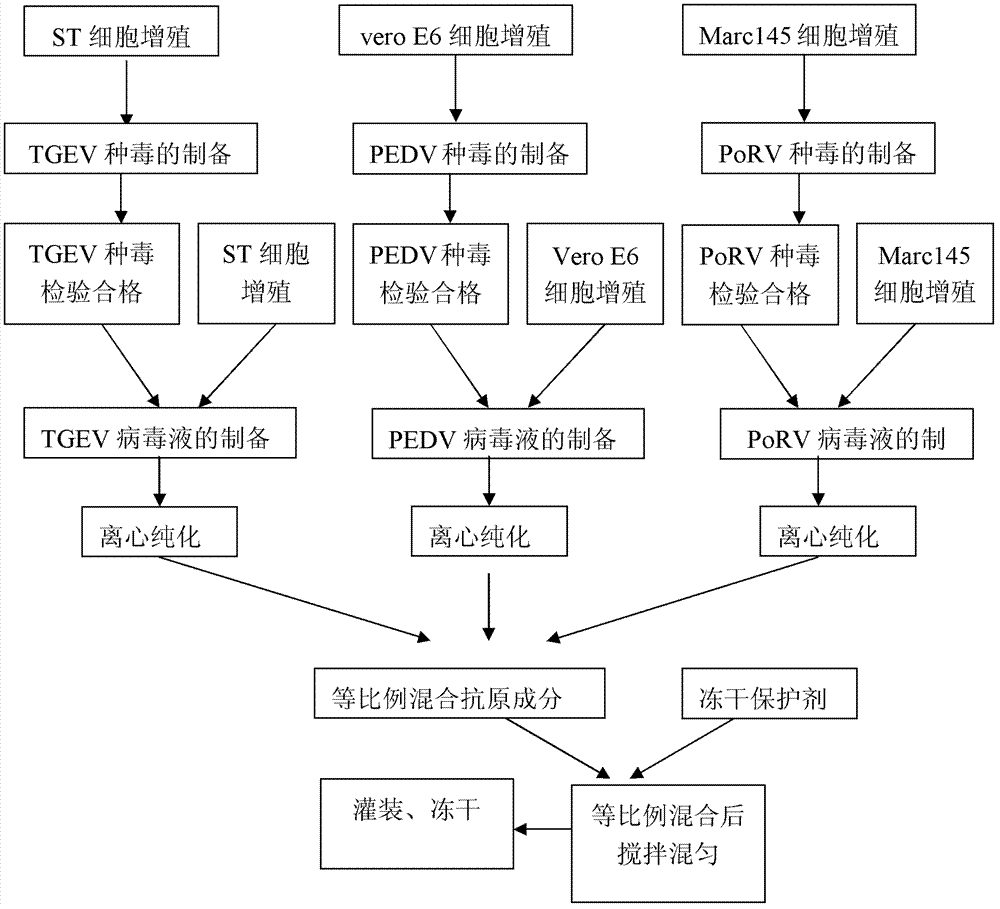
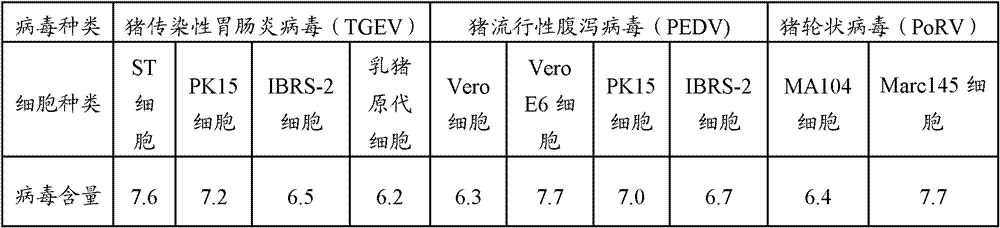
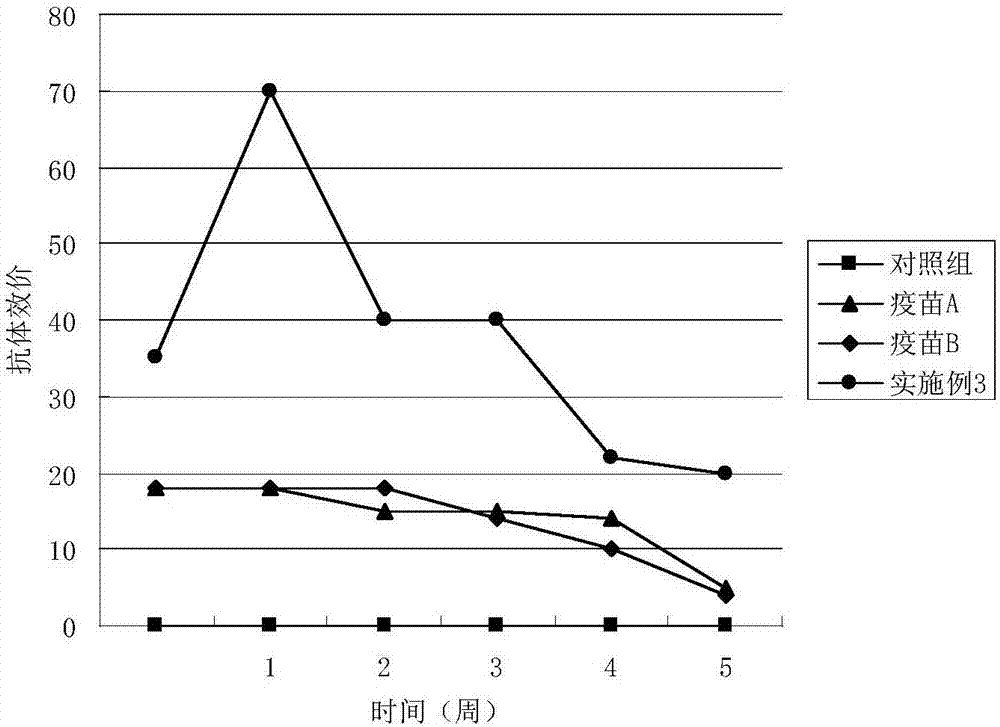
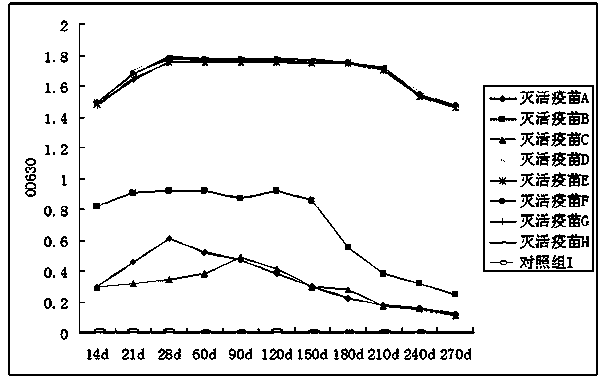
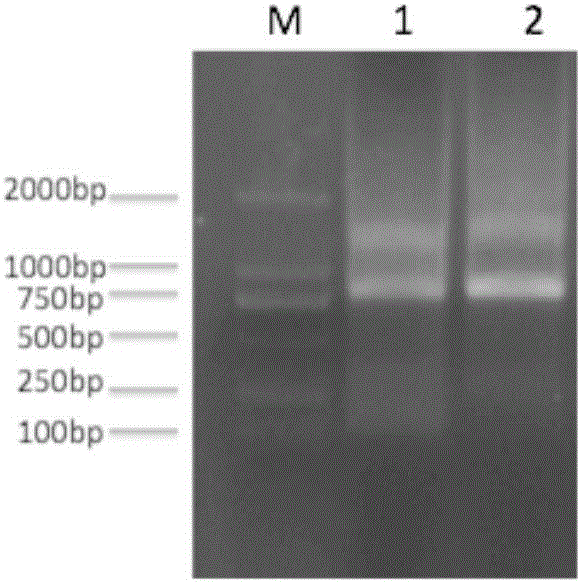
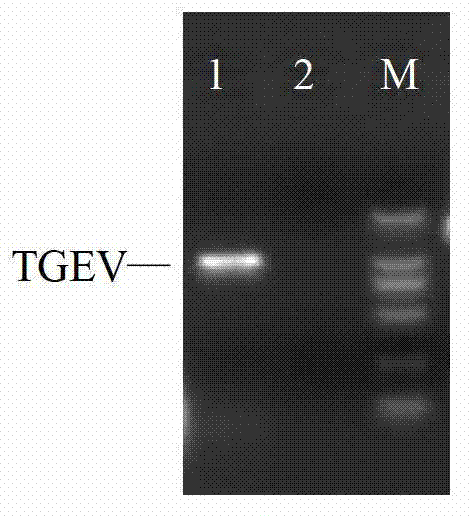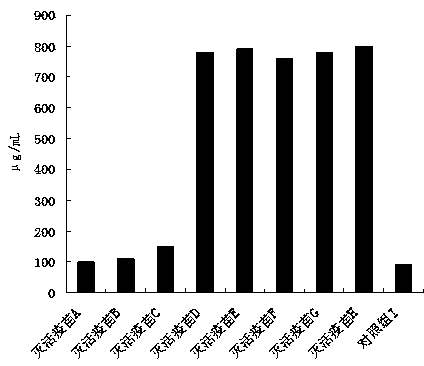Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
404 results about "Epidemic diarrhea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
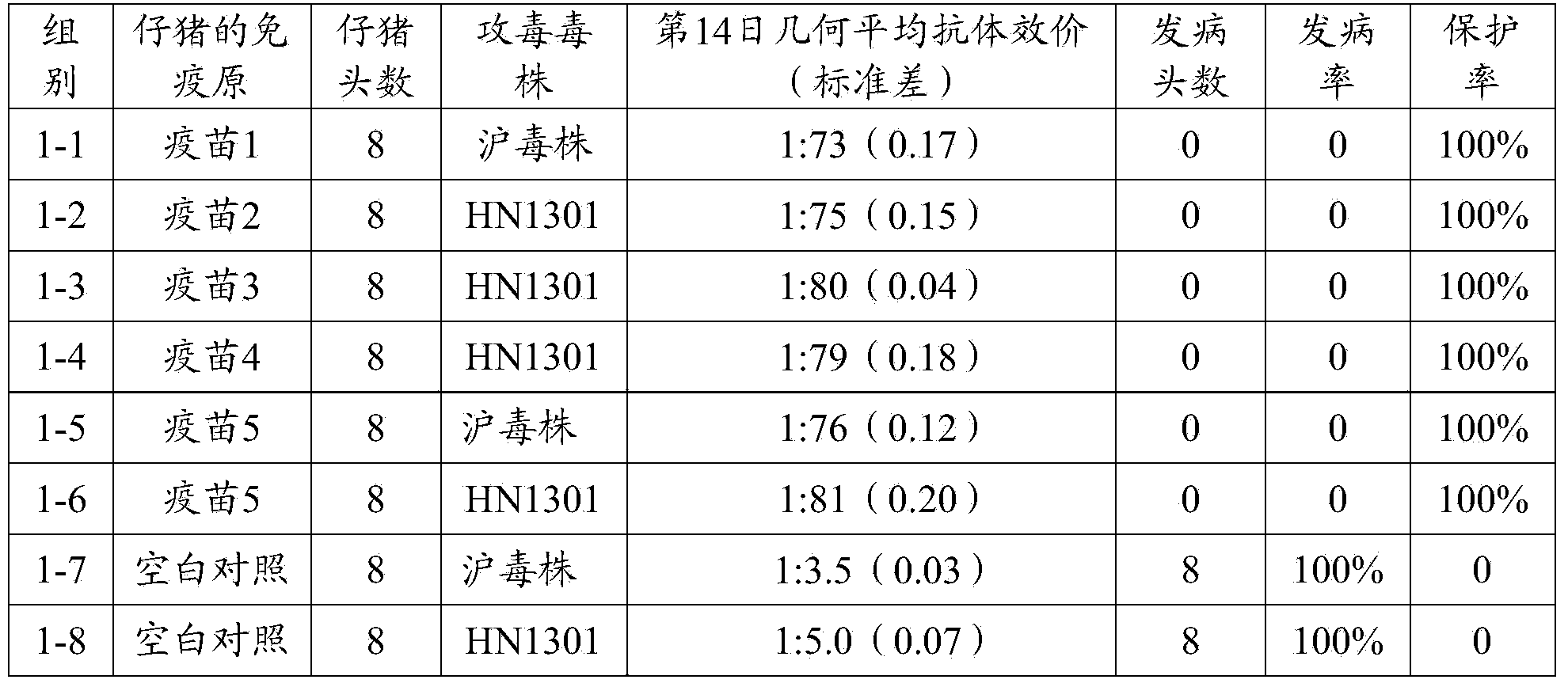
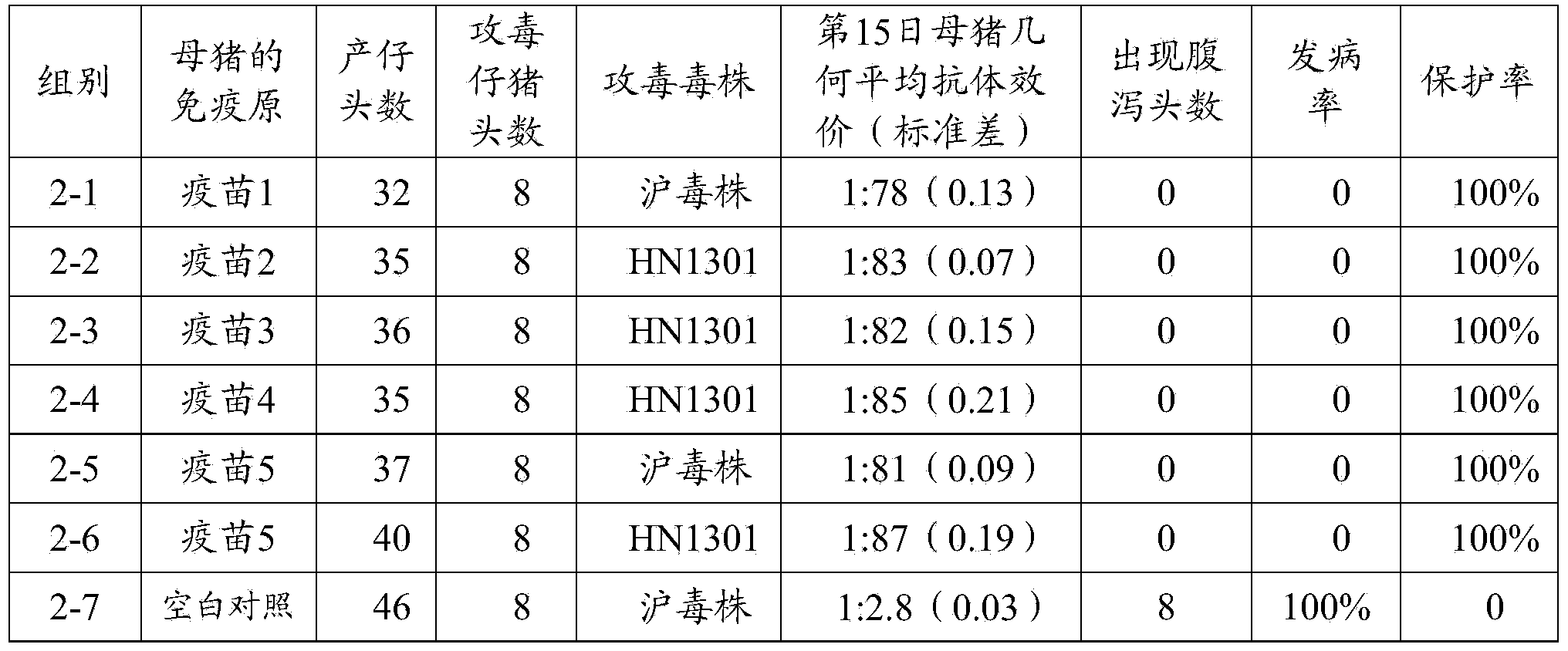
Porcine epizootic diarrhea virus strain, attenuated vaccine strain thereof and application thereof
