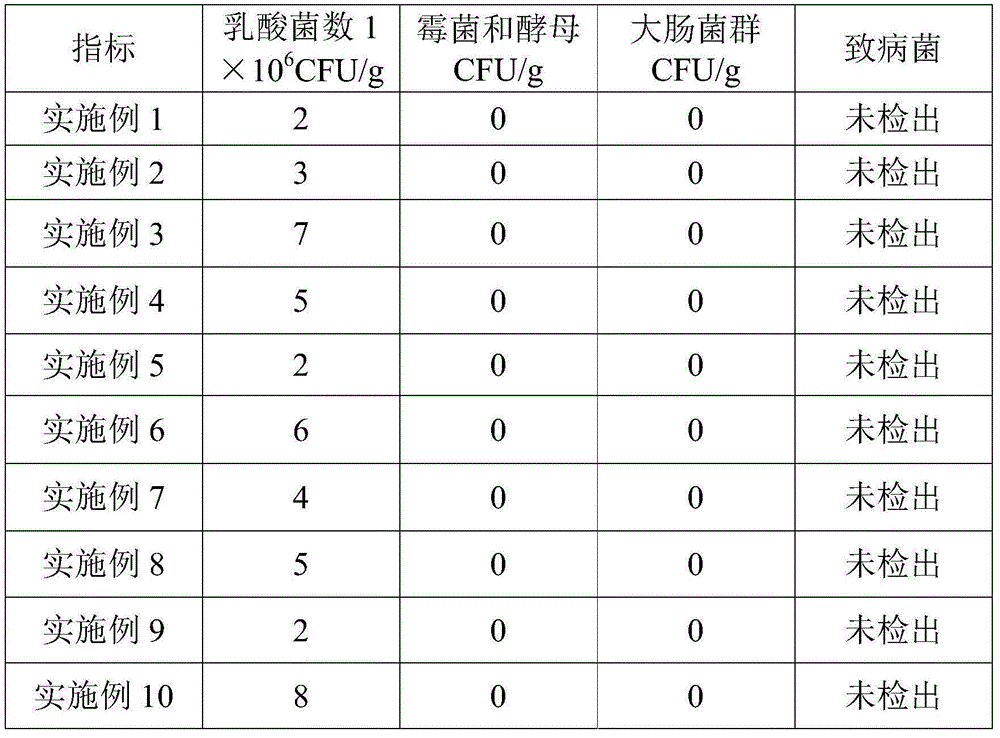
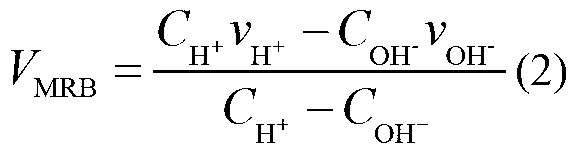Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
614 results about "Protein concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
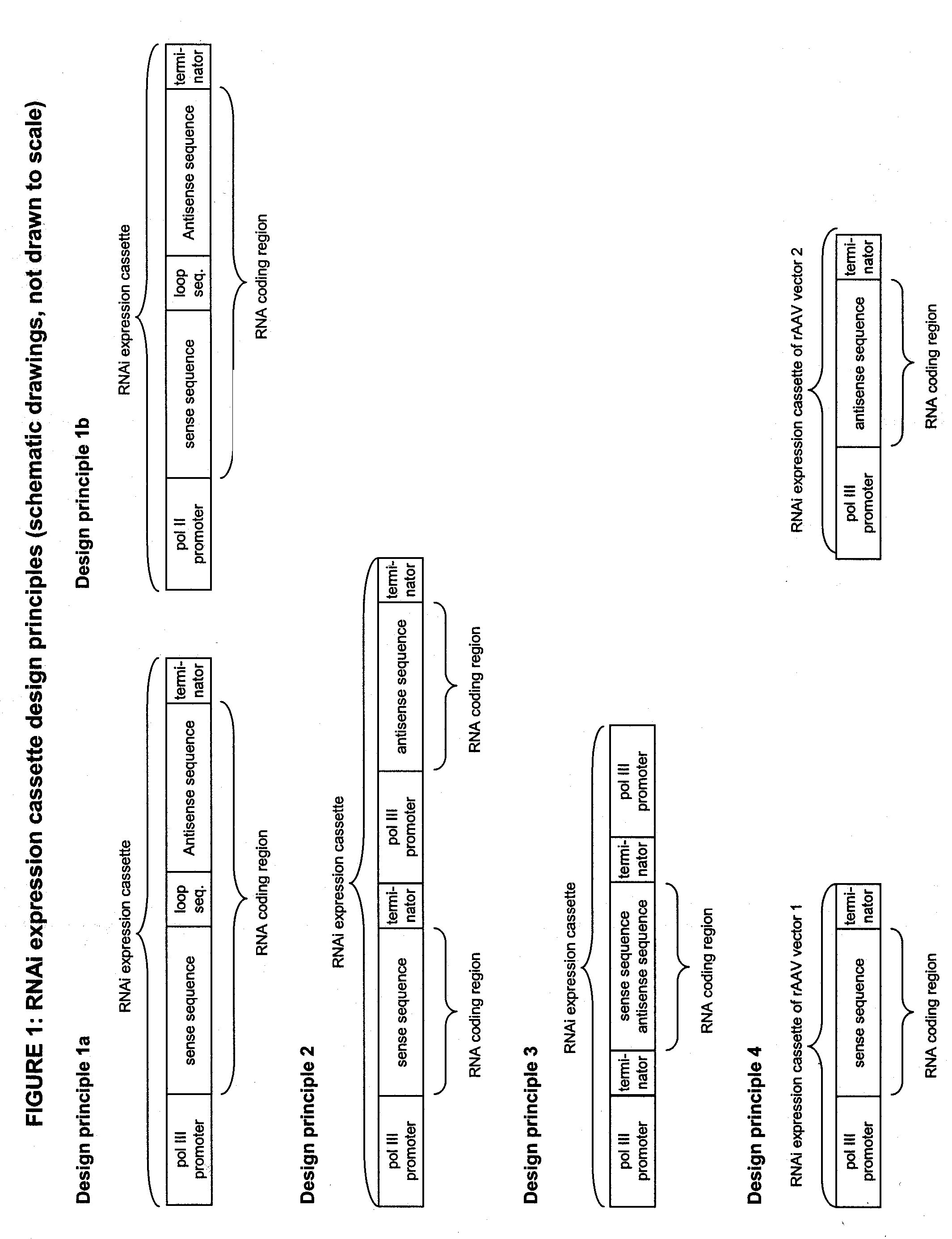
DECREASING GENE EXPRESSION IN A MAMMALIAN SUBJECT IN VIVO VIA AAV-MEDIATED RNAi EXPRESSION CASSETTE TRANSFER
InactiveUS20050019927A1High efficacyStrong specificityVectorsPeptide/protein ingredientsDecreased ConcentrationIn vivo
Decreasing the expression of genes in a mammalian subject has multiple applications ranging from cancer therapy to anti-infective therapy or treatment of autosomal dominant genetic disorders. Yet, there is still a lack of efficient technologies to achieve that goal in mammalian subjects in vivo. The present invention relates to methods for decreasing gene expression by administering to a mammalian subject a recombinant adeno-associated viral vector in vivo with said vector comprising an RNA interference (RNAi) expression cassette whose RNA expression products directly or indirectly lead to a decrease in expression of the corresponding RNAi target gene. Upon successful transduction with the recombinant adeno-associated viral vector, the RNA expression products of the RNAi expression cassette will decrease the cellular concentration of the mRNA transcripts of the RNAi target gene, thus resulting in decreased concentration of the protein encoded by the RNAi target gene.
Owner:HILDINGER MARKUS +1
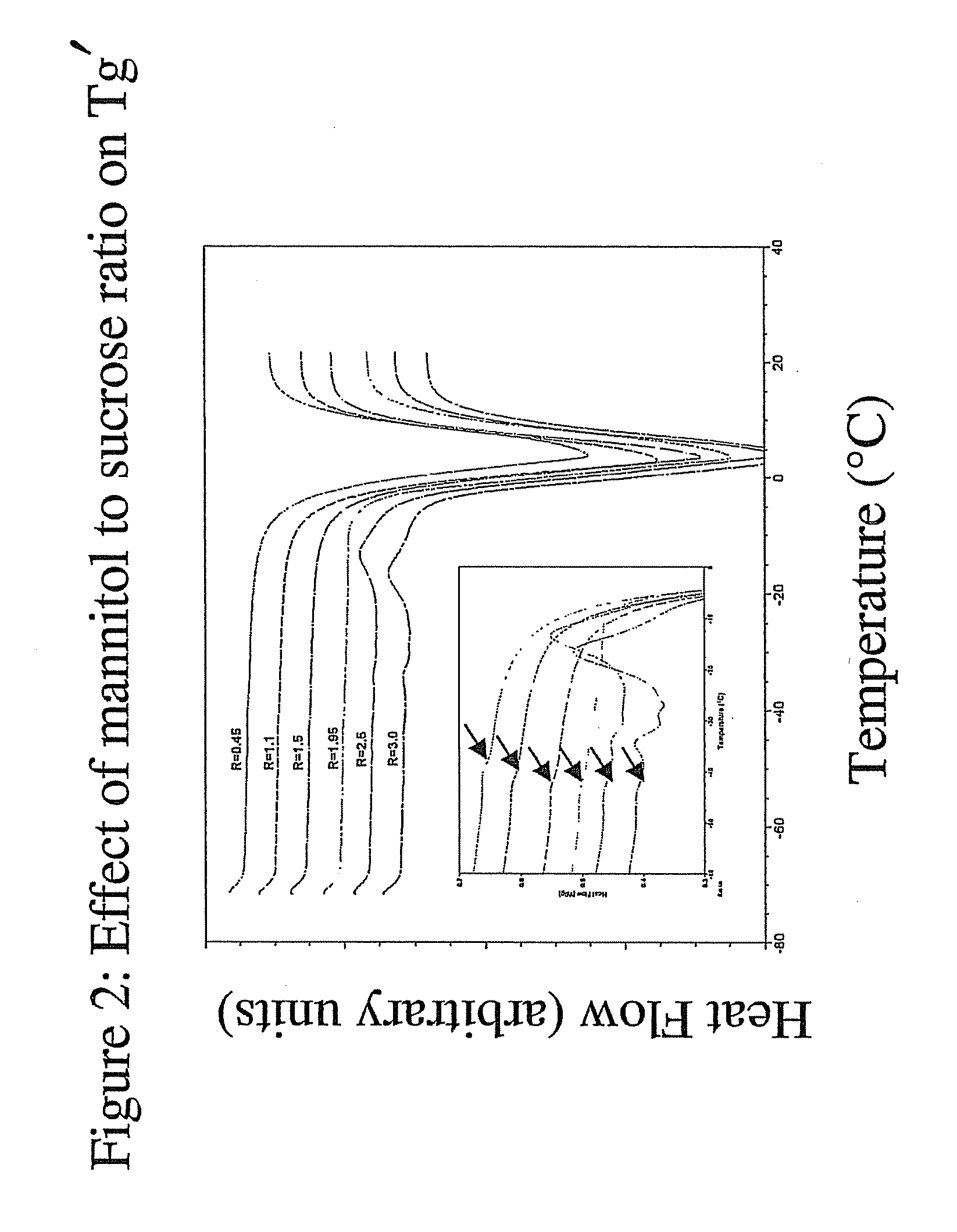
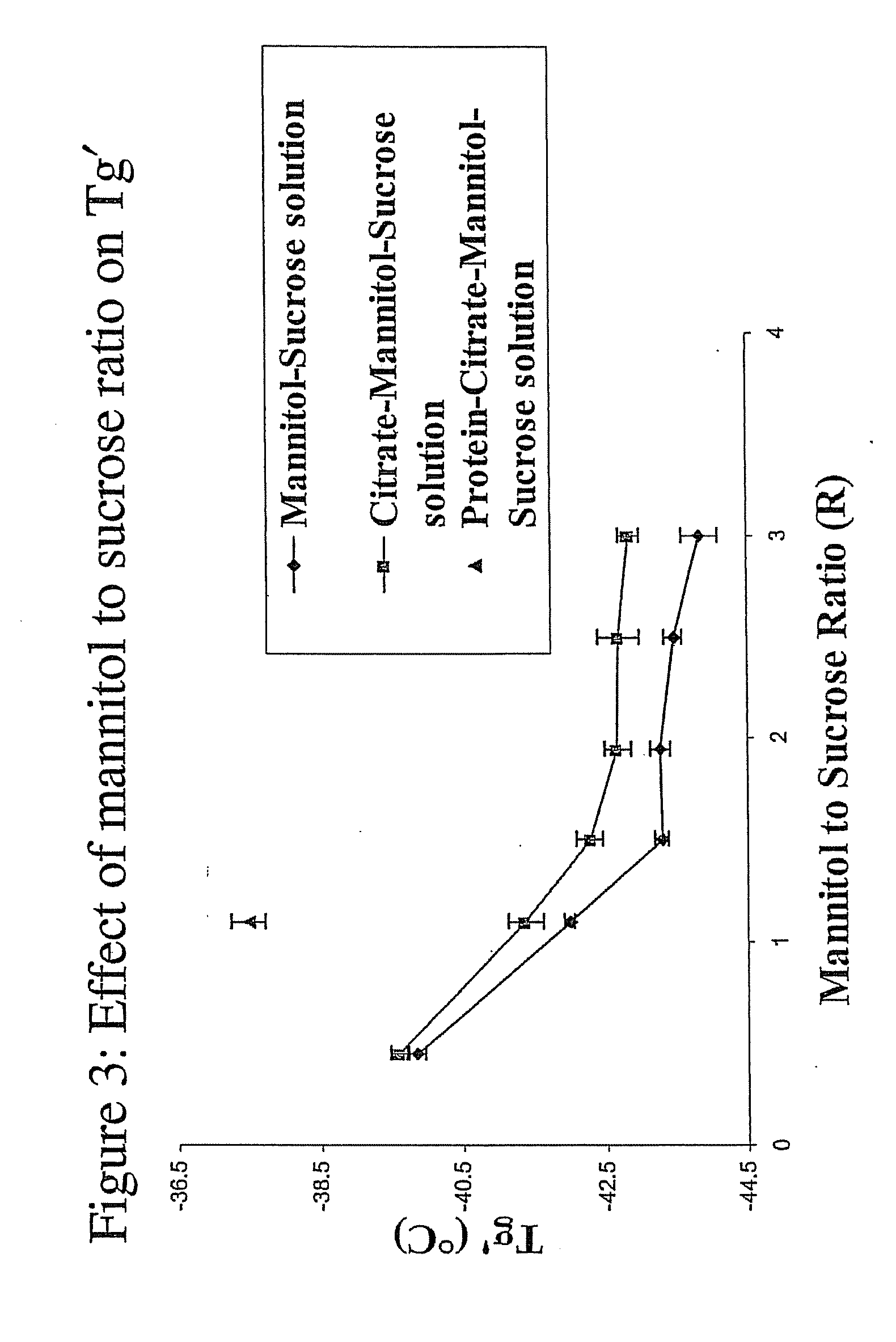
Pharmaceutical Formulation and Process
InactiveUS20070196364A1Increased Tg′Less-dramatic effectOrganic active ingredientsBiocideSucroseActive agent
A process for lyophilization or freeze-drying of a pharmaceutical product is provided and a liquid formulation suitable for lyophilization. In particular, a process for lyophilization or freeze-drying a liquid formulation that includes a protein active agent, a bulking agent and a saccharide stabilizing agent is provided. The saccharide to bulking agent ratio and the protein concentration of the formulation are important factors that affect crystallization of the bulking agent during lyophilization and storage as are some processing conditions. In one embodiment, the saccharide is a disaccharide, such as sucrose and the crystalline bulking agent is mannitol. The protein can be an antibody or a non-antibody protein.
