Kit for flavivirus quick typing and virus load detection
A viral load and flavivirus technology, which is applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, DNA/RNA fragments, etc., can solve the problems that cannot meet the detection requirements of flaviviruses, and facilitate prevention, control and treatment Measures, reducing sample size requirements, and improving the effect of cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, primers and probes
[0039] The invention provides a primer and a probe for rapid typing of flavivirus and detection of viral load.
[0040] Table 1 Primers and probes provided by the present invention
[0041]
Embodiment 2
[0042] Embodiment 2, the detection method that is used for the test kit of flavivirus rapid typing and viral load detection
[0043] 1. Prepare a kit including the following components: 1 tube of reaction solution I (20 μl / tube), detection reaction II (280 μl / tube), 1 tube of positive quality control (20 μl / tube), and 1 tube of negative quality control (20 μl / tube). tube) 1 tube, quantitative reference substances S1 ~ S4 (20μl / tube) 1 tube each.
[0044] 2. Specimen type: Serum, urine and saliva samples collected clinically.
[0045] 3. Nucleic acid extraction:
[0046] Commercial RNA extraction kits can be used, such as nucleic acid extraction reagents based on silica gel membrane spin column method or nucleic acid extraction reagents based on magnetic bead method, and operate according to the kit instructions, and finally collect 20-30 μl RNA solution for direct detection Or store at -20°C.
[0047] 4. Real-time fluorescence rapid PCR and quantitative detection:
[0048]...
Embodiment 3
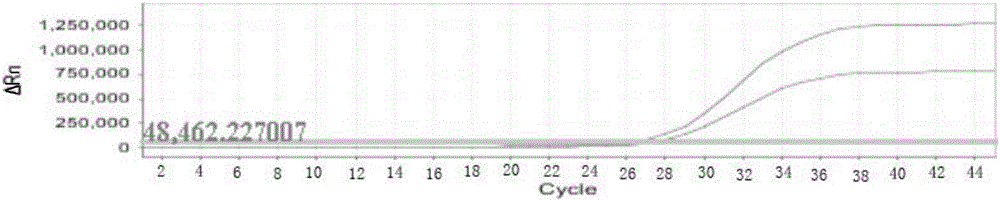
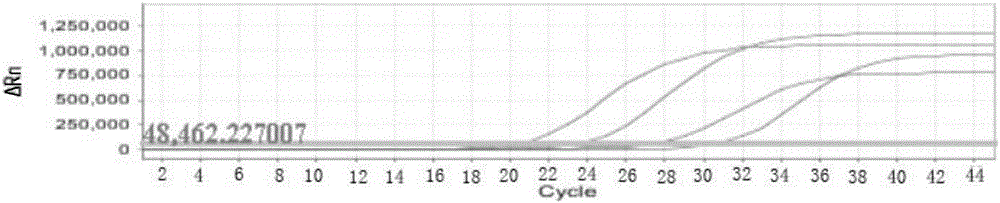
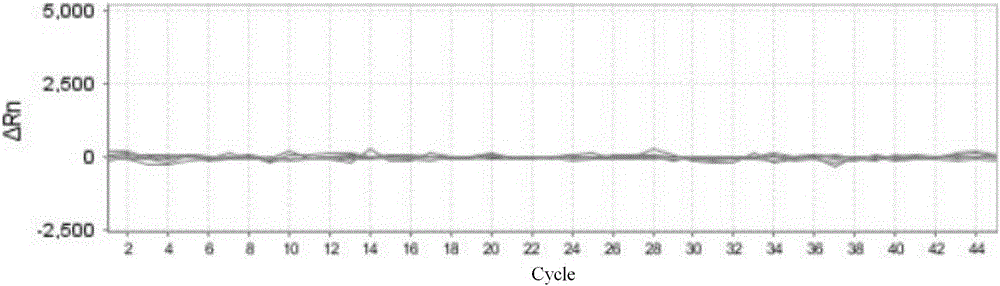
[0054] Embodiment 3, the detection clinical sample of using the kit of rapid identification of flavivirus and detection of viral load
[0055] 1. Select 1 case of serum and urine samples from positive cases of Zika virus infection confirmed by clinical and virus culture methods, and 1 case of saliva samples from Zika virus infection positive cases on the 2nd, 3rd, 4th, and 5th day of onset. Serum samples from positive cases identified as chikungunya virus, dengue virus type I, dengue virus type II, dengue virus type III, dengue virus type IV, and yellow fever virus were identified by clinical and virus culture methods. , as well as one case of serum samples from positive cases of measles virus, Hantaan virus, Seoul virus, fever with thrombocytopenia syndrome bunya virus infection, and one case of yellow fever virus vaccine strain. Nucleic acid extraction was performed on all samples, and the steps of on-board amplification detection and result analysis were carried out with re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com