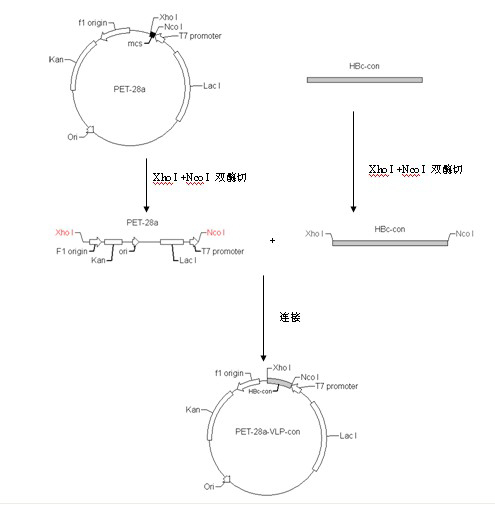
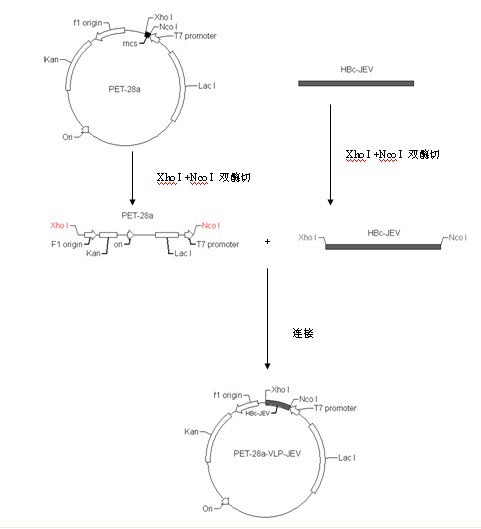
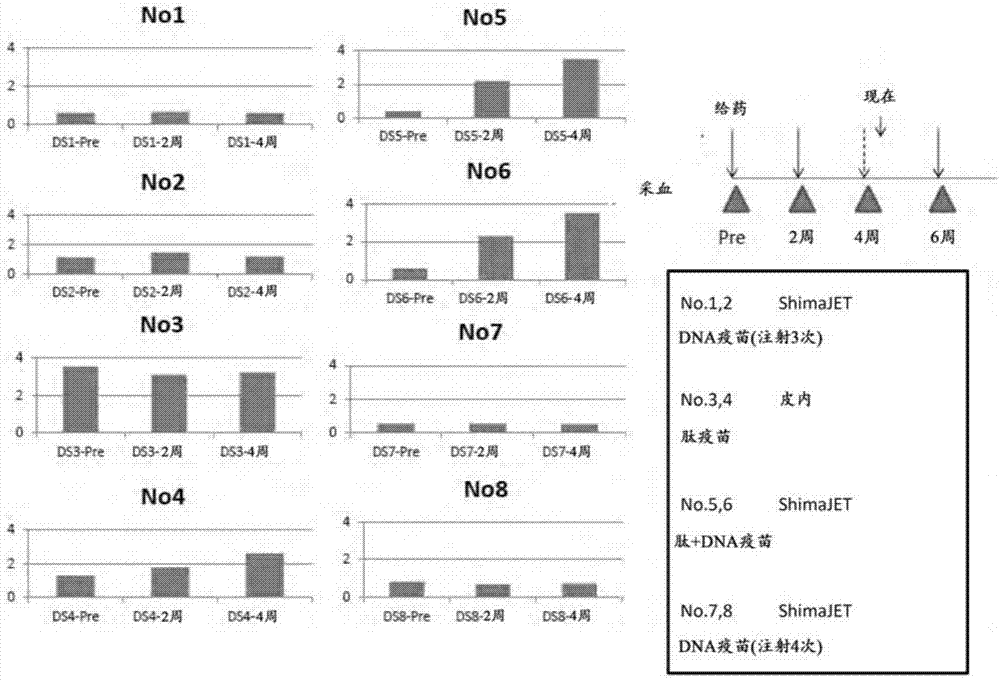
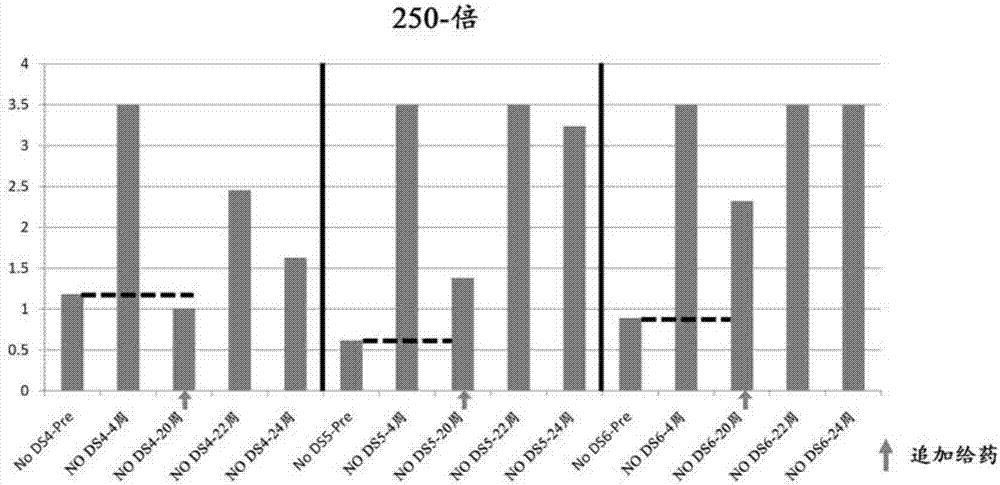
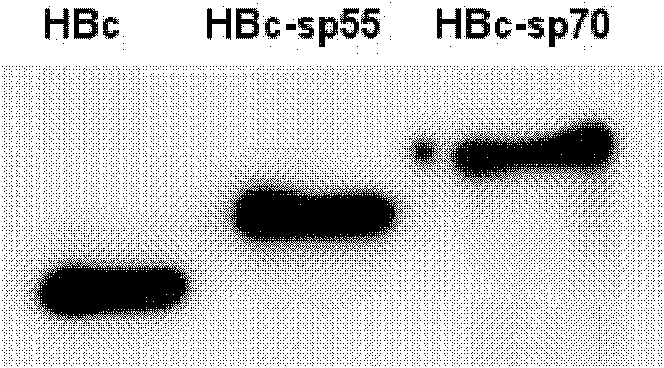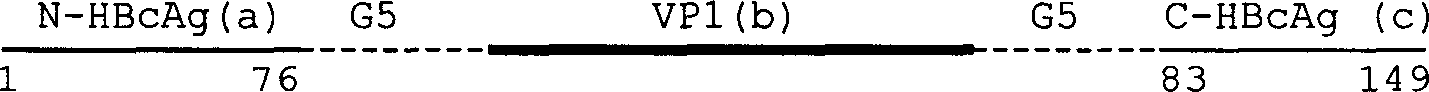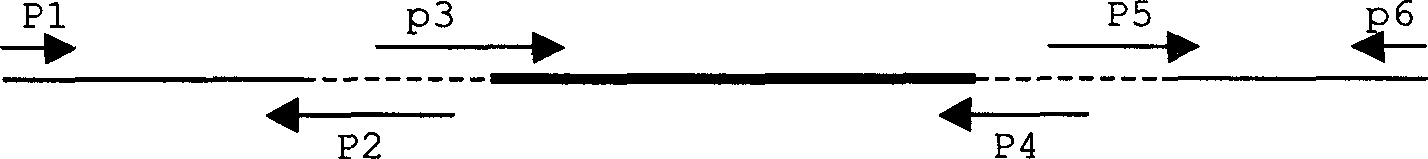Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "Hepatitis B virus core Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Codon-optimzed hepatitis b virus core antigen (HBCAG)
InactiveUS20130012865A1Increase ionic strengthReduce interactionAmpoule syringesElectrotherapyHepatitis B virus core AntigenPressure generation
