Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Corneal neovascularization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Corneal neovascularization (CNV) is the in-growth of new blood vessels from the pericorneal plexus into avascular corneal tissue as a result of oxygen deprivation. Maintaining avascularity of the corneal stroma is an important aspect of corneal pathophysiology as it is required for corneal transparency and optimal vision. A decrease in corneal transparency causes visual acuity deterioration. Corneal tissue is avascular in nature and the presence of vascularization, which can be deep or superficial, is always pathologically related.
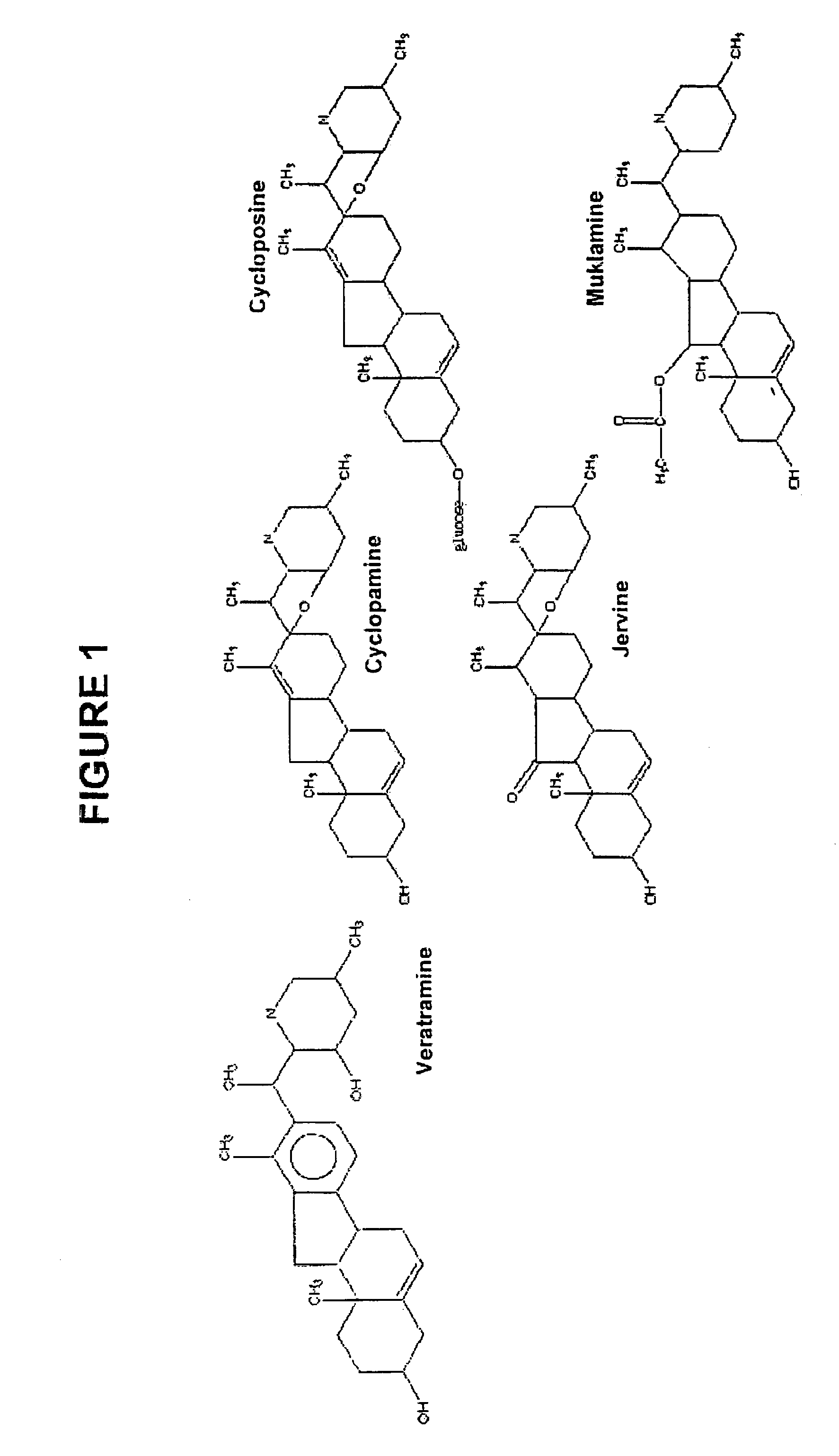
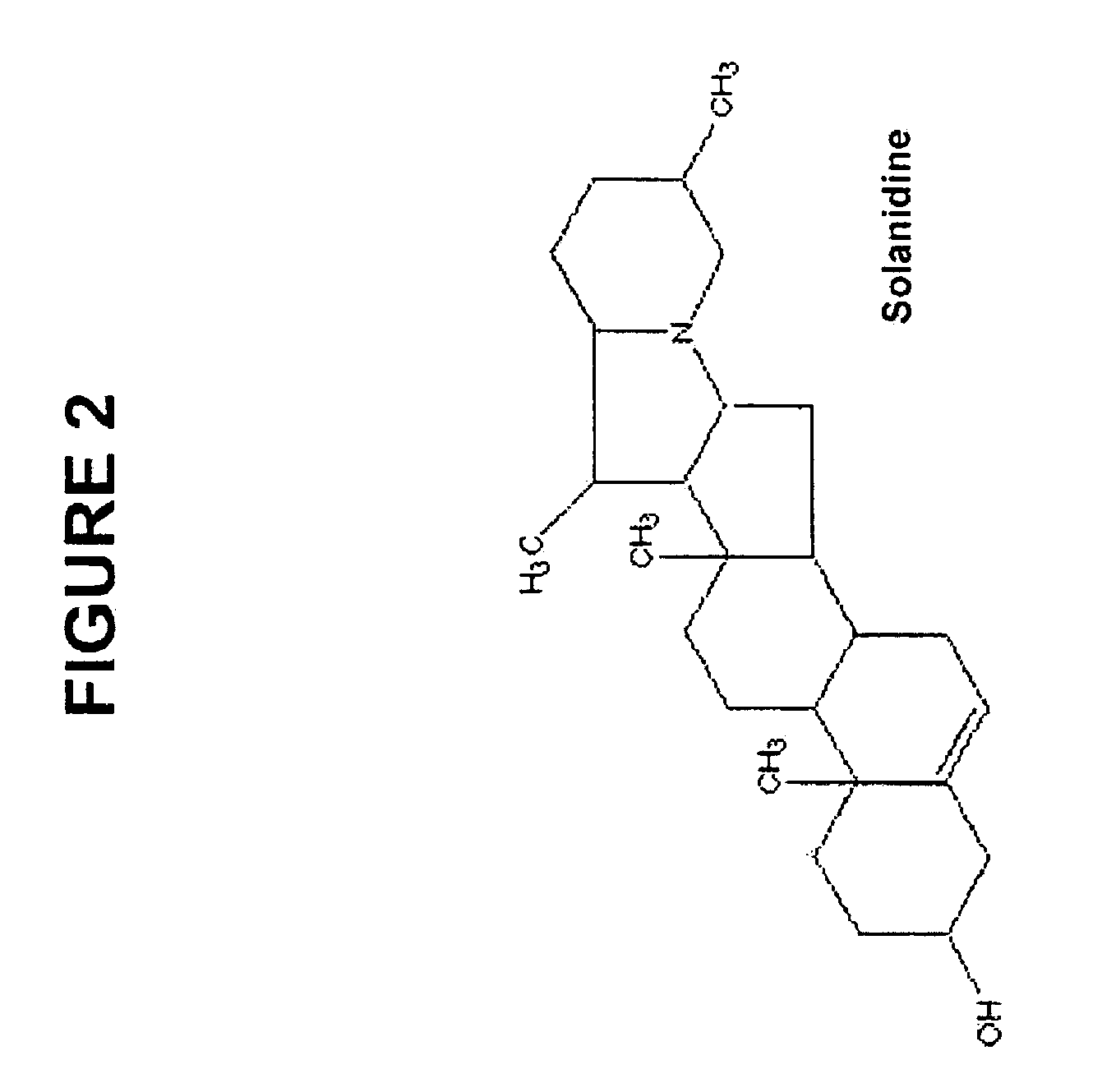
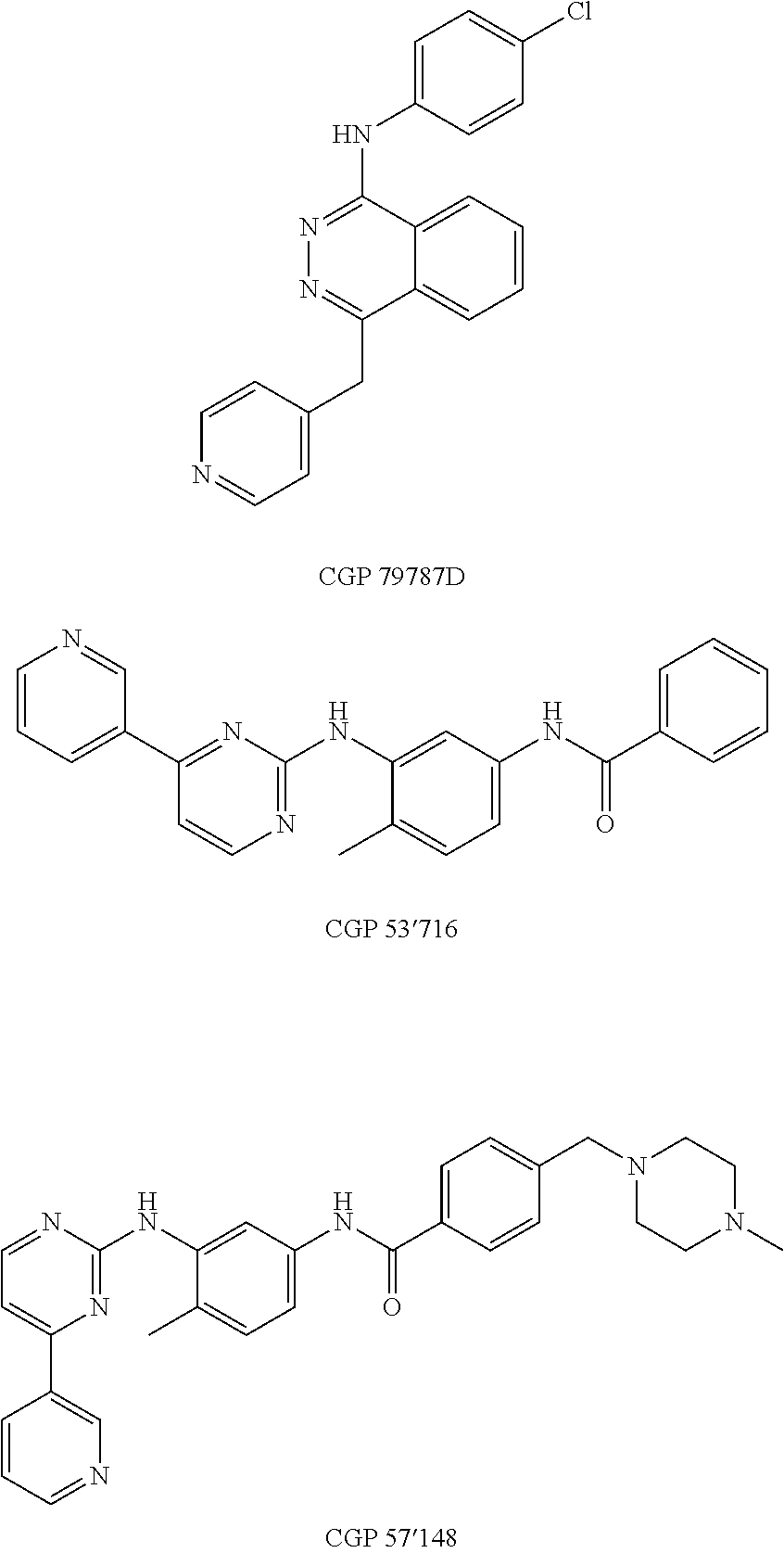
Use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and/or reversing ocular diseases related with ocular neovascularization
InactiveUS7517870B2High unmet needControl rateBiocideOrganic active ingredientsDiabetic retinopathyHedgehog signaling pathway
The present invention concerns the use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and / or reversing ocular diseases related with ocular neovascularization. Particularly, the above-mentioned diseases are (wet) age-related macular degeneration, (proliferative) diabetic retinopathy, neovascular glaucoma, retinal vein occlusion, or retinopathy of prematurity (ROP).
