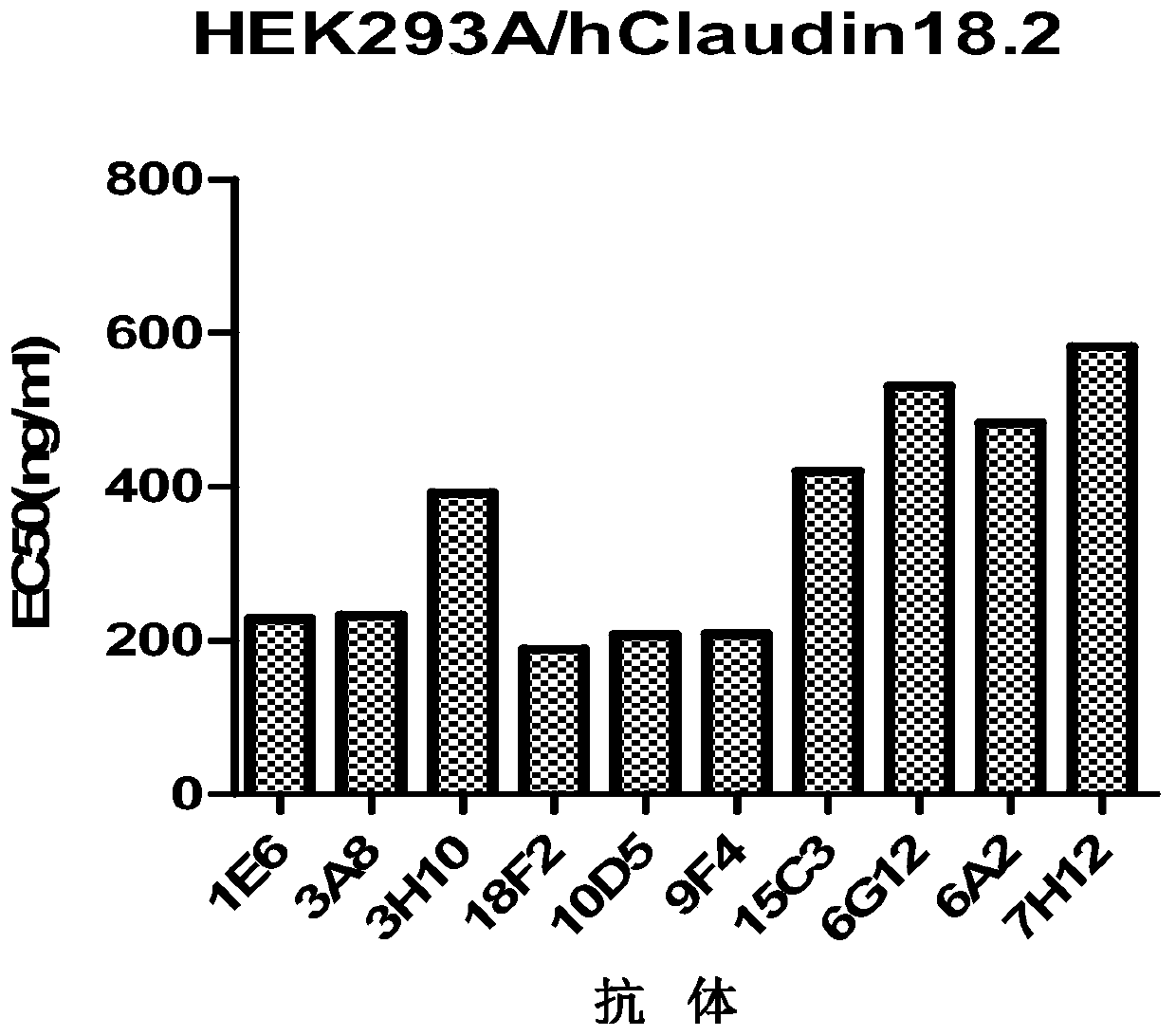
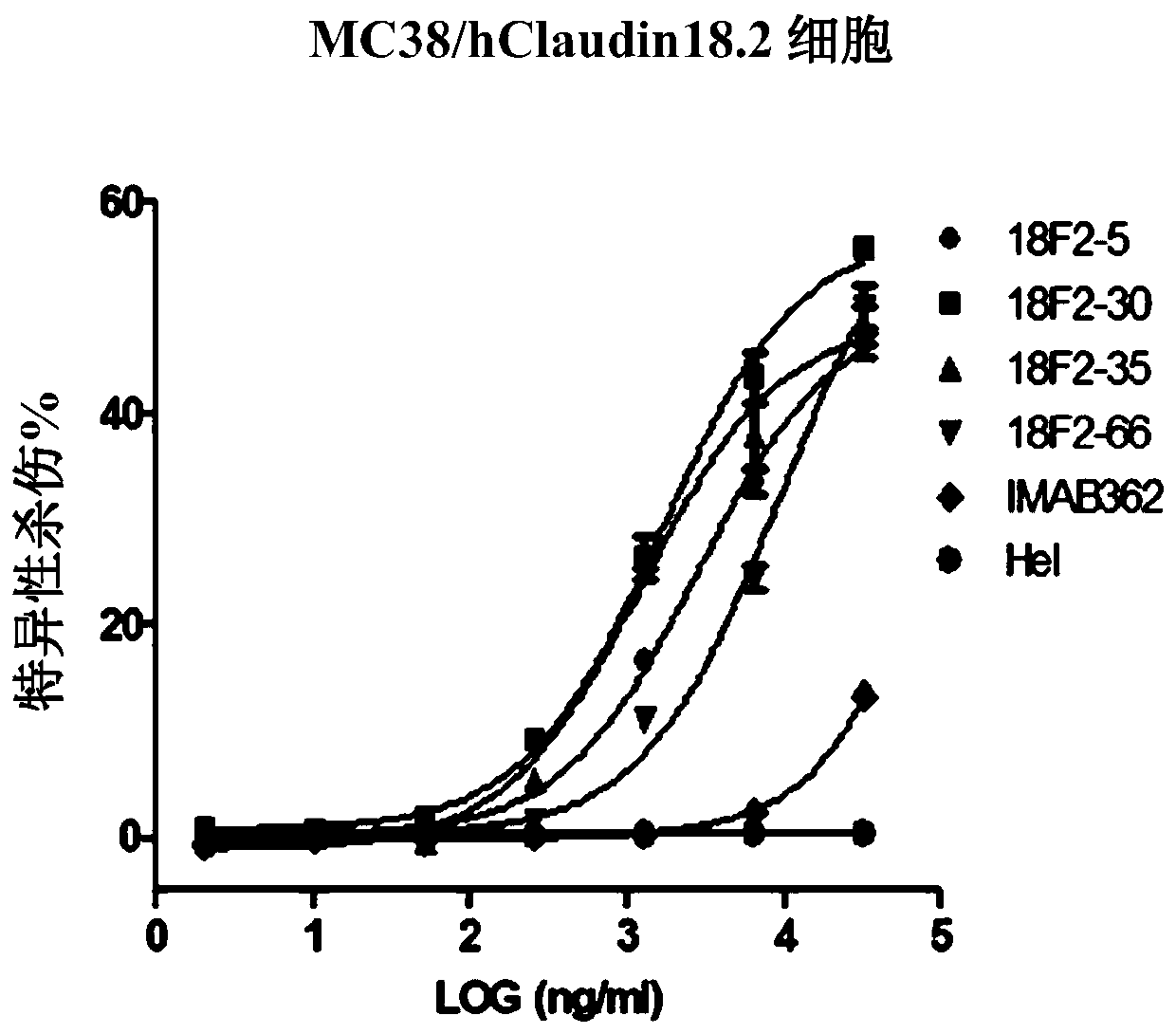
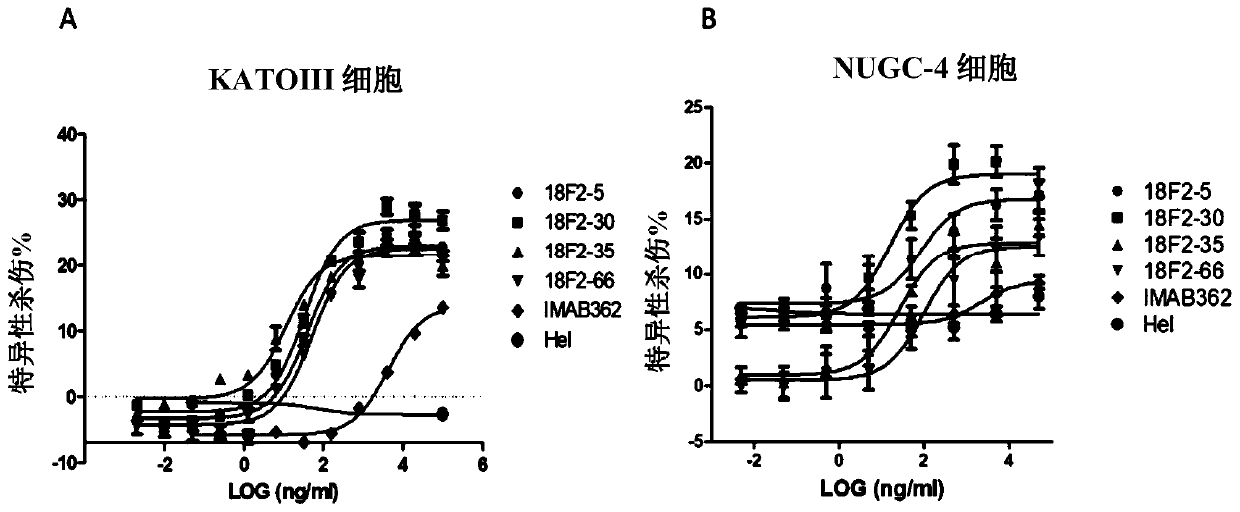
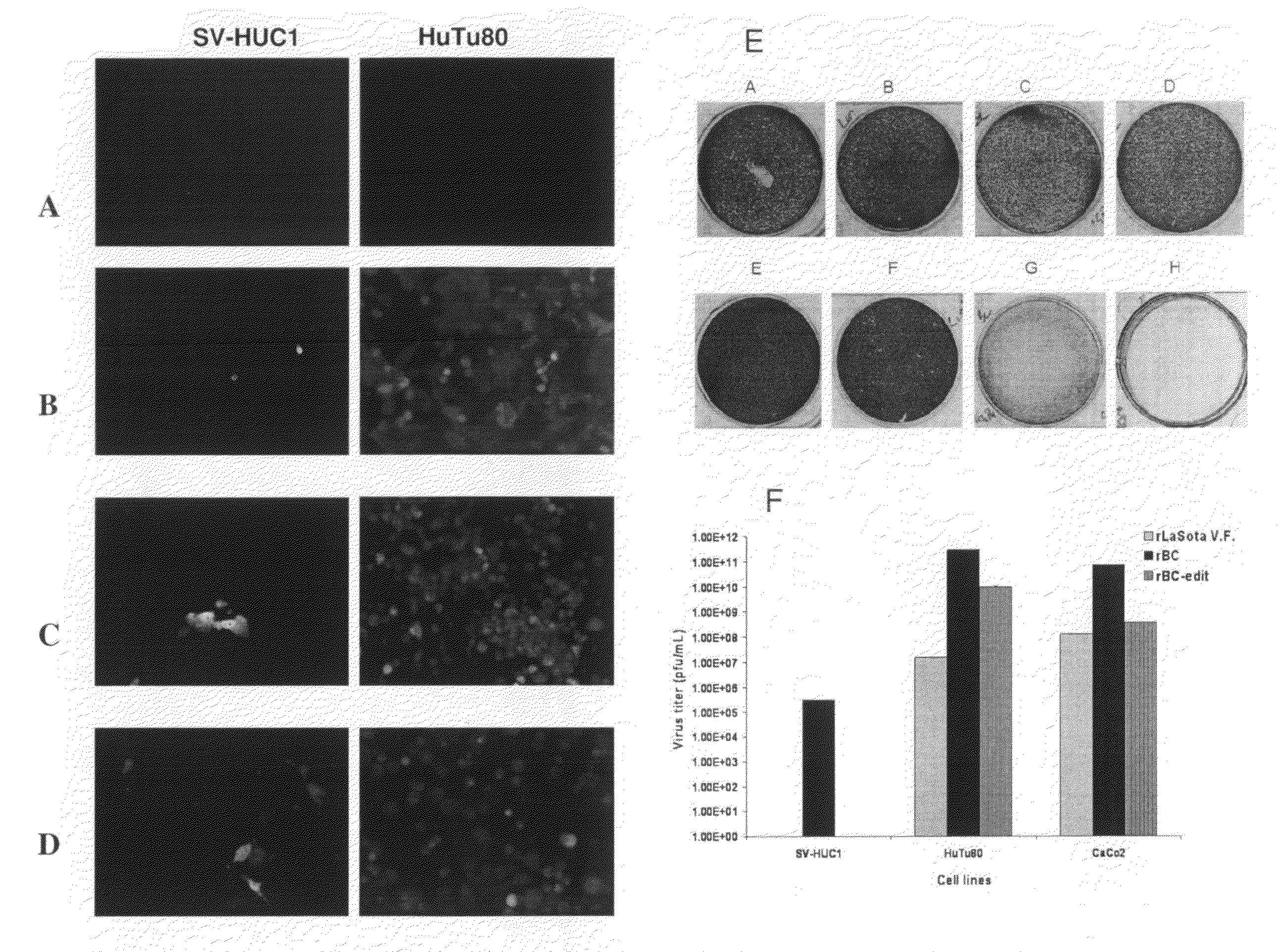
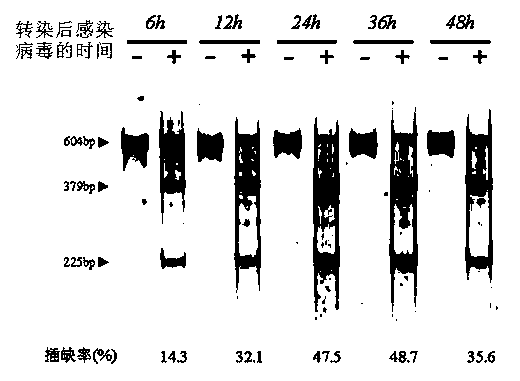
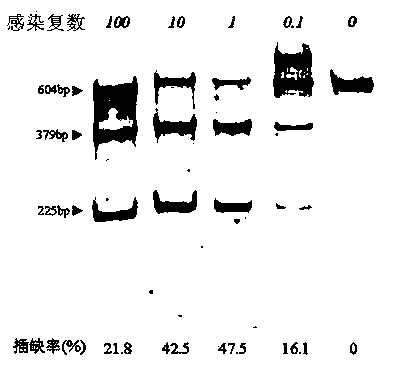
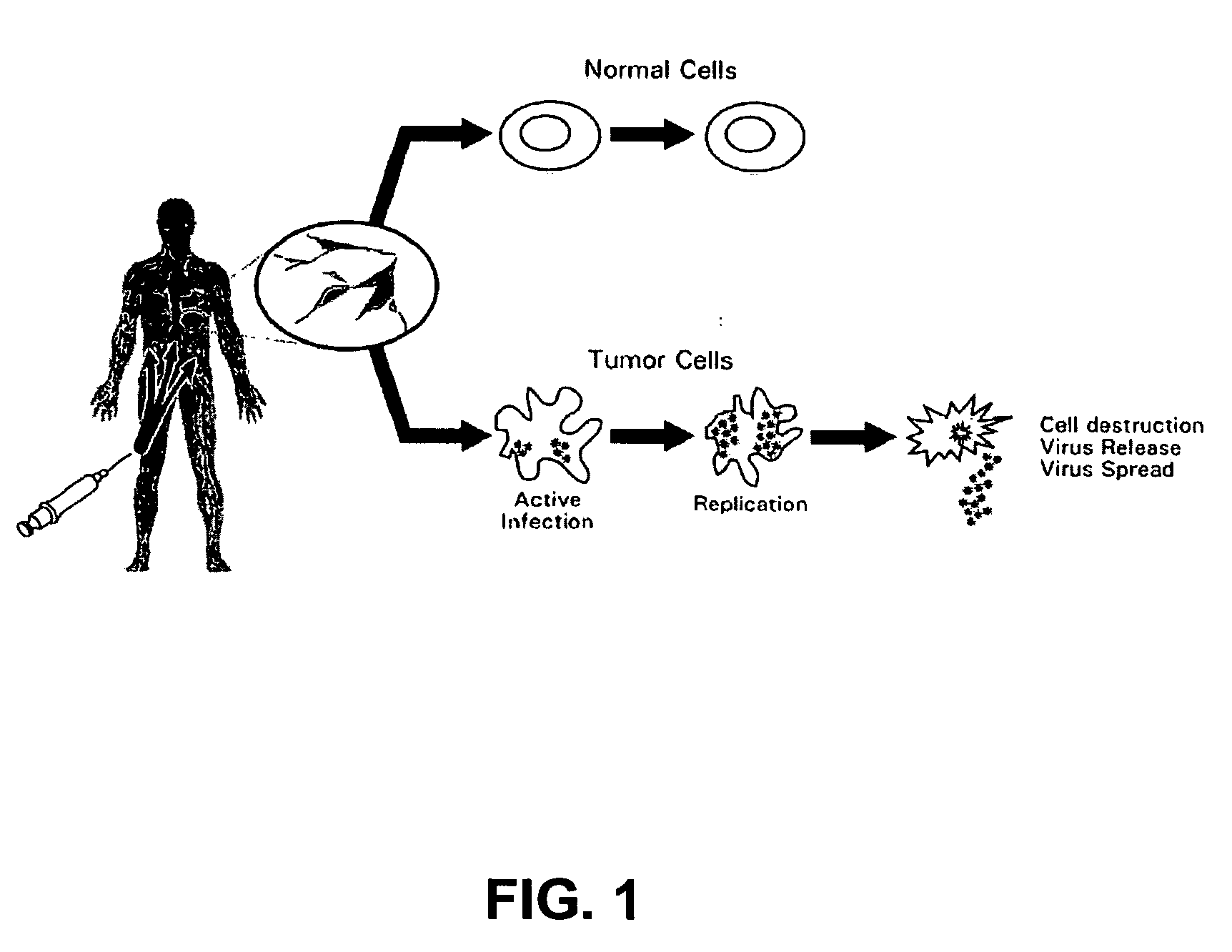
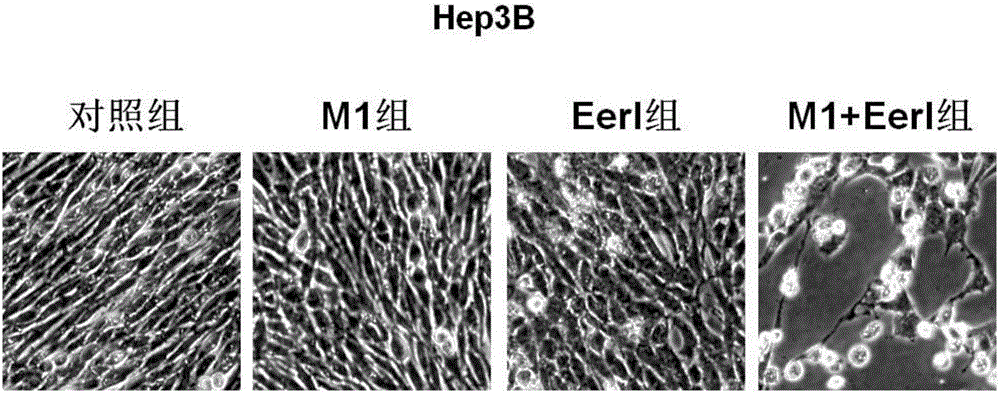
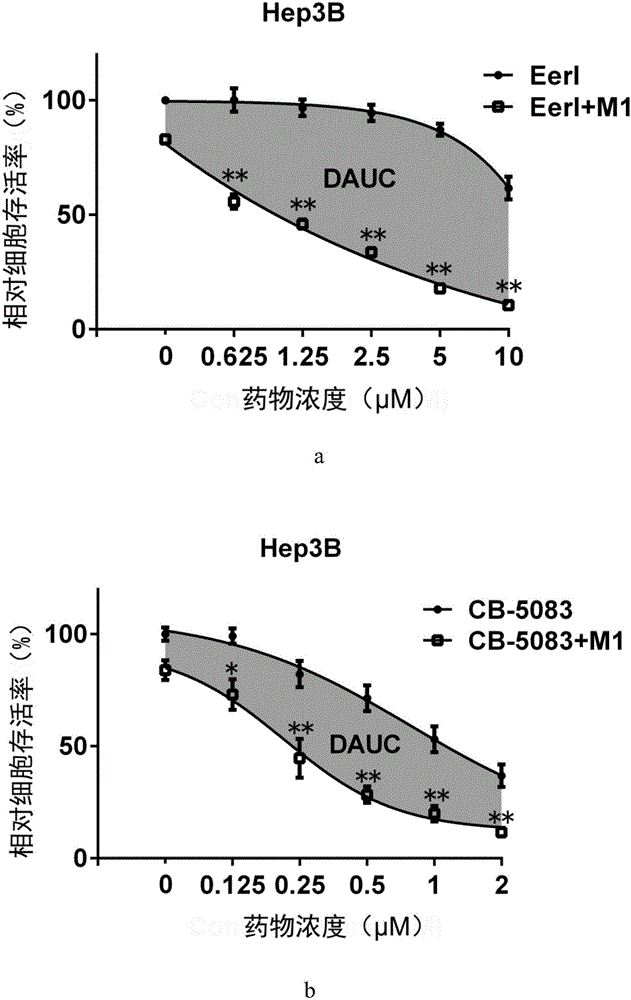
