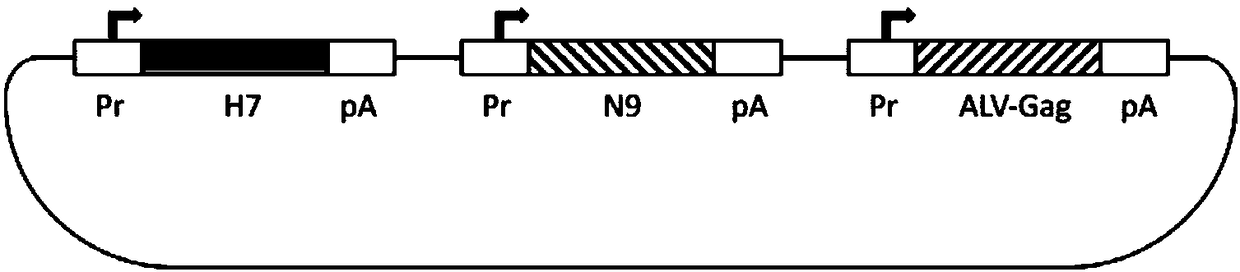
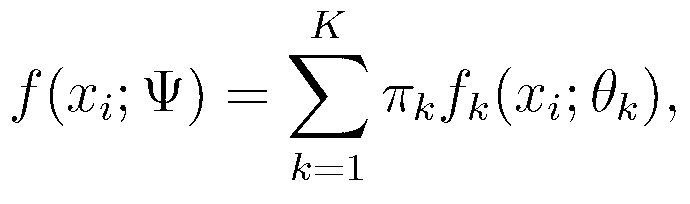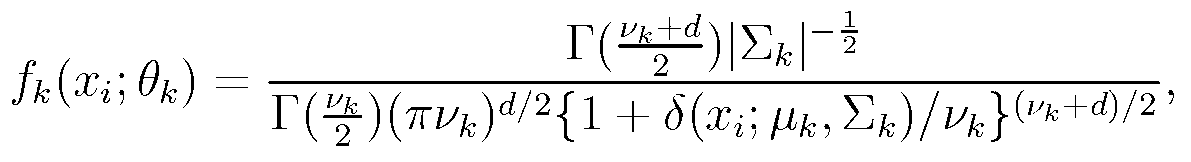Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
430 results about "Hypotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hypotype is a genus of moths of the family Noctuidae.
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
InactiveUS20050186621A1Minimal numberSsRNA viruses negative-senseFungiHeterologousVirus-like particle
Influenza virus-like particles (VLPs) comprising the structural proteins HA, NA, M1 and M2 are described. VLPs are also generated containing M1 alone, as are VLPs with M1 and any one or two of HA, NA and M2. VLPs with HA from one influenza subtype and NA from a different influenza subtype are also described, as are VLPs in which a portion or all of HA or NA is replaced by a heterologous moiety not produced by influenza virus, so as to comprise chimeric VLPs.
Owner:WYETH HOLDINGS LLC
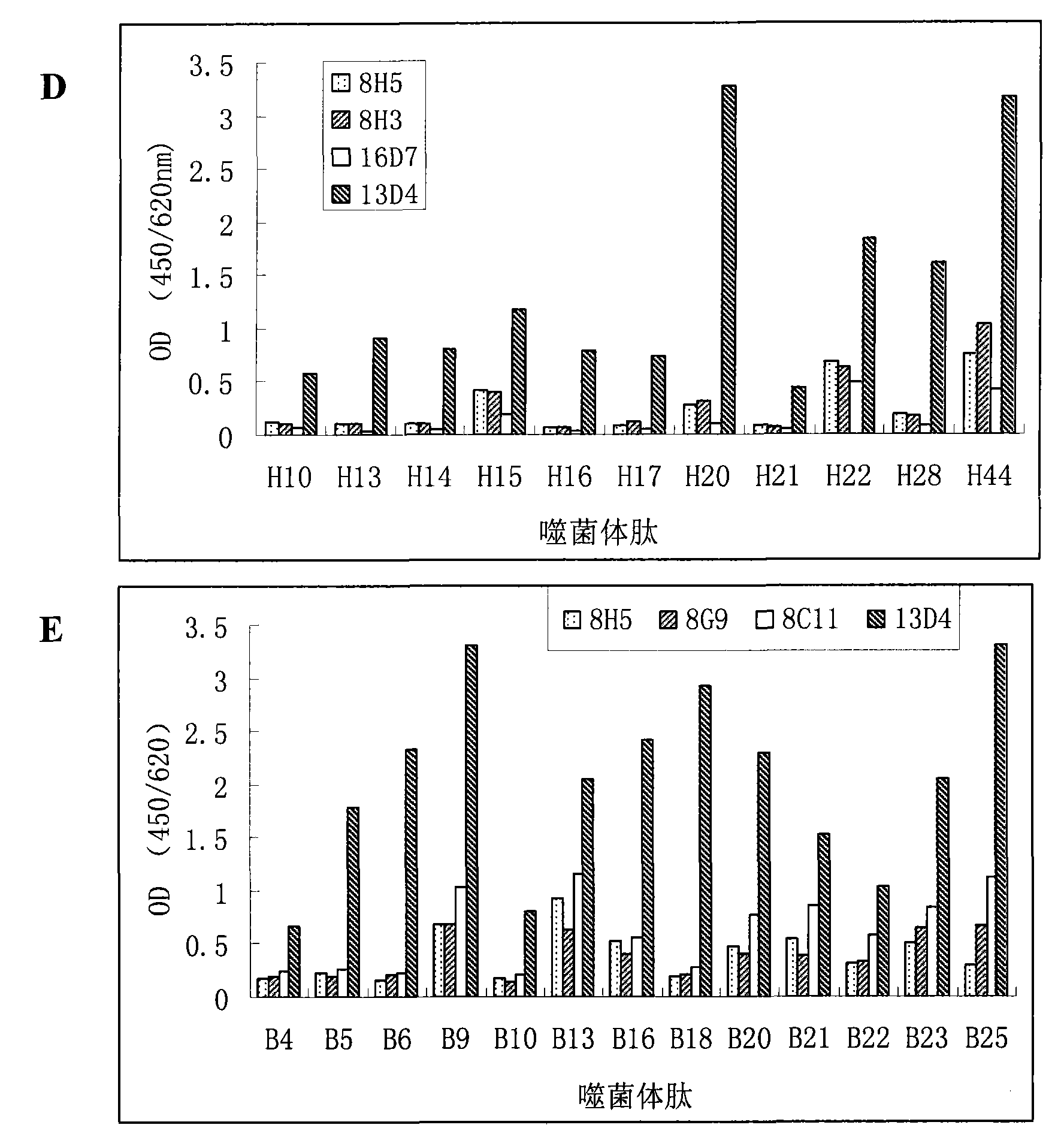
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
Primer, probe series and method for multiple real time fluorescent RT-PCR detection of H5, H7 and H9 subtype bird flu
InactiveCN1670221AGood fast sensitivityStrong specificityMicrobiological testing/measurementFowlHighly pathogenic
Owner:TAITAI GENOMICS +1
Avian influenza virus marking vaccine, preparation process and application thereof
The invention discloses a vaccine strain H5N1 / PR8-5B19 marked by H5N1 hypotype poultry influenza virus, which also provides the application to prevent and monitor poultry influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Primer system and method for detecting and analyzing avian influenza virus
InactiveCN101058833AQuick checkAccurate detectionMicrobiological testing/measurementDNA preparationFowlHypotype
The invention provides a method for detecting fowl influenza virus and parting primer system and augmenting fowl influenza virus RNA asymmetrically. The invention designs and screens 25 pairs multiple asymmetrical RT-PCR primers, a pair general primer, 52 specific probes and 3 quality control probes according to the report fowl influenza virus total hypotype genome sequence in Genebank, wherein every pair in 25 pairs multiple asymmetrical RT-PCR primers is fit for a hypotype of fowl influenza virus, the primer system comprising 25 pairs multiple asymmetrical RT-PCR primers and a pair general primer can be used for detecting and parting fowl influenza virus, the primer system builds the method of asymmetrically augmenting fowl influenza virus RNA and detecting and parting fowl influenza virus. The invention provides strong specificity, high sensibility, which also proceeds the first step application.
