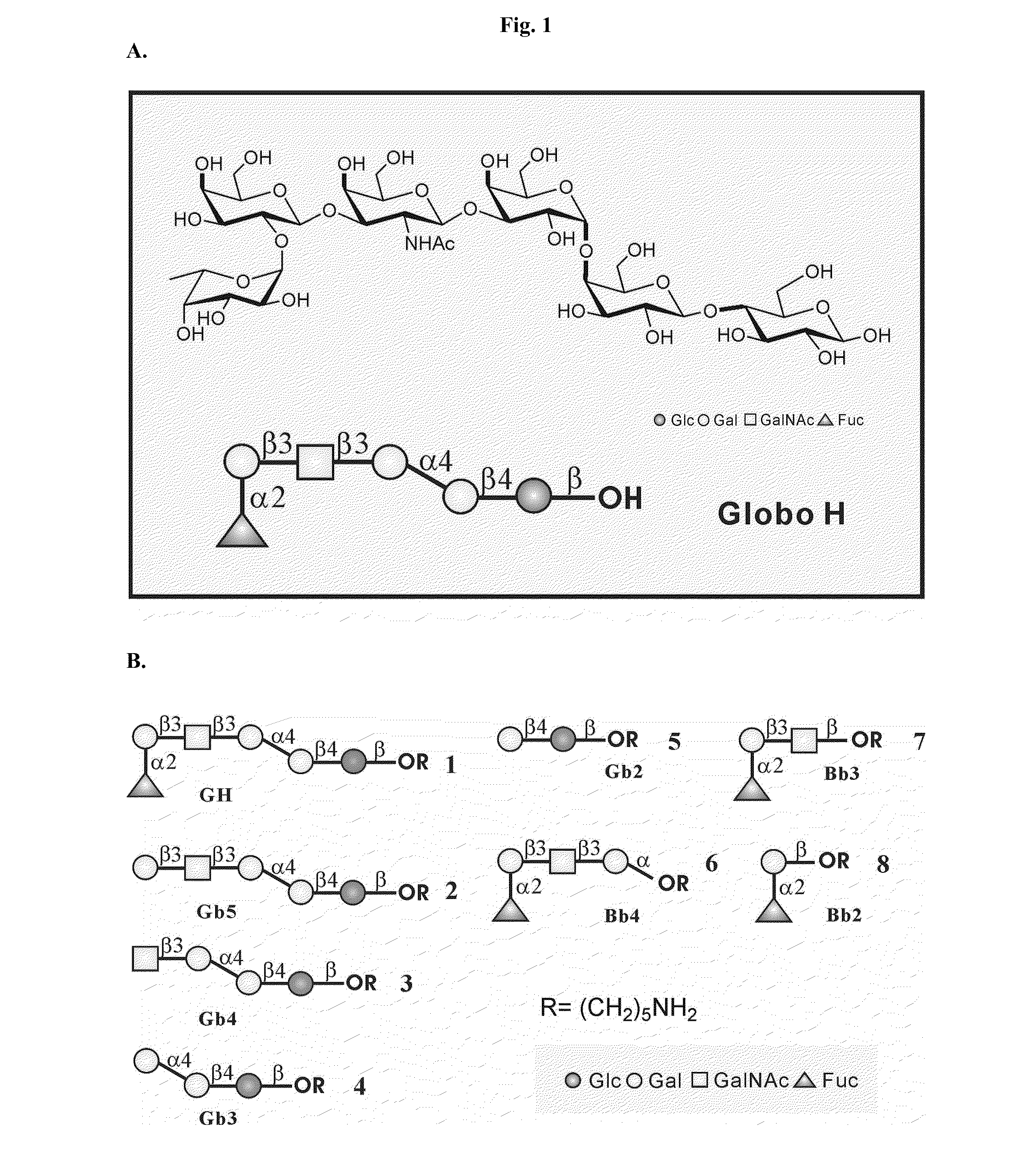
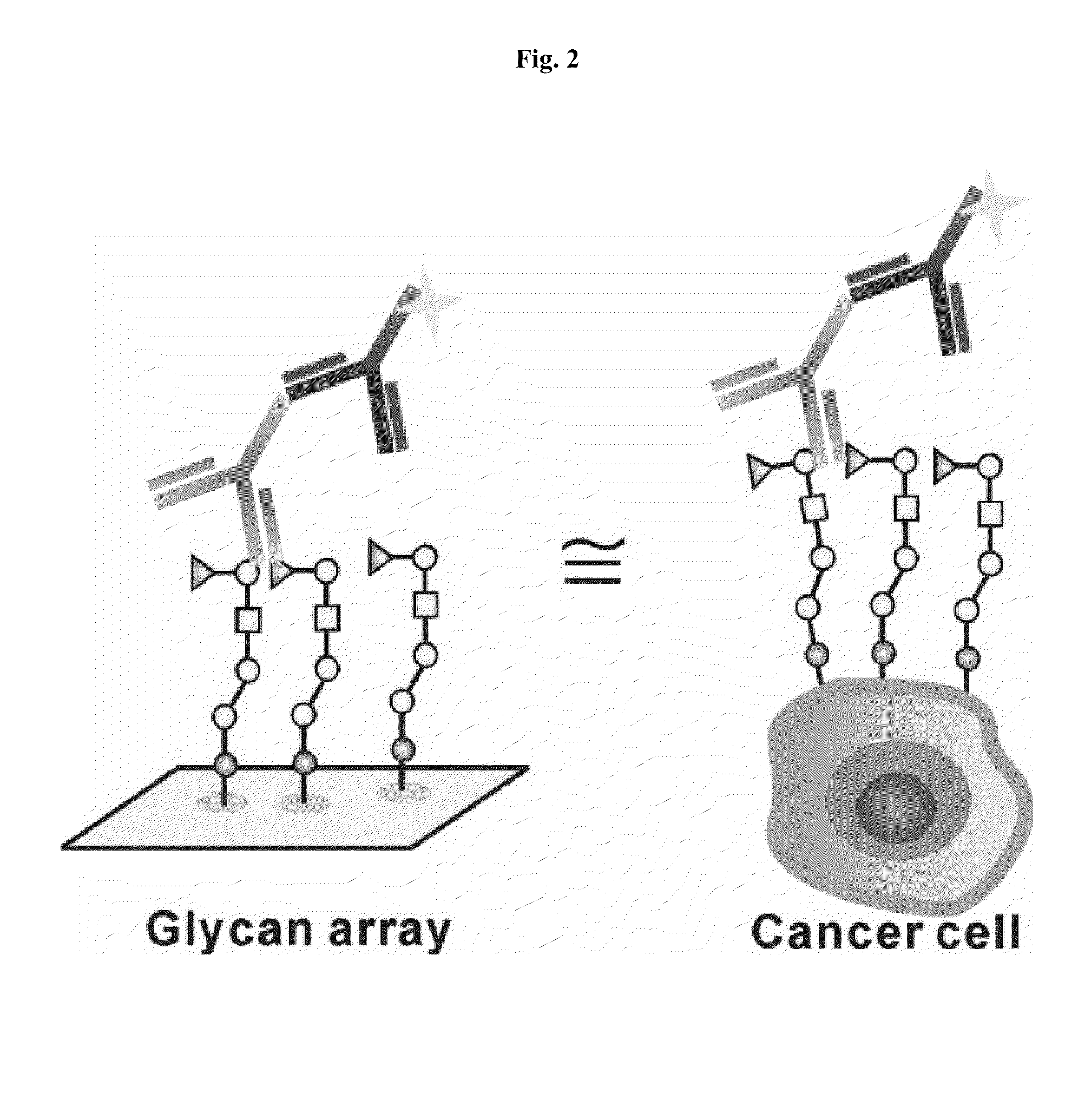
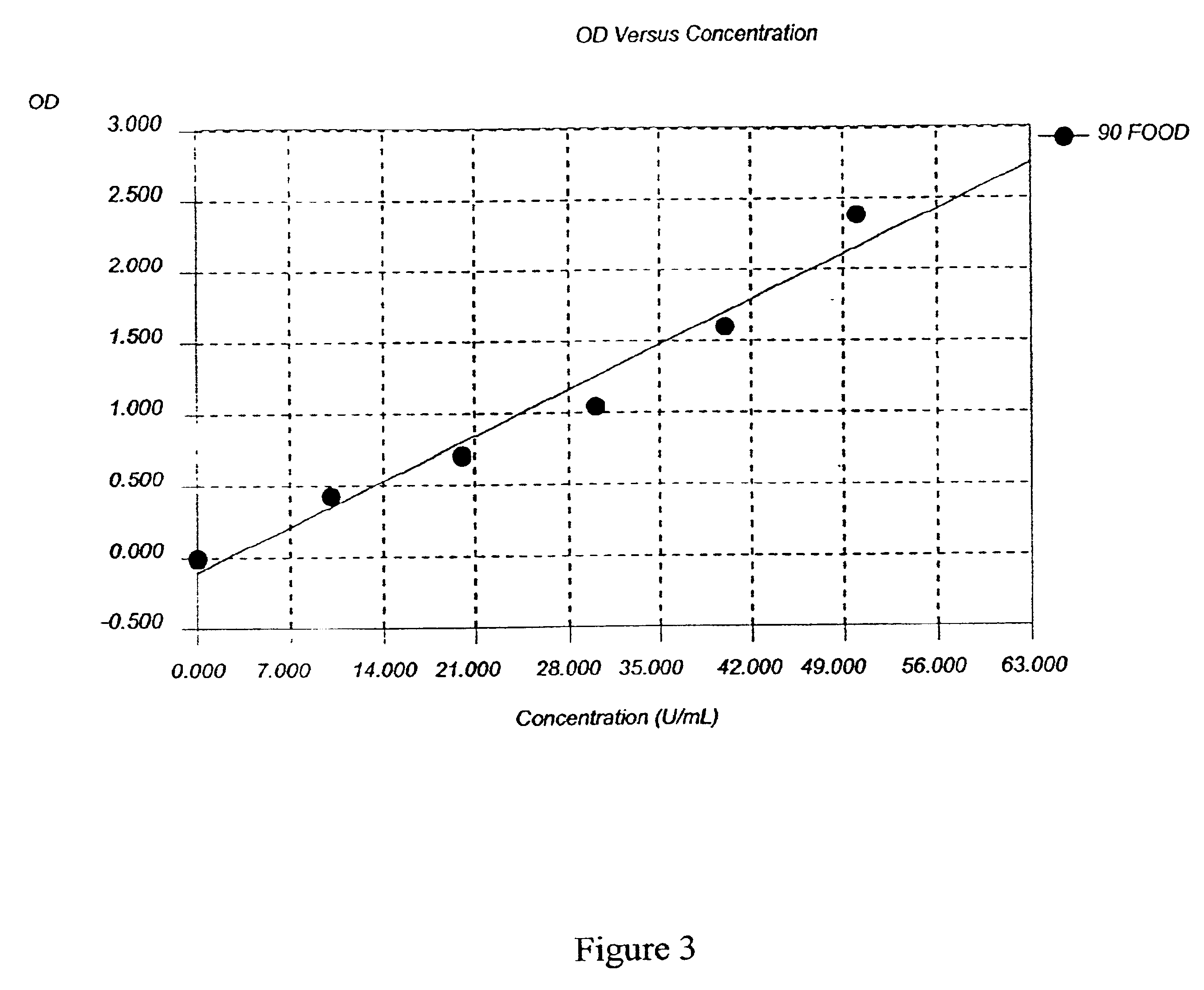
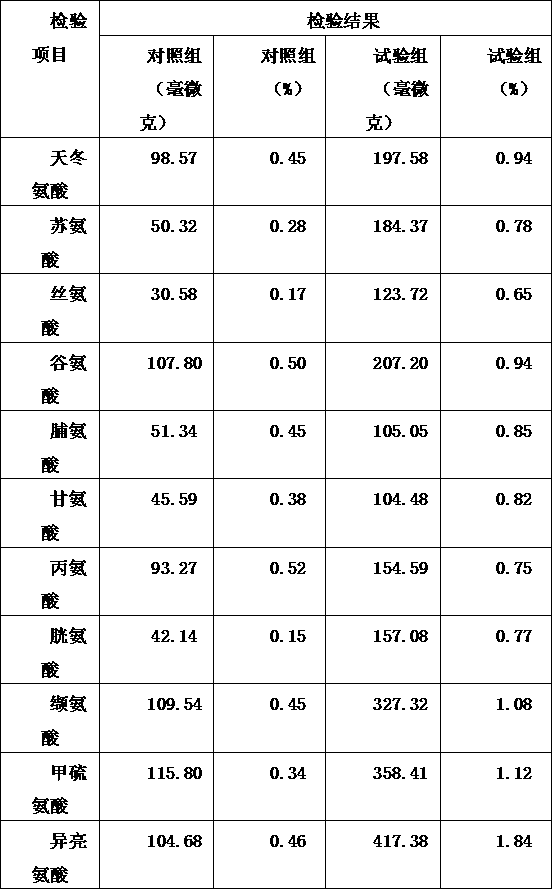
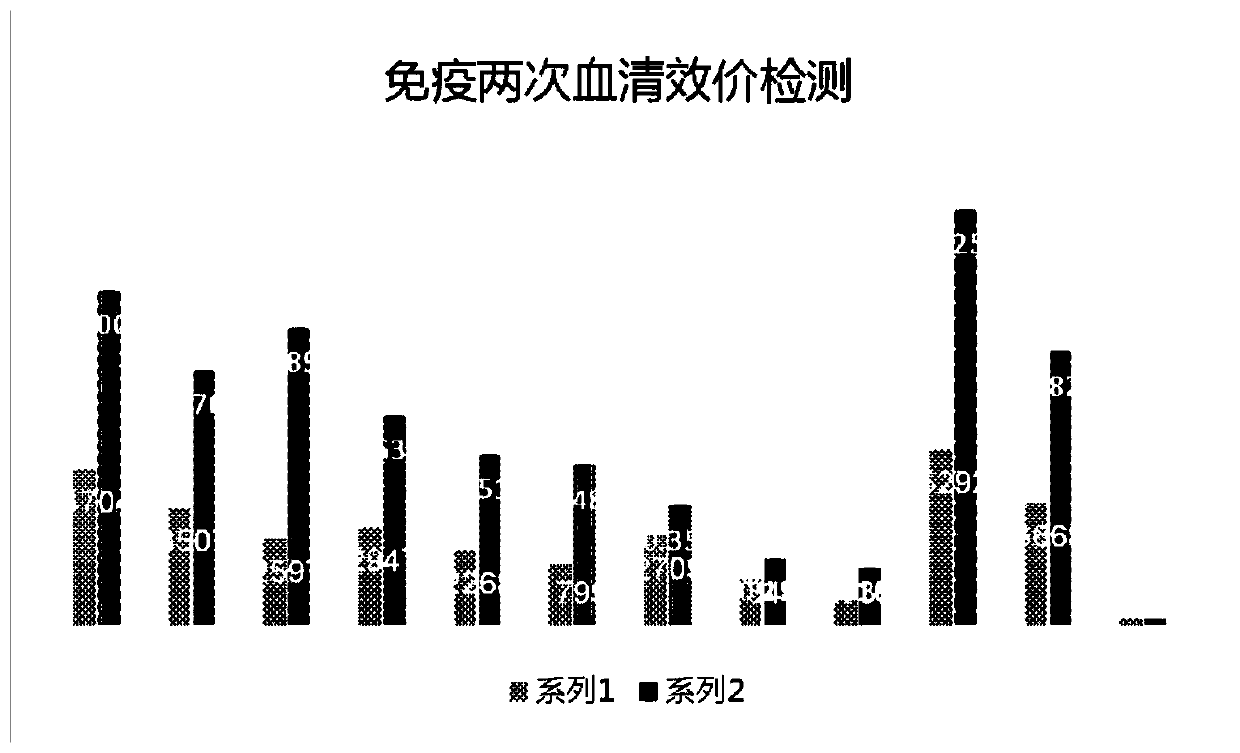
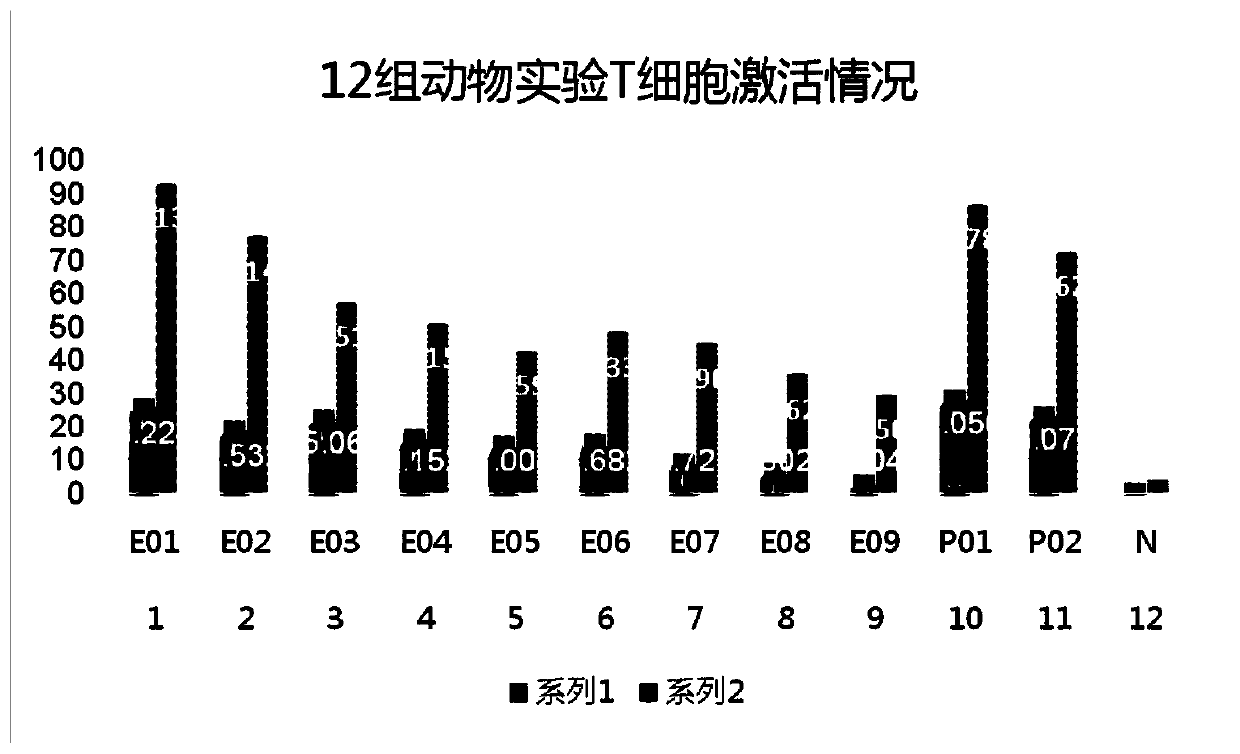
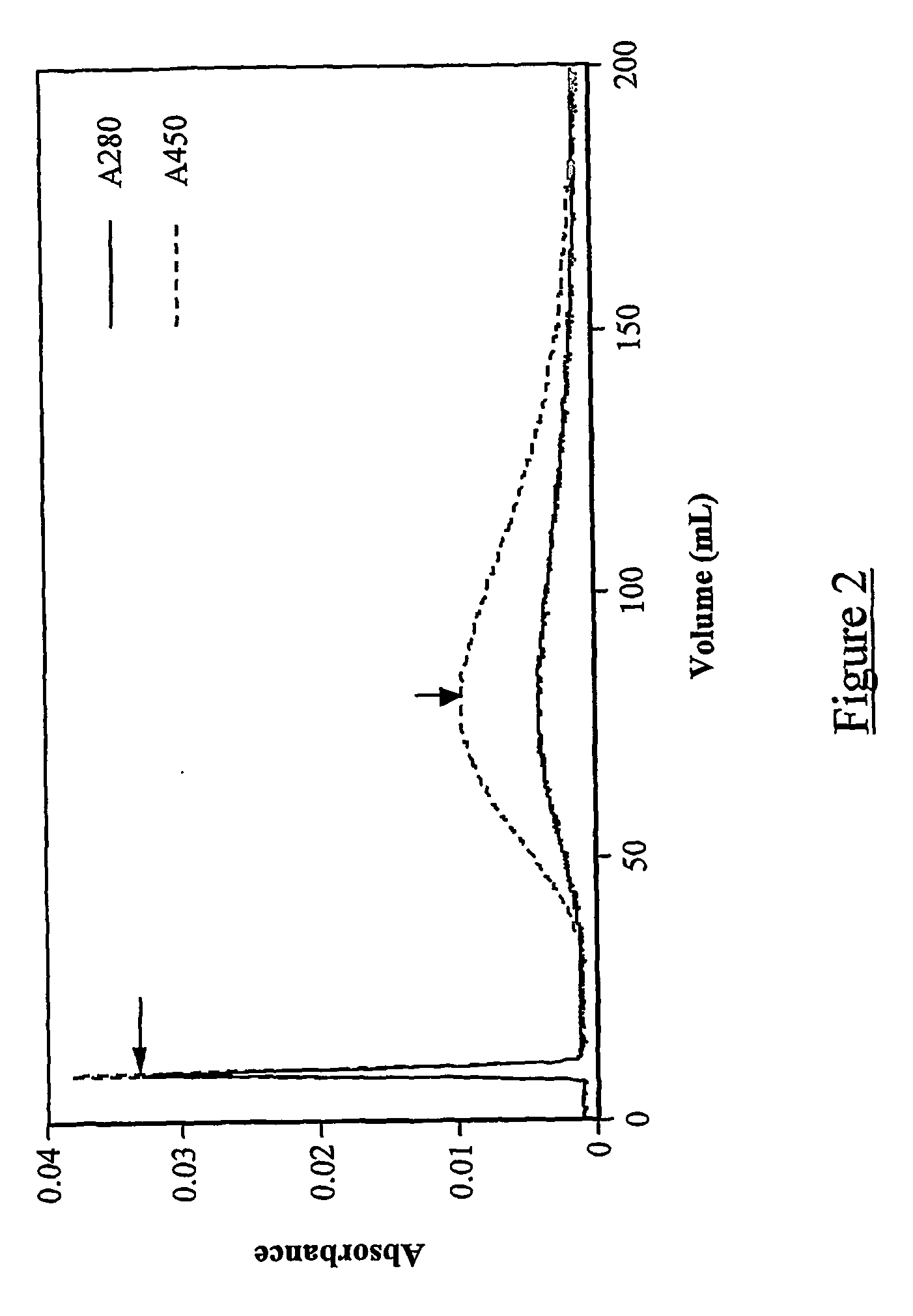
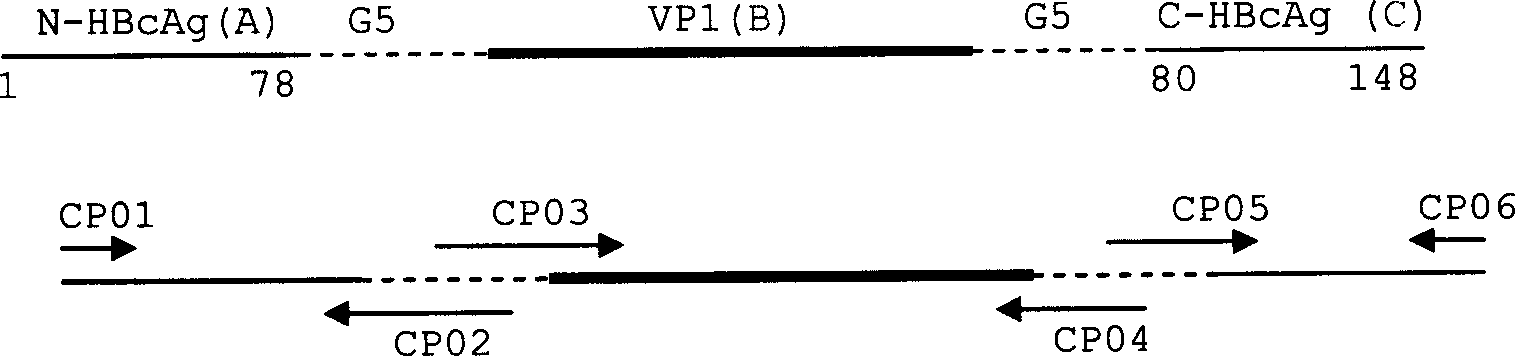
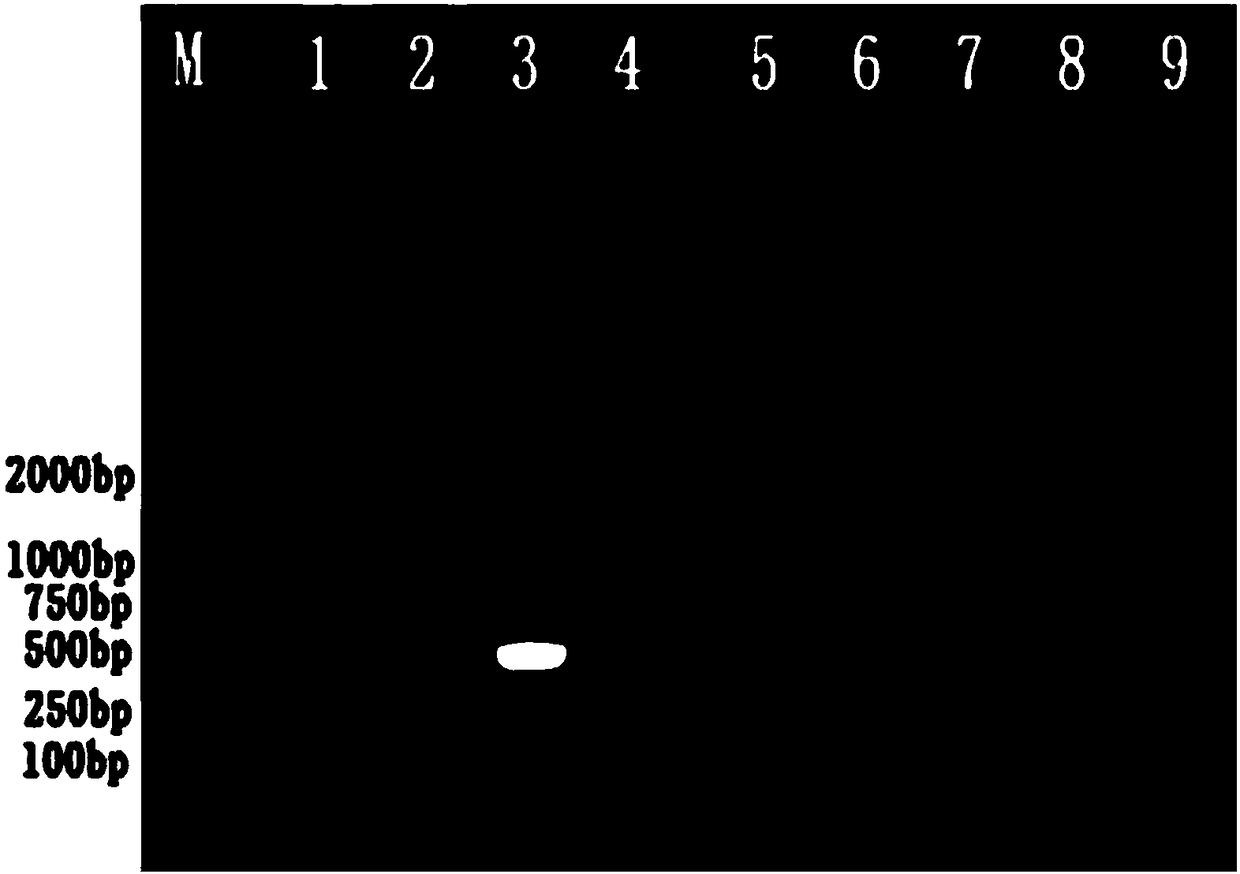
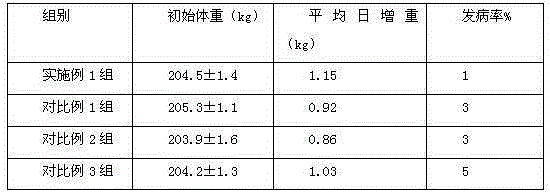
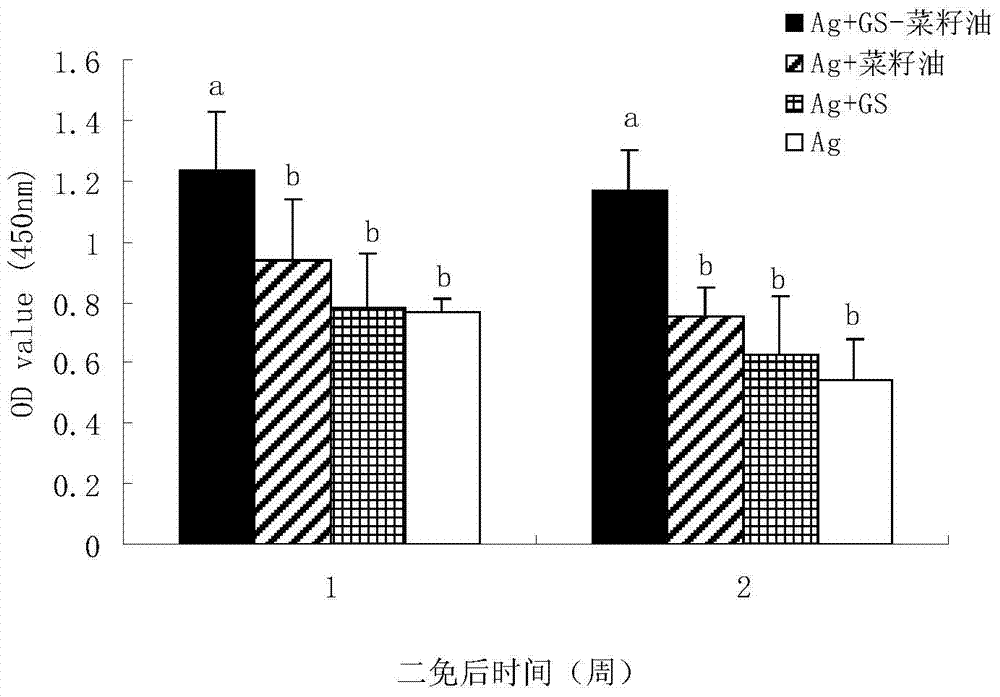
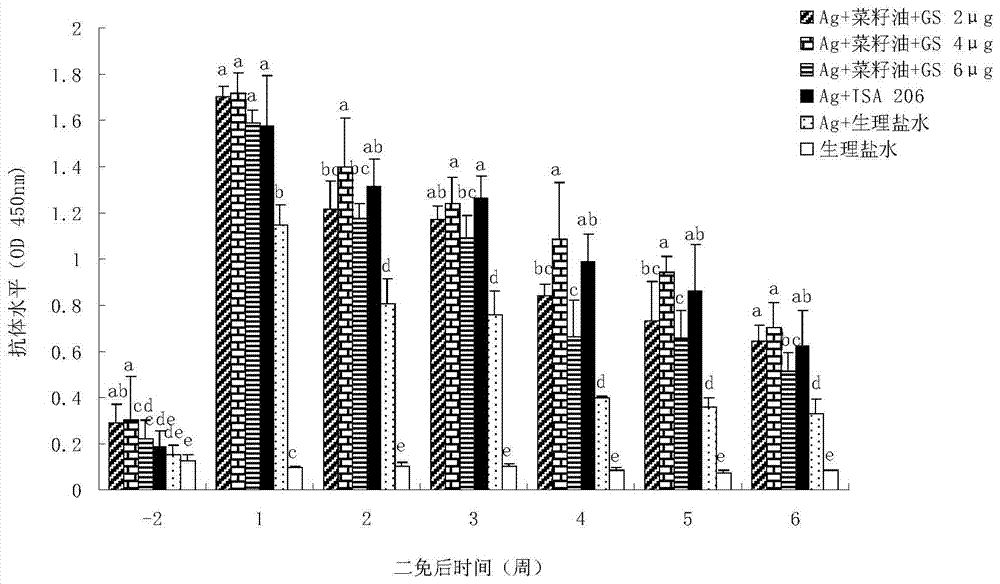
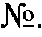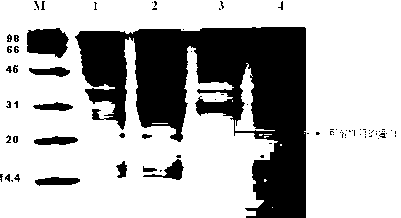Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
540 results about "Antibody level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody Test Results. Antibody levels can be determined by analyzing a blood sample. Normal values are as follows: TPO antibody: The measured serum level should be less than 9 IU/mL. Anti-Tg antibody: The measured serum level should be less than 4 IU/mL.
Saliva test for detection of food allergy and intolerance
