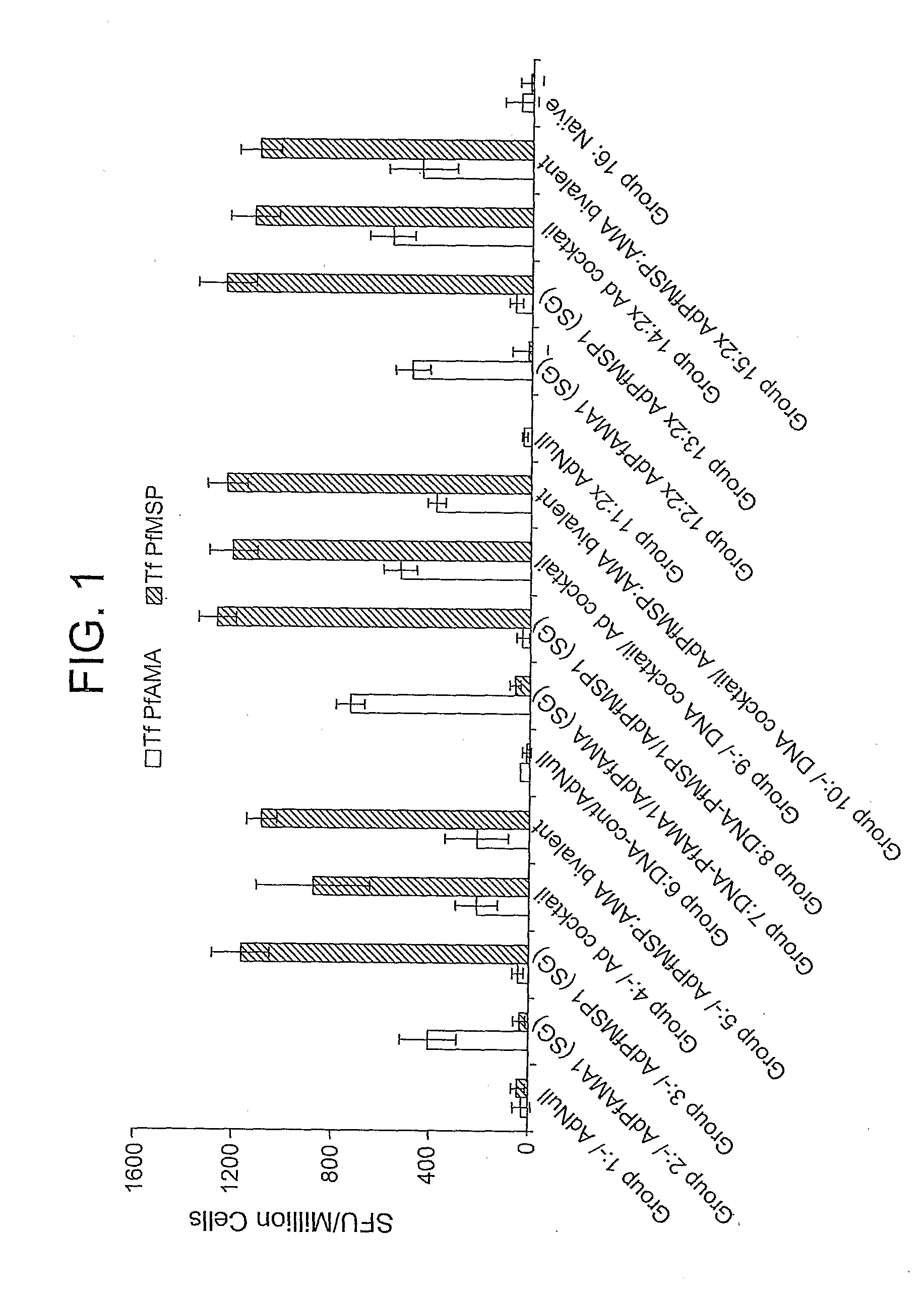
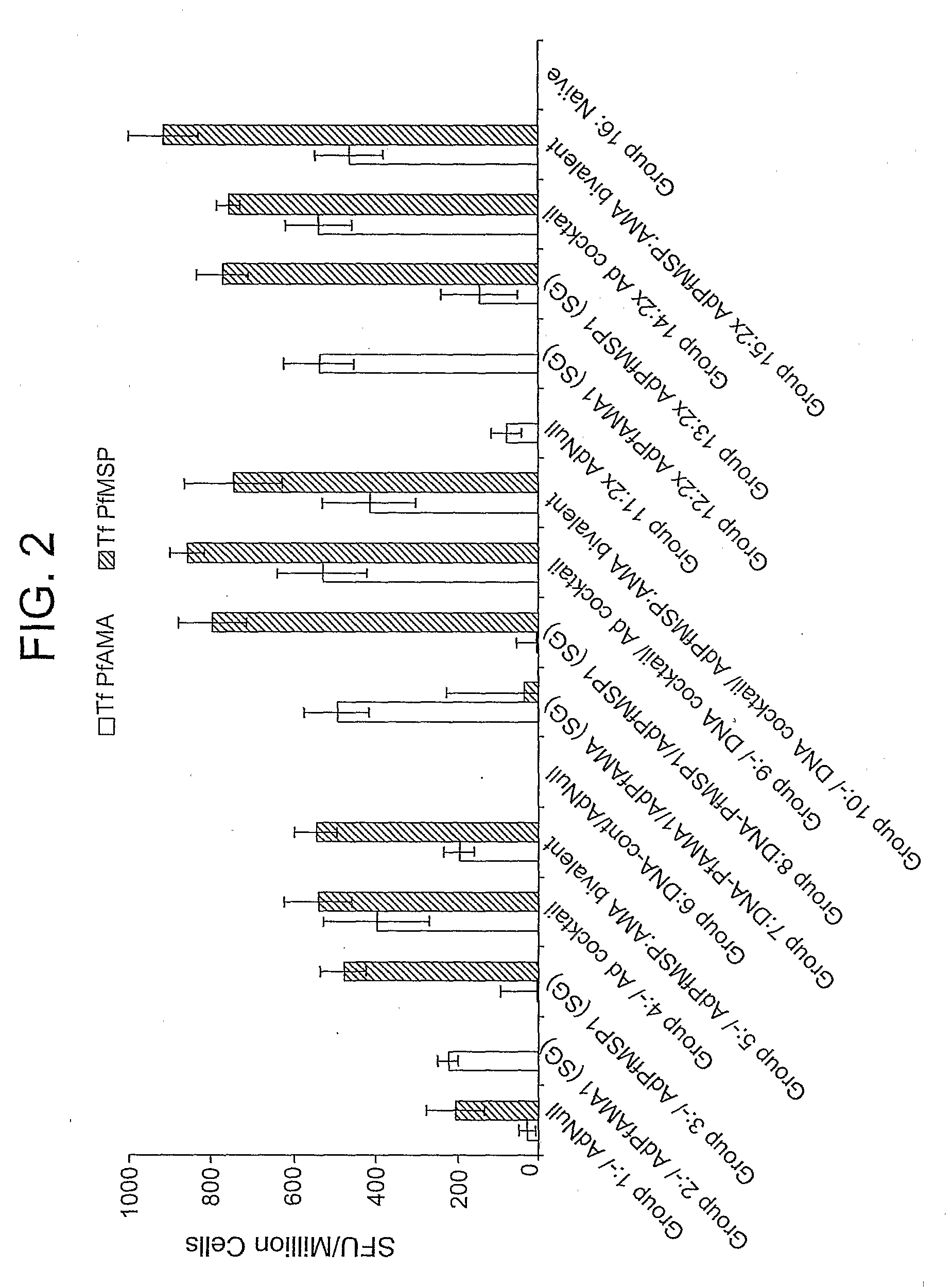
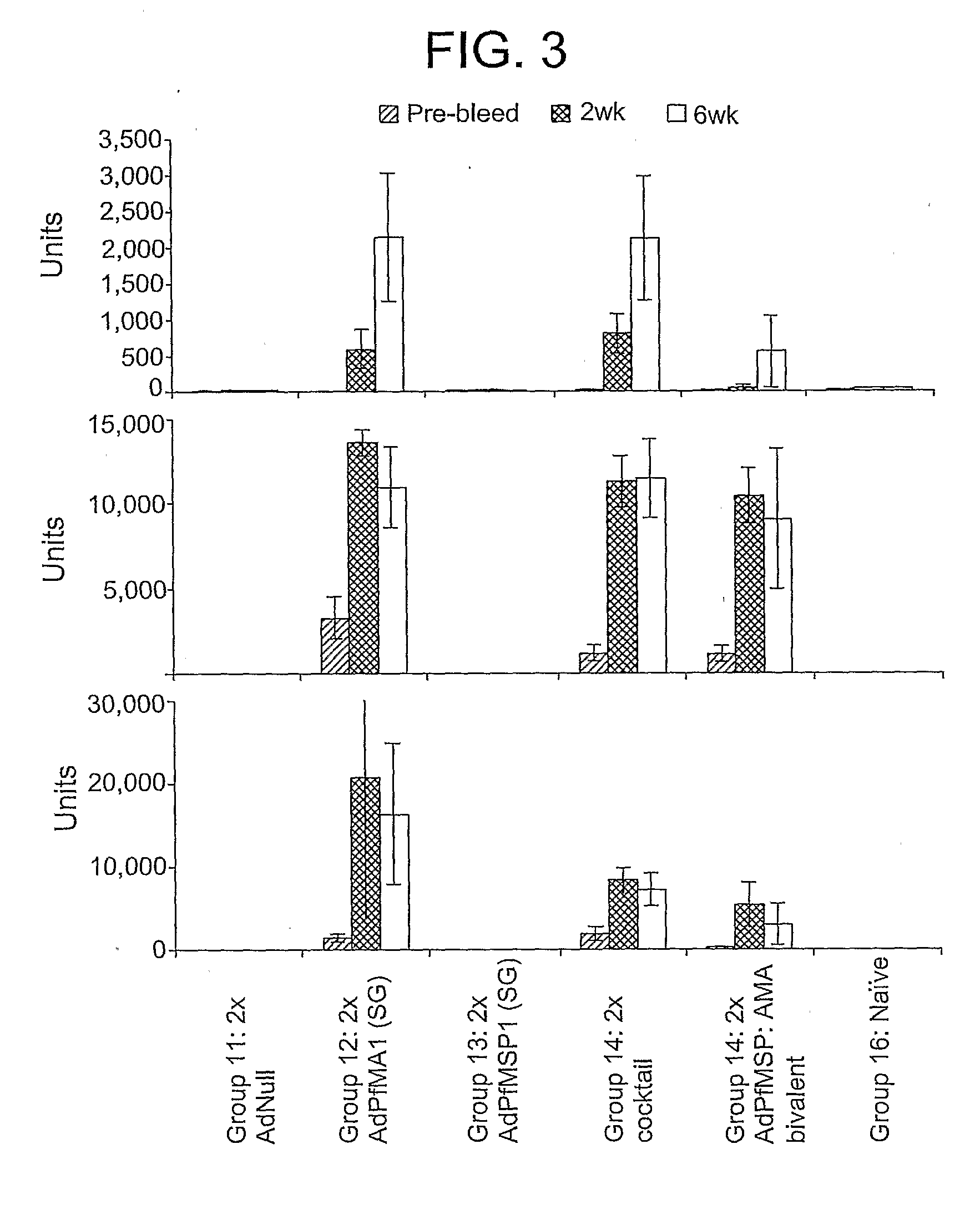
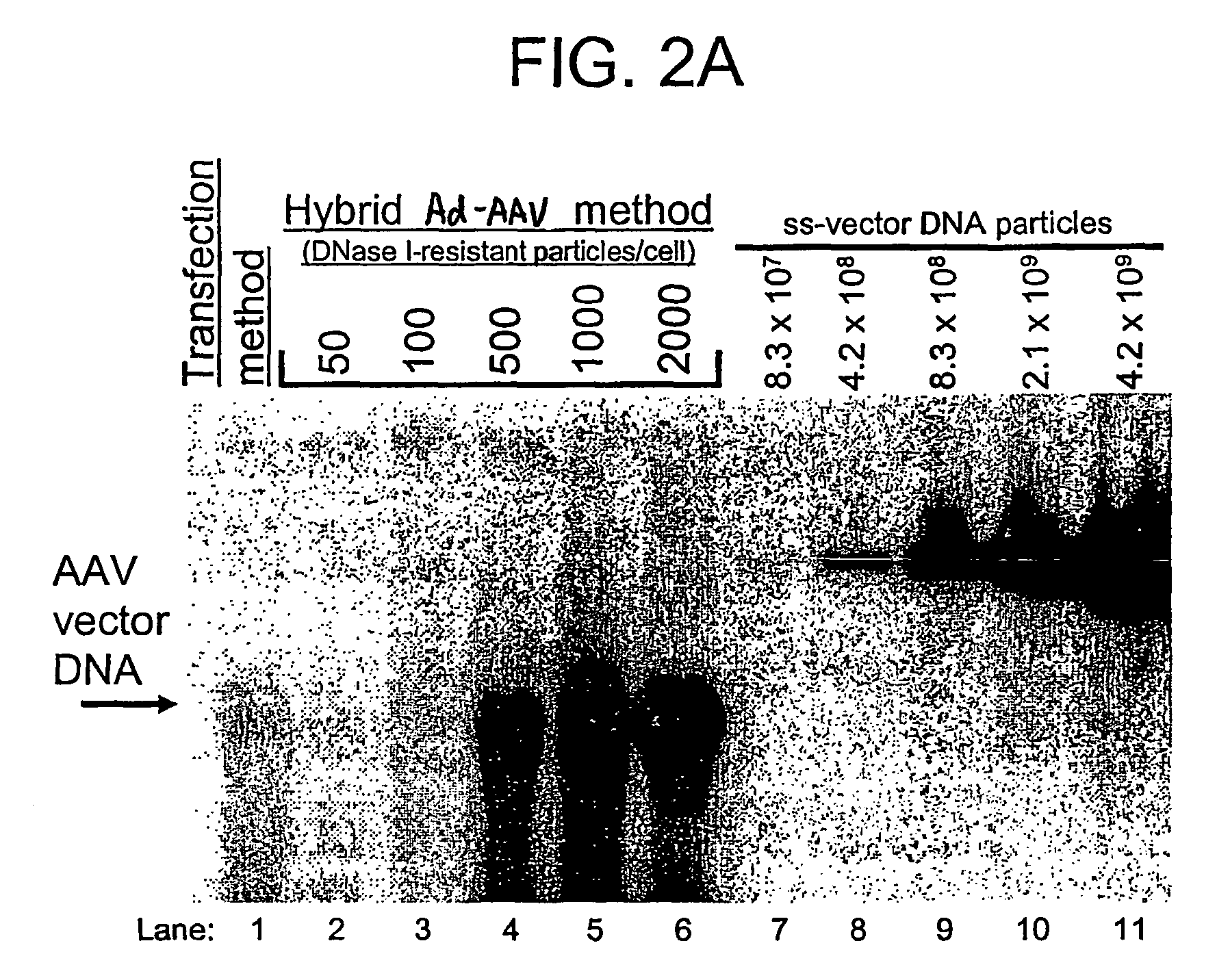
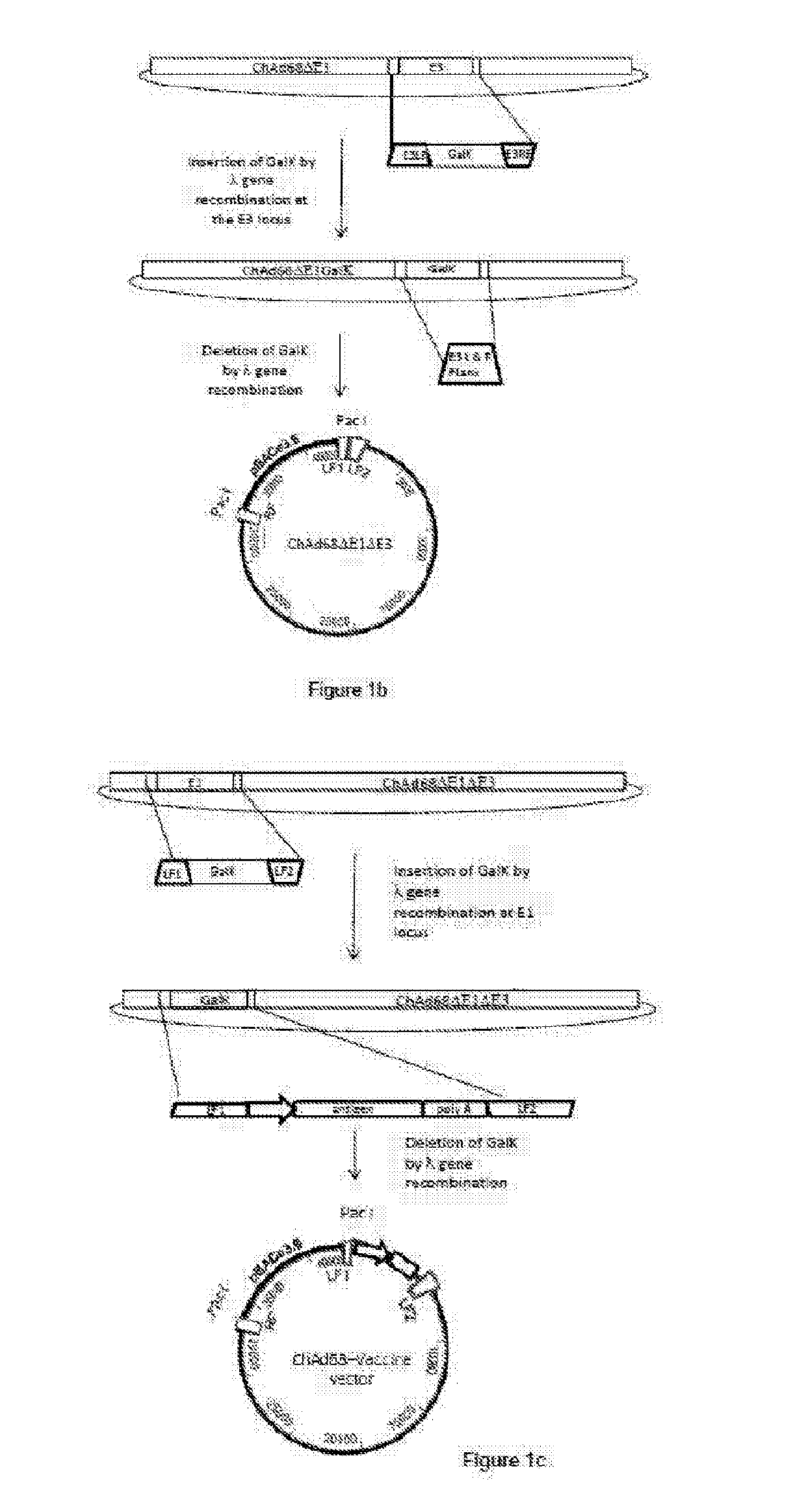
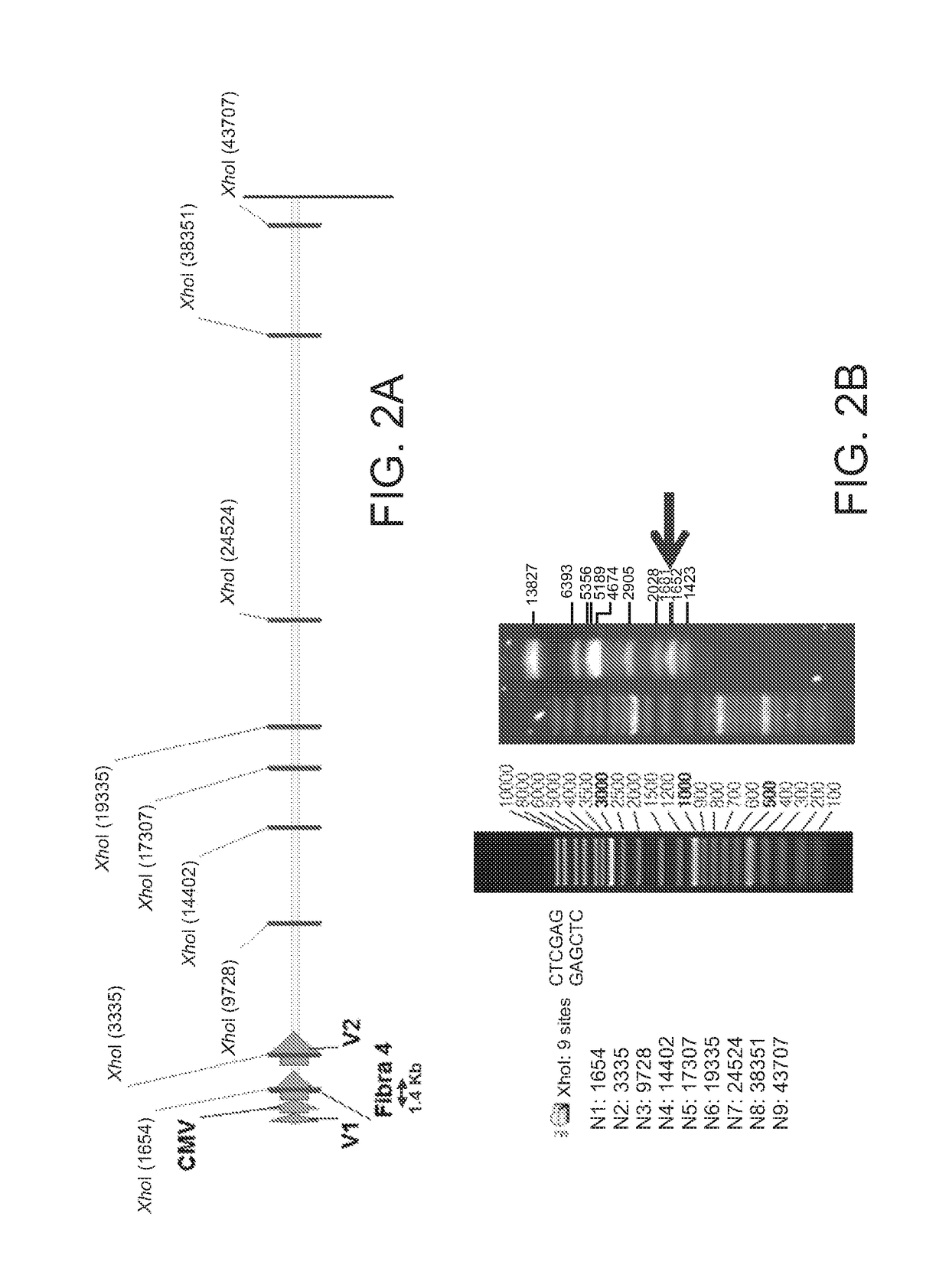
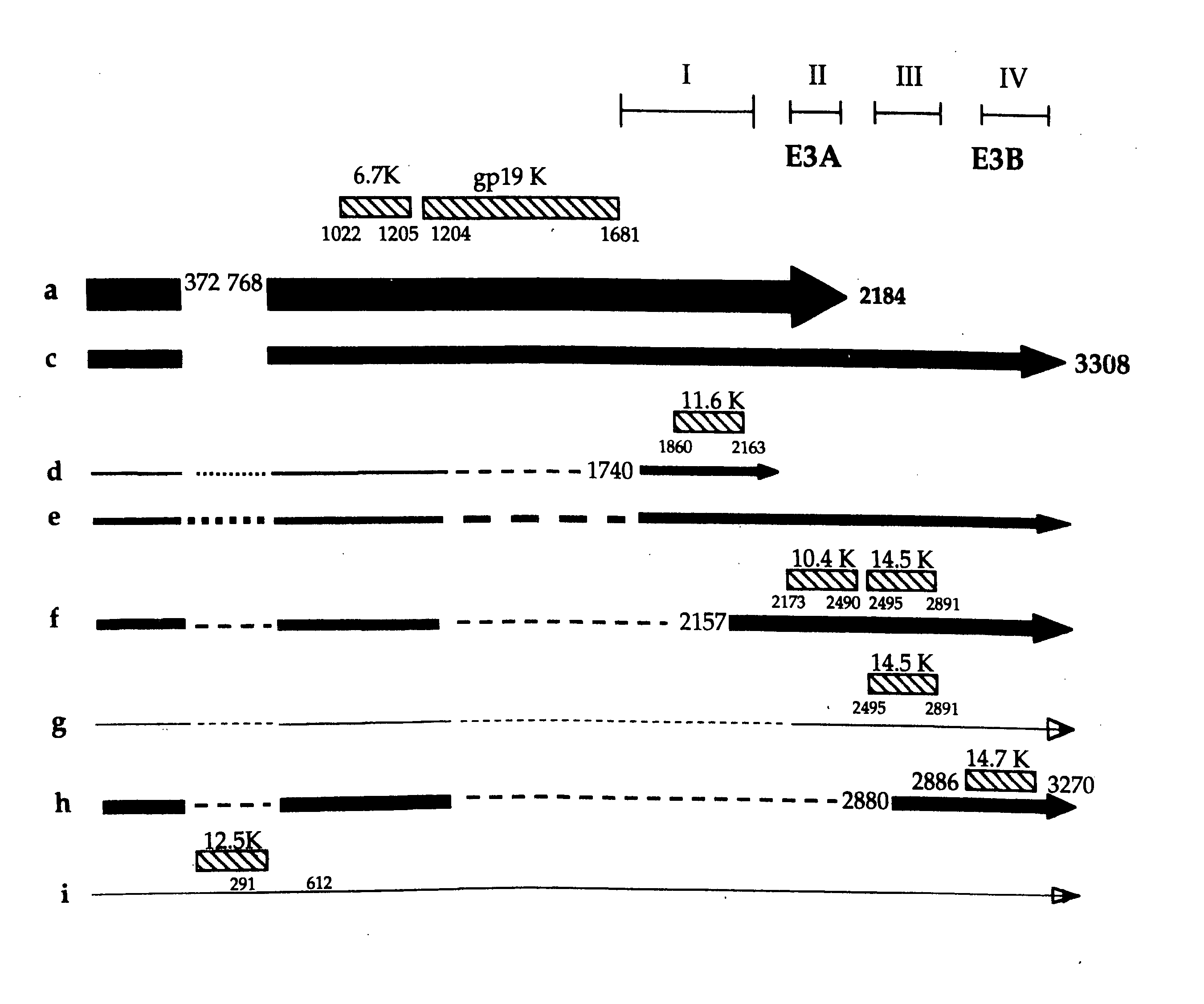
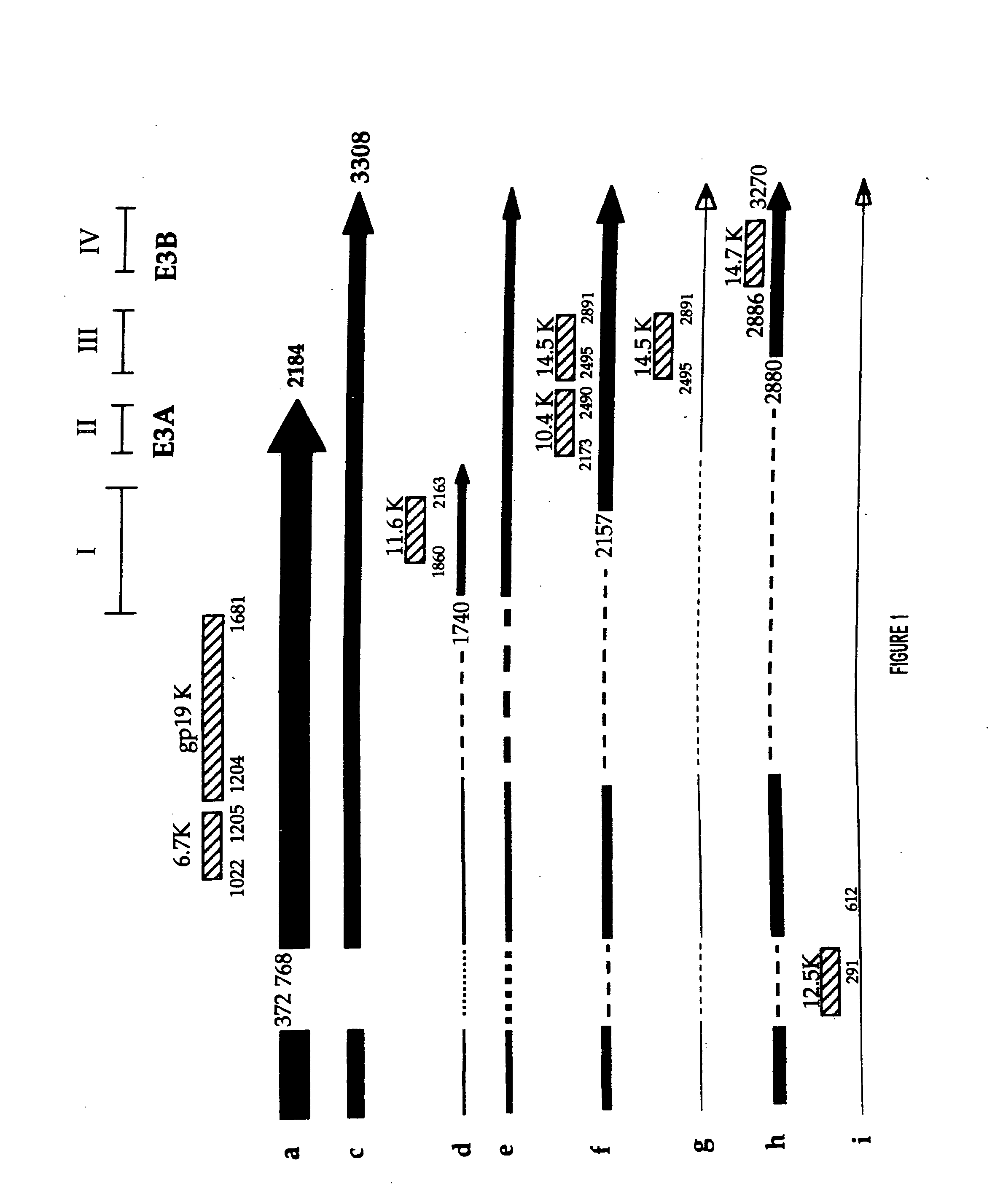
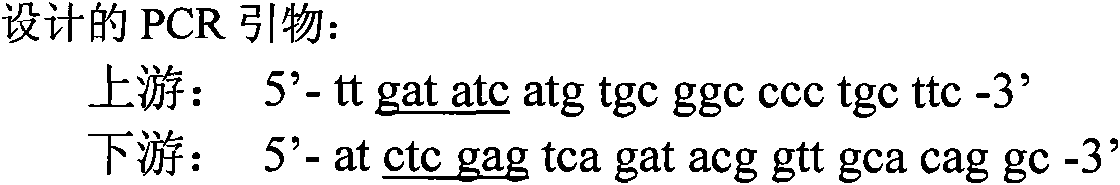Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Adenovirus genome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adenovirus genomes are linear, non-segmented double-stranded (ds) DNA molecules that are typically 26-46 Kbp long, containing 23-46 protein-coding genes. The example used for the following description is Human adenovirus E, a mastadenovirus with a 36 Kbp genome containing 38 protein-coding genes. While the precise number and identity of genes varies among adenoviruses, the basic principles of genome organization and the functions of most of the genes described in this article are shared among all adenoviruses.
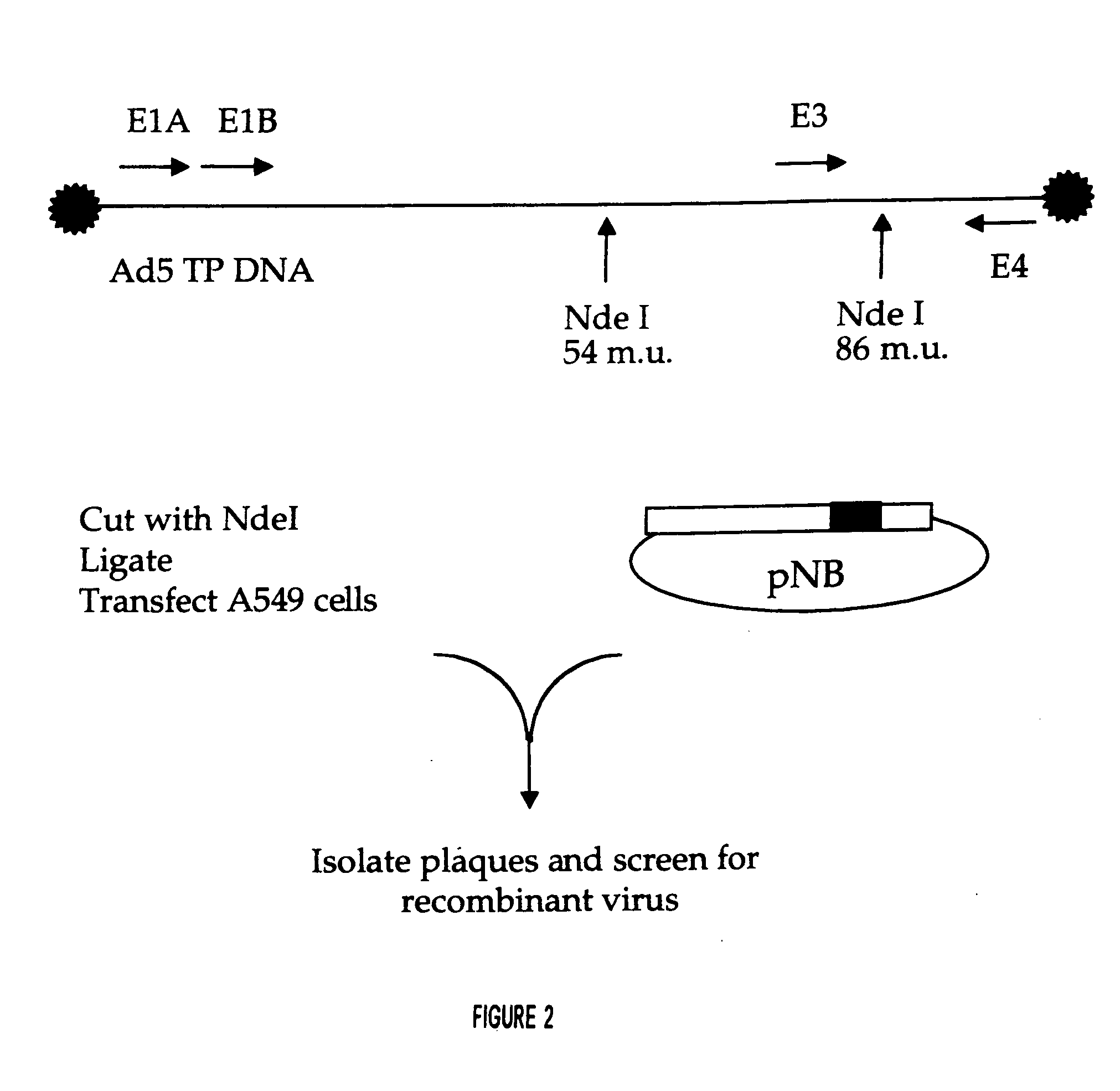
Adenoviral vector and methods for making and using the same
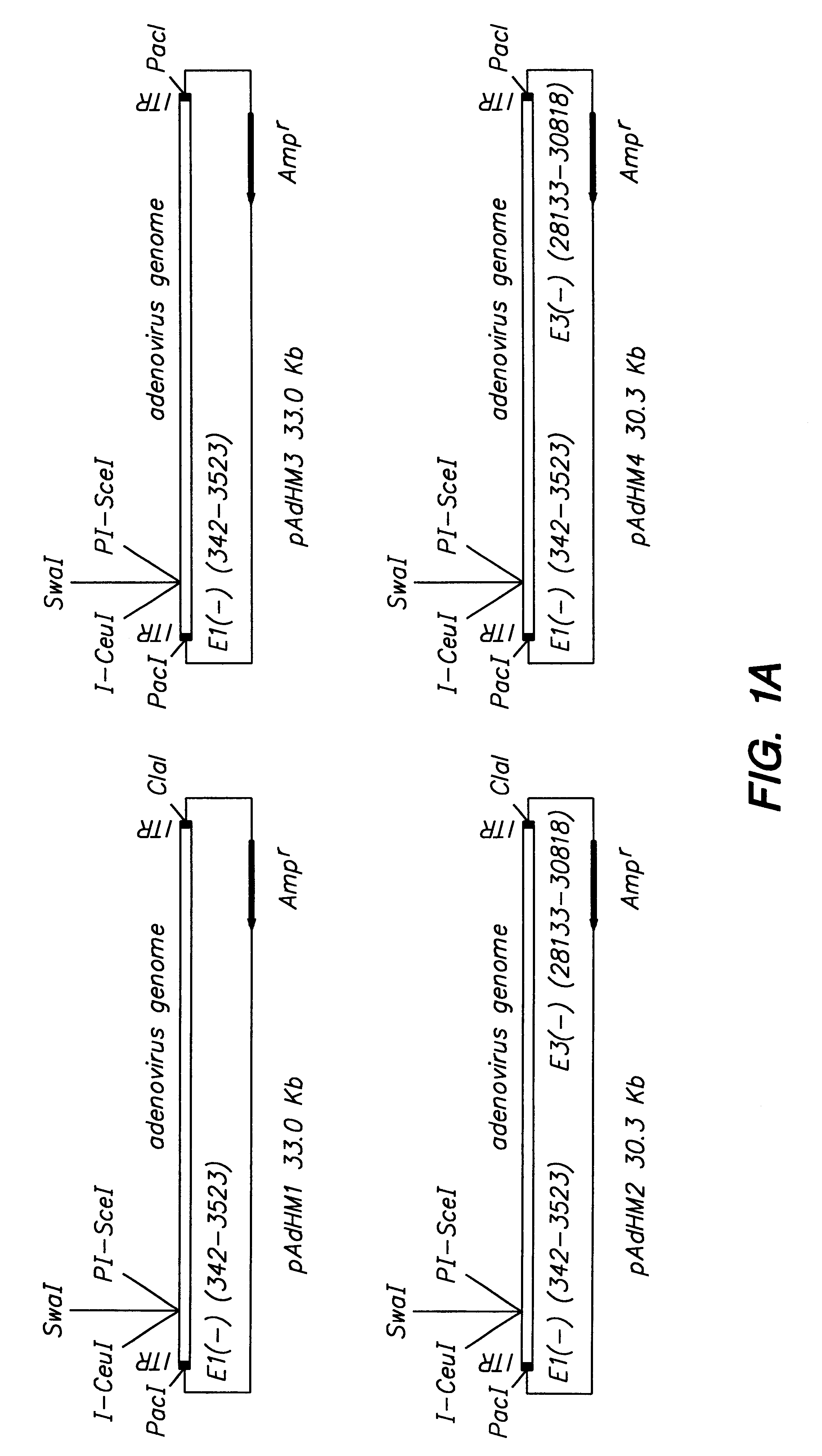
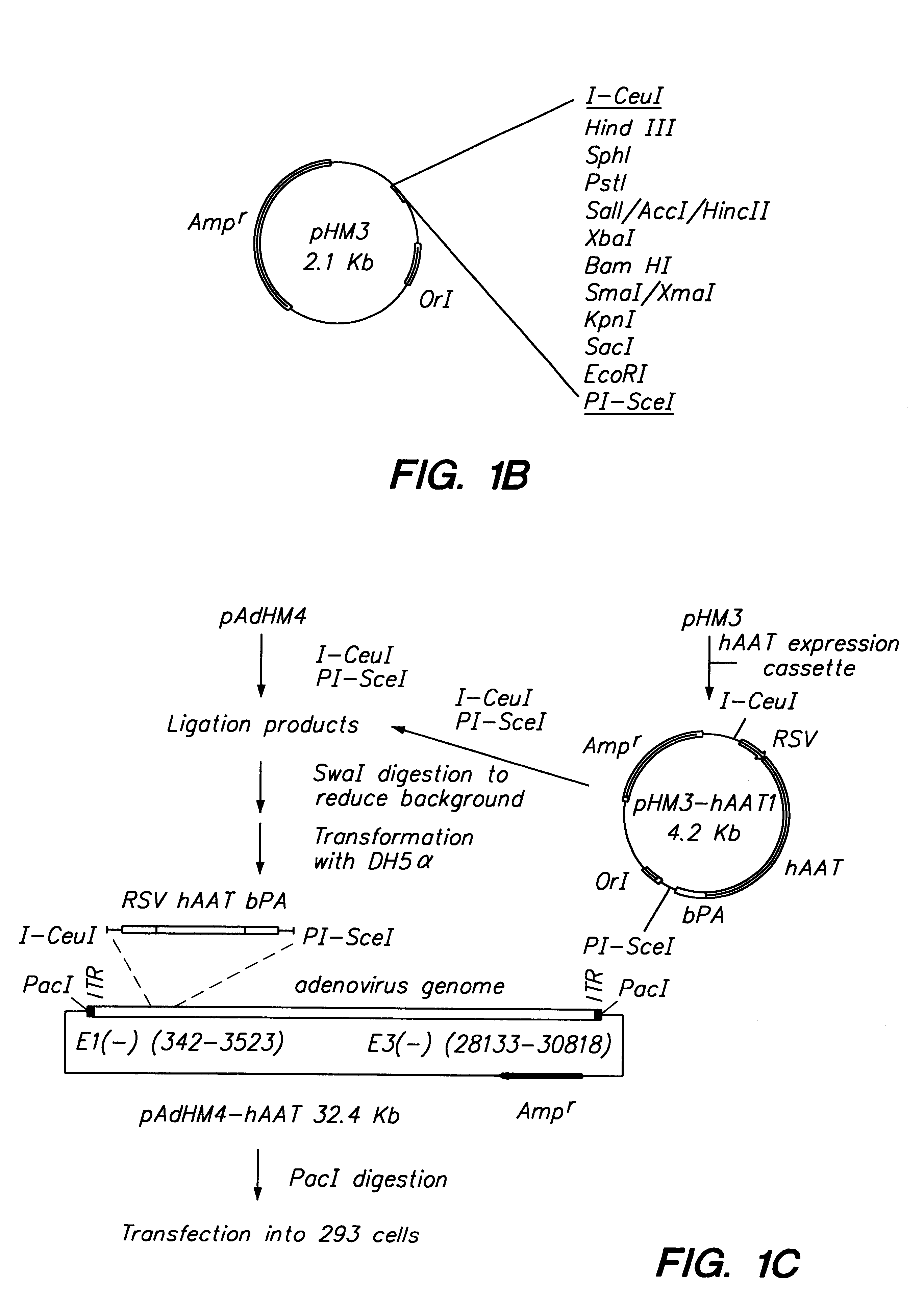
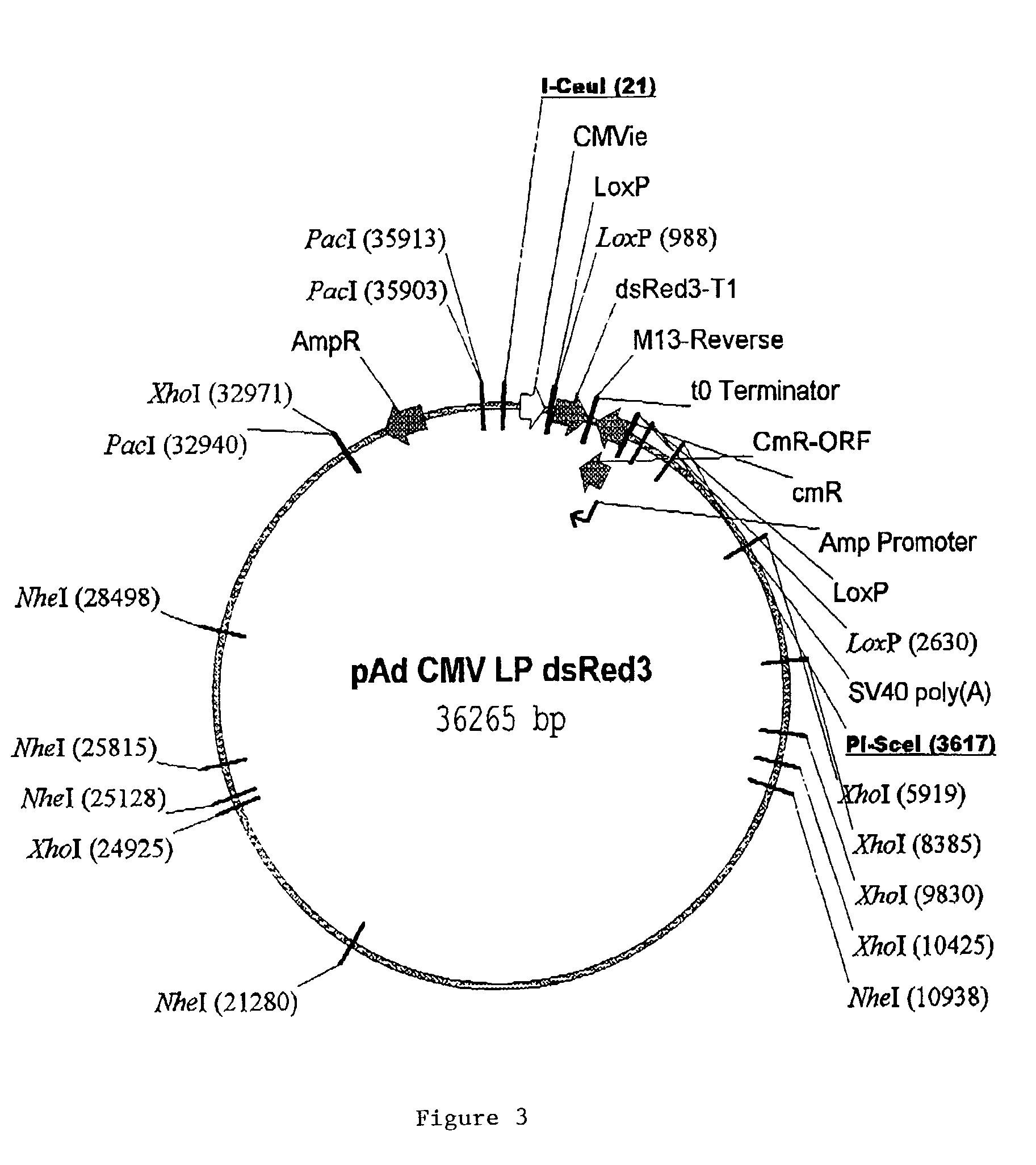
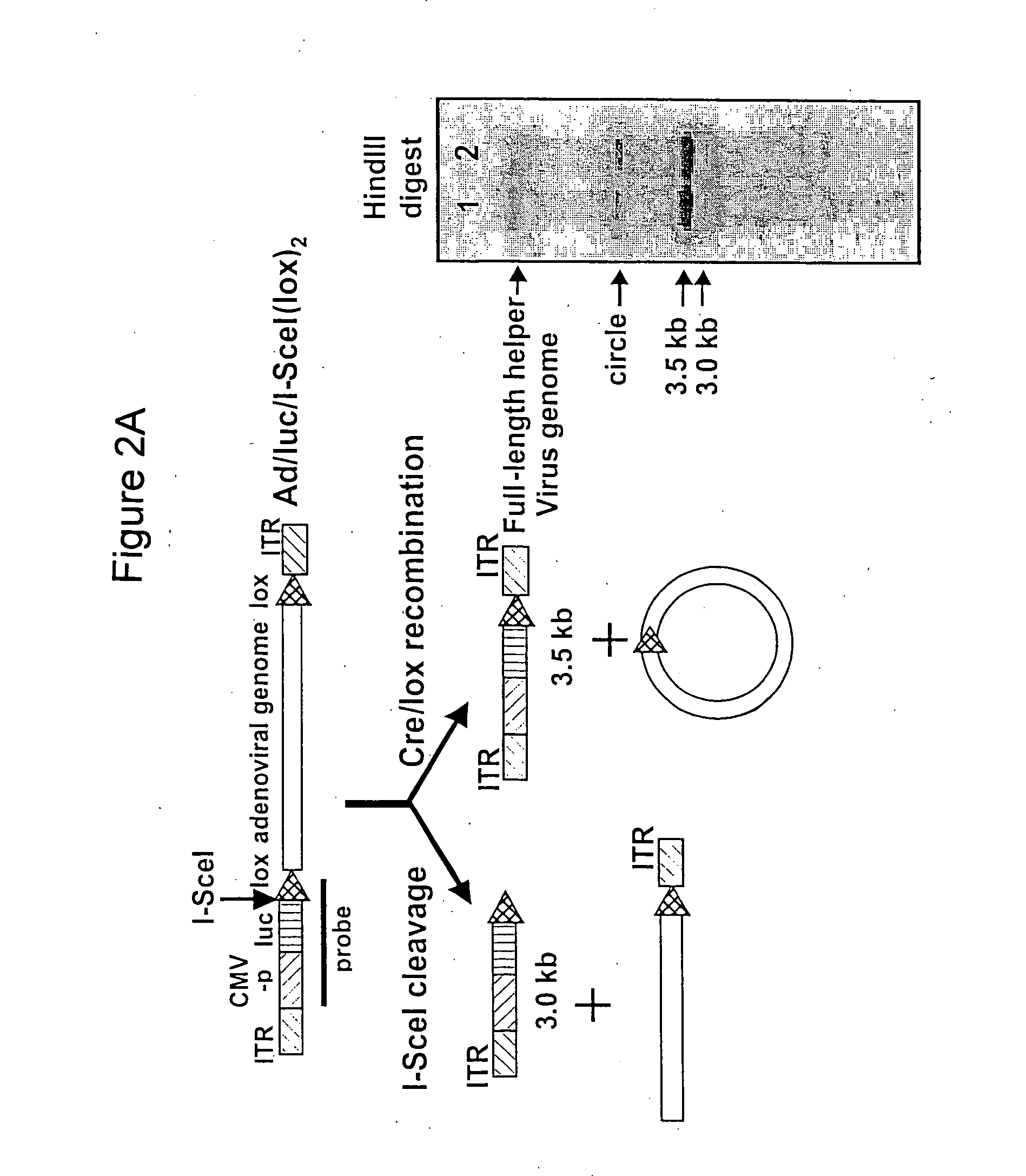
In vitro methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from first and second vectors. The first vector includes an adenoviral genome having an E region deletion and three different, non-adenoviral restriction endonuclease sites located in the E region. The second vector is a shuttle vector and includes an insertion nucleic acid flanked by two of the three different non-adenoviral restriction endonucleases sites present in the first vector. Cleavage products are prepared from the first and second vectors using the appropriate restriction endonucleases. The resultant cleavage products are then ligated to produce the subject recombinant adenovirus genome. The subject adenoviral genomes find use in a variety of application, including as vectors for use in a variety of applications, including gene therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Viral vectors and methods for producing and using the same