Owner:中国检验检疫科学研究院动植物检疫研究所
Method and detection kit used for detecting virus
InactiveCN102435746AEasy separationHigh fluorescence intensityFluorescence/phosphorescenceViral membraneHypotype
The invention relates to a method and a detection kit used for detecting a virus. The method comprises steps that: an antibody of the glycoprotein of a target virus adventitia is coupled with magnetic nano / micro-spheres, such that immunized magnetic nano / micro-spheres are obtained; the immunized magnetic nano / micro-spheres are used for catching the target virus in the sample, such that a magnetic sphere virus composition is obtained; the antibody of the glycoprotein of the adventitia of the biotinylated virus is incubated with the magnetic sphere virus composition; the obtained material is incubated with streptavidin-coupled quantum dots, such that a magnetic sphere-virus-quantum dot composition is obtained; and the virus is detected through the detection upon fluorescence signals of the magnetic sphere-virus-quantum dot composition. With the method, specific recognition, preconcentration, separation, and high-sensitivity detection of H9N2 subtype avian influenza active virus can be realized. The invention also provides a detection kit employed by the method. The kit and the method provided by the invention are characterized by simple operation, high specificity, high sensitivity, and the like. The whole detection process can be finished in one hour.
Owner:WUHAN UNIV
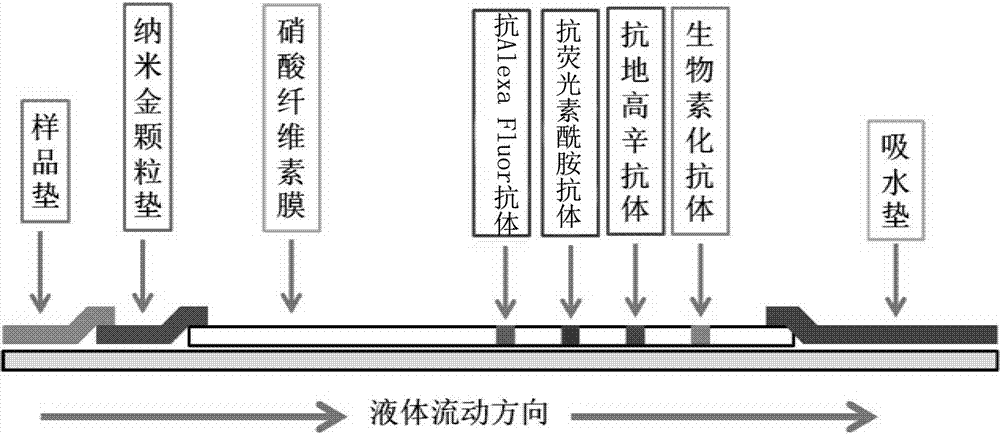
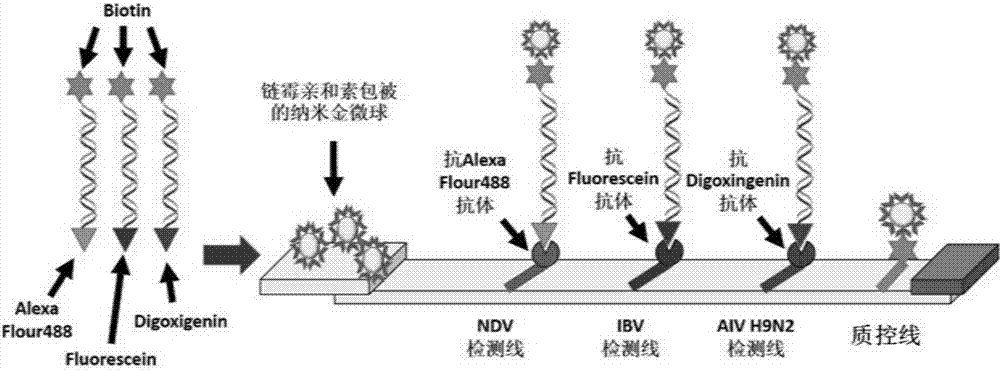
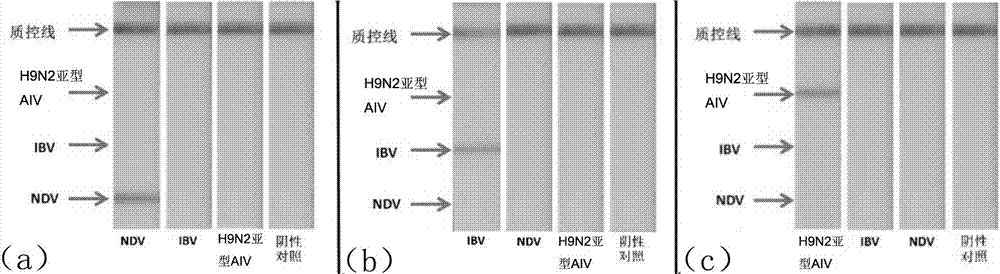
Multi-RPA primer and probe for simultaneously detecting NDV, IBV, H9N2-subtype AIV and detection method of multi-RPA primer and probe
ActiveCN107287349AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMultiplex pcrsRNA extraction