A method for determining the presence of food allergy or food intolerance and their cross-reactive tissue antigens is disclosed. The method includes determining a level of antibodies against a dietary antigen in a mucosal sample from the patient and comparing the level with normal levels of the antibodies. Dietary antigens that were tested include milk and milk products; eggs and egg products; meat and meat products; fish, mollusks, and crustaceans and their products; oils, fats, and their products; grains and grain products; pulses, seed, kernels, nuts, and their products; vegetable and vegetable products; fruit and fruit products; sugar, sugar product, chocolate products, and confectionary; and spices.
Owner:IMMUNOSCI LAB
Method for propagating Chinese medicine ducks
ActiveCN102696548AReduce drug residuesReduce odorFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention discloses a method for propagating Chinese medicine ducks, which comprises the following steps: I, selecting a Chinese medicine formulation; II, fermenting and processing the Chinese medicine formulation to obtain zymotic fluid and fermentation body; and III, applying the zymotic fluid and the fermentation body in a method for propagating the Chinese medicine ducks in different stages. Owing to the method for propagating Chinese medicine ducks, the propagation of the Chinese medicine ducks no longer needs hormone and antibiotic, zymotic Chinese medicine formulation elaborates drug action to the maximum extent, and large-scale propagation is realized; the method has the characteristics of simple operation, safety and environment-protection, natural state, zero pollution, drugand food isogeny, pure, zero side effect, absence of no drug resistance and the like, and can effectively reduce drug residual of duck production; the physique of the ducks can be remarkably enhanced, the immunity and antibody level are improved, and generation of diseases is reduced; and the effects of health care and treatment are achieved, the smell of dejection of the ducks is radiated, and the ecological environment is ameliorated.
