Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
377 results about "Vero cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vero cells are a lineage of cells used in cell cultures. The 'Vero' lineage was isolated from kidney epithelial cells extracted from an African green monkey (Chlorocebus sp.; formerly called Cercopithecus aethiops, this group of monkeys has been split into several different species). The lineage was developed on 27 March 1962, by Yasumura and Kawakita at the Chiba University in Chiba, Japan. The original cell line was named "Vero" after an abbreviation of verda reno, which means "green kidney" in Esperanto, while vero itself means "truth" in Esperanto.
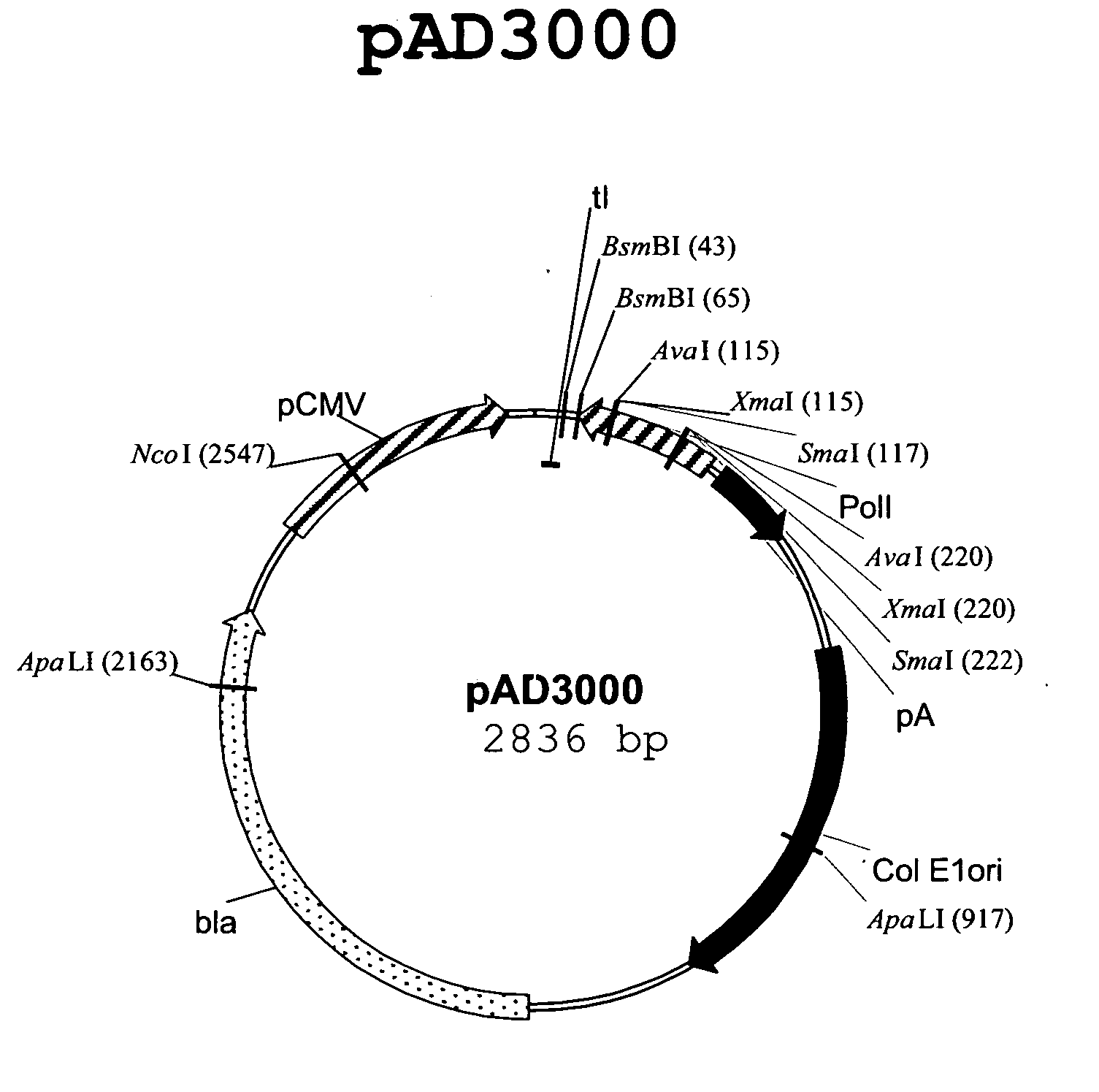
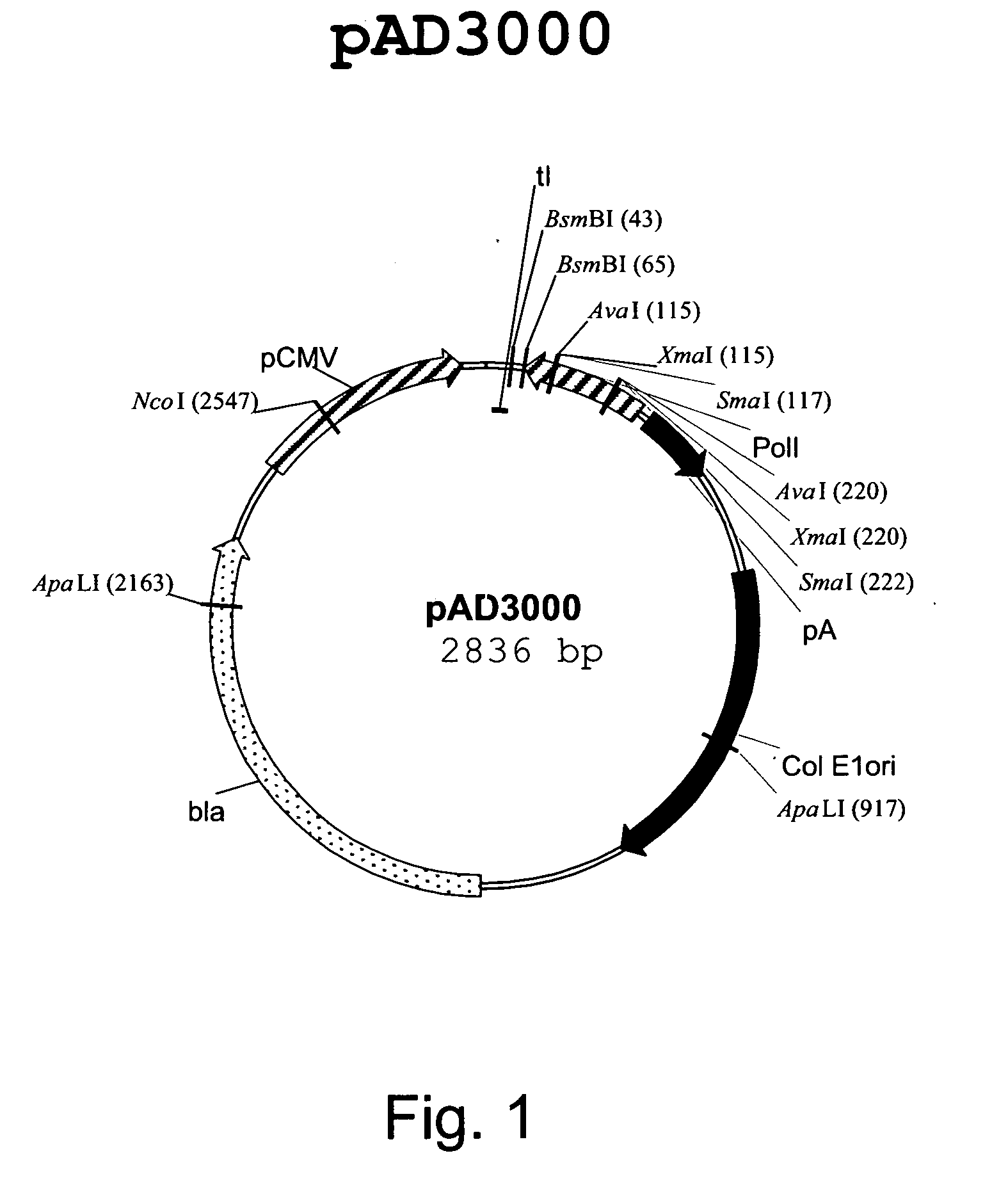
Multi plasmid system for the production of influenza virus
InactiveUS20050158342A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggEukaryotic plasmids
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. In addition, the present invention includes an improved method of rescue, wherein animal cells (e.g., SF Vero cells) are electroporated with plasmids and vectors of the invention.
