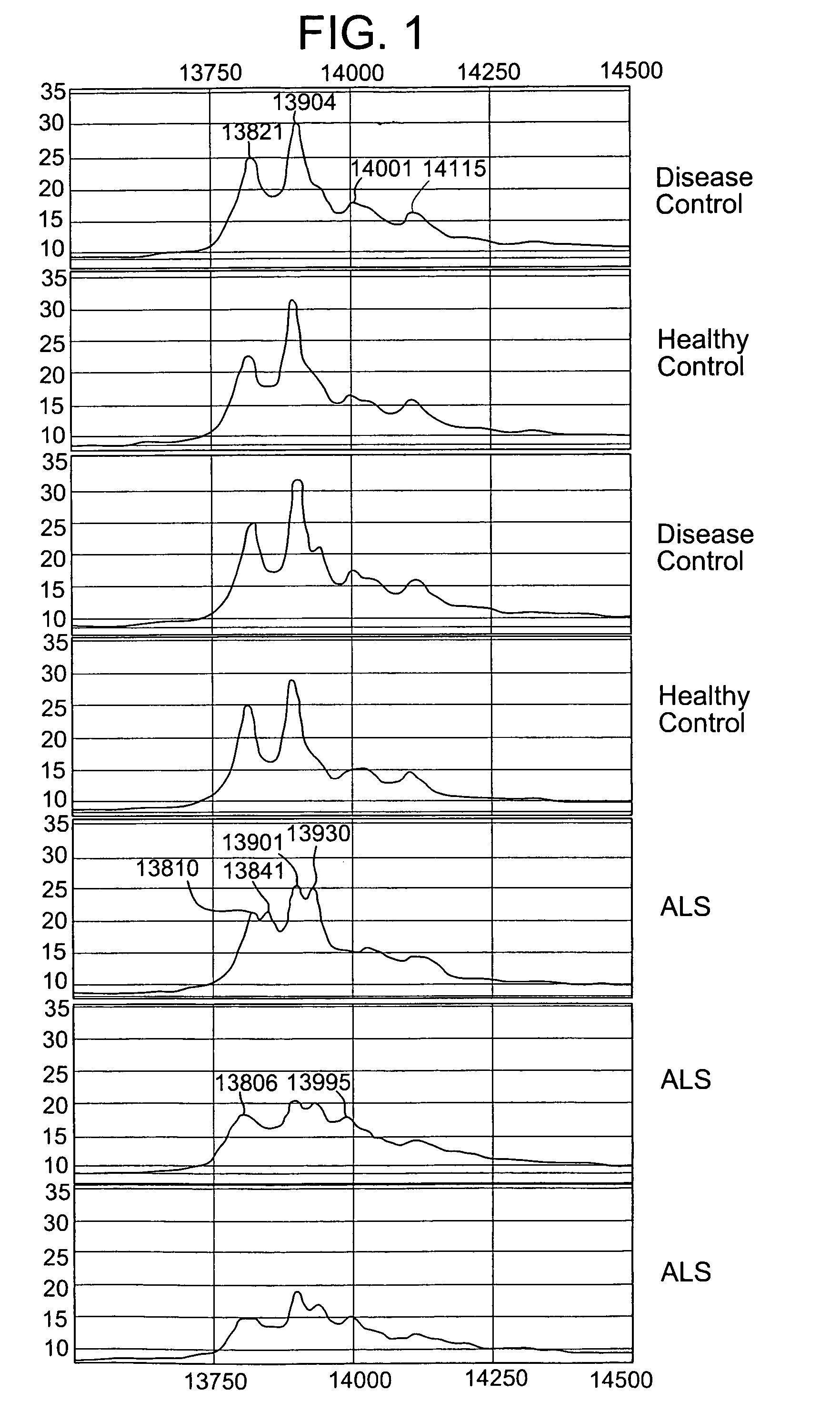
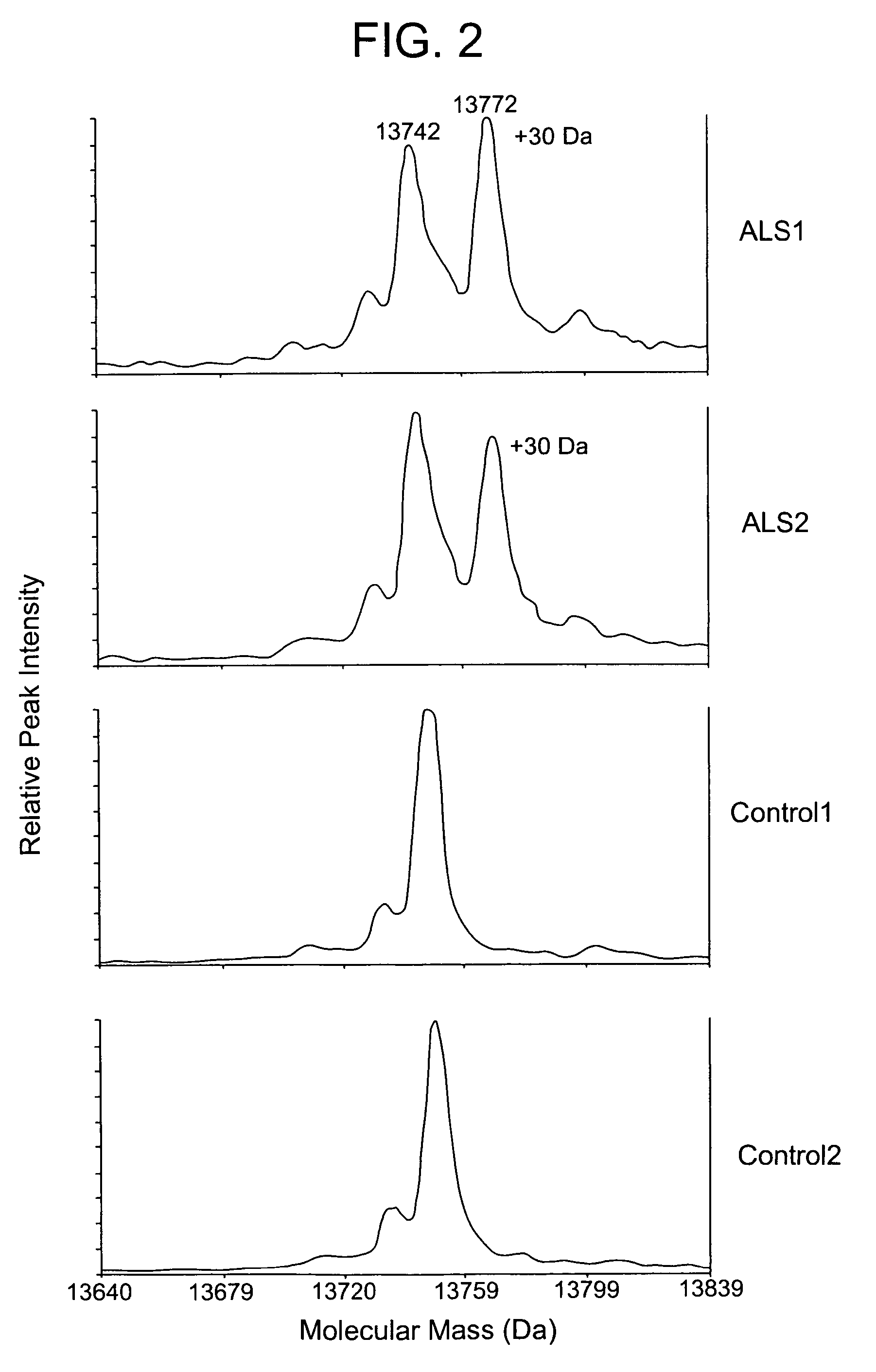
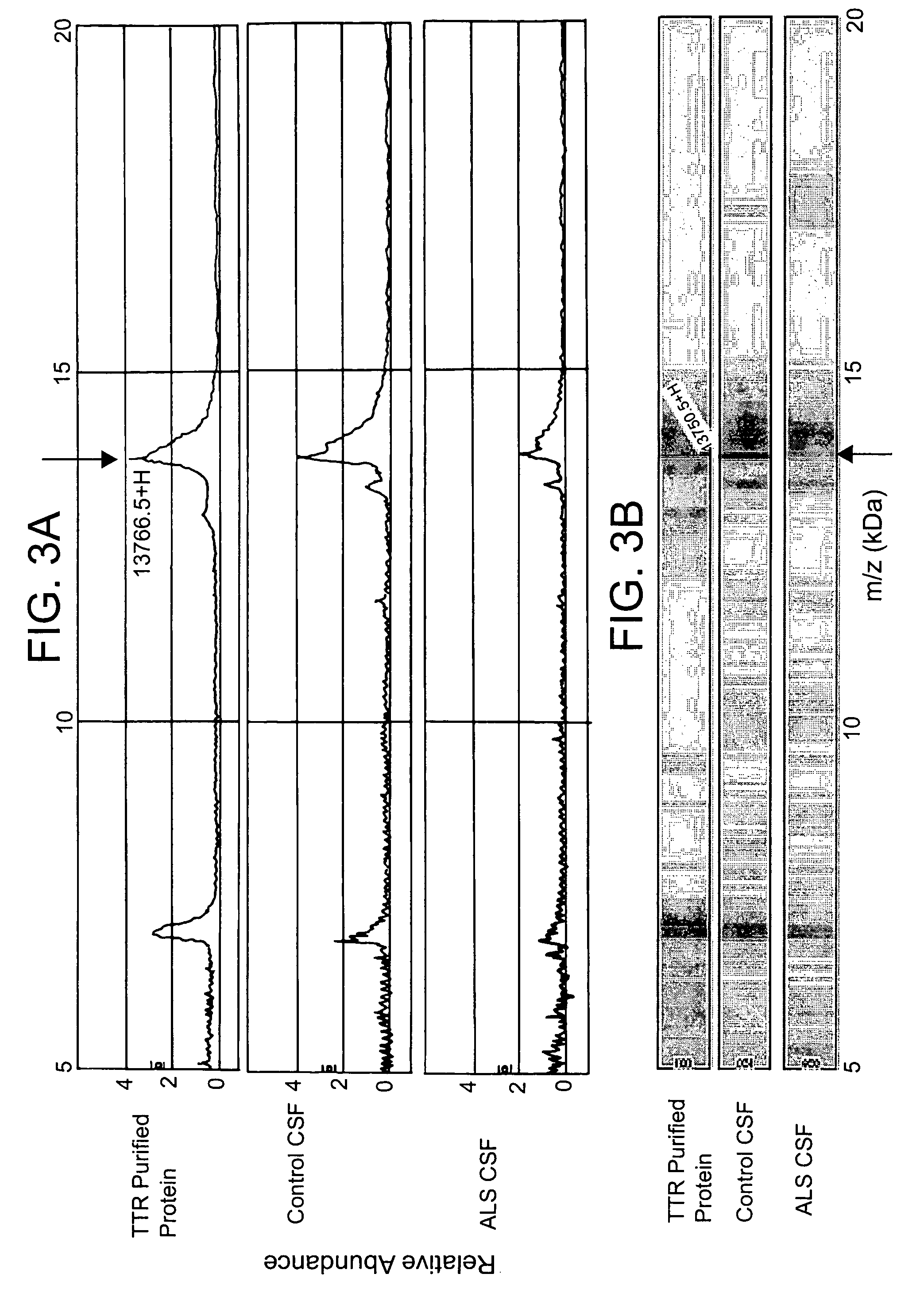
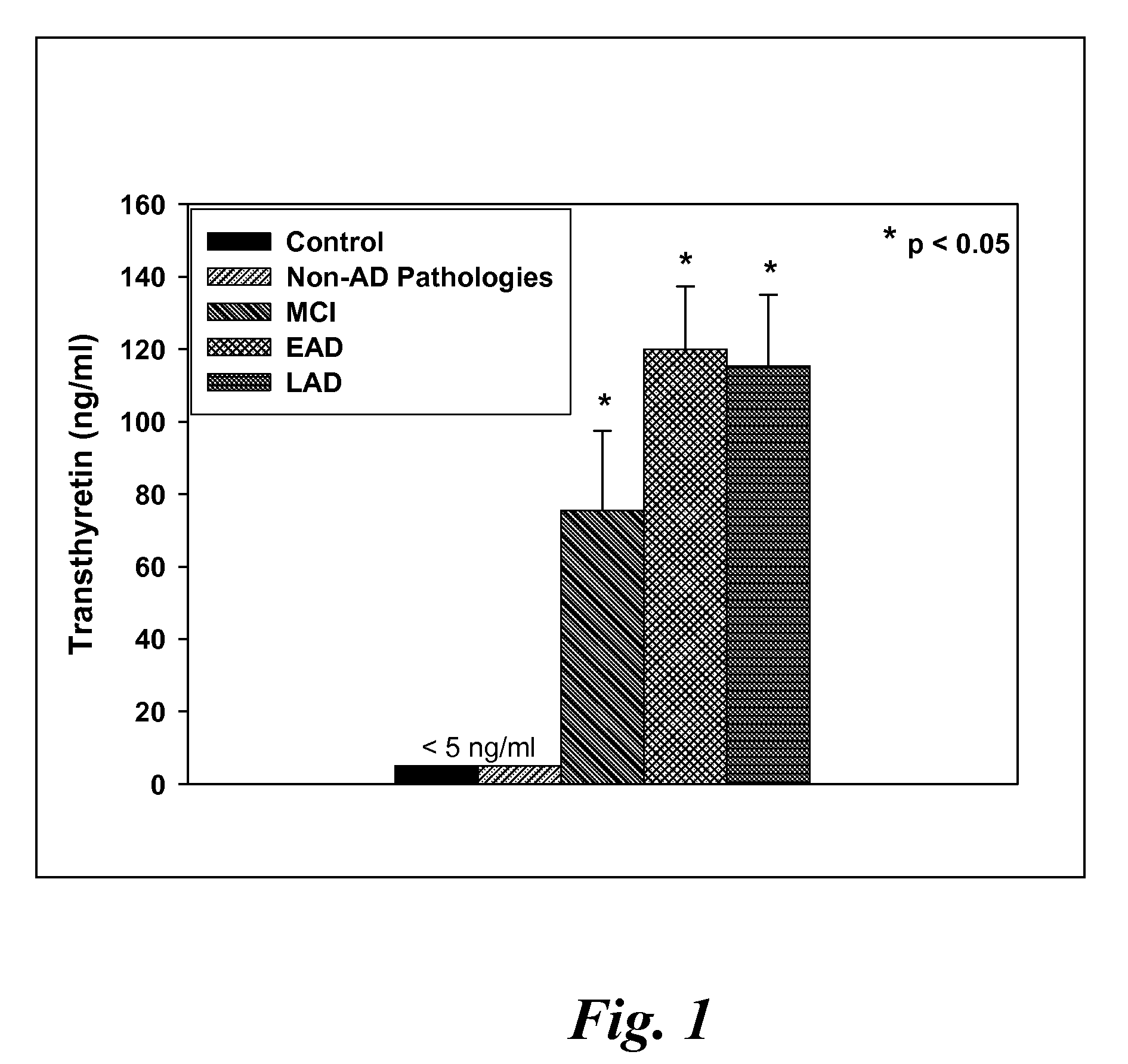
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Transthyretin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
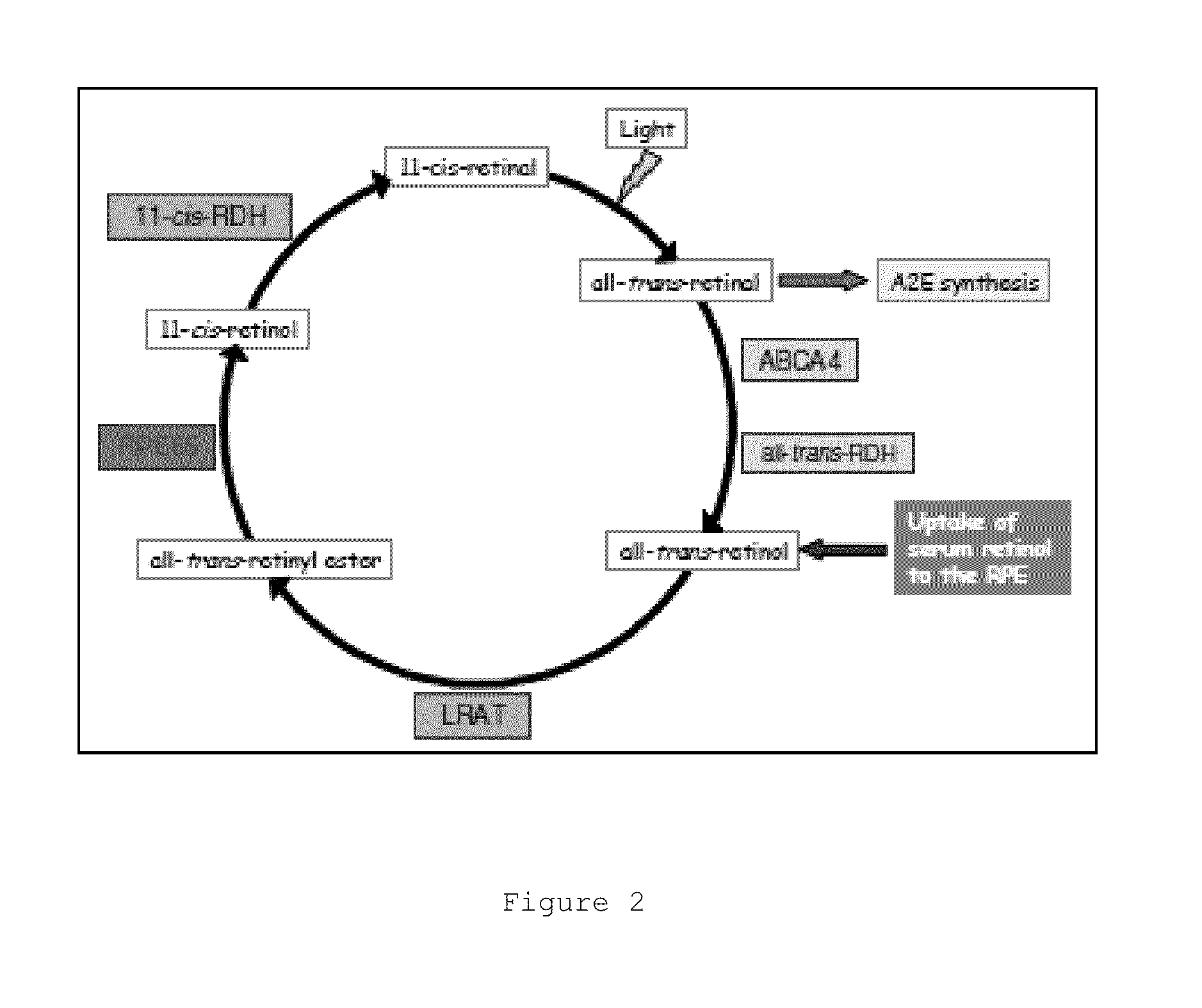
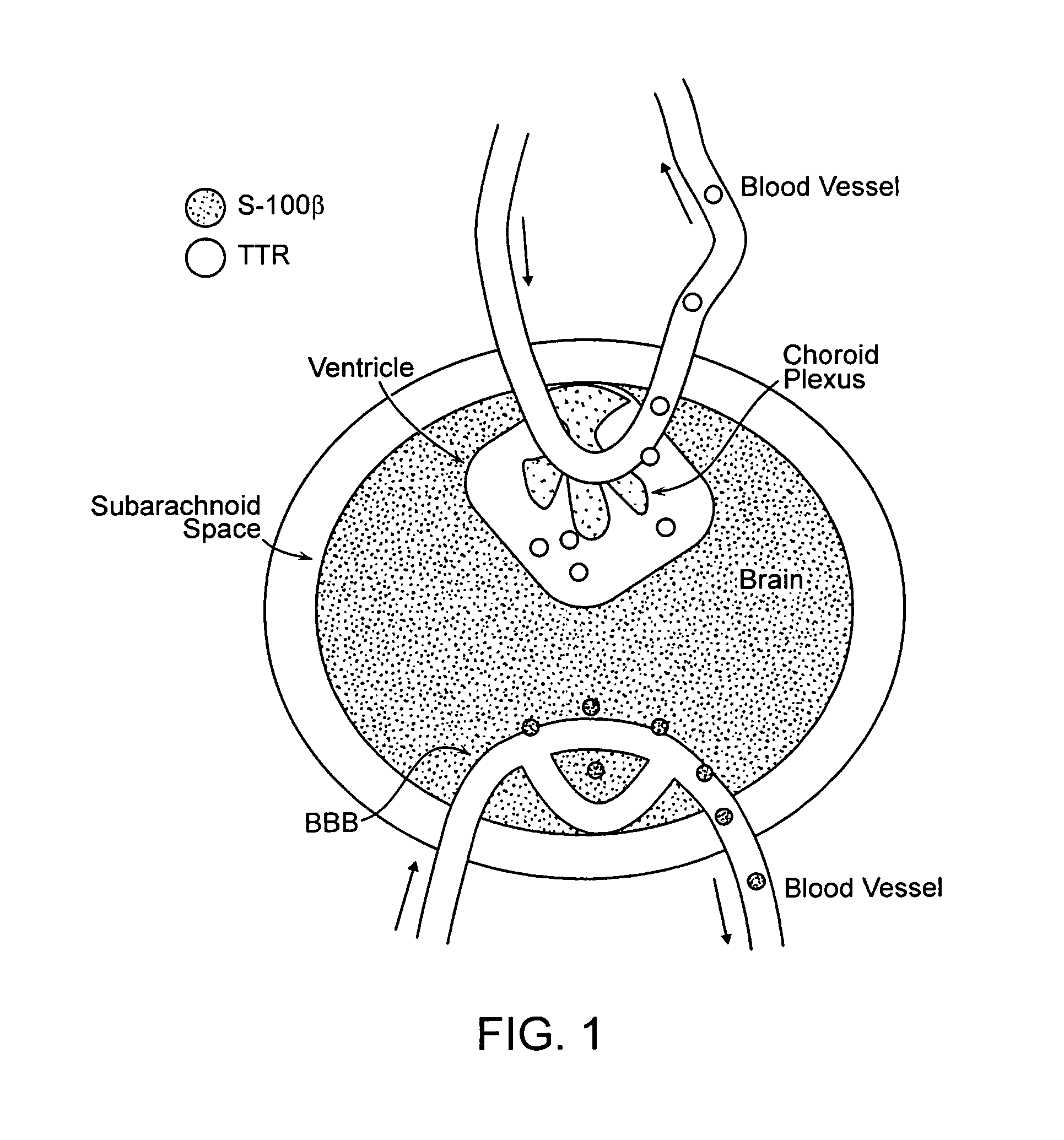
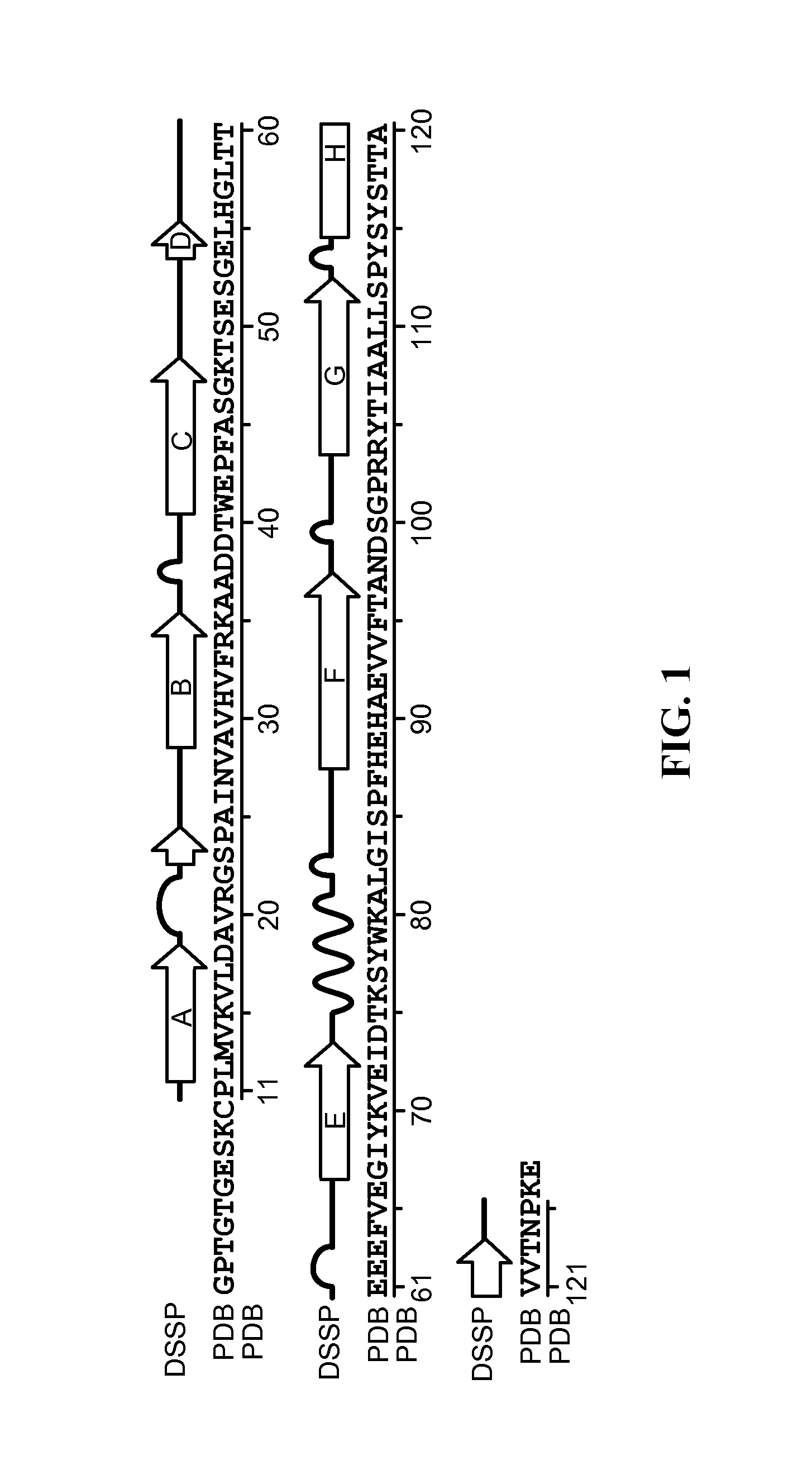
Transthyretin (TTR or TBPA) is a transport protein in the serum and cerebrospinal fluid that carries the thyroid hormone thyroxine (T₄) and retinol-binding protein bound to retinol. This is how transthyretin gained its name: transports thyroxine and retinol. The liver secretes transthyretin into the blood, and the choroid plexus secretes TTR into the cerebrospinal fluid.
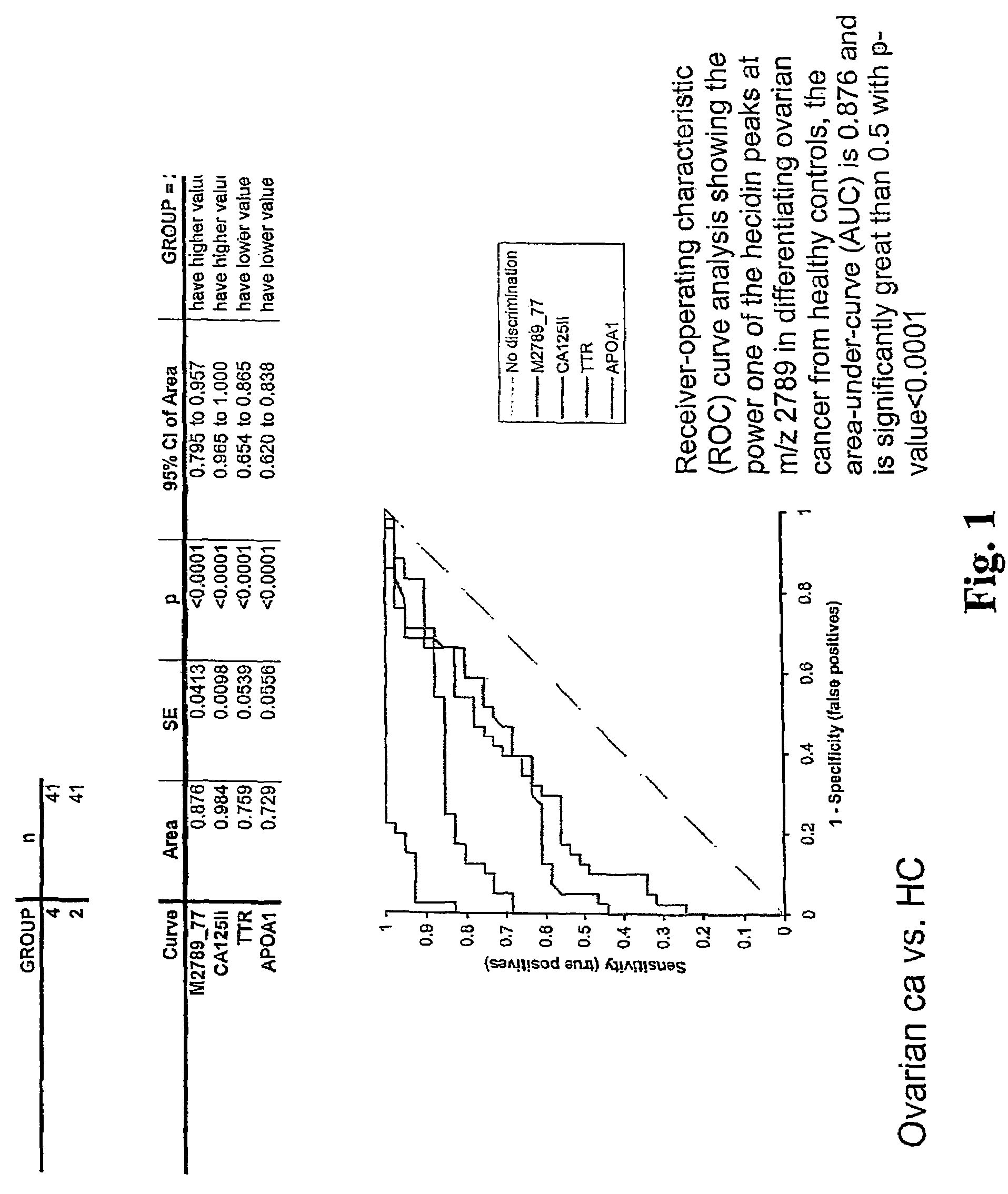
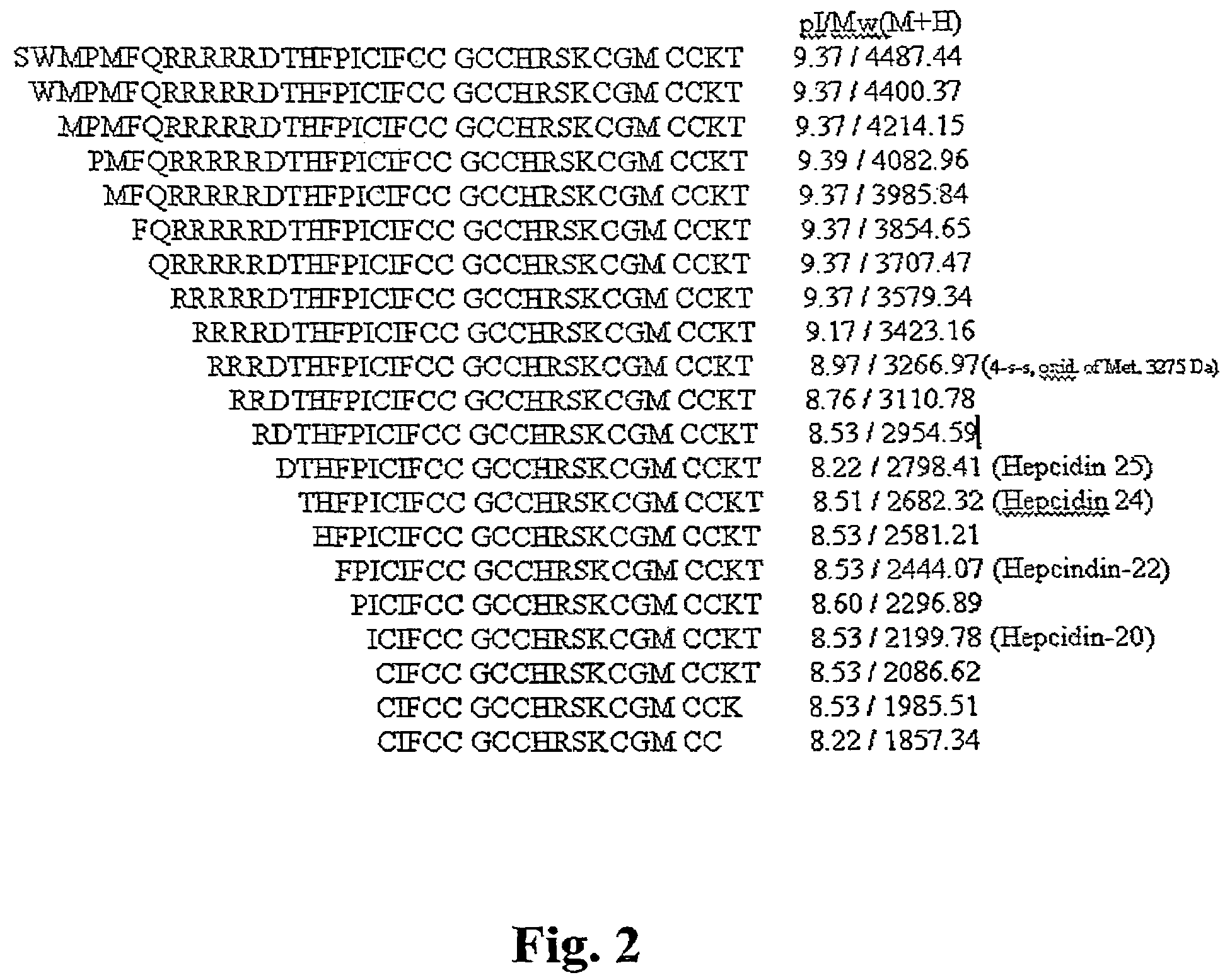
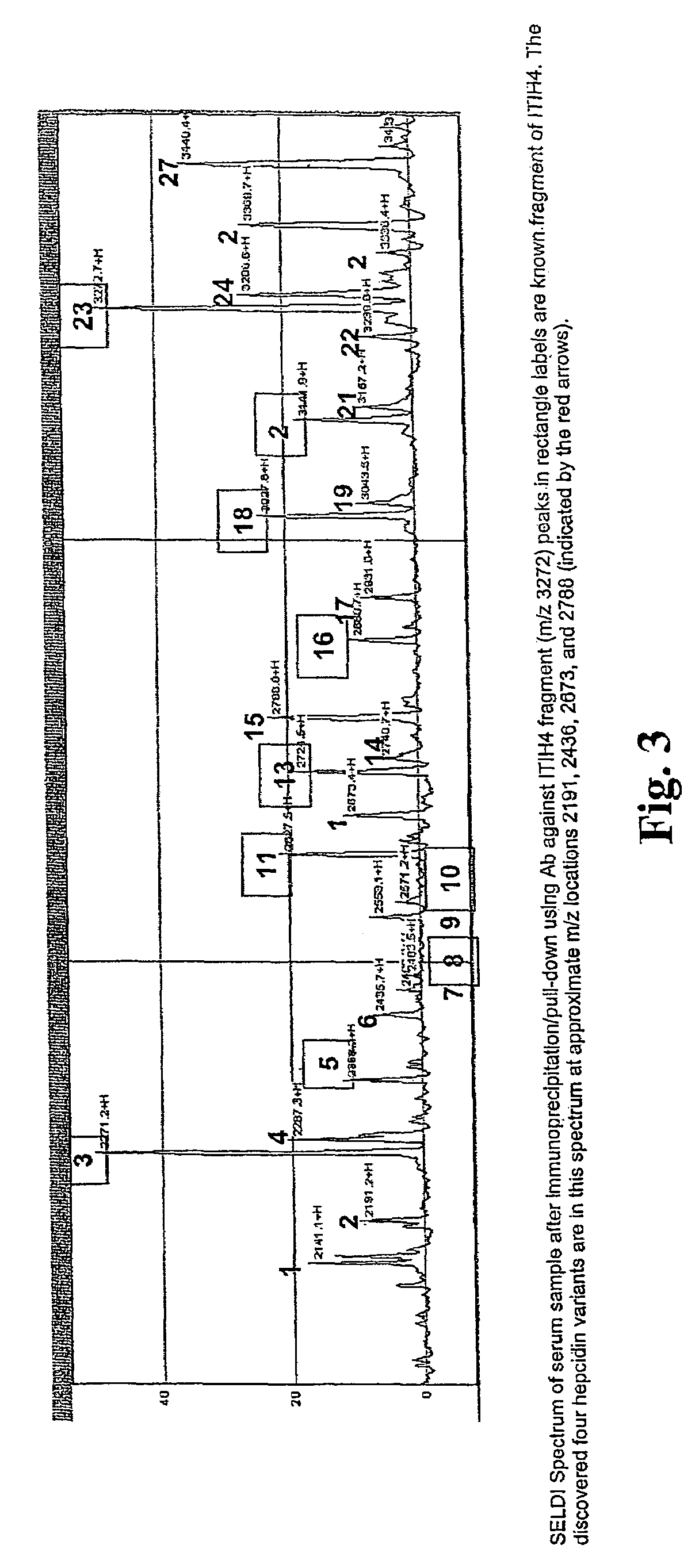
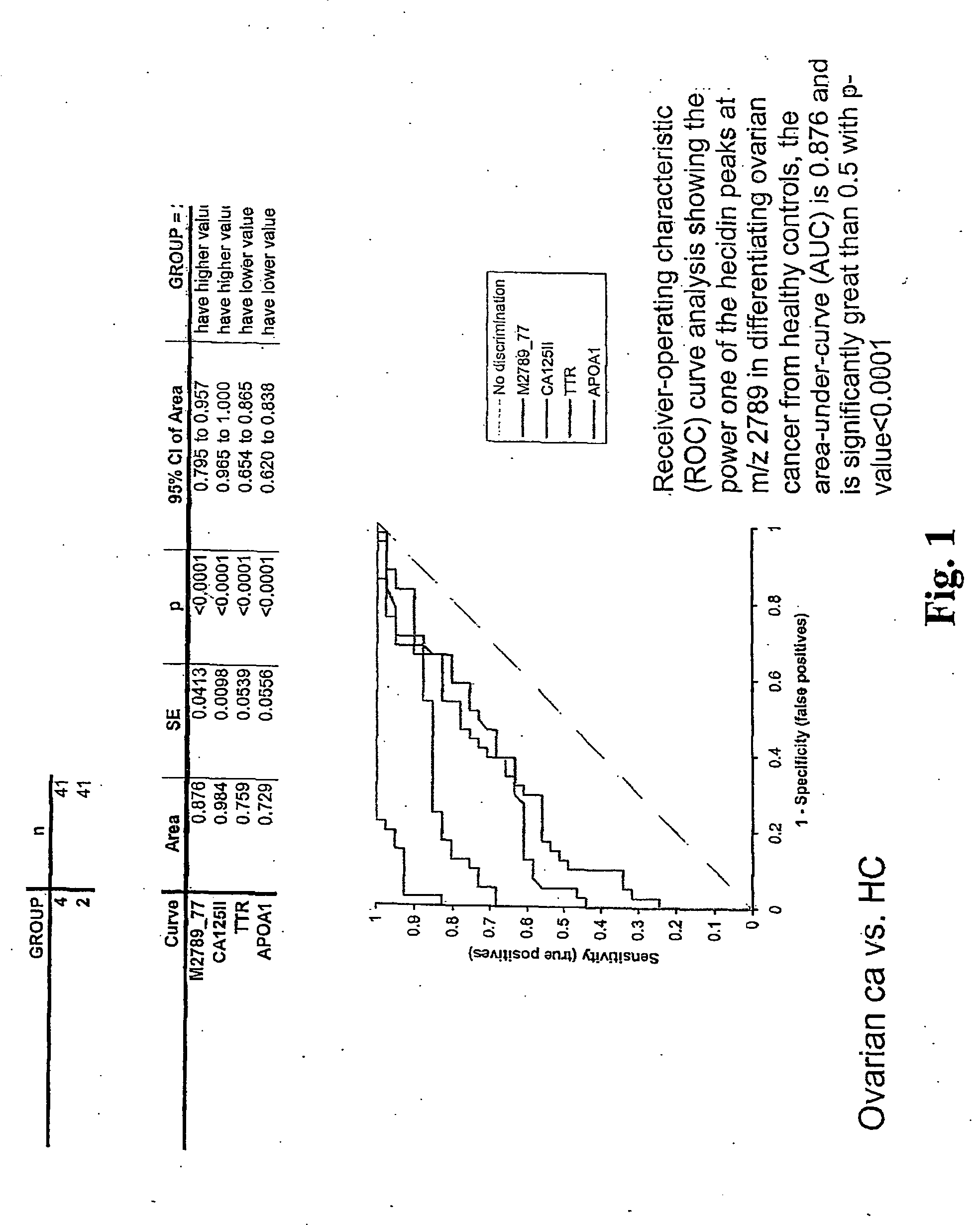
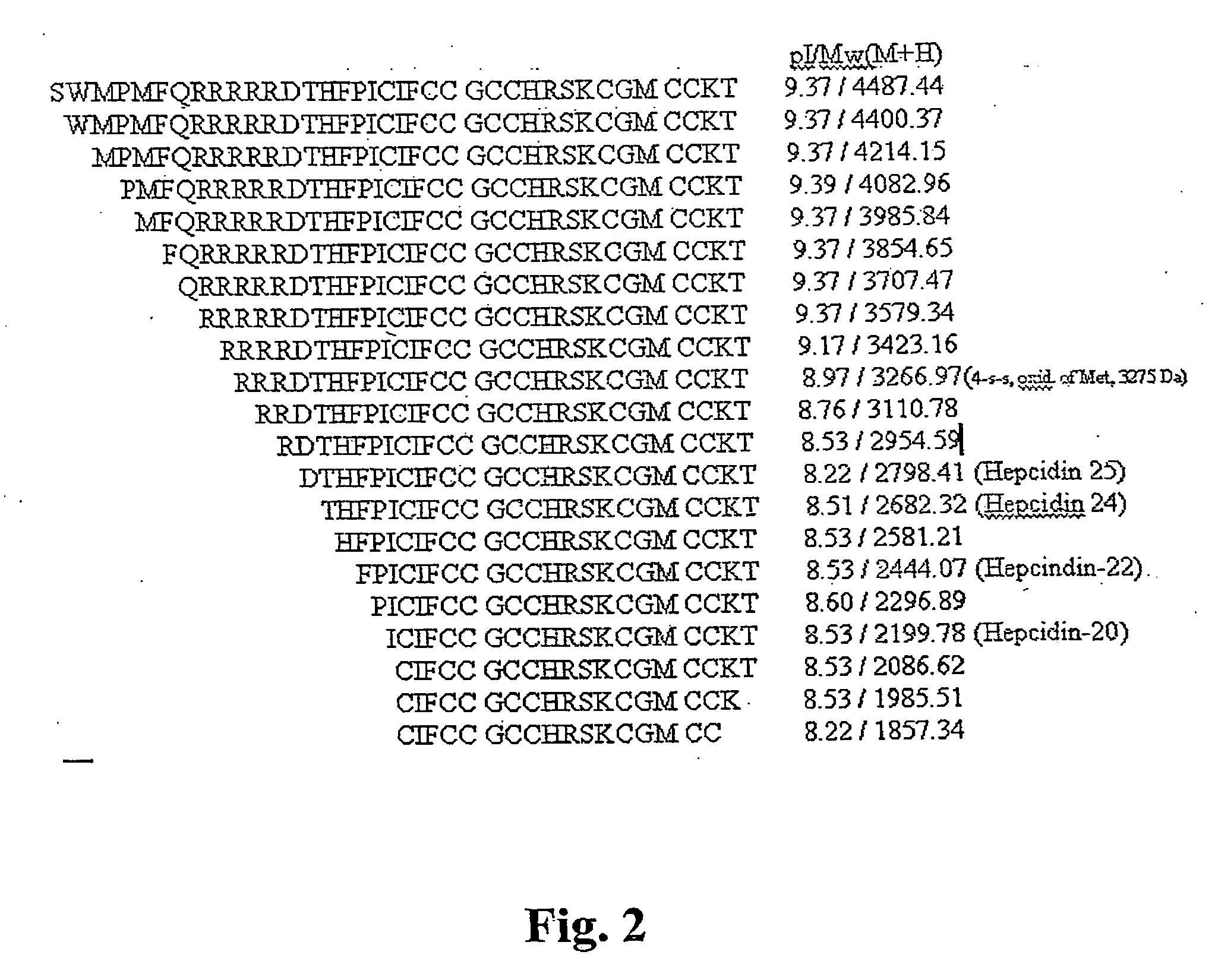
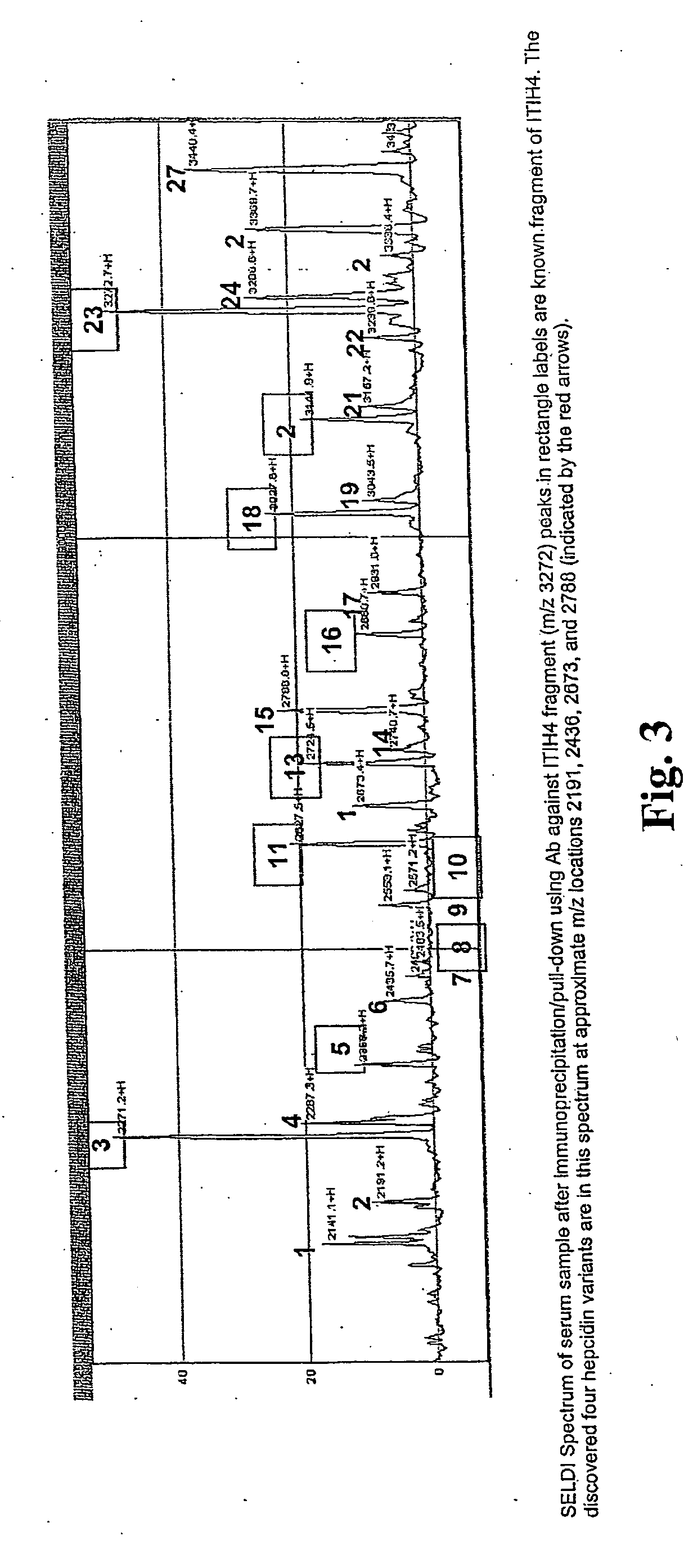
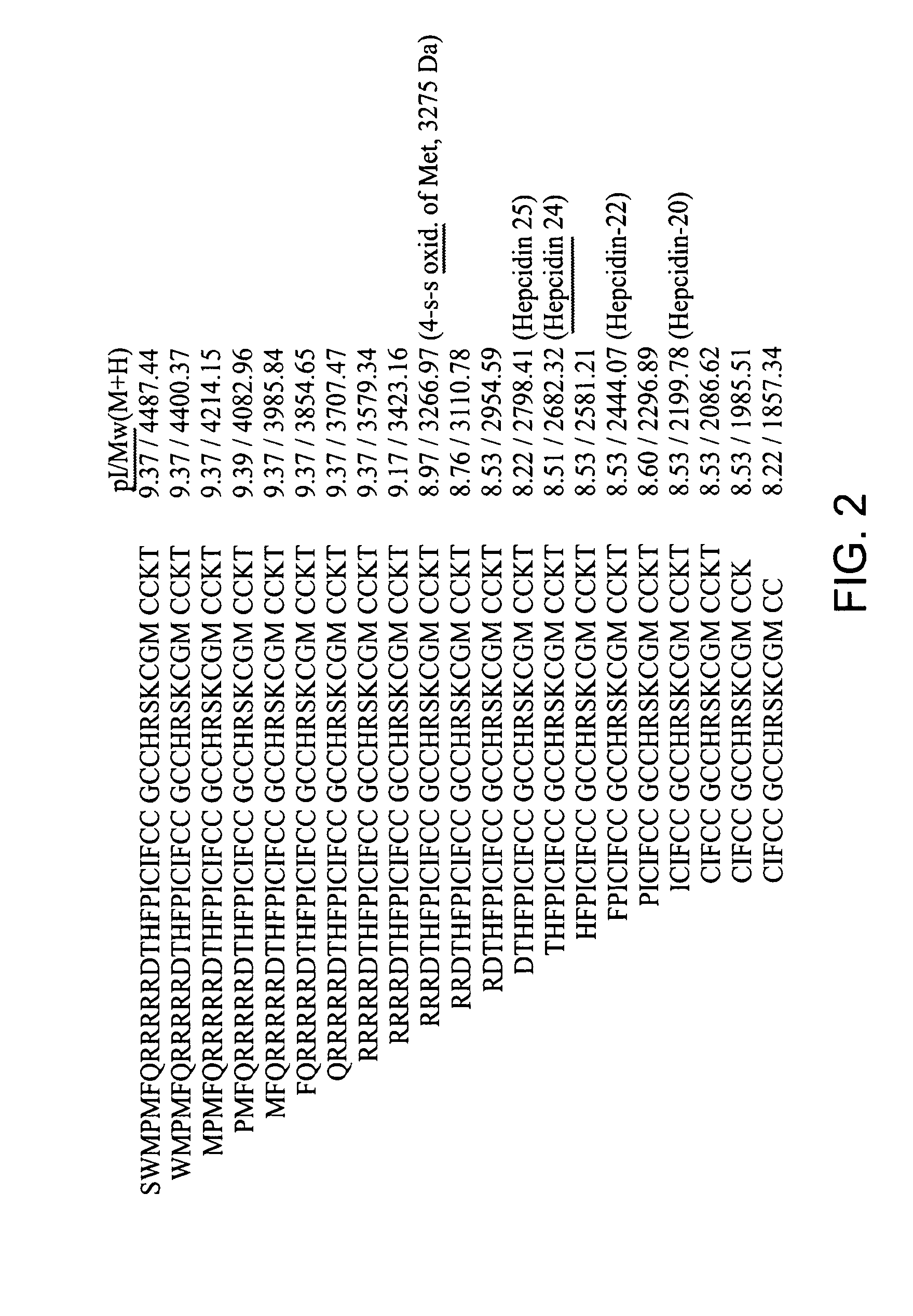
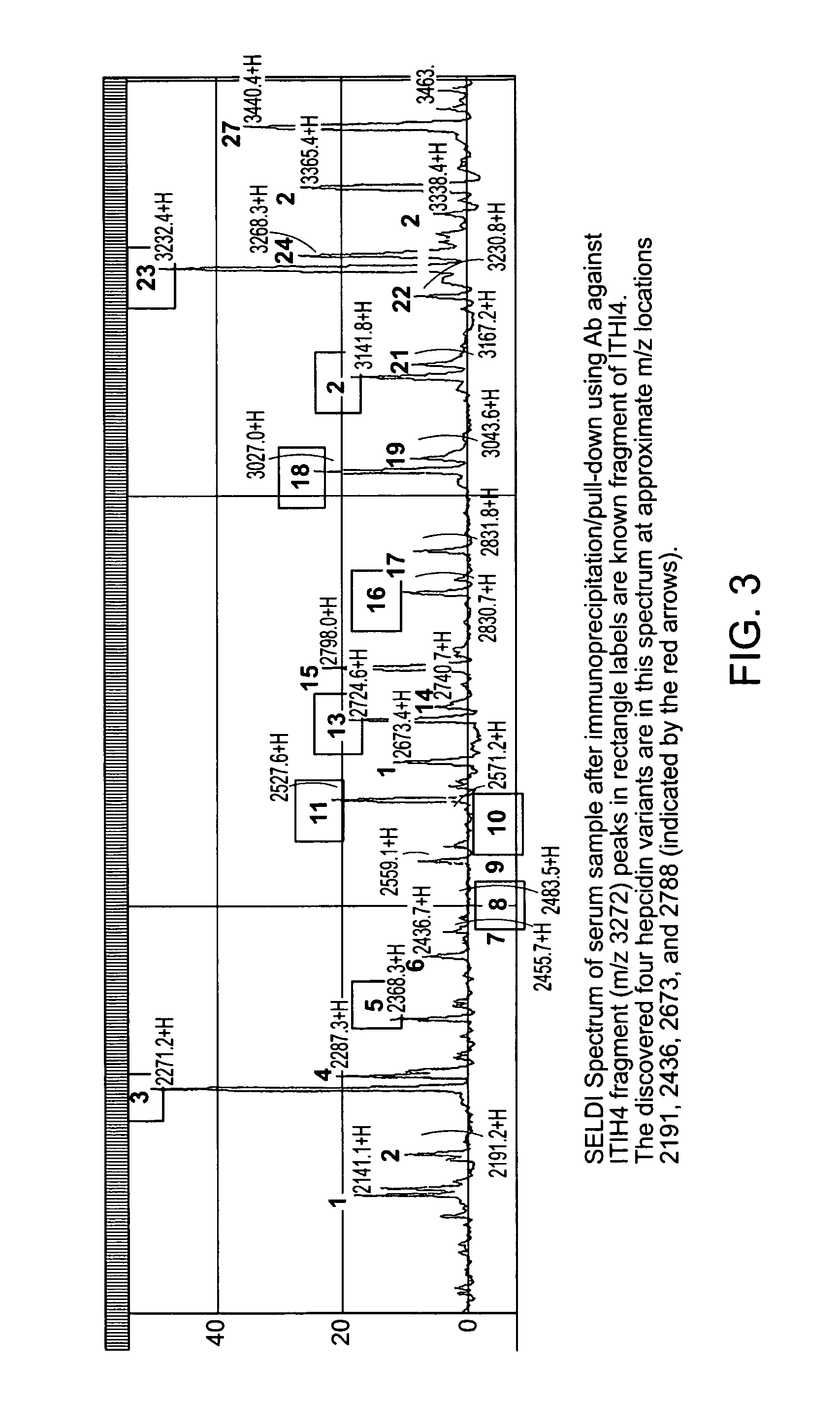
Biomarker for ovarian and endometrial cancer: hepcidin
ActiveUS7510842B2Improve diagnostic capabilitiesImprove the level ofBiocideCompound screeningThyroxine measurementMass spectrometry
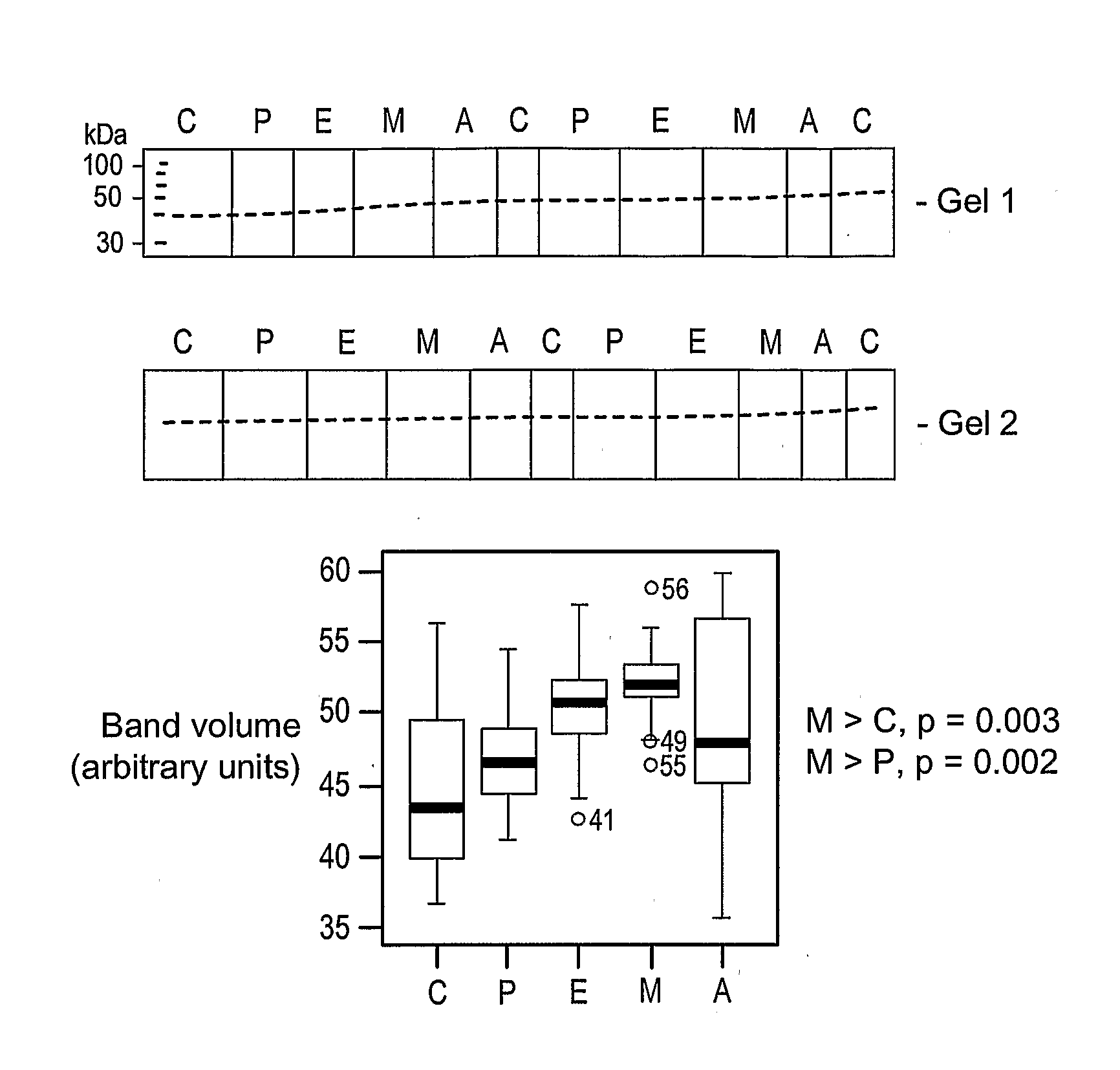
