Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1001 results about "Ocular disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
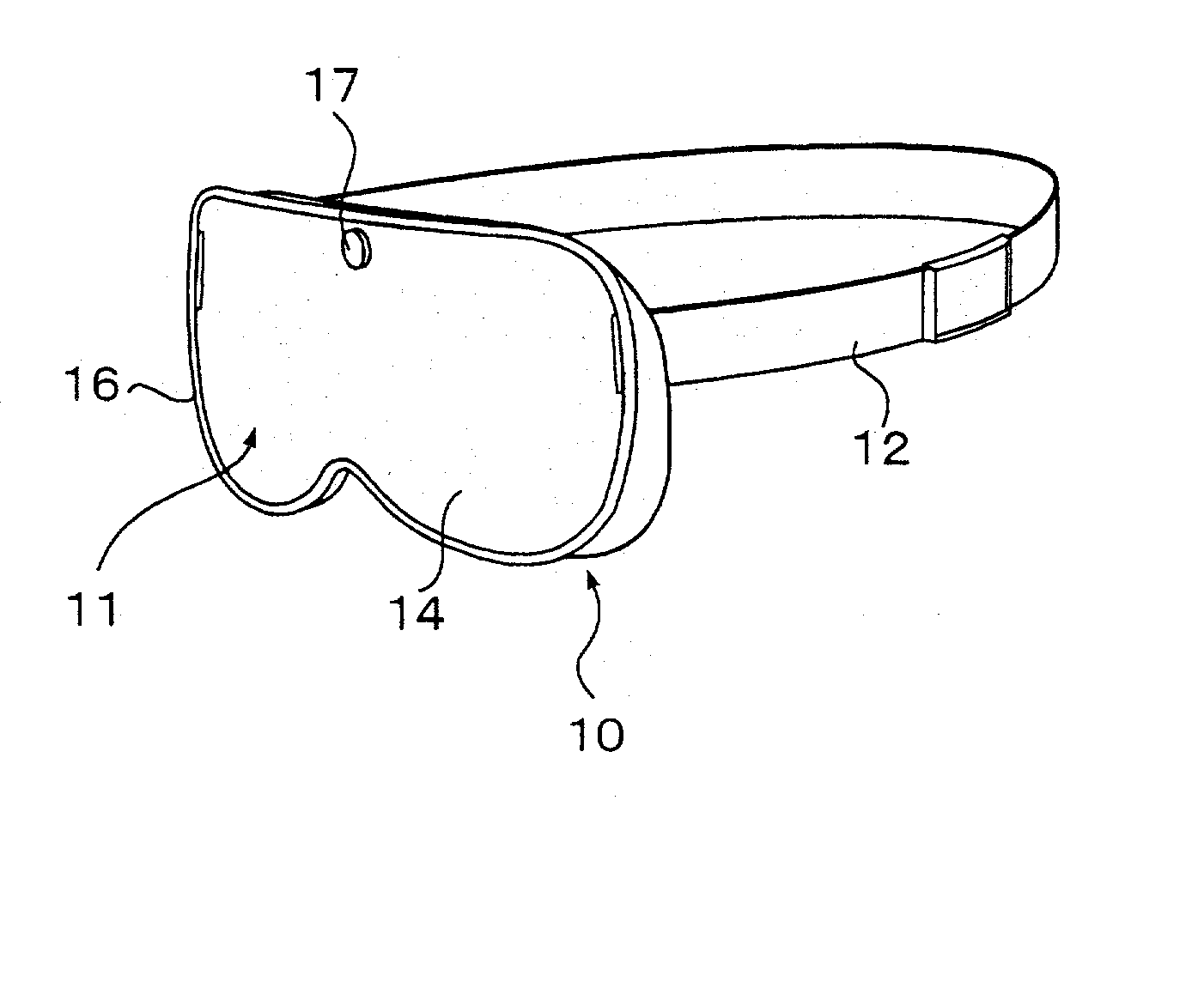
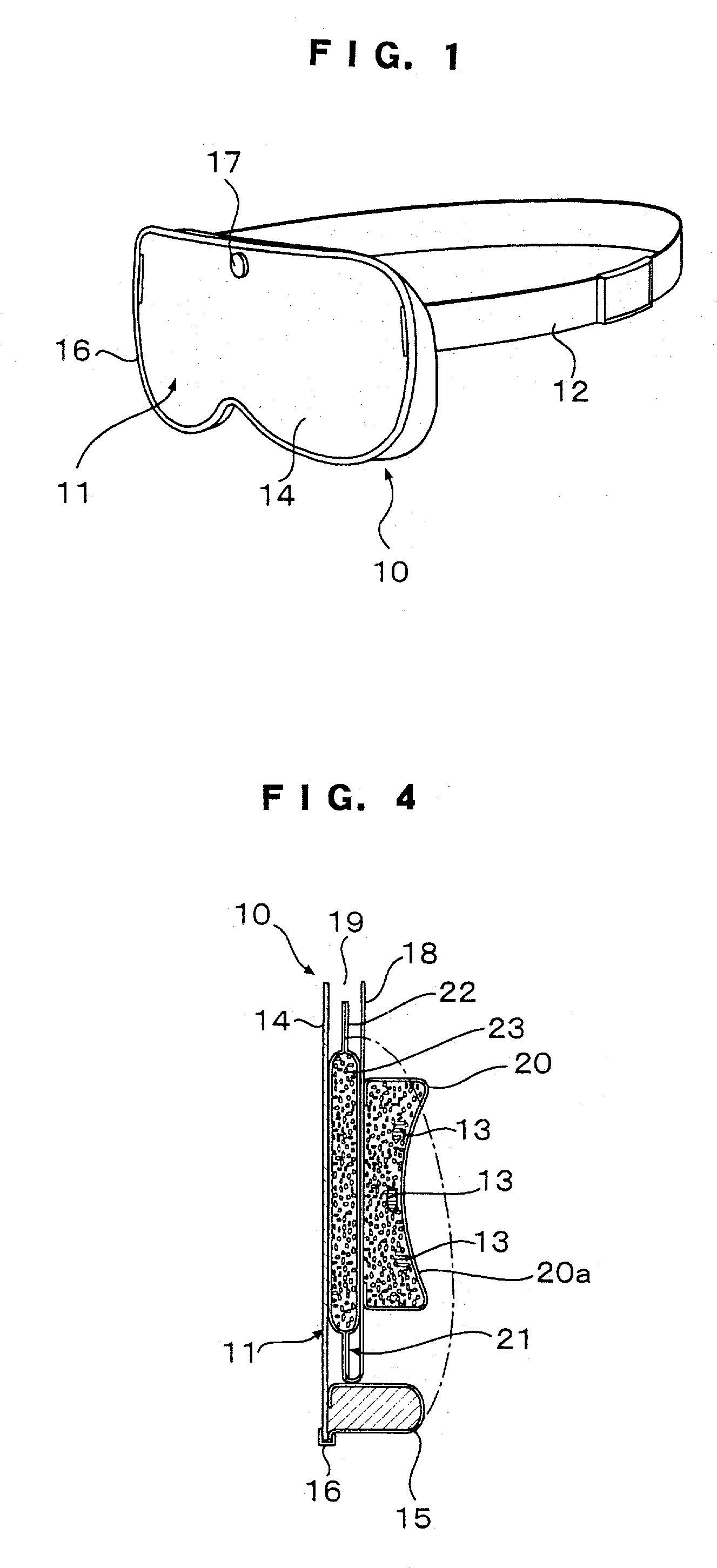
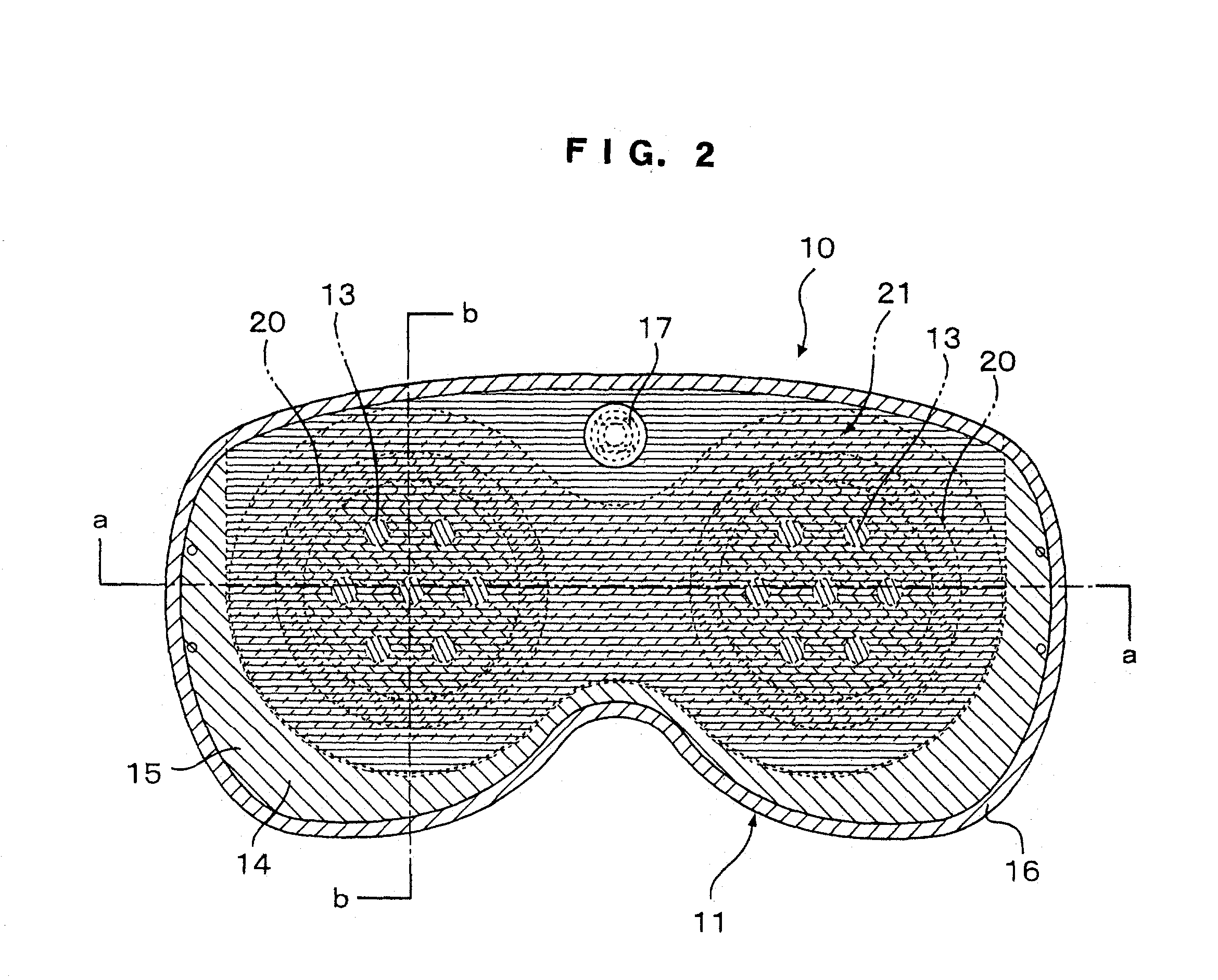
Eye mask
InactiveUS20030056281A1Recovering and restoringSimple structureElectrotherapyVibration massageDiseaseFarsightedness
An eye mask has magnetic bodies and self-heating warm members, which are inserted in eye pads on a mask member to be placed over eyeball parts. If required, vibrators and illumination bodies may be additionally placed in the eye pads. Thus, fatigue on the eyes and surroundings thereof can be relieved by the magnetic actions of the magnetic bodies and the warming effects of the warming member, in addition to expected effects of restoring ocular functions, recovering from various ocular diseases, and so on. Furthermore, the surface of each of the eye pads is gradually curved like the inner surface of a sphere. When the eye pads are press-contact to the eyeball parts at predetermined pressures for a long time, the cornea can be warmed by the warming members so that the shape of the cornea can be changed along the shape of the eye pad, resulting in the effects of recovering from eye sight disorder such as pseudo-myopia, moderate farsightedness, or moderate astigmatism.
Owner:HASEGAWA TOKUICHIRO
Janus kinase inhibitors for treatment of dry eye and other eye related diseases
InactiveUS20100113416A1High expressionIncrease productionBiocideOrganic active ingredientsDiseaseOcular disease
Owner:INCYTE
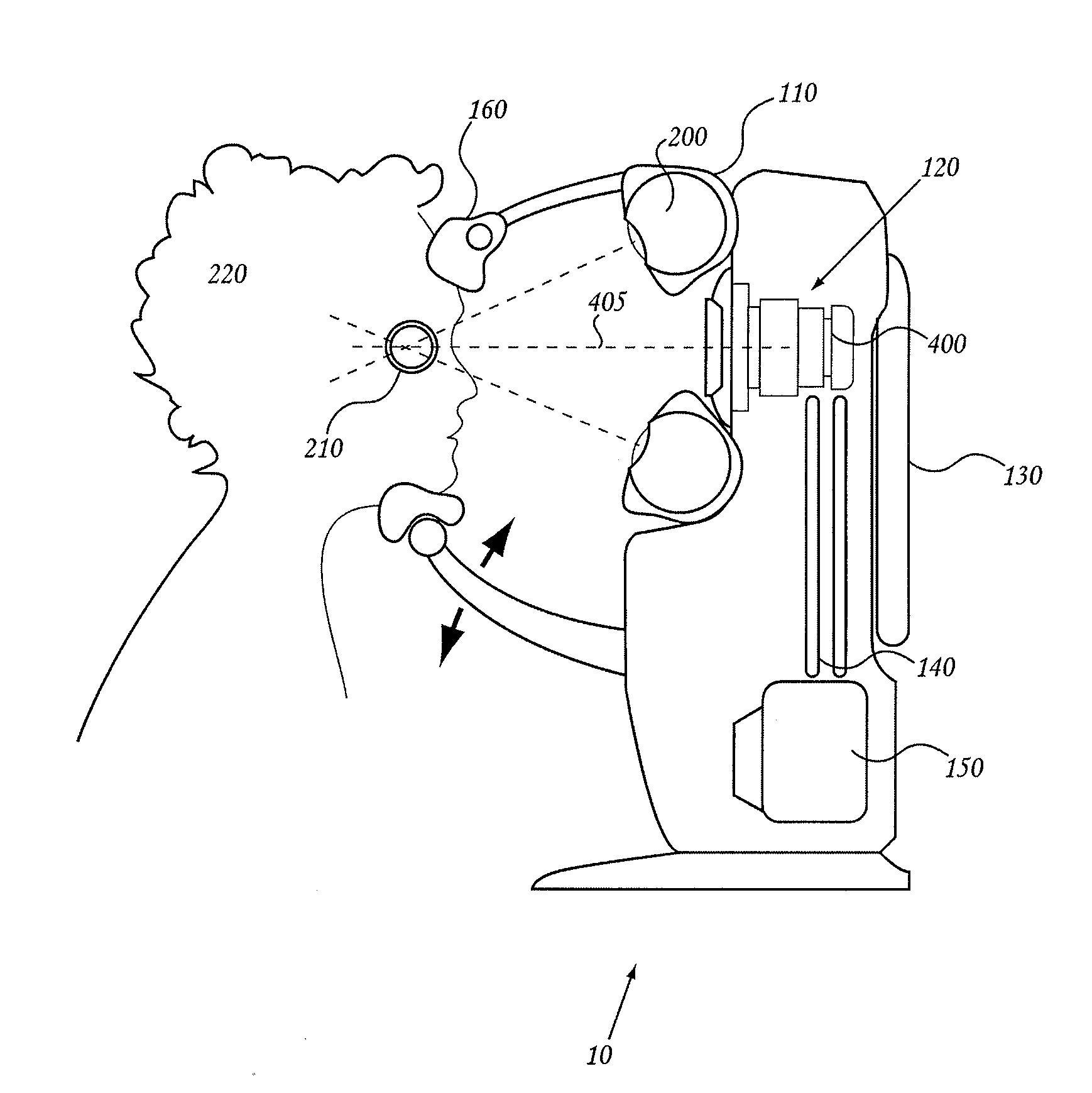
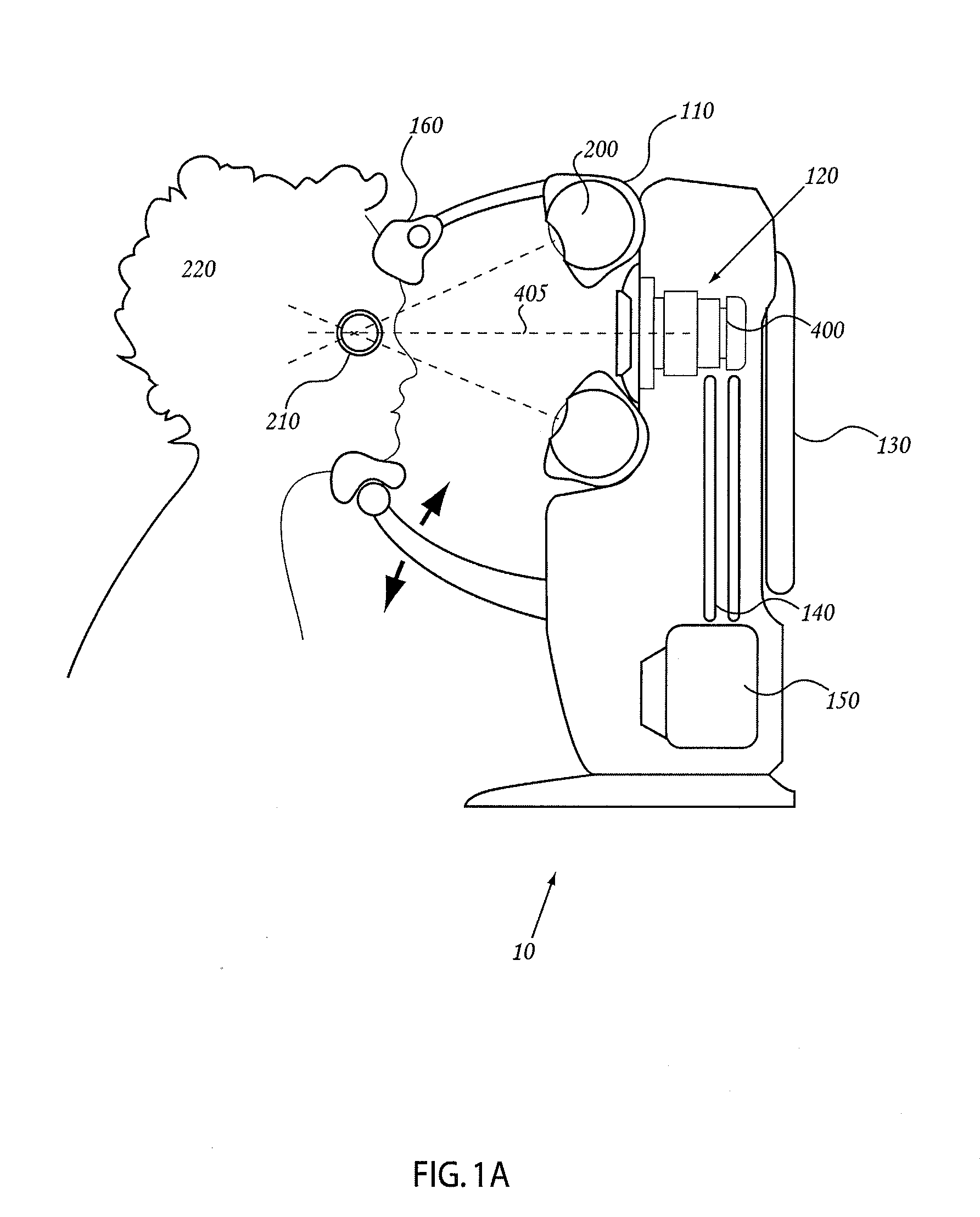
Orthovoltage radiotherapy
ActiveUS20080212738A1Dry up neovascular membraneStabilized and improved acuityHandling using diaphragms/collimetersRadiation beam directing meansRadiosurgeryBeam energy
A radiosurgery system is described that is configured to deliver a therapeutic dose of radiation to a target structure in a patient. In some embodiments, inflammatory ocular disorders are treated, specifically macular degeneration. In some embodiments, other disorders or tissues of a body are treated with the dose of radiation. In some embodiments, the target tissues are placed in a global coordinate system based on ocular imaging. In some embodiments, the target tissues inside the global coordinate system lead to direction of an automated positioning system that is directed based on the target tissues within the coordinate system. In some embodiments, a treatment plan is utilized in which beam energy and direction and duration of time for treatment is determined for a specific disease to be treated and / or structures to be avoided. In some embodiments, a fiducial marker is used to identify the location of the target tissues. In some embodiments, radiodynamic therapy is described in which radiosurgery is used in combination with other treatments and can be delivered concomitant with, prior to, or following other treatments.
