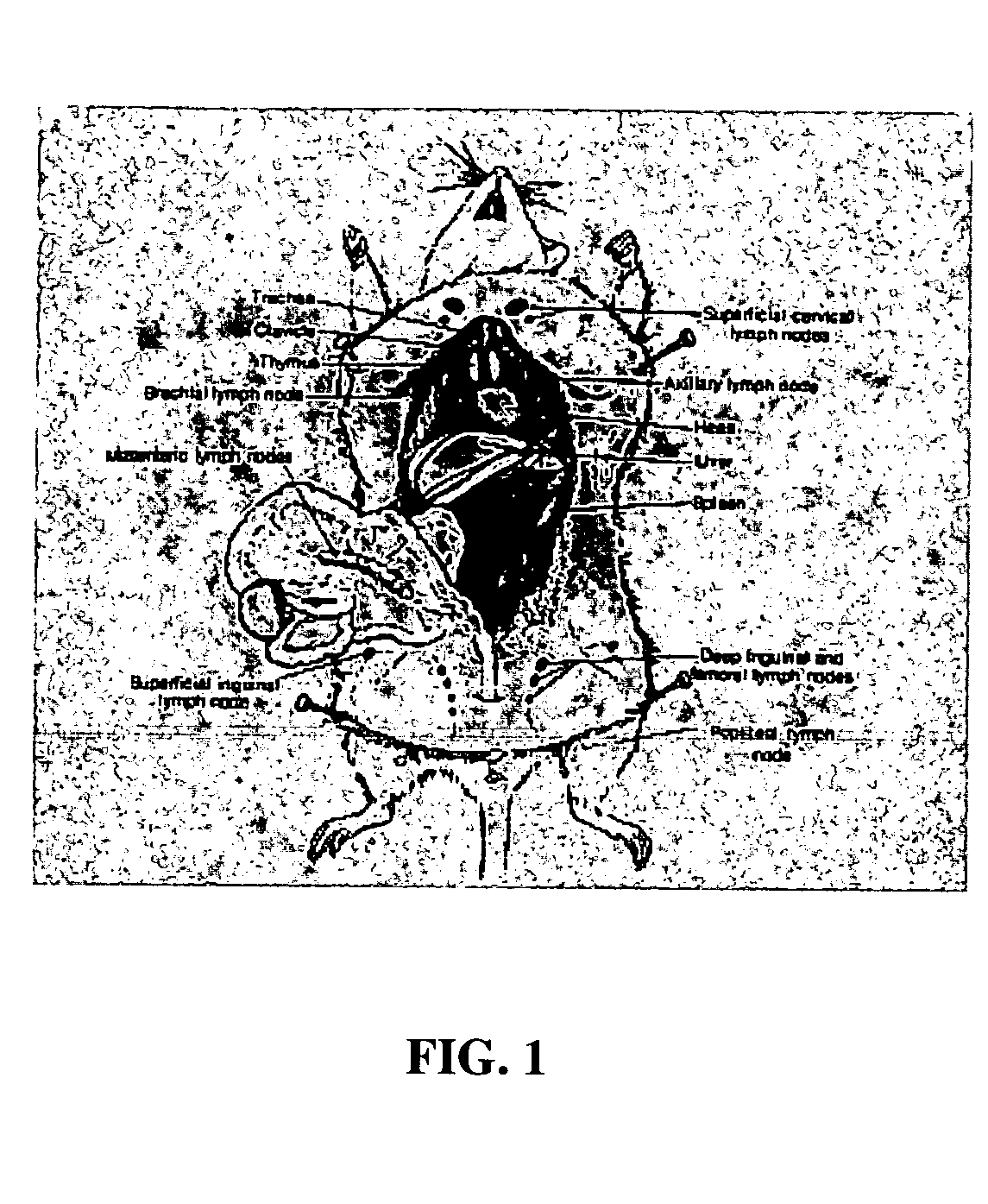
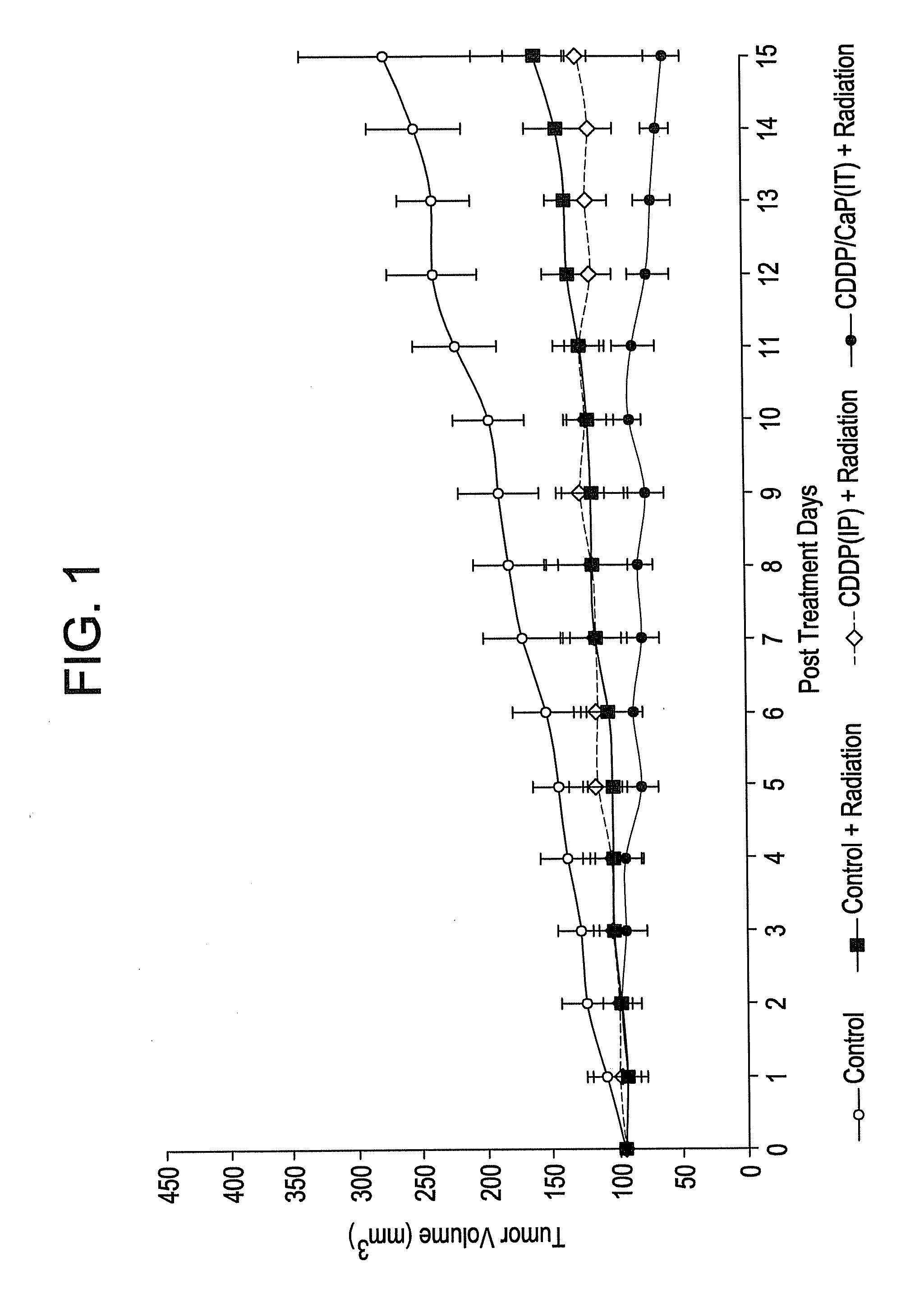
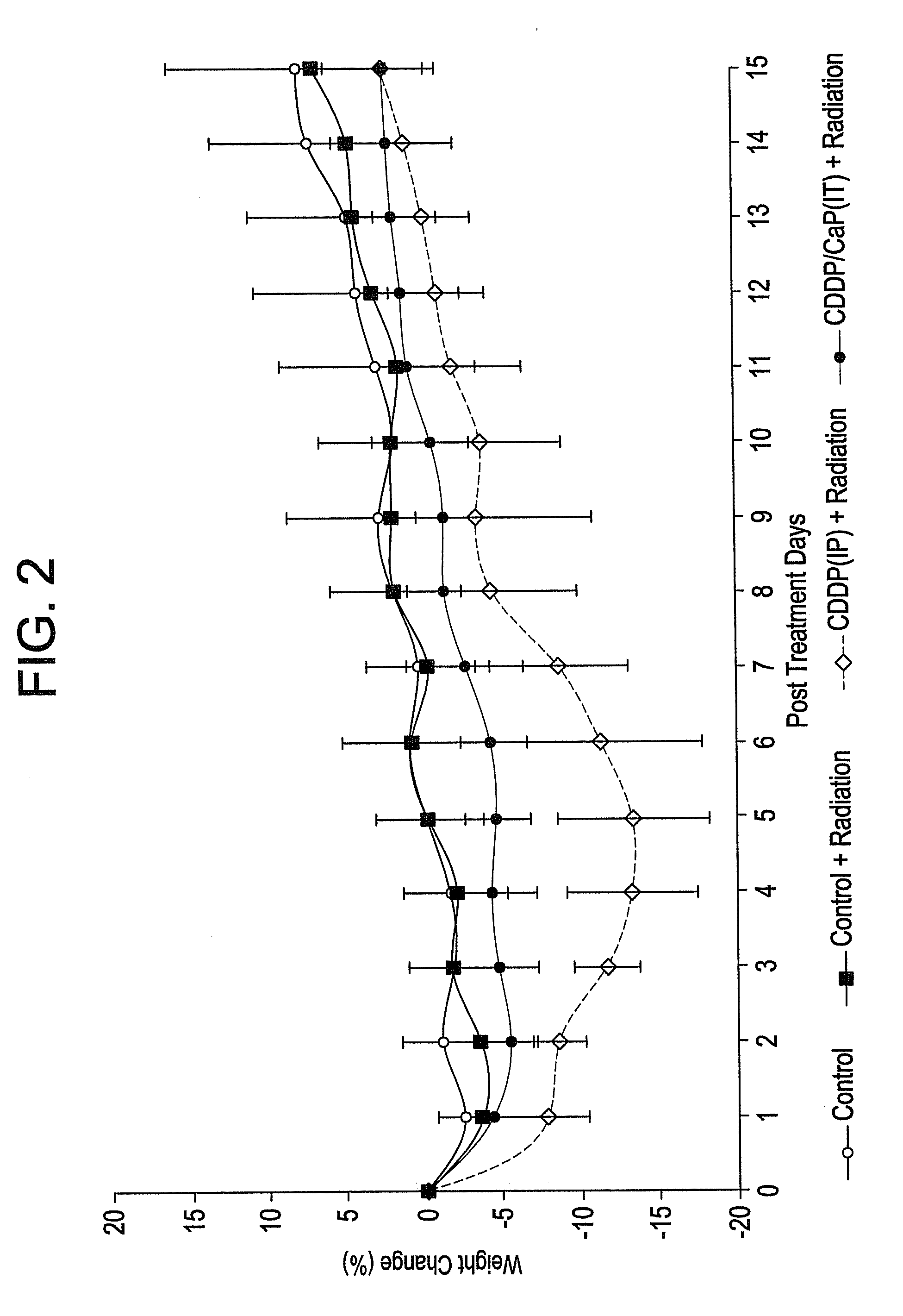
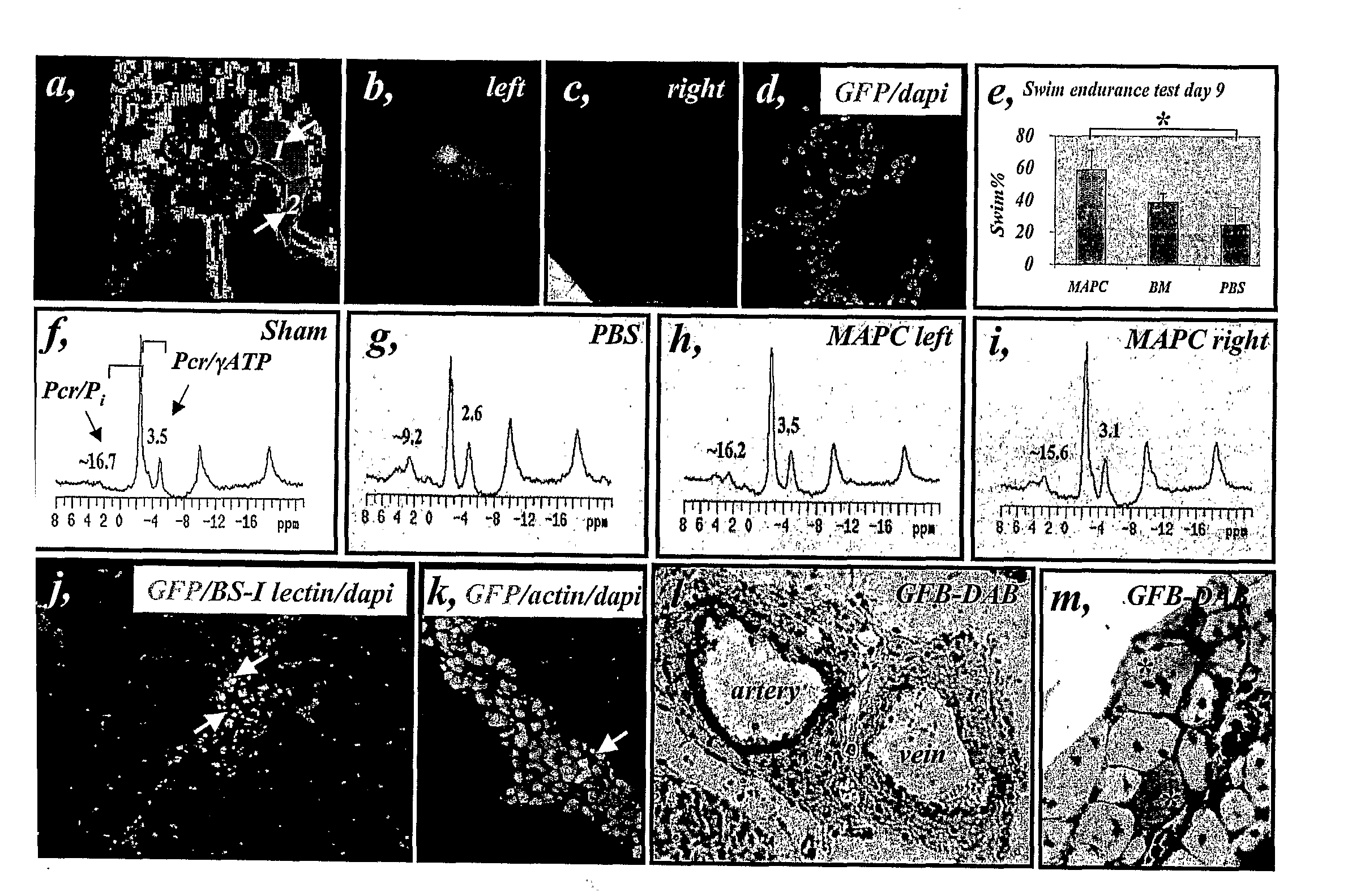
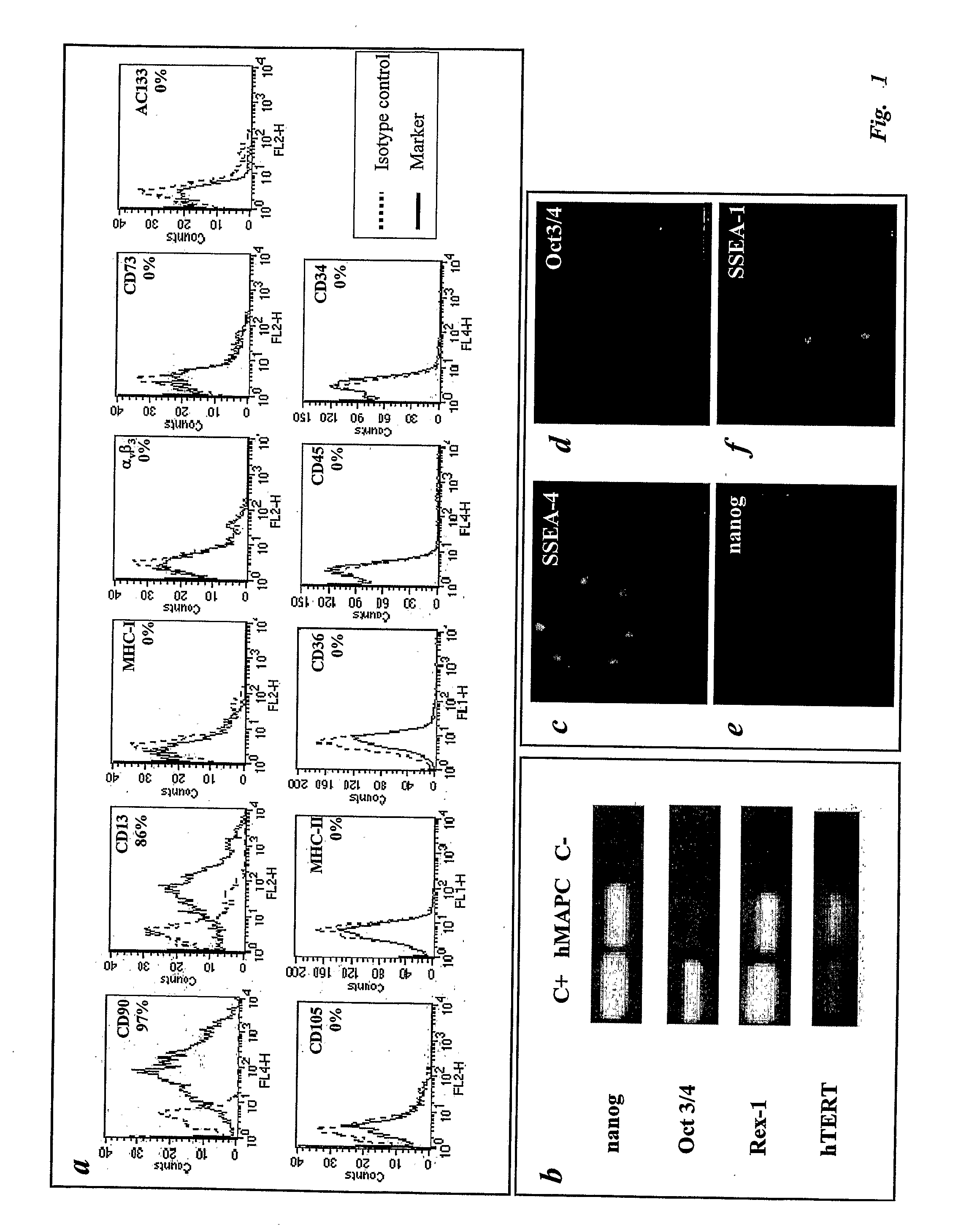
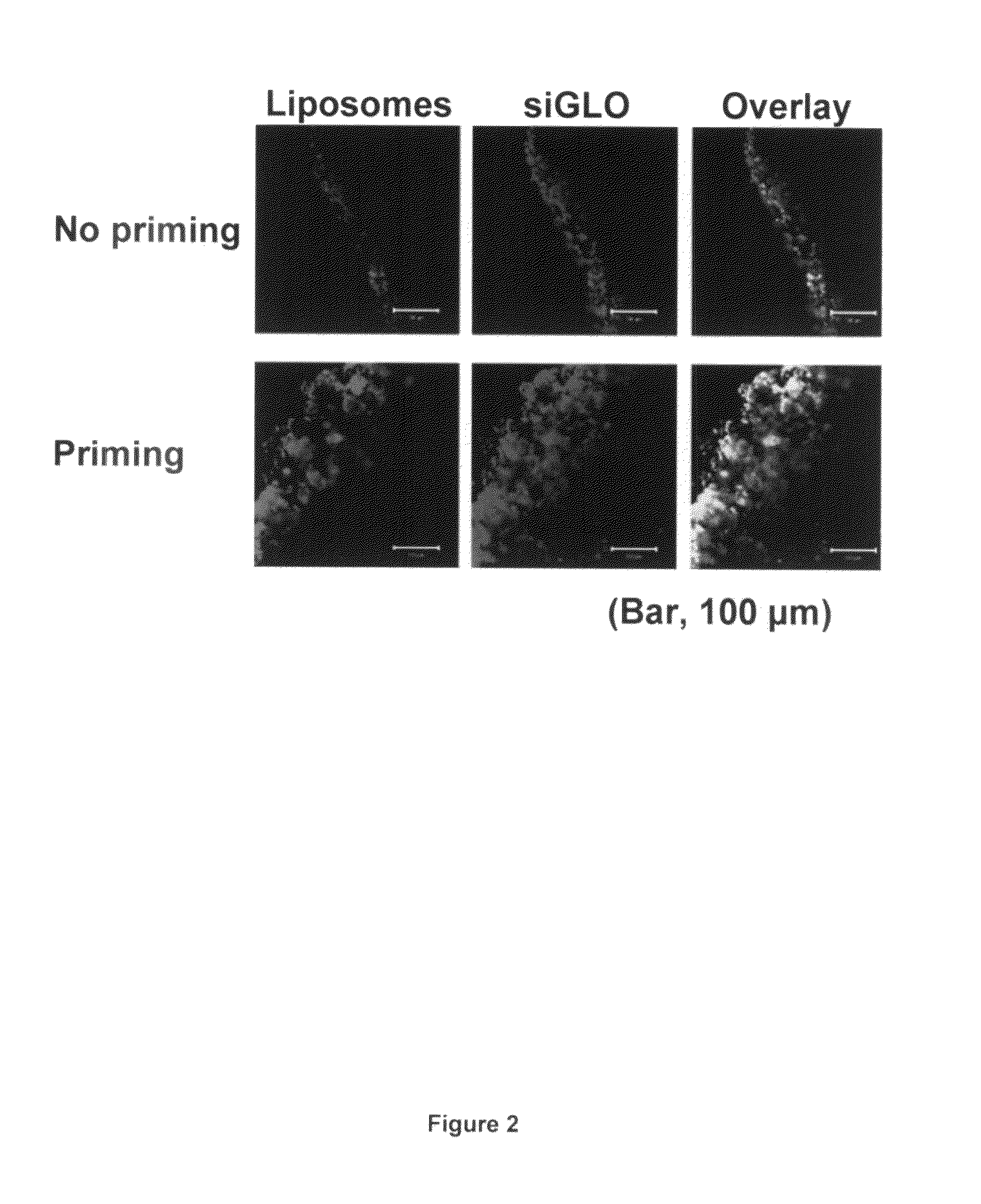
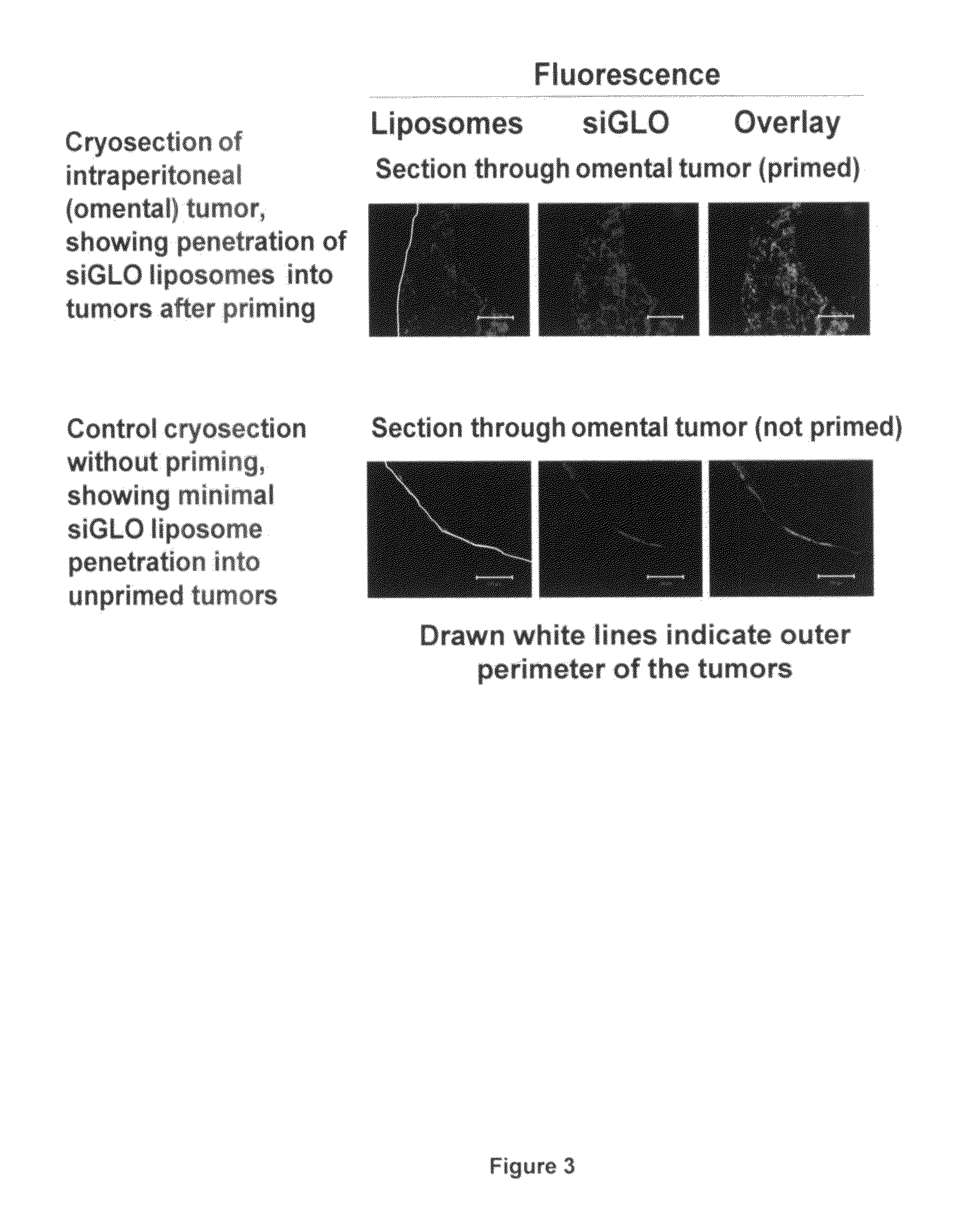
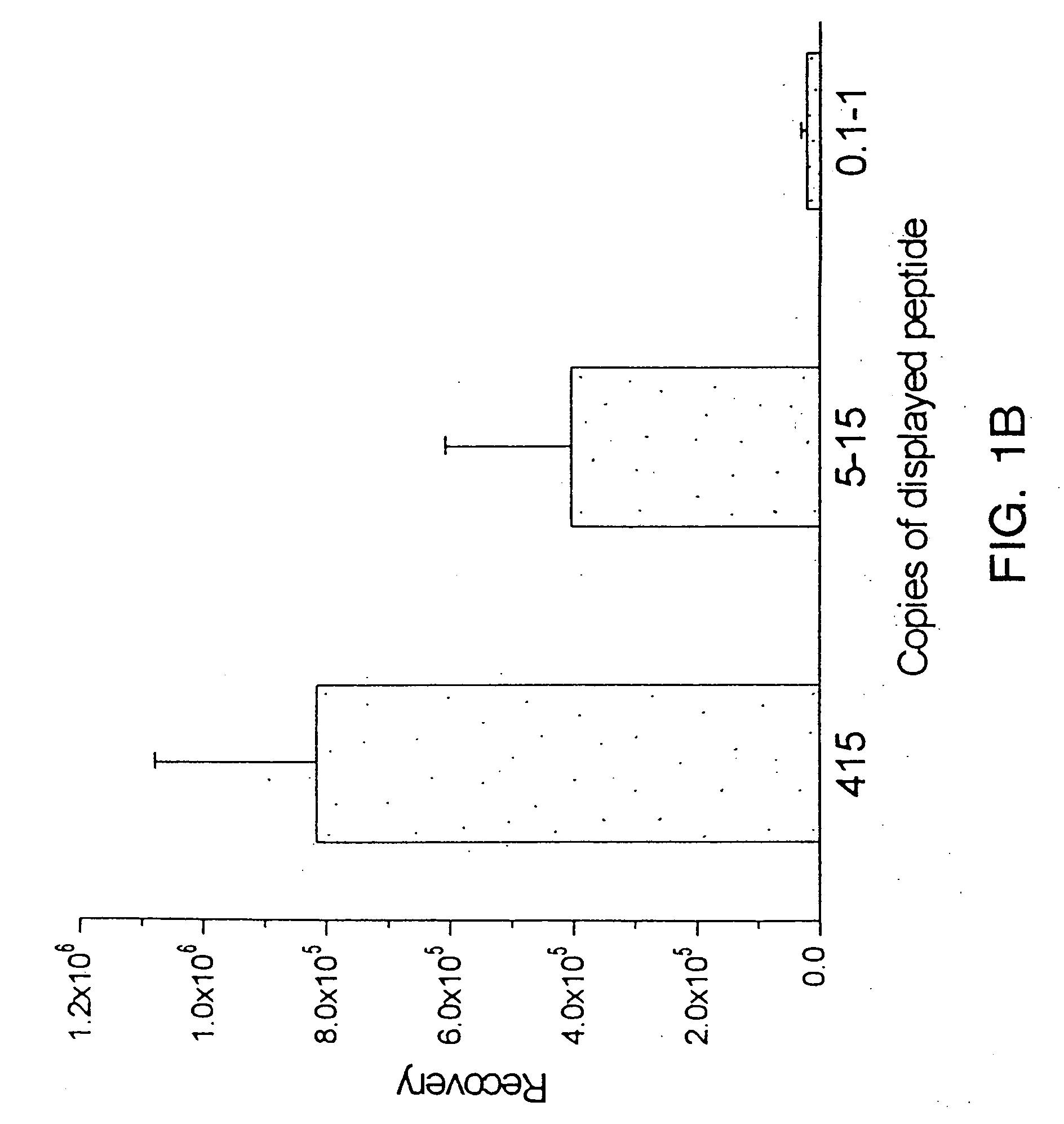
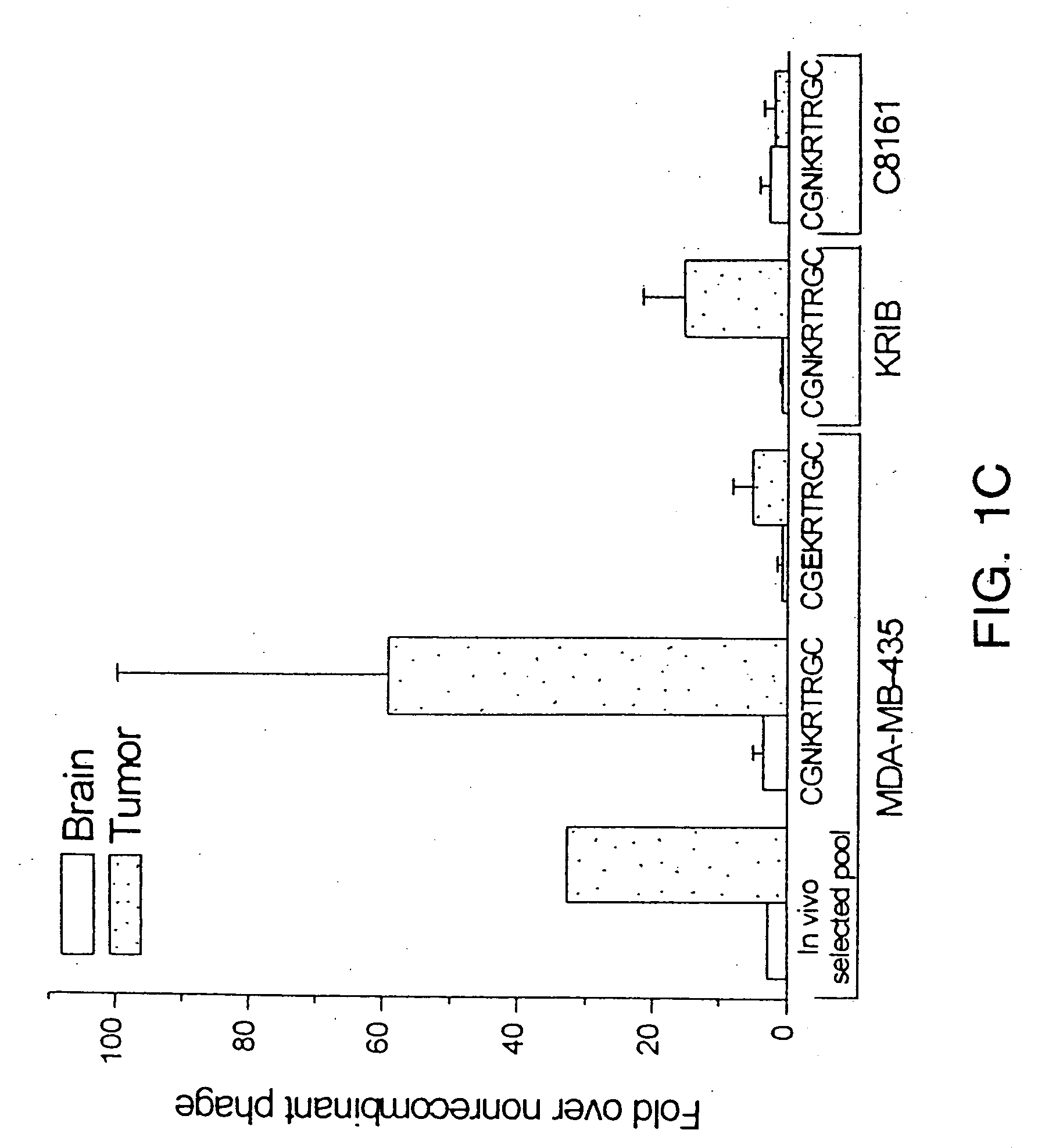
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
249 results about "Lymphatic vessel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The lymphatic vessels (or lymph vessels or lymphatics) are thin-walled vessels (tubes) structured like blood vessels, that carry lymph. As part of the lymphatic system, lymph vessels are complementary to the cardiovascular system. Lymph vessels are lined by endothelial cells, and have a thin layer of smooth muscle, and adventitia that binds the lymph vessels to the surrounding tissue. Lymph vessels are devoted to the propulsion of the lymph from the lymph capillaries, which are mainly concerned with absorption of interstitial fluid from the tissues. Lymph capillaries are slightly larger than their counterpart capillaries of the vascular system. Lymph vessels that carry lymph to a lymph node are called afferent lymph vessels, and those that carry it from a lymph node are called efferent lymph vessels, from where the lymph may travel to another lymph node, may be returned to a vein, or may travel to a larger lymph duct. Lymph ducts drain the lymph into one of the subclavian veins and thus return it to general circulation.
