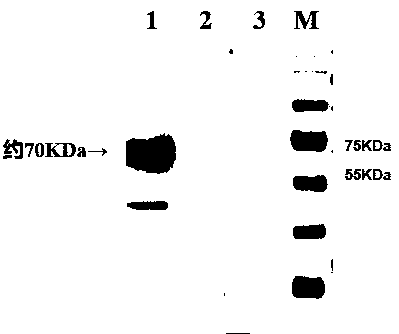
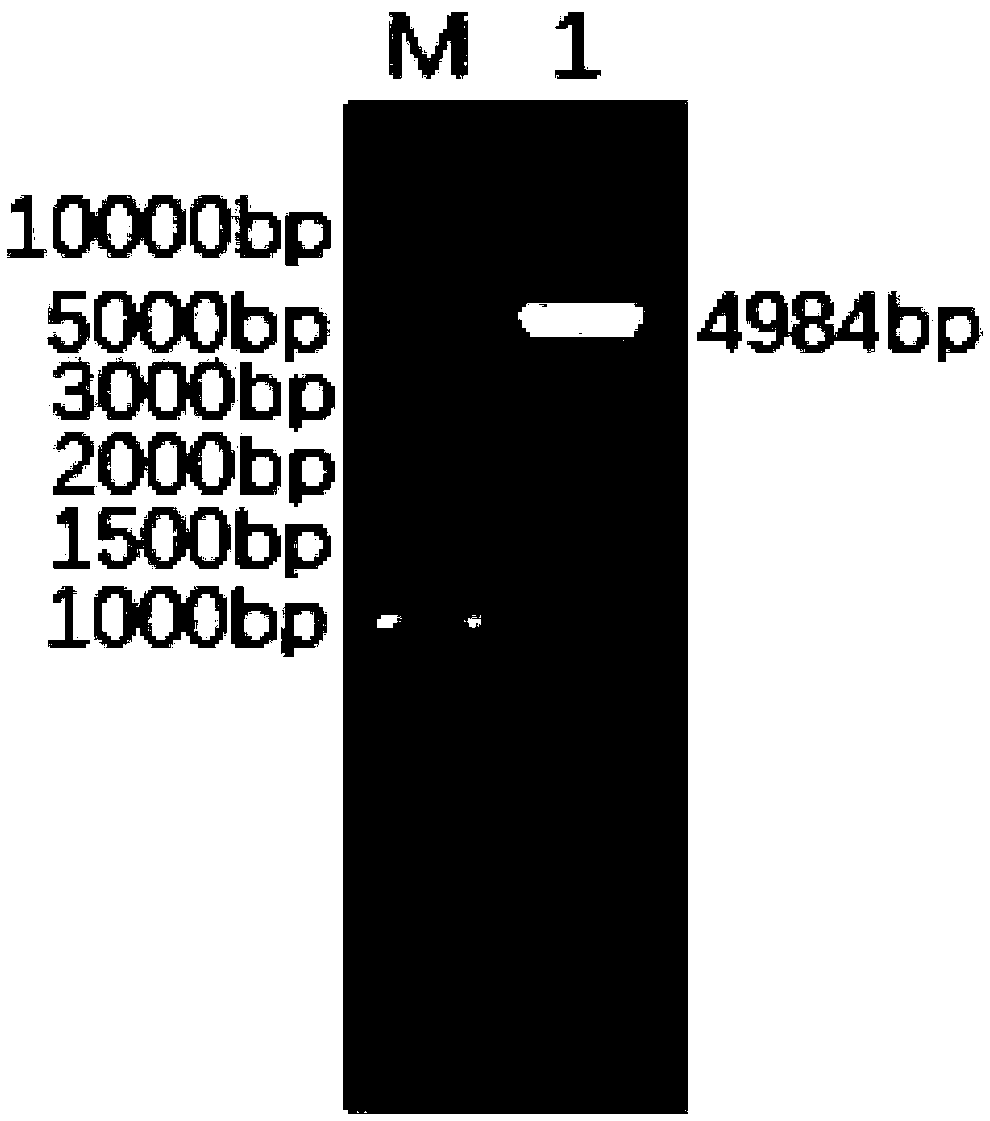
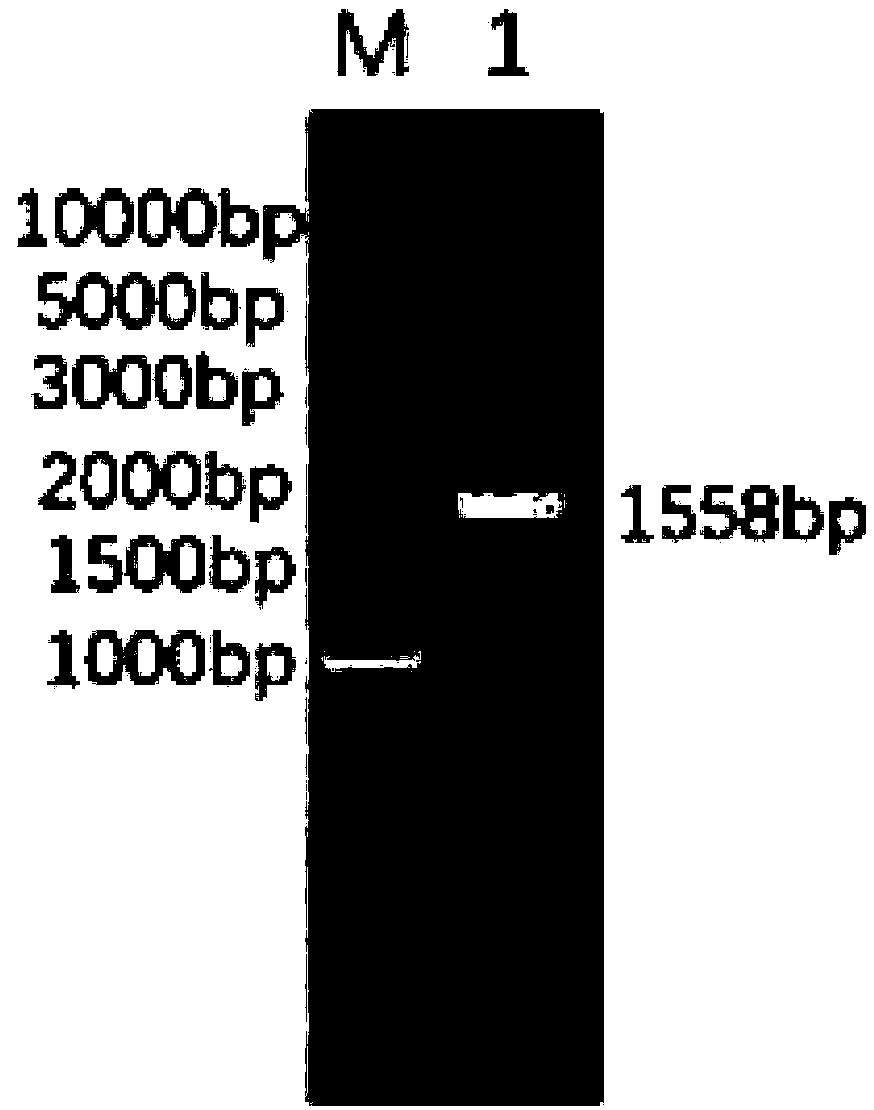
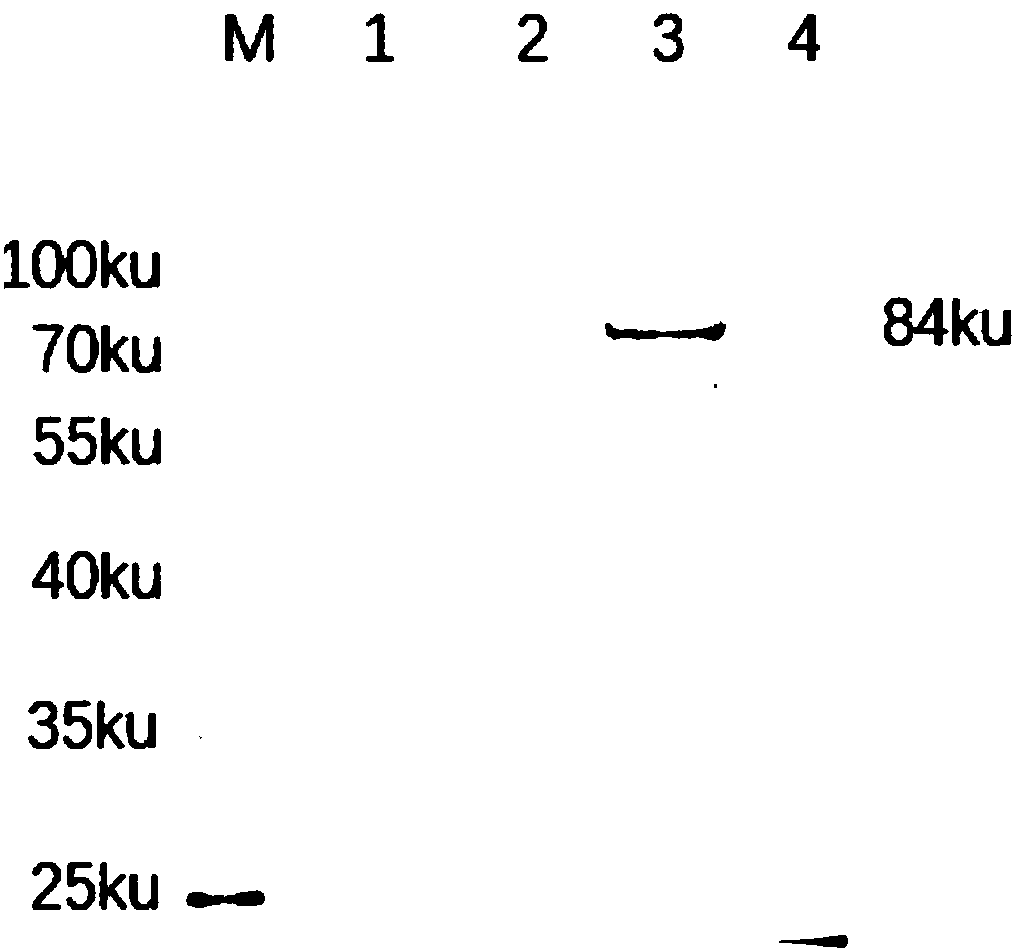
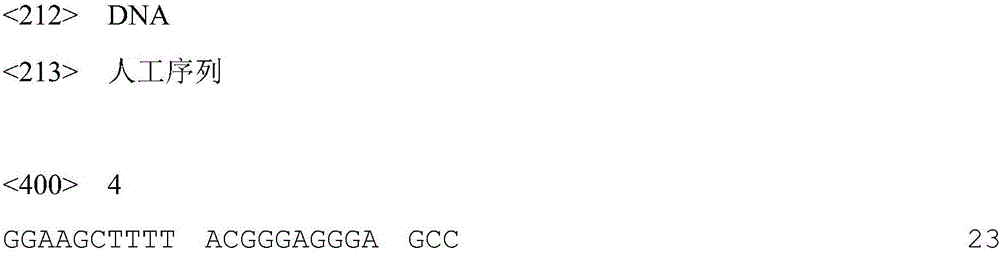Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117 results about "Avian adenovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
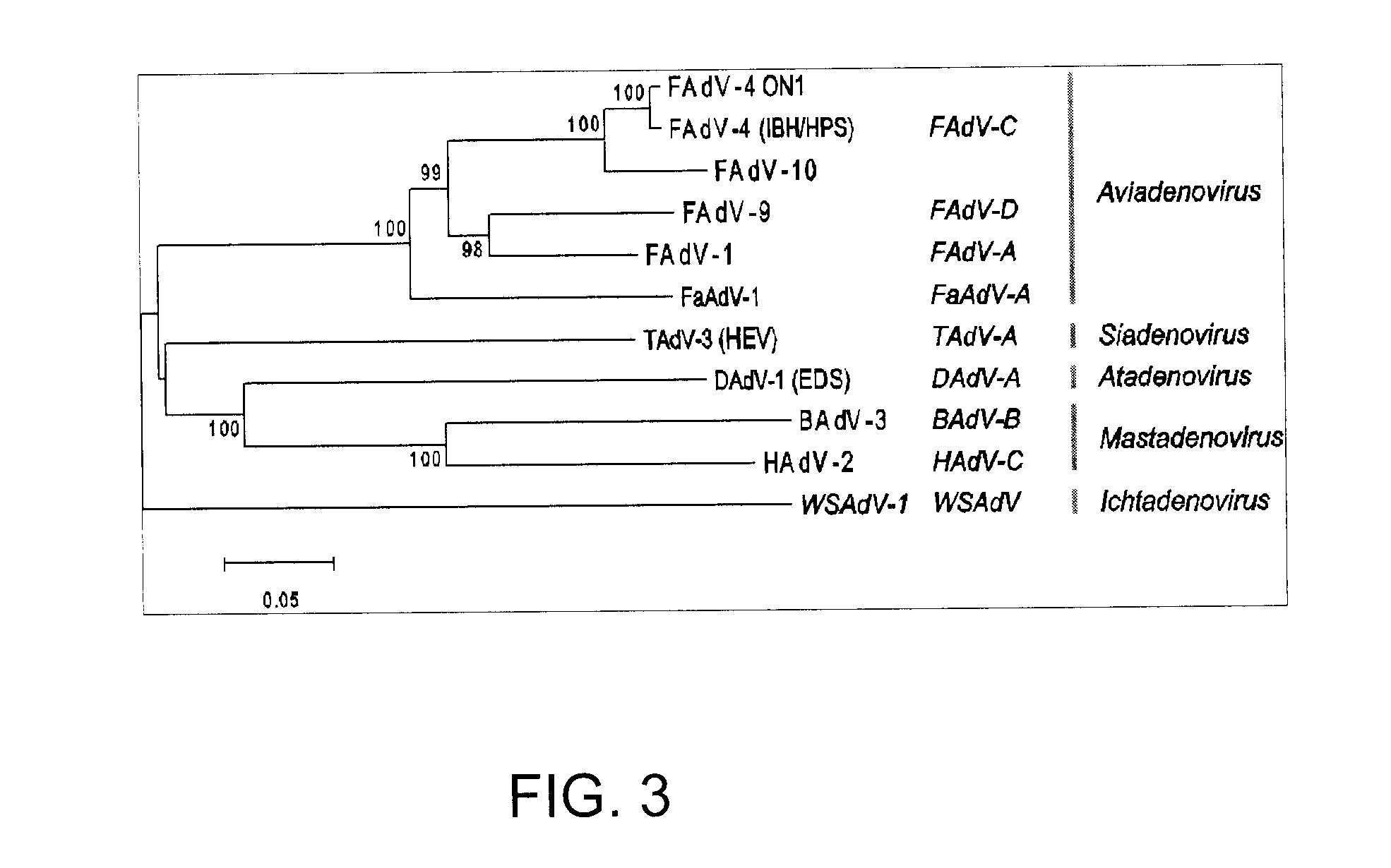
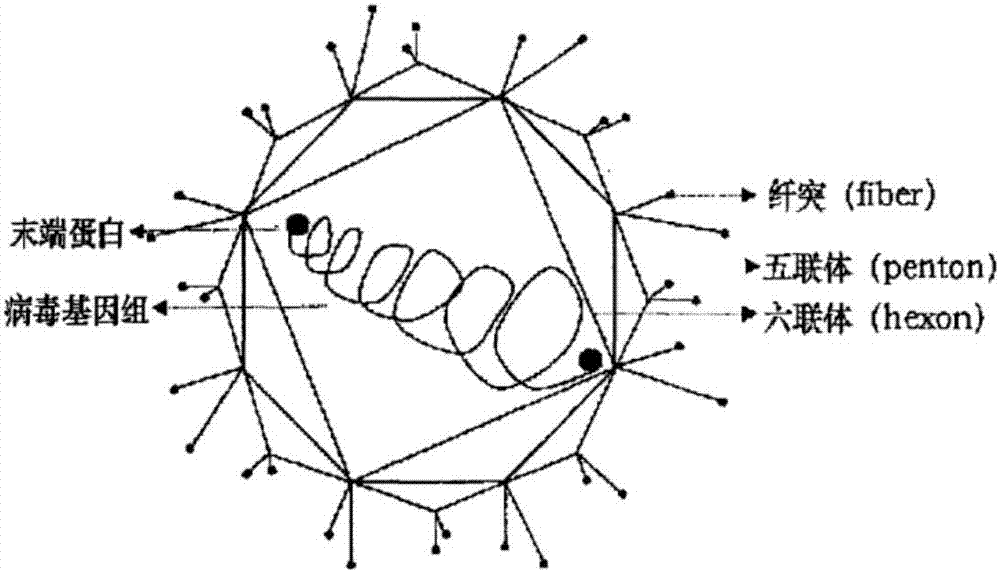
Aviadenoviruses are adenoviruses that affect birds - particularly chickens, ducks, geese, turkeys and pheasants. There are currently eight species in this genus including the type species Fowl aviadenovirus A. Viruses in this genus cause specific disease syndromes such as Quail Bronchitis (QB), Egg Drop Syndrome (EDS), Haemorrhagic Enteritis (HE), Pheasant Marble Spleen Disease (MSD), Falcon adenovirus A and Inclusion Body Hepatitis (IBH). Avian adenoviruses have a worldwide distribution and it is common to find multiple species on a single farm. The most common serogroups are serogroup 1, 2 and 3.
I-colony fowl adenovirus 4 strain and application thereof
ActiveCN105368795AImprove featuresImproving immunogenicityViral antigen ingredientsBiological material analysisInfected cellLaryngotracheitis virus
The invention aims at providing an I-colony fowl adenovirus 4 strain, which is preserved with preservation number of CCTCC No. V201541. The I-colony fowl adenovirus 4 YBAV-4 strain disclosed by the invention is excellent in specificity and immunogenicity; a specific precipitation line does not appear in specific positive serum chicken SPF chicken serum such as infected cell sap and egg drop syndrome resisting virus, chicken infectious bursal disease virus, Newcastle disease virus, chicken infectious laryngotracheitis virus, chicken Marek's disease virus, avian influenza and the like, while an obvious specific precipitation line appears in I-colony fowl adenovirus 4 specific serum. The strain disclosed by the invention, as a vaccine strain which is good in manufacturing effect, is capable of preventing chicken hydropericardium syndrome, and the strain is applicable to identification of virus serotype and investigation on epidemiology.