Owner:CARL ZEISS MEDITEC INC
Methods and devices for the treatment of ocular diseases in human subjects
InactiveUS20150258120A1Reduce in quantityReduce severityOrganic active ingredientsPowder deliveryDiseaseMicroparticle
Methods and devices are provided for targeted non-surgical administration of a drug formulation to the suprachoroidal space (SCS) of the eye of a human subject for the treatment of a posterior ocular disorder or a choroidal malady. In one embodiment, the method comprises inserting a hollow microneedle into the eye at an insertion site and infusing a drug formulation through the inserted microneedle and into the suprachoroidal space of the eye, wherein the infused drug formulation flows within the suprachoroidal space away from the insertion site during the infusion. In one embodiment, the fluid drug formulation comprises drug nanoparticles or microparticles.
Owner:CLEARSIDE BIOMEDICAL
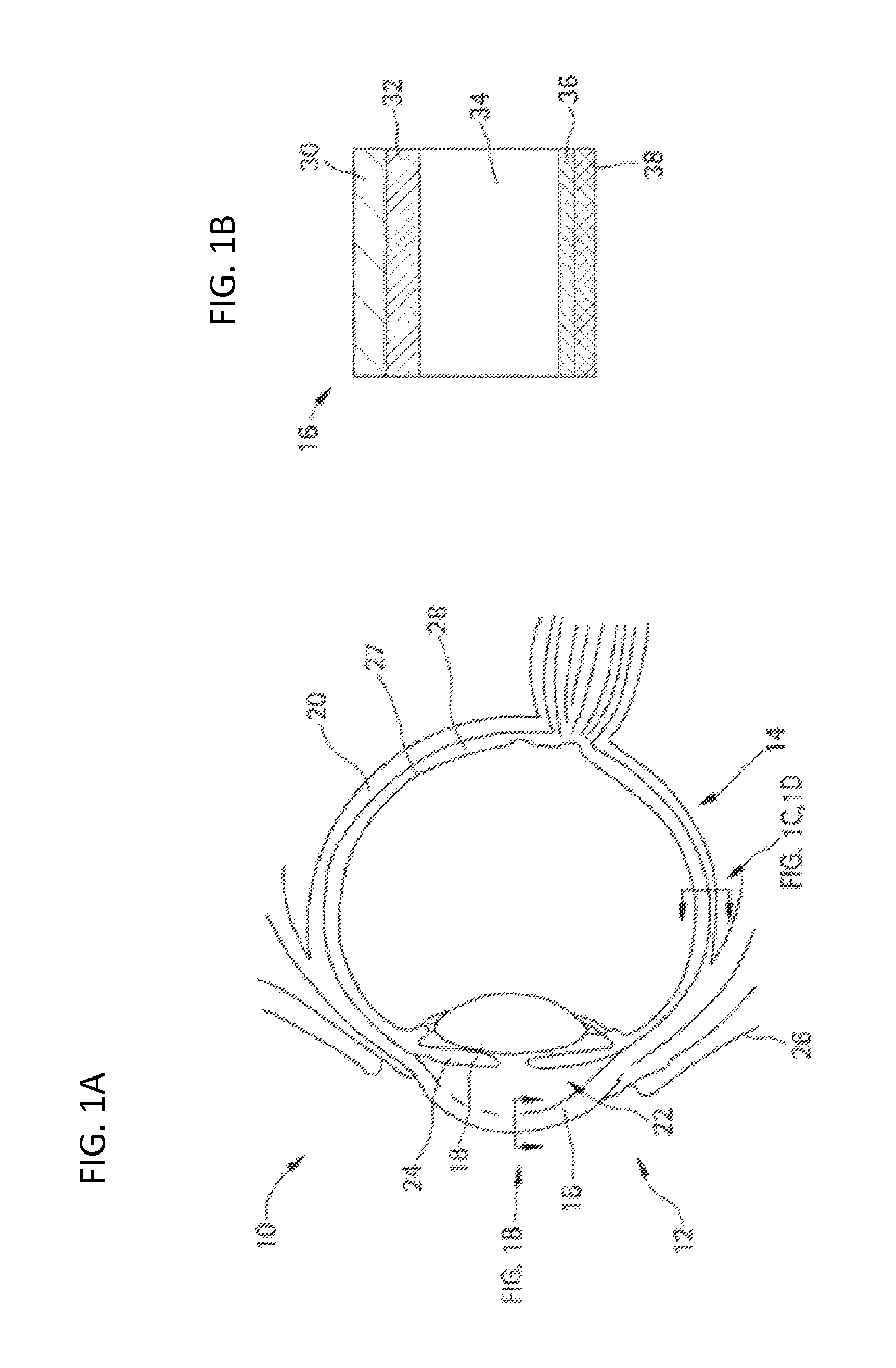
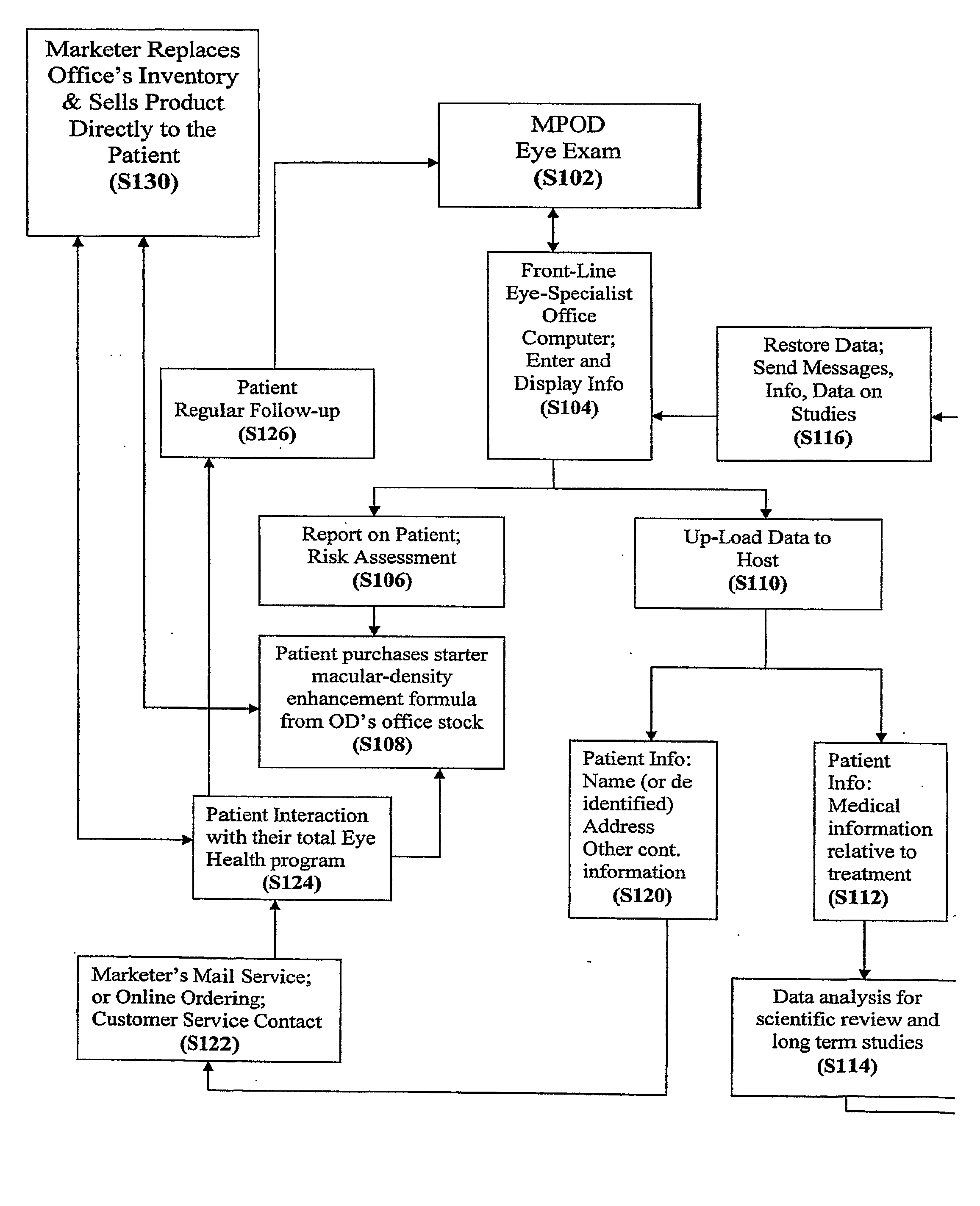
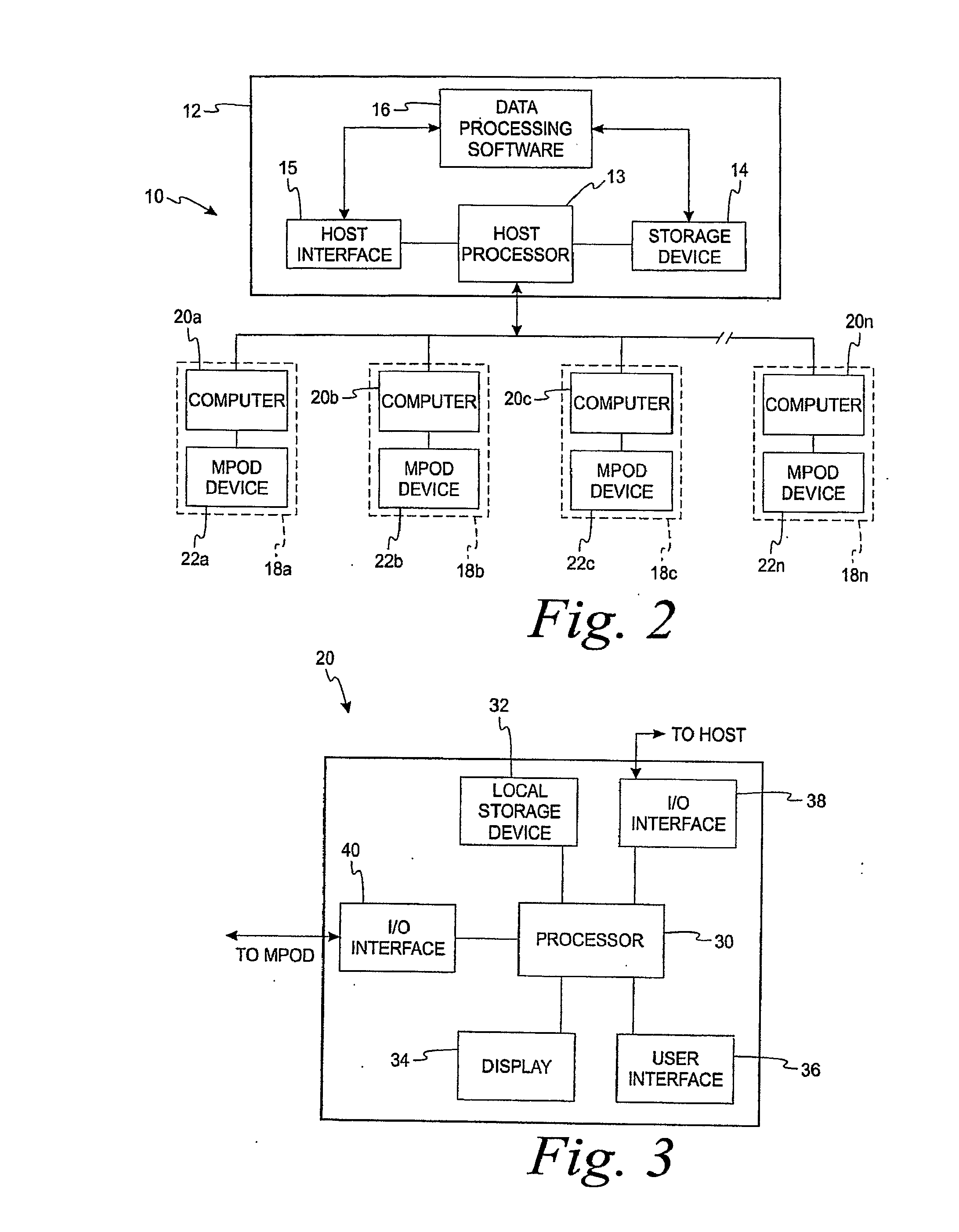
Diagnostic, Prescriptive, and Data-Gathering System and Method For Macular Pigment Deficits and Other Eye Disorders
ActiveUS20100241450A1Compact and inexpensive and well-suited for placement and operationInexpensive and convenientDrug and medicationsNutrition controlPatient dataMedical prescription
A macular health measurement and storage system comprises a plurality of macular-pigment measurement machine for measuring macular pigment density in humans, a plurality of computers each of which is associated with a corresponding one the macular-pigment measuring machines, and a central host. The plurality of macular-pigment measurement machines include a device for receiving macular pigment data from a patient, at least one data transfer port, and at least one processor that enables the transfer of the macular pigment data from the transfer port. The plurality of computers include a first port coupled to the data transfer port of the corresponding macular-pigment measurement machine for receiving the macular pigment data. Each of the computers includes a second port for transferring patient data. The central host is coupled to the second ports on each of the plurality of computers. The central host includes a storage device for storing the patient data.