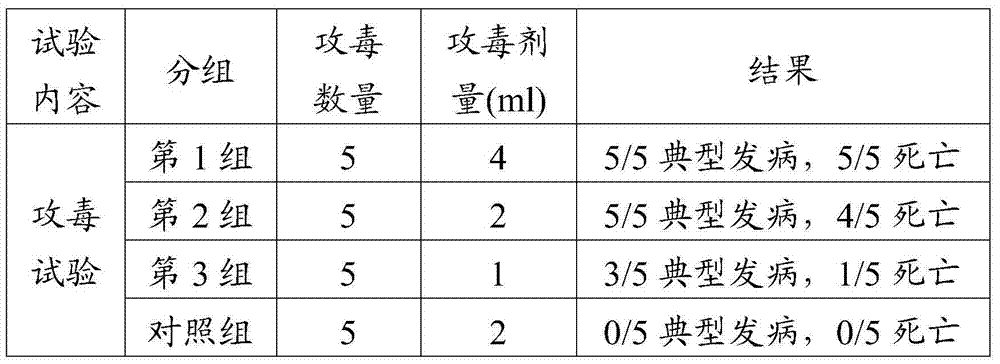
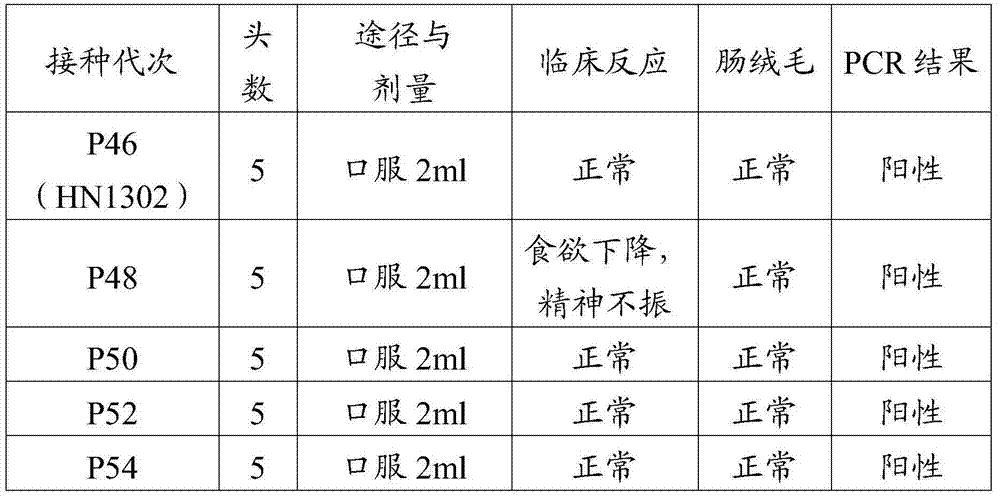
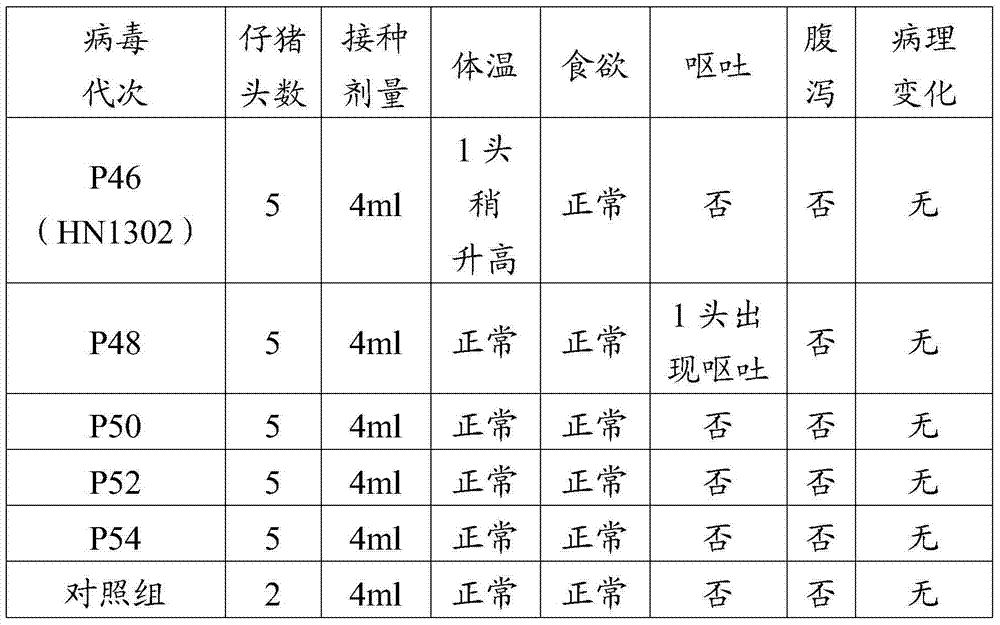
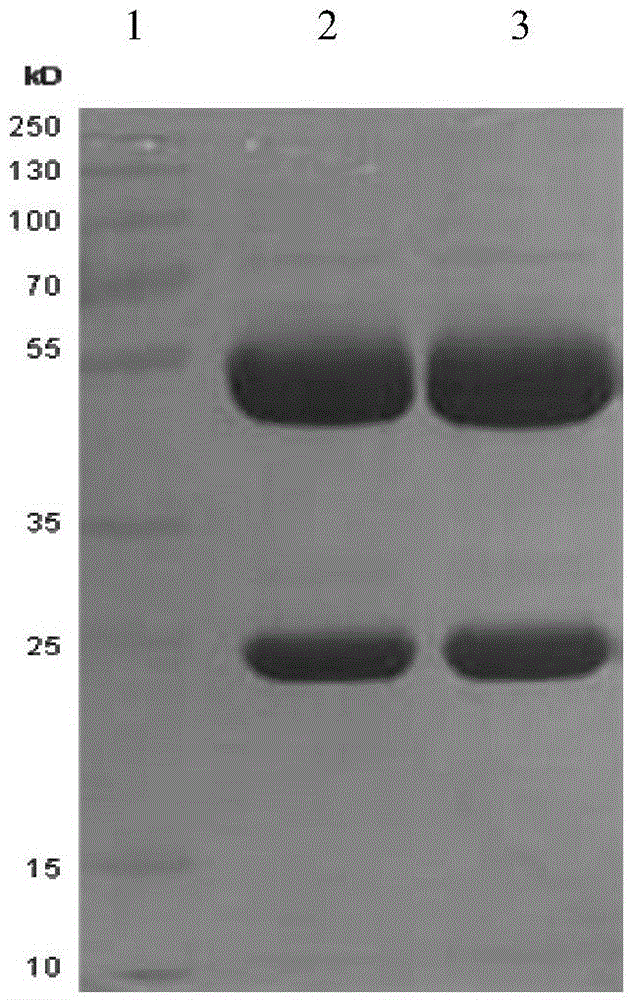
The invention discloses a porcine epizootic diarrhea virus strain (HN1301) and an attenuated vaccine strain (HN1302) attenuated by passage of the porcine epizootic diarrhea virus. The attenuated vaccine strain of the porcine epizootic diarrhea virus is assigned the accession number CCTCC-V201342. The attenuated vaccine strain (HN1302) is good in safety, is strong in immune protective capability and is high in immune efficacy.
