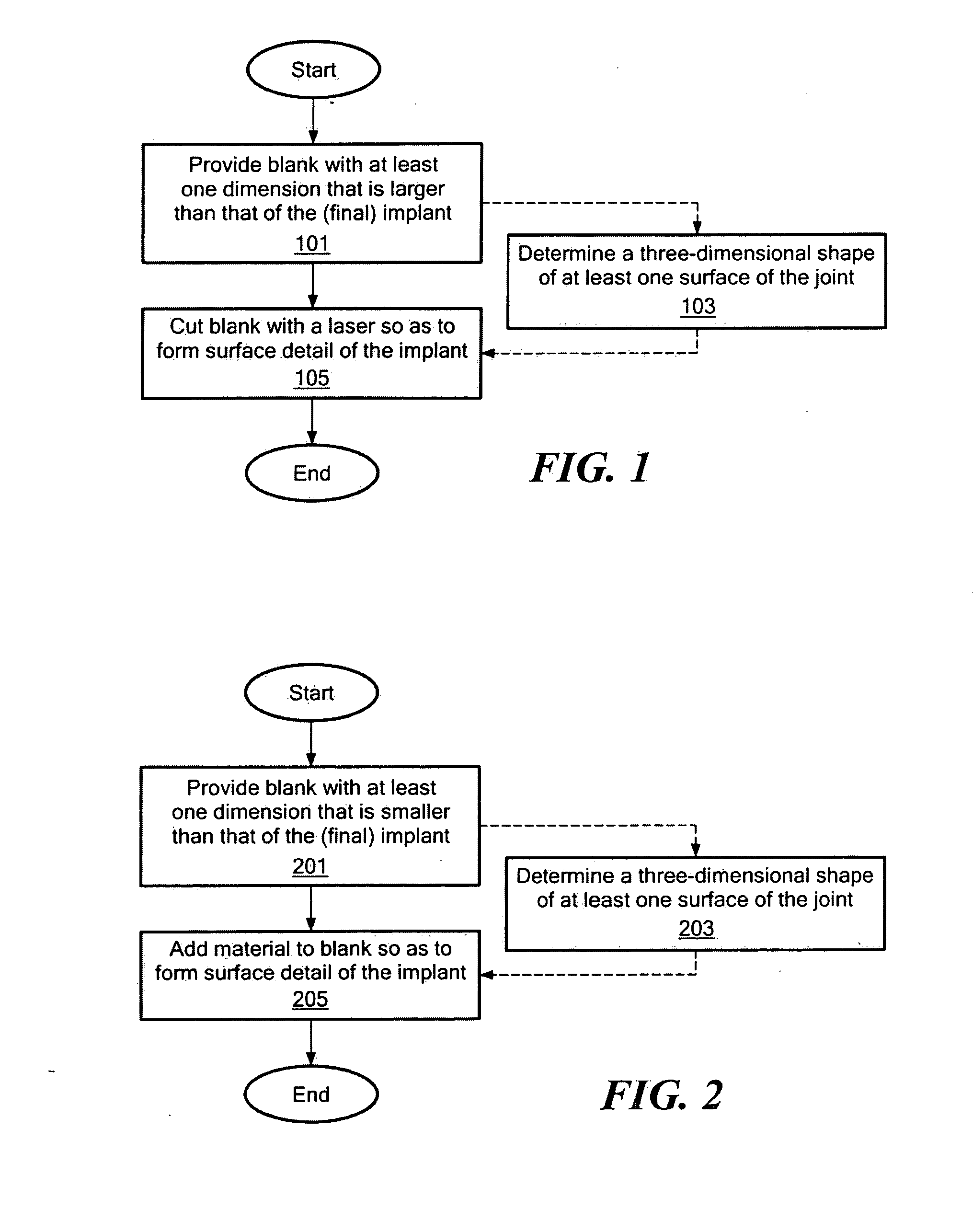
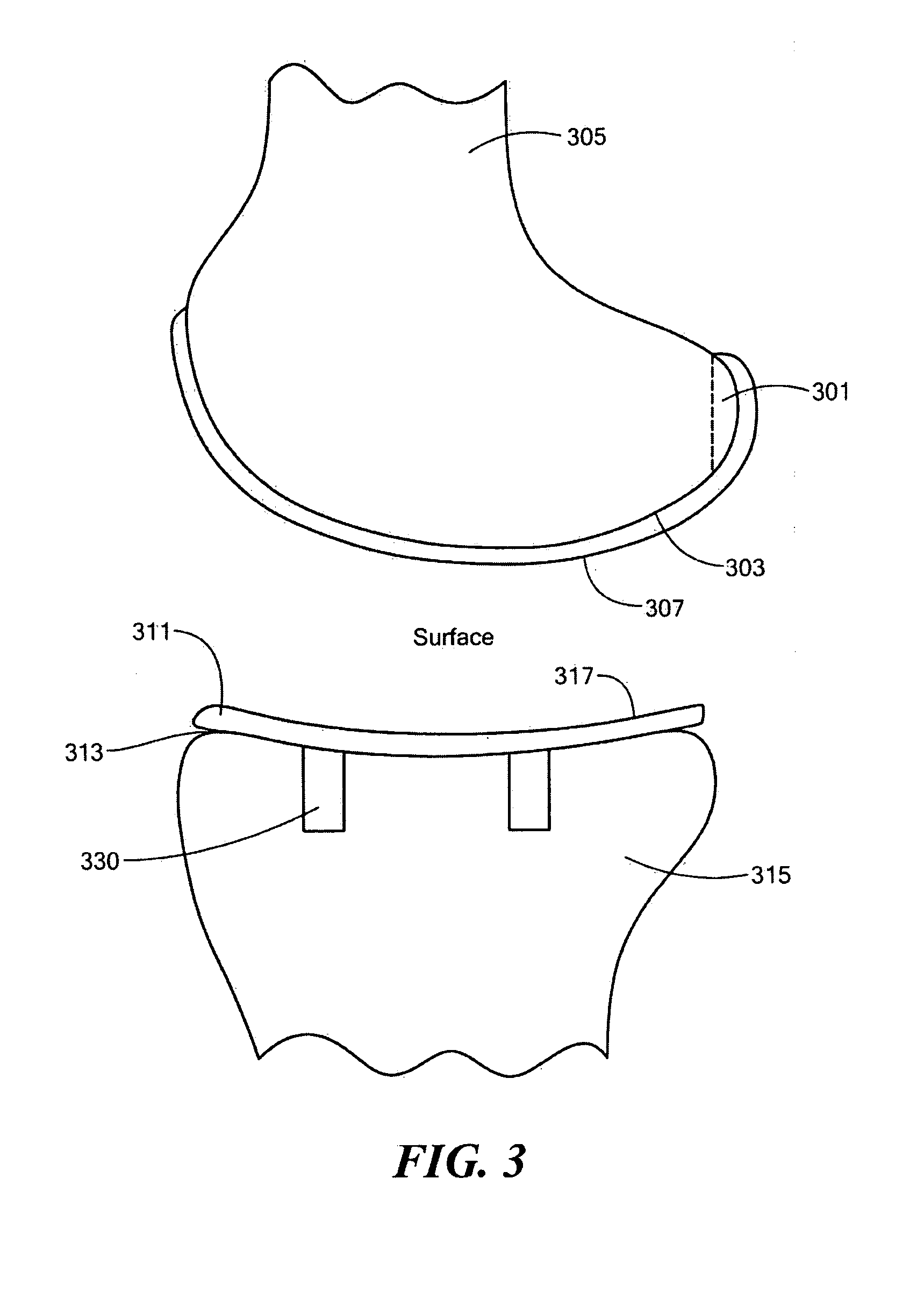
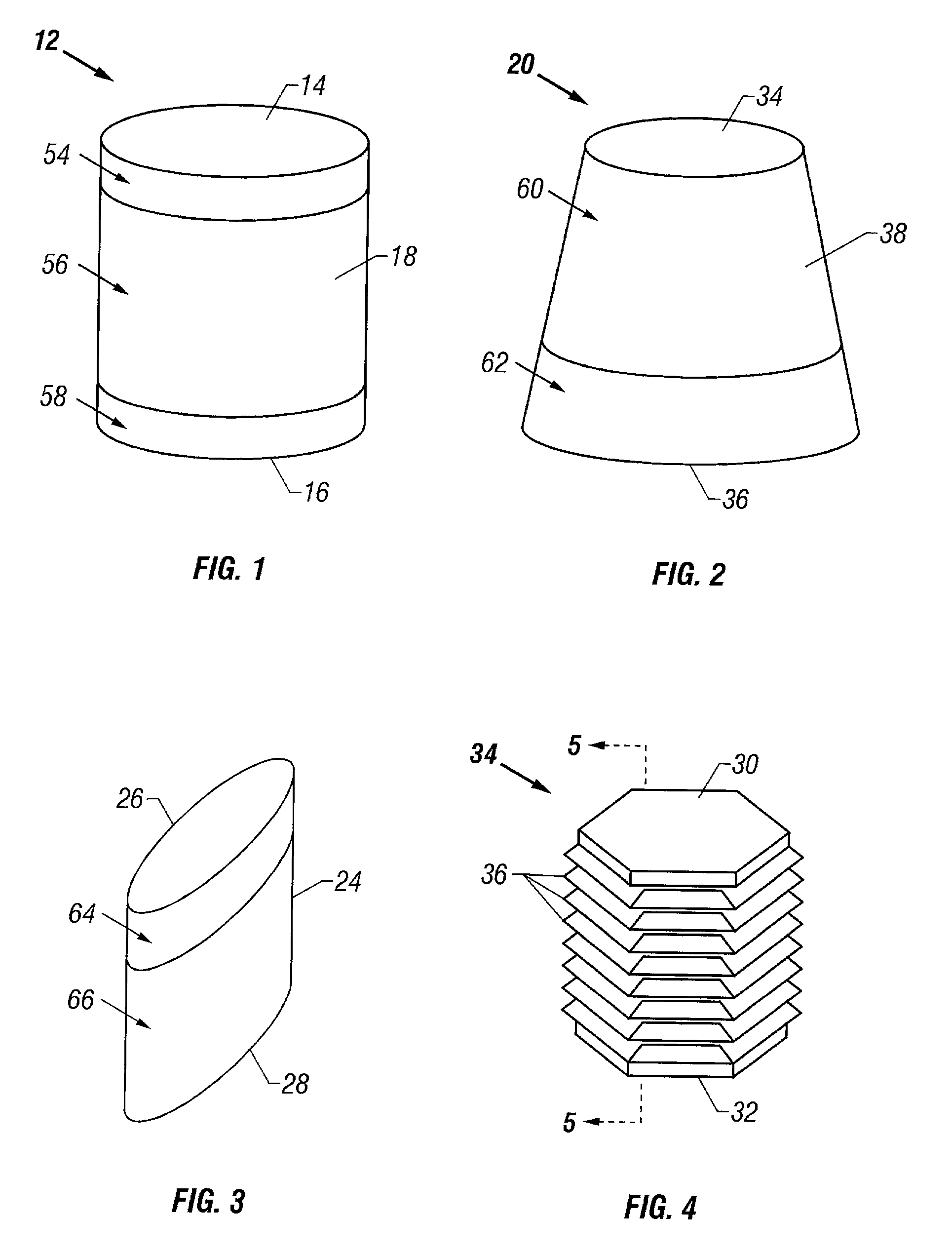
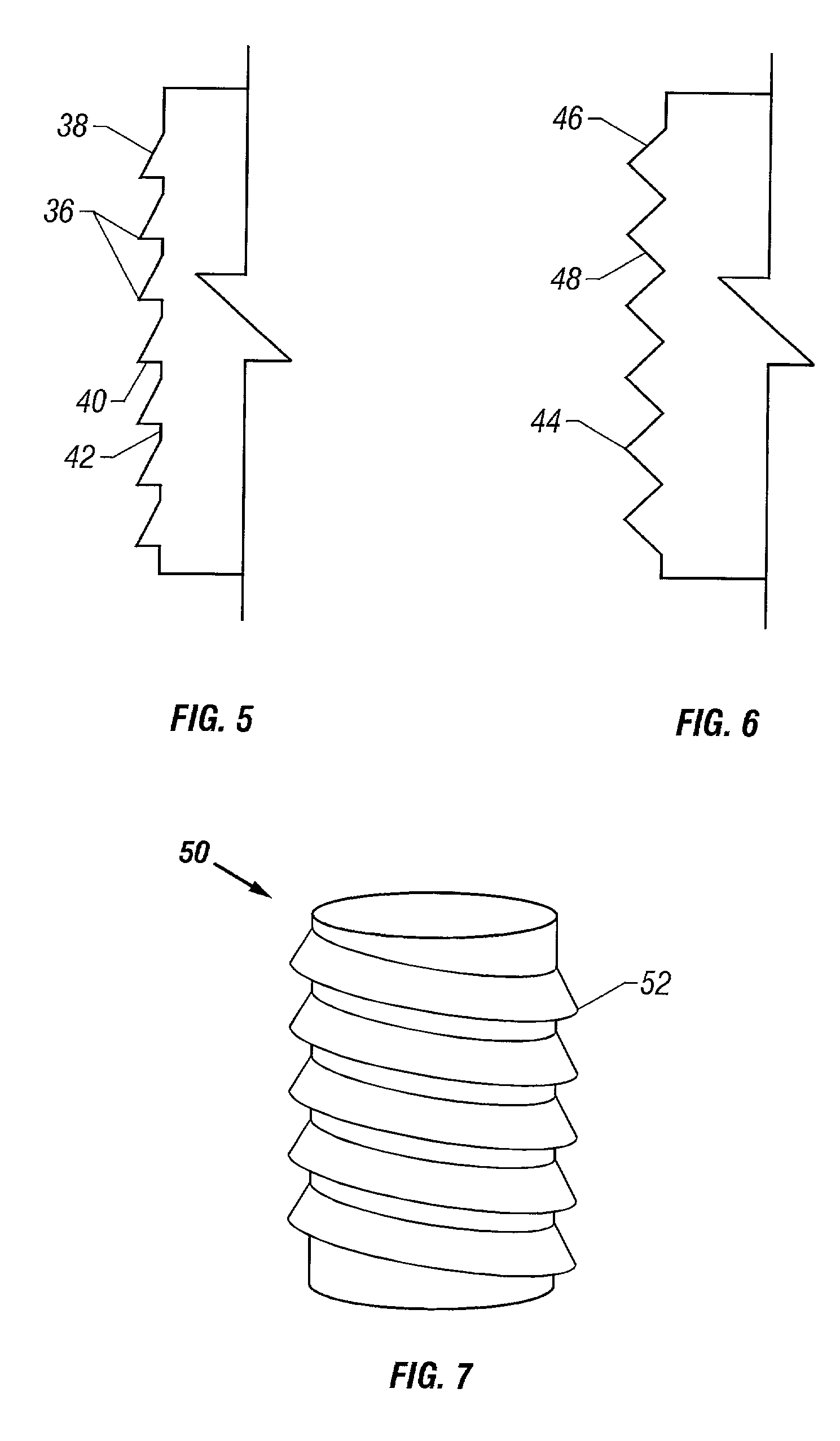
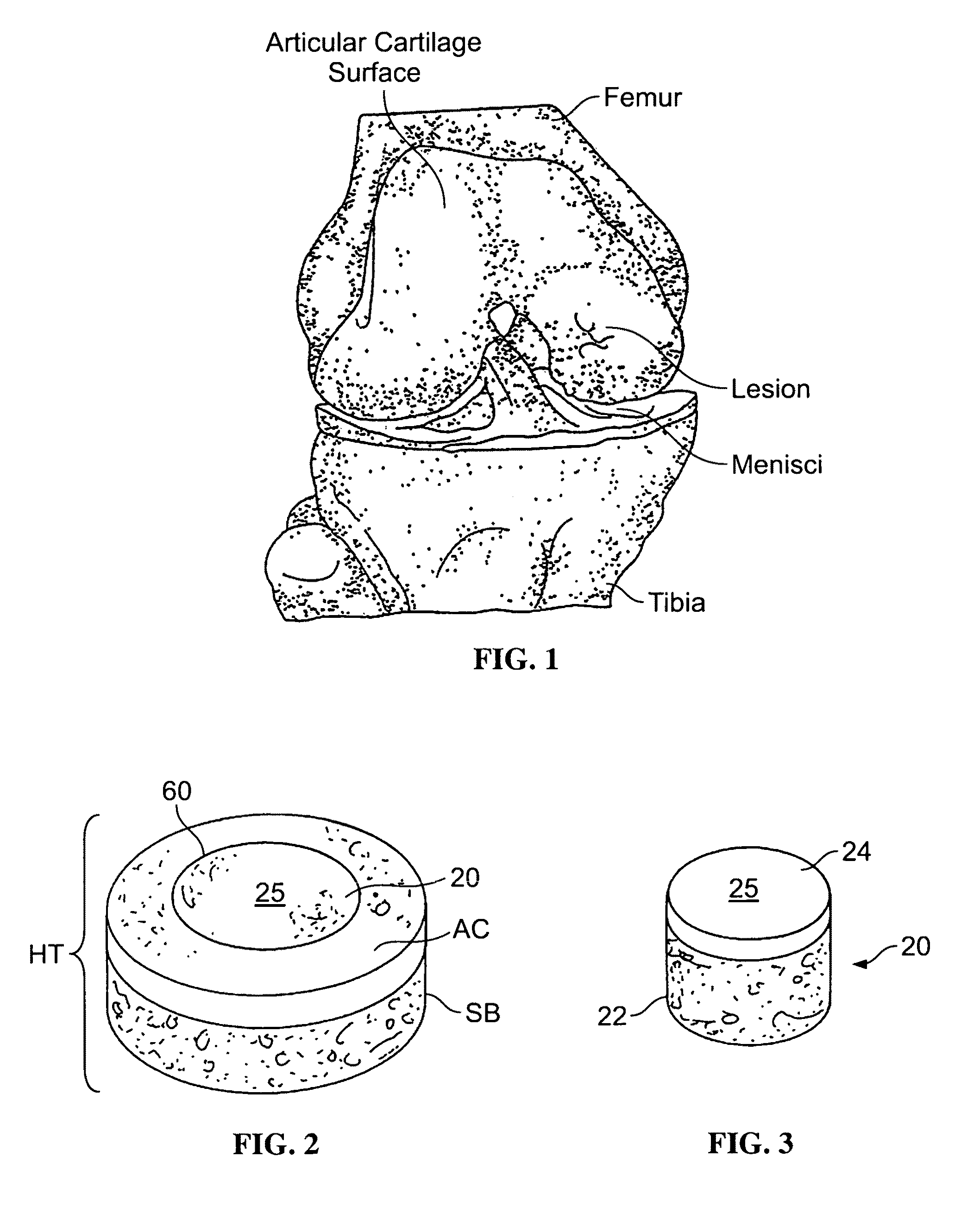
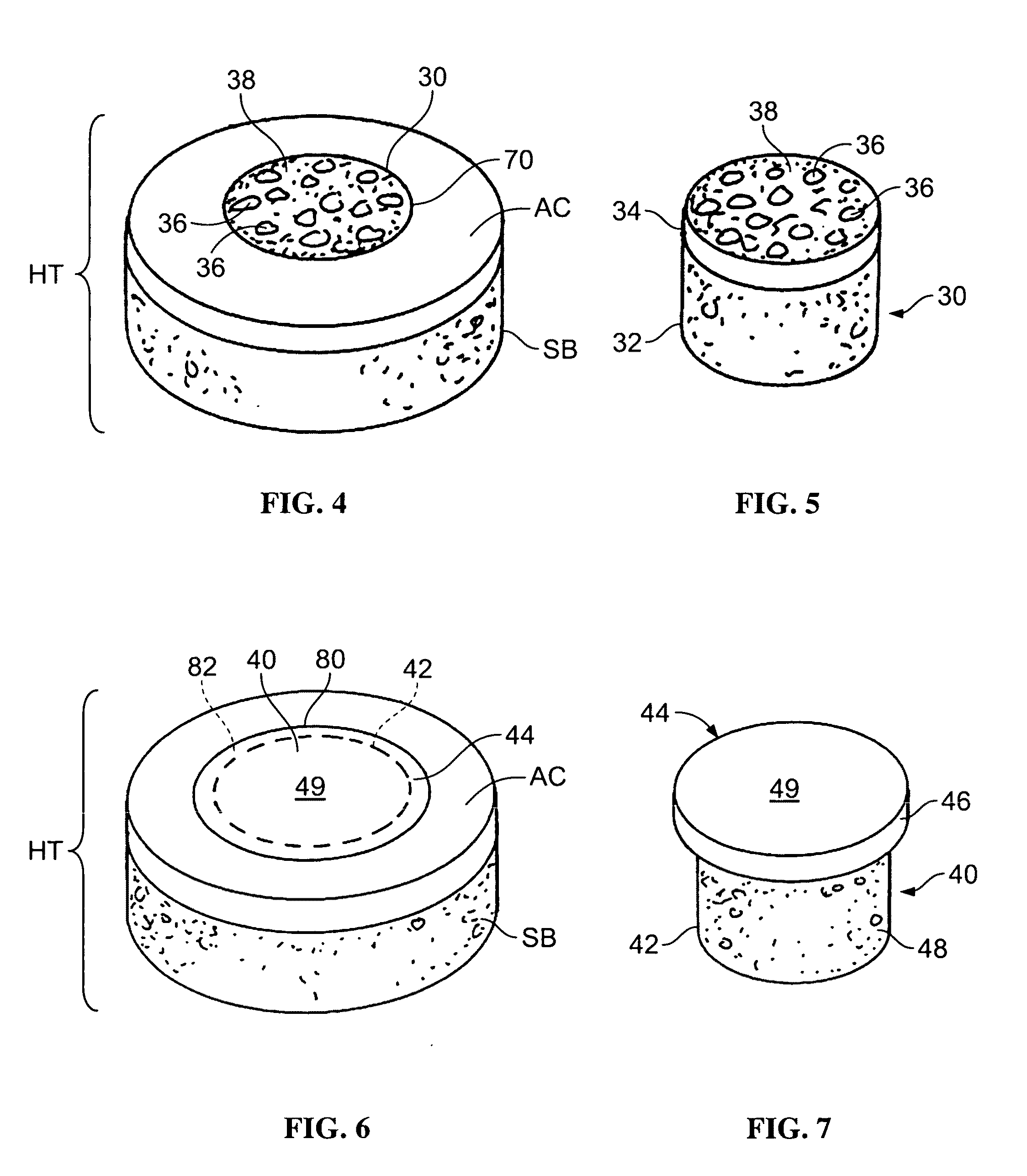
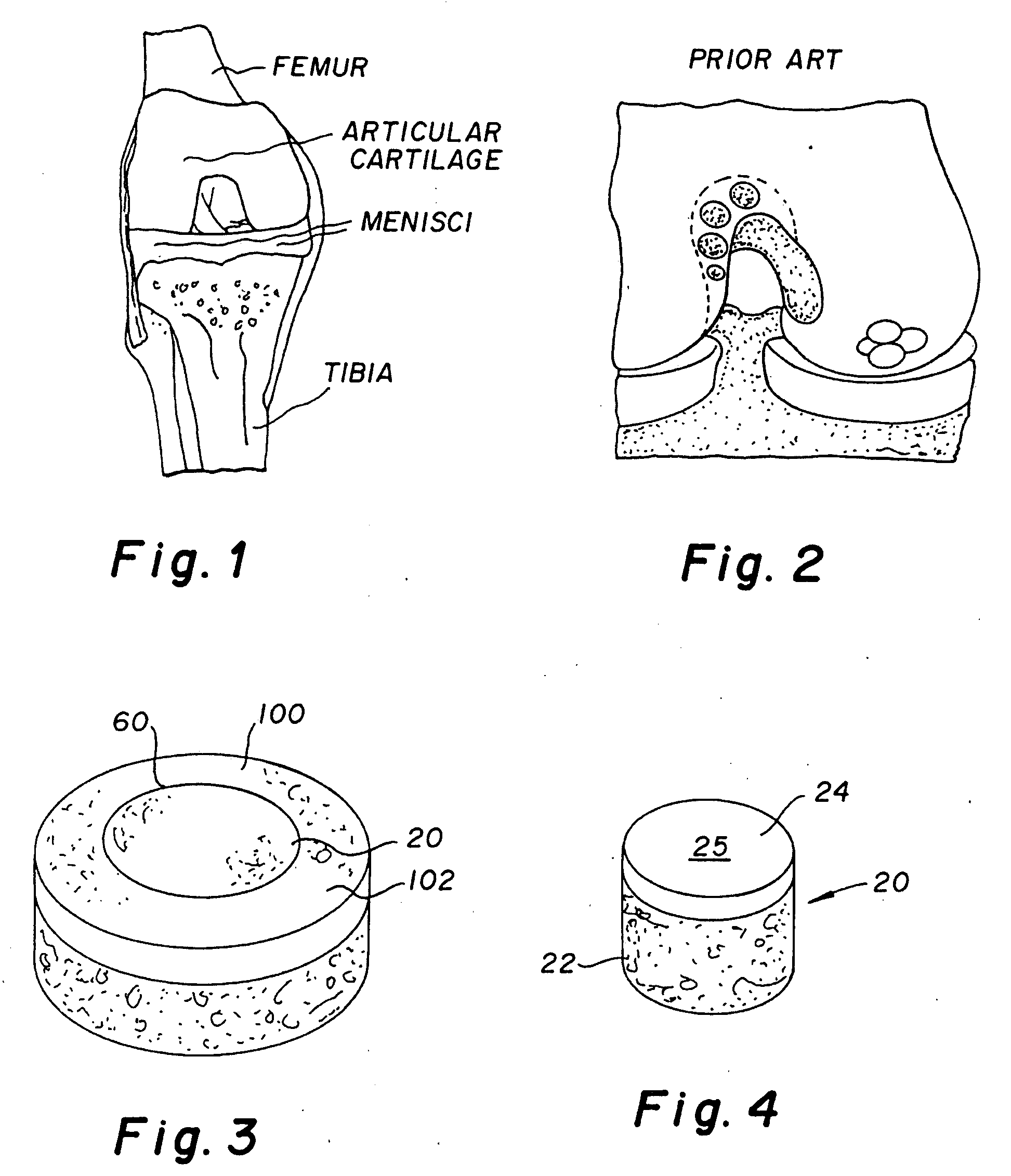
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