Owner:ZEAVISION LLC
Methods and Apparatuses for the Treatment of Glaucoma using visible and infrared ultrashort laser pulses
InactiveUS20120283557A1Short pulse durationLaser surgerySurgical instrument detailsDiseaseFemto second laser
Transcorneal and fiberoptic laser delivery systems and methods for the treatment of eye diseases wherein energy is delivered by wavelengths transparent to the cornea to effect target tissues in the eye for the control of intraocular pressure in diseases such as glaucoma by delivery systems both external to and within ocular tissues. External delivery may be effected under gonioscopic control. Internal delivery may he controlled endoscopically or fiberoptically, both systems utilizing femtosecond laser energy to excise ocular tissue. The femtosecond light energy is delivered to the target tissues to be treated to effect precisely controlled photodisruption to enable portals for the outflow of aqueous fluid in the case of glaucoma in a manner which minimizes target tissue healing responses, inflammation and scarring.
Owner:BERLIN MICHAEL S
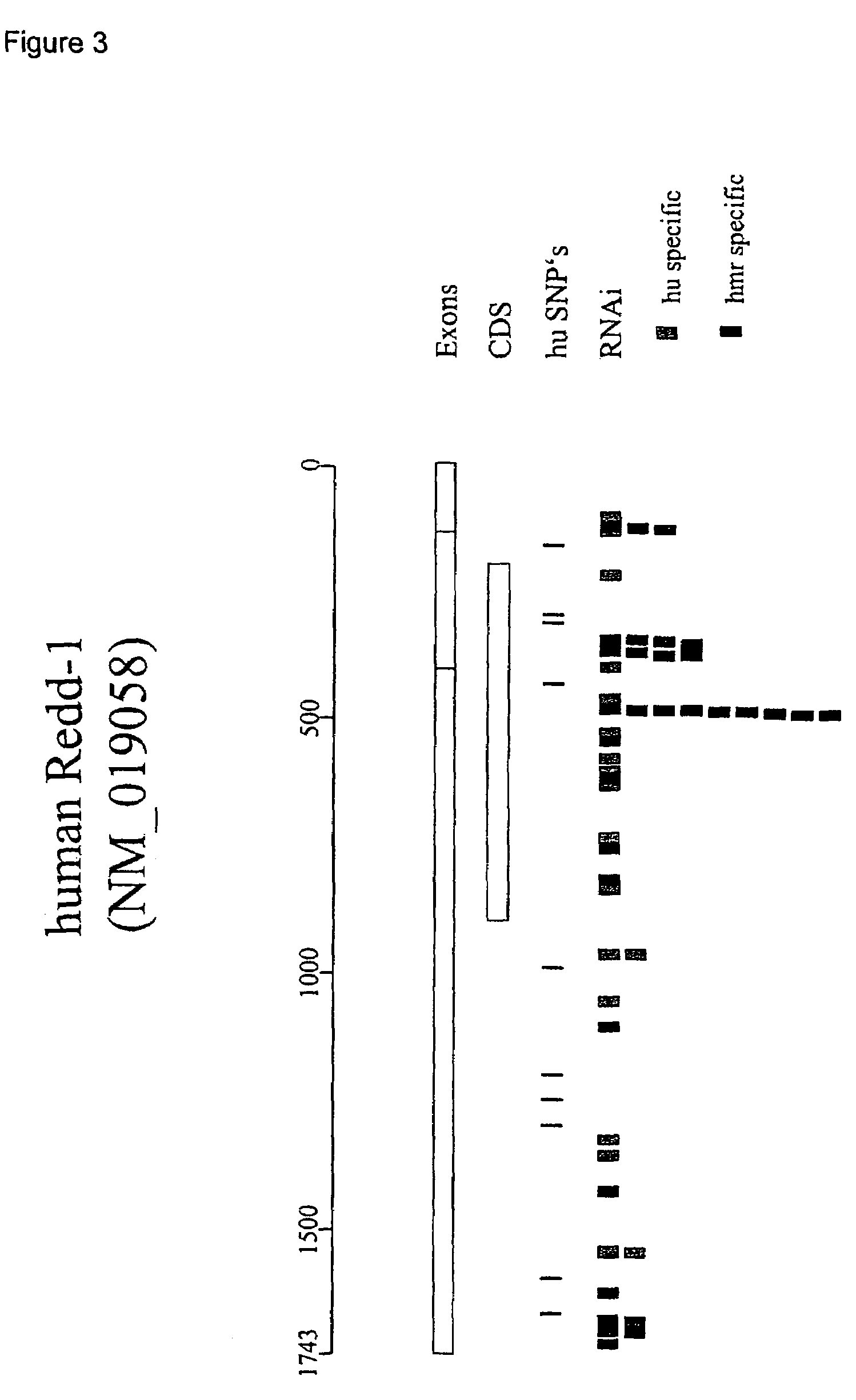
Therapeutic uses of inhibitors of RTP801
InactiveUS7741299B2Effect minimizedMinimize ToxicitySenses disorderNervous disorderTreatment useCancer research
Owner:QUARK FARMACUITIKALS INC
Wearable photoactivator for ocular therapeutic applications and uses thereof
The invention provides a wearable device for delivery of light of a desired wavelength and power to the cornea of a subject. The device includes a frame for attachment of a light source housing which includes a light source and a lens positioned in the housing to allow light to be directed to the eye of the subject, and the light source is operably linked to a power source. The invention provides method for the prevention and treatment of ocular disease including infection, neoplasia, and corneal dystrophies. The device of the invention can be used in conjunction with photoactive therapeutic agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and/or reversing ocular diseases related with ocular neovascularization
InactiveUS7517870B2High unmet needControl rateBiocideOrganic active ingredientsDiabetic retinopathyHedgehog signaling pathway
