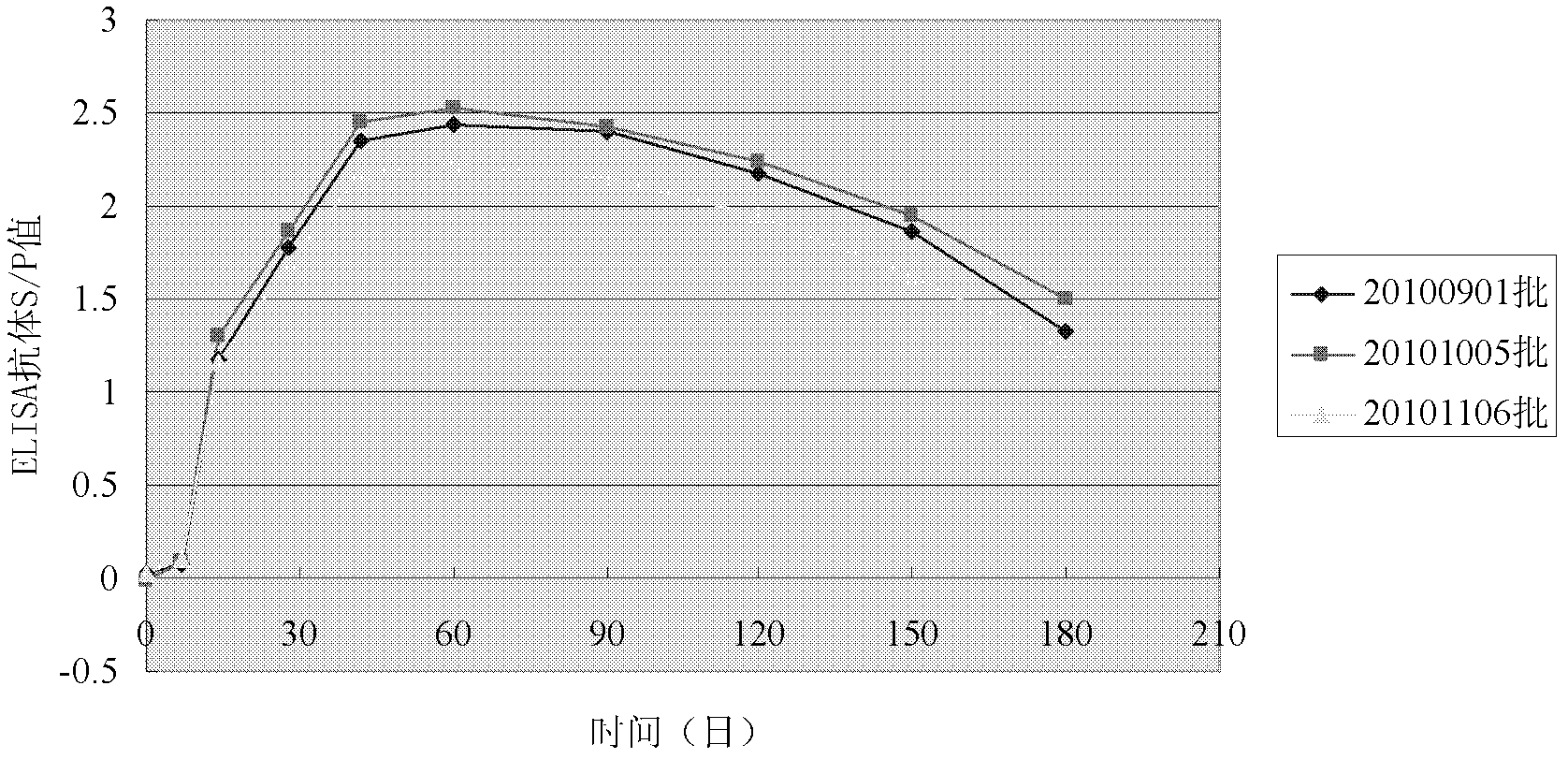
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44results about How to "Good immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Swine mycoplasma pneumoniae inactivated vaccine and preparation method thereof
ActiveCN103182076ASimple and efficient operationGood immunogenicityAntibacterial agentsBacterial antigen ingredientsImmunogenicityFilling-in
The invention belongs to the technical field of veterinary biological new medicine, and relates to a swine mycoplasma pneumoniae inactivated vaccine and a preparation method thereof. The vaccine adopts a bacterial strain with good immunogenicity, has an immunization persistent period of up to 6 months after intramuscular injection of a single dose of 2.0 ml once, and can effectively prevent the generation of swine mycoplasma pneumoniae diseases. The invention solves the problems of complex operation and difficult popularization of domestic live attenuated vaccines, and fills in the gaps of domestic independent research and development of swine mycoplasma pneumoniae inactivated vaccines.