Owner:FOND AZIONE TELETHON
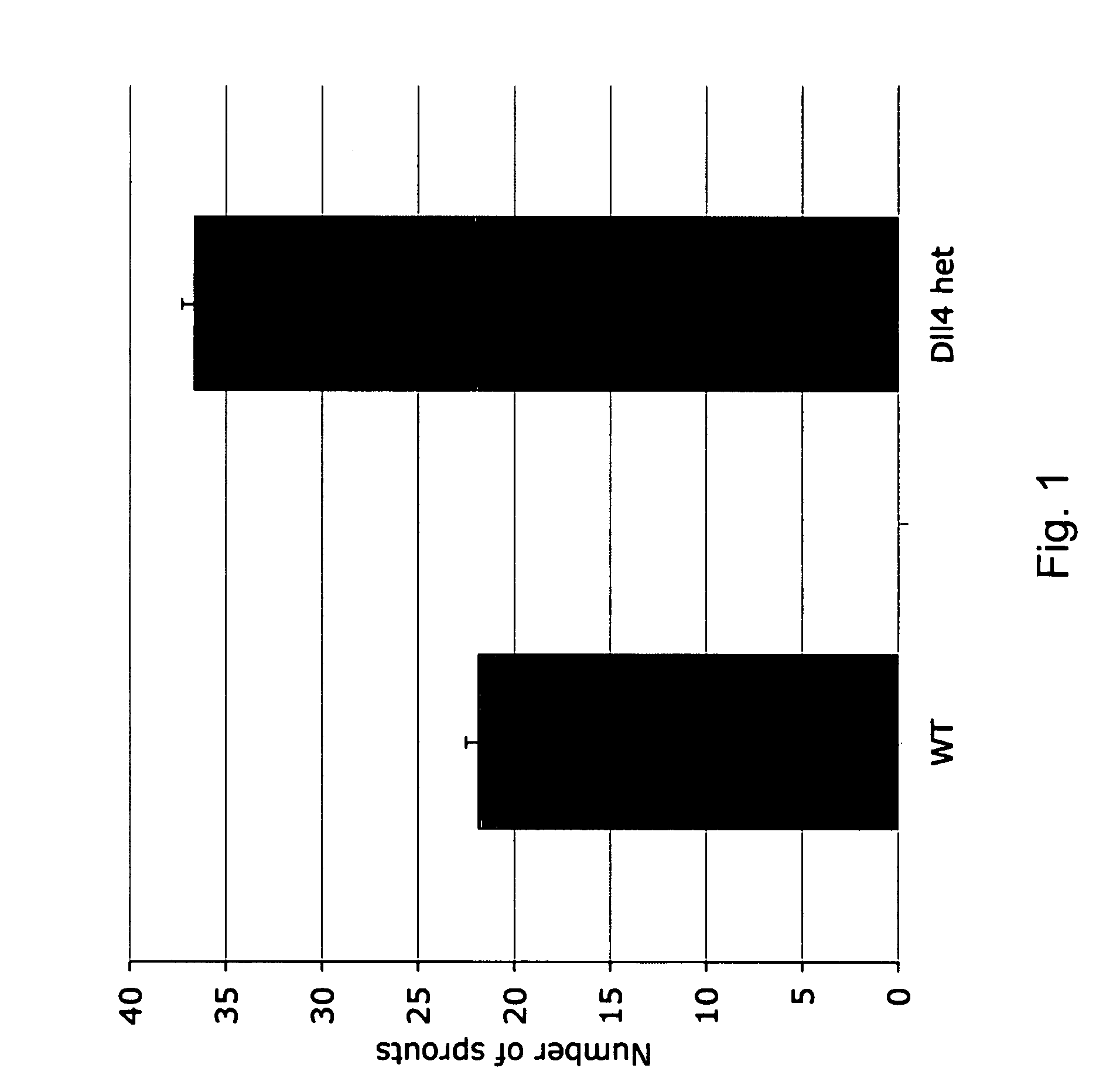
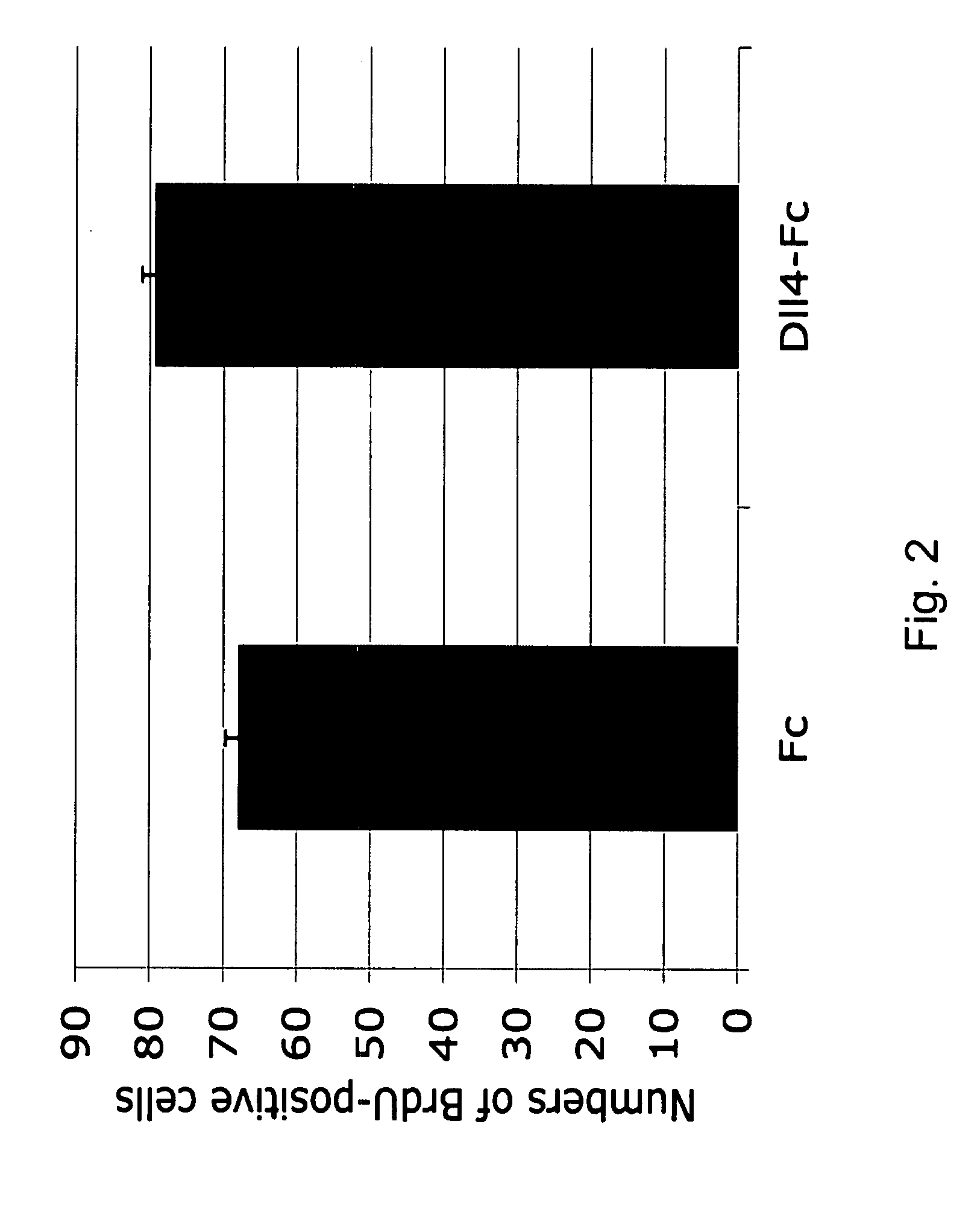
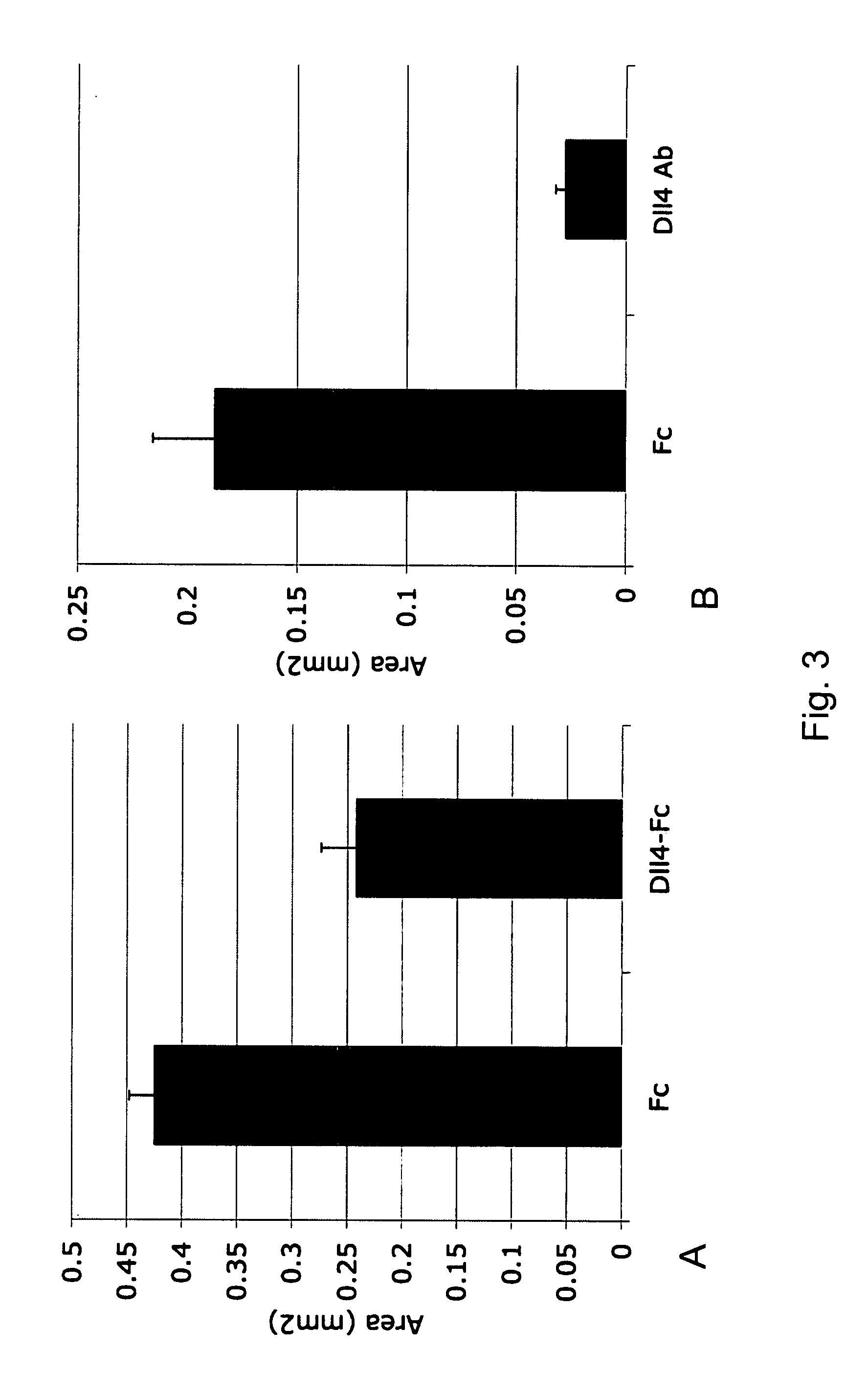
Therapeutic methods for treating vascular eye disorders with DII4 antagonists
InactiveUS20080181893A1Inhibition of developmentEnhanced regrowthSenses disorderPeptide/protein ingredientsDiabetic retinopathyIschemic retinopathy
A therapeutic method for treating ischemic or vascular disorders by administering an agent capable of inhibiting human delta-like ligand 4 (Dll4) activity to a subject in need thereof. In one embodiment, the agent is an anti-Dll4 antibody or antibody fragment capable of inhibiting the binding of Dll4 to a Notch receptor. The method of the invention is useful for treating eye disorders such as ischemic retinopathy, diabetic retinopathy, age related macular degeneration, corneal neovascularization, neovascular glaucoma, or retinopathy of prematurity. The method is also useful or treating ischemic or vascular disorders such as ischemic injury, cerebral ischemia, cardiac ischemia, ischemic conditions affecting the limbs and other organs or tissues, arteriovenous malformations, wound healing, organ or tissue transplantation, placental insufficiency, arterial narrowing and occlusion, atherosclerosis, and systemic or pulmonary hypertension.
Owner:REGENERON PHARM INC
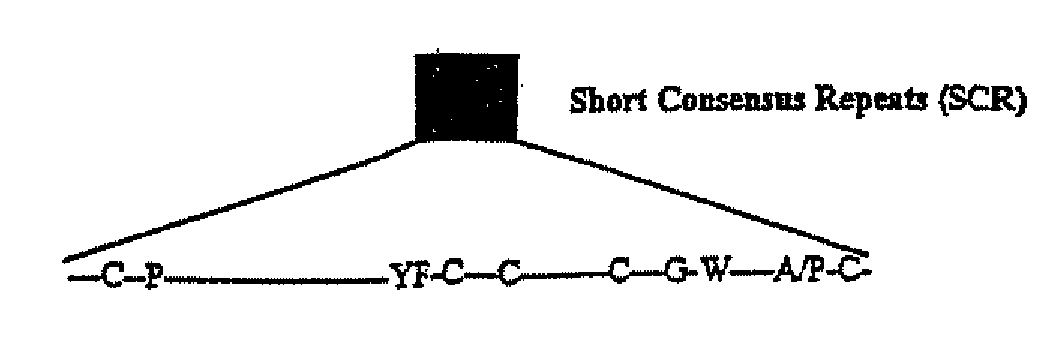
Viral Complement Control Proteins for Eye Disorders
InactiveUS20080075755A1Improve in vivo stabilityLow failure rateBiocideSenses disorderComplement control proteinOcular inflammation
The present invention provides compositions and methods for treating and / or preventing age related macular degeneration and other conditions involving macular degeneration or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the compositions comprise a poxvirus complement control protein or a complement binding fragment or variant thereof. Other compositions comprise a poxvirus complement control protein linked to a moiety that binds to a component present on or at the surface of cell or noncellular molecular entity, e.g., a component present in the eye of a subject at risk of or suffering from age related macular degeneration or a related condition or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the methods comprise administering a poxvirus complement control protein or complement binding fragment or variant thereof to a subject.
Owner:POTENTIA PHARMA INC
Oxygenated ophthalmic composition
The present invention relates to oxygenated ophthalmic topical compositions and methods of administering oxygenated ophthalmic topical compositions. The present invention also encompasses methods for making and administering the oxygenated ophthalmic topical compositions for use in treating or ameliorating pathologic conditions, complications, diseases and symptoms related to hypoxia of the anterior eye structure, including corneal neovascularization (CNV), edema, abrasions, infections, infiltrates, dry eye syndrome, contact lens intolerance; and redness, blurred vision, discomfort, foreign body sensation, photophobia, and irritation.