224 results about "Subchondral bone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
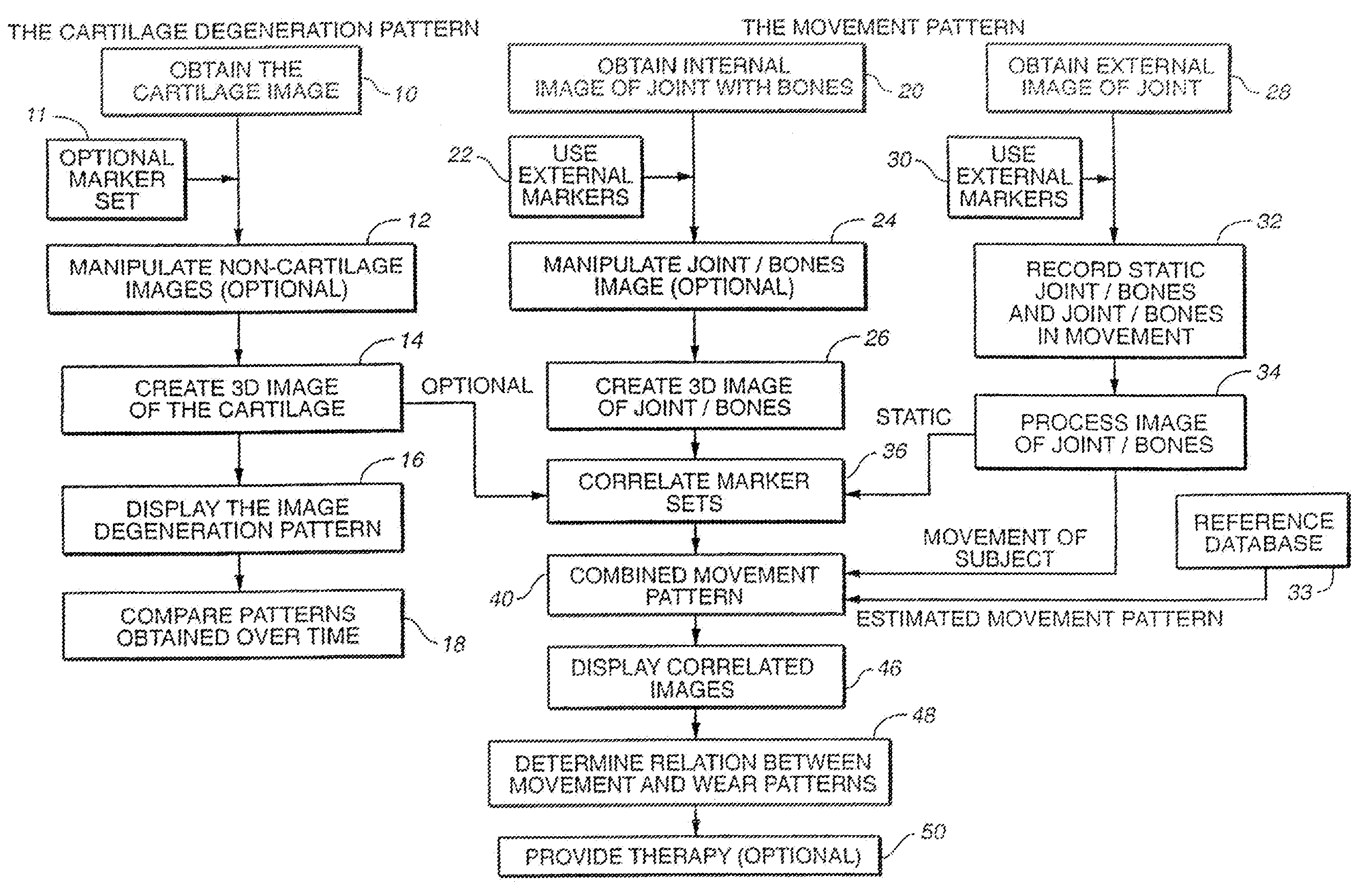
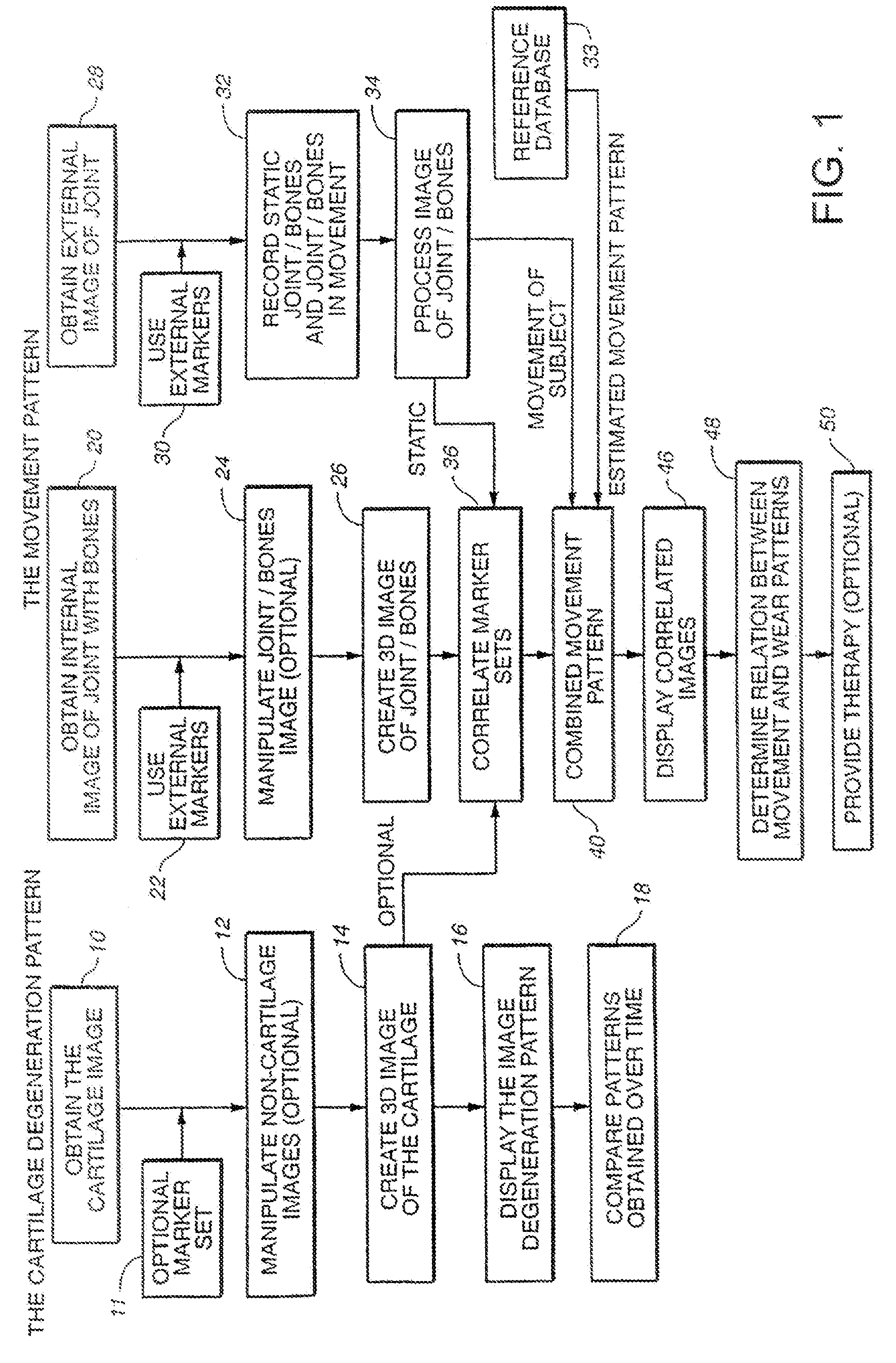
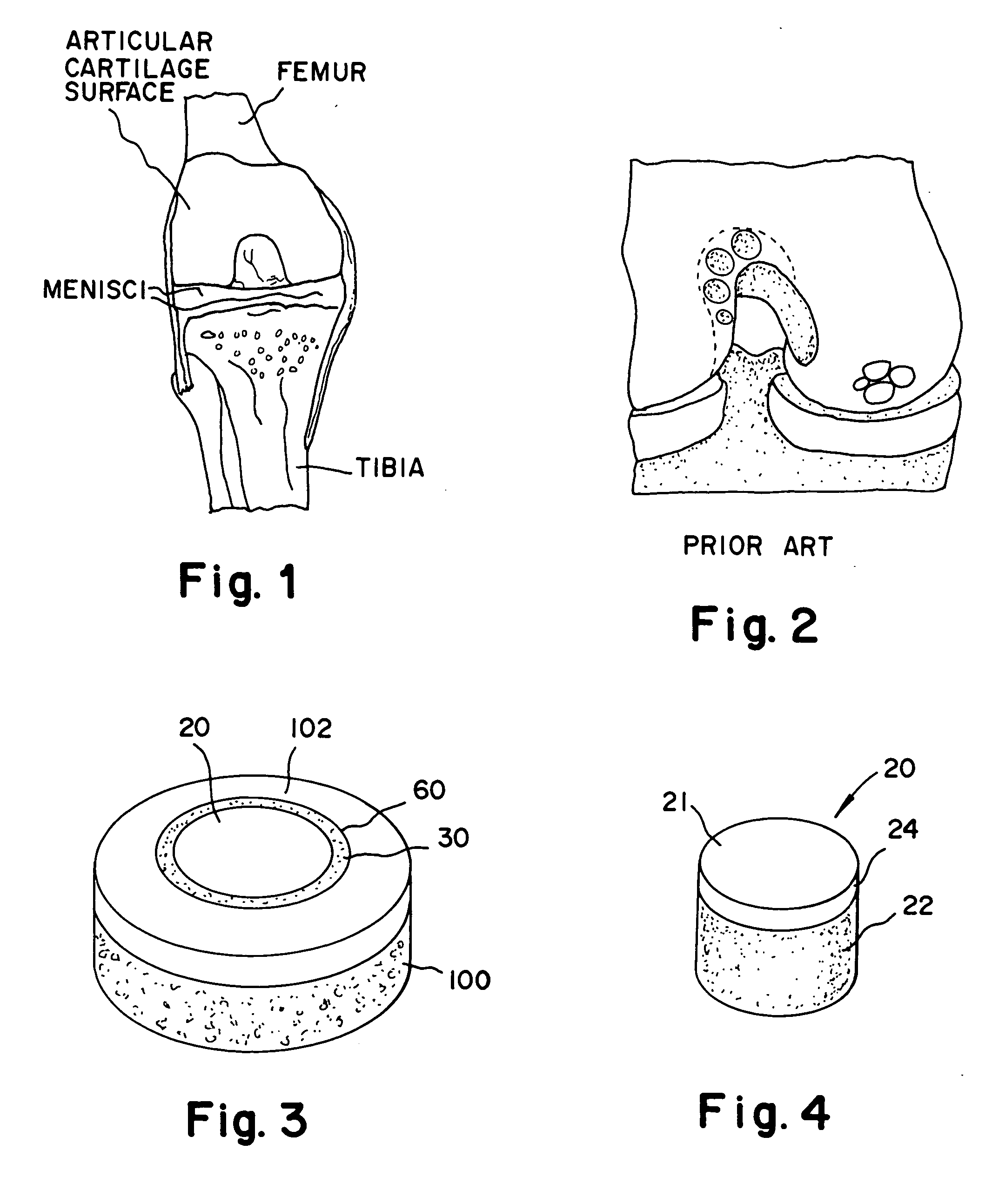
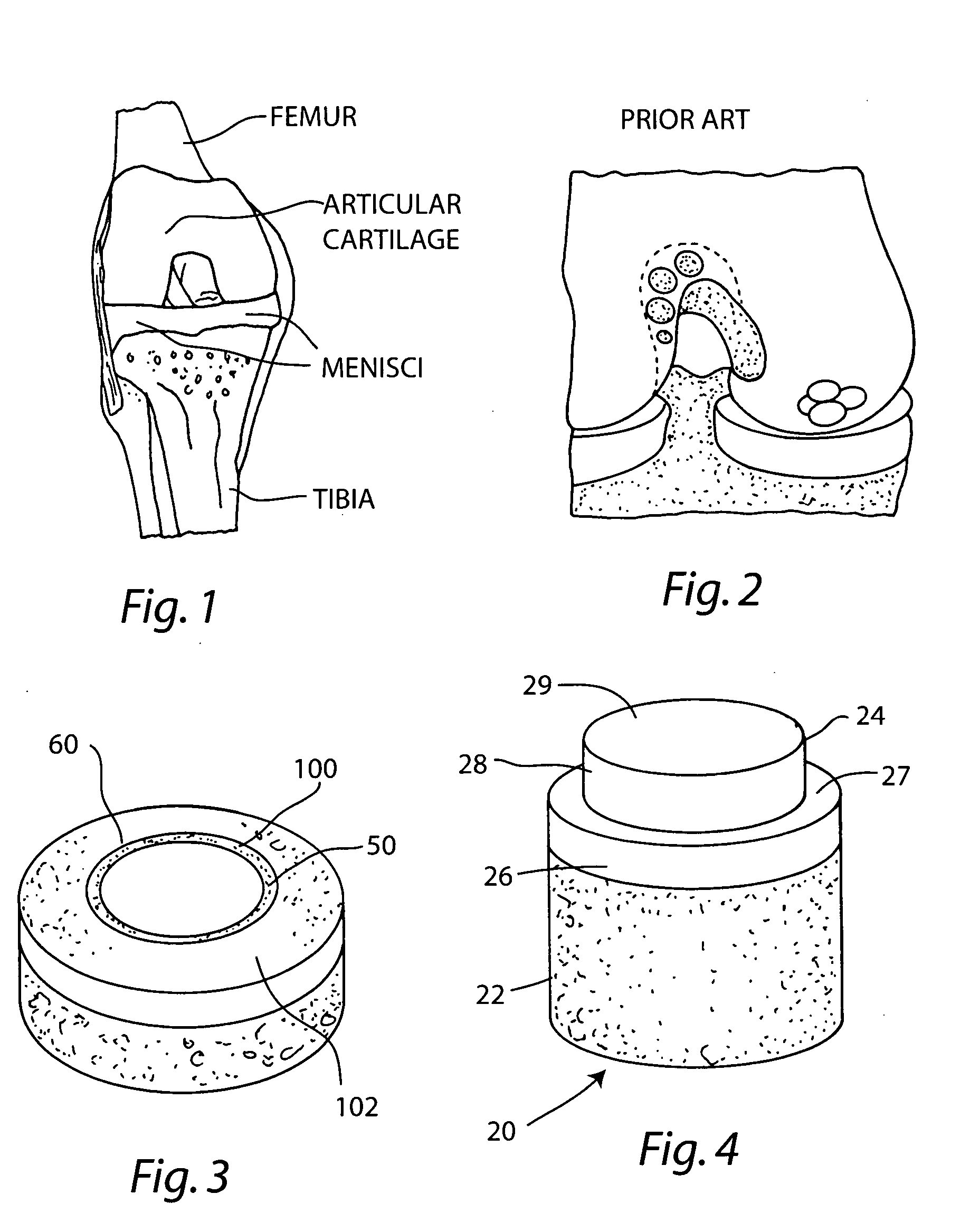
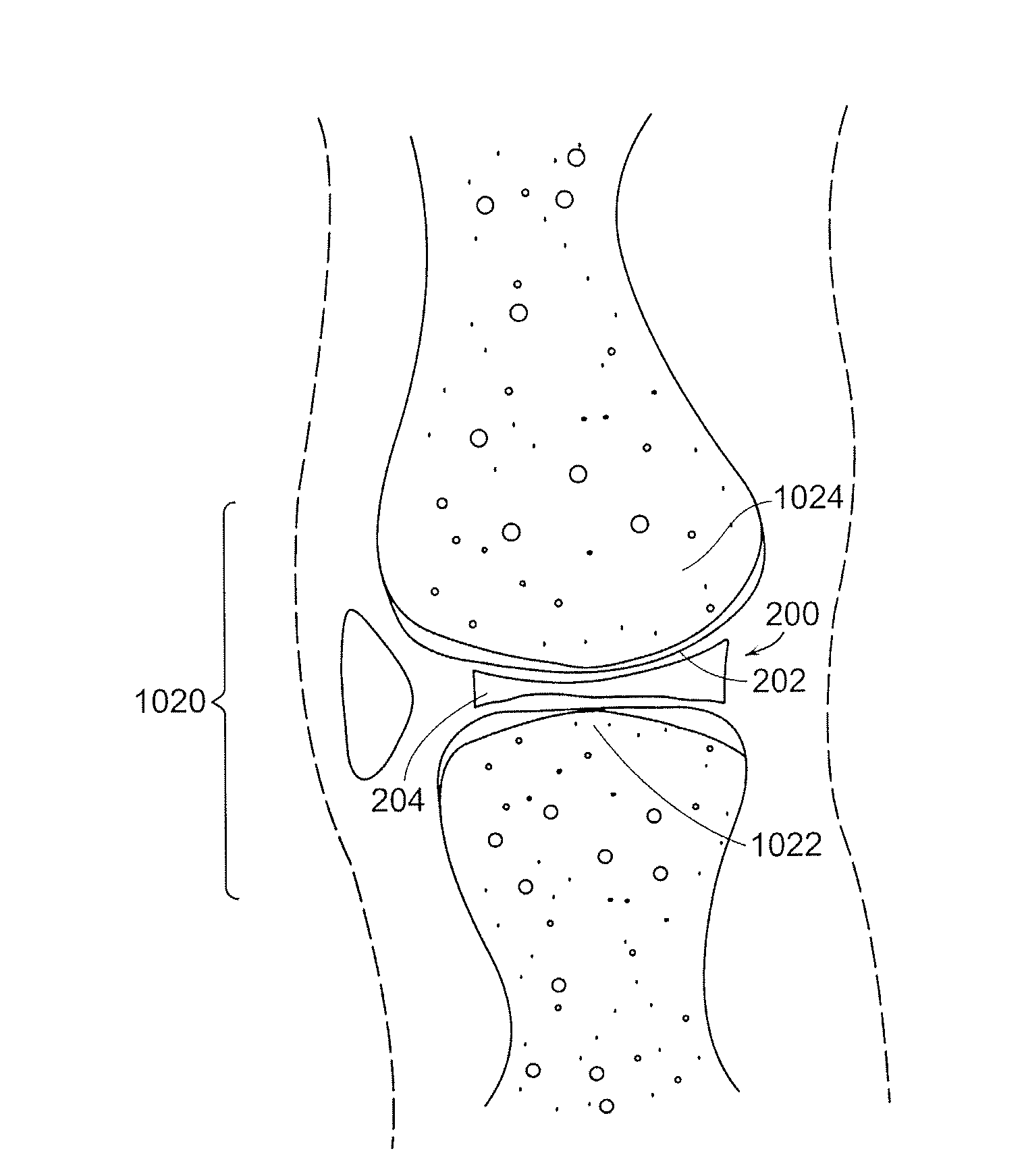
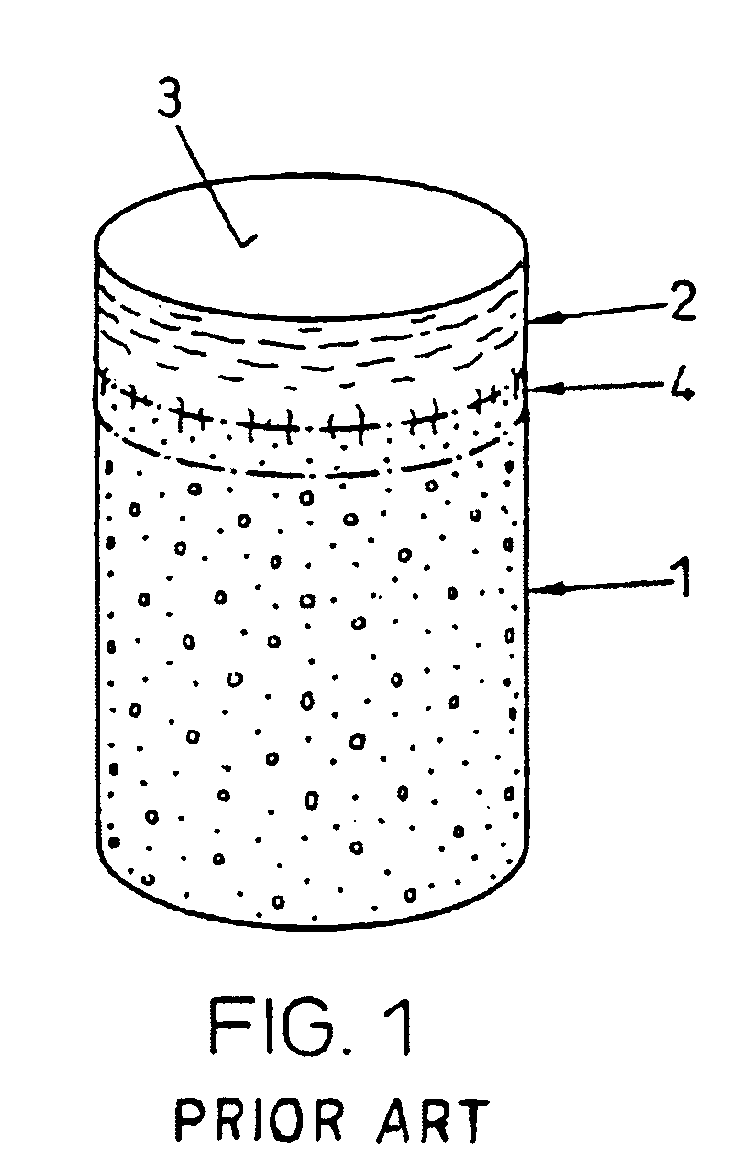
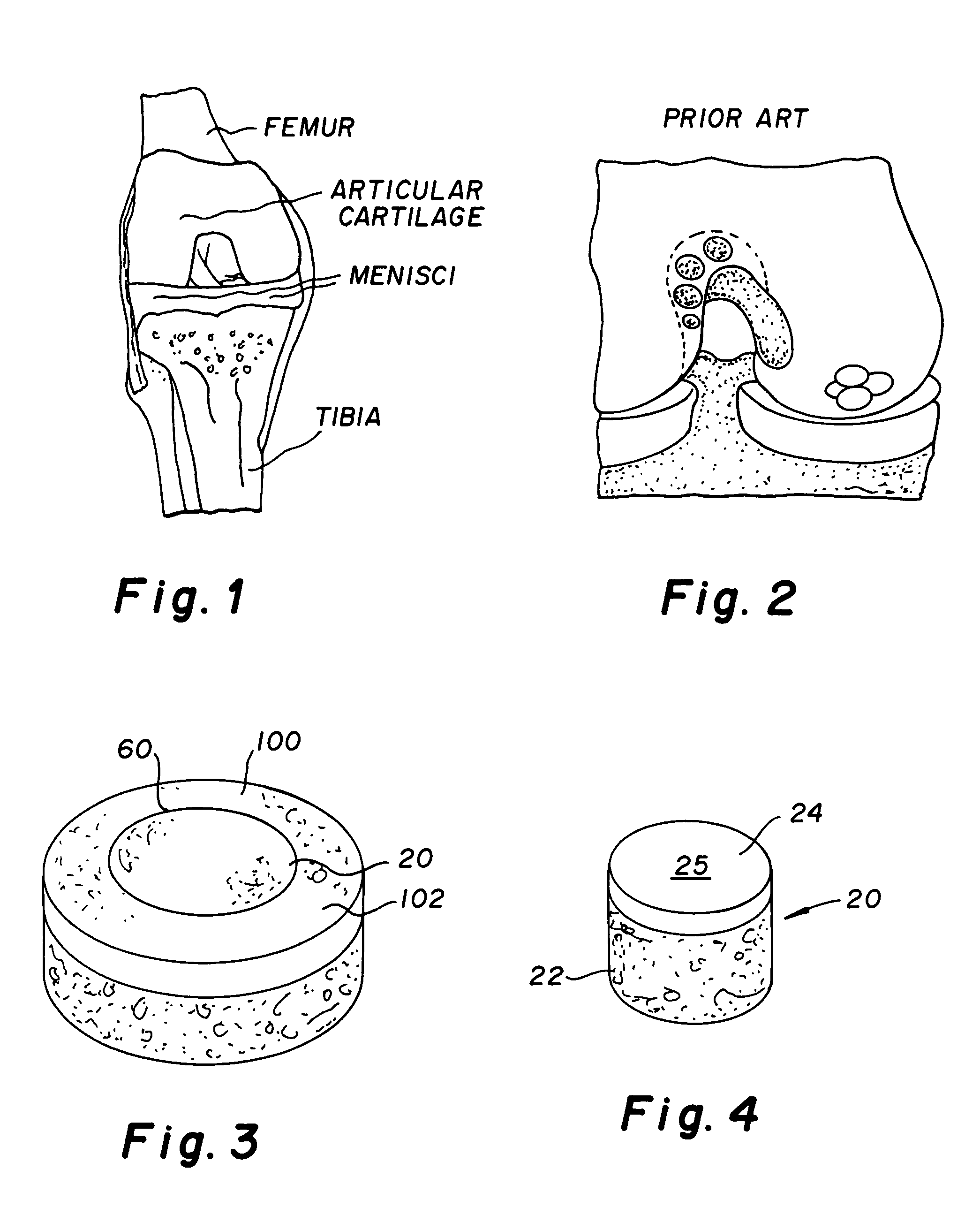
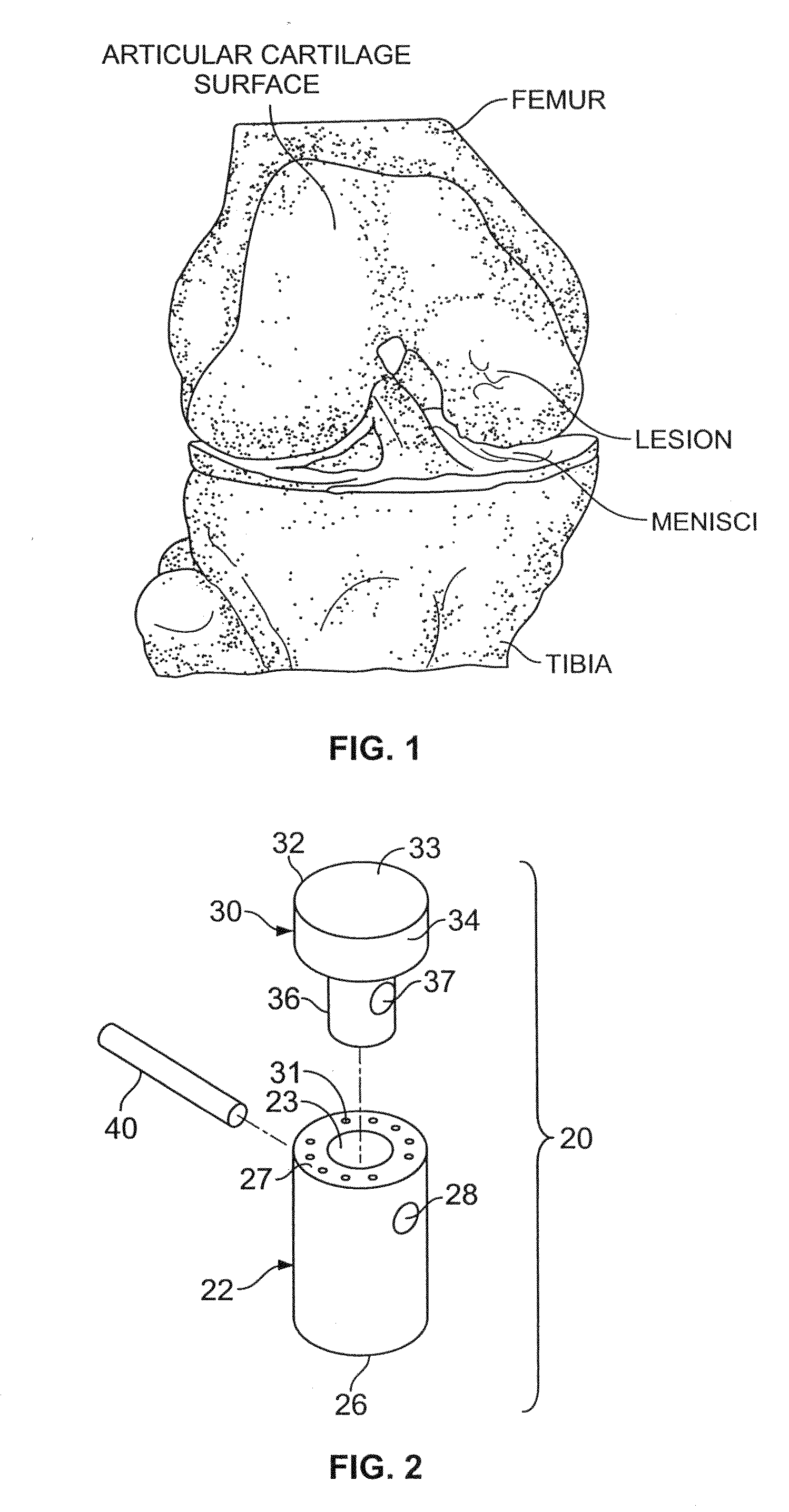