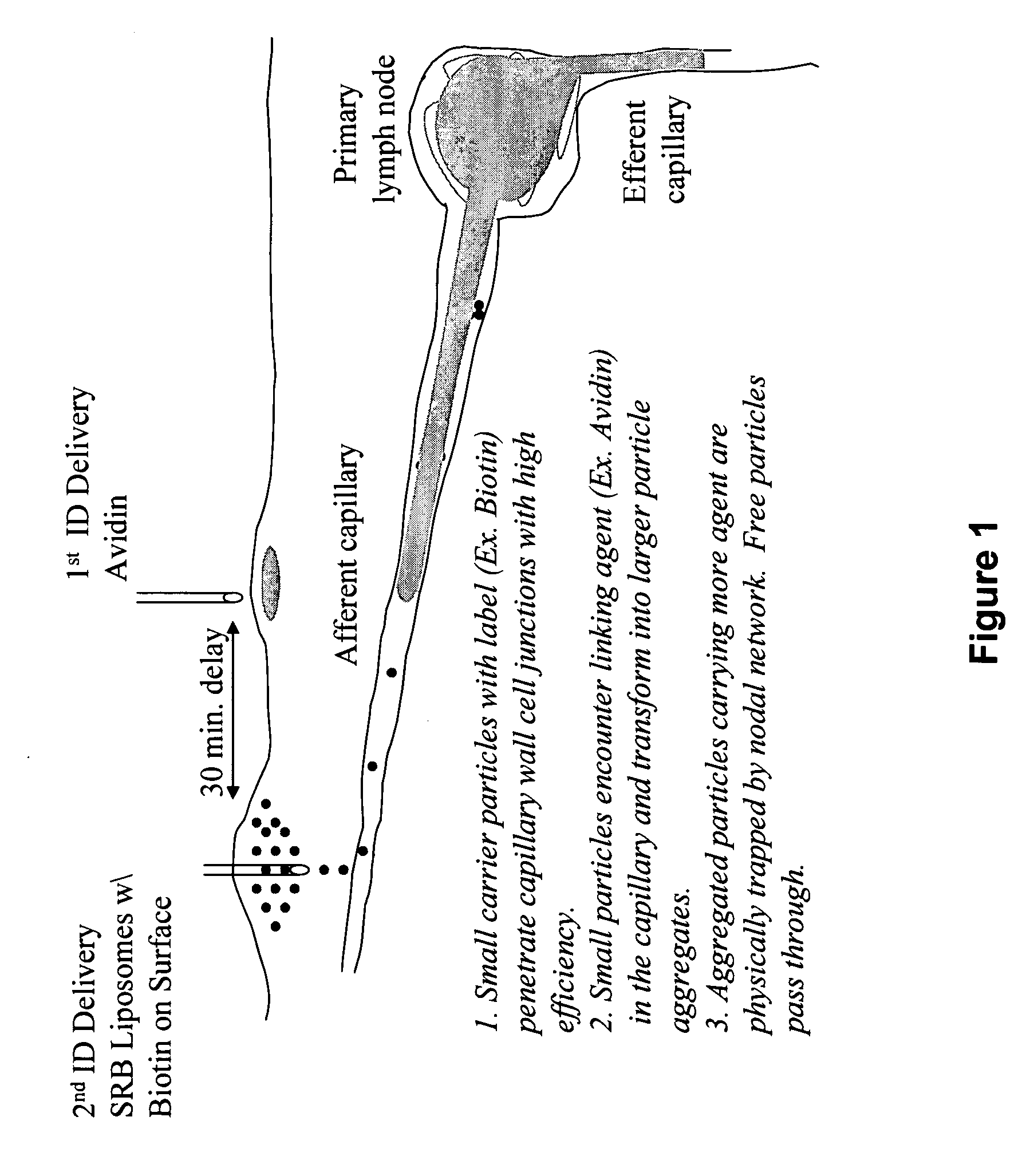
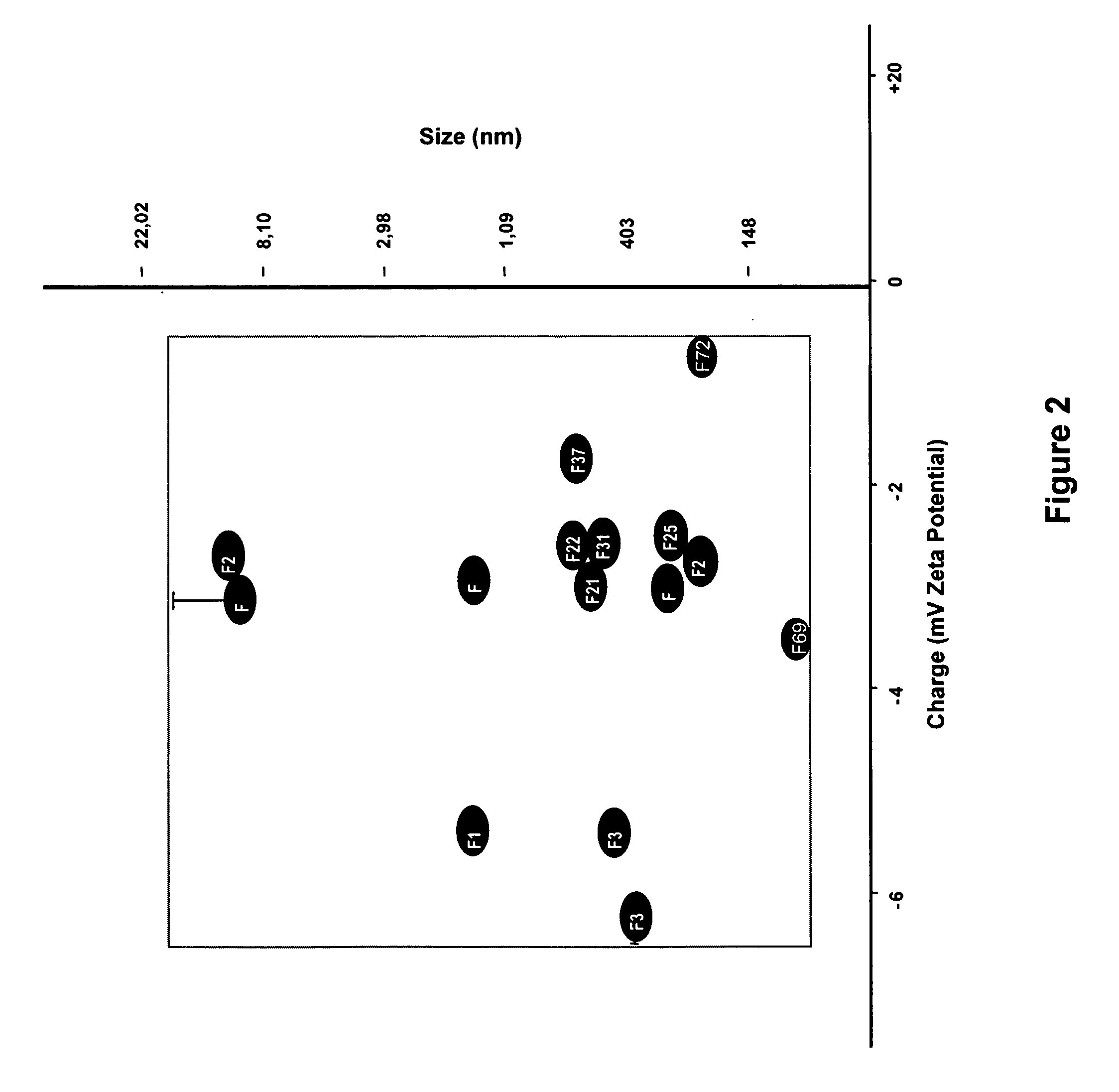
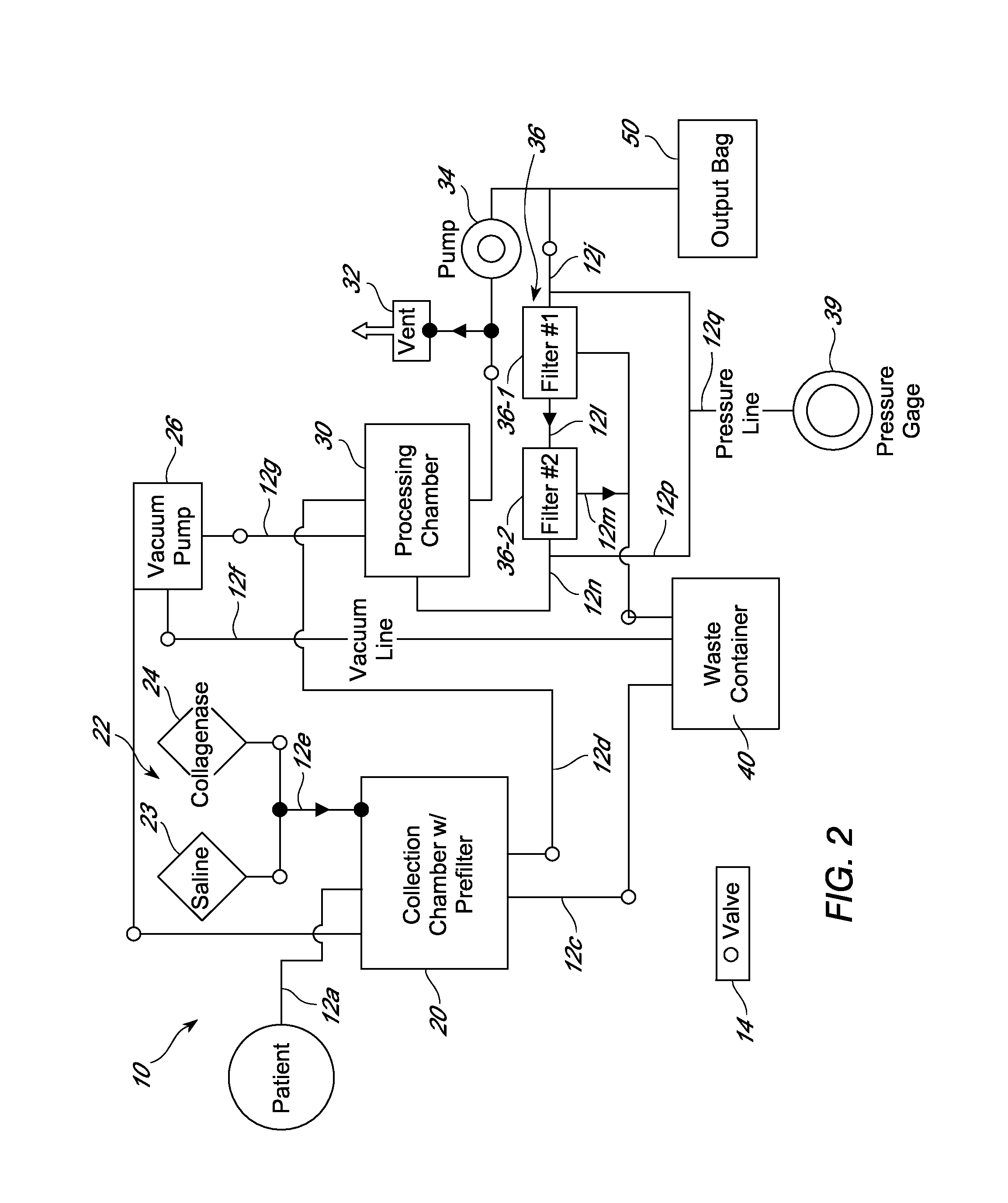
Intra-dermal delivery of biologically active agents
InactiveUS20050163711A1Rapid uptakeFast shippingMetabolism disorderDigestive systemLymphatic vesselDiagnostic agent
The present invention relates to methods and devices for delivering one or more biologically active agents, particularly a diagnostic agent to the intradermal compartment of a subject's skin. The present invention provides an improved method of delivery of biologically active agents in that it provides among other benefits, rapid uptake into the local lymphatics, improved targeting to a particular tissue, improved bioavailability, improved tissue bioavailability, improved tissue specific kinetics, improved deposition of a pre-selected volume of the agent to be administered, and rapid biological and pharmacodynamics and biological and pharmacokinetics. This invention provides methods for rapid transport of agents through lymphatic vasculature accessed by intradermal delivery of the agent. Methods of the invention are particularly useful for delivery of diagnostic agents.