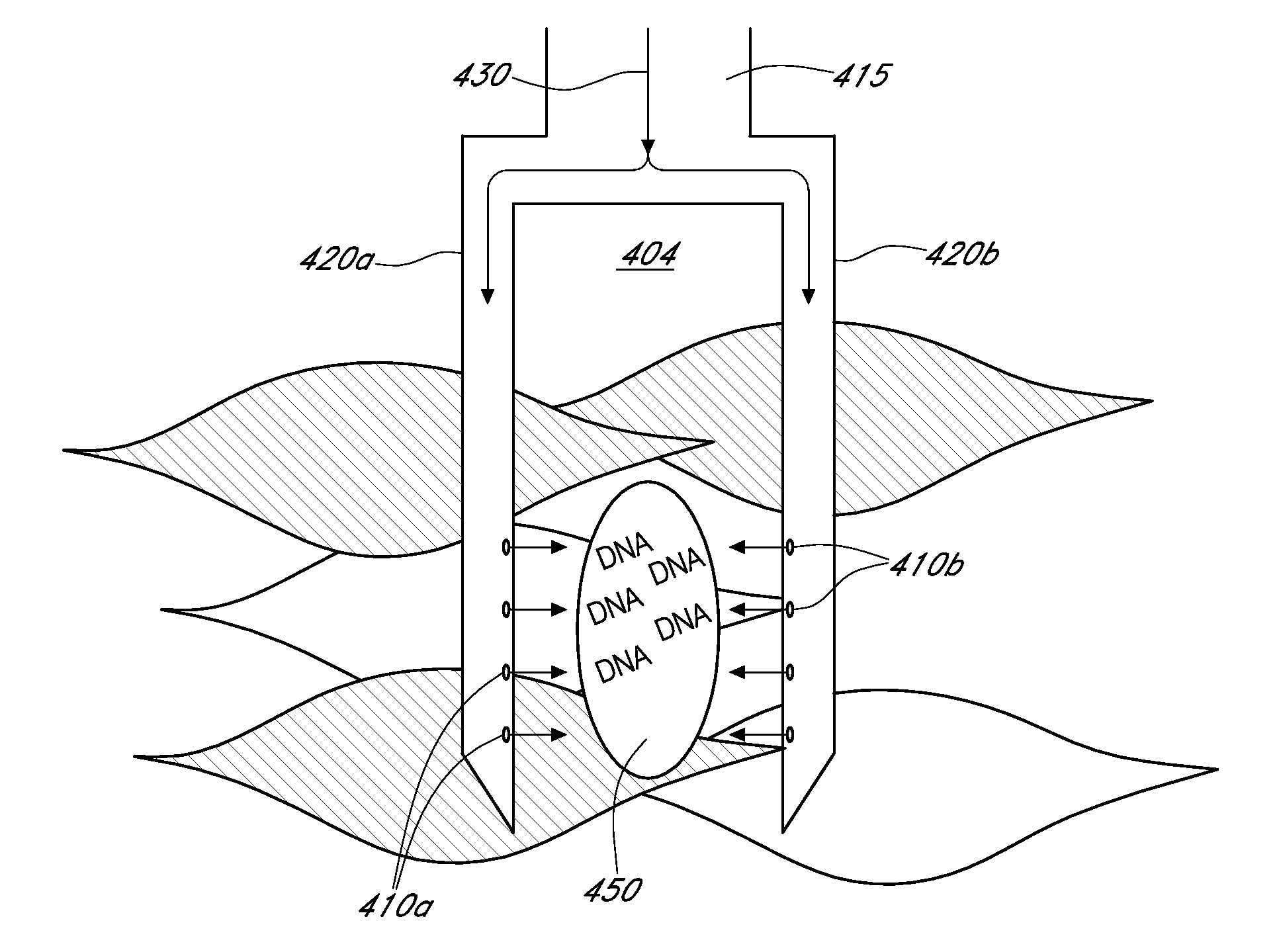
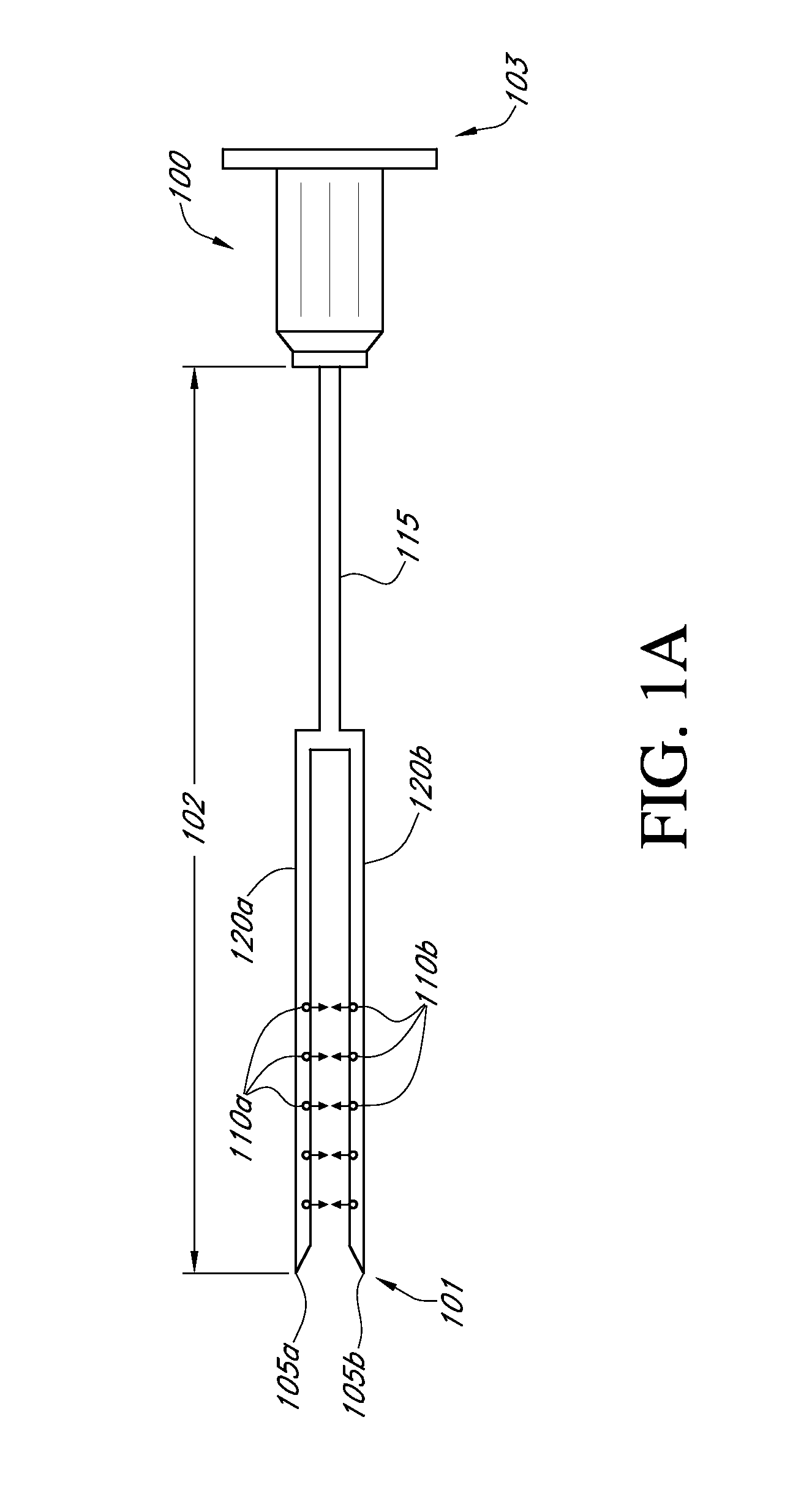
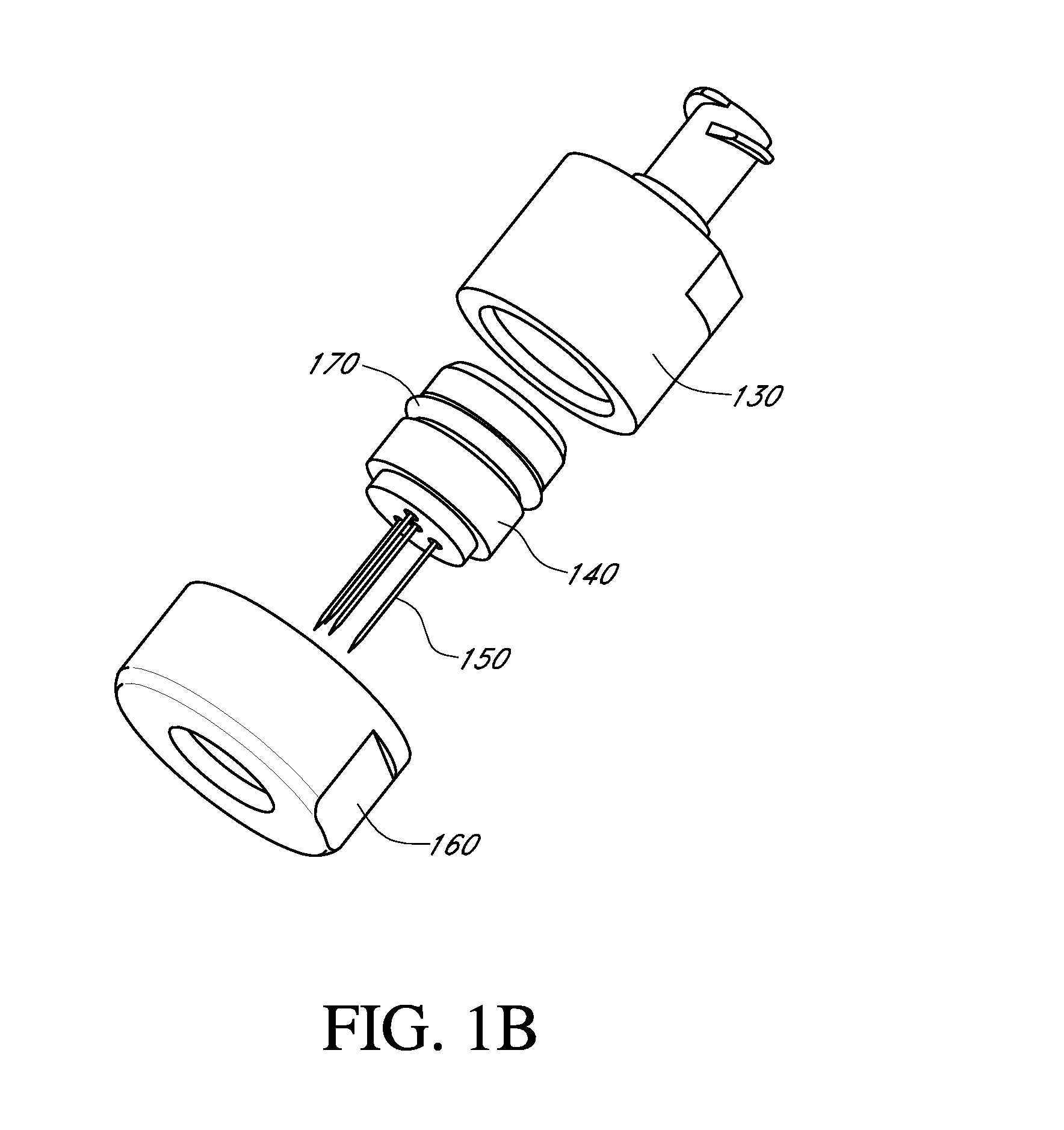
A needle device for the delivery of therapeutic material into tissue comprising a connection to a pressure generation element, a lumen adapted for the passage of a therapeutic material, and a needle barrel, wherein each needle barrel comprises an opening adapted to control and deliver a pressure transmitted from the pressure generation element into a tissue to cause an increase in the permeability of a cell membrane to the therapeutic material.