Owner:YEBIO BIOENG OF QINGDAO
Primer pair for preparing kit for detecting type-4 avian adenovirus and application thereof
ActiveCN105886502AHigh detection sensitivityThe result is accurateMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleotide
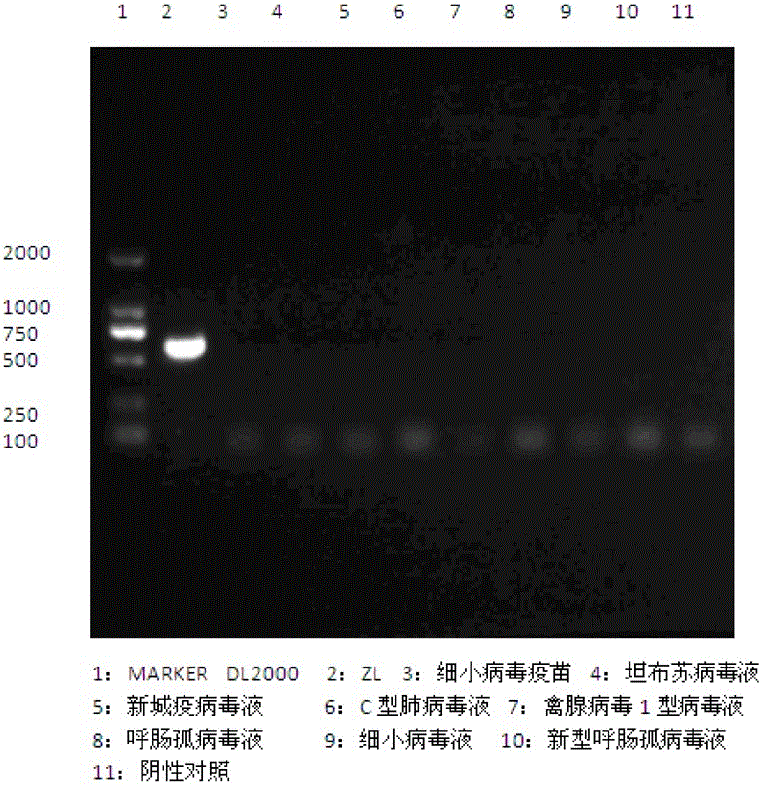
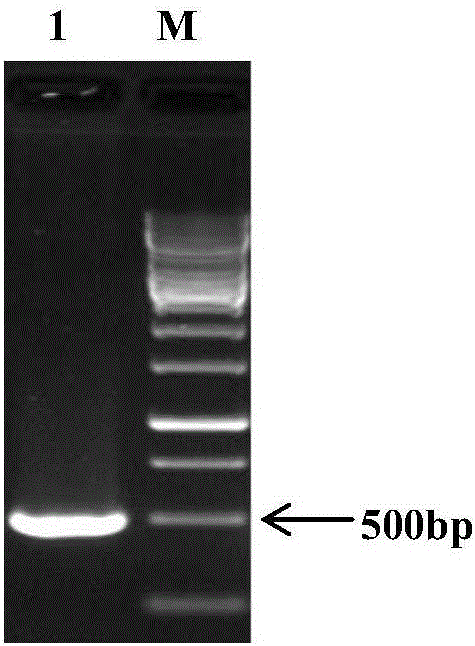
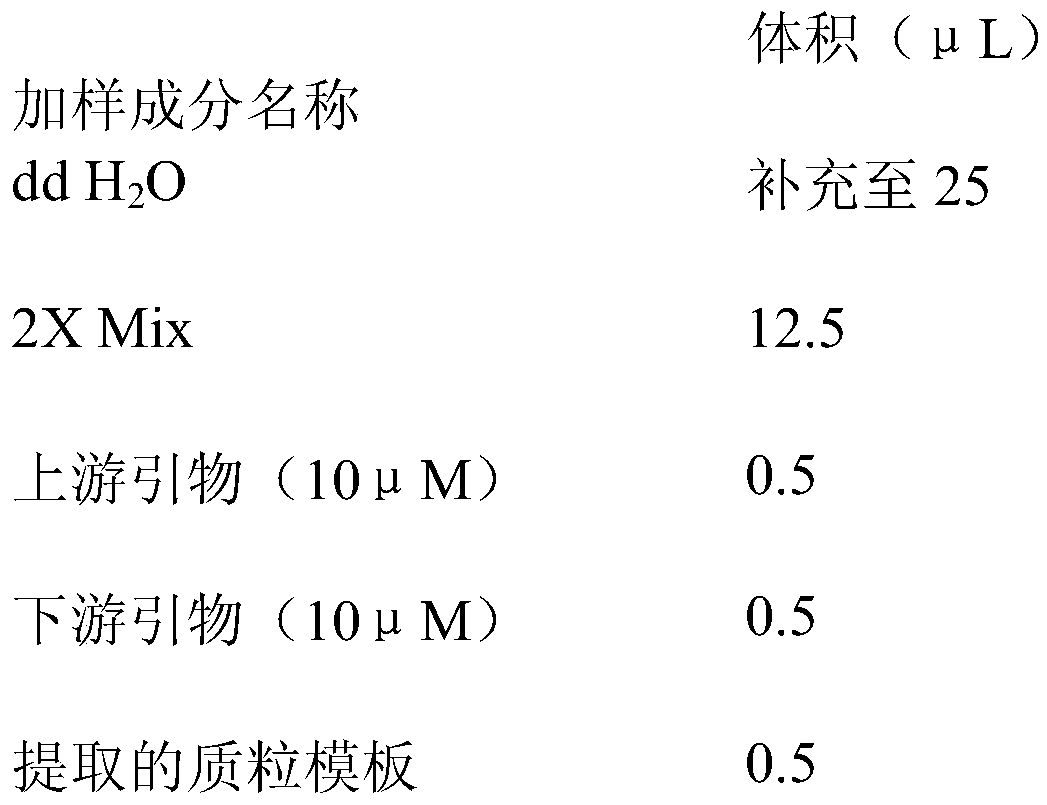
The invention discloses a primer pair for preparing a kit for detecting type-4 avian adenovirus and an application thereof. The primer pair comprises an upstream primer and a downstream primer, wherein the nucleotide sequence of the upstream primer is shown by SEQ ID No.1; and the nucleotide sequence of the downstream primer is shown by SEQ ID No.2. The invention also discloses a kit comprising the primer pair and a method for detecting the type-4 avian adenovirus. The primer pair can specifically amplify the Hexon gene segment of the type-4 avian adenovirus in a sample, the detection sensitivity is high, the lowest detection limit reaches 7.2*10<4>ng / mu L, the result is accurate, and the primer pair can be applied to the detection and identification of the type-4 avian adenovirus; the primer pair is universal in detecting different strains of the type-4 avian adenovirus; and the rapid, specific and accurate detection method of the type-4 avian adenovirus provides a detection tool for detecting and controlling the prevalence of the disease.
Owner:ZHEJIANG UNIV
Livestock adenovirus strain, vaccine composition and application thereof
ActiveCN107523556AImproving immunogenicityFully protectedViral antigen ingredientsMicroorganism based processesSerum igeAvian adenovirus
The invention provides a serum type-4 livestock adenovirus strain FAV-HN. The strain has good immunogenicity. A vaccine composition prepared from an antigen of the strain can rapidly generate antibodies after immune, and chickens can be completely protected by using a low-content vaccine composition. In addition, the antigen has combined action with multiple other antigens, and the immunity effects are not affected by the antigens.
Owner:PU LIKE BIO ENG
Avian adenovirus 4-type strain, vaccine composition and application of strain
ActiveCN107338226AImprove featuresImproving immunogenicityViral antigen ingredientsAntiviralsFowlMicroorganism
The invention discloses a newly isolated avian adenovirus 4-type HNJZ strain. The microbial preservation number of the newly isolated avian adenovirus 4-type HNJZ strain is CGMCC NO.13385, and the strain has good specificity and immunogenicity, can serve as an inactivated vaccine production strain and an inspection strain and is used for preventing hepatitis-hydropericardium syndromes of poultry. The invention further discloses a vaccine composition prepared from the avian adenovirus 4 type HNJZ strain. The vaccine composition contains the inactivated avian adenovirus 4-type HNJZ strain and pharmaceutically acceptable adjuvant, the vaccine composition has good immunogenicity, high immunity can be generated after immunization, the protection rate is high, prevalence and spread of avian adenoviruses can be effectively prevented, and application prospects are wide.
Owner:HENAN AGRICULTURAL UNIVERSITY
Primer pair and kit for detecting duck adenovirus II type
InactiveCN105039587AShort detection timeStrong specificityMicrobiological testing/measurementMicroorganism based processesTembusu virusTest sample
The invention discloses a primer pair and a kit for identifying duck adenovirus II type, wherein the nucleotide sequence of the primer pair is represented as the SEQ ID No.1-2. The primer pair is strong in specificity, can accurately and high-effectively identify whether a to-be-test sample contains the duck adenovirus II type or not, while other poultry viruses, comprising poultry adenovirus, parvovirus, Tembusu virus and Newcastle disease virus, cannot be amplified to form DNA fragments. The primer pair is high in sensitivity, can at least detect 0.2258 ng of sample DNA. The primer and the PCR detection kit are short in detection time, wherein the whole PCR process only lasts for about 2 h. The primer pair and the kit are simple and economical, are free of expensive experimental instrument and reagents, can be used for detecting whether ill ducks are infected with the virus or not timely, so that greater economic loss can be avoided by means of corresponding measures.
Owner:WENS FOOD GRP CO LTD
I-group 4-type aviadenovirus genetic engineering subunit vaccine and preparation method thereof
InactiveCN106344919AGood prospects for commercial developmentLow costViral antigen ingredientsVirus peptidesSequence analysisInclusion bodies
The invention provides an I-group 4-type aviadenovirus genetic engineering subunit vaccine and a preparation method thereof. According to the technical scheme, the preparation method comprises the following steps: cloning an encoding gene of fibrous protein C-terminal from an I-group 4-type aviadenovirus genome according to a PCR technology and performing sequence analysis; cloning the gene to an expression vector pET-32a, transforming escherichia coli, constructing engineering bacteria, and inducing the engineering bacteria by isopropyl-beta-D-thiogalactopyranoside to express the fibrous protein C-terminal; performing lysis on an engineering bacterial cell, performing centrifugal separation on an inclusion body of the engineering bacterial cell, dissolving urea and diluting for renaturation; preparing the vaccine according to the conventional preparation method of a mineral oil adjuvant inactivated vaccine. According to the I-group 4-type aviadenovirus genetic engineering subunit vaccine prepared by the method, the immune effect of the vaccine is evaluated by a serological method and an immunity challenge method, and the result indicates that the aviadenovirus inactivated vaccine prepared by the method can provide effective immunoprotection and has a good commercialized development prospect.
Owner:TIANJIN RINGPU BIO TECH
Methods and compositions for use of a coccidiosis vaccine
InactiveCN102333877ASuitable for mass vaccinationViruses/bacteriophagesAntiparasitic agentsAntigenHeterologous
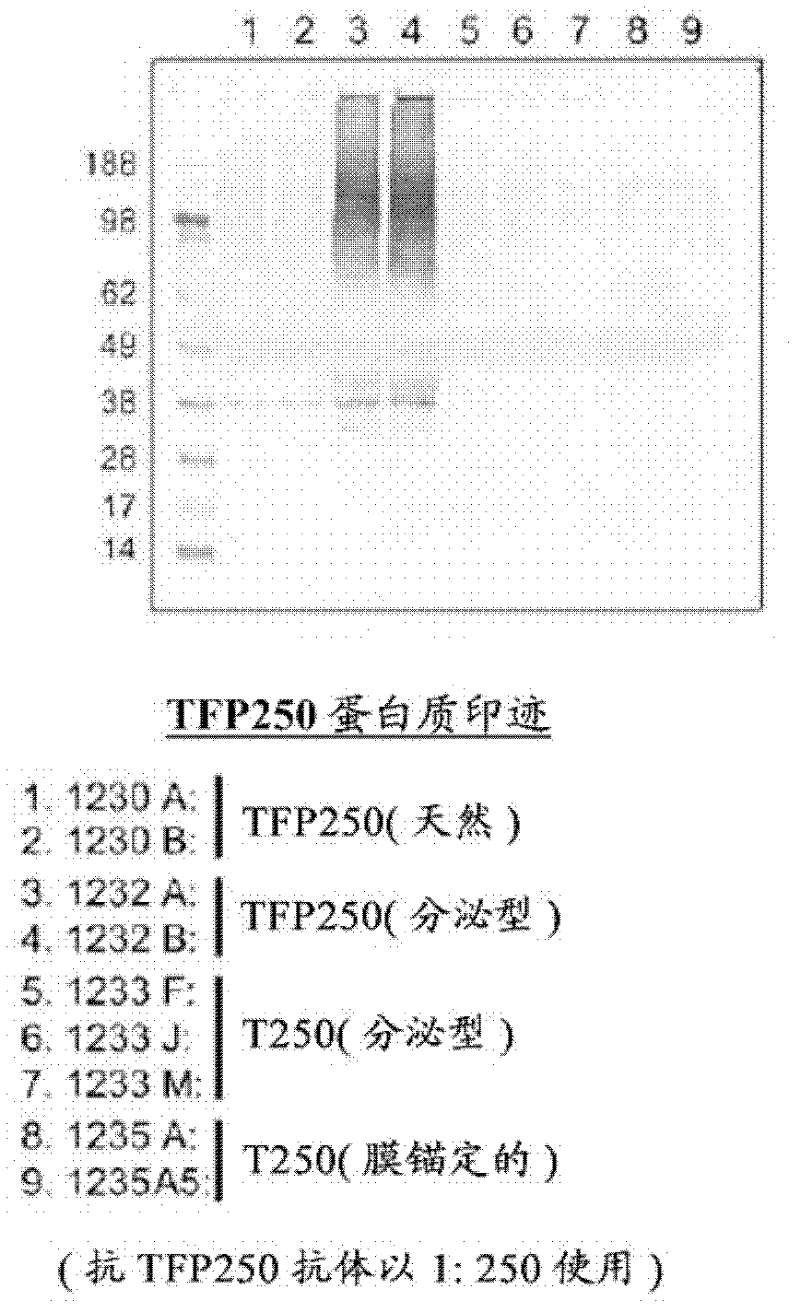
The present invention relates to a coccidiosis vaccine to protect poultry against Eimeria infection, comprising a recombinant avian adenovirus vector comprising in frame a (heterologous) promoter linked to a hydrophobic signal sequence for membrane anchoring, or linked to an hydrophobic secretion signal and a cleavage site to allow secretion; a multiple cloning site for in frame insertion of an Eimeria antigen ORF such as derived from the r56, 82 kDa, and / or TFP250 antigens; a polyadenylation signal; and an avian adenovirus genome.