Owner:BECTON DICKINSON & CO
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Particulate formulations for intradermal delivery of biologically active agents
InactiveUS20070088414A1Small sizeMinimizes accumulationNanoinformaticsNanomedicineLipid formationParticulates
The present invention relates to formulations, methods and devices for delivering one or more biologically active agents, particularly a diagnostic or therapeutic agent to the intradermal compartment of a subject's skin. The present invention provides an improved method of delivery of biologically active agents in that it provides among other benefits, rapid uptake into the local lymphatics, improved targeting to a particular tissue, improved bioavailability, improved tissue bioavailability, improved tissue specific kinetics, improved deposition of a pre-selected volume of the agent to be administered. This invention provides methods for rapid transport of agents through lymphatic vasculature accessed by intradermal delivery of the agent. Methods of the invention are particularly useful for delivery of diagnostic and therapeutic agents. The invention relates to the synergy gained in diagnosing and treating disease when intradermal delivery and controlled release materials are combined. Specifically, the synergy is achieved when intradermal delivery is combined with lipid based particles.
Owner:BECTON DICKINSON & CO
Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease
InactiveUS20110206646A1Ease and low morbidityAvoid contaminationBiocideMetabolism disorderProgenitorLymphatic vessel
Aspects of the invention provides methods for preparing and using adipose-tissue-derived stem and progenitor cells, adipose-tissue-derived lymphatic endothelial cells, and cells capable of differentiating into lymphatic endothelial cells to treat disorders of the lymphatic system and to modulate expansion, repair, and / or regeneration of the lymphatic system. The invention further provides using adipose-tissue-derived lymphatic endothelial cells and cells capable of differentiating into lymphatic endothelial cells for delivery of therapeutic agents to tumor cells as a means for treating malignant disease, and assays to screen for drugs that modulate lymphatic system expansion, repair or regeneration.
Owner:LOREM VASCULAR PTE LTD
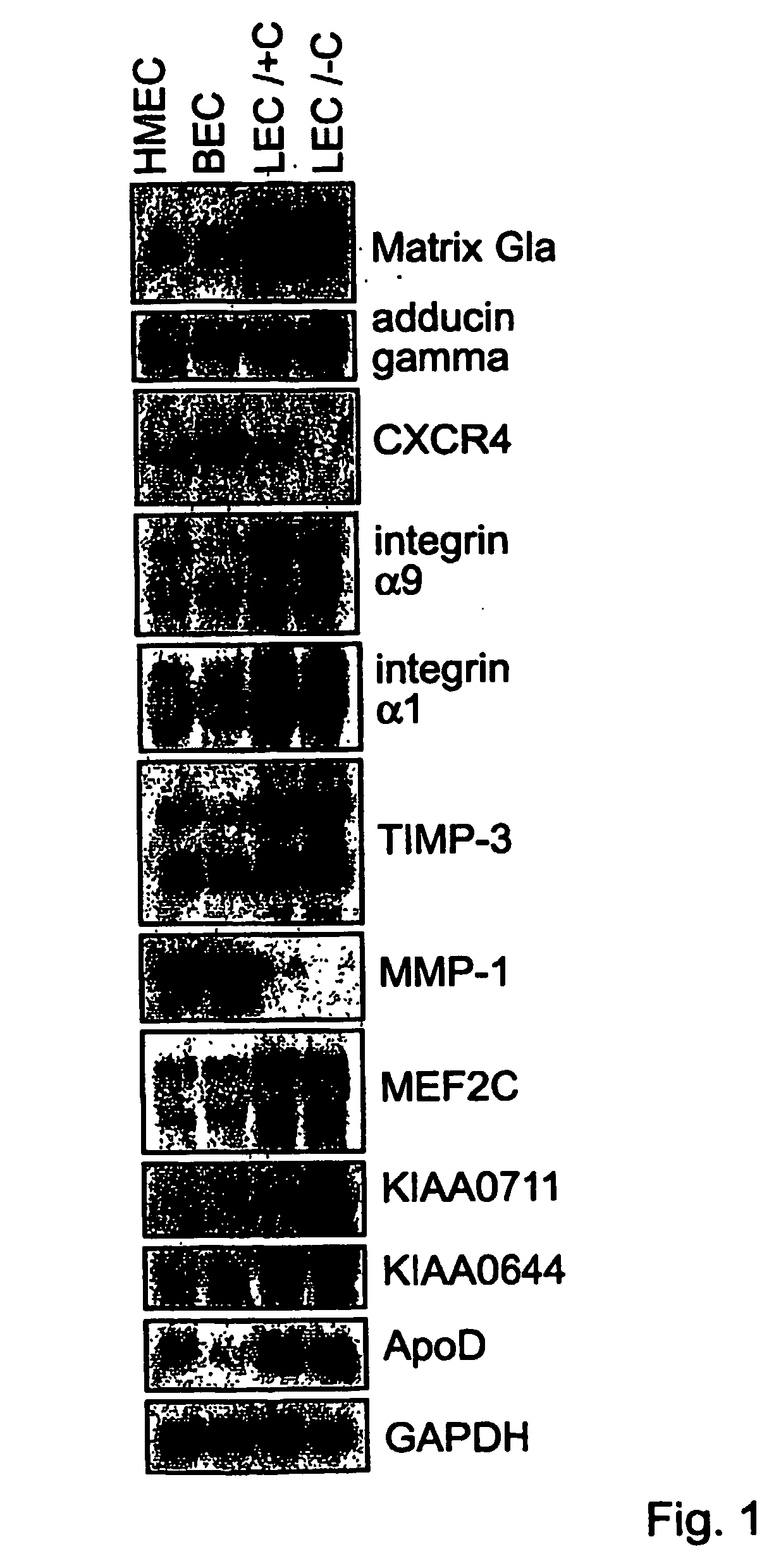
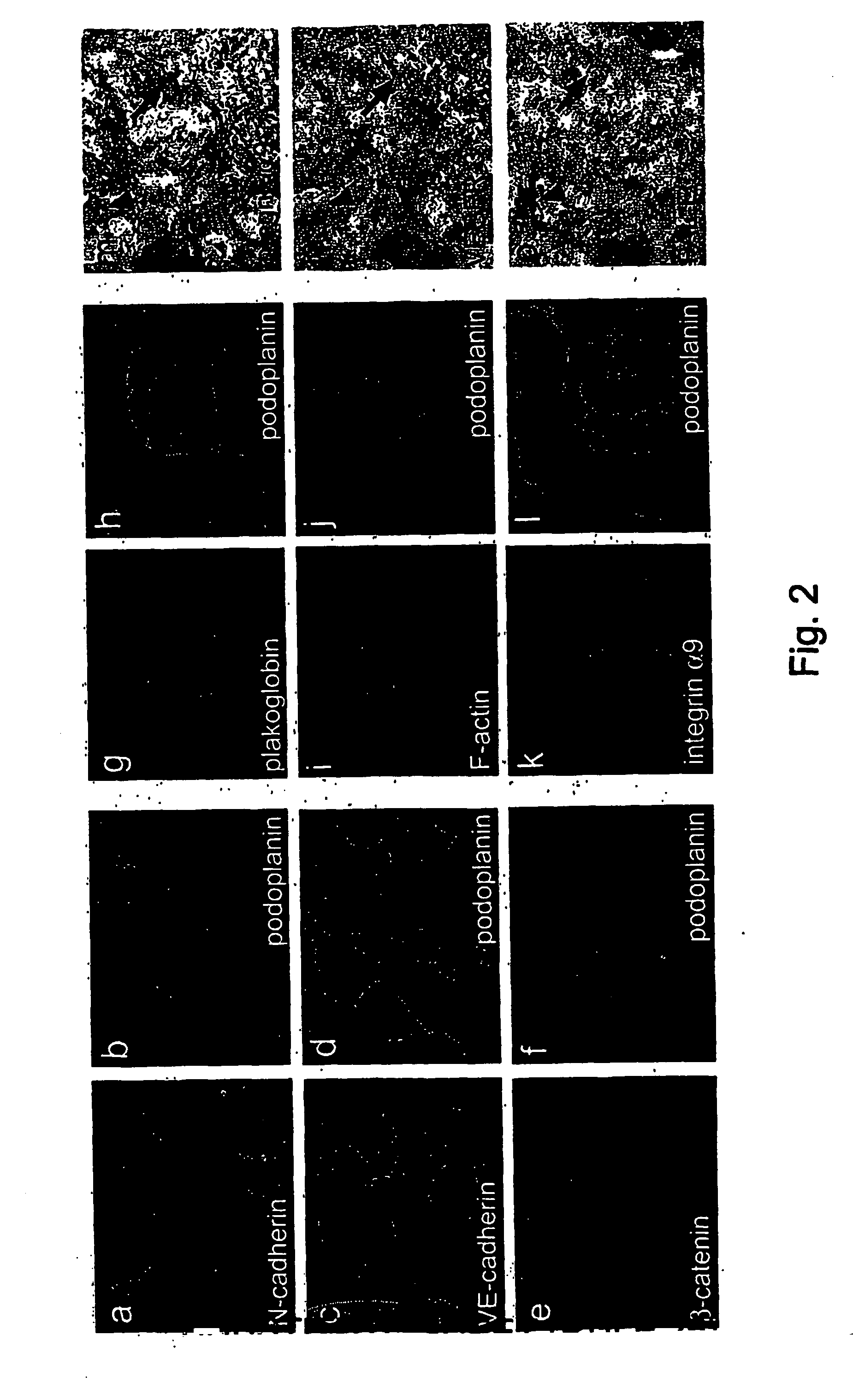
Lymphatic and blood endothelial cell genes
The invention provides polynucleotides and genes that are differentially expressed in lymphatic versus blood vascular endothelial cells. These genes are useful for treating diseases involving lymphatic vessels, such as lymphedema, various inflammatory diseases, and cancer metastasis via the lymphatic system.
Owner:VEGENICS PTY LTD
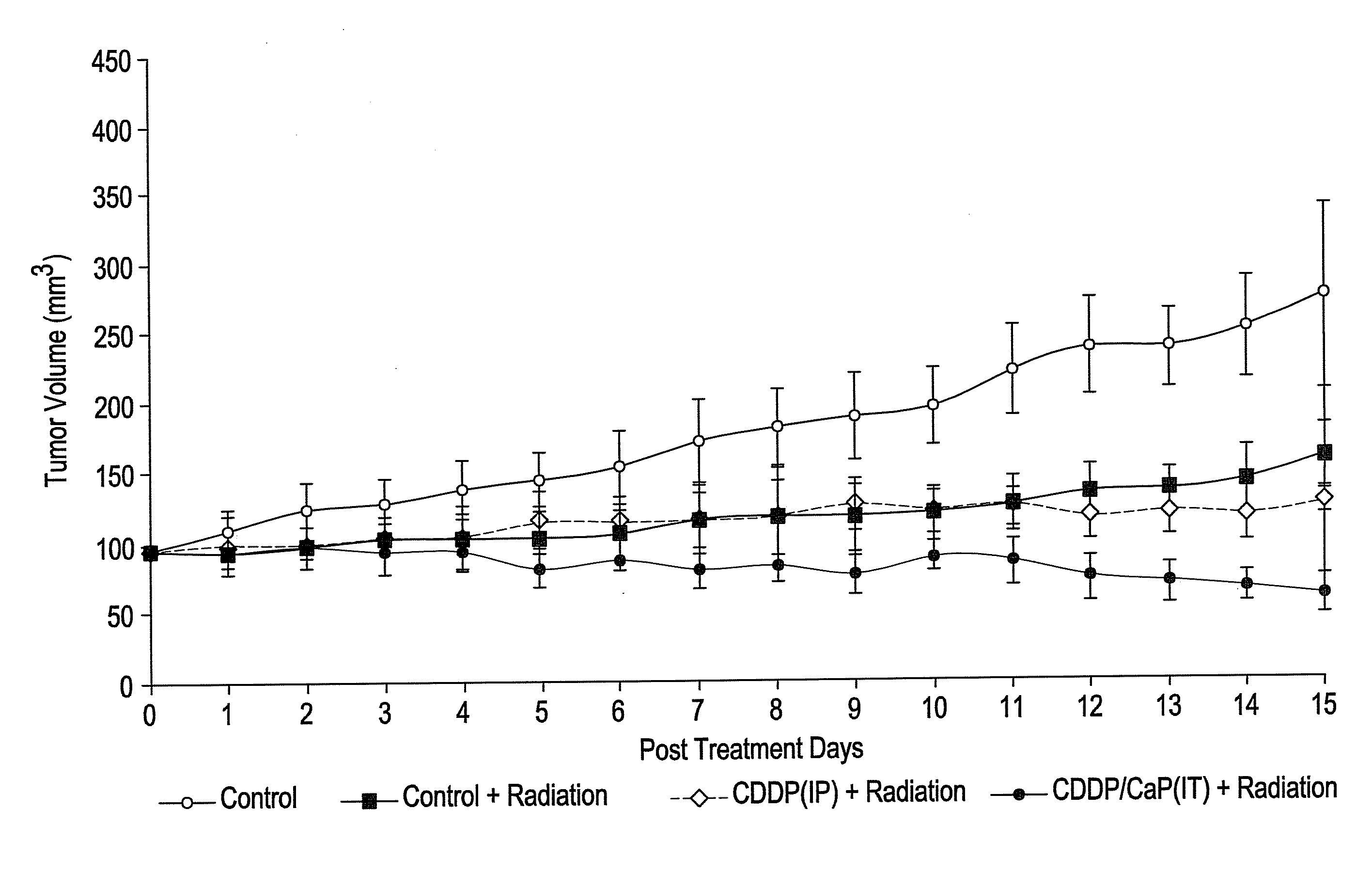
Targeted active agent delivery system based on calcium phosphate nanoparticles
InactiveUS20080241256A1Organic active ingredientsHeavy metal active ingredientsActive agentCalcium biphosphate
Calcium phosphate nanoparticle active agent conjugates are described. Specifically, anticancer agent conjugates are prepared which are suitable for targeted active agent delivery to tumor cells and lymphatics for the treatment of cancer and the treatment or prevention of cancer metastasis.
Owner:UNIV OF CONNECTICUT
Vascular/Lymphatic Endothelial Cells
Owner:PROYECTO DE BIOMEDICINA CIMA +1
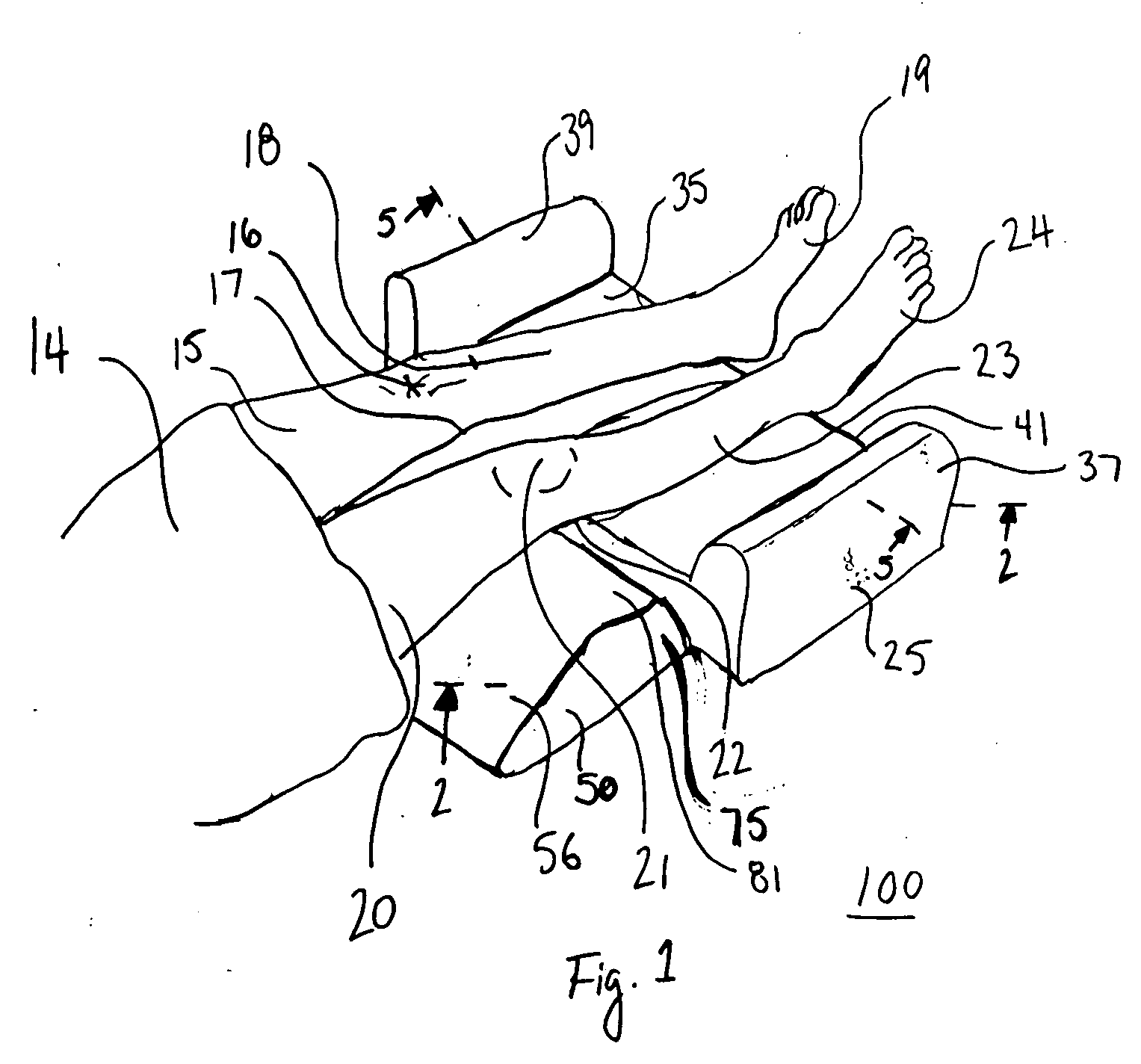
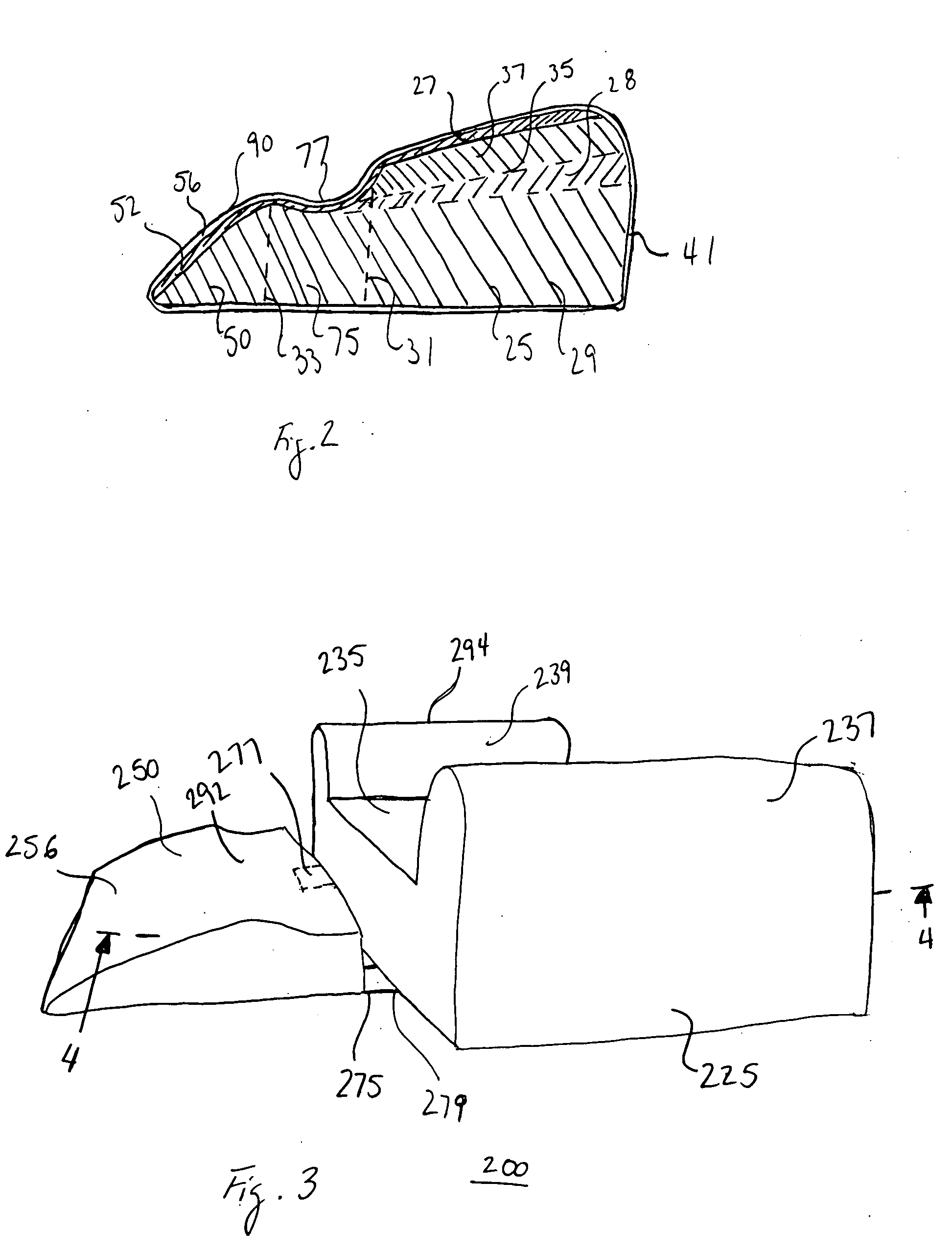
Thigh support with free space for popliteal fossa
An exemplary embodiment providing one or more improvements includes cushions which support a patient's thighs and lower legs and which have cut out areas in the areas of the patient's popliteal fossa at the back of the patient's knees. By avoiding contact with the popliteal fossa complications such as compression of the patient's nerves and blood vessels are avoided. This prevents the harmful effects of pressure on the popliteal fossa in loss of sensation in the lower legs and feet, and occlusion of blood and lymph vessels in the lower legs. A number of embodiment cushions are disclosed, some embodiments comprised of a relatively firm core foam material with a relatively softer viscoelastic foam in areas which are in contact with the patient's skin.