The invention discloses a multi-RPA primer and probe for simultaneously detecting a newcastle disease virus, an infectious bronchitis virus and an H9N2-subtype avian influenza virus. The multi-RPA primer and probe comprise a primer and probe for detecting the newcastle disease virus, a primer and probe for detecting the infectious bronchitis virus and a primer and probe for detecting the H9N2-subtype avian influenza virus. The multi-RPA primer and probe are high in specificity, high in sensitivity and accurate in detection result. The invention further discloses a method for simultaneously detecting the newcastle disease virus, the infectious bronchitis virus and the H9N2-subtype avian influenza virus through multi-RPA. The method comprises the steps of primer synthesis, template RNA extraction, reverse transcription, multi-PCR amplification and amplification product analysis. The detection method is simple in operation and good in stability, and a low-cost, fast and specific field diagnosis method is provided for effective detection and identification of mixed infection of common avian respiratory diseases.
Owner:HENAN AGRICULTURAL UNIVERSITY
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
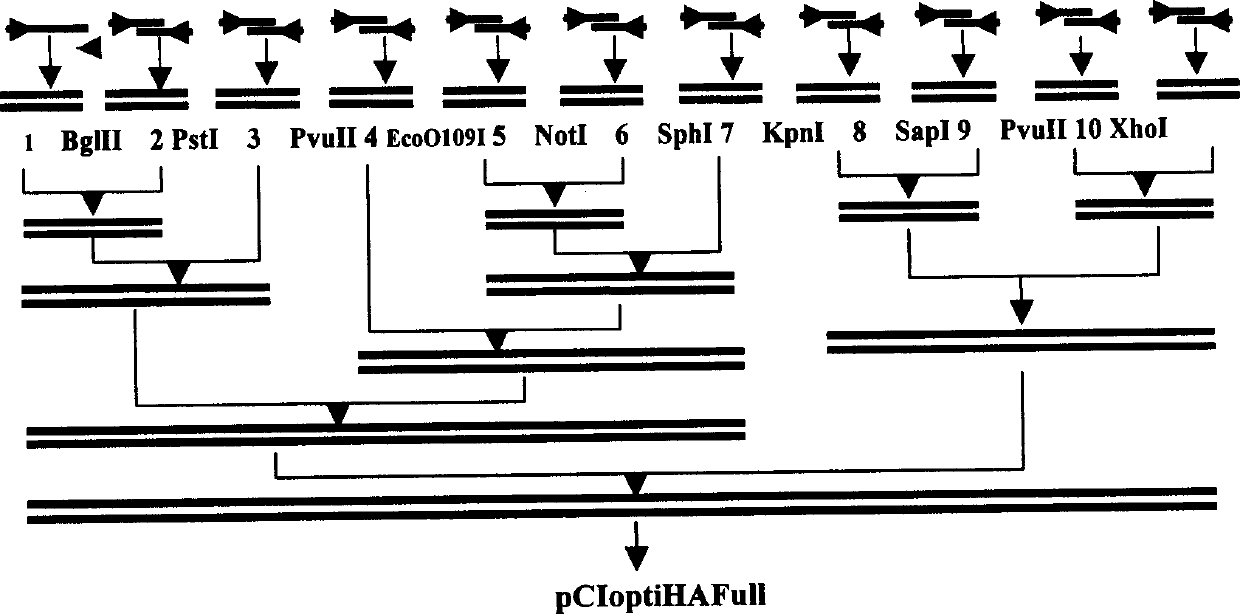
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting influenza A virus subtype H7N9
ActiveCN103275862AQuantitatively accurateLow costBioreactor/fermenter combinationsBiological substance pretreatmentsConserved sequenceInfluenza Viruses Type A
The invention provides a fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting an influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit can be used for detection of influenza A viruses and the influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit comprises a quantitative RT-PCR reaction solution, an enzyme mixed liquor, a primer and probe mixed liquor, standard substances of influenza A viruses, H7, N9 and RNaseP, positive reference substances of influenza A viruses, H7, N9 and RNaseP), and negative reference substances. Specific primers and probes are designed according to conserved sequences of influenza A viruses, H7 and N9. The RNaseP primers and probes are used as internal references. Through the one-step quadruple real-time fluorescent RT-PCR technology, the influenza A virus and the influenza A virus subtype H7N9 in the sample can be fast and accurately detected. The fluorescent quantitative RT-PCR kit has a reasonable design, very high singularity, sensitivity and repeatability, can be used for laboratory emergency diagnosis and fast screening of an epidemic disease caused by the influenza A virus subtype H7N9, and for an epidemiology study on the influenza A virus and the influenza A virus subtype H7N9 causing fever and respiratory tract syndrome.