Owner:CHYNN EMIL W +1
Angiogenic agents from plant extracts, gallic acid, and derivatives
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Use of a VEGF antagonist to treat angiogenic eye disorders
ActiveUS9254338B2Good treatment effectSenses disorderPeptide/protein ingredientsVein occlusionPharmacology
The present invention provides methods for treating angiogenic eye disorders by sequentially administering multiple doses of a VEGF antagonist to a patient. The methods of the present invention include the administration of multiple doses of a VEGF antagonist to a patient at a frequency of once every 8 or more weeks. The methods of the present invention are useful for the treatment of angiogenic eye disorders such as age related macular degeneration, diabetic retinopathy, diabetic macular edema, central retinal vein occlusion, branch retinal vein occlusion, and corneal neovascularization.
Owner:REGENERON PHARM INC
Eye drop and preparation method thereof
ActiveCN101829048ASimple ingredientsReliable ingredientsSenses disorderPeptide/protein ingredientsLymphatic vesselFiltration
The invention discloses an eye drop and a preparation method thereof, relating to an eye drop. The invention provides an eye drop with short action time, high purity and relatively good effect and capability of simultaneously playing a role of corneal neovascularization, lymphangiogenesis and cornea inflammation resistance and a preparation method thereof. The eye drop contains the following components by volume percent: 0.1-5% of bovine serum, 5-15% of thickening agent, 1-5% of acid-base regulating solution, 0.5-2% of antibiotics, 5-20% of recombinant protein and the balance of balanced salt solution. The eye drop contains the balanced salt solution, the thickening agent, the bovine serum, the antibiotics, the acid-base regulating solution and the recombinant protein. The preparation method of the eye drop comprises the following steps of: adding the thickening agent, the bovine serum, the antibiotics and the recombinant protein to the balanced salt solution, regulating a pH value by using the acid-base regulating solution and osmotic pressure by using an osmotic pressure buffering agent, and removing bacteria through membrane filtration; or preparing the bovine serum to sterile micropowder, dissolving the bovine serum into the balanced salt solution, regulating the pH value, removing the bacteria through the membrane filtration, and dissolving recombined powder into the solution so as to obtain the eye drop.
Owner:XIAMEN UNIV
Inhibition of choroidal neovascularization
InactiveUS20120301439A1Promotes cholesterol effluxReduce activationBiocideSenses disorderDiabetic retinopathyWhole body
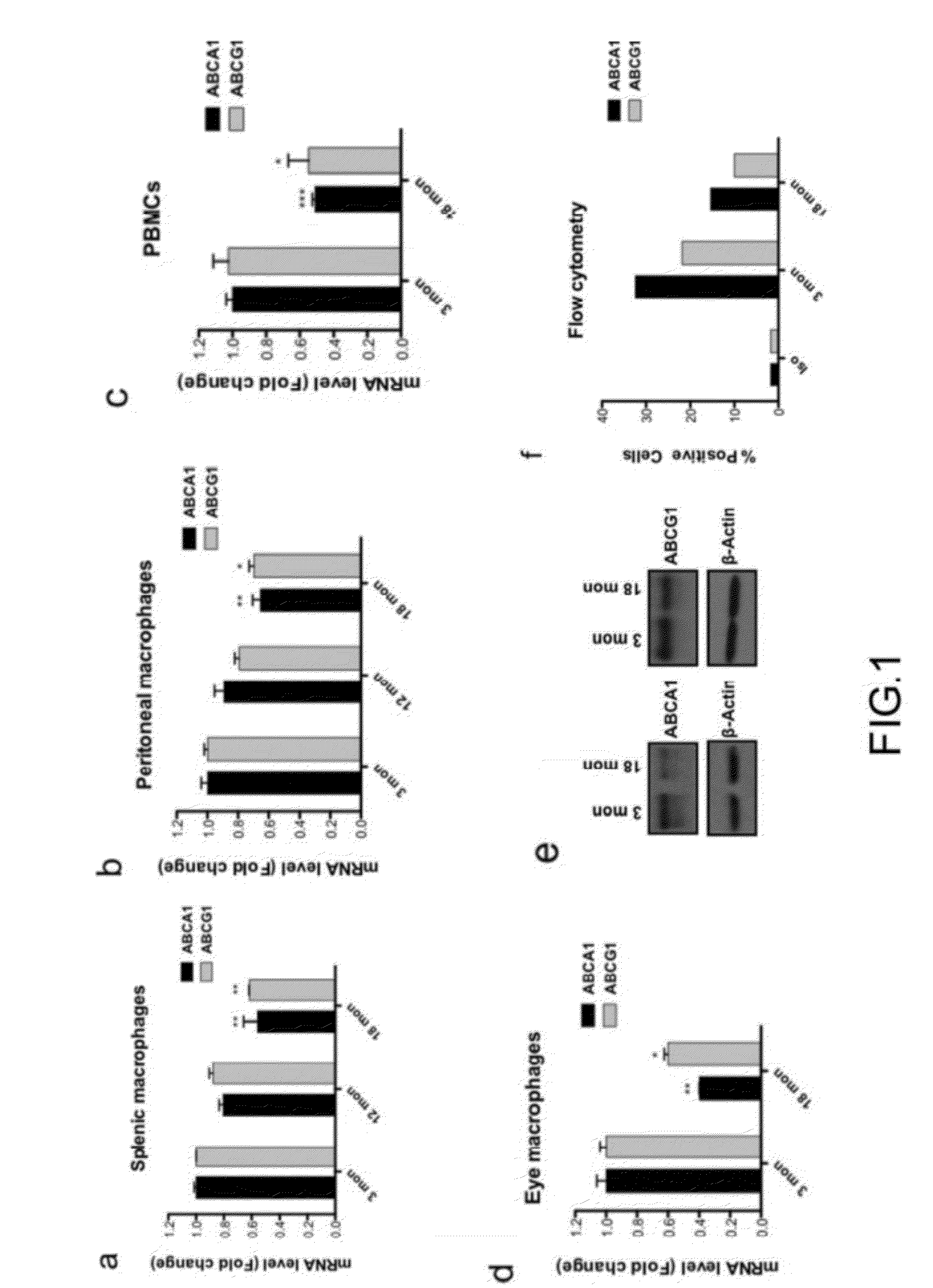
Methods of treatment of diseases that include or are characterized by inappropriate or pathological neovascularization are disclosed. These diseases include diseases of the eye, such as diabetic retinopathy, retinopathy of prematurity, and choroidal neovascularization which can occur in age-related macular degeneration (AMD). Disclosed methods include administering agents that cause directly or indirectly upregulation of the ABCA1 transporter protein in macrophages. These agents include, without limitation, LXR agonists. In some embodiments, inhibitors of CETP expression or activity can also be effective. Administration routes can include, without limitation, intraocular, periocular, or systemic administration.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Use of a VEGF antagonist to treat angiogenic eye disorders
ActiveUS20130295094A1Good treatment effectSenses disorderPeptide/protein ingredientsVein occlusionPharmacology
The present invention provides methods for treating angiogenic eye disorders by sequentially administering multiple doses of a VEGF antagonist to a patient. The methods of the present invention include the administration of multiple doses of a VEGF antagonist to a patient at a frequency of once every 8 or more weeks. The methods of the present invention are useful for the treatment of angiogenic eye disorders such as age related macular degeneration, diabetic retinopathy, diabetic macular edema, central retinal vein occlusion, branch retinal vein occlusion, and corneal neovascularization.
Owner:REGENERON PHARM INC
Eye drop with anti-cornea rebirth blood vessel function and preparation method thereof
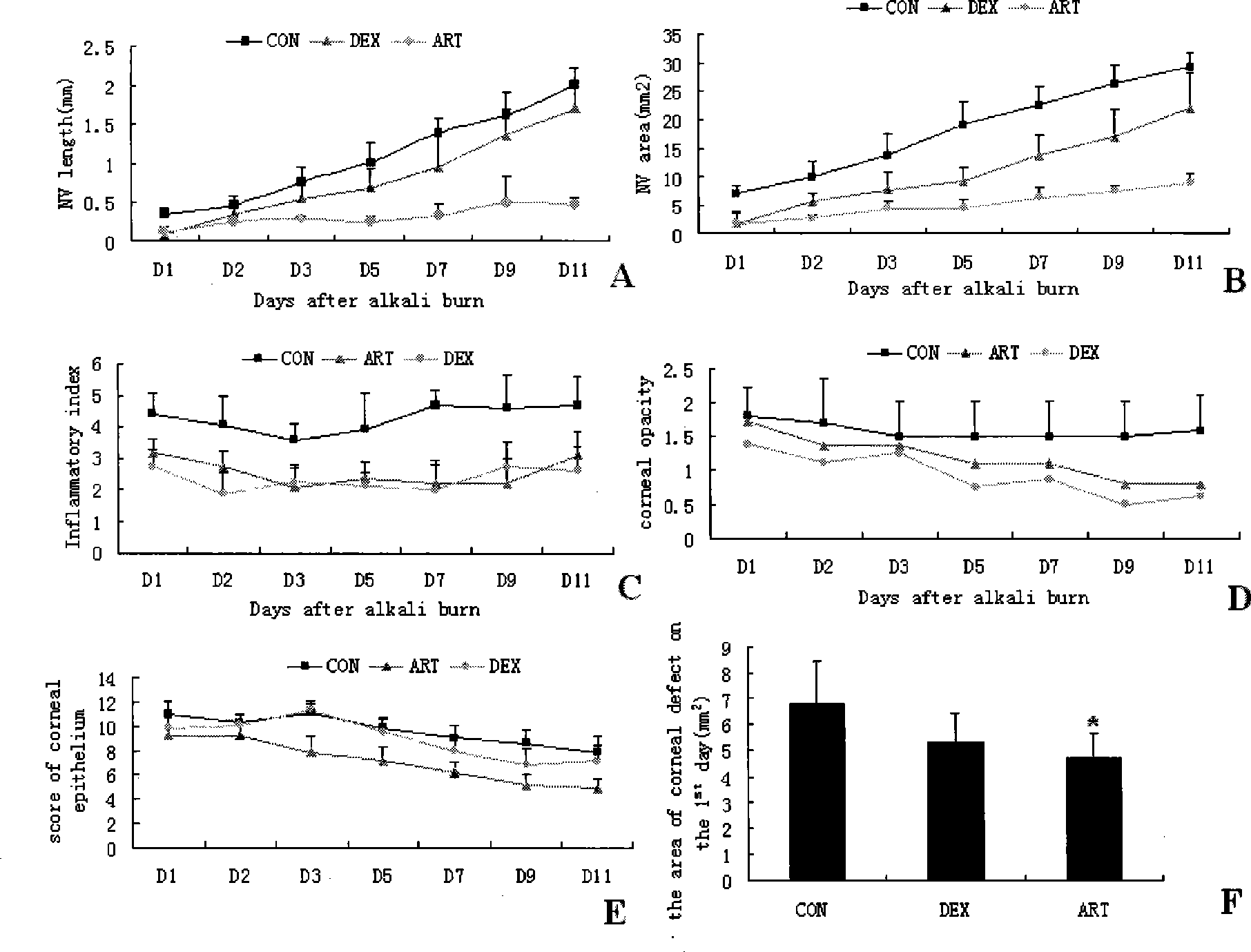
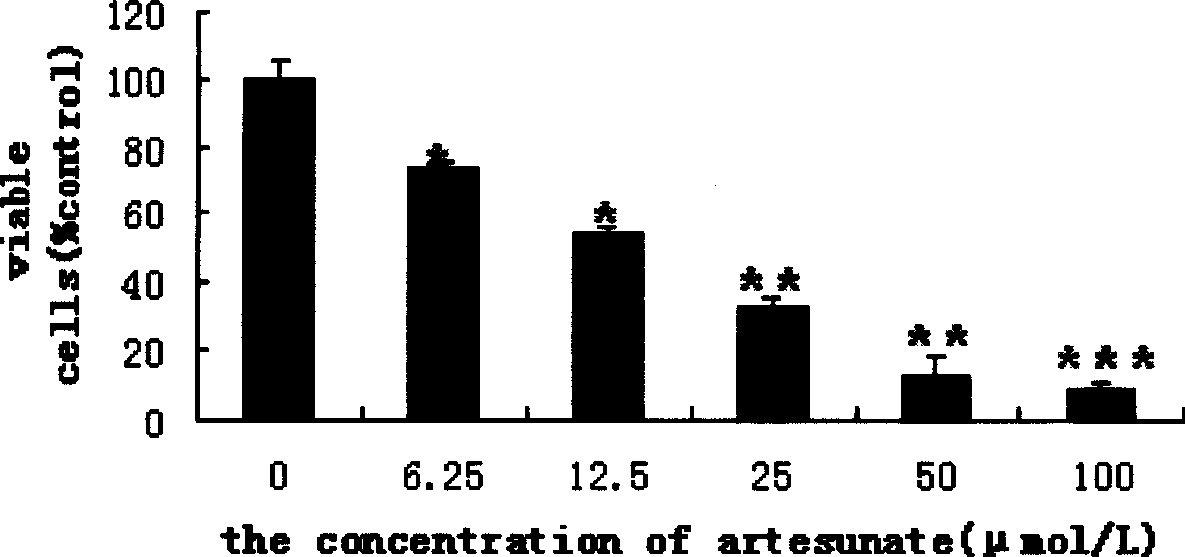
The invention relates to eye drops adopting arteannuinum succinate as the raw material for treating corneal neovascularization and a preparation method thereof. The arteannuinum succinate eye drops can be produced by adopting raw materials in the following parts by weight: 0.0096 to 0.0384 parts of arteannuinum succinate, 1.0032 to 12.028 parts of acid component in buffer solution, 0.106 to 18.0477 parts of alkaline component in buffer solution, 1.0 to 35.0 parts of thickener, 2.0 to 4.72 parts of isotonic reagent and 0.01 to 0.02 parts of antiseptics. The preparation method for the eye drops comprises the following steps: dissolving the arteannuinum succinate with NaHCO3, filtering and sterilizing for later use; dissolving appropriate amount of buffer solution, isotonic reagent and thickener into distilled water, sterilizing for 20 minutes with 100 to 120 DEG C high pressure to obtaining treatment fluid, and cooling the treatment fluid to room temperature for stand to by use; preparing the antiseptics with distilled water into high concentration storage solution, filtering, sterilizing and subpackaging for later use; in aseptic condition, adding the arteannuinum succinate into the treatment fluid according to the ratio of adding 16 to 66 Mul of arteannuinum succinate into 100 ml treatment fluid, uniformly blending, and at the same time, adding the antiseptics into the buffer solution according to the ratio of adding 50 to 100 Mul of antiseptics into 100 ml buffer solution, and subpackaging and producing eye drops in different specifications; preferably, the package per bottle is 0.5 ml.