Owner:DUDONIS MATT
Enhancing lymph channel development and treatment of lymphatic obstructive disease
InactiveUS20030211988A1Efficient deliveryExtended half-lifePeptide/protein ingredientsLymphatic vesselObstructive diseases
Disclosed and claimed are compositions and methods for therapy and / or prevention of lymphedema. The compositions can include an agent that induces development of lymphatic channels or lymphangiogenesis, such as, VEGF-C and / or that which stimulates VEGF-C expression and / or that which stimulates VEGF-C expression or that which stimulates its interaction or that which stimulates other pathways to so stimulate the development of lymphatic channels or lymphangiogenesis or that which stimulates along any point of or any molecules involved in the signal transduction pathway leading to lymphangiogenesis or lymph channel development (and / or vector(s) expressing one or more of these agent(s)). Embodiments can include kits.
Owner:EPSTEIN STEPHEN E
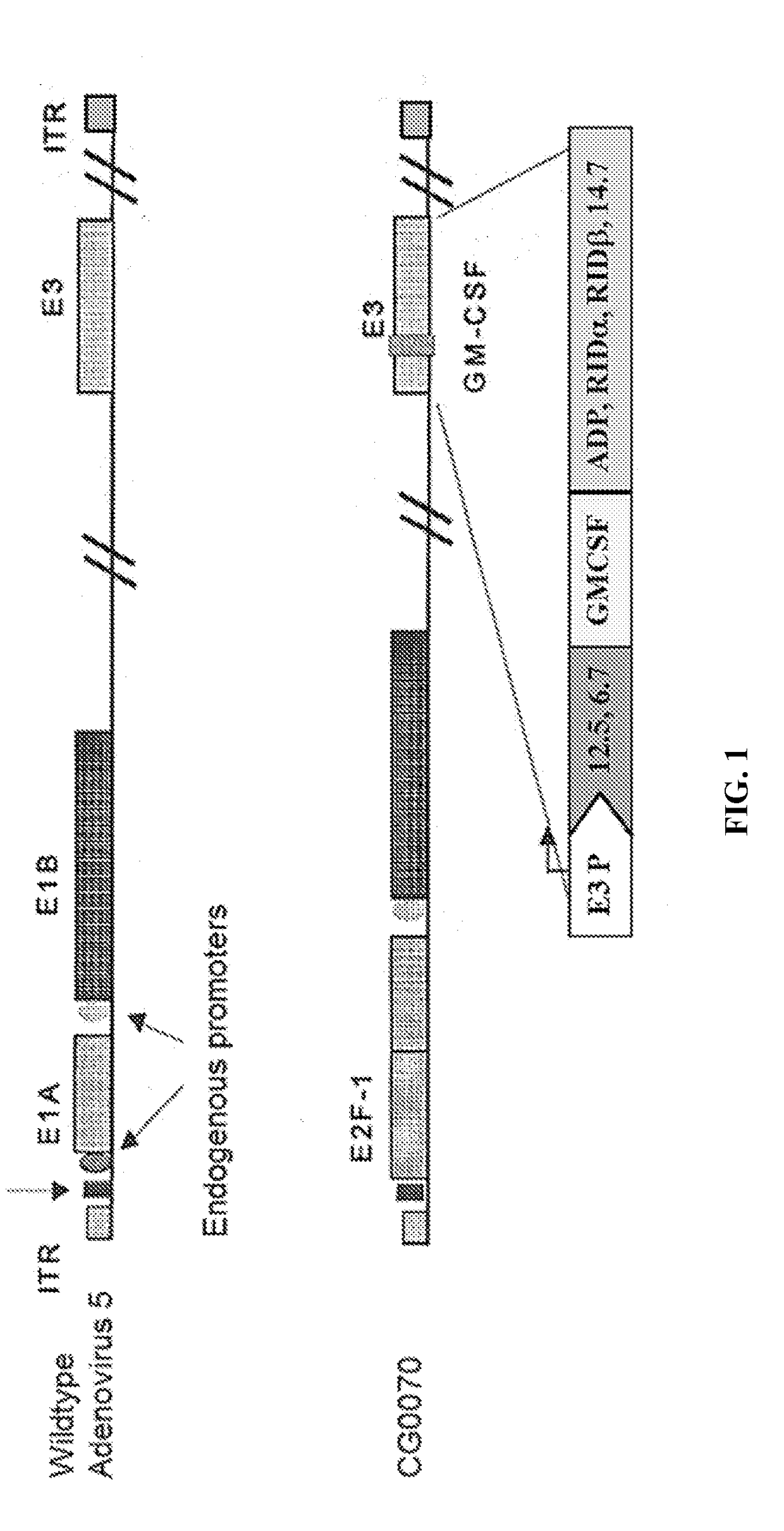
Methods of treating solid or lymphatic tumors by combination therapy
The present invention provides methods for treating an individual having solid or lymphatic tumor comprising locally administering to the site of the tumor an oncolytic virus, and systemically administering an immunomodulator (including a combination of immunomodulators). The methods may further comprise local administration to the site of the tumor a second immunomodulator (including a combination of immunomodulators). Also provided are compositions and kits for the cancer therapy methods.
Owner:CG ONCOLOGY INC
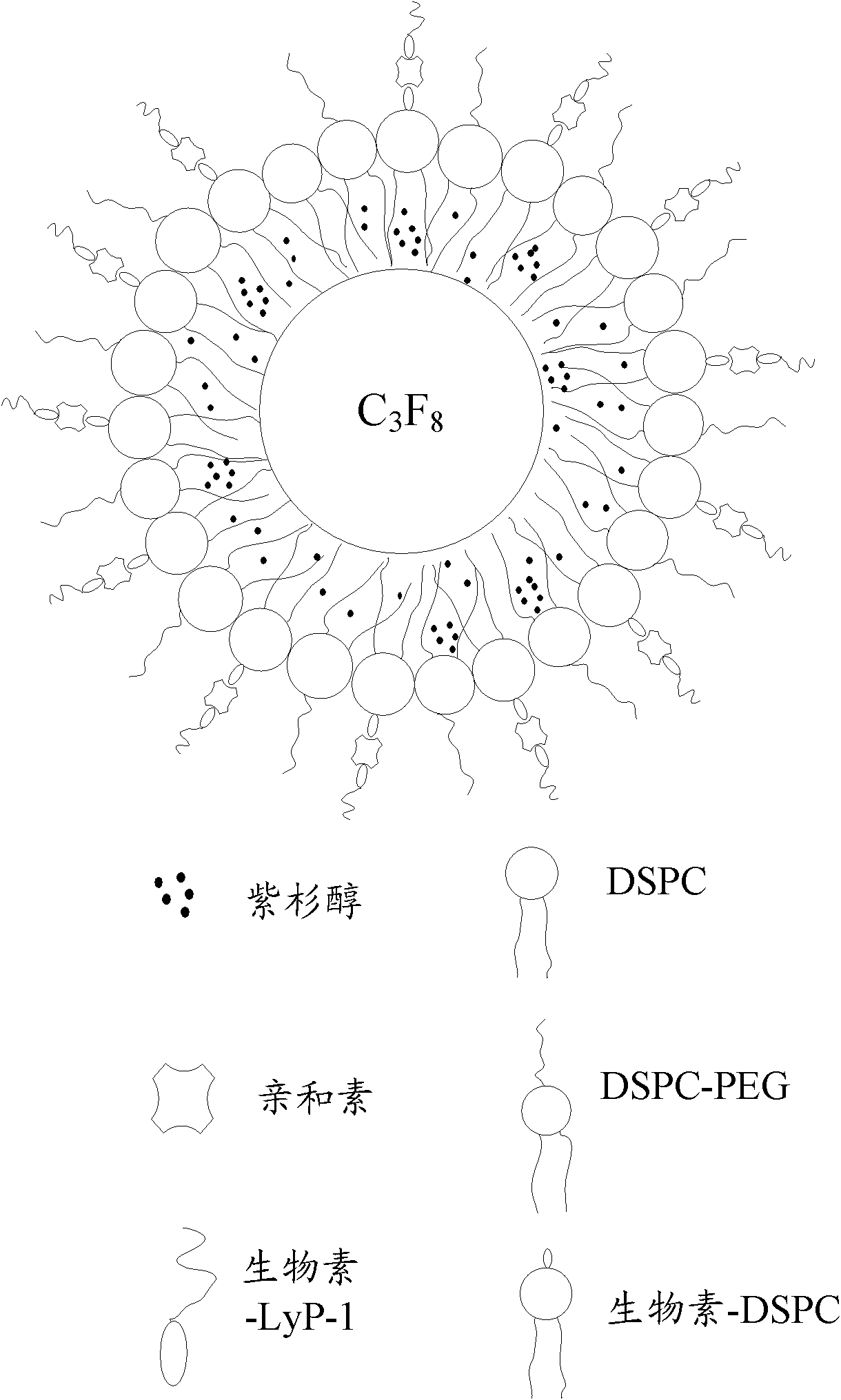
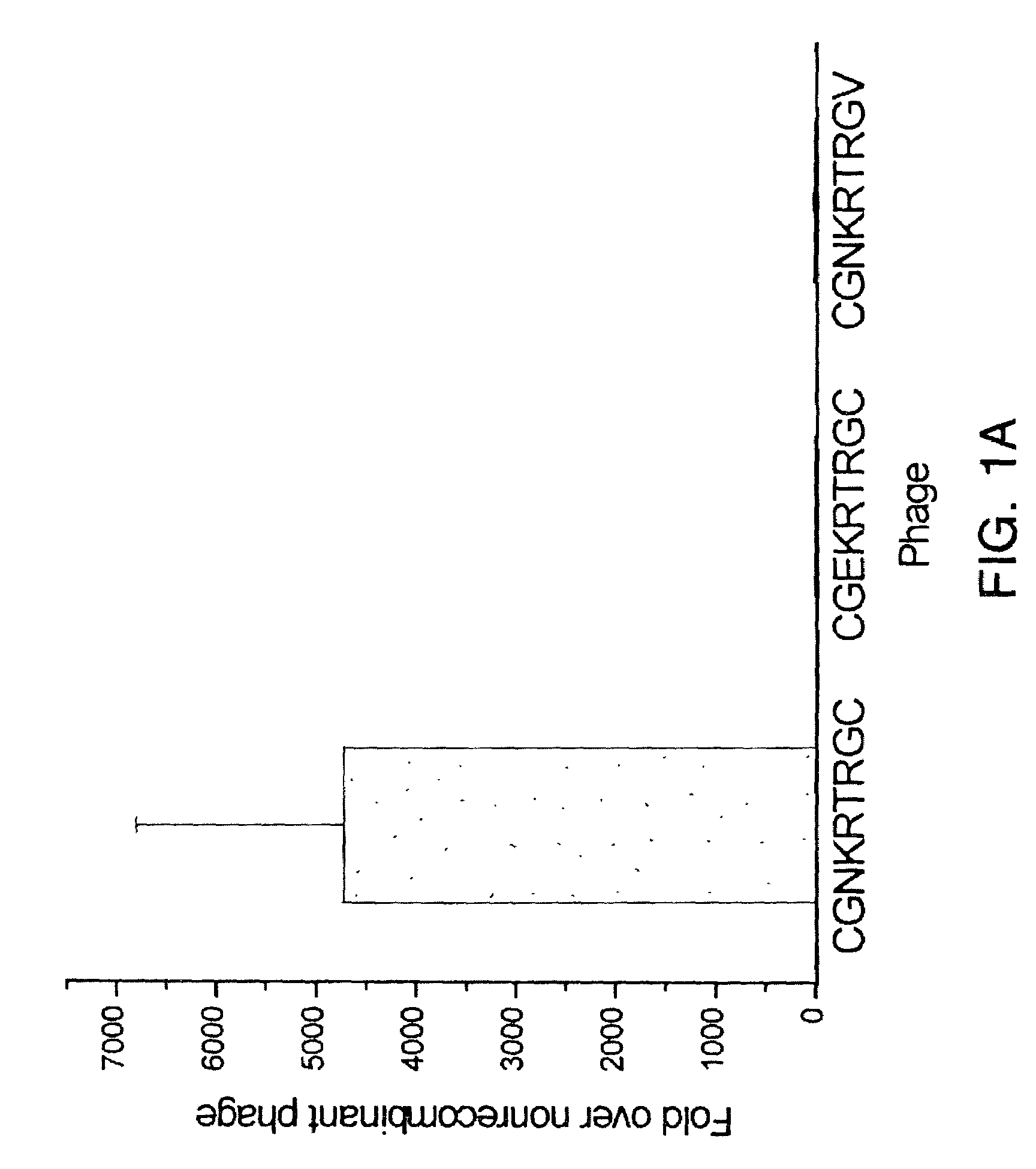
Targeted drug-bearing ultrasonic microbubble and preparation method thereof
InactiveCN102138889AOrganic active ingredientsGenetic material ingredientsTumor targetLymphatic vessel
The invention relates to a targeted drug-bearing ultrasonic microbubble comprising a lipide dimolecular layer outer shell, targeted polypeptide fixed at the outer side of the lipide dimolecular layer outer shell, a biological inert gas wrapped in the lipide dimolecular layer outer shell and medicament granules dispersed in the lipide dimolecular layer outer shell. The targeted polypeptide is a polypeptide or protein derivative containing an amino acid sequence CGNKRTRGC. By connecting the polypeptide or protein derivative containing a tumor targeted peptide sequence outside the lipide dimolecular layer outer shell, the obtained targeted drug-bearing ultrasonic microbubble can target the lymph vessels and the tumor cells of a tumor, can detect and diagnose the generation, the development and the curative effect on the tumor in real time through high-frequency ultrasonic imaging and can crush the microbubble through low-frequency ultrasound to release the medicament granules so as to achieve the aim of controllably and targetedly releasing the medicament, thereby having extremely important meanings to the prevention, diagnose and treatment of the tumor. In addition, the invention also relates to a preparation method of the targeted drug-bearing ultrasonic microbubble.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