Owner:TRIPEP
Mosaic type virus-like particle DNA vaccine
InactiveCN1903363AIncreased ability to elicit a complete immune responseResist infectionGenetic material ingredientsAntiviralsHepatitis B virus core AntigenT cell
A chimeric virus-like granular DNA vaccine able to generate stronger B cell activity, exciting high antibody level, improving cell's immunizing level by cross presentation, and activating T cell, toxic T cell and complete immunoreaction is a recombinant eucaryotic expression carrier of coding gene with chimeric protein. Its preparing process is also disclosed.
Owner:CHINA AGRI UNIV
Use of 15-methano-substituted-andrographolide derivative in preparing anti-hepatitis B medicine
ActiveCN101416958AExpand the range of optionsTo clarify the anti-HBV activity in vitroOrganic active ingredientsDigestive systemHepatitis B virus core AntigenMedicine
The invention discloses the medical application of a 15-methylene replaced andrographolide derivant as shown in general formula 1, more particularly relates to the application thereof in preparing anti-hepatitis B virus drugs, pertaining to the pharmaceutical chemistry field. HepG2.2.15 cells are used for detecting the secretory volumes of HBsAg and HBeAg and the HBV DNA level related to viral particles in the supernatant liquid of a nutrient solution, and the result shows that the 15-methylene replaced andrographolide derivant has good in-vitro anti-HBV effect. The 15-methylene replaced andrographolide derivant has better development and application prospect by being applied in preparing drugs used for treating and preventing Hepatitis B.
Owner:ZHENGZHOU UNIV
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
Immune enhanced gene vaccine of hepatitis B virus core antigen and preparation method thereof
InactiveCN101884793AEnhance immune responseEfficient induction of antiviral replication abilityGenetic material ingredientsDigestive systemAnti virusHepatitis B virus core Antigen
The invention relates to the technical field of gene engineering, in particular to an immune enhanced gene vaccine of a hepatitis B virus core antigen (HBcAg). The vaccine is an immune stimulating complex (ISCOM) vaccine consisting of HBcAg and OX40 ligand (OX40L) dual-gene co-expression recombinant eukaryotic vector, saponin Quil A, cholesterol and lecithin. The vaccine can promote immune response of specific T cells of the HBcAg and efficiently induce the anti-virus replication capacity of immune cells, and has good application prospect in the field of hepatitis B virus infection resistance. The invention also relates to a preparation method for the vaccine with simple and convenient operation and low cost.
Owner:ARMY MEDICAL UNIV
Recombinant fusion protein and use thereof
InactiveCN104341506AAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenAntigen epitope
The invention belongs to the biological medicine field, relates to recombinant fusion protein and use thereof, and in particular relates to recombinant fusion protein carrying hepatitis B virus therapeutic antigen epitopes which are inserted into hepatitis B core antigen protein particles or truncated fragments and use thereof. The recombinant fusion protein contains multiple epitope antigens of hepatitis B virus (HBV) and other immune stimulating epitope antigens and hepatitis B core antigen virus-like particles or truncated fragments thereof for preparation of chimeric antigen, the multiple antigen epitopes can be inserted into same or different sites of hepatitis B virus core antigen HBc or truncated fragments thereof in the manner of single epitope or multi epitope combination, and by combination with different adjuvants, HBV specific humoral and cellular immune functions can be strengthened.
Owner:FUDAN UNIV
ELISA measuring reagent kit for detecting hepatitis B virus kernel antigen in blood serum
ActiveCN101140285AAvoid interferenceImprove featuresMaterial analysisAntigenHepatitis B virus core Antigen
The present invention discloses an enzyme association immunity test reagent kit and its operation instruction to test hepatitis B core antigen. Wherein, the reagent kit mainly comprises a monoclonal hepatitis B core antigen prepackaged micro-perforated plate, sample processing agent and polyclone hepatitis B core antigens marked with horse radish peroxidase. During operation, a sample to be tested is firstly preprocessed with the sample processing agent and then added into the monoclonal hepatitis B core antigen prepackaged micro-perforated plate. And then, polyclone hepatitis B core antigens marked with horse radish peroxidase and zymolytes are added to colorize. Stop solution is added to stop reaction and finally the measuring result is confirmed through color comparison. The present invention can detect hepatitis B core antigen in blood serum, ensure quite high conformance ratio in aspect of HBV-DNA detecting result, achieve requirements of clinical examination, have characteristics of convenience, sensitivity and stability, supplement conventional hepatitis B virus detecting method and bring better clinical operation.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Application of HBcAg (hepatitis B core antigen) virus-like particle serving as cancer therapeutic vaccine carrier
InactiveCN105497886AImprove the level ofViral antigen ingredientsAntineoplastic agentsHepatitis B virus core AntigenEscherichia coli
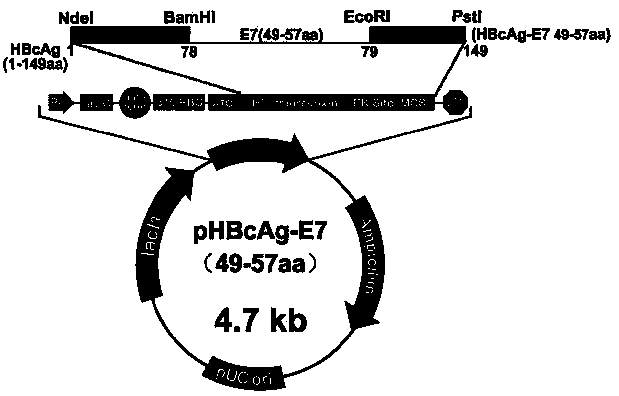
The invention relates to the field of molecular biology and immunology, in particular to an application of an HBcAg (hepatitis B core antigen) virus-like particle serving as a cervical cancer therapeutic vaccine carrier. A preparation method comprises steps as follows: an HPV16 E749-57CTLs epitope peptide fragment is selected, a DNA (deoxyribose nucleic acid ) fragment of the HPV16 E749-57CTLs epitope peptide fragment is inserted between 78 and 79 amino acids of the HBcAg through genetic recombination, an obtained recombinant plasmid pHBcAg-E749-57 is converted into Escherichia coli DH5alpha, and an HBcAg virus-like particle vaccine presenting E749-57 is obtained after induction expression and purification. After a tumor-bearing mouse is immunized with the virus-like particle vaccine, the body of the mouse can be induced to generate a higher HPV16E7 specific cellular immunologic response, and growth of tumors is remarkably inhibited.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Method for constructing IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma
ActiveCN104001168AEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDiseaseHepatitis B virus core Antigen
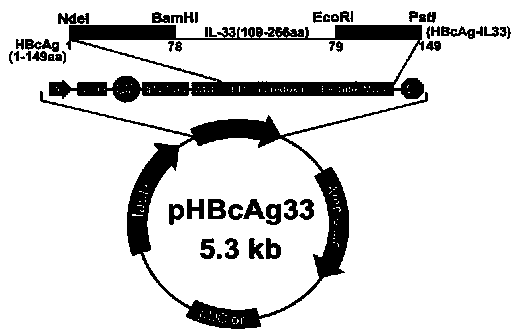
The invention provides a method for constructing an IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma. The method comprises the following steps: extracting IL-33 total RNA (Ribonucleic Acid) from a mouse; performing reverse transcription to obtain IL33 total cDNA (complementary deoxyribonucleic acid); performing PCR (Polymerase Chain Reaction) amplification on the obtained total cDNA with a designed specific primer to obtain a coded IL-33 mature segment gene; inserting the gene between 78-bit amino acid and 79-bit amino acid of a hepatitis B virus core antigen HBcAg to obtain a recombinant plasmid pHBcAg33; transferring the plasmid onto escherichia coli DH5alpha or a BL21 competent cell; inducing by using IPTG (isopropyl-beta-d-thiogalactoside) and purifying to obtain the IL-33 presentation VLP vaccine. A strong neutralizing antibody which is specific to own molecules and has a durable action can be induced by inoculating the vaccine repeatedly in order to regulate and control immune response, thereby fulfilling the aim of regulating and controlling the progress of asthma.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
DC vaccine for treating chronic hepatitis B
InactiveCN1981866AImprove efficiencyImprove immunityPharmaceutical delivery mechanismAntiviralsHepatitis B virus core AntigenChronic hepatitis
A therapeutic DC vaccine for preventing and treating chronic hepatitis B is a hepatitis B specific DC vaccine carried by both HBsAg and HBcAg. The dendritic cells coming from mononuclear cells are carried by both recombinant HBV surface antigen and recombinant HBV core antigen.