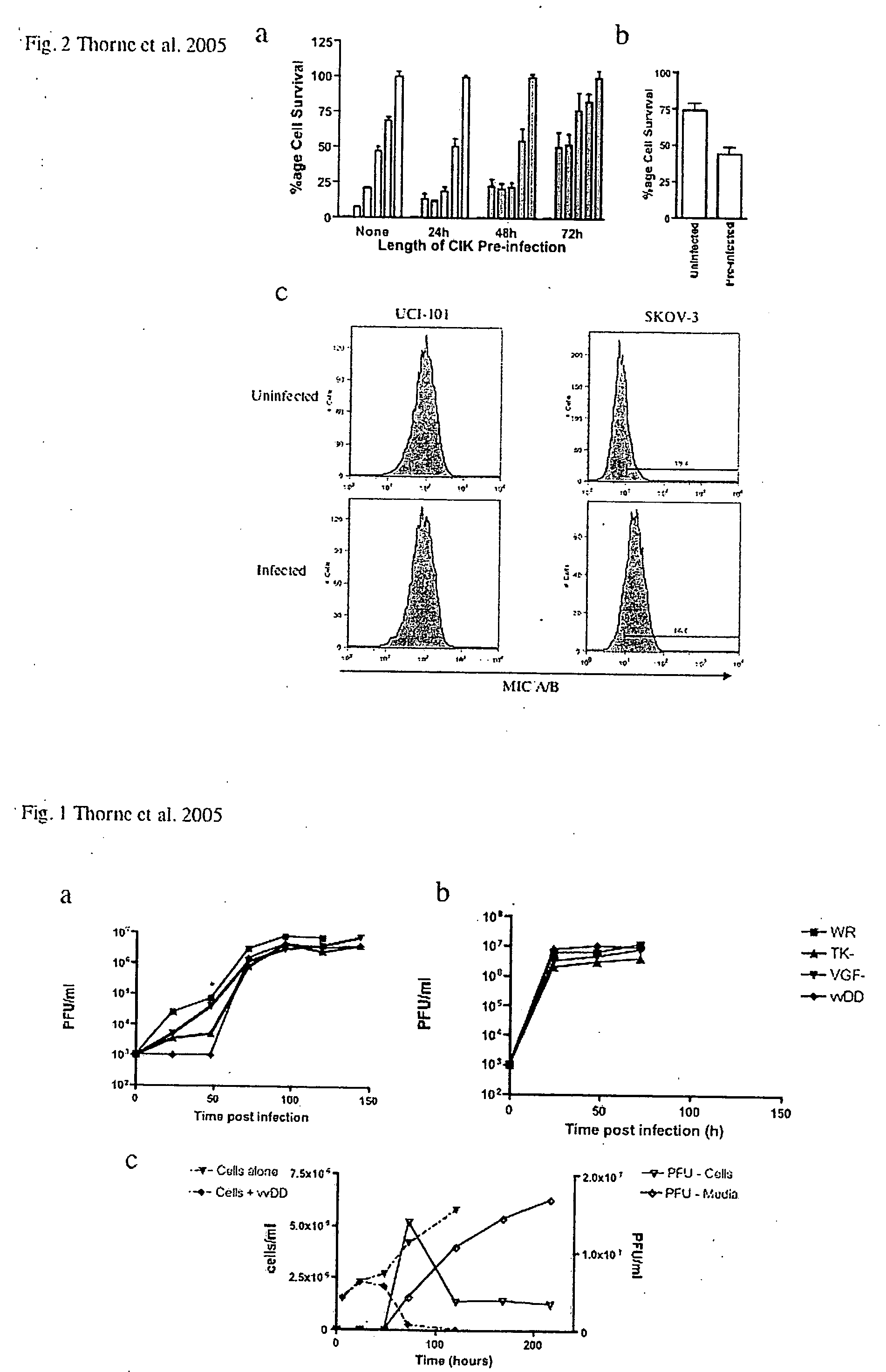
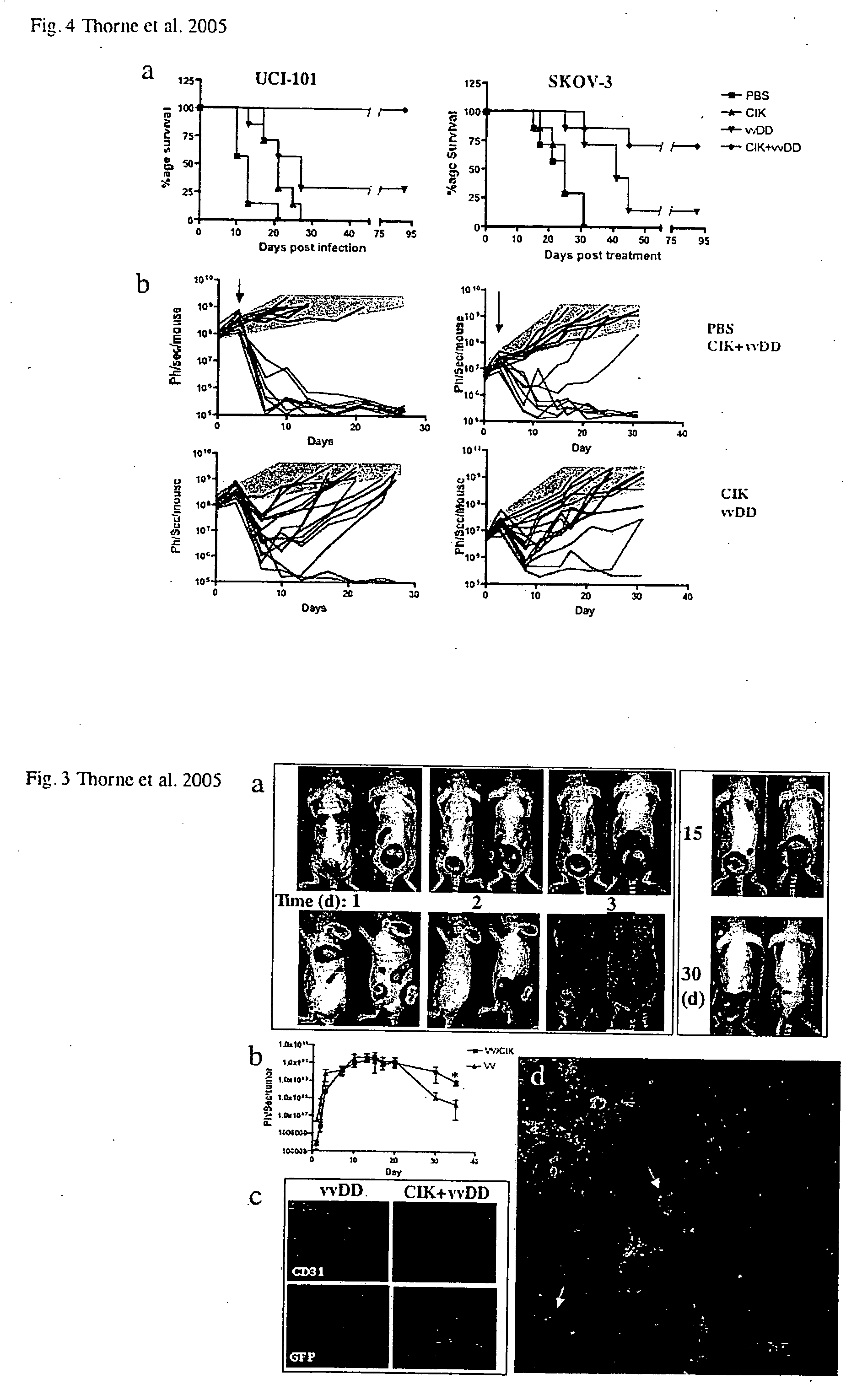
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
278 results about "Oncolytic virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
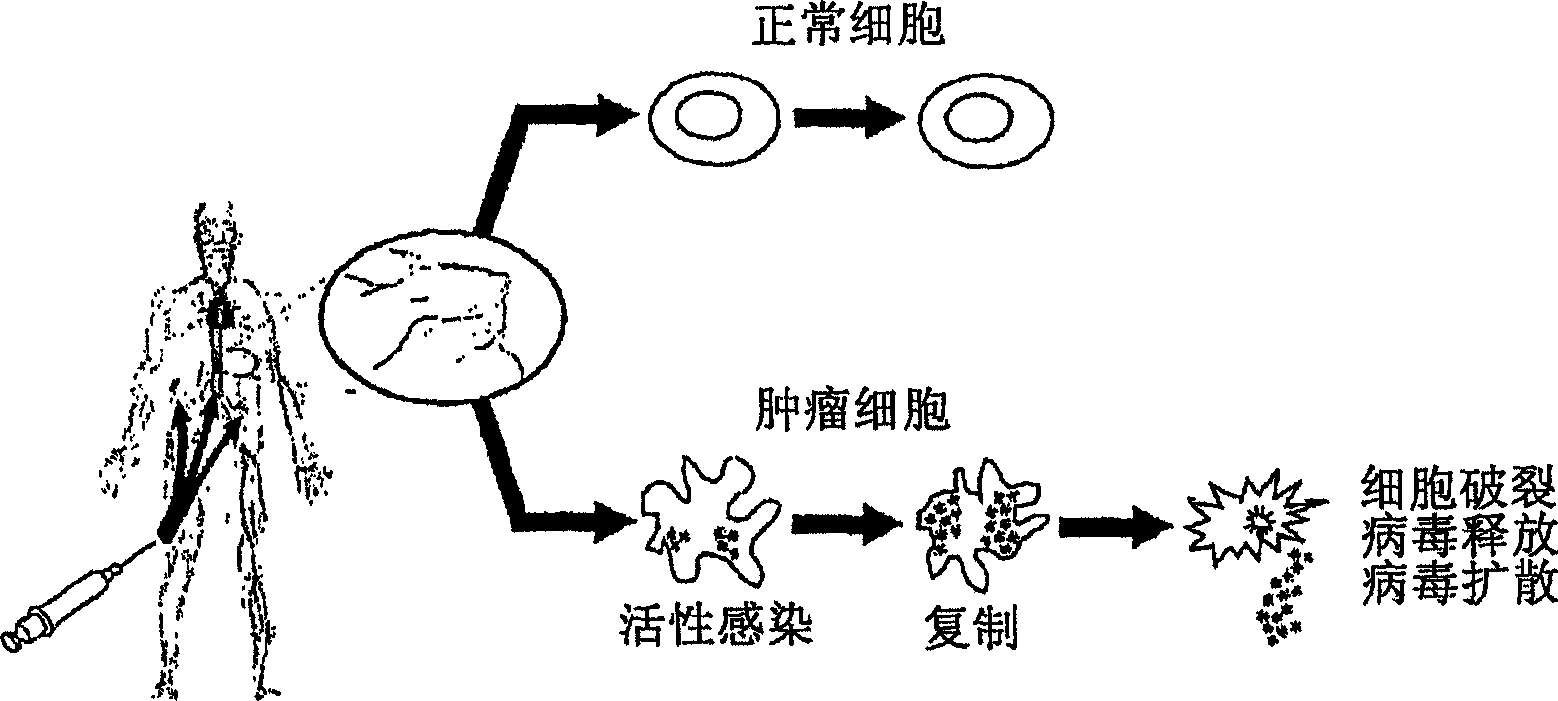
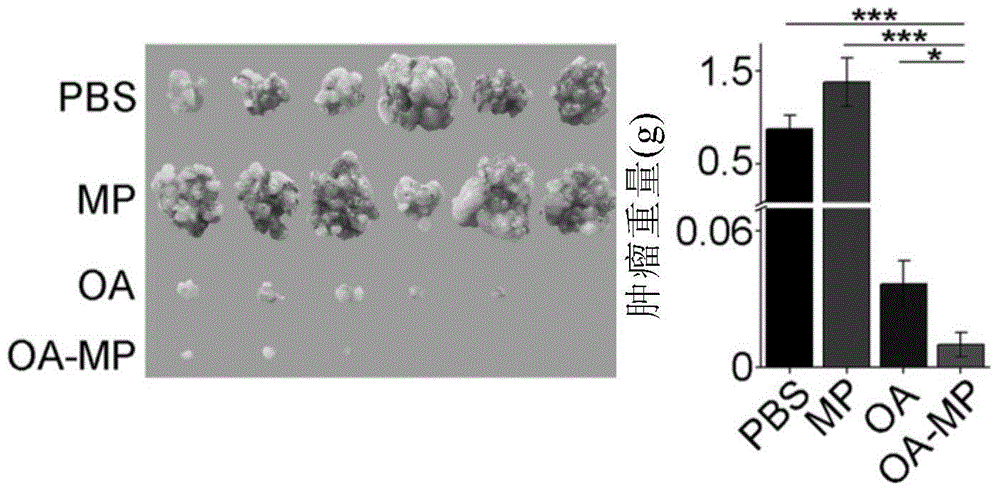
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses.
