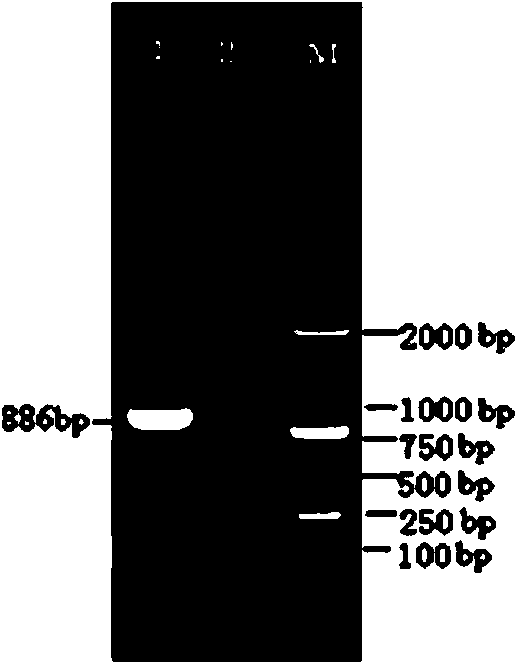
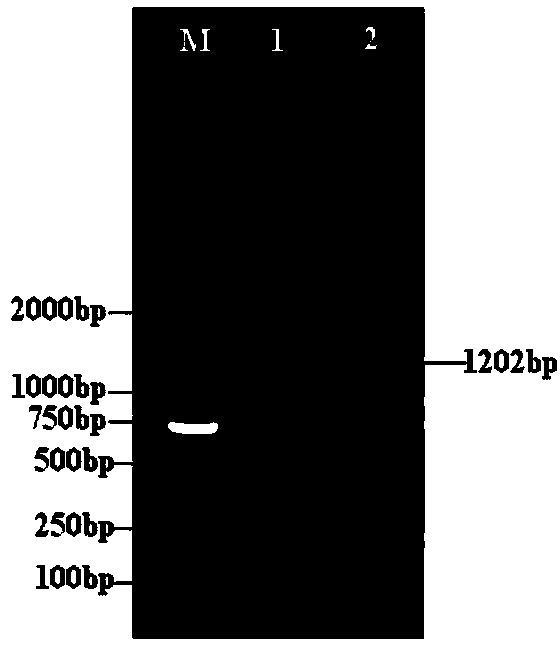
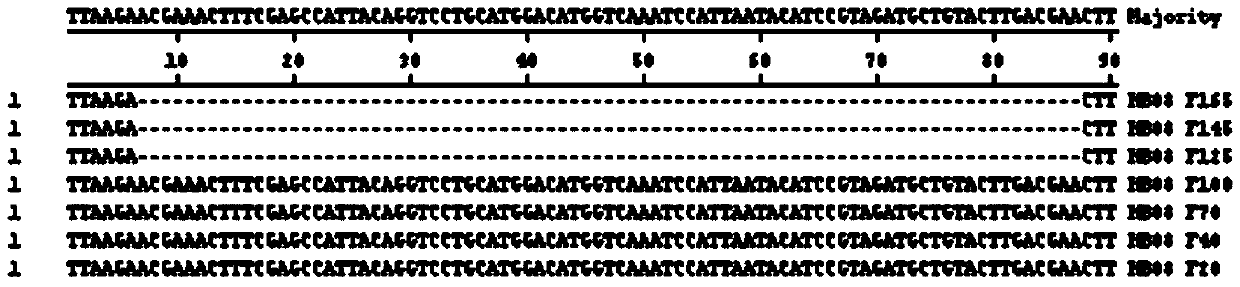
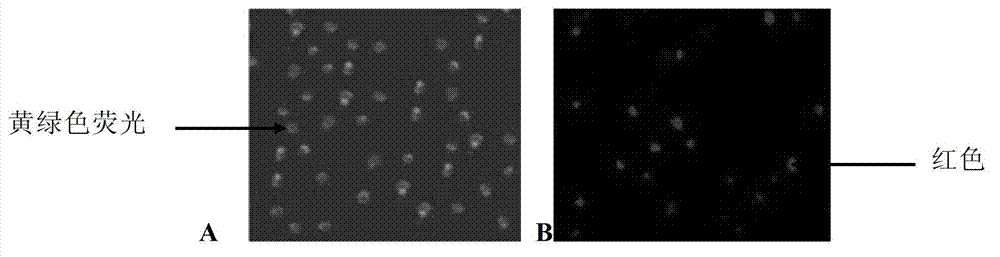
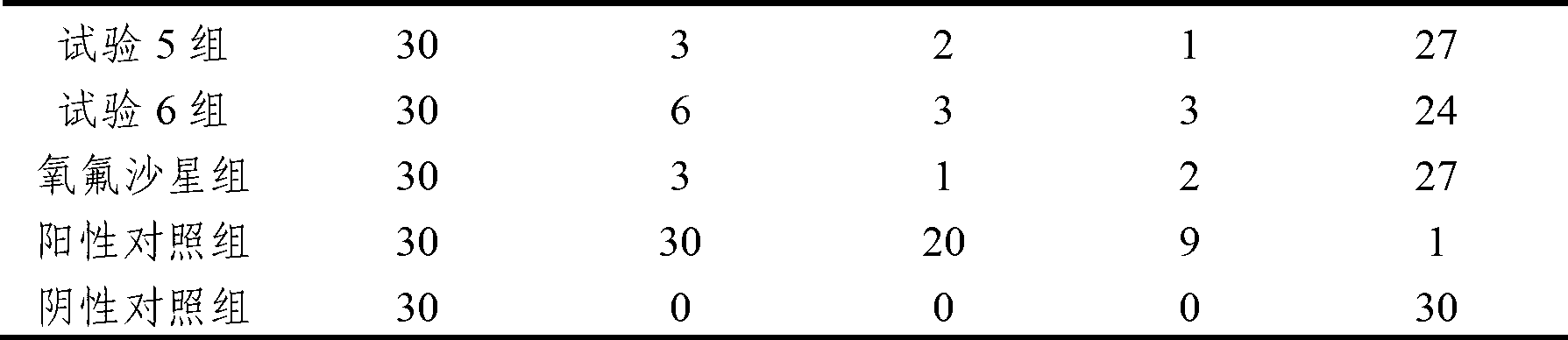Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Swine Transmissible Gastroenteritis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Etiology: Transmissible gastroenteritis (TGE) in swine is known to be one of the most significant diarrhea-produceing diseases in young pigs. The causative agent, TGE virus (TGEV) belongs to the genus Coronavirus of the family Coronaviridae.
Multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV
ActiveCN105154589AAvoid crossbreedingGuaranteed temperatureMicrobiological testing/measurementDNA/RNA fragmentationImmune profilingSwine Transmissible Gastroenteritis
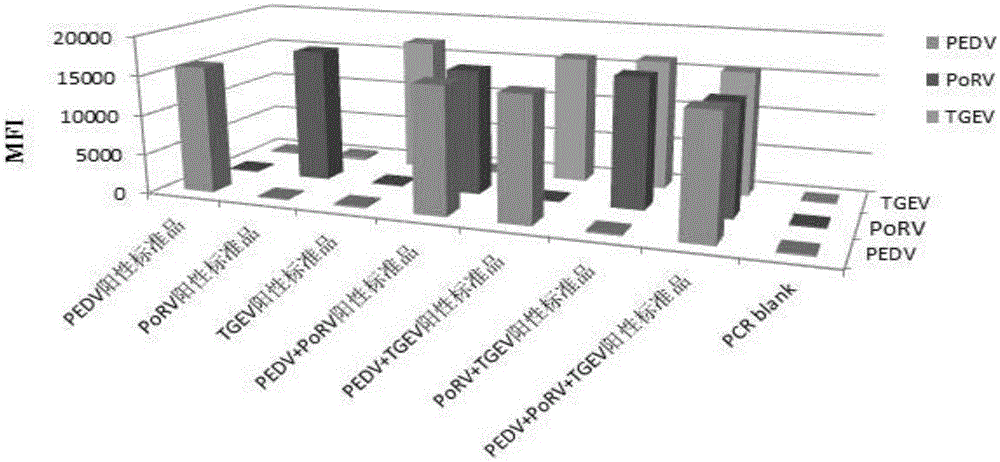
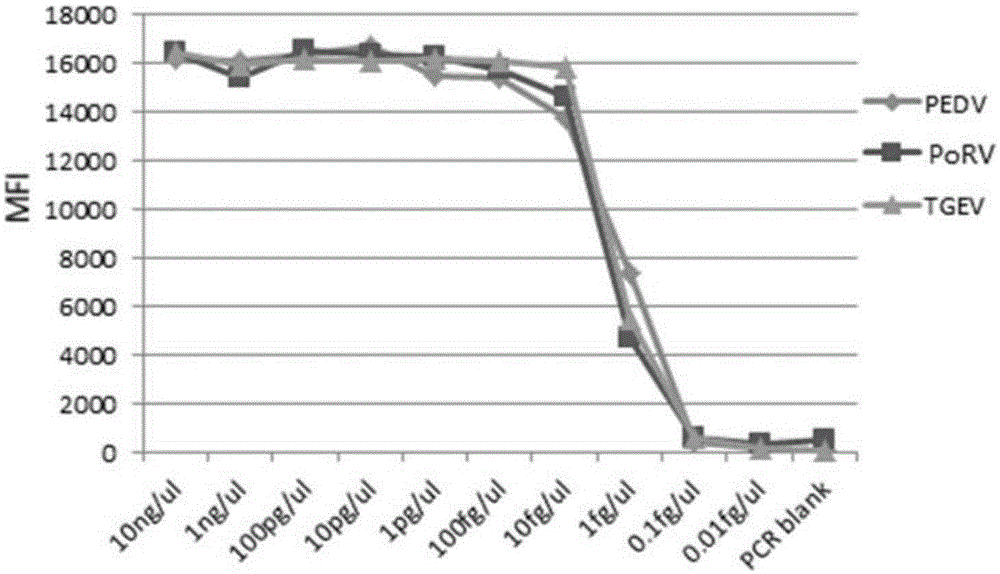
The invention discloses a multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV. The method is easy to operate, a target amplified fragment is obtained through PCR, then hybridization is conducted on an amplified product, fluorescence coded microspheres and streptavidin-phycoerythrin, the MFi value is read through a detection instrument, and different types of viruses are distinguished. By means of the method, porcine epizootic diarrhea, swine transmissible gastroenteritis and pig group A rotavirus can be accurately detected at the same time, the specificity is high, the sensitivity is high, and the repeatability is good. Compared with a traditional detection method, various molecules of different purposes in the same sample are detected at the same time, the sample consumption is little, operation is simple and fast, and the detection cost can be greatly lowered.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
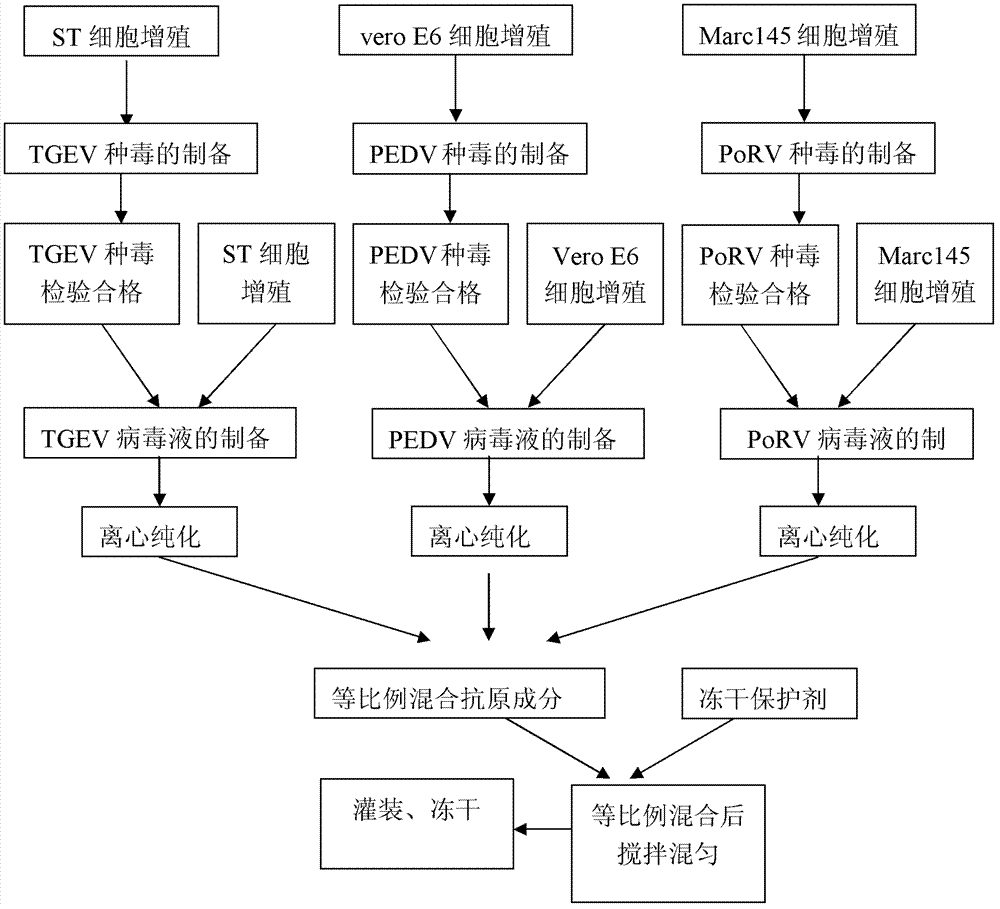
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
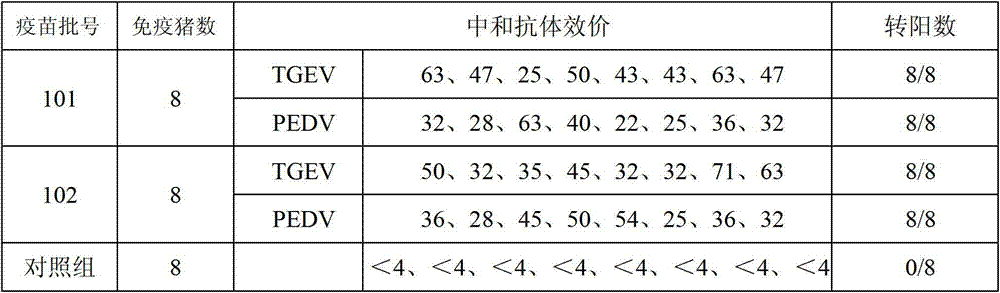
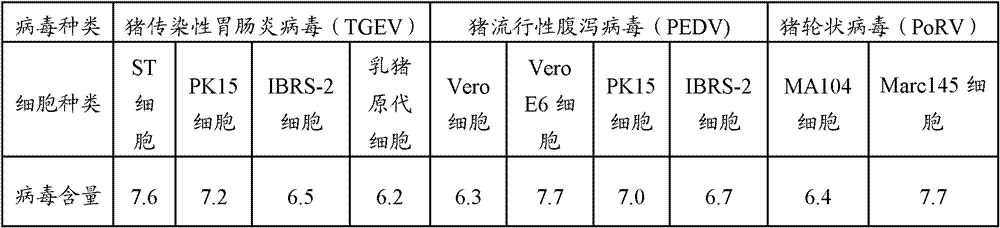
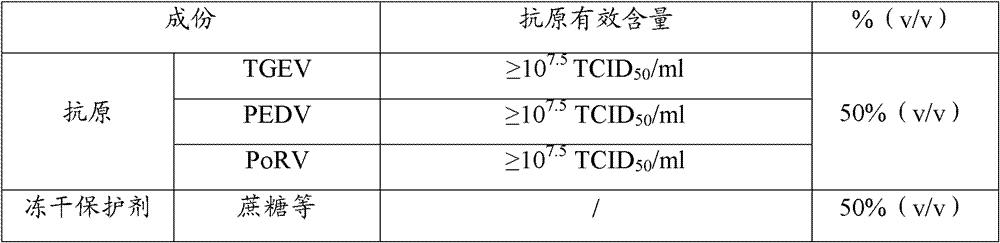
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody and preparation method thereof
ActiveCN104788561ANo growthGood physical propertiesEgg immunoglobulinsImmunoglobulins against virusesSwine Transmissible GastroenteritisArtificial infection