Owner:安徽笑果农牧产业科技有限公司
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Biological health padding and preparation method thereof
InactiveCN103088010AReduce usageReduce drug residuesMicroorganism based processesAnimal housingBiotechnologyMicrobial agent
The invention provides biological health padding and a preparation method thereof. The biological health padding includes: rice husk, sawdust, a compound microbial agent, dandelion, argy wormwood leaves, honeysuckle, mint, cogongrass, liquorice, isatis root and water. The padding is suitable for rural small-scale farming, helps to reduce livestock diseases, is beneficial to improvement of livestock and poultry antibody levels, prevention and control of animal digestive tract, respiratory tract and other diseases, and reduction of antibiotic drug residues in animal organism of livestock and poultry, and helps to promote the sustainable development of breeding industry.
Owner:HUNAN TAIGU BIOTECH +1
Novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses
PendingCN111426844ARapid Quantitative DetectionHigh sensitivityBiological testingImmunoassaysIgm antibodyStructural protein
The invention discloses a novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses. The test strip comprises a bottom plate, a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad, wherein the sample pad, the combination pad, the nitrocellulose membrane and the water absorption pad are sequentially connected end to end and adhered to the bottom plate; the combination pad is coated with an SARS-CoV-2 structural protein-marker and a goat anti-rabbit IgG-marker, and the nitrocellulose membrane is provided with a detection line T1 coated with a mouse anti-human IgG monoclonal antibody, a detection line T2 coated with a mouse anti-human IgM monoclonal antibody and a quality control line C coated withrabbit IgG. When the test strip is used for quantitatively detecting SARS-CoV-2 IgG and IgM antibodies, the detection sensitivity is high, and the specificity is good and can reach 96%; the batch-to-batch difference is small, and good repeatability is achieved; the test strip can be stored for half a year at normal temperature without reducing the sensitivity and has good stability; the test strip is simple to operate and low in cost, can quickly and quantitatively detect the levels of SARS-CoV-2 IgG and IgM antibodies in a human body, assists a nucleic acid detection means, and provides powerful support for epidemic situations.
Owner:NANJING AGRICULTURAL UNIVERSITY
Raising method for traditional Chinese medicine free-range chickens
The invention discloses a raising method for traditional Chinese medicine free-range chickens, which includes the following steps: (1) site selection and chicken house construction; (2) disinfection; (3) raising management: purebred, robust chicks are chosen; the temperature of the chick-raising houses is controlled at 32 DEG C to 33 DEG C for the age of 0 to 1 week, and is decreased by 1 DEG C to 2 DEG C each week, and at the age of 4 weeks, heating is stopped; the chicks drink water and are fed; drinking water vaccination is adopted at the previous stage, and an injection method is adopted for vaccination at the later stage; after being raised for 1 month, the chicks enter the later stage of raising, later-stage feed is granular feed, and the chicks are fed after 10 a.m. each day, fed once after 3 p.m. and fed once again before sleep; in the whole raising period, water is not stopped. The growing speed of the free-range chickens is high, constitution is strong, the immunity and antibody level of the free-range chickens are increased, the incidence rate of the free-range chickens is decreased, the raising method for traditional Chinese medicine free-range chickens is easy to operate, is safe, environment-friendly and pollution-free, and the traditional Chinese medicine free-range chickens can be raised on a large scale.
Owner:TONGLING SHUIZHIYU ECOLOGICAL FARMING CO LTD
Immunity enhancing agent, inactivated vaccine, and preparation method thereof
InactiveCN103083663AImprove immunityEnhance immune responseViral antigen ingredientsAntiviralsDipeptideOil phase
The invention provides an immunity enhancing agent, an inactivated vaccine, and a preparation method thereof. The invention relates to the field of biopharmaceutical. The immunity enhancing agent comprises 0.1-21mg / mL of monophosphoryl lipid A, 1.5-125mg / mL of muramyl dipeptide, and 0.7-4.5mg / mL of beta-glucan. The invention also provides the inactivated vaccine comprising the immunity enhancing agent, and a preparation method of the inactivated vaccine. According to the invention, the immunity enhancing agent is mixed with an inactivated antigen solution, such that a water-phase solution is obtained; and the water-phase solution is mixed with an oil-phase solution, such that the inactivated vaccine is obtained. According to the immunity enhancing agent provided by the invention, with a synergetic effect of the components, body immunity level can be improved, and immune response to antigen can be improved, such that antibody level after immunization can be increased, immune window period can be shortened, and vaccine immunization effect can be enhanced. According to the inactivated vaccine comprising the immunity enhancing agent, antibody level after immunization is high, a protection period is long, and immunization window period is short.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
Anti-diarrhea yelk antibody feed additive for swine and injection formulation, and preparation method
InactiveCN1484972ASimple preparation processLow costPharmaceutical delivery mechanismAnimal feeding stuffRotavirusAnti-diarrhea
A feed additive containing yolk antibodies against diarrhea in pigs. The antibodies are preparing from bacilluscoli, epidemic diarrhea or rotavirus, or immunizing non-immunitive hens laying eggs withcombined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens. A yolk antibody injecting agent, which is prepared from bacilluscoli, epidemic diarrheaor rotavirus, or immunizing non-immunitive hens laying eggs with combined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens.
Owner:HEBEI KEXING PHARMA
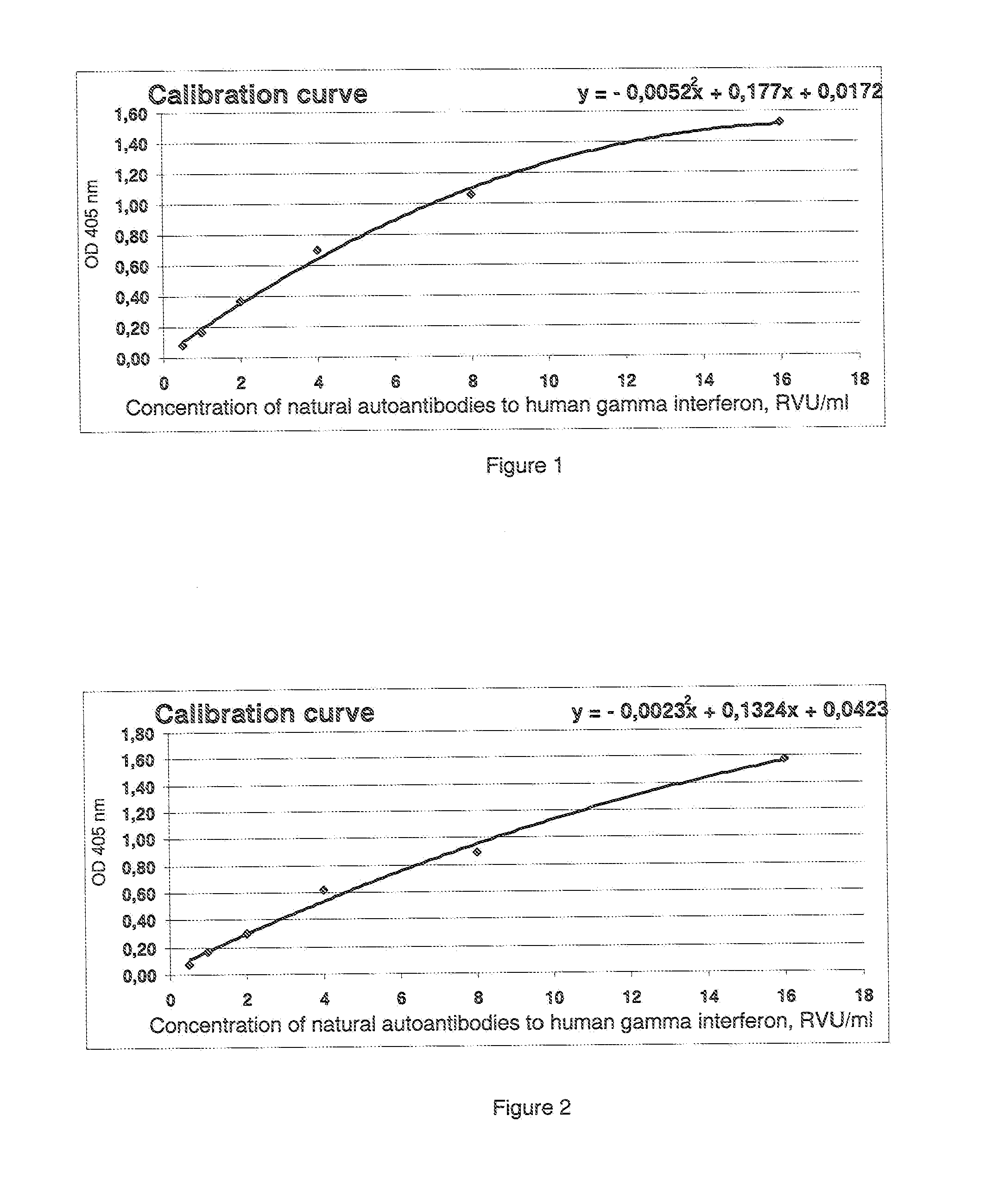
Method of determination of autoantibody level by means of enzyme immunoassay
InactiveUS20130189707A1High sensitivityReduce sensitivityBiological testingBiotin-streptavidin complexSolid phases
The method for quantitative determination of the level of natural autoantibodies in human biological fluids, when as a solid phase of physical sorption is used the solid phase of physical sorption, coated with streptavidin, and the solid phase of physical sorption is treated with preliminary biotinylated antigen and blocking agent for closing the sites of nonspecific binding at the solid phase of physical sorption, for which purpose are used proteins, biotinylated according to standard procedure. As the conjugate-containing solution are used enzyme-labeled monoclonal and polyclonal antibodies, which react with one or all isotypes of human immunoglobulins. In addition, the tested biological fluid is preliminary diluted in a buffer, containing proteins which are used for closing the sites of nonspecific binding at solid phase of physical sorption, and also substances protecting natural autoantibodies from destruction during heat treatment, and subjected to heat treatment. For each tested specimen of biological fluid, a control solid phase of physical sorption is used, and the number of natural autoantibodies is determined with the use of a calibration curve which is plotted using monoclonal or polyclonal antibodies to antigen.