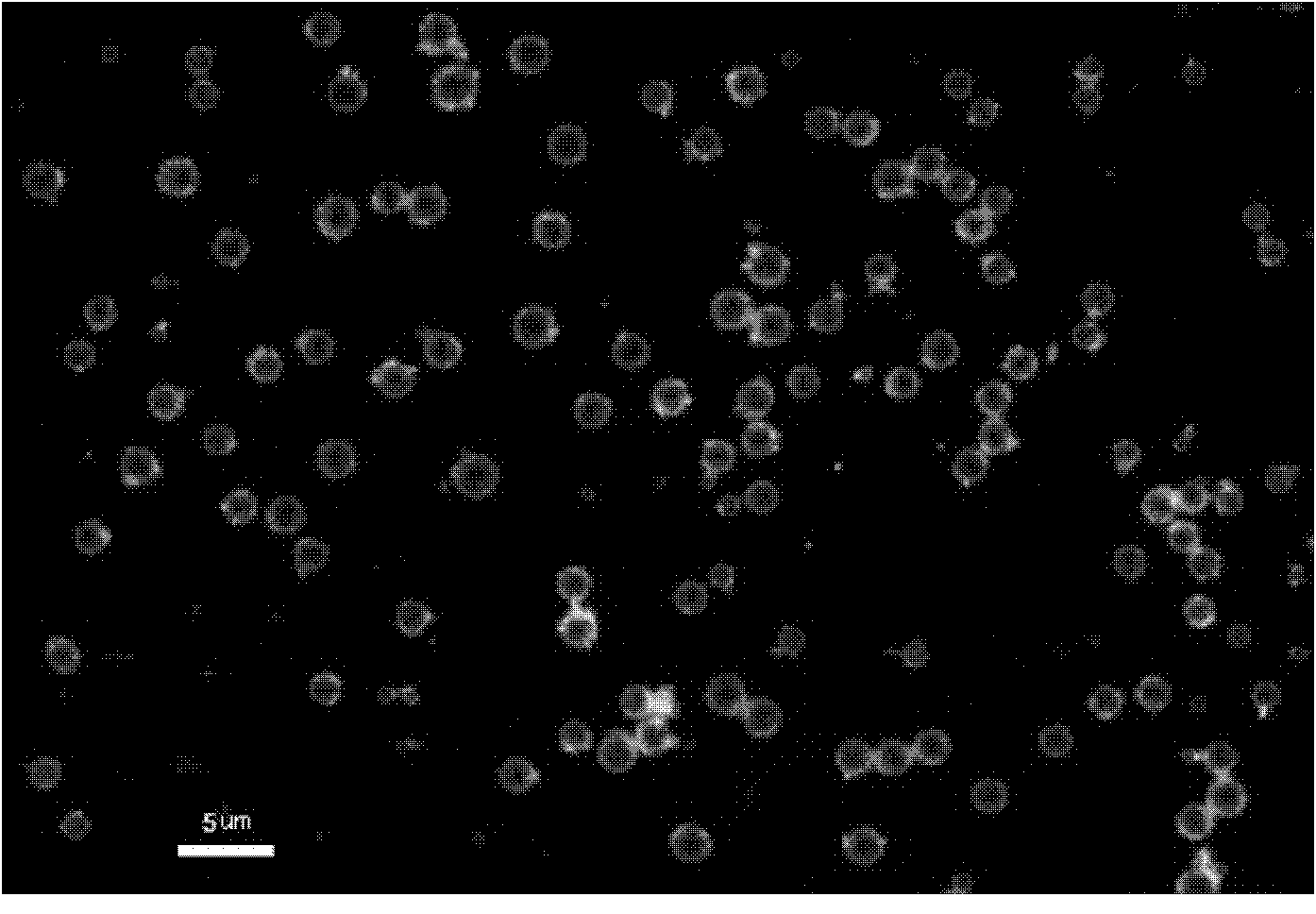
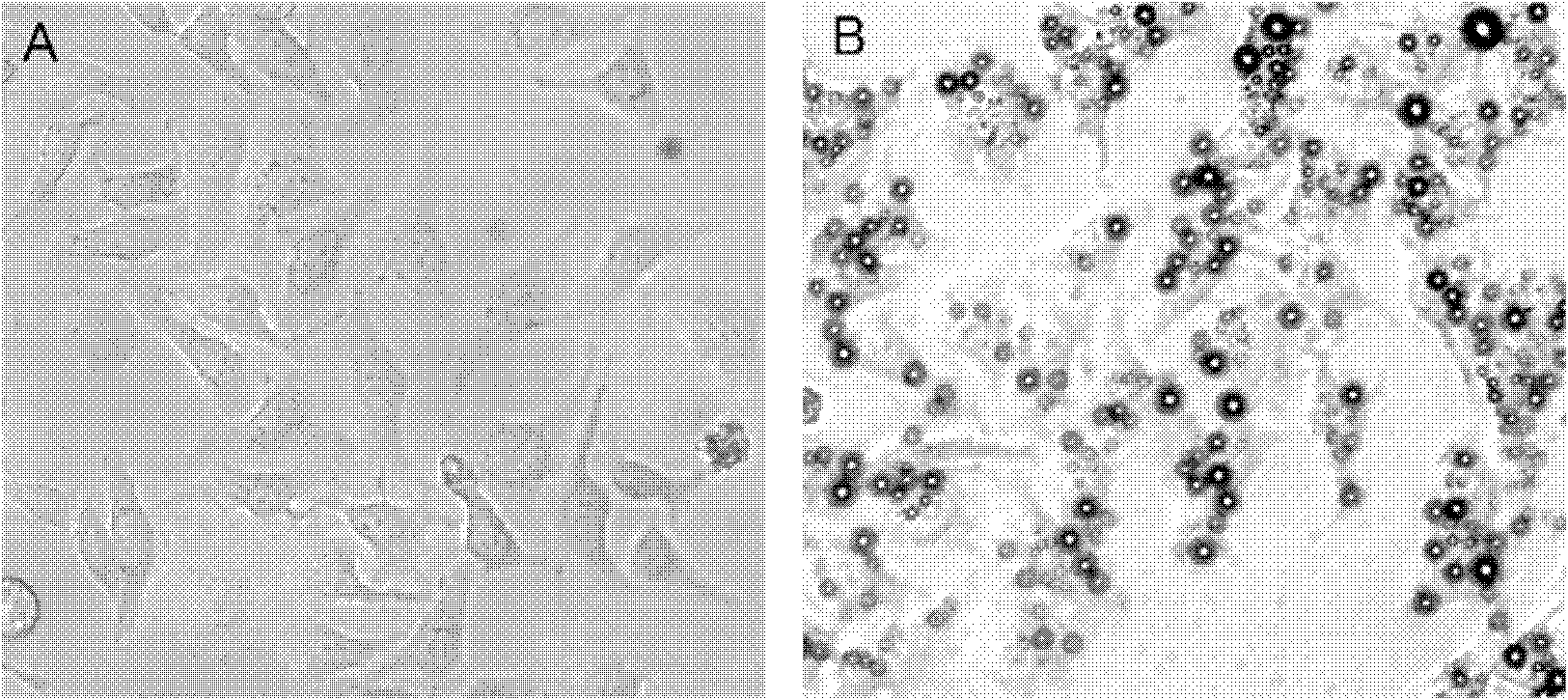
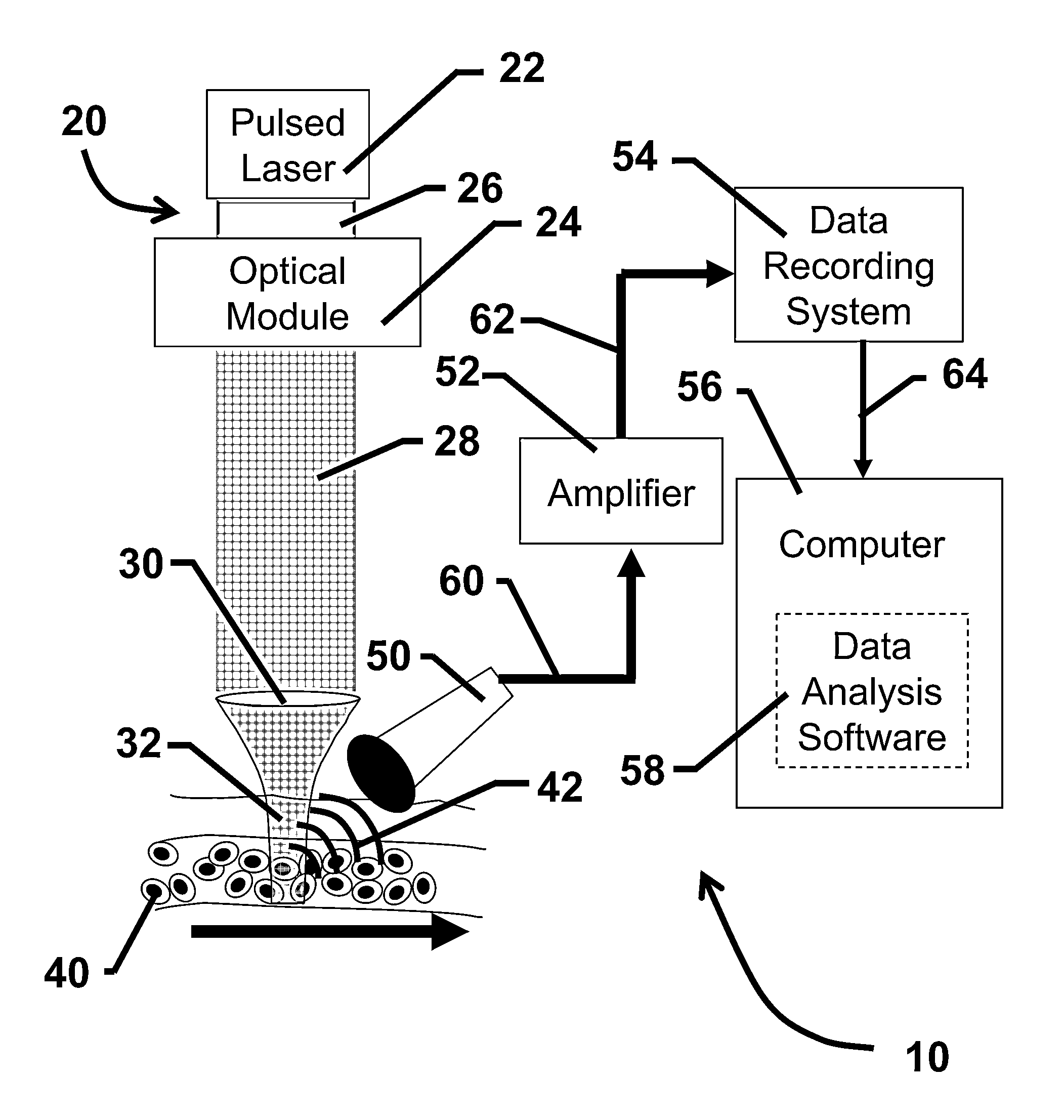
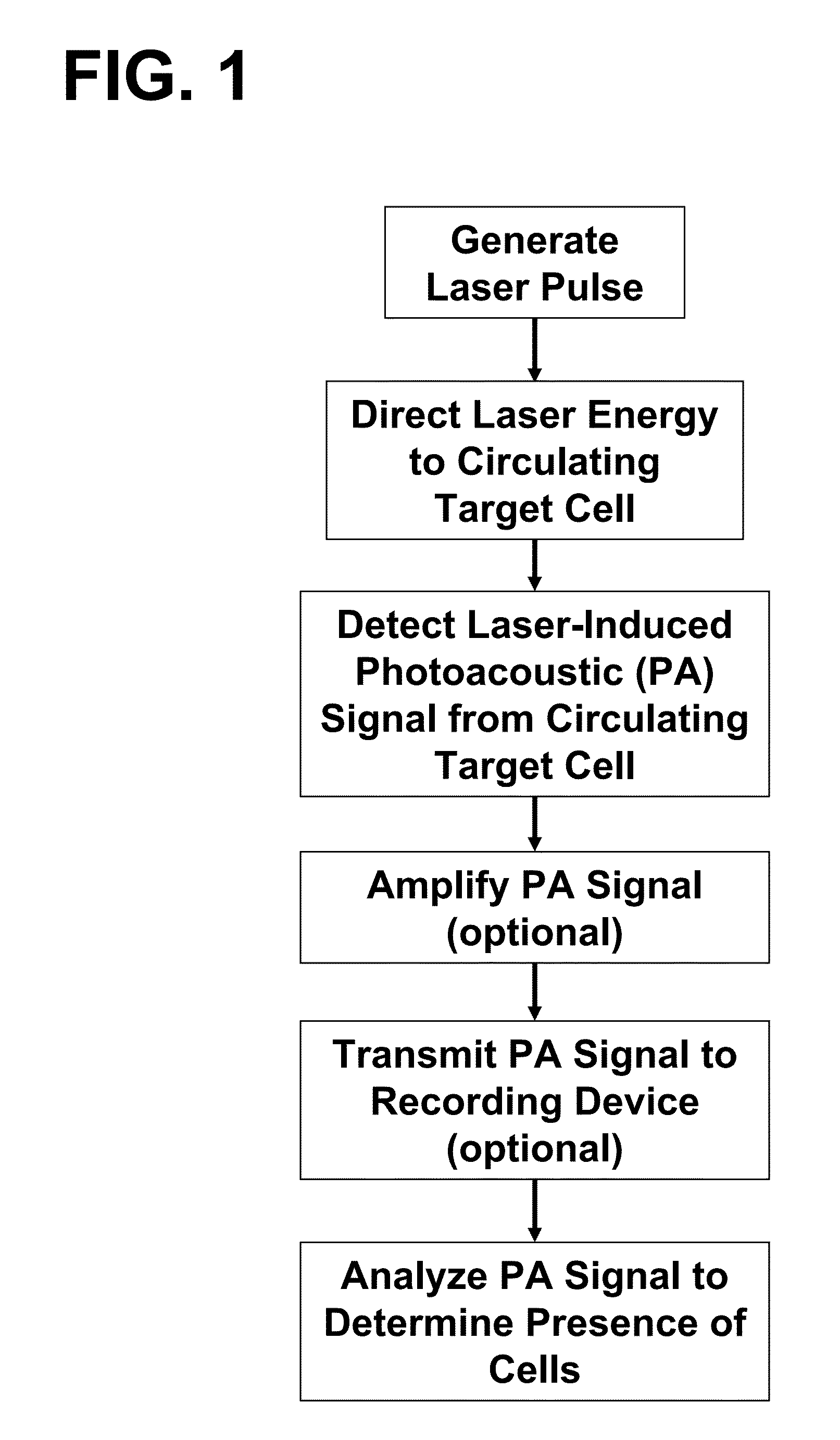
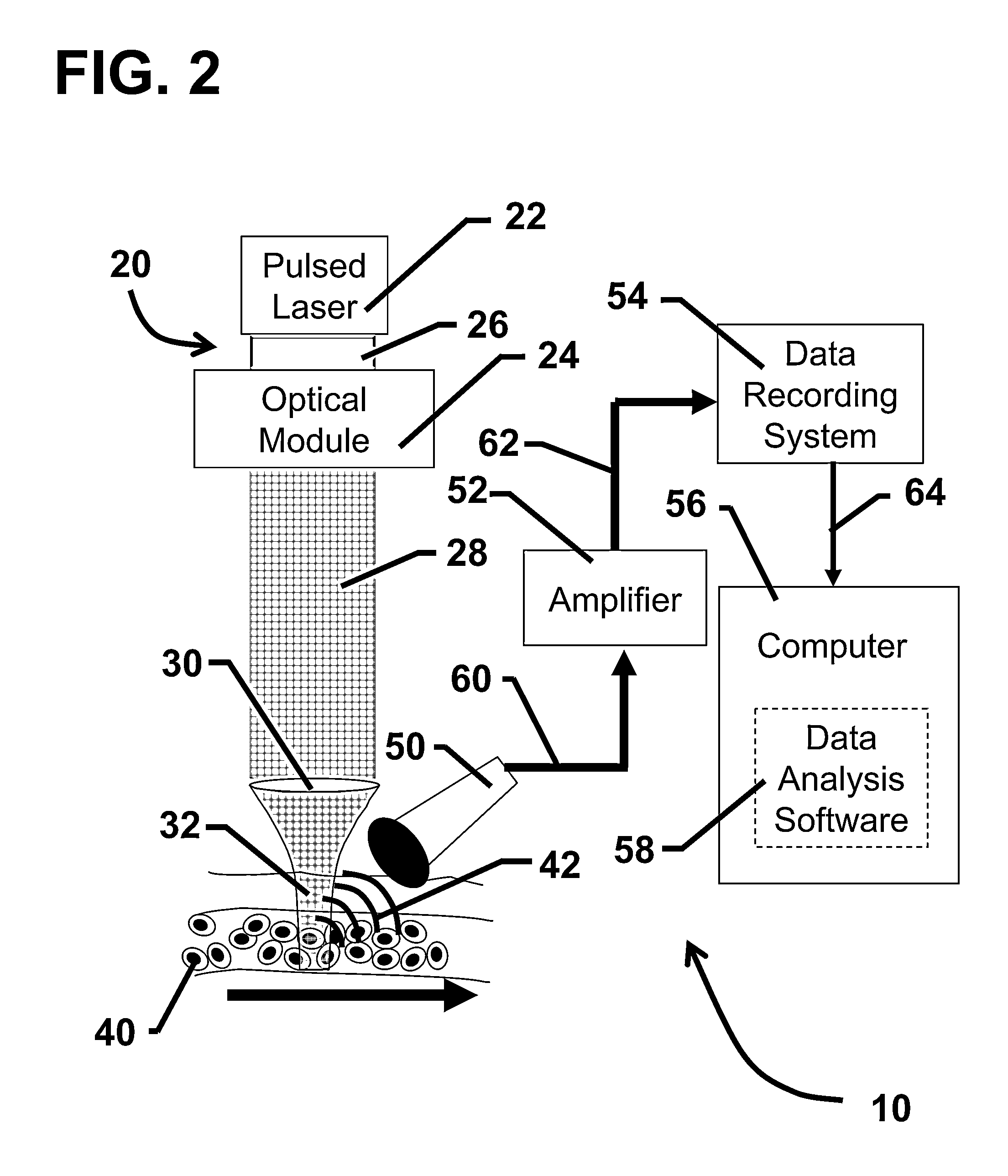
Device and method for in vivo flow cytometry using the detection of photoacoustic waves
ActiveUS20130060122A1Diagnostics using lightOrgan movement/changes detectionLymphatic vesselLaser light
A photoacoustic flow cytometry (PAFC) device for the in vivo detection of cells circulating in blood or lymphatic vessels is described. Ultrasound transducers attached to the skin of an organism detect the photoacoustic ultrasound waves emitted by target objects in response to their illumination by at least one pulse of laser energy delivered using at least one wavelength. The wavelengths of the laser light pulse may be varied to optimize the absorption of the laser energy by the target object. Target objects detected by the device may be unlabelled biological cells or cell products, contrast agents, or biological cells labeled with one or more contrast agents.
Owner:BIOVENTURES LLC
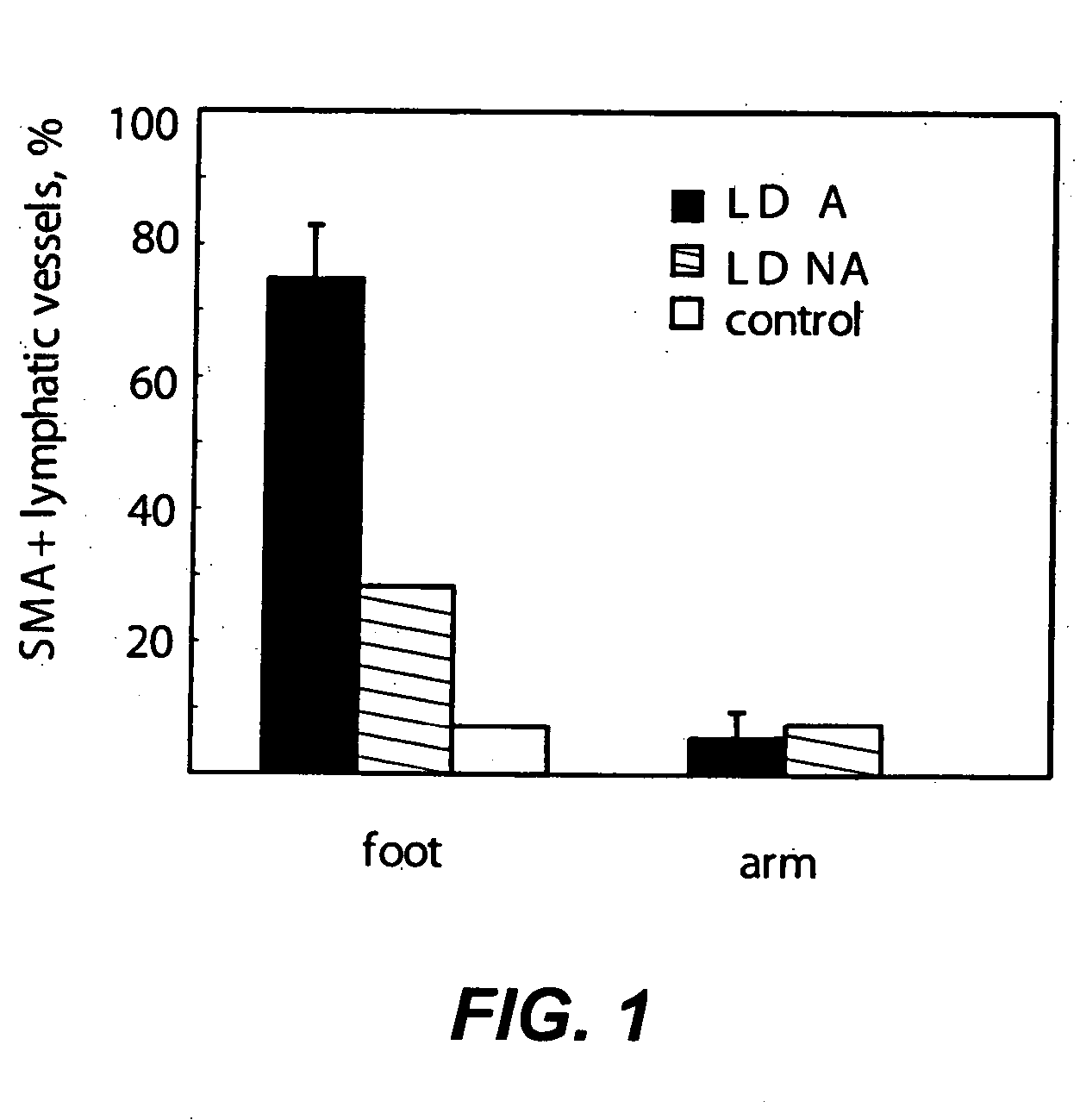
Compositions and methods for treatment of lymphatic and venous vessel arterialization
InactiveUS20060025338A1Promote healingReduce edemaOrganic active ingredientsVirusesSmooth muscleLymphatic vessel
The present invention is directed to methods and compositions that may be used in disrupting the association of smooth muscle cells with lymphatic endothelial cells and in correcting the valvular dysfunction in veins and lymphatic vessels. Such compositions are useful for therapeutic and prophylactic treatment of impaired lymphatic and venous function, particularly for the treatment of lymphedema distichiasis or chronic venous insufficiency.
Owner:VEGENICS PTY LTD
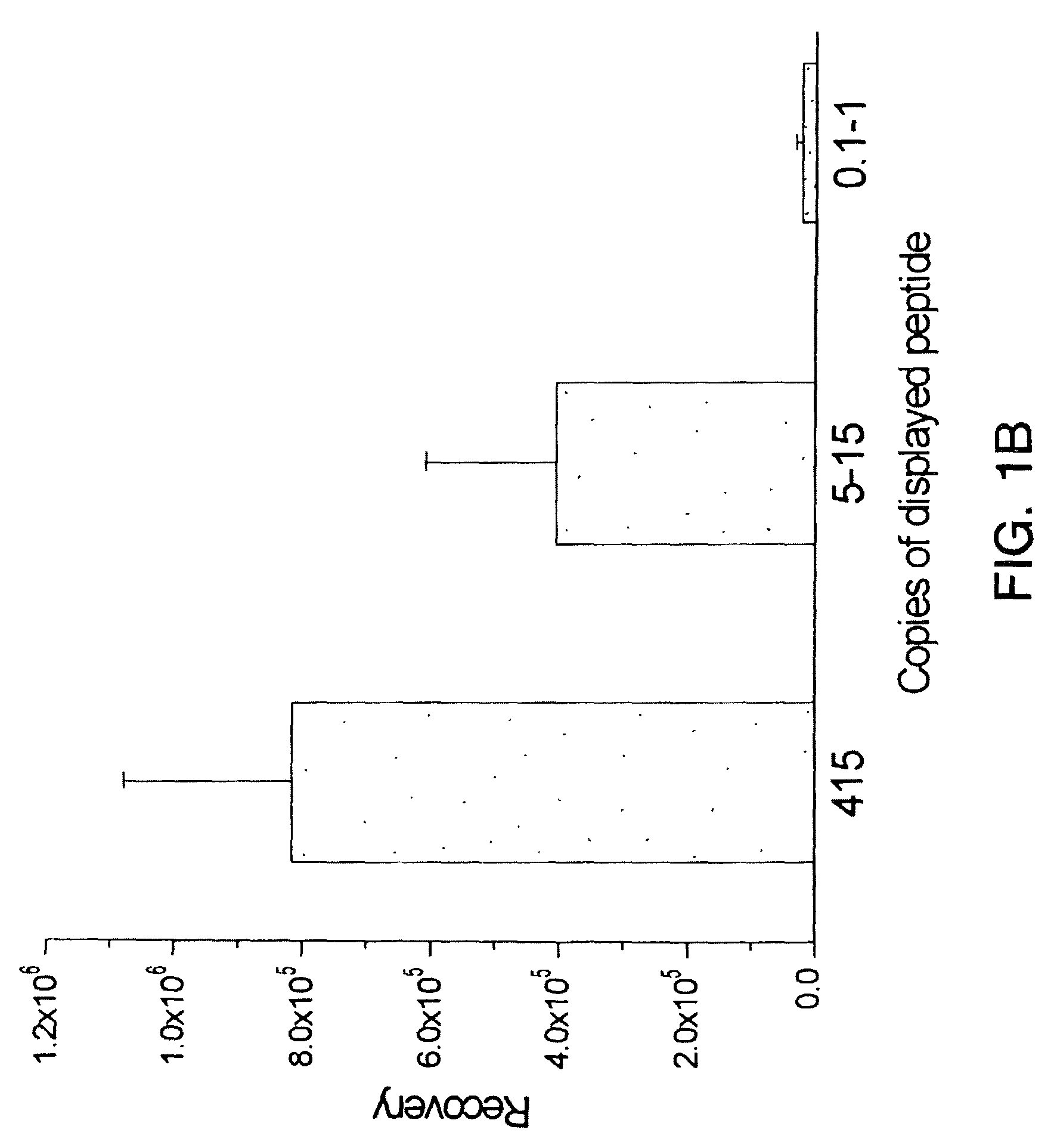
Peptides that home to tumor lymphatic vasculature and methods of using same
InactiveUS7192921B2Reduce in quantityReduce the numberPeptide-nucleic acidsAntibody mimetics/scaffoldsLymphatic vesselWilms' tumor
The present invention provides a conjugate containing a moiety linked to a homing molecule that selectively homes to tumor lymphatic vasculature. The invention also provides a method of directing a moiety to tumor lymphatic vasculature in a subject by administering to the subject a conjugate containing a moiety linked to a homing molecule that selectively homes to tumor lymphatic vasculature.
