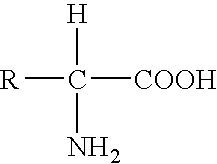
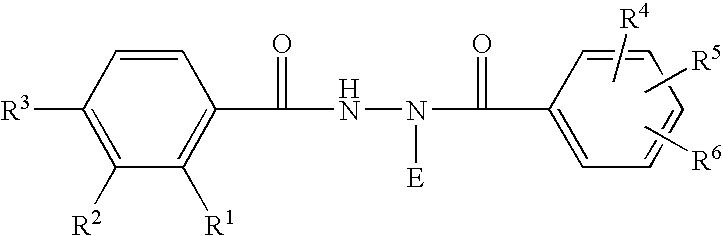
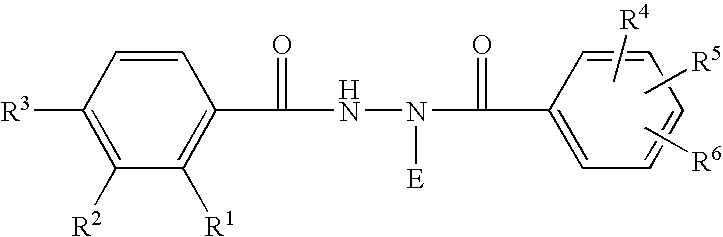
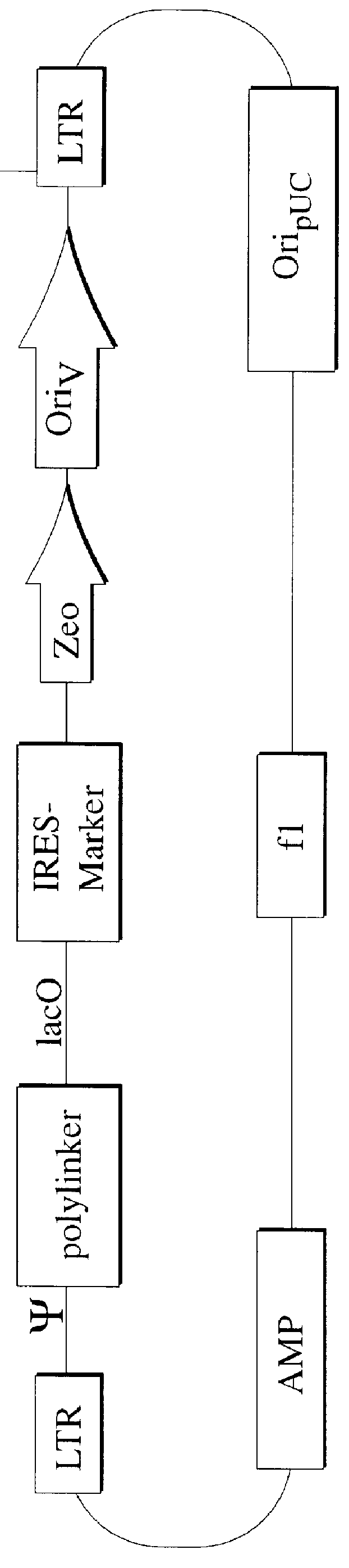
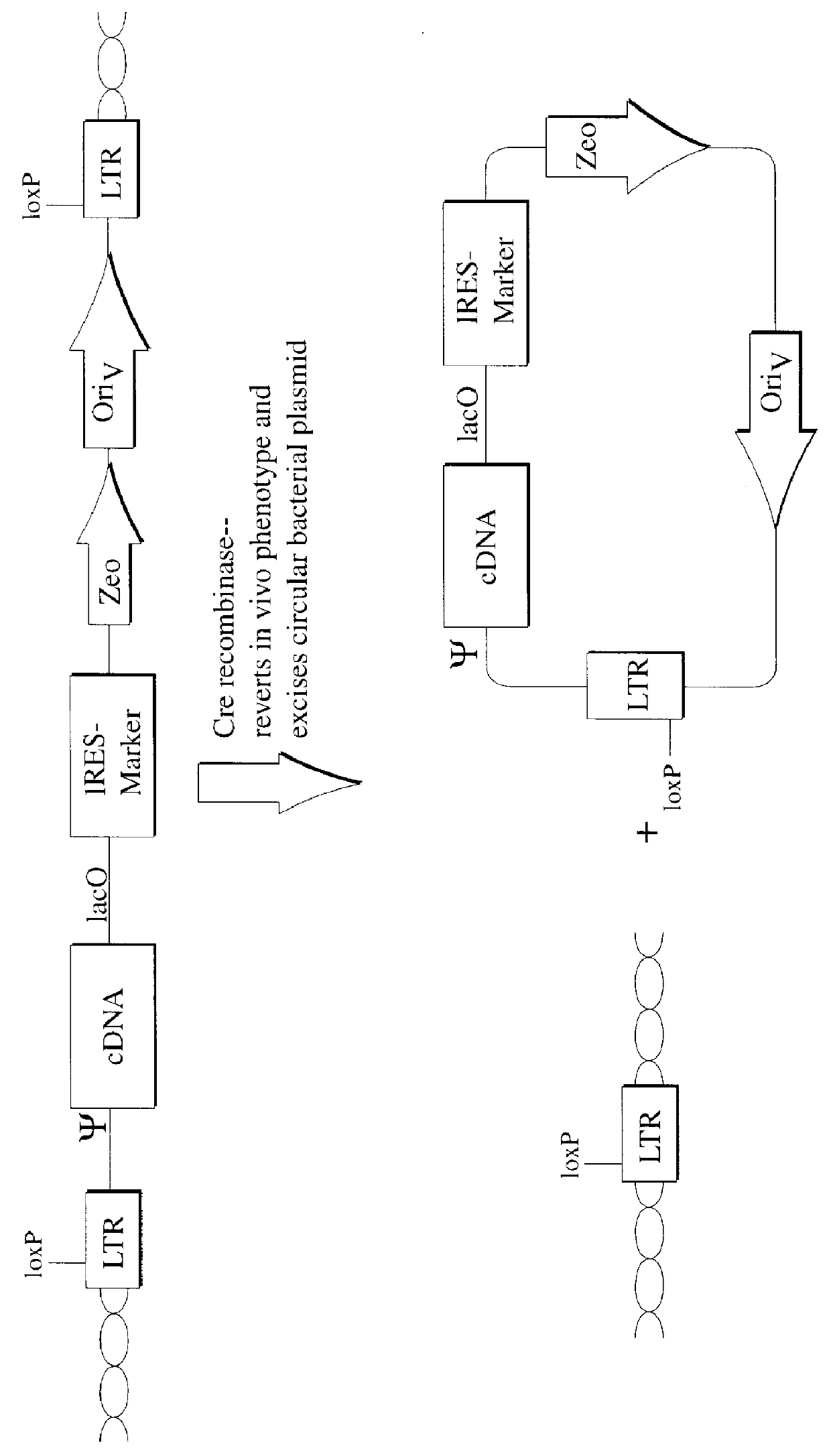
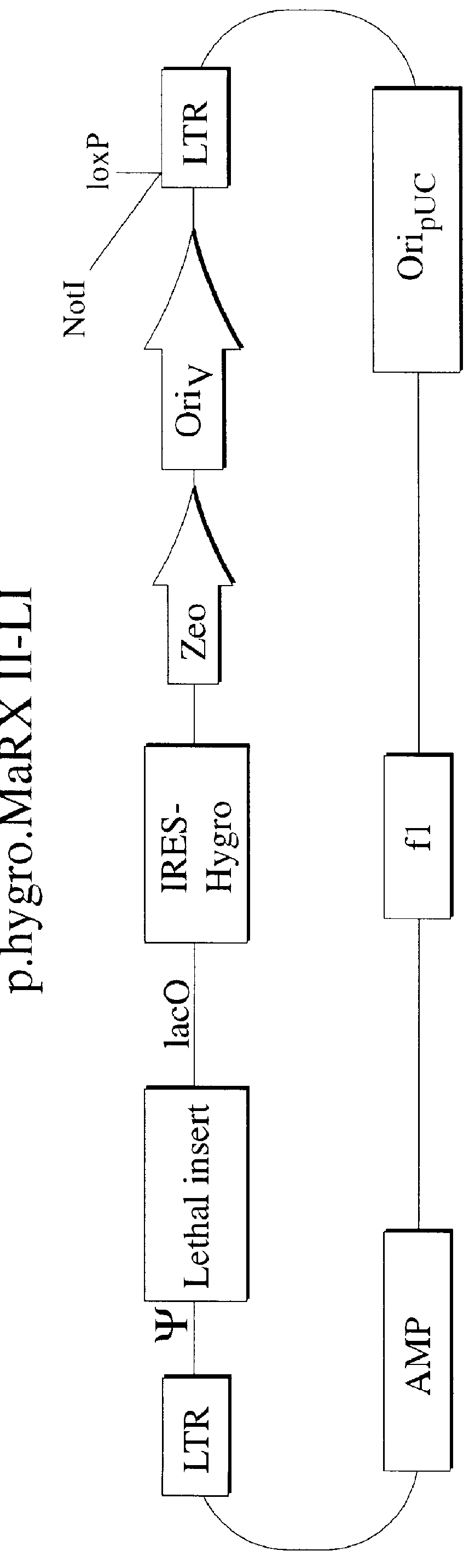
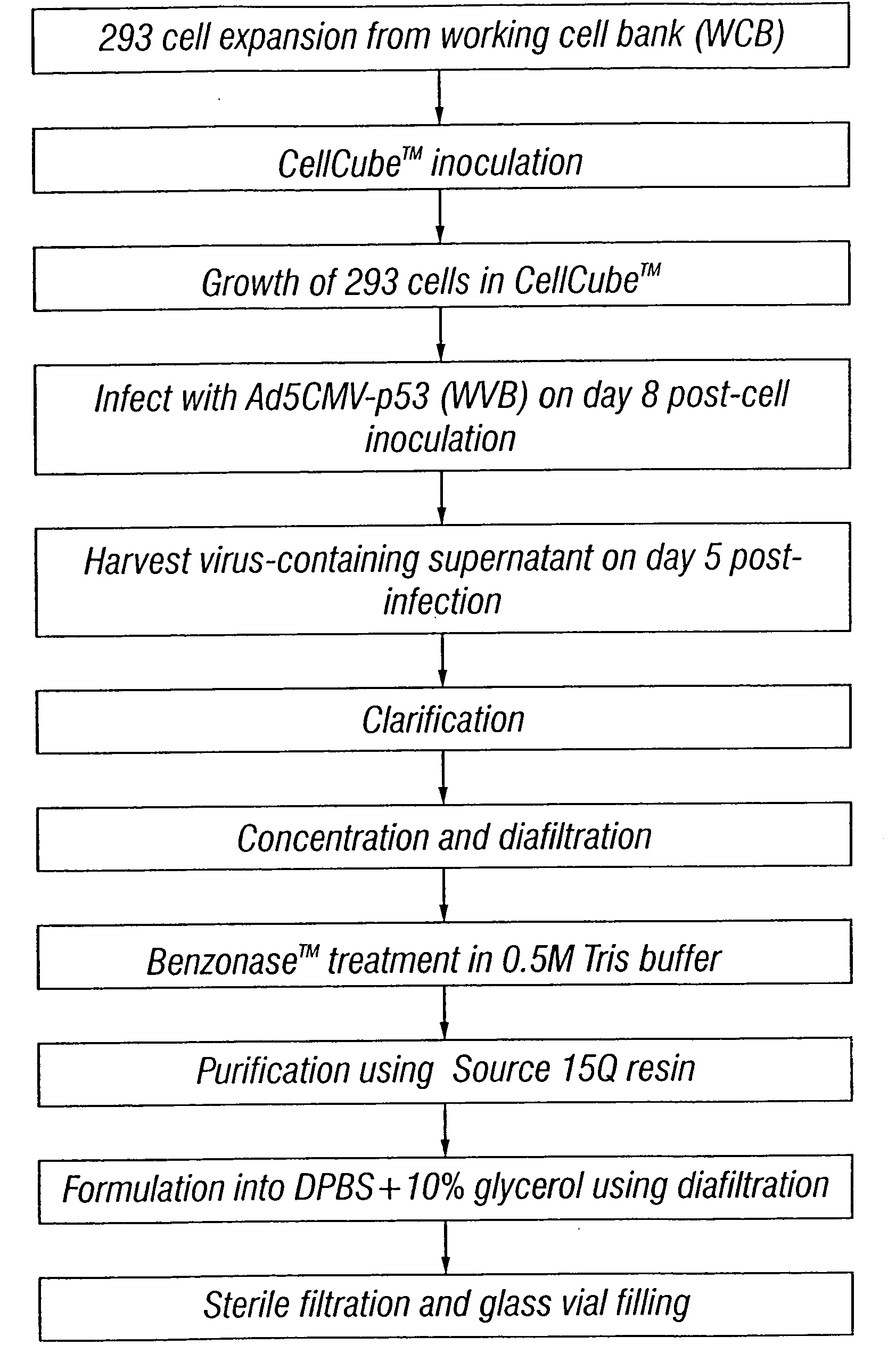
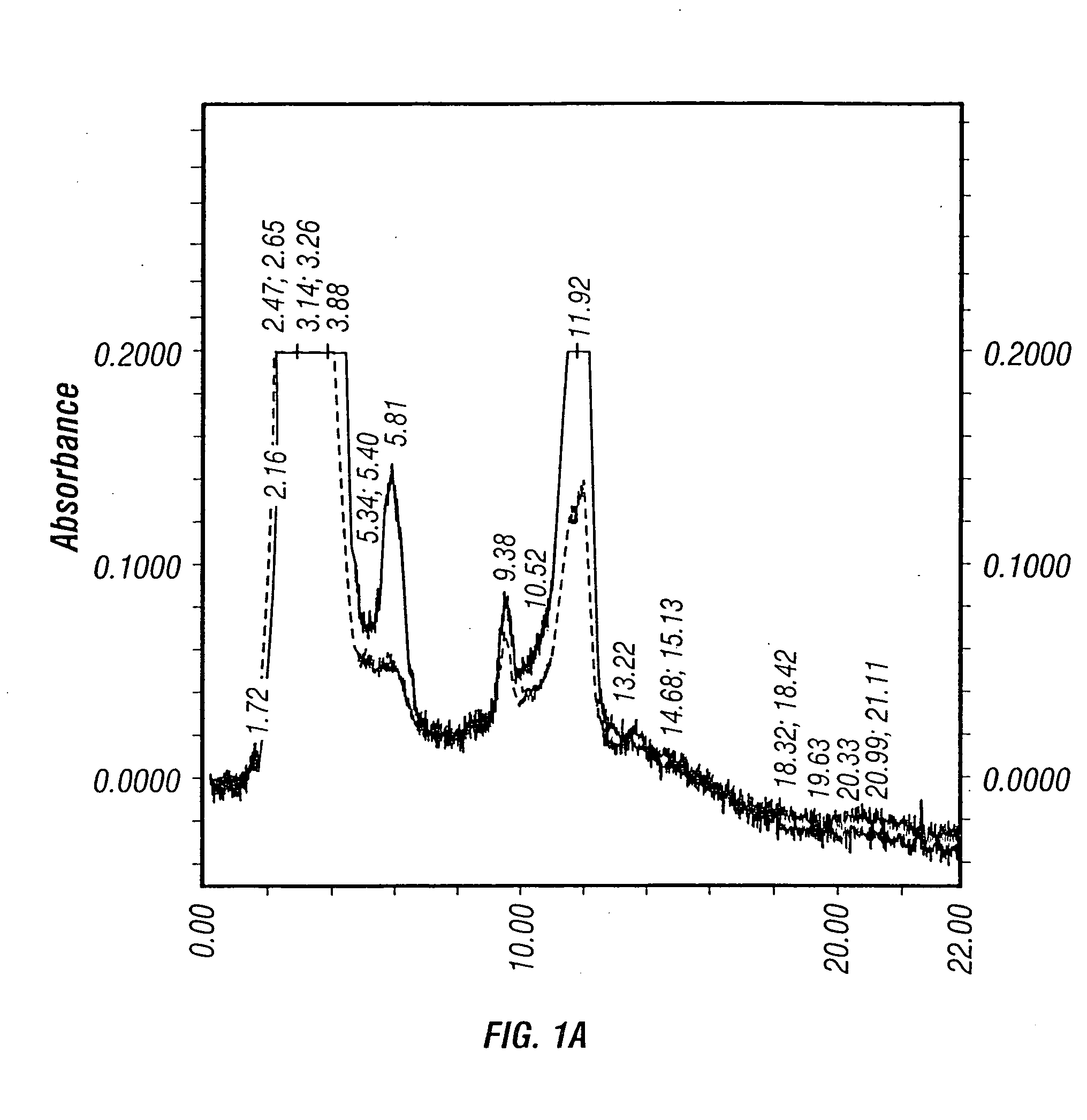
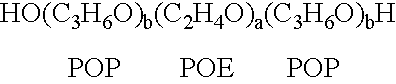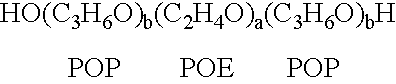Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7294results about "DsDNA viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
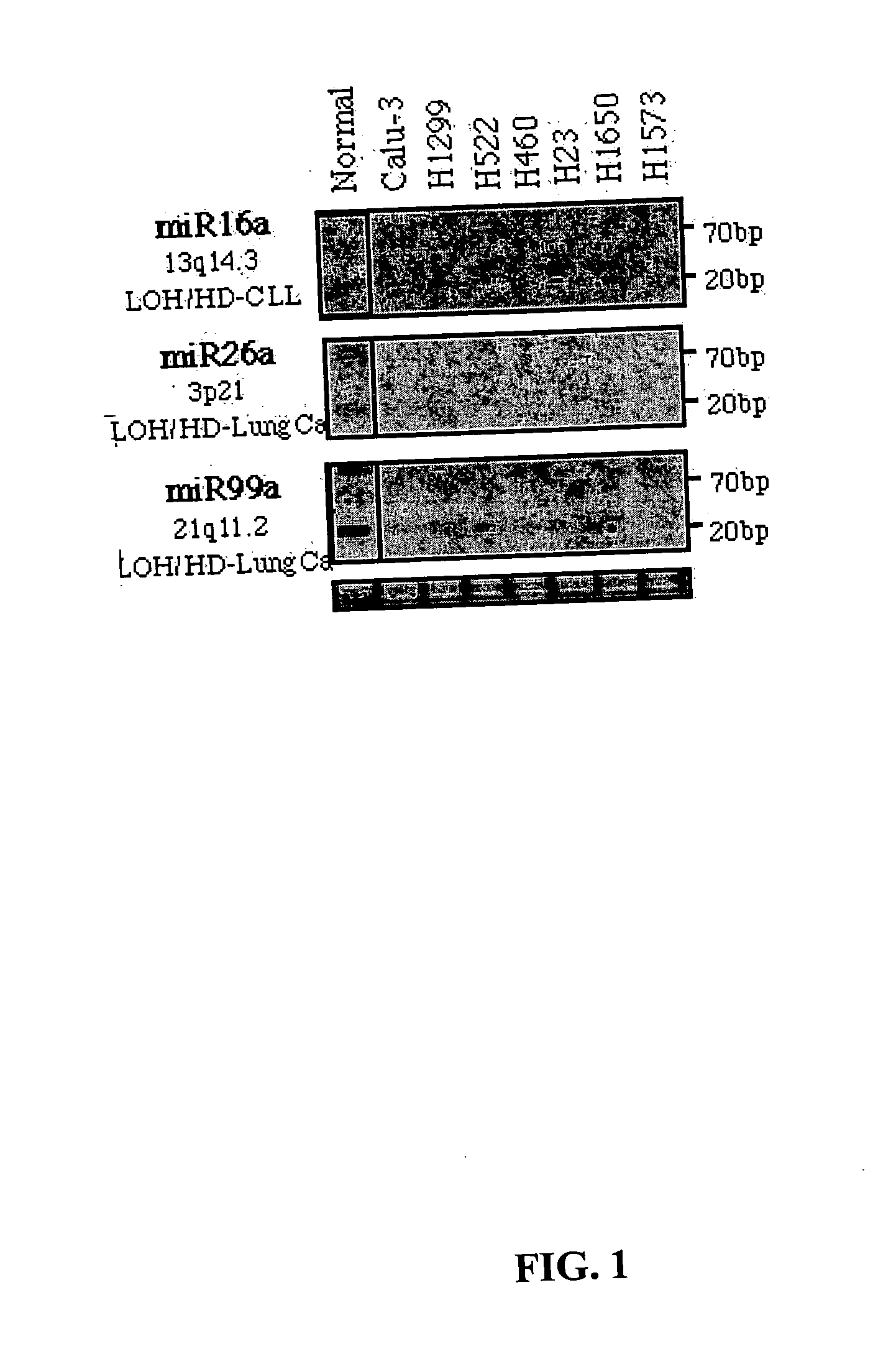
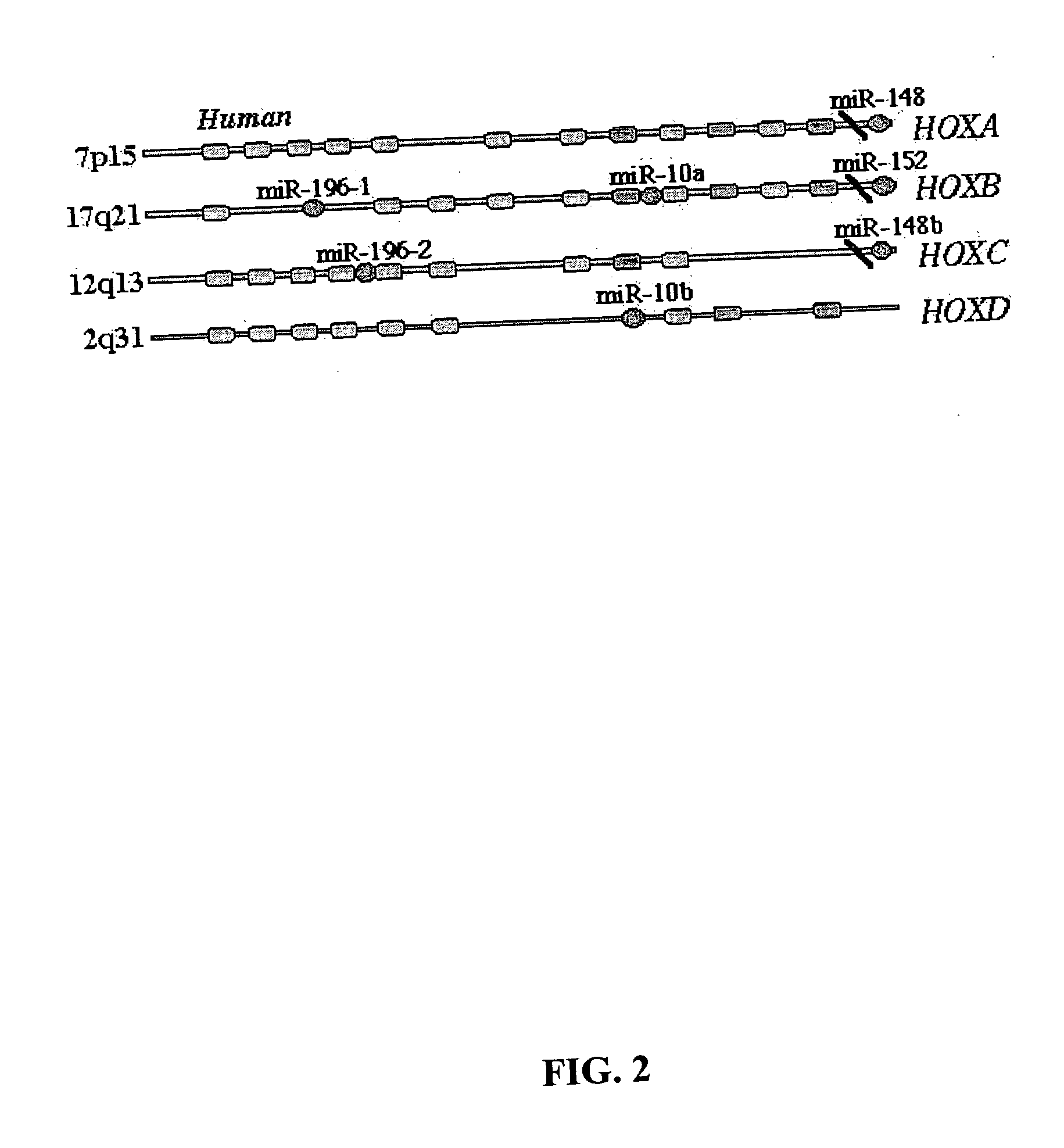
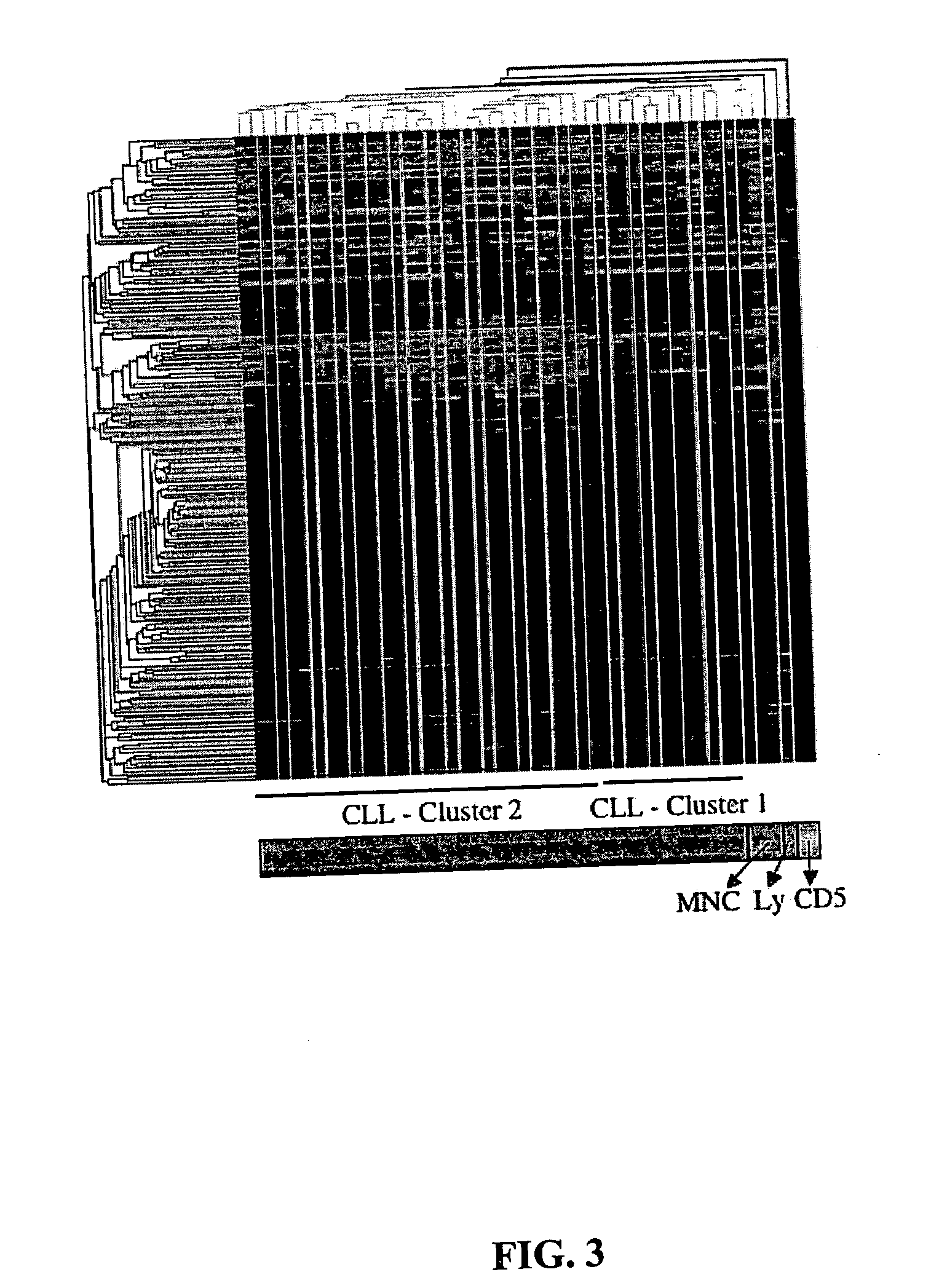
Diagnosis and treatment of cancers with microRNA located in or near cancer associated chromosomal features
InactiveUS20060105360A1Restore levelOrganic active ingredientsMicrobiological testing/measurementEtiologyMicroRNA Gene
MicroRNA genes are highly associated with chromosomal features involved in the etiology of different cancers. The perturbations in the genomic structure or chromosomal architecture of a cell caused by these cancer-associated chromosomal features can affect the expression of the miR gene(s) located in close proximity to that chromosomal feature. Evaluation of miR gene expression can therefore be used to indicate the presence of a cancer-causing chromosomal lesion in a subject. As the change in miR gene expression level caused by a cancer-associated chromosomal feature may also contribute to