InactiveUS20050220766A1Improve AAV production titerReduce and even essentially eliminate contaminationBiocidePeptide/protein ingredientsPolymerase LNucleic acid sequencing
A recombinant hybrid virus, including: (a) a deleted adenovirus vector genome comprising the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation, and further comprising a deletion in an adenovirus genomic region selected from the group consisting of: (i) the polymerase region, wherein said deletion essentially prevents the expression of a functional polymerase protein from said deleted region and said hybrid virus does not otherwise express a functional polymerase protein, (ii) the preterminal protein region, wherein said deletion essentially prevents the expression of a functional preterminal protein from said deleted region, and said hybrid virus does not otherwise express a functional preterminal protein, and (iii) both the regions of (i) and (ii); and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), said recombinant AAV vector genome comprising (i) AAV 5′ and 3′ inverted terminal repeats, (ii) an AAV packaging sequence, and (iii) a heterologous nucleic acid sequence, wherein said heterologous nucleic acid sequence is flanked by the 5′ and the 3′ AAV inverted terminal repeats of (i). Methods of making and using the recombinant hybrid virus are also disclosed.
Owner:DUKE UNIV
Site specific recombinase based method for producing adenoviral vectors
Site-specific recombinase based methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from donor and acceptor vectors that each include at least one site recombinase recognition site, where in certain preferred embodiments, one of the donor and acceptor vectors includes a single recombinase