The present invention concerns the use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and / or reversing ocular diseases related with ocular neovascularization. Particularly, the above-mentioned diseases are (wet) age-related macular degeneration, (proliferative) diabetic retinopathy, neovascular glaucoma, retinal vein occlusion, or retinopathy of prematurity (ROP).
Owner:FOND AZIONE TELETHON
Antibodies to Dkk-1
ActiveUS20060127393A1Block and reduce bindingHigh activitySenses disorderNervous disorderConformational epitopeOcular disease
The present invention provides antibodies and immunologically functional fragments thereof that specifically bind Dkk-1 polypeptides. The subject antibodies and fragments bind with high affinity to a conformational epitope located in the carboxy region of the Dkk-1 protein. Methods for preparing such antibodies or fragments thereof as well as physiologically acceptable compositions containing the antibodies or fragments are also provided. Use of the antibodies and fragments to treat various diseases including bone disorders, inflammatory diseases, neurological diseases, ocular diseases, renal diseases, pulmonary diseases and skin diseases are also disclosed.
Owner:AMGEN INC
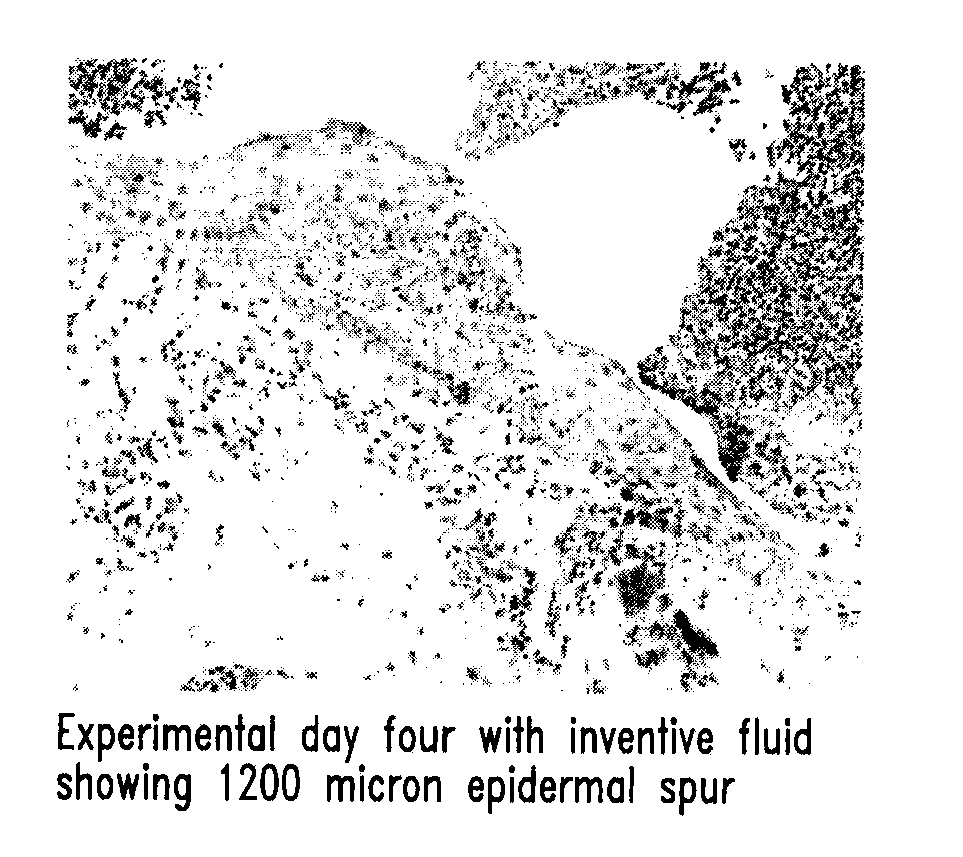
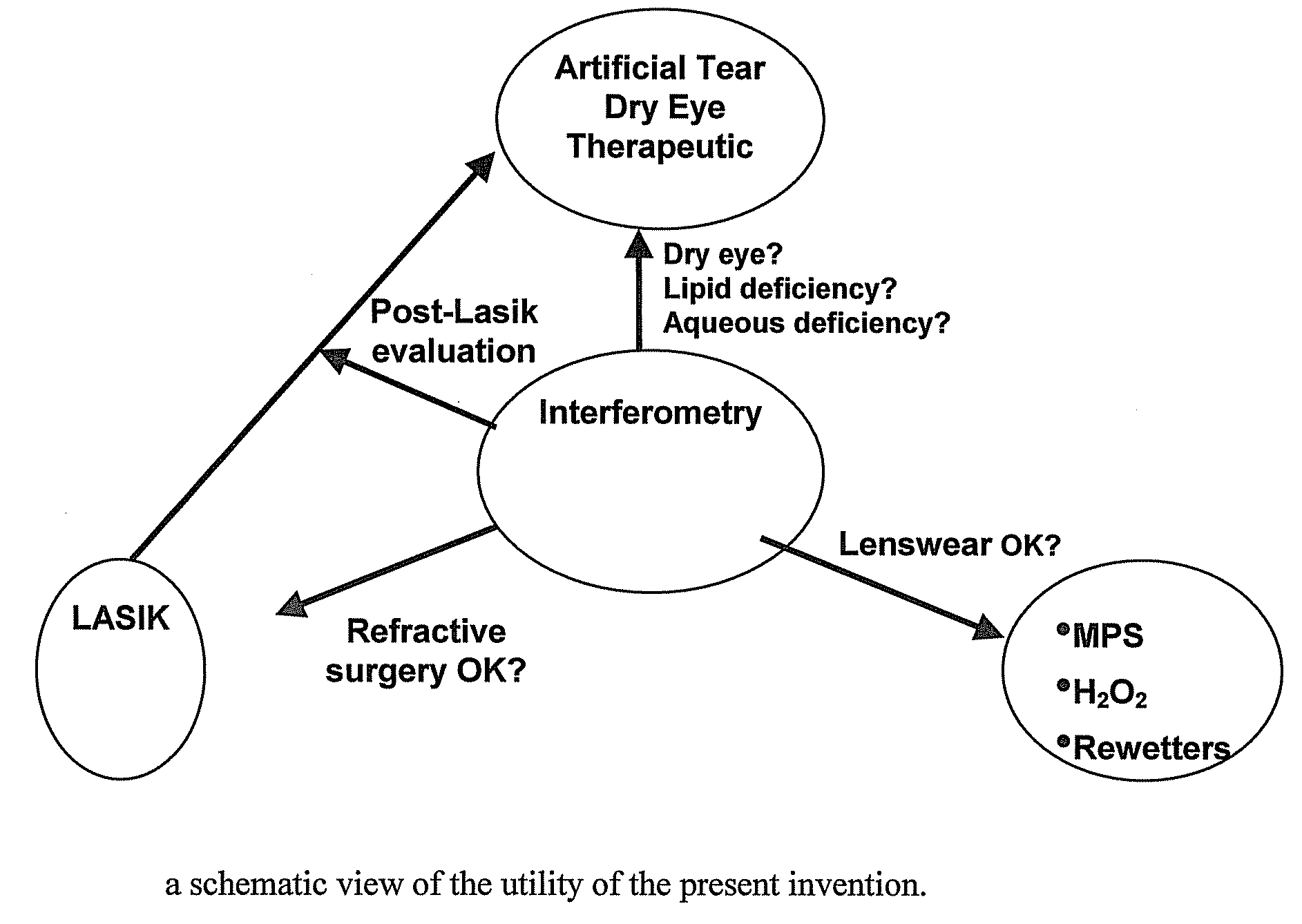
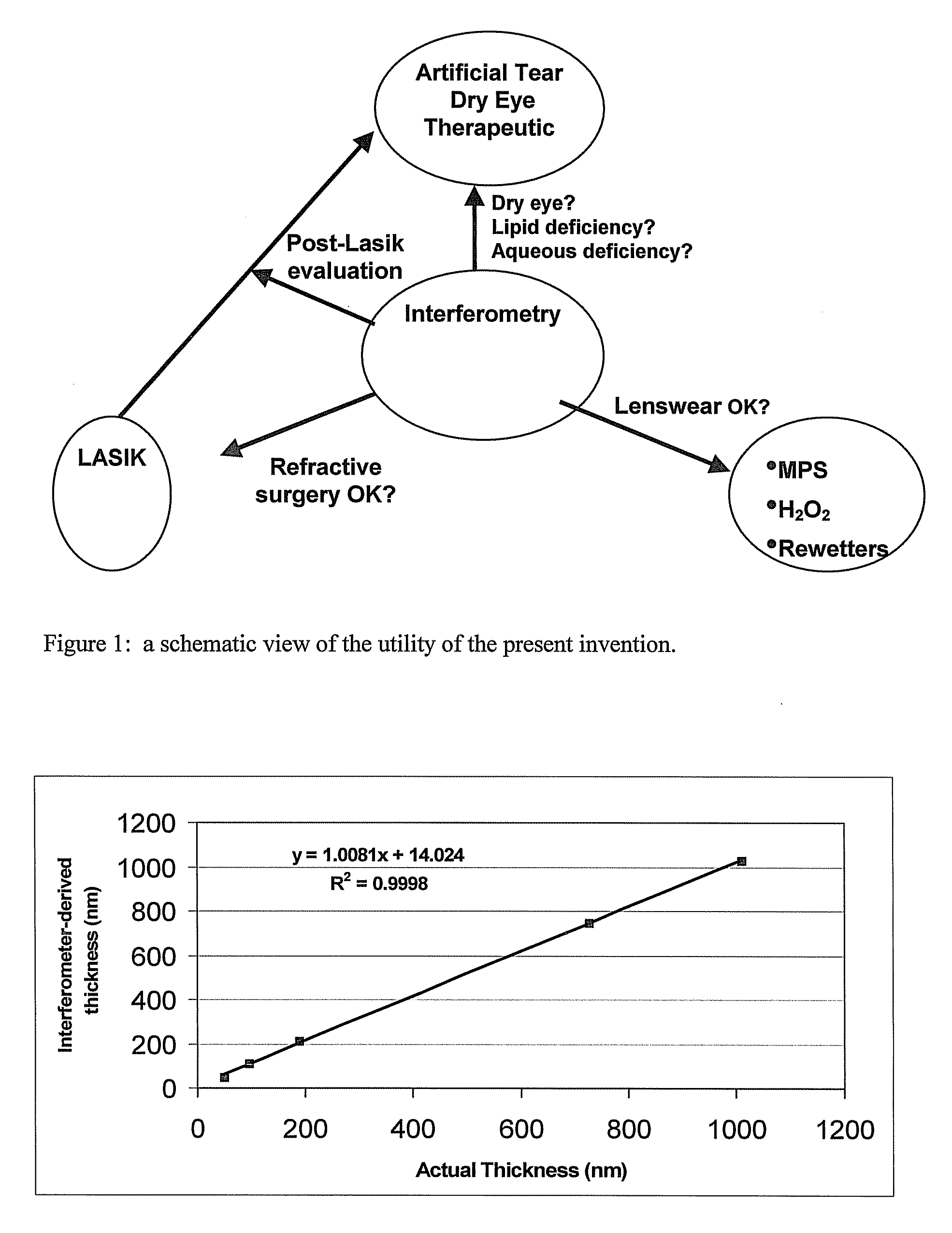
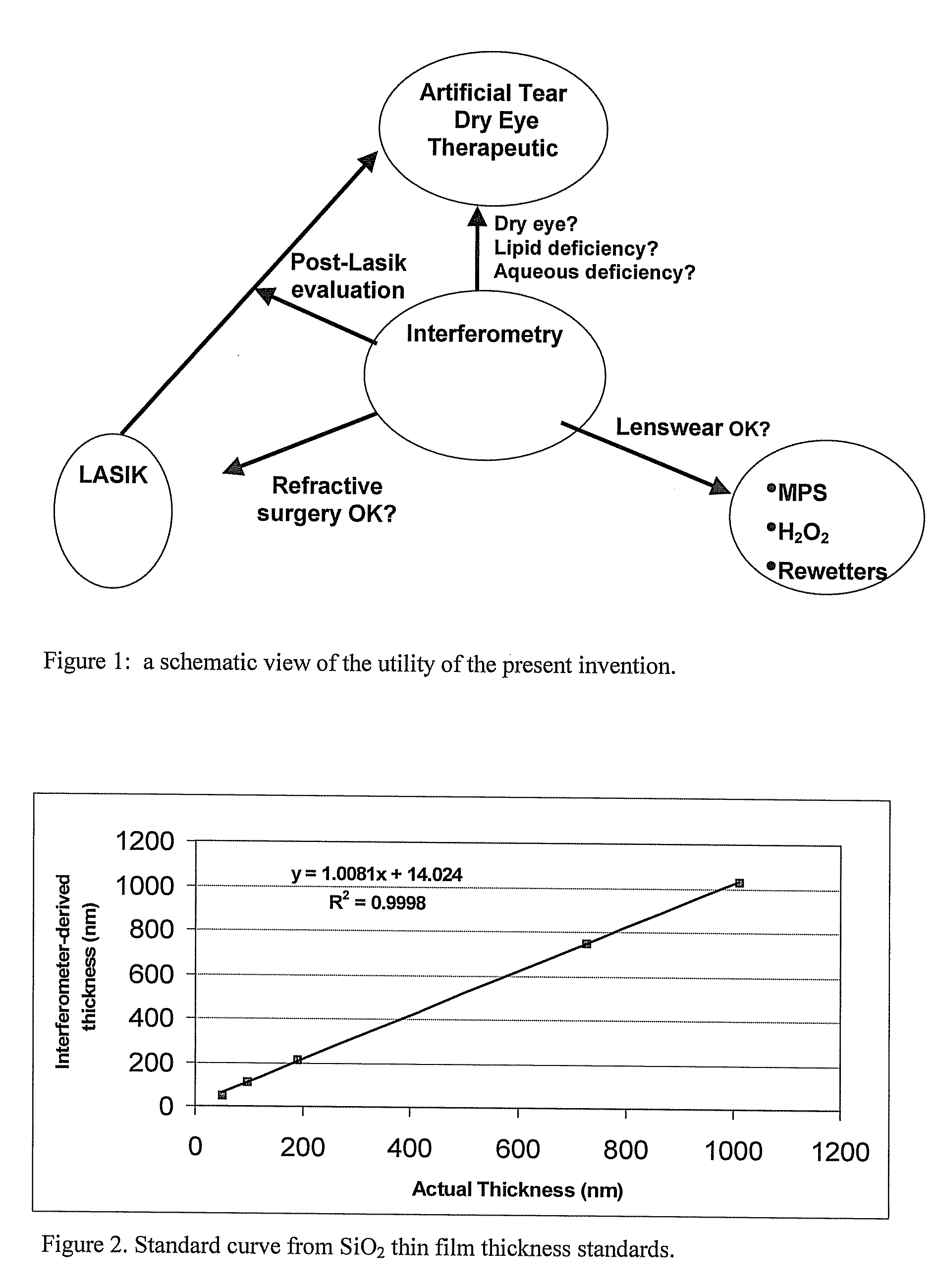
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
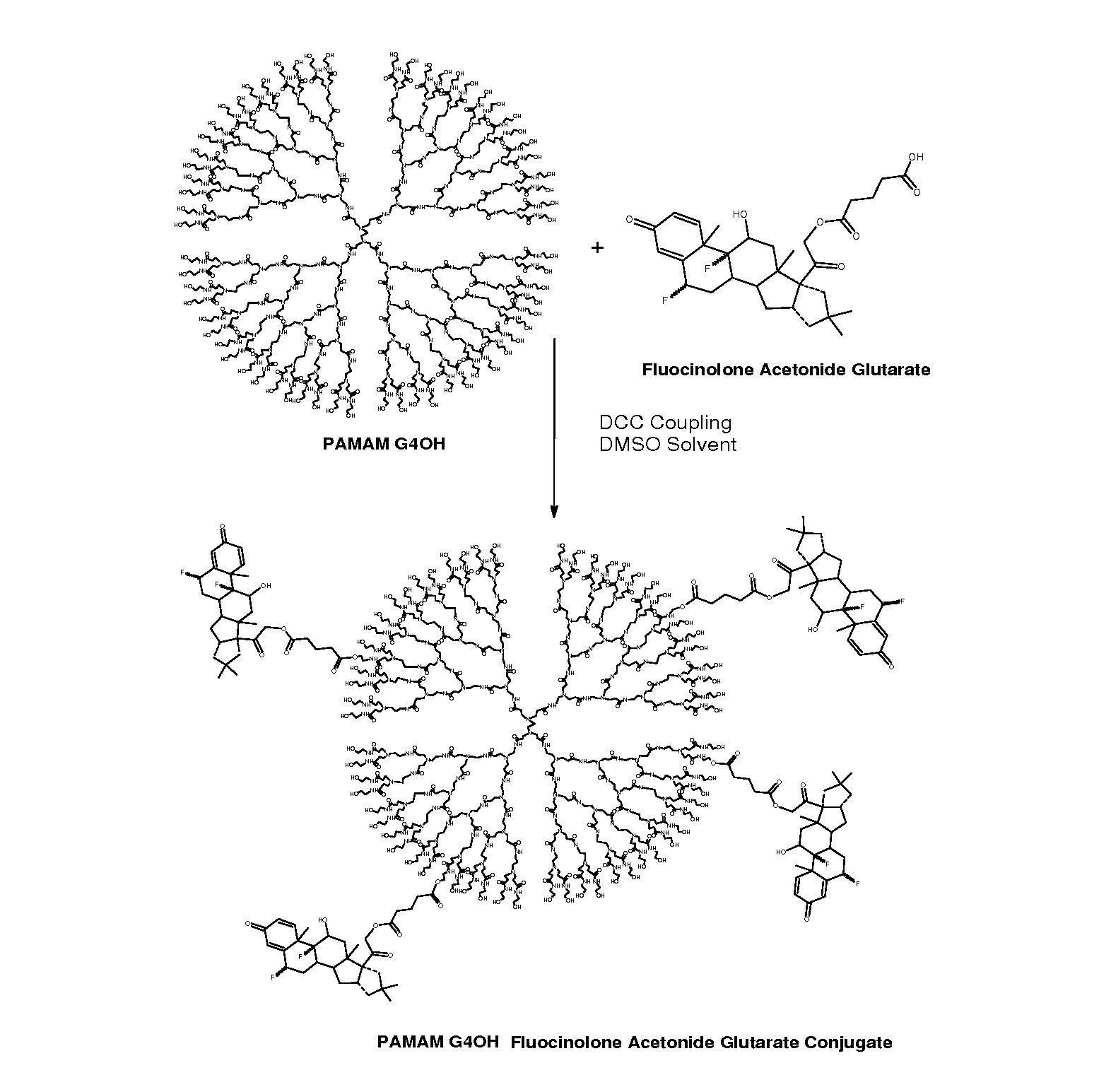
Dendrimers for sustained release of compounds
Dendrimer-based compositions and methods are provided, that are useful for administering pharmaceutical compositions to target cells and tissues for treatment of ocular diseases including macular degeneration, diabetic retinopathy, and retinitis pigmentosa.
Owner:WAYNE STATE UNIV
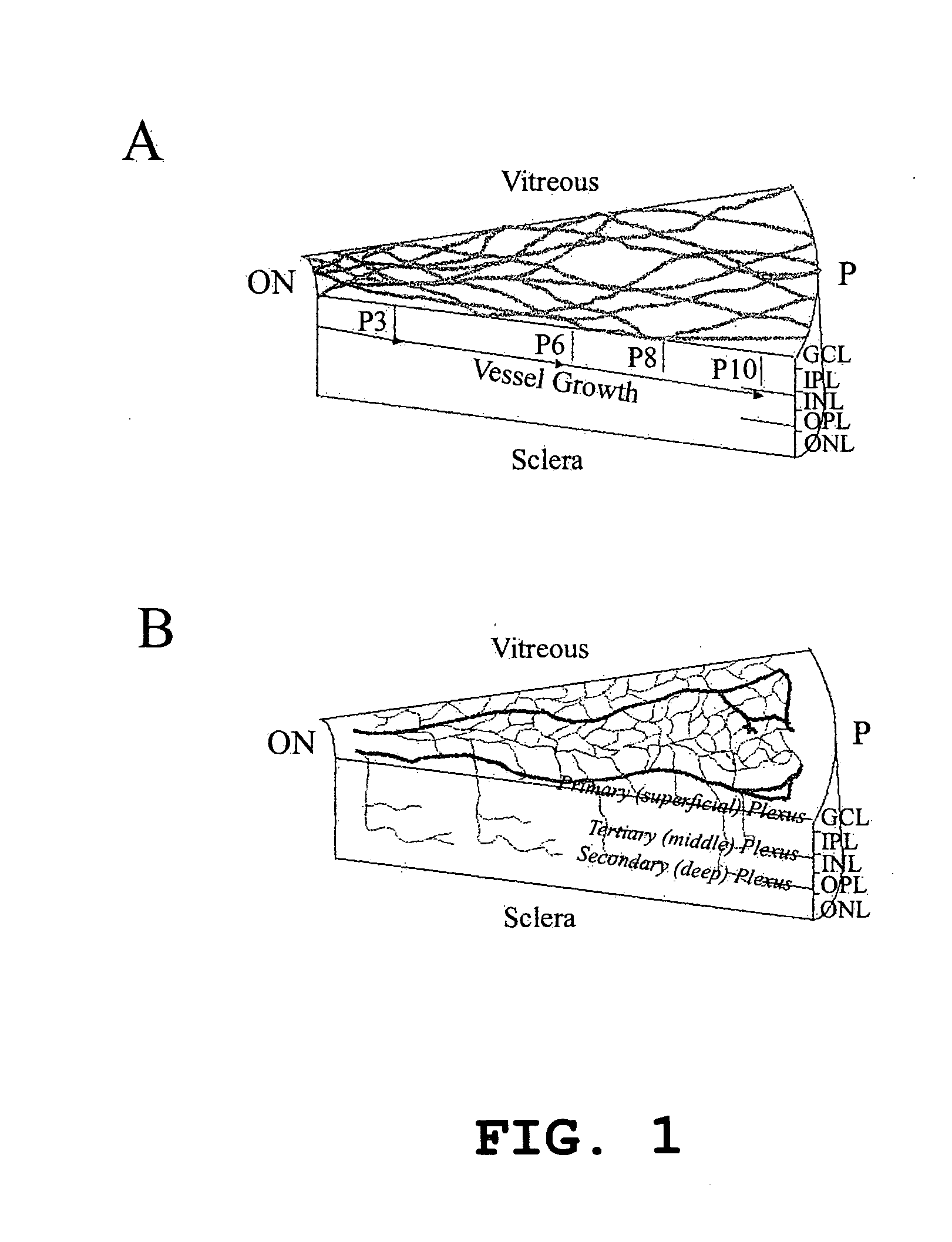
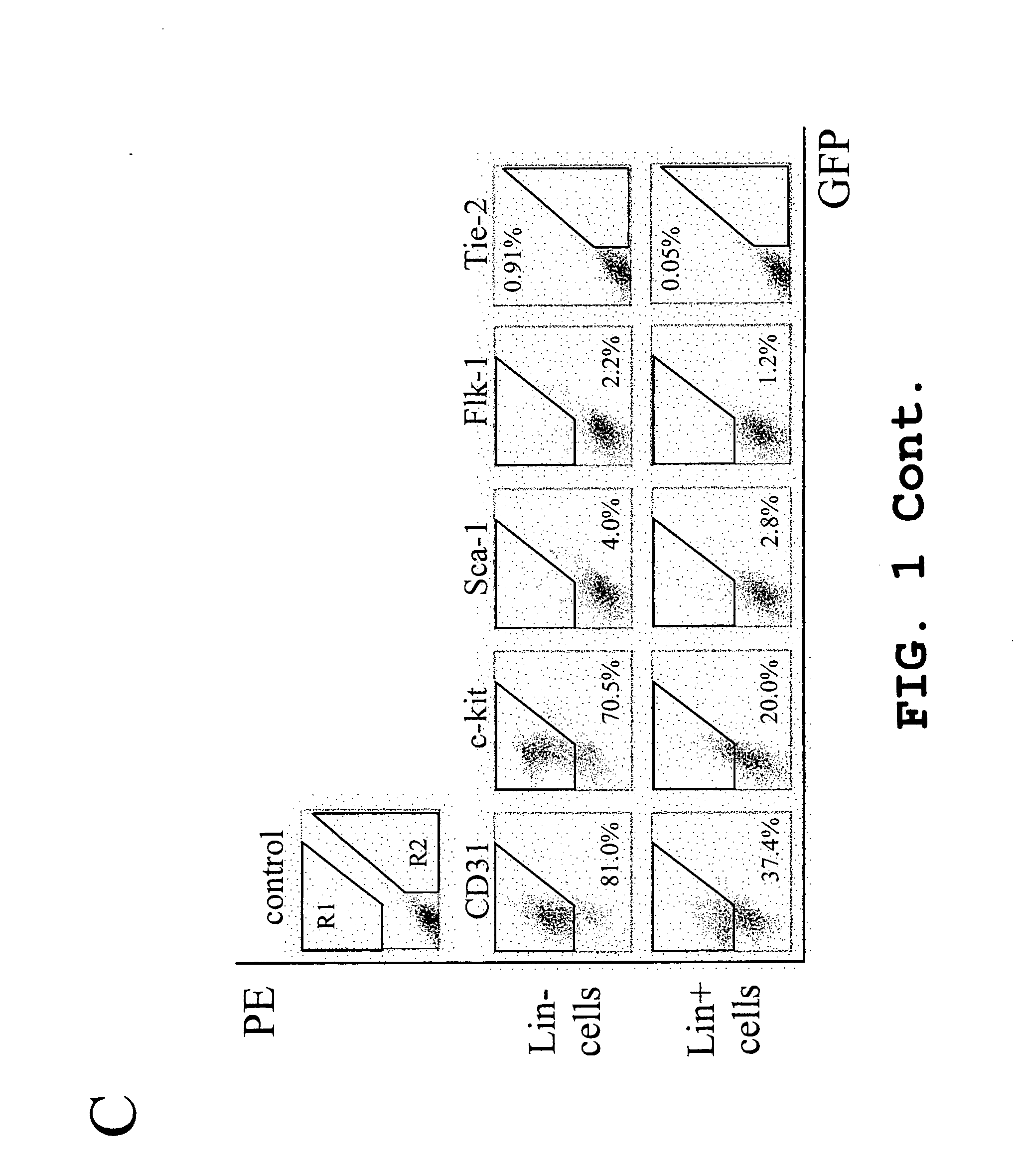
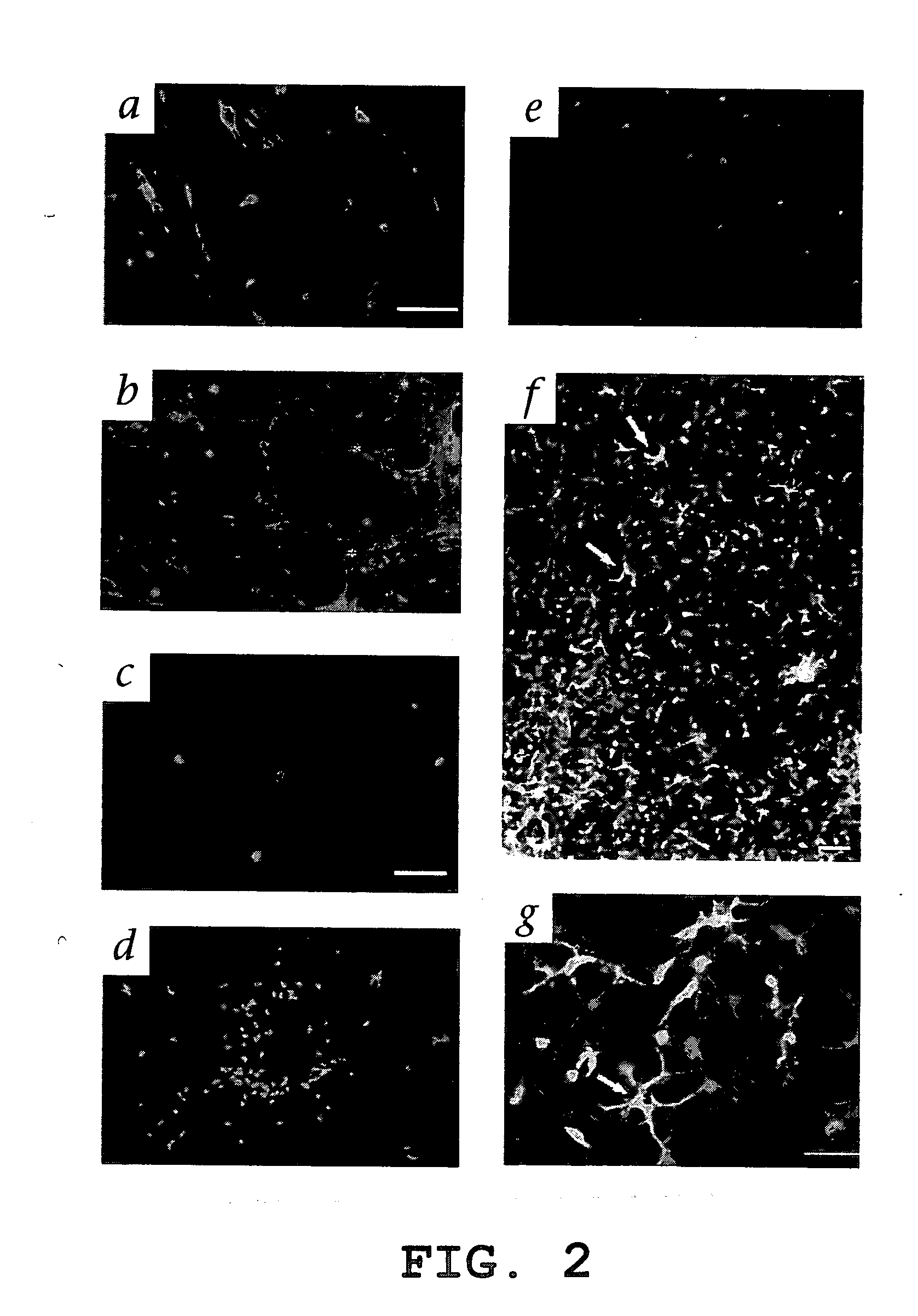
Hematopoietic stem cells and methods of treatment of neovascular eye diseases therewith
InactiveUS20050063961A1Stably incorporated into neovasculature of the eyePromote repairBiocideSenses disorderDiseaseProgenitor
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lin− HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. Isolated Lin− HSCs that have been transfected with therapeutically useful genes are also provided, and are useful for delivering genes to the eye for cell-based gene therapy. Methods of preparing isolated stem cell populations of the invention, and methods of treating ocular diseases and injury are also described.
