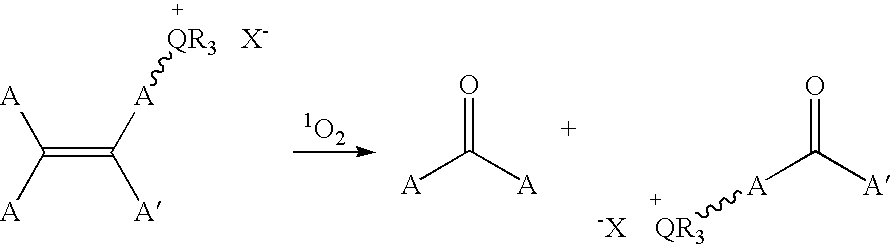
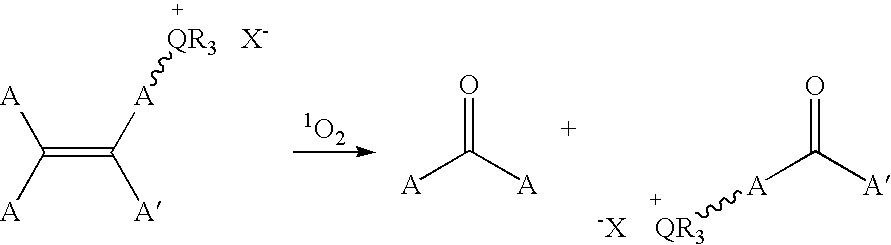
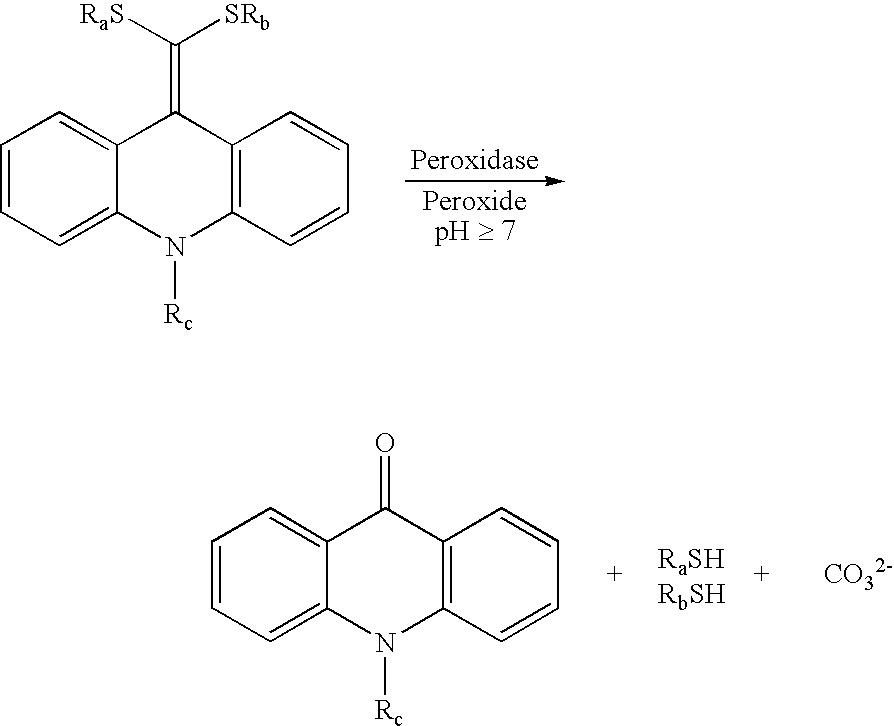
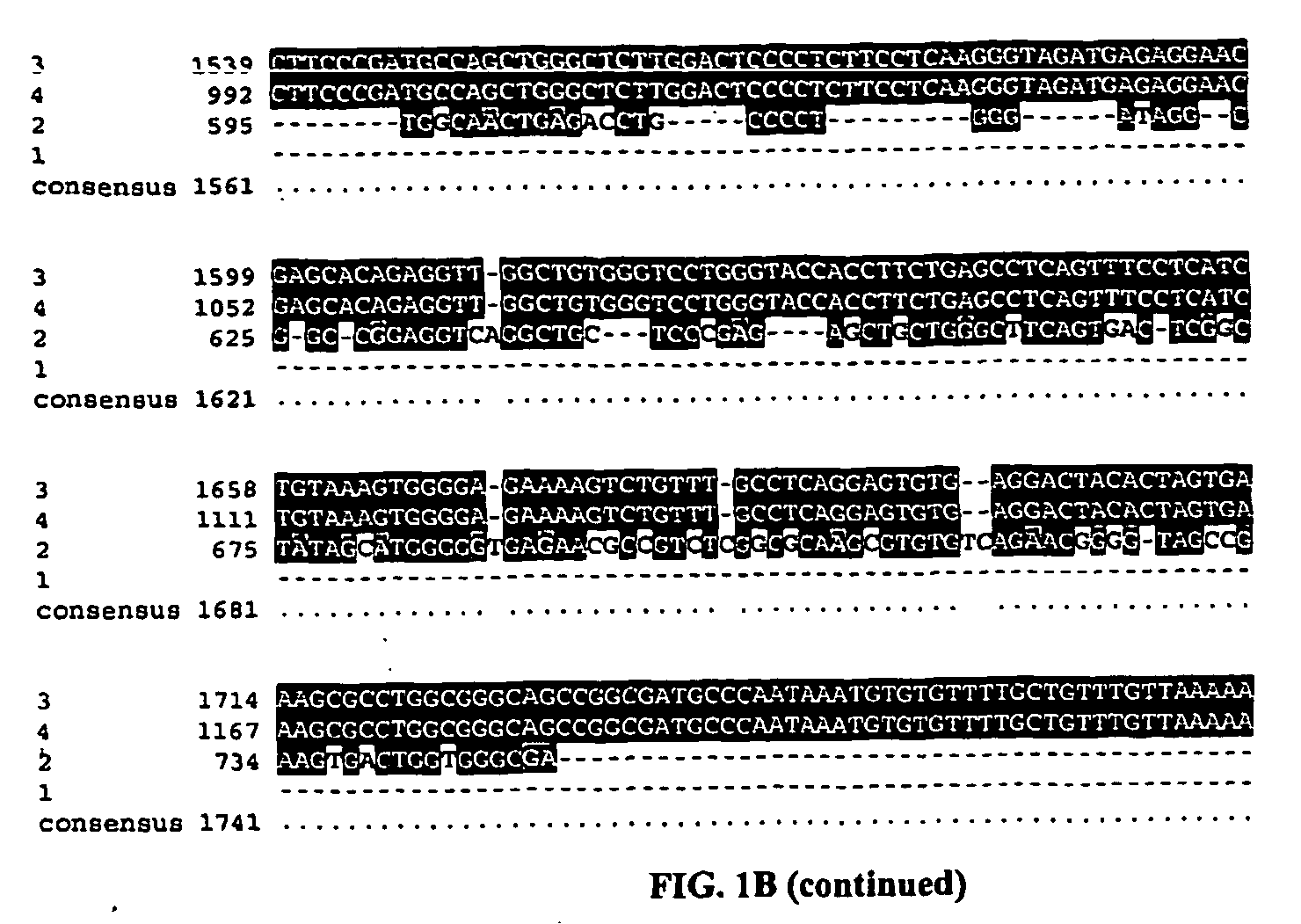
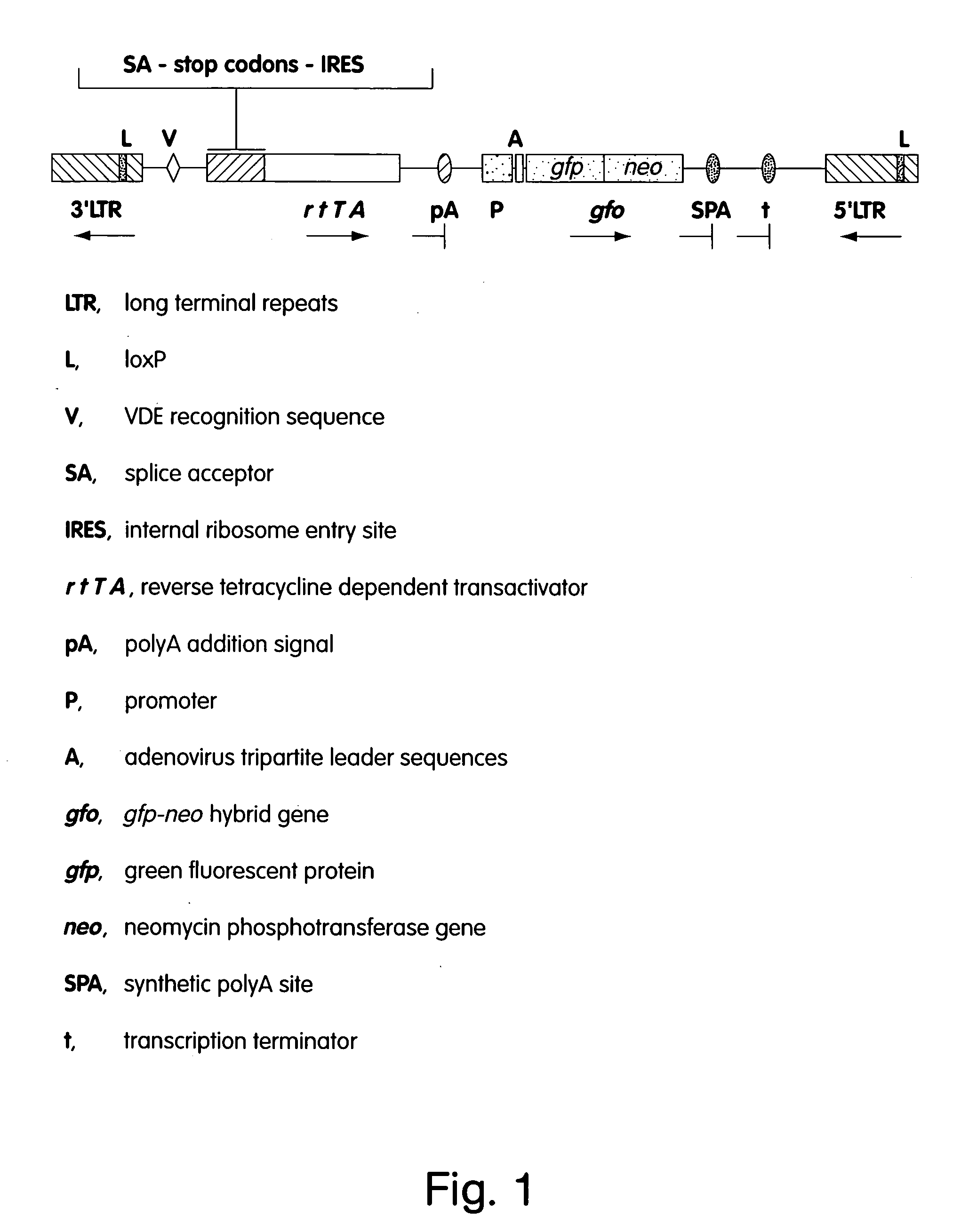
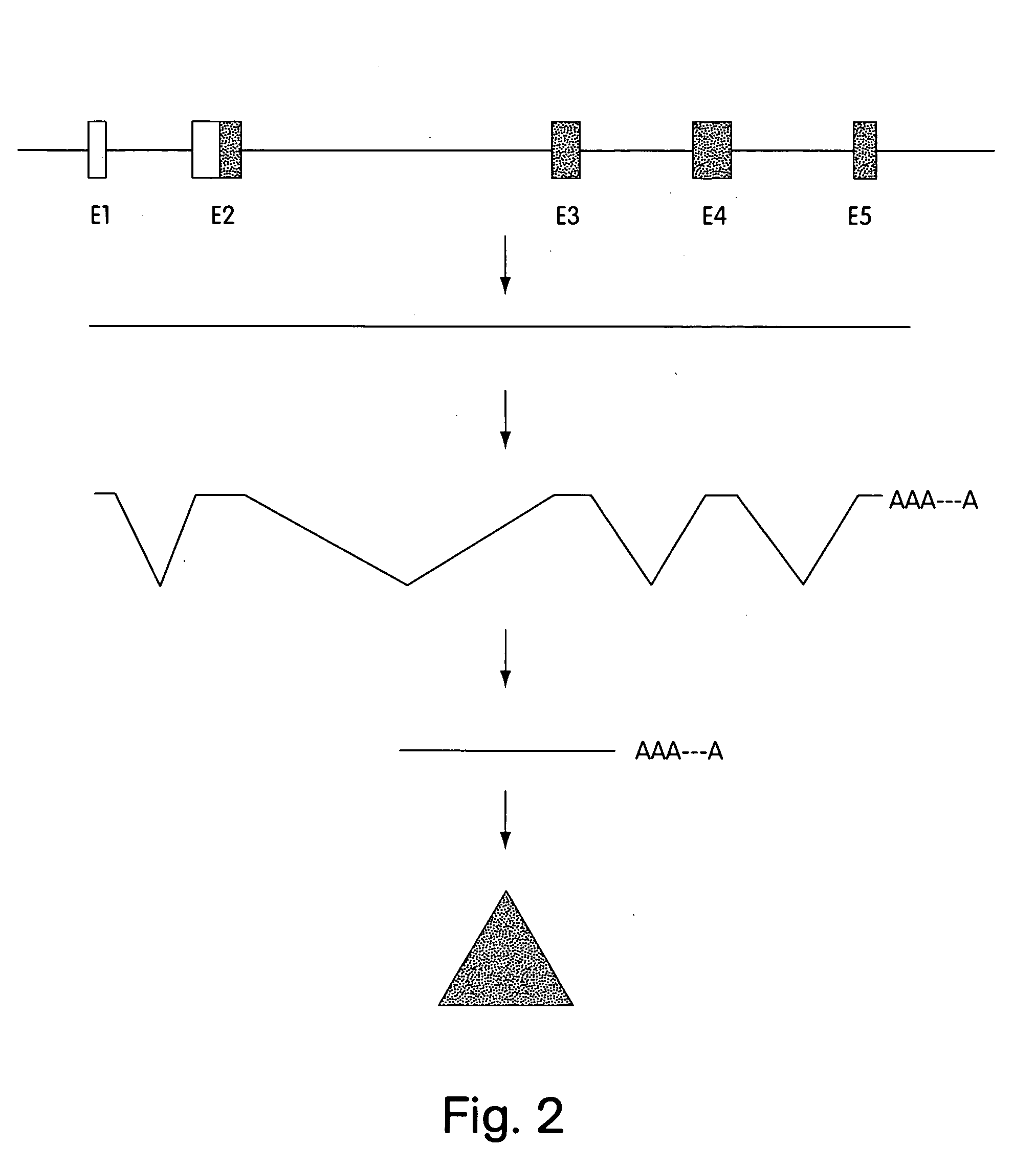
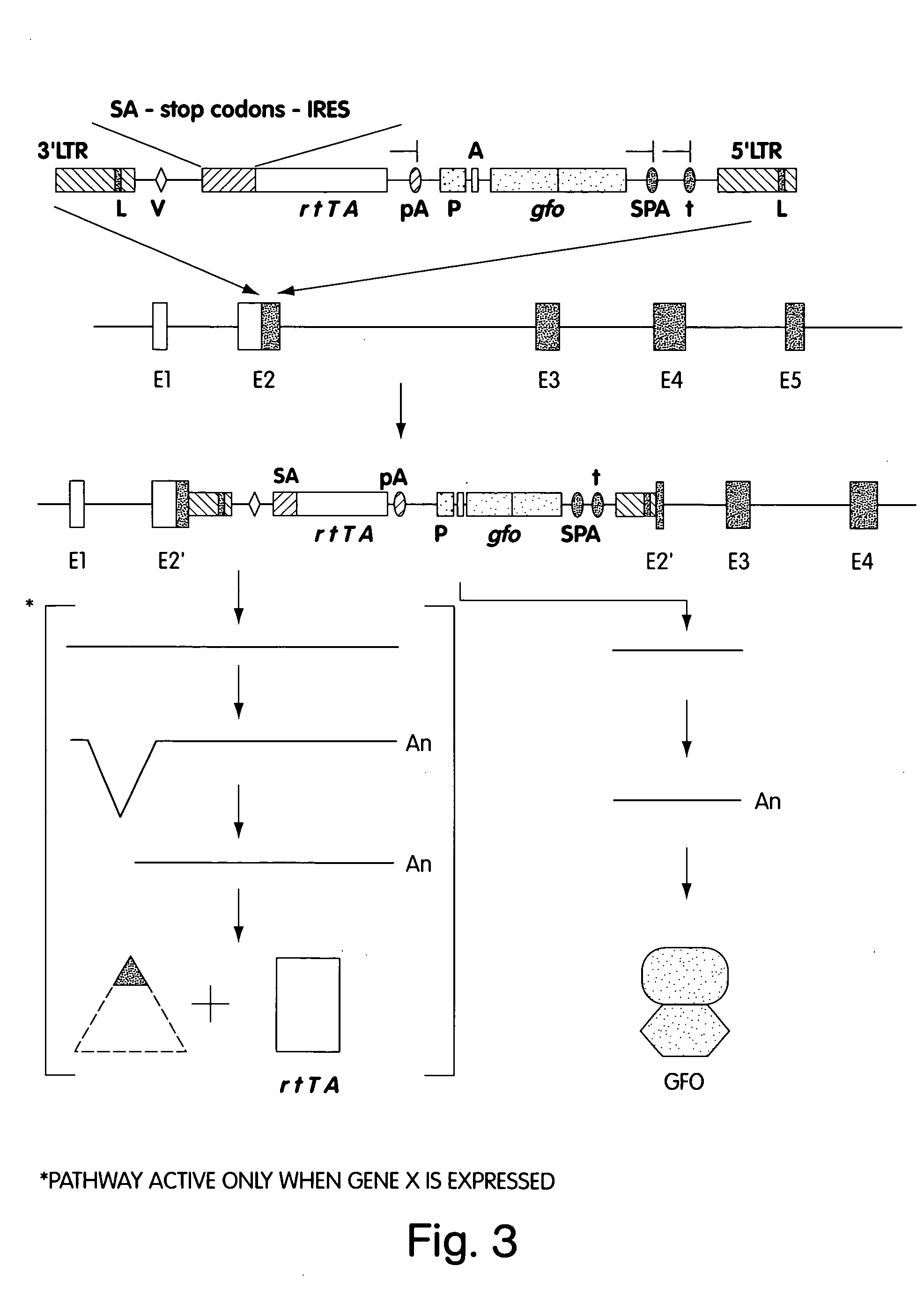
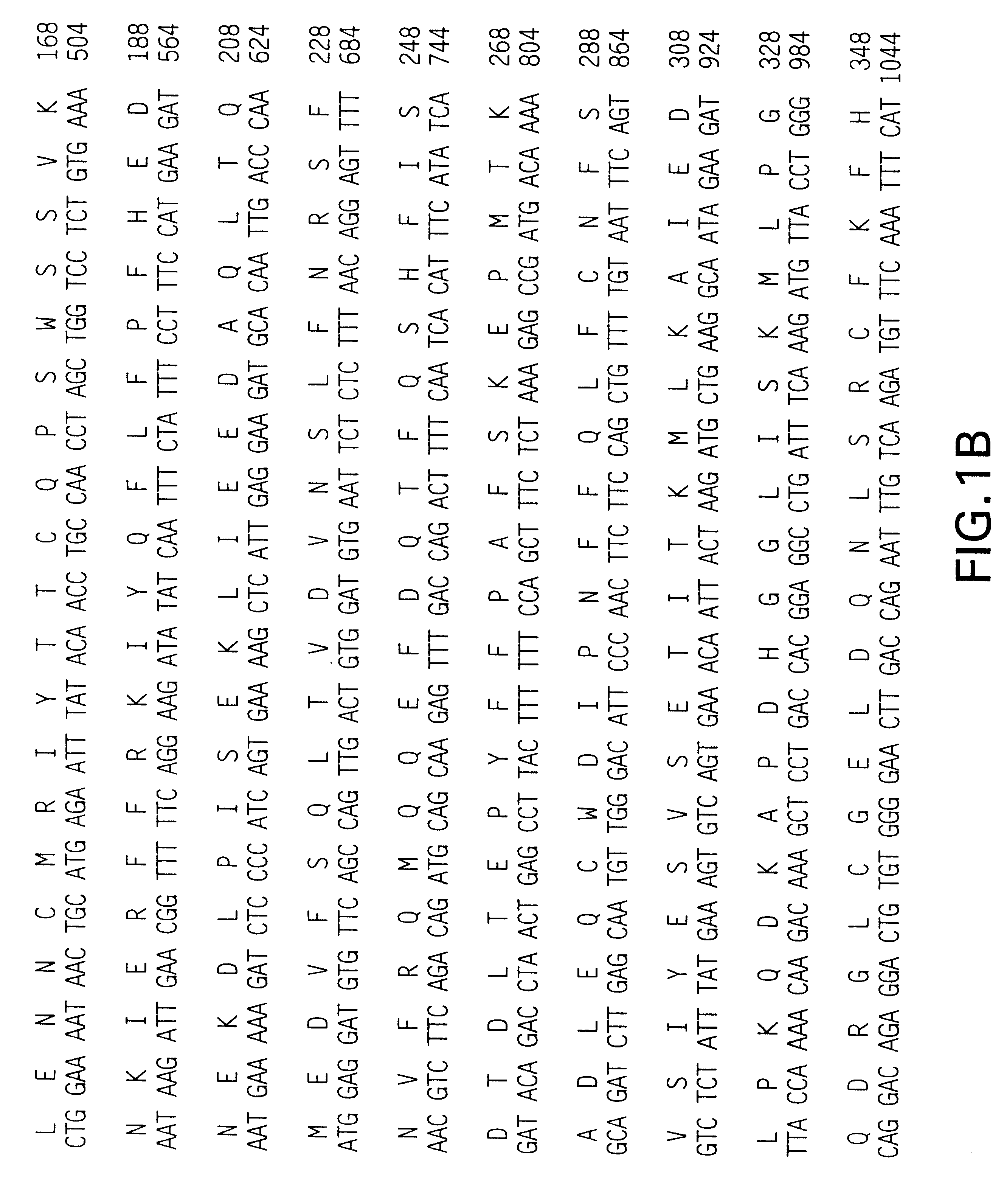
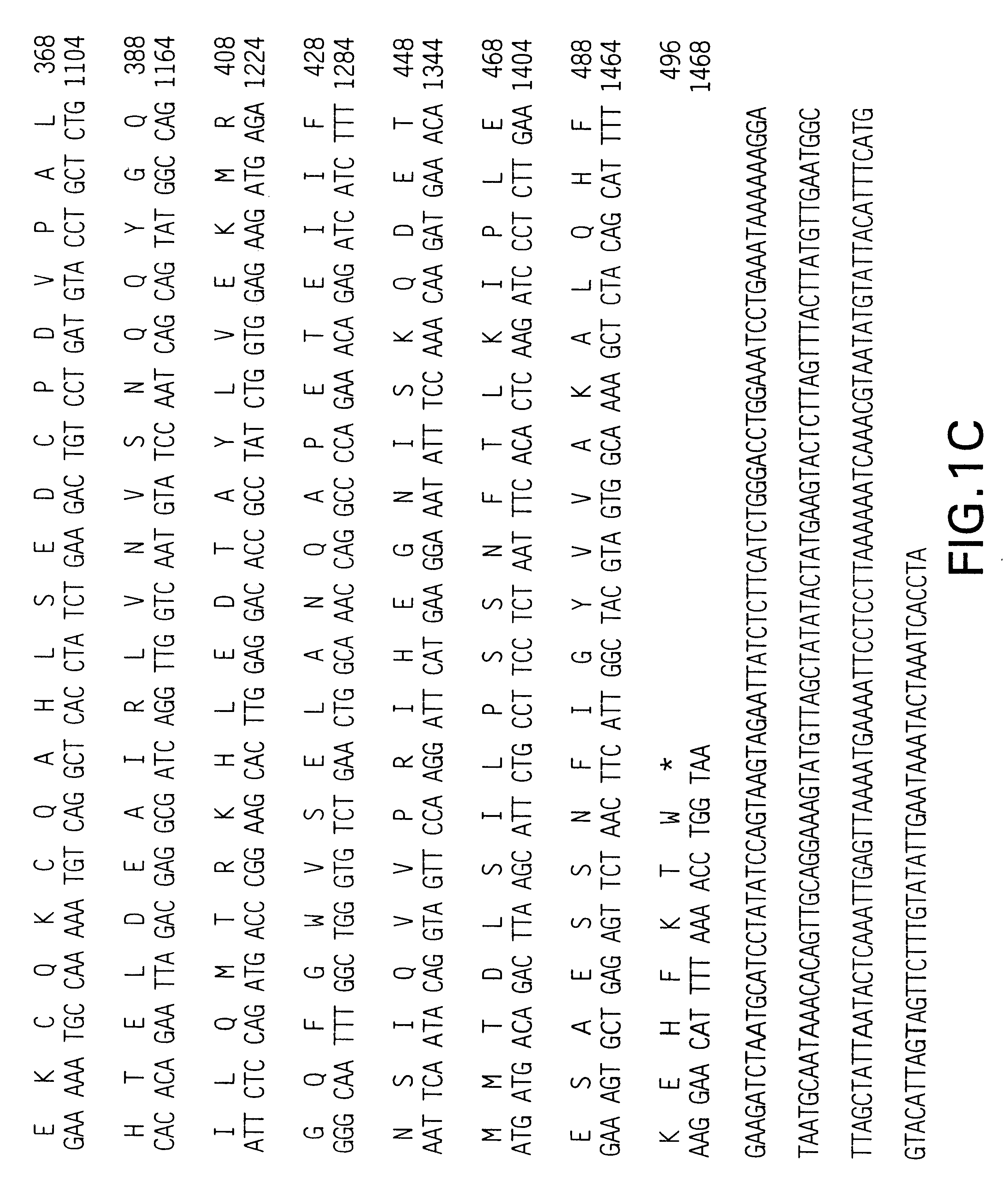
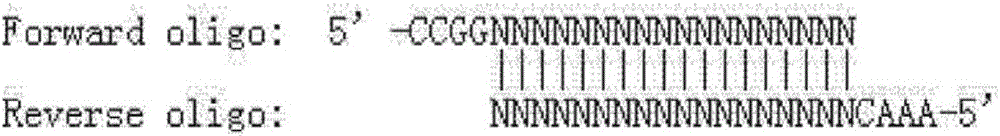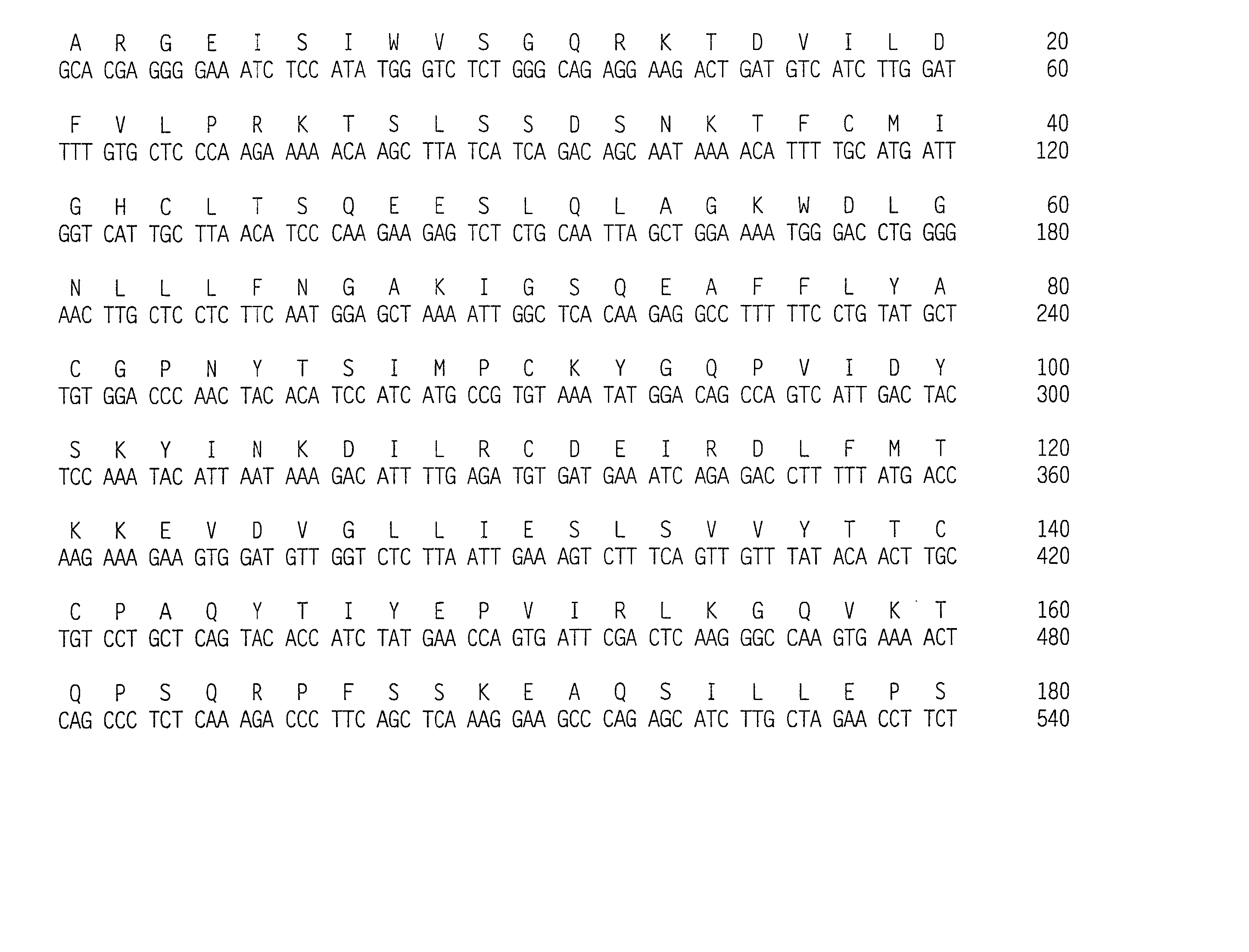Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Mammalian gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
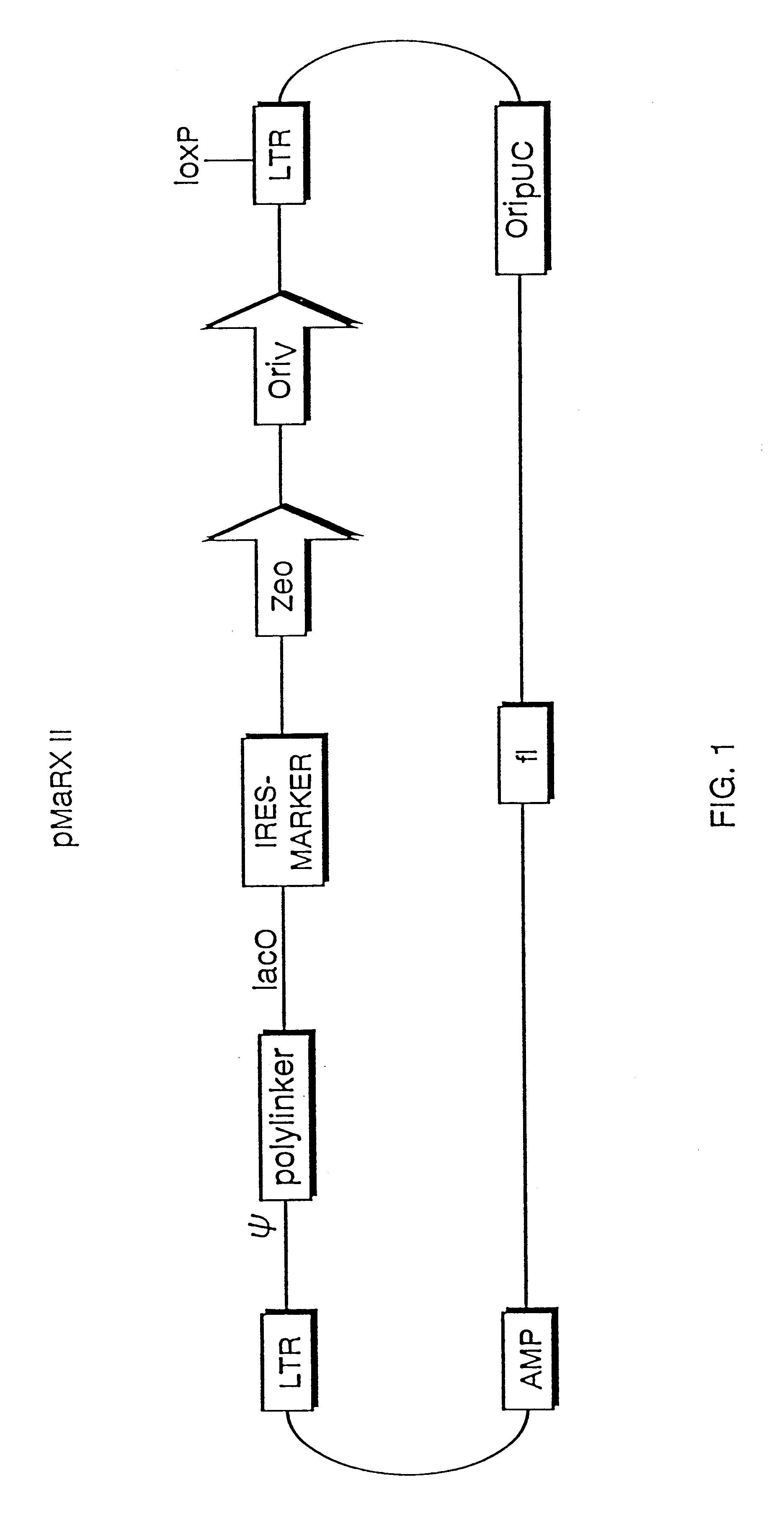
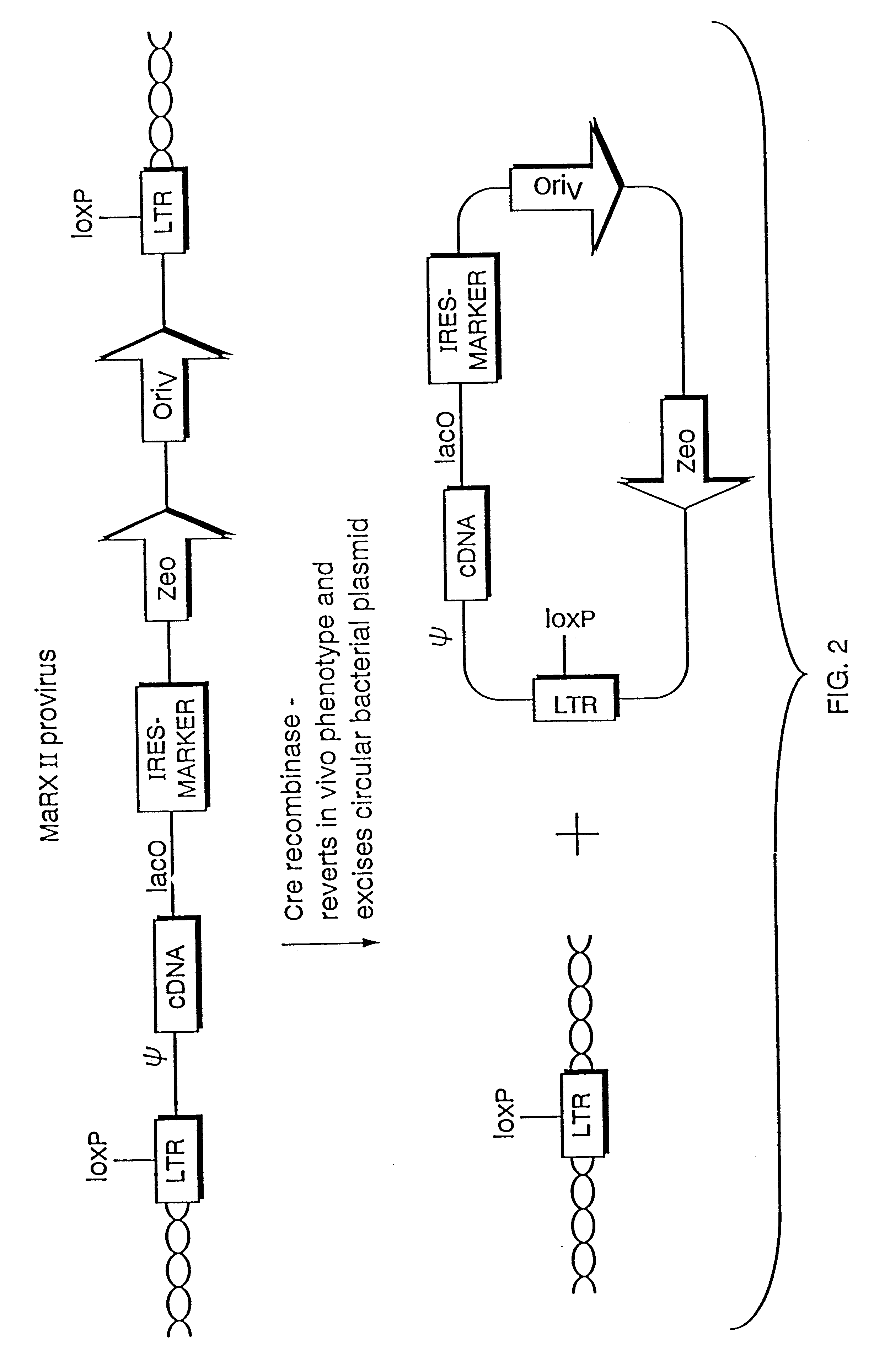
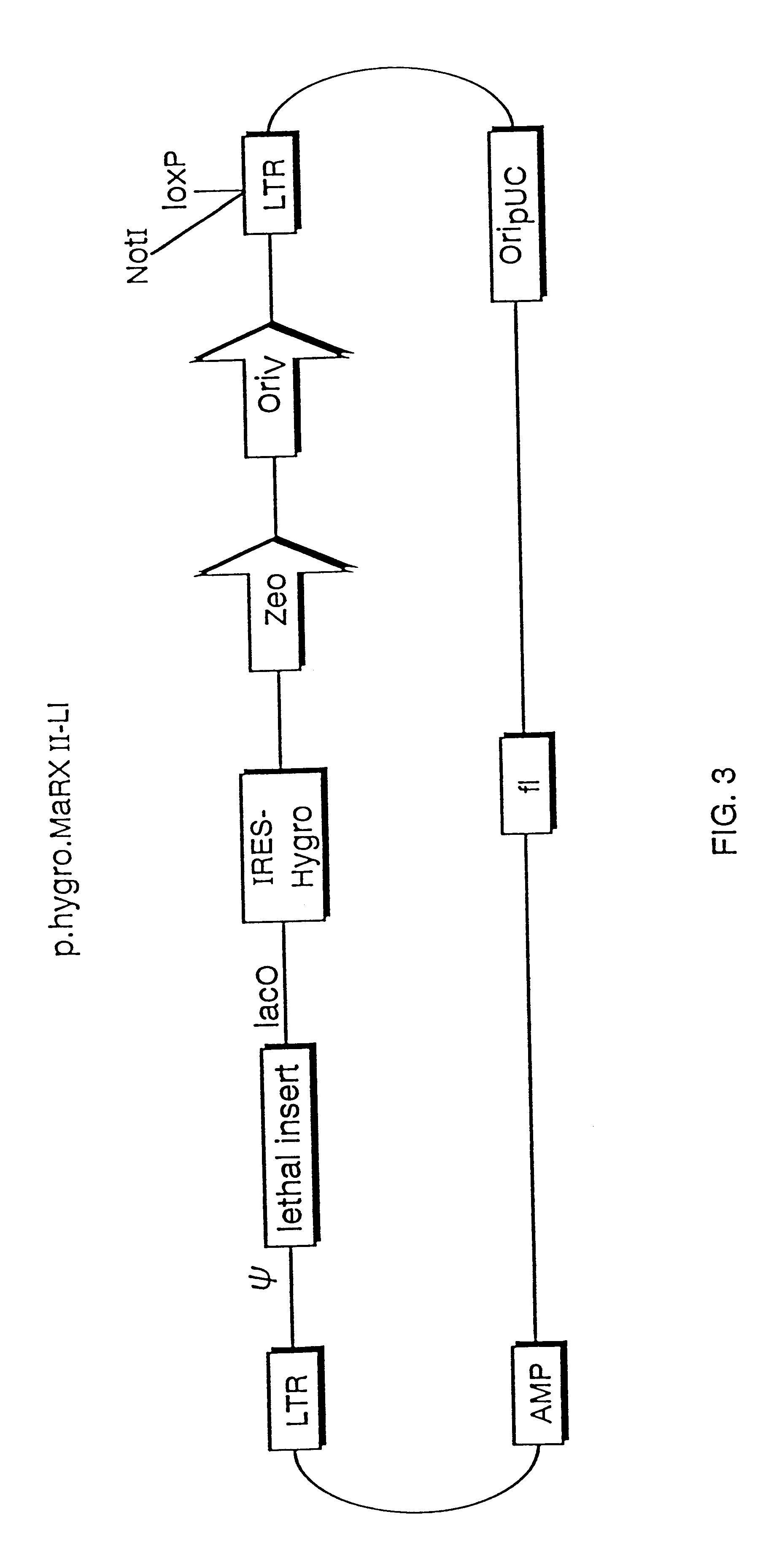
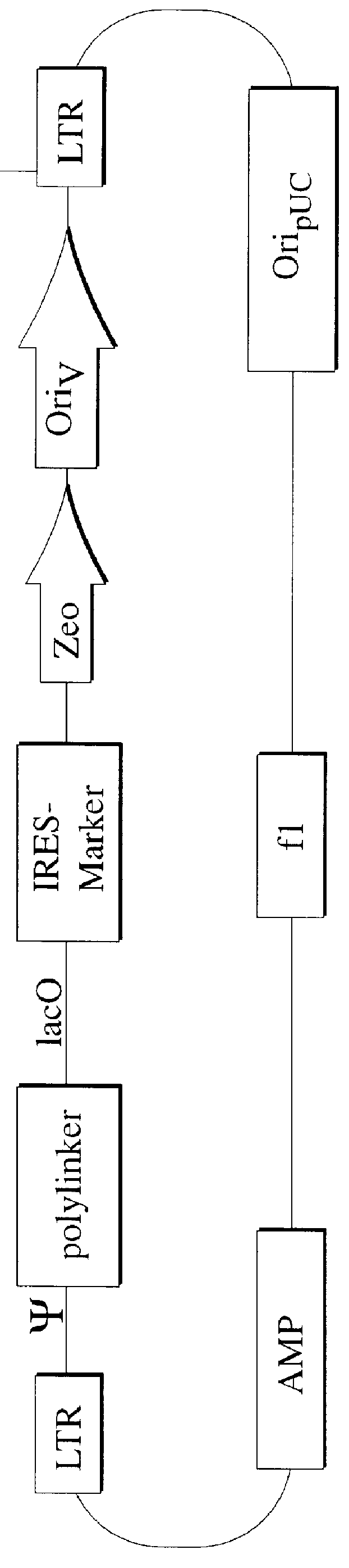
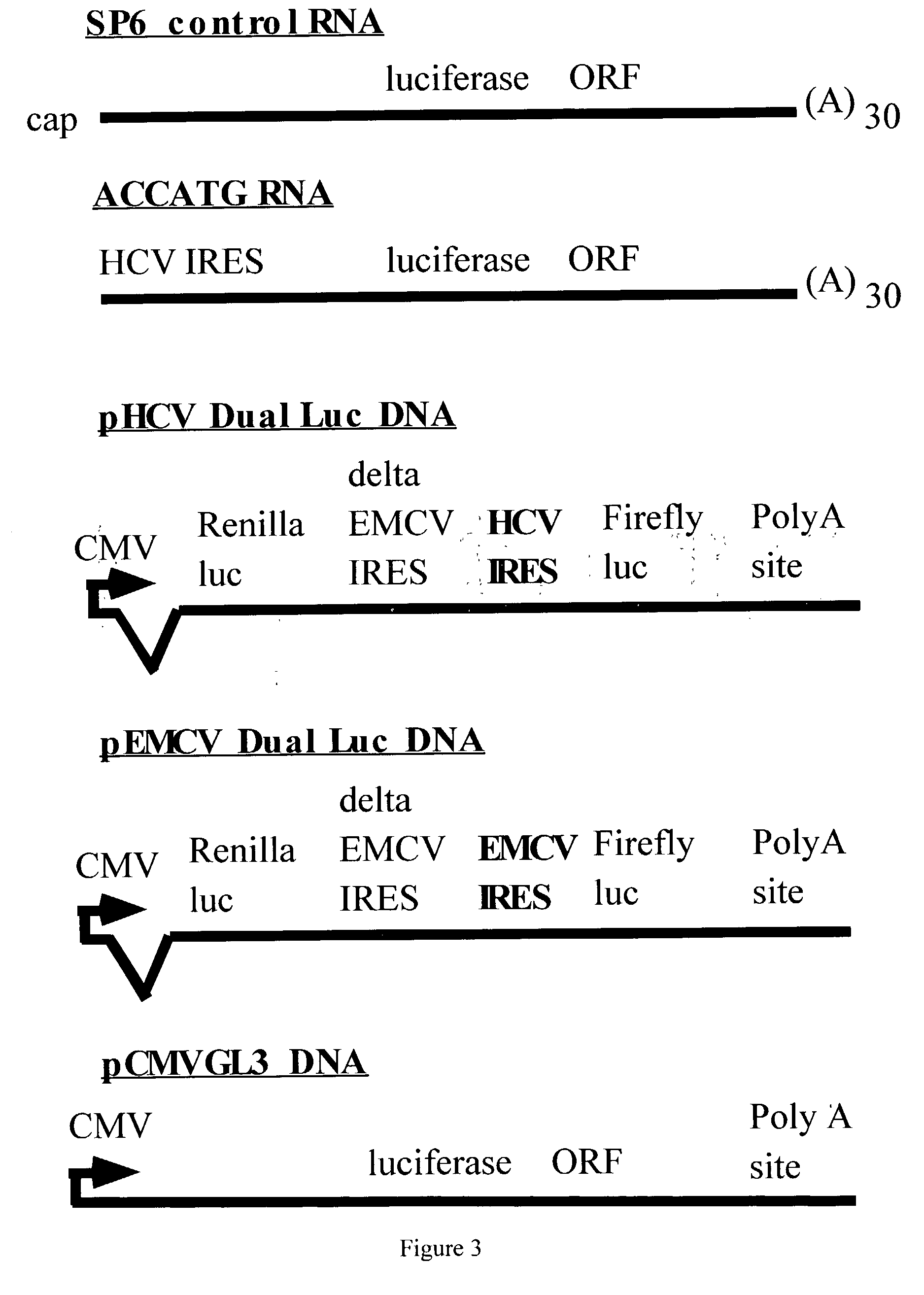
Mammalian viral vectors and their uses
InactiveUS6255071B1Stable episomal maintenanceMaintain relatively stableSugar derivativesMicrobiological testing/measurementRetroviral provirusMammal
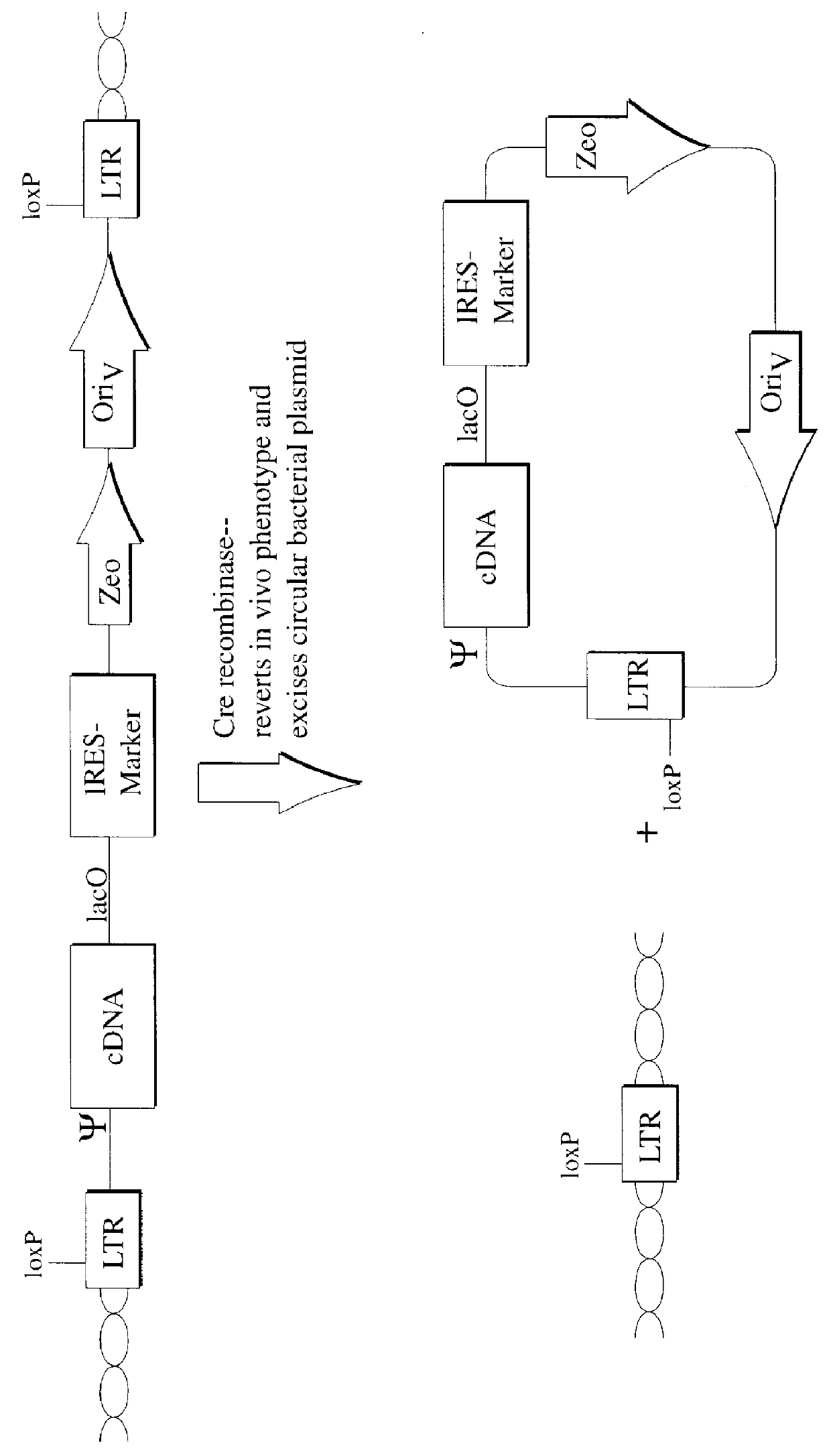
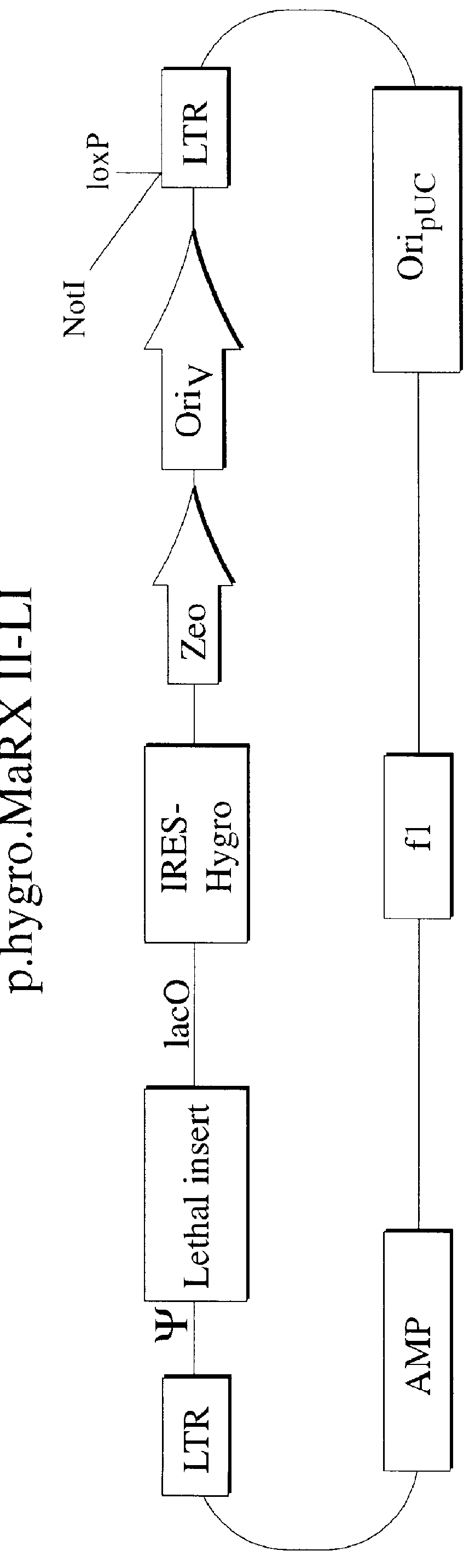
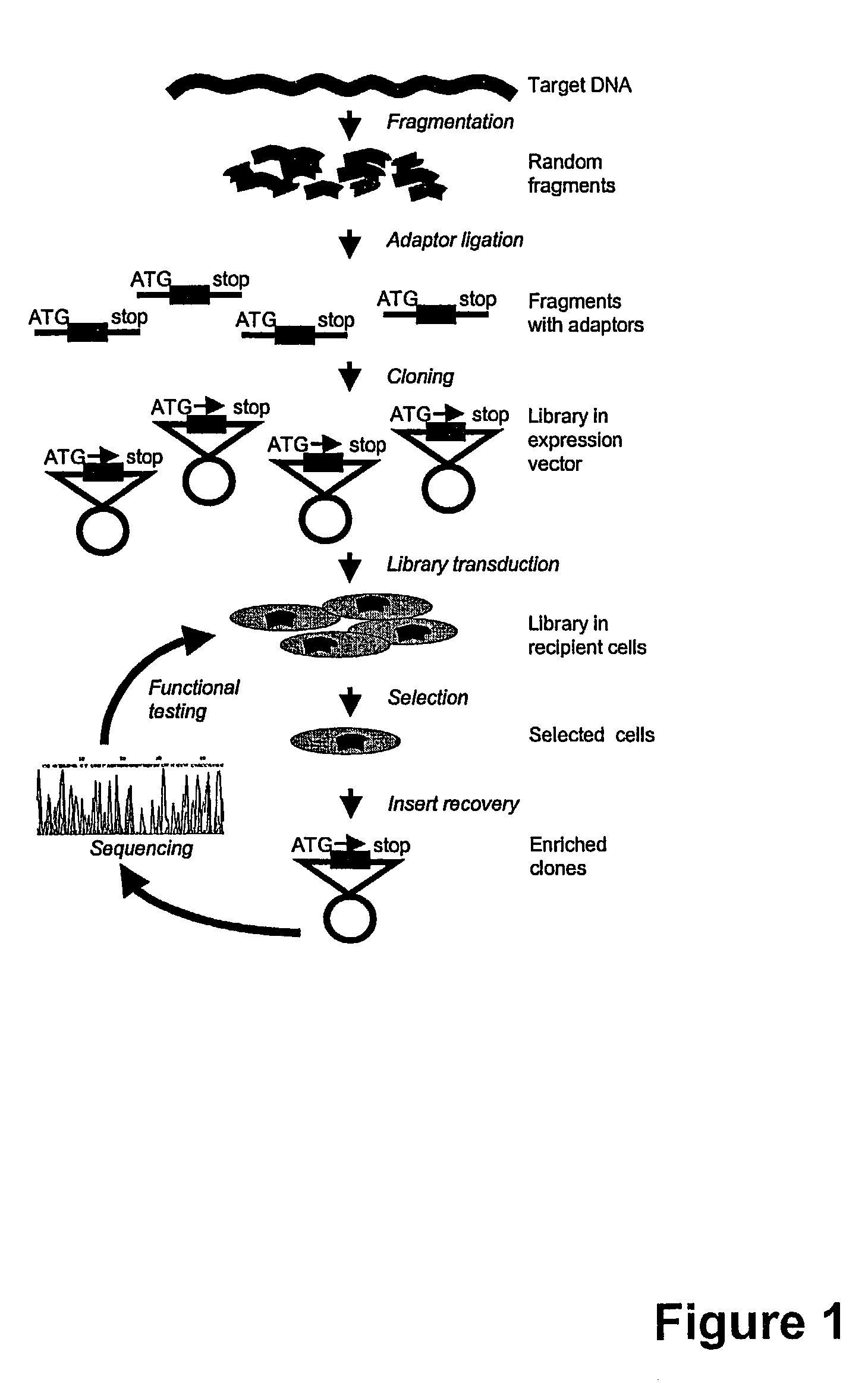