Owner:ZHEJIANG UNIV
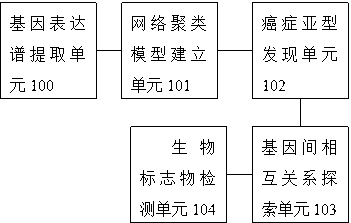
Cancer hypotype biomarker detecting system based on student t distribution
InactiveCN103268431ARobustEasy to handleSpecial data processing applicationsMaterial analysisNetwork structureOncology
The invention relates to a cancer hypotype biomarker detecting system based on student t distribution. The cancer hypotype biomarker detecting system comprises a gene expression profile extracting unit, a network cluster model establishing unit, a cancer hypotype finding unit, an intergenic mutual relation exploring unit and a biomarker detecting unit. The gene expression profile extracting unit is used for extracting an expression profile of a gene sample in a gene chip. When the extracted gene expression profile is input to the network cluster model establishing unit, the network cluster model establishing unit is used for establishing a mixture model and solving the gene expression profile, wherein the mixture model uses the diverse student t distribution to describe each component. The cancer hypotype finding unit is used for finding new cancer hypotypes by utilizing the mixture model and a clustering method. The intergenic mutual relation exploring unit regards each gene as a point inside a network, and is used for exploring the mutual relation among genes in different cancer hypotypes by exploring the network structure. The biomarker detecting unit is used for detecting a biomarker related to the cancer hypotypes according to the mutual relation among the genes in different cancer hypotypes. The cancer hypotype biomarker detecting system describes each component in the mixture model by using the diverse student t distribution, and can have robustness for abnormal points and noise in the clustering and variable selecting process.
Owner:SUN YAT SEN UNIV
Flu/human avian influenza virus detection gene chip and production method and use
InactiveCN101392302AShort detection timeImprove detection accuracyMicrobiological testing/measurementHighly pathogenicTyping
The invention discloses an influenza / human and avian influenza virus detection gene chip and a preparation method and an application thereof, 102 probes used for detecting A type, B type, H1N1 hipotype, H3N2 hipotype, H5N1 hipotype and H9N2 hipotype influenza viruses and 4 highly pathogenic differential diagnosis probes, as well as a process of sample treatment, hybridization, elution and chip identification which can carry out detection, typing or identification over the 6 types of viruses simultaneously and can identify the high pathogenicity of viruses. The invention not only greatly shortens the detection time of influenza viruses, but also improves the detection accuracy and provides a feasible technique support for the early warning mechanism of influenza epidemic situation in fields of clinical diagnosis, inspection quarantine, and the like.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Method for inhibiting influenza virus infection and medicament thereof
InactiveCN101186637APeptide/protein ingredientsGenetic material ingredientsHighly pathogenicHuman influenza
The invention relates to a method for restraining enveloped virus infection, relative polypeptide and protein drug, belonging to biological medicine technical field. The invention comprises a method for restraining influenza virus, particularly for restraining highly pathogenic influenza virus and human influenza virus (as H1N1 hypotype and H3N2 hypotype), relative polypeptide and protein, relative nucleic acid encoding the polypeptide and protein, and relative carriers and cells for representing the polypeptide and protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Artificially recombinant H9N2 hypotype influenza virus and its uses
The invention relates to an artificial recombination N9 hypotype influenza virus A / Harbin / Re-2 / 2003 (H9N2), artificial recombination H9 hypotype influenza virus A / Harbin / Re-2 / 2003(H9N2) characterized in that, it includes a HA of H9N2 hypotype fowl influenza virus A / chicken / Guangxi / 10 / 99(H9N2) and six internal genes of influenza virus A / PR / 8 / 34(H1N1), PB2, PB1, PA, NP, M and NS. its storage number is CCTCC-V200311.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Fast typing detection method of high-flux visualized HPV (Human Papilloma Virus) gene chip
Owner:SHANXI LIFEGEN
Detection method and detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus
InactiveCN101818207AIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesReaction conditionsTaqMan
The invention discloses a detection method and a detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus, which has the advantages of specificity, sensitivity, good repeatability, fastness and low cost. A specific primer and a TaqMan probe are designed through sequence alignment for searching a highly conserved area according to a North American variant strain M gene of Influenza A Viruse(H1N1)virus in 2009, an HA gene sequence and the HA gene sequence of Influenza A Viruse(H3N2) epidemic strain, which are published in GeneBank; key reagents such as the probe, the primer and positive control (to structure the influenza A virus, the H1N1 and the H3N2 subtype influenza virus gene recombination clone plasmid) in the detection method are developed; and the influenza A virus, H1N1 and H3N2 subtype influenza virus real-time fluorescence RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection method is established through the optimization of various reaction conditions and the tests on the specificity, the sensitivity and the repeatability. The detection method can be used for simultaneously and specifically detecting the influenza A virus, the H1N1 subtype influenza virus and the H3N2 subtype influenza virus in one test.