Owner:VECTOGEN
Non-pathogenic serotype 4 fowl adenovirus (fadv-4) and viral vector thereof
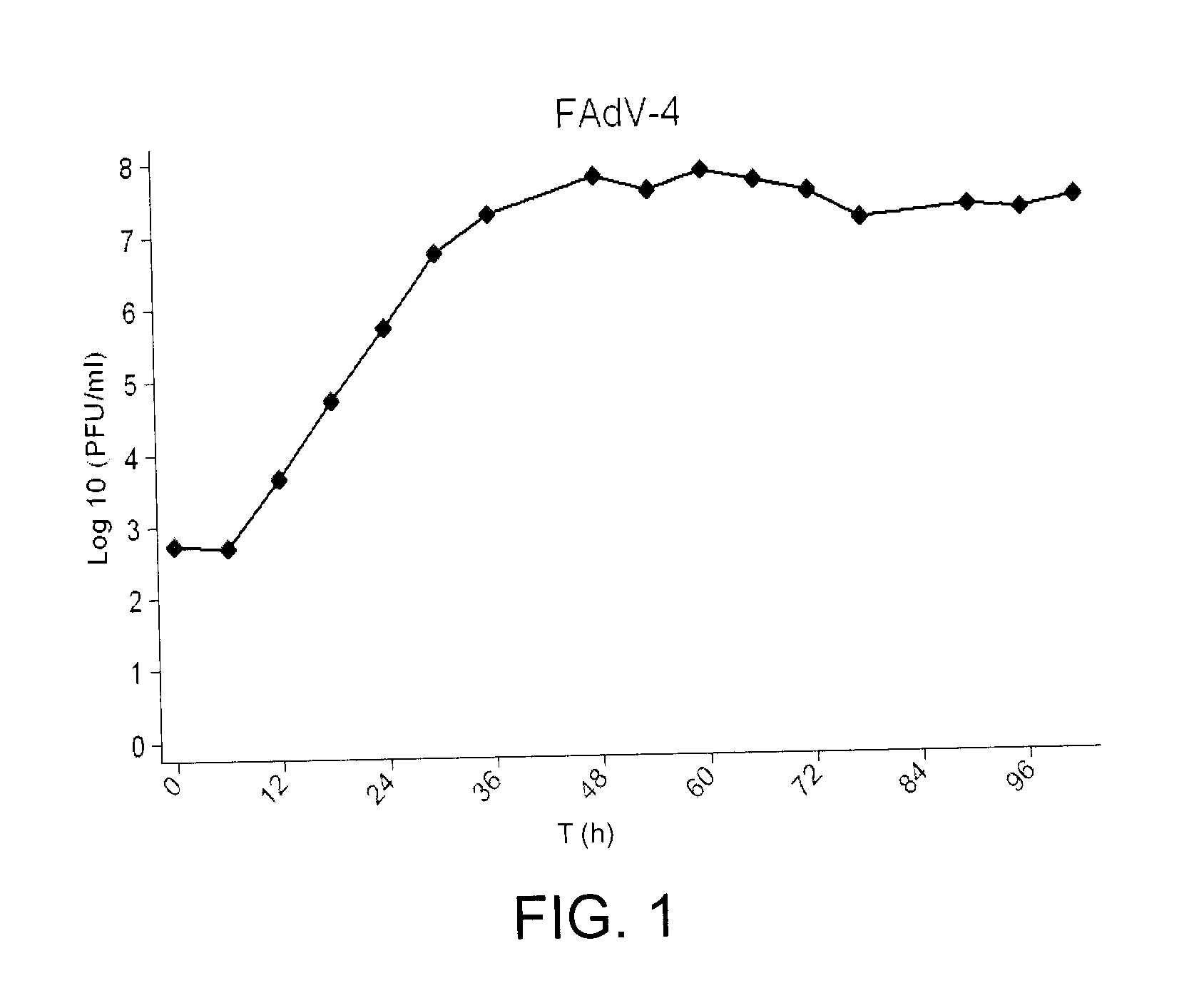
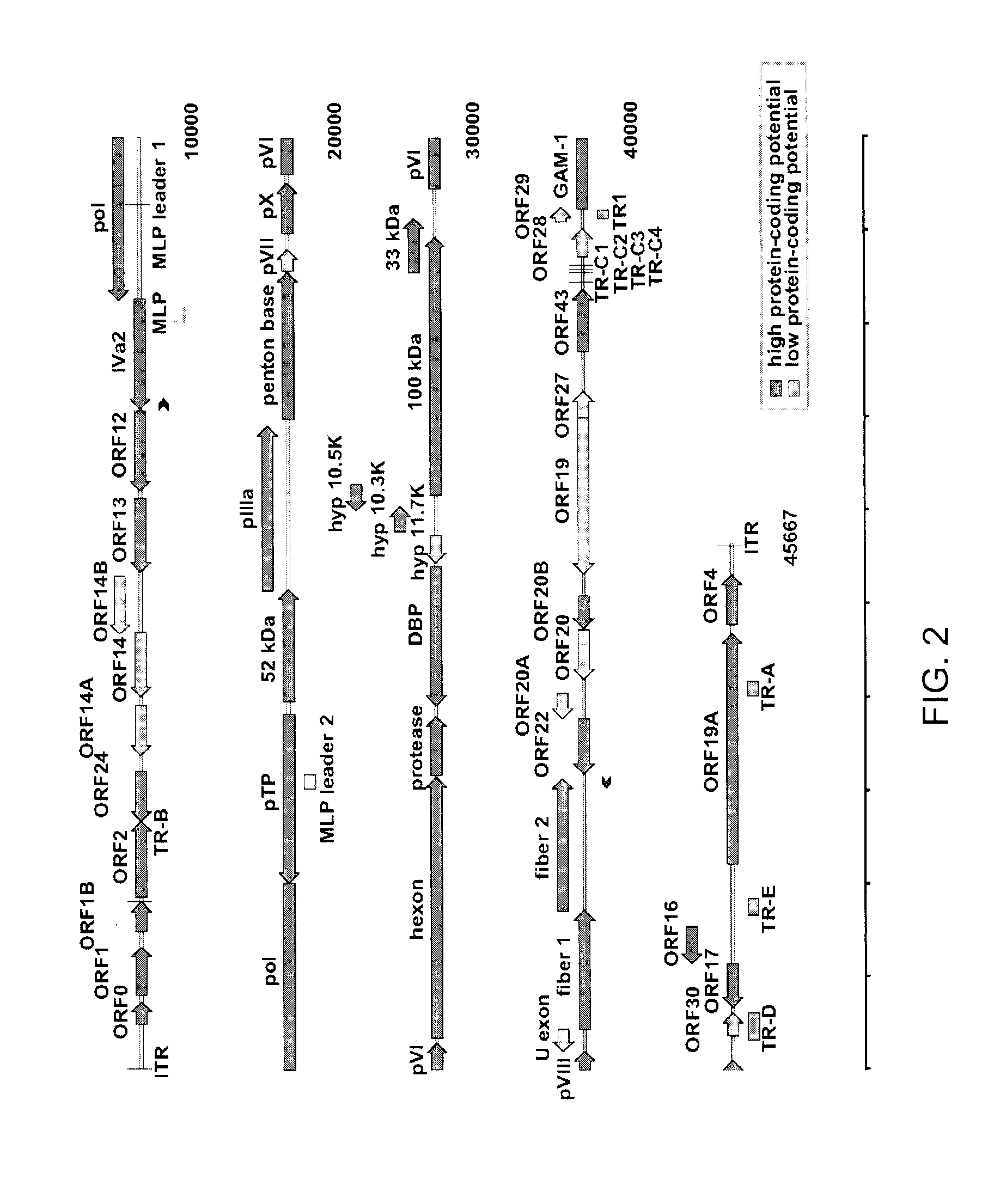
A high level replication fowl adenovirus (FAdV) isolate capable of reaching a viral titer of at least 3 log 10 is described. Said FAdV is a non-pathogenic strain of fowl adenovirus serotype 4, identified as FAdV-4 ON1. Additionally, the present disclosure also provides a viral vector comprising the fowl adenovirus, which has inserted an exogenous nucleotide sequence coding for at least one antigenic site of a disease of concern, as well as a method for obtaining said viral vector and an immunogenic composition comprising the same.