Owner:PU LIKE BIO ENG
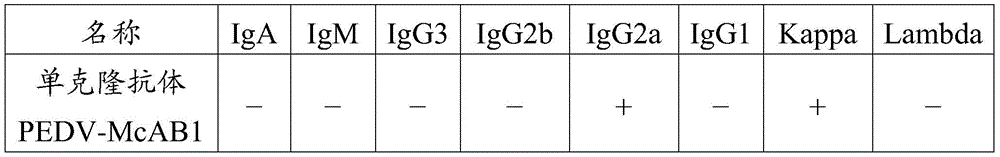
Monoclonal antibody for resisting porcine epidemic diarrhea viruses and application thereof
ActiveCN105461805AResolve infectionImmunoglobulins against virusesAntiviralsEpidemic diarrheaAntibody fragments
The invention provides a variable region sequence for a rat monoclonal antibody specially combined with porcine epidemic diarrhea viruses, a prepared antibody or an antibody fragment thereof, and vaccine and a kit prepared from the antibody. The medicine composition can effectively prevent and treat porcine epidemic diarrhea virus infection. The kit can effectively detect the porcine epidemic diarrhea viruses, is high in sensitivity, and carry out detecting fast.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
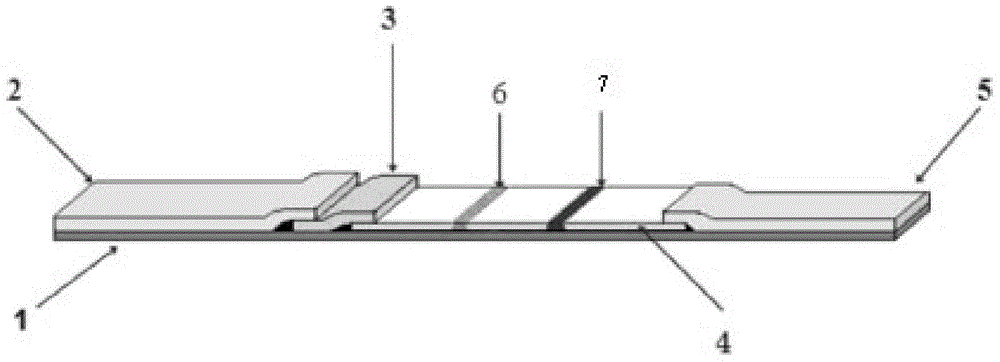
Immunofluorescence detection test strip and preparation method thereof for rapid quantitative detection of porcine epidemic diarrhea viruses
ActiveCN104804082AEasy to operateRealize quantitative detectionImmunoglobulins against virusesFluorescence/phosphorescenceAntigenEpidemic diarrhea
The invention discloses an immunofluorescence detection test strip and a preparation method thereof for rapid quantitative detection of porcine epidemic diarrhea viruses. The test strip comprises a sample cushion, a combination cushion, a chromatography film and a water-absorbing cushion, wherein the combination cushion is provided with a fluorescent microsphere labelled anti-PEDV (Porcine Epidemic Diarrhea Viruses) single-domain antibody; the single-domain antibody has high specificity and high sensibility against the antigen PEDV. The test strip is used for detecting PEVD viruses in breeding pig manure or PEDV pollution in feeding stuff plasma proteins on the basis of the newfound immunology principle of the specific single-domain antibody and antigen, can be used for on-site rapid detection or laboratory detection by simply taking 5-10 min; when the test strip is used in combination with a fluorescence quantitative detector, quantitative detection can be realized; the test strip is simple and convenient to operate, operators don't need professional training, a special laboratory is not needed, the limitation of the conventional detection method is overcome, and the test strip has good market prospects.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
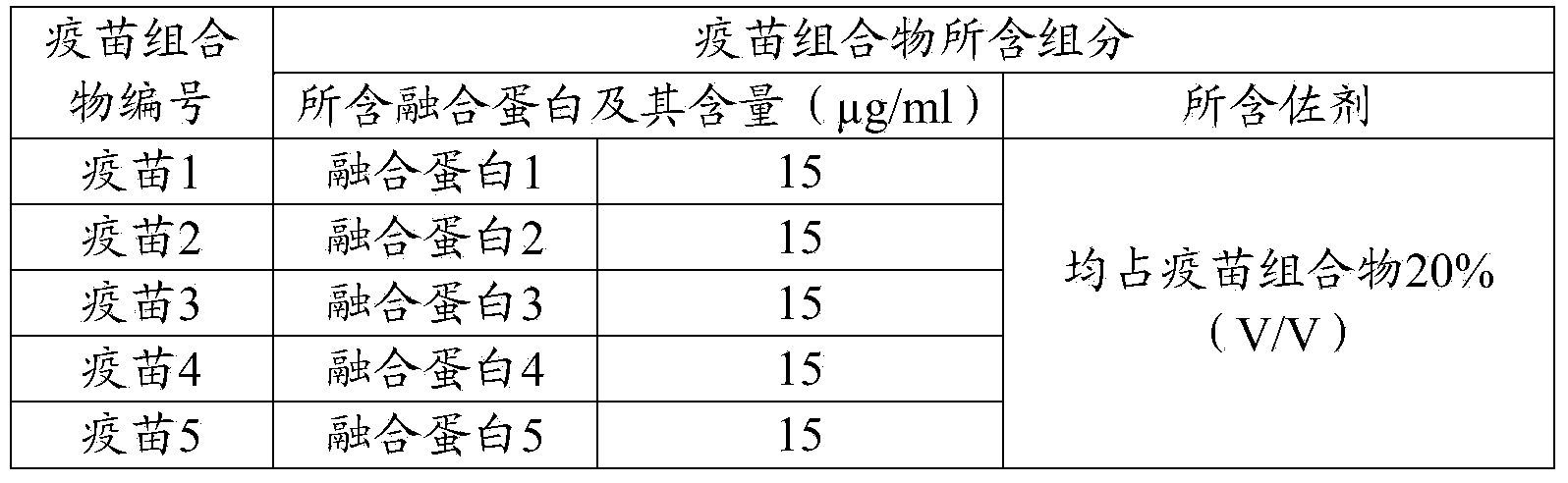
Preparation and application of fusion protein and vaccine composition thereof
ActiveCN104262488AShort timeEase of mass productionAntiviralsPharmaceutical non-active ingredientsDiseaseImmunoglobulin Fc Fragments
The invention provides a fusion protein, a porcine epidemic diarrhea vaccine composition containing the fusion protein and application thereof. The fusion protein contains porcine epidemic diarrhea virus antigenic protein and immunoglobulin Fc segment, wherein the porcine epidemic diarrhea virus antigenic protein contains a protein formed by series combination of porcine epidemic diarrhea virus S protein segments. The invention also provides a porcine epidemic virus vaccine composition which contains the fusion protein and a carrier. The invention also provides a preparation method of the vaccine composition and application of the vaccine composition in preparing drugs for preventing and / or treating diseases initiated by porcine epidemic diarrhea virus. The vaccine composition prepared from the fusion protein avoids the technical problem that the porcine epidemic diarrhea virus totivirus can not be easily separated and cultured in the traditional vaccine inactivation process. The fusion protein can utilize the gene engineering technique to perform abundant recombinant expressions, has the advantage of short time consumption, and is convenient for large-scale production.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea virus stain and application thereof
ActiveCN103725651AImprove securityGood immune protectionMicroorganism based processesAntiviralsEpidemic diarrheaMicroorganism
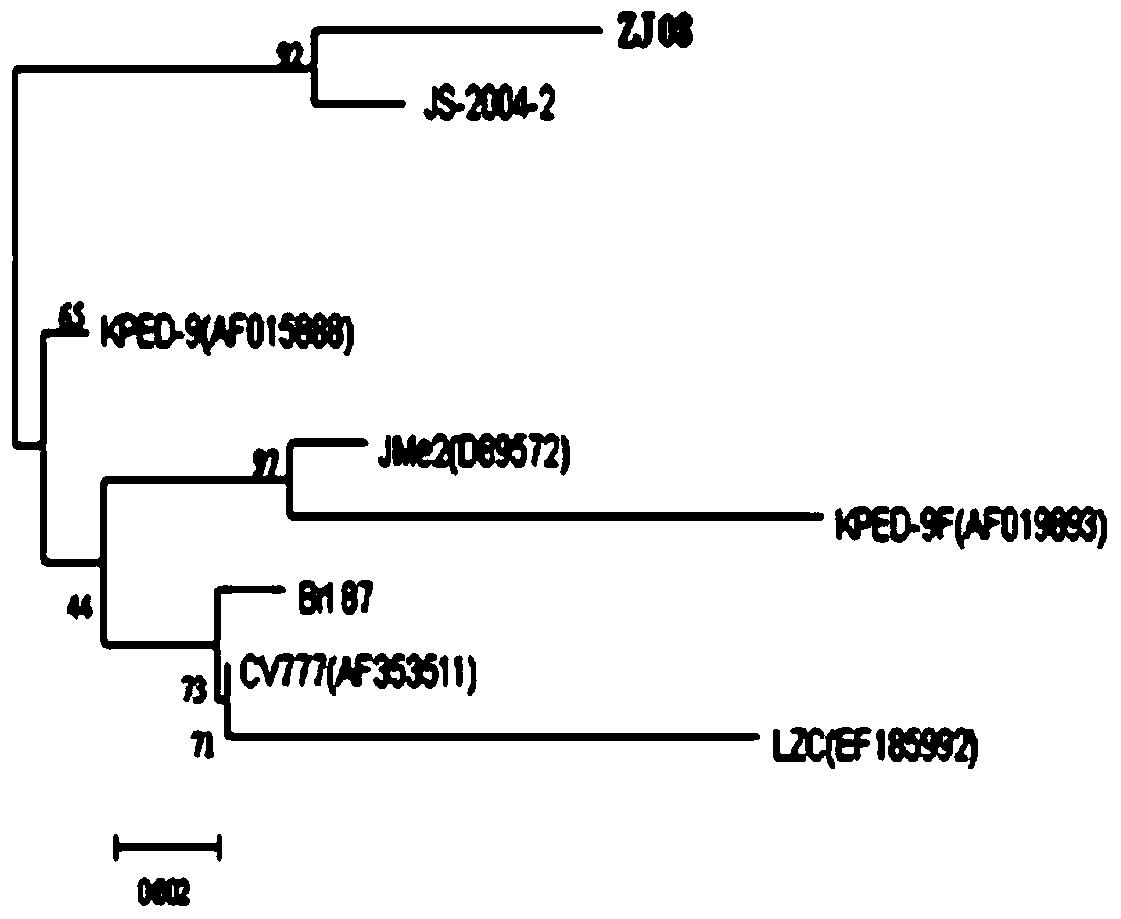
The invention discloses a porcine epidemic diarrhea virus low-virulent stain ZJ08 and application thereof. The microbial collection number of the low-virulent stain is CGMCC No.7806. The PEDV low-virulent stain has high safety, and is safe for pregnant sows, baby pigs and pigs in all ages. The active protection ratio of the low-virulent stain for 3-day baby pigs reaches 100%, and the passive protective ratio reaches higher than 94.7%, which indicates that the low-virulent stain has favorable immunoprotection effect on porcine epidemic diarrhea.