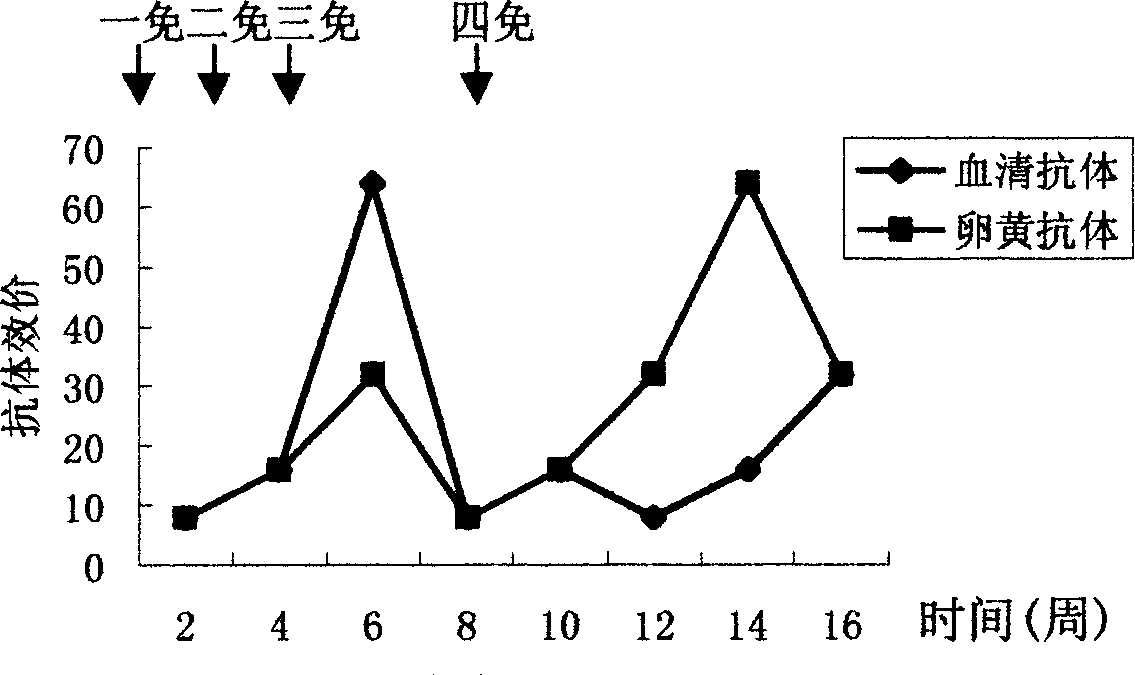
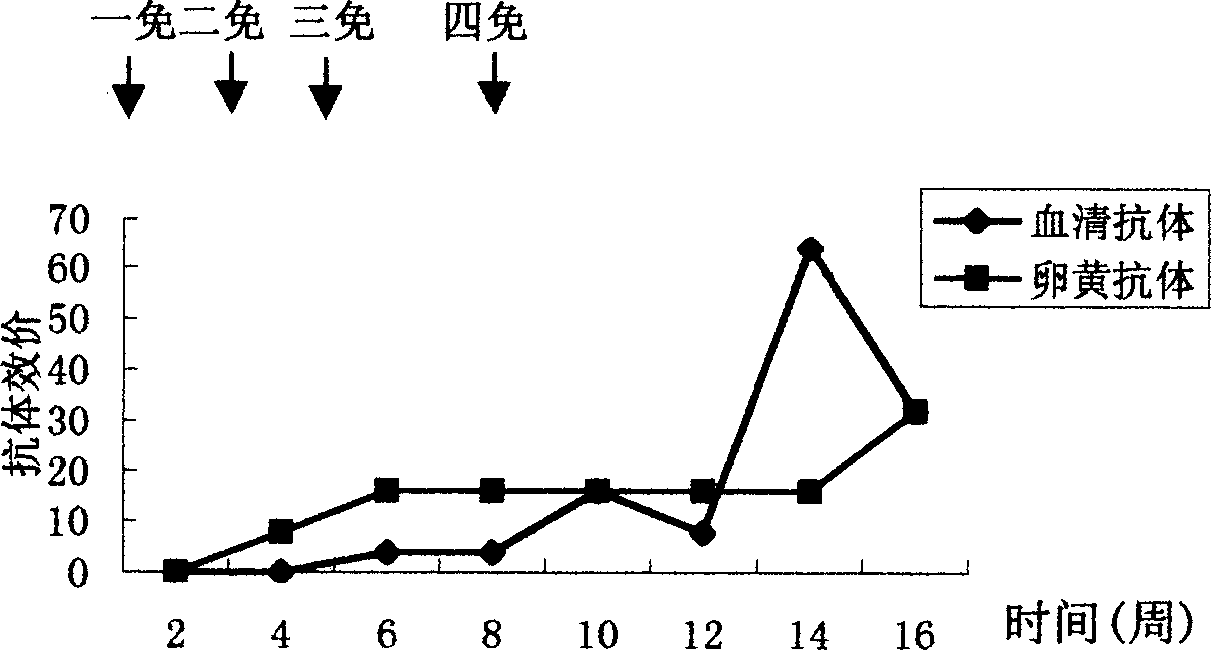
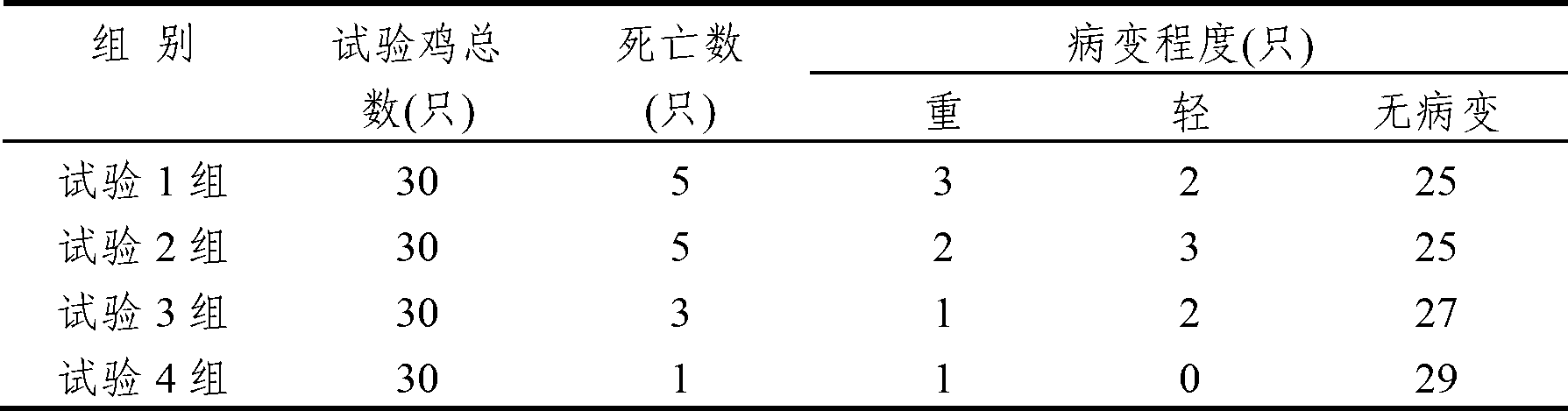
The invention discloses an anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody. Swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus combined inactivated vaccine is used as an immunogen to immune laying hens, and an egg-yolk antibody is purified from egg yolk, wherein the swine transmissible gastroenteritis virus is swine transmissible gastroenteritis virus HB08 with the collection number being CGMCC No.7807; and the porcine epidemic diarrhea virus is porcine epidemic diarrhea virus ZJ08 with the collection number being CGMCC No.7806. The prepared egg-yolk antibody has good and safe traits. Artificial infection cure rate of the prepared egg-yolk antibody reaches 100% and is obviously higher than cure rate of an egg-yolk antibody prepared from classical IBDV. Clinical case cure rate of the prepared egg-yolk antibody reaches 93.0%. In clinical preventive tests, incidence of diarrhea can be reduced by 17-24% for tested pigs.
Owner:兆丰华生物科技(南京)有限公司 +3
Yolk antibody and antigen of pig's infective enterogastritis virus and its preparing process
InactiveCN1382736AGood treatment effectNo toxic side effectsEgg immunoglobulinsVirus peptidesYolkAntigen
A yolk antibody for pig's infective enterogastritis virus is prepared through reproducing the said virus on pig's testis cell, applying it as immunogen to the layer, collecting yolk and purifying. When it is applied to pig, the comparison between experimental and reference groups shows that it has high effect on preventing and cuting pig's infective enterogastritis.