Owner:UNIVERSITY OF GUELPH
Group I type 4 aviadenovirus strain WZ and application of strain WZ
ActiveCN106754754AImprove featuresImproving immunogenicitySsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineAvian adenovirus
The invention discloses a group I type 4 aviadenovirus strain WZ. The strain has a preservation number of China Center for Type Culture Collection (CCTCC) No: V201662, is named as group I type 4 aviadenovirus strain WZ in a classified way, and is preserved in December 14, 2016; a group I type 4 viral vaccine of the aviadenovirus is prepared from the inactivated group I type 4 aviadenovirus strain which is taken as an active ingredient; the invention also provides application of the group I type 4 aviadenovirus strain WZ in preparation of an agar gel precipitating antigen, a hemagglutination inhibition (HI) antigen and positive serum antigen reagent which are used for diagnosing the group I type 4 virus of the aviadenovirus, and preparation of egg yolk antibody and antiserum which are used for treating the group I type 4 virus of the aviadenovirus. After the vaccine prepared by using the group I type 4 aviadenovirus strain is used for immunizing, the toxin attacking protection rate for the group I type 4 aviadenovirus strain WZ reaches 90-100%. As a vaccine strain with good manufacturing effect, the group I type 4 aviadenovirus strain WZ can be used for preventing avian inclusion body hepatitis and pericardial effusion syndrome, and also can be used for virus identification and epidemiological investigation.
Owner:HENAN AGRICULTURAL UNIVERSITY
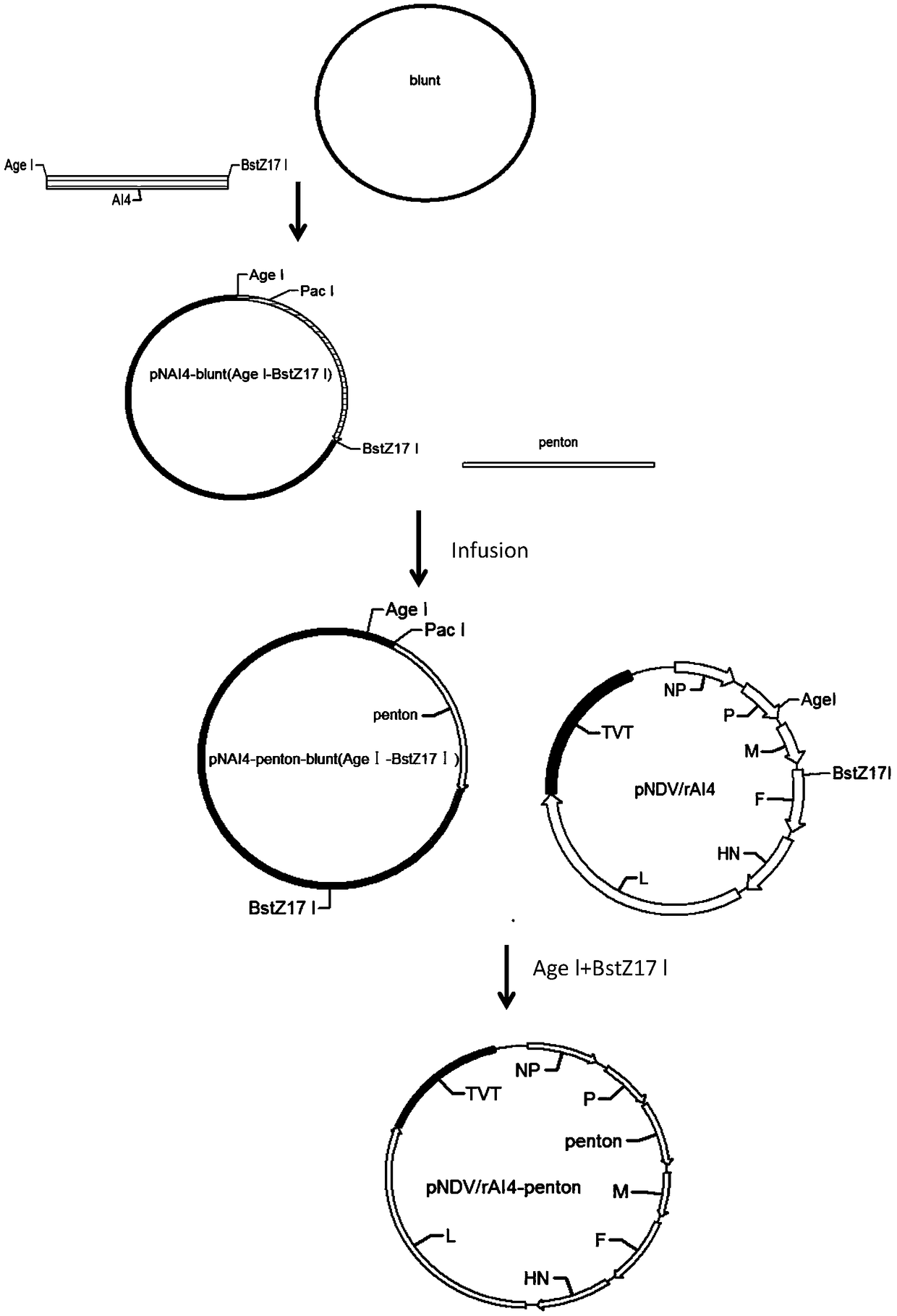
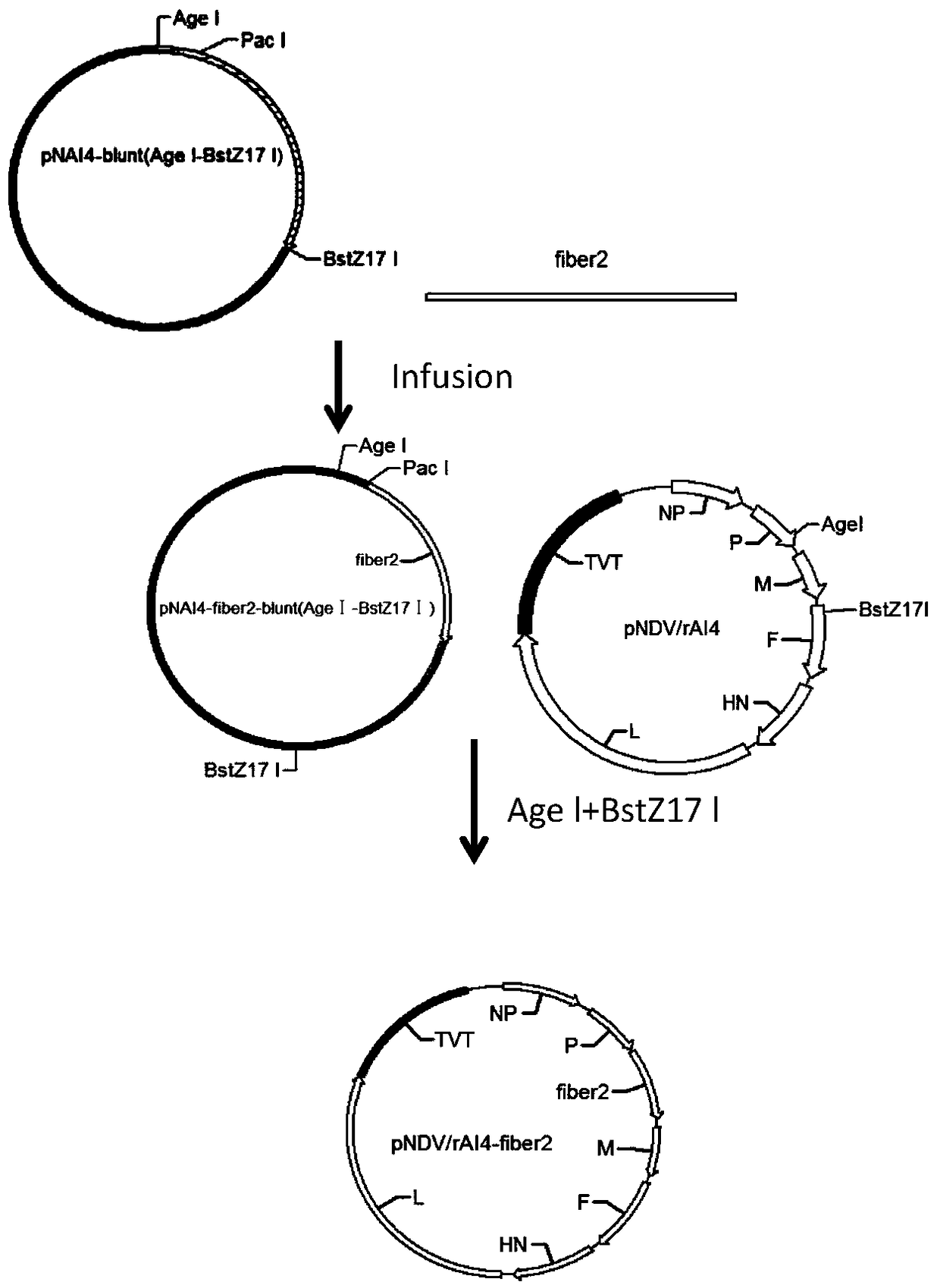
Recombinant newcastle disease vaccine candidate rAI4-penton to express avian adenovirus penton protein and construction method thereof
InactiveCN108728419ASuitable for mass productionImprove reproductive performanceFermentationDsDNA virusesEmbryoAvian adenovirus
The invention relates to recombinant newcastle disease vaccine candidate rAI4-penton to express avian adenovirus penton protein and a construction method thereof. newcastle viral strain rAI4-penton iscollected under CGMCC No: 15492. The construction method includes: using a reconstructed reverse genetics operating platform of a newcastle attenuated strain to penton gene sequence of avian adenovirus into genome full-length transcription vector pNDV / rAI4 of the strain AI4 so as to obtain recombinant newcastle viral genome full-length cDNA clone pNDV / rAI4-penton, and performing transfecting to obtain recombinant viral rAI4-penton that successfully express penton protein. The recombinant virus rAI4-penton has high breeding titer on chicken embryos, can stabilize the penton protein even aftercontinuous passage, is suitable for large-scale production of vaccines, and is suitable for making vaccines.
Owner:YANGZHOU UNIV
Extraction method of yolk antibody of aviadenovirus IV and application thereof
InactiveCN106749640AStrong specificityHigh potencyEgg immunoglobulinsImmunoglobulins against virusesAntigenYolk
The invention relates to the field of the preparation of yolk antibodies from avian eggs, and discloses an extraction method of a yolk antibody of aviadenovirus IV. The extraction method comprises the following steps of (1) using an oil-emulsion inactivated vaccine of the aviadenovirus IV as an antigen to immunize a health hen, and collecting an egg laid by the immunized hen; (2) separating yolk in the egg laid by the immunized hen to obtain water-soluble component liquor; (3) adding the water-soluble component liquor obtained by separating the yolk into an aqueous two-phase system, so as to obtain water-soluble component mixed liquor, wherein the aqueous two-phase system is prepared from 25wt% to 28wt% of polyethylene glycol and 20wt% to 25wt% of sodium citrate; (4) carrying out separation and purification on the water-soluble component mixed liquor. The invention provides the extraction method of the yolk antibody of the aviadenovirus IV, which is high in antibody purity and high in yield and does not influence activity.