Owner:兆丰华生物科技(南京)有限公司 +3
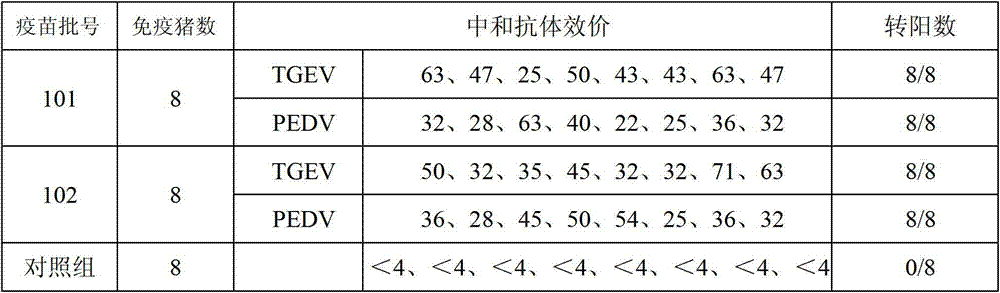
Porcine epidemic diarrhea vaccine composition, and preparation method and application thereof
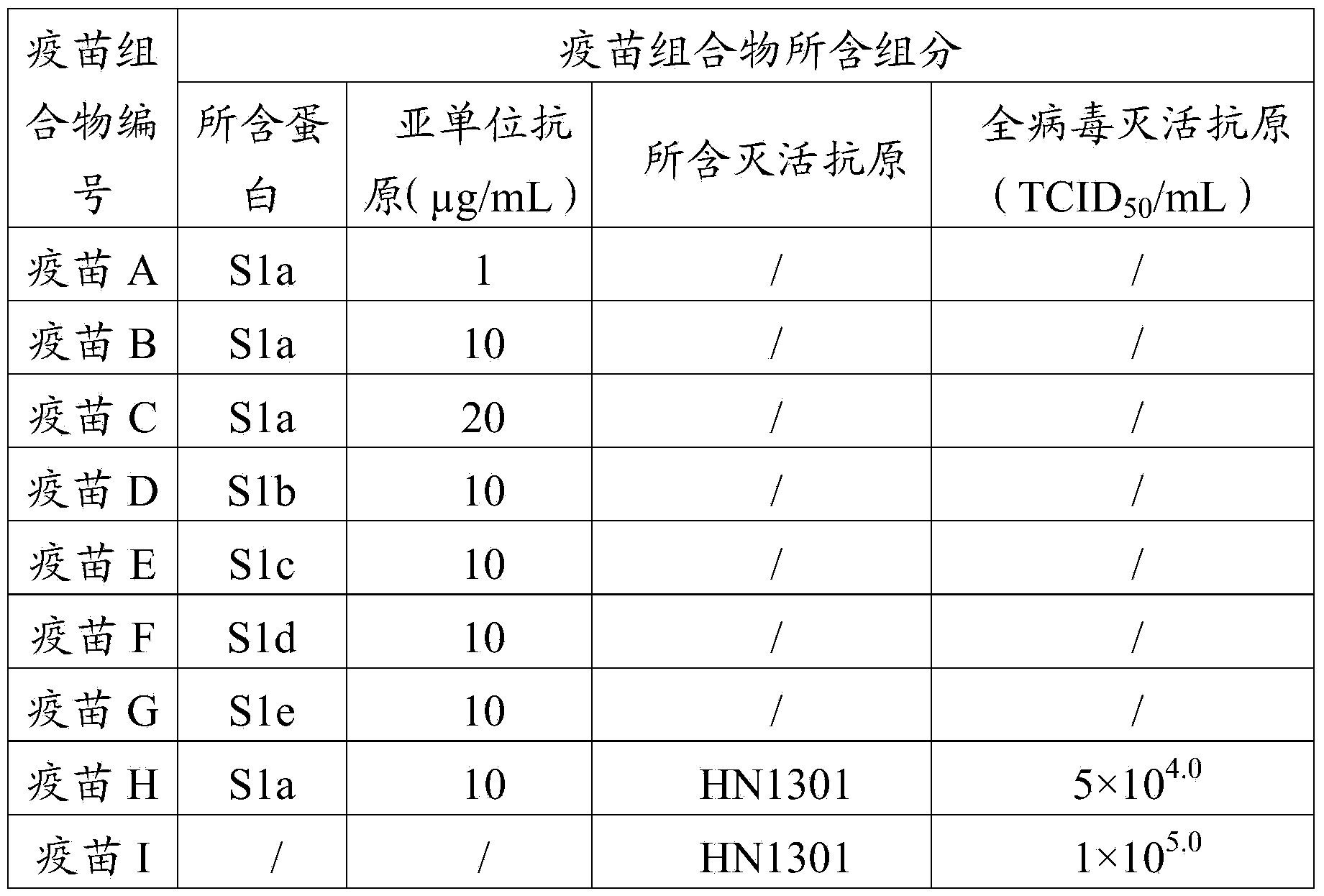
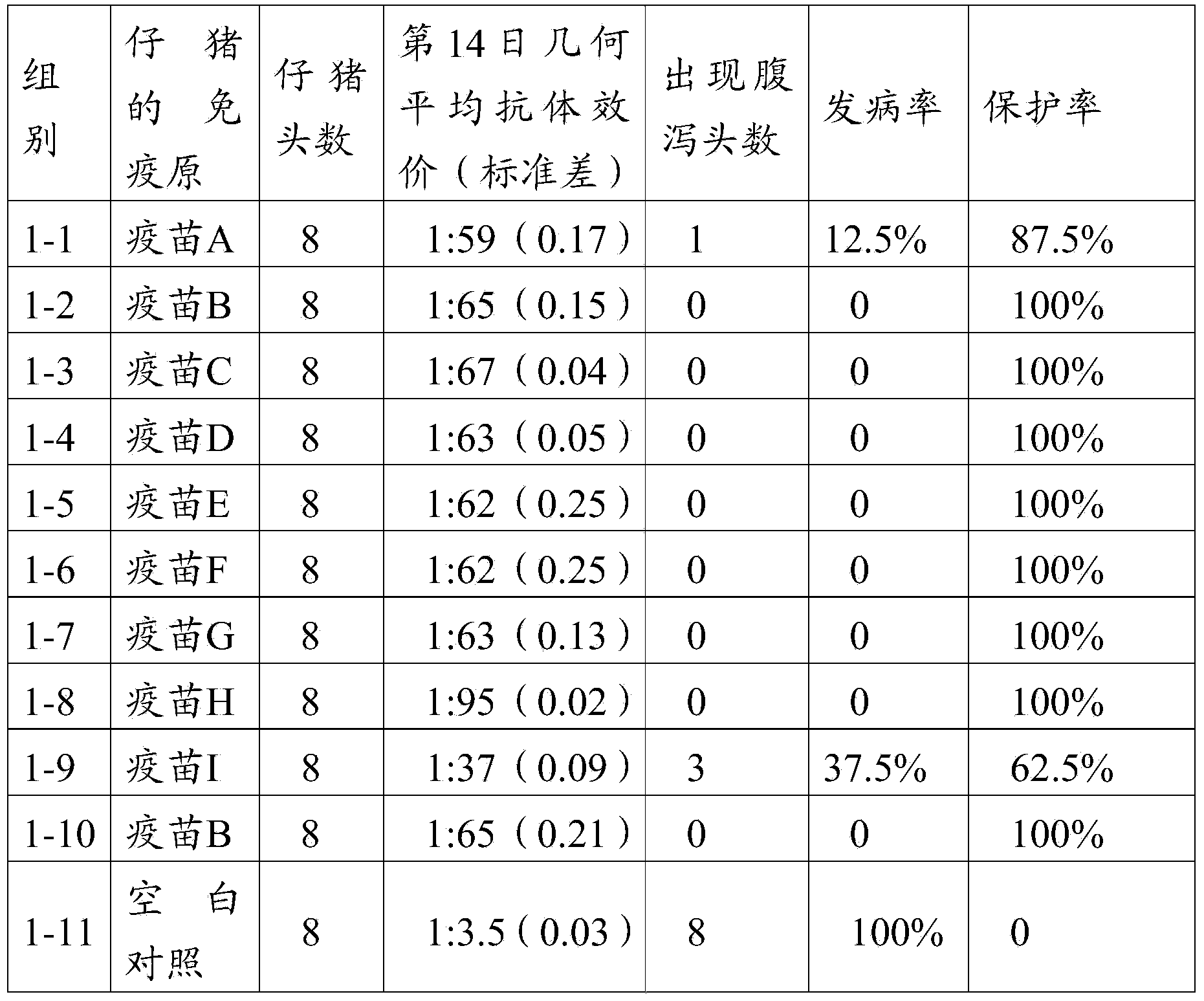
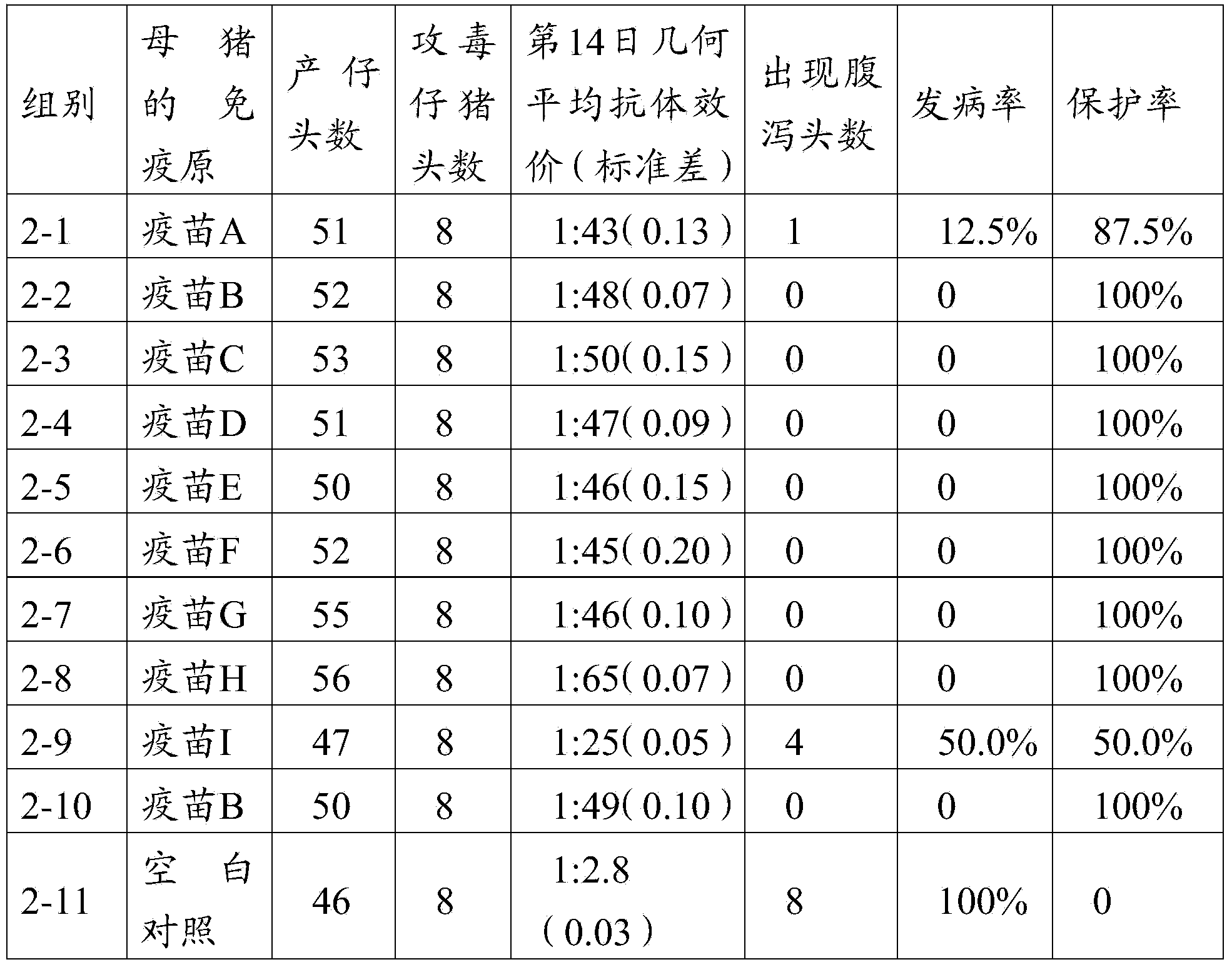
The invention provides a porcine epidemic diarrhea vaccine composition. The vaccine composition contains a porcine epidemic diarrhea virus subunit antigen and a vector. The invention also provides a porcine epidemic diarrhea vaccine composition, and the vaccine composition comprises a porcine epidemic diarrhea virus subunit antigen, a porcine epidemic diarrhea totivirus inactivated antigen and a vector. The invention also discloses a preparation method of the vaccine composition and application thereof to preparation of drugs for preventing and / or treating porcine epidemic diarrhea. An S1 protein in the vaccine composition can conduct large amount of recombinant expression of components in the vaccine composition by means of genetic engineering, so as to realize short time consumption as well as facilitate large-scale production. When the vaccine composition contains the S1 protein and porcine epidemic diarrhea totivirus inactivated antigen, the vaccine composition has better immune effect than single usage of totivirus inactivated antigen, and can effectively prevent diseases caused by variants of porcine epidemic diarrhea and common strains.
Owner:PU LIKE BIO ENG
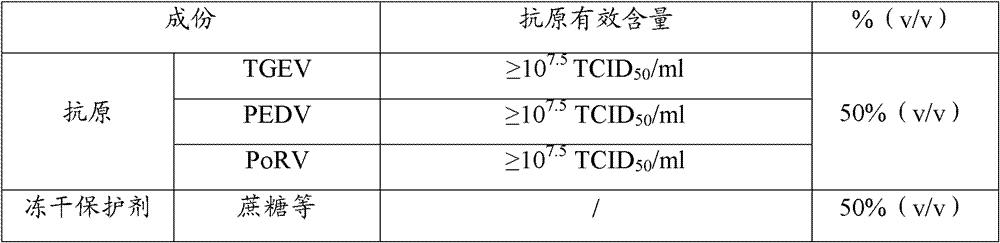
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Feed and traditional Chinese medicine for treating pigling epidemic diarrhea and preparation method thereof
InactiveCN102613412AImprove the immunityGood curative effectAnimal feeding stuffAccessory food factorsTherapeutic effectAristolochia debilis
The invention provides feed which comprises a puffing feed carrier, a micro-organism bacterial agent, a trace element additive, an amino acid additive and a traditional Chinese medicine additive, wherein the traditional Chinese medicine additive further comprises galangal, cumin, root of chinese barberry, herba lagotis, partrinia, phellodendron, astragalus, atractylodes, myrobalan, limonitum, dendrobium, liriope, hairy euphorbia, aristolochia debilis, coriolus versicolor, Kapok and schisandra chinensis. According to the invention, the traditional Chinese medicine composition has the efficacies that the treatment of epidemic diarrhea of pigling pays an attention to treating both principal and secondary aspect of epidemic diarrhea; the treatment effect is good; drug residue does not exist, functions of an organism can be aroused, the resistance of the organism is improved.
Owner:HAIMEN XINGWANG MEAT PROD
Indirect ELISA (enzyme linked immunosorbent assay) kit for detecting porcine epidemic diarrhea virus antibody
ActiveCN103675274AStrong specificityIncreased sensitivityBiological material analysisSerum igeEpidemic diarrhea