Owner:SERGEEVA SVETLANA ALEXANDROVNA +3
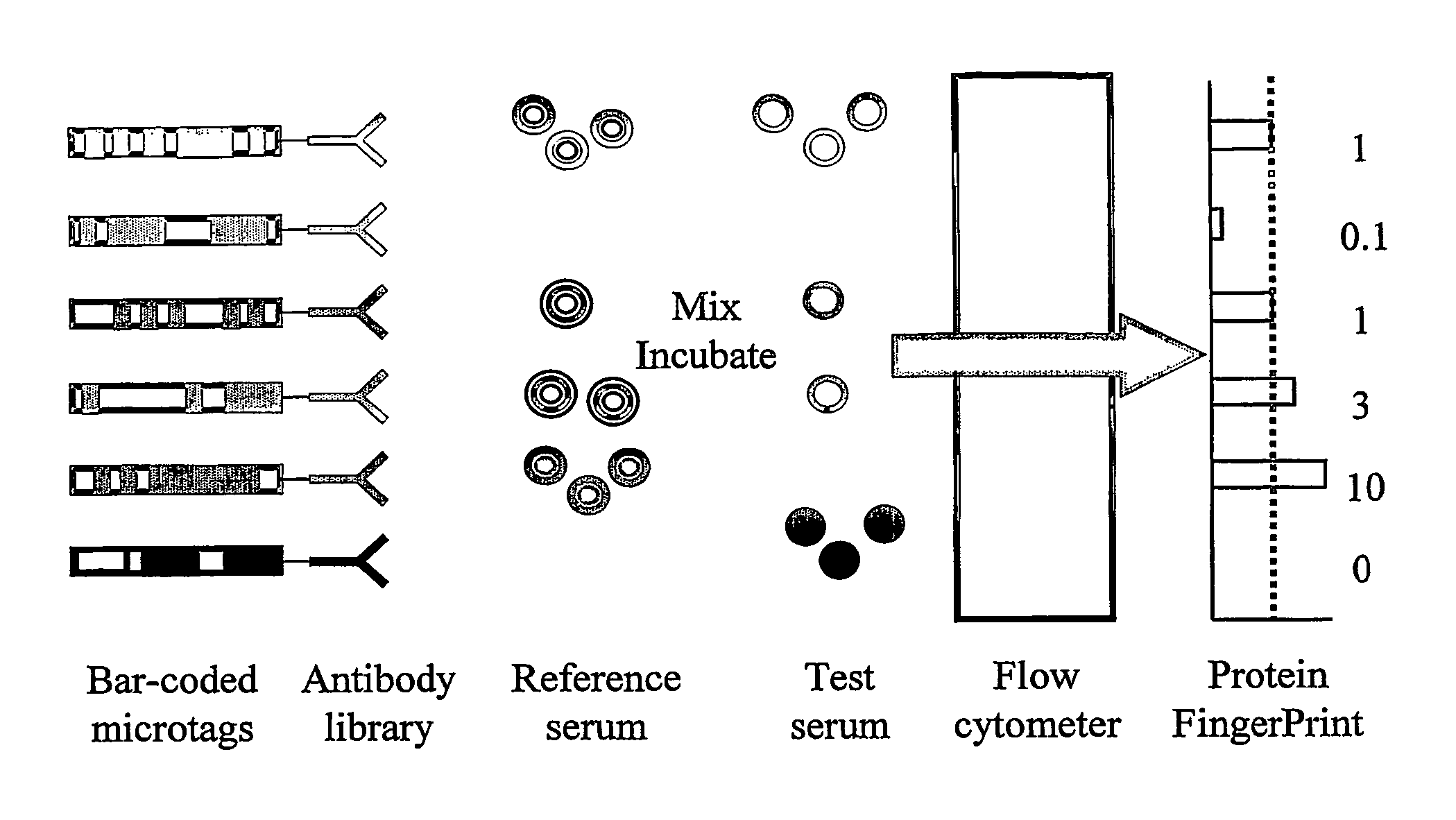
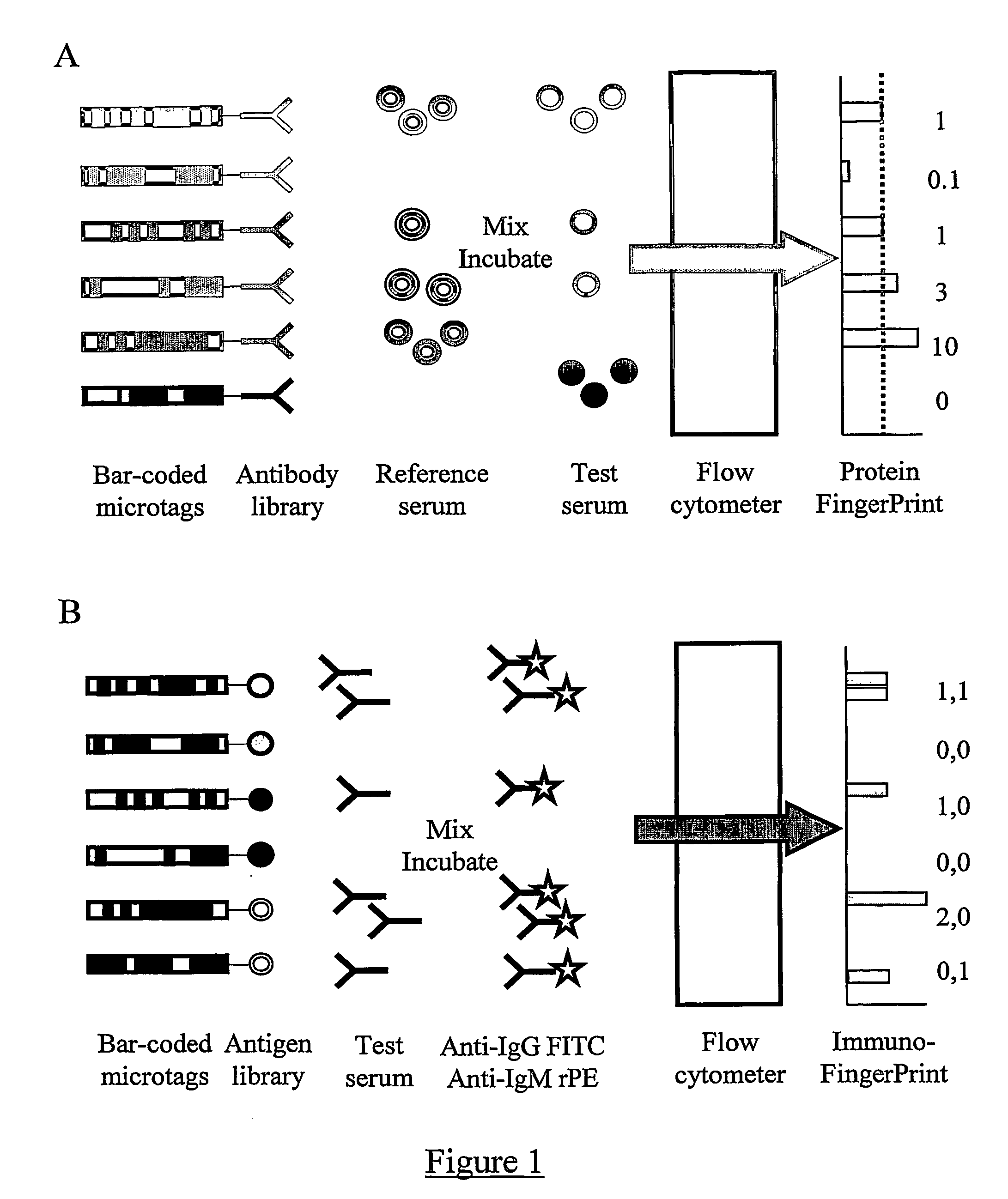
Immunoassay
InactiveUS20060073611A1Quickly determineQuick fixPeptide librariesLibrary screeningNatural abundanceTest sample
The present invention relates to methods of assaying the levels of proteins or antibodies in a test sample. In particular, the present invention relates to a method of determining the relative abundance of a plurality of proteins in a test sample compared to a reference sample, the method comprising: (a) providing a reference sample comprising a plurality of labelled proteins; (b) incubating a plurality of tagged antibodies capable of binding components of the reference sample with (i) a mixture of the labelled reference sample and the test sample and (ii) the reference sample alone, under conditions suitable for the binding of said antibodies to their targets; (c) comparing the amount of labelled protein bound to individual antibody tags in the presence and absence of the test sample.
Owner:PRONOSTICS LTD
Mosaic type virus-like particle DNA vaccine
InactiveCN1903363AIncreased ability to elicit a complete immune responseResist infectionGenetic material ingredientsAntiviralsHepatitis B virus core AntigenT cell
A chimeric virus-like granular DNA vaccine able to generate stronger B cell activity, exciting high antibody level, improving cell's immunizing level by cross presentation, and activating T cell, toxic T cell and complete immunoreaction is a recombinant eucaryotic expression carrier of coding gene with chimeric protein. Its preparing process is also disclosed.
Owner:CHINA AGRI UNIV
Reagent box for enzyme linked immunosorbent assay of EB virus protease and its preparation
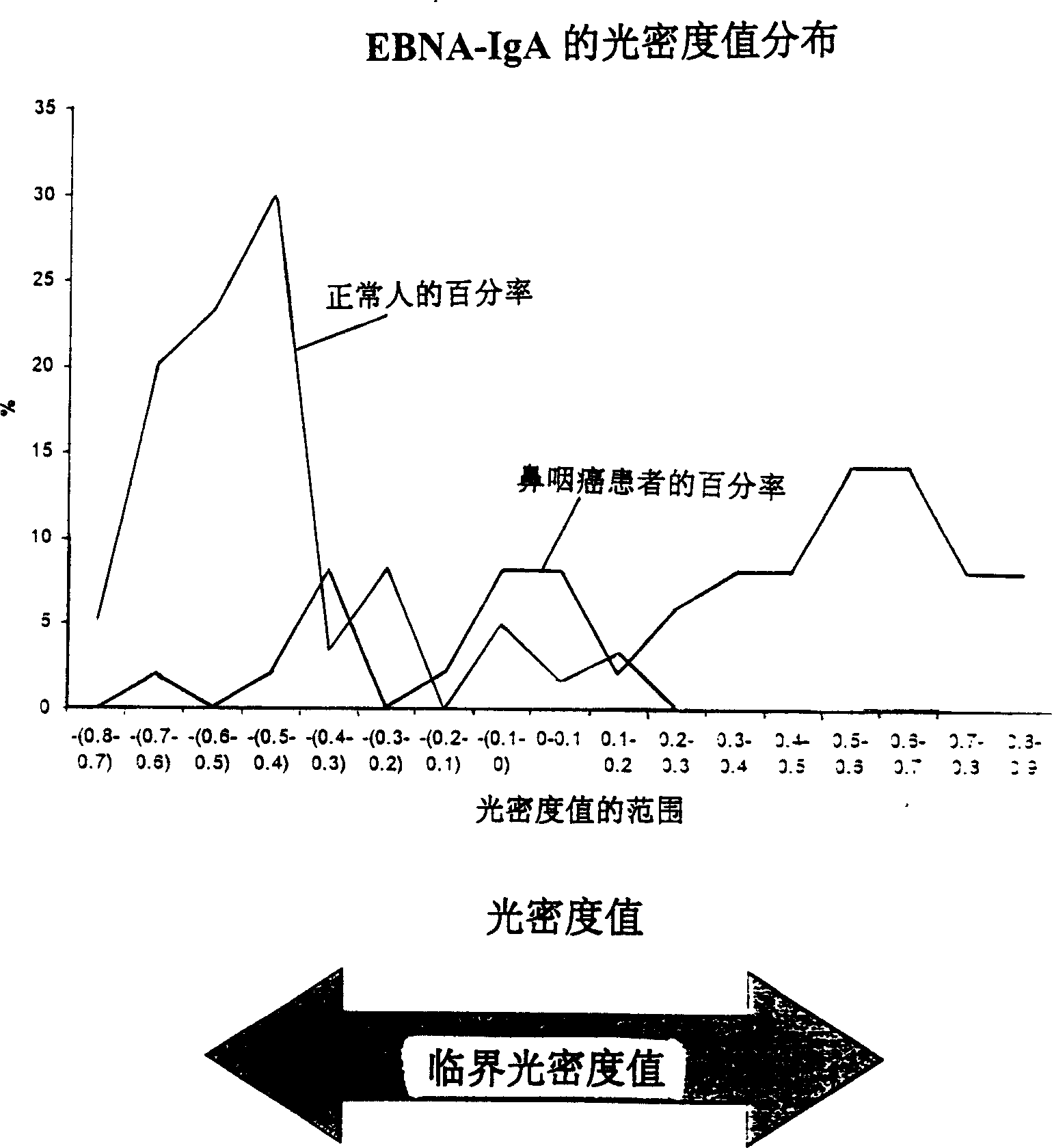
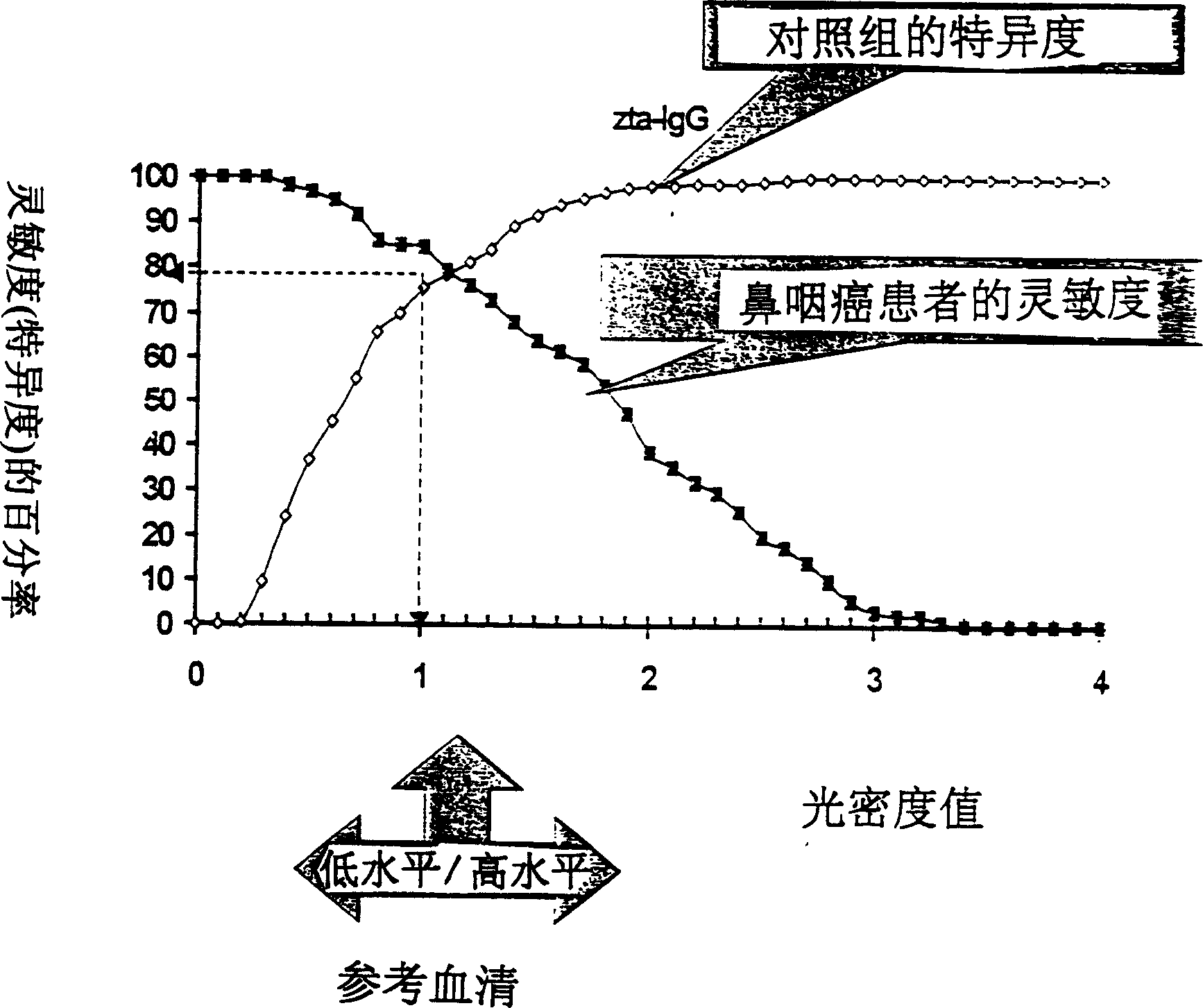
A method for preparing EB viral protein enzyme-linked immunosorbent diagnostic kit includes selectng EBNAl (BKRF1) prote, Zta (BZLF1) protein and VCA-p18 protin in EB viral protein for diagnosing nasopharyngeal carcinoma of serodiagnosis as target antigen for detecting antibody level in blood serum, using glutathione-transferase gene fusion system to carry on clone, presentation and purification of EB viral protein for creating diagnostic kit.
Owner:SINOCLONE LTD
Codon optimized porcine circovirus type 2 Cap protein coding gene and application thereof
InactiveCN103451196AStructuredImproving immunogenicityBacteriaViral antigen ingredientsPorcine CircovirusesImmunogenicity
The invention provides a codon optimized porcine circovirus type 2 Cap protein coding gene and application thereof, and belongs to the field of the molecular biology. The Cap protein coding gene is as shown in SEQ ID NO: 1. The invention also provides a recombinant vector containing the gene and recombinant bacteria containing the gene. A method for preparing porcine circovirus type 2 virus-like particles comprises the steps of inducing the recombinant bacteria to express a porcine circovirus type 2 Cap protein, lysing the recombinant bacteria, and taking the lysate for purification to obtain the porcine circovirus type 2 virus-like