recognition site while the other includes two recombinase recognition sites. The acceptor vector includes an adenoviral genome having an E region deletion. The donor vector includes an insertion nucleic acid. In the subject methods, the donor and acceptor vectors are combined in the presence of a recombinase to produce an adenoviral genome that includes the insertion nucleic acid. The subject adenoviral genomes find use in a variety of applications, including as vectors for use in a variety of applications.
Owner:LIFE TECH CORP
Adenoviral vector-based malaria vaccines
The invention provides adenoviral vectors comprising an adenoviral genome comprising heterologous antigen-encoding nucleic acid sequences, such as Plasmodium nucleic acid sequences, operably linked to promoters. The invention further provides a method of inducing an immune response against malaria in a mammal comprising administering the adenoviral vectors to the mammal.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Viral vectors and methods for producing and using the same
InactiveUS7858367B2Reduce and even essentially eliminate contaminationReduce dependenceBiocidePeptide/protein ingredientsHeterologousPolymerase L
A recombinant hybrid virus which includes: (a) a deleted adenovirus vector genome having the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation and a deletion in an adenovirus genomic region selected from the polymerase region and / or the preterminal protein region, wherein the deletion essentially prevents the expression of a functional polymerase and / or preterminal protein from the deleted region and the hybrid virus does not otherwise express a functional polymerase protein; and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), wherein the recombinant AAV vector genome includes an AAV packaging sequence and a heterologous nucleic acid sequence, wherein the heterologous nucleic acid sequence is flanked by 5′ and 3′ AAV inverted terminal repeats.