The present invention provides protein-based biomarkers and biomarker combinations that are useful in qualifying ovarian cancer status as well as endometrical cancer status in a patient. In particular, it has been found that hepcidin is a biomarker for both ovarian cancer and endometrial cancer and that a panel of biomarkers, including hepcidin, transthyretin and optionally other markers are useful to classify a subject sample as ovarian cancer or non-ovarian cancer. The biomarkers can be detected by SELDI mass spectrometry.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +2
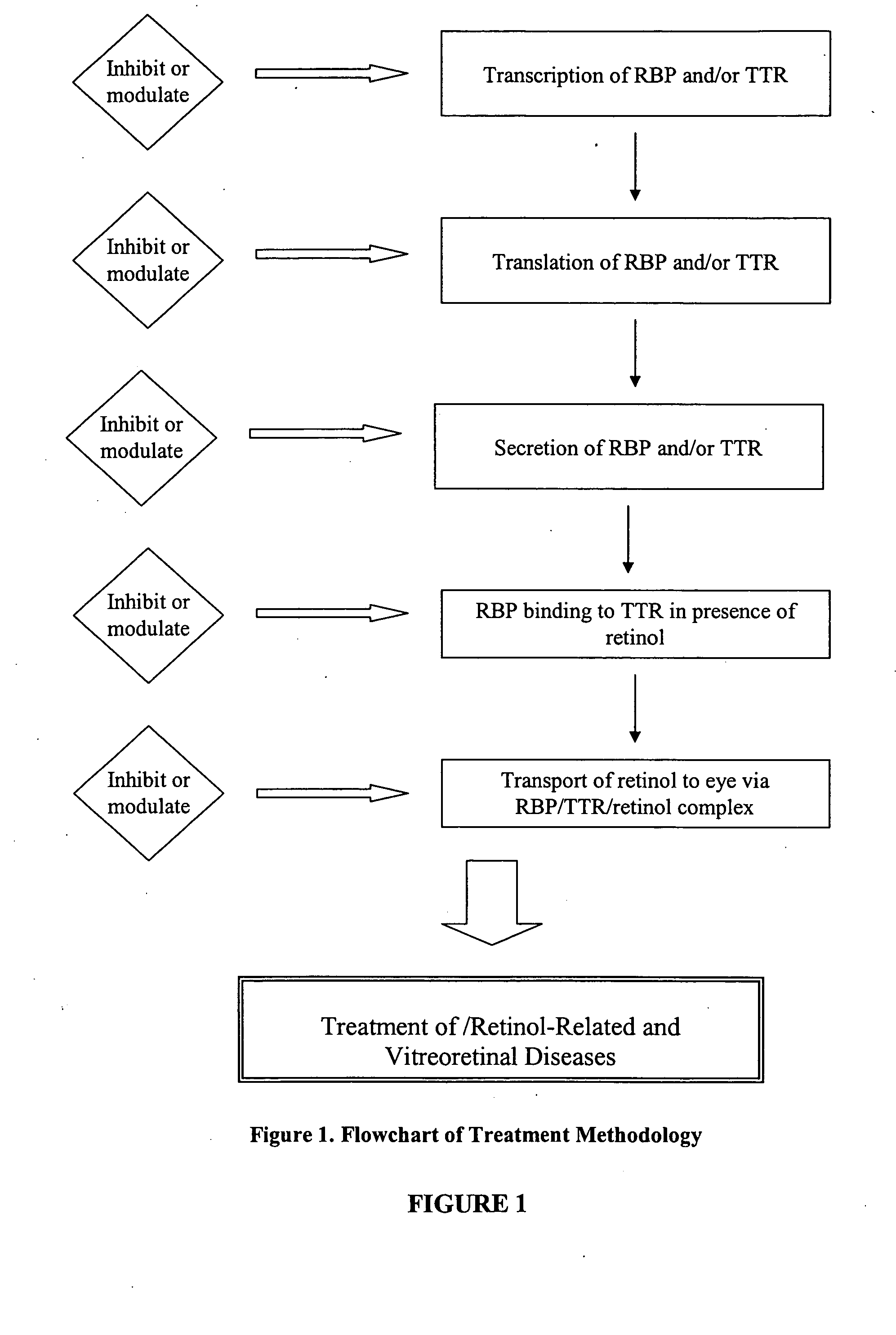
Methods, assays and compositions for treating retinol-related diseases
InactiveUS20060135460A1Reduce formationInhibit transcriptionBiocideSenses disorderDiseaseVitamin A Retinol
Described herein are methods and compositions for treating certain retinol-related diseases and conditions by modulation of transthyretin (TTR) and retinol binding protein (RBP) availability in the subject. For example, the methods and compositions provide for therapeutic agents for the treatment and / or prevention of age-related macular degeneration and / or dystrophies, metabolic disorders, idiopathic intracranial hypertension, hyperostosis, and protein misfolding and aggregation diseases. The compositions disclosed may be used as single agent therapy or in combination with other agents or therapies. In addition, described herein are methods and assays for selecting appropriate agents that can modulate the TTR and RBP availability in a subject.
Owner:ACUACELA INC
Modulation of transthyretin expression