Owner:INST OF ZOOTCHNICS & VERTERINARY SCI BEIJING ACAD OF AGRI & FORESTRY SCI
Veterinary drug for preventing livestock bowel diseases, preparation method of drug, and feed
ActiveCN103006792APrevention and treatment of infectious gastroenteritisPrevention and treatment of piglet yellow and white scourAntibacterial agentsPowder deliveryDiseaseSwine Transmissible Gastroenteritis
The invention discloses a veterinary drug for preventing livestock bowel diseases, a preparation method of the drug, and feed. The veterinary drug contains active ingredients which are prepared from purslane, humifuse euphorbia herb, plantain herb, acalypha australis and liquorice. The veterinary drug provided by the invention has the effects of clearing away heat and toxic materials, eliminating dampness to stop dysentery and astringing to stop bleeding, can be effectively used for preventing the livestock bowel diseases such as swine transmissible gastroenteritis, piglet diarrhea, swine dysentery, chicken salmonellosis, chicken colibacillosis and chicken stphyoococcl disease, and has the advantages of an extensive material source, safety, environment-friendliness, a simple preparation process, low production cost and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes
ActiveCN102943129AGuaranteed sensitivityGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMultiplex ligation-dependent probe amplification
The invention discloses a multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes. The multiplex ligation-dependent probes are shown in sequence tables SEQ ID NO:1 to SEQ ID NO:10; and the primers are shown in sequence tables SEQ ID NO:11 to SEQ ID NO:12. By using the primers, the probes and / or the multiplex ligation-dependent probe amplification detection kit containing the primers and the probes, five important swine disease pathogens such as a swine influenza virus, a swine reproductive and respiratory syndrome virus, a pseudorabies virus, a swine transmissible gastroenteritis virus and a foot-and-mouth disease virus can be simultaneously detected, thereby saving the detection time and cost and being beneficial to accurately diagnosing the epidemic diseases in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
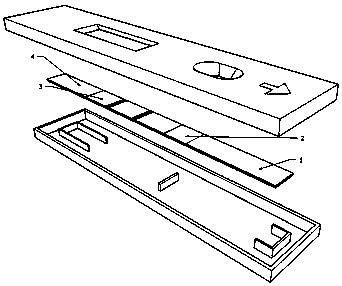
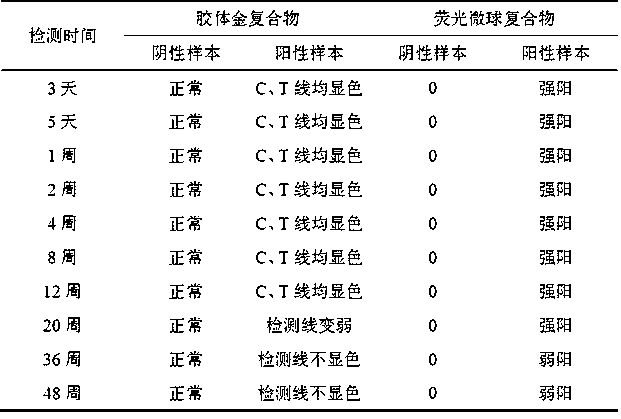
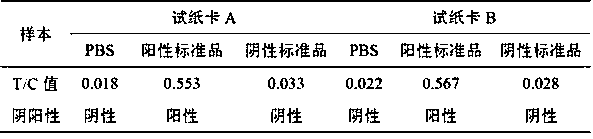
Test paper card for detecting swine transmissible gastroenteritis virus antibody and preparation and detection method of test paper card
InactiveCN107942061AImprove stabilityHigh sensitivityMaterial analysisCelluloseTransmissible gastroenteritis virus Antibody
The invention relates to a test paper card for detecting porcine transmissible gastroenteritis virus antibody, preparation and detection method thereof, and belongs to the field of immunological detection. The test paper card includes a jammed case and a test strip, and the test strip includes a bottom plate and sequentially lapped and pasted on the Absorbent pad, detection pad, binding pad and sample pad on the base plate; the detection pad is a nitrocellulose membrane with a quality control line C and a detection line T, the quality control line C is coated with goat anti-mouse monoclonal antibody, and the detection line T is Coated with inactivated porcine transmissible gastroenteritis virus; the binding pad is a glass cellulose membrane embedded with time-resolved fluorescent microsphere-labeled anti-E2 protein monoclonal antibody; the sample pad is dried glass after soaking in the sample treatment solution Cellulose film. The test paper card prepared by the invention has better stability and higher sensitivity, and can achieve the purpose of semi-quantitative detection through fluorescence signal analysis.
Owner:洛阳现代生物技术研究院有限公司
Domestic animal and aquatic product causal agent resistant specific IgY or compound IgY and application thereof
InactiveCN101081866AEgg immunoglobulinsAntibody ingredientsAquatic animalSwine Transmissible Gastroenteritis
The present invention relates to one kind of specific or compound IgY for antagonizing pathogen of livestock and aquatic animals, and features that the specific or compound IgY is one or combination of specific IgY for antagonizing pathogene of swine transmissible gastroenteritis and epidemic diarrhea, specific IgY for antagonizing virus of swine transmissible gastroenteritis and epidemic diarrhea, specific compound IgY for antagonizing aquatic animals' pathogene, specific compound IgY for antagonizing aquatic animals' fungi, specific compound IgY for antagonizing aquatic animals' viruses, specific IgY for antagonizing reovirus, specific IgY for antagonizing fishes'herpes virus, specific IgY for antagonizing pathogene of ox transmissible gastroenteritis and epidemic diarrhea, and specific IgY for antagonizing virus of ox transmissible gastroenteritis and epidemic diarrhea. The present invention relates to also the preparation process and application of the specific or compound IgY.
Owner:深圳雅臣生物科技有限公司
TGEV and PEDV combined live vaccine and preparation method thereof
The invention discloses a combined live vaccine of transmissible gastroenteritis virus of swine (TGEV) and porcine epidemic diarrhea virus (PEDV) and a preparation method thereof. An attenuated swine transmissible gastroenteritis virus HB08 and an attenuated porcine epidemic diarrhea virus ZJ08 which are self-separated, attenuated and stored respectively undergo viral multiplication on ST cells and VeroE6 cells, and seedling and freeze drying are then carried out by adding a freeze-drying protective additive into a virus solution which is qualified after inspected. The two diseases, transmissible gastroenteritis of swine and porcine epidemic diarrhea virus, which are epidemic in clinic at present, can be effectively prevented by the use of the combined live vaccine.
Owner:兆丰华生物科技(南京)有限公司 +3
Preparation and application method for treating swine viral diarrhea biological agent
InactiveCN103694348ALow costHigh recovery rateEgg immunoglobulinsDigestive systemSwine Transmissible GastroenteritisHigh activity
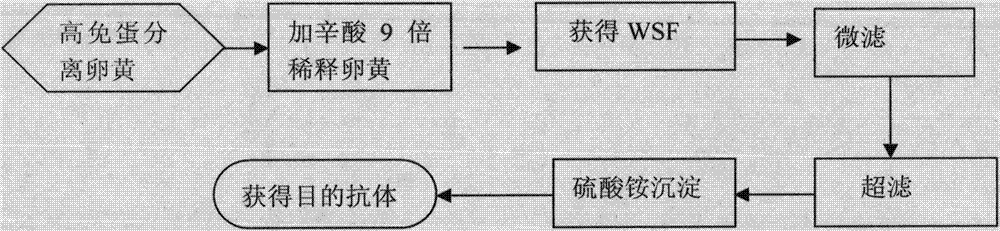
The invention relates to a preparation and application method for treating a swine viral diarrhea biological agent, and belongs to the technical field of biological agents. The method specifically comprises the following steps of: (1) preparing for immunogen preparation, namely, getting swine transmissible gastroenteritis and porcine epizootic diarrhea duplex inactivated vaccine which consists of two viral protein; (2) performing immune procedure; (3) detecting valence of antibody; (4) purifying an egg yolk antibody; and (5) identifying the egg yolk antibody. The preparation and application method has the advantages that the water diluting method is performed and the method is safe, reliable, out of pollution, and low in cost; the treated high-immunity egg can gain the egg yolk antibody with high activity, purity and output and stable performance; the operation method is simple, feasible, and reliable; the industrial production can be carried out conveniently.
Owner:刘聚祥
Drug for prevention and treatment of transmissible gastroenteritis of swine and epidemic diarrhea
ActiveCN103127416AEfficient killingEco-friendly formulaDigestive systemAntiviralsAngelica Sinensis RootSwine Transmissible Gastroenteritis
The invention relates to a drug for prevention and treatment of transmissible gastroenteritis of swine and epidemic diarrhea. The drug comprises the following components according to weight ratio: 10000 of rice powder, 25 of ligusticum wallichii, 30 of costus root, 35 of cortex cinnamomi, 25 of radix angelicae, 30 of fructus gleditsiae, 35 of Chinese wild ginger, 30 of ephedra, 25 of aconite root, 30 of radix scrophulariae, 30 of golden cypress, 25 of radix aconite, 30 of sophora flavescens, 30 of distiller yeast, 30 of cortex moutan, 30 of angelica sinensis, 50 of ginkgo leaves, 100 of pear tree bark and 3000 of white sand mud. The drug is prepared by pure Chinese herbal medicine, can effectively kill virus triggering porcine epizootic diarrhea and transmissible gastroenteritis of swine, and has the advantages of being environment-friendly in formula, simple and reasonable in technology, low in manufacture cost, good in prevention and treatment effect, and small in side effect.