Owner:HUMAN GENOME SCI INC
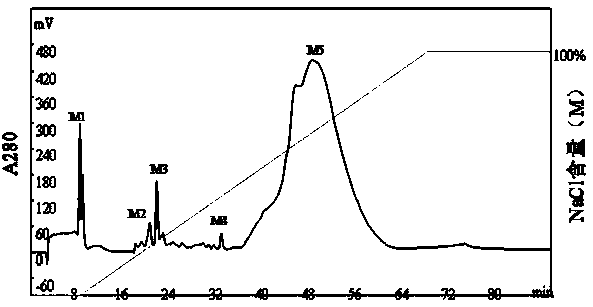
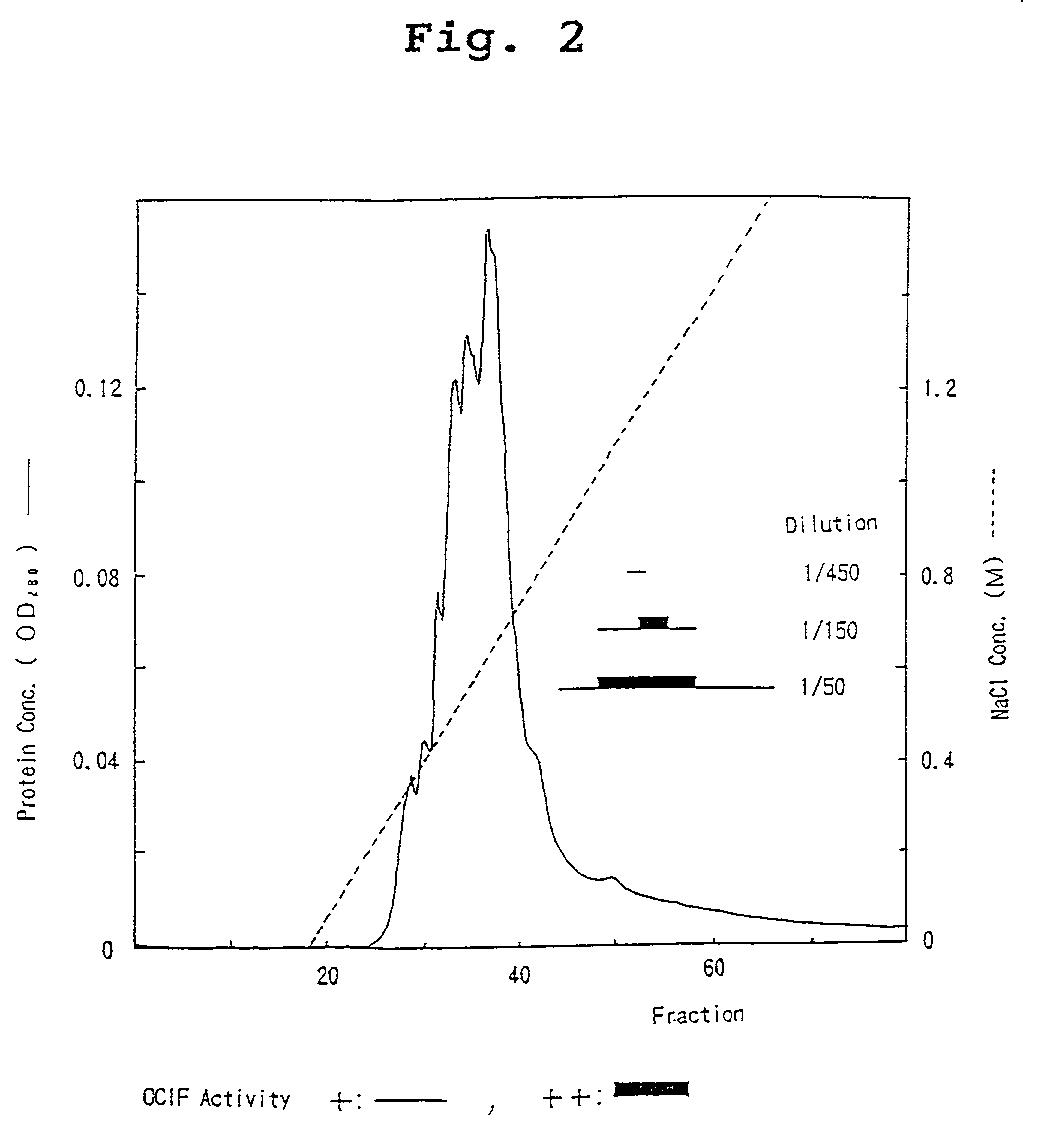
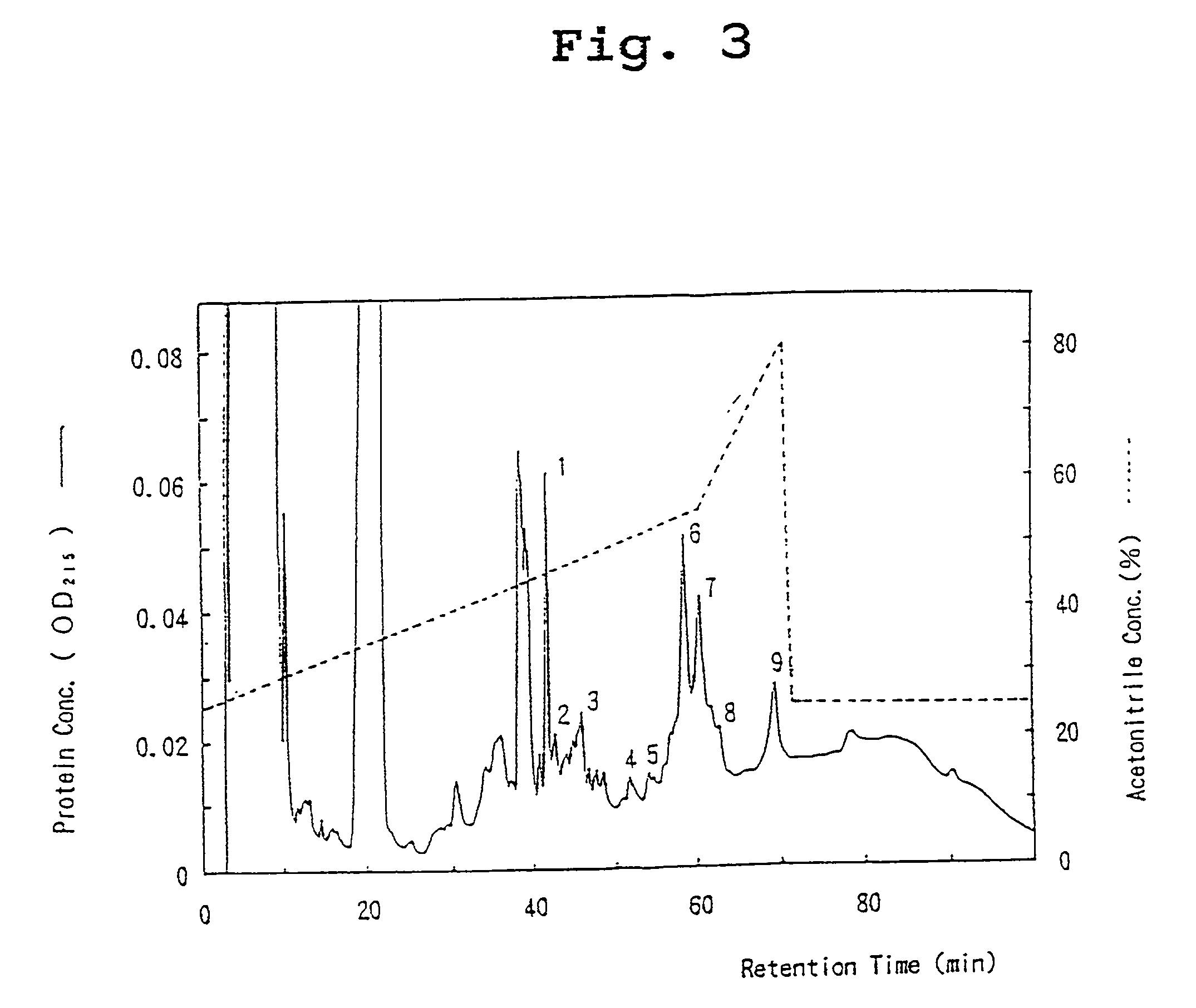
Monoclonal antibodies that bind OCIF
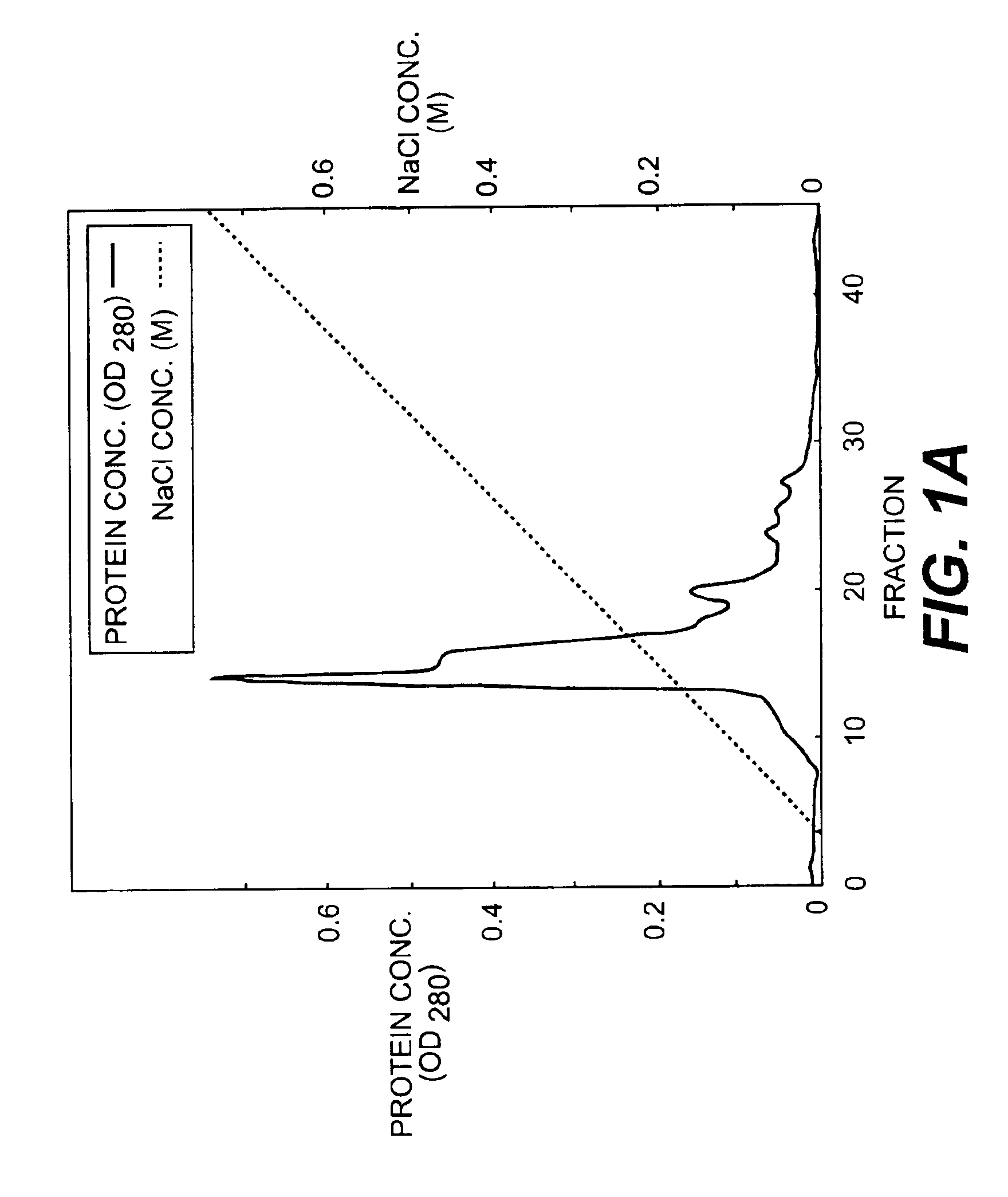
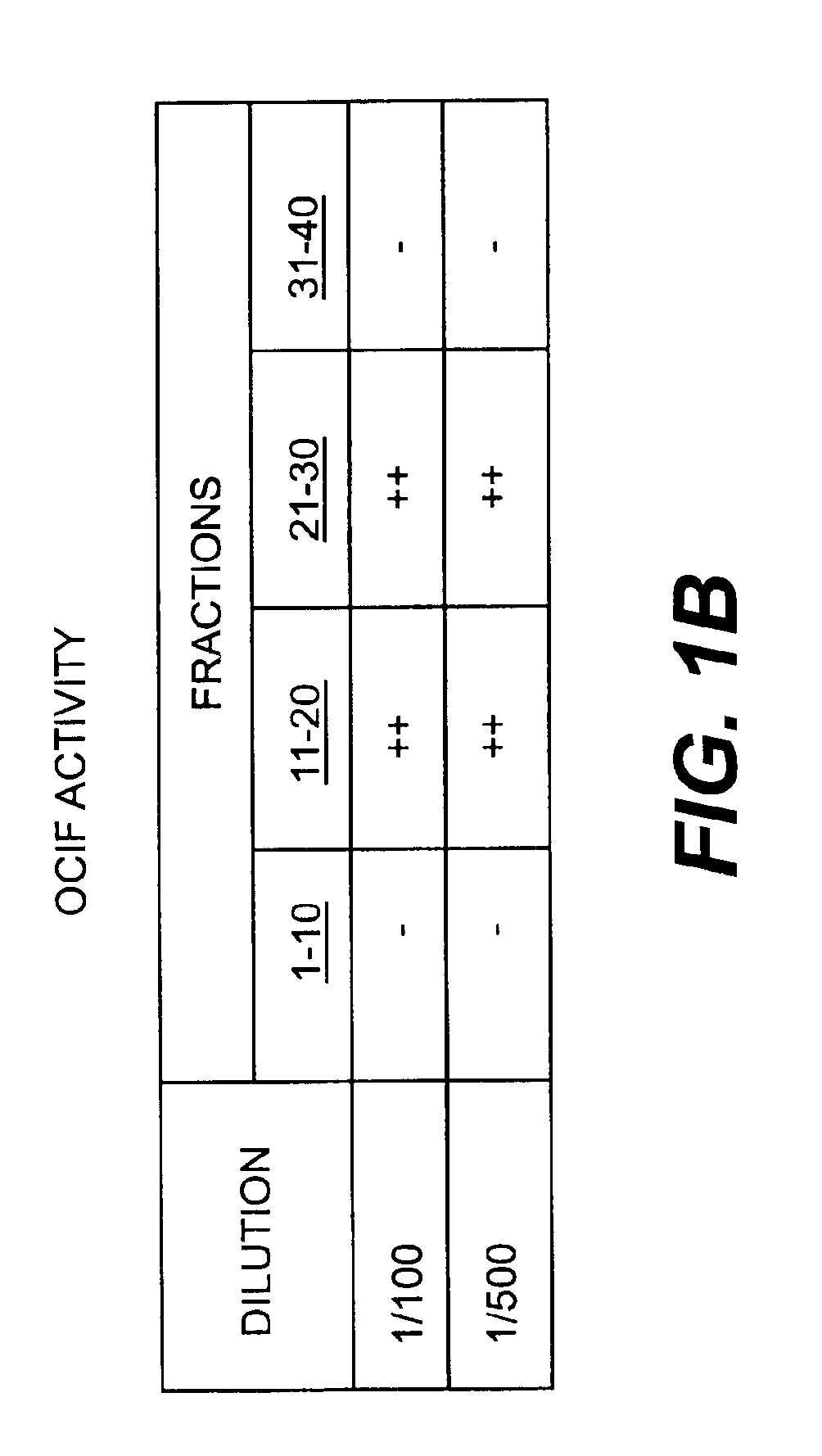
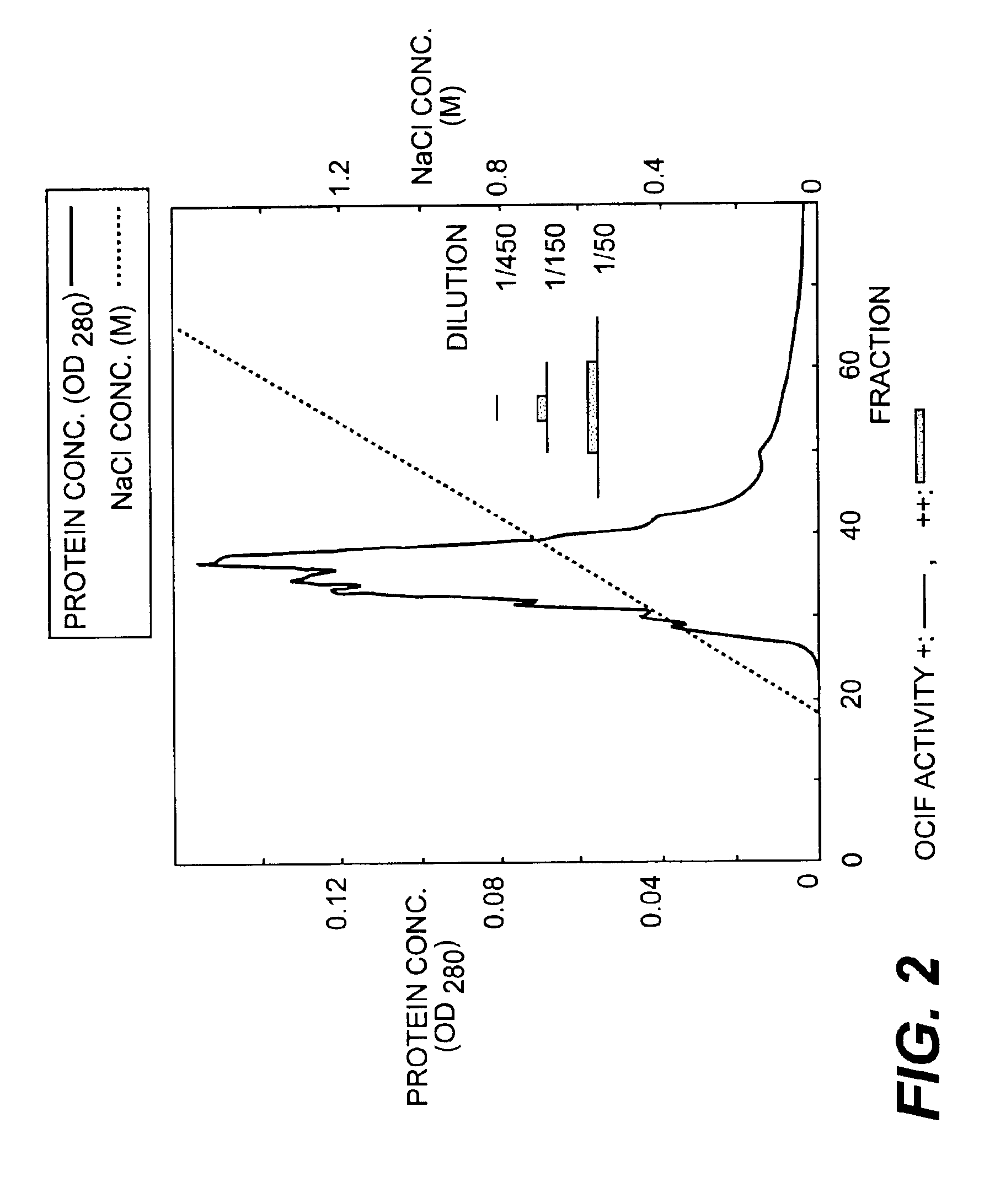
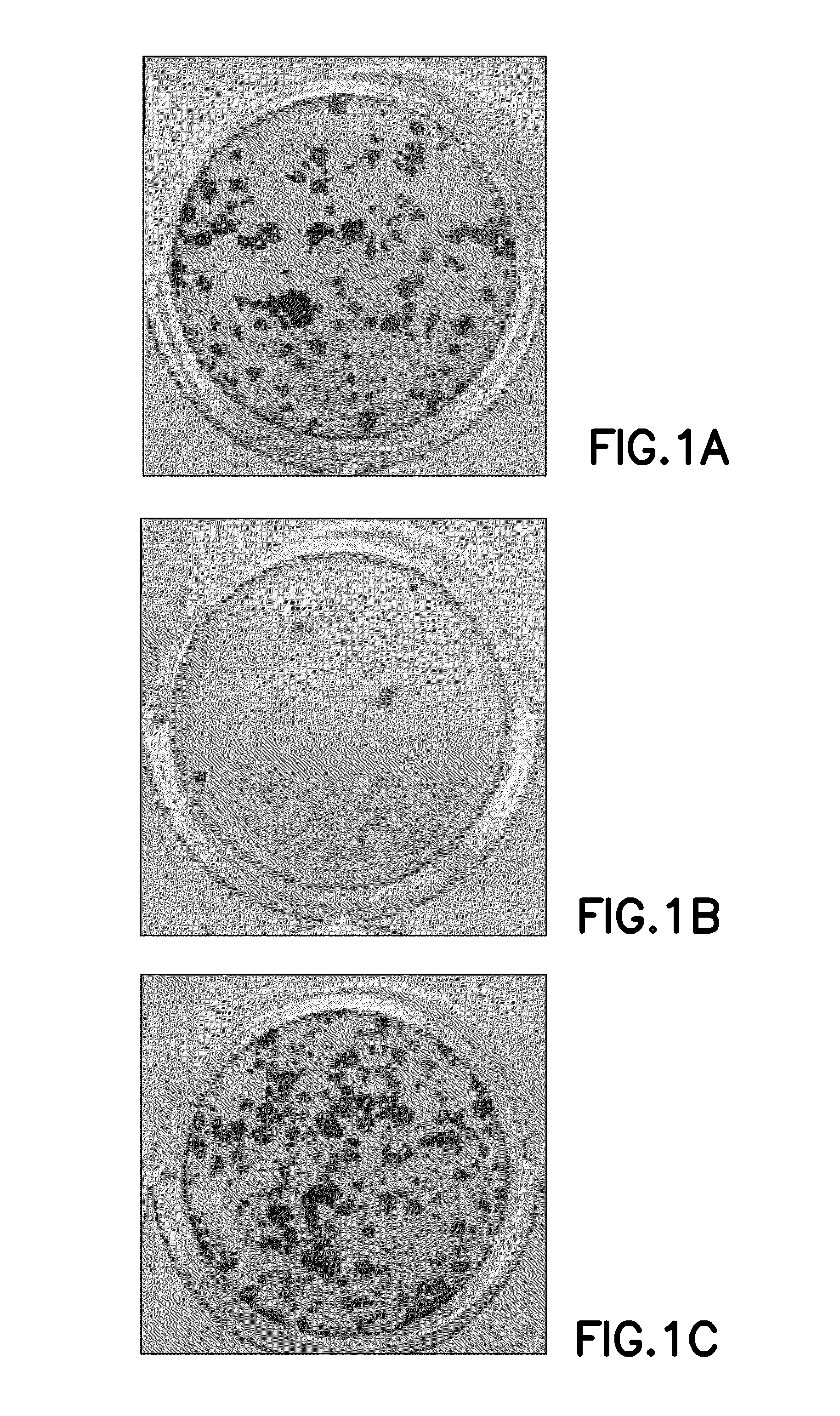
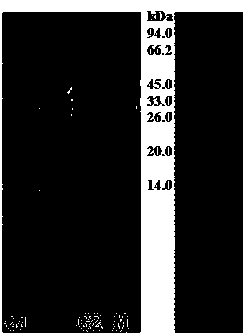
InactiveUS6919434B1Efficient productionMicrobiological testing/measurementEnzymologyADAMTS ProteinsEmbryo
A protein which inhibits osteoclast diffraction and / or maturation and a method for producing the protein. The protein is produced by human embryonic lung fibroblasts and has a molecular weight of about 60 kD and about 120 kD under non-reducing conditions and about 60 kD under reducing conditions an SDS-polyacrylamide gel electrophoresis. The protein can be isolated and purified from the culture medium of fibroblasts. Furthermore, the protein can be produced by gene engineering. The present invention includes cDNA for producing the protein by gene engineering, antibodies having specific affinity for the protein or a method for determining protein concentration using these antibodies.