Owner:THE SCRIPPS RES INST
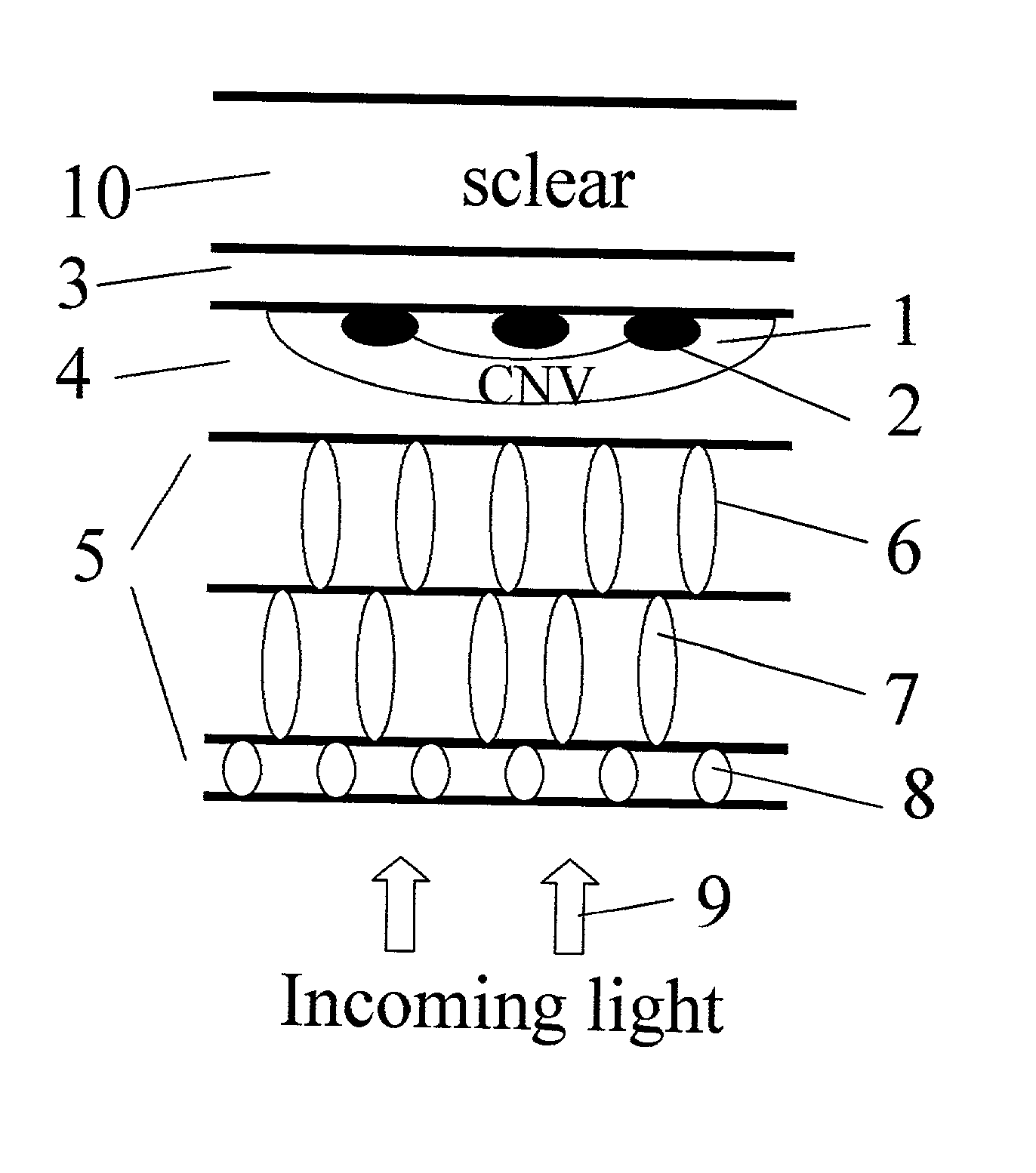
Apparatus and methods for prevention of age-related macular degeneration and other eye diseases
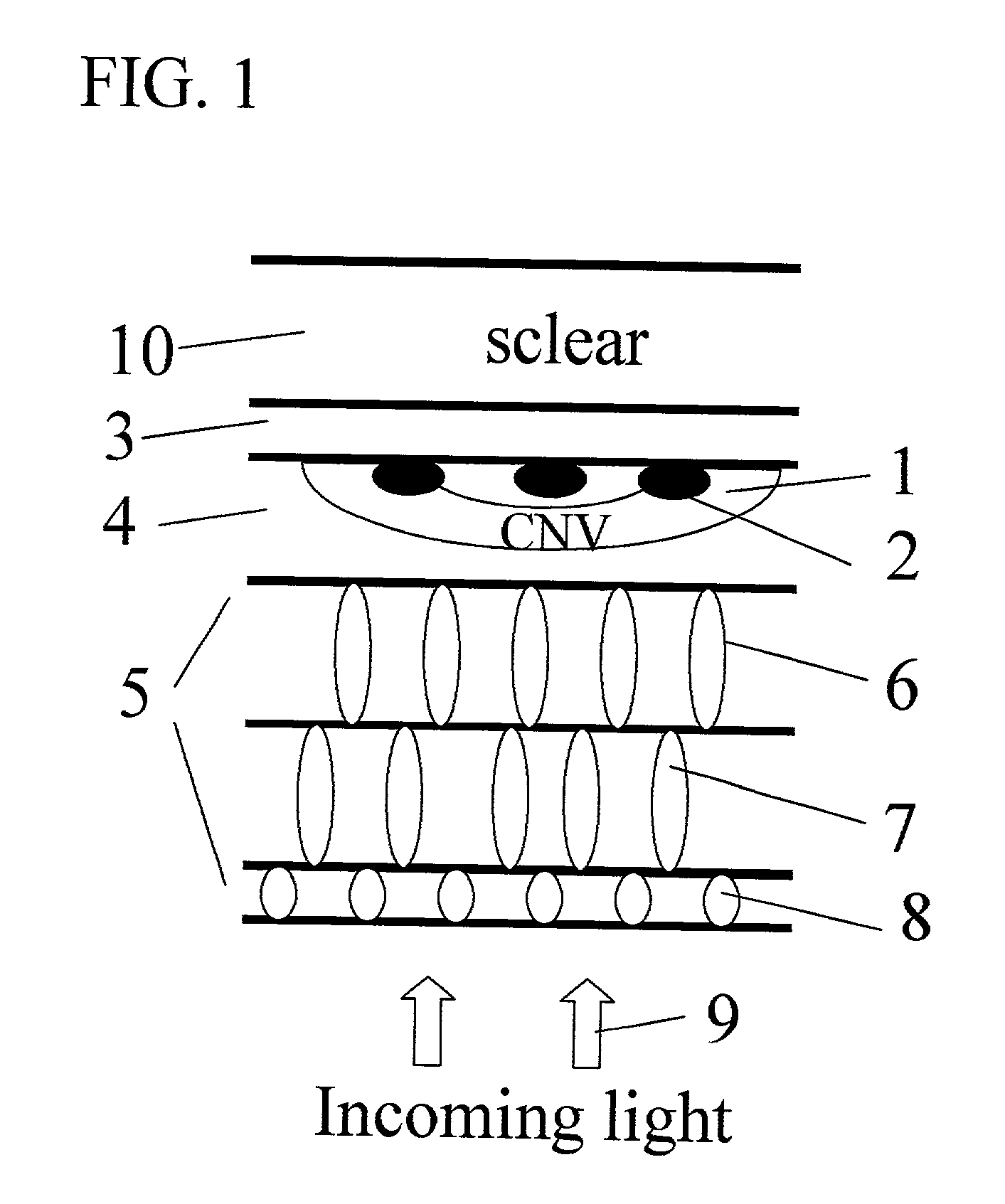
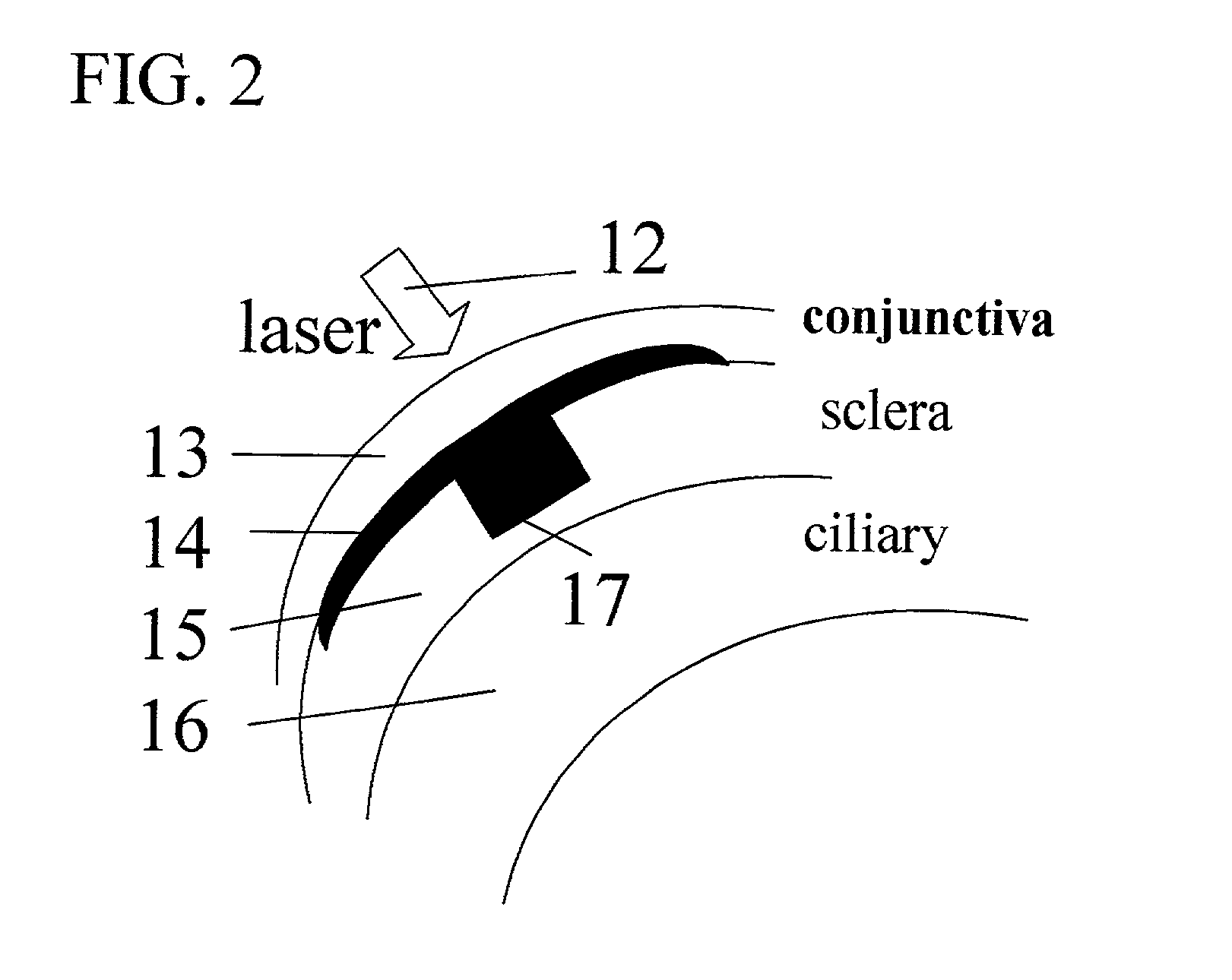
InactiveUS20030105456A1Minor side effectsIncrease flexibilityLaser surgerySurgical instruments for heatingRadio frequencyLaser beams
Surgical apparatus and surgical methods are proposed for the prevention of age-related macular degeneration (AMD) and choroidal neovascularization (CNV), and other eye diseases such as glaucoma by removal of the sclera tissue to reduce its rigidity and increase the flood flow and decrease pressure in the choriocapillaris. The disclosed preferred embodiments of the system consists of a tissue ablation means and a control means of ablation patterns and a fiber delivery unit. The basic laser beam includes UV lasers and infrared lasers having wavelength ranges of (0.15-0.36) microns and (0.5-3.2) microns and diode lasers of about 0.98, 1.5 and 1.9 microns. AMD and CNV are prevented, delayed or reversed