Owner:MEDIMMUNE LLC
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
Culture medium used for Vero cell and cultivation method thereof
InactiveCN101182490AFast growthArtificial cell constructsVertebrate cellsSerum free mediaMicrobiology
The invention relates to the preparation and application method of a medium, which is specifically related to a medium which is used for Vero cell and a culture method of the Vero cell. The invention belongs to the biological product area. The culture method of the invention is that the adhering Vero cells are suspend and cultured in SFM serum-free medium for the domestication and adaptation; the growing pattern of the Vero cell changes from adherence growth to suspension growth; the Vero cells are further amplified in Vero amplification CHEMICALLY DEFINED MEDIUM. The method of the invention has good effects; the growing pattern of the Vero cell is transformed from adherence growth to suspension growth, which changes the growth pattern of the cell and greatly increases the yield of the cell.
Owner:天津百若克医药生物技术有限责任公司
Live attenuated influenza vaccine
InactiveUS7494659B2Simple and efficient processOvercomes drawbackSsRNA viruses negative-senseViral antigen ingredientsAntigenSerum free
The invention relates to a simple and efficient process for isolating viruses from various sources and for producing live attenuated influenza vaccines in a serum-free Vero cell culture under conditions where alterations in the surface antigens of the virus due to adpative selection are minimized or prevented. The process does not require purification of the virus-containing supernatant harvested from the cell culture nor post-incubation treatment of the viruses for HA activation. The invention further relates to influenza A and B master strain candidates and to vaccines made thereof.
Owner:POLYMUN SCI IMMUNBIOLOGISCHE FORSCHUNG
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Method for producing influenza vaccine in large-scale by using bioreactor
InactiveCN101062411AImprove infection abilityAmount of antigenBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenContinuous perfusion
The invention discloses a method to mass produce flu vaccine with bioreactor, which comprises the following steps: proceeding passage adaptation on Africa green monkey kidney cell (Vero cell) with influenza virus; intensifying intrinsic appeal of influenza virus for Vero cell; fitting for Vero cell quickly; breeding the Vero cell with cell density above 10. 7deka / ml in bioreactor; seeding the influenza virus into bioreactor; adjusting various controlling parameter in the reactor; making the virus high effective breed in the reactor; proceeding continuous perfusion; harvesting virus liquid; deactivating the virus; condensing; purifying; cracking; getting the flu vaccine. This invention possesses the advantages of high antigen quantity and stable quality.