Owner:DUKE UNIV
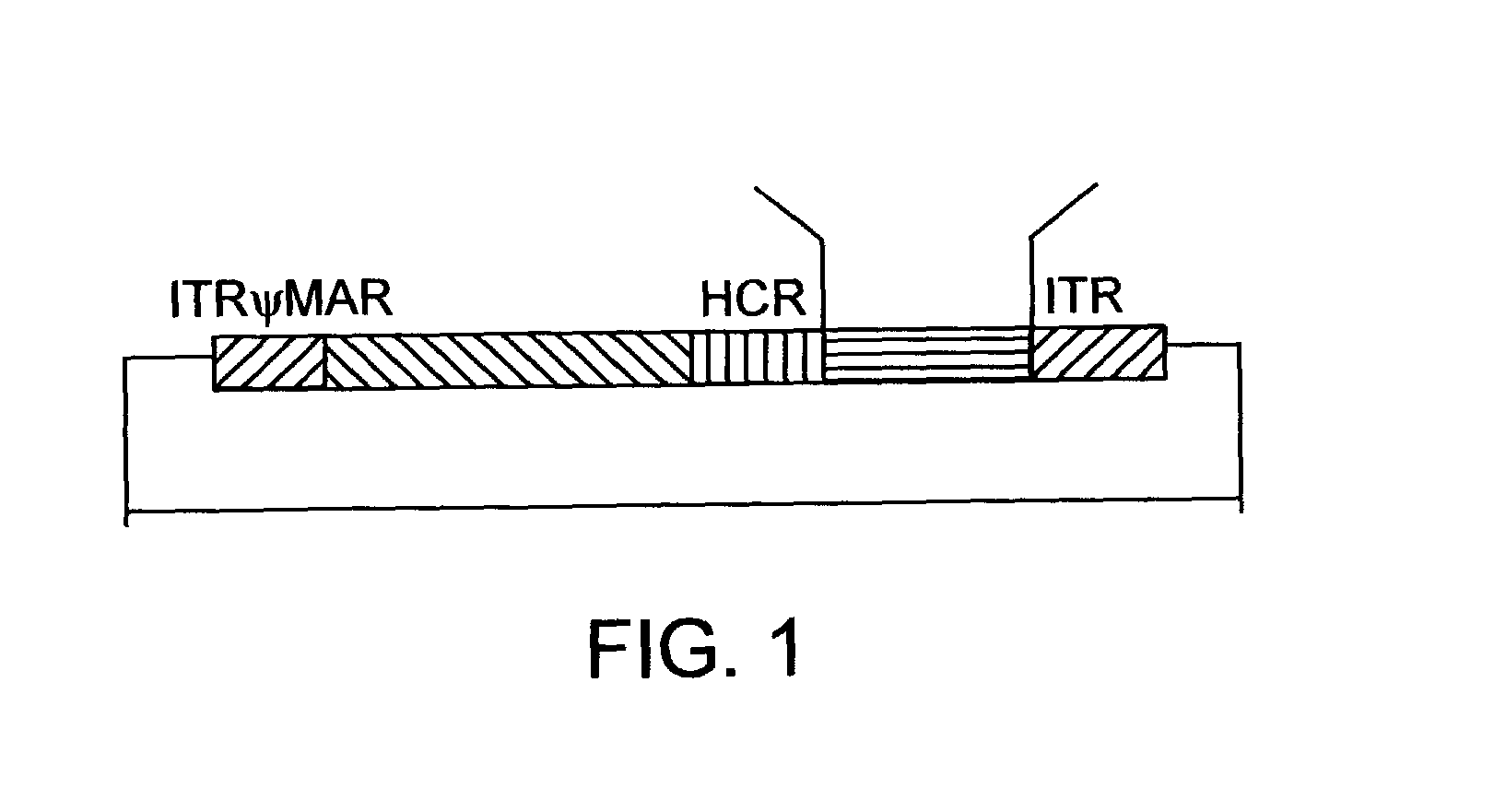
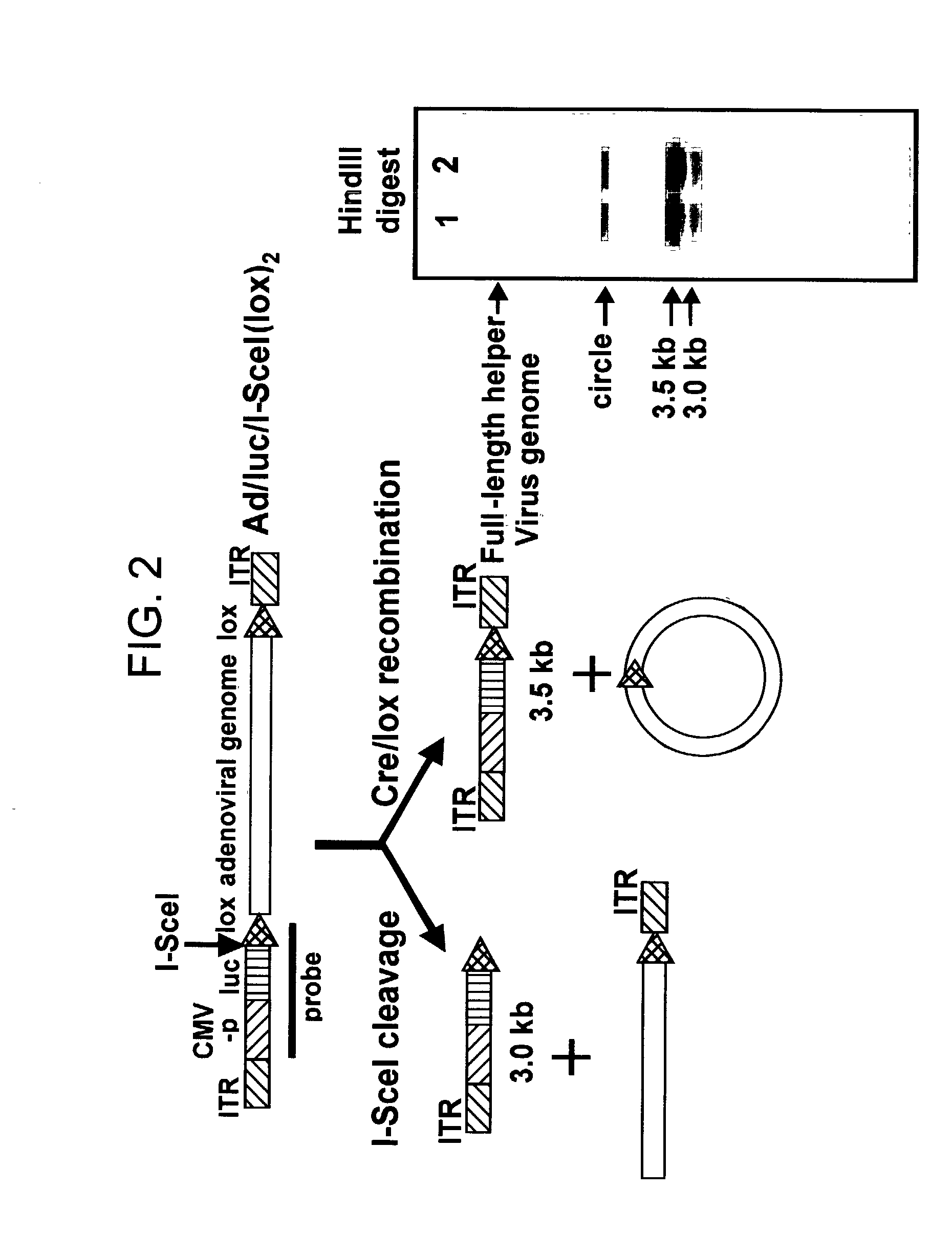
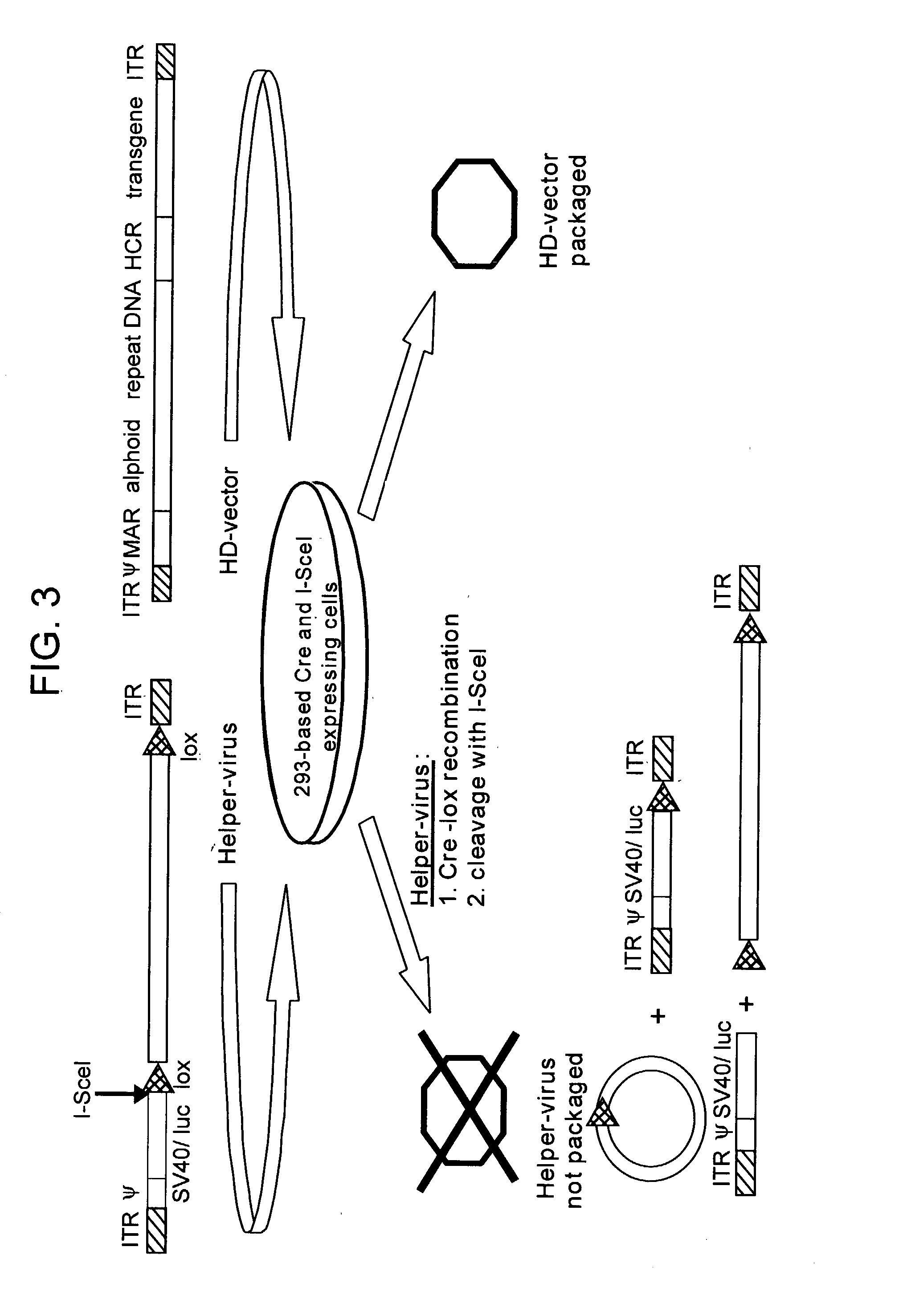
Helper dependent adenoviral vector system and methods for using the same
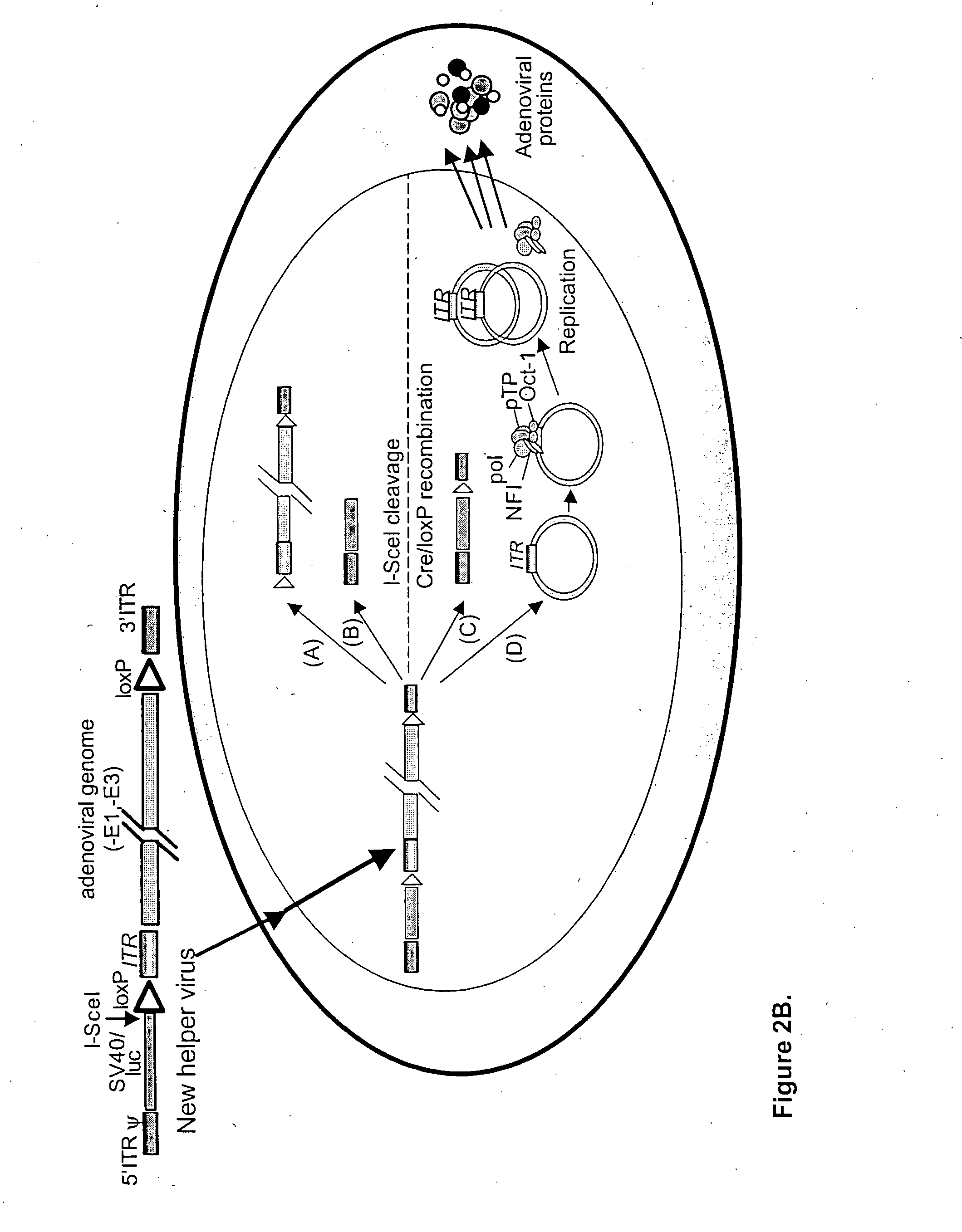
A helper dependent adenoviral vector system is provided. The subject helper dependent adenoviral vector system is made up of: (1) a "gutless" adenoviral vector that include cis-acting human stuffer DNA that provides for in vivo long term, high level expression of a coding sequence present on the vector; (2) an adenoviral helper vector that is characterized by having an adenoviral genome region flanked by recombinase recognition sites, where the helper vectors further include a non-mammalian endonuclease recognition site positioned outside of the adenoviral genome region; and (3) a mammalian cell that expresses the corresponding recombinase and endonuclease, as well as the adenoviral preterminal and polymerase proteins. Also provided are methods of using the subject systems to produce virions having the subject helper dependent adenoviral vectors encapsulated in an adenoviral capsid. In addition, kits for use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
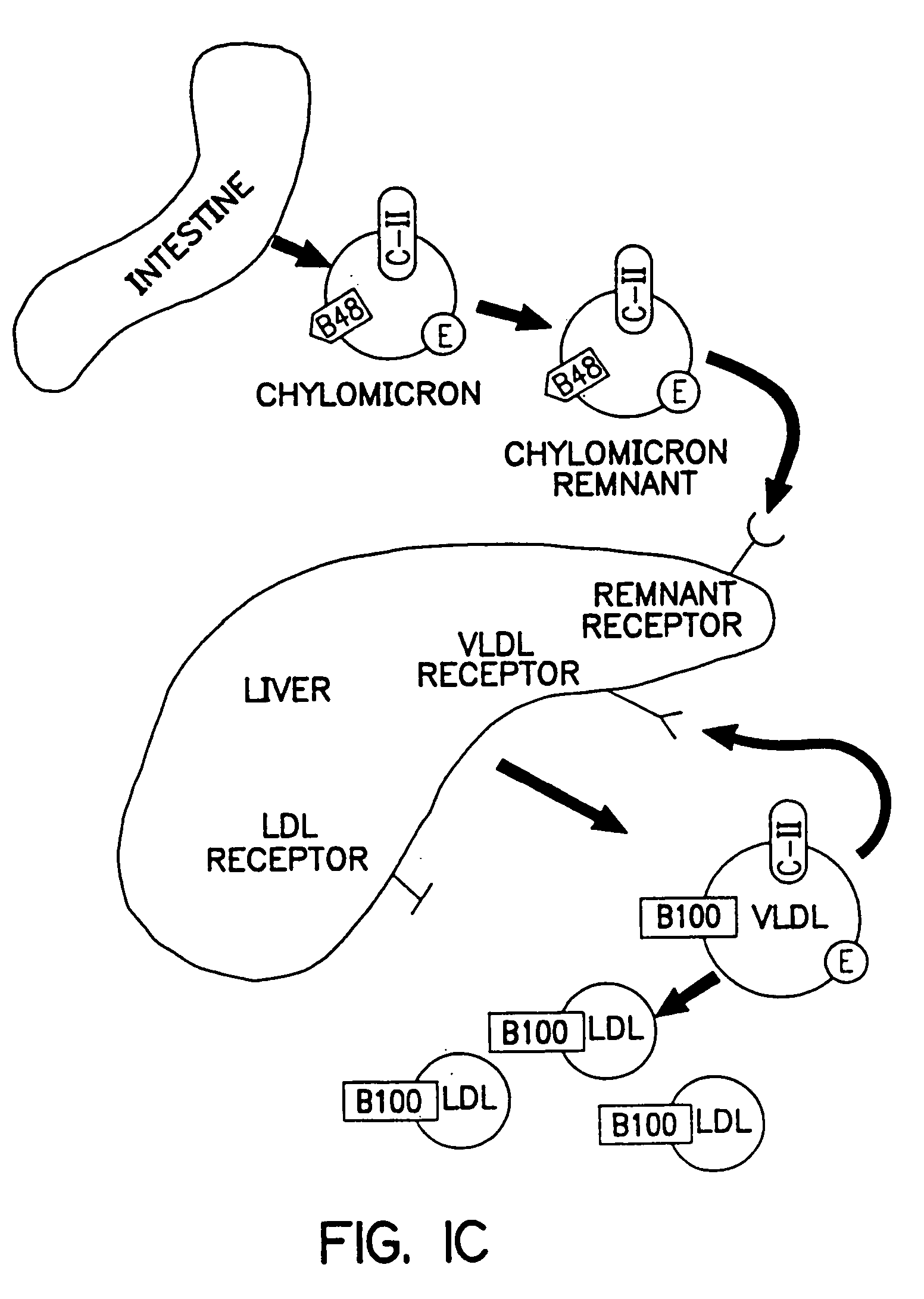
Methods and compositions for the treatment of defects in lipoprotein metabolism
InactiveUS20050112103A1BiocidePeptide/protein ingredientsVLDL receptorFamilial combined hyperlipidemia
The invention provides a recombinant viral vector comprising the DNA of, or corresponding to, at least a portion of the genome of an adenovirus, which portion is capable of infecting a hepatic cell; and a human VLDL receptor gene operatively linked to regulatory sequences directing its expression. The vector is capable of expressing the normal VLDL receptor gene product in hepatic cells in vivo or in vitro. This viral vector is useful in the treatment of metabolic disorders caused by the accumulation of LDL in plasma, such as familial hypercholesterolemia or familial combined hyperlipidemia.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Site specific recombinase based method for producing adenoviral vectors
Site-specific recombinase based methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from donor and acceptor vectors that each include at least one site recombinase recognition site, where in certain preferred embodiments, one of the donor and acceptor vectors includes a single recombinase recognition site while the other includes two recombinase recognition sites. The acceptor vector includes an adenoviral genome having an E region deletion. The donor vector includes an insertion nucleic acid. In the subject methods, the donor and acceptor vectors are combined in the presence of a recombinase to produce an adenoviral genome that includes the insertion nucleic acid. The subject adenoviral genomes find use in a variety of applications, including as vectors for use in a variety of applications.