Owner:SUN YAT SEN UNIV
Viral complement control proteins for eye disorders
InactiveUS8043609B2Improve in vivo stabilityLow failure rateBiocideSenses disorderComplement control proteinOcular inflammation
The present invention provides compositions and methods for treating and / or preventing age related macular degeneration and other conditions involving macular degeneration or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the compositions comprise a poxvirus complement control protein or a complement binding fragment or variant thereof. Other compositions comprise a poxvirus complement control protein linked to a moiety that binds to a component present on or at the surface of cell or noncellular molecular entity, e.g., a component present in the eye of a subject at risk of or suffering from age related macular degeneration or a related condition or choroidal neovascularization, ocular inflammation, or any combination of these. Certain of the methods comprise administering a poxvirus complement control protein or complement binding fragment or variant thereof to a subject.
Owner:POTENTIA PHARMA INC
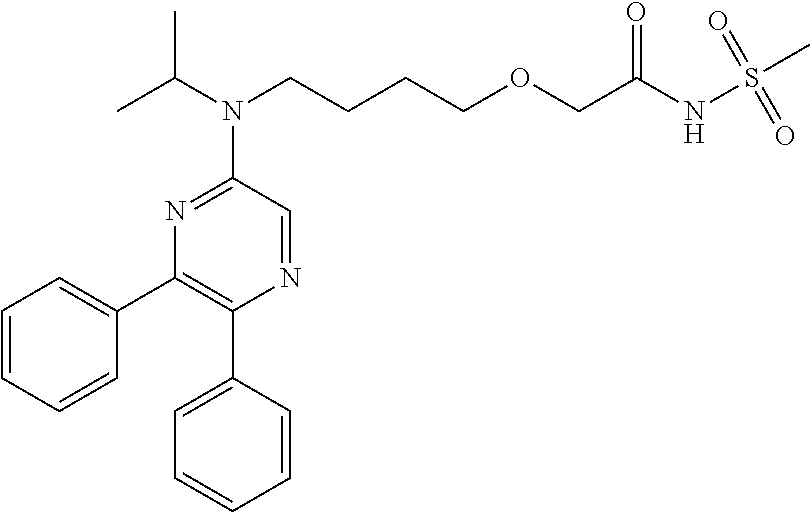
Sunitinib formulations and methods for use thereof in treatment of ocular disorders
InactiveUS20170273901A1Avoid nerve damageIncreasing encapsulation and incorporationOrganic active ingredientsSenses disorderDiseaseCytotoxicity
Methods for increasing the encapsulation or incorporation of Sunitinib into polymeric matrices have been developed. The resulting formulations provide for more sustained controlled release of sunitinib or its analog or a pharmaceutically acceptable salt thereof. Increased loading is achieved using an alkaline solvent system. The pharmaceutical compositions can be administered to treat or prevent a disease or disorder in or on the eye of a patient associated with vascularization, such as corneal neovascularization and acute macular degeneration. Upon administration, the sunitinib or its analog or salt is released over an extended period of time at concentrations which are high enough to produce therapeutic benefit, but low enough to avoid unacceptable levels of cytotoxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
