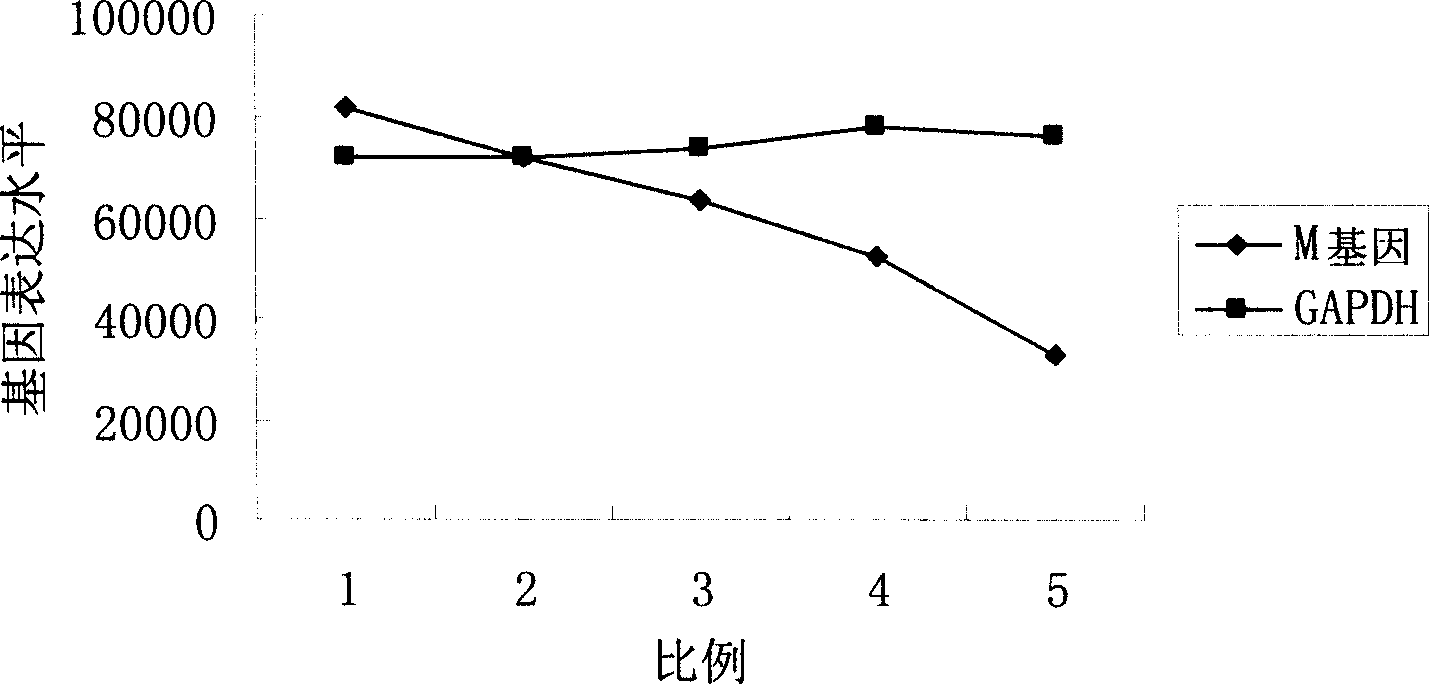
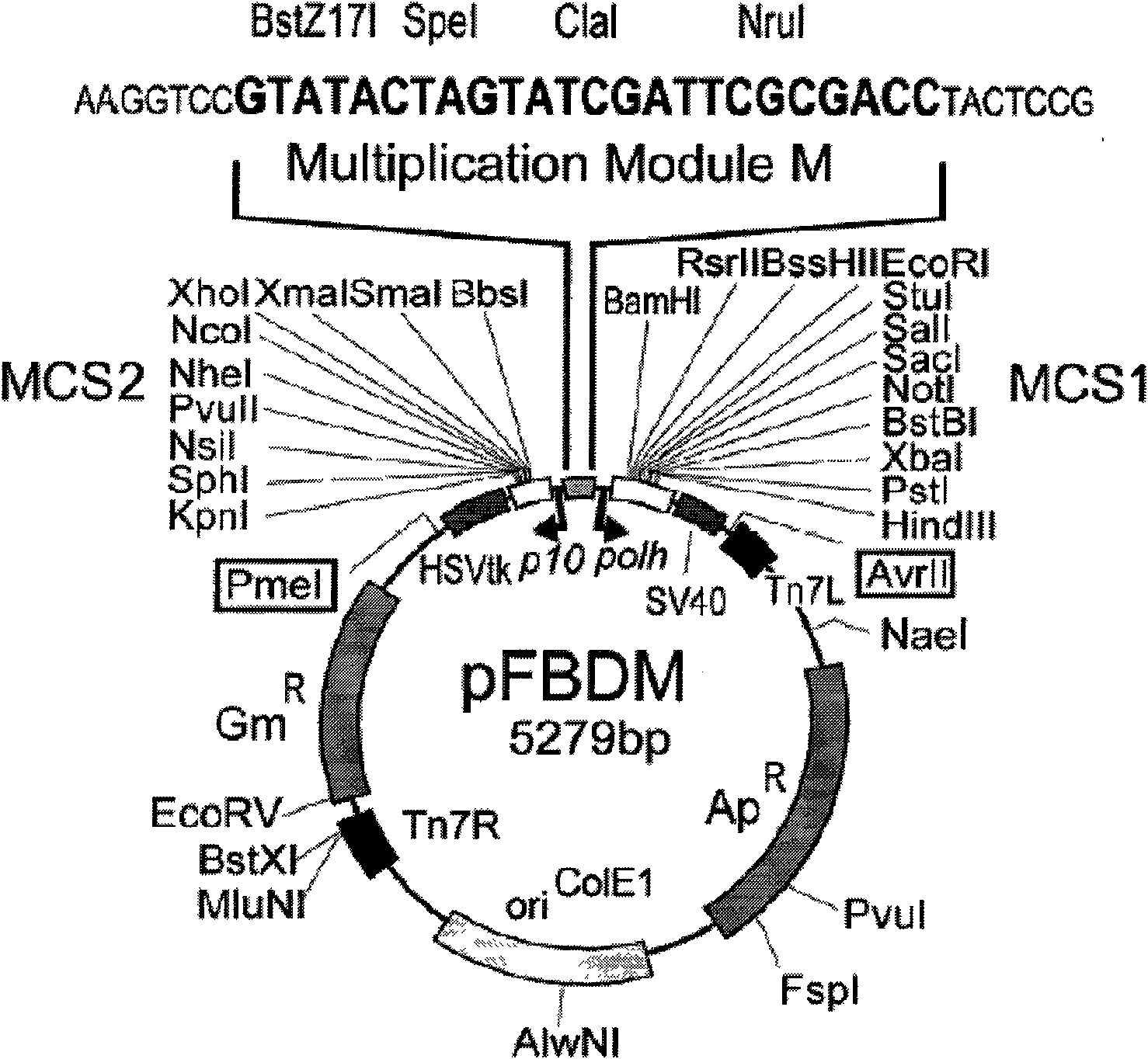
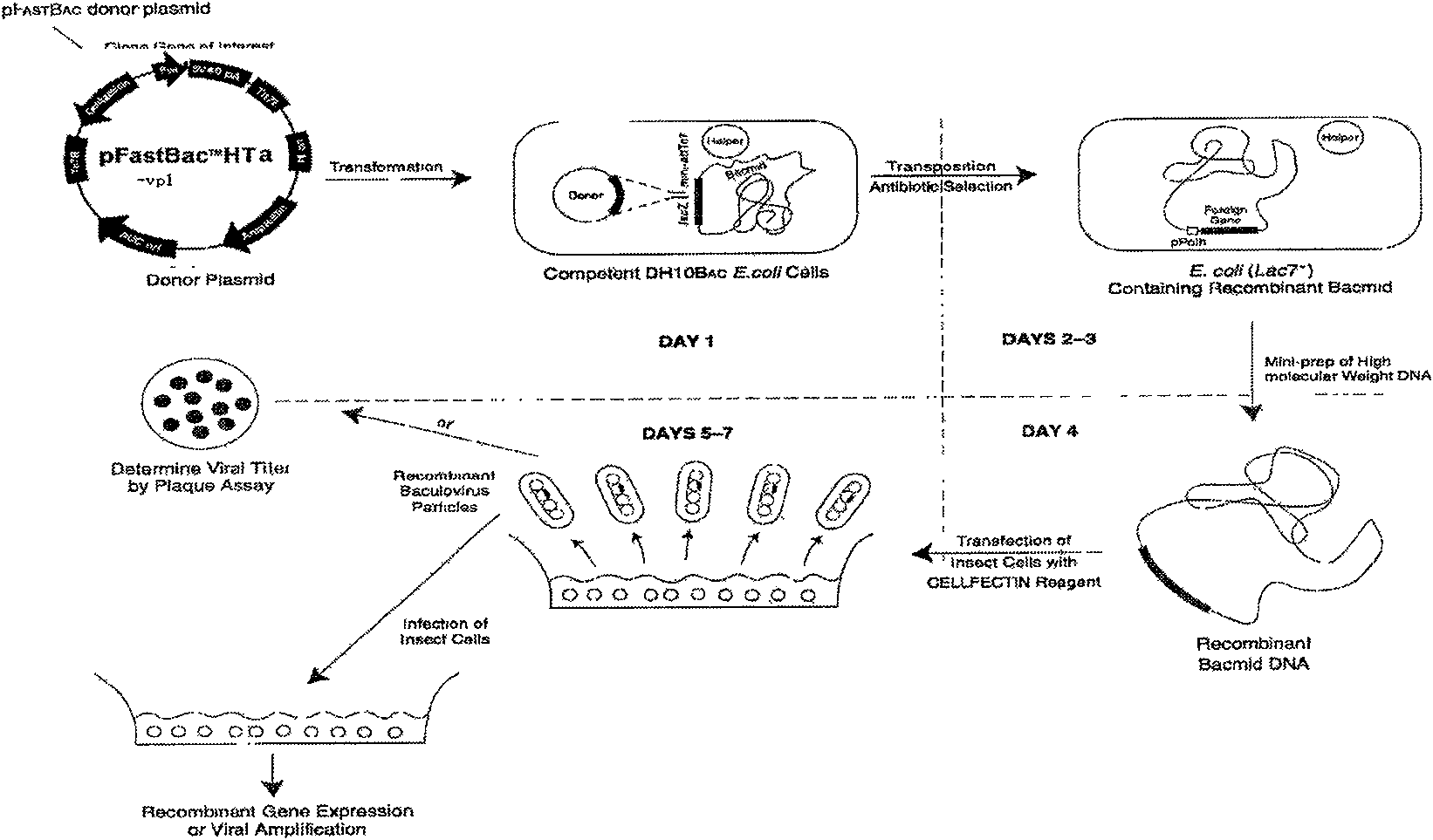
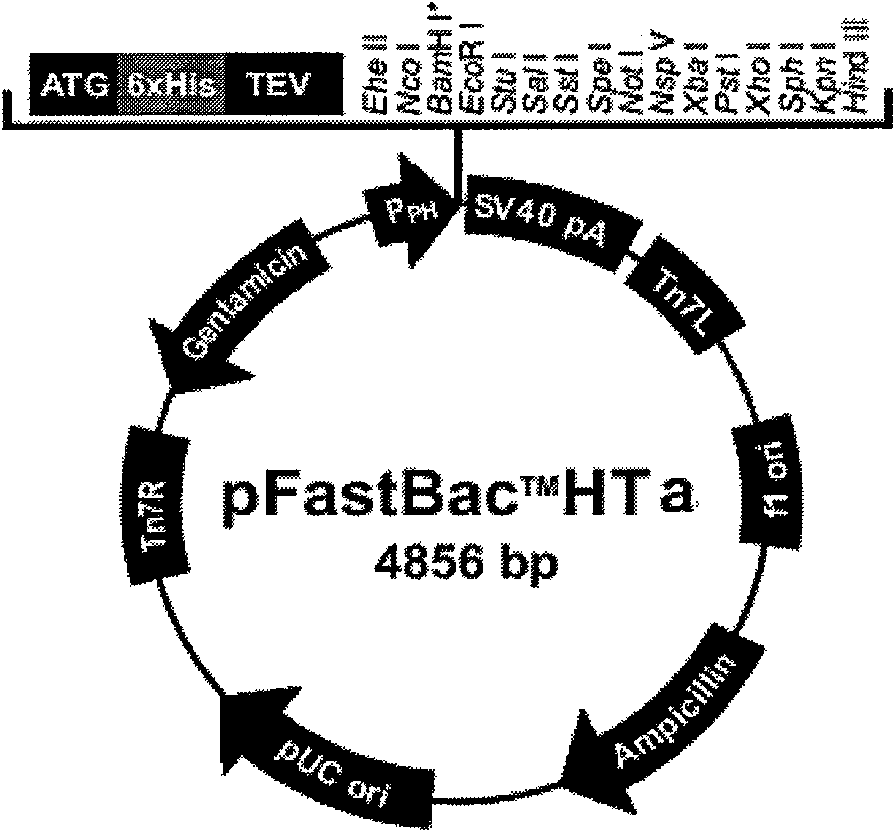
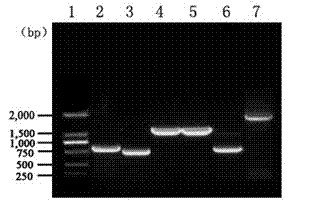
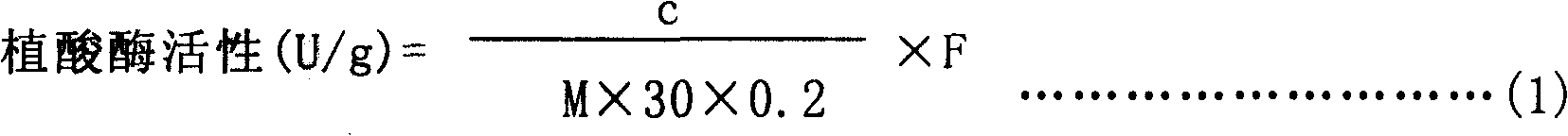Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Streptococcal M protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
M protein is a virulence factor that can be produced by certain species of Streptococcus. Viruses, parasites and bacteria are covered in protein and sugar molecules that help them gain entry into a host by counteracting the host's defenses. One such molecule is the M protein produced by certain streptococcal bacteria.