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli.In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
Modified retroviral vectors
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli. In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
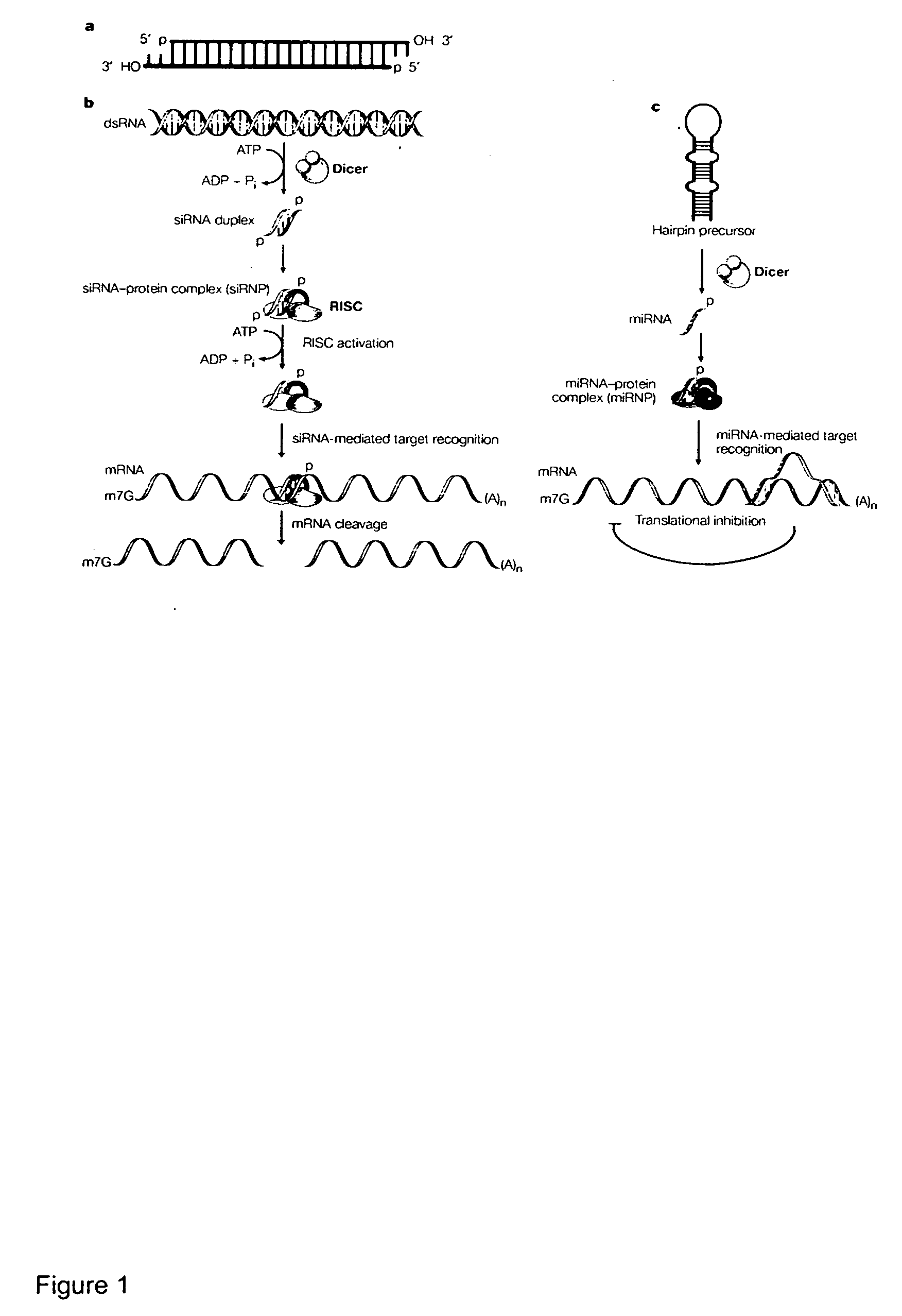
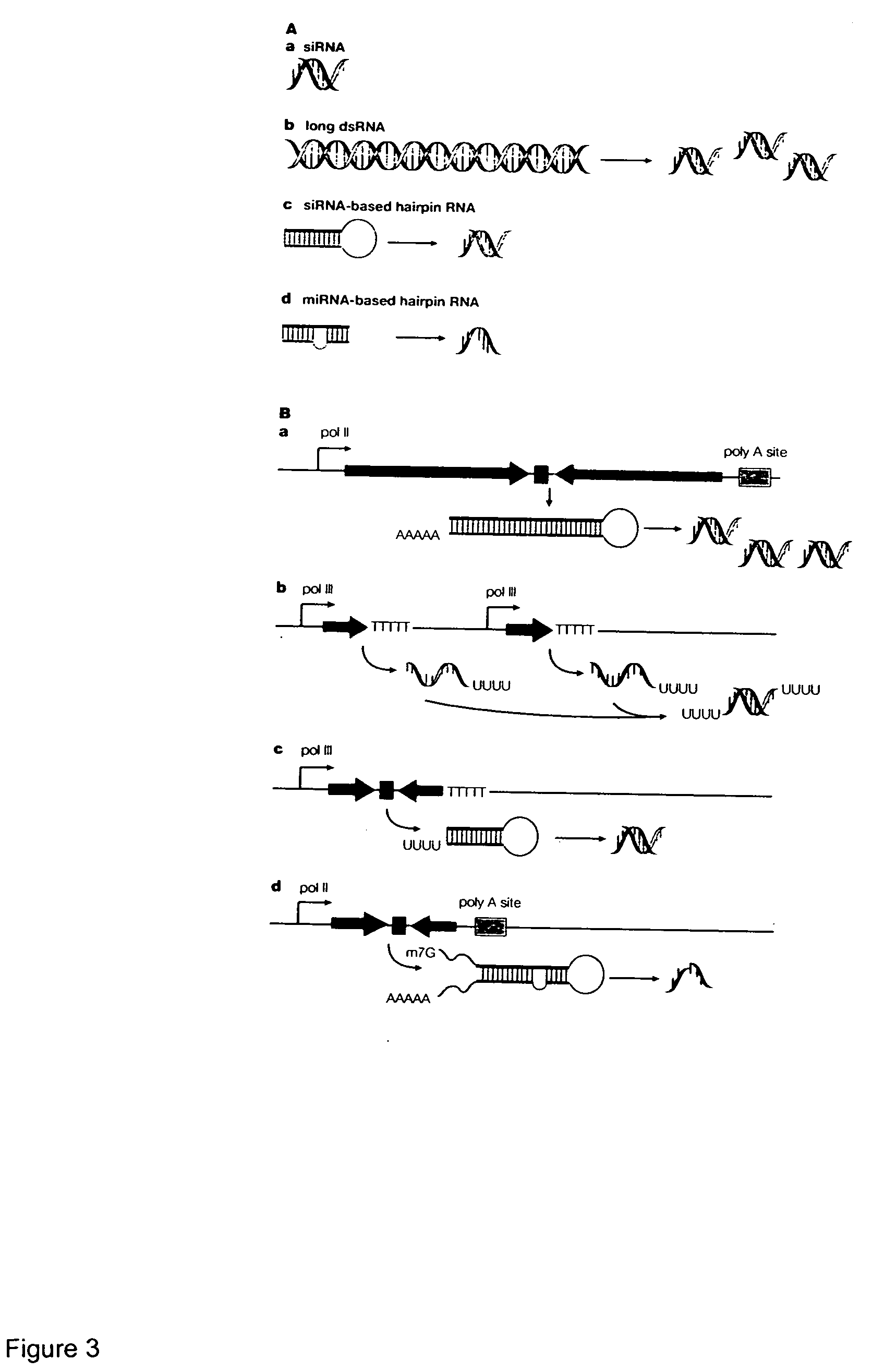
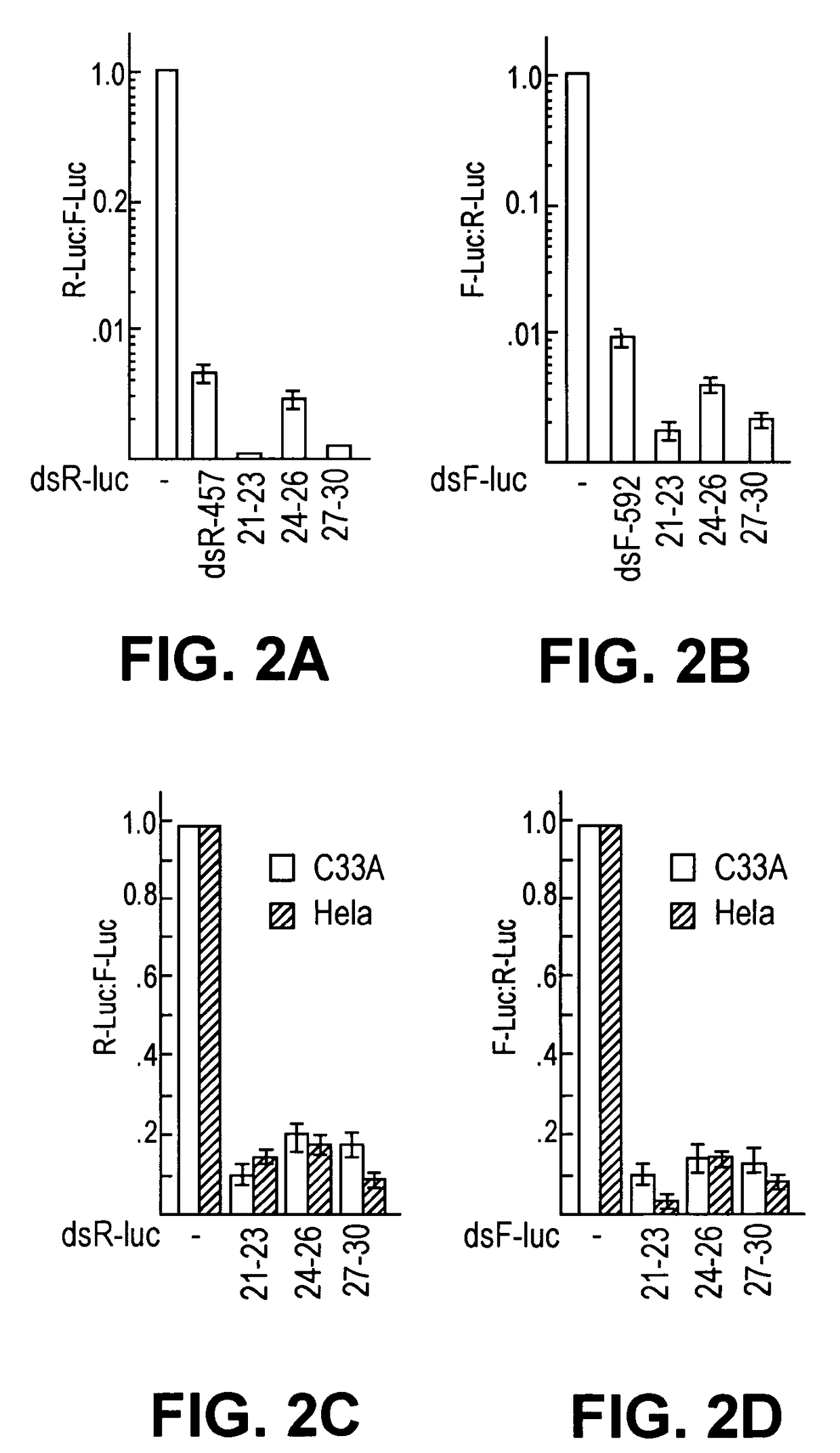
Methods and compositions for RNAi mediated inhibition of gene expression in mammals
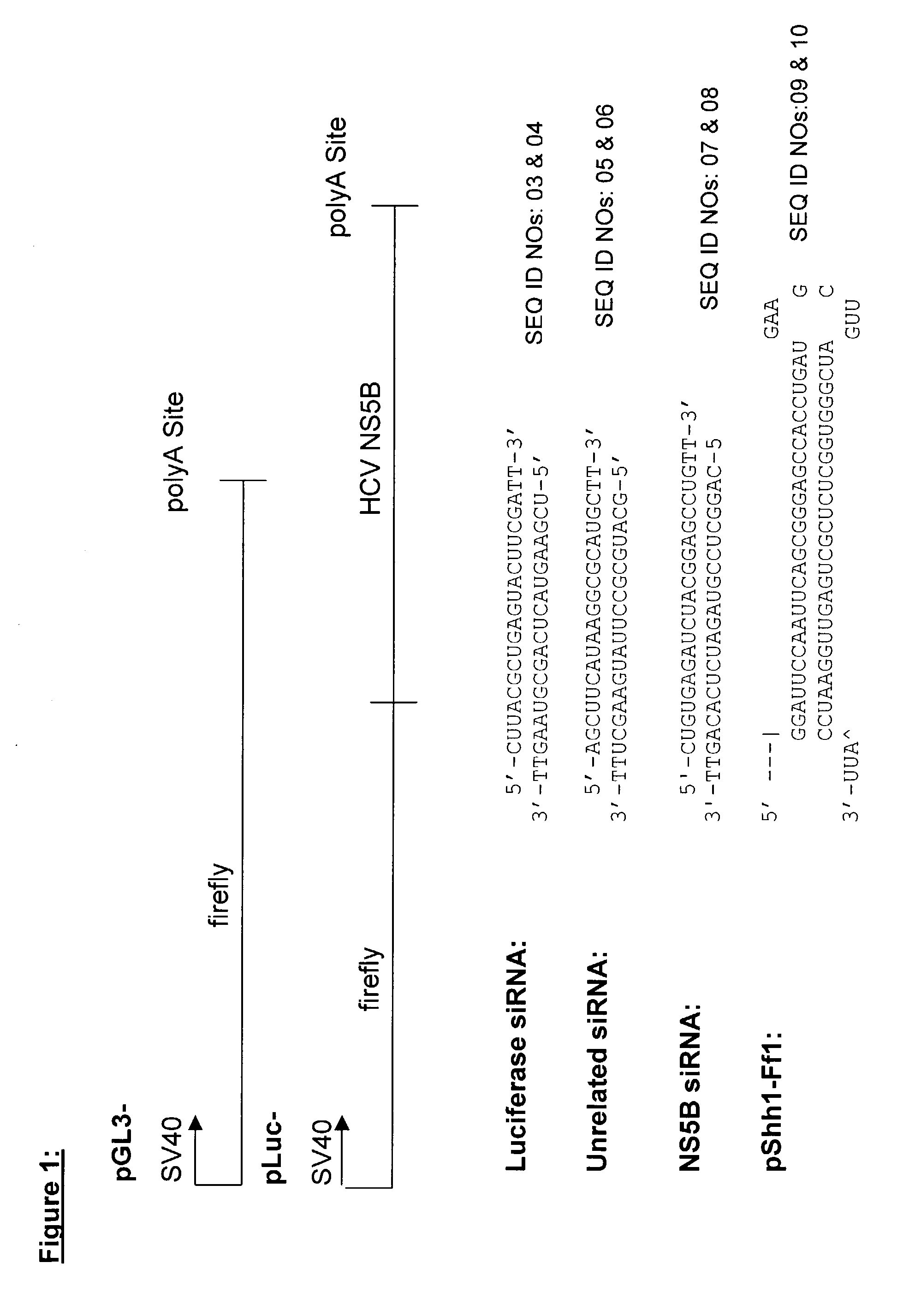
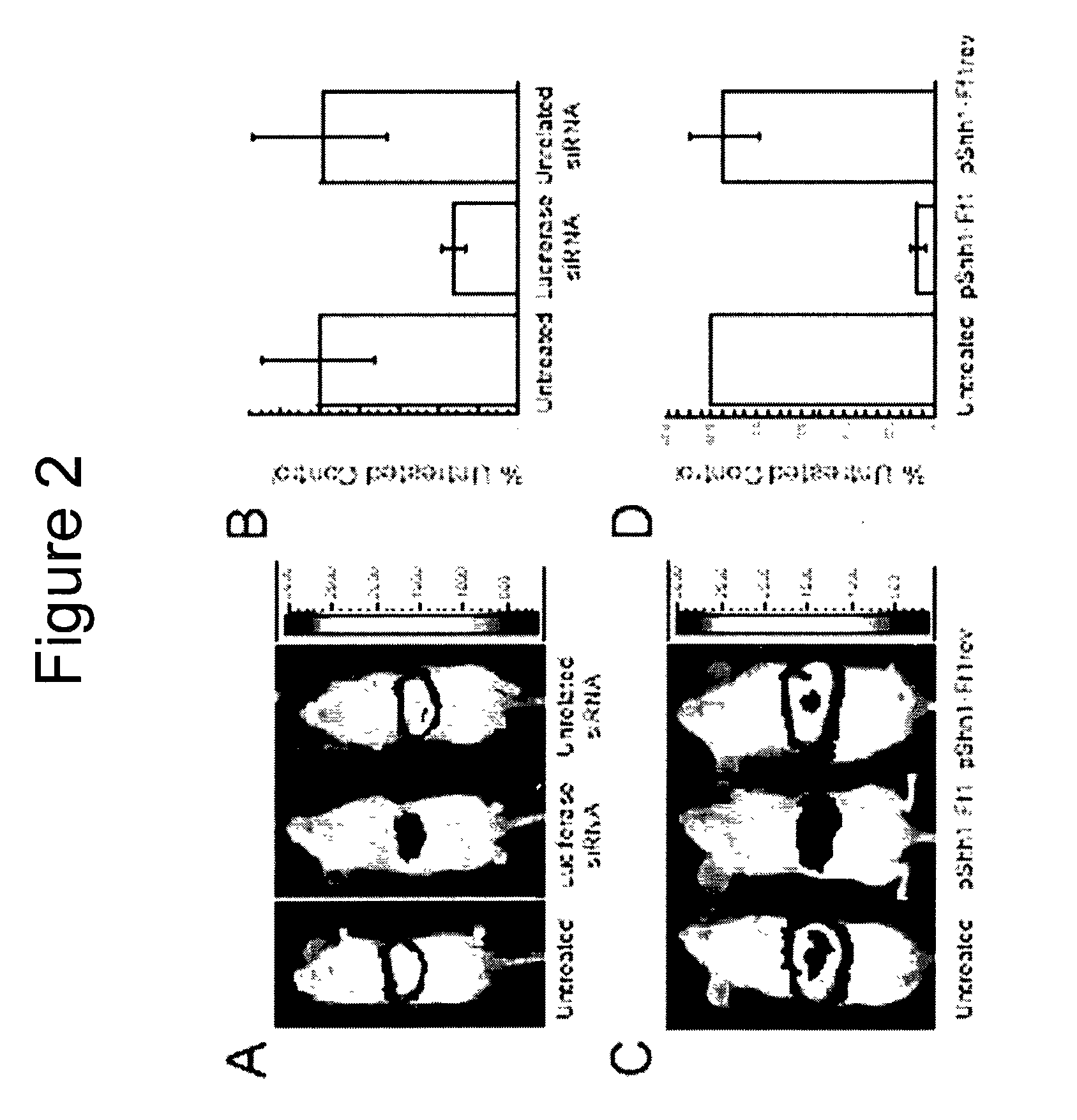
Methods and compositions are provided for modulating, e.g., reducing, coding sequence expression in mammals. In the subject methods, an effective amount of an RNAi agent, e.g., an interfering ribonucleic acid (such as an siRNA or shRNA) or a transcription template thereof, e.g., a DNA encoding an shRNA, is administered to a non-embryonic mammal, e.g., via a hydrodynamic administration protocol. Also provided are RNAi agent pharmaceutical preparations for use in the subject methods. The subject methods and compositions find use in a variety of different applications, including academic and therapeutic applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