Isolated monoclonal antibodies that specifically bind to human Claudin 18.2
ActiveCN109762067AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonal antibody 14G2AAntigen receptors
Owner:BEIJING MABWORKS BIOTECH
Genetically-engineered newcastle disease virus as an oncolytic agent, and methods of using same
Recombinant strains of avian paramyxovirus (APMV), such as Newcastle disease virus (NDV), are provided. Also provided are compositions comprising them, and methods of using them to lyse tumor cells and to treat cancer. In certain aspects, genetically-engineered viral strains that incorporate therapeutic transgenes are also provided. The recombinant viruses may be used in accordance with methods of providing enhanced oncolytic efficacy and delivering an oncolytic virus to tumors present in a patient. Also provided are methods for identifying a recombinant virus as an oncolytically-effective agent.
Owner:UNIV OF MARYLAND OFFICE OF TECH COMMLIZATION
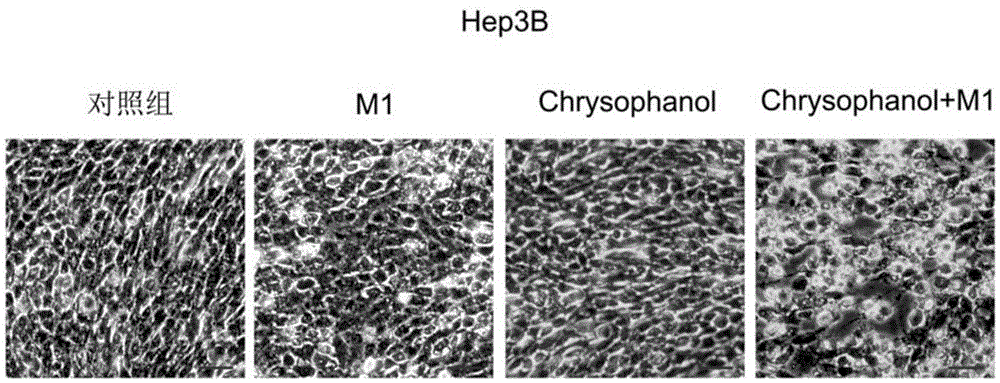
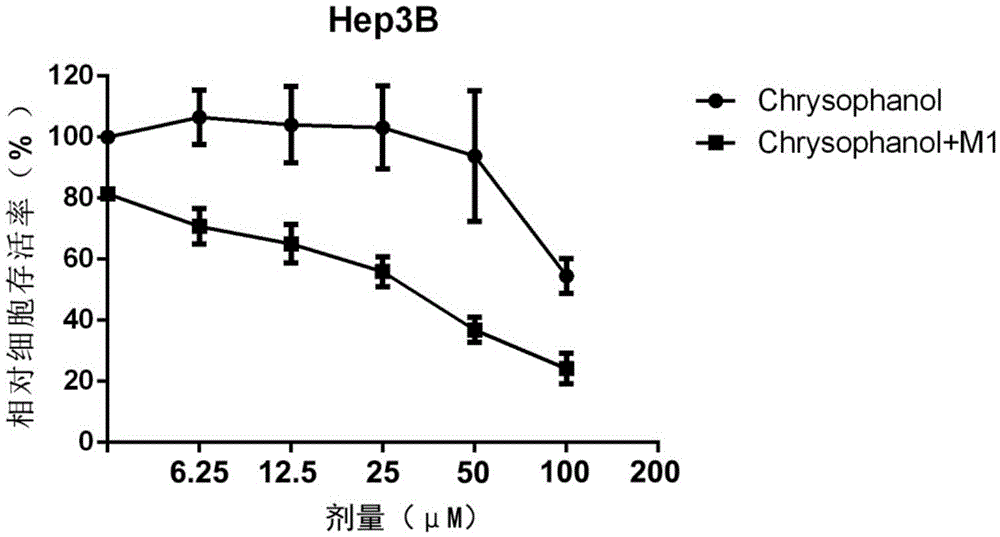
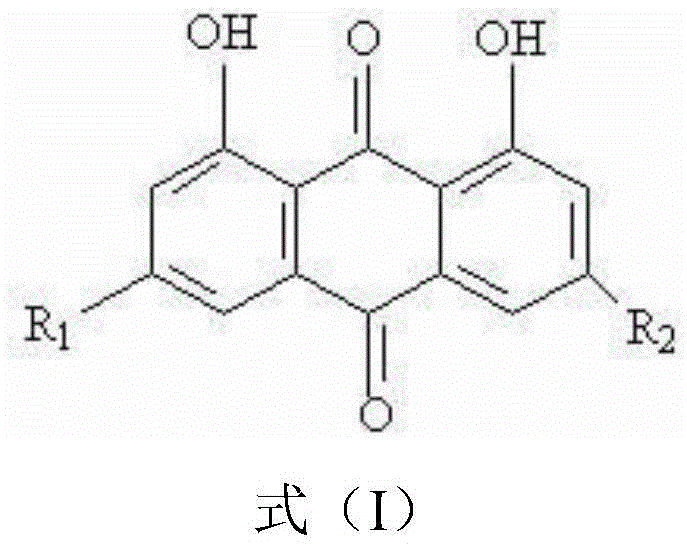
Applications of chrysophanol or derivative thereof and oncolytic virus in preparation of antitumor drugs
ActiveCN105456302AImprove anti-tumor effectImprove the effectiveness of treatmentOrganic active ingredientsUnknown materialsOncolytic adenovirusVirology
The invention belongs to the field of biomedicines, and relates to applications of chrysophanol or a derivative thereof and oncolytic virus in preparation of antitumor drugs. The invention finds that chrysophanol or the derivative thereof can be used for preparing oncolytic virus antitumor synergistic agents for the first time. Meanwhile, the invention also relates to a pharmaceutical composition containing chrysophanol and oncolytic virus, a medicine suit containing chrysophanol and oncolytic virus, and applications of chrysophanol and oncolytic virus in treatment of tumors, particularly tumors insensitive to the oncolytic virus.
Owner:GUANGZHOU VIROTECH PHARMA
Methods of treating solid or lymphatic tumors by combination therapy
The present invention provides methods for treating an individual having solid or lymphatic tumor comprising locally administering to the site of the tumor an oncolytic virus, and systemically administering an immunomodulator (including a combination of immunomodulators). The methods may further comprise local administration to the site of the tumor a second immunomodulator (including a combination of immunomodulators). Also provided are compositions and kits for the cancer therapy methods.
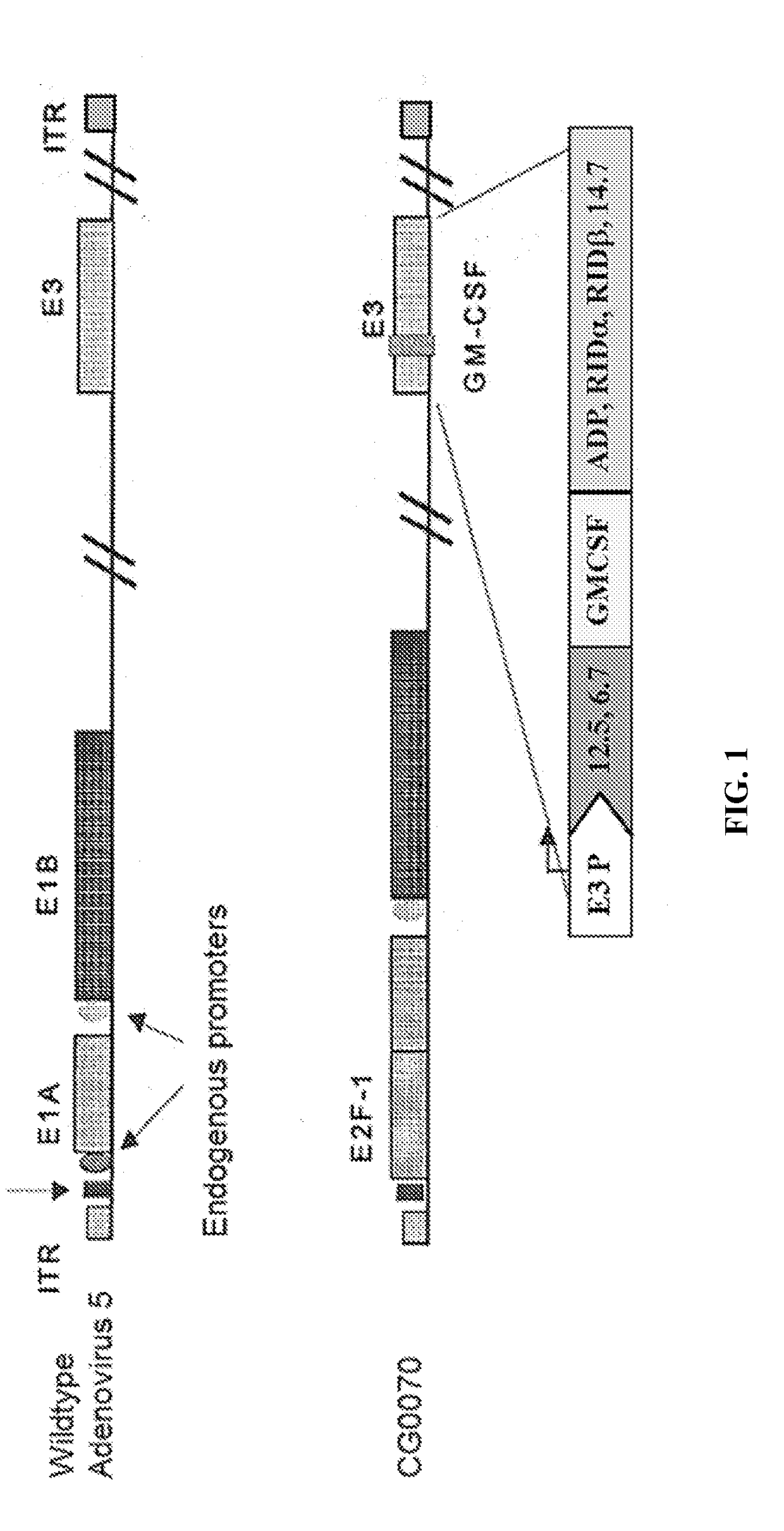
Owner:CG ONCOLOGY INC
Tetracycline repressor regulated oncolytic viruses
The present invention is directed oncolytic Herpes simplex-l viruses whose replication is controlled using a tetracycline operator / repressor system. The invention also includes DNA sequences used in making the viruses and methods in which these viruses are used in the treatment of cancer patients with solid tumors.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Oncolytic viruses as phenotyping agents for neoplasms
The present invention provides a method of diagnosing neoplasms having a particular phenotype by using oncolytic viruses that selectively replicate in neoplasms having the particular phenotype. For example, reovirus does not replicate in normal cells. However, reovirus selectively replicate in cells with an activated ras pathway, which leads to death of these cells. Therefore, a cell which becomes neoplastic due to, at least in part, elevated ras pathway activities can be diagnosed by its susceptibility to reovirus replication. This invention can further be applied, using other oncolytic viruses, to the diagnosis and / or treatment of other tumors, such as interferon-sensitive tumors, p53-deficient tumors and Rb-deficient tumors. Kits useful in the diagnosis or treatment disclosed herein are also provided.