Owner:LIFE TECH CORP
Kit for detecting B and E type human adenovirus nucleic acid and detection method thereof
ActiveCN105586439ARapid responseHigh sensitivityMicrobiological testing/measurementFreeze-dryingMagnesium salt
The invention discloses a kit for detecting B and E type human adenovirus nucleic acid and a detection method thereof. The kit for detecting the B and E type human adenovirus nucleic acid comprises RPA freeze-dried particles, an RPA reaction primer, an RPA reaction probe, an RPA buffer solution, a magnesium salt solution, a transverse flow test strip, a buffer solution, double distilled water and an adenovirus genome DNA. The kit for detecting the B and E type human adenovirus nucleic acid has the advantages that the transverse flow test strip is utilized for carrying out visual qualitative detection on an RPA nucleic acid amplification product, the whole reaction is carried out at the constant temperature of 38 DEG C, a stripe on the test strip can be observed by virtue of naked eyes within 5-20 minutes, results are read and discriminated, and a special detecting instrument is not needed during detection. The kit disclosed by the invention has the characteristics of high sensitivity, strong specificity, easiness in operation, quick reaction and portability of instruments and is especially applicable to bedside rapid diagnosis of adenovirus, epidemic prevention and disease control.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
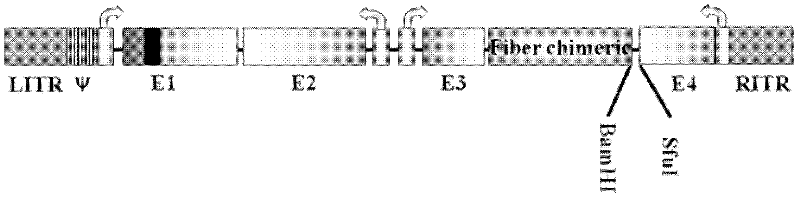
D24 fiber protein modified conditionally replicating adenovirus carrier with exogenous gene by one-step method and application of carrier
InactiveCN102586327AGood treatment effectGenetic material ingredientsFermentationFiberEnzyme digestion
The invention discloses a D24 fiber protein modified conditionally replicating adenovirus carrier with an exogenous gene by a one-step method and an application of the carrier. on the basis of an Ad56 D24 conditionally replicating adenovirus carrier, the construction efficiency of the carrier for inserting into the exogenous gene can be improved by two aspects: (1) a BamHi locus on an adenovirus gene group is mutated; and (2) BamHi and SfuI are introduced between fiber and E4 by a homologous recombination method so as to introduce the exogenous gene into the adenovirus gene group in one step by an enzyme digestion ligation method. The obtained D24 fiber protein modified conditionally replicating adenovirus carrier with an exogenous gene is subjected to the pesticide effect experiment to prove that the D24 fiber protein modified conditionally replicating adenovirus carrier with an exogenous gene can improve a treatment effect on turmor.
Owner:SHAANXI NORMAL UNIV
Helper dependent adenoviral vector system and methods for using the same
A helper dependent adenoviral vector system is provided. The subject helper dependent adenoviral vector system is made up of: (1) a "gutless" adenoviral vector, which in certain embodiments includes cis-acting human stuffer DNA that provides for in vivo long term, high level expression of a coding sequence present on the vector, where in certain embodiments the vector includes an integrating domain; (2) an adenoviral helper vector that is characterized by having an adenoviral genome region flanked by recombinase recognition sites, where the helper vectors further include a non-mammalian endonuclease recognition site positioned outside of the adenoviral genome region and in certain embodiments a third adenoviral inverted terminal repeat (ITR) sequence positioned between first and second terminal ITRs; and (3) a mammalian cell that expresses the corresponding recombinase and endonuclease, as well as the adenoviral preterminal and polymerase proteins. Also provided are methods of using the subject systems to produce virions having the subject helper dependent adenoviral vectors encapsulated in an adenoviral capsid. In addition, kits for use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
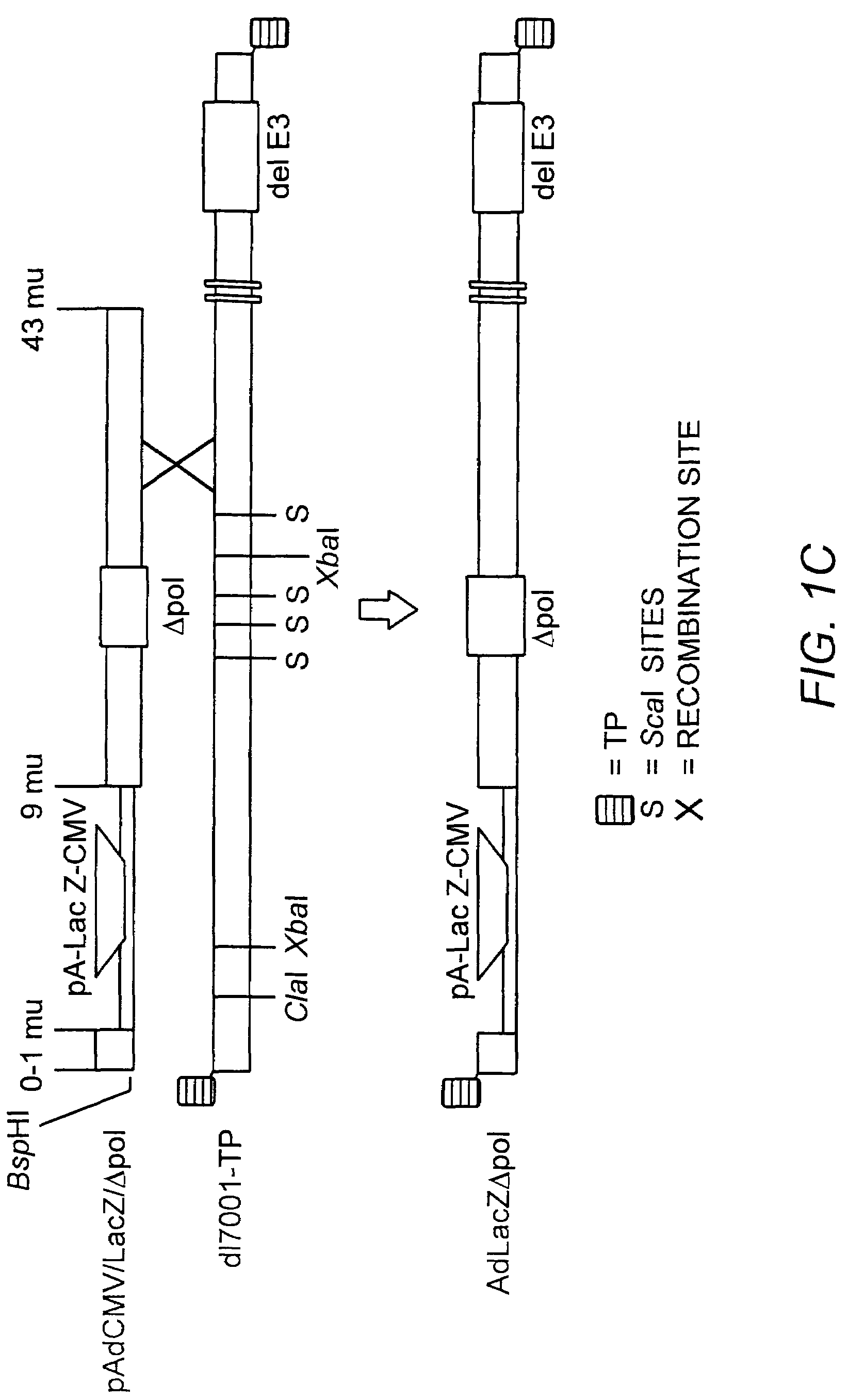
Deleted adenovirus vectors and methods of making and administering the same
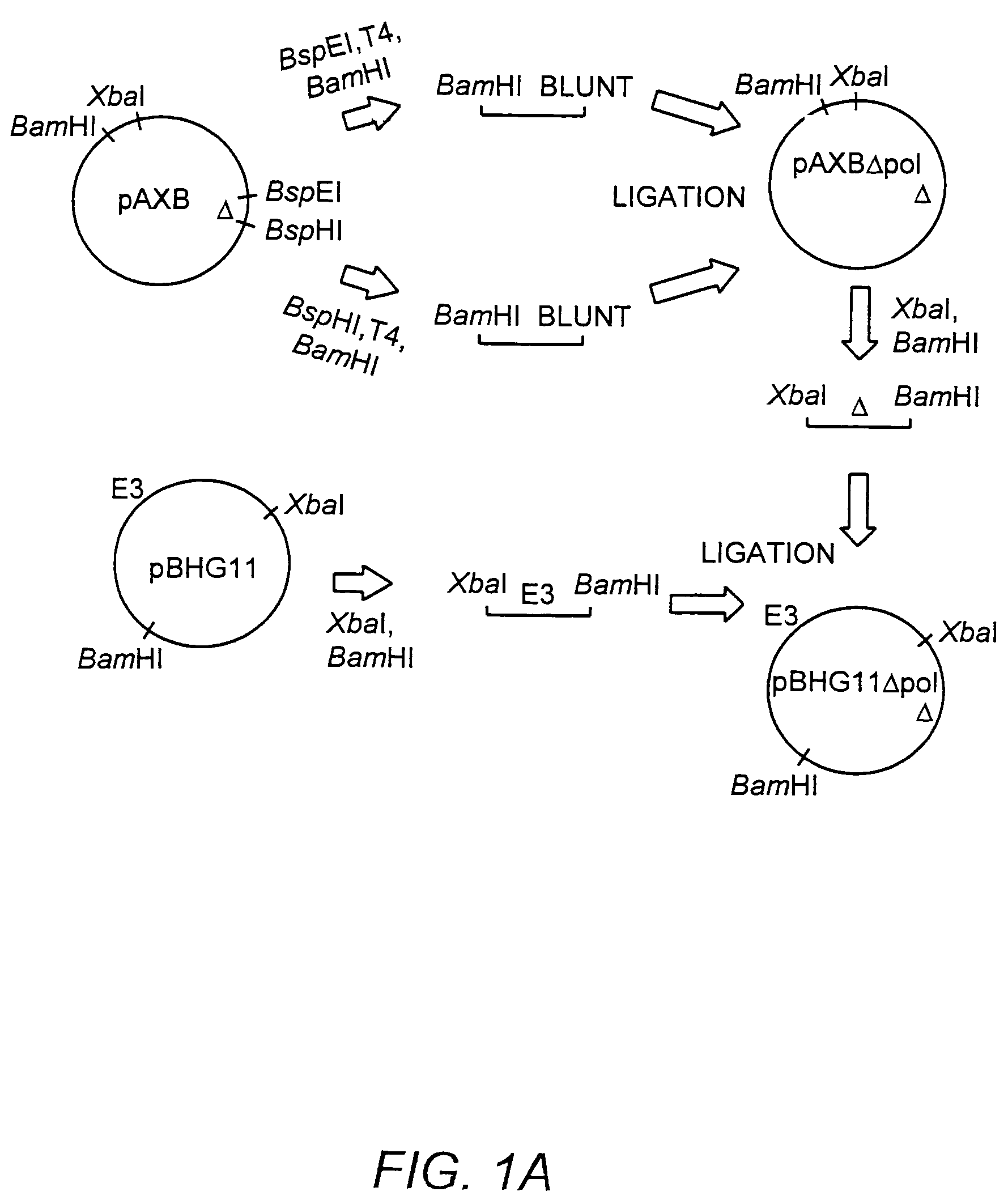
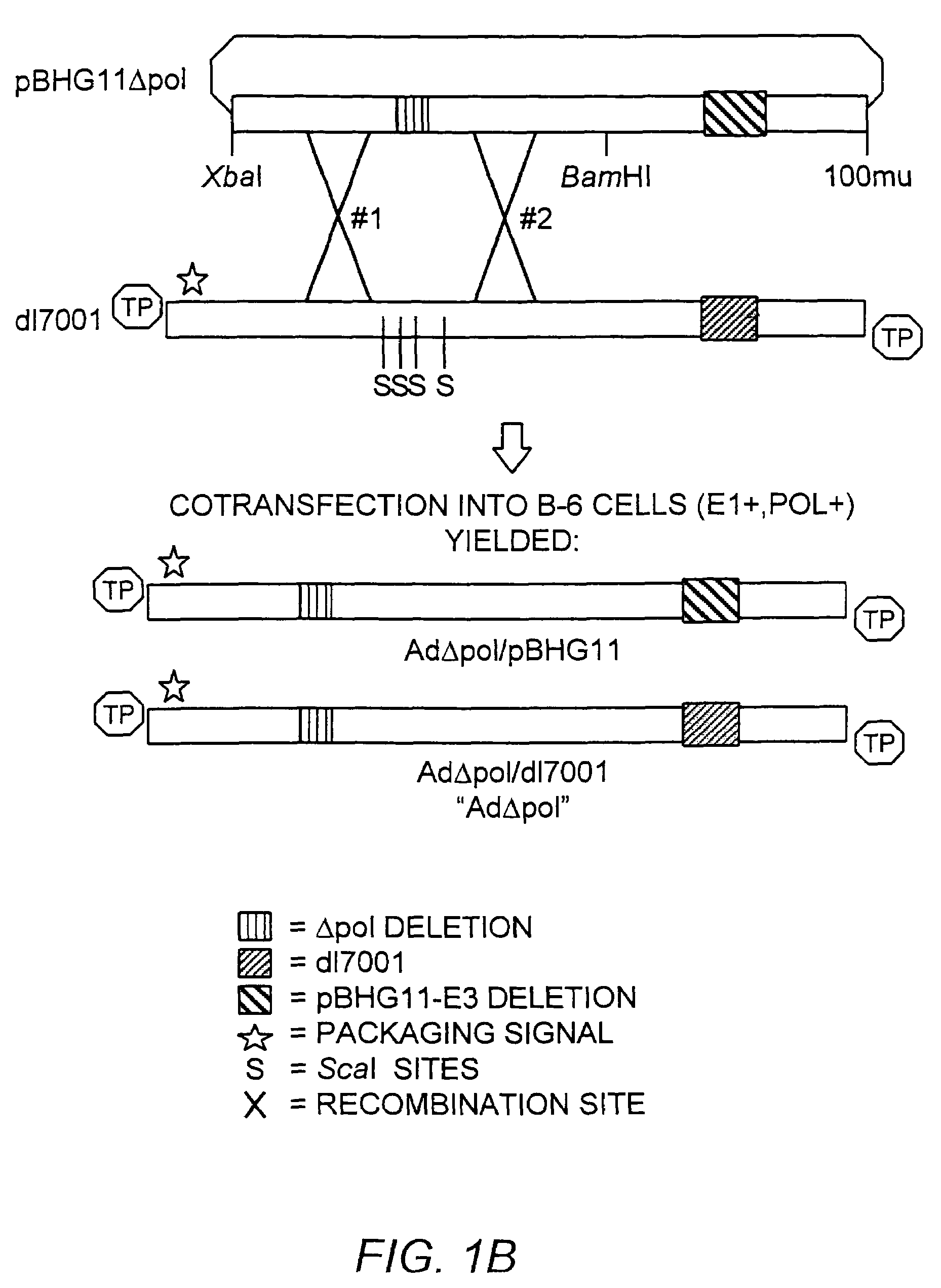
The present invention provides deleted adenovirus vectors. The inventive adenovirus vectors carry one or more deletions in the IVa2, 100K, polymerase and / or preterminal protein sequences of the adenovirus genome. The adenoviruses may additionally contain other deletions, mutations or other modifications as well. In particular preferred embodiments, the adenovirus genome is multiply deleted, i.e., carries two or more deletions therein. The deleted adenoviruses of the invention are “propagation-defective” in that the virus cannot replicate and produce new virions in the absence of complementing function(s). Preferred adenovirus vectors of the invention carry a heterologous nucleotide sequence encoding a protein or peptide associated with a metabolic disorder, more preferably a protein or peptide associated with a lysosomal or glycogen storage disease, most preferably, a lysosomal acid α-glucosidase. Further provided are methods for producing the inventive deleted adenovirus vectors. Further provided are methods of administering the deleted adenovirus vectors to a cell in vitro or in vivo.
Owner:DUKE UNIV
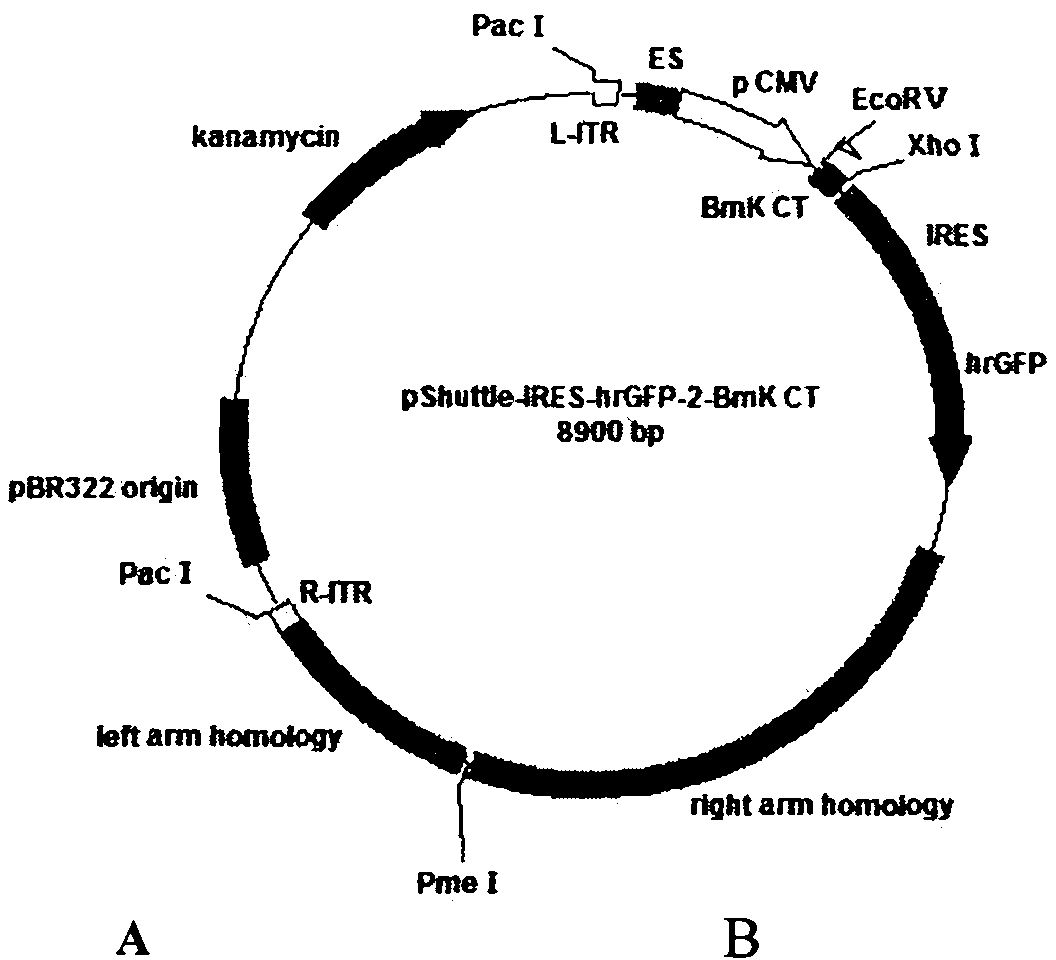
Recombinant adenovirus, preparation method and application thereof
InactiveCN101921769APrevent proliferationInhibited DiffusionNervous disorderGenetic material ingredientsEscherichia coliRecombinant virus
The invention provides recombinant adenovirus, comprising: using a mammal preference codon table to optimize and synthesize the gene of chloride ion channel toxin (BmK CT) of the scorpion; cloning to a intermediate vector of the adenovirus to obtain a recombinant plasmid pShuttle-IRES-hrGFP-2-BmK CT comprising the toxin gene; transforming the recombinant plasmid to the escherichia coli BJ5183 having the adenovirus skeleton genome, obtaining pAdEasy-1-BmK CT after homologous recombination; transfecting the adenovirus genome to the AD293 cell, obtaining the recombinant adenovirus particles AdEasy-1-BmK CT containing the gene of chloride ion channel toxin of the scorpion. The chloride ion channel toxin can inhibit the glioma; with the adenovirus as the carrier, the infection efficiency and the action time of the glioma are improved; the recombinant adenovirus has good application prospect in the gene treatment aspect of the glioma.