The invention discloses an indirect ELISA (enzyme linked immunosorbent assay) kit for detecting a porcine epidemic diarrhea virus antibody. The indirect ELISA kit comprises a coated ELISA plate, negative control serum, positive control serum, ELISA secondary antibody, a concentrated cleaning solution, a sample diluent, a developing liquid and a stop buffer, wherein the coated ELISA plate utilizes recombinant protein Nh as a coating antigen. Research and practice show that the indirect ELISA kit has the characteristics of high specificity and sensitiveness, simplicity in operation and the like, is easy to popularize and use in a large range, has a broad market prospect, and can be used for the fields of serological investigation, antibody monitoring and vaccine immunity effect evaluation and the like of epidemic diarrhea infection condition.
Owner:GUANGXI UNIV
Fluorescent quantitative PCR primers and probes for porcine epidemic diarrhea viruses
ActiveCN103509882AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaFluorescent quenching
The invention discloses fluorescent quantitative PCR (polymerase chain reaction) primers and probes for porcine epidemic diarrhea viruses. An upstream primer and a downstream primer are shown as SEQIDNO: 1-2; a probe P1 is shown as SEQIDNO: 3; a probe P2 is shown as SEQIDNO: 4; the 3' end of sequences of the probe 1 and the probe 2 are combined with a fluorescent quenching group; and the 5' end of sequences of the probe 1 and the probe 2 are combined with different fluorescent reporter groups. The primers are relatively high in specificity and sensitivity, can detect out variant PEDV (porcine epidemic diarrhea viruses), and can detect and differentiate a variety of clinical samples so as to quickly differentiate epidemic PEDV strains; the operation is simple and practical.
Owner:WENS FOODSTUFF GRP CO LTD
Pure traditional Chinese medicine recipe for curing various animal diarrhoeal diseases and preparation method of pure traditional Chinese medicine recipe
InactiveCN103006916AImprove stress responseReduce diarrhea rateDigestive systemPlant ingredientsEpidemic diarrheaDiarrhoeal disease
The invention discloses a pure traditional Chinese medicine recipe for curing various animal diarrhoeal diseases and a preparation method of the pure traditional Chinese medicine recipe. The pure traditional Chinese medicine recipe comprises the following ingredients: 1 g of white paeony root, 1 g of atractylis ovata, 1 g of Chinese pulsatilla root, 1 g of rhizoma atractylodis, 2 g of dried orange peel, 1 g of folium isatidis, 1 g of cortex phellodendri, 0.75 g of fructus forsythiae, 3 g of dandelion, 1.5 g of ash bark, 5 g of garden burnet, 1 g of pomegranate rind and 1 g of tuckahoe. The recipe has an effect on curing various animal diarrhoeal diseases such as piglet's yellow-white dysentery, epidemic diarrhea and transmissible gastroenteritis; the preparation method is simple and convenient to operate; and suitability for popularization and application is obtained.