Owner:ONCOLYTICS BIOTECH
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
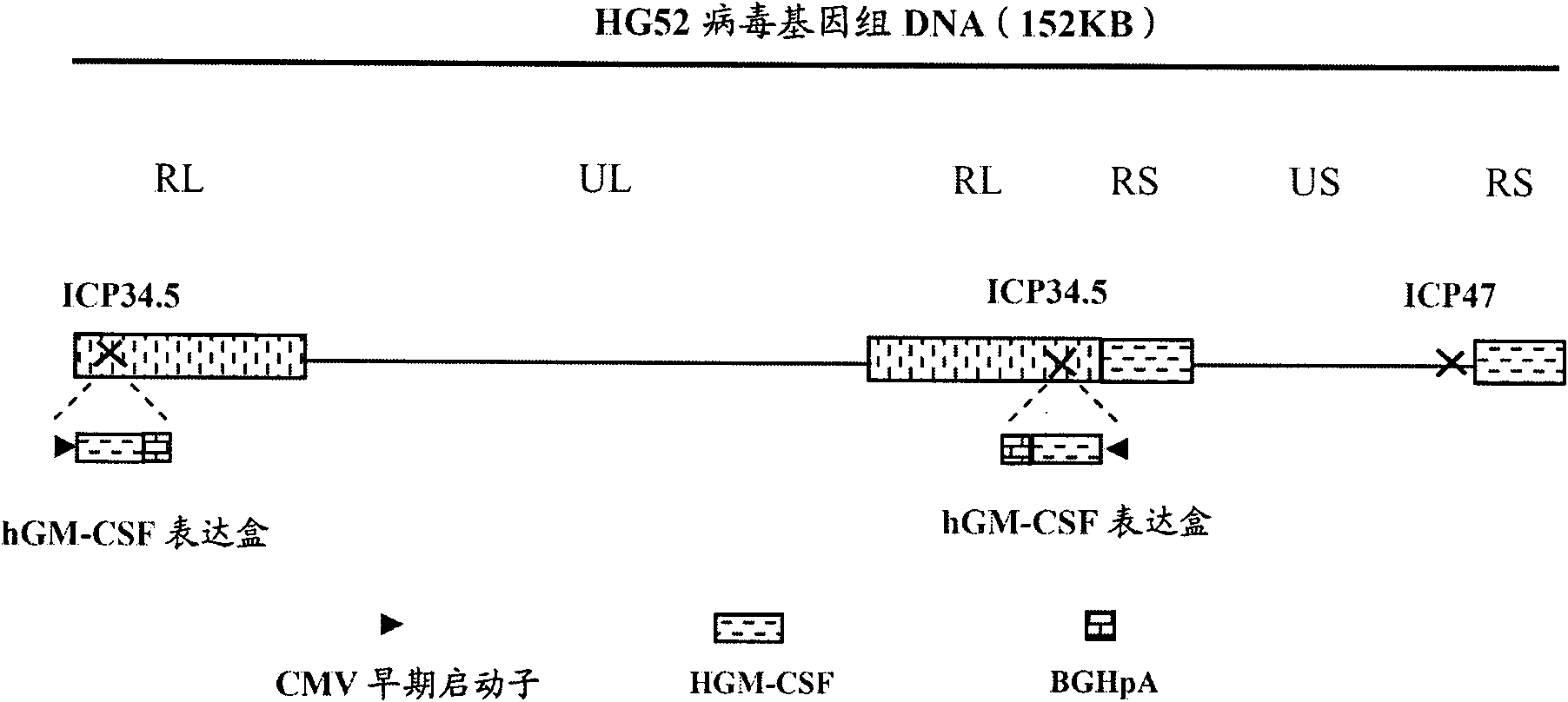
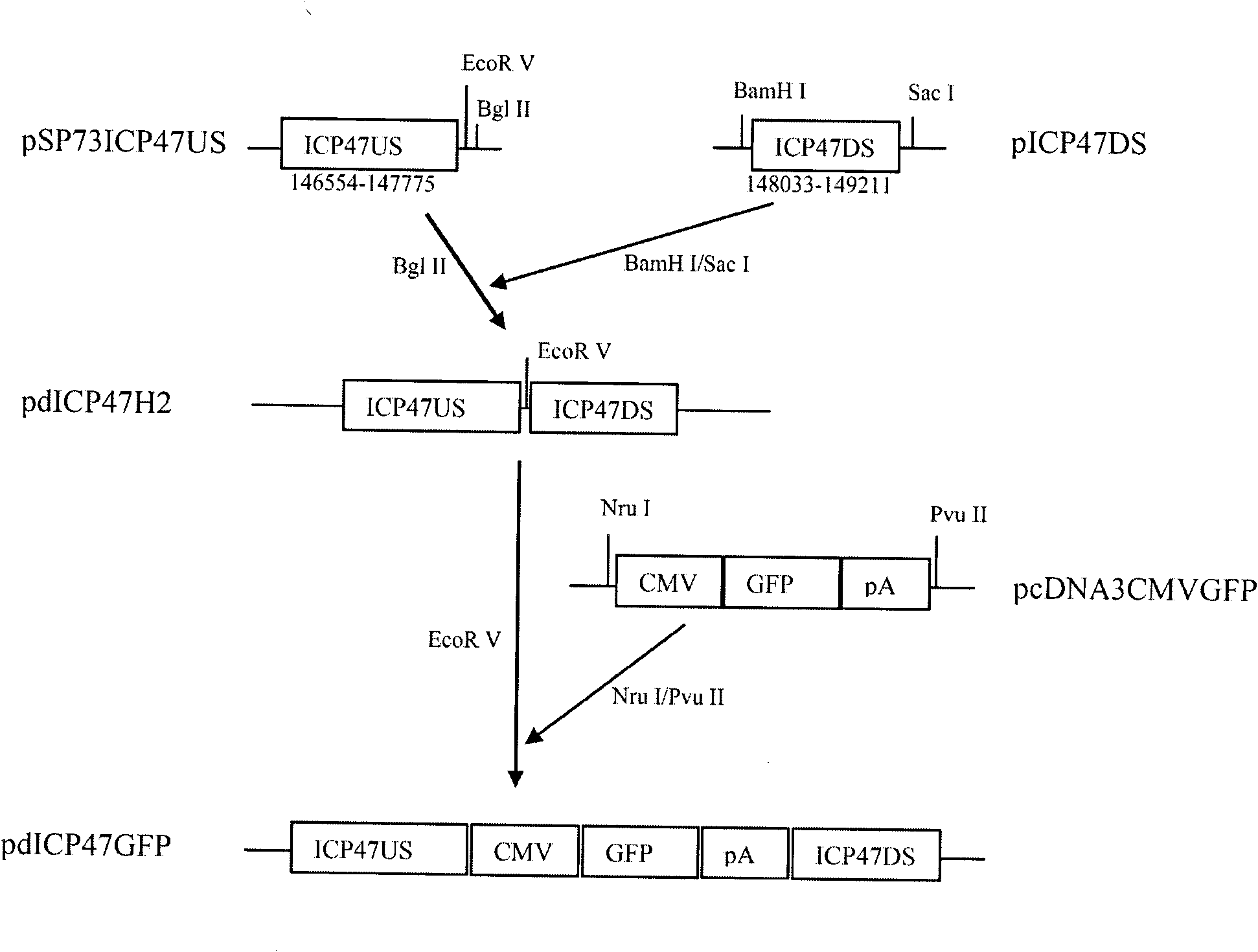
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
Site-directed modification method for DNA viral genome
ActiveCN103397018ARealize fixed-point transformationQuick insertRecombinant DNA-technologyFermentationFreeze thawingRecombinant virus
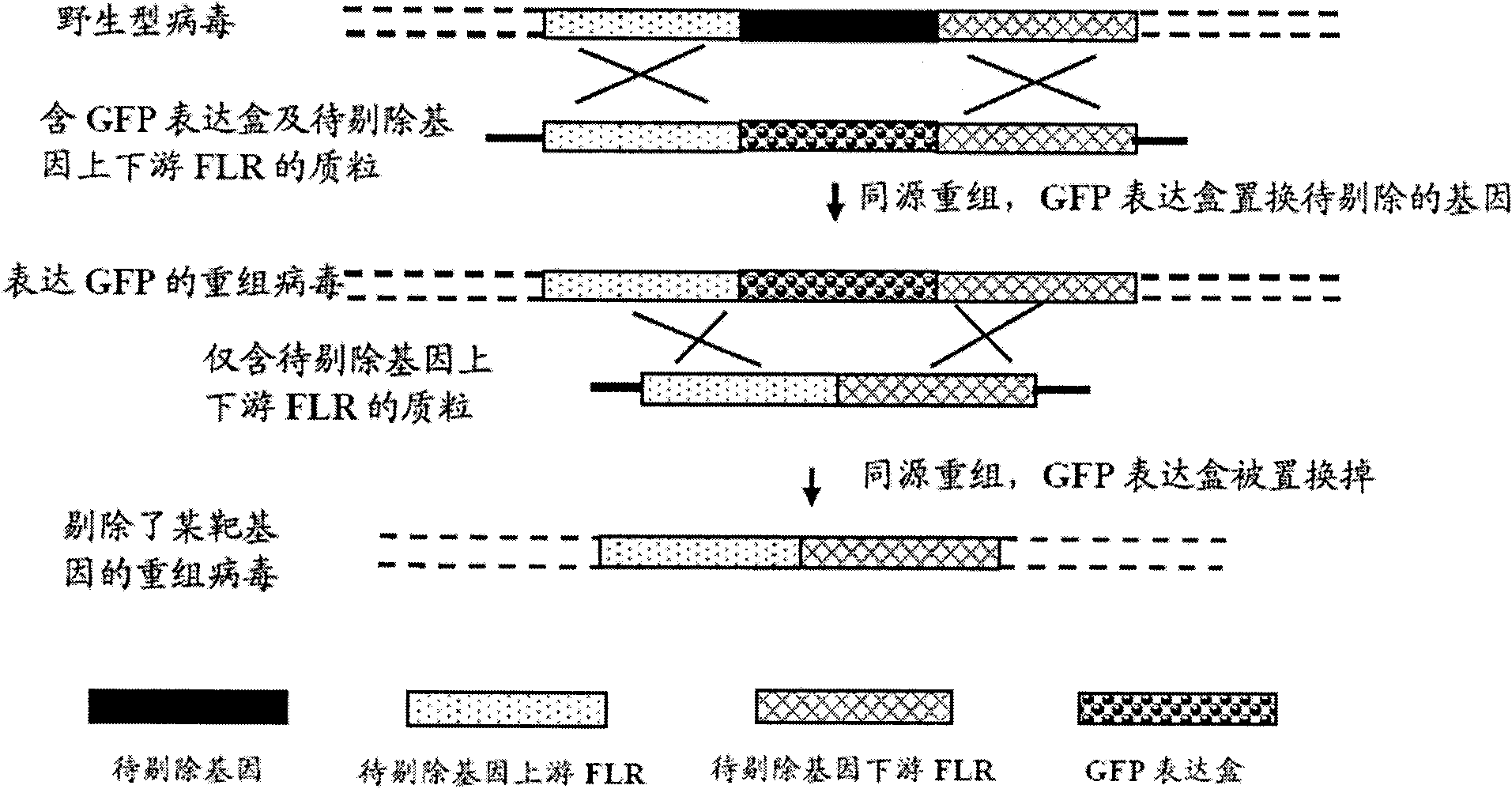
The invention provides a site-directed modification method for DNA viral genome, and the problems in the prior art are solved that induction of site-directed mutagenesis of DNA viral genome is difficult, the operation of inserting an exogenous fragment is complex, and recombination rate is lower. The site-directed modification method comprises: transfecting cells by a plasmid carrying a nuclease system, infecting by a virus, after the cells show pathological changes, collecting the cells with pathological changes, performing freeze-thaw or ultrasonic processing, and centrifuging, separating the liquid supernatant to obtain a progeny virus. The site-directed modification method is capable of realizing applications to screening of virus attenuated vaccine strains, construction of viral genetic carriers and an oncolytic virus, research on virus function sequences, and the like; during modification of the viral genome, the method helps to improve mutagenesis efficiency, accurately control DNA virus for genome site-directed mutagenesis and specific gene knockout, simplify operation steps of inserting the DNA virus carrier by an exogenous gene, and improve efficiency that the exogenous gene is integrated to the viral genome, so that the work of screening high-flux recombination viruses is convenient to conduct.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Seneca valley virus based compositions and methods for treating disease
ActiveUS20060159659A1High therapeutic indexSafe and effective and new lineBiocideSsRNA viruses positive-senseAbnormal tissue growthProtein detection
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus (“SVV”). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:PERCEIVER PHARMA +1
Application of VCP (valosin containing protein) inhibitor and oncolytic virus in preparation of antitumor drugs
The invention belongs to the field of biological medicine and relates to an application of a VCP (valosin containing protein) inhibitor and an oncolytic virus in preparation of antitumor drugs. The invention discovers that the VCP inhibitor can be used for preparing an oncolytic virus antitumor synergist. Meanwhile, the invention relates to pharmaceutical composition containing the VCP inhibitor and the oncolytic virus, a drug suit containing the VCP inhibitor and the oncolytic virus as well as an application of the VCP inhibitor and the oncolytic virus in treatment of tumors, especially tumors insensitive to the oncolytic virus. Besides, the invention relates to an antitumor medication system, and the system is characterized by comprising a VCP expression level detection reagent and the oncolytic virus.
Owner:GUANGZHOU VIROTECH PHARMA
Immune effector cells pre-infected with oncolytic virus
InactiveUS20070077231A1Improved biodistributionMinimal viral infectionBiocideGenetic material ingredientsAbnormal tissue growthImmune effector cell
Compositions and methods are provided for the treatment of cancer. An immune effector cell population is pre-infected with an oncolytic virus. The combined therapeutic is safe and highly effective, producing an enhanced anti-tumor effect compared to either therapy alone. The methods of the invention thus provide for a synergistic effect based on the combined biotherapeutics.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Generation of antibodies to tumor antigens and generation of tumor specific complement dependent cytotoxicity by administration of oncolytic vaccinia virus
The present invention relates to methods and compositions for use in inducing tumor-specific antibody mediated complement-dependent cytotoxic response in an animal having a tumor comprising administering to said animal a composition comprising a replication competent oncolytic virus wherein administration of the composition induces in the animal production of antibodies that mediate a CDC response specific to said tumor.
Owner:SILLAJEN +1
Application of IAP inhibitor and oncolytic virus in preparation of antitumor drug
The invention belongs to the field of biological medicines and relates to an application of combination of a Caspase activator and an oncolytic virus in preparation of an antitumor drug. The condition that the antitumor effect of the oncolytic virus can be improved with the Caspase activator is discovered for the first time, the combination of the Caspase activator and the oncolytic virus has a quite high synergistic effect, and an effective therapeutic schedule is provided for oncotherapy with low drug sensitivity.