Owner:兆丰华生物科技(南京)有限公司 +1
Preparation method of varicella-zoster virus glycoprotein E extracellular domain protein
InactiveCN107022559AGood immunogenicityBreeding is easyBacteriaVirus peptidesGlycoproteinGenetic engineering
The invention discloses a preparation method of varicella-zoster virus glycoprotein E extracellular domain protein, and relates to a recombinant vector of the varicella-zoster virus glycoprotein E extracellular domain protein, a transformant prepared by use of the recombinant vector, a protein formed by use of the transformant and application of the protein in the fields of research and development of varicella vaccines, zoster virus vaccines, combined vaccines and other related vaccines containing the protein, and detection of corresponding antigens and antibodies, and the like. A VZV-gE extracellular nucleic acid sequence is cloned and connected with prokaryotic expression vector pET-21b to obtain the recombinant vector by genetic engineering technology, the recombinant vector is transformed into Escherichia coli BL21 (DE3) to obtain the transformant, and the target protein is obtained by inducible expression of the transformant. The expression system can be used for efficient VZV-gE extracellular antigen protein expression, and the expressed VZV-gE extracellular antigen protein has good immunogenicity, and can be used in the fields of research and development of the related vaccines and detection of the corresponding antigens and antibodies.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
I-type canine adenovirus attenuated vaccine strain and application thereof
InactiveCN101914502AGood protectionGood immunogenicityNervous disorderDigestive systemCanine kidneyDog attack
The invention discloses an I-type canine adenovirus attenuated vaccine strain and application thereof. In the invention, the separated I-type canine adenovirus attenuated vaccine strain is carried out passage and cloned on MDCK (Madin-Darby Canine Kidney), and attenuated strains (CAV-HR) are cultured. The security and immunogenicity test result shows that the attenuated strains CAV-HR can provide good protection force for dogs attacked by isogeny virulent virus. The attenuated strains (CAV-HR) has favorable immunogenicity, can be prepared into a single seed or combined seeds, and can effectively prevent or treat the diseases caused by the I-type canine adenovirus. The attenuated vaccine strain has the advantages of stable hereditary, lasting immunity, good effect, security, reliability, long preservation period and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Construction of brucellosis A19 molecular marker vaccine strain and determination of virulence and immunogenicity
ActiveCN102776220AWeak toxicityGood immunogenicityBacteriaMicrobiological testing/measurementVaccine strainHomologous recombination
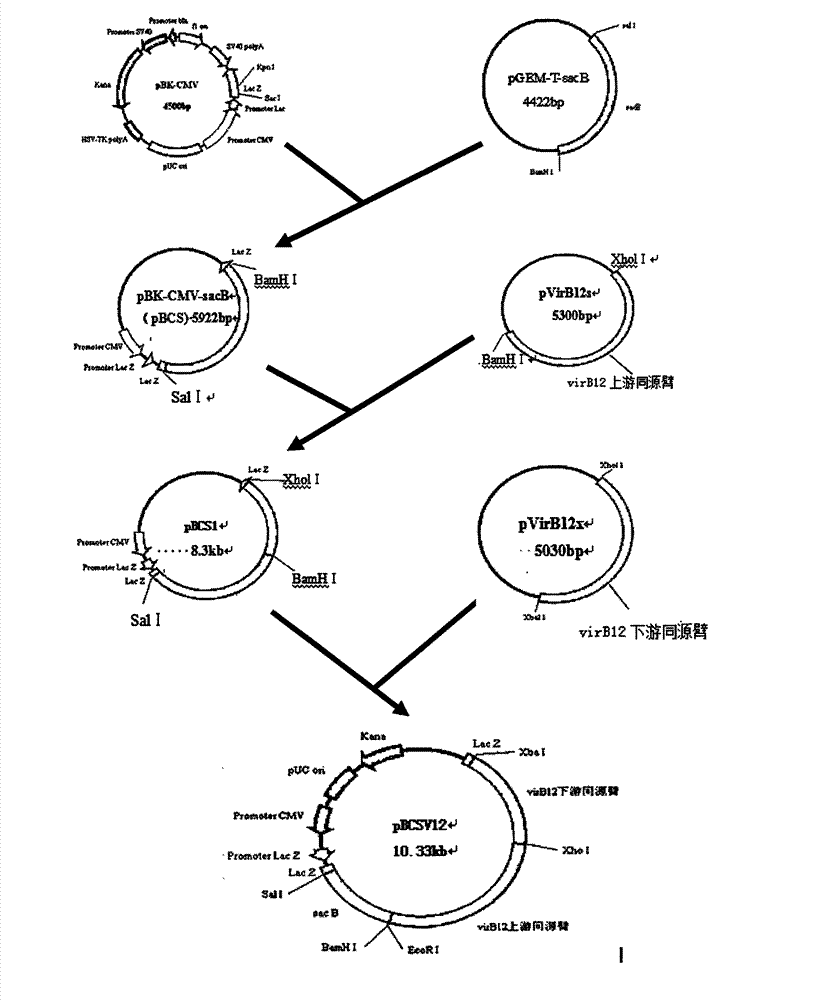
The invention relates to construction of a brucellosis A19 molecular marker vaccine strain and determination of virulence and immunogenicity. According to the invention, a suicide-plasmid-mediated homologous recombination technology is utilized to knock out a virulence factor VirB12 gene of a brucellosis tetratype secretory system of a brucellosis A19 vaccine strain, thus obtaining the A19-delta VirB12 molecular marker vaccine strain. Animal experiments show that the virulence of the A19 molecular marker vaccine strain is slightly weaker than that of a parent A19 vaccine strain, so that the strain safety is further improved, but the good immune effect on brucellosis is maintained; and moreover, the molecular marker vaccine strain has stable heredity. According to the invention, the brucellosis VirB12 gene is taken as a molecular marker, a polymerase chain reaction (PCR) method is applied to identify the brucellosis vaccine strain from wild viral strains, so that the molecular marker is provided for building a determination method for identifying vaccine immunization and natural infection, and the function of the original brucellosis A19 vaccine is improved, therefore, the brucellosis A19 molecular marker vaccine strain has an very important practical meaning on controlling the epidemic brucellosis, and has wide application prospect.
Owner:新疆维吾尔自治区畜牧科学院兽医研究所
Recombinant plasmid, recombinant virus vector, recombinant virus strain and application thereof, recombinant protein and subunit vaccine containing the same
InactiveCN105039373AGood immunogenicityViral antigen ingredientsVirus peptidesChallenge testsGenetic engineering
The invention relates to the technical field of animal genetic engineering and animal virology and especially relates to a recombinant plasmid, a recombinant virus vector, a recombinant virus strain and an application thereof, a recombinant protein and a subunit vaccine containing the same. In the invention, a recombinant baculovirus strain BacSC-Dual-GP5, which high-effectively expresses porcine reproductive and respiratory syndrome virus GP5 protein, is constructed, thereby achieving a better immunogenicity. The recombinant protein expressed by the recombinant virus strain is free of a fusion part and is very high in product expression yield. The PRRSV-GP5 subunit vaccine product in the invention is safe to animal. An immune efficacy test result of the vaccine proves that after 28 days that all vaccines are subjected to immunization, all antibody detection shows positive. A strong poison challenge test proves that both a 1 ml immune group and a 2 ml immune group achieve 100% protection while a 0.5 ml group achieves 80% (4 / 5) protection.