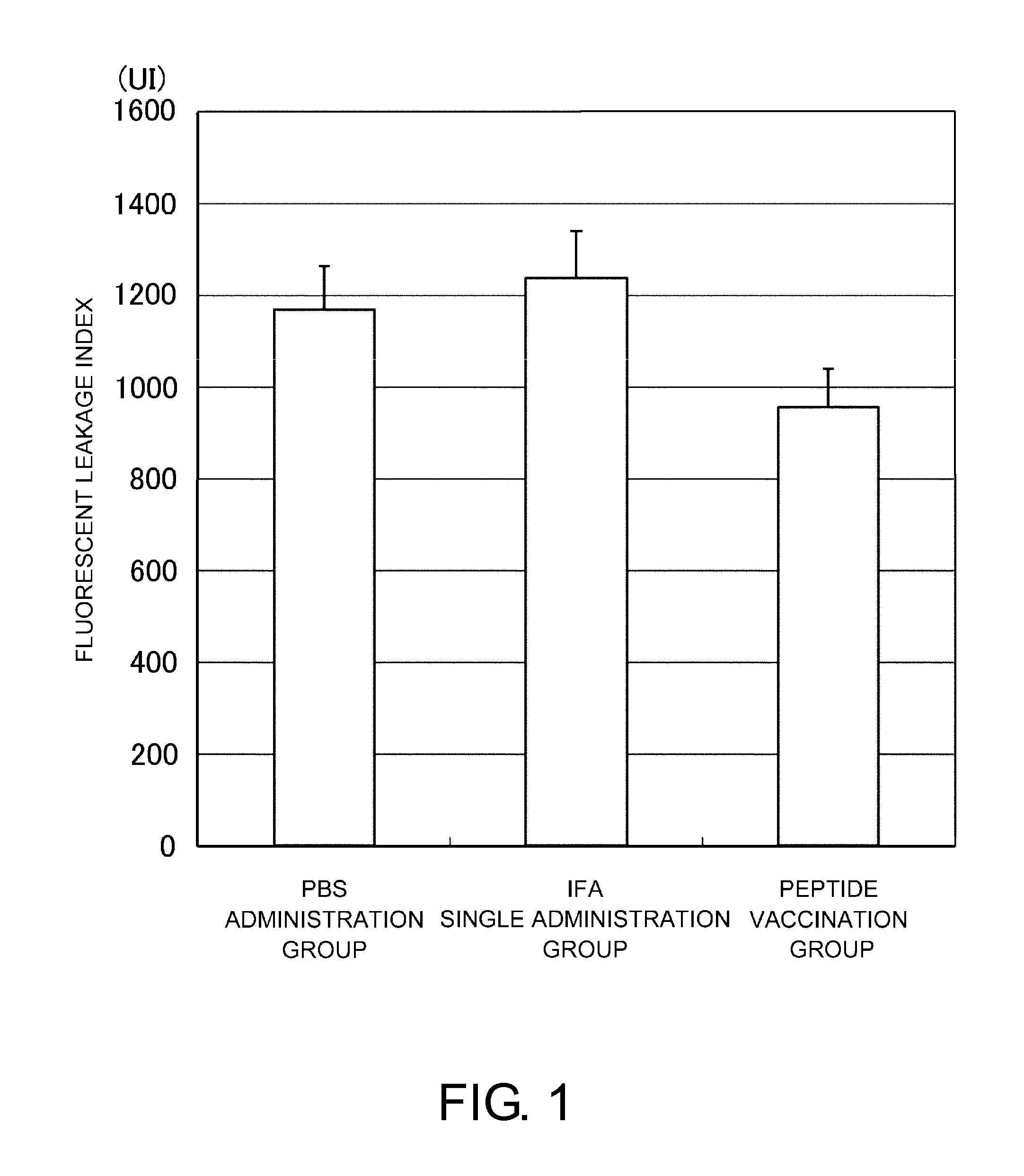
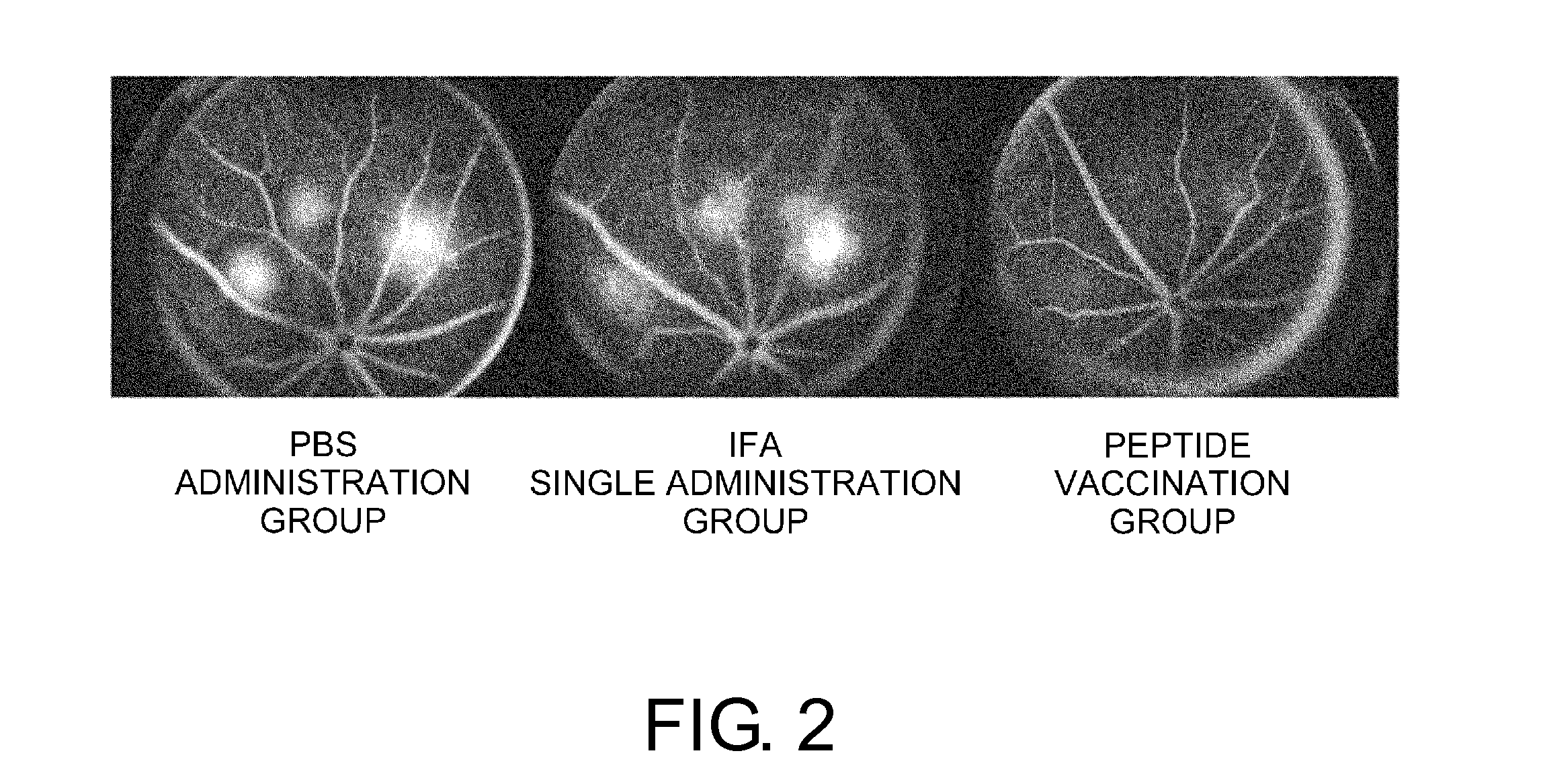
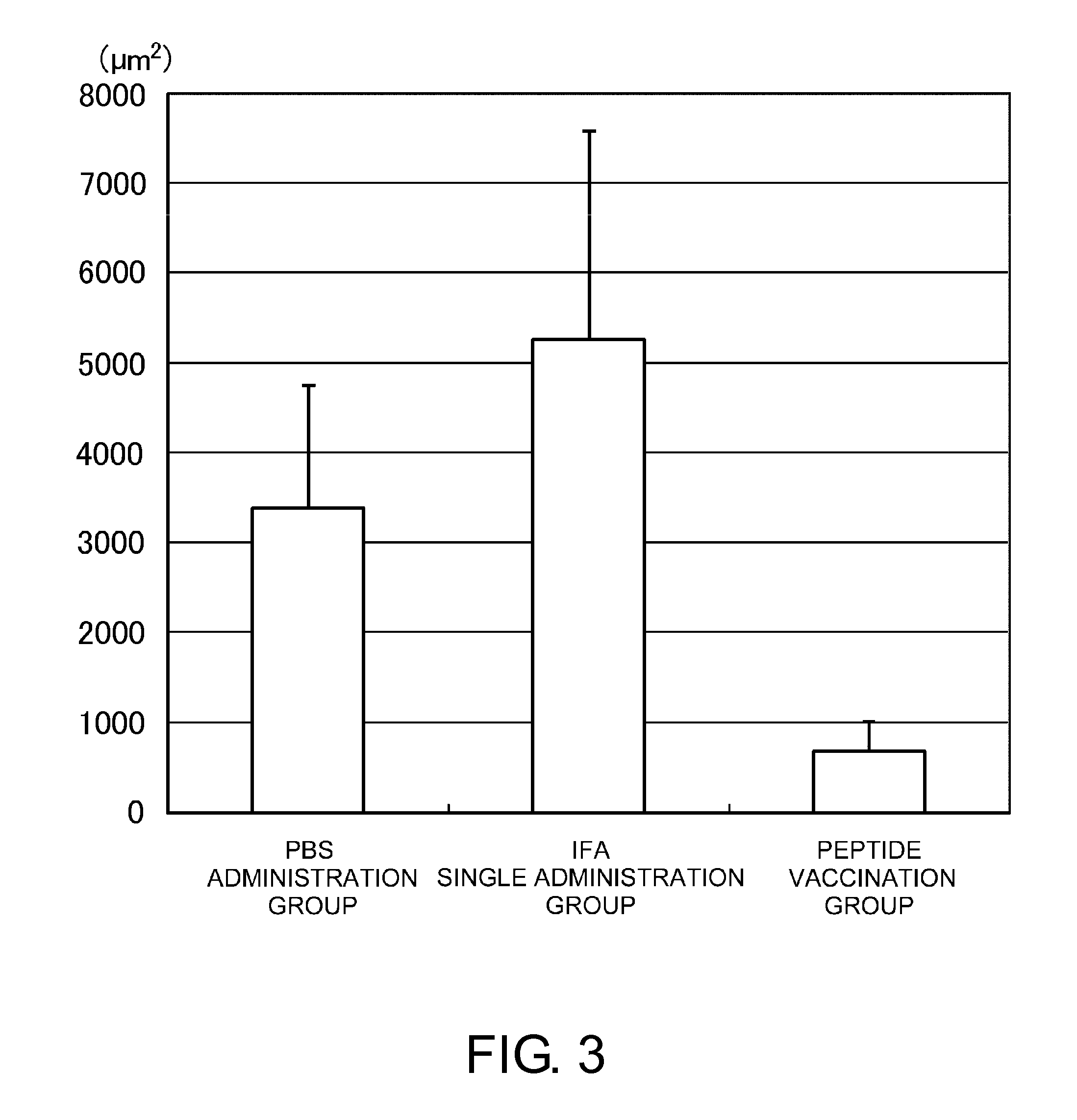
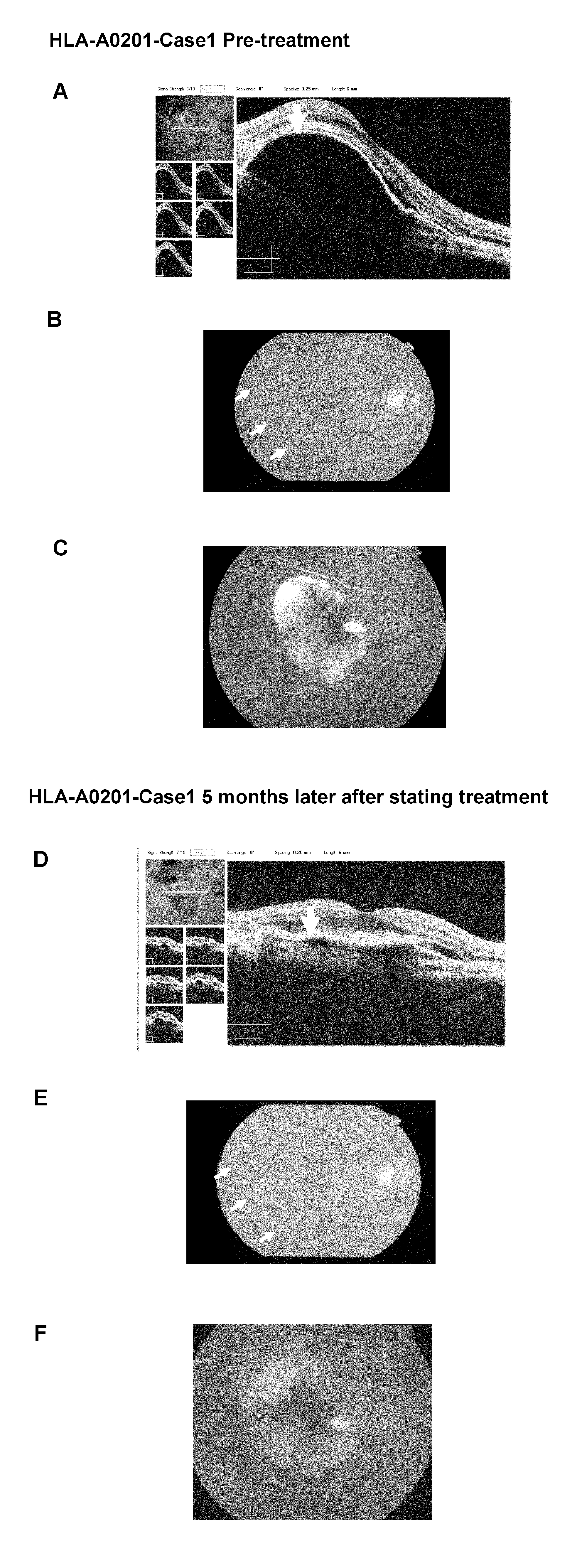
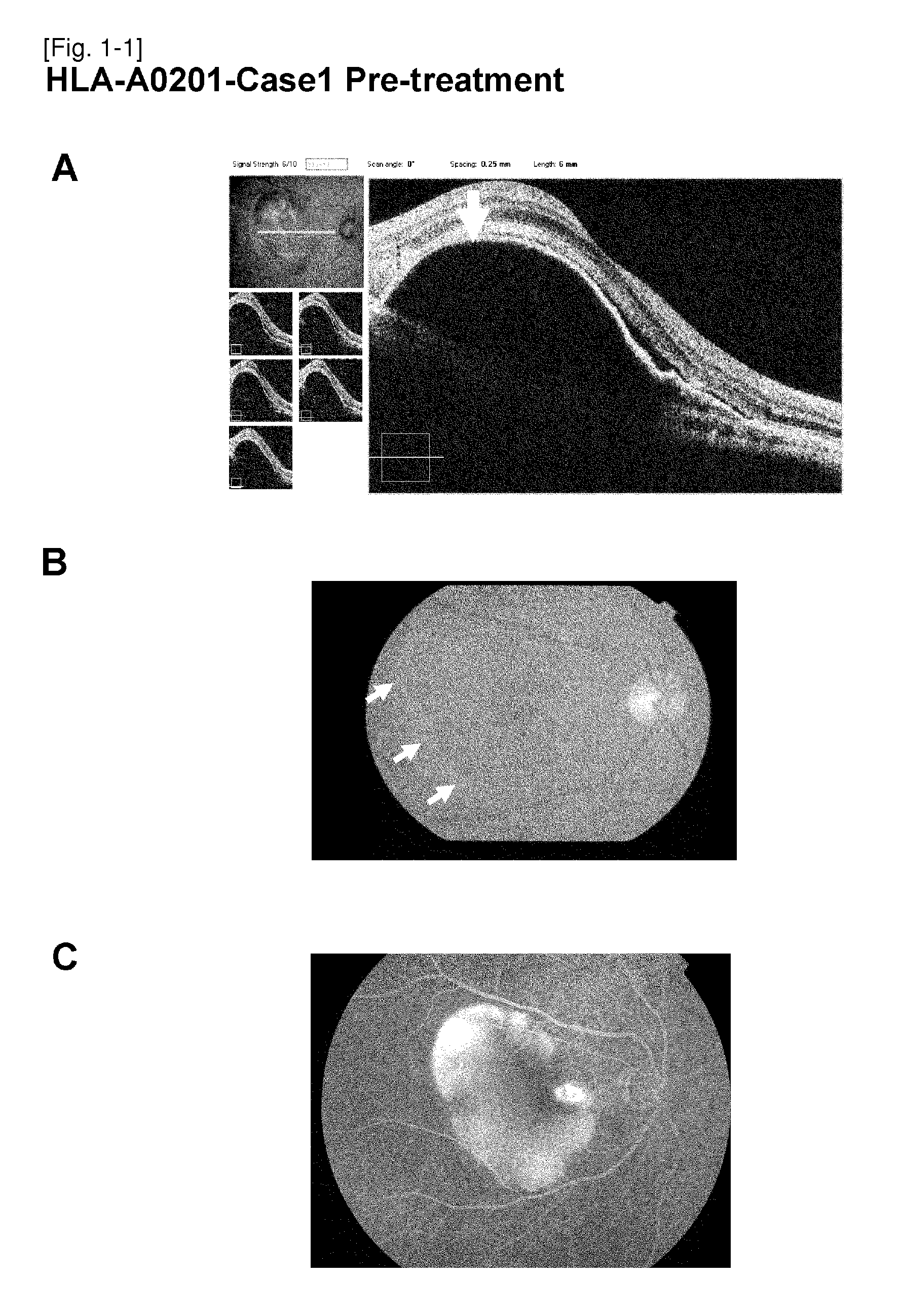
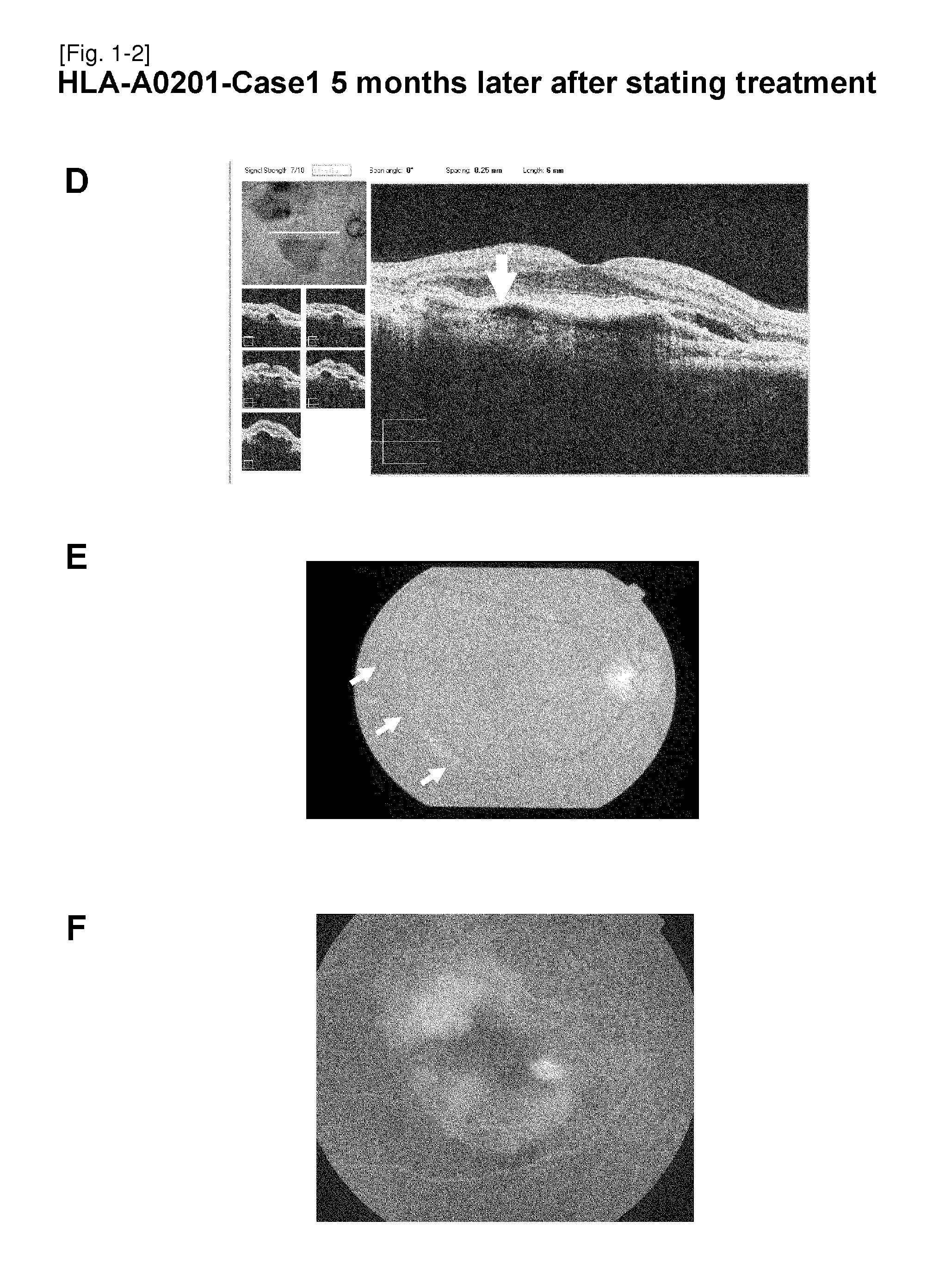
Vaccine therapy for choroidal neovascularization
The present inventors administered a peptide derived from VEGFR-2, which is known as one of the proteins involved in neovascularization, to model mice (A2 / Kb transgenic mice) expressing human HLA-A*0201, and tested whether or not the peptide has vaccine effect. As a result, the present inventors successfully discovered that vaccination using this peptide as an antigen is effective for inhibition of choroid neovascularization, and thereby completed the present invention. More specifically, the present invention provides vaccines for treatment and / or prevention of diseases caused by choroid neovascularization (neovascular maculopathy), which contain a VEGFR-2-derived peptide as an active ingredient.
Owner:ONCOTHERAPY SCI INC
Application of transthyretin to inhibition of ocular neovascularization
InactiveCN109481668AInhibitionInhibition of neogenesisAntibacterial agentsSenses disorderDiabetes mellitusOcular neovascularization
The invention discloses application of transthyretin (TTR) to the inhibition of ocular neovascularization and belongs to the field of biochemistry, molecular biology and medical science. The application finds out that mammal TTR is given and ocular new blood vessels in STZ (Streptozocin)-induced diabetes mellitus rat and mouse models are obviously reduced. An in-vivo experiment model of ocular neovascularization and brain neovascularization verifies that the TTR has the effect of inhibiting the neovascularization and a result shows that the TTR acts on ocular capillary endothelial cells and can be used for effectively inhibiting the formation of 90 percent of the blood vessels; and when the TTR acts in the rat and mouse models, high up to 90% of neovascularization can be effectively inhibited.