InactiveUS20050244869A1Modulate expressionSugar derivativesMicrobiological testing/measurementDiseaseOligonucleotide
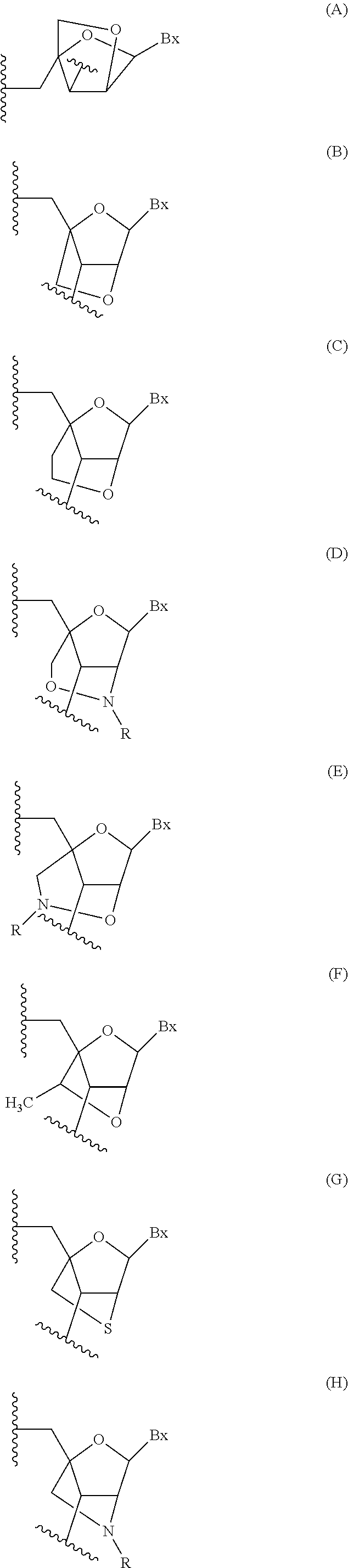
Compounds, compositions and methods are provided for modulating the expression of transthyretin. The compositions comprise oligonucleotides, targeted to nucleic acid encoding transthyretin. Methods of using these compounds for modulation of transthyretin expression and for diagnosis and treatment of diseases and conditions associated with expression of transthyretin are provided.
Owner:IONIS PHARMA INC
Modulation of transthyretin expression
Owner:IONIS PHARMA INC
Modulation of transthyretin expression
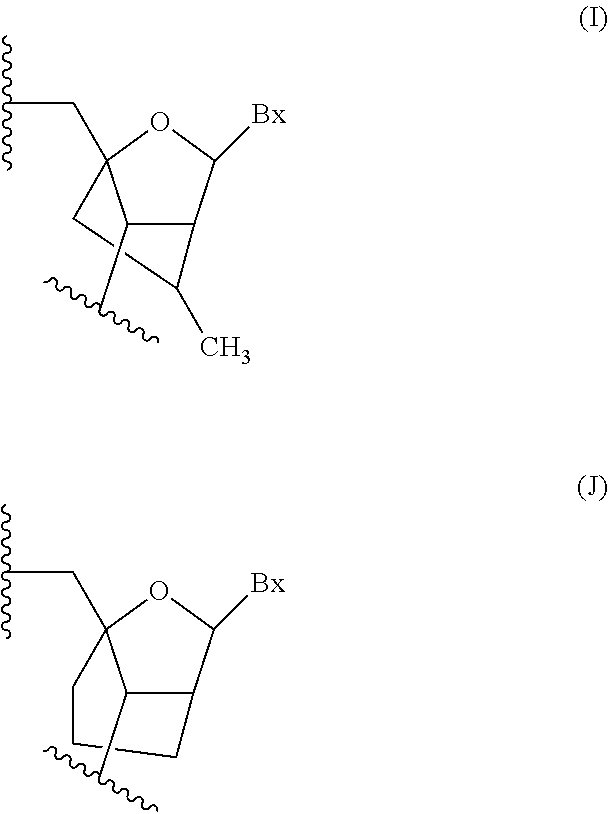
Provided herein are methods, compounds, and compositions for reducing expression of transthyretin mRNA and protein in an animal. Such methods, compounds, and compositions are useful to treat, prevent, delay, or ameliorate transthyretin amyloidosis, or a symptom thereof.
Owner:IONIS PHARMA INC
Modulation of transthyretin expression for the treatment of CNS related disorders
Compounds, compositions and methods are provided for modulating the expression of transthyretin in the brain, specifically the choroid plexus. The compositions comprise oligonucleotides, targeted to nucleic acid encoding transthyretin. Methods of using these compounds for modulation of transthyretin expression and for diagnosis and treatment of diseases and conditions associated with expression of transthyretin are provided.
Owner:IONIS PHARMA INC
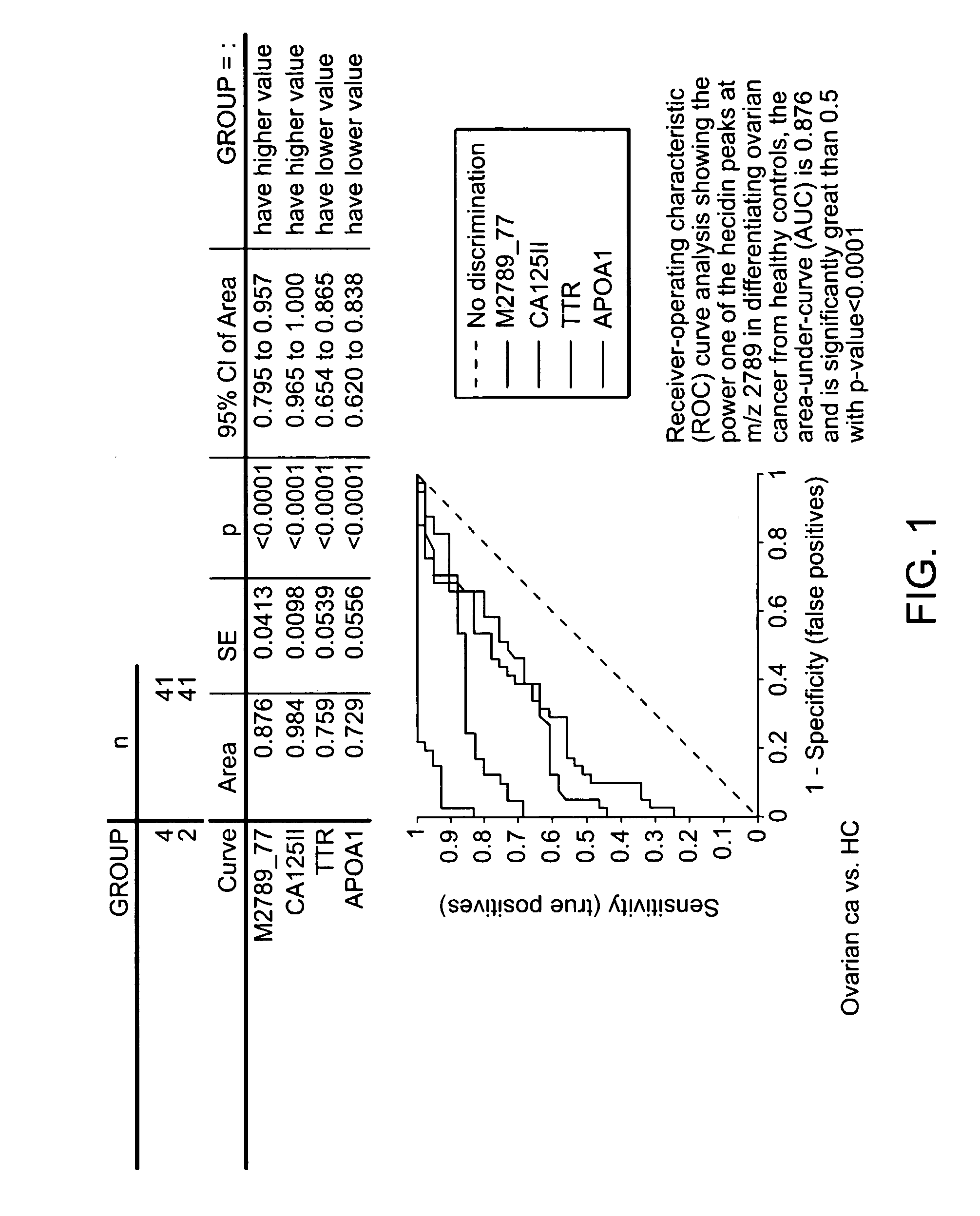