Owner:江苏全益生物科技股份有限公司
Serum-free culture medium without animal origin components for culturing Vero cell micro-carrier
InactiveCN101864393APromote growthSame densityVertebrate cellsArtificial cell constructsSerotoninVitamin C
The invention discloses a serum-free culture medium without animal origin components for culturing a Vero cell micro-carrier. The serum-free culture medium consists of a DMEM / F12 (1:1) culture medium and culture medium additive components such as epidermal growth factors, insulin, serotonin, aurin tricar-boxylic acid, biotin, vitamin C, amino acid, fructose, trehalose, trace elements, hydroxypropyl-beta-cyclodextrin and the like. The serum-free culture medium has the following advantages that: (1) the attachment growth of Vero cells in a tissue culture vessel and on the surface of the micro-carrier can be supported, and the cells can be transferred from a serum culture medium to the serum-free culture medium without a course of adaptation; (2) the serum-free culture medium contains no animal origin components, and has basically definite chemical components, and a low cost; and (3) the cells grow well, and the cellular morphology, the density and the vitality are basically the same as those of the serum culture medium.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Method for production of porcine epidemic diarrhea virus
The invention discloses a technology for the production of porcine epidemic diarrhea virus by means of the microcarrier culture of VREO cells using a bioreactor, and comprises the technology for the production of different porcine epidemic diarrhea virus strains. The technology comprises the following technical steps: (1) selection of VERO cells as cell line for vaccine; (2) passage and culture of cells for vaccine; (3) propagation of seed culture of the porcine epidemic diarrhea virus; (4) microcarrier suspension culture of the VERO cells in the bioreactor; (5) propagation of porcine epidemic diarrhea virus antigen; and (6) treatment of acquired virus antigen liquid. The production method can remarkably lower production cost and enhance output-input ratio by 5 to 10 times, and has the advantages of short production period, small occupied space, great easiness for enlarging production scale rapidly, little environmental pollution, easy processing, high automation degree, a small number of staff, easy implementation of even and stable quality, obviously lowered production cost and enhanced yield and quality of vaccine.
Owner:成都史纪生物制药有限公司
Culture medium applicable to suspension and magnification cultivation of Vero cell microcarriers and method for suspension magnification cultivation of Vero cell microcarriers
ActiveCN102827804AIncrease productivityEasy to separate and purifyArtificial cell constructsVertebrate cellsBottleCulture mediums
The invention discloses a culture medium applicable to suspension and magnification cultivation of Vero cell microcarriers. The culture medium comprises amino acid, inorganic salt, vitamins, protein hydrolysate, lipid, buffer components and additives. The culture medium not only is applicable to static cultivation in a Vero square bottle and cultivation in a roller bottle, but also is supportive for suspension cultivation of the microcarriers and magnification cultivation of the microcarriers in a reactor, and can be used as a serum-free virus maintenance medium to realize serum-free virus production.
Owner:苏州沃美生物有限公司
Multi plasmid system for the production of influenza virus
ActiveUS20090208527A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggElectroporation
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. In addition, the present invention includes an improved method of rescue, wherein animal cells (e.g., SF Vero cells) are electroporated with plasmids and vectors of the invention.
Owner:MEDIMMUNE LLC
Mass-production method of hydrophobic vaccine
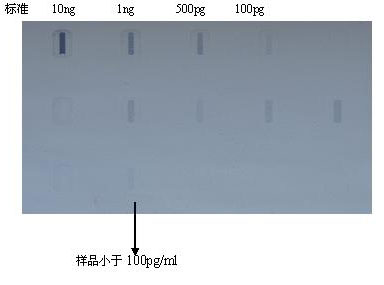
InactiveCN1966076AEasy to zoom inSmall pressure dropAntiviralsAntibody medical ingredientsUltrafiltrationFixed bed
The invention relates to a method for producing batch hydrophobia vaccine, wherein it comprises that: (1), in the biological reactor with fixed bed mixing system and Fibra-Cel disks carrier, using cell increment cultivate liquid to cultivate Vero cell; (2), when the Vero cell grows to some density, using cell hold cultivate liquid, seeding hydrophobia vaccine, and affecting cell; (3), increasing virus; (4), obtaining virus continuously; (5), ultra-filter concentrating and inactivating via beta-propanolide; (6), using protamine sulfate or DNA enzyme treatment to remove Vero cell DNA; (7), using Sepharose 4 Fast Flow as stuff to process chromatography purification; (8), adding human blood albumin and sugar as protector to be made into liquid agent; or adding human blood albumin, sugar, and gelatin, as protector and shaping agent to be made into dried agent. The inventive method can produce virus continuously in small biological reactor, to realize batch production.