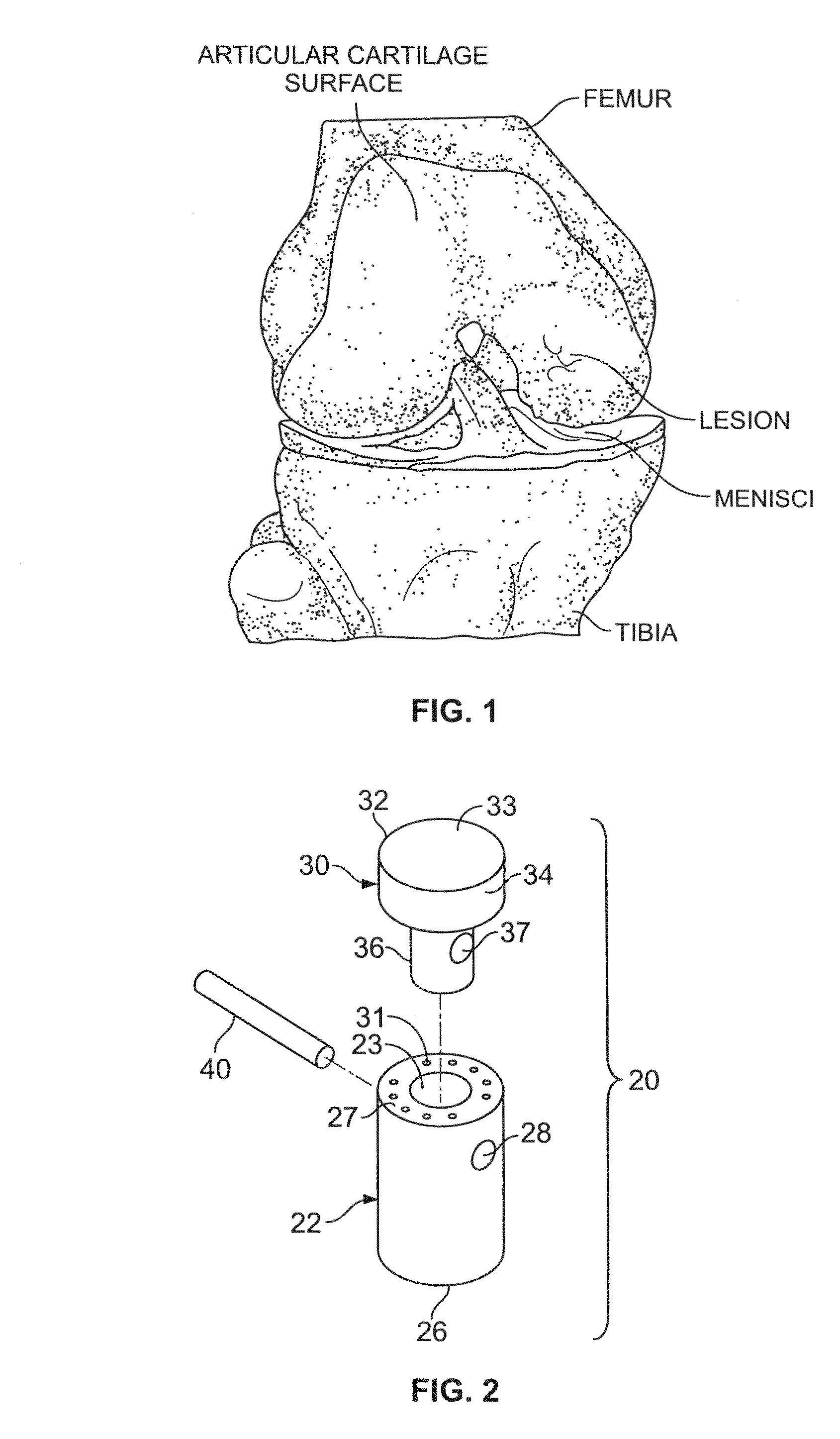
The subchondral bone acts as a shock absorber in weight-bearing joints, such as the knees, hips, and shoulders. The liquid inside subchondral bone cysts (SBCs) is hyaluronic acid, a component found in synovial fluid, which is the thick substance that lubricates joints, allowing the bones to slide past one another without friction.
