Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
436 results about "Virus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral vaccines contain either inactivated viruses or attenuated (alive but not capable of causing disease) viruses. Inactivated or killed viral vaccines contain viruses, which have lost their ability to replicate and in order for it to bring about a response it contains more antigen than live vaccines.
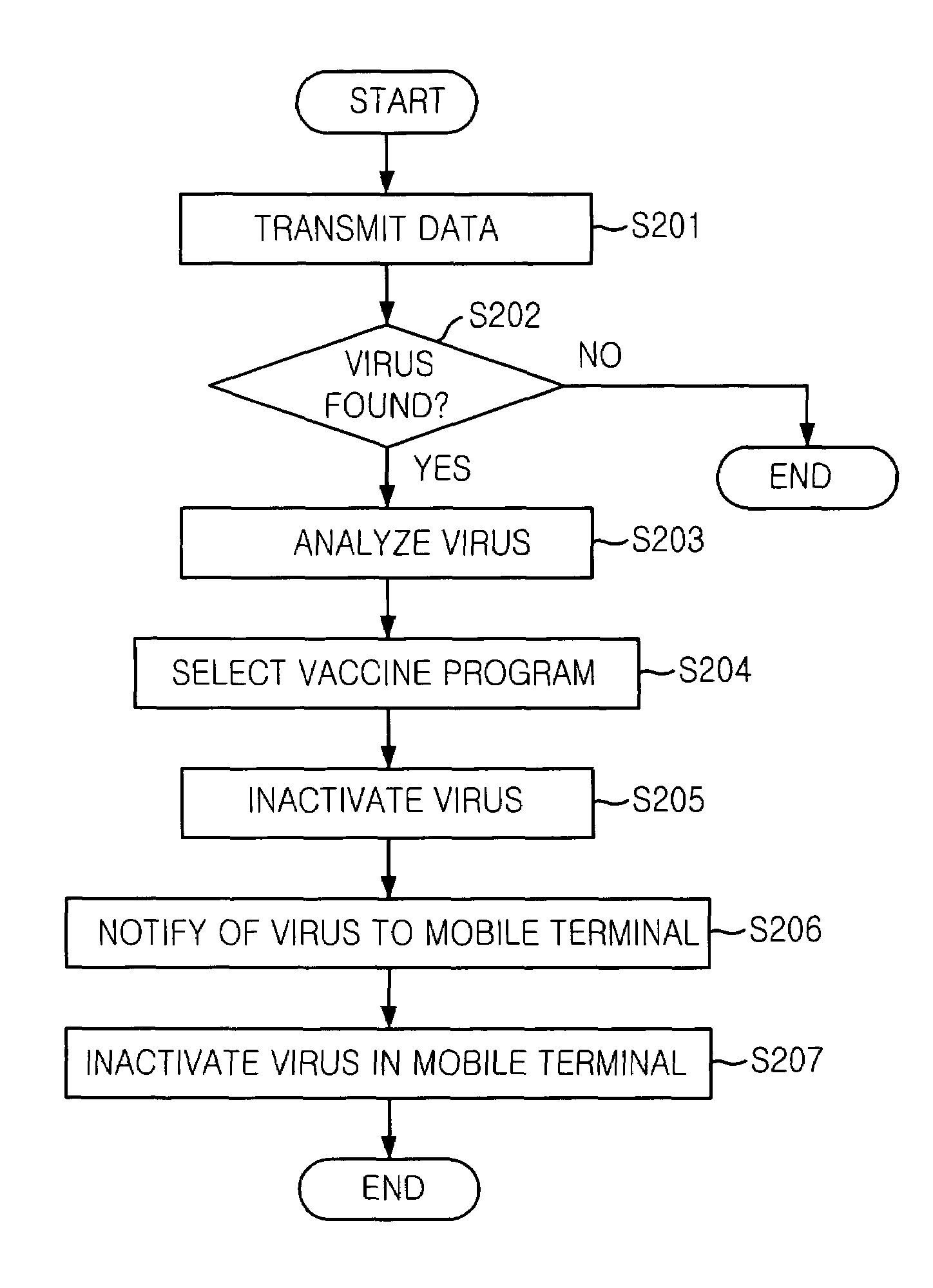
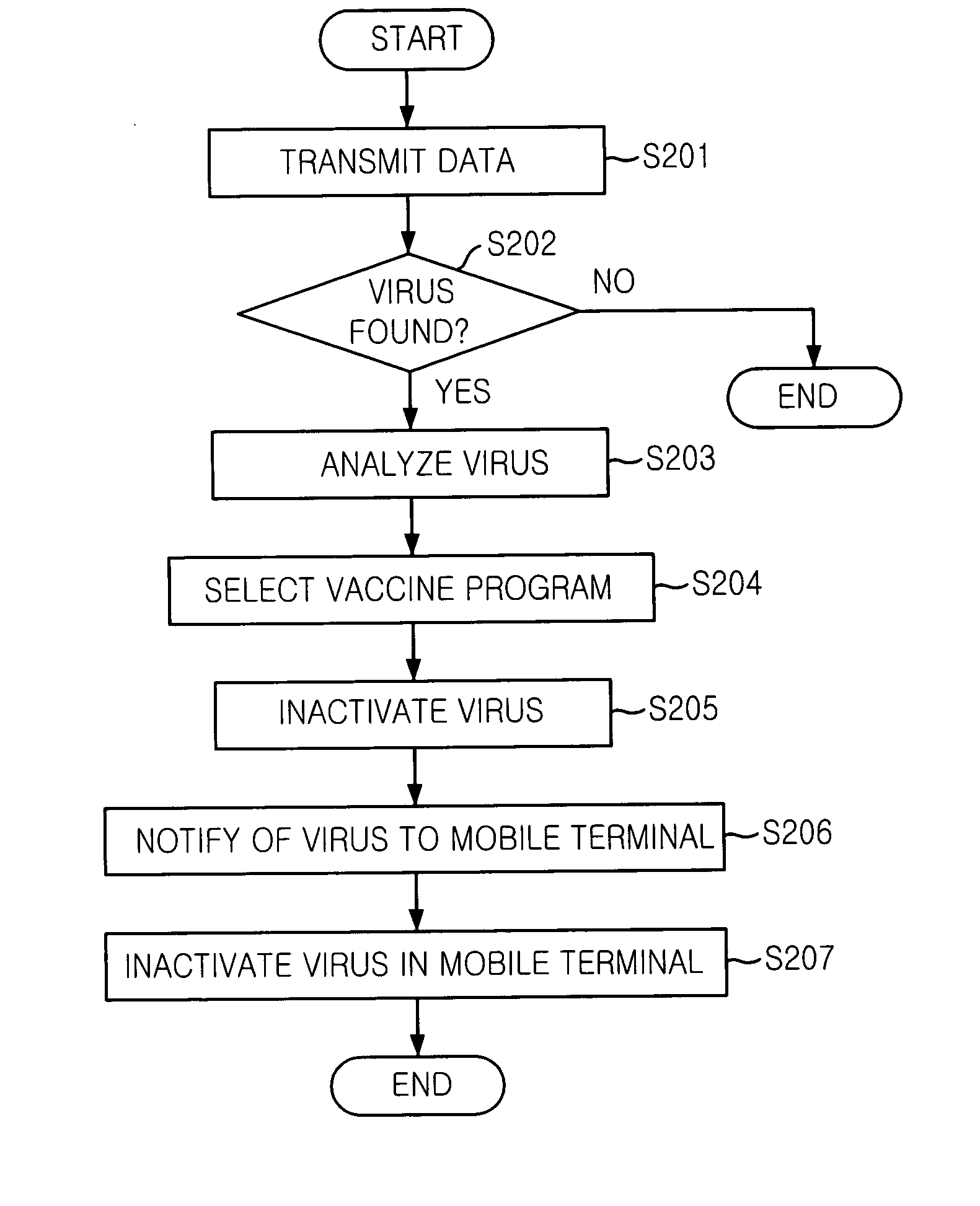
Mobile communication system and mobile terminal having function of inactivating mobile communication viruses, and method thereof
ActiveUS7386297B2Unauthorised/fraudulent call preventionMemory loss protectionMobile data terminalComputer terminal
The present invention provides a mobile communication system and method for inactivating or curing mobile communication viruses. The mobile communication system for inactivating a virus includes: a database associated with the mobile communication system, for storing at least one virus vaccine program; and a virus monitoring unit associated with the mobile communication system, for checking virus infection of received data, analyzing virus information, choosing one of virus vaccine programs that are stored in the database and inactivating the virus. Virus vaccine programs are timely updated over the air (OTA) whenever a new version of vaccine program is available.
Owner:PANTECH CORP
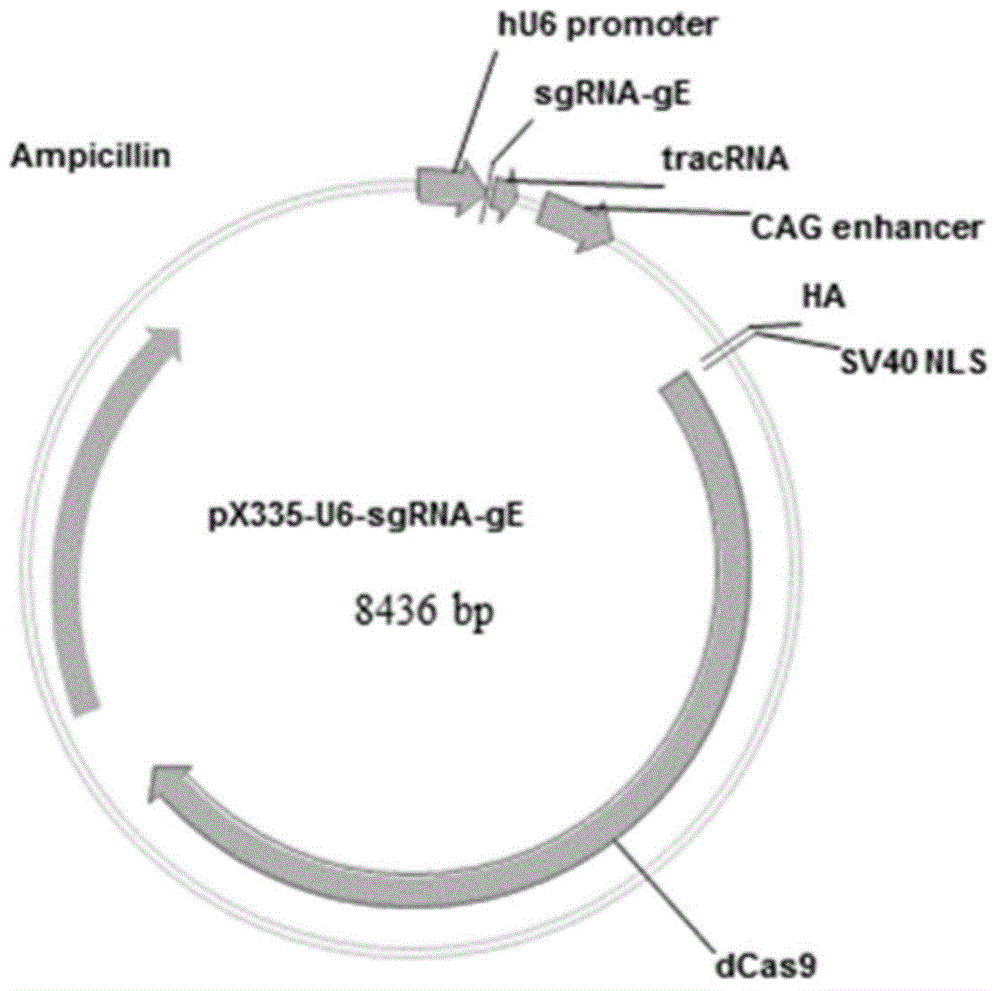
Method for preparing vaccine by editing pseudorabies virus genomes based on CRISPR/Cas9 and Cre/lox systems and application of method