Owner:DAIICHI SANKYO CO LTD
High protein edible composition and method of preparing the same
A dough composition for producing a baked, edible, high protein product having a protein concentration, based on calories, of at least 25%, comprising:(a) a mixture of high protein components,(b) flour,(c) leavening agent,(d) sweetener, and(e) water.
Owner:FOCUSED FOODS
Preparation method of human umbilical cord mesenchymal stem cell factor freeze-dried powder
InactiveCN106367386AHigh purityHigh protein contentCosmetic preparationsToilet preparationsFreeze-dryingFiltration
The invention relates to a preparation method of human umbilical cord mesenchymal stem cell factor freeze-dried powder, and aims to solve the problems of low cell factor yield and poor activity in the prior art. Umbilical cord mesenchymal stem cells are sequentially separated and subjected to passage, culture supernatant fluid is collected, filtered and concentrated, freeze-dried excipients and 50 DEG C deionized water are added, protein concentration is adjusted, and the materials are blended, sub-packaged and then freeze-dried under the freeze-drying condition of 50-100Pa pressure intensity and temperature ranging from -30 DEG C to -35 DEG C to obtain the human umbilical cord mesenchymal stem cell freeze-dried powder. The collected culture supernatant fluid is firstly frozen and stored, filtering and concentrating processes of the culture supernatant fluid include unfreezing the culture supernatant fluid at room temperature and filtering the cells by 0.45 micrometer filter membranes, ultra-filtration and concentration of 3KD filter membranes include concentrating the supernatant fluid to be 1 / 10 of the original volume of the supernatant fluid, and 0.22 micrometer filter membranes perform filtration sterilization to obtain the human umbilical cord mesenchymal stem cell freeze-dried powder stock solution for preparing the human umbilical cord mesenchymal stem cell freeze-dried powder. The prepared freeze-dried powder can effectively store various cell factors secreted by the human umbilical cord mesenchymal stem cell and with biological activity, and the obtained cell factors are high in yield, good in activity and easy to store and transport.
Owner:中卫华医(北京)生物科技有限公司 +1
Defined cell culturing surfaces and methods of use
ActiveUS20100021998A1Excellent Adhesive PropertiesAdvantageously avoidedBone marrow stroma cellsLiquid surface applicatorsCoated surfaceCell-Extracellular Matrix
In one aspect, there is provided a cell culturing substrate including: a cell culture surface having a film attached thereto, wherein the film includes one or more plasma polymerized monomers; and a coating on the film-coated surface, the coating deposited from a coating solution comprising one or more extracellular matrix proteins and an aqueous solvent, where the total extracellular matrix protein concentration in the coating solution is about 1 ng / mL to about 1 mg / mL.
Owner:CORNING INC
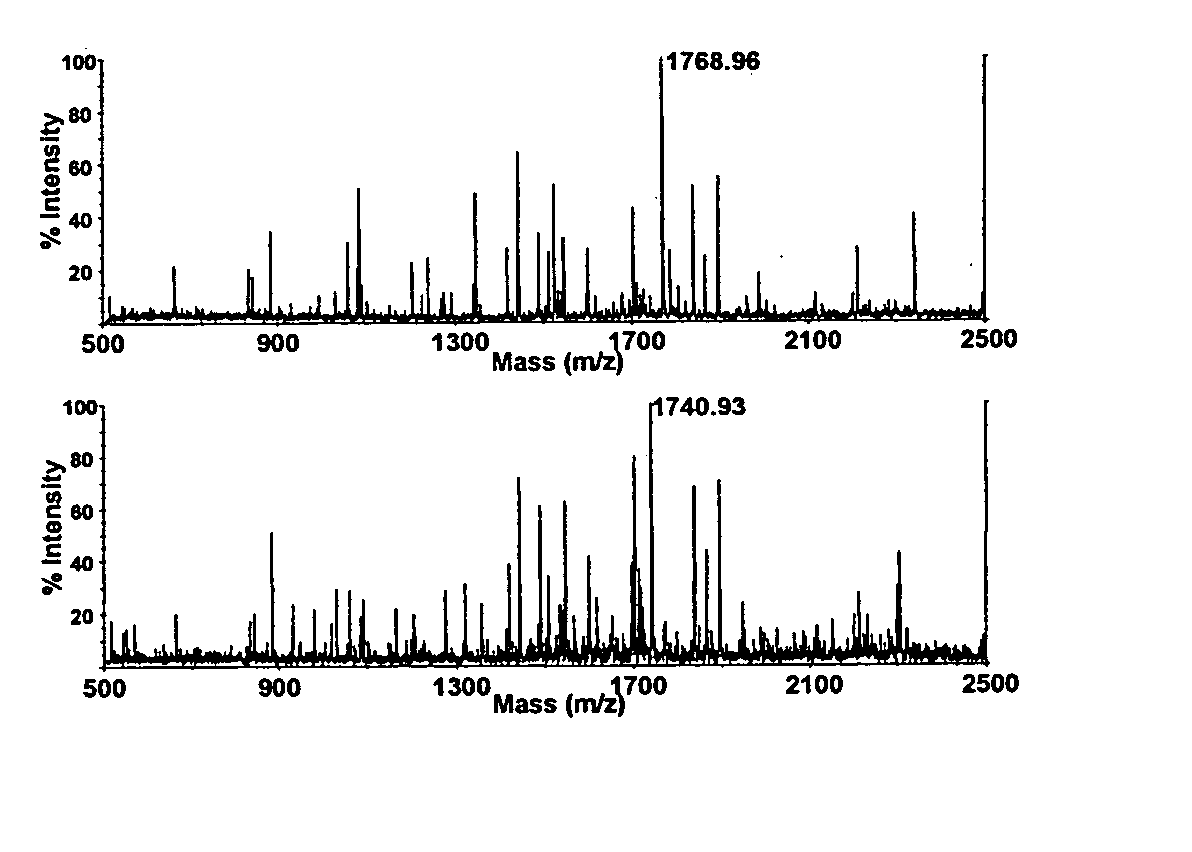
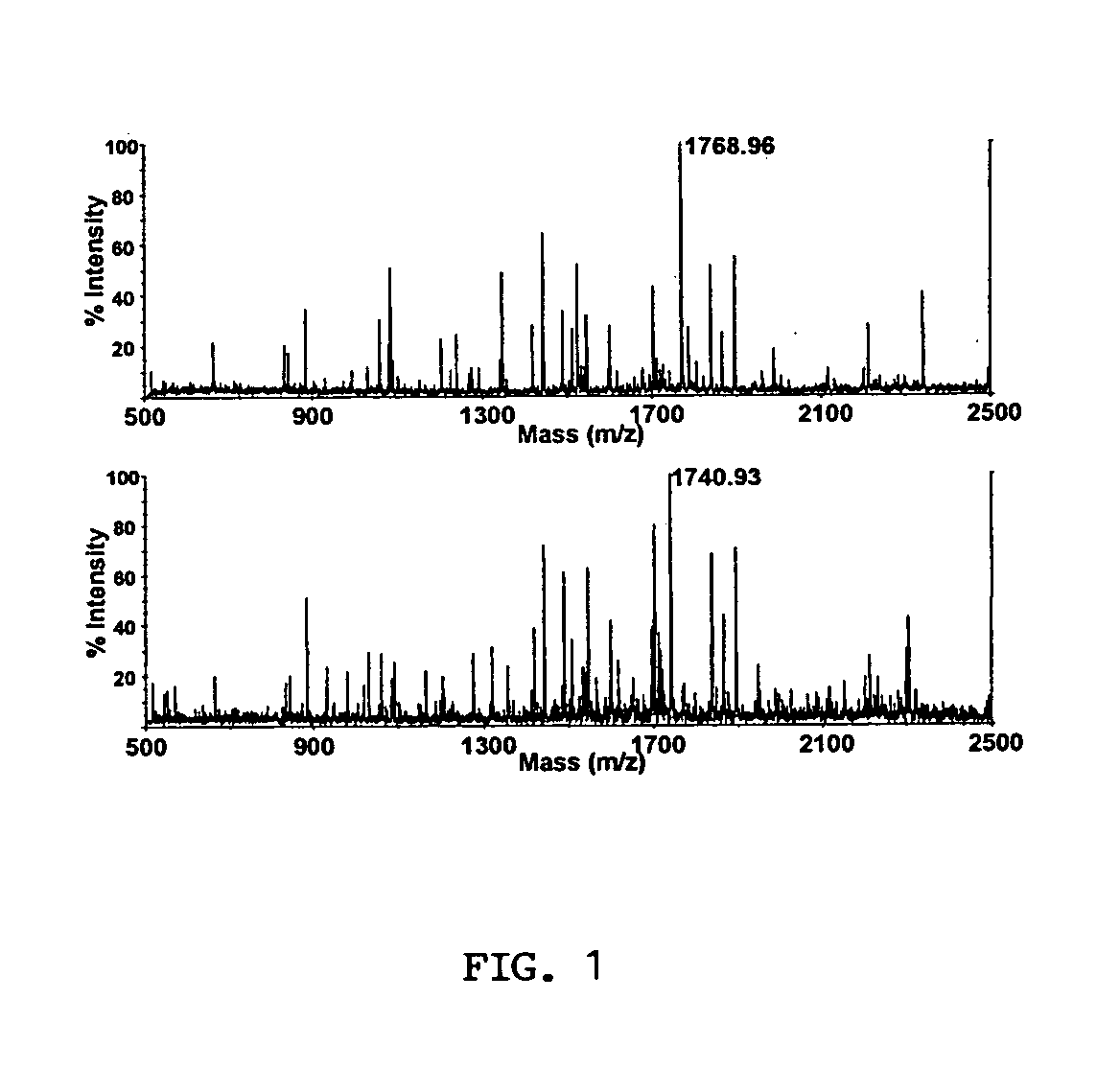
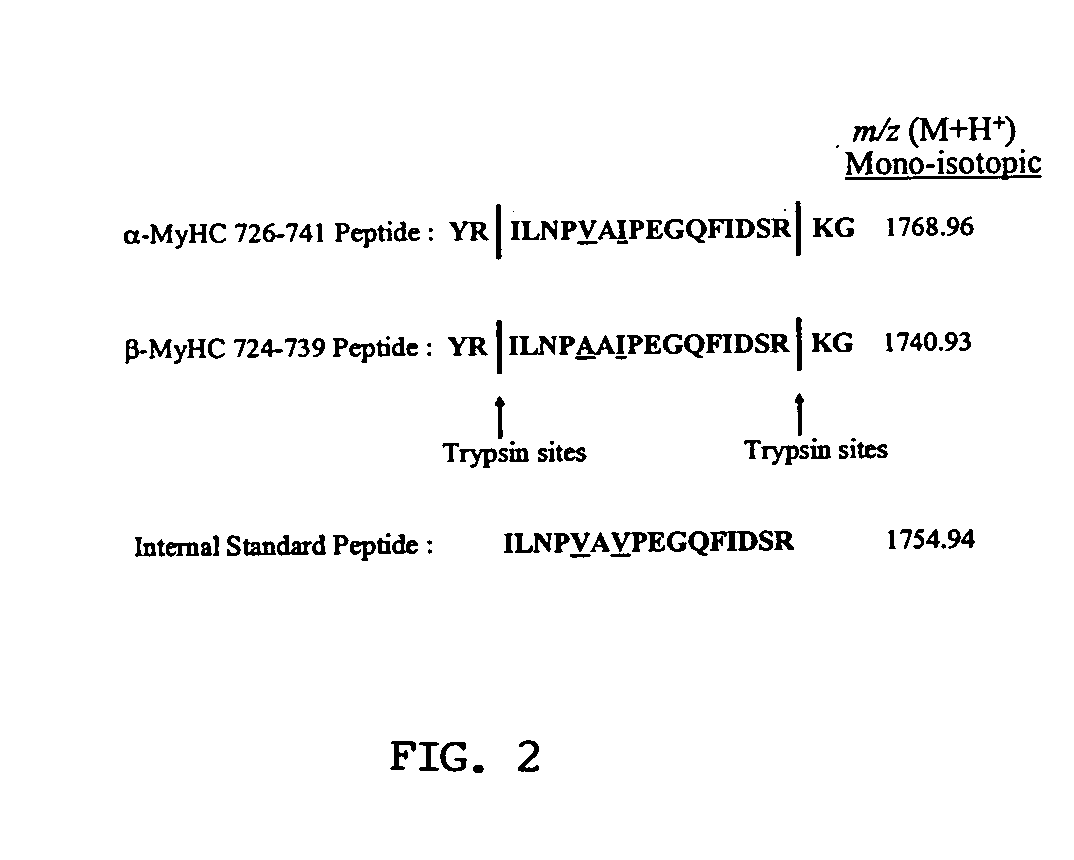
Quantitative analysis of protein isoforms using matrix-assisted laser desorption/ionization time of flight mass spectrometry
InactiveUS20040119010A1Particle separator tubesMicrobiological testing/measurementMass Spectrometry-Mass SpectrometryMatrix assisted laser desorption ionization time of flight
The present invention provides for methods of quantitating the amounts of proteins or peptides, including those that are closely related isoforms, using matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOF-MS). Measurement of protein concentrations in vivo has been extremely difficult and problematic, and protein concentrations have not been shown to correlate well with mRNA levels, the standard used in the past. The present invention overcomes the deficiencies of prior methodologies by taking advantage of MALDI-TOF-MS technology and applying it to proteins and peptides in a way that allows for accurate, quantitative measurement in vivo of protein or peptide concentrations.
Owner:UNIV OF COLORADO THE REGENTS OF
Edible film preparation method based on compound of protein and polysaccharide
InactiveCN103588997AGood gas and moisture barrierLow costFlexible coversWrappersPlasticizerMoisture resistance
The invention relates to an edible film preparation method based on compound of protein and polysaccharide. The edible film is prepared by components in percentage by mass as follows: 1%-5% of polysaccharide, 1%-3% of a plasticizer, 0.5%-2% of an emulsifier and 1%-5% of protein. According to the edible film preparation method based on compound of protein and polysaccharide, protein and polysaccharide are creatively compounded, preparation agents of the emulsifier, the plasticizer, the cell permeable agent and the like are added, so that the formed preservative film has good air and moisture resistance and is transparent and glossy, and the storage period can be remarkably improved; and raw materials for forming the film are edible, thus, the formed edible film is safe, non-toxic and pollution-free; and moreover, the method is simple in operation, low in cost and easy to popularize and apply, and has a very broad application prospect.