by using an ablative laser to ablate the sclera tissue in a predetermined patterns outside the limbus to increase the elasticity of the sclera tissue surrounding the eye globe The surgery apparatus also includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device
Owner:LIN J T
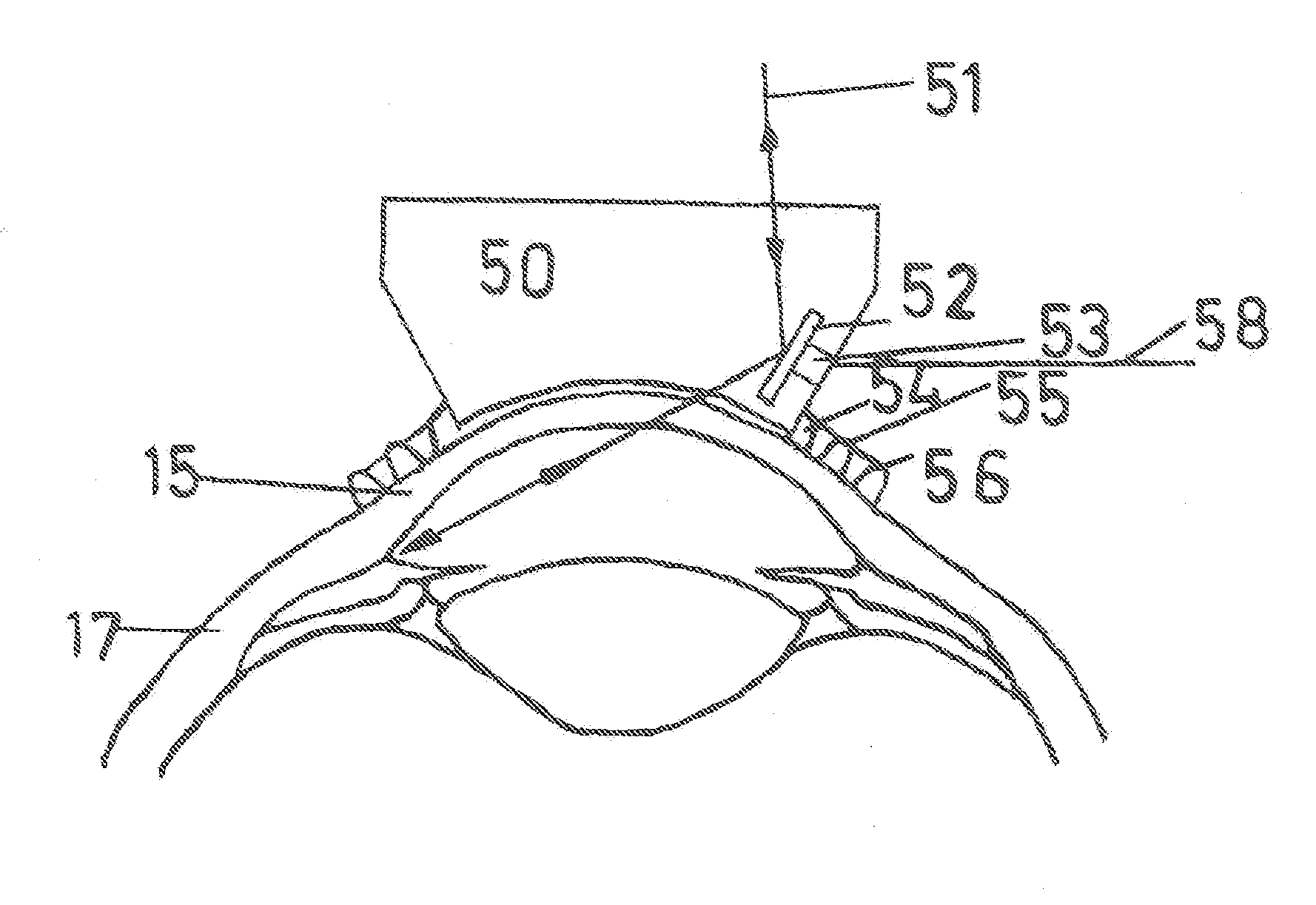
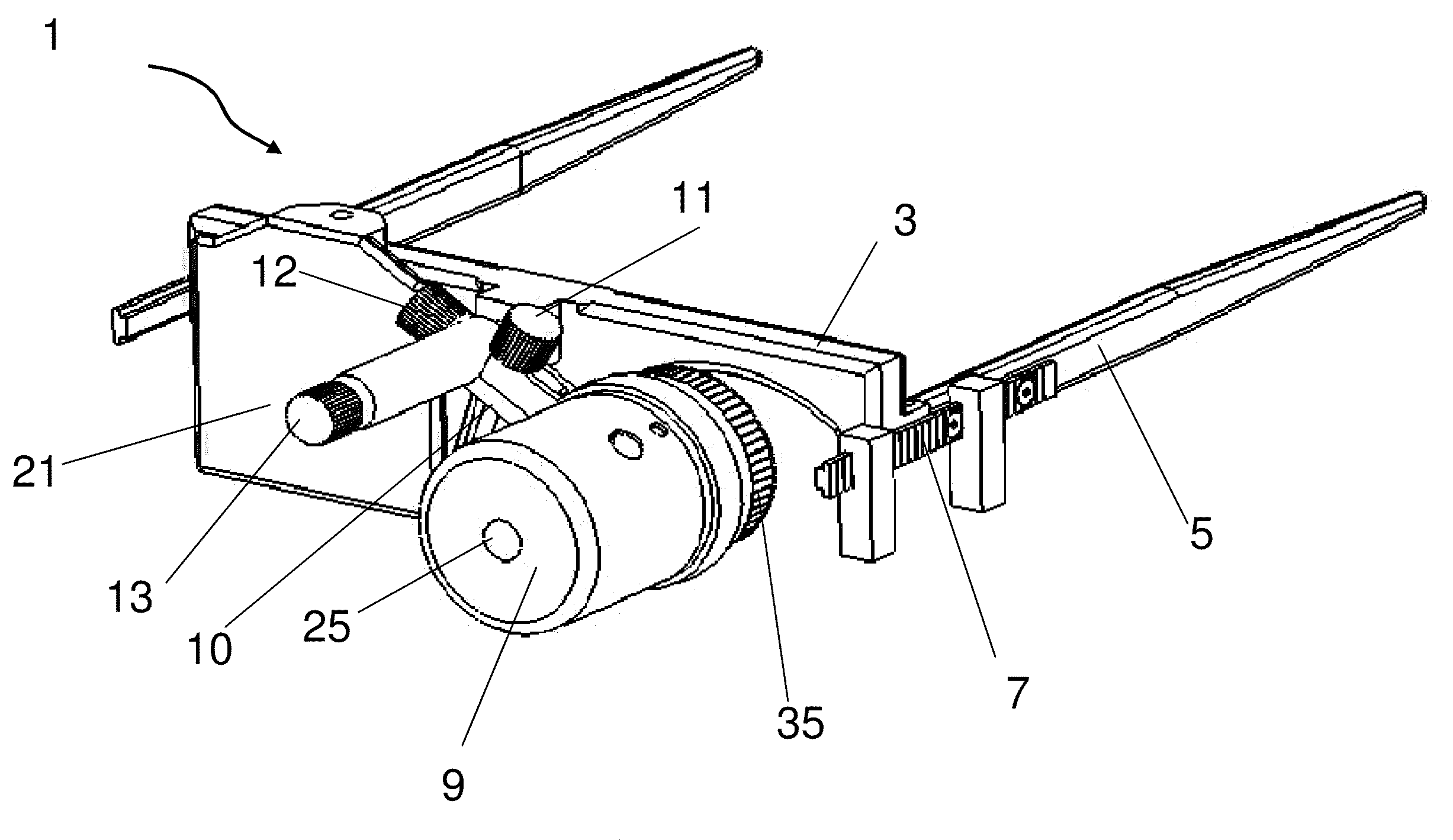
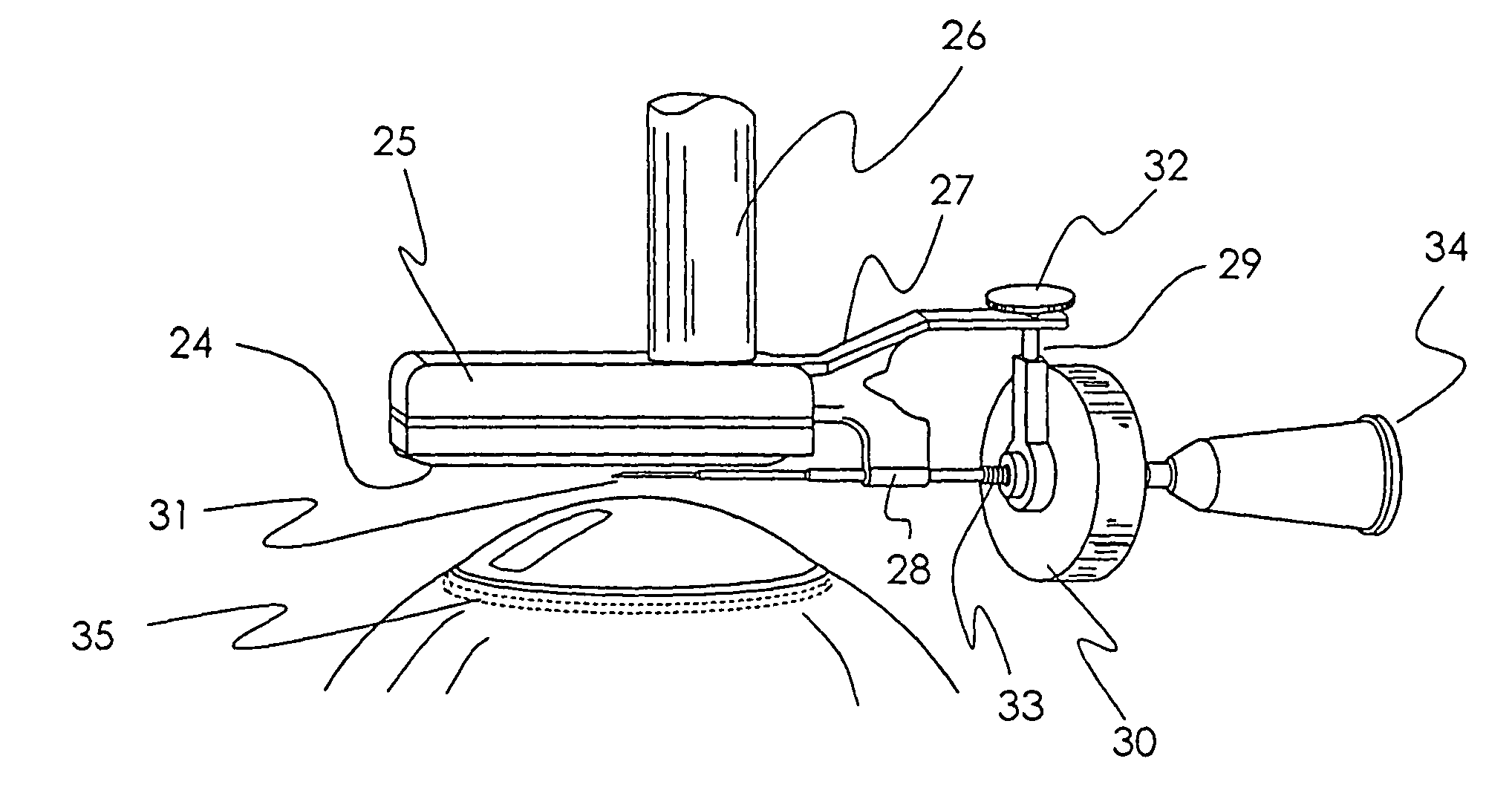
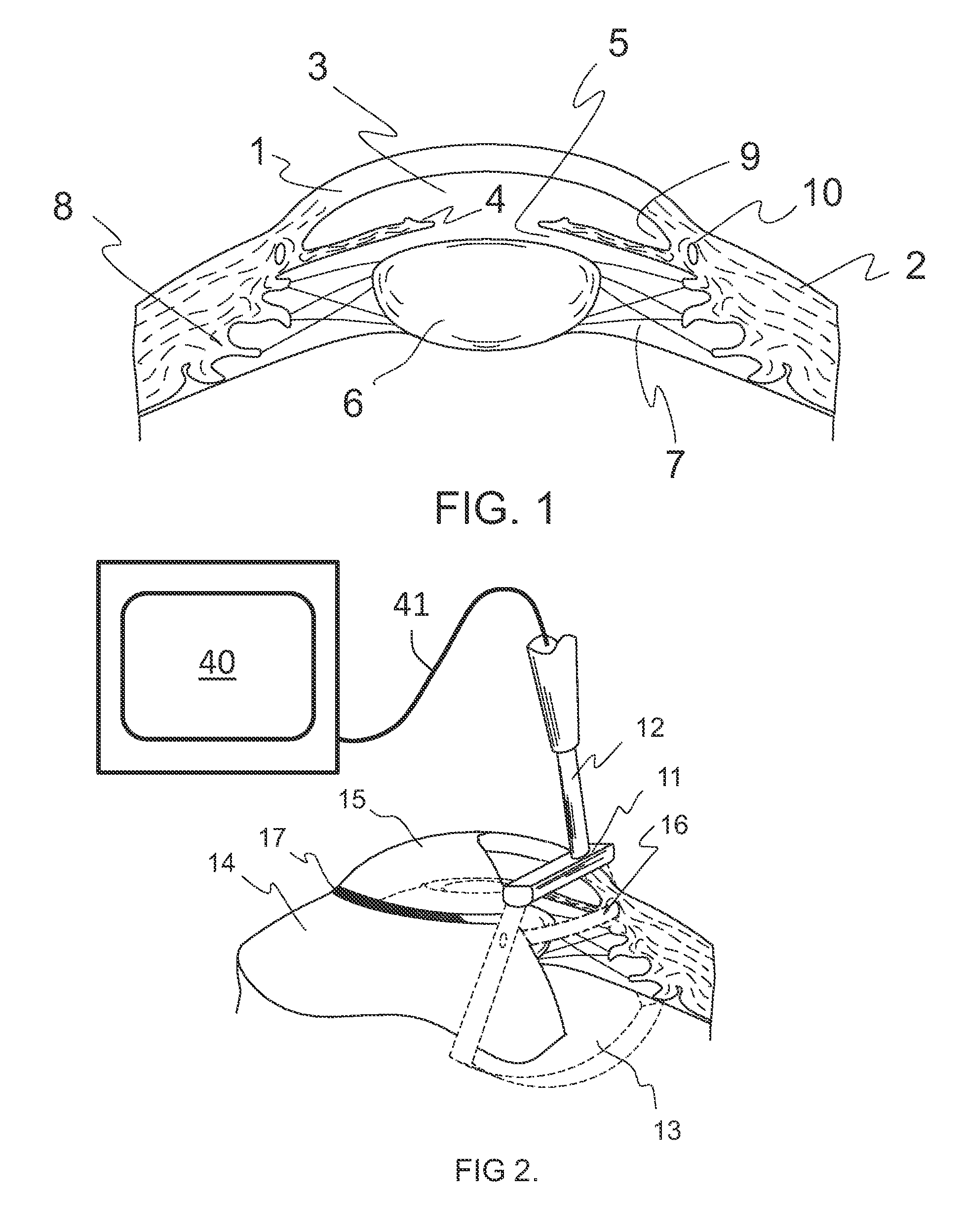
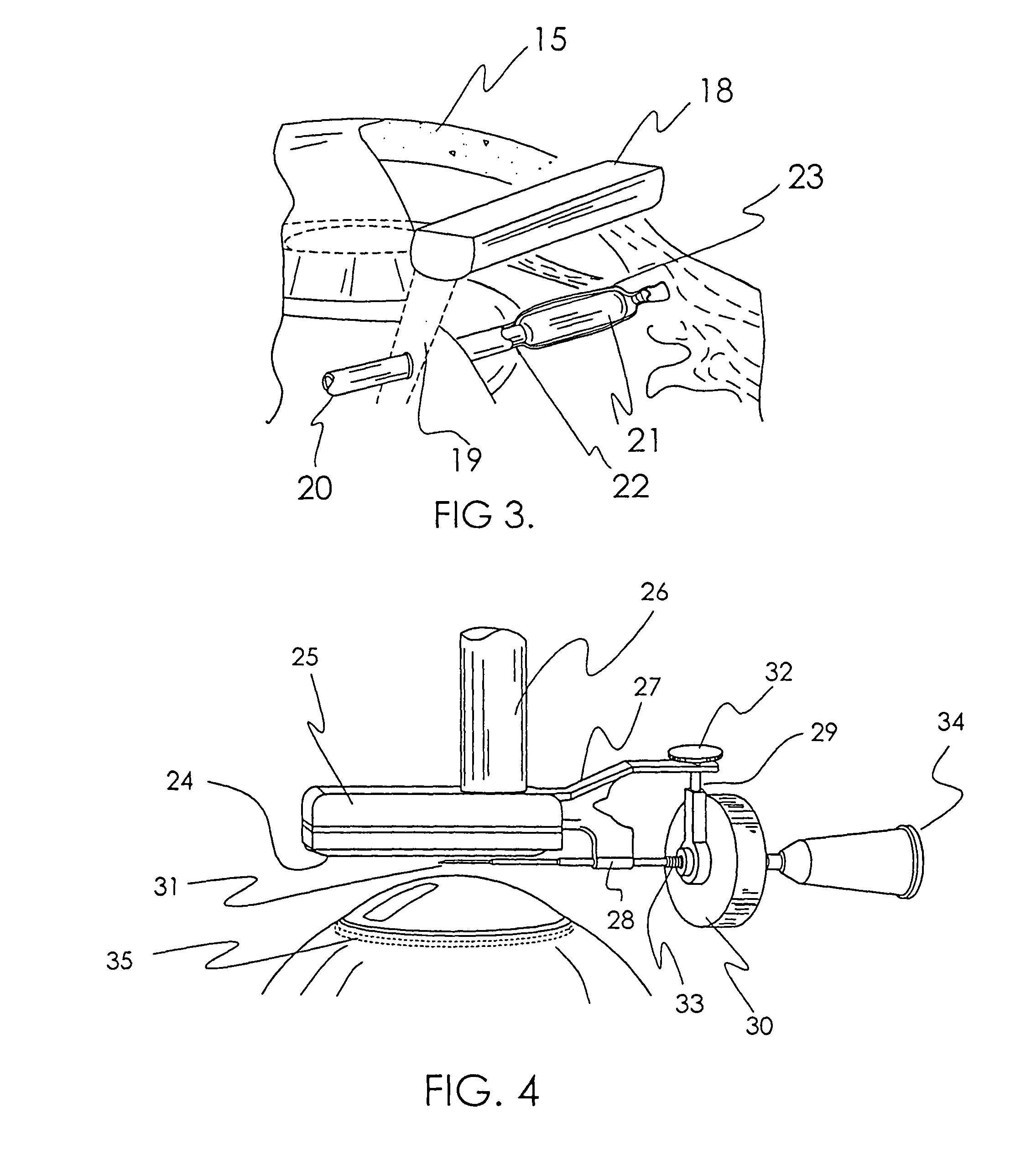
Treatment of ocular disease
The invention relates to a novel apparatus for the treatment of ocular disease, particularly glaucoma. The apparatus consists of a locating device to locate Schlemm's Canal within the anterior portion of the eye and a surgical tool to access the canal for treatment. The apparatus allows for guided, minimally invasive surgical access to Schlemm's Canal to enable surgical procedures to be performed on the canal and trabecular meshwork to reduce intraocular pressure. The apparatus may also deliver devices or substances to Schlemm's Canal in the treatment of glaucoma.