ActiveCN104894075AReduce disease lossEasy to operateAntiviralsViruses/bacteriophagesMCherry fluorescent proteinBiology
The invention discloses a method for quickly preparing a vaccine by editing pseudorabies virus genomes based on a CRISPR / Cas9 gene editing system and a Cre / lox recombination system and an application of the method. According to the method, the CRISPR / Cas9 gene editing system is used for synchronously and efficiently recombining a GFP gene and a mCherry gene to a pseudorabies virus gE gene site and a TK gene site respectively to obtain conditional deletion strains of a gE gene and a TK gene; after purification, the Cre / lox system is used for cutting off extraneous GFP and mCherry genes in the pseudorabies virus recombinant virus genome so as to perform purification quickly to obtain a live pseudorabies virus vaccine lack of gE / TK genes; multiple genes are operated at the same time, so that multiple rounds of flows for knocking out multiple genes in the conventional method are reduced to one round; and meanwhile, the efficient edition of virus genes by using the CRISPR / Cas9 and Cre / lox systems simplifies about thirty generations of plaque purification processes into 3-4 generations, so that the preparation efficiency of the virus vaccine is greatly improved, and the method provides a strong guarantee for effectively preventing and controlling the larger-range popularization of variant pseudorabies viruses and reducing heavy economic losses.