cancerigenesis, a given cancer can be treated by restoring the level of miR gene expression to normal. microRNA expression profiling can be used to diagnose cancer and predict whether a particular cancer is associated with an adverse prognosis. The identification of specific mutations associated with genomic regions that harbor miR genes in CLL patients provides a means for diagnosing CLL and possibly other cancers.
Owner:THOMAS JEFFERSON UNIV
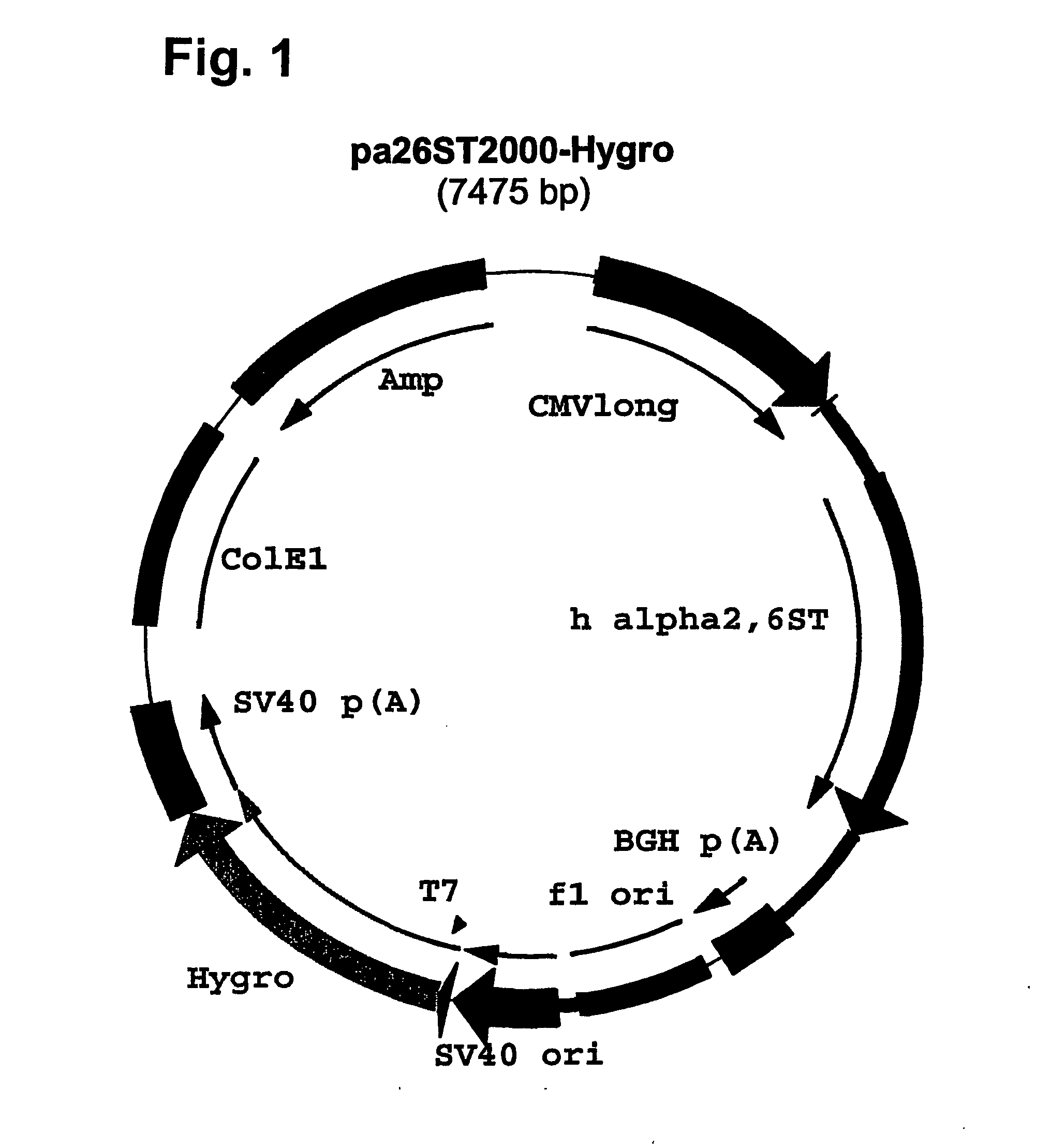
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
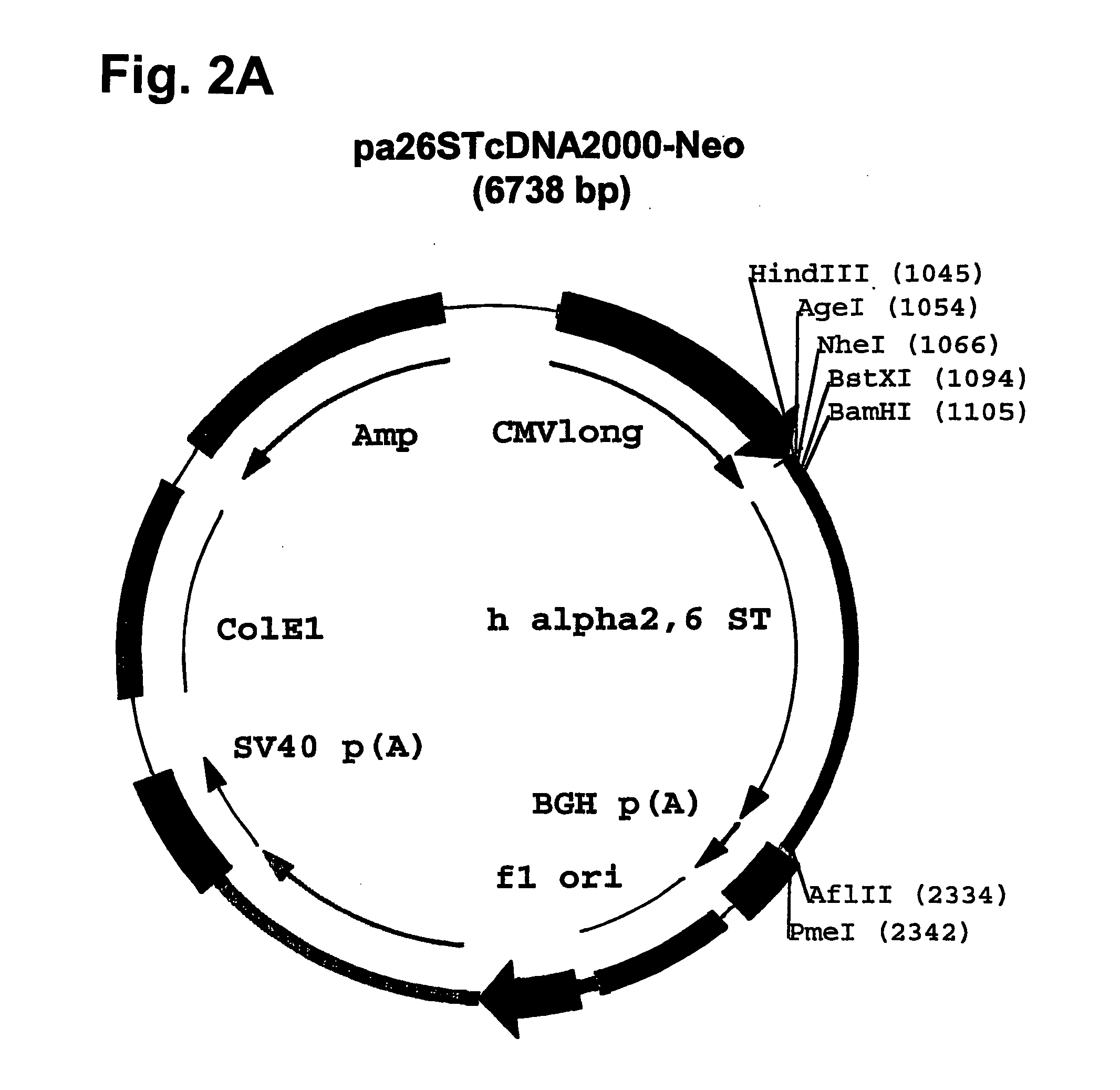
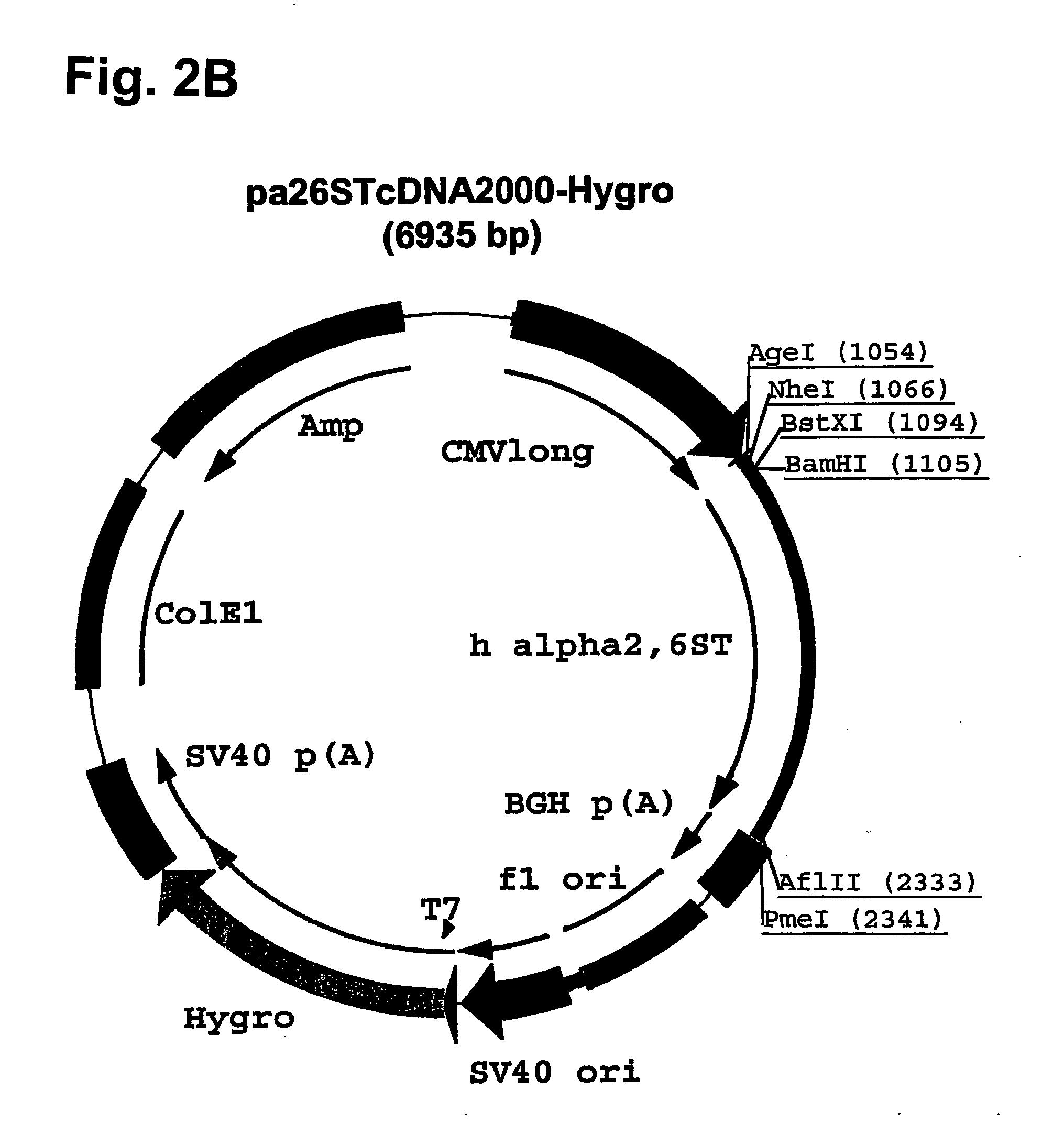
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
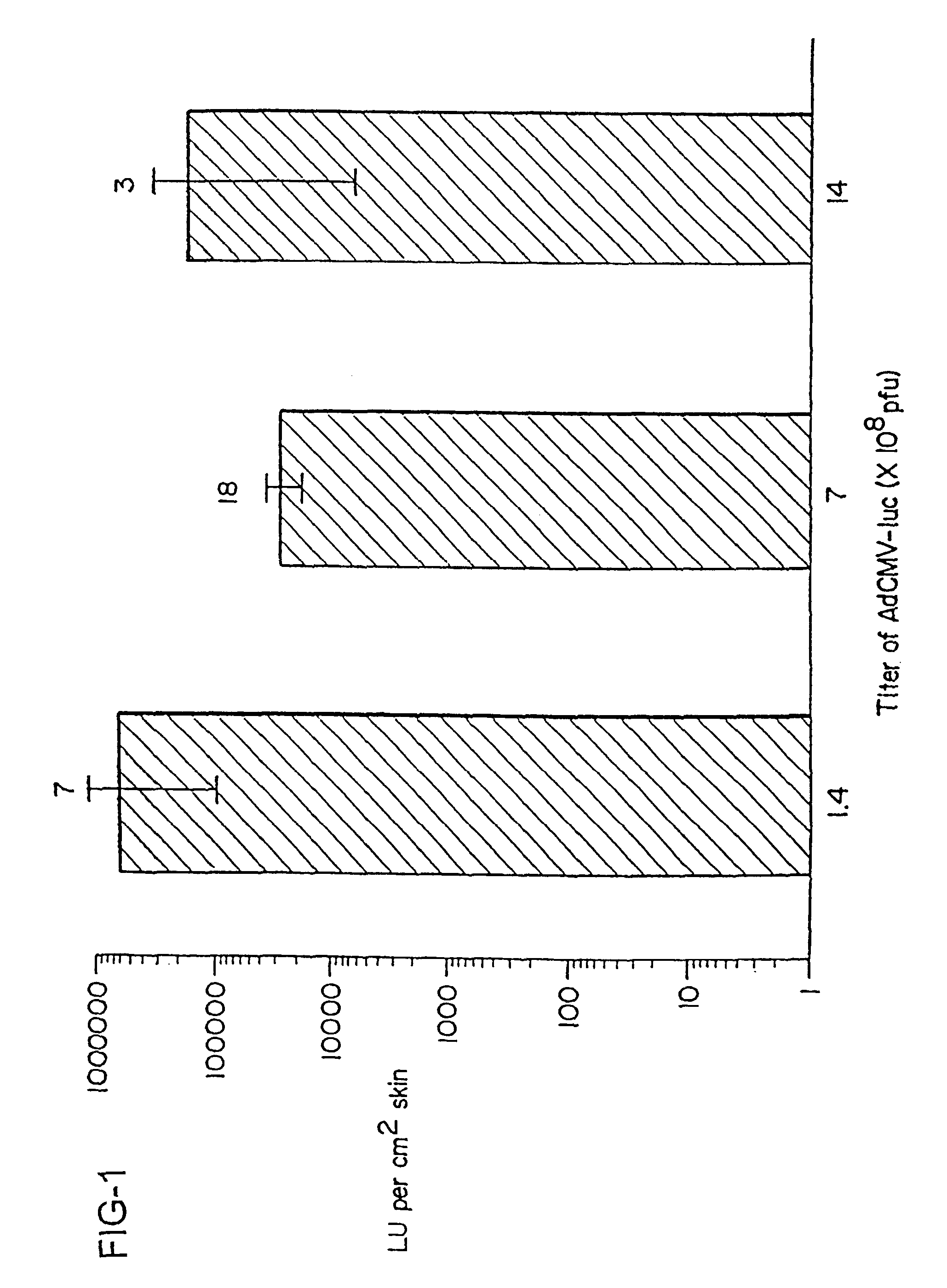
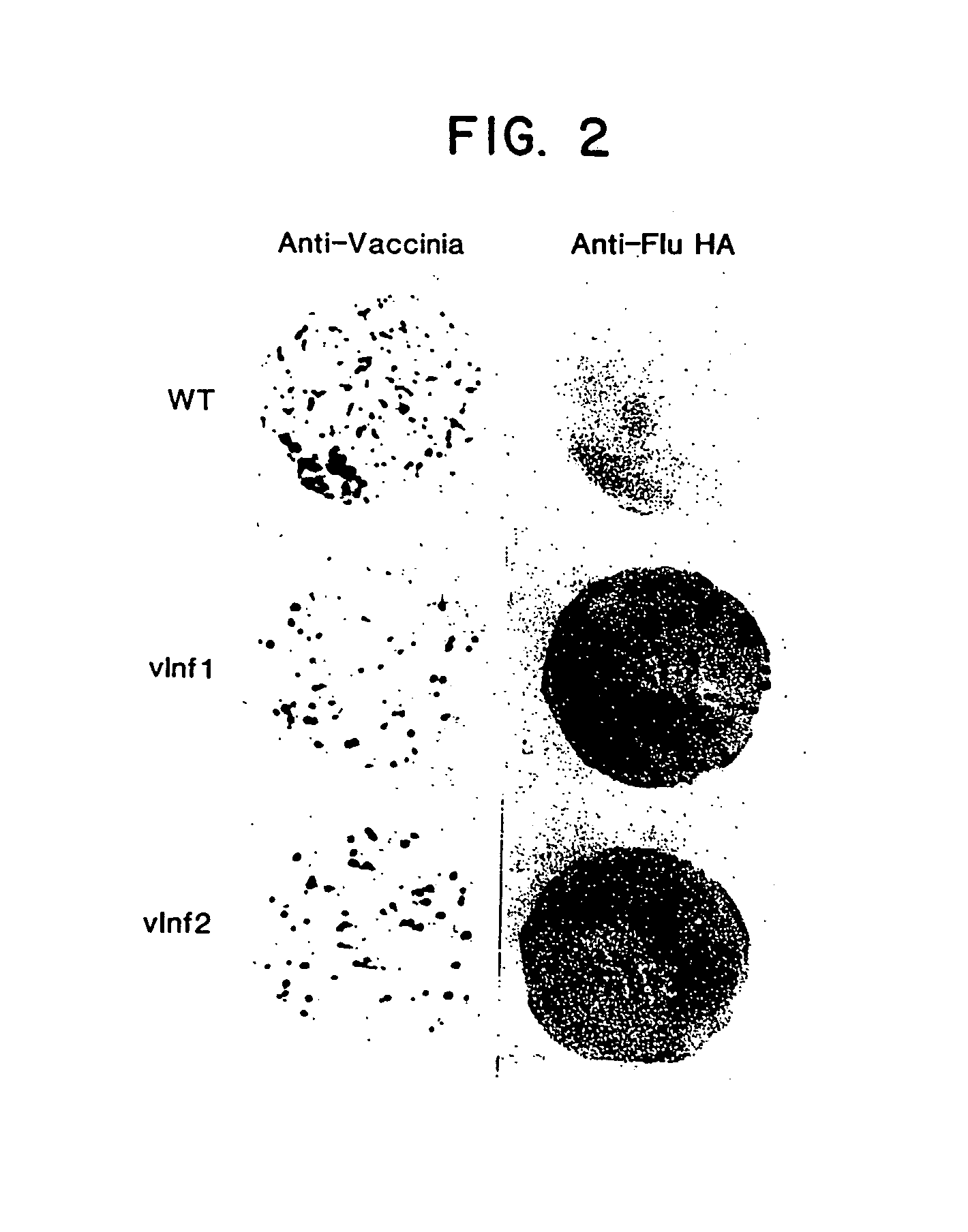
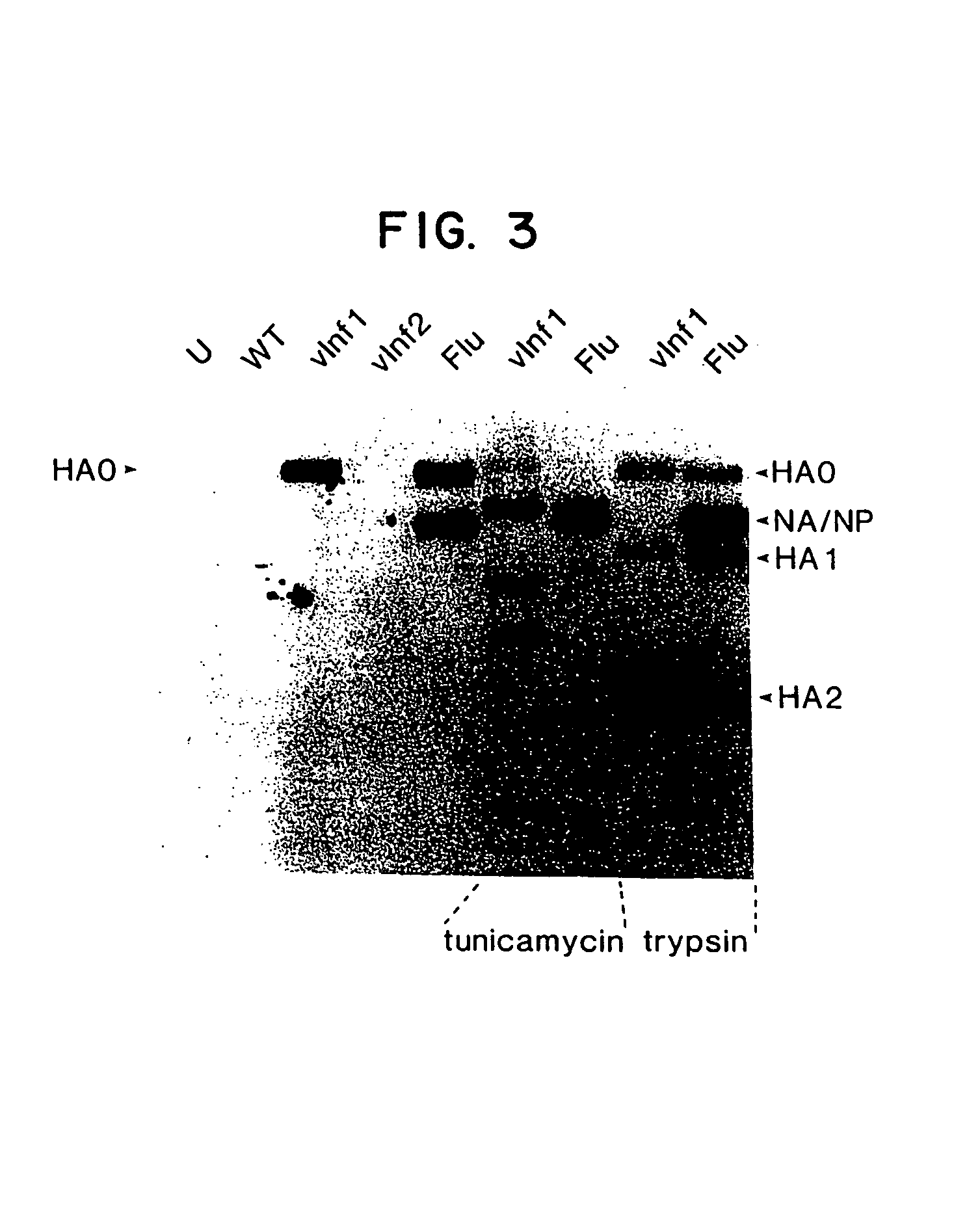
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Production of pseudotyped recombinant AAV virions
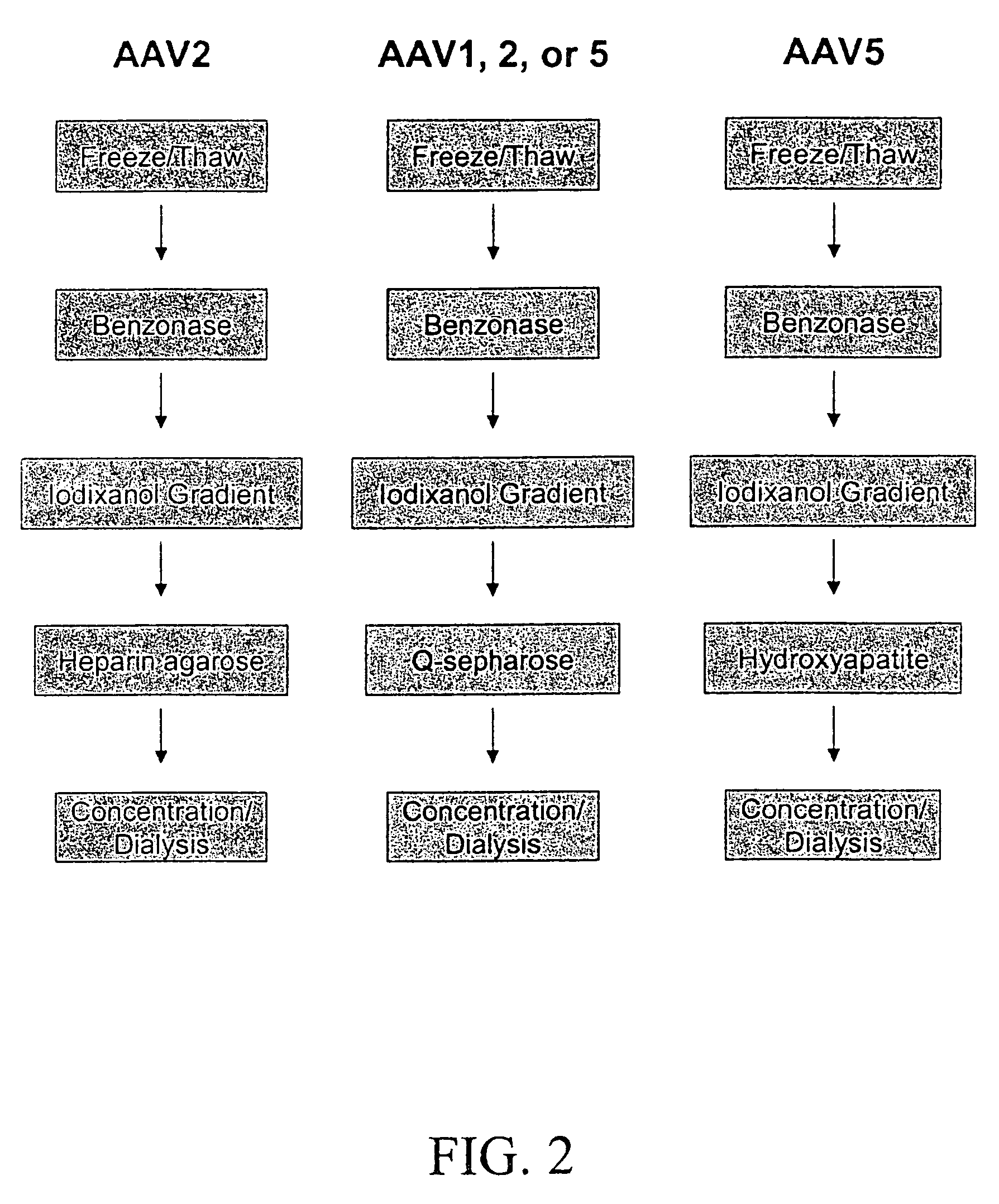
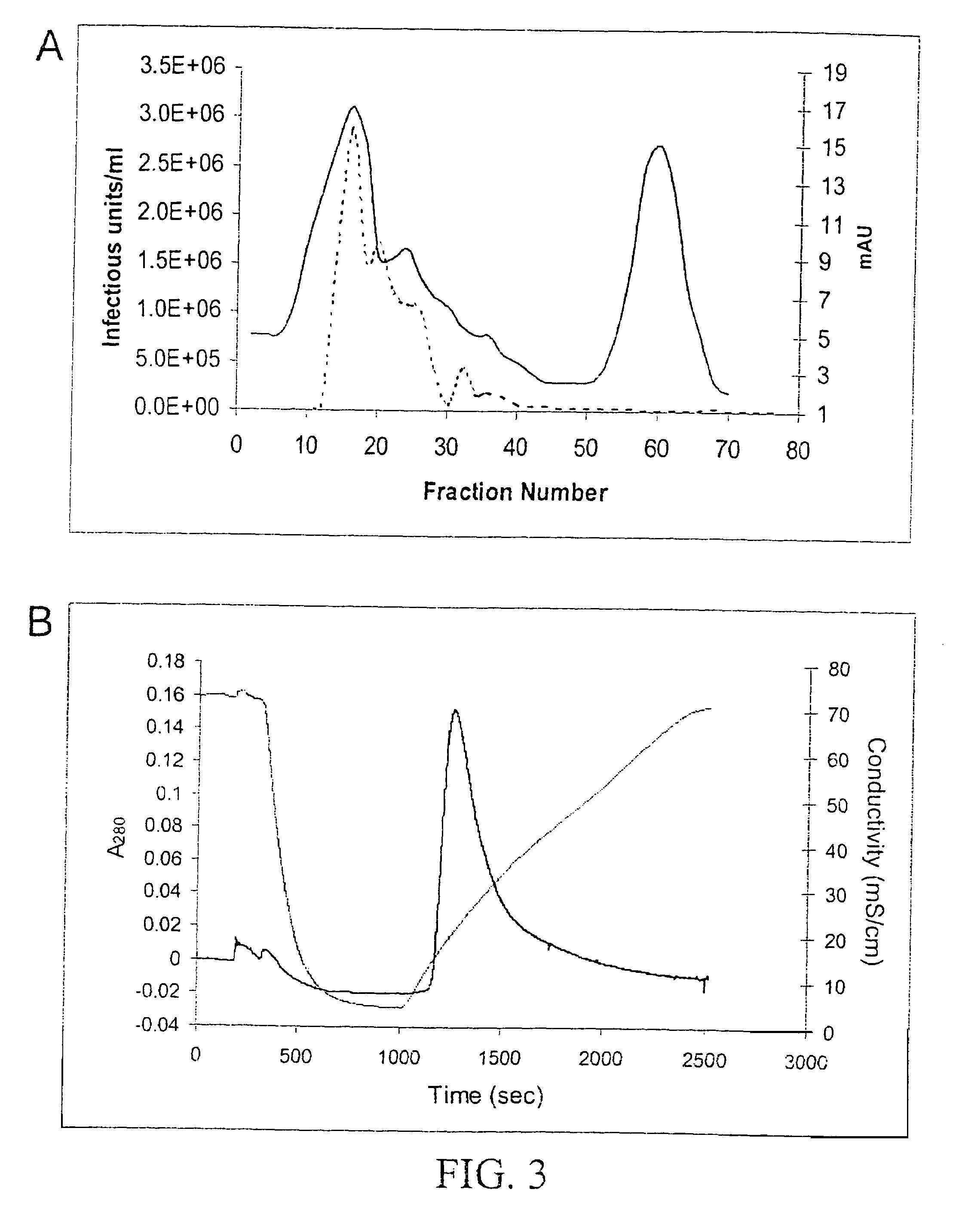
InactiveUS7094604B2Highly purified and concentratedEfficient and large-scale productionVectorsSugar derivativesPurification methodsSerotype
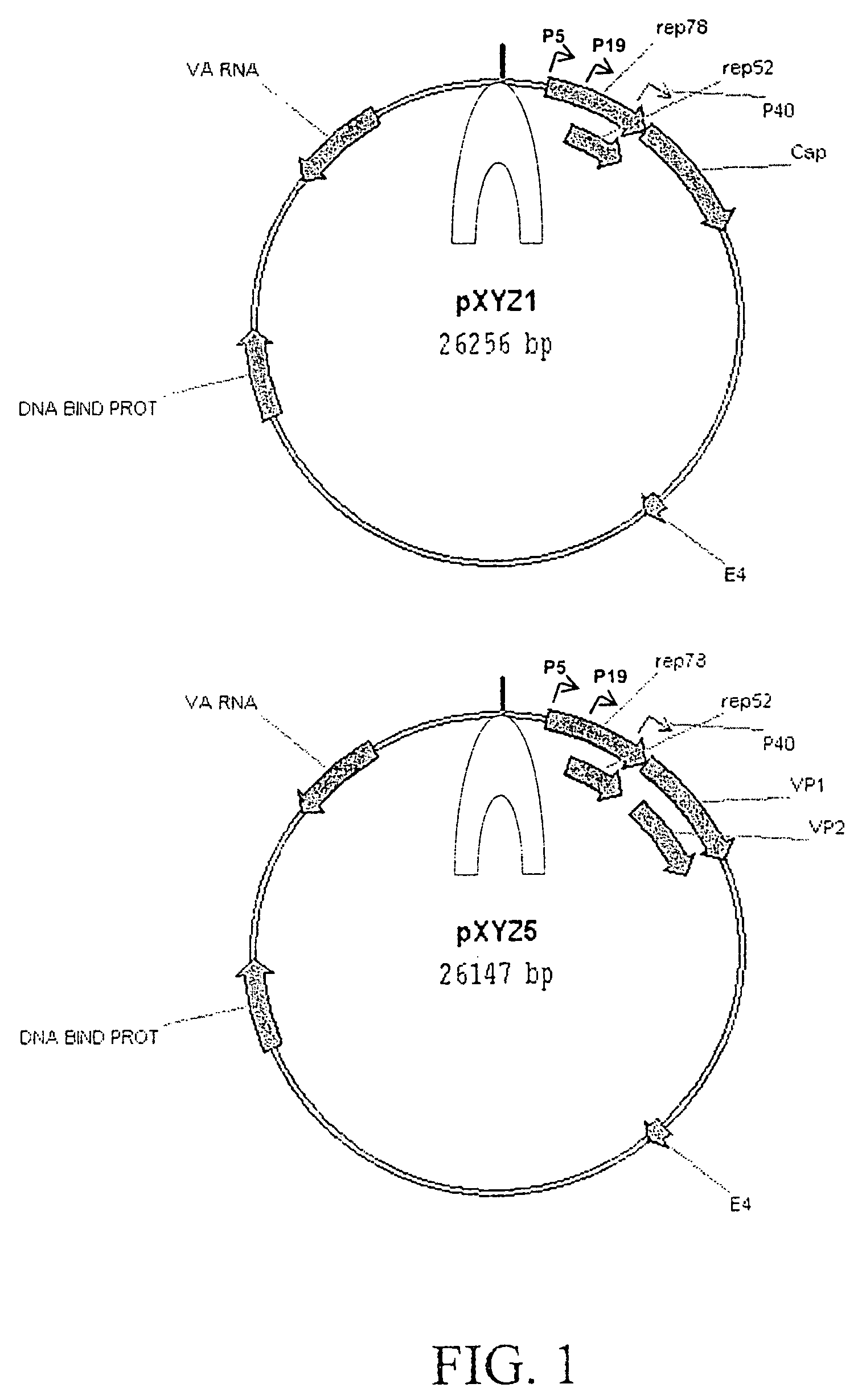
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012–1×1013 vector genomes / ml.
Owner:UNIVIRSITY OF FLORIDA RES FOUND INC
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Vectors having enhanced expression and methods of making and uses thereof
InactiveUS6130066AHigh expressionIncrease transcriptionVectorsSugar derivativesOpen reading frameVaccinia
Disclosed and claimed are vectors having enhanced expression and methods for making and using them. Enhancement of expression is from substantially co-temporal expression of at least one first nucleic acid molecule and at least one second nucleic acid molecule. The second nucleic acid molecule encodes a transcription factor or a translation factor or a transcription factor and a translation factor. The contemporaneous expression can be from operably linking the first and second nucleic molecules to a single promoter, or from operably linking the first nucleic acid molecule to a first promoter and the second nucleic molecule to a second promoter wherein the first and second promoters function substantially contemporaneously. Thus, the first and second nucleic acid molecules can be at the same locus in the vector, or at different loci. The second nucleic acid molecule can encode: one transcription factor or more than one transcription factor; or one translation factor or more than one translation factor; or at least one transcription factor and at least one translation factor. The transcription factor can be from vaccinia H4L, D6, A7, G8R, A1L, A2L, H5R, or combinations thereof. The translation factor can be from a K3L open reading frame, an E3L open reading frame, a VAI RNA, an EBER RNA, a sigma 3 open reading frame, a TRBP open reading frame, or combinations thereof. The vector can be a poxvirus such as an attenuated poxvirus, e.g., NYVAC, or ALVAC.
