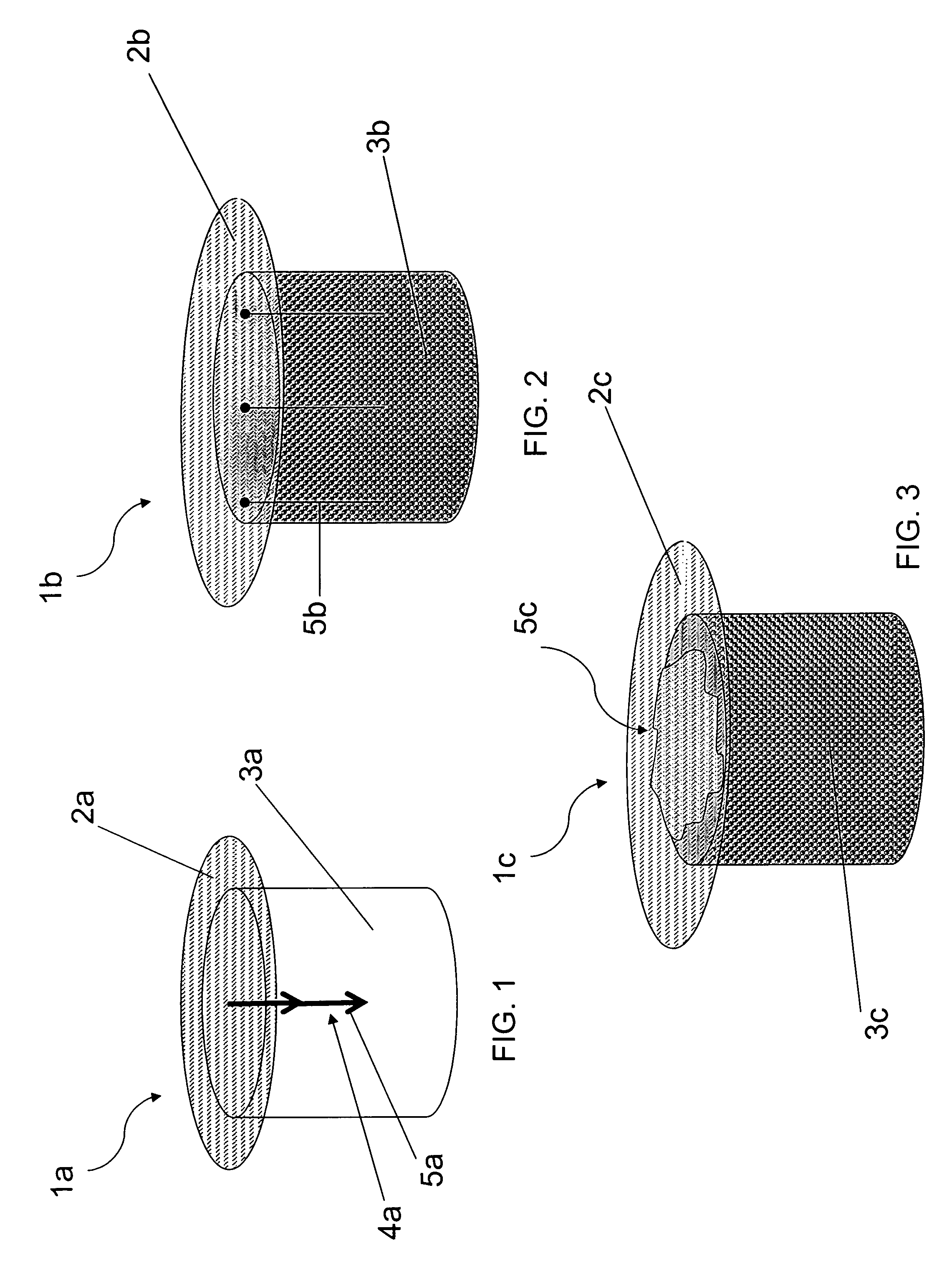
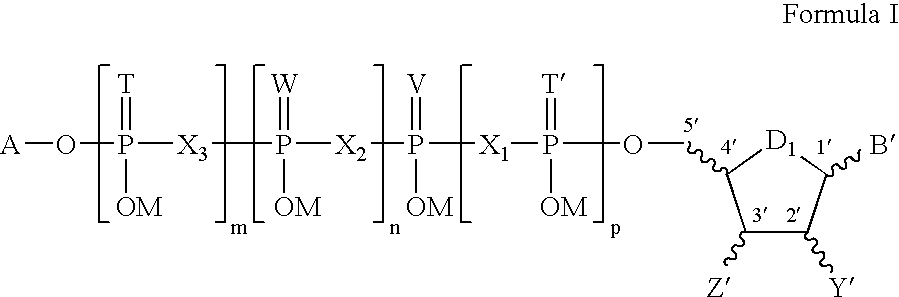Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
208 results about "Synovial fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
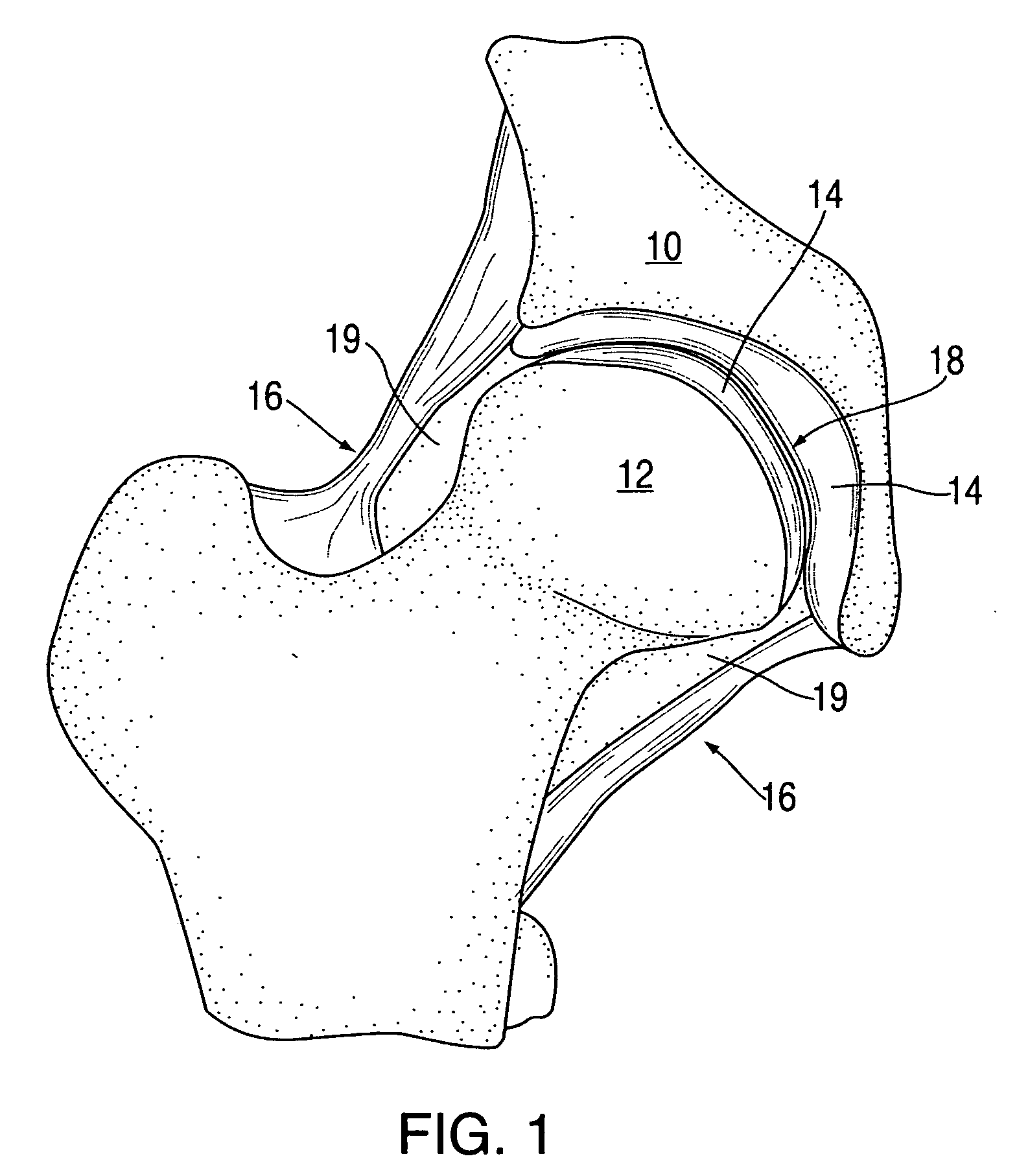
Synovial fluid, also called synovia, is a viscous, non-Newtonian fluid found in the cavities of synovial joints. With its egg white–like consistency, the principal role of synovial fluid is to reduce friction between the articular cartilage of synovial joints during movement. Synovial fluid is a small component of the transcellular fluid component of extracellular fluid.
Synovial fluid barrier
InactiveUS20060178743A1Improve regenerative healingImprove responseSuture equipmentsPeptide/protein ingredientsSynovial jointsSacroiliac joint
A composite tissue formed in situ is provided. The composite tissue includes a synovial joint tissue; and a barrier material adhered thereto for sealing the synovial joint tissue against synovial fluid. Also provided is a method for regenerating synovial joint tissue in situ by excluding synovial fluid therefrom. The method includes providing a synovial joint tissue having a defect; and placing a barrier material in intimate contact with the defect for sealing the defect against synovial fluid. The barrier material includes a curable protein copolymer. The method further includes curing the protein copolymer in situ. The barrier material can include a crosslinked network, or a self gelled network of repeating elastin-like and fibroin-like polymer chains.
Owner:SPINEWAVE
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
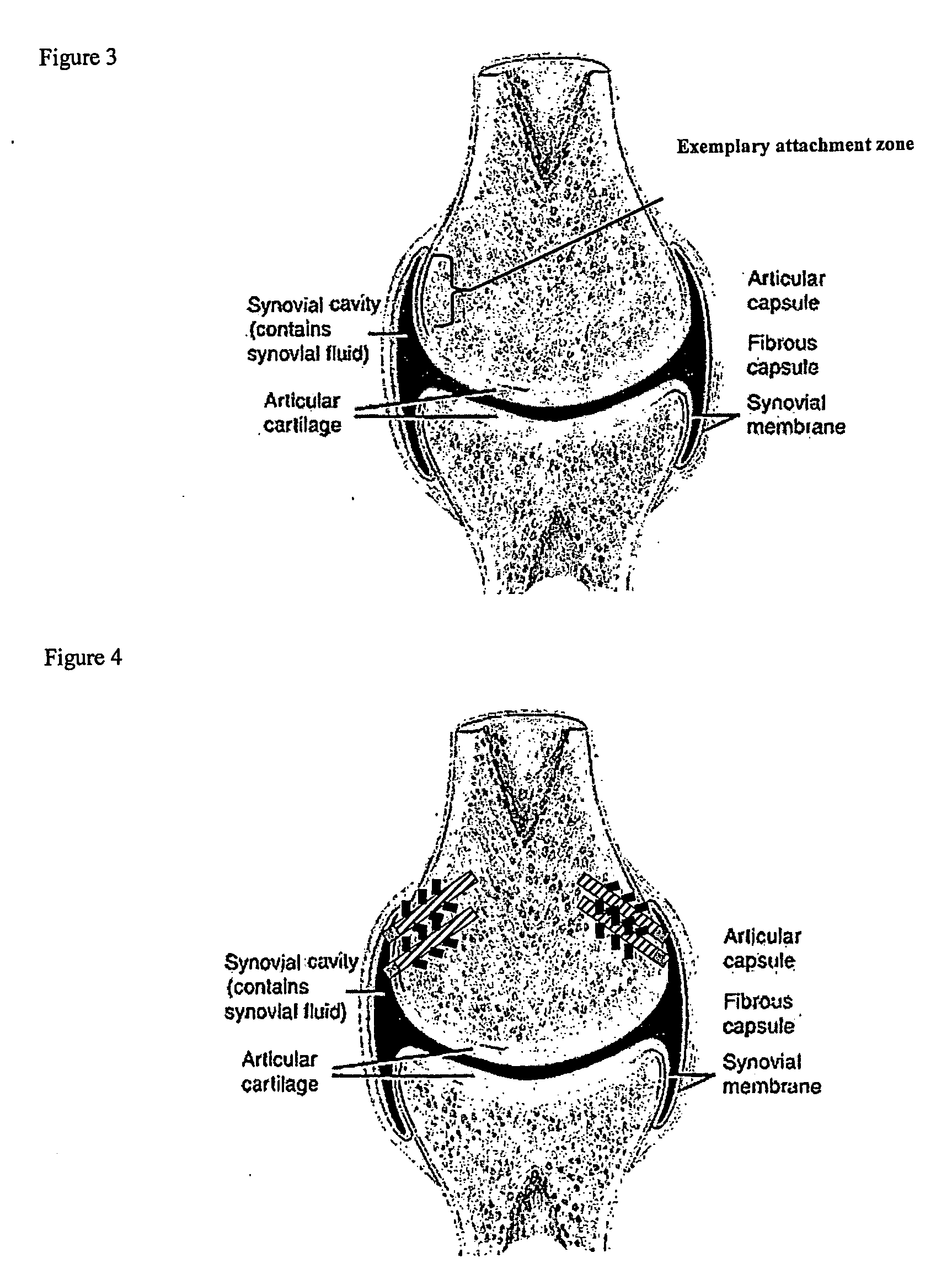
Hydrogel implants for replacing hyaline cartilage, with charged surfaces and improved anchoring
InactiveUS20050287187A1High strengthIncreased durabilityFinger jointsWrist jointsKnee JointVolumetric Mass Density
Hydrogel devices for surgical implantation to replace damaged cartilage in a mammalian joint (such as a knee, hip, shoulder, etc.) are disclosed, with one or more of the following enhancements: (1) articulating surfaces that have been given negative surface charge densities that emulate natural cartilage and that interact with positively charged components of synovial fluid; (2) anchoring systems with affixed pegs that will lock into accommodating receptacles, which will be anchored into hard bone before the implant is inserted into a joint; (3) a three-dimensional reinforcing mesh made of strong but flexible fibers, embedded within at least a portion of the hydrogel.