Owner:广州市嘉合生物技术有限公司
African swine fever virus georgia strain adapted to efficiently grow in the vero cell line
We have developed an ASFV Georgia strain adapted to grow in Vero cell line. The resulting virus, ASF-GVAV, efficiently grows in Vero cells although it still is able to significantly replicate in primary cell cultures of swine macrophages. ASF-GVAV virus was successfully used as parental virus to develop several recombinant ASF viruses. The development of an ASFV adapted to grow in an established cell line is a significant advance for research and development of vaccine candidate strains using genetic manipulation based in the process of homologous recombination. The GVAVS can be utilized as a basis for large scale production of ASF vaccines.
Owner:US SEC AGRI +1
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS20110110978A1Enhanced influenza virus replicationHigh pHSsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Vero cell-based influenza virus strains and vaccines
InactiveUS20090074804A1Increase productionSsRNA viruses negative-senseVirus peptidesVaccine ProductionMammal
The present invention relates to isolated influenza virus strains suitable for increased vaccine production for mammals. The influenza virus strains contain at least one modified influenza protein that results in increased production of the influenza virus from a mammalian host cell, such as a vero cell. The present invention also relates to the vaccines produced from the influenza virus strains. The present invention further relates to isolated modified influenza proteins and isolated nucleic acid molecules that encode for the modified influenza proteins.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
High titer recombinant influenza viruses with enhanced replication in mdck or vero cells or eggs
ActiveUS20170354730A1Improve scalabilitySignificantly higher viral titersSsRNA viruses negative-senseAntiviralsDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Vero cell domestication method suitable for serum-free culture system and application of vero cell domestication method
ActiveCN104862270AObvious cytopathicObvious lesionsVertebrate cellsArtificial cell constructsSerum free mediaSerum free
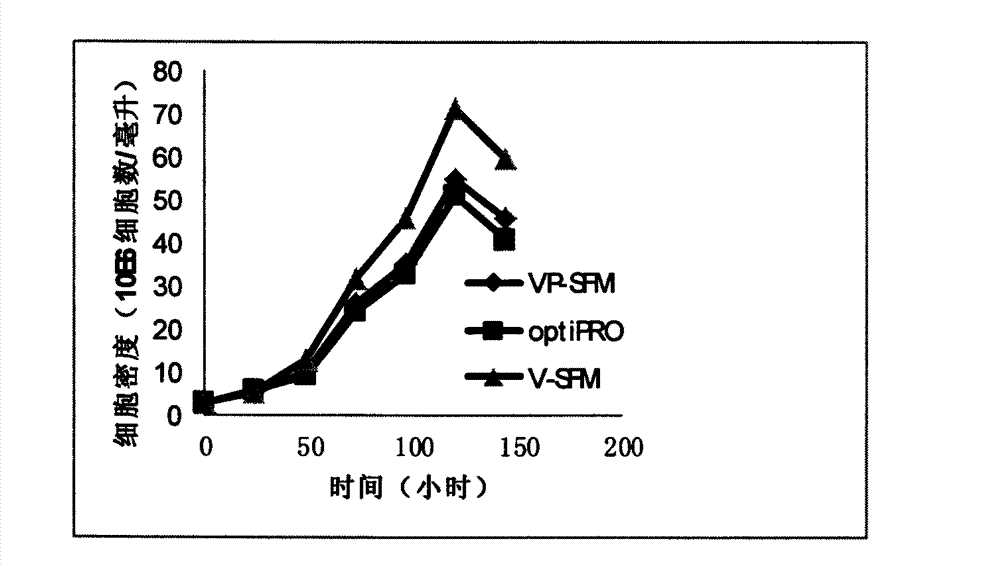
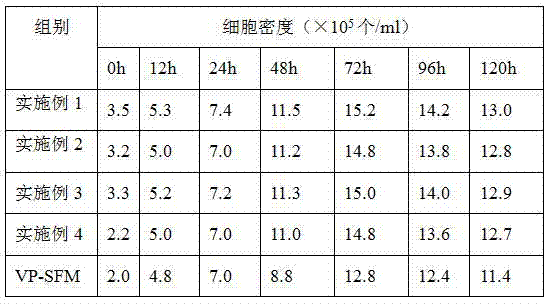
The invention discloses a vero cell domestication method suitable for a serum-free culture system. The vero cell domestication method comprises the following steps: reviving frozen vero cells; cultivating in a serum culture medium; and domesticating the vero cells by virtue of a domestication method of gradually reducing the serum content. The vero cells are domesticated and cultivated by virtue of the domestication method of gradually reducing the serum content, so that the vero cells can adapt to the serum-free culture environment; the vero cells which can adapt to the serum-free culture are obtained; and the domesticated vero cells can be continuously subcultured for at least 50 generations in a VP-SFM (serum-free medium).