Owner:武汉都为康生物科技有限公司
Stabilizing Excipient for Inactivated Whole Virus Vaccine
The invention relates to a vaccine composition comprising:a) inactivated whole virus, andb) a stabilizing excipient which comprises:i. a buffer solution,ii. a mixture of essential and nonessential amino acids,iii. a disaccharide,iv. a polyol,v. a chelating agent,vi. urea or a urea derivative, andvii. a nonionic surfactant.
Owner:SANOFI PASTEUR SA
Modified poxviruses, including modified smallpox virus vaccine based on recombinant drug-sensitive vaccinia virus, and new selection methods
The present invention provides recombinant poxviruses, such as vaccinia virus, that contain an integrated exogenous sequence, such as a foreign gene, encoding a prodrug converting polypeptide that can convert a prodrug to a drug that prevents virus replication or is otherwise toxic to the virus. The recombinant poxviruses can be suitable for use as vaccines. The invention also provides, among other things, methods of inhibiting virus replication, methods of vaccination and methods of treating vaccinated subjects showing signs or otherwise at risk for of vaccination-induced disease.
Owner:BAXTER HEALTHCARE SA +1
Methods and Compositions for Stabilization of a Virus Vaccine
InactiveUS20110243988A1Improve stabilityReduce pressureBacterial antigen ingredientsViral antigen ingredientsRoom temperatureAttenuated vaccine
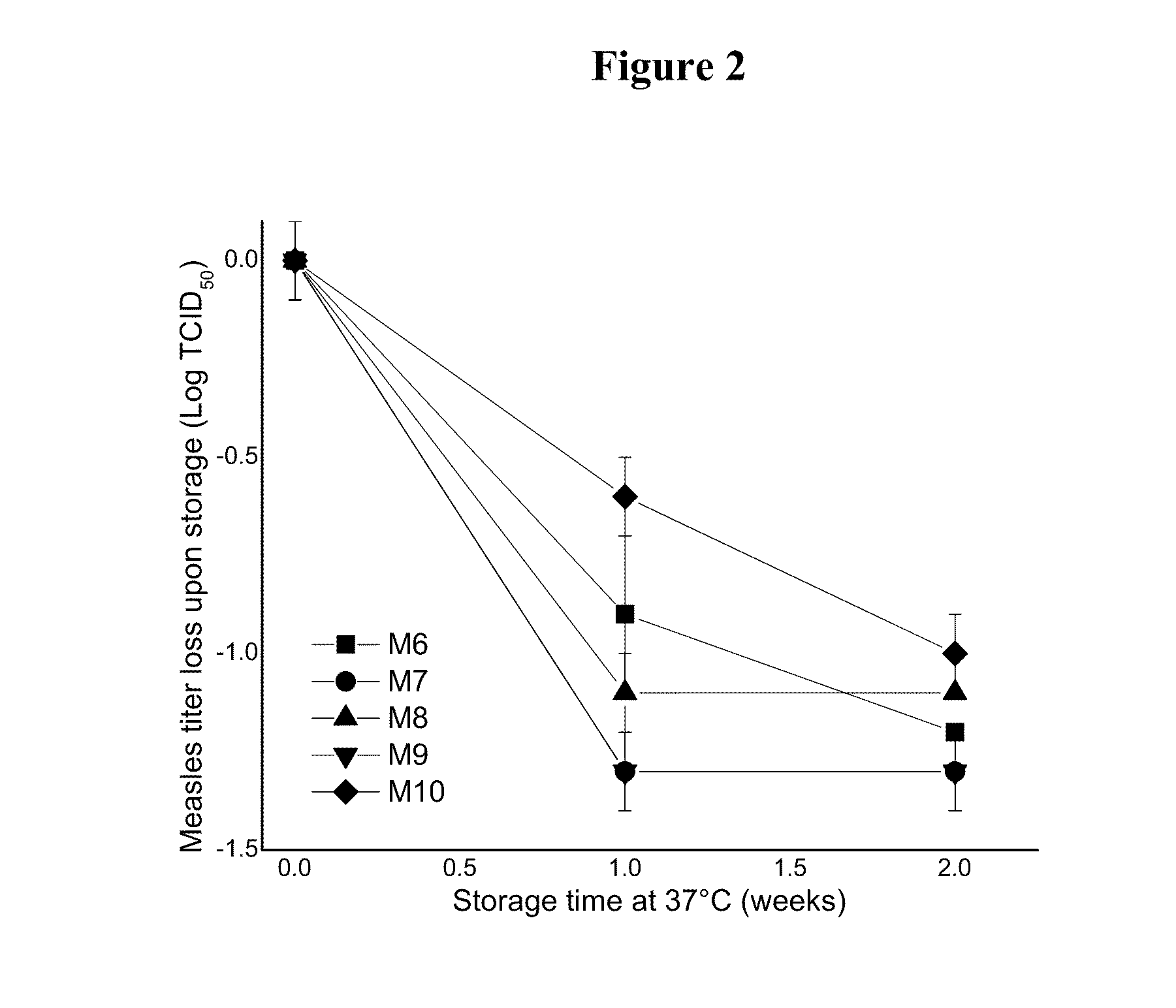
This invention provides compositions and methods for stabilizing a live attenuated virus in dried formulations. In particular, compositions and methods of preparing a dried vaccine are provided that stabilize the viability of live vaccines such as measles and adenovirus at room temperature.