Owner:解放军三〇二医院生物治疗研究中心
Compound of hepatitis B virus antigen and lewis oligosaccharide and preparation method and application thereof
InactiveCN101797381AStimulate immunityStimulate powerful immunityAntiviralsPharmaceutical non-active ingredientsHepatitis B Virus AntigenBiotin-streptavidin complex
The invention discloses a compound of a hepatitis B virus antigen and lewis oligosaccharide and a preparation method and application thereof. The compound is prepared mainly through combination of a core hepatitis B virus antigen and lewis oligosaccharides-x. The preparation method comprises the following steps: preparing a conjugate of the core hepatitis B virus antigen and streptavidin, and combining the conjugate with (lewis oligosaccharides-x)-sodium polyacrylate-biotin through high affinity of the streptavidin and the biotin, thereby preparing the compound of the hepatitis B virus antigen and the lewis oligosaccharide. The compound enhances the capability of recognition of a immune system to the hepatitis B virus due to the active targeting of dendritic cells through the high affinity of the lewis oligosaccharides-x and DC-SIGN, can activate specific CTL responses and induce strong anti-viral immunity, and can be applied in the preparation of vaccines for treatment of the hepatitis B.
Owner:ARMY MEDICAL UNIV
Hepatitis B virus dendritic cell therapeutic vaccine and preparation method thereof
InactiveCN101897965AEnhance immune responseAbility to efficiently induce antiviral replicationAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenDendritic cell
The invention discloses a hepatitis B virus dendritic cell therapeutic vaccine and a preparation method thereof. The preparation method thereof includes the steps that: HBcAg-Fc digenic co-expression recombinant eukaryotic vector is constructed; the obtained digenic co-expression recombinant eukaryotic vector is added with saponin Quil A and then is added with cholesterol and lecithin, ice-bath ultrasonic processing is carried out, so that solution is fully mixed and emulsified, PBS dialysis is carried out, and the obtained micro floccus is immunostimulating complex; and the obtained immunostimulating complex activates and simulates dendritic cell, thus obtaining the cell therapeutic vaccine. The vaccine carries out high efficiency transfection on dendritic cell, the generated fusion protein targets the dendritic cell in high efficiency by virtue of Fc segment, thus activating the dendritic cell, promoting immune response of hepatitis B virus core antigen specificity T cell and inducing the antivirus replication capability of immune cell in high efficiency, so that the vaccine can be taken as hepatitis B virus therapeutic vaccine.
Owner:ARMY MEDICAL UNIV
Recombination broad-spectrum vaccine specific to Human enterovirus 71
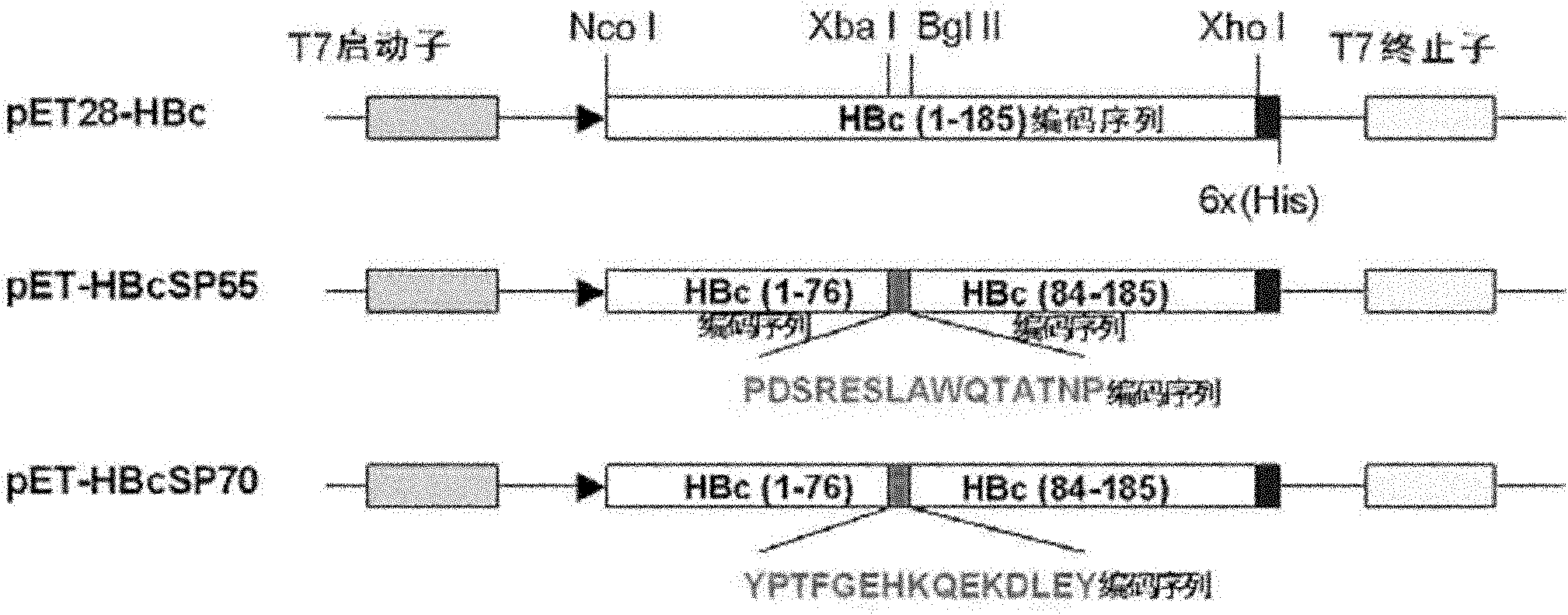
The invention relates to a recombination broad-spectrum vaccine specific to Human enterovirus 71. The invention constructs a fusion protein which comprises at least one copied peptide SP55 and / or SP70 of Human enterovirus 71 VP1 protein and a hepatitis B virus core antigen. The fusion protein can renature and assemble by self in a solubility manner after being expressed, denatured and purified, thereby forming virus-like particles with strong immunogenicity; and the virus-like particles can induce the generation of a broad-spectrum neutralizing antibody with high valence in vivo, thereby being capable of serving as the vaccine for preventing diseases related to Human enterovirus 71 infection. According to the invention, suitable carriers for forming the virus-like particles are found for antigens deriving from the enterovirus 71 VP1 protein, thereby the formed virus-like particles properly expose the antigens and increase the immunogenicity of the antigens.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Composition for treating and/or preventing hepatitis b virus infection and use thereof
PendingCN110944662AAvoid infectionReduce infectionOrganic active ingredientsPharmaceutical delivery mechanismHepatitis B virus core AntigenAntigen
A composition for treating and / or preventing Hepatitis B virus infection and Hepatitis B virus infection mediated diseases and the method thereof are provided. In some embodiments, the composition includes a polyriboinosinic acid-polyribocytidylic acid (PIC), at least one antibiotic or polyamide compound, at least one positive ion, and Hepatitis B virus surface antigen. In some embodiments, the composition includes PIC, at least one antibiotic or polyamide compound, at least one positive ion, Hepatitis B virus surface antigen and Hepatitis B virus core antigen. The present disclosure also relates to a method of treating and / or preventing Hepatitis B virus infection, particularly for treating chronic HBV infection.