Owner:SICHUAN AGRI UNIV
ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for porcine epidemic diarrhea viruses and preparation method thereof
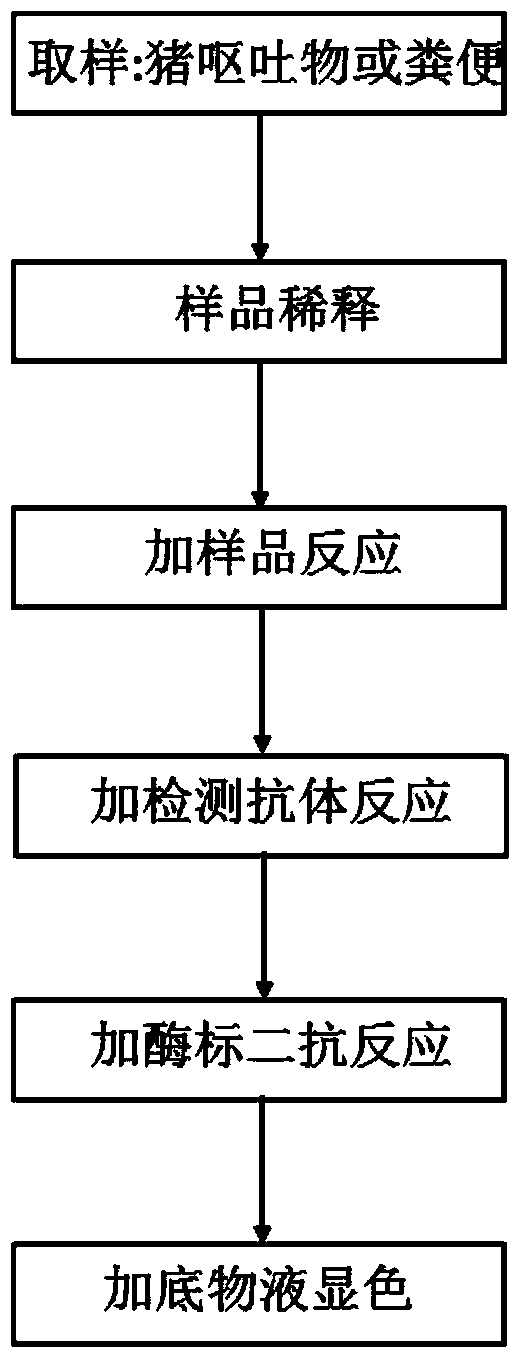
The invention relates to an ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for a porcine epidemic diarrhea virus (PEDV) and a preparation method of the ELISA detection kit. The ELISA detection kit for the porcine epidemic diarrhea virus comprises an ELISA plate, a detection antibody and an ELISA second antibody, wherein the ELISA plate is coated with a specific egg yolk antibody of the porcine epidemic diarrhea virus; the detection antibody is a polyclonal antibody of the porcine epidemic diarrhea virus; the ELISA second antibody is an antibody which is labeled by an enzyme and resists the detection antibody. The ELISA detection kit for the porcine epidemic diarrhea virus takes the PEDV of vomitus and excrement of diarrhea pigs as a detection object and can find PEDV infected pigs as soon as possible; meanwhile, the operation difficulty of the detection is reduced.
Owner:SHENZHEN BROSTIGER BIO PHARMA
Recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, vaccine and application
InactiveCN107619819AEase of mass productionGood antigenicityAntiviralsAntibody medical ingredientsEpidemic diarrheaAdjuvant
The invention discloses a recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, a vaccine and an application. The recombinant cell line is constructed by transfecting HEK-293T cells by virtue of recombinant plasmid which is constructed by carrying a target gene on a lentiviral vector, and then transfecting the HEK-293T cells by virtue of generated high-titer virus particles; and the recombinant cell line, which can achieve stable expression, can still keep an excellent protein expression level after several passages. The recombinant cell line for stable expression of the porcine epidemic diarrhea virus S1 protein provided by the invention has the characteristics of being easy for culture, rapid in proliferation, unlimited in expansion, stable in property and high in protein expression amount; and when the vaccine, which is prepared from the expression protein and adjuvants, is used for immunizing pigs, the generation of a high-titer porcine epidemicdiarrhea virus neutralizing antibody can be induced from animal bodies, and the piglets (the pigs) can resist strong attack of porcine epidemic diarrhea viruses.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
Antiviral live-hog feed
InactiveCN106107042ANutritional balanceImprove immunityFood processingAnimal feeding stuffInfection rateRadix Astragali seu Hedysari
The invention relates to the technical field of the breeding of live hogs, and particularly relates to antiviral live-hog feed, which is prepared from 90 percent of basal feed, 6 percent of Chinese herbal medicinal additive and 4 percent of growth promoter, wherein the basal feed is prepared from corn, peanut meal, bean meal, rice bran and wheat bran; the Chinese herbal medicinal additive is prepared from herba houttuyniae, pericarpium citri reticulatae, herba taraxaci, radix bupleuri, fructus forsythiae, radix astragali seu hedysari, flos lonicerae, caulis lonicerae japonicae, radix isatidis and the like; the growth promoter is prepared from shrimp shell powder, bone powder, fish meal, folic acid, choline, calcium lactate and the like. The antiviral live-hog feed is complete and balanced in nutrition; the immunity of a hog can be enhanced; the infection rate of a virus is reduced; the use of an antibiotic and the residue of a medicine are reduced. Through the compatibility of multiple traditional Chinese medicinal materials, the antiviral live-hog feed has the effects of clearing away heat and toxic materials and being obviously antiviral, has obvious inhibiting effects on all of epidemic diarrhea, a porcine reproductive and respiratory syndrome, influenza viral pneumonia and the like, and can be used for replacing a conventional antibiotic; the residue of the medicine is reduced; the economic benefit of a breeder is improved.
Owner:云南金碑康康生态科技有限公司
Porcine epidemic diarrhea virus inactivated vaccine and preparation method thereof
ActiveCN107050447AImproving immunogenicityPassive immunity is goodSsRNA viruses positive-sensePeptide/protein ingredientsEpidemic diarrheaMicroorganism
The invention provides a porcine epidemic diarrhea virus inactivated vaccine. The porcine epidemic diarrhea virus inactivated vaccine contains the inactivated porcine epidemic diarrhea virus and an adjuvant, wherein the adjuvant is prepared from the following components in percentage by weight: 5% of squalane, 1% of oleic acid, 1% of Tween 80, 92% of 0.005M sodium citrate buffer solution, and 1% of beta-glucan, the porcine epidemic diarrhea virus is prepared by inactivating the porcine epidemic diarrhea virus strain PEDV-KB2013-4, is assigned with the microbial accession number of CGMCC No.12663, has the classification name of Porcine Epidemic Diarrhea Virus (PEDV), and is preserved in the China General Microbiological Culture Collection Center (CGMCC) on Aug 23, 2016, and the preservation address is the Institute of Microbiology of Chinese Academy of Sciences located in 3, Courtyard 1, West Beichen Road, Chaoyang District, Beijing.
Owner:陕西诺威利华生物科技有限公司 +1
PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
ActiveCN104383528AEnhance immune responseImprove the level ofOrganic active ingredientsDipeptide ingredientsAntiendomysial antibodiesDipeptide
The invention provides a PED (Porcine Epedemic Diarrhea) inactivated vaccine and a preparation method thereof and relates to the field of biopharmacy. The PED inactivated vaccine comprises inactivated PEDV (Porcine Epedemic Diarrhea Virus), and is characterized in that the PED inactivated vaccine comprises 0.05-10 mg / mL Beta-glucosylceramide, 0.1-21 mg / mL monophosphoryl lipid A, 1.5-125 mg / mL muramyl dipeptide and 0.7-4.5 mg / mL Beta-glucan. According to the ingredients of the PED inactivated vaccine, Beta-glucosylceramide, monophosphoryl phosphoryl lipid A, muramyl dipeptide and Beta-glucan have the synergistic effect, the immune response of animals to antigens in the vaccine is significantly improved, the immune window phase is shortened, the antibody production duration of the animal body is obviously prolonged, the serum antibody level is improved, and the level of total intestinal mucosa secretory antibodies (the total SIgA) is improved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Domestic animal and aquatic product causal agent resistant specific IgY or compound IgY and application thereof
InactiveCN101081866AEgg immunoglobulinsAntibody ingredientsAquatic animalSwine Transmissible Gastroenteritis
The present invention relates to one kind of specific or compound IgY for antagonizing pathogen of livestock and aquatic animals, and features that the specific or compound IgY is one or combination of specific IgY for antagonizing pathogene of swine transmissible gastroenteritis and epidemic diarrhea, specific IgY for antagonizing virus of swine transmissible gastroenteritis and epidemic diarrhea, specific compound IgY for antagonizing aquatic animals' pathogene, specific compound IgY for antagonizing aquatic animals' fungi, specific compound IgY for antagonizing aquatic animals' viruses, specific IgY for antagonizing reovirus, specific IgY for antagonizing fishes'herpes virus, specific IgY for antagonizing pathogene of ox transmissible gastroenteritis and epidemic diarrhea, and specific IgY for antagonizing virus of ox transmissible gastroenteritis and epidemic diarrhea. The present invention relates to also the preparation process and application of the specific or compound IgY.
Owner:深圳雅臣生物科技有限公司
Chinese herb extract feed additive capable of effectively preventing and treating porcine epizootic diarrhea
ActiveCN103141702AImprove immunityPromote growthAntibacterial agentsBacteria material medical ingredientsFood additiveBiotechnology
The invention discloses a Chinese herb extract feed additive capable of effectively preventing and treating porcine epizootic diarrhea, and belongs to the field of feed additives. The feed additive is prepared from the main materials of snow grass meal, curcuma powder, semen cassiae powder, burdock powder, shallot white powder, Chinese herbal medicine preparation and curcuma extract, and toadstool and bacillus subtilis are scientifically compounded, so that the application amount of antibiotics medicine in cultivation of pigs is effectively reduced, the use amount of the antibiotic is reduced by above 60%, the drug property of the Chinese herbal medicine is improved, the porcine epizootic diarrhea caused by porcine epidemic diarrhea virus can be effectively prevented and treated, and the Chinese herb extract feed additive is safe and non-toxic. Besides, the amount of the additive in the feed is low, the quality is stable, an acting effect is improved by combining different Chinese herb extracts, the porcine epizootic diarrhea is reduced by 60-80%, and the taste and quality of animal products are obviously improved. A sense experimental evaluation proves that the evaluation score on the taste and quality of the animal products using the additive is improved by above 65%.