Owner:MANSMANN KEVIN A
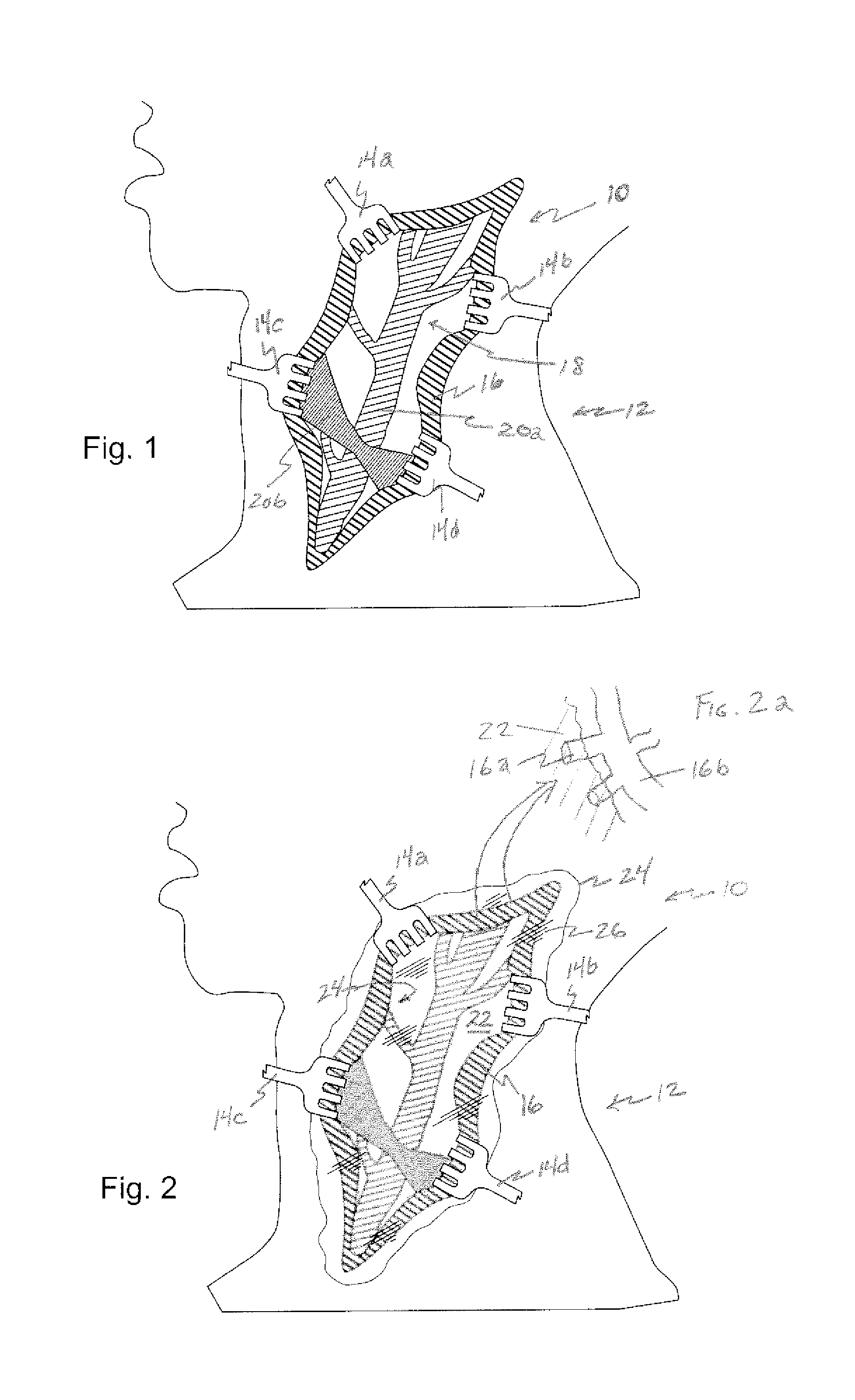
Drug delivery to a joint
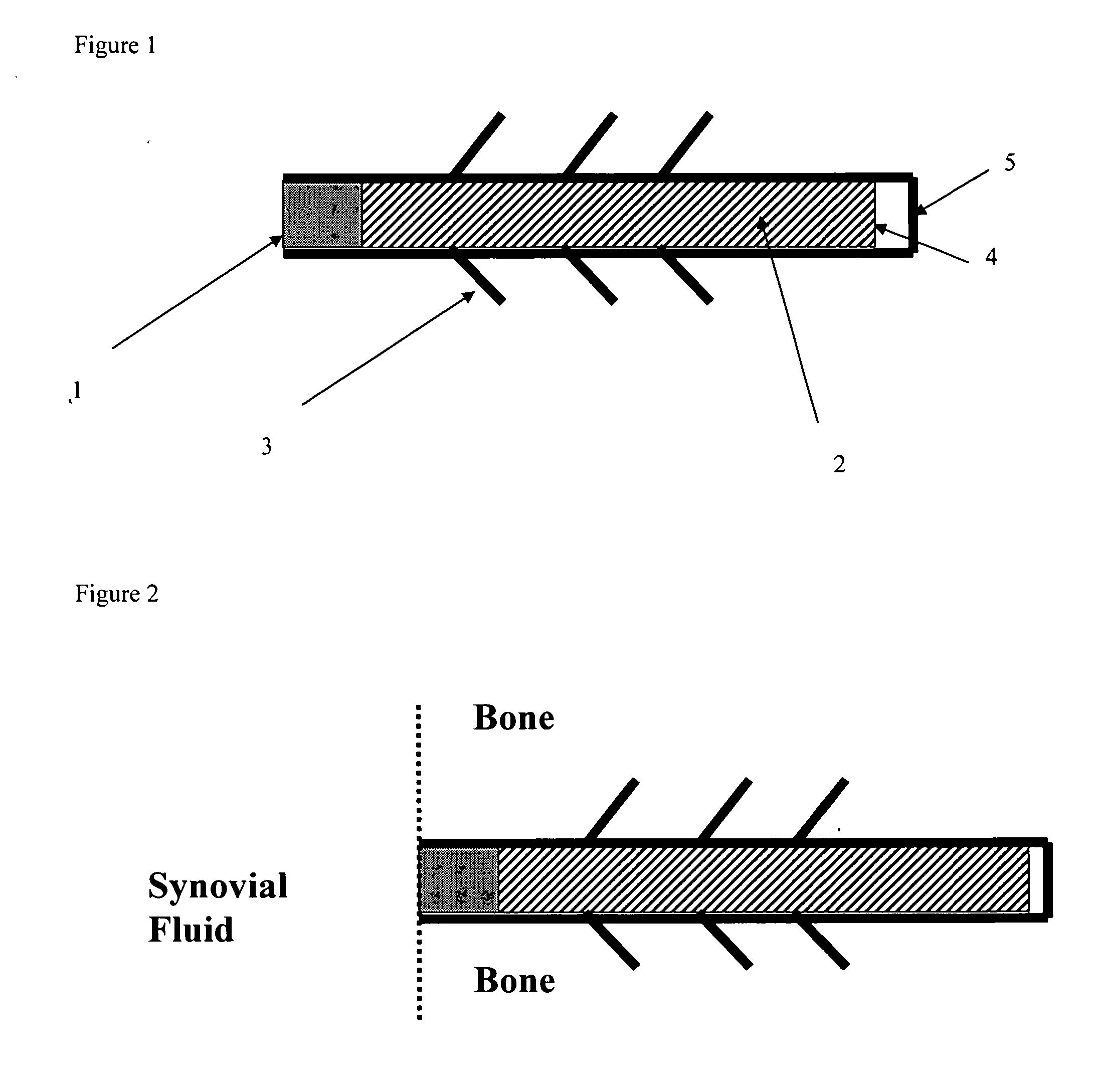
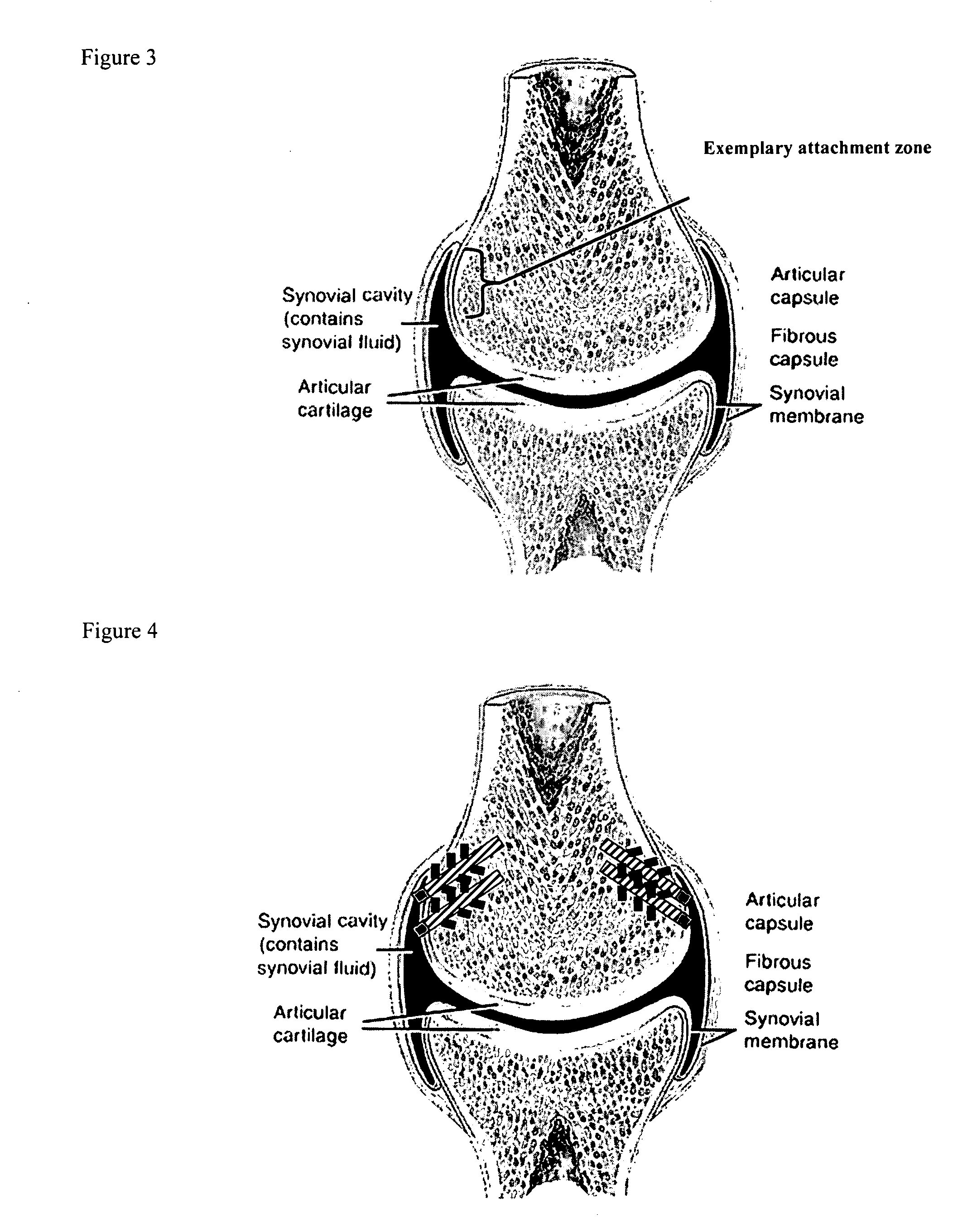
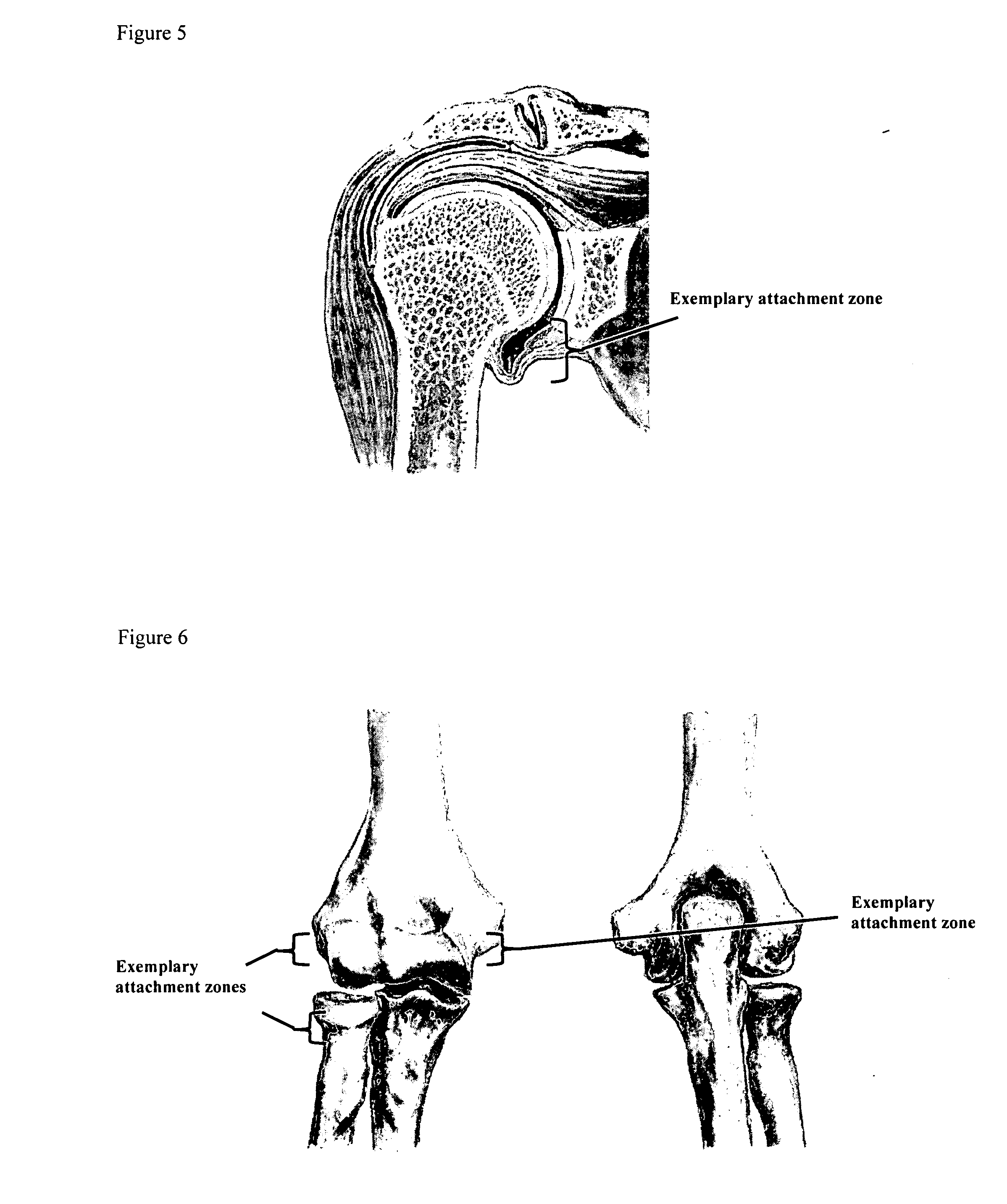
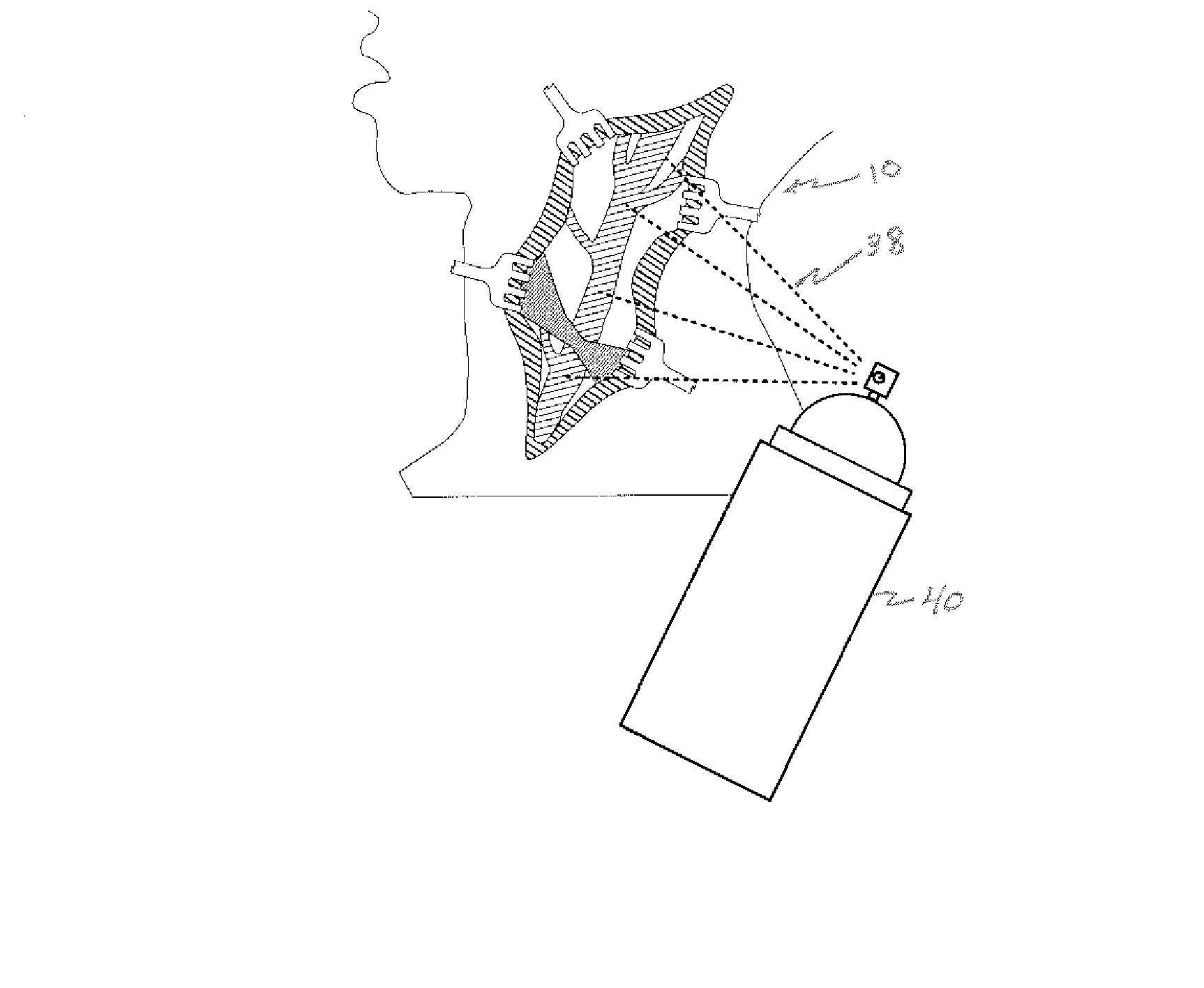
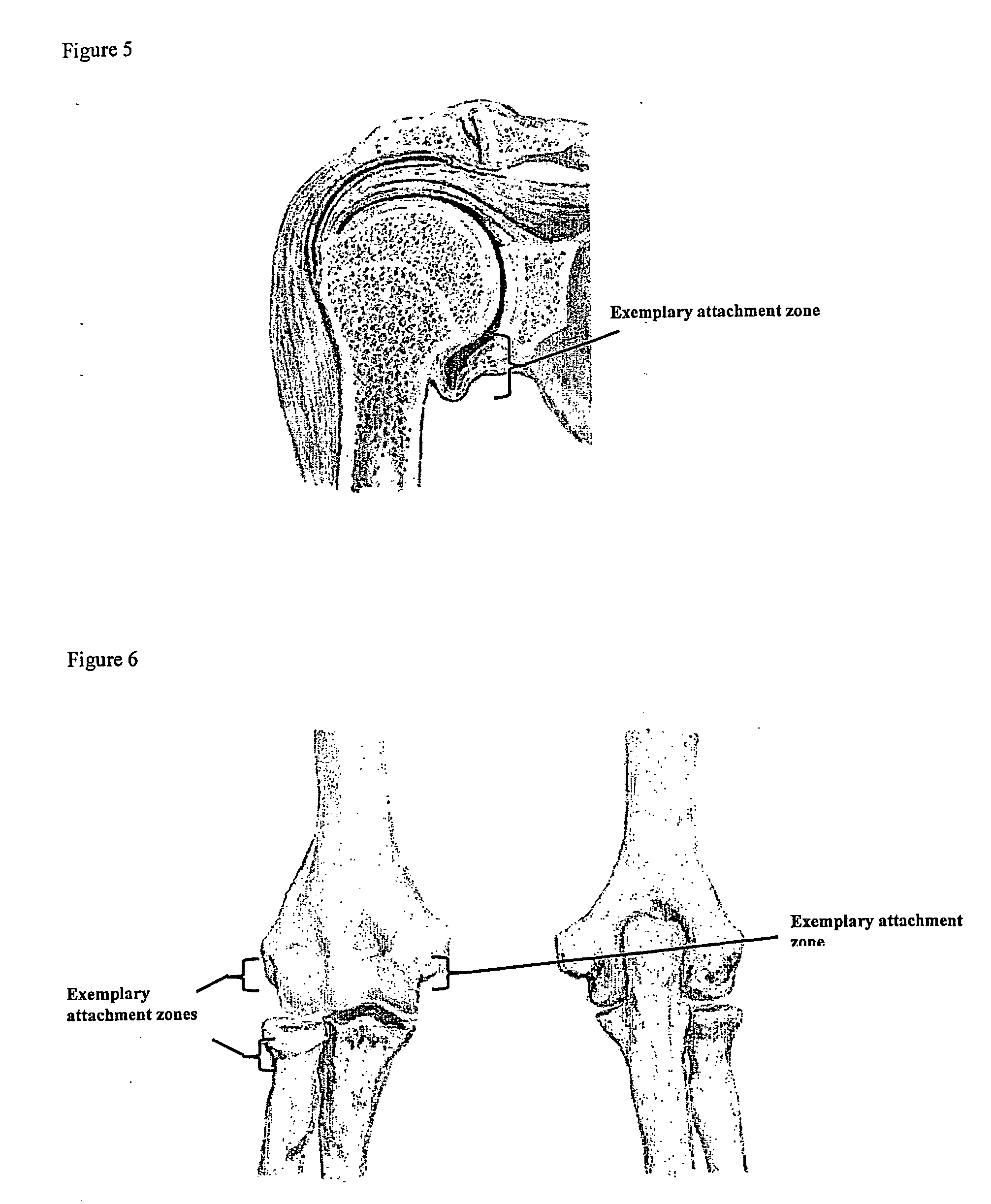
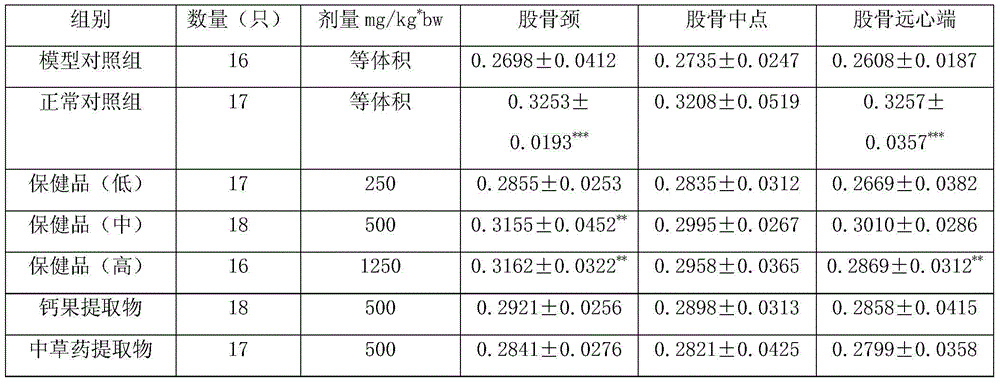
A method of intra-articular drug delivery may include selecting an attachment zone in a synovial joint; affixing a drug release device in the attachment zone, the drug release device comprising a base affixable in the attachment zone, a sustained-release drug carrier, and a drug, the device positioned so that the device releases the drug into the synovial fluid of the synovial joint, and so that agitation of the synovial fluid facilitates elution of the drug from the drug release device.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Method of inhibiting the formation of adhesions and scar tissue and reducing blood loss
InactiveUS20080069855A1Increased riskEasy to disassembleBiocideInternal osteosythesisWound siteBlood vessel
The present invention provides a method for inhibiting the formation of scar tissue and / or exogenous bone at a wound site in a body of a patient. The method includes administering an amount of a biologic agent to the wound site, wherein the biologic agent is synovial fluid or cerebrospinal fluid. A viscous substance applied to the wound site, advantageously incorporating the biologic agent, reduces the flow of blood from cut blood vessels, and contributes to a reduction in the formation of adhesions through action of the biologic agent, and through barrier properties introduced by the viscous substance.
Owner:P TECH
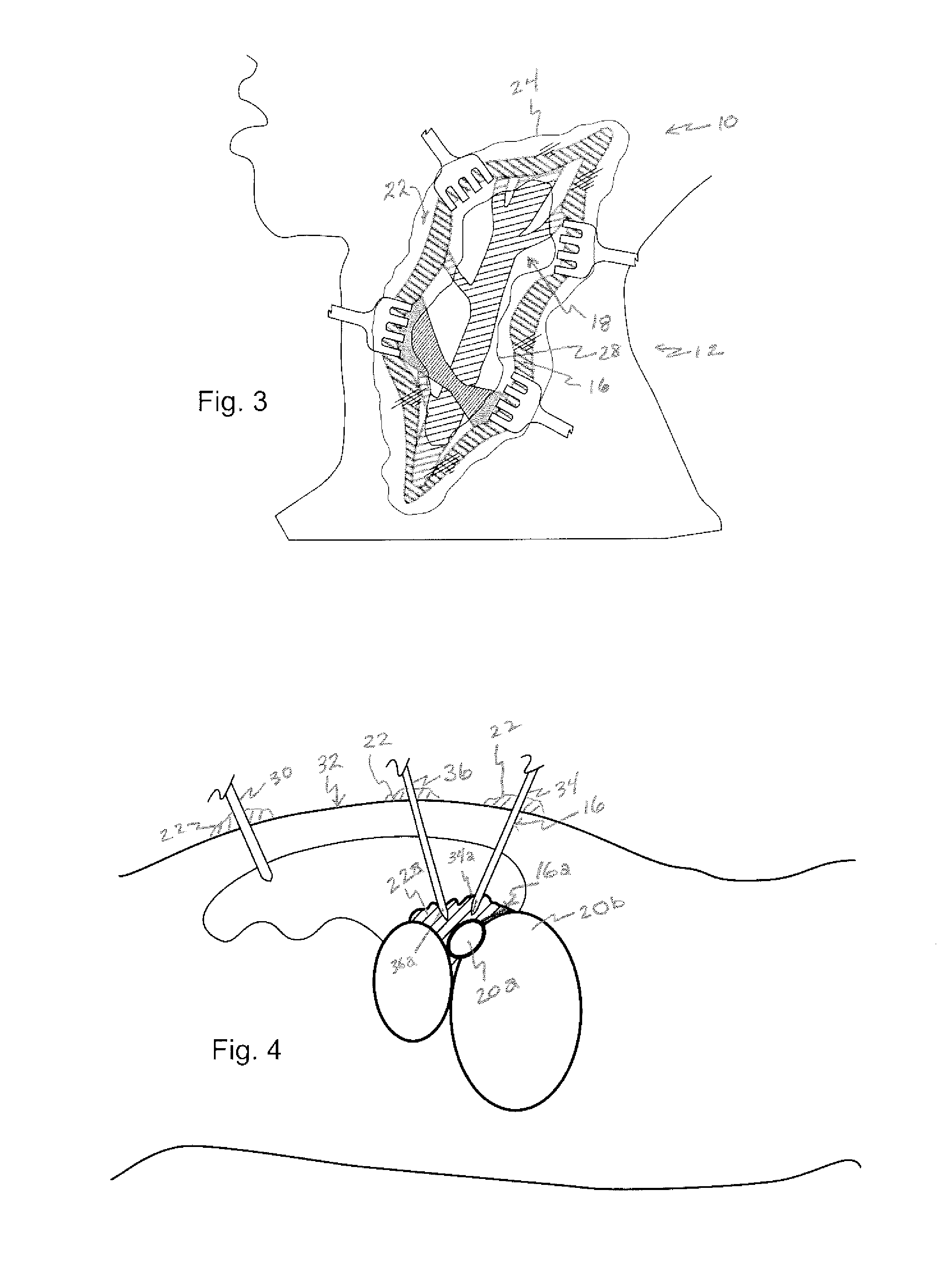
Drug delivery to a joint
InactiveUS20070053963A1Easy to eluteAntibacterial agentsPowder deliverySustained release drugElution