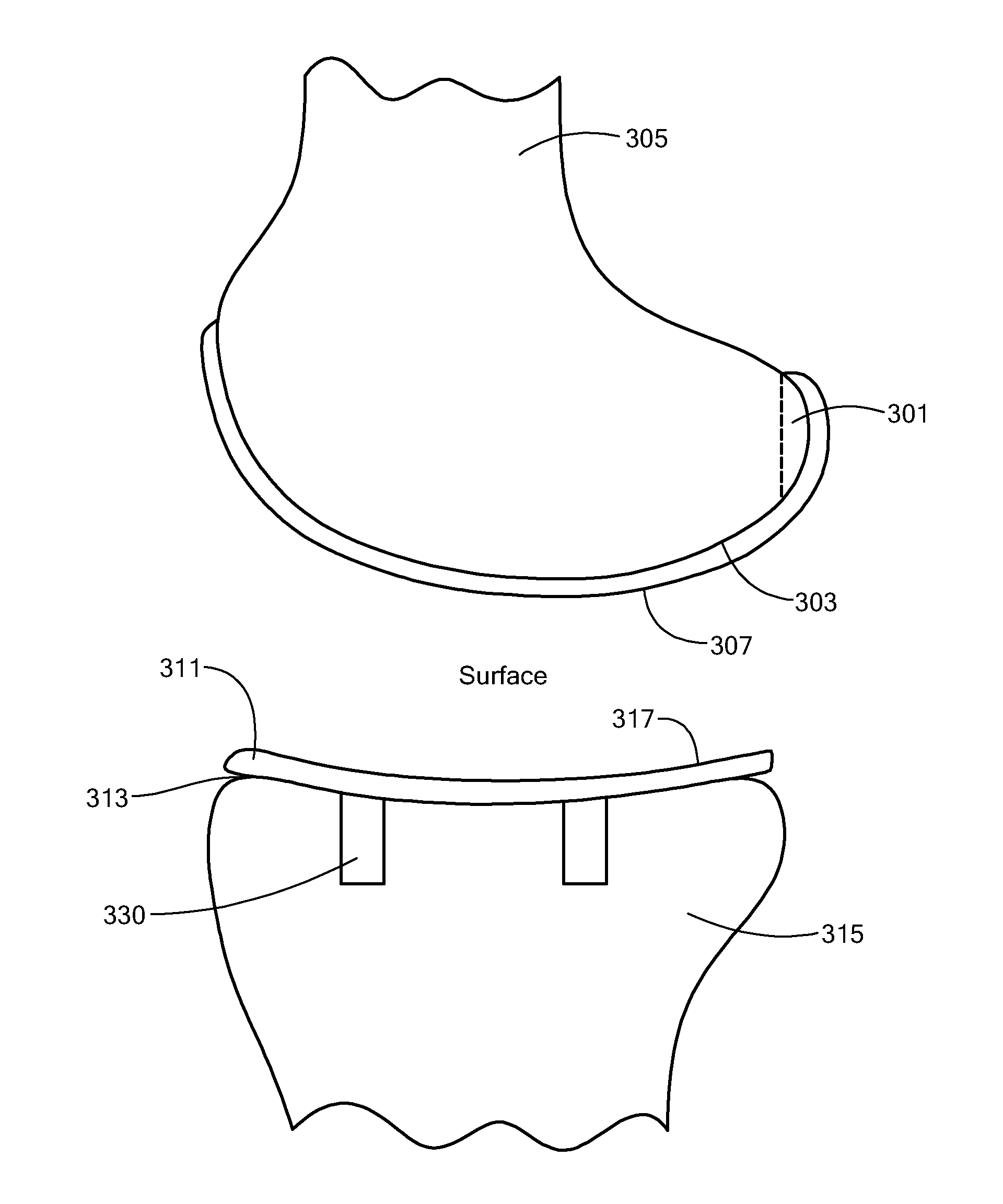
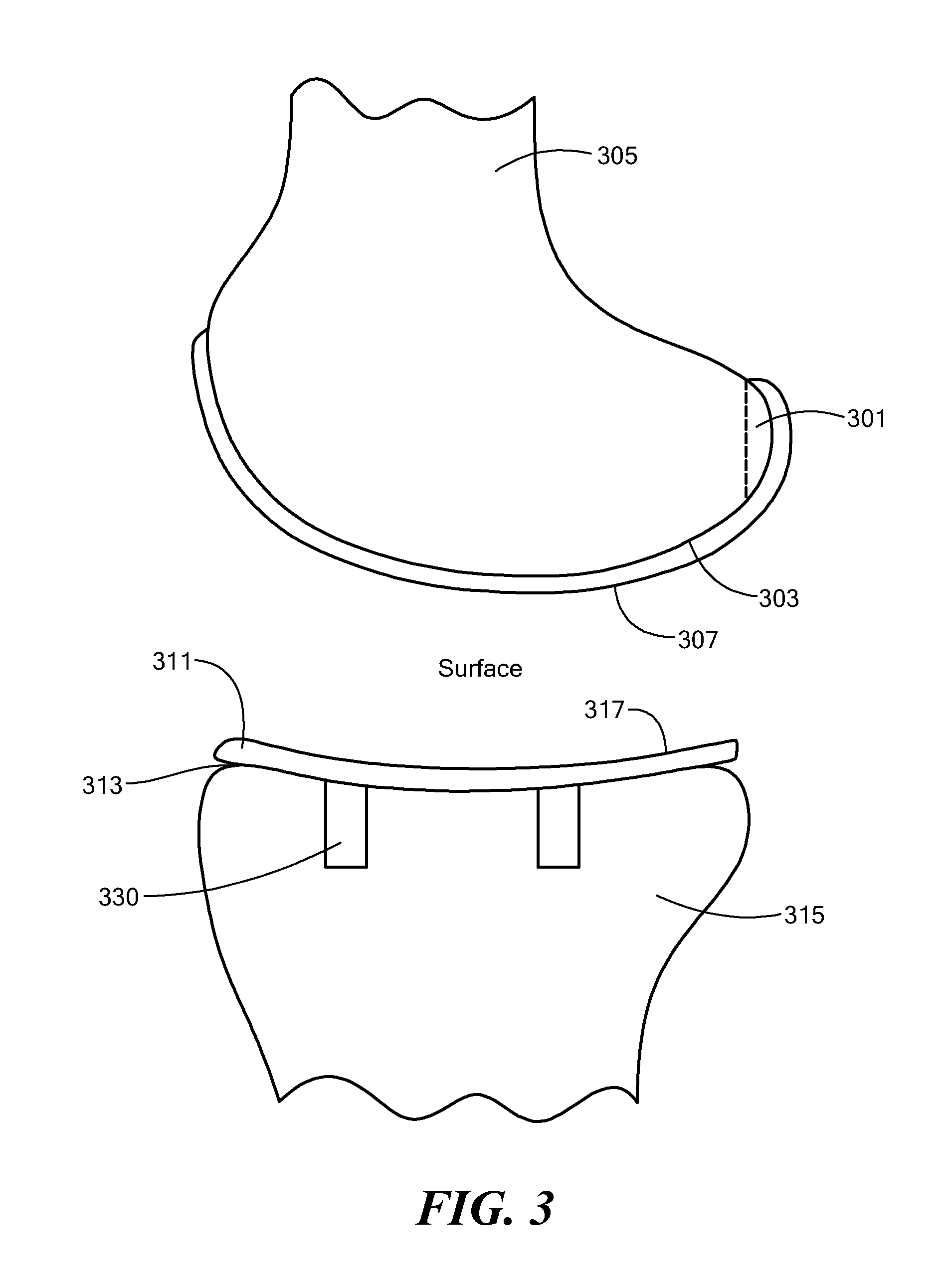
Joint Arthroplasty Devices
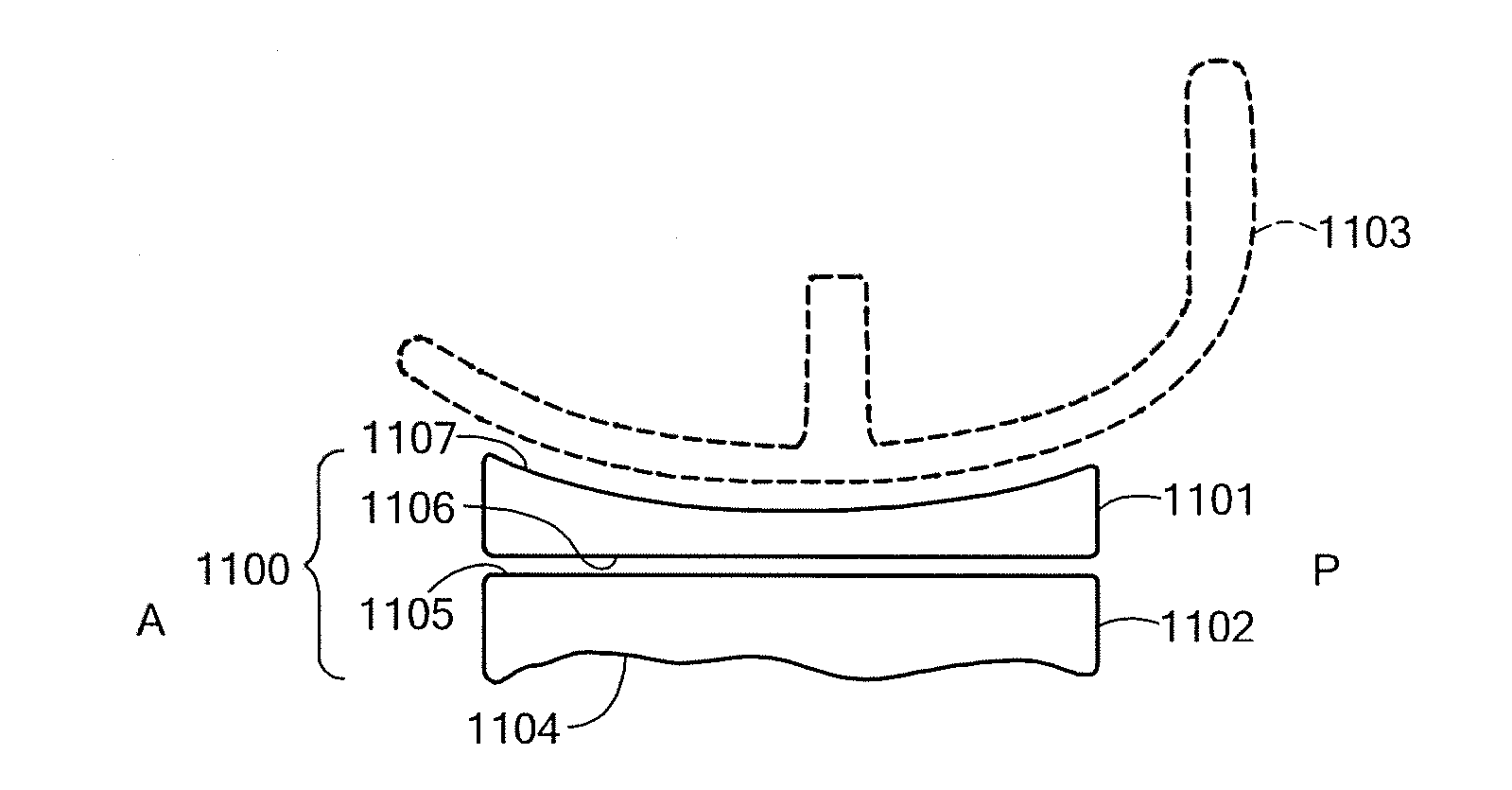
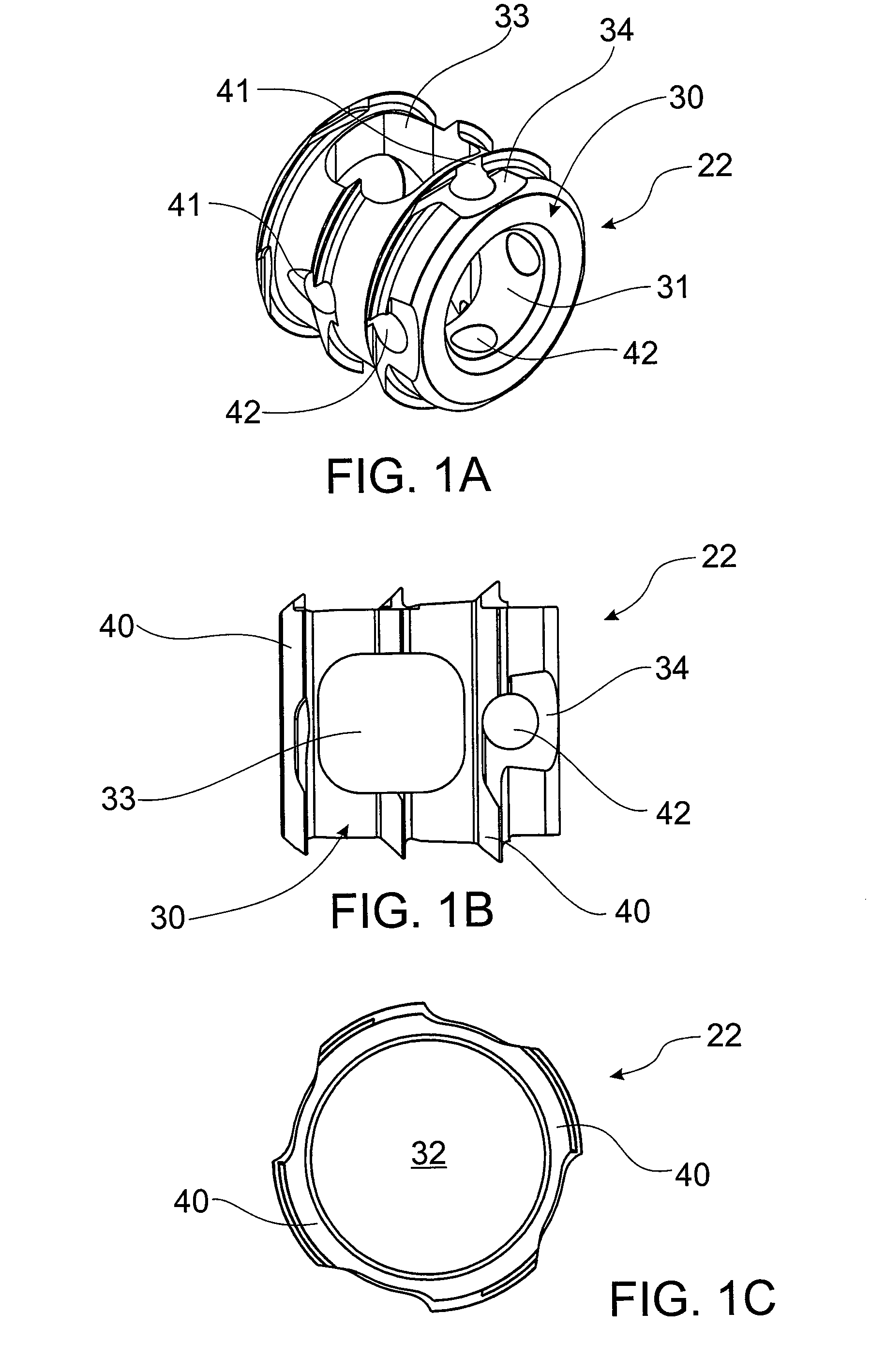
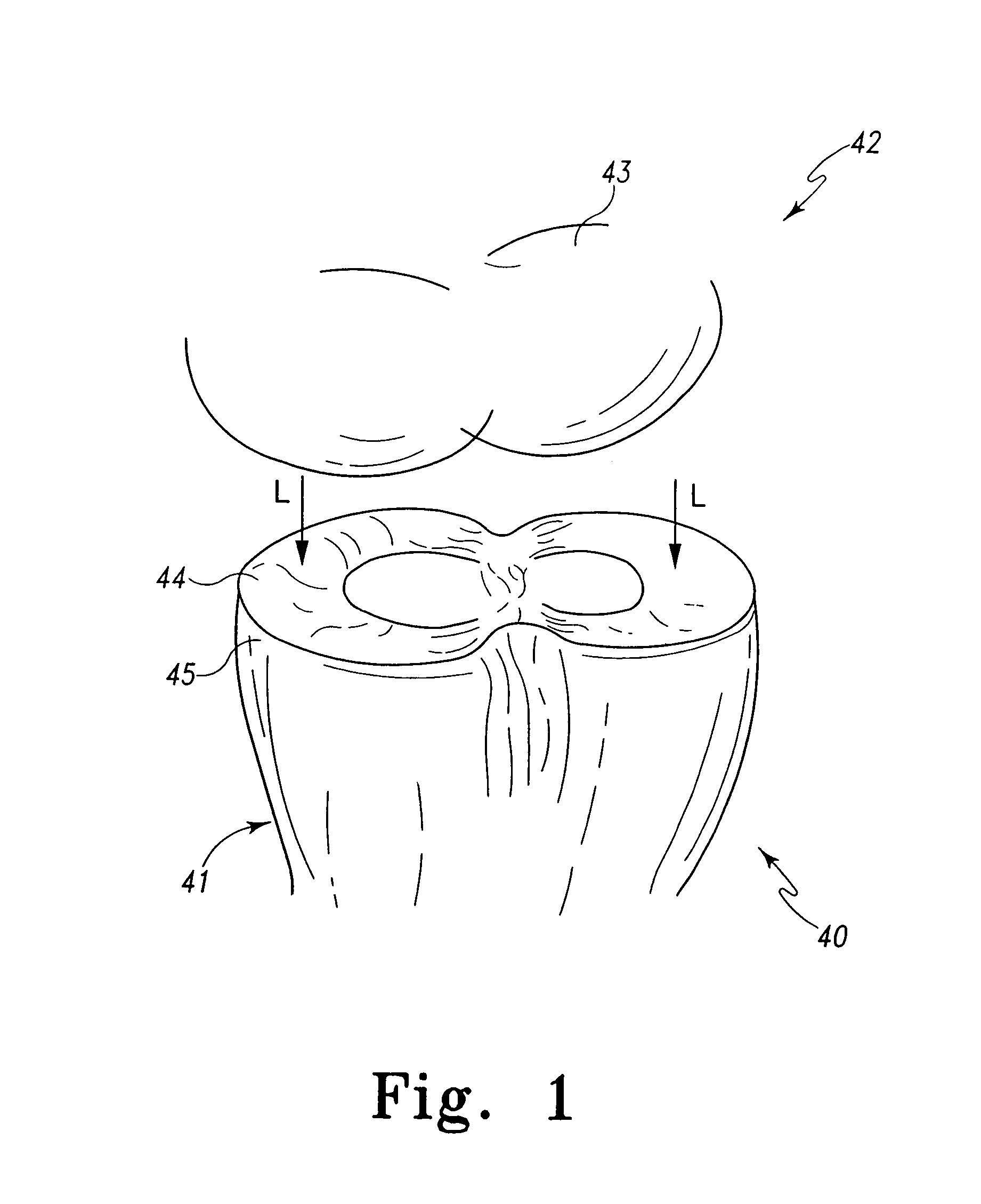
A mobile bearing implant includes a first component. The first component includes a bone facing surface for engaging one of a substantially uncut articular cartilage surface and a substantially uncut subchondral bone surface. The bone facing surface substantially matches the one of the articular cartilage surface and the subchondral bone surface. The mobile bearing implant further includes an external surface. A bearing component has a first surface for slidingly engaging the external surface of the first component, and a second surface for engaging at least one of a second component, bone, and cartilage.
Owner:CONFORMIS
Implant Device and Method for Manufacture
A knee implant includes a femoral component having first and second femoral component surfaces. The first femoral component surface is for securing to a surgically prepared compartment of a distal end of a femur. The second femoral component surface is configured to replicate the femoral condyle. The knee implant further includes a tibial component having first and second tibial component surfaces. The first tibial component surface is for contacting a proximal surface of the tibia that is substantially uncut subchondral bone. At least a portion of the first tibial component surface is a mirror image of the proximal tibial surface. The second tibial component surface articulates with the second femoral component surface.
Owner:CONFORMIS
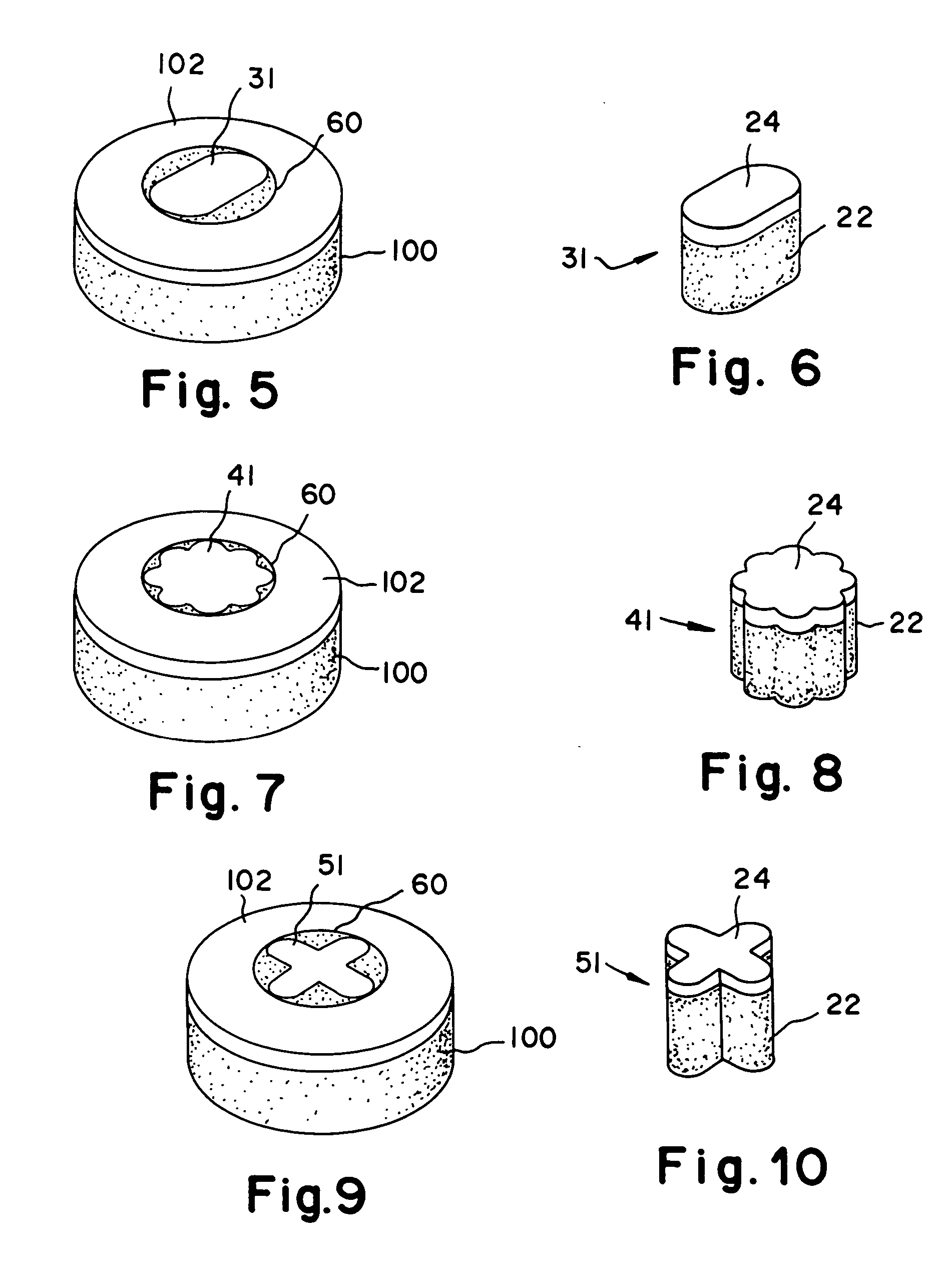
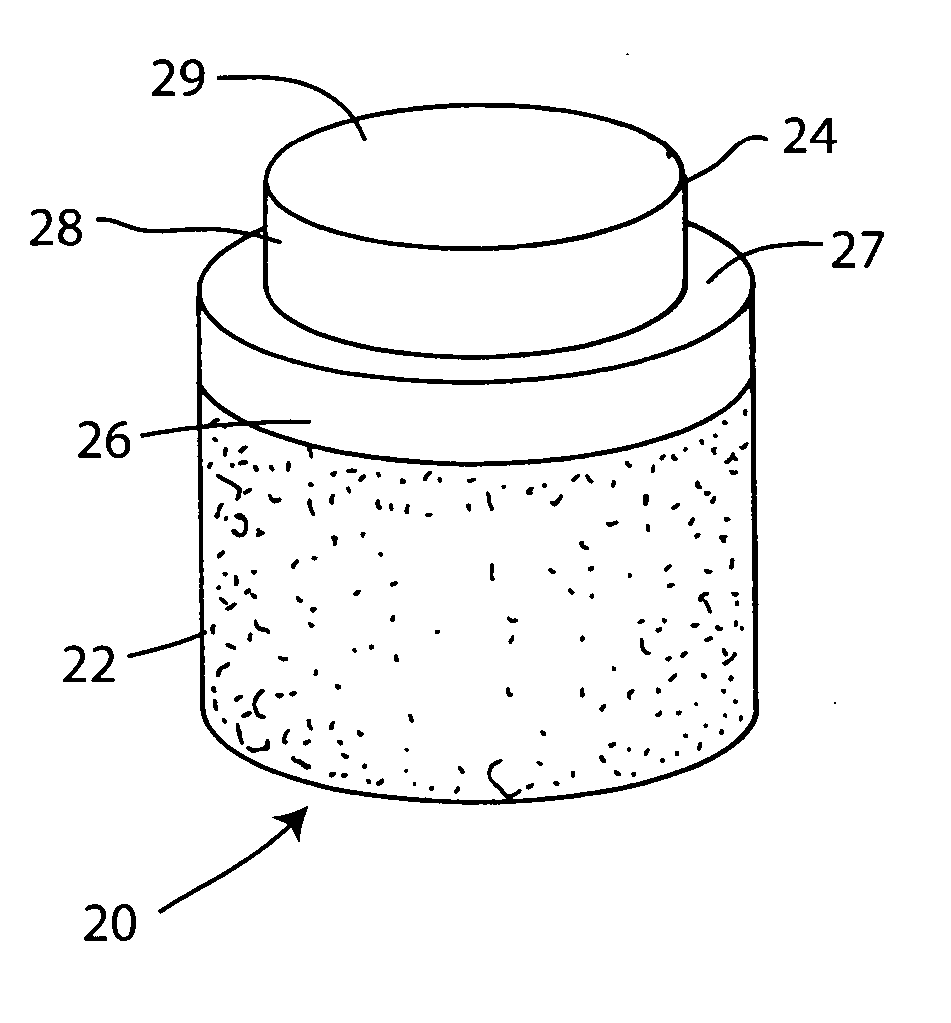
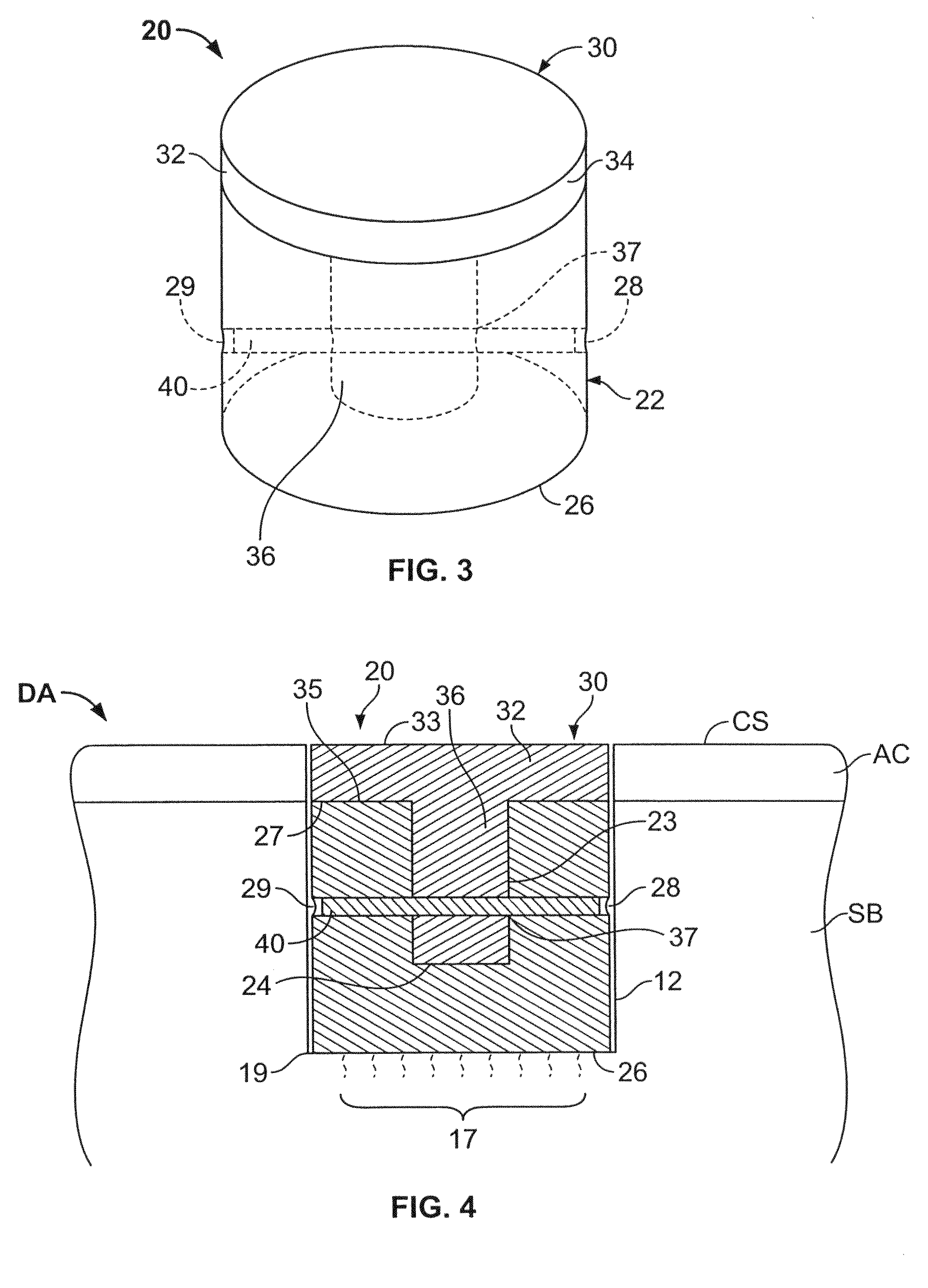
Cartilage repair plug
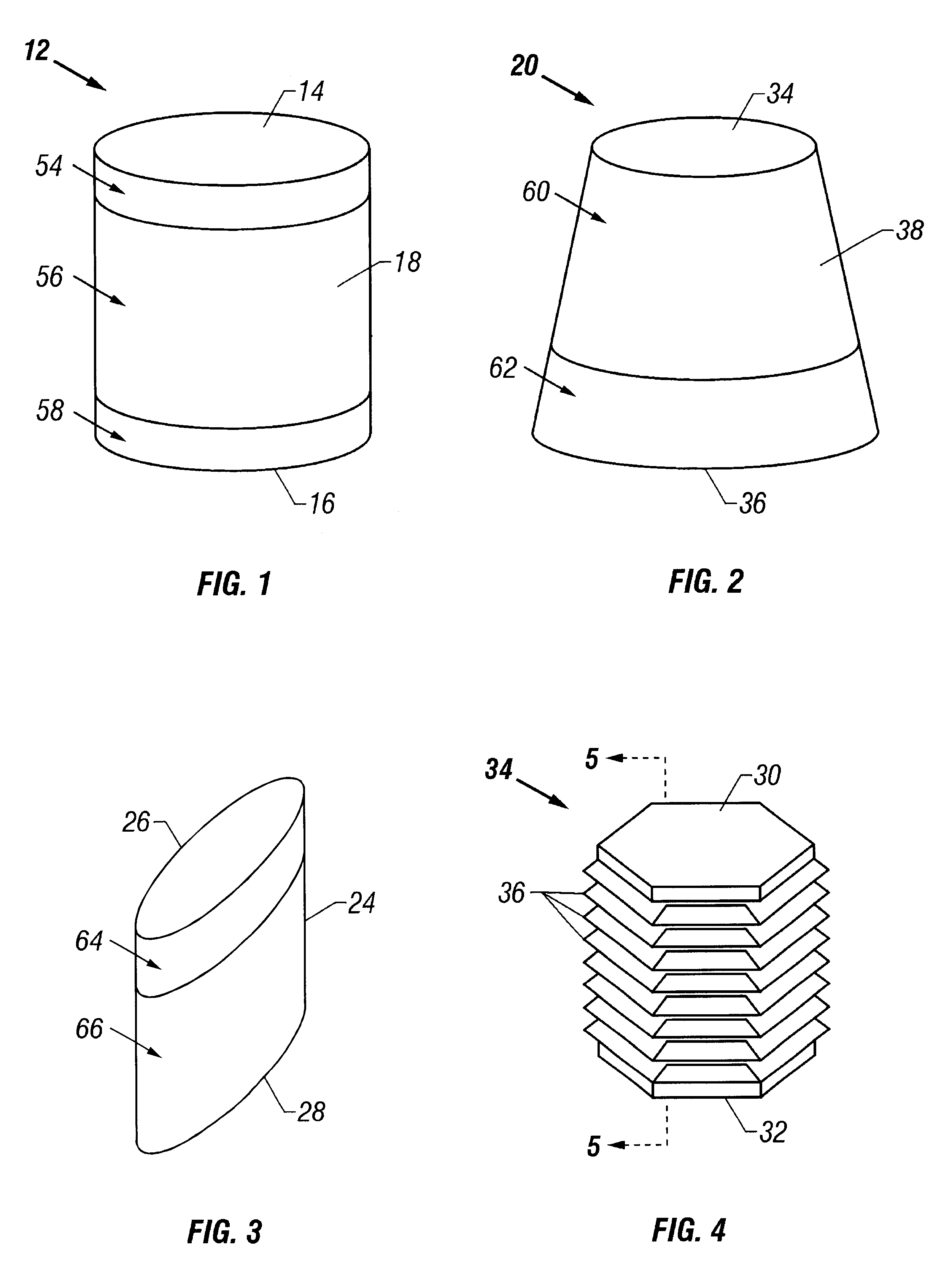
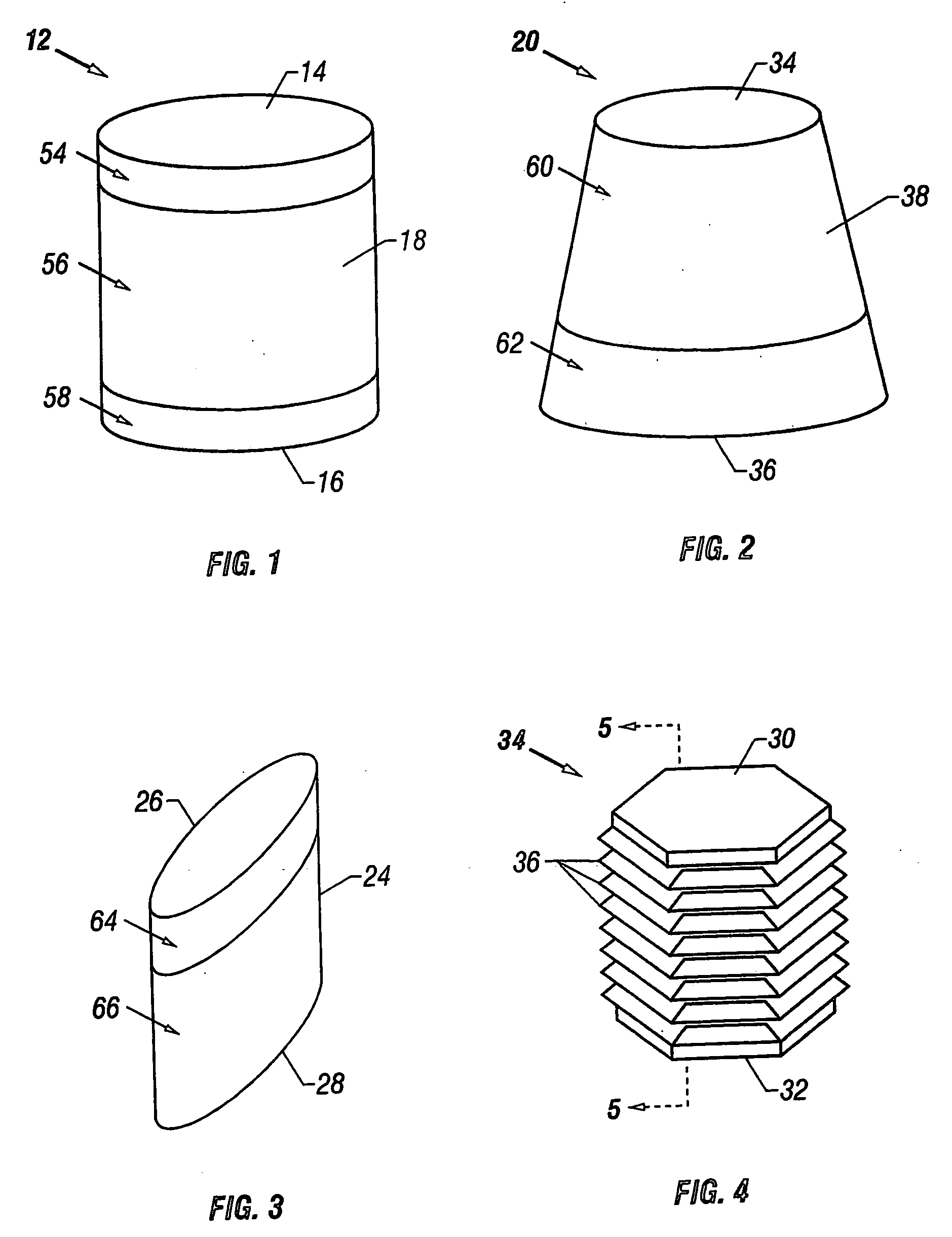
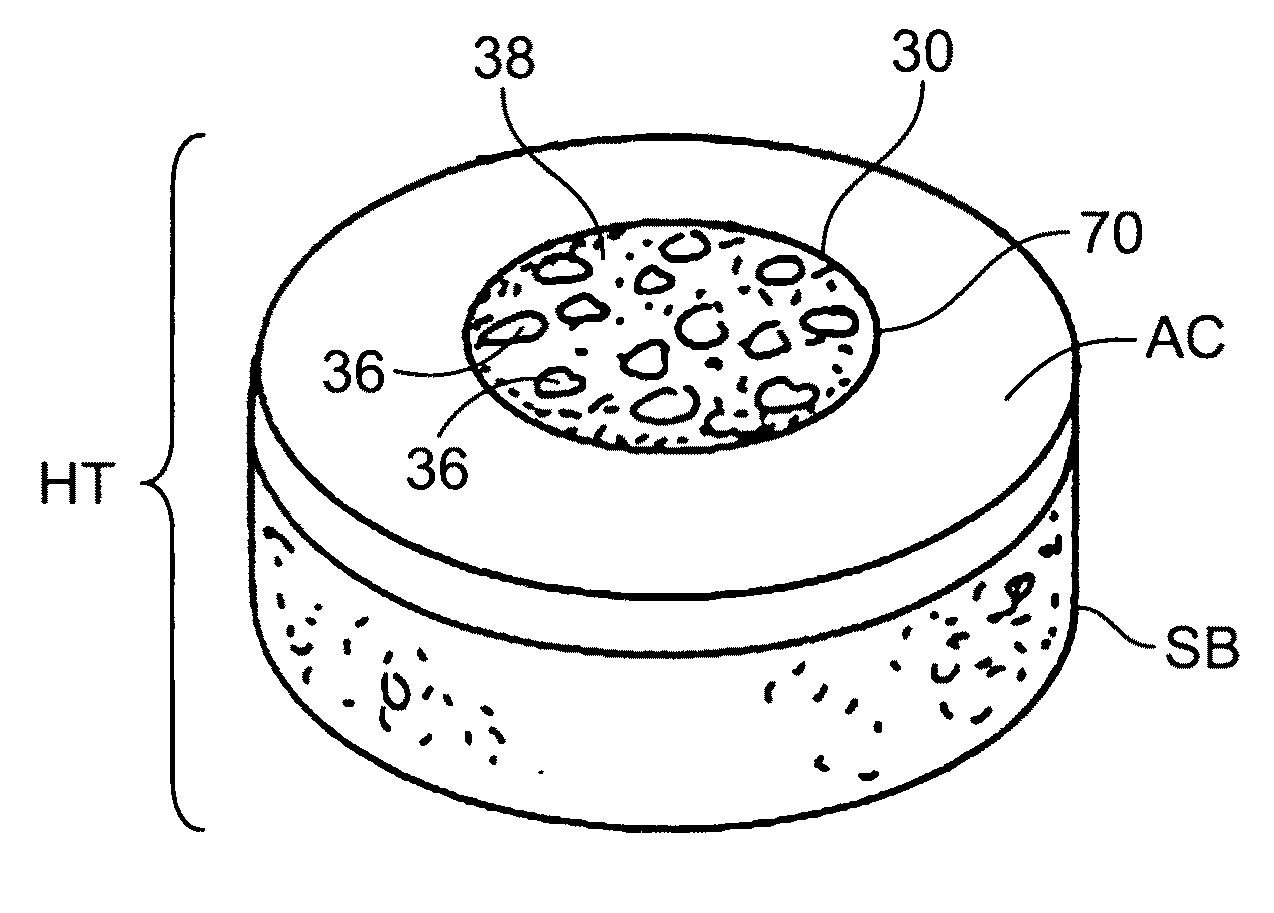
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. A plurality of anchoring elements may share a single upper layer.
Owner:ABS
Joint and Cartilage Diagnosis, Assessment and Modeling
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
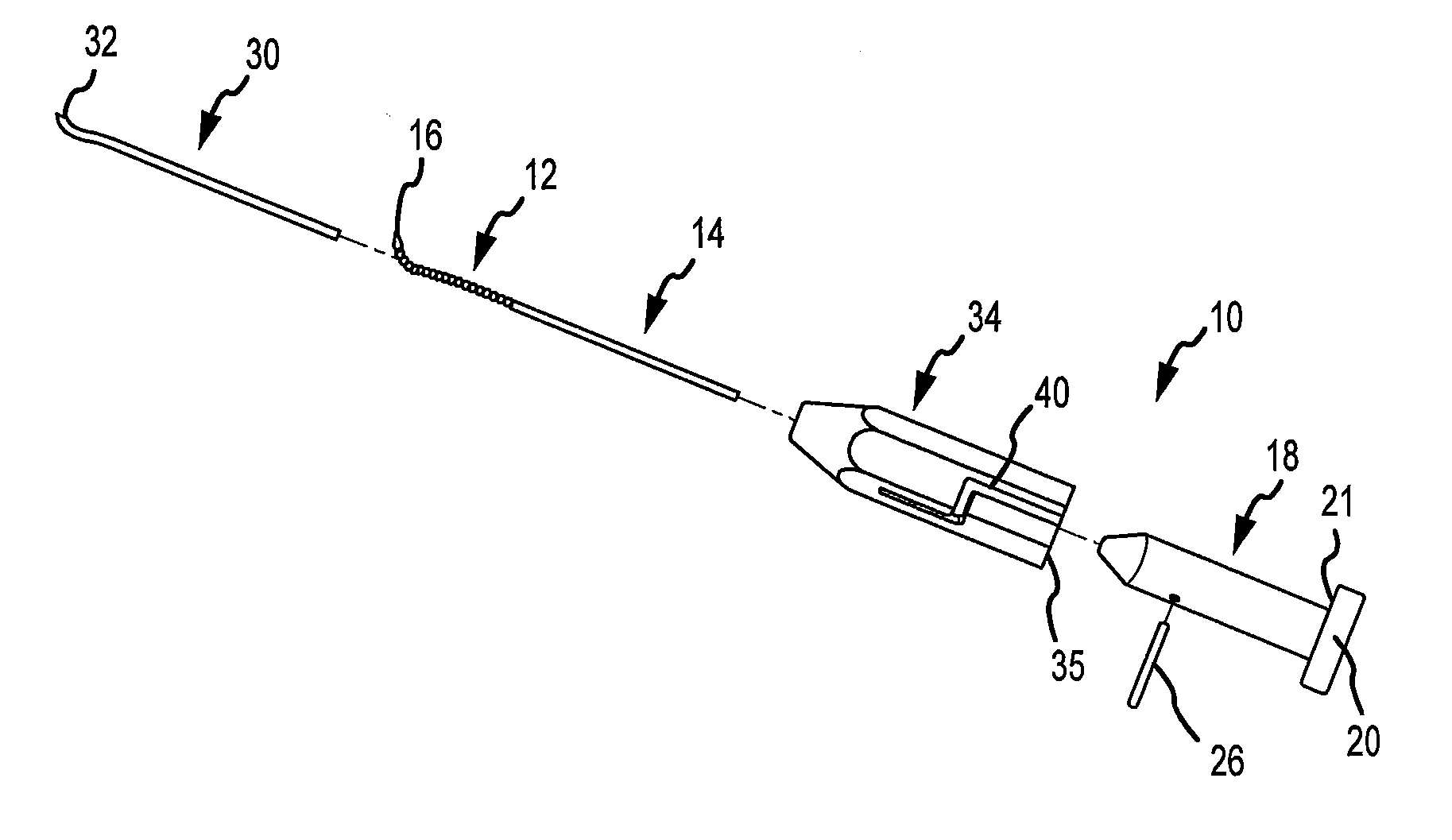
Articular cartilage repair implant delivery device and method of use