Owner:FUZHOU WEST FOOD LIMITED
CRP monoclonal antibody nanometer latex microsphere composition and preparation process thereof
ActiveCN103073642ASmall variance between different production batchesQuality improvementImmunoglobulins against animals/humansCarrier-bound/immobilised peptidesTherapy monitoringMicrosphere
The present invention discloses a CRP monoclonal antibody, a CRP antibody nanometer latex microsphere composition and a preparation process thereof. According to the CRP antibody nanometer latex microsphere composition, CRP monoclonal antibodies with different epitopes and carboxylated polystyrene microspheres with different particle sizes are subjected to covalent cross-linking to form conjugates, and then the different conjugates are mixed according to a certain ratio to prepare the CRP monoclonal antibody nanometer latex microsphere composition. The CRP monoclonal antibody nanometer latex microsphere composition can be applicable for automatic biochemical analyzers and special protein analyzers, can be provided for full measuring range determination of C-reactive protein concentration in human whole blood and serum, and can be used in differential diagnosis of bacterial infections and viral infections, drug therapy monitoring and cardiovascular disease risk assessments.
Owner:深圳伯美生物医药有限公司
Production technology of preparing aminosal using hydrolysed residual active sludge by quick lime and its equipment
A process for preparing hydrolytic protein by using the calcium lime to hydrolyze the residual active sludge includes such steps as proportionally mixing residual active sludge, water and calcium line, stirring, hydrolyzing reaction, filtering, using hydrochloric acid to regulate pH=7.0-8.5, concentrating and drying. Its apparatus is also disclosed.
Owner:武汉市城市排水发展有限公司 +1
Process for concentrating protein in soy protein wastewater by two-stage foam separation method
InactiveCN101870722AReduce processing loadHigh recovery ratePeptide preparation methodsSolubilitySeparation technology
The technical scheme of the invention belongs to the wastewater processing field, in particular to a process for concentrating protein in soy protein wastewater by a two-stage foam separation method, comprising the following steps: 1) collecting and detecting soy protein production wastewater; 2) preheating the soy protein production wastewater; and 3) performing two-stage foam separation processby utilizing a foam separation tower. In the process, the two-stage foam separation technology is applicable to concentrating the protein in the soy protein wastewater, the concentration ratio of theprotein in primary foam separation can reach 6.0-8.0, the protein in defoaming solution is dissolved out after the concentration thereof exceeds solubility, and the dissolved protein can be directly used as a production raw material of soy protein; and the protein recovery rate in secondary foam separation is as high as possible, and the defoaming solution and the initial wastewater are mixed andadded into feed of the primary foam separation. The two-stage foam separation process can greatly improve the protein concentration ratio and enhance the recovery rate.
Owner:HEBEI UNIV OF TECH
Additive-free high-protein yogurt and making method thereof
ActiveCN105010530AThe state of organization is smoothDelicate tissue stateMilk preparationSucroseLactase
The invention provides additive-free high-protein yogurt and a making method thereof. The method relates to raw materials including raw milk, single cream, a leavening agent and lactase. The method includes the steps that (1) the raw milk is degreased at the temperature of 40-50 DEG C, ultrafiltration and concentration are carried out after microfiltration sterilization, then the raw milk is mixed with the single cream, the protein content is made to be 4%-6% and the fat content is made to be 4%-7%; afterwards, homogeneity is performed, heat treatment is conducted for 5-10 minutes at the temperature of 90-95 DEG C, and cooling is conducted till the temperature is 38-45 DEG C; (2) 100-200 U / 1000 kg of leavening agent and 1000-3000 NLU / L of lactase are added to be stirred for 5-10 minutes, and the temperature rises to 38-45 DEG C so that filling can be conducted; (3) fermentation is carried out at the temperature of 38-45 DEG C and stops till the pH value is 4.4-4.55, curing is conducted for 12-24 hours after cold storage at the temperature of 2-6 DEG C is completed, and accordingly the additive-free high-protein yogurt is obtained. By the adoption of the method, the quality of the raw milk is greatly improved, the obtained yogurt is high in protein concentration and does not contain sucrose, and the yogurt further has good mouthfeel and flavors.