A method of intra-articular drug delivery may include selecting an attachment zone in a synovial joint; affixing a drug release device in the attachment zone, the drug release device comprising a base affixable in the attachment zone, a sustained-release drug carrier, and a drug, the device positioned so that the device releases the drug into the synovial fluid of the synovial joint, and so that agitation of the synovial fluid facilitates elution of the drug from the drug release device.
Owner:HOSPITAL FOR SPECIAL SURGERY
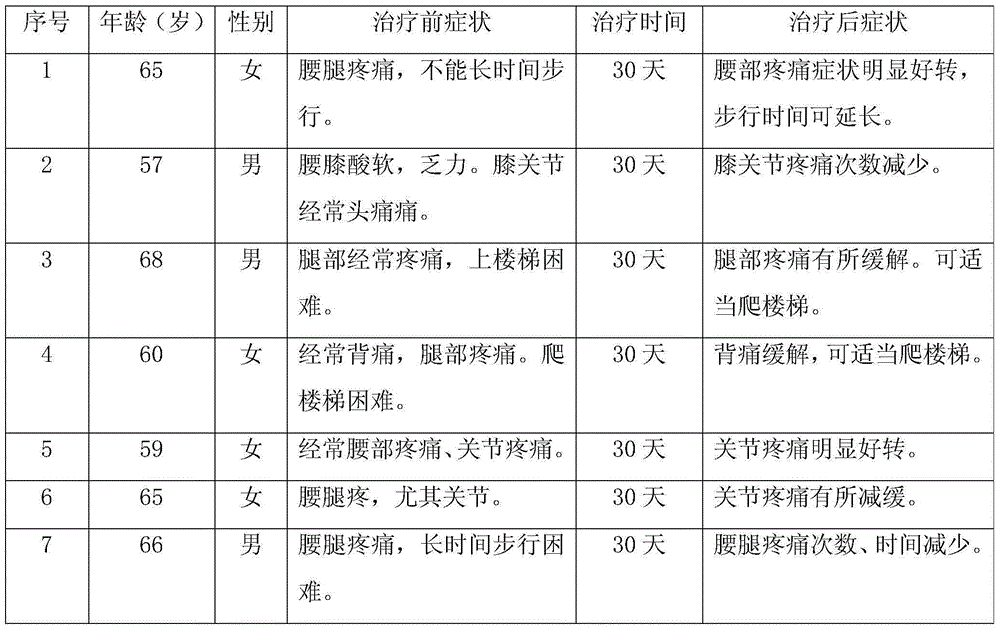
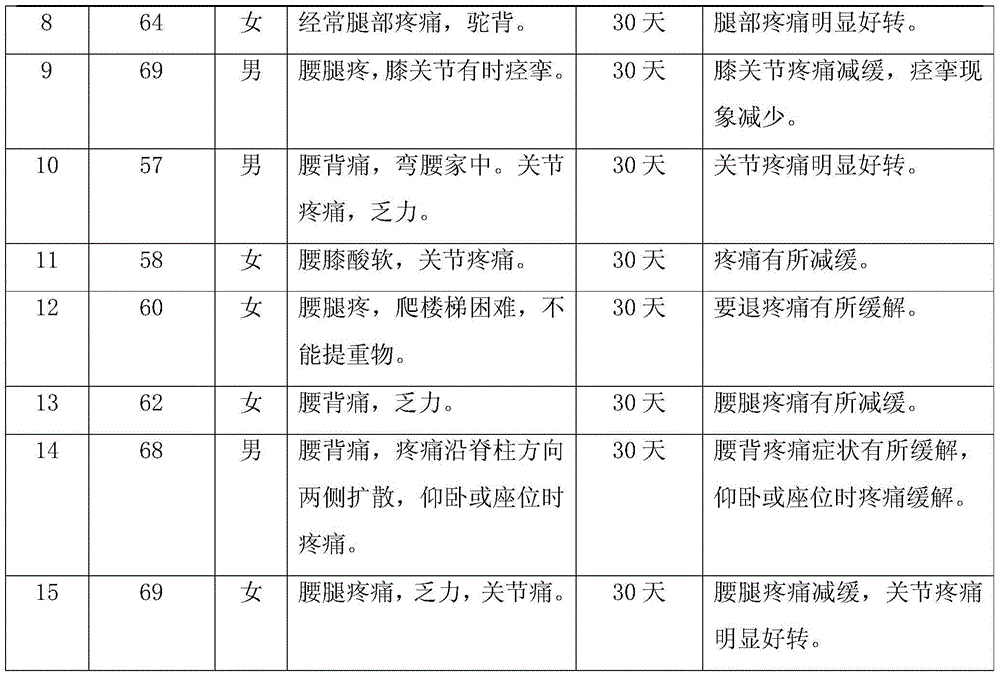
Health product for increasing bone density and preparation method of health product
InactiveCN104431684AImprove adsorption capacityStrong adsorption effect is stronger; 3) water holding capacityFood preparationRe absorptionIncreased Bone Density
The invention discloses a health product for increasing bone density and a preparation method of the health product. Chinese dwarf cherries, Chinese herbal medicine extracts refined by adopting a modern extraction technology and other raw materials having the function of increasing the bone density are scientifically compounded and are scientifically combined omnibearingly to play a synergistic effect, so as to effectively promote the calcium absorption in multiple aspects, repair and reconstruct the cartilago articularis, promote the generation of synovial fluid, promote the synthesis of collagen in cartilago articularis substrates, prevent the further degradation of cartilage, indirectly reduce the osteoclast function, inhibit the re-absorption of bones, increase the content of bone calcium and perfect the biomechanical properties of the bones, so that the product has a good function of increasing the bone density. A microwave drying technology in the preparation method can be used for further improving the integration and biological stability of the health product, and by means of the antioxidant activity of a grape seed extract in the raw materials, the strong adsorption and inclusion functions of modified dietary fibers and the antifungal propertie of the Chinese herbal medicine extracts, an efficient, safe, nutritional and stable health product having the function of increasing the bone density is prepared finally.