Owner:ARIDIS PHARMA INC
Cross-protective influenza vaccine
InactiveUS20120052082A1Broad and improved cross protectionSsRNA viruses negative-senseViral antigen ingredientsMultiple copyVirosome
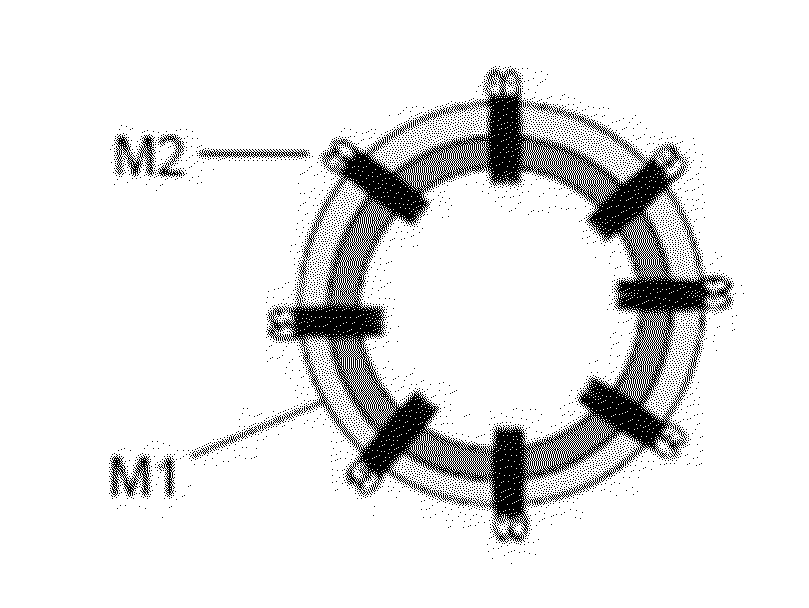
A cross-protective influenza virus vaccine has been designed based on the incorporation of the genetically engineered, highly conserved M2 influenza viral protein optionally in combination with an adjuvant such as a bacterial flagellin protein incorporated into the membrane of a virosome or virus-like particles. Immunogenicity and the breadth of cross protection efficacy are significantly enhanced using multiple copies of the influenza M2 protein as a membrane bound tetramer and / or in combination with a membrane bound adjuvant. A method for vaccinating a subject for influenza A has also been developed that results in broad and improved cross-protection against multiple subtypes of influenza A virus.
Owner:ZETRA BIOLOGICALS
Mobile communication system and mobile terminal having function of inactivating mobile communication viruses, and method thereof
ActiveUS20040127195A1Memory loss protectionUnauthorised/fraudulent call preventionComputer terminalMobile communication systems
The present invention provides a mobile communication system and method for inactivating or curing mobile communication viruses. The mobile communication system for inactivating a virus includes: a database associated with the mobile communication system, for storing at least one virus vaccine program; and a virus monitoring unit associated with the mobile communication system, for checking virus infection of received data, analyzing virus information, choosing one of virus vaccine programs that are stored in the database and inactivating the virus. Virus vaccine programs are timely updated over the air (OTA) whenever a new version of vaccine program is available.