Owner:JILIN HEYUAN BIOENG LIMITED
Helicobacter pylori urease B subunit B cell antigen epitope polypeptide, identification method and application
InactiveCN101033468AGood immunogenicityClear Helicobacter pylori infectionBacterial antigen ingredientsMicrobiological testing/measurementAnti-Helicobacter pylori IgGCarrier protein
This invention provides a B cell epitope peptide and its identification method to neutralize helicobacterpylori urease B subunit, determines the antigen epitope corresponding with the monoclonal antibody 6E6 of helicobacterpylori urease B subunit, and confirms this B cell epitope is specific for the 6E6. The invention also provides a chimeric epitope vaccine and its preparation method containing B cell epitope and carrier protein BSA, and the B cell epitope has strong antigenicity.
Owner:ARMY MEDICAL UNIV
Anti-TEGV (Transmissible Gastroenteritis Virus) high-immune serum and preparation method thereof
ActiveCN103709248AStable virulenceGood immunogenicitySerum immunoglobulinsImmunoglobulins against virusesLaboratory culturePorcine transmissible gastroenteritis virus
The invention discloses an anti-TEGV (Transmissible Gastroenteritis Virus) high-immune serum, which is prepared by separating serum from immunized pigs through taking the inactivated vaccine of a TEGV HB08 strain CGMCC (China General Microbiological Culture Collection Center) No.7807 as an immunogen. The prepared high-immune serum is excellent in character, has no bacteria, mould, mycoplasma and exogenous virus pollution, and is strong in specificity and high in safety, the cure rate of artificial infection can achieve 100%, and the neutralization titer of the serum is 29 above.
Owner:兆丰华生物科技(南京)有限公司 +3
Streptococcus suis and application thereof
InactiveCN106929451AStrong pathogenicityGood immunogenicityAntibacterial agentsBacterial antigen ingredientsRespiratory symptomLaboratory culture
The invention discloses a Streptococcus suis and application thereof. The Streptococcus suis is Streptococcus suis HNSS1 and is preserved in China Generall Microbiological Culture Collection Center with the preservation number of CGMCC NO:13334, the preservation data of November 22th, 2016 and the preservation address of No.3, Courtyard 1, Beichen West Road, Chaoyang District of Beijing. The strain HNSS1 is separated from a brain tissue of a nursery pig dead due to occurrence of typical respiratory symptoms and neurologic symptoms, no other bacteria grow during separation on a TSA solid culture medium, only streptococcus suis grows, and proliferation titer in a TSB liquid culture medium is high and reaches up to more than 10<9>CFU / mL. The strain HNSS1 disclosed by the invention has stronger pathogenicity on the nursery pig, results in pathogenesis and death of the nursery pig and has good immunogenicity.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Infectious bovine rhinotracheitis virus IBRV-JN03 isolate and application thereof
ActiveCN104928260AGood immunogenicityTargetedMicrobiological testing/measurementMicroorganism based processesGenetic engineeringLaboratory culture
The invention discloses an infectious bovine rhinotracheitis virus IBRV-JN03 isolate and application thereof. The preservation number of the infectious bovine rhinotracheitis virus is CGMCC No.10396 and the infectious bovine rhinotracheitis virus is preserved in the China General Microbiological Culture Collection Center of the China Committee for Culture Collection of Microorganisms, and has relatively high virus titer and good immunogenicity; after calves are immunized by an aluminium hydroxide adjuvant inactivated vaccine prepared through the infectious bovine rhinotracheitis virus, relatively high antibodies are generated, so that the infectious bovine rhinotracheitis virus IBRV-JN03 isolate is a good infectious bovine rhinotracheitis vaccine candidate virus strain. Therefore, an infectious bovine rhinotracheitis diagnostic reagent, inactivated vaccine, attenuated vaccine and genetic engineering vaccine prepared on the basis of the virus can be used for diagnosis and vaccine prevention and control of the endemic infectious bovine rhinotracheitis and a wide market application prospect is realized.