Owner:邵素英
Implant designs and methods of improving cartilage repair
InactiveUS20080125863A1Prevent influxOvercomes drawbackSuture equipmentsBone implantMedicineCartilage repair
The invention herein generally refers to an in situ cartilage repair implant. The implant promotes cartilage repair by providing a sealed barrier that prevents the flow of synovial fluid and inflammatory cytokines into a surgically prepared hole that accommodates the implant. Optionally, additives are associated with the implant to promote cartilage repair.
Owner:WARSAW ORTHOPEDIC INC
Device and Method for Treating Conditions of a Joint
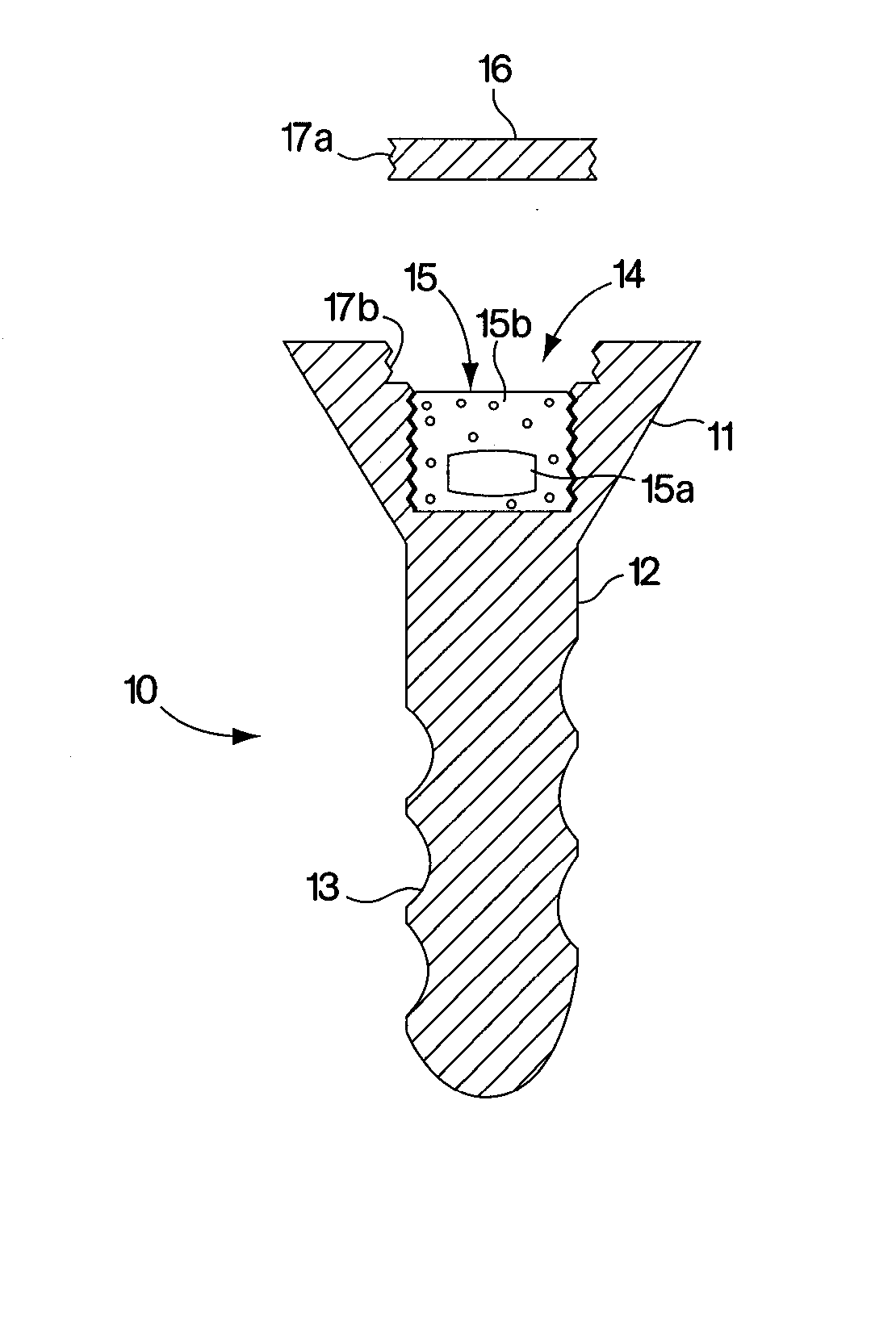
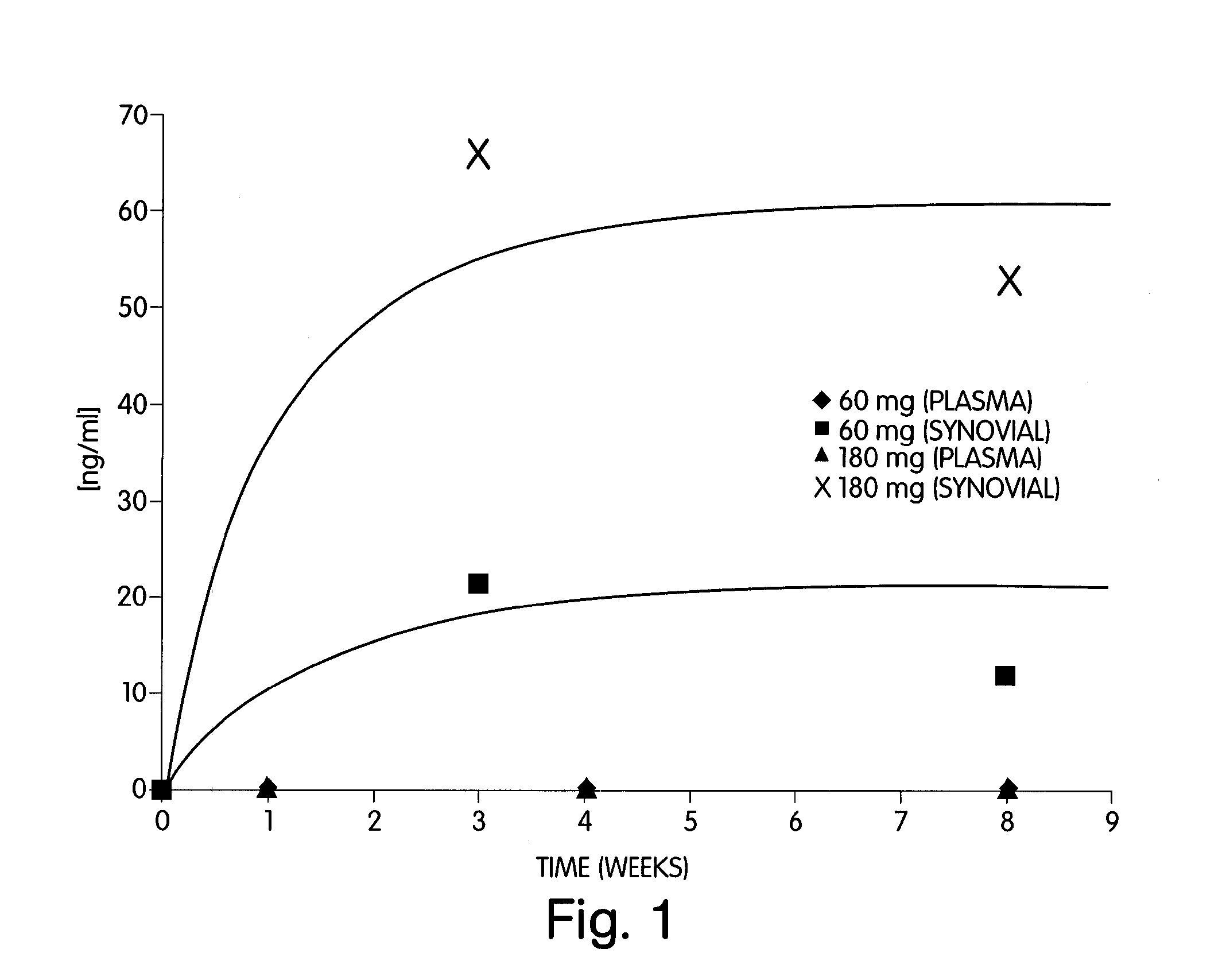
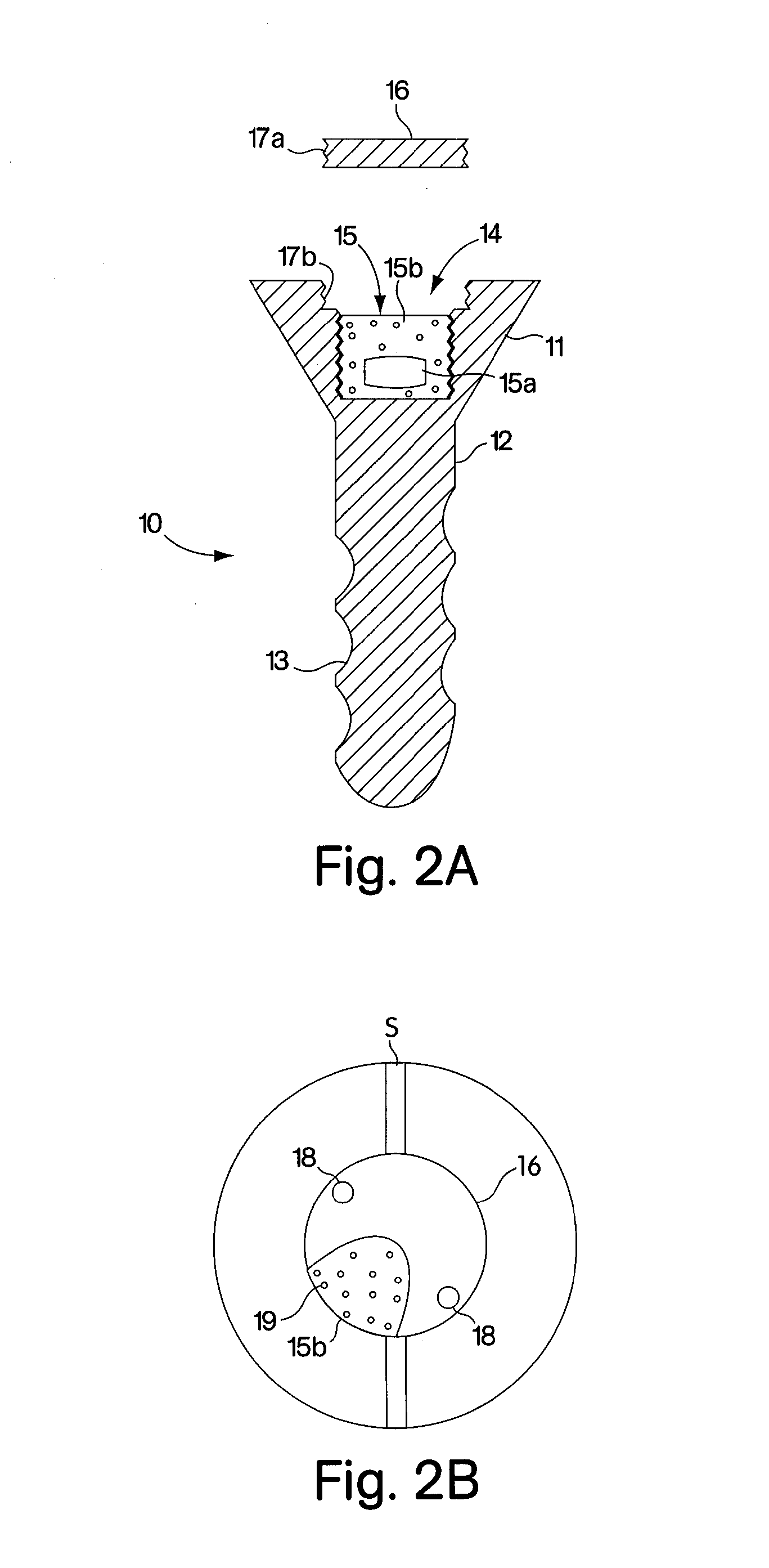
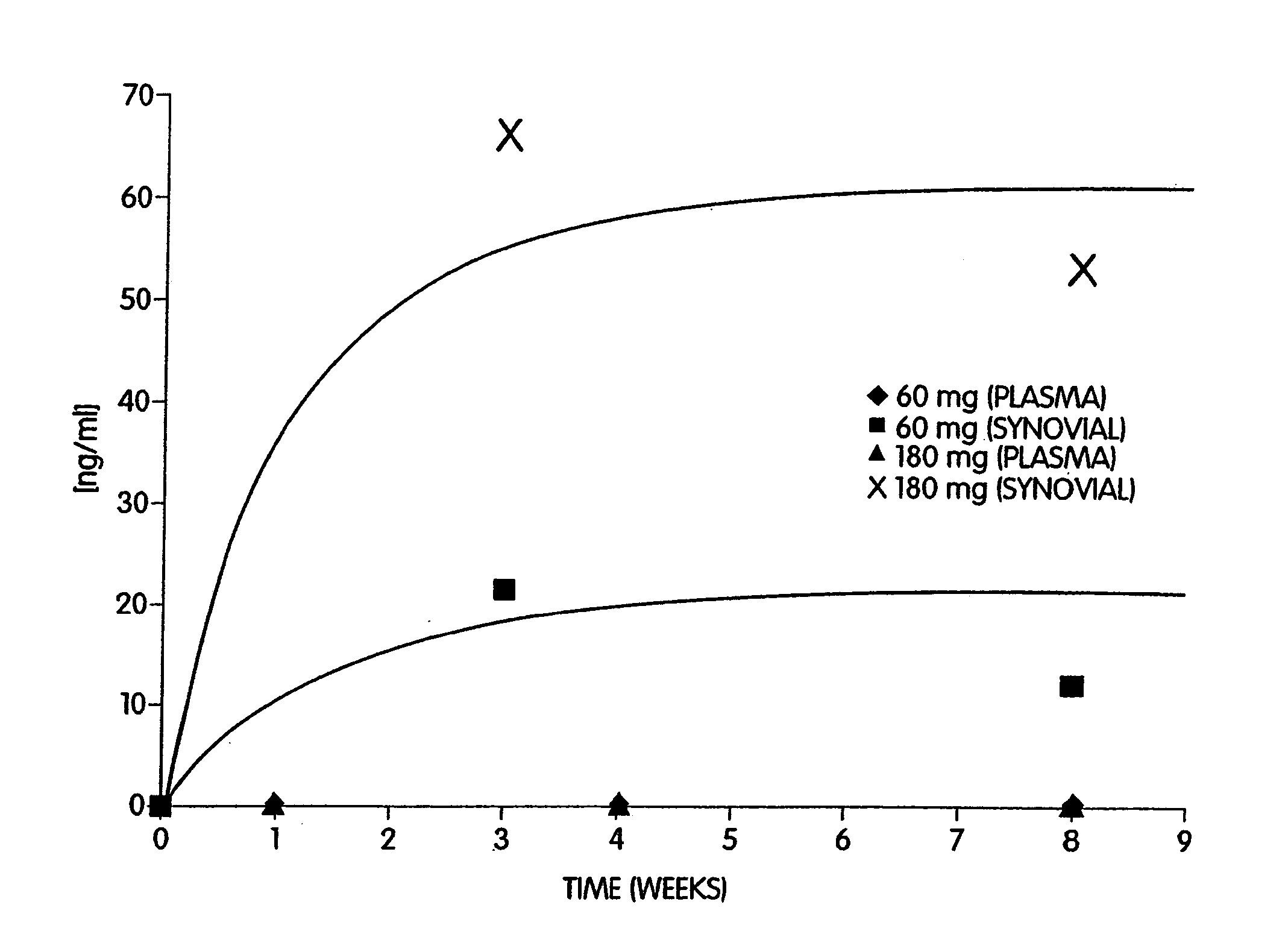
Abstract of Disclosure A therapeutically effective compound is locally administered by associating the compound with a piece of orthopedic hardware that is implanted at an appropriate site within a body. The compound is adapted, such as through a sustained release device, to administer an effective dosage continuously over an extended period of time. The compound may be administered, for example, to a joint of a mammal by intraarticularly implanting a sustained release device to deliver the therapeutically effective compound within a synovial capsule of the joint, such that synovial fluid concentration of the compound is greater than plasma concentration of the compound. A wide range of orthopedic hardware, such as bone screws and staples, may be adapted to use in the systems described herein to provide treatment for a variety of medical conditions.
