Preparation and application of baculovirus expression system-based duck tembusu virus subunit vaccine
A technology of baculovirus and recombinant baculovirus, applied in the direction of microorganism-based methods, applications, vaccines, etc., can solve the problems of immune effect being easily affected by various factors, poor protein biological activity, strong virulence, etc. , to achieve good protective effect, high biological safety and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Construction of recombinant baculovirus AC-prME expressing duck Tembusu PrM-E gene
[0079] 1. Construction of recombinant baculovirus AC-prME
[0080] 1. Acquisition of prME gene
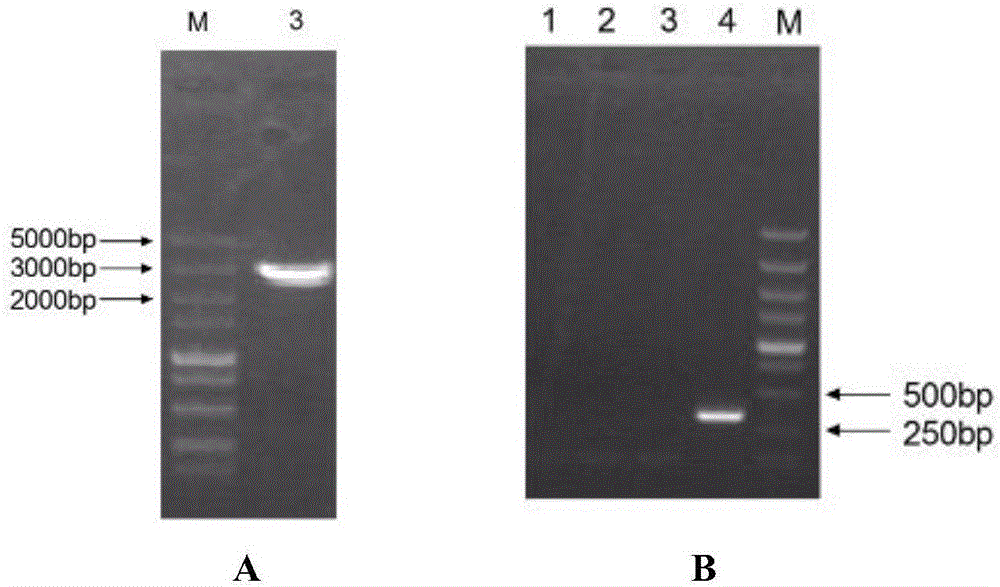
[0081] A 2100bp prME gene and its signal peptide sequence (GenBank ID: KJ489355) were artificially synthesized with reference to the prME gene and its signal peptide sequence of Duck Tembusu virus DF2 strain (Duck Tembusu virus DF2), and the sequence was cloned into the vector pUC-18 (Invitrogen Company) The recombinant plasmid pUC-prME was obtained. Use the plasmid as a template with a primer pair
[0082] Forward: 5'-TAGGCGGCCGCATGCAGATGCTCGACGGACTGAAT-3',
[0083] Reverse: 5'-AGCTGCAGTTAGGCATTGACATTTACTGC-3';
[0084] PCR amplification was carried out, and a prME gene fragment with a size of 2100 bp was recovered.
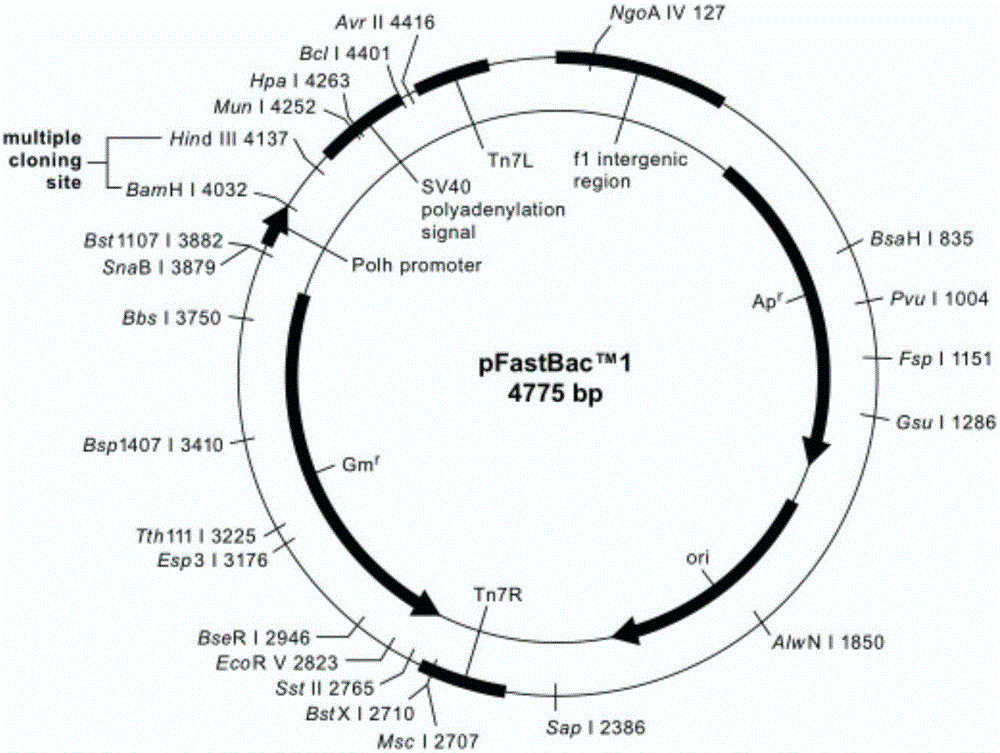
[0085] 2. Construction of baculovirus transfer vector pFastBac1-prME
[0086] Baculovirus universal vector pFastBac1 TM (Invitrogen Company) as the backbone,...