Owner:SHANDONG NORMAL UNIV
Preparation and application of baculovirus expression system-based duck tembusu virus subunit vaccine
InactiveCN106834351AGood immunogenicityGood protectionSsRNA viruses positive-senseViral antigen ingredientsSpecific immunityAdjuvant
The invention discloses preparation and application of a baculovirus expression system-based duck tembusu virus subunit vaccine. Tembusu prME protein is expressed by adopting a baculovirus expression system; WB shows that the prME protein is successfully expressed in an sf9 cell and a supernatant, and is partially cut into M protein and E protein after being maturely processed; existence of tembusu virus-like particles of which the diameters are about 30-50nm in the sf9 cell and secreted supernatant is observed through an electron microscope; and the virus-like particles are formed through self-assembly of the protein in the sf9 cell and have relatively high immunogenicity. The protein or the virus-like particles can be purified through two different modes and mixed with an adjuvant at the ratio to prepare the safe, stable and efficient duck tembusu virus subunit vaccine. The vaccine is capable of immunizing cherry valley ducks and egg-laying sheldrakes and then inducing the organism to generate a specific immune response, and 60% of egg-laying sheldrakes can be prevented from being attacked by a duck tembusu virus.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
Strain of duck viral hepatitis virus and application thereof
ActiveCN103525772AGood immunogenicityAvoid infectionEgg immunoglobulinsDigestive systemViral hepatitisImmunogenicity
The invention provides a strain of duck viral hepatitis virus and application thereof, and belongs to the field of biotechnology. The duck viral hepatitis virus 1 type DHV-JS strain has a microbial preservation number of CGMCC NO.8159. The invention also provides application of the duck viral hepatitis virus I type DHV-JS strain, duck viral hepatitis virus vaccines and egg yolk antibodies capable of resisting the duck viral hepatitis. The duck viral hepatitis virus 1 type DHV-JS strain has good immunogenicity for the current popular duck viral hepatitis virus and is capable of resisting viral attack of a duck viral hepatitis virus 1 type R85952 strain, a duck viral hepatitis virus 1 type A66 strain and the self virus strain. After the duck viral hepatitis virus vaccines are vaccinated on the duck, ducklings can be protected from the mother source to resist the inflection of the duck viral hepatitis virus. Another purpose of the invention is to provide the egg yolk antibodies capable of resisting the duck viral hepatitis. The egg yolk antibodies are capable of preventing and treating duck viral hepatitis.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Eimeria tenella calcium-dependent protein kinase gene and application thereof
The invention discloses an eimeria tenella gene. The nucleotide sequence of the gene coded calcium-dependent protein kinase comprises a DNA sequence for coding an SEQ ID NO.1 amino acid sequence or a DNA sequence for coding an amino acid sequence with calcium-dependent protein kinase activity obtained by deleting, adding, inserting or replacing one or a plurality of amino acids in the SEQ ID NO.1 amino acid sequence. The invention also discloses a protein coded by the gene. The eimeria tenella gene is a new gene highly expressed in a sporozoite phase of the eimeria tenella, and is related to the invasion of a to a host cell. The gene has high application value in developing new vaccines or new medicines for inhibiting the invasion of the sporozoite to the host cell and blocking the life cycle of the eimeria tenella.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Recombinant BCG vaccine for tuberculosis prevention
InactiveCN101822829AGood immunogenicityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsGenetic engineeringShuttle plasmid
The invention relates to construction of a BCG vaccine through chimeric expression of GMCSF-CFP10-ESAT6 protein with a human GM-CSF gene, and a CFP10 gene and an ESAT6 gene of mycobacterium tuberculosis and research on immunogenicity of the BCG vaccine. In the construction method, a human GM-CSF gene sequence and a CFP10 gene sequence and an ESAT6 gene sequence of the mycobacterium tuberculosis are inserted into one escherichia coli-mycobacterium tuberculosis shuttle plasmid pMV361 sequence through the genetic engineering technology to construct a recombinant shuttle plasmid rpMV361GMCSF-CFP10-ESAT6. Then the carrier is guided into a BCG vaccine through electroporation to construct a recombinant BCG vaccine rBCG: GMCSF-CFP10-ESAT6. The recombinant BCG vaccine constructed in the invention can stably express the GMCSF-CFP10-ESAT6 chimeric protein, and the immunogenicity of the recombinant BCG vaccine is superior to that of the immunogenicity of the traditional BCG vaccine. The inventionalso provides a preparation process of the recombinant BCG vaccine and research on the immunity of the recombinant BCG vaccine, which belong to the genetic engineering field and the tuberculosis vaccine field. Moreover, the invention can prevent the occurrence and spread of the tuberculosis more effectively.
Owner:SICHUAN UNIV
Application of EG-TPx and kit for diagnosing echinococcosis granulosa of livestock
InactiveCN108982849AGood immunogenicityHigh sensitivity and specificityBiological material analysisOxidoreductasesLivestockEnzyme
The invention discloses application of EG-TPx and a kit for diagnosing echinococcosis granulosa of livestock. The EG-TPx can serve as an immunizing antigen and can be recognized by positive sheep serum with natural infection of echinococcosis granulosa. When applied to indirect ELISA (Enzyme-Linked Immuno Sorbent Assay), the EG-TPx has excellent immunogenicity and high specificity and sensitivity,the sensitivity is 92.6%, the specificity is 99.0%, and the cross reaction with other taeniasis positive serum is low. The ELISA detection method established by an immunizing antigen prepared from echinococcus granulosus thioredoxin peroxidase has an excellent diagnosis effect, and can be applied to preliminary screening of echinococcosis granulosa of cattle and sheep in epidemic areas.
Owner:SICHUAN AGRI UNIV
Indirect ELISA method for detecting Xinxiang babesiosis of sheep
ActiveCN106568950AGood immunogenicityEfficient and specific detectionMaterial analysisElisa methodIndirect elisa
The invention discloses an ELISA diagnostic kit for detecting Xinxiang babesiosis of sheep. The kit comprises a recombinant RAP-1 protein, which is prepared by an escherichia coli expression system and is taken as the antigen for preventing specific antibody of Xinjiang babeisa in sheep.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
DNA vaccine with resistance to infectious spleen and kidney necrosis viruses
InactiveCN104096240AGood immunogenicityEasy to prepareGenetic material ingredientsAntiviralsAdjuvantNucleic acid sequence
The invention discloses a DNA vaccine with resistance to infectious spleen and kidney necrosis viruses (ISKNV). The nucleotide sequence of the DNA vaccine is the gene sequence of MCP (major capsid protein) of ISKNV. When the DNA vaccine is used together with an adjuvant QCDC, excellent immunogenicity is realized, the relative percent survival can reach 80% which is remarkably superior to the relative percent survival (64.3%) of the present MCP protein vaccine, and a novel vaccine with the resistance to ISKNV of siniperca chuatsi can be developed. A preparation method of the DNA vaccine is simple, easy to operate, low in cost and easy for popularization in the market.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Sheep A-type clostridium perfringens strain, as well as inactivated vaccine thereof and vaccine preparation method
PendingCN111500482AGood immunogenicityHigh in toxinsAntibacterial agentsBacterial antigen ingredientsClostridium bacteriaImmunogenicity
The invention discloses a sheep A-type clostridium perfringens strain as well as an inactivated vaccine and a vaccine preparation method thereof, and belongs to the field of biological medicines. Thesheep A-type clostridium perfringens strain screened by the method is relatively high in toxin production level, good in immunogenicity and stable in culture characteristic; a culture medium formula and culture conditions for culturing the sheep A-type clostridium perfringens strain are optimized, the culture stability and the basic antigen titer are improved, and a toxin bacterium solution with high antigen toxin content is obtained; a sheep A-type clostridium perfringens inactivated vaccine is prepared from the toxin bacterial liquid, the sheep A-type clostridium perfringens inactivated vaccine with reliable immune effect and low immune dose is obtained, and the sheep A-type clostridium perfringens inactivated vaccine can be used for effectively preventing and controlling sudden death caused by sheep A-type clostridium perfringens which are popular severely at present.