Owner:YISHENG BIOPHARMA SINGAPORE
Heat shock protein 65- multiple epitope hepatitis B virus core antigen recombinant protein ú¿HSP65-HBcAgú®
InactiveCN1737147AStrong ability to kill HBV-infected cellsPeptide/protein ingredientsVirus peptidesHepatitis B virus core AntigenT lymphocyte
The invention relates to a recombinant fusion protein HSP65-HbcAg, which is fused from heat shock protein 65 and multi-epitope hepatitis B virus HbcAg, in the recombinant fusion protein, the multi-epitope hepatitis B virus HbcAg comprises 5 different antigen epitopes through interconnection by mathematical combination modes. the recombinant fusion protein can make hepatitis B virus HbcAg specific cell toxic T lymphocyte CTL eradicate the cells infected by hepatitis B virus, thus achieving the goal of treating and / or preventing hepatitis B. The invention also provides the nucleic acid sequence encoding the recombination fusion protein.
Owner:BEIJING HYDVAX BIOTECH
Development and application of wide spectrum influenza vaccine
InactiveCN101015690AImprove immunitySolve the situation of insufficient production capacityAntiviralsRecombinant DNA-technologyHepatitis B virus core AntigenConserved sequence
This invention relates to a development study and application method of a new gene engineering synthetic peptide vaccine, specially relates to development study and application method of broad spectrum influenza vaccine by fusing conserved sequence of influenza virus stroma protein M2 and hepatitis B virus core antigen. The vaccine can immunize body to resist attacks from different hypotype influenza virus.
Owner:河南省生物工程技术研究中心
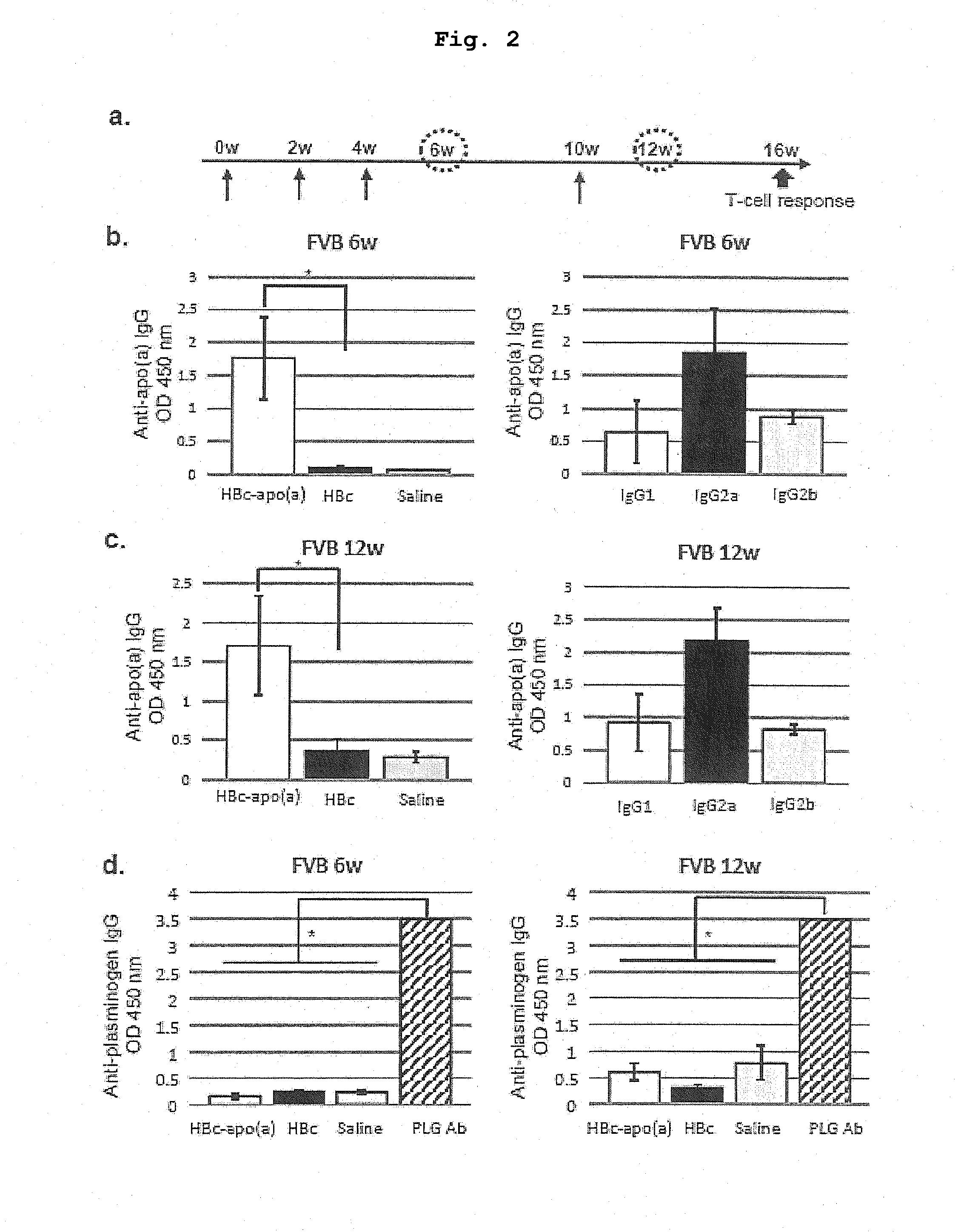
DNA VACCINE CONTAINING SPECIFIC EPITOPE OF APOLIPOPROTEIN (a)
InactiveUS20140086944A1Treating and preventing arteriosclerosisAvoid inductionApolipeptidesViral antigen ingredientsEpitopeHepatitis B virus core Antigen
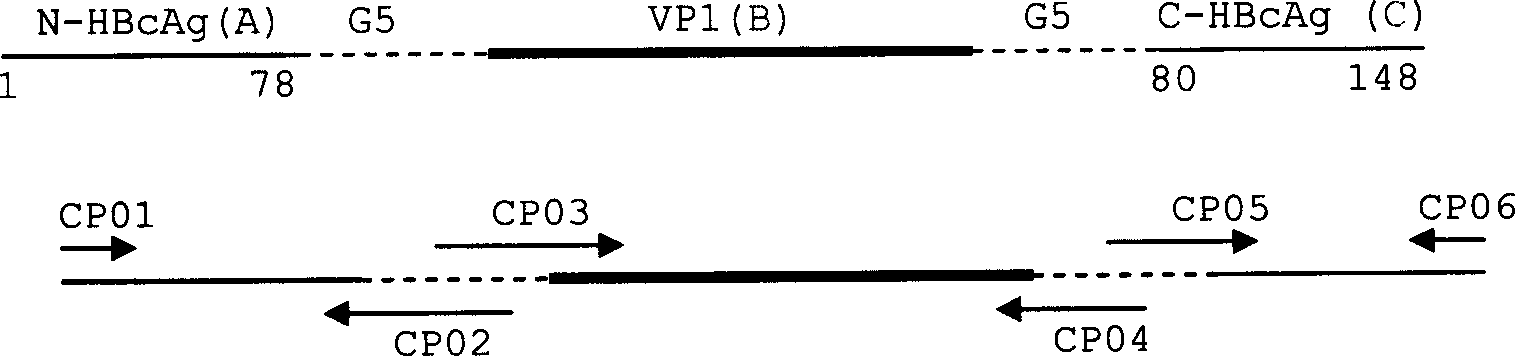
The present invention provides an agent for the treatment or prophylaxis of arteriosclerosis comprising an expression vector encoding a chimeric Hepatitis B virus core antigen polypeptide inserted with an amino acid sequence containing a specific epitope of apolipoprotein (a), wherein the amino acid sequence containing the specific epitope is inserted between the amino acid residues 80 and 81 of the hepatitis B virus core antigen polypeptide.
Owner:ANGES MG INC
DNA vaccine
ActiveUS20140099335A1Treatment or prophylaxis of a lifestyle-related diseaseHormone peptidesSsRNA viruses positive-senseEpitopeHepatitis B virus core Antigen
The present invention provides a therapeutic or improving agent for a lifestyle-related disease, containing an expression vector encoding a chimeric Hepatitis B virus core antigen polypeptide inserted with an amino acid sequence containing a specific epitope of the lifestyle-related disease-related factor, wherein the amino acid sequence containing the specific epitope is inserted between the amino acid residues 80 and 81 of the hepatitis B virus core antigen polypeptide.