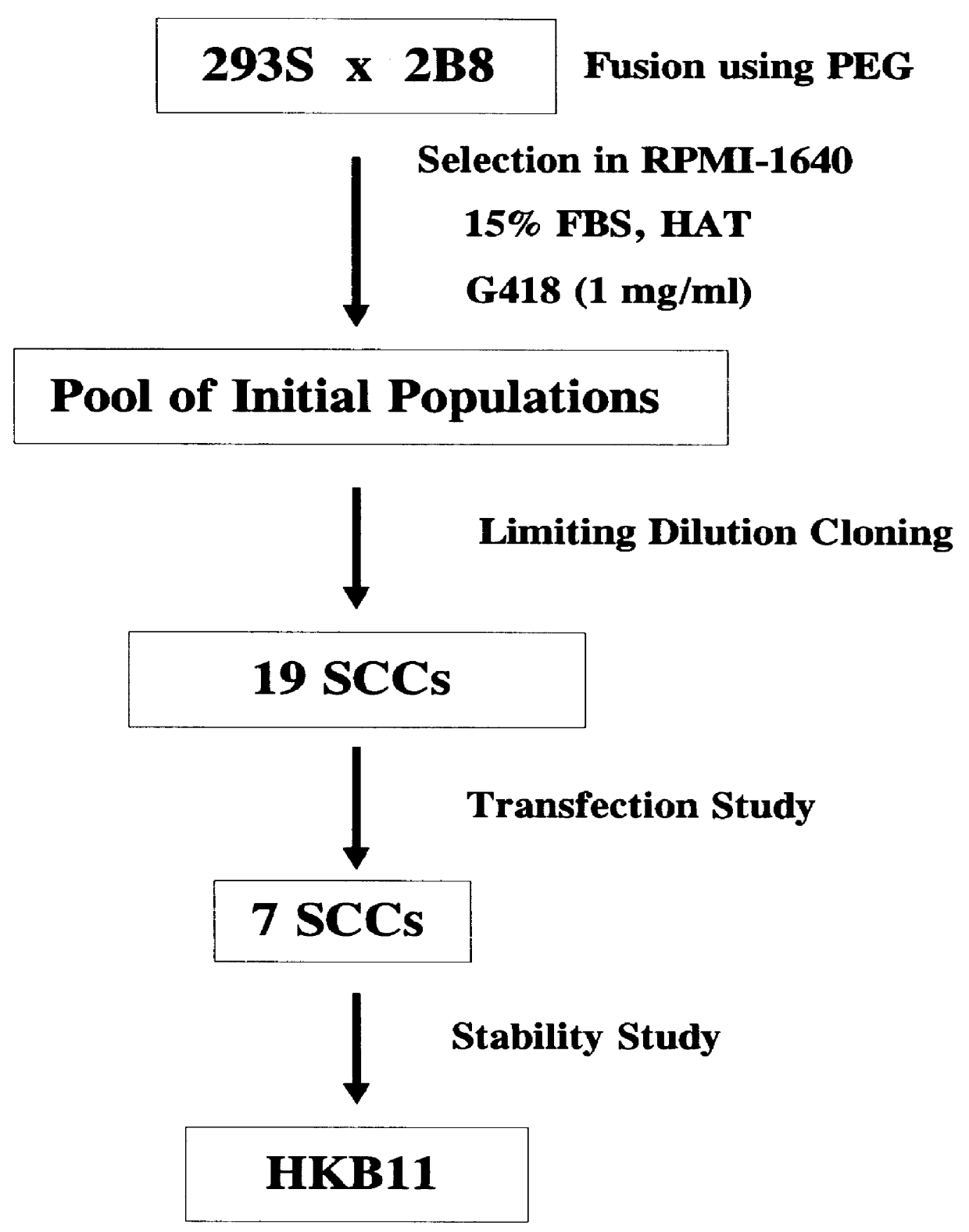
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
Reagents and methods for identification of RNAi pathway genes and chemical modulators of RNAi
InactiveUS20050266552A1Increased and decreased expressionCompound screeningApoptosis detectionMammalScreening method
The present invention provides reagents such as cells, cell lines, and vectors, that can be used to identify mammalian genes whose expression products (RNA or protein) play a role in RNA interference (RNAi) and / or to identify chemical modulators of RNAi, or for other purposes. The invention further provides a variety of methods for identifying such genes or modulators. In particular, the invention provides a mammalian cell comprising a nucleic acid that encodes a selectable marker and one or more nucleic acid templates for transcription of an RNAi-inducing agent integrated into the genome of the cell, wherein the RNAi-inducing agent reduces expression of the marker and is not naturally found in the cell. Additional cells and cell lines comprising nucleic acids that