Owner:SHANXI UNIV
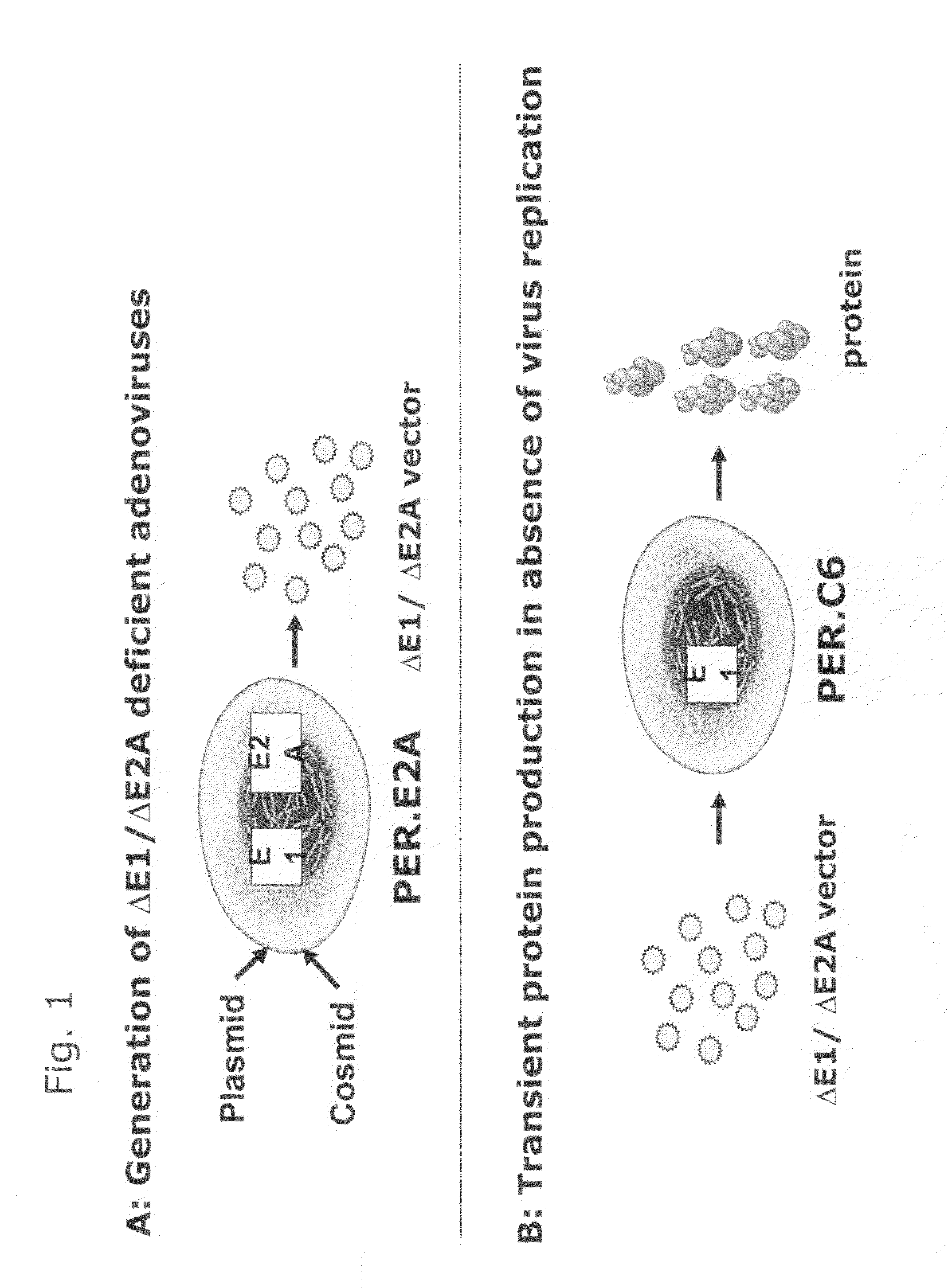
Transient protein expression methods
Described is a method for producing a protein of interest, the method comprising: a) providing a recombinant adenoviral vector comprising nucleic acid encoding the protein of interest under control of a promoter, wherein the adenoviral vector has deletions in a first region and in a second region of the adenovirus genome, wherein each of the first region and the second region is required for adenoviral genome replication and / or adenovirus particle formation, b) propagating the adenoviral vector in a first type of complementing cells that express proteins from the first and from the second region of the adenovirus genome so as to complement the deletions of the recombinant adenoviral vector, to obtain recombinant adenovirus particles, c) infecting a culture of a second type of complementing cells with the recombinant adenovirus particles, wherein the second type of complementing cells express protein from the first region of the adenovirus genome but not protein from the second region of the adenovirus genome, to produce the protein of interest, and d) harvesting the protein of interest.
Owner:JANSSEN VACCINES & PREVENTION BV
Adenoviral vector-based foot-and-mouth disease vaccine
ActiveUS8323663B2SsRNA viruses positive-senseViral antigen ingredientsAntigenNucleic acid sequencing
The invention is directed to an adenoviral vector comprising at least one nucleic acid sequence encoding an aphthovirus antigen and / or a cytokine operably linked to a promoter. The adenoviral vector is replication-deficient and requires at most complementation of both the E1 region and the E4 region of the adenoviral genome for propagation. The invention also is directed to a method of inducing an immune response in a mammal comprising administering to the mammal a composition comprising the aforementioned adenoviral vector.
Owner:US SEC AGRI +2
Helper dependent adenoviral vector system and methods for using the same
A helper dependent adenoviral vector system is provided. The subject helper dependent adenoviral vector system is made up of: (1) a “gutless” adenoviral vector that include cis-acting human stuffer DNA that provides for in vivo long term, high level expression of a coding sequence present on the vector; (2) an adenoviral helper vector that is characterized by having an adenoviral genome region flanked by recombinase recognition sites, where the helper vectors further include a non-mammalian endonuclease recognition site positioned outside of the adenoviral genome region; and (3) a mammalian cell that expresses the corresponding recombinase and endonuclease, as well as the adenoviral preterminal and polymerase proteins. Also provided are methods of using the subject systems to produce virions having the subject helper dependent adenoviral vectors encapsulated in an adenoviral capsid. In addition, kits for use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Conditionallly replication-competent adenovirus
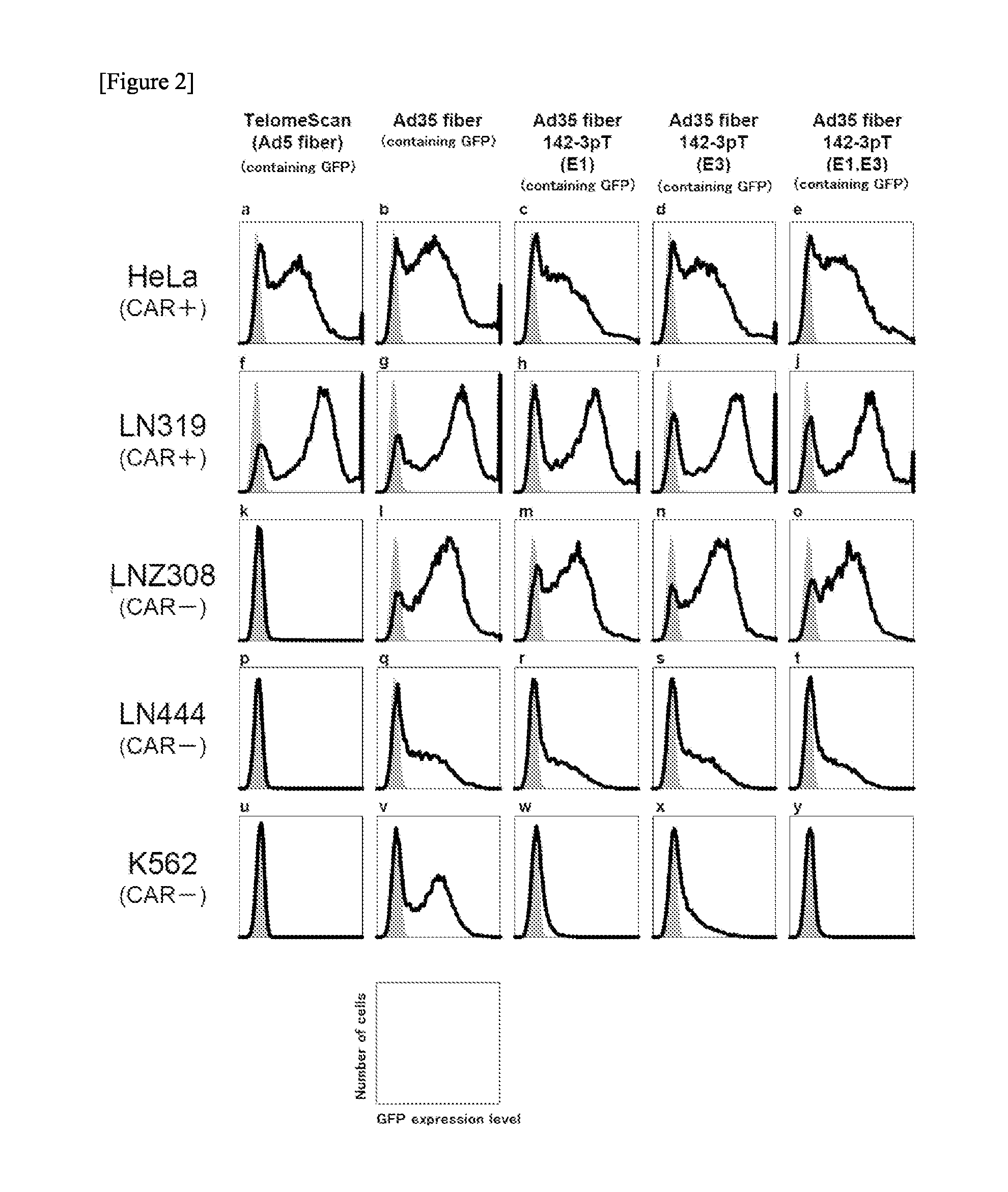
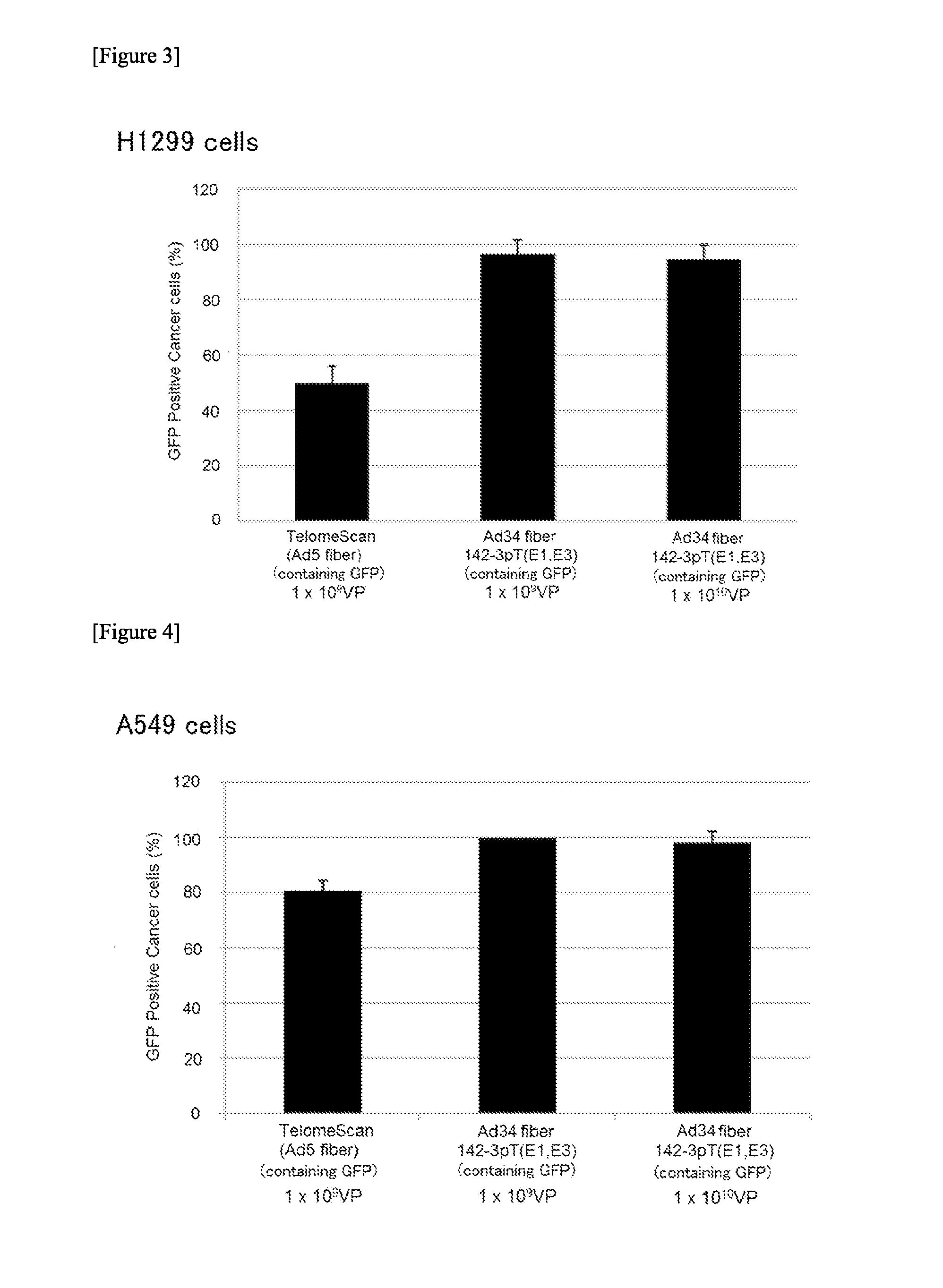
InactiveUS20140199688A1Simple and highly sensitive detectionAvoid False Positive ResultsVectorsMicrobiological testing/measurementCancer cellCancers diagnosis
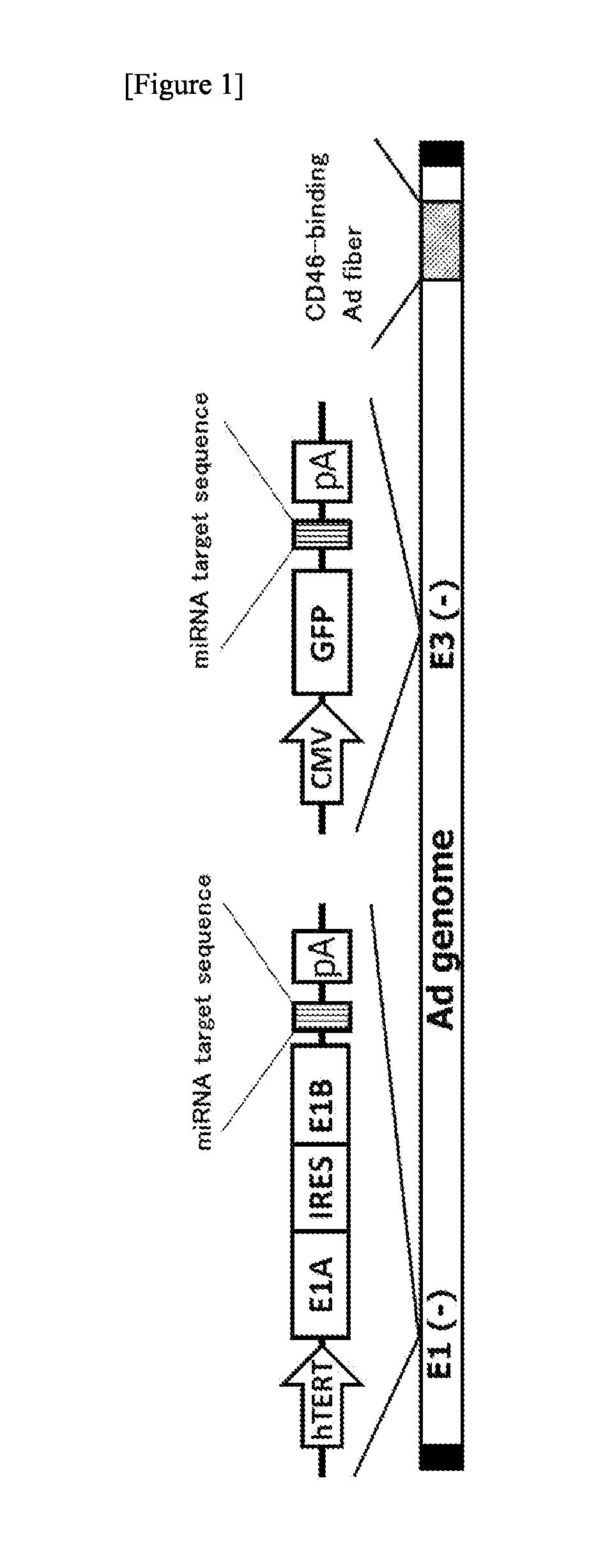
The object of the present invention is to provide a novel conditionally replicating adenovirus and a reagent comprising the same for cancer cell detection or for cancer diagnosis.The present invention provides a polynucleotide, which comprises human telomerase reverse transcriptase (hTERT) promoter, E1A gene, IRES sequence and E1B gene in this order and which comprises a target sequence of a first miRNA. The present invention also provides a recombinant adenovirus, which comprises a replication cassette comprising the above polynucleotide, wherein the replication cassette is integrated into the E1 region of the adenovirus genome.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION
Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
ActiveCN106318916ANo recombinationHigh neutralization potencyViral antigen ingredientsVirus peptidesHuman typeSerotype
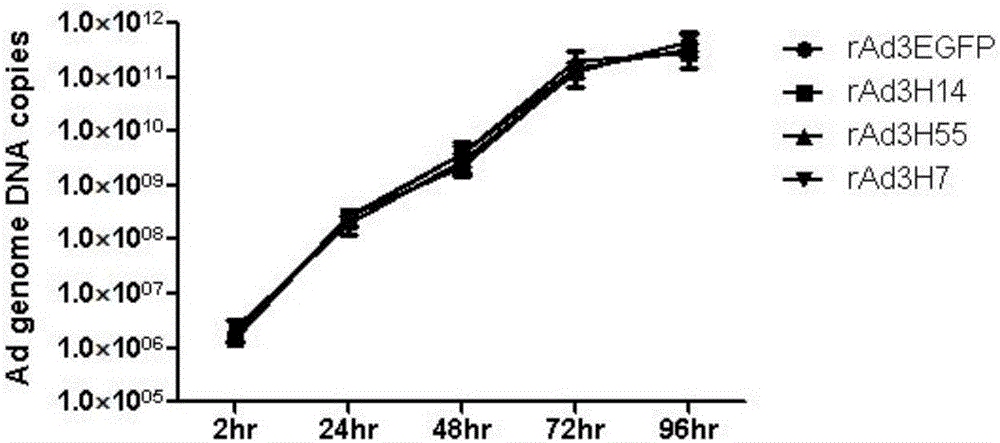
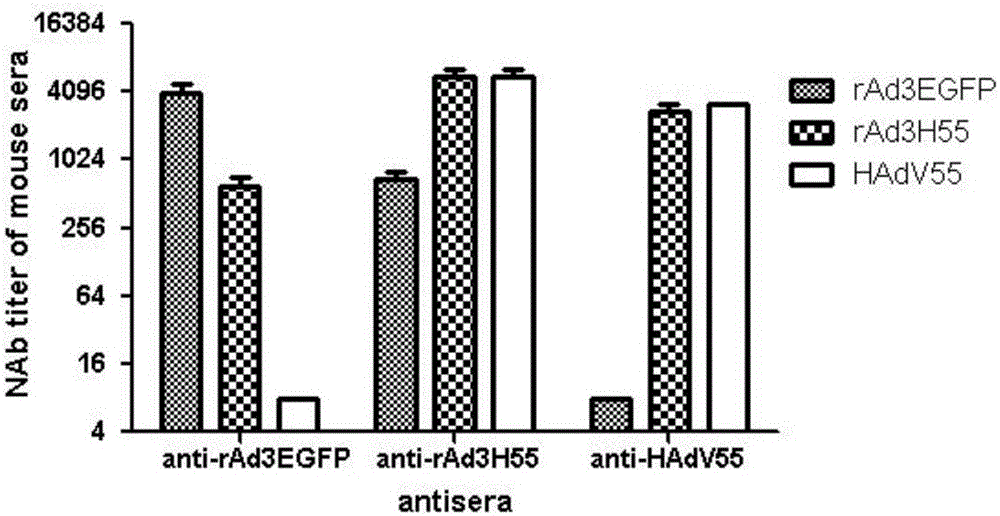
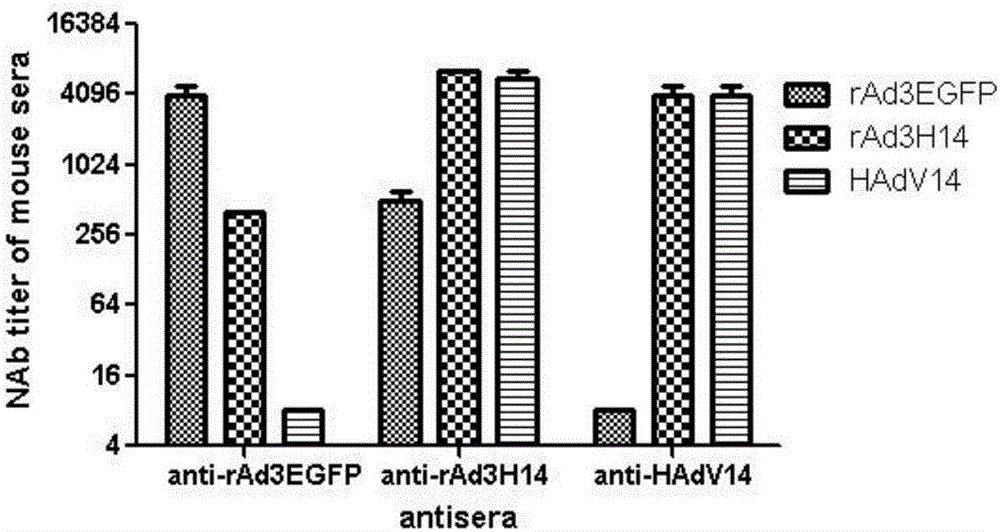
The invention discloses a recombinant adenovirus and tetravalent adenovirus vaccine and a preparation method thereof. The tetravalent recombinant adenovirus vaccine contains a recombinant type 3 adenovirus strain, a recombinant type 7 adenovirus strain, a recombinant type 14 adenovirus strain and a recombinant type 55 adenovirus strain. The preparation method disclosed by the invention comprises the following steps: preparing recombinant shuttle plasmids containing hexon gene segments, and performing in-bacteria homologous recombination with a recombinant human type 3 adenovirus strain, thereby obtaining a recombinant adenoviral genome in which the hexon gene segments are replaced by type 7, type 14 and type 55; transfecting cells, rescuing to obtain recombinant human type 3, 7, 14 and 55 recombinant adenoviruses with different main capsid protein-hexon proteins; purifying, mixing according to the same protein content, and inactivating by using beta-propiolactone, thereby obtaining the tetravalent adenovirus vaccine. The tetravalent adenovirus vaccine is capable of inducing neutralizing antibody responses to four types of serotype adenoviruses, and the neutralizing titer is 500-1000.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
Adenoviral vectors for treating disease
Adenoviral vectors, including mutant adenoviruses, that have restriction sites in the E3 region, that facilitate its partial or total deletion, or select genes contained therein, and compositions and methods for substituting heterologous gene(s), if desired, which gene(s) will exhibit an expression pattern, both in terms of timing and degree of expression, similar to the endogenous adenoviral gene that it replaces, and further optionally including mutations in other parts of the adenoviral genome, including certain E1B or E1A regions, and that have applications for diagnosing or treating disease, preferably disease involving unwanted cell growth, including cancer.