Owner:内蒙古金宇保灵生物技术研究院有限公司
Virus-like particles based type-A foot and mouth disease virus antibody detection kit
The invention discloses a virus-like particles based type-A foot and mouth disease virus antibody detection kit and a preparation method thereof. The virus-like particles based type-A foot and mouth disease virus antibody detection kit comprises an elisa plate coating the virus-like particles, HRP labeled rabbit antibody, a sample diluent containing phosphate buffer, a cleaning solution containingTween, a TMB substrate, positive control serum, negative control serum, and stop buffer mixed of concentrated sulfuric acid and water. Compared with the prior art, the kit has better safety, good immunogenicity and higher flexibility and specificity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
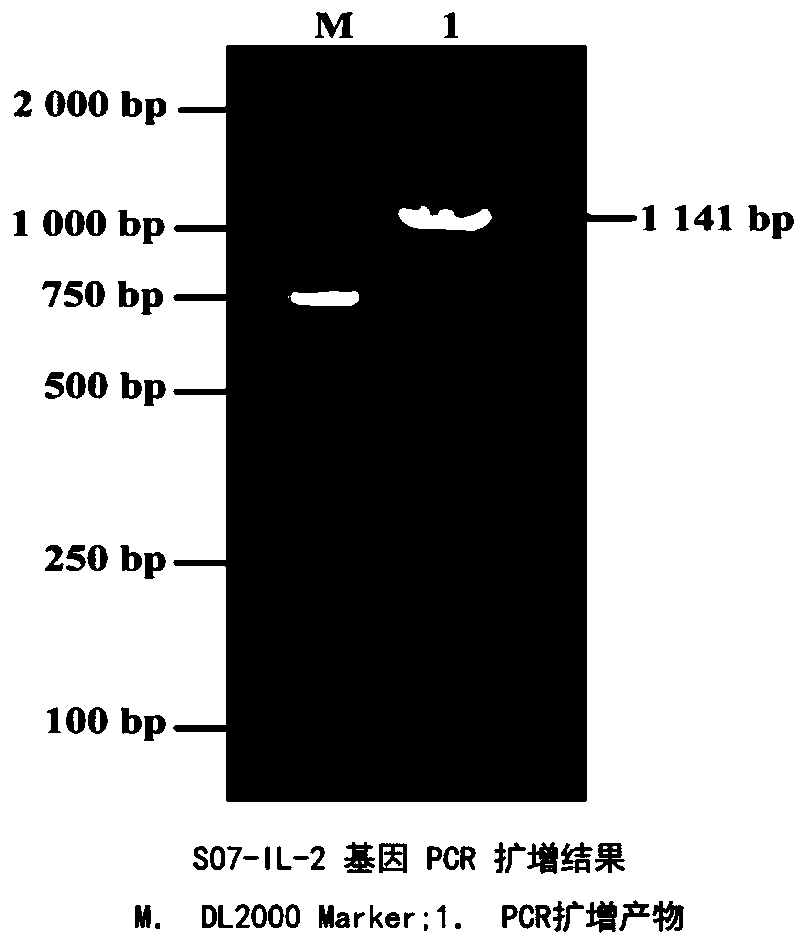
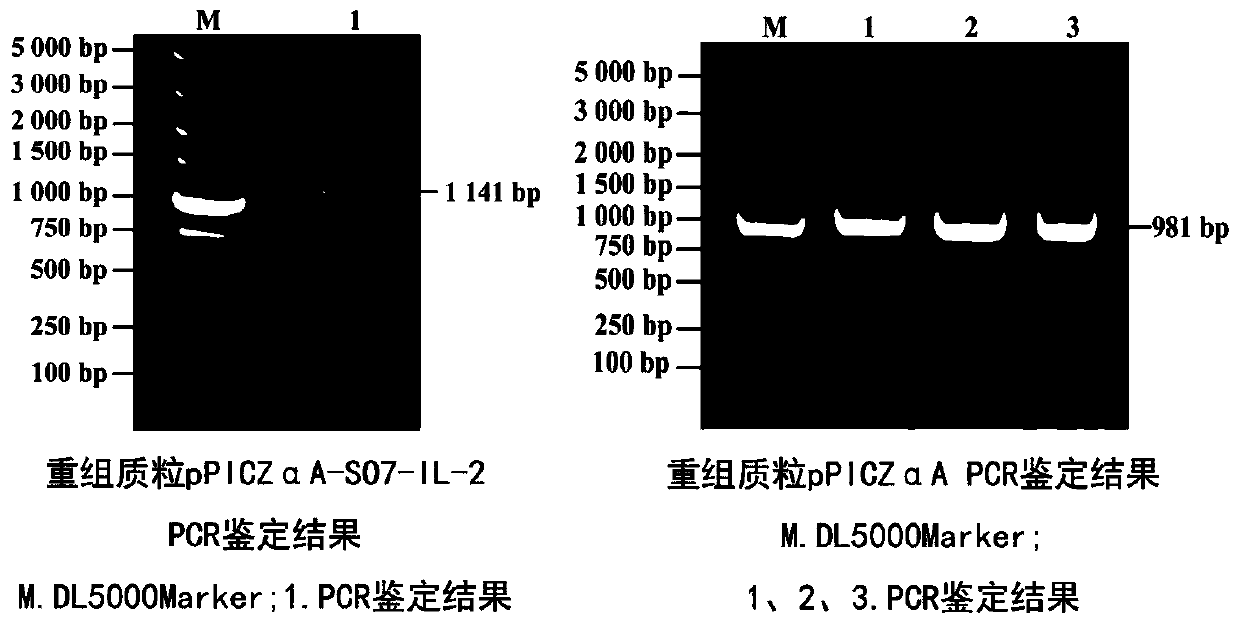
Eimeria tenella SO7-IL-2 protein and preparation method thereof
PendingCN110256576AStrong protective effectGood immunogenicityAntibody mimetics/scaffoldsMicroorganism based processesProtein totalAnimal science
The invention provides Eimeria tenella SO7-IL-2 protein and a preparation method thereof. The Eimeria tenella SO7-IL-2 protein based a Pichia pastoris vector is novel Eimeria tenella recombinant yeast protein. Chickens are immunized by protein obtained by recombinant yeast protein total crushing and induction expression, and the recombinant protein extracted by the total crushing method is high in protecting effect. By detecting humoral immunity and cellular immunity level, the results show that the immunity level of the chickens immunized by the protein obtained by recombinant yeast protein total crushing and induction expression is evidently higher than that of control, and the protein can be used for preventing Eimeria tenella diseases.