encode one or more additional markers are also provided. According to certain of the inventive methods cells such as these are mutagenized, transfected or infected with a library of genetic suppressor elements, or contacted with a test compound. Cells in which RNAi is inhibited or activated are identified using an appropriate selective condition or screening method. The identity of the mutated or inhibited gene or the identity of the compound is then determined.
Owner:MASSACHUSETTS INST OF TECH
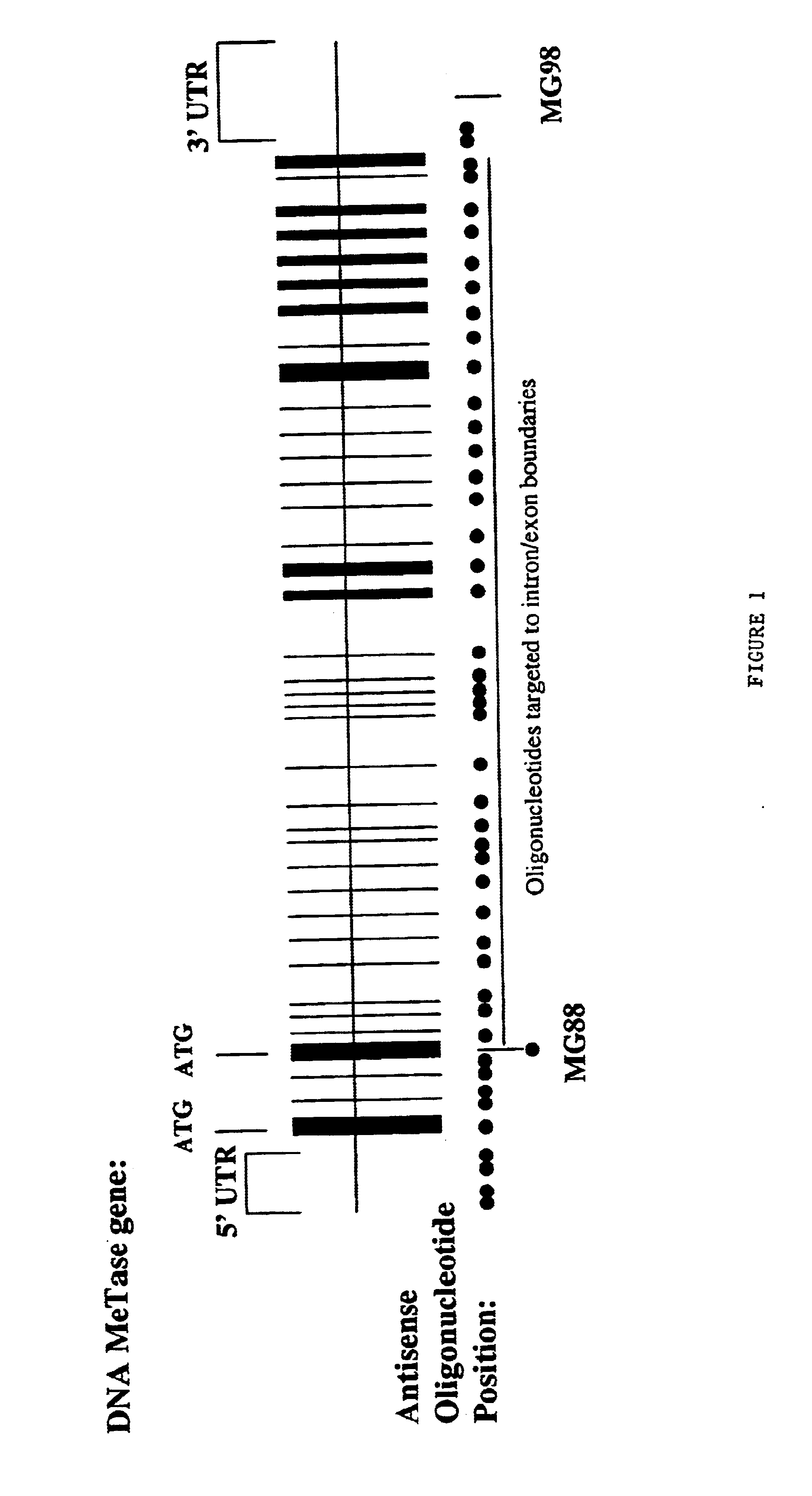
Modulation of gene expression by combination therapy
InactiveUS6953783B1Suppress gene expressionModulate methylation levelBiocideSugar derivativesCombined Modality TherapyGene product
The invention relates to the modulation of gene expression. In particular, the invention relates to compositions comprising antisense oligonucleotides which inhibit expression of a gene in operable association with protein effectors of a product of that gene, and methods of using the same.In addition, the invention relates to the modulation of mammalian gene expression regulated by methylation.