Owner:SHANGHAI CUTSEQ BIOMEDICAL TECH CO LTD
Application of celastrol to preparing eye drop preparation for inhibiting alkali burn corneal neovascularization and promoting corneal alkali burn healing
ActiveCN106265682ARealize the way of drug deliveryHigh drug loadingOrganic active ingredientsSenses disorderFiltrationSolvent
The invention belongs to the field of biomedical-material-and-medical crossover, and particularly discloses an application of celastrol to preparing an eye drop preparation for inhibiting alkali burn corneal neovascularization and promoting corneal alkali burn healing. 7.04 g-67.55 g of triblock copolymer PEG-PCL-PEi and 0.68 g-2.11 g of the celastrol are weighed and jointly dissolved into a mixed solvent prepared from methyl alcohol and acetonitrile in the volume ratio of 1:(1-3); under ultrasonic stirring, mixed liquid is dropwise added into dissolving equivalent amount of injection water, an organic solvent is removed in a vacuum-distillation mode, dissociative celastrol is removed in an ultra-filtration mode, a medicine loading polymeric micelle is obtained, injection water is additionally added till the volume is fixed to be 100 mL, and eye drop is obtained. According to the celastrol, the PEG-PCL-PEi firstly serves as a carrier, and loads the celastrol, the eye drop is prepared, the medicine feeding mode of eye-surface dropping is achieved, and the celastrol has the milepost-type significance.
Owner:河南省眼科研究所
Glucocorticoid-loaded nanoparticles for prevention of corneal allograft rejection and neovascularization
ActiveUS20170157147A1Severe side effectEasy to manageOrganic active ingredientsSenses disorderGlucocorticoidTransplant rejection
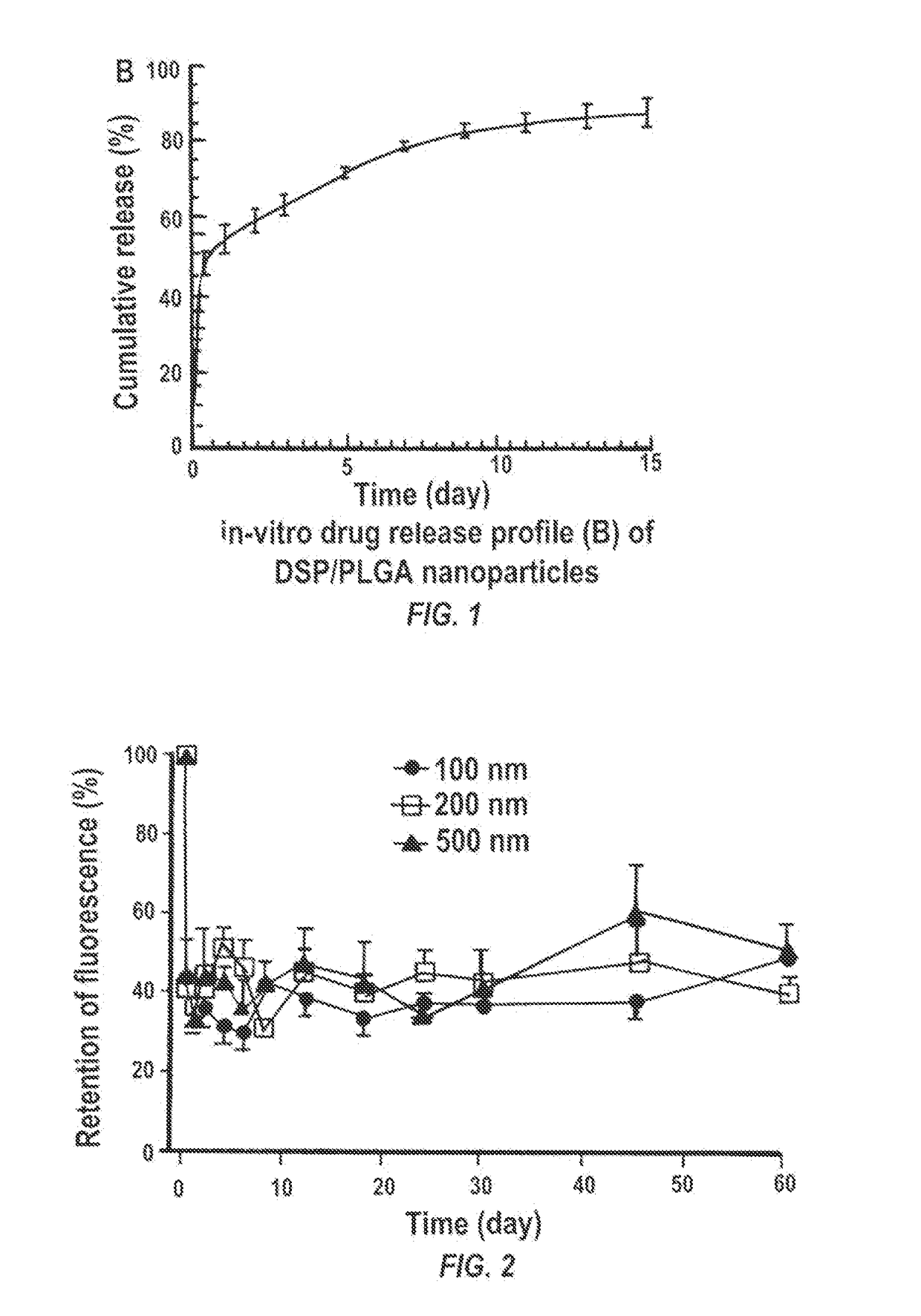
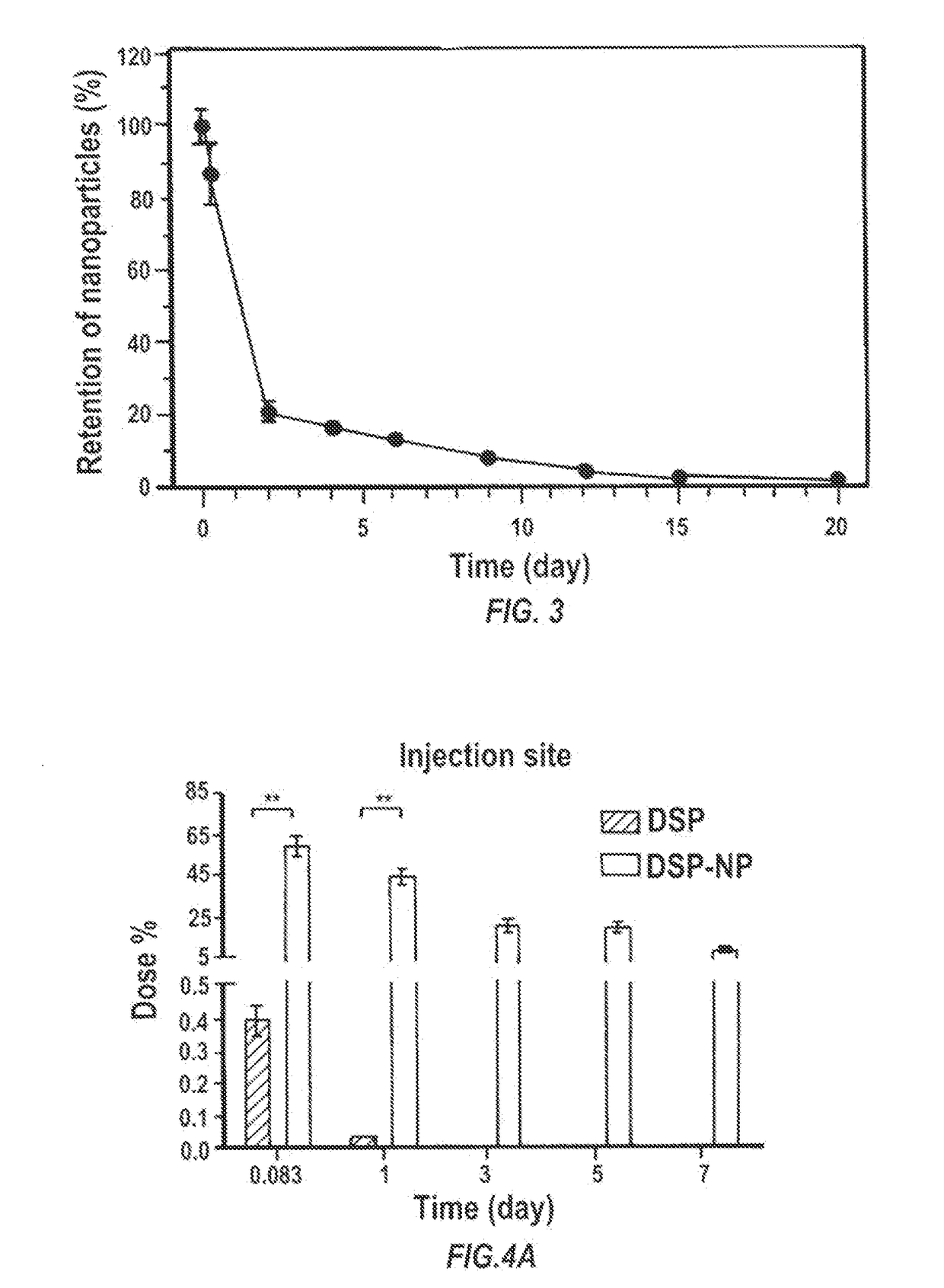
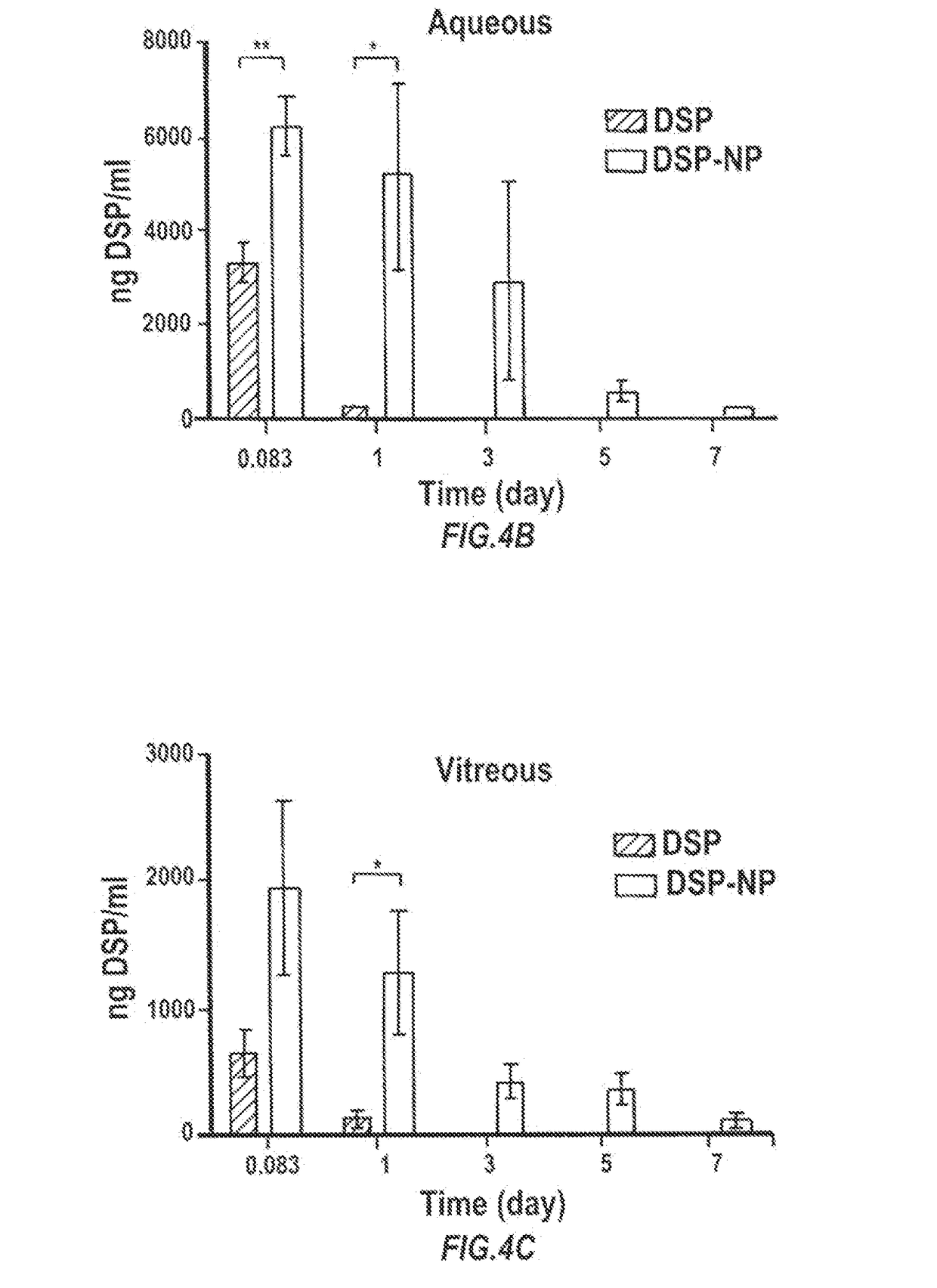
Particles encapsulating a glucocorticoid such as dexamethasone sodium phosphate (DSP) into a matrix such as biodegradable poly(lactic-coglycolic acid) (PLGA) which is densely coated with hydrophilic polymer such as PEG or PLURONIC® F127, exhibit sustained release of DSP for up to 7 days in vitro. These nanoparticles can be used to prevent corneal graft rejection or corneal neovascularization.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for treating neovascularization
The present invention describes an improved photodynamic treatment to treat subfoveal choroidal neovascularization (CNV).