Biomarker for ovarian and endometrial cancer: hepcidin
ActiveUS20070054329A1Improve diagnostic capabilitiesImprove the level ofBiocideCompound screeningMass spectrometryOncology
The present invention provides protein-based biomarkers and biomarker combinations that are useful in qualifying ovarian cancer status as well as endometrical cancer status in a patient. In particular, it has been found that hepcidin is a biomarker for both ovarian cancer and endometrial cancer and that a panel of biomarkers, including hepcidin, transthyretin and optionally other markers are useful to classify a subject sample as ovarian cancer or non-ovarian cancer. The biomarkers can be detected by SELDI mass spectrometry.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +2
Biomaker for ovarian and endometrial cancer: hepcidin
InactiveUS20100068724A1Improve diagnostic capabilitiesImprove the level ofCompound screeningBiocideThyroxine measurementMass spectrometry
The present invention provides protein-based biomarkers and biomarker combinations that are useful in qualifying ovarian cancer status as well as endometrical cancer status in a patient. In particular, it has been found that hepcidin is a biomarker for both ovarian cancer and endometrial cancer and that a panel of biomarkers, including hepcidin, transthyretin and optionally other markers are useful to classify a subject sample as ovarian cancer or non-ovarian cancer. The biomarkers can be detected by SELDI mass spectrometry.