Owner:VIROGENETICS
Oligopeptide-free cell culture media
InactiveUS20070212770A1Efficient expression of recombinantEfficient productionFactor VIIBacteriaCulture cellCell culture media
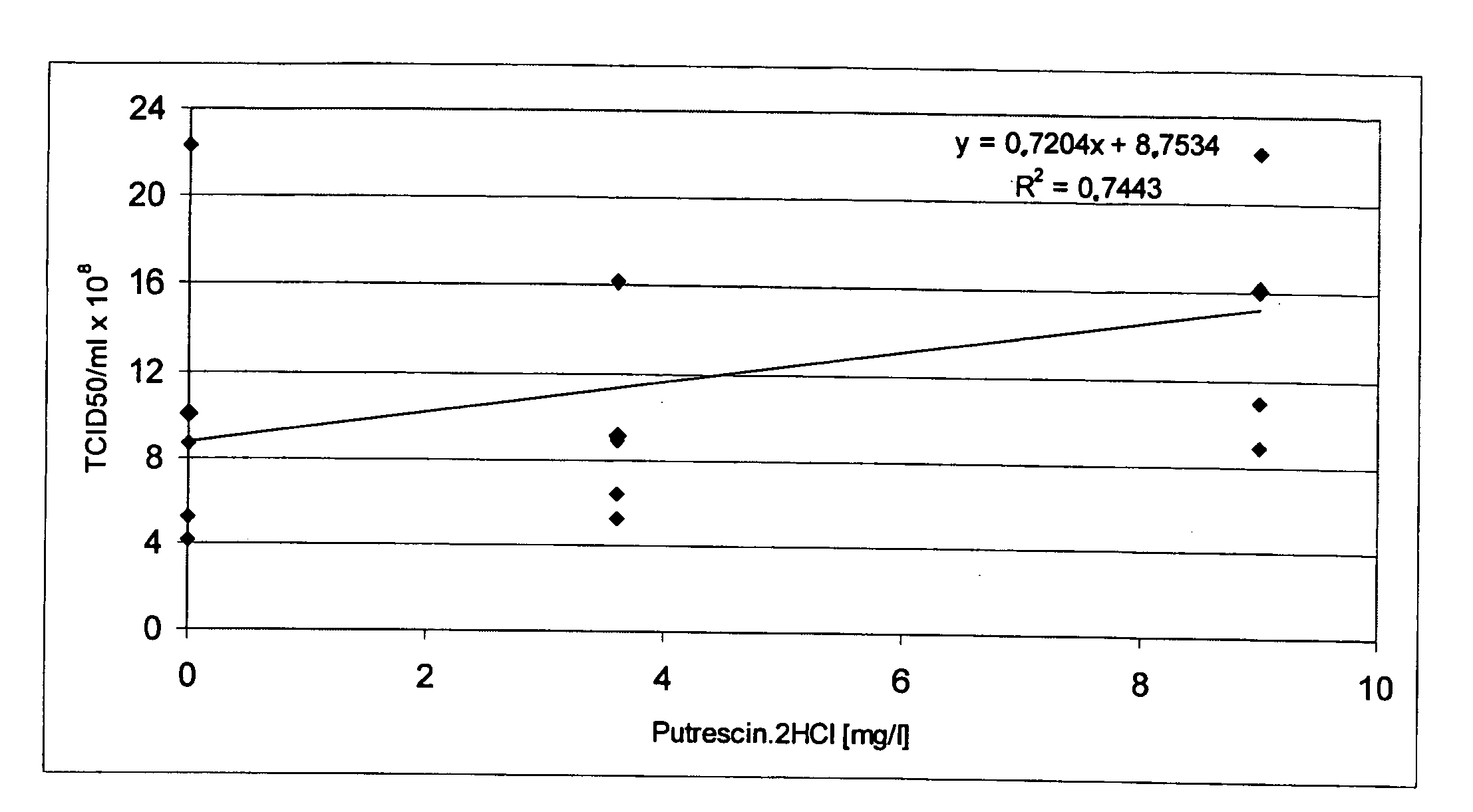
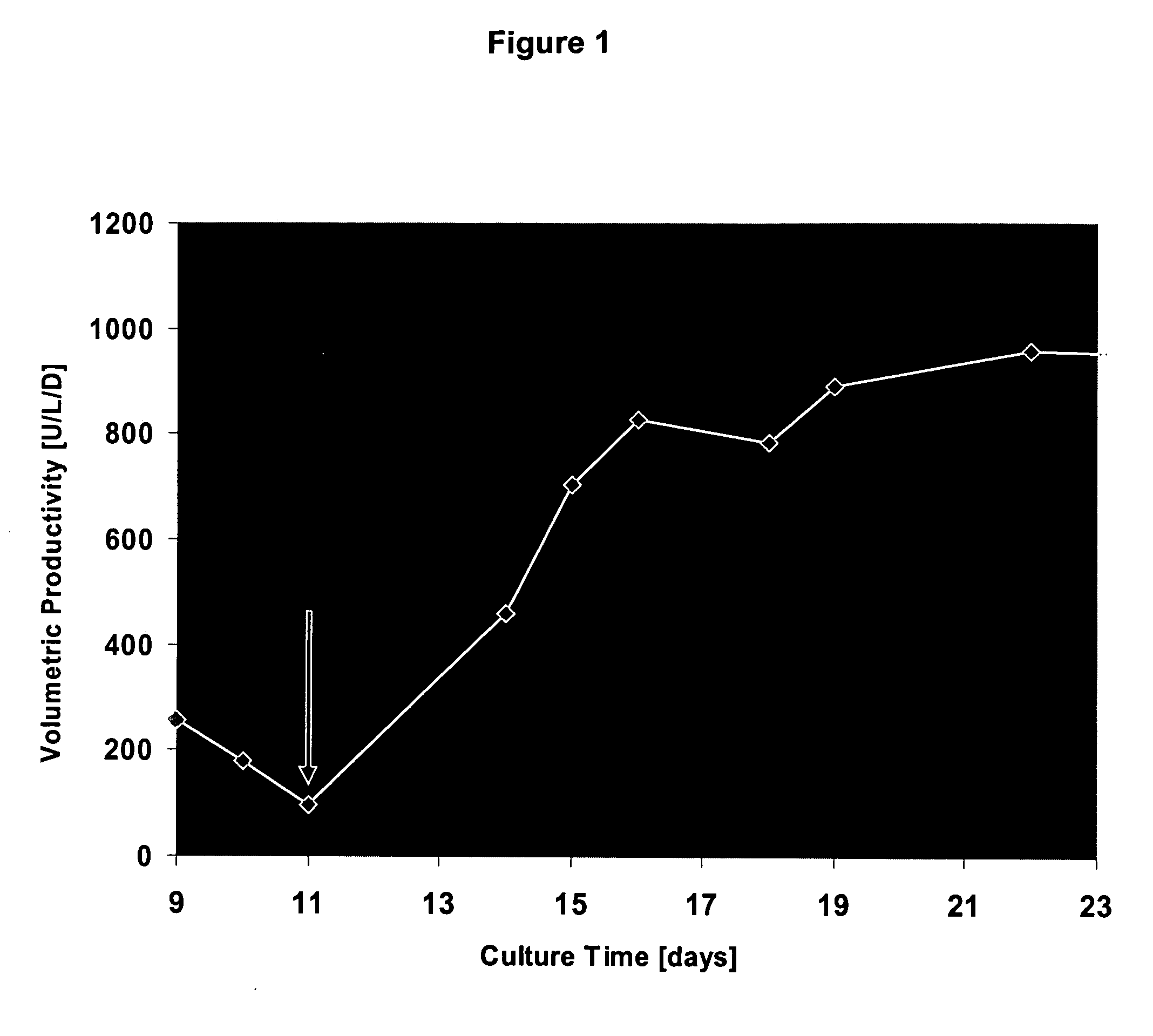
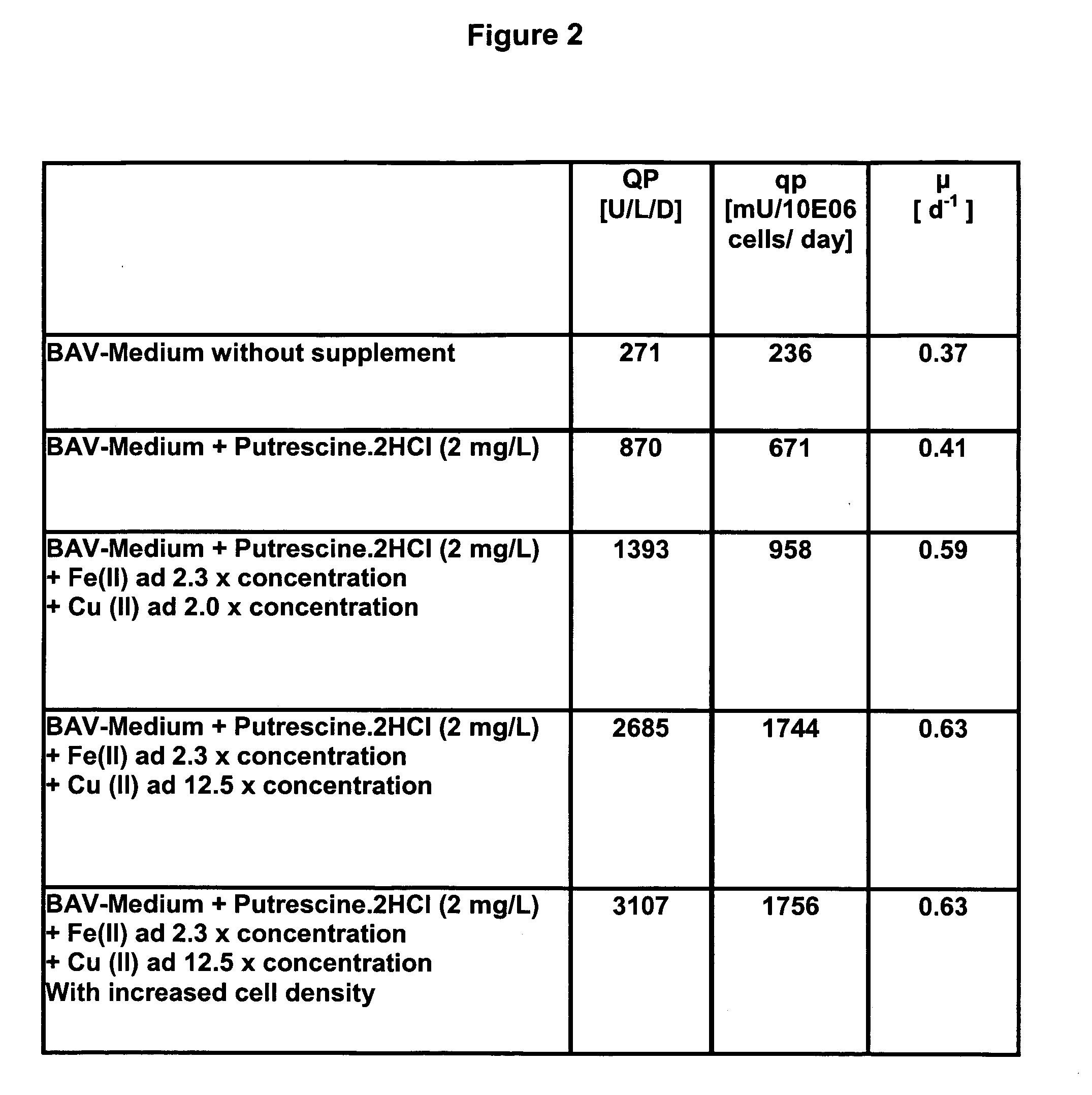
The present invention relates to oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine and to methods for cultivating cells in said oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine. The invention also relates to methods for expressing at least one protein in a medium comprising at least 0.5 mg / L of a polyamine and to methods for producing at least one virus in a medium comprising at least 0.5 mg / L of a polyamine.
Owner:BAXTER HEALTHCARE SA +1
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
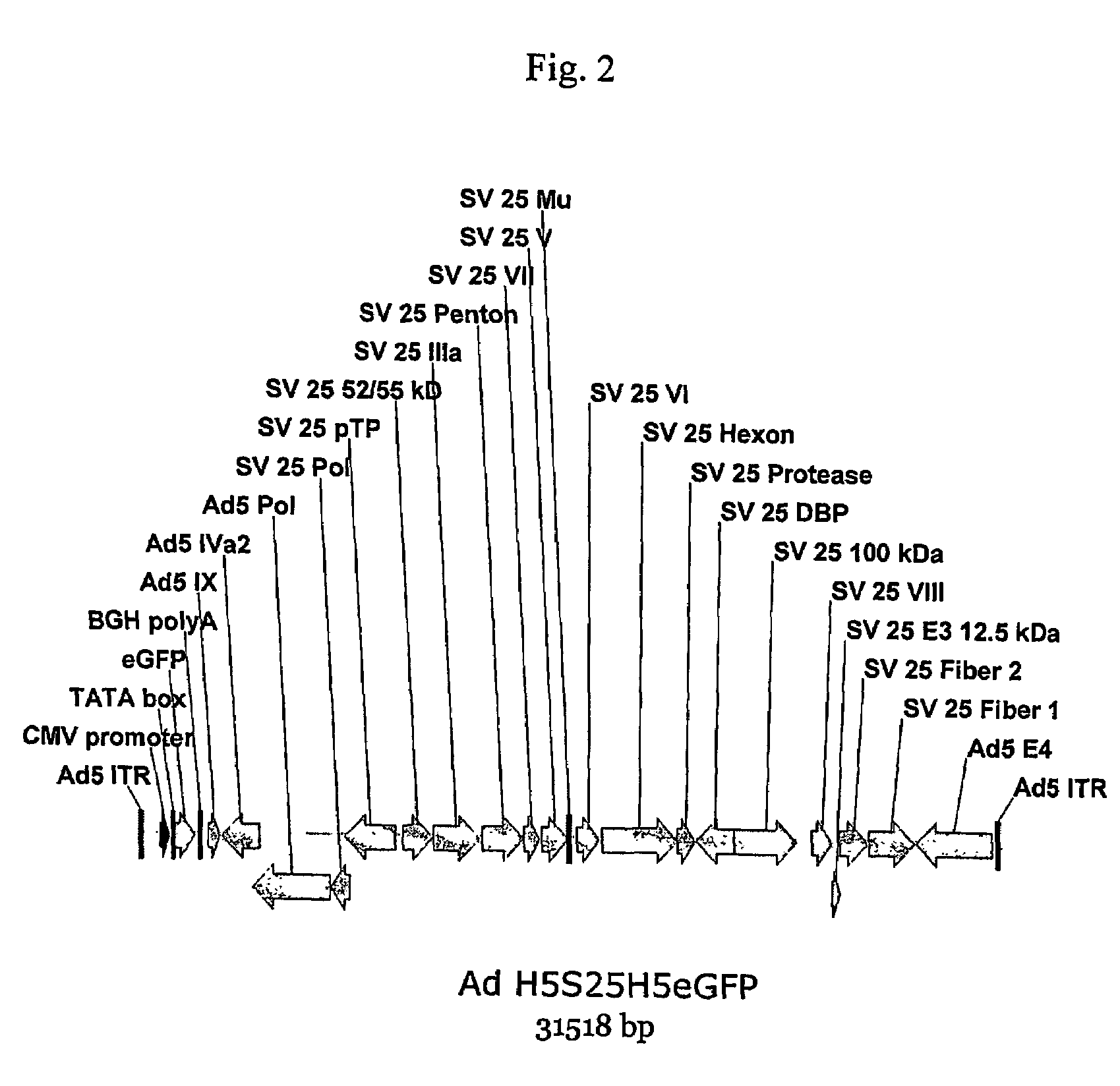
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
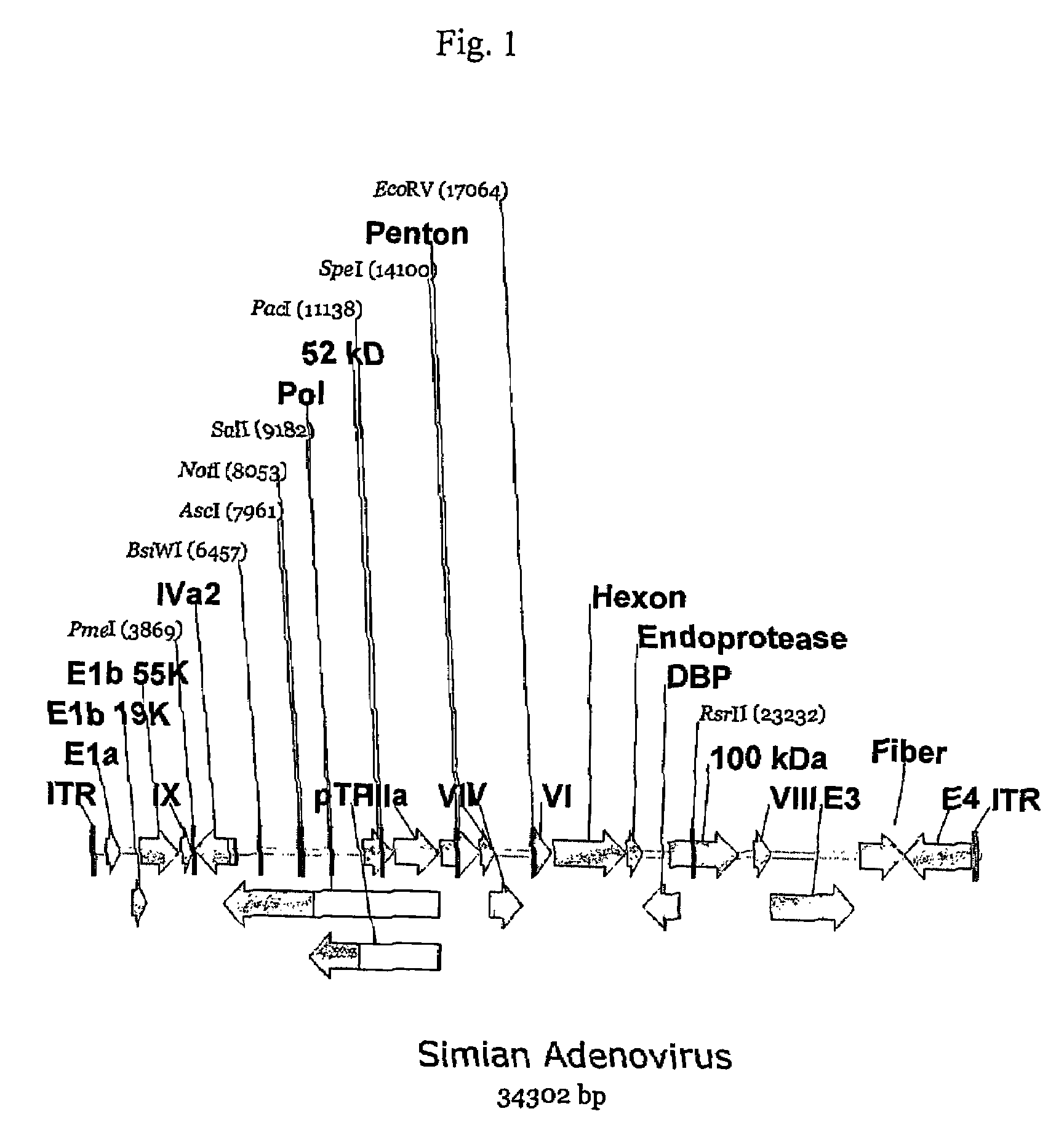
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Mammalian viral vectors and their uses
InactiveUS6255071B1Stable episomal maintenanceMaintain relatively stableSugar derivativesMicrobiological testing/measurementRetroviral provirusMammal
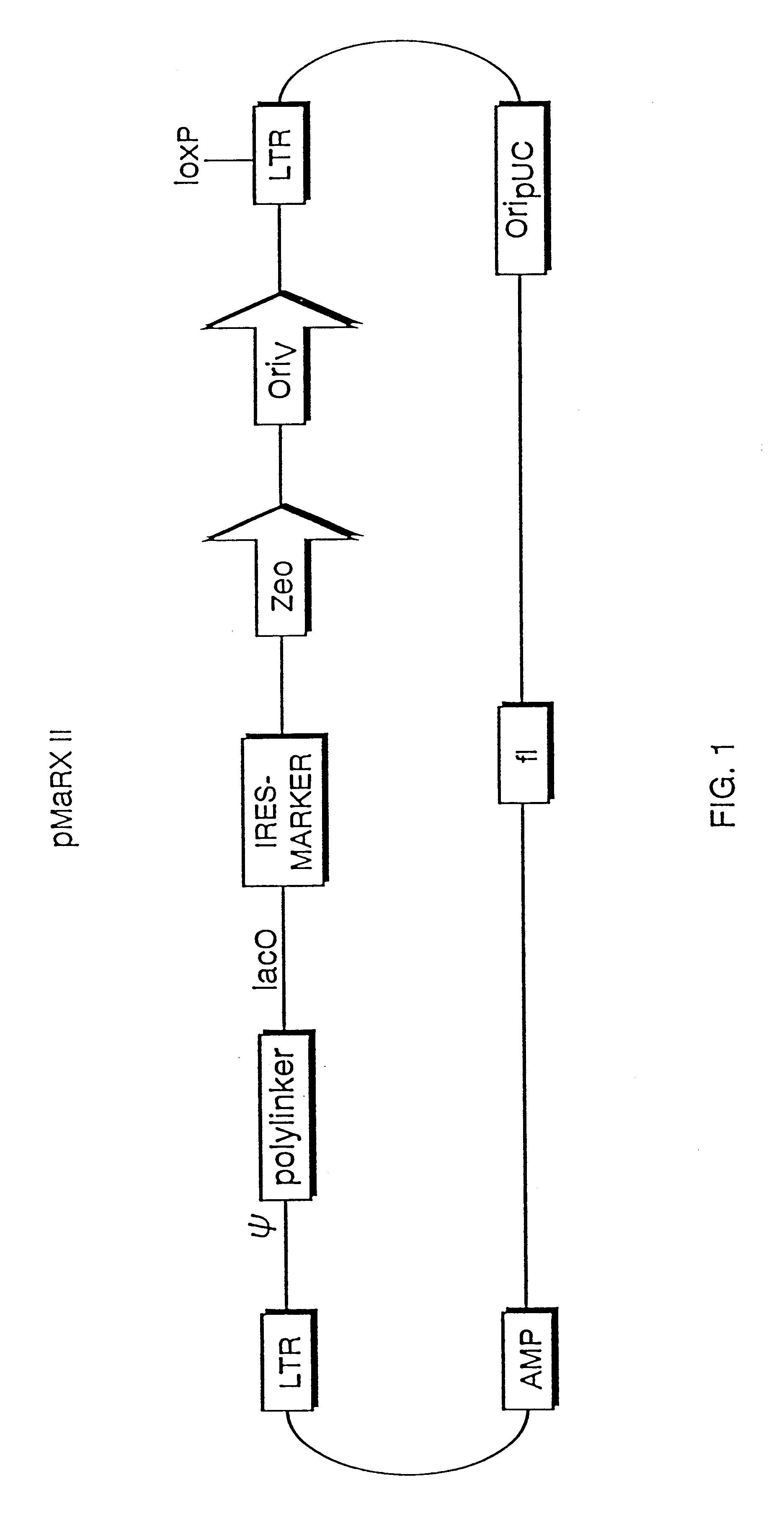
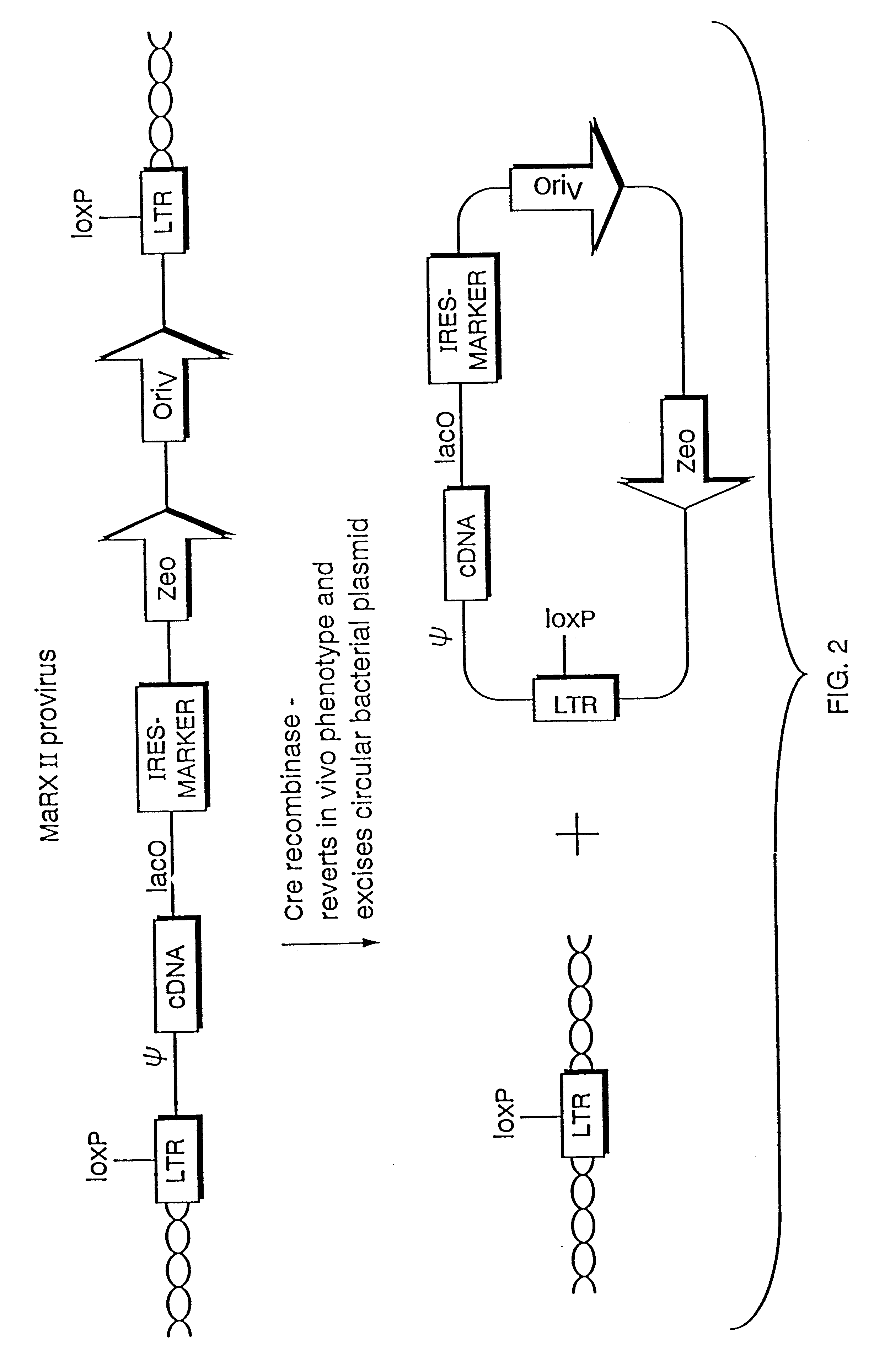
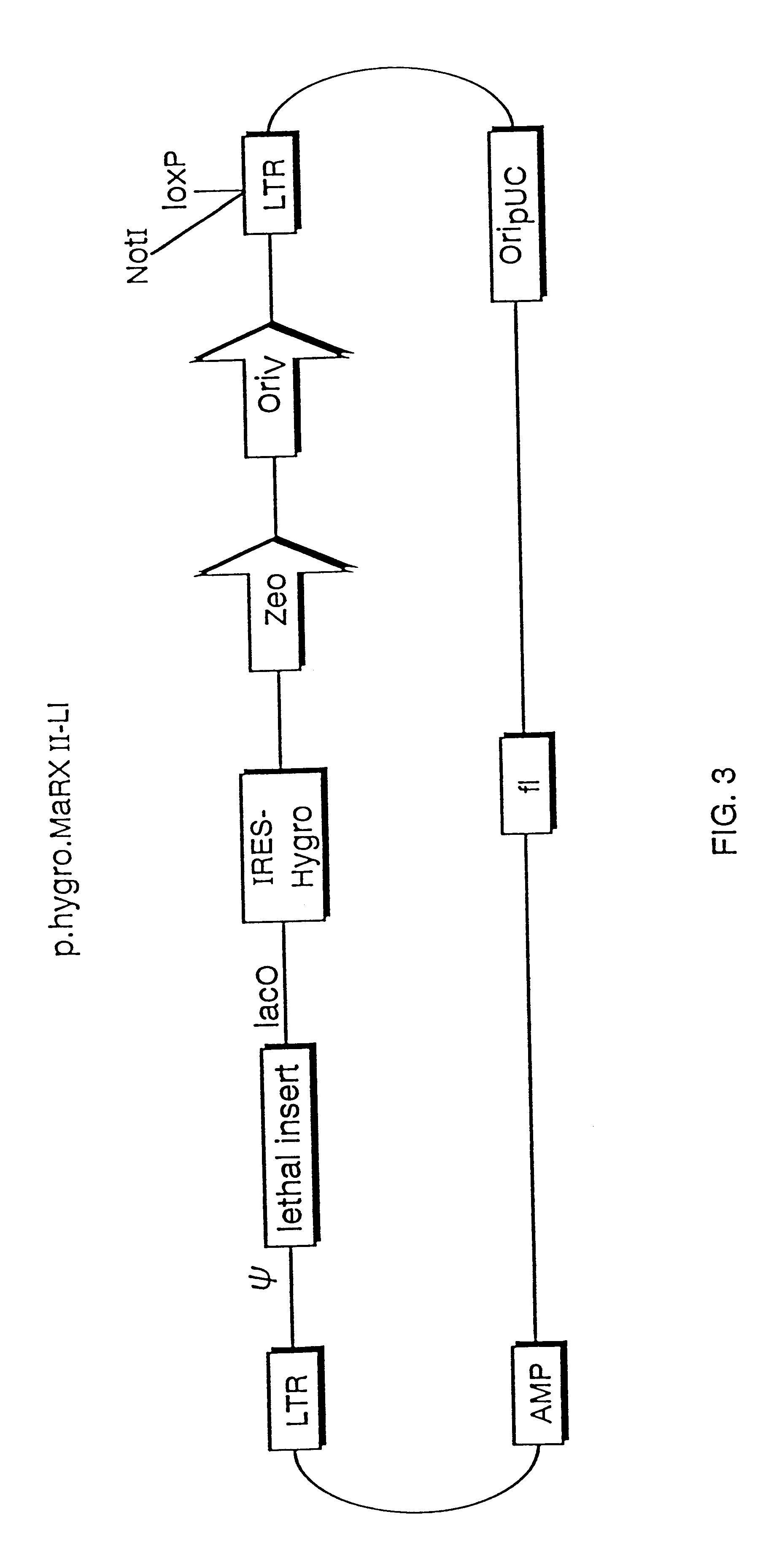
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli.In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Methods for the production of ips cells using non-viral approach