Owner:GUANGDONG WENS DAHUANONG BIOTECH
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671AStable titerImproving immunogenicitySerum immunoglobulinsMicroorganism based processesSequence analysisImmunogenicity
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
Methods for purification of viruses
The present invention provides methods for the purification of cell-associated viruses from adherent cells (e.g., MDCK or Vero cells). In particular, the present invention provides purification methods for the production of immunogenic compositions comprising a live attenuated cell-associated virus (e.g., an attenuated respiratory syncytial virus (RSV) or cold-adapted, and / or temperature sensitive influenza virus) that result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
Cell line capable of stably expressing protein P54 of African swine fever virus as well as preparation and application of cell line
ActiveCN107937349AStrong specificityGood passage stabilityVirus peptidesAntiviralsSerum igeAfrican swine fever
The invention provides a Vero cell line capable of stably expressing protein P54 of an African swine fever virus. The collection number of the cell line is CGMCC No.14316. The cell line not only can identify a polyclonal antibody of African swine fever, but also can generate specific reaction with positive serum of an African swine fever-infected pig. The monoclonal Vero cell line of an African swine fever P4 antigen can be used for stably expressing the protein P54 of the African swine fever virus, and can be used as a cell model or a virus infection model; the cell transmission stability ishigh, the growing speed is high, and culture conditions are simple; a large number of cell lines can be provided; the cell line has a good application prospect for researches on detection and pathogenesis mechanisms of the African swine fever.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Pseudorabies virus and application of pseudorabies virus
ActiveCN104805060AImprove securityHigh protection rateInactivation/attenuationMicroorganism based processesPseudorabies Virus PRVGenetic engineering
The invention provides a pig pseudorabies virus and the application of the pig pseudorabies virus. The preservation number of the pig pseudorabies virus is CGMCC No.10266. The selected pig pseudorabies virus has the characteristics of high safety and high protection rate. A pig pseudorabies virus PRV-QD strain is inoculated with a Vero cell to form cell venom, the DNA (deoxyribonucleic acid) of the strain is extracted, the separated strain is subjected to deletions of virulence gene TK, gE and gI gene by a genetic engineering method, the virus is propagated after cell retrieval, and after a protecting agent is added into the virus, a vaccine is formed. The protection rate of the manufactured bacterin to piglets is 100 percent, the efficiency is obviously more excellent than that of a Bartha-K61 group (20 percent), and the vaccine has the advantages of high efficiency and high safety.