Owner:NOVA EYE INC
Janus kinase inhibitors for treatment of dry eye and other eye related diseases
InactiveUS20120301464A1High expressionIncrease productionBiocideSenses disorderDiseaseOcular disease
Owner:INCYTE HLDG & INCYTE
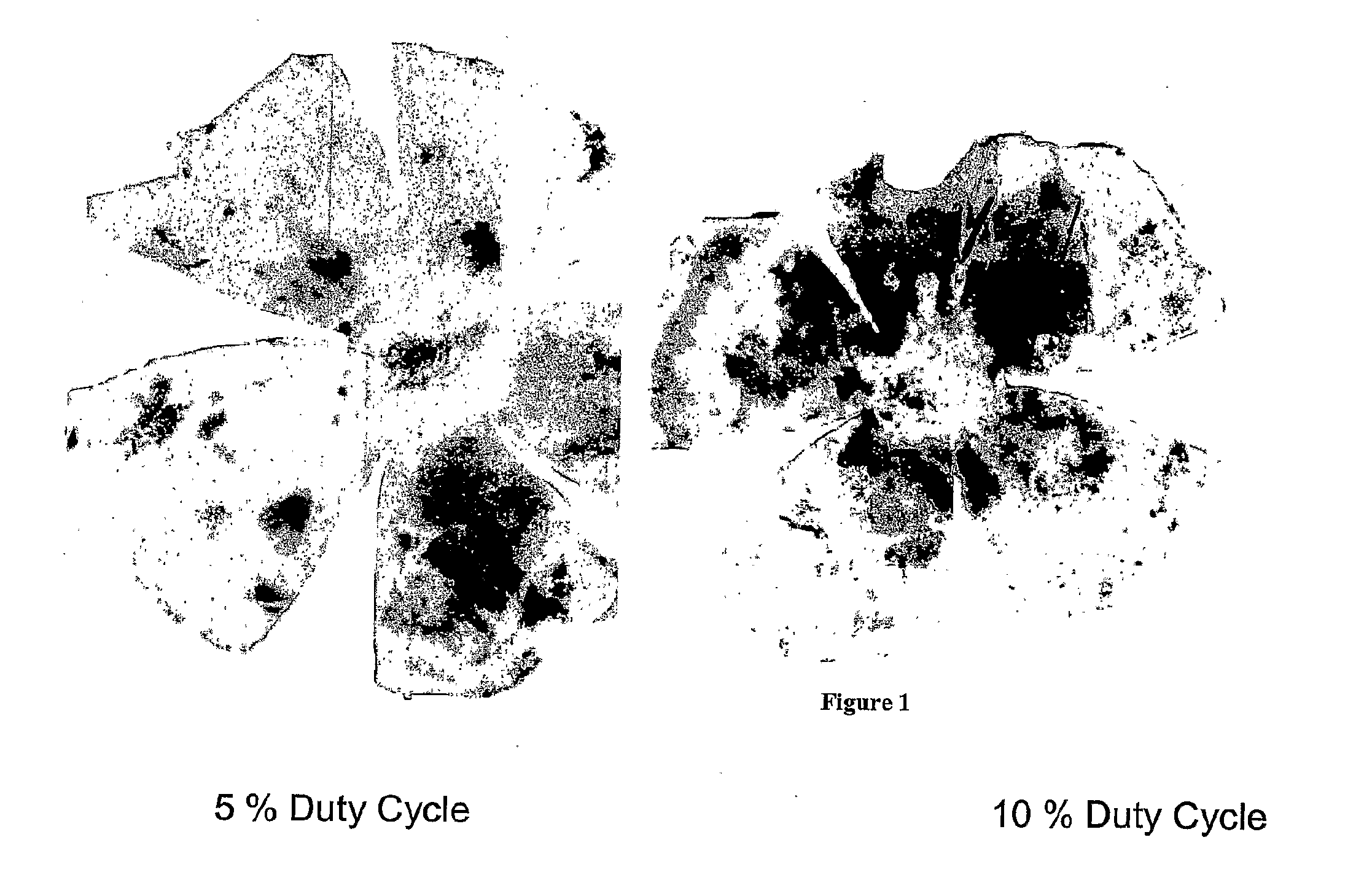
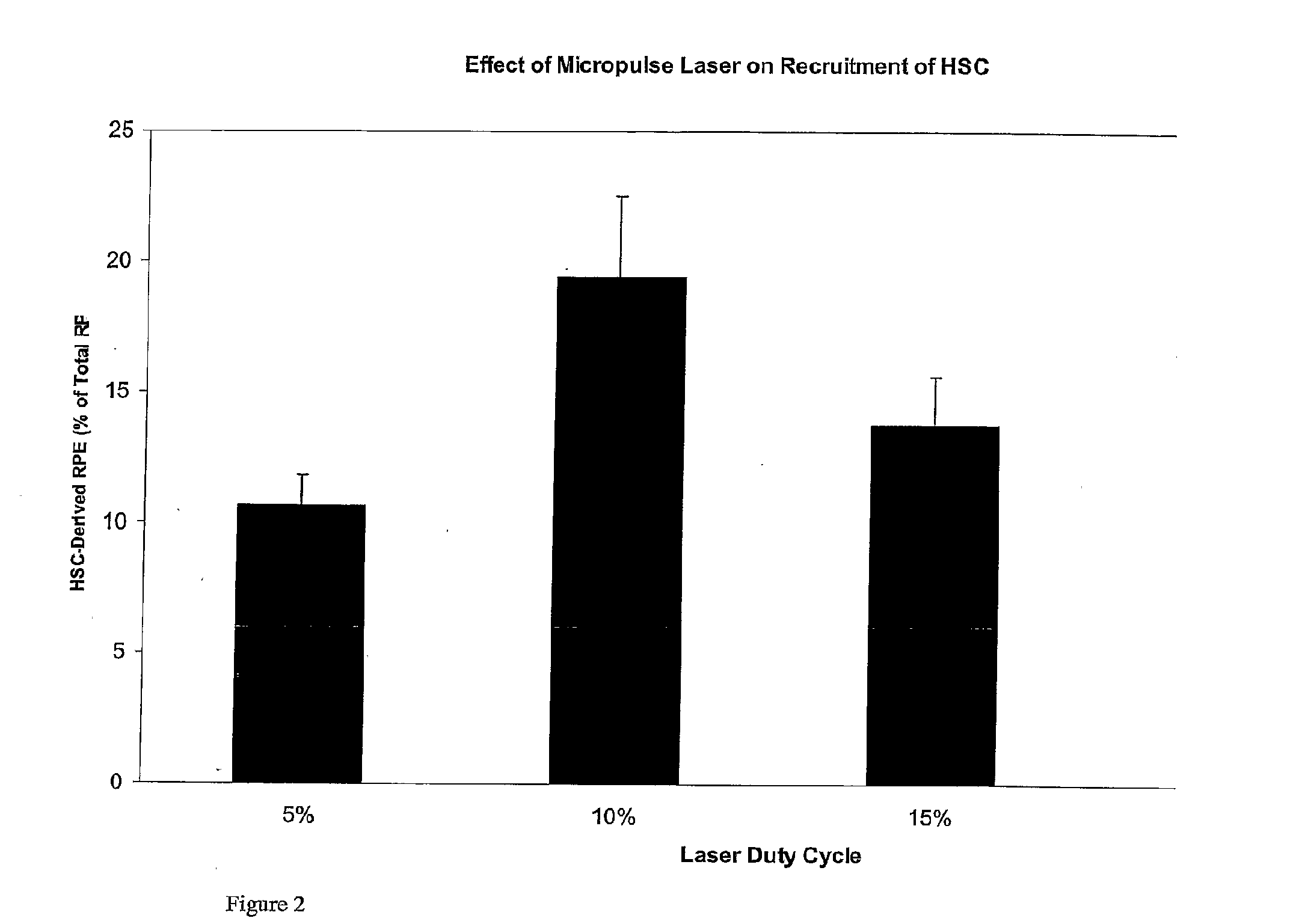
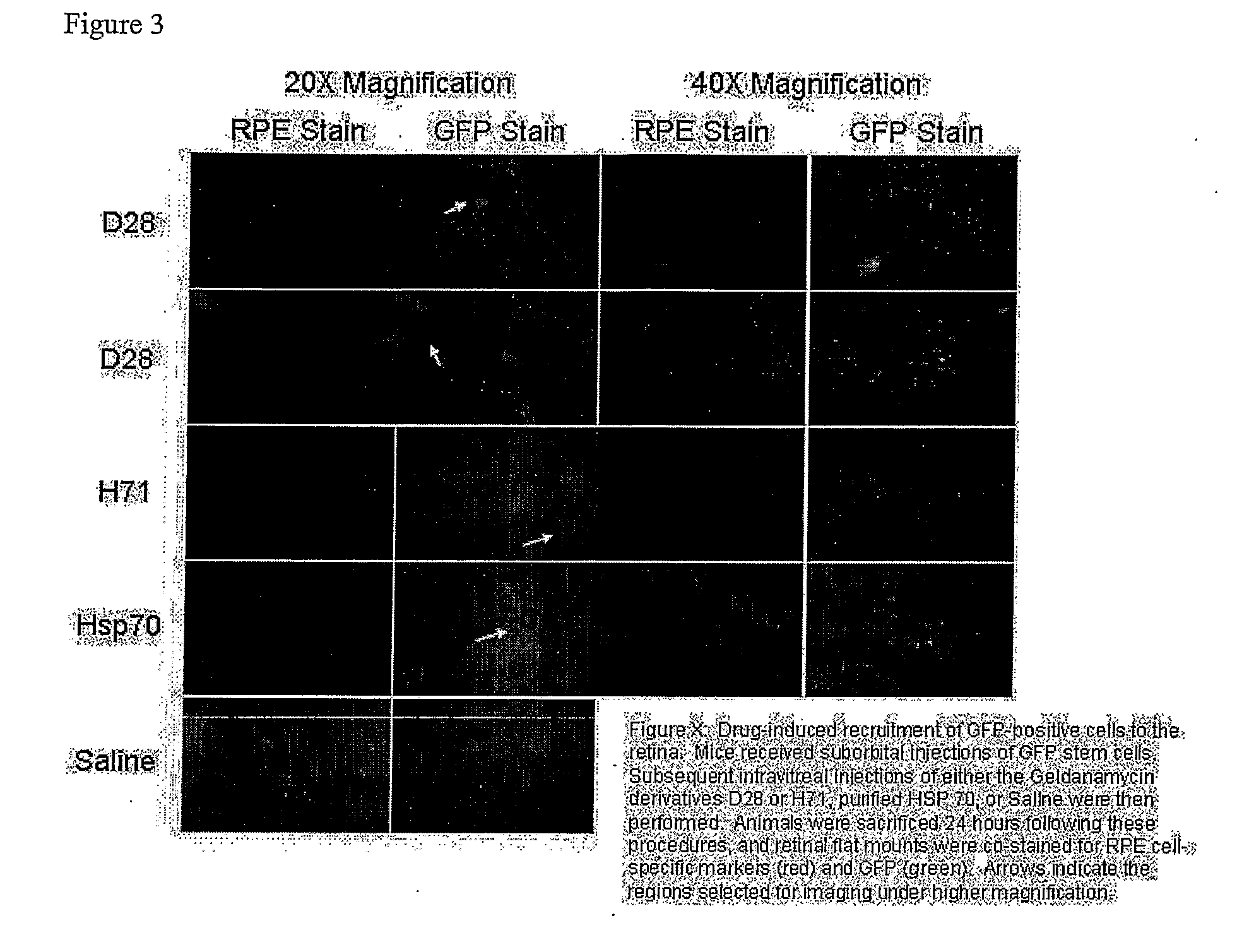
Use of heat shock to treat ocular disease
Owner:UNIV OF FLORIDA RES FOUNDATION INC
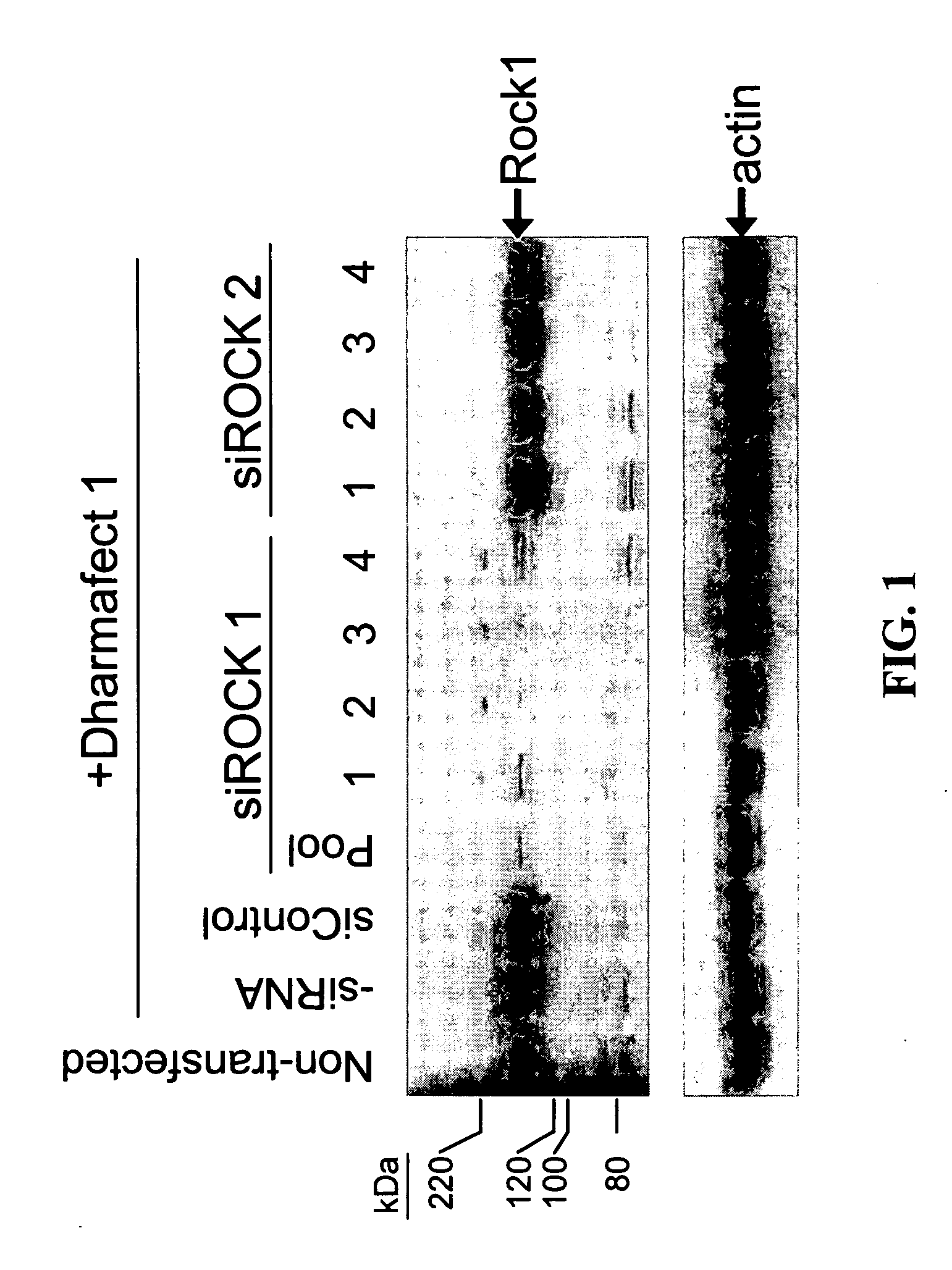
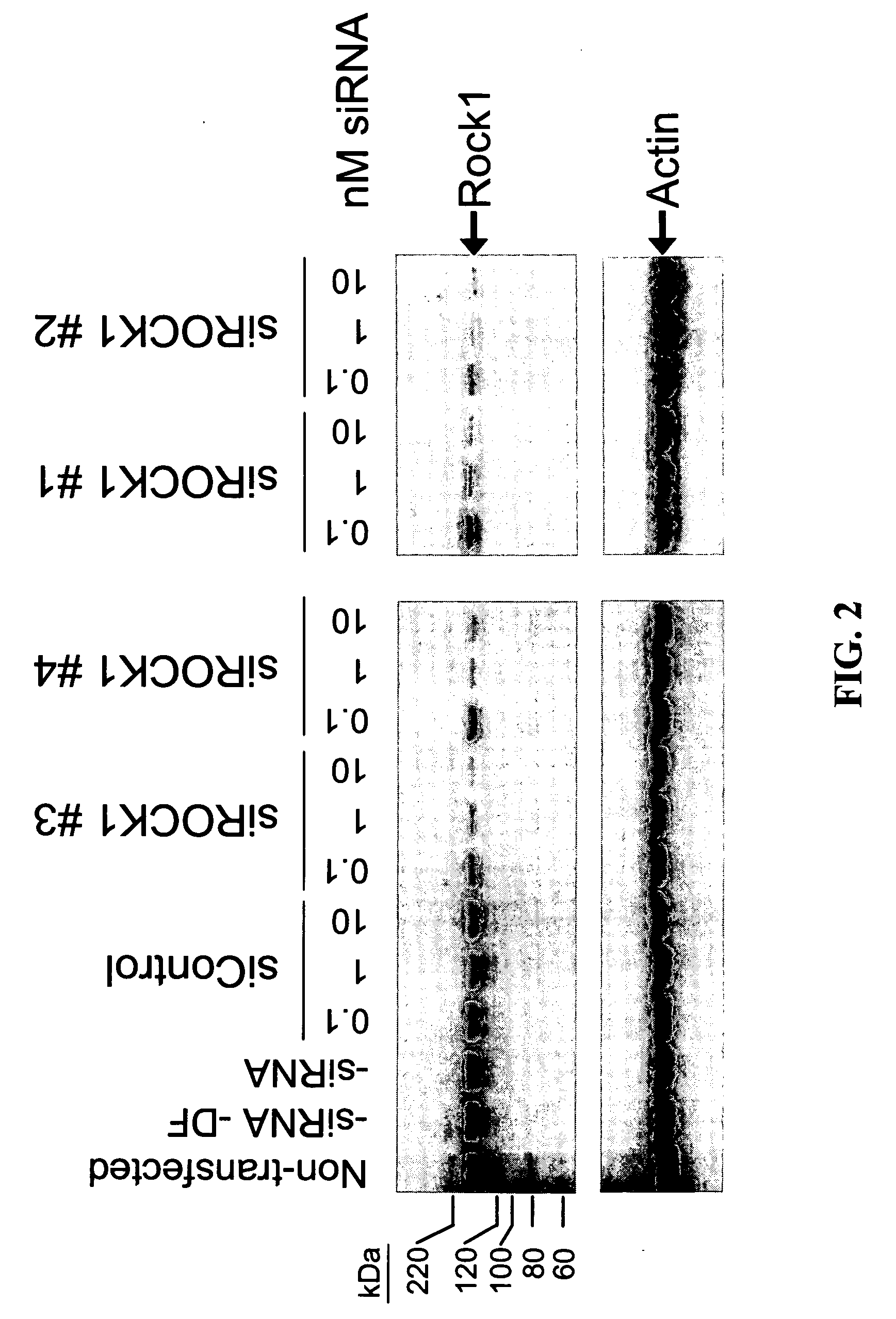
RNAi-mediated inhibition of RHO kinase for treatment of ocular disorders
RNA interference is provided for inhibition of Rho kinase mRNA expression for treating patients with ocular disorders, particularly for treating intraocular pressure, ocular hypertension and glaucoma. Rho kinase mRNA targets include mRNA for ROCK1 and ROCK2.
Owner:ALCON RES LTD
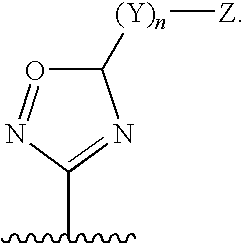
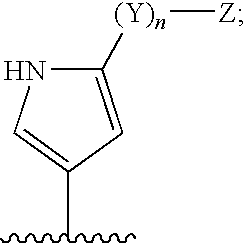
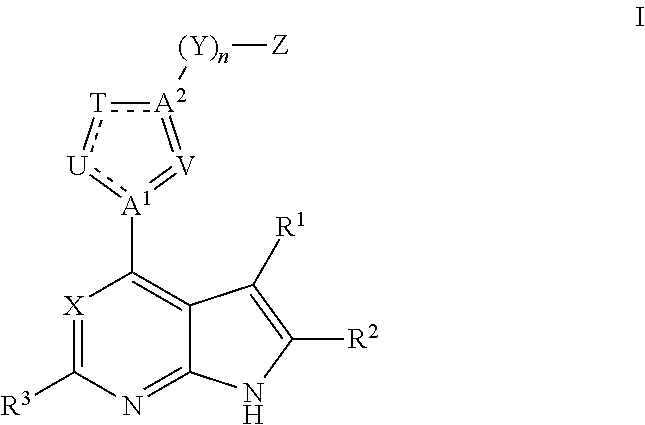
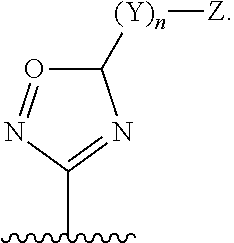
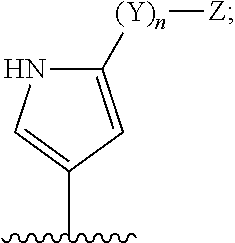
6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS
Methods for using 6-aminoimidazo[1,2-b]pyridazine analogs are disclosed herein to treat rho kinase-mediated diseases or rho kinase-mediated conditions, including controlling intraocular pressure and treating glaucoma, are disclosed. Ophthalmic pharmaceutical compositions useful in the treatment of eye diseases such as glaucoma, and additionally useful for controlling intraocular pressure, the compositions comprising an effective amount of 6-aminoimidazo[1,2-b]pyridazine analogs, are disclosed herein.
Owner:ALCON RES LTD
Methods of therapeutic treatment of eyes and other human tissues using an oxygen-enriched solution
Particular embodiments disclosed herein relate to electrokinetically altered gas-enriched fluids, methods of making the same, systems for making the same and / or methods of treatment utilizing the gas-enriched fluids for eye related conditions and / or diseases. In certain embodiments, the electrokinetically altered gas-enriched fluid is oxygen-enriched water. Certain embodiments relate to cosmetic and / or therapeutic fluids and / or methods of treatment utilizing the fluids to treat a cosmetic and / or therapeutic symptom related to eye conditions and / or diseases.
Owner:REVALESIO CORP
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Anti-Angiogenic Peptides and Methods of Use Thereof
Anti-angiogenic peptides that inhibit activation or proliferation of endothelial cells are disclosed. Such peptides maybe used to inhibit VEGF binding to the VEGFR2 receptor (also known as the kinase domain receptor or KDR) and bFGF binding to its receptor. Such peptides may also be used to inhibit, VEGF, bFGF, or integrin activation of endothelial cells in angiogenesis-associated diseases such as cancer, leukemia, multiple myeloma, inflammatory diseases, eye diseases and skin disorders.
Owner:SOPHERION THERAPEUTICS
Methods and compositions for treating eye disorders
InactiveUS20040234532A1Efficiently provideAntibacterial agentsBacterial antigen ingredientsUveitisViral Conjunctivitis
The present invention provides methods of treating an eye disorder. The methods comprise a step of locally administering a Clostridial toxin to the eye of a patient to treat the disorder. The eye disorder may be associated with an inflammation of the eye, including for example, bacterial conjunctivitis, fungal conjunctivitis, viral conjunctivitis, uveitis, keratic precipitates, macular edema, and inflammation response after intra-ocular lens implantation. The Clostridial toxin may be produced by a Clostridial beratti, a Clostridia butyricum, a Clostridial tetani bacterium and / or a Clostridial botulinum.
Owner:ALLERGAN INC
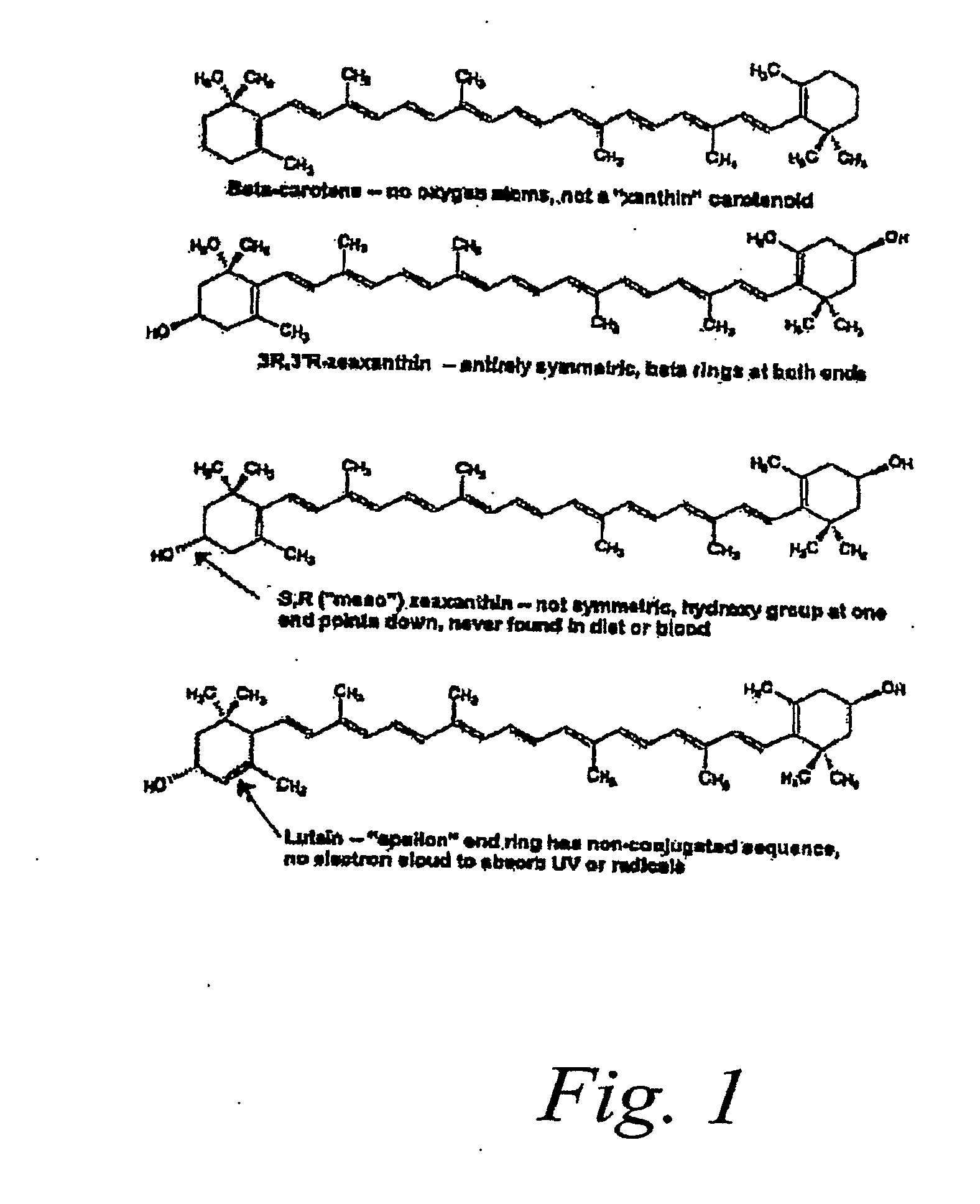
Nutritional supplement for treatment of ocular diseases