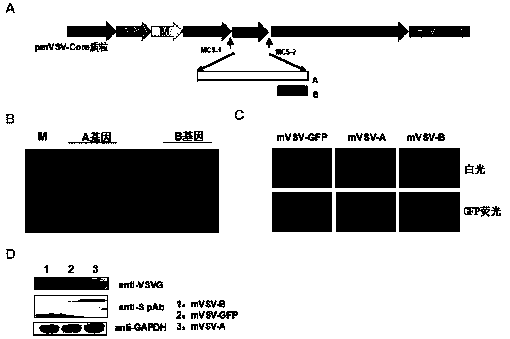
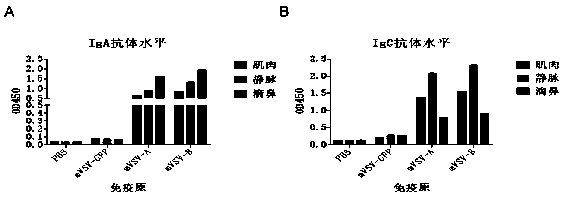
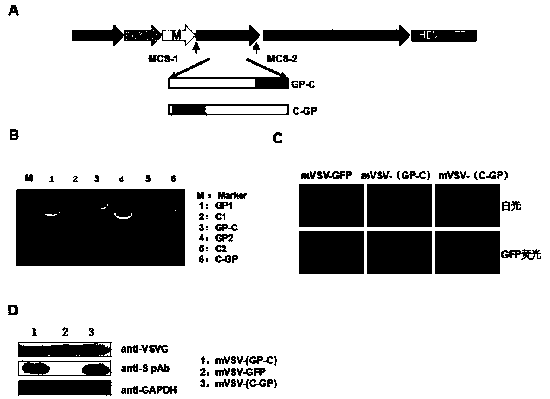
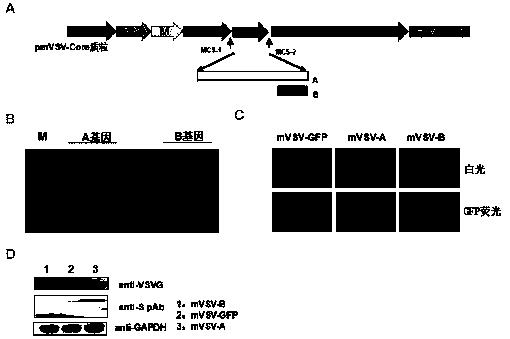
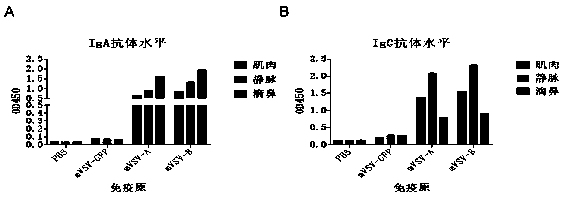
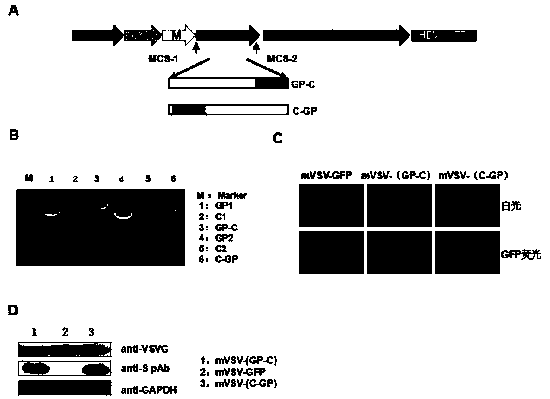
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus genetic engineering strain and application
InactiveCN101457215AAvoid infectionPrevention of swine pseudorabiesViral antigen ingredientsMicroorganism based processesAnimal virusAntigen
The invention belongs to the animal virus genetic engineering technique field, especially to a recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain construction, a vaccine preparation and applications. The recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain E001 lacks the pseudorabies virus main virulence gene TK, glycoprotein genes gG, gE and gI, does not express the functional glycoprotein gG and gE / gI of the pseudorabies virus; and simultaneously expresses a GP5m / M protein antigen of the porcine propagate and breath complex virus classical strain and the porcine propagate and breath complex virus internal variant GP5 protein and porcine circovirus ORF2. The strain can stimulate the porcine to produce protective immunity reaction for resisting pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus, effectively prevent the infection of the pseudorabies virus, the porcine propagate and breath complex virus and porcine circovirus. The invention also discloses a preparation and application of a tervalence genetic engineering vaccine.
Owner:HUAZHONG AGRI UNIV
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
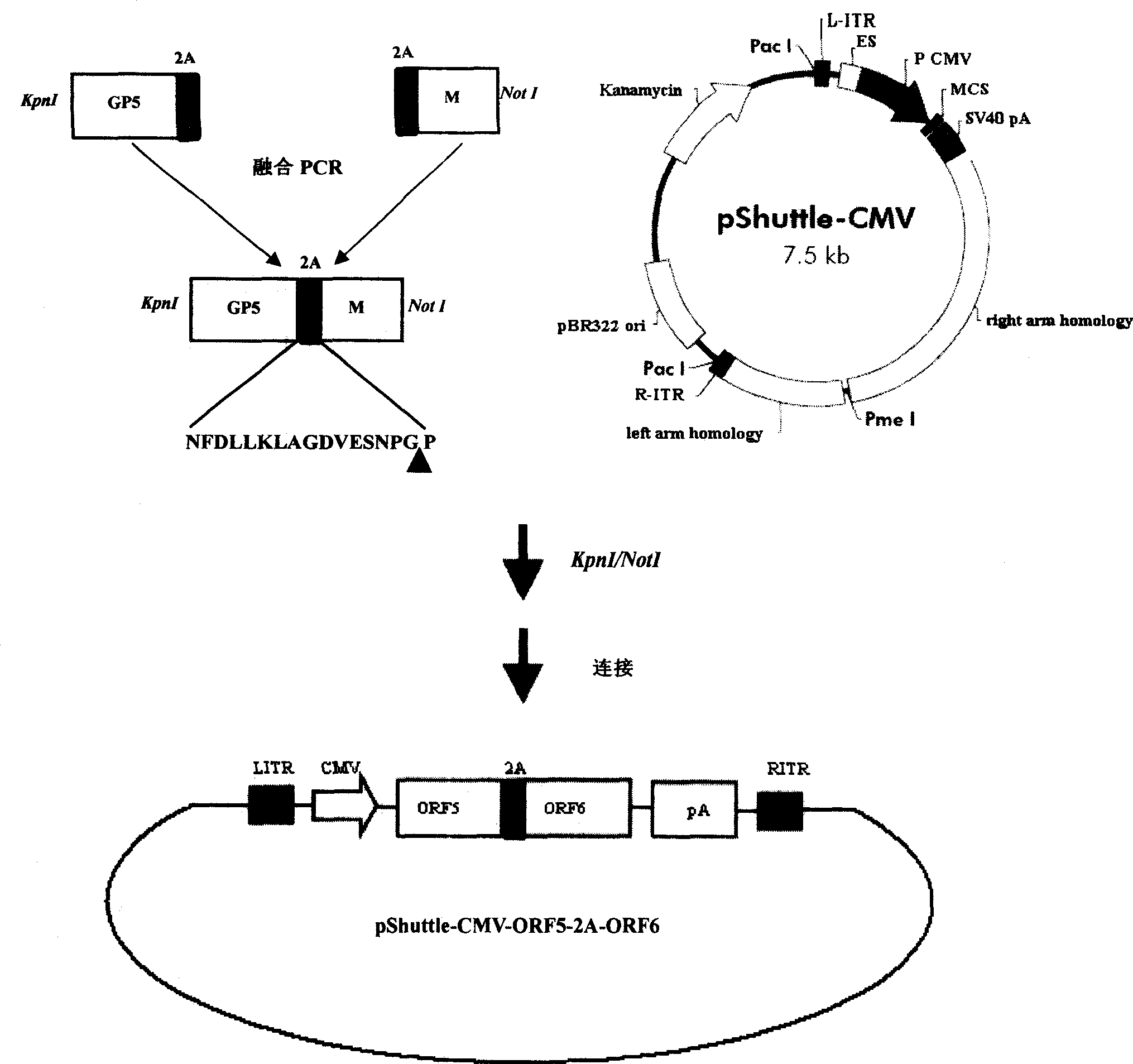
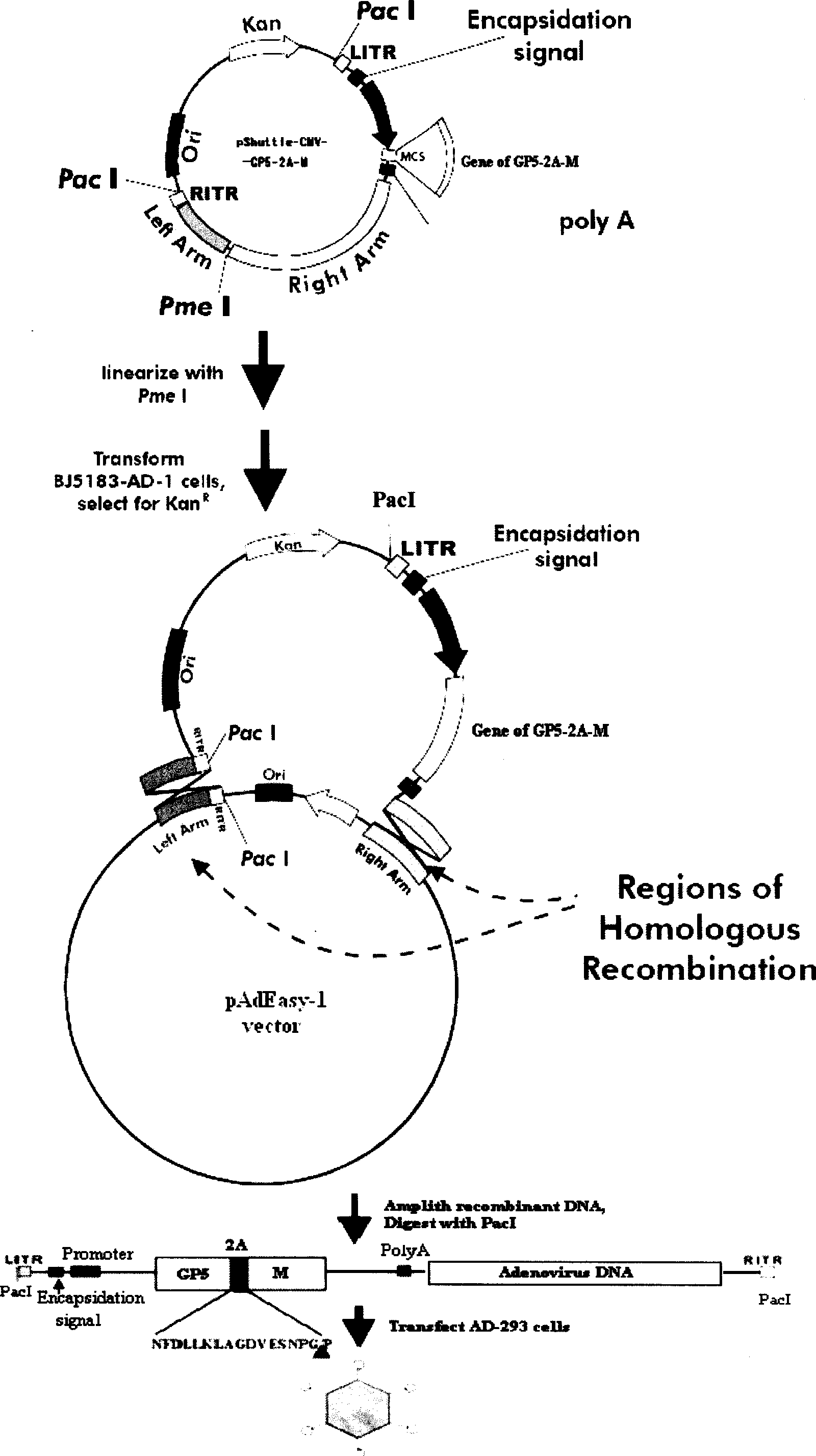