Owner:VERMILLION INC +2
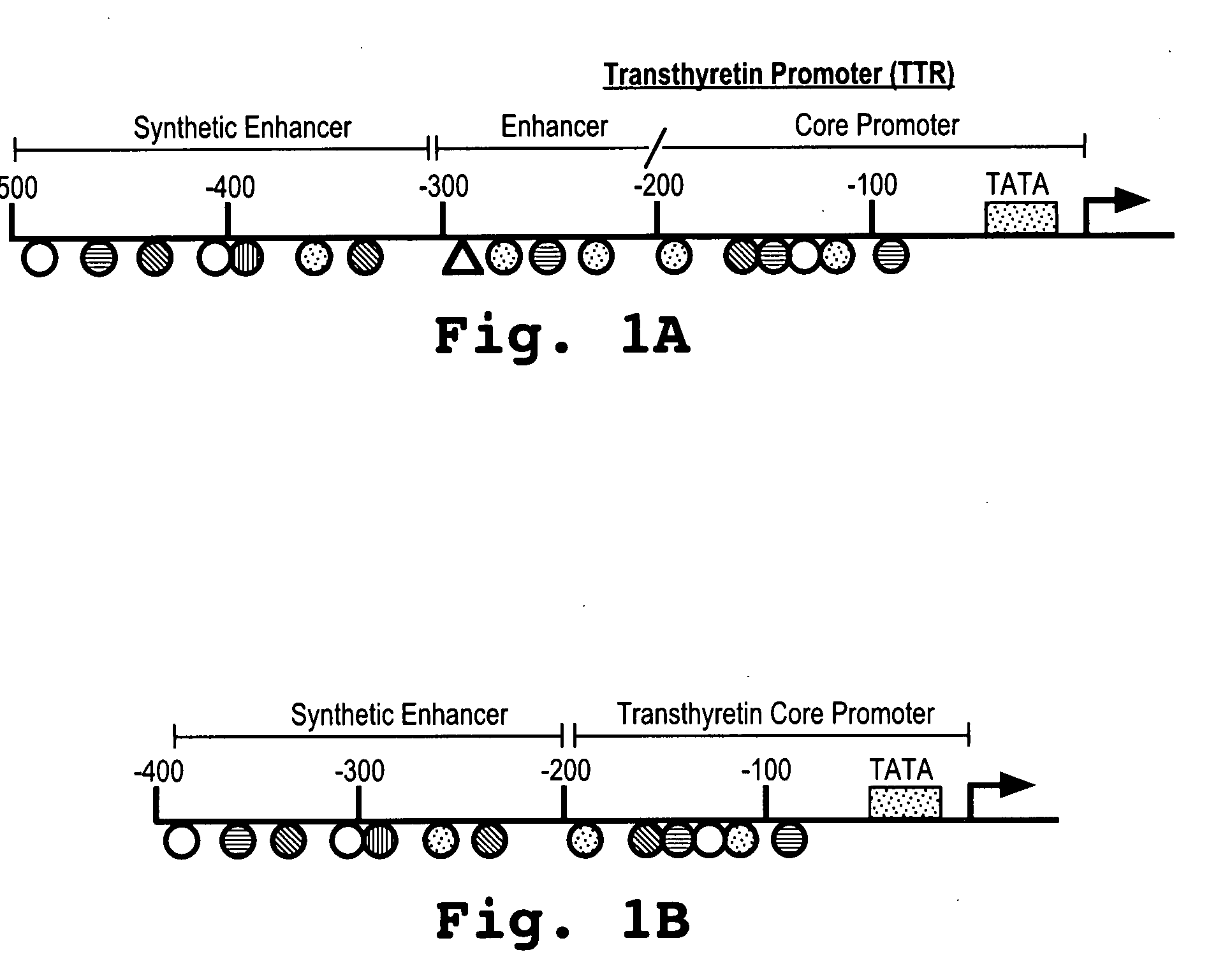
Minigene expression cassette
Methods and compositions for expressing a gene or nucleotide sequence of interest are provided. The compositions include an expression cassette that includes a synthetic enhancer, a transthyretin promoter, and a nucleotide sequence operably under the control of the synthetic enhancer and the transthyretin promoter. The expression cassette may be used in an adeno-associated viral (AAV) vector, such as a self-complementary AAV vector.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Modulation of the neuroendocrine system as a therapy for motor neuron disease
Owner:UNIVERSITY OF PITTSBURGH
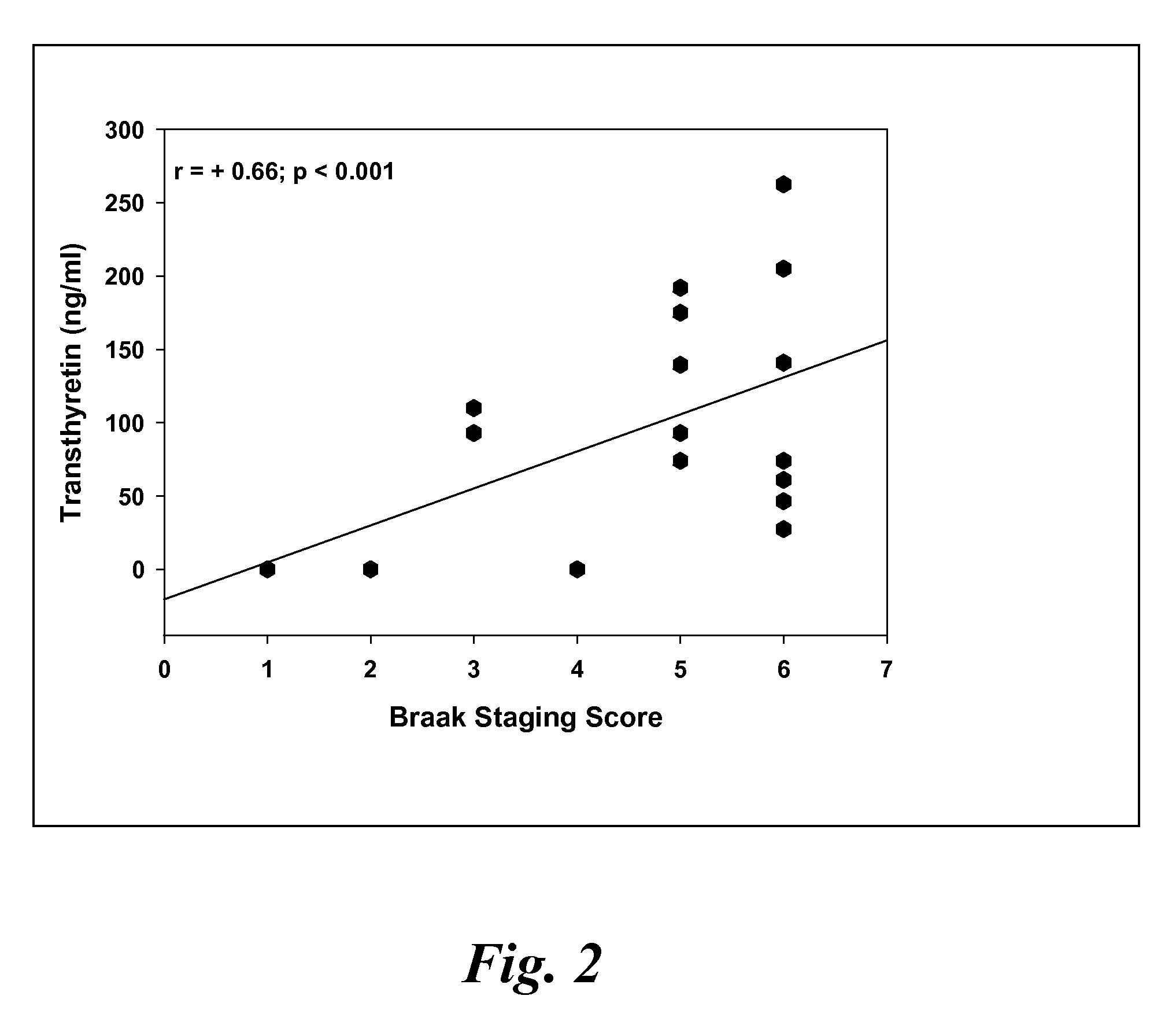
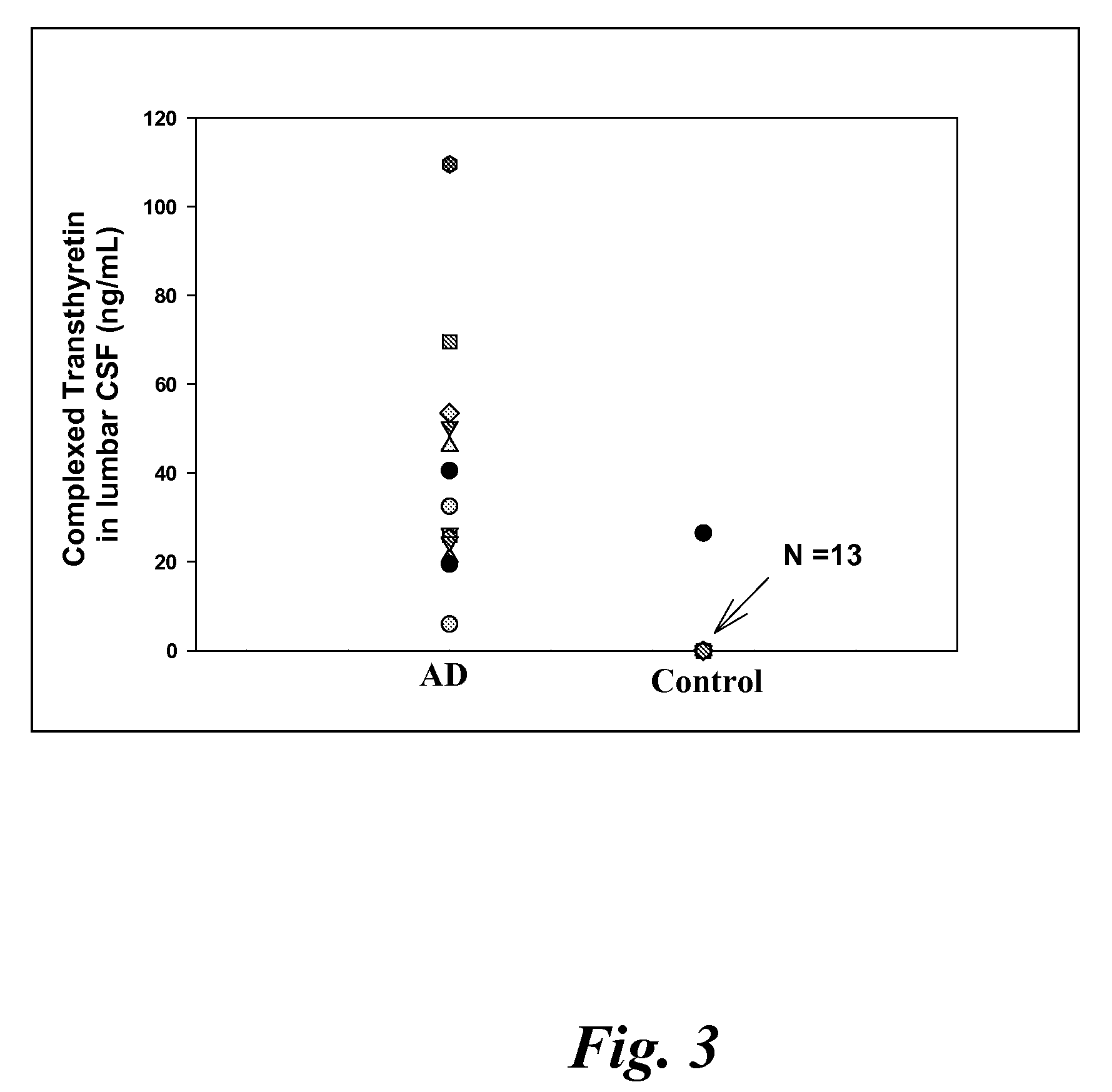
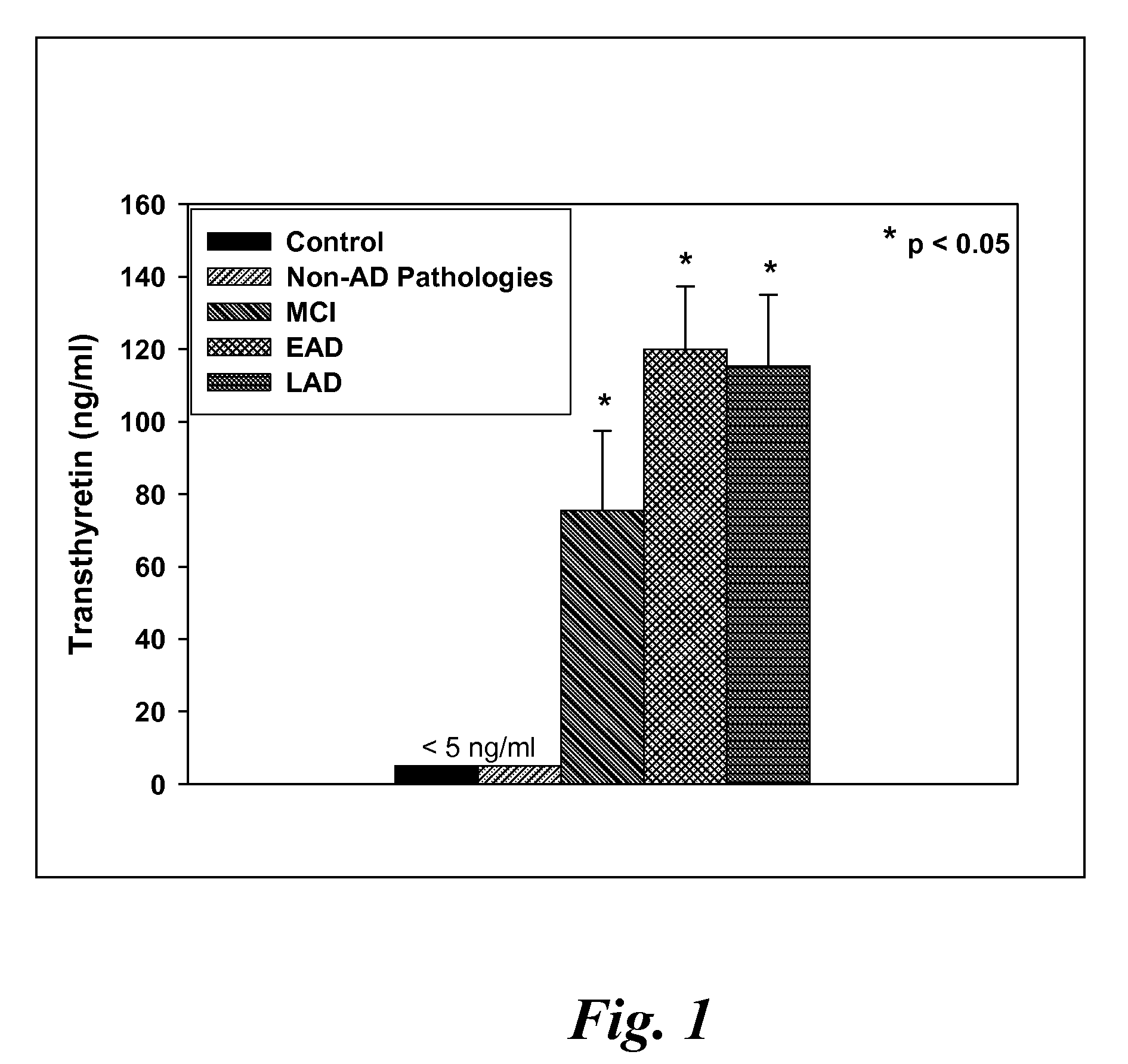
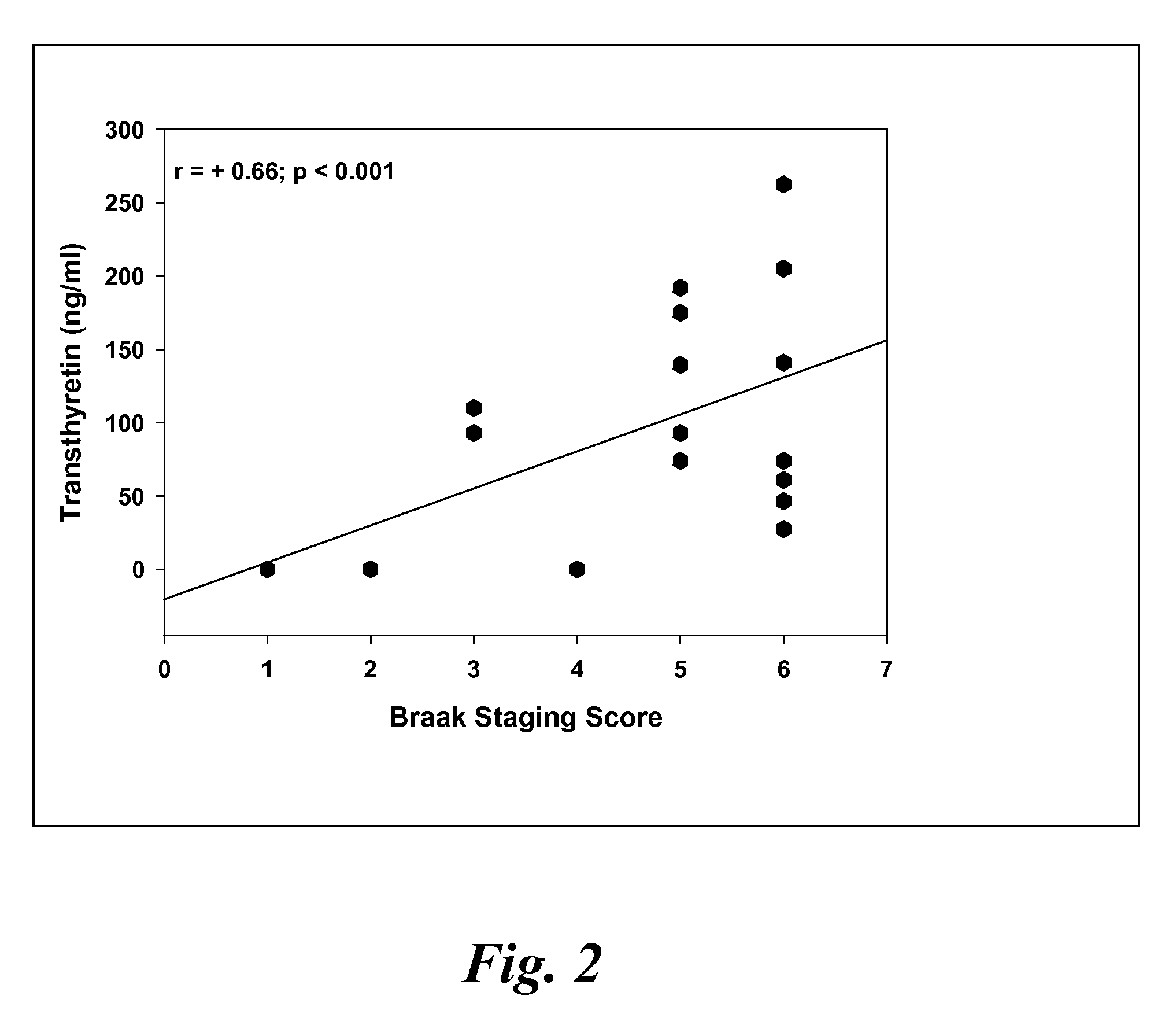
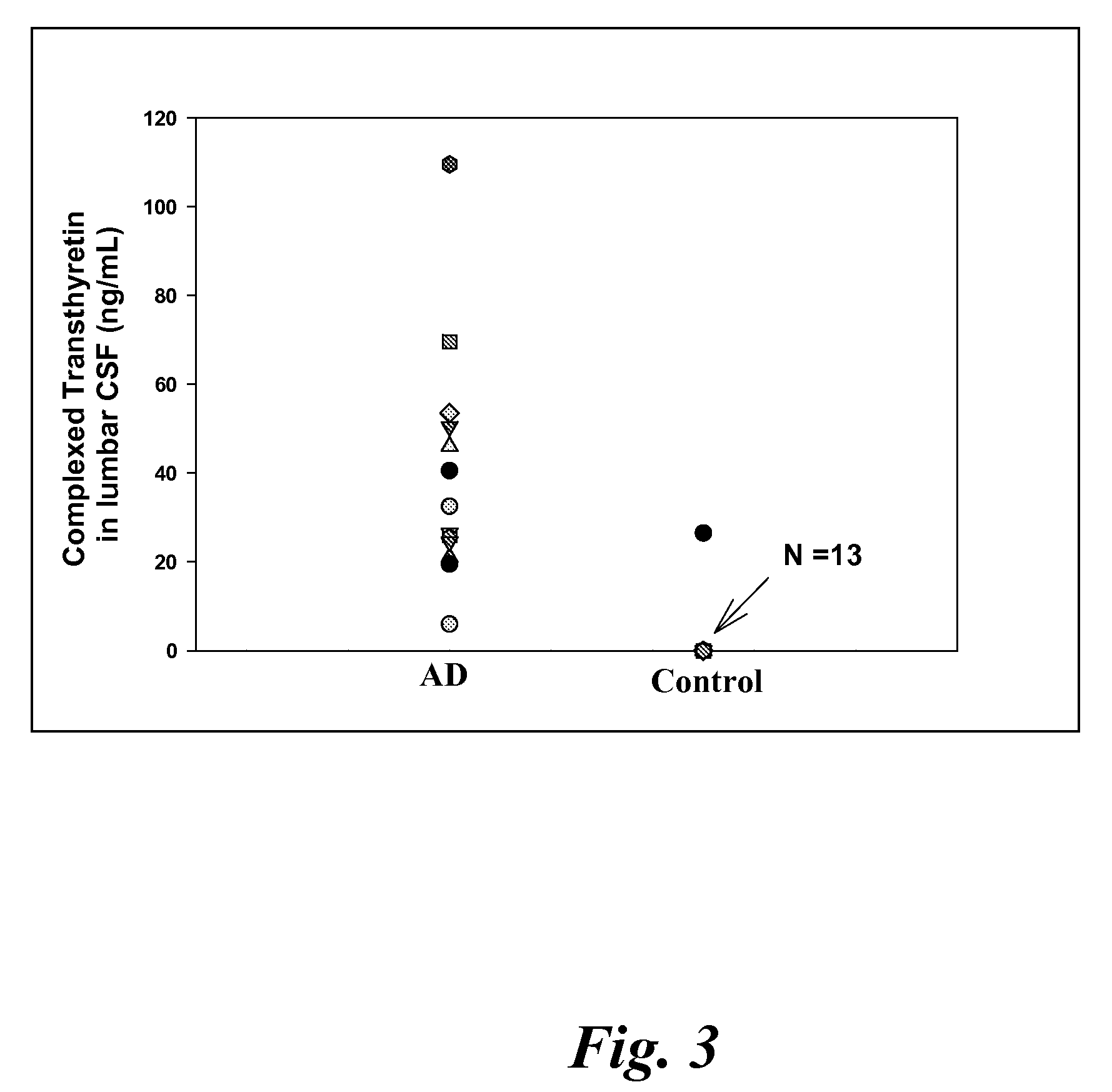
Biomarkers of mild cognitive impairment and alzheimer's disease
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein.