Owner:METHYLGENE
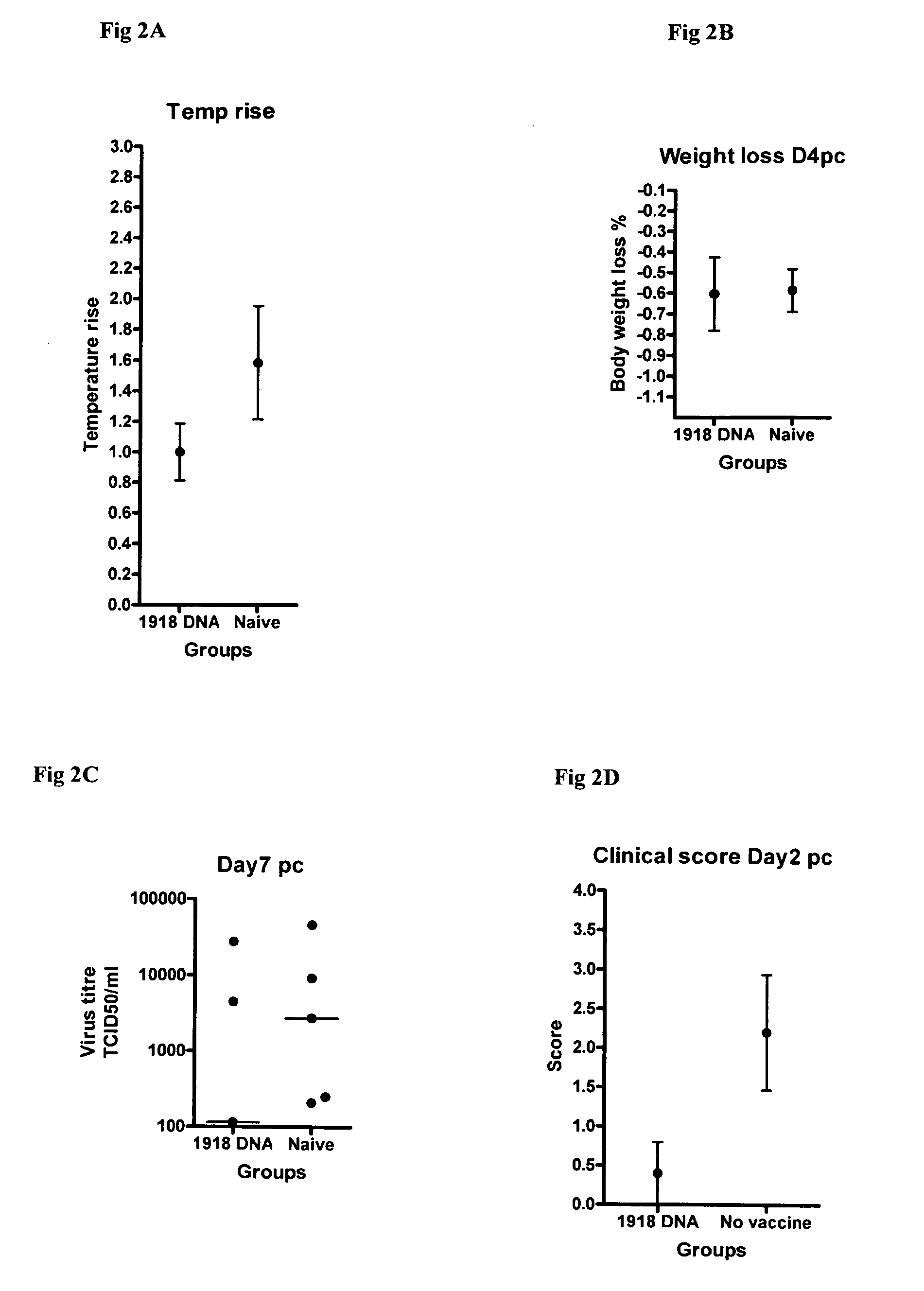
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
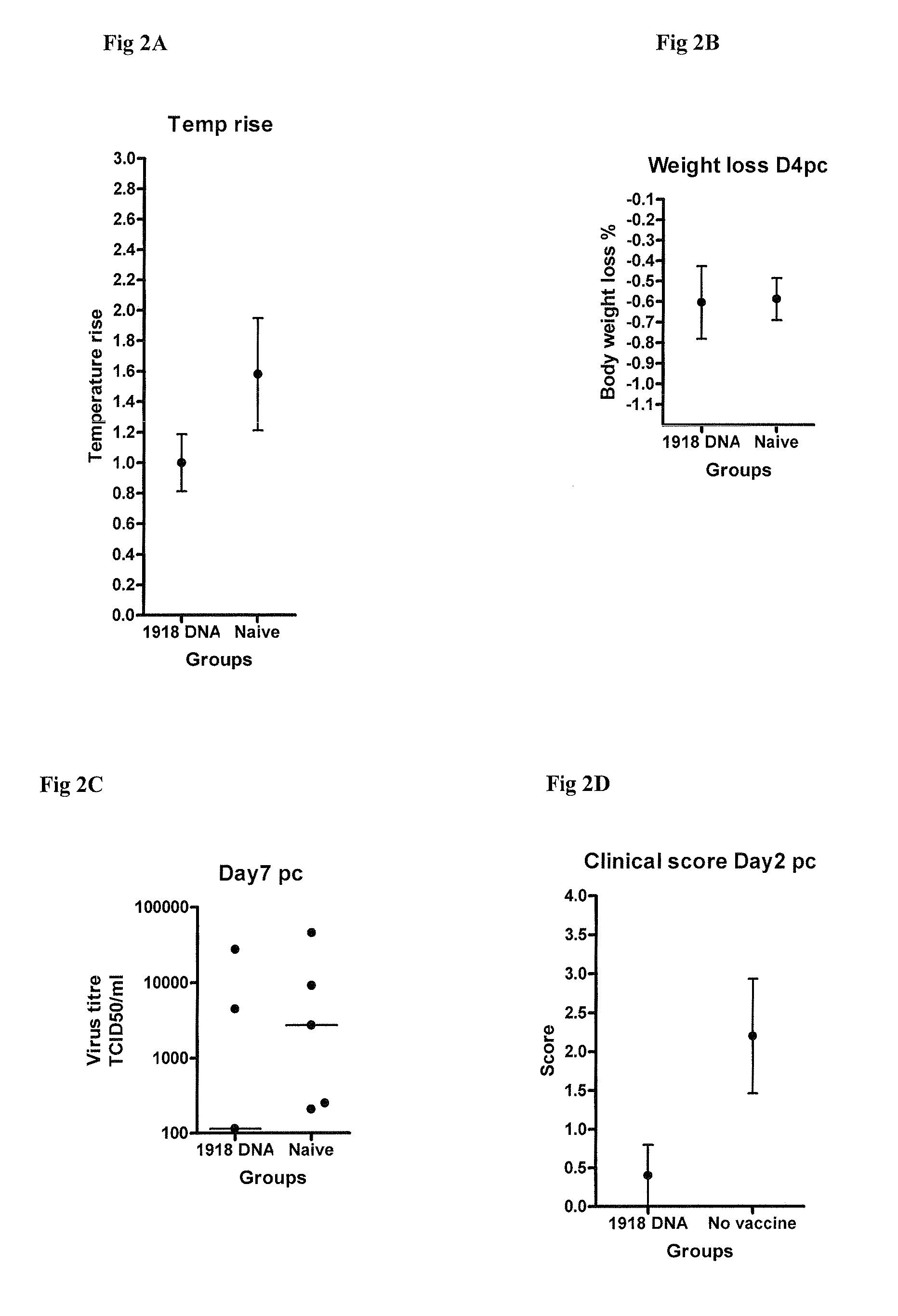
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
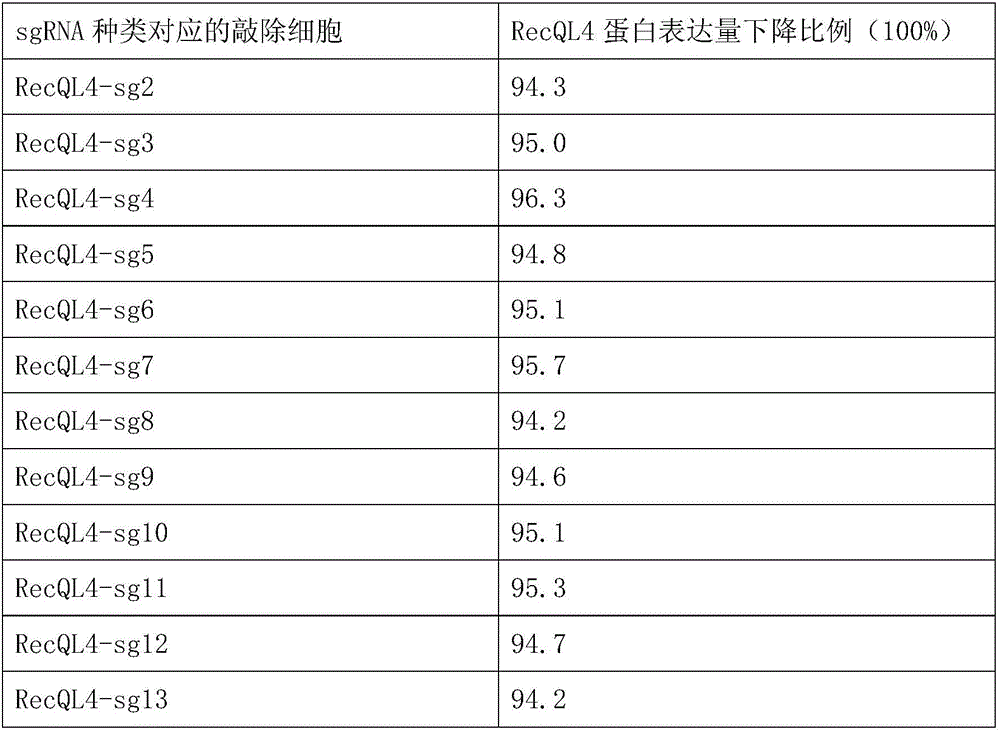
CRISPR-Cas9 system and application therefore in treating breast cancer diseases
ActiveCN106191071AThe identification rules are simpleEasy to analyzeOrganic active ingredientsNucleic acid vectorDiseaseGene targets
The invention provides a CRISPR-Cas9 system and an application thereof in treating breast cancer diseases, wherein by providing a plurality of sgRNAs (single guide RNAs), the efficient knockout of an RecQL4 gene can be achieved. In comparison with ZFNs and TALENs complex expression structures, siRNA is low in using complexity and efficiency; a Cas9 expression structure in the CRISPR-Cas9 system is fixed; for different genes, system building can be completed by interpolating recognition sequences into the sgRNA expression structure, so that operations are simplified and cost is reduced; and the system is applicable to mammal gene targeting in a large scale.
Owner:广东龄值生物科技有限公司
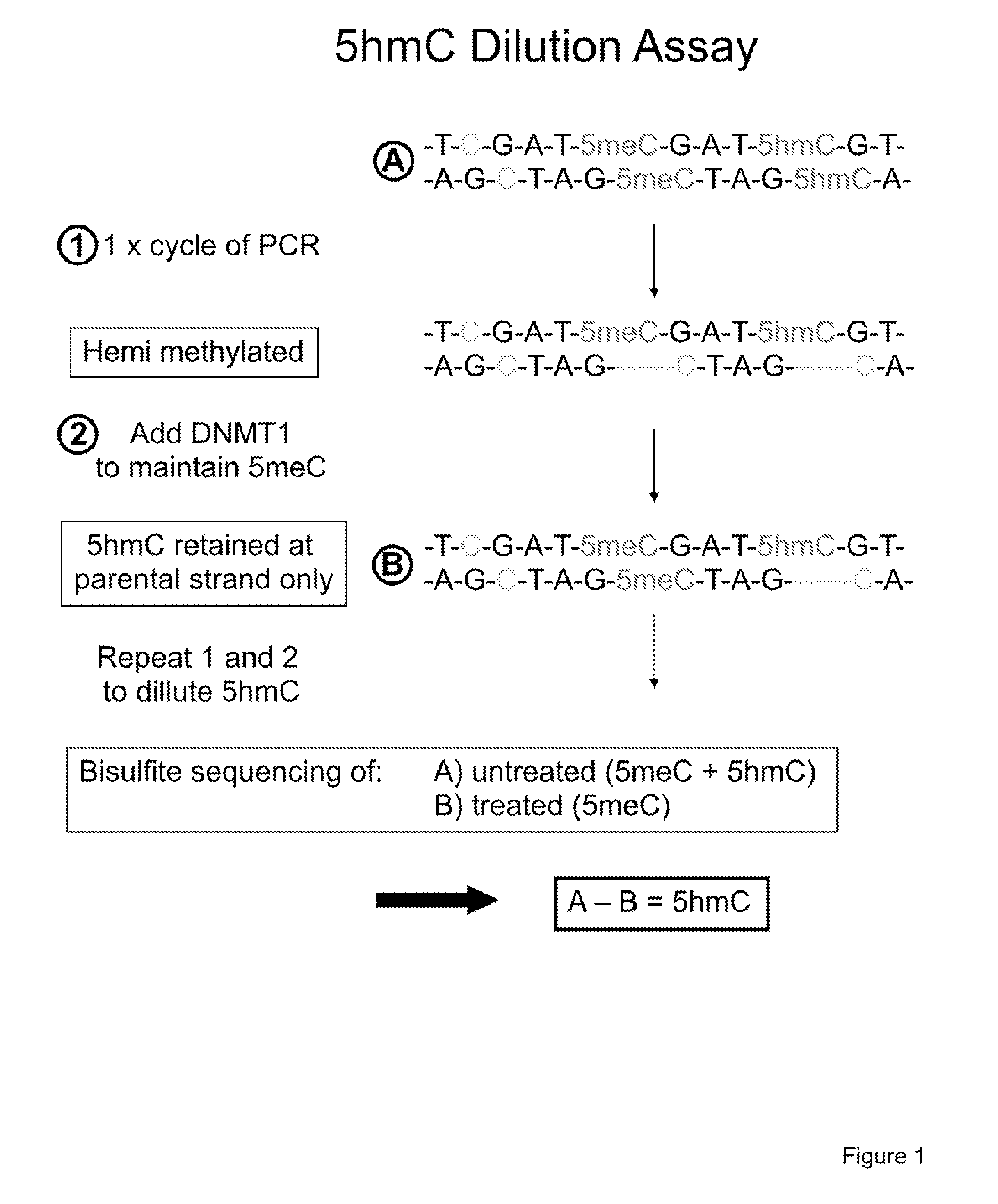
Methods and kits for detection of methylation status
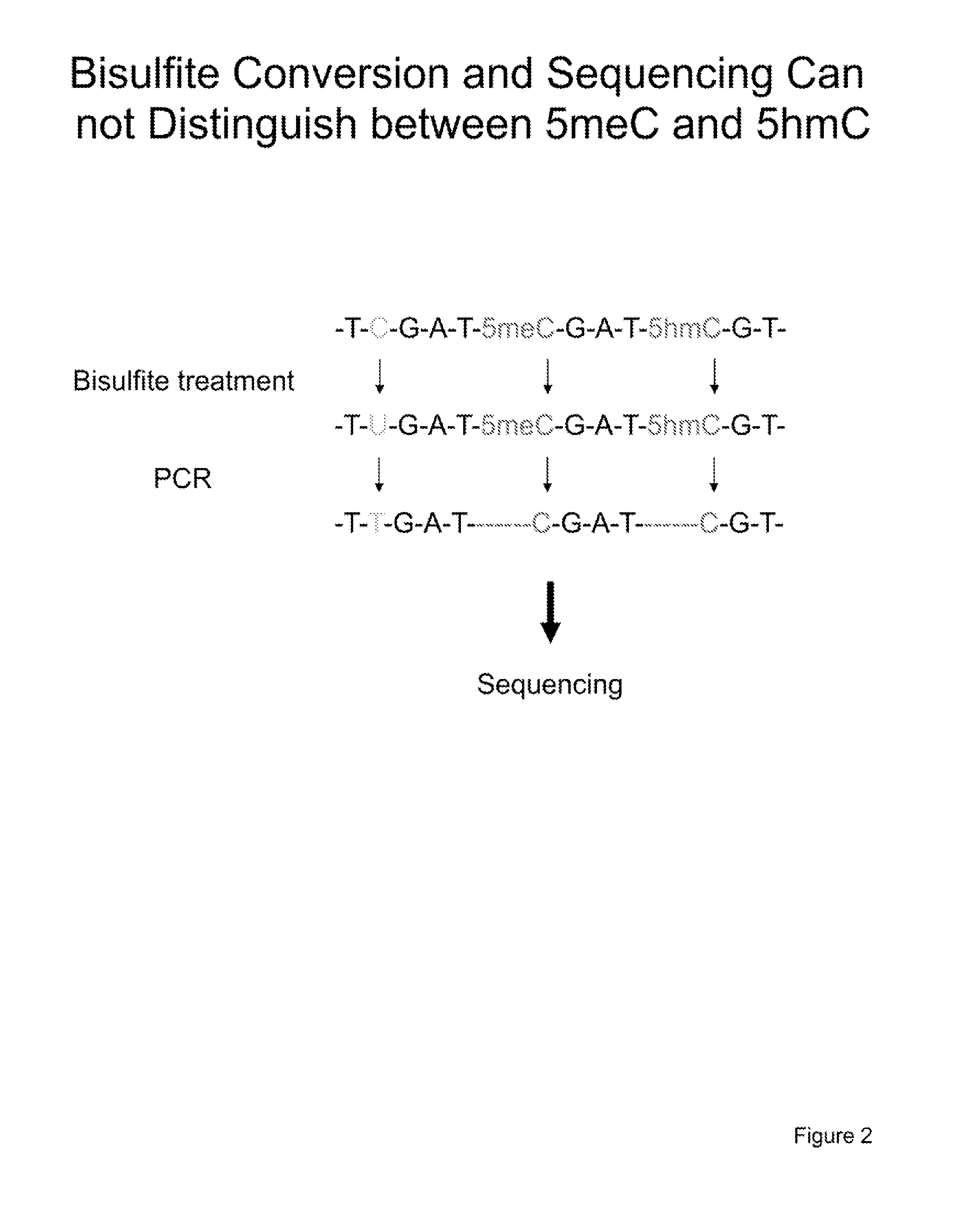
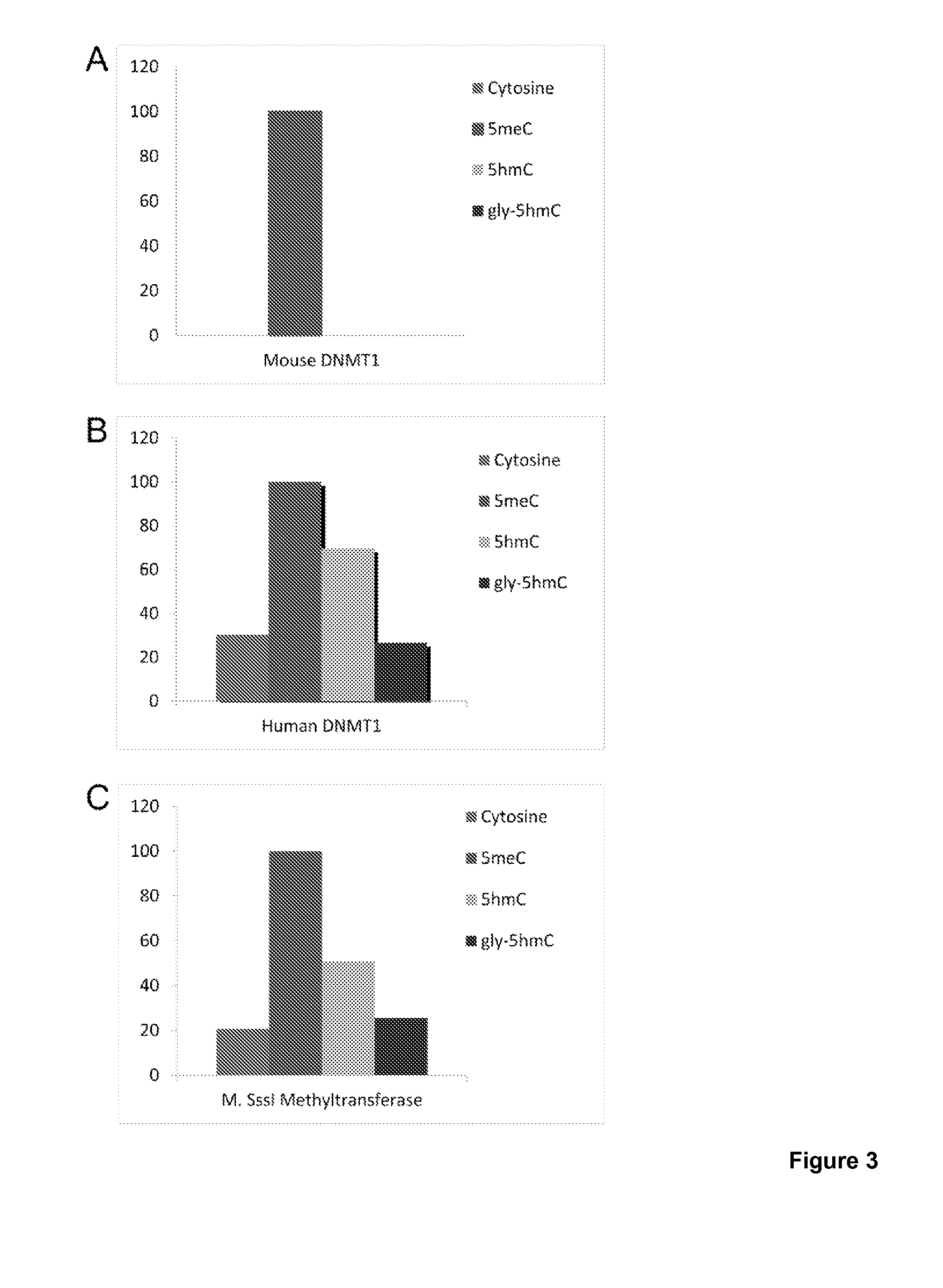
The present invention relates to methods and kits for the detection of 5-hydroxymethylcytosine (5hmC) and / or 5-methylcytosine (5meC). In some embodiments, the present invention relates to methods and kits for detection of 5hmC and / or 5meC in nucleic acid (e.g., DNA, RNA). In some embodiments, the present invention relates to detection of 5hmC in genomic DNA, e.g., mammalian genomic DNA
Owner:UNIV OSLO HF
Mammalian Genes Involved in Infection
InactiveUS20130323835A1Reduce infectionReduced activityOrganic active ingredientsAntimycoticsMammalNucleic acid sequencing
The present invention relates to nucleic acid sequences and cellular proteins encoded by these sequences that are involved in infection or are otherwise associated with the life cycle of one or more pathogens.