Owner:OSAKA UNIV +1
DNA vaccine
ActiveUS9446120B2Treatment or prophylaxis of a lifestyle-related diseaseHormone peptidesSsRNA viruses positive-senseEpitopeHepatitis B virus core Antigen
Owner:OSAKA UNIV +1
Purification method of HBcAg (hepatitis B virus core antigen)-VLP or HBcAg-VLP derivative
InactiveCN109134621AImprove stabilityTo achieve the purpose of purification and preparationBacteriaVirus peptidesHepatitis B virus core AntigenPurification methods
The invention provides a purification method for HBcAg (hepatitis B virus core antigen)-VLP or an HBcAg-VLP derivative. The method comprises the following steps: treating a loading solution containingHBcAg-VLP and a loading solution containing the HBcAg-VLP derivative by hydrophobic interaction chromatographic packing, and purified HBcAg or the purified HBcAg-VLP derivative is obtained. Accordingto the purification method, the problems of numerous steps, low yield, expensive consumables and the like of existing methods are solved, purification can be achieved by only one-step hydrophobic chromatography; when a gel filtration refining method is not used for treatment, the yield can reach up to 90% or higher, purity can reach up to about 86%, and the purification effect is greatly improved; if the purification method is supplemented with refining and purification methods such as further gel filtration, the purity can reach 99% or higher. The purification method has good application prospect and very high application value.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Immune dominant HLA-A3 super-type restrictive CTL epitope of hepatitis B virus core antigen and identification method and application thereof
InactiveCN101979405AImproving immunogenicityStrong specificityMicrobiological testing/measurementDigestive systemHepatitis B virus core AntigenCtl epitope
The invention relates to an antigen epitope, in particular to an immune dominant HLA-A3 (human leukocyte antigen A3) super-type restrictive CTL (cytotoxic T lymphocyte) epitope of a hepatitis B virus core antigen (HBcAg) and an identification method for the super-type CTL epitope. The super-type CTL epitope consists of the following amino acid sequence: Leu-Leu-Asp-Thr-Ala-Ser-Ala-Leu-Tyr-Arg. The method is also suitable for identification of other antigen super-type CTL epitopes, and can provide a useful tool for design and research of therapeutic polypeptide vaccines. The invention also relates to application of the super-type CTL epitope in preparing hepatitis B therapeutic polypeptide vaccines, and provides a new strategy and a new method for efficient research of the hepatitis B therapeutic polypeptide vaccines. The method is expected to break through hepatitis B immune tolerance, reconstruct the immune function of cells and efficiently inhibit and clear hepatitis B viruses.
Owner:ARMY MEDICAL UNIV
Connective tissue growth factor chimeric vaccine for treating liver fibrosis and application of connective tissue growth factor chimeric vaccine
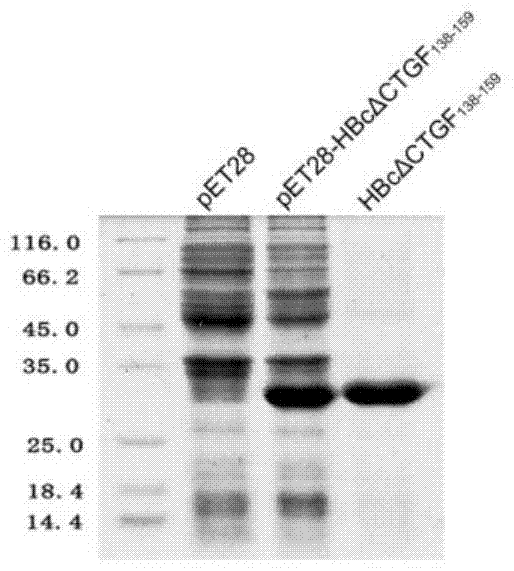
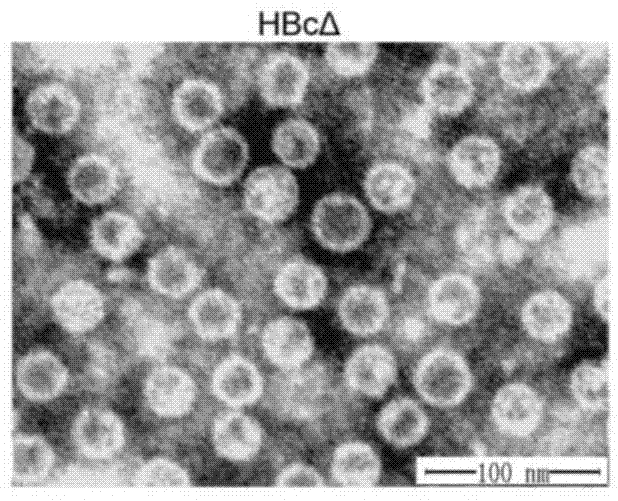
ActiveCN104740628ADegree of inhibitory activationReduce processDigestive systemAntibody medical ingredientsHepatitis B virus core AntigenCTGF
The invention discloses a CTGF (connective tissue growth factor) chimeric vaccine for treating liver fibrosis. The antigenic epitope of the CTGF is inserted into c / e1B cell epitope of hepatitis b virus core antigen to form the CTGF chimeric vaccine which can be assembled into hepatitis B core sample particles. Under the non-adjuvant assistance, the chimeric vaccine can stimulate a body to generate high-valence anti-CTGF neutral antibody. A mouse which is immunized by the chimeric vaccine is obviously relieved in fibrosis degree generated after carbon tetrachloride induction. According to the experimental verification, the CTGF chimeric vaccine can be used for obviously restraining the activation intensity of hepatic stellate cells in the mouse liver, and also can be used for stimulating liver cells to multiply and restraining liver apoptosis. Besides, the TGF-beta 1 and PDGF level in the immunized mouse is obviously reduced, so that the process of liver fibrosis can be relieved in a facilitated manner. According to the results, the CTGF chimeric vaccine can be used for successfully restraining the carbon-tetrachloride-induced mouse liver fibrosis. Therefore, the CTGF chimeric vaccine is expected to be developed into effective means for treating the liver fibrosis.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Recombinant virus-like granule and preparation method and application thereof
