compounds useful to modulate viral infection, replication, maturation or progression, or viral-related conditions or diseases. Further embodiments provide diagnostic and / or prognostic assays for viral infection, replication, maturation or progression. Preferably, the viruses all selected from the group consisting of retroviruses (e.g., human immunodeficiency virus (HIV), and viruses of the family Flaviviridae that includes the flaviviruses (e.g., West Nile virus (WNV), Japanese encephalitis virus (JEV), yellow fever virus (YFV) and Dengue fever virus (DEN)), and hepatitis C virus (HCV).
Owner:OREGON HEALTH & SCI UNIV
Multiple polymerase chain reaction (PCR) kit and method for detecting mosquito-borne pathogens
InactiveCN101979665AReliable informationEffective early warning dataMicrobiological testing/measurementAgainst vector-borne diseasesTissue fluidMultiplex pcrs
The invention provides a multiple polymerase chain reaction (PCR) kit for detecting mosquito-borne pathogens. The kit comprises six pairs of specific primers. The invention also provides a method for detecting the mosquito-borne pathogens. Electrophoresis is performed on a PCR-amplified product. Whether pathogens, such as encephalitis B virus, dengue fever virus, yellow fever virus, plasmodium falciparum, plasmodium vivax, plasmodium knowlesi, plasmodium ovale, plasmodium malariae, wuchereria malayi, wuchereria bancrofti and the like, exists or not is detected and identified according to the length of a PCR-amplified fragment. By the method, various reported mosquito-borne pathogens, and the yellow fever virus and west nile virus which come from other countries can be detected quickly, accurately and sensitively at the same time, and can be applied to the detection of various samples, such as mosquitoes, blood of patients, tissue fluid and the like. The invention provides a low-cost and high-efficient method for early monitoring and finding mosquito-borne disease prevalence for prevention and control work of mosquito-borne diseases in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
High yield yellow fever virus strain with increased propagation in cells
ActiveUS20110287519A1High yieldSpread the wordSsRNA viruses positive-senseViral antigen ingredientsYellow Fever Virus InfectionViral infection
The invention provides a an inactive, non-replicating vaccine comprising whole virion, chemically inactivated Yellow Fever virus which is inactivated using a method that ensures preservation of critical, neutralizing epitopes. The Yellow Fever virus has been adapted to propagate in cells to higher yields than the unadapted virus. The invention also provides methods for preventing Yellow Fever viral infection.
Owner:PNUVAX INC
Compositions and methods for detecting certain flaviviruses, including members of the japanese encephalitis virus serogroup
ActiveCN1795275AMicrobiological testing/measurementAgainst vector-borne diseasesSalmonella serotype typhiEncephalitis Viruses
The present invention provides rapid and accurate methods, primers, probes and kits for detecting certain flaviviruses in samples. Detectable flaviviruses include Japanese encephalitis virus serogroup members, dengue virus, St. Louis encephalitis virus, Montana myotis leukoencephalitis virus, Modoc virus, and yellow fever virus. The primers and probes of the present invention can hybridize to the region within the 3' untranslated region of the genome of the virus to be detected.
Owner:F HOFFMANN LA ROCHE & CO AG
Identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes
InactiveCN103911462AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesChikungunya feverRepeatability
The invention discloses an identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes. The identification and detection method comprises the step of rapidly, qualitatively and quantitatively detecting a yellow fever virus, a Japanese encephalitis virus, a chikungunya virus and a west Nile virus by combining polymerase chain reaction (PCR) and a liquid chip. The four pairs of primers and probes with high specificity, high sensitivity and good repeatability are provided in the method.
Owner:河北国际旅行卫生保健中心
Fulminating-infectious-disease pathogen detecting primer pair and kit
ActiveCN105483293AReduced Pollution ChancesShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesColor changesBiology
The invention discloses a fulminating-infectious-disease pathogen detecting primer pair and a kit. The primer pair comprises at least a pair of RT-LAMP primers of Ebola viruses, Lassa fever viruses, Marburg viruses, rift valley fever viruses, yellow fever viruses and Chikungunya fever viruses. By means of the primer system, the amplification reaction background is reduced, and sensitivity and specificity are quite good. The kit formed by the primer pair further comprises detecting liquid and a micro-fluidic chip; as an independent RT-PCR secondary amplification step of a detecting liquid system is omitted, detecting time is shortened; as the denaturation process and the renaturation process of nucleic acid do not exist, the polluted chance of RNA enzymes and the polluted chance of amplification nucleic acid are reduced, and the sensibility and the safety of detection are improved. By means of the constant-temperature sealed environment provided by a micro-fluidic chip system, rapid and constant-temperature amplification and automation result distinguishing of a nucleic acid extracting template are finally achieved, the requirement for test hardware is reduced, the use level of a reaction reagent is reduced, detection cost is reduced, and the result can be directly determined through color changes.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Dengue chimeric viruses
InactiveUS7968102B2High genetic stabilitySsRNA viruses positive-senseSugar derivativesPolymerase LYellow fever viruses
The invention relates to Dengue chimeric viruses which are less prone to accumulate point mutations and genetic variations. In these Dengue chimeric viruses, the NS5 gene, which encodes polymerase, has been replaced by the corresponding NS5 sequence of a Yellow Fever virus.
Owner:SANOFI PASTEUR SA
A new method for the detection of yellow fever virus by fluorescent quantitative PCR and a PCR system for the detection of yellow fever virus
ActiveCN102277446AQuick QualificationQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceEncephalitisFluorescence
The invention discloses a novel fluorescence quantitative PCR (Polymerase Chain Reaction) detection method for yellow fever viruses and a yellow fever virus detection PCR system. The yellow fever virus detection PCR system comprises primers and probes, a Premix Ex Taq reaction liquid, and sterilized Tris water, wherein the primers and the probes are good in detection specificity and high in sensitivity, is very suitable to bouquet fever viruses, and has no cross reactions with the yellow fever viruses and encephalitis B viruses.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Multiplex-fluorescence PCR (Polymerase Chain Reaction) detection kit and application thereof
InactiveCN103305633AGuaranteed heightGuaranteed FeaturesMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention relates to the technical field of biological detection, and in particular relates to a primer, a probe and a kit used for detecting dengue fever, chikungunya and yellow fever virus through a single tube by the multiplex-fluorescence PCR (Polymerase Chain Reaction). The primer and probe used for detecting the dengue fever, chikungunya and yellow fever virus through the multiplex-fluorescence PCR have nucleotide sequences as shown in SEQ ID NO: 1-9; the multiplex PCR detection kit for detecting the dengue fever, chikungunya and yellow fever virus comprises multiplex-fluorescence PCR detection mixed liquid which contains the primer and probe mentioned above. The kit, primer and probe can quickly detect the dengue fever virus, chikungunya virus and yellow fever virus, have the advantages of being simple and convenient to operate, high in sensitivity and outstanding in specificity, and can timely find out and confirm a suspect case so as to improve the monitoring level on such diseases.
Owner:浙江国际旅行卫生保健中心 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com