Owner:EYEPOINT PHARMA US INC
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
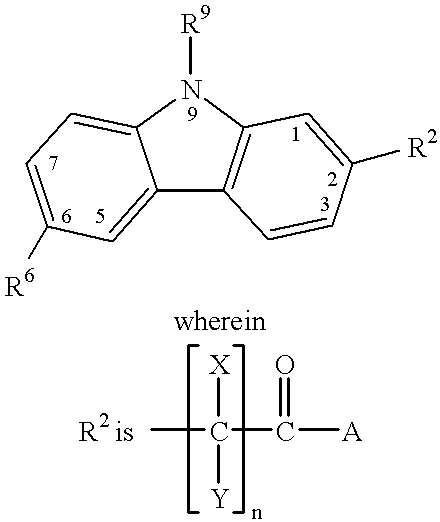
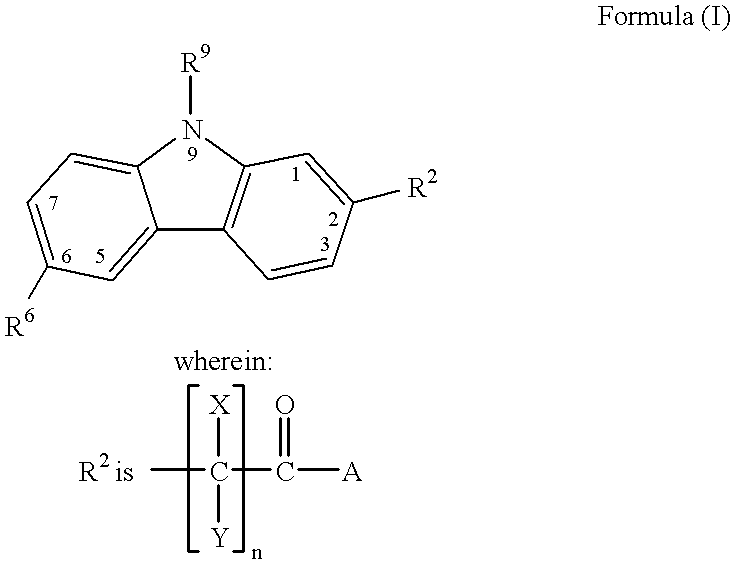
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
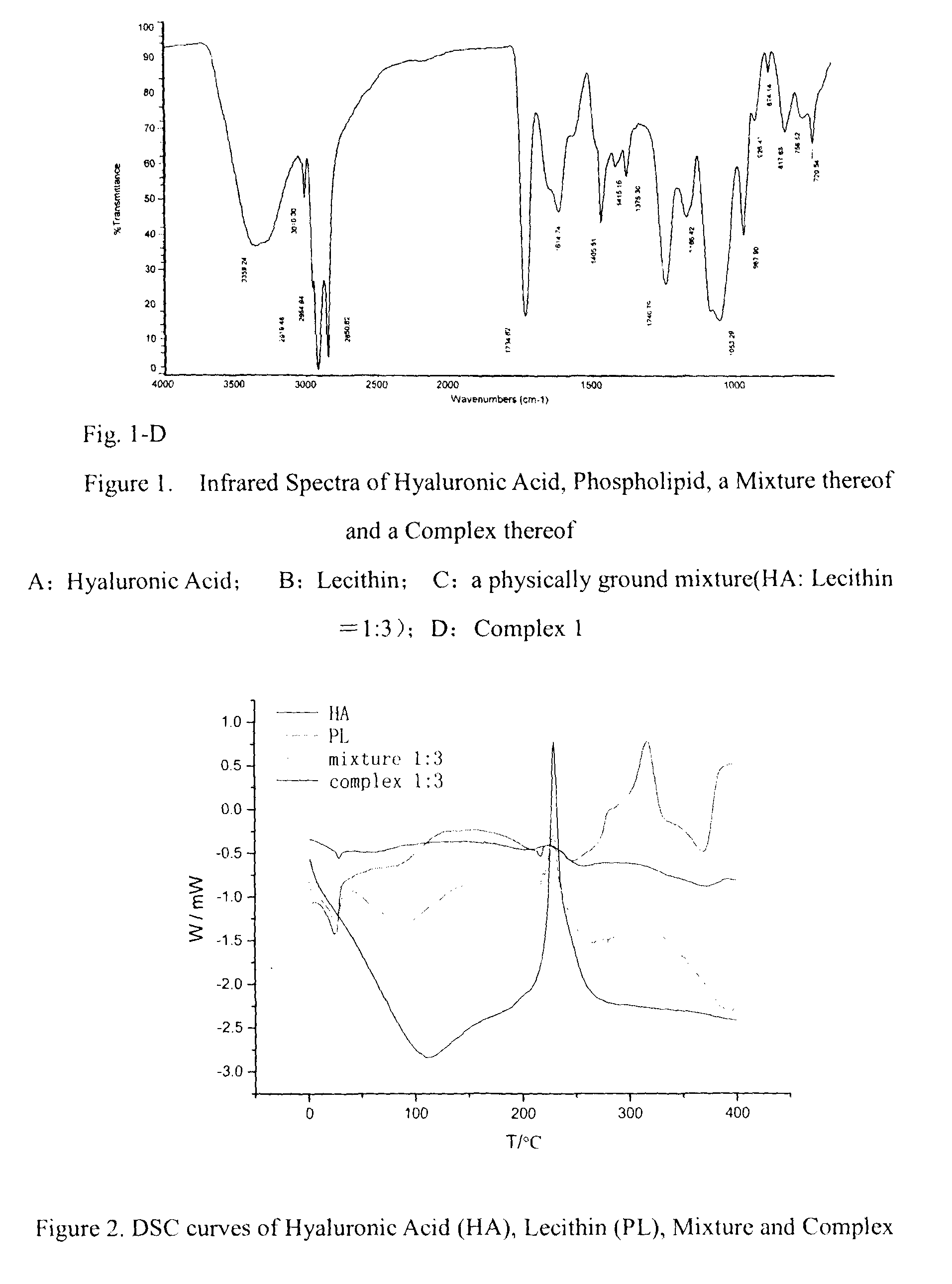
Oral agent for improving and protecting the function of joint comprising hyaluronic acid-phospholipid complexes
InactiveUS20090170808A1Improve concentrationImprove joint functionBiocideOrganic active ingredientsOral medicationArthritis
The present invention relates to an oral formulation comprising a hyaluronic acid-phospholipid complex, and to use of a hyaluronic acid-phospholipid complex for the manufacture of a joint function-improving and protecting agent for oral administration which can alleviate the arthritic symptoms in patients with arthritic conditions, increase the concentration of hyaluronic acid in synovial fluid, improve the lubrication of joints, as well as maintain and enhance the normal functions of joints.
Owner:LING PEIXUE
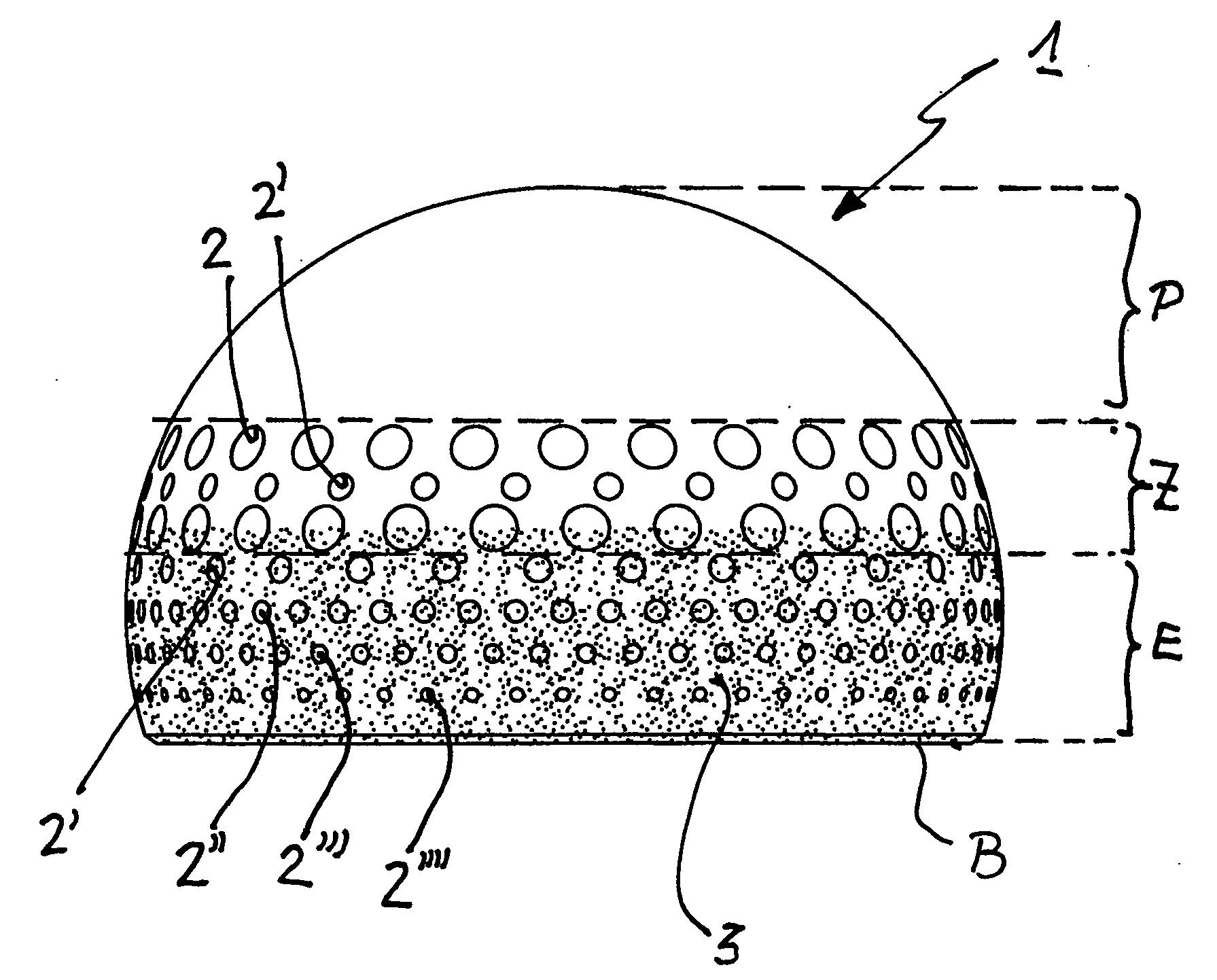
Ball joint or cap implant for an artificial hip joint
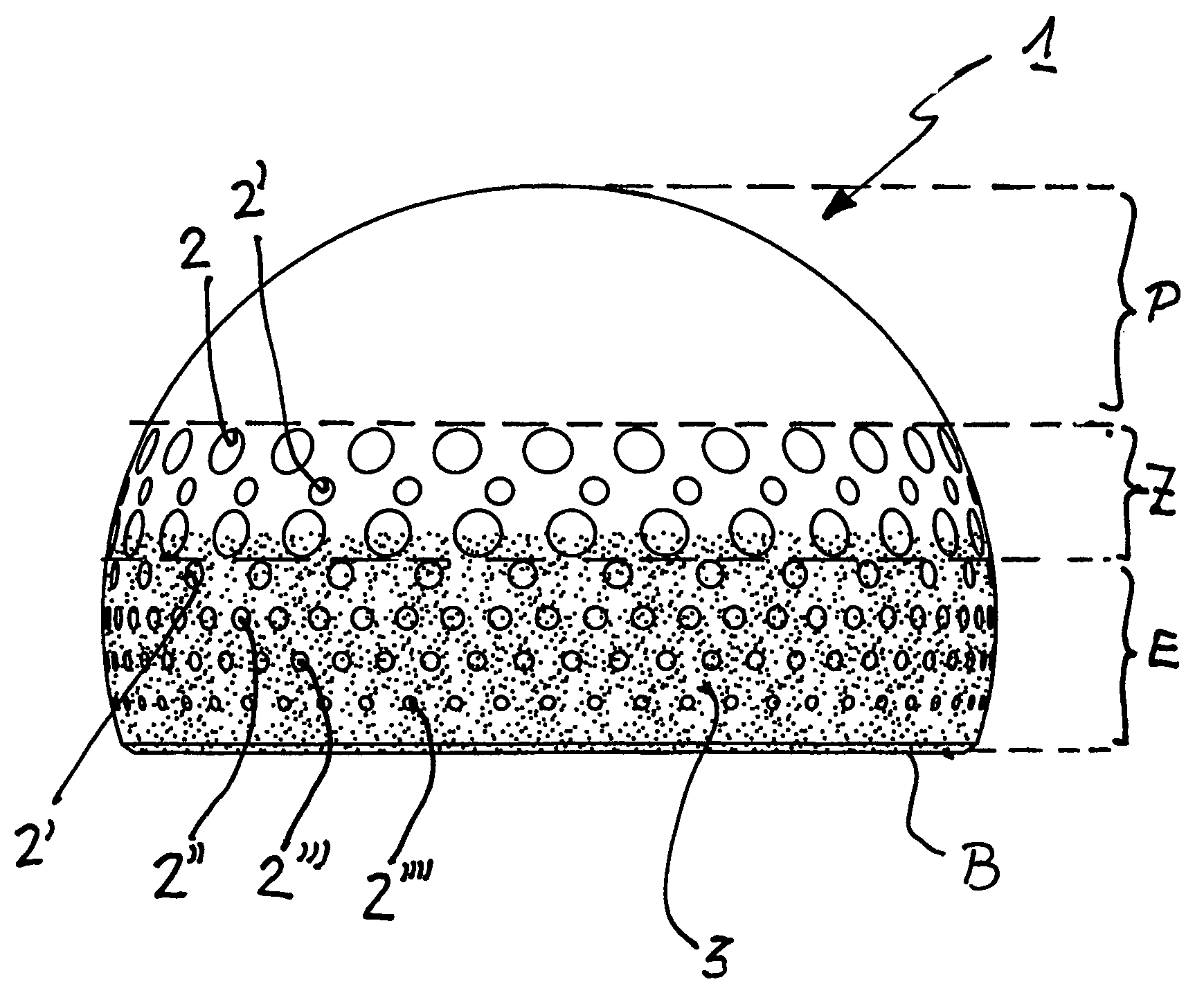
InactiveUS20090112330A1Easy to rinseEasy to replaceJoint implantsFemoral headsArtificial hip jointsSacroiliac joint
A ball joint or cap for an artificial hip joint is described that is suitable for carrying out a rotating or pivoting motion in an artificial hip joint socket, with a gap being provided for a natural fluid film (synovial fluid) between itself and the artificial hip joint socket, and with regular depressions without interconnecting channels between them being recessed in areas of its surface. It is proposed that its surface in the polar area is smooth and without depressions, that an area is provided in an intermediate area between the smooth polar area and its equator, in which depressions are recessed with hole widths between 1.0 mm to 3.0 mm, that adjacently in the intermediate area in the direction of its base edge, further depressions are recessed, the hole widths of which become steadily smaller, the closer they lie to the base edge, with hole widths between 0.5 mm and 1.0 mm.