ActiveUS20100003757A1Promote generationExpand the populationGenetically modified cellsVirus peptidesVector elementCell type
Methods and composition of induction of pluripotent stem cells and other desired cell types are disclosed. For example, in certain aspects methods for generating essentially vector-free induced pluripotent stem cells are described. Furthermore, the invention provides induced pluripotent stem cells and desired cell types essentially free of exogenous vector elements with the episomal expression vectors to express differentiation programming factors.
Owner:FUJIFILM CELLULAR DYNAMICS INC
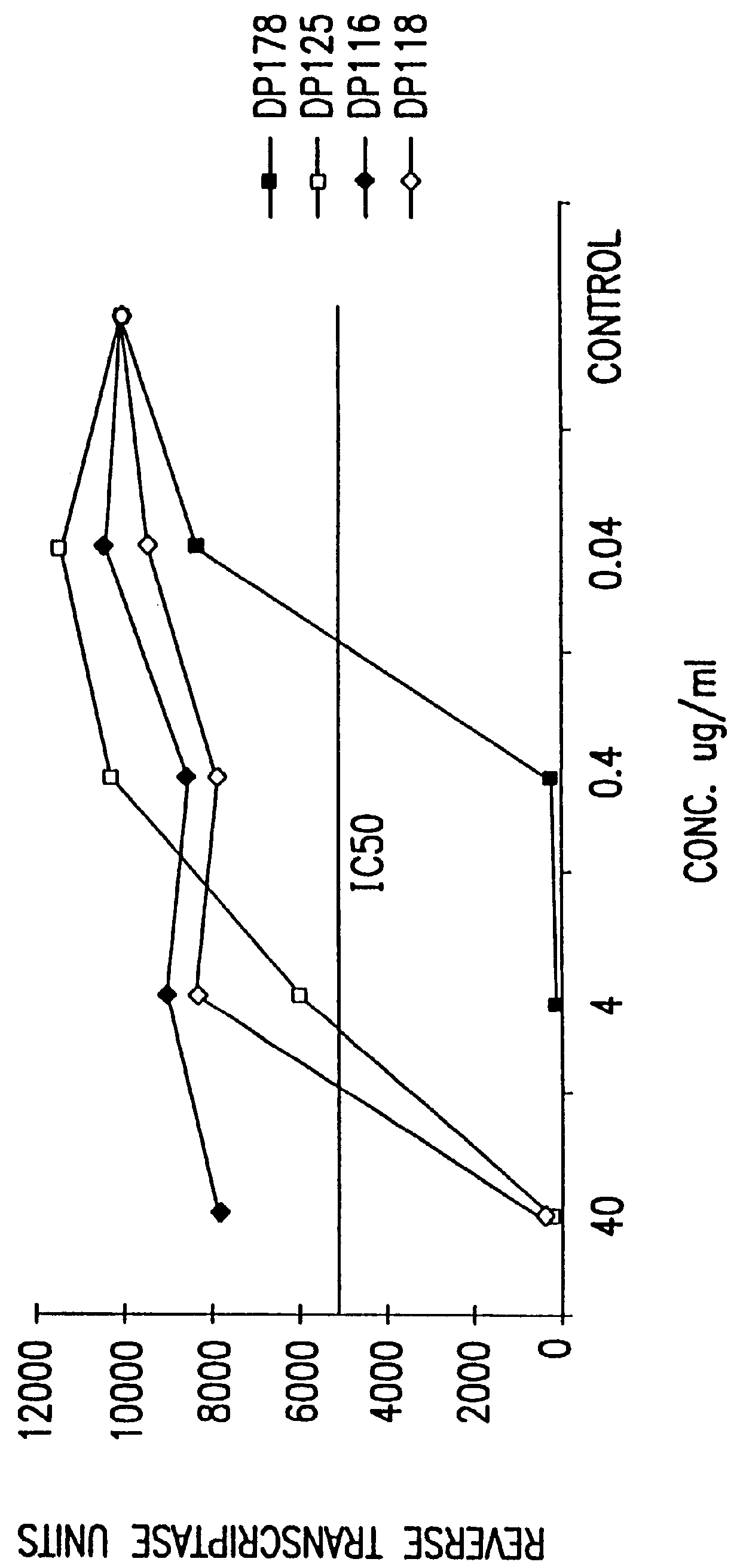
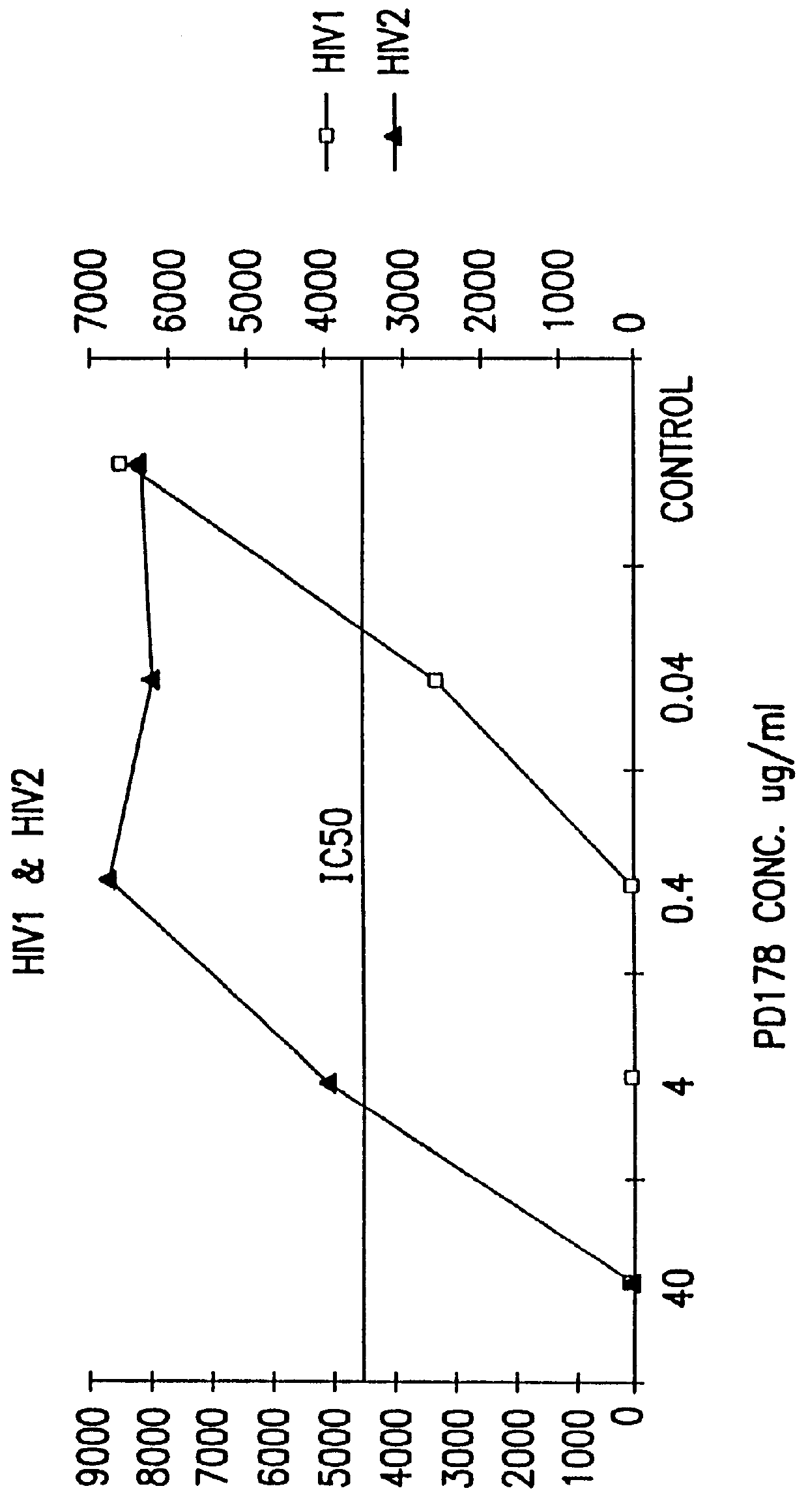
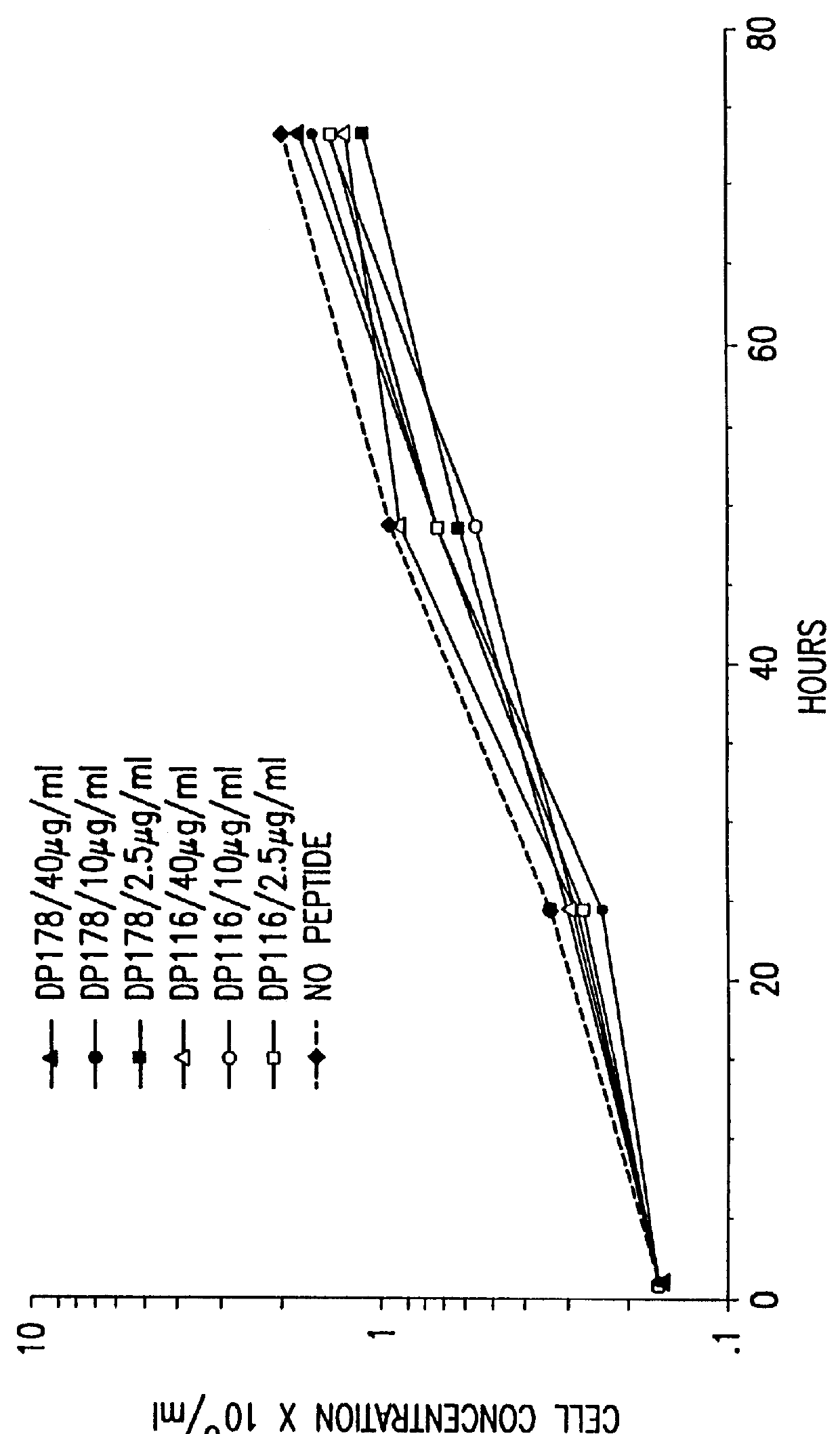
Simian immunodeficiency virus peptides with antifusogenic and antiviral activities
InactiveUS6017536ASsRNA viruses negative-sensePeptide/protein ingredientsSimian immunodeficiency viruses SIVDefective virus
The present invention relates to peptides which exhibit antifusogenic and antiviral activities. The peptides of the invention consist of a 16 to 39 amino acid region of a simian immunodeficiency virus (SIV) protein. These regions were identified through computer algorithms capable of recognizing the ALLMOTI5, 107x178x4, or PLZIP amino acid motifs. These motifs are associated with the antifusogenic and antiviral activities of the claimed peptides.