Owner:JILIN UNIV
Escherichia coli O157:H7 attenuated strain and application thereof
ActiveCN108018228AGood immunogenicitySimple methodAntibacterial agentsBacteriaAttenuated strainToxicity
The invention provides an Escherichia coli O157:H7 attenuated strain and an application thereof, and belongs to the field of biotechnology. The preservation number of the Escherichia coli O157:H7 attenuated strain JSC2 is CCTCC M 2017356. A solution of the attenuated strain is prepared, and a result of toxicity attack test of the BALB / c mice and young rabbits with the attenuated strain solution shows that the strain does not cause death in the mice and young rabbits and does not cause obvious histopathological changes. After a mouse is subcutaneously inoculated with the attenuated strain, a high-level IgG antibody can be induced, the antibody has a long duration, a good immune effect is provided for vaccinated animals, and the attenuated strain can be used for developing Escherichia coli O157:H7 vaccines and diagnostic reagents.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
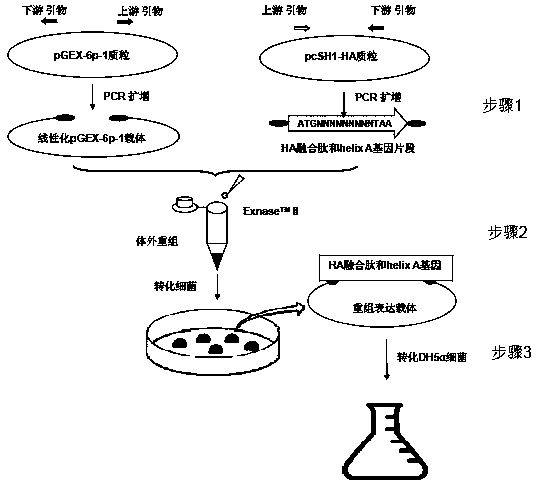
Preparation method, application and preparation primer of influenza virus HA fusion peptide and helix A protein
InactiveCN109082423AGood immunogenicityStimulationSsRNA viruses negative-senseAntibody mimetics/scaffoldsEnzymeExpression vector
The invention discloses a preparation method, application and preparation primer of an influenza virus HA fusion peptide and helix A protein expression vector. The principle and key technique herein are to scientifically design and amplify a pGEX-6P-1 linear vector and influenza virus HA fusion peptide-helix A gene fragment; commercial recombinant enzyme Exnase TM II is subjected directly to in-vitro quick recombinant cloning of HA fusion peptide-helix A without enzyme-cut and link-up connection; Escherichia coli is converted and expressed under IPTG induction, and fusion expression of influenza virus HA fusion peptide-helix A protein with GST is achieved; the purified HA fusion protein-helix A fusion protein is attained.
Owner:YANGZHOU UNIV
Structuring and preparation method of Ad5-M2m used as general influenza vaccine virus seed
InactiveCN106480095AHigh expressionGood immunogenicitySsRNA viruses negative-senseViral antigen ingredientsAntigenInfluenza vaccine
The invention discloses a structuring and preparation method of Ad5-M2m used as general influenza vaccine virus seed; the Ad5-M2m recombinant adenovirus is prepared as a general influenza vaccine virus seed; the preparation method includes steps of structuring multiple flu subtype conservative immunogen M2m recombinant adenovirus and establishing the virus seed; inoculating and cultivating Ad5-M2m; harvesting Ad5-M2m; preliminary immunizing of Ad5-M2m, and so on. The Ad5-M2m used as general influenza vaccine virus seed prepared by the invention is wide in antigen spectrum, stable in quality, high in purity, good in safety, low in cost and others; the method is applicable to large-scale production and has big application value.