Owner:ORTHODYNAMICS GMBH
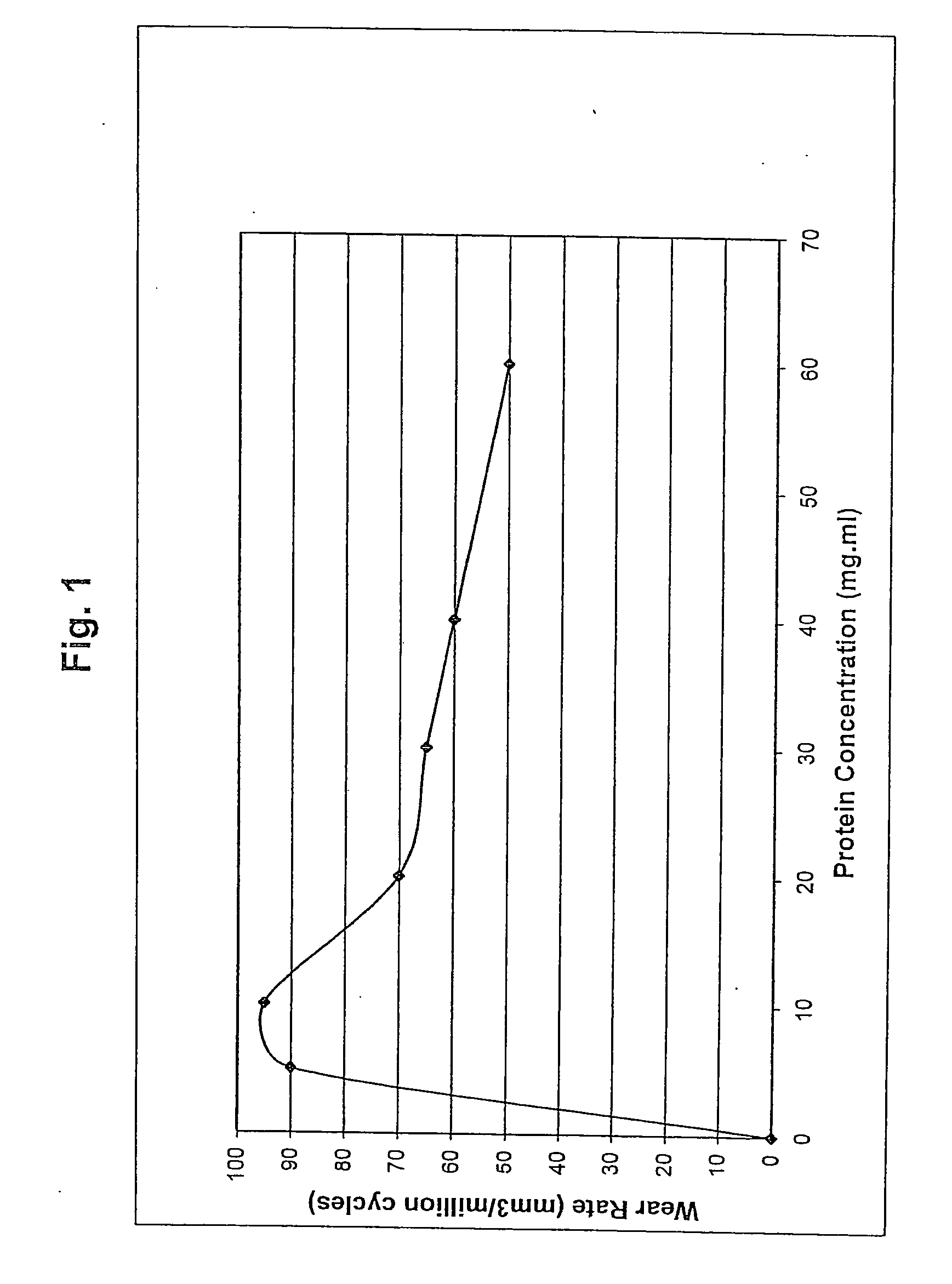
Bearing material of medical implant having reduced wear rate and method for reducing wear rate
Disclosed is a bearing material of a medical implant comprising a polymer such as UHMWPE and a surface active agent that is not covalently bonded to the polymer. The surface active agent includes a hydrophilic moiety or segment, such as an ethoxylated moiety, and a hydrophobic moiety or segment, such as an alkanol, alkenol, aromatic alcohol, alkylamine, alkenyl amine, alkyl aromatic alcohol, alkanoic acid, alkenoic acid, or any combination thereof. The bearing material has a reduced wear rate. Also disclosed is a method of reducing the wear rate of a polymeric bearing material of a medical implant when it articulates against a hard counterface in the presence of synovial fluid, the method comprising providing a surface active agent in the synovial fluid in close proximity to the bearing surface, the hard counterface, or both.
Owner:DEPUY PROD INC
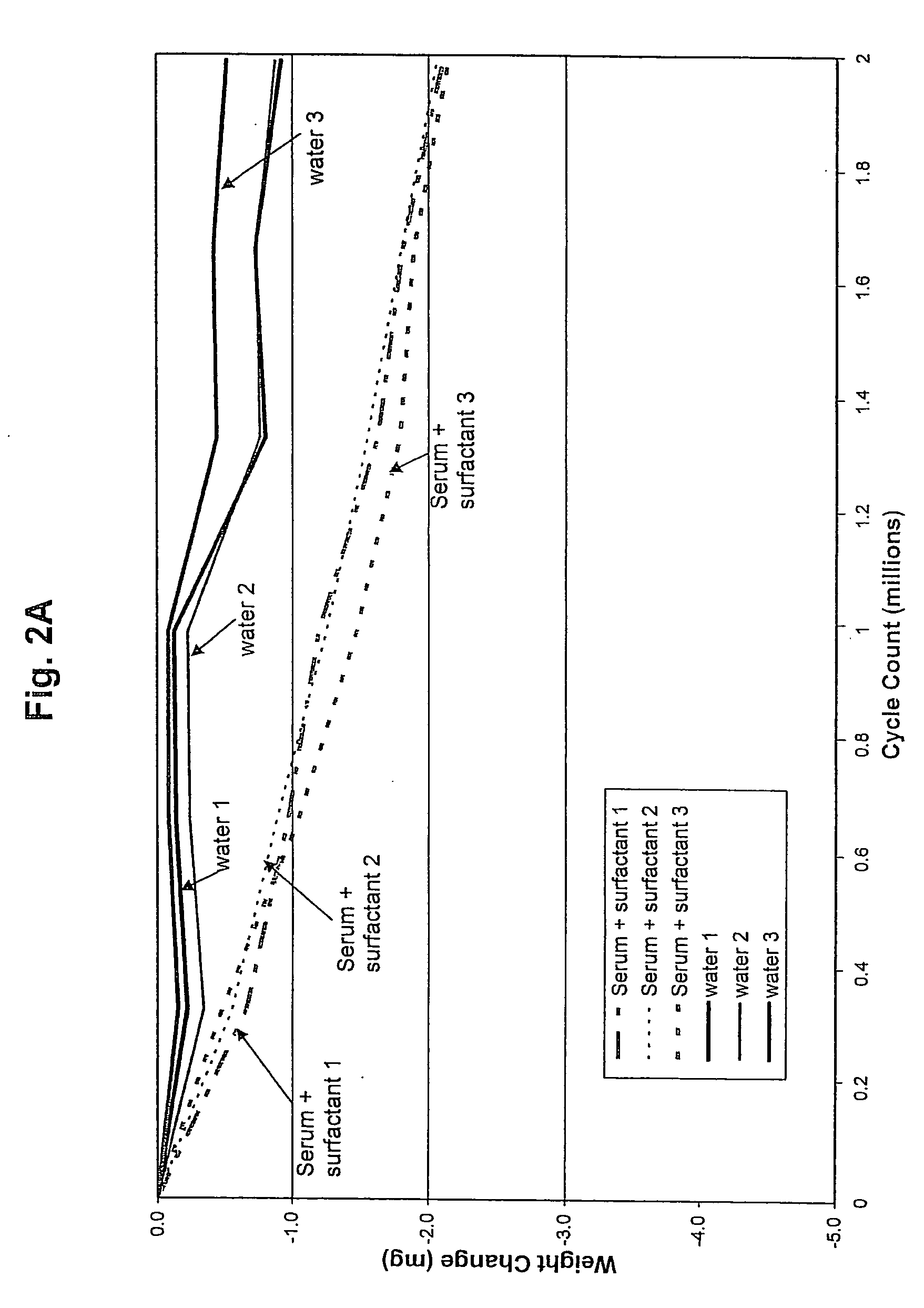
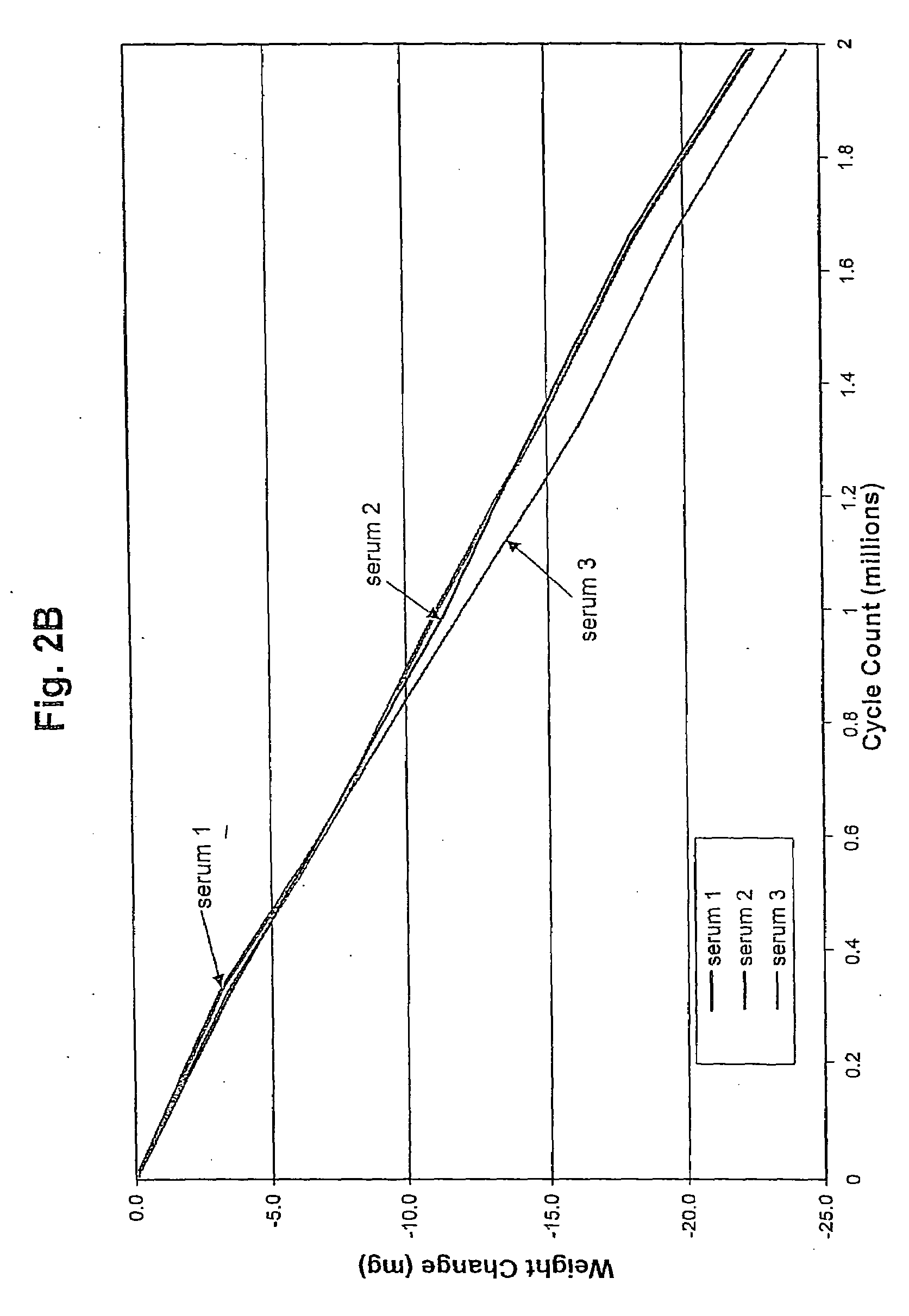
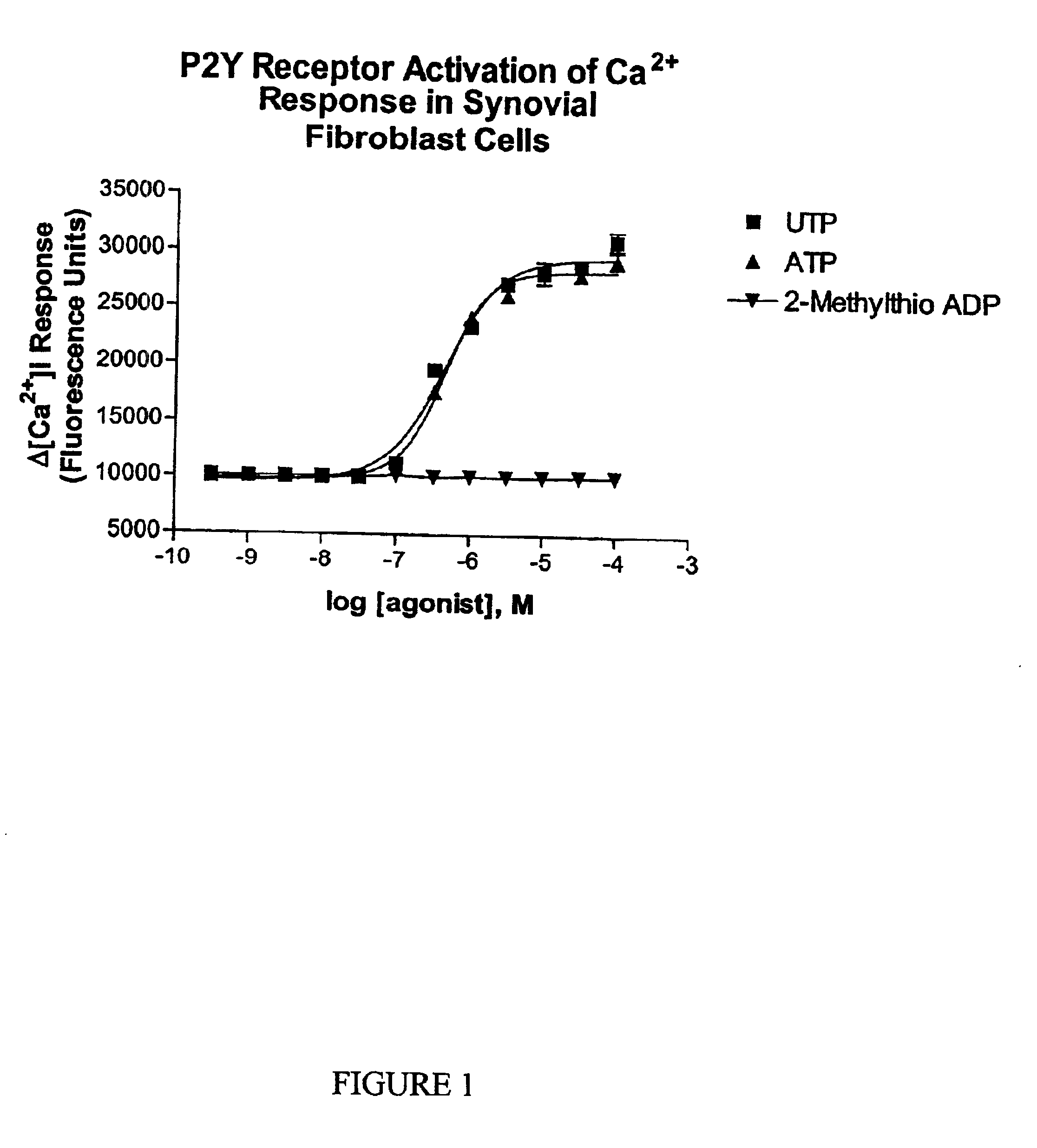
Joint lubrication with P2Y purinergic receptor agonists
InactiveUS7109181B2Enhance joint lubricationTreat osteoarthritisBiocideAerosol deliveryPhospholipidUridine Nucleotides
The present invention is directed to a method of altering the amount or composition of synovial fluids secreted from joints in a subject in need of such treatment. The method comprises administering to a subject a pharmaceutical composition comprising a P2Y purinergic receptor agonist in an amount effective to alter the amount or composition of synovial fluids. The P2Y purinergic receptor agonist is administered in an amount effective to stimulate secretion of synovial fluid, lubricin, hyaluronic acid, or surface-active phospholipids; to enhance joint lubrication; or to treat osteoarthritis. The pharmaceutical compositions useful in the present invention comprise a P2Y purinergic receptor agonist of Formula I and include, but are not limited to: uridine-, adenosine-, cytidine-5′-di- or triphosphates, dinucleoside polyphosphates, and analogs thereof. The invention is useful for treating conditions associated with reduced joint lubrication and joint stiffness, such as osteoarthritis.