Owner:ZIRUS
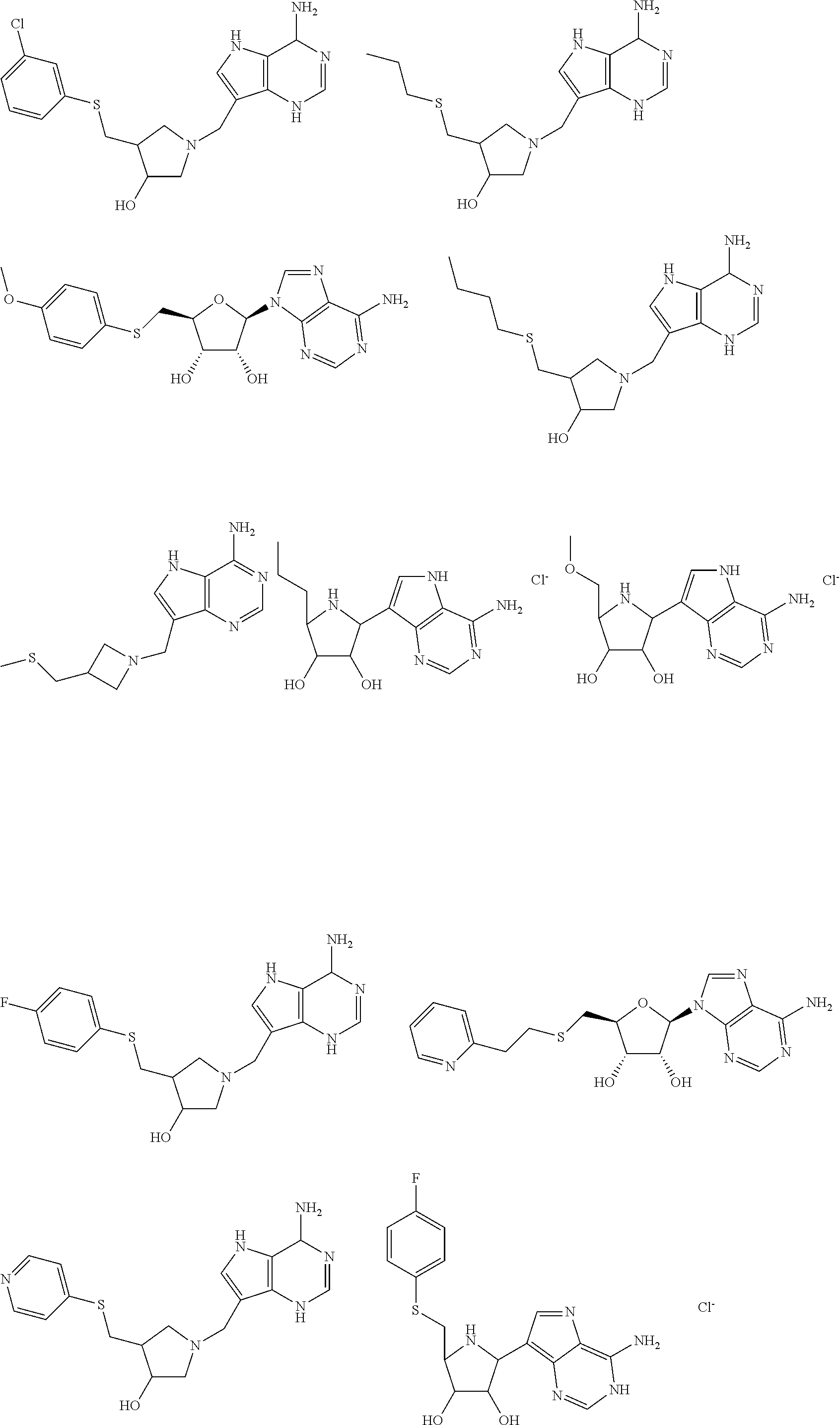
MORC gene compositions and methods of use
InactiveUS6632934B1Easy to storeInhibit microbial growthSugar derivativesAnimals/human peptidesPlant Germ CellsNucleotide
Disclosed are compositions and methods comprising a novel mammalian gene, designated MORC, that is expressed in male germ cells. Also disclosed are polynucleotide compositions comprising a MORC gene from human and murine sources, and polypeptides encoded by these nucleic acid sequences. Methods for preparing MORC polypeptides, transformed host cells, and antibodies reactive with MORC polypeptides are also provided. In certain embodiments, the invention describes methods for diagnosing and treating infertility or testicular cancer, as well as methods for identifying MORC-related polynucleotide and polypeptide compositions.< / PTEXT>
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Hmgi proteins in cancer and obesity
InactiveUS20030051260A1Peptide/protein ingredientsMicrobiological testing/measurementProtein regulationEmbryonic Stage
The present invention pertains to a method for treating obesity in a mammal which comprises reducing the biological activity of HMGI genes in the mammal. In another embodiment, the invention pertains to a method for treating a tumor in a patient by reducing the biological activity of normal HMGI genes which comprises administering to the patient a therapeutically effective amount of an inhibitor compound active against normal HMGI-C or HMGI(Y) genes. In another embodiment, the invention pertains to a method of producing a transgenic non-human mammal, the germ cells and somatic cells of which contain an inactivated HMGI gene sequence introduced into the mammal at an embryonic stage. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI proteins. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI genes. In another embodiment, the invention pertains to a method for detecting normal HMGI proteins as a diagnostic marker for a tumor using a probe. that recognizes normal HMGI proteins, which comprises the steps of (a) contacting normal HMGI proteins from a sample from a patient with a probe which binds to HMGI proteins; and (b) analyzing for normal HMGI proteins by detecting levels of the probe bound to the normal HMGI proteins, wherein the presence of normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to a method for detecting antibodies to normal HMGI proteins using a probe that recognizes antibodies to HMGI normal proteins, which comprises the steps of (a) treating a sample from a patient with a probe which binds to antibodies to normal HMGI proteins; and (b) analyzing for antibodies to HMGI proteins by detecting levels of the probe bound to the antibodies to HMGI proteins, wherein the presence of antibodies to normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to HMGI genes and proteins for use as a starting point to isolate downstream target genes regulated by the HMGI genes and proteins.
Owner:MEDICINE & DENTISTRY OF NEW YORK UNIV OF
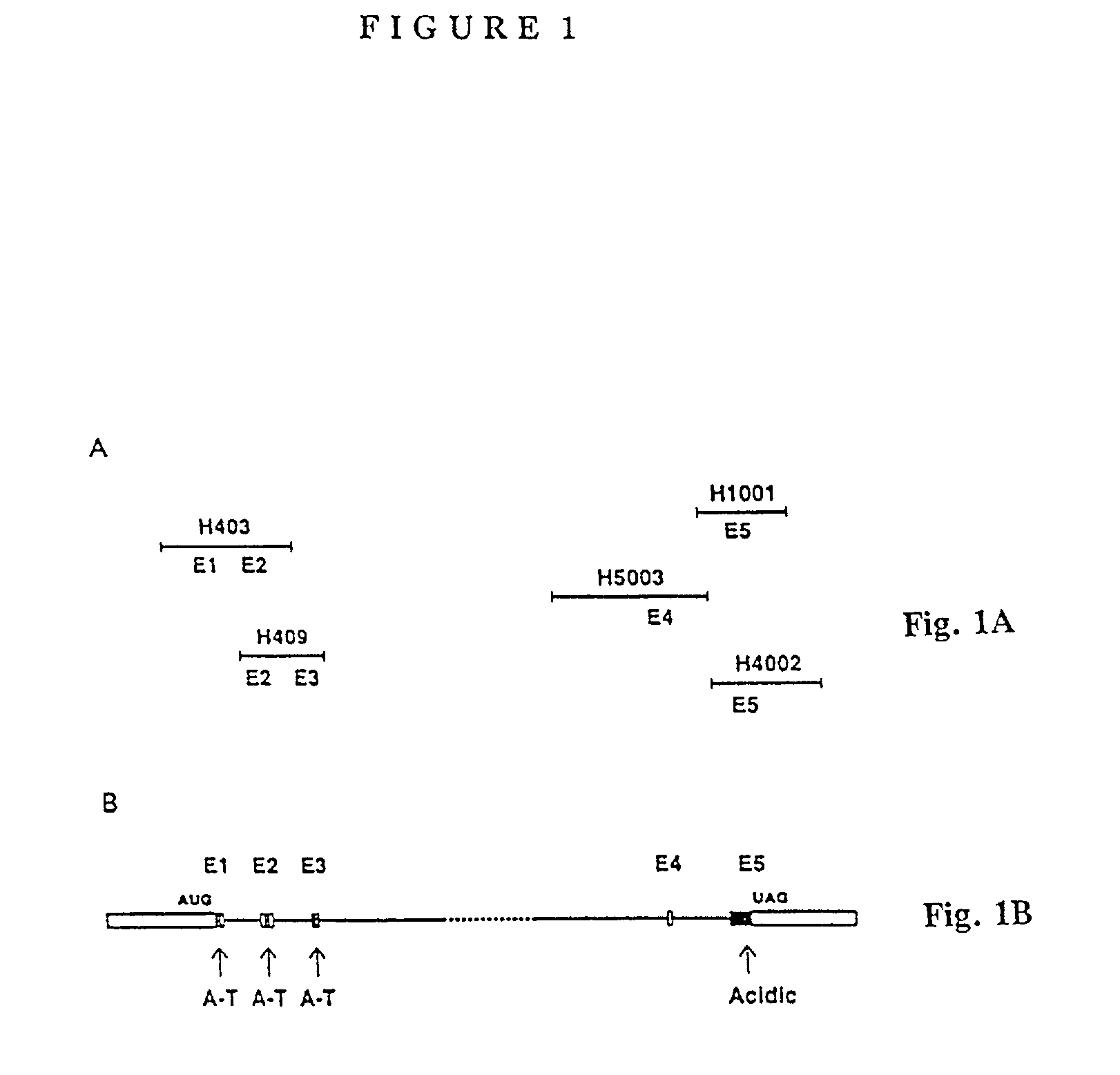
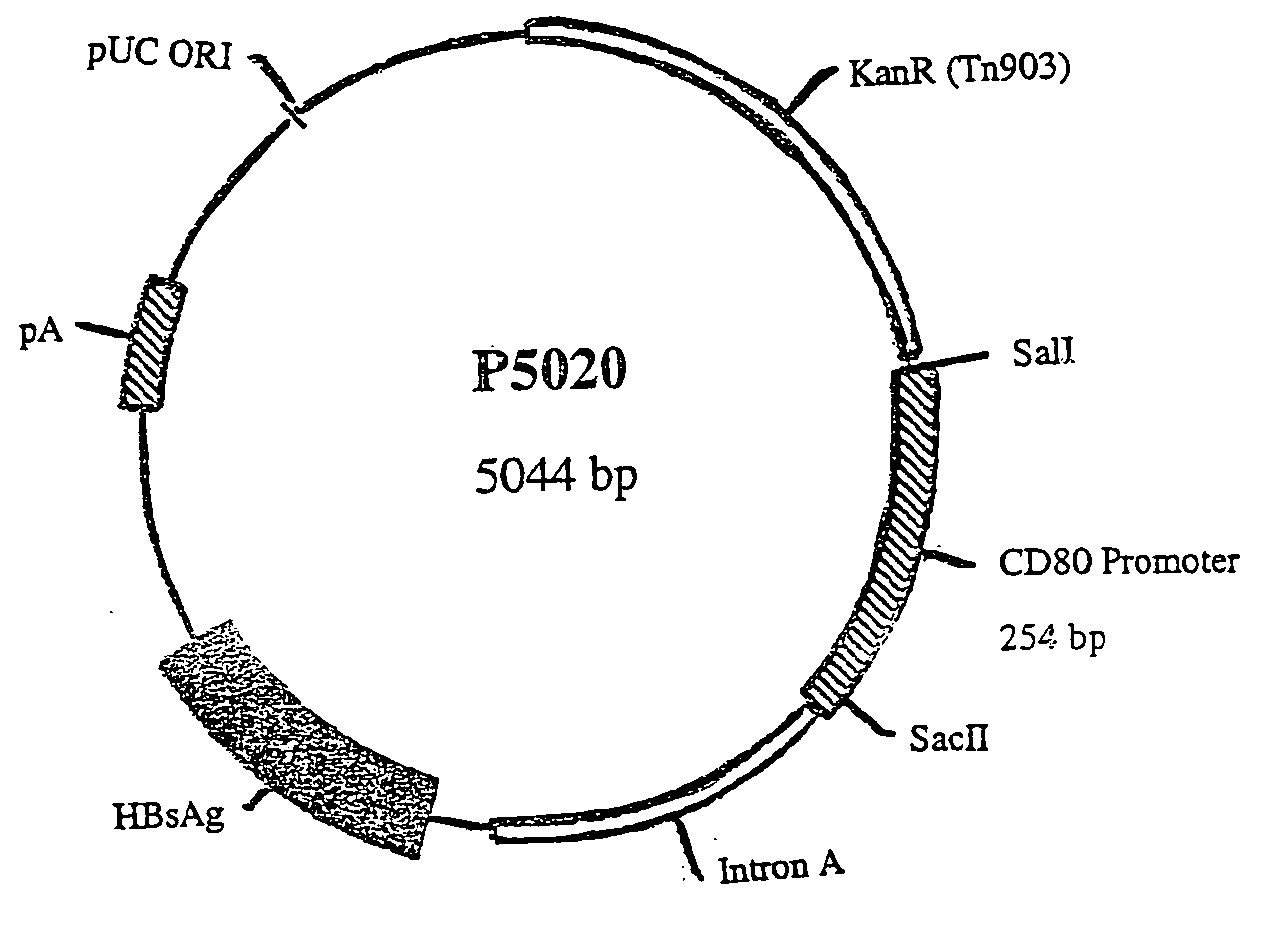
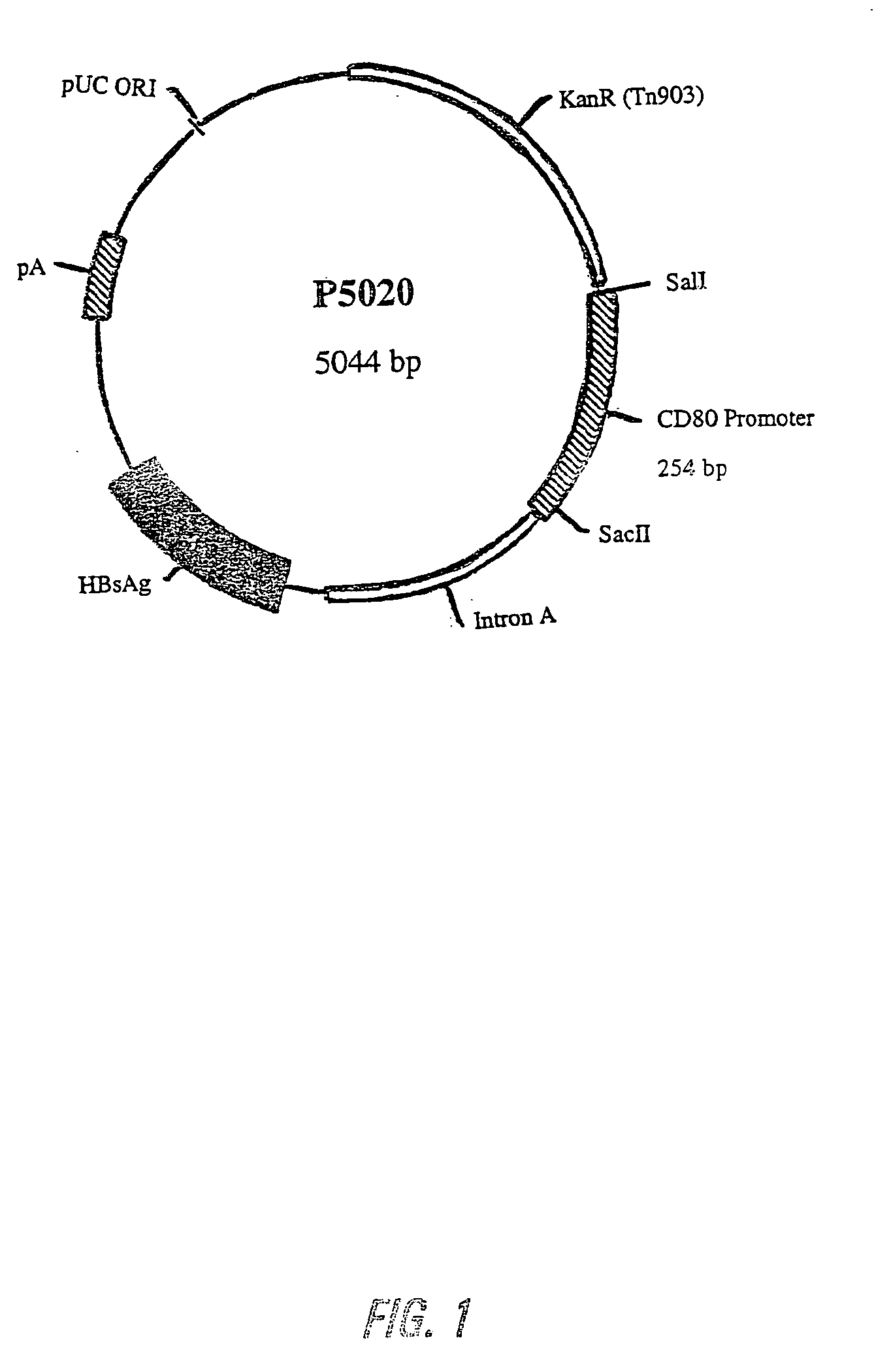
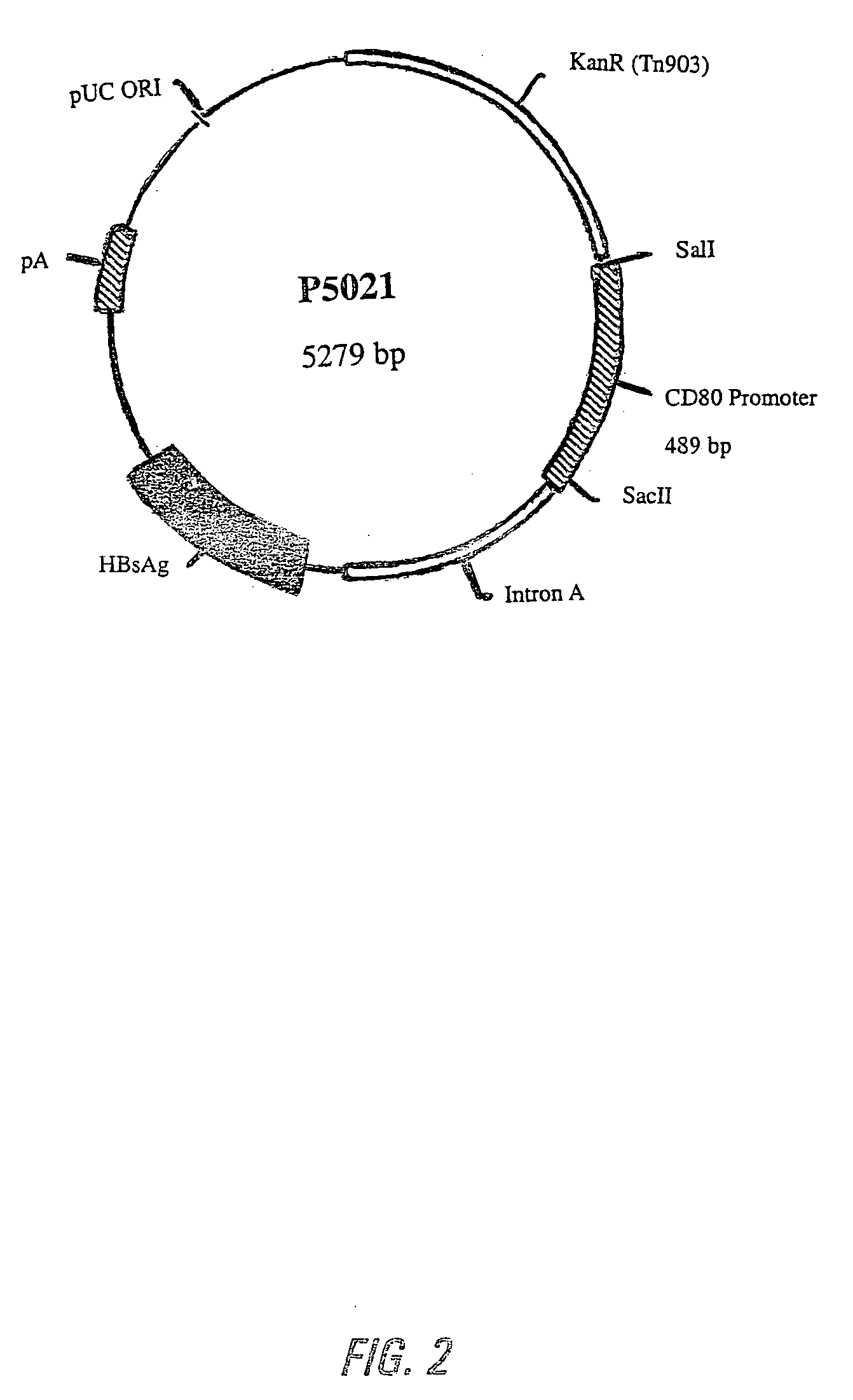
Nucleic acid vaccine compositions having a mammalian CD80/CD86 gene promoter driving antigen expression
Polynucleotides encoding at least one immunizing antigen whose expression is controlled by a promoter derived from a gene encoding a co-stimulatory molecule are provided. The polynucleotides may also encode adjuvants. Compositions comprising at least one immunizing agent and at least one cytokine that enhance dendritic cell stimulation and / or survival are also provided. Methods for eliciting an immune response against the immunizing agent are also provided. The method includes the steps of administering the polynucleotides and, optionally, co-administering an adjuvant.