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
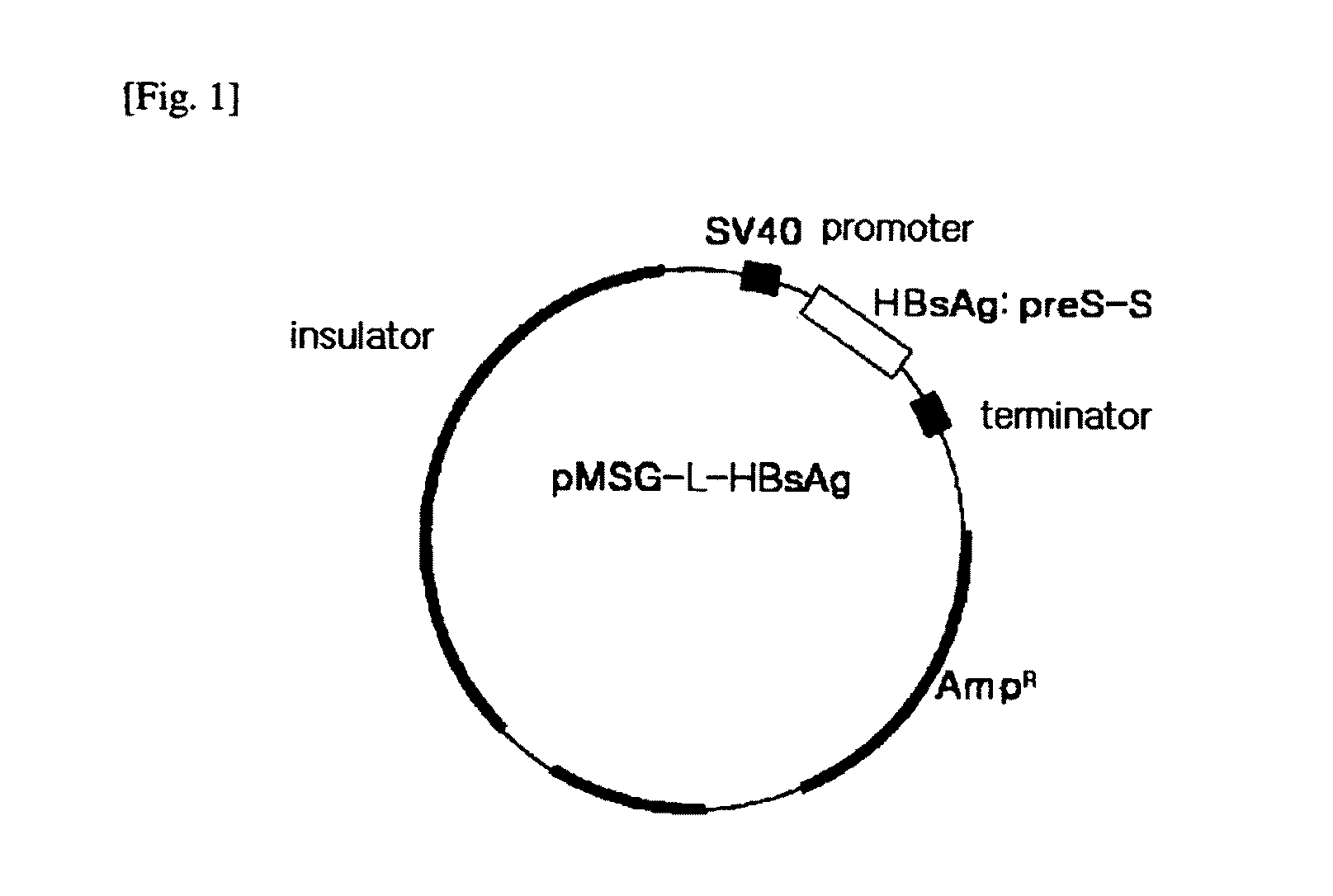
HBV vaccine and a process of preparing the same
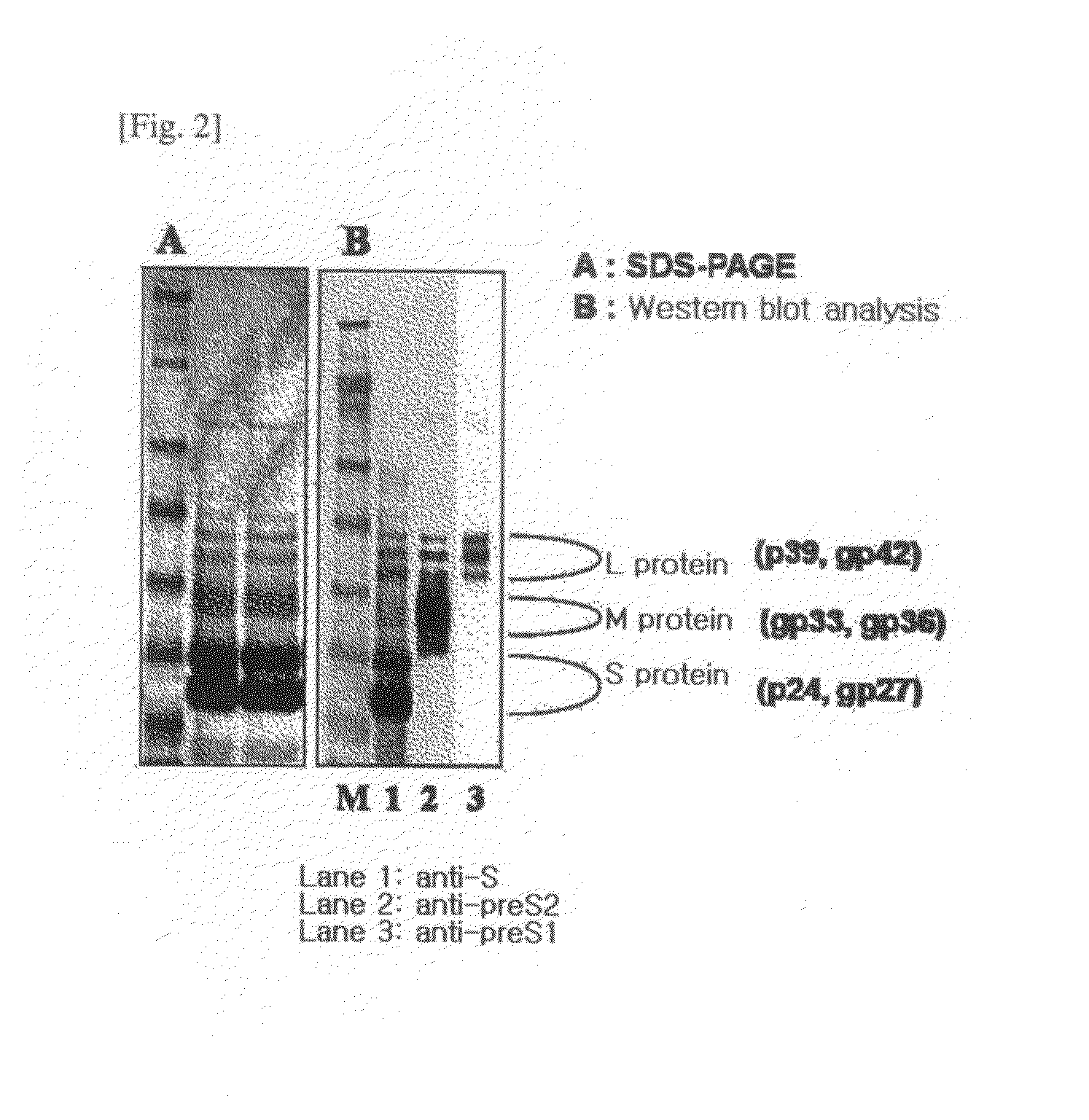
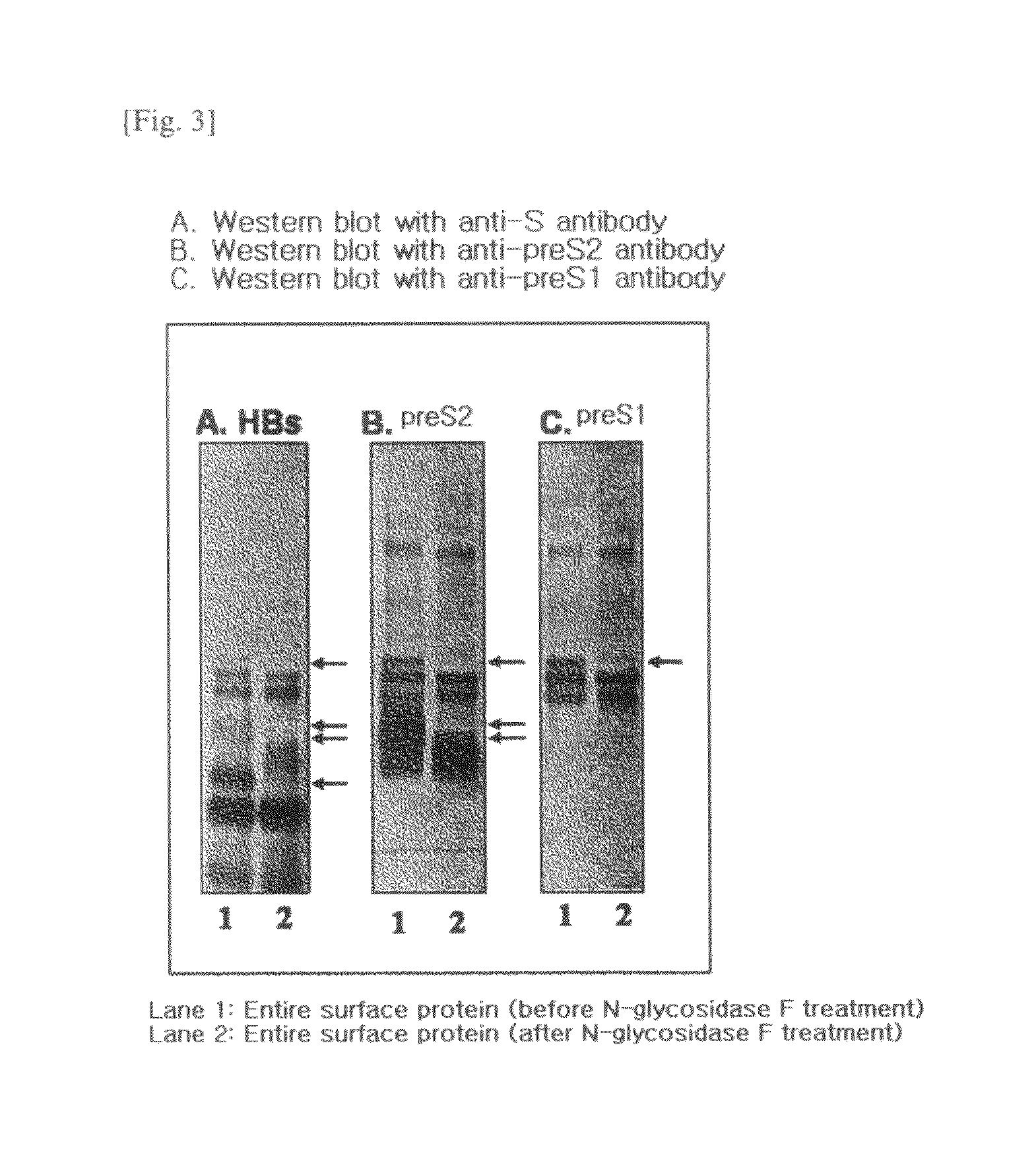
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
Respiratory syncytial virus sub-units vaccine, preparation and application
InactiveCN101264323ALimit developmentEnhance cellular immune responseDepsipeptidesAntiinfectivesEscherichia coliCtl epitope
The invention relates to a respiratory syncytial virus vaccine, and the preparation method and application, in particular to an application of escherichia coli to express and recombinant respiratory syncytial virus G protein and mutation G protein, and preparation vaccine with nontoxic typed escherichia coli heat labile enterotoxin, for human or animal preventive inoculation, and for respiratory syncytial virus resistance, belonging to the field of biotechnology. The respiratory syncytial virus vaccine is characterized in that: the respiratory syncytial virus subunits vaccine comprises lopped respiratory syncytial virus RSV protein G, and further comprises nontoxic typed escherichia coli heat labile enterotoxin LT adjuvant. The vaccines are lopped respiratory syncytial virus RSV protein G containing amino acid between aa130 and 230 of the original G protein, and substitutes the amino acid CAWIC (CX3C module ordered) between aa182 and 186 with the amino acid YLEKESIYY (CTL epitope) on the RSV M protein, forming the GCIL protein. The respiratory syncytial virus vaccine has the advantages of remarkable practical significance for preventing human respiratory syncytial virus infection.