Owner:SHANDONG SINDER TECH
Porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of cell and attenuation via drug screening and application thereof
ActiveCN106282128AImprove securityImprove protection efficiencyViral antigen ingredientsMicroorganism based processesLarge fragmentVariant strain
The invention discloses a porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and an application thereof. The attenuated vaccine strain is prepared through the following steps: based on a porcine pseudorabies virus variant strain (named as strain HeN1, of which the microbial preservation serial No. is CGMCC No. 6656), firstly, carrying out low-temperature passage and screening on a Vero cell to obtain large fragments of deleted viruses including gI, gE, Us9, Us2 and part of inverted repeated sequence which exist in zone US through, and then making the TK gene thereof partially deleted through drug screening. The gene-deleted attenuated vaccine strain is named as strain PRV TP, of which the microbial preservation serial No. is CGMCC No. 12300. A live vaccine or an inactivated vaccine (a single vaccine or combined vaccine) can be prepared from the attenuated vaccine strain disclosed by the invention, and can prevent porcine pseudorabies effectively, and a reagent for diagnosing or treating porcine pseudorabies can be prepared from the attenuated vaccine strain too. According to the porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and the application thereof, the porcine pseudorabies attenuated vaccine strain PRV TP has the advantages of good safety, efficient protection, convenient differential diagnosis and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS9109013B2Improve scalabilityEasy to copySsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Method for preparing rotavirus vaccine stock solution by using serum-free Vero cells and serum-free rotavirus vaccine product
InactiveCN106676076ARemove inhibitionHigh titerViral antigen ingredientsMicroorganism based processesRotavirus RNAFiltration
The invention provides a method for preparing a rotavirus vaccine stock solution by using serum-free Vero cells. The method comprises the following steps: culturing Vero cells by using a serum-free culture medium, thus obtaining serum-free culture medium adapted cell strains; establishing a serum-free Vero cell seed bank by utilizing the obtained serum-free culture medium adapted cell strains; establishing a serum-free rotavirus strain working seed bank by utilizing the obtained serum-free culture medium adapted cell strains; carrying out reviving, culturing, passage and amplification on cells in a Vero cell working seed bank by utilizing the serum-free culture medium, using the cells in the Vero cell working seed bank as basic cells cultured in a bioreactor, and carrying out continuous perfusion culture on high-density Vero cells by applying the bioreactor and a microcarrier and using the serum-free culture medium after cell amplification; after inoculating virus seeds in the rotavirus strain working seed bank, carrying out bioreactor-microcarrier serum-free culture, obtaining a virus solution when virus is amplified to the summit, obtaining liquid virus titer, and carrying out clarification and ultra-filtration concentration, thus obtaining a serum-free rotavirus stock solution for human.
Owner:AB&B BIO TECH CO LTD JS
Method for preparing Vero cell influenza virus vaccine
ActiveCN102078605ASimple purification processQuality standardsAntiviralsAntibody medical ingredientsInfluenza virus vaccineIon exchange
The invention provides a method for preparing a Vero cell influenza virus vaccine. Seasonal trivalent influenza vaccines and pandemic influenza storage vaccines with qualified quality can be prepared by improving a vaccine purification process, combining hydrophobic chromatography with ion exchange and auxiliarily adopting other purification methods.
Owner:吉林亚泰生物药业股份有限公司
Serum-free medium used for culturing Vero cells, and preparation method thereof
InactiveCN105441378AGood proliferation rateExcellent Cell PerformanceVertebrate cellsArtificial cell constructsAntioxidantProliferation rate