Owner:GUANGZHOU VIROTECH PHARMA
Attenuated, brightened and replication-controllable HSV recombinant virus, preparation method and applications thereof
InactiveCN107630009AEasy genetic manipulationEasy to insertViruses/bacteriophagesFermentationCell specificNervous system
The invention discloses attenuated, brightened and replication-controllable HSV recombinant virus, a preparation method and applications thereof. According to the present invention, thymidine kinase (TK) gene essential for replicating viruses in neurons and being a main virulence factor is knocked out by using a homologous recombination method, and subsequently a red or green fluorescent gene enhancement expression cassette is recombined into the genome of the virus to construct a series of novel recombinants viruses, wherein the toxicity is markedly low, the states of infected mice are good,the fluorescence signal is strong, and the expression of the recombinant viruses is limited at the injection site after the recombinant viruses are used in in-vivo animal center labeling; by combiningwith Cre-dependent AAV helper viruses capable of expressing TK in a compensated manner, the cell-specific transmonosynaptic loop tracing is achieved; and the recombinant HSV has wide application value in nervous system targeted gene transduction, neural network transsynaptic tracing, tumor disintegration, viral replication and pathogenesis mechanism, antiviral drug screening and other fields.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Seneca valley virus based compositions and methods for treating disease
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus ('SVV'). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:NOVARTIS PHARM AG
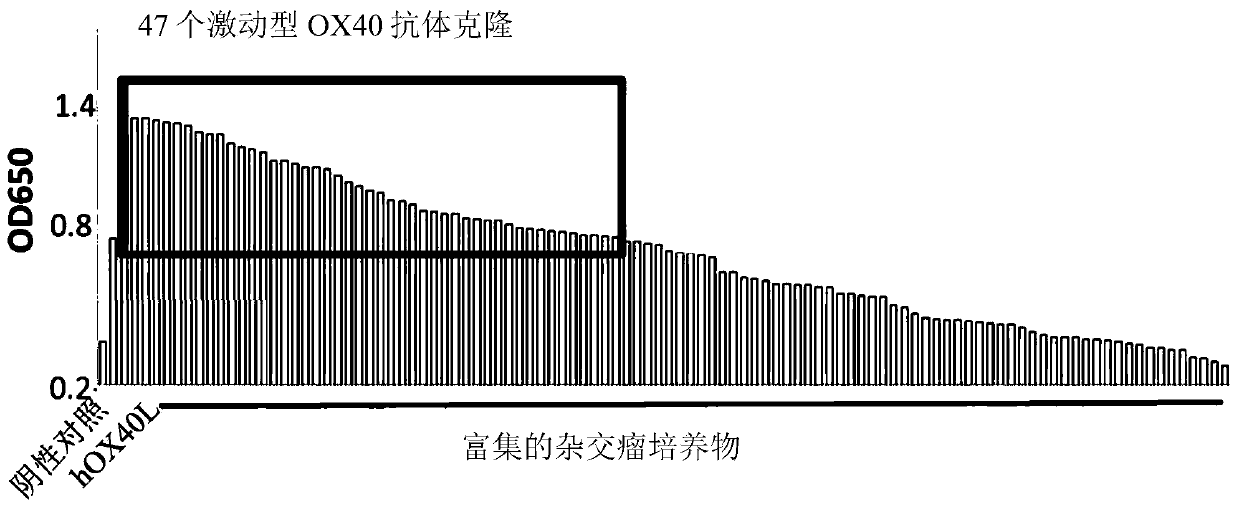
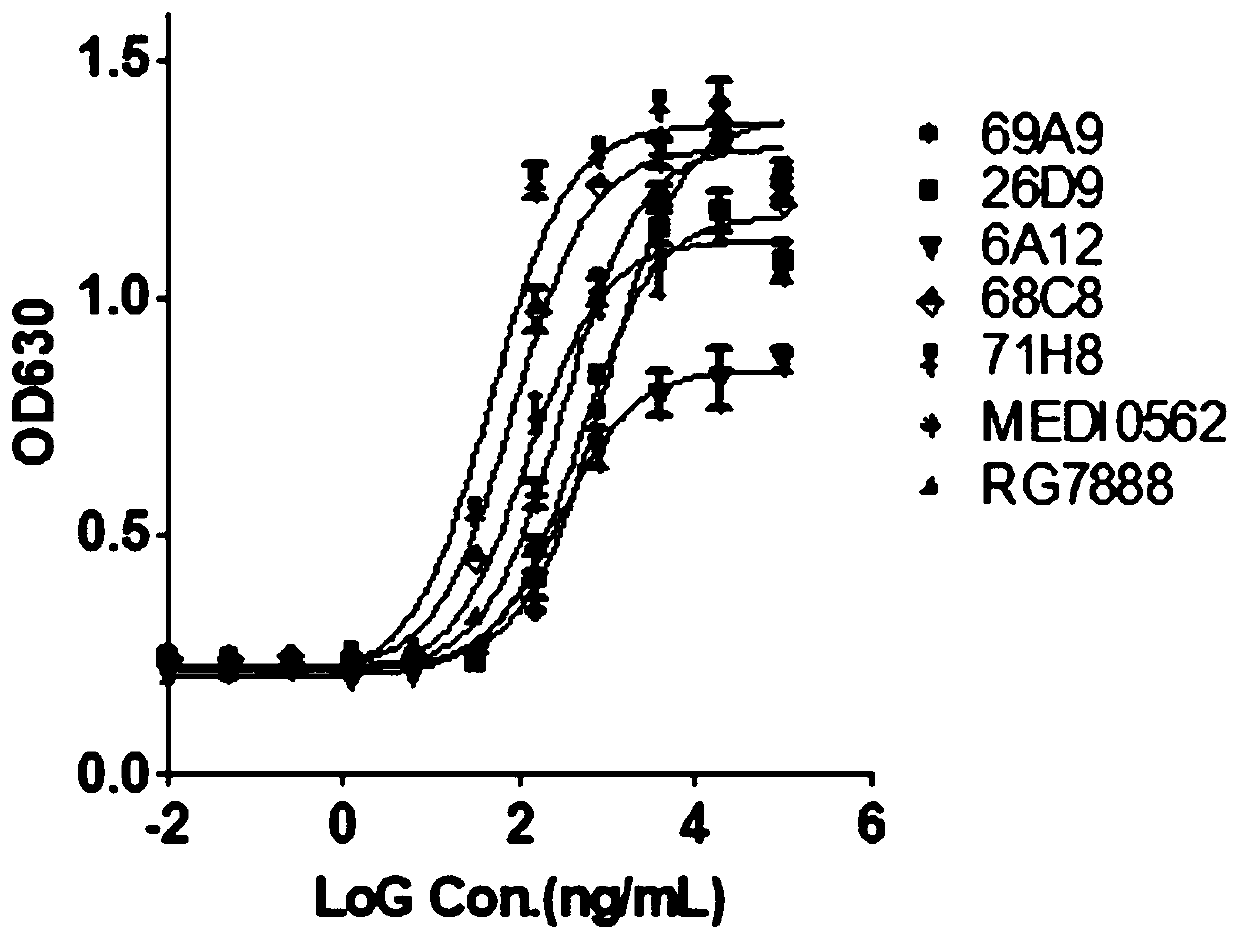
Antibody that binds to OX40 and use thereof
ActiveCN110078825AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen receptorsChimeric antigen receptor
The present invention provides an isolated monoclonal antibody that specifically binds to human OX40. Also provided are nucleic acid molecules encoding the antibody, an expression vector, a host celland a method for expressing the antibody. The invention also provides immunoconjugates, bispecific molecules, chimeric antigen receptors, oncolytic viruses, and pharmaceutical compositions comprisingthe antibody, as well as methods of treatment using the antibody of the invention.
Owner:BEIJING MABWORKS BIOTECH
Seneca valley virus based compositions and methods for treating disease
InactiveCN101448526ASimple and fast life cycleEasy to operateSugar derivativesGenetic material ingredientsViral nucleic acidTropism
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus ('SVV'). The invention provides isolated SW nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of rumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SW nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:纽特罗佩克斯公司 +1
Oncolytic virus preparation and preparing method thereof
InactiveCN104958324AConducive to achieveEvade attackSsRNA viruses negative-senseViral/bacteriophage medical ingredientsCell vesicleWilms' tumor
The invention provides an oncolytic virus preparation and a preparing method thereof. The oncolytic virus preparation comprises cell vesicles coming from apoptotic tumor cells and oncolytic viruses which are wrapped in the cell vesicles and serve as effective constituents. According to the oncolytic virus preparation, as the cell vesicles coming from the cells are used for wrapping the oncolytic viruses, the oncolytic viruses escape from attacks of an organism immune system and can reach the tumor treatment positions in a targeted manner, and the tumor killing effect is improved.
Owner:HUBEI SOUNDNY BIOLOGICAL TECH
Compositions and methods for enhancing antigen-specific immune responses
Methods for treating or preventing recurrence of hyper proliferating diseases, e.g., cancer and persistent viral infections, are described. A method may comprise priming a mammal by administering to the mammal an effective amount of a nucleic acid composition encoding an antigen or a biologically active homolog thereof and boosting the mammal by administering to the mammal an effective amount of an oncolytic virus comprising a nucleic acid encoding the antigen or the biologically active homolog thereof.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
In Vivo Enhancement of Immune System Recognition of Neoplasms Following Treatment with an Oncolytic Virus or Gene Therapy Vector
InactiveUS20070071723A1Increase killIncrease opportunitiesOrganic active ingredientsBiocideNeoplasmIn vivo
This invention provides novel methods of treating or alleviating neoplasms and enhancing the efficacy of oncolytic viruses by administering an oncolytic virus to a mammal suffering from a neoplasm and subsequently administering an immunostimulant. The invention also provides methods of increasing immunorecognition of neoplastic cells.
Owner:ONCOLYTICS BIOTECH
Modified Oncolytic Viruses
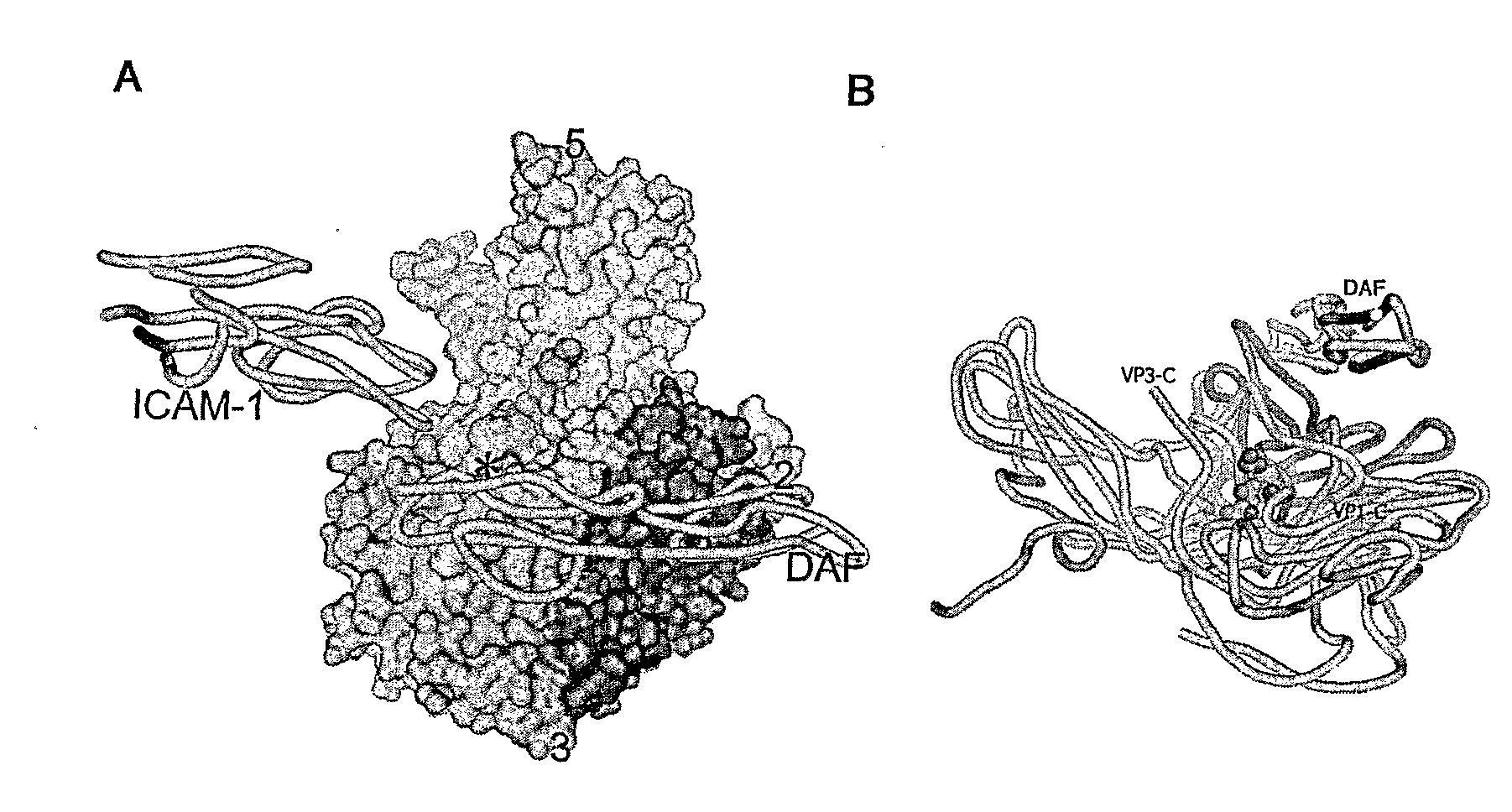
An isolated selected Picornavirus capable of lytically infecting or killing a cell substantially in the absence of intercellular adhesion molecule-1 (ICAM-1).