A technique for the arthroscopic delivery and fixation of an articular cartilage repair device or implant is provided. The technique includes the use of a cannula tube that functions as both a cartilage cutter and a guide to pass instruments into the body arthroscopically. One such instrument is an end-cutting reamer that both prepares the subchondral bone by re-surfacing it down to a specified depth and also simultaneously drills a pilot hole in the subchondral bone to accept the cartilage repair device. A delivery device is utilized to hold and deliver the cartilage repair device to the delivery site.
Owner:DEPUY PROD INC
Cartilage repair plug
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. A plurality of anchoring elements may share a single upper layer.
Owner:ABS
Cartilage repair plug
InactiveUS6632246B1Facilitate cell ingrowthMechanically fixedBone implantDiagnosticsCartilage repairSurgical department
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease is disclosed. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prebricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. Additionally, ridges may be formed about the periphery of each plug to facilitate its anchoring to surrounding cartilage, bone and / or adjacent plugs. A procedure for resecting damaged or diseased cartilage and for implanting a replacement plug or plugs according to this invention, as well as a set of instruments for effecting the procedure, and a self-contained system for orthopedic surgeons, which includes a variety of differently sized and shaped plugs, as well as a set of instruments for the procedure are also disclosed.< / PTEXT>
Owner:ABS
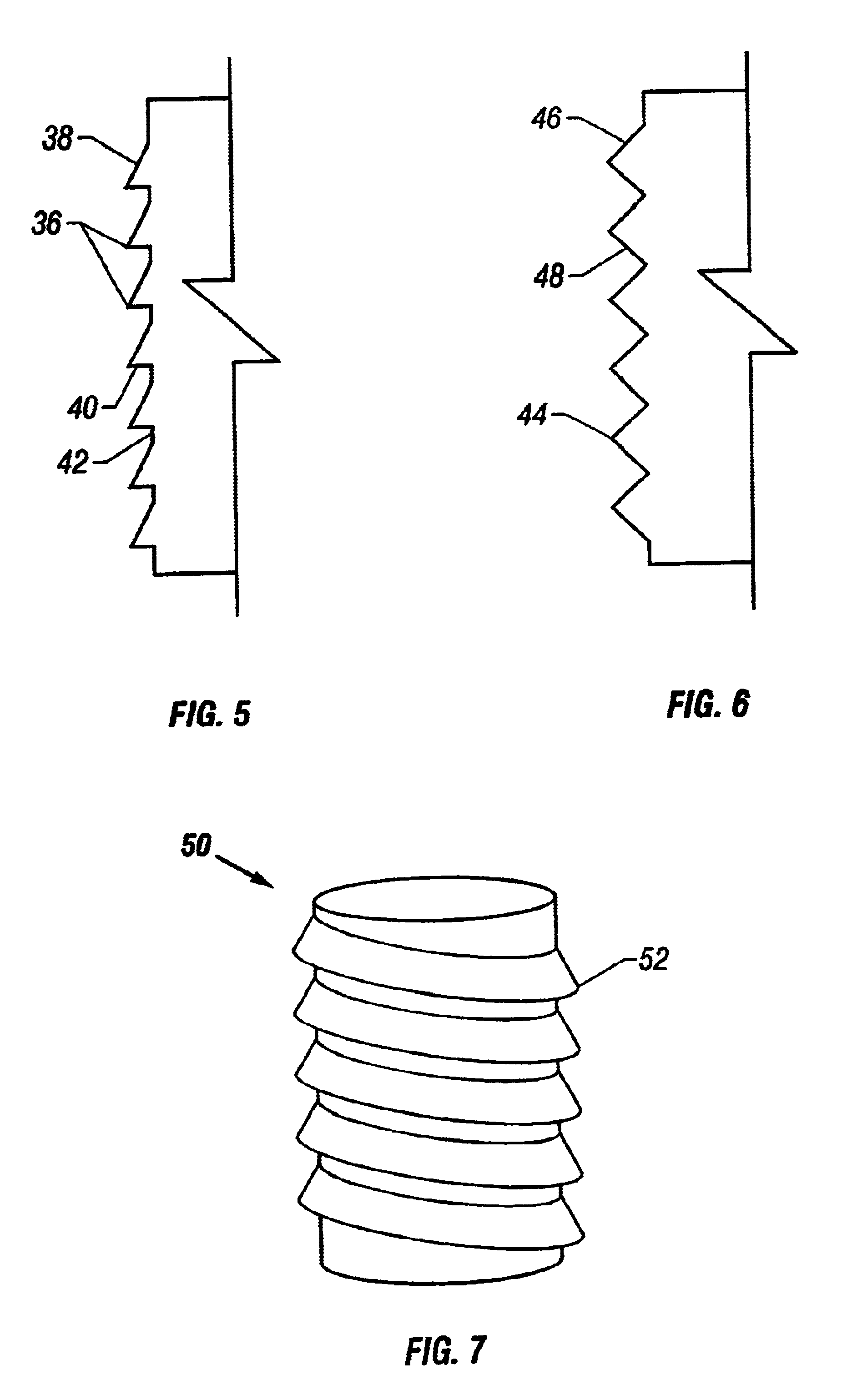
Implant device for cartilage regeneration in load bearing articulation regions
An implant device for cartilage regeneration in loading-bearing regions uses the osteochondral defect model. The implant is formed of resorbable polymeric materials. The implant is designed such that load is transmitted from the articulating surface of the bone platform through the implant to the entire area of subchondral bone of the bone platform. Application of load in this manner results in reduced subchondral bone resorption, leading to joint stabilization and maintenance of normal joint biomechanics. The implant allows for the incorporation therein of a resorbable scaffold or matrix material. The present implant solves the current inability to regenerate cartilage in load-bearing articulating surfaces using engineered scaffold devices.