Owner:中华人民共和国珠海出入境检验检疫局
Fluorescent quantitative PCR primer, probe, kit and detection method for detecting avian influenza subtype
ActiveCN106435024AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementAvian influenza virusRepeatability
The invention provides a multiplex fluorescent quantitative PCR primer and a probe for detecting H5, H7 and H9 subtype avian influenza viruses. With adoption of the primer and the probe, multiplex fluorescent quantitative PCR can be adopted to simultaneously detect which subtypes the H5, H7 and H9 subtype avian influenza viruses concretely are, no influences exist among the primers of different subtypes, the specificity is strong, the detection sensitivity is 10-50 copy numbers, a target sequence can be accurately detected quantitatively and qualitatively, the repeatability is good, and the reliability is high. The invention provides a multiplex fluorescent quantitative PCR kit for simultaneously detecting the H5, H7 and H9 subtype avian influenza viruses. The kit has the detection sensitivity being 10-50 copy numbers and is high in sensitivity and strong in specificity. The invention also provides a detection method which is good in stability. With adoption of plasmids with gene fusion as a positive standard substance, tedious operation of changing the positive standard substance for multiple times is avoided, the detection time is greatly shortened, the detection times are greatly reduced, and detection of a sample can be finished within 2h.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for detecting influenza virus by liquid phase chip
ActiveCN101717830AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescenceMicrosphere
The invention discloses a method for detecting influenza virus by a liquid phase chip. The method is used for carrying out homology analysis on all Non Structural nucleotide sequences of A type influenza virus and B type influenza virus as well as H1, H2, H3, H5, H7 and H9 of the A type influenza virus and hypotypes of N1 and N2, and primers and specific probes are designed aiming at the corresponding gene segments; an improved nest-PCR method is adopted for PCR amplification by using nested primers with different lengths; amination treatment is carried out the 5' end of the specific probe, and the specific probe is coupled with a fluorescent coded microballoon; incubation hybridization is carried out on the PCR amplification product and the mixed microballoon coupled with the specific probe; and finally Luminex 100 is used for detecting. The detection method has higher specificity and sensitivity as well as rapid detection speed, and can be used for indentifying and monitoring the influenza viruses.
Owner:SHANDONG ACV BIOTECH CO LTD
Multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing and application thereof
ActiveCN108192996AEasy to detectReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationTypingTrue positive rate
The invention belongs to the field of molecular biological detection, and relates to a multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing and application thereof.The multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing comprises an RT-RPA primer for detecting an influenza A virus Matrix gene, an H1 subtype HA gene and an H3 subtype HA gene, and the sequences are sequentially shown in SEQ ID NO.1-NO.6. The multiple RT-RPA primer combination can be used for detecting an influenza A virus and verifying H1 and H3 subtypes. According to the multiple RT-RPA primer combination, a multiple RT-RPA method is adopted for detecting the influenza A virus and verifying the H1 and H3 subtypes for the first time, and the combinationis short in consumed time, high in sensitivity and specificity and capable of being quickly and effectively used for influenza A virus detecting and H1 and H3 typing.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