Owner:SOUTH CHINA AGRI UNIV +2
Application of mycoplasma capricolum subsp. Capripneumoniae Mccp1801 in preparation of contagious caprine pleuropneumonia vaccine
ActiveCN112891525AToxicGood immunogenicityAntibacterial agentsBacterial antigen ingredientsVirulenceTiter
The invention provides application of mycoplasma capricolum subsp. Capripneumoniae Mccp 1801 in preparation of a contagious caprine pleuropneumonia vaccine, and relates to the technical field of biology. The invention further provides a vaccine based on the M1801 strain and a preparation method thereof. The vaccine can improve the immune protection effect, reduce immune side reactions and provide a good immune protection effect; meanwhile, the M1801 strain is high in virulence, good in immunogenicity and excellent in growth performance, a body can be induced to generate a high-level antibody when the strain is used for preparing a vaccine, and the antibody is early in generation time and high in titer; the vaccine safety is high; and immune sheep is protected by 4 / 5 or more, and symptoms such as body temperature rise, cough, sneezing, depressed spirit or tissue lesions can be obviously relieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
RTG fusion protein capable of enhancing immunogenicity and immune protection effect
PendingCN114524880AGood immunogenicityImprove abilitiesAntibacterial agentsBacterial antigen ingredientsCell immune responseStreptococcus agalactiae
The invention relates to an RTG fusion protein capable of enhancing immunogenicity and immune protection effect, which is formed by carrying out series fusion expression on a modified peptide ligand APLR106A obtained by mutating an epitope peptide of staphylococcus aureus TRAP and streptococcus agalactiae GapC1-150 through Linker-(GS) 4, and the amino acid sequence of the RTG fusion protein is as shown in SEQ ID No.1. The invention also discloses a function of the RTG fusion protein. According to the present invention, the APLR106A and the GapC1-150 are subjected to series fusion expression through the flexible Linker to obtain the recombinant fusion protein RTG, the APLR106A can significantly enhance the level of the cellular immune response and the humoral immune response induced by the GapC1-150, and the recombinant fusion protein RTG has good immunogenicity, and can simultaneously improve the streptococcus resistance and the staphylococcus aureus resistance of the body.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Mycobacterium tuberculosis inclusion body protein renaturation method and special renaturation buffer solution thereof
PendingCN114380897AGood immunogenicityImproving immunogenicityBacteriaMicroorganism based processesInclusion bodiesSplenic lymphocyte
The invention discloses a mycobacterium tuberculosis inclusion body protein renaturation method and a special renaturation buffer solution thereof. Experiments prove that the renaturation buffer solution provided by the invention can be used for renaturating mycobacterium tuberculosis protein W544, and the renaturated W544 protein can effectively stimulate splenic lymphocytes of a mouse infected by mycobacterium tuberculosis to generate immune response and does not stimulate splenic lymphocytes of a healthy mouse to generate immune response. Therefore, the renatured W544 protein has very good immunogenicity. The renaturation buffer solution and the mycobacterium tuberculosis inclusion body protein renaturation method established according to the renaturation buffer solution have important application value.
Owner:中国人民解放军总医院第八医学中心
Egg yolk antibody for treating novel reovirus of chicken and preparation method thereof
ActiveCN108659122AGood immunogenicityGood securityEgg immunoglobulinsImmunoglobulins against virusesAntibodyAnimal science
The invention discloses an egg yolk antibody for treating novel chicken reovirus and a preparation method thereof. The egg yolk antibody comprises an antibody resisting chicken reovirus strain; the preservation number of the chicken reovirus strain is CCTCC NO: V201817. The formed egg yolk antibody is good in safety, and any local or systemic side effect caused by the egg yolk antibody does not occur; infection of the novel chicken reovirus can be effectively prevented and / or treated, and the egg yolk antibody has a good commercial development prospect.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Asia 1 type foot and mouth disease virus antigen and preparation and application thereof
ActiveCN103382480BGood immunogenicityImprove the level ofAntiviralsAntibody medical ingredientsTransgenesisPathogen
The invention discloses a nucleotide sequence opti-LTB-Asia 1 / VP1 and a recombinant vector pC 1300 / opti-LTB-Asia 1 / VP1 containing the sequence. The invention further discloses fusion expression mucosal immune adjuvant Asia 1 type foot and mouth disease virus antigen prepared by using the recombinant vector pC 1300 / opti-LTB-Asia 1 / VP1 and an application thereof. The antigen expressed by transgenic tomatoes has good immunogenicity. The transgenic tomatoes only express sigmasubunit antigen, do not contain pathogenic microorganisms or potential pathogenic microorganisms and are safe for human and livestock and free of pathogen pollution. An extraction and purification process is not required by producing immunogen through transgenic plants, and the antigen, the preparation and the application have the advantages of being simple in process, good in immune effect and protective effect on animals and the like and are suitable for immunity of livestock.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Inactivated vaccine for preventing and controlling new chicken reovirus and preparation method thereof
ActiveCN108379574AGood immunogenicityGood securityViral antigen ingredientsMicroorganism based processesAntibody levelVirus inactivation
The invention discloses an inactivated vaccine for preventing and controlling a new chicken reovirus and a preparation method thereof. The inactivated vaccine contains inactivated chicken reovirus strain virus liquor with the effective dose on prevention or treatment. The preservation number of a chicken reovirus strain is CCTCC NO:V201817. The prepared inactivated vaccine for preventing and controlling the new chicken reovirus is good in safety, no any local or whole-body adverse reactions caused by the vaccine are generated, and detection of all indexes is stable and effective. After broilers are immunized by the inactivated vaccine, the broilers can obtain the high antibody level, the duration is long, infection and outburst of the new chicken reovirus can be pertinently prevented and controlled, and effective immunoprotection is provided for chicken flocks. Meanwhile, the production process of the vaccine is simplified, and large-scale production can be achieved.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
H9 subtype avian influenza virus isolate and application thereof
ActiveCN112574958AGood immunogenicityGood immune prophylaxisSsRNA viruses negative-senseSsRNA viruses positive-senseCombined VaccinesImmunogenicity
The invention provides an H9 subtype avian influenza HF strain and a vaccine composition prepared from the inactivated antigen of the H9 subtype avian influenza HF strain. The H9 subtype avian influenza HF strain has good immunogenicity, the existing H9 subtype avian influenza can be completely protected under the condition of low content, and the H9 subtype avian influenza HF strain can be used for preparing combined vaccines and compound vaccines together with various other antigens.