Owner:兴安双胞胎饲料有限公司
Swine-derived single-chain antibody resistant to porcine epidemic diarrhea viruses and preparing method thereof
The invention discloses a swine-derived single-chain antibody resistant to porcine epidemic diarrhea viruses and a preparing method thereof. The swine-derived single-chain antibody resistant to the porcine epidemic diarrhea viruses is formed by connecting a heavy chain variable region VH with a light chain variable region VL of an antibody through short connecting peptide Linker. The amino acid sequence of the light chain variable region VL is shown as SEQ ID No.1, and the amino acid sequence of the heavy chain variable region VH is shown as SEQ ID No.2. The molecular weight of the single-chain antibody resistant to the obtained porcine epidemic diarrhea viruses is about 28 kDa, the porcine epidemic diarrhea viruses can be specifically identified, and the single-chain antibody can be used for further blocking infection and invasion of the porcine epidemic diarrhea viruses.
Owner:SHANGHAI JIAO TONG UNIV
Porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application
InactiveCN102399806AReduce colonizationPromote accumulationBacteriaGenetic material ingredientsBacillus megateriumCell wall
The invention discloses a porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application. An antigen fusion gene shown as SEQ ID No. 1. is obtained by connecting an antigen locus of porcine epidemic diarrhea virus (PEDV) S glycoprotein and a cell wall anchoring sequence. The invention further constructs a secretion expression vector containing the antigen fusion gene, and secretion expression vector is converted into the bacillus megaterium to express recombinant protein on the cell wall or surface of the bacillus megaterium. Immunoblotting experiments indicate that the expressed recombinant protein can react with PEDV immune serum and has the same antigenicity as PEDV natural antigens. Immunofluorescent tests of live bacteria which are subjected to induced expression indicate that the expressed recombinant protein is positioned to the surfaces of the bacteria. Experiment results indicate that the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating porcine epidemic diarrhea.
Owner:WUHAN HUAYANG ANIMAL PHARMA
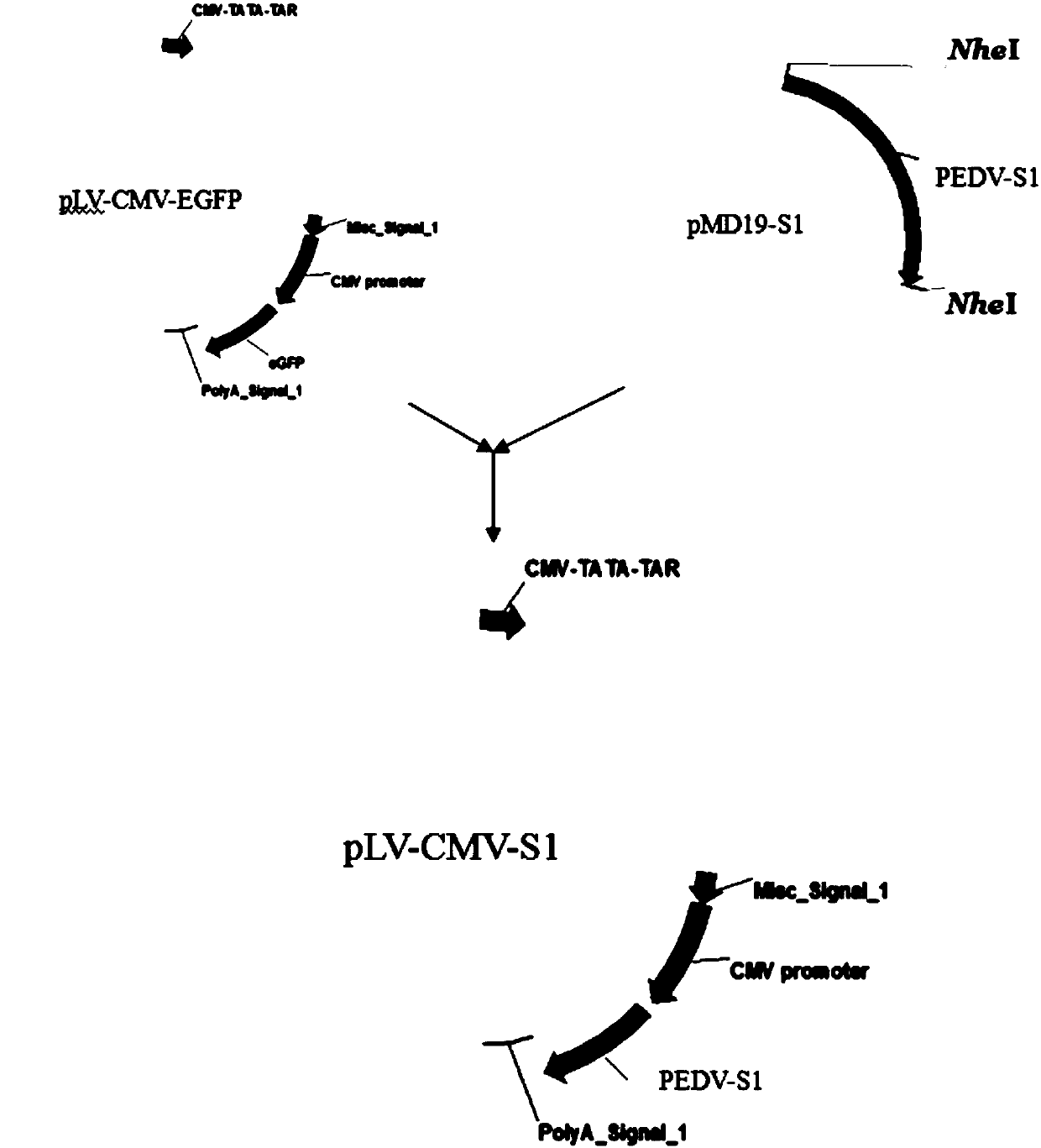
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
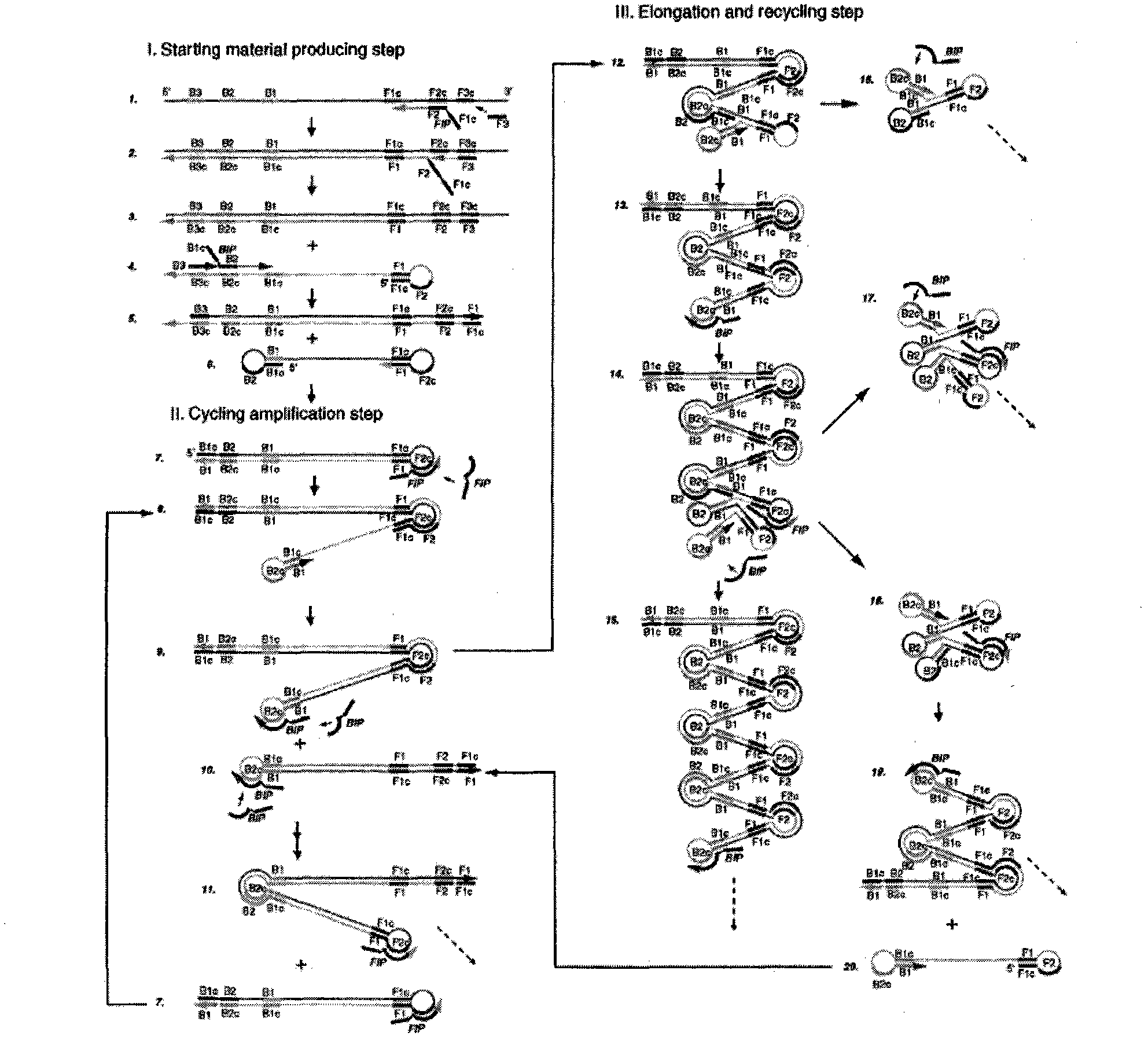
Method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification
InactiveCN102021249AQuick checkSensitive detectionMicrobiological testing/measurementReverse transcriptaseBiology
The invention provides a method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification. According to the conserved domain gene of PEDV (Porcine Epidemic Diarrhea Virus) N protein, three pairs of primers aiming at six areas on a target gene are designed, two RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primers are combined, and PED (Porcine Epidemic Diarrhea) viruses are identified by the RT-LAMP (Reverse Transcription-Loop-Mediated Isothermal Amplification) technology; RT-LAMP reacts; reverse transcription is performed on obtained RNA(Ribose Nucleic Acid); and the obtained cDNA is used for PCR reaction. The method provided by the invention ensures amplification specificity, has high amplification speed and can obtain a result within 30-60 minutes, and people can observe amplification effect with eyes without electrophoresis. Reverse transcription and nucleic acid amplification can be realized in 1 hour at the constant temperature of 65 DEG C by using enzyme mixture; RT-LAMP detection is carried out on the basis of LAMD amplification DNA; a reverse transcription enzyme is added to realize amplified detection of RNA; and reverse transcription and amplification are finished in one step so as to omit the reverse transcription step which is firstly carried out by the traditional RT-PCR.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Multiplex RT-PCR detection primer for porcine delta coronavirus, porcine epidemic diarrhea virus and porcine transmissible gastroenteritis virus
ActiveCN105483291ARapid differential diagnosisMicrobiological testing/measurementMicroorganism based processesPorcine parvovirusBovine parvovirus
The invention discloses a multiplex RT-PCR detection primer for a porcine delta coronavirus, a porcine epidemic diarrhea virus and a porcine transmissible gastroenteritis virus. The minimum detection capacity of the multiplex RT-PCR for the three viruses is 4.05*10<1> copies / microliter, 4.52*10<3> copies / microliter and 5.47*10<3> copies / microliter respectively. The amplification results for a porcine parvovirus (PPV) and a porcine pseudorabies virus (PRV) are both negative. The multiplex RT-PCR detection results of 57 clinical samples show that one sample is infected with the three viruses at the same time, 11 samples are infected with the PDCoV, 15 samples are infected with the PEDV, one sample is infected with the TGEV, five samples are infected with the PDCoV and the PEDV, and one sample is infected with the PDCoV and the TGEV.