Owner:ONYX PHARMA INC
Adenoviral Vector
ActiveUS20190175716A1Determine immunogenicityDetermine safetyAntibacterial agentsBacterial antigen ingredientsImmunogenicityCoding region
The present invention provides recombinant adenoviral vectors, immunogenic compositions thereof and their uses in medicine. In particular, the present invention provides an adenoviral vector comprising the genome of an adenovirus other than AdHu5 and AdY25, wherein the genome of the adenovirus has been modified such that the vector lacks the native E4 locus of the adenovirus and comprises heterologous E4Orf1, E4Orf2 and E4Orf3 coding regions from AdY25.
Owner:OXFORD UNIV INNOVATION LTD
Dynein mosaic type recombinant human type-B adenovirus and preparation method thereof
ActiveCN107267469AHigh infection efficiencyMicroorganism based processesFermentationGolden hamsterHuman type
The invention discloses a dynein mosaic type recombinant human type-B adenovirus and a preparation method thereof. The skeleton of the dynein mosaic type recombinant human type-B adenovirus is a human type-B adenovirus genome, and a base sequence which encodes a receptor binding domain of dynein is a base sequence which encodes a corresponding domain of a human type-C adenovirus. By a molecular cloning method, Ad5-knob gene fragments are cloned and replaced to recombinant shuttle plasmids, in-vitro recombinant on the Ad5-knob gene fragments and a recombinant human type-3 adenovirus genome is realized, obtained knob gene fragments are replaced into type-5 recombinant human type-3 adenovirus genome, and therefore, dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is obtained. The dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 can be infected with mouse primitive epithelial cells and golden hamster lung and kidney primitive cells in vitro, and the infection efficiency of the dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is close to that of Ad5, and is much higher than that of a parent strain rAd3E, in golden hamster cells, significant copying exists, and the dynein mosaic type recombinant human type-B adenovirus can be used for small animal model research of human type-3 adenovirus vaccines and antiviral drug evaluation.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Non-adenoviral gene product-based complementing cells for adenoviral vectors
The invention provides cells and methods of using the cells for the propagation of replication-deficient adenoviral vectors. The cells comprise at least one heterologous nucleic acid sequence which upon expression produces at least one non-adenoviral gene product that complements in trans for a deficiency in at least one essential gene function of one or more regions of an adenoviral genome so as to propagate a replication-deficient adenoviral vector comprising an adenoviral genome deficient in the at least one essential gene function of the one or more regions when present in the cell.
Owner:GEN VEC INC
Recombinant denovirus for expressing foot-and-mouth disease virus type A empty capsid, and application thereof
InactiveCN102533798AImprove securityDoes not interfere with immunityGenetic material ingredientsVirus peptidesEscherichia coliNeutralizing antibody
The invention discloses a recombinant denovirus for expressing foot-and-mouth disease virus type A empty capsid, and application thereof. A sub-genome for coding the foot-and-mouth disease virus type A empty capsid, which is shown as SEQIDNo.1, and a denovirus shuttle plasmid are operably connected to form a recombinant denovirus shuttle expression vector; the recombinant denovirus shuttle expression vector and a denovirus backbone vector plasmid co-transform Escherichia coli, and a cloned recombinant denovirus genome existing in a plasmid form is obtained; and the cloned recombinant denovirus genome is linearized and transfects cells, and the replication-deficient recombinant denovirus is obtained. The replication-deficient recombinant denovirus has high toxicity titer, and can form the foot-and-mouth disease virus type A empty capsid in the replication process and stably express foot-and-mouth disease virus empty capsid in the virus propagation process; the expressed foot-and-mouth disease virus empty capsid can constantly induce high-level neutralizing antibody in bodies of mice and resist the attack of the foot-and-mouth disease virus; and the recombinant denovirus can be prepared into a vaccine for controlling foot-and-mouth disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
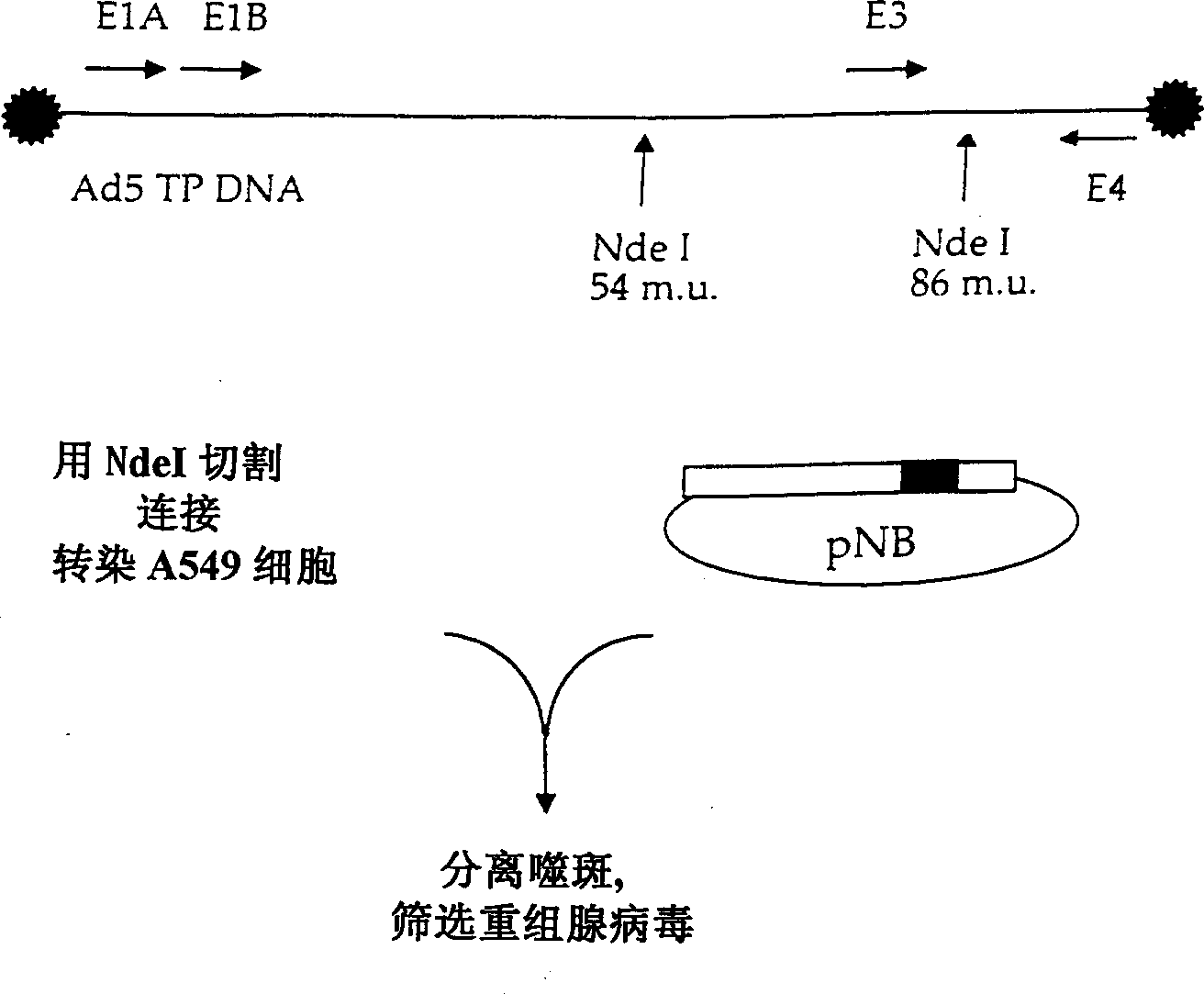
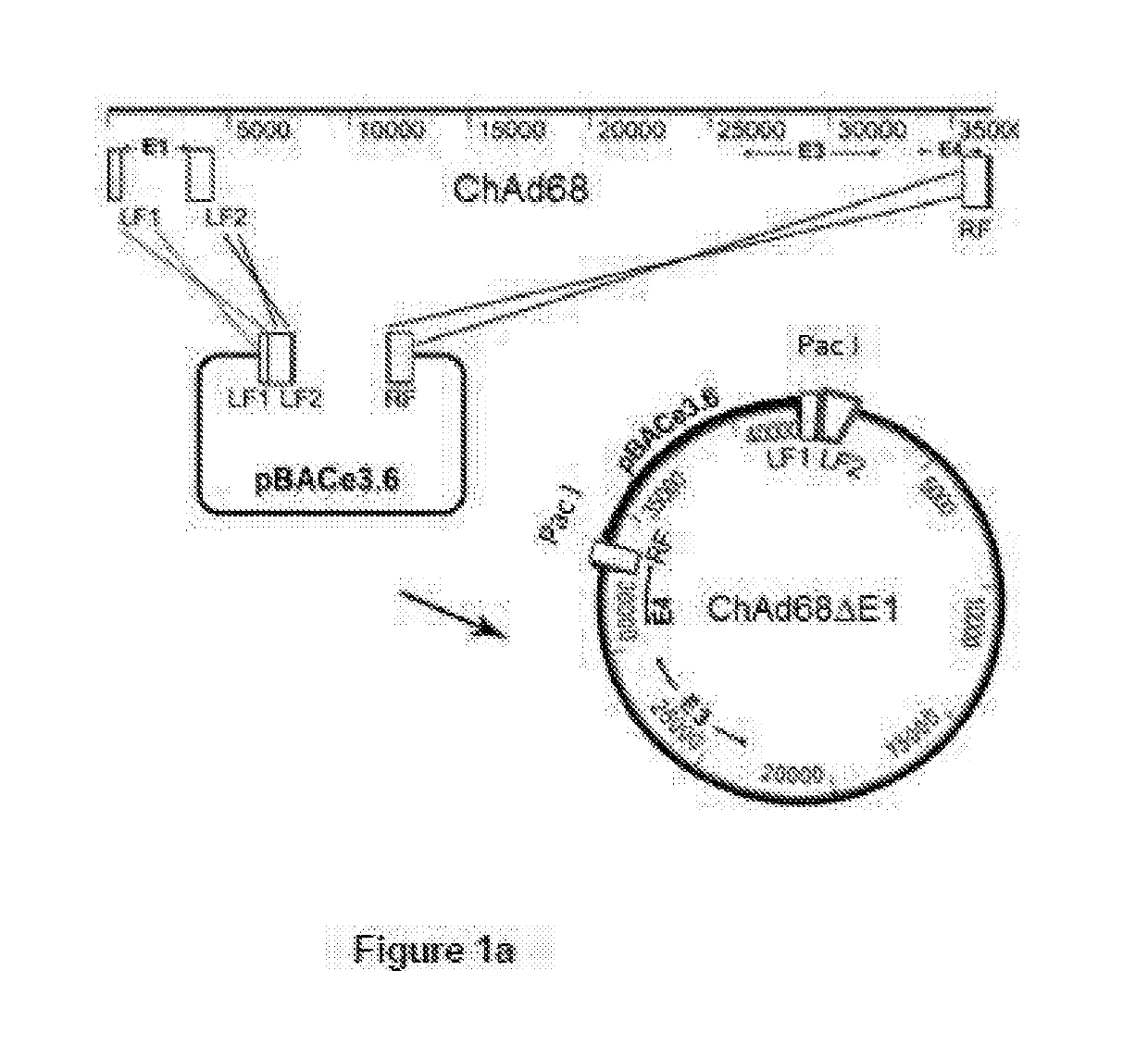
A method of making adenovirus and corresponding plasmids
ActiveUS20160319304A1Flexible operationGenetic material ingredientsNucleic acid vectorShuttle vectorRestriction site
The present disclosure relates to a method of making an adenovirus plasmid comprising a part or all of an adenovirus genome and one or more original restriction sites allowing rapid and flexible manipulation of the adenovirus genome, and methods of preparing adenovirus constructs, for example comprising a transgene. The disclosure also extends to novel intermediates employed in and generated by the method, to plasmids and shuttle vectors of the method and to adenoviruses or adenoviral vectors obtainable from the plasmid and / or method. The disclosure further relates to use of the viruses or vectors, for example obtained from a method disclosed herein, in therapy, such as use in the treatment of cancer.