Owner:PU LIKE BIO ENG +1
Recombinant FCV antigen and feline calicirus genetic engineering subunit vaccine
PendingCN113896773AStable structureGood immunogenicitySsRNA viruses positive-senseVirus peptidesGenetic engineeringSuspension culture
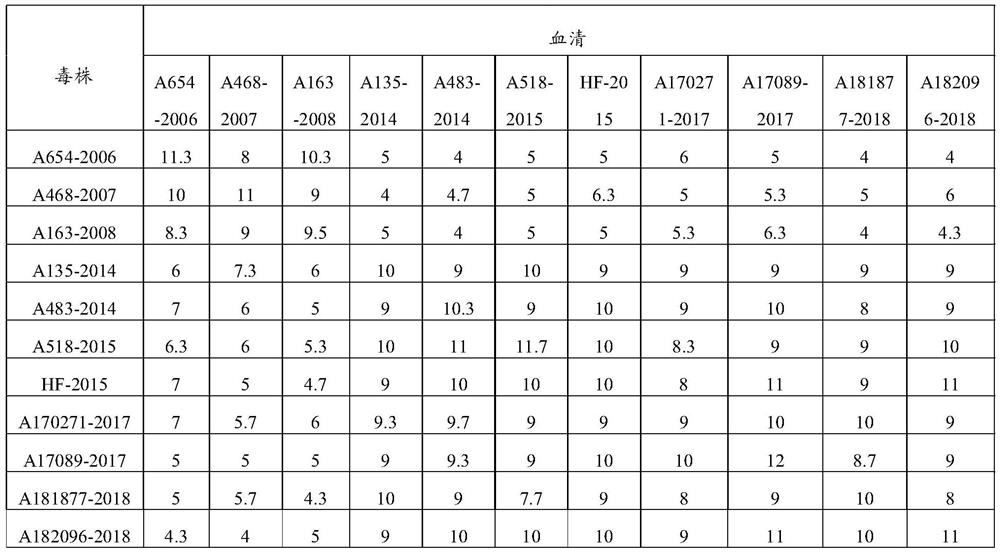
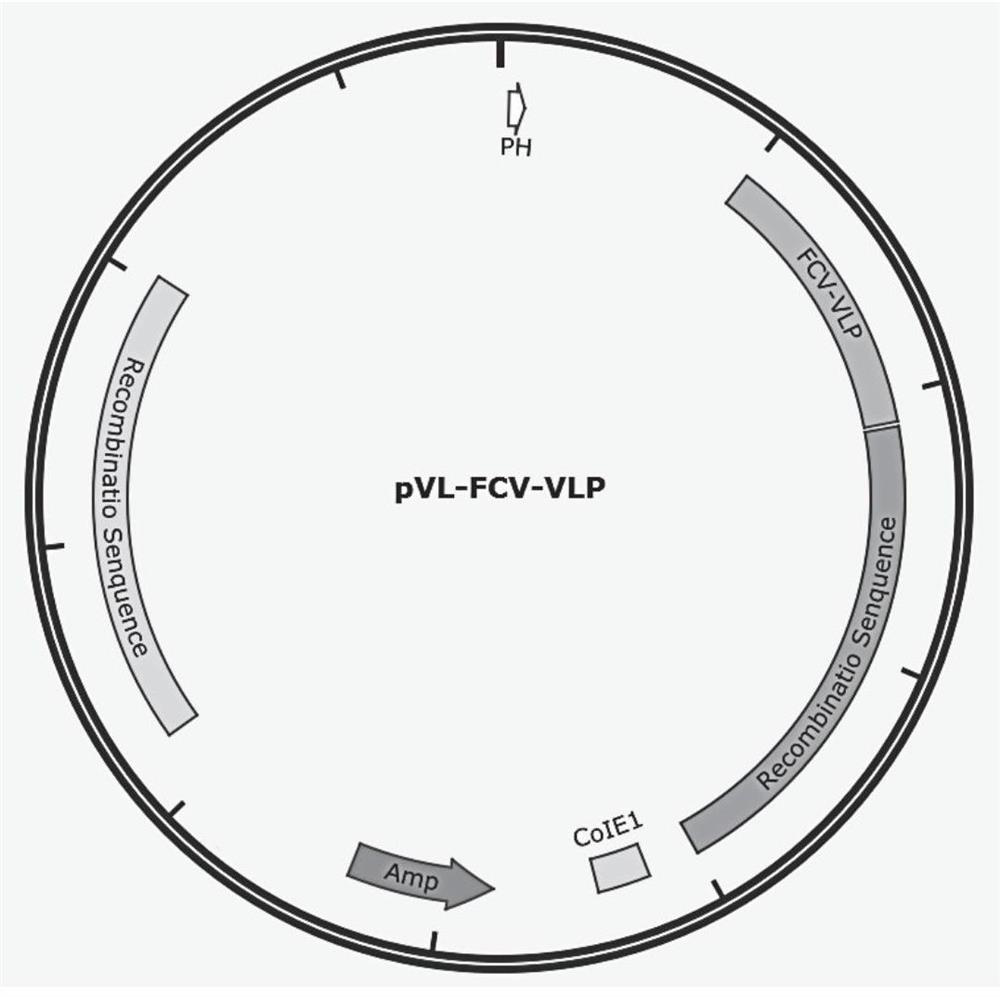
The invention discloses a recombinant FCV antigen and a Feline calicirus genetic engineering subunit vaccine. The recombinant FCV antigen comprises two proteins with the sequences shown as SEQ ID NO: 2 and SEQ ID NO: 3 respectively. The vaccine comprises the recombinant FCV antigen and a pharmaceutically acceptable carrier. The recombinant FCV antigen provided by the invention is easy for self-assembly to form a virus-like particle (VLP) with stable structure and excellent immunogenicity. The VLP has shape, stability and immunogenicity close to those of a wild virion, and can be prepared by large-scale serum-free suspension culture in a bioreactor through an insect cell expression system along with high expression level and good protein immunotherapy. The prepared vaccine is easy in quality control, stable in batches and low in production cost.
Owner:苏州世诺生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com