Owner:POWDERJECT RES LTD OXFORD (GB)
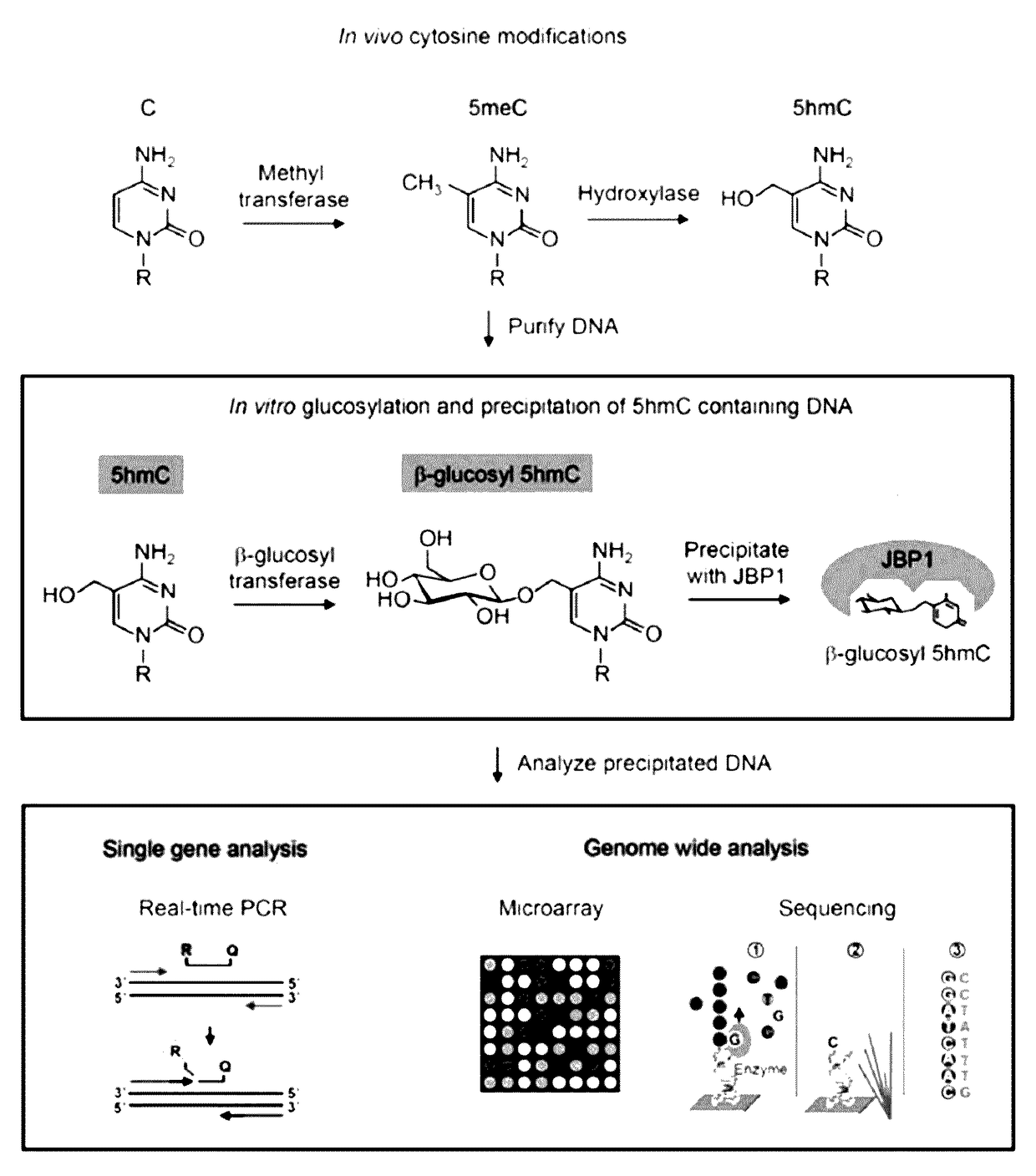
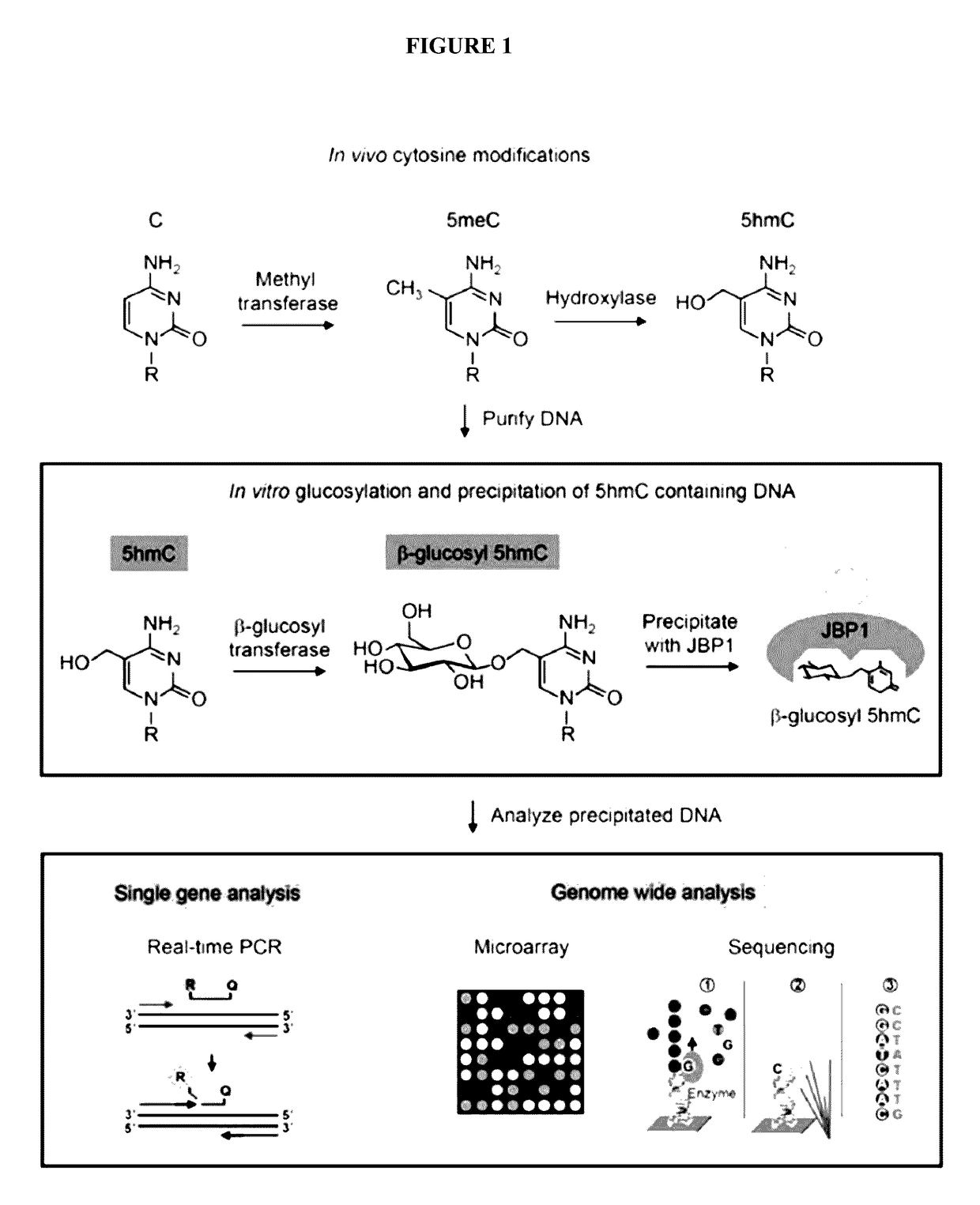
Methods and kits for detection of 5-hydroxymethylcytosine
ActiveUS9677128B2Rapid and inexpensive identificationEfficiently and selectively pulled downSugar derivativesMicrobiological testing/measurementGenomic DNABioinformatics
The present invention relates to methods and kits for the detection of 5-hydroxymethylcytosme (5hmC). In some embodiments, the present invention relates to methods and kits for detection of 5hmC in nucleic acid (e.g., DNA, RNA). In some embodiments, the present invention relates to detection of 5hmC in genomic DNA, e.g., mammalian genomic DNA.
Owner:UNIV OSLO HF
Methods and kits for detection of 5-hydroxymethylcytosine
ActiveUS20130323728A1Improve isolationRapid and inexpensive identificationMicrobiological testing/measurementDepsipeptidesGenomic DNAGenome
The present invention relates to methods and kits for the detection of 5-hydroxymethylcytosme (5hmC). In some embodiments, the present invention relates to methods and kits for detection of 5hmC in nucleic acid (e.g., DNA, RNA). In some embodiments, the present invention relates to detection of 5hmC in genomic DNA, e.g., mammalian genomic DNA.
Owner:UNIV OSLO HF
Methods of extracting RNA
InactiveUS20070185322A1Rapid and simple extractionRapid and simple and isolationSugar derivativesDNA preparationPresent methodLysis
Methods and materials are disclosed for rapid and simple extraction and isolation of RNA from a biological sample involving the use of an acidic solution and a solid phase binding material that has the ability to liberate nucleic acids from biological samples, including whole blood, without first performing any preliminary lysis to disrupt cells or viruses. No detergents or chaotropic substances for lysing cells or viruses are needed or used. Viral, bacterial and mammalian genomic RNA can be isolated using the method of the invention. RNA isolated by the present method is suitable for use in downstream processes such as RT-PCR.
Owner:NEXGEN DIAGNOSTICS LLC
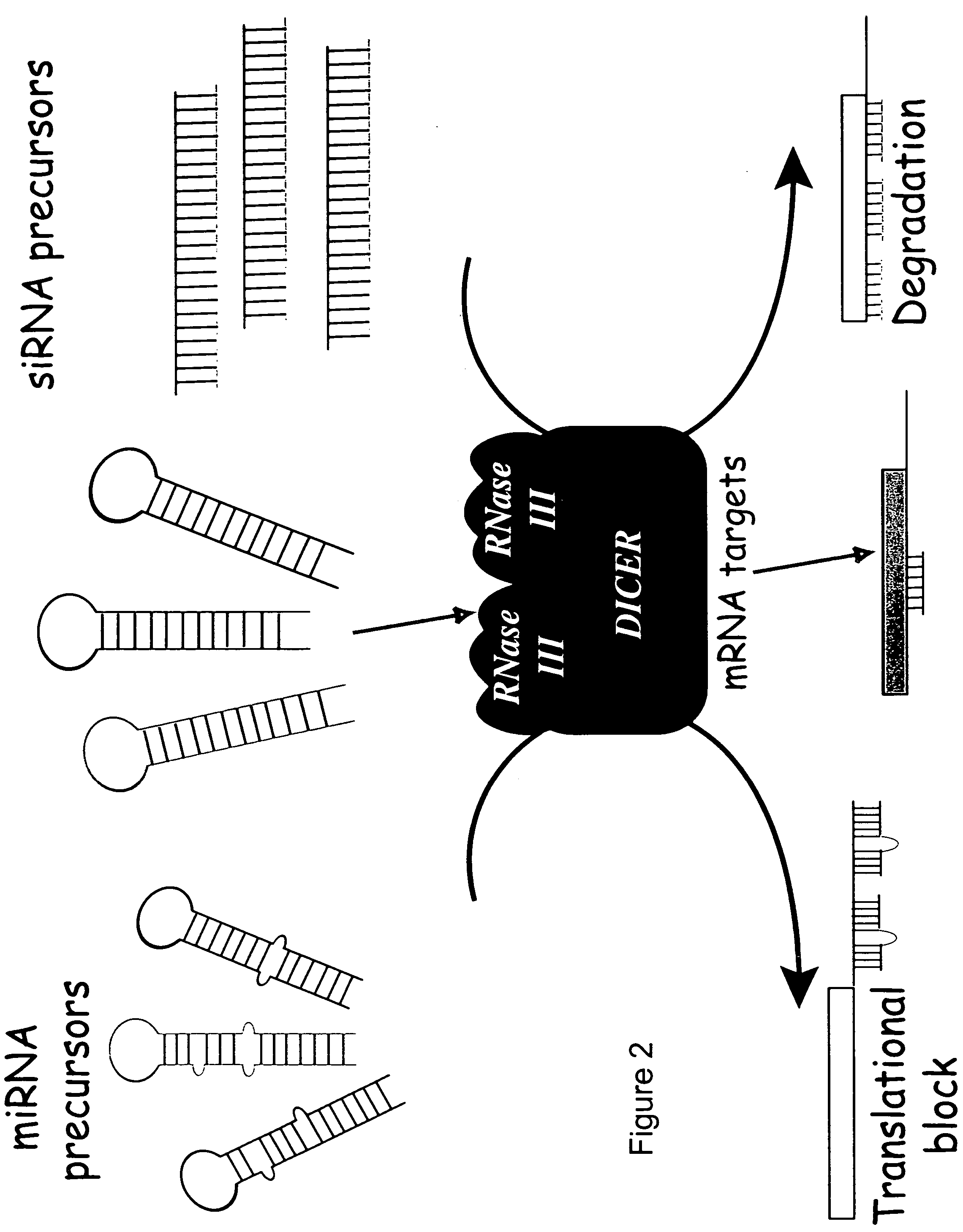
Method for efficient RNA interference in mammalian cells
The present invention relates to methods of making RNAi libraries using E. coli RNAse III for inhibition of mammalian gene expression.
Owner:RGT UNIV OF CALIFORNIA
Mammalian genes involved in toxicity and infection
InactiveUS20140242692A1Low toxicityReduced activityAntibacterial agentsOrganic active ingredientsMammalToxicology
The present invention relates to cellular proteins that are involved in toxicity and infection or are otherwise associated with the life cycle of one or more pathogens.
Owner:VANDERBILT UNIV
Mammalian genes involved in rapamycin resistance and tumorgenesis annexin XIII genes
InactiveUS20060052320A1High expressionReduce the binding forcePeptide/protein ingredientsMicrobiological testing/measurementDiseaseAnnexin XIII
The present invention provides methods and compositions for regulating rapamycin resistance and / or tumorgenesis by modulating the expression and / or the activity of annexin XIII gene. The invention also provides methods and compositions for treatment of diseases, e.g., cancers, by modulating the expression and / or activity of annexin XIII gene. The invention also provides methods and compositions for diagnosing and screening annexin XIII mediated rapamycin resistance and / or tumorgenesis in patients. The invention further provides host cells whose annexin XIII gene can be reversibly switched on or off, and to methods of using annexin XIII gene in evaluation and screening for drugs which regulate rapamycin resistance and / or tumorgenesis. The invention also provides methods for generating genetically modified cells having altered sensitivity to rapamycin by knocking out a gene which mediates rapamycin resistance.
Owner:FUNCTIONAL GENETICS
Influenza vaccines
ActiveUS20100160421A1Less protectionBroad and efficient immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininSaline injection
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
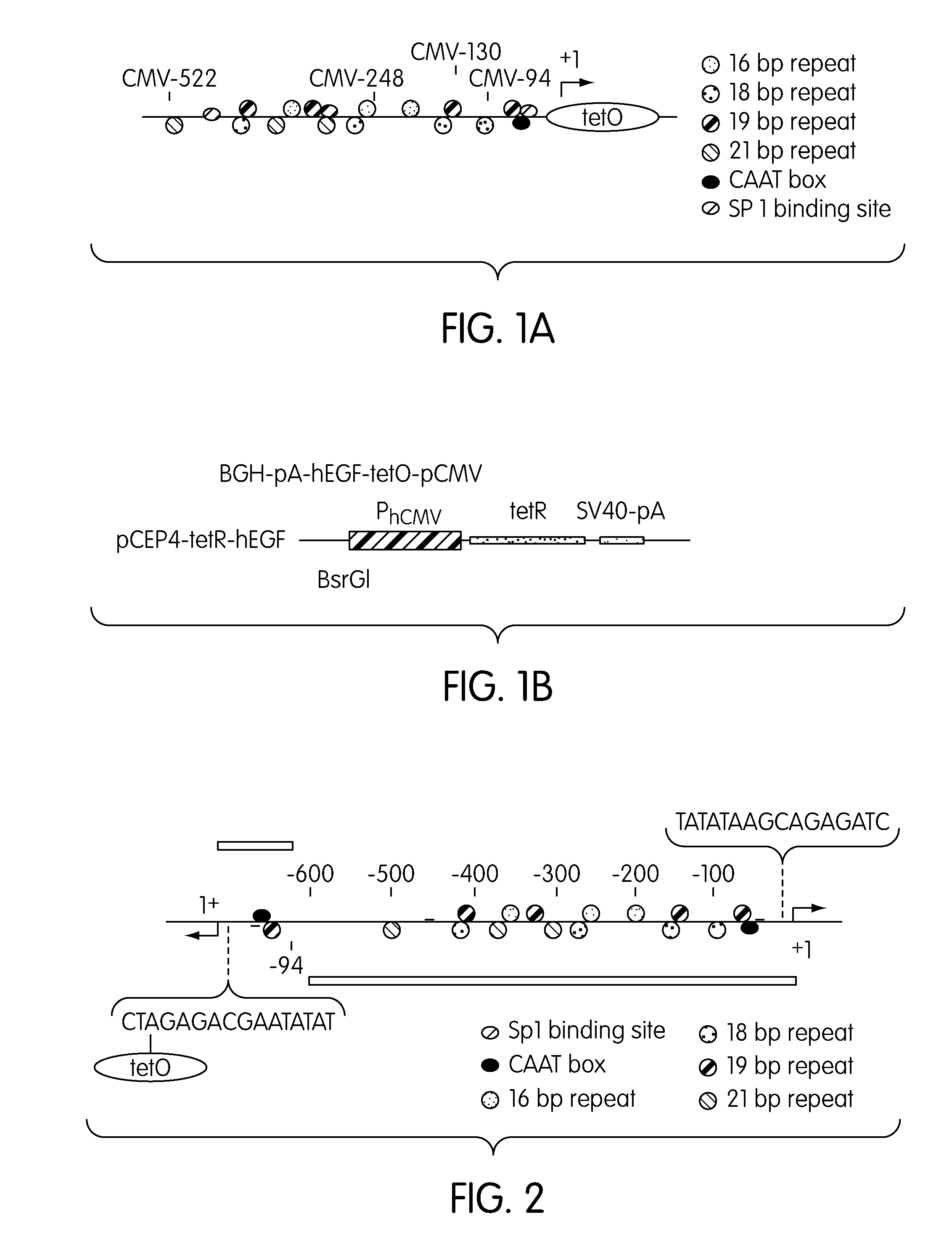
Promoter System for Regulatable Gene Expression in Mammalian Cells
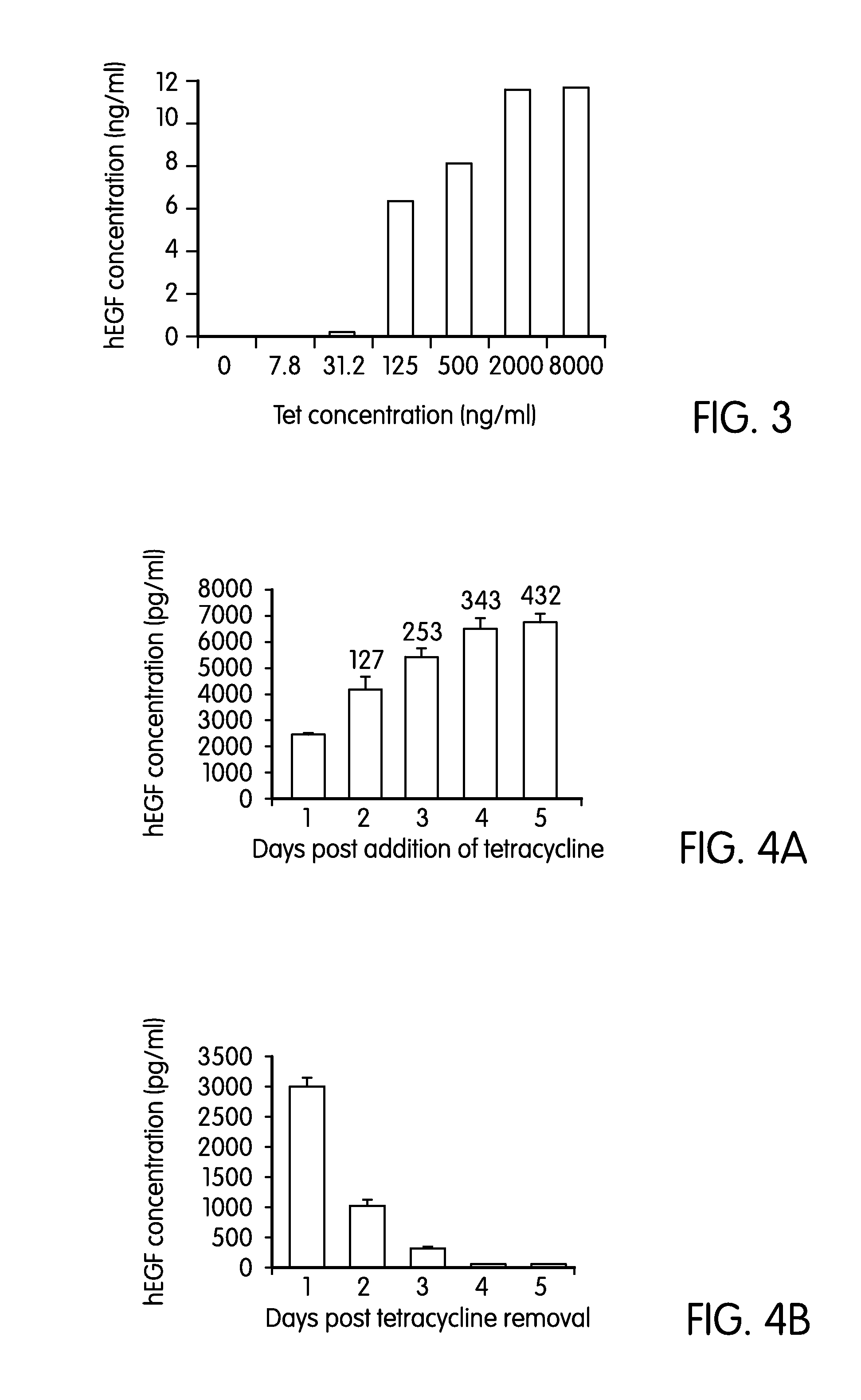
The present invention is directed to a bidirectional human cytomegalovirus (hCMV) promoter that can be used to promote transcription on both strands of a double stranded DNA molecule. When used as part of a system that includes tet operator and the gene coding for the tet repressor, the promoter can be used to induce mammalian gene expression in a highly regulated way.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC
Reagents and methods for identifying gene targets for treating cancer
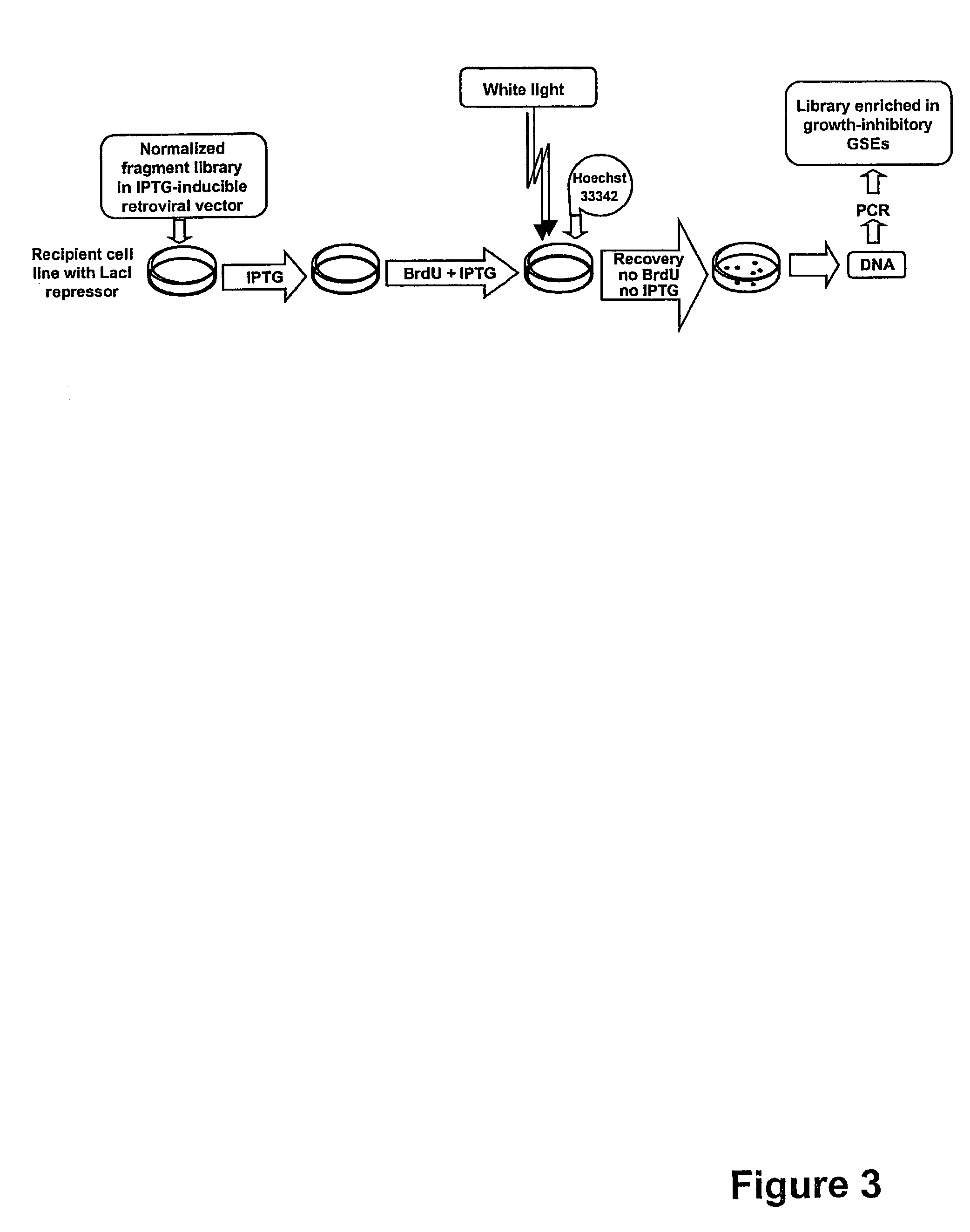
InactiveUS7235403B2Growth inhibitionImprove developmentCompound screeningApoptosis detectionGene targetsMammal
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
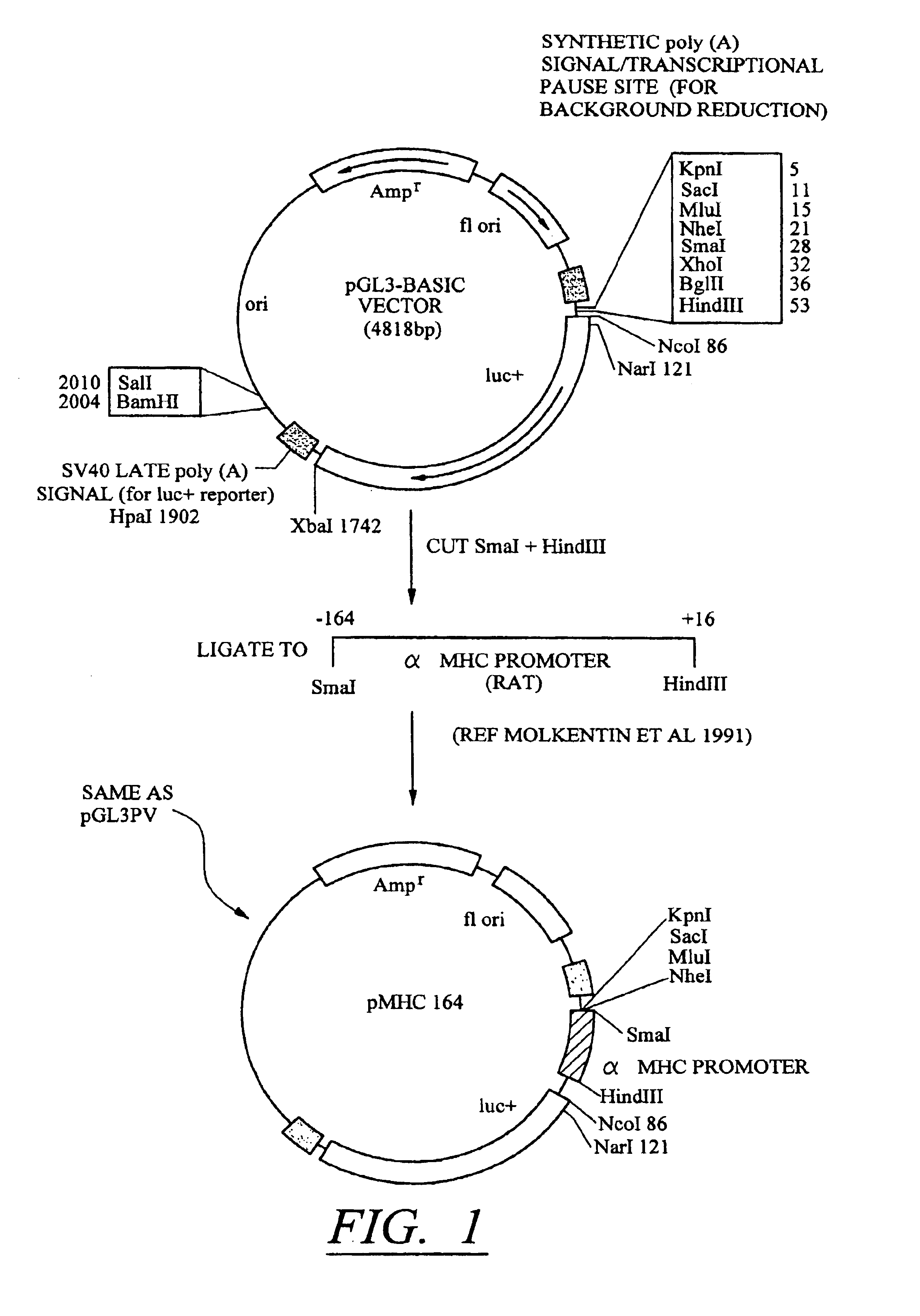
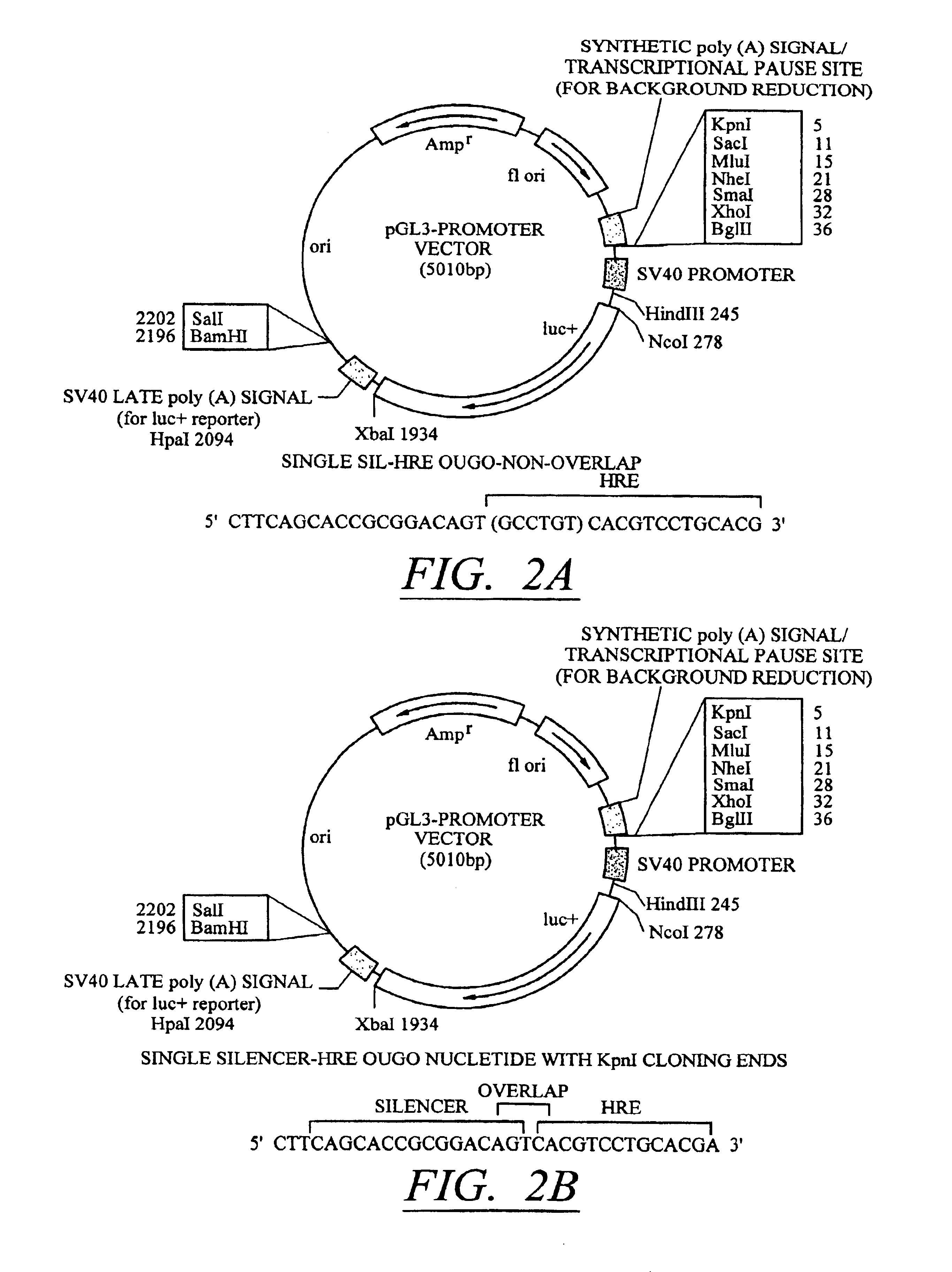
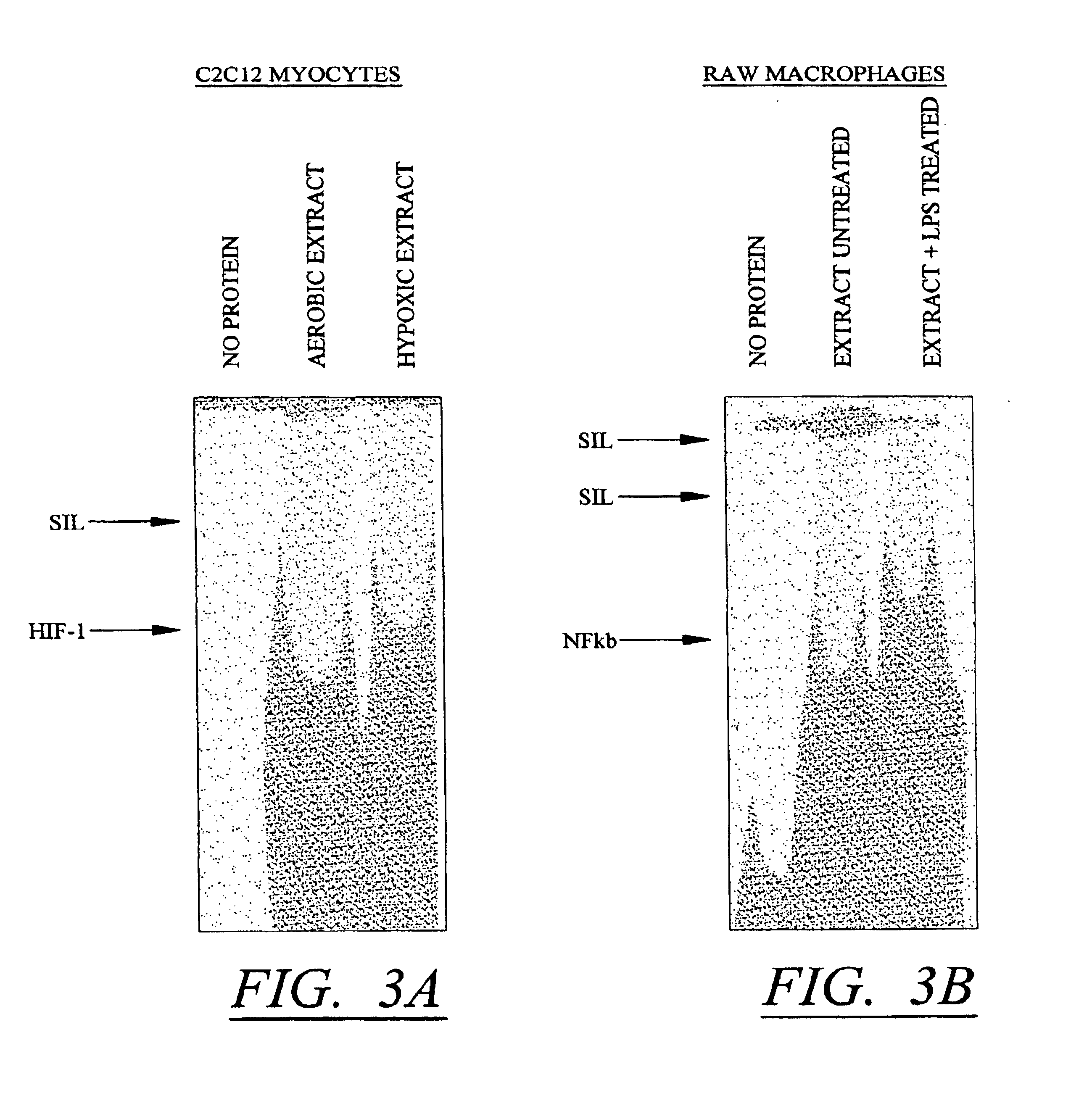
Molecular switch for regulating mammalian gene expression