Owner:MERCK SHARP & DOHME LLC
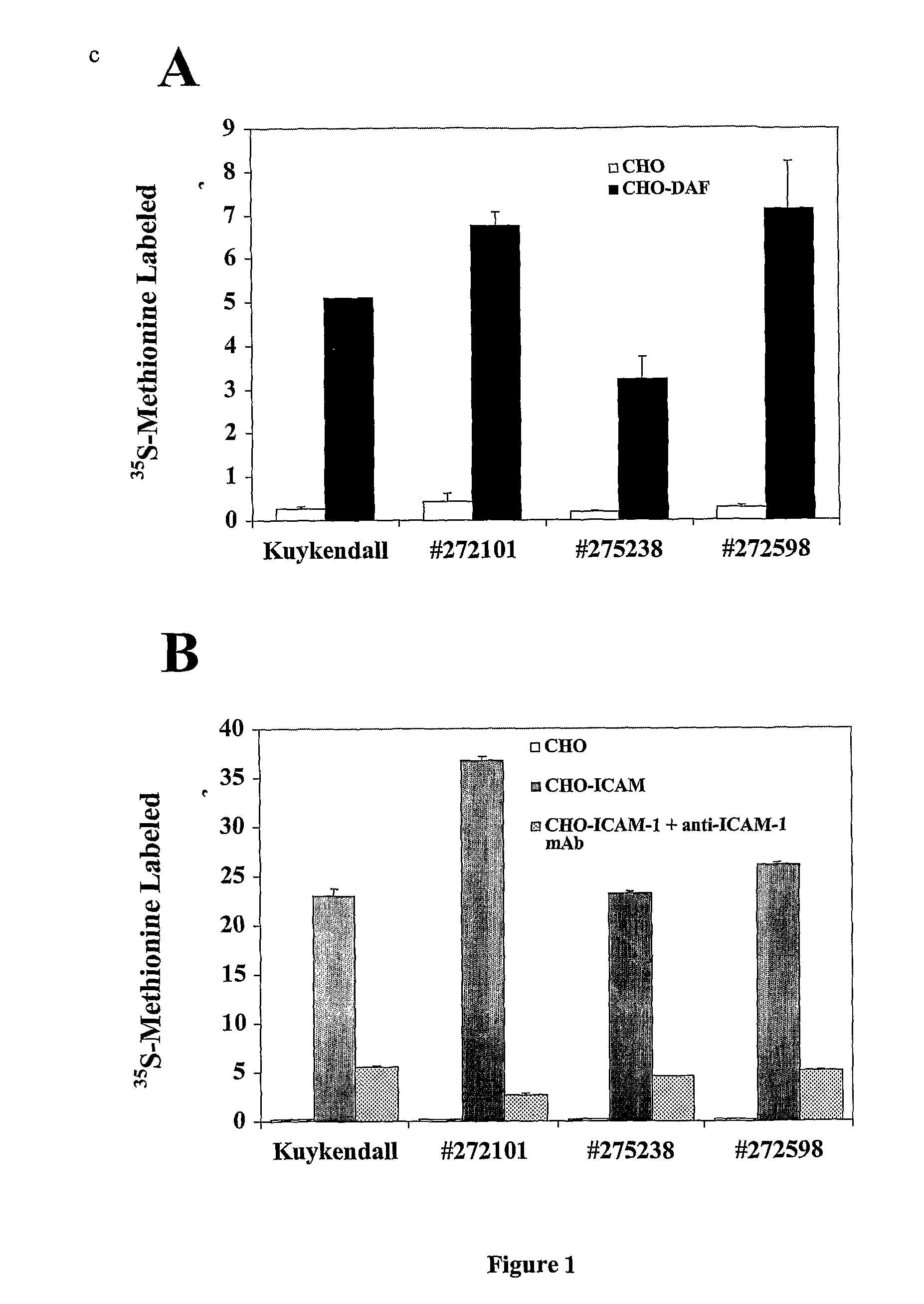
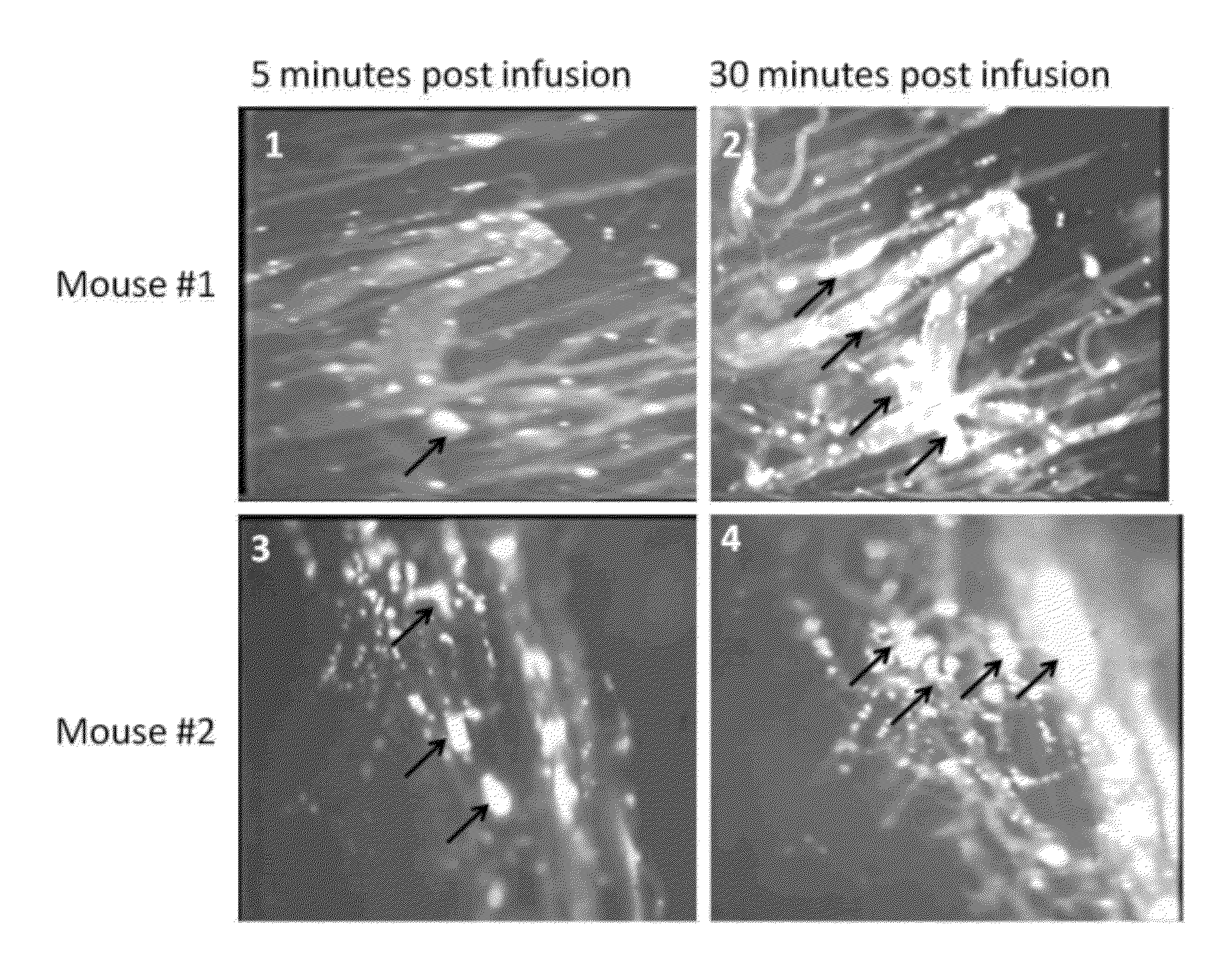
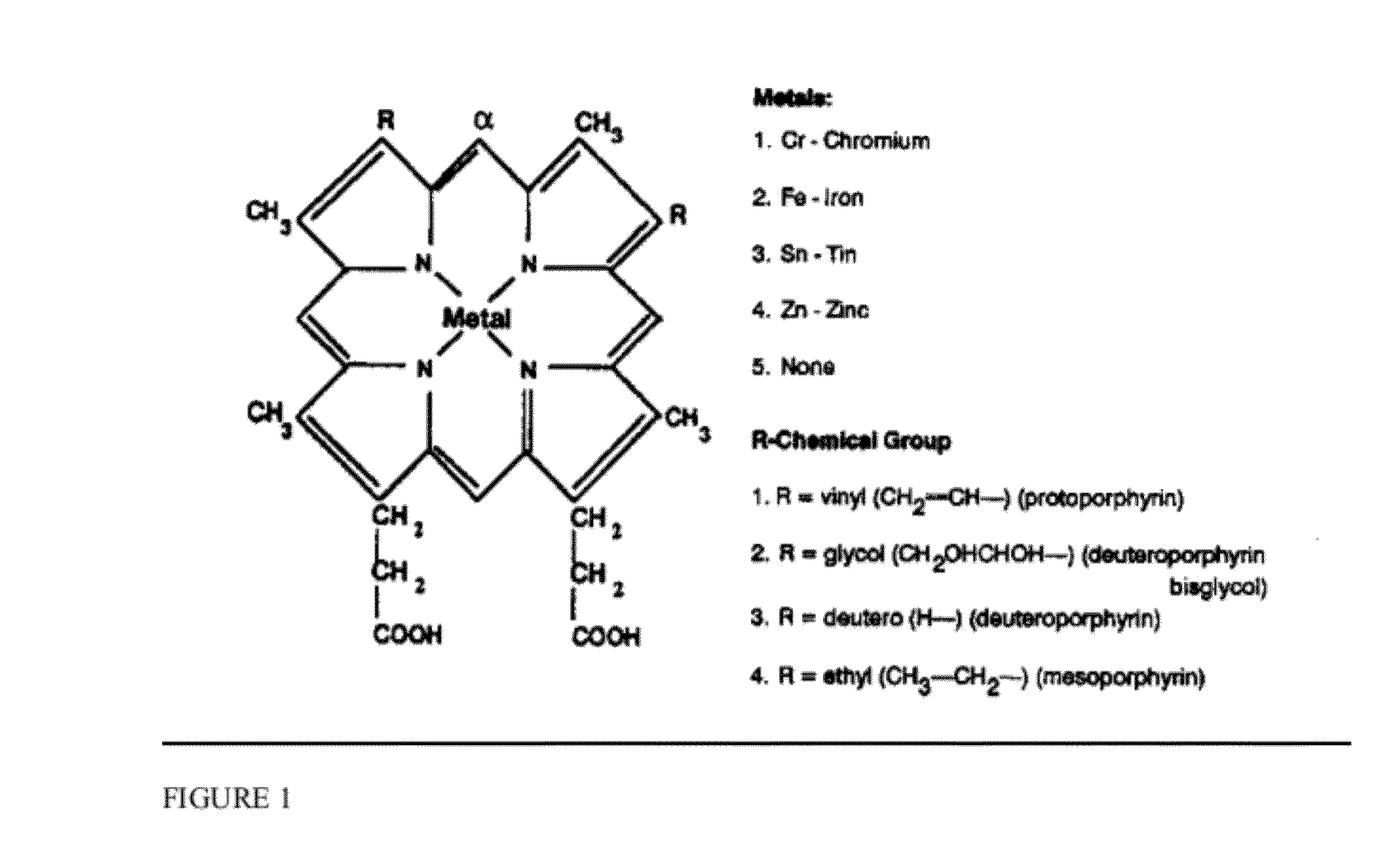
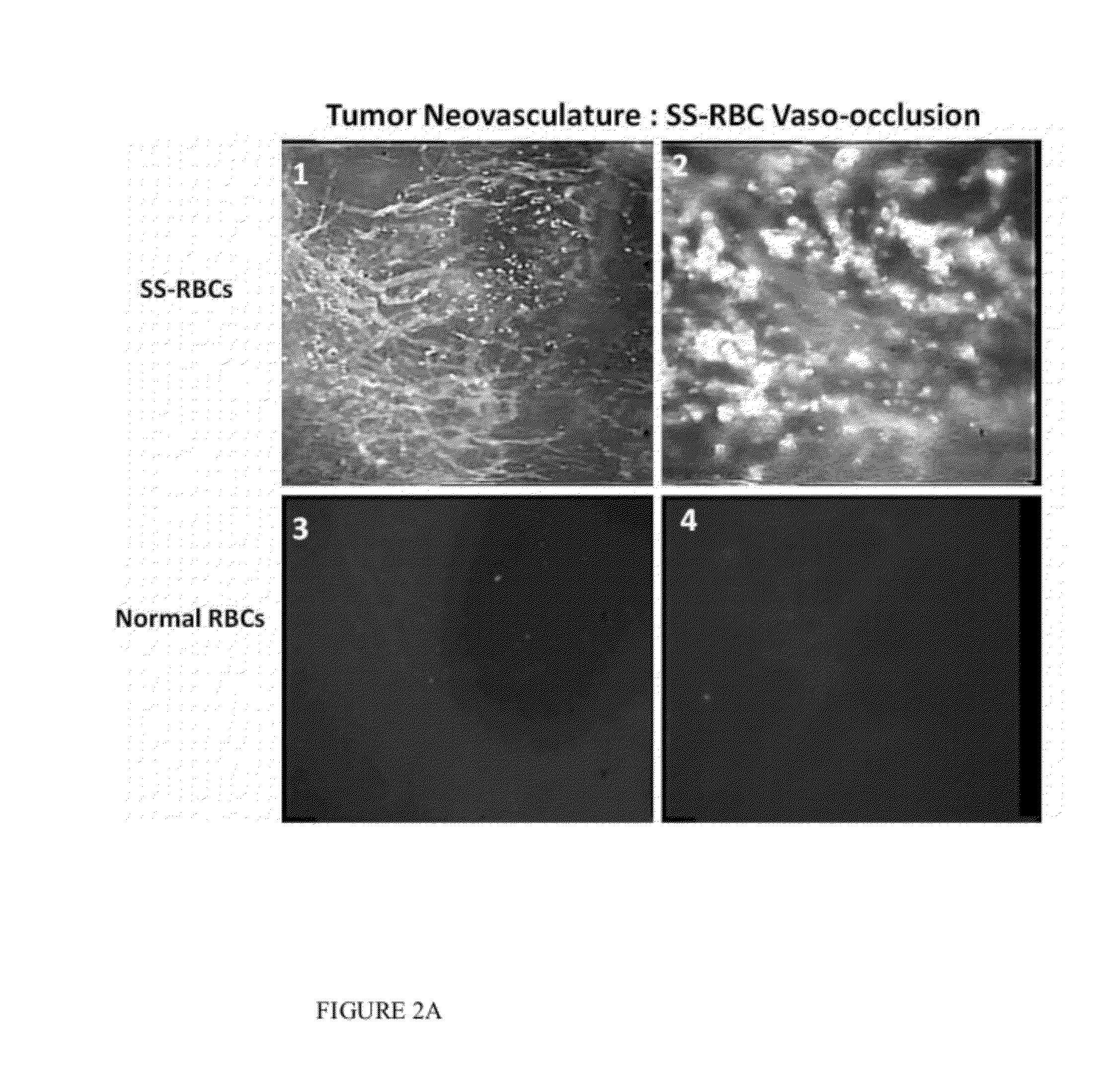
Sickled Erythrocytes with Anti-tumor Agents Induce Tumor Vaso-occlusion and Tumoricidal Effects
The present invention provides erythrocytes or nucleated erythrocyte precursors from animals or patients with at least one S hemoglobin allele which are capable of selectively localizing in tumor vasculature resulting in vaso-occlusion, hemolysis and heme release. A tumoricidal effect is achieved when these cells are administered in before during or after administration of (i) an agent(s) that interferes with degradation of reactive oxygen species, (ii) impairs glucose uptake and / or (iii) chemotherapy. These cells also carry oncolytic viruses, antitumor proteins, multidrug resistant proteins, chemotherapy, monoclonal antibodies, superantigens, superantigen conjugates and fusion proteins, siRNAs, plasmids and non-protein toxins and attenuated tumoricidal bacterial cells specifically into the tumors and induce a tumoricidal effect.
Owner:TERMAN DAVID S
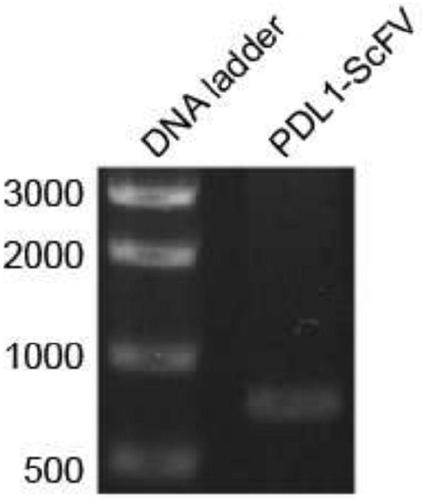
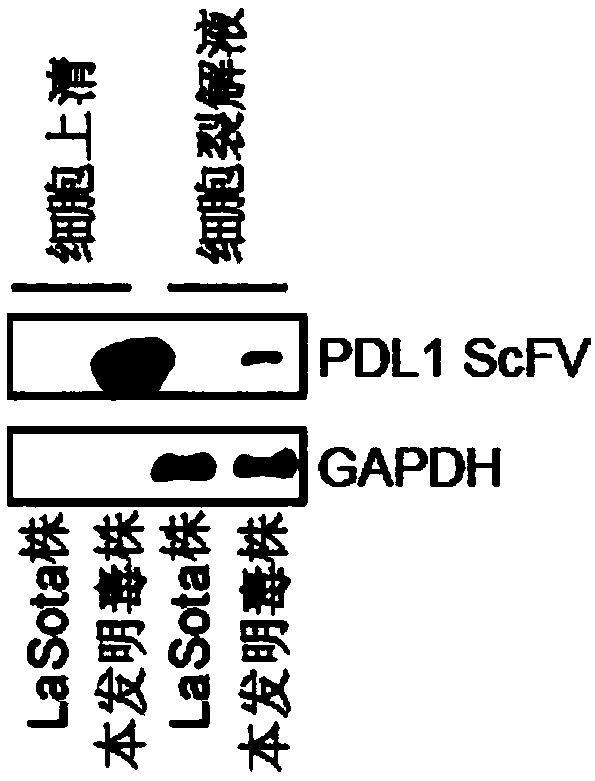
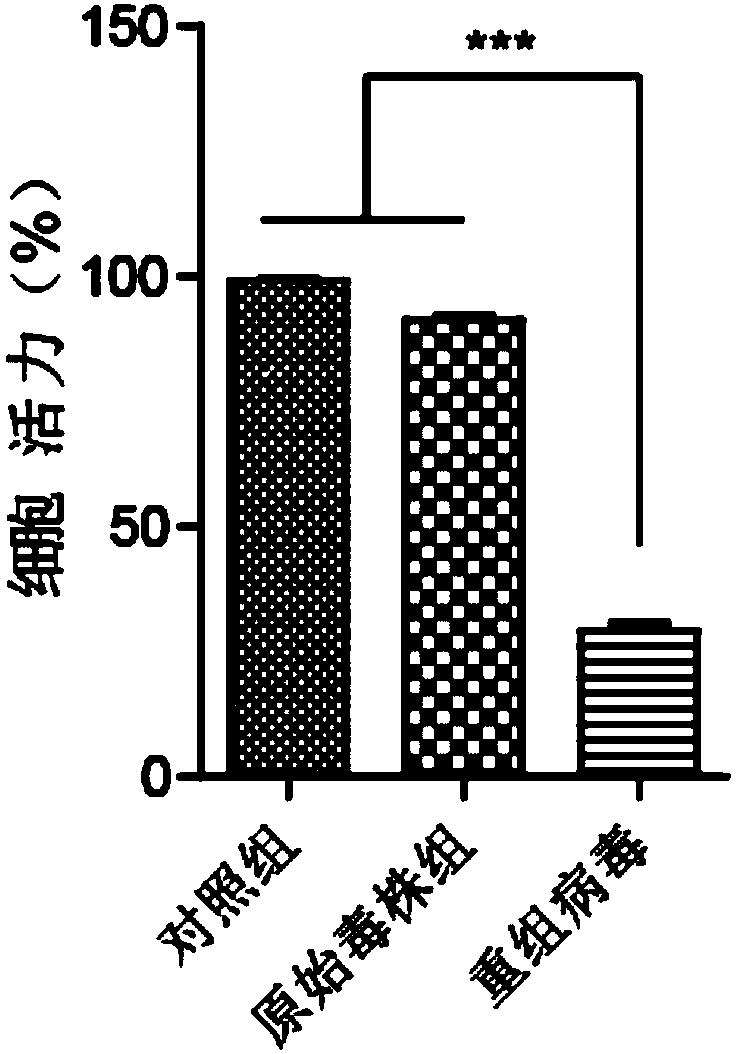
Preparation method and application of newcastle disease oncolytic virus expressing PD-L1 single chain antibody
InactiveCN109627336AOncolytic abilityFunctionalSsRNA viruses negative-senseStable introduction of DNASingle-Chain AntibodiesF protein
The invention discloses a preparation method and application of a newcastle disease oncolytic virus expressing PD-L1 single chain antibody and an application thereof. The amino acid residues at sits of 112-117 of F protein of mutant recombinant LaSota strain newcastle disease virus is mutated into 112-R-R-Q-R-F-117, so that the newcastle disease oncolytic virus has independent infection capability; a PD-L1 single chain antibody gene is inserted between a P gene and M gene of a full-length clone of the newcastle disease virus LaSota strain through homologous recombination to obtain a finally modified full-length clone pBRN-FL (112-RRQRRF-117)-PDL1(ScFV); the recombinant virus is purified by chick embryo proliferation and ultracentrifugation. The rNDV-LaSota (112-RRQRRF-117)-PDL1(ScFV) recombinant virus has safety comparable to that of a LaSota original strain, and also has tumor killing capability and immune checkpoint inhibition function of a virulent strain.