Owner:DEPUY PROD INC
Cartilage implant assembly and method for implantation
InactiveUS20050222687A1Recovery functionRelief the painBone implantSurgerySubchondral boneInterference fit
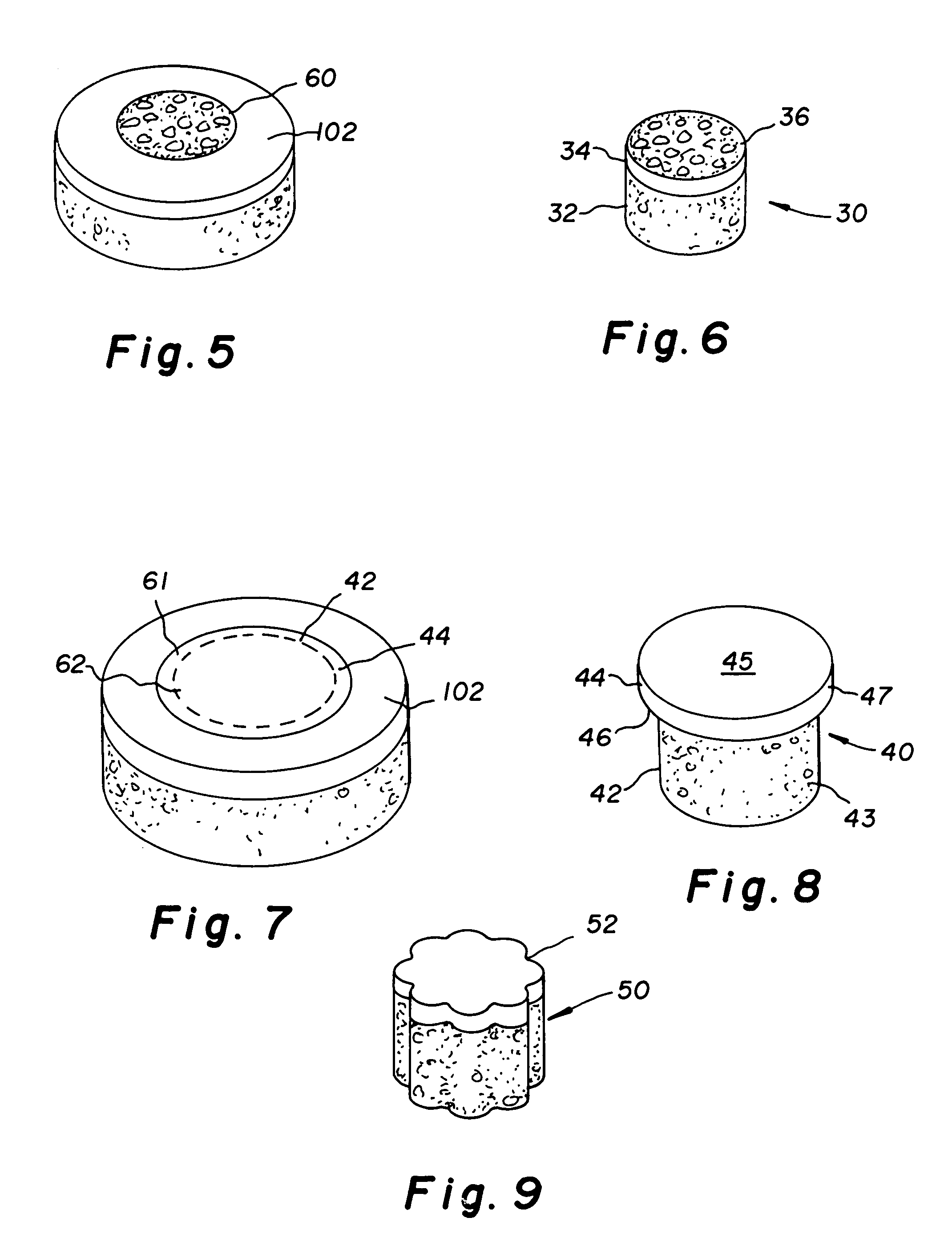
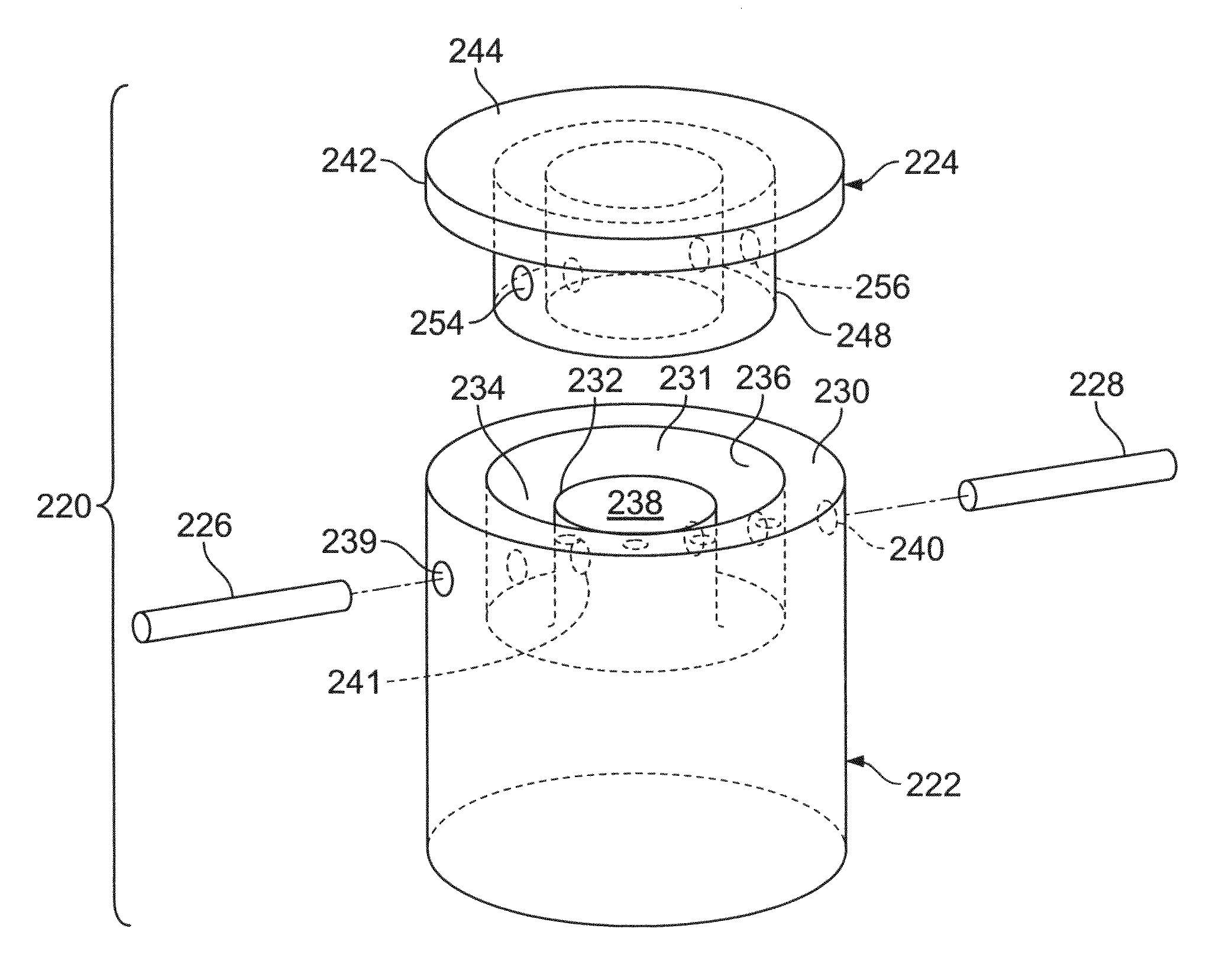
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore in an interference fit and is surrounded by milled cartilage and carrier. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
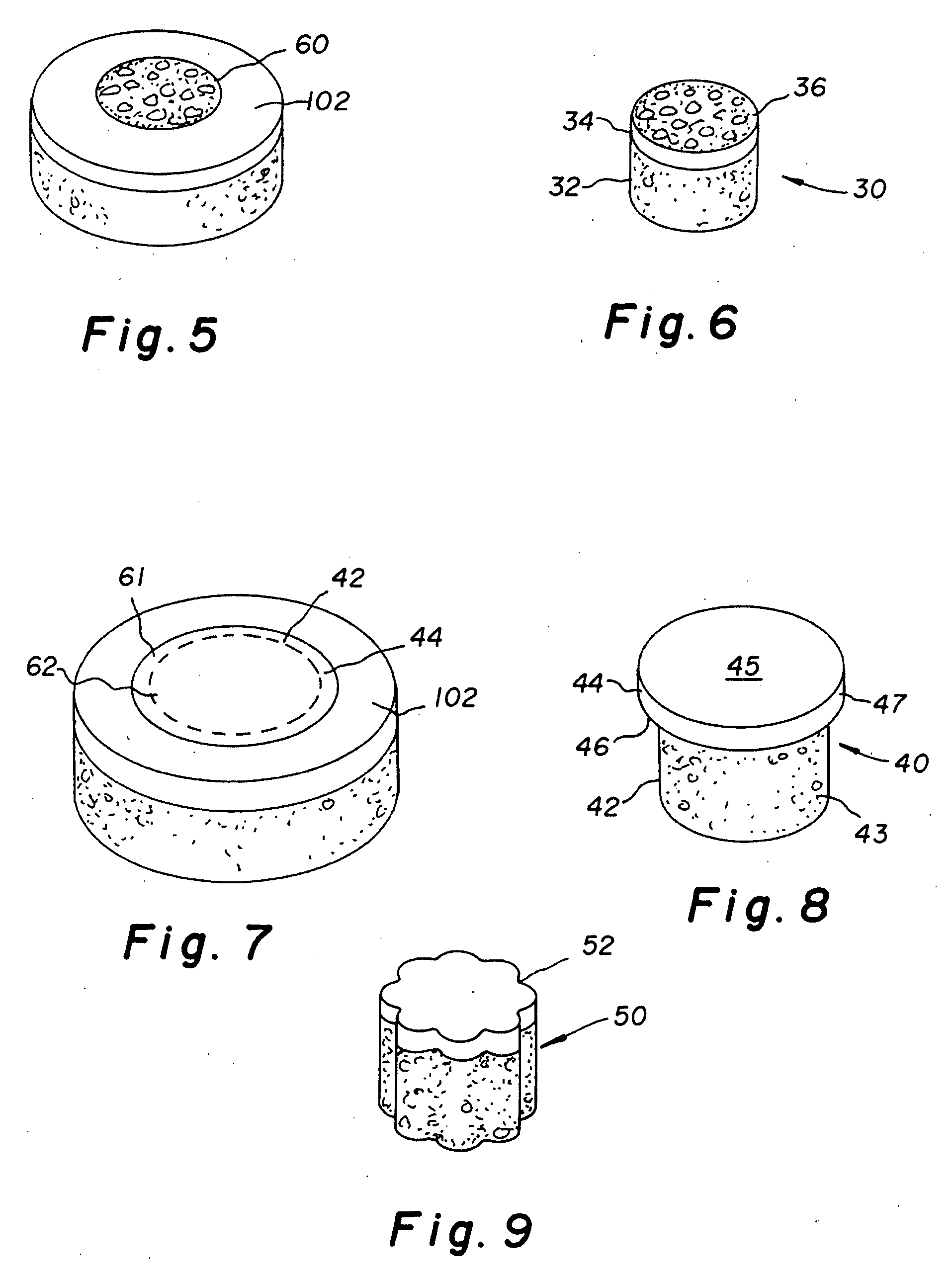
Cartilage allograft plug
InactiveUS20050251268A1Increase chondrocyte migrationIncreased proliferationBone implantLigamentsInterference fitSubchondral bone
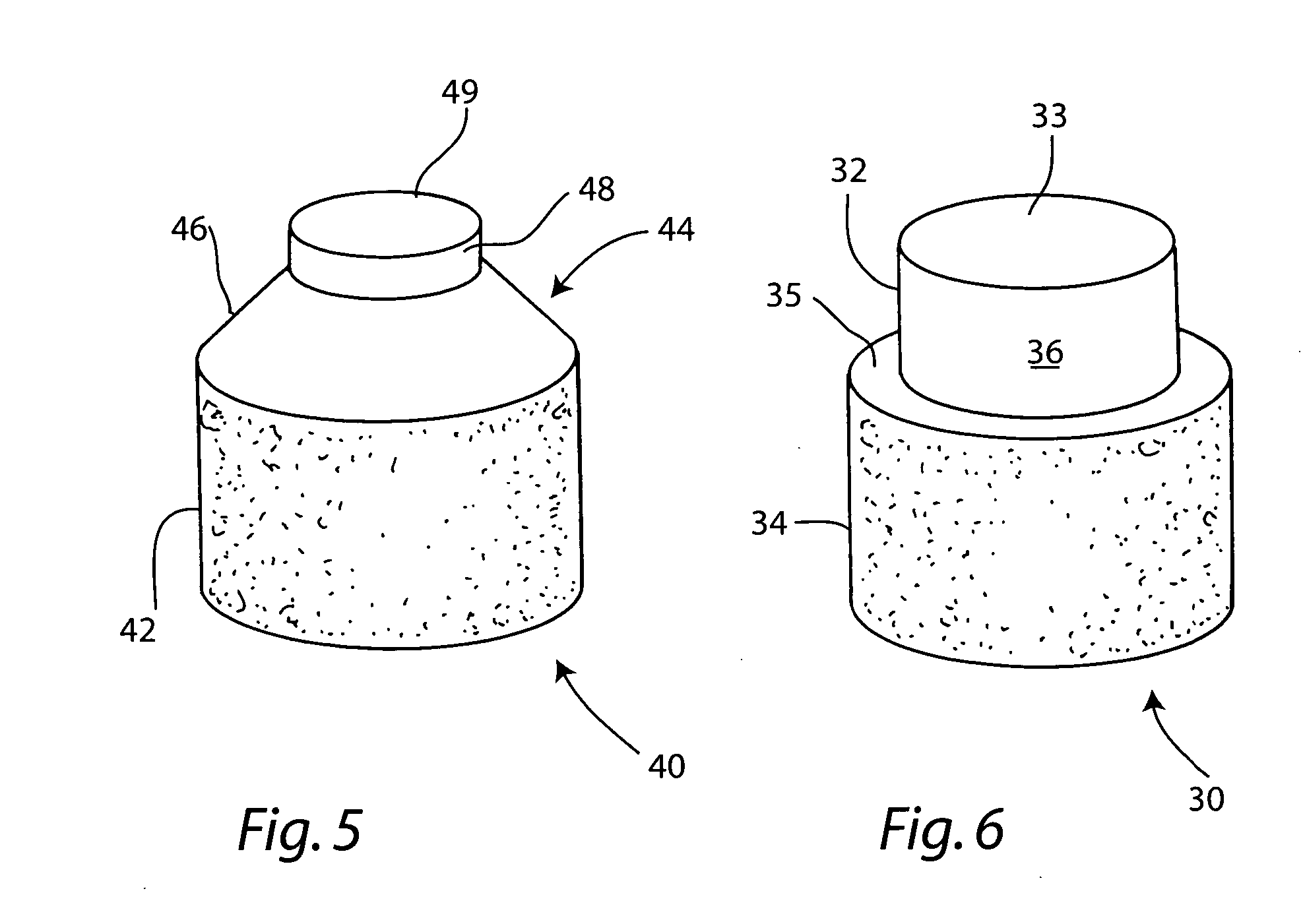
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Method for performing automated microfracture
The present invention is directed to a method for repairing defects in articular cartilage and, more particularly, to a new method for performing automated microfracture on subchondral bone to repair articular cartilage. The microfractured holes on the surface of the subchondral bone plate are formed with an automated process using a pneumatically driven orthopedic microfracture instrument. The instrument moves a fracture pin through the end of a guide tube until a sharp end of the fracture pin punctures or penetrates the subchondral bone plate and creates a microfracture or hole in the bone.
Owner:ZIMMER INC
Resorbable polymeric device for localized drug delivery
InactiveUS20050177118A1Good adhesionMinimizes procedural discomfortMedical devicesMammal material medical ingredientsOsteoblastHigh doses
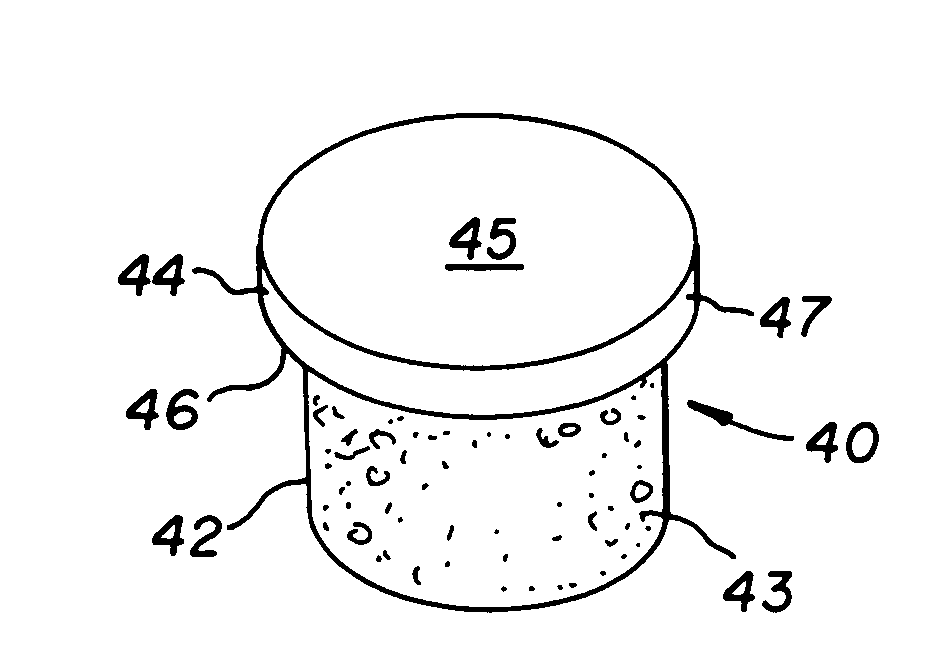
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. Another embodiment is arranged for the local delivery of therapeutic agent. A preferred embodiment is a porous resorbable implant, wherein the therapy delivery may be localized in nature, rather than systemic, such that higher doses at the target site may be allowed than would be tolerable by the body systemically.
Owner:KENSEY NASH CORP
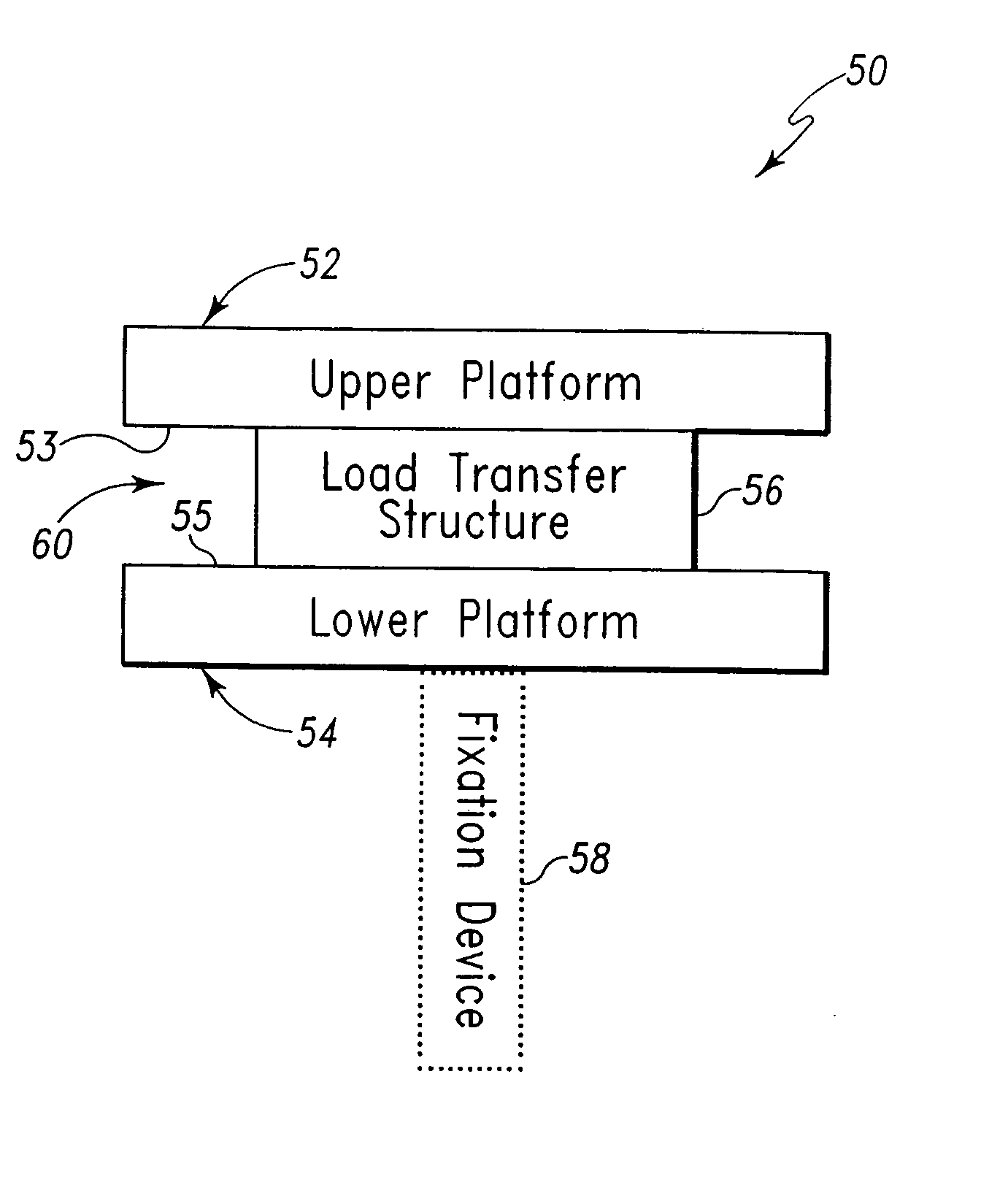
Joint implant and a surgical method associated therewith
InactiveUS20080154374A1Easy passAccurate placementFinger jointsAnkle jointsSubchondral boneJoint fusion
A method of performing surgery to enable joint fusion by preparing bony surfaces of a joint to create an enlarged space between sides of the joint in which subchondral bone of the joint is exposed, inserting a hollow structural implant, having at least two large fenestrations which are located on substantially opposite sides of the implant into the enlarged space so that the implant contacts the subchondral bone and orientating the implant so that the large fenestrations are located adjacent the subchondral bone on respective sides of the joint.
