Method for preparing rotavirus vaccine stock solution by using serum-free Vero cells and serum-free rotavirus vaccine product
A rotavirus, serum-free technology, applied in the directions of viral antigen components, microorganism-based methods, biochemical equipment and methods, etc. Vaccine production instability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
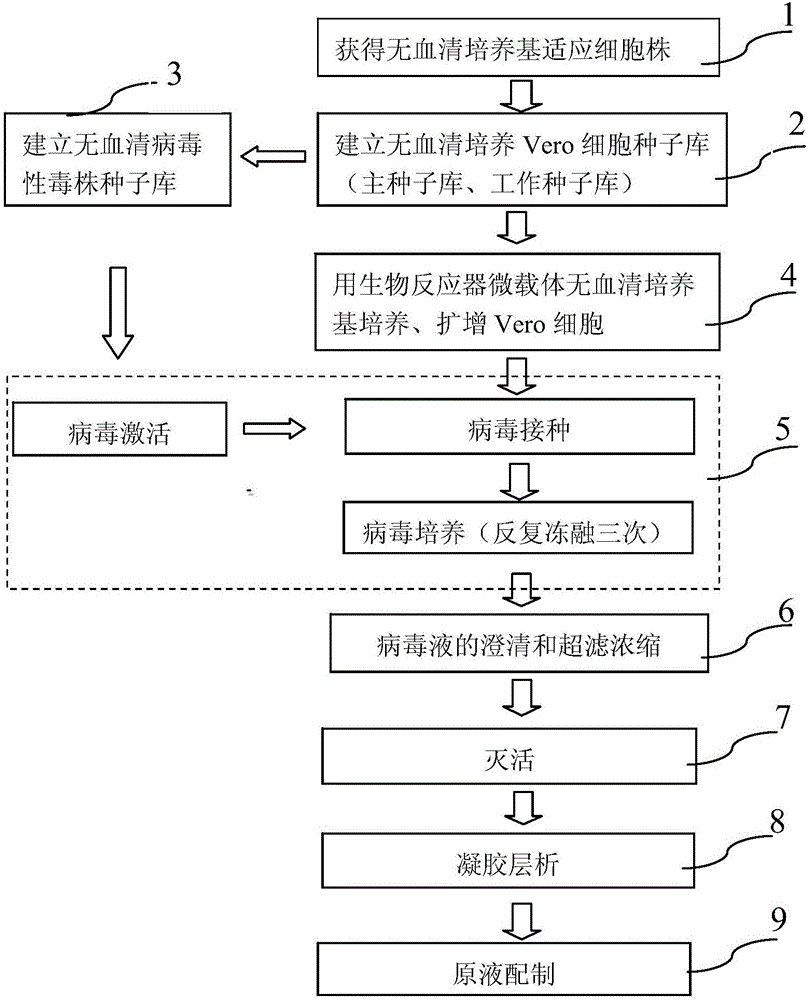
[0082] Embodiment one (bioreactor microcarrier serum-free culture Vero cell human-bovine reassortment rotavirus G1 serotype (D × UK) vaccine stock solution preparation)
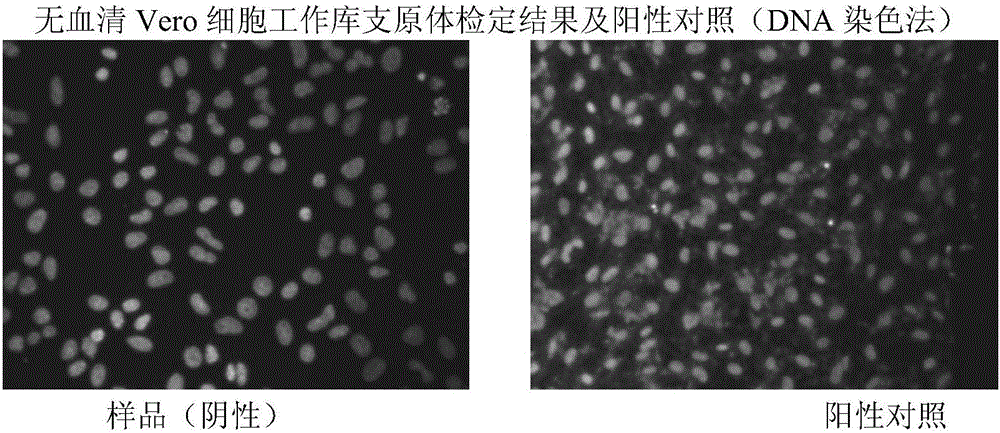
[0083] CCL-81Vero cells introduced from ATCC were directly cultured with serum-free medium VP-SFM produced by GIBCO Company to obtain serum-free medium-adapted strains. On this basis, the serum-free Vero cell seed bank and the serum-free human-bovine reassortant rotavirus G1 serotype (D×UK) strain seed bank (human-bovine reassortant rotavirus G1 serotype provided by NIH) were established. (D×UK) strain). Serum-free medium VP-SFM was used to resuscitate, culture, passage, and expand Vero cell working seed bank cells. After cell expansion, use NBS (New Brunswich Scientific) company 14L bioreactor (Celligen310 Bioreactor System With Cell Lift Impeller) and microcarrier cytodex-1 produced by GE company (General Electric Company), with 8-20g / L high density Microcarrier culture technology, using serum-free medium, ...
Embodiment 2
[0149] On the basis of Example 1, a bioreactor with a smaller capacity, such as a bioreactor with a capacity of less than 14 L, can be used for perfusion culture. Cells are maintained for a long time, and perfusion can be selected for virus culture in small-volume bioreactors. When the virus peak arrives, the virus liquid can be harvested for the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com