Owner:BRIGHT DAIRY & FOOD CO LTD
Intravenous immunoglobulin composition
ActiveUS20130011388A1Reduce riskEasy to managePeptide/protein ingredientsAntibody mimetics/scaffoldsSpecific antibodyHigh titre
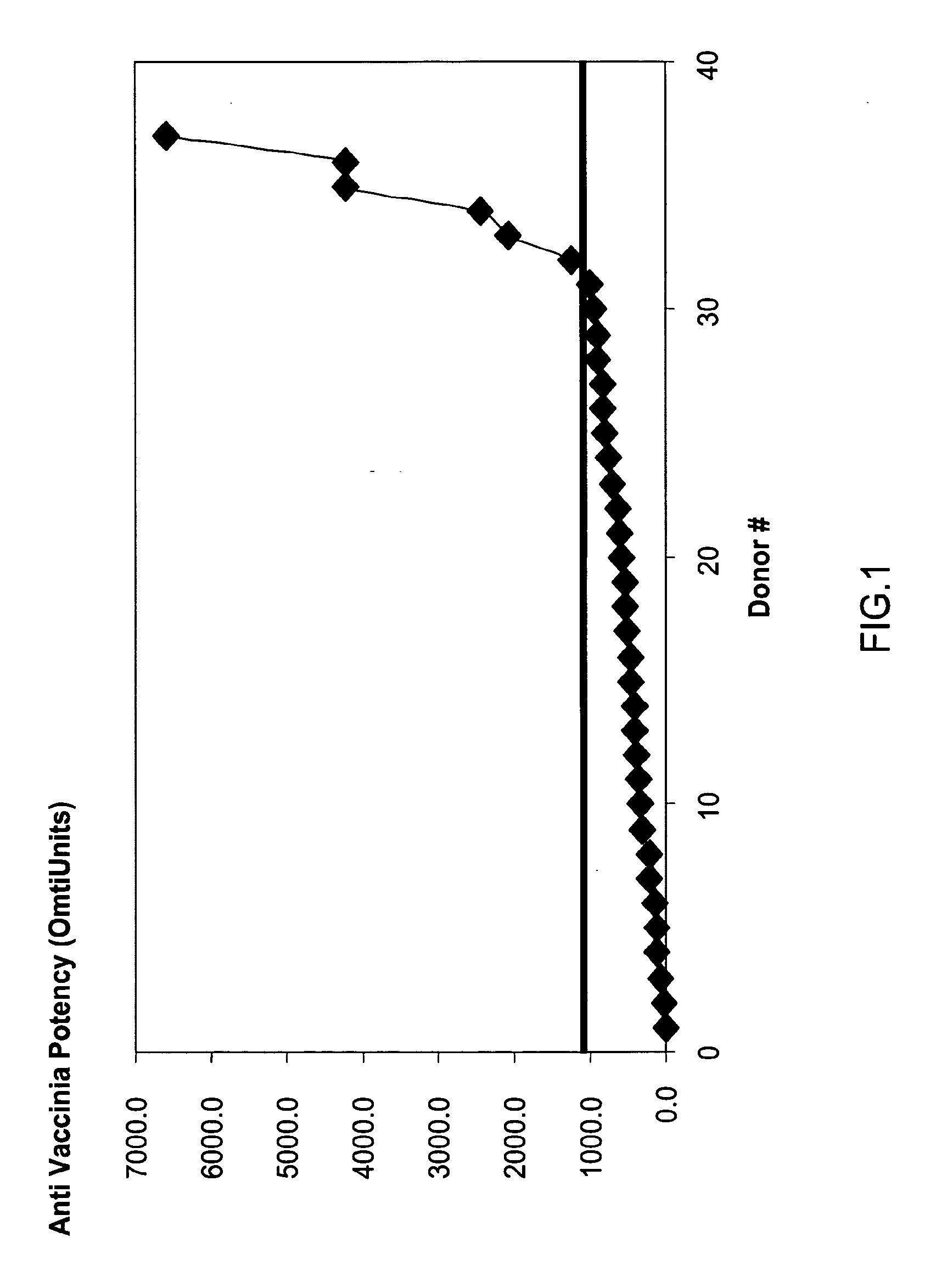
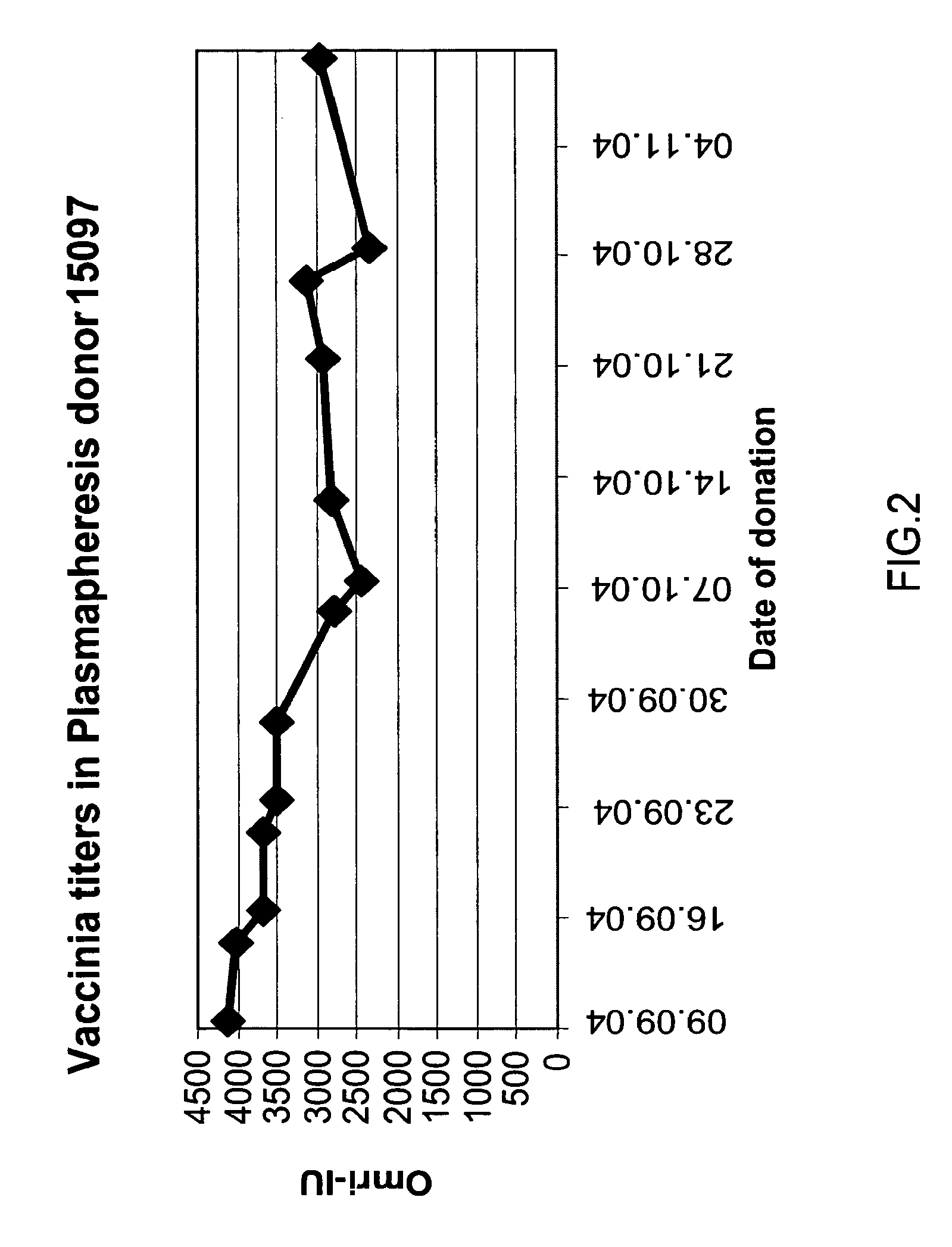
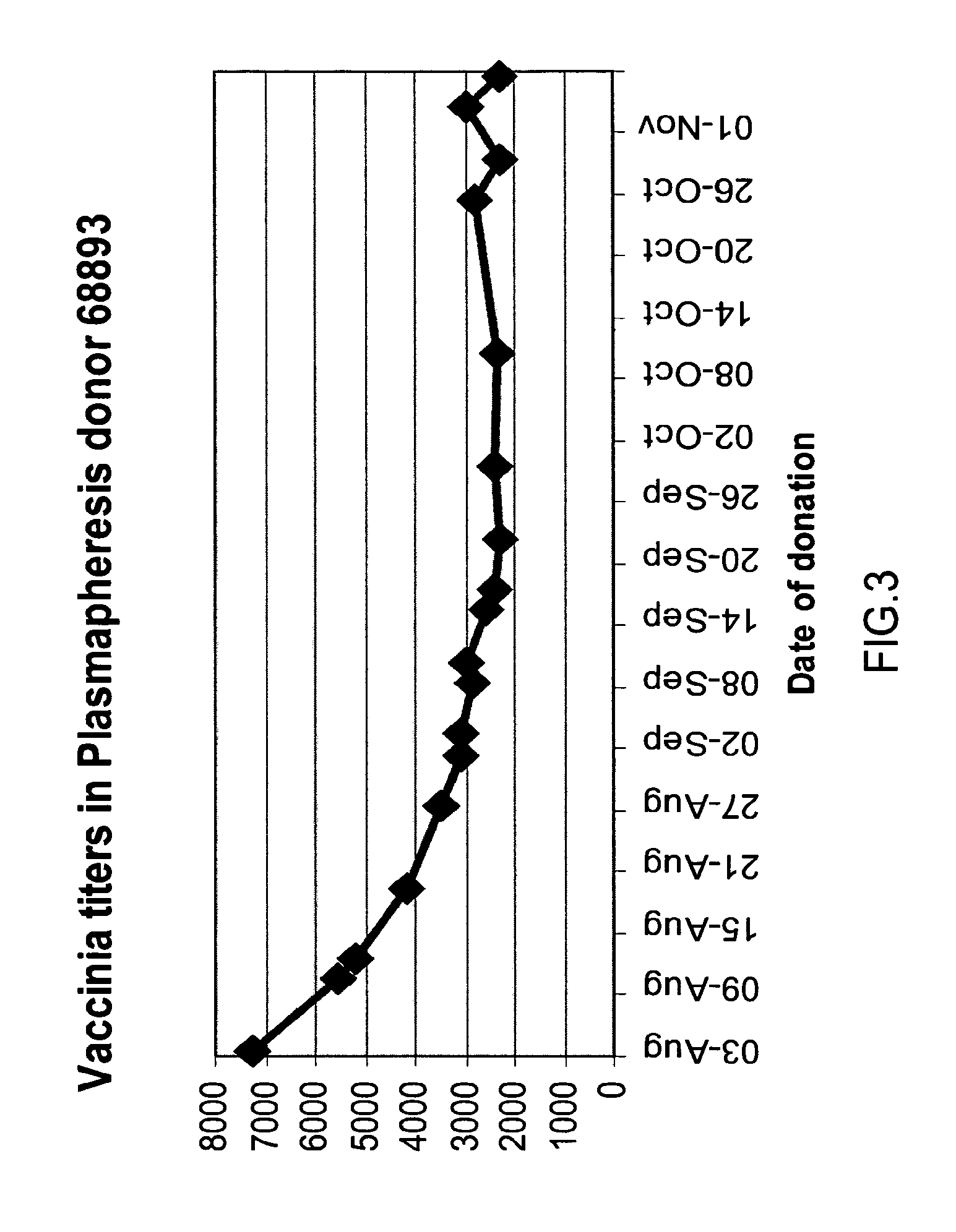
A concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, is made by (a) selecting a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism; (b) identifying very high titre individuals by determining the level of specific antibodies immunoreactive with the pathogenic microorganism in the blood of the individuals; (c) combining blood from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c). A concentrated immunoglobulin composition can include specific antibodies immunoreactive with a pathogenic microorganism, wherein the titre of specific antibodies is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates. The pathogenic microorganism is preferably smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
Method for providing protein microarrays
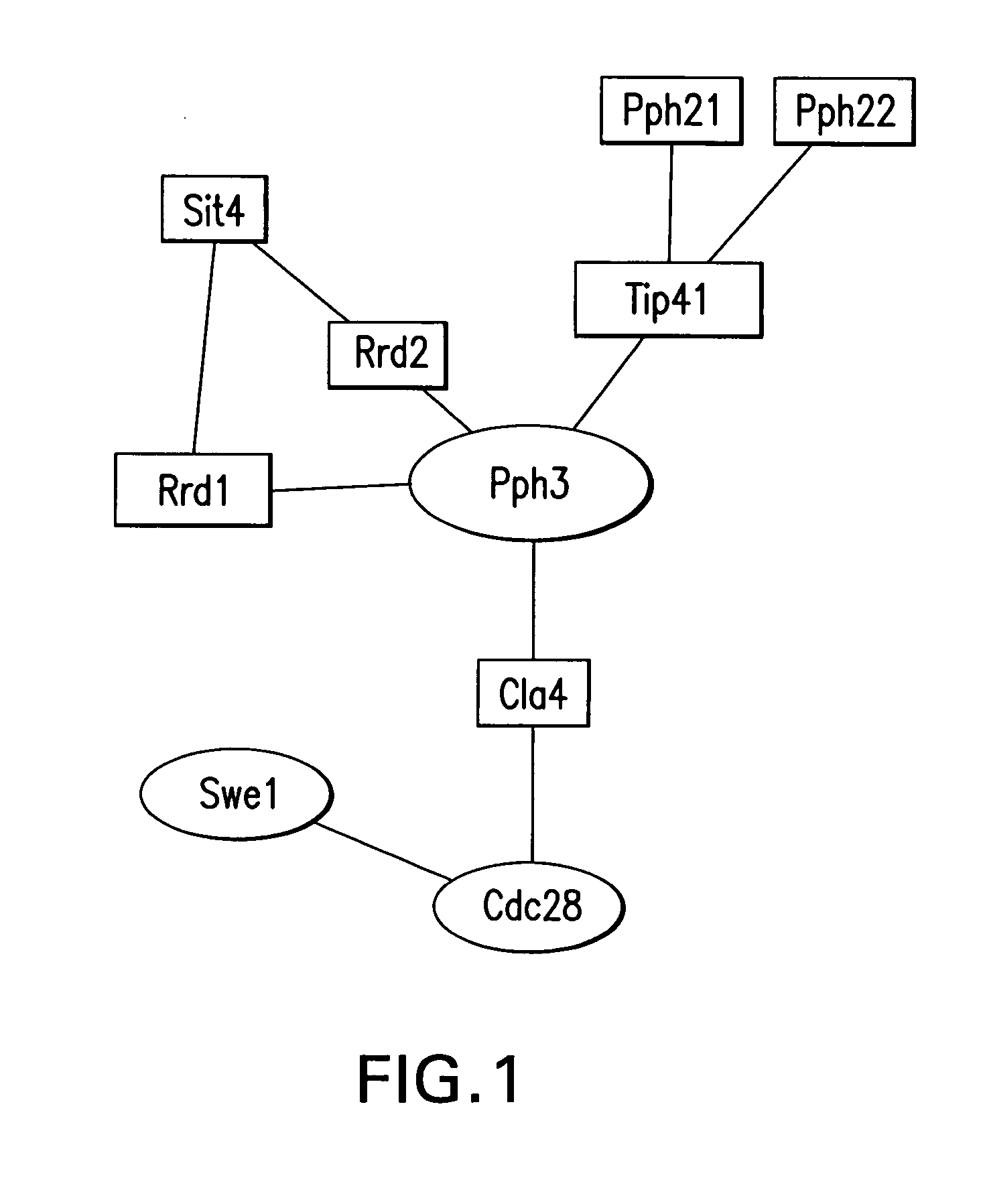
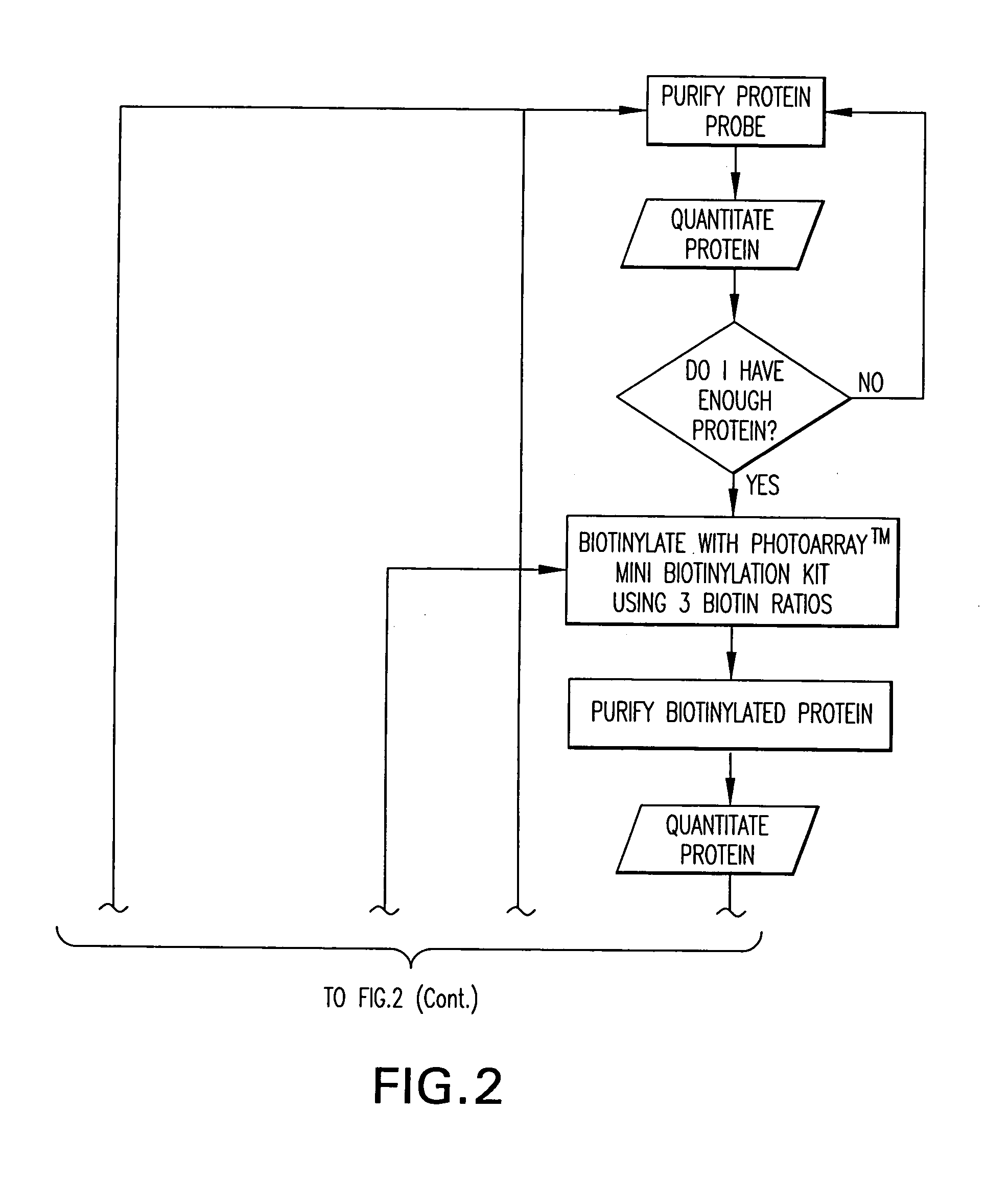
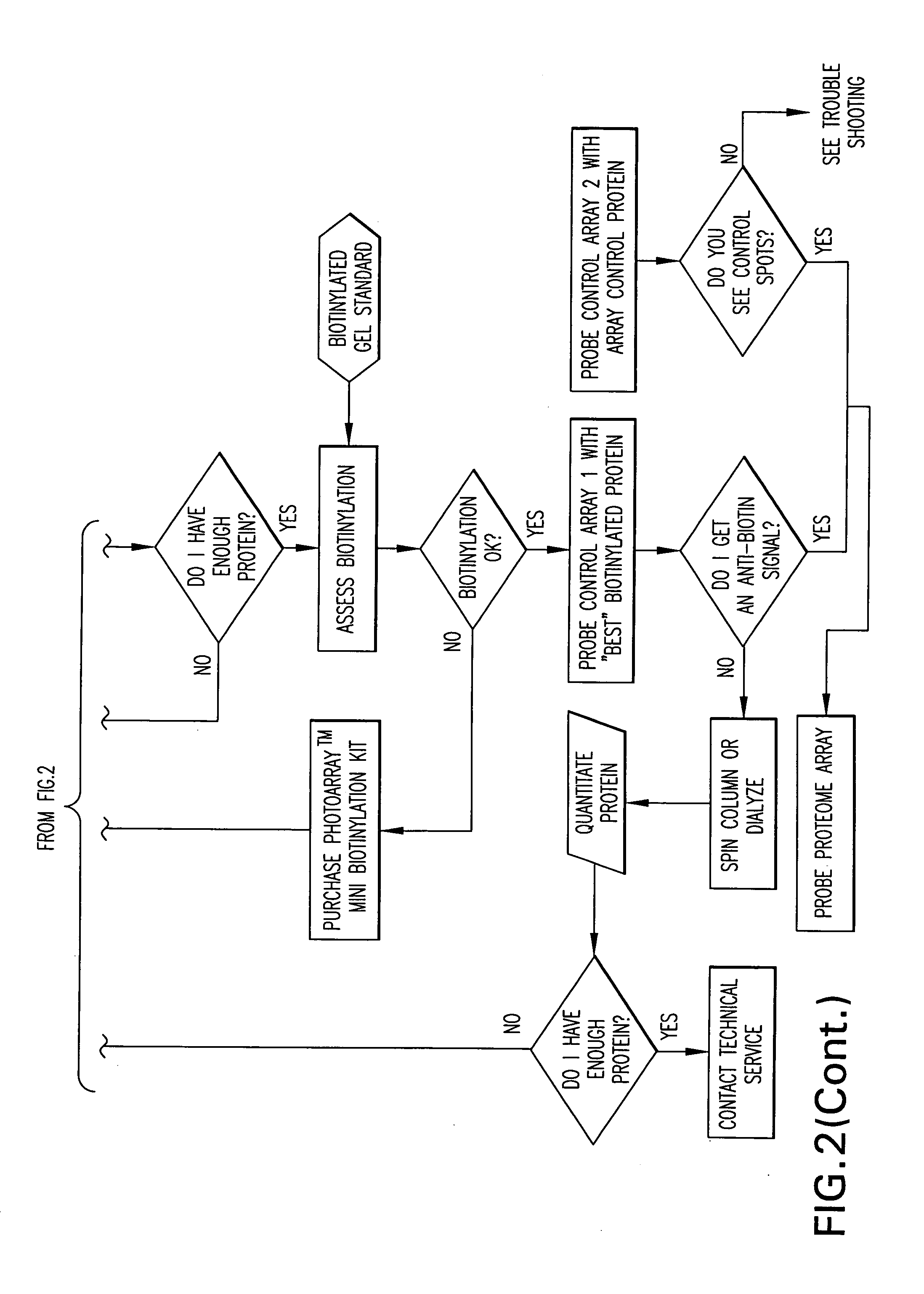
InactiveUS20060099704A1Further statistical confidence in values obtainedBioreactor/fermenter combinationsBiological substance pretreatmentsProtein microarrayBioinformatics
The present application relates to methods for providing a protein microarray product and related products and services to a customer, methods, kits, and systems for labeling a probe for a protein microarray, and methods for determining protein concentrations using a protein microarray. The methods for providing a protein micorarray can include, in certain aspects, a computer function for performing some of the steps of the methods. Methods and kits for labeling a probe can include a control array that includes a molecule that binds to a label of a probe.
Owner:PROTOMETRIX
Concentrated Protein Preparations of Bone Morphogenetic Proteins and Methods of Use Thereof
InactiveUS20100144631A1Efficient preparationHigh recovery rateSenses disorderNervous disorderDiseaseWhole body
Disclosed herein are heretofore undescribed preparations of highly concentrated, solubilized proteins, such as but not limited to, Bone Morphogenetic Proteins. Such protein preparations can be formulated in an aqueous carrier at protein concentrations in excess of 10 mg / ml when using the methods of manufacture taught herein. Such methods yield stable protein preparations in either solubilized or lyophilized form. The protein preparations of the present invention are particularly beneficial when administered either locally or systemically, in part, because low administration volumes can be accomplished. This is especially important for local treatment of certain anatomic locations such as, for example, the synovial fluid of a joint when treating osteoarthritis with BMP-7 or the intradiscal space when treating degenerative disc disease with BMP-7.
Owner:MARIEL THERAPEUTICS
Dairy product and process
InactiveUS20120114795A1Broad utilitySimple heating stepProtein composition from fishMilk preparationWhey protein isolateProtein concentration
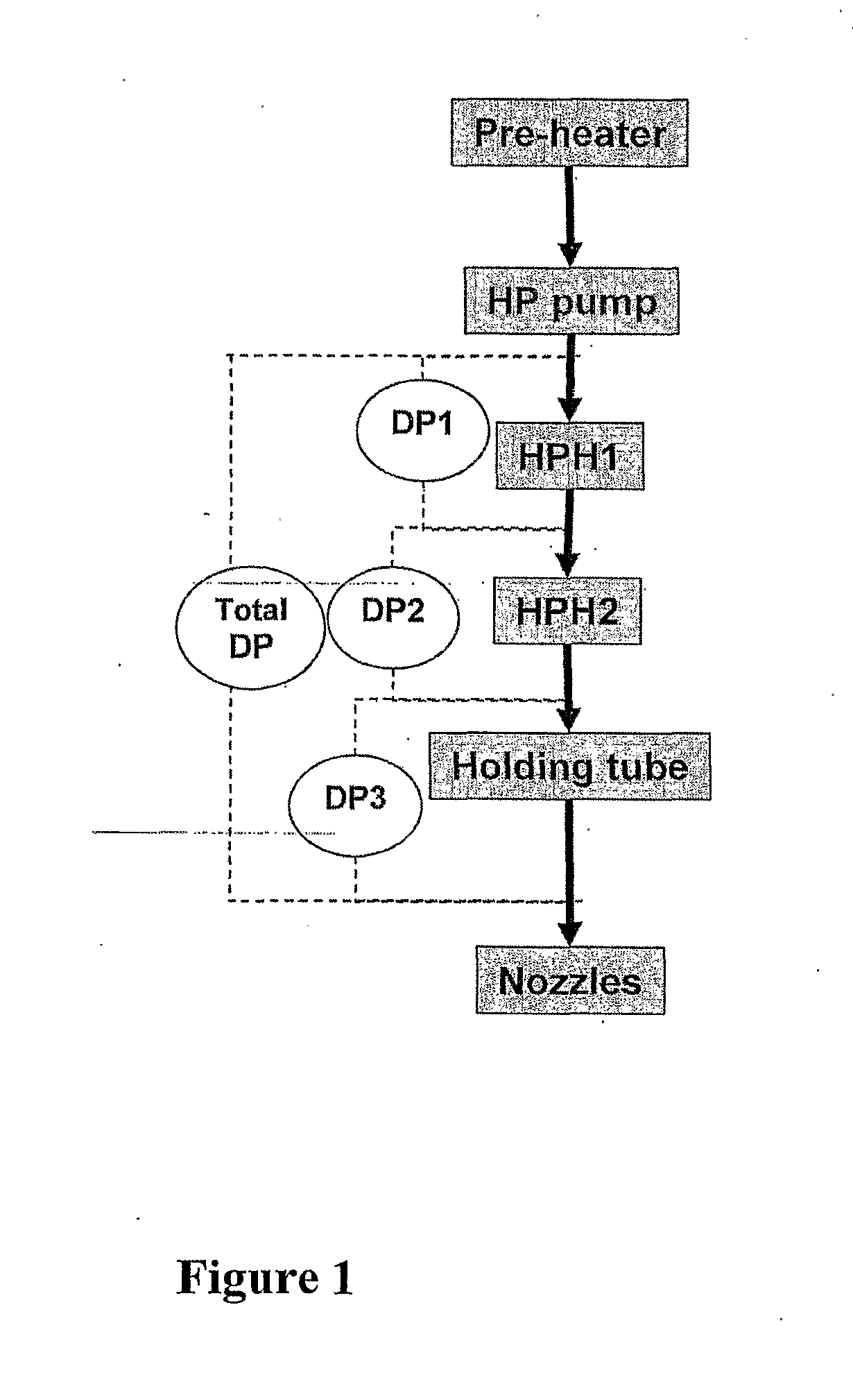
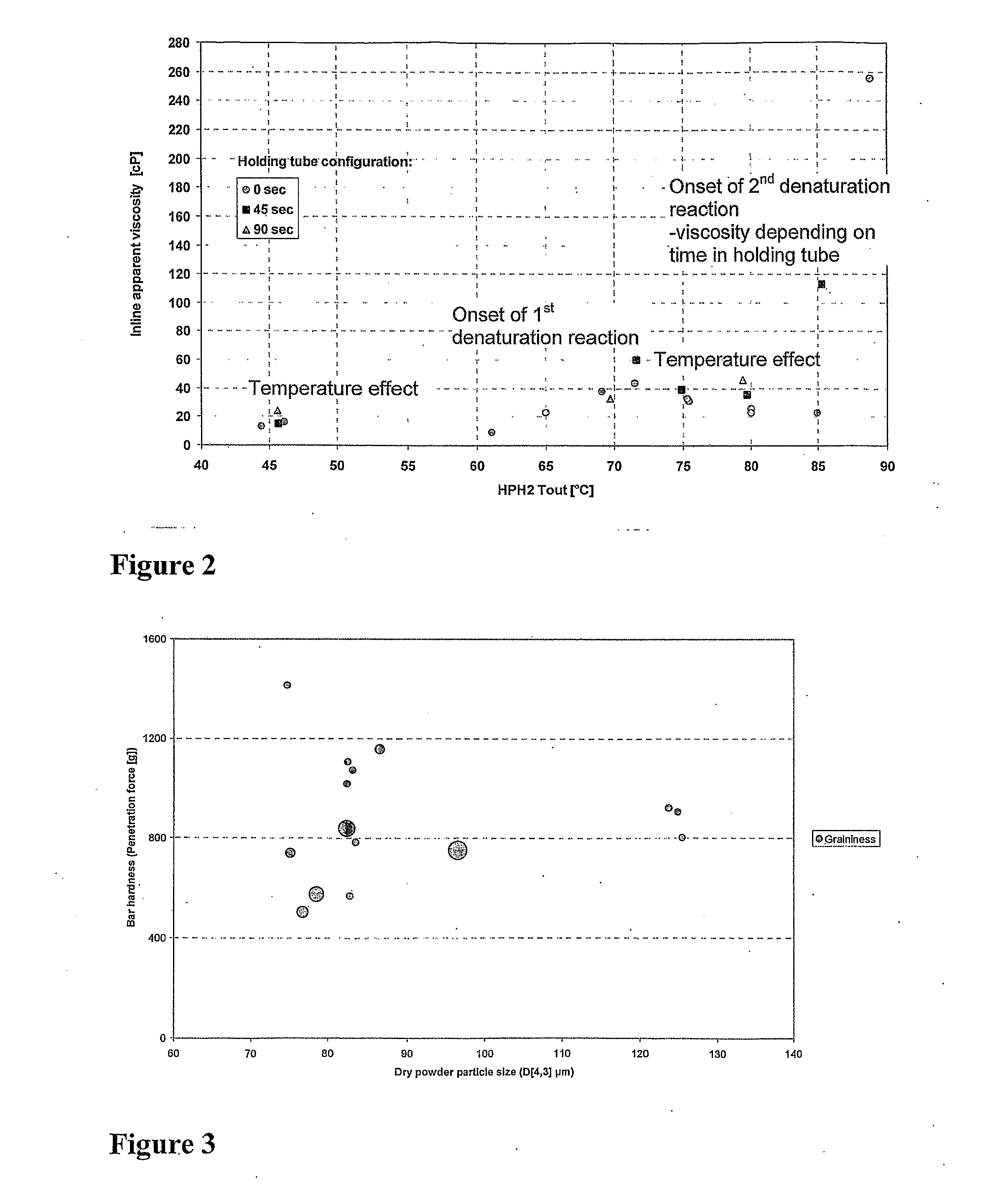
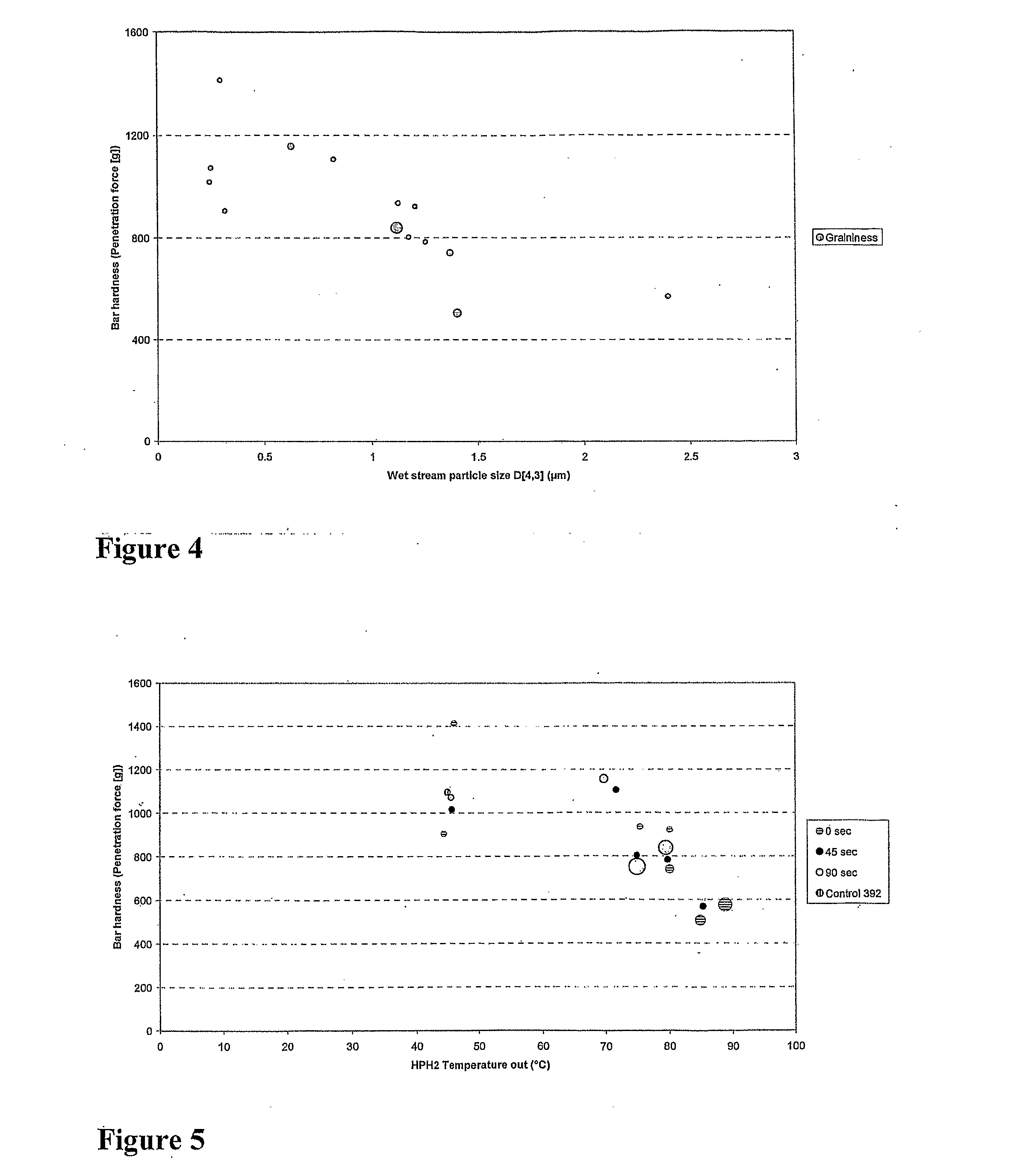
A method for preparing a modified whey protein concentrate (WPC) or whey protein isolate (WPI) is described. It involves (a) providing an aqueous WPC or WPI solution having a protein concentration of 15-50% (w / v), at a pH of 4.7-8.5; (b) heat treating the solution to more than 50° C., for a time that allows protein denaturation to occur; the heat treating comprising heating the solution while under conditions of turbulent flow. At the end of the heat treatment, the heat treated material may be promptly transferred to a drier or to be mixed with other ingredients. The heat-treated WPC or WPI is not subjected to a mechanical shear process prior to the transfer other than where liquid is converted into droplets to facilitate drying. The modified WPC is useful in the manufacture of food and drinks where a high protein content is desired without undesirable changes in texture.