Owner:刘荣彬
Multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis
InactiveCN102586409AIncreased sensitivityStrong specificityMicrobiological testing/measurementSwine Transmissible GastroenteritisPorcine epidemic diarrhoea virus
The invention relates to a multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis. An antibody is needed to be prepared by an euzymelinked immunosorbent assay, so that the period is longer, and external factors have larger interference on the diagnostic method of the euzymelinked immunosorbent assay. The method comprises the following steps of: designing and synthesizing a transmissible swine gastroenteritis virus gene primer; establishing a multiple reverse transcription polymerase chain reaction diagnostic method of the transmissible swine gastroenteritis viruses and porcine epidemic diarrhea viruses; optimizing concentration of the primer of a bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of magnesium oxide of the bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of triphosphoric acid base deoxynucleotide of the bigeminy reverse transcription polymerase chain reaction; determining the optimal annealing temperature of the bigeminy reverse transcription polymerase chain reaction; testing the sensitivity of the bigeminy reverse transcription polymerase chain reaction; and testing the specificity of the bigeminy reverse transcription polymerase chain reaction. The multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis is suitable for detecting swine transmissible gastroenteritis.
Owner:郑世民
Primer and multiple RT-PCR method for identifying and detecting swine diarrhea coronavirus
InactiveCN107557494AEfficient detection methodShorten detection timeMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaSwine Transmissible Gastroenteritis
Owner:HUAZHONG AGRI UNIV
Traditional Chinese medicament composition and preparation and preparation method thereof
ActiveCN103070949AUnique formulaSimple recipeAntibacterial agentsDigestive systemDiseaseSwine Transmissible Gastroenteritis
The invention discloses a traditional Chinese medicament composition, which consists of the following components in parts by weight: 50 to 100 parts of rhizoma atractylodis macrocephalae, 50 to 150 parts of radix scutellariae, 20 to 40 parts of sophora flavescens, 60 to 150 parts of patchouli, 60 to 120 parts of astragalus, 60 to 100 parts of Chinese pulsatilla root, 25 to 60 parts of gardenia and 60 to 140 parts of lonicera japonica. The traditional Chinese medicament composition has reasonable proportion, and the effects of strengthening body resistance and eliminating evil, invigorating the spleen and eliminating dampness and clearing heat and stopping dysentery. The invention further discloses a preparation containing the traditional Chinese medicament composition, and the preparation is convenient to use and simple to prepare, and can be used for treating moist heat dysentery of swine, transmissible gastroenteritis of swine, weakened immune system and other diseases, thereby having a good application prospect.
Owner:SOUTHWEST UNIVERSITY
RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection method for transmissible gastroenteritis of swine TGE
InactiveCN102912037AMicrobiological testing/measurementAgainst vector-borne diseasesSwine Transmissible GastroenteritisInfectivity
The invention provides an RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection method for transmissible gastroenteritis of swine TGE. The RT-LAMP detection method comprises the following steps: 1) designing an LAMP primer by using an online software according to a conserved region of a TGEVN (TGE Virus N) gene in a GenBank; 2) preparing a virus solution for use at the temperature of 80 DEG C below zero; 3) extracting RNA (Ribose Nucleic Acid) of TGEV; 4) taking the RNA of the TGEV obtained in the step 3) as a template for RT-LAMP reaction; and 5) detecting an RT-LAMP reaction result with agarose gel. Compared with the conventional method, the RT-LAMP detection method is simple in operation, quick, and high in efficiency, specificity and sensitivity.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
Vaccine diluent and preparing method and application thereof
InactiveCN106466295ASimple methodEasy to operateSsRNA viruses negative-senseOrganic active ingredientsDiseaseSwine Transmissible Gastroenteritis
The invention relates to the field of animal biological products, in particular to a vaccine diluent and a preparing method and application thereof. The vaccine diluent is prepared from 0.1-100g / L Carbomer and 0.1-200g / L levamisole; the adopted solvent is any one kind of water, normal saline or phosphate buffer. The vaccine diluent can solve the problems that a neutralizing antibody is low in potency after a vaccine is immunized, long in producing time and short in antibody maintenance time, and can be matched with a livestock and poultry attenuated live vaccine to use, such as a single vaccine or a mixed vaccine of viral vaccines of a swine fever live vaccine, a swine pseudorabies live vaccine, a porcine reproductive and respiratory syndrome live vaccine, a porcine epizootic diarrhea live vaccine, a swine transmissible gastroenteritis live vaccine, a porcine rotavirus disease live vaccine, a fowl pox live vaccine, a new castle disease live vaccine and the like.
Owner:SICHUAN HUASHEN ANIMAL BIOLOGICAL PRODS
Fluorogenic quantitative PCR kit for detecting swine transmissible gastroenteritis virus
InactiveCN104611465AEfficient detectionQuick checkMicrobiological testing/measurementMicroorganism based processesSwine Transmissible GastroenteritisGene
The invention discloses a fluorogenic quntitative PCR kit for detecting swine transmissible gastroenteritis virus. The fluorogenic quantitative PCR kit comprises primer pairs showed in SEQ ID NO: 1-2 for amplifying swine transmissible gastroenteritis virus genes. According to the kit disclosed by the invention, the swine transmissible gastroenteritis virus can be detected accurately and effectively, and the kit has the advantages of strong specificity, high sensitivity, short consumed time, high detecting speed and good application prospect.
Owner:SICHUAN AGRI UNIV
TaqMan fluorogenic quantitative PCR kit and detection method for porcine deltacoronavirus
InactiveCN107557497AGood linear relationshipStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesSwine Transmissible GastroenteritisPcr method