particles. The invention also provides a gene engineering subunit vaccine with the virus-like particles as an active ingredient. The codon optimized porcine circovirus type 2 Cap is capable of realizing abundant soluble expression. The virus-like particles (VLP) are assembled from the Cap proteins and have excellent immunogenicity. A vaccine prepared from the virus-like particles provided by the invention is capable of preventing porcine circovirus type 2 infection and has the advantages of good immunogenicity, high antibody level and effective protection after challenge.
Owner:JIANGSU ACAD OF AGRI SCI
African swine fever neutralizing epitope subunit vaccine
PendingCN111018996AImprove securityAvoid the risk of infectionViral antigen ingredientsVirus peptidesAfrican swine feverNeutralising antibody
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever neutralizing epitope subunit vaccine that is mainly composed of African swine fever neutralizing epitope fusion protein. The African swine fever neutralizing epitope fusion protein mainly comprises a B cell neutralizing epitope peptide fragment including at least one neutralizing epitopepeptide of p72 protein, at least one neutralizing epitope peptide of p54 protein and at least one neutralizing epitope peptide of p30 protein. With the African swine fever neutralizing epitope fusionprotein, the risk of accelerating virus infection possibly caused by non-neutralizing antibodies can be effectively eliminated and the immune safety of the fusion protein can be effectively improved.Besides, when the African swine fever neutralizing epitope fusion protein is used for immunizing pigs, the antibody level obviously higher than that of a control group can be generated after one immunization.
Owner:河南省生物工程技术研究中心 +1
Novel coronavirus detection test strip as well as preparation method and application thereof
InactiveCN111426840AImprove accuracySimple and fast operationBiological testingImmunoassaysIgm antibodyMonoclonal antibody agent
The invention provides a novel coronavirus detection test strip as well as a preparation method and application thereof. The test strip comprises a binding pad and an analysis membrane, and the binding pad is coated with a luminescent substance labeled 2019-nCoV recombinant antigen and a mouse anti-human HCG monoclonal antibody; a T2 detection line, a T1 detection line and a quality control line are sequentially arranged on the analysis membrane along the chromatography direction; the T2 detection line is coated with a mouse anti-human IgG monoclonal antibody, the T1 detection line is coated with a mouse anti-human IgA monoclonal antibody and a mouse anti-human IgM monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG polyclonal antibody. The test paper card can simultaneously determine the positive conditions of IgA antibody, IgM antibody and IgG antibody in serum of a patient, can more accurately detect the early antibody level condition in the body of the patient, assists in judging different periods of novel coronavirus infection of the patient, and improves the sensitivity and specificity of novel coronavirus detection.