Owner:QINGDAO YIYIHE AGRI & ANIMAL HUSBANDRY SCI & TECH LTD
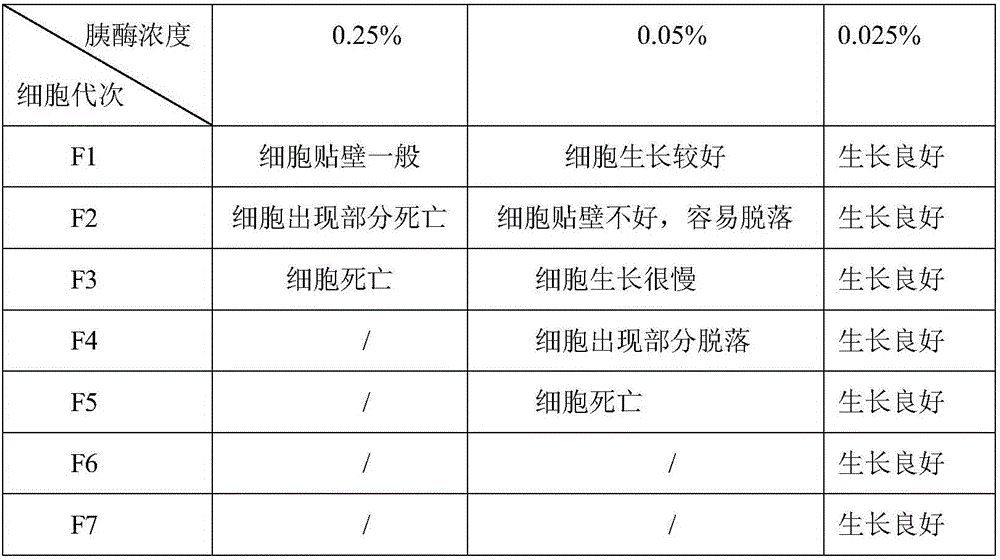
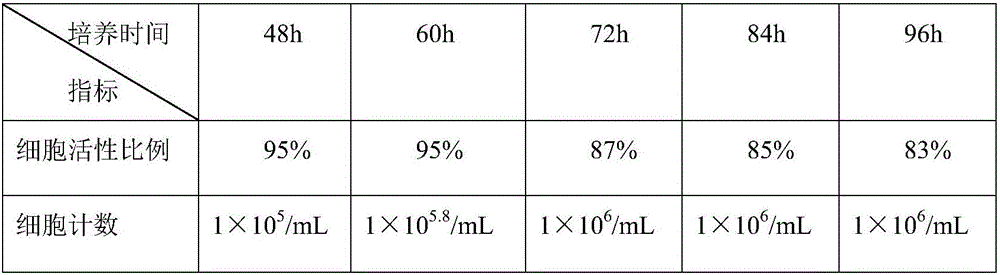
Method for producing avian adenovirus inactivated vaccine through LMH clone line
InactiveCN106798918AGood immune effectReduce manufacturing costViral antigen ingredientsInactivation/attenuationAntigenEmulsion
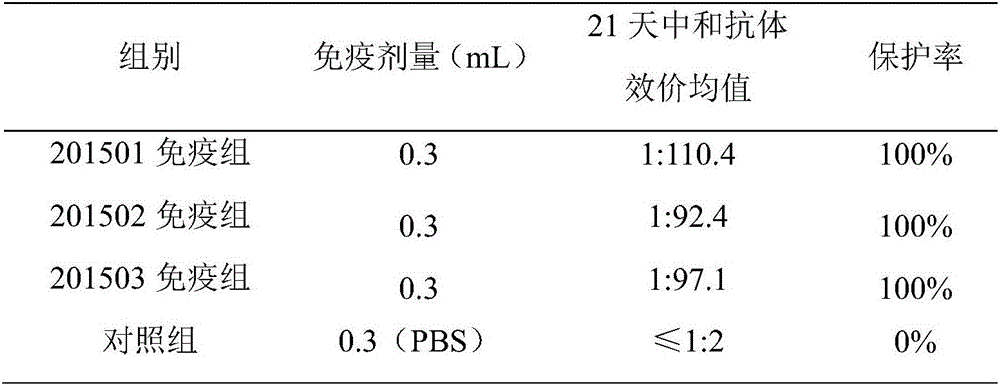
The invention relates to a method for producing avian adenovirus inactivated vaccine through an LMH clone line. The method comprises the following steps: firstly, preparation continuous cell line LMH cell; secondly, virus-seed reproduction; thirdly, virus collection, concentration and purification; fourthly, virus content determination; fifthly, inactivation and emulsification of virus, and forming emulsion inactivated vaccine. Compared with the traditional technical method for producing avian adenovirus through cell culture, the method has the advantages that high-quality inactivated vaccine with higher antigen titer through the optimization of the concentration of digestive LMH cell pancreatin-EDTA, the culture time of LMH cell and the inoculation concentration and harvest time of virus, injection immunization is performed at different doses, and all the vaccine protection rates reach 100 percent, so that the immune efficiency of the avian adenovirus type IV inactivated vaccine produced through the method is high, and the vaccine has complete immune protection effect on avian adenovirus type IV.
Owner:广州博恒生物科技有限公司
ELISA (enzyme-linked immunosorbent assay) detection method for identifying fowl adenovirus group I (FAVI) infection
The invention discloses an ELISA (enzyme-linked immunosorbent assay) detection method for identifying fowl adenovirus group I (FAVI) infection. The method is characterized by taking an FAVI 100K recombinant protein as the envelop antigen, chicken serum as the detection sample and horse radish peroxidase labeled goat anti-chicken IgG as the enzyme-labeled antibody to detect the antibody generated through FAVI infection. The method is strong in specificity, short in time and low in cost, is simple to operate, can be used for mass detection, can effectively get rid of interference of immunity of FAVI inactivated vaccines and can specifically detect FAVI infection, thus distinguishing FAVI infected animals from FAVI inactivated vaccine inoculated animals and providing a diagnostic tool with actual value for eliminating FAVI in China.
Owner:GUANGXI VETERINARY RES INST
Nano multiplex PCR method for distinguishing four kinds of serotype avian adenovirus I group
ActiveCN107841575AIncreased sensitivityStrong specificityMicrobiological testing/measurementInstabilitySerotype
The invention relates to a nano multiplex PCR method for distinguishing four kinds of serotype avian adenovirus I group. Various types of specific primers are designed and synthesized according to hexon gene sequences of 4, 8a, 8b and 11 type avian adenovirus I group disclosed by GenBank; after gene extraction, nano multiplex PCR amplification is performed, with the PCR reaction conditions of pre-degeneration for 5min at 95 DEG C and 30s at 94 DEG C, annealing for 1min at 53 DEG C and 1min49s at 72 DEG C, and 30 circulation; extension for 10min at 72 DEG C; and after nano multiplex PCR amplification reaction is ended, a PCR product is taken, and the size of an amplification fragment is observed by taking DL 2000 as a Maker and adopting 1% agarose gel electrophoresis, so as to determine theserotype of virus. According to the method disclosed by the invention, the detection time can be obviously shortened; by adding nano particles, under the condition of detecting four kinds of serotypeviruses, the sensitivity is at least increased by 10 times in comparison with common multiplex PCR; and the method disclosed by the invention is strong in specificity, high in sensitivity and good instability, and can quickly, sensitively and accurately classify serotype avian adenovirus I group which is popular mainly in China.
Owner:QINGDAO AGRI UNIV
Preparation method of newcastle disease, avian influenza virus and avian adenovirus trivalent inactivated vaccine
InactiveCN106729690AProduced fastReduce generationSsRNA viruses negative-senseViral antigen ingredientsFreeze thawingNewcastle disease virus NDV
The invention relates to a preparation method of a newcastle disease, avian influenza virus and avian adenovirus trivalent inactivated vaccine. The method comprises the following steps: (1) inoculating an avian adenovirus type 4 virus into an LMH cell and obtaining a vaccine-made virus liquid through the steps of culture, freeze thawing, centrifugation and concentration; (2) inoculating a newcastle disease virus La Sota strain and an H9 subtype avian influenza virus into a chick embryo allantoic cavity separately, culturing to obtain a chick embryo allantoic liquid and carrying out centrifugation and concentration to obtain a newcastle disease and H9 subtype avian influenza virus vaccine-made virus liquid; and (3) inactivating virus liquids of the three viruses, and carrying out isopyknic mixing and emulsification to obtain an emulsion-type inactivated vaccine. The trivalent inactivated vaccine provided by the invention has a complete immune protecting effect on the newcastle disease virus, the H9 subtype avian influenza virus and the avian adenovirus type 4 virus, and is good in security, stable, effective and long in protection period.
Owner:广州博恒生物科技有限公司
Serum type 4 fowl adenovirus inactivated vaccine and preparation method thereof
The invention belongs to the technical field of vaccine preparation, and in particular, relates to a serum type 4 fowl adenovirus inactivated vaccine and a preparation method thereof. The serum type 4 fowl adenovirus inactivated vaccine has an antigen which is an inactivated serum type 4 fowl adenovirus having the titer not less than 109 TCID50 / ml and obtained by a suspension culture method. With use of chicken liver cells, the serum type 4 fowl adenovirus is efficiently amplified by a riptide-type bioreactor suspension culture technology, and the high-titer serum type 4 fowl adenovirus inactivated vaccine is developed. The titer of the vaccine is 109 TCID50 / ml and is 100 times that of a traditional chick embryo vaccine. The preparation of the high-titer serum type 4 fowl adenovirus inactivated vaccine can greatly reduce the cost of the vaccine, provides the immune effect of the vaccine, and has a good prospect of application.
Owner:YANGZHOU UNIV +2
Avian adenovirus fiber protein subunit vaccine
InactiveCN110128508AHigh antigen stabilityHigh purityViral antigen ingredientsVirus peptidesEscherichia coliNucleotide
The invention provides an avian adenovirus fiber protein subunit vaccine. A new-type antigen fiber protein of an avian adenovirus is used, after a sequence is optimized, the fiber protein acquires soluble expression in escherichia coli, and an expression product is used for preparing the subunit vaccine, wherein an amino acid sequence of the avian adenovirus fiber antigen protein is SEQ ID NO: 4,and a nucleotide sequence of an encoding gene thereof is SEQ ID NO: 3. The subunit vaccine prepared by the avian adenovirus fiber protein has the characteristics of high antigen stability, high purity, strong specificity, no generation of other uncorrelated antibodies, and convenient and accurate detection method. A firm foundation is established for industrially producing the avian adenovirus subunit vaccine and diagnostic reagent.