Owner:TRIMERIS +1
Novel substitution mutant receptors and their use in an nuclear receptor-based inducible gene expression system
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to novel substitution mutant receptors and their use in a Group H nuclear receptor-based inducible gene expression system and methods of modulating the expression of a gene in a host cell for applications such as gene therapy, large scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:PRECIGEN INC
Modified retroviral vectors
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli. In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
Method for the production and purification of adenoviral vectors
InactiveUS20080050770A1Peptide/protein ingredientsGenetic material ingredientsSerum igeUltracentrifuge
Owner:JANSSEN VACCINES & PREVENTION BV
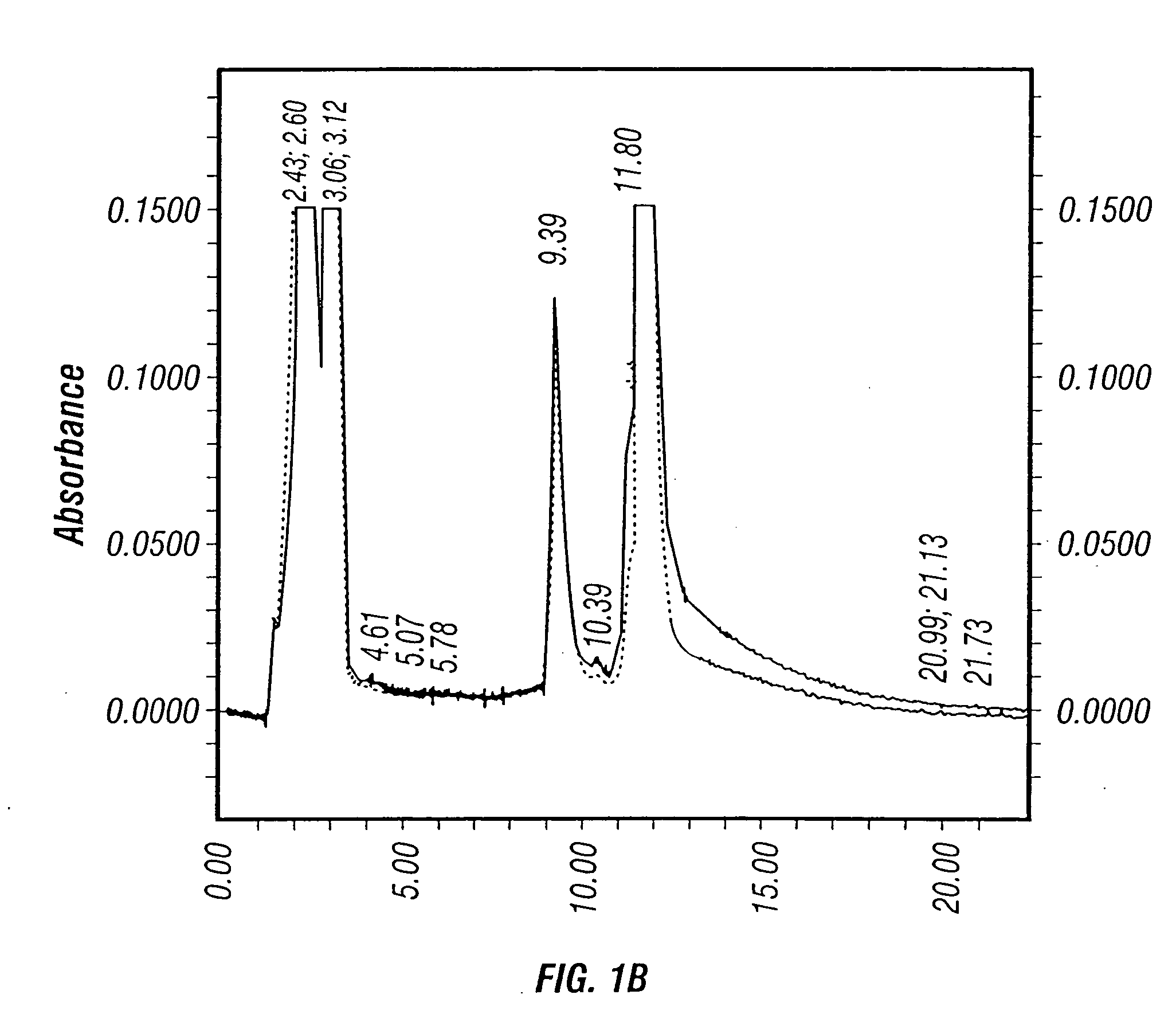
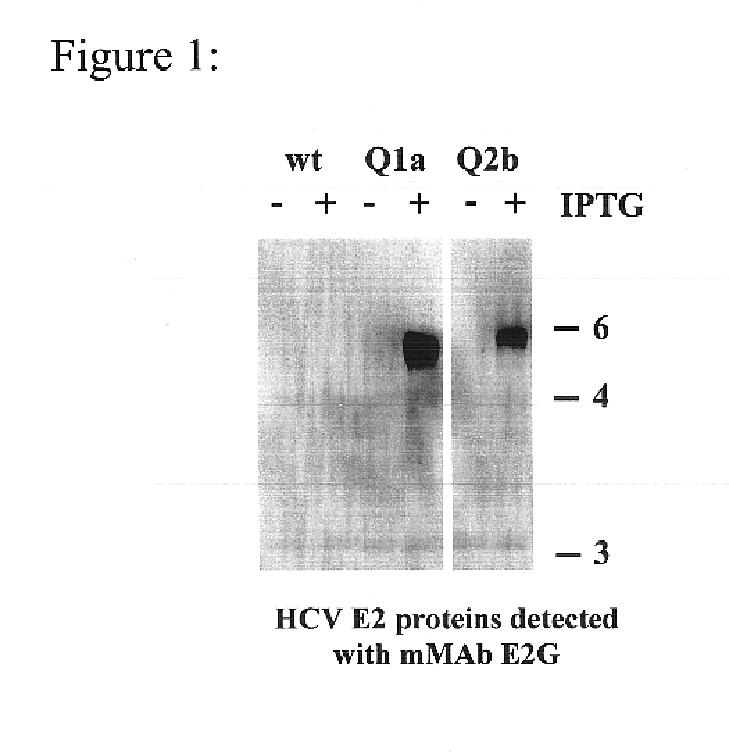
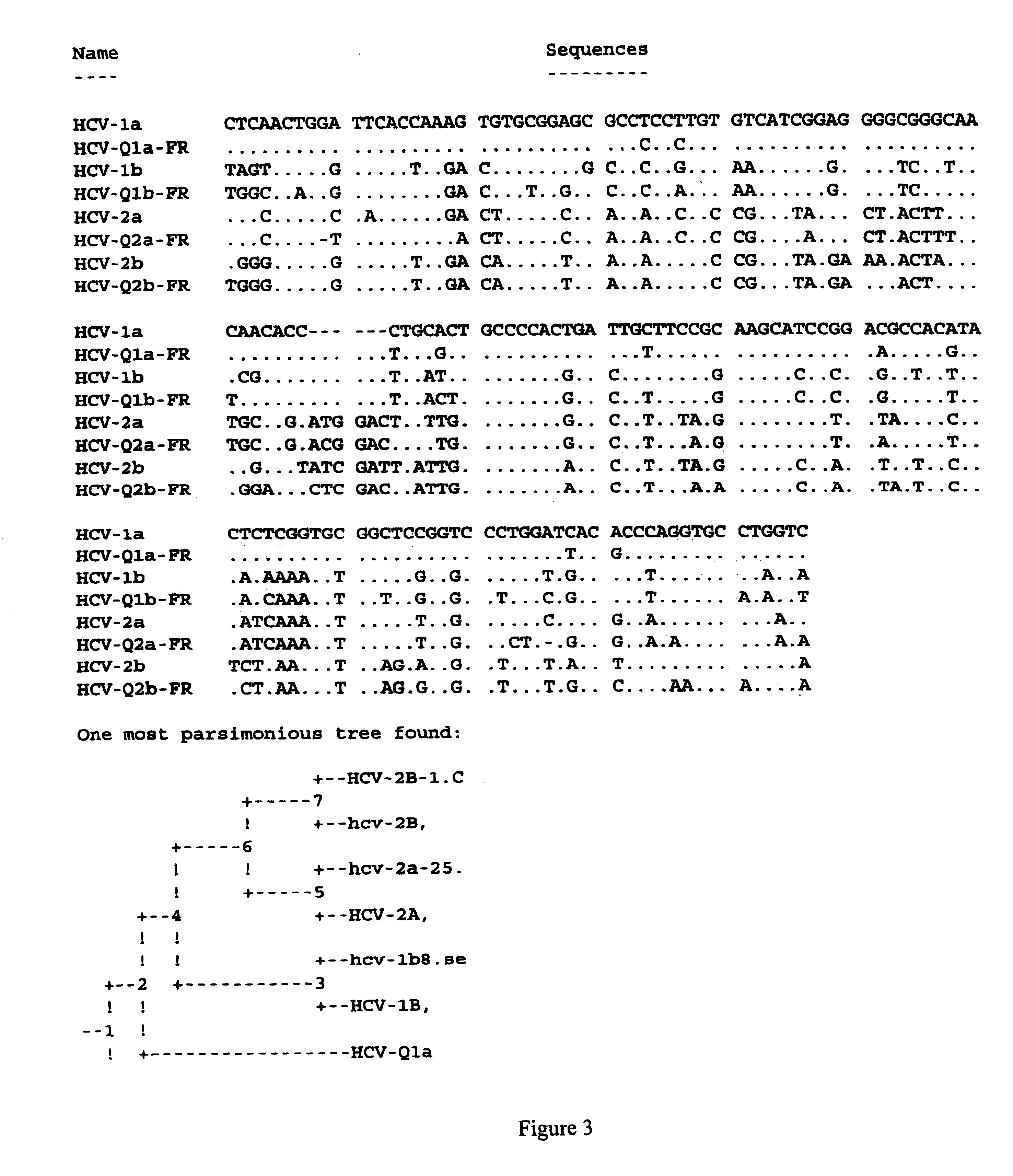
Prevention and treatment of HCV infection employing antibodies that inhibit the interaction of HCV virions with their receptor
Human monoclonal antibodies binding to epitopes common to type 1 and 2 HCV are provided, as well as conformationally conserved HCV E2 2a and 2b proteins. Compositions comprising the antibodies find use in diagnosis and therapy. The antibodies recognize conformational epitopes that are conserved across multiple genotypes of HCV. Thus the antibodies have the potential to be useful in the prevention and treatment of the majority of HCV infections. A subset of the antibodies (CBH-2, CBH-5, CBH-7, CBH-8C, CBH-8E, and CBH-11) have the ability to prevent the binding of HCV E2 proteins of multiple genotypes to human CD81, a possible co-receptor for HCV infection. A subset of the antibodies (CBH-2 and CBH-5) have been shown to inhibit the binding of HCV virions (as opposed to purified E2 protein) to human CD81. A further subset of the antibodies (CBH-4D, CBH4B, CBH-8C, and CBH-9) have been shown to prevent HCV envelope mediated fusion using an HCV psuedotype system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Retroviral vectors comprising a functional splice donor site and a functional splice acceptor site
InactiveUS7303910B2Efficient expressionIncrease rangeSsRNA viruses negative-senseFungiNucleotideNucleotide sequencing
A retroviral vector is described. The retroviral vector comprises a functional splice donor site and a functional splice acceptor site; wherein the functional splice donor site and the functional splice acceptor site flank a first nucleotide sequence of interest (“NOI”); wherein the functional splice donor site is upstream of the functional splice acceptor site; wherein the retroviral vector is derived from a retroviral pro-vector; wherein the retroviral pro-vector comprises a first nucleotide sequence (“NS”) capable of yielding the functional splice donor site and a second NS capable of yielding the functional splice acceptor site; wherein the first NS is downstream of the second NS; such that the retroviral vector is formed as a result of reverse transcription of the retroviral pro-vector.
Owner:OXFORD BIOMEDICA (UK) LTD
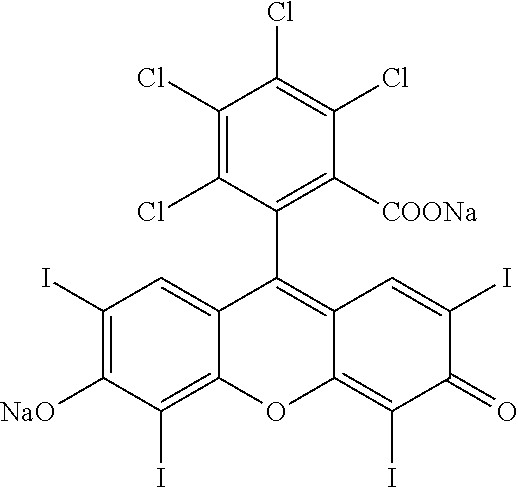
Combination of Local and Systemic Immunomodulative Therapies for Enhanced Treatment of Cancer
A method for the treatment of cancer comprising administration of a therapeutically effective amount of an intralesional chemoablative pharmaceutical composition, or variant of said composition, in combination with a therapeutically effective amount of a systemic immunomodulatory anticancer agent. A further method for the treatment of cancer comprising administration of a therapeutically effective amount of an intralesional chemoablative pharmaceutical composition, or variant of said composition, in combination with a therapeutically effective amount of a systemic targeted anticancer agent. The present invention is further directed to pharmaceutical compositions for treatment of cancer. The intralesional chemoablative pharmaceutical composition can comprise an IL chemoablative agent comprising primarily a halogenated xanthene.
Owner:PFIZER INC +1
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
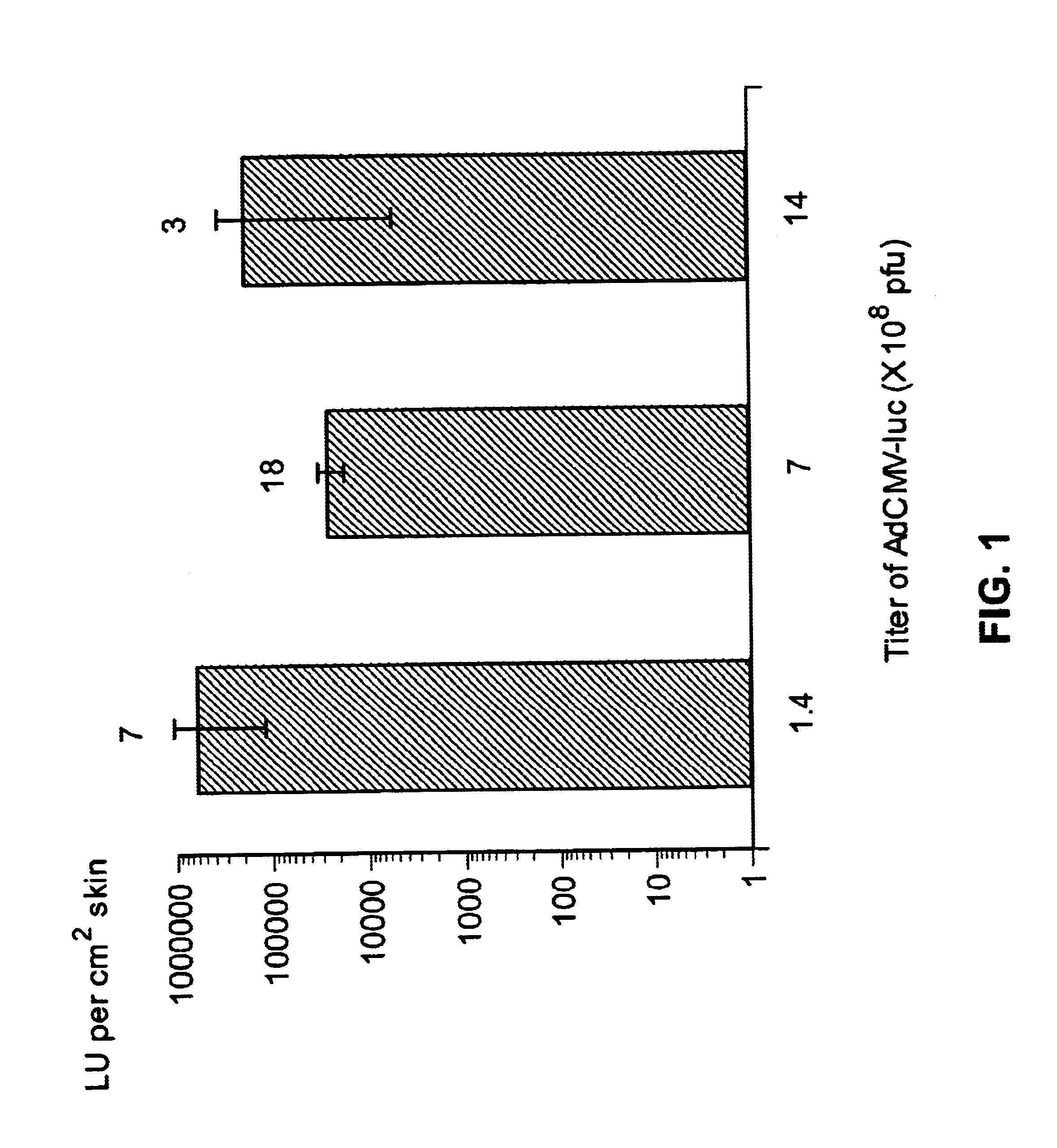
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapeutic delivery compositions and methods of use thereof
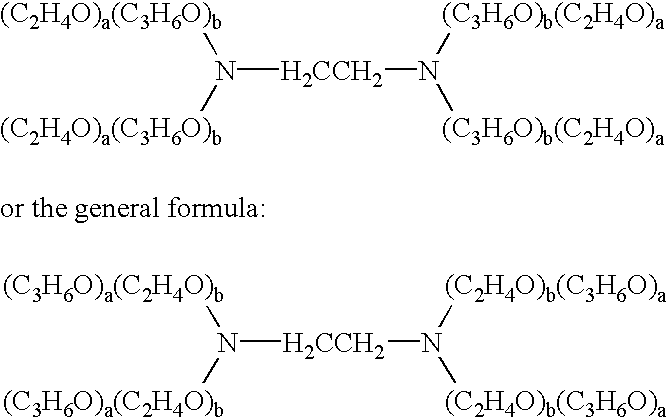
InactiveUS20020128218A1Reduce deliveryFacilitate transmission and introductionBiocidePeptide/protein ingredientsHydrophileNucleic acid sequencing
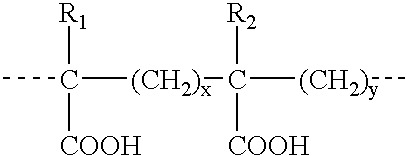
The present invention relates to compositions and methods for treating infectious diseases and genetic disorders through gene therapy and intracellular delivery of antisense oligonucleotides or other nucleic acid sequences. The present invention comprises a therapeutic delivery composition effective for treating a disease state comprising an administerable admixture of an effective amount of a therapeutic compound capable of altering nucleic acid sequence function and an effective amount of a block copolymer having the following general formula: 1 wherein: the mean aggregate molecular weight of the portion of the octablock copolymer represented by polyoxypropylene is between about 5000 and about 7000 Daltons; a is a number such that the portion represented by polyoxyethylene constitutes between about 10% to about 40% of the compound by weight; and b is a number such that the polyoxypropylene portion of the total molecular weight of the octablock copolymer constitutes between about 60% and about 90% of the compound by weight. The present invention also includes compositions and methods using biologically active nonionic reverse block copolymers. The reverse copolymers have an inner core of polyoxypropylene (POP) that is flanked on either end by polyoxyethylene (POE). The reverse block copolymers have the following formula: 2 wherein "b" represents a number such that the molecular weight of the hydrophobe (C.sub.3H.sub.6O).sub.b is between approximately 2,000 and 10,000, and "a" represents a number such that the percentage of hydrophile (C.sub.2H.sub.4O).sub.a is between approximately 5% and 30%.