Owner:HENAN AGRICULTURAL UNIVERSITY
Porcine epidemic diarrhea virus s protein and subunit vaccine thereof as well as method for preparing subunit vaccine and application thereof
ActiveUS20200188508A1Improve securityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaAdjuvant
The disclosure discloses a porcine epidemic diarrhea virus S protein and a subunit vaccine thereof as well as a method for preparing the subunit vaccine and application thereof. The vaccine contains 30˜220 μg of a recombinant porcine epidemic diarrhea virus S protein and a pharmaceutically acceptable ISA 201 VG adjuvant. A method for preparing the subunit vaccine comprises the following steps: (1) cloning the recombinant porcine epidemic diarrhea virus S protein; (2) expressing and purifying the recombinant porcine epidemic diarrhea virus S protein; (3) preparing the recombinant porcine epidemic diarrhea virus S protein prepared in (2) into a water phase; (4) emulsifying the water phase and the ISA 201 VG adjuvant in a volume ratio of 46:54 to obtain a vaccine. The vaccine is high in safety, good in immunogenicity, stable in batches, low in production cost and strong in immunogenicity. On the other hand, CHO cell strains suspending and stably and efficiently expressing the PEDV-S protein are successfully constructed and screened for the first time. The CHO cell strain can express the PEDV-S protein in high yield, the yield can reach 1 g / L, and the expressed PEDV-S protein is easy to purify.
Owner:NOVO BIOTECH CORP
Fusion protein and application thereof
ActiveCN107098974AAvoid separabilityAvoid technical difficulties of cultivationSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpidemic diarrheaNanoparticle
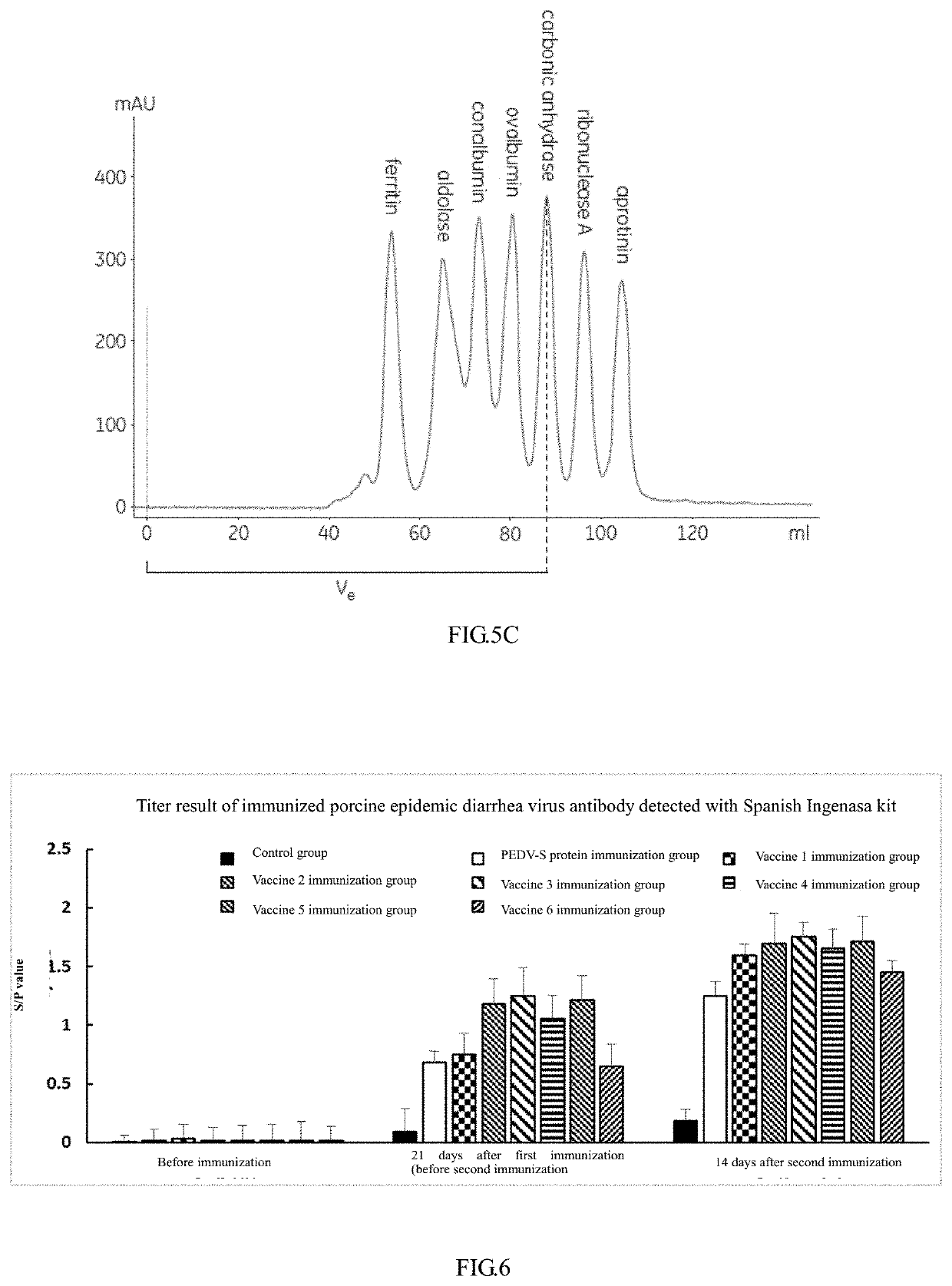
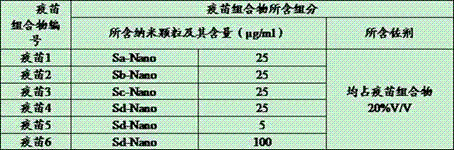
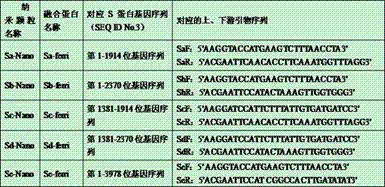
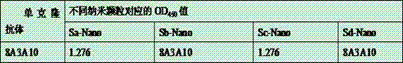
The invention provides fusion protein, which comprises monomeric ferritin subunit protein connected to porcine epidemic diarrhea virus antigenic protein, which includes porcine epidemic diarrhea virus S protein and / or fragments thereof, and further includes S1 protein, M protein, N protein and / or fragments thereof. The invention further provides nanoparticles containing the fusion protein. The invention further provides a vaccine composition containing the nanoparticles and / or a carrier. The invention further discloses a preparation method of the vaccine composition and an application of the vaccine composition to prepare a medicine for preventing and / or treating porcine epidemic diarrhea virus. The vaccine composition made from the nanoparticles overcomes the technical problem that whole viruses are hard to separate and culture in a process of manufacturing a conventional inactivation vaccine from porcine epidemic diarrhea whole viruses. The nanoparticles can be subjected to massive recombinant expression via genetic engineering technology. The time consumption is short, and the fusion protein is convenient for massive production.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea virus attenuated vaccine strain and its culture method and use
InactiveCN106591245AEfficient ProliferationNo pollution in the processSsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaDisease
The invention discloses a porcine epidemic diarrhea virus attenuated vaccine strain. The porcine epidemic diarrhea virus attenuated vaccine strain is an attenuated vaccine obtained by PEDV / CH / NB / 2014 passage attenuation and has an accession number of CGMCC No. 13299. The invention also discloses a use of the attenuated vaccine strain NB120 in prevention of porcine epidemic diarrhea diseases and the use belongs to the field of biotechnology. The attenuated vaccine strain NB120 can be processed to form a single vaccine or a multi-vaccine and the vaccine can effectively prevent porcine epidemic diarrhea diseases. The attenuated vaccine strain NB120 has the advantages of good safety and good immunogenicity. After applying to a sow or piglet, the vaccine strain can produce protective immune response to a prevalent strain of porcine epidemic diarrhea virus and thus the vaccine strain can effectively prevent infection caused by the prevalent strain of porcine epidemic diarrhea virus, can reduce a disease loss and has a good application prospect.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Ecological pig breeding method
The invention relates to the technical field of pig breeding, in particular to an ecological pig breeding method. The ecological pig breeding method includes the steps of timely mating, wherein stock boars are reasonably utilized; pigpen management, wherein a fermentation bed technology is adopted for improving the external environment of a farm; feeding management, wherein ecological pig feed is used for feeding pigs in a pigpen until the pigs are slaughtered; disease prevention management, wherein the pigs are subjected to injection immunization in spring and autumn, parasite expelling and environment disinfection are conducted periodically, and the pigs are fed with antiviral traditional Chinese medicine pills regularly; timely slaughtering. By means of the ecological pig breeding method, the immunity of the pigs can be enhanced, the viral infection rate can be reduced, and the antibiotic use level and drug residues can be reduced. By the adoption of Chinese herbal medicine additives, the antiviral traditional Chinese medicine pills have a heat-clearing and detoxicating effect and an obvious antiviral effect, have an obvious inhibiting effect on epidemic diarrhea, porcine reproductive and respiratory syndromes, influenza virus pneumonia and the like, and can replace traditional antibiotics and reduce drug residues.
Owner:永胜县土林生物科技开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com