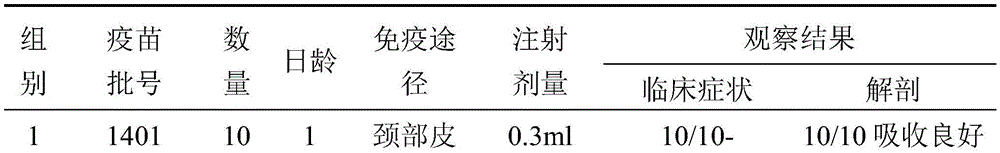
Combined inactivated vaccine for avian influenza virus and fowl adenovirus
A dual inactivated vaccine, avian influenza virus technology, applied in the direction of vaccines, viruses, antiviral agents, etc., can solve the problems of increasing the manpower and material burden of chicken farms, affecting production performance, and increasing the susceptibility of chickens to diseases. , to achieve the effect of good safety, good immunogenicity and high titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the screening of YBAV-4 strain strain
[0022] 1. Epidemiological survey Since 2010, some broiler breeders, laying hens and Ma chickens in Shandong, Jiangsu and other regions have experienced a disease characterized by high mortality and anatomical manifestations of hepatomegaly and hydropericardium. The disease was initially diagnosed as hydropericardium hepatitis syndrome caused by group I group C-4 avian adenovirus through clinical investigation and laboratory testing. In 2010, the inventor successfully isolated a virus strain from the liver of a dead chicken with typical symptoms of inclusion body hepatitis and hydropericardium in a farm in Zibo, Shandong.
[0023] 2. Virus isolation Take the liver of sick and dead chicken and grind it, add sterilized saline at a ratio of 1:5 to make a suspension; after repeated freezing and thawing 3 times, centrifuge at 3000r / min for 30min, take the supernatant; add penicillin and Streptomycin at 10,000 IU / ml each, ...
Embodiment 2
[0032] Embodiment 2: the preparation of YBAV-4 strain virus seed
[0033] (1) Study on optimal culture conditions of chicken hepatocytes
[0034] 1. The effect of cell density on cell growth. Five different densities (10,000 to 50,000 / ml, 50,000 to 100,000 / ml, 100,000 to 150,000 / ml, 150,000 to 200,000 / ml, 20 to 25 10,000 / ml) chicken liver cells were inoculated into the same batch of 25cm 2 Cell bottle, 225cm 2 In cell flasks, 3000ml spinner bottles, and 10-layer cell factories, use the same batch of DMEM nutrient solution to culture under the same conditions, inoculate 5 bottles / 2 cells at each density, culture under the same conditions, and observe that the cells grow into a dense monolayer The time required and the shape of the cells.
[0035] 2, the influence of newborn bovine serum content on cell growth with the newborn bovine serum produced by the same manufacturer, add in the DMEM medium by the ratio of 6%, 8%, 10%, 12% respectively, cultivate the same batch of chick...
Embodiment 3
[0045] Example 3: Preparation of H9 subtype avian influenza, avian adenovirus (group I, type 4) antigen
[0046] 1. The use, maintenance conditions and methods of use of the Millipore ultrafiltration concentrator should be carried out according to the instructions provided by the manufacturer.
[0047] 2. Detection of concentration effect
[0048] 2.1 Antigen titer (HA and / EID) in the virus liquid before concentration was determined by retaining sample before concentration. 50 / TCID 50 ), during the concentration process, take samples at any time to measure the antigen titer (HA) of the concentrated solution, determine the concentration multiple, retain the sample of the concentrated solution after concentration, and measure the antigen titer (HA and / EID) in the concentrated solution 50 / TCID 50 ).
[0049] 2.2 Filtrate Antigen Detection Take the filtrate when the concentration is about to end (at this time, the concentration of the antigen in the concentrate is the highe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com