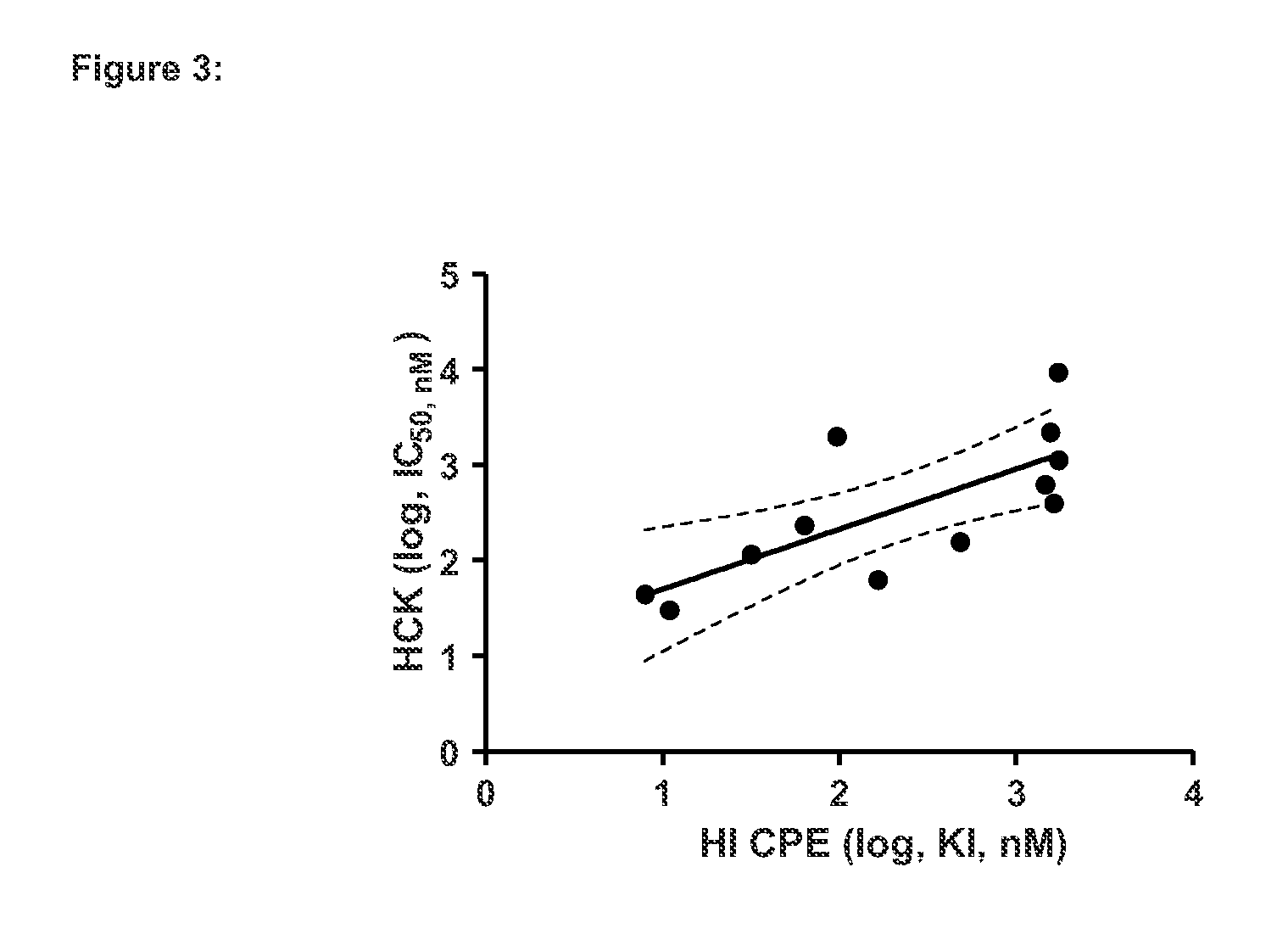
Inhibitors of Hemopoietic Cell Kinase (P59-HCK) and Their Use in the Treatment of Influenza Infection
The present invention relates inter alia to the treatment or prevention of influenza virus infection (including subtypes influenza A virus, influenza B virus, avian strain H5N1, A / H1N1, H3N2 and / or pandemic influenza) using compounds which inhibit the activity of p59-HCK and to a method of screening for a candidate drug substance intended to prevent or treat influenza virus infection in a subject, said method comprising identifying a test substance capable of inhibiting p59-HCK activity.
Owner:RESPIVERT
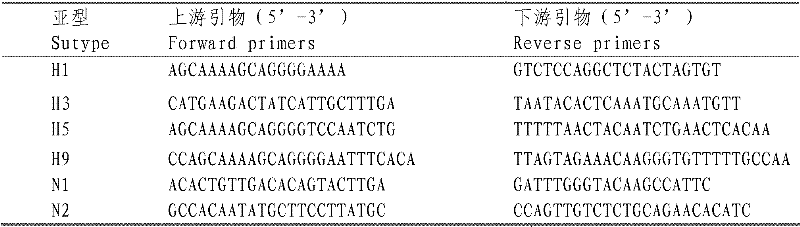
Conserved neutralizing epitope mimic peptide of H5 subtype avian influenza viruses and use thereof
The invention provides a hemagglutinin (HA) protein neutralizing epitope mimic peptide of H5 subtype avian influenza viruses, a conserved derivative or active fragment or mutant sequence thereof, related coding sequence thereof and the use of the mimic peptide or the conserved derivative or active fragment or mutant sequence thereof in prevention and diagnosis.
Owner:XIAMEN UNIV
Method for detecting different subtype avian influenza viruses and special kit thereof
InactiveCN101724713AImprove throughputEasy to operate and save timeMicrobiological testing/measurementFluorescence/phosphorescenceAgricultural scienceHigh flux
The invention discloses a method for detecting different subtype avian influenza viruses and a special kit thereof. The method comprises the following steps of:, carrying out multiple PCR using a primer pair B by a primer pair A by utilizing the cDNA of a biological sample to be tested as a template, carrying out agarose gel electrophoresis on products of the multiple PCR, and respectively carrying out gel scanning and analysis by a fluorescence imaging analyzer with the wavelength of 532 nm and 635 nm; if a fluorescence strip exists under the wavelength of 635nm, judging that the biological sample to be tested contains H3 subtype avian influenza virus; and if a fluorescence strip exists under the wavelength of 532nm, judging that the biological sample to be tested contains H6 subtype avian influenza virus. The primer pair A is a primer pair consisting of nucleotides represented by a sequence 11 and a sequence 12 in a sequence list, and the 5' end of the nucleotides represented by the sequence 12 is marked by Tamra. The primer pair B is a primer pair consisting of nucleotides represented by a sequence 3 and a sequence 4 in the sequence list, and the 5' end of the nucleotides represented by the sequence 4 is marked by Cy5. In the invention, the method has the advantages of high flux, high distinguishability, strong maneuverability, wide application range, time saving, test consumable saving, safety and the like.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Canine influenza virus and related compositions and methods of use
ActiveUS20070098742A1SsRNA viruses negative-sensePeptide/protein ingredientsImmunity responseHypotype
The present invention provides an isolated canine influenza virus of subtype H3N8 comprising an HA having SEQ ID NO: 4 or an amino acid sequence that is greater than 99% identical to SEQ ID NO: 4, with the proviso that the amino acids at positions 94 and 233 are identical to SEQ ID NO: 4; a composition comprising attenuated or inactivated virus; isolated or purified HA, NM, NP, M1, NS1, PA, PB1, and PB2 proteins and fragments thereof and compositions comprising same or nucleic acids, optionally as part of a vector, encoding same; and a method of inducing an immune response to canine influenza virus in an animal comprising administering to the animal an aforementioned composition.