Owner:北京中检安泰诊断科技有限公司
Panda source lactobacillus plantarum and application
InactiveCN106434411AProtection from damageReduce percentageAntibacterial agentsBacteriaEscherichia coliTLR4
The invention discloses a panda source lactobacillus plantarum and application. The strain was preserved in China Center for Type Culture Collection on May 4, 2016 and the preservation number is CCTCC NO: M2016245. The panda source lactobacillus plantarum provided by the invention can be used for improving antibody levels of IgA, IgM and IgG immune globulins of blood serum; a condition that the percent of lymphocytes is reduced and the percent of neutrophils is increased, caused by infection and induction of enterotoxigenic escherichia coli, is inhibited; expression levels of inflammation related factors IL-1beta, IL-8, IL-6, TLR4 and MyD88mRNA (messenger Ribonucleic Acid) are remarkably down-regulated, and intestinal acute inflammation response caused by the enterotoxigenic escherichia coli is alleviated.
Owner:SICHUAN AGRI UNIV +1
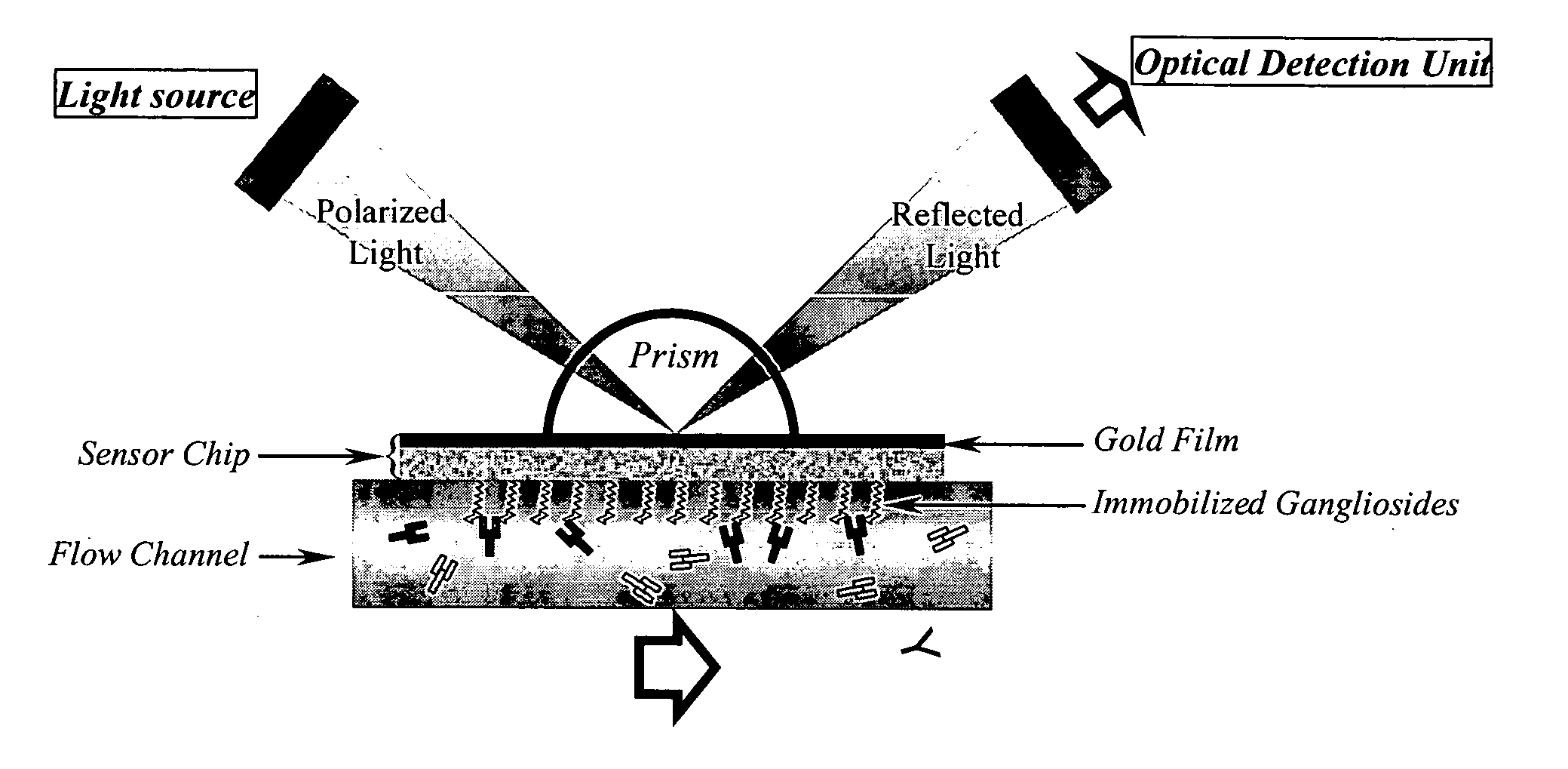
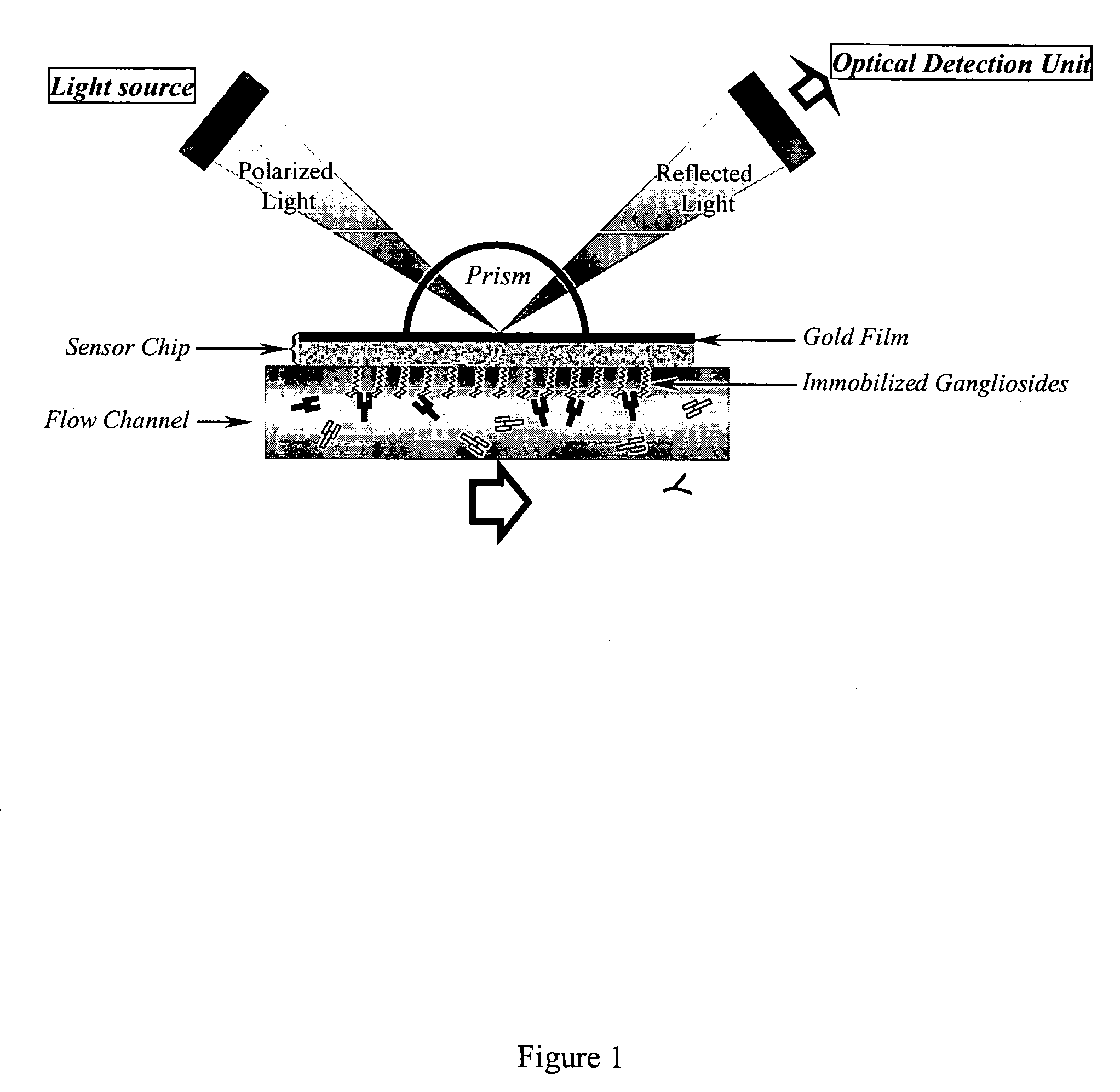
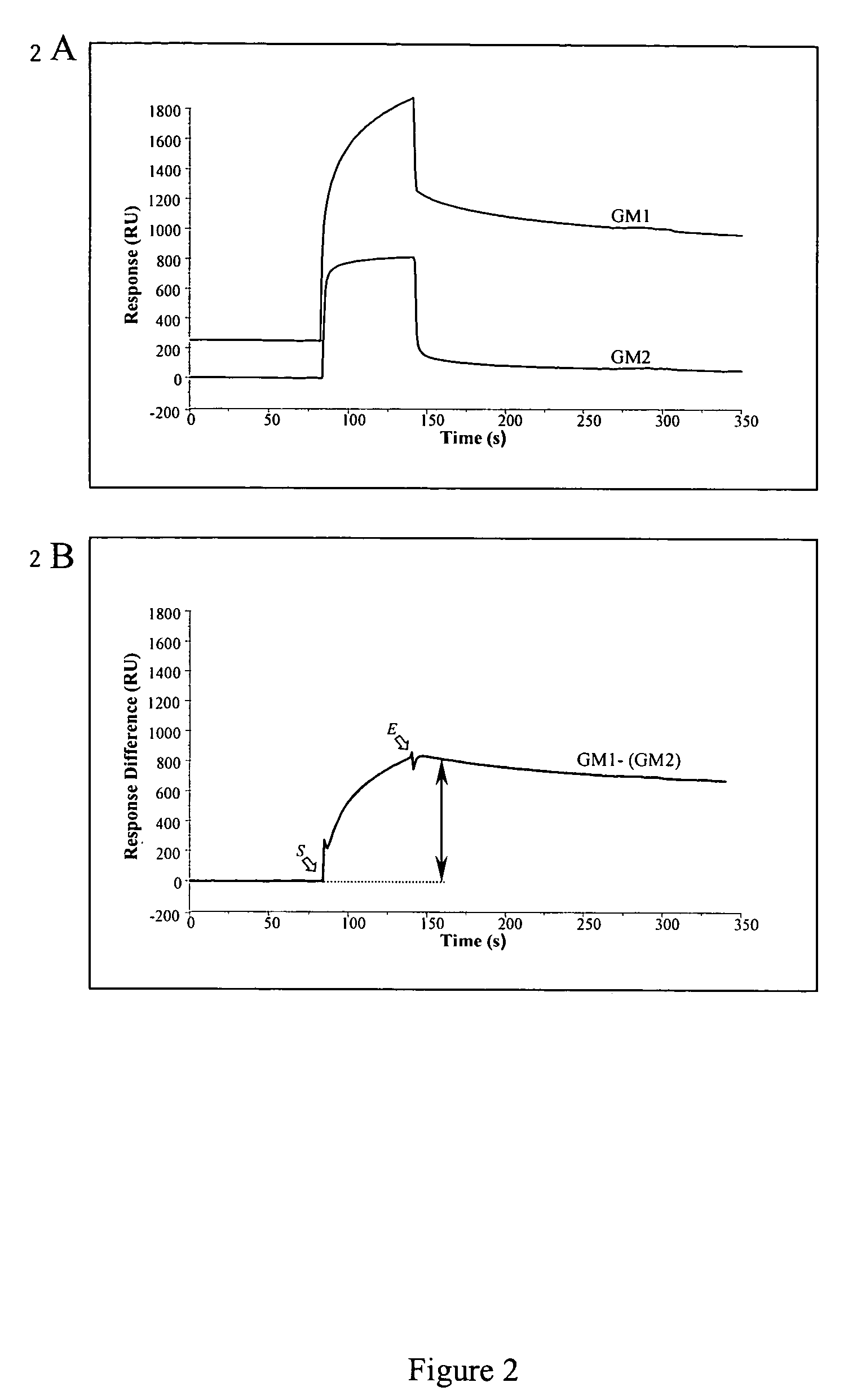
Surface plasmon resonance biosensor for measurement of anti-glycolipid antibody levels in neuropathy
InactiveUS7074621B2Increase in sizeAnalysis by subjecting material to chemical reactionBiological testingSerum igeDisease
The present invention provides a method of detecting human antibodies in a sera solution. The invention also provides a method of quantitating anti-glycolipid antibody levels in solutions. The invention provides a method of diagnosing disease states, including neurological diseases, by quantitating a subject's antibody levels.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Muscovy duck parvovirus and application thereof
ActiveCN103820397AEnhanced antibodyLow toxicityMicroorganism based processesImmunoglobulins against virusesFreeze thawingAdjuvant
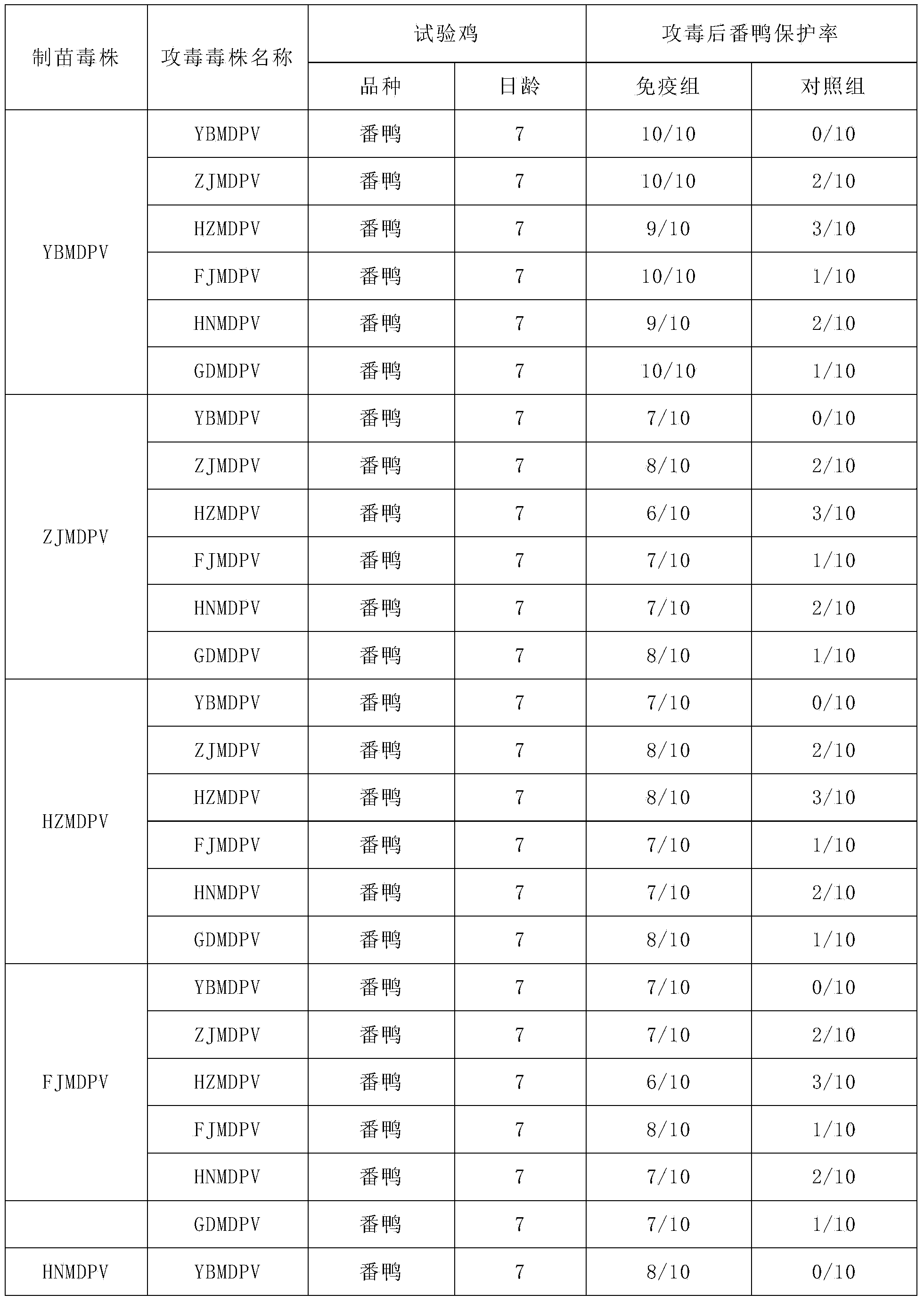
The invention aims to provide a Muscovy duck parvovirus. The Muscovy duck parvovirus is a YBMDV strain, and is preserved in the China General Microbiological Culture Collection Center of the China Committee Of Culture Collection For Microorganisms in the Institute of Microbiology Chinese Academy of Science at the address of No. 3, No. 1 yard, Beichen west road, Chaoyang District, Beijing, on November 11th, 2013; the preservation number is CGMCC No. 8504. The Muscovy duck parvovirus screened by the invention has the characteristics of low toxicity and high immunogenicity; dead embryo and embryo liquid can be obtained by vaccinating the Muscovy duck parvovirus to a Muscovy duck embryo; virus liquid can be collected after grinding and freeze thawing; a vaccine is prepared by ultrafiltration concentration, formalin inactivation, the addition of an adjuvant, and mixing and emulsification; the Muscovy duck parvovirus can improve antibody of the Muscovy duck, ensure parent source antibody level of an offspring, and prevent green duck parvovirus infection caused by Muscovy duck parvovirus; the vaccine has the advantages of high efficiency and good safety.