Embodiment 2
[0118] Embodiment 2: the subunit vaccine prepared by recombinant baculovirus Ac-prME
[0119] 1. A subunit vaccine prepared by recombinant baculovirus Ac-prME.
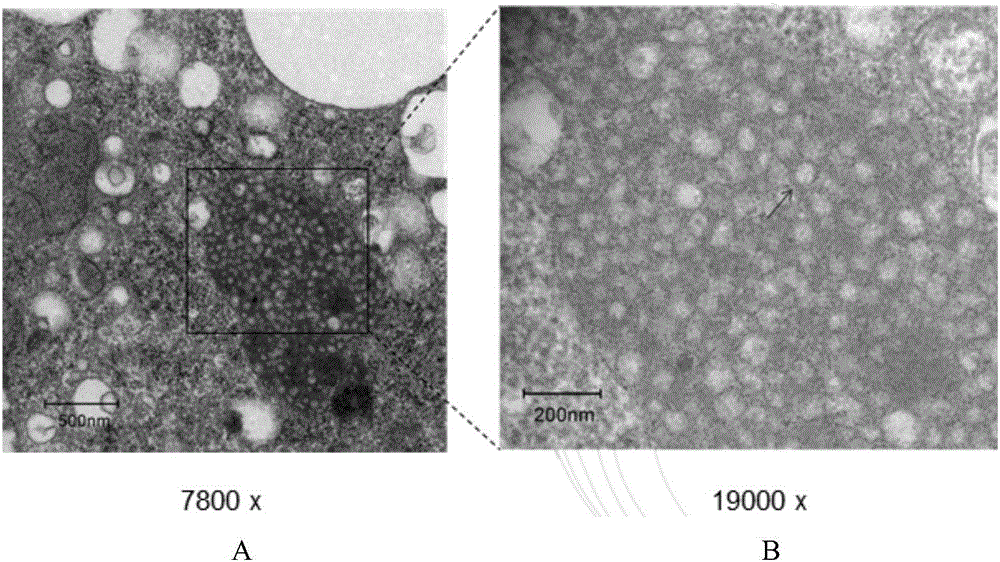
[0120] The recombinant baculovirus Ac-prME obtained in Example 1 was used to inoculate suspension-cultured insect cells sf9 at a multiplicity of infection dose of 2.0 MOI, and the cells and supernatant were collected after 72 hours, and the cells were purified according to the method described in 4 of Example 1 DTMUV-VLPs in the medium, after measuring the protein concentration, the water phase is composed of protein and Tween 80 according to the volume ratio of 97:3, and the water phase and the oil phase are mixed and emulsified according to the volume ratio of 1:2 to prepare a sub-vaccine, so that each ml The total protein content is 0.5 mg / ml, which is used in the following examples 3 and 4, and stored at 4 degrees.
[0121] 2. Subunit vaccine prepared by recombinant baculovirus Ac-prME.
Embodiment 3
[0123] Example 3: Subunit vaccine components and routine testing
[0124] Dosage form: water-in-oil (W / O)
[0125] Adjuvant composition: white mineral oil (Marcol52)
[0126] Tween 80 (CRILLET4)
[0127] Vaccine emulsification: the water phase is composed of protein and Tween 80 at a volume ratio of 97:3, and the water phase and oil phase are mixed and emulsified at a volume ratio of 1:2 to prepare a sub-vaccine.
[0128] Vaccine property test: after emulsification, the vaccine is dripped into clear water, the first drop will disperse, and the second drop will be in the form of oil beads, and the oil beads on the liquid surface will not break the emulsification.
[0129] Sterility test: Take a small amount of the vaccine and coat it on a TSA plate, and incubate in a 37°C incubator for 18 hours without colonies growing.
[0130] Stability test: Centrifuge at 3000rpm / min for 15 minutes, the emulsified vaccine is not separated after centrifugation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com