Expression vectors are disclosed that are comprised of (a) one or more silencer elements and conditionally inducible elements to form silencer-inducible regions and (b) promoters in operative linkage upstream of at least one expressed region. The expression vector thereby regulates expression of at least one downstream region by conditional silencing in which an expressed DNA region of a gene is transcribed to produce RNA transcripts, which may or may not be translated to produce polypeptides. Genetically engineered mammalian cells and non-human mammals can be made using such expression vectors through transfection and transgenesis techniques. Moreover, processes of making and using the aforementioned products are disclosed (e.g., the expression vector may be used diagnostically, therapeutically, or prophylactically).
Owner:KEITH WEBSTER +1
Mammalian genes involved in rapamycin resistance and tumorgenesis: rapr6 genes
InactiveUS20060099676A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMammalRegulator gene
The invention provides nucleotide sequence of a novel mammalian gene, the RapR6 gene, which is involved in resistance and tumorgenesis, and amino acid sequences of the encoded proteins. The present invention also provides methods and compositions for regulating rapamycin resistance and / or tumorgenesis by modulating the expression and / or the activity of RapR6 gene. The invention also provides methods and compositions for treatment of diseases, e.g., cancers, by modulating the expression and / or activity of RapR6 gene. The invention also provides methods and compositions for diagnosing and screening RapR6 mediated rapamycin resistance and / or tumorgenesis in patients. The invention further provides host cells a portion of whose RapR6 gene can be reversibly expressed, and to methods of using the RapR6 gene in evaluation and screening for drugs which regulate rapamycin resistance and / or tumorgenesis.
Owner:FUNCTIONAL GENETICS
Mammalian genes; related reagents and methods
InactiveUS20050142587A1HydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsMammalSpecific antibody
Purified genes encoding proteins from a mammal, reagents related thereto including purified proteins, specific antibodies, and nucleic acids encoding these molecules are provided. Methods of using said reagents and diagnostic kits are also provided.
Owner:SCHERING CORP
Rath genes and polypeptides and methods for the treatment and diagnosis of immune disorders
The present invention relates, first, to the identification of novel nucleic acid molecules, termed RATH genes and RATH gene products encoded by such nucleic acid molecules, or degenerate variants thereof, that participate in the regulation, control and / or modulation of G-protein-mediated signal transduction involved in T cell activation, including, but not limited to T helper (TH) cell and TH cell subpopulation activation. Specifically, the nucleic acid molecules of the present invention include the genes corresponding to the mammalian RATH genes, including the RATH1.1 genes. Sequence analysis indicates that the RATH genes are novel genes belonging to the RGS ("regulator of G-protein signalling") gene family, a gene family which encodes gene products involved in G-protein-mediated signal transduction.
Owner:MILLENNIUM PHARMA INC
Vectors and methods for the mutagenesis of mammalian genes
InactiveUS20050059078A1Increased cellular fluorescenceGood streamlineSugar derivativesMicrobiological testing/measurementMammalGenome
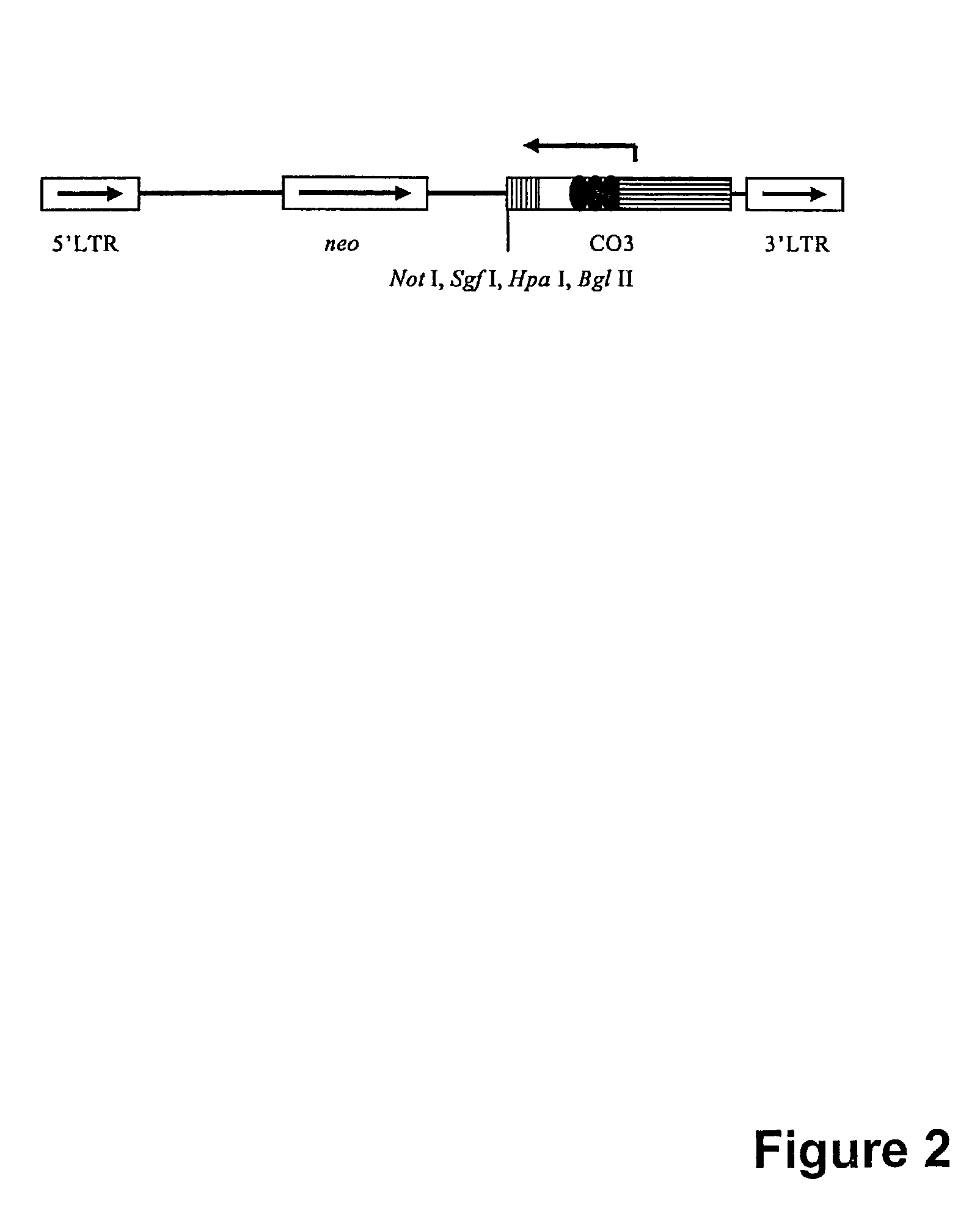
Disclosed herein are methods for mutagenizing a mammalian gene, the methods involving introducing into a mammalian cell a retroviral vector which includes a splice acceptor sequence, a transcription termination sequence, and retroviral packaging and integration sequences, the introducing step being carried out under conditions which allow the vector to integrate into the genome of the cell. Also disclosed are retroviral vectors for use in these methods as well as methods for the use of mutagenized cells.
Owner:OMEROS CORP
Compositions for the diagnosis and treatment of chediak-higashi syndrome
The present invention relates to the identification of novel nucleic acid molecules and proteins encoded by such nucleic acid molecules or degenerate variants thereof, that participate in the differentiation and / or function of intracellular vesicles. The nucleic acid molecules of the present invention represent the genes corresponding to the mammalian bg gene, a gene that, when mutated, is responsible for the human Chediak-Higashi syndrome.
Owner:MILLENNIUM
Methods and compositions for diagnosing and treating chromosome-18p related disorders
InactiveUS6342351B1Affect safetyAffect efficacyFungiNervous disorderSchizo-affective typeGenetic Screening (procedure)
The present invention relates of the mammalian HKNG1 gene, a gene associated with bipolar affective disorder (BAD) in humans. The invention relates, in particular, to methods for the diagnostic evaluation, genetic testing and prognosis of HKNG1 neuropsychiatric disorders including schizophrenia, attention deficit disorder, a schizoaffective disorder, a bipolar affective disorder or a unipolar affective disorder.
Owner:MILLENNIUM PHARMA INC +1
Method for extracting genome DNA from mammal solidification blood
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com