Owner:BURNHAM INST FOR MEDICAL RES
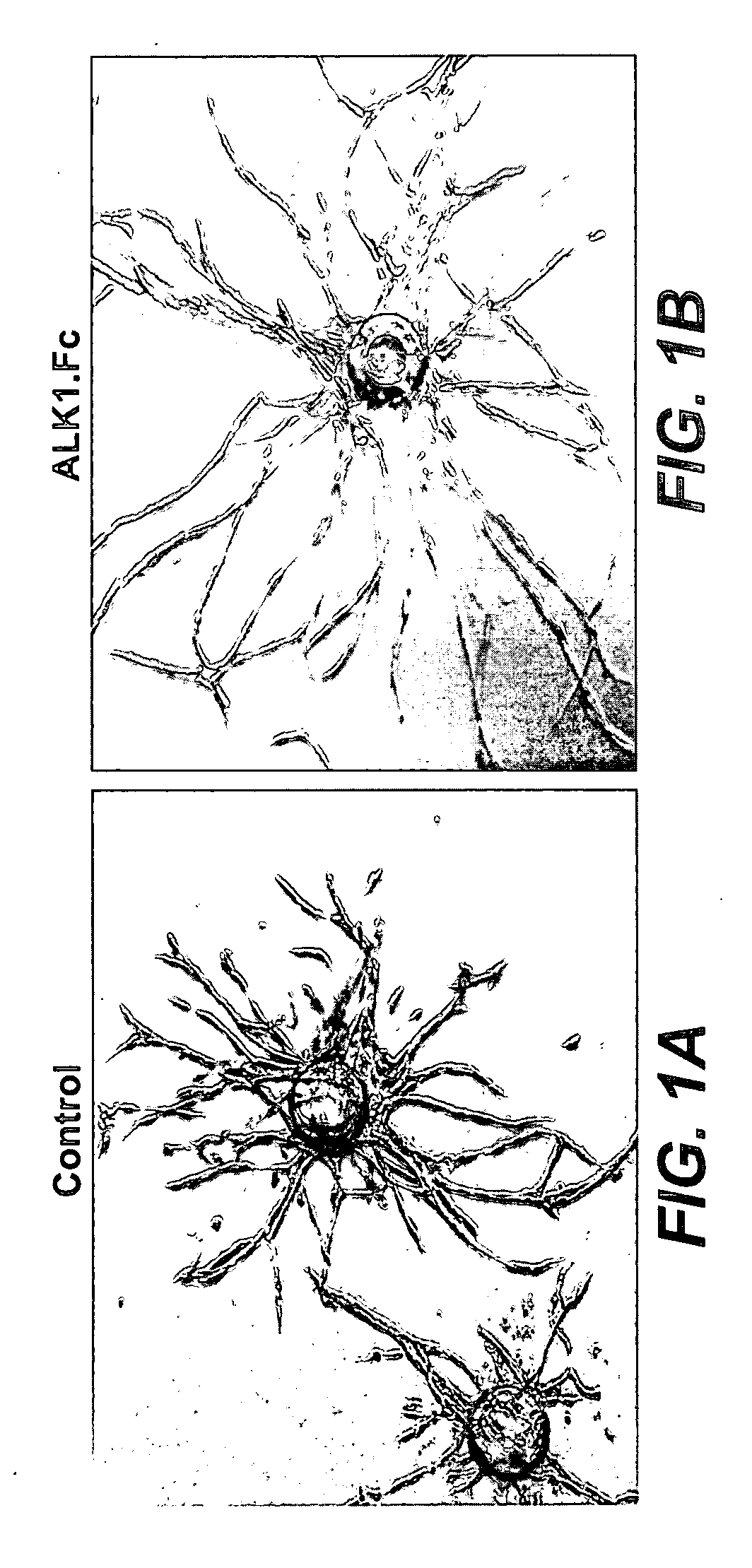
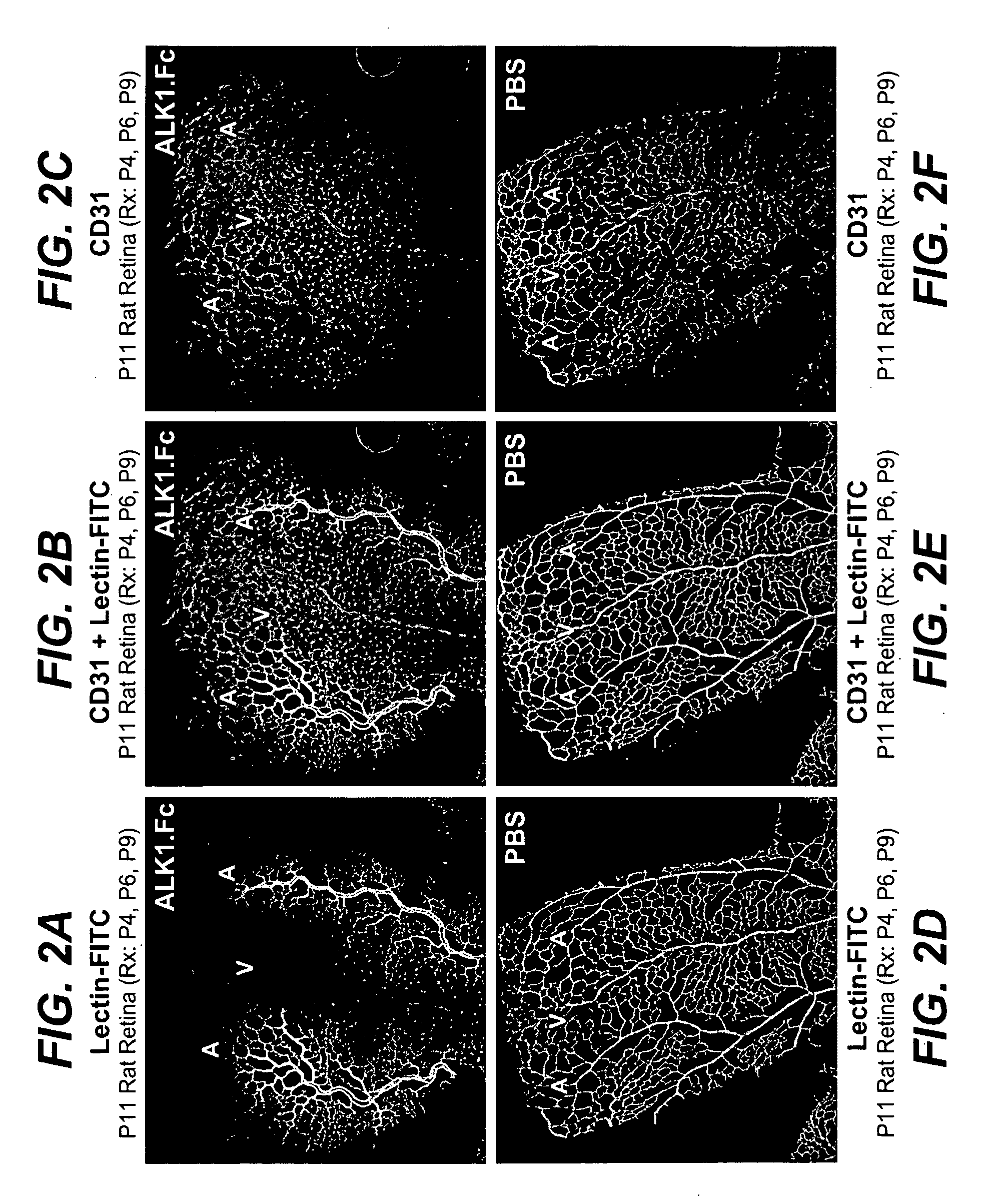
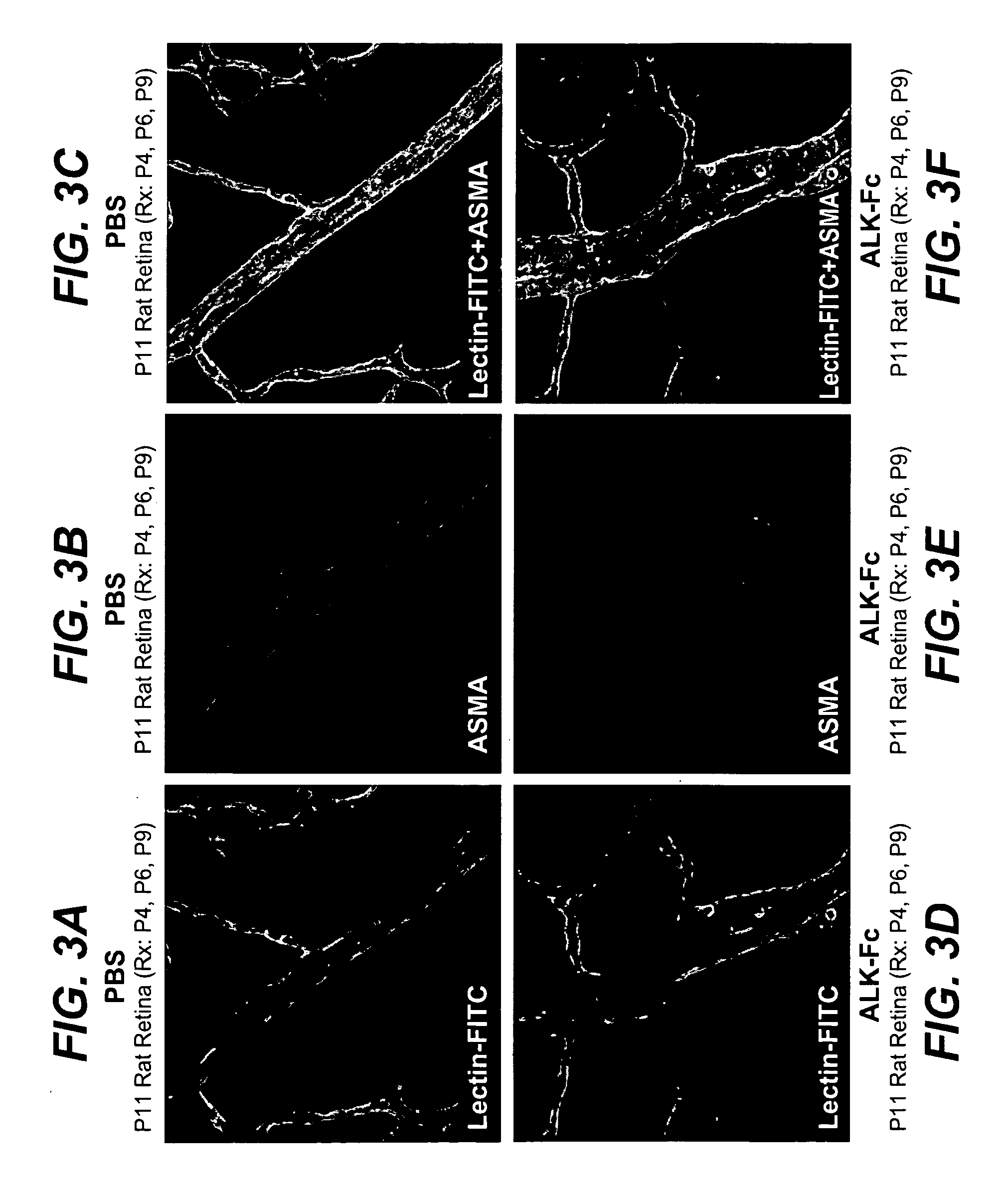
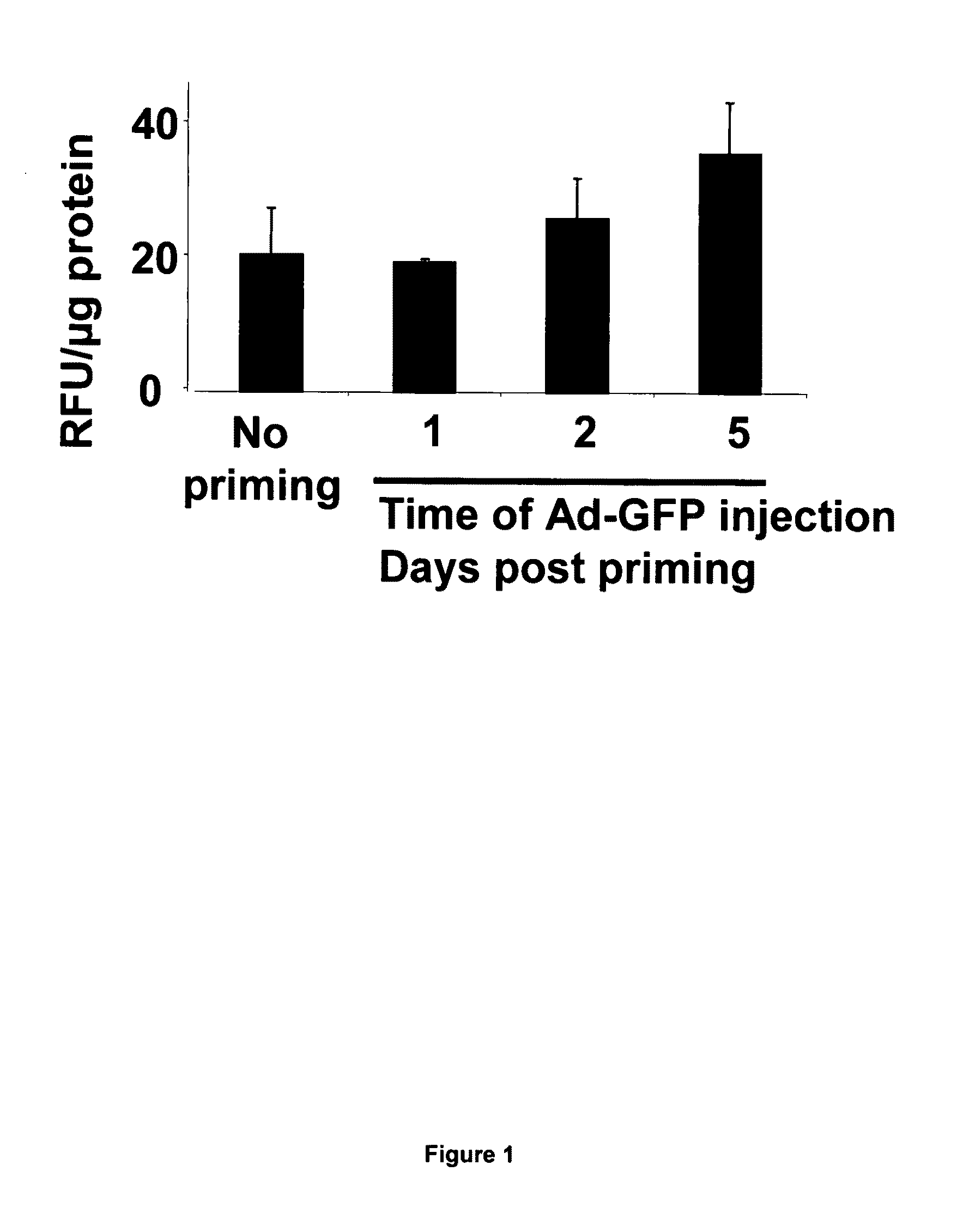
Activin receptor-like kinase-1 compositions and methods of use
InactiveUS20090226441A1Improve efficacyStrong inhibitory activitySenses disorderAntibody mimetics/scaffoldsLymphatic vesselAngiogenesis growth factor
Disclosed herein are methods and compositions for treating disorders associated with angiogenesis or lymphangiogenesis using ALK-1 antagonists.
Owner:GENENTECH INC
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Peptides that home to tumor lymphatic vasculature and methods of using same
InactiveUS20070149444A1Reduce in quantityReduce the numberAntibody mimetics/scaffoldsCyclic peptide ingredientsAbnormal tissue growthLymphatic vessel
Owner:BURNHAM INST FOR MEDICAL RES
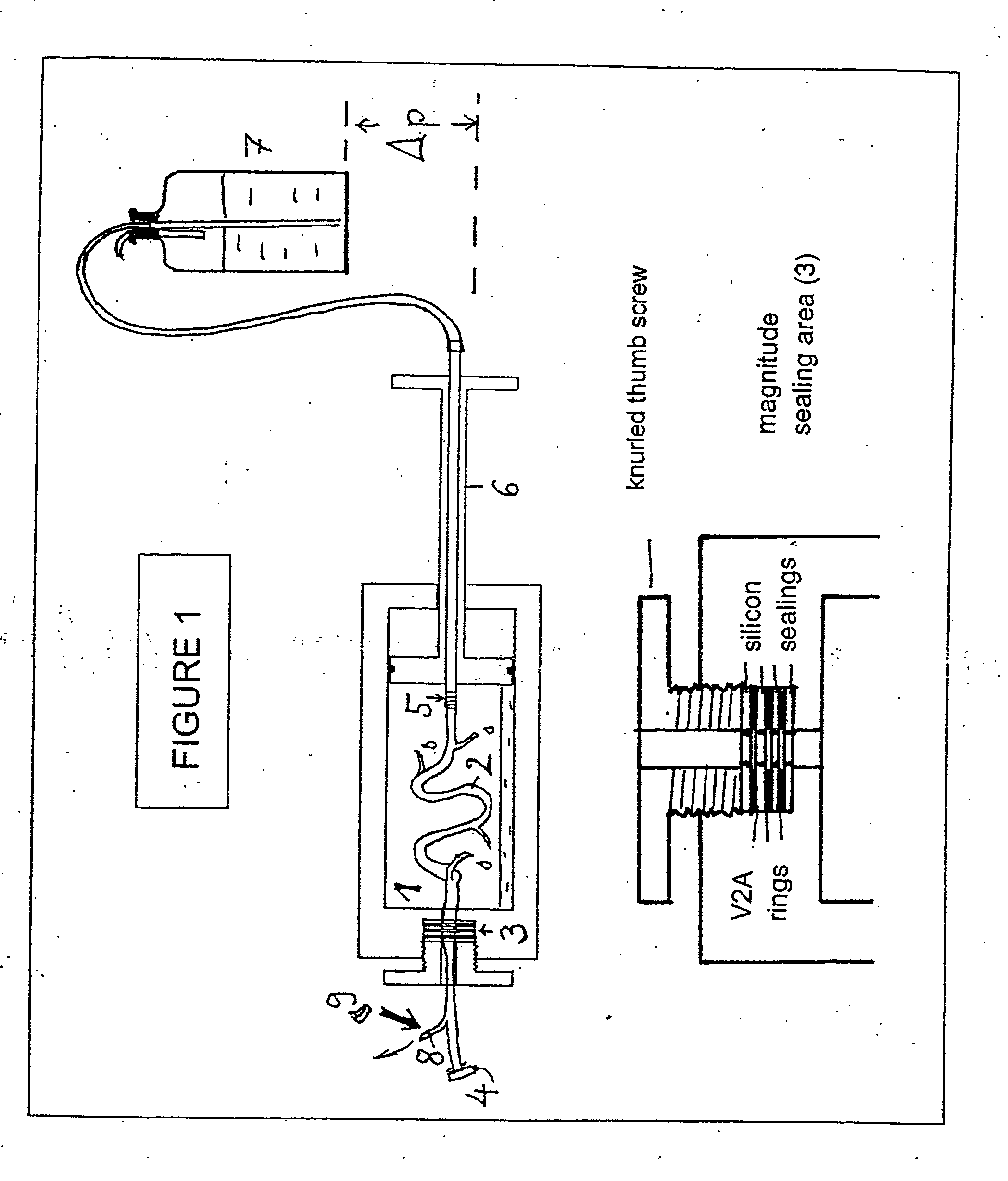
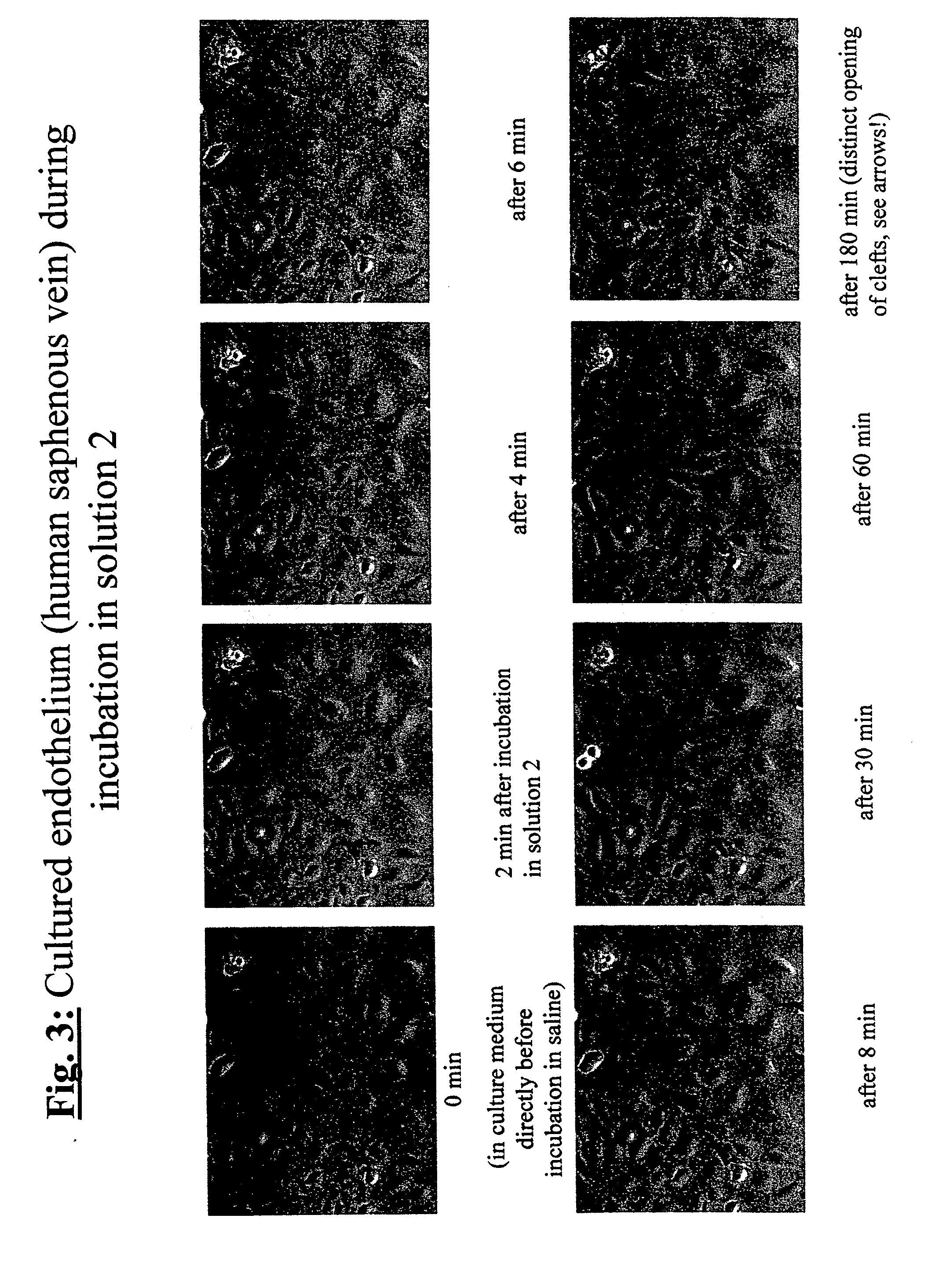
Methods And Apparatus For Preserving The Endothelium In Isolated Hollow Organs And Biological Vessels
InactiveUS20070202485A1Avoid ablationAvoid destructionSurgeryDead animal preservationVascular diseaseLymphatic vessel
The invention relates to a method and an apparatus for the endothelium-preserving treatment of isolated hollow organs, especially biological vessels such as blood vessels and lymphatic vessels by means of endothelium-protective perfusates or incubation solutions containing at least 0.1 percent by weight of native albumin and a nutrient substrate, preferably L-glutamine. Also disclosed are the use of the endothelium-protective perfusates for preparing hollow organs or biological vessels as a transplant for the treatment of organ diseases or vascular diseases, the use thereof for repairing endothelial lesions in isolated hollow organs and / or biological vessels, and the use thereof for preserving organs and / or vessels.