Owner:KUNMING UNIV OF SCI & TECH
Recombinant PRRSV virus-like particles having immunogenicity and preparation thereof
ActiveCN109385435ABroad-spectrum cross-immunogenicityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsSpecific immunityTransfer vector
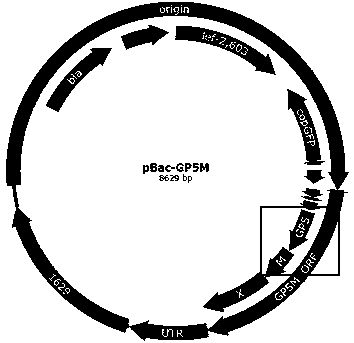
The invention discloses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) virus-like particles (VLP) and a preparation method and an application thereof. Based on comparative analysis of GP5 of a PRRSV epidemic strain and an M gene sequence, a GP5 and M tandem sequence GP5M is synthesized artificially, the synthesized GP5M gene sequence is cloned into a vector with a pBAC5 plasmid as a skeleton, the baculovirus transfer vector pBAC-PRRSVGP5M is obtained, the recombinant bacmid rBacmid-GP5M is obtained, sf9 cells are transfected with the bacmid, and the recombinant baculovirus Ac-PRRSVGP5M is obtained. The PRRSV GP5 and M protein are expressed efficiently by the recombinant baculovirus, and the virus-like particles are formed. A subunit vaccine prepared by the proteinexpressed by the recombinant baculovirus can induce a body to produce a specific immune response after immunizing animals and can protect the pig body against the strong poison attacking of porcine reproductive and respiratory syndrome virus.
Owner:陕西诺威利华生物科技有限公司
Preparation and application of baculovirus expression system-based duck tembusu virus subunit vaccine
InactiveCN106834351AGood immunogenicityGood protectionSsRNA viruses positive-senseViral antigen ingredientsSpecific immunityAdjuvant
The invention discloses preparation and application of a baculovirus expression system-based duck tembusu virus subunit vaccine. Tembusu prME protein is expressed by adopting a baculovirus expression system; WB shows that the prME protein is successfully expressed in an sf9 cell and a supernatant, and is partially cut into M protein and E protein after being maturely processed; existence of tembusu virus-like particles of which the diameters are about 30-50nm in the sf9 cell and secreted supernatant is observed through an electron microscope; and the virus-like particles are formed through self-assembly of the protein in the sf9 cell and have relatively high immunogenicity. The protein or the virus-like particles can be purified through two different modes and mixed with an adjuvant at the ratio to prepare the safe, stable and efficient duck tembusu virus subunit vaccine. The vaccine is capable of immunizing cherry valley ducks and egg-laying sheldrakes and then inducing the organism to generate a specific immune response, and 60% of egg-laying sheldrakes can be prevented from being attacked by a duck tembusu virus.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
Porcine reproductive and respiratory syndrome virus M protein CTL cell epitopes and application thereof
The invention discloses porcine reproductive and respiratory syndrome virus M protein CTL cell epitopes and application thereof. The identification of the epitopes comprises the following steps of: fusing and cloning a M protein gene of a porcine reproductive and respiratory syndrome virus CH-1a strain and a mouse ubiquitin gene to form a DNA vaccine and also form recombination viruses, expressing an M protein, of a WR strain vaccinia virus; immunizing a BALB / c mouse according to a Priming-Boosting policy, separating the mouse splenic lymphocyte, culturing and stimulating a CTL short peptide in vitro predicted and synthesized by a bioinformatic method, and using the flow cytometry and enzyme-linked immunospot technology to identify two CTL epitopes that are K93FITSRCRL and F57GYMTFVHF. The identification of the PRRSVM protein CTL cell epitopes lays a certain theoretical foundation for the PRRS cell immune mechanism and the novel epitope pepetide vaccine and has an instructing significance for the theoretical study on PRRS preventing and control technology.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