Owner:YEBIO BIOENG OF QINGDAO
Fowl adenovirus strain, inactivated vaccine and preparation method
ActiveCN106119212AImprove adaptabilityIncrease culture titerViral antigen ingredientsAntiviralsVirus-like particleSerotype
The invention provides a fowl adenovirus strain, an inactivated vaccine and a preparation method. The fowl adenovirus strain is named a fowl adenovirus TJ strain with a preservation number of CCTCC (China Center For Type Culture Collection) NO:V201632; the adenovirus strain is of race C in a fowl adenovirus I subgroup, the serotype is 4 type, viral nucleic acid is double stranded DNA (Deoxyribonucleic Acid), and no envelope is found; the diameter of virus particles is 70 to 90nm, and the virus particles are of icosahedral symmetrical structures; the inactivated vaccine is prepared by performing cell inoculation, venom harvesting, inactivating and emulsifying on a fowl adenovirus; a vaccine strain is a prevalent strain, and is good in immunogenicity and high in multiplication capacity; the prepared vaccine has the advantages of high safety, good stability, quick production of immune response, high virus challenge protecting rate, and the like.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Group I type 4 avian adenovirus subunit protein as well as preparation method and application thereof
The invention belongs to the technical field of biology, and discloses group I type 4 avian adenovirus subunit protein with an immunoprotection function. The subunit protein is group I type 4 avian adenovirus short fiber mutation protein spherical region truncated protein; the antigen from amino acid 283 site to amino acid 479 site determines the cluster amino acid sequence; better antigenicity and immunogenicity are realized. The group I type 4 avian adenovirus subunit protein and pharmaceutically acceptable adjuvant is prepared into vaccine; a preparation method of the vaccine mainly comprises the steps that firstly, the group I type 4 avian adenovirus short fiber mutation protein spherical region truncated protein nucleotide sequence subjected to codon optimization is cloned into the carrier; then, a recombinant vector is subjected to conversion, inducible expression and purification to obtain antigen protein; finally, the antigen protein and the adjuvant are emulsified to obtain the vaccine. The vaccine is safe and effective; the method for preparing the subunit vaccine is simple; the cost is low; the amplification is easy.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Group I avian adenovirus type 4 strain and application thereof
ActiveCN109207436AHigh toxin productionImproving immunogenicityViral antigen ingredientsDigestive systemDiseaseNewcastle disease virus NDV
The invention relates to the technical field of veterinary biological products, in particular to a group I avian adenovirus type 4 strain and application thereof. The group I avian adenovirus type 4 strain ZMXZAV-4 strain, with an accession number of CGMCC No. 14296, has the characteristics of strong cellular adaptability, high toxin production and strong immunogenicity. Newcastle disease virus (NDV), infectious bursal disease virus (IBDV) and avian adenovirus (AAV) are triple inactivated vaccines based on the virus strain, wherein the NDV is La Sota strain, the infectious bursal disease virus(IBDV) strain is LY23 with an accession number of CGMCC No. 11594; the Avian adenovirus is group I avian adenovirus type 4 ZMXZAV-4 strain, with an accession number of CGMCC No. 14296. The vaccine issafe and effective, and can be used for preventing Newcastle disease, infectious bursal disease and avian adenoviral disease, achieving the effect of preventing three diseases by one injection, and reducing immune cost and immune stress reaction.
Owner:乾元浩生物股份有限公司
Preparation method of avian influenza virus and fowl adenovirus combined inactivated vaccine
ActiveCN107050448APromote proliferationImprove vaccine effectivenessSsRNA viruses negative-senseViral antigen ingredientsBiotinAvian adenovirus
The invention belongs to the technical field of veterinary biological products and particularly relates to a preparation method of avian influenza virus subtype H9 and fowl adenovirus 4 combined inactivated vaccine. LHM (Leghorn male hepatocarcinoma) continuous cell line is used as carrier cells to perform viral multiplication, culture medium A composed of glutamine, recombinant human insulin, human serum albumin, transferrin, biotin and growth factors is used to perform culturing, and a viral liquid is collected and inactivated to prepare the vaccine. The multiplication of avian influenza virus subtype H9 and fowl adenovirus 4 by the LHM continuous cell line has no need for additional pancreatin, the background of LHM cells is clear, no extraneous pathogens occur, the multiplication is easy, the process can be effectively simplified, and the cost can be reduced; in addition, the avian influenza virus and fowl adenovirus combined inactivated vaccine has high viral content, good stability and high safety and is an ideal avian influenza virus and fowl adenovirus combined inactivated vaccine.
Owner:广东渔跃生物技术有限公司 +2
Newcastle disease virus, avian influenza virus and avian adenovirus triple inactivated vaccine
ActiveCN105664150AImprove securityHigh potencySsRNA viruses negative-senseViral antigen ingredientsDiseaseWhole body
The invention provides a newcastle disease virus, avian influenza virus and avian adenovirus triple inactivated vaccine. TCID50 of I-group 4-type avian adenovirus YBAV-4 new strains is high in tilter, good in immunogenicity and capable of resisting attack of isolated viruses of H9 sub-type and avian adenovirus diseases in various places. The prepared vaccine is good in safety, and no local or whole-body adverse effects caused by the vaccine occur. By means of analysis of character, safety test and potency test data in a preservation state test, compared with results of single vaccines of similar products, difference of the triple vaccine is not remarkable, and the triple vaccine is stable and effective; a potency test result proves that the triple vaccine and three kinds of single-vaccine antibodies are all kept at a high level, antibody generation is faster compared with similar products, and the antibody of a control group is negative.
Owner:YEBIO BIOENG OF QINGDAO
Egg yolk antibody of inclusion body hepatitis and preparation method thereof
ActiveCN106065030AWell developedEgg immunoglobulinsImmunoglobulins against virusesYolkInclusion bodies
The invention discloses an egg yolk antibody of inclusion body hepatitis (IBH) and a preparation method thereof. The egg yolk antibody comprises an IBH virus strain. The virus is avian adenovirus group-I serum 4-type virus and is called SDJ15-8, and is preserved at China Center for Type Culture Collection, Wuhan University, and is assigned the accession number CCTCC No.V201636. The preparation method comprises the steps of: 1) preparing an inactivated vaccine from the IBH virus strain SDJ15-8; 2) performing injection immunization to laying hens with the inactivated vaccine to produce high-immunized eggs; and 3) collecting yolks from the high-immunized eggs, and performing extraction and inactivation in an acidified water-octanoic acid manner to prepare the IBH egg yolk antibody. The egg yolk antibody can effectively prevent and treat the IBH and is more than 82% in curative rate.
Owner:CHONGQING SANJIE ZHONGXIN BIOLOGICAL ENG CO LTD
Subunits of the adenovirus fiber protein and uses thereof as vaccines
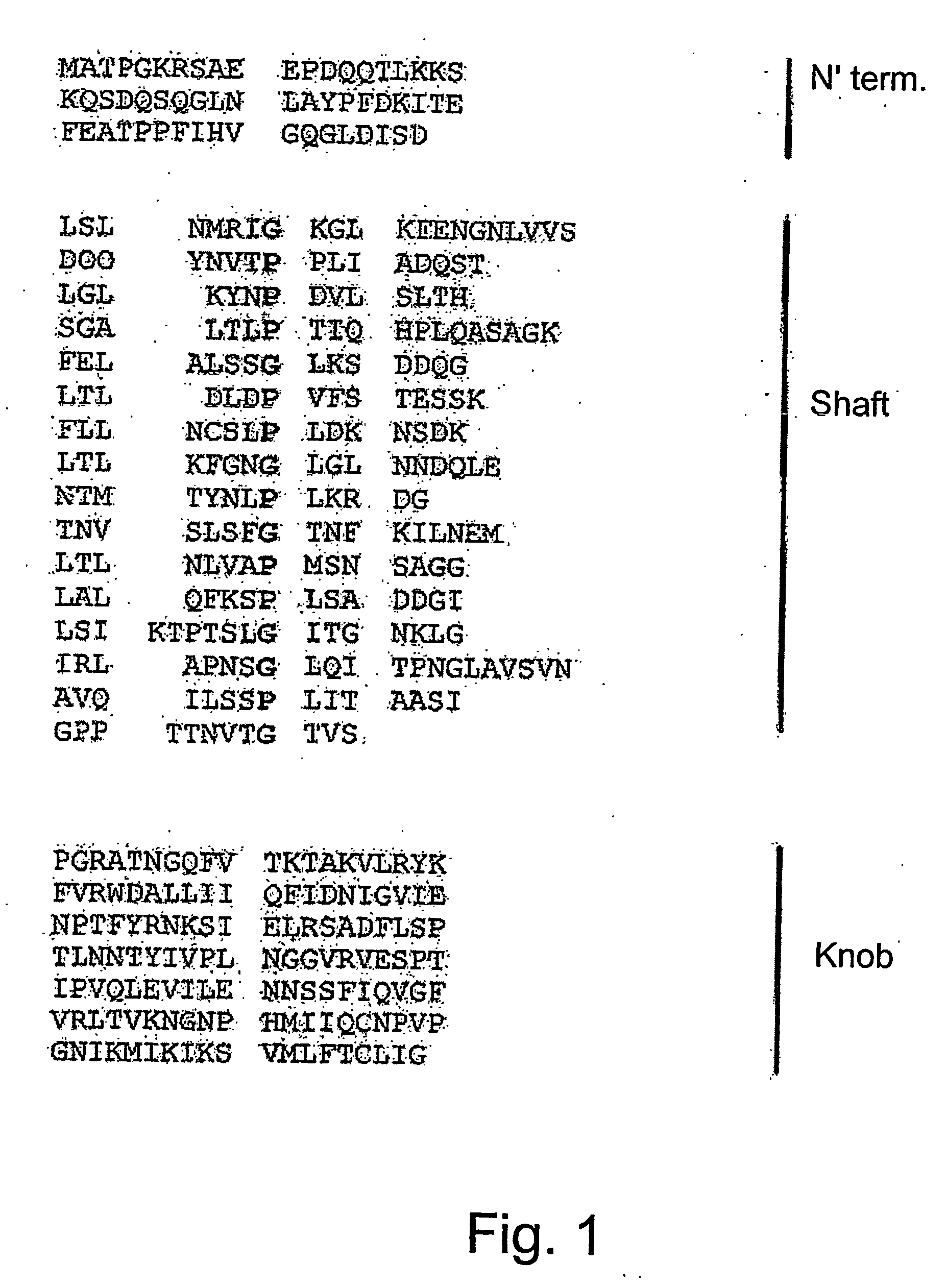
The present invention relates to a nucleic acid sequence encoding a fragment of the adenovirus fiber capsid protein, particularly, of the avian adenoviruses HEV or EDS. This sequence comprises the C-terminal knob and part of the shaft domain of the fiber protein of these adenoviruses. The invention further provides for DNA constructs comprising said DNA fragment and recombinant proteins encoded thereby. The recombinant proteins of the invention elicit in an animal, preferably in a domestic bird, protective immunity against a specific viral pathogen. The invention further relates to the use of these recombinant proteins as an active ingredient in vaccinating compositions for conferring to an animal immunity against a pathogenic infection by an adenovirus, and to methods for vaccinating a domestic bird against a pathogenic adenoviral infection.