Owner:BAUSCH LOMB IRELAND LTD
Inhibition of neovascularization by inhibition of prostanoid IP receptors
ActiveUS20140275238A1Reduce neovascularizationBiocideSenses disorderMedicineCorneal neovascularization
There are provided inter alia methods and compounds useful for decreasing choroidal neovascularization in a subject in need thereof.
Owner:ALLERGAN INC
VEGF (Vascular Endothelial Growth Factor) resistant antibody and application thereof
InactiveCN103739710AImprove bindingImprove biological activityImmunoglobulins against growth factorsAntibody ingredientsAntibody affinityHumanized antibody
The invention discloses a VEGF (Vascular Endothelial Growth Factor) resistant antibody belonging to the fields of biotechnology and immunology and application of the VEGF resistant antibody. Amino acid sequences of three complementary determining regions (CDR) of heavy chain variable regions of the antibody are respectively shown as SED ID NO. 1, SEQ ID NO. 2 and SEQ ID NO. 3; the amino acid sequences of the three complementary determining regions of light chain variable regions of the antibody are respectively shown as SEQ ID NO. 4, SEQ ID NO. 5 and SEQ ID NO. 6. According to the VEGF resistant humanized antibody, specific targeting combines with VEGF antigens, and an antibody affinity is 3.11*10<-10>, the binding capacity of the antibody and the antigens is high; the VEGF resistant humanized antibody has a good biological activity and can inhibit cell proliferation of vascular endothelial cells, obviously inhibit tumor growth, and effectively inhibit the growth of corneal neovascularization, and can be used for preparing targeted medicines of specific targeting VEGF antigens or angiogenesis inhibiting medicines.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Vaccine therapy for choroidal neovascularization
The present invention provides novel pharmaceutical agents and methods for treating or preventing diseases caused by neovascularization in human choroid (neovascular maculopathy). The present invention provides pharmaceutical compositions and vaccines for treating and / or preventing diseases caused by neovascularization in human choroid (neovascular maculopathy), comprising at least one type each of a peptide comprising an amino acid sequence derived from a VEGFR-1 protein and having an activity of inducing cytotoxic T cells, and a peptide comprising an amino acid sequence derived from a VEGFR-2 protein and having an activity of inducing cytotoxic T cells.
Owner:ONCOTHERAPY SCI INC
Compositions and methods for reducing ocular neovascularization
InactiveUS20190100582A1Prolonged and sustained releaseIncreased riskSenses disorderAntibody mimetics/scaffoldsOcular neovascularizationNucleic acid sequencing
The present disclosure provides pharmaceutical compositions and methods thereof for the prevention or treatment of ocular neovascularization, such as AMD, in a subject, by administering to the subject a pharmaceutical composition comprising a rAAV vector having a nucleic acid sequence that encodes an anti-VEGF agent.
Owner:ADVERUM BIOTECH INC
Factor vii conjugates for selectively treating neovascularization disorders
Methods and compositions are provided for the treatment of diseases such as exudative macular degeneration, diabetic retinopathy, retinopathy of prematurity, choroidal neovascularization, retinal neovascularization, iris neovascularization, corneal neovascularization, ocular tumors, and other disorders of the eye, cancer, and inflammatory disorders. The method involves administering a conjugate, referred to as fVIIPD, containing a photosensitizer and a targeting molecule such as factor VII (“fVII”), fVIIa, or modified fVII, which binds with high affinity and specificity to tissue factor (TF). TF is more highly expressed, abnormally expressed or specifically expressed on endothelial cells lining the luminal surface of pathological neovasculature, than on normal vasculature, thus providing a specific and accessible therapeutic target. Following administration of fVIIPD, the compound specifically binds to the pathological neovasculature of the eye by interaction of the targeting molecule with TF expressed by endothelial cells within abnormal blood vessels. The photosensitizer may then be activated with a non-thermal laser light for selective destruction of abnormal vasculature.
Owner:YALE UNIV
Use of compound triptolide in choroidal neovascularization
InactiveCN105560253AInhibition formationInhibit progressOrganic active ingredientsSenses disorderWhole bodyBuccal administration
The invention discloses a use of a compound triptolide in choroidal neovascularization. After oral administration, intravenous systemic administration and / or eye local administration, a triptolide clinical drug or a triptolide mixture clinical drug with the triptolide additive can act the whole human body and local lesions, inhibit a choroidal neovascularization formation related mechanism thereby inhibiting choroidal neovascularization formation and development, reduce choroidal neovascularization atrophy-caused scar and realize eyesight keeping and / or improvement.
Owner:金陈进
Liposome formulations
ActiveUS20190321467A1Improve permeabilityOrganic active ingredientsSenses disorderVascular endothelial growth factor bindingLiposome
The present invention relates to pharmaceutical formulations comprising an anti angiogenic compound such as a monoclonal antibody or fragment thereof selected from, for example, ranibizumab, which is a vascular endothelial growth factor binder which inhibits the action of VEGF, and a delivery agent selected from a pharmaceutically acceptable liposome. The formulations are useful in the treatment of a variety of angiogenic disorders and diseases in animals and people, and, preferably, in ophthalmic disorders selected from age-related macular degeneration, diabetic macular edema and corneal neovascularization.
Owner:OPKO PHARMA
Formula of medicament for treating non-infectious ocular inflammations, and inhibiting corneal neovascularization and anti-rejection reaction generated after corneal grafting
InactiveCN102210864AHigh activityEasy to storeSenses disorderPeptide/protein ingredientsMicrosphereRetention time