The invention discloses a TaqMan fluorogenic quantitative PCR kit and a detection method for porcine deltacoronavirus. The kit comprises specific primers and a probe, wherein primer sequences are as follow: the upstream primer is 5'-ACGTCGTAAGACCCAGCATC-3' and the downstream primer is 5'-CCCACCTGAAAGTTGCTCTC-3', and a probe sequence is 5'-FAM-GTATGGCTGATCCTCGCATCATGGC-BHQ1-3'. A gene part fragment648bp of the porcine deltacoronavirus is expanded, a lowest detection limit of the TaqMan real-time fluorogenic quantitative PCR disclosed by the invention is 26.6copies / mu L, and the detection results of the TaqMan real-time fluorogenic quantitative PCR to swine transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine kobuvirus, porcine reproductive and respiratory syndromevirus as well as foot and mouth disease virus are all feminine. The TaqMan probe real-time fluorogenic quantitative PCR method for detecting the porcine deltacoronavirus (PDCoV) disclosed by the invention has the advantages of strong specificity, high sensitivity and good inter-batch and intra-batch repeatability. When the TaqMan probe real-time fluorogenic quantitative PCR method disclosed by theinvention are used for detecting 194 clinical pig manure samples, a result shows that a PDCoV positive rate is 22.1% and is about one time higher than a 11.9% positive rate of detecting the clinicalpig manure samples by general RT-PCR.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
RT-qPCR detection method and primers and TaqMan probe for detecting swine transmissible gastroenteritis virus N gene
InactiveCN104673936AStrong conservativeHigh homologyMicrobiological testing/measurementMicroorganism based processesForward primerAgricultural science
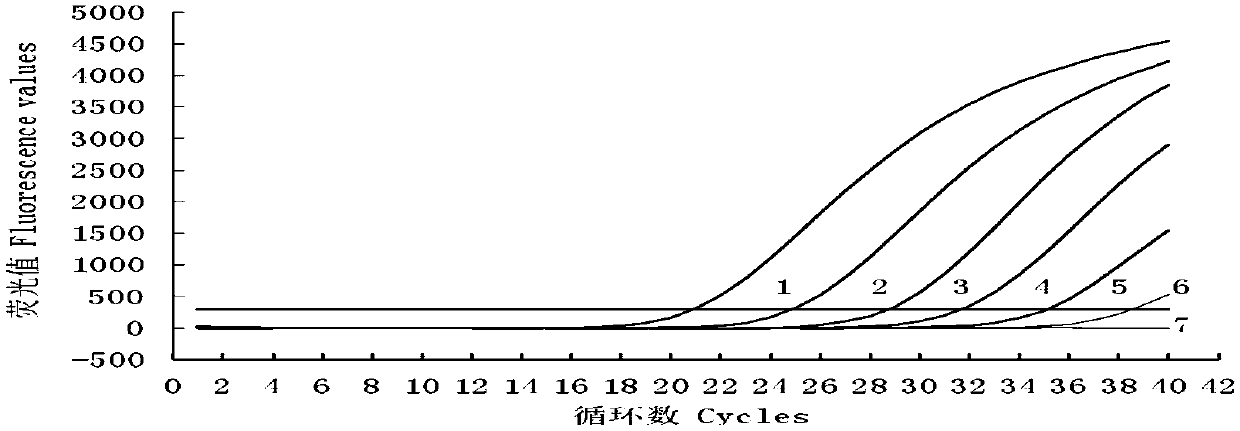
The invention provides an RT-qPCR detection method, primers and TaqMan probe for detecting a swine transmissible gastroenteritis virus N gene. Particularly, the invention provides a set of RT-qPCR detection method for detecting the swine transmissible gastroenteritis virus gene and a forward primer shown in SEQ ID No.1 in a sequence table, a reverse primer shown in SEQ ID No.2 in the sequence table, and a TaqMan probe sequence shown in SEQ ID No.3 in the sequence table. The invention provides an RT-qPCR detection method for detecting the swine transmissible gastroenteritis virus N gene on the basis of the primers and the TaqMan probe. The detection method has excellent detection sensitivity, and in the case of 1*10<5>copies / microliter to 1*10<1>copies / microliter, Ct values and fluorescence values show typical amplification curves. At the same time, the detection method has good repeatability, strong specificity and convenience in operation.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
Porcine Deltacoronavirus and swine transmissible gastroenteritis virus multiplex RT-PCR detection primer and detection method
ActiveCN105400910ARapid differential diagnosisMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceSwine Transmissible Gastroenteritis
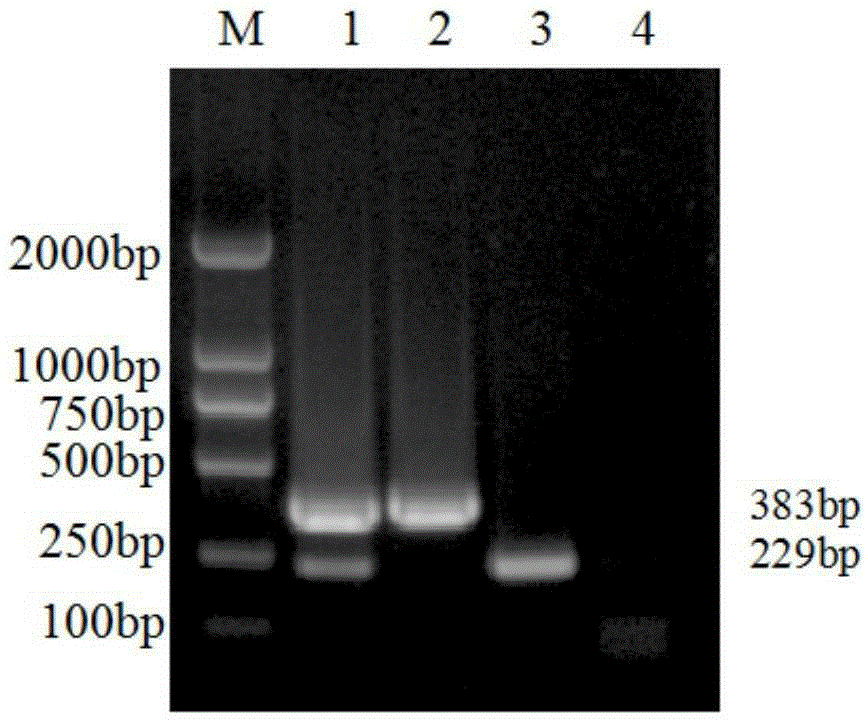
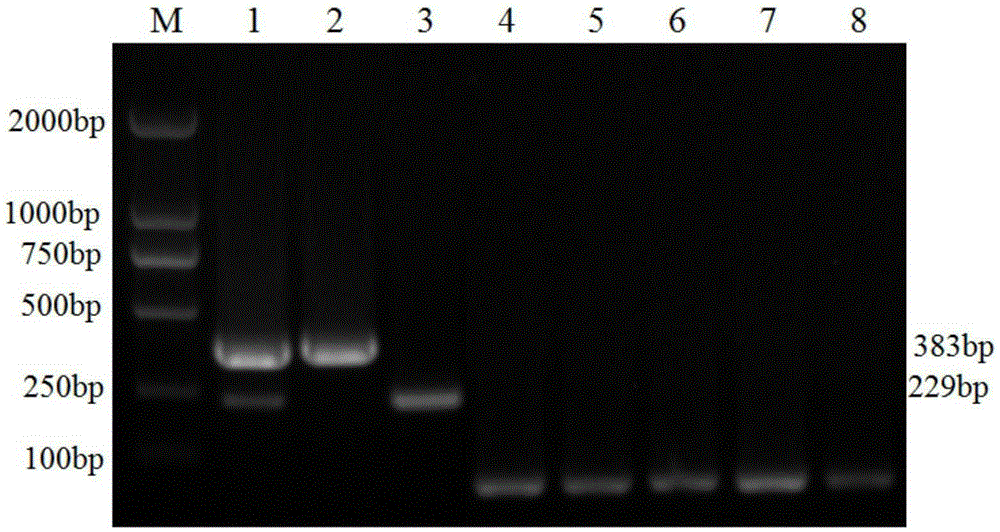
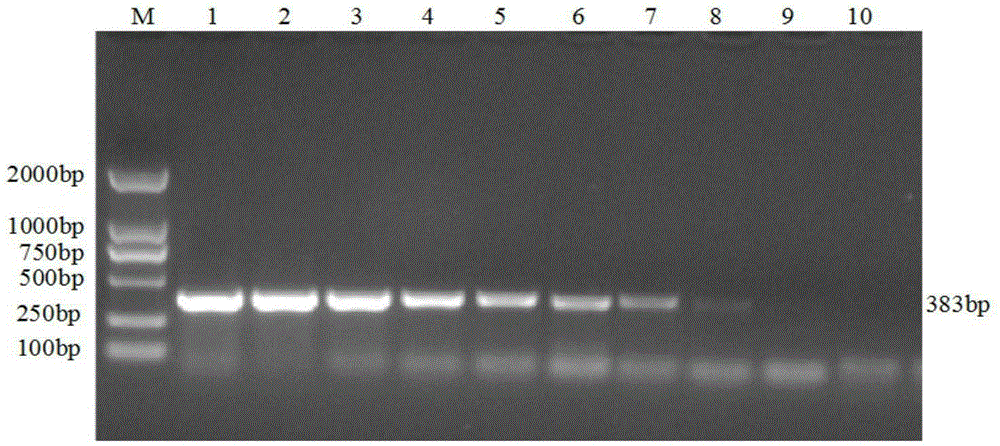
The invention discloses a porcine Deltacoronavirus (PDCoV) and swine transmissible gastroenteritis virus (TGEV) multiplex RT-PCR detection primer. The primer sequence of PDCoV is expressed as follows: upstream primer P1: 5'-ATGGCTACTGGCTGCGTTAC-3', downstream primer P2: 5'-GCGTTTCCTGGGCTGATT-3', and partial PDCoV gene segments are amplified by 383 bp. The primer sequence of TGEV is expressed as follows: upstream primer P3: 5'-CCCTCCAGCAAGGTTCAA-3', downstream primer P4: 5'-GCAACCCAGACAACTCCA-3', and partial TGEV gene segments are amplified by 229 bp. The detection limits of multiplex RT-PCR on PDCoV and TGEV are 4.05*101 copy per microliter and 5.47*102 copy per microliter respectively, and amplified results on porcine epidemic diarrhea virus, bocavirus, porcine reproductive and respiratory syndrome virus and porcine rotavirus are negative. Multiplex RT-PCR detection results of 57 clinical samples show that the positive rate of being infected with the two viruses at the same time is 1.75%, the PDCoV infection positive rate is 19.30%, and the TGEV infection positive rate is 1.75%.
Owner:HENAN AGRICULTURAL UNIVERSITY
Pharmaceutical composition for preventing and/or treating diseases caused by coronaviruses and/or rotaviruses
ActiveCN105832759AGood treatment effectReduce dosageDigestive systemAntiviralsDiseaseSwine Transmissible Gastroenteritis
Belonging to the technical field of disease prevention and treatment, the invention particularly discloses a pharmaceutical composition for preventing or treating diseases caused by coronaviruses and / or rotaviruses. The invention for the first time studies and finds that taurine can prevent or treat a series of diseases caused by coronaviruses or rotaviruses, like porcine epizootic diarrhea, swine transmissible gastroenteritis, rotaviruses caused rotavirus diarrhea and the like. Therefore, on the basis, the invention develops a pharmaceutical composition containing taurine, the pharmaceutical composition combines taurine, a vitamin compound and a flavoring agent, and the nutrient intake of animals is increased and the resistance of the body itself is strengthened to realize the purpose of assisting taurine in efficient treatment of diseases.
Owner:GENIFARM LAB INC +1
Swine-derived single-chain antibody capable of resisting swine transmissible gastroenteritis viruses and preparation method of swine-derived single-chain antibody
ActiveCN106632670AMicroorganism based processesImmunoglobulins against virusesSingle-Chain AntibodiesHeavy chain
The invention discloses a swine-derived single-chain antibody capable of resisting swine transmissible gastroenteritis viruses and a preparation method of the swine-derived single-chain antibody. The swine-derived single-chain antibody is formed by linking a heavy-chain variable region with a light-chain variable region through a short linker peptide, wherein an amino acid sequence of the light-chain variable region is shown as SEQ ID No.1, and an amino acid sequence of the heavy-chain variable region is shown as SEQ ID No.2. The swine-derived single-chain antibody capable of resisting the swine transmissible gastroenteritis viruses is about 28 kDa in molecular weight and capable of specifically recognizing the swine transmissible gastroenteritis viruses and can be used for further blocking infection and invasion of the swine transmissible gastroenteritis viruses.