Owner:FONTERRA COOP GRP LTD
Preparation process of intravenous injection human immunoglobulin
ActiveCN101972479AHigh yieldHigh purityAntibody ingredientsImmunoglobulinsUltrafiltrationPhosphoric acid
The invention relates to a preparation process of intravenous injection human immunoglobulin, belonging to the field of pharmaceuticals. On a basis of a traditional preparation process of intravenous injection human immunoglobulin with the low protein concentration of 5 percent, a filter pressing method is adopted instead of a centrifuging method in an extraction process. During hyperfiltration, the protein concentration is adjusted to 3-6 percent, a pH value is adjusted to 6.4-6.6 with 0.5 mol / L of NaOH; then, 1 mol / L phosphoric acid-NaOH buffer solution is added to adjust the electrical conductivity which is measured to be 0.175-0.205 s / m at a temperature T of 19 DEG C; and a chromatography method is adopted to carrying out column chromatography and purification by using upper ion exchange columns after the electrical conductivity is adjusted. The protein impurities can be effectively removed, the protein purify and the product yield are improved; maltose or glycin is used as a protector, which benefits the improvement of the stability of the intravenous injection human immunoglobulin; and the glycin is used as the protector, which satisfies the clinical use of diabetics. According to the invention, the intravenous injection human immunoglobulin with the protein concentration of 5-11 percent can be obtained.
Owner:华润博雅生物制药集团股份有限公司
Chip preparation method, DNA or protein immobilization method and chip
InactiveCN109610006AQuality improvementImprove efficiencyPeptide librariesMicrobiological testing/measurementProtein chipRepeatability
The invention discloses a chip preparation method, a DNA or protein immobilization method, and a chip. The chip preparation method of the invention comprises performing weak passivation treatment after immobilization treatment, wherein the weak passivation treatment comprises contacting a weak passivation reaction solution containing a catalyst with a chip subjected to immobilization treatment, soas to promote binding of DNA or protein to the surface of a substrate, and to enable the DNA or protein to be sufficiently immobilized on the surface of the substrate. The preparation method of the invention increases the weak passivation treatment step after the immobilization treatment, so that the DNA or protein can be more fully immobilized on the surface of the substrate, the quality and efficiency of DNA or protein immobilization are improved, also DNA or protein is more fully immobilized, the relationship between the amount of the DNA or protein immobilized on the chip and the concentration of the DNA or protein initially added is more closely, and thus the immobilization amount of the DNA or protein is highly controllable, the repeatability is good, and a foundation is laid for preparing high-quality DNA or protein chips.
Owner:GENEMIND BIOSCIENCES CO LTD
Separation method for plasma protein
ActiveCN1523038AReduce labor intensityFewer separation stepsAlbumin peptidesPeptide preparation methodsAlcoholIonic strength
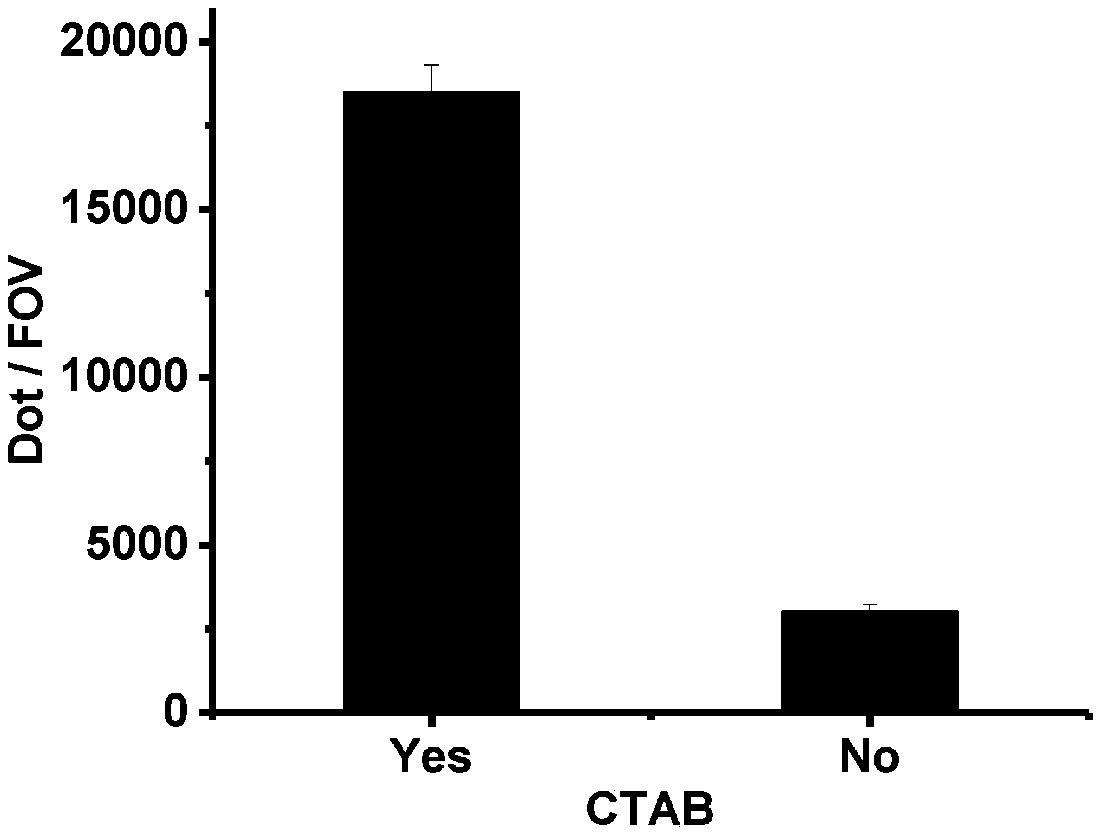
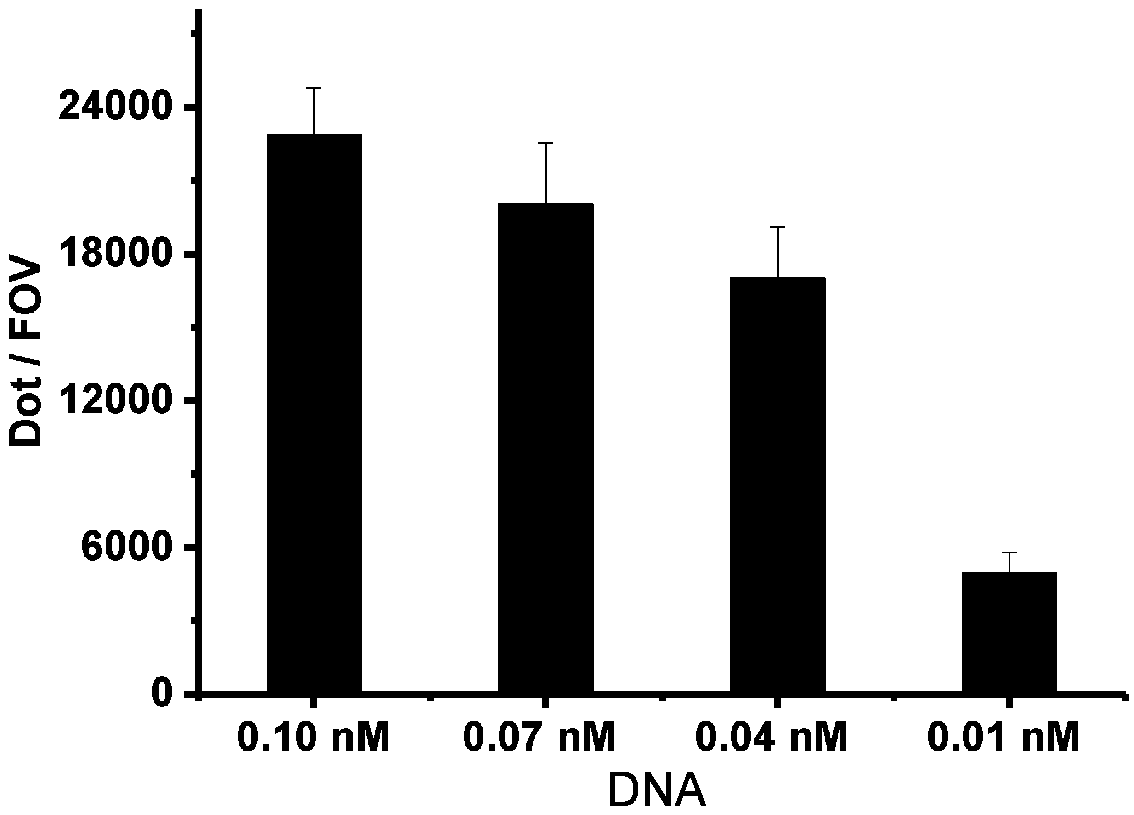
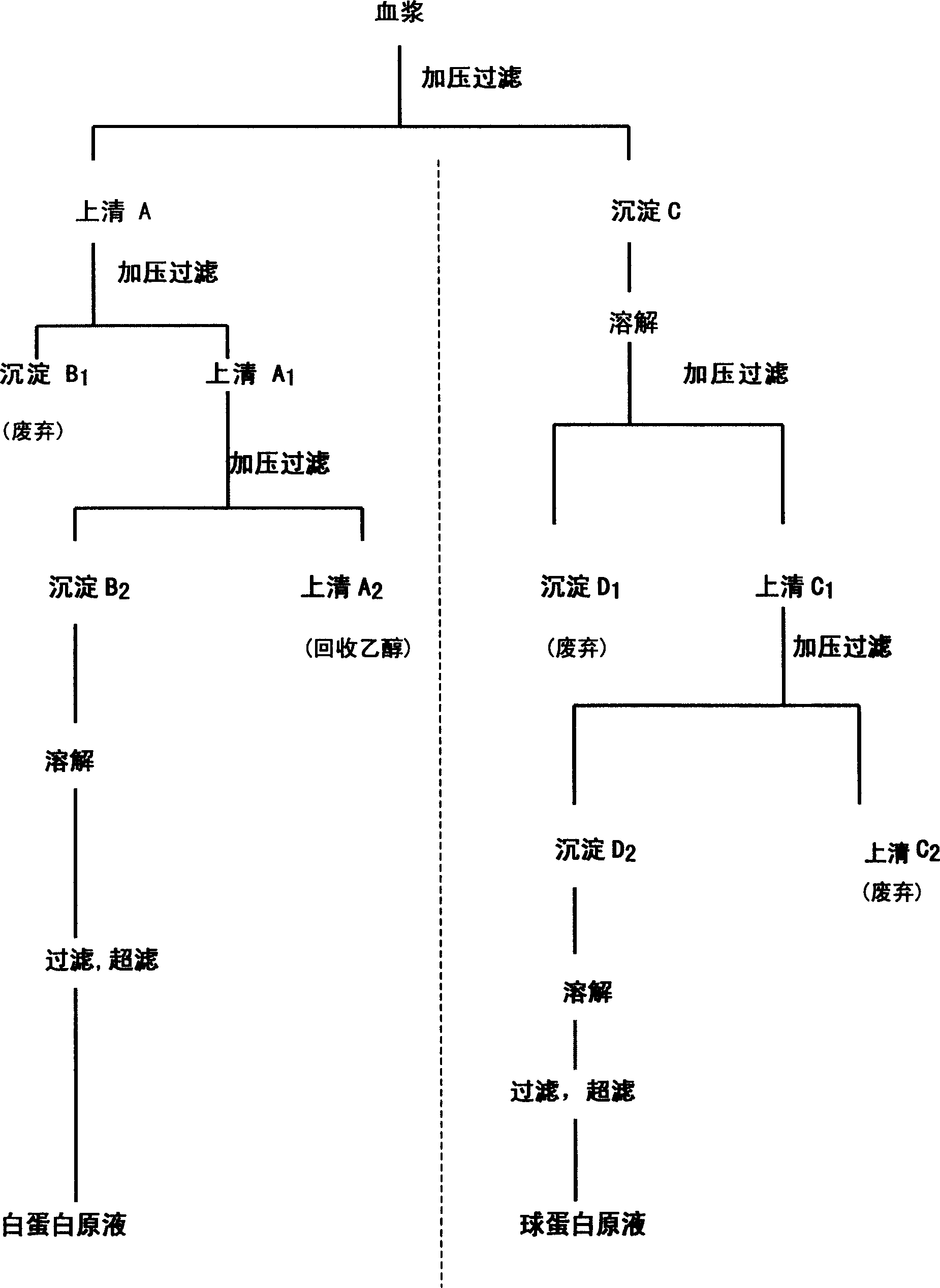
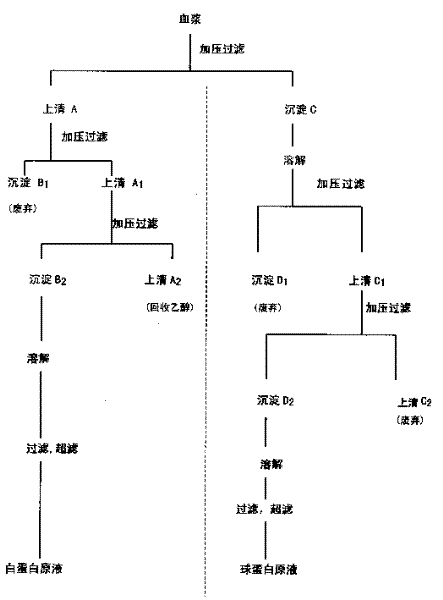
The present invention discloses a method for separating plasma protein, in particular, it relates to a separation method of albumin and globulin. Said method includes the following stps: regulating pH value of plasma ionic strength, protein concentration, ethyl alcohol concentration and temperature, and adopting pressure filtering mode to make separation and obtain supernatant fluid and precipitate. Its filtering process can be implemented in a closed pipeline, so that the separation step can be reduced. It can raise product quality and product yield.