The invention discloses a formula of a medicament for treating non-infectious ocular inflammations, and inhibiting the corneal neovascularization and the anti-rejection reaction generated after corneal grafting. Through the selection of an isotonizing agent and a buffering agent and the addition of an antiseptic agent, a release microsphere preparation, a recombinant human interleukin-8 receptor antagonist and a chemokine-like factors 1 (CKLF1), the activity of a recombinant human interleukin-1 receptor antagonist is enhanced, and the retention time thereof is extended. In the invention, the problem that in the prior art, because of having a high environmental requirement on the outside, the existing recombinant human interleukin-1 receptor antagonist is easy to inactivate is solved; and through adding the recombinant human interleukin-8 receptor antagonist and the chemokine-like factors 1 (CKLF1) into the medicament, a synergistic effect is generated among the recombinant human interleukin-8 receptor antagonist, the chemokine-like factors 1 (CKLF1) and the recombinant human interleukin-1 receptor antagonist, thereby reducing the dosage of the medicament.
Owner:BEIJING DAZHOU HEKANG BIO TECH
Use of 2,5-dihydroxybenzene for the treatment of ocular diseases
The present invention relates to the use of a compound of Formula (IIV) or pharmaceutically acceptable salt or solvate, isomer or prodrug thereof in the manufacturing of a medicament for the treatment and / or prophylaxis of any of the diseases selected from the group consisting of macular degeneration, corneal neovascularization or angiogenesis, iris neovascularization or angiogenesis, retinal neovascularization or angiogenesis, diabetic proliferative retinopathy and non-diabetic proliferative retinopathy.
Owner:CUEVAS SANCHEZ PEDRO +7
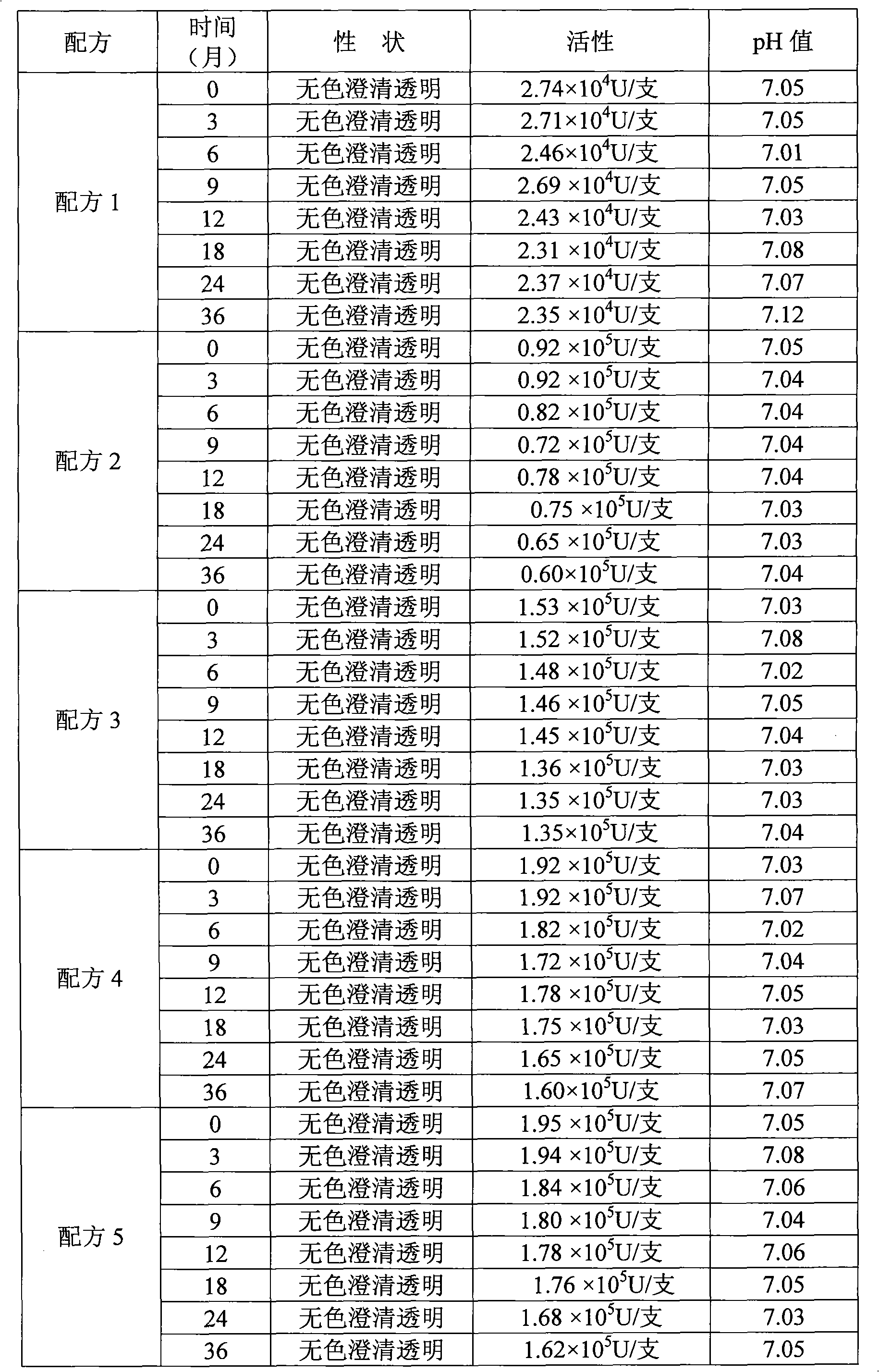
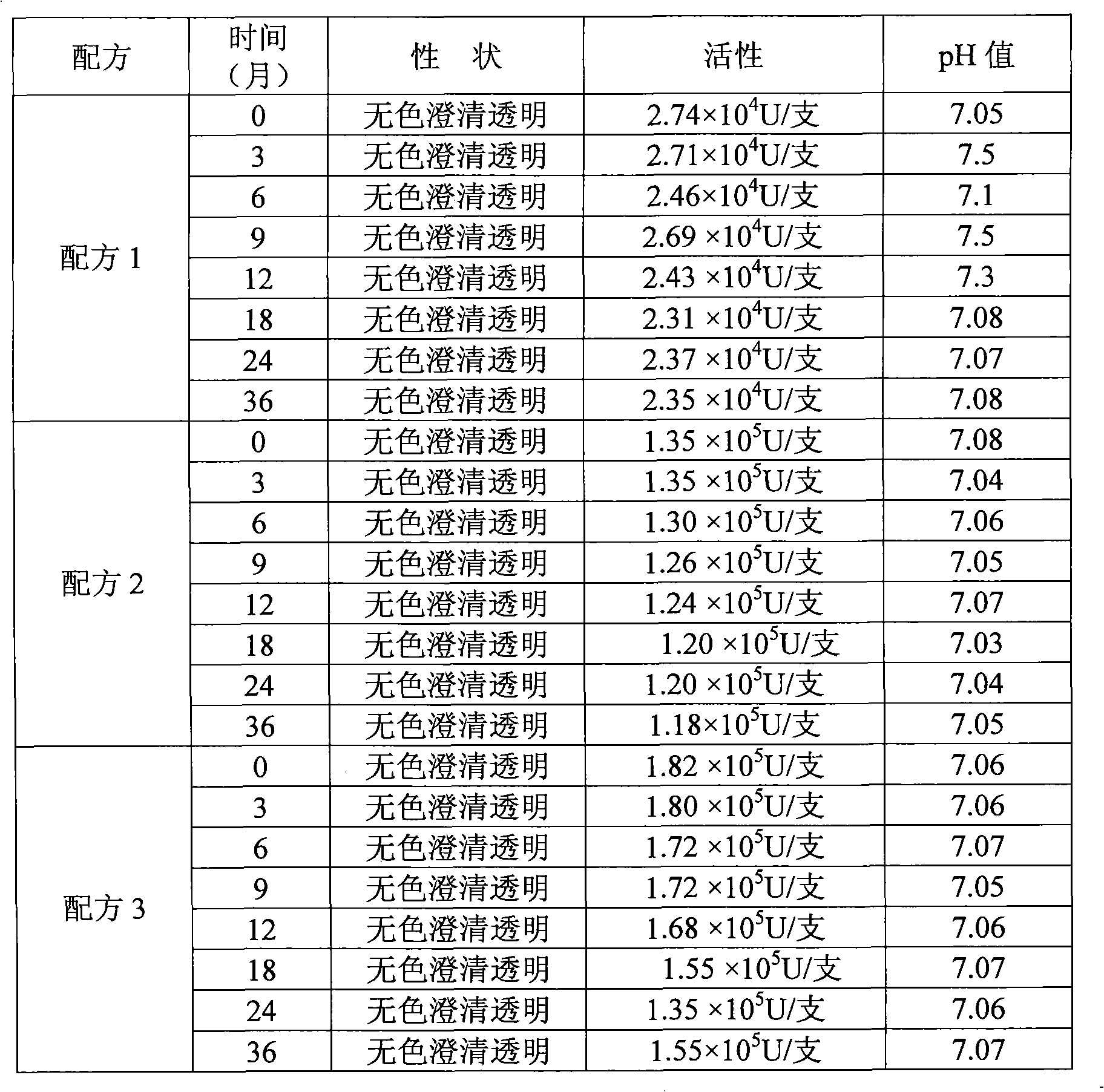
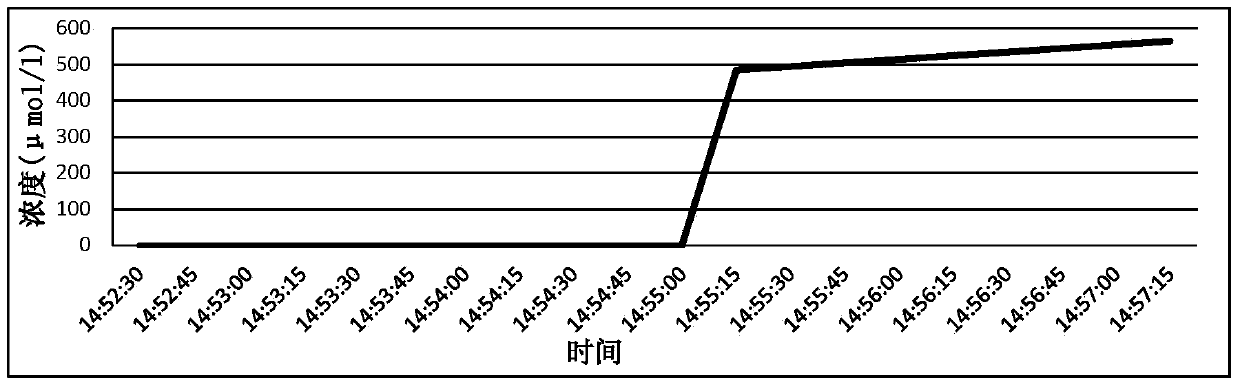
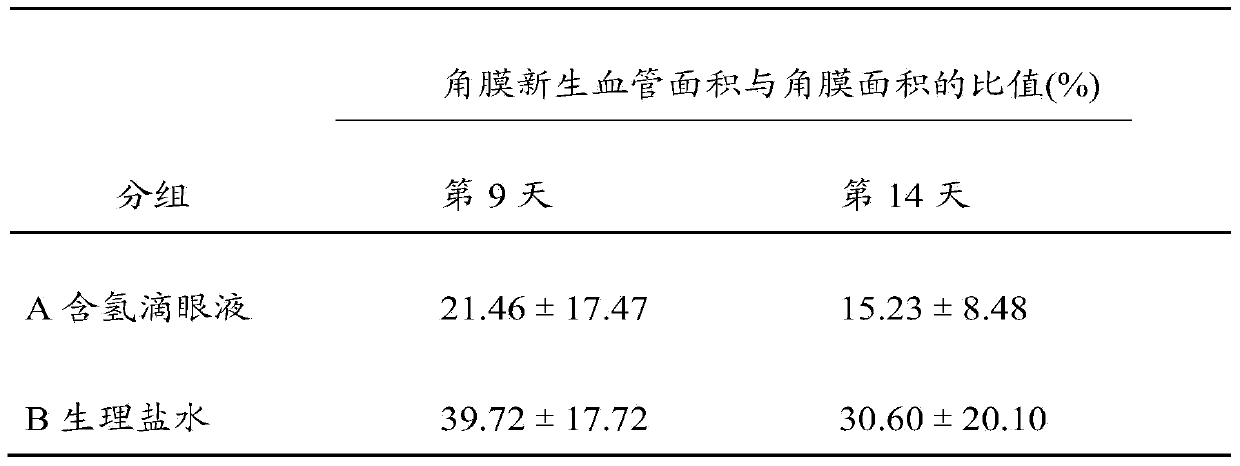
Hydrogen-containing eye drops and preparation method and application thereof
InactiveCN103417566AReduce generationInhibition of secretionSenses disorderInorganic active ingredientsTumor necrosis factor alphaBlood vessel
The invention discloses hydrogen-containing eye drops and a preparation method and application thereof. The hydrogen-containing eye drops comprise injection water and 0.5-1.3 mmol / l hydrogen. The preparation method of the hydrogen-containing eye drops includes: introducing hydrogen into the injection water in the tightness condition; after the hydrogen is dissolved and saturate in the injection water, stopping hydrogen introduction to obtain hydrogen-containing solution; filling and sealing to obtain the hydrogen-containing eye drops. The hydrogen-containing eye drops are applied to treating corneal neovascularization. The hydrogen-containing eye drops have the advantages that expression of an important VEGF (vascular endothelial growth factor) in corneal neovascularization growth factors is decreased by lowering inflammatory response factor interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-alpha); secretion of MMPs (matrix metalloproteinases) can be inhibited, and accordingly less corneal neovascularization occurs, keratitis response is lessened, and great benefit is brought to patients with corneal neovascularization.
Owner:BEIJING TONGREN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Recombinant vector, fibroin film modified by transgenic marrow stromal cells and application thereof
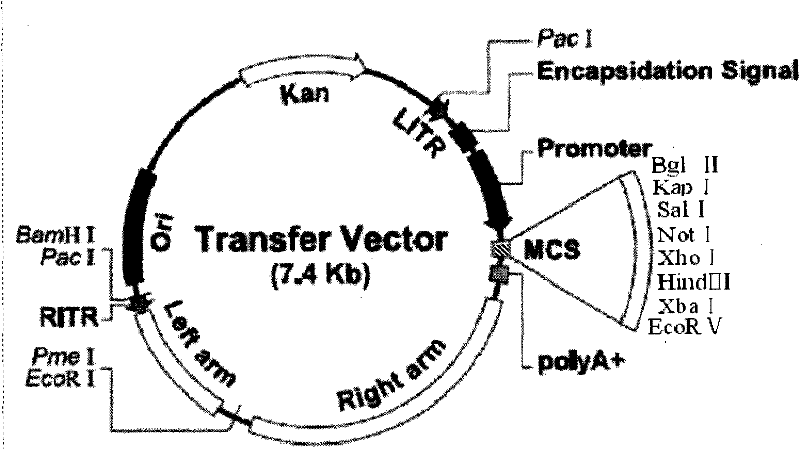
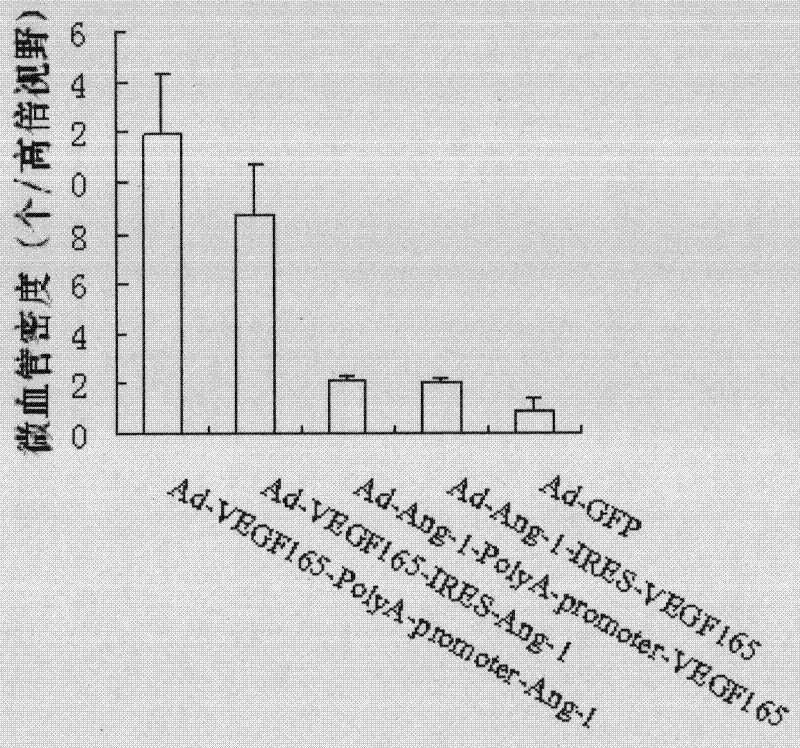
The invention relates to the biology field, and concretely discloses a recombinant plasmid pAdTrack-CMV-VEGF165-PolyA-promoter-Ang of VEGF165 and Ang-1 as well as a recombinant plasmid pAdTrack-CMV-VEGF165-IRES- Ang-1 of VEGF165 and Ang-1, wherein the preservation numbers are CCTCC No: M 2010221, CCTCC NO: M 2010220. The invention also discloses a homologous recombination of the plasmid and adenovirus and a recombinant adenovirus incased and generated from a QBI-293A cell. The invention also provides a fibroin film modified by transgenic marrow stromal cells. Rabbit corneal limbus injected with the recombination adenovirus provided in the invention is capable of successfully inducing the formation of corneal neovascularization; after the fibroin film modified by transgenic cells is implanted in a rabbit cornea layer, the neovascularization can be grown in a cornea of a fibroin film implanted area and the density of blood vessels is obviously increased, therefore, VEGF165, Ang-1, CD34 can be successfully expressed in a rabbit cornea layer. Wound repairing experiments of rats prove that the fibroin film modified by transgenic marrow stromal cells of the invention is capable of promoting the blood vessel generation of the wound organization and repairing wound, so that the recombinant adenovirus and the fibroin film modified by transgenic marrow stromal cells of the invention have medical application value.
Owner:SUZHOU UNIV
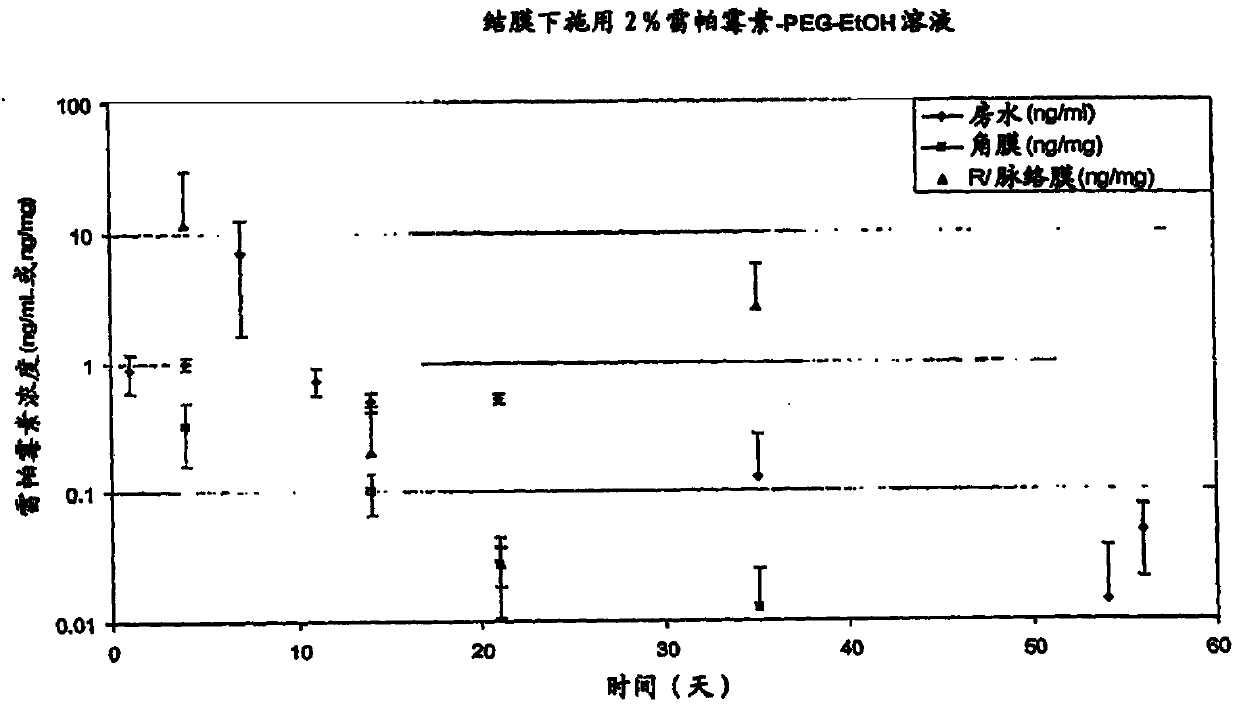
Liquid formulations for ocular treatment
ActiveCN104147005ASenses disorderHydroxy compound active ingredientsDiseaseCorneal neovascularization
Described herein are liquid rapamycin formulations. Described herein are methods of treating or preventing diseases or conditions, such as choroidal neovascularization, wet AMD and dry AMD, and preventing transition of dry AMD to wet AMD, using the liquid rapamycin formulations described herein.
Owner:SANTEN PHARMA CO LTD
Use of 2,5-dihydroxybenzene for the treatment of ocular diseases
The present invention relates to the use of a compound of Formula (IIV) or pharmaceutically acceptable salt or solvate, isomer or prodrug thereof in the manufacturing of a medicament for the treatment and / or prophylaxis of any of the diseases selected from the group consisting of macular degeneration, corneal neovascularization or angiogenesis, iris neovascularization or angiogenesis, retinal neovascularization or angiogenesis, diabetic proliferative retinopathy and non-diabetic proliferative retinopathy.
Owner:CUEVAS SANCHEZ PEDRO +7
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com