Owner:PANTECH CORP
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6083925AGood immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
Recombinant mva strains as potential vaccines against p. falciparum malaria
This invention relates to recombinant viruses based on MVA, which comprise at least one nucleic acid coding for a Plasmodium falciparum MSP-1 protein, a fragment or mutein of it. Furthermore, methods for the production of the recombinant viruses, virus-containing vaccines and the use of the recombinant viruses for the prophylaxis and / or therapy of malaria are provided.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT +1
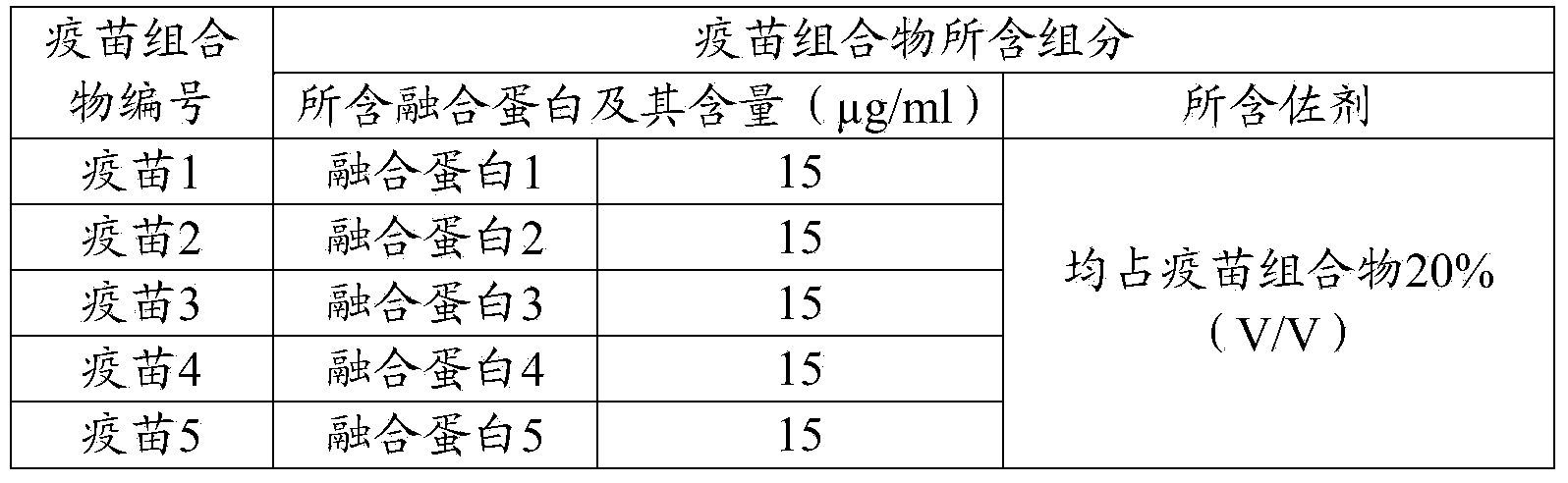
Preparation and application of fusion protein and vaccine composition thereof
ActiveCN104262488AShort timeEase of mass productionAntiviralsPharmaceutical non-active ingredientsDiseaseImmunoglobulin Fc Fragments
The invention provides a fusion protein, a porcine epidemic diarrhea vaccine composition containing the fusion protein and application thereof. The fusion protein contains porcine epidemic diarrhea virus antigenic protein and immunoglobulin Fc segment, wherein the porcine epidemic diarrhea virus antigenic protein contains a protein formed by series combination of porcine epidemic diarrhea virus S protein segments. The invention also provides a porcine epidemic virus vaccine composition which contains the fusion protein and a carrier. The invention also provides a preparation method of the vaccine composition and application of the vaccine composition in preparing drugs for preventing and / or treating diseases initiated by porcine epidemic diarrhea virus. The vaccine composition prepared from the fusion protein avoids the technical problem that the porcine epidemic diarrhea virus totivirus can not be easily separated and cultured in the traditional vaccine inactivation process. The fusion protein can utilize the gene engineering technique to perform abundant recombinant expressions, has the advantage of short time consumption, and is convenient for large-scale production.
Owner:PU LIKE BIO ENG
SARS-CoV virus structural protein infusion protein and its high yield expression and purification and uses
InactiveCN1884303ASsRNA viruses positive-senseAntibody mimetics/scaffoldsProtein tagStructural protein
The invention relates to fusion protein of SARS-Cov virus structural protein and its high expression and purification in mammalian cell and its usage. The construction of said fusion protein is X-Y-z, and X is S or M or E or N selected from SARS-CoV virus construction protein, or their arbitrary short form; Y is the connection part with 0-20 amino acid; Z is Fc and its modification or other protein tag. The invention also provides the method of expressing and purifying said fusion protein in mammalian cell for the batch preparation or industrial production. The fusion protein can be used to prepare genetic engineering vaccine preventing SARS-CoV virus infection, solvent box for checking SARS-CoV virus, and to sift drug anti SARS-CoV virus infection with S protein combined with its acceptor ACE2. The invention can detect combination of S protein of SARS-CoV virus with ACE2, which reduces ACE2 expression, and result in or exacerbate acute lung damage, then it modifies combined segment, which can improve safety of preventing SARS-CoV virus vaccine.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Detection reagent and method for identifying porcine pseudorabies virus vaccine strain and wild strain
InactiveCN104561374AHigh detection sensitivityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseVirology
The invention provides a detection reagent and a method for identifying a porcine pseudorabies virus vaccine strains and a wild strain, and belongs to the field of animal pathogen detection. The detection reagent comprises two primer pairs as shown in SEQ ID NO.1-4 and probes as shown in SEQ ID NO.5-6. The invention further provides a method for identifying a porcine pseudorabies virus vaccine strain gD gene and a wild strain gE gene by using the detection reagent. The porcine pseudorabies virus vaccine strains and the wild strain can be simultaneously identified through dual fluorogenic quantitative PCR, high-flux detection on large-scale samples can be achieved, the detection reagent has the characteristics of rapidness, specificity, sensitivity, accuracy, simplicity and convenience, provides an effective tool for surveying porcine pseudorabies infection sources and tracing infection environments and disease sources, and has relatively good application value in prevention and control on porcine pseudorabies.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Porcine pseudorabies virus vaccine composition and preparation method and application thereof
ActiveCN104248757AShort timeEase of mass productionAntiviralsAntibody medical ingredientsDiseaseImmune effects
The invention provides a porcine pseudorabies virus vaccine composition. The porcine pseudorabies virus vaccine composition contains a porcine pseudorabies virus subunit antigen, or a recombinant Newcastle disease virus, namely a porcine pseudorabies virus vector. The invention further provides the preparation method and application of the vaccine composition. The vaccine composition can effectively prevent related diseases of a porcine pseudorabies virus and related diseases infected by the porcine pseudorabies virus. The combination of immunogenicity antigens in the porcine pseudorabies virus vaccine composition can be induced to generate a synergetic immune effect, thereby having good immune effect, and further lowering the immune usage amount to lower the immunization cost.