Owner:DEPUY MOTION
Joint Arthroplasty Devices
A mobile bearing implant includes a first component. The first component includes a bone facing surface for engaging one of a substantially uncut articular cartilage surface and a substantially uncut subchondral bone surface. The bone facing surface substantially matches the one of the articular cartilage surface and the subchondral bone surface. The mobile bearing implant further includes an external surface. A bearing component has a first surface for slidingly engaging the external surface of the first component, and a second surface for engaging at least one of a second component, bone, and cartilage.
Owner:CONFORMIS
Implant device and method for manufacture
A knee implant includes a femoral component having first and second femoral component surfaces. The first femoral component surface is for securing to a surgically prepared compartment of a distal end of a femur. The second femoral component surface is configured to replicate the femoral condyle. The knee implant further includes a tibial component having first and second tibial component surfaces. The first tibial component surface is for contacting a proximal surface of the tibia that is substantially uncut subchondral bone. At least a portion of the first tibial component surface is a mirror image of the proximal tibial surface. The second tibial component surface articulates with the second femoral component surface.
Owner:CONFORMIS
Device for regeneration of articular cartilage and other tissue
InactiveUS20050074481A1Good adhesionMinimizes procedural discomfortBone implantMammal material medical ingredientsSubchondral boneOsteoblast
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. A hydrophobic barrier exists between the regions, of this embodiment. In one embodiment, the polyelectrolytic complex transforms to hydrogel, following the implant procedure.
Owner:KENSEY NASH CORP
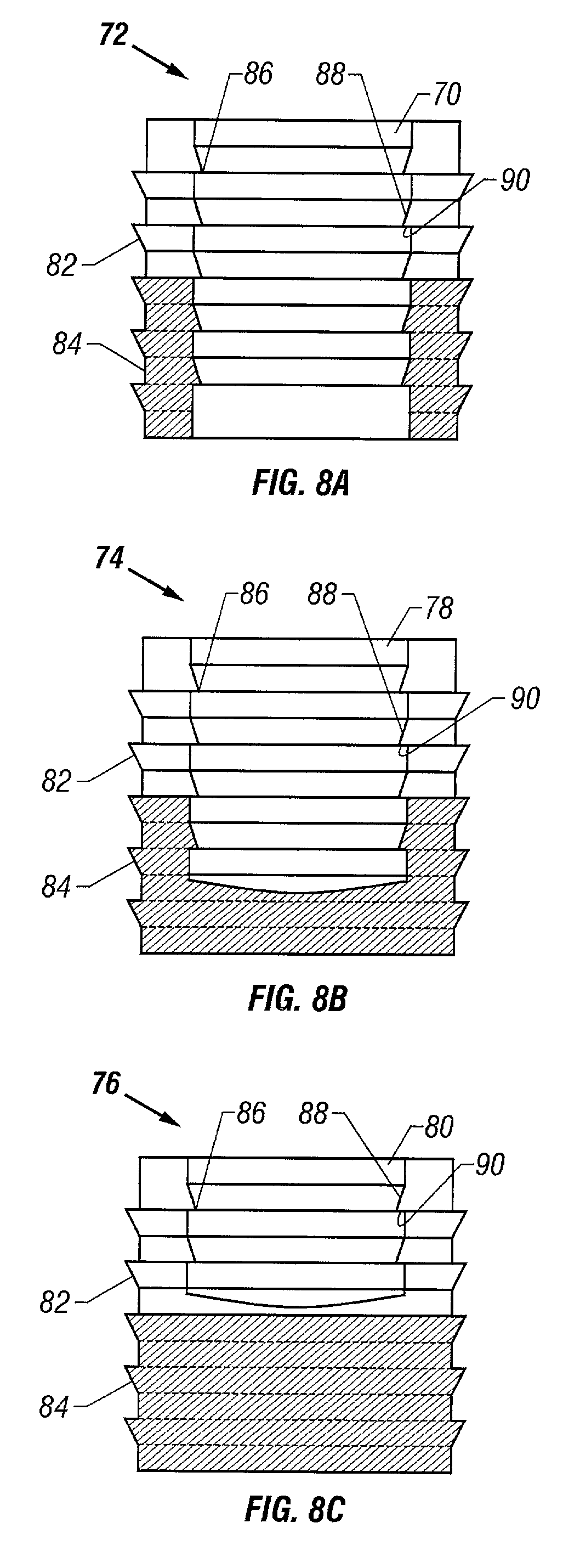
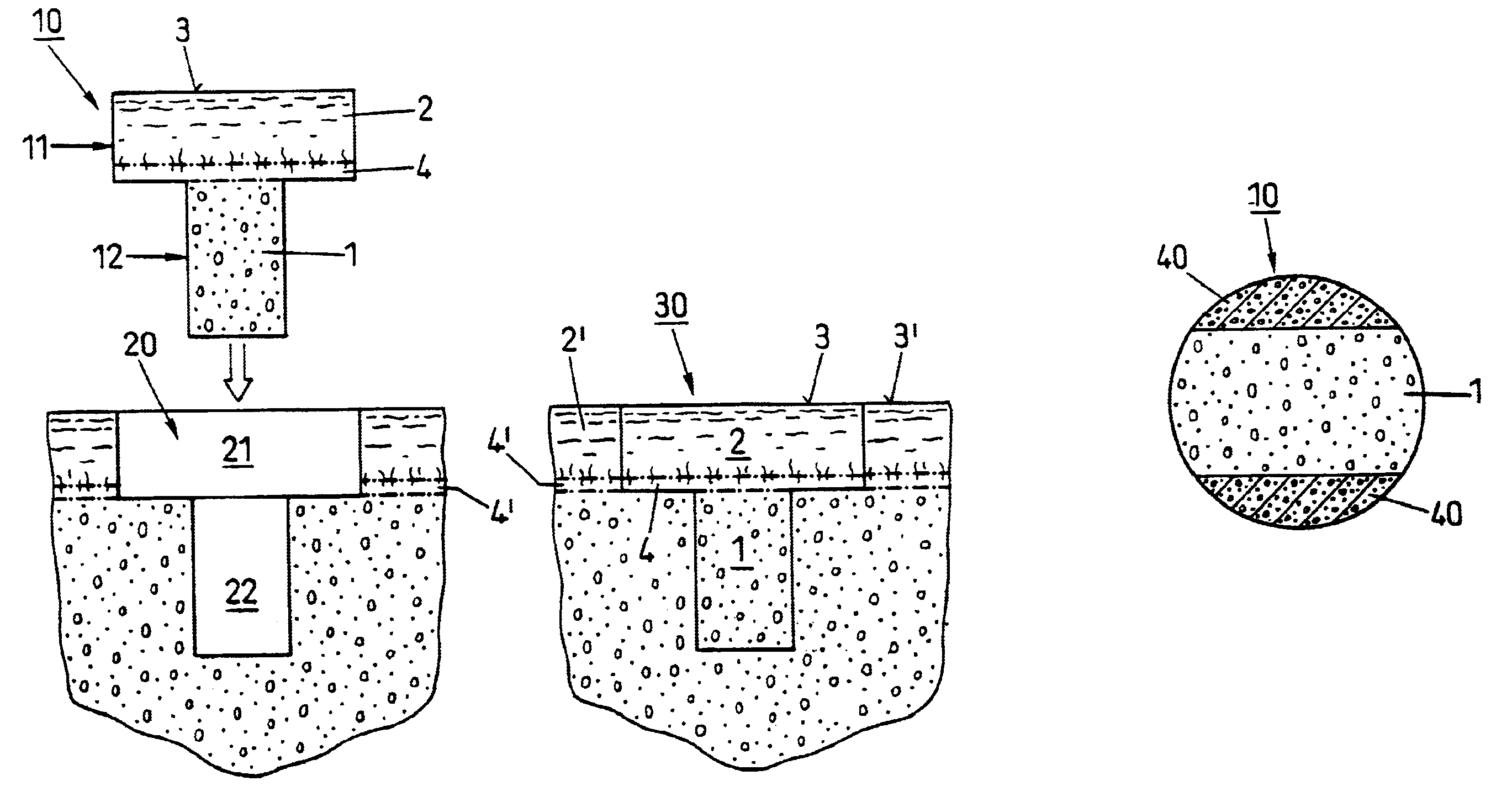
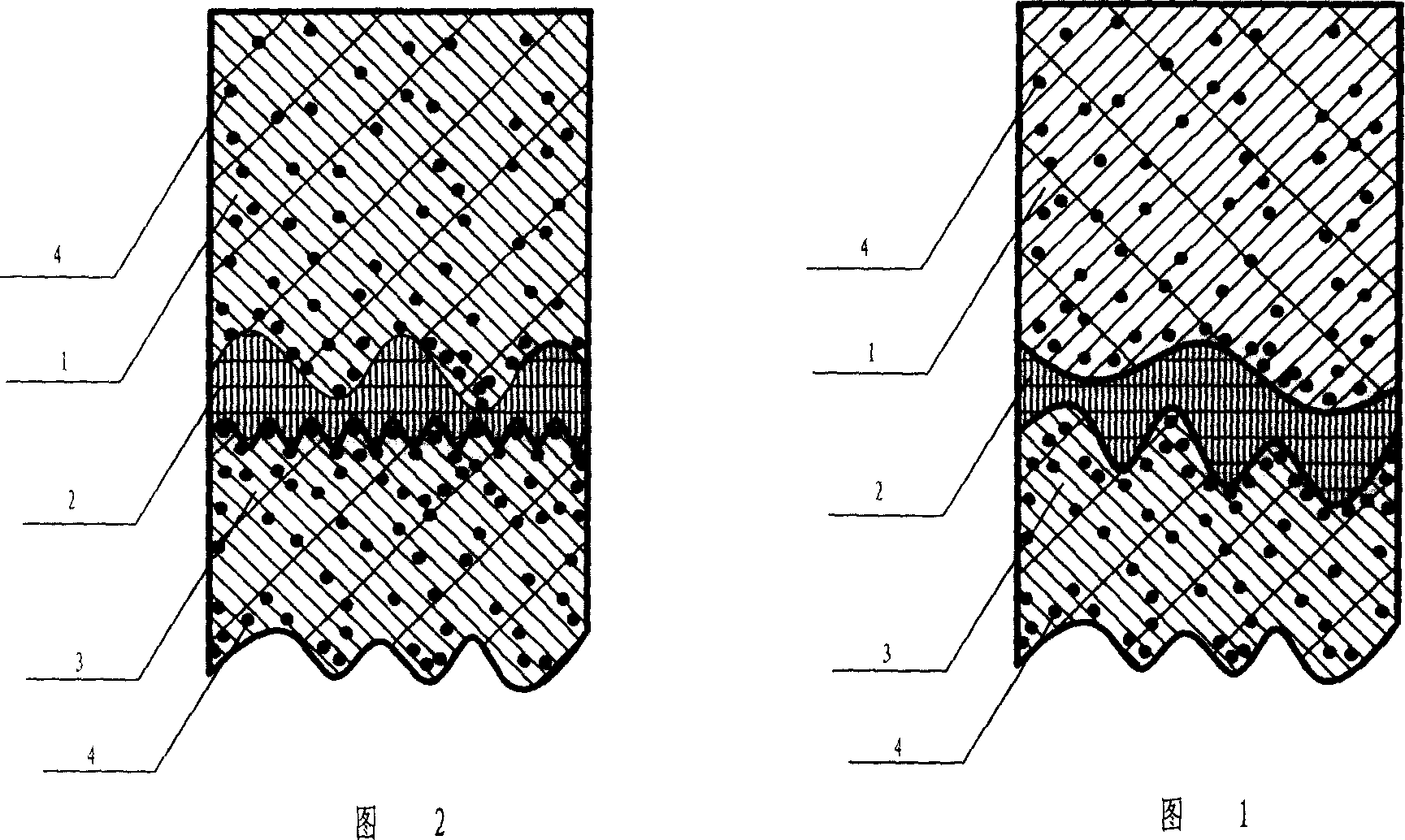
Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints
InactiveUS6858042B2Reduce absorptionResistant to resorptionBone implantJoint implantsRepair tissueCyst
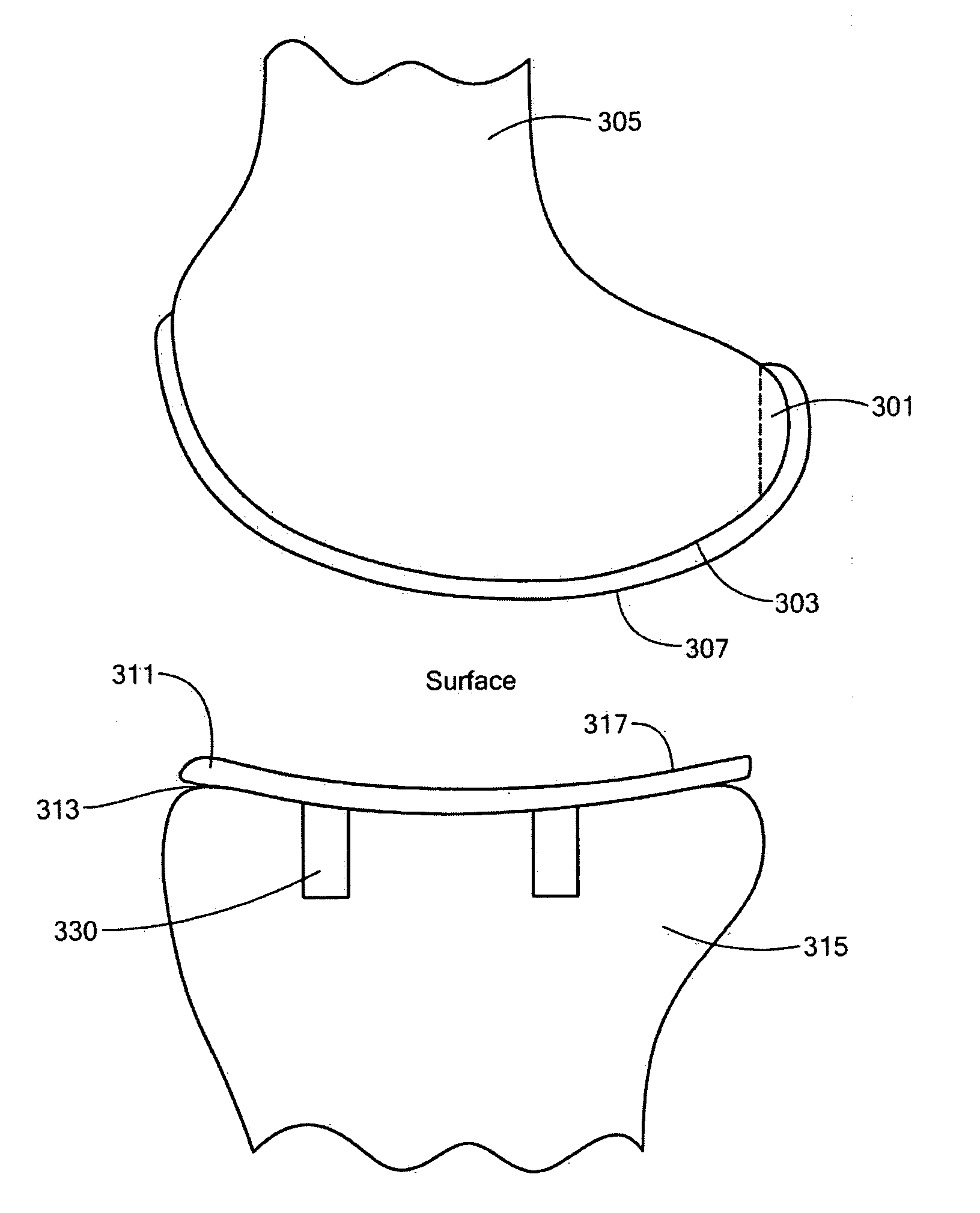
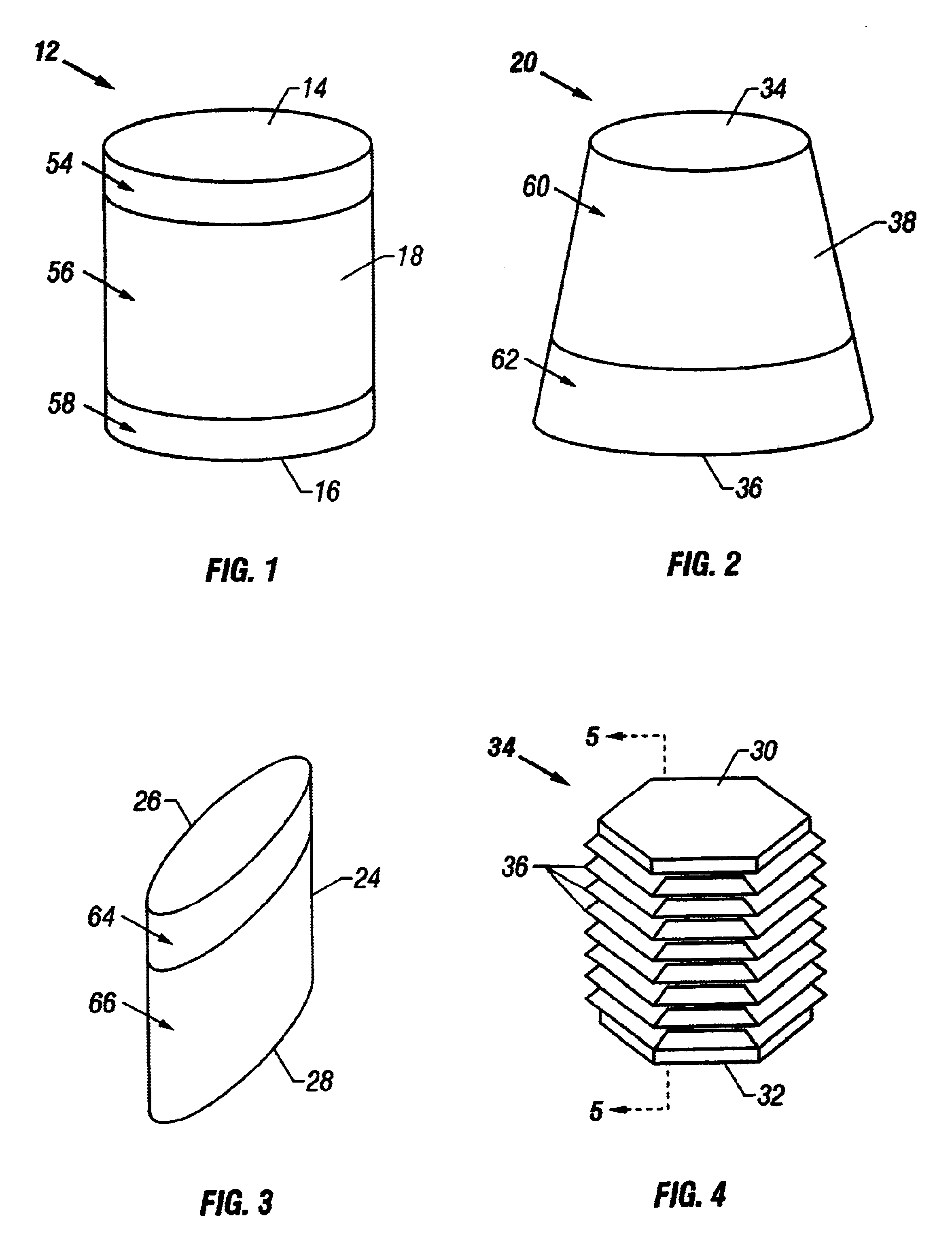
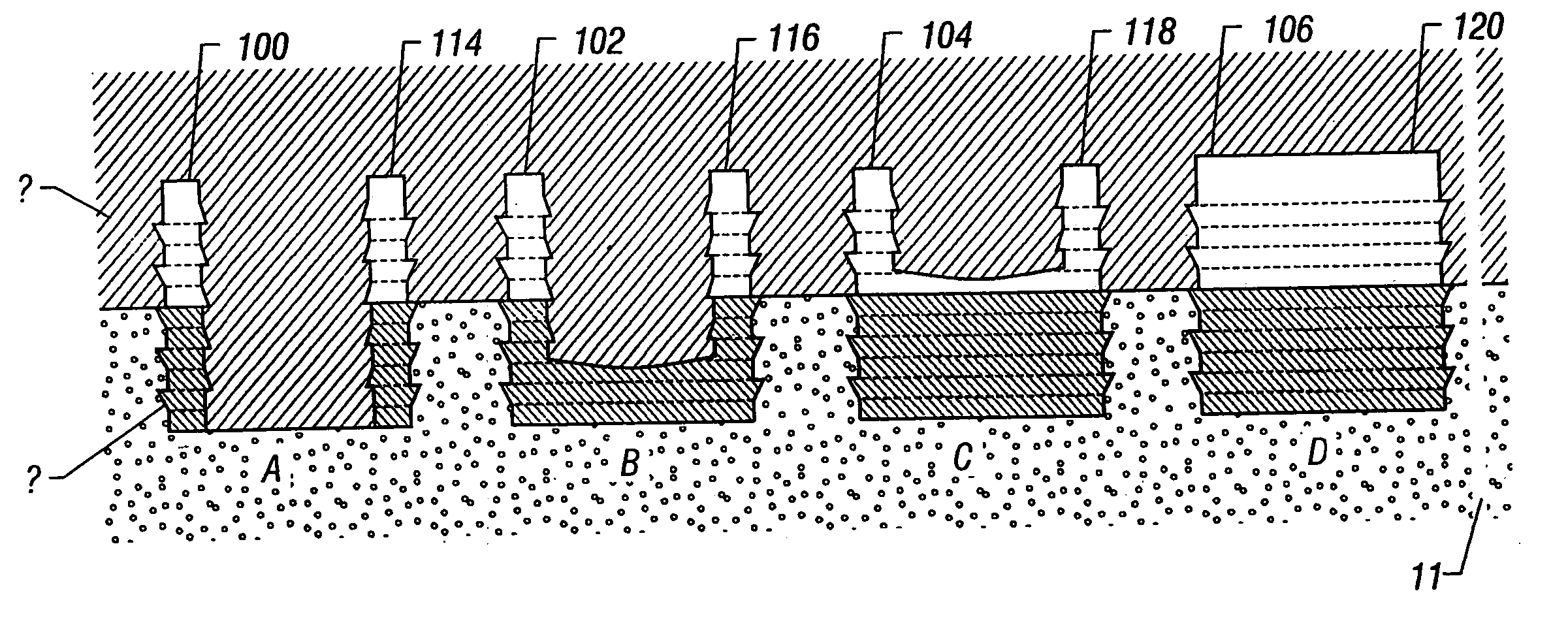
Repairs of cartilage defects or of cartilage / bone defects in human or animal joints with the help of devices including a bone part (1), a cartilage layer (2) and a subchondral bone plate (4) or an imitation of such a plate in the transition region between the cartilage layer (2) and the bone part (1). After implantation, the bone part (4) is resorbed and is replaced by reparative tissue only after being essentially totally resorbed. In a critical phase of the healing process, a mechanically inferior cyst is located in the place of the implanted bone part (1). In order to prevent the cartilage layer (2) from sinking into the cyst space during this critical phase of the healing process the device has a top part (11) and a bottom part (12), wherein the top part (11) consists essentially of the cartilage layer (2) and the subchondral bone plate (4) and the bottom part (12) corresponds essentially to the bone part (1) and wherein the top part (11) parallel to the subchondral bone plate (4) has a larger diameter than the bottom part (12). After implantation in a suitable opening or bore (20), the cartilage layer (2) and the subchondral bone plate (4) are supported not only on the bone part (1) but also on native bone tissue having a loading capacity not changing during the healing process. Therefore, the implanted cartilage layer cannot sink during the healing process.