The invention relates to a serum-free medium used for culturing Vero cells. The serum-free medium is composed of, by volume, 85 to 95% of a base culture solution, 2 to 5% of an amino acid solution, 2 to 7% of a serum alternative factor solution, 0.1 to 0.5% of an antioxidant solution, 0.8 to 2% of a yeast extract solution, and 0.1 to 0.5% of an ethanol amine solution. The invention also provides a preparation method of the serum-free medium. In the preparation method, the base culture solution, the amino acid solution, the serum alternative factor solution, the antioxidant solution, the yeast extract solution, and the ethanol amine solution are mixed at the above ratio so as to obtain the serum-free medium. The serum-free medium used for culturing Vero cells is capable of promoting rapid attachment of Vero cells; cell morphology of the Vero cells cultured with the serum-free medium can be maintainer preferably, and cell proliferation rate is higher; the composition of the serum-free medium is simple; cost is reduced; and preparation is convenient.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
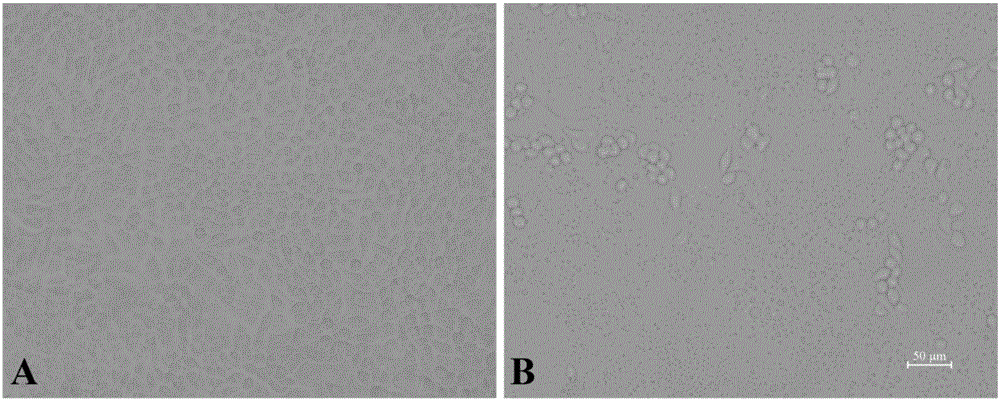
Attenuated strain YN150 of variant porcine epidemic diarrhea virus and applications thereof
InactiveCN105821006AVariation features are obviousEasy to makeSsRNA viruses positive-senseViral antigen ingredientsMicroorganismVero cell
The invention discloses an attenuated strain YN150 of variant porcine epidemic diarrhea virus and applications thereof. The attenuated strain is prepared by consecutively passing strain YN144 (microbial preservation number: CCTCC V201547) for six generations in Vero cells in the presence of pancreatin (10 [mu]g / mL). The PEDV attenuated strain is originated from variant porcine epidemic diarrhea virus, has good safety, and is safe to various pigs. The provided vaccine can stimulate the pigs to generate protective immune response so as to resist variant porcine epidemic diarrhea virus and effectively prevent the infection caused by variant porcine epidemic diarrhea virus.
Owner:HUAZHONG AGRI UNIV
The method of ibdv serum-free microcarrier suspension culture proliferation
InactiveCN102268411AImproving immunogenicityReduce the impactViruses/bacteriophagesVaccine ProductionEmbryo
The invention provides a method for IBDV serum-free microcarrier suspension culture proliferation, comprising the following steps: 1) Acclimatization of Vero cells in serum-free culture; 2) IBDV virus propagation: inoculate the acclimatized cells into 0.25% whey The serum-free medium of the protein hydrolyzate was inoculated with the IBDV virus when the cells adhered to the microcarrier and started to grow, and the virus was harvested after 90 hours. The present invention domesticates the Vero cells introduced from ATCC, cultivates them with reduced serum, finally obtains Vero cells under the condition of serum-free microcarrier culture, improves the culture medium composition during IBDV virus proliferation at the same time, reduces the influence of exogenous viruses, and improves IBDV Virus titers under serum-free microcarrier culture conditions. The titer of the IBDV virus propagated by optimizing the virus propagation medium is higher than that of the IBDV propagated by the unoptimized medium and the IBDV virus titer propagated by chicken embryo fibroblasts; it is equivalent to the IBDV cultured by Vero cells with serum; the immunogenicity experiment shows that : The virus has strong immunogenicity and is suitable for production as a vaccine.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Coxsackievirus A16-type virus strain and use thereof
ActiveCN103087994AGenetic stabilityStable titerSerum immunoglobulinsTransferasesSequence analysisDisease
The invention provides a coxsackievirus A16-type virus strain and a use thereof. The coxsackievirus A16-type virus strain has the preservation number of CGMCC No. 5371. The full-length sequence analysis and the mass spectrometry analysis on a VP1 protein produced by the coxsackievirus A16-type virus strain prove that the oxsackievirus A16-type virus strain is a good CA16 virus strain which is not polluted by allothigenes and has good immunogenicity. The CA16 virus strain can efficiently proliferate in Vero cells and has virus titer of 7.41g CCID 50 / ml. The CA16 virus strain or a vaccine prepared from the CA16 virus strain can be used for preventing diseases caused by CA16 viruses, and has the characteristics of stable titer, good immunogenicity and less immunizing dose.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com