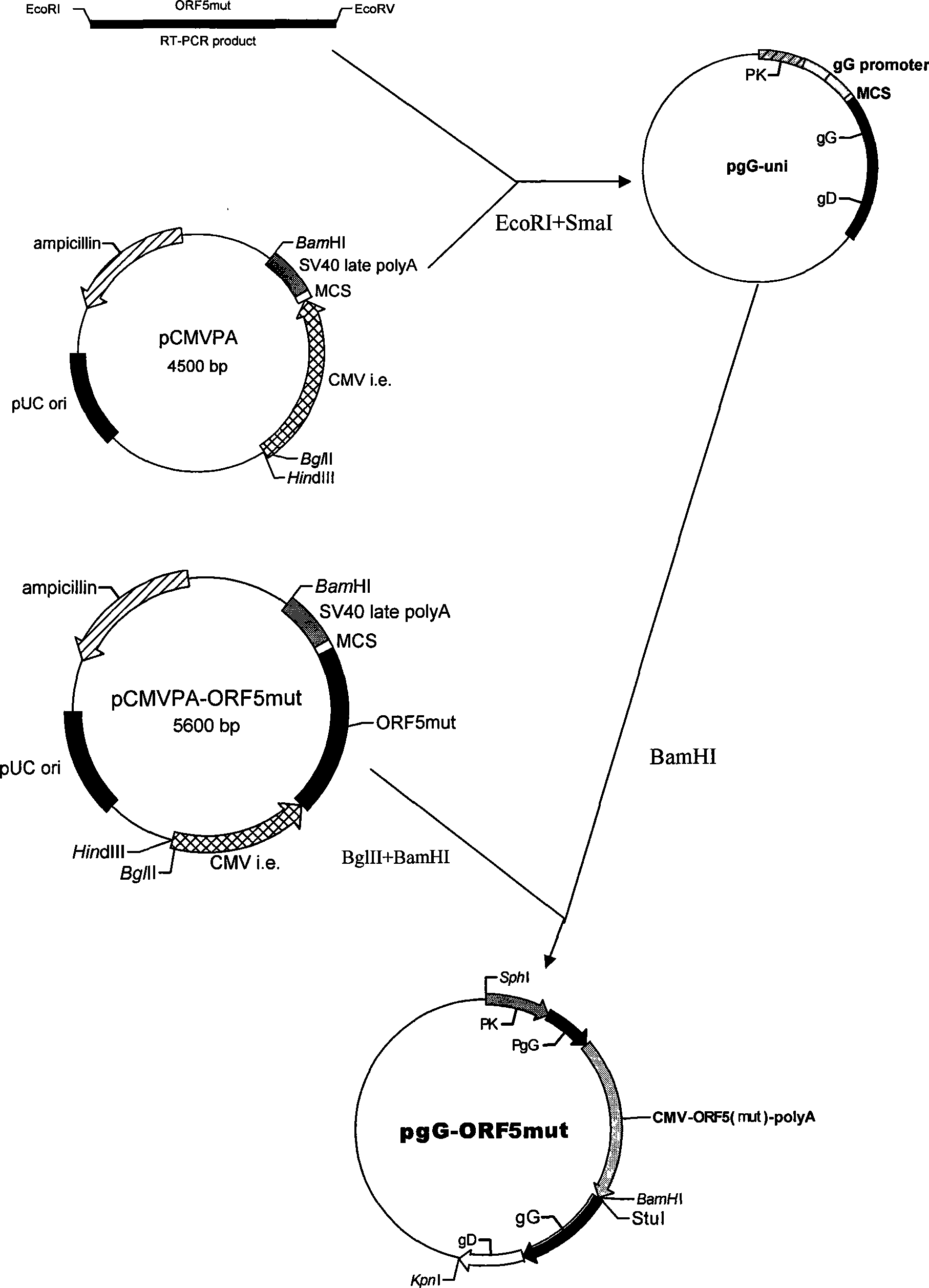
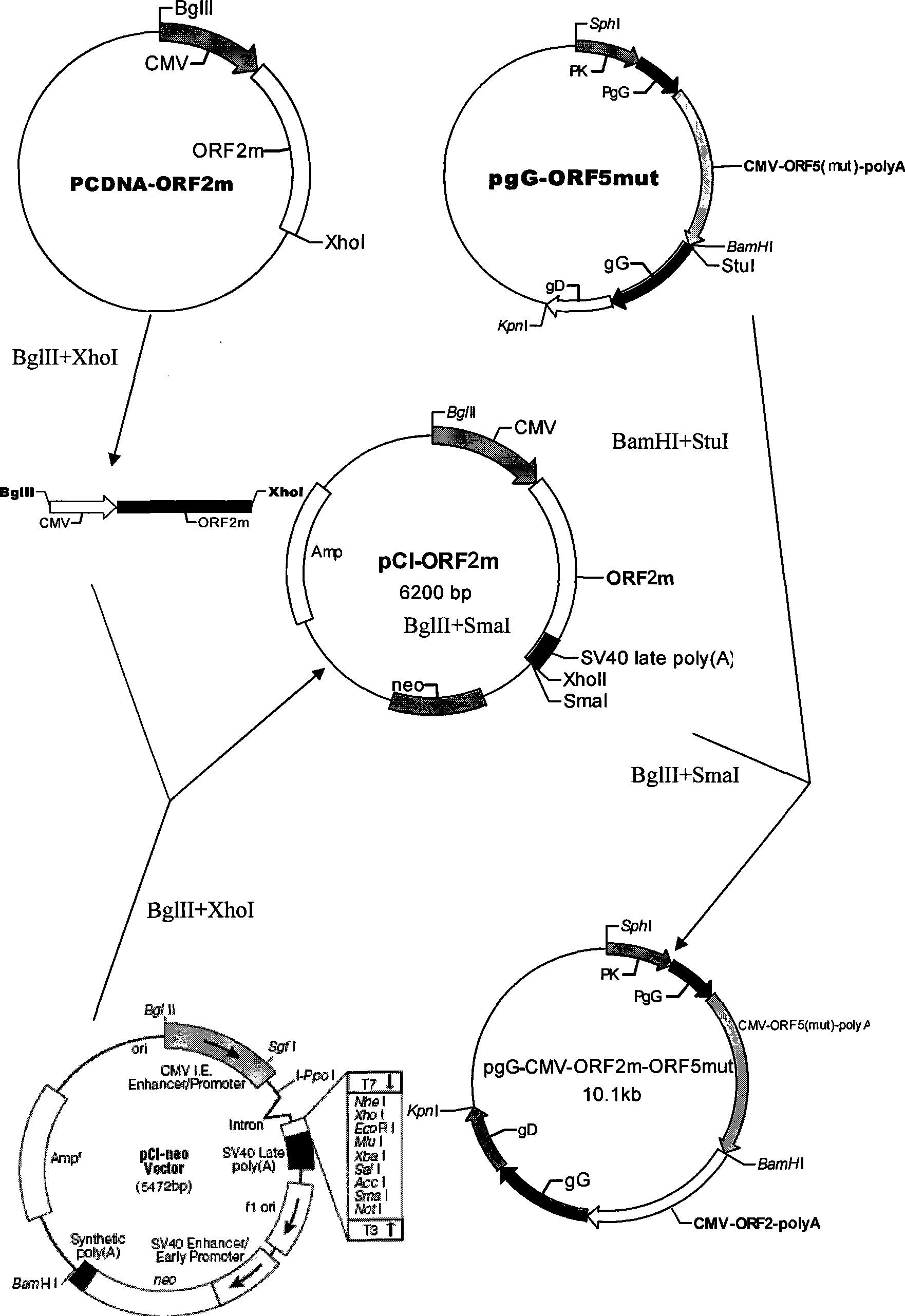
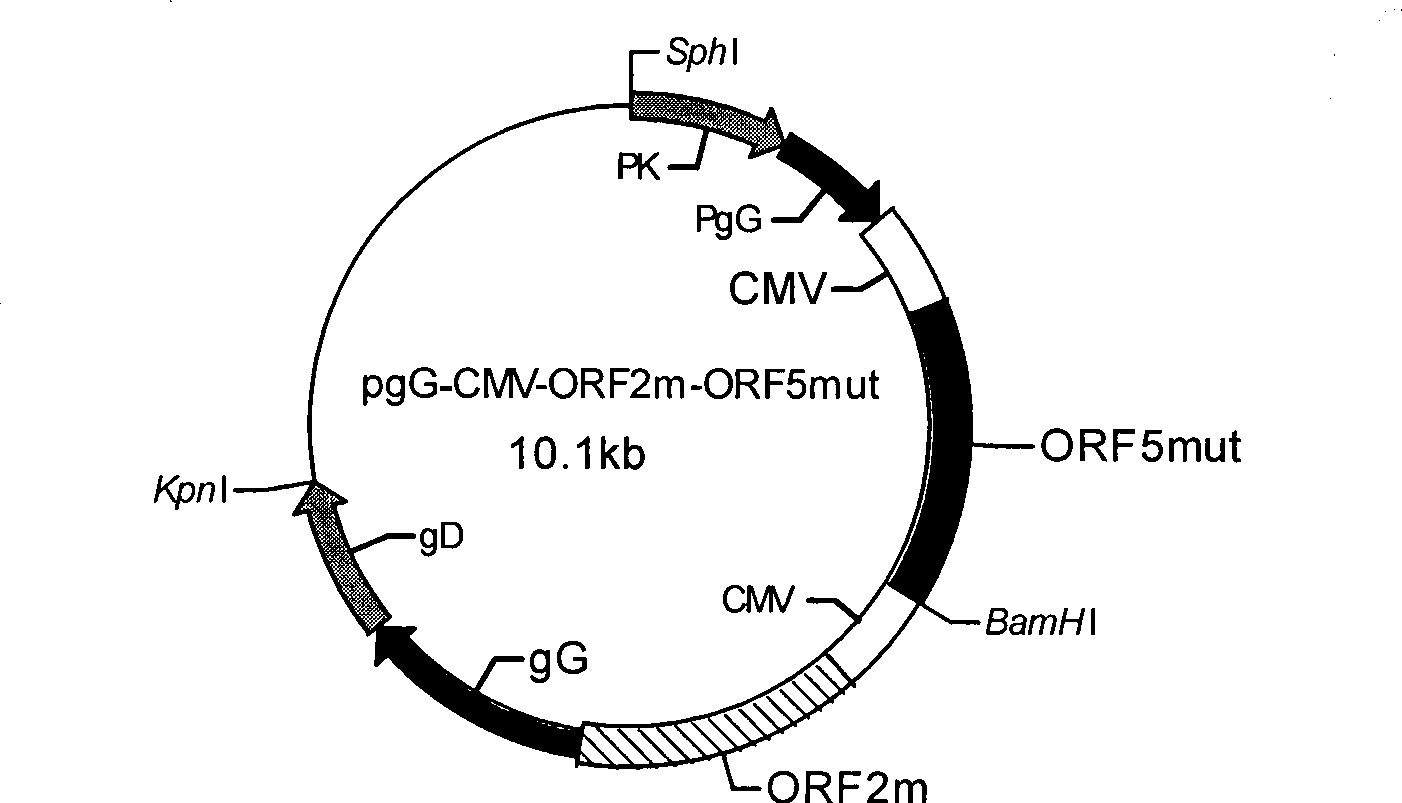
Method of preparing coexpression PRRSV ORF5 and ORF6 double-gene nucleic acid vaccine
InactiveCN101401937AMake up for the defect that can not produce cellular immunityBreak through limitationsAntiviralsRespiratory disorderWestern blotOrganism
The invention provides a co-expression porcine reproduction and a method for preparing a dual-gene nucleic acid vaccine of respiratory syndrome virus (PRRSV) ORF5 and ORF6. The method comprises the following steps: amplifying the whole gene sequences of the PRRSV ORF5 and the ORF6 respectively by an RT-PCR method, and inserting two genes into two multiple cloning sites of a mammalian eukaryotic expression vector pIRES to obtain recombinant plasmids pIRES-ORF5 and ORF6; transfecting the recombinant plasmids with CHO cells to establish a stable expression cell line; and detecting the transcription of the genes PRRSV ORF5 and ORF6 by an RT-PCR method, and detecting the expressions and the immune activities of GP5 and M proteins through the SDS-PAGE and the Western blot. The nucleic acid vaccine can generate GP5 proteins and M proteins in animals continuously so that organisms generate antibodies against both of the proteins continuously, thus the nucleic acid vaccine provides long-lasting immune protection for pig bodies, and has wide application prospect in PRRS prevention and control.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
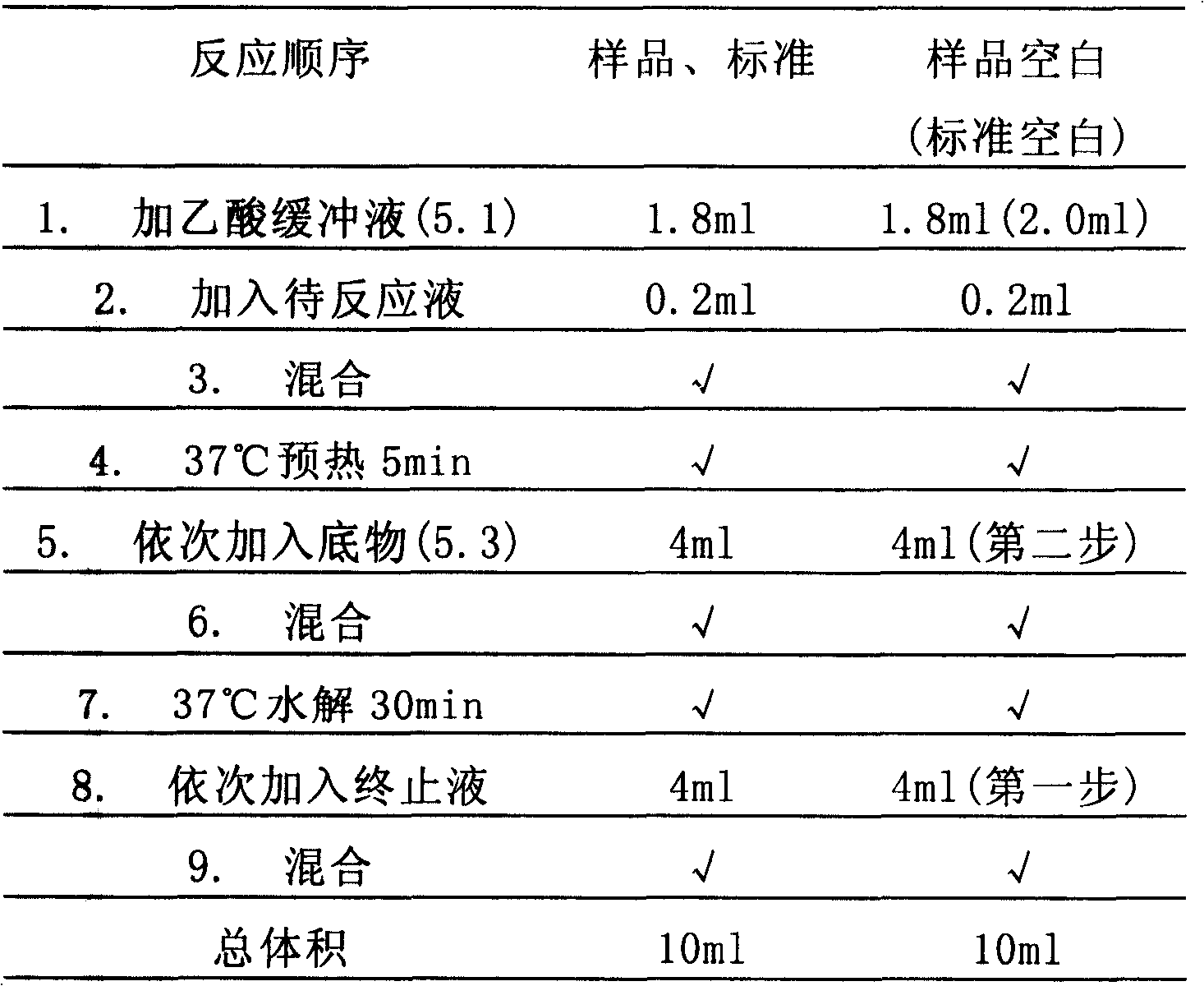
Heat-resistance phytase gene and application thereof
The invention discloses a novel PhyA-M protein and application of the same. The PhyA-M protein is a heat-resistant phytase and can be used for decomposition of phytic acids or phytates. The invention also discloses a gene for encoding the PhyA-M protein, and a carrier and a host cell which comprise the gene. The PhyA-M protein has wide application prospect in fields such as feedstuff additives and so on.
Owner:SICHUAN HEBEN BIOENG
CCV (canine corona virus) M protein monoclonal antibody and preparation method thereof, and preparation method of immune colloidal gold test strip
ActiveCN109970851ASimple methodFast wayImmunoglobulins against virusesBiological testingField testsEpidemiologic survey
The invention relates to a CCV (canine corona virus) M protein monoclonal antibody and a preparation method thereof, and a preparation method of an immune colloidal gold test strip and particularly relates to a CCV M protein monoclonal antibody, comprising CCV M protein specific monoclonal antibodies 2H11 and 2G3. The preparation method of the CCV M protein monoclonal antibody comprises: preparinga CCV monoclonal antibody with separated and purified inactivated CCV as an immunogen by means of a monoclonal antibody technique; verifying the monoclonal antibody for the M protein by means of western-blot test, performing genetic cloning on the CCV M protein by means of recombinase ExnaseTMII to obtain a linear pCDNA3.1 vector, transfecting with 293T cells to carry out M protein expression, and carrying out IFA test verification to obtain two CCV M protein specific monoclonal antibodies 2H11 and 2G3. The invention also discloses the preparation method of the immune colloidal gold test strip. The CCV M protein monoclonal antibody herein has high specificity, high sensitivity, good operational simplicity and low cost, is applicable to clinical and field tests and is applicable to CCV etiological diagnosis, epidemiological investigation and quick diagnosis in animal hospitals.