InactiveCN109666067APromote extracellular assemblyEfficient secondary assemblySsRNA viruses negative-senseSsRNA viruses positive-senseHepatitis B virus core AntigenDepolymerization
The invention provides a recombinant virus-like granule and a preparation method and application thereof. The method comprises the following steps that cells expressing the recombinant virus capsid protein are fermented, then fermentation products are collected, a 2-6-M urea solution is adopted for dissolving and depolymerization, and an intact virus-like granule of the recombinant viral capsid protein is obtained through extracellular secondary assembly. According to the preparation method of the recombinant hepatitis B virus core antigen granule, the recombinant capsid protein precipitated by ammonium sulphate is dissolved, depolymerized and purified under relatively mild conditions, and finally extracellular secondary assembling is conducted to form the virus-like granule with a naturalstructure. The method is simple and efficient, and the prepared recombinant hepatitis B virus core antigen granule has the advantages of being complete and uniform in structure and free of host nucleic acid and miscellaneous protein. The granule has the important significance and broad application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Hepatitis b immunisation regimen and compositions
PendingCN113573730AViral antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsHepatitis B virus core AntigenAdjuvant
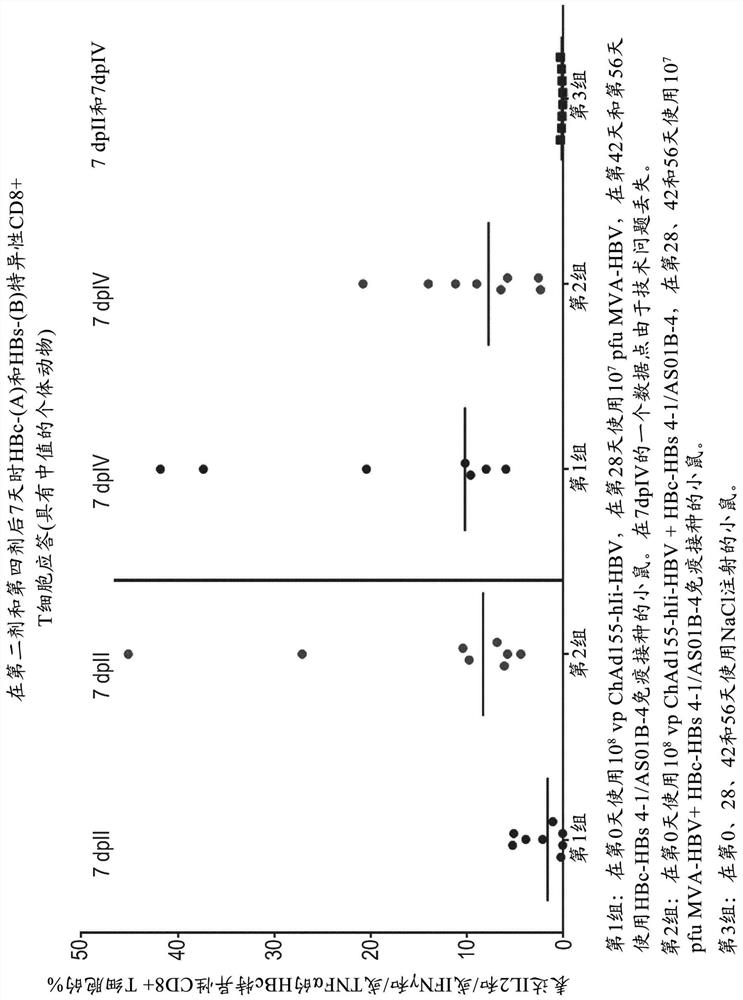
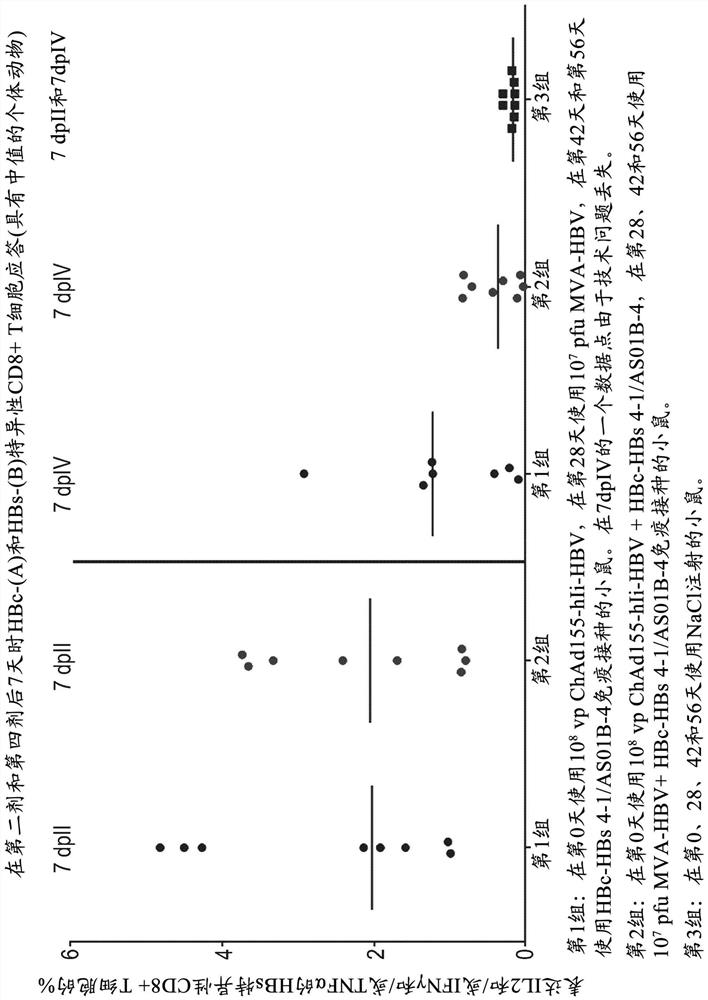
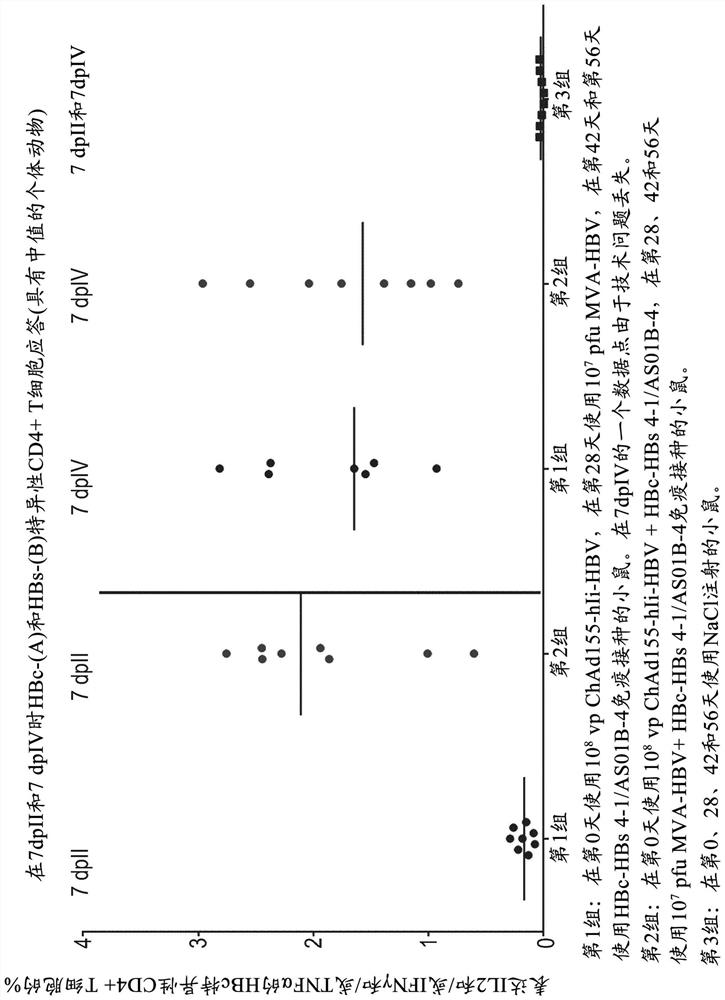
There is provided a method of treating chronic hepatitis B infection (CHB) and / or chronic hepatitis D infection (CHD) in a human, comprising the steps of: a) administering to the human a composition comprising an antisense oligonucleotide (ASO) 10 to 30 nucleosides in length, targeted to a HBV nucleic acid (an HBV ASO); b) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); c) administering to the human a composition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); and d) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, expression vector of immunity-enhanced type virus-like particle and preparation and application of mmunity-enhanced type virus-like particle
InactiveCN105219742AImproving immunogenicityHigh detection sensitivityInactivation/attenuationAllergen ingredientsHepatitis B virus core AntigenADAMTS Proteins
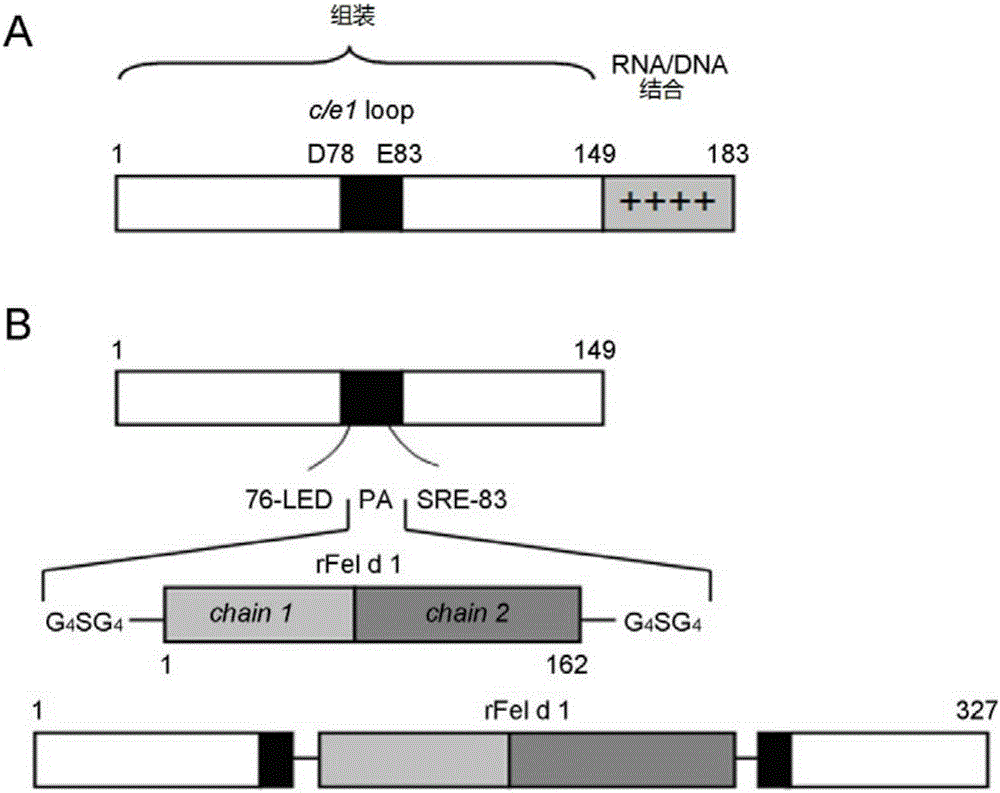
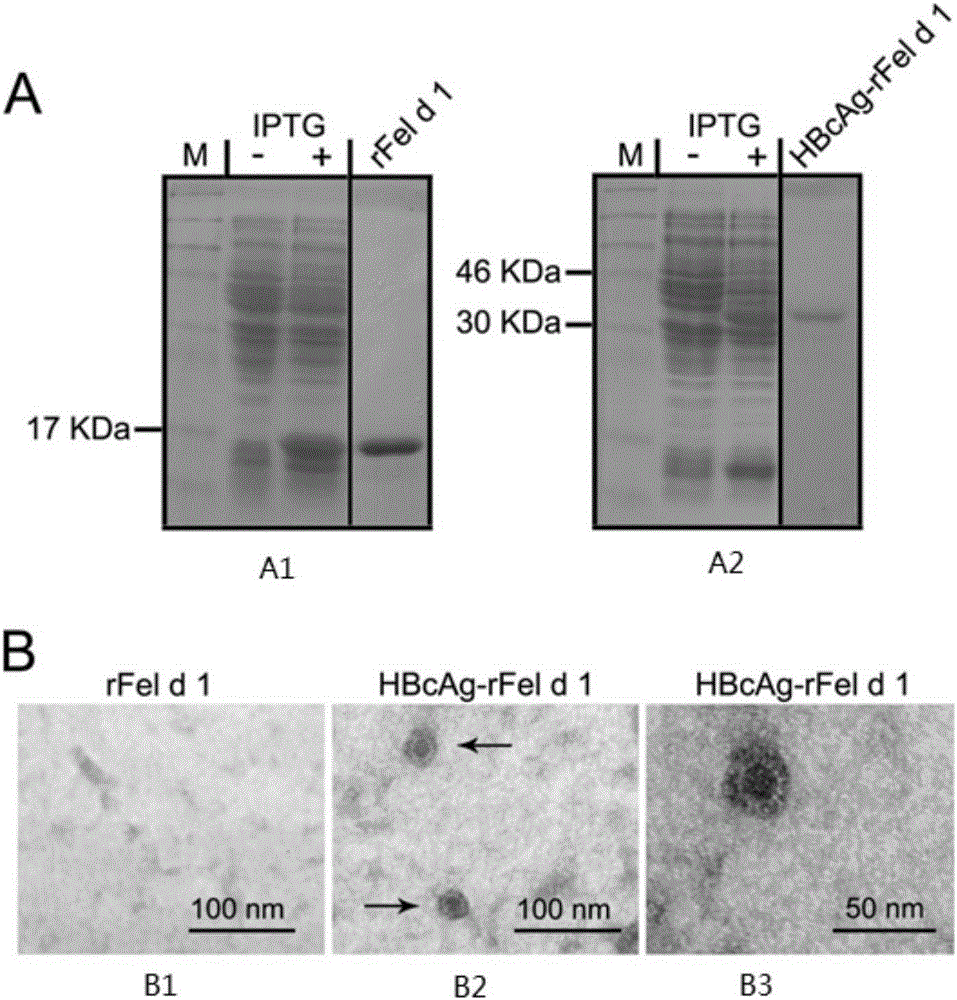
The invention provides an immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, an expression vector of the immunity-enhanced type virus-like particle and preparation and application of the immunity-enhanced type virus-like particle. The protein subunit forming the virus-like particle is fused protein of hepatitis B core antigen and polypeptides with the amino acid sequence of SEQ ID NO:1. The amino acid sequence of the fused protein is formed by inserting the sequence SEQ ID NO:1 of polypeptides between the 78th to 81st amino acid of hepatitis B core antigen and replacing the 79th to 80th amino acid of hepatitis B core antigen. By means of HBcAg-fFel d 1 fused protein, the immunogenicity of fFel d 1 can be remarkably improved, and therefore the detection sensitivity of fFel d 1 recombined and expressed in vitro in application of in-vitro diagnosis cat allergies is improved.
Owner:HAINAN UNIVERSITY
Vaccine preparation for fused virus-like granule, its production method and process of using
InactiveCN1481898AElicit a complete immune responseTrigger immune responseViral antigen ingredientsImmunological disordersHepatitis B virus core AntigenAdjuvant
The fusion virus-like particle contains protein capable of forming virus-like particle as carrier and fused protective target virus antigen. Specifically, human hepatitis B virus core antigen forming virus-like particle is used as the carrier, and protective target virus antigen gene segment is fused in to form fused virus-like particle, which is further mixed with adjuvant, if any, to form vaccine. The target antigen may display its surface to produce relatively high B cell activity and stimulate T cell to produce protective immunity. During use, the vaccine may be made to enter body via injection, spraying, oral taking, dropping to nose or eye, penetration, absorption or physical and chemical mediation.