Owner:南京昂科利医药科技创新研究院有限公司
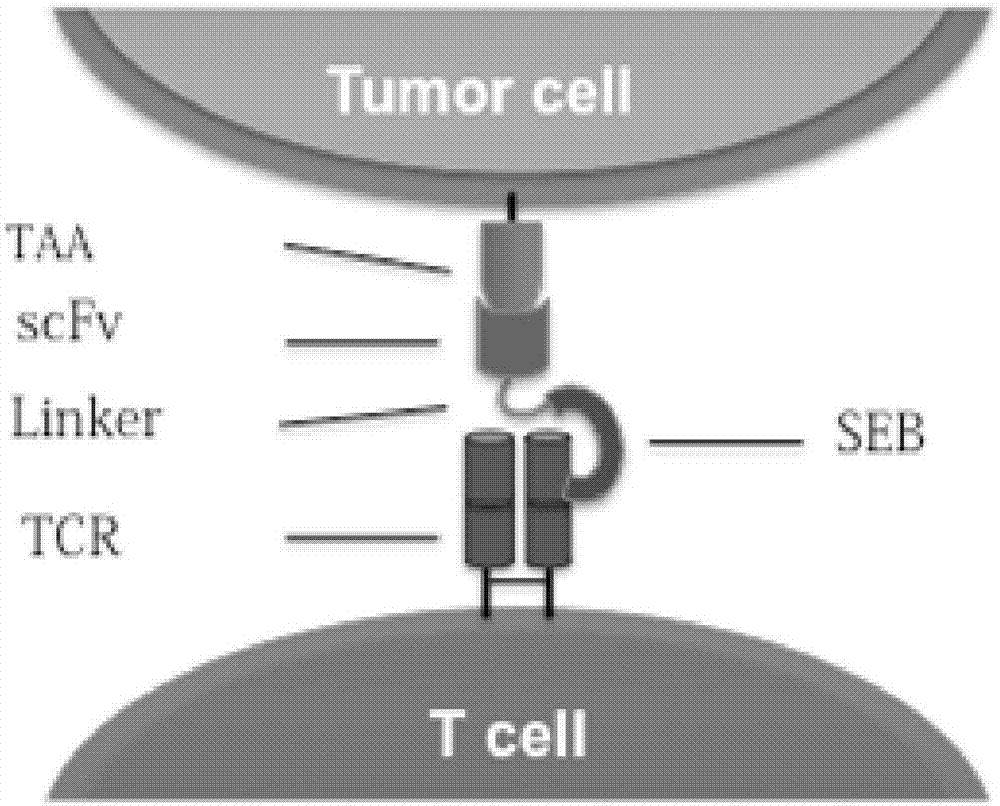
Recombinant oncolytic virus, and application thereof
PendingCN107164338AStrong targetingImprove securityViral/bacteriophage medical ingredientsImmunoglobulinsSingle-Chain AntibodiesTumor-specific antigen
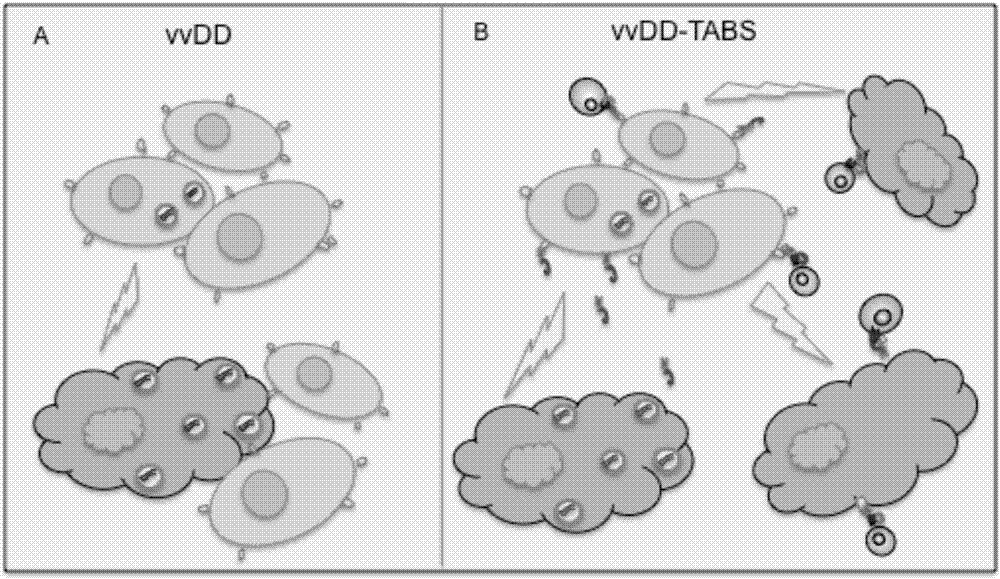
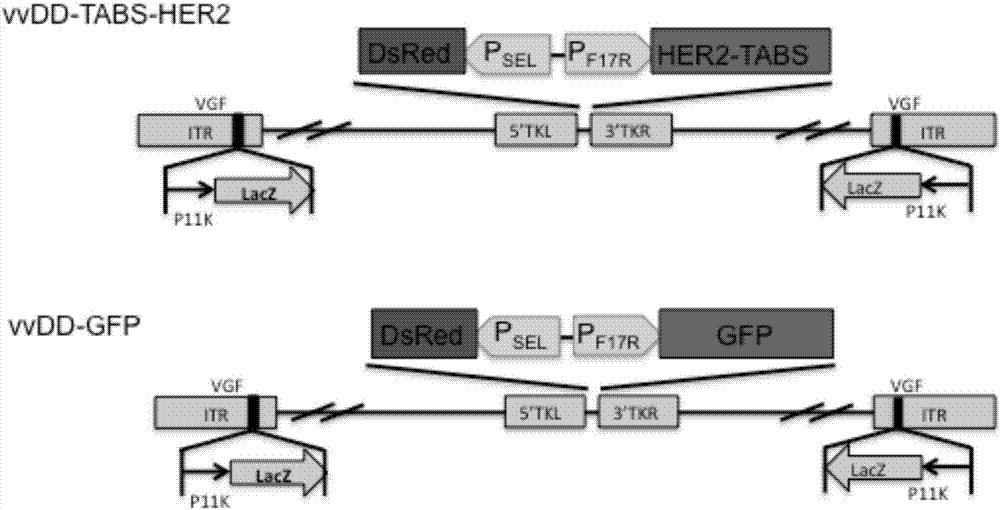
The invention belongs to the fields of biotechnology and gene therapy, and concretely to a recombinant oncolytic virus, and an application thereof. The recombinant oncolytic virus is a oncolytic virus carrying a TABS fusion protein gene, one end of the TABS fusion protein can specifically bind to tumor antigens, and the other end of the TABS fusion protein can specifically bind to the TCR of T cells. The single-chain fusion protein comprises a single chain antibody (scFv) which can specifically recognize a tumor associated antigen (TAA) or a tumor specific antigen (TSA), a linker polypeptide and a superantigen which specifically binds to a TCR complex or component from the amino terminal to the carboxy terminal, and a virus promoter in front of the TABS fusion protein gene is F17R. OVV-TABS prepared in the invention effectively combines the oncolytic effect of the virus and the anticancer effect of T cells, and has a remarkable anticancer effect, so the oncolytic virus has great clinical application prospect.
Owner:镇江市卫克生物科技有限公司
Novel oncolytic virus, as well as preparation method and application thereof
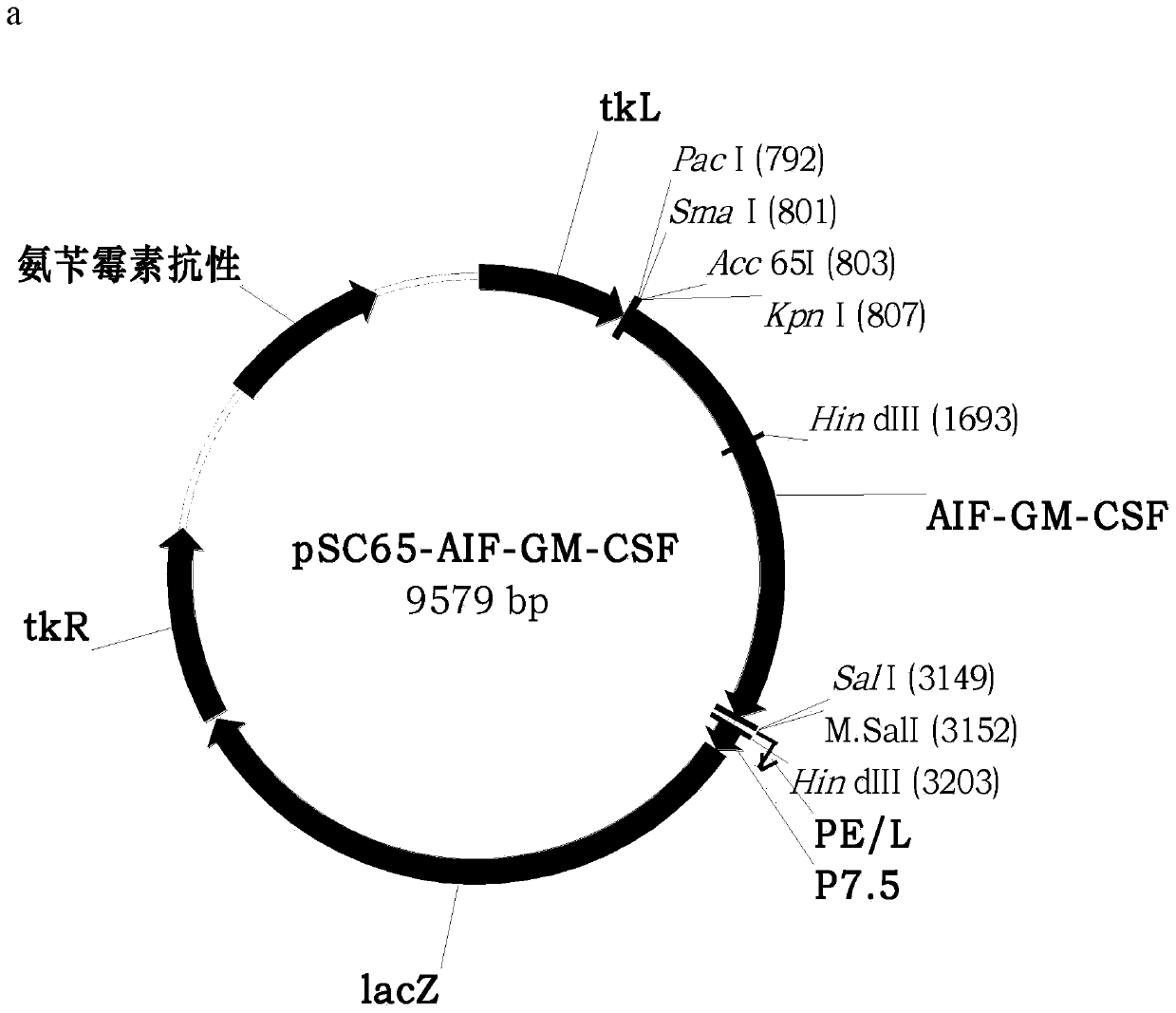
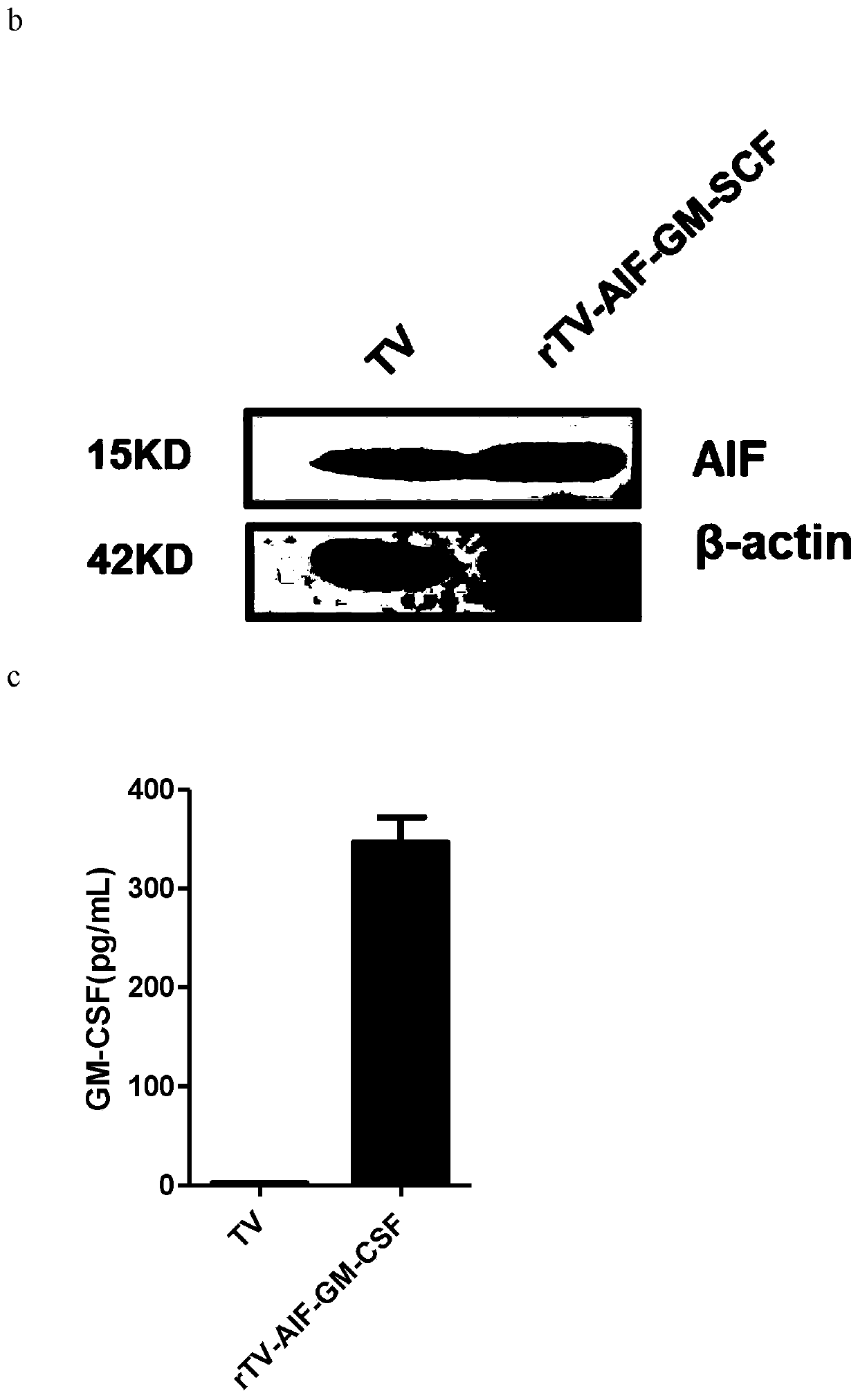
ActiveCN110499297APromote value-added differentiationIncrease lethalityMicroorganism based processesPeptidesNatural Killer Cell Inhibitory ReceptorsApoptosis
The invention discloses a novel oncolytic virus based on vaccinia virus Tiantan strain. The thymidine kinase (TK) region of the virus contains a coding sequence of AIF-GM-CSF as shown in SEQ ID NO.1.The oncolytic vaccinia virus which can efficiently expressing a human AIF-GM-CSF gene is prepared by effectively combining the tumor suppression effect of gene therapy and the oncolytic effect of viral therapy. While the oncolytic virus of the vaccinia virus Tiantan strain achieves the oncolytic effect to pyrolyze tumor cells, human AIF is massively expressed to cause apoptosis of a great number of infected tumor cells; and a great number of human GM-CSF can be expressed, and NK cells or DC cells can be recruited into the inside of a tumor to kill the tumor or effectively represent a tumor antigen, so that proliferation and differentiation of cytotoxicity t cells can be improved, and multiple anti-tumor effects can be achieved. Compared with simple gene therapy or virus therapy, the malignant tumor killing performance of the novel oncolytic virus is reinforced.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Programmable oncolytic virus vaccine system and application thereof
ActiveCN108064305AWide host rangeEffective treatmentPeptidesUnknown materialsCell specificRecognition sequence
The present invention provides an expression system, the system comprising: a first nucleic acid molecule having a cell specific promoter; a second nucleic acid molecule encoding a transcriptional activator; a third nucleic acid molecule having a first recognition sequence of the transcriptional activator; a fourth nucleic acid molecule having a first promoter and a first regulatory element; a fifth nucleic acid molecule encoding a first regulatory protein; a sixth nucleic acid molecule having a second recognition sequence of the transcriptional activator; a seventh nucleic acid molecule having a second promoter and a second regulatory element; an eighth nucleic acid molecule encoding a second regulatory protein; and: a ninth nucleic acid molecule configured to conditionally inhibit expression of the first regulatory protein; and a tenth nucleic acid molecule configured to conditionally inhibit expression of the second regulatory protein, wherein the first regulatory element is adaptedto inhibit the function of the first promoter by binding to the second regulatory protein, and the second regulatory element is adapted to inhibit the function of the second promoter by binding to the first regulatory protein.