Owner:UNIV OF KENTUCKY RES FOUND
Diagnosis and treatment of disease
Provided herein are methods, compounds, and compositions for reducing expression of transthyretin mRNA and protein in an animal. Such methods, compounds, and compositions are useful to treat, prevent, delay, or ameliorate transthyretin amyloidosis, or a symptom thereof.
Owner:IONIS PHARMA INC
Biomarkers of mild cognitive impairment and alzheimer's disease
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein;
Owner:UNIV OF KENTUCKY RES FOUND
Transthyretin ligands capable of inhibiting retinol-dependent rbp4-ttr interaction for treatment of age-related macular degeneration, stargardt disease, and other retinal disease characterized by excessive lipofuscin accumulation
A method for treating a disease characterized by excessive lipofuscin accumulation in the retina of a mammal afflicted therewith comprising administering to the mammal an effective amount of a transthyretin (TTR) ligand.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Markers of blood barrier disruption and methods of using same
InactiveUS7144708B2Easy to useBioreactor/fermenter combinationsBiological substance pretreatmentsDisease riskScreening method
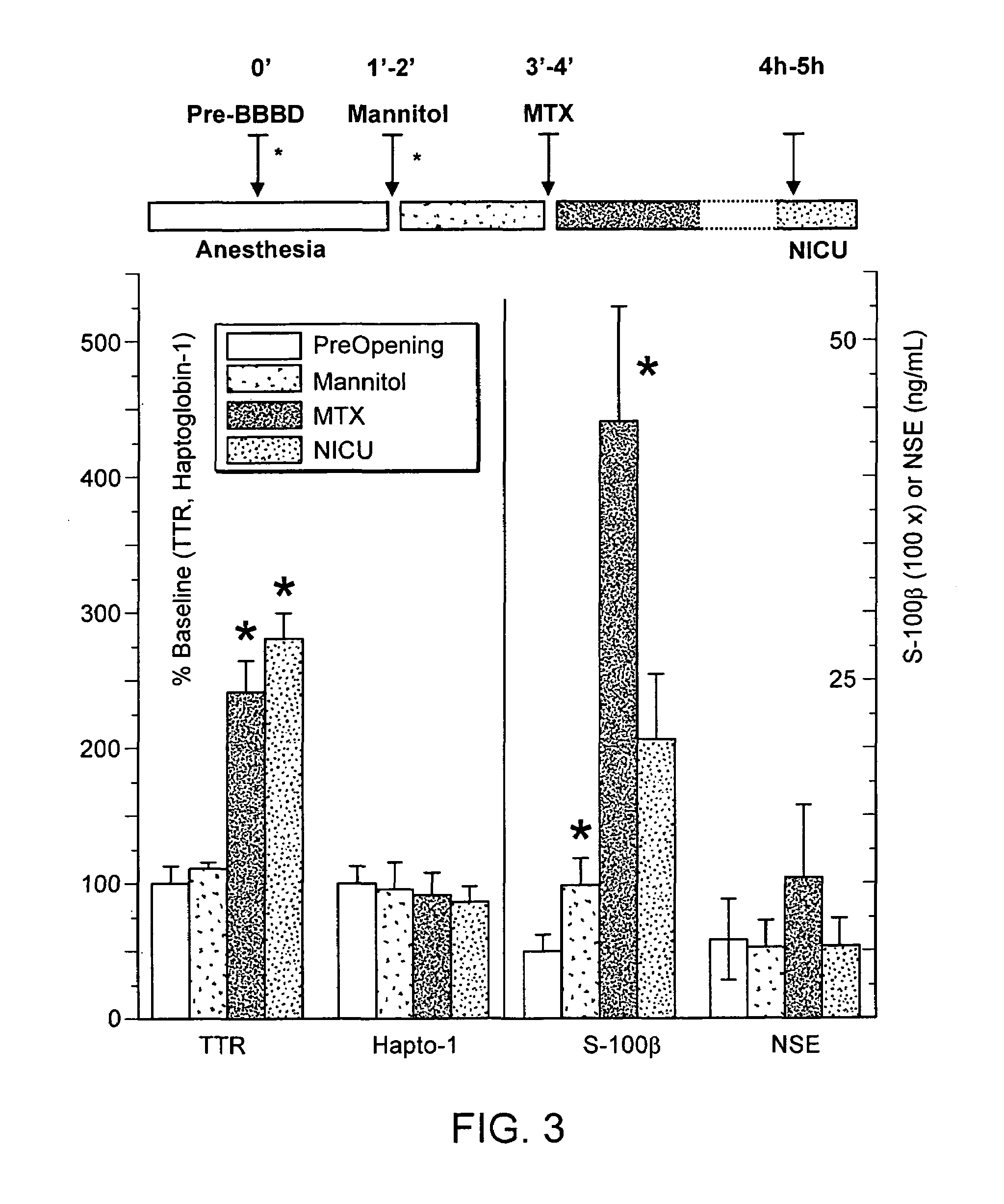
The present invention relates generally to a peripheral marker or markers of blood barrier integrity and methods of using same in the diagnosis, prognosis, and treatment of a variety of diseases. The peripheral marker(s) of the present invention are particularly useful in the differential diagnosis of diseased states. The preferred embodiments of the present invention relate to methods, compositions, kits, and assays useful in determining the integrity or permeability of either a blood CSF barrier or a blood brain barrier. The various embodiments of the present invention can be used to identify subjects at risk for developing a disease associated with increased permeability of the blood brain barrier, as well as to provide insight on the ability of an agent or agents to pass through the blood brain barrier. Embodiments of the present invention preferably involve the use of subject derived blood samples to determine the occurrence and level of circulating proteins indicative of blood brain barrier permeability or integrity. The embodiments of the present invention also provides screening methods for diagnosis, prognosis, susceptibility, or degree of permeability of penetration of the blood brain barrier by detecting the presence of serum Transthyretin either directly or through the use of antibodies.
Owner:THE CLEVELAND CLINIC FOUND
Transthyretin antibodies and uses thereof
The present invention provides compositions comprising anti-transthyretin antibodies. The compositions are particularly useful for diagnosis, prognosis and / or treatment of amyloid diseases or symptoms thereof.
Owner:PROTEGO BIOPHARMA INC
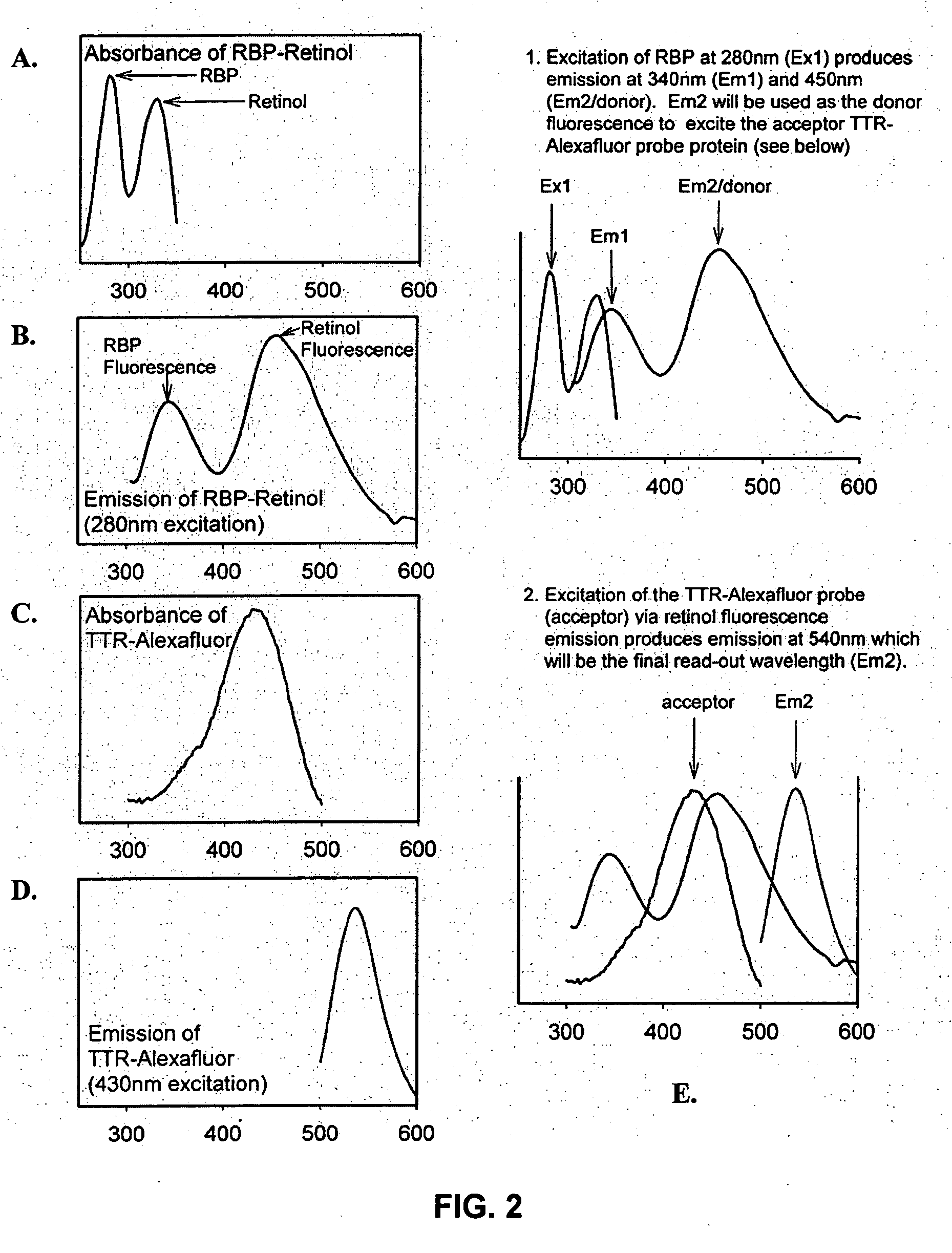
Modulators of retinol-retinol binding protein (RBP)-transthyretin (TTR) complex formation
Described herein are methods and compositions for the detection of transthyretin (TTR), retinol binding protein (RBP) and retinol complex formation. The methods and compositions described herein also provide for the screening of modulators of retinol-RBP-TTR complex formation. Furthermore, the methods and compositions provide for therapeutic agents for the treatment and / or prevention of age-related macular degeneration and / or dystrophies.
Owner:ACUACELA INC
Minigene expression cassette
Methods and compositions for expressing a gene or nucleotide sequence of interest are provided. The compositions include an expression cassette that includes a synthetic enhancer, a transthyretin promoter, and a nucleotide sequence operably under the control of the synthetic enhancer and the transthyretin promoter. The expression cassette may be used in an adeno-associated viral (AAV) vector, such as a self-complementary AAV vector.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Anti-transthyretin antibodies
ActiveUS20170058023A1Delay is slowImmunoglobulins against animals/humansRadioactive preparation carriersDiseaseAntibody
The invention provides antibodies that specifically bind transthyretin (TTR). The antibodies can be used for treating or effecting prophylaxis of diseases or disorders associated with TTR accumulation or accumulation of TTR deposits (e.g., TTR amyloidosis). The antibodies can also be used for diagnosing TTR amyloidosis and inhibiting or reducing aggregation of TTR, among other applications.
Owner:UNIV HEALTH NETWORK +1
Multiplexed biomarkers for monitoring the alzheimer's disease state of a subject
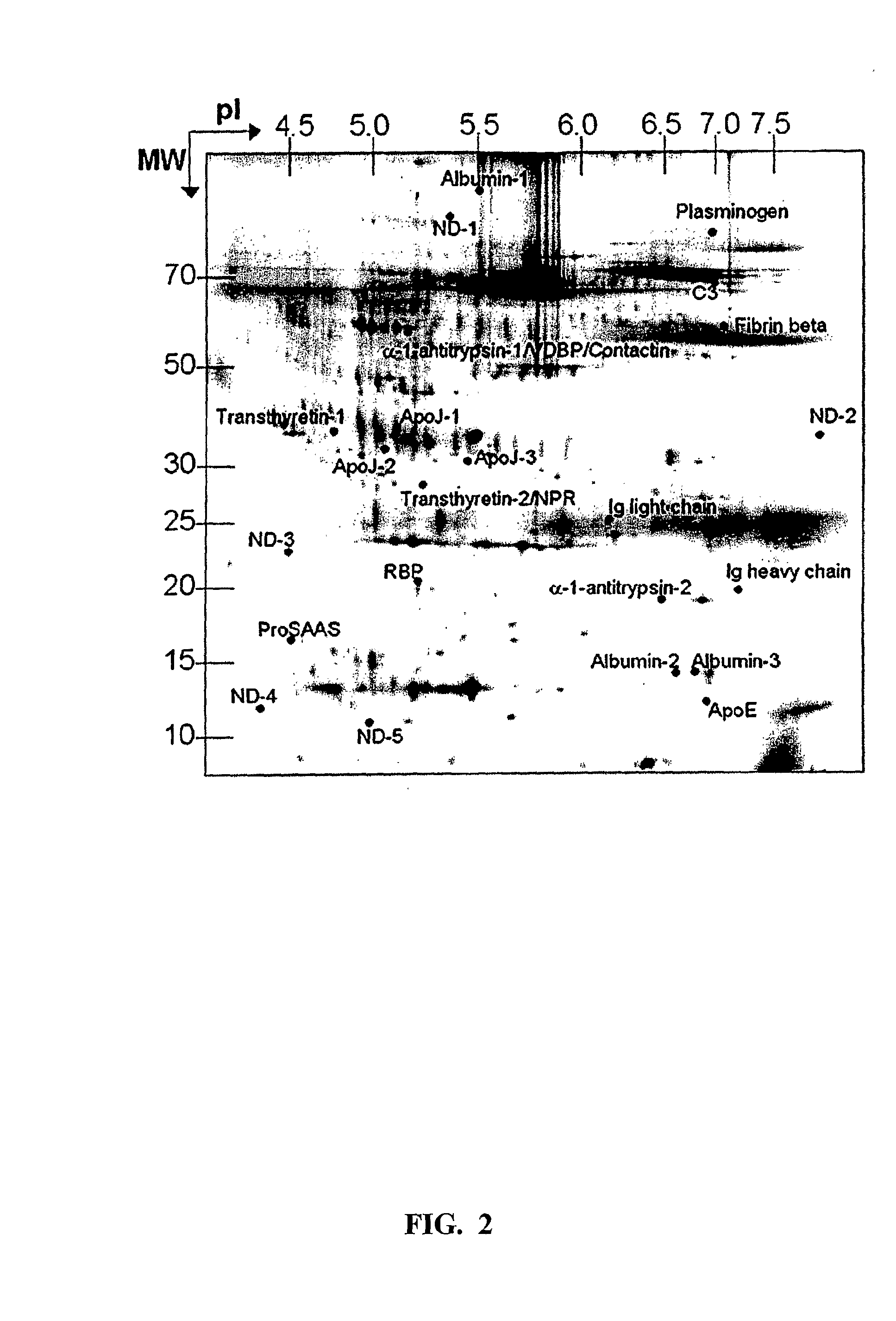
The present invention relates to a method for diagnosing a subject's Alzheimer's disease state. The method involves providing a database containing information relating to protein expression levels associated and not associated with Alzheimer's disease. The database includes information relating to at least a majority of the following proteins: albumin, alpha-1-antitrypsin, apolipoprotin E, apolipoprotein J, complement component 3, contactin, fibrin beta, Ig heavy chain, Ig light chain, neuronal pentraxin receptor, plasminogen, proSAAS, retinol-binding protein, transthyretin, and vitamin D binding protein. Information relating to proteins found in one or more cerebrospinal fluid samples from a subject is also provided and a database is used to analyze the information from the subject to diagnose the subject's Alzheimer's disease state. Also disclosed is a computer readable medium and a system, both useful in carrying out the present invention.