Owner:INSPIRE PHARMA
Methods for improving mobility and controlling cartilage matrix degradation of weight-bearing articular joints
ActiveUS8060210B1Improve mobilityImproving mobility and qualityElectrotherapyRange of motionMuscle group
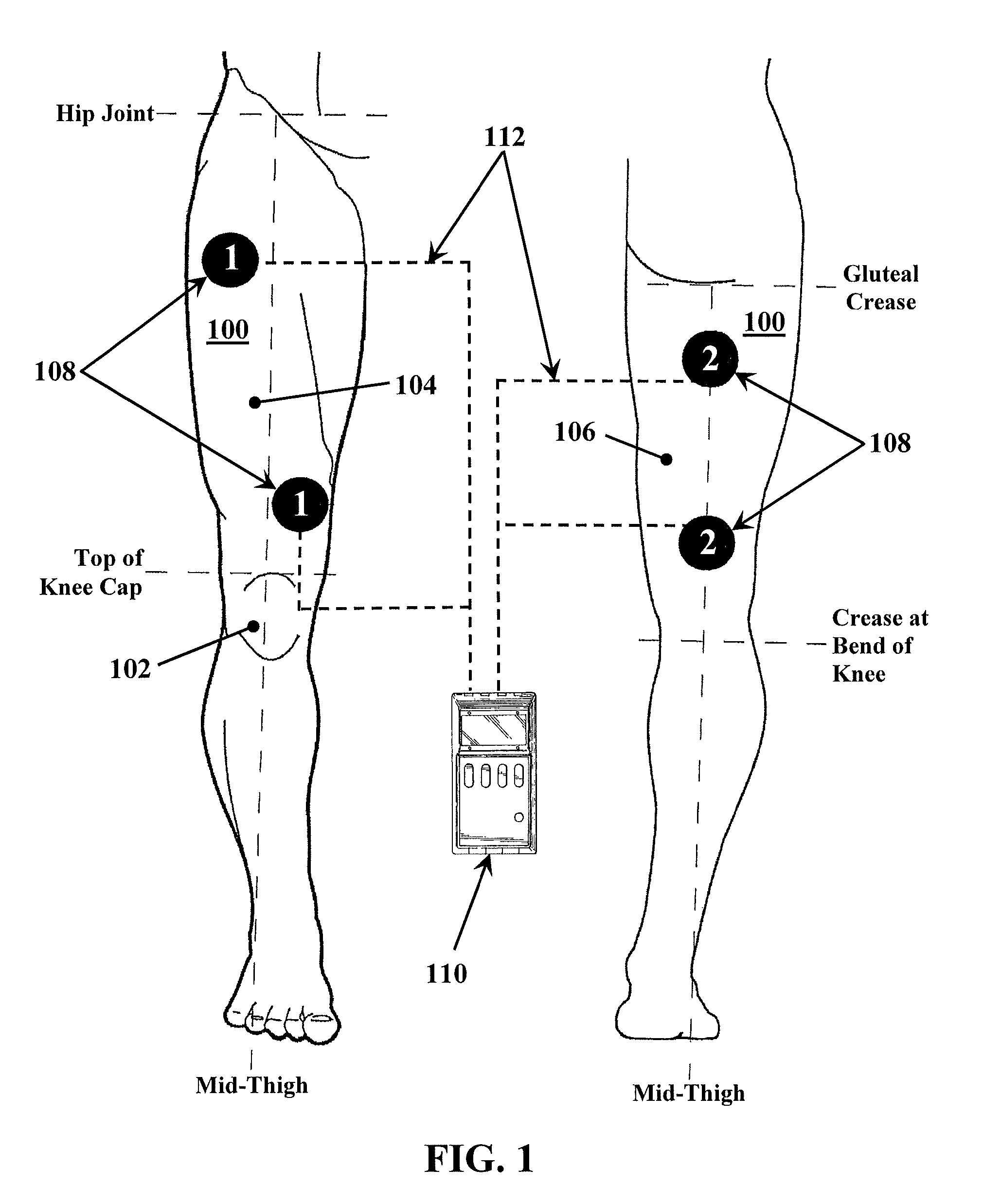
A method for improving mobility and / or the quality of synovial fluid of an affected articular joint, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The method includes positioning at least two first electrodes proximate to the at least first muscle group, positioning at least two second electrodes proximate to the at least second muscle group, and applying motor-level electrical stimulation to the at least first and second muscle groups via the at least two first and second electrodes in a multiphasic pattern corresponding to a sequence of electromyographic outputs.
Owner:MEAGAN MEDICAL
Method for the determination and the classification of rheumatic conditions
InactiveUS20100273671A1Nucleotide librariesMicrobiological testing/measurementRheumatismClassification methods
Methods for the determination and the classification of a rheumatic condition in a synovial sample of a subject afflicted with a rheumatic condition are disclosed. Methods include the steps of determining in the level of expression of at least 20 genes or gene fragments in the synovial sample and identifying whether the level of expression of the genes in the sample correlates with the presence of a rheumatic condition. Kits are also described for the determination and the classification of rheumatic conditions which contain a low density microarray having probes suitable for hybridizing with at least 20 genes or gene fragments. On the basis of the hybridization results, the rheumatic condition is determined.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN +1
Means and Methods for Detecting Bacteria in an Aerosol Sample
This disclosure provides a method for detecting and / or identifying uncultured bacteria. The sample is an aerosol sample selected from a group consisting of cough, sneeze, saliva, mucus, bile, urine, vaginal secretions, middle ear aspirate, pus, pleural effusions, synovial fluid, abscesses, cavity swabs, serum, blood and spinal fluid. The method comprises obtaining absorption spectra (AS) of the sample, extracting and processing the acquired data, thereby detecting and / or identifying the bacteria.
Owner:OPTICUL DIAGNOSTICS LTD
Apparatus and method for stabilizing, improving mobility, and controlling cartilage matrix degradation of weight-bearing articular joints
An apparatus and method for improving mobility and / or the quality of synovial fluid of an affected articular joint are disclosed, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The apparatus and method include an electro-medical device configured to apply motor-level electrical stimulation in a multiphasic pattern via at least a first channel and at least a second channel, the multiphasic pattern being programmed into the electro-medical device and corresponding to the sequence of an electromyographic output for the joint; at least two first electrodes connected to the at least first channel of said electro-medical device, the at least two first electrodes being positioned proximate to the at least first muscle group; at least two second electrodes connected to the at least second channel of said electro-medical device, the at least two second electrodes being positioned proximate to the at least second muscle group; and an applicator configured to be worn on the articular segment such that the at least two first electrodes and the at least two second electrodes are disposed between the applicator and the articular segment, the applicator being further configured to reduce compressive forces on at least one compartment of the affected joint.
Owner:MEAGAN MEDICAL
Device and method for treating conditions of a joint
A therapeutically effective compound is locally administered by associating the compound with a piece of orthopedic hardware that is implanted at an appropriate site within a body. The compound is adapted, such as through a sustained release device, to administer an effective dosage continuously over an extended period of time. The compound may be administered, for example, to a joint of a mammal by intraarticularly implanting a sustained release device to deliver the therapeutically effective compound within a synovial capsule of the joint, such that synovial fluid concentration of the compound is greater than plasma concentration of the compound. A wide range of orthopedic hardware, such as bone screws and staples, may be adapted to use in the systems described herein to provide treatment for a variety of medical conditions.
Owner:CONTROL DELIVERY SYST INC
Joint-protecting beverages and/or foods and their preparations
InactiveUS20040198695A1Overcome low absorptionPromote absorptionBiocideVitamin food ingredientsAdditive ingredientMedicine
A series of beverages and / or foods, which have protecting and enhancing effects on human's joints and bones and can therefore prevent and treat osteoarthritis, is provided. The beverages and / or foods comprise one or more cartilage- and synovial fluid-enhancing active ingredients or combinations of these ingredients with bone-enhancing calcium and phosphorus additives. Active ingredients in these beverages and / or foods are taken in liquid forms. So they can be easily absorbed and utilized thoroughly by human body.
Owner:LI ANHU +1
Method of treatment for osteoarthritis by local intra-articular injection of microparticles
A method of treatment of osteoarthritis is described, where a therapeutically effective amount of a composition having biodegradable microparticles in an aqueous vehicle is delivered into the intra-articular space of a joint. In one aspect, the microparticle-containing composition is injected into the synovial fluid-containing portion of an affected joint.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Concentrated Protein Preparations of Bone Morphogenetic Proteins and Methods of Use Thereof
InactiveUS20100144631A1Efficient preparationHigh recovery rateSenses disorderNervous disorderDiseaseWhole body
Disclosed herein are heretofore undescribed preparations of highly concentrated, solubilized proteins, such as but not limited to, Bone Morphogenetic Proteins. Such protein preparations can be formulated in an aqueous carrier at protein concentrations in excess of 10 mg / ml when using the methods of manufacture taught herein. Such methods yield stable protein preparations in either solubilized or lyophilized form. The protein preparations of the present invention are particularly beneficial when administered either locally or systemically, in part, because low administration volumes can be accomplished. This is especially important for local treatment of certain anatomic locations such as, for example, the synovial fluid of a joint when treating osteoarthritis with BMP-7 or the intradiscal space when treating degenerative disc disease with BMP-7.
Owner:MARIEL THERAPEUTICS
Device & method for restoring joints with artificial cartilage
ActiveUS20100125341A1Minimize surgical interventionMinimize human tissue resectionBone implantJoint implantsFemoral boneSacroiliac joint
An intra-articular device comprises a membrane shaped like a cap having a peripheral geometry similar to that of a head of a bone for a joint to be restored and an open end sized to be applied over the bone proximate the head, so that the open end can be stretched over the head of the bone and held in position on the bone interposed between the head and its corresponding articular component of the joint. The membrane is made of a polyether-urethane-urea material selected to have a property of absorbing the joint's own synovial fluid so as to swell and have a viscoelastic property similar to the body's own articular hyaline cartilage. In a preferred embodiment, the membrane cap is adapted for use on a femoral bone for restoring a hip joint. A related method of installing an intra-articular device as artificial cartilage comprises forming a membrane cap to be applied over the head of the bone of the joint, surgically exposing the head of the bone, installing the membrane cap over the head of the bone, then repositioning the capped head of the bone back in its place in the joint with the membrane interposed as artificial cartilage.
Owner:SOFTJOINT CORP
Semi-interpenetrating or interpenetrating polymer networks for drug delivery and tissue engineering
Compositions for tissue engineering and drug delivery have been developed based on solutions of two or more polymers which form semi-interpenetrating or interpenetrating polymer networks upon exposure to active species following injection at a site in a patient in need thereof. The polymers crosslink to themselves but not to each other; semi-interpenetrating networks are formed when only one of the polymers crosslink. The resulting viscous solutions retain the biologically active molecules or cells at the site of injection until release or tissue formation, respectfully, occurs.As a result of studies conducted with polymer-cell suspensions forming interpenetrating polymer networks, it has been determined that polymer solutions can be formulated wherein the active species is provided by exposure of the polymer solution to an exogenous source of active species, typically electromagnetic radiation, preferably light. Studies demonstrate that light will penetrate through skin, body fluids (such as synovial fluid) and membranes and polymerize the polymer solutions. The polymer solutions can be crosslinked ionically or covalently, to form a hydrogel, semi-interpenetrating polymer network or an interpenetrating polymer network.
Owner:UNIV OF COLORADO THE REGENTS OF
Method for classifying and counting bacteria in body fluids
InactiveUS20100021878A1Easy to distinguishRemove noise signalMicrobiological testing/measurementBiological particle analysisFluorescenceWhite blood cell
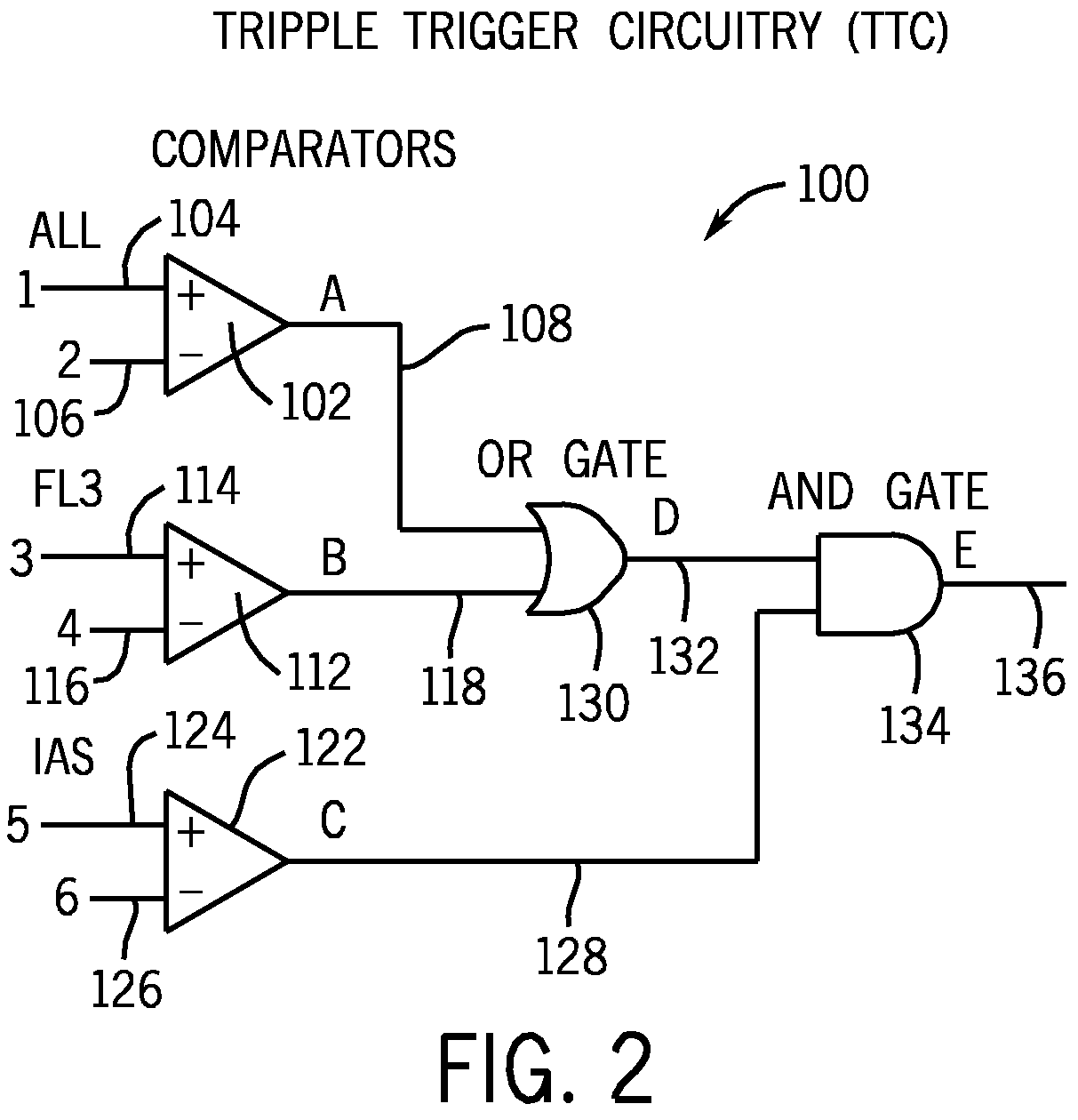
A method for distinguishing erythroblasts from bacteria by automated hematology analyzers, such as, for example, the CELL-DYN® 4000 automated hematology analyzer and the CELL-DYN® Sapphire™ automated hematology analyzer. Bacterial cells scatter light and fluoresce differently than do red blood cells, white blood cells, erythroblast nuclei, and platelets. Signals generated by bacteria are distinguishable from those of erythroblasts because the signals generated by erythroblast nuclei are sufficiently unique that erythroblast nuclei can be distinguished from signals generated by bacteria. Signals generated by platelets, lysed red blood cell ghosts, and other cell debris are blocked by the triple-trigger circuitry of the hematology analyzer, because all of the signals generated by noise are below the AND / OR thresholds. Algorithm(s) in the software of the system detect and count signals generated by bacteria by means of the location and the shape of the signals generated by bacteria and calculate the concentration of bacteria per unit of body fluid. In addition, certain body fluids, such as, for example, synovial fluid, can be pretreated with a viscosity reducing agent for a short period of time to reduce the viscosity of the body fluid prior to analyzing a sample of the body fluid by an automated hematology analyzer.