Owner:SHANGHAI JIAO TONG UNIV
Triple inactivated vaccine for porcine epidemic diarrhea, swine transmissible gastroenteritis and porcine delta coronavirus and preparation method of triple inactivated vaccine
ActiveCN107899007AImmunization method is simpleAddressing the absence of vaccinesSsRNA viruses positive-senseViral antigen ingredientsDiseaseEpidemic diarrhea
The invention provides a triple inactivated vaccine for porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) and a preparation method of the triple inactivated vaccine, before inactivation, the contents of the three viruses are greater than or equal to 10<7.0>TCID50 / mL, and after inactivation, the volume ratio of the antigens is 1 to 1to 1. With the triple inactivated vaccine disclosed by the invention, the problem that an effective multivalent vaccine for preventing and treating three diseases including porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) is not available on the market is solved, especially, the problem that the vaccine for the porcine delta coronavirus (PDCoV) epidemic in recent years is not available is solved. The triple inactivated vaccine provided by the invention is economical and practical, the immunizing procedure is simplified, the epidemic prevention cost can be effectively reduced, and a novel method for simultaneously preventing the occurrence of the three diseases is provided for domestic breeding enterprises.
Owner:YULIN UNIV +1
Multiplex RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer probe group for real-time fluorescent quantitative detection of four porcine diarrhea viruses, kit and detection method
PendingCN113462820AThe test result is accurateReal-time quantitative detectionMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexPig farms
The invention discloses a multiplex RT-PCR (reverse transcription-polymerase chain reaction) primer probe group for real-time fluorescent quantitative detection of four porcine epidemic diarrhea viruses. The multiplex RT-PCR primer probe group comprises an upstream primer, a downstream primer and a specific fluorescent probe of a porcine epidemic diarrhea virus M gene, an upstream primer, a downstream primer and a specific fluorescent probe of a swine transmissible gastroenteritis virus S gene; an upstream primer, a downstream primer and a specific fluorescent probe of a porcine rotavirus VP6 gene; and an upstream primer, a downstream primer and a specific fluorescent probe of a porcine D-type coronavirus M gene. The kit assembled on the basis of the primer probe group has the advantages of high sensitivity, high specificity, low pollution and real-time detection, and provides a reliable technology and product for early warning, early diagnosis and prevention and control monitoring of clinical diarrhea virus in a first-line pig farm.
Owner:HEBEI UNIV OF ENG
Swine transmissible gastroenteritis S/N protein fusion gene, recombinant lactococcus lactis and application
InactiveCN102399807AAntibacterial and antibacterialGood immune protectionBacteriaGenetic material ingredientsStreptococcus pyogenesSwine Transmissible Gastroenteritis
The invention discloses a swine transmissible gastroenteritis S / N protein fusion gene, recombinant lactococcus lactis and application. The invention provides an antigen fusion gene obtained by connecting A and D antigen loci in swine transmissible gastroenteritis virus (TGEV) S protein and an N321 antigen locus in N protein and a streptococcus pyogenes cell wall anchoring sequence, and a nucleotide sequence of the antigen fusion gene is shown as SEQ ID No. 1. The invention further provides an expression vector containing the antigen fusion gene and the recombinant lactococcus lactis strain containing the recombinant expression vector. The recombinant lactococcus lactis strain is induced by using nisin, the fusion gene can be expressed on the surface of the bacterial cell wall. Immunoblotting experiments indicate that the expressed recombinant protein can react with TGEV immune serum and has the same antigenicity with TGEV natural antigens, and the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating swine transmissible gastroenteritis.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Porcine transmissible gastroenteritis virus and application thereof
ActiveCN103740653AGood immune protectionMicroorganism based processesAntiviralsSwine Transmissible GastroenteritisMicrobacterium
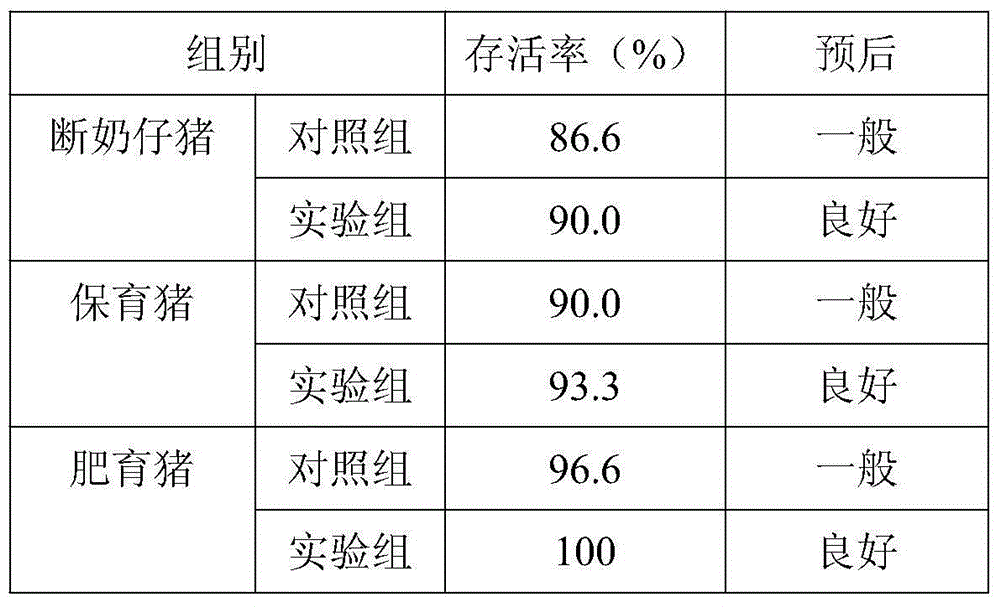
The invention discloses a low virulent strain HB08 of a porcine transmissible gastroenteritis virus (TGEV) and an application thereof. The microbial collection number of the low virulent strain is CGMCC No.7807. The low virulent strain of the TGEV is good in safety, and the virulent strain is safe to the pigs of all ages including pregnant sows and piglets. The low virulent strain has 100% active protection rate and 97.2% passive protection rate on three-day-old piglets, therefore, the low virulent strain HB08 of the porcine transmissible gastroenteritis virus is indicated to have excellent immunoprotection effect on the porcine transmissible gastroenteritis.
Owner:兆丰华生物科技(南京)有限公司 +3
Swine transmissible gastroenteritis virus S/N protein fusion gene and recombinant lactococcus lactis, and their use
ActiveCN103194471ABacteriaGenetic material ingredientsStreptococcus pyogenesSwine Transmissible Gastroenteritis
The invention discloses a swine transmissible gastroenteritis virus S / N protein fusion gene and recombinant lactococcus lactis, and their use. A and D antigen sites of a S protein of the swine transmissible gastroenteritis virus and an N321 antigen site of an N protein of the swine transmissible gastroenteritis virus are connected to a cell wall anchor sequence of streptococcus pyogenes so that the swine transmissible gastroenteritis virus S / N protein fusion gene is obtained and has a nucleotide sequence shown in the formula SEQ ID No.1. The invention further discloses an expression vector containing the swine transmissible gastroenteritis virus S / N protein fusion gene and the recombinant lactococcus lactis containing the expression vector. The recombinant lactococcus lactis is induced by Nisin so that the swine transmissible gastroenteritis virus S / N protein fusion gene can be expressed on the bacterial cell wall surface. A result of a western blot experiment shows that the expressed recombinant protein can react with TGEV immune serum so that it is proved that the expressed recombinant protein has the antigenicity the same as antigenicity of the TGEV natural antigen and thus the expressed recombinant protein can be prepared into a safe and effective mucosal immune live bacterium vaccine for preventing and treating swine transmissible gastroenteritis.