Owner:YEBIO BIOENG OF QINGDAO
Vitamin water aqua and preparation method thereof
ActiveCN101822320AActivate autoimmune functionAvoid lostMetabolism disorderAnimal feeding stuffVitamin K2Vitamin C
The invention relates to a vitamin water aqua. The raw materials of the water aqua comprise liposome, vitamin A, vitamin D, vitamin E, vitamin K, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin H, folic acid and solubilizer. In the invention, liposoluble vitamins are coated in a microcapsule structure of the liposome and form a homogenized and stable water aqua with water-soluble vitamins. The water aqua has stable storage character in a long time, smaller grain diameter of components and a cytoid structure and can activate the self immunologic function after entering an animal body to keep the immune level of organisms in a higher state; and meanwhile, a plurality of vitamins can be effectively stored in the organism, so that the invention avoids the problems of the loss and the insufficient utilization rate of the vitamins in the metabolic process, remarkably improves the utilization rate and the conversion rate of feed, improves the antibody level of animal organisms and enhances the disease resistant capability.
Owner:DINGZHENG XINXING BIOTECH TIANJIN
Feed additive comprising SOD
InactiveCN103315154AImprove immunity and disease resistanceImprove the immunityAnimal feeding stuffBiotechnologyDisease
The invention relates to a feed additive comprising SOD. The feed is characterized in comprising the following components, by weight: 0.8 parts of SOD, 0.6-0.8 parts of probiotics, 1-2 parts of multivitamins, and 7-8 parts of serum globulin powder. The activity of SOD is no lower than 6000u / mg, and protein is no lower than 4.0%. Compared to prior art, the feed additive provided by the invention has the advantages that: (1) the feed additive comprises no hormone or antibiotic, and is safe, green, healthy, and pollution-free; (2) with the additive, free radicals in pig bodies can be removed, pig immunity and disease resistance can be improved, pig resistance can be improved, and pig daily weight gain can be increased; (3) with the additive, pig antibody level can be increased, such that pig immunity can be improved, pig disease resistance can be improved, and pig survival rate can be improved; and (4) the additive provides necessary copper, manganese, multivitamins, proteins and immune growth promotion factors for pigs during a growth process, such that pig reproductive capacity and growth rate can be improved.
Owner:南京宝迪农业科技有限公司
Indirect ELISA (enzyme-linked immunosorbent assay) method for detecting PRRSV (porcine reproductive and respiratory syndrome virus) antibody through tandem repeat expression of GP5 dominant antigen epitopes
InactiveCN103275193AVirus peptidesMicroorganism based processesPorcine reproductive and respiratory syndrome virusEngineered genetic
The invention relates to an indirect ELISA (enzyme-linked immunosorbent assay) method for detecting a porcine reproductive and respiratory syndrome antibody, which comprises the following steps: by using a pGEX-6p-1 prokaryotic expression vector, performing tandem repeat on two epitopes to improve the antigen activity of an expressed protein, thus constructing a gene engineering bacterium BLpGEX-6p-GP5 capable of realizing secretory expression of the GP5 protein dominant antigen epitopes, wherein one epitope is a linear conservative neutralizing epitope (epitope B) of a screened PRRSV (porcine reproductive and respiratory syndrome virus) GP5 protein, which can be identified by a monoclonal antibody and can also be identified by porcine anti-PRRSV neutralizing serum, and the other epitope is a high-variability immunodominant epitope (A); and purifying and renaturing the expressed recombinant protein, and coating an ELISA plate, thus establishing the indirect ELISA method for detecting a PRRSV antibody to detect the PRRSV antibody level in porcine serum. Results show that the method has the characteristics of favorable repetitiveness and high specificity and can be used for PRRSV serological search.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Inactivated vaccine for preventing and treating novel goose astroviruses and preparation method thereof
ActiveCN108567974AImproving immunogenicityConducive to the prevention and control of infectionSsRNA viruses positive-senseViral antigen ingredientsWhole bodyOutbreak
The invention discloses an inactivated vaccine for preventing and treating novel goose astroviruses and a preparation method thereof. The inactivated vaccine is prepared from the effective dose of inactivated goose astrovirus strain virus liquid in prevention or treatment. A preservation number of the goose astrovirus strain is CCTCC NO: V201808. The prepared novel goose astrovirus inactivated vaccine is good in safety, any local or general adverse effects caused by the vaccine do not appear, and the detection of each index is stable and effective. After the inactivated vaccine is used for immunizing a young goose, the young goose can acquire the higher antibody level, and a persistent period is long. The infection and outbreak of the novel goose astroviruses can be prevented and treated specifically, and the effective immune protection is provided for a goose group. In addition, a production technology for the vaccine is simplified, and the large-scale production can be realized.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Porcine circovirus-II vaccine potency test method
InactiveCN101871941AShort detection timeShorten the timeBiological material analysisWhite mousePresent method
The invention belongs to the technical field of veterinary medicaments and new biological medicaments and relates to a porcine circovirus-II vaccine potency test method. The method comprises: immunizing a white mouse with a porcine circovirus-II inactivated vaccine; and determining the PCV2 specific antibody content of the serum of the white mouse to evaluate the potency of the vaccine. When the vaccine potency test method is used, and the problems of time-consuming and labor-consuming test pig screening, large inter-batch test result difference and the like of the conventional porcine circovirus-II inactivated vaccine potency test are solved. The test method of the invention has the advantages of short test period, simple operation, low cost, accurate result and high repeatability. The method has a high application value and a promising market prospect.
Owner:PU LIKE BIO ENG
Nanyang yellow cattle feed
ActiveCN105394371APromote absorptionHigh activityFood processingAnimal feeding stuffDiseasePeanut meal
The invention belongs to the field of feed and particularly relates to feed specially used for culturing Nanyang yellow cattle. The feed comprises corn flour, distillers grains of baijiu, oat powder, soybean meal, peanut meal, tenebrio molitor, cockroach powder, rapeseed cake, calcium carbonate, amino acids, folic acid, choline chloride, an antiseptic, enzyme preparations, plant extracts, probiotics, sodium alginate, citric acid and sodium carboxymethylcellulose. A plurality of the composite probiotics, the enzyme preparations and the plant extracts are added into the feed, thus providing a plurality of beneficial compounds for yellow cattle, maintaining intestinal tract ecological balance of the yellow cattle, improving trace element absorption, stimulating growth and development of intestinal tract immune organs, increasing the animal antibody level and macrophage activity, boosting humoral immunity and cell-mediated immunity of a body, timely killing invaded pathogenic bacteria, boosting resistance of animals to a plurality of diseases, and largely reducing the morbidity and the mortality.
Owner:门祥吉
H9 subtype avian influenza virus strain
ActiveCN104946600AGuaranteed immune effectImprove securityMicroorganism based processesAntiviralsImmune effectsOil adjuvant
The invention aims at providing an H9 subtype avian influenza virus strain. Vaccine is prepared by the virus strain to solve the problem that existing H9 subtype avian influenza virus vaccine is poor in immune effect caused by a novel virus. The H9 subtype avian influenza virus QDY strain (Avian Influenza Virus) is preserved in the typical culture preservation center in China in Wuhan University on April 29, 2015, and the preservation number is CCTCC NO: V201517. After the H9 subtype avian influenza virus (QDY strain) is inoculated to a chick embryo, a virus solution is collected, after ultrafiltration concentration and formaldehyde solution inactivation are performed, oil adjuvant is added, and then mixing and emulsifying are performed to prepare the vaccine. According to the prepared vaccine, the antibody level after immunization can be improved, the antibody uniformity after immunization can be improved, and the immune effect of the vaccine can be guaranteed; the vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Heat-resisting lyophilized protecting agent of live vaccine for animal and preparation method thereof
InactiveCN101474410ARepair damage quicklyRepair damagePowder deliveryLyophilised deliveryFreeze-dryingDistilled water
The invention provides a heat-resistant freeze-drying protective agent applied to animal freeze-dried live vaccine. The heat-resistant freeze-drying protective agent comprises 1%-3% of glutin, 1%-8% of lactose and the like, wherein, all substances are dry components, and distilled water takes all the rest percentage. The invention also provides a method for preparing the protective agent. ND live vaccine prepared by the protective agent of the invention can be conserved for more than 24 months at the temperature of 2 DEG C to 8 DEG C. Immunity test shows that the average antibody level of immunized chicken is 1 to 2 titers higher than the average antibody level of tested chicken immunized by the ND live vaccine which is freeze-dried by ordinary protective agent, and the antibody level is uniform. Therefore, the freeze-drying protective agent can be widely applied to the production and conservation of various animal live vaccines and has wide application prospect.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Ginsenoside-containing vegetable oil adjuvant and preparation method and application thereof
InactiveCN103751775ARaise antibody levelsHigh activityImmunological disordersAntibody medical ingredientsOil adjuvantVegetable oil
Owner:ZHEJIANG UNIV
Yolk antibody feed additive and injection for resisting canine distemper and canine parvovirus disease and preparation thereof
InactiveCN101259273ARelief of clinical symptomsReduce mortalityImmunoglobulins against virusesPharmaceutical delivery mechanismYolkDisease
The invention relates to a yolk antibody injection against canicola fever and canine parvovirus and a preparation method thereof. The yolk antibody injection against canicola fever and canine parvovirus is extracted and prepared by the collected yolk antibody of the immunized laying hens which are immunized by the bivalent vaccine of the canicola fever and canine parvovirus. The yolk antibody injection against canicola fever and canine parvovirus prevents the diseases by improving the antibody level of canine bodies to the canicola fever and the canine parvovirus. The yolk antibody injection against canicola fever and canine parvovirus and a preparation method thereof have the beneficial effect that the anti-canicola fever and canine parvovirus disease egg yolk antibody injection is provided with faster effect in the onset of canicola fever and canine parvovirus diseases. The yolk antibody injection against canicola fever and canine parvovirus and the preparation method thereof has the advantages of obvious efficacy, low cost, fast effect, no side effects, no generated drug resistance, simple preparation, wide raw material resource, convenient use and easy application.
Owner:TIANJIN SHENGJI GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com