Owner:YANGZHOU UNIV
Small interfering RNA for restraining SARS corona virus M protein gene expression, encoding gene and application thereof
The invention discloses a small interfering RNA limiting SARS coronavirus M protein gene expression and the coding gene and the application. The small interfering RNA limiting SARS coronavirus M protein gene expression is about at least one double stranded RNA sequence among the following 1) and 2): 1). Nucleotide sequence for sequence 1 in sequence list is arranged for the sense strand; nucleotide sequence for sequence 2 in sequence list is arranged for the antisense strand; 2). Nucleotide sequence for sequence 3 in sequence list is arranged for the sense strand; nucleotide sequence for sequence 4 in sequence list is arranged for the antisense strand. The invention has an advantage of playing an important role in preparing drugs using small interfering RNA limiting SARS coronavirus M protein gene expression or an expression vector carrying the coding gene for small interfering RNA limiting SARS coronavirus M protein gene expression as active component.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
COVID-19 pseudovirus as well as preparation method and application thereof
ActiveCN112553172AStrong infection abilityHigh sensitivitySsRNA viruses positive-senseMicrobiological testing/measurementStreptococcal M proteinViral infection
The invention relates to the technical field of biology, in particular to a COVID-19 pseudovirus as well as a preparation method and application thereof. The COVID-19 pseudovirus is formed by virus packaging of coat protein particles and helper plasmids, wherein the coat protein particles comprise plasmids for expressing a COVID-19 S protein, plasmids for expressing a COVID-19 M protein and plasmids for expressing a COVID-19 E protein. The COVID-19 pseudovirus is packaged by adopting a three-plasmid system, and the S / M / E protein is used for replacing expression a VSV-G protein, so that the COVID-19 pseudovirus is stronger in infection capability and higher in sensitivity compared with the pseudovirus only containing the S protein. Moreover, the COVID-19 pseudovirus carries two fluorescentreporter groups, and different fluorescent reporter groups can be applied to different scenes, so that the COVID-19 pseudovirus is simpler and more convenient to apply.
Owner:NOVOPROTEIN SCI INC
Method for expressing IBV-HN99 membrane protein gene in insect-rhabdovirus system
InactiveCN101560522AReduce cumbersomeServe as an indicatorDepsipeptidesFermentationBio engineeringTwo generation
The invention relates to a method for expressing an IBV-HN99 membrane protein gene in an insect-rhabdovirus system, which belongs to the field of biological engineering. The invention also discloses a recombinant expression carrier of the membrane protein gene, and the recombinant expression carrier simultaneously contains a maltose label and a green fluorescent protein gene named ACB acmid-egfp-MBP-M. The method for expressing the IBV-HN99 membrane protein gene in the insect-rhabdovirus system is as follows: an expression carrier Bacmid-egfp-MBP-M is used for transfecting an Sf9 cell by a lipidosome transfect method through two generations of blind passages. The construction of the method for expressing the IBV-HN99 membrane protein gene in the insect-rhabdovirus system has important significance for developing a gene engineered vaccine of an IBV M gene or an ELISA diagnostic reagent kit of the M protein.
Owner:HENAN AGRICULTURAL UNIVERSITY
DNA (deoxyribonucleic acid) level-based highly pathogenic blue-eared pig disease JEV (Japanese encephalitis virus) replicon vaccine and application thereof
InactiveCN102247606AEnhanced antibodyStrong proliferative responseViral antigen ingredientsGenetic material ingredientsDiseaseHighly pathogenic
The invention discloses a DNA (deoxyribonucleic acid) level-based highly pathogenic blue-eared pig disease JEV (Japanese encephalitis virus) replicon vaccine and an application thereof. In the invention, primer amplification is carried out to obtain GP5 and M genes of a highly pathogenic blue-eared pig disease virus XH strain, an FMDV-2A sequence is inserted between the two genes by utilizing a fusion PCR (polymer chain reaction) method to obtain a G-2A-M segment, and finally the G-2A-M segment is cloned into pJEV-REP by utilizing two restriction enzyme cutting sites SpeI and SalI; besides, an IRES (internal ribosome entry site) sequence (G-2A-M-IRES) is inserted into the downstream of the G-2A-M segment, thus M protein can produce a real N terminal; and the G-2A-M-IRES segment is inserted into the pJEV-REP by utilizing two restriction enzyme cutting sites SalI-HF and SpeI, and finally a highly pathogenic blue-eared pig disease vaccine which is based on a JEV replicon and can express GP5 and M proteins is constructed.
Owner:SOUTH CHINA AGRI UNIV
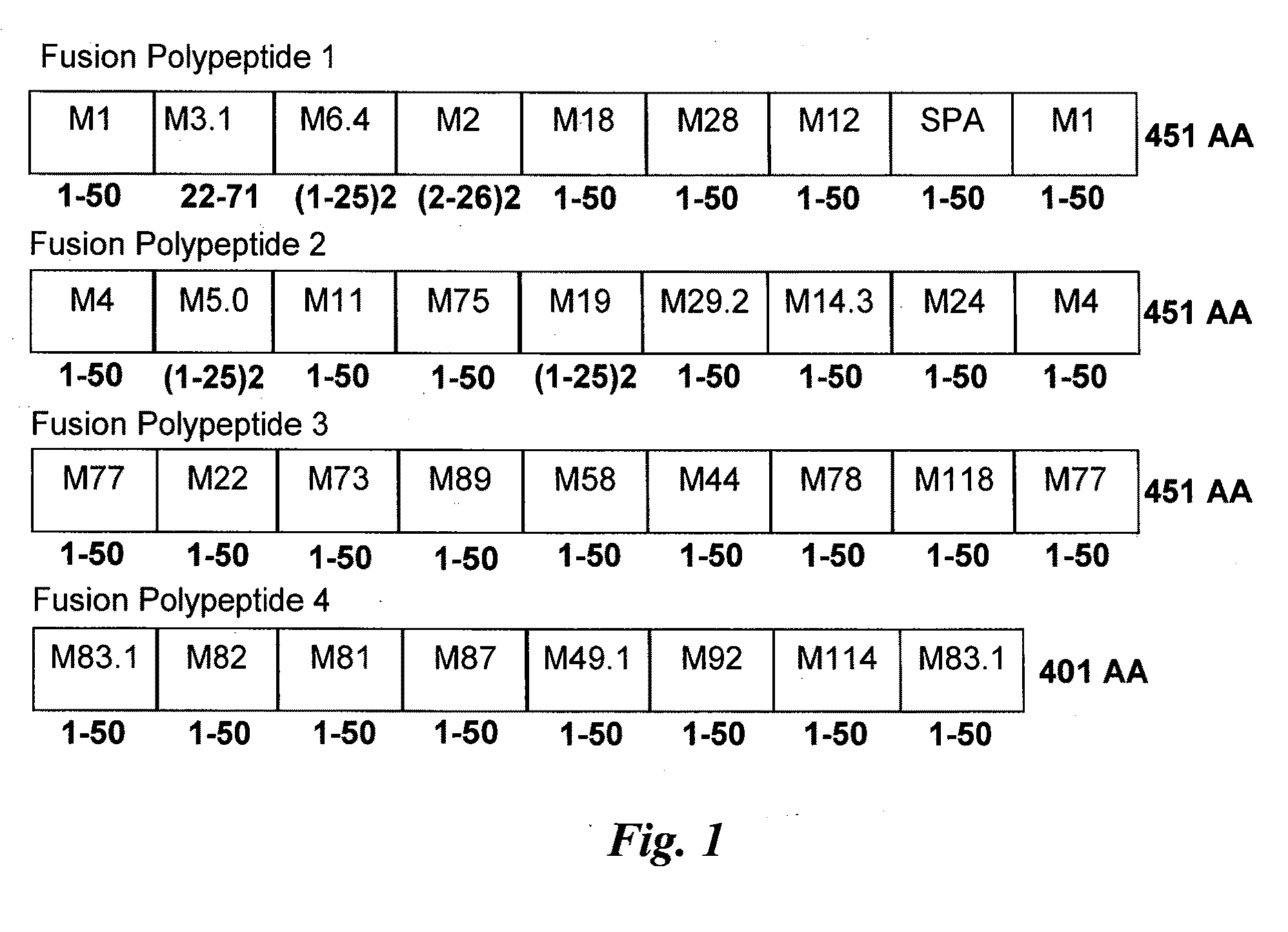
Group A Streptococcus multivalent vaccine
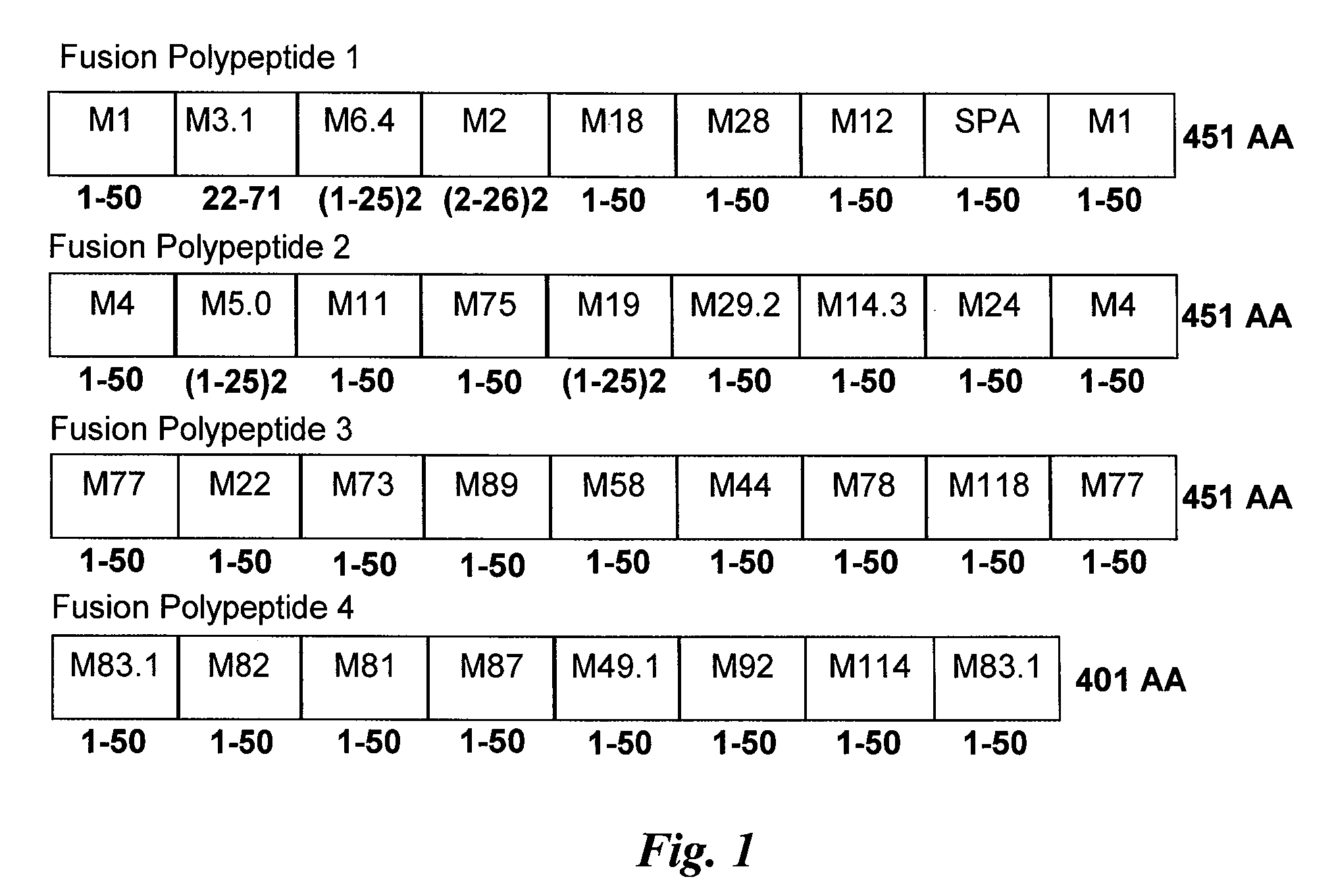
Immunogenic compositions are provided herein that are useful for inducing an immune response specific against group A streptococcus (GAS). Immunogenic compositions provided herein are multivalent and comprise a plurality of immunogenic peptides or fusion polypeptides comprising the immunogenic peptides that induce an immune response against GAS. The immunogenic compositions provided herein induce an immune response against the GAS serotypes represented by an immunogenic peptide (derived from an M protein or Spa protein) comprised within the immunogenic composition and also induce an immune response against serotypes that are unrepresented by any immunogenic peptide included in the immunogenic composition. Methods for using the compositions for inducing an immune response against GAS and for treating or reducing the likelihood of occurrence of a GAS infection are also provided.