Owner:CHINA AGRI UNIV
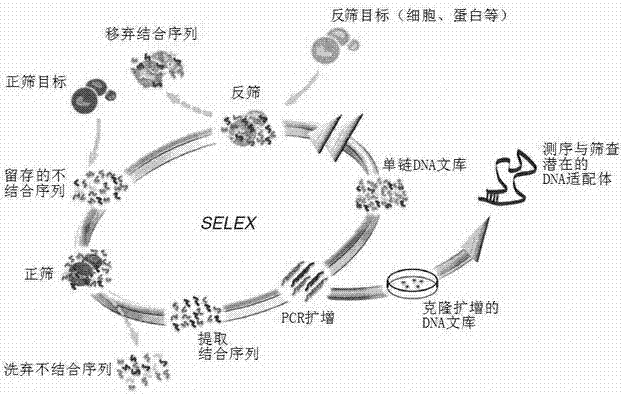
Aptamer sequence of hepatitis B virus (HBV) core antigen and application of nucleic aptamer sequence
ActiveCN102649961AMicrobiological testing/measurementGenetic material ingredientsHepatitis B virus core AntigenAptamer
The invention belongs to the field of molecular immunity and relates to an aptamer sequence for a hepatitis B virus (HBV) core antigen and an application of the nucleic aptamer sequence. According to the invention, a gene-recombination plasmid is adopted to express the core antigen, and an aptamer specially bound with the core antigen is screened, so as to prepare a characteristic sequence CATTT with the special binding HBV core antigen. The characteristic sequence is the base of the special binding of the aptamer and the HBc (hepatitis B core) antigen, can be used for drug design and the preparation of drugs and other products; and the aptamer with the characteristic sequence can be used as a probe or therapeutic target resisting to HBV to design and prepare drugs or agents resisting to HBV.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
HLA-DR9 restrictive regulatory T cell epitope of Hepatitis B virus core antigen and e antigen and application thereof
InactiveCN101942013ABreak immune toleranceClear specificityDigestive systemVirus peptidesHepatitis B virus core AntigenRegulatory T cell
The invention discloses an HLA-DR9 restrictive regulatory T cell epitope of Hepatitis B virus core antigen and e antigen, which is composed of the following amino acid sequence: SRDLVVNYVNTNMGLKIRQLLWFHI. The invention also discloses an application of a regulatory T cell epitope in preparing hepatitis B therapeutic vaccine containing the Hepatitis B virus core antigen and e antigen, namely when the hepatitis B therapeutic vaccine containing the Hepatitis B virus core antigen and / or e antigen is prepared, the regulatory T cell epitope with immunosuppressive action in the Hepatitis B virus coreantigen and / or e antigen is eliminated. The invention provides a new strategy and a method for development of the high-efficient hepatitis B therapeutic vaccine, is expected to break hepatitis B immune tolerance, rebuilds cellular immune function and effectively inhibits and eliminates the hepatitis B virus.
Owner:ARMY MEDICAL UNIV
Nanometer antibody for resisting hepatitis B virus core antigen and application of nanometer antibody
InactiveCN110759994ABiological material analysisImmunoglobulins against virusesHepatitis B virus core AntigenHepatitis B immunization
The invention discloses a nanometer antibody for resisting a hepatitis-B-virus core antigen (HBcAg). HBcAg is used for immunization of alpacas, and through screening, the nanometer antibody for resisting the HBcAg is obtained. The invention further relates to a complete antibody consisting of antibody fragments in fusion with Fc. The antibody is used for treatment of hepatitis-B-viruses and livercancer, and the invention provides a method for preparing the antibody.
Owner:SHENZHEN IMMUNOTHERAPY BIOTECH CO LTD
Dna-peptide combination vaccine
PendingCN107530408AEfficient inductionReduce the number of dosesViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenAntigen
The present invention provides a pharmaceutical preparation that is a combination pharmaceutical preparation for inducing a specific immune response to an antigenic peptide, wherein: the pharmaceutical preparation comprises (I) the antigenic peptide and (II) an expression vector that encodes a chimera hepatitis B virus core antigen polypeptide to which the antigenic peptide has been inserted or added, the antigenic peptide being inserted into the region of amino acid residues 74 to 87 or 130 to 138 of the hepatitis B virus core antigen polypeptide or added to the N terminal or C terminal of the hepatitis B virus core antigen polypeptide; and the antigenic peptide (I) and the expression vector (II) are given substantially simultaneously to an application subject.
Owner:ANGES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com