Owner:PU LIKE BIO ENG
O type foot-and-mouth disease virus variant as well as coding gene and application thereof
The invention discloses an O type foot-and-mouth disease virus variant as well as a coding gene and application thereof. In the invention, an O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 is firstly separated out, the nucleotide sequence of the O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 SEQ ID NO: 1, and the amino acid sequence is SEQ ID NO: 2. In comparison with a VP1 amino acid sequence, the virus strain has 7 variable sites, five of which are centralized in a G-H ring. Mutation of the sites ensures that the virus variant has the capability of escaping from host immunity so as to have the superiority for becoming a popular virus strain. Therefore, the variant can be employed to prepare an inactivated vaccine for prevention and treatment of the variant and relevant strains, dominant antigen epitope of the variant can be employed to prepare a synthetic peptide vaccine, and the variant can be employed to develop novel O type foot-and-mouth disease virus vaccines such as VLP vaccine and the like. Therefore, the invention has important value in controlling the popularity of O / YS / CHA / 05 and relevant variable strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Combined vaccines for prevention of porcine virus infections
ActiveUS20140093535A1SsRNA viruses positive-senseViral antigen ingredientsProtection sexProtective immunity
The present disclosure provides vaccine compositions comprising a PRRSV vaccine and a second porcine vaccine, which are substantially free from immuno-inhibition against each other. The second porcine virus vaccine can be CSFV and / or PRV. The preparation methods for the vaccines and the formulations are also provided. The vaccine compositions provided herein confer protective immunity to pigs against porcine reproductive and respiratory syndrome, classical swine fever, and / or pseudorabies.
Owner:SINOVET JIANGSU BIOPHARM CO LTD
Virus Vaccines Comprising Envelope-Bound Immunomodulatory Proteins and Methods of Use Thereof
InactiveUS20090214590A1Minimal toxicityMinimize potential damageSsRNA viruses negative-senseViral antigen ingredientsAdjuvantCytokine
The present invention provides novel virus vaccines with augmented, e.g., enhanced and / or extended immunogenicity. The virus vaccines of the invention comprise an envelope-bound immunomodulatory protein, e.g., a cytokine, chemokine or costimulatory molecule. The immunomodulatory protein serves as an adjuvant to augment, e.g., enhance or extend the immunogenicity of the virus vaccine, thereby augmenting, e.g., enhancing or extending immune response to the virus when administered to a subject.
Owner:WAYNE STATE UNIV
Genetically engineered swine influenza virus and uses thereof
ActiveUS8124101B2Reduce capacityLow toxicitySsRNA viruses negative-senseViral antigen ingredientsUltrasound attenuationGene product
The present invention relates, in general, to attenuated swine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated swine influenza viruses having modifications to a swine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +2
Development of dengue virus vaccine components
The invention is related to a dengue virus or chimeric dengue virus that contains a mutation in the 3′ untranslated region (3′-UTR) comprising a Δ30 mutation that removes the TL-2 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR that removes sequence in the 5′ direction as far as the 5′ boundary of the TL-3 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, or a replacement of the 3′-UTR of a dengue virus of a first serotype with the 3′-UTR of a dengue virus of a second serotype, optionally containing the Δ30 mutation and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR; and immunogenic compositions, methods of inducing an immune response, and methods of producing a dengue virus or chimeric dengue virus.
Owner:UNITED STATES OF AMERICA
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Liquid stable virus vaccines
ActiveUS20140056942A1Prevent diseaseOvercome deficienciesSsRNA viruses negative-senseViral antigen ingredientsSugarAmino acid
The present invention discloses liquid stable vaccines that comprise a live attenuated virus, 10-30% sugar additive, and an amino acid. The present invention also discloses the manufacture of such vaccines and methods of protecting an animal by administration of such vaccines.
Owner:INTERVET INC
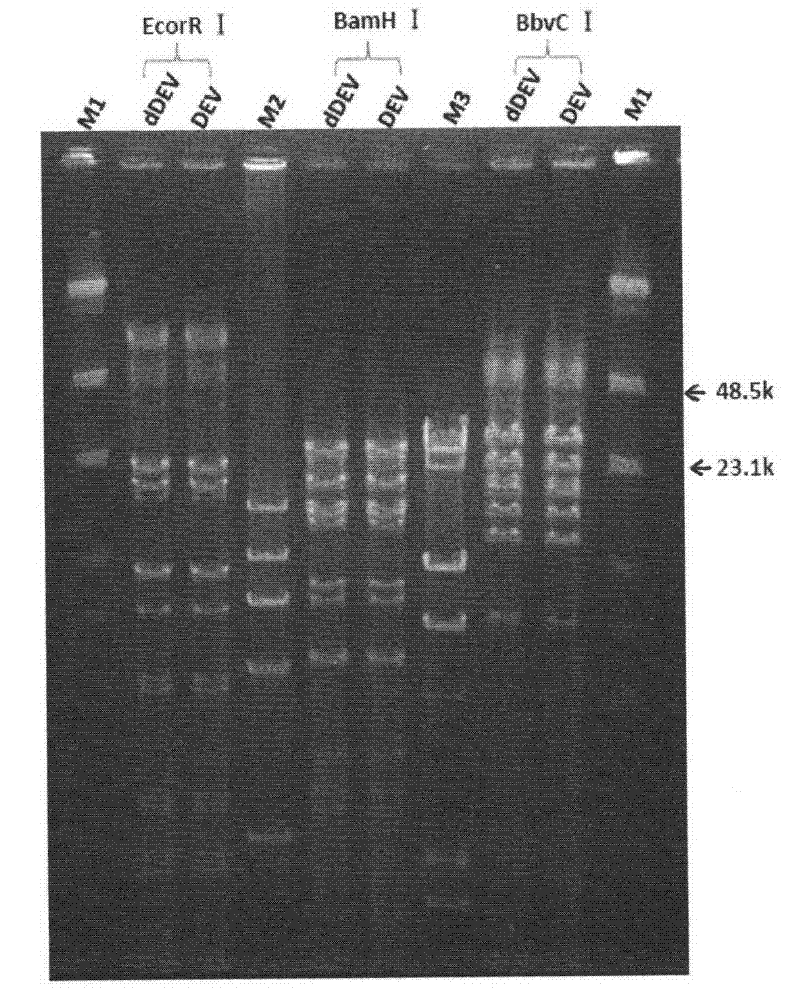
Recombinant duck virus enteritis virus vaccine strain (rDEVul41HA) for expressing avian influenza virus hemagglutinin (HA) genes and construction method as well as application thereof
The invention provides a recombinant duck virus enteritis virus vaccine strain CCTCC No.V201026, named as rDEVul41HA, for expressing avian influenza virus hemagglutinin (HA) genes and a construction method as well as application thereof. Specifically, gene segments SV40-HA containing avian influenza virus hemagglutinin HA genes and a SV40 promoter sequence are inserted into UL41 genes of duck virus enteritis virus to construct cosmids with SV40-HA expression cassette inserted into UL41 genes; and by the cosmids, the recombinant duck virus enteritis virus vaccine strain CCTCC V201026 for expressing the avian influenza virus hemagglutinin (HA) genes is saved and obtained. The invention also relates to a method for constructing the recombinant duck virus enteritis virus vaccine strain and application of the recombinant duck virus enteritis virus vaccine strain to preparing vaccines for preventing duck virus enteritis and avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Polynucleotide herpes virus vaccine