Owner:EMANUELE R MARTIN +3
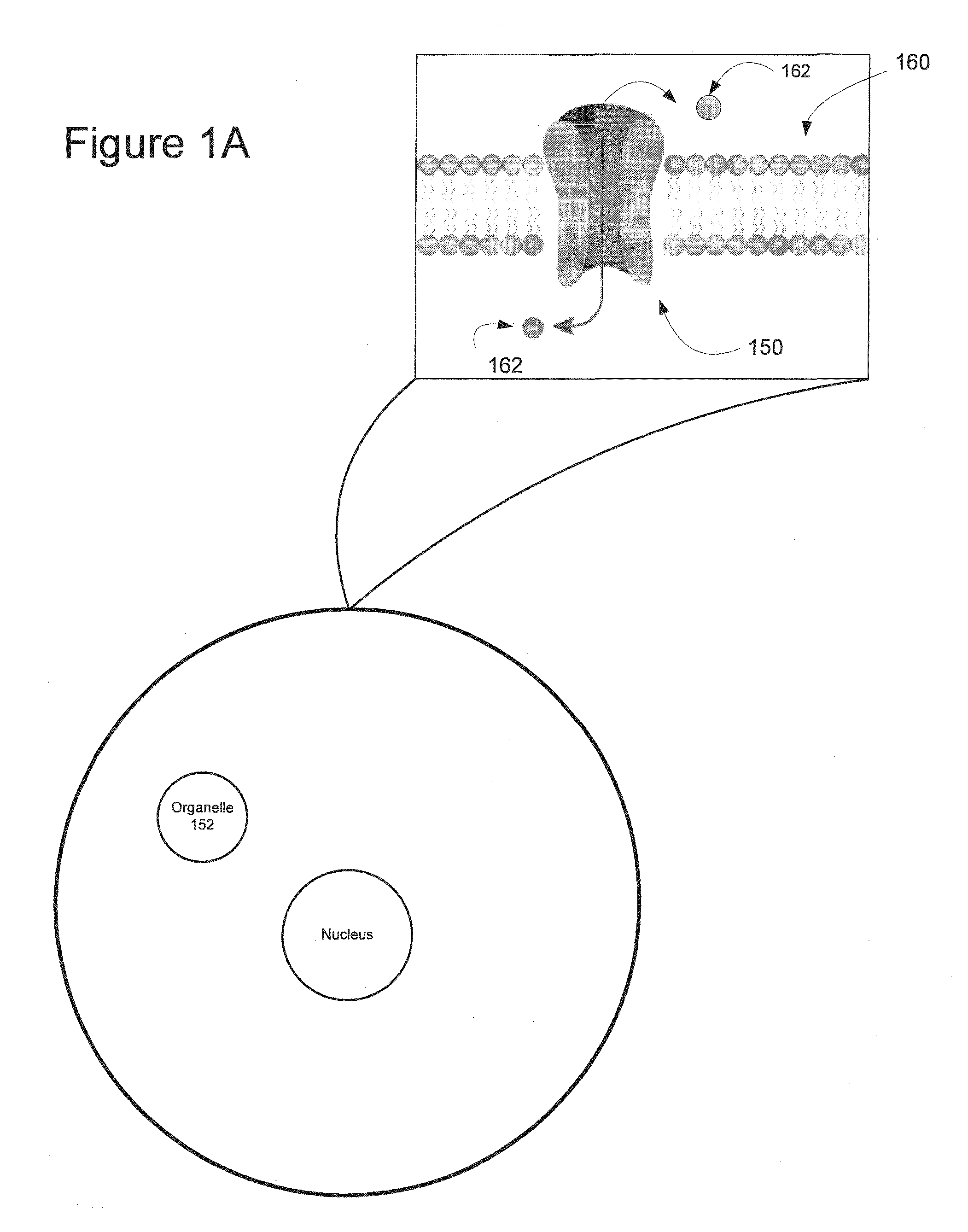
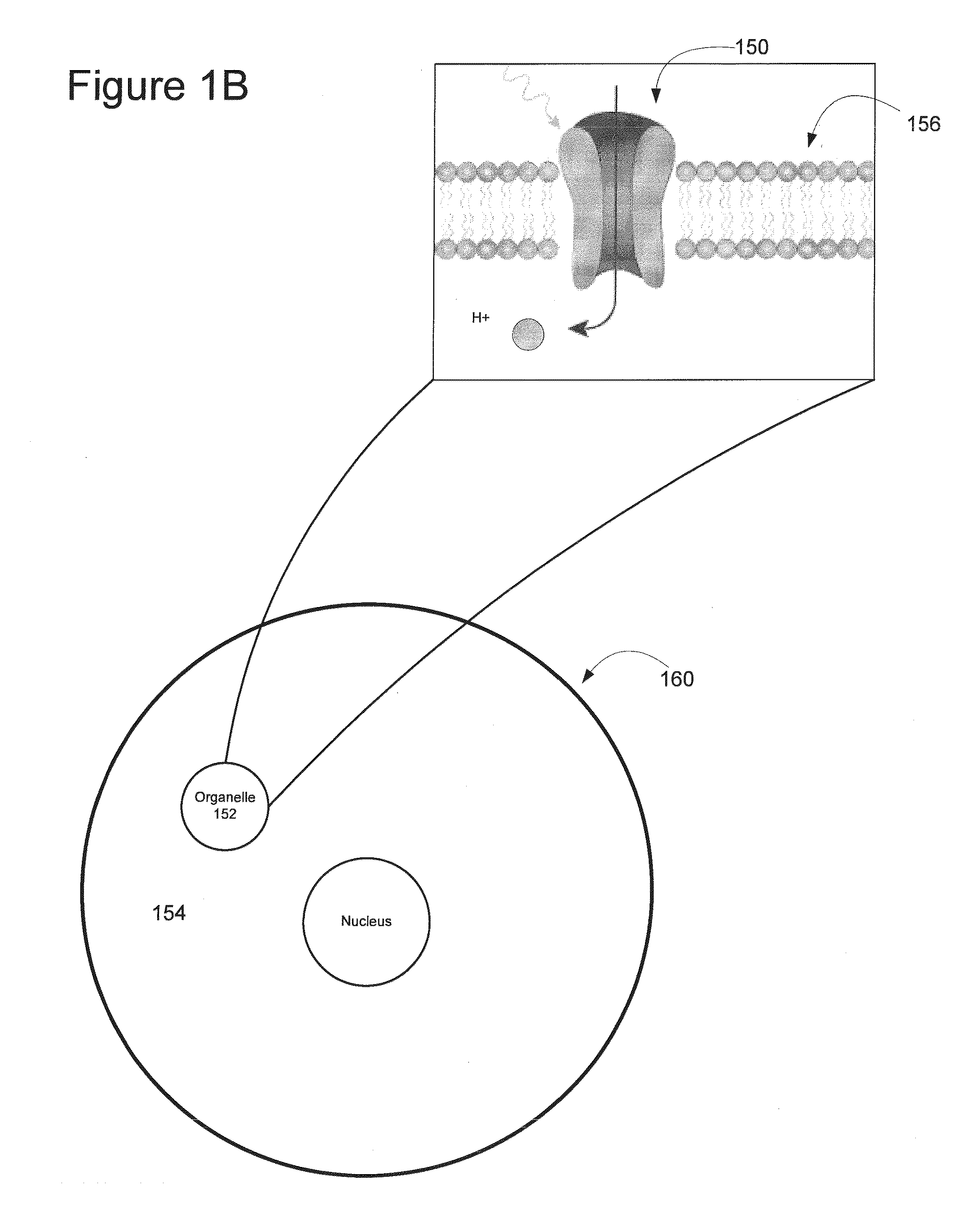
Light-Sensitive Ion-Passing Molecules
ActiveUS20130019325A1Dissuade depolarizationIncrease probabilityNervous disorderPeptide/protein ingredientsOptical stimulationLight activated
Disclosed are polynucleotides and methods for expressing light activated proteins in animal cells and altering an action potential of the cells by optical stimulation. The disclosure also provides animal cells and non-human animals comprising cells expressing the light-activated proteins.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
AAV vectors produced in insect cells
ActiveUS8163543B2Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
InactiveUS20070014810A1Reduce the possibilityImproving immunogenicitySugar derivativesViral antigen ingredientsEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:GENIMMUNE NV +1
Mutant cholera holotoxin as an adjuvant
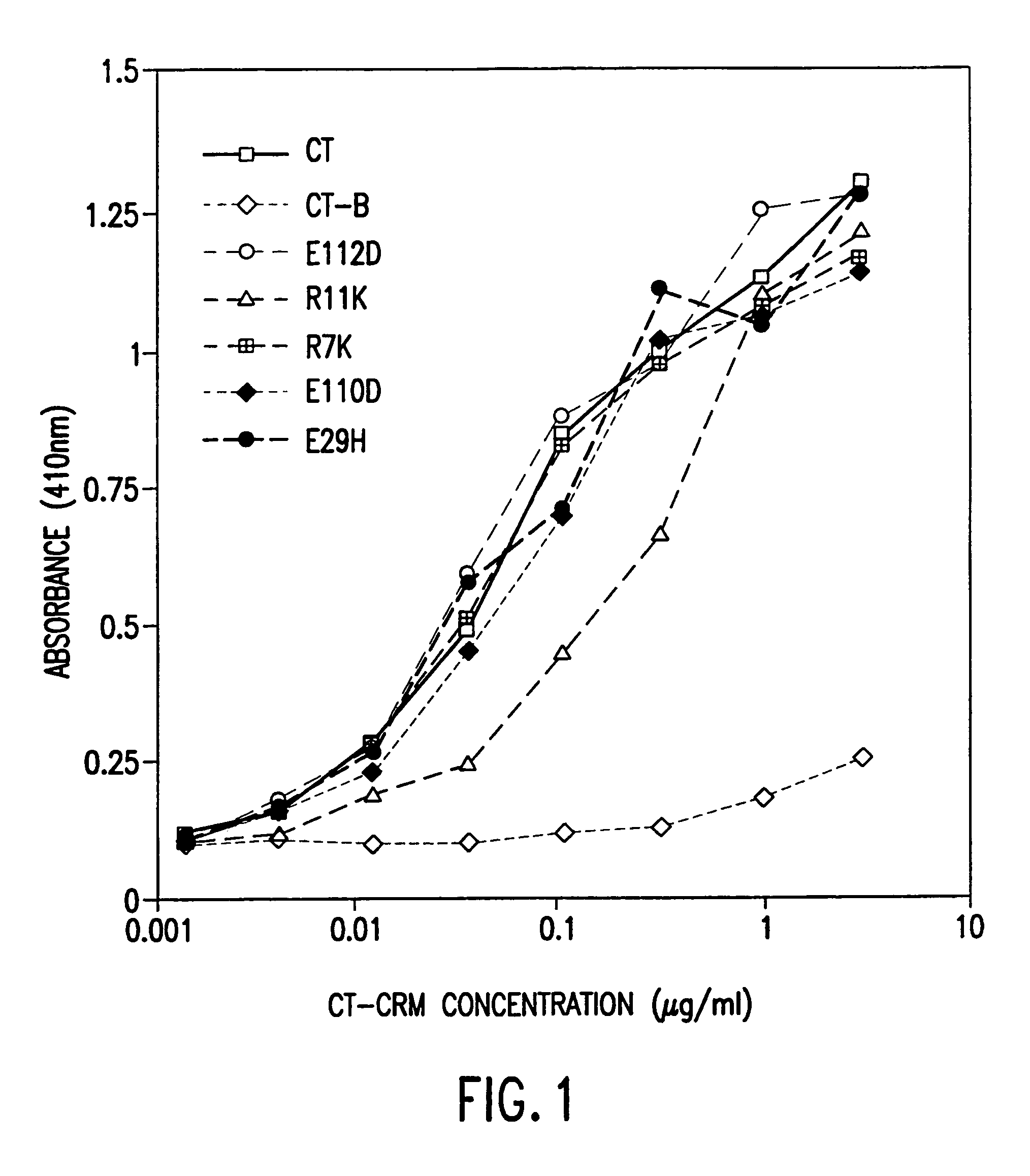
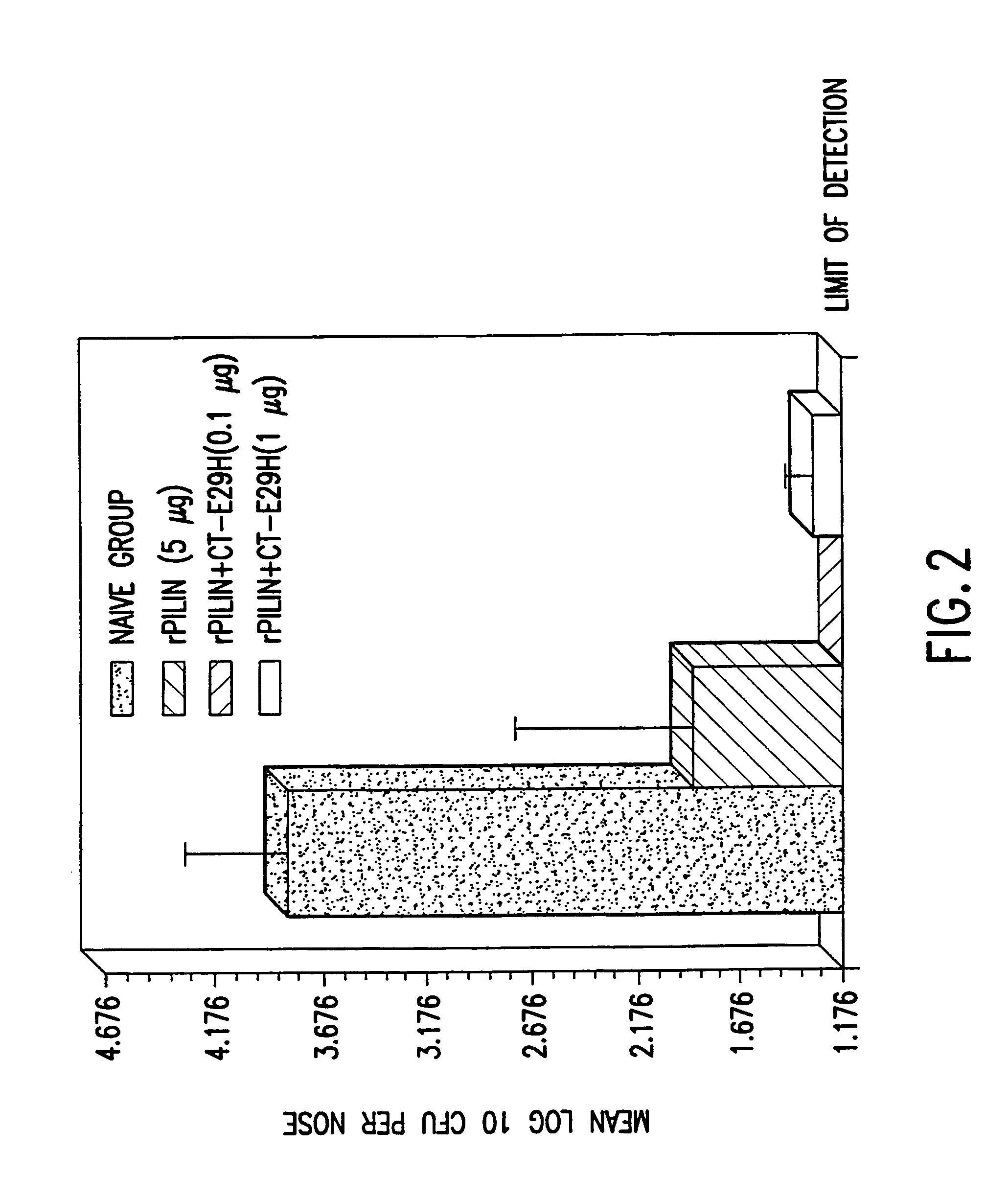
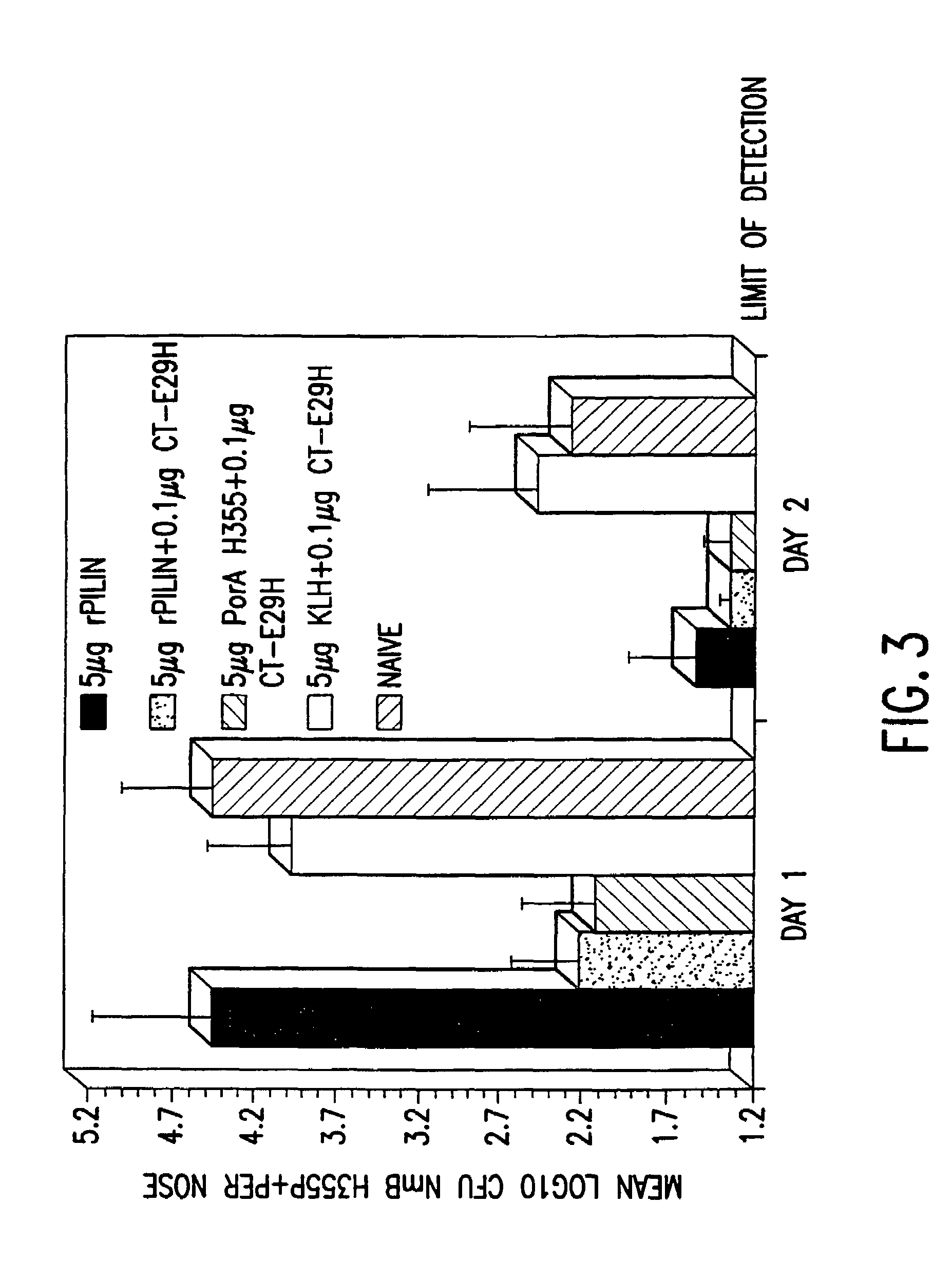
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Vaccine delivery compositions and methods of use
InactiveUS20080160089A1Easy to produceImprove efficiencySsRNA viruses negative-sensePowder deliveryPolyesterMHC class I
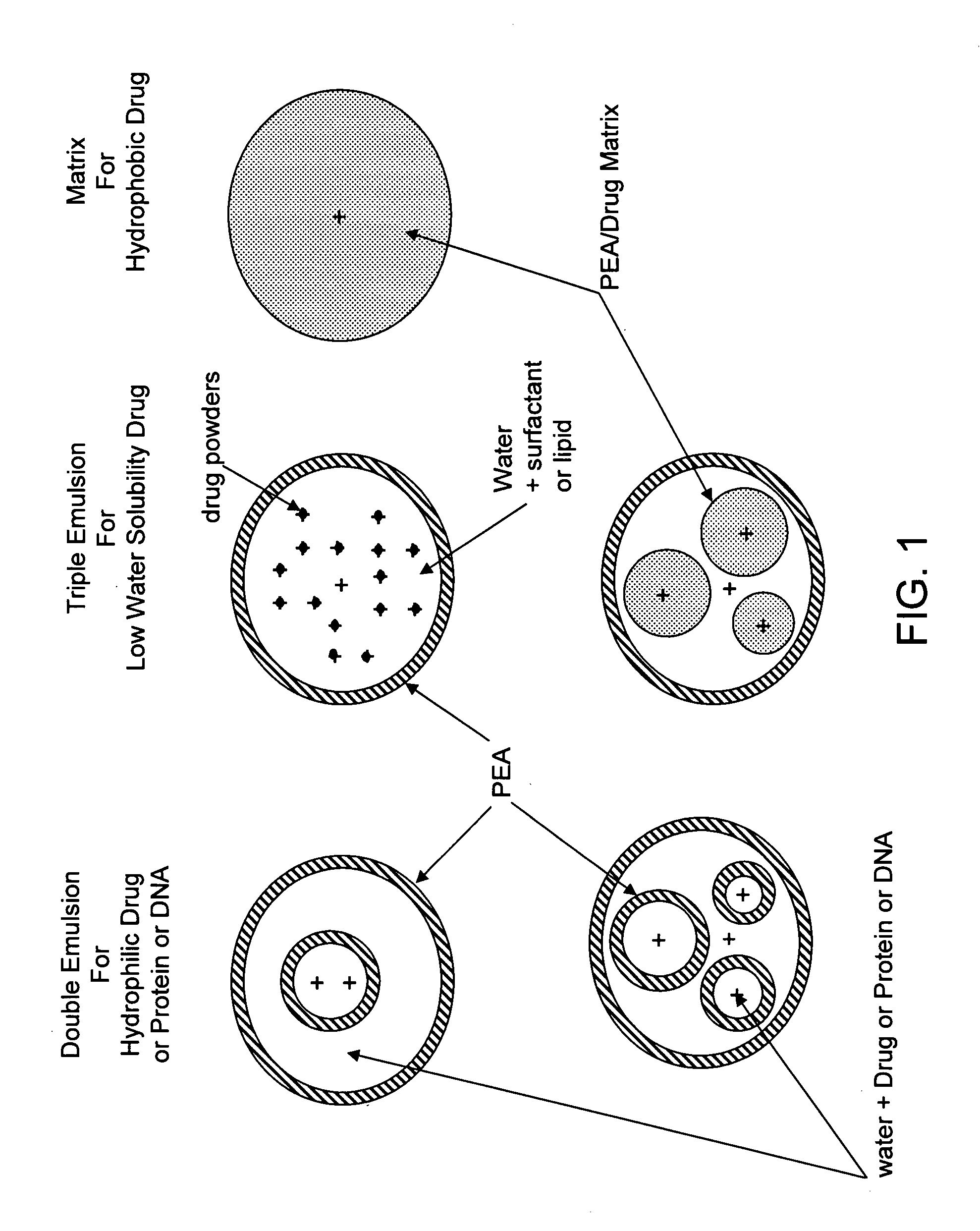
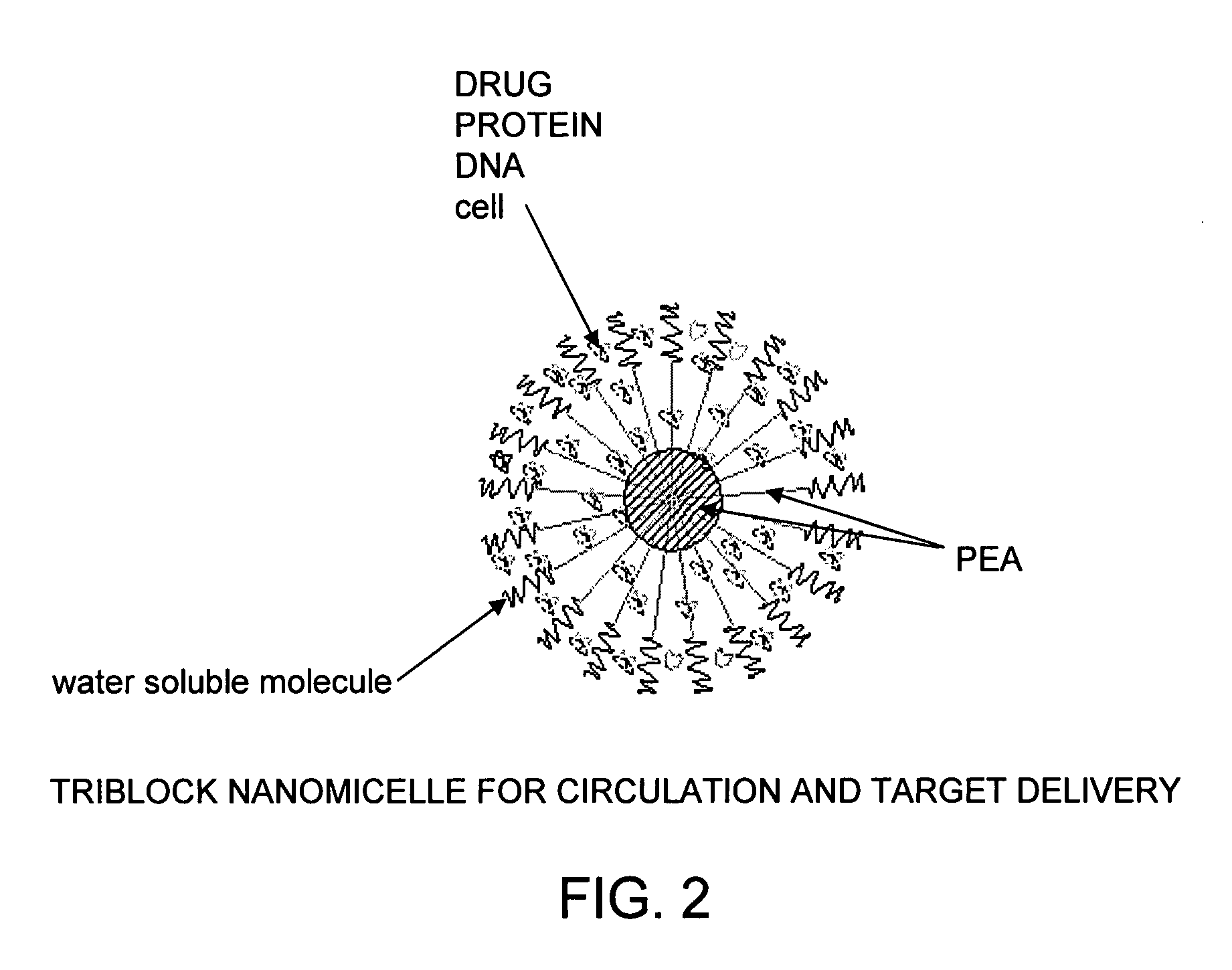
The present invention provides synthetic vaccines against a variety of pathogenic organisms and tumor cells in humans and other mammals based on biodegradable polymers containing polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) and immunostimulatory adjuvants. The vaccines can be formulated as a liquid dispersion of polymer particles or molecules in which are dispersed an immunostimulatory adjuvant, such as a TLR agonist, and whole protein or peptidic antigens containing MHC class I or class II epitopes derived from organism or tumor cell proteins. Methods of inducing an immune response via intracellular mechanisms to the pathogenic organism or tumor cells specific for the antigen in the invention compositions are also included.