Owner:CORNELL RES FOUNDATION INC
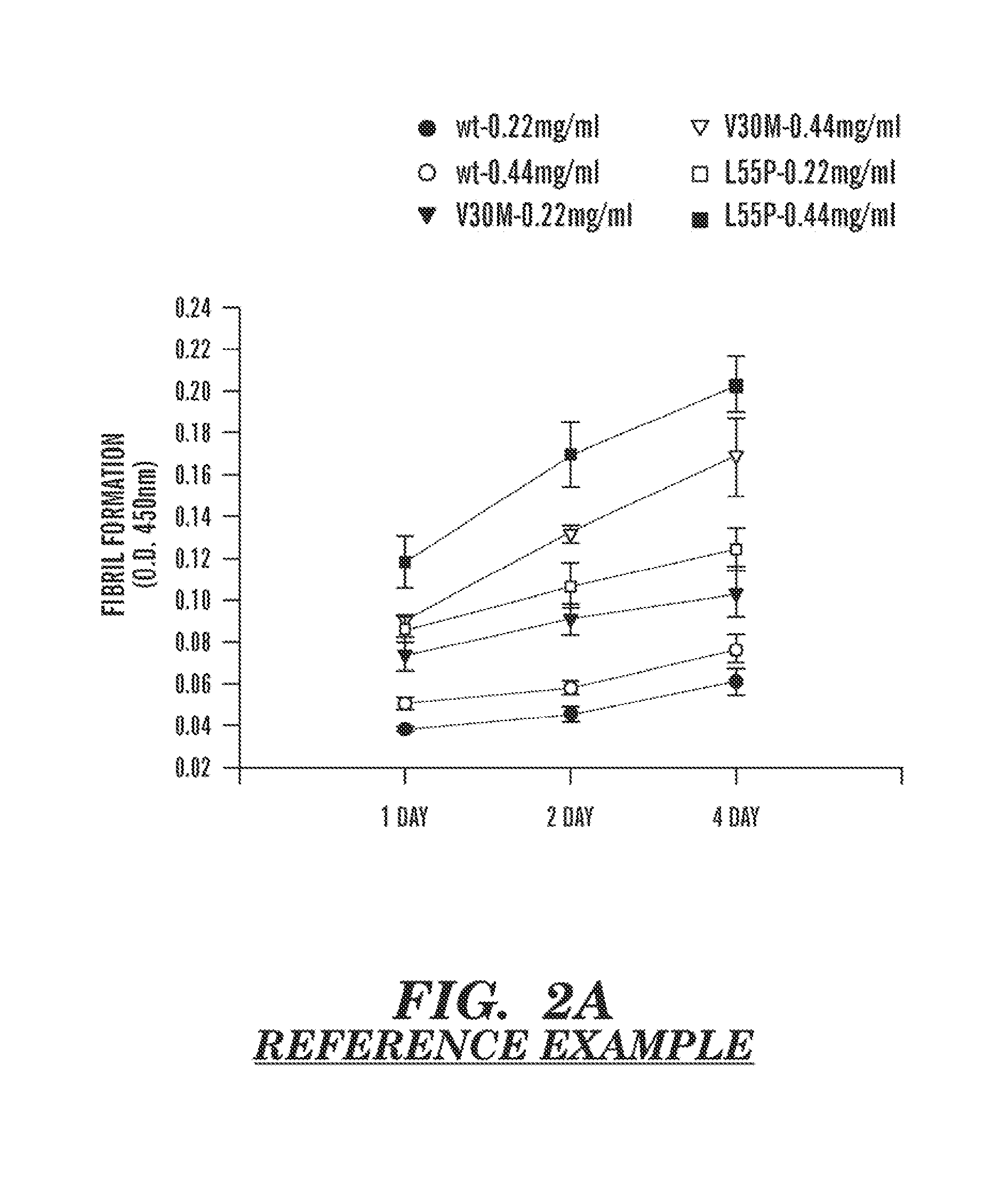
Mechanism-based inhibitors of transthyretin amyloidosis: studies with biphenyl ethers and structural templates
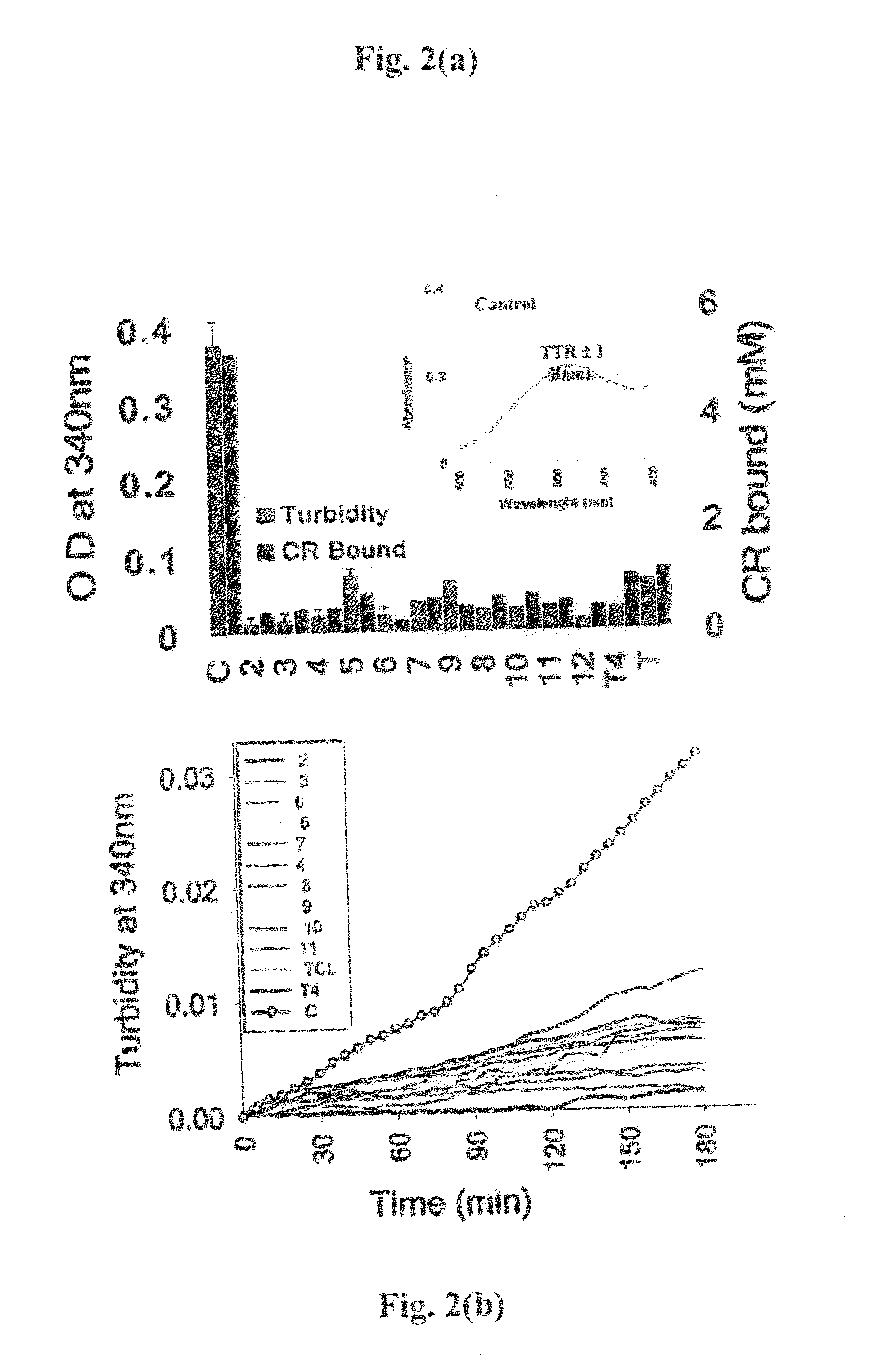
Transthyretin (TTR), a tetrameric thyroxine (T4) carrier protein, is associated with a variety of amyloid diseases. Derivative of biphenyl ethers (BPE), which are shown to interact with a high affinity to its T4 binding site thereby preventing its aggregation and fibrillogenesis. They prevent fibrillogenesis by stabilizing the tetrameric ground state of transthyretin. Two compounds (2-(5-mercapto-[1,3,4]oxadiazol-2-yl)-phenol and 2,3,6-trichloro-N-(4H-[1,2,4]triazol-3-yl) exhibit the ability to arrest TTR amyloidosis. The dissociation constants for the binding of BPEs and compound 11 and 12 to TTR correlate with their efficacies of inhibiting amyloidosis. They also have the ability to inhibit the elongation of intermediate fibrils as well as show nearly complete (>90%) disruption of the preformed fibrils. Biphenyl ethers and compounds 11 and 12 as very potent inhibitors of TTR fibrillization and inducible cytotoxicity.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Methods and biomarkers for diagnosing and monitoring psychotic disorders such as schizophrenia
InactiveCN101356446ALittle side effectsShorten the timeMagnetic measurementsBipolar mood disorderMulti analyte
The invention relates to methods of diagnosing or monitoring a psychotic disorder in a subject comprising providing a test biological sample from the subject, performing spectral analysis on said test biological sample to provide one or more spectra, and, comparing the one or more spectra with one or more control spectra. The invention also relates to methods for diagnosing or monitoring psychotic disorders such as schizophrenic or bipolar disorders, comprising measuring the level of one or more biomarkers present in a biological sample taken from a test subject, said biomarkers being selected from the group consisting of transthyretin, ApoA1, VLDL, LDL and aromatic species such as plasma proteins. The invention also relates to sensors, biosensors, multi-analyte panels, arrays, assays and kits for performing methods of the invention.
Owner:PSYNOVA NEUROTECH LTD
Diagnosis Of Neurodegenerative Diseases
InactiveUS20080286263A1Improve trustNervous disorderElectrolysis componentsHemoglobin Beta ChainApolipoprotein C-II
The invention relates to a method of diagnosis of Huntington's Disease in a diagnostic sample of a valid body tissue taken from a human subject, which comprises detecting an altered concentration of a protein in the diagnostic sample, compared with a sample of a control human subject, the protein being selected from: Swiss Prot accession number: Protein name; P10909: Clusterin precursor; P00738: Haptoglobin precursor; P01009: Alpha-1-antitrypsin precursor; P01024: Complement C3 precursor; P01620: 1 g kappa chain V-III region; P01834: 1 g kappa chain C region P01842: 1 g lambda chain C regions; P01857: 1 g gamma-1 chain C region; P01859: Ig gamma-2 chain C region; P01876: 1 g alpha-1 chain C region P02647: Apolipoprotein A-I precursor; P02649: Apolipoprotein E precursor; P02652: Apolipoprotein A-II precursor; P02655: Apolipoprotein C-II precursor; P02656: Apolipoprotein C-II precursor P02671: Fibrinogen alpha / alpha-E chain precursor; P02763: Alpha-1-acid glycoprotein 1 precursor; P02766: Transthyretin precursor; P02768: Serum albumin precursor; P02787: Serotransferrin precursor; P04196: Histidine-rich glycoprotein precursor; P06727: Apolipoprotein A-IV precursor; P19652: Alpha-1-acid glycoprotein 2 precursor; P68871 / P02042: Hemoglobin beta chain / Hemoglobin delta chain; P60709: Beta actin.
Owner:ELECTROPHORETICS LTD
Tumor marker for ovarian cancer diagnosis
InactiveUS20070134689A1Accurate detectionMicrobiological testing/measurementMaterial analysisAbnormal tissue growthCathepsin B
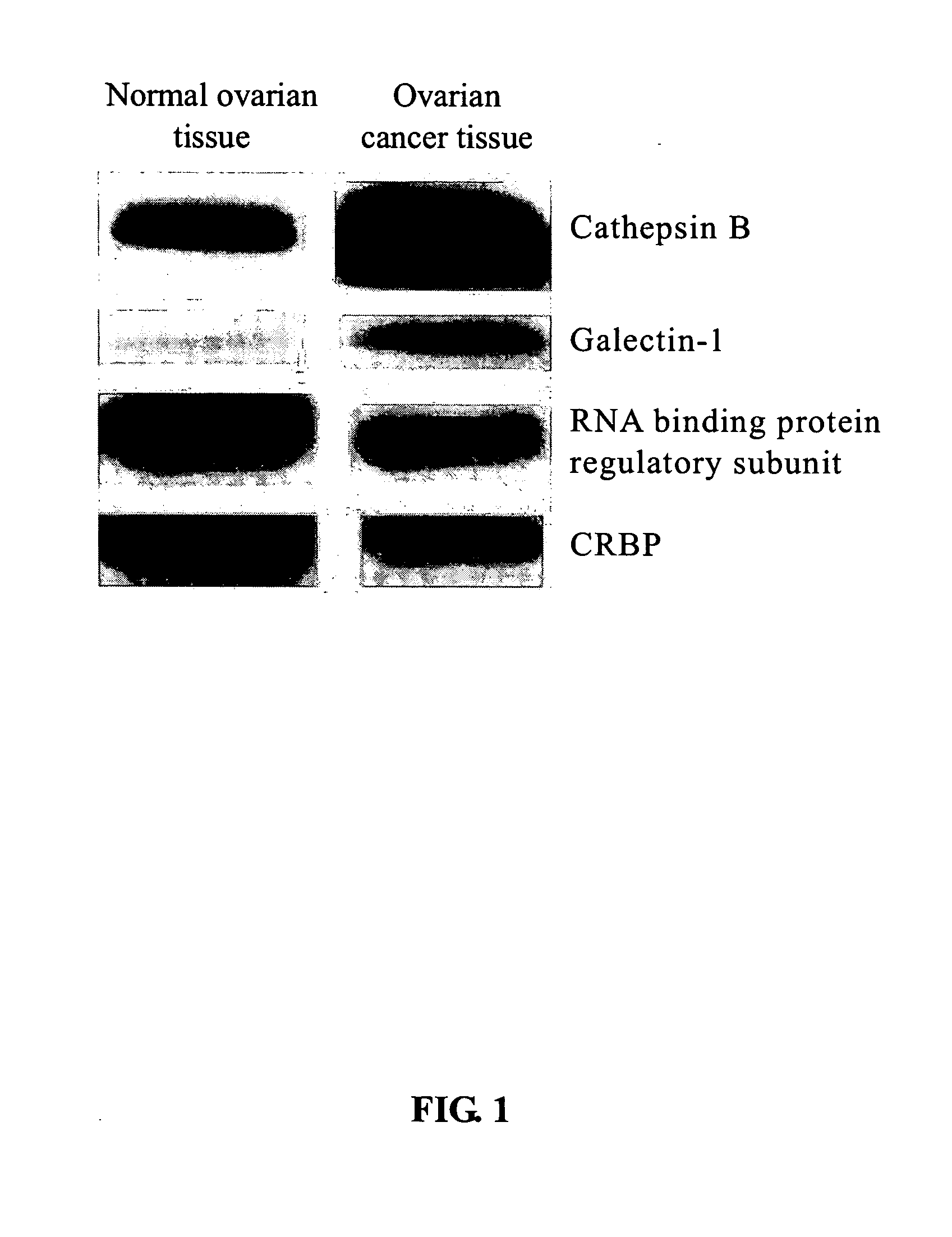
The present invention relates to a tumor marker for diagnosis of ovarian cancer, which is selected from the group consisting of alectin-1, cathepsin B, MHC class I antigen, heat shock protein (HSP) 27, ubiquitin carboxy-termal esterase L1, cellular retinol-binding protein (CRBP), transthyretin, SH3 binding glutamate-rich protein, tubulin-specific chaperone A, RNA binding protein regulatory subunit, γ-actin, tropomyosin and calcium / calmodulin-stimulated cyclic nucleotide phosphatase. The ovarian cancer is diagnosed effectively and efficiently based on detecting the expression levels of the tumor markers in the invention from the ovarian tissue sample of an individual to be diagnosed.
Owner:NAT TAIWAN UNIV
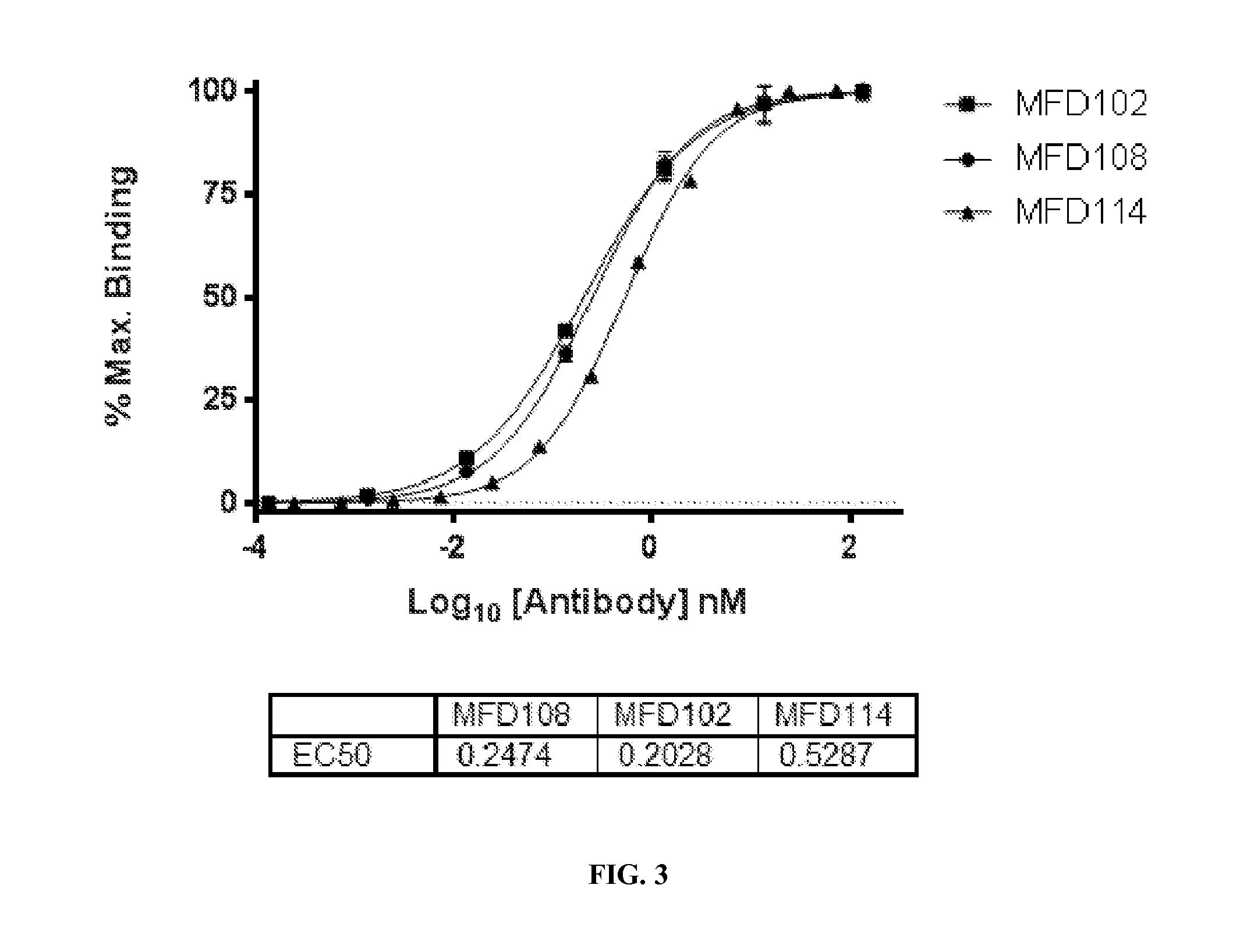
Antibody-based therapy of transthyretin (TTR) amyloidosis and human-derived antibodies therefor
Provided are novel human-derived antibodies specific for transthyretin (TTR), preferably capable of binding misfolded, misassembled, and / or aggregated TTR species, as well as methods related thereto. In addition, methods of diagnosing and / or monitoring diseases and treatments thereof which are associated with TTR amyloidosis are provided. Assays and kits related to anti-bodies specific for TTR or TTR deposits and aggregates are also disclosed. The novel anti-TTR antibodies can be used in pharmaceutical and diagnostic compositions for TTR targeted immunotherapy and diagnostics.