Owner:ZIMMER GMBH
Cartilage allograft plug
InactiveUS7901457B2Promote migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cartilage allograft plug
InactiveUS7488348B2Guaranteed functionEasily placed in defect areaBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
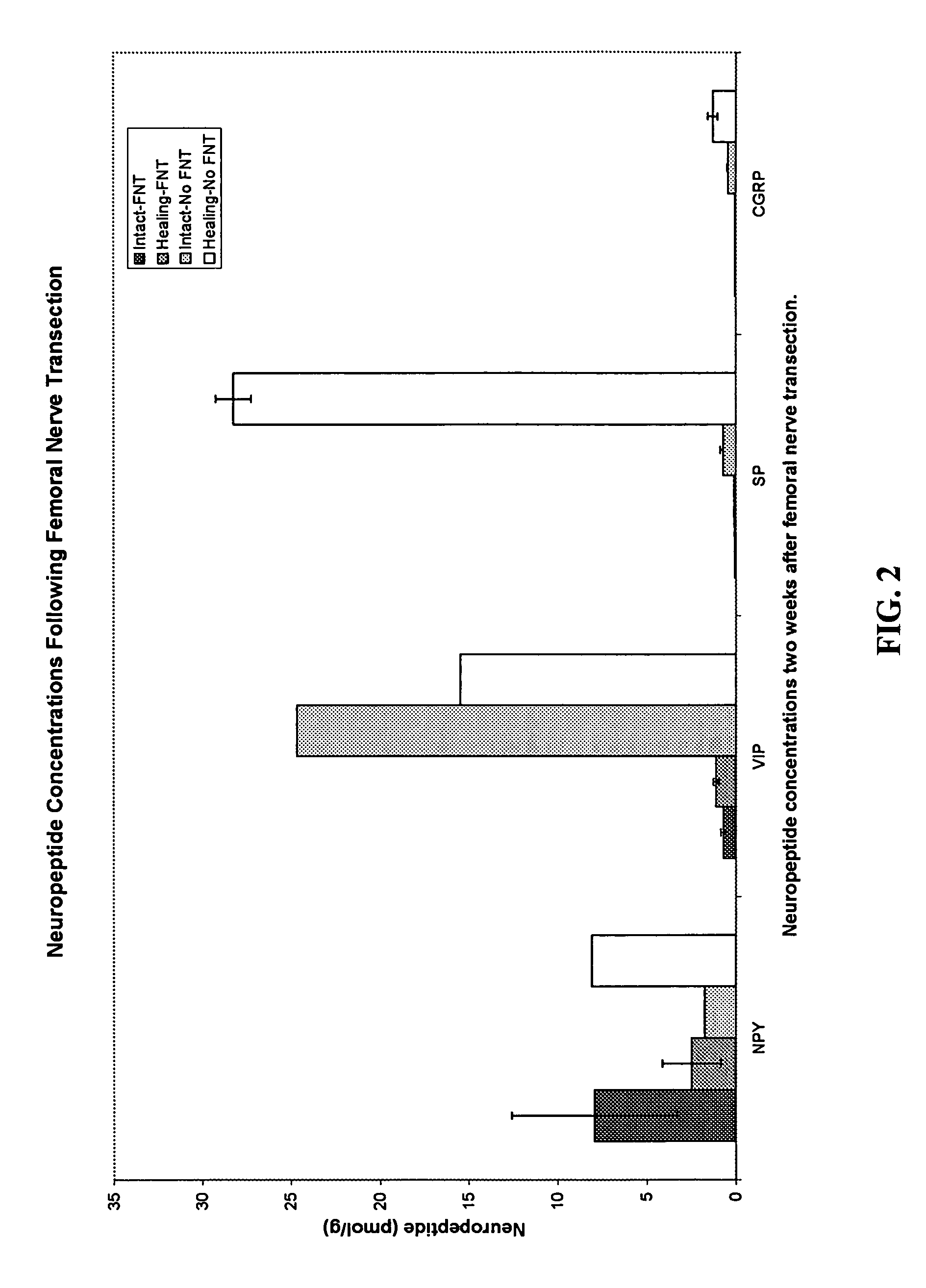
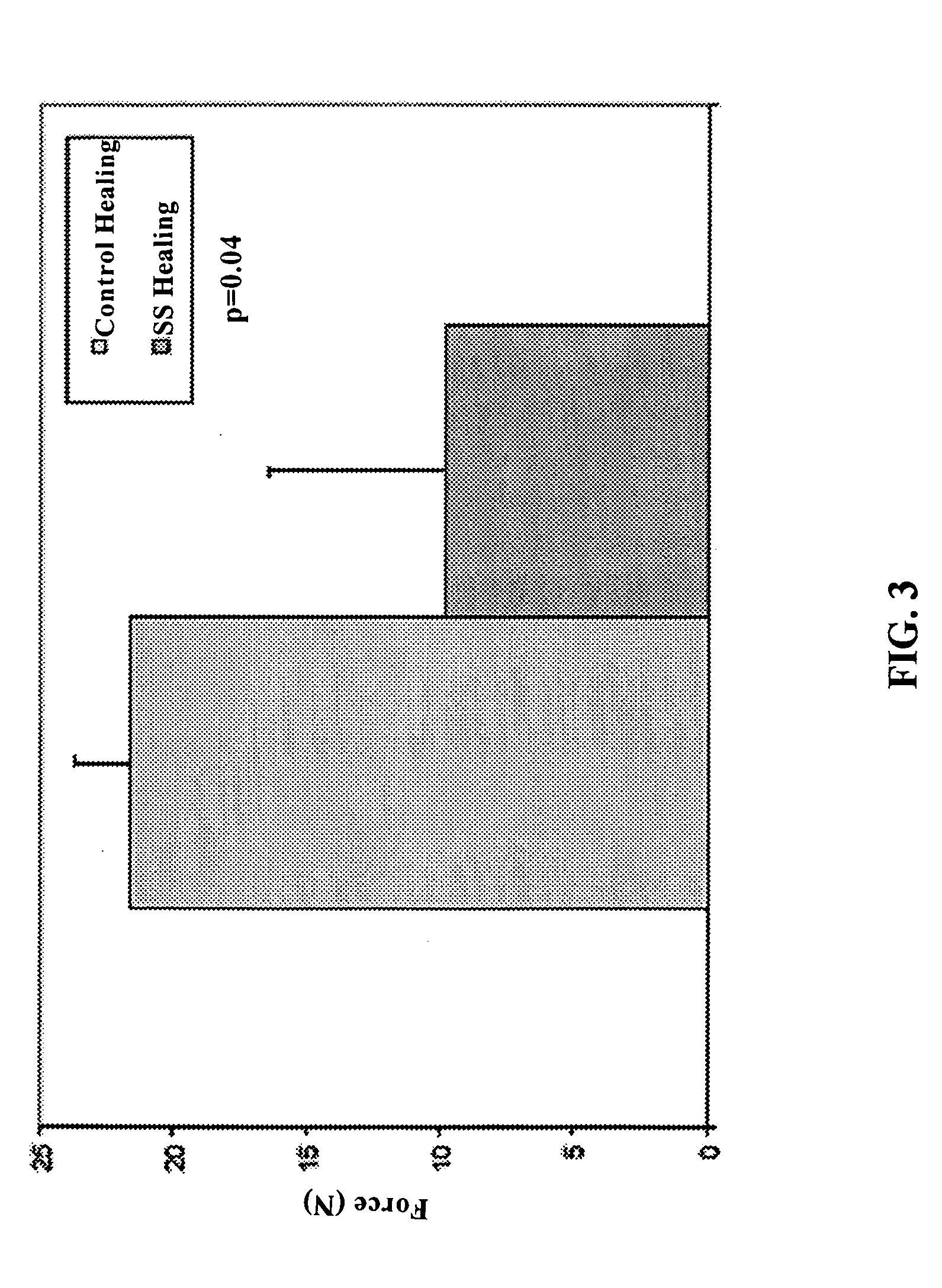
Use of neuropeptides for ligament, cartilage, and bone healing
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged cartilage and subchondral bone. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of cartilage and bone damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Joint support and subchondral support system
InactiveUS20100145451A1Increasing the thicknessImprove abilitiesSurgeryLigamentsVariable thicknessSupporting system
A joint support and subchondral support system for providing structural and dampening support to damaged subchondral bone in generalized or discrete arthritis includes a contoured, porous plate having a variable shaped inner surface, outer surface, and peripheral surface of variable thickness extending between the inner surface and the outer surface, suitable for insertion within the subchondral bone. The inner surface, outer surface and peripheral surface each have a concave portion and a convex portion. A guide pin hole or slot is located within the contoured, porous plate to aid in insertion and placement of the plate over at least one corresponding guide pin within the subchondral bone. The joint support and subchondral support system of the present invention is applicable to many parts of the joint as any area with cartilage disease has an adjoining subchondral component.
Owner:DEE DEREK
System and method for joint restoration by extracapsular means
InactiveUS7867235B2Restoration of joint spaceIncrease spaceInternal osteosythesisBone implantSubchondral boneEngineering
A system and method for joint restoration by extracapsular means includes an actuator operable to apply a force to a portion of a bone to effect a change in the joint space geometry. One embodiment of the system includes an actuator operable to apply a cyclic loading to subchondral bone of a femur, wherein loads of a predetermined magnitude are alternately applied and released. Between periods of cyclic loading, rest periods are provided where no load is applied. Over time, the femoral joint surface is remodeled in accordance with the location, direction, magnitude, and frequency of the loading.
Owner:FELL BARRY M +1
Cartilage repair plug
InactiveUS20040162622A1Easy inflowEasy to fixBone implantDiagnosticsCartilage repairSurgical department
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease is disclosed. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. Additionally, ridges may be formed about the periphery of each plug to facilitate its anchoring to surrounding cartilage, bone and / or adjacent plugs. A procedure for resecting damaged or diseased cartilage and for implanting a replacement plug or plugs according to this invention, as well as a set of instruments for effecting the procedure, and a self-contained system for orthopaedic surgeons, which includes a variety of differently sized and shaped plugs, as well as a set of instruments for the procedure are also disclosed.
Owner:ABS
Allograft osteochondral plug combined with cartilage particle mixture
InactiveUS20090291112A1Promoting cartilage cell migration intoPromoting proliferationBiocidePeptide/protein ingredientsInterference fitSubchondral bone
An allograft osteochondral plug is combined with a mixture that includes freeze-milled cartilage particles, and such combination is used to repair defects in articular cartilage. The plug includes an subchondral bone portion and an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans. At least a portion of the plug has a lateral dimension selected to form an interference fit against a tissue layer exposed as a result of a bore formed in a defect area in articular cartilage of a host. The cartilage particle mixture is placed adjacent at least a portion of the plug for promoting cartilage cell migration into (i.e., from the adjacent host cartilage) and proliferation in the bore, and for enhancing tissue integration between the plug and patient (i.e., host) tissue when the plug is inserted into the bore. Methods for surgical implantation of the plug into a patient are also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cartilage allograft plug
ActiveUS20090069901A1Increase chondrocyte migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Integral engineering rack of interface osteochondro tissue with bionic function
InactiveCN101020083AIncrease connection areaImprove connection strengthJoint implantsSubchondral boneBiocompatibility Testing
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles
Constructs that are at least partially constructed of allograft cancellous bone are disclosed, along with cartilage particles that may be used with the constructs for repairing articular cartilage defects. A multi-piece construct includes a base member, a cap member and at least one pin that secures the cap member to the base member. The base member may be constructed of mineralized cancellous bone, and is used to replace the subchondral bone removed when a surgeon cuts a bore in the area of an adjacent cartilage defect. The base member includes a blind bore and first and second through-going transverse bores in opposite sides of a wall of the base member. The cap member includes an upper section that has a thickness that is similar to that of a patient's surrounding articular cartilage layer and a stem depending from the upper section that is dimensioned to be received in and by the blind bore of the base member. The stem includes a transverse through-going bore, which may be aligned with the transverse through-going bores of the base member to receive the pin therein when the construct has been assembled. The cap member is at least partially formed of demineralized allograft cancellous bone, into which a mixture containing lyophilized, freeze-milled allograft cartilage particles may be infused for the repair of articular cartilage defects. The cartilage particles have a size within a range of from about 10 microns to about 210 microns.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
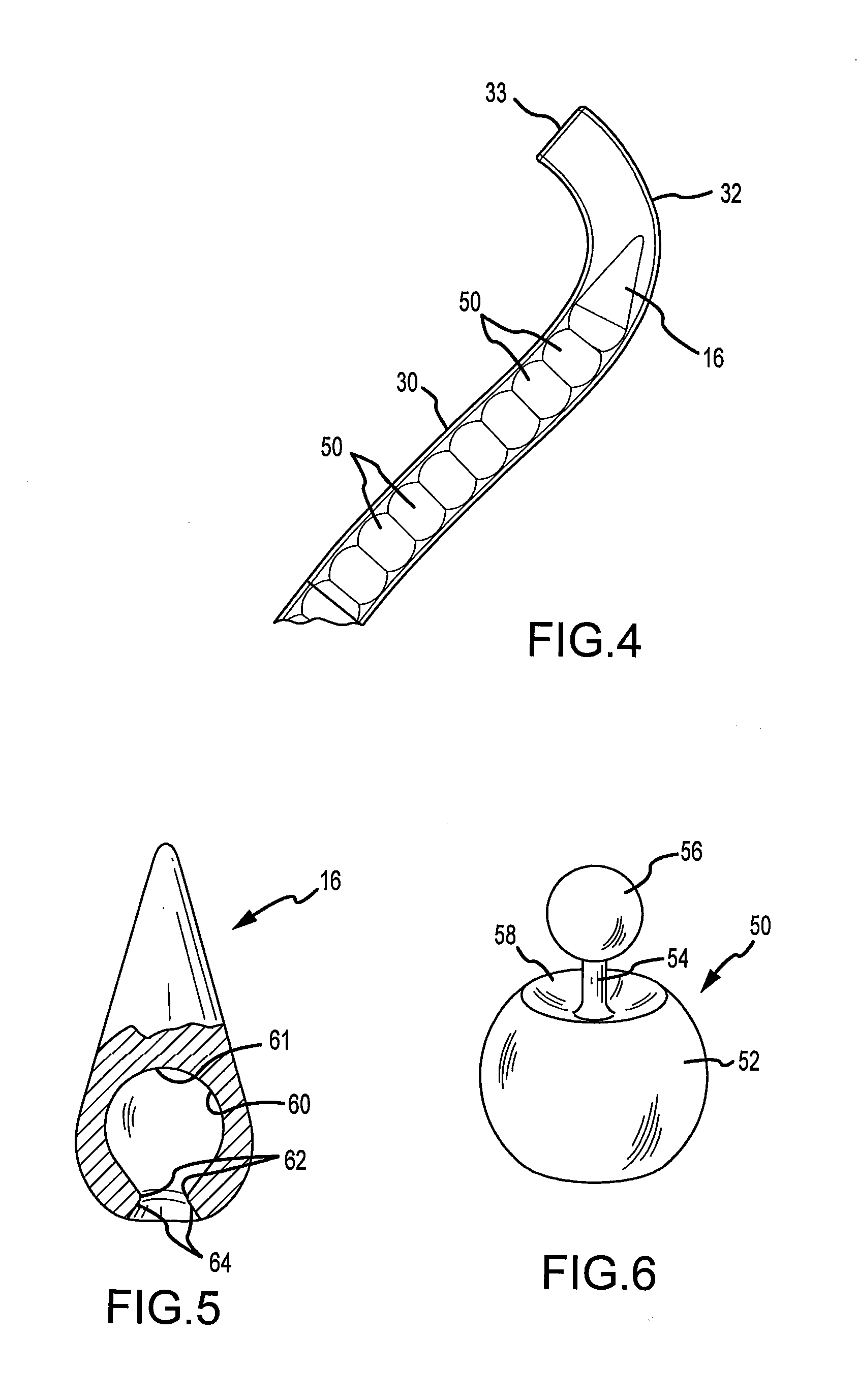
Microfracture awl
InactiveUS20120071876A1Cost of manufacturing be satisfiedCost of sterilization be satisfiedProsthesisOsteosynthesis devicesFibrocartilageBone marrow cell
A microfracture awl includes an articulating portion that enables the microfracture awl to strike a subchondral bone plate at a precise desired angle to prevent scuffing or undue scraping of the targeted subchondral bone plate. The articulating portion of the microfracture awl is received through a guide sleeve. A proximal end of the articulating portion is rigid and connects to a base. The articulating portion is placed through a guide sleeve and the base resides in a handle attached to the sleeve. The guide sleeve has a curved distal end. The practitioner strikes the rigid base of the microfracture awl and thereby transmits a force to the articulating portion of the awl however, the force is not transmitted to the guide sleeve or handle. Accordingly, the articulating portion of the awl may smoothly travel through the guide sleeve at the angle defined by the curved distal end of the guide sleeve, thereby angularly positioning the tip of the awl with the targeted bone plate. The articulating portion includes a plurality of articulating members configured in a series of ball and socket configurations. A method includes selection of a guide sleeve with the desired curvature and impacting the awl a number of times at the targeted area to produce a desired effect with respect to the release of bone marrow cells to induce growth of fibrocartilage tissue.
Owner:HONDA MOTOR CO LTD +1
Implant device for cartilage regeneration in load bearing articulation regions
An implant device for cartilage regeneration in loading-bearing regions uses the osteochondral defect model. The implant is formed of resorbable polymeric materials. The implant is designed such that load is transmitted from the articulating surface of the bone platform through the implant to the entire area of subchondral bone of the bone platform. Application of load in this manner results in reduced subchondral bone resorption, leading to joint stabilization and maintenance of normal joint biomechanics. The implant allows for the incorporation therein of a resorbable scaffold or matrix material. The present implant solves the current inability to regenerate cartilage in load-bearing articulating surfaces using engineered scaffold devices.
Owner:DEPUY PROD INC
Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles
InactiveUS20090319045A1Enhancing chondrogenesisMitigating fibrous tissue formationBone implantSurgerySubchondral boneChondral defect
Constructs that are at least partially constructed of allograft cancellous bone are disclosed, along with cartilage particles that may be used with the constructs for repairing articular cartilage defects. A multi-piece construct includes a base member, a cap member and at least one pin that secures the cap member to the base member. The base member may be constructed of mineralized cancellous bone, and is used to replace the subchondral bone removed when a surgeon cuts a bore in the area of an adjacent cartilage defect. The base member includes a blind bore and first and second through-going transverse bores in opposite sides of a wall of the base member. The cap member includes an upper section that has a thickness that is similar to that of a patient's surrounding articular cartilage layer and a stem depending from the upper section that is dimensioned to be received in and by the blind bore of the base member. The stem includes a transverse through-going bore, which may be aligned with the transverse through-going bores of the base member to receive the pin therein when the construct has been assembled. The cap member is at least partially formed of demineralized allograft cancellous bone, into which a mixture containing lyophilized, freeze-milled allograft cartilage particles may be infused for the repair of articular cartilage defects. The cartilage particles have a size within a range of from about 10 microns to about 210 microns.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com