Owner:BIOTEST SERUM INST GMBH
Polysaccharide macromolecular paramagnetic metal complex and synthesis method and application thereof
InactiveCN101619106AIncreased toxicityShort retention timeIn-vivo testing preparationsLymphatic vesselSynthesis methods
The invention relates to a polysaccharide macromolecular paramagnetic metal complex and a synthesis method and an application thereof. Polysaccharide macromolecules are used as carriers, and a ligand compound, the side chains of which contain open-chain or annular polyamino polycarboxylic compounds, acts with paramagnetic metal ions to form the paramagnetic metal complex, wherein the paramagnetic metal ions are positive divalent or trivalent ions of metal elements, the atomic numbers of which are 21-29, 42 and 44 or 57-71. The polysaccharide macromolecular paramagnetic metal complex can be used as a magnetic resonance imaging contrast agent for lymphatic organs, lymphatic vessels, lymphatic systems and cardiovascular systems of people or other mammals.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
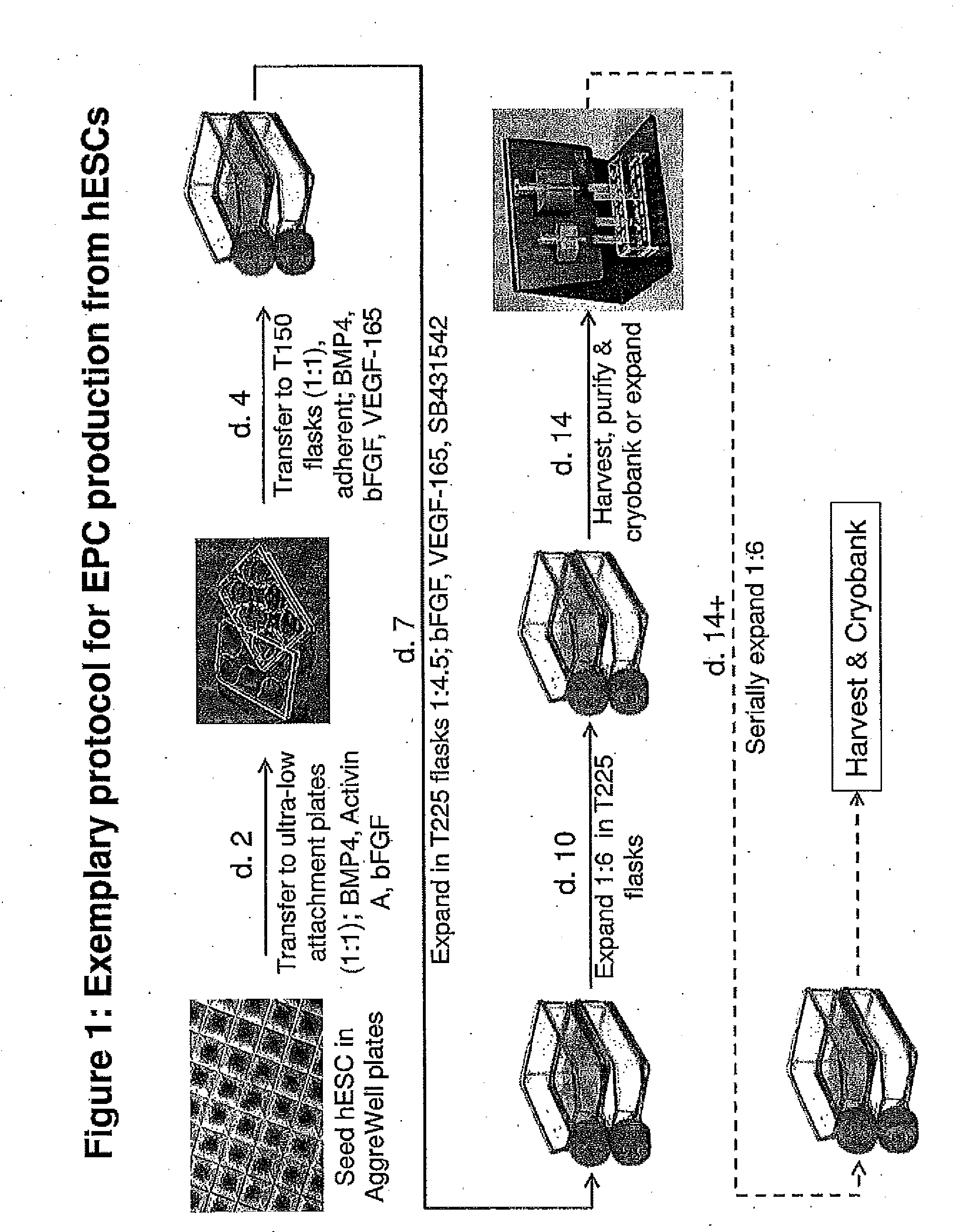
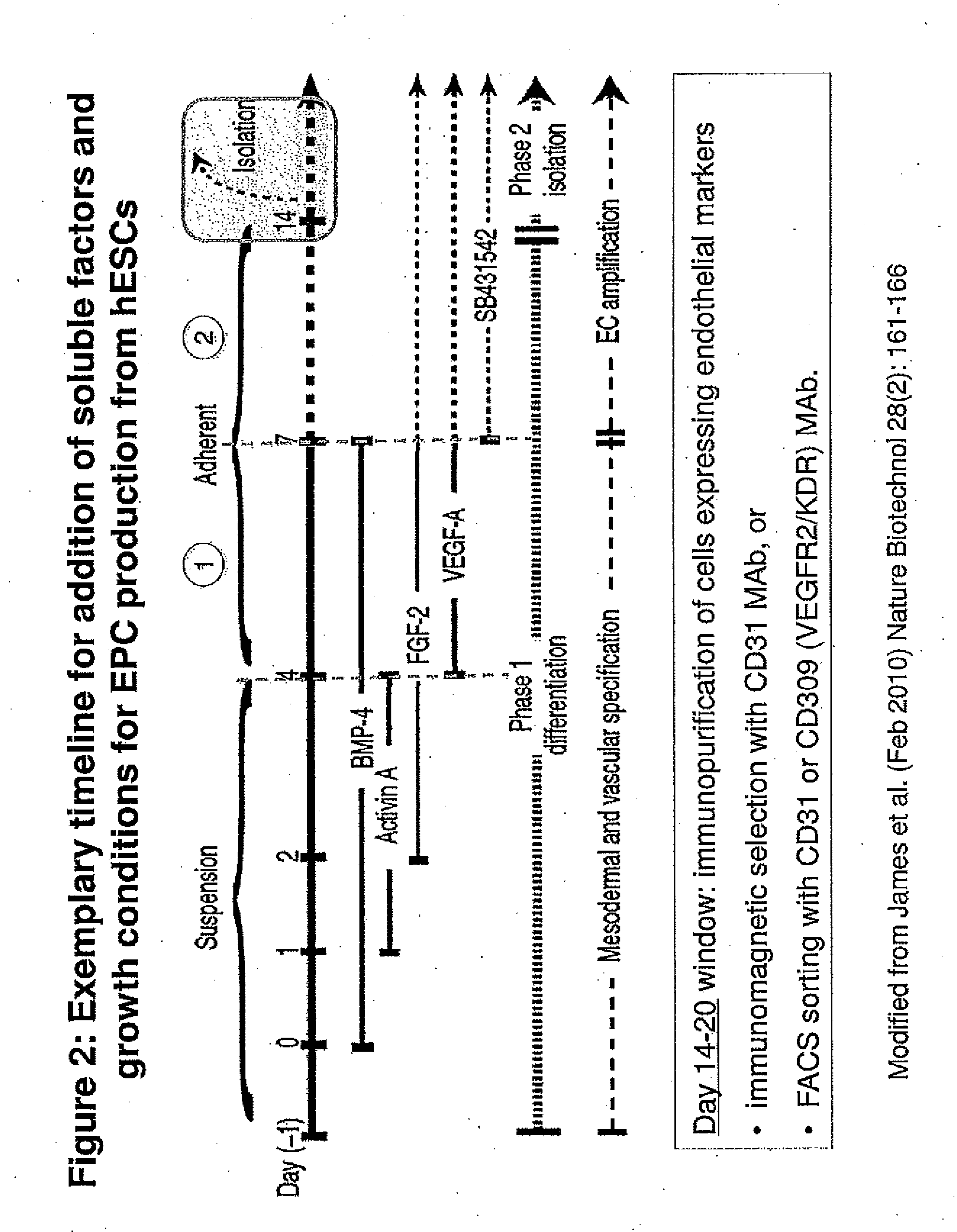
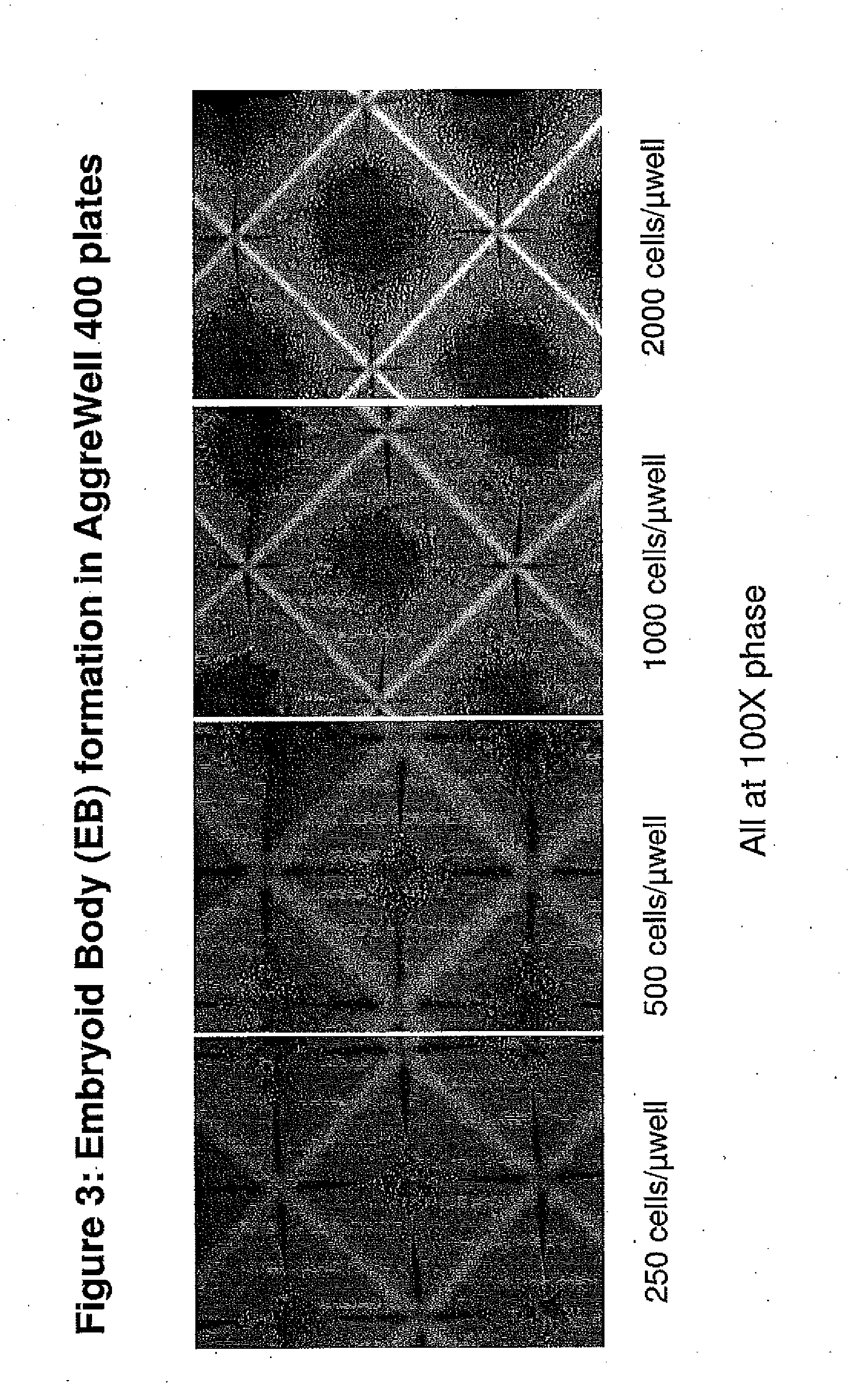
Methods and Compositions for Producing Endothelial Progenitor Cells from Pluripotent Stem Cells
InactiveUS20120295347A1Artificial cell constructsCell culture supports/coatingProgenitorLymphatic vessel
Aspects of the present invention are drawn to methods and compositions for producing endothelial progenitor cells (EPCs) in vitro from pluripotent stem cells and compositions containing such EPCs. The methods produce sufficient EPCs to use in therapeutic applications. In certain embodiments the EPCs are bipotent, giving rise to both vascular and lymphatic endothelial cells. In certain embodiments, EPCs express one or more of the following gene products: LYVE-1, PV-1 / PAL-E, CD31, and CD34.
Owner:RECYTE THERAPEUITCS
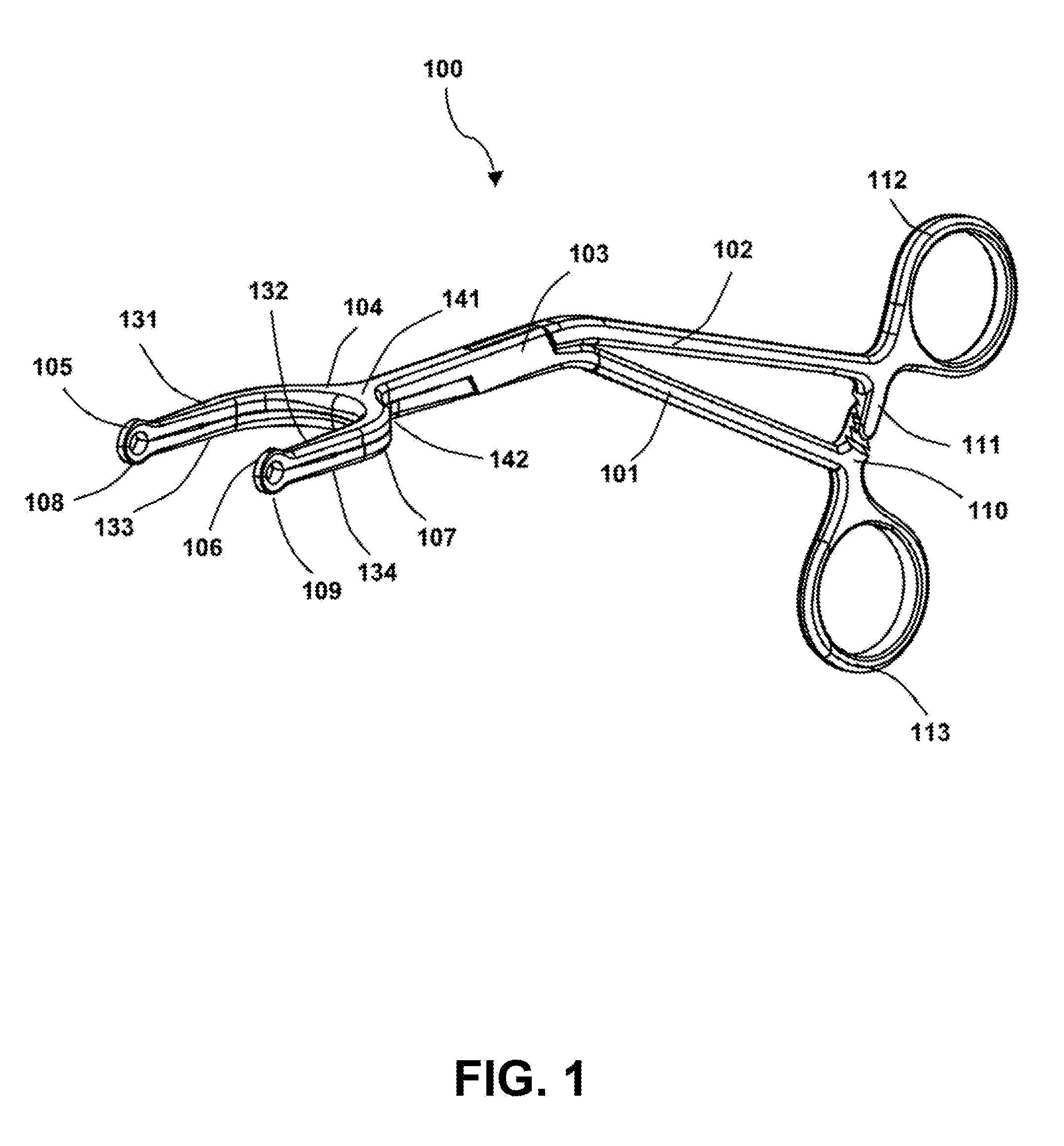
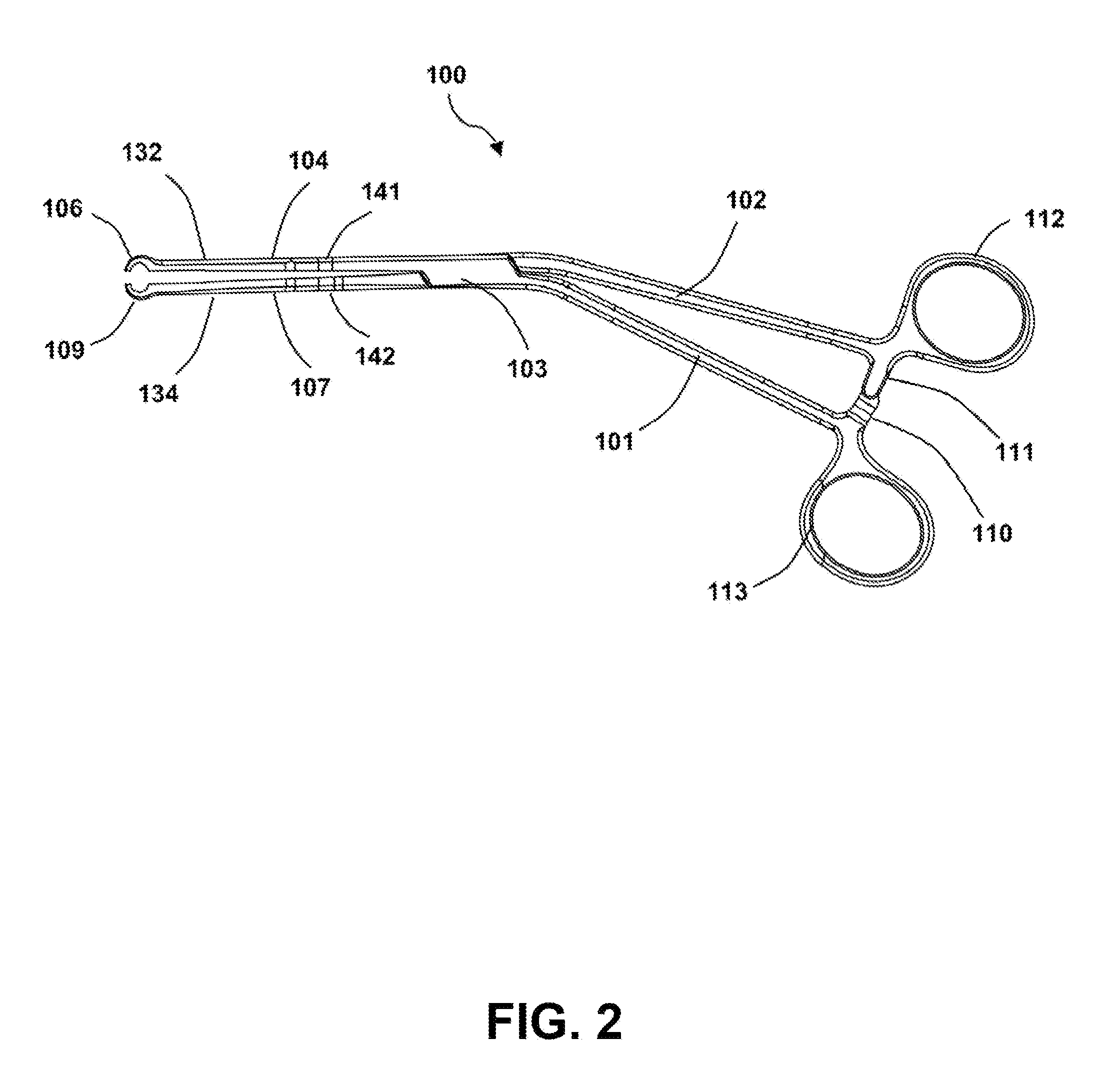
Surgical forceps
InactiveUS20130144313A1Comfortable and easy to maintainEnhanced surface contactFallopian occludersSurgical forcepsAnatomical structuresLymphatic vessel
This application presents a bifurcated, optimally-angled surgical forceps. In one example, this surgical forceps may enable a more natural maneuver for initial clamping of the vas deferens through the scrotal skin. This may be more comfortable for users and easier to maintain, and may provide greater tactile surface contact between the thumb and vas deferens. This device also may provide the surgeon with an entire segment of vas deferens upon which the vasectomy may be performed, thus reducing the need for frequent repositioning of instruments. The device may also be applied to other surgical procedures that may benefit from the features of the device and where a section of a tubular anatomical structure may need clamping at two points along its length. Examples include blood and lymphatic vessels, ducts of the digestive system, and large nerves or nerve bundles.
Owner:ALFRED E MANN INST FOR BIOMEDICAL ENG AT THE UNIV OF SOUTHERN CALIFORNIA
VEGFR-3 inhibitor materials and methods
InactiveUS7611711B2Extended shelf lifeImprove stabilityImmunoglobulinsCyclic peptide ingredientsDiseaseLymphatic vessel
The present invention relates to the diagnosis, evaluation, and therapeutic intervention of disorders mediated by the activity of cell surface receptor VEGFR-3, which activity often is stimulated by VEGFR-3 ligands VEGF-C and VEGF-D. More particularly, the present invention identifies novel methods and compositions for the inhibition of VEGF-C / D binding to VEGFR-3. The compositions of the present invention will be useful in the inhibition of angiogenesis and lymphangiogenesis.
Owner:VEGENICS PTY LTD
Substrate for cell transfer
ActiveUS20070015277A1Form evenlyLow wettabilityBioreactor/fermenter combinationsBiological substance pretreatmentsVessel networkLymphatic vessel
A main object of the present invention is to provide a cell transfer substrate capable of transplanting cells, maintaining a pattern as it is, on a living body tissue or the like, even when: the size of the cell sheet is extremely small; the cells are cultured sparsely; the cells are in a form of a small colony; or the cells are cultured in a pattern, for example, as a blood vessel, a vessel network such as a lymphatic vessel, or a nerve network, and to provide a substrate for cell transfer to be used for the cell transfer substrate. In order to achieve the above-mentioned object, the present invention provides a substrate for cell transfer comprising: a polymer base material; an intermediate layer formed on the polymer base material; and a cell transfer layer formed on the intermediate layer.
Owner:DAI NIPPON PRINTING CO LTD
Method of drug delivery to interstitial regions of the myocardium
InactiveUS20060078496A1Small sizeFast shippingIn-vivo radioactive preparationsInternal electrodesLymphatic vesselMicrosphere
A method of treating the heart and other body tissues by injecting a compound comprised of microsphere encapsulated macromolecule therapeutic agents into the myocardium, such that the microsphere size inhibits capillary transport of the compound but may permit lymphatic transport of the compound, and the compound releases therapeutic agents upon degradation of the microsphere. The compounds may be used in a method of treating the coronary arteries in which lymphatic transportable therapeutic agents are injected into the myocardium at a location distal to a target site in the coronary artery, after which they are taken up by the lymphatic vessels and transported proximally relative to the coronary artery, and migrate from the lymphatic vessel to the coronary blood vessel.
Owner:BIOCARDIA
Insulin intranasal inhalation powder spray
InactiveCN101428009AImprove stabilityImprove bioavailabilityPeptide/protein ingredientsMetabolism disorderNasal cavityLymphatic vessel
The invention provides insulin nasal dry powder inhalation which comprises the following components by contents (weight percentages): 1% to 100% of insulin freeze-dry powder with self emulsifying effect and 0% to 99% of carrier. In the insulin nasal dry powder inhalation, the dosage of grease is determined according to the surface area and the grain size of grease drops, and a large quantity of animal experiments prove that under the condition and in the proportion, the optimal drug treatment effect can be achieved. Compared with the liquid preparation, the stability of the dry powder is increased, and the dry powder can be automatically re-dissolved into nano-sized emulsion after being in contact with water; after the drug-containing compound enters the nasal cavity, the nano-size emulsion easily passes by the barrier of the nasal mucosa and enters the body via the rich capillaries and lymphatic vessels in the nasal mucosa to exert the efficacy, thereby remarkably improving the bioavailability of the drug and being rapidly absorbed, without stimulation to the nasal mucosa; in addition, the adoption of bio-adhesive increases the retention time of the drug-containing powder on the nasal mucosa, so that the absorption and the utilization of the drug are more complete.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Methods of using adipose tissue-derived cells in the treatment of the lymphatic system and malignant disease
InactiveUS8784801B2Ease and low morbidityAvoid contaminationBiocideMetabolism disorderProgenitorDisease
Aspects of the invention provides methods for preparing and using adipose-tissue-derived stem and progenitor cells, adipose-tissue-derived lymphatic endothelial cells, and cells capable of differentiating into lymphatic endothelial cells to treat disorders of the lymphatic system and to modulate expansion, repair, and / or regeneration of the lymphatic system. The invention further provides using adipose-tissue-derived lymphatic endothelial cells and cells capable of differentiating into lymphatic endothelial cells for delivery of therapeutic agents to tumor cells as a means for treating malignant disease, and assays to screen for drugs that modulate lymphatic system expansion, repair or regeneration.