Owner:NEURIMMUNE HLDG
Anti-transthyretin antibodies
ActiveUS20170121398A1Improve bindingNervous disorderImmunoglobulins against animals/humansAntibodyAmyloidosis
The invention provides antibodies that specifically bind to transthyretin (TTR). The antibodies can be used for treating or effecting prophylaxis of diseases or disorders associated with TTR accumulation or accumulation of TTR deposits (e.g., TTR amyloidosis). The antibodies can also be used for diagnosing TTR amyloidosis and inhibiting or reducing aggregation of TTR, among other applications.
Owner:UNIV HEALTH NETWORK +1
Anti-transthyretin antibodies
InactiveUS20160257736A1Improve bindingNervous disorderImmunoglobulins against animals/humansAmyloidosisDisease cause
The invention provides antibodies that specifically bind to transthyretin (TTR). The antibodies can be used for treating or effecting prophylaxis of diseases or disorders associated with TTR accumulation or accumulation of TTR deposits (e.g., TTR amyloidosis). The antibodies can also be used for diagnosing TTR amyloidosis and inhibiting or reducing aggregation of TTR, among other applications.
Owner:UNIV HEALTH NETWORK +1
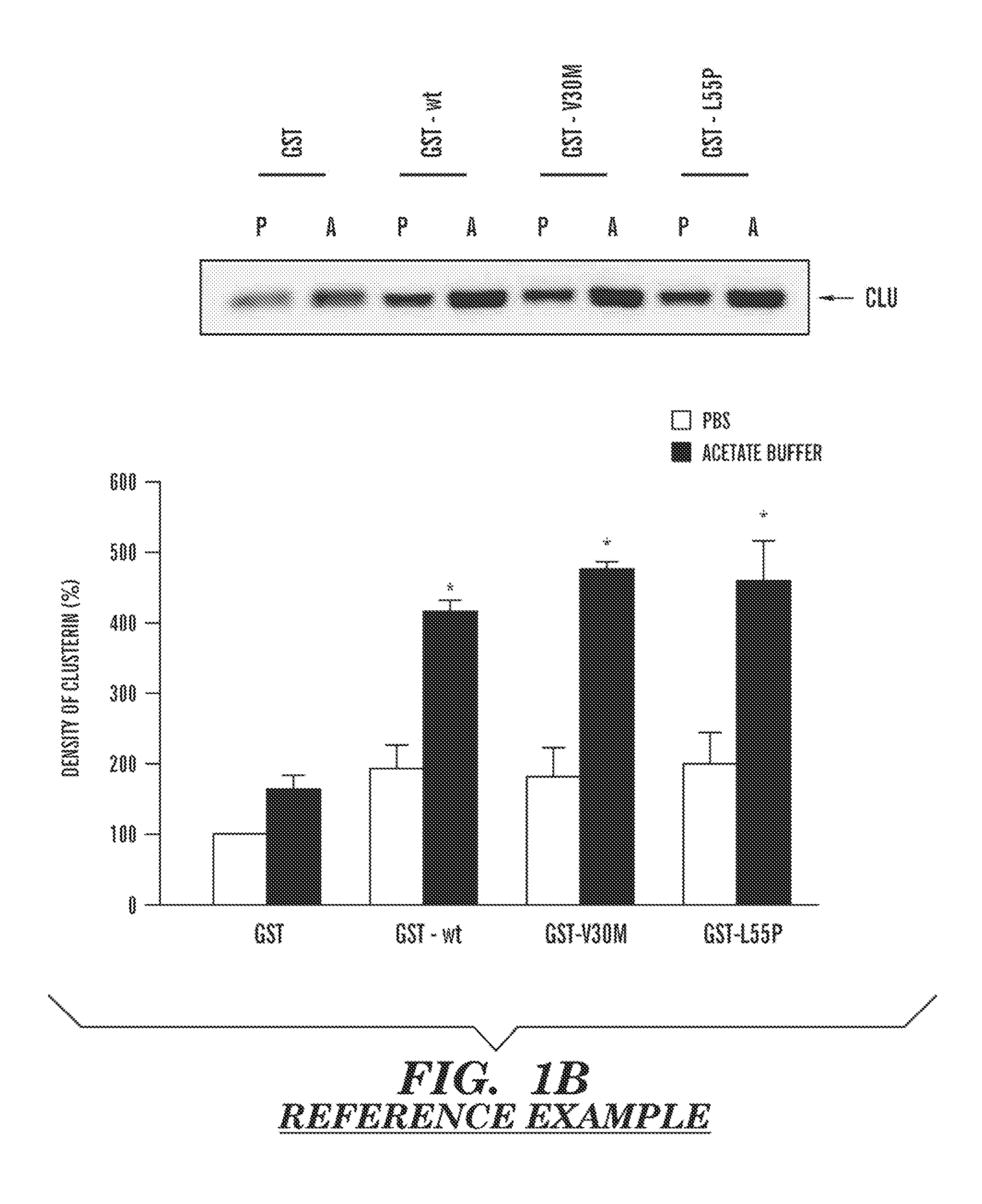
Serum clusterin levels in systemic amyloidosis featuring cardiomyopathy
InactiveUS20130203083A1Reduced cardiac amyloid depositImproved prognosisBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseDiagnostic methods
The present invention relates generally to diagnostic methods, systems, assays and kits for identification of subjects with cardiac amyloid deposits, where a low level of clusterin protein in a peripheral fluid sample, e.g., a serum sample from the subject, indicates the subject likely has cardiac amyloid deposits. Other aspects relate to methods of treatment of diseases or disorders characterized by cardiac amyloid deposits and transthyretin (TTR) amyloidosis, and more particularly to methods of treatment of cardiac-related amyloidosis and cardiac amyloid deposits in subjects with familial transthyretin (TTR), senile systemic amyloidosis (SSA), or familial amyloidodic polyneuropathy (FAP), or immunoglobulin light chain (AL) amyloidosis. Other aspects relate to methods and compositions comprising clusterin (CLU) or a clusterin agent (e.g. an agonist of clusterin activity or a biologically active fragment or derivative thereof), and their use in methods to treat a disease or disorder characterized by transthyretin (TTR) amyloidosis, e.g. senile systemic amyloidosis (SSA) or familial amyloidodic polyneuropathy (FAP), and their use in methods to treat amyloidotic cardiomyopathy associated with transthyretin (TTR) amyloidosis.
Owner:TRUSTEES OF BOSTON UNIV
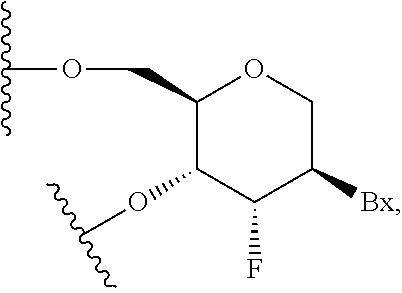
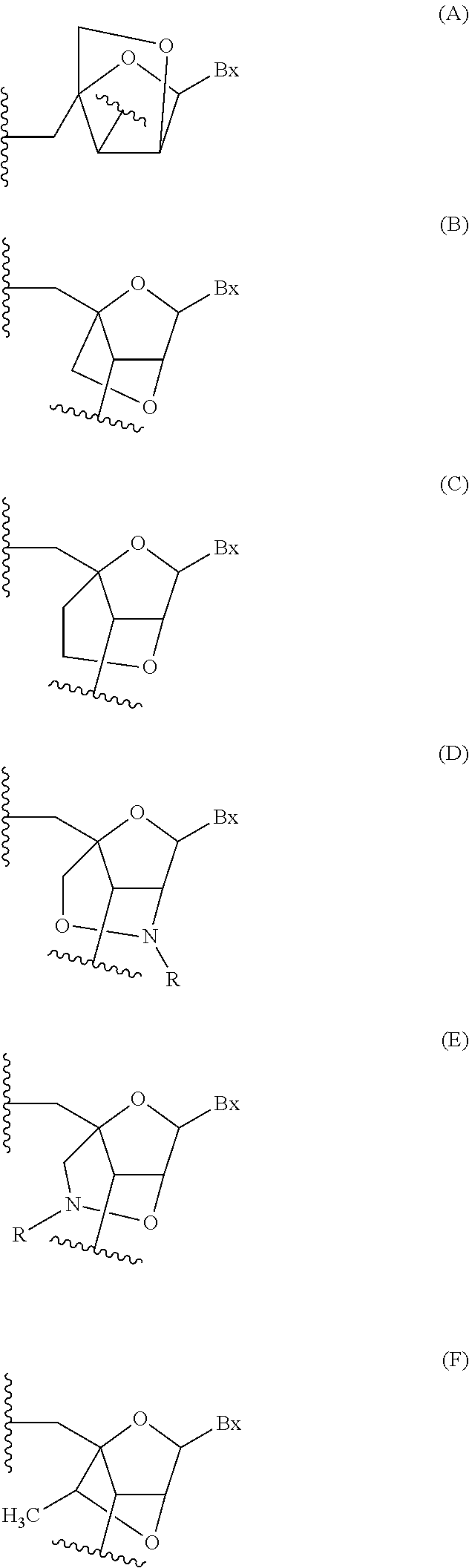
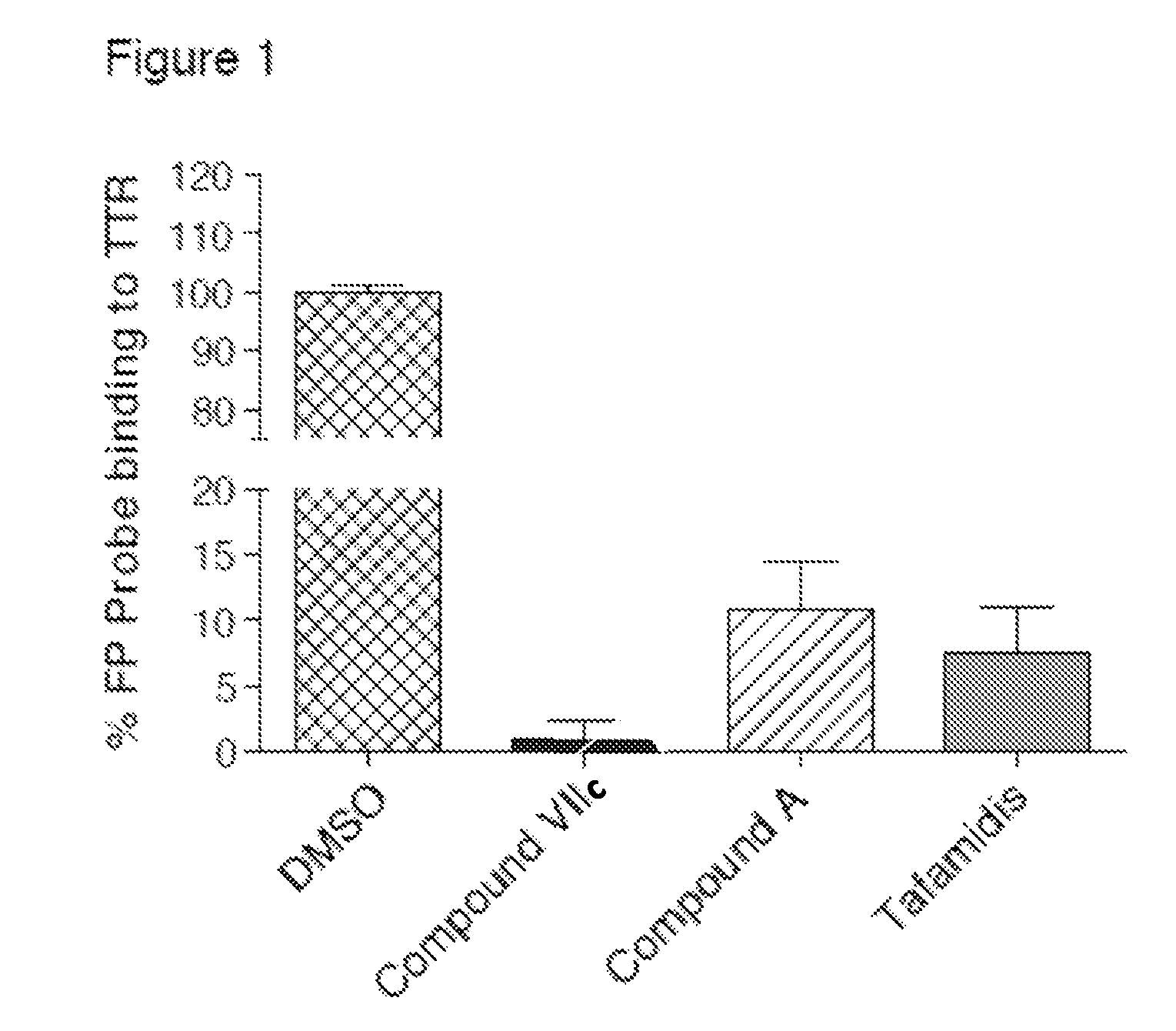
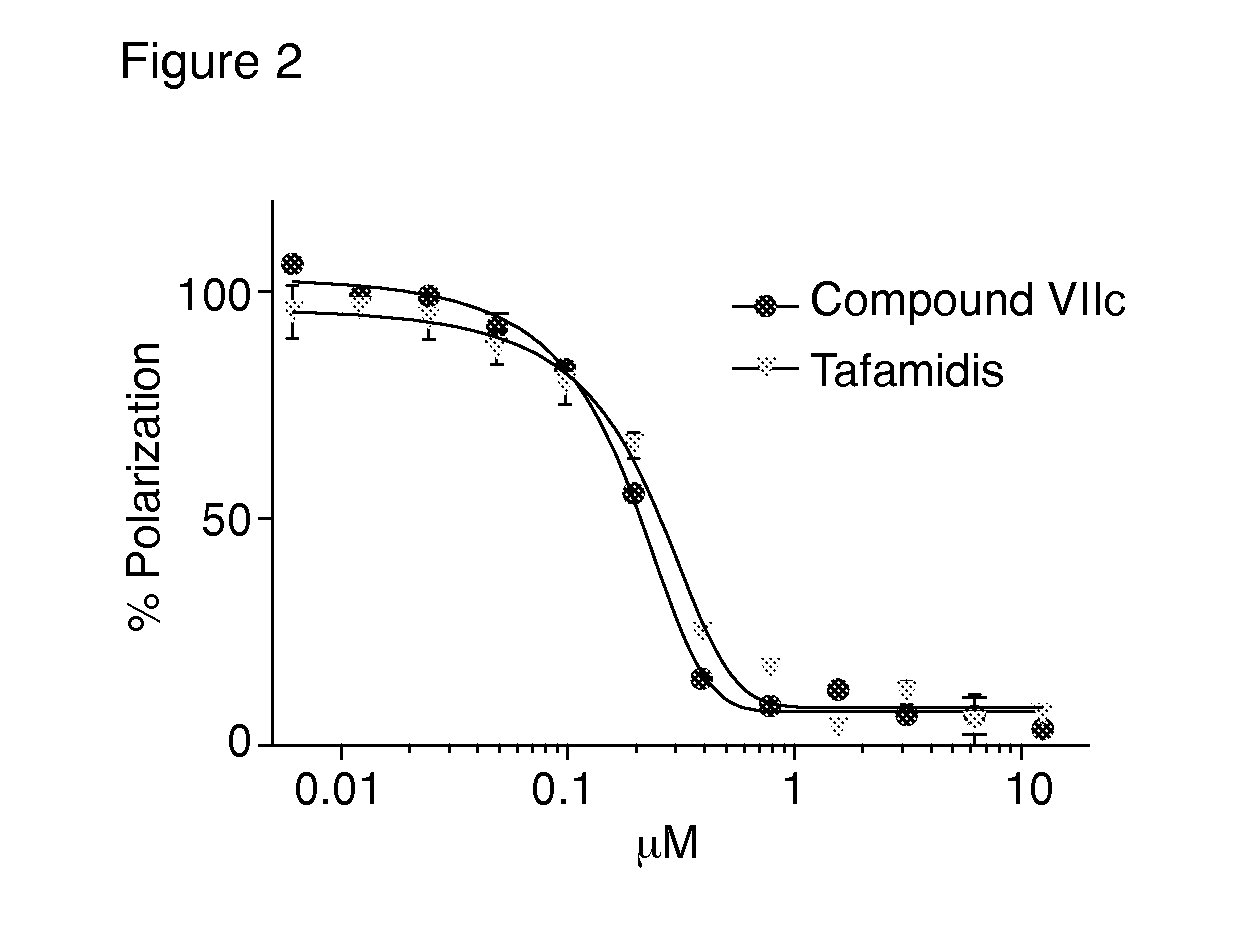
Compounds and compositions that bind and stabilize transthyretin and their use for inhibiting transthyretin amyloidosis and protein-protein interactions
ActiveUS9169214B2Improve stabilityDecreasing aggregate formationOrganic active ingredientsSenses disorderProtein targetOrganic chemistry
Disclosed herein are compounds and compositions thereof which find use in increasing stability of proteins particularly proteins that tend to misfold and form aggregates. Also provided herein are methods for using these compounds and compositions for increasing stability of proteins and thereby decreasing aggregate formation by these proteins. Also disclosed herein are heterobifunctional compounds that include a TTR binding compound connected to a targeting moiety via a linker, for use in disrupting PPIs of a target protein.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Biomarker of Parkinson disease
InactiveCN101661032AMicrobiological testing/measurementMaterial analysis by electric/magnetic meansDiagnosis earlyBiomarker (petroleum)
The invention relates to a biomarker of Parkinson disease, in particular to a method used for detecting pathogeny of the Parkinson disease and identifying the biomarker of the Parkinson disease (PD).The invention also provides a kit used for diagnosing or monitoring PD. A proteinic isoform or a monomer or polymer form thereof can be used for early diagnosing vulnerable subjects who are possible to suffer from PD. For example, the increase of the level of monomer transthyretin (TTR) in serum or the reduction of the level of dipolymer TTR can be used as a tool for diagnosing and monitoring PD.
Owner:生物远景技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com