A nutritional supplement composition that promotes visual health and reduces or reverses visual acuity loss by a reduced Vitamin E content from standard supplements with the addition of taurine, omega-3 fatty acids, and non proform Vitamin A carotenoids including lutein and zeaxanthin. The essential ingredients of the nutritional or dietary supplements are Vitamin C, no more than 300 IUs of Vitamin E, Vitamin A at least a portion of which is provided in the form of a proform Vitamin A carotenoid, omega-3 fatty acids, and non proform Vitamin A carotenoids including lutein and / or zeaxanthin. The essential ingredients are provided in a form suitable of oral ingestion or other forms of administration in one or more doses per day.
Owner:PAUL JR EDWARD L
Controlled release formulations for the delivery of HIF-1 inhibitors
ActiveUS8962577B2Controlled drug and drug release profileReduce solubilityBiocidePowder deliveryDiseaseSide effect
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Treatment of ocular disease
A formulation to treat ocular conditions such as dry eye disease, as well as other conditions, is disclosed. Rapamycin and / or ascomycin is administered intraocularly, such as by topical application, injection into the eye, or implantation in or on the eye. For example, a topical administration may contain between about 50 pg / ml drug to about 50 μg / ml drug in a formulation which may be applied at bedtime or throughout the day. For injection, a dose of about 50 pg / ml to about 200 μg / ml may be used. Rapamycin and / or ascomycin may also be administered in milligram quantities as a surgical implant, for example, in a diffusible walled reservoir sutured to the wall of the sclera, or may be contained within an inert carrier such as microspheres or liposomes to provide a slow-release drug delivery system.
Owner:PEYMAN GHOLAM A DR
Treatment of ocular disease
InactiveUS20050025810A1Prevent and decrease time of onsetReduce severityBiocideSenses disorderMicrosphereSurgical implant
A formulation to treat ocular conditions such as dry eye disease, as well as other conditions, is disclosed. Rapamycin and / or ascomycin is administered intraocularly, such as by topical application, injection into the eye, or implantation in or on the eye. For example, a topical administration may contain between about 50 pg / ml drug to about 50 μg / ml drug in a formulation which may be applied at bedtime or throughout the day. For injection, a dose of about 50 pg / mi to about 200 μg / ml may be used. Rapamycin and / or ascomycin may also be administered in milligram quantities as a surgical implant, for example, in a diffusible walled reservoir sutured to the wall of the sclera, or may be contained within an inert carrier such as microspheres or liposomes to provide a slow-release drug delivery system.
Owner:PEYMAN GHOLAM A DR
Intraocular delivery compositions and methods
The present invention relates to intraocular drug delivery for treating ocular diseases. Particularly, the invention relates to particles useful for the delivery of certain pharmacologically active agents to treat ocular diseases. The particles contain calcium phosphate core particles, particularly nanoparticles, as delivery agents and adjuvants. The invention also relates to methods of making such particles and to methods of treating ocular disease by delivery of a therapeutic drug to an ocular surface using the particles of this invention. The invention further relates to methods of regulating ocular pressure using certain formulations according to the present invention.
Owner:BIOSANTE PHARMA
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Methods and compositions for treating ocular disorders
InactiveUS20060293270A1Efficient ConcentrationEffective maintenanceGenetic material ingredientsGene therapyCompound (substance)Vascular endothelial growth factor
This invention relates to methods of treating ocular disease. The method of the invention is directed to the administration of an anti-vascular endothelial growth factor (anti-VEGF) compound to treat such disease.
Owner:EYETECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00001.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00002.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00003.png)