Owner:CHENGDU RONGSHENG PHARMA
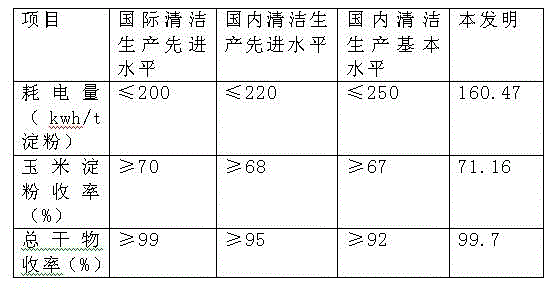
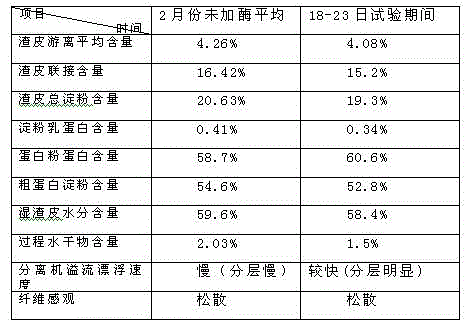
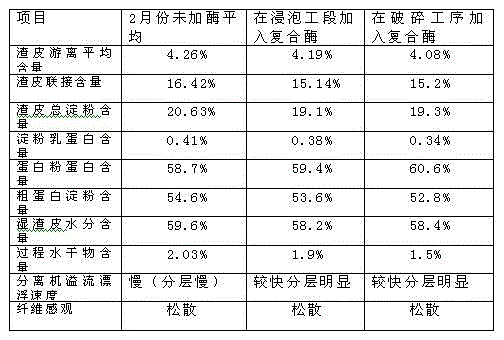
Production improvement method for corn starch
Belonging to the technical field of corn starch production, the invention in particular relates to a production improvement method for corn starch. The method consists of a purification process, an acid making process, a soaking process, a crushing section, germ separation drying, fiber washing and drying, protein concentration drying, starch milk refining, as well as starch dehydration and drying. The method is characterized in that the technical scheme of adding a compound enzyme composed of cellulose, xylanase, pectinase and mannanase into a crushed corn slurry plays a significant role in separating all corn components, thus facilitating production and processing of corn starch, and greatly improving the production level of corn starch.
Owner:SHANDONG XIWANG SUGAR
Liquorice protein nanoparticles and preparation method thereof
ActiveCN103739688AImprove securityIncrease loading capacityPeptide preparation methodsPlant peptidesNanoparticleFood safety
The invention discloses preparation methods of liquorice protein and nanoparticles of the liquorice protein. The liquorice protein with molecular weight of 31.0 kDa and isoelectric point of 4.5 is obtained through separation and purification. A primary structure sequence of N-end of the liquorice protein is NPDGLIACYCGQYCW. The liquorice protein nanoparticles are prepared by heating a saline solution of the liquorice protein at 100 DEG C under the conditions that the protein concentration is 1 mg / mL, and pH is 7.9 for 60 minutes, and the average particle size of the liquorice protein nanoparticles is 74.09+ / -0.69 nm. Freeze-dried products of the liquorice protein nanoparticles are good in liquidity and re-solubility, and easy to stably store. The liquorice protein disclosed by the invention derives from food, and is high in safety. The preparation method of the liquorice protein nanoparticles does not involve any crosslinking agent, finished products of the liquorice protein nanoparticles widely exist in various decoction using liquorice, are taken by the majority of people for many years, and are high in safety. The liquorice protein nanoparticles disclosed by the invention are expected to be applied to in-vivo delivery of various medicines.
Owner:研译(杭州)生物科技有限公司
Preparation method for human immunoglobulin for intravenous injection
ActiveCN103554253AShorten the production cycleHigh yieldSerum immunoglobulinsPeptide preparation methodsUltrafiltrationIon exchange
The invention discloses a preparation method for human immunoglobulin for intravenous injection. The preparation method comprises the following steps: (1) two-step filtering pressing: separating II+III precipitate from human plasma as a starting raw material, removing component III precipitate through low-temperature ethanol filter pressing, collecting supernatant, performing ultrafiltration and dialysis to the supernatant, and regulating the protein concentration; (2) two-step chromatography: performing upper column chromatography purification through a DEAF-Sepharose-FF ion exchange column, and collecting a first-step flow penetration liquid; performing ultrafiltration and dialysis to the collected first-step flow penetration liquid, regulating and performing chromatographic purification by using a Fractogel-EMD-TMA ion exchange column, and collecting a second-step flow penetration liquid; (3) performing ultrafiltration, dialysis and liquid preparation to the collected second-step flow penetration liquid; (4) inactivating virus, disinfecting, filtering and subpackaging to obtain a product. The product prepared by using the method is higher in bioactivity, yield and purity.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Novel proteins and methods for producing the proteins
InactiveUS20020051969A1Efficient productionSure easyBacteriaPeptide/protein ingredientsADAMTS ProteinsCulture mediums
A protein which inhibits osteoclast differentiation and / or maturation and a method of production of the protein. The protein is produced by human embryonic lung fibroblasts and has molecular weight of about 60 kD and about 120 kD under non-reducing conditions and about 60 kD under reducing conditions on SDS-polyacrylamide gel electrophoresis, respectively. The protein can be isolated and purified from culture medium of the said fibroblasts. Furthermore, the protein can be produced by gene engineering. The present invention includes cDNA for producing the protein by gene engineering, antibodies having specific affinity to the protein or a method for determination of the protein concentration using the antibodies.
Owner:DAIICHI SANKYO CO LTD
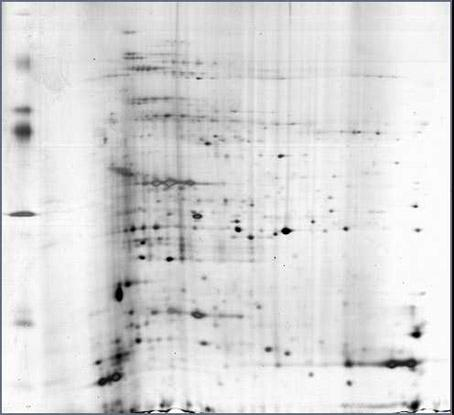
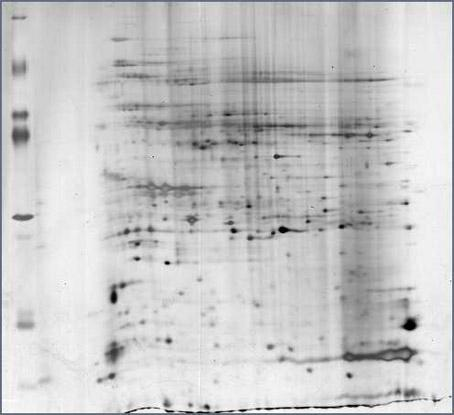
Extraction and dimensional electrophoresis method of mangrove plant total protein
InactiveCN102321149AClear backgroundEasy to separatePeptide preparation methodsElectrophoresisTannin
The invention provides an economical, simple, easily operated and rapid extraction and dimensional electrophoresis method of mangrove plant total protein. Repeated experiments have proved that a sample prepared by the method has good repeatability and an intelligible spectrum, and the technical scheme is especially suitable for an extraction and dimensional electrophoresis method of mangrove plant total protein. The invention employs 20% TCA-acetone solution to extract protein; 20% TCA can better remove salt ion, and acetone with a concentration of 80% has substantial effect on separating of a protein and condensed tannin compound, and can better separate the compound, remove tannin and increase protein concentration; the mangrove plant protein extracted by 20% TCA-acetone has high content and an intelligible 2-DE electrophoresis pattern; besides, the method is simply operated, with good repeatability and low reagent toxicity.
Owner:ZHEJIANG MARICULTURE RES INST
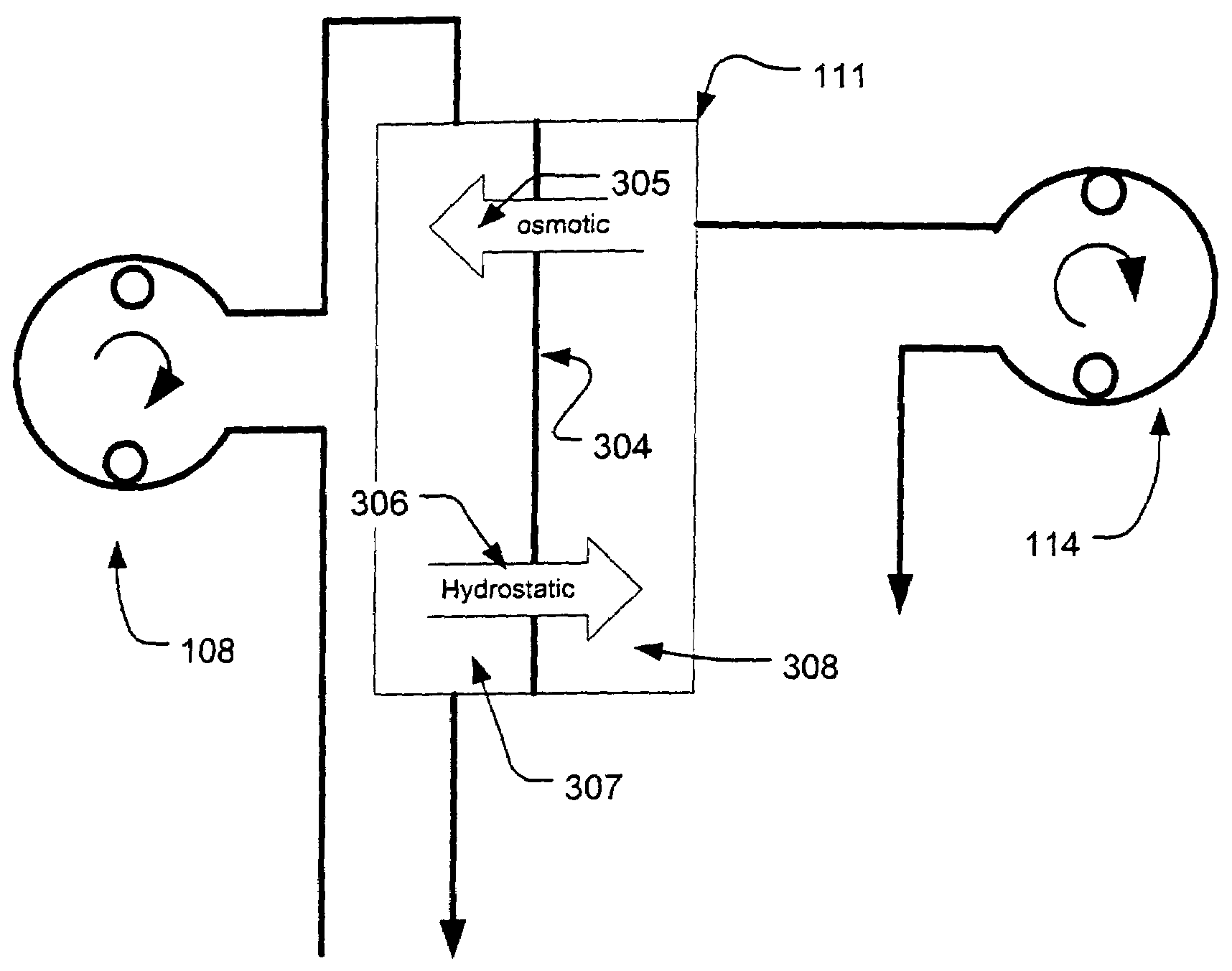
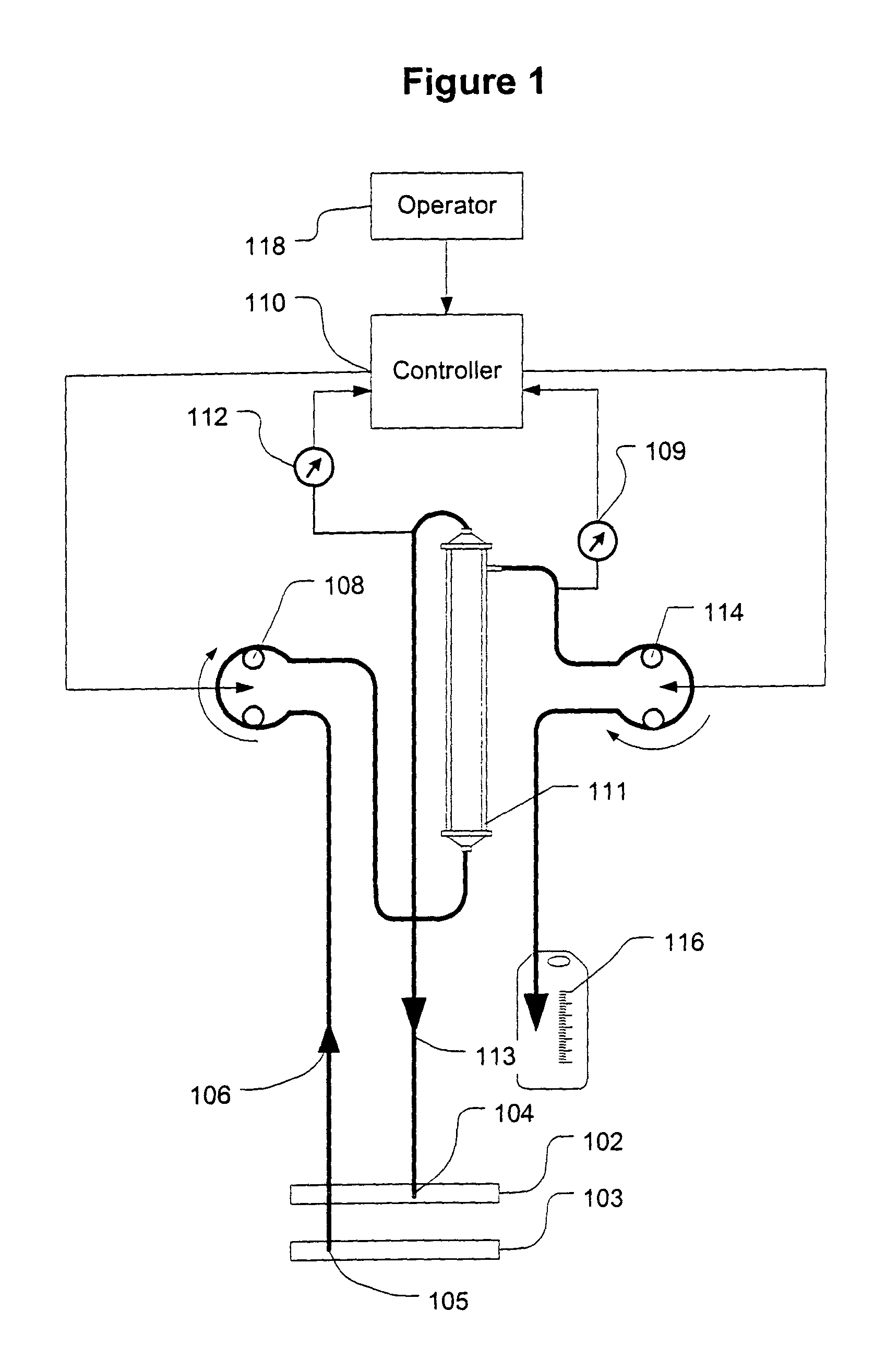
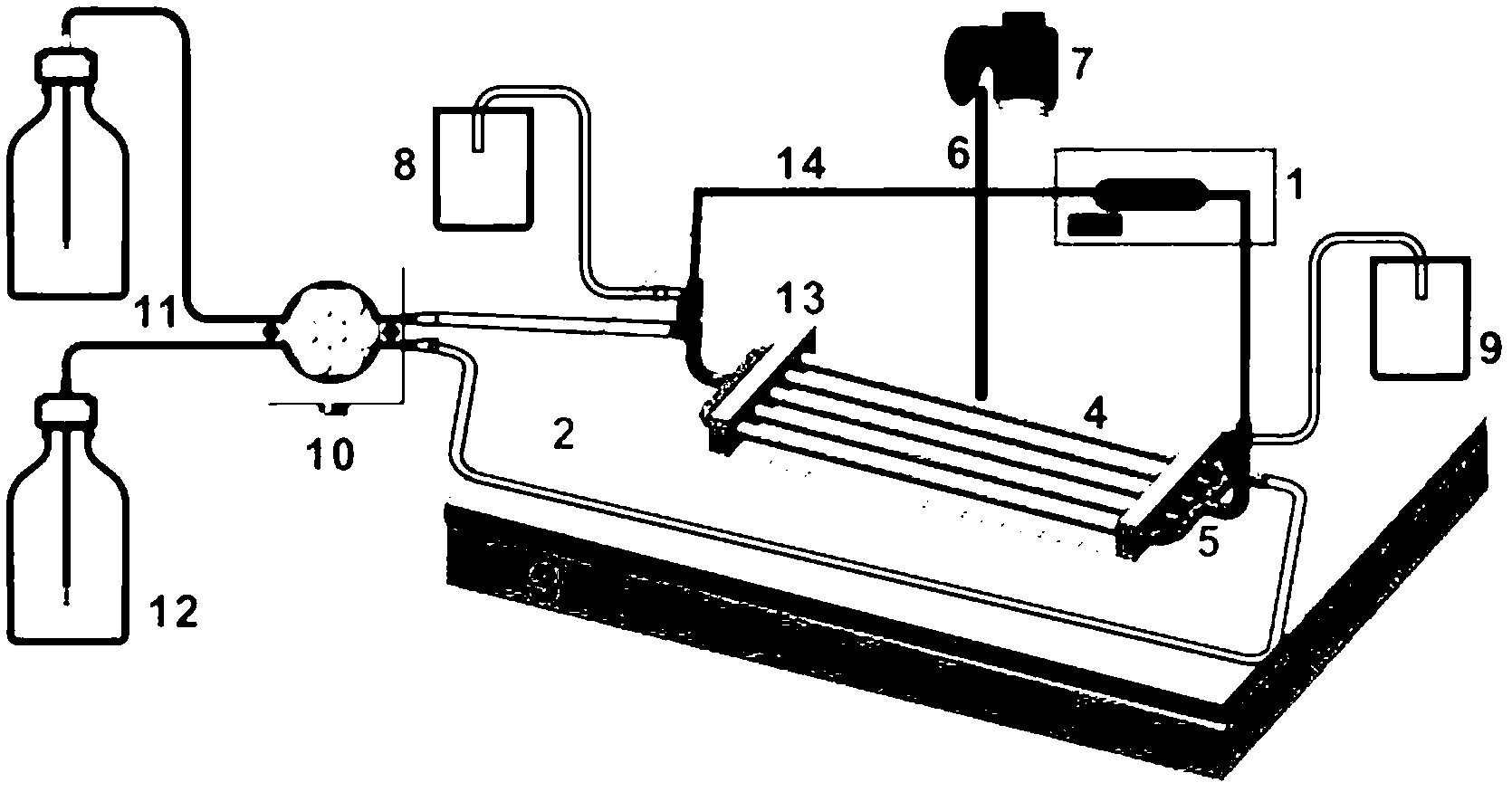
Controller for ultrafiltration blood circuit which prevents hypotension by monitoring osmotic pressure in blood
InactiveUS7399289B2Robust and inexpensiveSemi-permeable membranesSolvent extractionAutomatic controlUltrafiltration
A method and system for the extracorporeal treatment of blood to remove fluid from the fluid overloaded patient is disclosed that non-invasively measures osmotic pressure across a filter membrane of a blood filter. The filter is permeable to water and electrolytes, but not to blood protein. The osmotic pressure indicates the protein concentration in the blood. Osmotic pressure is used to detect when hypotension is about to occur in a patient, as a result of excessive blood volume reduction during treatment of the blood. Using the osmotic pressure measurement as a feedback signal, the rate of fluid extraction is automatically controlled to achieve the desired clinical outcome and avoid precipitating a hypotensive crisis in the patient.