Owner:IOWA STATE UNIV RES FOUND
Ordinary/chimeric virus-like particle of H7 subtype influenza virus H7N9, preparation method and application of ordinary/chimeric virus-like particle, and vaccine
ActiveCN108329379AGood effectFix security issuesSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral Vaccine
The invention provides an ordinary / chimeric virus-like particle of an H7 subtype influenza virus H7N9, a preparation method and application of the ordinary / chimeric virus-like particle, and a vaccine,and belongs to the technical field of bioengineering and viral vaccines. The ordinary / chimeric virus-like particle of the H7 subtype influenza virus is used for preparing the vaccine, and accordingly, the safety problems of existing vaccines are well solved; the obtained vaccine has high antibody valence and obvious effect; the ordinary / chimeric virus-like particle has wide application range andhigh actual application value.
Owner:NOVARTIS BIOTECH WUHAN +1
Combined inactivated vaccine for avian influenza virus and fowl adenovirus
ActiveCN105582533AImprove securityHigh potencySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityTGE VACCINE
The invention provides a combined inactivated vaccine for an avian influenza virus and a fowl adenovirus. An H9 subtype avian influenza virus QDY strain and an I-group 4-type aviadenovirus YBAV-4 new strain used by the vaccine are high in TCID50 / EID50 valence and good in immunogenicity and can well withstand attacks of the H9 avian virus and various local separate viruses. The vaccine prepared by the invention is good in safety, and free of local and whole-body adverse effects caused by the vaccine. Through analysis of characters, a safety test and efficacy test data in a storage-life test, the combined vaccine is free of an obvious difference from a single vaccine of a similar product in effect, and is stable and effective; the efficacy test result proves that the antibodies of the combined vaccine and two single vaccines are kept at a high level and are faster in antibody generation of similar products; and a control group antibody is negative.
Owner:YEBIO BIOENG OF QINGDAO
Human enterovirus(EV) fluorescence quantitative PCR detecting technology