Owner:UNIV OF TENNESSEE RES FOUND
Antigenic epitopes, antibodies, identification methods and applications of Newcastle disease virus matrix proteins
ActiveCN108840911AEasy identification systemHighly conservativeSsRNA viruses negative-senseViral antigen ingredientsGenotypeB cell
The invention discloses antigenic epitopes, antibodies, identification methods and applications of Newcastle disease virus (NDV) matrix (M) proteins, and relates to the technical field of biology. Theantigenic epitopes of the NDV M proteins provided by the invention has a sequence 1 of 77MIDDKP82 and a sequence 2 of 354HTLAKYNPFK363. B cell dominant linear epitopes of the two NDV M proteins provided by the invention can be used for detection antigen of serological analysis of NDV, and can be used as markers for constructing marker vaccines of the NDV; sequences of the B cell dominant linear epitopes of the two M proteins provided by the invention has a relatively high conservative property in the M proteins of all genotypes of the NDV; and epitope regions 77-82 and 354-363 of the M proteins are applicable to all NDV strains.
Owner:NORTHWEST A & F UNIV
MVSV virus vector and its virus vector vaccine, a novel coronavirus pneumonia vaccine based on MVSV
ActiveCN111088283BEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
mVSV virus vector, that is, the attenuated mVSV obtained after multiple modifications and mutations at the amino acid sites of the M protein of the wild Indiana strain VSV, and the preferred heterologous antigen gene is preferentially integrated into the double cloning site region of the mVSV packaging core plasmid pmVSV‑Core A kind of mVSV virus vector vaccine, including the heterologous antigen gene of fusion or chimeric target virus in the mVSV vector envelope G and L gene, the antigen gene comprises the antigen gene encoding the chimeric target virus envelope, Chimeric combined antigen gene or fused antigen gene; mVSV viral vector chimerized or fused the dominant antigen of the spike protein S of the COVID‑19 virus pathogen, and the dominant antigen is preferably from the receptor binding structure of the spike protein S The domain is RBD, forming a new crown pneumonia vaccine based on mVSV, which has a good preventive or therapeutic effect on patients infected with the new crown pneumonia virus.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
High specific activity phytase gene and application thereof
InactiveCN101260390AHigh activityImproves pH stabilityHydrolasesAnimal feeding stuffPhytaseDecomposition
The invention discloses a novel AppA-M protein and application of the same. The AppA-M protein is a phytase with high specific activity and can be used for decomposition of phytic acids or phytates. The invention also discloses a gene for encoding the AppA-M protein, and a carrier and a host cell which comprise the gene. The AppA-M protein has wide application prospect in fields such as feedstuff additives and so on.
Owner:SICHUAN HEBEN BIOENG
Antigen of hybrid M protein and carrier for group a streptococcal vaccine
InactiveUS20070053937A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsStreptococcal VaccinesProtection sex
Recombinant hybrid streptococcal M protein antigens are provided which elicit protective antibodies against Group A streptococci and prevent rheumatic fever. Recombinant hybrid genes that encode the antigen are provided. Vaccine compositions and methods of administering the compositions are provided to elicit immunity against Group A streptococci.
Owner:UNIV OF TENNESSEE RES FOUND
Porcine epidemic diarrhea virus fusion protein as well as coding gene and application thereof
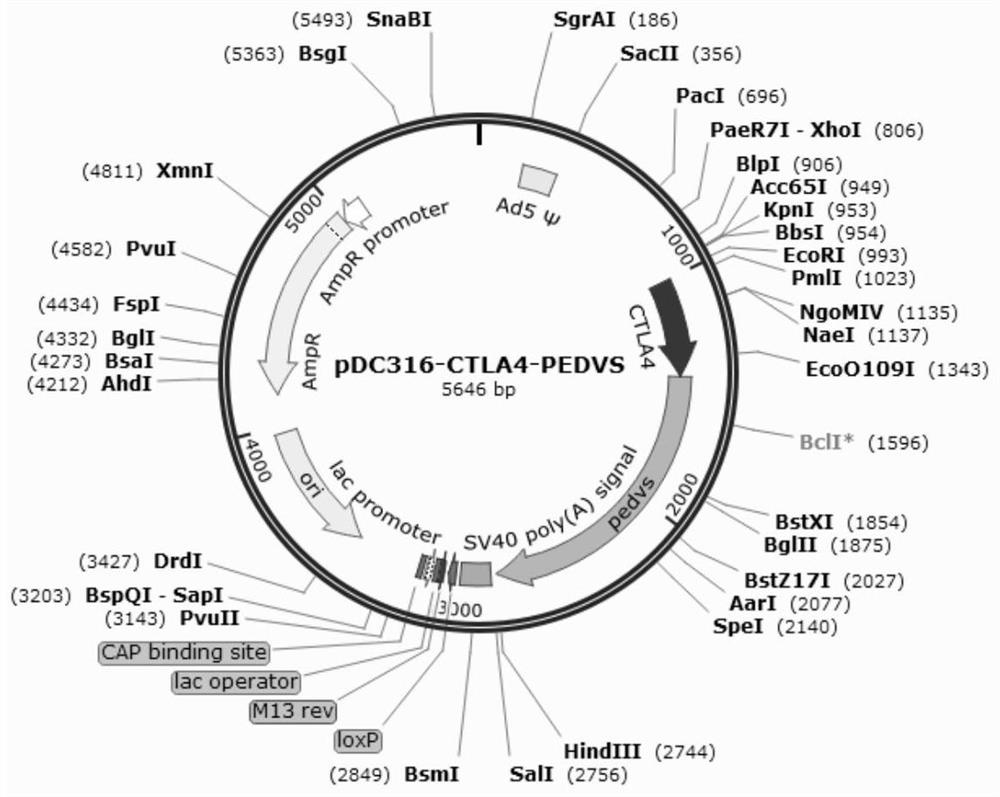
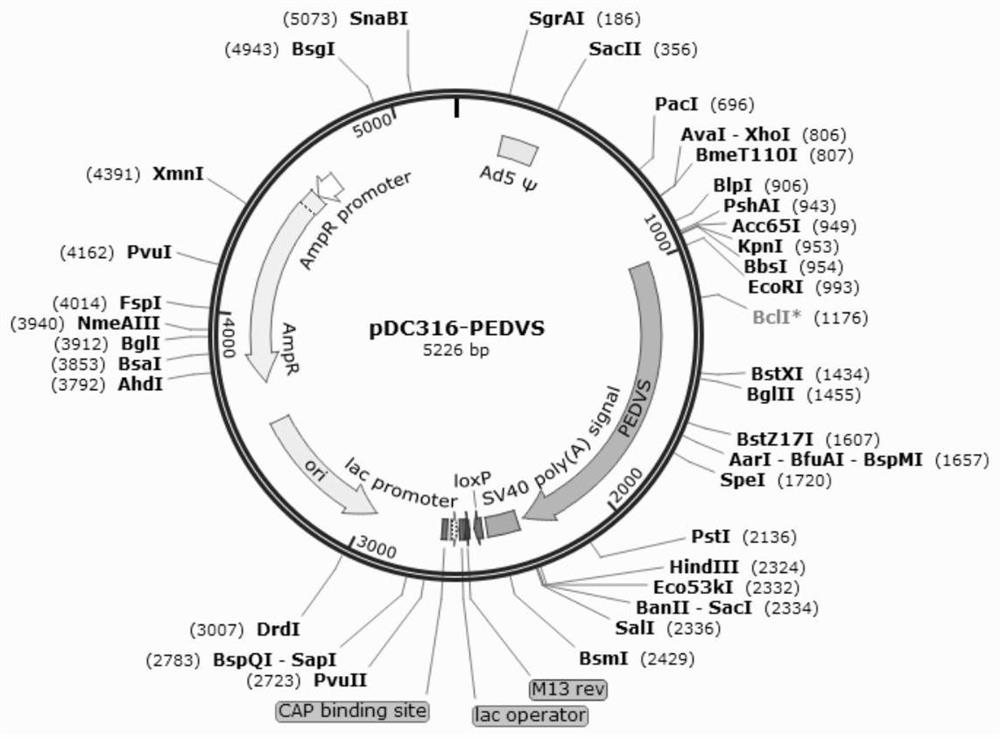
ActiveCN114395574AStrong immune responseGood effectSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpidemic diarrheaShuttle vector
The invention relates to a porcine epidemic diarrhea virus fusion gene as well as an encoding protein and application thereof. According to the invention, a porcine CTLA4 gene and a porcine epidemic diarrhea virus S gene are selected to synthesize a fusion gene CTLA4-PEDVS; the recombinant adenovirus vector rAd-CTLA4-PEDVS is obtained by cloning the fusion gene to an adenovirus shuttle vector plasmid and co-transfecting cells with an adenovirus packaging plasmid, and the recombinant adenovirus vector rAd-CTLA4-PEDVS can be safely used after being purified and can stimulate stronger cellular and humoral immune response; the recombinant adenovirus 5 rAd-CTLA4-PEDVS has good safety and high immune titer, can be immunized once a half a year, is suitable for large-scale production, can effectively prevent and treat the porcine epidemic diarrhea virus, does not generate M protein and other proteins and antibodies of the epidemic diarrhea virus, and is easy to distinguish infected animals.
Owner:长沙爱科博生物科技有限公司
Chimeric recombinant vaccine strain for porcine reproductive and respiratory syndrome and application thereof
ActiveCN113151195AInfection fromReduce pathogenicitySsRNA viruses positive-senseViral antigen ingredientsDiseaseRecombinant vaccines
The invention relates to the technical field of veterinary biological products, in particular to a chimeric recombinant vaccine strain for porcine reproductive and respiratory syndrome and application thereof. The chimeric recombinant vaccine strain for the porcine reproductive and respiratory syndrome is obtained by replacing a GP5 gene, an M protein gene and an N protein gene of a porcine reproductive and respiratory syndrome WH strain with corresponding genes of an NADC30-like strain. The chimeric recombinant vaccine strain for the porcine reproductive and respiratory syndrome provided by the invention is low in pathogenicity and stable in characters, does not cause diseases of piglets after 40 times of passage, and is relatively high in safety; and moreover, the chimeric recombinant vaccine strain has a good immune protection effect, can simultaneously immunize against the porcine reproductive and respiratory syndrome and NADC30-like infection, and has important value in the development of new bivalent vaccines.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Streptococcus m protein, immunogenic fragments, nucleic acids and methods of use
Isolated M proteins, or M-like proteins, of Streptococcus iniae, encoding nucleic acids, genetic constructs and antibodies which bind the isolated M proteins are provided. Also provided are isoforms and / or fragments of the M proteins, or M-like proteins, that display reduced fibrinogen binding. The isolated M proteins, antibodies and encoding nucleic acids maybe useful for diagnosis, immunization and / or therapy of Streptococcus iniae infections of animals such as fish and humans.