Owner:GAMBRO LUNDIA AB
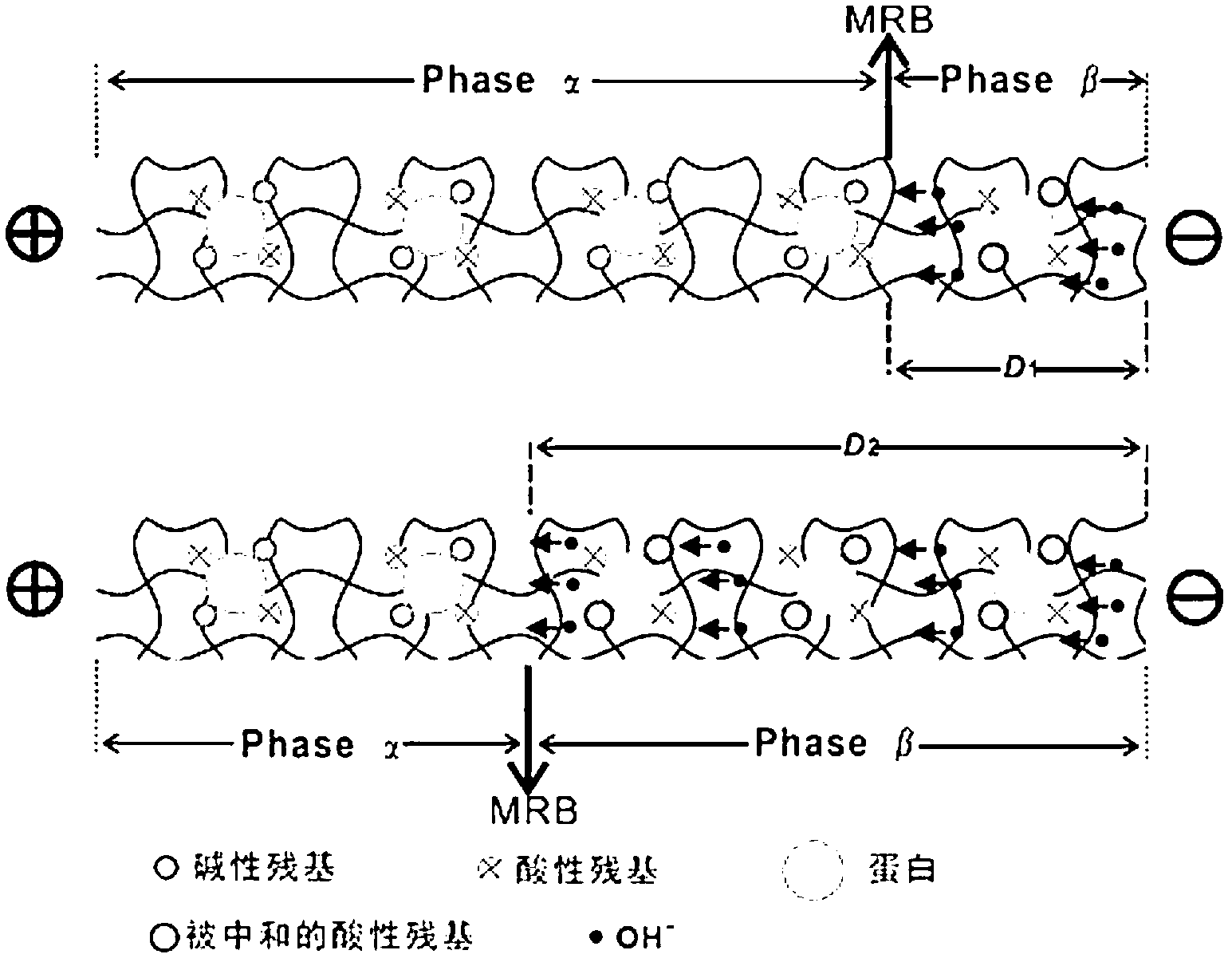
High-flux protein titration method based on moving reaction boundary electrophoresis
InactiveCN103175885AEasy to moveEasy to observeMaterial electrochemical variablesElectrophoresisHigh flux
The invention relates to a moving reaction boundary electrophoresis based on high-flux protein titration method of an electrochemical method analyzing material. The high-flux protein titration method comprises the following steps of: uniformly fixing protein molecules to be detected into an electrophoretic tube through gel by injecting the gel into the electrophoretic tube with a biological sample contained; then carrying out alkali titration; and finally establishing a standard curve of MRB (Moving Reaction Boundary) movement speed and protein concentration by utilizing the functional relation of the MRB movement speed and the protein concentration to obtain the MRB movement speed, and calculating to obtain the definite protein concentration. The high-flux protein titration method disclosed by the invention can be used for fast detecting the total protein quantity and is high in anti-interference capacity.
Owner:SHANGHAI JIAO TONG UNIV
Fermented milk without food additive and preparation method for fermented milk
ActiveCN103651784AReduce viscosity lossLittle acidificationMilk preparationFood additiveWhey protein powder
The invention discloses fermented milk without a food additive and a preparation method for the fermented milk, and belongs to the field of dairy product production. The fermented milk without the food additive comprises the following components by mass percent: 85-93 percent of milk, 0.5-5 percent of cheese powder, 0.5-2 percent of whey protein powder and 6-8 percent of sugar, wherein the inoculation amount of a fermenting agent is 1*10<6>-1*10<7>CFU / g, and the sum of the mass percentages of all the components is 100. According to the fermented milk, the protein concentration in the raw milk is increased by adding the natural cheese powder and the whey protein powder, and the problem of granular milk caused by the excessive adding amount of the whey protein powder can be solved; besides, nutrient substances in the cheese is easier to digest and absorb under the fermentation action of microorganisms; compared with the concentrated fermented milk with single flavor, the fermented milk is more easily accepted by customers; streptococcus thermophilus with weaker post-acidification or a yoghurt fermentation agent with weaker post-acidification is selected, so that the filling temperature can be increased, and the loss of viscosity of the fermented milk during filling is reduced.
Owner:武汉光明乳品有限公司
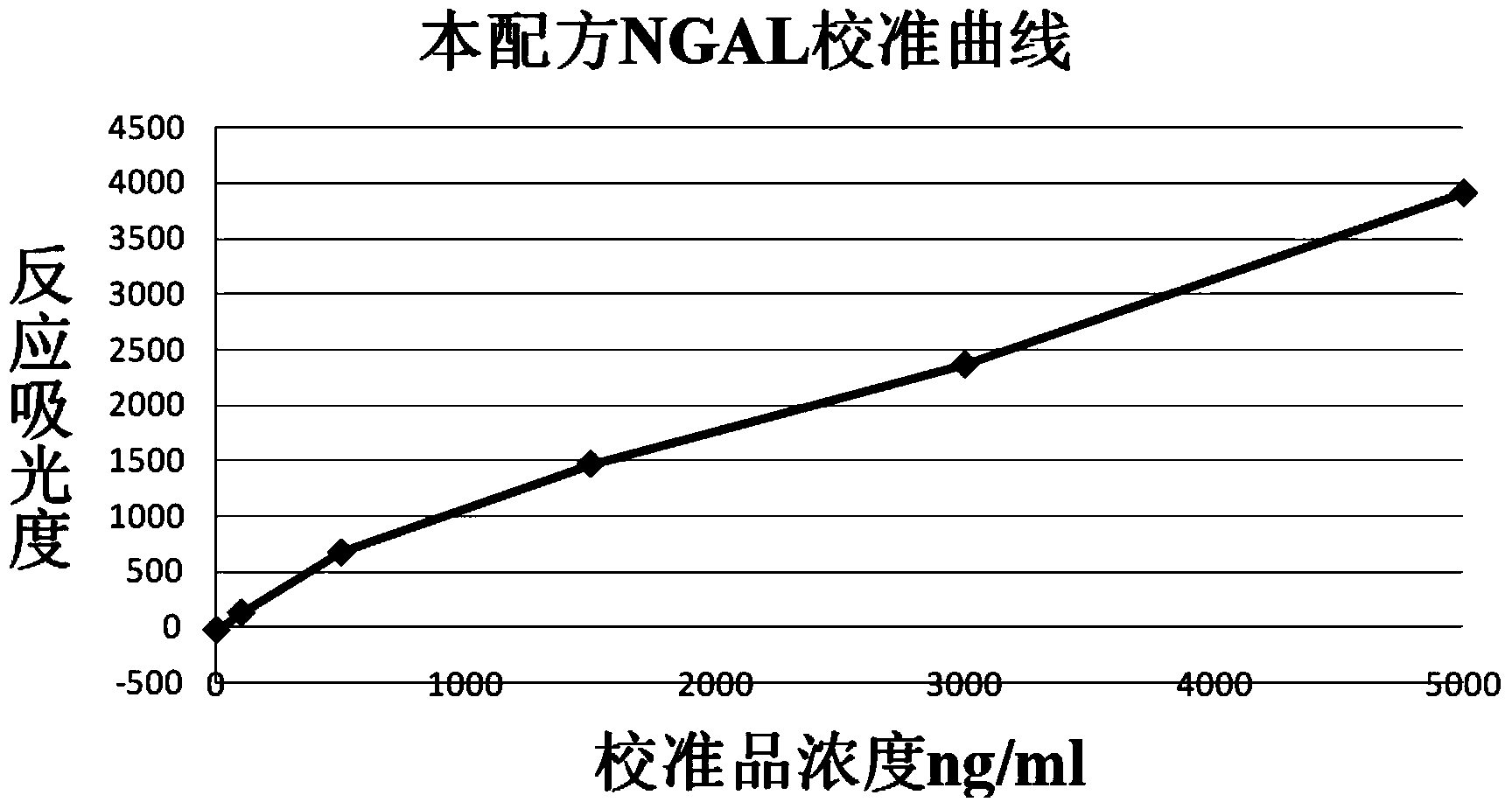
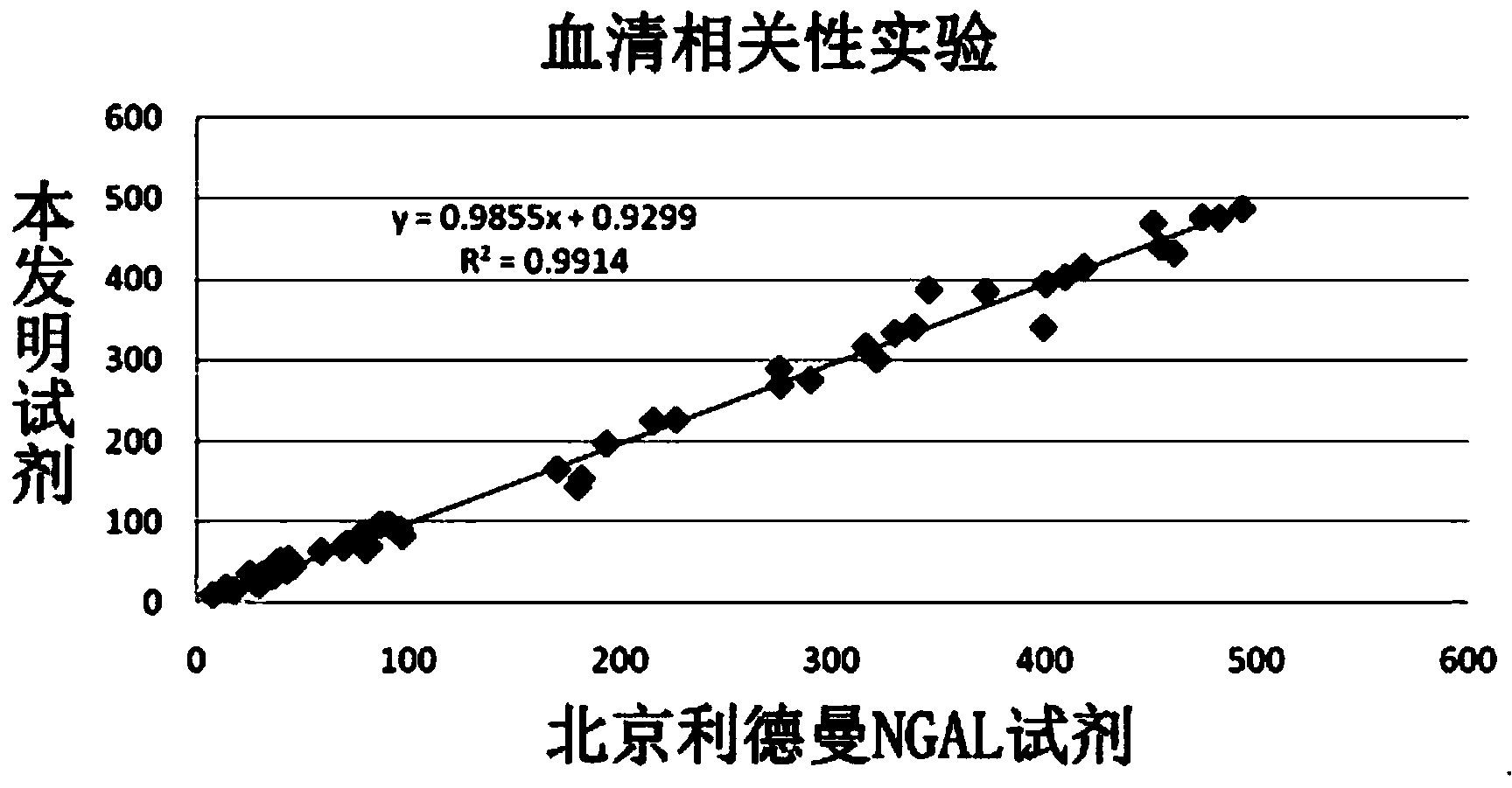
Latex enhanced immunoturbidimetry NGAL detection kit
InactiveCN104215769AGuaranteed uniformityGuaranteed stabilityBiological testingLatex particleNGAL Protein
The invention provides a latex enhanced immunoturbidimetry NGAL detection kit. The latex enhanced immunoturbidimetry NGAL detection kit comprises a reagent R1, a reagent R2 and a standard substance; the reagent R1 comprises a buffer solution, a surfactant, a chelating agent, an accelerator and an antiseptic; the reagent R2 comprises the buffer solution, NGAL antibody-coated latex particles, and an unrelated protein; and the standard substance is a solution containing 6 NGAL recombinant protein concentration gradients. Compared with kits in the prior art, the neutral granulocyte gelatinase related lipid transporter content detection kit used on a fully automatic biochemical analyzer has the advantages of rapid determination of the content of the NGAL in urine or blood plasma, guarantee of the sensitivity and the linearity, and improved sensitivity, accuracy and linearity of the determination result.
Owner:上海睿康生物科技有限公司
Detergent suitable for cleaning hollow fiber ultrafiltration membrane and method for producing the same
ActiveCN101219341AStrong targetingReduce cleaning frequencySemi-permeable membranesFiberUltrafiltration
The invention provides a cleaning agent that is applicable to clean hollow fiber ultrafiltration membrane, which comprises the compounds (percentage by weight): anhydrous sodium carbonate accounts for 25-45 percent, trisodium phosphate accounts for 5-20 percent, anhydrous sodium sulfate accounts for 2-5 percent, ethylene diamine tetraacetic acid tetrasodium salt (EDTA-4Na) accounts for 5-15 percent, sodium percarbonate accounts for 0-33 percent, emulsifying agent SAS accounts for 8-15 percent, peregal-25 accounts for 0-5 percent and dodecyl sodium sulfate accounts for 4-10 percent. The compounds are added into an enamel reactor with a stirrer and a grinder in sequence to be stirred for 2-3 hours, thus obtaining the cleaning agent through grinding the mixture evenly. The invention has simple preparation and is particularly applicable to clean hollow fiber ultrafiltration membrane such as ultrafiltration oily wastewater, organic wastewater, protein concentration, etc.; the invention has strong decontamination direction, high effect and low cleaning cost.
Owner:OCHEMATE MATERIAL TECH CO LTD
Mung bean pea edible separation protein and producing method thereof
InactiveCN101238846AHigh yieldReduce difficultyProtein composition from vegetable seedsProtein solutionFiber
The invention relates to a kind of isolating edible protein in the mung bean and pea and the producing method thereof, which belongs to the field of the edible protein producing method technology. It is characterized in that, the steps are as follows: a. alkali dissolving: adding alkali solution into the waste mud after starch processing of the mung bean and pea, adjusting pH to 9.0-11.0; b. impurity isolation: separating by separator, discharging parts of fiber and residues of the protein solution; c. acid precipitation: adding hydrochloric acid solution into acid tank for adjusting pH to 4.5-5.5; d. protein concentration: secondary separation, adding water for adjusting the density of the protein solution to 8-15%; e. neutralizing: adding alkali solution for adjusting pH up to 7.0; f. sterilizing, removing soy smell: high temperature sterilizing, flashing and then degassing and removing soy smell; g. homogenizing: homogenizing in homogenizer; h. spray drying: spray drying for acquiring edible isolating protein. The invention uses the seriflux produced by starch process as the raw materials to produce edible protein, the product has good appearance, good quality, and has solubility, emulsifying property, water absorbing and oil protecting ability, foamability and other good characteristics, and is environment protected.
Owner:王雪源
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com