This invention relates to one human intestinal canal virus fluorescence meter test technique in the field of virus nuclear test technique, which adopts common upstream object, common downstream object and common fluorescence detector for EV each hypotype, wherein, the detector 5'end is labeled with fluorescence emission base group 6- carboxyl fluorandiol and 3'labeled fluorescence quencher base group and 6-carboxyl tetramethyl rhodamine. Due to the test agent case, it tests each EV hypotype standard blood serum for online test and to get each parameter as the following: abnormal property for 100 percent; sensitivity for 95 percent, repetitiveness CV less than 10 percent and minimum content of EV as 500 copy each ml.
Owner:河南省生物工程技术研究中心
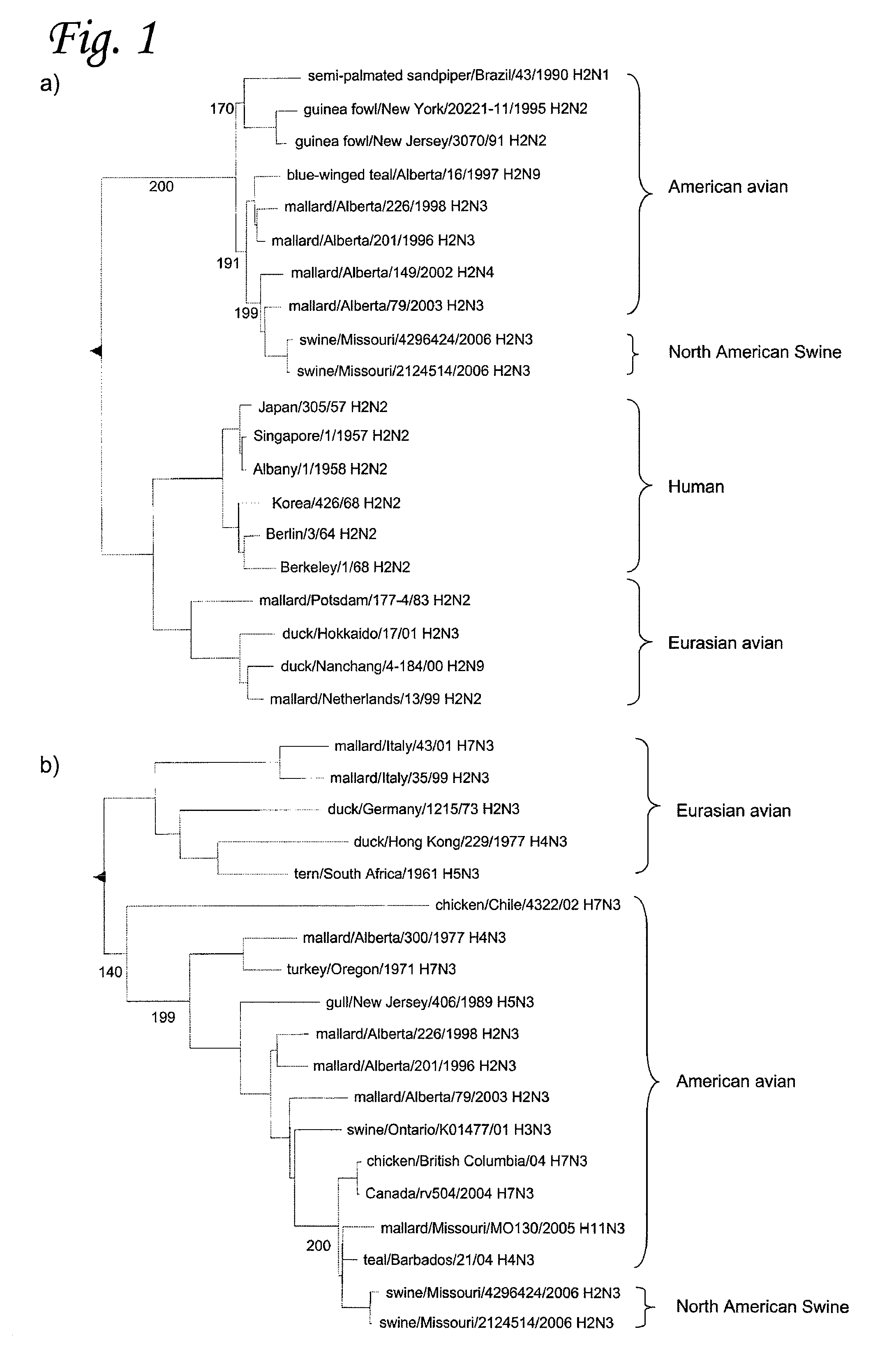
H2N3 influenza A viruses and methods of use
Owner:RGT UNIV OF MINNESOTA +2
Newcastle disease-H9 subtype avian influenza bivalent dual adjuvant inactivated vaccine and preparation method thereof
InactiveCN102416177APrevent immune failureAvoid immune failureViral antigen ingredientsAntiviralsImmune effectsOil adjuvant
The invention discloses a Newcastle disease-H9 subtype avian influenza bivalent dual-adjuvant inactivated vaccine and a preparation method thereof. The bivalent vaccine consists of a concentrated and inactivated ND and AI (H9) venom, levamisole hydrochloride and an oil adjuvant, wherein every milliliter of inactivated vaccine contains 0.5-16 mg of levamisole hydrochloride immunopotentiator. When the synergic ND-AI (H9) bivalent dual-adjuvant inactivated vaccine prepared with the method is applied to immunized chicken, the humoral immunity and cell immune levels of table poultry and laying hens can be raised effectively, the growth is promoted, and rate of live weight growth is increased. The bivalent dual-adjuvant inactivated vaccine has an immune effect which is remarkably superior to that of the conventional chicken ND-AI bivalent oil emulsion inactivated vaccine, and can be used for effectively protecting chicken flocks from being intruded by the ND virus and AI (H9) virus and preventing the Newcastle disease and H9 subtype avian influenza at the immune period.
Owner:TIANJIN RINGPU BIO TECH
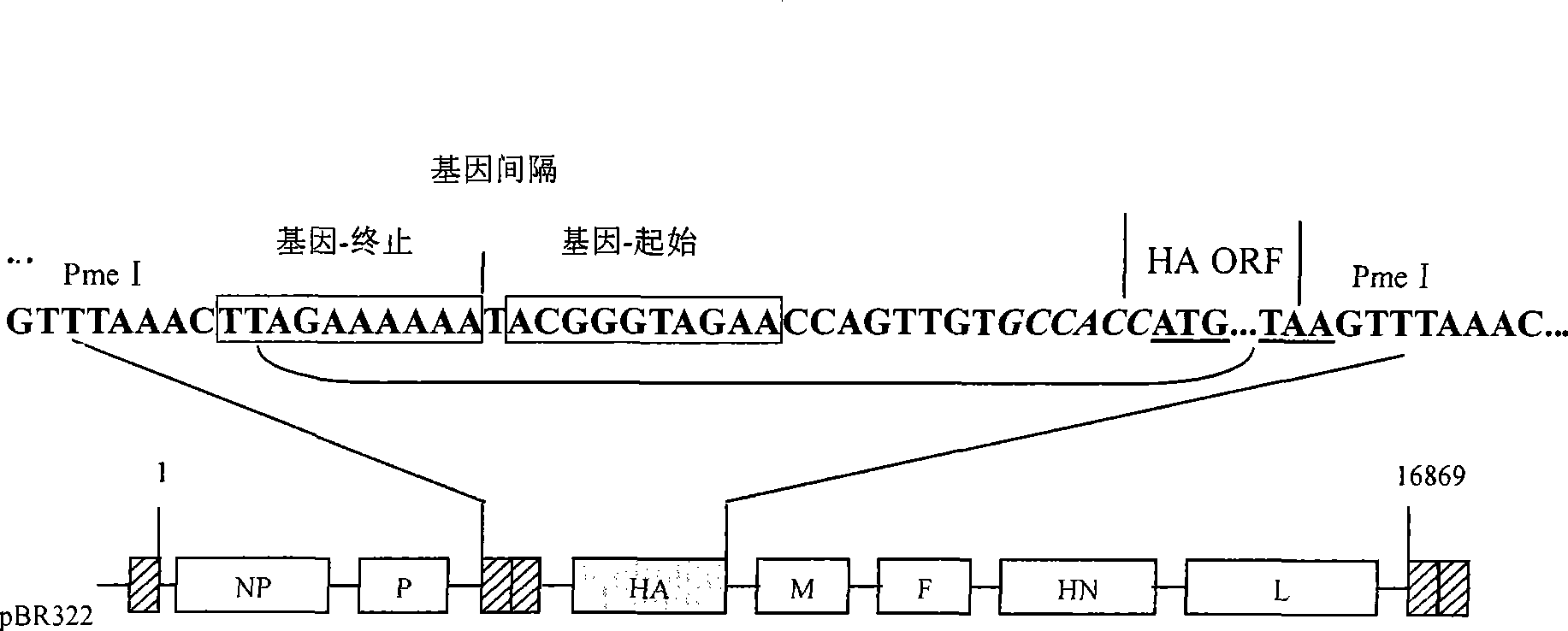
Recombined newcastle disease virus LaSota attenuated vaccine strain for expressing avian influenza virus H9 subtype HA protein
The invention relates to a recombinant newcastle pestilence LaSota HCLV expressing the bird flu virus H9 subtype hemagglutinin (HA) protein, in particular the recombinant newcastle pestilence LaSota is rL-H9HA. The invention further discloses a method for preparing the recombinant newcastle pestilence LaSota HCLV and the application of the HCLV in preparing a bivalent vaccine for preventing bird flu and newcastle pestilence.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com