Owner:LOREM VASCULAR PTE LTD
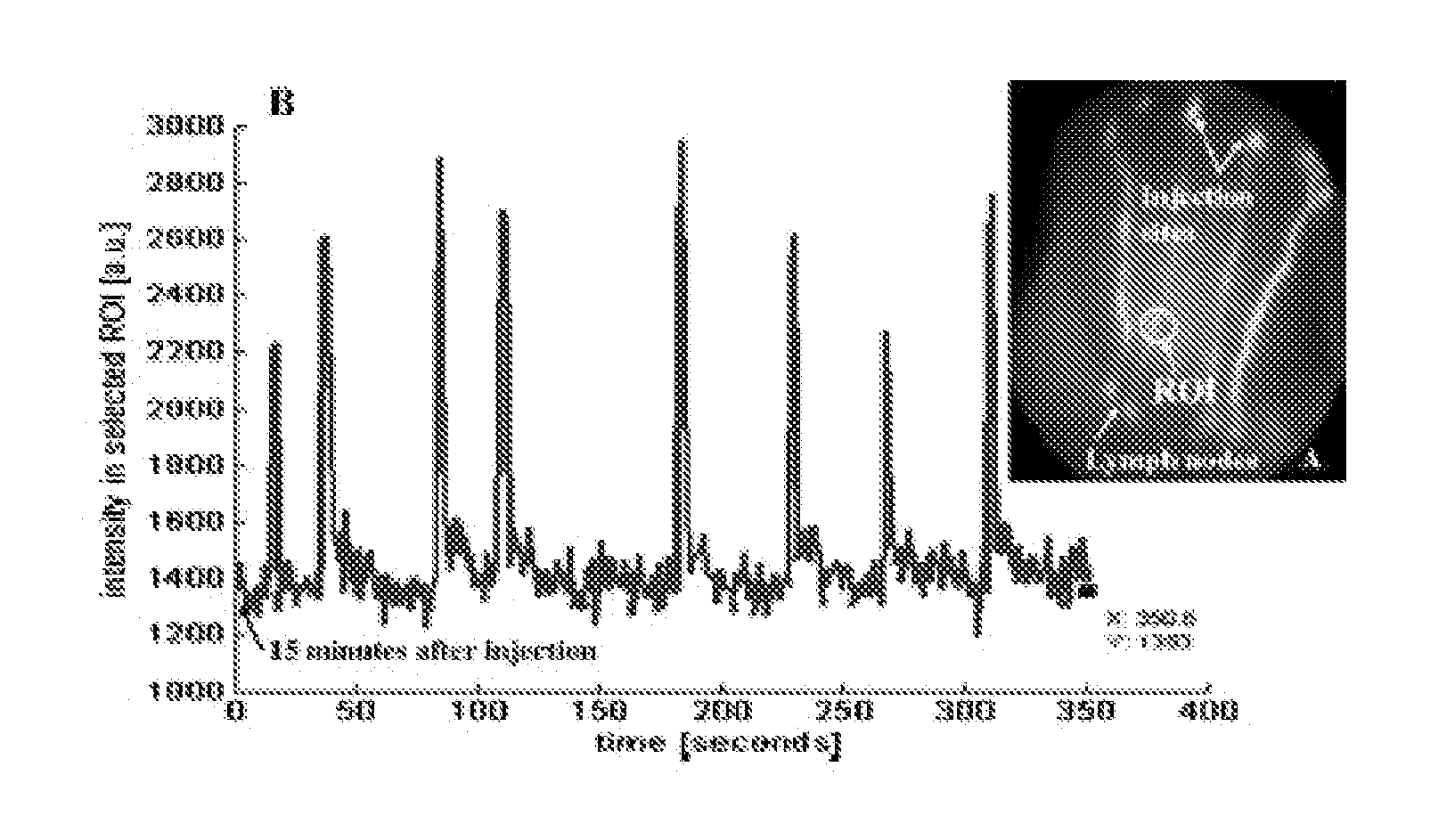
Imaging agents for functional imaging of lymphatic structures
InactiveUS20080056999A1Ultrasonic/sonic/infrasonic diagnosticsDiagnostics using lightLymphatic SpreadImaging agent
Novel imaging agents targeted to a lymph vascular cell receptor and a hyaluranon cell receptor are disclosed. The disclosed imaging agents incorporate biological molecules such as hyaluronic acid which bind to the receptors. Lymph vascular cell receptor expression may be related to the beginnings of tumor formation. As such, embodiments of the imaging agents may be used to stain lymph structures for detailed imaging of lymph architecture as well as serving as potential markers for tumor angiogenesis, tumor metastases, etc.
Owner:BAYLOR COLLEGE OF MEDICINE
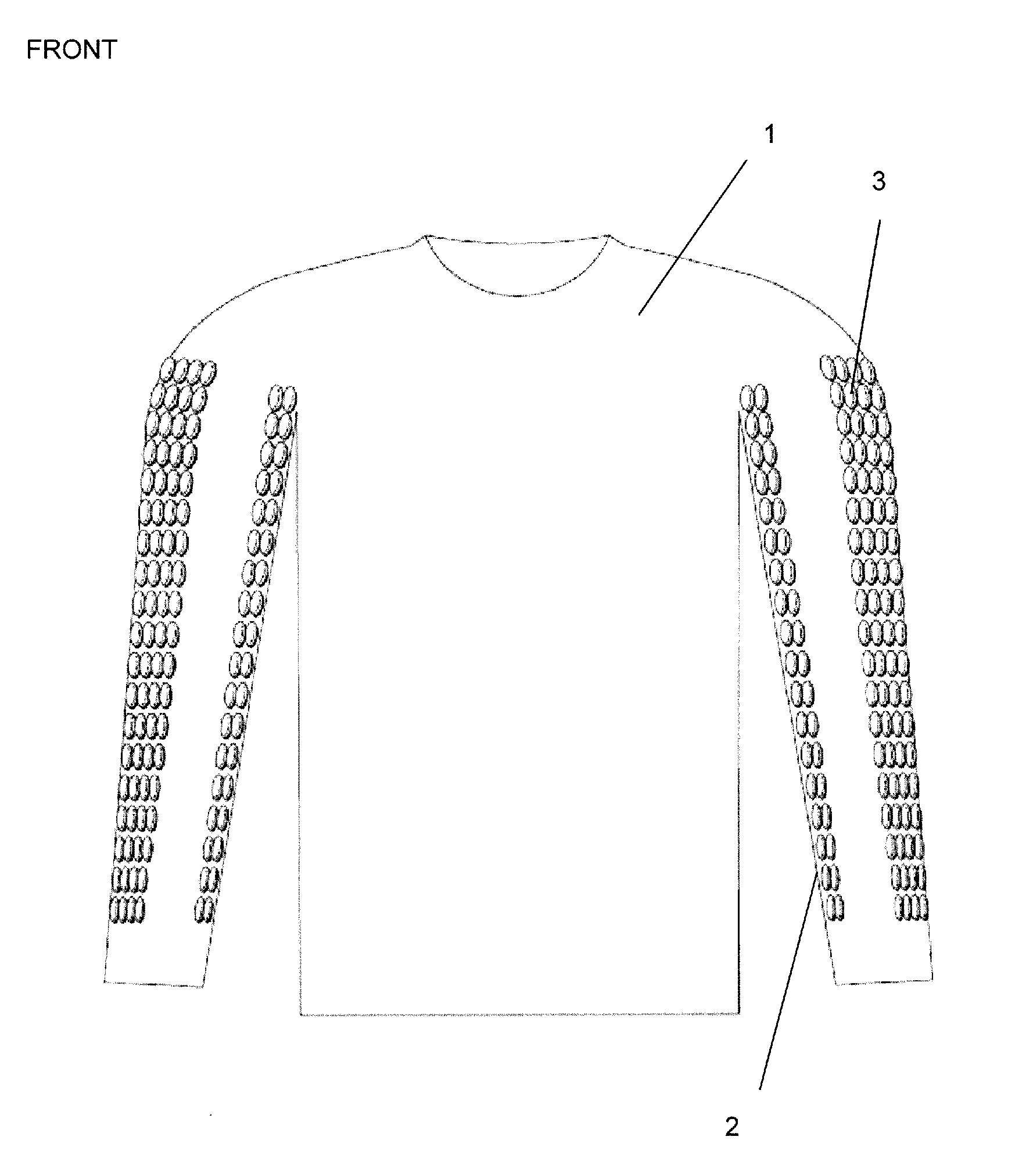
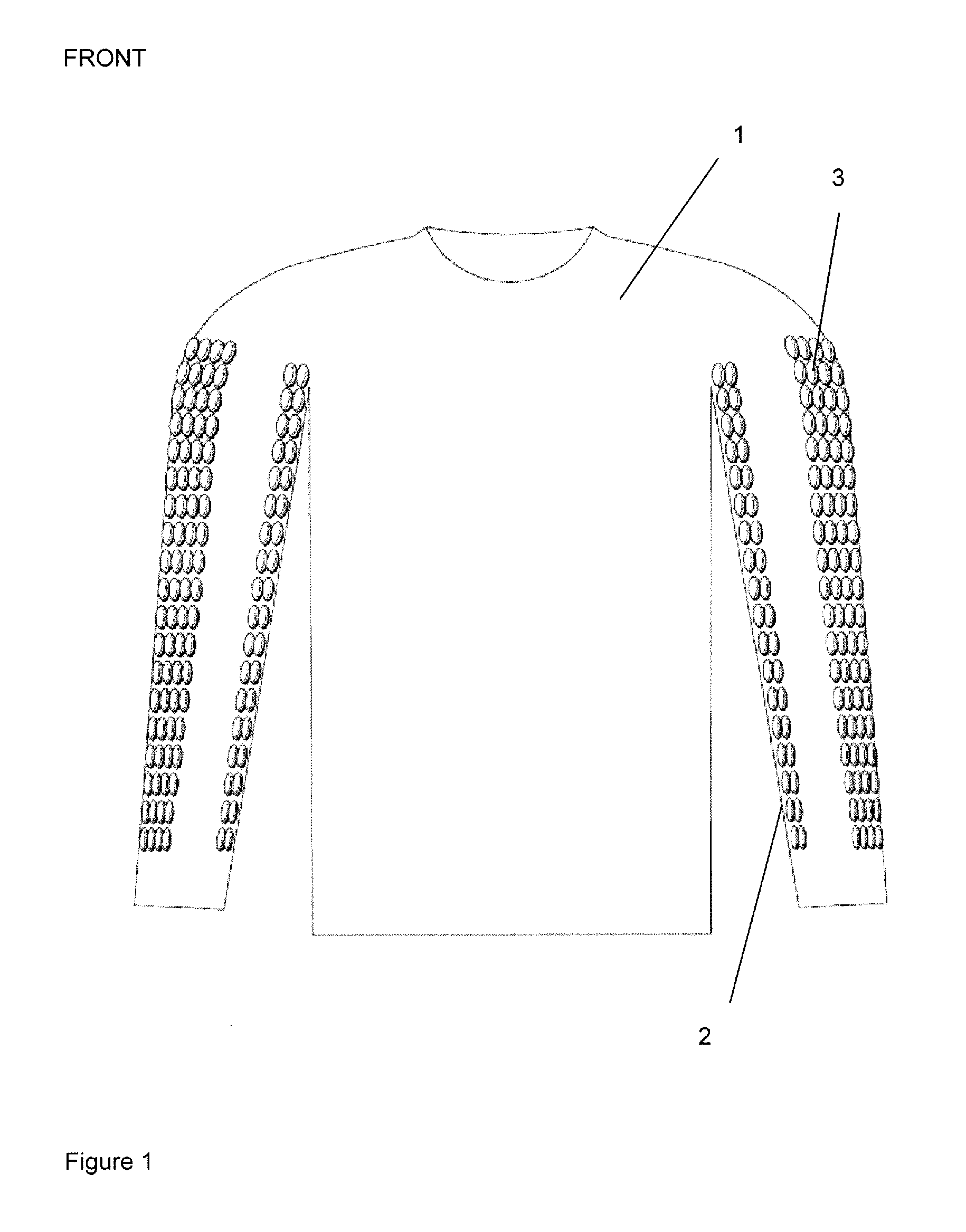
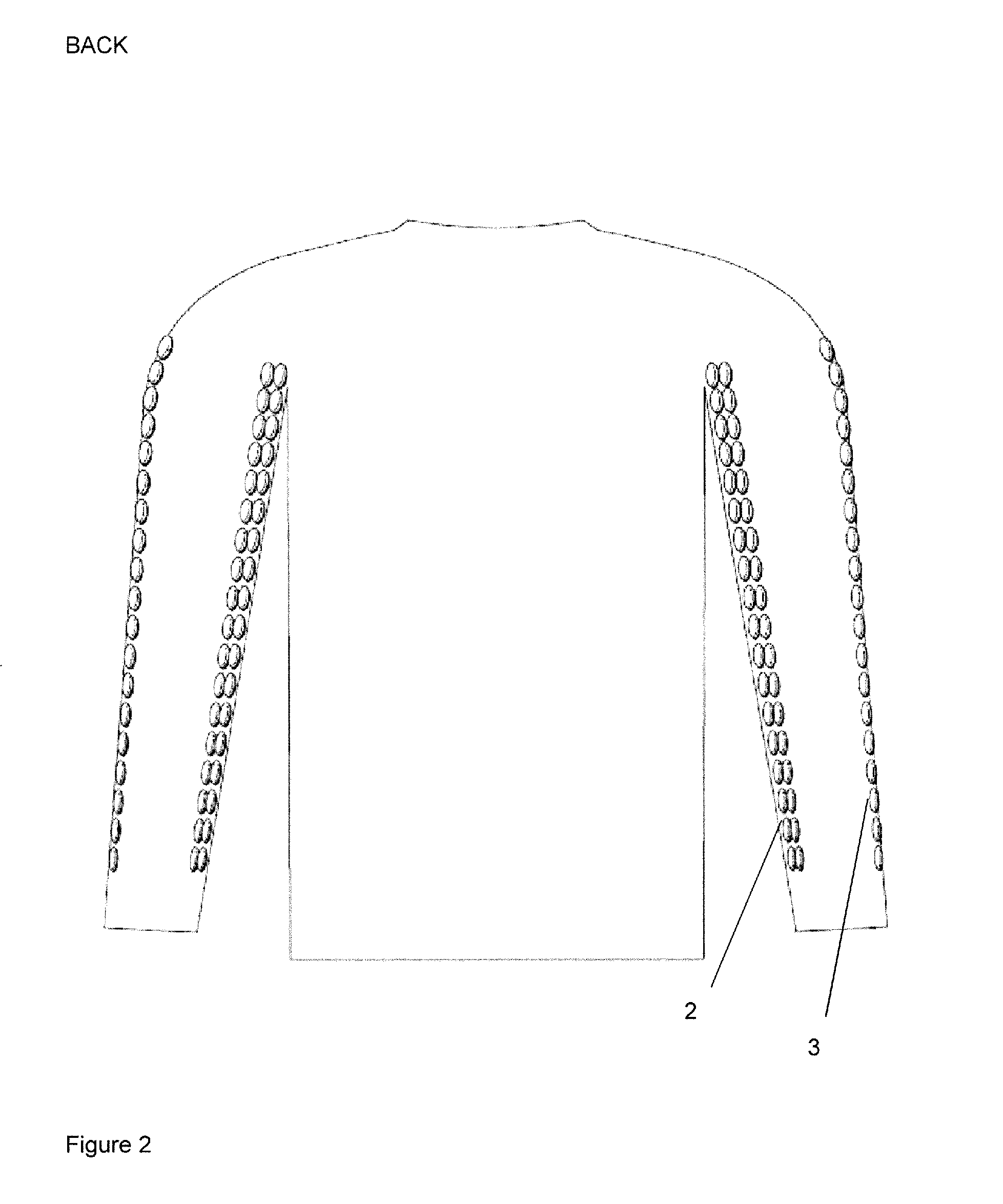
Anatomically targeted compression clothing
ActiveUS20150366735A1Reduce deliveryImprove skinGarment special featuresChiropractic devicesVeinDisease
An item of clothing is adapted to be worn against the skin, and comprises at least one panel adapted to provide targeted compression of at least 20% of the total length of a specific surface vein in the body, or adapted to provide targeted compression of at least 20% of a specific plexus of veins, a specific lymphatic plexus, drainage plexus or a collection of lymphatic vessels. The clothing is useful in a method of reducing recovery time in a human or other mammal, after a period of activity and in a method of enhancing performance, in particular sports performance, in a human or other mammal. It may also improve the conditioning of the skin and aid lymphatic drainage, and can be used in the treatment of certain medical conditions.
Owner:ANATOMIC FOCUS
Eye drop and preparation method thereof
ActiveCN101829048ASimple ingredientsReliable ingredientsSenses disorderPeptide/protein ingredientsLymphatic vesselFiltration
The invention discloses an eye drop and a preparation method thereof, relating to an eye drop. The invention provides an eye drop with short action time, high purity and relatively good effect and capability of simultaneously playing a role of corneal neovascularization, lymphangiogenesis and cornea inflammation resistance and a preparation method thereof. The eye drop contains the following components by volume percent: 0.1-5% of bovine serum, 5-15% of thickening agent, 1-5% of acid-base regulating solution, 0.5-2% of antibiotics, 5-20% of recombinant protein and the balance of balanced salt solution. The eye drop contains the balanced salt solution, the thickening agent, the bovine serum, the antibiotics, the acid-base regulating solution and the recombinant protein. The preparation method of the eye drop comprises the following steps of: adding the thickening agent, the bovine serum, the antibiotics and the recombinant protein to the balanced salt solution, regulating a pH value by using the acid-base regulating solution and osmotic pressure by using an osmotic pressure buffering agent, and removing bacteria through membrane filtration; or preparing the bovine serum to sterile micropowder, dissolving the bovine serum into the balanced salt solution, regulating the pH value, removing the bacteria through the membrane filtration, and dissolving recombined powder into the solution so as to obtain the eye drop.
Owner:XIAMEN UNIV
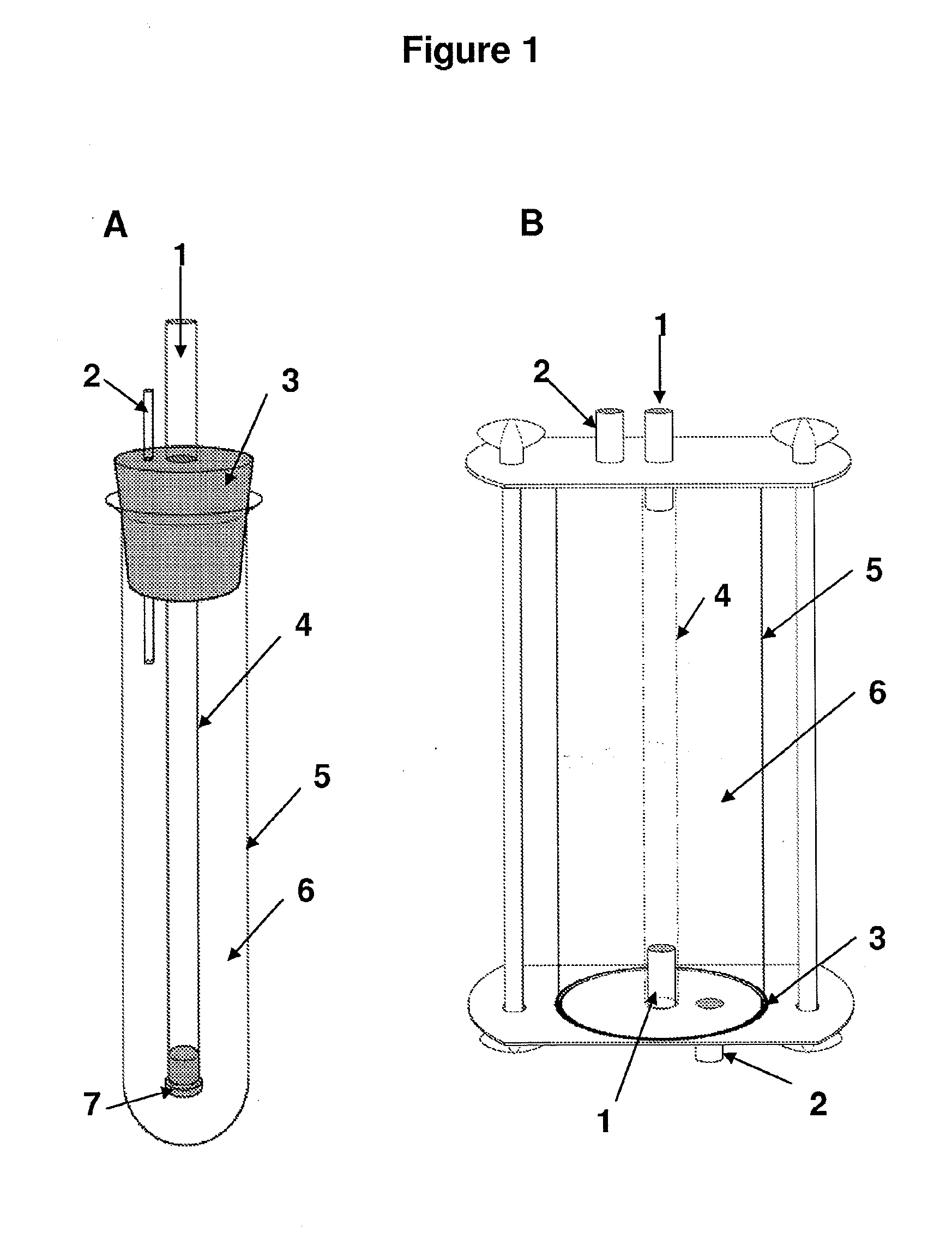
Preparation of hollow cellulose vessels
InactiveUS20100042197A1Good mechanical resistanceIncrease burst pressureEnvelopes/bags making machineryLayered productsHollow fibreLymphatic vessel
The present invention relates to an improved method for the preparation of hollow cellulose vessels produced by a microorganism, and hollow cellulose vessels prepared by this method. The method is characterized by the culturing of the cellulose-producing microorganisms being performed on the outer surface of a hollow carrier, and providing an oxygen containing gas on the inner side of the hollow carrier, the oxygen containing gas having an oxygen level higher than atmospheric oxygen. The hollow microbial cellulose vessels of the present invention are characterized by improved mechanical properties and can be used in surgical procedures to replace or repair an internal hollow organ such as the urethra, ureter, the trachea, a digestive tract, a lymphatic vessel or a blood vessel
Owner:ARTERION AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com