Owner:ABBOTT LAB INC
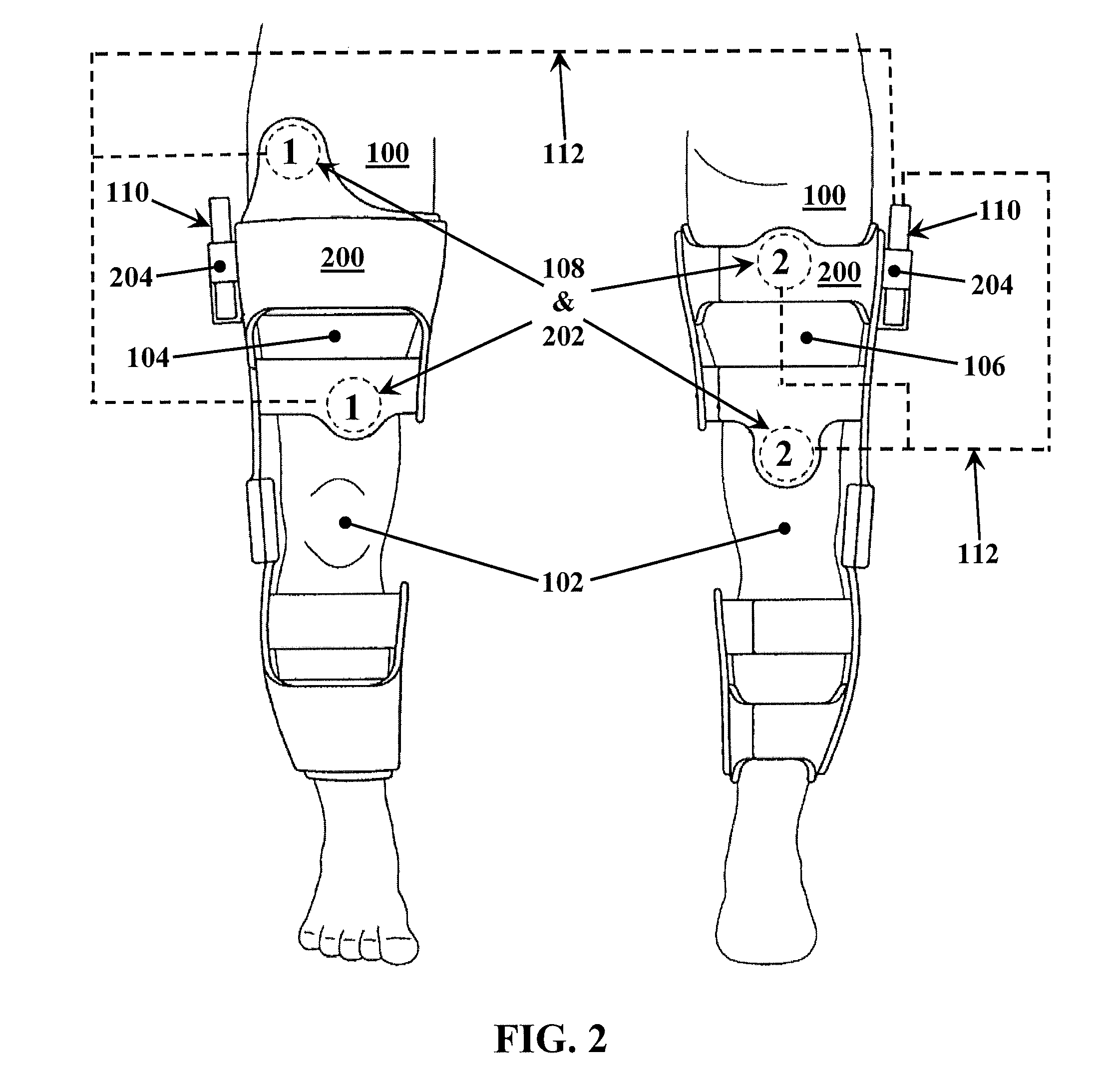
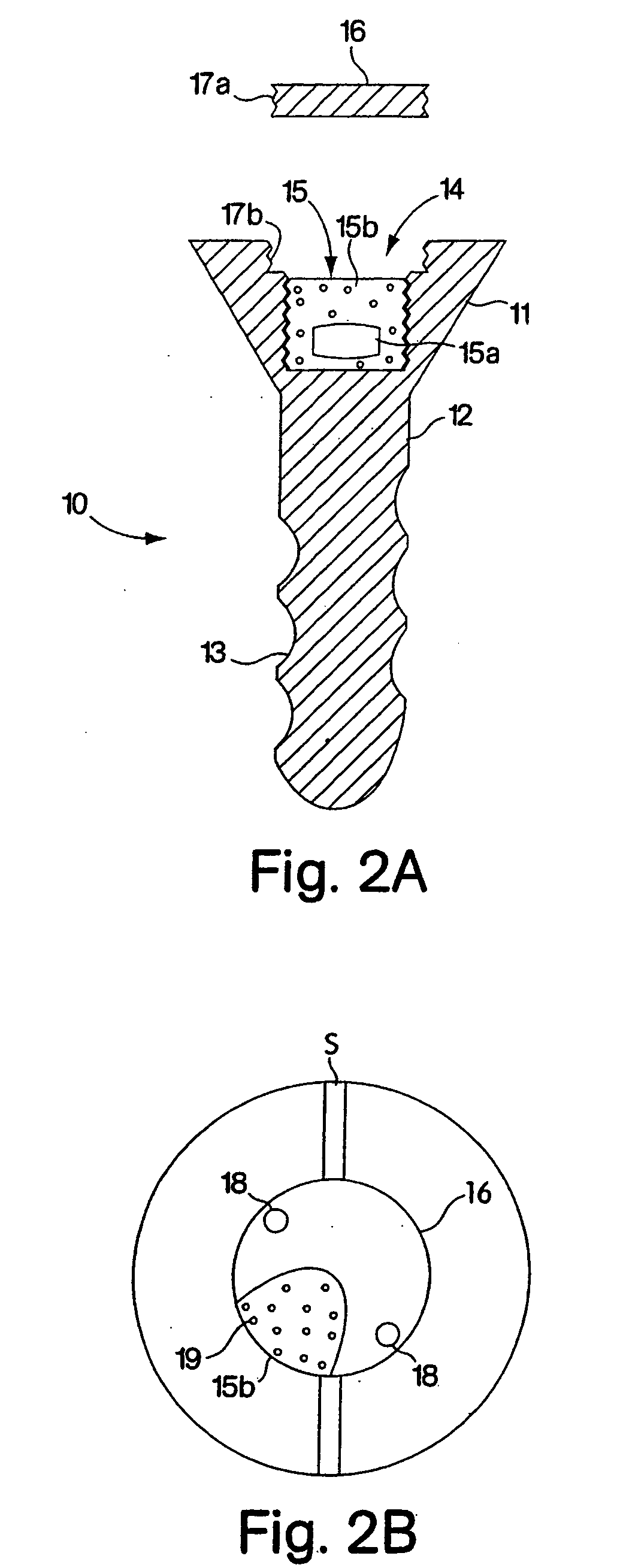
Bicortical tibial fixation of ACL grafts
A method of securing a graft in a bone tunnel, in which graft is secured within the tunnel at both the entrance and the exit ends of the tunnel to provide bicortical fixation of the graft in the bone. Interference screws or other fixation devices are used to secure the graft within the tunnel. For tibial tunnel fixation using an interference screw, the back end of the distal screw is angled so that it closely approximates the angle of the outer tibial tunnel rim. The distal screw is non-cannulated to prevent hematomas from being formed by blood flowing from the tibial tunnel into the surrounding soft tissue. The proximal screw has a restricted cannula to minimize the flow of synovial fluid entering the tibial tunnel. Advantageously, the space between the two screws fills with blood to promote faster healing and incorporation of the graft in the tibial tunnel.
Owner:ARTHREX
Method and apparatus for electromagnetic human and animal immune stimulation and/or repair systems activation
InactiveUS20100099942A1Decrease pathogen loadGood effectElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseAlertness
A method and device that can deliver a time-varying electric field non-invasively inside of biological tissue and / or biological fluid such as blood and / or lymph and / or synovial fluid and / or interstitial fluid and / or other fluids in-vivo and with magnitudes that do not cause a tolerable amount of neural motor and sensory action potential generation and frequency components below the megahertz range by means of the placement of more than one electrode on the skin with or without some intermediate biological, synthetic, or natural agent or substance between the electrode and the skin and / or by induction by means of a time-varying magnetic field or both with the novel purpose of activation and / or potentiation and / or normalization and / or regulation and / or stimulation and / or signaling and / or increase and / or regulation of the alertness and / or self tuning of the immune system or any of its parts or components directly or indirectly, locally and / or globally with the purpose of pathogen load reduction and / or reduction of the disease symptoms and / or clinical improvement and / or tumor size reduction and / or malignancy reduction or amelioration and / or improvement of the effects of an autoimmune disorder or any other related condition that could be treated or remised by its effects.
Owner:PORTELLI LUCAS
Method of treatment for osteoarthritis by local intra-articular injection of microparticles
A method of treatment of osteoarthritis is described, where a therapeutically effective amount of a composition having biodegradable microparticles in an aqueous vehicle is delivered into the intra-articular space of a joint. In one aspect, the microparticle-containing composition is injected into the synovial fluid-containing portion of an affected joint.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Apparatus and method for stabilizing, improving mobility, and controlling cartilage matrix degradation of weight-bearing articular joints
An apparatus and method for improving mobility and / or the quality of synovial fluid of an affected articular joint are disclosed, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The apparatus and method include an electro-medical device configured to apply motor-level electrical stimulation in a multiphasic pattern via at least a first channel and at least a second channel, the multiphasic pattern being programmed into the electro-medical device and corresponding to the sequence of an electromyographic output for the joint; at least two first electrodes connected to the at least first channel of said electro-medical device, the at least two first electrodes being positioned proximate to the at least first muscle group; at least two second electrodes connected to the at least second channel of said electro-medical device, the at least two second electrodes being positioned proximate to the at least second muscle group; and an applicator configured to be worn on the articular segment such that the at least two first electrodes and the at least two second electrodes are disposed between the applicator and the articular segment, the applicator being further configured to reduce compressive forces on at least one compartment of the affected joint.
Owner:MEAGAN MEDICAL
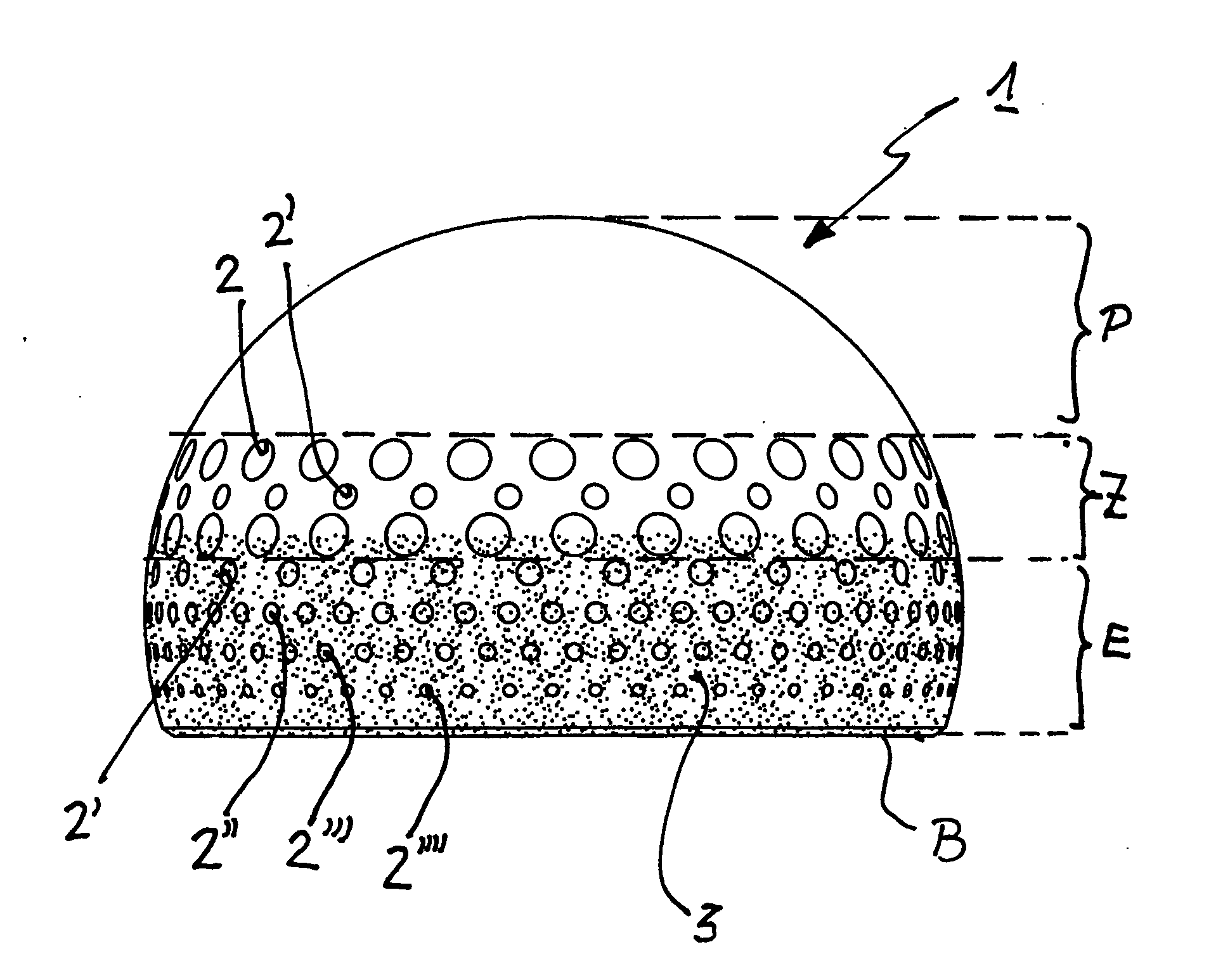
Ball joint or cap implant for an artificial hip joint
InactiveUS7771485B2Good rinsing and replacementJoint implantsFemoral headsArtificial hip jointsEngineering
A ball joint or cap for an artificial hip joint is described that is suitable for carrying out a rotating or pivoting motion in an artificial hip joint socket, with a gap being provided for a natural fluid film (synovial fluid) between itself and the artificial hip joint socket, and with regular depressions without interconnecting channels between them being recessed in areas of its surface. It is proposed that its surface in the polar area is smooth and without depressions, that an area is provided in an intermediate area between the smooth polar area and its equator, in which depressions are recessed with hole widths between 1.0 mm to 3.0 mm, that adjacently in the intermediate area in the direction of its base edge, further depressions are recessed, the hole widths of which become steadily smaller, the closer they lie to the base edge, with hole widths between 0.5 mm and 1.0 mm.
Owner:ORTHODYNAMICS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com