Owner:THE UNIV OF QUEENSLAND
IHNV genetically engineered oral microsphere vaccine and preparation method and application thereof
PendingCN110151985AImprove stabilityImprove uniformitySsRNA viruses negative-senseViral antigen ingredientsProtein targetMicrosphere
The invention provides an IHNV genetic engineering oral microsphere vaccine and a preparation method and application thereof. The vaccine is characterized in that the preparation method comprises thefollowing steps: construction of an expression vector pET-22b-m, expression of M protein in the expression vector pET-22b-m, and preparation of the oral microsphere vaccine. The microsphere vaccine has the advantages that adopted sodium alginate and chitosan are natural polysaccharides, safe and free of toxics and pollution, and can stably increase the stability and homogeneity of target protein,the chitosan itself can inhibit the bacterial activity, and a guarantee is provided for the long-term and stable preservation of the microsphere vaccine. The provided preparation method is simple in process and takes less time, and the success rate of the preparation is high. The provided IHNV genetic engineering oral microsphere vaccine has good release properties and acid resistance, and it is found through immune experiments that the vaccine has a certain immune protection effect on rainbow trout.
Owner:HEBEI NORMAL UNIV
A kind of porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application
InactiveCN103585625BImprove abilitiesPracticalMicroorganism based processesAntiviralsElisa kitEngineered genetic
The invention belongs to the technical field of biological vaccine preparation, in particular to a porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application. The present invention selects the S1 gene and M gene of the current new epidemic strain of PEDV as the reference sequence, uses the baculovirus expression system to express the S1 protein or part of the S1 protein and M protein, and prepares the obtained recombinant protein into a subunit vaccine for effective Control the occurrence of porcine epidemic diarrhea. The porcine epidemic diarrhea genetically engineered subunit vaccine prepared by the method of the present invention solves the defects of the current porcine epidemic diarrhea virus traditional vaccine, can be used to prevent and treat porcine epidemic diarrhea virus infection and related diseases caused by it, and can also It is also suitable for preparing the coating antigen of the ELISA kit for detecting porcine epidemic diarrhea virus antibody.
Owner:SOUTH CHINA AGRI UNIV
Group a streptococcus multivalent vaccine
Immunogenic compositions are provided herein that are useful for inducing an immune response specific against group A streptococcus (GAS). Immunogenic compositions provided herein are multivalent and comprise a plurality of immunogenic peptides or fusion polypeptides comprising the immunogenic peptides that induce an immune response against GAS. The immunogenic compositions provided herein induce an immune response against the GAS serotypes represented by an immunogenic peptide (derived from an M protein or Spa protein) comprised within the immunogenic composition and also induce an immune response against serotypes that are unrepresented by any immunogenic peptide included in the immunogenic composition. Methods for using the compositions for inducing an immune response against GAS and for treating or reducing the likelihood of occurrence of a GAS infection are also provided.
Owner:UNIV OF TENNESSEE RES FOUND
Vesicular stomatitis virus for prime boost vaccine
ActiveUS20140370043A1Reduce releaseAttenuated and avirulentSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenArginine
The present invention relates to vesicular stomatitis virus (VSV) matrix (M) protein mutants. One mutant M protein includes a glycine changed to a glutamic acid at position (21), a leucine changed to a phenylalanine at position (111) and a methionine changed to an arginine at position (51). Another M protein mutant includes a glycine changed to a glutamic acid at position (22) and a methionine changed to an arginine at positions (48) and (51). Yet another VSV M protein mutant includes a glycine changed to a glutamic acid at position (22), a leucine changed to a phenylalanine at position (110) and a methionine changed to an arginine at positions (48) and (51). The present invention is directed also to recombinant VSVs (rVSV) having these M mutants and to vaccines based on the rVSV having the M mutants of the present invention. These new rVSVs having the mutant M were significantly attenuated and lost virulence, including neurovirulence, and are capable of inducing an immune responses against an antigen of interest. In addition, a rVSV serotype Indiana having the first described M mutant is capable of efficient replication at 31° C., and of poor replication or incapable of replication at about 37° C. or higher.
Owner:UNIV OF WESTERN ONTARIO
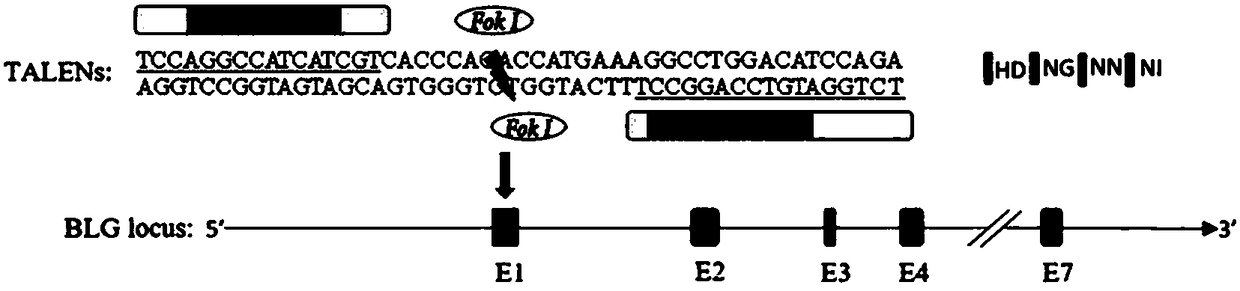
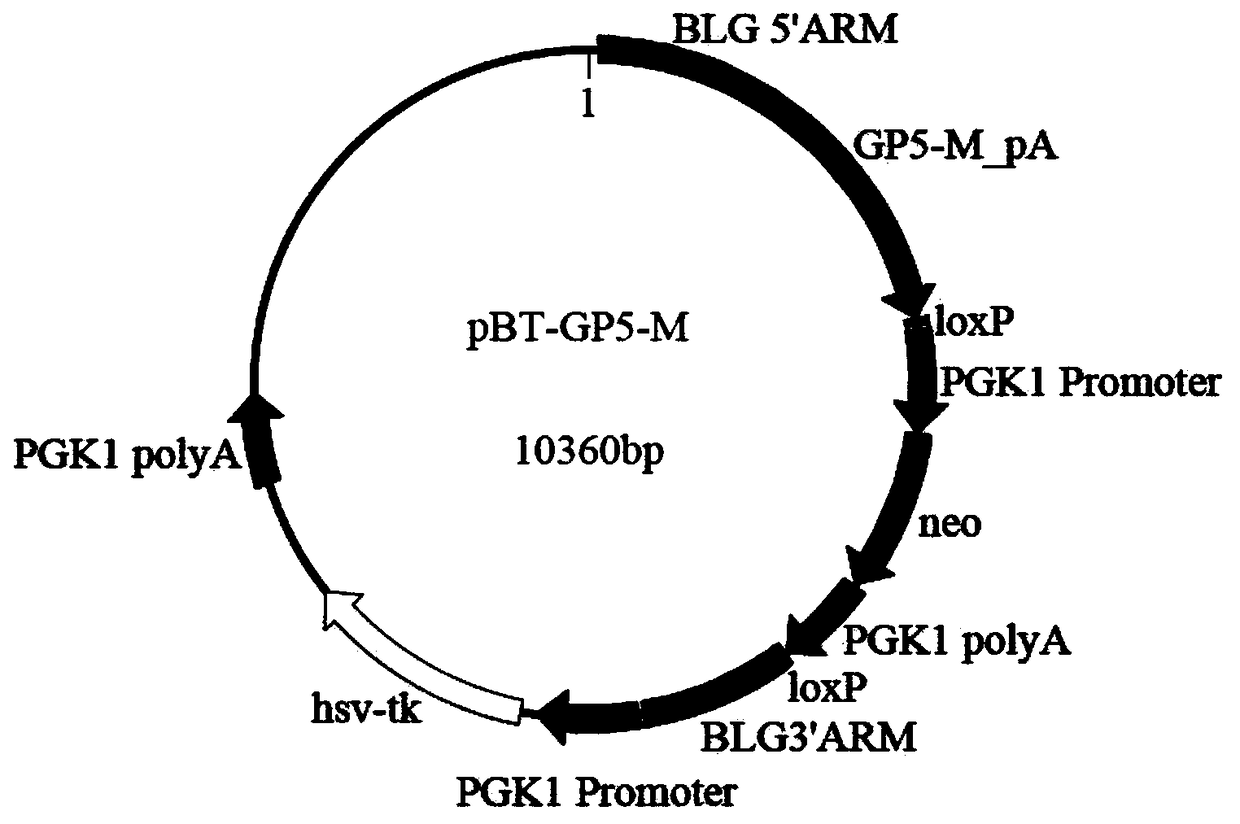
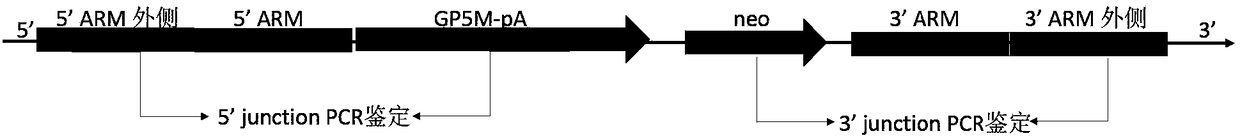
Preparation method of milk goat mammary gland bioreactor for expressing GP5-M protein in milk
InactiveCN108611371AImmunocompetentAvoid defectsSsRNA viruses positive-senseHydrolasesBiotechnologyAdjuvant
The invention discloses a preparation method of a milk goat mammary gland bioreactor for expressing a GP5-M protein in milk. A gene targeting vector of fusion expression of GP5-M is prepared and thenknocked into a first exon of beta-lactoglobulin of a milk goat to screen target cells; a transgenetic milk goat is prepared by using a somatic nucleus transplantation technology; the mammary gland bioreactor is used for producing milk containing a GP5-M fusion protein; and the pure GP5-M fusion protein is separated from the milk, and an adjuvant is added, so as to prepare a gene engineered subunitvaccine and fulfill the aim of preventing and controlling PRRS.
Owner:NORTHWEST A & F UNIV
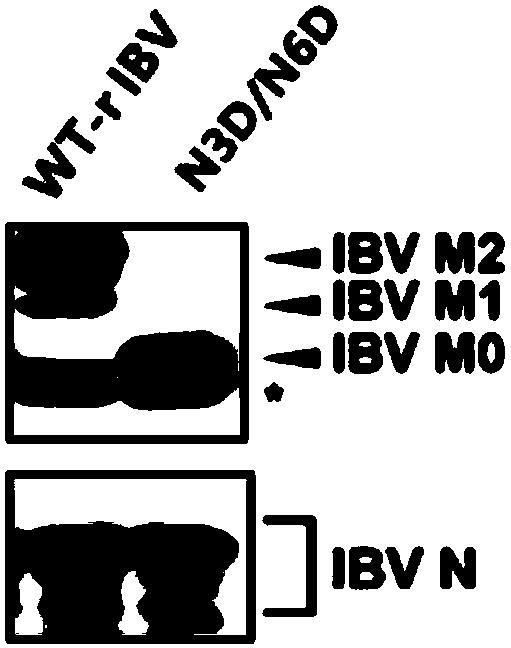
Infectious bronchitis virus with mutation of glycosylation site of M protein and preparation method and application thereof
InactiveCN109517808AWeak stressProliferation does not affectSsRNA viruses positive-senseViral antigen ingredientsInfectious bronchitis virusInfectious laryngotracheitis virus
The invention discloses an infectious bronchitis virus with the mutation of a glycosylation site of a M protein, a preparation method and application thereof. According to the preparation method, a full-length infectious clone of the IBV (infectious bronchitis virus) is obtained by utilizing a reverse genetics technology; then the N->D point mutation is carried out on the glycosylation site of theM protein of the IBV by using the full-length infectious clone as a template; and the mutated virus stain of the M protein of the IBV is obtained after the in vitro transcription of electrotransfection cells. The obtaining of the mutant strain is conductive to studying the influence of the mutation of the glycosylation site of the M protein of the IBV on the structure of the M protein, the proliferation of the virus and the endoplasmic reticulum pressure, to judging whether the replication capacity and pathogenicity of the virus change and to providing a reference method for the development of IBV attenuated vaccines.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com