Owner:ABIC BIOLOGICAL LAB
Group I serum type 4-serum type 8 avian adenovirus bivalent subunit vaccine and preparation method thereof
ActiveCN109721642AIncreased host expressionHigh purityAntiviralsDepsipeptidesSerum igeAvian adenovirus
The invention discloses a group I serum type 4-serum type 8 avian adenovirus bivalent subunit vaccine and a preparation method thereof. The vaccine comprises type-4 avian adenovirus fibrin with an amino acid sequence as shown in SEQ ID NO.1 and type-8 avian adenovirus fibrin with an amino acid sequence as shown in SEQ ID NO.2. The method adopts the full-length fibrin of avian adenovirus serum type4 and serum type 8 as an immunogen to prepare the bivalent subunit vaccine, and after optimization by a specific codon optimization mode, the expression quantity and the soluble expression quantity of serum type 4-serum type 8 fibrinare obviously improved. The bivalent subunit vaccine provided by the invention has higher safety, can quickly induce immune animals to generate high-level neutralizing antibodies, and realizes high-efficiency immune protection of avian adenovirus serum type 4 and serum type 8. Besides, the vaccine is simple in preparation process, low in cost and easy for large-scale production.
Owner:乾元浩生物股份有限公司
Avian adenovirus, quadruple vaccine and preparation method thereof
ActiveCN109097340AImproving immunogenicityExtended shelf lifeSsRNA viruses negative-senseViral antigen ingredientsDiseaseMicroorganism
The invention provides a novel avian adenovirus, a quadruple vaccine and a preparation method thereof. The novel avian adenovirus is avian adenovirus group I serum type 4 DC strain, which was preserved in General Microbiology Center of China Microbe Preservation Management Committee on April 25, 2018 and was assigned the accession number CGMCC No. 15589; the quadruple vaccine is a quadruple vaccine against Newcastle disease, avian influenza, infectious bursal disease and group I type 4 aviadenovirus DC strain. The quadruple vaccine has good immunogenicity, the antibody production is quick after immunity, the generated antibody titer is high, the maintenance time is long, the immunizing dose is small, a selected adjuvant is easy to inject, and four diseases can be prevented and controlled with one needle. The vaccine has the advantages of being efficient and good in safety.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
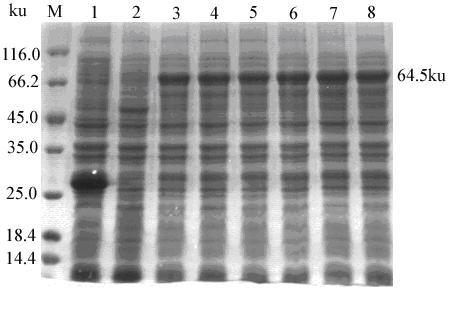
Construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2
InactiveCN111534547AImprove expression levelOptimize purification stepsFermentationDsDNA virusesCytopathic effectShuttle vector
The invention relates to a construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2. The construction method comprises the following steps: (1) constructing an FAdV4F2 gene recombinant baculovirus expression vector; (2) packaging and passing baculovirus for expressing recombinant FAdV4 protein; and (3) transfecting baculovirus genome carrying FAdV4F2 gene into Sf9 cells, culturing in an incubator at 27 DEG C for 57 days, and observing cytopathic effect; when cytopathic effect reaches 70%, collecting cell lysis buffer, centrifuging to removeprecipitate, and preserving as seed virus; passing virus, and carrying out enlarged culture as seed virus when passing to the third generation. According to the invention, a baculovirus expression system is utilized to construct a recombinant baculovirus shuttle vector containing F2 gene, the recombinant baculovirus shuttle vector is transfected into Sf9 cells to obtain recombinant baculovirus rBAF2, and expression of recombinant protein F2 is identified by virtue of indirect immunofluorescence assay (IFA) and Western blot.
Owner:YANGZHOU UNIV
Avian adenovirus transfer carrier and preparation method thereof
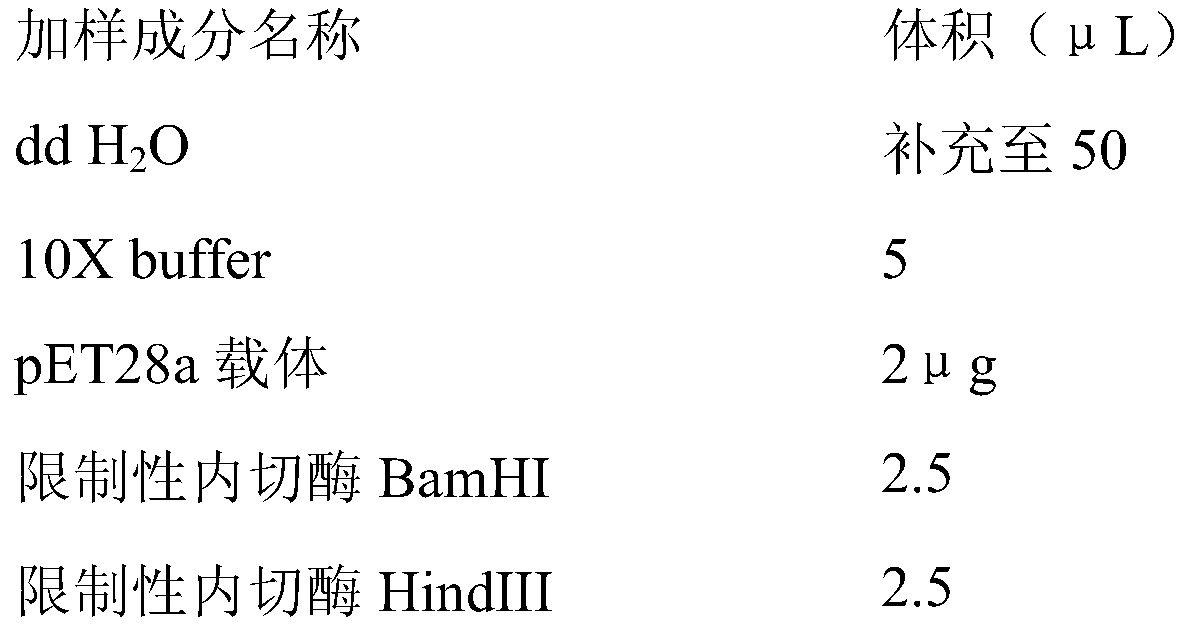
The invention discloses an avian adenovirus transfer carrier. PUC18 is regarded as a skeleton plasmid; left and right flanking sequences of an FAVI-AV208 strain genome unnecessary region are inserted in the PUC18 carrier for homologous recombination, but a replicative unessential region of 2980 bp between the left and right flanking sequences is deficient; and a gene expression box including polyA signals of hCMV, MCS, eGFP and SV40 early transcription is inserted between the left and right flanking sequences. The invention also provides a preparation method of the avian adenovirus transfer carrier; and a recombinant virus is also prepared successfully. By introducing a complete foreign gene expression box for one step and adding suitable digestion positions, a carrier construction method is improved, workload is extremely reduced, the replicative unessential region is deleted to a relatively great extent and greater fragmental foreign genes can be contained.
Owner:SHANGHAI RES CENT FOR MODEL ORGANISMS +1
Indirect ELISA (Enzyme-linked Immuno Sorbent Assay) kit for detecting blood serum 8 type avian adenovirus antibody based on spike glycoprotein F
ActiveCN108107208AStrong specificityMaterial analysis by observing effect on chemical indicatorBiological material analysisReticuloendothelial cell hyperplasiaElisa kit
The invention discloses an indirect ELISA (Enzyme-linked Immuno Sorbent Assay) kit for detecting a blood serum 8 type avian adenovirus antibody based on spike glycoprotein F. The indirect ELISA kit comprises an ELISA enzyme-linked plate which is coated with expression protein of blood serum 8 type avian adenovirus spike glycoprotein gene F, positive and negative controls, an HRP (Horseradish Peroxidase) labeled rabbit anti-chicken secondary antibody, a diluting solution, a color developing solution, a washing solution and a stopping solution. The ELISA kit disclosed by the invention can be used for specifically detecting a FAdV-8 antibody and does not react with anti-influenza virus blood serum, anti-Marek's virus blood serum, anti-Newcastle disease virus blood serum, anti-chicken infectious anemia virus blood serum, reticuloendotheliosis virus, ant-blood serum 4 type avian adenovirus blood serum, anti-egg drop syndrome virus blood serum and SFP chicken blood serum. Therefore, the kitdisclosed by the invention has good FAdV-8 specificity and can be used for FAdV-8 infection and immunization status epidemiological surveys.
Owner:YANGZHOU UNIV
Vaccine in the form of a recombinant sero type 9 avian adenovirus vector
ActiveUS20170232096A1Adequate responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenMaternal antibody
A recombinant vaccine comprising a serotype 9 fowl adenovirus vector (FAdV-9) having at least one exogenous nucleotide sequence inserted encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region, and a pharmaceutically acceptable vehicle, adjuvant and / or excipient, wherein the at least one exogenous nucleotide sequence encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region is located between the 491 and 2782 nucleotides.The vector of this vaccine is stable for industrial scale production. Likewise, even when administering this vaccine in combination with a vaccine against Marek's disease, both vaccines produce an adequate immune response which is not affected by interference between each other. In the same way, effectiveness of the recombinant vaccine is not affected by maternal antibodies, and is capable of inducing both an early and lasting protective response, even with only one application.
Owner:GRUPO IND PECUARIO S A DE CV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com