Owner:WUHAN HUAYANG ANIMAL PHARMA
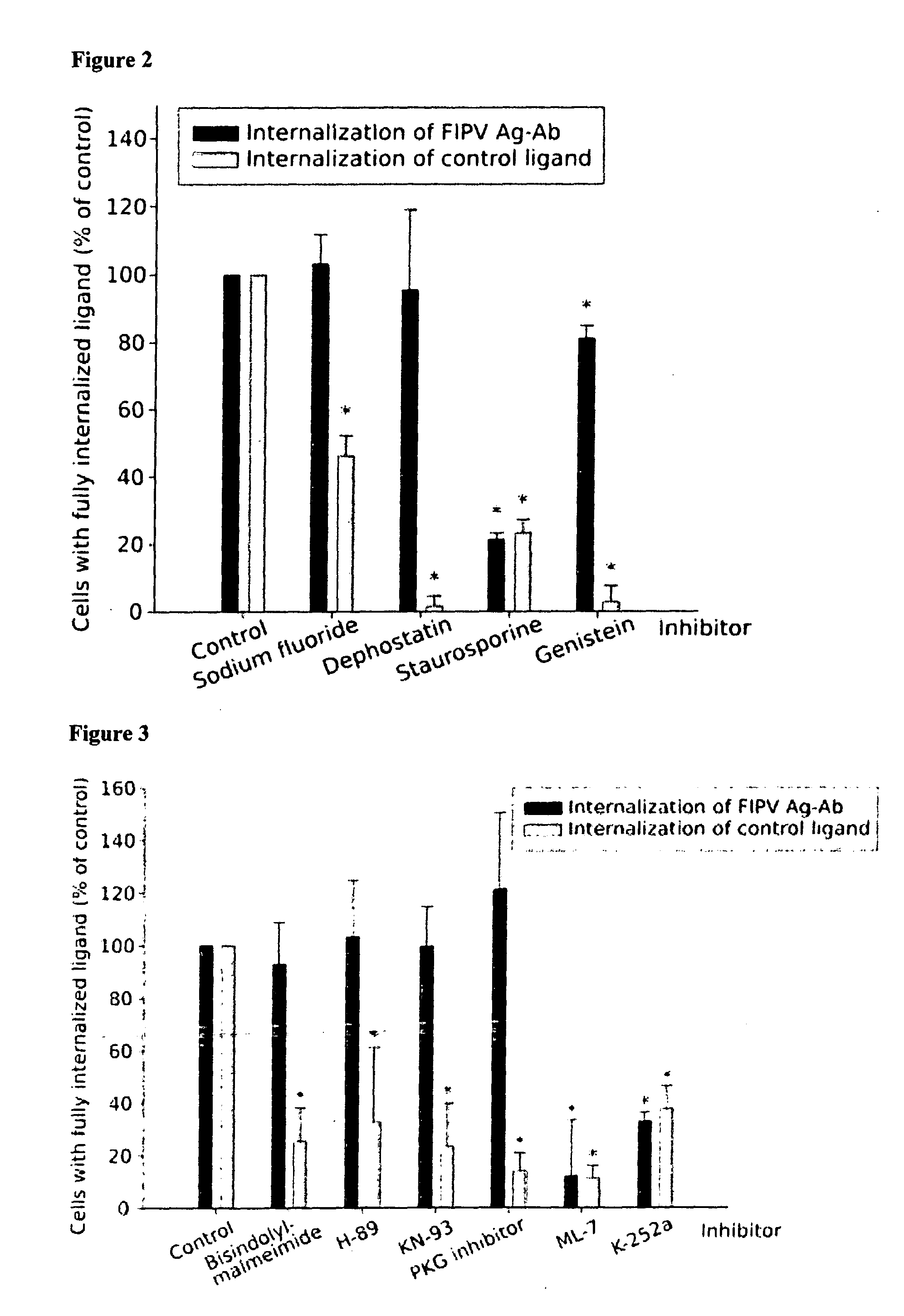
Methods and compositions in the treatment of coronaviruses
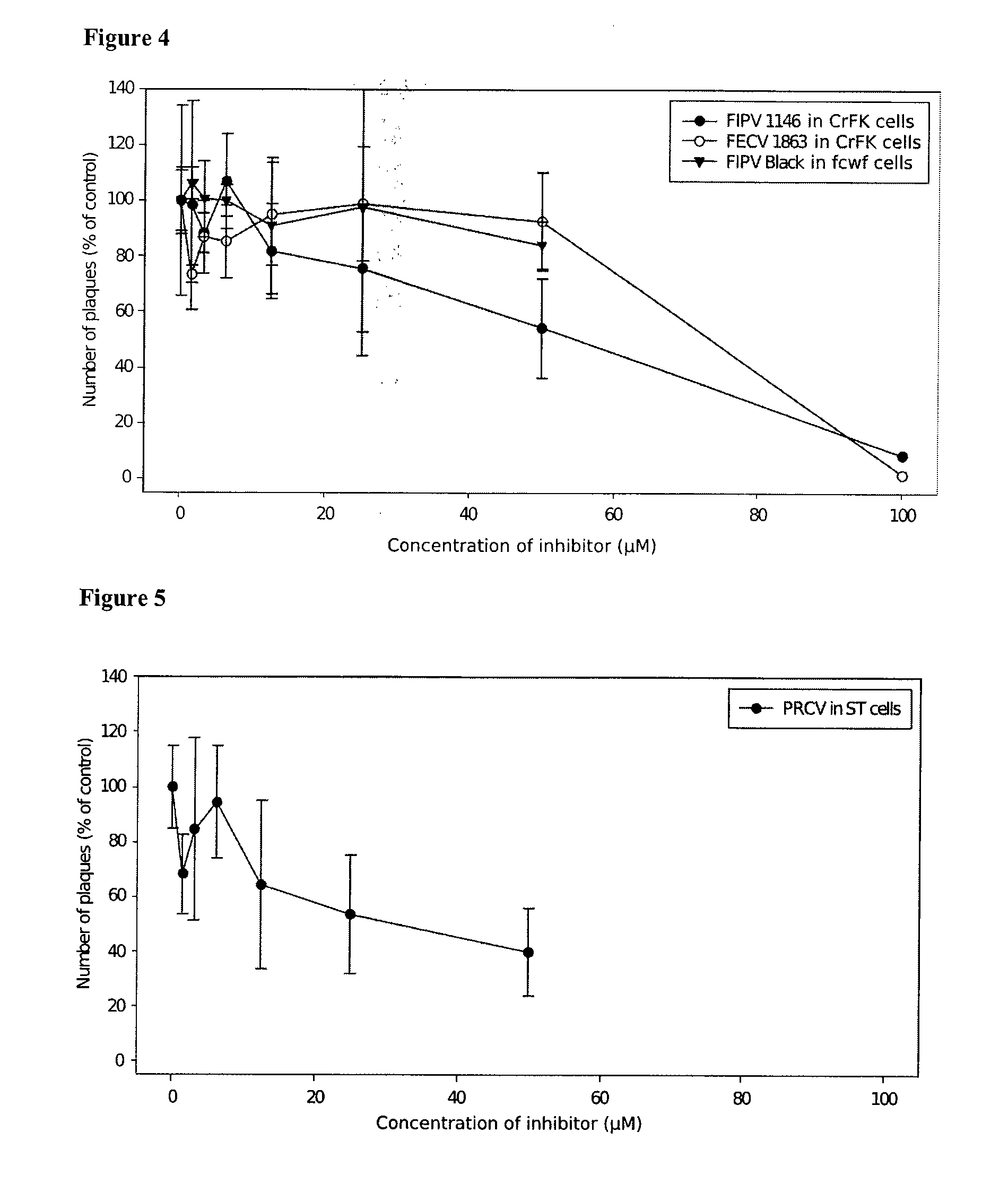
The invention relates generally to the field of virology, and relates to the identification and characterization of the targets involved in the evasion strategy of viruses and to the use thereof in methods to identify anti-viral compounds. More in particular to identify compounds which are modulators of myosin light chain kinase (MLCK), a target within said entry and immune-evasion strategy. Other aspects of the invention are directed to anti-viral compounds identified using the models and methods of the present invention, as well as to the use thereof in treating viral infections, such as for example caused by the feline infectious peritonitis virus (FIPV), a coronavirus which belongs to an antigenic group which comprises in particular feline enteric coronavirus (FECV), canine coronavirus (CCV), swine transmissible gastroenteritis coronavirus (TGEV), porcine respiratory coronavirus (PRCV) and human coronavirus (HCV), and which induces, in a host-dependent manner, a range of symptoms which range from mild enteritis to the severe debilitating disease, and, in some cases, up to death. In a particular aspect the present invention provides the use of MLCK inhibitors for the treatment of feline infectious peritonitis (FIP).
Owner:UNIV GENT
PR-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit and detection method of swine transmissible gastroenteritis virus
InactiveCN103740860AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlSwine Transmissible Gastroenteritis
The invention discloses a PR-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit of swine transmissible gastroenteritis virus, which comprises an RT reaction solution, a positive control, a negative control, DEPC (diethylpyrocarbonate) water, 2*Taq PCR Master Mix and a primer mixed solution. Compared with an existing detection technology, the PR-PCR detection kit of the swine transmissible gastroenteritis virus has the advantages of high sensitivity, good specificity, simple and practical and the like, and can be used for rapidly detecting transmissible gastroenteritis of swine virus in various clinical samples on a large scale.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com