Owner:TSINGHUA UNIV +1
Sensitization of neoplastic cells to radiation therapy with reovirus
ActiveUS7198783B2Good curative effectSmall sizeBiocideGenetic material ingredientsRadiation therapyIrradiation
The present invention relates to methods of sensitizing neoplastic cells to irradiation by using oncolytic viruses, particularly reoviruses. Also provided are methods of treating or ameliorating a tumor with a combination of oncolytic viruses and radiotherapy.
Owner:ONCOLYTICS BIOTECH
Recombinant oncolytic vaccinia virus and preparation method and application thereof
InactiveCN108165536AInhibitory activityIncrease lethalityUnknown materialsImmunoglobulinsWilms' tumorTumor cells
The invention discloses a recombinant oncolytic vaccinia virus and a preparation method and application thereof. The thymidine kinase (TK) region of the virus comprises a coding sequence of a PD1 full-length antibody shown in the formula of SEQ ID NO.1. Through effective combination of the anti-tumor effect of gene therapy and the oncolytic effect of virus treatment, the oncolytic vaccinia virus for efficiently expressing the PD1 full-length antibody gene is prepared. When the oncolytic vaccinia virus produces the oncolytic effect of splitting tumor cells, the PD1 full-length antibody is efficiently expressed, the activity of PD1 on the surfaces of T cells is inhibited, the T cell immune response is activated and the dual antitumor effects are produced. Compared with the single gene therapy or viral therapy, the method using the recombinant oncolytic vaccinia virus improves the effects of killing malignant B cell lymphoma. Through virus replication-related gene deletion, the TK regionof the vaccinia virus genome is deleted so that specific replication of the viruses in the abnormally proliferating tumor cells is ensured and the viruses can not replicate in normal cells, so that the safety of the oncolytic vaccinia virus vector is greatly improved.
Owner:ZHEJIANG UNIV
Oncolytic virus vaccine and medicine for treating tumors by combining oncolytic virus vaccine with immune cells
ActiveCN111286493AEffective treatmentHigh cure rateSsRNA viruses negative-senseMammal material medical ingredientsTumor therapyOncology
The invention belongs to the technical field of biology, and particularly relates to an oncolytic virus vaccine and a medicine for treating tumors by combining the oncolytic virus vaccine with immunecells. The invention provides a brand-new oncolytic virus attenuated strain by carrying out site-specific mutagenesis on the VSV wild type virus matrix protein M. The gene sequence of the matrix protein M is shown as SEQ ID NO 3. The attenuated strain can be independently used as a drug for treating tumors, and is superior to wild type viruses and other known attenuated strains in safety and curerate. On the basis of the oncolytic virus attenuated strain, NY-ESO-1 is inserted into the attenuated strain, and the invention further provides a vaccine capable of being applied to tumor treatment.The vaccine is high in cure rate and high in biological safety. On the basis of the vaccine, the vaccine and TCR-T cells are combined for application, and a medicine capable of efficiently treating various tumors is provided. On a mouse lung cancer model, the cure rate can reach the surprising rate of 95 percent.
Owner:JOINT BIOSCIENCES (SH) LTD
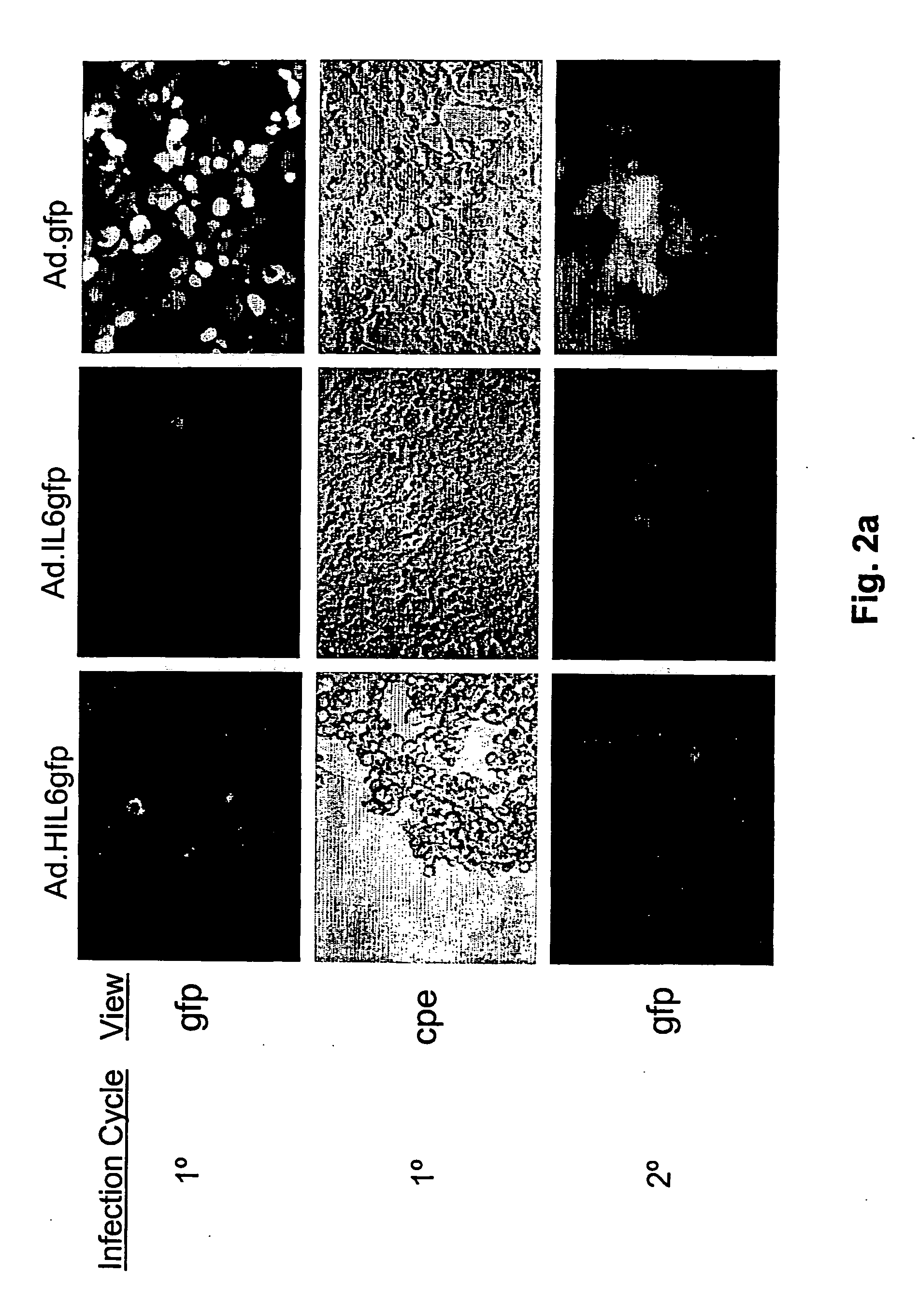
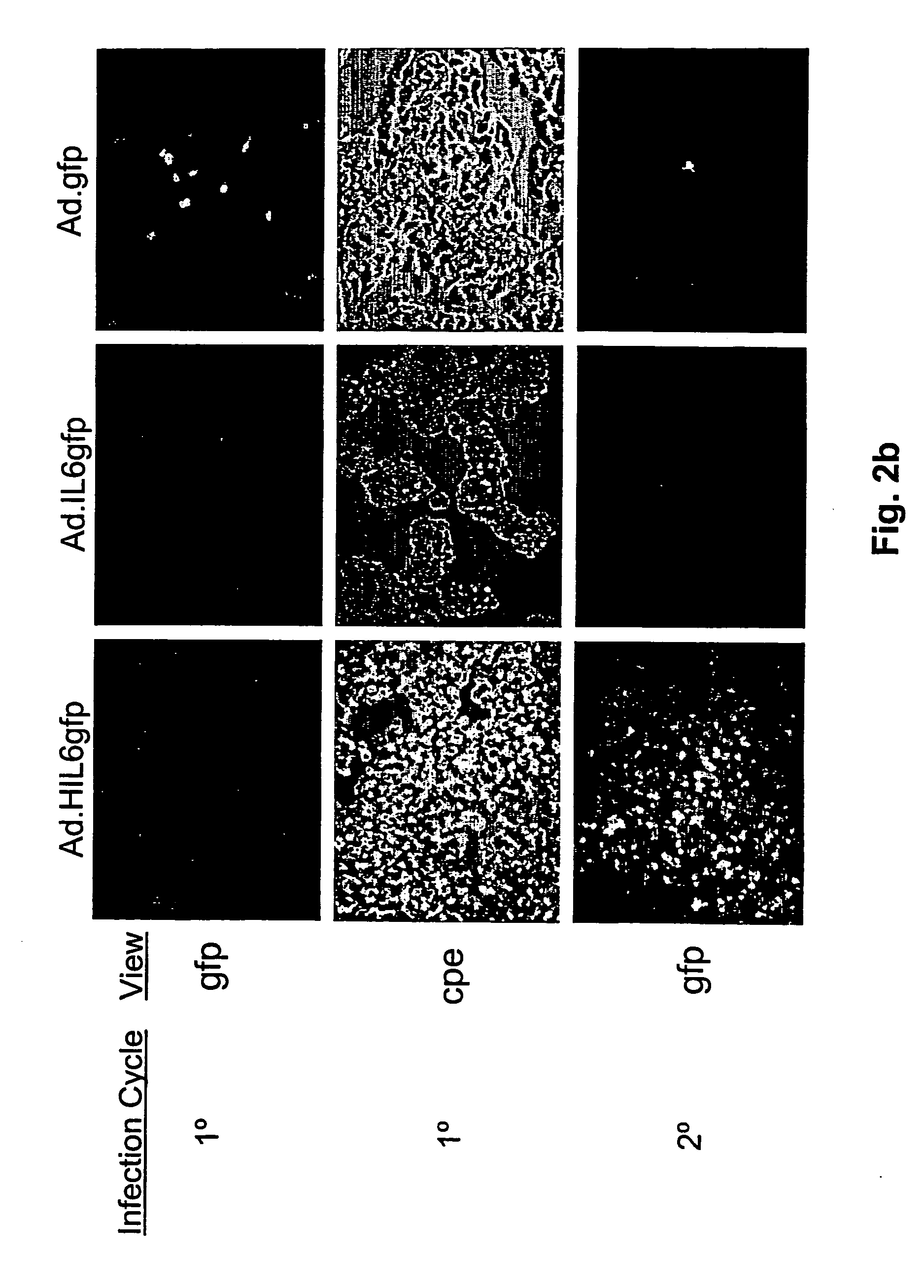
Compositions and methods for treating cancer with an oncolytic viral agent
The invention discloses lytic viruses as anti-neoplastic agents for specifically replicating and lysing tumor cells. According to the present invention, the agents preferably include E1A deficient adenoviral vectors, exemplified by Ad.HIL6gfp, encoding an IL-6 / sIL-6R complex, HIL-6, which is able to replicate, produce cytotoxic effects, and kill tumor cells in the absence of either E1A or exogenous IL-6 protein. These viral agents have utility as therapeutic vehicles for treating cancers of various types either as a single agent, or applied in combination with other therapeutic strategies.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com