Owner:PSIOXUS THERAPEUTICS LTD
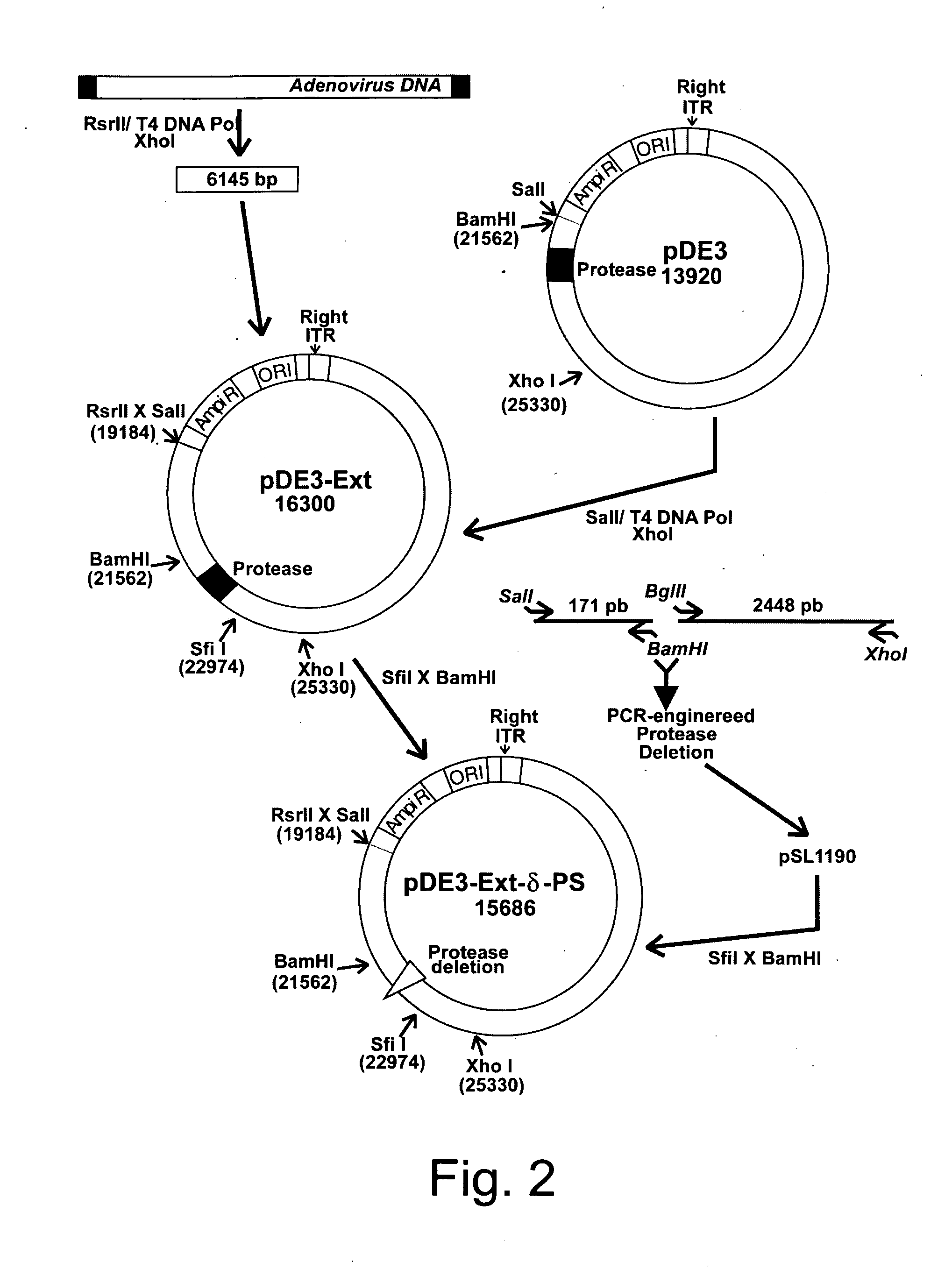
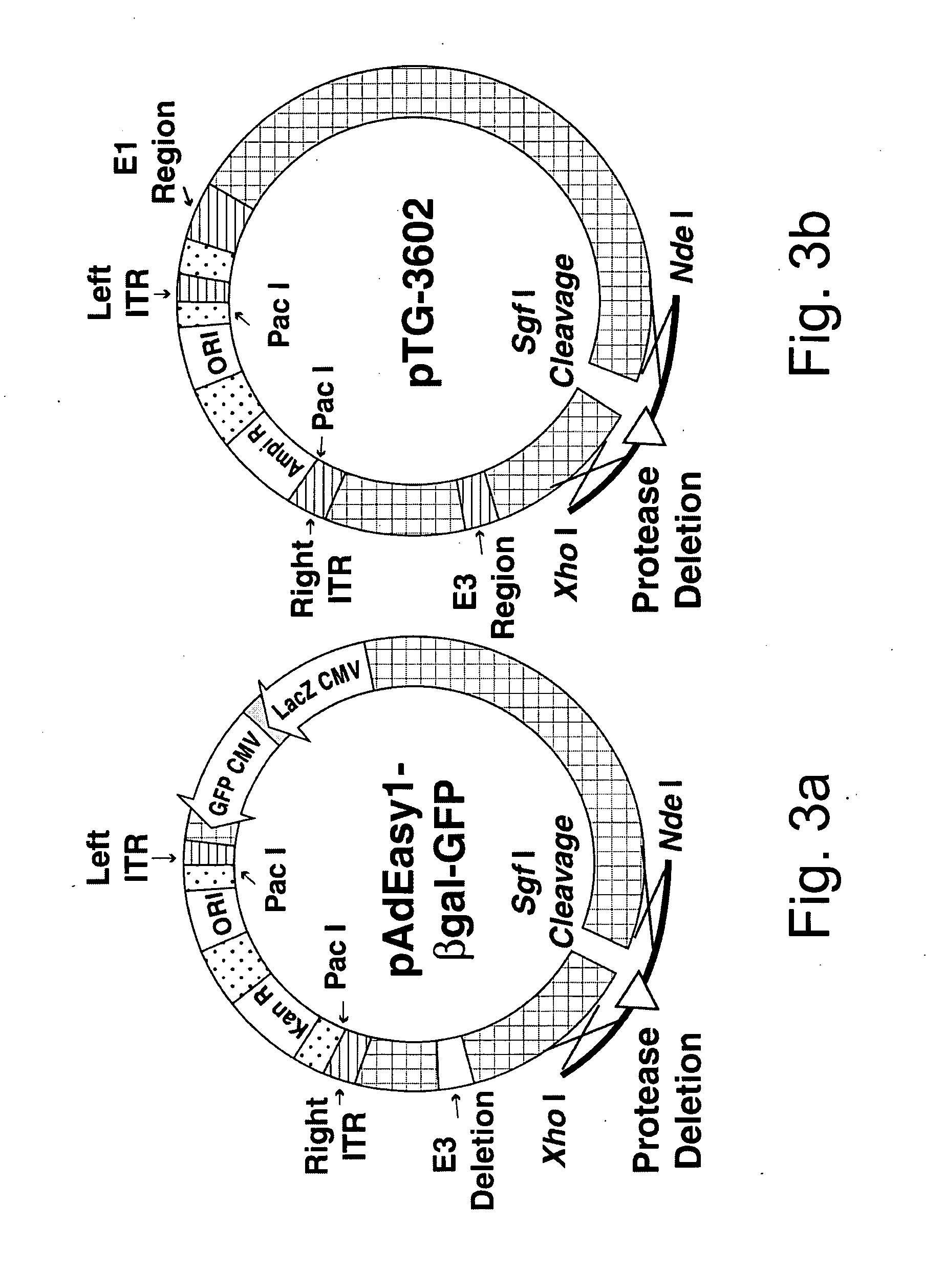
Efficient generation of adenovirus-based libraries by positive selection of adenoviral recombinants through ectopic expression of the adenovirus protease
InactiveUS20060210965A1Improve securityImprove suitabilityVectorsMicrobiological testing/measurementExpression LibraryShuttle vector
Disclosed is a new system for generating recombinant adenovirus vectors and adenovirus-based expression libraries, by positive selection of recombinants deleted for the endogenous protease gene, which gene is expressibly cloned into another region of the adenoviral genome. In a preferred embodiment, the invention allows positive selection of E1-deleted, protease-deleted recombinant adenovirus vectors comprising an exogenous gene or an expressible piece of exogenous DNA, by providing an expression cassette comprising the protease gene and the exogenous DNA inserted in place of E1 region in a shuttle vector. In vivo recombination of the shuttle vector with a protease-deleted adenoviral genome in suitable non-complementing cells generates viable recombinants only when rescuing the protease cloned in E1 region. Non-recombinant viral genomes are not able to grow due to the deletion of the protease gene, ensuring that only recombinant viral plaques are generated. This positive selection can be used for the generation of a large number of high purity recombinant adenovirus vectors and allows generation of adenovirus-based libraries with diversity exceeding 106 clones.
Owner:NAT RES COUNCIL OF CANADA
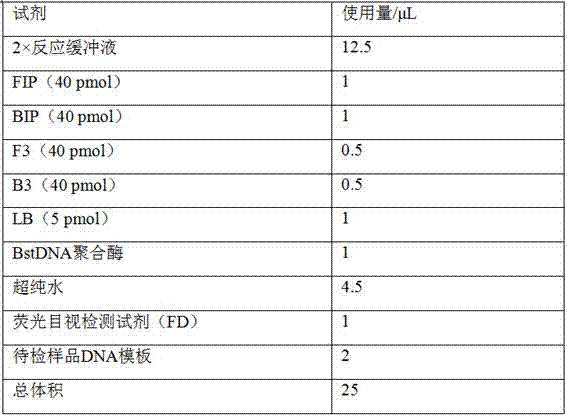
Duck type 2 adenovirus loop-mediated isothermal amplification detection primer group and kit
ActiveCN107400736AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMicrobiologyLoop-mediated isothermal amplification
The invention belongs to the field of animal infectious disease detection, and discloses a duck type 2 adenovirus loop-mediated isothermal amplification detection primer group and a kit. According to the method, the primer group is designed according to duck type 2 adenovirus genome conservative sequences and comprises outer primers F3 and B3, inner primers FIP and BIP as well as a loop primer LB. The particular sequences are as shown in SEQ ID NO.1 to SEQ ID NO.5 correspondingly. A loop-mediated isothermal amplification method for detecting the duck type 2 adenovirus is established according to the designed primers. Cross reaction of the detection method and the duck common infectious disease pathogeny is avoided. The detection method provided by the invention is high in specificity, high in accuracy and high in sensitivity, does not need expensive instruments and equipment, is convenient to use on primary clinical site, and is convenient and rapid.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Vaccine in the form of a recombinant sero type 9 avian adenovirus vector
ActiveUS20170232096A1Adequate responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenMaternal antibody
A recombinant vaccine comprising a serotype 9 fowl adenovirus vector (FAdV-9) having at least one exogenous nucleotide sequence inserted encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region, and a pharmaceutically acceptable vehicle, adjuvant and / or excipient, wherein the at least one exogenous nucleotide sequence encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region is located between the 491 and 2782 nucleotides.The vector of this vaccine is stable for industrial scale production. Likewise, even when administering this vaccine in combination with a vaccine against Marek's disease, both vaccines produce an adequate immune response which is not affected by interference between each other. In the same way, effectiveness of the recombinant vaccine is not affected by maternal antibodies, and is capable of inducing both an early and lasting protective response, even with only one application.
Owner:GRUPO IND PECUARIO S A DE CV
HSV1-TK (herpes simplex virus type 1-thymidine kinase) gene recombination oncolytic adenovirus, preparation method and application thereof
InactiveCN101768576AHigh transfection efficiencyImprove stabilityGenetic material ingredientsViral/bacteriophage medical ingredientsCytotoxic substancesOncolytic adenovirus
The invention discloses an HSV1-TK (herpes simplex virus type 1-thymidine kinase) gene recombination oncolytic adenovirus, a preparation method and an application thereof. The genome of the recombination oncolytic adenovirus sequentially comprises a promoter, an adenovirus E1 area gene and an HSV1-TK full length coding gene from upstream to downstream. The recombination oncolytic adenovirus effectively combines the characteristics of a suicide gene and an oncolytic virus and ensures that the oncolytic virus is limited in a tumor cell and efficiently replicated, and meanwhile, an expression product of the HSV1-TK gene converts a nontoxic prodrug into a cytotoxic substance to cause the condition that the tumor cell suicides. The recombination oncolytic adenovirus has the advantages of high transfection efficiency, good stability, wide tumor treatment spectrum, simple preparation method, and the like, can be used for preparing anti-tumor medicaments and has good development and application prospects in the field of tumor gene treatment.
Owner:ARMY MEDICAL UNIV
Adenoviral vectors for treating diseases
Adenoviral vectors, including mutant adenoviruses, that have restriction sites in the E3 region, that facilitate its partial or total deletion, or select genes contained therein, and optionally compositions and methods for substituting heterologous gene(s) in the partially or totally deleted E3 region(s), which heterologous gene(s) being operably linked to endogenous adenoviral transcriptional control sequences will exhibit an expression pattern, both in terms of timing and degree of expression, similar to the endogenous adenoviral gene(s) that it replaces, and further optionally including mutations in other parts of the adenoviral genome, including certain E1B or E1A regions, and that have applications for diagnosing or treating disease, preferably disease involving unwanted cell growth, including cancer.
Owner:ONYX PHARMA INC
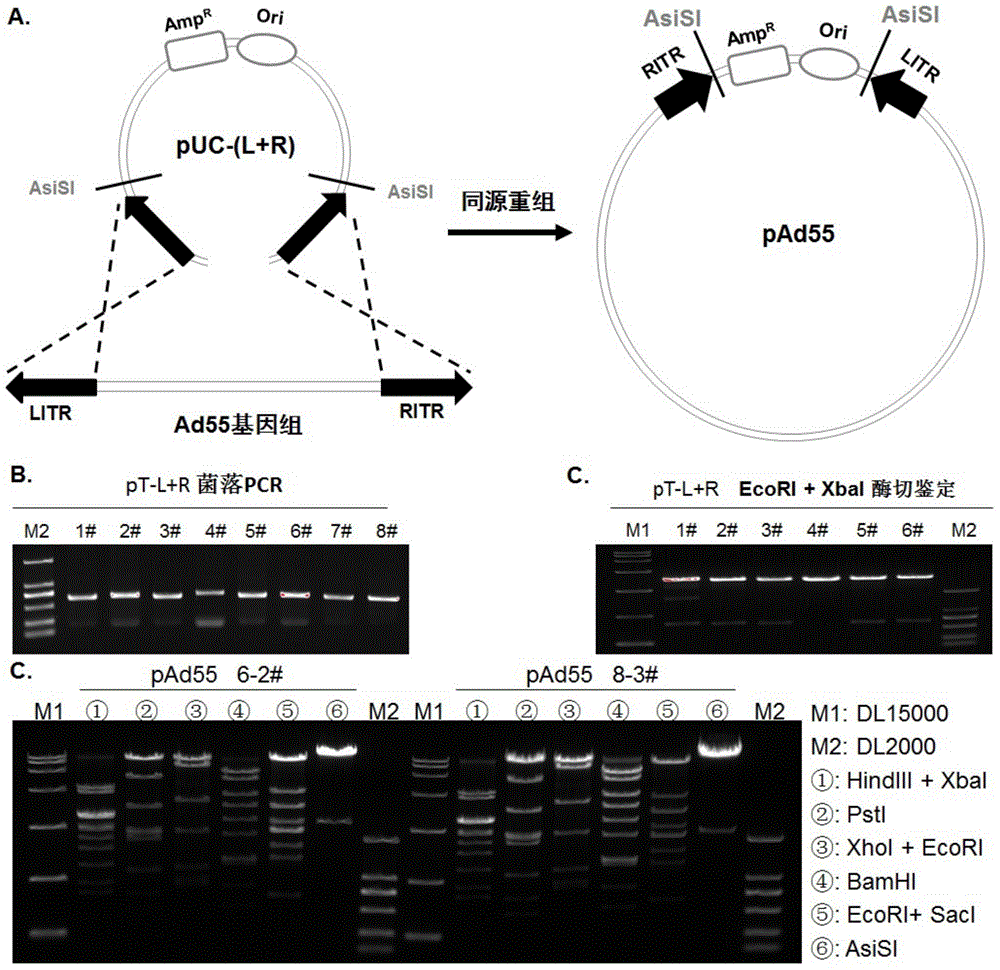
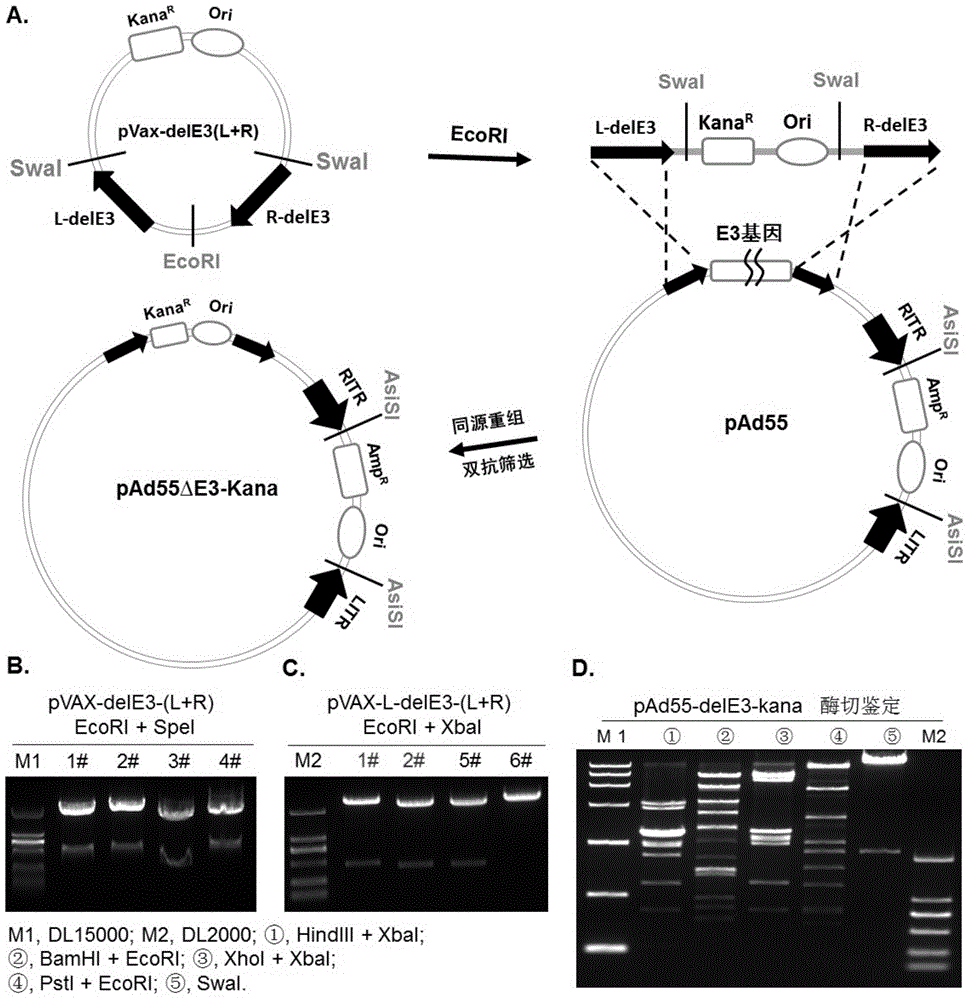
Replicative recombinant human 55-type Adenovirus vector and preparation method and application thereof
ActiveCN105274142AGood effectBreakthrough of pre-existing immunityGenetic material ingredientsImmunoglobulins against virusesNeutralizing antibodyBiology
The invention discloses a replicative recombinant human 55-type Adenovirus vector and a preparation method and application thereof. A human 55-type Adenovirus genome is cyclized through homologous recombination technology, E3 genes of Ad55 are knocked out through homologous recombination and double resistance screening technology, and an exogenous gene expression cassette can be integrated in; after Ad55 vector plasmids deleted in the E3 genes are linearized, successful rescue and mass production can be realized by transfecting mammalian cells; further, a purified recombinant Ad55 vector is obtained through density gradient centrifugation, efficient expression in target cells can be realized by using the vector to carry exogenous genes, and activity of exogenous reporter genes can reflect growth characteristics of virus. The replicative recombinant vector based on human Ad55 can be potentially applied to research and development of vaccines resistant to human 55-type Adenovirus, screening of drug and neutralizing antibodies resistant to the human 55-type Adenovirus, research and development of vaccines resistant to other pathogens and development of report tracer systems for biological study.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com