InactiveUS7094767B2Avoid infectionAmeliorate HSV-related diseaseSugar derivativesGenetic material ingredientsMammalSeroconversion
Genes encoding herpes simplex virus type 2 (HSV-2) proteins were cloned into eukaryotic expression vectors to express the encoded proteins in mammalian muscle cells in vivo. Animals were immunized by injection of these DNA constructs, termed polynucleotide vaccines or PNV, into their muscles. In a DNA titration, it was found that a single immunization of ≧0.5 μg of (one) PNV, gave >90% seroconversion by ten weeks post immunization. Immune antisera neutralized both HSV-2 and HSV-1 in cell culture. When animals were challenged with HSV-2, significant (p<0.001) protection from lethal infection was achieved following PNV vaccination. DNA constructs may be full-length, truncated and / or mutated forms and may be delivered along or in combination in order to optimize immunization and protection from HSV infection.
Owner:MERCK SHARP & DOHME CORP
Recombination newcastle disease LaSota weak virus vaccine for expressing poultry influenza virus H5 sub type HA protein
ActiveCN1869234AAvoid Biosafety HazardsSsRNA viruses negative-senseViral antigen ingredientsFowlWild type
The invention relates to reconstructing LaSota weak poison vaccine strain to express wild type or mutant type fowl influenza virus H5 HA albumen. Concretely, it is rL-QHwH5 and rL-QHmH5. The invention also discloses the method to make the LaSota weak poison vaccine and the application in defending fowl influenza virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Respiratory syncytial virus sub-units vaccine, preparation and application
InactiveCN101264323ALimit developmentEnhance cellular immune responseDepsipeptidesAntiinfectivesEscherichia coliCtl epitope
The invention relates to a respiratory syncytial virus vaccine, and the preparation method and application, in particular to an application of escherichia coli to express and recombinant respiratory syncytial virus G protein and mutation G protein, and preparation vaccine with nontoxic typed escherichia coli heat labile enterotoxin, for human or animal preventive inoculation, and for respiratory syncytial virus resistance, belonging to the field of biotechnology. The respiratory syncytial virus vaccine is characterized in that: the respiratory syncytial virus subunits vaccine comprises lopped respiratory syncytial virus RSV protein G, and further comprises nontoxic typed escherichia coli heat labile enterotoxin LT adjuvant. The vaccines are lopped respiratory syncytial virus RSV protein G containing amino acid between aa130 and 230 of the original G protein, and substitutes the amino acid CAWIC (CX3C module ordered) between aa182 and 186 with the amino acid YLEKESIYY (CTL epitope) on the RSV M protein, forming the GCIL protein. The respiratory syncytial virus vaccine has the advantages of remarkable practical significance for preventing human respiratory syncytial virus infection.
Owner:KUNMING UNIV OF SCI & TECH
Method of bioreactor micro-carrier for cultivating human diploid cell to produce viral vaccine
InactiveCN102559617AIncrease productionImprove consistencyImmobilised enzymesInactivation/attenuationBiotechnologyCell growth
The utility model provides a method of bioreactor micro-carrier for cultivating human diploid cell to produce viral vaccine. The method comprises the following steps: reviving human diploid cell; amplifying the culture of the human diploid cell and preparing the cell suspension; inoculating the obtained cell suspension to the bioreactor, fresh complete cell growth culture solution for sterilizing and micro-carrier are pre-added into the micro-carrier, the final density of the inoculated cell is 5*104-2*106 cell / ml, the monolayer cell of the micro-carrier is cultivated under the conditions that the temperature is 35 to 39 degrees, pH is 6.5 to 8.0, and the dissolved oxygen is 15 to 100%, and the cell density amounts to more than 1*106 cell / ml; and inoculating, breeding and harvesting virus. According to the method, utilizing the bioreactor for cultivating the human diploid cell to produce the virus vaccine in large scale becomes possible, the produced virus vaccine has high output and consistency, and the large-scale industrial production for the virus vaccine is effectively realized.
Owner:BEIJING SKYWING TECH CO LTD
Food grade lactic acid bacteria active carrier Group A rotavirus vaccine and preparation method thereof
InactiveCN103656633AWill not spreadNo horizontal transferViral antigen ingredientsDigestive systemEscherichia coliSerotype
The invention discloses food grade lactic acid bacteria active carrier Group A rotavirus vaccine and a preparation method thereof. The food grade lactic acid bacteria active carrier Group A rotavirus vaccine is characterized in that VP6 antigen protein from Group A virus, common serotype VP7 antigen protein (P serotype) and VP4 antigen protein (G serotype-expressed separately in the form of VP5* and VP8* protein subunit), vaccine adjuvant escherichia coli thermal unstable toxin B (LTB) and cholera toxin subunit B (CTB) are expressed and secreted by thyA gene deletion lactic acid bacteria cell or shown by the cell wall. Expressions of antigen protein and vaccine adjuvant protein are controlled by inducible or constitutive promoter, protein expression cassette is integrated onto the chromosome of the expression host lactic acid bacteria strain, and external antibiotics resistance gene introduced in gene manipulation is removed. The lactic acid bacteria active carrier rotavirus vaccine has the advantages of having wide serotype covering range, being easy to produce in large scale, and being safe and convenient to use without a refrigerator and a needle tubing.