Owner:MEDIVAS LLC
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
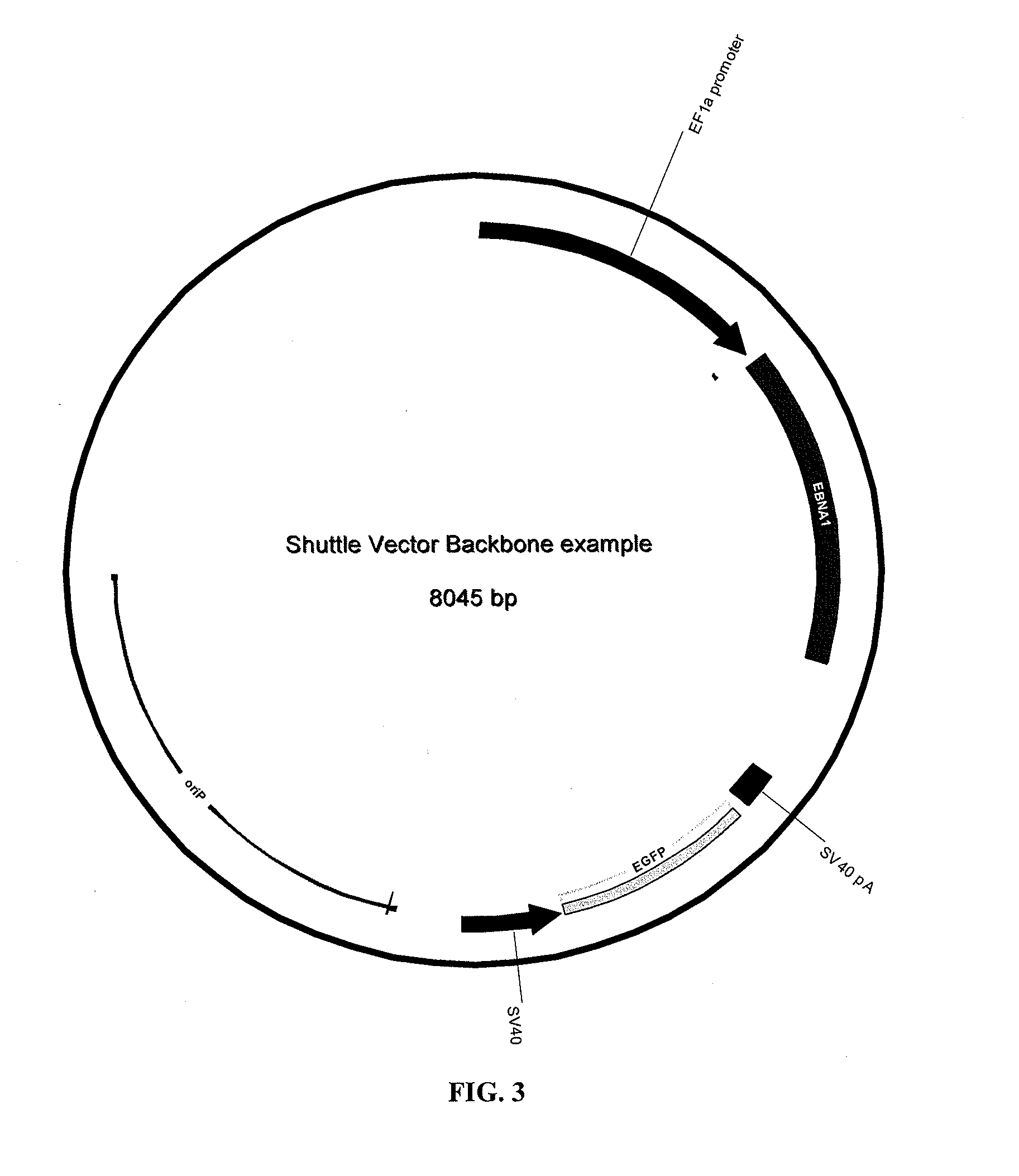
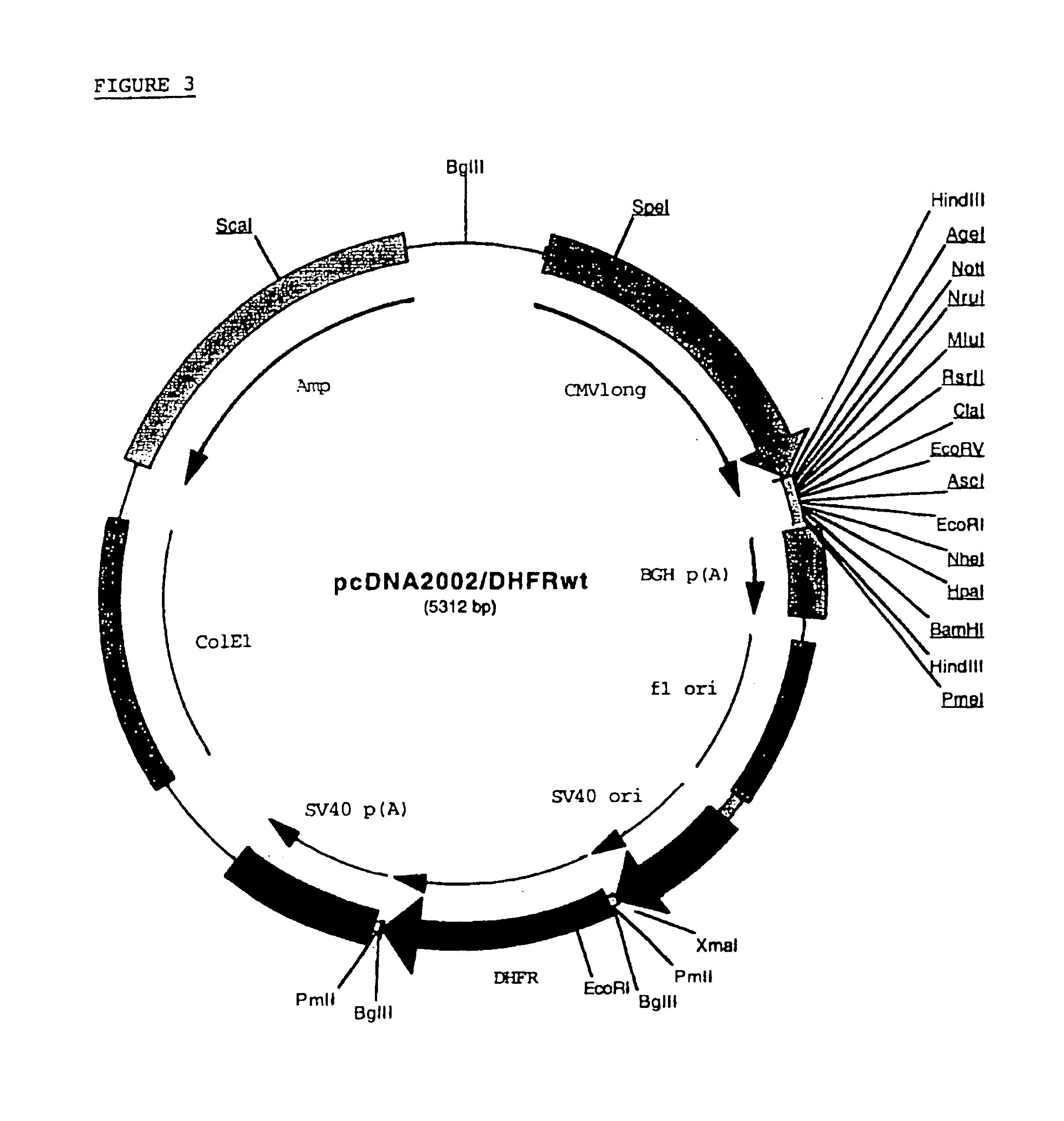
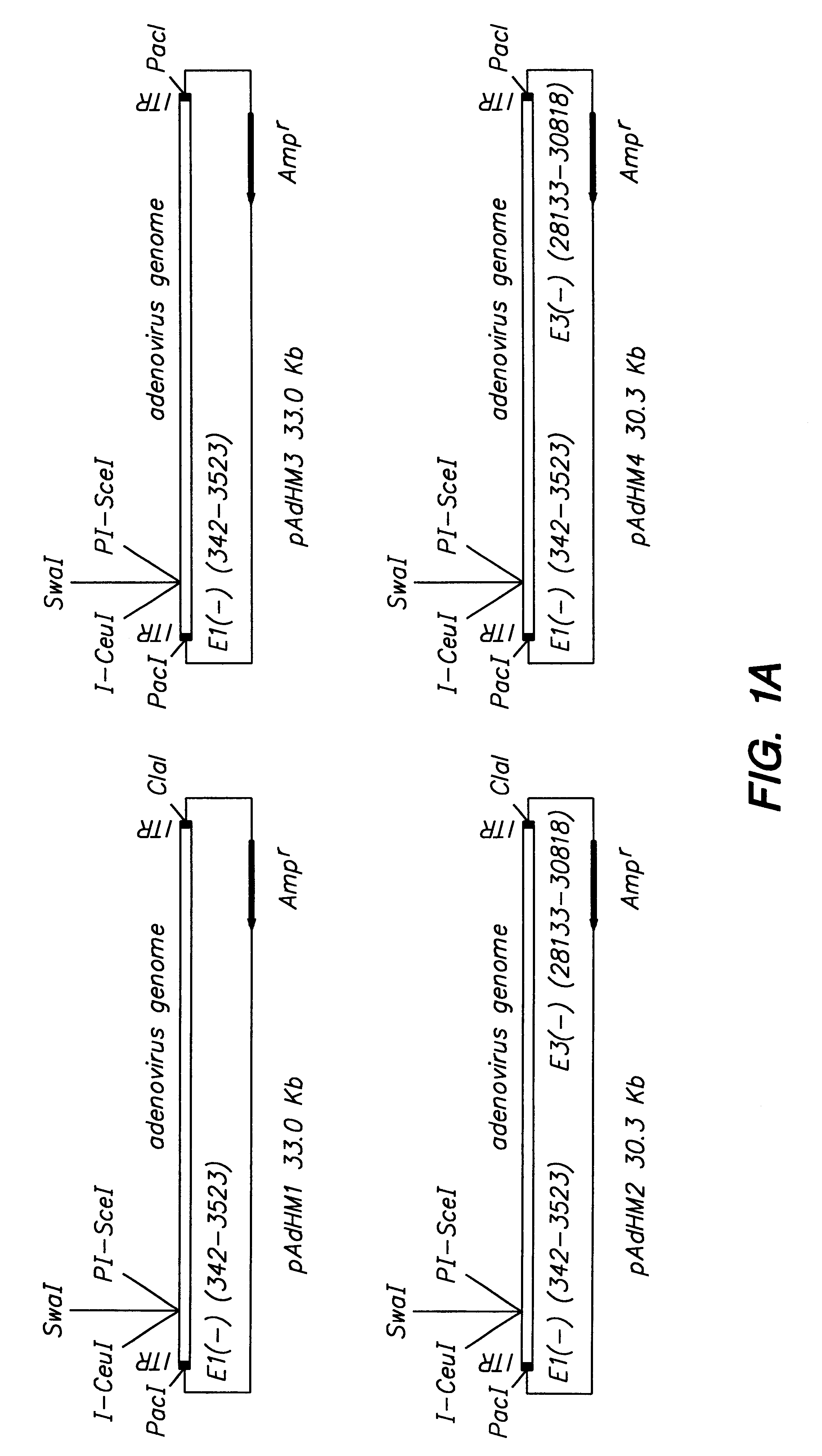
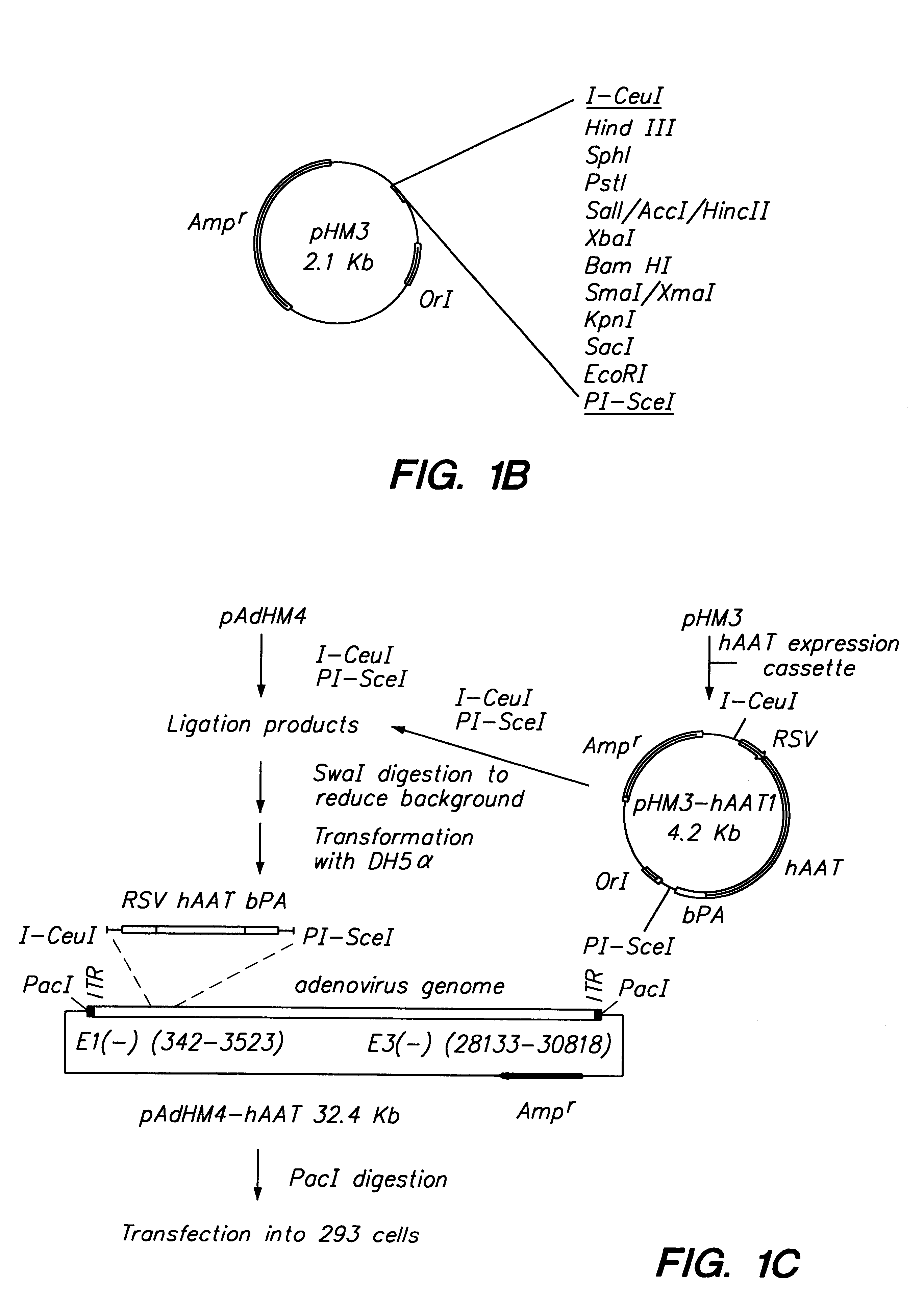
Adenoviral vector and methods for making and using the same
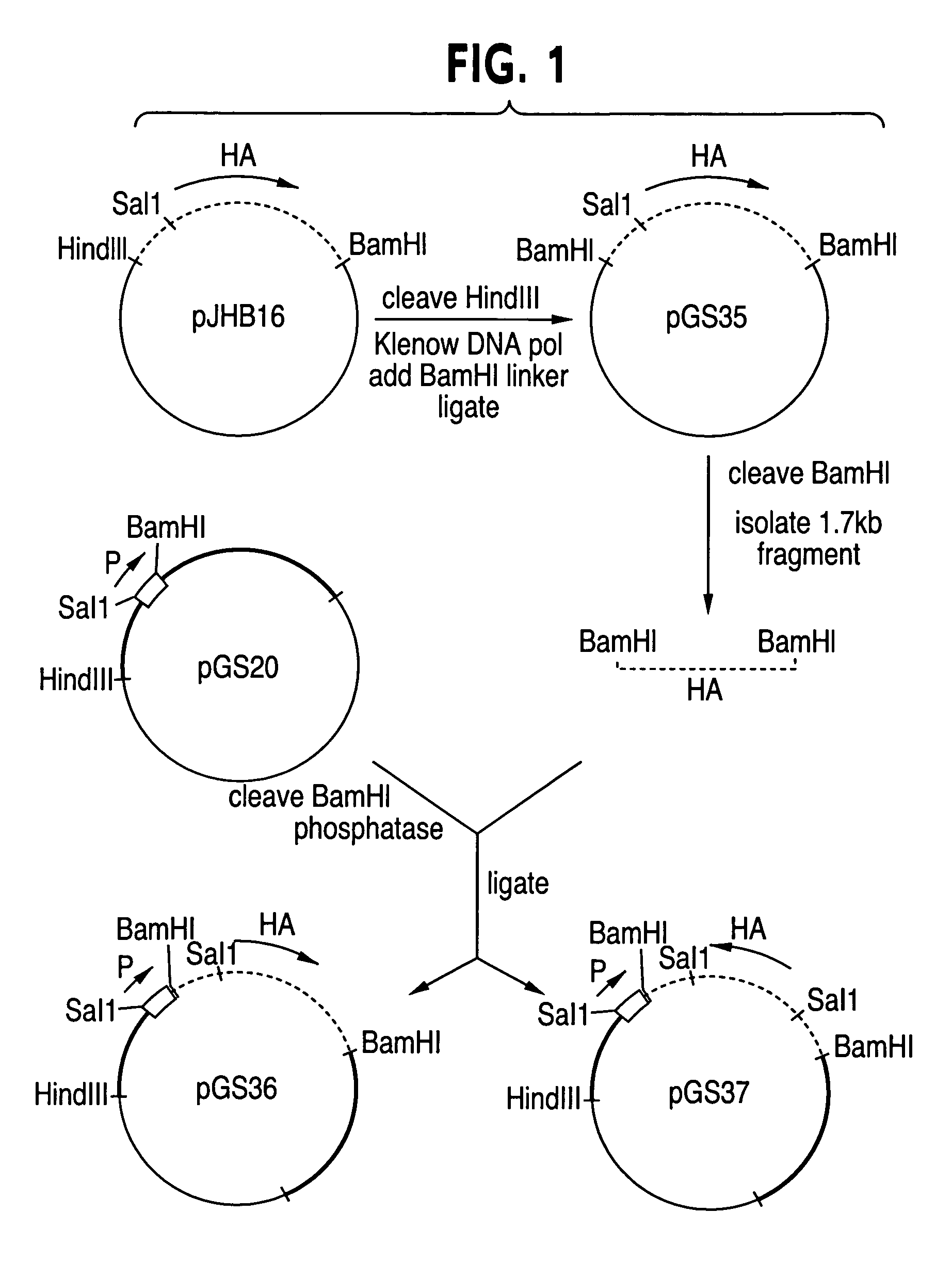
In vitro methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from first and second vectors. The first vector includes an adenoviral genome having an E region deletion and three different, non-adenoviral restriction endonuclease sites located in the E region. The second vector is a shuttle vector and includes an insertion nucleic acid flanked by two of the three different non-adenoviral restriction endonucleases sites present in the first vector. Cleavage products are prepared from the first and second vectors using the appropriate restriction endonucleases. The resultant cleavage products are then ligated to produce the subject recombinant adenovirus genome. The subject adenoviral genomes find use in a variety of application, including as vectors for use in a variety of applications, including gene therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com