Owner:刘占良 +2
Recombined new castle disease virus vaccine strain for expressing African swine fever virus p72 proteins
ActiveCN104962581AImprove protectionEffective protectionViral antigen ingredientsMicroorganism based processesDiseaseNewcastle disease virus NDV
The invention provides a preparation method of a recombined new castle disease virus vaccine strain which expresses African swine fever virus p72 proteins and a recombined new castle disease virus vaccine strain. The method provided by the invention comprises the following steps: constructing a recombinant transcriptional plasmid which is inserted with a p72 gene of African swine fever virus (ASFV); constructing a transcriptional helper plasmid system; carrying out a contransfection for the transcriptional plasmids and the transcriptional helper plasmids into host cells BHK-21 which can be duplicated in new castle disease attenuate strains; and saving and obtaining the recombined virus stain. The vaccine strain for expressing the African swine fever virus p72 proteins provided by the invention has important preservation and strategy meaning in the aspect of animal epidemic disease prevention and control, and can be applied to the treatment and prevention of African swine fever virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing Vero cell influenza virus vaccine
ActiveCN102078605ASimple purification processQuality standardsAntiviralsAntibody medical ingredientsInfluenza virus vaccineIon exchange
The invention provides a method for preparing a Vero cell influenza virus vaccine. Seasonal trivalent influenza vaccines and pandemic influenza storage vaccines with qualified quality can be prepared by improving a vaccine purification process, combining hydrophobic chromatography with ion exchange and auxiliarily adopting other purification methods.
Owner:吉林亚泰生物药业股份有限公司
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
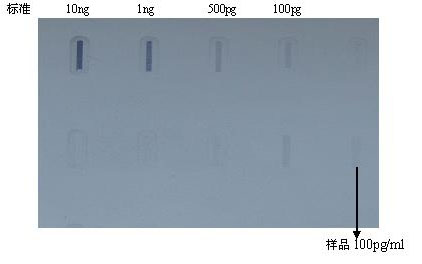
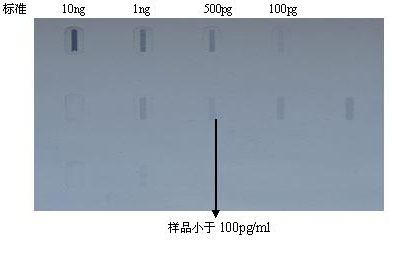
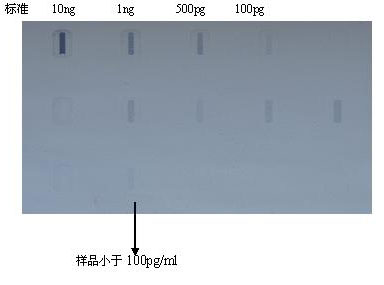
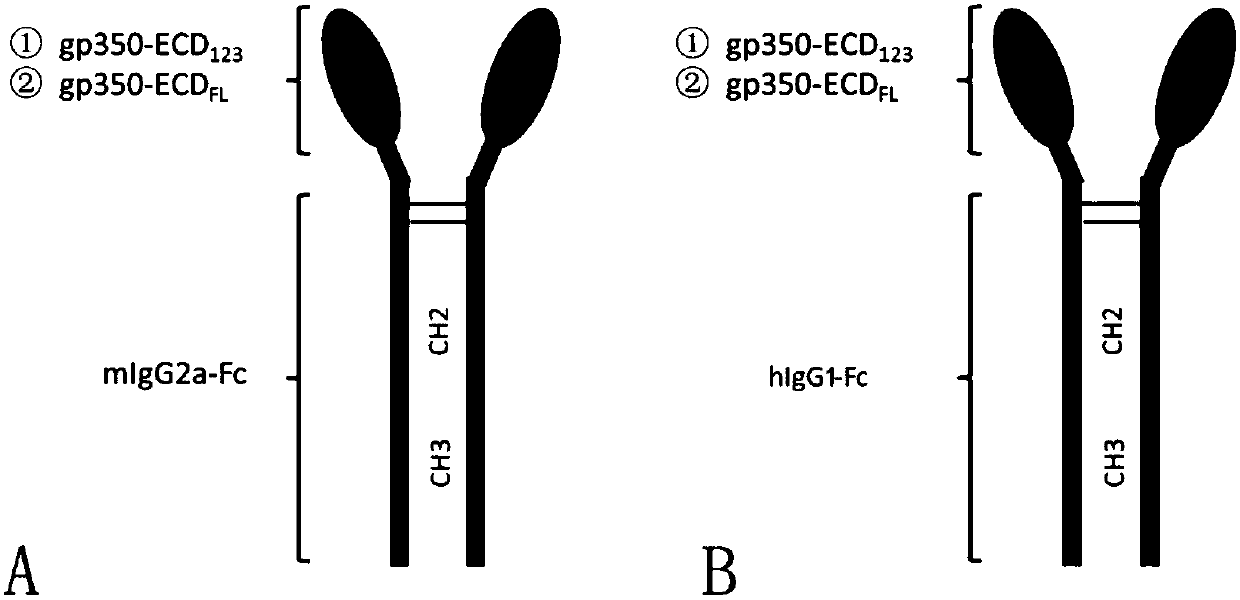
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
Genetically engineered equine influenza virus and uses thereof
ActiveUS8137676B2Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsVirus influenzaIn vivo
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com